Abstract
Background:
The phase III MONALEESA-7 trial (NCT02278120) assessed ribociclib + endocrine therapy (ET) versus ET in premenopausal women with HR+/HER2− advanced breast cancer (ABC). The relationship between work productivity loss (WPL) and domains of European Organisation for Research and Treatment of Cancer Quality of Life (EORTC QLQ-C30) and the breast cancer (BC)-specific module (QLQ-BR23) has not been explored in ABC. In this post hoc analysis (data cutoff, November 30, 2018), we assessed the correlation between the WPL component of the Work Productivity and Activity Impairment: General Health (WPAI:GH) questionnaire and EORTC QLQ-C30/BR23 domains.
Methods:
We analyzed EORTC and WPAI:GH data from 329 patients in both treatment arms of MONALEESA-7 who were employed during the trial. Separate univariable mixed-model repeated measures (MMRM) regression models were fitted for each domain, with WPL as dependent variable and each EORTC domain score as a single fixed-effect covariate. Linear and quadratic relationships were considered based on the Akaike information criterion. Next, two separate multivariable MMRM regression models were fitted with WPL a dependent variable and all QLQ-C30/BR23 domain scores as fixed-effect covariates. The strength of correlation between WPL and EORTC domains was assessed in terms of minimally important differences for the QLQ-C30/BR23 modules.
Results:
Our univariable analysis showed that greater WPL was statistically significantly associated with lower levels of overall quality of life (QoL) and other functional domains and with higher levels of all symptomatic domains of the QLQ-C30/BR23 modules. Our multivariable analysis determined that this correlation was primarily driven by changes in QoL; physical, role, social, and future perspective domains; and BC-specific symptomatic domains.
Conclusion:
This analysis determined the QoL domains that correlate with WPL in premenopausal patients with HR+/HER2− ABC. These results may inform prognostic tools to identify and characterize patients with greater risk for WPL and help design interventional strategies to minimize WPL.
Keywords: advanced breast cancer, breast cancer, premenopausal women, quality of life, ribociclib, work productivity loss
Introduction
Breast cancer (BC) is the most commonly diagnosed cancer (24.2%) and leading cause of cancer deaths (15.0%) among women worldwide. 1 While the majority of deaths due to BC occur in older women, the latest Surveillance, Epidemiology, and End Results (SEER) Program (2014–2018) reported that approximately 5.5% of BC-related deaths in the United States occur in women aged <45 years. 2 Hormone receptor–positive, human epidermal growth factor receptor 2–negative (HR+/HER2−) subtype is the most common type of BC comprising approximately 73% of BC cases. 3
In recent years, there has been an increased focus on self-reported quality of life (QoL) in patients with BC, particularly among younger women who are of working age. 4 Younger women (aged <50 years) with BC score worse on multiple functional and symptomatic domains of QoL than women aged ⩾ 50 years. 5 Furthermore, prognosis is worse in younger women with BC than older women.6,7 Deterioration of QoL is common with disease progression,8,9 and women whose disease had progressed to advanced breast cancer (ABC) have been shown to have lower QoL and higher symptom burden, including BC-related symptoms, compared with patients with early-stage BC.10,11
Disease progression is also associated with increased workplace hours missed, 12 which imposes an economic burden, especially in younger women. 13 A recent study showed that annual per-woman costs associated with lost productivity due to ABC was $5169 for younger women compared with $680 for older women. 13 Thus, there is a need to evaluate the various aspects of QoL in younger working women with HR+/HER2− ABC as they relate to changes in work productivity.
The international, randomized, double-blind, placebo-controlled, phase III MONALEESA-7 trial (NCT02278120) was the first phase III trial that assessed the cyclin-dependent kinase 4/6 inhibitor (CDK 4/6i) ribociclib (RIB) exclusively in premenopausal women with HR+/HER2− ABC.14,15 MONALEESA-7 reported statistically significant benefits in progression-free survival and overall survival with RIB plus endocrine therapy (ET) versus placebo plus ET in the intent-to-treat (ITT) population.14,16 MONALEESA-7 also reported that time to deterioration ⩾ 10% in pain and in global health-related quality of life (HRQoL) was significantly delayed, along with maintenance or improvement in work productivity, with RIB plus ET compared with placebo plus ET.17,18
The Work Productivity and Activity Impairment: General Health (WPAI:GH) questionnaire evaluates work productivity loss (WPL), which measures work impairment due to any health problems. 19 The European Organisation for Research and Treatment of Cancer Quality of Life (EORTC QLQ-C30) questionnaire evaluates QoL by functional and symptomatic domains in patients with cancer. 20 While the EORTC QLQ-C30 is aimed at a wide range of cancer patient populations, the questionnaire can be supplemented by the tumor-specific questionnaire module for patients with BC (QLQ-BR23). To our knowledge, the relationship between the QoL domains and WPL in patients with ABC has not been investigated. A comprehensive understanding of this relationship is crucial to implement strategic interventions to minimize WPL in patients with ABC.
Thus, the aim of this study was to understand how symptoms and functioning of premenopausal women with HR+/HER2− ABC, as assessed by the EORTC QLQ-C30/BR23 questionnaires, correlate with changes in WPL.
Methods
Overview
The MONALEESA-7 trial was a phase III, randomized, double-blind, placebo-controlled trial conducted in 188 centers in 30 countries and enrolled 672 patients with HR+/HER2− ABC. Briefly, patients were randomized in a 1:1 ratio to receive RIB or matching placebo with either a nonsteroidal aromatase inhibitor (NSAI) or tamoxifen; all patients also received goserelin. MONALEESA-7 was approved by each participating site’s institutional review board or independent ethics committee, and the trial was performed in accordance with the Good Clinical Practice guidelines and the Declaration of Helsinki. Written informed consent was obtained from all patients at enrollment. The study was registered with ClinicalTrials.gov (NCT02278120) on October 29, 2014, and patients were enrolled from December 17, 2014, to August 1, 2016. The complete MONALEESA-7 methodology has been described in detail previously. 14
This study was a post hoc correlational analysis of the MONALEESA-7 trial to assess the existence and strength of the relationship between the WPL component of the WPAI:GH questionnaire and all the functional and symptomatic domains of the EORTC QLQ-C30 and QLQ-BR23 questionnaires in premenopausal women with HR+/HER2− ABC. The data cutoff for the analysis was November 30, 2018.
