Abstract
Objective:
This study aimed to assess Severe Acute Malnutrition (SAM) associated mortality rate of children attending HIV/AIDS care in North West Ethiopia 2009–2019.
Methods:
Institutional-based retrospective cohort study was employed among 721 on antiretroviral therapy care seropositive children since 2009–2019. Data were entered using EpiData version 4.2 and exported to STATA (SE) R-14 version statistical software for further analysis. Beside, Besides, WHO (World Health Organization) Anthro Plus software was used to assess the nutritional status of the children. Bivariable and multivariable Cox regression analyses were conducted to identify the predictors of mortality. Finally, variables with p-value less than 0.05 were considered significant predictors of mortality.
Result:
Overall, 721 (N = 721) seropositive children were included with a mean (±SD) age of 118.4 ± 38.24 months. A median time of death was reported at 19.5 months (interquartile range = ±8.5). The overall mortality rate in this study was determined as 5.4 per 100 child-years (95% confidence interval: 3.6–5.8). Severe stunting (height for age Z-score <−3) (hazard ratio = 2.9, 95% confidence interval: 1.8–6.4), admission with septic shock (hazard ratio = 2.3, 95% confidence interval: 1.2–4.3, p < 0.008), CD4+ count below threshold (hazard ratio = 1.6, 95% confidence interval: 1.19–7.9), and World Health Organization Stages III and IV (hazard ratio = 2.9, 95% confidence interval: 1.8–6.4) were at high risk of mortality.
Conclusion:
A high rate of SAM associated mortality rate within short median (±SD) time to death was reported as compared with previous finding in Ethiopia. Seropositive children presenting with CD4 counts being below a threshold, World Health Organization Stages III and IV, and being nutritionally stunted during antiretroviral therapy initiation were at high risk of early mortality.
Keywords: SAM, children, HIV, mortality rate
Introduction
Malnutrition occurs when an individual fails to take or absorb enough essential nutrients due to different underlying factors, including infection as in HIV)/AIDS. It manifests in three forms—acute (wasting), chronic (stunting), or micronutrient. 1 HIV/AIDS is associated with biological and social factors that affect the individual’s ability to consume, utilize, and acquire food. 2 Once there is an infection with HIV, the patient’s nutritional status declines further, leading to its advanced clinical stage.3,4 Substantial reduction in CD4 count and T-cell concentration through HIV causes physiological changes of microbial translocation (16s DNA) and dysregulated lipid metabolism. 5
More than 2 million children globally are living with HIV infection, and 90% of these reside in sub-Saharan Africa (SSA). 3 Despite the remarkable effects of highly active antiretroviral therapy (HAART) in the reduction of morbidity and mortality rate, 6 all antiretroviral therapy (ART) users may not have equally responded to the therapy. In response to ART, there may be individual variability with some individuals having a slower progression in the recovery rate of immune function and remaining at higher risk of dying from opportunistic infections (OIs).2,7 Globally, 2.1 million of 33 million people living with HIV/AIDS are children aged <15 years,8,9 and 90% of these reside in SSA. 3 HIV increased poor nutrition as a result of poor food intake and fueled nutrient usage from the body 4 and increased the incidence of lethal OI for further hospitalization. 10 HIV-infected children with severe acute malnutrition (SAM) are more likely to present with comorbidities and complications, and lethal OIs such as tuberculosis, persistent diarrhea (due to villous atrophy, disaccharide or monosaccharide intolerance), and oral candidiasis, which contributes to anorexia and poor oral intake. 5
Epidemiologically, the incidence of stunting is declining too slowly, while wasting still has a great impact on too many young children worldwide for hospitalization and further risk of mortality. 11 In 2017, 13.8 million children were wasted, of whom 4 million were severely wasted and admitted for SAM treatment.8,12 In a recent review, more than 30% of severely malnourished children in SSA admitted to inpatient derangement with HIV. 2 Seropositive children with SAM had approximately nine times the risk of death compared with normal children. In cases in most developing countries, the fatality rate remains high after inpatient admissions (20%–60%).2,13 Evidence suggests that even relatively small losses in weight (5%) are associated with a decrease in the survival rate of HIV-infected children. 14
Malnutrition in Ethiopia has long been a silent killing crisis and contributing cause for death in infants and young children. 15 According to the Health and Health-Related Indicators (HHRI) of 2014, SAM was the third leading cause of mortality in Ethiopia and accounted for 8.1% of deaths and 20% of hospital admissions.16 –18 The prevalence of SAM-associated HIV mortality rate ranges from 12.3% to 46.8%.7,11 However, evidence on predictors for SAM-associated mortality of HIV-infected children <15 years of age remains sparse and inconclusive in Ethiopia. 14 Up-to-date and recent evidence on SAM-related mortality predictors after ART initiation is highly needed for nutritional-based programmatic intervention. Thus, this aimed to assess SAM-associated mortality rate of children attending HIV/AIDS care in Northwest Ethiopia, 2009–2019.
Methods
Study setting and area
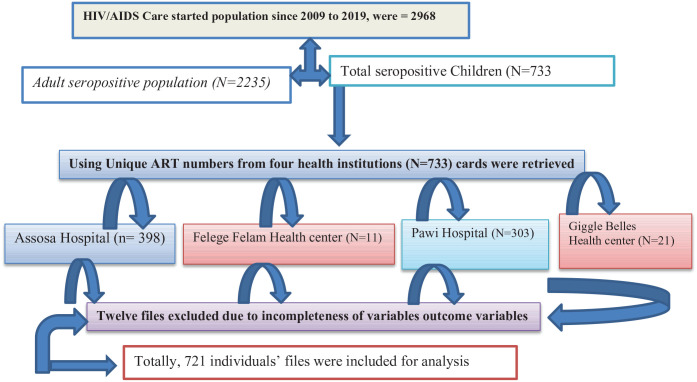
This study was conducted in the Benishangul Gumuz regional state (Assosa is the capital city of Benishangul Gumuz regions) and its surroundings, located about 700 km from Addis Ababa adjacent to the Ethio-Sudan border in the northwest of the country. The region is one of the nine regional administrative states of the country. It shares borders with the Amhara region in the north and northeast, the Sudan Republic in the west, the Gambella region in the south, and the Oromia region in the southeast. 19 About 30% of the population live below the national poverty level; hence, the region is characterized as emerging due to its limited infrastructure, including health service facilities. Like other regions in Ethiopia, the region is subdivided into three administrative zones, namely, Assosa, Metekel, and Kemashi. 1 Metekel zone is one of the three administrative zones found in the Benishangul Gumuz region in Northwest Ethiopia between 34°10′N and 37°40′E and in the latitude 09°17′N and 12°06′N. This zone had seven administrative districts with two primary and one referral hospitals.19,20 Gilgal Belles is the capital of Metekel zone, which is located at 565 and 394 km away from Addis Ababa and Assosa, Northwest of Ethiopia, on the way of Great Ethiopian Renaissance Dam (GERD) at Abay River.1,20 This is the largest covering 26,272 km2, which is half the area of the region. The region has two referral hospitals (Assosa and Pawe Referral hospitals) and 32 health centers. This study was conducted in two health centers (Gilgal Belese and Felege Selam Health center = 2007), and two referral hospitals (Assosa Referral Hospital from Assosa zone = 2007 and Pawe Referral Hospitals from Metekel zone = 2007) were included. Apart from other health services, all these health institutions have been providing ART care service since 2007 for an estimated 2968 catchment population19,20 (Figure 1).
