Introduction
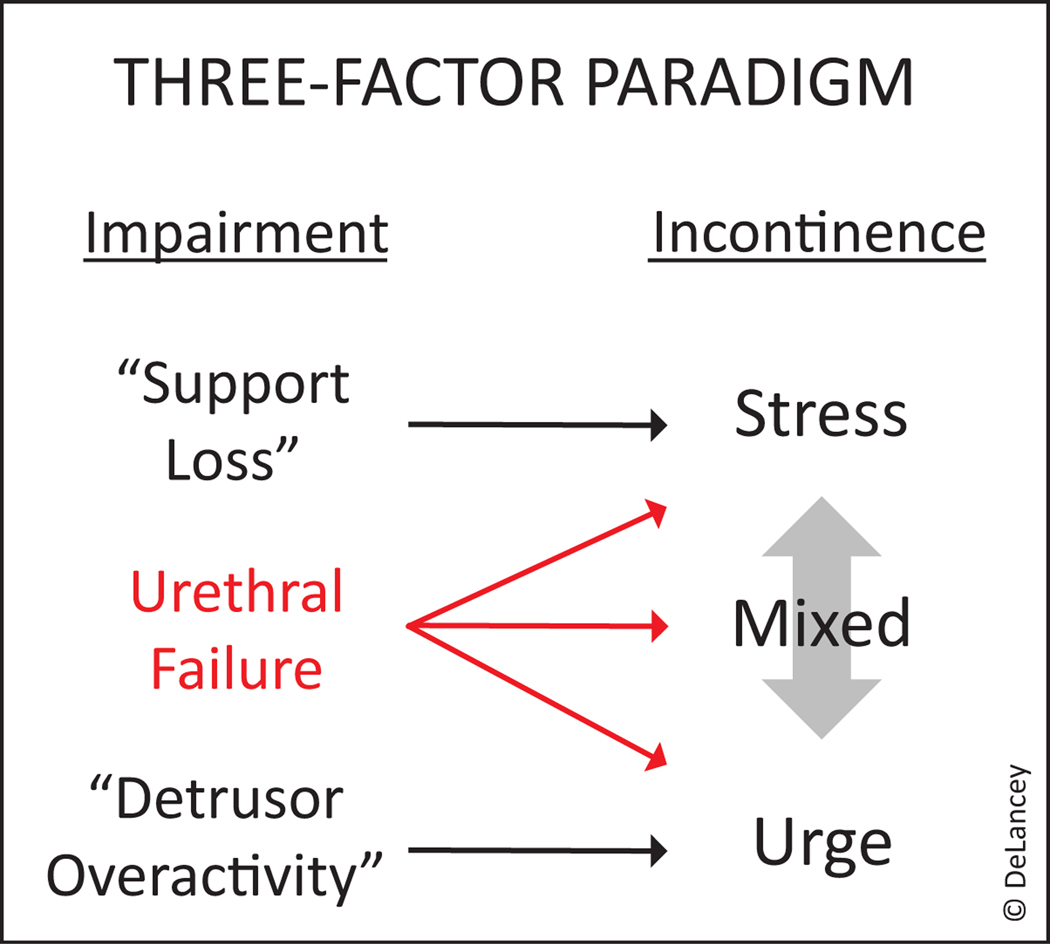
The NIDDK recently sponsored a seven-week virtual seminar series titled: “Female Urethral Function and Failure: Advancing Basic and Translational Research for Genitourinary Conditions.” In this multinational consultation, recent evidence revealing the key role played by the urethra was discussed, the state of urethral knowledge evaluated, and important knowledge gaps in our understanding this critical organ identified. An executive summary of the series, as well as key points and identified research gaps, are available as supplementary material. This international gathering of scientists underscored the need to re-evaluate the current two-factor theory that emphasizes abnormal detrusor function for urgency urinary incontinence (UUI) and poor urethral support for stress urinary incontinence (SUI) and to move to a three-factor theory that includes the role that urethral failure plays in both stress and urge incontinence (Figure 1). If the urethra is critical to maintaining continence, then the question becomes, “now what?”
Figure 1.
Three-factor theory of incontinence. Urethral function is as an important component of both stress and urge urinary incontinence.
