Abstract
Background:
Patients with diabetes and co-existing chronic kidney disease and/or cardiovascular disease have complex medical needs with multiple indications for different guideline-directed medical therapies and require high health care resource utilization. The Cardiac and Renal Endocrine Clinic (C.a.R.E. Clinic) is a multi- and interdisciplinary clinic offering a unique care model to this population to overcome barriers to optimal care.
Objective:
To describe the patient characteristics and clinical data of consecutive patients seen in the C.a.R.E. Clinic between 2014 and 2020, with a focus on the feasibility, strengths, and challenges of this outpatient care model.
Design:
Single-center retrospective cohort study.
Setting:
The C.a.R.E. Clinic is a multi- and interdisciplinary clinic at Toronto General Hospital in Toronto, Canada.
Patients:
We reviewed the charts of all 118 patients who had been referred to the C.a.R.E. Clinic with type 2 diabetes mellitus, co-existing renal disease, and/or cardiovascular disease.
Measurements:
Demographic data, medication data, clinic blood pressure measurements, and laboratory data were assessed at the first and last available clinic visit.
Methods:
Data were extracted via manual chart review of paper and electronic medical records.
Results:
First and last attended clinic visit data were available for descriptive analysis in 74 patients. There was a significant improvement in low-density lipoprotein (LDL) cholesterol (1.9 mmol/L vs 1.5 mmol/L, P < .01), hemoglobin A1C (7.5% vs 7.1%, P = .02), and the proportion of patients with blood pressure at target (52.7% vs 36.5%, P = .04), but not body mass index (29.7 kg/m² vs 29.6 kg/m², P = .15) between the last and first available clinic visits. There was higher uptake in evidence-based medication use including statins (93.2% vs 81.1%, P = .01), SGLT-2i (35.1% vs 4.1%, P < .01), and GLP-1 receptor agonists (13.5% vs 4.1%, P = .02), while RAAS inhibitor use was already high at baseline (81.8% vs 78.4%, P = .56). There remains a significant opportunity for therapy with sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 receptor agonists.
Limitations:
This is a retrospective chart review lacking a control group, therefore clinical improvements cannot be causally attributed to the clinic alone. New evidence and changes to guideline-recommended therapies also contributed to practice changes during this time period.
Conclusions:
A multi- and interdisciplinary clinic is a feasible and potentially effective way to improve evidence-based and patient-centered care for patients with diabetes, kidney, and cardiovascular disease.
Keywords: diabetes, chronic kidney disease, cardiovascular disease, multidisciplinary, interdisciplinary
Abrégé
Contexte:
Les patients diabétiques présentant une néphropathie chronique et/ou maladie cardiovasculaire co-existante ont des besoins complexes avec de multiples indications concernant différents traitements médicaux recommandés par les lignes directrices. En outre, ces patients nécessitent une utilisation élevée des ressources de santé. La clinique C.a.R.E. (Cardiac and Renal Endocrine Clinic) est une clinique interdisciplinaire et multidisciplinaire offrant un modèle de soins unique qui permet de surmonter les obstacles aux soins optimaux pour cette population.
Objectif:
Décrire les caractéristiques et les données cliniques des patients consécutifs suivis à la clinique C.a.R.E. entre 2014 et 2020, en se concentrant sur la faisabilité et sur les avantages et les défis de ce modèle de soins ambulatoires.
Type d’étude:
Étude de cohorte rétrospective menée dans un seul centre.
Cadre:
La clinique C.a.R.E. est une clinique multidisciplinaire et interdisciplinaire de l’Hôpital général de Toronto (Canada).
Sujets:
Nous avons examiné les dossiers des 118 patients diabétiques de type 2 atteints d’une néphropathie et/ou maladie cardiovasculaire qui ont été dirigés vers la clinique C.a.R.E. au cours de la période étudiée.
Mesures:
Les données démographiques, les données sur les ordonnances, les mesures cliniques de la pression artérielle et les données de laboratoire ont été évaluées pour la première et la dernière visite à la clinique disponibles.
Méthodologie:
Les données ont été extraites par un examen manuel des dossiers médicaux papier et électronique.
Résultats:
Les données d’intérêt pour la première et la dernière visite à la clinique étaient disponibles pour l’analyse descriptive chez 74 patients. Entre la première et la dernière visite disponible, on a observé une amélioration significative du taux de cholestérol LDL (1,9 mmol/L vs 1,5 mmol/L; p < 0,01), de l’hémoglobine A1c (7,5 % vs 7,1 %; p = 0,02) et de la proportion de patients avec une mesure de pression artérielle dans les valeurs cibles (52,7 % vs 36,5 %; p = 0,04) alors que l’indice de masse corporelle est demeuré inchangé (29,7 kg/m² vs 29,6 kg/m²; p = 0,15). Les ordonnances de thérapies fondées sur les données probantes ont été plus fréquentes, notamment pour les statines (93,2 % vs 81,1 %; p = 0,01), le SGLT-2i (35,1 % vs 4,1 %; p < 0,01) et les agonistes des récepteurs GLP-1 (13,5 % vs 4,1 %; p = 0,02); l’utilisation d’inhibiteurs du SRAA était déjà élevée au départ (81,8 % vs 78,4 %; p = 0,56). De grandes possibilités de traitement demeurent pour les inhibiteurs du cotransporteur-2 de sodium-glucose et les agonistes des récepteurs du peptide-1 de type glucagon.
