Abstract
Necrotic enteritis (NE) is a devastating disease that has seen a resurgence of cases following the removal of antibiotics from feed resulting in financial loss and significant animal health concerns across the poultry industry. The objective was to evaluate the efficacy of a microencapsulated blend of organic (25% citric and 16.7% sorbic) acids and botanicals (1.7% thymol and 1% vanillin [AviPlusP]) to reduce clinical NE and determine the signaling pathways associated with any changes. Day-of-hatch by-product broiler breeder chicks were randomly assigned to a control (0) or supplemented (500 g/MT) diet (n = 23–26) and evaluated in a NE challenge model (n = 3). Birds were administered 2X cocci vaccine on d 14 and challenged with a cocktail of Clostridium perfringens strains (107) on d 17 to 19. On d 20 to 21 birds were weighed, euthanized, and scored for NE lesions. Jejunal tissue was collected for kinome analysis using an immuno-metabolism peptide array (n = 5; 15/treatment) to compare tissue from supplement-fed birds to controls. Mortality and weight were analyzed using Student's t test and lesion scores analyzed using F-test two-sample for variances (P < 0.05). The kinome data was analyzed using PIIKA2 peptide array analysis software and fold-change between control and treated groups determined. Mortality in the supplemented group was 47.4% and 70.7% in controls (P = 0.004). Lesions scores were lower (P = 0.006) in supplemented birds (2.47) compared to controls (3.3). Supplement-fed birds tended (P = 0.19) to be heavier (848.6 g) than controls (796.2 g). Kinome analysis showed T cell receptor, TNF and NF-kB signaling pathways contributed to the improvements seen in the supplement-fed birds. The following peptides were significant (P < 0.05) in all 3 pathways: CHUK, MAP3K14, MAP3K7, and NFKB1 indicating their importance. Additionally, there were changes to IL6, IL10, and IFN- γ mRNA expression in tissue between control- and supplement-fed chickens. In conclusion, the addition of a microencapsulated blend of organic acids and botanicals to a broiler diet reduced the clinical signs of NE that was mediated by specific immune-related pathways.
Key words: Clostridium perfringens; feed additive, kinome, microencapsulated organic acids and botanicals, necrotic enteritis
INTRODUCTION
The poultry industry has moved away from using antibiotic growth promoters (AGP) in poultry production (Castanon, 2007), and this transition has created a burgeoning feed additive industry focused on providing natural alternatives that enhance performance and improve animal health. There are numerous products proven to be viable AGP alternatives for the poultry industry including, but not limited to, organic acids, essential oils, plant metabolites, medicinal herbs, amino acids, oligosaccharides, minerals, yeast cell wall components, and various by-products (Zeng et al., 2015; Gessner et al., 2017; Hashim et al., 2018; Micciche et al., 2018; Suresh et al., 2018; Yadav and Jha, 2019). For a feed additive to have an impact on performance and animal health, it must reach the gastrointestinal tract, particularly the small intestine, where most digestion and virtually all nutrient absorption occurs (Svihus, 2014a,b). The additive must be durable enough to tolerate the conditions of the gastrointestinal tract or it should be protected from those harsh conditions. One approach to protect the feed additive is to encapsulate the components with a lipid (or similar) matrix which will increase stability and will allow for a slow and targeted release (Grilli et al., 2007; Piva et al., 2007; Yang et al., 2016).
Clostridium perfringens (C. perfringens) is a spore forming, Gram-positive rod-shaped bacterium that is ubiquitous in nature, is an economically important poultry pathogen, and one of the known etiological agents associated with necrotic enteritis (NE) (Timbermont et al., 2011). Removal of AGP from the feed has contributed to a substantial increase of NE outbreaks across the poultry industry (Broom and Kogut, 2019; Adhikari et al., 2020). Globally, NE costs the poultry industry $2B USD due to reduced performance, disease treatment, and carcass condemnations (Van der Sluis, 2000; Paiva and McElroy, 2014); however, more recent estimates indicate the impact is closer to $5 to 6B USD (Wade and Keyburn, 2015). In addition to the economic impact, NE creates significant animal welfare concerns because of decreased appetite, depression, diarrhea, and severe necrosis of the intestinal tract (Ficken and Wages, 1997; Van Immerseel et al., 2004). A number of studies show the effectiveness of natural products including organic acids, essential oils, postbiotics, prebiotics, or probiotics to improve growth and performance, reduce mortality, lessen intestinal pathology, and alter gut microbial populations under experimental NE challenge (Keerqin et al., 2017; Yin et al., 2017; Calik et al., 2019; Johnson et al., 2019, 2020; Abdelli et al., 2020).
Necrotic enteritis is a complex and multifaceted disease and, as such, disease progression can be influenced by dietary composition, the immune status of the bird, or if there has been a sudden change to the gut microbial populations (Smith, 1965; Ficken and Wages, 1997; Timbermont et al., 2011; Paiva and McElroy, 2014). Understanding a complex disease such as NE will require a powerful tool to delineate the mechanism(s) employed by the host that either confers protection or susceptibility to disease. Evaluating the host kinome with peptide arrays provide site-specific protein information and a detailed picture of phosphorylation-mediated events making it a powerful instrument to define mechanism(s) (Manning et al., 2002; Ouyang et al., 2003; Wang, 2014). Our laboratory developed a chicken-specific kinome array (Arsenault and Kogut, 2012; Arsenault et al., 2013) enabling us to identify specific signaling pathways that are altered following administration of various feed additives; therefore, making it possible to better define their mechanism(s) of action (Arsenault et al., 2017; Hashim et al., 2018; Johnson et al., 2019, 2020; Swaggerty et al., 2020a; Bortoluzzi et al., 2021).
The objectives of this study were to determine if dietary supplementation with a microencapsulated blend of organic acids and botanicals reduced clinical signs of NE and altered jejunal kinome activity and signaling pathways compared to birds on the control diet.
MATERIALS AND METHODS
Experimental Chickens
Day-of-hatch by-product male broiler breeder chicks were obtained from a commercial hatchery and placed in floor pens containing wood shavings and provided supplemental heat and ad libitum access to water for the duration of the study. To ensure reproducibility, the experiments were conducted on 3 separate occasions with chicks from different flocks. Chicks were randomly assigned to either a control- or supplement-fed pen (n = 23–26 each). Those assigned to the control pen were allowed ad libitum access to a balanced, unmedicated corn and soybean meal-based starter diet that met or exceeded the established nutrient requirements (National Research Council, 1994). Chicks assigned to the supplement-fed pen were given free access to the same starter diet mixed with 500 g/metric ton (MT) of a microencapsulated blend of citric (25%) and sorbic (16.7%) acids, thymol (1.7%), and vanillin (1.0%) (AviPlus P, Vetagro S.p.A., Reggio Emilia, Italy). No medication or other therapeutic interventions were administered over the duration of the study. The experiments were conducted in accordance with the recommended code of practice for the care and handling of poultry and followed the ethical principles according to the Guide for the Care and Use of Agricultural Animals in Research and Training (AgGuide, 2020) and were overseen by the on-site veterinarian. All bird studies were under the approved experimental procedures outlined in protocol number 2019-002 and were approved by the USDA/ARS Institutional Animal Care and Use Committee operating under the Animal and Plant Health Inspection Service establishment number 334299.
