Abstract
Background: The current COVID-19 pandemic has put millions of people, especially children at risk of protein-energy malnutrition (PEM) by pushing them into poverty and disrupting the global food supply chain. The thymus is severely affected by nutritional deficiencies and is known as a barometer of malnutrition. Aim: The present commentary provides a novel perspective on the role of malnutrition-induced thymic dysfunction, involution and atrophy on the risk and severity of disease in children during the COVID-19 pandemic. Methods: A review of pertinent indexed literature including studies examining the effects of malnutrition on the thymus and immune dysfunction in COVID-19. Results: Protein-energy malnutrition and micronutrient deficiencies of zinc, iron and vitamin A are known to promote thymic dysfunction and thymocyte loss in children. Malnutrition- and infection-induced thymic atrophy and immune dysfunction may increase the risk of first, progression of COVID-19 disease to more severe forms including development of multisystem inflammatory syndrome in children (MIS-C); second, slow the recovery from COVID-19 disease; and third, increase the risk of other infections. Furthermore, malnourished children may be at increased risk of contracting SARS-CoV-2 infection due to socioeconomic conditions that promote viral transmission amongst contacts and create barriers to vaccination. Conclusion: National governments and international organizations including WHO, World Food Program, and UNICEF should institute measures to ensure provision of food and micronutrients for children at risk in order to limit the health impact of the ongoing COVID-19 pandemic.
Keywords: COVID-19, MIS-C, protein-calorie malnutrition, thymic atrophy, lymphocytopenia, micronutrients, zinc, starvation
What is known?
Protein-energy and micronutrient deficiencies in children induce thymic dysfunction and atrophy leading to immune suppression.
During the COVID-19 pandemic, rising poverty and disruption in global food supply chain promote malnutrition amongst children in developing countries.
What is new?
Malnutrition associated with the COVID-19 pandemic can induce thymic atrophy in children which may increase the risk of severe COVID-19 disease and mortality.
Ensuring adequate food and micronutrient supplies for children can help limit the impact on thymic health, and thereby on morbidity and mortality during this pandemic.
Introduction
The social impact of the COVID-19 pandemic has devastated the lives of millions, especially those from poor socio-economic background living in low- and middle-income countries (LMIC). The World Bank reports that the COVID-19 pandemic has pushed 97 million people globally into extreme poverty, measured at the international poverty line at $1.90 a day, while an additional 163 million people are living on less than $5.50 a day since before the pandemic (World Bank, 2021). Furthermore, economic recessions especially in Sub-Saharan Africa (Mahler et al., 2020) and increasing out-of-pocket health care costs during the pandemic have contributed to more than half a billion people being pushed into extreme poverty (WHO, 2021a).
Rising poverty rates and the economic impact from social distancing, full or partial lockdowns and quarantining raise the growing concern of malnutrition, especially in children during the COVID-19 pandemic (Huizar et al., 2021). UNICEF has reported that the COVID-19 pandemic has pushed 100 million children into poverty and hunger, and limited access to healthcare, vaccines and essential services for kids (UNICEF, 2021). Poverty rates of those aged 17 and under are estimated to have jumped by 10% since the start of 2020 and were projected to rise to 20% in a number of countries (Mahler et al., 2020).
Millions of children globally receive micronutrient powders comprising iron, vitamin A and zinc at points-of-use including homes, schools, nurseries and refugee camps (WHO, 2021b). This pandemic has exacerbated child hunger and malnutrition, forcing more than 1.6 billion children out of school in 199 countries, thereby depriving nearly 370 million children in 150 countries access to nutritious meals (US Global Leadership Coalition, 2021). It has been projected that by 2022, COVID-19 would cause an additional 9.3 million wasted children, 2.6 million stunted children, 168,000 additional child-deaths and 2.1 million children born to women with low body mass index (Osendarp et al., 2020).
Poor hygienic conditions, overcrowding, and lack of personal protective equipment in LMIC contribute to higher risk of contracting SARS-CoV-2 infection (unicef, 2020). Therefore, children from a low socio-economic background are at increased risk of both malnutrition and exposure to SARS-CoV-2 infection. In a multivariable random intercept logistic regression model from malnutrition and COVID-19 data in the United States, children with a history of malnutrition are at increased risk of severe COVID-19 compared to children without a history of malnutrition (Kurtz et al., 2021). Malnutrition is known to induce thymic atrophy and immune dysfunction in children, possibly contributing to increased risk of morbidity and mortality from COVID-19. In this review we highlight the potential health risks to socioeconomically disadvantaged children during the current pandemic and its implications for the society at large.
A. Protein-energy malnutrition, thymic atrophy and immune dysfunction
More than 12 million children die every year due to infections often complicated by concomitant malnutrition and cachexia (Chevalier et al., 1996). It is well known that malnutrition or protein-energy malnutrition (PEM) compromises the host immune response to infections. PEM and micronutrient deficiencies during the SARS-CoV-2 pandemic can have acute and long-term impact on the immune system as described hereunder.
Involution and atrophy of the thymus gland is closely related to pediatric malnutrition, hence the nickname, barometer of malnutrition (Prentice, 1999). Malnutrition with deficient intake of proteins, minerals or vitamins leads to thymic dysfunction, involution and atrophy secondary to apoptosis and reduced proliferation of thymocytes, particularly immature CD4+CD8+ cells.
