Abstract
Aims
Cytokines, soluble mediators of immunity, are key factors of the innate and adaptive immune system. They are secreted from and interact with various types of immune cells to manipulate host body’s immune cell physiology for a counter-attack on the foreign body. A study was designed to explore the mechanism of Toxoplasma gondii (T. gondii) resistance from host immune response.
Methods and results
The published data on aspect of host (murine and human) immune response against T. gondii was taken from Google scholar and PubMed. Most relevant literature was included in this study. The basic mechanism of immune response starts from the interactions of antigens with host immune cells to trigger the production of cytokines (pro-inflammatory and anti-inflammatory) which then act by forming a cytokinome (network of cytokine). Their secretory equilibrium is essential for endowing resistance to the host against infectious diseases, particularly toxoplasmosis. A narrow balance lying between Th1, Th2, and Th17 cytokines (as demonstrated until now) is essential for the development of resistance against T. gondii as well as for the survival of host. Excessive production of pro-inflammatory cytokines leads to tissue damage resulting in the production of anti-inflammatory cytokines which enhances the proliferation of Toxoplasma. Stress and other infectious diseases (human immunodeficiency virus (HIV)) that weaken the host immunity particularly the cellular component, make the host susceptible to toxoplasmosis especially in pregnant women.
Conclusion
The current review findings state that in vitro harvesting of IL12 from DCs, Np and MΦ upon exposure with T. gondii might be a source for therapeutic use in toxoplasmosis. Current review also suggests that therapeutic interventions leading to up-regulation/supplementation of SOCS-3, IL12, and IFNγ to the infected host could be a solution to sterile immunity against T. gondii infection. This would be of interest particularly in patients passing through immunosuppression owing to any reason like the ones receiving anti-cancer therapy, the ones undergoing immunosuppressive therapy for graft/transplantation, the ones suffering from immunodeficiency virus (HIV) or having AIDS. Another imortant suggestion is to launch the efforts for a vaccine based on GRA6Nt or other similar antigens of T. gondii as a probable tool to destroy tissue cysts.
Keywords: Toxoplasma gondii, cytokinomes, host immune response, host resistance, susceptibility
Introduction
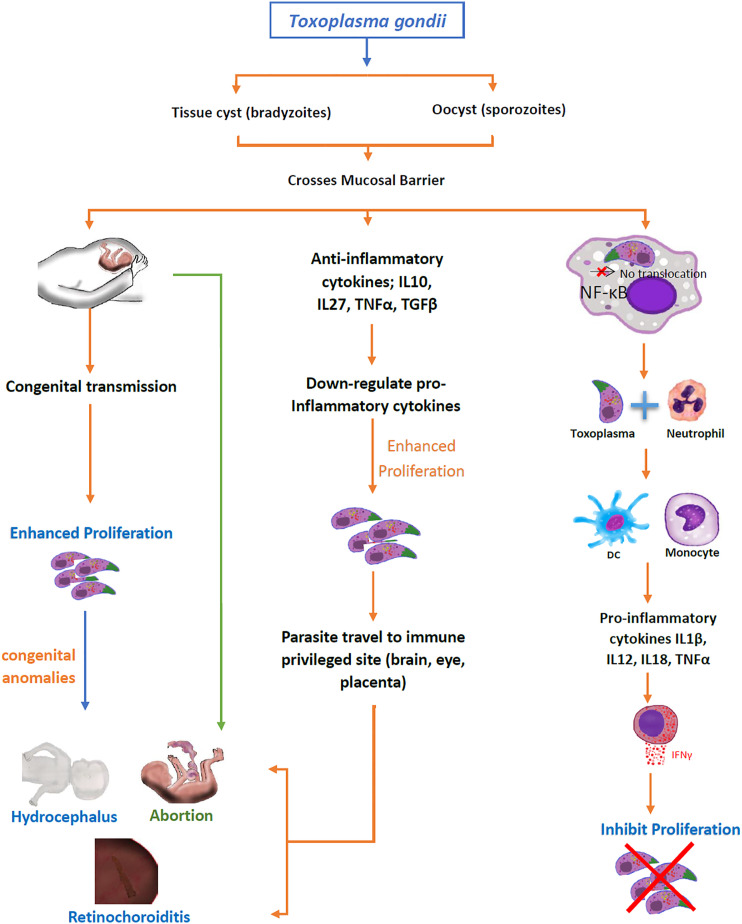
Toxoplasmosis is a zoonotic infectious disease caused by an intracellular protozoon pathogen called Toxoplasma gondii (T. gondii). It causes the abortion or congenital abnormalities (hydrocephalus and retinochoroiditis) in pregnant women which are more susceptible to Toxoplasma infection. 1 Generally, the immune system of immunocompetent individuals builds a protective immune response upon the interaction of antigen with antigen-presenting cells (APC) which induces the translocation of nuclear factor kappa (NF-κB) to initiate production of pro-inflammatory cytokines (IL1b, IL12, IL18, and IFNγ).2,3 The host lymphocytes and myeloid cells not only secrete a network of cytokines for signaling pathways upon exposure to antigen but also up-regulate certain chemokines (CXC, C, and CX3C) and toll-like receptors (TLR) on their surfaces for acting as signal-recipients against any antigen.3,4 In response to specific intracellular signals, various pro-inflammatory (IL1β, IL12, IL18, TNFα, IFNγ) and anti-inflammatory (IL4, IL10, TGFβ) cytokines (Table 1) give rise to a cytokinome that acts for specific immunological stimulus to develop immune response for susceptibility or resistance (Figure 1) to toxoplasmosis.4–7
Table 1.
This table illustrates the role of specific cytokines either in resistance or susceptibility against T. gondii. The cytokines’ source/s, main function/s, synergistic, and antagonistic relations with other cytokines.
| Name of cytokine | Immunity response | Source | Main functions | Synergistic relationship | Antagonistic relationship | References |
|---|---|---|---|---|---|---|
| INFγ | Resistant | CD4+, CD8+, and NK cells | Renders protection against T. gondii by activation of MΦ, NO, and GTPase signaling | TNFα and IL1β | IL4 and IL10 | [60] |
| TNFα | Resistant | MΦ, T cells, and basophils | Involves in acute inflammatory response | IFNγ and IL12 | IL4 and IL10 | [59-61] |
| IL1β | Resistant | Endothelial cells | Acute phase response mediator | TNFα | IL4 | [57] |
| IL2 | Resistant | CD4+ cells | Induces growth of T cells and the release of IFNγ, involved in the lytic activity of MΦ and NK cells | IFNγ | IL4 | [45] |
| IL4 | Susceptible | Basophils | Antagonizes the products of Th1 cells, long exposure leads to chronic toxoplasmosis | Th2 cytokines | Th1 cytokines | [53] |
| IL5 | Resistant (chronic) and susceptible (acute) | Mast cells | Plays a counter protective role in acute toxoplasmosis and protective role in chronic toxoplasmosis | IL4 (acute infection) | IL12 | [94] |
| IL6 | Resistant | MΦ, endothelial cells, monocytes, fibroblasts, myelomatous | Plays a pleotropic role in immunity; builds barriers in early ocular and encephalitis toxoplasmosis, enhanced activities of NK cells, and maturation of T and B cells | IL1β and TNFα | IL12, IFNγ, and IL27 | [97,111] |
| IL7 | Resistant | DCs, hepatocytes, endothelial cells | Plays a crucial role in the development of memory CD8+ T cells | IL15 | IL10 and IL4 | [113] |
| IL10 | Susceptible | CD4+ cells, MΦ, B cells, DCs, mastocytes | Controls hyper-inflammation, keeps check on protective functioning of CD4+ cells, and plays a suppressive microbicidal function for MΦ and Np | IL6 | IL12 and IFNγ | [201,202] |
| IL12 | Resistant | DCs, MΦ, Np | Central inducer of IFNγ | TNFα | IL4 and IL10 | [24] |
| IL15 | Resistant | Mononuclear phagocytes | Required for optimal role of NK cells, CD8+ cells, and IELs | IL12 and IL7 | IL10 and IL4 | [61] |
| IL17A | Resistant | CD8+, γδT cells, NK cells | Mainly involves in innate immunity by the recruitment of Np | IL12, IFNγ, and IL6 | IL10 and IL4 | [151,164] |
| IL18 | Resistant | MΦ and some other cells | Involves in production of IFNγ by NK cells and T cells | IL12 | IL10 and IL6 | [182] |
| IL23 | Resistant | MΦ and DCs | Stimulates NK cells and T cells more specifically in the absence of IL-12 | IL12 | IL10, IL4 and IL6 | [158,159) |
| TGFβ | Susceptible | Intraepithelial lymphocyte | Anti-inflammatory role in brain, eyes and intestine | IL10 | TNFα, TNFβ, IFNγ, IL6, and IL12 | [15] |
IFN (Interferon), IL (Interleukin), TNF (Tumor Necrosis Factor), TGF (Transforming Growth Factor).
Figure 1.
Outcome of toxoplasmosis in case of resistance and susceptibility.
Immunological studies on human infections have clearly concluded that cell-mediated immunity and IFNγ are paramount in the control of any infection particularly caused by the intracellular pathogen (T. gondii). 8 The inability of the humoral response alone (antibodies) to prevent Toxoplasma reactivation is evident by the fact that most of the HIV-infected people lack effective immunity hence exhibit symptoms of T. gondii infection and reactivation, even in the presence of high titers of specific IgG. 9
Pro-inflammatory cytokines are among the key factors to initiate and maintain innate as well as acquired immunity to restrict proliferation of Toxoplasma. A variety of cytokines are produced upon activation of APCs and cells of the adaptive immune system (B and T cells). The differences in cytokinome can be speculated at the different stages of infection, due to intra- or extra-cellular nature of pathogens as well as due to diversity of the host genetic makeup. 10 The indirect role of cytokines against T. gondii in leading to either resistance or susceptibility also depends upon the stage of parasite in the host and the induction and modulation of pro-inflammatory cytokines driven by the particular parasite strain as well as the robustness of the host’s immune profile. Different typical and atypical strains of T. gondii exist globally, and have been specifically studied in America and Europe. This parasite is identified having three distinct genotypic lineages in humans: type I strain (RH-88), type II strain (ME49, and DEG), and type III strains (CEP and VEG). 11 The genetic moieties in these strains result into a highly varied level of virulence7,12 which inflicts diverse pathological effects in host by a variety of cytokine pathways as well as owing to the wide range of interactions through a vast diversity of host and parasite molecules interacting each other, with some known and some unknown footprints. The known footprints include but not limited to inherent-oxidative stress, 13 a diversity of IRGs (Immunity Related GTPases) 12 of host with a locus on chromosome 11, 14 Z-DNA binding protein 1 (ZBP1), receptor-interacting serine/threonine-protein kinase 3 (RIPK3) of host. 15 Likewise, the wide range of parasite molecules includes a diversity of rhoptry proteins (e.g. ROP5, ROP18),12,16 dense granule proteins (e.g., GRA5, GRA12, GRA16, GRA24) 17 ; small GTPase immunity-associated proteins (GIMAPs 4, 5 & 6) 18 of T. gondii. This review describes the cytokinome (Table 1) in toxoplasmosis and their interactive role for development of host’s susceptibility and resistance toward T. gondii infection.
Database search
A search was conducted on Science Direct (https://www.sciencedirect.com/science/search) and PubMed (https://www.ncbi.nlm.nih.gov/pubmed) using “host immune response and resistance of toxoplasmosis” to achieve the relevant literature for this study. The searched period ranged from 1980 until 15 September 2021, yield 649 publications. The literature having mechanism of immune response and pathogen resistance were included in this study. Whereas for the last 5 years, the data were searched from PubMed using the same key words as mentioned above and majority of the articles with novel insights into the immune response mechanism as well as host parasite interactions were included in this review. Although maximum efforts have been done to review the literature on “host immune response and resistance of toxoplasmosis”, certainly there are limitations of this review. Hence, this should not be considered a review encompassing all the new literature on ‘Toxoplasma gondii” and “Toxoplasmosis”. This is because of the fact that 24,912 and 13,928 results are retrieved from Science Direct and PubMed, respectively, while using a key word “Toxoplasmosis” with the same time period as mentioned above. Likewise, 23,158 and 15,750 results are displayed from Science Direct and PubMed, respectively, with a key word “Toxoplasma gondii” for the same time period. Similarly, slightly modified key words, that is, “host immune response of toxoplasmosis” displays almost 7000 (6969) results in Science Direct.