Clostridium Perfringens Preparation
Four field isolates of wild-type C. perfringens (type A) were obtained from active and confirmed outbreaks of NE in Georgia (two isolates), Texas (one isolate), and Virginia (one isolate). The isolates were cultured separately in thioglycollate medium (Becton Dickinson Co., Sparks, MD) for 12 h then combined to yield the challenge stock as previously described (McReynolds et al., 2007).
Necrotic Enteritis Model
Birds were orally administered a 2X dose of a commercially available coccidiosis vaccine (1 mL, Coccivac-B52; Merck Animal Health, Kenilworth, NJ) on d14 and challenged orally (3 mL) with a stock culture containing the cocktail of four C. perfringens strains (107 cfu/mL) on d 17 to 19. For all three experimental replicates, chickens were euthanized by cervical dislocation and necropsied at 20- or 21-d-of-age.
At necropsy, birds were scored for intestinal lesions (see next section for additional details). A piece of jejunum (100 mg) was collected and rinsed with PBS to remove intestinal content, immediately flash frozen in liquid nitrogen to preserve kinase activity, and then transferred to −80°C until further analysis using the array. Additionally, a piece of jejunum (100 mg) was collected, rinsed with PBS to remove content, placed in RNAprotect tissue reagent (Qiagen; Germantown, MD), and stored at -20°C until quantitative real-time RT-PCR (qRT-PCR) performed. In relation to Meckel's diverticulum, the samples were collected approximately 10 cm proximal. All birds were scored for lesions while the first 5 necropsied from each line for each experiment were used for the kinome array.
Lesion Scores
To evaluate gross lesions associated with NE, the jejunum and ileum of the small intestine were examined and scored as previously described (Prescott, 1979). To eliminate bias, one person blindly scored all tissues for lesions. Lesions were scored on a scale of 0 to 4. A score of 0 indicated normal healthy tissue with no gross lesions; a score of 1 was characterized by thin-walled or friable tissue with a grey appearance; a score of 2 was thin-walled, had focal necrosis, and gray in appearance with small amounts of gas production; a score of 3 had thin walls with sizable patches of necrosis, gas-filled intestine, and small flecks of blood; and a score of 4 was defined by severe extensive necrosis, marked hemorrhage, and large amounts of gas in the intestine.
Kinome Array
Peptide array protocol was carried out as previously described (Arsenault et al., 2017) and summarized below using PepStar peptide microarrays from JPT Peptide Technologies GmbH (Berlin, Germany). A 40 mg sample of jejunum was homogenized by a Bead Ruptor homogenizer (Omni, Kennesaw, GA) in 100 μL of lysis buffer (20 mM Tris–HCl pH 7.5, 150 mM NaCl, 1 mM Ethylenediaminetetraacetic acid (EDTA), 1 mM ethylene glycol tetraacetic acid (EGTA), 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM Na3VO4, 1 mM NaF, 1 μg/mL leupeptin, 1 g/mL aprotinin and 1 mM Phenylmethylsulphonyl fluoride). All chemicals were purchased from Sigma-Aldrich, Co. (St. Louis, MO) unless specified otherwise. Arrays were then imaged using a Tecan PowerScanner microarray scanner (Tecan Systems, San Jose, CA) at 532 to 560 nm with a 580 nm filter to detect dye fluorescence.
Isolation of Total RNA for Quantitative Real-Time RT-PCR
Tissue samples were homogenized with a BeadBug homogenizer (Benchmark Scientific, Edison, NJ). Briefly, a piece jejunum (30–40 mg) was removed from RNAprotect, transferred to prefilled microtubes containing zirconium beads (1.5 mm; TriplePure M-Bio Grade; Benchmark Scientific), lysis buffer (350 µL; RNeasy Mini Kit; Qiagen) was added, and the tissue was homogenized for 2 min at maximum speed. Total RNA was isolated from the homogenized tissue according to the manufacturer's instructions, eluted with 50 μL RNase-free water, and stored at −80°C.
Quantitative Real-Time RT-PCR
Cytokine mRNA expression of jejunum tissue from control- and supplement-fed chickens (n = 12/treatment) was determined using qRT-PCR as described previously (Kaiser et al., 2000) using published probes and primer sets shown in Table 1 (Kaiser et al., 2004, 2005); Rothwell et al., 2004. Amplification and detection were carried out on the StepOnePlus Real-Time PCR System using Taqman RNA-to-CT 1 Step Kit (Applied Biosystems, Foster City, CA). Sample standardization was done using 28S RNA. Results were calculated as 40-cycle threshold (CT) for each tissue sample from control- and supplement-fed chickens and the data presented as fold-change from controls. Fold change was calculated as 2^(supplement-fed corrected mean – control-fed corrected mean).
Table 1.
Quantitative real-time RT-PCR primer and probe sequences.
| Target | Primer and probe sequence | Accession no.2 | |
|---|---|---|---|
| 28s | Forward Reverse Probe |
GGCGAAGCCAGAGGAAACT GACGACCGATTTGCACGTC 15´-(FAM)-AGGACCGCTACGGACCTCCACCA-(TAMRA)-3´ |
X59733 |
| IL6 | Forward Reverse Probe |
GCTCGCCGGCTTCGA GGTAGGTCTGAAAGGCGAACAG 5´-(FAM)-AGGAGAAATGCCTGACGAAGCTCTCCA-(TAMRA)-3´ |
AJ250838 |
| IL10 | Forward Reverse Probe |
CATGCTGCTGGGCCTGAA CGTCTCCTTGATCTG CTTGATG 5´-(FAM)-CGACGATTCGGCGCTGTCACC-(TAMRA)-3´ |
AJ621614 |
| IFN- γ | Forward Reverse Probe |
GTGAAGAAGGTGAAAGATATCATGGA GCTTTGCGCT GGATTCTCA 5´-(FAM)-TGGCCAAGCTCCCGATGAACGA-(TAMRA)-3´ |
Y07922 |
FAM Fluorescent reporter dye 5-carboxyfluorescein; TAMRA quencher N, N, N, N´-tetramethyl-6-carboxyrhodamine
Genomic DNA sequence.
Data and Statistical Analysis
Three separate challenges were conducted using chickens from a different flock for each trial and 23 to 26 birds were used for each group per experiment. A total of 72 birds were on the control diet and 74 birds were on the diet supplemented with the microencapsulated blend of organic acids and botanicals. The data from the three challenge trials were combined for statistical analysis and presentation. With the exception of the kinome data, all analyses were performed using Microsoft Excel for Mac version 16.50. Weight, mortality, and cytokine mRNA expression were analyzed using a Student t test while lesion scores were analyzed using the F-test 2-sample for variances. Significance was considered at P ≤ 0.05.