PEM associated thymic dysfunction is characterized by decreased serum thymulin, a nonapeptide produced by epithelial cells in the thymus involved in T-cell differentiation and enhancement of T and NK cell actions (Bach et al., 1977; Jambon et al., 1988). A decrease in thymic thymulin levels occurs in zinc deficiency and accounts at least partly for the immune dysfunction associated with zinc deficiency (Dardenne and Pleau, 1994). However, PEM-associated thymulin deficiency independent of zinc has also been reported in 58 malnourished Senegalese children who died of infection (Jambon et al., 1988). Malnutrition and wasting inhibits thymocyte proliferation, and causes massive thymocyte death, especially the loss of immature CD4 + CD8 + thymocytes (Savino et al., 2007). In severely malnourished Bolivian children, there were a significantly higher proportion of circulating immature T lymphocytes, a lower proportion of mature T lymphocytes, and severe involution of the thymus, all of which recovered in two months with diet rehabilitation (Chevalier et al., 1996). In other studies, severe thymic atrophy was found in the necropsies of 19 malnourished children and in 18 infants with marasmus (Lyra et al., 1993; Ruhräh, 1903).
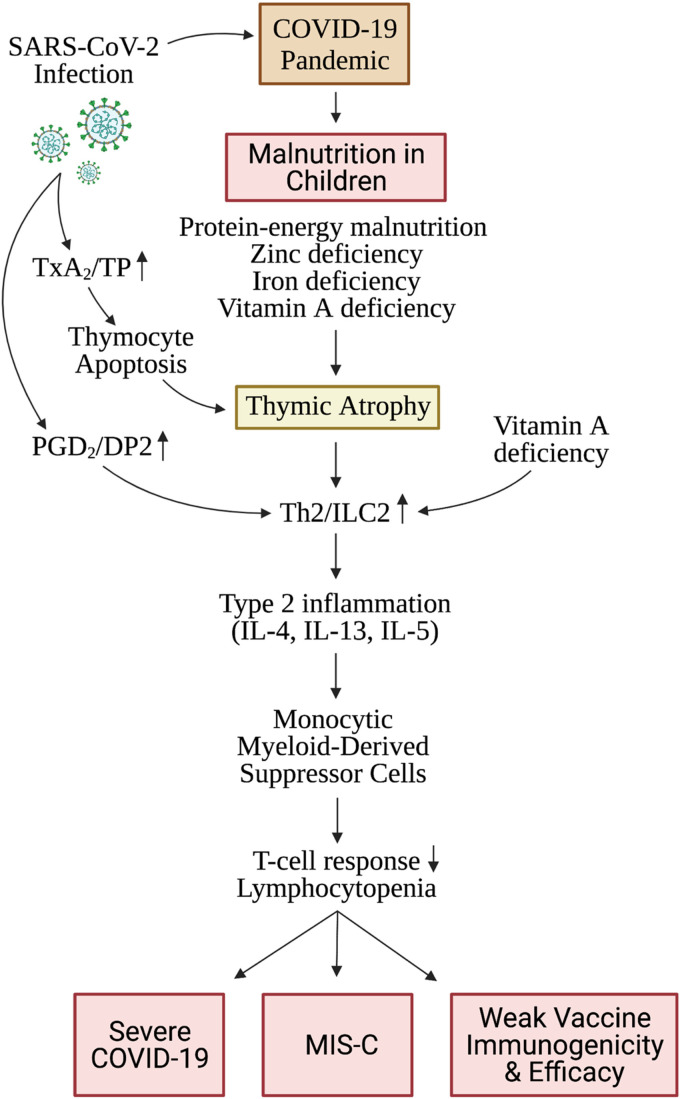
Malnutrition-induced thymic involution is associated with increased expression of prostaglandin D2 (PGD2) in the thymus, which leads to polarization of naïve T cells to a T helper 2 (Th2)-like phenotype and upregulation of type 2 innate lymphoid cells (ILC2) (Razali et al., 2020; Wilhelm et al., 2016). ILC2 release of type 2 cytokines including IL-13 and IL-4 enacts commitment of early thymic progenitors to the myeloid rather than the T cell lineage (Barik et al., 2017), and expansion of monocytic myeloid-derived suppressor cells (MDSC), which play a role in immunosuppression and lymphocytopenia (Figure 1) (Arima and Fukuda, 2011; Xue et al., 2005; Trabanelli et al., 2017). PEM-induced thymic atrophy leads to lymphocytopenia (Figure 1). In fact, PEM is the most common cause of lymphocytopenia worldwide (Long and Vodzak, 2018).
Figure 1.
Proposed mechanisms of malnutrition- and SARS-CoV-2 infection-induced thymic atrophy and maladaptive immune responses: The COVID-19 pandemic has contributed to increasing protein-energy malnutrition and micronutrient deficiencies in children. SARS-CoV-2 infection is associated with an inflammatory lipid mediator storm with a predominance of arachidonic acid metabolites, notably TxB2 >> PGD2. TxA2/TP signaling induces thymocyte apoptosis and thymic atrophy. PGD2/DP2 signaling promotes polarization of the immune response to a Th2/ILC2 phenotype leading to type 2 inflammation characterized by elevation in IL-4, IL-5 and IL-13. IL-13 induces expansion of monocytic myeloid-derived suppressor cells leading to suppression of T cell responses and lymphocytopenia. Malnutrition induces premature thymic atrophy which further promotes maladaptive type 2 immune responses and immune suppression. TxA2, thromboxane A2; TP, thromboxane prostanoid receptor; PGD2, prostaglandin D2; DP2, prostaglandin D2 receptor 2; Th2, T helper 2; ILC2, type 2 innate lymphoid cells; IL, interleukin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019; MIS-C, multisystem inflammatory syndrome in children. (created with BioRender.com).
