Abstract
Respiratory syncytial virus (RSV) is the single most important cause of lower respiratory tract infection during infancy and early childhood. Once RSV infection is established, the host immune response includes the production of virus-neutralizing antibodies and T-cell-specific immunity. The humoral immune response normally results in the development of anti-RSV neutralizing-antibody titers, but these are often suboptimal during an infant’s initial infection. Even when the production of RSV neutralizing antibody following RSV infection is robust, humoral immunity wanes over time. Reinfection during subsequent seasons is common. The cellular immune response to RSV infection is also important for the clearance of virus. This immune response, vital for host defense against RSV, is also implicated in the immunopathogenesis of severe lower respiratory tract RSV bronchiolitis. Many details of the immunology and immunopathologic mechanisms of RSV disease known at present have been learned from rodent models of RSV disease and are discussed in some detail. In addition, the roles of immunoglobulin E, histamine, and eosinophils in the immunopathogenesis of RSV disease are considered. Although the treatment of RSV bronchiolitis is primarily supportive, the role of ribavirin is briefly discussed. Novel approaches to the development of new antiviral drugs with promising anti-RSV activity in vitro are also described.
Respiratory syncytial virus (RSV) is the single most important cause of lower respiratory tract infection during infancy and early childhood worldwide (13, 22, 60, 111). Both the magnitude and intensity of infection and the host response to RSV infection determine the severity and intensity of disease. To begin unraveling the complexities of these host-virus interactions, we first discuss the epidemiology and pathogenesis of RSV disease and the humoral and cellular immune response mounted by the host. Once RSV infection is established, the host immune response includes the production of virus-neutralizing antibodies and T-cell-specific immunity. Much of what is known about the immunobiology of RSV infection has been learned from rodent models, with extrapolation, in many instances, to human disease. Vaccine-induced enhancement of RSV immunopathogenesis has been recognized since field trials of RSV vaccine were performed in the 1950s. Since this body of work has led to a greater understanding of RSV immunobiology in general, the observations made during those experiments, as well as the efforts to dissect out the reasons for this phenomenon, are summarized. Finally, the available treatment modalities for severe RSV infection are considered.
EPIDEMIOLOGY OF RSV DISEASE
Human RSV is a member of the Pneumovirus subfamily of the family Paramyxoviridae. It accounts for approximately 50% of all pneumonia and up to 90% of the reported cases of bronchiolitis in infancy (13, 22, 55, 60, 111). Outbreaks of RSV disease are abrupt in onset and can last up to 5 months (59, 62, 99). These epidemics occur annually at regular, predictable intervals. In geographical areas of temperate climate such as the continental United States, RSV activity usually peaks in the winter and extends into the spring (52, 59, 123). Although the “RSV season” is a predictable annual event, the severity of the outbreaks varies (74).
Fluctuations in the circulating strains of RSV may contribute to the variation seen in the severity of these annual outbreaks. The two major strain groups, A and B, circulate simultaneously during an outbreak, and it has been suggested that group A strains may be associated with more severe disease, although the correlation of strain group with clinical severity merits further investigation (74, 112, 134, 167, 176).
The public health problem caused by this virus is exemplified by the fact that approximately two-thirds of infants are infected with RSV in the first year of life; one-third of those infected develop lower respiratory tract disease, 2.5% are hospitalized (more than 90,000 children in the United States every year), and 0.1% die (88, 140).
PATHOGENESIS OF RSV BRONCHIOLITIS
The incubation period of RSV respiratory disease is estimated to be 5 days (95, 163). At the beginning of the illness, the virus replicates in the nasopharynx, reaching titers as high as 106 50% tissue culture infective doses per ml of nasal secretion in young infants (78, 79). The mechanism by which virus spreads from the upper respiratory tract to the lower airways is not clear, but it is presumed to be via direct spread along the respiratory epithelium and/or through the aspiration of nasopharyngeal secretions. RSV can spread from cell to cell, without emerging into the extracellular milieu, by inducing cell fusion and syncytium formation (156). In experimentally infected primates, the tracheal epithelium is sparsely and discontinuously infected, arguing that emergence into the extracellular space is necessary for viral spread in vivo in this animal model (148). Another possible mechanism for the spread of RSV to the lower respiratory tract is via infection of macrophages, with migration to the lower airways. Alveolar macrophages are infected with RSV in vivo (120, 138), and if high multiplicities of infection are used, monocyte-derived macrophages can be infected with RSV in vitro (105).
In infants, lower respiratory tract signs, such as tachypnea, wheezing, or rales, usually appear 1 to 3 days after the onset of rhinorrhea, representing viral spread into the bronchi and bronchioles. Lower respiratory tract viral titers are difficult to determine, but in autopsy studies of patients with fatal cases of lower respiratory tract RSV disease, viral antigens are much more plentiful in fatal RSV pneumonia than in fatal RSV bronchiolitis (56), suggesting that virus replication continues as respiratory failure progresses. This study also demonstrated that RSV antigens failed to penetrate deeper than the superficial layers of the respiratory epithelium.
Clinical recovery from RSV bronchiolitis may occur in the presence of continued virus shedding from the upper respiratory tract (78, 79). However, some studies have demonstrated that virus shedding stops coincident with the emergence of specific secretory immunoglobulin A (IgA) at the time of clinical recovery (117, 118).
The pathologic findings of severe RSV infection in infants are similar to those of bronchiolitis and/or pneumonia due to other respiratory viruses such as influenza virus, parainfluenza virus type 3, and adenovirus. During bronchiolitis, ciliated epithelial cells are destroyed, and in widespread bronchiolar epithelial necrosis is observed the most severe cases (1). In its severe form, lower respiratory tract disease caused by RSV involves peribronchiolar mononuclear cell infiltrates accompanied by submucosal edema and bronchorrhea. These events lead to bronchiolar obstruction with patchy atelectasis and areas of compensatory emphysema. Pneumonitis may occur as the alveoli become filled with fluid (1, 55, 56).
In milder cases of bronchiolitis, infection involves predominantly the lower airways, with various degrees of peribronchiolar and interstitial inflammation. Tristram et al. (171) recently demonstrated that RSV added ex vivo to primary respiratory cell cultures induced rapid and near complete ciliostasis, a phenomenon that may predispose to worsening obstructive symptoms in vivo. It is the tropism of this agent for respiratory epithelium and its propensity to induce the formation of large syncytia that give this virus its name (Fig. 1). It is unknown if such sheets of syncytia are formed in vivo as respiratory epithelial cells fuse, but sloughing of this cellular debris into the airway lumen certainly could exacerbate the obstructive process.
FIG. 1.
RSV-infected respiratory epithelial cells. Respiratory epithelial cells (HEp-2, laryngeal carcinoma cell line) on day 1 (A), day 3 (B), and day 5 (C) following infection with RSV. Magnification, ×400. After 1 day, the cells continue to appear healthy. After 3 days, many of the cells have fused, forming large syncytia. After 5 days, most of the syncytia have detached from the surface and the majority of the cells are dead.
Although RSV infection is considered to be confined to the respiratory epithelium, RSV antigens have been detected in circulating mononuclear cells (46) and the virus replicates in macrophages in vitro, albeit at very low titers (105). Moreover, RSV can induce mononuclear cells to produce cytokines (6). Despite these findings, viremia has never been described, suggesting that hematogenous spread of RSV does not occur in immunocompetent individuals. In contrast, immunocompromised individuals have experienced extrapulmonary spread of RSV to the liver and kidneys (121, 137). Prolonged viral shedding from the respiratory tract is observed in immunocompromised individuals, suggesting that intact cell-mediated immunity is critical in the eradication of RSV infection (19, 77).
IMMUNITY TO RSV
Properties of the Virus
RSV is an enveloped, nonsegmented, negative-sense RNA virus. The RSV genome is a single strand of RNA composed of approximately 15,000 nucleotides that are transcribed into 11 major subgenomic mRNAs (30, 32, 41) (Fig. 2). Each of the mRNAs encodes a major viral protein. Three of these proteins are transmembrane surface proteins (G, F, and SH). One protein is a nonglycosylated virion matrix protein (M), and four proteins associate with the genomic RNA to form the viral nucleocapsid (N, P, L, and M2 open reading frame 1 [M2 ORF-1]). M2 ORF-2 is a second, distinct protein transcribed from the M2 gene, which has defined properties in transcriptional regulation (33, 34) (see below). Finally, two proteins (NS1 and NS2) are nonstructural viral products that accumulate in infected cells but are present in only trace amounts in mature virions (30, 133). Table 1 summarizes the structural and functional properties of the known RSV proteins.
FIG. 2.
RSV RNA genome. Shown is a schematic of the 15.2-kb RSV genomic RNA. The genome is nonsegmented; the order of the genes from 3′ to 5′ is as depicted. RSV-specific proteins encoded by these genes, and their functions, when known, are listed in Table 1.
TABLE 1.
RSV-encoded proteins and their characteristics

The RSV F (for “fusion”) protein was identified by the observation that some F-specific monoclonal antibodies inhibited syncytium formation in tissue culture, emphasizing the importance of the F protein in these cell fusion events (177). F protein expression alone may not be sufficient to mediate cell fusion, since coexpression of F, G, and SH proteins was required for syncytium formation in one recombinant protein expression system (84). In a more recent study, a recombinant RSV lacking SH was shown to retain infectivity and the ability to form syncytia (15), ruling out the need for SH in cell-to-cell fusion. Perhaps the most clinically important characteristic of F protein is that it has been identified as one of the major virus-neutralizing antigens produced during viral replication; i.e., host cell antibodies produced against epitopes of the F protein are able to neutralize virus. This observation has led to production of virus-neutralizing monoclonal antibodies for use in passive immunization to prevent severe RSV disease in high-risk groups. This is discussed in detail in a later section.
The RSV G protein was identified as the viral attachment protein by the finding that antibodies specific to G protein, but not to F protein, inhibited the adsorption of virions to cells (108). There is some question whether G protein is the unique (or even the major) viral attachment protein, since recombinant RSV that lacks the G protein coding region remains fully viable in tissue culture (96). G protein is heavily glycosylated; the significance of the high sugar content is unknown (29, 180). It has been suggested that the glycosylation, being specified by the host, may protect the virus-specific peptide from being recognized as a foreign antigen (31). Variation in these glycosylation sites from strain to strain may also contribute to the antigenic heterogeneity demonstrated by RSV. The extracellular domain of G protein has a high degree of strain-to-strain diversity, but it also contains a 13-amino-acid region that is fully conserved among all RSV strains known to infect humans; this motif is certainly a candidate as a host receptor binding site (93). The identity of the host cell surface receptor(s) for RSV remains elusive, but it is assumed to be abundant based on the efficiency with which tissue culture cells bind purified G protein (178). Like F protein, G protein can induce the production of potent RSV-neutralizing antibodies by host cells.
The nucleocapsid-associated proteins include the major nucleocapsid protein (N), the phosphoprotein (P), and the RNA polymerase (L). Together, these proteins have been demonstrated to function as the RSV replicase (34, 72, 194). The M2 mRNA contains two overlapping translational open reading frames, ORF-1 and ORF-2 (33). M2 ORF-1 has historically been considered a second matrix protein (hence the abbreviation M2) but has recently been found to colocalize with the nucleocapsid proteins N and P (49, 54). M2 ORF-1 protein is a necessary component of the viral replicase, since it ensures efficient production of full-length mRNA (34, 81). M2 ORF-2 protein appears to be a negative regulatory factor (33, 34, 81). Collins et al. (34) have proposed that this negative regulatory function renders nucleocapsids synthetically quiescent prior to incorporation into virions. The functional significance of the nonstructural proteins (NS1 and NS2) remains unclear.
Protective Immunity
Protection against and recovery from RSV infection are mediated largely by the host immune system; secretory antibodies, serum antibodies, major histocompatibility complex class I-restricted cytotoxic T lymphocytes (CTLs), and, in the very young, maternally derived antibodies serve as specific effectors (8, 9, 23, 116, 149, 181). The importance of the host immune system in the eradication of RSV is exemplified by the observation that children with primary or acquired immunodeficiency diseases have difficulty in eradicating the virus and shed virus for many months rather than the typical interval of 1 to 3 weeks (19, 51, 166). Studies of RSV infection in mouse models have demonstrated that in previously naive animals, CTLs and antibodies both play a role in eradicating RSV from the respiratory tract.
