Abstract
It has been reported that the mammalian target of rapamycin (mTOR) pathway is involved in the pathogenesis of systemic lupus erythematosus (SLE), and increasing evidence has shown the effect of mTOR-targeted therapies with sirolimus in SLE. The objective of this study was to report the successful treatment of sirolimus in a Chinese patient with refractory lupus nephritis (LN) and anti-phospholipid antibody syndrome (APS). A 44-year-old female with a previous diagnosis of autoimmune hemolytic anemia (AIHA) and APS secondary to SLE presented with lupus nephritis refractory to cyclophosphamide and mycophenolate. Renal biopsy met the criteria of WHO class III LN complicated by acute tubular injury and immunofluorescence confirmed the activation of the mTOR pathway. Treatment with the mTOR inhibitor sirolimus was initiated in this patient. Complete remission (CR) was achieved after 6 months, and flare-free remission was maintained for the next 3.5 years. The literature on the efficacy of sirolimus in patients with LN was reviewed. Although the available evidence is limited to retrospective studies with small sample sizes, sirolimus appeared to be efficacious in some patients with refractory LN. Well-designed clinical trials are warranted, and pathology-guided precision medicine might assist in guiding physicians’ treatment decisions.
Keywords: antiphospholipid antibody syndrome (APS), lupus nephritis (LN), mammalian target of rapamycin (mTOR), refractory lupus nephritis, sirolimus, systemic lupus erythematosus (SLE)
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease. Lupus nephritis (LN) is one of the most common and severe manifestations of SLE. Despite extensive first-line treatment with induction therapy, there is still some proportion of LN patients who do not response to induction therapy, this condition is sometimes referred to as refractory LN. Patients with refractory LN are an increased risk of developing poorer outcomes, especially in end-stage renal disease (ESRD); however, management of these patients remains a challenge. 1 The current treatment options recommended by European League Against Rheumatism (EULAR/ERA-EDTA) for these patients include high-dose glucocorticoids and alternating induction treatments between cyclophosphamide (CTX) and mycophenolate acid derivates. As reported, B cell-targeted therapy, a multitarget regimen, plasma exchange (PLEX) or immunoadsorption (IAS) and stem cell transplantation could be considered. 2
Increasing evidence regarding the use of the mTOR inhibitor rapamycin (also called sirolimus) as a new potential treatment regimen for LN has been gained from animal models since 2008 3 , 4 and patient trials since 2009.5 –9 A single-arm, open-label, phase 1/2 trial and a real-world study confirmed the safety and efficacy of oral sirolimus in the treatment of SLE.10,11 Retrospective studies reported LN patients who responded to sirolimus as an induction or maintenance therapy. Additionly, in lupus nephritis (class III or IV) patients with secondary antiphospholipid syndrome nephropathy (APSN), marked activation of the mTORC pathway was found in the vascular endothelial cells of renal tissue. Compared with the control group, sirolimus treatment protected patients from renal failure in patients with APSN who had already received transplantation (11% (3/27) vs. 70% (7/10), p = 0.001). 12 In the first case of APSN in a nontransplant patient treated with sirolimus, inhibition of the AKT/mTORC pathway were observed in a repeat kidney biopsy. 13
Here, we report a Chinese SLE patient with a diagnosis of both refractory LN and secondary anti-phospholipid antibody syndrome (APS) who was successfully treated with sirolimus. Endothelial mTORC activation in the kidney was indicated by immunofluorescence. Literature on the efficacy of sirolimus in patients with LN was also reviewed.
