Abstract
The odour emitted from the high-tannin fab bean flour (Vicia faba var. minor), was characterized by headspace solid-phase microextraction/gas chromatography-mass spectrometry (HS-SPME/GC–MS). The relative odour activity value (ROAV) was used to monitor the changes in key volatile compounds in the flour during short-term storage at different temperature conditions. The key flavour compounds of freshly milled flour included hexanal, octanal, nonanal, decanal, 3-methylbutanal, phenyl acetaldehyde, (E)-2-nonenal, 1-hexanol, phenyl ethyl alcohol, 1-octen-3-ol, β-linalool, acetic acid, octanoic acid, and 3-methylbutyric acid; these are oxidative degradation products of unsaturated fatty acids and amino acids. Despite the low lipid content of faba beans, the abundances of aldehydes arising during room temperature storage greatly contributed to the flavour of the flour due to their very low odour thresholds. Two of the key volatiles responsible for beany flavour in flour (hexanal, nonanal) increased greatly after 2 weeks of storage at room temperature or under refrigerated conditions. These volatile oxidation products may arise as a result of enzymatic activity on unsaturated fatty acids, and was seen to be arrested by freezing the flour.
Keywords: Faba bean flour, aroma components, sensory properties, quality ingredients, beany flavour
Introduction
Pulses can be consumed as cooked whole grains, or they can be processed into flours, or fractionated into ingredients, such as starch, fibre, or protein isolates. Bakery products, soups, pasta, noodles and canned products are all examples of pulse and pulse flours based foods (Abu-Ghannam and Gowen, 2011).
One of the impediments to further broadening the use of pulses as functional food ingredients is the distinct beany flavour often associated with them (Kaneko et al., 2011). Beany, green, grassy, earthy, rancid, and muddy are the main beany-related flavours of pulses described by consumers (Bott and Chambers, 2006). Beany flavours in raw pulse flours are thought to be mainly derived from hydrolytic and oxidative degradation of lipids and degradation of amino acids (MacLeod et al., 1988). Despite the relative low content of lipids in pea (up to 4%) (Coxon and Wright, 1985) and faba bean (up to 3%) (Nitsan, 1971), these pulses can still have a distinctive beany flavour, and also may impart other non-volatile flavours contributing to bitterness after baking (Hinchcliffe et al., 1977). As a result, both pulses are still under-utilised in many countries.
As is well known, many foods contain components that can degrade into off-flavours caused by lipid oxidation, non-enzymatic browning, enzymatic changes, or photo-catalyzed reactions (Reineccius, 2006). Certain of these volatile flavour compounds can be used as key markers of the beany flavour of pulses. Examples of these include hexanal, (E,E)-2,4-nonadienal, (E,E)-2,4-decadienal, 3-methyl-1-butanol, 1-hexanol, and 2-pentyl-furan in pea flour (Xu et al., 2019); also 2-methylfuran, 2-pentylfuran, hexanal, nonanal, and 2-heptanone in faba bean flour (Jiang et al., 2016). Some of these volatiles are indicators of enzymatic activities, such as the actions of peroxidases and lipoxygenases on unsaturated fatty acids. In fact, it has been suggested that the very high lipid hydrolysing activity that is present in faba bean may affect not only lipids from the seeds, but also other lipids in food products (Yang et al., 2017).
Most pulses can be stored for a long time without spoiling yet still retaining their nutrients, when stored in a cool and dry place. However, the abundances of nonanal and hexanal have been observed to significantly increase over storage time when pulse flour was kept at room temperature (Akkad et al., 2019). The permeability of the packaging material can also have a substantial effect on flavour, since foods can lose aroma and flavour components by absorption into, or permeation through, the packaging material; it also governs moisture and oxygen permeation into the food. The use of plastics has replaced paper, glass, and metal in many food packaging applications (Geueke et al., 2018) and pulse flours are commonly sold in plastic packages. In a previous study of the high-tannin faba bean flour (Akkad et al., 2019), storage time as well as packaging greatly influenced the resulting profile of volatile compounds. These changes included a significant difference in the contents of both aldehydes and alcohols, as shown by comparisons between stored and freshly milled flours. In addition, traces of phthalates derived from the plastic bags were evident.
Storing faba bean flour at different conditions of temperature and humidity will have a great impact on the key volatile compounds that largely contribute to the volatile flavour profile of the flour. HS-SPME/GC-MS is a suitable technique to elucidate the volatile flavour profiles of faba bean flour (Akkad et al., 2019), and was chosen to reveal changes occurring during storage at different conditions. Thus, the novelty of the study is in monitoring changes that take place when faba bean flours are stored for short periods under differing conditions and in studying how these changes affect the quality of faba bean flours as food ingredients. In this new study, packaging in polyethylene zipper-closure bags was used, since these are commonly available for domestic re-packaging. In order to investigate changes contributing to the beany flavour of high-tannin faba bean flour, headspace volatiles measurements were performed over two months of storage at room temperature (RT), under refrigeration, and in a deep freezer.