Humoral immunity.
Specific RSV-neutralizing antibodies are present in the sera of all full-term newborns by virtue of the transplacental transfer of maternal antibodies (12, 140). The measurable antibody titers in the newborn are similar to the maternal levels and decline slowly during the first few months of life. After 7 months of age, detectable neutralizing antibody is usually the result of natural infection (12). Breast-fed infants have the added benefit of maternal antibodies in the form of colostrum (23, 116, 149, 181).
Both serum and secretory antibodies are produced in response to RSV infection, even in very young infants (116, 117). In infants, however, the antibody titers that develop are usually low. This blunted antibody response may be secondary to the relative immunologic immaturity of the infants and/or to a suppressive effect of maternally transmitted transplacental antibodies (126). Evidence for such a suppressive effect of passively transferred antibodies has been shown in an animal model of RSV infection (128). In addition, recent studies with RSV protein vaccines suggest that the presence of RSV-neutralizing antibodies is much more important than the absolute titer of anti-F and/or anti-G antibody present in the serum (172, 173).
In mice, RSV-specific antibodies do not appear to play a major role in the resolution of infection (65), although administration of physiologic amounts of RSV-specific antibodies during periods of peak RSV replication can accelerate resolution of the infection. Data from the Canadian collaborative group of pediatric infectious disease investigators demonstrated that the frequency of lower respiratory tract infections caused by RSV is significantly lower among children with RSV-neutralizing antibody titers greater than 1:100 than among those with lower titers (107). Evidence from animal models, including neonatal ferrets, cotton rats, and owl monkeys, also indicated that antibody protects against RSV disease (85, 145, 164). Once infection is established, however, it is the cellular immune response that promotes viral clearance.
Cellular immunity.
Cellular immune responses are thought to play an important role in clearing RSV. Immunocompetent infants infected with RSV stop shedding virus within 21 days following infection; by contrast, children with deficient cellular immunity can shed virus for several months (51, 77). In the murine model of RSV infection, CD8+ CTLs are capable of clearing RSV infection but may induce an enhanced inflammatory response (5, 8, 18, 169). RSV-specific CTLs are easily detected in peripheral blood from previously infected adults (9), in whom CTL responses are associated with decreased clinical symptoms (90).
Limited data from studies of infants with primary RSV infection suggest that a cellular immune response with specific CTLs is initiated within 10 days of infection (26, 91). The N, F, and M2 proteins are targets for CTLs in rodents and humans (9, 16, 24, 131, 136, 141); SH, M, and NS2 proteins were recognized by human CTLs. The protective effects associated with a (vaccine-induced) immune response to N and M2 appear to be CTL mediated but are short-lived (37, 38). It has been suggested that the failure of G protein to induce a CTL response may be an important factor in RSV disease pathogenesis (161).
The contribution of the different T-cell subsets to recovery from RSV infection remains a focus of investigation by a number of laboratories. The response to RSV infection in mice has been characterized as a Th1 response with subsequent production of gamma interferon, interleukin-2 (IL-2), and IgG2a. The production of RSV-specific CTLs follows close behind (7, 9, 16, 17, 124). In addition, RSV-specific CTL cell lines infused into normal or nude mice resulted in viral clearance, emphasizing their importance (5, 17, 18, 66, 124). Graham et al. characterized the T-cell subset responses and their functional contributions in mice infected with RSV (66–68). Mice depleted of either their CD4+-T-cell or CD8+-T-cell subsets still eradicated the virus; however, viral shedding was prolonged. Mice that were previously infected with RSV and thus were immune to the virus did not have prolonged shedding when rechallenged with virus under conditions of CD4+-T-cell or CD8+-T-cell depletion. Passive transfer of RSV-specific CD4+ or CD8+ T cells to infected mice resulted in decreased pulmonary shedding of RSV but in some studies resulted in increased pulmonary damage (3, 18, 124). In RSV-infected adult mice, the combined effects of CD4+ and CD8+ lymphocytes were necessary for full antiviral activity (68). The proliferative lymphocyte responses to RSV antigens in vitro have been measured during and after natural RSV infection in infants. Two separate studies demonstrated that lymphocytes collected from patients with bronchiolitis and asthma possess enhanced stimulation induces early in convalescence, a phenomenon not observed in lymphocytes collected from patients with upper respiratory infections or pneumonia (39, 185).
As discussed in detail in the next section on immunopathogenesis of RSV infection, if RSV antigen (as opposed to natural wild-type virus infection) is used in primary immunization, different T-helper cytokine expression patterns are evoked. Immunization with live virus stimulates a Th1 type response, whereas immunization with inactivated virus results in a Th2 cytokine expression pattern (65). The importance of this distinction becomes clear in the next section.
In direct contrast to both parainfluenza and influenza virus infections, RSV infection frequently fails to induce a detectable level of interferon in nasal secretions of infected patients (80, 114). This observation supports a minor role, if any, for interferon in recovery from RSV infection.
ROLE OF IMMUNITY IN PATHOGENESIS OF RSV DISEASE
The potential cytopathic effects of RSV on respiratory epithelial cells explains many of the pathologic findings associated with lower respiratory tract RSV infection. However, evidence implicating the host cell immune response as a direct participant in RSV pathophysiologic changes is also compelling. The early observations that severe RSV disease is most likely to occur in infants between 2 and 6 months of age, when maternally derived anti-RSV neutralizing antibodies persist at high concentrations, prompted investigators to consider the involvement of anti-RSV antibodies in immunopathologic mechanisms in RSV bronchiolitis (20, 21, 115). However, there are equally compelling arguments for a protective rather than an immunopathologic role for maternally derived anti-RSV antibodies, as discussed above. Further evidence for a protective role of maternally derived antibodies was provided by the recognition of an association between a low umbilical cord blood RSV antibody titer and serious RSV disease (61). There is also relative sparing of serious RSV disease in infants younger than 6 weeks, an age when maternally derived antibodies would be at their peak (88, 140). The parenteral administration of RSV-neutralizing antibodies in animal models and more recently in high-risk infants prevented rather than enhanced RSV disease (69, 145), arguing against antibody involvement in disease immunopathogenesis.
Role of Cell-Mediated Immunity
The most dramatic support for an immunopathologic mechanism in RSV disease emerged from field studies where infants were immunized with a formalin-inactivated, alum-precipitated, parenterally administered RSV vaccine. Although these infants developed complement-fixing and neutralizing antibodies, an unusually large number of vaccinees later developed natural RSV infection that was more severe than is usually seen without immunization (27, 53, 94, 99, 125).
The present understanding of the immunopathologic mechanisms in RSV disease has been largely advanced through animal studies. In these studies, cotton rats or mice were immunized with inactivated RSV vaccine and then challenged with intranasal inoculations of wild-type RSV. This model system faithfully reproduced the results of the field studies in humans, since the animals developed enhanced pulmonary histopathologic findings including florid pulmonary eosinophilia with associated hemorrhagic necrosis (144). The most widely accepted explanation for this immune system-mediated enhancement of RSV disease is that there is an imbalance in the Th1- and Th2-lymphocyte response to the vaccine. Normally, Th1-type responses with gamma interferon and IL-2 production are seen with viral disease. The pulmonary histologic findings in the mice previously immunized with the inactivated RSV vaccine are more consistent with a Th2-type response, an ineffective immune response to viral pathogens.
There are a number of other reasons for the inefficacy of the early vaccine trials. First, because the vaccine is administered parenterally, it is very unlikely to induce appreciable local secretory IgA antibodies, thereby leaving vaccinees susceptible to infection. Moreover, there was a dissociation between neutralizing-antibody and glycoprotein antibody responses in serum in vaccine recipients (127). Specifically, vaccinees immunized with formalin-inactivated RSV developed high antibody titers to the F glycoprotein of the virus, but these antibodies had only marginal virus-neutralizing ability. The antigenicity of the protective epitopes on the F glycoprotein appeared to be reduced during the process of formalin inactivation. The immunogenicity of RSV G protein epitopes was likewise adversely affected. When the immunization phase of the early vaccine trial was completed, it was noted that lymphocytes from young vaccinees exhibited an exaggerated response to RSV antigens in vitro (100). The idiosyncratic response of these infants to the formalin-inactivated vaccine is highly artificial but plays an important role in our understanding of normal RSV immunobiology. For this reason, the current understanding of this phenomenon is described in some detail.
With the identification of discrete lymphocyte subsets, studies with the mouse models of RSV infection demonstrated that the formalin-inactivated vaccine induced a high level of virus-specific memory lymphocytes, probably of the CD4+ lineage, but did not induce an RSV-specific CD8+ CTL response. Indeed, CD4+ cells are the predominant lymphocyte type in the pulmonary infiltrate of infected, previously immunized mice (65). Of the CD4+ cells present, cells with the Th2 phenotype predominated in these mice, whereas Th1 cells were present in largest numbers during natural, wild-type RSV infection. Again, this suggests that vaccinees experienced imbalanced cell-mediated immune responses favoring a Th2-type over a Th1-type response (132).
Studies with mice also provided evidence that RSV-specific CD8+ lymphocytes may play a role in the pathogenesis of RSV disease. RSV-specific CTLs can clear virus from persistently infected, immunodeficient (irradiated) mice, but acute pulmonary damage occurs under these experimental conditions and the mice die. Similarly, CTLs accelerate the clearance of RSV from immunocompetent mice, but virus clearance is associated with acute and sometimes fatal pulmonary disease (18). Further work with splenocytes enriched in either CD4+ or CD8+ lymphocytes indicated that both phenotypes decreased the pulmonary virus titer, with CD4+ cells being more efficient (3). The CD4+ cells in this study were of the Th2 phenotype, secreting IL-4, and IL-5 but only low levels of IL-2. Histopathologic testing revealed intense pulmonary eosinophilia associated with this Th2 response. A series of elegant studies with mice in which either CD4+ or CD8+ cells were depleted indicated that both subsets of cells were involved in both recovery and the pathologic response (68) but that the CD8+ cells were more efficient in both functions. Clearly, a fine balance exists between the protective and disease-producing effects of T cells; it may be impossible to completely dissociate the two elements.
Taken together, these data strongly support the hypothesis that recipients of the inactivated vaccine were susceptible to infection because the vaccine did not allow the production of local protective IgA responses and did not result in adequate levels of serum neutralizing antibodies. Because the RSV-specific CD4+ lymphocytes were present, they underwent rapid amplification in response to RSV antigens. The resulting effector functions of these cells, in addition to the recruitment of other cells by inflammatory cytokines, causes considerable damage to the bronchioles and alveoli. These immunopathologic mechanisms of tissue injury would continue, especially in the absence of protective serum or neutralizing antibodies, and inadequate CTL response. While cellular immunopathologic events are clearly implicated in the vaccine-induced pulmonary pathologic findings reported in the 1960s, it is also likely that subtle alterations in the natural cellular immune responses to RSV can contribute to the immunopathologic findings of wild-type RSV infection. This may explain why some infants with RSV lower respiratory tract disease are “happy wheezers” while others develop hypoxemia leading to respiratory failure.
Immunoglobulin E, Histamine, and Eosinophils
The clinical similarities between viral bronchiolitis and reactive airway disease (asthma) has led to the speculation that the two disease states have similar pathophysiologic mechanisms. Because of this, investigators have begun to explore the roles of IgE, histamine, and eosinophils in RSV disease.
The concept that IgE might be involved in the pathogenesis of RSV disease has been suggested by a number of studies (184, 186, 187). RSV-specific IgE was detected in the secretions of infants during the recovery phase of RSV bronchiolitis (184, 187). Levels of IgE were high in infants with bronchiolitis and correlated with the degree of hypoxemia during acute RSV disease. Interestingly, the levels of IgE found in such patients correlated inversely with the number of circulating CD8+ cells (186). In addition, histamine, presumably released during the interaction of IgE, RSV, and mast cells in the respiratory epithelium, was detected in the secretions of patients with bronchiolitis and RSV-induced asthma but was not found in the secretions of patients with RSV pneumonia or upper respiratory infection (14).
