Abstract
Background: Phytomedicine is becoming more acceptable as an alternative medicinal approach in the modern era.
Objectives: The current study examined the antioxidant capacity and in vitro response of phytochemical constituents of Withania somnifera (Ashwagandha) on standard parameters of healthy volunteer semen.
Methods: The phytochemicals and their pharmacological response in a hydroethanolic (30:70 v/v) extract of W. somnifera roots were determined using standard protocols.
Results: The constituents included flavonoids, phenolic acids, alkaloids, and terpenoids were reported. High-performance liquid chromatography and Fourier-transform infrared spectroscopy determined a diverse array of biologically active chemical constituents in the extract. The extract of W. somnifera exhibits substantial antioxidant properties, including total antioxidant capacity, 2,2-diphenyl-1-picrylhydrazyl inhibition, H2O2 scavenging, and Fe3+ reducing potential (P < .05). The analysis of essential natural minerals explored adequate levels determined using atomic absorption spectrophotometer. Cytotoxic studies revealed significant thrombolytic, RBC membrane stabilization, and DNA damage protection activity (P < .05) while remaining non-mutagenic against Salmonella typhi TA98 and TA100. The best protective response of W. somnifera extract on human semen parameters (n = 30), such as total motility, progressive motility, and viability, demonstrated a significant (P < .05) improvement, particularly at the dose of 25 μg/mL and 50 μg/mL.
Conclusion: The study concludes that W. somnifera possesses favorable in vitro characteristics that could aid in the preservation of sperm during intrauterine insemination and in vitro fertilization.
Keywords: antioxidants, ashwagandha, Indian ginseng, intrauterine insemination, sperm preservation, in vitro fertilization, phytomedicine
Introduction
It is widely accepted that more than 80% of the habitats of developing countries cannot afford allopathic medicines. However, those are dependent on traditional herbal drugs to treat various disorders, as reported by World Health Organization (WHO) (2003). 1 Potential herbal sources are also used to generate revenue worldwide, so the demand for medicinal plants has been increased day by day due to their therapeutic value for the ailment of many veterinary and medical complications. 2 The side effects of herbal drugs are very few when compared with allopathic medicines. Most Asian countries are blessed with a wide range of medicinal plants where the medicinal plants are still a long-standing being practiced to date. About 2000 species of medicinal plants in Pakistan have been identified and about 400 plants are practiced extensively as folk medicines having various therapeutic active ingredients.3,4
Withania somnifera in the family of Solanaceae is usually called ashwagandha, Indian ginseng, poisonous gooseberry, Kaknaje in Hindi, or winter cherry. Several different species are morphologically similar in the variety Withania. The herb in Ayurvedic medicine is regularly used as a medicinal plant. It is accepted to treat various diseases affecting human health. 5 The W. somnifera is commonly located in hot and dry climates (including Pakistan, India, and Iran). 6 Leaves, roots, seeds, fruits, and flowers are used to treat impotence and enhance sexual desire with other conventional plants, either alone or in the polyherbal formulation.7,8 Several extracted phytochemicals with varying pharmacological and biological properties have been identified. 9
This plant contains over 80 typical phytochemicals, including steroidal, alkaloids, saponins, glycosides, and volatile oil. Among these chemical ingredients, sitoindosides and withaferin A had the leading role in WS therapeutic effects.10-14 Ashwagandha has the potential to treat joint inflammation, nervous system disorders, diabetes, hyperlipidemia, attention deficit hyperactivity disorder, hiccup, obsessive-compulsive disorder, bronchitis, back pain, sleep deprivation, tumors, Parkinson’s disease, tuberculosis, menstrual irregularities, and chronic liver disease. 15 Ashwagandha roots possess antioxidant properties and treat infertility, the immune system and cardiovascular disease. Dried roots of the plant are generally utilized to treat nervous and sexual issues, and because of anti-inflammatory properties, ashwagandha improves sperm quality. 16 The present study aimed to explore the in vitro antioxidant potential of phytochemical constituents of W. somnifera plant roots and its impact on spermatozoa parameters collected from healthy volunteers.
Material and Methods
Selection and Collection of Plant Material
The roots of W. somnifera were obtained from the Local market of Faisalabad-Pakistan and were identified and authenticated taxonomically from the Department of Botany, Government College University, Faisalabad-Pakistan (Ref#Bot-2019-7787). Roots were washed thoroughly using distilled water, dried under shade and then grinded into a fine powder with the help of an electric grinder machine (Model CB 222, Cambridge, UK). Aqueous ethanol (30:70 v/v) extract was prepared following the protocol described by Sulaiman et al., 17 with some modifications. The extract was weighed and the final yield as a percentage yield of extract was calculated as
Phytochemical Analysis
Analysis for identification of the major phytoconstituents was carried out as follow:
Qualitative Analysis
Various phytochemicals, including alkaloids, flavonoids, tannins, saponins, glycosides, steroids, and triterpenoids, were detected in the hydroethanolic extract by standard methods.18-20
Quantitative Analysis
Total Phenolic Contents Estimation
The hydroethanolic extract was processed to estimate the total phenolic contents (TPC) using the Folin-Ciocalteu method described by Jain et al. 18 Gallic acid was used to construct the standard curve, and a reading at 765 nm wavelength was taken to measure the intensity of the color complex. Total phenolic contents present in extracts was measured as mg equivalent to gallic acid (GAE) per mL of extract using the following formula
where C = concentration of gallic acid (mg/mL) taken from standard; V = volume of extract used in mL; M = weight of plant extract in grams.
Total Flavonoids Contents
The quantification of total flavonoids contents (TFC) was done following the protocol described by Pranuthi et al., 19 and optical density was measured using 510 nm wavelength. The TFC present in the hydroethanolic extract was calculated using a linear regression curve of Catechin (CE) as μg CE/g of dried plants material.
High-Performance Liquid Chromatography for Phenolic Compounds
High-performance liquid chromatography (HPLC) for the determination of different phenolic compounds present in hydroethanolic plant extract was used following the method as described by Yue et al., 21 with minor modifications. Liquid chromatography having C18 column (250 × 4.6 mm internal diameter) of 5 μm film thickness and with an oven set at 30°C was used. Chromera HPLC system (Perkin Elmer, USA.) attached with Flexer Binary LC pump, UV/Vis LC Detector (Shelton CT, 06 484 USA) controlled by software V. 4.2. 6410 was used to analyze the data. Solvent A (70:30), composed of acetonitrile: methanol, respectively, and solvent B consisted of double distilled water with .5% glacial acetic acid, were used as mobile phase. Wavelength 275 nm was used to identify different phytochemical compounds using standards to compare the retention times and spiking.