Limites:
Il s’agit d’un examen rétrospectif des dossiers sans groupe témoin; les améliorations cliniques ne peuvent être attribuées de façon causale à la clinique seule. Pendant la période étudiée, de nouvelles données probantes et des changements aux traitements recommandés par les lignes directrices ont également entraîné des changements dans la pratique.
Conclusion:
Une clinique multidisciplinaire et interdisciplinaire est une solution viable et potentiellement efficace pour améliorer les soins axés sur les patients et les traitements fondés sur les données probantes pour les patients diabétiques atteints de néphropathie et/ou de maladies cardiovasculaires.
Background
Cardiovascular disease (CVD), chronic kidney disease (CKD), and type 2 diabetes (T2D) are common and often co-existing problems. More than 40% of CKD patients have T2D, and CVD is the leading cause of death in people with CKD and people with diabetes.1,2 Patients with T2D and co-existing CKD and/or CVD have complex medical needs with multiple indications for different guideline-directed medical therapies, barriers to care and also require high health care resource utilization. 3 The prevalence of comorbid clinical conditions including hypertension, dyslipidemia, and obesity is also very high in patients with T2D, 4 which may lead to competing clinical priorities. The last decade has seen new pharmacological advances with overlapping mechanisms and clinical benefits in the realms of diabetes management, and in the primary and secondary prevention of both CVD and CKD. 5 The historical underutilization of evidence-based therapies (such as inhibitors of the renin-angiotensin-aldosterone system—RAAS) in high-risk populations highlights the need to prioritize the timely adoption of newer therapies. 6
Shared-care clinics involving multiple specialist physicians are a potential solution to the challenge of integrating care for patients with complex medical issues.7,8 A combined multicare clinic in patients with T2D, CVD, and CKD was shown to be noninferior to separate specialist physician care in patients with advanced CKD who were already involved in a multidisciplinary kidney care clinic, with significant estimated cost-savings per patient. 9 Preliminary data of 60 patients followed in a multidisciplinary cardiometabolic clinic for 4 months demonstrated superior clinical endpoints and use of guideline-directed therapy compared with propensity-matched controls. 10 A multidisciplinary care model has also been promoted as a more patient-centered approach to subspecialty care. 11 It remains unknown whether a multi- and interdisciplinary clinic can lead to long-term changes in clinical endpoints in patients with CKD, diabetes, and/or cardiac disease.
The Cardiac and Renal Endocrine Clinic (C.a.R.E. Clinic) is a once-monthly, multi-, and interdisciplinary clinic at Toronto General Hospital in Toronto, Canada. The clinic is multidisciplinary in that individuals from separate disciplines may consult on a single patient on a single appointment day (eg, a dietician and a chiropodist). The clinic is also interdisciplinary in that separate disciplinary approaches are integrated (by a cardiologist, nephrologist, and endocrinologist) into a single consultation, developing a single management plan with the patient. 12 Since 2014, 118 patients with T2D and co-existing renal disease and/or CVD were referred to the C.a.R.E. Clinic with the objective of providing integrated and patient-centered evidence-based care. Our objective was to describe the patient characteristics and clinical data of consecutive patients seen in the C.a.R.E. Clinic between 2014 and 2020, with a focus on the feasibility, strengths, and challenges of this outpatient care model.
Methods
Clinic Description
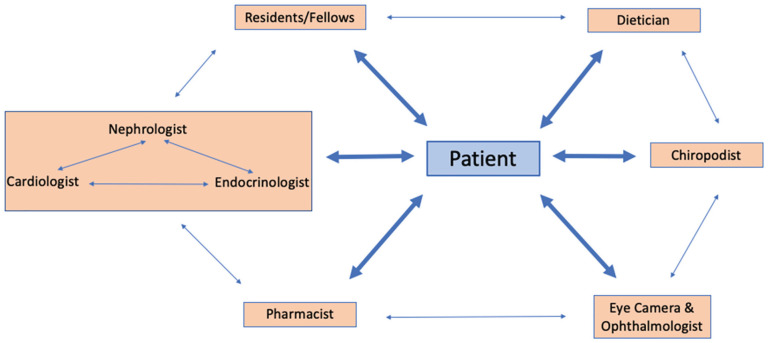
Patients are referred to the C.a.R.E. Clinic by their primary care or specialist physician and are not eligible for the clinic if they are already involved in a multidisciplinary kidney care clinic caring for patients with advanced CKD, thereby including patients with less advanced renal disease. The C.a.R.E. Clinic has both multi- and interdisciplinary components (Figure 1). 12 A resident or clinical fellow in cardiology, nephrology, endocrinology, or internal medicine assesses each patient and performs a history and physical examination, noting salient features relevant to all 3 disciplines. The medical plan is then reviewed by the trainee with the relevant attending physicians (based on the patient’s medical history) simultaneously—a cardiologist, nephrologist, and endocrinologist, and a cohesive plan is made and then reviewed with the patient regarding further investigations and medication changes. Patients are referred for further laboratory investigations as needed, as well any necessary cardiac investigations (such as echocardiograms, stress echocardiograms, and computed tomographic [CT] coronary angiograms). Patients are also informed of any relevant clinical trials for which they may be eligible to participate and invited to speak with a study coordinator if interested. All patients are assessed in clinic by a pharmacist for reconciliation of medications, and on an as-needed basis for medication education. Patients are assessed at the initial visit (as allowed by time) and on an as-needed basis by a dietician for counseling on weight loss and/or kidney/diabetes-specific diets, and a chiropodist to assess for foot pathology and provide education on diabetic foot disease. A diabetes nurse educator is available on an as-needed basis for counseling on glycemic management. There is also the opportunity for an on-site ophthalmology assessment for patients who have not had screening in the past 6 months. The fundus and optical coherence tomography (OCT) images are obtained by a technician at the time of clinic visit, uploaded to an electronic server, and then reviewed by an ophthalmologist to be graded for evidence of diabetic retinopathy or diabetic macular edema. At the conclusion of the clinic, members of the multi- and interdisciplinary care team review the bottom-line plans for each of the assessed patients, to ensure consistent messaging and care plans.
