Abstract
Aim
To describe the presenting features, bone characteristics and molecular genetics in a large monocentric cohort of children and young adults with idiopathic primary osteoporosis.
Methods
Sixty-six patients (19 children, 47 adults; 28 males, 38 females; age at referral: 3.8 to 65 years) diagnosed with primary osteoporosis were included in this study; patients with features of osteogenesis imperfecta or other known syndromes associated with osteoporosis were excluded. For each patient, the following data were collected by retrospective chart review: family and personal history of fracture and osteoporosis, mineral homeostasis parameters and markers of bone formation and resorption, bone mineral density (BMD) of the lumbar spine (LS-BMD), the total body less head (TB-BMD), and total hip levels (TH-BMD) measured by DXA. As part of the initial assessment process, a bone fragility gene panel sequencing was performed in all of these patients.
Results
There was a higher predominance of males in the children (63%) and of females in the adults (66%) (p = 0.030). Compared to the adults, the children had a significantly lower frequency of vertebral fractures (26 vs 57%, p = 0.022) and a higher frequency of peripheral fractures (84 vs 53%; p = 0.019). Bone fragility gene panel sequencing allowed the identification of the heterozygous pathogenic variant in 27% of patients (most frequently in LRP5, WNT1 and COL1A1 or 2 genes) and the heterozygous p.(Val667Met) LRP5 variant in 11% of them. The frequency of pathogenic variants tended to be higher in the children compared to the adults without reaching statistical significance (42 vs 19%; p = 0.053). The frequency of the p.(Val667Met) LRP5 variant was similar in children and adults. No significant differences were found regarding the various clinical, biological and radiological characteristics of the patients according to genotype.
Conclusion
In this study, we reported the presenting features and bone characteristics in a large cohort of children and young adults with idiopathic primary osteoporosis. Bone fragility gene panel sequencing allowed the identification of genetic variants in a significant proportion of these patients. Molecular diagnosis in these patients is important in order to be able to offer genetic counselling and organise patient management.
Keywords: Bone mineral density, DXA, Early-onset osteoporosis, Idiopathic juvenile osteoporosis, Primary osteoporosis
1. Introduction
Osteoporosis is a systemic bone disorder characterised by low bone mass and/or impaired bone tissue microarchitecture that results in reduced bone strength and increased risk of fragility fractures. The high morbidity and mortality associated with fragility fractures - especially in the elderly population - make osteoporosis a major public health concern (NIH consensus development panel on osteoporosis prevention, diagnosis, and therapy, March 7-29, 2001). Although osteoporosis mainly affects the elderly population, the disease is increasingly recognised in children and young adults.
In elderly people, the risk of fracture is correlated with the bone mineral density (BMD) values measured by dual-energy X-ray absorptiometry (DXA), with a 2-fold increase in fracture risk for every one standard deviation decrease in BMD (Marshall et al., 1996). Consequently, the World Health Organization defines adult osteoporosis in postmenopausal women and men over the age of 50 years as the presence of low BMD values on bone densitometry (BMD T-score < −2.5 at the lumbar spine or hip) (3). Since more than half of fragility fractures occur in patients with a BMD T-score > −2.5 (Siris et al., 2004; Wainwright et al., 2005), a clinical diagnosis of osteoporosis can also be considered in adult patients with a fragility fracture in the spine or hip, independently of BMD results (Siris et al., 2014). In children and adolescents, the predictive value of isolated low BMD values for the risk of fractures is uncertain and a “fracture threshold” for BMD has not been established (Clark et al., 2006). In addition, fragility fractures can also occur in patients with BMD values within the normal range for age. Consequently, the definition of paediatric osteoporosis is primarily based on pathological symptoms: presence of one or more vertebral fractures occurring without significant trauma regardless of BMD values or history of clinically significant long bone fractures, plus a low BMD for age (standard deviation for age, Z-score, <−2) (Baim et al., 2008). In contrast to children/adolescents and postmenopausal/elderly patients, the diagnosis of osteoporosis in young adults between 20 and 50 years of age remains poorly defined.
The causes of osteoporosis are complex and involve multiple interactions between genetic variations and environmental factors. Twin studies have suggested that genetic factors account for up to 80% of the variation in bone mass among individuals (Pocock et al., 1987). Although more than 220 genetic loci have been linked to osteoporosis, most of them present a small genetic effect size, explaining less than 20% of bone mass variations (Yuan et al., 2019). However, several monogenic forms of primary osteoporosis have been described in children and young adults (Kämpe et al., 2015). The most prevalent form of primary osteoporosis is osteogenesis imperfecta (OI), mainly caused by the pathogenic variant in the collagen type 1 alpha 1 or 2 (COL1A1 or 2) genes. Although the OI phenotype varies in severity, most patients display a variety of extra-skeletal features such as blue sclerae, dentinogenesis imperfecta, joint hypermobility and hearing impairments. Other rare syndromic conditions (such as Bruck syndrome, osteoporosis-pseudoglioma syndrome, Ehlers-Danlos syndrome, Marfan syndrome and homocystinuria) are also associated with primary osteoporosis. A small proportion of children and young adults present with apparently isolated primary bone fragility; this clinical entity was formerly referred to as “idiopathic juvenile osteoporosis (IJO)” in children (Dent, 1977; Lorenc, 2002) and “early-onset osteoporosis (EOO)” in young adults. Recent genetic studies have shown that some of these children and young adults with IJO and EOO harbour pathogenic variants in bone-related genes such as the low-density lipoprotein receptor-related protein 5 or 6 (LRP5 or LRP6) (Butscheidt et al., 2018; Hartikka et al., 2005; Korvala et al., 2012; Stürznickel et al., 2021; Fahiminiya et al., 2013), WNT family member 1 (WNT1) (Laine et al., 2013; Mäkitie et al., 2016; Keupp et al., 2013) or plastin 3 (PLS3) (Laine et al., 2015) genes. Apart from the pathogenic variants of the LRP5 gene, two variants of this gene, p.Val667Met (c.2047G > A, population frequency 0% to 5.3%) and p.(Ala1330Val) (c.4037C > T, population frequency 4.7% to 22%), have been associated with low peak bone mass (Koller et al., 2005; Saarinen et al., 2007), decreased BMD and fragility fractures in adults (Koller et al., 2005; Ferrari et al., 2005; Kiel et al., 2007; van Meurs et al., 2008; Koay et al., 2004; Richards et al., 2008; van Meurs et al., 2006). Even in the absence of extra-skeletal features of OI, some patients may harbour a mutation in the COL1A1 or 2 genes (Butscheidt et al., 2018; Rolvien et al., 2018).
The aim of the present study was to describe the presenting features, bone characteristics and molecular genetics in a large monocentric cohort of children and young adults with idiopathic primary osteoporosis.
2. Subjects and methods
2.1. Patient population
This retrospective cross-sectional study comprised all patients, children and adults, reffered for idiopathic primary osteoporosis between 2014 and 2020 at the regional reference centre for rare bone diseases at Toulouse University Hospital. These patients were referred by primary or secondary health care physicians to complete the investigation, notably gene panel sequencing that can only be performed by a tertiary health care centre (i.e. University Hospital) in France.