Figure 1.
Schematic presentation of sampling procedures for the study of seropositive children.
Study design
A multicenter institution-based retrospective cohort study was conducted in 721 HIV-infected children <15 years of age registered on nutritional SMART care service in two hospitals and two health centers from 2009 to 2019.
Study populations
All recoded pediatrics patients below the age of 15 years who received ART and started care in four selected public health facilities were considered as source population. All HIV-infected children who had at least 1 month of ART follow-up on the undernutrition registration book from 1 January 2009 to 31 December 2019 were included. Individual files with incomplete data on nutritional status, such as weight, height, and age, and/or ART treatment outcome were excluded from the study. The intake form registration or follow-up form that lacks the aforementioned variables was excluded. Cases, which transferred to other facilities (transferee out and confirmed accidental death due to injury, unrelated competing causes of death), were excluded.
Sample size determination and sampling techniques
The sample size was calculated using the formula for survival analysis by Moore and Moore 21 considering two-sided significance level (α = 5%); Za/2 = Z value at 95% confidence interval (CI) = 1.96, power (ZB) = 80%, and P = % cumulative occurrence of death rate, 1.73 HR 22
| (1) |
where alpha (α) = 0.05, beta (β) = 0.2, AHR = hazard ratio, E = number of event, N (sample size) = E/P (E), where P (E) = probability of event and P = cumulative occurrence of treatment failure valued from reference for sample size calculation. 23 The final sample size was determined as 512.5 after adding 15% contingency for incompleteness. However, from 30 January 2016 to 31 December 2020, 738 charts were there in four institutions. Since it is manageable in resources, we set up and included all 721 charts without the sampling procedure.
Outcome ascertainment
The dependent variable was the death of SAM-admitted children after HIV/AIDS care started, where it is not due to accidental causes. Those records or ART clients who developed death without SAM admission were not included as event of interest for this study; rather, they were counted as censored data. The independent variables included age and sex of children, residence, marital status of caregiver, family size, educational status of the caregiver, World Health Organization (WHO) clinical staging, CD4 count, Hgb, viral load, functional infection and OI, ART regimens types, isoniazid preventive therapy (IPT), cotrimoxazole preventive therapy (CPT), and adherence status of children, weight for age (WFA), height for age (HFA), and weight for height (WFH).
Operational words
SAM is defined anthropometrically by the WHO as a WFH ⩽ −3 Z-score, or mid-upper-arm circumference (MUAC) < 115 mm, or the presence of bilateral edema with failed appetite test. 24 Undernutrition is defined as a child having one of the following descriptions: H/Age Z-score < −2, or W/Age Z-score < −2, or W/H Z-score < −2 SD.8,25 Moreover, the detailed description is given here: moderate underweight was defined as children having W/Age Z-score < −2 SD or severe underweight as children having W/Age Z-score < −3 SD. Moderate stunting was defined as children having H/Age Z-score < −2 SD. Severe stunting was defined as children having H/Age Z-score < −3 SD.8,18 Moderate wasting was defined as children having W/H Z-score < −2 SD. Severe wasting was defined as children having W/H Z-score < −3 SD.8,25 A CD4 count: CD4 below the threshold level was classified based on the age of the child (i.e. infants, CD4 < 1500 mm−3; 12–35 months, <750 mm−3; 36–59 months, <350 mm−3; and ⩾5 years, <200 mm−3). 26 Seropositive children: According to the Federal Ministry of Health (FMOH) Continuum of HIV services referring to a comprehensive package of HIV prevention, diagnostic, treatment, care, and support services provided for people at risk of HIV infection or those living with HIV and their families, children aged < 15 years were classified as seropositive children.26,27
Data collection instruments
A standard and pretested data extraction form was used to extract the required information from Ethiopia’s Federal Ministry of Health Pediatrics ART follow-up. 28 Before the actual data collection, the prepared checklists were pretested on 28 case notes of follow-up of HIV-positive children from Jawi Hospital. The 2-day training was given for six diploma nurses and three degree public health officers on the objective of the study, variables of interest, and maintaining data confidentiality. Strict follow-up and supervision were carried out during data collection by the principal investigators, and feedback was given on a daily basis. The collected data were checked for inconsistencies, coding errors, completeness, accuracy, and missing values.
Data collection procedures and quality assurance
To assure the quality of data, data collectors and supervisors were trained about how and what information they should collect from the medical records for 1 day. The checklist was pretested on 5% of randomly selected charts, which were not included in the actual study. After the pretest, necessary modification of the data collection tool was made. Strict follow-up and supervision were carried out during data collection by the principal investigators, and feedback was given daily. Individual records with incomplete data during data collection were excluded. The collected data were first being checked and cleaned for completeness.
Statistical analyses
Data were entered using EpiData version 4.2 statistical software and exported to STATA (SE) R-14 version statistical software for further analysis. The WHO AnthroPlus Version 1.04 and Emergency Nutrition Assessment (ENA) for SMART Software were used to generate the Z-score (weight for age Z-score (WAZ), height for age Z-score (HAZ), weight for height Z-score (WHZ)/BAZ) to define the nutritional status of seropositive children. We used the Cox regression hazards model to estimate mortality incidence and predictors during risky successive follow-up. Kaplan–Meier survival analysis was used to determine the cumulative probability of death for all seropositive children and the mean time to death. Variable with p-value less than 0.25 in bivariable Cox regression analysis was included in the multivariable Cox regression model. We tested the assumptions of Cox proportional hazards models using Schoenfeld residuals. Variables with an adjusted hazard ratio and 95% CI and a p-value less than 0.05 were claimed as significant predictors of SAM-associated mortality rate of seropositive children.