Case report
A 44-year-old woman was admitted to the hospital because of refractory proteinuria for two years. She was diagnosed with autoimmune hemolytic anemia (AIHA) secondary to systemic lupus erythematosus (SLE) 22 years ago. The disease remained in remission after treatment with prednisone. There was a flare of AIHA 4 years ago when she was giving birth to a baby. Oral prednisone (0.5 mg/kg/d) and hydroxychloroquine were prescribed to her. Soon after that, she experienced a deep venous thrombosis in her lower right leg without other precipitating factors. Warfarin, a vitamin K antagonist, was administered to the patient, and the prothrombin time and international normalized ratio were monitored. In 2015, she presented to our department after an upper respiratory infection with proteinuria. The laboratory examination revealed that her 24-hour urine protein (24hUP) was 12 g/d with a normal creatinine level. The serologic antibodies were positive for ANA, anti-nucleosome antibody, anti-double-stranded DNA, as well as a high level of IgG type of anti-cardiolipin antibody and anti-β2-glycoprotein 1. However, she had no history of miscarriages or thrombocytopenia. A diagnosis of ‘systemic lupus erythematosus, lupus nephritis and the antiphospholipid syndrome’ was made, and 1 mg/kg/d prednisone was prescribed to her. However, she began to have symptoms of progressive systemic edema, oliguria, severe hypertension and shortness of breath. Repeated examination of 24hUP was 20 g/d with an elevated creatinine level of 126 µmol/L and albumin level of 16 g/L. In addition, ultrasound showed a new venous thrombosis in her left femoral vein. She received a high dose of methylprednisolone and hydroxychloroquine followed by 0.75 g/m2 body surface area cyclophosphamide once (0.4 g) as well as continuous renal replacement therapy. She did not receive renal biopsy due to anticoagulant therapy. Her symptoms and decreased urine output resolved, and renal replacement therapy was discontinued, but an impaired liver function limited the use of cyclophosphamide. Then, she tried 1.5 g/d mycophenolate mofetil for two months and another round of cyclophosphamide for another four months (2.2 g), but she experienced recurrent infections, including soft tissue infection in her right lower limb, baric abscess and pneumonia. Her 24hUP remained more than 10 g/d. Therefore, she received a renal biopsy after suspension of anticoagulants. The pathology revealed lupus nephritis Class III (A), with acute tubular injury.
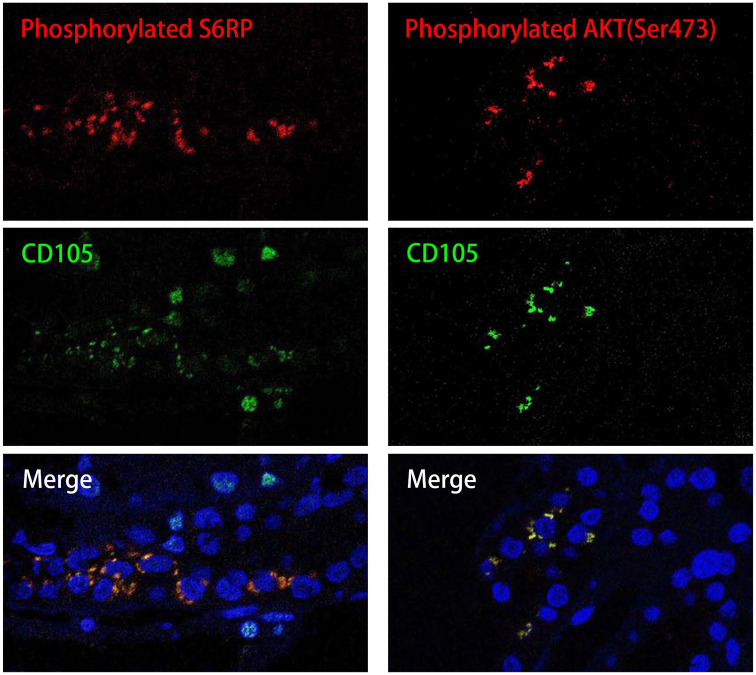
Given the previous evidence of sirolimus treatment in patients with active LN and APSN, we tried to explore whether mTORC was activated and whether an mTORC inhibitor could be effective in this case since both refractory LN and secondary APS were present. We then performed immunofluorescence and observed that S6RP and AKT (Ser473) were activated in renal vascular endothelial cells that expressed CD105, indicating the activation of the mTORC pathway (Figure 1). Sirolimus 2 mg/d was administered to her, as well as warfarin. Her 24hUP resolved (<0.5 g) after 6 months. After 1 year, sirolimus was tapered to 1 mg/d for another half year and then discontinued. Her lupus nephritis remained in remission with a low dose of prednisone (lowest 2.5 mg/d) even after discontinuation of sirolimus for another 3.5 years. During the period of sirolimus, she had two courses of upper respiratory infection, and no serious adverse events were reported. The main clinical parameters and therapeutic interventions are depicted in Figure 2.