Why has the urethra escaped scientific scrutiny for so long? It is probably because the widely held two-factor theory of incontinence paralleled the treatments we had available at the time. However, starting in the 1960’s evidence began accumulating that urethral function was much lower in incontinent women (2,3) and many studies of this parameter followed. However, the fact that urethral support operations for SUI were reasonably successful without changing urethral pressure (4,5) and the fact that measuring urethral pressure didn’t change outcomes (6) led to the false assumption that urethral failure was not clinically important. Similarly, UUI is strongly associated in our minds with detrusor overactivity (i.e. bladder dysfunction), despite observations that involuntary detrusor contractions occur regularly in continent asymptomatic women while the bladder is filling (7) and a failure to reliably demonstrate detrusor overactivity in women with UUI. However, despite the enigmatic nature of detrusor overactivity, bladder specific treatments (e.g., anticholinergics, B3-agonists, Botox) do reduce UUI episodes, at least partially. Continued belief in the theory that SUI was caused by poor support, and UUI by detrusor overactivity, and that the urethra did not play a role, is an example of what Nobel Prize winning psychologist Daniel Kahneman described “theory induced blindness” wherein we discount our observations when they disagree with a theory we believe. In this way, our two-factor theory blinded us to the data showing the role of urethral function in incontinence. In addition, because we did not have interventions to increase urethral closure pressures, making a measurement to identify something you couldn’t treat seemed futile. Ironically, it should have been obvious, even to lay people, that an organ’s sphincter would play a role in holding fluid in. Now that treatment success based on the two-factor theory has plateaued and is not improving despite the myriad of interventions based on it, seeking a more complete understanding of urethral function, and identifying new therapeutic targets, is warranted.
There are many factors suggesting an effort to understand causal mechanisms should be successful. The female urethra is smaller than your little finger, is easily accessible for investigation, and only contains a limited number of tissue types. What we need is a plan.
A common approach in science when something is believed to be important (i.e., the urethra for maintaining continence) is simply to “study it more” with whatever techniques are at hand. For example, there is evidence that an altered microbiome may be a new explanation for UUI (8). Further work remains to clarify to what extent, if any, the microbiome plays in incontinence, and considerable work has begun to investigate this area. This approach of studying a topic in more depth to improve our understanding obviously has value and will be highlighted in other follow-up articles to this series while focusing on topics such as urethral motor and sensory function. However, this general approach may prevent us from delivering the best possible incontinence care to patients. In the following sections we identify and advocate for two complimentary approaches for moving this field forward.
Systems Diagrams and Computational Modeling
To be successful, our current knowledge must be pushed beyond current considerations and approaches. An overall, evidence-based understanding of how the various elements involved in generating urethral closure pressure at rest and during stressor events is needed. There are nine structural components in urethral wall (9) that work in a coordinated way to maintain a “continence margin.” A considerable amount of information is available for the striated muscle, yet other tissue types, such as connective tissue, presumably deteriorate with age and are largely unstudied. Neuromuscular control systems that modulate urethral closure are mapped, but how, on a minute by minute basis, they adjust closure has typically not been part of the discussion despite their proven role in lower urinary tract symptoms (10). Since urethral closure pressure can be increased during a pelvic muscle contraction, and decreased by relaxation (11), observed pressure is not simply the result of the maximal muscle effect. The exact mechanics of what determines the set-point are not known. We must develop a mechanistic understanding of the interactions, control systems, and structure specific failures that occur.
