Abstract
Aims
We hypothesize that in patients with paroxysmal atrial fibrillation (AF), verapamil is associated with lower AF progression compared to beta blockers or no rate control.
Methods and results
In this pre-specified post hoc analysis of the RACE 4 randomized trial, the effect of rate control medication on AF progression in paroxysmal AF was analysed. Patients using Vaughan-Williams Class I or III antiarrhythmic drugs were excluded. The primary outcome was a composite of first electrical cardioversion (ECV), chemical cardioversion (CCV), or atrial ablation. Event rates are displayed using Kaplan–Meier curves and multivariable Cox regression analyses are used to adjust for baseline differences. Out of 666 patients with paroxysmal AF, 47 used verapamil, 383 used beta blockers, and 236 did not use rate control drugs. The verapamil group was significantly younger than the beta blocker group and contained more men than the no rate control group. Over a mean follow-up of 37 months, the primary outcome occurred in 17% in the verapamil group, 33% in the beta blocker group, and 33% in the no rate control group (P = 0.038). After adjusting for baseline characteristics, patients using verapamil have a significantly lower chance of receiving ECV, CCV, or atrial ablation compared to patients using beta blockers [hazard ratio (HR) 0.40, 95% confidence interval (CI) 0.19–0.83] and no rate control (HR 0.64, 95% CI 0.44–0.93).
Conclusion
In patients with newly diagnosed paroxysmal AF, verapamil was associated with less AF progression, as compared to beta blockers and no rate control.
Keywords: Atrial fibrillation, Atrial fibrillation progression, Rate control, Rhythm control, RACE 4 study
What’s new.
Patients using verapamil demonstrate significantly less progression of atrial fibrillation (AF) than patients using beta blockers or no rate control medication.
Patients using verapamil require fewer electrical cardioversions compared to patients using beta blockers and no rate control.
There is no difference in progression of AF between patients using beta blockers or no rate control.
Introduction
Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia, with a lifetime risk of 23%.1,2 Atrial fibrillation has a tendency to progress to more persistent forms over time.3 Progression from paroxysmal AF to persistent or permanent AF is associated with increased disease burden, and hospitalization rates.4 Progression of AF might be related to structural and electrical remodelling of the atria.5–7 There is growing evidence that continued calcium influx during rapid atrial rates plays a major role in electrical remodelling.8 Several studies have shown that non-dihydropyridine calcium channel antagonists (CCA), such as verapamil, reduce tachycardia-induced electrical remodelling in AF.9–12 Furthermore, in contrast to beta blockers, verapamil increases sympathetic tone.13 In patients with vagal paroxysmal AF, verapamil has shown to reduce progression to persistent AF compared to digoxin and beta blockers.14 Considering the above effects, rate control with non-dihydropyridine CCA in the general AF population may reduce progression of AF.
We investigated this hypothesis in paroxysmal AF patients who were included in the RACE 4 trial.15 We identified patients with paroxysmal AF using verapamil, beta blockers, or no rate control medication at their first outpatient visit and compared their effect on AF progression. For the purpose of the present analysis, AF progression was defined as the need for atrial ablation, electrical cardioversion (ECV), or chemical cardioversion (CCV), all an expression of progressive symptoms as well as AF persistence.
Methods
The RACE 4 trial is a multi-centre study in which nurse-led AF care was compared to routine care. A detailed description of the methods, intervention, and follow-up has been described by Petra Wijtvliet et al.15 In short, 1375 AF patients were enrolled in the study in 8 hospitals in the Netherlands, from 2012 through 2017. Inclusion criteria were ≥18 years of age and presenting with first detected AF. Patients with unstable heart failure or acute coronary disease in the 3 months prior to screening were excluded. The patients were randomized to either nurse-led care or routine care.
For the present study, we selected all patients with paroxysmal AF, and classified them according to treatment with a beta blocker, verapamil, or no rate control medication. Patients using Class I or Class III antiarrhythmic drugs, including sotalol, were excluded. Patients were divided into a verapamil group, a beta blocker group, and a no rate control group based on their discharge medication after the first outpatient clinic visit. The primary outcome of this study was the occurrence of first ECV, CCV, or atrial ablation whichever came first. The secondary outcomes were the individual components of the primary outcome and a composite of cardiovascular death, hospital admission for arrhythmias, heart failure, thromboembolic events, major bleeding, acute coronary syndrome, or life-threatening effects of drugs. In RACE 4, patients had a maximum follow-up of 5 years and 10 months.