WPAI:GH Questionnaire
Patients were asked to complete the WPAI:GH version 2.0 questionnaire throughout the trial, namely, at screening (day −28 to day 1), subsequent cycles (every 8 weeks during the first 18 months after randomization and every 12 weeks thereafter), and at the end-of-treatment visit, which occurred within 15 days of permanent treatment discontinuation. The questionnaire focuses on the number of hours missed from work and usual daily activities as well as the extent to which these were limited over the past 7 days due to one’s overall health (including physical or emotional problems). 19 The WPAI:GH scores obtained from the questionnaire thus characterize absenteeism (work time missed), presenteeism (degree of impairment while working/reduced on-the-job effectiveness), WPL (overall work impairment/absenteeism plus presenteeism), and activity impairment (impact on usual daily activities). 19 These four scores are expressed as ‘impairment percentages’, in which high numbers are associated with greater impairment and decreased productivity. For example, a WPL of 40% at any time point (e.g. screening) would suggest that 40% of work hours over the past 7 days were lost due to either absenteeism or presenteeism. A change from baseline of, for example, +20% would suggest a 20% reduction in work productivity from baseline over time.
EORTC QLQ-C30/EORTC QLQ-BR23
The EORTC QLQ-C30 and QLQ-BR23 questionnaires were administered according to the same schedule as the WPAI:GH questionnaire during the treatment period as well as at the safety follow-up visit and throughout the efficacy follow-up period.
The EORTC QLQ-C30 questionnaire consists of nine multi-item domains: five functional domains (physical, role, cognitive, emotional, and social), three symptom domains (fatigue, pain, and nausea/vomiting), and a global health status (GHS)/QoL domain. 20 In addition, there are five single-symptom item domains: dyspnea, insomnia, appetite loss, constipation, and diarrhea, along with the perceived financial impact of the disease. The BC-specific module (QLQ-BR23) supplements the QLQ-C30 with five additional multi-item domains (systemic therapy side effects, arm symptoms, breast symptoms, body image, and sexual functioning) and three single-item domains (sexual enjoyment, upset by hair loss, and future perspective).
A high score on functional domains represents a high/healthy level of functioning or QoL; a high score on symptomatic domains represents more severe symptoms or worse problems. 20
Minimally important differences
Minimally important differences (MIDs) are defined as the smallest change in a QoL score that is perceived as important by a patient or clinician, which may indicate a change in patient management. 21 It is important to interpret QoL scores in terms of MIDs, as a statistically significant change may not reflect a change that is perceived by the patient as important or lead a patient or clinician to consider a change in management. 21 The strength of the correlation between WPL and EORTC domains was assessed in terms of published MIDs for the EORTC QLQ-C30 and EORTC QLQ-BR23 questionnaires (Supplementary Table 1). MIDs were selected from suitable publications within the literature and were primarily specific to ABC or BC.22 –24 Where more than one MID was available for the same domain from multiple sources, the most relevant source was selected. To our knowledge, no published MIDs are available for the WPAI:GH questionnaire.
Statistical analysis
The correlation between WPL and domains of the EORTC QLQ-C30 and QLQ-BR23 was explored through post hoc regression analyses of 329 patients who were in paid work (including part time and self-employed) at any time during the MONALEESA-7 trial and had at least one complete response in the relevant EORTC QLQ-C30/QLQ-BR23 domain and the WPAI:GH WPL questionnaire. Separate univariable mixed-model repeated measures (MMRM) regression models were fitted with the work productivity component (absenteeism plus presenteeism combined) of the WPAI:GH questionnaire as the dependent variable and each domain score of the EORTC QLQ-C30 and QLQ-BR23 questionnaires as a fixed-effect covariate. For each regression model, the EORTC questionnaire domain scores were rescaled such that the interpretation of the regression coefficients was in terms of published MIDs for deterioration in each domain. Both linear and quadratic models were fitted, and the favored model was chosen using the Akaike information criterion (AIC). 25 Linear models, which offer a simpler interpretation, were favored when AICquadratic – AIClinear ⩾ –2; quadratic models were selected otherwise. 26
Furthermore, two different multivariable MMRM regression models were fitted with the work productivity component of the WPAI:GH questionnaire as the dependent variable. In the first model, all domain scores of the EORTC QLQ-C30 questionnaire were used as fixed-effect covariates. In the second model, all domain scores of the EORTC QLQ-BR23 questionnaire were used as fixed-effect covariates. The multivariable regression analysis determined the specific domains of the EORTC QLQ-C30/QLQ-BR23 modules that may be independently driving the correlations between the EORTC domains and WPL.
Next, the predictive accuracy of the multivariable model, including all EORTC QLQ-C30 domains, was assessed in terms of prediction of WPL via 10-fold cross-validation. This consisted of a sequential procedure whereby one randomly selected subset (one-tenth) of the data set was set aside for validation. Based on the multivariable model fitted to the rest of the data, WPL in the validation subset of the data was predicted and the predictions compared against the observed scores. This process was repeated 10 times, with a separate subset (distinct from all previous validation subsets) of the data set that was set aside for validation at each step.
All analyses were performed in the ITT population of the MONALEESA-7 trial, which also included patients who were taking tamoxifen. The univariable analyses were also conducted in the NSAI subgroup of MONALEESA-7 using identical methods as per the ITT population. All analyses were conducted using statistical software R (version 4.0.4). 27
Results
EORTC QLQ-C30 correlation analyses
Univariable analyses in the ITT population for the QLQ-C30 module
The favored model (linear or quadratic) for each domain of the EORTC QLQ-C30 module was selected based on comparing the linear and quadratic AIC values (Supplementary Table 2). Linear models were favored by an a priori criterion based on the AIC (see ‘Methods’) for the following QLQ-C30 domains in the ITT population: GHS, role, cognitive, emotional, and social functional domains; nausea/vomiting, fatigue, insomnia, constipation, and diarrhea symptomatic domains; and the financial difficulties domain (Figure 1, Table 1).
