Abstract
Ferroptosis is an iron-dependent, nonapoptotic form of regulated cell death triggered by impaired redox and antioxidant machinery and propagated by the accumulation of toxic lipid peroxides. A compendium of experimental studies suggests that ferroptosis is tumor-suppressive. Sensitivity or resistance to ferroptosis can be regulated by cell-autonomous and non-cell-autonomous metabolic mechanisms. This includes a role for ferroptosis that extends beyond the tumor cells themselves, mediated by components of the tumor microenvironment, including T cells and other immune cells. Herein, we review the intrinsic and extrinsic factors that promote the sensitivity of cancer cells to ferroptosis and conclude by describing approaches to harness the full utility of ferroptotic agents as therapeutic options for cancer therapy.
Keywords: ferroptosis, cancer, tumor microenvironment, metabolism, tumor immunity
Abbreviations: 4-HNE, 4-Hydroxynonenal; 8-OHG, 8-hyroxyguanosine; αKG, alpha ketoglutarate; ACC, Acetyl-CoA carboxylase; ACSL3, acyl-CoA synthetase long-chain family member 3; ACSL4, acyl-CoA synthetase long-chain family member 4; AGER, advanced glycosylation end-product specific receptor; AMPK, 5′ adenosine monophosphate-activated protein kinase; ATM, ataxia-telangiectasia mutated; ATP, adenosine triphosphate; BH2, dihydrobiopterin; BH4, tetrahydrobiopterin; CAF, cancer associated fibroblasts; CD36, cluster of differentiation 36; CHAC1, ChaC Glutathione Specific Gamma-Glutamylcyclotransferase 1; CoA, coenzyme A; CoQ10, Coenzyme Q10; CoQ10H2, Coenzyme Q10 or ubiquinol; CPX, ciclopirox; CTLA4, cytotoxic T-lymphocyte-associated protein 4; DFO, deferoxamine; DHFR, dihydrofolate reductase; DHODH, dihydroorotate dehydrogenase; ePL-PUFA, ether phospholipid-polyunsaturated fatty acids; ETC, electron transport chain; FSP1, ferroptosis suppressor protein1; GCH1, GTP cyclohydrolase 1; GOT1, glutamate oxaloacetate transaminase 1; GPX4, glutathione peroxidase 4; GSH, glutathione; GSSG, glutathione (oxidized); HCAR1, Hydroxycarboxylic acid receptor 1; HMGB1, high mobility group box protein 1; IFNγ, interferon gamma; IFNR, interferon receptor; IKE, imidazole ketone erastin; JAK, Janus kinase; KEAP1, Kelch-like ECH-associated protein 1; LIP, labile iron pool; LOXs, lipoxygenases; LPCAT3, lysophosphatidylcholine acyltransferase 3; MCT1, monocarboxylate transporter 1; MDA, Malondialdehyde; ML162, molecular libraries 162; MUFA, monounsaturated fatty acid; MUFA-PL, monounsaturated fatty acid phospholipid; NRF2, nuclear factor erythroid 2-related factor 2; oxLDL, oxidized low density lipoprotein; OXPHOS, oxidative phosphorylation; P, Phosphate; PD-L1, Programmed death-ligand 1; PLOO, phospholipid hydroperoxyl radical; PLOOH, phospholipid hydroperoxide; PTGS2, Prostaglandin-Endoperoxide Synthase 2; PUFA, polyunsaturated fatty acid; PUFA-PLs, polyunsaturated fatty acid phospholipids; ROS, reactive oxygen species; RSL3, Ras selective lethal 3; SAPE-OOH, oxidized phospholipid, 1-steaoryl-2-15-HpETE-sn-glycero-3-phosphatidylethanolamine; SCD1, stearoyl-CoA desaturase enzyme 1; Se, Selenium; STAT1, signal transducer and activator of transcription 1; STING1, stimulator of interferon genes 1; TAZ, transcriptional co-activator with PDZ-binding motif; TCA, tricarboxylic acid cycle; TLR2, toll-like receptor 2; TME, tumor microenvironment; Ub, Ubiquitin; Vit E, Vitamin E; YAP, Yes-associated protein 1
Cancer cells are subjected to and robustly adapt to more oxidative stress than nonmalignant cells (1). Higher oxidative stress in tumors is thought to be caused by altered mitochondrial function and increased activity of reactive oxygen species (ROS)-generating enzymes such as cyclooxygenases, lipoxygenases, and NADPH oxidases, most of which are modulated by tumor-intrinsic factors including increased growth factor and oncogenic signaling (e.g., Ras signaling) and loss of tumor suppressor function (e.g., p53). In addition, external factors such as chemotherapy and radiation also contribute to redox stress in cancer cells. However, if not carefully controlled, unabated ROS can damage macromolecular structures, including proteins and membrane lipids, which can result in cell death or senescence. Therefore, cancer cells rely on endogenous antioxidant networks to maintain redox homeostasis (2, 3, 4).
Lipid ROS is a form of ROS generated by biochemical reactions between oxidant radicals and membrane-lipid polyunsaturated fatty acids (5). This results in oxidative lipid damage that can lead to cell death by ferroptosis. Ferroptosis is a term that describes a nonapoptotic form of cell death associated with the perturbation of redox and antioxidant mechanisms, and propagation of lipid peroxidation reactions. This cell death requires labile active iron and is morphologically, phenotypically, and biochemically distinct from other cell death programs such as apoptosis, necroptosis, pyroptosis, and necrosis (6, 7, 8, 9). Dysregulated metabolism is at the core of ferroptosis, characterized by three hallmarks: (i) impaired antioxidant machinery, (ii) availability of redox-active iron, and (iii) the propagation of toxic lipid hydroperoxides (10).
The importance of ferroptosis in cancer is indicated by its tumor-inhibitory capacity in both primary and drug-resistant cancer cells (11, 12). Much of the now classic work on ferroptosis in cancer has focused on cell-autonomous effects in the tissue culture setting. These studies put forth the idea that ferroptosis may serve a tumor suppressor mechanism, akin to apoptosis or senescence. Contemporary studies have begun to test this idea in animal models, studying cancer ferroptosis and how this is impacted by the tumor microenvironment (TME). The TME contains a myriad of distinct cell types and factors including the tumor cells, stromal cells (e.g., cancer associated fibroblasts), immune cells, vasculature, extracellular matrix (e.g., collagen, fibronectin, laminin), and secreted molecules (e.g., metabolites, cytokines). This collective milieu influences all aspects of tumor biology. For example, oxidative stress is among the many factors influenced by the TME, and this cross talk promotes cancer cell proliferation and survival, cell migration and invasion, wound healing, tumor vascularization defects, and treatment resistance (4, 13). However, there is a delicate balance relating to the impact of ROS in the TME, wherein moderate levels promote tumor growth, while higher levels tilt the balance to increased oxidative damage to macromolecules and cell death (14). In line with this concept, the production of lipid ROS and, by extension the initiation of ferroptosis, could have multifaceted and complex impact on the TME, tumor growth, and therapeutic response.
In this review, we start by setting the stage for ferroptosis as a metabolic form of cell death. We describe early studies that elucidated the mechanisms that form the basis of our understanding of cell-autonomous ferroptosis as tumor-suppressive. Further, we discuss recent studies describing the impact of ferroptosis on the TME. We round out the review by highlighting the therapeutic utility and current challenges mitigating the use of classic ferroptotic agents in preclinical and clinical setting and close by outlining perspectives for future investigation.
Ferroptosis: When lipid ROS formation outpaces removal
Ferroptosis occurs when lipid ROS production exceeds the capacity of the lipid ROS detoxification machinery. Lipid ROS is generated by redox active labile iron via Fenton chemistry, and the availability of iron has been shown to be crucial to the initiation of ferroptosis (6) (Fig. 1). The lipid ROS are then spontaneously propagated across unsaturated lipids in the membrane. Specifically, peroxidation of membrane polyunsaturated fatty acid (PUFA) phospholipids (PUFA-PLs) drives the ferroptosis process (15). Studies have identified phosphatidylethanolamine (PE)-containing arachidonic acid (AA; C20:4) and adrenic acid (AdA; C22:4) phospholipids as key substrates of phospholipid peroxidation in ferroptosis (16). The presence of highly oxidizable methylene double bonds makes them major substrates for phospholipid peroxidation (17). The direct connection between accumulation of lipid peroxides and cell death manifests in the fact that ferroptosis can be effectively inhibited by lipophilic antioxidants such as alpha-tocopherol (vitamin E) and its analogs, ferrostatin-1, and liproxstatin-1, all of which serve as radical trapping antioxidants that neutralize lipid ROS and therefore prevent cell death (18, 19).
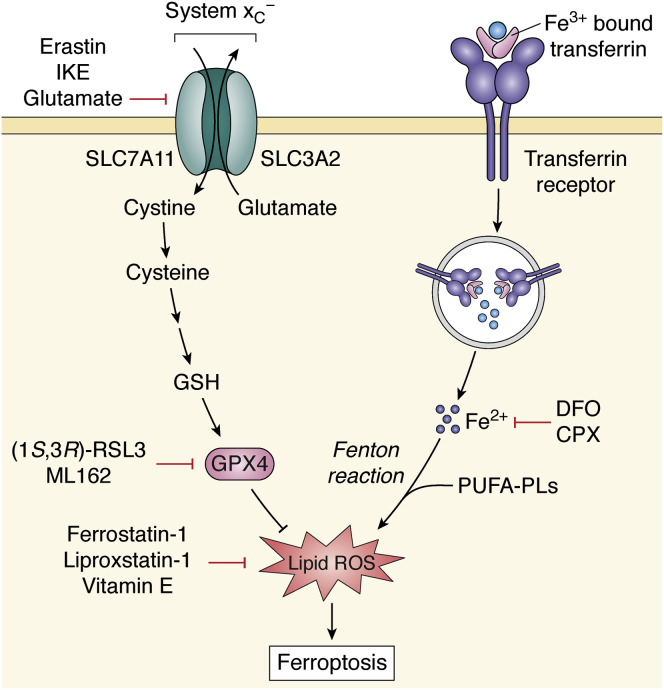
Figure 1.
The first and well-characterized pathway governing ferroptosis. Ferroptosis is mediated by an iron-dependent accumulation and propagation of lipid peroxidation reactions. Iron (Fe3+) bound to transferrin is internalized into cells via receptor-mediated endocytosis and subsequently released as redox-reactive iron (Fe2+) required for Fenton chemistry. Upstream ferroptosis induction can occur as a result of cysteine starvation via inhibition of system xC− using small-molecule inhibitors (Erastin, IKE, Glutamate), genetic deletion of SLC7A11, or cystine withdrawal, which result in diminished GSH levels, and impaired GPX4 activity. In addition, direct inhibition of GPX4 with small-molecule compounds ((1S,3R)-RSL3, ML162) or GPX4 depleting class of ferroptosis compounds, can impair antioxidant system and result in the accumulation of lipid ROS leading to ferroptosis. Accordingly, ferroptosis is blocked by iron chelators, DFO and CPX, as well as radical trapping antioxidants, Ferrostatin-1, Liproxstatin-1, and Vitamin E. CPX, ciclopirox; DFO, deferoxamine; GPX4, glutathione peroxidase 4; GSH, glutathione; IKE, imidazole ketone erastin; ML162, molecular libraries 162; PUFA-PLs, polyunsaturated fatty acid phospholipids; ROS, reactive oxygen species; RSL3, Ras selective lethal 3.
Detoxification of cellular lipid ROS, which consequently inhibits ferroptosis, can occur through multiple pathways. In fact, many of these pathways have been illuminated by observing that their inhibition promotes ferroptotic cell death. For example, a small-molecule screen for compounds selectively lethal in oncogenic Ras-expressing cells identified what is now considered to be a classic inducer of ferroptosis: i.e., Erastin, a system xC− inhibitor (20). System xC− is a heterodimeric cystine-glutamate antiporter encoded by SLC3A2 (CD98 subunit) and the transporter-specific gene SLC7A11 (xCT subunit) (21, 22). Cystine is the dimeric and oxidized form of cysteine, a nonessential amino acid and the rate-limiting component of glutathione (GSH). GSH is an antioxidant tripeptide and a primary mediator of cellular redox balance. Extracellular cysteine in serum (and tissue culture media) is predominantly present as cystine, and cancer cells employ a variety of mechanisms to increase levels of xCT to obtain cysteine (23). This provides the rationale as to why cancer cells are more vulnerable to ferroptosis initiated by system xC− inhibition.
Shortly after the description of system xC− inhibitors (24), a second class of ferroptosis-inducing compounds were described that inhibit glutathione peroxidase 4 (GPX4) (20). GPX4 is a GSH-dependent and selenocysteine-containing lipid peroxidase, in which direct or indirect inhibition, e.g., GSH depletion, leads to ferroptosis (20, 25). A notable inhibitor of GPX4 is the small-molecule compound, (1S,3R)-Ras Selective Lethal 3, abbreviated as RSL3. RSL3 acts by binding covalently to GPX4 and inducing the latter’s inactivation and consequent accumulation of lipid ROS in the cell (25). Thus, these two nodes (system xC− and GPX4) converge to promote ferroptosis by way of cysteine depletion and GPX4 inhibition. More recently, other mechanisms have been uncovered that arise from genetic, transcriptional, or metabolic dysregulation that predict sensitivity or resistance to ferroptosis. Some of these directly act on the cysteine-GSH-GPX4 axis, and others work entirely in parallel. In addition, there is now a growing appreciation that the mechanisms regulating ferroptosis in isolated cancer cells in culture are not readily recapitulated in vivo. Put another way, the in vivo tumor microenvironment can protect cancer cells from ferroptosis through non-cell-autonomous functions and activities. In the following sections, we will discuss these metabolic factors and pathways from a cell-autonomous perspective followed by a description of the ways these pathways are circumvented by factors in the TME.
Intrinsic regulation of ferroptosis
The importance and therapeutic potential of ferroptosis in cancer and other diseases have recently received considerable attention. In line with this, so has the list of cell autonomous mechanisms reported to govern ferroptotic sensitivity and resistance (Fig. 2). The section that follows provides a succinct overview of the cell autonomous mechanisms that regulate ferroptosis, as more detailed descriptions have been provided in several excellent reviews (10, 15, 26, 27). Notably, we highlight the literature examples that form the basis of the well-appreciated mechanisms regulating ferroptosis in order to prepare the reader for our more detailed discussion of the non-cell-autonomous mechanisms that follow.