Result
Sociodemographic characteristics of seropositive children
As depicted in Figure 1, 732 (N = 733) seropositive children who were initiated ART care were reviewed. During the data extraction process, 12 pediatrics files were excluded due to incomplete documentation, and the overall response rate of the extracted data was 721 (99.6%). Among the 721 records included in the final analysis, about half (384–53.26%) were females, and the majority (510–70.74%) were from urban areas. The largest percentage of children was categorized under the age group of ⩾10 years which accounted for 389 (53.96%) of the total subjects with a mean (±SD) age of 9.83 ± 3.3 years. The majority (290–79.89%) of the children living with HIV were in urban residences. The larger proportion—that is, 381 (52.84%) and 337 (46.74%) of caregivers—had both parents alive and occupationally were merchants, respectively. Regarding the family size of children, the majority—462 (64.08%) and 32 (4.44%)—of the participants’ children live in with 3–4 and ⩾4 family members, respectively (Table 1).
Table 1.
Baseline sociodemographic characteristic of HIV-positive children attending ART care in a selected public health facility in Benishangul Gumuz, Northwest Ethiopia (n = 721).
| Variables | Categories | Frequency | % |
|---|---|---|---|
| Sex | Male | 337 | 46.7 |
| Female | 384 | 53.3 | |
| Age of children (years) | ⩾5 | 78 | 10.8 |
| 6–10 | 254 | 35.2 | |
| 11–15 | 389 | 53.9 | |
| HIV disclosure status of children | Disclosed | 158 | 21.9 |
| Not disclosed | 563 | 78.1 | |
| Age of caregivers (years) | ⩽45 | 244 | 38.8 |
| <45 | 477 | 66.1 | |
| Resident | Urban | 510 | 70.8 |
| Rural | 211 | 29.3 | |
| Marital status of the caregiver | Single | 115 | 15.9 |
| Married | 498 | 69.1 | |
| Divorced | 82 | 11.4 | |
| Widowed | 26 | 3.6 | |
| Family size of caregivers | ⩽2 | 227 | 31.4 |
| 3–5 | 462 | 64.1 | |
| ⩾6 | 32 | 4.4 | |
| HIV status of caregivers | Positive | 550 | 76.3 |
| Negative | 91 | 12.6 | |
| Unknown | 80 | 11.1 | |
| Religions of caregivers | Orthodox | 381 | 52.8 |
| Muslim | 152 | 21.2 | |
| Protestant | 139 | 19.3 | |
| Catholics | 49 | 6.8 | |
| Occupational status of caregivers | Farmer | 99 | 13.7 |
| Merchant | 337 | 46.7 | |
| Employer | 124 | 17.2 | |
| Laborer worker | 161 | 22.3 | |
| Parental status of care children | Both alive | 381 | 52.8 |
| Paternal orphan | 135 | 18.2 | |
| Maternal orphan | 108 | 14.9 | |
| Both orphaned | 97 | 13.5 |
Clinical and medication characteristics
From the total 721 children participants, more than one-third, 258 (35.78%), of cases had at least one form of medical OI. The most frequent OIs were diarrheal disease and bacterial pneumonia in 107 (41.47%) and 100 (38.7%) children, respectively. About 156 (64.2%) children started ART based on the WHO criteria of being below 15 years of age, while others were admitted based on CD4 or WHO staging criteria with a median age of ART starting at the age of 5 years. The majority of children, 293 (40.64%), were on AZT-3TC-NVP of ART regimen with a mean baseline hemoglobin (Hgb) of 11.08 g dL−1 and CD4 count of 669.456 cells mm−3. A total of 237 (33.1%) and 202 (28.02%) children on ART were on WHO Stages I and II during enrollment in chronic HIV care, while the remaining 282 (39.1%) cases were on Stages III and IV. Concerning the ART adherence at the time of the last ART visit, 356 (49.4%) had good ART adherence, while 188 (26.3%) of them had poor adherence. Nearly two in five, 295 (40.1%), cases had 36–72 months of follow-up. More than half, 419 (58.2%), of the participant children took CPT, whereas 270 (37.45%) did not take IPT. Initially, nearly one-fifth (19.9%) of participant children were moderately underweight, 21.40% were moderately stunted, and 16.6% were moderately wasted. In addition, the proportion of cases presenting with nutritional were classified as severe stunting (19.7%), severe wasting (9.9%), and severe underweight (11.8%) (Table 2).
Table 2.
Clinical and hematologic characteristics of seropositive children attending ART care in Benishangul Gumuz public health facility from 2009 up to 2020.
| Variables | Categories | No. | Frequency |
|---|---|---|---|
| Dietary counseling during follow-up | Yes | 465 | 64.5 |
| No | 256 | 35.51 | |
| Admission history of SAM | Yes | 124 | 17.35 |
| No | 597 | 82.6 | |
| Opportunistic infection (OIs at baseline) | Yes | 258 | 35.8 |
| No | 453 | 64.2 | |
| Types of ART regiment | D4t-3TC-NVP | 48 | 6.66 |
| D4t-3TC-EFV | 26 | 3.6 | |
| AZT-3TC-NVP | 293 | 40.6 | |
| AZT-3TC-EFV | 165 | 22.8 | |
| TDF-3TC-EFV | 104 | 14.43 | |
| AZT-3TC-LPV/R | 36 | 4.99 | |
| ABC-3TC-NVP | 25 | 3.5 | |
| ABC-3TC-EFV | 24 | 3.3 | |
| ART regimen change | Yes | 211 | 29.3 |
| No | 510 | 70.74 | |
| Functional status (age ⩽5 years) | Appropriate | 69 | 71.98 |
| Delay | 15 | 15.63 | |