Statistical analysis
Normally distributed variables are reported as mean ± standard deviation, non-normally distributed variables as median (25–75% range), and categorical variables as count (percentage of patients). Differences between variables were tested using one-way ANOVA for normally distributed variables and Kruskal–Wallis for non-normally distributed variables. The Fisher’s exact test was used to test for differences between categorical variables. Cumulative incidences over time were displayed using Kaplan–Meier curve, the P-value for between-group differences was calculated using the log rank test. Multivariable Cox regression analysis was performed to adjust for differences in baseline characteristics. The proportional hazard assumptions for this model were tested. The characteristics used for this analysis were the individual parameters used in the HATCH score:4 age, heart failure, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, and a history of a stroke in addition to sex and the presence of symptoms at first presentation. All tests performed were two-sided. Overall a P-value <0.05 was considered to be statistically significant. Bonferroni correction was used to correct for multiple testing in post hoc analysis. Data analysis was performed using R statistics (version 4.0.0).
Results
Baseline characteristics
A total of 666 patients with paroxysmal AF without Class I or Class III antiarrhythmic medication were included in the present analysis. Forty-seven patients used verapamil at baseline, 383 patients used beta blockers, and 236 patients used no rate control. The verapamil group was significantly younger than the beta blocker group and contained fewer men than the no rate control group (Table 1). Patients in the verapamil and the no rate control groups used fewer oral anticoagulation drugs compared to the beta blocker groups. There were no other differences in co-medication. At baseline, patients in the verapamil group had slightly higher heart rates, less often heart failure and a lower HATCH score compared to the other two groups. These differences were not significant.
Table 1.
Characteristics of the patients at baseline
| Verapamil (n = 47) | Beta blocker (n = 383) | No rate control (n = 236) | P-value | |
|---|---|---|---|---|
| Age (years) | 60 (55–68)a | 66 (59–72)b | 63 (55–69) | <0.01 |
| Male sex, n (%) | 23 (49) | 234 (61)b | 170 (72)c | <0.01 |
| Symptoms at first visit, n (%) | 22 (47) | 178 (46) | 91 (39) | 0.141 |
| Heart rate at first visit (beats per minute) | 76 (65–91) | 71 (61–94) | 73 (65–91) | 0.75 |
| Hypertension, n (%) | 20 (43) | 198 (52)b | 89 (38) | <0.01 |
| History of heart failure, n (%) | 1 (2) | 37 (9) | 11 (5) | 0.03 |
| Diabetes, n (%) | 2 (4) | 41 (11) | 18 (8) | 0.25 |
| Ischaemic stroke of transient ischaemic attack, n (%) | 2 (4) | 27 (7) | 14 (6) | 0.76 |
| Haemorrhagic stroke, n (%) | 0 0 | 7 (2) | 5 (2) | 1 |
| Coronary artery disease, n (%) | 3 (6) | 28 (7) | 9 (4) | 1 |
| Valvular heart disease, n (%) | 1 (2) | 18 (5) | 6 (3) | 0.37 |