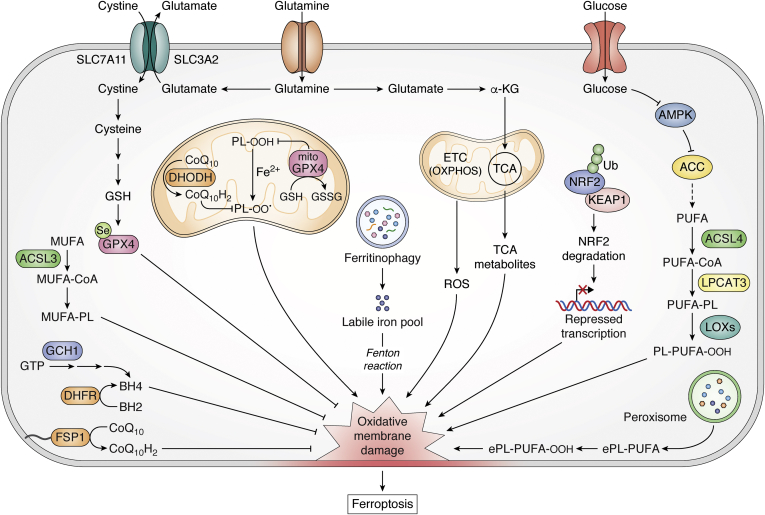
Figure 2.
Additional pathways governing the intrinsic regulation of ferroptosis. Several cell-autonomous or intrinsic mechanisms modulate cancer cell sensitivity to ferroptosis. This nonexhaustive list includes metabolic pathways that regulate PUFA and MUFA levels, mitochondria respiration and bioenergetics, ferritinophagy, NRF2 antioxidant system, and other direct lipid peroxide neutralizing pathways, e.g., FSP1 and DHODH, which are independent and parallel to GSH-GPX4. αKG, alpha ketoglutarate; ACC, Acetyl-CoA carboxylase; ACSL3, acyl-CoA synthetase long-chain family member 3; ACSL4, acyl-CoA synthetase long-chain family member 4; AMPK, 5′ adenosine monophosphate-activated protein kinase; BH2, dihydrobiopterin; BH4, tetrahydrobiopterin; CoA, coenzyme A; CoQ10, Coenzyme Q10; CoQ10H2, Coenzyme Q10 or ubiquinol; DHODH, dihydroorotate dehydrogenase; DHFR, dihydrofolate reductase; ePL-PUFA, ether phospholipid-polyunsaturated fatty acids; ETC, electron transport chain; FSP1, ferroptosis suppressor protein1; GCH1, GTP cyclohydrolase 1; GPX4, glutathione peroxidase 4; GPX4mito, mitochondrial GPX4; GSH, glutathione (reduced); GSSG; glutathione (oxidized); KEAP1, Kelch-like ECH-associated protein 1; LIP, labile iron pool; LOXs, lipoxygenases; LPCAT3, lysophosphatidylcholine acyltransferase 3; MUFA, monounsaturated fatty acid; MUFA-PL, monounsaturated fatty acid phospholipid; NRF2, nuclear factor erythroid 2-related factor 2; OXPHOS, oxidative phosphorylation; PLOO, phospholipid hydroperoxyl radical; PLOOH, phospholipid hydroperoxide; PUFA, polyunsaturated fatty acid; PUFA-PL, polyunsaturated fatty acid phospholipid; ROS, reactive oxygen species; Se, Selenium; TCA, tricarboxylic acid cycle; Ub, Ubiquitin.
ACSL4 and LPCAT3 axis
Two of the earliest characterized genes in the regulation of GPX4-inhibition mediated ferroptosis are the lipid metabolic genes: acyl-coenzyme A synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3) (16, 28, 29, 30). ACSL4 and LPCAT3 were found to be critical proferroptotic genes, which encode proteins that remodel the cellular membrane lipid architecture to execute ferroptosis. The protein encoded by ACSL4 functions in the acylation of long-chain fatty acids, a step that is crucial for the biosynthesis of long-chain PUFA-CoA, including arachidonic acid-CoA (AA-CoA) and adrenic acid-CoA (AdA-CoA). While the protein encoded by LPCAT3, on the other hand, mediates the incorporation of these and other acylated AA into membrane phospholipids (16, 28, 29, 30).
ACSL4 expression directly correlates with sensitivity of cancer cells to ferroptosis; deletion of ACSL4 suppresses ferroptosis sensitivity, while overexpression sensitizes cells to ferroptosis. Accordingly, ACSL4 transcript levels were found to be downregulated in ferroptosis-resistant cancer cell lines (29). Deletion of ACSL4 and LPCAT3 prevented the integration of PUFA into the membrane bilayer, thereby depleting the substrate for oxidative lipid damage (30). Remarkably cells with ACSL4 and GPX4 codeleted maintained their viability (29), indicating the interconnection between the two enzymes. Hence, the membrane remodeling enzymes are key regulatory nodes of ferroptosis susceptibility.
PUFAs and MUFAs
PUFAs are the site of oxidative lipid damage and are required for the execution of ferroptosis (31). The abundance of PUFAs determines the extent of available lipid peroxidation sites and thus ferroptosis susceptibility. Moreover, high concentration of PUFAs on lipid membranes has been shown to be positively correlated with increased dependency on GPX4 and enhanced sensitivity to iron-dependent oxidative lipid damage (32). Exogenous supplementation with long chain PUFAs enhances ferroptosis sensitivity, even under conditions of ACSL4 deletion (28). Additionally, exogenous PUFA in the form of DGLA (dihomo-γ-linolenic acid) was recently described as a metabolic inducer of ferroptosis in human cancer cells, and this could be blocked by ferrostatin-1 or endogenous ether lipids (33). The proferroptotic activity of DGLA appeared to be unique to this class of PUFA, as a similar phenotype was not shared by other PUFAs such as AA, eicosapentaenoic acid (EPA). and docosahexaenoic acid (DHA) (33).
Exogenous monounsaturated fatty acids (MUFAs), such as oleic acid (OA, C18:1), display the opposite effect(s) of PUFAs by potently suppressing ferroptosis. Mechanistically, this involves the competitive decrease of PUFA incorporation into membranes, which blocks the propagation of lipid ROS at the plasma membrane (31, 34). This lipid peroxidation-suppressing and ferroptosis-inhibitory role of MUFA is dependent on the activity of the protein encoded by the acyl-coenzyme A synthetase long-chain family member 3 (ACSL3) gene, which allows for the selective incorporation of MUFAs into membrane lipids. Treating cells with exogenous MUFAs decreased cellular PUFA-PLs levels, ultimately leading to ferroptosis resistance. Accordingly, low expression of ACSL3 was correlated with increased ferroptosis sensitivity (34). Therefore, the differential incorporation of PUFAs or MUFAs into membranes increases or decreases sensitivity to ferroptosis, respectively.
FSP1, CoQ10, and NADPH
The ferroptosis suppressor protein 1 (FSP1) was recently characterized as a novel lipid peroxidation neutralizing pathway distinct and parallel to the GPX4 pathway. FSP1 was identified in genetic screens from two independent research groups that sought to determine pathways that govern resistance to GPX4 inhibition-induced ferroptosis in cancer lines. It was discovered that FSP1 provided resistance to ferroptosis under conditions of GPX4 deletion (35, 36). Specifically, FSP1 is recruited to the plasma membrane where it uses NAD(P)H to reduce coenzyme Q10 (CoQ10) to its radical trapping antioxidant form, ubiquinol (CoQ10-H2). This then blocks oxidative phospholipid damage and directly mitigates lipid peroxidation. Deletion of FSP1 in cancer cells results in increased lipid ROS even in the presence of functional GPX4, and overexpression of FSP1 blocks propagation of lipid ROS in RSL3-treated cells. These collective results illustrate that FSP1 serves as a complementary pathway to GPX4 to block lipid peroxidation (Fig. 2).
BH4 and DHFR
A recent metabolism-centered genetic screen identified a novel role of the cofactor tetrahydrobiopterin (BH4) and the enzyme dihydrofolate reductase (DHFR) in the protection against ferroptosis (37). BH4 was found to be a potent endogenous radical-trapping antioxidant that regulates sensitivity to ferroptosis induced by GPX4-inhibition, but not cysteine depletion. The radical-trapping antioxidant role of BH4 was distinct from its function as a cofactor to enzymes involved in hydroxylation of aromatic amino acids. The biosynthesis of BH4 from GTP involves several steps catalyzed by the rate-limiting enzyme, GTP cyclohydrolase 1 (GCH1). Genetic deletion of GCH1 reduced intracellular levels of BH4 and decreased the antioxidant capacity of the cells. These BH4-deficient cells showed enhanced sensitization to GPX4 inhibition-induced ferroptosis. Consistently, treating BH4-deficient cells with exogenous BH4 in the form of dihydrobiopterin (BH2), the dehydrogenated product of BH4, protected against and rescued RLS3 and ML162-induced ferroptosis, but not Erastin-induced ferroptosis. In this setting, DHFR was involved in the restoration of BH4 from BH2 (37) (Fig. 2).
PUFA-ether phospholipids (PUFA-ePLs)
Contrasting results have been presented on the role of PUFA-ether phospholipids (PUFA-ePLs) in ferroptosis regulation, thus suggesting the involvement of various cellular and genetic contexts in ferroptosis. Perez et al. (33) showed that ether lipids resist the induction of lipid peroxidation and ferroptosis induced by exogenous dihomo-γ-linolenic acid (DGLA) in Caenorhabditis elegans and human cancer cells (33). This observation was consistent with the role of endogenous ether lipids as antioxidants that protect PUFAs from lipid peroxidation (38, 39). In contrast, Zou et al. (40) found that PUFA-ePLs, synthesized by peroxisomes, have a proferroptotic function. In this study (40), it was revealed that PUFA-ePLs served as substrates for lipid ROS propagation and sensitized cells to ferroptosis (40). Whole-genome CRISPR-Cas9 screens with GPX4 inhibitors identified several peroxisomal genes (e.g., PEX3, PEX10) and ether-lipid biosynthesis genes (e.g., AGPS, FAR1), which when deleted, promoted resistance to ferroptosis induced by GPX4-inhibition (40). Thus, this work suggests that PUFA-ePLs synthesized by peroxisomes could represent a vulnerability wherein cells downregulate PUFA-ePLs as a strategy to evade ferroptosis and upregulate PUFA-ePLs as substrates of lipid ROS to promote sensitivity to ferroptosis (40) (Fig. 2). Further investigation will be required to shed more light on the role of ether lipids in ferroptosis susceptibility.
Autophagy and ferritinophagy
Autophagy is an evolutionarily conserved cellular degradation pathway (41). Selective forms of autophagy exist in which discrete subcellular compartments can be targeted for degradation. Relevant here is the regulation of iron availability by ferritinophagy—an autophagy-mediated degradation of iron-bound ferritin (42). In ferritinophagy, NCOA4 is the selective cargo receptor mediating autophagic degradation of ferritin to release iron into the intracellular labile iron pools (LIP) (43, 44). The availability of LIP increases the iron-dependent Fenton lipid peroxidation reactions that trigger ferroptosis (Fig. 2). Reciprocally, increases in ferritin hinder ferroptosis by decreasing the LIP, while ferritinophagy sensitizes cells to ferroptosis via the NCOA4-mediated degradation of ferritin. In mouse embryonic fibroblasts (MEF) (45, 46), human pancreatic cancer cells (46), and human sarcoma cells (45, 46), genetic or pharmacological inhibition of autophagy impaired Erastin-induced accumulation of lipid ROS and ferroptosis. On the other hand, increased autophagic flux was associated with enhanced Erastin- and cysteine deprivation-induced tumor ferroptosis (45, 46). Mechanistically, overexpression of NCOA4 diminished ferritin levels, increased cellular LIP, and consequently enhanced Erastin-induced ferroptosis. Conversely, the knockdown of NCOA4 increased levels of ferritin, decreased labile iron, and diminished sensitivity of cancer cells to Erastin-induced ferroptosis (46). Furthermore, NCOA4-mediated ferritinophagy is modulated by the metabolic state of the cell. For example, inhibition of glutamate oxaloacetate transaminase 1 (GOT1) in pancreatic cancer cells induced energetic stress, marked by the induction of autophagy and ferritinophagy. This led to increased intracellular LIP and sensitized pancreatic cancer cells to various inducers of ferroptosis (47). The regulation of ferroptosis by ferritinophagy is, therefore, via modulating the availability of cellular labile iron for lipid peroxidation reactions.
NRF2 regulation
The transcription factor nuclear factor erythroid 2-related factor 2 (NRF2) is a master regulator of redox homeostasis and signaling, which modulates the cellular antioxidant mechanisms in addition to other functions in xenobiotic metabolism and cell proliferation. Under homeostatic conditions, levels of NRF2 are kept comparatively low by the activity of E3-ubiquitin ligase complexes, including Kelch-like ECH-associated protein 1-Cullin 3 Ring box 1 (KEAP1-CUL3-RBX1) (48). However, when cells are exposed to oxidant stressors or under conditions of gene alterations, chemical inhibition, or disruption of binding of the ligase complexes to NRF2 protein, NRF2 is released from its interaction with the ligase complex, is stabilized, and is translocated into the nucleus where it activates transcription of antioxidant genes. Several proteins and enzymes that impinge on ferroptosis are encoded by NRF2 target genes, these include proteins involved in iron homeostasis (ferritin, ferroportin, heme-oxygenase) (49, 50) and antioxidant functions (system xC−, glutathione synthase, GPX4) (48, 50, 51).
NRF2 is upregulated in several cancer types where it serves a tumor-promoting function and confers poor prognosis (50, 52, 53). Consistent with its antioxidant role and upregulation of ferroptosis target genes, NRF2 generally promotes ferroptosis resistance, while genetic silencing or pharmacological inhibition of its activity increases sensitivity to ferroptosis agents (50, 52, 54) (Fig. 2). The hyperactivation of NRF2 was revealed to be critical to the growth of non-small-cell lung cancer (NSCLC) spheroids as it suppressed the induction of ferroptosis in the core of the cancer spheroids (51, 55). Importantly, the combination of GPX4 inhibition and NRF2 silencing efficiently eradicated the tumor cells within spheroids, highlighting the potential for dual targeting of NRF2 and ferroptosis in solid tumors.