Figure 1.
Ca.R.E. clinic structure.
Study Design
We conducted a single-center retrospective cohort study to characterize the patient characteristics and clinical data relevant to the care of this population with T2D and co-existing CVD and/or CKD. We manually reviewed paper and electronic clinical documentation and laboratory data from the first and last patient visit for consecutive patients seen in the C.a.R.E. Clinic between the time of its inception in July 2014 and February 2020. This project was approved by the University Health Network Quality Improvement Review Committee.
All consecutive patients seen in the C.a.R.E. clinic at Toronto General Hospital between 2014 and February 2020 were included in this analysis. Charts were reviewed for all patients seen at least one time in the clinic. The number of patients without a follow-up visit was recorded, with the reason for no follow-up recorded if available. We gathered demographic data, data on relevant past medical history, most recent laboratory data available prior to the clinic visit, medication data, and clinic blood pressure (BP) readings (measured as an attended automated office BP) for consecutive patients at their first clinic visit and their last attended clinic visit prior to February 2020. Patients seen as a first visit after October 31, 2019 were excluded from the study given the proximity to the cut-off date of February 2020 would indicate insufficient time to meaningfully impact care. All included patient visits were in-person, as virtual care was not initiated until March 2020 in the context of the COVID-19 pandemic.
We identified the proportion of patients meeting clinical practice guideline-directed therapies such as BP target of less than or equal to 130/80, and clinically indicated statin and RAAS inhibitor therapy according to the 2018 clinical practice guidelines from Diabetes Canada. 13 Indications for RAAS inhibitors included T2D and any of the following: macrovascular or any microvascular complication (retinopathy, neuropathy, nephropathy defined as albumin to creatinine ratio>2 mg/mmol), CKD (glomerular filtration rate [GFR]<60 mL/min/1.73 m² on 2 occasions 3 months apart), and hypertension, and age >55 and any additional risk factors. Indications for statin therapy included T2D and age >40, T2D of any age, and any microvascular complication (retinopathy, neuropathy, nephropathy) or macrovascular complication of diabetes. 13 Although age >30 and diabetes duration >15 years is an additional indication for statin therapy, we did not include this criterion as diabetes duration could not reliably be ascertained in the chart review.
To calculate statistical significance between the paired data from the first and last visits, we used the Wilcoxon signed rank test and McNemar test for continuous and categorical variables, respectively. All statistical analyses evaluating treatment effects used a 5% significance level and were 2-sided. All analyses were performed using SAS 9.4 for Windows (SAS Institute, Cary, NC).
Results
Clinic Attendance
A total of 118 patients were referred to the clinic between July 2014 and February 2020. Twenty patients who were referred to the clinic did not attend their first in-patient visit and were excluded from further analysis. Four patients were excluded due to the first visit being after October 31, 2019. Of the remaining 94 patients, 20 patients did not attend their first follow-up visit (of which 3 patients were either discharged from the clinic or transferred to another specialized nephrology clinic after the first clinic visit), reflecting an 18% first follow-up nonattendance (Figure 2). First and last visit data were therefore available for descriptive analysis in 74 patients (Table 2). The mean ± SD length of follow-up duration was 27.7 ± 19.6 months, median (interquartile range) 24 (10.8-43.3). Three patients died during the follow-up period. We had complete medical history and medication data for all patients. We had missing data in various components of laboratory or BP data in 5% to 9% of patients. We had incomplete data to calculate body mass index (BMI) for 9% of patients at the initial visit and 24% at the last visit.
Figure 2.