In children (<18 years old), osteoporosis was defined by clinically significant long bone fractures (≥2 long bone fractures before age 10 and ≥3 fractures before age 19) and BMD Z-score < −2 or ≥1 vertebral fracture without significant trauma regardless of the BMD values. In adults (<55 years old and menopause for less than 5 years in females), osteoporosis was defined by BMD T-score < −2.5 whether or not associated with fractures or ≥1 clinically significant fracture. Clinically significant fractures are defined by their mechanism (fractures secondary to mild to moderate trauma) and their location (long bones or vertebrae, excluding fractures of the nose, fingers and toes). Only index cases were included.
Patients with clinical features of OI (such as blue sclerae, dentinogenesis imperfecta, joint hypermobility and hearing impairments) or other known syndromes associated with primary osteoporosis were excluded. Similarly, patients with osteoporosis caused by associated diseases (such as malignant diseases, chronic inflammatory diseases, malnutrition or hormonal deficiencies,) and patients taking medication that interferes with bone metabolism - such as long-term corticosteroid therapy - were also excluded.
Finally, the cohort reported in this study included 66 patients (19 children, 47 adults).
According to the French law on ethics, the patients were informed that their codified data was to be used for the study. According to the French ethical and regulatory law (public health code), retrospective studies based on the exploitation of routine care data do not have to be submitted at an ethics committee, but they must be declared or covered by the reference methodology of the French National Commission for Informatics and Liberties (CNIL). The personal and medical data were collected and computer-processed to analyse the results of the research. Toulouse University Hospital signed a commitment of compliance to the reference methodology MR-004 of the French National Commission for Informatics and Liberties (CNIL). After evaluation and validation by the data protection officer and in compliance with the General Data Protection Regulation (Regulation 2016/679 of the European Parliament and of the Council of 27 April 2016), this study was deemed to fulfil all the criteria and was registered in the retrospective study register of the Toulouse University Hospital (registration number: RnIPH 2021-123) and covered by the MR-004 (CNIL number: 2206723 v 0).
2.2. Personal history and clinical evaluation
For each patient, the following data were collected by retrospective chart review: family history of fracture and osteoporosis, fracture history (age at first fracture, number and site), personal history, mineral homeostasis and bone marker levels before any treatment, bone parameters evaluated by DXA and genetic analysis.
Vertebral fractures were defined as >20% vertebral height loss involving the anterior, posterior, and/or middle vertebral body.
French reference data were used to convert height, weight and BMI measurements to age and gender-specific Z-scores.
2.3. Biochemical measurements
Fasting blood samples were obtained as part of the patients' clinical follow-up. Total serum calcium, phosphate and alkaline phosphatase were measured using colorimetric methods. Serum concentrations of 25-hydroxy vitamin D (25-OHD) and serum carboxy-terminal collagen crosslinks (CTX) were measured using automated chemiluminescence immunoassay systems (Cobas 8000 modular analyser series, Roche Diagnostics; iSYS, Immunodiagnostic Systems). The Quidel enzyme immunoassay (San Diego, CA, USA) was used to determine bone alkaline phosphatase (BAP) levels. BAP and CTX serum concentrations were converted to age- and gender-specific Z-scores based on published reference data (Koay et al., 2004). The following cut-off points were used for the serum 25-OHD levels: sufficiency above 75 nmol/L (>30 ng/mL), insufficiency between 50 and 75 nmol/L (20–30 ng/mL) and deficiency below 50 nmol/L (<20 ng/mL).
2.4. Dual-energy X-ray absorptiometry
Bone mineral density (BMD) of the lumbar spine (LS-BMD), the total body less head (TB-BMD), and total hip levels (TH-BMD) were measured via DXA using a Lunar Prodigy device (GE Healthcare). The BMD results were converted to age-specific Z-scores using data provided by the densitometer manufacturer and published reference data (Fan et al., 2014).
2.5. Genetic analysis
As part of the initial assessment process in our regional reference centre, a bone fragility gene panel sequencing was performed in all patients with idiopathic primary osteoporosis.
All molecular analyses were performed as described in a previous publication (Collet et al., 2017). Briefly, after DNA extraction from peripheral leukocytes, all DNA was screened using a targeted gene sequencing panel for the genes reported in primary osteoporosis: ALPL (NM_000478), COL1A1 (NM_000088), COL1A2 (NM_000089), LRP5 (NM_002335), LRP6, PLS3 (NM_005023), TNFRSF11A (NM_001270949), TNFRSF11B (NM_002546), WNT1 (NM_005430), WNT3A (NM_033131), IFITM5 (NM_001025295), The NGS technology was based on the surelect QXT kit (Agilent, Les Ulis, France) for library preparation and the hybrid capture system for sequencing on a Miseq sequencer (Illumina, Paris, France). Fastq files were generated by using Miseqreporter (Illumina) and then aligned with SeqNext (JSI Medical Systems, Ettenheim, Germany) based on BWA aligner and GATK HaplotypeCaller (Broad Institute (GATK 3.8.1)). The Copy Number Variations (CNV) were also determined using this software and a multiplex ligation-dependent probe amplification (MLPA) reagent for LRP5, COL1A1 and COL1A2 on the ABI3130 sequencer with Coffalyser software (MRC, Amsterdam, Netherlands). Variant pathogenicity was evaluated using the prediction software Polyphen (http://genetics.bwh.harvard.edu/pph2), CADD (https://cadd.gs.washington.edu) Mutation taster, and SIFT (http://sift.bii.a-star.edu.sg). The known pathogenic mutations were searched for in the professional Human Gene Mutation database (HGMD-pro) or Leiden Open Variation Database (LOVD). All novel variants will be reported in the ClinVar database. Identified variants were classified according to the American College of Medical Genetics (ACMG) classification: classes 1 and 2 of the ACMG were considered as non-pathogenics, class 3 as a variant of unknown significance (VUS) and classes 4 and 5 as pathogenics. If a patient had two variants, the most pathogenic was taken into account when classifying the mutations.
2.6. Statistical analyses
The raw results were transformed into age- and gender-specific Z-scores from the average result in the reference population using the published reference data cited above in the description of the measurement techniques. The quantitative variables are expressed in medians (range) and the qualitative variables in percentages.
Patients were stratified into a number of different subgroups according to age (child and adult groups) and genotype (absent or non-pathogenic variant, pathogenic variant, variant of unknown significance, LRP5 p.(Val667Met) variant) for the statistical analysis.
The differences between the groups were tested for significance using the Mann-Whitney's U test for pairwise group comparisons and the Kruskal-Wallis test for comparisons between more than two groups. The group differences in the dichotomous variables were tested for significance using the chi square test. The associations are given as Pearson correlations or Spearman rank correlations, as appropriate.
All tests were two-tailed and throughout the study p < 0.05 was considered significant. These calculations were performed using SPSS software, version 11.5 for Windows (SPSS Inc., Chicago, IL, USA).