Kinome array images were gridded using GenePix Pro software (Molecular Devices, San Jose, CA), and the spot intensity signal was collected as the mean of pixel intensity using local feature background intensity calculation with scanner 50% gain level. The data was then analyzed using the Platform for Intelligent Integrated Kinome Analysis (PIIKA2) peptide array analysis software as previously described (Trost et al., 2013). Using the normalized data set, comparisons between treatment and control groups were performed, calculating fold-change and a significance P-value. The P value is calculated by conducting a one-sided paired t test between treatment and control values for a given peptide which allows for a statistically robust analysis. The resulting data was imported in Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases to pinpoint changes in protein–protein interactions and signal transduction pathways as previously described (Hashim et al., 2018).
RESULTS
Necrotic Enteritis Challenge Model
Day-old chicks were assigned to one of 2 groups (23–26 chicks/group): control diet without supplementation or a diet supplemented with 500 g/MT of a microencapsulated blend of organic acids and botanicals. Both groups were evaluated in a laboratory model for NE (n = 3 separate trials [n = 72 total chicks on the control diet; n = 74 total chicks on the supplemented diet]). At the conclusion of each experimental trial, birds were weighed and each chick was scored for intestinal lesions representative of NE. Additionally, mortality was recorded in both groups over the duration of the study.
Weights: There were no differences in chick weights at placement (control = 44.0 g; supplement = 43.7 g; data not shown). At termination, group weights were measured, and the supplement-fed chicks tended to be numerically heavier than the chicks on the control diet (848.6 ± 26.6 g and 796.2 ± 3.8 g, respectively), but the difference was not statistically significant (Table 2; P = 0.19).
Mortality: Mortality was monitored over the duration of the study and the results are presented in Table 2. Any bird that died within the first 3 d after placement was not included in the mortality data. The mortality for birds on the control diet (72.2% or 52/72) was significantly higher (P = 0.004) than birds on the diet supplemented with the microencapsulated blend of organic acids and botanicals (47.3% or 35/74).
Lesion scores: The jejunum and ileum were scored for NE-associated lesions on a scale of 0 to 4, and the data are shown in Table 3. There were 2 birds on the control diet that had a score of 0 whereas 11 birds on the diet supplemented with the microencapsulated blend of organic acids and botanicals had a score of 0. There were 16 birds on the control diet that had a lesion score of 1 or 2 while 26 birds on the supplemented diet had similar lesion scores. The number of birds with a lesion score of 3 or 4 was 54 in the control group and 37 in the supplement treated group. The overall average lesion score for birds on the control diet was 3.3 ± 0.14 while birds on the diet supplemented with the microencapsulated blend of organic acids and botanicals had a significantly lower average lesion score of 2.47 ± 0.18 (P = 0.006).
Table 2.
Final weight and mortality of chickens on a control diet compared to those on a diet supplemented with a microencapsulated blend of organic acids and botanicals in a necrotic enteritis challenge model.
| Treatment | No. of trials | Weight (g) | Mortality (%) |
|---|---|---|---|
| Control diet | 3 | 796.2 ± 3.8 | 52/72 (72.2) |
| Supplemented diet1 | 3 | 848.6 ± 26.6 | 35/74 (47.3a) |
Birds fed the control diet supplemented with 500 g/MT of a microencapsulated blend of organic acids and botanicals (AviPlus P).
Columns with different subscripts are significantly different (P = 0.004).
Table 3.
Distribution of lesion scores of chickens on a control diet compared to those on a diet supplemented with a microencapsulated blend of organic acids and botanicals in a necrotic enteritis challenge model.
| Lesion score3 |
||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | n | 0 | 1 | 2 | 3 | 4 | Mean lesion score ± SEM | P-value |
| Control diet | 72 | 22 | 8 | 8 | 2 | 52 | 3.30 ± 0.14 | 0.006 |
| Supplemented diet1 | 74 | 11 | 17 | 9 | 0 | 37 | 2.47 ± 0.18 | |
Birds fed the control diet supplemented with 500 g/MT of a microencapsulated blend of organic acids and botanicals (AviPlus P).
Actual number of chickens in the experiment with a specific lesion score.
Lesions were scored on a scale of 0 to 4. A score of 0 indicated normal healthy tissue with no gross lesions; a score of 1 was characterized by thin-walled or friable tissue with a grey appearance; a score of 2 was thin-walled, had focal necrosis, and grey in appearance with small amounts of gas production; a score of 3 had thin walls with sizable patches of necrosis, gas-filled intestine, and small flecks of blood; and a score of 4 was defined by severe extensive necrosis, marked hemorrhage, and large amounts of gas in the intestine (Prescott, 1979).
KEGG Signaling Pathways
KEGG pathways for jejunal samples collected from control- and supplement-fed chickens subjected to a laboratory model to induce NE were generated using the STRING database. In total, 163 distinct signaling pathways were significantly (P < 0.05) different between the tissue collected from birds on the control diet compared to those on the diet supplemented with a microencapsulated blend of organic acids and botanicals. Signaling pathways with 5 or fewer significant peptides and those associated with cancer and viral infections were excluded, and the remaining 73 signaling pathways that were significant between the two treatments are detailed in Table 4.
Table 4.
Kyoto Encyclopedia of Genes and Genomes (KEGG) signaling pathways altered in the jejunum of chickens on a control diet compared to those on a diet supplemented with a microencapsulated blend of organic acids and botanicals in a necrotic enteritis challenge model.
