Abstract
Human reproductive organs are of vital importance to the life of an individual and the reproduction of human populations. So far, traditional methods have a limited effect in recovering the function and fertility of reproductive organs and tissues. Thus, aim to replace and facilitate the regrowth of damaged or diseased tissue, various biomaterials are developed to offer hope to overcome these difficulties and help gain further research progress in reproductive tissue engineering. In this review, we focus on the biomaterials and their four main applications in reproductive tissue engineering: in vitro generation and culture of reproductive cells; development of reproductive organoids and models; in vivo transplantation of reproductive cells or tissues; and regeneration of reproductive tissue. In reproductive tissue engineering, designing biomaterials for different applications with different mechanical properties, structure, function, and microenvironment is challenging and important, and deserves more attention.
Keywords: Biomaterials, Reproductive tissue engineering, Organoids and models
Graphical abstract
In this review, we mainly focus on the biomaterial strategies for three main applications in reproductive tissue engineering including in-vitro germ cell generation and culture, the biomaterials for repairing reproductive organs, and developing reproductive organoids.
Highlights
-
•
Various biomaterials have been developed and used in reproductive tissue engineering.
-
•
3D culture systems can lead to better cell-cell interactions for in vitro production of reproductive cells.
-
•
Reproductive organoids and models are formed by biomaterials to simulate the environment of natural reproductive organs.
-
•
Biomaterials should promote vascular regeneration and resist inflammation for in-situ reproductive tissue regeneration.
1. Introduction
Human reproductive organs and tissues comprise male and female reproductive organs or tissues, including the ovaries, uterus, vagina, penis, and testes, that are vital importance to the life of an individual and the reproduction of human populations [1]. Inflammation, aggressive tumors, trauma, infectious disease, and acquired or congenital abnormalities may cause degenerative damage to reproductive organs or loss of reproductive capacity [[2], [3], [4]]. Traditional repair methods mainly revolve around hormone therapy, surgical methods and organ transplantation [[5], [6], [7]]. These methods have a limited effect in recovering the function and fertility due to the lack of regenerative ability and the risk of serious rejection in reproductive organs and tissues [8]. Therefore, new therapeutical strategies to recover the reproductive capacity and repair the defect of reproductive tissues need to be developed.
Aim to replace and facilitate the regrowth of damaged or diseased tissue, biomaterials are designed and synthesized to offer a hope to overcome the difficulties in reproductive tissue engineering. The biomaterials are required to be non-toxic and biocompatible to the organs and tissues in the body at first. In tissue engineering, some biomaterials can not only directly combine and interact with biological organisms but also guide the reconstruction of the basic structure and morphology of organs and accelerate tissue regeneration. In addition, biomaterials are usually loaded with cells and collaborated with growth factors and drugs in most researches rather than act alone. They can markedly improve the local and durable cell retention rates in the body and also served as a carrier for drugs and growth factors [[9], [10], [11]].
For different organs and tissues in reproductive tissue engineering, biomaterials need to be designed so that can be harmonious with their specific mechanical performance, structure, degradation, function, and physiological environment [12]. For example, the testis and ovary have complex structures and precise systems as the organs produce germ cells and mature oocytes in individual organisms. The age-related and environmental stimuli-associated organ decline and infertility occurred frequently in humans with uncertain mechanisms and was hard to reverse [5,8]. Thus, in vitro spermatogenesis and oocyte maturation are significant treatments for fertility restoration [13]. The complex physiological processes of spermatogenesis and follicular development and ovulation have involved the interaction of stratified epithelium and gametogenic cells, and cyclical changes of sex hormone levels [[14], [15], [16]]. Therefore, some special requirements are imposed on the biomaterials to simulate the microenvironment of testis and ovary. In this case, biomaterials often require to have the low internal pressure and mechanical rigidity, a microporous structure that support sufficient contact between the developing cells and surrounding cell, as well as a highly hydrophilic environment that provides efficient mass transfer capabilities for the diffusion and transfer of hormones and cytokines. In contrast to testis and ovary, male penis and female vagina and endometrium might have a trauma caused by sexual intercourse or surgery [[17], [18], [19]]. Biomaterials applied for in-situ regeneration are more likely to have better mechanical strength and ability to promote vascular regeneration and resist inflammation [20,21].
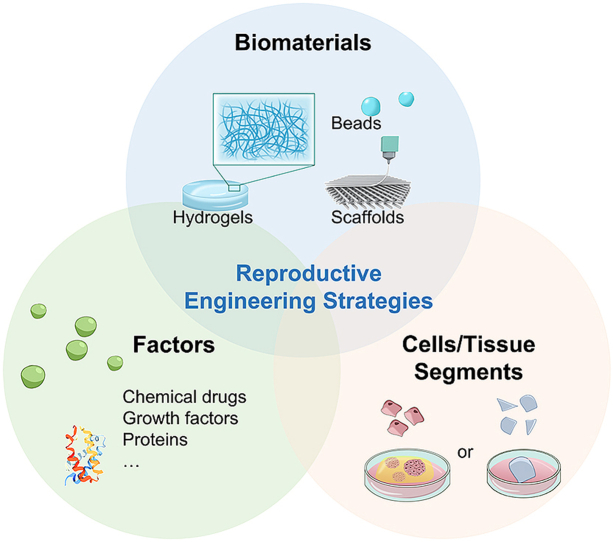
In this review, we mainly focus on the biomaterials and their reproductive tissue engineering, including in vitro generation and culture of reproductive cells, development of reproductive organoids, in vivo transplantation of reproductive cells or tissues, and reproductive tissue regeneration. (Fig. 1).
Fig. 1.
Diagram of biomaterial for the applications of reproductive tissue engineering.
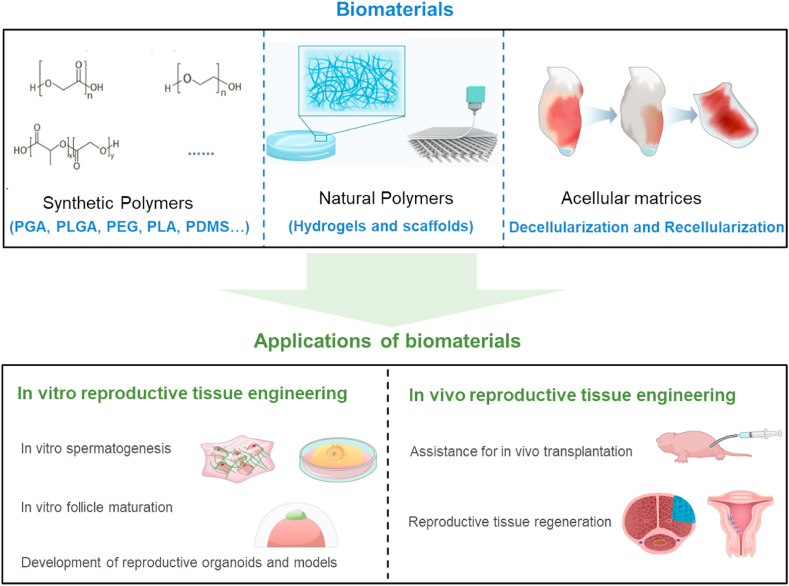
2. Classification of biomaterials in reproductive tissue engineering
The main biomaterials used in reproductive tissue engineering can be divided into synthetic polymers and natural polymers (hydrogels and scaffolds), and decellularized matrices [1]. Here, we summarize the advantages and shortfalls of different biomaterials and the main typical application cases. Table 1 outlines the biomaterials that are discussed in this review.
Table 1.
Biomaterials in reproductive tissue engineering and their applications.
| Classification | Merits and demerits | Biomaterials | Applications | Ref. |
|---|---|---|---|---|
| Synthetic polymers | Inexpensive and can be manipulated easily; tunable mechanical properties and high malleability; lack of or limited biocompatibility Foreign body response; adverse immunologic reactions; lack of adhesion to living tissues |
PLA | Combined with autologous chondrocytes to construct penile prostheses | [22] |
| PGA | Combined with cells to reconstruct the smooth muscle tissue of the cavernous body | [23] | ||
| PDMS | Coculture of embryonic stem cells and testicular cells; Microfluidic devices | [26,90] | ||
| PLGA | In vitro spermatogenesis of immature spermatogenic germ cells | [27] | ||
| PEG |
3D follicular culture |
[92] |
||
| Natural polymers (hydrogels) | High-water content; excellent biodegradability and biocompatibility; environmental stresses similar to tissue; can be easily loaded with other factors; maintain the 3D culture environment; ensure the effect of cell–cell interactions; Insufficient mechanical strength |
Hyaluronan-based hydrogels | In vitro maturation of follicles | [29] |
| Gelatin-based hydrogel | Constructing placental barrier models | [31] | ||
| Soft agar hydrogel | Coculture of spermatogonia and somatic cells | [81] | ||
| Alginate-based hydrogels | In vitro follicular culture; in vivo transplantation of isolated preantral follicles and ovarian cells; 3D culture system for testicular cells | [32,54] | ||
| Fibrin hydrogels; fibrin-alginate hydrogels; fibrin-collagen composites | Primordial follicle transplantation | [57,58] | ||
| Collagen-based hydrogel | In vitro oocyte maturation of ovary follicles | [51] | ||
| Matrigels |
Generation of functional spermatids from human SSCs in vitro; testicular, ovarian, and endometrial organoids |
[[40], [41], [42], [43], [44], [45],55] |
||
| Natural polymers (scaffolds) | Porous structure, good tissue integration; can be loaded with cell growth factors and drugs; improve angiogenesis Insufficient mechanical strength |
Collagen scaffolds | Loaded with human umbilical cord-derived mesenchymal stem cells/bone marrow mesenchymal stem cells for endometrial regeneration | [28,117] |
| Alginate-based macroporous scaffolds | Culture and growth of primitive follicles | [126] | ||
| Gelatin-based scaffolds |
Create a bioprosthetic ovary; endometrial repair; functional reconstruction of injured corpus cavernosa |
[30,59,119] |
||
| Acellular matrices | Retention of the bioactive matrix; Structural integrity with better mechanical performance Need improvements in terms of the morphology and precise structure of the original tissue after decellularization; cell filling rate during the recellularization process needs to be improved |
Acellular porcine small intestinal submucosa graft | Cervicovaginal reconstruction | [127] |
| Corpora collagen matrices | Functional restoration of the penis | [62,66] | ||
| Autologous cartilage rods | Penile prostheses | [67] | ||
| Amniotic membrane | Penile reconstruction (treatment of Peyronie's disease) | [128] | ||
| Acellular uterus | Recellularized in vitro with primary uterine cells (Bioengineered uterine tissue) | [[68], [69], [70], [71]] | ||
| Bovine pericardium | Potential scaffold for testicular repair | [72] | ||
| Ovarian scaffolds | Artificial ovaries | [63,64] | ||
| Decellularized placental scaffold | 3D dynamic culture of mouse embryonic fibroblasts | [70] | ||
| Acellular testis | Testicular organoid construction | [[73], [74], [75]] |
2.1. Synthetic polymers
Synthetic polymers are inexpensive and can be easily manipulated to satisfy specific structural requirements of organs and tissues. In reproductive tissue engineering, they are usually used to repair the defect of male penis and female reproductive tract, and endometrium caused by sexual intercourse or surgery. For instance, the rods, films, and textile materials formed by biodegradable synthetic polymers, including poly (lactic-co-glycolic acid) (PLGA), polyglycolic acid (PGA), and polylactic acid (PLA), can be used in penile reconstruction [22,23]. However, the disadvantages of synthetic polymers as implant biomaterials are associated with the risk of a foreign body response, adverse immunologic reactions, and lack of adhesion to living tissues [24]. During degradation and reabsorption, some polymers such as PGA may activate macrophages, reactive oxygen intermediates, and dehydrogenases at the interface between cells and biomaterials [25]. Most synthetic polymers cannot provide an advantageous microenvironment for the culture and development of gametogenic cells and Sertoli cells compared with natural polymers and acellular matrices. However, the surface of polydimethylsiloxane (PDMS) substrates after being treated with oxygen plasma to increase surface wettability and cell adhesion can also support the culture and testicular cord formation of testicular cells [26]. Also, PLGA-based hydrogel provides a favorable environment for spermatogenic germ cells to proliferate and differentiate into mature spermatids [27].