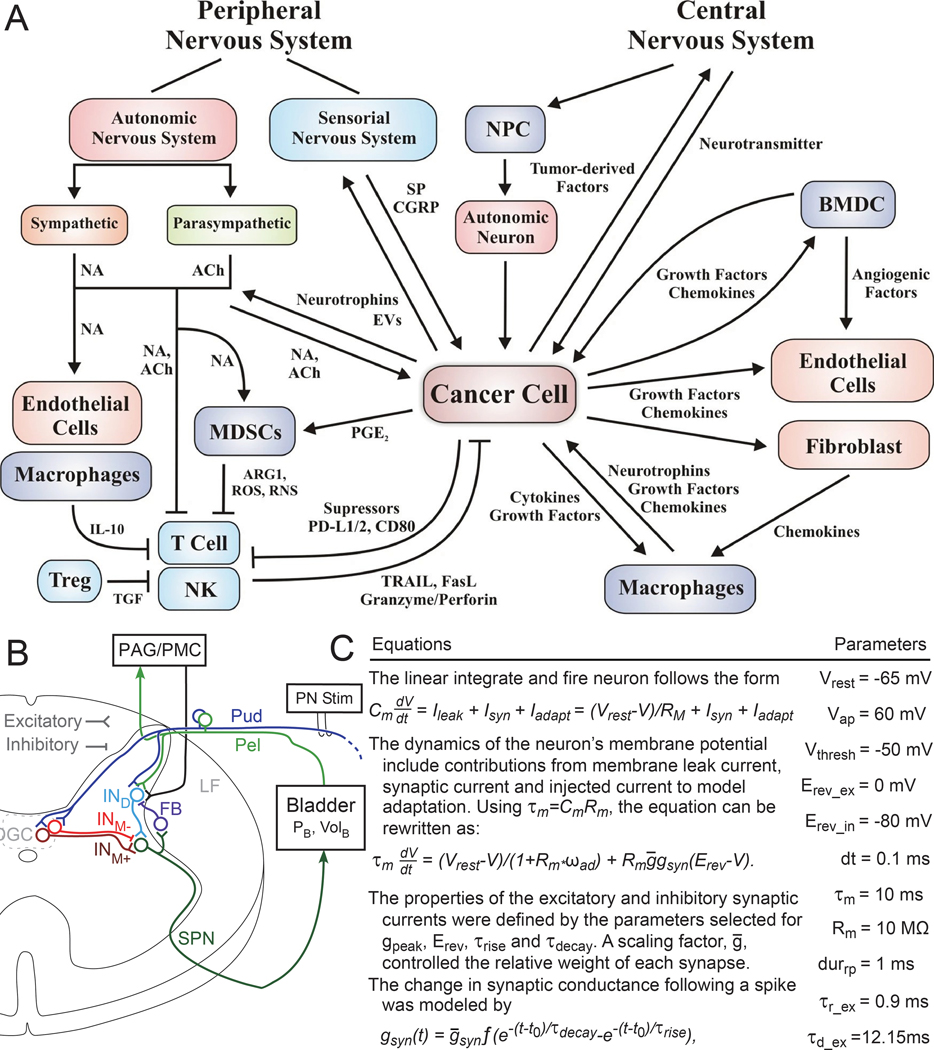
The first step in such a process is to enumerate relevant factors. This is often done via a systems diagram that outlines the interactions between different aspects (components) of the system of interest (e.g., lower urinary tract) and provides a common conceptual framework for multiple researchers to work from. An example systems diagrams is shown in Figure 2A. Different investigators can use these diagrams to test specific hypotheses regarding the interaction of specific components, e.g., does longitudinal smooth muscle modulate urethral pressure during bladder filling? Crucially, these diagrams give investigators a place to plug in their components of interest, to integrate knowledge, and to identify knowledge gaps. Physiological tests no longer need to stand on their own but can contribute towards a common shared understanding.
Figure 2:
Systems Diagrams and Computational Models. A) Example systems diagram demonstrating communication networks between a cancer cell and neuro-immune-vascular systems (17)(reproduced with permission). Such diagrams provide a place for integrating knowledge and identifying knowledge gaps. B) Another example diagram showing neural connections in the cat spinal cord(18)(reproduced with permission). In the original manuscript the connections in the diagram are modeled using linear integrate and fire neurons. Some of the equations and parameters used are shown in (C). This computational model was used to explore how the bladder responds to different stimulation patterns delivered on the pudendal (Pud) nerve. Additional details are available in the original text. Computational models are lacking and underutilized in this field.
Many of these systems diagrams start with minimal complexity and only become more detailed as experiments provide data about specific hypotheses. One component may interact with another component, and often that interaction is denoted as being excitatory or inhibitory -- positive or negative. In some cases, it may prove useful to turn these diagrams into detailed computational models (Figure 2B, 2C) that make more explicit hypotheses or numerical assertions. For example, a biomechanical model may try to replicate urethral pressure observed during filling as a function of multiple components (smooth muscle, striated muscle, blood flow, etc.). Errors in these models indicate gaps in knowledge to fill in.