Mitochondria
The first indication that mitochondria were involved in ferroptosis came from evidence of alterations in their morphology in ferroptotic cancer cells, including smaller size, enlarged cristae, and increased membrane density (6, 28, 56). Yet, Erastin treatment effectively induced ferroptosis in mitochondria-DNA depleted cancer cells that were unable to respire (6). Moreover, mitochondria are not essential for the ferroptotic inhibitory function of ferrostatin-1 (18). On the other hand, studies have linked mitochondrial glutamine metabolism to ferroptosis, as glutaminolysis promoted serum-dependent oxidative cell damage and ferroptosis under conditions of either full amino acid or cysteine deprivation (57).
The role of mitochondria was investigated by Gao et al. (58) in a study that implicated the mitochondria in cysteine deprivation-induced ferroptosis. Targeting the mitochondria diminished cysteine deprivation, but not GPX4 inhibition-induced, ferroptosis (58). Metabolite intermediates of mitochondrial tricarboxylic acid cycle (TCA) pathway such as α-ketoglutarate, fumarate, succinate, malate, and the components of the electron transport chain (ETC) were revealed to modulate sensitivity of cells to cysteine deprivation-induced ferroptosis. Impairment of the TCA cycle or ETC diminished sensitivity to cysteine deprivation or Erastin treatment (Fig. 2). Interestingly, the loss of function of fumarate hydratase (FH), a tumor suppressor gene in renal cancer, was found to promote ferroptosis resistance. This was posited to be an underlying mechanism of its tumor suppressive function, wherein the loss of function mutation in FH results in tumor growth benefits.
In another study (59), a genome-wide CRISPR screen in tandem with small-molecule mitochondrial inhibitors revealed a synthetic lethal interaction between GPX4 deletion and mitochondrial dysfunction. The loss of GPX4 enhanced the toxic effects of mitochondrial inhibitors and sensitized cancer cells to ferroptosis. Indeed, the synthetic lethal interaction and ferroptosis phenotype were reversed when mitochondrial specific GPX4 was overexpressed in the GPX4 KO cells (59). Moreover, GPX4 expression was shown to be induced in response to mitochondrial dysfunction in vitro and in vivo. This study (59) supported a potential role of mitochondria as an important site of ferroptosis regulation. Similarly, the inner mitochondria membrane-localized enzyme, dihydroorotate dehydrogenase (DHODH), was shown to modulate ferroptosis sensitivity wherein the deletion or inhibition of the enzyme promoted or sensitized cancer cells to ferroptosis (60). Interestingly, the combination of GPX4 silencing and DHODH deletion, but not either alone, induced robust mitochondrial lipid peroxidation, which could be blocked by mitochondrial specific radical trapping antioxidants (60). To reconcile some of the contrasting findings on the role of mitochondria in ferroptosis, it is worth noting that there are differences in the model of mitochondria elimination in the different studies. These included mitochondria DNA deletion (6) versus eradication of the entire organelle (58) versus small-molecule inhibition of the ETC (59). Altogether, the context-dependent roles of the mitochondria and mitochondrial metabolism will require future studies.
Energy metabolism and AMPK
As discussed above, cellular energy metabolism has been linked to ferroptosis susceptibility. Recent work from Lee et al. (61, 62) revealed that energetic stress induced by depletion of intracellular glucose or ATP protected cells from ferroptosis. AMPK is a sensor of the cellular bioenergetic state, which is activated upon energetic stress resulting from an increase in AMP/ATP ratio. The phosphorylation-induced activation of AMPK leads to the inhibition of ATP-utilizing anabolic biosynthetic pathways and the activation of ATP-generating catabolic pathways. In MEFs, glucose starvation, subsequent ATP depletion, and activation of AMPK were found to mitigate lipid peroxidation and protect cells from ferroptosis induced by classic ferroptosis inducers (i.e., cystine depletion, Erastin, RSL3). In a spectrum of cancer cell lines, high AMPK levels correlated with increased ferroptosis resistance. Moreover, the molecular mechanism of ferroptosis blockade by AMPK activation did not involve mTORC activation, regulation of iron levels, or autophagy. However, the mechanism was mediated in part by the AMPK phosphorylation and inactivation of downstream acetyl-CoA carboxylase (ACC). ACC is a rate-limiting enzyme in the fatty acid biosynthetic pathway, and its phosphorylation inhibits fatty acid synthesis. In sum, the activation of ACC by AMPK led to the decreased generation of PUFAs, the substrate that drives ferroptosis.
In a related study (63), Song et al. (63) found that inhibition of pyruvate dehydrogenase kinase 4 (PDK4) promoted cysteine depletion-induced ferroptosis in pancreatic cancer cells. Through siRNA screening, PDK4 was identified as a key gene that mediated metabolic resistance to ferroptosis. PDK4 acted by blocking pyruvate entry into the TCA cycle, thereby diminishing fatty acid biosynthesis and PUFA production, in a manner akin to that described for activation of AMPK.
Extrinsic regulation of ferroptosis
In this section, we distinguish extrinsic regulators of ferroptosis from the intrinsic pathways discussed in the preceding section as those that occur through interaction with other cells or capture of micronutrients from the serum (Fig. 3). These classifications are somewhat arbitrary in nature and are meant to serve as a bridge between the cell-autonomous pathways that regulate ferroptosis and the non-cell-autonomous mechanisms at play in the TME. Furthermore, the intrinsic and extrinsic pathways are not mutually exclusive.
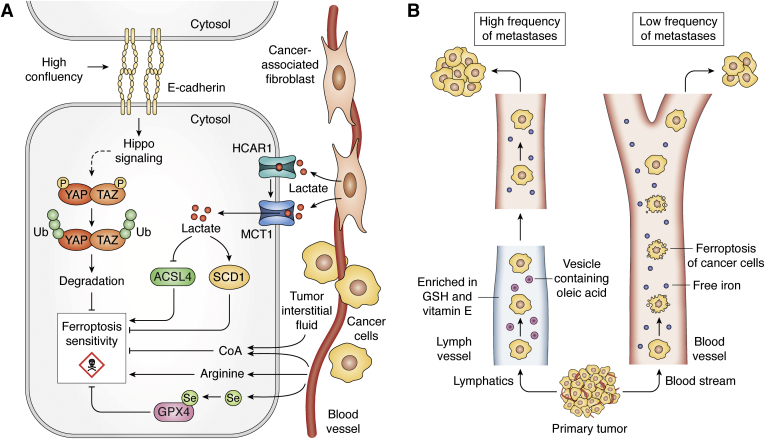
Figure 3.
Extrinsic regulation of ferroptosis.A, E-cadherin/hippo signaling and the downstream YAP/TAZ pathways mediate ferroptosis insensitivity with increased cell contact; MCT1-mediated uptake of lactate (red dots) regulates ferroptosis via ACSL4 and SCD1; availability of Arginine promotes sensitivty to ferroptosis, while CoA diminishes sensitivity to ferroptosis; and supplementation with Selenium (Se) enables ferroptosis resistance through GPX4. B, unlike blood, which contains greater levels of free iron, the lymph provides a reducing and antioxidant-rich environment with higher levels of GSH, Vit E, and vesicles containing oleic acid, all of which protect metastasizing tumor cells from ferroptosis. ACSL4, acyl-CoA synthetase long-chain family member 4; CAF, cancer associated fibroblasts; CoA, coenzyme A; GSH, Glutathione; HCAR1, Hydroxycarboxylic acid receptor 1; MCT1, monocarboxylate transporter 1; P, Phosphate; SCD1, stearoyl-CoA desaturase enzyme 1; TAZ, transcriptional co-activator with PDZ-binding motif; U, Ubiquitin; Vit E, Vitamin E; YAP, Yes-associated protein 1.
Cell-cell contact
High cell density, and the resultant increase in cell-to-cell contact, diminishes sensitivity to ferroptosis induced by various strategies (e.g., cysteine depletion, GPX4 inhibition). This process is linked to cell–cell interactions mediated by E-cadherin (64) (Fig. 3A).Higher levels of E-cadherin and downstream Hippo pathway signaling negatively regulated ferroptosis across a spectrum of cancer cell lines. YAP and TAZ are two transcriptional coactivators downstream of Hippo signaling regulated by cell contact (65). High cell confluency results in the retention of YAP/TAZ in the cytosol and their targeted degradation by the proteasome. Conversely, low cell density promotes the translocation of YAP/TAZ to the nucleus, which consequently enables the transcriptional activation of genes regulating cellular growth, survival, and migration (65, 66, 67).
In colon cancer cells, the effector molecules downstream of YAP signaling that mediate ferroptosis sensitivity were found to include transferrin receptor (TRFC) and ACSL4. In line with this model, the absence of YAP abolished ferroptosis sensitivity, while increased expression of TRFC and ACSL4 enhanced ferroptosis in confluent cells previously insensitive to ferroptosis (64). In a model of renal cell carcinoma, the molecular mechanism for ferroptosis resistance in highly confluent cells involved TAZ-induced regulation of epithelial membrane protein 1 (EMP-1) and NADPH oxidase 4 (NOX4). In a low-cell-density state, the presence of TAZ increased the expression of EMP1, which subsequently modulated the levels and activity of the lipid ROS regulator, NOX4, driving ferroptosis (68). Taken together, these studies demonstrate that ferroptosis regulation in the context of cell–cell interaction is modulated similarly upstream by Hippo signaling but differently downstream, via YAP/TAZ, in a tumor-type-dependent manner (64, 68).
Serum-derived nutrients
Selenium (Se) is a metal and micronutrient obtained from the blood that is required for the biosynthesis of the amino acid selenocysteine. Selenocysteine is incorporated into a class of proteins known as selenoproteins, which includes GPX4 (Fig. 3A) (69). The critical role of selenocysteine for the catalytic activity of GPX4 was demonstrated by replacing the codon for selenocysteine with that of cysteine in the GPX4 active site. Under these circumstances, the mutant GPX4 did not recapitulate the antiferroptotic and cell-survival effects of the wild-type protein. Mechanistically, it was observed that the wild-type protein displayed intrinsic resistance to peroxidation upon exposure to peroxides. In contrast, the cysteine mutant was irreversibly oxidized when exposed to peroxides, and this led to the inactivation of the enzyme and cell death by ferroptosis (70). Moreover, the observations that selenium supplementation upregulated GPX4 expression and enhanced resistance to ferroptosis further corroborate the modulatory role of selenium in ferroptosis (71, 72).
Arginine is a nonessential amino acid that has numerous important roles in metabolism outside of its proteinogenic function, including the regulation of cell growth through mTOR and immune cell function (73, 74). A recent study demonstrated that depletion of arginine repressed Erastin-induced, but not GPX4-induced, ferroptosis across a spectrum of cancer cell lines (75). This is consistent with previous observations that ferroptosis induced by inhibition of system xC− or cysteine depletion displays distinct mechanisms from those induced by GPX4 inhibition or its gene deletion (37). Arginine deprivation-induced suppression of ferroptosis occurred in a manner independent of its function as sensor for mTORC1 or the GCN2/ATF4 pathway (75). The precise mechanistic roles remain to be determined, a finding that is of great interest for cancer therapy. For instance, arginine is degraded by various cell types in the TME, in part to limit immune function (74, 76), while the presence of arginine in the TME promotes metabolic fitness and survival of T cells, factors that are important for antitumor responses (77). These findings suggest that the depletion of arginine in the TME may also impair the ability to induce tumor ferroptosis and thereby enable cancer cell persistence.
In a recent study elucidating the cysteine dependency of pancreatic cancer, it was demonstrated that depletion of GSH was necessary but not sufficient to induce ferroptosis (78). The authors then used mass-spectrometry-based metabolomics profiling of heavy carbon (13C)-labeled cystine to determine other fates of cysteine (78). This demonstrated a rapid accumulation of cystine-derived carbon into coenzyme A (CoA). Under conditions of system xC− inhibition, exogenous supplementation of CoA was able to rescue ferroptosis in pancreatic cancer cells. Furthermore, combined inhibition of coenzyme A and GSH biosynthesis potently induced ferroptosis, where each agent alone had modest impact (78). Interestingly, CoA has also been implicated in the ferroptosis resistance seen in MEFs harboring the African specific S47 variant of the p53 tumor suppressor gene (79). Together, these results extend the role of cysteine metabolism beyond GSH and raise interesting questions about the role of CoA in ferroptosis. CoA is also present in the tumor interstitial fluid of pancreatic tumors (80), and recent work has described how it can enter cells (Fig. 3A) (81). However, its downstream functions in ferroptosis remain to be determined but may include inputs into saturated fatty acid and/or sterol (i.e., CoQ10) biosynthesis (78).
Lactate is an important component of the tumor milieu (82). In hepatocellular carcinoma, lactate was demonstrated to be a negative regulator of ferroptosis. Both exogenous lactate and cancer-associated fibroblasts (CAFs)-conditioned media enriched in lactate were shown to rescue Erastin- and RSL3-induced lipid peroxidation and ferroptosis (83). The blockade of lactate uptake and signaling, via genetic silencing or with the pharmacological agent, AZD3965, enhanced lipid ROS and ferroptosis in vitro and in tumor-bearing immunodeficient mice (83). Mechanistically, high lactate levels upregulated the pyruvate/lactate transporter monocarboxylate transporter 1 (MCT1) and the lactate receptor hydroxycarboxylic acid receptor 1 (HCAR1). The elevated MCT1 resulted in the downregulation of ACSL4, and upregulation of stearoyl-CoA desaturase enzyme 1 (SCD1)—an enzyme critical for MUFA synthesis (83) (Fig. 3A). Thus, the effects of lactate occur via perturbation of lipid metabolism products, PUFAs and MUFAs, which modulate ferroptosis sensitivity.
