Abstract
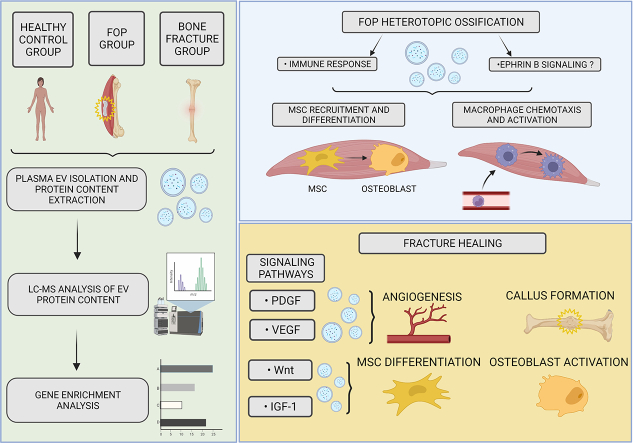
Fibrodysplasia ossificans progressiva (FOP) is an extremely rare disease in which bone tissue forms in extraskeletal sites, which is known as heterotopic ossification (HO). Extracellular vesicles (EVs) are small phospholipid-enclosed particles released by various cells which have an emerging, but not completely understood role in various (patho)physiological processes. In order to further study the pathophysiology of FOP we conducted a small observational study comparing the proteomic profiles of EV cargo, derived from pooled plasma of four patient groups: FOP patient (N = 1) during active disease phase (flare-up), FOP patients during remission (N = 2), patients after long bone fracture (N = 20) and healthy controls (N = 10). After isolation of EVs – their protein cargo was determined using liquid chromatography / mass spectrometry, after which a functional gene enrichment analysis was performed. Our results show a sizeable difference of the proteomics profiles in which EVs from the bone fracture group show significant activity of integrin interactions, Wnt, VEGF, IGF-1 and PDGF pathways; conversely, FOP patients' EVs indicate that HO occurs via processes of innate immunity and the Ephrin B signaling pathway. We hypothesize that the Ephrin B signaling (expressed in EVs) contributes to HO by aiding in mesenchymal stem cell recruitment and osteogenic differentiation, as well as by contributing to the inflammatory response, including macrophage chemotaxis and activation. This is, to our knowledge, the first published analysis of EV protein cargo in FOP.
Keywords: Extracellular vesicles, Microvesicles, Fibrodysplasia ossificans progressiva, Myositis ossificans, Heterotopic ossification, Bone fractures, Ephrin B signaling
Graphical abstract
Highlights
-
•
Proteomics-based analysis of extracellular vesicles’ protein cargo in FOP patients, bone fracture healing and controls.
-
•
Marked differences in signaling pathways expressed in extracellular vesicles in FOP vs. patients with bone fractures.
-
•
Ephrin B signaling pathway expressed in extracellular vesicles identified as a likely cogwheel in heterotopic ossification.
1. Introduction
Bone fractures are the most common large-organ traumatic injuries, and they heal by regeneration (Einhorn and Gerstenfeld, 2015). This process is similar to embryonal development, as it includes a cascade of consecutive events that take place with significant overlaps. The repair starts by blood clotting at the injury site, followed by an inflammatory phase, a fibrocartilaginous phase (including callus formation), primary bone formation and secondary bone remodeling (Morgan et al., 2014). Inflammation is a key initiator of this cascade that activates the innate immune system, thus triggering the secretion of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and several interleukins (IL-1, IL-6, IL-11, IL-23) (Hauser et al., 1997; Schmidt-Bleek et al., 2014). These in turn attract to the injury site the immune cells involved in fracture healing, such as macrophages, monocytes and lymphocytes. The recruited cells remove necrotic tissue and secrete growth and signaling factors, important prerequisites for the transition between different phases of fracture healing (Einhorn and Gerstenfeld, 2015; Morgan et al., 2014; Schmidt-Bleek et al., 2014; Bahney et al., 2019; Hankenson et al., 2015). In response, mesenchymal stem/progenitor cells (MSCs) are recruited to the callus, where they modulate skeletal repair. Many of these cells will foster bone forming osteoblasts and chondrocytes, and initiate endochondral and intramembranous ossification (Hankenson et al., 2015). These processes are regulated by a number of known signaling pathways, such as BMP, TGF-β, Wnt, VEGF, IGF-1, PDGF and, more recently implicated, Eph tyrosine kinase receptors (forward signaling) and their ephrin ligands (reverse signaling) (Hankenson et al., 2015; Kan et al., 2018; Hsu and Keenan, 2010; Matsuo and Otaki, 2012; Arthur and Gronthos, 2021; Arthur et al., 2011). In addition to bone healing and development, studies have linked these pathways to immune response regulation, such as in immune cell development, activation, and migration (Darling and Lamb, 2019). Therefore, all of these mechanisms work in concert to aid the process of bone healing and form new bone tissue at the fracture site.
In contrast to physiological bone formation, heterotopic ossification (HO) is a pathological process that results in spontaneous bone formation at extraskeletal sites, such as muscles and soft tissues (Meyers et al., 2019). It can occur after trauma, surgery or as a result from a rare, genetic disease, such as fibrodysplasia ossificans progressiva (FOP). This disease has a prevalence of about one in every two million, and leads to a lifelong, progressive ossification of patients' soft tissues, such as skeletal muscles, tendons, ligaments, fascia, and aponeuroses (Kaplan et al., 2008; McCarthy and Sundaram, 2005; Pignolo et al., 2011). Unfortunately, there are no successful treatment options available and the patients are fated to periods of disease exacerbations called flare-ups that drive further bone formation (Pignolo et al., 2011). The accumulating HO leads to decreased mobility, cumulative and progressive disability, as well as thoracic insufficiency syndrome, which over time often becomes fatal (Kaplan et al., 2008; Kaplan and Glaser, 2005). Disease flare-ups are reported to be triggered by several factors, such as small tissue injuries, intramuscular injections, muscle fatigue and even viral infections (Kaplan et al., 2008; Scarlett et al., 2004; Lanchoney et al., 1995; Grgurevic et al., 2021). As inflammation is an important component of HO, innate and adaptive immunity, as well as inflammatory cytokines have an important role in HO occurrence and progression (Matsuo et al., 2019; Grgurević et al., 2019). Furthermore, the signaling pathways related to bone physiology, such as BMP/TGF-β, are believed to have a central role in the HO of FOP (Kan et al., 2018).
