Abstract
Over thousands of years, natural bioactive compounds derived from plants (bioactive phytocompounds, BPCs) have been used worldwide to address human health issues. Today, they are a significant resource for drug discovery in the development of modern medicines. Although many BPCs have promising biological activities, most of them cannot be effectively utilized in drugs for therapeutic applications because of their inherent limitations of low solubility, structural instability, short half-life, poor bioavailability, and non-specific distribution to organs. Researchers have utilized emerging nanoformulation (NF) technologies to overcome these limitations as they have demonstrated great potential to improve the solubility, stability, and pharmacokinetic and pharmacodynamic characteristics of BPCs. This review exemplifies NF strategies for resolving the issues associated with BPCs and summarizes recent advances in their preclinical and clinical applications for imaging and therapy. This review also highlights how innovative NF technologies play a leading role in next-generation BPC-based drug development for extended therapeutic applications. Finally, this review discusses the opportunities to take BPCs with meaningful clinical impact from bench to bedside and extend the patent life of BPC-based medicines with new formulations or application to new adjacent diseases beyond the primary drug indications.
Keywords: Natural products, Phytocompound, Nanoformulation, Drug delivery, Phototherapy
Graphical abstract
Highlights
-
•
Natural bioactive phytocompounds derived from plants have been used worldwide to address human health issues. However, most of them cannot be effectively utilized in drugs for therapeutic applications because of their inherent limitations.
-
•
Nanoformulation approach has recently been underlined as an emerging pharmaceutical strategy to overcome the intrinsic drawbacks of bioactive phytocompounds.
-
•
Various types of nanoformulation and their up-to-date applications for targeted delivery, phototherapy, and imaging are reviewed. Finally, their clinical implications for the repurposing of bioactive phytocompounds are deliberated.
1. Introduction
Throughout human history, natural products have been an invaluable resource as therapeutics for diseases [[1], [2], [3]]. Since the first written records on the medicinal applications of approximately 1000 plants in Mesopotamia in 2600 BCE, there has been an exponential growth in natural product-based research for medicinal uses [4]. A wide variety of natural products originate from diverse natural resources, such as plants, bacteria, fungi, and marine organisms. Among them, plants are a crucial source of pharmacologically active compounds, including many plant-derived blockbuster drugs, such as paclitaxel, morphine, and artemisinin. Currently, over 100 active phytochemicals are used for medicinal purposes, and almost 80% of the world's population depends on plant-derived extracts for primary health care [5,6].
Phytochemicals with various molecular sizes, chemotypes, and structural features have been collected in the form of extracts, fractions, or bioactive compounds through purification processes [7]. Among the rest, the bioactive compounds, referred to as bioactive phytocompounds (BPCs) in this Review, are in turn isolated from the bioactive fractions by bioassay-guided purification. Although many reports have demonstrated promising biological activities of various extracts and fractions, there is consensus that identifying and isolating BPCs by their biological activities is imperative for the use of phytochemicals as safe and effective phytomedicines [8,9].
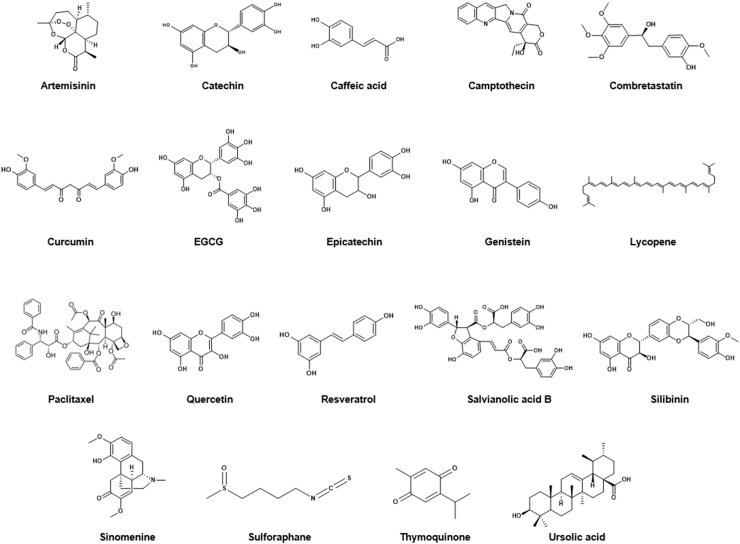
In many studies, BPCs have shown promising anticancer, anti-inflammatory, antioxidant, antibacterial, and anti-neurodegenerative effects for treating or preventing several diseases (Fig. 1) [[10], [11], [12], [13]]. Many bioactive compounds have been evaluated in clinical studies and are currently prescribed clinically (Table 1).
Fig. 1.
Chemical structures of representative bioactive phytocompounds.
Table 1.
Characteristics of bioactive phytocompounds and their clinical statuses.
| Natural compound | Natural resource | Solubility (log P)a | Biological activity | Statusb |
|---|---|---|---|---|
| Artemisinin | Artemisia annua | 3.11 | Anticancer | Phase 3 |
| Catechin | Green tea, beans | 1.80 | Antioxidant | Phase 4 |
| Caffeic acid | Coffee, Eucalyptus | 1.53 | Anticancer, antioxidant, anti-inflammatory | Phase 3 |
| Camptothecin | Stem wood of the Chinese tree Camptotheca acuminate | 1.74 | Anticancer | Phase 1 |
| Combretastatin | Bark of Combretum caffrum | 2.34 | Anticancer | Phase 2 |
| Curcumin | Tumeric | 4.12 | Inhibition of tumor cell proliferation, anti-inflammatory | Phase 4 |
| EGCG | Green tea, white tea, black tea | 3.08 | Antioxidant, chemopreventive | Phase 3 |
| Epicatechin | Woody plants | 1.8 | Antioxidant | Phase 2 |
| Genistein | Plants (lupins, fava beans, soybeans) | 3.08 | Anticancer, anti-inflammatory | Phase 3 |
| Lycopene | tomato | 11.93 | Antioxidant, anticancer | Phase 4 |
| Paclitaxel | Bark of Pacific yew tree | 3.00 | Mitotic inhibitor in cancer chemotherapy | Approved (Taxol®): FDA (1998) |
| Quercetin | Fruits, red onions, kale | 2.16 | Anti-inflammatory, anticancer | Phase 3 |
| Resveratrol | Grapes, blueberries, raspberries, mulberries | 3.4 | Antioxidant, anti-inflammatory, cardioprotective, anti-carcinogenic | Phase 4 |
| Salvianolic acid B | Red sage | 4.99 | Antioxidant, angiogenetic | Phase 2 |
| Silibinin | Milk thistle, coffee | 2.63 | Hepatoprotective, anticancer | Phase 4 |
| Sinomenine | Roots of Sinomenium acutum | 1.83 | Anti-inflammatory, anti-rheumatic | Phase 3 |
| Sulforaphane | Brassica vegetables | 0.22 | Anticancer, antioxidant, antimicrobial, anti-inflammatory | Phase 2 |
| Thymoquinone | Herbs, spices | 2.55 | Hepatoprotective, antioxidant, anticancer | Phase 2 |
| Ursolic acid | Fruits (waxes of apples, pears) | 6.58 | Antitumor, antioxidant | Phase 2 |
log P was obtained from ChemAxon.
Clinical status was obtained from http://clinicaltrials.gov (top clinical status in drugs of intervention category).
For instance, the US Food and Drug Administration (FDA) approved vinblastine for breast cancer in 1965 and paclitaxel (Taxol®) for ovarian cancer in 1992. Since then, many BPCs have been approved. Accordingly, phytochemicals and their derivatives currently account for approximately one-third of the drugs approved by the FDA [3,14]. However, the clinical use of BPCs has often been hampered by their poor solubility, bioavailability, and stability [[15], [16], [17]]. These inherent limitations of BPCs have led to critical issues in clinical studies, including insufficient intravenous dosing, reduced biological action in physiological conditions, and deficient plasma levels of administered BPC families, such as polyphenols, alkaloids, and flavonoids [[18], [19], [20], [21]]. For this reason, the number of new BPC-based drug approvals has remained stationary despite the increasing expenditure and quantity of scientific reports published [22,23].
To overcome the drawbacks of BPC-based therapeutics, researchers from multidisciplinary research fields, such as pharmacology, materials sciences, and nanoscience, have begun utilizing nanoformulation (NF) techniques to encapsulate BPCs. NFs based on nanocarriers composed of lipids, proteins, polymers, and polysaccharides have shown great promise in overcoming the inherent drawbacks of BPCs [[24], [25], [26], [27], [28]]. Owing to their unique physicochemical properties, such as the large surface area to volume ratio, high reactivity, and tissue-specific distribution, Nanocarriers can alter the physico-biochemical properties of BPCs and increase their therapeutic efficacy for human diseases.
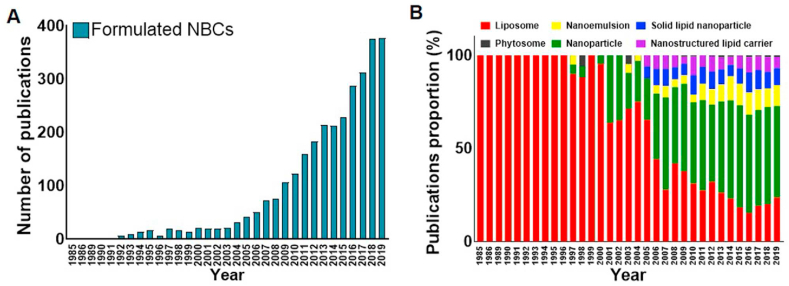
Several BPC-based nanoformulations (BPC-NFs) have been approved for clinical use by the FDA and are prescribed clinically. Examples include liposomal vincristine for acute lymphoid leukemia (Marqibo®), paclitaxel-bound albumin (Abraxane®), and paclitaxel-loaded polyethylene glycol-polylactic acid (PEG-PLA) polymeric micelle (Genexol-PM®) for breast and lung cancer (Table 2). Many other BPC-NFs are also currently under clinical trials. The enormous potential of BPC-NFs is evident from the publication trends over the last decade (Fig. 2). Since the first report of a nano-sized liposomal formulation in 1985, over 3000 publications on BPC-NFs had been published as of 2019.
Table 2.
Representative clinical trials of BPC-based nanomedicine.
| Name | Formulation type | Indicationa | Clinical statusa |
|---|---|---|---|
| Camptothecin | PEG–drug conjugate | Gastric cancer | Phase 2 |
| Polyglutamic acid–drug conjugate | Colon cancer, ovarian cancer | Phase1/2 | |
| Cyclodextrin NP | Solid tumors, renal cell carcinoma, rectal cancer, non-small-cell lung cancer | Phase 1/2 | |
| HPMA–drug conjugate | Solid tumors | Phase 1 | |
| Fleximer–drug conjugate | Gastric cancer, lung cancer | Phase 1 | |
| Curcumin | Liposome | Advanced cancer | Phase1 |
| Irinotecan | Liposome | Metastatic pancreatic cancer | Onivyde® Approved: FDA (2015) |
| Paclitaxel | NPs albumin-bound | Breast cancer, non-small-cell lung cancer, pancreatic cancer | Abraxane® Approved: FDA (2005, 2012, 2013) |
| Micelle | Ovarian cancer, primary peritoneal cancer | Apealea® Approved: EMA (2018) |
|
| PEG-PLA polymeric micelle | Breast cancer, lung cancer | Genexol-PM® Approved: marketed in Korea and Europe (2007) |
|
| Polymeric micelle | Advanced breast cancer | Phase 3 | |
| Polyglutamic acid–drug conjugate | Lung cancer, ovarian cancer | Phase 3 | |
| DHA–drug conjugate | Melanoma, liver cancer, adenocarcinoma, kidney cancer, non-small-cell lung cancer | Phase 2/3 | |
| PEG-PAA polymeric micelle | Gastric cancer, breast cancer | Phase 2/3 | |
| Liposome | Triple-negative breast cancer | Phase 2 | |
| Liposome | Solid tumors, gastric cancer, metastatic breast cancer | Phase 2 | |
| Liposome | Ovarian cancer, breast cancer, lung cancer | Phase1/2 | |
| Polymeric NPs | Peritoneal neoplasms | Phase 1 | |
| Polymeric micelle | Ovarian cancer | Phase 1 | |
| HPMA–drug conjugate | Solid tumor | Phase 1 | |
| Vincristine | Liposome | Acute lymphoid leukemia | Marqibo® Approved: FDA (2012) |
Indication and clinical status were obtained from http://clinicaltrials.gov (top clinical status in drugs of intervention category).
Fig. 2.
A) Number of publications related to formulated BPCs per year. B) Publication proportions of formulated BPCs from 1985 to 2019. The data were obtained from PubMed using the following keywords: natural compound, natural product, liposome, nanoemulsion, solid lipid nanoparticle, phytosome, nanoparticle, nanostructured lipid carrier, formulation.
Among them, NFs based on nanoparticles, nanoemulsions, or lipid-based nanocarriers other than studies on liposomal BPC-NFs account for almost one-third. The remainders are BPC-liposomes, such as solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs). Nanoparticle-based BPC-NFs have been studied since 1997. The number of publications on NFs has steeply increased recently, outnumbering the publications on liposome-based BPC-NFs from 2007, totaling 1876 by 2019. Other types of BPC-NFs have also been widely studied and published in almost 900 articles as of 2019. Publication trends indicate the growing research interest in BPC-NFs and changes in NF technology for BPCs.
The following sections review recent biomedical applications of BPC-NFs from targeted drug delivery to imaging to phototherapy. This review particularly focuses on NF strategies to resolve the inherent limitations of phytochemical BPC drugs. The role of innovative NF technologies in next-generation phytochemical-based drug development for extended therapeutic applications is also discussed.