Lymphatic system
Beyond circulating metabolites and intercellular communication, recent evidence implicates the lymphatic system in providing an antiferroptotic environment for metastasizing tumor cells (84). In contrast to the blood, the lymph was found to be enriched with higher levels of GSH, vitamin E, and lower amounts of free iron. This reducing and antioxidant-rich environment of the lymph inhibited oxidative stress and lipid ROS production, prevented ferroptosis of the trafficking melanoma cells, and thus promoted tumor survival and metastasis to lymph nodes and distal sites. Mechanistically, oleic acid (a ferroptosis-inhibitory MUFA) was found to be present at higher levels in the lymph compared with blood and was a key determinant of the survival of melanoma cells trafficking through the lymph. The origin of the oleic acid in the lymph, and the attendant protective effects, was mechanistically linked to triacyl-glycerides (TAGs) trafficked within ApoB+ vesicles (Fig. 3B). Additionally, expression of ACSL3, which facilitates incorporation of MUFAs such as oleic acid into membranes, was found to be essential for promoting metastasis (84). Altogether, this study (84) illuminated how the unique milieu of the lymph protects tumor cells from ferroptosis and thereby allows and promotes tumor metastasis.
Ferroptosis and tumor immunity
The role of the TME in regulating ferroptosis, especially in the context of tumor immunity, is an area of considerable recent interest and therapeutic potential. This is because the nature and properties of the heterogenous immune cells in the TME shape tumor progression, metastasis, and response to therapy by altering the balance between protumor and antitumor immune responses (85). Moreover, how the cells in the TME are influenced by ferroptosis, or mediate ferroptosis sensitivity, determines the efficacy of ferroptosis induction in vivo. This understanding is crucial for the development and translation of ferroptosis therapeutics, including rational combination with immunotherapy. In the section that follows, we highlight recent examples from the literature on the role of ferroptosis in tumor immunity, contextualize the emergent data, and discuss their limitations. Of note, many of the in vivo studies examining ferroptosis to date have been performed in immune-deficient xenograft models. These transplant models lack the native architecture of a tumor, including the complex stroma, as well as bona fide immune system. This made it impossible to study the impact of antitumor immunity, which some studies have indicated both act through and respond to ferroptosis (86, 87). Reciprocally, data from other models have also suggested that ferroptotic cells release danger signals that could enable evasion of immune surveillance (88, 89). The influence of these factors is not accurately considered in the absence of a complete immune system, and where such limitations are evident, these will again be noted and discussed.
Release of DAMPs
Damage-associated molecular patterns (DAMPs) are endogenous molecules released or exposed by dying cells and in response to tissue damage. Such factors include ATP, high-mobility group box protein 1 (HMGB1), cell-surface calreticulin, and interferon alpha/beta (IFNα/β). These DAMPs act as chemoattractants or adjuvants that activate innate immune cells such as professional antigen presenting cells—macrophages and dendritic cells (90), which then go on to activate CD4 T and CD8 T cells of the adaptive immune system. Early evidence of DAMP release from ferroptotic cells indicated that MEFs and cancer cells released HMGB1 in a mechanism dependent on the activation of the autophagic machinery (91). The HMGB1 released by ferroptotic cancer cells activated innate immune cells and triggered an inflammatory response that was dependent on HMGB1 binding to the cognate receptor, advanced glycosylation end product-specific receptor (AGER), and not toll-like receptor 4 (TLR4) on macrophages (Fig. 4A).
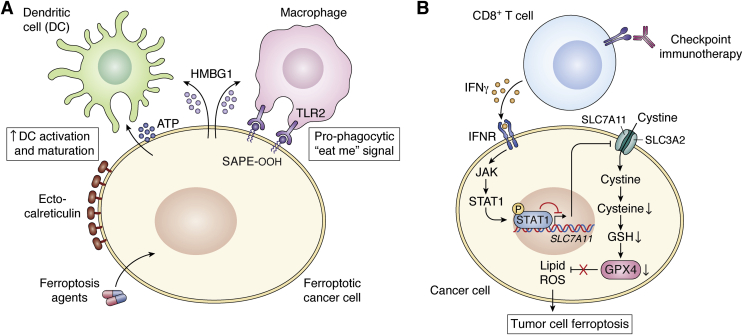
Figure 4.
Antitumor roles of ferroptosis in the tumor immune microenvironment.A, immune-stimulatory effects of ferroptosis. Ferroptosis agents induce the release of immunogenic damage associated molecular patterns (DAMPs) such as ATP, HMGB1, and exposure of calreticulin on cell surface (ecto-CRT) from cancer cells. Early- but not late-stage released/exposed DAMPs result in the activation and maturation of professional antigen presenting cells (APCs), such as dendritic cells and macrophages, leading to activation of cytotoxic T cells. In addition, ferroptotic cells release/expose SAPE-OOH, an oxidized phospholipid molecule, which serves as a pro-phagocytic or “eat-me” signal by binding to its cognate receptor, TLR2, on macrophages. B, immune-checkpoint activated CD8+ T cell-mediated ferroptosis of tumor cells. Activated CD8+ T cells secrete IFNγ, which binds to interferon receptor on tumor cells activating the JAK/STAT pathway and causing the translocation of phosphorylated STAT1 into the nucleus where it represses transcription of SLC7A11. The decreased level of the cystine importer leads to cysteine depletion, diminished GSH levels, and impaired GPX4 activity. Loss of GPX4 activity eventually results in the accumulation of lipid ROS and tumor ferroptosis. ATP, adenosine triphosphate; ecto-CRT, cell-surface calreticulin; GPX4, Glutathione peroxidase 4; GSH, Glutathione; HMGB1, high mobility group box protein 1; IFNγ, interferon gamma; IFNR, interferon receptor, JAK, Janus kinase; P, Phosphate; ROS, reactive oxygen species; SAPE-OOH, oxidized phospholipid, 1-steaoryl-2-15-HpETE-sn-glycero-3-phosphatidylethanolamine; STAT1, signal transducer and activator of transcription 1; TLR2, toll-like receptor 2.
Oxidized phospholipids have recently been classified as DAMPs (92, 93), and these are uniquely enriched in ferroptotic cells. A novel role for a ferroptosis specific-oxidized phospholipid DAMP, cell-surface oxidized phospholipid, 1-steaoryl-2-15-HpETE sn-glycero-3-phosphatidylethanolamine (SAPE-OOH), was shown to mediate cell clearance by macrophages. Cell-surface SAPE-OOH served as an “eat me” signal that allowed the recognition and phagocytic uptake of ferroptotic cells by macrophages (94). This process occurred via binding of SAPE-OOH to its identified cognate receptor, toll-like receptor 2 (TLR2), on macrophages (Fig. 4A).
Immunogenicity of ferroptotic tumor cells
Recent evidence has shown that ferroptotic tumor cells can elicit antitumor immune responses, implicating ferroptosis as a potential form of immunogenic cell death (ICD). ICD in cancer therapy is a cell death process characterized by the release of immunogenic DAMPs resulting in a robust and durable activation of the adaptive immune system to eliminate tumor cells (95). Interestingly, RSL3 treatment of fibrosarcoma and glioma cells displayed in vitro hallmarks of ICD, which included release of DAMPs, ATP, and HMGB1, phagocytosis of ferroptotic tumor cells by bone-marrow-derived dendritic cells (BMDCs) as well as the maturation of BMDCs (96) (Fig. 4A). This immunogenic feature of ferroptotic tumor cells was further confirmed using an immunocompetent in vivo prophylactic vaccination model. Herein, subcutaneous vaccination with ferroptotic tumor cells in the flanks of mice led to a protective adaptive antitumor immune response (96). While these findings provided evidence that ferroptotic tumor cells exhibited features of ICD, the antitumor immune response was observed only with cells that had experienced early ferroptosis (i.e., less than 3 h of drug treatment). In contrast, analysis of late ferroptotic cells in vitro and in vivo did not yield similar outcomes. Rather, the immunogenic phenotype was lost as the late ferroptotic cells neither induced maturation of dendritic cells in vitro nor elicited protective antitumor immunity in vivo. This study (96) therefore indicates a temporal-specific immunogenicity of ferroptotic cancer cells predicated on the time of treatment and phase of cell death. This finding is also in marked contrast with the immunogenicity induced by apoptosis-inducing ICD agents, which can be maintained from early-stage through late-stage apoptosis (97). This observation therefore sets the stage for more rigorous investigations to define the contextual features for immunogenicity of ferroptosis.
CD8 T-cell-mediated ferroptosis
It has been demonstrated that CD8 T cells mediate direct tumor cell killing through the induction of ferroptosis of tumor cells. This pioneering work adds to a growing body of mechanisms by which T cells can kill cancer cells, and the therapeutic potential is expounded upon in the section that follows. Mechanistically, the authors (86) showed that activated CD8 T cells released the effector cytokine, interferon gamma (IFNγ), which upon binding to cancer cells triggered the downregulation of SLC7A11 expression, thereby impairing cystine uptake and resulting in lipid peroxidation and tumor ferroptosis (86) (Fig. 4B). This activity could be potentiated by depleting cystine/cysteine levels in the TME via an engineered degrading enzyme, Cyst(e)inase, the effects of which were further enhanced when combined with checkpoint immunotherapy. Of particular relevance to clinical data, the authors (86) found that the expression of SLC7A11 was inversely correlated with the frequency of tumor infiltrating CD8 T cells in human melanoma tumor tissues and that the ferroptosis signature was positively correlated with CD8 T cell effector capacity. Although this remarkable observation was made in the context of immunotherapy-activated CD8 T cells, it raises the possibility of ferroptosis being an intrinsic antitumor CD8 T cell pathway, analogous to traditional pathways such as the granzyme-mediated killing of tumor cells. In addition, it further highlights a potential role for ferroptosis in T-cell-mediated immune surveillance under physiological settings. Indeed, these exciting, albeit hypothetical, concepts warrant extensive investigation.
Oxidized lipids and T cell ferroptosis
As discussed above, T-cell-mediated ferroptosis of tumor cells is beneficial in that it can elicit antitumor immunity and enhance the effect of immunotherapy. However, T cells are also sensitive to ferroptosis. Recent evidence has shown that cytotoxic T cells in the TME can accumulate lipid ROS and undergo ferroptosis, leading to decreased T cell effector function and impaired antitumor immunity. For example, high cholesterol in the TME promoted CD8 T cell exhaustion, while decreased levels of cholesterol in the TME restored CD8 T cell effector function (98). Cholesterol accumulation promoted increased levels of the fatty acid importer CD36, which was associated with increased fatty acid uptake, increased lipid peroxidation, and ferroptosis. Reciprocally, genetic deletion of CD36 on T cells rescued their function and blocked ferroptosis, a feature that was conserved in both murine and human CD8T cells (87). In line with these findings, elevated lipids in the TME have been shown to induce CD8 T cell dysfunction marked by increased CD36 expression and increased uptake of oxidized low-density lipoprotein (oxLDL), which resulted in the induction of lipid peroxidation and impaired CD8 T cell function (Fig. 5). Overexpression of GPX4 in CD8 T cells blocked lipid peroxidation, restored CD8 T cell effector cytokine production, and promoted antitumor immune responses in vivo (99). Taken together, these observations suggest an interconnection between the inhibition of lipid peroxidation in maintaining functional CD8 T cell responses and the likelihood of diminished CD8 T effector function as a result of ferroptosis induction in the TME.
Figure 5.
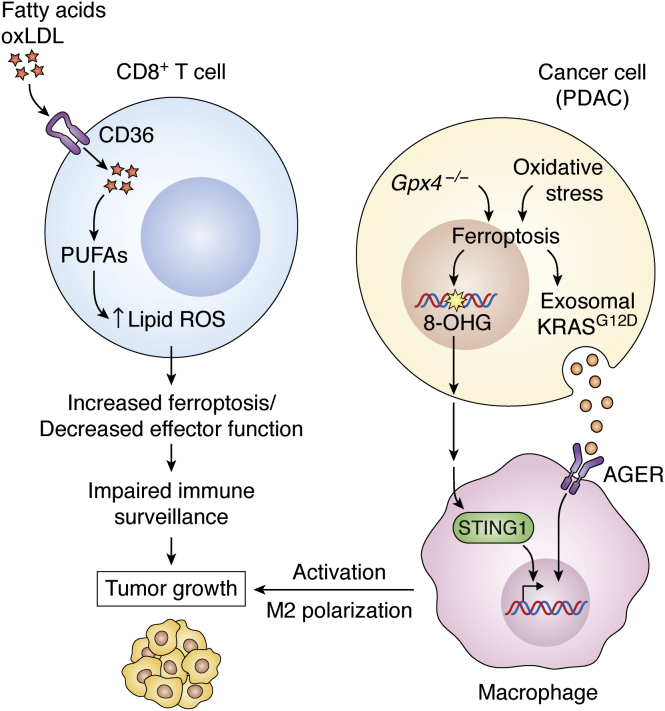
Protumor roles of ferroptosis in the tumor microenvironment. The accumulation of lipids, including cholesterol, fatty acids, or oxLDLs, in the TME induces the upregulation of CD36 on CD8+ T cells, which leads to further increased uptake of fatty acids and oxLDLs. Elevated fatty acid uptake by CD36 results in higher levels of PUFAs, increased lipid ROS, and enhanced ferroptosis. Ferroptosis of CD8+ T cells decreases effector function, impairs immune surveillance, and promotes tumor growth. Moreover, Gpx4 deletion or high-iron diet promotes pancreatic tumorigenesis via the release of oxidized nucleobases, e.g., 8-OHG, which activates the STING pathway resulting in increased macrophage activation and infiltration and tumor growth. Similarly, oxidative stress in pancreatic cancer cells results in autophagy-induced ferroptosis and the release of KRASG12D packaged in exosomes. Binding of exosomes containing KRASG12D to AGER receptors on macrophages induced polarization of macrophages to “M2” phenotype and increased tumorigenesis. 8-OHG, 8-hyroxyguanosine; AGER, advanced glycosylation end-product specific receptor; CD36, cluster of differentiation 36; Gpx4, glutathione peroxidase 4; oxLDL, oxidized low density lipoprotein; PUFA, polyunsaturated fatty acids; ROS, reactive oxygen species; STING1, stimulator of interferon genes 1.