| Peripheral vascular disease, n (%) | 1 (2) | 13 (3) | 8 (3) | 1 |
| Hyperthyroidism, n (%) | 0 0 | 8 (2) | 1 0 | 0.27 |
| Chronic obstructive pulmonary disease, n (%) | 5 (11) | 22 (6) | 9 (4) | 0.13 |
| CHA2DS2-VASc,dn (%) | b | <0.01 | ||
| 0 | 18 (38) | 80 (21) | 83 (35) | |
| 1 | 9 (19) | 68 (18) | 48 (20) | |
| ≥2 | 20 (42) | 235 (61) | 105 (44) | |
| HATCH score,en (%) | b | <0.01 | ||
| 0 | 24 (51) | 142 (37) | 128 (54) | |
| 1 | 19 (40) | 154 (40) | 79 (33) | |
| ≥2 | 4 (9) | 87 (23) | 29 (12) | |
| HASBLED >3, n (%) | 5 (11) | 37 (10) | 16 (7) | 0.36 |
| Body mass index (kg/m2) | 28 (±5) | 28 (±5) | 27 (± 5) | 0.58 |
| Systolic blood pressure (mmHg) | 141 (±20) | 141 (±21) | 141 (±19) | 0.98 |
| Echocardiographic left atrial size, long axis (mm) | 38 (±5) | 39 (±6) | 39 (± 6) | 0.30 |
| Left ventricular ejection fraction (%) | 60 (55–60) | 55 (52–60) | 57 (55–60) | 0.06 |
| RAAS inhibition, n (%) | 7 (15) | 79 (21) | 39 (17) | 0,35 |
| ACE inhibitor | 4 (9) | 61 (16) | 25 (11) | 0,10 |
| Angiotensin receptor blocker | 3 (6) | 18 (5) | 14 (6) | 0,67 |
| Dihydropyridine calcium channel blockers, n (%) | 1 (2) | 23 (6) | 10 (4) | 0.497 |
| Diuretics, n (%) | 3 (6) | 40 (10) | 14 (6) | 0,14 |
| Statins, n (%) | 5 (11) | 50 (13)b | 14 (6) | 0,01 |
| Digoxin, n (%) | 1 (2) | 16 (4)b | 0 0 | <0.01 |
| Oral anticoagulation, n (%) | 26 (55)a | 289 (75)b | 119 (50) | <0.01 |
| Direct oral anticoagulation, n (%) | 18 (38) | 169 (44) | 63 (27) | |
| Coumarines, n (%) | 8 (17) | 120 (31) | 56 (24) |
ACE, angiotensin-converting enzyme; RAAS, renin–angiotensin–aldosterone system.
Significant differences between groups after correction for multiple testing using the Bonferroni correction.
Significant difference between verapamil and beta blocker.
Significant difference between beta blocker and no rate control.
Significant difference between no rate control and verapamil.
Congestive heart failure, hypertension, an age of 65–74 years, diabetes, and vascular disease are each assigned one point, and previous stroke or transient ischaemic attack and an age >75 years are assigned two points.
Age above 75 years, hypertension and chronic obstructive pulmonary disease are each assigned one point and heart failure and previous stroke or transient ischaemic attack are each assigned two points.
Primary outcome
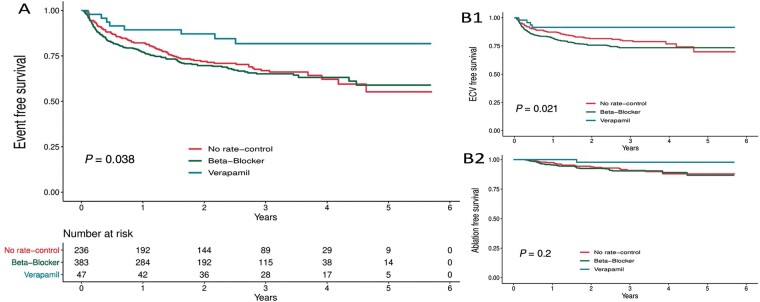
Over a mean follow-up of 37 months, the primary outcome occurred in 8 out of 47 patients (17%) in the verapamil group, 126 out of 383 patients (33%) in the beta blocker group, and 78 out of 236 patients (33%) in the no rate control group (P = 0.038, Figure 1A). After adjusting for baseline characteristics, patients in the verapamil group had a significantly lower chance of receiving ECV, CCV, or atrial ablation compared to patients in the beta blocker group [hazard ratio (HR) 0.40, 95% confidence interval (CI) 0.19–0.83] and no rate control (HR 0.64, 95% CI 0.44–0.93).
Figure 1.
Rate control medication and event free survival from (A) ECV-CCV-Ablation, (B1) ECV and (B2) ablation.