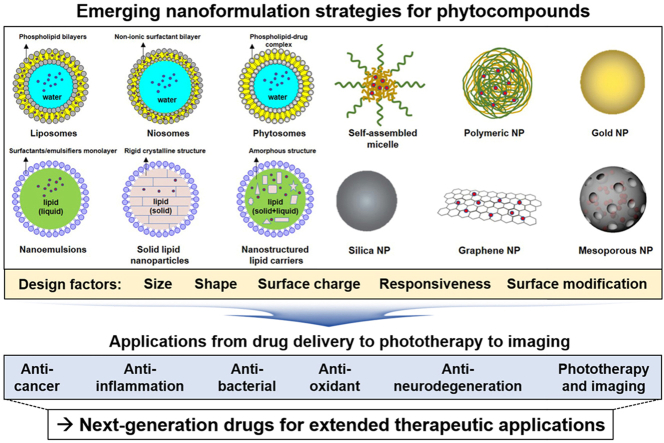
2. Structural design of nanoformulations
The main goal in designing NFs is to manage particle size, surface properties, and release profiles of bioactive compounds to accomplish the site-specific accumulation and action of the drugs at the therapeutically optimal rate and dose regimen. To improve the properties of bioactive compounds, NFs, typically 10–200 nm in diameter, are synthesized through encapsulation, i.e., entrapment of drugs inside the nanocarriers, or complexation, i.e., covalent attachment of drug molecules to nanocarriers. Such NFs have been employed in drug delivery research to improve the bioavailability, solubility, prolonged blood circulation, and targeted delivery of drugs while minimizing their side effects. NFs can enter the body via inhalation, ingestion, skin penetration, or injection and potentially interact with biological systems [29]. NF design helps increase the possibility of reaching the site of action after administration, protecting the cargo from environmental factors such as pH, enzymes, and biochemical degradation. Moreover, NFs can lower administration doses by targeted delivery, site-specific release, and in turn, improved therapeutic effects of drugs [30]. On the other hand, the NFs can be designed to be responsive to exogenous stimuli (e.g., temperature, light, magnetic/electric field, and ultrasound) or endogenous stimuli (e.g., changes in pH, redox potential, and concentration of enzymes). BPC release from the stimuli-responsive NFs can be controlled by specific environmental conditions or external energy input [[31], [32], [33], [34], [35], [36]].
Based on these advantages, NF approaches have shown great potential to improve the pharmacokinetic and pharmacodynamic properties of BPCs and help overcome their inherent limitations by enhancing their solubility, bioavailability, stability, and targeted delivery [[37], [38], [39]]. The diverse methods for the preparation of BPC-NFs can be applied by thin-film hydration, emulsification, high-pressure homogenization, ionic gelation, opposite charge ions precipitation, nanoprecipitation, coacervation, microfluidic mixing, and self-assembly. As is illustrated in Fig. 3, this section highlights various NF platforms for BPCs, such as liposomes, SLNs, NLCs, phytosomes, nanoparticles, and nanoemulsions.
Fig. 3.
Schematic illustration of representative NF designs for BPCs.
2.1. Liposomes
Since Bangham et al. first reported a synthetic liposome in 1961, liposomes have offered the potential to enhance blood half-life, increase drug concentration in the target tissue and reduce the related adverse effects (e.g., cardiotoxicity) [40,41]. Liposomes, which are biodegradable and non-toxic vehicles, can encapsulate both hydrophilic and hydrophobic compounds due to the polar lipid structure of their concentric bilayer [[42], [43], [44]]. Liposomal NFs have several advantages, such as excellent biocompatibility, simple synthesis, encapsulation versatility for hydrophilic, amphiphilic, and lipophilic molecules, and prolonged circulation in the bloodstream [[45], [46], [47]]. Based on these characteristics, various BPCs formulated with liposomes have been studied, as summarized in Table 3.
Table 3.
Formulations and characteristics of bioactive phytocompound-based nanomedicine.
| Type | Natural compound | Formulation method | L.C (%) /L.E (%) |
Size (nm) | Features | Ref |
|---|---|---|---|---|---|---|
| Liposomes | ART | Thin-film hydration | -/90 | 76 ± 10 |
|
[96] |
| CCM | -/50 | 100 ± 23 |
|
[97] | ||
| -/76 | 100 |
|
[98,99] | |||
| Cuc E | -/98 | 140–350 |
|
[100] | ||
| EB | -/85 | 500 -734 |
|
[57] | ||
| MA | -/40 | 293 |
|
[101] | ||
| Melanin | 9/89 | 300 |
|
[102] | ||
| Quercetin | -/90 | 182 ± 1 |
|
[103] | ||
| RVT | -/78 | 122–140 |
|
[104] | ||
| SA | -/96 | 21 ± 1 |
|
[105] | ||
| TMP | -/86 | 168 ± 14 |
|
[106] | ||
| Vitamin C | -/48 | 67 ± 6 |
|
[107] | ||
| Nano Emulsions |
BCL | Emulsification and high-pressure homogenization | -/99 | 90 |
|
[91] |
| CCM | Emulsification | -/91 | 103 ± 11 |
|
[108] | |
| Imperatorin | Ionic gelation | -/90 | 168 ± 2 |
|
[109] | |
| Lycopene | High-pressure homogenization | -/51 | 184 ± 20 |
|
[110] | |
| β-sitosterol | Emulsification | -/72-87 | 163–258 |
|
[90] | |
| TQ | Ionic gelation | -/99 | 95 ± 7 |
|
[92] | |
| Self-assembled NPs or other NPs | CCM | Ionic gelation | -/50 | 70–90 |
|
[[111], [112], [113]] |
| Opposite charge ion precipitation | ∼8/- | 63 |
|
[80] | ||
| Physical encapsulation | 7/85 | 37 ± 6 |
|
[114] | ||
| -/89 | 100 |
|
[115] | |||
| EGCG | Physical encapsulation | -/- | 109 ± 30 |
|
[84] | |
| Gossypol | Nanoprecipitation | 91/97 | 43 ± 2 |
|
[116] | |
| Lycopene | Chemical conjugation | 9/89 | 152 ± 32 |
|
[85] | |
| PTX | Solvent evaporation | -/42 | 43 |
|
[81] | |
| QCT | Nanoprecipitation | 29/81 | 100 |
|
[117] | |
| Vitamin E | Physical interaction | -/99 | 130–350 |
|
[82] | |
| NLCs | Bixin | High-pressure homogenization | 17.9/>99 | 136–353 |
|
[118] |
| ZER | High-shear homogenization | /99 | 52 |
|
[119] | |
| Niosomes | Boswellic acid | Co-acervation phase separation | -/98 | 708 |
|
[120] |
| CCM | -/59 | 71 ± 1 |
|
[121] | ||
| α-M | Thin-film hydration | -/99 | 180 ± 30 |
|
[122] | |
| OXY | Microfluidic mixing | >5/>84 | 96–108 |
|
[70] | |
| Phytosomes | Apigenin | Sequential co-loading prior to thin film evaporation | 31/99 | 361–400 |
|
[83] [123] |
| CCM | -/>75 | |||||
| Kaempferol | 34/99 | |||||
| QCT | High-shear homogenization | 31/98 | 376 ± 34 |
|
[83] | |
| SLNs | CCM | Hot homogenization | -/49 | 58 ± 13 |
|
[124] |
ART: artemisinin; BCL: baicalein; CuC E: cucurbitacin E; CCM: curcumin; DOX: doxorubicin; DPA: dopamine; EB: embelin; EGCG: epigallocate-chin-3-O-gallate; LDH: layered double hydroxide; LF: lactoferrin; MA: madecassoside; MSN: mesoporous silica nanoparticles; NE: nanoemulsion; NF: nanoformulation; NLC: nanostructured lipid carriers; NP: nanoparticle; OEGCG: oligomerized (−)-epigallocatechin-3-O-gallate; OXY: oxyreseveratol; PLGA: poly(lactic-co-glycolic) acid; PTX: paclitaxel; PPB: pheophorbide; QCT: quercetin; RVT: resveratrol; SLN: solid lipid nanoparticles; SFN: sulforaphane; SA: syringic acid; TMP: tetramethylpyrazine; TQ: thymoquinone; ZER: zerumbone; α-M: α-mangostin.
Liposomal NFs have also been modified with functional moieties to improve their performance, such as prolonged blood circulation, target-specific delivery, and enhanced cellular penetration [45,48,49]. The representative approach is PEGylation, which is the conjugation of polyethylene glycol (PEG) molecules with NFs. The clearance of NFs by the mononuclear phagocytic system (MPS) in the bloodstream can be affected by their physicochemical properties. In particular, PEGylation impedes the clearance followed by opsonization and phagocytosis, which subsequently improves the pharmacokinetics of NFs [[50], [51], [52]].
Pegylated liposomes have been employed as a formulation platform in pegylated liposomal doxorubicin (Doxil®), the first clinical NF approved by the FDA for treating some types of cancers in 1995. Pegylated liposomes have also been used for the delivery of an BPC derivative, irinotecan. The FDA approved the use of pegylated liposomal irinotecan (Onivyde®) to treat metastatic pancreatic ductal adenocarcinoma in 2015. Many preclinical and clinical studies have demonstrated that pegylated liposomal formulations improve pharmacokinetics, increase drug concentration in tumor tissues, and reduce side effects.
Targeting or cell-penetrating molecules, such as antibodies, antigen-binding fragments (Fab’), peptides, and small molecules, have been further conjugated onto pegylated liposomes to construct targeted liposomes that actively bind to or penetrate target tissues or cells. In a clinical study, pegylated liposomal doxorubicin was functionalized with EGFR Fab’ to treat patients with EGFR-overexpressing advanced solid tumors that were no longer amenable to standard treatment [53]. In another study, pegylated liposomes were dual-functionalized with a tandem peptide conjugation of cell-penetrating peptide R8 and a tumor-targeting peptide cyclic RGD to bypass the blood-brain barrier (BBB), target glioma tissues, and penetrate the tumor tissue [54].
Liposomal NFs have also been engineered by changing the chemical composition of their bilayer components for drug delivery responsive to stimuli, such as enzymes, redox, and light [45,48,49]. An example is a lysolipid-based thermosensitive liposome, which showed improved bioavailability, thermoresponsiveness, and fast drug release behavior. ThermoDOX®, a doxorubicin-loaded thermoresponsive liposome, is currently under Phase III clinical trials [55,56].
Recently, niosomes have been studied as drug delivery nanocarriers. Unlike liposomes consisting of double-chain phospholipids, niosomes are comprised of uncharged single-chain surfactants and cholesterol. This unique structure enables niosomes to encapsulate both hydrophobic and hydrophilic compounds within the nanostructure and improve their pharmacokinetics and organ distribution [[57], [58], [59]]. Niosomes are considered nanocarriers that can overcome the limitations of liposomes owing to their lower cost, improved chemical stability, and purer phospholipid contents of single-chain phospholipids over double-chain phospholipids [60,61].
2.2. Solid lipid nanoparticles and nanostructured lipid carriers
In addition to traditional liposomal formulations, diverse lipid-based nanocarriers, including SLNs and NLCs, have been developed and employed as NFs. SLNs and NLCs can be prepared reproducibly using high-pressure homogenization or high-speed stirring without the use of toxic organic solvents [62,63]. The solid matrices of SLNs and NLCs allow the entrapment of hydrophilic and hydrophobic drugs with higher entrapment efficiencies than those of liposomes, improving the stability of BPCs [64]. Furthermore, SLNs and NLCs enable controlled drug release and can be functionalized with appropriate ligands to target specific tissues [65]. Therefore, SLNs and NLCs have been widely utilized for topical, oral, intranasal, pulmonary, and parenteral applications.
SLNs are composed of a solid lipid core stabilized by surfactants in an aqueous dispersion and are considered the first generation of lipid nanoparticles with a solid matrix. SLNs have proven to be an efficient and attractive alternative to colloidal drug carriers due to their excellent biocompatibility, protective effects on labile drugs in the bloodstream or GI tract, controlled drug release, and enhanced drug absorption [65,66]. SLNs can load lipophilic and hydrophilic drug molecules, such as liposomes and niosomes. A recent report demonstrated the formulation of a BPC, baicalin, with SLNs by the coacervation separation technique based on fatty acid precipitation from stearate sodium salt micelles in the presence of polymeric nonionic surfactants [67]. The SLN carrier had a mean diameter of 347 nm (PDI: 0.169) and an entrapment efficiency of over 88%. An in vivo pharmacokinetic study showed that baicalin-loaded SLN carriers increase oral absorption compared with a baicalin suspension, improving the bioavailability of baicalin following oral administration.
However, these systems generally show a low drug payload capacity and risk drug expulsion while in storage [68]. NLCs, the second generation of lipid particles, have been developed to address these issues. NLCs are composed of a mixture of solid and liquid lipids. Incorporating a liquid lipid into a solid matrix increases imperfections in the solid matrix core, leading to unique nanostructures with improved drug incorporation and sustained release properties compared to those of SLNs [64,69]. For example, an BPC, oxyresveratrol, was formulated in NLCs and SLNs via a high-shear homogenization technique to improve its oral bioavailability [70]. The oxyresveratrol-loaded NLC had a smaller and more uniform nanoparticle size, significantly higher efficiency for oxyresveratrol entrapment (89%), and better stability than SLN by a 12-month storage period. In in vivo absorption studies, oxyresveratrol-loaded NLC increased the relative bioavailability of oxyresveratrol by 177% compared with unformulated oxyresveratrol.
2.3. Phytosomes
A considerable proportion of bioactive phytocompounds are polar and water-soluble molecules, and water-soluble phytocompounds are poorly absorbed in phospholipid structures because of their intrinsic limitation of relatively large molecular size, which interferes with absorption by passive diffusion and low lipid solubility, limiting their ability to pass across the lipid-rich biological membrane, resulting in poor encapsulation efficacy and bioavailability. To solve these problems, researchers have developed phytosomes to enhance the bioavailability of water-soluble BPCs [71,72].