Materials and methods
Flour filling and storage conditions
Seeds of high-tannin faba beans (Vicia faba var. minor, from the 2016 crop year) were grown in Alberta, Canada. The seeds were milled in the laboratory using a Micro-Mill (Science ware, Bel-Art Products, USA) at speed of 10,000 RPM for 60 s. A water-cooled system was applied to protect the mill from overheating. Portions of freshly milled flour (15 g) of whole high-tannin seeds (WHT) were filled into 3 polyethylene zipper-closure bags (Durable and transparent polyethylene bags (PE), Thermo Fisher Scientific, USA) and stored in darkness, and in a solvent-free location under the following conditions:
Room temperature of 22°C and 19% RH
Refrigerated at 4°C and 9% RH
Frozen at –21°C
The storage temperatures and relative humidity (RH) were measured using a Traceable Remote Alarm RH/Temperature Monitor (Thermo Fisher Scientific, USA). The volatile flavour profile of each sample packed in bags as described above was determined after 7, 14, 28, and 60 days and compared to the profile of the freshly ground faba bean seeds (day 0, FG). All measurements were performed in triplicate.
Sample preparation and characterization
The volatile flavour profiles of faba bean flours stored under different conditions over 2 months were analysed using headspace solid-phase microextraction gas chromatography-mass spectrometry (HS-SPME/GC-MS). Full details and optimization of this method are as described in our previous report (Akkad et al., 2019).
Data analysis
After subtraction of background peaks that were found to be present in a blank sample (including any fibre and column bleed), the GC peaks representing volatiles of all samples were identified using electron ionization (EI) mass spectrometry (MS) and matching spectra via a mass spectral library search (NIST library, National Institutes of Standards and Technology, ver. 2.0, 2017, Gaithersburg, MD, USA). The identified volatiles were then categorized into 9 different groups including aromatic hydrocarbons, aldehydes, alkanes, alkenes, alcohols, ketones, organic acids, esters, and others. The levels of these volatile components were determined from the average of triplicate measurements and expressed as peak areas (total area counts, arbitrary units). The relative peak area (RPA) of each volatile compound was expressed as a percentage of the total peak area (TPA, the sum of the peak areas of all volatile compounds, after background subtraction) which allowed a semi-quantitative comparison to be made (Azarnia et al., 2011):
Total peak area for each group (G-TPA, %) = ∑ RPA in each group
Relative odour activity value (ROAV) was determined to measure the contribution of each volatile compound towards the entire aroma profile and calculated using the following equation described in (Liu et al., 2008):
Where OAV i is the odour activity value, C i is the concentration of compound i in the sample (here is RPA (%)), and OT i is its odour detection threshold concentration measured in air, found in literature (Van Gemert, 2011). OAV max the highest value of OAV i amongst all compounds, in each measurement. Compounds with ROAV ≥1 significantly contribute to aroma and are considered key volatile components, whereas compounds with 0.1 ≤ROAV <1 also contribute to the whole flavour. Analysis of variance (ANOVA) and Post Hoc test (Tukey HSD, 95% Confidence Interval) were performed using IBM SPSS 18.0.