Figure 1.
Renal biopsy proved mTORC pathway activation in endothelial cells from an LN patient with APS.
The confocal microscopy on kidney-biopsy specimens showed positive phosphorylated ribosomal protein S6 (S6RP red), AKT phosphorylated on serine 473 (Ser473 red) and the endothelial cell marker CD105 (green) with merge image (yellow) on the bottom. These demonstrated the recruitment of mTORC1 and mTORC2, respectively, in vascular endothelial cells. Nuclei are blue (Dapi stain).
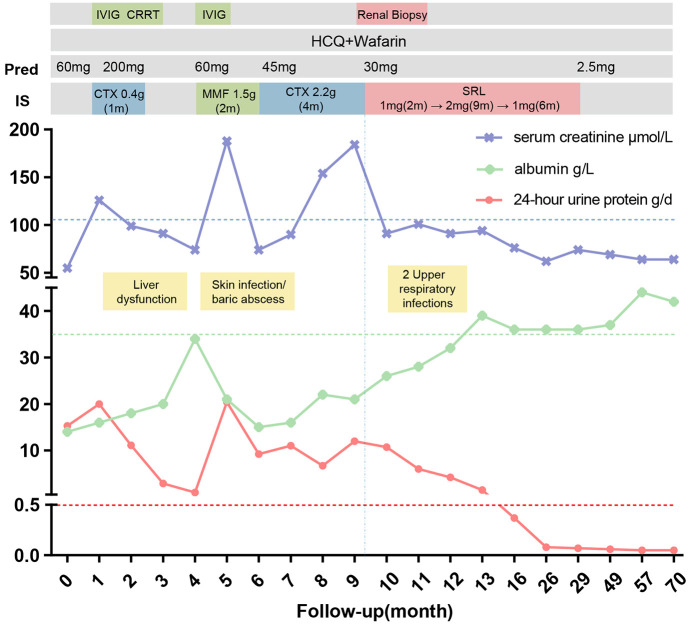
Figure 2.
Laboratory findings and therapeutic interventions in a patient with LN and APS.
The numbers displayed on the Horizontal axis represent months. The patient presented to the department of rheumatology in 2015 with diagnosis of LN with APS. She received high dose of methylprednisolone and hydroxychloroquine followed by 0.4 g CTX for once as well as renal replacement therapy for elevated creatine. With impaired liver function, she shifted to MMF for 2 months, and another turn of CTX for 4 months. Side-effect showed and 24hUP remained more than 10 g/d. Renal biopsy demonstrated the mTOR pathway activation and then sirolimus 2mg/d was administrated to her as well as warfarin. Her 24hUP resolved (<0.5g/d) after 6 months. (IVIG, intravenous immunoglobulin; CRRT, continuous renal replacement therapy; HCQ, hydroxychloroquine; CTX, cyclophosphamide; MMF, mycophenolate mofetil; SRL, sirolimus).
Methods
We have de-identified all the details of this patient. This case was reported according to the CARE guidelines (checklist in supplementary materials). 14 Paraffin-embedded renal sections were incubated with P-AKT (Ser473) antibodies, anti-P-S6RP antibodies, and anti-CD105 antibodies after appropriate antigen retrieval. The primary antibodies were revealed with the appropriate FITC- or AF568-conjugated secondary antibodies. Immunofluorescence staining was visualized using a Zeiss LSM 800 confocal microscope. The patient’s informed consent to treatment was obtained. Institutional review board approval by the Ethics Committee of Renji Hospital was waived for case report. We have de-identified all the details of this patient.