We postulate that SARS-CoV-2 infection of malnourished children with thymic dysfunction increases risk of progression to severe disease including development of multisystem inflammatory syndrome in children (MIS-C) as discussed below.
B. Malnutrition in children and risk of severe COVID-19 and MIS-C
Effective control of viral infections requires the host adaptive T cell immune response. Though lymphocytosis is considered a characteristic feature, some viral infections such as tuberculosis, HIV, viral hepatitis, SARS-CoV, and most recently SARS-CoV-2 are known to cause lymphocytopenia lasting for months and potentially years following the acute infection (Zhou et al., 2020; Diao et al., 2020; Warny et al., 2018; He et al., 2005; Herbinger et al., 2016). Lymphocytopenia is defined as the number of peripheral lymphocytes in the blood totaling under 1000/µL in adults and 1500/µL in children (Diao et al., 2020; Davids; Coates).
Lymphocytopenia, a common characteristic feature of moderate to severe COVID-19, is a determinant of morbidity and mortality. Lymphocytopenia has been observed in approximately 1/3 of children with COVID-19 (Zhu et al., 2020; Chang et al., 2020). About 10% of children with SARS-CoV-2 infection have severe lymphocytopenia defined as lymphocyte counts <1000/µL, similar to the prevalence of lymphocytopenia in children with SARS-CoV 2003 infection (Chang et al., 2020). In a meta-analysis of 371 children with mild COVID-19, lymphocytopenia/leukocytopenia was present in about 30% (Ding et al., 2020), while about 75% amongst 186 children with MIS-C had lymphocytopenia (Feldstein et al., 2020). Therefore, lymphocytopenia seems to correlate with severity of SARS-CoV-2 infection in children. The acute phase of MIS-C is characterized by differential T and B cell subset lymphocytopenia along with elevated levels of proinflammatory cytokines (Carter et al., 2020). The five-year observational study called “Long-TerM Outcomes after the Multisystem Inflammatory Syndrome in Children: MUSIC” should clarify the cause and effect relationship between nutrition status, lymphocytopenia and course/severity of this disease (Study, 2020).
Acute 2003 SARS-CoV infection was associated with lymphocytopenia which was prolonged for up to two years in a minority of patients (Li et al., 2006). In approximately 7–12% of ‘long-haul’ COVID-19 patients (Mandal et al., 2021; Varghese et al., 2021), lymphocytopenia appears to persist throughout a 40- to 50-day period follow-up, despite clinical recovery and gradual normalization of IL-6 and IL-8 levels (Zhang et al., 2020). Any protracted lymphocytopenia in children with COVID-19 may increase the risk of contracting other infections after recovery from acute COVID-19 (Warny et al., 2018; Li et al., 2006; Zhou et al., 2020). A longitudinal meta-analysis of 98,344 individuals found to have lymphocytopenia unrelated to coronavirus infections had a 1.4-fold increased risk of hospitalization with infection and a 1.7-fold increased risk of infection-related death in the general population (Warny et al., 2018).
C. Nutritional deficiencies associated with thymic atrophy
Thymocyte depletion, characterized by loss of immature CD4+CD8+ cells (Savino, 2002), is a recurrent finding during both acute and chronic malnutrition, secondary to diets deficient in protein, vitamins, or metal elements including zinc, magnesium and iron as discussed below. (Chandra, 1992; Kuvibidila et al., 1990; Malpuech-Brugère et al., 1999).
Protein-energy malnutrition (PEM)
It is well established that severe forms of PEM lead to immune deficiency which increases susceptibility to infections, and is associated with high mortality observed in malnourished subjects, particularly in the young (Suskind, 1988; Chandra, 1983). A histological study of the thymuses from 234 fetuses and young children who died after a short period of acute illness revealed significant correlation between acute thymus involution and duration of acute illness (van Baarlen et al., 1988). Thymus dysfunction and impaired immunity was characterized by macrophages in the cortex, increase of interlobular interstitium and lymphodepletion of the cortex (van Baarlen et al., 1988). Although all compartments of the immune system may be affected by malnutrition, the effect on cell-mediated immunity (CMI) is most profound (Parent et al., 1994).
Thymus appears to be a key mediator of PEM-induced dysfunction in CMI (Chandra, 1979; Haroun, 2018). Severe thymic atrophy with cortical thymocyte depletion is a consistent finding in necropsies of malnourished subjects (Chandra, 1992; Lyra et al., 1993). Indeed, by means of echography, atrophy of the organ was also observed in vivo in severely malnourished children (Parent et al., 1994). In a cross-sectional study of 366 children aged 6–59 months with severe acute malnutrition, thymus size positively correlated with current breastfeeding and anthropometric measurements, and negatively correlated with >2 weeks duration of sickness (Nabukeera-Barungi et al., 2021). PEM-related thymocyte depletion seems to result from both decreased thymocyte proliferation and enhanced thymocyte death. Energy-depletion associated with malnutrition may lead to thymocyte loss due to high energy requirements for thymocyte generation (Domínguez-Gerpe and Rey-Méndez, 2003; Harenberg et al., 2005). Conversely, nutritional rehabilitation restores thymic health and immunity (Chevalier et al., 1996).