Results and discussion
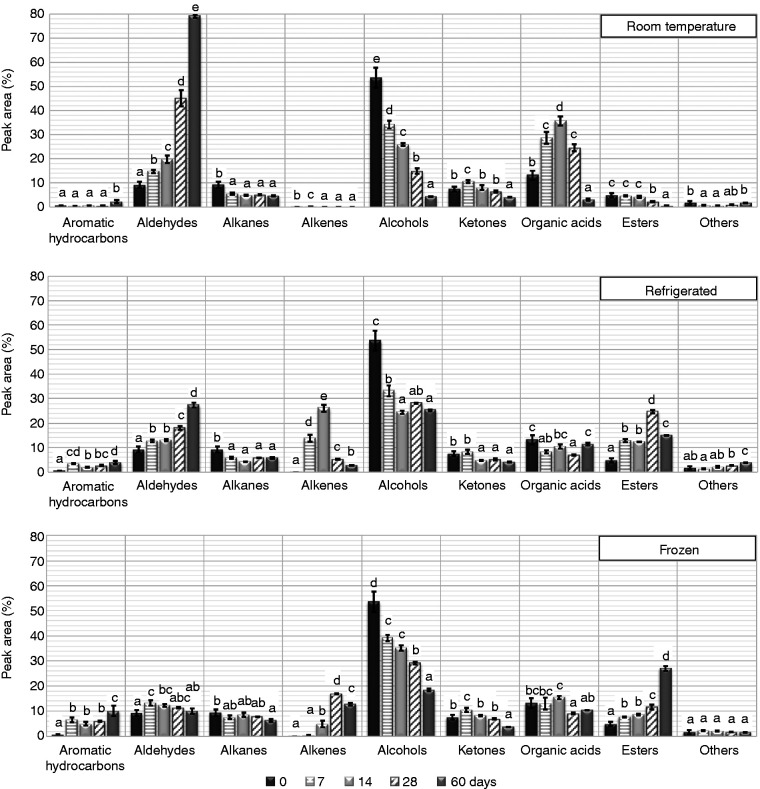
In general, reactions occurring within food can be retarded by storing at low temperatures, i.e., in refrigerators, or even inhibited if frozen for later use. The changes that occur during the storage of food depend on the components present in the food itself and their activities in response to the storage conditions, such as exposure to sunlight, temperature, and relative humidity (Nummer et al., 2013). Figure 1 shows the volatile profile of faba bean flour during storage under different temperature conditions. Regardless of the storage condition, the abundance of most of the volatiles, especially the aldehydes, alcohols, and organic acids, have significantly changed, even after only one week of storage. Although after one week, the aldehyde content was similar for all conditions of storage, it significantly increased after two weeks’ storage at RT and under refrigeration. After 2 months, the total volatile aldehydes increased by about 80% at RT, and by 28% under refrigeration, whereas in the freezer, its abundance increased slightly for the first 2 weeks, then decreased. Alcohols decreased significantly under all conditions but at different rates; similar changes were seen for ketones but to a much lower extent. The notable increase in the organic acids content at RT was due to the presence of a high concentration of 3-methyl butyric acid. This was also the dominant volatile organic acid under the other storage conditions, but at a lower concentration. The total organic acids content has showed no clear trend during storage at lower temperatures. Alkanes were found at low concentrations with a generally decreasing trend over storage time at all conditions; their abundance was slightly higher in frozen flour (Figure 1). The total abundance of alkenes significantly increased during low-temperature storage, dominated by the presence of D-limonene (Table S1).
Figure 1.
The total volatile flavour profile of whole high-tannin faba bean flour (WHT) stored in zipper-closure bags at different temperatures, over two months storage. Data were represented in total peak area for each group (G-TPA, %). Error bars show standard deviation (n = 3). Different letters above bars represent statistical significant differences established by the Post Hoc test (Tukey HSD, 95% Confidence Interval).
Surprisingly, at RT this volatile was not observed, and hence the alkene content was negligible. Esters were present at low abundance which steadily decreased over time at RT, whereas, they generally increased during low-temperature storage.
The concentration of aromatic hydrocarbons was significantly higher in the headspace of refrigerated and frozen samples than at RT, (Figure 1). This class was dominated by the presence of toluene and ethylbenzene, both of which were present under all storage conditions and increased with storage time. These are likely environmental pollutants, which can be taken up by plants in small amounts (Irwin et al., 1997). It should be noted that the volatiles profile observed by SPME is partly a result of competitive adsorption onto the fibre, so for low abundance compounds, such as the aromatic hydrocarbons at RT, there is less chance of observing these minor components (e.g., xylenes, styrene, etc., Table S1) than at lower storage temperatures where their abundances are relatively higher (Figure 1). The natural presence of such compounds as styrene in the volatile flavour of faba beans was reported in an earlier study (Oomah et al., 2014).
The volatile compounds that have been used as markers to quantify the beany flavour formation in the flours of chickpea, lentil, and yellow pea (Xu et al., 2019), include hexanal, (E,E)-2,4-nonadienal, (E,E)-2,4- decadienal, 1-hexanol, and 2-pentylfuran, are derived from oxidation of the unsaturated fatty acids linoleic and linolenic (Jeleń and Wasowicz, 2012). For faba beans, 2-methylfuran, 2-pentylfuran, hexanal, nonanal, and 2-heptanone are thought to contribute to the beany flavour (Jiang et al., 2016), and are also derived from oxidation of linoleic and linolenic acids.
Aroma, which is important in assessing food quality, is often strongly affected by lipid oxidation, which can lead to the formation of potent odorants including saturated and unsaturated aldehydes (Belitz et al., 2009). In plant foods, lipases and lipooxygenases are also responsible for the formation of characteristic rancid flavours, including aldehydes, alcohols, acids, etc. Lipid oxidation processes are also affected by the storage temperature, oxygen levels, heat treatments, homogenization, and packaging. Food deterioration marked by off-flavours is therefore often a result of lipid oxidation, even when the lipid content is very low. The odour activity of a compound can be expressed as the ratio of its concentration in an extract to its aroma threshold. Thus, during food storage, the odour activity resulting from carbonyl (or other) compounds may increase, along with the increase in its abundance (Frankel, 2005).
