Abstract
The most critical intervention for large hemothorax is draining the blood out of the pleural cavity by placing a thoracostomy tube but it can be disastrous if done without due consideration. We report a rare case of spontaneous hemothorax due to posterior intercostal artery aneurysm and implication of diagnostic evaluation on its management.
Keywords: Spontaneous hemothorax, Posterior intercostal artery aneurysm, Von Recklinghausen disease
1. Introduction
Hemothorax is usually caused by trauma, coagulopathy, or iatrogenic issues such as central line insertion, thoracocentesis, pleural biopsies. It is defined as a pleural fluid hematocrit greater than 50% of the patient's blood [1]. Spontaneous hemothorax (SH) is blood accumulation in the pleural cavity in the absence of trauma or iatrogenic cause. Management of spontaneous hemothorax encompasses conservative and surgical approaches, with attention to etiology-specific treatments [2]. The usual treatment of significant hemothorax involves draining the blood out of the pleural cavity by aspiration or largely by placing a tube into the pleural cavity.
2. Case
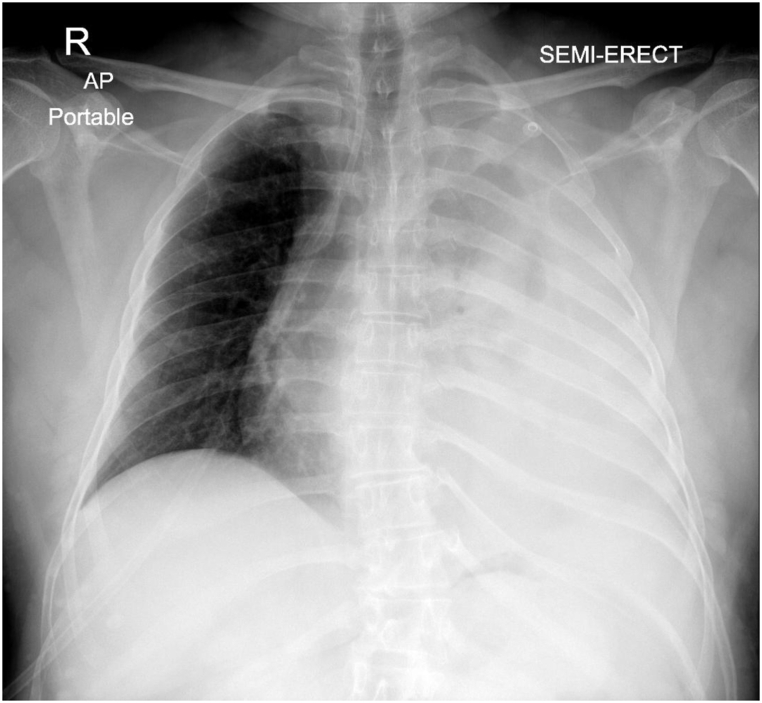
A 47-year-old man presented to the emergency department of our hospital with a history of left-sided chest pain and mild breathlessness for the last 3–4 days and a history of 2 episodes of vomiting one day before the presentation. There was no history of fever, loss of appetite, weight loss, trauma, apparent bleeding, or medication. Past medical history was not significant except for the presence of neurofibromatosis type 1 (NF 1) which is also known as Von Recklinghausen disease since the age of 12 years. He was a non-smoker. On arrival, he was conscious, with a respiratory rate around 27 per minute, heart rate 110 per minute regular, and blood pressure 110/60 mmHg. On further evaluation, the diagnosis of massive left pleural effusion was made, and diagnostic pleural tapping was done which revealed the presence of blood in the left pleural cavity. His coagulation profile was normal. CT chest with contrast was done to evaluate the possible etiology. His haemoglobin value decreased to 12 gm/dl from the initial value of 14 gm/dl in the next 12 hours. We planned to place a thoracostomy tube in the pleural cavity given the mediastinal shift to the right side while evaluating the cause of spontaneous hemothorax (Fig. 1).
Fig. 1.
Chest X-ray depicting large pleural collection on left side with right tracheal deviation.