Structurally, phytosomes use phospholipid molecules containing phosphatidylcholine to form complexes with BPCs. Conventional liposomes are obtained by simply mixing water-soluble BPCs with phospholipids with no chemical bond formation, while phytosomes are assembled via phyto-phospholipid complexes-forming hydrogen bonds by reacting a stoichiometric amount of the phospholipid polar heads with the functionalities of the polar BPCs in a suitable solvent. Therefore, unlike liposomes where BPCs are distributed in the central space or the layers of the membrane, phytosomes have an essential part where BPCs are stabled through the hydrogen bond to the polar head of the phospholipids. Given the stable incorporation of BPCs in phytosomes, phytosomes can significantly improve the permeability of BPCs to the outer membrane of target cells as well as provide robust stability over conventional liposomes [73]. Therefore, phytosomes are more readily absorbed into the body and achieve higher bioavailability than free BPCs.
Based on these superior properties, NFs using phytosomes have been applied to various kinds of BPCs, such as ginkgo, milk thistle, green tea, curcumin, and silibinin [74]. Phytosomes anchoring BPCs can boost absorption through oral administration, increasing the bioavailability and reducing the required dose. In addition, phytosomes can also increase the percutaneous absorption of BPCs, improving skin penetration [75].
2.4. Self-assembled nanoparticles or other nanoparticles
In recent decades, there has been growing research interest in nanoparticle-based drug formulations for biomedical applications [76,77]. Exemplary nanoparticle formulations are summarized in Table 3. Nanoparticle-based drug delivery nanocarriers can be constructed based on polymers, proteins, lipids, carbohydrates, and inorganic materials. Notably, the main materials of nanoparticles determine their physicochemical and biological properties. For instance, carbohydrates are mostly hydrophilic, many inorganic materials are hard and bio-inert, and the characteristics of polymers can be tailored by controlling the types of repeating units and their molecular weight. The types of nanoparticles include i) self-assembled nanoparticles (e.g., polymeric solid nanoparticles, self-assembled micelles or aggregates based on polymers, lipids, proteins, or carbohydrates, and polymersomes) and ii) inorganic nanoparticles (e.g., gold, iron oxide, silver, and silica-based nanoparticles). Depending on nanoparticle type, drugs can be encapsulated in nanoparticles or conjugated with their surface.
Given the low bioavailability of many BPCs, prolonged in vivo circulation is critical for improving the delivery efficiency of BPCs into target tissues and increasing their therapeutic potential. Nanoparticulate formulations increase drug solubility, protect BPCs from premature chemical or enzymatic degradation in the body, improve the bioavailability of BPCs compared with the corresponding crude drugs, and reduce medicinal doses [78,79]. Many BPCs, such as quercetin, paclitaxel, curcumin, and vitamin E, have been encapsulated within nanoparticulate formulations to enhance their solubility, bioavailability, pharmacokinetic, and pharmacodynamic properties by improving biodistribution and facilitating entry into the target tissue [[80], [81], [82], [83]]. However, BPCs themselves have also been exploited as a hydrophobic core to form self-assembled nanoparticles. Recent studies have demonstrated that chemical modification of the hydrophobic BPC epigallocatechin gallate with biopolymers, such as chitosan and hyaluronic acid, allows the nanoparticle formulation to protect BPCs from degradation and deliver them to the target tissues with high efficiency [84,85].
2.5. Nanoemulsions
Nanoemulsions are heterogeneous systems in which one liquid with nano-sized droplets forms a dispersed phase, while another surrounding liquid forms a continuous phase [86]. Generally, nanoemulsions have droplet sizes between 20 and 500 nm, and their types are determined by the class of the dispersed and continuous phases; for example, single emulsions include oil-in-water (O/W) and water-in-oil (W/O), and double emulsions include W/O/W and O/W/O. In terms of fabrication, nanoemulsions can be relatively easily prepared with biocompatible and biodegradable ingredients, including FDA-approved oils. Because these nanoemulsions are non-equilibrium and kinetically stable, surfactants or emulsifiers can be used to impart colloidal stability in their formation. Nanoemulsions have evolved as attractive carriers for the delivery of drugs, including BPCs, nucleic acids, and imaging agents, owing to their favorable features, such as ease of production, enhanced loading capacities for poorly water-soluble drugs, in vivo long-term stability, protection and controlled release of cargo, and ability to accumulate in pathological areas with defective vasculatures [[87], [88], [89]].
Some studies have demonstrated that BPCs, such as baicalein, β-sitosterol, and thymoquinone, can be stabilized by forming nanoemulsions and sustainedly releasing them from the nanoemulsions [[90], [91], [92]]. For targeted drug delivery, intelligent nanoemulsion systems with unique structures have been used for targeted drug delivery [93]. For example, perfluorooctyl bromide and camptothecin were co-loaded into poly(lactic-co-glycolic acid) (PLGA) nanocapsules with ultrathin silicon coating [94]. PLGA dispersed in chloroform solution containing perfluorooctyl bromide, camptothecin, and cetyltrimethylammonium bromide to form an oil-water two-phase emulsion and followed by ultrasonic emulsification and solvent evaporation. The nanoemulsions exhibited a very high loading capacity of up to 19% for the camptothecin. Owing to the structural advantage of ultrathin silica, the release rate of camptothecin is very low under physiological conditions, while high-intensity focused ultrasound triggering can induce an accelerated release of camptothecin. Such distinct drug release provides a synergistic effect of high-intensity focused ultrasound-enhanced ablation and chemotherapy for tumor tissue, inhibiting tumor recurrence.
Furthermore, a transformer-like nanoemulsion was fabricated by self-emulsification in the intestinal lumen after oral administration of an enteric-coated gelatin capsule containing an acid initiator, forming an agent, surfactant, and curcumin [95]. The transformer-like nanoemulsions with diameters of 300–900 nm and 80–90% curcumin encapsulation efficiency could be transcytosed into intestinal M cells, resulting in high accumulation in pancreatic tissue via the intestinal lymphatic system. In this regard, the nanoemulsion strategy provides increased absorption of lipophilic BPCs within the body, indicating targeted delivery and prolonged action for BPCs, thereby improving their bioavailability.
3. Therapeutic applications of BPC-NF
Over time, plants have evolved to biosynthesize various BPCs to survive and protect themselves against environmental challenges. These BPCs have shown potential as therapeutic agents against cancer, inflammatory diseases, microbial infection, oxidative stress, and neurological disorders. However, their biomedical applications have been significantly hampered by several obstacles, including their poor solubility, low stability, low bioavailability, rapid clearance from the body, and off-target toxicity. For this reason, various formulation strategies have been developed to increase their solubility and stability and facilitate controlled release at the target site, thereby improving the bioavailability of BPCs in a physiological environment. This section reviews recent BPC-NFs for anticancer, anti-inflammatory, antibacterial, antioxidant, and anti-neurodegenerative applications (Table 4).
Table 4.
Therapeutic application of bioactive phytocompound-based nanomedicine for disease treatment.
| Category | Type | Therapeutic agent | Target | Therapeutic outcome | Refs |
|---|---|---|---|---|---|
| Anticancer | Liposome | CCM | Breast cancer |
|
[97] |
| Breast/lung cancer |
|
[98,99] | |||
| Celastrol | Prostate cancer |
|
[187] | ||
| GEVPG | Breast/lung cancer |
|
[188] | ||
| GCK | Lung cancer |
|
[189] | ||
| QCT | Breast cancer |
|
[190] | ||
| SFN | Breast cancer |
|
[191] | ||
| WG | Liver cancer |
|
[192] | ||
| Nano emulsion | Catechin | Prostate cancer |
|
[193] | |
| PTX | Brain cancer |
|
[194] | ||
| Tocotrienol | Epithelial cancer |
|
[195] | ||
| Self-assembled NPs or other NPs | Betulin | Cervical cancer |
|
[196] | |
| CCM | Prostate cancer |
|
[124] | ||
| Breast cancer |
|
[197] | |||
| CHE | Liver cancer |
|
[198] | ||
| DI | Cervical cancer |
|
[199] | ||
| Gossypol | Prostate cancer |
|
[116] | ||
| Hesperetin | Colon cancer |
|
[200] | ||
| QCT | Gastric cancer |
|
[117] | ||
| Breast/lung cancer |
|
[201] | |||
| Shikonin | Ovarian cancer |
|
[202] | ||
| PTX | Ovarian cancer |
|
[81] | ||
| Breast cancer |
|
[203] | |||
| Prostate cancer |
|
[204] | |||
| NLC | TQ | Liver/breast cancer |
|
[[205], [206], [207]] | |
| ZER | Leukemia |
|
[119] | ||
| Niosome | LAW | Breast cancer |
|
[58] | |
| Phytosome | RVT | Prostate cancer |
|
[124] | |
| Anti-inflammation | Liposome | SA | Hepatic Injury |
|
[105] |
| Self-assembled NPs or other NPs | BBR | Diabetes |
|
[208] | |
| EB | Ulcerative colitis |
|
[209] | ||
| EGCG | Psoriasis |
|
[210] | ||
| Paeonol | Pigment disorders |
|
[211] | ||
| Paeonol Salidroside | |||||
| SCU | Retinopathy |
|
[212] | ||
| NLC | TQ | Gastric ulcer |
|
[213] | |
| Niosome | CCM | Odema |
|
[59] | |
| EB | Diabetes |
|
[57] | ||
| Phytosome | Sinigrin | Skin wound |
|
[214] | |
| QCT | Menopause |
|
[215] | ||
| SLN | Bixingin | Hepatic injury |
|
[118] | |
| SG | Gastric ulcer |
|
[216] | ||
| Anti-bacterial | Liposome | Allicin | Food preservation |
|
[217] |
| Phytosome | Gingerol | Respiratory infection |
|
[218] | |
| Anti-oxidant | Nano emulsion | TQ | Cerebral Ischemia |
|
[92] |
| Self-assembled NPs or other NPs | Eugenol | Cerebral Ischemia |
|
[219] | |
| Rg3 | Myocardial ischemia-reperfusion |
|
[220] | ||
| RU | Cerebral Ischemia |
|
[221] | ||
| Anti-neuro degeneration | Self-assembled NPs or other NPs | RU | Alzheimer's diseases |
|
[222] |
| Schisantherin A | Parkinson's diseases |
|
[186] |
BBR: berberine; CB: celecoxib; CHE: chelerythrine; CCM: curcumin; DI: 15,16-dihydrotanshinone I; DOX: doxorubicin; EB: embelin; EGCG: epigallocate-chin-3-O-gallate; GCK: ginsenoside compound K; Rg3: ginsenoside Rg3; GA: glycyrrhetinic acid; GEVPG: glucoevatromonoside; LAW: lawsone; NLC: nanostructured lipid carriers; PTX: paclitaxel; PD: Parkinson's disease; PK: pharmacokinetic; RVT: resveratrol; RU: Rutin; SG: sanguinarine; SCU: scutellarin; SLN: solid lipid nanoparticles; SFN: sulforaphane; SA: syringic acid; TQ: thymoquinone; WG: wogonin; ZER: zerumbone.
3.1. Anticancer
BPCs have been explored as a major source of cancer therapeutics and offered new chemical entities with selective activity against cancer-related molecular targets for anticancer drug discovery [[125], [126], [127]]. The anticancer effects of BPCs include many different mechanisms of action, such as the inhibition of tumor cell growth, induction of apoptosis, DNA damage, and inhibition of topoisomerases I and II. Moreover, to overcome the inherent limitations of BPCs for clinical use, researchers have developed BPC-NFs, which can selectively deliver BPCs to tumor tissue and improve the bioavailability and therapeutic outcome.
Epigallocatechin-3-O-gallate, a major constituent of green tea, is one of the most potent and promising chemopreventive agents [128,129]. Recently, epigallocatechin-3-O-gallate was used as a carrier for biological molecules and as an anticancer drug to achieve synergistic anticancer effects [130]. The sequential self-assembly of the epigallocatechin-3-O-gallate derivative with anticancer proteins such as Herceptin and interferon α-2a led to the formation of micellar nanocomplexes, which exhibited robust stability in serum for 15 days without a change in size. In vitro experiments indicated that Herceptin-loaded micellar nanocomplexes showed an excellent inhibitory effect on HER2-overexpressing human breast cancer cells but an insignificant suppression effect on normal cells. Interferon α-2a-loaded micellar nanocomplexes also displayed a superior inhibitory effect on human liver cancer cells than free Interferon α-2a. These enhanced anticancer effects were supported by in vivo results, which demonstrated that both Herceptin- and Interferon α-2a-loaded micellular nanocomplexs exhibit longer blood half-life, higher tumor selectivity, and better growth reduction compared with free Herceptin and free Interferon α-2a.
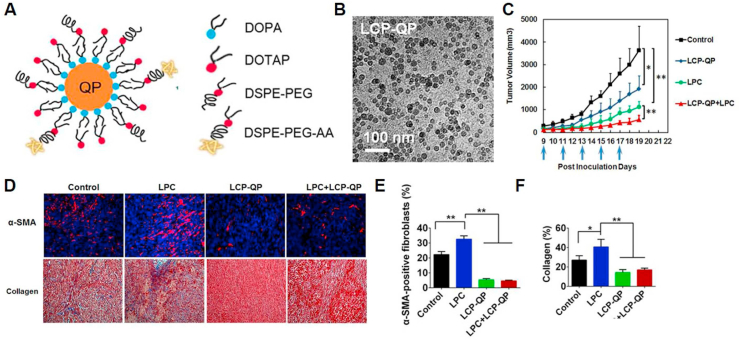
In addition to using BPCs as carrier molecules for anticancer therapies, the phosphorylation approach has been used to improve the solubility of natural compound-based prodrugs [131,132]. For example, researchers have developed a quercetin prodrug via phosphorylation of the hydroxyl groups of quercetin (Fig. 4) [133].
Fig. 4.