3. Results
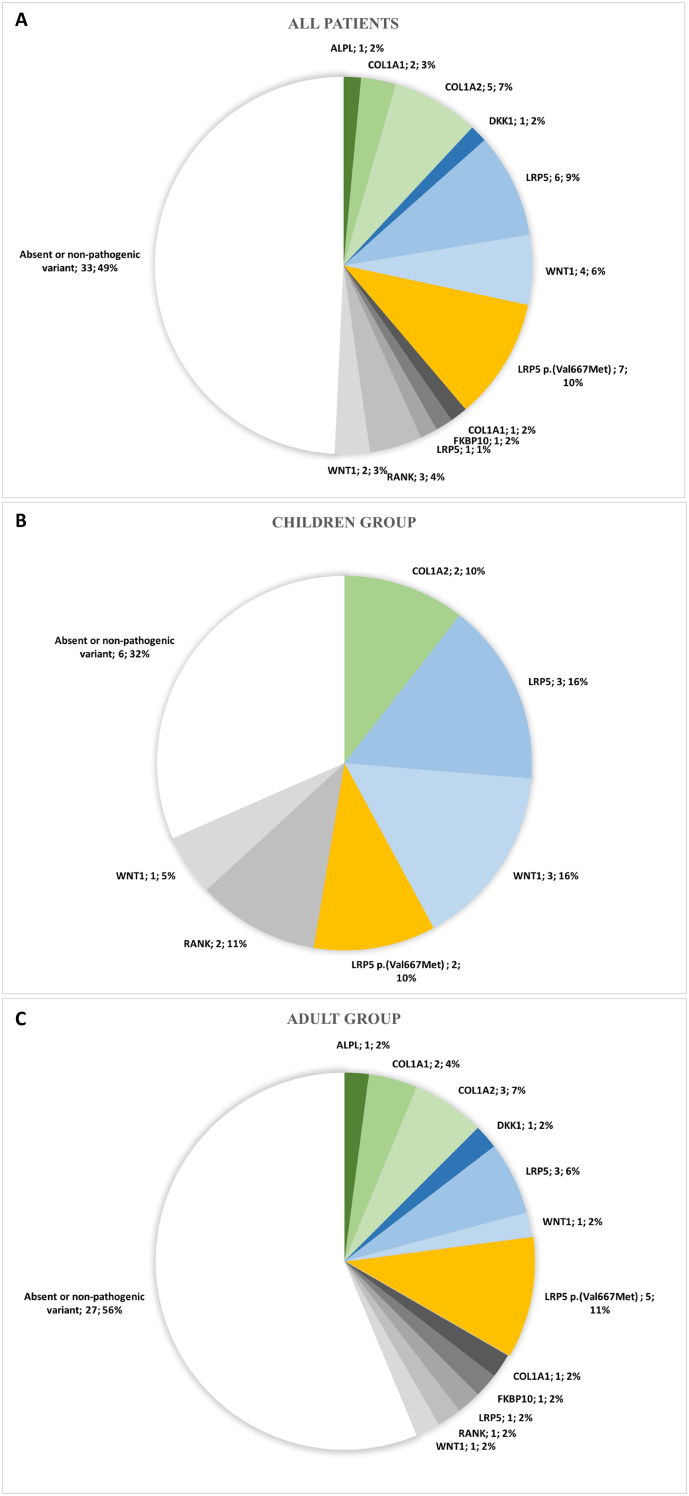
No significant differences were found between genders for the clinical, biochemical and radiological bone parameters; thus, the results recorded for males and females were analysed as a single group. The clinical, biological and radiological characteristics of patients according to age and genotype are reported in Table 1. The distribution of gene variants is given in Fig. 1.
Table 1.
Clinical, biological and radiological characteristics of patients according to age group and genotype.
| Children | Adults | P-value | Absent or non-pathogenic variants | Variants of unknown significance | Pathogenic variants | LRP5 p.(Val667Met) | P-value | |
|---|---|---|---|---|---|---|---|---|
| Number | 19 | 47 | – | 33 | 8 | 18 | 7 | – |
| Gender ratio (M/F) | 1.7 (12/7) | 0.5 (16/31) | 0.030 | 0.8 (15/18) | 0.6 (3/5) | 0.8 (8/10) | 0.4 (2/5) | 0.853 |
| Family history of osteoporosis (n (%)) | 5 (26) | 16 (34) | 0.542 | 8 (24) | 2 (25) | 8 (44) | 3 (43) | 0.430 |
| Age at first fracture (years) | 7.0 (2.0; 15.0) | 33.0 (3.0; 60.0) | <0.0001 | 25.5 (3.0; 60.0) | 15.0 (2.0; 52.0) | 10.0 (2.0; 52.0) | 23.5 (7.0; 54.0) | 0.728 |
| Age at referral (years) | 10.7 (3.8; 15.9) | 46.0 (20.0; 65.0) | <0.0001 | 40.0 (3.8; 60.0) | 30.5 (9.8; 52.0) | 28.0 (5.9; 65.0) | 35.0 (10.7; 54.0) | 0.566 |
| Height (Z-score) | 0.4 (−2.2; 2.5) | 0.5 (−2.7; 2.6) | 0.791 | 0.9 (−2.1; 2.3) | 0.3 (−1.7; 1.2) | −0.7 (−2.7; 2.5) | −0.3 (−1.3; 1.8) | 0.224 |
| BMI (Z-score) | −0.4 (−1.9; 3.5) | 0.6 (−2.4; 2.3) | 0.055 | 0.5 (−2.4; 2.1) | 1.2 (−1.3; 2.1) | 0.3 (−1.9; 3.5) | −0.6 (−2.2; 2.3) | 0.282 |
| Vertebral fractures (n (%)) | 5 (26) | 27 (57) | 0.022 | 17 (52) | 1 (13) | 11 (61) | 3 (43) | 0.138 |
| Peripheral fractures (n (%)) | 16 (84) | 25 (53) | 0.019 | 19 (58) | 6 (75) | 10 (56) | 6 (86) | 0.417 |
| LS-BMD (Z-score) | −2.1 (−3.7; −0.7) | −2.7 (−4.7; −1.0) | 0.012 | −2.4 (−4.7; −1.4) | −2.4 (−4.5; −2.0) | −2.2 (−3.7; −0.7) | −2.8 (−3.6; −2.4) | 0.449 |
| LS-BMD (T-score) | – | −3.3 (−4.9; 1.2) | – | −3.0 (−4.7; 1.2) | −3.4 (−4.7; −2.1) | −3.4 (−4.9; −2.3) | −2.7 (−4.3; −2.0) | 0.432 |
| TB-BMD (Z-score) | −1.5 (−3.0; 0.4) | – | – | −1.0 (−1.3; −0.3) | −2.0 (−2.5; −0.8) | −2.2 (−3.0; 0.4) | −1.6 (−1.6; −1.6) | 0.511 |
| TH-BMD (Z-score) | – | −1.6 (−3.5; 0.7) | – | −1.6 (−3.5; −0.1) | −2.1 (−2.1; −2.1) | −0.9 (−2.4; 0.7) | −0.9 (−0.9; −0.9) | 0.692 |
| TH-BMD (T-score) | – | −2.0 (−4.2; 1.6) | – | −1.9 (−4.2; 1.6) | −1.9 (−2.3; −1.8) | −2.5 (−3.1; −1.0) | −1.9 (−2.5; −0.5) | 0.502 |
| BAP (Z-score) | −0.7 (−2.7; 1.9) | 0.0 (−2.9; 1.5) | 0.760 | 0.0 (−2.7; 1.9) | −1.1 (−2.9; 0.0) | −0.0 (−1.6; 1.5) | −0.1 (−1.0; 1.2) | 0.193 |
| CTX (Z-score) | 0.4 (−1.3; 3.3) | −0.2 (−1.2; 6.2) | 0.053 | −0.3 (−1.2; 3.3) | 0.1 (−1.1; 1.3) | 0.3 (−1.3; 6.2) | 0.8 (−1.1; 1.7) | 0.280 |
| No or non-pathogenic variants (n (%)) | 6 (32) | 27 (56) | 0.057 | – | – | – | – | – |
| VUS (n (%)) | 3 (16) | 5 (10) | 0.562 | – | – | – | – | – |
| Pathogenic variant (n (%)) | 8 (42) | 10 (19) | 0.053 | – | – | – | – | – |
| LRP5 p.Val667Met (n (%)) | 2 (11) | 5 (15) | 0.801 | – | – | – | – | – |
If a patient had two mutations, the most pathogenic was taken into account when classifying the mutations.