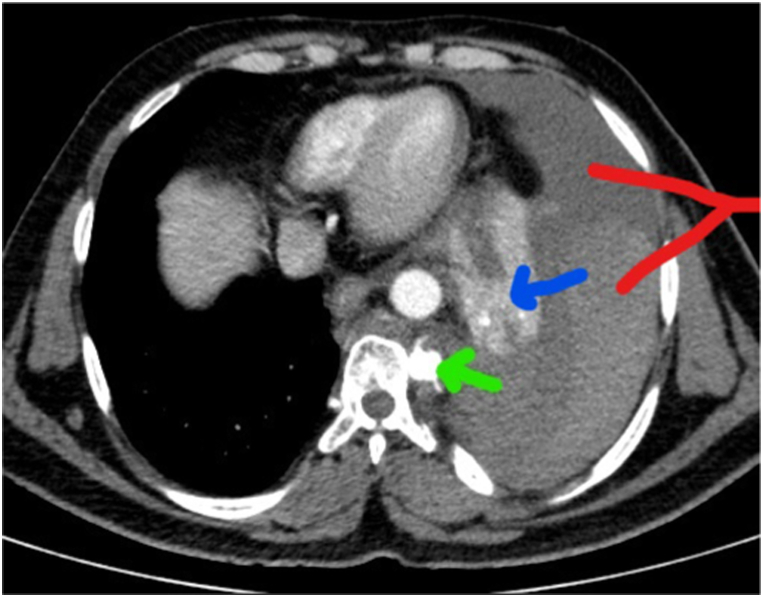
The preliminary findings on the computed tomography (CT) of the chest did not reveal the underlying etiology of the SH, but upon further discussion with the radiologist revealed the presence of a posterior intercostal artery aneurysm (Fig. 2). Thereafter, we did not place the thoracostomy tube and transferred the patient to a higher center with the facility of interventional radiology, vascular surgery, and thoracic surgery. He underwent aneurysmal coiling and subsequent placement of intercostal drainage at the higher center.
Fig. 2.
Green arrow pointing towards posterior intercostal artery aneurysm, Blue arrow showing collapsed lung due to hemothorax. Red arrow indicates hematocrit sign (Two different densities in collection because cellular components in effusion settle to dependant region) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
3. Discussion
Hemothorax in the absence of trauma or iatrogenic cause is labelled as spontaneous. It can be seen in association with many disease conditions, but it is an uncommon finding in all of them [1]. The causes include pneumothorax, coagulopathy, aortic dissection, and pulmonary vascular malformation [3]. While many malignant carcinomas of the thorax can cause hemorrhagic pleural effusions, true hemothorax is uncommon. The initial diagnostic evaluation should focus on the potentially fatal causes, including aortic aneurysm, pulmonary arteriovenous malformation, and coagulopathy. Once the cause of spontaneous hemothorax has been identified, specific therapy should be directed toward the underlying disorder.
Hemothorax, if significant may result in either tension physiology or hypovolemic shock. The large collection should be drained with a thoracostomy tube to monitor blood loss and to decrease the risk of fibrothorax. In the setting of hemothorax refractory to chest tube drainage, hemodynamic instability, or massive bleeding, thoracoscopy or thoracotomy should be considered [3]. Any hemodynamic instability due to hypovolemic shock should be managed on the line of hemorrhagic shock and it includes appropriate intravenous access, hemodynamic assessment, judicious use of blood and its product and surgical intervention if required.