Several groups have shown that during RSV infection, eosinophils are recruited to and degranulate in the lung parenchyma (35, 58, 82, 135, 158). Wheezing during RSV infection was associated with increased concentrations in respiratory secretions of leukotriene C4 (175) and eosinophil cationic protein (58), both of which are mediators produced and secreted by activated eosinophils. Stark et al. (162) showed that cultured respiratory epithelial cells infected with RSV support an increased adherence of activated eosinophils. Kimpen et al. (101, 102) presented evidence for the direct activation of eosinophils by RSV in vitro, and Saito et al. (155) demonstrated that human epithelial cells upregulated the expression of the eosinophil chemoattractant RANTES in response to infection with RSV. Recently, we confirmed the upregulation of RANTES in respiratory epithelial cell lines in response to RSV infection and, further, have demonstrated the upregulation of another eosinophil chemoattractant, macrophage inflammatory protein 1α (MIP-1α), during ongoing RSV replication (45). We have also demonstrated elevated MIP-1α levels in lower respiratory tract specimens from intubated infants with RSV bronchiolitis (82). Although the mechanism(s) by which these chemokines are upregulated merits further investigation, our preliminary studies confirmed that RANTES and MIP-1α are upregulated via distinct signaling pathways (45). The observation that RSV-infected epithelial cells upregulate and secrete eosinophil chemoattractants supports the hypothesis that eosinophils are recruited to and participate in the immunopathogenesis of RSV disease.
Eosinophils have also been associated with the pathophysiologic findings of RSV infection when children were previously immunized with inactivated vaccine (27). As mentioned above, children who were previously vaccinated with the formalin-inactivated RSV vaccine and who subsequently developed natural RSV infection had increased eosinophil counts in blood (27) and massive eosinophil infiltrates were observed in postmortem specimens from vaccinated children who died of RSV pneumonia (98). Clearly, the infiltration of eosinophils into the lungs of these patients participated in the immunopathogenesis of their disease.
Together, these studies demonstrated that recruitment of eosinophils to the respiratory tract does occur in response to RSV infection and, when exaggerated, may lead to a more severe form of RSV disease. Recruitment of eosinophils to the lungs during RSV infection may also represent a primary immune system mechanism designed to blunt viral replication, since eosinophils and eosinophil RNases have been demonstrated to possess antiviral activity (42, 43, 44, 103). We speculate that eosinophils play beneficial roles in RSV disease but, when overrecruited, exacerbate the immunopathologic process, a “double-edged sword” reminiscent of the role of the neutrophil in adult respiratory distress syndrome.
While the immunopathologic mechanisms associated with enhanced RSV disease following vaccination with the inactivated vaccine are compelling and the involvement of immune system-mediated components in the pathogenesis of RSV bronchiolitis and pneumonia is likely, it is important to emphasize that much of the damage is probably a result of direct cytotoxicity. Moreover, the clinical presentation of bronchiolitis, with its resemblance to asthma in older patients, is probably a combination of multiple factors including immunologic mechanisms, the tropism of RSV for respiratory epithelium, and the anatomy of airways, which are obstructed easily in the face of necrosis and edema, especially in very young infants.
TREATMENT OF RSV DISEASE
Ribavirin
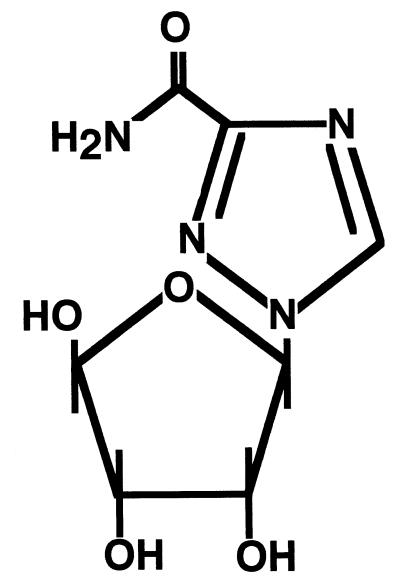
While significant advances in our knowledge of RSV biology, immunology, pathophysiology, and epidemiology have been made in the past 40 years, there continues to be a fair amount of controversy about the optimum management of infants and children with RSV infection (179). Ribavirin, a synthetic nucleoside (Fig. 3) delivered as a small-particle aerosol, was approved for the treatment of RSV lower respiratory tract infection and licensed in 1985 for use in hospitalized children in the United States. The precise mechanism of action of its antiviral properties is unknown.
FIG. 3.
Structural formula of ribavirin. Ribavirin is a synthetic nucleoside with the empirical formula C8H12N4O5. While the mechanism of action of its antiviral properties remain unknown, the reversal of the in vitro antiviral activity by guanosine or xanthosine that suggests ribavirin may act as an analogue of these cellular metabolites.
The lack of consensus about the management of RSV-induced bronchiolitis in infancy cannot be attributed to a paucity of literature. The results of at least 14 trials of bronchodilators, 3 trials of adjunctive systemic glucocorticoid treatment, and 11 randomized trials of ribavirin have been published (11, 70, 75, 76, 107, 118, 122, 146, 152, 160, 165, 179, 189). In six controlled, randomized studies, ribavirin treatment was found to reduce the severity of RSV illness by decreasing viral shedding, improving oxygen saturation, and improving clinical scores (11, 75, 76, 152, 160, 165). Recently, a systematic overview of these studies, highlighting potential flaws, has been published (147). The results of this meta-analysis were discouraging but were consistent with the clinical experience of pediatricians. Ribavirin treatment did not lead to significant improvement in clinically important outcomes. The growing concern about the efficacy of ribavirin and the high cost of its use (50) have led to the recent wording change in the American Academy of Pediatrics recommendation for its usage from “should be used” to “may be considered” for selected infants and young children at high risk for serious RSV disease (36).
Studies on the long-term outcome of bronchiolitis in infancy have focused on the incidence of recurrent wheezing and the possible effect of ribavirin therapy (57, 110, 113, 154, 182, 183). A recent retrospective sample of children with and without ribavirin treatment of RSV lower respiratory tract infection in infancy found no differences in wheezing or other pulmonary function measures 6 to 8 years later (104).
Future multi-institutional, prospective, randomized trials may help curb the controversy about the use of ribavirin and may provide information about the contexts in which it may provide clinical benefits. Animal experiments have already shown that ribavirin combined with RSV immune globulin (RSVIG) given either parenterally or as an aerosol was more effective than therapy with either alone (64, 73). In addition, clinical trials with children and immunocompromised adults are planned that have been designed to evaluate the efficacy of humanized anti-RSV monoclonal antibody alone and in combination with ribavirin.
Prospects for New Therapies
New therapies designed to combat moderate to severe RSV disease are clearly needed. Categories of agents currently under investigation include sulfated polysaccharide compounds, miscellaneous organic compounds, a variety of proteins (some with known enzymatic activities), and nucleosides other than ribavirin, including antisense oligodeoxyribonucleotides.
Sulfated polysaccharides extracted from marine algae (83), sulfonic acid polymers (89), and derivatized dextrans (130) each have anti-RSV activity in vitro, presumably by inhibiting viral attachment to and/or internalization into the host cell. Until the host cell receptor (and/or coreceptors) for RSV is identified, it will be impossible to determine if these compounds are interfering at a virus-receptor binding site.
A number of organic compounds have also received attention as anti-RSV agents. Benzylimidazotriazine derivatives have antiviral activity, but many forms are too cytotoxic to develop as clinically useful agents (63). Polyoxometalates (10) and pyridobenzazoles (25) are less toxic in vitro, and SP-303, a natural polyphenolic polymer, reduced pulmonary RSV titers when used in the cotton rat model of RSV lower respiratory tract disease (193).
A variety of peptides and proteins have also been tested for their ability to impair RSV replication in vitro. Speculation that inhibiting cell-to-cell fusion might lessen the severity of clinical disease has led to attempts at developing fusion inhibitors as therapeutic agents. Synthetic peptides derived from separate domains of the F protein blocked RSV-induced syncytium formation when used at concentrations as low as 0.015 μM. The high selectivity of these peptides for RSV make them candidates for further development (106). Other proteins recently demonstrated to have potent anti-RSV activity include crude plant protein extracts (2), some forms of superoxide dismutase (192), an l-aspartate transcarbamoylase inhibitor (191), and eosinophil-derived neurotoxin, a potent generalized RNase (44).
Nucleoside derivatives other than ribavirin have generated scant interest from investigators in this field (174); the development of antisense technology has been more aggressive. Specific cleavage of genomic RSV target RNA has been detected at antisense oligonucleotide-binding sites (92). Because of the pattern of RNA cleavage observed, it was suggested that endogenous RNase H participated in RSV genomic degradation. Other RNases have been studied for their antiviral properties, and the ability of these proteins to degrade viral genomic RNA has led to some elegant studies. For example, recruitment and activation of RNase L to an RSV mRNA by using 2-5A-antisense oligonucleotide technology showed potent inhibition of RSV replication (28, 170).
These novel strategies designed to overcome RSV replication have been executed almost exclusively in vitro. Perhaps one or a combination of these approaches will eventually emerge as beneficial in the clinical realm.
Passive-Immunization Therapy
Intravenous immunoglobulin therapy to prevent RSV disease.
The use of hyperimmune globulins in the treatment and prevention of RSV infections was the focus of a recent review in this journal (86). For an extensive discussion of this topic, the reader is referred to that state-of-the-art review. Here we summarize the information and add details of recent developments including the current recommendations for the clinical use of RSVIG.
RSVIG is an emerging therapeutic modality, now attracting attention as both a treatment adjunct and an immunoprophylactic modality. Parenteral administration of RSV-neutralizing antibodies in animal models and, more recently, in high-risk infants resulted in prevention of RSV disease. This is a critical observation, given the exacerbation of natural RSV disease seen following vaccination with the formalin-inactivated, alum-precipitated formulation in the 1960s (27).
In the cotton rat model, a preparation of RSVIG showing a high titer of antibody to RSV afforded superior protection to standard intravenous immunoglobulin (IVIG) against challenge with RSV (157). The observation that passive antibodies to RSV were protective in animal models prompted studies with humans. A placebo-controlled trial of IVIG involving 49 children at high risk for severe RSV disease observed 6 infections in each group (119). It was concluded that the achievable RSV-neutralizing antibody concentrations after administration of IVIG were too low, and subsequent trials were performed with RSVIG (69, 143). In this study, pooled Ig containing high titers of anti-RSV neutralizing antibodies (RSVIG), given as monthly infusions during RSV season, reduced the frequency of lower respiratory tract infections, hospitalizations, and total days of hospitalization. However, an increased number of unexplained deaths occurred in RSVIG recipients who suffered from congenital heart disease. This worrisome phenomenon was also observed in another study, leading to the recommendation that RSVIG not be used in patients with underlying heart disease (159).
The compelling results of the aforementioned studies led to approval of RSVIG by the U.S. Food and Drug Administration for the prevention of RSV infection in high-risk infants, but there is considerable controversy about specific indications. Problems associated with the use of RSVIG include the potential for transmission of blood-borne pathogens, the need to administer a large volume of product (15 ml/kg of body weight), and the associated fever and desaturation that can accompany the administration of any blood product. Additional practical problems associated with monthly administration of this preparation during RSV season include the necessity for intravenous access, long infusion times (which can strain hospital resources), and high cost. Prober and Wang (146) calculated that to prevent a single hospitalization, 12 infants with bronchopulmonary dysplasia (BPD) would have to receive RSVIG prophylaxis. In contrast, to prevent a single hospitalization, 63 premature infants younger than 6 months would have to be treated. Based on these findings, these authors proposed that only infants with BPD who are receiving supplemental oxygen therapy be given prophylaxis. Recommendations of the American Academy of Pediatrics assert that RSVIG prophylaxis “be considered” for infants and children younger than 2 years with BPD who are currently receiving oxygen therapy or have received oxygen therapy in the last 6 months. They also contend that infants with a gestational age of 32 weeks or less at birth may benefit from prophylaxis until 6 to 12 months of age (4).