Values are expressed as median (min; max) or number (%). Differences between the groups were tested for significance using the Mann-Whitney's U test for pairwise group comparisons and the Kruskal-Wallis test for comparisons between more than two groups. Group differences in dichotomous variables were tested for significance using the chi square test.
BMI: bone mass index; BMD: bone mineral density; BAP: bone alkaline phosphatase; CTX: carboxy-terminal collagen crosslinks; BMD: bone mineral density; LS-BMD: lumbar spine BMD; TB-BMD: total body BMD; TH-BMD: total hip BMD; VUS: variant of unknown significance.
Fig. 1.
Distribution of gene variants in all patients (A) children (B) and adults (C) with idiopathic primary osteoporosis
Absence or presence of non-pathogenic variants are represented in white, variant of unknown significance (VUS) in grey, p.(Val667Met) LRP5 variant in orange and pathogenic variants in blue/green. If a patient had two variants, the most pathogenic was taken into account when classifying the mutations.
3.1. Children group
Nineteen children and adolescents were included in the study, with a greater proportion of males (12 males, 63%). The median age at referral for suspicion of bone fragility was 10.7 years (range: 3.8 to 15.9 years), about three years after the first fractures (median age: 7.0 years; range: 2.0 to 15.0 years). In all but one child (95%), the diagnosis of bone fragility was suspected because of multiple long bones or vertebral fractures; only one child was referred for investigation of excessive bone transparency on X-rays but had previously displayed 2 tibia fractures without significant trauma. Most children (16 children, 84%) displayed long bones fractures with a median number of three fractures per child (range: 2 to 8 fractures). Five children (26%) had vertebral fractures, which were isolated in three of them. All but one child was of normal height (defined as height Z-score above −2). All the children were normally active. A positive family history of low trauma fractures was found in 5 children (26%).
The BMD Z-score values at the lumbar spine were significantly lower than those of the total body (−2.1 vs − 1.5; p = 0.003).
The mineral homeostasis parameters (i.e. total serum calcium, phosphate and alkaline phosphatase) were in the normal range for all the children. Six children (32%) had a low urinary calcium/creatinine ratio (defined as a ratio < 0.20 mmol/mmol). Despite a median serum 25-OHD concentration of 62 nmol/L falling within the normal range, 4 patients (21%) presented 25-OHD deficiency (<50 nmol/L) and 8 patients (42%) presented 25-OHD insufficiency (between 50 and 75 nmol/L).
The bone fragility gene panel sequencing identified pathogenic variants in 8 children (42%; 3 in LRP5, 3 in WNT1 and 2 in COL1A2 genes) and a p.(Val667Met) LRP5 variant in 3 children (16%; isolated in 2 children and associated with a pathogenic variant of LRP5 in one child) (Table 2 and Fig. 1). The positivity rate of genetic variants was higher in children with vertebral fractures; thus, among the five children with vertebral fractures, 3 (60%) had a pathogenic variant and one (20%) a p.(Val667Met) LRP5 variant. Four children had VUS, which were isolated in 3 children and associated with pathogenic variants in 1 child.
Table 2.
Description of pathogenic variant, p.(Val667Met) LRP5 variant and variant of unknown significance.
| N° | Gene | cDNA | Protein | Gender | Age at first fracture (years) | Age at diagnosis (years) | Peripheral fractures | Vertebral fractures | LS-BMD (Z-score) | Other diseases |
|---|---|---|---|---|---|---|---|---|---|---|
| Pathogenic variant | ||||||||||
| 1 | ALPL | c.77C > T | p.(Pro26Leu) | F | 28.0 | 28.0 | Ribs, metacarpal | Yes | −2.8 | |
| 2 | COL1A1 | c.2792C > A | p.(Ala931Asp) | F | 32.0 | 50.0 | Yes | Yes | −3.4 | |
| 3 | COL1A2 | c.577G > A | p.(Gly193Ser) | M | 7.0 | 7.2 | No | Yes | −2.4 | |
| 4 | COL1A2 | c.3305G > C | p.(Gly1102Ala) | F | 2.0 | 7.9 | Radius-ulna, humerus | No | −2.0 | |
| 5 | COL1A2a | c.2821C > A | p.(Gln941Lys) | M | 10.0 | 28.0 | No | Yes | −2.4 | Auto-immune thyroiditis, prolactin adenoma |
| 6 | COL1A2 | c.1666G > A | p.(Gly556Ser) | F | 47.0 | 47.0 | No | Yes | −4.5 | |
| 7 | COL1A2 | c.946G > A | p.(Gly316Ser) | F | ND | 52.0 | No | Yes | −4.3 | Uveitis, otospongiosis, ankylosing spondylitis HLAB27- |
| 8 | DKK1b | c.359G > T | p.Arg120Leu | F | ND | 53.0 | Radius, metatarsal | No | −3.4 | |
| 9 | LRP5 | c.1425G > A | p.(Trp475*) | F | 5.0 | 5.9 | Radius-ulna | No | −0.7 | |
| 10 | LRP5 | c.2225 T > G | p.(Ile742Ser) | M | 3.0 | 8.8 | Tibia, femur, radius | No | −3.4 | |
| 11 | LRP5a | c.2273G > A | p.(Arg758Lys) | M | ND | 13.5 | Wrist, ankle | No | −1.9 | Spondylolisthesis L5-S1 |
| 12 | LRP5 | c.4105_4106delAT | p.(Met1369Valfs*2) | F | 47.0 | 47.0 | Pelvis | No | −4.9 | Auto-immune thyroiditis |
| 13 | LRP5 | c.4502C > T | p.(Pro1501Leu) | F | ND | 60.0 | Metatarsal | Yes | −3.7 | |
| 14 | LRP5 | c.1393G > A | p.(Ala465Thr) | M | 48.0 | 65.0 | No | Yes | −2.3 | |
| 15 | WNT1c | c.711_726del | p.(Thr238Alafs*150) | M | 7.0 | 7.3 | Femur | No | −3.7 | Charcot-Marie-tooth disease with normal mobility |
| 16 | WNT1 | c.403delG | p.Val135SerfsTer64 | M | 7.0 | 8.4 | Wrist, femur, fibula | Yes | −2.3 | Moderate asthma without treatment |