A) Preparation procedure for lipid calcium phosphate-quercetin phosphate (LCP-QP.) B) Transmission electron microscopy image of LCP-QP final particles. C) Tumor volume changes for LCP-QP, cisplatin nanoparticles (LPC), and LCP-QP + LPC on a stroma-rich UMUC3 bladder cancer xenograft model after five IV injections (blue arrows, four mice per group. D), E), F) Effects of different treatments on the inhibition of fibroblast growth and Masson's trichrome stain for collagen and quantification results expressed as a percentage of total cell number. **p < 0.01, *p < 0.05, n = 5. Adapted with permission [133]. Copyright 2017, American Chemical Society.
As a result, quercetin phosphate increased its solubility in aqueous conditions. Moreover, it facilitated its precipitation with calcium to be encapsulated into lipid calcium phosphate nanoparticles with a high loading efficiency (26.6% w/w). The nanoparticles protected the quercetin phosphate from degradation and improved its tumor accumulation via the enhanced permeability and retention effects. In particular, quercetin phosphate released from the nanoparticles was replaced with the parent quercetin in response to abundant phosphatases in the tumor tissue. Furthermore, when the nanoparticles were intravenously co-injected with cisplatin nanoparticles, they induced synergistic antitumor effects, decreasing the active fibroblasts and collagen content in the tumor microenvironment. These results suggest that BPC-NFs are a safe and effective way to modulate the tumor microenvironment and assist in traditional chemotherapy for cancer treatment.
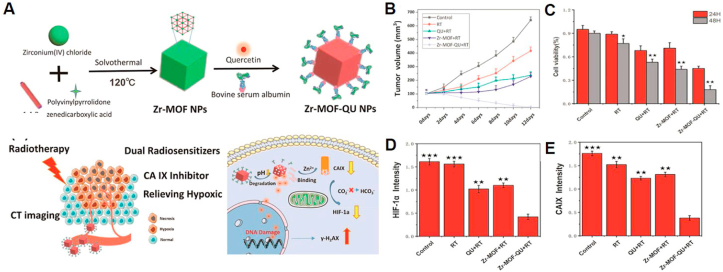
Quercetin has also been employed for tumor radiotherapy because of its ability to enhance the sensitivity to radiotherapy and kill cancer cells [134,135]. For instance, researchers prepared quercetin-loaded Zr-metal-organic framework nanoparticles to improve the irradiation energy absorption and downregulate hypoxic tumor conditions (Fig. 5) [136]. The nanoparticle was fabricated using Zr-metal-organic framework as a carbonic anhydrase IX inhibitor and quercetin as a radiosensitizer for dual sensitization of radiotherapy in hypoxic tumors. Owing to its large surface area and porous structure, the nanoparticles contained a large quantity of quercetin (20.7%) inside the nanostructure. After intravenous administration to A549 tumor-bearing mice, the nanoparticles effectively suppressed carbonic anhydrase IX expression by 1,4-benzenedicarboxylic acid generated from the nanoparticles and boosted the sensitivity of tumor tissues by quercetin, showing no significant side effects during radiation therapy. These examples suggest that BPC-NFs are potential anticancer therapeutics for treating various cancers with high efficiency and low off-target toxicity.
Fig. 5.
A) Principle scheme of the synthesis of quercetin-modified Zr-MOF nanoparticle (Zr-MOF-QU) nanocarriers and schematic illustration of the nanoparticle-based therapeutic principle for dual sensitization radiation therapy of tumors and the mechanism of relieving hypoxia in the tumor microenvironment B) Tumor volumes of different groups in A549 tumor-bearing mice. C) Representative results of an A549 MTT assay in different groups, including a control, RT, QU + RT, Zr-MOF + RT, and Zr-MOF-QU + RT. The results from 24 to 48 h show a statistically significant difference. The p values were calculated by Student's t-test. *p < 0.05, **p < 0.01, ***p < 0.001. D), E) Intensity expression levels of HIF-1a and CA IX in control, RT, QU + RT, Zr-MOF + RT, and Zr-MOF-QU + RT groups. *p < 0.05, **p < 0.01, ***p < 0.001. Adapted with permission [136]. Copyright 2019, American Chemical Society.
3.2. Antiinflammation
BPCs have long been explored as a folk remedy for inflammatory diseases, as seen in the first description of the four classic signs of inflammation and the use of BPCs to relieve them [137]. With our increasing understanding of inflammation, many BPCs with anti-inflammatory activity have been investigated to treat many severe disorders, including cancer, diabetes, gastrointestinal disorders, and liver disorders and eventually enter the commercial market. For example, plants containing salicylate have led to the production of major anti-inflammatory drugs, such as aspirin [138].
NFs have shown great promise for treating inflammatory diseases by optimizing the properties such as size, surface charge, or decorating their surface with coating group (PEG), specific antibodies, or other affinity ligands (small molecules, peptides, aptamers) [139]. In addition, well-designed NFs can target effector cells, such as antigen-presenting cells, membrane receptors on inflammatory cells such as Toll-like receptors, and macrophages through phagocytosis, and regulate the expression of pro- and anti-inflammatory molecules [140,141].
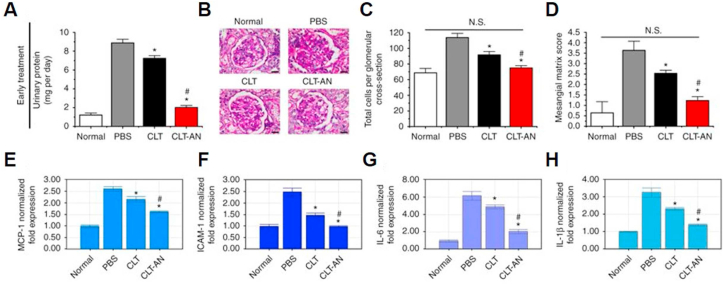
Celastrol, a pentacyclic triterpene extracted from Thunder God Vine, is a potent immunosuppressive, anti-inflammatory, and anticancer agent [142]. In a recent study, researchers fabricated celastrol-loaded albumin nanoparticles to treat mesangioproliferative glomerulonephritis using albumin with excellent biocompatibility and high binding affinity with celastrol (Fig. 6) [143]. Ex vivo distribution data demonstrated that FITC-labeled nanoparticle was extensively distributed throughout the kidneys and displayed the highest renal accumulation compared to control groups. These results were supported by the enhanced therapeutic efficacy after treatment with nanoparticles in the rat anti-Thy1.1 nephritis model. In addition, the nanoparticles altered the biodistribution behavior of celastrol with reduced drug accumulation in the heart, brain, and liver, minimizing celastrol-related off-target toxicity.
Fig. 6.
A) Effects of early CLT and CLT-AN (Celastrol-albumin) treatment on 24-h urinary protein excretion in anti-Thy1.1 nephritic rats on day 5 after disease induction. B) Glomerular histology revealed by PAS staining of kidney tissue sections from anti-Thy1.1 nephritic rats on day 5 after early treatment with CLT and CLT-AN; the scale bars represent 20 μm. C) Effects of early CLT and CLT-AN treatment on total glomerular cellularity on day 5 after disease induction. D) Effects of early CLT or CLT-AN treatment on ECM deposition on day 5 after disease induction. For each animal group, 150 glomeruli were analyzed and ECM deposition was graded semi-quantitatively, as described in Methods. Anti-inflammatory effects of CLT and CLT-AN in anti-Thy1.1 nephritic rats. Real-time PCR analysis of renal mRNA levels of MCP-1 E), ICAM-1 F), IL-6 G), IL-1β H) in anti-Thy1.1 nephritic rats on day 1 after treatment with CLT or CLT-AN. Data are shown as normalized fold expressions relative to normal group using β-actin mRNA as internal control. The data in A), C), and H) are mean ± s.d. (n = 5), and those in I) are mean ± s.d. (n = 3). Adapted with permission [143]. Copyright 2017, Springer Nature.
Berberine, an isoquinoline quaternary alkaloid extracted from berberis spp., has been associated with anti-inflammatory effects [144] and cardioprotective properties. In contrast, its utilization has been hampered because of its low solubility and short half-life in the body after administration. To address these limitations, researchers have developed a long-circulating, berberine-encapsulated liposome that can reduce adverse cardiac remodeling after myocardial infarction and protect heart function in a myocardial infarction mouse model (Fig. 7) [145]. When berberine-loaded liposomes were intravenously administered, they significantly preserved the cardiac ejection fraction at day 28 compared to a control group and free berberine injection. NF strategies using liposomal encapsulation significantly enhanced berberine's water-solubility and therapeutic efficacy to protect cardiac function in an animal model of myocardial infarction.
Fig. 7.
A) Schematic illustration of berberine-loaded liposome (BB-lips) for treatment of myocardial infarction. B) Masson's trichrome stain to indicate the infarcted area. C) Infiltration of macrophages into infarcted heart tissue. MAC3 staining is shown in brown. D) Liposomes visualized with confocal microscopy. Cy5.5 is shown in green and merged with heart tissue obtained under a bright field. The infarct region is indicated by a dashed line (B–D). E) Co-localization of liposomes (green) and macrophages (CD68 staining in red) in infarct area in the heart indicated by white arrowheads, as observed by confocal microscopy. Nuclear staining was performed with DAPI (blue). F) Accumulation of control liposomes in infarcted tissue visualized with IVIS spectrum imaging system. G) Accumulation of Cy5.5-liposomes in the infarcted tissue visualized with IVIS spectrum imaging system. Infarct region is indicated by arrowheads (F–G). Adapted with permission [145]. Copyright 2017, Elsevier.
In one study, researchers demonstrated H2O2-responsive copolyoxalate-loaded vanillyl alcohol (PVAX) nanoparticles as curcumin carriers to treat ischemic diseases, such as peripheral artery disease [146]. Curcumin, a highly potent antioxidant, anti-inflammatory, and angiogenic BPC, was encapsulated in PVAX nanoparticles by a single emulsion method. The nanoparticles had mean diameters of 360 nm (PDI: 0.152) and contained 10 wt% curcumin in the nanostructure. When the nanoparticles were treated with RAW 264.7 cells in the presence of H2O2 stimulation, the expression levels of TNF-α and IL-1β were significantly downregulated, indicating their anti-inflammatory activity. Interestingly, the nanoparticles generate CO2 bubbles in response to H2O2 and release both vanillyl alcohol and curcumin; thus, the nanoparticles could be used to increase ultrasound contrast in the ischemic site associated with a high level of H2O2. The nanoparticles also suppressed the expression of pro-inflammatory cytokines in a mouse model of ischemic injury. Of particular note, this study demonstrated that BPC-NFs hold potential as theranostic agents for imaging and treating ischemic diseases.
Rosmarinic acid has also been considered as an anti-inflammatory, anticancer, and antibacterial BPC. Rosmarinic acid-based nanoparticles were developed as anti-inflammatory nanomedicine for the treatment of inflammatory bowel disease in a dextran sulfate sodium (DSS)-induced acute colitis mouse model [147]. The nanoparticle with a mean diameter of 64 ± 4 nm showed high colloidal stability for up to 2 weeks in a physiological environment and effectively protected CHO–K1 cells from H2O2-induced damage by effectively scavenging H2O2. Moreover, the nanoparticles exhibited significantly prolonged circulation and preferential accumulation in inflamed colon tissue. In addition to this, the nanoparticles showed improved therapeutic efficacy in the colitis model by encapsulating dexamethasone in the nanostructure, implying synergistic effects with a conventional drug. These results suggest that BPC-based NFs warrant further consideration as potential therapeutic nanomedicines for treating various inflammatory diseases, including inflammatory bowel disease.
3.3. Antibacterial
BPCs have been recognized as a valuable resource for the development of antibacterial agents. Moreover, the rapid emergence of resistant bacterial strains has forced the re-evaluation of BPCs as a route to identify novel chemical skeletons with antibiotic activity. Nevertheless, the contributions of BPCs in the development of new antibiotics have been mostly through semisynthetic tailoring of BPCs discovered in the middle of the 20th century.
Fortunately, advances in nanotechnology and pharmaceutics have led to the development of NF strategies to improve the antibiotic effects of BPCs and overcome resistance issues [148]. BPC-NFs have been utilized to combat multidrug-resistant organisms by enhancing their antibiotic effects and inhibiting biofilm formation, as well as by overcoming common resistance mechanisms, such as enzyme inactivation, decreased cell permeability, modification of target sites/enzymes, and increased efflux via overexpression of efflux pump [149,150].
Quercetin, a highly abundant flavonoid found in fruits and vegetables, has been used to treat several diseases. To achieve synergistic antibacterial effects, researchers have developed silver nanoparticle-decorated quercetin nanoparticles [151]. The nanoparticles have displayed enhanced antibacterial activities and cellular uptake against drug-resistant E. coli and S. aureus compared to quercetin or silver nanoparticles alone. Furthermore, in vivo studies using infected mice demonstrated that bacteria gradually decreased over time and were at almost similar levels to the control group on day 5 after administration of the nanoparticles. These enhanced antibacterial activities of the nanoparticles could be attributed to the morphological changes of E. coli and S. aureus, leading to bacterial death by direct cell membrane damage by silver nanoparticles and quercetin dissociated from the nanoparticles trapped in bacteria.
Reactive oxygen species, such as hydroxyl radicals (•OH), have been used to reduce bacterial resistance [152,153]. Ascorbic acid, a pro-oxidant and potent antioxidant, acts as a prodrug of H2O2 to treat drug-resistant infections, improving antibacterial efficiency in the presence of peroxidase. In one study, researchers prepared a targeted “on-demand” prodrug ascorbic acid delivery system, consisting of hyaluronic acid-capped graphene-mesoporous silica nanosheets as the carrier, ferromagnetic nanoparticles as a peroxidase-like catalyst, and ascorbic acid as a prodrug for the treatment of bacterial infection. Ascorbic acid treatment displayed minor antibacterial activity due to the slow decomposition of the generated H2O2. In contrast, the antibacterial ability of the nanocarriers was notably improved at a much lower concentration due to their high catalytic activity. The nanocarriers exhibited excellent antibacterial activity against E. coli and S. aureus, given the high targetability of HA, •OH generated from ferromagnetic nanoparticles and the photothermal effect from graphene mesoporous silica nanosheets. These studies substantiated that targeted “on-demand” BPC-NFs could help treat bacterial infections and overcome bacterial resistance.