New anti-RSV monoclonal antibodies are under development (40, 168). An advantage of this approach is that highly concentrated RSV-neutralizing antibody preparations can be delivered in much smaller volumes than the polyclonal RSVIG preparations, thereby shortening the administration time. Data on the efficacy of these preparations in the prevention and treatment of RSV disease led to Food and Drug Administration approval for their use in the 1998 to 1999 RSV season.
Intravenous immunoglobulin therapy to treat RSV disease.
The role of RSVIG as therapy for severe RSV-induced lower respiratory tract disease in high-risk infants and young children has begun to receive some attention. In a recent study (153), 105 children were randomized to receive RSVIG (54 children) or placebo (51 children). Interestingly, there was no significant difference in duration of hospitalization, duration of intensive-care unit stay, mechanical ventilation, supplemental oxygen use, or adverse effects between the two groups. Although RSVIG was demonstrated to be safe, it did not appear to be efficacious in the attenuation of the disease process. At first glance, it seems paradoxical that RSVIG has some protective benefit in preventing the frequency and consequences of RSV infection in high-risk infants but lacks therapeutic efficacy. One explanation is that by the time respiratory symptoms are present, the virus has already penetrated the respiratory epithelium and is sequestered from host humoral immunity (146). Prophylactic administration of RSVIG may neutralize infectious virions before cellular penetration occurs, thereby preventing disease at the outset. Antibodies applied topically to the airways of RSV-infected animals appear to be more beneficial than antibodies delivered systemically. Topical application of high-titer antibody preparations resulted in a 30-fold greater reduction in pulmonary viral titer than that produced by preparations low in anti-RSV titer (86). Direct delivery of antibodies to the mucosal surface via aerosolization has also been demonstrated to be safe when used in infants (150); however, one placebo-controlled randomized trial with 65 patients showed no benefit to its administration (151).
Combination therapy with ribavirin and systemically administered Ig has also been tried with some success in the cotton rat model (73). An anecdotal report of combined aerosolized ribavirin and systemic Ig treatment in bone marrow transplant recipients who developed RSV disease showed a 22% mortality among patients treated before the onset of respiratory failure and 100% mortality among patients who went untreated or in whom treatment was initiated within 24 h of respiratory failure (188). Intravenous ribavirin for the treatment of RSV respiratory disease in bone marrow transplant recipients has also been tried, but the results have been variable (109, 188).
Finally, mouse, humanized-mouse, and human monoclonal antibodies to RSV have been developed and tested for their potential prophylactic and therapeutic benefits (40, 168, 190). Preliminary results in human trials designed to evaluate the ability of the monoclonal-F product in preventing severe RSV disease in premature infants appear promising.
PREVENTION OF RSV DISEASE: THE VACCINE EFFORT
The morbidity and mortality associated with bronchiolitis, coupled with its frequency and worldwide distribution, make RSV a prime target for the development of a vaccine that can be administered early in life. The reasons why an RSV vaccine is not yet available stem from multiple problems with its development. First, and most important, is the possibility that vaccination will potentiate naturally occurring RSV disease, as observed with the aforementioned formalin-inactivated vaccine (27, 53, 94, 98). Second, newborns and very young infants may not mount a protective immune response because of relative immunologic immaturity or because of suppression of their immune response due to circulating maternally derived anti-RSV antibodies (31, 47, 129). Another important consideration in the development of an effective RSV vaccine is the need to provide protection against multiple antigenic strains of RSV in the two major groups, A and B. A number of strategies have been implemented recently to generate safe and effective subunit, inactivated, and live attenuated virus vaccines (47). Vaccine development is ongoing. Currently, the two most promising candidate vaccines to be studied in clinical trials are an RSV F subunit vaccine (for immunization of patients who have already had their primary RSV infection, such as the elderly and older RSV-seropositive children with conditions predisposing them to severe RSV disease) (48, 71, 87, 139, 142, 172, 173) and cold-passaged, temperature-sensitive (cpts) attenuated RSV strains (97). While this cpts mutant appears immunogenic, retained virulence has been observed in older children, precluding the study of this variant in infants. However, progress with this technology continues, and other cpts variants which are slightly more attenuated are under study. Another possibility is to immunize infants with an attenuated RSV vaccine to optimize the Th1-type response and then to immunize them with a subunit vaccine to boost both anti-F and anti-G neutralizing antibody production. As progress on the vaccine front continues, an optimal immunization schedule, possibly with different forms of the RSV vaccine, will probably emerge.
REFERENCES
- 1.Ahern W, Bird T, Court S D M. Pathologic changes in virus infections of the lower respiratory tract in children. J Clin Pathol. 1970;23:7–18. doi: 10.1136/jcp.23.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad A, Davies J, Randall S, Skinner G R. Antiviral properties of extract of Opuntia streptacantha. Antiviral Res. 1996;30:75–85. doi: 10.1016/0166-3542(95)00839-x. [DOI] [PubMed] [Google Scholar]
- 3.Alwan W H, Record F M, Openshaw P J. CD4+ T cells clear virus but augment disease in mice infected with respiratory syncytial virus. Comparison with the effects of CD8+ T cells. Clin Exp Immunol. 1992;88:527–536. doi: 10.1111/j.1365-2249.1992.tb06482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics, Committee on Infectious Diseases, Committee on Fetus and Newborn. Respiratory syncytial virus immune globulin intravenous: indications for use. Pediatrics. 1997;99:645–650. [PubMed] [Google Scholar]
- 5.Anderson J J, Norden J, Saunders D, Toms G L, Scott R. Analysis of the local and systemic immune responses induced in BALB/c mice by experimental respiratory syncytial virus infection. J Gen Virol. 1990;71:1561–1570. doi: 10.1099/0022-1317-71-7-1561. [DOI] [PubMed] [Google Scholar]
- 6.Anderson L J, Tsou C, Potter C. Cytokine response to respiratory syncytial virus stimulation of human peripheral blood mononuclear cells. J Infect Dis. 1994;174:1201–1208. doi: 10.1093/infdis/170.5.1201. [DOI] [PubMed] [Google Scholar]
- 7.Bangham C R M, Askonas B A. Murine cytotoxic T cells specific to respiratory syncytial virus recognize different antigenic subtypes of the virus. J Gen Virol. 1986;67:623–629. doi: 10.1099/0022-1317-67-4-623. [DOI] [PubMed] [Google Scholar]
- 8.Bangham C R M, Cannon M J, Karzon D T, Askonas B A. Cytotoxic T-cell response to respiratory syncytial virus in mice. J Virol. 1985;56:55–59. doi: 10.1128/jvi.56.1.55-59.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bangham C R M, Openshaw P J M, Ball L A, King A M Q, Wertz G W, Askonas B A. Human and murine cytotoxic T-cells specific to respiratory syncytial virus recognize the viral nucleoprotein (N), but not the major glycoprotein (G), expressed by vaccinia virus recombinants. J Immunol. 1986;137:3973–3977. [PubMed] [Google Scholar]
- 10.Barnard D L, Hill C L, Gage T, Matheson J E, Huffman J H, Sidwell S W, Otto M I, Schinazi R F. Potent inhibition of respiratory syncytial virus by polyoxometalates of several structural classes. Antiviral Res. 1997;34:27–37. doi: 10.1016/s0166-3542(96)01019-4. [DOI] [PubMed] [Google Scholar]
- 11.Barry W, Cockburn F, Cornall R, Price J F, Sutherland G, Vardag A. Ribavirin aerosol for acute bronchiolitis. Arch Dis Child. 1985;61:593–597. doi: 10.1136/adc.61.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beem M, Egerer R, Anderson J. Respiratory syncytial virus neutralizing antibodies in persons residing in Chicago, Illinois. Pediatrics. 1964;34:761–770. [PubMed] [Google Scholar]
- 13.Brandt C D, Kim H W, Arrobio J O. Epidemiology of respiratory syncytial virus infection in Washington, D.C. III. Composite analysis of eleven consecutive yearly epidemics. Am J Epidemiol. 1973;98:355–364. doi: 10.1093/oxfordjournals.aje.a121565. [DOI] [PubMed] [Google Scholar]
- 14.Bui R H, Molinar G A, Kettering J D, Heiner D C, Imagawa D T, St. Geme J M., Jr Virus-specific IgE and IgG4 antibodies in serum of children infected with respiratory syncytial virus. J Pediatr. 1987;110:87–90. doi: 10.1016/s0022-3476(87)80295-0. [DOI] [PubMed] [Google Scholar]
- 15.Bukreyev A, Whitehead S S, Murphy B R, Collins P L. Recombinant respiratory syncytial virus from which the entire SH gene has been deleted grows efficiently in cell culture and exhibits site-specific attenuation in the respiratory tract of the mouse. J Virol. 1997;71:8973–8982. doi: 10.1128/jvi.71.12.8973-8982.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannon M J, Bangham C R M. Recognition of respiratory syncytial virus fusion protein by mouse cytotoxic T-cell clones and a human cytotoxic T cell line. J Gen Virol. 1989;70:79–87. doi: 10.1099/0022-1317-70-1-79. [DOI] [PubMed] [Google Scholar]
- 17.Cannon M J, Scott E J, Taylor G, Askonas B A. Clearance of persistent respiratory syncytial virus infections in immunodeficient mice following transfer of primed T-cells. Immunology. 1987;62:133–138. [PMC free article] [PubMed] [Google Scholar]
- 18.Cannon M J, Openshaw P J M, Askonas B A. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988;168:1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chadwani S, Borkowsky W, Krasinski K, Lawrence R, Welliver R. Respiratory syncytial virus infection in human immunodeficiency virus-infected children. J Pediatr. 1990;117:251–254. doi: 10.1016/s0022-3476(05)80539-6. [DOI] [PubMed] [Google Scholar]
- 20.Chanock R M, Kapikian A Z, Parrot R H. Possible role of immunologic factors in pathogenesis of RS virus lower respiratory tract disease. Perspect Virol. 1968;6:125–139. [Google Scholar]
- 21.Chanock R M, Kapikian A Z, Mills J, Kim H W, Parrot R H. Influence of immunologic factors in respiratory syncytial virus disease. Arch Environ Health. 1970;21:347–356. doi: 10.1080/00039896.1970.10667249. [DOI] [PubMed] [Google Scholar]
- 22.Chanock R M, Parrott R H. Acute respiratory disease in infancy and childhood: present understanding and prospects for prevention. Pediatrics. 1965;36:21–39. [PubMed] [Google Scholar]
- 23.Chanock R M, Kim H W, Vargosko A J. Respiratory syncytial virus. I. Virus recovery and other observations during 1960 outbreak of bronchiolitis, pneumonia, and minor respiratory diseases in children. JAMA. 1961;176:647–653. [PubMed] [Google Scholar]