Indeed, early studies on the role of GPX4 on T cell activity established that GPX4 is critical to antigen-specific CD4 T and CD8 T cells by functioning to, not only prevent T cell-ferroptosis but to also, confer effective protection against viral and parasitic infections (100). Additionally, the accumulation of oxidized lipids in dendritic cells has been shown to diminish the expression of peptide-MHC I complex and impair cross-presentation of exogenous tumor antigen (101). Moreover, oxidized lipids generated via the activity of lipid oxidizing enzyme 12/15-LOX have been found to inhibit the maturation of dendritic cells via the activation of NRF2 (84). Thus, it is conceivable that increased ferroptosis, accompanied by release of oxidized lipids, could impair DC cross-presentation and CD8 T cell priming in the TME. These observations raise the possibility that systemic GPX4 inhibition could yield confounding or adverse effects on T-cell-mediated antitumor immunity.
Protumor roles for ferroptosis
Contrasting the previous studies, ferroptosis has also been shown to support tumor growth in certain contexts (Fig. 5). For example, tumor-associated neutrophils were reported to induce lipid peroxidation and iron-dependent tumor cell death in glioblastoma multiforme (GBM) in vivo (102). This led to increased tumor killing, yet it also increased aggressiveness of the disease. The mechanism for ferroptotic killing of tumor cells by neutrophils involved intercellular transfer of myeloperoxidase encased in granules to the tumor cells. Reciprocally, overexpression of GPX4 or silencing of ACSL4 in the tumors suppressed ferroptosis and diminished disease aggressiveness (102).
Similar roles have been reported for other immune cells in the TEM. For example, in a study of carcinogen-induced intestinal tumor progression in mice, deletion of Gpx4 in myeloid cells induced the accumulation of ROS and promoted carcinogen-induced intestinal tumor invasion (103). The authors (103) observed increased recruitment of macrophages to the tumor, which released and elevated levels of peroxide in the TME. This resulted in global DNA mutation events in the intestinal cells that promoted tumor progression (103). These findings illustrate how ferroptosis in immune cells can function to promote tumor initiation and disease progression.
Turning our attention to the malignant cells from a pancreatic tumorigenesis model, ferroptosis induction was found to promote tumor formation and progression. The authors (104) employed an autochthonous model of pancreatic tumor initiation in which the KrasG12D oncogene is expressed in a pancreas-specific manner. Combination with Gpx4 deletion or the administration of high-iron diet accelerated pancreatic tumorigenesis, as characterized by an elevated stromal response, increased tumor invasion and metastasis, and decreased survival (104). Mechanistically, both Gpx4 deletion and high-iron diets were found to promote the release of the oxidized nucleoside base, 8-hyroxyguanosine (8-OHG), which subsequently activated the stimulator of interferon genes (STING) pathway. The STING pathway is an innate immune inflammatory response that senses cytosolic DNA leading to a type I interferon-mediated inflammatory response (105). In this Kras-driven pancreatic cancer tumorigenesis model, the STING response heightened the infiltration and polarization of macrophages into a protumor phenotype. The protumorigenic phenotypes and decreased survival were rescued by the ferroptosis inhibitor, liproxstatin-1, depleting macrophages, or inhibiting the STING pathway (Fig. 5). In a different study using a pancreatic cancer disease model (106), it was found that ferroptosis promoted autophagy-dependent exosomal release of KRASG12D protein from pancreatic cancer cells. The extracellular KRASG12D was bound by and taken up by AGER/RAGE receptors on macrophages and resulted in the tumorigenic polarization of macrophages and promoted pancreatic tumor growth (106).
Collectively, these emerging data on the role of ferroptosis in the TME suggest a dual effect, where ferroptosis is either tumor-promoting or tumor-suppressive, in a cell-type, model, disease state, and cancer-type-dependent manner. Therefore, in order to translate ferroptosis-regulating agents as cancer therapies, there is a need for more rigorous and systematic investigation of ferroptosis that considers the full spectrum of the TME landscape. This will assist in determining conditions under which ferroptosis promotes versus inhibits tumor growth in vivo. Furthermore, the evidence of a duality of ferroptosis in the TME speaks to the importance of compartmentalizing the induction of ferroptosis to specific cell types.
Emerging opportunities for targeting ferroptosis in cancer therapy
Employing ferroptosis as a treatment strategy has been hampered by the limited methods to induce and monitor ferroptosis in vivo. These drawbacks include the lack of potent, specific drugs with suitable pharmacokinetic and pharmacodynamic profiles, and challenges in defining specific biomarkers for assessing tumor ferroptosis, relative to other forms of tumor cell death, in vivo. These shortcomings notwithstanding, several experimental approaches have been developed to test the therapeutic potential of ferroptosis in tumors.
First, genetic strategies have been employed to circumvent the paucity of in vivo ready drugs. For example, deletion of the gene encoding xCT has been reported to inhibit tumor growth in both pancreatic cancer xenograft models (107) and established tumors in a Kras and p53-driven transgenic mouse model of pancreatic cancer (78). In the latter study, Badgley et al. developed a seven-allele genetically engineered mouse model of pancreatic cancer in which tumors could be initiated using the Flp-Frt recombination system and whole body Slc7a11 deletion could be initiated by tamoxifen administration. These animals were monitored for endogenous tumors by ultrasound and then scheduled for a tamoxifen treatment. Slc7a11 knockout led to inhibition of tumor growth and increased survival in mice (78). Given Slc7a11 deletion is safe (108), and considering it does not compromise antitumor immune response in vivo (109), xCT inhibition is now posed as a promising therapeutic approach for pancreatic cancer.
Second, new generations of pharmacological system xC− inhibitors are being developed with better in vivo properties. For example, the Erastin analog, imidazole ketone erastin (IKE) is a highly effective and metabolically stable inhibitor of system xC−. When applied in a diffuse large B cell lymphoma (DLBCL) tumor xenograft model, IKE induced hallmarks of ferroptosis and suppressed growth (110).
Third, a novel, orthogonal strategy for inducing cysteine depletion was recently repurposed to induce and study ferroptosis in vivo. Cyst(e)inase is an enzyme engineered to breakdown extracellular cysteine and cystine. Cyst(e)inase exhibits good pharmacokinetic and toxicology profile and remarkable efficacy, depleting circulating cyst(e)ine levels by more than 99% (111). The therapeutic efficacy of Cyst(e)inase has recently been evaluated in a number of in vivo tumor models. For example, Cyst(e)inase was shown to induce ferroptosis and suppress tumor growth in human xenograft (112) and endogenous murine tumor models of pancreatic cancer (78), prostate cancer xenografts (111), and breast cancer xenografts (111). Furthermore, it was found that pancreatic cancer cells resistant to Cyst(e)inase treatment upregulated thioredoxin 1 as mechanism of escape. Consequently, treating resistant pancreatic tumor xenografts with auranofin (a thioredoxin reductase inhibitor) sensitized the tumors to Cyst(e)inase treatment and suppressed tumor growth without overt systemic toxicity (112). Interestingly, Cyst(e)inase synergized with radiotherapy to inhibit tumor growth in the B16 melanoma model (113) and also synergized with immune checkpoint therapy (i.e., anti-PDL1) to promote cytotoxic CD8 T cell antitumor immune response in ovarian-tumor-bearing mice (86).
Fourth, beyond evaluating ferroptosis inducers as potential single drug agents in treating cancer, the evidence presented above illustrates that such agents could also serve as effective sensitizers to radiation and immune-based therapies. Although single-agent radiation therapy (RT) has been demonstrated to suppress lung cancer xenografts via ferroptosis (114, 115), this effect can be further enhanced in combination with system xC− inhibitors, IKE, and sulfasalazine, which in combination strongly suppressed tumor growth in sarcoma xenografts, glioma xenografts, and lung cancer xenografts (114, 115, 116). Further, the genetic deletion of SLC7A11 in various preclinical tumor models acts synergistically with immune checkpoint immunotherapeutic agents, such as anti-CTLA4, to suppress tumor growth (109).
In addition to classic ferroptosis-inducing agents, certain conventional chemotherapy drugs have been shown to either sensitize cancer cells to ferroptosis, e.g., Statins (117, 118), or to exert their antitumor effects fully or in part via ferroptosis, e.g., Cisplatin (119). The combination of radiotherapy and immunotherapy has also been shown to induce ferroptosis in a variety of preclinical tumor models (113). The mode of action of this combinatorial strategy was linked to the suppression of xCT levels and consequent limited cysteine uptake in the tumor cells. This cysteine deprivation was engendered by combined effects of immunotherapy-generated IFNγ and radiation therapy-activated ATM pathway. Ultimately, tumors exposed to radiation and immunotherapy underwent extensive lipid peroxidation and ferroptosis resulting in decreased tumor burden. A summary of ferroptosis agents that have been tested in preclinical models is presented in Table 1. Also included is a synopsis of preclinical testing of the combinatorial application of ferroptosis with other chemotherapeutic agents, radiation therapy, or immunotherapy (Table 2).
Table 1.
Ferroptosis inducing agents tested in vivo, their mechanism of action, and clinical status
| Ferroptosis agents | Mechanism | Cancer model | Used in clinic | Ref |
|---|---|---|---|---|
| Artesunate | Lipid peroxidation | Pancreatic cancer | Yes | (128) |
| Buthionine Sulfoximine (BSO) | γ-glutamylcysteine synthetase inhibitor | Clear cell renal cell carcinoma | Yes | (129) |
| Cyst(e)inase | Depletes extracellular cysteine/cystine | Pancreatic, prostate, breast carcinoma | No | (78, 111, 112) |
| Dihydroartemisinin | Degradation of ferritin, lipid ROS | Lung cancer | Yes | (130) |
| Imidazole Ketone Erastin (IKE) | System xC− inhibitor | Diffuse large B cell lymphoma (DLBCL) | No | (110) |
| JQ1 | Elevated iron, lipid ROS | Lung cancer | Yes | (131) |
| Piperazine Erastin (PE) | System xC− inhibitor | Fibrosarcoma | No | (25) |
| Sulfasalazine | System xC− inhibitor | Head and neck cancer | Yes | (126) |
| Sorafenib | System xC− inhibitor | Hepatocellular carcinoma | Yes | (132) |
| Withaferin A | Depletion and inactivation of GPX4 | Neuroblastoma | No | (133) |
| Ionizing Radiation (IR) | ROS, lipid ROS, increased ACSL4 | Lung cancer | Yes | (114, 115) |
| Immune checkpoint blockade: anti-PD-L1, anti-CTLA4 | IFNγ secretion, xCT suppression, lipid ROS | Melanoma, ovarian cancer | Yes | (86) |
Abbreviations: ACSL4, acyl-CoA synthetase long-chain family member 4; CTLA4, cytotoxic T-lymphocyte-associated protein 4; GPX4, glutathione peroxidase 4; GSH, glutathione; IFNγ, interferon gamma, PD-L1, programmed death-ligand 1; ROS, reactive oxygen species.
Table 2.
Ferroptosis sensitizing combinatorial strategies tested in cancer models in vivo
| Ferroptosis combinatorial strategies | Mechanism | Cancer model(s) | Ref | |
|---|---|---|---|---|
| Cisplatin | Erastin | Inhibition of GSH synthesis, induction of ferritinophagy | Ovarian cancer | (134) |
| Sulfasalazine | GSH depletion, lipid ROS accumulation | Head and Neck Cancer | (135) | |
| BSO | GOT1 deletion | GSH depletion | Pancreatic cancer | (47) |
| IKE | Dichloroacetate | System xC− and PDK4 inhibition | Pancreatic cancer | (63) |
| Dihydroartemisinin | GPX4 deletion | Degradation of ferritin, lipid ROS | Lung cancer | (130) |
| Cisplatin | Degradation of GPX4, accumulation of iron | Pancreatic cancer | (136) | |
| Artesunate | GRP78 deletion | Lipid peroxidation | Pancreatic cancer | (128) |
| Brequinar | GPX4 silencing | Lipid peroxidation | Fibrosarcoma | (60) |
| Sulfasalazine | ||||
| Rapamycin | GPX4 deletion | Lipid peroxidation | Pancreatic cancer | (137) |
| Sorafenib | Sigma-1 receptor depletion | Lipid peroxidation, Fe2+ elevation | Hepatocellular carcinoma | (138) |
| Sulfasalazine | Pioglitazone | xCT inhibition and Fe2+ elevation | Head and Neck cancer | (126) |
| Metformin | Fe2+ elevation, lipid ROS | Breast cancer | (139) | |
| Sorafenib | Ferritinophagy, xCT inhibition | Pancreatic cancer | (140) | |
| IFNγ | xCT suppression, lipid ROS | Fibrosarcoma | (86) | |
| Oxyfedrine | Sulfasalazine | GSH depletion, 4-HNE accumulation | Colon cancer | (127) |
| Radiation therapy | IKE, Sorafenib | Lipid peroxidation, decreased GSH | Fibrosarcoma | (116) |
| Sulfasalazine | Decreased GSH | Melanoma, Lung Cancer | (114, 115, 141) | |
| Cyst(e)inase, Anti-CTLA4, Anti- PD-L1 | IFN release, xCT suppression, ATM activation, lipid peroxidation | Melanoma | (113) | |
| X-ray irradiation | Erastin | Decreased GPX4 and GSH | Non-small cell lung cancer (NSCLC) | (142) |
| Immune checkpoint blockade: anti-PD-L1, anti-CTLA4 | Cyst(e)inase | xCT suppression, cysteine depletion, lipid ROS | Melanoma | (86) |
Abbreviations: 4-HNE, 4-Hydroxynonenal; ATM, ataxia-telangiectasia mutated; BSO, buthionine sulfoximine; CTLA4, cytotoxic T-lymphocyte-associated protein 4; GOT1, glutamate oxaloacetate transaminase 1; GPX4, glutathione peroxidase 4; GSH, glutathione; IFNγ, interferon gamma; IKE, imidazole ketone erastin; PD-L1, programmed death-ligand 1; PDK4, pyruvate dehydrogenase kinase 4; ROS, reactive oxygen species.