In order to determine which volatiles have the greatest aroma impact on faba bean flour, the relative odour activity values (ROAV) for all volatiles of the freshly milled flour were calculated, using literature values of the odour thresholds (Van Gemert, 2011). All compounds for which ROAV ≥ 0.1, are listed in Table 1. The key volatile compounds (ROAV ≥ 1) that were identified are labeled with asterisks. These can be grouped according to each volatile’s origin into: 1) volatiles originating from unsaturated fatty acids, including hexanal, octanal, nonanal, decanal, (E)-2-nonenal, 1-hexanol, 1-octen-3-ol, and octanoic acid; 2) volatiles originating from amino acids (Spinnler, 2012), including 3-methylbutanal, phenyl acetaldehyde, phenyl ethyl alcohol, acetic acid, and 3-methylbutyric acid; and 3) volatiles of other origins, including β-linalool. At longer storage times, some volatiles including 3-methyl-1-butanol, 1-nonanol, and nonanoic acid become key compounds (ROAV ≥ 1), changing the overall volatile profile at that time. Overall, it can be seen from Table 1 that the flavour profile of faba bean flour is most negatively affected by volatiles originating from fatty acids. For example, the ROAV values for nonanal, decanal and 2-nonenal are all high and increasing with time. However, volatiles originating from amino acids, such as 3-methylbutyric acid, are also clearly significant. Other key volatiles, some of which individually may have more pleasant descriptors, such as 3-methylbutanal or β-Linalool, also contribute.
Table 1.
Odour contribution of volatile components (ROAV) of faba bean flour under different temperature conditions over storage time.
| FG |
Room temperature |
Refrigerated |
Frozen |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Volatile compound/day | 0 | 7 | 14 | 28 | 60 | 7 | 14 | 28 | 60 | 7 | 14 | 28 | 60 | TOVa | Key odorantb | |
| UFA | Hexanal* | 6.93 | 2.78 | 3.01 | 12.26 | 17.80 | 22.91 | 11.00 | 7.40 | 6.05 | 11.07 | 8.09 | 9.72 | 3.61 | 0.20 | Fat, grass, tallow |
| Heptanal | 0.71 | 0.45 | 0.64 | 1.47 | 1.49 | 2.62 | 2.23 | 1.59 | 1.25 | nd | nd | nd | nd | 0.18 | Fat, citrus, rancid | |
| Octanal* | 4.30 | 5.05 | 14.01 | 48.14 | 31.77 | 23.35 | 32.59 | 13.76 | 21.12 | 9.65 | 8.96 | 13.34 | 9.36 | 0.01 | Fat, soap, lemon, green | |
| Nonanal* | 34.89 | 24.59 | 27.92 | 100 | 100 | 98.22 | 98.66 | 100 | 100 | 55.84 | 47.99 | 90.92 | 58.33 | 0.05 | Fat, citrus, green | |
| Decanal* | 4.82 | 8.03 | 13.12 | 37.20 | 22.37 | 19.87 | 25.15 | 30.51 | 31.87 | 7.27 | 8.30 | 14.58 | 16.18 | 0.04 | Soap, orange peel, tallow | |
| (E)-2-Octenal | 0.24 | 0.09 | 0.08 | 0.19 | 0.12 | 0.49 | 0.87 | 0.18 | 0.12 | 0.24 | 0.23 | 0.41 | 0.25 | 0.52 | Green, nut, fat | |
| (E)-2-Nonenal* | 4.53 | 4.46 | 4.16 | 19.56 | 22.78 | 21.27 | 12.85 | 9.70 | 6.82 | 6.91 | 5.92 | 8.46 | 6.97 | 0.02 | Cucumber, fat, green | |