Predictive Statistical Modeling
A complementary approach is to simply try and fit clinical data to treatment outcomes or observations. For example, we may wish to examine whether a patient will respond positively to sacral neuromodulation or whether a patient is at risk of developing cognitive issues from taking an anticholinergic. This approach is known as predictive modeling (and sometimes as machine learning, artificial intelligence, personalized/precision medicine, etc.).
In predictive modeling, a treatment outcome (e.g., reduction in urinary incontinence episodes) or observation (e.g., presence or absence of stress incontinence) is related to a set of measurements or factors. (N.B. This approach is similar to yet different from risk/predictive factor analysis) For example, risk factor analysis might tell us that low maximal urethral closure pressure increases the odds of incontinence or the chance of not responding to sling surgery. However, it is generally difficult to integrate this information with other factors to guide therapy selection or to understand how much a factor, on its own, is driving our observation of interest. For example, low urethral pressure may increase the odds of treatment failure by 40% (the risk factor approach) whereas a predictive model will integrate other measurements to give a patient specific probability of treatment success (e.g., you have a 30% chance of benefiting from surgery). Similarly, we may measure different parameters (e.g., maximum urethral closure pressure and urethral support) in continent and incontinent women and assign risk factors to each of these parameters. For example, a woman with low urethral pressure but poor urethral support may be cured by surgery while one with similar urethral function but a well-supported urethra might not. However, it is unclear if these parameters are sufficient for accurately identifying continence status. In contrast a predictive model could integrate these and other parameters and report how well it is able to distinguish continent from incontinent women remembering that we may not be measuring yet-to-be discovered phenomena. Using this approach revealed that maximum urethral closure pressure and urethral support only accounted for 61% of the variance in a model (with maximum urethral closure pressure alone describing 50%), leaving 39% unexplained (12); an obvious opportunity for discovery research.
Importantly, the predictive modeling approach forms a framework into which other measurements can be added. For example, we may find that a neurological test that helps understand the minute to minute fluctuations that occur in urethral pressure (10), in addition to the maximum urethral pressure measurement, significantly improves our ability to identify women with or without stress incontinence. In the case of predicting treatment outcomes, this approach can also help with patient counseling and therapy selection. In patients where our predictions are uncertain and end up not matching reality, we can study these patients further for other factors that may be leading to the observed outcomes. In patients for whom therapy fails, we can begin to investigate what set of factors are associated with failure. As each of these factors is identified and validated, they provide opportunities to develop novel therapies that can offer greater improvements than currently available. This personalized medicine approach more precisely targets to the abnormality present in a specific patient, not just the “average” patient. Patients in whom current therapies are not successful may also be good candidates for testing of new therapies that are not yet widely used clinically, rather than only resorting to trying novel approaches after failing all existing approaches. This approach could save money and lessen patients’ frustrations with trying and failing multiple therapies.
Moving Forward
With these two approaches in mind, we briefly highlight areas where increased effort should be devoted.
First, we needed expanded efforts towards generating systems diagrams and computational models related to incontinence so that each element of the diagram can be evaluated to see if there is reliable data to support it or if it needs further experimental verification. There needs to be a generally agreed upon accurate structural map and detailed control system including both nerves and blood vessels. This will require the effort of scientists from several different disciplines.
Second, for predictive models involving forecasting treatment outcomes, we need to quantify the measurements of interest, as well as treatment outcomes. It is relatively common to measure a physiological parameter and to summarize how it compares amongst different cohorts (12,13), but less common to follow patients through treatment. Without knowing how these measurements relate to treatment outcomes, we can’t develop predictive models to predict treatment outcomes based on these measurements.