Lastly, simple and robust methods to definitively identify ferroptosis in vivo, akin to cleaved caspase 3 as a marker for apoptosis, are not currently available. Early studies utilized select pharmacodynamic markers to evaluate the degree of ferroptotic lesions in tumor samples. These markers included the expression of PTGS2 and CHAC1, immunofluorescence, or histological staining of 8-OHdG, MDA adducts, and 4-HNE (25, 78, 110, 120, 121). However, the application of these assays is still limited by the context-dependent utility and the lack of ferroptosis specificity sufficient to distinguish ferroptotic lesions from other cell death programs, oxidative-stress-related damage, or inflammatory conditions. For instance, PTGS2 is not a highly sensitivity pharmacodynamic marker when tumors are treated with low concentration of system xC− inhibitor. Moreover, PTSG2 can be upregulated in other inflammatory conditions; CHAC1 is upregulated in system xC− inhibition but not GPX4 inhibition; and MDA-adducts can be detected under certain conditions of general oxidative stress not related to ferroptosis (25, 110, 120, 121).
More recent studies have employed combinations of orthogonal approaches and additional biomarkers to more definitively call ferroptotic cell death. A comprehensive analysis of ferroptotic tumor lesions was described by Badgley et al., (78) who examined Cyst(e)inase-treated or autochthonous Slc7a11-deleted pancreatic tumors. In this study (78), ferroptosis was classified based on histopathological hallmarks of ferroptosis, such as the accumulation of lipid peroxidation products (4HNE, MDA) and lipid droplet formation with oil red O staining; mitochondrial anomalies and the accumulation of lipid droplet structures by transmission electron microscopy; gene expression changes by RNA sequencing on laser capture micro-dissected tumor sections; and the absence of tumor-specific cleaved caspase 3. This approach, while conclusive, is time- and cost-intensive. In an effort to identify reliable and robust ferroptosis-selective markers without the need for a panoply of orthogonal assays, Feng et al. performed a screen for antibodies from mice immunized with membranes from cells undergoing ferroptosis. This led to the identification of 3F3-FMA and its target antigen, transferrin receptor 1 (TfR1), which called ferroptosis in tissue sections across diverse human and mouse models (122). It is expected that future investigations will take advantage of the combination of ferroptosis markers, including anti-TfR1, 3F3-FMA, anti-MDA, anti-4HNE, to establish the role of ferroptosis in vivo. The paucity of biomarkers calls for more efforts to identify additional ferroptosis-specific biosignatures for use in preclinical and clinical settings.
Conclusion and perspectives
Since the term ferroptosis was coined by the Stockwell lab in 2012 (6), numerous studies detailing genetic, transcriptional, and metabolic mediators of this regulated cell death process have emerged. Ferroptosis has been implicated in various pathologies including renal disease, ischemia/reperfusion injury, neurodegenerative diseases, and cancer (123). Whether there are physiological stimuli of ferroptosis and whether ferroptosis play a role in physiological processes such as in tissue development, immune surveillance, or maintenance of cellular homeostasis is largely unknown—a topic requiring considerable attention. Moreover, much of what we know about ferroptosis in the context of cancer biology and cancer therapy has been delineated with cancer cells in culture, conditions that are largely often considered nonphysiologic. Cancer cells in tumors do not exist in isolation as in culture, and their behaviors, properties, and ferroptosis susceptibility are highly influenced by their in vivo microenvironment. As an example, it was reported that targeting the ferroptosis gene, SLC7A11, in both tumor cells and CAFs, but not in tumors cells alone, decreased tumor growth and metastatic burden in an orthotopic model of human pancreatic ductal adenocarcinoma (PDAC) (124). Indeed, more recent studies are beginning to shed light on the role of non-cell-autonomous regulatory mechanisms, including those from the serum, TME, and immune system. Nevertheless, significant gaps in our knowledge of the impact of ferroptosis in the TME remain with contrasting reports describing a duality of protumor or antitumor effect(s) in a context-dependent manner. Therefore, more rigorous investigations are required to evaluate the impact of tumor ferroptosis on stroma cells such as CAFs, myeloid cells, tumor-associated macrophages, and T lymphocytes, all of which have remained largely underexplored. Such a detailed understanding will be required to harness cell autonomous and non-cell-autonomous ferroptotic mechanisms to design the most effective cancer therapies
Another area of future investigation and of clinical significance is whether metabolic signatures of ferroptosis can find utility as biomarkers and consequently stratify tumor types based on ferroptosis susceptibility. This will be crucial for the application of precision medicine-based approaches that leverage the next generation of ferroptosis agents. In addition, it is pertinent to determine whether the metabolic regulators (or pathways) that predict ferroptosis sensitivity will also provide an adequate therapeutic window. Preclinical studies with Cyst(e)inase (78, 111, 112) and the lack of overt phenotypes in the Slc7a11 null mouse models (108, 125) suggest that therapeutic window could be readily achievable. Finally, single-agent engagement of ferroptosis will undoubtedly lead to therapeutic resistance, as has been seen in preclinical models (78, 126, 127). Thus, achieving ferroptosis in vivo would most likely require combination approaches such as those detailed in Table 2.
Conflict of interest
C. A. L. has received consulting fees from Astellas Pharmaceuticals and Odyssey Therapeutics and is an inventor on patents pertaining to KRAS-regulated metabolic pathways, redox control pathways in cancer, and targeting the GOT1-pathway as a therapeutic approach.
Acknowledgments
We would like to thank Dr Luigi Franchi and members of the Lyssiotis laboratory, including Dr Zeribe Nwosu, Dr Megan Radyk, Hanna Hong, and Samuel Kerk for their feedback.
Author contributions
N. E. M. resources; N. E. M. and C. A. L. writing—original draft.
Funding and additional information
C. A. L. is supported by grants from the NIH/NCI (R37CA237421, R01CA248160, R01CA244931). N. E. M. was supported by grants from the Michael Mosier Defeat DIPG Foundation and ChadTough Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Edited by Alex Toker
References
- 1.Fiaschi T., Chiarugi P. Oxidative stress, tumor microenvironment, and metabolic reprogramming: A diabolic liaison. Int. J. Cell Biol. 2012;2012:762825. doi: 10.1155/2012/762825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 3.Aboelella N.S., Brandle C., Kim T., Ding Z.-C., Zhou G. Oxidative stress in the tumor microenvironment and its relevance to cancer immunotherapy. Cancers (Basel) 2021;13:986. doi: 10.3390/cancers13050986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perillo B., Di Donato M., Pezone A., Di Zazzo E., Giovannelli P., Galasso G., Castoria G., Migliaccio A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020;52:192–203. doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., Morrison B., Stockwell B.R. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao J.Y., Dixon S.J. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 2016;73:2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conrad M., Kagan V.E., Bayir H., Pagnussat G.C., Head B., Traber M.G., Stockwell B.R. Regulation of lipid peroxidation and ferroptosis in diverse species. Genes Dev. 2018;32:602–619. doi: 10.1101/gad.314674.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaschler M.M., Andia A.A., Liu H., Csuka J.M., Hurlocker B., Vaiana C.A., Heindel D.W., Zuckerman D.S., Bos P.H., Reznik E., Ye L.F., Tyurina Y.Y., Lin A.J., Shchepinov M.S., Chan A.Y., et al. FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat. Chem. Biol. 2018;14:507–515. doi: 10.1038/s41589-018-0031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon S.J., Stockwell B.R. The hallmarks of ferroptosis. Annu. Rev. Cancer Biol. 2019;3:35–54. [Google Scholar]
- 11.Hangauer M.J., Viswanathan V.S., Ryan M.J., Bole D., Eaton J.K., Matov A., Galeas J., Dhruv H.D., Berens M.E., Schreiber S.L., McCormick F., McManus M.T. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551:247–250. doi: 10.1038/nature24297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y., Yu C., Luo M., Cen C., Qiu J., Zhang S., Hu K. Ferroptosis in cancer treatment: Another way to Rome. Front. Oncol. 2020;10:571127. doi: 10.3389/fonc.2020.571127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes J.D., Dinkova-Kostova A.T., Tew K.D. Oxidative stress in cancer. Cancer Cell. 2020;38:167–197. doi: 10.1016/j.ccell.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J., Fulda S., Gascón S., Hatzios S.K., Kagan V.E., Noel K., Jiang X., Linkermann A., Murphy M.E., Overholtzer M., et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kagan V.E., Mao G., Qu F., Angeli J.P.F., Doll S., Croix C.S., Dar H.H., Liu B., Tyurin V.A., Ritov V.B., Kapralov A.A., Amoscato A.A., Jiang J., Anthonymuthu T., Mohammadyani D., et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin H., Xu L., Porter N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011;111:5944–5972. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- 18.Gaschler M.M., Hu F., Feng H., Linkermann A., Min W., Stockwell B.R. Determination of the subcellular localization and mechanism of action of ferrostatins in suppressing ferroptosis. ACS Chem. Biol. 2018;13:1013–1020. doi: 10.1021/acschembio.8b00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skouta R., Dixon S.J., Wang J., Dunn D.E., Orman M., Shimada K., Rosenberg P.A., Lo D.C., Weinberg J.M., Linkermann A., Stockwell B.R. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J. Am. Chem. Soc. 2014;136:4551–4556. doi: 10.1021/ja411006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang W.S., Stockwell B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008;15:234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassi M.T., Gasol E., Manzoni M., Pineda M., Riboni M., Martín R., Zorzano A., Borsani G., Palacín M. Identification and characterisation of human xCT that co-expresses, with 4F2 heavy chain, the amino acid transport activity system xc- Pflugers Arch. 2001;442:286–296. doi: 10.1007/s004240100537. [DOI] [PubMed] [Google Scholar]
- 22.Bannai S., Kitamura E. Transport interaction of L-cystine and L-glutamate in human diploid fibroblasts in culture. J. Biol. Chem. 1980;255:2372–2376. [PubMed] [Google Scholar]
- 23.Koppula P., Zhang Y., Zhuang L., Gan B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun. 2018;38:12. doi: 10.1186/s40880-018-0288-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dolma S., Lessnick S.L., Hahn W.C., Stockwell B.R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3:285–296. doi: 10.1016/s1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 25.Yang W.S., SriRamaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S., Cheah J.H., Clemons P.A., Shamji A.F., Clish C.B., Brown L.M., Girotti A.W., Cornish V.W., Schreiber S.L., Stockwell B.R. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang X., Stockwell B.R., Conrad M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021;22:266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim M.J., Yun G.J., Kim S.E. Metabolic regulation of ferroptosis in cancer. Biology (Basel) 2021;10:83. doi: 10.3390/biology10020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doll S., Proneth B., Tyurina Y.Y., Panzilius E., Kobayashi S., Ingold I., Irmler M., Beckers J., Aichler M., Walch A., Prokisch H., Trümbach D., Mao G., Qu F., Bayir H., et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan H., Li X., Zhang X., Kang R., Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem. Biophys. Res. Commun. 2016;478:1338–1343. doi: 10.1016/j.bbrc.2016.08.124. [DOI] [PubMed] [Google Scholar]
- 30.Dixon S.J., Winter G.E., Musavi L.S., Lee E.D., Snijder B., Rebsamen M., Superti-Furga G., Stockwell B.R. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem. Biol. 2015;10:1604–1609. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang W.S., Kim K.J., Gaschler M.M., Patel M., Shchepinov M.S., Stockwell B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E4966–E4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tousignant K.D., Rockstroh A., Poad B.L.J., Talebi A., Young R.S.E., Taherian Fard A., Gupta R., Zang T., Wang C., Lehman M.L., Swinnen J.V., Blanksby S.J., Nelson C.C., Sadowski M.C. Therapy-induced lipid uptake and remodeling underpin ferroptosis hypersensitivity in prostate cancer. Cancer Metab. 2020;8:11. doi: 10.1186/s40170-020-00217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez M.A., Magtanong L., Dixon S.J., Watts J.L. Dietary lipids induce ferroptosis in caenorhabditiselegans and human cancer cells. Dev. Cell. 2020;54:447–454.e4. doi: 10.1016/j.devcel.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magtanong L., Ko P.-J., To M., Cao J.Y., Forcina G.C., Tarangelo A., Ward C.C., Cho K., Patti G.J., Nomura D.K., Olzmann J.A., Dixon S.J. Exogenous monounsaturated fatty acids promote a ferroptosis-resistant cell state. Cell Chem. Biol. 2019;26:420–432.e9. doi: 10.1016/j.chembiol.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bersuker K., Hendricks J.M., Li Z., Magtanong L., Ford B., Tang P.H., Roberts M.A., Tong B., Maimone T.J., Zoncu R., Bassik M.C., Nomura D.K., Dixon S.J., Olzmann J.A. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doll S., Freitas F.P., Shah R., Aldrovandi M., da Silva M.C., Ingold I., Goya Grocin A., Xavier da Silva T.N., Panzilius E., Scheel C.H., Mourão A., Buday K., Sato M., Wanninger J., Vignane T., et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 37.Soula M., Weber R.A., Zilka O., Alwaseem H., La K., Yen F., Molina H., Garcia-Bermudez J., Pratt D.A., Birsoy K. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat. Chem. Biol. 2020;16:1351–1360. doi: 10.1038/s41589-020-0613-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi X., Tarazona P., Brock T.J., Browse J., Feussner I., Watts J.L. A Caenorhabditis elegans model for ether lipid biosynthesis and function. J. Lipid Res. 2016;57:265–275. doi: 10.1194/jlr.M064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engelmann B. Plasmalogens: Targets for oxidants and major lipophilic antioxidants. Biochem. Soc. Trans. 2004;32:147–150. doi: 10.1042/bst0320147. [DOI] [PubMed] [Google Scholar]