3.4. Antioxidation
Oxidative stress is the process of cell damages caused by reactive oxygen or nitrogen species (RONS), such as •OH, H2O2, nitric oxide, peroxide, peroxynitrite, singlet oxygen (1O2), and superoxides [154]. Generally, plants contain many biological compounds, such as ascorbic acid and glutathione, an antioxidant that blocks oxidative stress [155]. Based on these innate defense mechanisms, many natural compounds have been used to treat oxidative stress-associated diseases or to reduce the side effects of drugs that generate RONS [156].
Recently, NFs have been employed as antioxidant delivery vesicles or antioxidants to enhance antioxidant activity under oxidative stress conditions and provide targeted delivery of certain antioxidants with poor permeation across cell membranes and cell internalization. To date, there have been many studies on BPCs with antioxidant effects and their potential in diverse biomedical applications.
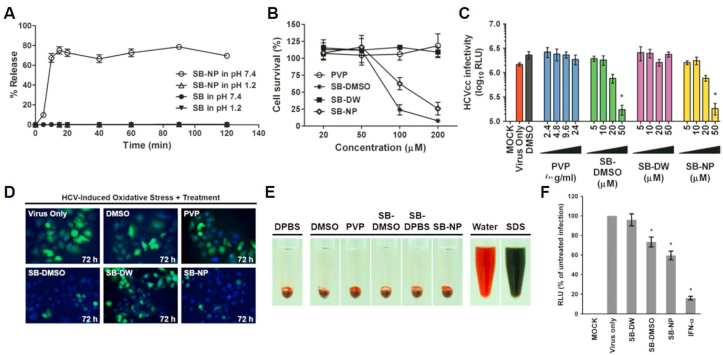
Hepatitis C is a viral infection that causes oxidative stress and leads to liver fibrosis, liver cirrhosis, and hepatocellular carcinoma [157,158]. As hepatitis C treatment, researchers utilized silibinin, a flavonolignan isolated from Silybum, which is known to be an excellent hepatoprotective antioxidant [159,160]. A recent study demonstrated the encapsulation of silibinin into hydrophilic polyvinylpyrrolidone via a simple nano-emulsification technique to improve the solubility and bioavailability of silibinin (Fig. 8) [161].
Fig. 8.
A) Dissolution test of silibinin (SB) (filled circle: pH 7.4; filled triangle: pH 1.2) and SB nanoparticles (SB-NPs) (open circle: pH 7.4; open triangle: pH 1.2) by high-performance liquid chromatography analysis. Data shown are mean ± SEM or representative analysis from three independent experiments. B) Cytotoxicity evaluation of SB-NPs on Huh-7 cells by 2,3-bis[2-methoxy-4-nitro-5- sulfophenyl]-2H-tetrazolium-5- carboxyanilide inner salt assay. All data represent mean ± SEM (*p < 0.05) from three independent experiments. C) Determination of antiviral dose–response effect of SB-NPs against reporter cell culture-derived HCV particle (HCVcc) infection (multiplicity of infection (MOI) = 0.01) by luciferase assay. All data represent mean ± SEM (*p < 0.05) from three independent experiments. D) Effect of SB-NPs on HCV-induced oxidative stress in Huh-7 cells by 2’,7′-dichlorofluorescin diacetate (H2DCFDA) staining analysis. E) Haemolytic analysis of SB-NP treatment effect on human erythrocytes. F) SB-NPs inhibit cell culture-derived HCVcc infection of primary human hepatocytes. Primary human hepatocytes inoculated with reporter HCVcc (MOI = 0.01) were treated with clarified insoluble suspension of silibinin in water (SB-DW), silibinin dissolved in dimethyl sulfoxide (SB-DMSO), and SB-NPs and analyzed for luciferase reporter activity following incubation. Interferon (IFN)-α (1000 IU/mL) served as a positive control. Data shown are mean ± SEM (*p < 0.05) from three independent experiments. RLU: relative light units. Adapted with permission [161]. Copyright 2017, BMJ journals.
Silibinin-loaded polyvinylpyrrolidone nanoparticles increased the solubility of silibinin by over 75% while maintaining anti-hepatitis C virus and antioxidant activities, resulting in remarkable suppression of hepatitis C virus-induced oxidative stress in primary human hepatocytes. After oral administration of silinibinin-loaded nanoparticles in an animal model, the serum level and biodistribution to the liver were significantly enhanced compared with non-modified free silibinin, indicating the enhanced bioavailability of the nanoparticles.
In addition to hepatitis C, elevated RONS levels have been implicated in developing RONS-associated diseases, such as brain ischemia. During brain ischemia, the generated RONS can dramatically aggravate an oxidative injury and lead to RONS-induced inflammation in the brain tissue [162,163]. Melanin, a heterogeneous biological polymer, has attracted increasing attention in photoacoustic imaging, magnetic resonance imaging, and photothermal therapy [[164], [165], [166], [167]]. Interestingly, some reports have shown the antioxidant effects of melanin as a radical scavenger [[168], [169], [170]]. Given its radical scavenging activities against multiple RONS, researchers have developed polyethylene glycol-conjugated melanin nanoparticles with high physiological stability and antioxidant activity (Fig. 9) [171].
Fig. 9.
A) Transmission electron microscopy image of pegylated melanin nanoparticles (PEG-MeNPs). B) Effect of PEG, SOD, MeNPs, and PEG-MeNPs on EPR signals of DEPMPO-OOH. C) O2 production from KO2 solution (100 μM) with vs without PEG-MeNPs. The inset is a digital image of the PEG-MeNP solution before vs after KO2 addition. The assay was performed in triplicate. D) O2 production from KO2 solution (100 μM) catalyzed by SOD vs PEG-MeNPs. The concentration of PEG-MeNPs for catalysis is 0.1 nM. Assay was performed in triplicate. E) Levels of ROS in LPS-stimulated macrophages with different PEG-MeNP concentrations. F) Representative images of TTC-stained brain slices from different groups (*p < 0.05 and **p < 0.01 vs saline control). Adapted with permission [171]. Copyright 2017, American Chemical Society.
The nanoparticles reduced oxidative damage as a potent RONS scavenger by broad antioxidative activities against multiple toxic RONS, including O2 •−, H2O2, • OH, ONOO−, and • NO. In addition, the nanoparticles exhibited neuroprotective and anti-inflammatory effects in Neuro 2A and activated macrophage cells. In particular, in a rat model of ischemic stroke, pre-treatment of the nanoparticle decreased the infarct area by a factor of two compared to the control group and suppressed the generation of O2•− in the brain tissue. Altogether, these findings demonstrate that polyethylene glycol-conjugated melanin nanoparticles can be useful as antioxidants and neuroprotective agents for treating RONS-associated diseases.
Catechin, a highly bioactive polyphenol, has been widely studied due to its excellent ROS scavenging activity, inhibition of xanthine oxidase and many proteinases associated with ROS generation [172,173]. However, the practical application of catechin has been hindered by its poor bioavailability, pro-oxidant activity, and easy degradation by basic solutions or light [172]. To address these issues, researchers have developed polymerized catechin-capped gold nanoparticles via self-polymerization and self-assembly to treat dry eye disease, a ROS-induced inflammatory disease [174]. In the nanostructure, polymerized catechin acts as a drug carrier to deliver anti-inflammatory drugs and as a drug itself to attenuate ROS-induced damage. In vitro results demonstrated that polymerized catechin-capped gold nanoparticles acted as an antioxidant by suppressing ROS and the COX-2 enzyme and served as a drug carrier of amfenac to have a synergistic effect on dry eye conditions. When the nanoparticles were topically administered, they exhibited superior recovery efficacy in a rabbit DED model through long retention time in the blood and dual suppression effects on ROS and inflammation compared to the commercially available cyclosporine A. From these results, the nanoparticles have shown significant potential as dual-functional BPC-NFs for the simultaneous treatment of anti-inflammatory and anti-oxidative diseases. These studies indicate that BPC-based NFs can be used as potent antibiotic nanomedicines to treat RONS-associated diseases.
3.5. Anti-neurodegeneration
Neurodegradative diseases such as Alzheimer's disease, Parkinson's disease, and Huntington's disease are incurable and debilitating conditions. Although various therapeutic strategies have been introduced to modulate neurodegenerative diseases, current therapies are limited because of the poor permeability of drugs through the blood-brain barrier. Therefore, there is an urgent need to develop effective therapeutic strategies to deliver drugs to the central nervous system [175,176]. Blood-brain barrier penetration strategies, such as adsorptive and receptor/transporter-mediated transcytosis and targeting specific domains within brain tissues, can enhance the delivery efficiency of NFs to brain lesions. With the development of brain-targeted drug delivery systems, NFs can interact with target cells and tissues in the brain to induce the preferred physiological response while lowering harmful side effects.
Induction of neurogenesis, which is a process of generating new neurons, can be a potential target for treating neurodegradative diseases [177]. Curcumin, a natural polyphenol compound derived from the rhizome of the Indian spice turmeric, has neuroprotective and antioxidant effects through activation of the transcription factor Nrf2, promotion of neurite outgrowth, and proliferation of neural stem cells via activation of the ERK and MAP kinase pathways [[178], [179], [180]]. Recently, researchers reported curcumin-encapsulated biodegradable poly(lactic-co-glycolic acid) nanoparticles that can induce neural stem cell proliferation and neuronal differentiation by neurogenesis [181]. Curcumin-loaded nanoparticles were developed by encapsulating curcumin in PLGA nanoparticles using the emulsion method. The nanoparticles have a mean diameter of 200 ± 20 nm and entrapment efficiency of 77%, leading to the increased water solubility of curcumin. Moreover, these nanoparticles increased neuronal differentiation by activating the Wnt/β-catenin pathway and reversed learning and memory impairments in an amyloid beta-induced rat model with AD-like phenotypes compared to free curcumin. These results could be ascribed to the localization of curcumin-loaded nanoparticles in the hippocampus across the blood-brain barrier.
Accumulation of amyloid-β (Aβ) in the brain has been implicated in the pathogenesis of Alzheimer's disease. For this reason, a strategy for Aβ clearance is currently the primary therapeutic target for treating Alzheimer's disease [177,182]. It was reported that α-mangostine, a polyphenolic xanthone derivative from mangosteen tree, upregulated low-density lipoprotein receptor (LDLR) expression, leading to the clearance of Aβ via inhibition of amyloid deposition in the brain. To facilitate in vivo applications of α-mangostine, α-mangostine was encapsulated in the core of the poly(ethylene glycol)-poly(l-lactide) nanoparticles [183] (Fig. 10). Compared to the free α-mangostine solution, the α-mangostine-encapsulated nanoparticles remarkably delayed the blood clearance of α-mangostine and improved biodistribution profiles in the blood, brain, and liver by 48.8-, 2.29-, and 9.22-fold, respectively. Moreover, these nanoparticles increased the clearance of Aβ and reduced Aβ deposition in the brain tissue, resulting in amelioration of neurological changes and neuroinflammatory responses in Alzheimer's disease model mice via α-mangostine-mediated modulation of LDLR expression. These findings demonstrate that the nanoparticles-mediated LDLR modulation might offer an efficient disease-modifying therapy for Alzheimer's disease by the improved biodistribution of α-mangostine.
Fig. 10.
A) Nanoformulated α-mangostine (NP(α-M)) selectively enhanced LDLR expression in microglia B) rather than astrocytes. C). Seven-month-old APP/PS1 mice were treated daily with NP(α-M) at an α-M dose of 1 mg/kg intravenously via the tail vein for 4 weeks. CD11b and GFAP were used as markers of microglia and astrocytes, respectively. The scale bar represents 20 μm. NP(α-M) rescued memory deficits in SAMP8 mice. Seven-month-old SAMP8 mice were treated daily with α-M formulations at an α-M dose of 1 mg/kg intravenously via the tail vein for 4 weeks with age-matched SAMP8 and SAMR1 mice given saline as the negative and normal controls, respectively. D) Escape latency, ⁎p < 0.05, ⁎⁎⁎p < 0.001, significantly different from that of the negative control by Student's t-test. E) Number of times crossing the escape platform used to locate. F) Percentage of time spent in the quadrant of the escape platform used to locate. Adapted with permission [183]. Copyright 2017, Elsevier.
BPCs can also treat Parkinson's disease caused by dopaminergic neuron death in the substantia nigra [184]. Schisantherin A, a major active component isolated from Schisandra Chinensis (Turcz.) Baill. has been investigated as a potential hepatoprotective and neuroprotective agent [185,186]. To improve cellular uptake in the brain and prolong circulation in the bloodstream, researchers have developed schisantherin A-loaded PEG-PLGA nanoparticles [186]. These nanoparticles have a mean particle size of 70 ± 4 nm and exhibit high encapsulation efficiency (91%) and drug loading (28%). When the nanoparticles were orally administered, schisantherin A was released from the nanoparticles in a sustained manner for an extended period, resulting in enhanced bioavailability. FRET imaging using zebrafish demonstrated that the nanoparticles improved cross-barrier transportation and afforded a sustained drug release profile. In addition, these nanoparticles notably exerted strong neuroprotective effects in zebrafish and an MPP-induced SH-SY5Y cell injury model by activating the Akt/Gsk3β pathway. These studies demonstrated that BPC-NFs can be designed to target and deliver BPCs to brain disorders and treat neurodegenerative diseases.