Discussion
In this case of refractory Class III LN and secondary APS, although the histology of the renal biopsy was not a typical APSN, activation of the mTORC pathway in kidney endothelial cells was found. Therefore, mTORC-targeted monotherapy was administered to the patient accordingly, the patient attaining and maintaining complete remission. To the best of our knowledge, this is the first case of refractory LN accompanied by APS guided by biopsy-proven activation of the mTORC pathway effectively treated with sirolimus as a monotherapy.
The treatment effect of sirolimus in LN can be multifaceted. First, it has been reported that IgG antibodies from patients with APS can cause mTORC activation in endothelial cells, 12 which indicates that the treatment outcomes might partially be attributed to additional regulation of vascular dysfunction. Second, mTOR is crucial for the proliferation and differentiation regulation of immune cells. 15 In a phase 1/2 trial, sirolimus treatment for 12 months expanded regulatory T cells and CD8 + memory T cell populations and inhibited interleukin-4 and interleukin-17 production by CD4 + and CD4-CD8- double-negative T cells in SLE patients. 10 Studies have shown that ramamycin can effectively inhibit the pathogenic memory B cells 16 from SLE. Moreover, rapamycin can protect against podocyte injury by inducing autophagy. 17
Systemic literature review of the treatment of sirolimus in LN was performed and 5 publications were identified. The earliest report about the effect of sirolimus in LN was from Changi Hospital, Singapore. A 34-year-old Chinese woman with SLE/APS experienced a disease flare manifesting as LN, and she was unresponsive to tacrolimus, cyclosporin, or mycophenolate. After sirolimus was administered, and complete remission was achieved with a reduction in the 24hUP from 1.29 g/d to 0.3 g/d. 6
Two retrospective studies about the effect of sirolimus from Queen Mary Hospital, Hong Kong were quite encouraging. In 2012, they reported 3 patients with active LN who received sirolimus as induction therapy achieving renal response and another 2 LN patients with sirolimus as maintenance therapy attaining flare-free status for 18 months. 7 In 2018, the same group reported long-term data about sirolimus in 16 LN patients, with a mean follow-up of 45 months, and only 1 patient had a renal flare. 8
Another real-world study from Peking Union Medical College Hospital (PUMCH) showed discrepant results. Among 17 active LN patients who were treated with sirolimus, 7 had a renal response by 3 to 9 months, whereas another 10 patients experienced worsening of proteinuria. 9 The discrepancy in the treatment response might be explained by the different inclusion criteria. PUMCH enrolled refractory patients with either intolerance or a poor response to the previous immunosuppressants.
Recently, a Caucasian LN cohort reported 12 patients taking sirolimus with a mean follow-up of 35.5 months. 5 All the patients had previously received first-line induction therapy but transitioned to sirolimus because of renal flare or intolerance. At 12 months and 24 months, 4 (33%) and 7 (58%) out of 12 patients achieved CR, respectively. Another 5 patients had no response to sirolimus.
There are some limitations of this study. First, this is a retrospective case report. Second, a thorough overview of pathology was not feasible due to a lack of electron microscopic manifestations. Third, a repeat kidney biopsy could not be conducted according to the patient’s will to confirm the efficacy of sirolimus.
In conclusion, sirolimus might be a promising remedy for unresponsive proliferative lupus nephritis. Further clinical studies with a larger population are needed. Molecular pathology may guide personalized treatment.
Supplemental Material
Supplemental material, sj-jpg-1-tab-10.1177_1759720X221079253 for Successful treatment of sirolimus in a Chinese patient with refractory LN and APS: a case report by Danting Zhang, Fangfang Sun and Shuang Ye in Therapeutic Advances in Musculoskeletal Disease
Footnotes
Author contributions: Danting Zhang: Conceptualization; Data curation; Visualization; Writing – original draft
Fangfang Sun: Conceptualization; Data curation; Funding acquisition; Methodology; Visualization; Writing – original draft
Shuang Ye: Funding acquisition; Project administration; Supervision; Writing – review & editing
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research is supported by grants from the Clinical Research Plan of Shanghai Hospital Development Center (Project No. SHDC2020CR1015B) and Shanghai Municipal Health Commission (No. 202040291).