| 17 | WNT1 | c.962C > T | p.(Ala321Val) | F | 10.0 | 8.9 | Yes | −1.3 | Beta thalassemia | |
| 18 | WNT1 | c.610G > T | p.(Glu204*) | M | 52.0 | 44.0 | Yes | −3.0 | ||
| p.(Val667Met) LRP5 variant | ||||||||||
| 19 | LRP5 | c.1999G > A | p.(Val667Met) | F | 7.0 | 10.7 | Radius-cubitus, radius, femur | No | −2.8 | Learning difficulties, albinism |
| 20 | LRP5 | c.1999G > A | p.(Val667Met) | F | ND | 11.9 | No | Yes | −2.4 | Learning difficulties |
| 21 | LRP5 | c.1999G > A | p.(Val667Met) | F | 31.0 | 31.0 | Yes | Yes | −2.9 | |
| 22 | LRP5 | c.1999G > A | p.(Val667Met) | F | 16.0 | 35.0 | Femur | No | −2.5 | Migraine |
| 23 | LRP5 | c.1999G > A | p.(Val667Met) | M | 15.0 | 50.0 | Tibia, elbow, clavicula, ribs, phalanges | No | −2.0 | Breast cancer, mild form of inflammatory rheumatic disorders |
| 24 | LRP5 | c.1999G > A | p.(Val667Met) | M | 42.0 | 53.0 | No | Yes | −4.3 | |
| 25 | LRP5 | c.1999G > A | p.(Val667Met) | F | 54.0 | 54.0 | Femur | No | −2.7 | |
| Variant of unknown significance | ||||||||||
| 26 | COL1A1 | c.1875 + 3G > T | p.(?) | F | 20.0 | Femur | −2.1 | |||
| 27 | FKBP10 | c.926G > A | p.(Arg309His) | F | 42.0 | 42.0 | Ribs | No | −4.7 | |
| 28 | LRP5 | c.3977G > A | p.(Arg1326His) | F | ND | 46.0 | No | No | −3.4 | Mild form of rheumatoid arthritis |
| 29 | RANK | c.718A > G | p.(Lys240Glu) | F | 2.0 | 9.8 | Wrist, radius, femur, radius | No | −2.1 | Moderate asthma without treatment, pyelonephritis |
| 30 | RANK | c.1660A > G | p.(Thr554Ala) | M | 7.0 | 11.9 | Elbow, radius | No | −2.0 | Horseshoe kidney |
| 31 | RANK | c.544G > A | p.(Val1182Ile) | M | ND | 41.0 | No | Yes | −3.4 | |
| 32 | WNT1 | c.1013C > T | p.(Thr338Met) | M | 15.0 | 15.9 | Tibia | No | −2.4 | Algodystrophy |
| 33 | WNT1 | c.793C > A | p.(Arg265Ser) | F | 52.0 | 52.0 | Ribs | No | −4.0 | Breast cancer, low-grade chondrosarcoma |
LS-BMD: lumbar spine BMD; ND: non-determined; VUS: variant of unknown significance.
Associated with a p.(Val667Met) LRP5 variant.
Associated with a VUS in ALPL gene (c.1574delG, p.(Ter525=)).
Associated with a VUS in OPG gene (c.1012G > A, p.(Asp338Asn)).
Fifteen children (75%) were treated with intravenous bisphosphonates.
3.2. Adult group
Forty-seven adults were included in the study, with a greater proportion of females (31 females, 66%). The median age at referral was 46 years (range: 20 to 65 years). In most of the adults (36 adults, 75%), the diagnosis of osteoporosis was suspected due to clinically significant long bone or vertebral fractures. Twelve adults were referred for low BMD values (defined by BMD T-score < −2.5) with minor fractures (6 patients with rib or metatarsal fractures) or bone pain (6 patients). Unlike the children, vertebral fractures were predominant (27 adults, 57%) and more often isolated (26 adults, 54%). Six adults had isolated long bones fractures and 3 had pelvic fractures.
A positive family history of low trauma fractures was found in 16 adults (34%).
The median BMD T-score was −3.3 (range: −4.9 to 1.2) at the lumbar spine and − 2.0 (range: −4.2 to 1.6) at the hip.
The mineral homeostasis parameters were in the normal range for all but one adult; one patient with a mutation in the ALPL gene had low phosphatase alkaline levels. Among the 28 adults with available data, 7 patients (25%) presented 25-OHD deficiency (<50 nmol/L) and 11 patients (39%) presented 25-OHD insufficiency (between 50 and 75 nmol/L).
The genetic analyses found a pathogenic variant in 10 adults (19%; 4 in COL1A1/2, 3 in LRP5, 1 in WNT1, 1 in ALPL and 1 in DKK1 genes) and a p.Val667Met LRP5 variant in 5 (15%) (Table 2 and Fig. 1). The frequency of variants was similar in the 28 patients with vertebral fractures (6 [21%] pathogenic variant and 4 [21%] p.(Val667Met) LRP5 variant).
Nine patients had VUS, which were isolated in 8 adults and associated with pathogenic variants in 1 adult.
Thirty-nine adults (83%) received a bone-specific therapy. The main drugs were oral or intravenous bisphosphonates (18 patients), teriparatide (13 patients), denosumab (2 patients) and raloxifene (6 patients).
3.3. General comparison between age groups and genotypes
There was a higher predominance of males in the children (63%) and of females in the adults (66%) (p = 0.030). Compared to the adults, the children had a significantly lower frequency of vertebral fractures (26 vs 57%, p = 0.022) and a higher frequency of peripheral fractures (84 vs 53%; p = 0.019). The frequency of pathogenic variants tended to be higher in children compared to adults without reaching statistical significance (42 vs 19%; p = 0.053). The frequency of p.(Val667Met) LRP5 variant and VUS was similar in children and adults.
Four groups of patients were identified according to the genetic results (absent or non-pathogenic variant, VUS, pathogenic variant and LRP5 p.(Val667Met) variant). We observed no significant differences regarding the various clinical, biological and radiological characteristics of the patients according to genotype.
Regression analysis showed a significant positive association between age at referral and age at first fracture (r = 0.776, p < 0.0001) and a significant negative association between age at referral and BMD values at the lumbar spine (r = −0.309, p = 0.005).