2.2. Natural polymers
In contrast to synthetic polymers, natural polymers have excellent biodegradability and biocompatibility and can be advantageous biomaterials in the reproductive tissue engineering field [28]. These natural polymers mainly include hyaluronan [29], gelatin [30,31], alginate [32,33], fibrin [24,[34], [35], [36]], collagen [[37], [38], [39]], soft agar [13], and Matrigel [[40], [41], [42], [43], [44], [45]]. Natural polymers can form natural hydrogels and scaffolds by chemical cross-linking, and physical cross-linking based on their formation methods. The natural polymers that have a lot of hydroxyl groups on the molecules (e.g. hyaluronan, gelatin, collagen) can be modified with photopolymerizable methacrylate (MA) groups to allow the natural matrix to be chemical covalently cross-linked by UV light [46]. As a linear anion polysaccharide, alginate, chitosan and agar can be cross-linked by Ca2+ to form the hydrogels, films and macroparticles [47]. Besides, enzyme reactions have been used to drive the polymerization of gelation and collagen-based hydrogels by generating no cytotoxic byproducts [48,49].
Because of the similar mechanical environment and similar components to human tissue, natural hydrogels and scaffolds have been widely used for 3D cell culture systems of cells and tissues in vitro. 3D hydrogels culture systems can ensure the effect of cell–cell interactions among different cells, which is vital for reproductive tissue engineering [[50], [51], [52], [53]]. For example, an alginate-based 3D culture system can support the interactions between germ cells and somatic cells to promote the self-organization of testicular cells into seminiferous tubules [54]. In reproductive tissue engineering, hydrogel culture systems like Matrigel, which is composed of ECM proteins and growth factors, have been used to mimic the 3D environment of testis and ovary [[40], [41], [42], [43], [44], [45],55]. However, inherent shortcomings such as uncertainty of biosafety, mixed components, and lack of physical and chemical versatility hinder the widespread use of Matrigel [56]. Other natural hydrogels can supply the required biological and mechanical signals by loading other factors (cytokines, growth factors, proteins or collagen components) [57,58]. Moreover, the natural scaffolds with the porous structure can be obtained by 3D printing or lyophilized preparation method. The natural scaffolds have a good tissue integration and can load some cell growth factors (e.g. VEGF) and drugs to improve angiogenesis and tissue regeneration when implanted into the body [59,60]. Nevertheless, the insufficient mechanical strength of natural hydrogels and scaffolds, especially the poor tensile strength, limits their application in reproductive tissue reconstruction.
2.3. Acellular matrices
Acellular matrices are obtained by chemically removing all cells while retaining the bioactive matrix. It can provide a bionic structure consisting of collagens, elastin, and bioactive proteins and has a similar mechanical performance to native tissue [61,62]. Acellular matrices are usually obtained via sodium lauryl ester sulfate (SLES)-treated or sodium dodecyl sulfate (SDS)-treated processes [[63], [64], [65]].
To date, acellular matrices, including corpora collagen matrices [62,66], autologous cartilage rods [67], acellular uterine tissue [[68], [69], [70], [71]], and bovine pericardium [72], have been used as the main methods to regenerate the penis, uterus, and vagina. Acellular ovarian scaffolds [63,64] and acellular matrix testes [[73], [74], [75]] can provide a suitable environment to culture ovarian and testicular tissue fragments and related cells for follicle growth and spermatogenesis after recellularization. For example, the acellular matrices of the ovary can maintain the specific orientation and structure of collagen fibers and biological activity after decellularization, and the natural elasticity of natural collagen can protect the formation of follicles [63]. Moreover, the composition and nanostructure of the biomaterials affect the morphological and physiological behavior of testicular cells. Artificial scaffolds usually lack or have limited biocompatibility and tissue specificity and result in disorganized testicular cells. Since the testicular matrix is rich in tissue-specific ECM proteins, it has been considered the best scaffold for testicular recellularization [73,76]. They have the functional structure to separate seminiferous tubules, provide a closed environment for spermatogenesis and are responsible for mediating the intercellular communication and transportation of biologically active molecules.
At present, the developed acellular matrix materials must be improved in terms of the morphology and precise structure of the original tissue after decellularization, and the subsequent recellularization strategy must be improved to preserve the ultrastructural characteristics of the matrix and increase the cell filling rate during the recellularization process. To improve the cellular content, a dynamic seeding approach was reported. The biomaterials were placed in the center of the perfusion bioreactors with a stirring speed of 40 rpm to keep the cells in suspension, seed on the biomaterials and continuously penetrate into the scaffold pores, and the flow perfusion rate can modulate the cell deposition [77,78].
3. Biomaterials of in vitro reproductive tissue engineering
3.1. In vitro spermatogenesis
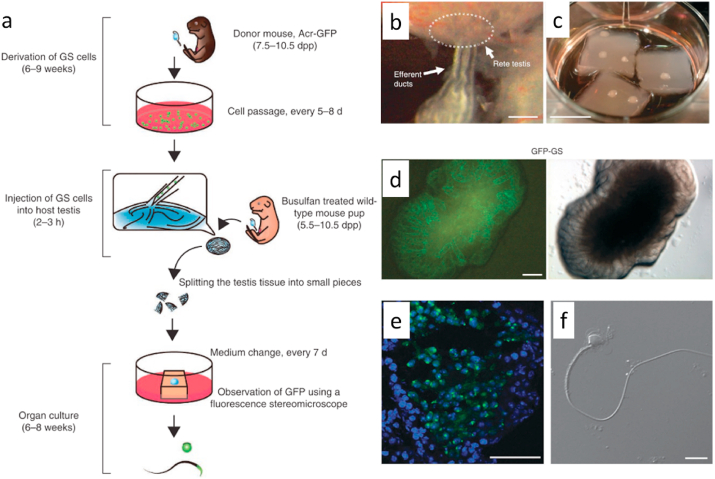
In vitro spermatogenesis is a significant treatment for sperm deformity and azoospermia for male fertility. This technique is crucial in many clinical applications, hopefully including exploring the cellular mechanisms during the generation of male germ cells and developing new strategies to produce male germ cells in vitro [13]. Spermatogenesis can be achieved by culturing testicular tissue fragments, prepubertal testicular cells, or ESCs in vitro (Table 2). In 2011, Sato et al. placed testicular tissue fragments in the gas-liquid interphase of agarose gel and successfully reconstituted mouse sperm in vitro [79,80] (Fig. 2). Also, Stukenborg et al. developed a soft agar culture system to provide the physical support and environmental features that enable the clonal expansion of male germ cells in coculture of spermatogonia and somatic cells [81]. Spermatogonial stem cells (SSCs) are located in seminiferous tubules and have close contact with Sertoli cells in the testis [50,82]. The success of spermatogenesis depends on the correct spatial arrangement of SSCs and Sertoli cells, which organize in seminiferous tubules in the testis [50]. Based on previous studies, 2D culture systems may lose the effect of cell–cell interactions between SSCs and Sertoli cells and also have a limited potential in the formation of testicular microtubules [39,83]. Therefore, 3D culture systems have been developed with biomaterials to support the cell–cell interactions among different cells in the process of in vitro spermatogenesis (Table 2). Natural hydrogels and scaffolds are the main choice. For instance, Baert et al. achieved mouse in vitro spermatogenesis in weeks following prepubertal testicular cells seeding on alginate-based 3D-bioprinted scaffolds to produce testicular constructs [84]. An in vitro 3D culture system of testicular cells formed by collagen hydrogel enable the spermatocytes from patients with nonobstructive azoospermia to differentiate into spermatids [39]. Moreover, the hydrogel microspheres also can serve as a 3D culture model to encapsulate mouse ESCs and improve the differentiation process to primordial germ cells [85]. As we know, the mechanical environment of hydrogels for sperm generation are important and usually be very soft in those previous works [43,86,87]. And suggested that testicular cells embedded into the very soft decellularized testicular dECM gel (storage modulus <20 Pa) undergo better seminiferous tubule organization, which is superior to the reference culture in collagen hydrogels (storage modulus 75–100 Pa) [88]. However, the effect of the mechanical environment on sperm generation has not been systematically studied.
Table 2.