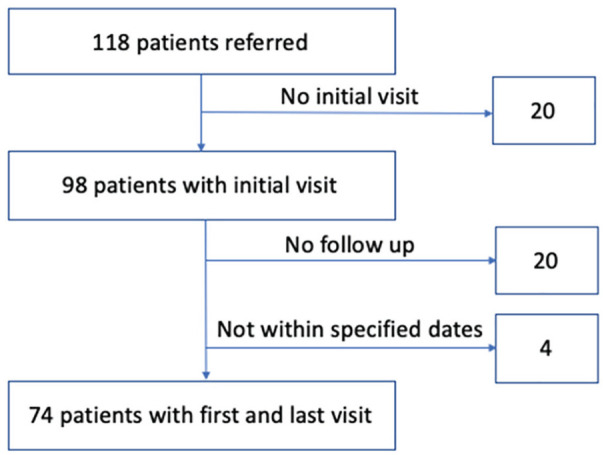
Patients included in retrospective chart review.
Table 2.
Clinical Data at First Clinic Visit Versus Last Clinic Visit (N = 74).
| First visit data | Last visit data | P value | |
|---|---|---|---|
| Body mass index, kg/m² | 29.7 (26.7, 33.9) | 29.6 (26.7, 33.6) | .15 |
| Systolic blood pressure, mm Hg | 132.0 (120.8, 154.8) | 129.0 (119.3, 140.0) | .03 |
| Diastolic blood pressure, mm Hg | 76.0 (70.0, 84.0) | 74.0 (67.0, 77.0) | .04 |
| Blood pressure ≤130/80—No. (%) | 27 (36.5) | 39 (52.7) | .04 |
| Hemoglobin A1C, % | 7.5 (6.6, 8.2) | 7.1 (6.3, 8.1) | .02 |
| eGFR, mL/min/1.73 m² a | 45.0 (33.0, 59.0) | 40.0 (30.0, 54.9) | <.01 |
| No albuminuria—No. (%) b | 12 (17.6) | 11 (16.2) | |
| Moderately increased proteinuria—No. (%) b | 24 (35.3) | 32 (47.1) | |
| Severely increased proteinuria—No. (%) b | 32 (47.1) | 25 (36.8) | |
| Low-density lipoprotein, mmol/L | 1.9 (1.5, 2.3) | 1.5 (1.2, 1.9) | <.01 |
| Aspirin and/or clopidogrel use—No. (%) | 37 (50.0) | 38 (51.4) | .71 |
| Renin-angiotensin-aldosterone system inhibitor use—No. (%) | 58 (78.4) | 61 (81.8) | .56 |
| Statin use—No. (%) | 60 (81.1) | 69 (93.2) | .01 |
| Sodium-glucose cotransporter-2 inhibitor use—No. (%) | 3 (4.1) | 26 (35.1) | <.01 |
| Glucagon-like peptide-1 receptor agonist use—No. (%) | 3 (4.1) | 10 (13.5) | .02 |
Note. Continuous data are presented as median (IQR); categorical data are presented as No. (%). eGFR = estimated glomerular filtration rate.
eGFR calculated using Chronic Kidney Disease Epidemiology Collaboration equation.
Proteinuria data available by either albumin:creatinine ratio or 24-hour urine collection for n = 68 at both first and last visits.
Demographic and Clinical Characteristics
Demographic and clinical data between patients who attended and who did not attend their first follow-up visit were similar between the 2 groups with the exception of higher baseline BPs in the lost-to-follow up group (Supplementary Table 1). The mean age at the first clinic visit was 66 ± 14 years, two thirds of patients were male, and most of the patients were overweight (34.3%) or obese (47.7%). In all, 68 (92%) patients had T2D, 2 patients had type 1 diabetes, 3 had impaired fasting glucose, and 1 patient did not have diabetes. In all, 31 of 74 patients (41.9%) had an established diagnosis of a macrovascular complication of diabetes (history of coronary artery disease, cerebrovascular accident, or peripheral vascular disease). In all, 29 patients (39.2%) and 24 patients (32.4%) had a previous diagnosis of diabetic retinopathy and neuropathy, respectively. In all, 71 patients (95.9%) had an established diagnosis of CKD, with a mean (SD) eGFR of 51.4 ± 24.17 mL/min/m2 prior to the first clinic visit (Table 1 and Figure 3).
Table 1.
Baseline Clinical Characteristics at the First Clinic Visit (N = 74).
| First visit data | |
|---|---|
| Age, y | 70.5 (59.0, 75.8) |
| No. (%) male | 49 (66) |
| Estimated glomerular filtration rate, mL/min/1.73 m² | 45.0 (33.0, 59.0) |
| No. (%) with chronic kidney disease | 71 (95.9) |
| No. (%) with retinopathy | 29 (39.2) |
| No. (%) with neuropathy | 24 (32.4) |
| No. (%) with coronary artery disease | 24 (32.4) |
| No. (%) with peripheral vascular disease | 8 (10.8) |
| No. (%) with stroke/transient ischemic attack | 6 (8.1) |
| No. (%) with indication for renin-angiotensin-aldosterone system inhibitor | 72 (97.3) |
| No. (%) with indication for statin | 72 (97.3) |
| No. (%) with indication for sodium-glucose cotransporter-2 inhibitor | 46 (62.2) |
| No. (%) indication for glucagon-like peptide-1 receptor agonist | 21 (28.4) |
Note. Continuous data are presented as median (IQR); categorical data are presented as No. (%).
Figure 3.