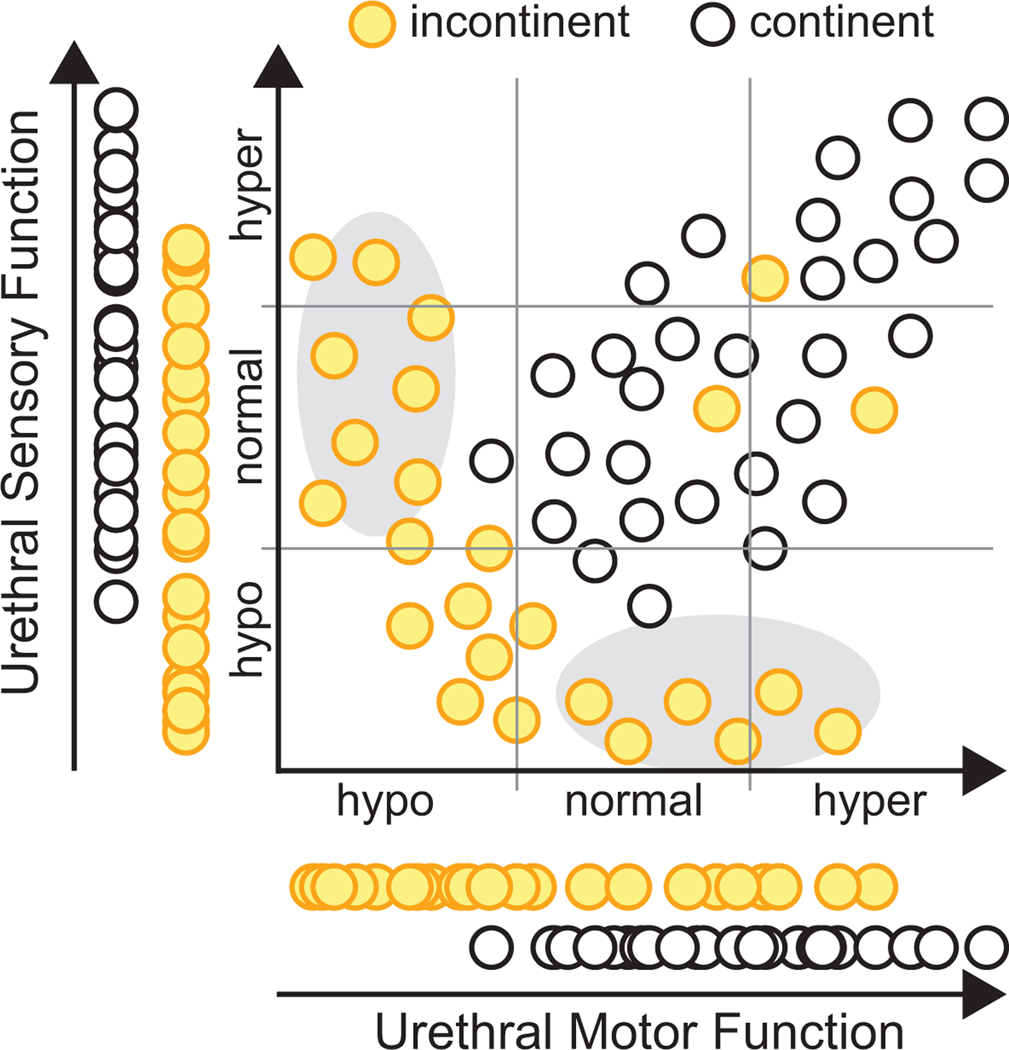
Third, when measuring physiological data, particularly in humans, we need to improve how it is collected. In particular, we advocate that data concerning several factors should all be collected in the same people to better understand how the measured parameters interact and covary. The common alternative is to focus on one parameter at a time to understand its impact. This is fine for understanding that parameter, but it gives an incomplete picture of dysfunction. For example, one group might measure urethral sensation and another might measure urethral motor function. However, if we measure both simultaneously, we might find that is common to observe declines of both sensory and motor function in the same person. In others we may see a sensory or motor specific deficiency, suggesting a different treatment approach may be needed as compared to the common case (Figure 3). Alternatively, we may find normal sensory and motor function (as tested by urethral pressure profile), but that someone is still incontinent, indicating dysfunction elsewhere. In the absence of a single parameter that completely explains lower urinary tract function we need to measure multiple parameters, simultaneously, to better understand why disease occurs and how these parameters are associated with treatment outcomes. Note, this may require developing new and reproducible techniques to quantify factors not currently measured.
Figure 3:
Interpretation of urinary dysfunction benefits from measurement of multiple parameters in the same subjects. Hypothetical example in which urethral motor function (e.g., maximum urethral closure pressure) and urethral sensory function (e.g., current perception threshold testing) are tested in the same individuals, along with continence status. Either parameter on its own (left and bottom), as is commonly studied, fails to clearly delineate continent from incontinent subjects. However, when measured together a clearer picture emerges on how these two parameters contribute to continence. Note, this information could also relate to treatments, as it may turn out subjects with more sensory or motor specific failures (gray ovals) may require different therapies than those with combined sensorimotor impairments. Additionally, some subjects are still incontinent despite good urethral sensory and motor function (3 points in upper right), suggesting additional measurements are needed to help understand factors that may be contributing to their incontinence.