Secondary outcomes
When ECV, CCV, and ablation are analysed separately, patients using verapamil had a significantly lower probability of requiring an ECV (P = 0.021, Figure 1B). Electrical cardioversion was performed in 4 of 47 (9%) patients in the verapamil group, 95 of 383 (25%) patients in the beta blocker group, and 50 of 236 (21%) in the no rate control group. When adjusted for baseline characteristics, patients in the verapamil group had a significantly lower probability of requiring ECV compared to the beta blocker group (HR 0.31, 95% CI 0.11–0.85) and the no rate control group (HR 0.59, 95% CI 0.35–1.00). ECV or CCV, whichever came first, was performed in 189 (28%) out of 666 patients overall. There was no difference in ECV and CCV free overall survival (Supplementary material online, Figure S1). But similar to ECV alone, when adjusted for baseline characteristics, the verapamil group had a significantly lower probability of receiving ECV or CCV compared to the beta blocker group (HR 0.41, 95% CI 0.19–0.89) and the no rate control group (HR 0.65, 95% CI 0.44–0.97). In total, 56 (8%) atrial ablations were performed, 1 (2%) was performed in the verapamil group, 34 (9%) were performed in the beta blocker group, and 21 (9%) were performed in the no rate control group. There were no significant differences in atrial ablations performed between groups (P = 0.20, Figure 1B) even when adjusted for baseline characteristics (Table 2). We performed a sensitivity analysis excluding the patients who underwent ablation. This did not change the results on AF progression as expressed by ECV and CCV free survival (data not shown).
Table 2.
Cox regression analysis of the effect of different rate control drugs
| Hazard ratio | 95% confidence interval | |
|---|---|---|
| Composite outcome | ||
| Verapamil vs. beta blockers | 0.4 | 0.19–0.83 |
| Verapamil vs. no rate control | 0.64 | 0.44–0.93 |
| Beta blocker vs. no rate control | 1.09 | 0.81–1.47 |
| ECV | ||
| Verapamil vs. beta blockers | 0.31 | 0.11–0.85 |
| Verapamil vs. no rate control | 0.59 | 0.35–1.00 |
| Beta blocker vs. no rate control | 1.32 | 0.92–1.90 |
| ECV and CCV | ||
| Verapamil vs. beta blockers | 0.41 | 0.19–0.89 |
| Verapamil vs. no rate control | 0.65 | 0.44–0.97 |
| Beta blocker vs. no rate control | 1.1 | 0.81–1.52 |
| Atrial ablation | ||
| Verapamil vs. beta blockers | 0.18 | 0.02–1.33 |
| Verapamil vs. no rate control | 0.46 | 0.17–1.28 |
| Beta blocker vs. no rate control | 1.18 | 0.67–2.10 |
Adjusted for the individual components of HATCH score: age, heart failure, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, and a history of a stroke in addition to sex and presents of symptoms at first presentation.
CCV, chemical cardioversion; ECV, electrical cardioversion.
The secondary composite outcome of cardiovascular death, hospital admission for arrhythmias, heart failure, thromboembolic events, major bleeding, acute coronary syndrome, or life-threatening effects of drugs occurred in 11 patients (23%) in the verapamil group, 113 (30%) in the beta blocker group, and 62 (26%) times in the no rate control group, this was not significantly different (P = 0.52, Table 3). The secondary outcome was mostly driven by hospital admissions for arrhythmic events which occurred in 8 patients (17%) in the verapamil group, 82 patients (24%) in the beta blocker group, and 56 patients (24%) in the no rate control group (P = 0.61). There were also no significant differences between any of the other components of the composite secondary outcome. For a full overview of the secondary outcome see Table 3.
Table 3.
Secondary outcomes
| Verapamil (n = 47) | Beta blocker (n = 383) | No rate control (n = 236) | P-value | |
|---|---|---|---|---|
| Composite of secondary outcome, n (%) | 11 (23) | 113 (30) | 62 (26) | 0.52 |
| Cardiovascular death, n (%) | ||||
| Cardiac | 0 (0) | 0 (0) | 0 (0) | 1 |
| Vascular non-cardiac | 1 (2) | 2 (1) | 1 0 | 0.27 |
| Hospitalizations for arrhythmic events, n (%) | 8 (17) | 92 (24) | 56 (24) | 0.61 |
| Heart failure, n (%) | 1 (2) | 7 (2) | 5 (2) | 0.91 |
| Acute coronary syndrome, n (%) | 0 (0) | 6 (2) | 3 (1) | 1 |
| Ischaemic thromboembolic complications, n (%) | 1 (2) | 10 (3) | 1 (0) | 0.1 |
| Major bleeding, n (%) | 0 (0) | 9 (2) | 3 (1) | 0.57 |
| Life-threatening effects of drugs, n (%) | 1 (2) | 2 (1) | 0 (0) | 0.155 |
Discussion
This pre-defined post hoc analysis from the RACE 4 trial suggests that in patients with newly diagnosed paroxysmal AF verapamil may reduce the progression to persistent AF.