Fourier-Transform Infrared Spectroscopy
Fourier-Transform Infrared Spectrophotometer (FTIR) was used to identify different chemical bonds or functional groups in the phytochemicals. Specific wavelength light absorbed by specific chemical bonds is the salient feature of the chemical bonds representing different functional groups. FTIR spectrometer (Model Bruker Platinum ATR with accessories A225/Q Platinum ATR Multiple Crystals CRY diamond and having interferogram size of 10 550 points) in the frequency range of 400–4000/cm available in the Central Hi-Tech Laboratory, Government College University, Faisalabad-Pakistan, was used as described in our previous study. 22
Trace Elements and Heavy Metals Estimation
An atomic absorption spectrophotometer determined the concentration of important trace elements and heavy metals in medicinal plants. The procedure recommended by the AOAC (2000) 23 consisted of two phases and nitric-perchloric acid was used to digest the sample. After digestion, Colagar et al. 24 protocol was used to determine trace and heavy metals (ppm) including Cu, Fe, Cd, Pb, Mg, Co, Zn, Ni, and Mn by an atomic absorption spectrometer (Aurora, Canada).
Determination of Antioxidant Potential of Selected Herb Using Different Assays
Total Antioxidant Capacity (Phosphomolybdenum Method)
Phosphomolybdenum assay was used to measure the TAC of selected plant hydroethanolic extract spectrophotometrically following the protocol described by Prieto et al. 25 The obtained results of TAC were expressed as ascorbic acid (used as standard) equivalents mg/g of the dry plant. Butylated hydroxytoluene (BHT) was used as reference controls.
2, 2-diphenyl-1-picrylhydrazyl Radical Scavenging Activity
Begum et al. 26 method was used to evaluate the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay to determine the antioxidant capacity of plant extract against Vitamin C and BHT as standard. The following formula was used to calculate the percent DPPH inhibition after triplicate measurements of each selected medicinal plant.
Hydrogen Peroxide (H2O2) Scavenging Potential
Ruch et al. 27 method to measure the H2O2 scavenging potential of W. somnifera hydroethanolic extract was used taking absorbance at 230 nm. Phosphate buffer saline (PBS) as blank and Vitamin C as standard were used to determine the percent hydrogen peroxide scavenging as
where AS= absorbance in the presence of the extract sample or standard.
Reducing Power Assay (FRAP Method)
To evaluate the antioxidant potential of the selected medicinal plant, the ferric reducing power assay (FRAP) method was used in which the reducing potential of substance Fe3+ (CN)6 into Fe2+ (CN)6 by direct electron donation is measured. Yadav et al. 28 protocol was used to determine the reducing potential of plant extract. Different concentrations of selected plant hydroethanolic extract as 25, 50, 100, 150, 200, 250, 300, 350, and 400 (μg/mL) were used to determine the dose-response effect. Wavelength 700 nm was used to take the absorbance of the mixture and the reducing potential of the plant extract is directly proportional to the absorbance of the reaction mixture.
In Vitro Evaluation of Toxicity of W. somnifera Extract
Hemolytic Assay to Determine Cytotoxic Potential of Plant Extract
To evaluate the safety of hydroethanolic plant extract, a hemolytic assay was used in accordance with Powell et al. 29 protocol with some modifications. Prior to the assay, RBCs were suspended in PBS at a concentration of 7.0 x 108 RBCs/mL (using a hemocytometer). Absorbance against Triton X-100 (.1%) treated RBCs as positive control and PBS as a negative control was taken using Microwell Plate Reader BioTeK, μ Quant (BioTek, Winooski, VT, USA) set at 576 nm wavelength and calculated as
where Ae= the absorbance of plant extract; Ap= the absorbance of PBS; Ad=, the absorbance of DMSO (20%), used to make plant dilution.
Clot Lysis Assay for Thrombolytic Potential of Plant Extract
To evaluate the clot-dissolving potential of selected medicinal plant in vitro clot lysis assay was used following Rahman et al. 30 Fresh 1 mL venous blood from healthy volunteers (n = 10) (excluded those taking any type of anticoagulant) was taken into pre-weighed micro-centrifuge tubes as instructed by Institutional Research Scrutiny Committee (Ref. No. GCUF/DAS/19/1534). Streptokinase vial (1 500 000 IU) available in the market was used as positive control while PBS as a negative control for comparison and clot-dissolving potential was calculated as
Mutagenicity/Genotoxicity Evaluation (AMES Test)
Because of its simplicity and relatively low cost, the Bacterial Reverse Mutation Test (Ames test) is recommended by several regulatory agencies (Organization for Economic Co-operation and Development, Food and Drug Administration, International Conference on Harmonization) to preclude genotoxic activity. It is used as a screening method in drug development. Bruce Ames developed the AMES test in the 1970s. 31 Auxotrophic bacterial strains are commonly used to investigate the capability of a substance for genotoxicity through reverse mutations. Salmonella typhimurium two strains TA98 and TA100 were used using the fluctuation method to evaluate the genotoxicity of selected herb extract on incubating with the bacterial cell for up to 5 days in 96 well microplates. Revertant characteristic of genotoxic substance was expressed as change in color from blue (due to purple bromocresol) to yellow, representing the growth and metabolic activities of bacterial reverted strain. The results were represented as the number of revertants compared with controls. A probability test was performed to evaluate the results statistically.
DNA Damage Prevention Test
The DNA damage protection assay was used to determine the genoprotective capacity of the examined plant’s aqueous ethanolic extract, following the protocol of Tian and Hua, 32 with some modifications. Calf thymus DNA (Ct DNA) (.5 μg/3 μl) was treated with 5 μl of Fenton reagent [30% (v/v) hydrogen peroxide (4 μl), with 3 µ ferrous sulfates (2 mM)] and with 5 μl of studied medicinal plant extract (100 μg/ml), respectively. Then, the DNA damage protection capacity of selected plant extract was evaluated by running the DNA on agarose gel electrophoresis and control DNA. Syngene GeneGenius Gel Light Imaging System was used for gel documentation.
In Vitro Spermatozoa Parameters
For in vitro spermatozoa parameters determination, healthy volunteers (time to pregnancy of 12 months) were selected (n = 30) for the collection of semen samples after taking written informed consent and semen samples were processed according to WHO protocol. Normal samples were selected for current analysis according to the WHO reference range. 33 Different doses of extract as 25 μg/mL, 50 μg/Ml, and 100 μg/mL were used to determine the impact of selected medicinal Crude aqueous ethanolic extract. Physiological saline was used to dissolve the extracts in labeled Eppendorf’s and kept overnight at room temperature. Kuma et al. 34 protocol with some modification was used to determine the in vitro effect of medicinal plant on semen parameters, including motility total (%), progressive motility (%) and viability (%). After collecting the semen and evaluating for standard parameters, equal volume (1:1) of semen was mixed with plant extracts dissolved in .9% physiological saline. Then mix and incubate for 30 seconds at room temperature, then sperm motility total (%), as well as progressive motility (%), were examined under the light microscope (40X) at specific intervals like 0 min, 15 min, 30 min, 45 min, 60 min, and 120 min. The impact of studied medicinal plant on viability (%) of spermatozoa was also investigated using .1% Eosin Y stain in .9% physiological saline by incubating the semen, extract and stain (1:1:1) for up to 120 min following the protocol of Prakash et al. and Cheesbrough.35,36
Statistical Analysis
The obtained results were reported as Mean ± SEM and were interpreted statistically using the one-way ANOVA test. Minitab 17 statistical software was used for the Probability test. 37
Results
Alternative medicines for treating general health problems are becoming more popular and receiving more attention in drug development. W. somnifera roots gave extract yield as 22.19 ± 1.03 g/100 g dry weight of plant material on preparing the extract using 70% ethanol in water.