4. Applications for phototherapy and imaging
Light has been used as a source for treating and imaging various diseases, such as psoriasis, vitiligo, and skin cancer [223]. Since our systematic understanding of photochemical and photophysical processes began in the last century, phototherapy has been used to treat many diseases, such as cheilitis and cancers. Due to its high efficacy and minimal invasiveness, phototherapy has been used as a stand-alone treatment or combination treatment with traditional chemotherapy to produce synergistic effects [[224], [225], [226]]. Phototherapy can generally be divided into photodynamic therapy (PDT) and photothermal therapy (PTT). For phototherapy, light with a fixed wavelength should be used to activate photoactive molecules. In PDT, photosensitizers activated by visible light generate ROS to kill target cells. Photothermal agents convert photo-energy into thermal energy under light excitation, leading to the thermal ablation of target cells. Recently, various BPCs isolated from plants have been identified and used as alternatives to conventional phototherapeutic agents in cancer therapy because of their low systemic cytotoxicity towards normal tissues and selective therapeutic action against diseased tissues [227]. This section introduces several plant-derived BPCs as agents for phototherapy, imaging, and their applications.
Melanin, a naturally occurring pigment in many organisms, has been extensively investigated as a biomarker for melanoma imaging and as a probe for molecular imaging [228]. In a recent report, researchers fabricated melanin nanoparticles, a biopolymer with excellent biocompatibility, biodegradability, intrinsic photoacoustic properties, and binding ability to drugs as an efficient drug carrier for imaging-guided chemotherapy [229]. To enhance the water solubility of melanin, researchers have developed ultrasmall PEG-functionalized melanin nanoparticles (Fig. 11). Through the π-π interaction between aromatic rings on sorafenib and melanin under high surface-to-volume conditions, sorafenib was successfully loaded onto the nanoparticles.
Fig. 11.
A) Schematic illustration of the process of imaging-guided therapy of HepG2 tumor in vivo by Sorafenib-loaded PEG-MNPs (SRF-MNPs). B) Representative decay-corrected coronal (top) and transaxial (bottom) small-animal PET images (upper layer) and overlaid CT (grey) and PET (color) images (bottom layer) of HepG2 tumor (region enveloped by white dotted line) acquired at 2, 4, and 24 h after tail vein injection of 64Cu-radiolabled (70.0 ± 5.0 μCi) SRF-MNPs. C) PA images, ultrasound (US) images, and overlaid PA (green) and US images (grey) of HepG2 tumor (region enveloped by yellow dotted line) before and after tail vein injection of 200 μL of SRF-MNP (SRF: 10 mg kg ˉ1; MNP: 40 mg kg ˉ1) in living mice (excitation wavelength = 680 nm for PAI). D) Histological sections of tumor tissue with TUNEL staining ( × 10). Nuclei are stained blue (DAPI staining, × 10), and apoptotic cells are stained green (TUNEL staining, × 10). The scale bar represents 100 μm. Adapted with permission [229]. Copyright 2015, Wiley Online Library.
PEG-functionalized melanin nanoparticles had a hydrodynamic diameter of around 7.5 nm, while these nanoparticles containing sorafenib formed larger nanoparticles with a hydrodynamic diameter of 60 nm under aqueous conditions by self-aggregation. In addition, sorafenib-loaded nanoparticles exhibited a gradual and linear release of sorafenib without the influence of pH. Owing to its native photoacoustic property and chelating capability with Cu2+, the in vivo biodistribution of nanoparticles was successfully monitored by photoacoustic and PET imaging. Furthermore, the nanoparticles showed long-term retention in a tumor, guaranteeing the gradual and efficient release of sorafenib from the nanoparticles in the tumors. Intravenously injected nanoparticles showed better therapeutic effects compared to free sorafenib. These results demonstrated the great potential of BPC-NFs for image-guided tumor therapy.
Melanin also has multimodal functions for magnetic resonance imaging and photothermal therapy [165,166]. One report introduced melanin-based liposomes with a unique core/shell structure consisting of PEG as the shell and melanin as the core [102]. The liposomes showed high performance as a contrast agent for photoacoustic imaging and magnetic resonance imaging in tumor-bearing mice, guiding the therapeutic process. Furthermore, upon 808 nm laser irradiation, liposomes with high melanin encapsulation efficiency (>89%) exhibited a remarkable photothermal effect for the complete eradication of tumors in breast cancer-bearing mice, mitigating the potential toxicity of melanin at a high dose (100 mg/kg). Another report demonstrated that melanin extracted from natural sesame was used as a natural staining agent for sentinel lymph node mapping [230]. Liposomes containing melanin showed high accumulation in the SLNs of tumor-bearing mice. Moreover, intratumorally injected liposomes significantly suppressed tumor growth upon 808 nm laser irradiation. These results obtained from melanin-based NFs provide a new strategy for creating desirable theranostic agents with high biocompatibility and satisfactory theranostic performance.
Pheophorbide A, the product of chlorophyll breakdown, was used as a photosensitizer to treat microbes. Due to the singlet oxygen generated from pheophorbide A, erythromycin-loaded liposomes coated with pullulan-pheophorbide A conjugate exerted antimicrobial activity against P. acnes under a 671 nm laser irradiation [231]. In another study, pheophorbide A-conjugated glycol chitosan nanoparticles acted as a theranostic agent for treating and imaging human colon cancer [232]. These nanoparticles, under aqueous conditions, maintained photo-inactivity and a quenched state by self-quenching effects. On the other hand, under intracellular reductive conditions, the nanoparticles rapidly recovered their photoactivity, exhibiting higher phototoxicity toward cells under laser irradiation. Furthermore, the in vivo biodistribution monitored by a non-invasive fluorescence imaging system indicated that pheophorbide A-conjugated glycol chitosan nanoparticles exhibited prolonged circulation in the whole body and enhanced accumulation in tumor tissue compared with free pheophorbide A after systemic administration.
Researchers have also fabricated berberine chloride with aggregation-induced emission characteristics as a theranostic agent for cancer cells and bacteria [233]. Given the emission peak at 530 nm when it forms aggregates and its mitochondria-targeting ability by the positively charged structure, berberine chloride was used as a fluorescence imaging agent for HeLa cells and gram-positive S. aureus. Moreover, berberine chloride ablated cancer cells more effectively than normal cells via the photodynamic therapy pathway. It exerted an excellent photodynamic antibacterial effect against gram-positive bacteria in vitro and in vivo under white light irradiation.
Hypericin-encapsulated NLCs have been studied to investigate the PDT efficacy of hypericin in human cervical carcinoma cells. The NLCs generated singlet oxygen under a 580 nm laser treatment, leading to remarkable phototoxicity towards the cells [234]. Hypericin was also encapsulated in SLNs, which showed cellular uptake increased by 30% and an improvement of 26% in phototoxicity compared with free hypericin [235]. In addition, liposomal and polymeric nanoparticle formulations containing curcumin derivatives have also been developed as photodynamic agents. These curcumin-loaded liposomes and nanoparticles exhibited a formulation-dependent selective photodynamic effect on human ovarian cancer cells and microbes (S. mutans, C. albicans, and methicillin-resistant S. aureus). The phototherapeutic effects of hypericin and curcumin are responsible for generating singlet oxygen species upon irradiation with visible light [236].
As described above, several BPCs with photoproperties are classified as phototherapeutic agents, but numerous BPCs must still be explored (Fig. 12). Although the detailed photopharmacological effects of BPCs have only been investigated with selected BPCs, such as melanin, curcumin, hypericin, and berberine, the workflows for the isolation and property evaluation of new BPCs will further increase through the revolution of irradiation techniques using xenon lamps with precise monochromatic light or LEDs with narrow-band emission. These new applications will help to fight numerous human diseases through phototherapy and imaging.
Fig. 12.
Chemical structures of potential bioactive phytocompounds discussed in this review.
5. Conclusions and perspectives
For millennia, phytochemicals have been a fruitful source for the development of successful drugs. They are recognized as a crucial pool of pharmacologically active compounds that have been used to derive many blockbuster drugs. Progress in phytochemical-based drug discovery has also led to advancements in technologies for identifying and characterizing natural products. However, the pharmaceutical applications of BPCs have been clinically limited due to their poor solubility, instability, low bioavailability, off-targetability, and consequent toxicity after administration. With advances in nanotechnology, NF strategies have been used as an alternative approach to address these inherent problems. NF strategies in pharmaceutical applications have played an essential role in i) increasing the water solubility of lipophilic BPCs, ii) improving the chemical stability of BPCs under physiological conditions, iii) preventing the aggregation of BPCs, iv) enhancing the bioavailability of hydrophilic BPCs to transport across lipid-rich biological membranes, v) facilitating targeted delivery to the desired site, and vi) sustained and stimuli-responsive release of BPCs. For example, the therapeutic and diagnostic potential of BPCs have been greatly improved through NFs based on liposomes, lipid nanoparticles, phytosomes, and organic/inorganic nanoparticles. Because the physicochemical properties of BPCs, such as molecular weight, water solubility, hydrophobicity (log p), and the number of ring structures, vary significantly, suitable nanocarriers and formulation techniques should be selected for matching with BPC properties. Many BPCs with therapeutic potential have been approved for clinical use in many countries or are under clinical study. Moreover, dozens of nanoformulated BPCs are currently in clinical trials. With increasing interest in nanomedicine and the clarification of nanotechnology-specific medical regulations, BPC-NFs are expected to help overcome the drawbacks of BPCs in providing safer and more efficacious clinical treatment options.
NF strategies can also facilitate the repurposing of BPCs. Drug development is a challenging process that requires enormous costs and long-term investment with a high risk of failure. To reduce time-to-market, development costs, and risk, investigators and scientists have proposed alternative approaches. Above all, drug repurposing has become a mainstream strategy for reducing the chance of failure in drug development [283]. Drug repurposing is the process of identifying a new use of an existing drug with an indication outside the range of its initial indication. This includes new therapeutic uses for already known drugs (repositioning) and different formulations of the same drug (reformulation). The repurposing of old drugs can shorten development time and reduce costs and risks for pharma because drug repurposing commonly starts with pre-tested compounds at a satisfactory level of safety and human tolerability.
Researchers in pharmaceutics and nanotechnology consider using nanoformulation techniques for the reformulation of BPCs with poor solubility, stability, and undesirable toxicity. This allows BPCs to gain a wide therapeutic window through targeted delivery and their controlled release. As shown in Table 5, phytochemical BPCs have been widely explored, and their potent biological activities have been unveiled. Reformulating potential BPCs with NF techniques can improve solubility, stability, and bioavailability while avoiding side effects. Moreover, indications of BPCs formulated with NFs can be extended to various diseases beyond the primary drug indication. In this regard, NF techniques may guide advancements in drug repurposing.
Table 5.
Potential BPCs for treatment of various human diseases.
| Natural compound | Natural resource | Solubility (logP)a | Biological activity | Refs |
|---|---|---|---|---|
| Allicin | Garlic | 2.02 | Antimicrobial, anticancer | [237] |
| Apigenin | Parsley, celery, celeriac, chamomile tea | 2.71 | Antimicrobial, anticancer | [238] |
| Arborinine | Herbs, spices (Ruta graveolens) | 3.15 | Anticancer | [239] |
| Baicalein | Roots of Scutellaria baicalensis and Scutellaria lateriflora | 2.71 | Antioxidant, anti-inflammatory | [240,241] |
| Berberine | Berberis | −1.3 | Antiarrhythmic action, anti-inflammatory, antiangiogenic | [145,208] |
| Betulin | Bark of birch trees | 6.17 | Anticancer | [196] |
| Bixin | Annatto | 5.53 | Apoptotic, antioxidant | [242,243] |
| Boswellic acid | Boswellia | 6.58 | Apoptosis of cancer cells, anti-inflammatory | [120] |
| Celastrol | Root extracts of Tripterygium wilfordii | 5.33 | Antioxidant, anti-inflammatory, anti-cancer | [187,244] |
| Chelerythrine | Chelidonium majus, Zanthoxylum clava-herculis | −0.88 | Antibacterial | [245] |
| Cinnamaldehyde | Bark of cinnamon trees | 1.98 | Antibacterial | [246] |
| Curcubitacin E | Cucurbitaceae | 3.48 | Anticancer, anti-inflammatory | [247] |
| 15,16-Dihydrotanshinone I | Salvia miltiorrhiza | – | Antiproliferative | [199,248] |
| Gambogic acid | Garcinia hanburyi | 7.78 | Antitumor | [249] |
| Ellagic acid | Fruits, vegetables (pomegranate) | 2.32 | Antiproliferative, antioxidant | [250] |
| Embelin | Embelia ribes | 4.18 | Anti-inflammatory | [209] |
| Eugenol | Clove oil, basil, cinnamon, nutmeg | 2.61 | Antimicrobial, anti-inflammatory | [219] |
| 6-gingerol (Gingerol) | Ginger | 3.62 | Tumor suppressive | [251] |
| Ginsenoside compound K | Panax ginseng | – | Antidementia, antitumor, anti-inflammatory | [189,252] |
| Hesperetin | Peppermint, lemon, sweet orange | 2.68 | Antioxidant, anti-inflammatory, anti-allergic, anticarcinogenic | [200,253] |
| Hydroxytyrosol | Extra virgin olive oil | 0.89 | Antioxidant, anti-inflammatory, cancer preventive | [254,255] |
| Imperatorin | Parsnips, lovages, parsley | 3.15 | Antioxidant, anti-inflammatory, antiallergic | [256,257] |
| Kaempferol | Plants (Pteridophyta, Pinophyta, Angiospermae) | 2.46 | Anticancer | [258] |
| Luteolin | Celery, broccoli, green pepper, parsley | 2.4 | Antioxidant, anti-inflammatory, chemopreventive | [71,259] |
| Madecassoside | Centella asiatica | −1.8 | Anti-inflammatory, skin hydrating | [101,260] |
| α-Mangostin | Mangosteen tree (Garcinia mangostana) | 6 | Anti-proliferative, antioxidant, anticancer | [122,261] |
| Melanin | Chestnut shell | −0.27 | Antioxidant | [164,230] |
| Morin | Osage orange, guava, bilberry | 2.16 | Antioxidant, antihypertensive, neuroprotective, anti-inflam matory | [262,263] |
| Naringenin | Grapefruit, bergamot, sour orange | 2.84 | Antioxidant, free radical scavenger, anti-inflammatory | [264] |
| Oleuropein | Green olives, olive leaves, organ oil | 0.11 | Antioxidant, anticancer | [265] |
| Oxyresveratrol | Artocarpus lakoocha, white mulberry | 0.83 | Antioxidant, potent tyrosinase inhibitor | [266] |
| Paeonol | Peonies (Paeonia suffruticosa) | 1.72 | Anti-inflammatory, anticancer | [211,267] |
| Pheophorbide | Plants with chlorophyll | A 3.43, B 2.62 | Photosensitizer, therapeutic | [232,268] |
| Rosmarinic acid | Rosemary, mint, sage, thyme, lemon balm | 3.00 | Antitumor, antimicrobial neuroprotective, osteiarthritis pain reduction | [147] |
| Rutin | Citrus fruit | −0.87 | Preventive effect of blood clotting | [221,222] |
| Salidroside | Rhodiola rosea | −0.58 | Antidepressant, anxiolytic | [211,269] |
| Sanguinarine | Sanguinaria canadensis | −0.94 | Antifungal, anticarcinogenic | [270,271] |
| Schisantherin A | Schisandra chinensis | 4.9 | Anti-parkinsonian, anti-inflammatory | [186,272] |
| Scutellarin | Scutellaria barbata | – | Antitumor | [273] |
| Shikonin | Lithospermum erythrorhizon | 2.12 | Antibacterial, anti-inflammatory, anticancer | [202,274] |
| Sinigrin | Brassica vegetables | −3.2 | Anticancer, antibacterial, antioxidant | [275,276] |
| β-sitosterol | Wheat germ, rice bran, flax seeds, peanuts, soybeans | 7.84 | Cholesterol-lowering | [277] |
| Syringic acid | Ardisia elliptica | 1.01 | Antioxidant | [278] |
| Tanshinone I | Herbs (Salvia miltiorrhiza Bunge) | 4.00 | Anticancer, neuroprotective | [199,248] |
| Taxifolin | Wood (Siberian larch), cloudberry, macadamia nut | 1.82 | Antioxidant, anti-inflammatory, cardiovascular, hepatoprotective | [279] |
| Tetramethylpyrazine | Natto, fermented cocoa bean | 0.063 | Anti-inflammatory, antiplatelet | [106,280] |
| Tetrandrine (Isotetrandrine) | Root of Radix stephania tetrandrae | 6.48 | Anti-inflammatory, anticancer | [281] |
| Tyrosol | Wine, virgin olive oil | 1.19 | Antioxidant | [255] |
| Zerumbone | Zingiber zerumbet Smith | 4.57 | Chemopreventive | [282] |
log P was obtained from ChemAxon.