4. Discussion
In the present study, we reported the different presenting features between children and adults with idiopathic primary osteoporosis. Bone fragility gene panel sequencing allowed the identification of a heterozygous pathogenic variant in 27% of patients. The positivity rate of pathogenic variants was twice as frequent in children compared to adults (42 vs 19%). We observed no significant differences regarding the various clinical, biological and radiological characteristics of the patients according to genotype.
The children included in our study fulfilled the diagnostic criteria for IJO (Dent, 1977; Lorenc, 2002). IJO is classically diagnosed in previously healthy children of both genders who present in the prepubertal period with bone pain followed by low-impact long bone and vertebral fractures with an absence of extra-skeletal features of OI or secondary osteoporosis. Although a spontaneous remission with the progression of puberty is usually reported (Lorenc, 2002), only a small amount of data is available on the evolution of the disease in adulthood. The clinical characteristics of the children included in this study are comparable with those of previous published studies (Lorenc, 2002; Bacchetta et al., 2013; Franceschi et al., 2015; Smith, 1995), with a median age at first fracture of 7 years and fractures mainly located at the sites of weight-bearing bones (spine and lower limbs). As previously reported, there was a delay of about 3 years in the diagnosis of bone fragility with respect to the first skeletal signs. The predominance of males found in our study was also reported by some studies (Bacchetta et al., 2013), but not by others (Lorenc, 2002; Laine et al., 2012). Although defective bone formation was reported on bone histomorphometric studies (Bacchetta et al., 2013; Franceschi et al., 2015; Rauch et al., 2000; Rauch et al., 2002), the specific cause of IJO long remained unclear. In the past decade, pathogenic variants in bone-related genes have been identified in children with IJO. Our study confirms the benefit of performing genetic analysis on these children. Indeed, through bone fragility gene panel sequencing we were able to identify a pathogenic variant or p.(Val667Met) LRP5 variant in half of all children and in 80% of the children with at least one vertebral fracture.
In adults, the definition of EOO is less well defined. This diagnosis is often suspected in young adults who present bone fragility fractures associated with decreased BMD values. In fact, there is a continuum in IJO and EOO, which represent the phenotypical variability of the same disease since both are illustrated by the involvement of the same bone-related genes (i.e. LRP5, WNT1 and COL1A1 or 2 genes). Similarly to what happens in OI (Forlino and Marini, 2016), it is likely that the fracture risk, which decreases at the end of the pubertal growth spurt, may rise during adulthood due to aging and the presence of additional risk factors for low bone mass such as pregnancy (Butscheidt et al., 2018), menopause, immobilisation, drug side effects and inflammatory disease. This hypothesis is strengthened by the high proportion of comorbidities in our adult patients and the age-related decrease in BMD values. Low calcium intake and vitamin D deficiency may also contribute to decreased bone mass. In this study, one third of the children and adults had a low urinary calcium/creatinine ratio, suggesting low calcium intake, and two thirds of them had inadequate vitamin D levels (i.e. vitamin D deficiency or insufficiency). This highlights the importance of ensuring sufficient calcium and vitamin D intake in these patients in order to ensure optimal bone mass.
As previously described, the most frequent genes involved in idiopathic primary osteoporosis are LRP5, WNT1 and COL1A1/2. LRP5 and WNT1 are involved in the WNT signaling pathway, which plays a key role in regulating skeletal homeostasis, especially for osteoblast differentiation and subsequent bone formation. The frequency of the LRP5 pathogenic variant (about 10%) was similar to that reported in similar cohorts (Hartikka et al., 2005; Korvala et al., 2012). Moreover, about 10 to 15% of our patients harboured a p.(Val667Met) LRP5 variant; the clinical and radiological phenotypes of these patients were comparable to those of patients carrying a pathogenic variant of LRP5. In Caucasians, this variant has been associated with decreased BMD and increased risk fractures in young adults (Koller et al., 2005; Ferrari et al., 2005; Kiel et al., 2007; van Meurs et al., 2008). The frequency of this variant was also overrepresented in patients with primary osteoporosis (Collet et al., 2017; Laine et al., 2012). Functional studies demonstrated that this LRP5 variant induces reduced activation of canonical WNT signaling (Collet et al., 2017). This variant, which cannot currently be classified in one of the different categories described by ACMG classification, could be considered as a “susceptibility variant” for osteoporosis. In accordance with previous studies (Stürznickel et al., 2021; Laine et al., 2012), no pathogenic variant in the LRP6 gene was found in our cohort, which suggests that this gene plays a minor role in primary osteoporosis. This is in accordance with previous studies that failed to find any association between variants of the LRP6 gene and BMD values (van Meurs et al., 2008; van Meurs et al., 2006). Interestingly, even in the absence of extra-skeletal features of OI, one quarter to one third of our patients (children and adults) harbour a pathogenic variant in the COL1A1/2 gene, suggesting that a mild form of OI can be mistaken. This has already been reported in previous reports (Butscheidt et al., 2018; Rolvien et al., 2018) and emphasises the phenotypic heterogeneity of OI.
Although our study is one of the largest series reporting systematic molecular analysis in a cohort of children and adults with idiopathic primary osteoporosis, it has several limitations. Firstly, due to the rarity of the disease, the patient numbers were small for each gene group which prevented us from performing genotype-phenotype correlations. Moreover, the absence of genetic testing in all the parents of the patients (especially in adult patients) prevented us from reclassifying some VUS. Secondly, there could be a selection bias as this study only included patients who were referred to our regional reference centre to complete the investigation, notably genetic analyses. Unfortunately, it was not possible to determine the number of patients with primary osteoporosis who were not referred by primary or secondary care physicians. However, it must be emphasized that gene panel sequencing can only be performed by a tertiary health care centre (i.e. University Hospital) in France and our reference centre is the only one in our region. Moreover, bone fragility gene panel sequencing was performed in all patients with idiopathic primary osteoporosis referred to our centre as part of the initial assessment process. Finally, due to the retrospective design of this study, it was not possible to assess important predictors of bone mass, notably lifestyle factors, calcium intake, muscle strength and physical activity.
5. Conclusion
In this study, we reported the presenting features and bone characteristics in a large cohort of children and young adults with idiopathic primary osteoporosis. Bone fragility gene panel sequencing allowed us to identify genetic variants in a significant proportion of these patients. Molecular diagnosis in these patients is important in order to be able to offer genetic counselling and organise patient management.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bonr.2022.101176.
Appendix A. Supplementary data
The following is the supplementary data related to this article.
Description of variants.