Qualitative and Quantitative Phytochemical Constituents Analysis
The results of different phytochemical constituents in the hydroethanolic extract given in Table 1 revealed the presence of flavonoids, tannins, alkaloids, saponins, steroids, glycosides and triterpenoids in the selected plant extract. The results of total flavonoid and phenolic contents determined during current research work are given in Table 2. Significant (P<.05) concentration of TFC and TPC was found in the hydroethanolic extracts of W. somnifera as (148.79 ± 6.39 μg CE/g) and (372.81 ± 7.71 mg GAE/g), respectively.
Table 1.
Qualitative phytochemical constituents present in hydroethanolic extract of the studied medicinal plant.
| Plants/phytochemicals | Withania somnifera |
|---|---|
| Alkaloids | + |
| Flavonoids | + |
| Tannins | + |
| Saponins | − |
| Glycosides | + |
| Steroids | + |
| Triterpenoids | + |
“+” indicates the detection of phytoconstituent; “−” indicates non detection of phytoconstituent present.
Table 2.
Phytochemical constituents and antioxidant activities of W. somnifera hydroethanolic extract as mean ± SEM of multiple determinations.
| Plants\contents | W. somnifera | Vitamin C |
|---|---|---|
| TPC (mg GAE/g dry plants material) | 372.81 ± 7.71 | – |
| TFC (μg CE/g dry plants material) | 148.79 ± 6.39 | – |
| H2O2 scavenging activity (%) | 21.65 ± .71 | 48.70 ± 2.91A |
| DPPH inhibition (%) | 49.04 ± 1.03 | 90.15 ± 5.93A |
“–” indicates not tested.
High-Performance Liquid Chromatography for Phenolic Compounds
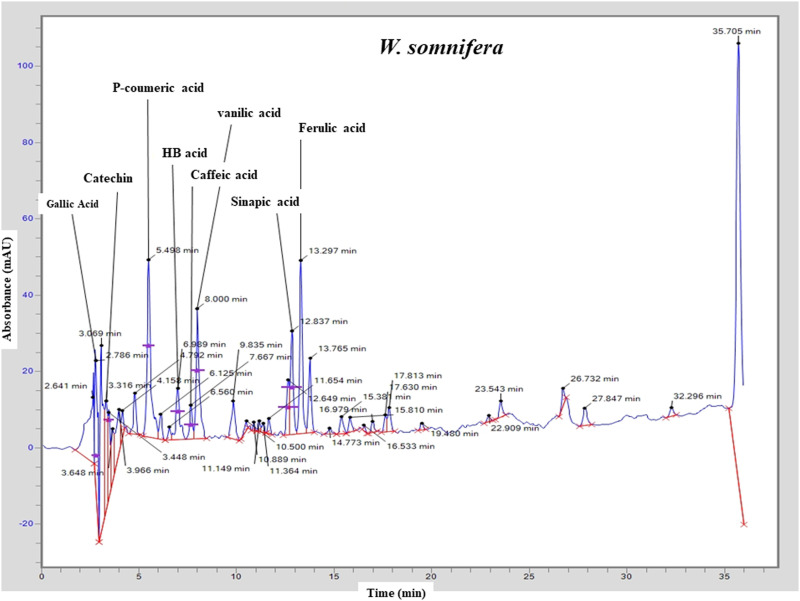
The results of HPLC-UV showed that various phenolic compounds including gallic acid (Rt = 2.786 min), catechin (Rt = 3.316 min), P-coumeric acid (Rt = 5.498 min), HB acid (Rt = 6.989 min), caffeic acid (Rt = 7.667), vanilic acid (Rt = 8.00 min), sinapic acid (Rt = 12.837) and ferulic acid (Rt = 13.297) are present in the roots extract of W. somnifera (Table 3 and Figure 1). All the phytochemical constituents identification was carried out by comparing the retention times (Rt) with the chromatographic peaks of reference standards. The chromatograph also represented some peaks with varying retention times, and these peaks were not identifiable. However, some unknown flavonoids might be present in the extracts, based on their chromatographic behaviors and UV spectra.
Table 3.
Different phytochemical constituents identified using HPLC in the hydroethanolic extract of selected medicinal plant.
| Selected Medicinal plants | Phytochemical constituents | Retention time (min) | Area under curve | Height |
|---|---|---|---|---|
| W. somnifera | Gallic acid | 2.786 | 364982.0 | 33353.0 |
| Catechin | 3.316 | 297540.5 | 28849.9 | |
| P-coumeric acid | 5.498 | 612715.3 | 46027.5 | |
| HB acid | 6.989 | 183444.9 | 13449.1 | |
| Caffeic acid | 7.667 | 85820.3 | 8763.4 | |
| Vanilic acid | 8.000 | 397856.8 | 33322.8 | |
| Sinapic acid | 12.837 | 282621.8 | 26896.2 | |
| Ferul ic acid | 13.297 | 450336.2 | 44925.1 |
Figure 1.
Chromatogram representing different phytochemical constituents identified using HPLC in the hydroethanolic extract of W. somnifera.
Fourier-Transform Infrared Spectroscopy
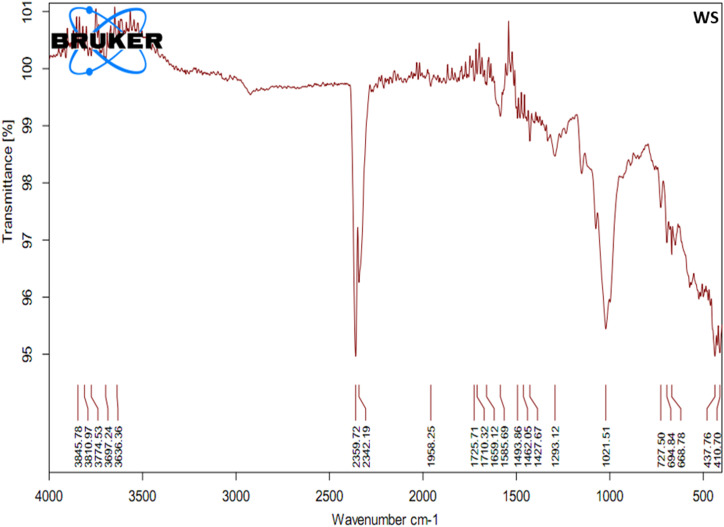
According to infrared spectroscopy in figure 2 and Table 4, the absorption peaks at range 4000 to 2500/cm was associated with the stretching vibration of single bond formed by hydrogen and other elements like O-H, N-H and C-H; peaks at 2500 to 2000/cm explored the absorption of light due to triple bond, for example, C≡C, C≡N; peaks at 2000–1500/cm represent the presence of a compound with double bonds including C=C, C=O while absorption peaks at 1500 to 400/cm consisted of many complicated bands and this part of the spectrum is unique to each compound also called fingerprint region. It is rarely used to identify particular functional groups like C-C, C-N, C-O, C-Cl, C-I, S-S, and N=O*.
Figure 2.