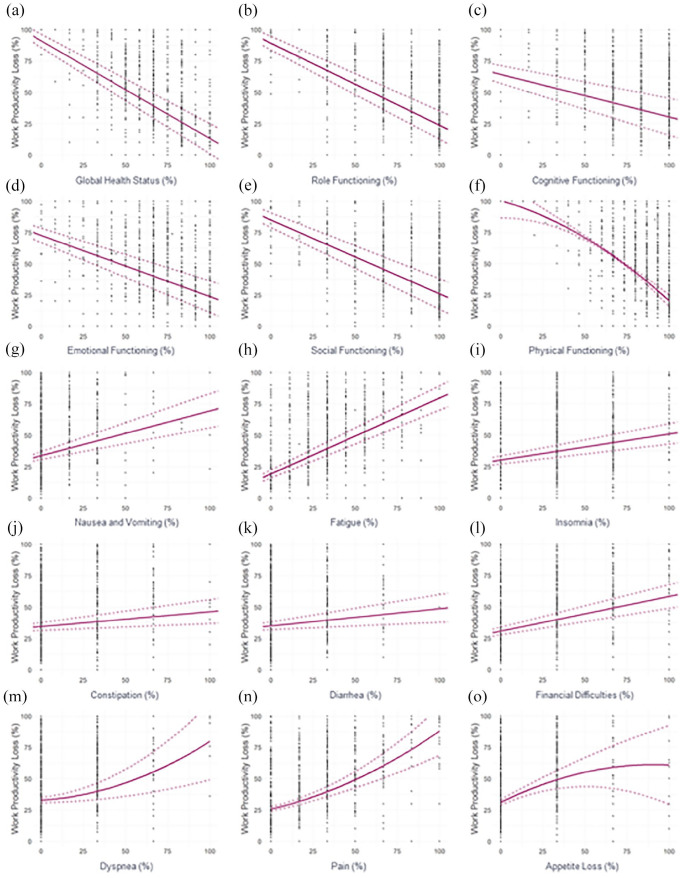
Figure 1.
Univariable regression plots showing WPL (%) in relation to the EORTC QLQ-C30 functional (a–f) and symptomatic (g–o) domain scores (%) in the ITT population. EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life questionnaire; ITT, intent to treat; WPL, work productivity loss.
Table 1.
Regression coefficients and p values for the EORTC QLQ-C30 domains in which linear models were favored in the ITT population, with WPL as the dependent variable.
| EORTC QLQ-C30 item/domain | MID for deterioration | Regression coefficient (95% CI) |
p value of regression coefficient |
|---|---|---|---|
| Global Health Status | 8 | −6.24 (−6.78, −5.70) |
<0.001 |
| Role Functioning | 6 | −3.92 (−4.28, −3.57) |
<0.001 |
| Emotional Functioning | 6 | −2.93 (−3.34, −2.52) |
<0.001 |
| Cognitive Functioning | 4 | −1.35 (−1.66, −1.04) |
<0.001 |
| Social Functioning | 7 | −4.12 (−4.56, −3.68) |
<0.001 |
| Fatigue | 8 | 4.84 (4.31, 5.37) |
<0.001 |
| Nausea and Vomiting | 11 | 3.93 (2.76, 5.09) |
<0.001 |
| Insomnia | 15 | 3.11 (2.38, 3.84) |
<0.001 |
| Constipation | 3 | 0.36 (0.17, 0.55) |
<0.001 |
| Diarrhea | 4 | 0.55 (0.23, 0.87) |
<0.001 |
| Financial Difficulties | 5 | 1.38 (1.06, 1.70) |
<0.001 |
CI, confidence interval; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life questionnaire; ITT, intent to treat; MID, minimally important difference; WPL, work productivity loss.
Domains in white background are functional components, and those in gray background are symptomatic components. Statistically significant p values are in bold text.
Univariable analyses showed that all EORTC QLQ-C30 domains in which the linear model was favored were statistically significantly associated with WPL (all p values <0.001; Table 1). As expected, functional domains were negatively associated with WPL (higher levels/scores in functional domains were associated with lower WPL; Figure 1(a)–(e), Table 1), whereas symptomatic domains were positively associated with WPL (higher levels/scores in symptomatic domains were associated with greater WPL; Figure 1(g)–(l), Table 1). The estimates provided in Table 1 can be interpreted, for example, as follows: a worsening in GHS by 8 points, which is considered the MID for GHS, was associated with an expected increase in WPL of 6.24% [95% confidence interval (CI): 5.70, 6.78; Figure 1(a), Table 1]. Similarly, a worsening in fatigue by 8 points, which is considered the MID for fatigue, was associated with an expected increase in WPL of 4.84% (95% CI: 4.31, 5.37; Figure 1(h), Table 1).
Quadratic models were favored by the AIC for the following QLQ-C30 domains in the ITT population: physical functioning (p = 0.009; Figure 1(f), Table 2), dyspnea, pain, and appetite loss (p values <0.001; Figure 1(m)–(o), Table 2). For these domains, the expected change in WPL given a deterioration in the EORTC domain displayed was dependent on the starting value of the respective EORTC domain. For example, a 17-point increase (MID value) in pain from 40 to 57 points versus 10 to 27 points was associated with an expected increase in WPL of 12.51% versus 9.32%, respectively (Figure 1(n), Supplementary Text).
Table 2.
Regression coefficients and p values for the EORTC QLQ-C30 domains in which quadratic models were favored in the ITT population, with WPL as the dependent variable.
| Linear coefficient (β1) |
Quadratic coefficient (β2) |
||||
|---|---|---|---|---|---|
| EORTC QLQ-C30 item/domain | MID for deterioration | Regression coefficient (95% CI) |
p value | Regression coefficient (95% CI) |
p value |
| Physical Functioning | 10 | −11.08 (−12.43, −9.73) |
<0.001 | −0.41 (−0.72, −0.10) |
0.009 |
| Pain | 17 | 7.37 (6.08, 8.66) |
<0.001 | 0.90 (0.42, 1.37) |
<0.001 |
| Dyspnea | 8 | 1.30 (0.46, 2.14) |
0.002 | 0.25 (0.10, 0.39) |
<0.001 |
| Appetite Loss | 14 | 8.49 (6.82, 10.17) |
<0.001 | −0.72 (−1.13, −0.31) |
<0.001 |
CI, confidence interval; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life questionnaire; ITT, intent to treat; MID, minimally important difference; WPL, work productivity loss.
Domains in white background are functional components, and those in gray background are symptomatic components. Statistically significant p values are in bold text.