ORCID iDs: Danting Zhang  https://orcid.org/0000-0001-7205-8795
https://orcid.org/0000-0001-7205-8795
Fangfang Sun  https://orcid.org/0000-0003-2570-4576
https://orcid.org/0000-0003-2570-4576
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Danting Zhang, Department of Rheumatology, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Fangfang Sun, Department of Rheumatology, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Shuang Ye, Department of Rheumatology, Renji Hospital, Shanghai Jiao Tong University School of Medicine, 2000 Jiangyue Road, Shanghai, 201112, China.
References
- 1. Chen YE, Korbet SM, Katz RS, et al. Value of a complete or partial remission in severe lupus nephritis. Clin J Am Soc Nephrol 2008; 3: 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kronbichler A, Brezina B, Gauckler P, et al. Refractory lupus nephritis: when, why and how to treat. Autoimmun Rev 2019; 18: 510–518. [DOI] [PubMed] [Google Scholar]
- 3. Lui SL, Yung S, Tsang R, et al. Rapamycin prevents the development of nephritis in lupus-prone NZB/W F1 mice. Lupus 2008; 17: 305–313. [DOI] [PubMed] [Google Scholar]
- 4. Lui SL, Tsang R, Chan KW, et al. Rapamycin attenuates the severity of established nephritis in lupus-prone NZB/W F1 mice. Nephrol Dial Transplant 2008; 23: 2768–2776. [DOI] [PubMed] [Google Scholar]
- 5. Piranavan P, Perl A. Improvement of renal and non-renal SLE outcome measures on sirolimus therapy – a 21-year follow-up study of 73 patients. Clin Immunol 2021; 229: 108781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoon KH. Proliferation signal inhibitors for the treatment of refractory autoimmune rheumatic diseases: a new therapeutic option. Ann N Y Acad Sci 2009; 1173: 752–756. [DOI] [PubMed] [Google Scholar]
- 7. Yap DYH, Ma MK, Tang CS, et al. Proliferation signal inhibitors in the treatment of lupus nephritis: preliminary experience. Nephrology 2012; 17: 676–680. [DOI] [PubMed] [Google Scholar]
- 8. Yap DYH, Tang C, Chan GCW, et al. Longterm data on sirolimus treatment in patients with lupus nephritis. J Rheumatol 2018; 45: 1663–1670. [DOI] [PubMed] [Google Scholar]
- 9. Peng L, Wu C, Hong R, et al. Clinical efficacy and safety of sirolimus in systemic lupus erythematosus: a real-world study and meta-analysis. Ther Adv Musculoskelet Dis 2020; 12: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lai Z-W, Kelly R, Winans T, et al. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: a single-arm, open-label, phase 1/2 trial. Lancet 2018; 391: 1186–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eriksson P, Wallin P, Sjöwall C. Clinical experience of sirolimus regarding efficacy and safety in systemic lupus erythematosus. Front Pharmacol 2019; 10: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Canaud G, Bienaimé F, Tabarin F, et al. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N Engl J Med 2014; 371: 303–312. [DOI] [PubMed] [Google Scholar]
- 13. Dufour I, Venot Q, Aydin S, et al. mTORC pathway activation and effect of sirolimus on native kidney antiphospholipid syndrome nephropathy: a case report. Am J Kidney Dis 2020; 76: 288–291. [DOI] [PubMed] [Google Scholar]
- 14. Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case report guideline development. J Clin Epidemiol 2014; 67: 46–51. [DOI] [PubMed] [Google Scholar]
- 15. Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol 2009; 9: 324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu C, Fu Q, Guo Q, et al. Lupus-associated atypical memory B cells are mTORC1-hyperactivated and functionally dysregulated. Ann Rheum Dis 2019; 78: 1090–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou XJ, Klionsky DJ, Zhang H. Podocytes and autophagy: a potential therapeutic target in lupus nephritis. Autophagy 2019; 15: 908–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-jpg-1-tab-10.1177_1759720X221079253 for Successful treatment of sirolimus in a Chinese patient with refractory LN and APS: a case report by Danting Zhang, Fangfang Sun and Shuang Ye in Therapeutic Advances in Musculoskeletal Disease