| Term ID | Pathway description1 | Peptide count | FDR2 |
|---|---|---|---|
| hsa04152 | AMPK signaling pathway | 24 | 3.07E-21 |
| hsa04010 | MAPK signaling pathway | 31 | 1.07E-20 |
| hsa04910 | Insulin signaling pathway | 24 | 1.07E-20 |
| hsa04660 | T cell receptor signaling pathway | 21 | 1.65E-19 |
| hsa04722 | Neurotrophin signaling pathway | 22 | 1.65E-19 |
| hsa04931 | Insulin resistance | 21 | 4.91E-19 |
| hsa04920 | Adipocytokine signaling pathway | 17 | 6.92E-17 |
| hsa04150 | mTOR signaling pathway | 21 | 1.26E-16 |
| hsa04068 | FoxO signaling pathway | 20 | 1.61E-16 |
| hsa04140 | Autophagy - animal | 19 | 1.13E-15 |
| hsa04218 | Cellular senescence | 20 | 2.89E-15 |
| hsa04066 | HIF-1 signaling pathway | 17 | 6.03E-15 |
| hsa04922 | Glucagon signaling pathway | 17 | 7.65E-15 |
| hsa04211 | Longevity regulating pathway | 16 | 1.99E-14 |
| hsa04064 | NF-kappa B signaling pathway | 16 | 4.09E-14 |
| hsa04151 | PI3K-Akt signaling pathway | 25 | 8.39E-14 |
| hsa04510 | Focal adhesion | 20 | 1.17E-13 |
| hsa04012 | ErbB signaling pathway | 15 | 1.40E-13 |
| hsa04668 | TNF signaling pathway | 16 | 2.76E-13 |
| hsa04919 | Thyroid hormone signaling pathway | 16 | 6.39E-13 |
| hsa04370 | VEGF signaling pathway | 13 | 7.77E-13 |
| hsa01521 | EGFR tyrosine kinase inhibitor resistance | 14 | 8.81E-13 |
| hsa04932 | Non-alcoholic fatty liver disease | 17 | 1.49E-12 |
| hsa04371 | Apelin signaling pathway | 16 | 3.60E-12 |
| hsa04662 | B cell receptor signaling pathway | 13 | 4.81E-12 |
| hsa04213 | Longevity regulating pathway | 12 | 1.74E-11 |
| hsa04659 | Th17 cell differentiation | 14 | 1.96E-11 |
| hsa04664 | Fc epsilon RI signaling pathway | 12 | 4.15E-11 |
| hsa04714 | Thermogenesis | 18 | 7.24E-11 |
| hsa04072 | Phospholipase D signaling pathway | 15 | 1.15E-10 |
| hsa05418 | Fluid shear stress and atherosclerosis | 14 | 4.24E-10 |
| hsa04210 | Apoptosis | 14 | 4.77E-10 |
| hsa04071 | Sphingolipid signaling pathway | 13 | 8.69E-10 |
| hsa04921 | Oxytocin signaling pathway | 14 | 1.43E-09 |
| hsa04062 | Chemokine signaling pathway | 15 | 1.63E-09 |
| hsa04014 | Ras signaling pathway | 16 | 3.84E-09 |
| hsa04666 | Fc gamma R-mediated phagocytosis | 11 | 7.73E-09 |
| hsa04657 | IL-17 signaling pathway | 11 | 1.04E-08 |
| hsa04520 | Adherens junction | 10 | 1.28E-08 |
| hsa01522 | Endocrine resistance | 11 | 1.33E-08 |
| hsa04620 | Toll-like receptor signaling pathway | 11 | 2.48E-08 |
| hsa05145 | Toxoplasmosis | 11 | 4.48E-08 |
| hsa04926 | Relaxin signaling pathway | 11 | 2.37E-07 |
| hsa04144 | Endocytosis | 14 | 3.39E-07 |
| hsa04360 | Axon guidance | 12 | 4.47E-07 |
| hsa04658 | Th1 and Th2 cell differentiation | 9 | 8.20E-07 |
| hsa04912 | GnRH signaling pathway | 9 | 8.20E-07 |
| hsa04650 | Natural killer cell mediated cytotoxicity | 10 | 1.33E-06 |
| hsa04622 | RIG-I-like receptor signaling pathway | 8 | 1.71E-06 |
| hsa04015 | Rap1 signaling pathway | 12 | 2.11E-06 |
| hsa04621 | NOD-like receptor signaling pathway | 11 | 2.12E-06 |
| hsa04530 | Tight junction | 11 | 2.22E-06 |
| hsa04810 | Regulation of actin cytoskeleton | 12 | 2.22E-06 |
| hsa04310 | Wnt signaling pathway | 10 | 3.99E-06 |
| hsa04670 | Leukocyte transendothelial migration | 9 | 4.52E-06 |
| hsa04110 | Cell cycle | 9 | 9.10E-06 |
| hsa04611 | Platelet activation | 9 | 9.10E-06 |
| hsa04750 | Inflammatory mediator/regulation TRP channel | 8 | 9.55E-06 |
| hsa04915 | Estrogen signaling pathway | 9 | 1.55E-05 |
| hsa04270 | Vascular smooth muscle contraction | 8 | 5.18E-05 |
| hsa04540 | Gap junction | 7 | 5.75E-05 |
| hsa04022 | cGMP-PKG signaling pathway | 9 | 5.97E-05 |
| hsa04630 | Jak-STAT signaling pathway | 9 | 5.97E-05 |
| hsa04720 | Long-term potentiation | 6 | 9.60E-05 |
| hsa04024 | cAMP signaling pathway | 9 | 0.00024 |
| hsa04141 | Protein processing in ER | 8 | 0.00035 |
| hsa04728 | Dopaminergic synapse | 7 | 0.0005 |
| hsa04390 | Hippo signaling pathway | 7 | 0.0012 |
| hsa01200 | Carbon metabolism | 6 | 0.0016 |
| hsa04145 | Phagosome | 6 | 0.0046 |
| hsa01100 | Metabolic pathways | 22 | 0.0051 |
| hsa04217 | Necroptosis | 6 | 0.0061 |
| hsa04020 | Calcium signaling pathway | 6 | 0.0113 |
Pathways with five or fewer significant peptides or those related to cancer and viral infections were removed.
False discovery rate.
Specific KEGG Signaling Pathways and Common Peptides
Further evaluation of three highly significant pathways (P < 2.7 × 10−13) showed that the T cell receptor pathway, TNF signaling pathway, and NF-B signaling pathways shared common peptides that were significantly different between jejunal samples from chickens on the control diet compared to those on the supplemented diet Table 5. shows the significant peptides within the T cell receptor pathway, TNF signaling pathway, and NF-κB signaling pathways. The common peptides across these pathways are listed in bold and include CHUK (IKK-⍺), MAP3K14, MAP3K7, and NFKB1.
Table 5.
Select Kyoto Encyclopedia of Genes and Genomes (KEGG) signaling pathways and the specific peptides that are different between jejunum tissue from chickens on a control diet compared to those on a diet supplemented with a microencapsulated blend of organic acids and botanicals in a necrotic enteritis challenge model.
| T cell receptor pathway | TNF signaling pathway | NF-κB signaling pathway |
|---|---|---|
| AKT1 | AKT1 | BIRC3 |
| AKT3 | AKT3 | BTK |
| CARD11 | BIRC3 | CARD11 |
| CHUK1 | CASP8 | CHUK |
| FYN | CEBPB | CSNK2A1 |
| GSK3B | CHUK | LCK |
| ITK | MAP2K1 | MAP3K14 |
| LCK | MAP3K14 | MAP3K7 |
| MAP2K1 | MAP3K5 | NFKB1 |
| MAP2K2 | MAP3K7 | PLCG1 |
| MAP3K14 | MAPK14 | PLCG2 |
| MAP3K7 | NFKB1 | PRKCQ |
| MAPK14 | RPS6KA5 | TAB3 |
| NFATC1 | SOCS3 | TRAF2 |
| NFATC3 | TAB3 | TRAF6 |
| NFKB1 | TRAF2 | ZAP70 |
| PDPK1 | ||
| PLCG1 | ||
| PRKCQ | ||
| SOS1 | ||
| ZAP70 |
Peptides in bold are significantly different in each of these signaling pathways.
Cytokine mRNA Expression
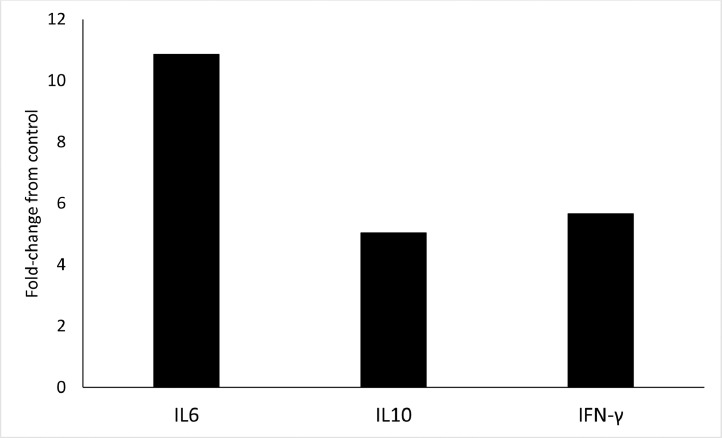
The mRNA expression levels of relevant cytokines in the T cell receptor pathway, TNF signaling pathway, or NF-B signaling pathways were measured to validate the kinome array. Jejunum samples were compared between control- and supplement-fed chickens and IL6, IL10, and IFN-γ mRNA expression was determined. The corrected 40-Ct for each cytokine was determined and in each instance was significantly (P < 0.002) higher in the tissue from chickens on the dietary supplement (data not shown). Additionally, the fold-change was determined and is shown in Figure 1. The mRNA expression of IL6 was 10.9-fold higher in jejunum collected from chickens on a diet supplemented with the microencapsulated blend of organic acids and botanicals while IL10 and IFN-γ were 5.0- and 5.7-fold greater, respectively, than the tissue sampled from chickens on the control diet.