These results are in agreement with earlier findings in the EURO Heart Survey, where it was demonstrated that in patients with vagal AF, verapamil reduced the progression to persistent AF, compared to digoxin or beta blocker treated patients.14 Even in patients with persistent AF requiring ECV, verapamil showed an increase in spontaneous conversion to sinus rhythm and reduced the amount of ECV’s required to achieve sinus rhythm compared to digoxin.16 Furthermore, supplementing Class 1C and Class III antiarrhythmic drug therapy with verapamil reduced AF recurrences after ECV.17 These results could be due to the electrophysiological effects of verapamil. Rapid atrial rates lead to calcium loading in the sarcoplasmic reticulum and increased calcium leakage from the sarcoplasmic reticulum. This calcium leak from the sarcoplasmic reticulum promotes cellular arrhythmogenesis in paroxysmal AF patients by inducing delayed afterdepolarization and triggered activity.18 Furthermore, calcium-handling abnormalities may play a role in AF perpetuation through conduction slowing and shortening of the refractory period, thereby promoting re-entry.12 In several animal studies, verapamil has been shown to prevent electrical remodelling and reduce shortening of the refractory period during rapid atrial rates.11,19 This effect is particularly strong early after onset of rapid atrial rates and may dissipate after long periods of rapid atrial rates.20 This may explain why in our patients with paroxysmal AF, verapamil was associated with less arrhythmia progression, whereas this was less clear in patients with persistent AF.16,21 By increasing triggers and promoting re-entry, calcium-handling abnormalities could play a major role in the progression of AF. The decrease in AF progression in the verapamil group might be explained by verapamil preventing or delaying electrical remodelling. In contrast, digoxin has been shown to decrease recovery from electrical remodelling.22 However, since concomitant use of digoxin was low and equally distributed among the groups, it is unlikely that digoxin use contributed to the results of the present analysis.
On the other hand, the result could also be explained by verapamil being superior for rate control,23,24 especially in patients with paroxysmal AF, thereby reducing symptoms and the need for a cardioversion or ablation. This can be explained by the fact that verapamil specifically lowers the heart rate during AF, but not during sinus rhythm. In contrast, the dose of beta blockers and their rate controlling effect during paroxysms of AF is limited by the heart rate during sinus rhythm. Therefore, heart rate during AF episodes in the beta blocker patients may have been more rapid, with the risk of ventricular dysfunction and subsequent atrial structural remodelling and AF progression.22 Furthermore, lower heart rates during sinus rhythm can promote atrial extra systoles, potentially triggering AF.
The patients receiving verapamil in this analysis were generally younger and had fewer comorbidities that are known to increase AF progression and disease burden, such as hypertension, history of heart failure, diabetes mellitus, and a previous stroke.4 It has been confirmed that targeted treatment of these comorbidities improves sinus rhythm maintenance,25 and therefore it has received a prominent place in the ESC guidelines.26 The differences in comorbidities could, in part, be responsible for differences in AF progression. Even after statistically adjusting for these comorbidities, residual confounding could still be present. These results justify further study of the effects of verapamil on AF progression in patients with paroxysmal AF with a randomized controlled trial.
Limitations
In the RACE 4 trial rate control treatment was not randomized, so no statements can be made about causality between rate control strategy and symptomatology or AF progression. Due to the different contraindications and side effects of verapamil and beta blockers there is the possibility of confounding factors or residual confounding after adjusting for baseline characteristics. The verapamil group is also much smaller than the beta blocker and the no rate control groups, therefore these data cannot be extrapolated to clinical practice and should be seen as hypotheses generating. Since medication persistence was no objective of the RACE 4 trial, we could not adjust for this factor.
Due to low event rates, the present analysis was underpowered for some of the study outcomes, mainly atrial ablation, heart failure, or death, which have a low incidence overall.
Conclusion
In patients with newly diagnosed paroxysmal AF, verapamil was associated with less AF progression, as compared to beta blockers and no rate control medication.
Funding
This research was supported by Netherlands healthcare insurance companies (DSW, ACHMEA, and CZ), Boehringer Ingelheim, Bayer, Pfizer, Bristol-Myers Squibb, and Daiichi Sankyo.