Extracellular vesicles (EV) as a group of phospholipid enclosed vesicles released by cells have recently triggered interest as important, naturally released paracrine mediators of health and disease. The functionally relevant cargo dispatched in EVs can be efficiently delivered to neighbouring or remote cells receiving these molecular messages. This process has also been implicated in skeletal metabolism, in particular in bone formation and bone regeneration following trauma or disease (Roy et al., 2018; Li et al., 2015; Haffner-Luntzer and Ignatius, 2020; Carnino et al., 2019; Rappsilber et al., 2007). Despite being researched extensively, to the best of our knowledge, there are no reports on the possible roles of EVs in HO or FOP. In order to further our understanding of fractured bone healing, we compared the dynamics of uncontrolled ectopic bone formation to normal bone remodeling. Therefore, we conducted a small prospective observational study in order to observe the differences and similarities in plasma-derived EV protein content that is found in physiological fracture healing in comparison to pathological HO in FOP patients. These findings will hopefully contribute toward a better understanding of the differences in cellular mechanisms and pathways involved in these processes.
2. Participants and methods
2.1. Study outline
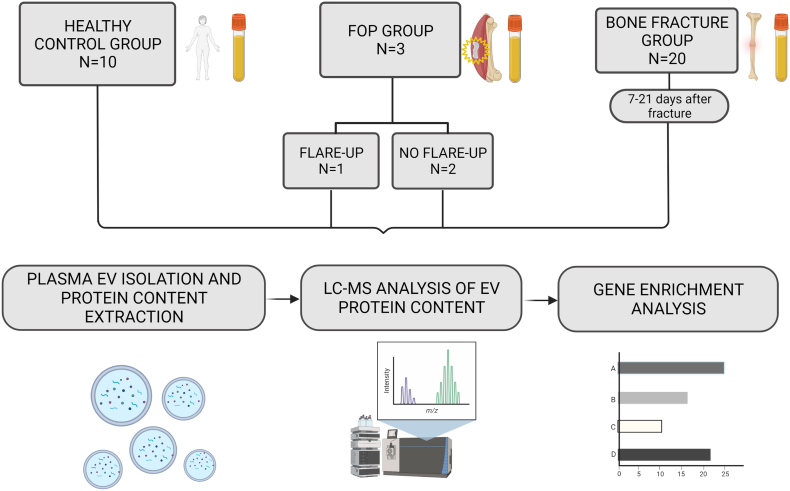
This prospective observational study was approved by the Ethics Committee of the Sisters of Charity (“Sestre milosrdnice”) University Hospital Center (SCUHC) (EP-003-06/20–03/023). The study included three subject groups: i) patients with FOP (N = 3), ii) patients with long bone fractures (N = 20) and iii) healthy control group (volunteers; N = 10) (Fig. 1). Blood plasma samples were obtained from different subject groups and used to isolate EVs, followed by further isolation of their protein content, liquid chromatography-mass spectrometry (LC-MS) and bioinformatic analysis, as outlined in Fig. 1. Subjects were enrolled in the study at the SCUHC, Zagreb. All participants provided a signed informed consent and had to meet the following inclusion criteria: 18–70 years of age, have clinical and radiological verification of fracture (for the fracture group), had to be free of any malignant disease, active infection or osteoporosis and could not be immunocompromised. A total of 20 patients treated for bone fractures were included in the study in the Traumatology Hospital of SMUHC, admitted between January 1st and March 31st, 2021. The long bone fractures included metaphyseal or diaphyseal part of the radius, ulna, humerus, femur, tibia or fibula. These types of fractures were selected for their presumed similarity to animal fracture models used in the existing studies (Li et al., 2015; Haffner-Luntzer and Ignatius, 2020). Blood samples were drawn between the 7th and 21st day after fracture occurrence, a timeframe that was presumed to reflect the transition period from the inflammatory to the fibrocartilaginous phase of fracture healing: we therefore presumed that at this time the patients would have peak blood expression levels of growth and signaling factors (Einhorn and Gerstenfeld, 2015; Morgan et al., 2014; Bahney et al., 2019; Hankenson et al., 2015). Due to the extreme rarity of the disease, only three (N = 3) patients treated for FOP at the SMUHC were included in this study. Two patients provided blood samples during disease remission and one patient during a disease flare-up. The control group consisted of blood samples provided by ten (N = 10) healthy women aged 20–70, free of any active infections, malignant or other life-endangering disease. Further information regarding participants' characteristics is shown in Supplementary Table 1.
Fig. 1.
Study outline showing subject groups and the methodological approach. The EVs were isolated from patient plasma samples, and their protein content was released and analysed by LC-MS. For EV analysis, samples were divided into 4 groups which were used to form sample pools (except for the FOP flare-up group, which consisted only of one sample). EV – extracellular vesicles, FOP – fibrodysplasia ossificans progressiva, LC-MS – liquid chromatography – mass spectrometry. Created with BioRender.com.
2.2. Methods
2.2.1. Plasma sample collection
Blood samples (~10 mL per participant) were drawn by venipuncture and stored in two 5 mL vacuette blood collection tubes containing 3.8% sodium citrate (blood to anticoagulant ratio 1:9). Plasma was isolated by centrifugation at 1500g for 15 min and stored at −80 °C until further analysis. For FOP patients, gentle baby-systems were used with utmost care to reduce tissue injury and prevent potential secondary ossification.
2.2.2. Extracellular vesicle isolation
Plasma samples were pooled into 5 groups (Fig. 1) and EVs were isolated by centrifugation (35 min/21,000 g). Vesicle membranes were lysed by sonication (5 min / 75% amplitude). The released proteins were precipitated by acetone (1 h / -80 °C), centrifuged (10 min / 16,000 g) and resuspended in 8 M urea. Protein concentrations of isolates were determined by Lowry assay (BioRad RC DC Proteins Assay) and a plate reader (SpectraMax i3x - Molecular Devices LLC.) (Carnino et al., 2019).