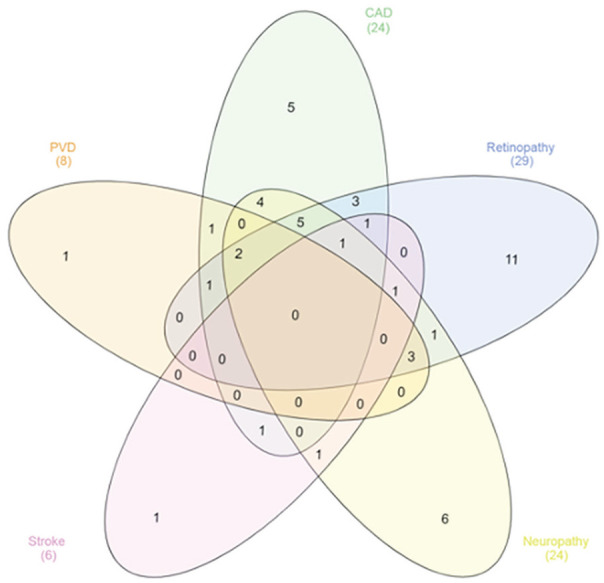
Venn diagram demonstrating the overlap of comorbid medical conditions in clinic patients.
Note. 95.9% of patients also had chronic kidney disease, not included for diagram simplicity. PVD = peripheral vascular disease; CAD = coronary artery disease.
Changes in Clinical Characteristics Between the First and Last Visits
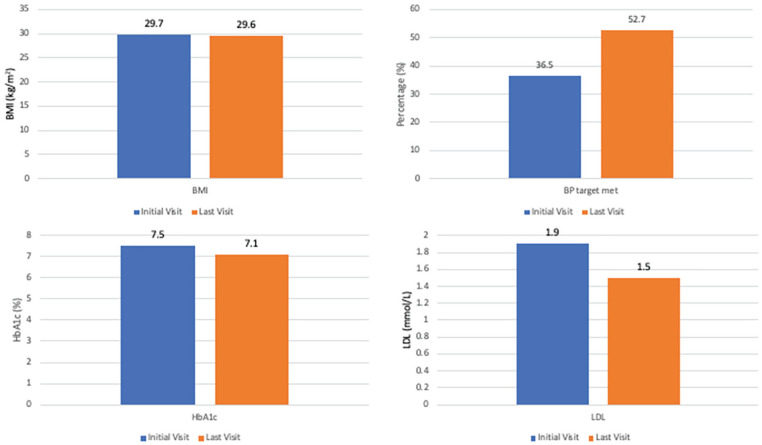
Clinical data between the first and last study visits are presented in Table 2, Figure 4, and Figure 5. Clinic BP improved between the first and last clinic visits, systolic BP 132 mm Hg (120.8, 154.8) versus 129 mm Hg (119.3, 140), P = .03 and diastolic BP 76 mm Hg (70, 84) versus 74 mm Hg (67, 77), P = .04, as did the proportion of patients meeting a BP target of ≤130/80 mm Hg, 27 patients (36.5%) at the first clinic visit versus 39 patients (52.7%) at the last clinic visit, P = .04, without a significant increase in the average number of BP medications (2.3 versus 2.5 at first and last visits, respectively, P = .08). Initial and last visit BMI data were available for 67 and 56 patients, respectively. There was no significant change in BMI between the first and last clinic visits.
Figure 4.
Clinical parameters at initial and last clinic visit.
Note. BMI = body mass index; BP = blood pressure; HbA1c = hemoglobin A1c; LDL= low-density lipoproteins.
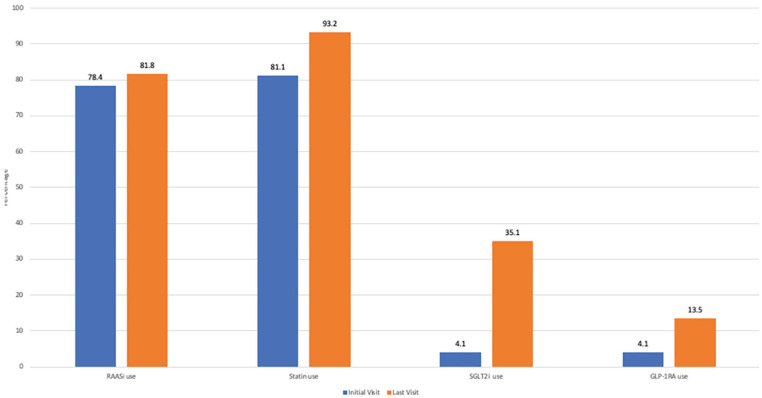
Figure 5.
Medication usage at initial and last clinic visits.
Note. RAASi = renin-angiotensin-aldosterone system inhibitor; SGLT2i = sodium-glucose cotransporter-2 inhibitor; GLP-1RA = glucagon-like peptide-1 receptor agonist.
Seventy-two patients (97.3%) patients had a clinical indication for RAAS inhibition. There was no significant difference between the proportion of patients on RAAS inhibitor therapy at the first and last visits, 58 patients (78.4%) versus 51 patients (81.8%), P = .56. At the last study visit, 14 patients remained off of RAAS inhibitors, 12 of which had a clinical indication for therapy based on clinical practice guidelines. Reasons for not being on RAAS inhibitors were identified in 10 patients and included acute kidney injury (3), hyperkalemia (2) hypotension (1), vomiting (1), stopped by primary care physician (1), stopped by patient (1), and not initiated due to advanced age (1).