1) Virulence of T. gondii strains
In humans, the regulatory cytokines profile depends on the T. gondii strain. Among three notable strains (type I, II and III) of T. gondii, the type I is more virulent as compared to type II and type III strains. In type I (RH strain) infected cells, the translocation of NF-κB does not take place resulting in the production of anti-inflammatory (IL10, IL27, and TGFβ1) cytokines which are higher as compared to uninfected cells.19–23 These anti-inflammatory cytokines enhance the proliferation of T. gondii. It was found that with a significantly high level of TGFβ1 in the blood as well as in the aqueous humor of the acutely intraocular Toxoplasma-infected host, it may adversely interfere with the effective cellular immune response leading to an increased mortality and extensive ocular tissue damage with tachyzoites observed in the pigment epithelium layers. 24 Consequently, Th2 and Treg responses are enhanced in comparison with a primary ocular infection. 25 ME49 is a type II strain that induces the translocation of NF-κB-light-chain-enhancer from the cytoplasm to the nucleus of activated B cells, splenocytes 26 and bone marrow–derived MΦ 27 which induce the production of pro-inflammatory cytokines (IL1β, IL12, IL18, TNFα, IFNγ, and IL12p40) by thioglycolated MΦ/cell lines19,28. Whereas, the production of anti-inflammatory cytokines are lower in ME49-infected cells than that of uninfected cells 21 speculating CD36-mediated engagement of low virulence strains, with macrophages. 29 Recent evidence also demonstrates the role of Toxoplasma’s parasitophorous vacuole-membrane–associate dense granule proteins in modulating parasitic virulence while interacting the host body’s resistance mechanisms like GRA12 of Toxoplasma was identified as a major virulence factor to counter the host’s IFNγ. 30
Host immune response
A) Innate immune response
In early stages of infection, dendritic cells (DC), macrophages (MΦ), natural killer (NK), and neutrophils (Np) interact in a coordinated way to provide the first line of defense in the form of innate immune response leading to develop adaptive immunity.31-33 The innate immune response is elicited against toxoplasmosis in the form of IL12 production upon interaction with antigen (Ag). The release of IL12 from MΦ, DCs, and Np is essential for the release of IFNγ from NK cells (innate immune response) and T lymphocytes (adaptive immune response) via antigen presentation. 34 IFNγ has been shown to induce guanylate binding proteins (GBPs) in a murine model of toxoplasmosis, thereby, these GBPs accumulate on the surface of intracellular parasites potentially causing parasitic destruction, thus displaying an active role of intracellular autonomous immunity. 35 The increased susceptibility toward T. gondii infection is due to the depletion of NK cells, MΦ, or DCs which have a significant involvement for innate immune response against the infection. 36 The mechanism of innate immune response initiate upon interaction of toll-like receptors (TLRs) with ligands expressed on T. gondii surface, thereby starting intracellular signaling pathways through engagement of the myeloid differentiation domain-88 (MyD88). These are the universal adaptor proteins involved in signaling of all TLRs except TLR-3. The study on MyD88 deficient mice model was impaired to induce primary protection in acute infection of T. gondii (RH strain). 37 Studies on mouse with targeted inactivation of MyD88 showed that DCs work as antigen-presenting cells (APC) and are responsible for the increased susceptibility to T. gondii infection. However, there was no effect on MΦ and Np. The MyD88 deficient mice masked the production of IL12 from DCs and IFNγ by NKs to initiate innate immune response. It explains a central role of DCs in the coordination of innate immune response against T. gondii infection and predicts the increased susceptibility towards infection if DCs are recognized defective in early encounter of T. gondii infection. 38
B) Cellular immune response
In T. gondii infection, the strong resistance to re-infection as well as the hindrance to reactivation of chronic infection is based on the host’s cell-mediated immunity.39,40 The synergistic role of CD4+ and CD8+ T cells for the development of acquired immunity was understood from targeted experiments on C57BL/6 mice vaccinated with temperature sensitive mutant strain of T. gondii (ME49).41,42 The development of complete immunity against a virulent strain (type I) is dependent on IFNγ synthesis from NK and T cells. The immunocompetent host activates either T cells or NK cells for encountering parasitic invasion. In a previous study, the MHC I (lack of CD8+ cell stimulation) impaired mice (beta 2m-deficient mice) surprisingly showed high resistance against T. gondii following vaccination. This enhanced immunological response in the absence of CD8+ cells showed the involvement of NK cells activated by IL12 upon parasitic invasion. 43
Generally, the CD8+ T cells are the major source of IFNγ production against most of the T. gondii strain. 44 The c-Rel expression regulated by NF-κB is widely dominant in hematopoietic cells, 45 which play a critical role in the development of resistance against T. gondii (ME49) infection. 46 The role of c-Rel in influencing the CD8+ cell response was investigated in mice model (c-Rel–/– mice) with a special infection of T. gondii strain (replication-deficient strain) in C57BL/6, CD45.1, and Thy1.1 mice. The CD8 + cells impair to replicate in the absence/deficiency of c-Rel. Likewise, the c-Rel deficient mice remained unable to survive during infection with the replicating pathogen.47,48
CD8+ T immune cells having a TCR Vβ8.1, 8.2+ phenotype produce protection against tissue cyst development in mouse model. Transfer of CD8+ cells induced by N-terminal of dense granule protein-6 (GRA6Nt) of parasite has been shown to clear T. gondii cysts from the brains of infected mice that were deficient in T cells (Sa et al. 49 2017), further highlighting the role of cytotoxic T cells in the induction of protective immunity against T. gondii. A genetically resistant strain of mice having a H-2d haplotype helped discover these specific type of cytotoxic T cells, whereas H-2Ld was found as a major antigen-presenting molecule to CD8+ T cells to achieve this objective of tissue cyst elimination.
The immune response of CD8+ cells is more dominant alike effector cells than CD4+ cells. Nevertheless, CD4+ helper T cells are direly required for an effective functioning of CD8+ cells.50,51 The correlation of CD4+ and CD8+ `cells proved to be the main scaffold of cytokine trafficking in mice host.
Regulatory T (T reg) cells are required for the maintenance of immunological self-tolerance and immune homeostasis by actively suppressing the pathological and physiological immune responses.52,53 An IL2 knocked-out mouse model orally infected with lethal dose of T. gondii, showed highly Th1 cell type-polarized mucosal immune response. Such effect contributed to the incapacity of T reg cells to perform effector responses and consequently led to immuno-pathogenesis.54,55 Besides this, T reg cells of infected mice expressed lower levels of Bcl-2 and increased levels of apoptotic markers than that of naive mice. It is suggested that de-regulation in the T reg cells is a consequence of these impaired cells turnover. 54 Besides this, there was found a gradual weight loss and significant delayed mortality in T reg-transferred toxoplasmosis-infected mice associated with lower level of IFNγ and TNFα. Additionally, higher cyst number and parasite load in brain of those mice were observed. 56 Furthermore, activity of T reg cells results in the death of proliferating T cells which favors the multiplication of pathogen in the murine model. 57 In a pregnant T. gondii-infected mouse model, numbers of splenic CD4+CD25+-T reg cells and placental Foxp3+ cells decreased synchronously. During infection, the reduction of splenic CD4+CD25+-T reg cells was associated with apoptosis (Bcl-2) induced by proliferating T cells. 58 Additionally, injection of pregnant mice with excretory–secretory antigens (ESA) of T. gondii also causes fetal death associated with apoptosis of CD4+CD25+ T reg cells by down-regulating their Bcl-2 expressions and Bcl-2/Bax ratio. It could be partly prevented by adoptive transfer of CD4+CD25+ T reg cells from normal to infected pregnant mice. 59
The high level of Th1 cytokines (IL2 and IFNγ) were reported to be produced by CD4+ cells upon interaction with tachyzoite. 50 It is found that MyD88 is effectively involved in the development of Th1 response. 60 Generally, the Th1 cell-mediated immunity builds the resistance against the T. gondii infection by IFNγ production via Th1 effector cells. 61 The role of Th1 cytokines (IFNγ, IL12, and TNFα) for susceptibility to toxoplasmosis has been witnessed with the absence of any of these pro-inflammatory mediators as previously studied.42,62 Moreover, other cytokines like IL2, IL6, IL7, IL15, IL18, and IL23 are also associated with the development of strong immunogenic response. 60 Recent evidence demonstrates TLR-11–independent activation of inflammasome for driving CD4+ T-cell–derived IFNγ-mediated host resistance to T. gondii. 7 GRA24-driven protective immunity mediated through p38 MAPK activation, IL12 production, and independent of MyD88 pathway has been evidenced, recently, through use of bicistronic IL12YFP reporter mice on MyD88+/+ and MyD88-/- genetic backgrounds, MyD88+/+ and MyD88-/- bone marrow–derived macrophages as well as exploiting parasites species named as uracil auxotrophic Type-I stain of T. gondii cps1-1 and cps1-1:Δgra24. 63
In contrast, various experiments have demonstrated that the cytokines involved in Th2 response also play a detrimental role for enhancing the susceptibility to T. gondii infection. 64 The modulation of Th2 response is mainly carried out by IL4 and IL10. Both cytokines increase the host susceptibility to T. gondii in early infection. 103 Nevertheless, the regulatory function of Th2 cytokines has been unveiled. The evidence from T. gondii infection (Type II stain) to the IL4-knockout mice resulted in less susceptibility to toxoplasmosis. 65 Shoot-up levels of inflammatory cytokines were detected in IL10 knockout mice causing early resistance to Toxoplasma.64,66
C) Cytokines and other inflammatory mediators playing a role against T. gondii
Interferon-γ: Interferon-γ (IFNγ) is reckoned as the main pillar of cytokines induced by T cells (CD4+ and CD8+), γδT cells, and NK cells as protective immunity against either the acute or chronic phase of T. gondii infection.42,67,68 The neutralization of INFγ (anti-INFγ antibodies) in in vivo makes the mice (Swiss-Webster) susceptible to the primary infection (acute infection) and reactivate parasite (ME49 strain) in chronic infection. 69 The IFNγ is produced from activated MΦs to exhibit its specific immunological functions against T. gondii (C56 strain). 70 The latest evidence demonstrating the CD4+, CD8+, γδT cells, and NK cells as the main producers of IFNγ during toxoplasmosis was based on the use of a newly developed mouse line named as “GREVEN” an IFNγ reporter mouse having a fusion protein of Venus and NanoLuc to analyze IFNγ producing cells. 67 Likewise, the Lck-Cre/Ifngfl/fl mice were found highly susceptible to toxoplasmosis, further strengthening the role of T-cell-IFNγ in protection against toxoplasmosis. 67 At the site of infection, the maintenance of IFR8+ inflammatory DCs is essential provision of host resistance against intracellular T. gondii, whereas this requires the production of IFNγ by ILC1 and NK cells through T-bet involvement. 68 The extensive role of IFNγ was partly dependent on the release of TNFα by activated MΦs. 71 In the host, most documented immunological pathway against T. gondii (virulent RH strain) is nitric oxide–dependent. 22 It involves the collective role of IFNγ and TNFα for the production of nitric oxide that curtails the development of microbial pathogens. 72 IFNγ is involved in an alternative mechanism via inducing Ag-specific CD8+ (CTL) cells-dependent immunity. 73 INFγ directs the antimicrobial response such as STAT-1, TNFα, IL1β, and CD40L by activating the transcription factor (NF-κβ) signaling pathway.74,75 This signaling pathway has been believed to rely mainly through involvement of inducible nitric oxide synthase (iNOS) and immunity related GTPase (IRGs) which make grounds to resist T. gondii (ME49) infection, 76 the nitric oxide-independent intracellular resistance mechanism has been evidenced recently through use of IFNγ-stimulated bone marrow–derived macrophages (BMDM). 77 Likewise, recent evidence has shown IFNγ as an inducing agent of guanylate binding proteins (GBP) that accumulate on surface of intracellular stage of T. gondii; thus, mediating the role of an intracellular check on the excessive growth of this parasite that may become lethal to mouse host in the absence of GBP as has been demonstrated by the early death of the mice deficient in murine guanylate binding protein-7 (mGBP7), by T. gondii infection. 35 The intracellular pathogen (T. gondii) has been highly adapted for combating host immune response by interfering with NF-κβ signaling pathway.78,79 It also has the ability to inhibit IFNγ signaling by curtailing the function of STAT-1 and by augmenting the levels of IFNγ signaling suppressor molecules, named as suppressor of cytokine signaling molecule-1 (SOCs1).80–82 Most of the Th2 cytokines function antagonistically to IFNγ.