2.2.3. Liquid chromatography–mass spectrometry (LC-MS) analysis
Protein pools (40 μg) were further processed in 10-kDa centrifugal filter units. After alkylation with 55 mM iodoacetamide in 8 M urea (20 min / RT / dark) and digestion by 0.8 μg of trypsine (ON / 37 °C; Worthington, TPCK treated), the obtained peptides were purified using Stage tips (Rappsilber et al., 2007). Peptides were then separated on a 15 cm C18 nano-column by HPLC (Ultimate 3000, Thermo Fischer Scientific) and injected to an LTQ Orbitrap Discovery (Thermo Fischer Scientific) mass spectrometer. Raw data was processed using MaxQuant software version 1.5.1.2. (Max Planck Institute of Biochemistry) using the default settings. Samples were analysed in technical triplicates.
2.2.4. Data analysis
Functional enrichment analysis of the identified EV proteins across sample pools was performed using FunRich 3.1.3 software, which utilizes gene enrichment analysis (Fonseka et al., 2021). The software used for analysis identified a majority of protein isoforms identified in each group, using the protein IDs as input data (additional information shown in Supplementary Table 2). Some of the sequenced peptides were consistent with more than one protein isoform, in which case multiple isoforms were included in the analysis. Gene enrichment analysis was performed and the results were deemed significant if the p-values calculated by the software were p ≤ 0.05, both by hypergeometric test and after Bonferroni correction. The data was analysed for cellular component, biological process, and biological pathway of the isolated EV cargo proteins.
3. Results
3.1. Identified EV proteins and determination of EV protein origin
Separation of plasma EVs and further analysis of their protein content yielded different amounts of isolated EV proteins. Analysis of the isolated peptides by LC-MS and bioinformatics led to the identification of a varying number of proteins in each group (Supplementary Table 2). In order to ensure the validity of the results, we first analysed the cellular compartment of origin of the identified proteins. The majority of the identified proteins were of EV origin, as they were significantly associated with the term “exosomes” (Supplementary Table 2). The mass spectrometry proteomics data were deposited at the ProteomeXchange Consortium via the PRIDE partner repository and are available via ProteomeXchange with identifier PXD030478. Additional details about differential expression of identified proteins in their respective subject groups can be found in the “Supplementary material - differentially expressed proteins” which shows data obtained by LC-MS and subsequent bioinformatic analysis.
3.2. Biological processes and pathways driven by the identified EV proteins
Using the FunRich software, the EV proteins identified by LC-MS were associated to the biological processes and pathways that were designated as statistically significant. The number of identified proteins was expressed as percentage of proteins identified from the total number of proteins involved in these biological processes or pathways. Proteins from all subject groups were significantly associated to cell growth or maintenance, which seemed to be the most active process in the control group. The fracture group was the only one to contain proteins involved in the transport process, and conversely both FOP groups had proteins identified that were associated to immune response and protein metabolism (Fig. 2).
Fig. 2.
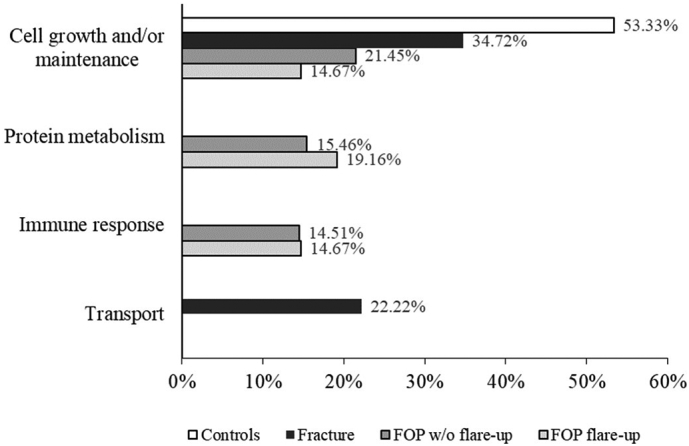
Bar chart showing subject groups and biological processes to which the respective pathways were associated by FunRich 3.1.3. software with statistical significance, according to proteins identified by LC-MS. Percentages represent relative ratios of identified proteins involved in respective biological processes; some proteins are involved in multiple signaling networks and overlap.
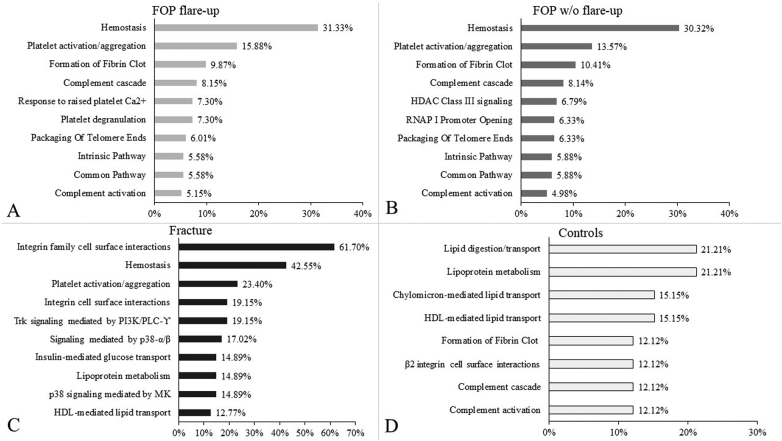
In order to interpret the data in more detail, we broadened the analysis to include the biological pathways the identified proteins were involved in. The significant biological pathways, as well as the percentage of proteins involved in them are shown in Fig. 3. Further analysis uncovered that the majority of pathways in both FOP groups were related to hemostasis (including platelet functions) and the complement system (Fig. 3 A and B). The fracture group, beside pathways related to hemostasis, showed a sizeable ratio of proteins (61.7%) to be involved in integrin family cell surface interactions, as well as substantial signaling by phosphoinositide 3-kinase (PI3K), phospholipase C (PLC) and p38 (Fig. 3C). The control group showed that the identified pathways were predominantly related to lipid and lipoprotein metabolism, clotting and complement cascade pathways (Fig. 3D).