The thymus is the site of T cell differentiation and maturation by direct cell to cell contact at the level of the epithelial network, and/or through secretion of lymphocyte-differentiating hormones by epithelial cells (Purtilo and Connor, 1975; Bach, 1979; Jambon et al., 1981). Thymic involution in severely malnourished children is associated with a high degree of T lymphocyte immaturity (Parent et al., 1994). In vivo, the main disorders are delayed cutaneous hypersensitivity test responses, depressed responses to T-specific mitogens, and modifications in circulating T lymphocyte populations (Whitehead, 1981; McMurray, 1984; Chandra, 1979; Keusch et al., 1987; Parent et al., 1994). Therefore, PEM-induced thymocyte depletion and immune dysfunction may increase the risk of severe disease and MIS-C in children with COVID-19.
Zinc
Zinc is an important micronutrient for supporting thymic function (Dardenne and Pleau, 1994). Zinc deficiency causes thymic involution, atrophy, and lymphocytopenia, especially in the young (Rytter et al., 2017; Wong et al., 2009; Golden et al., 1977), occurring in 17% to 20% of residents of Sub-Sahara Africa and Asia (Wessells and Brown, 2012).
Zinc converts thymulin into its active form and is important for thymulin's biological activity (Dardenne et al., 1982; Dardenne and Pleau, 1994). Reduced serum thymulin levels in both zinc deficient mice and humans is corrected by zinc supplementation (Prasad et al., 1988; Dardenne and Pleau, 1994) which increases the number of thymocytes by about 50% (Wong et al., 2009). African children with severe acute malnutrition and thymic atrophy exhibit sluggish rates of thymic recovery on nutrient deficient diets (Rytter et al., 2017). Therefore, zinc supplementation in zinc deficient and malnourished children can promote thymopoiesis and thymic recovery.
Consistent with the above, zinc deficiency results in impaired host immune response and increases susceptibility to infection (Fraker et al., 2000; Warny et al., 2018). Zinc-deficient mice infected with T.musculi infection exhibited a delay in production of protective antibodies and harbored >3 times as many parasites as control mice (Lee et al., 1983; Shankar and Prasad, 1998). This is likely due to impaired B cell activation from T cell deficiency during thymic atrophy. Therefore, zinc repletion may be necessary to ensure adequate immune response to SARS-CoV-2 infection and prevent superinfection (Figure 1).
Iron
Iron promotes thymic health by binding to transferrin receptors on thymocytes and promoting thymocyte proliferation and differentiation (Bowlus, 2003). Iron deficiency and iron deficiency anemia is present in more than 50% of children in some Sub-Saharan African countries (Lemoine and Tounian, 2020), and may contribute to thymic dysfunction and atrophy. Iron supplementation can restore immune function and decrease morbidity and mortality (Lemoine and Tounian, 2020).
Vitamin A
Vitamin A deficiency is common in prolonged PEM due to poor dietary intake and defective storage and transport of vitamin A (Version, 2020). Vitamin A deficiency is also endemic in areas such as southern and eastern Asia, where rice, devoid of β-carotene, is the staple food (Version, 2020).
Vitamin A is important for normal immune function, and vitamin A deficiency is associated with profound defects in adaptive immunity and thereby infections (Green and Mellanby, 1928; El-Zayat et al., 2018). Hence, vitamin A has been described as “the anti-infective vitamin” (Green and Mellanby, 1928; El-Zayat et al., 2018; Wilhelm et al., 2016).
β-carotene, a precursor for vitamin A, promotes thymus growth and lymphocyte blastogenesis (Seifter et al., 1981; Alexander et al., 1985; Chew and Park, 2004). On the other hand, vitamin A deficiency promotes ILC2s expansion with selective IL-13 production via increased acquisition and utilization of fatty acids (Spencer et al., 2014; Wilhelm et al., 2016), leading to immunosuppression. Therefore, vitamin A deficiency is detrimental to thymic regeneration and adaptive immune responses (Figure 1). Interestingly, severe COVID-19 is also associated with polarization of the immune response from a Th1 to a Th2 response characterized by increased plasma type 2 cytokines produced by Th2 and ILC2 cells, including IL-4, IL-13 and IL-5 (Yang et al., 2020; Lucas et al., 2020; Perlman, 2020). This raises the possibility that vitamin A deficiency can further promote the maladaptive immune response in severe COVID-19.
D. Malnutrition and response to vaccines
Decreased thymic function may impair response to vaccines and reduce duration of immunity (Al-Sukaiti et al., 2010). In fact, thymic function may help predict a patient's response to vaccines (Kellogg and Equils, 2020), since CD4+ T cell population correlates with response to vaccination (Lewin et al., 2002; Roux et al., 2000).
In children with impaired response to vaccination, thymic health and function should be taken into consideration. Patients with insufficient thymic response may better respond to high dose or adjuvanted vaccines (Lee et al., 2018; Tregoning et al., 2018).
E. Viral infections associated with thymic atrophy
Viral, bacterial, parasitic or fungal infections are associated with pathological thymic atrophy (Luo et al., 2021). Notably, the thymus is one of the target organs of viruses (Luo et al., 2021). Infections caused by influenza A and human immunodeficiency viruses, among others, cause thymic atrophy via direct or indirect mechanisms (Duan et al., 2015; Fiume et al., 2015). To our knowledge, there are no reports documenting the impact of SARS-CoV-2 infection on the thymus. However, thymic atrophy in COVID-19 disease has been proposed (Lins and Smaniotto, 2021). Moreover, the COVITHYM case-control observational study will evaluate the thymic function of hospitalized COVID-19 patients compared to patients hospitalized for other reasons (ClinicalTrials.gov, 2021).