Tumor necrosis factor-α (TNFα): TNFα is a pyrogenic factor, produced by MΦs, T lymphocytes and basophils, found to be responsible for the production of acute inflammatory response. It has the ability for microbicidal activity in MΦs via the production of IFNγ from NK cells. 83 TNFα functions synergistically with IFNγ for the development of resistance against T. gondii (C56 strain) infection.71,84 Hence, it is suggested to have a crucial role in the protective immunity against toxoplasmosis. However, certain researchers explained the role of TNFα to be doubtful. TNFα has also been reported to elicit cerebral and hepatic autoimmunity.85,86 It has also been found to assist in intra-cerebral dissemination of T. gondii (ts-4 strain and virulent RH) in mice ((TNF(-/-), LTalpha(-/-), and TNF/LTalpha(-/-)). 87
Interleukin-1: It is an acute phase response mediator cytokine that plays a synergistic role with TNFα enhancing inflammation during infection with T. gondii.90,91 TNFα has been found to be associated with IL1β in regulating endothelial cells for the immunological and inflammatory role. In vitro studies elucidated an effect of these cytokines in hindering the intracellular multiplication of T. gondii in murine peritoneal MΦs or human fibroblast.71,90 However, in vivo studies on mice showed the protective role of recombinant TNFα and/or recombinant IL1β during infection with tachyzoites of T. gondii (C56 strain and RH strain). 71 In female mouse models (BALB/c, and Swiss-Webster), IFNγ induced the anti-toxoplasmic activity by augmenting the production of TNFα when treated with recombinant cytokines (TNFα and IL1β).
Interleukin-2: Interleukin-2 is exclusively produced by CD4+ T cells. It is initially regarded as the primary T cell growth factor having significant role in the proliferation as well as in the development of antigen stimulated CD8+ T cells. 91 Various studies carried out on viral infections revealed an indispensable role of IL2 during primary response of T cells via effector cytotoxic T cell development and restoration of CD8+ memory T cells.92,95 IL2 has also been found to induce T cells proliferation for IFN-γ production during the infection with T. gondii (virgin).96–98 Whereas, the role of IL2 in secondary immune response to T. gondii (ME49) was disclosed by Sa et al., (2013) on CD8+ T cell hybridoma clones from the spleens of chronically infected female mice (BALB/c, BALB/c-background Rag1−/−, and Swiss-Webster). They found an increased production of INFγ with exogenous input of IL2 to CD8+ T cell hybridomas. 99 Various studies on murine models proved the protective role of IL2 against the infection with T. gondii. Increased survival rates and reduced number of cysts in the brains of mice were observed when treated with recombinant IL2. 96 It also triggered the lytic activity of MΦs and NK cells. 100
Interleukin-4: Interleukin-4 is a Th type 2 cytokine that down-regulates the effect of Th type 1 cytokines. The progressive toxoplasmic encephalitis found to be linked with the presence of mRNA transcripts in the brains of infected mice (C57BL/10 ScSn). 101 In acute infection of T. gondii, IL4 exerted a protective role by antagonizing the products of Th1 cells that reduced the number of mortalities. However, the prolonged exposure of IL4 made the mice (IL-42/2) susceptible to chronic toxoplasmosis with increased multiplication of parasite cysts in brain. 102
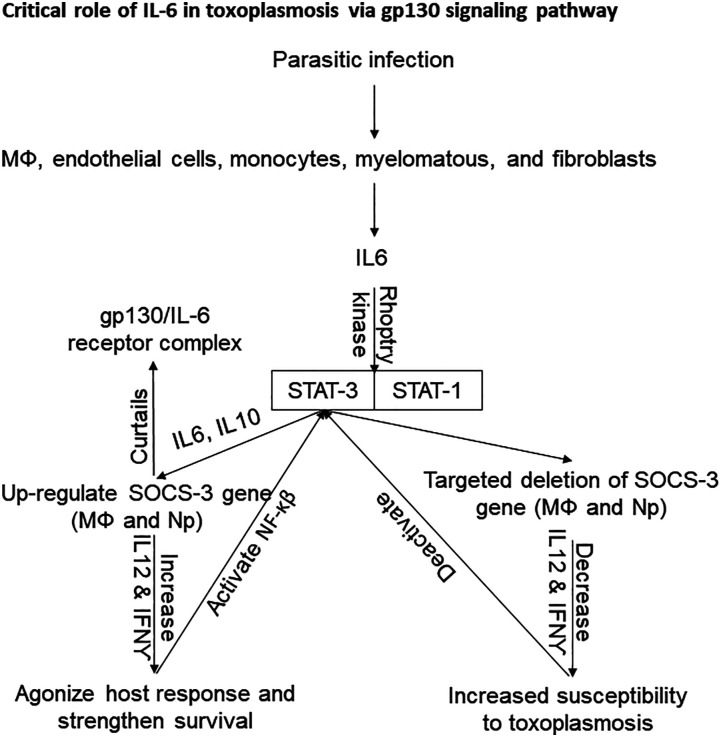
Interleukin-6: Mainly, it is involved in early development of acute phase response, maintenance of hematopoiesis 103 and immune barriers in ocular 104 as well as cerebral Toxoplasmosis. 105 It makes the NK cells to increment their cytotoxic activity and is also involved in the maturation of antibody secreting B lymphocytes and the differentiation of T lymphocytes. 106 Different immune cells such as MΦs, endothelial cells, monocytes, myelomatous, and fibroblasts are involved in the production of IL6. This cytokine functions synergistically with IL1β and TNFα. Hence, it is regarded as a remarkable pyrogenic factor mediating dominantly the production of hepatocyte based acute inflammatory proteins. The signaling pathway of IL6 is associated with gp130 signal transducing component that leads toward the activation of STAT1 and STAT3 via the signaling of JAK1, JAK2, and TYk2. This pathway is referred as gp130-mediated JAK/STAT signaling pathway 107 which is most effectively regulated by socs-3 signaling.108,109 The host transcription factor STAT3, antagonizes host response and strengthens parasite survival which is activated by the cytokines (IL6 and IL10) during infection with certain intracellular pathogens.110–112 Interestingly, T. gondii (ROP16-deficient type I) has been found to activate this factor by phosphorylation caused by rhoptry kinase.20,113 This event is accompanied with impaired production of IL12p40 and TNFα response. To investigate the mechanism involved in the invasion of immune strategies, it was found that socs-3 up-regulates IL6 and IL10 which stimulate activation of STAT-3. The role of IL6 is more critical as in normal defense mechanism, socs-3 can curtail function of this cytokine by formation of gp130/IL6 receptor complex. 114 In the work conducted by Whitmarsh et al., (2011), the mice deletion with socs-3 in MΦs and neutrophils unexpectedly resulted in increased susceptibility to toxoplasmosis by reducing the levels of IL12 and IFN-γ. This study suggested more pronounced anti-inflammatory role of IL6 in MΦs particularly in the absence of socs-3. 115
Moreover, the gp130 transducing component has common structural features to IL6 and IL27. 116 This IL27 is critically involved in curtailing the infection induced inflammatory pathology and functions antagonistically to IL6. The mice lacking gp130 signal transducer (gp130 Y757F mice) upon infection with T. gondii (ME49), showed high parasite burdens and increased mortality having low IL12 and IFNγ titer. 117 IL6 is a cytokine that more pronouncedly functions to resist the Toxoplasma-induced encephalitis in murine model. 118 Previously, the protective role of IL6 was questioned in various studies that illustrated the role of IL6 in increased intracellular multiplication of T. gondii. 110 Later studies defined the critical role of IL6 in progression of T. gondii infection as the IL6-deficient mice rapidly switched to severe states of toxoplasmosis such as Toxoplasma encephalitis 119 (Figure 2).
Figure 2.
Pathways for host susceptibility and resistance in human Toxoplasmosis, particularly as a result of IL6.
Interleukin-7: It plays a crucial role for the development of memory CD8+ T cells 120 which are the key producers of INF-γ in acquired immunity. 41 The IL2, IL7, and IL15 fall in the family of γ-chain cytokines that are involved in building CD8+ memory T cell.121,122 The development of memory cells in the form of CD8+ T cells has been disclosed to be dependent on the significant role of IL7 as well as IL15. The in vivo neutralization of IL15 affected CD8+ memory T cells productions which are more susceptible to T. gondii (76K) re-infection. 123 In memory cells development process, IL7 is recruited to provide the survival signals to naïve and memory CD8+ T cells. 124 In a study conducted by Bhadra et al., (2010), the synergistic role of IL7 and IL15 was explored. Their findings indicated a severe impairment of memory cells in case of the absence of both of these cytokines. However, the absence of any of these cytokines resulted in minimal impact on the maturation of splenic CD8+ T cells. 125
Interleukin-10: It induces pleiotropic effect on cells and is found to be a suppressive cytokine that is purely an anti-inflammatory in its action. 126 Immune cells including MΦs, CD4+, B cells, DCs, and mastocytes are the source of this cytokine.127–129 The rapid production of IL10 by localized MΦs was reported upon infection with high doses of virulent (RH) strain in mice. 130 However, it keeps a check on the protective functioning of CD4+ T cells in acute phase infection.131,132 IL10 is also reported to be responsible for suppressive microbicidal activity of MΦs and neutrophils by minimizing the function of nitric oxide (NO) synthase enzyme, IFNγ and IL12. The NO inhibits the production of O2 free radicals and prostaglandins.133–135 The simultaneous production of these antagonizing cytokines may lead to demolish the defensive mechanism. Hence, it was reported that the production of IL10 is supported by an “activation signal” after the release of IL12. The IL12 lets the production of IFNγ by Th1 cells not only triggering the effector cells to play protective role but also passing a signal for the reactivation of IL10 gene expression. The study conducted by Gaddi et al., 136 (2007) explained the negative feedback mechanism for the poised state of these pathogenic and regulatory cytokines via CD4+ T cell lineage. In the mice (BALB/c) susceptible to T. gondii, the increased levels of IL10 were found in their lymph nodes and central nervous system causing chronic toxoplasmic encephalitis. 134 In a study, the detrimental role of IL10 was disclosed as to promote the development of intracellular tachyzoites by inhibiting MΦ L–arginine-based killing. 133 However, making a comparison, severe combined immune-deficient (SCID) IL10 knockout T. gondii (RH strain) infected mice lived longer than the infected SCID mice. 66
Interleukin-12: In innate immune response, certain cell populations (DCs, MΦ, and Np) are reported to produce IL12 in vitro against T. gondii (a virulent strain).130,137,138 It is the central inducer of IFNγ in developing the protective immunity against T. gondii. It is evident from various experiments that the complete absence of immunity in INFγ as well as IL12p40, IL12p35, and STAT4 deficient mice leads to deaths in early acute infection.139–141 The increased mortalities were reported when diphtheria toxin were used to deplete DCs in T. gondii (ME49 strain) infected organism which abolish the production of IL12. 142 The absence of p40 chain in IL12 heterodimer is responsible for the poor production of IFNγ in mice (IL12-deficient). 143 During chronic Toxoplasmosis, the 12/15 lipoxygenase (12/15-LOX) is involved in the oxidation of unsaturated fatty acids in MΦs which deprives the production of IL12. In addition, the 12/15-LOX deficient mice were enabled to produce comparable levels of IL12 when stimulated with lipopolysaccharide (LPS). This finding was explained by the involvement of neutrophils and particularly DCs in inducing IL12 production in acute phase infection. 144
Interleukin-15: It belongs to Th1 immune response and plays an effective role in enhancing the function and development of CD8+ T cells. 145 IL15 serves to play a key role in the development of various lymphocyte population more importantly NK, CD8+ T cells and intraepithelial lymphocytes (IELs). 146 The memory (CD8+) T cell response was found inadequate identified in IL15 knockout mice.147,148 However, a study conducted by Lieberman et al., (2004) reported no role of IL15 for development of memory immune response. They found the mice deficient with this cytokine withstood the severe T. gondii (ME49) infection. 149
Interleukin-17: The IL17A, IL17F, and IL22 are secreted from Th17 cells reported by Wu et al., (2018). But a variety of immune cells including CD8+,150,151 γδ, 152 and NK cells153,154 also secrete IL17. It is an inflammatory cytokine that provides innate immunity from the recruitment of neutrophils 155 which make the host resistant against T. gondii infection. 156 A subset of CD4+ T cells has been identified which produces IL6, IL17A, IL17F, and TNF in response to IL23.157,158 In a study conducted by Kelly et al., (2005), IL17 knockout mice remained successful in developing a normal acquired immunity against T. gondii.152,161,162 A study carried out on T and B lymphocytes deficient mice revealed that NK cells are the major IL17 producers. These cells are influenced to produce IL17 in the same manner as T cells are triggered. In addition, the researchers revealed a key role of IL6 to target NK cells for secretion of IL17. 161 The IL17A neutralization by antibodies had a partial protective effect against fatal T. gondii–associated inflammation. 162 Severe South American ocular toxoplasmosis is associated with decreased level of intraocular IFNγ and IL17A. 163 But in ocular toxoplasmosis (with less severe clinical presentation and infected by non-virulent strains) in France, the IL17A level was augmented in toxoplasmic uveitis. 164 Neutralizing IL17A decreased intraocular inflammation and parasite load in mice (Swiss-Webster). It is suggested that the local IL17A production by resident cells plays a central role in the pathology of ocular toxoplasmosis. 25 Finally, there was observed a lower level of IL17 expressing CD4+ and CD8+ T lymphocytes in cells cultures from sero-negative and seropositive pregnant and non-pregnant women, respectively upon stimulation with tachyzoites. 165 A recent study demonstrates an essential role of T cells expressing class I-restricted T cell-associated molecule (CRTAM), for IL17 production during toxoplasmosis. 166 The study also highlights the importance of IL17 in regulating immunopathology whereby deficiency of IL17 can cause dysbiosis through the production of antimicrobial peptides as well as through translocating gut-bacterial flora to spleen and mesenteric lymph nodes. 166 Overall, these results suggest that IL17-mediated responses may be useful for both protective and pathogenic effects.