Fig. 3.
Bar chart showing biological pathways of the identified proteins in each subject group with most statistical significance, listed from largest to smallest by ratio of proteins involved in each respective pathway. X - axis represents the relative ratio (defined as percentage) of isolated proteins that are associated to a specific pathway, Y - axis shows names of identified specific pathways. A/ FOP group with current flare-up, B/ FOP group without ongoing flare-up, C/ group with bone fracture, D/ control group. Percentages represent relative ratio of identified proteins involved in respective biological pathways; some proteins are involved in multiple signaling networks and overlap.
3.3. Bone metabolism related biological pathways
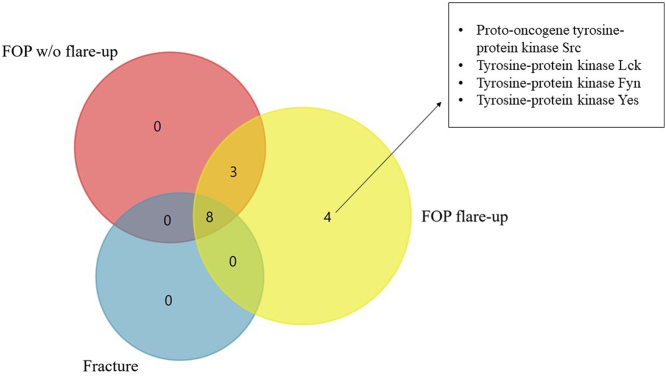
Selected biological pathways were analysed for presence of signaling cascades with known relation to fracture healing, endochondral ossification and HO, such as BMP, TGF-β, Wnt, VEGF, IGF-1 and PDGF (Hankenson et al., 2015; Kan et al., 2018; Hsu and Keenan, 2010). Significant associations were found only in the fracture group, which showed expression of proteins involved in the Wnt, VEGF, IGF-1 and PDGF pathways, while none of the selected pathways were active in other analysed groups. Proteins isolated from the EVs of patients in the fracture group showed that a sizeable ratio (57.5%) was related to the VEGF/VEGFR and PDGF receptor signaling network and the IGF-1 pathway, while 21.3% of isolated proteins were involved in the Wnt signaling network. To further compare the processes of fracture healing and HO that is evident in FOP, we analysed the Ephrin-B pathway that is enriched in HO and has important implications in it, such as enhancing endochondral ossification in fracture repair (Arthur et al., 2013; Yang et al., 2021). Ephrin-B activation was identified in the FOP and fracture groups, but not in the control group. Eight common proteins associated with the Ephrin-B pathway were identified in all three groups, three were present in both FOP groups, while only the FOP flare-up group showed four outliers which were identified as Src kinase family members (Fig. 4) (Boggon and Eck, 2004). Specifically, Ephrin-B reverse signaling (through the ephrin ligand itself) was identified only in the FOP flare-up and fracture group.
Fig. 4.
Venn diagram depicting proteins associated to Ephrin-B signaling in the FOP and fracture groups and corresponding proteins of FOP flare-up outliers. Eight proteins were present in all three groups, whereas three appear only in the FOP groups, and the FOP flare-up group shows four unique mediators. Ephrin-B reverse signaling in FOP flare-up and fracture groups is not shown.
4. Discussion
In this study we analysed for the first time the protein content of plasma derived EVs from FOP patients compared to patients that had fractured bones in the process of healing. Differences in the EV protein cargo in two similar, albeit different, conditions were established, since the pathophysiological background of HO was compared to normal bone regeneration. The analysis of EVs remains a challenge, due to the variability in isolation and analysis techniques, as well as vesicle nomenclature (Ramirez et al., 2018; Xu et al., 2016; Subedi et al., 2019). EVs isolated from different sources display varying content profiles, depending on their origin, sampling time point, patient age and other factors (Newman et al., 2021; Abramowicz et al., 2016). Due to their heterogeneity and small size, characterization and especially separation of these vesicles still presents a significant challenge. A consensus methodological approach for their isolation and study is therefore far from evident, as some insights can be gained through the use of immunological EV markers by flow cytometry or Western blotting, and others from direct visualization by electron microscopy (Linares et al., 2017; Torres Crigna et al., 2021). Our results showed a high relative ratio of identified proteins was of EV origin, which confirmed the validity of our experimental approach. Proteins of all subject groups were significantly associated to cell growth or maintenance, which is an important EV function (Eitan et al., 2015; Ogorevc et al., 2013; Desrochers et al., 2016). Additionally, the plasma EVs from the bone fracture group contained growth factors important for bone remodeling, osteogenesis and angiogenesis during bone healing (Qiao et al., 2018). In contrast, the EVs of both FOP groups were significantly associated with immune response. This finding further confirms the importance of immune regulation in FOP pathogenesis, as inflammation established and sustained by the innate immune system seems to be a key trigger of disease flare-ups (Grgurević et al., 2019; Kaplan et al., 2011; Haviv et al., 2019). Due to the fact that the ratio of involved proteins is comparable in participants with and without an ongoing flare-up, it could be argued that immune system activity is upregulated in FOP even in between flare-ups, although this needs to be further explored on a larger sample size.