Coronavirus infections, including SARS-CoV 2003 and SARS-CoV-2 viruses, are known to cause a robust upregulation in lipid mediators derived from arachidonic acid. Interestingly, SARS-CoV 2003 infection of mice led to significant increase in lung thromboxane A2 (TxA2) in younger compared to older mice (Vijay et al., 2015). Similarly, severe adult COVID-19 patients had marked increases in fatty acid levels as well as an accompanying inflammatory lipid storm with a predominance of arachidonic acid metabolites, notably TxB2 >> PGE2 > PGD2 (Archambault et al., 2021). TxA2 induces apoptosis of double-positive thymocytes and actin polymerization via the thromboxane prostanoid receptor, which is highly expressed on immature thymocytes (Harenberg et al., 2005). TxA2 levels in children with COVID-19 remain to be measured. Therefore, SARS-CoV-2 infection may exacerbate TxA2 production in malnourished children, thereby promoting early thymic involution and impaired immunity (Figure 1).
Conclusions
During the COVID-19 pandemic, malnutrition in children as a result of poverty and disruption in food supply is a potential cause of thymic dysfunction and atrophy leading to immune dysfunction (Naja and Hamadeh, 2020). In addition to PEM, several other factors including zinc, iron and vitamin deficiencies may cause thymic dysfunction or atrophy in children with malnutrition. Following recovery from COVID-19, malnutrition-induced thymic dysfunction and atrophy can predispose children to other respiratory or gastrointestinal infections. It is critical that governments and international organizations such as WHO, World Food Program, and UNICEF take appropriate steps to ensure adequate food and micronutrient supplies in order to limit and contain the health impact of this pandemic on children, especially in low- and middle-income countries.
Acknowledgements
We are grateful to Dr. Sudir Gupta, Division of Immunology, University of California Irvine for a critical review of the manuscript.
Abbreviations
- SARS-CoV
severe acute respiratory syndrome coronavirus
- COVID-19
coronavirus disease 2019
- PEM
Protein-energy malnutrition
- WFP
World Food Program
- UNICEF
United Nations International Children's Fund
- WHO
World Health Organization
- IL
interleukin
- ILC2
type 2 innate lymphoid cells
- Th2
T helper 2
- PGD2
prostaglandin D2
- TxA2
thromboxane A2
- MDSC
myeloid-derived suppressor cells
- CMI
cell-mediated immunity
- MIS-C
multisystem inflammatory syndrome in children
- LMIC
low- and middle-income countries
Footnotes
Author contributions: A. Gupta and K.C. Chiang conceptualized and created the framework for the manuscript and wrote the original draft. All authors reviewed and edited.
Consent for publication: Yes
Ethical approval: None declared.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ajay Gupta https://orcid.org/0000-0001-9182-3499
References
- Al-Sukaiti N, Reid B, Lavi S, et al. (2010) Safety and efficacy of measles, mumps, and rubella vaccine in patients with DiGeorge syndrome. Journal of Allergy and Clinical Immunology 126(4): 868–869. [DOI] [PubMed] [Google Scholar]
- Alexander M, Newmark H, Miller RG. (1985) Oral beta-carotene can increase the number of OKT4 + cells in human blood. Immunology Letters 9(4): 221–224. [DOI] [PubMed] [Google Scholar]
- Archambault AS, Zaid Y, Rakotoarivelo V, et al. (2021) High levels of eicosanoids and docosanoids in the lungs of intubated COVID–19 patients. The FASEB Journal 35(6): e21666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arima M, Fukuda T. (2011) Prostaglandin D2and TH2 inflammation in the pathogenesis of bronchial asthma. The Korean Journal of Internal Medicine 26(1): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach J, Bardenne M, Pleau J, et al. (1977) Biochemical characterisation of a serum thymic factor. Nature 266(5597): 55–57. [DOI] [PubMed] [Google Scholar]
- Bach JF. (1979) Thymic hormones. Journal of Immunopharmacology 1(3): 277–310. [DOI] [PubMed] [Google Scholar]
- Barik S, Miller MM, Cattin-Roy AN, et al. (2017) IL-4/IL-13 signaling inhibits the potential of early thymic progenitors to commit to the T cell lineage. The Journal of Immunology 199(8): 2767–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlus CL. (2003) The role of iron in T cell development and autoimmunity. Autoimmunity Reviews 2(2): 73–78. [DOI] [PubMed] [Google Scholar]
- Carter MJ, Fish M, Jennings A, et al. (2020) Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nature Medicine 26(11): 1701–1707. [DOI] [PubMed] [Google Scholar]
- Chandra RK. (1979) T and B lymphocyte subpopulations and leukocyte terminal deoxynucleotidyl-transferase in energy-protein undernutrition. Acta Paediatrica SCandinavica 68(6): 841–845. [DOI] [PubMed] [Google Scholar]
- Chandra RK. (1983) Nutrition, immunity, and infection: present knowledge and future directions. Lancet (London, England) 1(8326 Pt 1): 688–691. [DOI] [PubMed] [Google Scholar]
- Chandra RK. (1992) Protein-energy malnutrition and immunological responses. Journal of Nutrition 122(3 Suppl): 597–600. [DOI] [PubMed] [Google Scholar]
- Chang T-H, Wu J-L, Chang L-Y. (2020) Clinical characteristics and diagnostic challenges of pediatric COVID-19: A systematic review and meta-analysis. Journal of the Formosan Medical Association 119(5): 982–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier P, Sevilla R, Zalles L, et al. (1996) [Immuno-nutritional recovery of children with severe malnutrition]. Sante 6(4): 201–208. [PubMed] [Google Scholar]
- Chew BP, Park JS. (2004) Carotenoid action on the immune response. The Journal of Nutrition 134(1): 257S–261S. [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov (2021) Thymic Function in Patients with COVID-19 (COVITHYM). Available at: https://clinicaltrials.gov/ct2/show/NCT04716907 (accessed January 10).