Figure 1.
Fold-change of cytokine mRNA expression of jejunum samples from chickens on a diet supplemented with a microencapsulated blend of organic acids and botanicals compared to tissue from chickens on a control diet. Four tissues from control- and supplement-fed chickens were evaluated by quantitative real-time RT-PCR (n = 3 replicate experiments for a total of n = 12 chickens for each dietary treatment). Results were calculated as 40-cycle threshold (CT) for each tissue sample from control- and supplement-fed chickens and the data presented as fold-change from controls. Fold change was calculated as 2^(supplement-fed corrected mean – control-fed corrected mean).
DISCUSSION
The microencapsulated blend of organic acids and botanicals used in the current study (AviPlus P; identification number 4d3) is approved by the European Union Commission and European Food Safety Authority (EFSA) to enhance growth and feed efficiency in healthy chickens (EFSA, 2012). Previous studies evaluating this microencapsulated blend of organic acids and botanicals has shown that in addition to enhancing performance, it is also immunomodulatory and primes peripheral blood leukocyte functional activities in young chicks (Swaggerty et al., 2020b), alters key intestinal immune and metabolic signaling pathways (Swaggerty et al., 2020a) and microbial populations (Feye et al., 2020), and reduces colonization of important foodborne pathogens including Campylobacter (Grilli et al., 2013) and Salmonella (Grilli et al., 2011) in chickens.
With the re-emergence of NE outbreaks across the poultry sector, we sought to determine if the microencapsulated blend of organic acids and botanicals was also efficacious at reducing clinical signs of this devastating poultry disease, and if so, to determine the mode-of-action at the site of infection. Body weight at necropsy was measured, and despite the lack of significance (P > 0.05), the supplement-fed birds were still over 50 g heavier than their control counterparts (Table 2). The lack of significance may be due to the fact that weights were determined by pen as the birds were not individually weighed. A study using probiotics and phytobiotics to mitigate Clostridium-induced NE observed similar trends in final body weight that lacked significance (Hussein et al., 2020). To ensure the most accurate measurements, future studies will weigh birds individually and not whole pens.
The mortality (Table 2) and lesion scores (Table 3) observed in these studies indicate the NE model employed produced clinical NE rather than the milder sub-clinical disease often reported in other laboratorty studies (Swaggerty et al., 2016; Johnson et al., 2019; Kan et al., 2021). Despite the severity of disease observed across the 3 experimental trials, dietary supplementation with the microencapsulated blend significantly decreased both mortality and lesion scores compared to what was observed in the birds on the control diet. These findings are in agreement with other studies showing feed additives including organic acids and essential oils (Abdelli et al., 2020; Hofacre et al., 2020), citral (Yang et al., 2016), postbiotics (Johnson et al., 2019), or a combination of several additives (Calik et al., 2019; Du and Guo, 2021) have the potential to lessen the impact of NE in the absence of antibiotics or other medications. Most of the abovementioned studies improved gut health by reducing lesion scores and altering the microbial ecology, but not all were able to reduce mortality as we observed. This difference could be due, in part, to the specific organic acids and botanicals we used or the targeted delivery of the products. We and others have shown encapsulating the components with a lipid (or similar) matrix increases stability and allows for a slow and targeted release (Grilli et al., 2007; Piva et al., 2007; Yang et al., 2016) thereby increasing effectiveness.
To begin to understand the mechanism(s) of the microencapsulated blend of organic acids and botanicals used herein, our laboratory recently performed a kinome analysis of jejunal tissues collected from supplement-fed birds compared to tissues from control-fed birds in the absence of a microbial challenge (Swaggerty et al., 2020a). The kinome arrays can also be used to dissect signaling pathways and identify biomarkers associated with animal diseases such as bovine viral diarrhea virus (Van Wyk et al., 2016) and Johne's disease in cattle (Arsenault et al., 2012) and Salmonella in chickens (Arsenault et al., 2013; Kogut et al., 2016; Swaggerty et al., 2017) demonstrating the usefulness of the technology to provide detailed mechanistic insight into the host:pathogen interaction. Several studies have begun to identify specific host signaling pathways impacted by NE including MAPK signaling (Pham et al., 2020), JAK-STAT signaling (Truong et al., 2017a,b), and peroxisome proliferator-1 activated receptors (PPAR) signaling (Gharib-Naseri et al., 2021) pathways. Despite experimental model variations and the diversity of the tools used to determine the mode-of-action, all of these signaling pathways were also significantly different in the current study (Table 4) suggesting their overall importance in the underlying disease and the host:pathogen interaction(s) associated with NE in broilers.
To determine the seminal signaling pathways associated with increased livability and decreased intestinal pathology associated with NE, a section of the jejunum was analyzed using the kinome peptide array. Of the top 15 KEGG signaling pathways observed in our previous nonchallenge study (Swaggerty et al., 2020a), all those pathways were also significantly different in the NE model evaluated herein (Table 4). However, only 6 of the pathways remained in the top 15 under a NE challenge model indicating clinical NE dramatically altered the host response to combat the disease. The top KEGG signaling pathways observed in nonchallenged conditions (Swaggerty et al., 2020a) and the NE challenge were MAPK signaling, insulin signaling, neurotrophin signaling, mTOR signaling, FoxO signaling, and HIF-1 signaling pathways suggesting the feed additive is the main factor contributing to these changes and to a lesser extent NE; however, a deeper evaluation of the specific peptides would be required to fully understand changes within each pathway. To the best of our knowledge, there are limited publications using kinome array technology to delineate mechanistic pathways associated with NE and Clostridium challenges in chickens (Hashim et al., 2018; Johnson et al., 2019, 2020). Comparison of the most important KEGG pathways showed little overlap between those observed in the study by Johnson and colleagues (Johnson et al., 2019) compared to what was observed in the present study. The disparities are likely due to the different feed additive used (postbiotic compared to the blend of organic acids and botanicals), but other contributing factors could be the slight modifications to the approach to induce NE, or the genetic line of chickens used. Additionally, as previously suggested, NE could be “a syndrome, rather than a cause-effect linear infectious disease” (Johnson et al., 2020), but additional studies are required to support that hypothesis. Regardless of the differences between the studies, both show the effectiveness of antibiotic alternative feed additives to mitigate the adverse effects of NE and that the kinome array is an important tool that provides mechanistic insight into the pathways and peptides that are influencing the beneficial outcome(s).