3D culture systems of reproductive cells and tissue fragments in vitro.
| Reproductive cell culture and transplantation | Biomaterials and cells/tissue fragments | Highlights | Ref. |
|---|---|---|---|
| In vitro spermatogenesis | PLGA hydrogel (SD rat testicular cells) | Provide a favorable environment for spermatogenic germ cells to proliferate and differentiate into mature spermatids | [27] |
| Collagen gel matrix (human testicular cells) | Culture of spermatogenic cells from nonobstructive azoospermic patients to produce germ cells | [39] | |
| Agarose gels (mouse testicular tissue fragments, gas–liquid interphase) | Successfully reconstituted mouse sperm in vitro maintained over 2 months | [79,80] | |
| Alginate-based bioprinted scaffolds (mouse prepubertal testicular cells) | Successful in-vitro spermatogenesis in 80% of cultivated prepubertal tissue fragments in 3D-printed hydrogel scaffold | [84] | |
| Collagen IV-alginate microspheres (mouse ESCs) | Producing mature germ cells from mouse ESCs differentiation | [85] | |
| Matrigel (Sprague Dawley rat testicular cells) |
Generate testicular organoids with a functional BTB |
[41,42] |
|
| In vitro follicle culture | Hyaluronic acid-based hydrogel (mouse preantral follicles) | Support follicle growth, estradiol secretion and resumption of meiosis in 3D culture | [29] |
| 3D-printed microporous gelatin scaffolds (secondary follicles from 16-day-old mice) | Producing normal levels of hormones | [30] | |
| Alginate-based hydrogel (microencapsulation of rat ovarian cells) | Achieve stable secretion of hormones during 90 days | [34] | |
| Collagen-based hydrogel (ovarian follicles from rats) | Type I collagen hydrogel under different environmental stresses affect development of ovarian follicles | [51] | |
| Fibrin-based hydrogel (mouse primordial and primary ovarian follicles) | Transplantation into adult mice to obtain live births | [57] | |
| Fibrin-based hydrogel (ovarian tissue from young mice) | VEGF-loaded hydrogel promoted angiogenesis and enhanced engraftment and function of the tissue | [58] | |
| Cross-linked hydrogel of 4-arm PEG and difunctional peptide (immature secondary follicles from 14- to 15-day-old mice) | Synthetic hydrogels with tunable properties; support a 17-fold volumetric expansion of follicles | [92] | |
| Fibrin–alginate matrices (two-layered secondary follicles from young mice) | The rate of producing competent oocytes raised to 82% | [129,130] |
Fig. 2.
Testicular tissue fragments cultured in the gas–liquid interphase of an agarose gel to reconstitute mouse sperm in vitro. (a) Schematic diagram of the protocols of the gas–liquid interphase culture method. (b) Efferent ducts and rete testis, Scale bars: 0.5 cm. (c) The testis tissue fragments were placed on agarose gel and half-soaked in the medium. (Scale bars: 1 cm) (d) GFP-expressing germline stem cells (GFP-GS). (e) Immunostaining of the host testis tissue. GFP (green), Hoechst 33342 dye (blue). Scale bars: 50 μm. (f) The flagellated sperm were found after 57 days of culture of mouse testicular tissue fragments. Scale bars: 10 μm. Reproduced with permission [80]. Copyright 2013, Nature Protocols.
Still, the efficiency and duration of in vitro spermatogenesis were much lower than those in vivo [89]. To improve the efficiency and duration of sperm cell production, a microfluidic device has been adopted to culture testis tissues and ensure that nutrients and oxygen are efficiently and evenly provided [[89], [90], [91]].
3.2. In vitro follicle maturation
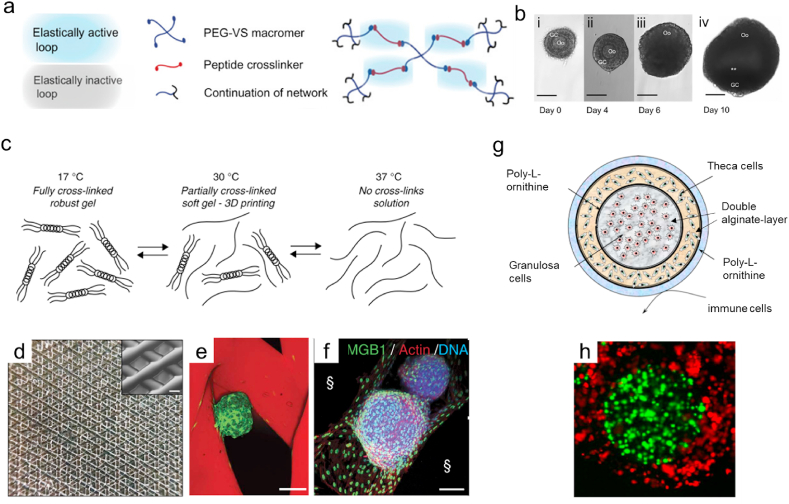
In vitro production of oocytes and fertilization can be an appropriate treatment for infertility in females and an advanced method to study the mechanism of follicle maturation in vivo [4,51]. The biophysical microenvironment can determine the cell fate and development in the process of ovarian follicle maturation [51]. A mature follicle is enclosed by theca and granulosa cells as supporting cells in the ovarian follicle. Thus, remaining in the spheroid shape of ovarian follicles and maintaining the cell-cell communication between support cells and oocytes are critical in the proliferation and maturation process [34,53]. Similar to in vitro spermatogenesis, 3D culture systems that formed by hydrogels and scaffolds also have the advantages of maintaining the cell-cell interactions and the normal shape of follicles (Table 2). Previous studies have shown that the physical environment and scaffold architectures of biomaterials and other loaded factors (cytokines, growth factors, proteins or collagen components) in hydrogels can impact the survival, phenotype and functional maintenance of follicles [30,51,53]. First, hydrogels or scaffolds can modulate the environmental stresses for the oocyte development by adjusting the concentration of the matrix. For example, Joo et al. altered the concentration of type I collagen hydrogel from 1% to 7% (weight/volume) to generate a 3D culture system for oocyte maturation of ovary follicles under different environmental stresses [51]. Ariella et al. designed the peptides crosslinked PEG hydrogels with a tunable physical properties that can support 17-fold volumetric expansion of follicles in the hydrogel network (Fig. 3a and b) [92]. The two studies demonstrated that suitable mechanical environment in ovarian follicle culture is vital for the survival, phenotype and functional maintenance of follicles. In addition, Laronda et al. reported a 3D-printed gelatin-based microporous scaffold to create a bioprosthetic ovary to restore the ovarian function of sterile mice (Fig. 3c–f) [30]. In their work, the 3D-printed microporous scaffold can support the adhesion of isolated follicles, and optimizing the porosity and pore geometry can improve the angiogenesis, survival and function of mouse follicles. Additionally, cytokines and growth factors can be introduced to improve the hydrogel culture environment for maintaining their respective phenotypes of follicles and oocyte generation [34,58]. The development of biomaterials for in vitro oocyte maturation will be a new direction to change the current solutions for fertility preservation [93,94].
Fig. 3.
Hydrogels and scaffolds designed for a 3D follicle culture. (a–b) PEG-based hydrogels with a difunctional peptide as the cross-linker for an ovarian follicle culture. (b) i-iii Morphology of ovarian follicles cultured in PEG hydrogel within 10 days. Scale bars: 100 mm. Reproduced with permission [92]. 2011, Biomaterials. (c–f) 3D-printed microporous gelatin scaffolds seeded with follicles. (c) Schematic of the thermoreversible properties of gelatin. (d) Photographs of a five-layered 3D-printed scaffold. Scale bar: 250 mm. (e) Follicles were seeded in a 60° scaffold, and confocal fluorescence images (f) of follicles cultured for 2 days. Scale bars: 100 mm. Reproduced with permission [30]. Copyright 2017, Nature Communication. (g) Schematic of the ovarian constructs. The ovarian constructs were fabricated using a cross-linked alginate layer and poly-l-ornithine (PLO) layer encapsulated with granulosa and theca cells to replicate the key structure of ovarian follicles. (h) Different cells and granulosa cells were labeled with CellTracker Green, and theca cells were labeled with CellTracker orange within the constructs. Reproduced with permission [34]. Copyright 2012, Reproductive Biology and Endocrinology.
3.3. Construction of reproductive organoids and models
Organoids are an in vitro culture model of 3D source tissue (from cells to tissue fragments) and have some level of tissue structure and function [95,96]. In reproductive organ tissue engineering, the study of in vitro organoids can be programmed and provide a support matrix and appropriate physical and biochemical cues to highly simulate the natural environment of cells in living organisms to study the mechanism and interactions in reproductive organs [53,[97], [98], [99]]. Here, we will introduce the biomaterials to construct various reproductive organoids and models, including testicular organoids, ovarian organoids, and other organoids and models.
3.3.1. Testicular organoids
Testicular organoids are an in vitro model of functional testicles to study the mechanisms of spermatogenesis and are a predictive first-tier drug-screening tool for the treatment of human spermatogenic failure [50,76]. The construction of a complete male microenvironment involves reaggregating testicular cells, performing testicular tubulogenesis, and forming the blood-testis barrier.
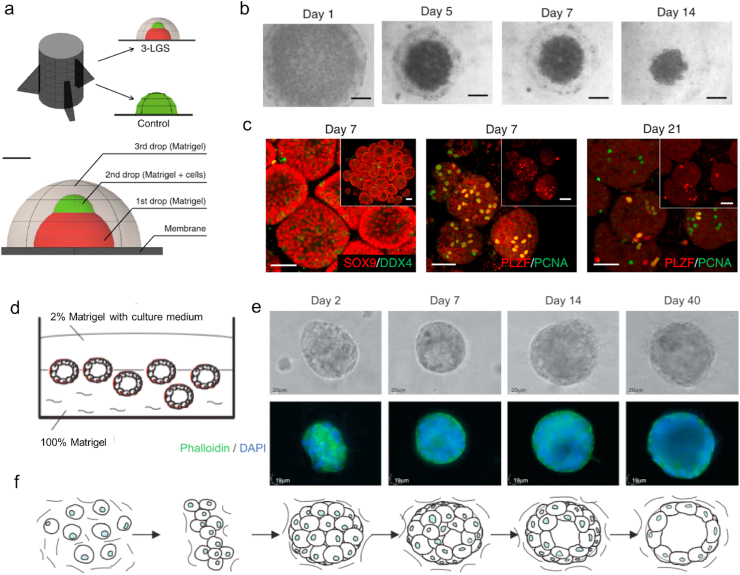
3D testicular culture systems can provide complex spatial conditions and microenvironments similar to the testis in vivo, which enables germ cell tissue to form dense cellular clusters, provides and maintains potentially important contact between germ cells and Sertoli cells during sperm information [50,85,100]. 3D biohydrogels such as soft agar [13], methylcellulose, collagen, and Matrigel [41] are the main 3D matrices to mimic the microenvironment of testicular cells to construct testicular organoids. In the testis, the blood-testis barrier (BTB) is a vital part of male reproductive function to defend the testis against foreign pathogens and environmental alien organisms and separate apical (adluminal) compartments of the seminiferous epithelium to provide a specialized microenvironment for hormonal regulation and spermatogenesis [101,102]. João Lee and colleagues reported a three-layer gradient system; specifically, a layer of Matrigel/testicular cells was placed between two layers of acellular Matrigel to generate testicular organoids with a functional BTB [41,42] (Fig. 4a–c). These testicular organoids are robust and reproducible models that can be applied to track and quantify cell migration in testicular organoids, study the role of the growth factors and study the toxic effects of drugs and environmental pollutants on the integrity of the BTB and maintenance of germ cells [42]. BTB formation can support cell epithelialization in these testicular organoids [41]. In addition, reducing the matrix size [103] is beneficial to the nutrient and oxygen supply for organoid culture, which improves the spermatogenic function of the testis and promotes the differentiation of germ cells in tubule-like structures.