| 1-Pentanol | 0.14 | 0.05 | 0.04 | 0.04 | 0.02 | 0.33 | 0.15 | 0.10 | 0.04 | 0.20 | 0.13 | 0.19 | 0.08 | 5.55 | Balsamic | |
| 1-Hexanol* | 6.67 | 0.33 | 0.18 | 0.15 | 0.02 | 1.91 | 0.94 | 0.67 | 0.24 | 1.15 | 0.99 | 1.36 | 0.48 | 2.39 | Resin, flower, green | |
| 1-Octanol | 0.40 | 0.07 | 0.13 | 0.13 | 0.05 | 0.69 | 0.54 | 0.42 | 0.28 | 0.49 | 0.46 | 0.76 | 0.42 | 0.94 | Chemical, metal, burnt | |
| 1-Nonanol | 0.48 | 0.24 | 0.17 | 0.24 | 0.13 | 1.65 | 1.07 | 1.00 | 0.61 | 0.71 | 0.61 | 1.18 | 1.09 | 0.90 | Fat, green | |
| 1-Octen-3-ol* | 4.37 | 1.72 | 0.45 | 0.50 | 0.22 | 9.71 | 4.98 | 3.79 | 1.90 | 7.63 | 5.72 | 6.67 | 0.90 | 0.19 | Mushroom | |
| 2-Heptanone | 0.17 | 0.08 | 0.08 | 0.09 | 0.05 | 0.44 | 0.18 | 0.16 | 0.15 | 0.31 | 0.26 | 0.20 | 0.18 | 0.75 | Soap | |
| Octanoic acid* | 11.07 | 5.90 | 18.70 | 3.24 | nd | 18.53 | 61.38 | nd | nd | 10.59 | 27.25 | nd | nd | 0.01 | Sweat, cheese | |
| Nonanoic acid | 0.73 | 0.25 | 1.56 | 1.48 | 0.12 | 1.80 | 4.88 | 0.97 | 0.75 | 0.73 | 2.62 | 0.79 | nd | 0.25 | Fat, green | |
| 2-Pentyl furan | 0.22 | 0.05 | 0.03 | 0.06 | 0.03 | 0.32 | 0.20 | 0.14 | 0.06 | 0.39 | 0.30 | 0.42 | 0.20 | 3.36 | Green bean, butter | |
| AA | 3-Methylbutanal* | 2.49 | 1.58 | 1.39 | 1.78 | 0.85 | 9.29 | 3.80 | 2.62 | 1.48 | 6.89 | 3.56 | 4.54 | 2.01 | 0.10 | Malt |
| Benzaldehyde | 0.19 | 0.13 | 0.13 | 0.11 | 0.04 | 0.17 | 0.12 | 0.09 | 0.06 | 0.26 | 0.21 | 0.16 | 0.18 | 2.31 | Almond, burnt sugar | |
| Phenyl acetaldehyde* | 1.50 | 1.30 | 1.11 | 1.22 | 0.29 | 4.19 | 2.86 | 1.93 | 1.01 | 2.39 | 2.20 | 2.44 | 0.96 | 0.12 | Hawthorne, honey, sweet | |
| 3-Methyl-1-butanol | 0.97 | 0.66 | 0.47 | 0.29 | 0.01 | 2.66 | 1.29 | 0.78 | 0.31 | 1.76 | 1.27 | 1.53 | 0.57 | 1.69 | Malt, burnt, whiskey | |
| Phenyl ethyl alcohol* | 6.06 | 4.19 | 3.56 | 3.01 | 0.38 | nd | nd | nd | nd | nd | nd | nd | nd | 0.07 | Honey, spice, rose, lilac | |
| Acetic acid* | 2.42 | 1.30 | 1.31 | 2.30 | 0.17 | 5.94 | 2.60 | 1.83 | 0.77 | 3.49 | 2.35 | 3.35 | 1.10 | 0.41 | Sour | |
| 3-Methylbutyric acid* | 100 | 100 | 100 | 87.33 | 6.87 | 100 | 100 | 48.20 | 58.92 | 100 | 100 | 100 | 100 | 0.05 | Sweat, acid, rancid | |
| Methyl 3-methylbutyrate | 0.46 | 0.17 | 0.05 | nd | nd | 0.82 | 0.33 | 0.17 | 0.05 | 0.47 | 0.32 | 0.37 | 0.13 | 2.32 | Apple | |
| OTH | β-Linalool* | 6.34 | 4.66 | 2.28 | 1.53 | 0.24 | 13.87 | 7.95 | 4.49 | 1.91 | 8.81 | 7.73 | 9.49 | 3.62 | 0.06 | Flower, lavender |
| 6-Methyl-5-hepten-2-one | 0.19 | 0.11 | 0.10 | 0.26 | 0.15 | nd | nd | nd | nd | nd | nd | nd | nd | 1.22 | Citrus, mushroom, pepper | |
Asterisks refer to key volatile compounds (ROAV ≥ 1) in fresh ground (FG) flour. aThreshold odour values in ppb (Van Gemert, 2011). bKey odorants were retrieved from Flavournet (Acree and Arn, 2004). nd, non-detectable. Only flavour components with ROAV ≥ 0.1 in FG flour samples are presented. Volatiles are separated according to their origin, either unsaturated fatty acids (UFA), amino acids (AA), or others (OTH).