Further examination of the T cell receptor, TNF, and NF-κB signaling pathways showed a small number of peptides that were significant in each pathway including CHUK (inhibitory-κB kinase [IKK] α), MAP3K14 (NFKB-inducing kinase [NIK]), MAP3K7 (transforming growth factor-β-activated kinase 1 [TAK1], and NFKB1 (Table 5). KEGG diagrams for these signaling pathways indicate phosphorylation changes in each of the aforementioned proteins (peptides) can result in cytokine production (KEGG, 2021a,b,c). NIK plays a pivotal role in immunity and inflammation across species (Pflug and Sitcheran, 2020) and TAK1 is involved in cell survival in chickens subjected to viral and bacterial challenge (Hashemi et al., 2021). Similarly, a study evaluating jejunal tissues from probiotic-fed birds under subclinical NE conditions shows activation of NF-κB highlighting the importance of this pathway in the intestinal immunological response to NE disease (Kan et al., 2021). To validate the kinome array, IL6, IL10, and IFN-γ cytokine mRNA expression was measured (Figure 1). In each instance, the observed mRNA expression level of each cytokine was higher in jejunal samples from chickens on the diet supplemented with the microencapsulated blend of organic acids and botanicals compared to tissue from those on the control diet confirming activation of the T cell receptor, TNF, and NF-κB signaling pathways. A change in cytokine expression is not surprising as NE is often accompanied by alterations in diverse cytokines including, but not limited to, IL2, IL4, IL5 (Truong et al., 2017b), IL17 (Kan et al., 2021), IL6, IL10, IL1-β (Emami et al., 2019; Zhang et al., 2019), and IFN-γ (Pham et al., 2020; Gharib-Naseri et al., 2021), several of which were upregulated in the present study. Ultimately, the challenge model, disease state (subclinical vs. clinical NE) and any therapeutic interventions or feed additives such as probiotics or essential oils will likely be the driving forces that influence the specific cytokine expression profile.
In conclusion, addition of the microencapsulated blend of organic (citric and sorbic) acids and botanicals (thymol and vanillin) to a broiler diet significantly reduced mortality and intestinal lesion scores associated with clinical NE. Mechanistically, the improvements were mediated, in part, by changes within the T cell receptor, TNF, and NF-κB signaling pathways and the accompanying cytokine responses.
ACKNOWLEDGMENTS
The authors thank Denise Caldwell, Jackie Kotzur, and M. Reiley Street, Jr. (U.S. Department of Agriculture, Agricultural Research Service [USDA/ARS], College Station, TX) for outstanding technical support and assistance with daily animal care. This research was supported, in part, by Vetagro S.p.A (Agreement number 58-3091-8-005) and the USDA/ARS. The USDA is an equal opportunity provider and employer. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. AP and EG are Vetagro employees but did not participate in the analysis of this study. All other authors declare no conflict of interest.
DISCLOSURES
AP and EG are Vetagro employees but did not participate in the analysis of this study. All other authors declare no conflict of interest.
REFERENCES
- Abdelli N., Perez J.F., Vilarrasa E., Cabeza Luna I., Melo-Duran D., D'Angelo M., Sola-Oriol D. Targeted-release organic acids and essential oils improve performance and digestive function in broilers under a necrotic enteritis challenge. Animals (Basel) 2020;10:259. doi: 10.3390/ani10020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari P., Kiess A., Adhikari R., Jha R. An approach to alternative strategies to control avian coccidiosis and necrotic enteritis. J. Appl. Poult. Res. 2020;29:515–534. [Google Scholar]
- AgGuide. 2020. Guide for the care and use of agricultural animals in research and teaching. Accessed Feb. 2022. https://www.aaalac.org/pub/?id=E900BDB6-CCCF-AB13-89B6-DA98A4B52218.
- Arsenault R.J., Kogut M.H. Chicken-specific peptide arrays for kinome analysis: flight for the flightless. Curr. Top. Biotechnol. 2012;7:79–89. [Google Scholar]
- Arsenault R.J., Lee J.T., Latham R., Carter B., Kogut M.H. Changes in immune and metabolic gut response in broilers fed beta-mannanase in beta-mannan-containing diets. Poult. Sci. 2017;96:4307–4316. doi: 10.3382/ps/pex246. [DOI] [PubMed] [Google Scholar]
- Arsenault R.J., Li Y., Bell K., Doig K., Potter A., Griebel P.J., Kusalik A., Napper S. Mycobacterium avium subsp. paratuberculosis inhibits gamma interferon-induced signaling in bovine monocytes: insights into the cellular mechanisms of Johne's disease. Infect. Immun. 2012;80:3039–3048. doi: 10.1128/IAI.00406-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault R.J., Napper S., Kogut M.H. Salmonella enterica Typhimurium infection causes metabolic changes in chicken muscle involving AMPK, fatty acid and insulin/mTOR signaling. Vet. Res. 2013;44:35. doi: 10.1186/1297-9716-44-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoluzzi C., Lahaye L., Perry F., Arsenault R.J., Santin E., Korver D.R., Kogut M.H. A protected complex of biofactors and antioxidants improved growth performance and modulated the immunometabolic phenotype of broiler chickens undergoing early life stress. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom L.J., Kogut M.H. Deciphering desirable immune responses from disease models with resistant and susceptible chickens. Poult. Sci. 2019;98:1634–1642. doi: 10.3382/ps/pey535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calik A., Omara II., White M.B., Evans N.P., Karnezos T.P., Dalloul R.A. Dietary non-drug feed additive as an alternative for antibiotic growth promoters for broilers during a necrotic enteritis challenge. Microorganisms. 2019;7:257. doi: 10.3390/microorganisms7080257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon J.I.R. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007;86:2466–2471. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- Du E., Guo Y. Dietary supplementation of essential oils and lysozyme reduces mortality and improves intestinal integrity of broiler chickens with necrotic enteritis. Anim. Sci. J. 2021;92:e13499. doi: 10.1111/asj.13499. [DOI] [PubMed] [Google Scholar]