Fig. 4.
Formation of testicular and ovarian organoids using Matrigel. (a–c) Scheme of testicular organoids using a three-layer gradient system (3-LGS). (b) Formation of 3-LGS testicular organoids after days 1, 5, 7, and 21 by bright-field microscopy. (c) Cells that were positive for SOX9 (Sertoli cell marker) and DDX4 (germ cell marker) in spherical–tubular structures of the testicular organoids after days 7 and 21 in culture. Reproduced with permission [42]. Copyright 2018, Nature Protocol. (d) Schematic diagram of the in vitro ovarian organoid model with spheroids of normal HOSE cells cultured in 3D matrix. (e) Phase-contrast images and fluorescence staining of F-actin and nuclei in the spheroids of normal HOSE cells. (f) Schematic diagram of morphogenesis in the spheroids of normal HOSE cells. Reproduced with permission [45]. Copyright 2009, Neoplasia.
3.3.2. Ovarian organoids
Ovarian follicles function as globular multicellular aggregates. The oocytes in ovarian follicles must maintain contact with the supporting cells until maturation and preparation for ovulation. The development of female ovarian 3D organoid technology has opened up a new approach to study its biology, pathology, and pregnancy physiology, and it has great potential in ovarian cancer drug screening. In the construction of ovarian organoids, the function of the tissue (support follicular growth, maturation, and release into the fallopian tubes) and basic morphological characteristics are required [104].
The combination of Matrigel and tissue fragments or isolated cells can construct a 3D cell culture model system as ovarian organoids [44,45]. For example, Kopper et al. constructed ovarian cancer organoids by isolating tumor cells from tumor pieces suspended in basement membrane extract for drug screening and investigation of the subtype responses of different tumors [44,105]. In exploring the relationship between ovarian cancer and chronic inflammation, as the origin of ovarian epithelial tumors, spheroids of human ovarian surface epithelial (HOSE) cells were harvested and seeded in ovarian organoids formed by the growth factor–reduced Matrigel. Then, whether the protumor inflammatory cytokine tumor necrosis factor α would introduce a malignant phenotype in normal HOSE cells was determined [45] (Fig. 4d–f).
3.3.3. Other organoids and models
Other organoids and models that have adopted biomaterials in reproductive tissue engineering are limited. Firstly, the placental barrier regulates the maternofetal exchange of endogenous and exogenous substances, which plays an essential role in fetal development [106]. Therefore, many scientists have focused on constructing placental barrier models [107] with great potential for testing interventions for diseases, improving the pregnancy process, identifying better treatment options, and achieving more accurate diagnoses. Kuo et al. reported a dynamic 3D placental model constructed by bioprinted gelatin hydrogels with a patent central lumen to model the spiral arteriole and study interactions between trophoblasts and endothelial cells and investigate the trophoblast-endothelium signaling involving angiogenesis and apoptosis [31]. Nanofilm biomarkers have also been used to construct a human placental barrier model with bilayer structures similar to the placental barrier in early pregnancy [106]. In this work, collagen and laminin nanofilms were used to mimic the basement membrane and encapsulate primary fibroblasts and endothelial cells. The nanofilms could support cell adhesion and function, cell communication, and gap junction formation [106]. The endometrial model is a good platform for studying embryo gestation and dynamic changes during the menstrual cycle. In the work of Boretto and his co-workers, processed tissue fragments of uterine horns have been cultured in 70% Matrigel, as a 3D extracellular matrix, to develop an endometrial organoid [55] and Bishop et al. then followed this research to model chlamydia infection in endometrium [108]. Besides, Matrigel is also the main biomaterials used in other organoids to study the human female reproductive tract like fallopian tubes and cervix [104,108,109].
4. Biomaterials of in vivo reproductive tissue engineering
In vivo reproductive tissue engineering includes the in vivo transplantation and functional regeneration of reproductive organs. Biomaterials for in vivo reproductive tissue engineering must provide similar mechanical properties to those of original tissue with biodegradability and nontoxic byproduct generation in vivo and bionomic structures.
4.1. Biomaterials for in vivo transplantation of reproductive tissue
In previous studies, in vivo transplantation of artificial ovaries, primordial follicles or testicular tissue cells can recover the normal levels of hormones in experimental animals, while some biomaterials can be adapted to assist in vivo transplantation to enhance the success rates [30,37,38,57].
Hydrogels based on natural polymers can also be a potential matrix for in vivo transplantation of isolated preantral follicles and ovarian cells to construct transplantable artificial ovaries [[32], [33], [34], [35], [36], [37], [38],57,58,65]. For example, isolated mouse follicles were inserted into microporous gelatin scaffolds and embedded into ovary-removed mice, which results in the production of normal levels of hormones [30]. Kniazeva et al. encapsulated the primordial and primary ovarian follicles into fibrin-alginate, fibrin-collagen, and fibrin for subsequent transplantation into adult mice [57]. In a further study, the results showed that VEGF highly increased the probability of live births among women with ovarian tissue transplantation [58]. Acellular ovarian scaffolds, which maintains the monodisperse pores of the matrix and ovarian vascular structures, have been implanted into pubertal mice that have been ovariectomized and increased the levels of serum estradiol and inhibin A [65].
Moreover, testicular tissue has limited survival after in vivo transplantation as an ectopic xenograft [110]. Hui Gao et al. used different hydrogels to encapsulate mouse testicular cells and transplanted them into the dorsal region of recipient nude mice to study self-organized seminiferous tubule processes [86]. Among them, collagen hydrogels and Matrigel can promote the formation of a seminiferous tubule structure and sustained for 12 weeks. Conversely, the encapsulation of CELLINK hydrogel that based on cellulose-alginate, would prevent the proliferation of embedded testicular cells [86].
4.2. Biomaterials for reproductive tissue regeneration
Biomaterials have also been widely used in reproductive tissue regeneration. The majority of research cases on regenerative repair are related to the male penis and female uterus and endometrium. Biomaterials applied for in-situ regeneration and repair of penis and vagina are more likely to have better mechanical strength and the ability to promote vascular regeneration [21,60].
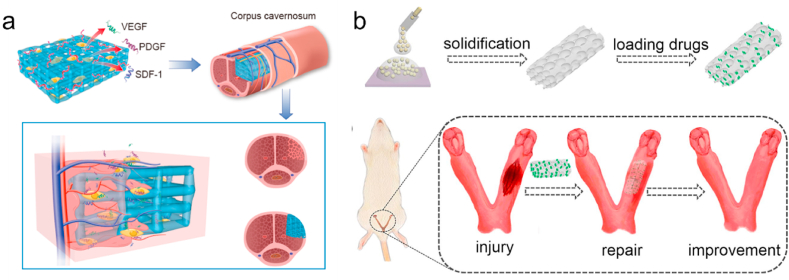
Traditional penile repair treatments, including prosthesis replacement and allogeneic transplantation, cannot meet the requirements of functional penile restoration. Synthetic polymer-based biomaterials are used for penile repair in the early years [22,23]. PGA mesh, combined with cells, can be used to reconstruct the smooth muscle tissue of the cavernous body [23]. To date, with good mechanical strength, acellular matrices have been used as the main method of repairing corpus cavernosum, including autologous cartilage rods [67], acellular corporal collagen matrices [62,66], and other acellular matrices, such as the porous bladder acellular matrix [111]. To address the challenges associated with the functional recovery of corpus cavernosum, decellularized matrices seeded with cells led to a more effective approach [61]. It has been demonstrated that corpora collagen matrices loaded with smooth muscle cells (SMCs) and endothelial cells (ECs) can recover a short segment of the penile corporal body and achieve structural and functional restoration of the penis [62]. As for burgeoning 3D bioscaffold, we recently developed a gelatin-based hydrogel scaffold by 3D printing. Seeded with stem cells to improve neovascularization, the scaffold achieved a positive effect on penile reconstruction and recovered the reproductive capability of males [59] (Fig. 5a).
Fig. 5.
3D scaffolds were implanted for reproductive tissue regeneration in vivo. (a) 3D-printed methacrylated gelatin hydrogels seeded with stem cells for penile reconstruction [59]. Copyright 2020, Nature Communications. (b) Drug-loaded porous scaffolds (prepared from methacrylated gelatin and Na-alginate) were developed by the droplet microfluidics method for endometrial repair [119]. Copyright 2019, Acta Biomaterialia.
To reconstruct uterine tissue, the acellular uterus can serve as a bioengineered scaffold that has been recellularized by primary uterine cells or other MSCs [[68], [69], [70], [71]]. Miyazaki et al. recellularized and regenerated uterine tissue by placing acellular uterine stroma on a partially resected uterus [112]. Thermal dehydration cross-linked collagen scaffolds can maintain the normal cavity structure and induce the proliferation of inherent endometrial cells and epithelial cells, thereby improving the ability of the regenerated endometrium to receive embryos [28]. However, no effective method or corresponding biological material can be used to treat patients with complete loss of uterine structure or function.
Hydrogels [[113], [114], [115]], films [116], and scaffolds [[117], [118], [119]] based on natural or synthetic polymers are used as biological supports and cell or drug delivery systems to resist inflammation and promote regeneration of vascular and endometrial [120]. For example, a synthetic pluronic-F127 hydrogel delivered with BMSCs and vitamin C (Vc) molecules can be used to regenerate the endometrium in an intrauterine adhesion rat model [114]. Additionally, Yunlang et al. developed drug-loaded porous gelatin-based scaffolds by droplet microfluidics for endometrial repair, which can repair damaged endometrium and improve neovascularization [119] (Fig. 5b).
However, there is no successful case of using biomaterials to repair the testis and ovary because of their complex structures, components and functions. Nonetheless, with the development of biomaterials and reproductive tissue engineering, acellular matrix and bioscaffolds are potential biomaterials for testis repair [[73], [74], [75]]. In the future, the technology to print complex living-tissue constructs within seconds has led to hopes for future use in regenerating ovarian and testicular tissues [121,122].