Interleukin-18: It is a pleiotropic cytokine produced in a non-specific manner. IL18 is a potent cytokine involved in the production of IFNγ by NK cells and T lymphocytes. Hence, it is involved in building innate as well as acquired immune response against T. gondii infection. Attributable to its identical role in developing resistance, IL18 is referred as a potential enhancer of IL12 activity.167,168 Structurally, it is closely related to IL1β cytokine family. 169 Moreover, similar to IL1β signaling pathway, IL18 precede the activation of NF-κβ170,171 that requires STAT4 factor for its activation.172,173 The impaired role of IFNγ was observed in IL18 deficient mice with intracellular infection.174–176 Interestingly, NK cell has certain receptors for IL18 which has synergistic role with IL12 in developing innate immunity against T. gondii infection.167,177–179 However, endogenous role of IL18 on SCID mice was demonstrated to be trivial having less influence on IFNγ production when the infected mice were treated with anti-IL18. In contrary, the exogenous role of IL18 was reported to increment the production of IFNγ ultimately boosting the resistance against T. gondii. 179 IL18 reported to be involved in the immunopathology of intestine in mice accompanied by IL12. 180
Interleukin -33: The host damage protein IL-33 has recently been shown to play an important role in the immune response by affecting the local environment of brain in the favour of both host and parasite survival through engagement of astrocytes via IL-33 Receptor. IL-33 is a host damage protein that is produced locally in the brain tissue from the oligodendrocytes and astrocytes. The evidence to this effect is very strong as it is based on the use of mice having IL:-33 receptor-deficient astrocytes. 181
Transforming Growth Factor-β: Transforming growth factor-β (TGFβ) is another immunosuppressant cytokine that plays a critical role in antagonizing the action of TNFα, TNFβ, IFNγ, and IL12.182,183 Moreover, it is considered to be involved in limiting the immune-pathologies incited by Th1 cytokines specifically in central nervous system (CNS) as well as in intestine.184,185 TGFβ has been reported to induce immunopathological effects on retinal cell line in an in vitro study by increasing the replication of T. gondii. 186 The intraepithelial lymphocytes are the main producers of TGFβ that is involved in the down-regulation of pro-inflammatory cytokines (IFNγ, TNFα, IL1β, IL12, IL15, and IL18) in case of pathogenic lamina propria lymphocytes (LPL) response (hyper-Th1 response). When wild type mice were treated with active transfer of IELs, they showed no sign of ileitis. This study revealed the modulating role of IELs for LPL produced Th1 response in the intestine. 187
In spite of gastrointestinal sites, TGFβ also has a dominant role as an anti-inflammatory agent in brain and eyes. 188 TGFβ signals the spleen cells to secrete anti-inflammatory cytokine such as IL10 that is synergistically involved in checking the pro-inflammatory secretions from NK cells and CD4+ T cells in these immune-privileged sites (eyes, brain, and placenta). 189 The production of IL6 by innate immune response functions antagonistically to TGFβ and suspends its protective role for immune privileged sites (eyes and brain) susceptible to hyper-inflammation. 190
CCL22: T. gondii induces expression of CCL22, a chemokine that has been linked to Toxoplasma-induced activation of Wnt/beta-catenin signaling pathway, for resisting cellular check on parasite replication and favoring the parasite survival within Toxoplasma-exposed naïve BMDMs. This cellular-check (induced by IFNγ and independent of nitric oxide) significantly reduced intracellular parasite load when Wnt/beta-catenin signaling pathway was chemically antagonized using IWR-1-endo in naïve BMDMs, thus strengthening the hypothesis that Toxoplasma induces this signaling pathway to survive intracellular anti-parasitic immunity. This got further support when a significant reduction in the intracellular parasite load of RHASP5, a mutant strain of T. gondii lacking ability to secrete dense proteins into the host cell, was seen in naïve BMDMs. Additionally, T. gondii invading the BMDMs pre-stimulated with IFNγ, switched on its bradyzoite gene profile. 77
2) Toxoplasmosis and pregnancy
Toxoplasmosis is more important in pregnant women and immune compromised patients with respect to abortion, hydrocephalus, and retinochoroiditis. In pregnancy, Th2 immune response becomes activated which favors the proliferation of Toxoplasma. Briefly, in acute phase of T. gondii infection, certain cytokines (TGFβ1, TNFα, IL4, IL5 IL7, IL10, and IL17A) and chemokines (CXC, C, and CX3C families) play an important role as protective immune response.191,192 These pro-inflammatory cytokines down-regulate anti-inflammatory cytokines which travel to immune-privileged sites (brain, eyes, and placenta) to favor the existence of corpus luteum in the presence of low progesterone and 17β estradiol in pregnant women. 23 Apoptosis of placental cells may end up in fetal resorption, congenital anomalies (hydrocephalus and retinochoroiditis), or abortion 191 (Figure 1). Briefly, toxoplasmosis with lymphadenitis has been reported with higher levels of chemokines (CXCL8/IL8, CXCL9, and CXCL10) in pregnant women. Additionally, levels of VCAM1, CCL2, and CCL5 are lower in pregnant than in non-pregnant women. 193 The levels of ICAM1, CXCL9, CXCL10, MCSF, and TNFβ were up-regulated in acutely toxoplasmosis-infected Colombian pregnant women. Whereas, the levels of Eotaxin (Et), TGFβ, TNFα, IFNγ, IL2, IL4, IL15, CXCL1, and stem cell factor (SCF) were down-regulated in pregnant American acute cohorts. 194 In congenital toxoplasmosis, it was found that serum levels of IFNγ and IL5 were greatly increased during active stage of retinochoroiditis. In contrast, IL10 production was low during inflammatory stage and significantly higher in patients with inactive lesions. 195 The cytokine profile of acute toxoplasmosis-infected patients varies with geographical localities.
4) Perspectives for immunomodulation, therapy, vaccine, and other anti-parasitic challenges
The exploration of deep knowledge on the role of cytokines in toxoplasmosis should open new avenues for therapeutic measures based on immunomodulation. For instance, the use of IL17A antagonist inhibited the ocular toxoplasmosis in European patients. 196 Similarly, inhibition of parasite kinases in South American toxoplasmosis patients enhances the expression of IFNγ.163,197,198 This difference in inhibition sites might be strain dependent. Recent analysis of the cytokines profile in congenital toxoplasmosis199,200 indicates that modulation of cytokines through immuno-modulatory peptides could be assayed as immune adjuvants. 201 Such approaches need to be explored for the control of toxoplasmosis in humans. Etanercept (a soluble TNF-receptor fusion protein), widely used to treat autoimmune disease, activates the conversion of bradyzoites (chronic toxoplasmosis) to tachyzoites (acute toxoplasmosis) through down-regulation of pro-inflammatory cytokines (TNF, IL-1beta, and IL6). 202 It would be interesting to try to achieve a sterile immunity in an experimental model of chronic toxoplasmosis, at first transforming bradyzoites to tachyzoites through use of Etanercept but not too long after this, treating the tachyzoites to eliminate the parasite from the host body.
A recently identified drug target for T. gondii is an endonuclease named as cleavage and polyadenylation specificity factor subunit-3 (CPSF3) that has a role in mRNA processing in eukaryotes. This has been demonstrated by strong in vitro anti-parasitic activity by use of benzoxaborole (AN3661), a drug molecule that targets wild-type CPSF3. The parasites that were found resistant to this drug molecule displayed mutations in the TgCPSF3. Recapitulation of the similar resistant phenotype of the parasite through generation of mutations in the wild-type CPSF3 while exploiting CRISPR/Cas9, further strengthened the importance of this new therapeutic target against T. gondii. 203
One of the most exciting areas of research is to explore the means and effects of intervention strategies on how various strains of T. gondii can modulate host’s transcriptome 204 and non-coding RNAs including mircoRNA and long non-coding RNA. 205 Similarly, exploring how T. gondii exploits exosomes in modulating host immune response 206 as well as how therapeutic interventions designed for heme-deficient conditions affect infection outcome, 207 remains interesting areas of research.
Given the assumed fact that around one third population of world is harboring Toxoplasma in chronic form, that is, tissue cysts, why not to plan a vaccine to eliminate the tissue cysts from human population and other hosts seropositive to this infection, with a vaccine (based on GRA6Nt or other similar antigens) 49 that should be capable of eliminating tissue cysts.
Conclusions
Different factors are responsible for the pathogenesis of Toxoplasmosis and the survival of host. These factors include versatile genetic makeup of different strains of T. gondii, complicated immunological background of hosts, biochemical interaction among certain cytokines, invasion strategies of parasite as well as the immunogenicity of antigens encountered with host’s immune cells. The type of cytokines production depends on the strain of Toxoplasma. The IL10 and TGFβ1 production were higher in type I strain and lower in type II and III strain of toxoplasmosis. The production of IL12 was higher upon exposure of pathogen to DCs, MΦ, Np, NK cells, and T cells which is essential for the release of IFNγ. The production of IL12 switches the NK cells for release of IFNγ which develops resistance against T. gondii infection in host. Impairment in the production of IL12 may lead to demolish IFNγ resulting to develop host sensitivity for T. gondii infection. Moreover, IL6 also has critical role for gp130 signaling pathway for the up- and down-regulation of SOCS-gene which is responsible for the susceptibility and resistance of toxoplasmosis (Figure 2). The basic switching of pro- and anti-inflammatory cytokines in acute and chronic phases of toxoplasmosis is direly required for understanding the development of disease. Such cytokines are involved in the development of resistance and susceptibility of Toxoplasma in host. Agonist and antagonist effect of host cytokines network leads to the chronic condition of disease. In vivo up- and down-regulation of desired cytokines (IFNγ, IL6, IL12, and SOCS-3) could be helpful to boost up the immune response of host for the control of toxoplasmosis. Moreover, the synergistic and antagonistic relations among cytokines need to be comprehended on molecular and biochemical basis. The most compelling results are related with a Th2-deviated response associated to virulent strains in South American patients. Type II strain has the ability to translocate NF-κB in the nucleus of mouse splenocytes and bone marrow–derived MΦ. It is the reason that Toxoplasma type I strain survives from host immune response rather than type II and III but the complete defeat of host’s immune response is not in the favor of parasite’s survival in the ecosystem. The survival of the host after entry of T. gondii, is essential for ensuring existence of both the host and the parasite as if parasite defeats the host’s immune response, it not only marks the death of the host but also of the parasite as parasite needs a viable host to ensure its own survival as well as for its transmission to next generations of the same host as well as to other host species. The current review findings state that in vitro harvesting of IL12 from DCs, Np and MΦ upon exposure with T. gondii might be a source for therapeutic use in toxoplasmosis. Current review suggests that therapeutic interventions leading to up-regulation/supplementation of SOCS-3, IL12, and IFNγ to the infected host could be a solution to sterile immunity against T. gondii infection. This would be of interest particularly in patients passing through immunosuppression owing to any reason like the ones receiving anti-cancer therapy, the ones undergoing immunosuppressive therapy for graft/transplantation, the ones suffering from immunodeficiency virus (HIV) or having AIDS.