References
- Bacchetta J., Wesseling-Perry K., Gilsanz V., Gales B., Pereira R.C., Salusky I.B. Idiopathic juvenile osteoporosis: a cross-sectional single-centre experience with bone histomorphometry and quantitative computed tomography. Pediatr. Rheumatol. 2013;11(6) doi: 10.1186/1546-0096-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baim S., Binkley N., Bilezikian J.P., Kendler D.L., Hans D.B., Lewiecki E.M., Silverman S. Official positions of the International Society for Clinical Densitometry and executive summary of the 2007 ISCD position development conference. J. Clin. Densitom. 2008;11(1):75–91. doi: 10.1016/j.jocd.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Butscheidt S., Rolvien T., Kornak U., Schmidt F.N., Schinke T., Amling M., Oheim R. Clinical significance of DXA and HR-pQCT in autosomal dominant osteopetrosis (ADO II) Calcified Tissue International. 2018;102:41–52. doi: 10.1007/s00223-017-0332-x. [DOI] [PubMed] [Google Scholar]
- Clark E.M., Tobias J.H., Ness A.R. Association between bone density and fractures in children: a systematic review and meta-analysis abbreviations DXA-dual-energy x-ray absorptiometry BMC-bone mineral content BA-bone area BMD-bone mineral density QCT-quantitative computed tomography QUS-quantitative ultrasound SMD-standardized mean difference CI-confidence interval. Pedioatrics. 2006;117:e291–e297. doi: 10.1542/peds.2005-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet C., Ostertag A.Es, Ricquebourg M., Delecourt M., Tueur G., Isidor B., Guillot P., Schaefer E., Javier R.-M., Funck-Brentano T., Orcel P., Laplanche J.-L., Cohen-Solal M. Primary osteoporosis in young adults: genetic basis and identification of novel variants in causal genes. J. Bone Miner. Res. Plus. 2017;2(1):12–21. doi: 10.1002/jbm4.10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent C.E. Osteoporosis in childhood. Postgrad. Med. J. 1977;53:450–456. doi: 10.1136/pgmj.53.622.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahiminiya S., Majewski J., Roughley P., Roschger P., Klaushofer K., Rauch F. Whole-exome sequencing reveals a heterozygous LRP5 mutation in a 6-year-old boy with vertebral compression fractures and low trabecular bone density. Bone. 2013;57(1):41–46. doi: 10.1016/j.bone.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Fan B., Shepherd J.A., Levine M.A., Steinberg D., Wacker W., Barden H.S., Ergun D., Wu X.P. National Health and nutrition examination survey whole-body dual-energy X-ray absorptiometry reference data for GE lunar systems. J. Clin. Densitom. 2014;17(3):344–377. doi: 10.1016/j.jocd.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Ferrari S.L., Deutsch S., Baudoin C., Cohen-Solal M., Ostertag A., Antonarakis S.E., Rizzoli R., De Vernejoul M.C. LRP5 gene polymorphisms and idiopathic osteoporosis in men. Bone. 2005;37(6):770–775. doi: 10.1016/j.bone.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Forlino A., Marini J.C. Osteogenesis Imperfecta. Lancet Publishing Group; 2016. pp. 1657–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi R., Vincenzi M., Camilot M., Antoniazzi Franco, Anthony J., Freemont J.E., Adams C., Laine O., Makitie M.Z.Mughal. Idiopathic juvenile osteoporosis: clinical experience from a single centre and screening of LRP5 and LRP6 genes. Calcif. Tissue Int. 2015;96:575–579. doi: 10.1007/s00223-015-9983-7. [DOI] [PubMed] [Google Scholar]
- Hartikka H., Mäkitie O., Männikkö M., Doria A.S., Daneman A., Cole W.G., Ala-Kokko L., Sochett E.B. Heterozygous mutations in the LDL receptor-related protein 5 (LRP5) gene are associated with primary osteoporosis in children. J. Bone Miner. Res. 2005;20(5):785–789. doi: 10.1359/JBMR.050101. [DOI] [PubMed] [Google Scholar]
- Kämpe A.J., Mäkitie R.E., Mäkitie O. New genetic forms of childhood-onset primary osteoporosis. Horm. Res. Paediatr. 2015;84:361–369. doi: 10.1159/000439566. [DOI] [PubMed] [Google Scholar]
- Keupp K., Beleggia F., Kayserili H., Barnes A.M., Steiner M., Semler O., Fischer B., Yigit G., Janda C.Y., Becker J., Breer S., Altunoglu U., Grunhagen J., Krawitz P., Hecht J., Schinke T., Makareeva E., Lausch E., Cankaya T., Caparros-Martin J.A., Lapunzina P., Temtamy S., Aglan M., Zabel B., Eysel P., Koerber F., Leikin S., Garcia K.C., Netzer C., Schonau E., Ruiz-Perez V.L., Mundlos S., Amling M., Kornak U., Marini J., Wollnik B. Mutations in WNT1 cause different forms of bone fragility. Am. J. Hum. Genet. 2013;92(4):565–574. doi: 10.1016/j.ajhg.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel D.P., Ferrari S.L., Cupples L.A., Karasik D., Manen D., Imamovic A., Herbert A.G., Dupuis J. Genetic variation at the low-density lipoprotein receptor-related protein 5 (LRP5) locus modulates wnt signaling and the relationship of physical activity with bone mineral density in men. Bone. 2007;40(3):587–596. doi: 10.1016/j.bone.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koay M.A., Woon P.Y., Zhang Y., Miles L.J., Duncan E.L., Ralston S.H., Compston J.E., Cooper C., Keen R., Langdahl B.L., MacLelland A., O'Riordan J., Pols H.A., Reid D.M., Uitterlinden A.G., Wass J.A., Brown M.A. Influence of LRP5 polymorphisms on normal variation in BMD. J. Bone Miner. Res. 2004;19(10):1619–1627. doi: 10.1359/JBMR.040704. [DOI] [PubMed] [Google Scholar]
- Koller D.L., Ichikawa S., Johnson M.L., Lai D., Xuei X., Edenberg H.J., Conneally P.M., Hui S.L., Johnston C.C., Peacock M., Foroud T., Econs M.J. Contribution of the LRP5 gene to Normal variation in peak BMD in women. J. Bone Miner. Res. 2005;20:75–80. doi: 10.1359/JBMR.041019. [DOI] [PubMed] [Google Scholar]
- Korvala J., Jüppner H., Mäkitie O., Sochett E., Schnabel D., Mora S., Bartels C.F., Warman M.L., Deraska D., Cole W.G., Hartikka H., Ala-Kokko L., Männikkö M. Mutations in LRP5 cause primary osteoporosis without features of OI by reducing wnt signaling activity. BMC Med. Genet. 2012;13(26) doi: 10.1186/1471-2350-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine C.M., Koltin D., Susic M., Varley T.L., Daneman A., Moineddin R., Cole W.G., Mäkitie O., Sochett E. Primary osteoporosis without features of OI in children and adolescents: clinical and genetic characteristics. American Journal of medical genetics. 2012;158A:1252–1261. doi: 10.1002/ajmg.a.35278. [DOI] [PubMed] [Google Scholar]