| Regression | 12 | 12.5 | |
| Developmental history (age >5 years) | Working | 488 | 77.96 |
| Ambulatory | 87 | 13.90 | |
| Bedridden | 51 | 8.15 | |
| Adherence | Good | 356 | 49.4 |
| Fair | 177 | 24.55 | |
| Poor | 188 | 26.1 | |
| WHO clinical stage | I | 237 | 32.87 |
| II | 202 | 28.1 | |
| III | 170 | 23.6 | |
| IV | 112 | 15.5 | |
| IPT | Yes | 451 | 62.5 |
| No | 270 | 37.45 | |
| CPT | Yes | 419 | 58.1 |
| No | 302 | 41.9 | |
| CD4 count or percent | Below the threshold | 308 | 42.7 |
| Above threshold | 413 | 57.3 | |
| Hemoglobin level (g dL−1) | ⩽10 | 229 | 31.78 |
| >10 | 492 | 68.24 | |
| ART eligibility criteria | Immunologic | 81 | 11.3 |
| Clinical stage | 96 | 13.3 | |
| WHO clinical stage | 79 | 10.96 | |
| By CD4 threshold | 199 | 27.60 | |
| Testing and treat | 266 | 36.89 | |
| Types of opportunistic other than TB | Bacterial pneumonia | 79 | 30.6 |
| Diarrhea | 74 | 28.8 | |
| Meningitis | 9 | 3.6 | |
| PCP | 6 | 2.33 | |
| Skin dermatitis | 7 | 2.7 | |
| Kaposi’s sarcoma | 5 | 1.9 | |
| Acute/chronic otitis media | 9 | 3.5 | |
| Others | 3 | 1.18 | |
| TB | 66 | 9.15 | |
| Duration on ART (months) | ⩽36 | 223 | 30.93 |
| 36–72 | 295 | 40.92 | |
| ⩾72 | 203 | 28.16 | |
| Current status of children | On follow-up | 539 | 74.76 |
| Transferred into the adult cohort | 68 | 9.43 | |
| Lost from follow-up | 13 | 1.8 | |
| Drop | 11 | 1.53 | |
| Died | 90 | 12.48 | |
| Maternal PMTC follow-up history | Yes | 487 | 67.6 |
| No | 234 | 32.4 | |
| MUSIC (CM) | ⩽11.5 | 270 | 37.45 |
| >11.5 | 451 | 62.55 | |
| Tuberculosis treatment history | Yes | 66 | 9.15 |
| No | 655 | 90.85 | |
| Underweight (WFA) | Normal | 491 | 68.1 |
| WAZ < −2 | 144 | 19.9 | |
| WAZ < −3 | 86 | 11.93 | |
| Stunting (HFA) | Normal | 428 | 59.4 |
| HAZ < −2 | 154 | 21.4 | |
| HAZ < −3 | 139 | 19.2 | |
| Wasting (WFH) | Normal | 531 | 73.5 |
| WHZ < BAZ−2 | 119 | 16.6 | |
| WHZ or BAZ < −3 | 71 | 9.9 | |
| Underweight (WFA) | Normal | 491 | 68.1 |
| WAZ < −2 | 144 | 19.9 | |
| WAZ < −3 | 86 | 11.93 | |
| Survival status of children | Died | 90 | 12.07 |
| Survived | 634 | 87.93 |
SAM: severe acute malnutrition; ART: antiretroviral therapy; WHO: World Health Organization; WFA: weight for age; WFH: weight for height; HAZ: height for age Z-score; WAZ: weight for age Z-score; WHZ: weight for height Z-score; WHO: World Health Organization.
SAM-associated mortality rate of seropositive children
Nearly 1 (17.2%) in 5 of 124 participant cases had SAM diagnosed and admitted in stabilizing centers for inpatient treatment. Of the total 124 SAM with HIV cases, 72.58% (N = 90/124) were dead. Of the total 124 SAM cases, the majority, 52.4% (N = 64/124), of them were male in gender and 34.8% (N = 42/124) were within 5–10 years of age. The overall SAM-associated HIV-infected children’s mortality rate was determined as 5.4 per 100 child-years (95% CI: 3.6–5.8) (Table 3).
Table 3.
Incidence density rate of SAM-associated mortalityon selected soci-odemographic variables among among HIV infected children received ARTat selected Health institutionattending ART from 2009–2020 North west Ethiopia (N = 721).
| Variables | Categories | SAM with HIV admitted cases (N = 124) | SAM with HIV associated Death (N = 90) | Survivors (n = 631) | PPM = (16,668.1M) | IDR/100 months | 95% CI |
|---|---|---|---|---|---|---|---|
| Sex | Male | 65/124 (52.4%) | 50 (55.5%) | 289 (45.8%) | 8288.7363 | 0.60322 | 0.4578–0.7983 |
| Female | 59/124 (47.6%) | 40 (44.4%) | 342 (54.2%) | 8379.3403 | 0.46543 | 0.340–0.6372 | |
| Resident | Urban | 70/124 (56.45%) | 26 (28.8%) | 480 (76.1%) | 11397.498 | 0.22812 | 0.15532–0.33504 |
| Rural | 54/124 (43.5%) | 64 (71.1%) | 151 (23.9%) | 5270.579 | 1.19531 | 0.9337–1.5301 | |
| Age (years) | ⩽5 | 33/124 (26.6%) | 37 (41.1%) | 56 (8.81%) | 2760.6287 | 1.34027 | 0.97108–1.84982 |
| 6–10 | 42/124 (33.8%) | 26 (28.8%) | 247 (39.2%) | 6070.364 | 0.41183 | 0.27828–0.60949 | |
| 11–15 | 49/124 (39.5%) | 27 (30%) | 328 (51.9%) | 7837.0839 | 0.34451 | 0.2362–0.50237 |
SAM: severe acute malnutrition; CI: confidence interval, PPM: person per month, IDR: Incidence density rate.
Survival and hazard estimate of cohorts
The study participants were followed for 16,668.077 Person per Month (PMOS) of total analysis of mortality under risk observation. At the end of the follow-up, more than half (74.79%, n = 539) were on follow-up, 1.86% (n = 13) were lost to follow-up, 9.40% (n = 68) were transferred into adult cohort, 1.53% (n = 11) dropped out, and the remaining 12.48% (n = 88) died. A median time of death reported and median survival probability were determined as 19.5 months (interquartile range (IQR) = ±8.5 months) and 92.9% (CI: 91.59–95.6), respectively. Regarding the time for death, 11.2%, 48.2%, and 81.6% mortality propertions of particicpant cases was reported 1, 12, 24, 48, and 72 months after ART initiated, respectively.