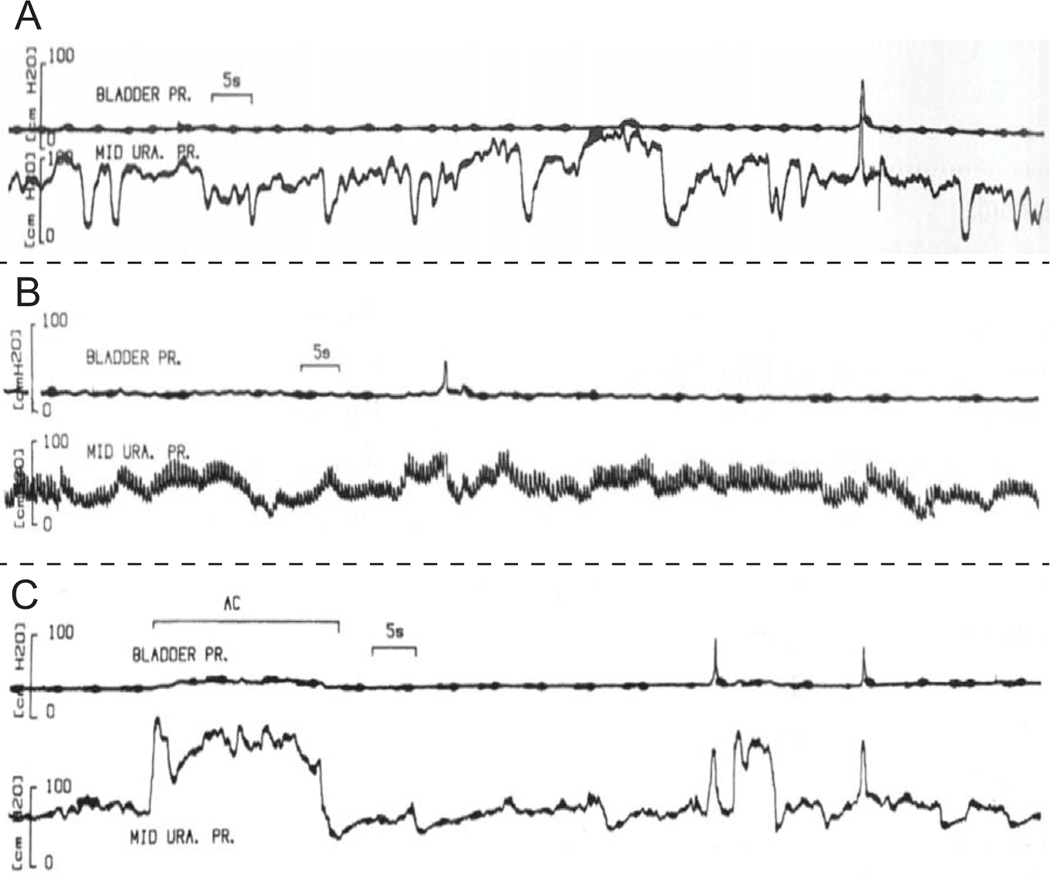
The fourth and final point is that it is critical to setup an infrastructure and process for sharing data amongst groups, similar to what has been done in genetics research. In predictive modeling more data generally means better results and more insight. As such we need to improve the way we share data. Once it becomes possible to get relevant and reproducible data, accumulating data from many teams can become useful as it has in genetics research. This also means sharing data continuously, not just over a small-time window. This includes access to raw data (e.g., raw urodynamics data, raw imaging data), not just interpreted or summarized data. This is especially important for signals where it is difficult to describe in words what a signal is or isn’t. For example, what exactly is urethral instability (Figure 4)? When uninhibited urethral relaxation occurs (14,15), what does it look like? Are there multiple types? Are urethral instability and uninhibited urethral relaxation the same thing? How do any of these signals relate to things we care about (e.g., treatment outcomes)? For computational models, access to raw data will more clearly convey what was observed, including variability and interactions, than any published paper will alone. We expect sharing data will, 1) facilitate standardization/interpretation, 2) facilitate the ability to reanalyze data to gain additional insight and form new hypotheses, 3) improve model performance, and 4) ultimately provide better results for patients. Of course, there are numerous technical issues to work out with such a proposal like protocol standardization and documentation, technical issues associated with data transfer and hosting, as well as patient privacy issues.