When considering repurposing BPCs, the rational selection of proper types of nanocarriers depending on the physicochemical properties of BCPs is imperative. In this regard, researchers or pharmaceuticals should have an in-depth understanding of the intrinsic characteristics of nanocarriers. For instance, liposomes are suitable for encapsulating hydrophilic BPCs or their derivatives in their hydrophilic inner core. Although they can also load hydrophobic molecules in the lipid bilayers, the encapsulation efficiency and capacity are limited. In particular, hydrophobic BPCs with bulky chemical structures and large molecular weight are not good candidates for liposomes.
On the other hand, self-assembled polymeric nanoparticles or micelles can only incorporate hydrophobic BPCs, not hydrophilic ones. Those types of NFs also have concerns about their long-term stability, low BPC loading efficiency/capacity, and unwanted burst release. Meanwhile, relatively low biodegradability and long-term organ toxicity are the concerns about the inorganic nanocarriers. Overall, the short shelf-life and high production costs of BPC-NFs remain the critical drawbacks to be addressed.
Furthermore, despite the considerable advancement of NF techniques for BPCs, the clinical use of BPC-NFs to date is still impeded due to the following issues. Standard protocols for the physico-biochemical characterization of BPC-NFs, including safety and toxicity, are still lacking. Various factors, such as size, shape, surface properties, BPC release profiles, stability, and interactions with biological systems, should be fully characterized for clinical use of the BPC-NFs. Moreover, although several BPC-NFs are already approved for clinical use, the specific regulatory guidelines for approval processes for certain types of BPC-NFs are still lacking, hampering their clinical applications. Therefore, significant efforts should be dedicated to coming up with standard characterization protocols and specific regulations for the clinical use of BPC-NF.
Overall, emerging NF strategies for BPCs can play a pivotal role in the development of next-generation drugs for extended therapeutic applications. Therefore, great efforts are required from researchers and industry to develop various NF techniques and optimize them to maximize human healthcare benefits. In doing so, there is a significant opportunity to take BPC-NFs from bench to bedside and make a meaningful clinical impact with rational design.
CRediT authorship contribution statement
Hwa Seung Han: Conceptualization, Investigation, Writing – original draft. Song Yi Koo: Investigation. Ki Young Choi: Conceptualization, Funding acquisition, Project administration, Writing – original draft, Supervision.
Declaration of competing interest
The authors have no competing interests to declare.
Acknowledgments
This research was supported by Basic Science Research Programs through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2017R1D1A1B03034888, 2021R1A6A3A01086719 and 2015R1A6A3A04059033) and a Korea Institute of Science and Technology (KIST) intramural research grant.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Li J.W.H., Vederas J.C. Drug discovery and natural products: end of an era or an endless frontier? Science. 2009;325:161–165. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]
- 2.Shen B. A new golden age of natural products drug discovery. Cell. 2015;163:1297–1300. doi: 10.1016/j.cell.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harvey A.L., Edrada-Ebel R., Quinn R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015;14:111–129. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- 4.Borchardt J.K. The beginnings of drug therapy: ancient mesopotamian medicine. Drug News Perspect. 2002;15:187–192. doi: 10.1358/dnp.2002.15.3.840015. [DOI] [PubMed] [Google Scholar]
- 5.Sandhya B., Thomas S., Isabel W., Shenbagarathai R. Ethnomedicinal plants used by the valaiyan community of piranmalai hills (reserved forest), tamilnadu, India. A pilot study. Afr. J. Tradit., Complementary Altern. Med. 2006;3:101–114. [Google Scholar]
- 6.Veeresham C. Natural products derived from plants as a source of drugs. "J. Adv. Pharm. Technol. Research"" (JAPTR)". 2012;3:200–201. doi: 10.4103/2231-4040.104709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cos P., Vlietinck A.J., Berghe D.V., Maes L. Anti-infective potential of natural products: how to develop a stronger in vitro ‘proof-of-concept. J. Ethnopharmacol. 2006;106:290–302. doi: 10.1016/j.jep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Kellogg J.J., Todd D.A., Egan J.M., Raja H.A., Oberlies N.H., Kvalheim O.M., Cech N.B. Biochemometrics for natural products research: comparison of data analysis approaches and application to identification of bioactive compounds. J. Nat. Prod. 2016;79:376–386. doi: 10.1021/acs.jnatprod.5b01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katiyar C., Gupta A., Kanjilal S., Katiyar S. Drug discovery from plant sources: an integrated approach. Ayu. 2012;33:10–19. doi: 10.4103/0974-8520.100295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanishlieva N.V., Marinova E., Pokorný J. Natural antioxidants from herbs and spices. Eur. J. Lipid Sci. Technol. 2006;108:776–793. [Google Scholar]
- 11.Gordaliza M. Natural products as leads to anticancer drugs. Clin. Transl. Oncol. 2007;9:767–776. doi: 10.1007/s12094-007-0138-9. [DOI] [PubMed] [Google Scholar]
- 12.Albarracin S.L., Stab B., Casas Z., Sutachan J.J., Samudio I., Gonzalez J., Gonzalo L., Capani F., Morales L., Barreto G.E. Effects of natural antioxidants in neurodegenerative disease. Nutr. Neurosci. 2012;15:1–9. doi: 10.1179/1476830511Y.0000000028. [DOI] [PubMed] [Google Scholar]
- 13.Moloney M.G. Natural products as a source for novel antibiotics. Trends Pharmacol. Sci. 2016;37:689–701. doi: 10.1016/j.tips.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasconcelos T., Sarmento B., Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today. 2007;12:1068–1075. doi: 10.1016/j.drudis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Kalepu S., Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm. Sin. B. 2015;5:442–453. doi: 10.1016/j.apsb.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savjani K.T., Gajjar A.K., Savjani J.K. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012:1–10. doi: 10.5402/2012/195727. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thilakarathna S., Rupasinghe H. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients. 2013;5:3367–3387. doi: 10.3390/nu5093367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manach C., Williamson G., Morand C., Scalbert A., Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 20.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 21.Sharma R.A. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004;10:6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 22.Ward D.J., Martino O.I., Simpson S., Stevens A.J. Decline in new drug launches: myth or reality? Retrospective observational study using 30yearsof data from the UK. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hay M., Thomas D.W., Craighead J.L., Economides C., Rosenthal J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 2014;32:40–51. doi: 10.1038/nbt.2786. [DOI] [PubMed] [Google Scholar]
- 24.Sercombe L., Veerati T., Moheimani F., Wu S.Y., Sood A.K., Hua S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kataoka K., Harada A., Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv. Drug Deliv. Rev. 2012;64:37–48. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z., Jiao Y., Wang Y., Zhou C., Zhang Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008;60:1650–1662. doi: 10.1016/j.addr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Sarker D.K. Engineering of nanoemulsions for drug delivery. Curr. Drug Deliv. 2005;2:297–310. doi: 10.2174/156720105774370267. [DOI] [PubMed] [Google Scholar]
- 28.Kataoka K., Harada A., Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001;47:113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 29.Jeevanandam J., Chan Y.S., Danquah M.K. Nano-formulations of drugs: recent developments, impact and challenges. Biochimie. 2016;128–129:99–112. doi: 10.1016/j.biochi.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., Rodriguez-Torres M.d.P., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S., Habtemariam S., Shin H.-S. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 2018;16:71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen W., Liu C., Ji X., Joseph J., Tang Z., Ouyang J., Xiao Y., Kong N., Joshi N., Farokhzad O.C., Tao W., Xie T. Stanene-based nanosheets for β-elemene delivery and ultrasound-mediated combination cancer therapy. Angew. Chem. Int. Ed. 2021;60:7155–7164. doi: 10.1002/anie.202016330. [DOI] [PubMed] [Google Scholar]
- 32.Han H.S., Choi K.Y., Ko H., Jeon J., Saravanakumar G., Suh Y.D., Lee D.S., Park J.H. Bioreducible core-crosslinked hyaluronic acid micelle for targeted cancer therapy. J. Contr. Release. 2015;200:158–166. doi: 10.1016/j.jconrel.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 33.Han H.S., Lee J., Kim H.R., Chae S.Y., Kim M., Saravanakumar G., Yoon H.Y., You D.G., Ko H., Kim K., Kwon I.C., Park J.C., Park J.H. Robust PEGylated hyaluronic acid nanoparticles as the carrier of doxorubicin: mineralization and its effect on tumor targetability in vivo. J. Contr. Release. 2013;168:105–114. doi: 10.1016/j.jconrel.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 34.Han H.S., Thambi T., Choi K.Y., Son S., Ko H., Lee M.C., Jo D.-G., Chae Y.S., Kang Y.M., Lee J.Y., Park J.H. Bioreducible shell-cross-linked hyaluronic acid nanoparticles for tumor-targeted drug delivery. Biomacromolecules. 2015;16:447–456. doi: 10.1021/bm5017755. [DOI] [PubMed] [Google Scholar]
- 35.Khatoon S., Han H.S., Jeon J., Rao N.V., Jeong D.-W., Ikram M., Yasin T., Yi G.-R., Park J.H. Hypoxia-responsive mesoporous nanoparticles for doxorubicin delivery. Polymers. 2018;10 doi: 10.3390/polym10040390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baeza A., Guisasola E., Ruiz-Hernández E., Vallet-Regí M. Magnetically triggered multidrug release by hybrid mesoporous silica nanoparticles. Chem. Mater. 2012;24:517–524. [Google Scholar]
- 37.Swierczewska M., Han H.S., Kim K., Park J.H., Lee S. Polysaccharide-based nanoparticles for theranostic nanomedicine. Adv. Drug Deliv. Rev. 2016;99:70–84. doi: 10.1016/j.addr.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi C., Guo D., Xiao K., Wang X., Wang L., Luo J. A drug-specific nanocarrier design for efficient anticancer therapy. Nat. Commun. 2015;6:7449. doi: 10.1038/ncomms8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medina O.P., Zhu Y., Kairemo K. Targeted liposomal drug delivery in cancer. Curr. Pharmaceut. Des. 2004;10:2981–2989. doi: 10.2174/1381612043383467. [DOI] [PubMed] [Google Scholar]
- 40.Bangham A.D., Horne R.W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964;8 doi: 10.1016/s0022-2836(64)80115-7. 660-IN610. [DOI] [PubMed] [Google Scholar]
- 41.Riaz M.K., Riaz M.A., Zhang X., Lin C., Wong K.H., Chen X., Zhang G., Lu A., Yang Z. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: a review. Int. J. Mol. Sci. 2018;19:195. doi: 10.3390/ijms19010195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benech R.O., Kheadr E.E., Laridi R., Lacroix C., Fliss I. Inhibition of Listeria innocua in cheddar cheese by addition of nisin Z in liposomes or by in situ production in mixed culture. Appl. Environ. Microbiol. 2002;68:3683. doi: 10.1128/AEM.68.8.3683-3690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akbarzadeh A., Rezaei-Sadabady R., Davaran S., Joo S.W., Zarghami N., Hanifehpour Y., Samiei M., Kouhi M., Nejati-Koshki K. Liposome: classification, preparation, and applications. Nanoscale Res. Lett. 2013;8:102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shehata T., Ogawara K.-i., Higaki K., Kimura T. Prolongation of residence time of liposome by surface-modification with mixture of hydrophilic polymers. Int. J. Pharm. 2008;359:272–279. doi: 10.1016/j.ijpharm.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Al-Jamal W.T., Kostarelos K. Liposomes: from a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc. Chem. Res. 2011;44:1094–1104. doi: 10.1021/ar200105p. [DOI] [PubMed] [Google Scholar]
- 46.Allen T.M., Cullis P.R. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 47.Torchilin V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 48.Fenske D.B., Cullis P.R. Liposomal nanomedicines. Expert Opin. Drug Deliv. 2008;5:25–44. doi: 10.1517/17425247.5.1.25. [DOI] [PubMed] [Google Scholar]