Fourier-transform infrared spectrophotometer (FTIR) graph representing different functional groups and possible phytochemical constituents identified using FTIR in the hydroethanolic extract of W. somnifera.
Table 4.
Different functional groups and possible phytochemical constituents identified using FTIR in the hydroethanolic extract of W. somnifera.
| Wavenumber (cm−1)/possible functional groups | W. somnifera |
|---|---|
| 4000–2500/cm O-H, N-H, C-H Stretch vibration |
3845 |
| 3810 | |
| 3774 | |
| 3697 | |
| 3636 | |
| 2500–2000/cm C≡C, C≡N Stretch vibration |
2359 |
| 2342 | |
| 2000–1500/cm C=C, C=O, C=N Stretch vibration |
1958 |
| 1725 | |
| 1710 | |
| 1659 | |
| 1585 | |
| 1500–400/cm C-C, C-N, C-O, C-Cl, C-I, S-S, N=O* Stretch, bend, or scissoring, rock vibration |
1493 |
| 1462 | |
| 1427 | |
| 1293 | |
| 1021 | |
| 727 | |
| 694 | |
| 668 | |
| 437 | |
| 410 |
Trace Elements and Heavy Metals Estimation
In the current research work, mineral contents, both essential and toxic elements including Cu, Fe, Cd, Pb, Mg, Co, Zn, Ni, and Mn were also measured. The results revealed that the selected medicinal plant contained a significant (P<.05) concentration (ppm) of essential minerals. The concentration of Cu in W. somnifera roots was 63.743 ± 4.33, Iron (Fe) 768.051 ± 11.53, Zinc (Zn) 39.284 ± 3.36, Magnesium (Mg) level 59.207 ± .88, Cobalt (Co) 313.461 ± 4.10, Nickle (Ni) 70.698 ± 3.04, Cadmium (Cd) 4.507 ± .35, and Lead (Pb) 8.565 ± 1.03 are given in Table 5.
Table 5.
Mineral contents in the roots of W. somnifera as mean ± SEM of multiple determinations.
| Plants\ contents | W. somnifera |
|---|---|
| Copper (ppm) | 63.743 ± 4.33 |
| Iron (ppm) | 768.051 ± 11.53 |
| Zinc (ppm) | 39.284 ± 3.36 |
| Magnesium (ppm) | 59.207 ± .88 |
| Manganese (ppm) | 80.384 ± 1.85 |
| Cobalt (ppm) | 313.461 ± 4.10 |
| Nickle (ppm) | 70.698 ± 3.04 |
| Cadmium (ppm) | 4.507 ± .35 |
| Lead (ppm) | 8.565 ± 1.03 |
Antioxidant Activities of Selected Medicinal Plant Extract
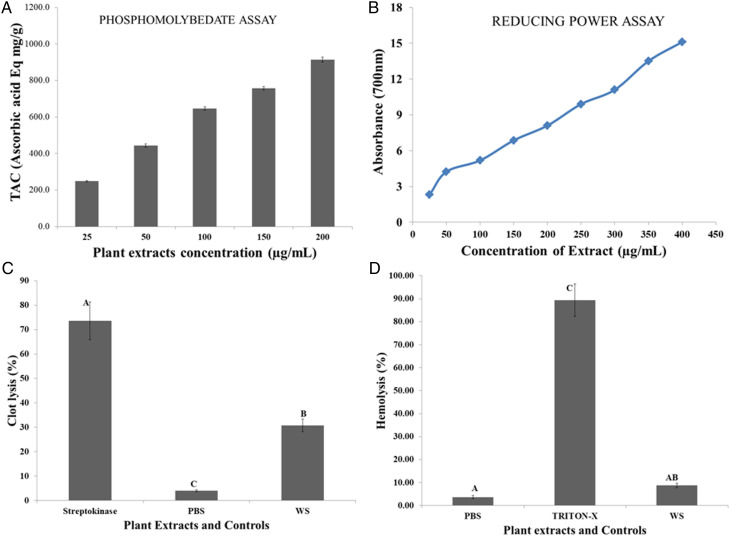
The DPPH and H2O2 are given in Table 2, while figure 3A represents the phosphomolybdenum scavenge assay results. The potential of hydroethanolic extract to scavenge DPPH (%) and H2O2 (%) explored that this herb has significant (P<.05) potential to neutralize the free radicals. The phosphomolybdenum assay results revealed that increasing extract dose concentrations directly correlated with TAC calculated using the standard curve of Vitamin C and represented as ascorbic acid Eq mg/g in Figure 3A. Vitamin C was used as positive control that showed 90.15 ± 5.93% and 48.70 ± 2.91% percent DPPH radical and H2O2 scavenging activities, respectively (Table 2). The results of the ferric reducing antioxidant power (FRAP) assay was represented in Figure 3B, which revealed that the selected medicinal plant has significant (P<.05) potential to reduce the ferric ion to ferrous ion. Results also explored that the reducing capacity also increased with increasing the concentrations of extracts (Figure 3B).
Figure 3.
Graphical representation of (A) total antioxidant capacity (TAC) by phosphomolybdenum method. (B) Reducing potential investigated by FRAP (C). Thrombolytic activities (%) of selected medicinal plant. (D) Hemolytic activity (%)evaluated against washed RBCs. Results are mean ± SEM of multiple determinations and controls. Different alphabets on the bars represent significance (P<.05) differences among tested plant extract and controls. WS= Withania somnifera.
In Vitro Evaluation of Toxicity of W. somnifera Extract
Cytotoxic Activity and Thrombolytic Activities
In vitro hemolytic analysis revealed that W. somnifera hydroethanolic extract has non-significant hemolysis (P >.05) as compared to a positive control (Triton X-100), which showed 90.37 ± 6.955% hemolysis. On the other hand, a negative control phosphate buffer saline causes 4.16 ± .575% RBCs hemolysis (Figure 3C). When comparing medicinal plant extracts to triton X-100, statistical analysis demonstrated that medicinal plant extract cause significantly (P < .001) less hemolysis of RBCs (Figure 3C). The results of clot lysis assay revealed that hydroethanolic extract of W. somnifera has significant (P < .001) clot lysis activity (20.72 ± 2.55%) on compared to a positive control (73.6 ± 7.70%) and negative control (4.00 ± .35%) on incubating for 90 min at 37°C. The results of thrombolytic activities as mean ± SEM are shown in Figure 3D.