Log-rank test for the mortality difference
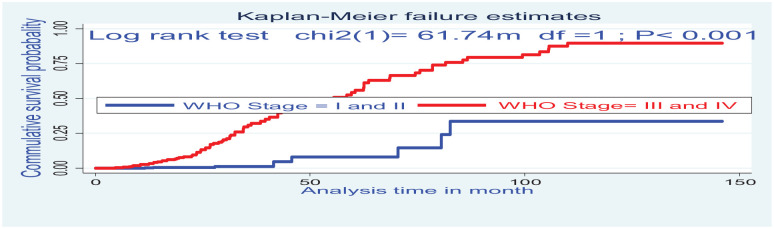
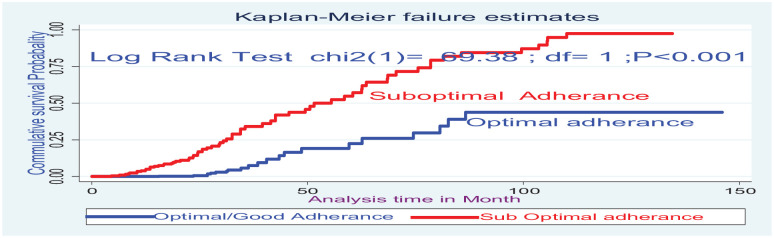
The Kaplan–Meier survival curve together with the log-rank test shows differences in hazards of death on different covariates. Cases at baseline with anemia (Hgb <10 mg dL−1), WHO clinical stage (III and IV), and CD4 below threshold had statistical survival differences between undernourished and counter groups (Figures 2 and 3).
Figure 2.
The Kaplan–Meier failure estimate curves to compare the hazard of death for HIV-positive children on ART on WHO clinical stages, Northwest Ethiopia, 2020.
Figure 3.
The Kaplan–Meier failure estimate curves to compare the hazard of death for HIV-positive children on ART on ART adherence, Northwest, Ethiopia, 2021.
Bivariable and multivariable Cox regression analysis
In variables, adjusted regression analysis factors with a p-value ⩽0.25 were sex, age, CD4, WHO clinical stage, adherence to ART, Hgb, family size, duration of follow-up, CPT, IPT, wasting, stunting, MUAC, OI, regiment type, caregiver income, and dietary diversity counseling which was a candidate for multivariable Cox regression. Finally, after adjustment for certain confounding, four variables were independently coupled with SAM-associated mortality incidence of seropositive children. Hence, participant children with baseline CD4+ count below the threshold were 2.6 (AHR = 2.6, 95% CI: 1.2–7.9) times at increased risks of death compared with children with CD4+ count above the threshold. Similarly, cases who presented with WHO Stages III and IV were 2.9 (AHR = 2.9, 95% CI: 1.8–6.4) times at increased hazard of death during successive follow-up compared with WHO clinical Stages I and II. Moreover, the risk of mortality for malnutrition-diagnosed seropositive children with baseline septicemia shock had fold (AHR = 2.3, 95% CI: 1.2–4.3, p < 0.008) increased the risk of death compared with counter groups. Participant cases presenting with severe stunting at the beginning of ART had 2.9 (AHR = 2.9, 95% CI: 1.8–6.4) times death hazard compared with non-stunted children (Table 4).
Table 4.
Bivariable and multivariate Cox regression analysis for SAM-associated mortality rate of children attending HIV/AIDS care in Northwest Ethiopia, 2009–2019.
| Variables | Categories | Survival status | CHR | AHR | p | |
|---|---|---|---|---|---|---|
| Died | Censored | |||||
| Sex | Male | 35 | 302 | Ref. | Ref. | |
| Female | 56 | 328 | 1.3 (0.88–2.07) | 1.7 (0.7–1.7) | 0.28 | |
| Age (years) | ⩽5 | 29 | 49 | 2.8 (1.7–4.6) | 1.7 (0.79–3.2) | 0.17 |
| 6–10 | 23 | 231 | 1.2 (0.79–2.11) | 2.5 (0.93–4.5) | 0.052 | |
| 11–15 | 38 | 351 | Ref. | Ref. | ||
| Residence | Urban | 61 | 449 | 1.16 (0.73–1.87) | 1.12 (0.7–1.85) | 0.65 |
| Rural | 29 | 182 | Ref. | Ref. | ||
| CD4 count | Below threshold | 70 | 238 | 3.12 (1.9–7.2) | 2.6 (1.2–7.85) | 0.018* |
| Above threshold | 20 | 393 | Ref. | Ref. | ||
| WHO stage | I and II | 8 | 435 | Ref. | Ref. | |
| III and IV | 82 | 196 | 10.9 (5.2–22.7) | 4.5 (2.8–8.4) | 0.001* | |
| Adherence | Optimal adherence | 21 | 489 | Ref. | Ref. | |
| Suboptimal adherence | 69 | 142 | 6.3 (3.8–10.3 | 1.7 (0.6–2.9) | 0.53 | |
| Dietary counseling | Yes | 44 | 421 | Ref. | Ref. | |
| No | 46 | 210 | 1.8 (1.5–2.5) | 1.9 (0.5–1.5) | 0.19 | |
| Disclosure status | Yes | 54 | 104 | 3.3 (2.3–5.1) | 1.7 (0.59–2.8) | 0.43 |
| No | 36 | 527 | Ref. | Ref. | ||
| Duration of follow-up (months) | ⩽36 | 6 | 217 | Ref. | Ref. | |
| 36–72 | 19 | 276 | 2.1 ( 0.9–5.1) | 2.1 (0.7–5.7) | 0.18 | |
| ⩾72 | 65 | 138 | 7.1 (3.1–16.3) | 2.6 (0.8–3.6) | 0.07 | |
| Wasting | Normal | 70 | 461 | Ref. | Ref. | |
| WHZ or BAZ < −2 | 18 | 101 | 9.6 (1.28–12.6) | 0.8 (0.18–4.3) | 0.58 | |
| WHZ or BAZ < −3 | 2 | 69 | 7.8 (1.8–16.3) | 1.07 (0.5–1.9) | 0.25 | |
| Underweight | Normal | 34 | 457 | Ref. | Ref. | |
| WAZ < −2 | 18 | 126 | 1.6 (0.91–2.91) | 0.6 (0.3–1.17) | 0.13 | |
| WAZ < −3 | 38 | 48 | 2.9 (1.8–4.82) | 1.2 (0.6–2.1) | 0.69 | |
| Stunting | Normal | 18 | 410 | Ref. | Ref. | |
| HAZ < −2 | 29 | 125 | 5.6 (2.5–8.4) | 1.6 (0.8–3.2) | 0.15 | |
| HAZ < −3 | 43 | 96 | 4.6 (3.2–9.9) | 2.9 (1.8–6.4) | 0.01* | |
| MUAC (mm) | ⩽11.5 | 56 | 214 | 4.5 (2.1–6.1) | 1.5 (0.6–4.2) | 0.11 |
| >11.5 | 34 | 417 | Ref. | Ref. | ||
| CPT | Yes | 17 | 402 | Ref. | Ref. | |
| No | 73 | 229 | 2.5 (1.6–7.6) | 1.2 (0.43–4.5) | 0.29 | |
| Septic shock | Yes | 46 | 57 | 3.8 (2.5–5.8) | 2.3 (1.2–4.3) | 0.008* |
| No | 44 | 574 | Ref. | Ref. | ||
WHO: World Health Organization; CPT: cotrimoxazole preventive therapy; MUAC: mid-upper-arm circumference.
indicate stastistically significant variables.