Faba bean flour has a low content of lipids at about 1.1% (Akkad et al., 2019). The measured proportions of both linoleic (54–56%) and oleic (22–23.6%) acids in faba beans are comparable to those found in soybean oil, which typically has around 52% linoleic acid and 24% oleic acid (Dubois et al., 2007). A recent stability study of soybean oil, demonstrated that most of the flavour of the oil was due to aldehydes which formed following the measured rise in peroxide values. These aldehydes were found at relatively high abundance in the oils’ headspace, and have low odour thresholds in oils (Xu et al., 2017). In particular, pentanal, hexanal, octanal, nonanal, trans-2-heptenal, and benzaldehyde were identified as key flavour compounds that were formed due to lipid oxidation in the edible oils tested.
The formation of lipid-derived off-flavours is one of the main issues associated with low consumer acceptance of pulse-based food products. Some of the off-flavours develop immediately through the action of enzymes, whereas others occur only after long-term storage. Lipoxygenases (LOX) are believed to be the key enzymes responsible for the formation of lipid hydroperoxides, which can in turn further react to form volatile off-flavours, such as hexanal. In order to evaluate the enzymatic contribution in formation of lipid-derived off-flavours, the activities of lipase, lipoxygenase (LOX) and peroxygenase (POX) were measured in faba bean samples (Yang et al., 2017). In that study, the enzyme activities were studied in seeds from several cultivars and growing seasons. Faba bean samples were shown to have lipase and LOX activities that varied significantly among the cultivars and the cultivation years. Hence, these should be taken into account when processing faba bean varieties, despite their low lipid content.
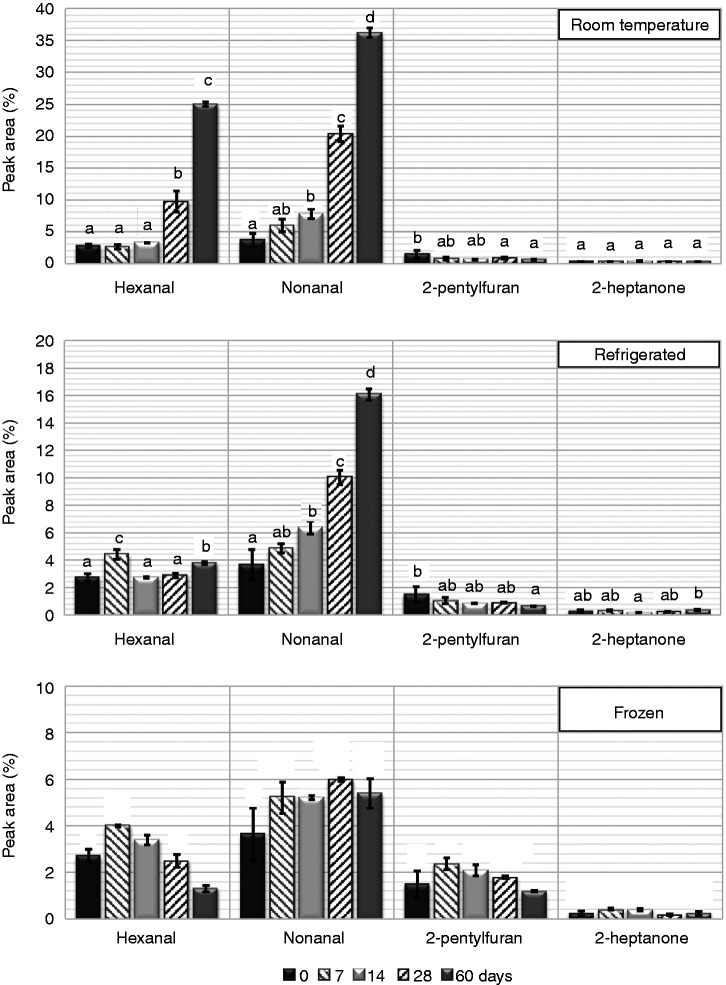
The volatile compounds that have been previously described as the key indicators of beany flavour in faba bean flour (Jiang et al., 2016), were also observed in the present study, with the exception of 2-methylfuran (Figure 2). Only hexanal and nonanal were identified as key volatile compounds (Table 1). Both of the minor volatiles, 2-pentylfuran and 2-heptanone, have similar ROAV values (about 0.2%, Table 1), but show no significant change over time in either their aroma impacts or in their concentrations (Table S1). In fact, 2-heptanone does not have any significant change over time regardless to the storage condition (Figure 2). The measured abundance of 2-pentylfuran decreased slightly over time, except for an increase after one week in the freezer. The changes in measured hexanal and nonanal abundances over storage time differ between the 3 storage temperatures. While both increased significantly with storage at room temperature, only nonanal showed rapid increase under refrigeration, although to a lower level (∼16% at day 60 under refrigeration compared to ∼36% at RT). For frozen faba bean flour, both headspace hexanal and nonanal increase over the first week, but then hexanal decreases whilst nonanal remains approximately constant over the 2-month study.