Thirty-one patients (41.9%) had a clinical indication for antiplatelet therapy for secondary prevention of CVD. There was no significant difference in the use of aspirin and/or clopidogrel at the first and last visits, 37 (50.0%) versus 38 (51.4%), P = .71. On further analysis, there was a small increase in use of antiplatelet agents in secondary prevention and small decrease in their use in primary prevention; however, we could not account for the use of warfarin and direct oral anticoagulants due to a lack of clear documentation of indications for use, which may have further affected these numbers.
Low-density lipoprotein-cholesterol improved significantly between the first and last clinic visits, 1.9 mmol/L (1.5, 2.3) versus 1.5 mmol/L (1.2, 1.9), P < .01. Sixty patients (81.1%) versus 69 patients (93.2%) were treated with statin therapy at the first and last visits, respectively (P = .01). Seventy-two patients (97.3%) seen in the clinic had a clinical indication for statin therapy—60 patients (83.3%) of which were on therapy at the first clinic visit, versus 69 patients (95.8%) at the last clinic visit (P = .02).
Hemoglobin A1C also improved between the first and last study visits from 7.5% (6.6, 8.2) to 7.1% (6.3, 8.1) (P = .02). Three patients (4.1%) were treated with a sodium-glucose cotransporter-2 inhibitor (SGLT2i), and 3 patients (4.1%) with a glucagon-like peptide-1 receptor agonist (GLP-1RA) prior to the first clinic visit. At the last clinic visit, a total of 26 patients (35.1%) were taking SGLT2i and 10 patients (13.5%) GLP-1RA as part of their routine care. In addition, 12 patients (16.2%) involved in the C.a.R.E. Clinic were enrolled in clinical trials involving SGLT2i during this time period with 8.1% still actively enrolled in clinical trials at the end of our study period. Patients were also considered for enrolment in trials involving GLP-1RA.
Discussion
We have described demographic and clinical data of a retrospective single-center cohort of consecutive patients seen at a combined C.a.R.E. Clinic in Toronto, Canada. Our purpose is to describe the feasibility, strengths, and challenges of this outpatient care model and identify areas for quality improvement (QI) initiatives in our clinic which may also be applicable to other subspecialty clinics. Our data are limited by the before- and after-nature of our retrospective review, in that we cannot with certainty attribute clinical improvements to the care provided in this clinic alone. We did not have data on medication adherence or health behavior changes, making it challenging to ascertain what component of our interventions was the most effective. Our results are also limited by a lack of control group, which are difficult to find in a general nephrology clinic given the high prevalence of multi-morbid conditions in our population (Figure 3). Moreover, a comparison between the CaRE clinic and other single-discipline clinics (ie, kidney or cardiovascular or endocrine clinic) would be difficult due to profound differences in patient characteristics. Future work should consider including a comparison in outcomes between Ca.R.E. clinic patients and those who are waitlisted to strengthen the internal validity of our results.
Our analysis revealed a first visit nonattendance rate of 17% of all referrals and a first follow-up nonattendance rate of 18%. A broad and diverse set of patient-, provider-, and disease-related factors have been associated with follow-up nonattendance in patients with T2D and hypertension. 14 Understanding the specific reasons for follow-up nonattendance in our patient population is complex but an important step for future QI work. The female:male ratio for patients seen in the clinic was 1:2, despite the absence of established sex-specific prevalence discrepancies regarding diabetes or diabetic kidney disease. 15 We did not have data on ethnicity or socioeconomic status of referred patients. Collaboration with referring physicians and primary care teams is an important step to ensuring the effective, efficient, and equitable use of this resource-intensive clinic.
Regarding clinical parameters, we did not find an improvement in BMI with clinic follow-up, reflecting the challenging nature of obesity management. Given the widespread presence of obesity-related complications in this population, our data suggest that a protocolized, dedicated obesity management pathway, including guideline-based medical, surgical, and psychological interventions, should be considered. 16 SGLT2i and GLP-1 RA are associated with weight loss, and it is possible with greater ongoing uptake of these medications we may see reduction in BMI. We found an improvement in clinic BP readings between the first and last clinic visits, corresponding with an increase in the proportion of patients meeting the recommended BP target. Our control rate of 52.6% at the last study visit is close to recent Canadian data reporting hypertension control rate of 58.6% 17 in patients with diabetes, and the co-existence of CKD in our population makes hypertension control more challenging. Importantly, masked uncontrolled hypertension is highly prevalent in the CKD population (26%-56%, depending on the definition used), 18 highlighting the importance of out-of-office BP measurements in our population. Home BP measurements were referenced in the reviewed clinical notes of 30% of patients, and 24-hour ambulatory BP measurements referenced in <5%. Our clinic is in the process of incorporating an interactive e-medicine model which is being adapted from its original use in the heart failure population. 19 This program integrates home BP, pulse rate, weight, and symptoms to remotely monitor patients so we can more safely titrate medications 19 and is an important step to monitoring out-of-office BP readings in this high-risk population.