5. Outlook
Although there are relatively few cases of developed biomaterials being introduced into the research of reproductive tissue engineering compared with other tissues. The development of biomaterials still help gain further research progress in reproductive organ tissue engineering. We believe that designing biomaterials for reproductive tissue engineering is an important research direction and deserves more attention. Also, due to the complex structure and function of organs containing specific reproductive hormones, especially the ovary and testis, reliable biomaterials have not been realized to promote the structural regeneration and functional recovery of these reproductive glands and have numerous obstacles.
Fortunately, various advanced biomaterial biomanufacturing technologies are rising and have a great potential in reproductive tissue engineering: 3D bioprinting technology, material modification and microfluidic technologies [14,[123], [124], [125]]. The emerging 3D bioprinting (printing with cells) has obvious strengths in developing complex 3D culture systems for producing germ cells and oocytes, as well as constructing bioactive 3D-printed scaffolds for the repair of reproductive organs and tissues. Also, microfluidic physiological models, chip platforms and other advanced technologies have shown certain advantages for the construction of reproductive organoids and models. They can be combined with novel biomaterials of biomimetic reproductive organ tissue to gain further research progress in future studies.
In the meanwhile, further mechanism studies are required to fill theoretical knowledge gaps in terms of the mechanism and interactions between cells, hormones, chemicals, and other factors in reproductive organs and tissues before designing materials to regenerate the ovary and testis in vivo. In further mechanism studies, organoids in vitro might replace the use of experimental animals and provide standard tools to simulate the microenvironment of stromal cells and germ cells in organs and tissue and study physiological and pathological mechanisms in different reproductive organs.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2018YFA0703000), National Natural Science Foundation of China (32022041), Key Research and Development Program of Guangzhou (202007020002), Youth Science and Technology Innovation Talent of Guangdong TeZhi Plan (2017TQ04R046).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Liang Gao, Email: gaoliang@gdut.edu.cn.
Xuetao Shi, Email: shxt@scut.edu.cn.
References
- 1.Gargus E.S., Rogers H.B., McKinnon K.E., Edmonds M.E., Woodruff T.K. Engineered reproductive tissues. Nat. Biomed. Eng. 2020;4:381–393. doi: 10.1038/s41551-020-0525-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poels J., Van Langendonckt A., Many M.C., Wese F.X., Wyns C. Vitrification preserves proliferation capacity in human spermatogonia. Hum. Reprod. 2013;28:578–589. doi: 10.1093/humrep/des455. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim M., Richardson M.K. Beyond organoids: in vitro vasculogenesis and angiogenesis using cells from mammals and zebrafish. Reprod. Toxicol. 2017;73:292–311. doi: 10.1016/j.reprotox.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Concepts C., Jeruss J.S., Woodruff T.K. Preservation of fertility in patients with cancer. Assessment. 2009;360:245–251. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laronda M.M. Engineering a bioprosthetic ovary for fertility and hormone restoration. Theriogenology. 2020;150:8–14. doi: 10.1016/j.theriogenology.2020.01.021. [DOI] [PubMed] [Google Scholar]
- 6.Poels J., Abou-Ghannam G., Herman S., Van Langendonckt A., Wese F.X., Wyns C. In search of better spermatogonial preservation by supplementation of cryopreserved human immature testicular tissue xenografts with N-acetylcysteine and testosterone. Front. Surg. 2014;1:47. doi: 10.3389/fsurg.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohta H., Wakayama T. Generation of normal progeny by intracytoplasmic sperm injection following grafting of testicular tissue from cloned mice that died Postnatally1. Biol. Reprod. 2005;73:390–395. doi: 10.1095/biolreprod.105.041673. [DOI] [PubMed] [Google Scholar]
- 8.Bedaiwy M.A., Shahin A.Y., Falcone T. Reproductive organ transplantation: advances and controversies. Fertil. Steril. 2008;90:2031–2055. doi: 10.1016/j.fertnstert.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Cai L., Dewi R.E., Heilshorn S.C. Injectable hydrogels with in situ double network formation enhance retention of transplanted stem cells. Adv. Funct. Mater. 2015;25:1344–1351. doi: 10.1002/adfm.201403631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cervello I., Gil-sanchis C., Santamaria X., Cabanillas S., Diaz A., Faus A., Pellicer A., Simon C. Human CD133+ bone marrow-derived stem cells promote endometrial proliferation in a murine model of Asherman syndrome. Fertil. Steril. 2015;104:1552–1560. doi: 10.1016/j.fertnstert.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 11.Li J., Mooney D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016;1:1–18. doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X., Jia Z., Zheng Y., Wang Y. Bioadaptability of biomaterials: aiming at precision medicine. Materials. 2021;4:2648–2650. [Google Scholar]
- 13.Stukenborg J.B., Schlatt S., Simoni M., Yeung C.H., Elhija M.A., Luetjens C.M., Huleihel M., Wistuba J. New horizons for in vitro spermatogenesis? An update on novel three-dimensional culture systems as tools for meiotic and post-meiotic differentiation of testicular germ cells. Mol. Hum. Reprod. 2009;15:521–529. doi: 10.1093/molehr/gap052. [DOI] [PubMed] [Google Scholar]
- 14.Xiao S., Coppeta J.R., Rogers H.B., Isenberg B.C., Zhu J., Olalekan S.A., McKinnon K.E., Dokic D., Rashedi A.S., Haisenleder D.J., Malpani S.S., Arnold-Murray C.A., Chen K., Jiang M., Bai L., Nguyen C.T., Zhang J., Laronda M.M., Hope T.J., Maniar K.P., Pavone M.E., Avram M.J., Sefton E.C., Getsios S., Burdette J.E., Kim J.J., Borenstein J.T., Woodruff T.K. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat. Commun. 2017;8:1–13. doi: 10.1038/ncomms14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J., Yu Q., Huang H., Deng W., Cao X., Adu-Frimpong M., Yu J., Xu X. Human chorionic plate-derived mesenchymal stem cells transplantation restores ovarian function in a chemotherapy-induced mouse model of premature ovarian failure. Stem Cell Res. Ther. 2018;9:1–9. doi: 10.1186/s13287-018-0819-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou R., Wu J., Liu B., Jiang Y., Chen W., Li J., He Q., He Z. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell. Mol. Life Sci. 2019;76:2681–2695. doi: 10.1007/s00018-019-03101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapoor D., Leech J., Yap W. Use of tunica vaginalis patch graft for repair of traumatic testicular rupture. Urology. 1992;40:374–375. doi: 10.1016/0090-4295(92)90394-c. [DOI] [PubMed] [Google Scholar]
- 18.Molokwu C.N., Doull R.I., Townell N.H. A novel technique for repair of testicular rupture after blunt trauma. Urology. 2010;76:1002–1003. doi: 10.1016/j.urology.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Leprince S., Huberlant S., Allegre L., Warembourg S., Leteuff I., Bethry A., Paniagua C., Taillades H., De Tayrac R., Coudane J., Letouzey V., Garric X. Preliminary design of a new degradable medical device to prevent the formation and recurrence of intrauterine adhesions. Commun. Biol. 2019;2:1–10. doi: 10.1038/s42003-019-0447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu F., Hu S., Wang S., Cheng K. Cell and biomaterial-based approaches to uterus regeneration. Regen. Biomater. 2019;6:141–148. doi: 10.1093/rb/rbz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Vocht D.E.C.M., de Kemp V., Iljas J.D., Bosch J.L.H.R., de Kort L.M.O., de Graaf P. A systematic review on cell-seeded tissue engineering of penile corpora. J. Tissue Eng. Regen. Med. 2018;12:687–694. doi: 10.1002/term.2487. [DOI] [PubMed] [Google Scholar]
- 22.Yoo J.J., Park H.J., Lee I., Atala A. Autologous engineered cartilage rods for penile reconstruction. J. Urol. 1999;162:1119–1121. doi: 10.1016/S0022-5347(01)68090-X. [DOI] [PubMed] [Google Scholar]
- 23.Kershen R.T., Yoo J.J., Moreland R.B., Krane R.J., Atala A. Reconstitution of human corpus cavernosum smooth muscle in vitro and in vivo. Tissue Eng. 2002;8:515–524. doi: 10.1089/107632702760184754. [DOI] [PubMed] [Google Scholar]
- 24.Saini M. Implant biomaterials: a comprehensive review. World J. Clin. Cases. 2015;3:52. doi: 10.12998/wjcc.v3.i1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrew T.W., Kanapathy M., Murugesan L., Muneer A., Kalaskar D., Atala A. Towards clinical application of tissue engineering for erectile penile regeneration. Nat. Rev. Urol. 2019;16:734–744. doi: 10.1038/s41585-019-0246-7. [DOI] [PubMed] [Google Scholar]
- 26.Pan F., Chi L., Schlatt S. Effects of nanostructures and mouse embryonic stem cells on in vitro morphogenesis of rat testicular cords. PLoS One. 2013;8:2–9. doi: 10.1371/journal.pone.0060054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fomby P., Cherlin A.J., Hadjizadeh A., Doillon C.J., Sueblinvong V., Weiss D.J., Bates J.H.T., Gilbert T., Liles W.C., Lutzko C., Rajagopal J., Prockop D.J., Chambers D., Giangreco A., Keating A., Kotton D., Lelkes P.I., Wagner D.E., Prockop D.J. Evaluation of in vitro spermatogenesis using poly(D,L-lactic-co-glycolic acid) (PLGA)-based macroporous biodegradable scaffolds. Ann. Am. Thorac. Soc. 2010;12:181–204. [Google Scholar]