Analysis of significant biological pathways showed a substantial number of pathways related to hemostasis, platelet functions and the complement cascade in both FOP groups. These findings could in part be explained by the fact that ~25% of total blood EVs are considered to be platelet derived; and EVs provide a way for the platelet to participate in various physiological maintenance functions, such as hemostasis and immunity (Yáñez-Mó et al., 2015). Our results indicate that EV involvement in immunity in FOP patients is mainly through innate immunity, namely the complement system, which Convente et al. have previously described as a factor in HO (Convente et al., 2015; Janeway et al., 2001). The complement system has also been shown to influence the differentiation of human MSCs into mature osteoblasts, regulate physiological bone development and skeletal growth and modulate bone healing (Mödinger et al., 2018). The EVs from the fracture group showed the biggest ratio of analysed proteins (61.7%) belonging to integrin cell surface interactions. This further underlines the conclusions of studies which have shown the roles of integrin and cadherin adhesion molecules in the control of osteogenesis, bone mechanotransduction, and in mesenchymal cell osteogenic differentiation (Marie et al., 2014). Signaling by PI3K, PLC and p38 was also shown to be significant in the fracture group, which might be due to these molecules being part of signaling pathways important to osteogenesis, angiogenesis and fracture healing, such as PDGF signaling (Hankenson et al., 2015; Evrova and Buschmann, 2017; Albulescu et al., 2019; Dong et al., 2020). Analysis of pathways specifically associated to fracture healing and endochondral ossification revealed that EV proteins from the fracture group were significantly associated to Wnt, PDGF, IGF-1 and VEGF signaling pathways, while they were not significant in the FOP group. These pathways are well-established as crucial in fracture healing, while the VEGF and IGF-1 pathways are also known EV-based crosstalk mechanisms during this process (Hankenson et al., 2015; Qiao et al., 2018; Locatelli and Bianchi, 2014). These findings are in line with known phases of fracture healing in relation to our timeframe of sampling, as VEGF and PDGF stimulate angiogenesis and proliferation of the callus, and activation of Wnt signaling in osteoblasts stimulates bone formation (Einhorn and Gerstenfeld, 2015; Hankenson et al., 2015). Among others, these processes lead to the transition of soft callus unmineralized tissue to hard callous or secondary bone, which usually occurs between 5 and 21 days after the fracture (Einhorn and Gerstenfeld, 2015). The results from the FOP patients were somewhat surprising, as several well-known signaling pathways related to bone metabolism, namely BMP/TGF-β signaling (which are thought to be crucial for HO in FOP) were not among the significant pathways: Our results suggest that EVs utilize other mechanisms and pathways, possibly mediated by the immune system, which might be of greater importance than previously thought (Kan et al., 2018).
Ephrin-B signaling, which was identified in the FOP and fracture groups, is of significant interest in this setting, as bones cannot properly form or be maintained without cell-cell interactions through ephrin ligands and Eph receptors (Matsuo and Otaki, 2012). Especially interesting is its role in bone cell differentiation from precursor cells, as migration, attachment and spreading of mesenchymal stem cells are regulated by ephrin-B ligands and EphB receptors (Matsuo and Otaki, 2012). Macrophage accumulation, also known to be important for HO in FOP, might in part be driven by ephrin signaling, which has been found to modulate the activation and chemotaxis of monocytes and macrophages (Darling and Lamb, 2019; Huang et al., 2021). Furthermore, activation of ephrin-B molecules expressed by MSCs was found to increase osteogenic and promote chondrogenic differentiation (Arthur et al., 2011). Osteogenic and chondrogenic differentiation of MSCs is one of the factors necessary for the occurrence and development of HO, FOP being no exception (Huang et al., 2021). Not much is known about the connection between Ephrin signaling and HO, but the Ephrin-B3 gene has previously been found to be intensively enriched in HO by gene expression analysis (Matsuo et al., 2019; Yang et al., 2021). Our results show that the FOP flare-up group showed expression of most Ephrin-B associated proteins, compared to other analysed groups. Furthermore, the FOP flare-up group showed 4 outliers, which were identified to encode members of the Src family of protein tyrosine kinases (Parsons and Parsons, 2004). Src tyrosine kinases have been shown to be involved in regulation of MSC recruitment and mineral formation (Arthur et al., 2011; Strzelecka-Kiliszek and Bożycki, 2017). We hypothesize that the Ephrin-B pathway might contribute to the MSC recruitment and osteogenic differentiation in FOP, as well as contribute to the inflammatory response, as it has been shown to play part in immune cell development, activation, and migration (Darling and Lamb, 2019). Substantial evidence is missing to form solid conclusions on the importance of Ephrin-B signaling in FOP pathogenesis, but present evidence makes it an interesting avenue for further exploration.
In order to draw valid conclusions, the limitations of this study must be taken into account. One of them is the relatively small number of participants, especially in the FOP groups, which are limited due to the extreme rarity of the disease. Another limitation of the study is sample pooling across experimental groups which prevents the analysis of individual patient EV cargo compositions. This was done to ameliorate the sampling and inter individual patient variability present in the expression of bone metabolism mediators and EV cargo proteins (Newman et al., 2021; Corrales et al., 2008; Sousa et al., 2015). Sample pooling allowed for the analysis of a “biological average” in each group, thus not losing focus on inter-individual within-group differences. An additional limitation of this pilot study is the lack of characterization of discrete EV subtypes. Despite these limitations, we are confident that our analysis accurately identified EV cargo proteins (Supplementary Table 2) and the biological phenomena related to the roles of EVs in these study groups. This is, to the best of our knowledge, the first study to explore the contents and functions of EVs in FOP patients. The importance of this study lies in making a step forward in the ongoing research of physiological bone formation and HO in FOP, as it underlined the importance of immunity and inflammation in FOP, and contrary to expectation, showed no connection of EV proteins to BMP/TGF-β signaling in FOP subjects, but showed a novel connection to Ephrin B signaling.
5. Conclusion
In the present study, we compared physiological bone healing to HO in FOP patients by analysing plasma derived EV protein cargo. Taken together, our findings suggest that EVs indeed influence and participate in bone healing and metabolism by transporting components of various signaling pathways. Their potential involvement in the development of HO in FOP is therefore not to be overlooked, as it is possibly an important contributing factor in HO pathophysiology. Our findings suggest sizeable differences between mechanisms of bone formation involved in fracture healing and FOP (at the site of EVs): the former seems to utilize well-known mechanisms of cell growth and bone-related signaling pathways, while immune response seems to have great importance in the latter. The fracture group showed an EV protein profile associated to specific, fracture healing related pathways, suggesting direct involvement of EV proteins in bone healing through integrin interactions, Wnt, VEGF, IGF-1 and PDGF pathways. The novel association with Ephrin B signaling in FOP and fracture groups, together with its known association with immunity and bone cell recruitment from stem cells, warrants further research and interest in Ephrin signaling in these settings. In general, it can be concluded that localized processes of bone healing and HO likely influence EV protein content and functionality. These results shed new light on the similarities and differences in certain aspects of HO in FOP and physiological bone healing. As EVs and their protein cargo likely present some of the many cogwheels which move these processes, these topics demand further research in order to fully understand and potentially treat FOP.