Univariable analyses in the NSAI subgroup for the QLQ-C30 module
Identical univariable analyses when conducted in patients who received an NSAI (NSAI subgroup) showed that all EORTC QLQ-C30 domains were statistically significantly associated with WPL (all p values <0.05; Supplementary Tables 3 and 4). Linear models were favored over quadratic models by the AIC for all functional domains in the NSAI subgroup (p < 0.001; Supplementary Table 3), five symptomatic domains, and the financial difficulties domain [p < 0.001 except constipation (p = 0.025); Supplementary Table 3]. As seen in the ITT population, functional domains were negatively associated with WPL, whereas symptomatic domains were positively associated with WPL (Supplementary Table 3). Quadratic models were favored by the AIC for three symptomatic domains in the NSAI subgroup: pain, dyspnea (p < 0.001 for both), and appetite loss (p = 0.01; Supplementary Table 4), with the expected change in WPL dependent on the starting value of the respective EORTC domain.
Multivariable analysis in the ITT population for the QLQ-C30 module
The multivariable analysis showed that the correlation between WPAI:GH WPL and the EORTC QLQ-C30 domains was primarily driven by the GHS, physical functioning, role functioning, social functioning, and appetite loss domains (Table 3).
Table 3.
Regression coefficients and p values for the EORTC QLQ-C30 domains from the multivariable correlation analysis in the ITT population (with corresponding MID values for deterioration), with WPL as the dependent variable.
| EORTC QLQ-C30 item/domain | MID for deterioration | Regression coefficient (95% CI) | p value of regression coefficient |
|---|---|---|---|
| Global Health Status | 8 | −3.03 (−3.69, −2.37) | <0.001 |
| Physical Functioning | 10 | −2.47 (−3.68, −1.26) | <0.001 |
| Role Functioning | 6 | −1.34 (−1.83, −0.86) | <0.001 |
| Emotional Functioning | 6 | −0.38 (−0.83, 0.07) | 0.097 |
| Cognitive Functioning | 4 | −0.02 (−0.31, 0.28) | 0.92 |
| Social Functioning | 7 | −0.78 (−1.32, −0.24) | 0.0047 |
| Fatigue | 8 | 0.20 (−0.48, 0.88) | 0.57 |
| Nausea and Vomiting | 11 | −0.33 (−1.42, 0.77) | 0.56 |
| Pain | 17 | 1.06 (−0.15, 2.26) | 0.086 |
| Dyspnea | 8 | 0.33 (−0.21, 0.87) | 0.23 |
| Insomnia | 15 | 0.02 (−0.69, 0.72) | 0.96 |
| Appetite Loss | 14 | 1.73 (0.78, 2.68) | <0.001 |
| Constipation | 3 | −0.04 (−0.21, 0.13) | 0.65 |
| Diarrhea | 4 | 0.23 (−0.05, 0.52) | 0.11 |
| Financial Difficulties | 5 | 0.21 (−0.09, 0.51) | 0.16 |
CI, confidence interval; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life questionnaire; ITT, intent to treat; MID, minimally important difference; WPL, work productivity loss.
Domains in white background are functional components, and those in gray background are symptomatic components. Statistically significant p values are in bold text.
Consistent with previous results, GHS (p < 0.001) and the functional domains (physical functioning (p < 0.001), role functioning (p < 0.001), and social functioning (p = 0.0047)) showed a statistically significant negative correlation with WPL, and appetite loss displayed a statistically significant positive correlation (p < 0.001) with WPL while accounting for all other domains of the EORTC QLQ-C30 (Table 3). Although not statistically significant, pain (p = 0.086) and emotional functioning (p = 0.097) displayed trends of a positive and negative correlation with WPL, respectively, while accounting for all other domains of the EORTC QLQ-C30 (Table 3).
When using the model as a predictive formula, 10-fold cross-validation showed that 48.75% and 66.45% of predictions from the QLQ-C30 multivariable model were within ± 15% and ± 20% of the observed WPL scores, respectively. That is, 48.75% (or 66.45%) of predictions were less than 0.75 (or 1) working day(s) per week away from the patients’ observed WPL scores.
EORTC QLQ-BR23 correlation analyses
Univariable analyses in the ITT population for the QLQ-BR23 module
The favored model (linear or quadratic) for each domain was selected based on comparing the linear and quadratic AIC values (Supplementary Table 5). Linear models were favored over quadratic models by the AIC for the following QLQ-BR23 domains in the ITT population: body image, future perspective, sexual enjoyment, breast symptoms, arm symptoms, and upset by hair loss (Figure 2(a)–(g), Table 4).
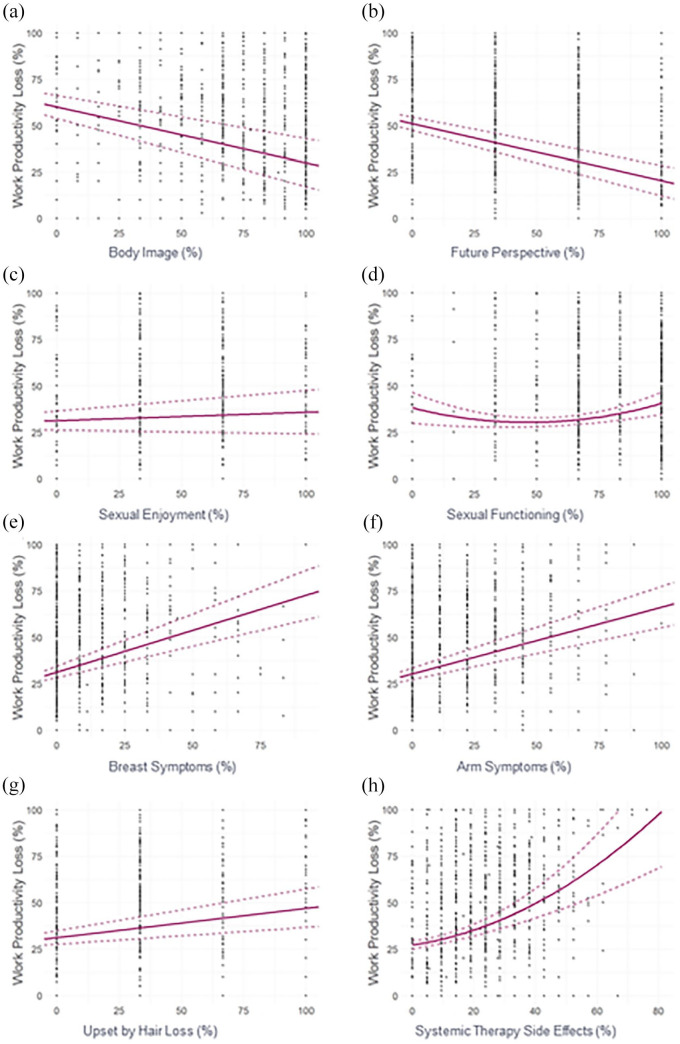
Figure 2.