Figure 4:
Examples (A)(B)(C) of different types of urethral instability as classified by the author (17)(reproduced with permission). Similar to detrusor overactivity, variation in the way these signals present themselves, and difficulty in describing the signals, makes it difficult to understand the importance of these signals as there is potentially a large amount of interpretation and information filtering being done by the authors. We argue that one path forward in improving our understanding of these signals is to share them electronically, so that computer algorithms can be created to consistently evaluate these signals across multiple studies. Secondly, once that has been accomplished, we need to relate quantification of these signals to treatment outcomes and symptoms. In other words, rather than worrying about defining an arbitrary threshold (of pressure change), we can begin to examine questions like, “How does a subject’s response to treatment vary as a function of the magnitude and frequency of urethral instability?” (along with any other parameters we measure – refer back to importance of multiple measurements in Figure 3).
Conclusion
Incontinence is a major public health problem and causes suffering and isolation for many women. Although we have reduced side-effects and operative morbidity, our treatment success has been stagnant for decades. We have gathered evidence from randomized trials of the relative performance of different treatments, yet these treatments often fail to produce the outcomes women want. There is a reduction of how often women have UUI with medications, but most subjects have fewer incontinent episodes but are not continent. Similarly, operations reduce SUI in 80% of women but 40% continue to be symptomatically incontinent (18). The opportunity exists to utilize the tools of modern science (i.e., systems diagrams, computational models, predictive models) to move this field forward, to uncover yet to be discovered diseases mechanisms, and to provide new approaches for going beyond current success rates.
Supplementary Material
Acknowledgements:
On behalf of the Organizing Committee for the “Female Urethral Function and Failure” series we wish to thank the staff of The Scientific Consulting Group, Inc., for their coordination of the meeting logistics and writing services, helping us to work towards improved incontinence treatments. The SCG science writers of the workshop summaries were: Amanda Cenname, Ph.D., science writer/editor; Robyn Engel, D.V.M., science writer; Carolyn Fisher, Ph.D., senior science writer/director of writing services; and Sally Paustian, M.A.L.S., writer/editor.
Funding Statement: Funding for this work provided by the NIDDK to Dr. Hokanson (K01 DK 121866 and to Dr. DeLancey (RC2 DK 122379).
Grant support: RC2DK122379 to DeLancey and K01DK121866 to Hokanson
Conflict of Interest Disclosure: Dr. Hokanson reports grant support from NIDDK during the conduct of the study. Dr. DeLancey reports grant support from NIDDK during the conduct of the study and other support from Hologic outside the submitted work.
Footnotes
Ethics of Approval Statement: NA
Patient Consent Statement: NA
Permission to Reproduce Material from Other Sources: Obtained. Figures 1 and 3 are original. Fig 2A is open access. Fig 2B,2C was purchased from Springer. Figure 4 was acquired from Karger.