- 28.Xin L., Lin X., Pan Y., Zheng X., Shi L., Zhang Y., Ma L., Gao C., Zhang S. A collagen scaffold loaded with human umbilical cord-derived mesenchymal stem cells facilitates endometrial regeneration and restores fertility. Acta Biomater. 2019;92:160–171. doi: 10.1016/j.actbio.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Desai N., Abdelhafez F., Calabro A., Falcone T. Three dimensional culture of fresh and vitrified mouse pre-antral follicles in a hyaluronan-based hydrogel: a preliminary investigation of a novel biomaterial for in vitro follicle maturation. Reprod. Biol. Endocrinol. 2012;10:1–12. doi: 10.1186/1477-7827-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laronda M.M., Rutz A.L., Xiao S., Whelan K.A., Duncan F.E., Roth E.W., Woodruff T.K., Shah R.N. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat. Commun. 2017;8:1–10. doi: 10.1038/ncomms15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo C.Y., Shevchuk M., Opfermann J., Guo T., Santoro M., Fisher J.P., Kim P.C.W. Trophoblast–endothelium signaling involves angiogenesis and apoptosis in a dynamic bioprinted placenta model. Biotechnol. Bioeng. 2019;116:181–192. doi: 10.1002/bit.26850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanacker J., Luyckx V., Dolmans M.M., Des Rieux A., Jaeger J., Van Langendonckt A., Donnez J., Amorim C.A. Transplantation of an alginate-matrigel matrix containing isolated ovarian cells: first step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials. 2012;33:6079–6085. doi: 10.1016/j.biomaterials.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 33.Vanacker J., Dolmans M.M., Luyckx V., Donnez J., Amorim C.A. First transplantation of isolated murine follicles in alginate. Regen. Med. 2014;9:609–619. doi: 10.2217/rme.14.33. [DOI] [PubMed] [Google Scholar]
- 34.Sittadjody S., Saul J.M., McQuilling J.P., Joo S., Register T.C., Yoo J.J., Atala A., Opara E.C. In vivo transplantation of 3D encapsulated ovarian constructs in rats corrects abnormalities of ovarian failure. Nat. Commun. 2017;8:1–13. doi: 10.1038/s41467-017-01851-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith R.M., Shikanov A., Kniazeva E., Ramadurai D., Woodruff T.K., Shea L.D. Fibrin-mediated delivery of an ovarian follicle pool in a mouse model of infertility. Tissue Eng. A. 2014;20:3021–3030. doi: 10.1089/ten.tea.2013.0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiti M.C., Dolmans M.M., Mortiaux L., Zhuge F., Ouni E., Shahri P.A.K., Van Ruymbeke E., Champagne S.D., Donnez J., Amorim C.A. A novel fibrin-based artificial ovary prototype resembling human ovarian tissue in terms of architecture and rigidity. J. Assist. Reprod. Genet. 2018;35:41–48. doi: 10.1007/s10815-017-1091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amorim C.A., Shikanov Ariella. The artificial ovary: current status and future perspectives. Future Oncol. 2016;12:2323–2332. doi: 10.2217/fon-2016-0202. [DOI] [PubMed] [Google Scholar]
- 38.Telfer E., Torrance C., Gosden R.G. Morphological study of cultured preantral ovarian follicles of mice after transplantation under the kidney capsule. J. Reprod. Fertil. 1990;89:565–571. doi: 10.1530/jrf.0.0890565. [DOI] [PubMed] [Google Scholar]
- 39.Lee J.H., Gye M.C., Choi K.W., Hong J.Y., Lee Y.B., Park D.W., Lee S.J., Min C.K. In vitro differentiation of germ cells from nonobstructive azoospermic patients using three-dimensional culture in a collagen gel matrix. Fertil. Steril. 2007;87:824–833. doi: 10.1016/j.fertnstert.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 40.Sun M., Yuan Q., Niu M., Wang H., Wen L., Yao C., Hou J., Chen Z., Fu H., Zhou F., Li C., Gao S., Gao W.Q., Li Z., He Z. Efficient generation of functional haploid spermatids from human germline stem cells by three-dimensional-induced system. Cell Death Differ. 2018;25:747–764. doi: 10.1038/s41418-017-0015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alves-Lopes J.P., Söder O., Stukenborg J.B. Testicular organoid generation by a novel in vitro three-layer gradient system. Biomaterials. 2017;130:76–89. doi: 10.1016/j.biomaterials.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 42.Alves-Lopes J.P., Söder O., Stukenborg J.B. Use of a three-layer gradient system of cells for rat testicular organoid generation. Nat. Protoc. 2018;13:248–259. doi: 10.1038/nprot.2017.140. [DOI] [PubMed] [Google Scholar]
- 43.Hadley M.A., Weeks B.S., Kleinman H.K., Dym M. Laminin promotes formation of cord-like structures by sertoli cells in vitro. Dev. Biol. 1990;140:318–327. doi: 10.1016/0012-1606(90)90082-t. [DOI] [PubMed] [Google Scholar]
- 44.de Witte C.J., Espejo Valle-Inclan J., Hami N., Lõhmussaar K., Kopper O., Vreuls C.P.H., Jonges G.N., van Diest P., Nguyen L., Clevers H., Kloosterman W.P., Cuppen E., Snippert H.J.G., Zweemer R.P., Witteveen P.O., Stelloo E. Patient-Derived ovarian cancer organoids mimic clinical response and exhibit heterogeneous inter- and intrapatient drug responses. Cell Rep. 2020;31 doi: 10.1016/j.celrep.2020.107762. [DOI] [PubMed] [Google Scholar]
- 45.Kwong J., Franky L.C., Wong K.K., Birrer M.J., Archibald K.M., Balkwill F.R., Berkowitz R.S., Mok S.C. Inflammatory cytokine tumor necrosis factor α confers precancerous phenotype in an organoid model of normal human ovarian surface epithelial cells. Neoplasia. 2009;11:529–541. doi: 10.1593/neo.09112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grigoryan B., Paulsen S.J., Corbett D.C., Sazer D.W., Fortin C.L., Zaita A.J., Greenfield P.T., Calafat N.J., Gounley J.P., Ta A.H., Johansson F., Randles A., Rosenkrantz J.E., Louis-Rosenberg J.D., Galie P.A., Stevens K.R., Miller J.S. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science. 2019;364:458–464. doi: 10.1126/science.aav9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aijaz A., Li M., Smith D., Khong D., LeBlon C., Fenton O.S., Olabisi R.M., Libutti S., Tischfield J., V Maus M., Deans R., Barcia R.N., Anderson D.G., Ritz J., Preti R., Parekkadan B. Biomanufacturing for clinically advanced cell therapies. Nat. Biomed. Eng. 2018;2:362–376. doi: 10.1038/s41551-018-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu C., Ma X., Zhu W., Wang P., Miller K.L., Stupin J., Koroleva-Maharajh A., Hairabedian A., Chen S. Scanningless and continuous 3D bioprinting of human tissues with decellularized extracellular matrix. Biomaterials. 2019;194:1–13. doi: 10.1016/j.biomaterials.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jee E., Bánsági T., Taylor A.F., Pojman J.A. Temporal control of gelation and polymerization fronts driven by an autocatalytic enzyme reaction. Angew. Chem. 2016;128:2167–2171. doi: 10.1002/ange.201510604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pendergraft S.S., Sadri-Ardekani H., Atala A., Bishop C.E. Three-dimensional testicular organoid: a novel tool for the study of human spermatogenesis and gonadotoxicity in vitro. Biol. Reprod. 2017;96:720–732. doi: 10.1095/biolreprod.116.143446. [DOI] [PubMed] [Google Scholar]
- 51.Joo S., Oh S.H., Sittadjody S., Opara E.C., Jackson J.D., Lee S.J., Yoo J.J., Atala A. The effect of collagen hydrogel on 3D culture of ovarian follicles. Biomed. Mater. 2016;11 doi: 10.1088/1748-6041/11/6/065009. [DOI] [PubMed] [Google Scholar]
- 52.Rezaei Topraggaleh T., Rezazadeh Valojerdi M., Montazeri L., Baharvand H. 2019. A Testis-Derived Macroporous 3D Scaffold as a Platform for the Generation of Mouse Testicular Organoids. [DOI] [PubMed] [Google Scholar]
- 53.Sakib S., Uchida A., Valenzuela-Leon P., Yu Y., Vallii-pulaski H., Orwig K., Ungrin M., Dobrinski I. Formation of organotypic testicular organoids in microwell culture. Biol. Reprod. 2019;100:1648–1660. doi: 10.1093/biolre/ioz053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuan L., Kusuda T. Adsorption of ammonium and nitrate ions by poly(N-isopropylacrylamide) gel and poly(N-isopropylacrylamideco-chlorophyllin) gel in different states. J. Appl. Polym. Sci. 2005;96:2367–2372. [Google Scholar]
- 55.Chumduri C., Gurumurthy R.K., Berger H., Dietrich O., Kumar N., Koster S., Brinkmann V., Hoffmann K., Drabkina M., Arampatzi P., Son D., Klemm U., Mollenkopf H.J., Herbst H., Mangler M., Vogel J., Saliba A.E., Meyer T.F. Opposing Wnt signals regulate cervical squamocolumnar homeostasis and emergence of metaplasia. Nat. Cell Biol. 2021;23:184–197. doi: 10.1038/s41556-020-00619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dargaville T.R., Hollier B.G., Shokoohmand A., Hoogenboom R. Poly(2-oxazoline) hydrogels as next generation three-dimensional cell supports. Cell Adhes. Migrat. 2014;8:88–93. doi: 10.4161/cam.28205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kniazeva E., Hardy A.N., Boukaidi S.A., Woodruff T.K., Jeruss J.S., Shea L.D. Primordial follicle transplantation within designer biomaterial grafts produce live births in a mouse infertility model. Sci. Rep. 2015;5:1–11. doi: 10.1038/srep17709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shikanov A., Zhang Z., Xu M., Smith R.M., Rajan A., Woodruff T.K., Shea L.D. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng. A. 2011;17:3095–3104. doi: 10.1089/ten.tea.2011.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.An G., Guo F., Liu X., Wang Z., Zhu Y., Fan Y., Xuan C., Li Y., Wu H., Shi X., Mao C. Functional reconstruction of injured corpus cavernosa using 3D-printed hydrogel scaffolds seeded with HIF-1α-expressing stem cells. Nat. Commun. 2020;11:1–12. doi: 10.1038/s41467-020-16192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peng G., Liu H., Fan Y. Biomaterial scaffolds for reproductive tissue engineering. Ann. Biomed. Eng. 2017;45:1592–1607. doi: 10.1007/s10439-016-1779-z. [DOI] [PubMed] [Google Scholar]