Univariable regression plots showing WPL (%) in relation to the EORTC QLQ-BR23 functional (a–d) and symptomatic (e–h) domain scores (%) in the ITT population. EORTC QLQ-BR23, European Organisation for Research and Treatment of Cancer breast cancer–specific module; ITT, intent to treat; WPL, work productivity loss.
Table 4.
Regression coefficients and p values for the EORTC QLQ-BR23 domains in which linear models were favored in the ITT population, with WPL as the dependent variable.
| EORTC QLQ-BR23 item/domain | Sample size | MID for deterioration | Regression coefficient (95% CI) |
p value of regression coefficient |
|---|---|---|---|---|
| Body Image | 329 | 11 | −3.30 (−4.05, −2.56) |
<0.001 |
| Sexual Enjoyment | 233 | 15 | 0.69 (−0.30, 1.67) |
0.17 |
| Future Perspective | 329 | 0.7 | −0.21 (−0.25, −0.18) |
<0.001 |
| Breast Symptoms | 327 | 6 | 2.72 (2.05, 3.39) |
<0.001 |
| Arm Symptoms | 327 | 11 | 3.96 (3.07, 4.84) |
<0.001 |
| Upset by Hair Loss | 237 | 10 | 1.58 (0.91, 2.25) |
<0.001 |
CI, confidence interval; EORTC QLQ-BR23, European Organisation for Research and Treatment of Cancer breast cancer–specific module; ITT, intent to treat; MID, minimally important difference; WPL, work productivity loss.
Domains in white background are functional components, and those in gray background are symptomatic components. Statistically significant p values are in bold text.
A statistically significant correlation was observed with WPL (p values <0.001; Table 4) for all domains of the EORTC QLQ-BR23 in which linear models were favored, except for sexual enjoyment (p = 0.17; Table 4). The functional domains were negatively associated with WPL (Figure 2(a) and (b)), whereas symptomatic domains were positively associated with WPL (Figure 2(e)–(g)).
Quadratic models were favored by the AIC for the following QLQ-BR23 domains in the ITT population: sexual functioning and systemic therapy side effects (Figure 2(d) and (h), Table 5). Both sexual functioning and systemic therapy side effects were statistically significantly associated with WPL (p values ⩽0.001; Table 5), and the expected change in WPL was dependent on the starting value of the respective EORTC domain. Although statistically significant, the relationship between sexual functioning and WPL was somewhat unclear (Figure 2(d)).
Table 5.
Regression coefficients and p values for the EORTC QLQ-BR23 domains in which quadratic models were favored in the ITT population, with WPL as the dependent variable.
| Linear coefficient (β1) |
Quadratic coefficient (β2) |
|||||
|---|---|---|---|---|---|---|
| EORTC QLQ-BR23 item/domain | Sample size | MID for deterioration | Regression coefficient (95% CI) |
p value | Regression coefficient (95% CI) |
p value |
| Sexual Functioning | 325 | 11 | 2.50 (1.58, 3.42) |
<0.001 | 0.44 (0.21, 0.67) |
<0.001 |
| Systemic Therapy Side Effects | 329 | 10 | 5.31 (4.08, 6.55) |
<0.001 | 0.80 (0.32, 1.28) |
0.001 |
CI, confidence interval; EORTC QLQ-BR23, European Organisation for Research and Treatment of Cancer breast cancer–specific module; ITT, intent to treat; MID, minimally important difference; WPL, work productivity loss.
Domains in white background are functional components, and those in gray background are symptomatic components. Statistically significant p values are in bold text.
Univariable analyses in the NSAI subgroup for the QLQ-BR23 module
Linear models were favored by the AIC for six QLQ-BR23 domains in the NSAI subgroup (Supplementary Table 6). A statistically significant association was observed with WPL (p < 0.001; Supplementary Table 6) for all domains of the EORTC QLQ-BR23 in which linear models were favored, except for sexual enjoyment (p = 0.15; Supplementary Table 6).
Quadratic models were favored by the AIC for the following QLQ-BR23 domains in the NSAI subgroup: sexual functioning and systemic therapy side effects (Supplementary Table 7). Both domains were statistically significantly associated with WPL (p ⩽ 0.001; Supplementary Table 7).
Multivariable analysis in the ITT population for the QLQ-BR23 module
As for EORTC QLQ-C30, a multivariable analysis was also conducted in the ITT population for EORTC QLQ-BR23 to explore which domains may be independently driving the correlations observed in the univariable analyses.
Multivariable analysis showed that the correlation between WPAI:GH WPL and the EORTC QLQ-BR23 domains was primarily driven by the systemic therapy side effects (p = 0.0038), breast symptoms (p = 0.035), arm symptoms (p = 0.019), and future perspective (p = 0.029) domains in the ITT population (Table 6). The functional domain (future perspective) was negatively associated with WPL, whereas symptomatic domains (systemic therapy side effects, breast symptoms, and arm symptoms) were positively associated with WPL (Table 6). In addition, it is worth noting that perhaps due to the relatively small MID (0.7) of the future perspective domain, the expected change in WPL (0.07%) due to a deterioration in future perspective domain was relatively small (Table 6).
Table 6.
Regression coefficients and p values for the EORTC QLQ-BR23 domains from the multivariable correlation analysis of the ITT population (with corresponding MID values for deterioration), with WPL as the dependent variable.
| EORTC QLQ-BR23 item/domain | MID for deterioration | Regression coefficient (95% CI) |
p value of regression coefficient |
|---|---|---|---|
| Body Image | 11 | −1.10 (−2.75, 0.54) | 0.19 |
| Sexual Functioning | 11 | 0.66 (−0.94, 2.26) | 0.42 |
| Sexual Enjoyment | 15 | −0.62 (−2.30, 1.07) | 0.47 |
| Future Perspective | 0.7 | −0.07 (−0.14, −0.01) | 0.029 |
| Systemic Therapy Side Effects | 10 | 3.35 (1.09, 5.60) | 0.0038 |
| Breast Symptoms | 6 | 1.35 (0.10, 2.61) | 0.035 |
| Arm Symptoms | 11 | 2.16 (0.36, 3.96) | 0.019 |
| Upset by Hair Loss | 10 | 0.53 (−0.39, 1.46) | 0.26 |
CI, confidence interval; EORTC QLQ-BR23, European Organisation for Research and Treatment of Cancer breast cancer–specific module; ITT, intent to treat; MID, minimally important difference; WPL, work productivity loss.