Conflict of interest: R.G.T. reports grants and personal fees from Boehringer Ingelheim, Bristol-Meyers-Squibb, Pfizer, and Daiichi Sankyo and grants from Bayer during the conduct of the study. N.A.H.A.P. reports grants from Boehringer Ingelheim, Medtronic, Bayer, Pfizer, Bristol-Myers-Squibb, and Daiichi Sankyo and grants from Netherlands Health Insurance companies DSW, ACHMEA, and CZ, during the conduct of the study, all to the institution. M.R. reports grants from Netherlands Cardiovascular Research Initiative funded by Dutch Heart Foundation, outside the submitted work. J.G.L.M.L. reports grants from the Netherlands Cardiovascular Research Initiative funded by the Dutch Heart Foundation, grants from Boehringer Ingelheim, Medtronic, Abbott, Bayer, Pfizer, Bristol-Myers Squibb, Daiichi Sankyo, and Biotronik, and grants from the Netherlands Health Insurance companies DSW, Achmea, and VGZ, all to the institution, and personal fees from Medtronic, outside the submitted work. H.J.G.M.C., I.C.V.G., and M.R. report grants from the Netherlands Cardiovascular Research Initiative: an initiative with support of the Dutch Heart Foundation, CVON 2014–9: Reappraisal of Atrial Fibrillation: interaction between hyperCoagulability, Electrical remodelling, and Vascular destabilization in the progression of AF (RACE V) outside the submitted work; and grants from Boehringer Ingelheim, Medtronic, Abbott, Bayer, Pfizer, Bristol-Myers-Squibb and Daiichi Sankyo, and from the Netherlands Health Insurance companies DSW, Achmea, and VGZ, during conduct of the study, and all to the institution. All other authors declared no conflict of interest.
Data availability
The data underlying this article cannot be shared publicly due to privacy reasons of the participants. Researchers can request the data by submitting a reasonable proposal to the corresponding author.
Supplementary Material
References
- 1. Lippi G, Sanchis-Gomar F, Cervellin G.. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke 2021;16:217–21. [DOI] [PubMed] [Google Scholar]
- 2. Heeringa J, Van Der Kuip DAM, Hofman A, Kors JA, Van Herpen G, Stricker BHC. et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 2006;27:949–53. [DOI] [PubMed] [Google Scholar]
- 3. Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA. et al. ; Document Reviewers. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace 2016;18:1455–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJS. et al. Progression from paroxysmal to persistent atrial fibrillation. Clinical correlates and prognosis. J Am Coll Cardiol 2010;55:725–31. [DOI] [PubMed] [Google Scholar]
- 5. Dobrev D, Wehrens XHT.. Calcium-mediated cellular triggered activity in atrial fibrillation. J Physiol 2017;595:4001–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tieleman RG, Crijns HJ.. The “second factor” of tachycardia-induced atrial remodeling. Cardiovasc Res 2000;46:364–6. [DOI] [PubMed] [Google Scholar]
- 7. Wijffels MCEF, Kirchhof CJHJ, Dorland R, Allessie MA.. Atrial fibrillation begets atrial fibrillation: a study in awake chronically instrumented goats. Circulation 1995;92:1954–68. [DOI] [PubMed] [Google Scholar]
- 8. Nattel S, Harada M.. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J Am Coll Cardiol 2014;63:2335–45. [DOI] [PubMed] [Google Scholar]
- 9. Daoud EG, Knight BP, Weiss R, Bahu M, Paladino W, Goyal R. et al. Effect of verapamil and procainamide on atrial fibrillation-induced electrical remodeling in humans. Circulation 1997;96:1542–50. [DOI] [PubMed] [Google Scholar]
- 10. Kinebuchi O, Mitamura H, Shiroshita-Takeshita A, Kurita Y, Ieda M, Ohashi N. et al. Oral verapamil attenuates the progression of pacing-induced electrical and mechanical remodeling of the atrium. Circ J 2004;68:494–500. [DOI] [PubMed] [Google Scholar]
- 11. Tieleman RG, De Langen CDJ, Van Gelder IC, De Kam PJ, Grandjean J, Bel KJ. et al. Verapamil reduces tachycardia-induced electrical remodeling of the atria. Circulation 1997;95:1945–53. [DOI] [PubMed] [Google Scholar]