Discussion
SAM is one of the most common problems for children living with HIV/AIDS and contributes to premature death and escalated the incidence of lethal OI. 6 At the end of this study, more than half (74.79%, n = 539) of the participant cases were on follow-up, 1.86% (n = 13) absconded or were lost to follow-up, 9.40% (n = 68) were transferred into the adult cohort, 1.53% (n = 11) dropped out, and the remaining 12.48% (n = 90) died.
The overall crude death rate of HIV-infected SAM cases (N = 90/721) in our study (12.48%; n = 90) was lower compared to other studies in Ethiopia, such as in Amhara regional hospitals (30%), 22 Northwest Ethiopia (46.2%), 8 Woldia hospital (30.8%), 17 and Burkina Faso (39.7%). 13 Nevertheless, the finding of this report is higher than the result in Wolkite Hospital (5.3%). 7 Similar associations with contemporary clinical trial among African seropositive children with severe edematous malnutrition were significantly increased with subsequent ART initiation. In the Anti-Retroviral Research for Watoto (ARROW) clinical trial, 3.03% of 1207 African children were hospitalized for SAM treatment within 2 months after ART initiation.5,29 The immune recovery usually occurred rapidly in HIV-infected children after ART initiation and experienced profound benefit from ART in a subset clinically worsen of IRIS recovery—a paradoxical infectious or inflammatory condition in a patient recovering from their severe immune reconstitution deficiency. This is also directly correlated with our reports; at 24 months after ART, more than half of (46/90, 51.2%) the death were reported with a median time for death reported at 19.5 months (IQR = ±8.5).
The relation of malnutrition and HIV/AIDS-associated mortality was more complex and modified on factor escalated for mortality rate. Similar to the finding registered in Abdu et al. 7 and Animut et al., 30 a high burden of malnutrition-associated death for seropositive children of male gender and rural resident was observed; a majority of 50 (55.5%) and 64 (71.1%) participants’ mortality cases in our research also occurred in male and rural children, respectively. Furthermore, the inpatient mortality incidence rate (IDR) of SAM among HIV-infected children in our report was 5.4 per 100 child-years (95% CI: 3.6–5.8), which was higher than in Amhara regional hospitals (3.2%), 30 but comparable with finding in Northwest Ethiopia (4.4%) 8 and national data reviewing of 24 months (5%–8%).7,26 This might be mortality incidence after SAM hospitalization for children living with HIV/AIDS can precipitate from several factors—the infection itself, with lack of immunological and virological controls. 31 However, this report is inconsistent with 11.2% of findings of Southern Ethiopia 7 and 8.8% in central Addis Ababa. 8 This difference might be due to the differences in the discrepancy of the sample size of the study population or the health professional’s poor adherence to inpatients; SAM therapeutic guidelines 32 might also have worthless increased 50% inpatient death. 33 Alternatively, it could be due to disparity of socioeconomic besides home feeding styles of different ethnic groups of the country and healthcare service differences in different health institutions. Mortality relative risks of malnutrition-associated seropositive children have positive association. In this study, participant case having CD4 cell count below the threshold had 2.6 (AHR = 2.6, 95% CI: 1.2–7.9) times increased death hazard compared with children with CD4 cell count above the threshold at baseline. This is consistent with another report in Ethiopia,7,11,30,34 which all indicated that below threshold CD4 count after ART initiation was significantly associated with mortality incidence of SAM within AIDS co-infected children. Moreover, consistent with a study finding from Burkina Faso 13 and Nigeria, 35 the risk of mortality for SAM of HIV-diagnosed children at admission who developed septic shock had nearly twofold (AHR = 1.7, 95% CI: 1.6–2.9, p < 0.013) increased hazards of death than their negative (counter) peers. Multiple infections and metabolic complications have synergism on death occurrence in severely malnourished children. Rationally, malnutrition was one of the preliminary causes for incidence of OI, which hastened a sharp reduction in CD4 count and survival probabilities. 20 Preferentially when nutritionally the body became malnourished, CD4+ and CD8+ T-cells are important in controlling HIV infection; they will be deleted, and stimulations of cytokines, such as TNFα, IL-6, IL-10, and IFNγ, from the lymphoid tissue, paradoxically, will help propagation and advancement of HIV infection. Another finding in this study, death hazard for HIV-positive children with advanced clinical diseases (WHO Stage III and IV), was significantly associated with mortality incidence. This is consistent with the finding in West Shewa, Ethiopia, 36 Malawi, 37 South Africa, 38 and Eastern India. 39 The risk of developing and recurrence of OI are double-fold increased with the advancement of HIV disease; furthermore, fatal incidence of OIs and then death will be inevitable. Malnutrition in HIV-positive children is rapidly ameliorated if they are given adequate foods in the right frequency and diversity with ART. 11 However, household food insecurity undermines such efforts; even in food- and nutrition-rich area, people have undernutrition and are inevitably exposed to be stunted.3,40 In this study, baseline severe stunting was another crucial predictor of mortality incidence for both HIV/AIDS and SAM co-infected children. Children who were severely stunted after ART had higher risks of mortality incidence compared with seropositive children who do not have both infections. This is consistent with the finding in Northwest Ethiopia, 8 Tanzania, 41 and Burkina Faso. 13 Rationally, malnutrition is an inevitable end-stage complication of HIV infection. Severe stunting is associated with a weakened immune system and complication of the late treatment of intestinal parasite, further resulting in long-term complication on the ability to absorb various nutrients (dysregulated lipid metabolism and proteolysis) into the body. In some instance of this study, children who had recurrent oral lesions were more likely to be stunted and highly exposed to death. This is because children with oral lesions have difficulty swallowing, which leads to nutritional imbalance less than body requirement; the gastrointestinal tract is the largest lymphoid organ in the body and is directly affected by HIV infection and causes damage to the intestinal cells by causing villous flattening and decreased D-xylose absorption. This leads to carbohydrate and fat malabsorption, thereby affecting fat-soluble vitamins, such as vitamins A and E, which are important for the proper functioning of the immune system. Whereas larger amounts of nutrients are required during fever and infections that accompany an HIV infection, they are utilized poorly by the body. This leads to loss of weight and lean muscle tissue, further causing damage to the immune system and reducing food intake and nutrient absorption, also causing disruption of metabolism, chronic infections, muscle wasting, or loss in lean body tissue.3,36
Limitation of the study
This study should be seen in light of some limitations, namely, the secondary nature and missed important variables, such as household food security status of the caregiver, and had a high impact on malnutrition assessment of children. A further prospective study on a large size of study participants or randomized clinical trials is needed to better understand the actual predictors of undernourishment—both macro- and micronutrients among children living with HIV for evidence-based action.