- Laine C.M., Joeng K.S., Campeau P.M., Tarkkonen K., Grover M., Lu J.T., Pekkinen M., Wessman M., Heino T.J., Nieminen V. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N. Engl. J. Med. 2013;368(19):1809–1816. doi: 10.1056/NEJMoa1215458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine C.M., Wessman M., Toiviainen-Salo S., Kaunisto M.A., Mäyränpää M.K., Laine T., Pekkinen M., Kröger H., Välimäki V.V., Välimäki M.J., Lehesjoki A.E., Mäkitie O. A novel splice mutation in PLS3 causes X-linked early onset low-turnover osteoporosis. J. Bone Miner. Res. 2015;30(3):437–445. doi: 10.1002/jbmr.2355. [DOI] [PubMed] [Google Scholar]
- Lorenc R.S. Idiopathic juvenile osteoporosis. Calcif. Tissue Int. 2002;70:395–397. doi: 10.1007/s00223-001-0045-y. [DOI] [PubMed] [Google Scholar]
- Mäkitie R.E., Haanpää M., Valta H., Pekkinen M., Laine C.M., Lehesjoki A.E., Schalin-Jäntti C., Mäkitie O. Skeletal characteristics of WNT1 osteoporosis in children and young adults. J. Bone Miner. Res. 2016;31(9):1734–1742. doi: 10.1002/jbmr.2841. [DOI] [PubMed] [Google Scholar]
- Marshall D., Johnell O., Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. Br. Med. J. 1996;312(7041) doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH consensus development panel on osteoporosis prevention, diagnosis, and therapy, March 7-29, 2000: highlights of the conference. South Med J. 2001;94(6):569–573. [PubMed] [Google Scholar]
- Pocock N.A., Eisman J.A., Hopper J.L., Yeates M.G., Sambrook P.N., Eberi S. Genetic determinants of bone mass in adults a twin study. J. Clin. Investig. 1987;80:706–710. doi: 10.1172/JCI113125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch F., Travers R., Norman M.E., Taylor A., Parfitt A.M., Glorieux F.H. Deficient bone formation in idiopathic juvenile osteoporosis: a histomorphometric study of cancellous iliac bone. J. Bone Miner. Res. 2000;15(5):957–963. doi: 10.1359/jbmr.2000.15.5.957. [DOI] [PubMed] [Google Scholar]
- Rauch F., Travers R., Norman M.E., Taylor A., Parfitt A.M., Glorieux F.H. The bone formation defect in idiopathic juvenile osteoporosis is surface-specific. Bone. 2002;31(1):85–89. doi: 10.1016/s8756-3282(02)00814-1. [DOI] [PubMed] [Google Scholar]
- Richards J.B., Rivadeneira F., Inouye M., Pastinen T.M., Soranzo N., Wilson S.G., Andrew T., Falchi M., Gwilliam R., Ahmadi K.R., Valdes A.M., Arp P., Whittaker P., Verlaan D.J., Jhamai M., Kumanduri V., Moorhouse M., van Meurs J.B., Hofman A., Pols H.A., Hart D., Zhai G., Kato B.S., Mullin B.H., Zhang F., Deloukas P., Uitterlinden A.G., Spector T.D. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet. 2008;371(9623):1505–1512. doi: 10.1016/S0140-6736(08)60599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolvien T., Stürznickel J., Felix N., Schmidt S., Butscheidt T., Schmidt B., Busse S., Mundlos T., Schinke U., Kornak M., Amling R.Oheim. Comparison of bone microarchitecture between adult osteogenesis imperfecta and early-onset osteoporosis. Calcif. Tissue Int. 2018;103:512–518. doi: 10.1007/s00223-018-0447-8. [DOI] [PubMed] [Google Scholar]
- Saarinen A., Valimaki V.V., Valimaki M.J., Loyttyniemi E., Auro K., Uusen P., Kuris M., Lehesjoki A.E., Makitie O. The A1330V polymorphism of the low-density lipoprotein receptor-related protein 5 gene (LRP5) associates with low peak bone mass in young healthy men. Bone. 2007;40(4):1006–1012. doi: 10.1016/j.bone.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Siris E.S., Chen Y.-T., Abbott T.A., Barrett-Connor E., Miller P.D., Wehren L.E., Berger M.L. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch. Intern. Med. 2004;164:1108–1112. doi: 10.1001/archinte.164.10.1108. [DOI] [PubMed] [Google Scholar]
- Siris E.S., Adler R., Bilezikian J., Bolognese M., Dawson-Hughes B., Favus M.J., Harris S.T., Jan de Beur S.M., Khosla S., Lane N.E., Lindsay R., Nana A.D., Orwoll E.S., Saag K., Silverman S., Watts N.B. The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporos. Int. 2014;25(5):1439–1443. doi: 10.1007/s00198-014-2655-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. Idiopathic juvenile osteoporosis: experience of twenty-one patients. Rheumatology. 1995;34(1) doi: 10.1093/rheumatology/34.1.68. [DOI] [PubMed] [Google Scholar]
- Stürznickel J., Rolvien T., Delsmann A., Butscheidt S., Barvencik F., Mundlos S., Schinke T., Kornak U., Amling M., Oheim R. Clinical phenotype and relevance of LRP5 and LRP6 variants in patients with early-onset osteoporosis (EOOP) J. Bone Miner. Res. 2021;36(2):271–282. doi: 10.1002/jbmr.4197. [DOI] [PubMed] [Google Scholar]
- van Meurs J.B., Rivadeneira F., Jhamai M., Hugens W., Hofman A., van Leeuwen J.P., Pols H.A., Uitterlinden A.G. Common genetic variation of the low-density lipoprotein receptor-related protein 5 and 6 genes determines fracture risk in elderly white men. J. Bone Miner. Res. 2006;21(1):141–150. doi: 10.1359/JBMR.050904. [DOI] [PubMed] [Google Scholar]
- van Meurs J.B., Trikalinos T.A., Ralston S.H., Balcells S., Brandi M.L., Brixen K., Kiel D.P., Langdahl B.L., Lips P., Ljunggren O., Lorenc R., Obermayer-Pietsch B., Ohlsson C., Pettersson U., Reid D.M., Rousseau F., Scollen S., Hul W.Van, Agueda L., Akesson K., Benevolenskaya L.I., Ferrari S.L., Hallmans G., Hofman A., Husted L.B., Kruk M., Kaptoge S., Karasik D., Karlsson M.K., Lorentzon M., Masi L., McGuigan F.E., Mellstrom D., Mosekilde L., Nogues X., Pols H.A., Reeve J., Renner W., Rivadeneira F., van Schoor N.M., Weber K., Ioannidis J.P., Uitterlinden A.G., Study G. Large-scale analysis of association between LRP5 and LRP6 variants and osteoporosis. JAMA. 2008;299(11):1277–1290. doi: 10.1001/jama.299.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright S.A., Marshall L.M., Ensrud K.E., Cauley J.A., Black D.M., Hillier T.A., Hochberg M.C., Vogt M.T., Orwoll E.S. Hip fracture in women without osteoporosis. J. Clin. Endocrinol. Metab. 2005;90(5):2787–2793. doi: 10.1210/jc.2004-1568. [DOI] [PubMed] [Google Scholar]
- Yuan J., Tickner J., Mullin B.H., Zhao J., Zeng Z., Morahan G., Xu J. Advanced genetic approaches in discovery and characterization of genes involved with osteoporosis in mouse and human. Front. Genet. 2019;10:288. doi: 10.3389/fgene.2019.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Report of a WHO Study Group. 1994. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis; pp. 1–129. (World Health Organ Tech Rep Ser 843). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of variants.