Acknowledgements
N. Cardona and M. Murillo, Centro de Investigaciones Biomedicas, Universidad del Quindio, Avenida Bolivar 12N, Armenia (Q), Colombia, South America helped to add some important studies in the manuscript. Mr. Adeel Mumtaz Abbasi (MPhil Student) is acknowledged for drawing of figures of the manuscript.
Appendix.
Abbreviation
- Ag
Antigen
- APC
Antigen-presenting cells
- BMDM
bone marrow–derived macrophages
- CNS
Central nervous system
- CPSF3
Cleavage and polyadenylation specificity factor subunit-3
- DCs
Dendritic cells
- ESA
Excretory–secretory antigens
- GBPs
Guanylate Binding Proteins
- GIMAPs
small GTPase immunity-associated proteins
- HIV
Human immune deficiency virus
- IELs
Intraepithelial lymphocyte
- iNOS
Inducible nitric oxide synthase
- LPL
Lamina propria lymphocytes
- IRGs
Immunity-related GTPases
- LPS
Lipopolysaccharide
- MyD88
Myeloid differentiation domain-88
- MΦ
Macrophages
- NK
Natural killer
- NP
Neutrophils
- RIPK3
receptor-interacting serine/threonine-protein kinase 3
- SCF
Stem cell factor
- SCID
Severe combined immune-deficient
- SOCs1
Suppressor of cytokine signaling molecule-1
- T reg
Regulatory T
- T. gondii
Toxoplasma gondii
- TGFβ
Transforming growth factor-β
- Th2
T helper cell 2
- Th17
T helper cell 17
- TLR
Toll-like receptors
- ZBP1
Z-DNA binding protein-1
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Imran Rashid https://orcid.org/0000-0002-0280-9335
Haroon Akbar https://orcid.org/0000-0002-8294-5862
References
- 1.Chen XQ, Zhou CX, Elsheikha HM, et al. (2017) Profiling of the perturbed metabolomic state of mouse spleen during acute and chronic toxoplasmosis. Parasites Vectors 10(1): 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White MW, Radke JR, Radke JB. (2014) Toxoplasma development - turn the switch on or off? Cell Microbiol 16(4): 466–472. [DOI] [PubMed] [Google Scholar]
- 3.Fisch D, Clough B, Frickel E-M. (2019) Human immunity to Toxoplasma gondii. PLoS Pathogens 15(12): e1008097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costantini S, Castello G, Colonna G. (2010) Human Cytokinome: a new challenge for systems biology. Bioinformation 5(4): 166–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarciron M, Gherardi A. (2000) Cytokines involved in Toxoplasmic encephalitis. Scand J Immunol 52(6): 534–543. [DOI] [PubMed] [Google Scholar]
- 6.Akbar H, Germon S, Berthon P, et al. (2012) Depletion of CD25+ cells during acute toxoplasmosis does not significantly increase mortality in Swiss OF1 mice. Memórias do Instituto Oswaldo Cruz 107(2): 155–162. [DOI] [PubMed] [Google Scholar]
- 7.López-Yglesias AH, Camanzo E, Martin AT, et al. (2019) TLR11-independent inflammasome activation is critical for CD4+ T cell-derived IFN-γ production and host resistance to Toxoplasma gondii. PLoS Pathogens 15(6): e1007872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan WJ, Jr., Jeffers V. (2012) Mechanisms of Toxoplasma gondii persistence and latency. FEMS Microbiol Rev 36(3): 717–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vidal JE, Hernandez AV, de Oliveira AC, et al. (2005) Cerebral toxoplasmosis in HIV-positive patients in Brazil: clinical features and predictors of treatment response in the HAART era. AIDS Patient Care STDS 19(10): 626–634. [DOI] [PubMed] [Google Scholar]
- 10.Schmitt N, Ueno H. (2015) Regulation of human helper T cell subset differentiation by cytokines. Curr Opin Immunol 34: 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saeij JP, Boyle JP, Boothroyd JC. (2005) Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host. Trends Parasitol 21(10): 476–481. [DOI] [PubMed] [Google Scholar]
- 12.Murillo-León M, Müller UB, Zimmermann I, et al. (2019) Molecular mechanism for the control of virulent Toxoplasma gondii infections in wild-derived mice. Nat Commun 10(1): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witola WH, Kim CY, Zhang X. (2017) Inherent oxidative stress in the Lewis rat is associated with resistance to toxoplasmosis. Infect Immun 85(10): e00289–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassan MA, Olijnik A-A, Frickel E-M, Saeij JP. (2019) Clonal and atypical Toxoplasma strain differences in virulence vary with mouse sub-species. Int J Parasitol 49(1): 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cervantes PW, Martorelli Di Genova B, Erazo Flores BJ, et al. (2021) RIPK3 facilitates host resistance to oral Toxoplasma gondii infection. Infect Immun 89(5): e00021–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rashid I, Moiré N, Héraut B, et al. (2017) Enhancement of the protective efficacy of a ROP18 vaccine against chronic toxoplasmosis by nasal route. Medical microbiology and immunology 206(1): 53–62. [DOI] [PubMed] [Google Scholar]
- 17.Hu L-Y, Zhang N-Z, Zhang F-K, et al. (2017) Resistance to Chronic Toxoplasma gondii infection induced by a DNA vaccine expressing GRA16. BioMed Research International 2017: 1295038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim CY, Zhang X, Witola WH. (2018) Small GTPase immunity-associated proteins mediate resistance to Toxoplasma gondii infection in Lewis rat. Infect Immun 86(4): e00582–e00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butcher BA, Kim L, Johnson PF, et al. (2001) Toxoplasma gondii tachyzoites inhibit proinflammatory cytokine induction in infected macrophages by preventing nuclear translocation of the transcription factor NF-kappa B. J Immunol 167(4): 2193–2201. [DOI] [PubMed] [Google Scholar]
- 20.Butcher BA, Kim L, Panopoulos AD, et al. (2005) IL-10-independent STAT3 activation by Toxoplasma gondii mediates suppression of IL-12 and TNF-alpha in host macrophages. J Immunol 174(6): 3148–3152. [DOI] [PubMed] [Google Scholar]
- 21.Angeloni MB, Guirelli PM, Franco PS, et al. (2013) Differential apoptosis in BeWo cells after infection with highly (RH) or moderately (ME49) virulent strains of Toxoplasma gondii is related to the cytokine profile secreted, the death receptor Fas expression and phosphorylated ERK1/2 expression. Placenta 34(11): 973–982. [DOI] [PubMed] [Google Scholar]
- 22.Butcher BA, Kim L, Johnson PF, et al. (2001) Toxoplasma gondii tachyzoites inhibit proinflammatory cytokine induction in infected macrophages by preventing nuclear translocation of the transcription factor NF-κB. J Immunol 167(4): 2193–2201. [DOI] [PubMed] [Google Scholar]
- 23.Iqbal J, Al-Awadhi M. (2016) Toxoplasmosis role of cytokines in disease modulation & tissue pathology. Ann Clin Pathol 4(7): 1090. [Google Scholar]
- 24.Iqbal J, Al-Awadhi MA, Raghupathy RG. (2016) TGF-beta1 levels and intraocular tissue alterations in mice infected with a virulent type I RH Toxoplasma gondii strain. Exp Parasitol 162: 57–63. [DOI] [PubMed] [Google Scholar]
- 25.Sauer A, Pfaff AW, Villard O, et al. (2012) Interleukin 17A as an effective target for anti-inflammatory and antiparasitic treatment of toxoplasmic uveitis. J Infect Dis 206(8): 1319–1329. [DOI] [PubMed] [Google Scholar]
- 26.Dobbin CA, Smith NC, Johnson AM. (2002) Heat shock protein 70 is a potential virulence factor in murine toxoplasma infection via immunomodulation of host NF-kappa B and nitric oxide. J Immunol 169(2): 958–965. [DOI] [PubMed] [Google Scholar]
- 27.Robben PM, Mordue DG, Truscott SM, et al. (2004) Production of IL-12 by macrophages infected with Toxoplasma gondii depends on the parasite genotype. J Immunol 172(6): 3686–3694. [DOI] [PubMed] [Google Scholar]
- 28.Schade B, Fischer HG. (2001) Toxoplasma gondii induction of interleukin-12 is associated with acute virulence in mice and depends on the host genotype. Vet Parasitol 100(1–2): 63–74. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Y, Reyes J, Rovira-Diaz E, et al. (2021) Cutting Edge: CD36 Mediates Phagocyte Tropism and Avirulence of Toxoplasma gondii. J Immunol 207(6): 1507–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox BA, Guevara RB, Rommereim LM, et al. (2019) Toxoplasma gondii parasitophorous vacuole membrane-associated dense granule proteins orchestrate chronic infection and GRA12 underpins resistance to host gamma interferon. mBio 10(4): e00589–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sayles PC, Johnson LL. (1996) Exacerbation of toxoplasmosis in neutrophil-depleted mice. Nat Immun 15(5): 249–258. [PubMed] [Google Scholar]
- 32.Hunter CA. (1996) How are NK cell responses regulated during infection? Exp Parasitol 84(3): 444–448. [DOI] [PubMed] [Google Scholar]
- 33.Denkers EY, Butcher BA, Del Rio L, et al. (2004) Neutrophils, dendritic cells and Toxoplasma. Int J Parasitol 34(3): 411–421. [DOI] [PubMed] [Google Scholar]
- 34.Denkers EY, Bzik DJ, Fox BA, et al. (2012) An inside job: hacking into Janus kinase/signal transducer and activator of transcription signaling cascades by the intracellular protozoan Toxoplasma gondii. Infect Immun 80(2): 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steffens N, Beuter-Gunia C, Kravets E, et al. (2020) Essential role of mGBP7 for survival of Toxoplasma gondii infection. mBio 11(1): e02993–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldszmid RS, Bafica A, Jankovic D, et al. (2007) TAP-1 indirectly regulates CD4+ T cell priming in Toxoplasma gondii infection by controlling NK cell IFN-gamma production. J Exp Med 204(11): 2591–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scanga CA, Aliberti J, Jankovic D, et al. (2002) Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol 168(12): 5997–6001. [DOI] [PubMed] [Google Scholar]
- 38.Hou B, Benson A, Kuzmich L, et al. (2011) Critical coordination of innate immune defense against Toxoplasma gondii by dendritic cells responding via their Toll-like receptors. Proc Natl Acad Sci U S A 108(1): 278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frenkel JK. (1988) Pathophysiology of toxoplasmosis. Parasitol Today 4(10): 273–278. [DOI] [PubMed] [Google Scholar]
- 40.Gomez Marin JE, Pinon JM, Bonhomme A, et al. (1997) Does human toxoplasmosis involve an imbalance in T1/T2 cytokines? Med Hypotheses 48(2): 161–169. [DOI] [PubMed] [Google Scholar]
- 41.Gazzinelli R, Xu Y, Hieny S, et al. (1992) Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol 149(1): 175–180. [PubMed] [Google Scholar]
- 42.Denkers EY, Gazzinelli RT. (1998) Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev 11(4): 569–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denkers EY, Gazzinelli RT, Martin D, et al. (1993) Emergence of NK1.1+ cells as effectors of IFN-gamma dependent immunity to Toxoplasma gondii in MHC class I-deficient mice. J Exp Med 178(5): 1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki Y, Sa Q, Gehman M, et al. (2011) Interferon-gamma- and perforin-mediated immune responses for resistance against Toxoplasma gondii in the brain. Expet Rev Mol Med 13: e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grumont RJ, Gerondakis S. (1990) The murine c-rel proto-oncogene encodes two mRNAs the expression of which is modulated by lymphoid stimuli. Oncogene Research 5(4): 245–254. [PubMed] [Google Scholar]
- 46.Mason NJ, Artis D, Hunter CA. (2004) New lessons from old pathogens: what parasitic infections have taught us about the role of nuclear factor-kappaB in the regulation of immunity. Immunol Rev 201: 48–56. [DOI] [PubMed] [Google Scholar]