- Coates T. Approach to the child with lymphocytosis or lymphocytopenia. In: Newburger P (ed) UpToDate. UpToDate, Waltham, MA. (Accessed on July 1, 2020).
- Dardenne M, Pleau J-M. (1994) Interactions between zinc and thymulin. Metal-Based Drugs 1(2-3): 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardenne M, Pleau JM, Nabarra B, et al. (1982) Contribution of zinc and other metals to the biological activity of the serum thymic factor. Proceedings of the National Academy of Sciences of the United States of America 79(17): 5370–5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids M. Approach to the adult with lymphocytosis or lymphocytopenia. In: Newburger P (ed) UpToDate. UpToDate, Waltham, MA. (Accessed on July 1, 2020).
- Diao B, Wang C, Tan Y, et al. (2020) Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Frontiers in Immunology 11: 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Yan H, Guo W. (2020) Clinical characteristics of children with COVID-19: a meta-analysis. Frontiers in Pediatrics 8: 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Gerpe L, Rey-Méndez M. (2003) Evolution of the thymus size in response to physiological and random events throughout life. Microscopy Research and Technique 62(6): 464–476. [DOI] [PubMed] [Google Scholar]
- Duan X, Lu J, Zhou K, et al. (2015) NK-cells are involved in thymic atrophy induced by influenza A virus infection. Journal of General Virology 96(11): 3223–3235. [DOI] [PubMed] [Google Scholar]
- El-Zayat SR, Sibaii H, Mahfouz NN, et al. (2018) Effect of vitamin A deficiency on thymosin-β4 and CD4 concentrations. Journal of Genetic Engineering and Biotechnology 16(1): 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein LR, Rose EB, Horwitz SM, et al. (2020) Multisystem inflammatory syndrome in U.S. children and adolescents. New England Journal of Medicine 383(4): 334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiume G, Scialdone A, Albano F, et al. (2015) Impairment of T cell development and acute inflammatory response in HIV-1 Tat transgenic mice. Scientific Reports 5(1): 13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker PJ, King LE, Laakko T, et al. (2000) The dynamic link between the integrity of the immune system and zinc status. The Journal of Nutrition 130(5): 1399S–1406S. [DOI] [PubMed] [Google Scholar]
- Golden MH, Jackson AA, Golden BE. (1977) Effect of zinc on thymus of recently malnourished children. Lancet (London, England) 2(8047): 1057–1059. [DOI] [PubMed] [Google Scholar]
- Green HN, Mellanby E. (1928) Vitamin A as an anti-infective agent. British Medical Journal 2(3537): 691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harenberg A, Girkontaite I, Giehl K, et al. (2005) The Lsc RhoGEF mediates signaling from thromboxane A2 to actin polymerization and apoptosis in thymocytes. European Journal of Immunology 35(6): 1977–1986. [DOI] [PubMed] [Google Scholar]
- Haroun HS. (2018) Aging of thymus gland and immune system. MOJ Anatomy & Physiology 5(2): 178–181. [Google Scholar]
- He Z, Zhao C, Dong Q, et al. (2005) Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. International Journal of Infectious Diseases 9(6): 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbinger K-H, Hanus I, Schunk M, et al. (2016) Lymphocytosis and lymphopenia induced by imported infectious diseases: a controlled cross-sectional study of 17,229 diseased German travelers returning from the tropics and subtropics. The American Journal of Tropical Medicine and Hygiene 94(6): 1385–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizar MI, Arena R, Laddu DR. (2021) The global food syndemic: The impact of food insecurity, Malnutrition and obesity on the healthspan amid the COVID-19 pandemic. Progress in Cardiovascular Diseases 64: 105–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambon B, Montagne P, Bene MC, et al. (1981) Immunohistologic localization of "facteur thymique serique" (FTS) in human thymic epithelium. The Journal of Immunology 127(5): 2055–2059. [PubMed] [Google Scholar]
- Jambon B, Ziegler O, Maire B, et al. (1988) Thymulin (facteur thymique serique) and zinc contents of the thymus glands of malnourished children. American Journal of Clinical Nutrition 48(2): 335–342. [DOI] [PubMed] [Google Scholar]
- Kellogg C, Equils O. (2020) The role of the thymus in COVID-19 disease severity: implications for antibody treatment and immunization. Human Vaccines & Immunotherapeutics 17(3): 638–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch GT, Cruz JR, Torun B, et al. (1987) Immature circulating lymphocytes in severely malnourished Guatemalan children. Journal of Pediatric Gastroenterology and Nutrition 6(2): 265–270. [DOI] [PubMed] [Google Scholar]
- Kurtz A, Grant K, Marano R, et al. (2021) Long-term effects of malnutrition on severity of COVID-19. Scientific Reports 11(1): 14974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuvibidila S, Dardenne M, Savino W, et al. (1990) Influence of iron-deficiency anemia on selected thymus functions in mice: Thymulin biological activity, T-cell subsets, and thymocyte proliferation. American Journal of Clinical Nutrition 51(2): 228–232. [DOI] [PubMed] [Google Scholar]
- Lee CM, Humphrey PA, Aboko-Cole GF. (1983) Interaction of nutrition and infection: effect of zinc deficiency on immunoglobulin levels in trypanosoma musculi infection. Journal of the National Medical Association 75(7): 677–682. [PMC free article] [PubMed] [Google Scholar]
- Lee JKH, Lam GKL, Shin T, et al. (2018) Efficacy and effectiveness of high-dose versus standard-dose influenza vaccination for older adults: a systematic review and meta-analysis. Expert Review of Vaccines 17(5): 435–443. [DOI] [PubMed] [Google Scholar]
- Lemoine A, Tounian P. (2020) Childhood anemia and iron deficiency in sub-Saharan Africa – risk factors and prevention: A review. Archives de Pédiatrie 27(8): 490–496. [DOI] [PubMed] [Google Scholar]
- Lewin SR, Heller G, Zhang L, et al. (2002) Direct evidence for new T-cell generation by patients after either T-cell-depleted or unmodified allogeneic hematopoietic stem cell transplantations. Blood 100(6): 2235–2242. [PubMed] [Google Scholar]
- Li T, Xie J, He Y, et al. (2006) Long-term persistence of robust antibody and cytotoxic T cell responses in recovered patients infected with SARS coronavirus. PLoS ONE 1(1): e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lins MP, Smaniotto S. (2021) Potential impact of SARS-CoV-2 infection on the thymus. Canadian Journal of Microbiology 67(1): 23–28. [DOI] [PubMed] [Google Scholar]
- Long S, Vodzak J. (2018) Laboratory Manifestations of Infectious Diseases. pp.1447–1459.e1444.