- EFSA Scientific opinion on the safety and efficacy of AviPlus®as feed additive for chickens and minor avian species for fattening and reared for laying and minor porcine species (weaned) EFSA J. 2012;10:2670. [Google Scholar]
- Emami N.K., Calik A., White M.B., Young M., Dalloul R.A. Necrotic enteritis in broiler chickens: the role of tight junctions and mucosal immune responses in alleviating the effect of the disease. Microorganisms. 2019;7:231. doi: 10.3390/microorganisms7080231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feye K.M., Swaggerty C.L., Kogut M.H., Ricke S.C., Piva A., Grilli E. The biological effects of microencapsulated organic acids and botanicals induces tissue-specific and dose-dependent changes to the Gallus gallus microbiota. BMC Microbiol. 2020;20:332. doi: 10.1186/s12866-020-02001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficken M.D., Wages D.P. In: Pages 255–274 in Diseases of Poultry. Calnek B.W., editor. Iowa State Univ. Press; Ames, IA: 1997. Clostridial diseases. [Google Scholar]
- Gessner D.K., Ringseis R., Eder K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. (Berl) 2017;101:605–628. doi: 10.1111/jpn.12579. [DOI] [PubMed] [Google Scholar]
- Gharib-Naseri K., de Las Heras-Saldana S., Kheravii S., Qin L., Wang J., Wu S.B. Necrotic enteritis challenge regulates peroxisome proliferator-1 activated receptors signaling and beta-oxidation pathways in broiler chickens. Anim. Nutr. 2021;7:239–251. doi: 10.1016/j.aninu.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli E., Bodin J.C., Gatta P.P., Tedeschi M., Piva A. Microencapsulation allows slow release of organic acids in the GI tract of broilers. Proc. 16th Eur. Symp. Poult. Nutr.; Strasbourg, France, CABI Publishing: 2007. [Google Scholar]
- Grilli E., Tugnoli B., Formigoni A., Massi P., Fantinati P., Tosi G., Piva A. Microencapsulated sorbic acid and nature-identical compounds reduced Salmonella Hadar and Salmonella Enteritidis colonization in experimentally infected chickens. Poult. Sci. 2011;90:1676–1682. doi: 10.3382/ps.2011-01441. [DOI] [PubMed] [Google Scholar]
- Grilli E., Vitari F., Domeneghini C., Palmonari A., Tosi G., Fantinati P., Massi P., Piva A. Development of a feed additive to reduce caecal Campylobacter jejuni in broilers at slaughter age: from in vitro to in vivo, a proof of concept. J. Appl. Microbiol. 2013;114:308–317. doi: 10.1111/jam.12053. [DOI] [PubMed] [Google Scholar]
- Hashemi S., Hosseini S.M., Ghalyanchilangeroudi A., Sheikhi N. Transcriptome based analysis of apoptosis genes in chickens co-infected with avian infectious bronchitis virus and pathogenic Escherichia coli. Iran. J. Microbiol. 2021;13:17–22. doi: 10.18502/ijm.v13i1.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashim M.M., Arsenault R.J., Byrd J.A., Kogut M.H., Al-Ajeeli M., Bailey C.A. Influence of different yeast cell wall preparations and their components on performance and immune and metabolic pathways in Clostridium perfringens-challenged broiler chicks. Poult. Sci. 2018;97:203–210. doi: 10.3382/ps/pex290. [DOI] [PubMed] [Google Scholar]
- Hofacre C.L., Mathis G.F., Lumpkins B.S., Sygall R., Vaessen S., Hofacre C.S., Smith J.A., Clanton E. Efficacy of butyric and valeric acid esters in a necrotic enteritis challenge model. Avian Dis. 2020;64:407–414. doi: 10.1637/aviandiseases-D-19-00124. [DOI] [PubMed] [Google Scholar]
- Hussein E.O.S., Ahmed S.H., Abudabos A.M., Suliman G.M., Abd El-Hack M.E., Swelum A.A., Alowaimer A.N. Ameliorative effects of antibiotic-, probiotic- and phytobiotic-supplemented diets on the performance, intestinal health, carcass traits, and meat quality of Clostridium perfringens-infected broilers. Animals (Basel) 2020;10:669. doi: 10.3390/ani10040669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.N., Hashim M.M., Bailey C.A., Byrd J.A., Kogut M.H., Arsenault R.J. Feeding of yeast cell wall extracts during a necrotic enteritis challenge enhances cell growth, survival and immune signaling in the jejunum of broiler chickens. Poult. Sci. 2020;99:2955–2966. doi: 10.1016/j.psj.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.N., Kogut M.H., Genovese K., He H., Kazemi S., Arsenault R.J. Administration of a postbiotic causes immunomodulatory responses in broiler gut and reduces disease pathogenesis following challenge. Microorganisms. 2019;7:268. doi: 10.3390/microorganisms7080268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser P., Rothwell L., Galyov E.E., Barrow P.A., Burnside J., Wigley P. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis, and Salmonella gallinarum. Microbiology. 2000;146:3217–3226. doi: 10.1099/00221287-146-12-3217. [DOI] [PubMed] [Google Scholar]
- Kaiser P., Rothwell L., Goodchild M., Bumstead N. The chicken proinflammatory cytokines interleukin-1á and interleukin-6: differences in gene structure and genetic location compared with their mammalian orthologues. Anim. Genet. 2004;35:169–175. doi: 10.1111/j.1365-2052.2004.01121.x. [DOI] [PubMed] [Google Scholar]
- Kaiser P., Yeow-Poh T., Rothwell L., Avery S., Balu S., Pathania U.S., Hughes S., Goodchild M., Morrell S., Watson M., Bumstead N., Kaufman J., Young J.R. A genomic analysis of chicken cytokines and chemokines. J. Interferon Cytokine Res. 2005;25:467–484. doi: 10.1089/jir.2005.25.467. [DOI] [PubMed] [Google Scholar]
- Kan L., Guo F., Liu Y., Pham V.H., Guo Y., Wang Z. Probiotics Bacillus licheniformis improves intestinal health of subclinical necrotic enteritis-challenged broilers. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.623739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keerqin C., Morgan N.K., Wu S.B., Svihus B., Choct M. Reintroduction of microflora from necrotic enteritis-resistant chickens reduces gross lesion and improves performance of necrotic enteritis-challenged broilers. J. Appl. Poult. Res. 2017;26:449–457. [Google Scholar]
- KEGG 2021a. NF-Kappa B signaling pathway. Accessed Sept. 2021. https://www.genome.jp/kegg-bin/show_pathway?hsa04064.
- KEGG 2021b. T cell receptor signaling pathway. Accessed Sept. 2021. https://www.genome.jp/kegg-bin/show_pathway?hsa04660.
- KEGG 2021c. TNF signaling pathway. Accessed Sept. 2021. https://www.genome.jp/kegg-bin/show_pathway?hsa04668.