- 47.Dzierszinski F, Pepper M, Stumhofer JS, et al. (2007) Presentation of Toxoplasma gondii antigens via the endogenous major histocompatibility complex class I pathway in nonprofessional and professional antigen-presenting cells. Infect Immun 75(11): 5200–5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jordan KA, Dupont CD, Tait ED, et al. (2010) Role of the NF-kappaB transcription factor c-Rel in the generation of CD8+ T-cell responses to Toxoplasma gondii. Int Immunol 22(11): 851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sa Q, Ochiai E, Tiwari A, et al. (2017) Determination of a key antigen for immunological intervention to target the latent stage of Toxoplasma gondii. J Immunol 198(11): 4425–4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gazzinelli RT, Denkers EY, Sher A. (1993) Host resistance to Toxoplasma gondii: model for studying the selective induction of cell-mediated immunity by intracellular parasites. Infect Agents Dis 2(3): 139–149. [PubMed] [Google Scholar]
- 51.Shirahata T, Yamashita T, Ohta C, et al. (1994) CD8+ T lymphocytes are the major cell population involved in the early gamma interferon response and resistance to acute primary Toxoplasma gondii infection in mice. Microbiol Immunol 38(10): 789–796. [DOI] [PubMed] [Google Scholar]
- 52.Ohkura N, Kitagawa Y, Sakaguchi S. (2013) Development and maintenance of regulatory T cells. Immunity 38(3): 414–423. [DOI] [PubMed] [Google Scholar]
- 53.Akbar H, Dimier-Poisson I, Moiré N. (2015) Role of CD4+ Foxp3+ regulatory T cells in protection induced by a live attenuated, replicating type I vaccine strain of Toxoplasma gondii. Infect Immun 83(9): 3601–3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oldenhove G, Bouladoux N, Wohlfert EA, et al. (2009) Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity 31(5): 772–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benson A, Murray S, Divakar P, et al. (2012) Microbial infection-induced expansion of effector T cells overcomes the suppressive effects of regulatory T cells via an IL-2 deprivation mechanism. J Immunol 188(2): 800–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olguin JE, Fernandez J, Salinas N, et al. (2015) Adoptive transfer of CD4(+)Foxp3(+) regulatory T cells to C57BL/6J mice during acute infection with Toxoplasma gondii down modulates the exacerbated Th1 immune response. Microb Infect 17(8): 586–595. [DOI] [PubMed] [Google Scholar]
- 57.Salinas N, Olguin JE, Castellanos C, et al. (2014) T cell suppression in vitro during Toxoplasma gondii infection is the result of IL-2 competition between Tregs and T cells leading to death of proliferating T cells. Scand J Immunol 79(1): 1–11. [DOI] [PubMed] [Google Scholar]
- 58.Ge YY, Zhang L, Zhang G, et al. (2008) In pregnant mice, the infection of Toxoplasma gondii causes the decrease of CD4+CD25+ -regulatory T cells. Parasite Immunol 30(9): 471–481. [DOI] [PubMed] [Google Scholar]
- 59.Chen JL, Ge YY, Zhang J, et al. (2013) The dysfunction of CD4(+)CD25(+) regulatory T cells contributes to the abortion of mice caused by Toxoplasma gondii excreted-secreted antigens in early pregnancy. PLoS One 8(7): e69012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen M, Aosai F, Norose K, et al. (2002) Involvement of MyD88 in host defense and the down-regulation of anti-heat shock protein 70 autoantibody formation by MyD88 in Toxoplasma gondii-infected mice. J Parasitol 88(5): 1017–1019. [DOI] [PubMed] [Google Scholar]
- 61.Hunter CA, Villarino A, Artis D, et al. (2004) The role of IL-27 in the development of T-cell responses during parasitic infections. Immunol Rev 202: 106–114. [DOI] [PubMed] [Google Scholar]
- 62.Alexander J, Hunter CA. (1998) Immunoregulation during toxoplasmosis. Chem Immunol 70: 81–102. [DOI] [PubMed] [Google Scholar]
- 63.Mercer HL, Snyder LM, Doherty CM, et al. (2020) Toxoplasma gondii dense granule protein GRA24 drives MyD88-independent p38 MAPK activation, IL-12 production and induction of protective immunity. PLoS Pathog 16(5): e1008572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gazzinelli RT, Wysocka M, Hieny S, et al. (1996) the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol 157(2): 798–805.In [PubMed] [Google Scholar]
- 65.Suzuki Y, Yang Q, Yang S, et al. (1996) IL-4 is protective against development of toxoplasmic encephalitis. J Immunol 157(6): 2564–2569. [PubMed] [Google Scholar]
- 66.Neyer LE, Grunig G, Fort M, et al. (1997) Role of interleukin-10 in regulation of T-cell-dependent and T-cell-independent mechanisms of resistance to Toxoplasma gondii. Infect Immun 65(5): 1675–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nishiyama S, Pradipta A, Ma JS, et al. (2020) T cell-derived interferon-γ is required for host defense to Toxoplasma gondii. Parasitol Int 75: 102049. [DOI] [PubMed] [Google Scholar]
- 68.López-Yglesias AH, Burger E, Camanzo E, et al. (2021) T-bet-dependent ILC1-and NK cell-derived IFN-γ mediates cDC1-dependent host resistance against Toxoplasma gondii. PLoS Pathog 17(1): e1008299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suzuki Y, Conley FK, Remington JS. (1989) Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J Immunol 143(6): 2045–2050. [PubMed] [Google Scholar]
- 70.McCabe RE, Luft BJ, Remington JS. (1984) Effect of murine interferon gamma on murine toxoplasmosis. J Infect Dis 150(6): 961–962. [DOI] [PubMed] [Google Scholar]
- 71.Chang HR, Grau GE, Pechere JC. (1990) Role of TNF and IL-1 in infections with Toxoplasma gondii. Immunology 69(1): 33–37. [PMC free article] [PubMed] [Google Scholar]
- 72.Green SJ, Nacy CA, Meltzer MS. (1991) Cytokine-induced synthesis of nitrogen oxides in macrophages: a protective host response to Leishmania and other intracellular pathogens. J Leukoc Biol 50(1): 93–103. [DOI] [PubMed] [Google Scholar]
- 73.Sharma DP, Ramsay AJ, Maguire DJ, et al. (1996) Interleukin-4 mediates down regulation of antiviral cytokine expression and cytotoxic T-lymphocyte responses and exacerbates vaccinia virus infection in vivo. J Virol 70(10): 7103–7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andrade RM, Portillo JA, Wessendarp M, et al. (2005) CD40 signaling in macrophages induces activity against an intracellular pathogen independently of gamma interferon and reactive nitrogen intermediates. Infect Immun 73(5): 3115–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lieberman LA, Banica M, Reiner SL, et al. (2004) STAT1 plays a critical role in the regulation of antimicrobial effector mechanisms, but not in the development of Th1-type responses during toxoplasmosis. J Immunol 172(1): 457–463. [DOI] [PubMed] [Google Scholar]
- 76.Martens, Parvanova I, Zerrahn J, et al. (2005) Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. PLoS Pathogens 1(3): e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gossner A, Hassan MA. (2020) Transcriptional analyses identify genes that modulate bovine macrophage response to Toxoplasma infection and immune stimulation. Frontiers in Cellular and Infection Microbiology 10: 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Butcher BA, Greene RI, Henry SC, et al. (2005) p47 GTPases regulate Toxoplasma gondii survival in activated macrophages. Infect Immun 73(6): 3278–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shapira S, Harb OS, Caamano J, et al. (2004) The NF-kappaB signaling pathway: immune evasion and immunoregulation during toxoplasmosis. Int J Parasitol 34(3): 393–400. [DOI] [PubMed] [Google Scholar]
- 80.Zimmermann S, Murray PJ, Heeg K, et al. (2006) Induction of suppressor of cytokine signaling-1 by Toxoplasma gondii contributes to immune evasion in macrophages by blocking IFN-gamma signaling. J Immunol 176(3): 1840–1847. [DOI] [PubMed] [Google Scholar]
- 81.Luder CG, Walter W, Beuerle B, et al. (2001) Toxoplasma gondii down-regulates MHC class II gene expression and antigen presentation by murine macrophages via interference with nuclear translocation of STAT1alpha. Eur J Immunol 31(5): 1475–1484. [DOI] [PubMed] [Google Scholar]
- 82.Kim SK, Fouts AE, Boothroyd JC. (2007) Toxoplasma gondii dysregulates IFN-gamma-inducible gene expression in human fibroblasts: insights from a genome-wide transcriptional profiling. J Immunol 178(8): 5154–5165. [DOI] [PubMed] [Google Scholar]
- 83.Tripp CS, Wolf SF, Unanue ER. (1993) Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci U S A 90(8): 3725–3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson LL. (1992) A protective role for endogenous tumor necrosis factor in Toxoplasma gondii infection. Infect Immun 60(5): 1979–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Black CM, Israelski DM, Suzuki Y, et al. (1989) Effect of recombinant tumour necrosis factor on acute infection in mice with Toxoplasma gondii or Trypanosoma cruzi. Immunology 68(4): 570–574. [PMC free article] [PubMed] [Google Scholar]
- 86.Marshall AJ, Brunet LR, van Gessel Y, et al. (1999) Toxoplasma gondii and Schistosoma mansoni synergize to promote hepatocyte dysfunction associated with high levels of plasma TNF-alpha and early death in C57BL/6 mice. Journal of immunology (Baltimore, Md 163(4): 2089–2097. [PubMed] [Google Scholar]
- 87.Grau GE, Tacchini-Cooler F, Piguet PF. (1992) Is TNF beneficial or deleterious in toxoplasmic encephalitis? Parasitol today 8(10): 322–324. [DOI] [PubMed] [Google Scholar]
- 88.Le J, Vilcek J. (1987) Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest 56(3): 234–248. [PubMed] [Google Scholar]
- 89.Beutler B, Cerami A. (1986) Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature 320(6063): 584–588. [DOI] [PubMed] [Google Scholar]
- 90.De Titto EH, Catterall JR, Remington JS. (1986) Activity of recombinant tumor necrosis factor on Toxoplasma gondii and Trypanosoma cruzi. Journal of immunology (Baltimore, Md 137(4): 1342–1345. [PubMed] [Google Scholar]
- 91.Boyman O, Sprent J. (2012) The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 12(3): 180–190. [DOI] [PubMed] [Google Scholar]
- 92.Williams MA, Tyznik AJ, Bevan MJ. (2006) Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 441(7095): 890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bachmann MF, Wolint P, Walton S, et al. (2007) Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur J Immunol 37(6): 1502–1512. [DOI] [PubMed] [Google Scholar]
- 94.Mitchell DM, Ravkov EV, Williams MA. (2010). , 184, pp. 6719–6730.Distinct roles for IL-2 and IL-15 in the differentiation and survival of CD8+ effector and memory T cells J Immunol 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pipkin ME, Sacks JA, Cruz-Guilloty F, et al. (2010) Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity 32(1): 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sharma SD, Hofflin JM, Remington JS. (1985). In vivo recombinant interleukin 2 administration enhances survival against a lethal challenge with Toxoplasma gondii. J Immunol, 135: 4160–4163. [PubMed] [Google Scholar]
- 97.Benedetto N, Auriault C, Darcy F, et al. (1991) Effect of rIFN-gamma and IL-2 treatments in mouse and nude rat infections with Toxoplasma gondii. Eur Cytokine Netw 2(2): 107–114. [PubMed] [Google Scholar]
- 98.Shirahata T, Muroya N, Ohta C, et al. (1993) Enhancement by recombinant human interleukin 2 of host resistance to Toxoplasma gondii infection in pregnant mice. Microbiol Immunol 37(7): 583–590. [DOI] [PubMed] [Google Scholar]