Mutagenicity/Genotoxicity and DNA Damage Prevention Tests
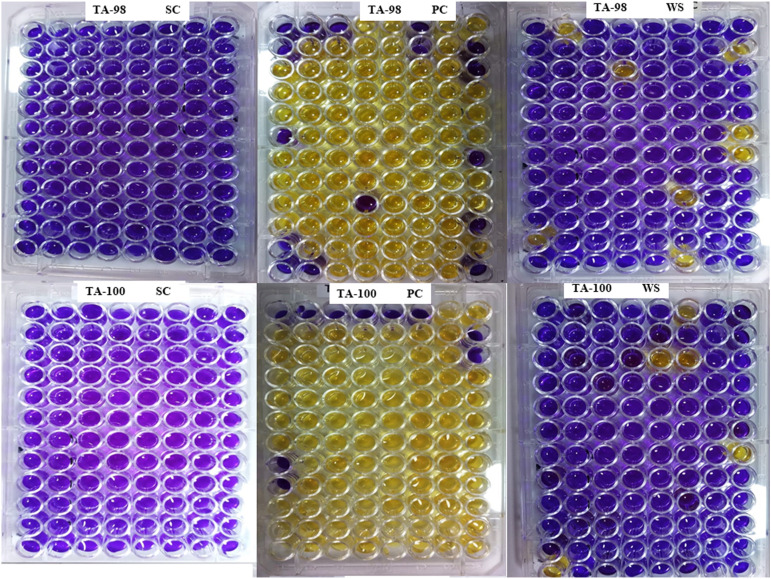
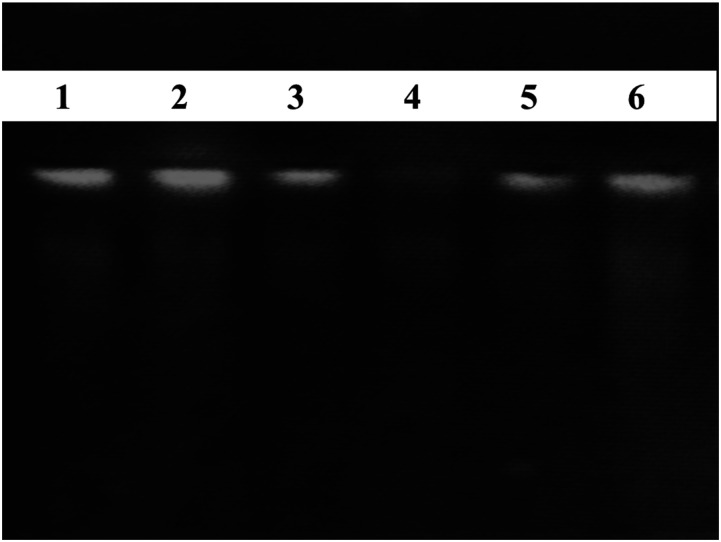
The results of the mutagenicity analysis of studied medicinal plants against TA98 and TA100 are given in Table 6 and Figure 4. The results of the Ames test explored that selected medicinal plants did not possess mutagenic activity or, in other words, W. somnifera is non-mutagenic. According to the Fold rule, to declare that extract is mutagenic, the number of positive wells should be double the number of positive wells in the background plate. 38 The results revealed that W. somnifera plates showed minimum reversion of both S typhi TA98 and TA100 compared with positive controls (Table 6 and Figure 4). Moreover, results revealed comparatively to controls noticeable DNA damaging prevention potential. Figure 5 represented the electrophoresis pattern of calf thymus (Ct) DNA after being treated with Fenton reagent and extract of selected herb to investigate DNA damage prevention activity against oxidative DNA damage.
Table 6.
Mutagenic activity of selected medicinal plants against S. typhi TA98 and TA100.
| Etahnolic extracts and bacterial strains | Number of positive wells/total number of wells | Results |
|---|---|---|
| Mutagenic activity against TA98 | ||
| (a) Background | 20/96 | - |
| (b) Standard (K2Cr2O7) | 80/96 | + |
| (c) W. somnifera | 8/96 | - |
| Mutagenic activity against TA100 | ||
| (a) Background | 25/96 | - |
| (b) Standard (NaN3) | 86/96 | + |
| (c) W. somnifera | 5/96 | - |
+, significant increase in the number of positive wells compared to the related control (P < .05); -, non-significant (P>.05) effect observed.
Figure 4.
Mutagenicity evaluation of selected medicinal plant using S. typhi TA 98 and TA 100. SC= sterility control, PC= positive control, WS= W. somnifera; blue color indicates no bacterial convergent (no bacterial growth) and yellow color indicates bacterial convergent (bacterial growth).
Figure 5.
DNA damage prevention potential of W. somnifera roots extract using Ct DNA (calf thymus DNA). Lane 1 = untreated DNA, Lane 2= 2 mM FeSO4, 30% H2O2 + DNA + 1 mM Quercetin, Lane 3 = 30% H2O2 + DNA, Lane 4 = 2 mM FeSO4, 30% H2O2 + DNA, Lane 5 = 2 mM FeSO4 + DNA, Lane 6 =2 mM FeSO4, 30% H2O2 + DNA + W. somnifera extract.
In Vitro Spermatozoa Parameters
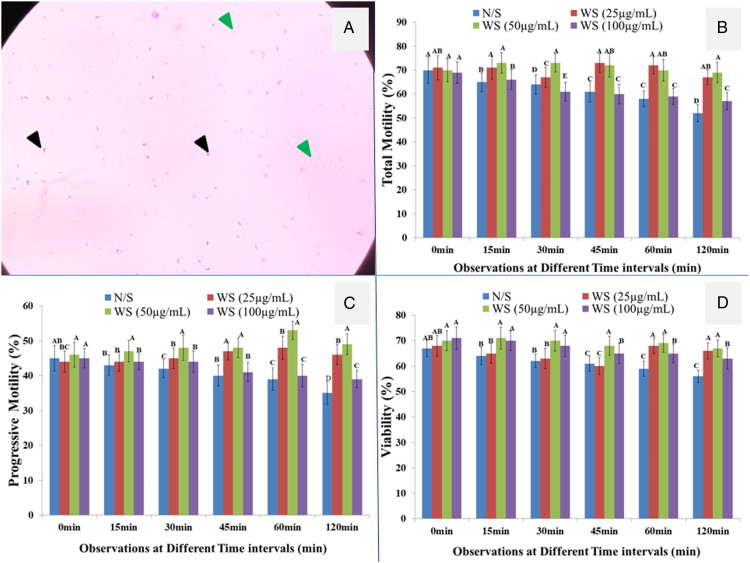
Results of different doses of plant extract on selective spermatozoa parameters revealed that there were significant (P < .05) differences among the control and plant extract (Figures 6B and C). Moreover, non-significant (P > .05) spermicidal activity was observed on incubating the semen with hydroethanolic extract of W. sominifera tested at different time intervals and normal saline as control (Figures 6A and D). Moreover, it was also reported that no significant (P>.05) reduction in motility of spermatozoa was observed in control-treated semen. It could be noted from figures 6B–D that incubation of the semen with the extracts of W. sominifera enhanced the motility and preserved the viability of spermatozoa for a longer time than control. Furthermore, comparing different doses of extract 50 μg/mL concentration has a more beneficial impact on motility and viability. At the same time, it was also found that a high dose of 100 μg/mL has toxic effects on selective spermatozoa (Figures 6B–D).
Figure 6.
In vitro response of different concentrations of W. somnifera extract on Spermatozoa parameters. (A) Sperms stained with .5% eosin Y black color arrow heads represent the non-viable (pink color) sperms while green represent the viable sperms (un-stain). (B) Total motility. (C) Progressive motility. (D) Viability of spermatozoa treated with extract and normal saline as control at different time intervals. The values are mean ± SEM of replicate determinations and bars share different alphabets are significantly (P < .05). N/S= normal saline (.89% sodium chloride); WS = W. somnifera.