- 40.Zou Y., Henry W.S., Ricq E.L., Graham E.T., Phadnis V.V., Maretich P., Paradkar S., Boehnke N., Deik A.A., Reinhardt F., Eaton J.K., Ferguson B., Wang W., Fairman J., Keys H.R., et al. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature. 2020;585:603–608. doi: 10.1038/s41586-020-2732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glick D., Barth S., Macleod K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santana-Codina N., Mancias J.D. The role of NCOA4-mediated ferritinophagy in health and disease. Pharmaceuticals (Basel) 2018;11:114. doi: 10.3390/ph11040114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dowdle W.E., Nyfeler B., Nagel J., Elling R.A., Liu S., Triantafellow E., Menon S., Wang Z., Honda A., Pardee G., Cantwell J., Luu C., Cornella-Taracido I., Harrington E., Fekkes P., et al. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat. Cell Biol. 2014;16:1069–1079. doi: 10.1038/ncb3053. [DOI] [PubMed] [Google Scholar]
- 44.Mancias J.D., Wang X., Gygi S.P., Harper J.W., Kimmelman A.C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509:105–109. doi: 10.1038/nature13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao M., Monian P., Pan Q., Zhang W., Xiang J., Jiang X. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26:1021–1032. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hou W., Xie Y., Song X., Sun X., Lotze M.T., Zeh H.J., III, Kang R., Tang D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kremer D.M., Nelson B.S., Lin L., Yarosz E.L., Halbrook C.J., Kerk S.A., Sajjakulnukit P., Myers A., Thurston G., Hou S.W., Carpenter E.S., Andren A.C., Nwosu Z.C., Cusmano N., Wisner S., et al. GOT1 inhibition promotes pancreatic cancer cell death by ferroptosis. Nat. Commun. 2021;12:4860. doi: 10.1038/s41467-021-24859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dodson M., Castro-Portuguez R., Zhang D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107. doi: 10.1016/j.redox.2019.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kerins M.J., Ooi A. The roles of NRF2 in modulating cellular iron homeostasis. Antioxid. Redox Signal. 2017;29:1756–1773. doi: 10.1089/ars.2017.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun X., Ou Z., Chen R., Niu X., Chen D., Kang R., Tang D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi N., Cho P., Selfors L.M., Kuiken H.J., Kaul R., Fujiwara T., Harris I.S., Zhang T., Gygi S.P., Brugge J.S. 3D culture models with CRISPR screens reveal hyperactive NRF2 as a prerequisite for spheroid formation via regulation of proliferation and ferroptosis. Mol. Cell. 2020;80:828–844.e6. doi: 10.1016/j.molcel.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fan Z., Wirth A.-K., Chen D., Wruck C.J., Rauh M., Buchfelder M., Savaskan N. Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis. 2017;6 doi: 10.1038/oncsis.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu S., Lu H., Bai Y. Nrf2 in cancers: A double-edged sword. Cancer Med. 2019;8:2252–2267. doi: 10.1002/cam4.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roh J.-L., Kim E.H., Jang H., Shin D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017;11:254–262. doi: 10.1016/j.redox.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu W.L., Papagiannakopoulos T. The center cannot hold: NRF2 battles ferroptosis in the 3rd dimension. Mol. Cell. 2020;80:760–761. doi: 10.1016/j.molcel.2020.11.022. [DOI] [PubMed] [Google Scholar]
- 56.Wang H., Liu C., Zhao Y., Gao G. Mitochondria regulation in ferroptosis. Eur. J. Cell Biol. 2020;99:151058. doi: 10.1016/j.ejcb.2019.151058. [DOI] [PubMed] [Google Scholar]
- 57.Gao M., Monian P., Quadri N., Ramasamy R., Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell. 2015;59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao M., Yi J., Zhu J., Minikes A.M., Monian P., Thompson C.B., Jiang X. Role of mitochondria in ferroptosis. Mol. Cell. 2019;73:354–363.e3. doi: 10.1016/j.molcel.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.To T.-L., Cuadros A.M., Shah H., Hung W.H.W., Li Y., Kim S.H., Rubin D.H.F., Boe R.H., Rath S., Eaton J.K., Piccioni F., Goodale A., Kalani Z., Doench J.G., Root D.E., et al. A compendium of genetic modifiers of mitochondrial dysfunction reveals intra-organelle buffering. Cell. 2019;179:1222–1238.e17. doi: 10.1016/j.cell.2019.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mao C., Liu X., Zhang Y., Lei G., Yan Y., Lee H., Koppula P., Wu S., Zhuang L., Fang B., Poyurovsky M.V., Olszewski K., Gan B. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593:586–590. doi: 10.1038/s41586-021-03539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee H., Zandkarimi F., Zhang Y., Meena J.K., Kim J., Zhuang L., Tyagi S., Ma L., Westbrook T.F., Steinberg G.R., Nakada D., Stockwell B.R., Gan B. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 2020;22:225–234. doi: 10.1038/s41556-020-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee H., Zhuang L., Gan B. Energy stress inhibits ferroptosis via AMPK. Mol. Cell. Oncol. 2020;7:1761242. doi: 10.1080/23723556.2020.1761242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song X., Liu J., Kuang F., Chen X., Zeh H.J., Kang R., Kroemer G., Xie Y., Tang D. PDK4 dictates metabolic resistance to ferroptosis by suppressing pyruvate oxidation and fatty acid synthesis. Cell Rep. 2021;34:108767. doi: 10.1016/j.celrep.2021.108767. [DOI] [PubMed] [Google Scholar]
- 64.Wu J., Minikes A.M., Gao M., Bian H., Li Y., Stockwell B.R., Chen Z.-N., Jiang X. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019;572:402–406. doi: 10.1038/s41586-019-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zanconato F., Cordenonsi M., Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hsiao C., Lampe M., Nillasithanukroh S., Han W., Lian X., Palecek S.P. Human pluripotent stem cell culture density modulates YAP signaling. Biotechnol. J. 2016;11:662–675. doi: 10.1002/biot.201500374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao B., Wei X., Li W., Udan R.S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L., Zheng P., Ye K., Chinnaiyan A., Halder G., Lai Z.-C., et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang W.-H., Ding C.-K.C., Sun T., Rupprecht G., Lin C.-C., Hsu D., Chi J.-T. The Hippo pathway effector TAZ regulates ferroptosis in renal cell carcinoma. Cell Rep. 2019;28:2501–2508.e4. doi: 10.1016/j.celrep.2019.07.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Labunskyy V.M., Hatfield D.L., Gladyshev V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014;94:739–777. doi: 10.1152/physrev.00039.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ingold I., Berndt C., Schmitt S., Doll S., Poschmann G., Buday K., Roveri A., Peng X., Porto Freitas F., Seibt T., Mehr L., Aichler M., Walch A., Lamp D., Jastroch M., et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell. 2018;172:409–422.e21. doi: 10.1016/j.cell.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 71.Alim I., Caulfield J.T., Chen Y., Swarup V., Geschwind D.H., Ivanova E., Seravalli J., Ai Y., Sansing L.H., Ste Marie E.J., Hondal R.J., Mukherjee S., Cave J.W., Sagdullaev B.T., Karuppagounder S.S., et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell. 2019;177:1262–1279.e25. doi: 10.1016/j.cell.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 72.Voorde J.V., Ackermann T., Pfetzer N., Sumpton D., Mackay G., Kalna G., Nixon C., Blyth K., Gottlieb E., Tardito S. Improving the metabolic fidelity of cancer models with a physiological cell culture medium. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aau7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolfson R.L., Sabatini D.M. The dawn of the age of amino acid sensors for the mTORC1 pathway. Cell Metab. 2017;26:301–309. doi: 10.1016/j.cmet.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim S.-H., Roszik J., Grimm E.A., Ekmekcioglu S. Impact of l-arginine metabolism on immune response and anticancer immunotherapy. Front. Oncol. 2018;8:67. doi: 10.3389/fonc.2018.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Conlon M., Poltorack C.D., Forcina G.C., Armenta D.A., Mallais M., Perez M.A., Wells A., Kahanu A., Magtanong L., Watts J.L., Pratt D.A., Dixon S.J. A compendium of kinetic modulatory profiles identifies ferroptosis regulators. Nat. Chem. Biol. 2021;17:665–674. doi: 10.1038/s41589-021-00751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Albaugh V.L., Pinzon-Guzman C., Barbul A. Arginine metabolism and cancer. J. Surg. Oncol. 2017;115:273–280. doi: 10.1002/jso.24490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geiger R., Rieckmann J.C., Wolf T., Basso C., Feng Y., Fuhrer T., Kogadeeva M., Picotti P., Meissner F., Mann M., Zamboni N., Sallusto F., Lanzavecchia A. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. 2016;167:829–842.e13. doi: 10.1016/j.cell.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Badgley M.A., Kremer D.M., Maurer H.C., DelGiorno K.E., Lee H.-J., Purohit V., Sagalovskiy I.R., Ma A., Kapilian J., Firl C.E.M., Decker A.R., Sastra S.A., Palermo C.F., Andrade L.R., Sajjakulnukit P., et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368:85–89. doi: 10.1126/science.aaw9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leu J.I.-J., Murphy M.E., George D.L. Mechanistic basis for impaired ferroptosis in cells expressing the African-centric S47 variant of p53. Proc. Natl. Acad. Sci. U. S. A. 2019;116:8390–8396. doi: 10.1073/pnas.1821277116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sullivan M.R., Danai L.V., Lewis C.A., Chan S.H., Gui D.Y., Kunchok T., Dennstedt E.A., Vander Heiden M.G., Muir A. Quantification of microenvironmental metabolites in murine cancers reveals determinants of tumor nutrient availability. Elife. 2019;8 doi: 10.7554/eLife.44235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Srinivasan B., Baratashvili M., van der Zwaag M., Kanon B., Colombelli C., Lambrechts R.A., Schaap O., Nollen E.A., Podgoršek A., Kosec G., Petković H., Hayflick S., Tiranti V., Reijngoud D.-J., Grzeschik N.A., et al. Extracellular 4’-phosphopantetheine is a source for intracellular coenzyme A synthesis. Nat. Chem. Biol. 2015;11:784–792. doi: 10.1038/nchembio.1906. [DOI] [PubMed] [Google Scholar]
- 82.Lyssiotis C.A., Kimmelman A.C. Metabolic interactions in the tumor microenvironment. Trends Cell Biol. 2017;27:863–875. doi: 10.1016/j.tcb.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao Y., Li M., Yao X., Fei Y., Lin Z., Li Z., Cai K., Zhao Y., Luo Z. HCAR1/MCT1 regulates tumor ferroptosis through the lactate-mediated AMPK-SCD1 activity and its therapeutic implications. Cell Rep. 2020;33:108487. doi: 10.1016/j.celrep.2020.108487. [DOI] [PubMed] [Google Scholar]
- 84.Ubellacker J.M., Tasdogan A., Ramesh V., Shen B., Mitchell E.C., Martin-Sandoval M.S., Gu Z., McCormick M.L., Durham A.B., Spitz D.R., Zhao Z., Mathews T.P., Morrison S.J. Lymph protects metastasizing melanoma cells from ferroptosis. Nature. 2020;585:113–118. doi: 10.1038/s41586-020-2623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M., Coussens L.M., Gabrilovich D.I., Ostrand-Rosenberg S., Hedrick C.C., Vonderheide R.H., Pittet M.J., Jain R.K., Zou W., Howcroft T.K., et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang W., Green M., Choi J.E., Gijón M., Kennedy P.D., Johnson J.K., Liao P., Lang X., Kryczek I., Sell A., Xia H., Zhou J., Li G., Li J., Li W., et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma X., Xiao L., Liu L., Ye L., Su P., Bi E., Wang Q., Yang M., Qian J., Yi Q. CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability. Cell Metab. 2021;33:1001–1012.e5. doi: 10.1016/j.cmet.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tang R., Xu J., Zhang B., Liu J., Liang C., Hua J., Meng Q., Yu X., Shi S. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J. Hematol. Oncol. 2020;13:110. doi: 10.1186/s13045-020-00946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Friedmann Angeli J.P., Krysko D.V., Conrad M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer. 2019;19:405–414. doi: 10.1038/s41568-019-0149-1. [DOI] [PubMed] [Google Scholar]
- 90.Hernandez C., Huebener P., Schwabe R.F. Damage-associated molecular patterns in cancer: A double-edged sword. Oncogene. 2016;35:5931–5941. doi: 10.1038/onc.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wen Q., Liu J., Kang R., Zhou B., Tang D. The release and activity of HMGB1 in ferroptosis. Biochem. Biophys. Res. Commun. 2019;510:278–283. doi: 10.1016/j.bbrc.2019.01.090. [DOI] [PubMed] [Google Scholar]