- 24.Cherrie A H, Anderson K, Wetrz G W. Human cytotoxic T-cells stimulated by antigen on dendritic cells recognize the N, SH, F, M, 22K, and 1b proteins of respiratory syncytial virus. J Virol. 1992;66:2102–2110. doi: 10.1128/jvi.66.4.2102-2110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiba T, Shigeta S, Numazaki Y. Inhibitory effect of pyridobenzazoles on virus replication in vitro. Biol Pharm Bull. 1995;18:1081–1083. doi: 10.1248/bpb.18.1081. [DOI] [PubMed] [Google Scholar]
- 26.Chiba Y, Higashidato Y, Suga K. Development of cell-mediated cytotoxic immunity to respiratory syncytial virus in human infants following naturally acquired infection. J Med Virol. 1989;28:133–139. doi: 10.1002/jmv.1890280304. [DOI] [PubMed] [Google Scholar]
- 27.Chin J, Maggoffin R L, Shearer L A, Schieble J H, Lennette E H. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969;89:449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 28.Cirino N M, Li G, Xiao W, Torrence P F, Silverman R H. Targeting RNA decay with 2′,5′ oligoadenylate-antisense in respiratory syncytial virus-infected cells. Proc Natl Acad Sci USA. 1997;94:1937–1942. doi: 10.1073/pnas.94.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins P L, Mottet G. Oligomerization and post-translational processing of glycoprotein G of human respiratory syncytial virus: altered O-glycosylation in the presence of brefeldin A. J Gen Virol. 1992;73:849–863. doi: 10.1099/0022-1317-73-4-849. [DOI] [PubMed] [Google Scholar]
- 30.Collins P L, Wertz G W. Human respiratory syncytial virus genome and gene products. In: Notkins A L, Oldstone M B A, editors. Concepts in viral pathogenesis. Vol. 2. New York, N.Y: Springer-Verlag; 1986. pp. 40–46. [Google Scholar]
- 31.Collins P L, McIntosh K, Chanock R M. Respiratory syncytial virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 2nd ed. Vol. 2. Philadelphia, Pa: Lippencott-Raven Publishers; 1996. pp. 1313–1351. [Google Scholar]
- 32.Collins P L, Dickens L E, Buckler-White A, Olmsted R A, Spriggs M K, Camargo E, Coelingh K V W. Nucleotide sequences for the gene junctions of human respiratory syncytial virus reveal distinctive features of intergenic structure and gene order. Proc Natl Acad Sci USA. 1986;83:4594–4598. doi: 10.1073/pnas.83.13.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins P L, Hill M G, Johnson P R. The two open reading frames of the 22K mRNA of human respiratory syncytial virus: sequence comparisons of antigenic subgroups A and B and expression in vitro. J Gen Virol. 1990;71:3015–3020. doi: 10.1099/0022-1317-71-12-3015. [DOI] [PubMed] [Google Scholar]
- 34.Collins P L, Hill M G, Cristina J, Grosfeld H. Transcription elongation factor of respiratory syncytial virus, a nonsegmented negative-strand RNA virus. Proc Natl Acad Sci USA. 1996;93:81–85. doi: 10.1073/pnas.93.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colocho Zelaya E A, Orvell C, Strannegard O. Eosinophil cationic protein in nasopharyngeal secretions and serum of infants infected with respiratory syncytial virus. Pediatr Allergy Immunol. 1994;5:100–106. doi: 10.1111/j.1399-3038.1994.tb00225.x. [DOI] [PubMed] [Google Scholar]
- 36.Committee on Infectious Disease, American Academy of Pediatrics. Reassessment of the indications for ribavirin therapy in respiratory syncytial virus infections. Pediatrics. 1996;97:137–140. [PubMed] [Google Scholar]
- 37.Connors M, Kulkarni A B, Collins P L. Resistance to respiratory syncytial virus (RSV) challenge induced by infection with a vaccinia virus recombinant expressing the RSV M2 protein (Vac-M2) is mediated by CD8+ T cells, while that induced by Vac-F or Vac-G recombinants is mediated by antibodies. J Virol. 1992;66:1277–1281. doi: 10.1128/jvi.66.2.1277-1281.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Connors M, Collins P L, Firestone C Y. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J Virol. 1991;65:1634–1637. doi: 10.1128/jvi.65.3.1634-1637.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cranage M P, Gardner P S. Systemic cell mediated and antibody responses in infants with respiratory syncytial virus infections. J Med Virol. 1980;5:161–170. doi: 10.1002/jmv.1890050210. [DOI] [PubMed] [Google Scholar]
- 40.Crowe J E, Murphy B R, Chanock R M. Recombinant human respiratory syncytial virus (RSV) monoclonal antibody Fab is effective therapeutically when introduced directly into the lungs of RSV-infected mice. Proc Natl Acad Sci USA. 1994;91:1386–1390. doi: 10.1073/pnas.91.4.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dickens L E, Collins P L, Wertz G W. Transcriptional mapping of human respiratory syncytial virus. J Virol. 1984;52:364–369. doi: 10.1128/jvi.52.2.364-369.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Domachowske J B, Rosenberg H F. Eosinophils inhibit retroviral transduction of human target cells by a ribonuclease-dependent mechanism. J Leukoc Biol. 1997;62:363–368. doi: 10.1002/jlb.62.3.363. [DOI] [PubMed] [Google Scholar]
- 43.Domachowske J B, Dyer K D, Adams A G, Leto T L, Rosenberg H F. Eosinophil cationic protein/RNase 3 is another Rnase A-family ribonuclease with direct antiviral activity. Nucleic Acids Res. 1998;26:3358–3363. doi: 10.1093/nar/26.14.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Domachowske J B, Dyer K D, Bonville C A, Rosenberg H F. Recombinant human eosinophil-derived neurotoxin/Rnase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis. 1998;177:1458–1464. doi: 10.1086/515322. [DOI] [PubMed] [Google Scholar]
- 45.Domachowske J B, Dyer K D, Rosenberg H F. Multiple signaling pathways are involved in respiratory syncytial virus induced chemokine expression. Clin Infect Dis. 1997;25:390. [Google Scholar]
- 46.Domurat F, Roberts N J, Jr, Walsh E E, Dagan R. Respiratory syncytial virus infection of human mononuclear leukocytes in vitro and in vivo. J Infect Dis. 1985;152:895–902. doi: 10.1093/infdis/152.5.895. [DOI] [PubMed] [Google Scholar]
- 47.Falsey A R, Walsh E E. Safety and immunogenicity of a respiratory syncytial virus subunit vaccine (PFP-2) in ambulatory adults over age 60. Vaccine. 1996;14:1214–1218. doi: 10.1016/s0264-410x(96)00030-8. [DOI] [PubMed] [Google Scholar]
- 48.Falsey A R, Walsh E E. Safety and immunogenicity of a respiratory syncytial virus subunit vaccine (PFP-2) in the institutionalized elderly. Vaccine. 1997;15:1130–1132. doi: 10.1016/s0264-410x(97)00002-9. [DOI] [PubMed] [Google Scholar]
- 49.Fearns R, Peebles M E, Collins P L. Increased expression of the N protein of respiratory syncytial virus stimulates minigenome replication but does not alter the balance between the synthesis of mRNA and antigenome. Virology. 1997;236:188–201. doi: 10.1006/viro.1997.8734. [DOI] [PubMed] [Google Scholar]
- 50.Feldstein T, Swegarden J, Atwood G. Ribavirin therapy: implementation of hospital guidelines and effect on usage and cost of therapy. Pediatrics. 1995;96:14–17. [PubMed] [Google Scholar]
- 51.Fishaut M, Tubergen D, McIntosh K. Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity. J Pediatr. 1980;96:179–186. doi: 10.1016/s0022-3476(80)80799-2. [DOI] [PubMed] [Google Scholar]
- 52.Foy H M, Cooney M K, Maletzky A J. Incidence and etiology of pneumonia, croup, and bronchiolitis in preschool children belonging to a prepaid medical care group over a four-year period. Am J Epidemiol. 1973;97:80–92. doi: 10.1093/oxfordjournals.aje.a121492. [DOI] [PubMed] [Google Scholar]
- 53.Fulginetti V A, Eller J J, Seiber O F, Joyner J W, Minamitani M, Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines: an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969;89:435–448. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- 54.Garcia J, Garcia-Barreno B, Vivo A, Melero J A. Cytoplasmic inclusions of respiratory syncytial virus-infected cells: formation of inclusion bodies in transfected cells that coexpress the nucleoprotein, the phosphoprotein, and the 22K protein. Virology. 1993;195:243–247. doi: 10.1006/viro.1993.1366. [DOI] [PubMed] [Google Scholar]
- 55.Gardner P S. How etiologic, pathologic, and clinical diagnoses can be made in a correlated fashion. Pediatr Res. 1977;11:254–261. [PubMed] [Google Scholar]
- 56.Gardner P S, McQuillin J, Court S D M. Speculation on pathogenesis in death from respiratory syncytial virus infection. Br Med J. 1970;1:327–330. doi: 10.1136/bmj.1.5692.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garofalo R, Welliver R C, Ogra P. Modulation of leukotriene (LT) release with ribavirin during infection with respiratory syncytial virus. Pediatr Res. 1989;25:163a. . (Abstract.) [Google Scholar]
- 58.Garofalo R, Kimpen J L L, Welliver R C, Ogra P L. Eosinophil degranulation in the respiratory tract during naturally acquired respiratory syncytial virus infection. J Pediatr. 1992;120:28–32. doi: 10.1016/s0022-3476(05)80592-x. [DOI] [PubMed] [Google Scholar]
- 59.Glezen W P. Pathogenesis of bronchiolitis: epidemiologic considerations. Pediatr Res. 1977;11:239–243. [PubMed] [Google Scholar]
- 60.Glezen W P. Incidence of respiratory syncytial and parainfluenza type 3 viruses in an urban setting. Pediatr Virol. 1987;2:1–4. [Google Scholar]
- 61.Glezen W P, Paredes A, Allison J E. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group and maternal antibody level. J Pediatr. 1981;98:708–715. doi: 10.1016/s0022-3476(81)80829-3. [DOI] [PubMed] [Google Scholar]
- 62.Glezen W P, Denny F W. Epidemiology of acute lower respiratory disease in children. N Engl J Med. 1973;288:498–505. doi: 10.1056/NEJM197303082881005. [DOI] [PubMed] [Google Scholar]
- 63.Golankiewicz B, Januszczyk P, Ikeda S, Balzarini J, DeClercq E. Synthesis and antiviral activity of benzyl-substituted imidazo[1,5-a]-1,3,5-triazine(5,8-diaza-7,9-dideazapurine) derivatives. J Med Chem. 1995;38:3558–3565. doi: 10.1021/jm00018a015. [DOI] [PubMed] [Google Scholar]
- 64.Graham B S, Davis T, Tang Y, Gruber W. Immunoprophylaxis and immunotherapy of respiratory syncytial virus-infected mice with respiratory syncytial virus-specific immune serum. Pediatr Res. 1993;34:167–172. doi: 10.1203/00006450-199308000-00013. [DOI] [PubMed] [Google Scholar]
- 65.Graham B S, Henderson G S, Tang Y W, Lu X, Neuzil K M, Colley D J. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151:2032–2040. [PubMed] [Google Scholar]
- 66.Graham B S, Bunton L A, Rowland J. Respiratory syncytial virus infection in anti-μ treated mice. J Virol. 1991;65:4936–4942. doi: 10.1128/jvi.65.9.4936-4942.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Graham B S, Bunton L A, Wright P F. Reinfection of mice with respiratory syncytial virus. J Med Virol. 1991;34:7–13. doi: 10.1002/jmv.1890340103. [DOI] [PubMed] [Google Scholar]
- 68.Graham B S, Bunton L A, Wright P F, Karzon D T. Role of T-lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991;88:1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Groothius J R, Simoes E A F, Levin M J. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. N Engl J Med. 1993;329:1524–1530. doi: 10.1056/NEJM199311183292102. [DOI] [PubMed] [Google Scholar]