Funding
Scientific Center of Excellence for Reproductive and Regenerative Medicine (project “Reproductive and regenerative medicine—exploration of new platforms and potentials”, Grant Agreement KK.01.1.1.01.0008 which is funded by the European Union through the European Regional Development Fund.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Hospital Centre Sisters of Charity's Ethical Committee (EP-003-06/20–03/023).
Contributions
Conceptualization: LG, SH; Investigation, Methodology: RN, SH, GS, TV, SG; Formal analysis; Visualization: GS, RN, LG, SH; Supervision, Validation: LG, RN; Writing–original draft: LG, GS, SH, RN; Writing–review & editing: All authors. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
We thank all study participants, especially our FOP patients on their significant contribution to science, their active involvement and a great desire to help in finding appropriate therapeutic solution for this rare bone disease. We would also like to thank Josko Bilandzic for providing technical support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bonr.2022.101177.
Appendix A. Supplementary data
Supplementary tables
Mass spectrometry proteomics data - proteins identified across the experimental samples.
References
- Abramowicz A., Widlak P., Pietrowska M. Proteomic analysis of exosomal cargo: the challenge of high purity vesicle isolation. Mol. BioSyst. 2016;12(5):1407–1419. doi: 10.1039/c6mb00082g. Apr. [DOI] [PubMed] [Google Scholar]
- Albulescu R., Popa A.-C., Enciu A.-M., Albulescu L., Dudau M., Popescu D., et al. Comprehensive in vitro testing of calcium phosphate-based bioceramics with orthopedic and dentistry applications. Materials (Basel). 2019;(12):3704. doi: 10.3390/ma12223704. Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur A., Gronthos S. Eph-ephrin signaling mediates cross-talk within the bone microenvironment. Front. Cell Dev. Biol. 2021;9:147. doi: 10.3389/fcell.2021.598612. Available from https://www.frontiersin.org/article/10.3389/fcell.2021.598612. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur A., Panagopoulos R.A., Cooper L., Menicanin D., Parkinson I.H., Codrington J.D., et al. EphB4 enhances the process of endochondral ossification and inhibits remodeling during bone fracture repair. J. Bone Miner. Res. 2013;28(4):926–935. doi: 10.1002/jbmr.1821. Apr. [DOI] [PubMed] [Google Scholar]
- Arthur A., Zannettino A., Panagopoulos R., Koblar S.A., Sims N.A., Stylianou C., et al. EphB/ephrin-B interactions mediate human MSC attachment, migration and osteochondral differentiation. Bone. 2011;48(3):533–542. doi: 10.1016/j.bone.2010.10.180. Available from https://www.sciencedirect.com/science/article/pii/S8756328210020004. [Internet] [DOI] [PubMed] [Google Scholar]
- Bahney C.S., Zondervan R.L., Allison P., Theologis A., Ashley J.W., Ahn J., et al. Cellular biology of fracture healing. J. Orthop. Res. 2019;37(1):35–50. doi: 10.1002/jor.24170. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggon T.J., Eck M.J. Structure and regulation of Src family kinases. Oncogene. 2004;23(48):7918–7927. doi: 10.1038/sj.onc.1208081. Available from: [DOI] [PubMed] [Google Scholar]
- Carnino J.M., Lee H., Jin Y. Isolation and characterization of extracellular vesicles from broncho-alveolar lavage fluid: a review and comparison of different methods. Respir. Res. 2019;20(1):240. doi: 10.1186/s12931-019-1210-z. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convente M.R., Wang H., Pignolo R.J., Kaplan F.S., Shore E.M. The immunological contribution to heterotopic ossification disorders. Curr. Osteoporos. Rep. 2015;13(2):116–124. doi: 10.1007/s11914-015-0258-z. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales L.A., Morshed S., Bhandari M., Miclau T., 3rd. Variability in the assessment of fracture-healing in orthopaedic trauma studies. J. Bone Joint Surg. Am. 2008;90(9):1862–1868. doi: 10.2106/JBJS.G.01580. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling T.K., Lamb T.J. Emerging roles for Eph receptors and ephrin ligands in immunity. Front. Immunol. 2019;10:1473. doi: 10.3389/fimmu.2019.01473. Jul 4. Available from: https://pubmed.ncbi.nlm.nih.gov/31333644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrochers L.M., Antonyak M.A., Cerione R.A. Extracellular vesicles: satellites of information transfer in cancer and stem cell biology. Dev. Cell. 2016;37(4):301–309. doi: 10.1016/j.devcel.2016.04.019. Available from https://www.sciencedirect.com/science/article/pii/S1534580716302398. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Xu X., Zhang Q., Yuan Z., Tan B. The PI3K/AKT pathway promotes fracture healing through its crosstalk with Wnt/β-catenin. Exp. Cell Res. 2020;394(1) doi: 10.1016/j.yexcr.2020.112137. Sep. [DOI] [PubMed] [Google Scholar]
- Einhorn T.A., Gerstenfeld L.C. Fracture healing: mechanisms and interventions. Nat. Rev. Rheumatol. 2015;11(1):45–54. doi: 10.1038/nrrheum.2014.164. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan E., Zhang S., Witwer K.W., Mattson M.P. Extracellular vesicle–depleted fetal bovine and human sera have reduced capacity to support cell growth. J. Extracell. Vesicles. 2015;4(1) doi: 10.3402/jev.v4.26373. Jan 1. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrova O., Buschmann J. In vitro and in vivo effects of PDGF-BB delivery strategies on tendon healing: a review. Eur. Cells Mater. 2017;(34):15–39. doi: 10.22203/eCM.v034a02. Jul 17. [DOI] [PubMed] [Google Scholar]