We report a significant improvement in the use of clinically indicated statins, improvement in LDL and HbA1c over time in patients attending the clinic. We did not have access to individualized HbA1c or LDL targets. RAAS inhibitor prescriptions were overall stable over the study period and saturated at referral to the clinic; most patients not on therapy at the last study visit had a clinically relevant reason to not be on therapy. We did not examine RAAS inhibitor dosing, and the improvement in BP control might suggest that doses were in fact optimized over the study period. SGLT2i and GLP-1RA were low at the initial clinic visits, but use increased over the study period in our clinic population, in concurrence with an increasing body of evidence supporting the benefits of these drug classes in an expanding population.20,21 We did not ascertain what percentage of patients were appropriately on drug therapy given the evolving nature of the evidence over the study period. We identified that at the time of the first clinic visit, 62.2% of the clinic population had a strong clinical indication for SGLT2i use based on either a history of CVD or CKD with severely increased albuminuria and GFR 30-90 mL/min/1.73 m, 2 and 28.4% had a strong indication for GLP-1RA use based on clinical history of CVD and HbA1c above glycemic target. This highlights an urgent need to appropriately identify eligible patients and safely prescribe these medications in this and similar patient populations.
There is a high burden of coronary artery disease in this patient group. In the C.a.R.E. Clinic, we integrate cardiovascular tests to optimize patient care and risk factor management. These tests include echocardiograms, stress echocardiograms, and CT coronary angiograms. Data regarding the number of tests ordered and the results of these tests were not collected in this analysis. We also did not examine patient-reported outcomes. One of the key benefits of this clinic is a “one-stop-shop” approach with multiple specialists and members of the multidisciplinary team giving a co-ordinated and cohesive message to patients with multiple comorbid conditions crossing multiple specialties. This was outside of the scope of this audit, but future qualitative research may be warranted to examine patient and provider satisfaction.
Conclusions
We have reported real-world data to demonstrate the feasibility of a unique multi- and interdisciplinary clinic, with advantages including cohesive patient care resulting in improvement in evidence-based clinical targets, opportunities for recruitment to clinical trials, and unique training opportunities for learners. We have identified potential areas for future QI work including a dedicated obesity management pathway, an active home BP telemonitoring system, and continued increased uptake of new evidence-based cardiorenal medications.
Supplemental Material
Supplemental material, sj-docx-1-cjk-10.1177_20543581221081207 for A Unique Multi- and Interdisciplinary Cardiology-Renal-Endocrine Clinic: A Description and Assessment of Outcomes by Lisa Dubrofsky, Jason F. Lee, Parisa Hajimirzarahimshirazi, Hongyan Liu, Alanna Weisman, Patrick R. Lawler, Michael E. Farkouh, Jacob A. Udell and David Z. Cherney in Canadian Journal of Kidney Health and Disease
Footnotes
Ethics Approval and Consent to Participate: This study was approved by the University Health Network Quality Improvement Review Committee.
Consent for Publication: All authors provided their consent for publication of the manuscript.
Availability of Data and Materials: No additional data and materials are available.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: D.Z.C. has received honoraria from Boehringer Ingelheim-Lilly, Merck, AstraZeneca, Sanofi, Mitsubishi-Tanabe, Abbvie, Janssen, Bayer, Prometic, BMS, Maze, CSL-Behring, and Novo-Nordisk and has received operational funding for clinical trials from Boehringer Ingelheim-Lilly, Merck, Janssen, Sanofi, AstraZeneca, and Novo-Nordisk. J.A.U. has received speaker/consulting honoraria from Amgen, Boehringer Ingelheim, Janssen, Merck, Novartis, and Sanofi and has received grant support to his institutions from AstraZeneca, Bayer, Boehringer Ingelheim, Janssen, Novartis, and Sanofi. M.F. has received research grant support from Amgen, Novartis, and Novo-Nordisk. The other authors report no disclosures.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: D.Z.C. is supported by a Department of Medicine, University of Toronto Merit Award and receives support from the CIHR, Diabetes Canada and the Heart and Stroke Richard Lewar Centre of Excellence, and the Heart and Stroke Foundation of Canada. L.D. and J.L. were supported by the UHN CaRE Fellowship. J.A.U. is supported by an Ontario Ministry of Colleges and Universities Early Researcher Award (ER15-11-037); University of Toronto Department of Medicine Merit Award; Women’s College Research Institute and Department of Medicine, Women’s College Hospital; and the Peter Munk Cardiac Centre, Toronto General Hospital.