- 70.Groothius J R, Woodin K A, Katz R. Early ribavirin treatment of respiratory syncytial virus infection in high-risk children. J Pediatr. 1990;117:792–798. doi: 10.1016/s0022-3476(05)83347-5. [DOI] [PubMed] [Google Scholar]
- 71.Groothius J R, King S J, Hogerman D A, Paradiso P R, Simoes E A. Safety and immunogenicity of a purified F protein respiratory syncytial virus (PFP-2) vaccine in seropositive children with bronchopulmonary dysplasia. J Infect Dis. 1998;177:467–469. doi: 10.1086/517377. [DOI] [PubMed] [Google Scholar]
- 72.Grosfeld H, Hill M G, Collins P L. RNA replication by respiratory syncytial virus (RSV) is directed by the N, P, and L proteins: transcription also occurs under these conditions but requires RSV superinfection for efficient synthesis of full-length mRNA. J Virol. 1995;69:5677–5686. doi: 10.1128/jvi.69.9.5677-5686.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gruber W C, Wilson S Z, Throop B J, Wyde P R. Immunoglobulin administration and ribavirin therapy: efficacy in respiratory syncytial virus infection in the cotton rat. Pediatr Res. 1987;21:270–274. doi: 10.1203/00006450-198703000-00013. [DOI] [PubMed] [Google Scholar]
- 74.Hall C B, Walsh E E, Schnabel K C. The occurrence of groups A and B of respiratory syncytial virus over 15 years. The associated epidemiologic and clinical characteristics in hospitalized and ambulatory children. J Infect Dis. 1990;162:1283–1290. doi: 10.1093/infdis/162.6.1283. [DOI] [PubMed] [Google Scholar]
- 75.Hall C B, McBride J T, Walsh E E. Aerosolized ribavirin treatment of infants with respiratory syncytial virus infection. N Engl J Med. 1983;308:1443–1447. doi: 10.1056/NEJM198306163082403. [DOI] [PubMed] [Google Scholar]
- 76.Hall C B, McBride J T, Gala C L, Hildreth S W, Schnabel K C. Ribavirin treatment of respiratory syncytial viral infection in infants with underlying cardiopulmonary disease. JAMA. 1985;254:3047–3051. [PubMed] [Google Scholar]
- 77.Hall C B, Powell K R, MacDonald N E. Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med. 1986;315:77–81. doi: 10.1056/NEJM198607103150201. [DOI] [PubMed] [Google Scholar]
- 78.Hall C B, Douglas R G, Geiman J M. Quantitative shedding patterns of respiratory syncytial virus in infants. J Infect Dis. 1975;132:151–156. doi: 10.1093/infdis/132.2.151. [DOI] [PubMed] [Google Scholar]
- 79.Hall C B, Douglas R G, Geiman J M. Respiratory syncytial virus infection in infants: quantitation and duration of shedding. J Pediatr. 1976;89:1–15. doi: 10.1016/s0022-3476(76)80918-3. [DOI] [PubMed] [Google Scholar]
- 80.Hall C B, Douglas R G, Geiman J M. Interferon production in children with respiratory syncytial virus, influenza, and parainfluenza infections. J Pediatr. 1978;93:28–32. doi: 10.1016/s0022-3476(78)80594-0. [DOI] [PubMed] [Google Scholar]
- 81.Hardy R W, Wertz G W. The production of the respiratory syncytial virus M2 gene ORF1 enhances readthrough of intergenic junctions during viral transcription. J Virol. 1998;72:520–526. doi: 10.1128/jvi.72.1.520-526.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harrison, A. M., C. A. Bonville, H. F. Rosenberg, and J. B. Domachowske. RSV-induced chemokine expression in the lower airways: eosinophil recruitment and degranulation. Am. J. Resp. Crit. Care Med., in press. [DOI] [PubMed]
- 83.Hasui M, Matsuda M, Okutani K, Shigeta S. In vitro activities of sulfated polysaccharides from a marine microalga (Cochlodinium polykrikoides) against human immunodeficiency virus and other enveloped viruses. Int J Biol Macromol. 1995;17:293–297. doi: 10.1016/0141-8130(95)98157-t. [DOI] [PubMed] [Google Scholar]
- 84.Heminway B R, Yu Y, Tanaka Y, Perrine K G, Gustafson E, Bernstein J M, Galinski M S. Analysis of respiratory syncytial virus F, G, and SH proteins in cell fusion. Virology. 1994;200:801–805. doi: 10.1006/viro.1994.1245. [DOI] [PubMed] [Google Scholar]
- 85.Hemming V G, Prince G A, Horswood R L. Studies of passive immunotherapy for infections of respiratory syncytial virus in the respiratory tract of a primate model. J Infect Dis. 1985;152:1083–1087. doi: 10.1093/infdis/152.5.1083. [DOI] [PubMed] [Google Scholar]
- 86.Hemming V G, Prince G A, Groothius J R, Siber G R. Hyperimmune globulins in prevention and treatment of respiratory syncytial virus infections. Clin Microbiol Rev. 1995;8:22–33. doi: 10.1128/cmr.8.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hildreth S W, Paradiso P. Immunogenicity and safety of respiratory syncytial virus subunit vaccine in seropositive children 18–36 months old. J Infect Dis. 1993;167:191–195. doi: 10.1093/infdis/167.1.191. [DOI] [PubMed] [Google Scholar]
- 88.Holberg C J, Wright A L, Martinez F D, Ray C G, Taussig L M, Leibowitz M D. Risk factors for respiratory syncytial virus-associated lower respiratory illnesses in the first year of life. Am J Epidemiol. 1991;133:1135–1151. doi: 10.1093/oxfordjournals.aje.a115826. [DOI] [PubMed] [Google Scholar]
- 89.Ikeda S, Neyts J, Verma S, Wickramasinghe A, Mohan P, DeClercq E. In vitro and in vivo inhibition of ortho- and paramyxovirus infections by a new class of sulfonic acid polymers interacting with virus-cell binding and/or fusion. Antimicrob Agents Chemother. 1994;38:256–259. doi: 10.1128/aac.38.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Isaacs D. Viral subunit vaccines. Lancet. 1991;337:1223–1224. doi: 10.1016/0140-6736(91)92893-7. [DOI] [PubMed] [Google Scholar]
- 91.Isaacs D, Bangham C R M, McMichael A J. Cell-mediated cytotoxic response to respiratory syncytial virus in infants with bronchiolitis. Lancet. 1987;2:769–771. doi: 10.1016/s0140-6736(87)92502-5. [DOI] [PubMed] [Google Scholar]
- 92.Jairath S, Vargas P B, Hamlin H A, Field A K, Kilkuskie R E. Inhibition of respiratory syncytial virus replication by antisense oligodeoxyribonucleotides. Antiviral Res. 1997;33:201–213. doi: 10.1016/s0166-3542(96)01015-7. [DOI] [PubMed] [Google Scholar]
- 93.Johnson P R, Spriggs M K, Olmsted R A, Collins P L. The G glycoprotein of human respiratory syncytial virus of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc Natl Acad Sci USA. 1987;84:5625–5629. doi: 10.1073/pnas.84.16.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kapikian A Z, Mitchell R H, Chanock R M, Shvedoff R A, Stewart C E. An epidemiologic study of altered reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 95.Kapikian A Z, Bell R J A, Mastrota F M, Johnson K M, Huebner R J, Chanock R M. An outbreak of febrile illness and pneumonia associated with respiratory syncytial virus infection. Am J Hyg. 1961;74:234–248. doi: 10.1093/oxfordjournals.aje.a120216. [DOI] [PubMed] [Google Scholar]
- 96.Karron R A, Buonagurio D A, Georgiu A F, Whitehead S S, Adamus J E, Clements-Mann M L, Harris D O, Randolph V B, Udem S A, Murphy B R, Sidhu M S. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc Natl Acad Sci USA. 1997;94:13961–13966. doi: 10.1073/pnas.94.25.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karron R A, Wright P F, Crowe J E, Jr, Clements-Mann M L, Thompson J, Makhene M, Casey R, Murphy B R. Evaluation of two live, cold passaged, temperature-sensitive respiratory syncytial virus (RSV) vaccines in chimpanzees, and in human adults, infants and children. J Infect Dis. 1997;176:1428–1436. doi: 10.1086/514138. [DOI] [PubMed] [Google Scholar]
- 98.Kim H W, Canchola J G, Brandt C D, Pyles G, Chanock R M, Jensen K, Parrott R H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 99.Kim H W, Arrobio J O, Brandt C D. Epidemiology of respiratory syncytial virus infection in Washington, D.C. I. Importance of the virus in different respiratory disease syndromes and temporal distribution of infection. Am J Epidemiol. 1973;98:216–225. doi: 10.1093/oxfordjournals.aje.a121550. [DOI] [PubMed] [Google Scholar]
- 100.Kim H W, Leikin S L, Arrobio J O, Brandt C D, Chanock R M, Parrott R H. Cell mediated immunity to respiratory syncytial virus induced by inactivated vaccine or by infection. Pediatr Res. 1976;10:75–78. doi: 10.1203/00006450-197601000-00015. [DOI] [PubMed] [Google Scholar]
- 101.Kimpen J L L, Garofalo R, Welliver R C, Ogra P L. Activation of human eosinophils in vitro by respiratory syncytial virus. Pediatr Res. 1992;32:160–164. doi: 10.1203/00006450-199208000-00007. [DOI] [PubMed] [Google Scholar]
- 102.Kimpen J L L, Garofalo R, Welliver R C, Fujihara K, Ogra P L. An ultrastructural study of the interaction of human eosinophils with respiratory syncytial virus. Pediatr Allergy Immunol. 1996;7:48–53. doi: 10.1111/j.1399-3038.1996.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 103.Klebanoff S J, Coombs R W. Virucidal effect of stimulated eosinophils on human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1996;12:25–29. doi: 10.1089/aid.1996.12.25. [DOI] [PubMed] [Google Scholar]
- 104.Krilov L R, Mandel F S, Barone S R. Follow-up of children with respiratory syncytial virus bronchiolitis in 1986 and 1987: potential effect of ribavirin on long term pulmonary function. Pediatr Infect Dis J. 1997;16:273–276. doi: 10.1097/00006454-199703000-00004. [DOI] [PubMed] [Google Scholar]
- 105.Krilov L R, Hendry R M, Godfrey E, McIntosh K. Respiratory virus infection of peripheral blood monocytes: correlation with aging of cells and interferon production in vitro. J Gen Virol. 1987;68:1749–1753. doi: 10.1099/0022-1317-68-6-1749. [DOI] [PubMed] [Google Scholar]
- 106.Lambert D M, Barney S, Lambert A L, Guthrie K, Medinas R, Bucy T, Erickson J, Merutka G, Petteway S R., Jr Peptides from conserved regions of paramyxovirus fusion (F) proteins are potent inhibitors of viral fusion. Proc Natl Acad Sci USA. 1996;93:2186–2191. doi: 10.1073/pnas.93.5.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Law, B., E. E. L. Wang, and N. MacDonald. 3 March 1997, posting date. Does ribavirin impact on the hospital course of children with respiratory syncytial virus infection? An analysis using the Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) RSV database. Pediatrics 99. [Online.] http://www.pediatrics.org/cgi/content/full/99/3/e7. [26 February 1999, last date accessed.] [DOI] [PubMed]
- 108.Levine S, Klaiber-Franco R, Paradiso P R. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J Gen Virol. 1987;68:2521–2524. doi: 10.1099/0022-1317-68-9-2521. [DOI] [PubMed] [Google Scholar]
- 109.Lewinsohn D M, Bowden R A, Mattson D, Crawford S W. Phase 1 study of intravenous ribavirin treatment of respiratory syncytial virus pneumonia after marrow transplantation. Antimicrob Agents Chemother. 1996;40:2555–2557. doi: 10.1128/aac.40.11.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Long C E, Voter K Z, Barker W, Hall C. Long term follow-up of children hospitalized with respiratory syncytial virus lower respiratory tract infection and randomly treated with ribavirin or placebo. Pediatr Infect Dis J. 1997;16:1023–1028. doi: 10.1097/00006454-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 111.Macasaet F F, Kidd P A, Balano C R. The etiology of acute respiratory infections. III. The role of viruses and bacteria. J Pediatr. 1968;72:829–839. doi: 10.1016/s0022-3476(68)80436-6. [DOI] [PubMed] [Google Scholar]