- 61.Kwon T.G., Yoo J.J., Atala A. Autologous penile corpora cavernosa replacement using tissue engineering techniques. J. Urol. 2002;168:1754–1758. doi: 10.1097/01.ju.0000030103.53181.7d. [DOI] [PubMed] [Google Scholar]
- 62.Chen K.L., Eberli D., Yoo J.J., Atala A. Bioengineered corporal tissue for structural and functional restoration of the penis. Proc. Natl. Acad. Sci. U.S.A. 2010;107:3346–3350. doi: 10.1073/pnas.0909367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hassanpour A., Talaei-Khozani T., Kargar-Abarghouei E., Razban V., Vojdani Z. Decellularized human ovarian scaffold based on a sodium lauryl ester sulfate (SLES)-treated protocol, as a natural three-dimensional scaffold for construction of bioengineered ovaries. Stem Cell Res. Ther. 2018;9:1–13. doi: 10.1186/s13287-018-0971-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eivazkhani F., Abtahi N.S., Tavana S., Mirzaeian L., Abedi F., Ebrahimi B., Montazeri L., Valojerdi M.R., Fathi R. Evaluating two ovarian decellularization methods in three species. Mater. Sci. Eng. C. 2019;102:670–682. doi: 10.1016/j.msec.2019.04.092. [DOI] [PubMed] [Google Scholar]
- 65.Laronda M.M., Jakus A.E., Whelan K.A., Wertheim J.A., Shah R.N., Woodruff T.K. Initiation of puberty in mice following decellularized ovary transplant. Biomaterials. 2015;50:20–29. doi: 10.1016/j.biomaterials.2015.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song L.J., Xu Y.M., Li C., Fu Q., Cui L., Hu X.Y. Construction of cavernosum smooth muscle using umbilical artery smooth muscle cells seeded on acellular corporal collagen matrices. Int. J. Androl. 2009;32:514–523. doi: 10.1111/j.1365-2605.2008.00888.x. [DOI] [PubMed] [Google Scholar]
- 67.Kim B.S., Yoo J.J., Atala A. Engineering of human cartilage rods: potential application for penile prostheses. J. Urol. 2002;168:1794–1797. doi: 10.1097/01.ju.0000028238.60060.59. [DOI] [PubMed] [Google Scholar]
- 68.Campo H., Baptista P.M., Nuria L., Sim C. De- and recellularization of the pig uterus: a bioengineering pilot study. Biol. Reprod. 2017;96:34–45. doi: 10.1095/biolreprod.116.143396. [DOI] [PubMed] [Google Scholar]
- 69.Hellström M., Moreno-Moya J.M., Bandstein S., Bom E., Akouri R.R., Miyazaki K., Maruyama T., Brännström M. Bioengineered uterine tissue supports pregnancy in a rat model. Fertil. Steril. 2016;106:487–496.e1. doi: 10.1016/j.fertnstert.2016.03.048. [DOI] [PubMed] [Google Scholar]
- 70.Barreto R.S.N., Romagnolli P., Fratini P., Mess A.M., Miglino M.A. Mouse placental scaffolds: a three-dimensional environment model for recellularization. J. Tissue Eng. 2019;10:1–11. doi: 10.1177/2041731419867962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Daryabari S.S., Kajbafzadeh A.M., Fendereski K., Ghorbani F., Dehnavi M., Rostami M., Azizi Garajegayeh B., Tavangar S.M. Development of an efficient perfusion-based protocol for whole-organ decellularization of the ovine uterus as a human-sized model and in vivo application of the bioscaffolds. J. Assist. Reprod. Genet. 2019;36:1211–1223. doi: 10.1007/s10815-019-01463-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marchalik D., Triest J.A., Wright H.C., Bandi G. Use of “off the shelf” extracellular matrix graft materials for repair of testicular rupture: a novel technique. Urology. 2014;84:719–721. doi: 10.1016/j.urology.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 73.Baert Y., Stukenborg J.B., Landreh M., De Kock J., Jörnvall H., Söder O., Goossens E. Derivation and characterization of a cytocompatible scaffold from human testis. Hum. Reprod. 2015;30:256–267. doi: 10.1093/humrep/deu330. [DOI] [PubMed] [Google Scholar]
- 74.Vermeulen M., del Vento F., de Michele F., Poels J., Wyns C. Development of a cytocompatible scaffold from pig immature testicular tissue allowing human Sertoli cell attachment, proliferation and functionality. Int. J. Mol. Sci. 2018;19:227. doi: 10.3390/ijms19010227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akbarzadeh A., Kianmanesh M., Fendereski K., Ebadi M., Daryabari S.S., Masoomi A., Ghazisaeedi F., Seyyed Hossein Beigi R., Sheikh R., Kajbafzadeh A.M. Decellularised whole ovine testis as a potential bio-scaffold for tissue engineering. Reprod. Fertil. Dev. 2019;31:1665–1673. doi: 10.1071/RD19070. [DOI] [PubMed] [Google Scholar]
- 76.Yuan Y., Li L., Cheng Q., Diao F., Zeng Q., Yang X., Wu Y., Zhang H., Huang M., Chen J., Zhou Q., Zhu Y., Hua R., Tian J., Wang X., Zhou Z., Hao J., Yu J., Hua D., Liu J., Guo X., Zhou Q., Sha J. In vitro testicular organogenesis from human fetal gonads produces fertilization-competent spermatids. Cell Res. 2020;30:244–255. doi: 10.1038/s41422-020-0283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eberli D., Susaeta R., Yoo J.J., Atala A. A method to improve cellular content for corporal tissue engineering. Tissue Eng. A. 2008;14:1581–1589. doi: 10.1089/ten.tea.2007.0249. [DOI] [PubMed] [Google Scholar]
- 78.Campos Marín A., Brunelli M., Lacroix D. Flow perfusion rate modulates cell deposition onto scaffold substrate during cell seeding. Biomech. Model. Mechanobiol. 2018;17:675–687. doi: 10.1007/s10237-017-0985-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sato T., Katagiri K., Gohbara A., Inoue K., Ogonuki N., Ogura A., Kubota Y., Ogawa T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011;471:504–508. doi: 10.1038/nature09850. [DOI] [PubMed] [Google Scholar]
- 80.Sato T., Katagiri K., Kubota Y., Ogawa T. In vitro sperm production from mouse spermatogonial stem cell lines using an organ culture method. Nat. Protoc. 2013;8:2098–2104. doi: 10.1038/nprot.2013.138. [DOI] [PubMed] [Google Scholar]
- 81.Stukenborg J.B., Wistuba J., Luetjens C.M., Elhija M.A., Huleihel M., Lunenfeld E., Gromoll J., Nieschlag E., Schlatt S. Coculture of spermatogonia with somatic cells in a novel three-dimensional Soft-Agar-Culture-System. J. Androl. 2008;29:312–329. doi: 10.2164/jandrol.107.002857. [DOI] [PubMed] [Google Scholar]
- 82.Inigo J. 2016. Modelling of Mouse Spermatogonial Stem Cell Niche for Designing 3D Scaffolds for in Vitro Spermatogenesis. [Google Scholar]
- 83.Staub C. A century of research on mammalian male germ cell meiotic differentiation in vitro. J. Androl. 2001;22:911–926. doi: 10.1002/j.1939-4640.2001.tb03430.x. [DOI] [PubMed] [Google Scholar]
- 84.Baert Y., Dvorakova-Hortova K., Margaryan H., Goossens E. Mouse in vitro spermatogenesis on alginate-based 3D bioprinted scaffolds. Biofabrication. 2019;11 doi: 10.1088/1758-5090/ab1452. [DOI] [PubMed] [Google Scholar]
- 85.Mansouri V., Salehi M., davood Omrani M., Niknam Z., Ardeshirylajimi A. Collagen-alginate microspheres as a 3D culture system for mouse embryonic stem cells differentiation to primordial germ cells. Biologicals. 2017;48:114–120. doi: 10.1016/j.biologicals.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 86.Gao H., Liu C., Wu B., Cui H., Zhao Y., Duan Y., Gao F., Gu Q., Wang H., Li W. Effects of different biomaterials and cellular status on testicular cell self-organization. Adv. Biosyst. 2020;4:1–12. doi: 10.1002/adbi.201900292. [DOI] [PubMed] [Google Scholar]
- 87.Yokonishi T., Sato T., Katagiri K., Komeya M., Kubota Y., Ogawa T. In vitro reconstruction of mouse seminiferous tubules supporting germ cell differentiation. Biol. Reprod. 2013;89:1–6. doi: 10.1095/biolreprod.113.108613. [DOI] [PubMed] [Google Scholar]
- 88.Vermeulen M., Del Vento F., Kanbar M., Ruys S.P.D., Vertommen D., Poels J., Wyns C. Generation of organized porcine testicular organoids in solubilized hydrogels from decellularized extracellular matrix. Int. J. Mol. Sci. 2019;20:5476. doi: 10.3390/ijms20215476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Komeya M., Kimura H., Nakamura H., Yokonishi T., Sato T., Kojima K., Hayashi K., Katagiri K., Yamanaka H., Sanjo H., Yao M., Kamimura S., Inoue K., Ogonuki N., Ogura A., Fujii T., Ogawa T. Long-term ex vivo maintenance of testis tissues producing fertile sperm in a microfluidic device. Sci. Rep. 2016;6:1–10. doi: 10.1038/srep21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Komeya M., Hayashi K., Nakamura H., Yamanaka H., Sanjo H., Kojima K., Sato T., Yao M., Kimura H., Fujii T., Ogawa T. Pumpless microfluidic system driven by hydrostatic pressure induces and maintains mouse spermatogenesis in vitro. Sci. Rep. 2017;7:1–8. doi: 10.1038/s41598-017-15799-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Komeya M., Yamanaka H., Sanjo H., Yao M., Nakamura H., Kimura H., Fujii T., Sato T., Ogawa T. In vitro spermatogenesis in two-dimensionally spread mouse testis tissues, Reprod. Med. Biol. 2019;18:362–369. doi: 10.1002/rmb2.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shikanov A., Smith R.M., Xu M., Woodruff T.K., Shea L.D. Hydrogel network design using multifunctional macromers to coordinate tissue maturation in ovarian follicle culture. Biomaterials. 2011;32:2524–2531. doi: 10.1016/j.biomaterials.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Telfer E.E. Future developments: in vitro growth (IVG) of human ovarian follicles. Acta Obstet. Gynecol. Scand. 2019;98:653–658. doi: 10.1111/aogs.13592. [DOI] [PubMed] [Google Scholar]
- 94.Marin D., Yang M., Wang T. In Vitro growth of human ovarian follicles for fertility preservation. Reprod. Dev. Med. 2018;2:230–236. [Google Scholar]
- 95.Takebe T., Wells J.M. Organoids by design. Science. 2019;364:956–959. doi: 10.1126/science.aaw7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moroni L., Burdick J.A., Highley C., Lee S.J., Morimoto Y., Takeuchi S., Yoo J.J. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat. Rev. Mater. 2018;3:21–37. doi: 10.1038/s41578-018-0006-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li M., Izpisua Belmonte J.C. Organoids — preclinical models of human disease. N. Engl. J. Med. 2019;380:569–579. doi: 10.1056/NEJMra1806175. [DOI] [PubMed] [Google Scholar]