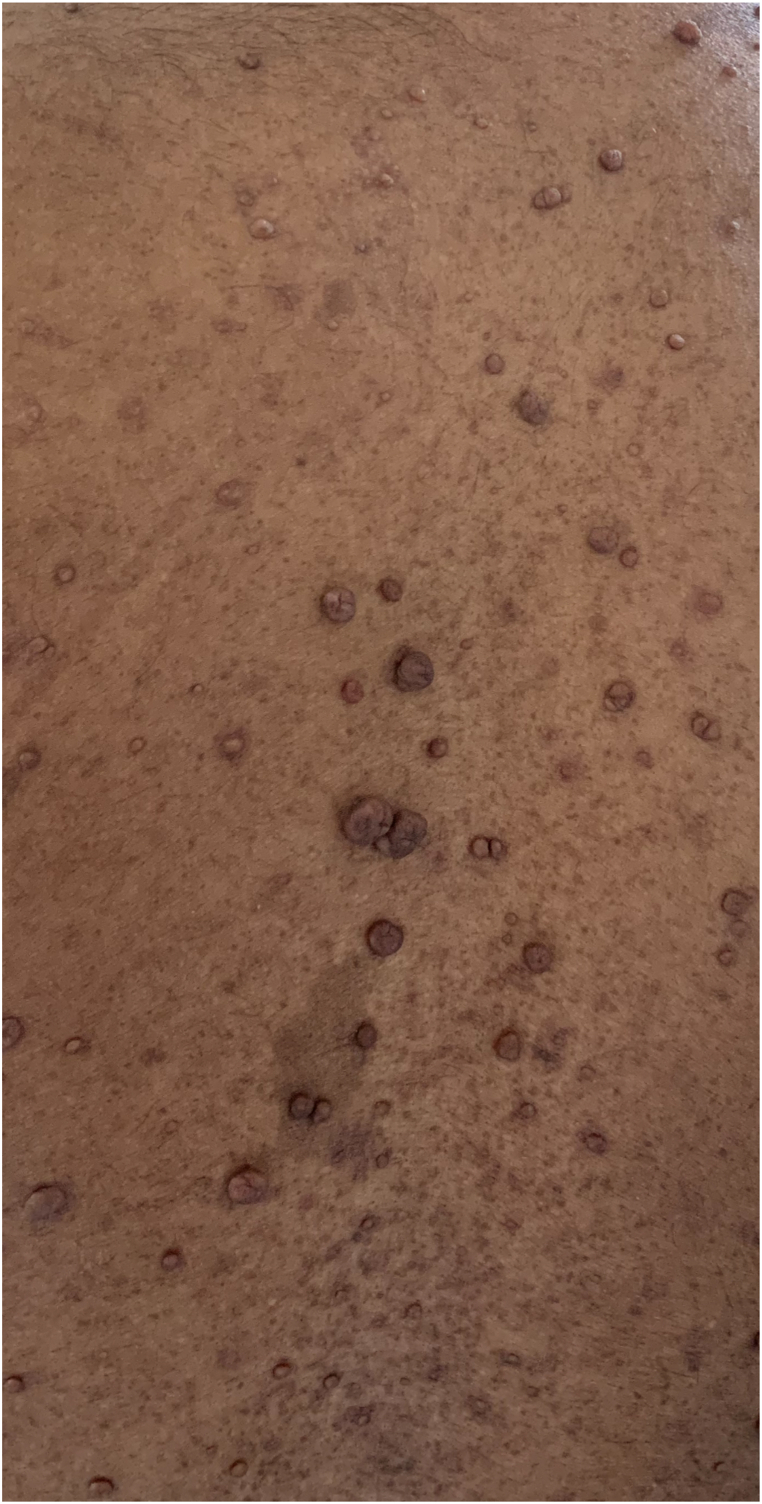
Our patient was a case of NF 1, and it is known to cause SH. It is an autosomal dominant disease with an occurrence rate of 1 in 3000 [4]. It is usually diagnosed in adulthood since its clinical characteristics develop throughout life. This entity can affect any organ system, especially connective, nervous, and vascular tissue, and is characterized by skin tumors and abnormal skin pigmentation. The pigmentation manifests itself both in the diagnostic café au lait spots and in common freckling of the skin in regions that are barely exposed to sunlight(Fig. 3). Two main pathogenetic mechanisms have been advocated for vasculopathy associated with NF 1: (I) direct vascular invasion from adjacent tumors such as schwannoma, neurofibroma, or neurofibrosarcoma [5]; and (II) vascular dysplasia with thickening and concomitant reduced strength of the vessel wall and aneurysm formation [6]. Invasive tissue leads to compression of the vasa vasorum with subsequent ischemia and weakness of blood vessels. Small vessel damage is due to dysplasia of the wall due to proliferation of the intimae and muscolaris, loss of the media, and fibrosis of the adventitia. This initiates stenosis of the vessel wall, potentiating vessel wall weakness and friability with subsequent risk of rupture. Vascular dysplasia often causes thickening and concomitant reduced strength of the vessel wall leading to aneurysm formation. Treatment options are dependent on the patient's hemodynamic stability. Endovascular embolization is indicated if there is hemodynamic stability. As an alternative, thoracotomy with surgical ligation is indicated in cases of active bleeding, with associated hemodynamic compromise. Regarding the prognosis of NF-1 in patients with hemothorax, the disease mortality is 36% and postoperative mortality is 33%. Recently published cases suggested that coil embolization offers the best results [4].