- 112.McConnochie K M, Hall C B, Walsh E E. Variation in severity of respiratory syncytial virus infection with subtype. J Pediatr. 1990;117:52–62. doi: 10.1016/s0022-3476(05)82443-6. [DOI] [PubMed] [Google Scholar]
- 113.McConnochie K M, Mark J D, McBride J T, Hall W J, Brooks J G. Normal pulmonary function measurements and airway reactivity in childhood after mild bronchiolitis. Pediatrics. 1985;107:54–58. doi: 10.1016/s0022-3476(85)80614-4. [DOI] [PubMed] [Google Scholar]
- 114.McIntosh K. Interferon from nasal secretions from infants with viral respiratory tract infections. J Pediatr. 1978;93:33–36. doi: 10.1016/s0022-3476(78)80595-2. [DOI] [PubMed] [Google Scholar]
- 115.McIntosh K, Fishaut J M. Immunopathologic mechanisms in lower respiratory tract disease of infants due to respiratory syncytial virus. Prog Med Virol. 1980;26:94–118. [PubMed] [Google Scholar]
- 116.McIntosh K, Masters H B, Orr I, Chao R K, Barkin R M. The immunologic response to infection with respiratory syncytial virus in infants. J Infect Dis. 1978;138:24–32. doi: 10.1093/infdis/138.1.24. [DOI] [PubMed] [Google Scholar]
- 117.McIntosh K, McQuillin J, Gardner P S. Cell-free and cell-bound antibody in nasal secretions from infants with respiratory syncytial virus infection. Infect Immun. 1979;23:276–281. doi: 10.1128/iai.23.2.276-281.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Meert K L, Sarnaik A P, Gelmini M J, Lieh-Lai M W. Aerosolized ribavirin in mechanically ventilated children with respiratory syncytial virus lower respiratory tract disease: a prospective, double blind, randomized trial. Crit Care Med. 1994;22:566–572. doi: 10.1097/00003246-199404000-00010. [DOI] [PubMed] [Google Scholar]
- 119.Meissner H C, Fulton D R, Groothius J R. Controlled trial to evaluate protection of high-risk infants against respiratory syncytial virus disease by using standard intravenous immune globulin. Antimicrob Agents Chemother. 1993;37:1655–1658. doi: 10.1128/aac.37.8.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Midulla F, Huang Y T, Gilbert I A, Cirino N M, McFadden E R, Jr, Panuska J R. Respiratory syncytial virus infection of human cord and adult blood monocytes and alveolar macrophages. Am Rev Respir Dis. 1989;140:771–777. doi: 10.1164/ajrccm/140.3.771. [DOI] [PubMed] [Google Scholar]
- 121.Milner M E, de la Monte S M, Hutchins G M. Fatal respiratory syncytial virus infection in severe combined immunodeficiency. Am J Dis Child. 1985;135:1111–1114. doi: 10.1001/archpedi.1985.02140130049028. [DOI] [PubMed] [Google Scholar]
- 122.Moler F W, Steinhart C M, Ohmit S E, Stidham G L the Pediatric Critical Care Study Group. Effectiveness of ribavirin in otherwise well infants with respiratory syncytial virus-associated respiratory failure. J Pediatr. 1996;128:422–428. doi: 10.1016/s0022-3476(96)70294-9. [DOI] [PubMed] [Google Scholar]
- 123.Mufson M A, Levine H D, Wasil R E. Epidemiology of respiratory syncytial virus infection among infants and children in Chicago. Am J Epidemiol. 1973;98:88–95. doi: 10.1093/oxfordjournals.aje.a121542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Muñoz J L, McCarthy C A, Clark M E. Respiratory syncytial virus infection in C57BL/6 mice: clearance of virus from the lungs with virus-specific cytotoxic T cell. J Virol. 1991;65:4494–4497. doi: 10.1128/jvi.65.8.4494-4497.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Murphy B R, Sotnikov A, Lawrence L, Banks S, Alling D, Prince G. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3–6 months after immunization. Vaccine. 1990;8:497–502. doi: 10.1016/0264-410x(90)90253-i. [DOI] [PubMed] [Google Scholar]
- 126.Murphy B R, Alling D W, Snyder M. Effect of age and preexisting antibody on serum antibody response of infants and children to the F and G glycoproteins during respiratory syncytial virus infection. J Clin Microbiol. 1986;24:894–898. doi: 10.1128/jcm.24.5.894-898.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Murphy B R, Prince G A, Walsh E E. Dissociation between serum neutralizing and glycoprotein antibody responses of infants and children who received inactivated respiratory syncytial virus vaccine. J Clin Microbiol. 1986;24:197–202. doi: 10.1128/jcm.24.2.197-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Murphy B R, Olmsted R A, Collins P L, Chanock R M, Prince G A. Passive transfer of respiratory syncytial virus (RSV) antiserum suppresses the immune response to the RSV fusion (F) and large (G) glycoproteins expressed by recombinant vaccinia viruses. J Virol. 1988;62:3907–3910. doi: 10.1128/jvi.62.10.3907-3910.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Murphy B R, Hall S L, Kulkarni A B, Crowe J E, Collins P L, Connors M, Karron R A, Chanock R M. An update on the approaches to the development of respiratory syncytial virus (RSV) and parainfluenza virus type 3 (PIV3) vaccines. Virus Res. 1994;32:13–36. doi: 10.1016/0168-1702(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 130.Neyts J, Reymen D, Letourneur D, Jozefonvicz J, Scols D, Este J, Adrei G, McKenna P, Witvrouw M, Ikeda S. Differential antiviral activity of derivatized dextrans. Biochem Pharmacol. 1995;50:743–751. doi: 10.1016/0006-2952(95)00193-4. [DOI] [PubMed] [Google Scholar]
- 131.Nicholas J A, Rubino K L, Levely M E. Cytolytic T-lymphocyte responses to respiratory syncytial virus: effector cell phenotype and target proteins. J Virol. 1990;64:4232–4241. doi: 10.1128/jvi.64.9.4232-4241.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nicholas J A, Rubino K L, Levely M E, Meyer A L, Collins P L. Cytotoxic T cell activity against the 22-kDa protein of human respiratory syncytial virus (RSV) is associated with a significant reduction in pulmonary RSV replication. Virology. 1991;182:664–672. doi: 10.1016/0042-6822(91)90607-d. [DOI] [PubMed] [Google Scholar]
- 133.Olmsted R A, Collins P L. The 1A protein of respiratory syncytial virus is an integral membrane protein present as multiple, structurally distinct species. J Virol. 1989;63:2019–2029. doi: 10.1128/jvi.63.5.2019-2029.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Opavsky M A, Stevens D, Wang E E. Testing models predicting severity of respiratory syncytial virus infection on the PICNIC RSV database. Arch Pediatr Adolesc Med. 1995;149:1217–1220. doi: 10.1001/archpedi.1995.02170240035005. [DOI] [PubMed] [Google Scholar]
- 135.Openshaw P J. Immunity and immunopathology to respiratory syncytial virus. The mouse model. Am J Respir Crit Care Med. 1995;152:59–62. doi: 10.1164/ajrccm/152.4_Pt_2.S59. [DOI] [PubMed] [Google Scholar]
- 136.Openshaw P J M, Anderson K, Wertz G W. The 22,000-kilodalton protein of respiratory syncytial virus is a major target for Kd-restricted cytotoxic T lymphocytes from mice primed by infection. J Virol. 1990;64:1683–1689. doi: 10.1128/jvi.64.4.1683-1689.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Padman R, Bye M R, Schidlow D V, Zaeri N. Severe RSV bronchiolitis in an immunocompromised child. Clin Pediatr. 1985;24:719–721. doi: 10.1177/000992288502401210. [DOI] [PubMed] [Google Scholar]
- 138.Panuska J R, Hertz M I, Taraf H, Villani A, Cirino N M. Respiratory syncytial virus infection of alveolar macrophages in adult transplant patients. Am Rev Respir Dis. 1992;145:934–939. doi: 10.1164/ajrccm/145.4_Pt_1.934. [DOI] [PubMed] [Google Scholar]
- 139.Paradiso P R, Hildreth S W, Hogerman D A, Speelman D J, Oren J, Smith D H. Safety and immunogenicity of a subunit respiratory syncytial virus vaccine in children 24–48 months old. Pediatr Infect Dis J. 1994;13:792–798. doi: 10.1097/00006454-199409000-00008. [DOI] [PubMed] [Google Scholar]
- 140.Parrot R H, Kim H W, Arrobio J O. Epidemiology of respiratory syncytial virus infection in Washington, D.C. II. Infection and disease with respect to age, immunologic status, race and age. Am J Epidemiol. 1973;98:289–300. doi: 10.1093/oxfordjournals.aje.a121558. [DOI] [PubMed] [Google Scholar]
- 141.Pemberton R M, Cannon M J, Openshaw P J. Cytotoxic T cell specificity for respiratory syncytial virus proteins: Fusion protein is an important target antigen. J Gen Virol. 1987;68:2177–2182. doi: 10.1099/0022-1317-68-8-2177. [DOI] [PubMed] [Google Scholar]
- 142.Piedra P A, Grace S, Jewell A, Spinelli S, Bunting D, Hogerman D A, Malinoski F, Hiatt P W. Purified fusion protein vaccine protects against lower respiratory tract illness during respiratory syncytial virus season in children with cystic fibrosis. Pediatr Infect Dis J. 1996;15:23–31. doi: 10.1097/00006454-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 143.PREVENT Study Group. Reduction of respiratory syncytial virus hospitalization among premature infants and infant with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics. 1997;99:93–99. doi: 10.1542/peds.99.1.93. [DOI] [PubMed] [Google Scholar]
- 144.Prince G A, Jensen B A, Hemming V G. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactivated virus. J Virol. 1986;57:721–728. doi: 10.1128/jvi.57.3.721-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Prince G A, Hemming V G, Horswood R L, Chanock R M. Immunoprophylaxis and immunotherapy of respiratory syncytial virus infection in the cotton rat. Virus Res. 1985;3:193–206. doi: 10.1016/0168-1702(85)90045-0. [DOI] [PubMed] [Google Scholar]
- 146.Prober C G, Wang E E L. Reducing the morbidity of lower respiratory tract infections caused by respiratory syncytial virus: still no answer. Pediatrics. 1997;99:472–475. doi: 10.1542/peds.99.3.472. [DOI] [PubMed] [Google Scholar]
- 147.Randolph A G, Wang E E L. Ribavirin for respiratory syncytial virus lower respiratory tract infection: a systematic overview. Arch Pediatr Adolesc Med. 1996;150:942–947. doi: 10.1001/archpedi.1996.02170340056011. [DOI] [PubMed] [Google Scholar]
- 148.Richardson L S, Belshe R B, Sly D L. Experimental respiratory syncytial virus pneumonia in cebus monkeys. J Med Virol. 1978;2:45–59. doi: 10.1002/jmv.1890020108. [DOI] [PubMed] [Google Scholar]
- 149.Richardson L S, Yolken R H, Belshe R B. Enzyme linked immunosorbant assay for measurement of serologic response to respiratory syncytial virus infection. Infect Immun. 1978;23:660–664. doi: 10.1128/iai.20.3.660-664.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rimensberger P C, Schaad U B. Clinical experience with aerosolized immunoglobulin treatment of respiratory syncytial virus infection in infants. Pediatr Infect Dis J. 1994;13:328–330. doi: 10.1097/00006454-199404000-00018. [DOI] [PubMed] [Google Scholar]
- 151.Rimensberger P C, Burek-Kozlowska A, Morell A. Aerosolized immunoglobulin treatment of respiratory syncytial virus infection in infants. Pediatr Infect Dis J. 1996;15:209–216. doi: 10.1097/00006454-199603000-00007. [DOI] [PubMed] [Google Scholar]
- 152.Rodriguez W J, Kim H W, Brandt C D. Aerosolized ribavirin in the treatment of patients with respiratory syncytial virus disease. Pediatr Infect Dis J. 1987;6:159–163. doi: 10.1097/00006454-198702000-00004. [DOI] [PubMed] [Google Scholar]