- 99.Sa Q, Woodward J, Suzuki Y. (2013). IL-2 produced by CD8+ immune T cells can augment their IFN-gamma production independently from their proliferation in the secondary response to an intracellular pathogen. J Immunol, 190(5):2199–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Subauste CS, Koniaris AH, Remington JS. (1991). Murine CD8+ cytotoxic T lymphocytes lyse Toxoplasma gondii-infected cells. J Immunol, 147(11): 3955–3959. [PubMed] [Google Scholar]
- 101.Hunter CA, Roberts CW, Alexander J. (1992) Kinetics of cytokine mRNA production in the brains of mice with progressive toxoplasmic encephalitis. Eur J Immunol 22(9): 2317–2322. [DOI] [PubMed] [Google Scholar]
- 102.Roberts CW, Ferguson DJ, Jebbari H, et al. (1996) Different roles for interleukin-4 during the course of Toxoplasma gondii infection. Infect Immun 64(3): 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Akira S, Taga T, Kishimoto T. (1993) Interleukin-6 in biology and medicine. Adv Immunol 54: 1–78. [DOI] [PubMed] [Google Scholar]
- 104.Lyons RE, Anthony JP, Ferguson DJ, et al. (2001) Immunological studies of chronic ocular toxoplasmosis: up-regulation of major histocompatibility complex class I and transforming growth factor beta and a protective role for interleukin-6. Infect Immun 69(4): 2589–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suzuki Y, Yang Q, Conley FK, et al. (1994) Antibody against interleukin-6 reduces inflammation and numbers of cysts in brains of mice with toxoplasmic encephalitis. Infect Immun 62(7): 2773–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Filisetti D, Candolfi E. (2004) Immune response to Toxoplasma gondii. Ann Ist Super Sanita 40(1): 71–80. [PubMed] [Google Scholar]
- 107.O'Shea JJ, Gadina M, Schreiber RD. (2002) Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell 109(Suppl): S121–S131. [DOI] [PubMed] [Google Scholar]
- 108.Nicholson SE, De Souza D, Fabri LJ, et al. (2000) Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130. Proc Natl Acad Sci U S A 97(12): 6493–6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Babon JJ, Sabo JK, Soetopo A, et al. (2008) The SOCS box domain of SOCS3: structure and interaction with the elonginBC-cullin5 ubiquitin ligase. J Mol Biol 381(4): 928–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Beaman MH, Hunter CA, Remington JS. (1994). Enhancement of intracellular replication of Toxoplasma gondii by IL-6. Interactions with IFN-gamma and TNF-alpha. J Immunol, 153(10):. 4583–4587. [PubMed] [Google Scholar]
- 111.Bermudez LE, Wu M, Petrofsky M, et al. (1992) Interleukin-6 antagonizes tumor necrosis factor-mediated mycobacteriostatic and mycobactericidal activities in macrophages. Infect Immun 60(10): 4245–4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Murray PJ. (2007) The JAK-STAT signaling pathway: input and output integration. J Immunol 178(5): 2623–2629. [DOI] [PubMed] [Google Scholar]
- 113.Yamamoto M, Standley DM, Takashima S, et al. (2009) A single polymorphic amino acid on Toxoplasma gondii kinase ROP16 determines the direct and strain-specific activation of Stat3. J Exp Med 206(12): 2747–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kubo M, Hanada T, Yoshimura A. (2003) Suppressors of cytokine signaling and immunity. Nat Immunol 4(12): 1169–1176. [DOI] [PubMed] [Google Scholar]
- 115.Whitmarsh RJ, Gray CM, Gregg B, et al. (2011) A critical role for SOCS3 in innate resistance to Toxoplasma gondii. Cell Host & Microbe 10(3): 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Taga T, Kishimoto T. (1997) Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol 15: 797–819. [DOI] [PubMed] [Google Scholar]
- 117.Silver JS, Stumhofer JS, Passos S, et al. (2011). , 187, pp. 350–360.IL-6 mediates the susceptibility of glycoprotein 130 hypermorphs to Toxoplasma gondii J Immunol 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Deckert-Schluter M, Albrecht S, Hof H, et al. (1995) Dynamics of the intracerebral and splenic cytokine mRNA production in Toxoplasma gondii-resistant and -susceptible congenic strains of mice. Immunology 85(3): 408–418. [PMC free article] [PubMed] [Google Scholar]
- 119.Suzuki Y, Rani S, Liesenfeld O, et al. (1997) Impaired resistance to the development of toxoplasmic encephalitis in interleukin-6-deficient mice. Infect Immun 65(6): 2339–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kasper LH, Matsuura T, Khan IA. (1995). IL-7 stimulates protective immunity in mice against the intracellular pathogen, Toxoplasma gondii. J Immunol, 155(10): 4798–4804. [PubMed] [Google Scholar]
- 121.Ma A, Koka R, Burkett P. (2006) Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol 24: 657–679. [DOI] [PubMed] [Google Scholar]
- 122.Schluns KS, Williams K, Ma A, et al. (2002). Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol, 168(10): 4827–4831. [DOI] [PubMed] [Google Scholar]
- 123.Combe CL, Moretto MM, Schwartzman JD, et al. (2006) Lack of IL-15 results in the suboptimal priming of CD4+ T cell response against an intracellular parasite. Proc Natl Acad Sci U S A 103(17): 6635–6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Prlic M, Lefrancois L, Jameson SC. (2002) Multiple choices: regulation of memory CD8 T cell generation and homeostasis by interleukin (IL)-7 and IL-15. J Exp Med 195(12): F49–F52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bhadra R, Guan H, Khan IA. (2010) Absence of both IL-7 and IL-15 severely impairs the development of CD8 T cell response against Toxoplasma gondii. PLoS One 5(5): e10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Adib-Conquy M, Petit AF, Marie C, et al. (1999) Paradoxical priming effects of IL-10 on cytokine production. Int Immunol 11(5): 689–698. [DOI] [PubMed] [Google Scholar]
- 127.Mosmann TR. (1994) Properties and functions of interleukin-10. Adv Immunol 56: 1–26. [PubMed] [Google Scholar]
- 128.Moore KW, de Waal Malefyt R, Coffman RL, et al. (2001) Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19: 683–765. [DOI] [PubMed] [Google Scholar]
- 129.Levings MK, Bacchetta R, Schulz U, et al. (2002) The role of IL-10 and TGF-beta in the differentiation and effector function of T regulatory cells. Int Arch Allergy Immunol 129(4): 263–276. [DOI] [PubMed] [Google Scholar]
- 130.Bliss SK, Butcher BA, Denkers EY. (2000) Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. Journal of immunology (Baltimore, Md 165(8): 4515–4521. [DOI] [PubMed] [Google Scholar]
- 131.de Waal Malefyt R, Yssel H, Roncarolo MG, et al. (1992) Interleukin-10. Curr Opin Immunol 4(3): 314–320. [DOI] [PubMed] [Google Scholar]
- 132.Rennick D, Berg D, Holland G. (1992) Interleukin 10: an overview. Prog Growth Factor Res 4(3): 207–227. [DOI] [PubMed] [Google Scholar]
- 133.Gazzinelli RT, Oswald IP, James SL, et al. (1992) IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. Journal of immunology (Baltimore, Md 148(6): 1792–1796. [PubMed] [Google Scholar]
- 134.Hunter CA, Litton MJ, Remington JS, et al. (1994) Immunocytochemical detection of cytokines in the lymph nodes and brains of mice resistant or susceptible to toxoplasmic encephalitis. J Infect Dis 170(4): 939–945. [DOI] [PubMed] [Google Scholar]
- 135.Schluter D, Kaefer N, Hof H, et al. (1997) Expression pattern and cellular origin of cytokines in the normal and Toxoplasma gondii-infected murine brain. Am J Pathol 150(3): 1021–1035. [PMC free article] [PubMed] [Google Scholar]
- 136.Gaddi PJ, Yap GS. (2007) Cytokine regulation of immunopathology in toxoplasmosis. Immunol Cell Biol 85(2): 155–159. [DOI] [PubMed] [Google Scholar]
- 137.Gazzinelli RT, Wysocka M, Hayashi S, et al. (1994) Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol 153(6): 2533–2543. [PubMed] [Google Scholar]
- 138.Bliss SK, Zhang Y, Denkers EY. (1999) Murine Neutrophil Stimulation by Toxoplasma gondii Antigen Drives High Level Production of IFN-γ-Independent IL-12. J Immunol 163(4): 2081–2088. [PubMed] [Google Scholar]
- 139.Scharton-Kersten TM, Wynn TA, Denkers EY, et al. (1996) the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J Immunol 157(9): 4045–4054.In [PubMed] [Google Scholar]
- 140.Yap G, Pesin M, Sher A. (2000) Cutting Edge: IL-12 Is Required for the Maintenance of IFN-γ Production in T Cells Mediating Chronic Resistance to the Intracellular Pathogen, Toxoplasma gondii. J Immunol 165(2): 628–631. [DOI] [PubMed] [Google Scholar]
- 141.Lieberman LA, Cardillo F, Owyang AM, et al. (2004). IL-23 provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. J Immunol, 173(3): 1887–1893. [DOI] [PubMed] [Google Scholar]
- 142.Liu CH, Fan YT, Dias A, et al. (2006) Cutting edge: dendritic cells are essential for in vivo IL-12 production and development of resistance against Toxoplasma gondii infection in mice. J Immunol 177(1): 31–35. [DOI] [PubMed] [Google Scholar]
- 143.Magram J, Connaughton SE, Warrier RR, et al. (1996) IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity 4(5): 471–481. [DOI] [PubMed] [Google Scholar]
- 144.Middleton MK, Rubinstein T, Pure E. (2006) Cellular and molecular mechanisms of the selective regulation of IL-12 production by 12/15-lipoxygenase. J Immunol 176(1): 265–274. [DOI] [PubMed] [Google Scholar]
- 145.Khan IA, Kasper LH. (1996) IL-15 augments CD8+ T cell-mediated immunity against Toxoplasma gondii infection in mice. J Immunol 157(5): 2103–2108. [PubMed] [Google Scholar]
- 146.Fehniger TA, Caligiuri MA. (2001) Interleukin 15: biology and relevance to human disease. Blood 97(1): 14–32. [DOI] [PubMed] [Google Scholar]
- 147.Lodolce JP, Boone DL, Chai S, et al. (1998) IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 9(5): 669–676. [DOI] [PubMed] [Google Scholar]
- 148.Kennedy MK, Glaccum M, Brown SN, et al. (2000) Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med 191(5): 771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lieberman LA, Villegas EN, Hunter CA. (2004) Interleukin-15-deficient mice develop protective immunity to Toxoplasma gondii. Infect Immun 72(11): 6729–6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Intlekofer AM, Banerjee A, Takemoto N, et al. (2008) Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science 321(5887): 408–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tajima M, Wakita D, Noguchi D, et al. (2008) IL-6-dependent spontaneous proliferation is required for the induction of colitogenic IL-17-producing CD8+ T cells. J Exp Med 205(5): 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lockhart E, Green AM, Flynn JL. (2006) IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. Journal of immunology (Baltimore, Md 177(7): 4662–4669. [DOI] [PubMed] [Google Scholar]
- 153.Michel ML, Keller AC, Paget C, et al. (2007) Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med 204(5): 995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rachitskaya AV, Hansen AM, Horai R, et al. (2008). Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol, 180(8): 5167–5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xue ML, Thakur A, Willcox M. (2002) Macrophage inflammatory protein-2 and vascular endothelial growth factor regulate corneal neovascularization induced by infection with Pseudomonas aeruginosa in mice. Immunol Cell Biol 80(4): 323–327. [DOI] [PubMed] [Google Scholar]
- 156.Kelly MN, Kolls JK, Happel K, et al. (2005) Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect Immun 73(1): 617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Aggarwal S, Ghilardi N, Xie MH, et al. (2003) Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem 278(3): 1910–1914. [DOI] [PubMed] [Google Scholar]