- 92.Miller Y.I., Choi S.-H., Wiesner P., Fang L., Harkewicz R., Hartvigsen K., Boullier A., Gonen A., Diehl C.J., Que X., Montano E., Shaw P.X., Tsimikas S., Binder C.J., Witztum J.L. Oxidation-specific epitopes are danger associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weismann D., Binder C.J. The innate immune response to products of phospholipid peroxidation. Biochim. Biophys. Acta. 2012;1818:2465–2475. doi: 10.1016/j.bbamem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Luo X., Gong H.-B., Gao H.-Y., Wu Y.-P., Sun W.-Y., Li Z.-Q., Wang G., Liu B., Liang L., Kurihara H., Duan W.-J., Li Y.-F., He R.-R. Oxygenated phosphatidylethanolamine navigates phagocytosis of ferroptotic cells by interacting with TLR2. Cell Death Differ. 2021;28:1971–1989. doi: 10.1038/s41418-020-00719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kroemer G., Galluzzi L., Kepp O., Zitvogel L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 96.Efimova I., Catanzaro E., Van der Meeren L., Turubanova V.D., Hammad H., Mishchenko T.A., Vedunova M.V., Fimognari C., Bachert C., Coppieters F., Lefever S., Skirtach A.G., Krysko O., Krysko D.V. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J. Immunother. Cancer. 2020;8 doi: 10.1136/jitc-2020-001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou J., Wang G., Chen Y., Wang H., Hua Y., Cai Z. Immunogenic cell death in cancer therapy: Present and emerging inducers. J. Cell. Mol. Med. 2019;23:4854–4865. doi: 10.1111/jcmm.14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ma X., Bi E., Lu Y., Su P., Huang C., Liu L., Wang Q., Yang M., Kalady M.F., Qian J., Zhang A., Gupte A.A., Hamilton D.J., Zheng C., Yi Q. Cholesterol induces CD8+ T-cell exhaustion in the tumor microenvironment. Cell Metab. 2019;30:143–156.e5. doi: 10.1016/j.cmet.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu S., Chaudhary O., Rodríguez-Morales P., Sun X., Chen D., Zappasodi R., Xu Z., Pinto A.F.M., Williams A., Schulze I., Farsakoglu Y., Varanasi S.K., Low J.S., Tang W., Wang H., et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8+ T cells in tumors. Immunity. 2021;54:1561–1577.e7. doi: 10.1016/j.immuni.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matsushita M., Freigang S., Schneider C., Conrad M., Bornkamm G.W., Kopf M. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 2015;212:555–568. doi: 10.1084/jem.20140857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cao W., Ramakrishnan R., Tuyrin V.A., Veglia F., Condamine T., Amoscato A., Mohammadyani D., Johnson J.J., Zhang L.M., Klein-Seetharaman J., Celis E., Kagan V.E., Gabrilovich D.I. Oxidized lipids block antigen cross-presentation by dendritic cells in cancer oxidized lipids and DCs in cancer. J. Immunol. 2014;192:2920–2931. doi: 10.4049/jimmunol.1302801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yee P.P., Wei Y., Kim S.-Y., Lu T., Chih S.Y., Lawson C., Tang M., Liu Z., Anderson B., Thamburaj K., Young M.M., Aregawi D.G., Glantz M.J., Zacharia B.E., Specht C.S., et al. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat. Commun. 2020;11:5424. doi: 10.1038/s41467-020-19193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Canli Ö., Nicolas A.M., Gupta J., Finkelmeier F., Goncharova O., Pesic M., Neumann T., Horst D., Löwer M., Sahin U., Greten F.R. Myeloid cell-derived reactive oxygen species induce epithelial mutagenesis. Cancer Cell. 2017;32:869–883.e5. doi: 10.1016/j.ccell.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 104.Dai E., Han L., Liu J., Xie Y., Zeh H.J., Kang R., Bai L., Tang D. Ferroptotic damage promotes pancreatic tumorigenesis through a TMEM173/STING-dependent DNA sensor pathway. Nat. Commun. 2020;11:6339. doi: 10.1038/s41467-020-20154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Corrales L., McWhirter S.M., Dubensky T.W., Gajewski T.F. The host STING pathway at the interface of cancer and immunity. J. Clin. Invest. 2016;126:2404–2411. doi: 10.1172/JCI86892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dai E., Han L., Liu J., Xie Y., Kroemer G., Klionsky D.J., Zeh H.J., Kang R., Wang J., Tang D. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16:2069–2083. doi: 10.1080/15548627.2020.1714209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Daher B., Parks S.K., Durivault J., Cormerais Y., Baidarjad H., Tambutte E., Pouysségur J., Vučetić M. Genetic ablation of the cystine transporter xCT in PDAC cells inhibits mTORC1, growth, survival, and tumor formation via nutrient and oxidative stresses. Cancer Res. 2019;79:3877–3890. doi: 10.1158/0008-5472.CAN-18-3855. [DOI] [PubMed] [Google Scholar]
- 108.Sato H., Shiiya A., Kimata M., Maebara K., Tamba M., Sakakura Y., Makino N., Sugiyama F., Yagami K., Moriguchi T., Takahashi S., Bannai S. Redox imbalance in cystine/glutamate transporter-deficient mice. J. Biol. Chem. 2005;280:37423–37429. doi: 10.1074/jbc.M506439200. [DOI] [PubMed] [Google Scholar]
- 109.Arensman M.D., Yang X.S., Leahy D.M., Toral-Barza L., Mileski M., Rosfjord E.C., Wang F., Deng S., Myers J.S., Abraham R.T., Eng C.H. Cystine–glutamate antiporter xCT deficiency suppresses tumor growth while preserving antitumor immunity. Proc. Natl. Acad. Sci. U. S. A. 2019;116:9533–9542. doi: 10.1073/pnas.1814932116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang Y., Tan H., Daniels J.D., Zandkarimi F., Liu H., Brown L.M., Uchida K., O'Connor O.A., Stockwell B.R. Imidazole ketone erastin induces ferroptosis and slows tumor growth in a mouse lymphoma model. Cell Chem. Biol. 2019;26:623–633.e9. doi: 10.1016/j.chembiol.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cramer S.L., Saha A., Liu J., Tadi S., Tiziani S., Yan W., Triplett K., Lamb C., Alters S.E., Rowlinson S., Zhang Y.J., Keating M.J., Huang P., DiGiovanni J., Georgiou G., et al. Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat. Med. 2017;23:120–127. doi: 10.1038/nm.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kshattry S., Saha A., Gries P., Tiziani S., Stone E., Georgiou G., DiGiovanni J. Enzyme-mediated depletion of L-cyst(e)ine synergizes with thioredoxin reductase inhibition for suppression of pancreatic tumor growth. NPJ Precis. Oncol. 2019;3:16. doi: 10.1038/s41698-019-0088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lang X., Green M.D., Wang W., Yu J., Choi J.E., Jiang L., Liao P., Zhou J., Zhang Q., Dow A., Saripalli A.L., Kryczek I., Wei S., Szeliga W., Vatan L., et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 2019;9:1673–1685. doi: 10.1158/2159-8290.CD-19-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lei G., Zhang Y., Hong T., Zhang X., Liu X., Mao C., Yan Y., Koppula P., Cheng W., Sood A.K., Liu J., Gan B. Ferroptosis as a mechanism to mediate p53 function in tumor radiosensitivity. Oncogene. 2021;40:3533–3547. doi: 10.1038/s41388-021-01790-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lei G., Zhang Y., Koppula P., Liu X., Zhang J., Lin S.H., Ajani J.A., Xiao Q., Liao Z., Wang H., Gan B. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020;30:146–162. doi: 10.1038/s41422-019-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ye L.F., Chaudhary K.R., Zandkarimi F., Harken A.D., Kinslow C.J., Upadhyayula P.S., Dovas A., Higgins D.M., Tan H., Zhang Y., Buonanno M., Wang T.J.C., Hei T.K., Bruce J.N., Canoll P.D., et al. Radiation-induced lipid peroxidation triggers ferroptosis and synergizes with ferroptosis inducers. ACS Chem. Biol. 2020;15:469–484. doi: 10.1021/acschembio.9b00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shimada K., Skouta R., Kaplan A., Yang W.S., Hayano M., Dixon S.J., Brown L.M., Valenzuela C.A., Wolpaw A.J., Stockwell B.R. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat. Chem. Biol. 2016;12:497–503. doi: 10.1038/nchembio.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Viswanathan V.S., Ryan M.J., Dhruv H.D., Gill S., Eichhoff O.M., Seashore-Ludlow B., Kaffenberger S.D., Eaton J.K., Shimada K., Aguirre A.J., Viswanathan S.R., Chattopadhyay S., Tamayo P., Yang W.S., Rees M.G., et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547:453–457. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guo J., Xu B., Han Q., Zhou H., Xia Y., Gong C., Dai X., Li Z., Wu G. Ferroptosis: A novel anti-tumor action for cisplatin. Cancer Res. Treat. 2018;50:445–460. doi: 10.4143/crt.2016.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dixon S.J., Patel D.N., Welsch M., Skouta R., Lee E.D., Hayano M., Thomas A.G., Gleason C.E., Tatonetti N.P., Slusher B.S., Stockwell B.R. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3 doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen X., Comish P.B., Tang D., Kang R. Characteristics and biomarkers of ferroptosis. Front. Cell Dev. Biol. 2021;9:637162. doi: 10.3389/fcell.2021.637162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Feng H., Schorpp K., Jin J., Yozwiak C.E., Hoffstrom B.G., Decker A.M., Rajbhandari P., Stokes M.E., Bender H.G., Csuka J.M., Upadhyayula P.S., Canoll P., Uchida K., Soni R.K., Hadian K., et al. Transferrin receptor is a specific ferroptosis marker. Cell Rep. 2020;30:3411–3423.e7. doi: 10.1016/j.celrep.2020.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yan H., Zou T., Tuo Q., Xu S., Li H., Belaidi A.A., Lei P. Ferroptosis: Mechanisms and links with diseases. Signal Transduct. Target. Ther. 2021;6:1–16. doi: 10.1038/s41392-020-00428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sharbeen G., McCarroll J.A., Akerman A., Kopecky C., Youkhana J., Kokkinos J., Holst J., Boyer C., Erkan M., Goldstein D., Timpson P., Cox T.R., Pereira B.A., Chitty J.L., Fey S.K., et al. Cancer-associated fibroblasts in pancreatic ductal adenocarcinoma determine response to SLC7A11 inhibition. Cancer Res. 2021;81:3461–3479. doi: 10.1158/0008-5472.CAN-20-2496. [DOI] [PubMed] [Google Scholar]
- 125.McCullagh E.A., Featherstone D.E. Behavioral characterization of system xc- mutant mice. Behav. Brain Res. 2014;265:1–11. doi: 10.1016/j.bbr.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 126.Kim E.H., Shin D., Lee J., Jung A.R., Roh J.-L. CISD2 inhibition overcomes resistance to sulfasalazine-induced ferroptotic cell death in head and neck cancer. Cancer Lett. 2018;432:180–190. doi: 10.1016/j.canlet.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 127.Otsuki Y., Yamasaki J., Suina K., Okazaki S., Koike N., Saya H., Nagano O. Vasodilator oxyfedrine inhibits aldehyde metabolism and thereby sensitizes cancer cells to xCT-targeted therapy. Cancer Sci. 2020;111:127–136. doi: 10.1111/cas.14224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang K., Zhang Z., Wang M., Cao X., Qi J., Wang D., Gong A., Zhu H. Role of GRP78 inhibiting artesunate-induced ferroptosis in KRAS mutant pancreatic cancer cells. Drug Des. Devel. Ther. 2019;13:2135–2144. doi: 10.2147/DDDT.S199459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Miess H., Dankworth B., Gouw A.M., Rosenfeldt M., Schmitz W., Jiang M., Saunders B., Howell M., Downward J., Felsher D.W., Peck B., Schulze A. The glutathione redox system is essential to prevent ferroptosis caused by impaired lipid metabolism in clear cell renal cell carcinoma. Oncogene. 2018;37:5435–5450. doi: 10.1038/s41388-018-0315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen G.-Q., Benthani F.A., Wu J., Liang D., Bian Z.-X., Jiang X. Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death Differ. 2020;27:242–254. doi: 10.1038/s41418-019-0352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sui S., Zhang J., Xu S., Wang Q., Wang P., Pang D. Ferritinophagy is required for the induction of ferroptosis by the bromodomain protein BRD4 inhibitor (+)-JQ1 in cancer cells. Cell Death Dis. 2019;10:331. doi: 10.1038/s41419-019-1564-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Louandre C., Marcq I., Bouhlal H., Lachaier E., Godin C., Saidak Z., François C., Chatelain D., Debuysscher V., Barbare J.-C., Chauffert B., Galmiche A. The retinoblastoma (Rb) protein regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma cells. Cancer Lett. 2015;356:971–977. doi: 10.1016/j.canlet.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 133.Hassannia B., Wiernicki B., Ingold I., Qu F., Van Herck S., Tyurina Y.Y., Bayır H., Abhari B.A., Angeli J.P.F., Choi S.M., Meul E., Heyninck K., Declerck K., Chirumamilla C.S., Lahtela-Kakkonen M., et al. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J. Clin. Invest. 2018;128:3341–3355. doi: 10.1172/JCI99032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cheng Q., Bao L., Li M., Chang K., Yi X. Erastin synergizes with cisplatin via ferroptosis to inhibit ovarian cancer growth in vitro and in vivo. J. Obstet. Gynaecol. Res. 2021;47:2481–2491. doi: 10.1111/jog.14779. [DOI] [PubMed] [Google Scholar]
- 135.Roh J.-L., Kim E.H., Jang H.J., Park J.Y., Shin D. Induction of ferroptotic cell death for overcoming cisplatin resistance of head and neck cancer. Cancer Lett. 2016;381:96–103. doi: 10.1016/j.canlet.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 136.Du J., Wang X., Li Y., Ren X., Zhou Y., Hu W., Zhou C., Jing Q., Yang C., Wang L., Li H., Fang L., Zhou Y., Tong X., Wang Y. DHA exhibits synergistic therapeutic efficacy with cisplatin to induce ferroptosis in pancreatic ductal adenocarcinoma via modulation of iron metabolism. Cell Death Dis. 2021;12:705. doi: 10.1038/s41419-021-03996-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu Y., Wang Y., Liu J., Kang R., Tang D. Interplay between MTOR and GPX4 signaling modulates autophagy-dependent ferroptotic cancer cell death. Cancer Gene Ther. 2021;28:55–63. doi: 10.1038/s41417-020-0182-y. [DOI] [PubMed] [Google Scholar]
- 138.Bai T., Lei P., Zhou H., Liang R., Zhu R., Wang W., Zhou L., Sun Y. Sigma-1 receptor protects against ferroptosis in hepatocellular carcinoma cells. J. Cell. Mol. Med. 2019;23:7349–7359. doi: 10.1111/jcmm.14594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang J., Zhou Y., Xie S., Wang J., Li Z., Chen L., Mao M., Chen C., Huang A., Chen Y., Zhang X., Khan N.U.H., Wang L., Zhou J. Metformin induces ferroptosis by inhibiting UFMylation of SLC7A11 in breast cancer. J. Exp. Clin. Cancer Res. 2021;40:206. doi: 10.1186/s13046-021-02012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang K., Zhang Z., Tsai H.-I., Liu Y., Gao J., Wang M., Song L., Cao X., Xu Z., Chen H., Gong A., Wang D., Cheng F., Zhu H. Branched-chain amino acid aminotransferase 2 regulates ferroptotic cell death in cancer cells. Cell Death Differ. 2021;28:1222–1236. doi: 10.1038/s41418-020-00644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nagane M., Kanai E., Shibata Y., Shimizu T., Yoshioka C., Maruo T., Yamashita T. Sulfasalazine, an inhibitor of the cystine-glutamate antiporter, reduces DNA damage repair and enhances radiosensitivity in murine B16F10 melanoma. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shibata Y., Yasui H., Higashikawa K., Miyamoto N., Kuge Y. Erastin, a ferroptosis-inducing agent, sensitized cancer cells to X-ray irradiation via glutathione starvation in vitro and in vivo. PLoS One. 2019;14 doi: 10.1371/journal.pone.0225931. [DOI] [PMC free article] [PubMed] [Google Scholar]