- Lucas C, Wong P, Klein J, et al. (2020) Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 584(7821): 463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Xu L, Qian Z, et al. (2021) Infection-associated thymic atrophy. Frontiers in Immunology 12: 652538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyra JS, Madi K, Maeda CT, et al. (1993) Thymic extracellular matrix in human malnutrition. The Journal of Pathology 171(3): 231–236. [DOI] [PubMed] [Google Scholar]
- Mahler GD, Lakner C, Aguilar RAC, et al. (2020) The impact of COVID-19 (Coronavirus) on global poverty: Why Sub-Saharan Africa might be the region hardest hit. In: World Bank Blogs. Available at: https://blogs.worldbank.org/opendata/impact-covid-19-coronavirus-global-poverty-why-sub-saharan-africa-might-be-region-hardest
- Malpuech-Brugère C, Nowacki W, Gueux E, et al. (1999) Accelerated thymus involution in magnesium-deficient rats is related to enhanced apoptosis and sensitivity to oxidative stress. British Journal of Nutrition 81(5): 405–411. [PubMed] [Google Scholar]
- Mandal S, Barnett J, Brill SE, et al. (2021) ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 76(4): 396–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray DN. (1984) Cell-mediated immunity in nutritional deficiency. Progress in Food & Nutrition Science 8(3-4): 193–228. [PubMed] [Google Scholar]
- Nabukeera-Barungi N, Lanyero B, Grenov B, et al. (2021) Thymus size and its correlates among children admitted with severe acute malnutrition: a cross-sectional study in Uganda. BMC Pediatrics 21(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naja F, Hamadeh R. (2020) Nutrition amid the COVID-19 pandemic: a multi-level framework for action. European Journal of Clinical Nutrition 74: 1117–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osendarp S, Akuoku J, Black R, et al. (2020) The potential impacts of the COVID-19 crisis on maternal and child undernutrition in low and middle income countries. Research Square.
- Parent G, Chevalier P, Zalles L, et al. (1994) In vitro lymphocyte-differentiating effects of thymulin (Zn-FTS) on lymphocyte subpopulations of severely malnourished children. American Journal of Clinical Nutrition 60(2): 274–278. [DOI] [PubMed] [Google Scholar]
- Perlman S. (2020) COVID-19 poses a riddle for the immune system. Nature 584(7821): 345–346. [DOI] [PubMed] [Google Scholar]
- Prasad AS, Meftah S, Abdallah J, et al. (1988) Serum thymulin in human zinc deficiency. The Journal of Clinical Investigation 82(4): 1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice AM. (1999) The thymus: a barometer of malnutrition. British Journal of Nutrition 81(5): 345–347. [PubMed] [Google Scholar]
- Purtilo DT, Connor DH. (1975) Fatal infections in protein-calorie malnourished children with thymolymphatic atrophy. Archives of Disease in Childhood 50(2): 149–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razali N, Hohjoh H, Inazumi T, et al. (2020) Induced prostanoid synthesis regulates the balance between Th1- and Th2-producing inflammatory cytokines in the thymus of diet-restricted mice. Biological and Pharmaceutical Bulletin 43(4): 649–662. [DOI] [PubMed] [Google Scholar]
- Roux E, Dumont-Girard F, Starobinski M, et al. (2000) Recovery of immune reactivity after T-cell-depleted bone marrow transplantation depends on thymic activity. Blood 96(6): 2299–2303. [PubMed] [Google Scholar]
- Ruhräh J. (1903) The relation of the thymus gland to marasmus. The Lancet 162(4174): 602–603. [Google Scholar]
- Rytter MJH, Namusoke H, Ritz C, et al. (2017) Correlates of thymus size and changes during treatment of children with severe acute malnutrition: a cohort study. BMC Pediatrics 17: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino W. (2002) The thymus gland is a target in malnutrition. European Journal of Clinical Nutrition 56(Suppl 3): S46–S49. [DOI] [PubMed] [Google Scholar]
- Savino W, Dardenne M, Velloso LA, et al. (2007) The thymus is a common target in malnutrition and infection. British Journal of Nutrition 98(S1): S11–S16. [DOI] [PubMed] [Google Scholar]
- Seifter E, Rettura G, Levenson SM. (1981) Carotenoids and cell-mediated immune responses. In: Charalambous G, Inglett G. (eds) The Quality of Foods and Beverages. New York: Academic Press, pp.335–347. [Google Scholar]
- Shankar AH, Prasad AS. (1998) Zinc and immune function: the biological basis of altered resistance to infection. The American Journal of Clinical Nutrition 68(2): 447S–463S. [DOI] [PubMed] [Google Scholar]
- Spencer SP, Wilhelm C, Yang Q, et al. (2014) Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science (New York, N.Y.) 343(6169): 432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Study CM. (2020) Long-Term Outcomes after the Multisystem Inflammatory Syndrome In Children. Available at: https://covidmusicstudy.com/ (accessed March 7).