Conclusion
In our study, high SAM-associated mortality rate of HIV-infected children was reported compared with previous findings in Ethiopia. Participant cases presenting with a baseline CD4 count below the threshold, WHO clinical Stages III and IV, being severely stunted, and having a history of shocked during admission had a higher risk of mortality. This calls the government to give due attention and strengthen HIV/AIDS treatment, care, and time-relevant nutritional support for the risky individual.
Research Data
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (https://creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
sj-docx-2-smo-10.1177_20503121221081337 for Severe Acute Malnutrition (SAM) associated mortality rate of children attending HIV/AIDS care in North West Ethiopia, 2009–2019 by Fassikaw Kebede in SAGE Open Medicine
Supplemental material, sj-dta-1-smo-10.1177_20503121221081337 for Severe Acute Malnutrition (SAM) associated mortality rate of children attending HIV/AIDS care in North West Ethiopia, 2009–2019 by Fassikaw Kebede in SAGE Open Medicine
Acknowledgments
The success of this study was not thinkable without the help of my family members in financial support during data collection. We would like also to thank the data collectors and supervisors for their unreserved cooperation during data collection. We would like to thank Pawe referral Hospital administrative staff members, especially Mr. Nemera Ethicha Bekonjo (chief manager of the Pawe hospitals) for his invaluable and unreserved cooperation during data collection. Finaly we/ I would like to thanks Gilgal Belese Health center, Felege SelamHealth center Assosa Hospitals and Pawe Hospitals; administrativestaffe members for their permission forthe data extractions of this research.
Footnotes
Author contributions: F.K. conceived the study and supervised the data during the collection processes. In addition, F.K. analyzed the software and wrote the original article. Finally, the authors commented, edited the draft, and approved the final version to be submitted.
Availability of data and materials: All relevant data within the article are in the hand of the main author with a reasonable request.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: The institutional review board (IRB) of Debre Markos University College of Medicine and Health Science School of Public Health graduate class approved this study by Ref. No: HSC/984/16/12. A formal letter was submitted for all selected health institution for data collection permission with meeting number 001/2910.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from every legally authorized representative of participant cases after the purpose and risks fully explained by their local language prior to the start of the study.
ORCID iD: Fassikaw Kebede  https://orcid.org/0000-0002-6117-5272
https://orcid.org/0000-0002-6117-5272
Supplemental material: Supplemental material for this article is available online.
References
- 1. Fassikaw K, Nemera E, Belete N, et al. Predictors for a cure rate of severe acute malnutrition 6-59 month children in Stabilizing Center at Pawe General Hospital, Northwest Ethiopia: retrospective cohort study. Int J Child Health Nutr 2021; 10: 34–43. [Google Scholar]
- 2. Maximilian M, Michael HRavesh S, Julia R, et al. Malnutrition in HIV-infected children is an indicator of severe disease with an impaired response to antiretroviral therapy. AIDS Res Hum Retrov 2018; 34(1): 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Daka DW, Ergiba MS. Prevalence of malnutrition and associated factors among adult patients on antiretroviral therapy follow-up care in Jimma Medical Center, Southwest Ethiopia. PLoS ONE 2020; 15(3): e0229883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andrew J, Prendergasta B, Chipo B, et al. Inflammatory biomarkers in HIV-infected children hospitalized for severe malnutrition in Uganda and Zimbabwe. Conc Commun 2019; 33: 1485–1490. [DOI] [PubMed] [Google Scholar]
- 5. Philippa MM, Pamela F. Severe malnutrition and metabolic complications of HIV-infected children in the antiretroviral era: clinical care and management in resource-limited settings. Am J Clin Nutr 2011; 94(Suppl): 1716S–1720S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Penda CI, Moukoko ECE, Nolla NP, et al. Malnutrition among HIV infected children under 5 years of age at the Laquintinie hospital Douala, Cameroon. Pan Afr Med J 2018; 30: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abdu O, Mina EK, Berhanu AM. Malnutrition as predictor of survival from anti-retroviral treatment among children living with HIV/AIDS in Southwest Ethiopia: a survival analysis. BMC Pediatr 2019; 19: 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alebe A, Cheru T, Getiye D, et al. Effects of undernutrition on survival of human immunodeficiency virus positive children on antiretroviral therapy. Italian J Pediat 2018; 44: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yetbarek T, Geteye D, Belisty T, et al. Nearly one in every six HIV-infected children lost from ART follow-up at Debre Markos Referral Hospital, Northwest Ethiopia: a 14- year retrospective follow-up study. PLoS ONE 2020; 15(9): e0239013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collins J, Ruth A, Esther LL, et al. Survival and nutritional status of children with severe acute malnutrition, six months post-discharge from outpatient treatment in Jigawa state, Nigeria. PLoS ONE 2018; 13(6): e0196971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsegu HG—, Haftea H.Mekonen; Kbrom Gemechu K; Undernutrition and associated factors among adult HIV/AIDS patients receiving antiretroviral therapy in the eastern zone of Tigray, Northern Ethiopia: a cross-sectional study. Arch Publ Health 2021; 78: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tesfay W, Abay M, Hintsa S, et al. Length of stay to recover from severe acute malnutrition and associated factors among under-five years children admitted to public hospitals in Aksum, Ethiopia. PLoS ONE 2020; 15(9): e0238311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Léon G, Savadogo B, Philippe D, et al. Impact of HIV/AIDS on mortality and nutritional recovery among hospitalized severely malnourished children before starting antiretroviral treatment. Open J Pediat 2013; 3: 340–345. [Google Scholar]