- 98.Takahashi Toshio. New trends and perspectives in the function of non-neuronal acetylcholine in crypt–villus organoids in mice. Organoids. 2016:145–155. doi: 10.1007/7651_2016_1. [DOI] [PubMed] [Google Scholar]
- 99.Kaushik G., Ponnusamy M.P., Batra S.K. Concise review: current status of three-dimensional organoids as preclinical models. Stem Cell. 2018;36:1329–1340. doi: 10.1002/stem.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Richer G., Baert Y., Goossens E. In-vitro spermatogenesis through testis modelling: toward the generation of testicular organoids. Andrology. 2020;8:879–891. doi: 10.1111/andr.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yan Cheng C., Mruk D.D. The blood-testis barrier and its implications for male contraception. Pharmacol. Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Legendre A., Froment P., Desmots S., Lecomte A., Habert R., Lemazurier E. An engineered 3D blood-testis barrier model for the assessment of reproductive toxicity potential. Biomaterials. 2010;31:4492–4505. doi: 10.1016/j.biomaterials.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 103.Kojima K., Nakamura H., Komeya M., Yamanaka H., Makino Y., Okada Y., Akiyama H., Torikai N., Sato T., Fujii T., Kimura H., Ogawa T. Neonatal testis growth recreated in vitro by two-dimensional organ spreading. Biotechnol. Bioeng. 2018;115:3030–3041. doi: 10.1002/bit.26822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alzamil L., Nikolakopoulou K., Turco M.Y. Organoid systems to study the human female reproductive tract and pregnancy. Cell Death Differ. 2021;28:35–51. doi: 10.1038/s41418-020-0565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kopper O., de Witte C.J., Lõhmussaar K., Valle-Inclan J.E., Hami N., Kester L., Balgobind A.V., Korving J., Proost N., Begthel H., van Wijk L.M., Revilla S.A., Theeuwsen R., van de Ven M., van Roosmalen M.J., Ponsioen B., Ho V.W.H., Neel B.G., Bosse T., Gaarenstroom K.N., Vrieling H., Vreeswijk M.P.G., van Diest P.J., Witteveen P.O., Jonges T., Bos J.L., van Oudenaarden A., Zweemer R.P., Snippert H.J.G., Kloosterman W.P., Clevers H. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat. Med. 2019;25:838–849. doi: 10.1038/s41591-019-0422-6. [DOI] [PubMed] [Google Scholar]
- 106.Nishiguchi A., Gilmore C., Sood A., Matsusaki M., Collett G., Tannetta D., Sargent I.L., McGarvey J., Halemani N.D., Hanley J., Day F., Grant S., Murdoch-Davis C., Kemp H., Verkade P., Aplin J.D., Akashi M., Case C.P. In vitro placenta barrier model using primary human trophoblasts, underlying connective tissue and vascular endothelium. Biomaterials. 2019;192:140–148. doi: 10.1016/j.biomaterials.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 107.Blundell C., Tess E.R., Schanzer A.S.R., Coutifaris C., Su E.J., Parry S., Huh D. A microphysiological model of the human placental barrier. Lab Chip. 2016;16:3065–3073. doi: 10.1039/c6lc00259e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bishop R.C., Boretto M., Rutkowski M.R., Vankelecom H., Derré I. Murine endometrial organoids to model Chlamydia infection. Front. Cell. Infect. Microbiol. 2020;10:1–9. doi: 10.3389/fcimb.2020.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chumduri C., Turco M.Y. Organoids of the female reproductive tract. J. Mol. Med. 2021;99:531–553. doi: 10.1007/s00109-020-02028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schlatt S., Honaramooz A., Ehmcke J., Goebell P.J., Rübben H., Dhir R., Dobrinski I., Patrizio P. Limited survival of adult human testicular tissue as ectopic xenograft. Hum. Reprod. 2006;21:384–389. doi: 10.1093/humrep/dei352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang J.W., Xie M.K., Zhang Y., Wei G.J., Li X., Bin Li H., Wang J.H., Zhu W.D., Li C., Xu Y.M., Song L.J. Reconstruction of penile urethra with the 3-dimensional porous bladder acellular matrix in a rabbit model. Urology. 2014;84:1499–1505. doi: 10.1016/j.urology.2014.07.044. [DOI] [PubMed] [Google Scholar]
- 112.Miyazaki K., Maruyama T. Partial regeneration and reconstruction of the rat uterus through recellularization of a decellularized uterine matrix. Biomaterials. 2014;35:8791–8800. doi: 10.1016/j.biomaterials.2014.06.052. [DOI] [PubMed] [Google Scholar]
- 113.Zhang S.S., Xia W.T., Xu J., Xu H.L., Lu C.T., Zhao Y.Z., Wu X.Q. Three-dimensional structure micelles of heparin-poloxamer improve the therapeutic effect of 17β-estradiol on endometrial regeneration for intrauterine adhesions in a rat model. Int. J. Nanomed. 2017;12:5643–5657. doi: 10.2147/IJN.S137237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang H., Wu S., Feng R., Huang J., Liu L., Liu F., Chen Y. Vitamin C plus hydrogel facilitates bone marrow stromal cell-mediated endometrium regeneration in rats. Stem Cell Res. Ther. 2017;8:1–10. doi: 10.1186/s13287-017-0718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xu H.L., Xu J., Shen B.X., Zhang S.S., Jin B.H., Zhu Q.Y., ZhuGe D.L., Wu X.Q., Xiao J., Zhao Y.Z. Dual regulations of thermosensitive heparin-poloxamer hydrogel using ε-polylysine: bioadhesivity and controlled KGF release for enhancing wound healing of endometrial injury. ACS Appl. Mater. Interfaces. 2017;9:29580–29594. doi: 10.1021/acsami.7b10211. [DOI] [PubMed] [Google Scholar]
- 116.Cai H., Li H., He Y. Interceed and estrogen reduce uterine adhesions and fibrosis and improve endometrial receptivity in a rabbit model of intrauterine adhesions. Reprod. Sci. 2016;23:1208–1216. doi: 10.1177/1933719116632923. [DOI] [PubMed] [Google Scholar]
- 117.Ding L., Li X., Sun H., Su J., Lin N., Péault B., Song T., Yang J., Dai J., Hu Y. Transplantation of bone marrow mesenchymal stem cells on collagen scaffolds for the functional regeneration of injured rat uterus. Biomaterials. 2014;35:4888–4900. doi: 10.1016/j.biomaterials.2014.02.046. [DOI] [PubMed] [Google Scholar]
- 118.Xiao B., Yang W., Lei D., Huang J., Yin Y., Zhu Y., You Z., Wang F., Sun S. PGS scaffolds promote the in vivo survival and directional differentiation of bone marrow mesenchymal stem cells restoring the morphology and function of wounded rat uterus. Adv. Healthc. Mater. 2019;8 doi: 10.1002/adhm.201801455. [DOI] [PubMed] [Google Scholar]
- 119.Cai Y., Wu F., Yu Y., Liu Y., Shao C., Gu H., Li M., Zhao Y. Porous scaffolds from droplet microfluidics for prevention of intrauterine adhesion. Acta Biomater. 2019;84:222–230. doi: 10.1016/j.actbio.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 120.Kou L., Jiang X., Xiao S., Zhao Y.Z., Yao Q., Chen R. Therapeutic options and drug delivery strategies for the prevention of intrauterine adhesions. J. Contr. Release. 2020;318:25–37. doi: 10.1016/j.jconrel.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 121.Bernal P.N., Delrot P., Loterie D., Li Y., Malda J., Moser C., Levato R. Volumetric bioprinting of complex living-tissue constructs within seconds. Adv. Mater. 2019;31 doi: 10.1002/adma.201904209. [DOI] [PubMed] [Google Scholar]
- 122.Song K.H., Highley C.B., Rouff A., Burdick J.A. Complex 3D-printed microchannels within cell-degradable hydrogels. Adv. Funct. Mater. 2018;28:1–10. [Google Scholar]
- 123.Sakib S., Yu Y., Voigt A., Ungrin M., Dobrinski I. Generation of porcine testicular organoids with testis specific architecture using microwell culture. J. Vis. Exp. 2019 doi: 10.3791/60387. [DOI] [PubMed] [Google Scholar]
- 124.Abbas Y., Oefner C.M., Polacheck W.J., Gardner L., Farrell L., Sharkey A., Kamm R., Moffett A., Oyen M.L. A microfluidics assay to study invasion of human placental trophoblast cells. J. R. Soc. Interface. 2017;14 doi: 10.1098/rsif.2017.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dabaghi M., Fusch G., Saraei N., Rochow N., Brash J.L., Fusch C., Ravi Selvaganapathy P. An artificial placenta type microfluidic blood oxygenator with double-sided gas transfer microchannels and its integration as a neonatal lung assist device. Biomicrofluidics. 2018;12 doi: 10.1063/1.5034791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Felder S., Masasa H., Orenbuch A., Levaot N., Shachar Goldenberg M., Cohen S. Reconstruction of the ovary microenvironment utilizing macroporous scaffold with affinity-bound growth factors. Biomaterials. 2019;205:11–22. doi: 10.1016/j.biomaterials.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 127.Ding J.X., Chen X.J., Zhang X.Y., Zhang Y., Hua K.Q. Acellular porcine small intestinal submucosa graft for cervicovaginal reconstruction in eight patients with malformation of the uterine cervix. Hum. Reprod. 2014;29:677–682. doi: 10.1093/humrep/det470. [DOI] [PubMed] [Google Scholar]
- 128.Salehipour M., Izadpanah K., Safaei A., Kamranpoor M., Farsiani M.R. Application of human amniotic membrane in canine penile tunica albuginea defect: first step toward an innovating new method for treatment of peyronie's disease. Int. Braz J. Urol. 2014;40:400–407. doi: 10.1590/S1677-5538.IBJU.2014.03.15. [DOI] [PubMed] [Google Scholar]
- 129.Shikanov A., Xu M., Woodruff T.K., Shea L.D. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials. 2009;30:5476–5485. doi: 10.1016/j.biomaterials.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rios P.D., Kniazeva E., Lee H.C., Xiao S., Oakes R.S., Saito E., Jeruss J.S., Shikanov A., Woodruff T.K., Shea L.D. Retrievable hydrogels for ovarian follicle transplantation and oocyte collection. Biotechnol. Bioeng. 2018;115:2075–2086. doi: 10.1002/bit.26721. [DOI] [PMC free article] [PubMed] [Google Scholar]