Clinical Trial Registration: NA
Data Availability Statement: Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1.Advancing Basic and Translational Research for Genitourinary Conditions: Female Urethral Function and Failure [Internet]. 2020. Available from: https://www.niddk.nih.gov/news/meetings-workshops/2020/advancing-basic-translational-research-benign-genitourinary-conditions-female-urethra
- 2.Enhorning G. Simultaneous recording of intravesical and intra-urethral pressure. A study on urethral closure in normal and stress incontinent women. Acta chirurgica Scandinavica Supplementum [Internet]. 1961;Suppl 276:1–68. Available from: http://www.ncbi.nlm.nih.gov/pubmed/13696922 [PubMed] [Google Scholar]
- 3.Hilton P, Stanton SL. Urethral pressure measurement by microtransducer: the results in symptom-free women and in those with genuine stress incontinence. British journal of obstetrics and gynaecology [Internet]. 1983;90(10):919–33. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6684949 [DOI] [PubMed] [Google Scholar]
- 4.Kobata SA, Girão MJBC, Sartori MGF, Baracat EC, De Rodrigues Lima G. Urodynamic and ultrasonographic evaluation after continence surgery. International Urogynecology Journal. 1999;10(5):321–4. [DOI] [PubMed] [Google Scholar]
- 5.Lo TS, Wang AC, Horng SG, Liang CC, Soong YK. Ultrasonographic and urodynamic evaluation after tension free vagina tape procedure (TVT). Acta Obstetricia et Gynecologica Scandinavica. 2001;80(1):65. [DOI] [PubMed] [Google Scholar]
- 6.Nager CW, Brubaker L, Litman HJ, Zyczynski HM, Varner RE, Amundsen CL, et al. A randomized trial of urodynamic testing before stress-incontinence surgery. The New England journal of medicine [Internet]. 2012. May 24;366(21):1987–97. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22551104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heslington K, Hilton P. Ambulatory monitoring and conventional cystometry in asymptomatic female volunteers. British journal of obstetrics and gynaecology [Internet]. 1996. May;103(5):434–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8624316 [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Phan MD, Bates LJ, Peters KM, Mukerjee C, Moore KH, et al. The urinary microbiome in patients with refractory urge incontinence and recurrent urinary tract infection. International Urogynecology Journal. 2018;29(12):1775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huisman AB. Aspects on the anatomy of the female urethra with special relation to urinary continence. Contributions to gynecology and obstetrics [Internet]. 1983;10:1–31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6685603 [PubMed] [Google Scholar]
- 10.Kulseng-Hanssen S. Prevalence and pattern of unstable urethral pressure in one hundred seventy-four gynecologic patients referred for urodynamic investigation. American journal of obstetrics and gynecology [Internet]. 1983;146(8):895–900. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6683931 [DOI] [PubMed] [Google Scholar]
- 11.Baessler KK, Miska K, Draths R, Schuessler B. Effects of voluntary pelvic floor contraction and relaxation on the urethral closure pressure. International urogynecology journal and pelvic floor dysfunction [Internet]. 2005;16(3):187–90; discussion 190–1. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15448884 [DOI] [PubMed] [Google Scholar]
- 12.DeLancey JOL, Trowbridge ER, Miller JM, Morgan DM, Guire K, Fenner DE, et al. Stress urinary incontinence: relative importance of urethral support and urethral closure pressure. The Journal of urology [Internet]. 2008. Jun;179(6):2286–90; discussion 2290. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2673985&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenton KS, Lowenstein L, Simmons J, Brubaker LT. Aging and overactive bladder may be associated with loss of urethral sensation in women. Neurourology and urodynamics [Internet]. 2007;26(7):981–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17626276 [DOI] [PubMed] [Google Scholar]
- 14.Low JA. Urethral behavior during the involuntary detrusor contraction. American journal of obstetrics and gynecology [Internet]. 1977;128(1):32–42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/557902 [DOI] [PubMed] [Google Scholar]
- 15.Hindmarsh JR, Gosling PT, Deane AM. Bladder instability. Is the primary defect in the urethra? British journal of urology [Internet]. 1983. Dec;55(6):648–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6652433 [DOI] [PubMed] [Google Scholar]
- 16.Richter HE, Albo ME, Zyczynski HM, Kenton KS, Norton PA, Sirls LT, et al. Retropubic versus transobturator midurethral slings for stress incontinence. The New England journal of medicine [Internet]. 2010. Jun 3;362(22):2066–76. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20479459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cervantes-Villagrana RD, Albores-García D, Cervantes-Villagrana AR, García-Acevez SJ. Tumor-induced neurogenesis and immune evasion as targets of innovative anti-cancer therapies. Signal Transduction and Targeted Therapy [Internet]. 2020;5(1). Available from: 10.1038/s41392-020-0205-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGee MJ, Grill WM. Modeling the spinal pudendo-vesical reflex for bladder control by pudendal afferent stimulation. Journal of Computational Neuroscience [Internet]. 2016;40(3):283–96. Available from: 10.1007/s10827-016-0597-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vereecken RL. Physiological and pathological urethral pressure variations. Urologia internationalis [Internet]. 1996;57(3):145–50. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8912442 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.