- 158.Langrish CL, Chen Y, Blumenschein WM, et al. (2005) IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 201(2): 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Amadi-Obi A, Yu CR, Liu X, et al. (2007) TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med 13(6): 711–718. [DOI] [PubMed] [Google Scholar]
- 160.Stumhofer JS, Laurence A, Wilson EH, et al. (2006) Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol 7(9): 937–945. [DOI] [PubMed] [Google Scholar]
- 161.Passos ST, Silver JS, O'Hara AC, et al. (2010) IL-6 promotes NK cell production of IL-17 during toxoplasmosis. Journal of immunology (Baltimore, Md 184(4): 1776–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guiton R, Vasseur V, Charron S, et al. (2010) Interleukin 17 receptor signaling is deleterious during Toxoplasma gondii infection in susceptible BL6 mice. J Infect Dis 202(3): 427–435. [DOI] [PubMed] [Google Scholar]
- 163.de-la-Torre A, Sauer A, Pfaff AW, et al. (2013) Severe South American ocular toxoplasmosis is associated with decreased Ifn-gamma/Il-17a and increased Il-6/Il-13 intraocular levels. PLoS Neglected Tropical Diseases 7(11): e2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sauer A, Villard O, Creuzot-Garcher C, et al. (2015) Intraocular levels of interleukin 17A (IL-17A) and IL-10 as respective determinant markers of toxoplasmosis and viral uveitis. Clin Vaccine Immunol : CVI 22(1): 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Silva JL, Rezende-Oliveira K, da Silva MV, et al. (2014) IL-17-expressing CD4(+) and CD8(+) T lymphocytes in human toxoplasmosis. Mediat Inflamm 2014: 573825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cervantes-Barragan L, Cortez VS, Wang Q, et al. (2019) CRTAM protects against intestinal dysbiosis during pathogenic parasitic infection by enabling Th17 maturation. Front Immunol 10: 1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hunter CA, Timans J, Pisacane P, et al. (1997) Comparison of the effects of interleukin-1 alpha, interleukin-1 beta and interferon-gamma-inducing factor on the production of interferon-gamma by natural killer. Eur J Immunol 27(11): 2787–2792. [DOI] [PubMed] [Google Scholar]
- 168.Kim YM, Talanian RV, Li J, et al. (1998) Nitric oxide prevents IL-1beta and IFN-gamma-inducing factor (IL-18) release from macrophages by inhibiting caspase-1 (IL-1beta-converting enzyme). Journal of immunology (Baltimore, Md 161(8): 4122–4128. [PubMed] [Google Scholar]
- 169.Bazan JF, Timans JC, Kastelein RA. (1996) A newly defined interleukin-1? Nature 379(6566): 591. [DOI] [PubMed] [Google Scholar]
- 170.Matsumoto S, Tsuji-Takayama K, Aizawa Y, et al. (1997) Interleukin-18 activates NF-kappaB in murine T helper type 1 cells. Biochem Biophys Res Commun 234(2): 454–457. [DOI] [PubMed] [Google Scholar]
- 171.Thomassen E, Bird TA, Renshaw BR, et al. (1998) Binding of interleukin-18 to the interleukin-1 receptor homologous receptor IL-1Rrp1 leads to activation of signaling pathways similar to those used by interleukin-1. J Interferon Cytokine Res 18(12): 1077–1088. [DOI] [PubMed] [Google Scholar]
- 172.Cai G, Radzanowski T, Villegas EN, et al. (2000)., 165, pp. 2619–2627.Identification of STAT4-dependent and independent mechanisms of resistance to Toxoplasma gondii J Immunol 5 [DOI] [PubMed] [Google Scholar]
- 173.Cai G, Kastelein R, Hunter CA. (2000) Interleukin-18 (IL-18) enhances innate IL-12-mediated resistance to Toxoplasma gondii. Infect Immun 68(12): 6932–6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kanakaraj P, Ngo K, Wu Y, et al. (1999) Defective interleukin (IL)-18-mediated natural killer and T helper cell type 1 responses in IL-1 receptor-associated kinase (IRAK)-deficient mice. J Exp Med 189(7): 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Takeda K, Tsutsui H, Yoshimoto T, et al. (1998) Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity 8(3): 383–390. [DOI] [PubMed] [Google Scholar]
- 176.Wei XQ, Leung BP, Niedbala W, et al. (1999) Altered immune responses and susceptibility to Leishmania major and Staphylococcus aureus infection in IL-18-deficient mice. Journal of immunology (Baltimore, Md 163(5): 2821–2828. [PubMed] [Google Scholar]
- 177.Hyodo Y, Matsui K, Hayashi N, et al. (1999) IL-18 up-regulates perforin-mediated NK activity without increasing perforin messenger RNA expression by binding to constitutively expressed IL-18 receptor. Journal of immunology (Baltimore, Md 162(3): 1662–1668. [PubMed] [Google Scholar]
- 178.Walker W, Aste-Amezaga M, Kastelein RA, et al. (1999). IL-18 and CD28 use distinct molecular mechanisms to enhance NK cell production of IL-12-induced IFN-gamma. J Immunol, 162(10): 5894–5901. [PubMed] [Google Scholar]
- 179.Zhang T, Kawakami K, Qureshi MH, et al. (1997) Interleukin-12 (IL-12) and IL-18 synergistically induce the fungicidal activity of murine peritoneal exudate cells against Cryptococcus neoformans through production of gamma interferon by natural killer cells. Infect Immun 65(9): 3594–3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vossenkamper A, Struck D, Alvarado-Esquivel C, et al. (2004) Both IL-12 and IL-18 contribute to small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii, but IL-12 is dominant over IL-18 in parasite control. Eur J Immunol 34(11): 3197–3207. [DOI] [PubMed] [Google Scholar]
- 181.Still KM, Batista SJ, O’Brien CA, et al. (2020) Astrocytes promote a protective immune response to brain Toxoplasma gondii infection via IL-33-ST2 signaling. PLoS Pathogens 16(10): e1009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hunter CA, Bermudez L, Beernink H, et al. (1995) Transforming growth factor-beta inhibits interleukin-12-induced production of interferon-gamma by natural killer cells: a role for transforming growth factor-beta in the regulation of T cell-independent resistance to Toxoplasma gondii. Eur J Immunol 25(4): 994–1000. [DOI] [PubMed] [Google Scholar]
- 183.Langermans JA, Nibbering PH, Van Vuren-Van Der Hulst ME, et al. (2001) Transforming growth factor-beta suppresses interferon-gamma-induced toxoplasmastatic activity in murine macrophages by inhibition of tumour necrosis factor-alpha production. Parasite Immunol 23(4): 169–175. [DOI] [PubMed] [Google Scholar]
- 184.Buzoni-Gatel D, Debbabi H, Mennechet FJ, et al. (2001) Murine ileitis after intracellular parasite infection is controlled by TGF-beta-producing intraepithelial lymphocytes. Gastroenterology 120(4): 914–924. [DOI] [PubMed] [Google Scholar]
- 185.Schluter D, Bertsch D, Frei K, et al. (1998) Interferon-gamma antagonizes transforming growth factor-beta2-mediated immunosuppression in murine Toxoplasma encephalitis. J Neuroimmunol 81(1–2): 38–48. [DOI] [PubMed] [Google Scholar]
- 186.Nagineni CN, Detrick B, Hooks JJ. (2002) Transforming growth factor-beta expression in human retinal pigment epithelial cells is enhanced by Toxoplasma gondii: a possible role in the immunopathogenesis of retinochoroiditis. Clin Exp Immunol 128(2): 372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mennechet FJ, Kasper LH, Rachinel N, et al. (2004) Intestinal intraepithelial lymphocytes prevent pathogen-driven inflammation and regulate the Smad/T-bet pathway of lamina propria CD4+ T cells. Eur J Immunol 34(4): 1059–1067. [DOI] [PubMed] [Google Scholar]
- 188.Okamoto S, Hara Y, Streilein JW. (1995) Induction of anterior chamber-associated immune deviation with lymphoreticular allogeneic cells. Transplantation 59(3): 377–381. [PubMed] [Google Scholar]
- 189.Lu F, Huang S, Kasper LH. (2003) Interleukin-10 and pathogenesis of murine ocular toxoplasmosis. Infect Immun 71(12): 7159–7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ohta K, Yamagami S, Taylor AW, et al. (2000) IL-6 antagonizes TGF-beta and abolishes immune privilege in eyes with endotoxin-induced uveitis. Invest Ophthalmol Vis Sci 41(9): 2591–2599. [PubMed] [Google Scholar]
- 191.Rezende-Oliveira K, Silva N, Mineo J, et al. (2012) Cytokines and Chemokines production by mononuclear cells from parturient women after stimulation with live Toxoplasma gondii. Placenta 33(9): 682–687. [DOI] [PubMed] [Google Scholar]
- 192.Zlotnik A, Yoshie O. (2000) Chemokines: a new classification system and their role in immunity. Immunity 12(2): 121–127. [DOI] [PubMed] [Google Scholar]
- 193.Pomares C, Holmes TH, Estran R, et al. (2017) Cytokine profiles in patients with toxoplasmic lymphadenitis in the setting of pregnancy. Cytokine 90: 14–20. [DOI] [PubMed] [Google Scholar]
- 194.Pernas L, Ramirez R, Holmes TH, et al. (2014) Immune profiling of pregnant Toxoplasma-infected US and Colombia patients reveals surprising impacts of infection on peripheral blood cytokines. J Infect Dis 210(6): 923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.de Araujo TE, Coelho-Dos-Reis JG, Bela SR, et al. (2017) Early serum biomarker networks in infants with distinct retinochoroidal lesion status of congenital toxoplasmosis. Cytokine 95: 102–112. [DOI] [PubMed] [Google Scholar]
- 196.Lahmar I, Abou-Bacar A, Abdelrahman T, et al. (2009) Cytokine profiles in toxoplasmic and viral uveitis. J Infect Dis 199(8): 1239–1249. [DOI] [PubMed] [Google Scholar]
- 197.Alvarez C, de-la-Torre A, Vargas M, et al. (2015) Striking Divergence in Toxoplasma ROP16 Nucleotide Sequences From Human and Meat Samples. J Infect Dis 211(12): 2006–2013. [DOI] [PubMed] [Google Scholar]
- 198.de-la-Torre A, Pfaff AW, Grigg ME, et al. (2014) Ocular cytokinome is linked to clinical characteristics in ocular toxoplasmosis. Cytokine 68(1): 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Torres-Morales E, Taborda L, Cardona N, et al. (2014) Th1 and Th2 immune response to P30 and ROP18 peptides in human toxoplasmosis. Med Microbiol Immunol 203(5): 315–322. [DOI] [PubMed] [Google Scholar]
- 200.Carneiro AC, Machado AS, Bela SR, et al. (2016) Cytokine signatures associated with early onset, active lesions and late cicatricial events of retinochoroidal commitment in infants with congenital toxoplasmosis. J Infect Dis 213(12): 1962–1970. [DOI] [PubMed] [Google Scholar]
- 201.Gomez Marin JE. (2016) Possibilities for immunomodulation in congenital toxoplasmosis. J Infect Dis 214(4): 656. [DOI] [PubMed] [Google Scholar]
- 202.Yang J, Wang L, Xu D, et al. (2018) Risk assessment of etanercept in mice chronically infected with Toxoplasma gondii. Front Microbiol 9: 2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Palencia A, Bougdour A, Brenier-Pinchart M-P, et al. (2017) Targeting Toxoplasma gondii CPSF3 as a new approach to control toxoplasmosis. EMBO Mol Med 9(3): 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Garfoot AL, Cervantes PW, Knoll LJ, et al. (2019) Transcriptional Analysis Shows a Robust Host Response to Toxoplasma gondii during Early and Late Chronic Infection in Both Male and Female Mice. Infect Immun 87(5): e00024–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Menard KL, Haskins BE, Denkers EY. (2019) Impact of Toxoplasma gondii Infection on Host Non-coding RNA Responses. Frontiers in Cellular and Infection Microbiology 9(132). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Varikuti S, Jha BK, Holcomb EA, et al. (2020) The role of vascular endothelium and exosomes in human protozoan parasitic diseases. Vessel Plus 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kloehn J, Harding CR, Soldati-Favre D. (2021) Supply and demand-heme synthesis, salvage and utilization by Apicomplexa. FEBS J 288(2): 382–404. [DOI] [PubMed] [Google Scholar]