Fig. 3.
Patient of Von Recklinghausen disease with cafe au lait spot.
Although aspiration of blood typically prompts the placement of a thoracostomy tube in a patient with traumatic hemothorax, we believe that this case highlights the importance of evaluating the source and cause of spontaneous hemothorax and addressing it before going for standard therapy of placement of thoracostomy tube. It could be disastrous, if done without identifying the source of bleeding, underlying etiology, and focused intervention for the same. In our case, the decompression of the pleural cavity by placing a thoracostomy tube, at our center could very well lead to incessant bleeding and its concomitant risk and putting the patient's life at risk.
4. Conclusion
The complexities described in this case serve to highlight the need for a thoughtful, reasoned, and multidisciplinary approach to the care of these patients. Careful integration of clinical and radiographic information and coordination with an interventional radiologist, thoracic surgeon, and vascular surgeon as well as other support is essential and critical in providing a personalized and best treatment plan. If the clinician encounters any such situation where the cause of spontaneous hemothorax is not apparent, the placement of thoracostomy should be delayed if a patient does not have tension physiology while further management is being planned. If bleeding remains unceasing after placing the thoracostomy tube in a patient with spontaneous hemothorax, the patient should be provided with hemodynamic support with blood and clamping of thoracostomy tube while the supplementary plan is under consideration along with re-evaluating the underlying etiology.
Declaration of competing interest
The authors have no affiliation with any organization with a direct or indirect financial interest in the subject matter discussed in the manuscript
References
- 1.Patrini D., Panagiotopoulos N., Pararajasingham J., Gvinianidze L., Iqbal Y., Lawrence D.R. Etiology and management of spontaneous hemothorax. J. Thorac. Dis. 2015;7:520–526. doi: 10.3978/j.issn.2072-1439.2014.12.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan C.K., Bashoura L., Balachandran D., Faiz S.A. vol. 12. Annals of the American Thoracic Society; 2015. (Spontaneous Hemothorax). [DOI] [PubMed] [Google Scholar]
- 3.Mehta S., Klinger J. Spontaneous hemothorax. Clin. Pulm. Med. 1995;5:303–309. [Google Scholar]
- 4.Rodriguez G.M., Gallegos C.B., Vicente A.S., Fernandez O.I., Zapatero G.J., Villar A.F. Spontaneous hemothorax in a patient with von Recklinghausens's disease. J. Clin. Med. Res. 2014;6:149–152. doi: 10.14740/jocmr1692w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chew D.K., Muto P.M., Gordon J.K., Straceski A.J., Donaldson M.C. Spontaneous aortic dissection and rupture in a patient with neurofibromatosis. J. Vasc. Surg. 2001;34:364–366. doi: 10.1067/mva.2001.116141. [DOI] [PubMed] [Google Scholar]
- 6.Miura H., Taira O., Uchida O., et al. Spontaneous hemothorax associated with von Recklinghausen's disease: review of occurrence in Japan. Thorax. 1997;52:577–578. doi: 10.1136/thx.52.6.577. [DOI] [PMC free article] [PubMed] [Google Scholar]