ORCID iDs: Lisa Dubrofsky  https://orcid.org/0000-0002-6995-2064
https://orcid.org/0000-0002-6995-2064
David Z. Cherney  https://orcid.org/0000-0003-4164-0429
https://orcid.org/0000-0003-4164-0429
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–2045. doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Palsson R, Patel UD. Cardiovascular complications of diabetic kidney disease. Adv Chronic Kidney Dis. 2014;21(3):273–280. doi: 10.1053/j.ackd.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Levin A, Chaudhry MR, Djurdjev O, Beaulieu M, Komenda P. Diabetes, kidney disease and cardiovascular disease patients. Assessing care of complex patients using outpatient testing and visits: additional metrics by which to evaluate health care system functioning. Nephrol Dial Transplant. 2009;24(9):2714–2720. doi: 10.1093/ndt/gfp180. [DOI] [PubMed] [Google Scholar]
- 4. Iglay K, Hannachi H, Joseph Howie P, et al. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin. 2016;32(7):1243–1252. doi: 10.1185/03007995.2016.1168291. [DOI] [PubMed] [Google Scholar]
- 5. Saxon DR, Reiter-Brennan C, Blaha MJ, Eckel RH. Cardiometabolic medicine: development of a new subspecialty. J Clin Endocrinol Metab. 2020;105(7). doi: 10.1210/clinem/dgaa261. [DOI] [PubMed] [Google Scholar]
- 6. Tuttle KR, Cherney DZ, Diabetic Kidney Disease Task Force of the American Society of N. Sodium glucose cotransporter 2 inhibition heralds a call-to-action for diabetic kidney disease. Clin J Am Soc Nephrol. 2020;15(2):285–288. doi: 10.2215/CJN.07730719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Watts BV, Shiner B, Pomerantz A, Stender P, Weeks WB. Outcomes of a quality improvement project integrating mental health into primary care. Qual Saf Health Care. 2007;16(5):378–381. doi: 10.1136/qshc.2007.022418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tyler KH, Haverkos BM, Hastings J, et al. The role of an integrated multidisciplinary clinic in the management of patients with cutaneous lymphoma. Front Oncol. 2015;5:136. doi: 10.3389/fonc.2015.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weber C, Beaulieu M, Djurdjev O, et al. Towards rational approaches of health care utilization in complex patients: an exploratory randomized trial comparing a novel combined clinic to multiple specialty clinics in patients with renal disease-cardiovascular disease-diabetes. Nephrol Dial Transplant. 2012;27(suppl 3):iii104–iii110. doi: 10.1093/ndt/gfr292. [DOI] [PubMed] [Google Scholar]
- 10. Thomas M, Magwire M, O’Keefe JH, Nassif M, Kosiborod MN. Abstract 369: secondary risk reduction in patients with type 2 diabetes and cardiovascular disease: experience from a cardiometabolic center of excellence. Circ: Cardiovasc Qual Outcomes. 2020;13(suppl_1):a369–a369. doi: 10.1161/hcq.13.suppl_1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spaak J. Novel combined management approaches to patients with diabetes, chronic kidney disease and cardiovascular disease. J R Coll Physicians Edinb. 2017;47(1):83–87. doi: 10.4997/JRCPE.2017.118. [DOI] [PubMed] [Google Scholar]
- 12. Jessup RL. Interdisciplinary versus multidisciplinary care teams: do we understand the difference? Aust Health Rev. 2007;31(3):330–331. doi: 10.1071/ah070330. [DOI] [PubMed] [Google Scholar]
- 13. Diabetes Canada Clinical Practice Guidelines Expert Committee, Stone JA, Houlden RL, Lin P, Udell JA, Verma S. Cardiovascular protection in people with diabetes. Can J Diabetes. 2018;42(suppl 1):S162–S169. doi: 10.1016/j.jcjd.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 14. Lee RRS, Samsudin MI, Thirumoorthy T, Low LL, Kwan YH. Factors affecting follow-up non-attendance in patients with Type 2 diabetes mellitus and hypertension: a systematic review. Singapore Med J. 2019;60(5):216–223. doi: 10.11622/smedj.2019042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maric-Bilkan C. Sex differences in diabetic kidney disease. Mayo Clin Proc. 2020;95(3):587–599. doi: 10.1016/j.mayocp.2019.08.026. [DOI] [PubMed] [Google Scholar]
- 16. Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192(31):E875–E891. doi: 10.1503/cmaj.191707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leung AA, Williams JVA, McAlister FA, Campbell NRC, Padwal RS, Hypertension Canada’s Research and Evaluation Committee. Worsening hypertension awareness, treatment, and control rates in canadian women between 2007 and 2017. Can J Cardiol. 2020;36(5):732–739. doi: 10.1016/j.cjca.2020.02.092. [DOI] [PubMed] [Google Scholar]
- 18. Agarwal R, Pappas MK, Sinha AD. Masked uncontrolled hypertension in CKD. J Am Soc Nephrol. 2016;27(3):924–932. doi: 10.1681/ASN.2015030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seto E, Leonard KJ, Cafazzo JA, Barnsley J, Masino C, Ross HJ. Mobile phone-based telemonitoring for heart failure management: a randomized controlled trial. J Med Internet Res. 2012;14(1):e31. doi: 10.2196/jmir.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 21. Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–785. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cjk-10.1177_20543581221081207 for A Unique Multi- and Interdisciplinary Cardiology-Renal-Endocrine Clinic: A Description and Assessment of Outcomes by Lisa Dubrofsky, Jason F. Lee, Parisa Hajimirzarahimshirazi, Hongyan Liu, Alanna Weisman, Patrick R. Lawler, Michael E. Farkouh, Jacob A. Udell and David Z. Cherney in Canadian Journal of Kidney Health and Disease