Discussion
It was well reported that the therapeutic applications like analgesic, antibacterial and antispasmodic were due to the alkaloids present or derived from medicinal plants 39 and the significant antioxidant potential is due to the presence of phenolic contents. 40 Most important groups like flavonoids as natural antioxidants are the critical natural phenolic agents. Findings revealed that reactive oxygen species' detoxifying capacity is due to these natural flavonoids, which also can inhibit the low-density lipoproteins oxidations. 41 The results of our study revealed the presence of various active constituents in W. somnifera extract. Gulati et al. 42 reported the presence of alkaloids and tannins in the root extract of W. somnifera. Saxena et al. 43 also reported many other pharmacologically important constituents like alkaloids, steroids, starch, reducing sugars, amino acids, volatile oil, glycosides, withaniol, hentriacontane, and dulcitol in the roots.
It was well reported that oxidative stress generated due to the defective natural antioxidant defense system associated with different diseased conditions. 44 So for the prevention of such conditions and to improve the natural antioxidant activities, synthetic chemicals or plants derived molecules like Vitamins A, C, E, carotenoids, and flavonoids with other supplementary foods are used. 45 Flavonoids and phenolic are important phytochemical constituents due to which medicinal plants have many therapeutic activities, including antioxidant potential. W. somnifera antioxidant potential is due to the presence of phytochemical compounds including ascorbic acid, phenolic compounds, alkaloids, anthocyanin, steroidal lactones, flavonoids, and tannin. 46
According to infrared spectroscopy in Figure 2, selected medicinal plant extract FTIR analysis revealed absorption peaks at 3500–4000/cm, which means hydrogen bonds in intra- and inter molecules. The absorption peaks at 3845, 3810, 3774, 3697, and 3636/cm were attributed to the asymmetrically stretching vibration of O-H, N-H, and C-H. The peak at 2359 and 2342/cm might be related to stretching vibration of C≡C and C≡N. The absorption peaks at 1958, 1725, 1710, and 1659/cm were attributed to the symmetrically stretching vibration of C=O. The absorption peaks at 1585/cm might be related to the stretching vibration of C=N and C=C. The peaks at 1400–1500/cm (1493, 1462, and 1427/cm) might be attributed to the strong to medium vibration of C=C and N=O functional groups. The absorption peak at 1293/cm was due to the stretching vibration of C-O. The peaks at 1021, 727, and 694/cm were attributed to the asymmetrically stretching vibration of C-O-C. The absorbance peak at 668/cm might be represented aliphatic C-I stretching, while the peak at 437 and 410/cm might be related to S-S stretching vibration (Table 4 and Figures 2). 22
Bioelements are classified in different groups as Group I–Group V based on requirements and applications. Group, I included the elements that are the components to generate macromolecules like C, H, O, and N. Group II included the nutritionally vital minerals and principal elements. Group III elements are also known as Trace elements whose daily requirement is less than 100 mg. However, these are essential elements and serious diseases can develop in the deficiency of these elements. Group IV elements are called additional trace elements whose pet role is unknown and possibly essential. Group V elements are toxic to the body and such elements enter the body through contaminated air, soil, water or food substances, including As, CN, and Hg. 47 The current research results explored an adequate amount of trace and essential elements in the roots of W. somnifera, which could be an important natural supplement to manage diseases due to the deficiency of such vital ingredients.
Antioxidant Activities of Selected Medicinal Plant Extract
The phytochemical constituents acting as antioxidants can prevent the oxidation of substrates and help detoxify the free radicals like reactive oxygen species, which might be produced within the human body due to oxidation processes. When the human body cannot manage the oxidant agents due to a defective antioxidant system, oxidative stress is generated. Such conditions resulted in different metabolic and other healthcare disorders. 44 The findings are directly in the correlation of total phenolic and total flavonoid compounds in quantitative estimations. It was reported that the radical scavenging assay reactions primarily depend on hydroxylation and structure stability due to the donation of hydrogen by antioxidant compounds present in medicinal plants. 48 The results explored that the highest antioxidant potential of the extract might act as a natural antioxidant agent in the development of pharmaceutical preparations in foods and cosmetics as additives. Paul, 46 found that W. somnifera exhibited significant activities to scavenge the free radicals like DPPH, ABTS.+ radical, nitric oxide scavenging. Our research findings agree with Paul, 46 who determined that the ferric ions (Fe3+) reducing the ability of WSI root extract was higher than the WSF root.46,49
In Vitro Evaluation of Toxicity of W. somnifera Extract
Worldwide, herbal remedies have long been regarded harmless due to their natural origin, but new research demonstrates that this is not the case. So concern related to the safety of medicinal plants is becoming one of the emerging interests for researchers and the investigation of toxic aspects of herbs that might be associated with their usage, therefore, avoiding potential harmful effects. 50 The results of cytotoxic activity suggested that the selected medicinal plant has significant (P<.05) membrane-stabilizing (antihemolytic) potential. The safety of W. somnifera regarding toxicity, side effects and dosing is not yet well established, so the current study was the first time to explore the in vitro cytotoxic potential. As a result, it revealed that W. somnifera extract is non-toxic. It also described previously that W. somnifera has no herb to herb or drug interactions in practical doses.51-53 Different thrombolytic drugs are used, but many of them were reported with a wide range of side effects like bleeding and embolism. 53 The clot-dissolving activities of the selected medicinal plant might be due to the presence of the secondary compounds. In phytochemical constituents analysis, a wide range of phytocompounds was identified. 54 Shahriar et al. 55 investigated the membrane-stabilizing and thrombolytic activities of the root extracts of W. somnifera. Sai Sandeep et al. 56 reported that B. monnieri Linn ethanolic extract leaf extracts have maximum (50.51%) thrombolytic activity.
According to the literature review, the selected medicinal plant was screened out for mutagenic activities for the first time. However, Khanam and Devi, 57 found that Ashwagandha root extract has a significant role in preventing DNA damage and ultimately replenishing the effect of oxidative stress imposed by a mutagen to prevent mutagenicity in mice.
In Vitro Spermatozoa Parameters
Due to the high antioxidant capacity and diversity of physiologically active constituents in selected herbs, their hydroethanolic extracts were evaluated in vitro for their effect on spermatozoa parameters. The beneficial properties of selected medicinal plants on selective spermatozoa parameters might be due to their potent antioxidant potential. Moreover, the presence of phytochemical constituents and an adequate amount of antioxidant minerals in W. somnifera has made its importance in treating infertility problems. According to the literature review, it was the first time investigating the in vitro improvement in the motility and viability of sperms using W. somnifera extract. However, many in vitro studies like Acanthopanacis senticosi and Astragalus membranaceus extracts also showed improvement in the motility and viability of sperms collected from infertile males. 58 Our results also agree with the findings of Peng et al. 59 who used medicinal plants like Rhizoma curculiginis and Radix Morindae officinalis. The results revealed in vitro improvement in motility and the stabilization of sperm membranes. In vitro positive characteristics of natural herbs may help to restore the sperm functions prior to intrauterine insemination (IUI) and in vitro fertilization (IVF).