Figure 2.
Volatile beany flavours of whole high-tannin faba bean flour (WHT) under different storage temperatures over two-months storage. Data were represented in relative peak area of each compound (RPA, %) Error bars show standard deviation (n = 3). Different letters above bars represent statistical significant differences established by the Post Hoc test (Tukey HSD, 95% Confidence Interval).
Thus, the formation of the beany flavour is highly affected by storage time and conditions; not surprisingly it is greater at room temperature than with refrigerated or frozen flour storage. It could be concluded that storing flour is acceptable for up two weeks under all conditions, but if longer time is desired, then freezing flour would be highly recommended to block the enzymatic activities that may lead to formation of rancid flavours (Figure 2).
Conclusions
Although faba bean flours have different volatile flavour profiles when stored under different temperature conditions the identified key volatiles with ROAV ≥1 were mostly present in the freshly ground flour as well as after storage. When ground faba bean was directly frozen, little or no loss of volatiles was observed, so that the headspace concentrations of most volatile compounds were similar in frozen flour and in freshly ground faba bean, unlike that observed at higher storage temperatures.
Despite the very low lipid content, faba bean has relatively high lipase and LOX activities that lead to the formation of off-flavour volatiles. These include aldehydes that have very low odour thresholds and were observed at increasing concentrations over time. The rate of formation of these aroma compounds changes according to storage temperature. The volatiles that are responsible for the beany flavour include hexanal, nonanal, 2-pentylfuran, and 2-heptanone, which appear at different rates during storage. A large increase in concentration of hexanal and nonanal was observed, whereas 2-pentylfuran slightly decreased and 2-heptanone remained almost constant. However, the total concentration of these compounds most significantly increase after 2 weeks of storage, except when frozen. Hence, after milling of faba beans, the total beany flavour in the flour likely remains acceptable for up to two weeks under RT or refrigerated storage in polyethylene zipper-closure bags. To minimise undesirable changes in the faba bean volatile profile, frozen storage is necessary.
Supplemental Material
Supplemental material, sj-pdf-1-fst-10.1177_1082013221998843 for The effect of short-term storage temperature on the key headspace volatile compounds observed in Canadian faba bean flour by Rami Akkad, Ereddad Kharraz, Jay Han, James D House and Jonathan M Curtis in Food Science and Technology International
ACKNOWLEDGEMENTS
Financial support for this research was awarded by the Alberta Pulse Growers Commission (APG) grant 2017F108R. The authors expressed many thanks to the Institute of International Education Scholar Rescue Fund (IIE-SRF, NY, USA) and the University of Alberta International for the kindly granted scholarship of Rami Akkad.
Footnotes
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jonathan M Curtis https://orcid.org/0000-0002-8863-5611
SUPPEMENTAL MATERIAL: Supplemental material for this article is available online.
References
- Abu-Ghannam N, Gowen A. (2011). Pulse-based food products. In: Tiwari BK, Gowen A, McKenna B. (eds) Pulse Foods: Processing, Quality and Nutraceutical Applications. 1st ed. Cambridge, MA: Academic Press, pp.249–282. [Google Scholar]
- Acree T, Arn H. (2004). Flavornet and human odor space. Available at: www.flavornet.org/flavornet.html (accessed 15 February 2021).