- Suskind RM. (1988) Malnutrition and the immune response. Beitrage Zu Infusionstherapie Und Klinische Ernahrung 19: 1–25. [PubMed] [Google Scholar]
- Trabanelli S, Chevalier MF, Martinez-Usatorre A, et al. (2017) Tumour-derived PGD2 and NKp30-B7H6 engagement drives an immunosuppressive ILC2-MDSC axis. Nature Communications 8(1): 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregoning JS, Russell RF, Kinnear E. (2018) Adjuvanted influenza vaccines. Human Vaccines & Immunotherapeutics 14(3): 550–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- unicef (2020) Novel Coronavirus (COVID-19): UNICEF support to the Middle East and North Africa. Available at: https://www.unicef.org/mena/media/8281/file/MENA%20COVID-19%20HAC_APRIL%202020.pdf.pdf (accessed 30 May).
- UNICEF (2021) COVID-19 is biggest threat to child progress in UNICEF’s 75-year history. Available at: https://news.un.org/en/story/2021/12/1107422#:∼:text=COVID%2D19%20has%20pushed%20a,when%20the%20pandemic%20was%20declared. (accessed Jan 26).
- US Global Leadership Coalition (2021) COVID-19 Brief: Impact on Food Security. Available at: https://www.usglc.org/coronavirus/global-hunger/ (accessed October 3).
- van Baarlen J, Schuurman HJ, Huber J. (1988) Acute thymus involution in infancy and childhood: a reliable marker for duration of acute illness. Human Pathology 19(10): 1155–1160. [DOI] [PubMed] [Google Scholar]
- Varghese J, Sandmann S, Ochs K, et al. (2021) Persistent symptoms and lab abnormalities in patients who recovered from COVID-19. Scientific Reports 11: 12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Version MMP (2020) Vitamin A Deficiency (Retinol Deficiency). Available at: https://www.merckmanuals.com/professional/nutritional-disorders/vitamin-deficiency-dependency-and-toxicity/vitamin-a-deficiency (accessed April 26).
- Vijay R, Hua X, Meyerholz DK, et al. (2015) Critical role of phospholipase A2 group IID in age-related susceptibility to severe acute respiratory syndrome-CoV infection. Journal of Experimental Medicine 212(11): 1851–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warny M, Helby J, Nordestgaard BG, et al. (2018) Lymphopenia and risk of infection and infection-related death in 98,344 individuals from a prospective Danish population-based study. PLOS Medicine 15(11): e1002685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells KR, Brown KH. (2012) Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE 7(11): e50568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead RG. (1981) Immunology of Nutritional Disorders. R. K. Chandra (1980). London: Edward Arnold (Publishers) Ltd., 110 DD.; illus. (B/W). ISBN 0 7131-4362-2. Price £9.95. Transactions of The Royal Society of Tropical Medicine and Hygiene 75(1): 118–118. [Google Scholar]
- WHO (2021a) More than half a billion people pushed or pushed further into extreme poverty due to health care costs. Available at: https://www.who.int/news/item/12-12-2021-more-than-half-a-billion-people-pushed-or-pushed-further-into-extreme-poverty-due-to-health-care-costs (accessed January 27).
- WHO (2021b) Multiple micronutrient powders for point-of-use fortification of foods consumed by infants and children. Available at: https://www.who.int/elena/titles/guidance_summaries/micronutrientpowder_infants/en/ (accessed March 7).
- Wilhelm C, Harrison OJ, Schmitt V, et al. (2016) Critical role of fatty acid metabolism in ILC2-mediated barrier protection during malnutrition and helminth infection. Journal of Experimental Medicine 213(8): 1409–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CP, Song Y, Elias VD, et al. (2009) Zinc supplementation increases zinc status and thymopoiesis in aged mice. The Journal of Nutrition 139(7): 1393–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank (2021) COVID-19 leaves a legacy of rising poverty and widening inequality. Available at: https://blogs.worldbank.org/developmenttalk/covid-19-leaves-legacy-rising-poverty-and-widening-inequality (accessed January 27).
- Xue L, Gyles SL, Wettey FR, et al. (2005) Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells. The Journal of Immunology 175(10): 6531–6536. [DOI] [PubMed] [Google Scholar]
- Yang L, Liu S, Liu J, et al. (2020) COVID-19: Immunopathogenesis and immunotherapeutics. Signal Transduction and Targeted Therapy 5: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tan Y, Ling Y, et al. (2020) Viral and host factors related to the clinical outcome of COVID-19. Nature 583: 437–440. [DOI] [PubMed] [Google Scholar]
- Zhou F, Yu T, Du R, et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet 395(10229): 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Ji P, Pang J, et al. (2020) Clinical characteristics of 3,062 COVID-19 patients: a meta-analysis. Journal of Medical Virology 92: 1902–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]