Conclusion
Medicinal plants with a wide range of therapeutic and nutraceutical properties are used mainly in developing countries to manage different disorders. The current study also revealed that W. somnifera has significant (P<.05) antioxidant activities due to a wide range of phytochemical constituents. Moreover, safety concerns in vitro study explored that hydroethanolic extract of the selected herb is non-toxic and non-mutagenic with membrane-stabilizing characteristics. By supplying varying amounts of the extract to spermatozoa, it has been observed that W. somnifera is a potential candidate for retaining spermatozoa parameters. However, the current findings revealed that W. somnifera has a beneficial in vitro impact. This natural herb might be helpful in storing/restoring the sperm functions for intrauterine insemination and in vitro fertilization. However, a trial at a large scale and In Vivo study is required to declare the W. somnifera as an aphrodisiac medicinal plant.
Acknowledgments
We acknowledged Prof. Dr Zahed Mahmood for providing technical supervision and guidance in Clinical Biochemistry Laboratory, Department of Biochemistry, Government College University Faisalabad–Pakistan to complete this research work.
The author(s) gratefully acknowledge the Pakistan Science Foundation (PSF) for funding the research through PSF research project # PSF/NSLP/P-GCUF (710).
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Naveed Munir https://orcid.org/0000-0003-0380-1332
Muhammad Riaz https://orcid.org/0000-0002-5524-7735
Muhammad Akram https://orcid.org/0000-0002-7457-8572
References
- 1.World Health Organization . Traditional medicine. Fact sheet No 134. 2003.http://www.who.int/mediacentre/factsheets/2003/fs134/en/, Accessed 7 March 2016.
- 2.Pandey S. Preliminary evaluation for phytochemicals in tribulus terrestris and its antibacterial activity. Rev J Microbiol Virol. 2014;4(3):1-6. [Google Scholar]
- 3.Taid TC, Rajkhowa RC, Kalita JC. A study on the medicinal plants used by the local traditional healers of Dhemaji district, Assam, India for curing reproductive health related disorders. Adv Appl Sci Res. 2014;5(1):296-301. [Google Scholar]
- 4.Kensa VM, Yasmin S. Phytochemical screening and antibacterial activity on Ricinus communis L. Plant Sciences. 2011;1(9):167-173. [Google Scholar]
- 5.Puri HS. Rasayana: Ayurvedic Herbs for Longevity and Rejuvenation. CRC press; 2002.https://www.taylorfrancis.com/books/mono/10.1201/b12602/rasayana-puri. Accessed 17 October 2002 [Google Scholar]
- 6.Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev. 2000;5(4):334-346. [PubMed] [Google Scholar]
- 7.Dar NJ, Hamid A, Ahmad M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell Mol Life Sci. 2015;72(23):4445-4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain R, Kachhwaha S, Kothari SL. Phytochemistry, pharmacology, and biotechnology of Withania somnifera and Withania coagulans: A review. J Med Plants Res. 2012;6(41):5388-5399. [Google Scholar]
- 9.Saiyed A, Jahan N, Majeedi SF, Roqaiya M. Medicinal properties, phytochemistry and pharmacology of Withania somnifera: An important drug of Unani Medicine. J Sci Innov Res. 2016;5(4):156-160. [Google Scholar]
- 10.Pal AJ, Bhushan BH, Khanum FA, Govil JN, Kaushik G, Rai N. Therapeutic uses of Withania somnifera (Ashwagandha). Recent progress in medicinal plants (RPMP). 2012;34:97-118. [Google Scholar]
- 11.Shukla KK, Mahdi AA, Mishra V, et al. Withania somnifera improves semen quality by combating oxidative stress and cell death and improving essential metal concentrations. Reprod Biomed Online. 2011;22(5):421-427. [DOI] [PubMed] [Google Scholar]
- 12.Shaikh NH, Deshmukh VM, Walvekar MV. Alteration in testicular morphology and sperm count due to Glycowithanolides treatment during aging. Asian J Pharmaceut Clin Res. 2015;8(3):72-77. [Google Scholar]
- 13.Walvekar M, Shaikh N, Sarvalkar P. Effects of glycowithanolides on lipid peroxidation and lipofuscinogenesis in male reproductive organs of mice. Iran J Reproductive Med. 2013;11(9):711-716. [PMC free article] [PubMed] [Google Scholar]
- 14.Imtiyaz S, Aslam M, Tariq M, Chaudhary SS. Withania somnifera: A potent unani aphrodisiac drug. Int Res J Pharmaceut Appl Sci. 2013;3(4):59-63. [Google Scholar]
- 15.Kulkarni SK, Akula KK, Dhir A. Effect of Withania somnifera Dunal root extract against pentylenetetrazol seizure threshold in mice: Possible involvement of GABAergic system. Indian Journal of Experimental Biology. 2008;46(6):465-469. [PubMed] [Google Scholar]
- 16.Patil D, Gautam M, Jadhav U, et al. Physicochemical stability and biological activity ofWithania somniferaExtract under real-time and accelerated storage conditions. Planta Medica. 2010;76(05):481-488. [DOI] [PubMed] [Google Scholar]
- 17.Sulaiman SF, Sajak AA, Ooi KL, Seow EM. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. J Food Compos Anal. 2011;24(4-5):506-515. [Google Scholar]
- 18.Jain S, Jain A, Vaidya A, Kumar D, Jain V. Preliminary phytochemical, pharmacognostical and physico-chemical evaluation of Cedrus deodara heartwood. J Pharmacogn Phytochem. 2014;3(1). [Google Scholar]
- 19.Pranuthi EK, Narendra K, Swathi J, et al. Qualitative assessment of bioactive compounds from a very rare medicinal plant ficus dalhousiae miq. J Pharmacogn Phytochem. 2014;3(1). [Google Scholar]
- 20.Ayaz M, Junaid M, Ahmed J, et al. Phenolic contents, antioxidant and anticholinesterase potentials of crude extract, subsequent fractions and crude saponins from Polygonum hydropiper L. BMC Complementary and Altern Med. 2014;14(1):145-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yue X, Hao Y, Jian-Fei W, et al. Simultaneous determination of nine phenolic acids in dendranthema morifolium (Ramat) Tzvel. cv. Chuju samples by high performance liquid chromatography. Chin J Anal Chem. 2013;41(3):383-388. [Google Scholar]
- 22.Munir N, Mahmood Z, Yameen M, Mustafa G. Therapeutic response of epimedium gandiflorum's different doses to restore the antioxidant potential and reproductive hormones in male albino rats. Dose-response. 2020;18(3):1559325820959563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Association of Agriculture chemists (AOAC) . Official Methods of Analysis. 17th ed. Galthersburg, MD, USA: The Association of Official Analytical Chemists; 2000. [Google Scholar]
- 24.Colagar AH, Marzony ET, Chaichi MJ. Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr Res. 2009;29(2):82-88. [DOI] [PubMed] [Google Scholar]