- Fonseka P., Pathan M., Chitti S.V., Kang T., Mathivanan S. FunRich enables enrichment analysis of OMICs datasets. J. Mol. Biol. 2021;433(11) doi: 10.1016/j.jmb.2020.166747. May. [DOI] [PubMed] [Google Scholar]
- Grgurevic L., Novak R., Hrkac S., Salai G., Grazio S. Post-COVID-19 exacerbation of fibrodysplasia ossificans progressiva with multiple flare-ups and extensive heterotopic ossification in a 45-year-old female patient. Rheumatol. Int. 2021;41(8):1495–1501. doi: 10.1007/s00296-021-04911-6. Aug. Available from: https://pubmed.ncbi.nlm.nih.gov/34110466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grgurević L., Novak R., Trkulja V., Hamzić L.F., Hrkač S., Grazio S., et al. Elevated plasma RANTES in fibrodysplasia ossificans progressiva – a novel therapeutic target? Med. Hypotheses. 2019;131(July) doi: 10.1016/j.mehy.2019.109313. [DOI] [PubMed] [Google Scholar]
- Haffner-Luntzer M., Ignatius A. Animal models for studying metaphyseal bone fracture healing. Eur. Cell Mater. 2020;40:172–188. doi: 10.22203/eCM.v040a11. Oct. [DOI] [PubMed] [Google Scholar]
- Hankenson K.D., Gagne K., Shaughnessy M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv. Drug Deliv. Rev. 2015;94:3–12. doi: 10.1016/j.addr.2015.09.008. Nov. [DOI] [PubMed] [Google Scholar]
- Hauser C.J., Zhou X., Joshi P., Cuchens M.A., Kregor P., Devidas M., et al. The immune microenvironment of human fracture/soft-tissue hematomas and its relationship to systemic immunity. J. TraumaInj. Infect. Crit. Care. 1997;42(5):895–904. doi: 10.1097/00005373-199705000-00021. May. [DOI] [PubMed] [Google Scholar]
- Haviv R., Moshe V., De Benedetti F., Prencipe G., Rabinowicz N., Uziel Y. Is fibrodysplasia ossificans progressiva an interleukin-1 driven auto-inflammatory syndrome? Pediatr. Rheumatol. 2019;17(1):1–7. doi: 10.1186/s12969-019-0386-6. Available from http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L627973654%0Ahttp://dx.doi.org/10.1186/s12969-019-0313-x. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J., Keenan M. Current review of heterotopic ossification. J. Orthop. 2010;20:126–130. [Google Scholar]
- Huang Y., Wang X., Zhou D., Zhou W., Dai F., Lin H. Macrophages in heterotopic ossification: from mechanisms to therapy. npj Regen. Med. 2021;6(1):70. doi: 10.1038/s41536-021-00178-4. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C.A., Jr., Travers P., Walport M., et al. Immunobiology: The Immune System in Health And Disease. 5th edition. Garland Science; New York: 2001. The complement system and innate immunity. [Google Scholar]
- Kan C., Chen L., Hu Y., Ding N., Lu H., Li Y., et al. Conserved signaling pathways underlying heterotopic ossification. Bone. 2018;109:43–48. doi: 10.1016/j.bone.2017.04.014. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan F.S., Glaser D.L. Thoracic insufficiency syndrome in patients with fibrodysplasia ossificans progressiva. Clin. Rev. Bone Miner. Metab. 2005;3(3–4):213–216. doi: 10.1385/BMM:3:3-4:213. Available from: [DOI] [Google Scholar]
- Kaplan F.S., Le Merrer M., Glaser D.L., Pignolo R.J., Goldsby R.E., Kitterman J.A., et al. Fibrodysplasia ossificans progressiva. Best Pract. Res. Clin. Rheumatol. 2008;22(1):191–205. doi: 10.1016/j.berh.2007.11.007. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan F.S., Lounev V.Y., Wang H., Pignolo R.J., Shore E.M. Fibrodysplasia ossificans progressiva: a blueprint for metamorphosis. Ann. N. Y. Acad. Sci. 2011;1237:5–10. doi: 10.1111/j.1749-6632.2011.06195.x. Available from https://pubmed.ncbi.nlm.nih.gov/22082359. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanchoney T.F., Cohen R.B., Rocke D.M., Zasloff M.A., Kaplan F.S. Permanent heterotopic ossification at the injection site after diphtheria-tetanus-pertussis immunizations in children who have fibrodysplasia ossificans progressiva. J. Pediatr. 1995;126(5):762–764. doi: 10.1016/s0022-3476(95)70408-6. [DOI] [PubMed] [Google Scholar]
- Li Y., Chen S.K., Li L., Qin L., Wang X.L., Lai Y.X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Transl. 2015;3(3):95–104. doi: 10.1016/j.jot.2015.05.002. Available from https://www.sciencedirect.com/science/article/pii/S2214031X15000388. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares R., Tan S., Gounou C., Brisson A.R. Imaging and quantification of extracellular vesicles by transmission electron microscopy. Methods Mol. Biol. 2017;1545:43–54. doi: 10.1007/978-1-4939-6728-5_4. [DOI] [PubMed] [Google Scholar]
- Locatelli V., Bianchi V. Effect of GH/IGF-1 on bone metabolism and osteoporsosis. Int. J. Endocrinol. 2014;23(2014) doi: 10.1155/2014/235060. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie P.J., Haÿ E., Saidak Z. Integrin and cadherin signaling in bone: role and potential therapeutic targets. Trends Endocrinol. Metab. 2014;25(11):567–575. doi: 10.1016/j.tem.2014.06.009. Available from: Nov 1. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Chavez R.D., Barruet E., Hsiao E.C. Inflammation in fibrodysplasia ossificans progressiva and other forms of heterotopic ossification. Curr. Osteoporos. Rep. 2019;17(6):387–394. doi: 10.1007/s11914-019-00541-x. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K., Otaki N. Bone cell interactions through Eph/ephrin: bone modeling, remodeling and associated diseases. Cell Adh. Migr. 2012;6(2):148–156. doi: 10.4161/cam.20888. [Internet]. 2012/03/01. Available from: https://pubmed.ncbi.nlm.nih.gov/22660185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy E.F., Sundaram M. Heterotopic ossification: a review. Skeletal Radiol. 2005;34(10):609–619. doi: 10.1007/s00256-005-0958-z. Available from: [DOI] [PubMed] [Google Scholar]