Domains in white background are functional components, and those in gray background are symptomatic components. Statistically significant p values are in bold text.
Discussion
This post hoc analysis of the phase III MONALEESA-7 trial assessed the correlation between the WPL component of the WPAI:GH questionnaire and the functional and symptomatic domains of the EORTC QLQ-C30 and QLQ-BR23 questionnaires. To our knowledge, no previous study has explored the correlation between WPL (derived from the WPAI:GH questionnaire) and domains of the EORTC QLQ-C30 or QLQ-BR23 module in patients with ABC. Our univariable analysis showed that greater WPL was associated with lower levels of overall QoL and other functional domains, and with higher levels in symptomatic domains, as measured by the EORTC core module (QLQ-C30). Multivariable analysis determined this correlation to be primarily driven by the GHS, physical, role, and social functioning domains, and the appetite loss symptomatic domain of the QLQ-C30. These results show that any clinically important change in the levels/scores of each of these QLQ-C30 domains was independently associated with a change in WPL. Subgroup univariable analyses of the NSAI subgroup were consistent with those of the ITT population, which also included patients receiving tamoxifen.
Cross-validation of the multivariable model explored prediction of WPL that was based on the EORTC QLQ-C30 domains alone. Although producing better predictions using the multivariable model may be desirable, the threshold of accuracy that would be acceptable is subjective given that, to our knowledge, no MID has been published for the WPAI:GH. Furthermore, the multivariable model is an interpretable model that identifies an important relationship. Such explanatory models with simple interpretation need not necessarily perform well at prediction, and, similarly, good predictive models may not be easily interpretable. 28 Further efforts to identify patients at increased risk of WPL based on their QoL could more accurately factor in additional variables such as patient demographics and on-treatment outcomes via out-of-sample prediction methods.
When considering the EORTC BC-specific module (QLQ-BR23), our univariable analysis showed that, except for sexual enjoyment, the functional domains were negatively associated with WPL, whereas symptomatic domains were positively associated with WPL. Our multivariable analysis found that greater WPL was primarily correlated with worsening of breast symptoms, arm symptoms, and systemic therapy side effects as well as a decrease in future perspective functional score.
The results of this analysis are particularly important for young women with BC. Younger women show worse HRQoL and lower emotional, social, and cognitive functioning scores along with more fatigue and insomnia compared with older women with BC and female survivors of other cancers.4,5 Poor body image and decreased sexual functioning during BC treatment are also common among young women with BC. 29 Furthermore, as many of these women are likely to be employed, deterioration in work productivity due to BC affects them to a greater extent than older women.12,13
In addition, identification of the functional and symptomatic domains of QoL that are specifically associated with work productivity, as attempted in this study, will help oncologists address QoL issues that relate to WPL through focused discussions with these patients. This will help them to attend to the needs of the patients that relate to WPL and can form the basis for tailoring different interventional strategies. Finally, an increased awareness among employers regarding the QoL domains that affect their employees’ work productivity can help them design employment strategies and workplace modifications that could support these patients for as long they are able to work and sustain their livelihoods.
Hence, a comprehensive understanding of the relationship between changes in QoL and WPL in this patient population, through studies like this that address these questions, is important to help lay the foundation for designing strategies that can minimize WPL in these at-risk patients.
Strengths
A major strength of this study is that, to our knowledge, this is the first study of its kind to evaluate the correlation between QoL domains and WPL in patients with ABC, a relatively common disease, using univariable and multivariable analysis, with potential application for other types of cancers. 30 The univariable analysis approach used here explored both linear and quadratic relationships between WPL and domains of the EORTC QLQ-C30 and QLQ-BR23 modules to allow for nonlinear relationships between WPL and QoL domains. For simplicity of interpretation, the multivariable analyses did not consider nonlinear relationships; however, they were able to identify which domains may independently drive the correlation between EORTC modules and WPL. Correlation between domains of the QLQ-C30 (and, separately, the QLQ-BR23) module likely had a confounding effect on the univariable correlations with WPL; therefore, the multivariable analyses are important when interpreting the results of these analyses.
Finally, all results were interpreted with reference to published MID values for deterioration to enhance clinical interpretation of the results. MIDs were selected from the literature and were primarily specific to ABC or BC. When multiple sources reported MIDs for the same domain, studies in patients most similar to the MONALEESA-7 trial were selected. In particular, MIDs reported in the contemporary study by Musoro et al. 22 in ABC were prioritized.
Limitations
One limitation of this study is that we did not determine the directionality of the association between EORTC domains and WPL. While it is plausible that, for example, better physical functioning may result in improved work productivity, or improved work productivity may result in improved overall QoL or future perspective, the analyses only explored correlations between these variables and not causality. Further research is needed to determine whether there are any causal relationships in either direction between QoL and WPL.
Another limitation of the analyses was that there is no published MID for WPL, which would have further augmented the clinical interpretation of these results. Also, the MID specified for upset by hair loss domain in the QLQ-BR23 was taken from the work by Osoba, 24 whose original MID calculations applied to the QLQ-C30 questionnaire only and not to the QLQ-BR23. This approach was taken as, to our knowledge, no other MID estimate has been published for this domain, and a similar approach has been taken elsewhere. 31 Furthermore, while most MIDs were specific to BC, the MID for upset by hair loss domain included patients with lung cancer along with those with BC due to lack of an alternative. 24 Similarly, the published MID for diarrhea 23 may have been unreliable due to a limitation in the data used to generate the MID – a number of the scores contained zero values, as noted in the work by Ousmen. 23
In addition, factors such as support/benefits while away from work and within work in different countries, along with the type of work, may have impacted the patients’ threshold for stopping work during treatment. These variables were not captured, and thus were not accounted for during the analysis. Furthermore, participation in a clinical trial is more time-consuming for patients than receiving standard of care, which could affect the general applicability of the results in a real-world setting.
Finally, the analyses were not adjusted for multiple testing; therefore, some statistically significant findings may be false-positive due to chance alone. For example, the univariable association between WPL and sexual functioning showed a peculiar nonlinear relationship but was statistically significant.