- 153.Rodriguez W J, Gruber W, Welliver R C, Groothius J R, Simoes E A F, Meissner H C, Hemming V G, Hall C B, Lepow M L, Rosas A J, Robertsen C, Kramer A A for the Respiratory Syncytial Virus Immune Globulin Study Group. Respiratory syncytial virus (RSV) immunoglobulin therapy for RSV lower respiratory tract infection in infants and young children at high risk for severe RSV infections. Pediatrics. 1997;99:454–461. doi: 10.1542/peds.99.3.454. [DOI] [PubMed] [Google Scholar]
- 154.Rosner I, Welliver R C, Edelson P, Geraci-Ciardullo K, Sun M. Effect of ribavirin therapy on RSV-specific IgE and IgA responses after infection. J Infect Dis. 1987;155:1043–1047. doi: 10.1093/infdis/155.5.1043. [DOI] [PubMed] [Google Scholar]
- 155.Saito, T., R. W. Deskin, A. Casola, H. Haeberle, B. Olszewska, P. B. Ernst, R. Alam, P. L. Ogra, and R. Garofalo. 1997. Respiratory syncytial virus induces selective production of the chemokine RANTES by upper airway epithelial cells. 175:497–504. [DOI] [PubMed]
- 156.Shigeta H, Hinuma Y, Suto T. The cell to cell infection of respiratory syncytial virus in HEp-2 monolayer cultures. J Gen Virol. 1968;3:129–131. doi: 10.1099/0022-1317-3-1-129. [DOI] [PubMed] [Google Scholar]
- 157.Siber G R, Leombruno R D, Leszczynski J. Comparison of antibody concentrations and protective activity of respiratory syncytial virus immune globulin and conventional immune globulin. J Infect Dis. 1994;169:1368–1373. doi: 10.1093/infdis/169.6.1368. [DOI] [PubMed] [Google Scholar]
- 158.Sigurs N, Bjarnason R, Sigurbergsson F. Eosinophil cationic protein in nasal secretion and in serum and myeloperoxidase in serum in respiratory syncytial virus bronchiolitis: relation to asthma and atopy. Acta Paediatr. 1994;83:1151–1155. doi: 10.1111/j.1651-2227.1994.tb18269.x. [DOI] [PubMed] [Google Scholar]
- 159.Simoes E A F, Sondheimer H M, Meissner H C. Respiratory syncytial virus immunoglobulin as prophylaxis against respiratory syncytial virus in children with congenital heart disease. Pediatr Res. 1996;662:113A. doi: 10.1016/s0022-3476(98)70056-3. . (Abstract.) [DOI] [PubMed] [Google Scholar]
- 160.Smith D W, Frankel L R, Mathers L H, Tang A T S, Ariaggno R L, Prober C G. A controlled trial of aerosolized ribavirin in infants receiving mechanical ventilation for severe respiratory syncytial virus infection. N Engl J Med. 1991;325:24–29. doi: 10.1056/NEJM199107043250105. [DOI] [PubMed] [Google Scholar]
- 161.Srikiatkhachorn A, Braciale T J. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med. 1997;186:421–432. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Stark J M, Godding V, Sedgewick J B, Busse W W. Respiratory syncytial virus infection enhances neutrophil and eosinophil adhesion to cultured respiratory epithelial cells. J Immunol. 1996;156:4774–4782. [PubMed] [Google Scholar]
- 163.Sterner G, Wolontis S, Bloth B, DeHevesy G. Respiratory syncytial virus: an outbreak of acute respiratory illnesses in a home for infants. Acta Paedriatr Scand. 1966;55:273–279. doi: 10.1111/j.1651-2227.1966.tb17654.x. [DOI] [PubMed] [Google Scholar]
- 164.Suffin S C, Prince G A, Muck K B, Porter D D. Immunoprophylaxis of respiratory syncytial virus in the infant ferret. J Immunol. 1979;123:10–14. [PubMed] [Google Scholar]
- 165.Taber L H, Knight V, Gilbert B E. Ribavirin aerosol treatment of bronchiolitis associated with respiratory syncytial virus infection in infants. Pediatrics. 1983;72:613–618. [PubMed] [Google Scholar]
- 166.Taylor C E, Craft A W, Kernahan J. Local antibody production and respiratory syncytial virus infection in children with leukemia. J Med Virol. 1990;30:277–281. doi: 10.1002/jmv.1890300409. [DOI] [PubMed] [Google Scholar]
- 167.Taylor C E, Morrow S, Scott M. Comparative virulence of respiratory syncytial virus subgroups A and B. Lancet. 1989;i:777–778. doi: 10.1016/s0140-6736(89)92592-0. [DOI] [PubMed] [Google Scholar]
- 168.Taylor G, Furze J, Tempest P R. Humanized monoclonal antibody to respiratory syncytial virus. Lancet. 1991;337:1411–1422. doi: 10.1016/0140-6736(91)93091-m. [DOI] [PubMed] [Google Scholar]
- 169.Taylor G, Scott E J, Hayle A J. Cytotoxic lymphocytes in the lungs of mice infected with respiratory syncytial virus. J Gen Virol. 1985;66:2533–2588. doi: 10.1099/0022-1317-66-12-2533. [DOI] [PubMed] [Google Scholar]
- 170.Torrence P F, Xiao W, Li G, Cramer H, Player M R, Silverman R H. Recruiting the 2-5A system for antisense therapeutics. Antisense Nucleic Acid Drug Dev. 1997;7:203–206. doi: 10.1089/oli.1.1997.7.203. [DOI] [PubMed] [Google Scholar]
- 171.Tristram D A, Welliver R C, Hicks W L, Jr, Hard R. Ciliated human respiratory epithelium: a new model for respiratory syncytial virus (RSV) infection. Pediatr Res. 1997;41:131A. . (Abstract.) [Google Scholar]
- 172.Tristram D A, Welliver R C, Hogerman D A, Hildreth S W, Paradiso P. Second-year surveillance of recipients of a respiratory syncytial virus (RSV) F protein subunit vaccine, PFP-1: evaluation of antibody persistence and possible disease enhancement. Vaccine. 1994;12:551–556. doi: 10.1016/0264-410x(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 173.Tristram D A, Welliver R C, Mohar K, Hogerman D A, Hildreth S W, Paradiso P. Immunogenicity and safety of respiratory syncytial virus subunit vaccine in seropositive children 18–36 months old. J Infect Dis. 1993;167:191–195. doi: 10.1093/infdis/167.1.191. [DOI] [PubMed] [Google Scholar]
- 174.VanAerschot A A, Mamos P, Weyns N J, Ikeda S, DeClercq E, Herdewijn P A. Antiviral activity of C-alkylated purine nucleosides obtained by cross-coupling with tetraalkylating reagent. J Med Chem. 1993;36:2938–2942. doi: 10.1021/jm00072a013. [DOI] [PubMed] [Google Scholar]
- 175.Volovitz B, Faden H, Ogra P L. Release of leukotriene C4 in respiratory tract during acute viral infection. J Pediatr. 1988;112:218–222. doi: 10.1016/s0022-3476(88)80058-1. [DOI] [PubMed] [Google Scholar]
- 176.Walsh E E, McConnochie K M, Long C E, Hall C B. Severity of respiratory syncytial virus infection is related to virus strain. J Infect Dis. 1997;175:814–820. doi: 10.1086/513976. [DOI] [PubMed] [Google Scholar]
- 177.Walsh E E, Hruska J. Monoclonal antibodies to respiratory syncytial virus proteins: identification of the fusion protein. J Virol. 1983;47:171–177. doi: 10.1128/jvi.47.1.171-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Walsh E E, Schlesinger J J, Brandriss M W. Purification and characterization of GP90, one of the enveloped glycoproteins of respiratory syncytial virus. J Gen Virol. 1984;65:761–767. doi: 10.1099/0022-1317-65-4-761. [DOI] [PubMed] [Google Scholar]
- 179.Wang E E L, Law B J, Boucher F D. Pediatric investigators collaborative network in infections in Canada (PICNIC) study of admission and management variation in patients hospitalized with respiratory syncytial lower respiratory tract infection. J Pediatr. 1996;129:390–395. doi: 10.1016/s0022-3476(96)70071-9. [DOI] [PubMed] [Google Scholar]
- 180.Wathen M W, Aeed P A, Elhammer A P. Characterization of oligosaccharide structures on a chimeric respiratory syncytial virus protein expressed in insect cell line Sf9. Biochemistry. 1991;30:2863–2868. doi: 10.1021/bi00225a019. [DOI] [PubMed] [Google Scholar]
- 181.Watt P J, Zardis M, Lambden P R. Age related IgG subclass response to respiratory syncytial virus fusion protein in infected infants. Clin Exp Immunol. 1986;64:503–509. [PMC free article] [PubMed] [Google Scholar]
- 182.Weiss S T, Tager I B, Speizer F E, Rosner B. Persistent wheeze: its relation to respiratory illness, cigarette smoking, and level of pulmonary function in a population sample of children. Am Rev Respir Dis. 1980;122:697–707. doi: 10.1164/arrd.1980.122.5.697. [DOI] [PubMed] [Google Scholar]
- 183.Welliver R. The relationship of RSV-specific immunoglobulin E antibody responses in infancy, recurrent wheezing, and pulmonary function at age 7–8 years. Pediatr Pulmonol. 1993;15:19–27. doi: 10.1002/ppul.1950150104. [DOI] [PubMed] [Google Scholar]
- 184.Welliver R C, Wong D T, Sun M, Middleton E, Vaughn R S, Ogra P L. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841–846. doi: 10.1056/NEJM198110083051501. [DOI] [PubMed] [Google Scholar]
- 185.Welliver R C, Kaul L A, Ogra P L. Cell-mediated immune response to respiratory syncytial virus infection: relationship to the development of reactive airway disease. J Pediatr. 1979;94:370–375. doi: 10.1016/s0022-3476(79)80573-9. [DOI] [PubMed] [Google Scholar]
- 186.Welliver R C, Kaul T N, Sun M, Ogra P L. Defective regulation of immune responses in respiratory syncytial virus infection. J Immunol. 1984;133:1925–1930. [PubMed] [Google Scholar]
- 187.Welliver R C, Kaul T N, Ogra P L. The appearance of cell bound IgE in respiratory tract epithelium after respiratory syncytial virus infection. N Engl J Med. 1980;303:1198–1202. doi: 10.1056/NEJM198011203032103. [DOI] [PubMed] [Google Scholar]
- 188.Whimbey E, Champlin R E, Englund J A, Mirza N Q, Piedra P A, Goodrich J M, Przepiorka D, Luna M A, Morice R C, Neumann J L. Combination therapy with aerosolized ribavirin and intravenous immunoglobulin for respiratory syncytial virus disease in adult bone marrow transplant recipients. Bone Marrow Transplant. 1995;16:393–399. [PubMed] [Google Scholar]
- 189.Wheeler J G, Wofford J, Turner R B. Historical cohort evaluation of ribavirin efficacy in respiratory syncytial virus infection. Pediatr Infect Dis J. 1993;12:209–213. doi: 10.1097/00006454-199303000-00007. [DOI] [PubMed] [Google Scholar]
- 190.Wyde P R, Moore D K, Hepburn T, Silverman C L, Porter T G, Gross M, Taylor G, Demuth S G, Dillon S B. Evaluation of the protective efficacy of reshaped human monoclonal antibody RSHZ19 against respiratory syncytial virus in cotton rats. Pediatr Res. 1995;38:543–550. doi: 10.1203/00006450-199510000-00012. [DOI] [PubMed] [Google Scholar]
- 191.Wyde P R, Moore D K, Pimentel D M, Blough H A. Evaluation of the antiviral activity of N-(phosphonoacetyl)-l-aspartate against paramyxoviruses in tissue culture and against respiratory syncytial virus in cotton rats. Antiviral Res. 1995;27:59–69. doi: 10.1016/0166-3542(94)00080-r. [DOI] [PubMed] [Google Scholar]
- 192.Wyde P R, Moore D K, Pimentel D M, Gilbert B E, Nimrod R, Panet A. Recombinant superoxide dismutase (SOD) administered by aerosol inhibits respiratory syncytial virus infection in cotton rats. Antiviral Res. 1996;31:173–184. doi: 10.1016/0166-3542(95)06967-4. [DOI] [PubMed] [Google Scholar]
- 193.Wyde P R, Ambrose M W, Meyerson L R, Gilbert B E. The antiviral activity of SP-303, a natural polyphenolic polymer, against respiratory syncytial and parainfluenza type 3 viruses in cotton rats. Antiviral Res. 1993;20:145–154. doi: 10.1016/0166-3542(93)90004-3. [DOI] [PubMed] [Google Scholar]
- 194.Yu Q, Hardy R W, Wertz G W. Functional cDNA clones of the human respiratory syncytial (RS) virus N, P, and L proteins support replication of RS virus genomic RNA analogs and define minimal trans-acting requirements for RNA replication. J Virol. 1995;69:2412–2419. doi: 10.1128/jvi.69.4.2412-2419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]