- Kogut M.H., Swaggerty C.L., Byrd J.A., Selvaraj R., Arsenault R.J. Chicken-specific kinome array reveals that Salmonella enterica serovar Enteritidis modulates host immune signaling pathways in the cecum to establish a persistence infection. Int. J. Mol. Sci. 2016;17:1207. doi: 10.3390/ijms17081207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G., Whyte D.B., Martinez R., Hunter T., Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- McReynolds J.L., Byrd J.A., Genovese K.J., Poole T.L., Duke S.E., Farnell M.B., Nisbet D.J. Dietary lactose and its effect on the disease condition of necrotic enteritis. Poult. Sci. 2007;86:1656–1661. doi: 10.1093/ps/86.8.1656. [DOI] [PubMed] [Google Scholar]
- Micciche A.C., Foley S.L., Pavlidis H.O., McIntyre D.R., Ricke S.C. A review of prebiotics against Salmonella in poultry: current and future potential for microbiome research applications. Front. Vet. Sci. 2018;5:191. doi: 10.3389/fvets.2018.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Ouyang Z., Takats Z., Blake T.A., Gologan B., Guymon A.J., Wiseman J.M., Oliver J.C., Davisson V.J., Cooks R.G. Preparing protein microarrays by soft-landing of mass-selected ions. Science. 2003;301:1351–1354. doi: 10.1126/science.1088776. [DOI] [PubMed] [Google Scholar]
- Paiva D., McElroy A. Necrotic enteritis: applications for the poultry industry. J. Appl. Poult. Res. 2014;23:557–566. [Google Scholar]
- Pflug K.M., Sitcheran R. Targeting NF-kappaB-inducing kinase (NIK) in immunity, inflammation, and cancer. Int. J. Mol. Sci. 2020;21:8470. doi: 10.3390/ijms21228470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham T.T., Ban J., Hong Y., Lee J., Vu T.H., Truong A.D., Lillehoj H.S., Hong Y.H. MicroRNA gga-miR-200a-3p modulates immune response via MAPK signaling pathway in chicken afflicted with necrotic enteritis. Vet. Res. 2020;51:8. doi: 10.1186/s13567-020-0736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piva A., Pizzamiglio V., Morlacchini M., Tedeschi M., Piva G. Lipid microencapsulation allows slow release of organic acids and natural identical flavors along the swine intestine. J. Anim. Sci. 2007;85:486–493. doi: 10.2527/jas.2006-323. [DOI] [PubMed] [Google Scholar]
- Prescott J.F. The prevention of experimentally induced necrotic enteritis in chickens by Avoparcin. Avian Dis. 1979;24:1072–1074. [PubMed] [Google Scholar]
- Rothwell L., Young J.R., Zoorob R., Whittaker C.A., Hesketh P., Archer A., Smith A.L., Kaiser P. Cloning and characterization of chicken IL-10 and its role in the immune response to Eimeria maxima. J. Immunol. 2004;173:2675–2682. doi: 10.4049/jimmunol.173.4.2675. [DOI] [PubMed] [Google Scholar]
- Smith H.W. The development of the flora of the alimentary tract in young animals. J. Patholog. Bacteriol. 1965;90:495–513. [PubMed] [Google Scholar]
- Suresh G., Das R.K., Kaur Brar S., Rouissi T., Avalos Ramirez A., Chorfi Y., Godbout S. Alternatives to antibiotics in poultry feed: molecular perspectives. Crit. Rev. Microbiol. 2018;44:318–335. doi: 10.1080/1040841X.2017.1373062. [DOI] [PubMed] [Google Scholar]
- Svihus B. Function of the digestive system. J. Appl. Poult. Res. 2014;23:306–314. [Google Scholar]
- Svihus B. Starch digestion capacity of poultry. Poult. Sci. 2014;93:2394–2399. doi: 10.3382/ps.2014-03905. [DOI] [PubMed] [Google Scholar]
- Swaggerty C.L., Arsenault R.J., Johnson C., Piva A., Grilli E. Dietary supplementation with a microencapsulated blend of organic acids and botanicals alters the kinome in the ileum and jejunum of Gallus gallus. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaggerty C.L., He H., Genovese K.J., Callaway T.R., Kogut M.H., Piva A., Grilli E. A microencapsulated feed additive containing organic acids, thymol, and vanillin increases in vitro functional activity of peripheral blood leukocytes from broiler chicks. Poult. Sci. 2020;99:3428–3436. doi: 10.1016/j.psj.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaggerty C.L., Kogut M.H., He H., Genovese K.J., Johnson C., Arsenault R.J. Differential levels of cecal colonization by Salmonella enteritidis in chickens triggers distinct immune kinome profiles. Front. Vet. Sci. 2017;4:1–14. doi: 10.3389/fvets.2017.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaggerty C.L., McReynolds J.L., Byrd J.A., Pevzner I.Y., Duke S.E., Genovese K.J., He H., Kogut M.H. Selection for pro-inflammatory mediators produces chickens more resistant to Clostridium perfringens-induced necrotic enteritis. Poult. Sci. 2016;95:370–374. doi: 10.3382/ps/pev348. [DOI] [PubMed] [Google Scholar]
- Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 2011;40:341–347. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- Trost B., Kindrachuk J., Maattanen P., Napper S., Kusalik A. PIIKA 2: an expanded, web-based platform for analysis of kinome microarray data. PLoS One. 2013;8:e80837. doi: 10.1371/journal.pone.0080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong A.D., Rengaraj D., Hong Y., Hoang C.T., Hong Y.H., Lillehoj H.S. Analysis of JAK-STAT signaling pathway genes and their microRNAs in the intestinal mucosa of genetically disparate chicken lines induced with necrotic enteritis. Vet. Immunol. Immunopathol. 2017;187:1–9. doi: 10.1016/j.vetimm.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Truong A.D., Rengaraj D., Hong Y., Hoang C.T., Hong Y.H., Lillehoj H.S. Differentially expressed JAK-STAT signaling pathway genes and target microRNAs in the spleen of necrotic enteritis-afflicted chicken lines. Res. Vet. Sci. 2017;115:235–243. doi: 10.1016/j.rvsc.2017.05.018. [DOI] [PubMed] [Google Scholar]
- Van der Sluis W. Clostridial enteritis is an often underestimated problem. Worlds Poult. Sci. J. 2000;126:42–43. [Google Scholar]
- Van Immerseel F., De Buck J., Pasmans F., Huyghebaert G., Haesebrouck F., Ducatelle R. Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. 2004;33:537–549. doi: 10.1080/03079450400013162. [DOI] [PubMed] [Google Scholar]
- Van Wyk B., Snider M., Scruten E., van Drunen Littel-van den Hurk S., Napper S. Induction of functional interferon alpha and gamma responses during acute infection of cattle with non-cytopathic bovine viral diarrhea virus. Vet. Microbiol. 2016;195:104–114. doi: 10.1016/j.vetmic.2016.09.015. [DOI] [PubMed] [Google Scholar]
- Wade, B., and A. Keyburn 2015. The true cost of necrotic enteritis. Accessed Sept. 2021. https://www.poultryworld.net/Meat/Articles/2015/10/The-true-cost-of-necrotic-enteritis-2699819W/.
- Wang X.-H. Progress in chemical proteomics-based kinome study. J. Intl. Pharmaceut. Res. 2014;41:259–267. [Google Scholar]
- Yadav S., Jha R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019;10:2. doi: 10.1186/s40104-018-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Wang Q., Diarra M.S., Yu H., Hua Y., Gong J. Functional assessment of encapsulated citral for controlling necrotic enteritis in broiler chickens. Poult. Sci. 2016;95:780–789. doi: 10.3382/ps/pev375. [DOI] [PubMed] [Google Scholar]
- Yin D., Du E., Yuan J., Gao J., Wang Y., Aggrey S.E., Guo Y. Supplemental thymol and carvacrol increases ileum Lactobacillus population and reduces effect of necrotic enteritis caused by Clostridium perfringes in chickens. Sci. Rep. 2017;7:7334. doi: 10.1038/s41598-017-07420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z., Zhang S., Wang H., Piao X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: a review. J. Anim. Sci. Biotechnol. 2015;6:7. doi: 10.1186/s40104-015-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Gan L., Shahid M.S., Lv Z., Fan H., Liu D., Guo Y. In vivo and in vitro protective effect of arginine against intestinal inflammatory response induced by Clostridium perfringens in broiler chickens. J. Anim. Sci. Biotechnol. 2019;10:73. doi: 10.1186/s40104-019-0371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]