Generalizability
These analyses were conducted in premenopausal women (median age range, 25–58 years) with HR+/HER2− ABC. 14 These patients are of working age and are a suitable sample population for an analysis investigating correlation with WPL in patients with this most frequently occurring BC subtype. Moreover, as treatment with RIB in premenopausal women with HR+/HER2− ABC has demonstrated longer maintenance of QoL along with maintenance or improvement in work productivity in MONALEESA-7, investigating the relationship between QoL and WPL is pertinent to understanding the clinical benefits of RIB in these patients.17,18 MONALEESA-7 did not have a high proportion of Black or Hispanic women as participants, and no men were eligible to participate in the trial as the trial was dedicated to premenopausal women. Thus, further research is required to determine whether these results are generalizable to other populations, including patients in different socioeconomic brackets, patients in other age brackets, those with early BC or different BC subtypes, and those with different types of cancers. To achieve that, there is a need for such analysis to be a crucial component of other therapeutic cancer trials.
Conclusion
In this study, we determined the QoL factors that are associated with loss in work productivity in premenopausal patients with HR+/HER2− ABC by utilizing patient-reported outcomes collected during the MONALEESA-7 trial. This post hoc analysis may inform prognostic tools to characterize the patient population that is at greater risk for WPL. Finally, a comprehensive understanding of the factors that drive WPL will help design strategies, interventional or otherwise, to minimize WPL in this patient population, including supporting patients with practical help and selecting therapies associated with minimal WPL.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359221081203 for Correlation between work productivity loss and EORTC QLQ-C30 and -BR23 domains from the MONALEESA-7 trial of premenopausal women with HR+/HER2− advanced breast cancer by Debu Tripathy, Tristan Curteis, Sara Hurvitz, Denise Yardley, Fabio Franke, K. Govind Babu, Paul Wheatley-Price, Young-Hyuck Im, Radost Pencheva, Lucy A. Eddowes, Pierre-Alexandre Dionne, David Chandiwana, Purnima Pathak, Brad Lanoue and Nadia Harbeck in Therapeutic Advances in Medical Oncology
Acknowledgments
We thank the patients who participated in this trial, their families, and their caregivers; members of the data monitoring committee; members of the study steering committee; staff members who helped with the trial at each site; and Shashank Tandon of MediTech Media, Ltd, for medical editorial assistance with this manuscript. Ribociclib was discovered by Novartis Institutes for BioMedical Research in collaboration with Astex Pharmaceuticals.
Footnotes
Author contributions: Debu Tripathy: Conceptualization; Formal analysis; Visualization; Writing – original draft; Writing – review & editing
Tristan Curteis: Data curation; Formal analysis; Visualization; Writing – original draft; Writing – review & editing
Sara Hurvitz: Visualization; Writing – review & editing
Denise Yardley: Investigation
Fabio Franke: Visualization; Writing – review & editing
K. Govind Babu: Visualization; Writing – review & editing
Paul Wheatley-Price: Visualization; Writing – review & editing
Young-Hyuck Im: Visualization; Writing – review & editing
Radost Pencheva: Visualization; Writing – review & editing
Lucy A. Eddowes: Formal analysis; Visualization; Writing – original draft; Writing – review & editing
Pierre-Alexandre Dionne: Writing – original draft; Writing – review & editing
David Chandiwana: Writing – original draft; Writing – review & editing
Purnima Pathak: Formal analysis; Writing – original draft; Writing – review & editing
Brad Lanoue: Formal analysis; Writing – original draft; Writing – review & editing
Nadia Harbeck: Formal analysis; Writing – original draft; Writing – review & editing
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: D.T. reports grants and consulting fees from Novartis and Polyphor; consulting fees from Pfizer, GlaxoSmithKline, AstraZeneca, OncoPep, Sellas Life Sciences Group, Exact Sciences, and Immunomedics; unpaid Scientific Advisory Board member from Puma Biotechnology. T.C., R.P., and L.A.E. are employees of Costello Medical, which was commissioned by Novartis to design and conduct the statistical analysis for this study. S.H. reports grants from Ambryx, Amgen, Bayer, Obi Pharma, Biomarin, Cascadian, Daiichi Sankyo, Dignitana, Genentech, Glaxo-Smith Kline, Lilly, Macrogenics, Medivation, Merrimack, Novartis, Pfizer, Pieris, Puma Biotechnology, Roche, Seattle Genetics; travel from Lilly, Novartis, Obi Pharma. D.Y. reports consulting/advisory fees from Biotheranostics, Bristol-Myers Squibb, Celgene, NanoString Technologies; grants and consulting/advisory fees from Daiichi Sankyo/Lilly, Eisai; consulting/advisory fees, Speakers’ Bureau member, research funding, and travel from Genentech/Roche and Novartis; grants from Abbvie, AstraZeneca, Clovis Oncology, Immunomedics, InventisBio, Lilly, MedImmune, Medivation, Merck, Oncothyreon, Pfizer, Syndax, Tesaro. F.F., K.G.B., and Y-H.I. have nothing to disclose. P.W-P. reports advisory fees from Roche, Abbvie, Takeda, Merck, Bristol-Myers Squibb, Novartis, Astra Zeneca, EMD Serono, Pfizer. D.C., B.L., P.P., and P-A.D. report employment and stock ownership from Novartis. N.H. reports consulting fees from Novartis, Lilly, Pfizer, and AstraZeneca.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was sponsored by Novartis.
ORCID iD: Nadia Harbeck  https://orcid.org/0000-0002-9744-7372
https://orcid.org/0000-0002-9744-7372
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Debu Tripathy, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe, Unit 1354, Houston, TX 77030, USA.
Tristan Curteis, Costello Medical, Cambridge, UK.
Sara Hurvitz, UCLA Jonsson Comprehensive Cancer Center, Los Angeles, CA, USA.
Denise Yardley, Sarah Cannon Research Institute, Nashville, TN, USA; Tennessee Oncology, PLLC, Nashville, TN, USA.
Fabio Franke, Hospital de Caridade de Ijuí, CACON, Ijuí, Brazil.
K. Govind Babu, HCG Curie Centre of Oncology and Kidwai Memorial Institute of Oncology, Bangalore, India.
Paul Wheatley-Price, University of Ottawa, Ottawa, ON, Canada.
Young-Hyuck Im, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea.
Radost Pencheva, Costello Medical, Cambridge, UK.
Lucy A. Eddowes, Costello Medical, Cambridge, UK
Pierre-Alexandre Dionne, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
David Chandiwana, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Purnima Pathak, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Brad Lanoue, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Nadia Harbeck, Breast Center, Department OB&GYN, LMU University Hospital, Munich, Germany.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Surveillance, Epidemiology, and End Results Program. Cancer stat facts: female breast cancer, 2021, https://seer.cancer.gov/statfacts/html/breast.html (accessed 27 September 2021).