- Meyers C., Lisiecki J., Miller S., Levin A., Fayad L., Ding C., et al. Heterotopic ossification:a comprehensive review. JBMR Plus. 2019;3(4) doi: 10.1002/jbm4.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mödinger Y., Löffler B., Huber-Lang M., Ignatius A. Complement involvement in bone homeostasis and bone disorders. Semin. Immunol. 2018;37:53–65. doi: 10.1016/j.smim.2018.01.001. Available from https://www.sciencedirect.com/science/article/pii/S1044532317300787. [Internet] [DOI] [PubMed] [Google Scholar]
- Morgan E.F., De Giacomo A., Gerstenfeld L.C. Overview of skeletal repair (fracture healing and its assessment) Methods Mol. Biol. 2014;1130:13–31. doi: 10.1007/978-1-62703-989-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman L.A., Fahmy A., Sorich M.J., Best O.G., Rowland A., Useckaite Z. Importance of between and within subject variability in extracellular vesicle abundance and cargo when performing biomarker analyses. Cells. 2021;10(3) doi: 10.3390/cells10030485. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogorevc E., Kralj-Iglic V., Veranic P. The role of extracellular vesicles in phenotypic cancer transformation. Radiol. Oncol. 2013;47(3):197–205. doi: 10.2478/raon-2013-0037. Available from https://pubmed.ncbi.nlm.nih.gov/24133383. Jul 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons S.J., Parsons J.T. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23(48):7906–7909. doi: 10.1038/sj.onc.1208160. Available from: [DOI] [PubMed] [Google Scholar]
- Pignolo R.J., Shore E.M., Kaplan F.S. Fibrodysplasia ossificans progressiva: clinical and genetic aspects. Orphanet. J. Rare Dis. 2011;6:80. doi: 10.1186/1750-1172-6-80. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Z., Greven J., Horst K., Pfeifer R., Kobbe P., Pape H.C., et al. Fracture healing and the underexposed role of extracellular vesicle-based cross talk. Shock. 2018;49(5):486–496. doi: 10.1097/SHK.0000000000001002. May. [DOI] [PubMed] [Google Scholar]
- Ramirez M.I., Amorim M.G., Gadelha C., Milic I., Welsh J.A., Freitas V.M., et al. Technical challenges of working with extracellular vesicles. Nanoscale. 2018;10(3):881–906. doi: 10.1039/c7nr08360b. Jan. [DOI] [PubMed] [Google Scholar]
- Rappsilber J., Mann M., Ishihama Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007;2(8):1896–1906. doi: 10.1038/nprot.2007.261. Available from: [DOI] [PubMed] [Google Scholar]
- Roy S., Hochberg F.H., Jones P.S. Extracellular vesicles: the growth as diagnostics and therapeutics; a survey. J. Extracell. Vesicles. 2018;7(1):1438720. doi: 10.1080/20013078.2018.1438720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlett R.F., Rocke D.M., Kantanie S., Patel J.B., Shore E.M., Kaplan F.S. Influenza-like viral illnesses and flare-ups of fibrodysplasia ossificans progressiva. Clin. Orthop. Relat. Res. 2004;423:275–279. doi: 10.1097/01.blo.0000129557.38803.26. [DOI] [PubMed] [Google Scholar]
- Schmidt-Bleek K., Schell H., Lienau J., Schulz N., Hoff P., Pfaff M., et al. Initial immune reaction and angiogenesis in bone healing. J. Tissue Eng. Regen. Med. 2014;8(2):120–130. doi: 10.1002/term.1505. Available from: Feb 1. [DOI] [PubMed] [Google Scholar]
- Sousa C.P., Dias I.R., Lopez-Peña M., Camassa J.A., Lourenço P.J., Judas F.M., et al. Bone turnover markers for early detection of fracture healing disturbances: a review of the scientific literature. An. Acad. Bras. Cienc. 2015;87(2):1049–1061. doi: 10.1590/0001-3765201520150008. [DOI] [PubMed] [Google Scholar]
- Strzelecka-Kiliszek A., Bożycki Ł. Cross-talk between Src kinases and Rho small GTPases regulates biomineralization and simplify imaging of the mineralization process. Postepy Biochem. 2017;63(2):93–109. [PubMed] [Google Scholar]
- Subedi P., Schneider M., Philipp J., Azimzadeh O., Metzger F., Moertl S., et al. Comparison of methods to isolate proteins from extracellular vesicles for mass spectrometry-based proteomic analyses. Anal. Biochem. 2019;584 doi: 10.1016/j.ab.2019.113390. Nov. [DOI] [PubMed] [Google Scholar]
- Torres Crigna A., Fricke F., Nitschke K., Worst T., Erb U., Karremann M., et al. Inter-laboratory comparison of extracellular vesicle isolation based on ultracentrifugation. Transfus. Med. Hemotherapy. 2021;48(1):48–59. doi: 10.1159/000508712. Available from https://www.karger.com/DOI/10.1159/000508712. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R., Greening D.W., Zhu H.J., Takahashi N., Simpson R.J. Extracellular vesicle isolation and characterization: toward clinical application. J. Clin. Invest. 2016;126(4):1152–1162. doi: 10.1172/JCI81129. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez-Mó M., Siljander P.R.M., Andreu Z., Zavec A.B., Borràs F.E., Buzas E.I., et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015;2015(4):1–60. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Liu D., Guan R., Li X., Wang Y., Sheng B. Potential genes and pathways associated with heterotopic ossification derived from analyses of gene expression profiles. J. Orthop. Surg. Res. 2021;16(1):499. doi: 10.1186/s13018-021-02658-1. Aug 14. Available from: https://pubmed.ncbi.nlm.nih.gov/34389038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables
Mass spectrometry proteomics data - proteins identified across the experimental samples.