- 25.Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem. 1999;269(2):337-341. [DOI] [PubMed] [Google Scholar]
- 26.Begum S, Shadrack DM, Joseph FM, Ndensendo VMK. Molecular dynamics simulation of bioactive compounds of Withania somnifera leaf extract as DNA gyrase inhibitor. J Biomol Struct Dyn. 2021:1-8. [DOI] [PubMed] [Google Scholar]
- 27.Ruch RJ, Cheng S-J, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10(6):1003-1008. [DOI] [PubMed] [Google Scholar]
- 28.Yadav M, Yadav A, Yadav JP. In vitro antioxidant activity and total phenolic content of endophytic fungi isolated from Eugenia jambolana Lam. Asian Pac. j. trop. med. 2014;7:S256-S261. [DOI] [PubMed] [Google Scholar]
- 29.Powell WA, Catranis CM, Maynard CA. Design of self-processing antimicrobial peptides for plant protection. Lett Appl Microbiol. 2000;31(2):163-168. [DOI] [PubMed] [Google Scholar]
- 30.Rahman MA, Sultana R, Bin Emran T, et al. Effects of organic extracts of six Bangladeshi plants on in vitro thrombolysis and cytotoxicity. BMC Complementary and Alternative Medicine. 2013;13(1):25-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ames BN, Durston WE, Yamasaki E, Lee FD. Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection. Proc Natl Acad Sci Unit States Am. 1973;70(8):2281-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian B, Hua Y. Concentration-dependence of prooxidant and antioxidant effects of aloin and aloe-emodin on DNA. Food Chem. 2005;91(3):413-418. [Google Scholar]
- 33.Kandil H, Agarwal A, Saleh R, et al. Editorial commentary on draft of world health organization sixth edition laboratory manual for the examination and processing of human semen. World J Mens Health. 2021:39(4): 577-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar L, Sarswat A, Lal N, et al. Design and synthesis of 3-(azol-1-yl)phenylpropanes as microbicidal spermicides for prophylactic contraception. Bioorg Med Chem. 2011;21(1):176-181. [DOI] [PubMed] [Google Scholar]
- 35.Prakash S, Ravikumar S, Reddy KVR, Kannapiran E. Spermicidal activity of Indian seaweeds: Anin vitrostudy. Andrologia. 2014;46(4):408-416. [DOI] [PubMed] [Google Scholar]
- 36.Cheesbrough M. District Laboratory Practice in Tropical Countries, Part 2. Cambridge University Press; 2005:313-318. [Google Scholar]
- 37.Montgomery DC, Myers RH, Carter WH, Jr, Vining GG. The hierarchy principle in designed industrial experiments. Qual Reliab Eng Int. 2005;21(2):197-201. [Google Scholar]
- 38.Ames BN, McCann J, Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res Environ Mutagen Relat Subj. 1975;31(6):347-363. [DOI] [PubMed] [Google Scholar]
- 39.Okwu DE, Okwu ME. Chemical composition of Spondias mombin Linn plant parts. J Sustain Agric Environ. 2004;6(2):140-147. [Google Scholar]
- 40.Zheng X, Liu B, Li L, Zhu X. Microwave-assisted extraction and antioxidant activity of total phenolic compounds from pomegranate peel. J Med Plants Res. 2011;5(6):1004-1011. [Google Scholar]
- 41.Leake JR. Is diversity of ectomycorrhizal fungi important for ecosystem function? New Phytologist. 2001;152:1-3. [DOI] [PubMed] [Google Scholar]
- 42.Gulati S, Madan VK, JANGRA SS, Yadav IS. Determination of total phenolics, total flavonoids and evaluation of DPPH free radical scavenging activity of Ashwagandha (Withania somnifera L.) roots. Asian J Chem. 2017;29(8):1660-1664. [Google Scholar]
- 43.Saxena M, Faridi U, Srivastava SK, et al. A cytotoxic and hepatoprotective agent from Withania somnifera and biological evaluation of its ester derivatives. Nat Prod Commun. 2007;2(7):1934578X0700200714. [Google Scholar]
- 44.Smolskaitė L, Venskutonis PR, Talou T. Comprehensive evaluation of antioxidant and antimicrobial properties of different mushroom species. LWT-Food Sci Technol. 2015;60(1):462-471. [Google Scholar]
- 45.Jayakumar R, Chennazhi KP, Srinivasan S, Nair SV, Furuike T, Tamura H. Chitin scaffolds in tissue engineering. Int J Mol Sci. 2011;12(3):1876-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paul RK. In vitro Antioxidant activity of Withania somnifera root. Int J Adv Res Comput Sci. 2016;3(3):45-56. [Google Scholar]
- 47.Chatterjea MN, Shinde R. Textbook of Medical Biochemistry. 8th ed. New Delhi: Jaypee Brothers Medical Publications; 2011:100-111. [Google Scholar]
- 48.Woldegiorgis AZ, Abate D, Haki GD, Ziegler GR. Antioxidant property of edible mushrooms collected from Ethiopia. Food Chemistry. 2014;157:30-36. [DOI] [PubMed] [Google Scholar]
- 49.Yadav AK, Rai DC. In vitro screening of Ashwagandha root extracts for the maximum functional components. J Pharm Innov. 2018;7(2): 12-16. [Google Scholar]
- 50.Srinivasa Rao B, Chandrasekaran CV, et al. Mutagenicity and acute oral toxicity test for herbal poultry feed supplements. J Toxicol. 2018;2018:9412167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel SB, Rao NJ, Hingorani LL. Safety assessment of Withania somnifera extract standardized for Withaferin A: Acute and sub-acute toxicity study. J Ayurveda Integr Med. 2016;7(1):30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prabu PC, Panchapakesan S, Raj CD. Acute and sub-acute oral toxicity assessment of the hydroalcoholic extract of Withania somnifera roots in wistar rats. Phytother Res. 2013;27(8):1169-1178. [DOI] [PubMed] [Google Scholar]
- 53.Capstick T, Henry MT. Efficacy of thrombolytic agents in the treatment of pulmonary embolism. Eur Respir J. 2005;26(5):864-874. [DOI] [PubMed] [Google Scholar]
- 54.Fuentes E, Guzmán L, Alarcón M, Moore R, Palomo I. Thrombolytic/Fibrinolytic Mechanism of Natural Products. sine loco: IntechOpen; 2014:107-121. [Google Scholar]
- 55.Shahriar M, Alam F, Uddin MM. Membrane stabilizing and thrombolytic activity of Withania somnifera root. Am J Phytomed Clin Ther. 2014;2(2):252-256. [Google Scholar]
- 56.Sai Sandeep Y, Panigrahi M, Divya GC, Beena DB. Evaluation of in vitro thrombolytic activity of phytochemicals in Bacopa monnieri Linn. J Pharm Res. 2012;5(1):100-101. [Google Scholar]
- 57.Khanam S, Devi K. Antimutagenic activity of ashwagandha. J Nat Remedies. 2005;5(2):126-131. [Google Scholar]
- 58.Liu J, Liang P, Yin C, et al. Effects of several Chinese herbal aqueous extracts on human sperm motility in vitro. Andrologia. 2004;36(2):78-83. [DOI] [PubMed] [Google Scholar]
- 59.Peng SJ, Lu RK, Yu LH. [Effects of semen Cuscutae, rhizoma Curculiginis, radix Morindae officinalis on human spermatozoan's motility and membrane function in vitro]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1997;17(3):145-147. [PubMed] [Google Scholar]