- 3. American Cancer Society. Breast cancer facts & figures 2019-2020, 2018, https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf (accessed 27 September 2021).
- 4. Bardia A, Hurvitz S. Targeted therapy for premenopausal women with HR(+), HER2(-) advanced breast cancer: focus on special considerations and latest advances. Clin Cancer Res 2018; 24: 5206–5218. [DOI] [PubMed] [Google Scholar]
- 5. Li J, Humphreys K, Eriksson M, et al. Worse quality of life in young and recently diagnosed breast cancer survivors compared with female survivors of other cancers: a cross-sectional study. Int J Cancer 2016; 139: 2415–2425. [DOI] [PubMed] [Google Scholar]
- 6. Sheridan W, Scott T, Caroline S, et al. Breast cancer in young women: have the prognostic implications of breast cancer subtypes changed over time? Breast Cancer Res Treat 2014; 147: 617–629. [DOI] [PubMed] [Google Scholar]
- 7. Azim HA, Jr, Partridge AH. Biology of breast cancer in young women. Breast Cancer Res 2014; 16: 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muller V, Nabieva N, Haberle L, et al. Impact of disease progression on health-related quality of life in patients with metastatic breast cancer in the PRAEGNANT breast cancer registry. Breast 2018; 37: 154–160. [DOI] [PubMed] [Google Scholar]
- 9. Rugo HS, Dieras V, Gelmon KA, et al. Impact of palbociclib plus letrozole on patient-reported health-related quality of life: results from the PALOMA-2 trial. Ann Oncol 2018; 29: 888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamer J, McDonald R, Zhang L, et al. Quality of life (QoL) and symptom burden (SB) in patients with breast cancer. Support Care Cancer 2017; 25: 409–419. [DOI] [PubMed] [Google Scholar]
- 11. De Mello Ramirez Medina J, De Araujo Trugilho I, Mendes GNB, et al. Advanced clinical stage at diagnosis of breast cancer is associated with poorer health-related quality of life: a cross-sectional study. Eur J Breast Health 2019; 15: 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yin W, Horblyuk R, Perkins JJ, et al. Association between breast cancer disease progression and workplace productivity in the United States. J Occup Environ Med 2017; 59: 198–204. [DOI] [PubMed] [Google Scholar]
- 13. Trogdon JG, Liu X, Reeder-Hayes KE, et al. Productivity costs associated with metastatic breast cancer in younger, midlife, and older women. Cancer 2020; 126: 4118–4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol 2018; 19: 904–915. [DOI] [PubMed] [Google Scholar]
- 15. Tripathy D, Bardia A, Sellers WR. Ribociclib (LEE011): mechanism of action and clinical impact of this selective cyclin-dependent kinase 4/6 inhibitor in various solid tumors. Clin Cancer Res 2017; 23: 3251–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Im SA, Lu YS, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med 2019; 381: 307–316. [DOI] [PubMed] [Google Scholar]
- 17. Harbeck N, Franke F, Villanueva-Vazquez R, et al. Health-related quality of life in premenopausal women with hormone-receptor-positive, HER2-negative advanced breast cancer treated with ribociclib plus endocrine therapy: results from a phase III randomized clinical trial (MONALEESA-7). Ther Adv Med Oncol 2020; 12: 1758835920943065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harbeck N, Hurvitz S, Bardia A, et al. Abstract P1-19-06: patient-reported outcomes, including work productivity, from the MONALEESA-7 trial of ribociclib plus endocrine therapy in patients with HR+/HER2− advanced breast cancer. Cancer Res 2020; 80: P1-19-06. [Google Scholar]
- 19. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993; 4: 353–365. [DOI] [PubMed] [Google Scholar]
- 20. European Organisation for Research and Treatment of Cancer. EORTC QLQ-C30 scoring manual, version 3, 2001, https://www.eortc.org/app/uploads/sites/2/2018/02/SCmanual.pdf (accessed 27 September 2021).
- 21. Johnston BC, Ebrahim S, Carrasco-Labra A, et al. Minimally important difference estimates and methods: a protocol. BMJ Open 2015; 5: e007953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Musoro JZ, Coens C, Fiteni F, et al. Minimally important differences for interpreting EORTC QLQ-C30 scores in patients with advanced breast cancer. JNCI Cancer Spectr 2019; 3: pkz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ousmen A, Conroy T, Guillemin F, et al. Impact of the occurrence of a response shift on the determination of the minimal important difference in a health-related quality of life score over time. Health Qual Life Outcomes 2016; 14: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998; 16: 139–144. [DOI] [PubMed] [Google Scholar]
- 25. Akaike H. Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csaki F. (eds) Proceeding of the 2nd international symposium on information theory. Budapest: Akadémiai Kiadó, 1973, pp. 267–281. [Google Scholar]
- 26. Burnham KP, Anderson DR. Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res 2004; 33: 261–304. [Google Scholar]
- 27. R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, 2017. [Google Scholar]
- 28. Shmueli G. To explain or to predict? Stat Sci 2010; 25: 289–310. [Google Scholar]
- 29. Fobair P, Stewart SL, Chang S, et al. Body image and sexual problems in young women with breast cancer. Psychooncology 2006; 15: 579–594. [DOI] [PubMed] [Google Scholar]
- 30. Mariotto AB, Etzioni R, Hurlbert M, et al. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol Biomarkers Prev 2017; 26: 809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ettl J, Quek RGW, Lee KH, et al. Quality of life with talazoparib versus physician’s choice of chemotherapy in patients with advanced breast cancer and germline BRCA1/2 mutation: patient-reported outcomes from the EMBRACA phase III trial. Ann Oncol 2018; 29: 1939–1947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359221081203 for Correlation between work productivity loss and EORTC QLQ-C30 and -BR23 domains from the MONALEESA-7 trial of premenopausal women with HR+/HER2− advanced breast cancer by Debu Tripathy, Tristan Curteis, Sara Hurvitz, Denise Yardley, Fabio Franke, K. Govind Babu, Paul Wheatley-Price, Young-Hyuck Im, Radost Pencheva, Lucy A. Eddowes, Pierre-Alexandre Dionne, David Chandiwana, Purnima Pathak, Brad Lanoue and Nadia Harbeck in Therapeutic Advances in Medical Oncology