- 14. Alebel A, Wagnew F, Tesema C, et al. Effects of undernutrition on mortality and morbidity among adults living with HIV in sub-Saharan Africa: a systematic review and meta-analysis. BMC Infect Dis 2021; 21: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Habtemu J, Aabdul haliks W, Fishehay A. Survival status and predictors of mortality in severely malnourished children admitted to Jimma University Specialized Hospital from 2010 to 2012, Jimma, Ethiopia: a retrospective longitudinal study. BMC Pediatrics 2015; 15: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wagnew F, Tesgera D, Mekonnen M, et al. Predictors of mortality among under-five children with severe acute malnutrition, Northwest Ethiopia: an institution-based retrospective cohort study. Arch Publ Health 2018; 76: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Getahun MB, Teshome GS, Fenta FA, et al. Determinants of severe acute malnutrition among HIV-positive children receiving HAART in public health institutions of North Wollo Zone, Northeastern Ethiopia: unmatched case–control study. Pediat Health Med Ther 2020; 11: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abate BB, Aragie TG, Tesfaw G. Magnitude of underweight, wasting and stunting among HIV positive children in East Africa: a systematic review and meta-analysis. PLoS ONE 2020; 15(9): e0238403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kebede F, Kebede T, Kebede B, et al. Time to develop and predictors for incidence of tuberculosis among children receiving antiretroviral therapy. Tuberc Res Treat 2021; 2021: 686019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kebede F, Kebede B, Kebede T, et al. Effect of isoniazid preventive therapy on the incidence of tuberculosis among seropositive children attending HIV/AIDS care in two general hospitals, Northwest Ethiopia, 2021. J Trop Med 2021; 2021: 996953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moore DF, Moore DF. Applied survival analysis using R. Piscataway, NJ: Springer, 2015. [Google Scholar]
- 22. Dessalegn N, Birhanu S, Birhanu M, et al. Undernutrition and its associated factors among human immunodeficiency virus-infected children on follow up in Amhara region referral hospitals, Ethiopia, 2020. Glob Pediatr Health 2021; 8: 39640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Birtukan Aklog Y, Gety DK, Cheru TL. Incidence and predictors of treatment failure among children on first-line antiretroviral therapy in Amhara Region Referral Hospitals, northwest Ethiopia 2018: a retrospective study. PLoS ONE 2019; 14(5): e0215300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization. Updates on the management of severe acute malnutrition in infants and children. Geneva: WHO, 2013. [PubMed] [Google Scholar]
- 25. World Health Organization. Guideline updates on the management of severe acute malnutrition in infants children: Treatment Manual. Geneva: WHO, 2021. [PubMed] [Google Scholar]
- 26. FMOH. National consolidated guidelines for comprehensive HIV prevention, care and treatment. 2018, https://www.afro.who.int/publications/national-consolidated-guidelines-comprehensive-hiv-prevention-care-and-treatment
- 27. Ethiopia F. Guidelines for paediatric HIV/AIDS care and treatment in Ethiopia, federal HIV/AIDS prevention and Control Office Federal Ministry of Health July 2007 (Manual), 2007. [Google Scholar]
- 28. FMOH. National guidelines for comprehensive hiv prevention, care and treatment, Manual, 2017. [Google Scholar]
- 29. Boulware DR, Callens S, Pahwa S. Pediatric HIV immune reconstitution inflammatory syndrome. Curr Opin HIV AIDS 2008; 3(4): 461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Animut A, Eshetu H, Mengistu MK, et al. Mortality rate among HIV-positive children on ART in Northwest Ethiopia: a historical cohort study. BMC Publ Health 2020; 20: 1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Flávia JA, Cristiane K, Marco A, et al. Influence of the antiretroviral therapy on the growth pattern of children and adolescents living with HIV/AIDS. J Pediatr 2018; 95(S1): S95–S101. [Google Scholar]
- 32. Tadele G, MesfineK Befikadu T. Incidence and predictors of mortality among severe acute malnourished under five children admitted to Dilla University Referal Hospital: a retrospective longitudinal study. J Biol Agri Healthc 2016; 6(13), https://core.ac.uk/download/pdf/234662065.pdf [Google Scholar]
- 33. Girum T, Kote M, Tariku B, et al. Survival status and predictors of mortality among severely acute malnourished children <5 years of age admitted to stabilization centers in Gedeo Zone: a retrospective cohort study. Therap Clin Risk Manag 2017; 13: 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Daniel M. Nutritional status and associated factors among adult HIV/AIDS clients in Felege Hiwot Referral Hospital, Bahir Dar, Ethiopia. Sci J Publ Health 2013; 1(1): 24. [Google Scholar]
- 35. Chiaha A. Burden of HIV infection in children with severe acute malnutrition at the University of Abuja teaching Hospital Gwagwalada, Nigeria. J HIV Clin Sci Res 2015; 2(2): 55–61. [Google Scholar]
- 36. Gebremichael DY, Hadush KT, Kebede EM, et al. Food insecurity, nutritional status, and factors associated with malnutrition among people living with HIV/AIDS attending antiretroviral therapy at public health Facilities in West Shewa Zone, Central Ethiopia. Biomed Res Int 2018; 2018: 913534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buck WC, Olson D, Kabue MM, et al. Risk factors for mortality in Malawian children with human immunodeficiency virus and tuberculosis co-infection. Int J Tuberc Lung Dis 2013; 17(11): 1389–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Muzigaba M, Sanders D. The impact of HIV infection and disease stage on the rate of weight gain and duration of refeeding and treatment in severely malnourished children in rural South African hospitals. Research 2017; 11(2), http://www.sajch.org.za/index.php/SAJCH/article/view/1374 [Google Scholar]
- 39. Bhowmik A, Bhandari S, Rajyasree D, et al. Predictors of mortality among HIV-infected patients initiating anti retroviral therapy at a tertiary care hospital in Eastern India. Asian Pacific J Trop Med 2012; 5: 986–990. [DOI] [PubMed] [Google Scholar]
- 40. Tekelehaimanot AN, Lemma TB, Gudina EK, et al. Predictors of under nutrition and its implication toward HIV continuum care among adult people living with HIV in Jimma Zone Public Hospitals, Southwest Ethiopia: a mixed method study. J Int Assoc Provid AIDS Care 2020; 19: 976254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sunguya BF, Poudel KC, Mlunde LB, et al. Poor nutrition status and associated feeding practices among HIV-positive children in a food secure region in Tanzania: a call for tailored nutrition training. PLoS ONE 2014; 9(5): e98308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (https://creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
sj-docx-2-smo-10.1177_20503121221081337 for Severe Acute Malnutrition (SAM) associated mortality rate of children attending HIV/AIDS care in North West Ethiopia, 2009–2019 by Fassikaw Kebede in SAGE Open Medicine
Supplemental material, sj-dta-1-smo-10.1177_20503121221081337 for Severe Acute Malnutrition (SAM) associated mortality rate of children attending HIV/AIDS care in North West Ethiopia, 2009–2019 by Fassikaw Kebede in SAGE Open Medicine