- 12. Heijman J, Wehrens XHT, Dobrev D.. Atrial arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia–is there a mechanistic link between sarcoplasmic reticulum Ca(2+) leak and re-entry? Acta Physiol (Oxf) 2013;207:208–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stern HC, Matthews JH, Belz GG.. Intrinsic and reflex actions of verapamil and nifedipine: assessment in normal subjects by noninvasive techniques and autonomic blockade. Eur J Clin Pharmacol 1986;29:541–7. [DOI] [PubMed] [Google Scholar]
- 14. de Vos CB, Nieuwlaat R, Crijns HJGM, Camm AJ, LeHeuzey J-Y, Kirchhof CJ. et al. Autonomic trigger patterns and anti-arrhythmic treatment of paroxysmal atrial fibrillation: data from the Euro Heart Survey. Eur Heart J 2008;29:632–9. [DOI] [PubMed] [Google Scholar]
- 15. Petra Wijtvliet EPJ, Tieleman RG, Van Gelder IC, Pluymaekers NAHA, Rienstra M, Folkeringa RJ. et al. Nurse-led vs. usual-care for atrial fibrillation. Eur Heart J 2020;41:634–41. [DOI] [PubMed] [Google Scholar]
- 16. Hemels MEW, Van Noord T, Crijns HJGM, Van Veldhuisen DJ, Veeger NJGM, Bosker HA. et al. Verapamil versus digoxin and acute versus routine serial cardioversion for the improvement of rhythm control for persistent atrial fibrillation. J Am Coll Cardiol 2006;48:1001–9. [DOI] [PubMed] [Google Scholar]
- 17. De Simone A, De Pasquale M, De Matteis C, Canciello M, Manzo M, Sabino L. et al. VErapamil plus antiarrhythmic drugs reduce atrial fibrillation recurrences after an electrical cardioversion (VEPARAF study). Eur Heart J 2003;24:1425–9. [DOI] [PubMed] [Google Scholar]
- 18. Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M. et al. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation 2014;129:145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goette A, Honeycutt C, Langberg JJ.. Electrical remodeling in atrial fibrillation: time course and mechanisms. Circulation 1996;94:2968–74. [DOI] [PubMed] [Google Scholar]
- 20. Lee SH, Yu WC, Cheng JJ, Hung CR, Ding YA, Chang MS. et al. Effect of verapamil on long-term tachycardia-induced atrial electrical remodeling. Circulation 2000;101:200–6. [DOI] [PubMed] [Google Scholar]
- 21. Saoudi N, Cosio F, Waldo A, Chen SA, Iesaka Y, Lesh M. et al. Classification of atrial flutter and regular atrial tachycardia according to electrophysiologic mechanism and anatomic bases: a statement from a Joint Expert Group from the working group of Arrhythmias of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. J Cardiovasc Electrophysiol 2001;12:852–66. [DOI] [PubMed] [Google Scholar]
- 22. Tieleman RG, Blaauw Y, Van Gelder IC, De Langen CDJ, De Kam PJ, Grandjean JG. et al. Digoxin delays recovery from tachycardia-induced electrical remodeling of the atria. Circulation 1999;100:1836–42. [DOI] [PubMed] [Google Scholar]
- 23. Ulimoen SR, Enger S, Carlson J, Platonov PG, Pripp AH, Abdelnoor M. et al. Comparison of four single-drug regimens on ventricular rate and arrhythmia-related symptoms in patients with permanent atrial fibrillation. Am J Cardiol 2013;111:225–30. [DOI] [PubMed] [Google Scholar]
- 24. Tsuneda T, Yamashita T, Fukunami M, Kumagai K, Niwano S, Okumura K. et al. Rate control and quality of life in patients with permanent atrial fibrillation: the Quality of Life and Atrial Fibrillation (QOLAF) Study. Circ J 2006;70:965–70. [DOI] [PubMed] [Google Scholar]
- 25. Rienstra M, Hobbelt AH, Alings M, Tijssen JGP, Smit MD, Brügemann J. et al. ; for the RACE 3 Investigators. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. Eur Heart J 2018;39:2987–96. [DOI] [PubMed] [Google Scholar]
- 26. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C. et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to privacy reasons of the participants. Researchers can request the data by submitting a reasonable proposal to the corresponding author.