- Akkad R, Kharraz E, Han J, House JD, Curtis JM. (2019). Characterisation of the volatile flavour compounds in low and high tannin faba beans (Vicia faba var. minor) grown in Alberta. Food Research International 120: 285–294. [DOI] [PubMed] [Google Scholar]
- Azarnia S, Boye JI, Warkentin T, Malcolmson L, Sabik H, Bellido AS. (2011). Volatile flavour profile changes in selected field pea cultivars as affected by crop year and processing. Food Chemistry 124(1): 326–335. [Google Scholar]
- Belitz H-D, Grosch W, Schieberle P. (2009). Lipids. In: Food Chemistry. 4th ed. Berlin, Heidelberg: Springer-Verlag, pp.158–247. [Google Scholar]
- Bott L, Chambers E. (2006). Sensory characteristics of combinations of chemicals potentially associated with beany aroma in foods. Journal of Sensory Studies 21(3): 308–321. [Google Scholar]
- Coxon DT, Wright DJ. (1985). Analysis of pea lipid content by gas chromatographic and microgravimetric methods. genotype variation in lipid content and fatty acid composition. Journal of the Science of Food and Agriculture 36(9): 847–856. [Google Scholar]
- Dubois V, Breton S, Linder M, Fanni J, Parmentier M. (2007). Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. European Journal of Lipid Science and Technology 109(7): 710–732. [Google Scholar]
- Frankel EN. (2005). Foods. In: Frankel EN. (ed.) Lipid Oxidation. Oily Press Lipid Library Series. 2nd ed. Bridgewater: Woodhead Publishing, pp.299–354. [Google Scholar]
- Geueke B, Groh K, Muncke J. (2018). Food packaging in the circular economy: Overview of chemical safety aspects for commonly used materials. Journal of Cleaner Production 193: 491–505. [Google Scholar]
- Hinchcliffe C, McDaniel M, Vaisey M, Eskin NAM. (1977). The flavor of fababeans as affected by heat and storage. Canadian Institute of Food Science and Technology Journal 10(3): 181–184. [Google Scholar]
- Irwin RJ, Mouwerik MV, Stevens L, Seese MS, Basham W. (1997). Environmental contaminants encyclopedia entry. National Park Service Water Resources Divisions, Colorado, 1 July, pp.1–62. Colorado, US: National Park Service, Fort Collins. [Google Scholar]
- Jeleń H, Wasowicz E. (2012). Lipid-derived flavor compounds. In: Jeleń H. (ed.) Food Flavors: Chemical, Sensory and Technological Properties. 1st ed. Boca Raton: CRC Press, pp.65–93. [Google Scholar]
- Jiang ZQ, Pulkkinen M, Wang YJ, Lampi AM, Stoddard FL, Salovaara H, et al. (2016). Faba bean flavour and technological property improvement by thermal pre-treatments. LWT –Food Science and Technology 68: 295–305. [Google Scholar]
- Kaneko S, Kumazawa K, Nishimura O. (2011). Studies on the key aroma compounds in soy milk made from three different soybean cultivars. Journal of Agricultural and Food Chemistry 59(22): 12204–12209. [DOI] [PubMed] [Google Scholar]
- Liu D, Zhou G, Xu X. (2008). ‘ ROAV’ method: A new method for determining key odor compounds of Rugao ham. Food Science 29: 370–374. [Google Scholar]
- MacLeod G, Ames J, Betz NL. (1988). Soy flavor and its improvement. Critical Reviews in Food Science and Nutrition 27(4): 219–400. [DOI] [PubMed] [Google Scholar]
- Nitsan Z. (1971). Vicia faba beans vs. Soybean meal as a source of protein. Journal of the Science of Food and Agriculture 22(5): 252–255. [Google Scholar]
- Nummer B, Washburn C, Hunsaker T. (2013). A Guide to Food Storage for Emergencies. Logan, UT, USA: Utah State University Extension. [Google Scholar]
- Oomah BD, Razafindrainibe M, Drover JCG. (2014). Headspace volatile components of Canadian grown low-tannin faba bean (Vicia faba L.) genotypes. Journal of the Science of Food and Agriculture 94(3): 473–481. [DOI] [PubMed] [Google Scholar]
- Reineccius G. (2006). Flavor Chemistry and Technology. 2nd ed. Boca Raton: CRC Press. [Google Scholar]
- Spinnler H-E. (2012). Flavors from amino acids. In: Jeleń H. (ed.) Food Flavors – Chemical, Sensory and Technological Properties. 1st ed. Boca Raton: CRC Press, pp.121–136. [Google Scholar]
- Van Gemert LJ. (2011). Odour Thresholds: Compilations of Odour Threshold Values in Air, Water and Other Media. 2nd ed. Utrecht, The Netherlands: Oliemans Punter & Partners BV. [Google Scholar]
- Xu L, Yu X, Li M, Chen J, Wang X. (2017). Monitoring oxidative stability and changes in key volatile compounds in edible oils during ambient storage through HS-SPME/GC–MS. International Journal of Food Properties 20(Suppl. 3): S2926–S2938. [Google Scholar]
- Xu M, Jin Z, Lan Y, Rao J, Chen B. (2019). HS-SPME-GC-MS/olfactometry combined with chemometrics to assess the impact of germination on flavor attributes of chickpea, lentil, and yellow pea flours. Food Chemistry 280: 83–95. [DOI] [PubMed] [Google Scholar]
- Yang Z, Piironen V, Lampi AM. (2017). Lipid-modifying enzymes in oat and faba bean. Food Research International (Ottawa, Ont.) 100(Pt 1): 335–343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-fst-10.1177_1082013221998843 for The effect of short-term storage temperature on the key headspace volatile compounds observed in Canadian faba bean flour by Rami Akkad, Ereddad Kharraz, Jay Han, James D House and Jonathan M Curtis in Food Science and Technology International