- 49.Bozzuto G., Molinari A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015;10:975–999. doi: 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitchell M.J., Billingsley M.M., Haley R.M., Wechsler M.E., Peppas N.A., Langer R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021;20:101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Onoue S., Yamada S., Chan H.-K. Nanodrugs: pharmacokinetics and safety. Int. J. Nanomed. 2014;9:1025–1037. doi: 10.2147/IJN.S38378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Owens D.E., 3rd, Peppas N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 53.Mamot C., Ritschard R., Wicki A., Stehle G., Dieterle T., Bubendorf L., Hilker C., Deuster S., Herrmann R., Rochlitz C. Tolerability, safety, pharmacokinetics, and efficacy of doxorubicin-loaded anti-EGFR immunoliposomes in advanced solid tumours: a phase 1 dose-escalation study. Lancet Oncol. 2012;13:1234–1241. doi: 10.1016/S1470-2045(12)70476-X. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y., Ran R., Chen J., Kuang Q., Tang J., Mei L., Zhang Q., Gao H., Zhang Z., He Q. Paclitaxel loaded liposomes decorated with a multifunctional tandem peptide for glioma targeting. Biomaterials. 2014;35:4835–4847. doi: 10.1016/j.biomaterials.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 55.Al-Jamal W.T., Al-Ahmady Z.S., Kostarelos K. Pharmacokinetics & tissue distribution of temperature-sensitive liposomal doxorubicin in tumor-bearing mice triggered with mild hyperthermia. Biomaterials. 2012;33:4608–4617. doi: 10.1016/j.biomaterials.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 56.Al-Ahmady Z., Kostarelos K. Chemical components for the design of temperature-responsive vesicles as cancer therapeutics. Chem. Rev. 2016;116:3883–3918. doi: 10.1021/acs.chemrev.5b00578. [DOI] [PubMed] [Google Scholar]
- 57.Alam M.S., Ahad A., Abidin L., Aqil M., Mir S.R., Mujeeb M. Embelin-loaded oral niosomes ameliorate streptozotocin-induced diabetes in Wistar rats. Biomed. Pharmacother. 2018;97:1514–1520. doi: 10.1016/j.biopha.2017.11.073. [DOI] [PubMed] [Google Scholar]
- 58.Barani M., Mirzaei M., Torkzadeh-Mahani M., Nematollahi M.H. Lawsone-loaded Niosome and its antitumor activity in MCF-7 breast Cancer cell line: a Nano-herbal treatment for Cancer. DARU J. Pharm. Sci. 2018;26:11–17. doi: 10.1007/s40199-018-0207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sadeghi Ghadi Z., Ebrahimnejad P. Curcumin entrapped hyaluronan containing niosomes: preparation, characterisation and in vitro/in vivo evaluation. J. Microencapsul. 2019;36:169–179. doi: 10.1080/02652048.2019.1617360. [DOI] [PubMed] [Google Scholar]
- 60.Kazi K.M., Mandal A.S., Biswas N., Guha A., Chatterjee S., Behera M., Kuotsu K. Niosome: a future of targeted drug delivery systems. "J. Adv. Pharm. Technol. Research"" (JAPTR)". 2010;1:374–380. doi: 10.4103/0110-5558.76435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yeo P.L., Lim C.L., Chye S.M., Kiong Ling A.P., Koh R.Y. Niosomes: a review of their structure, properties, methods of preparation, and medical applications. Asian Biomed. 2017;11:301–314. [Google Scholar]
- 62.Duong V.-A., Nguyen T.-T.-L., Maeng H.-J. Preparation of solid lipid nanoparticles and nanostructured lipid carriers for drug delivery and the effects of preparation parameters of solvent injection method. Molecules. 2020;25 doi: 10.3390/molecules25204781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wissing S.A., Kayser O., Müller R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004;56:1257–1272. doi: 10.1016/j.addr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 64.Joshi M.D., Müller R.H. Lipid nanoparticles for parenteral delivery of actives. Eur. J. Pharm. Biopharm. 2009;71:161–172. doi: 10.1016/j.ejpb.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 65.Mehnert W., Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv. Drug Deliv. Rev. 2012;64:83–101. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 66.zur Mühlen A., Schwarz C., Mehnert W. Solid lipid nanoparticles (SLN) for controlled drug delivery – drug release and release mechanism. Eur. J. Pharm. Biopharm. 1998;45:149–155. doi: 10.1016/s0939-6411(97)00150-1. [DOI] [PubMed] [Google Scholar]
- 67.Hao J., Wang F., Wang X., Zhang D., Bi Y., Gao Y., Zhao X., Zhang Q. Development and optimization of baicalin-loaded solid lipid nanoparticles prepared by coacervation method using central composite design. Eur. J. Pharmaceut. Sci. 2012;47:497–505. doi: 10.1016/j.ejps.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 68.Westesen K., Bunjes H., Koch M.H.J. Physicochemical characterization of lipid nanoparticles and evaluation of their drug loading capacity and sustained release potential. J. Contr. Release. 1997;48:223–236. [Google Scholar]
- 69.Pardeike J., Hommoss A., Müller R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009;366:170–184. doi: 10.1016/j.ijpharm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 70.Sangsen Y., Wiwattanawongsa K., Likhitwitayawuid K., Sritularak B., Wiwattanapatapee R. Modification of oral absorption of oxyresveratrol using lipid based nanoparticles. Colloids Surf. B Biointerfaces. 2015;131:182–190. doi: 10.1016/j.colsurfb.2015.04.055. [DOI] [PubMed] [Google Scholar]
- 71.Khan J., Alexander A., Ajazuddin, Saraf S., Saraf S. Luteolin–phospholipid complex: preparation, characterization and biological evaluation. J. Pharm. Pharmacol. 2014;66:1451–1462. doi: 10.1111/jphp.12280. [DOI] [PubMed] [Google Scholar]
- 72.Khan J., Alexander A., Ajazuddin, Saraf S., Saraf S. Recent advances and future prospects of phyto-phospholipid complexation technique for improving pharmacokinetic profile of plant actives. J. Contr. Release. 2013;168:50–60. doi: 10.1016/j.jconrel.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 73.El-Menshawe S., Ali A., Rabeh M., Khalil N. Nanosized soy phytosome-based thermogel as topical anti-obesity formulation: an approach for acceptable level of evidence of an effective novel herbal weight loss product. Int. J. Nanomed. 2018;13:307–318. doi: 10.2147/IJN.S153429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saraf S., Kaur C.D. Phytoconstituents as photoprotective novel cosmetic formulations. Pharmacogn. Rev. 2010;4:1–11. doi: 10.4103/0973-7847.65319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu M., Qiu Q., Luo X., Liu X., Sun J., Wang C., Lin X., Deng Y., Song Y. Phyto-phospholipid complexes (phytosomes): a novel strategy to improve the bioavailability of active constituents. Asian J. Pharm. Sci. 2019;14:265–274. doi: 10.1016/j.ajps.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brannon-Peppas L., Blanchette J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2004;56:1649–1659. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 77.Peer D., Karp J.M., Hong S., Farokhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 78.Jia L. Nanoparticle formulation increases oral bioavailability of poorly soluble drugs: approaches experimental evidences and theory. Curr. Nanosci. 2005;1:237–243. doi: 10.2174/157341305774642939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wais U., Jackson A.W., He T., Zhang H. Nanoformulation and encapsulation approaches for poorly water-soluble drug nanoparticles. Nanoscale. 2016;8:1746–1769. doi: 10.1039/c5nr07161e. [DOI] [PubMed] [Google Scholar]
- 80.Wang J., Wang Y., Liu Q., Yang L., Zhu R., Yu C., Wang S. Rational design of multifunctional dendritic mesoporous silica nanoparticles to load curcumin and enhance efficacy for breast cancer therapy. ACS Appl. Mater. Interfaces. 2016;8:26511–26523. doi: 10.1021/acsami.6b08400. [DOI] [PubMed] [Google Scholar]
- 81.Chen X., Ling X., Zhao L., Xiong F., Hollett G., Kang Y., Barrett A., Wu J. Biomimetic shells endow sub-50 nm nanoparticles with ultrahigh paclitaxel payloads for specific and robust chemotherapy. ACS Appl. Mater. Interfaces. 2018;10:33976–33985. doi: 10.1021/acsami.8b11571. [DOI] [PubMed] [Google Scholar]
- 82.Pereira G.G., Detoni C.B., Balducci A.G., Rondelli V., Colombo P., Guterres S.S., Sonvico F. Hyaluronate nanoparticles included in polymer films for the prolonged release of vitamin E for the management of skin wounds. Eur. J. Pharmaceut. Sci. 2016;83:203–211. doi: 10.1016/j.ejps.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 83.Karthivashan G., Masarudin M.J., Kura A.U., Abas F., Fakurazi S. Optimization, formulation, and characterization of multiflavonoids-loaded flavanosome by bulk or sequential technique. Int. J. Nanomed. 2016;11:3417–3434. doi: 10.2147/IJN.S112045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bae K.H., Tan S., Yamashita A., Ang W.X., Gao S.J., Wang S., Chung J.E., Kurisawa M. Hyaluronic acid-green tea catechin micellar nanocomplexes: fail-safe cisplatin nanomedicine for the treatment of ovarian cancer without off-target toxicity. Biomaterials. 2017;148:41–53. doi: 10.1016/j.biomaterials.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 85.Li W., Yalcin M., Lin Q., Ardawi M.-S.M., Mousa S.A. Self-assembly of green tea catechin derivatives in nanoparticles for oral lycopene delivery. J. Contr. Release. 2017;248:117–124. doi: 10.1016/j.jconrel.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 86.McClements D.J., Decker E.A., Weiss J. Emulsion-based delivery systems for lipophilic bioactive components. J. Food Sci. 2007;72:R109–R124. doi: 10.1111/j.1750-3841.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- 87.Yukuyama M.N., Ghisleni D.D.M., Pinto T.J.A., Bou-Chacra N.A. Nanoemulsion: process selection and application in cosmetics – a review. Int. J. Cosmet. Sci. 2016;38:13–24. doi: 10.1111/ics.12260. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Y., Shang Z., Gao C., Du M., Xu S., Song H., Liu T. Nanoemulsion for solubilization, stabilization, and in vitro release of pterostilbene for oral delivery. AAPS PharmSciTech. 2014;15:1000–1008. doi: 10.1208/s12249-014-0129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu H., Huang Q. Improving the oral bioavailability of curcumin using novel organogel-based nanoemulsions. J. Agric. Food Chem. 2012;60:5373–5379. doi: 10.1021/jf300609p. [DOI] [PubMed] [Google Scholar]
- 90.Lacatusu I., Badea N., Stan R., Meghea A. Novel bio-active lipid nanocarriers for the stabilization and sustained release of sitosterol. Nanotechnology. 2012;23:455702. doi: 10.1088/0957-4484/23/45/455702. [DOI] [PubMed] [Google Scholar]
- 91.Yin J., Xiang C., Wang P., Yin Y., Hou Y. Biocompatible nanoemulsions based on hemp oil and less surfactants for oral delivery of baicalein with enhanced bioavailability. Int. J. Nanomed. 2017;12:2923–2931. doi: 10.2147/IJN.S131167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ahmad N., Ahmad R., Alam M.A., Samim M., Iqbal Z., Ahmad F.J. Quantification and evaluation of thymoquinone loaded mucoadhesive nanoemulsion for treatment of cerebral ischemia. Int. J. Biol. Macromol. 2016;88:320–332. doi: 10.1016/j.ijbiomac.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 93.Singh Y., Meher J.G., Raval K., Khan F.A., Chaurasia M., Jain N.K., Chourasia M.K. Nanoemulsion: concepts, development and applications in drug delivery. J. Contr. Release. 2017;252:28–49. doi: 10.1016/j.jconrel.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 94.Ma M., Xu H., Chen H., Jia X., Zhang K., Wang Q., Zheng S., Wu R., Yao M., Cai X., Li F., Shi J. A drug–perfluorocarbon nanoemulsion with an ultrathin silica coating for the synergistic effect of chemotherapy and ablation by high-intensity focused ultrasound. Adv. Mater. 2014;26:7378–7385. doi: 10.1002/adma.201402969. [DOI] [PubMed] [Google Scholar]
- 95.Chuang E.-Y., Lin K.-J., Huang T.-Y., Chen H.-L., Miao Y.-B., Lin P.-Y., Chen C.-T., Juang J.-H., Sung H.-W. An intestinal “transformers”-like nanocarrier system for enhancing the oral bioavailability of poorly water-soluble drugs. ACS Nano. 2018;12:6389–6397. doi: 10.1021/acsnano.8b00470. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Y.J., Zhan X., Wang L., Ho R.J.Y., Sasaki T. pH-responsive artemisinin dimer in lipid nanoparticles are effective against human breast cancer in a xenograft model. J. Pharm. Sci. 2015;104:1815–1824. doi: 10.1002/jps.24407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dhule S.S., Penfornis P., Frazier T., Walker R., Feldman J., Tan G., He J., Alb A., John V., Pochampally R. Curcumin-loaded gamma-cyclodextrin liposomal nanoparticles as delivery vehicles for osteosarcoma. Nanomedicine. 2012;8:440–451. doi: 10.1016/j.nano.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hardiansyah A., Yang M.C., Liu T.Y., Kuo C.Y., Huang L.Y., Chan T.Y. Hydrophobic drug-loaded PEGylated magnetic liposomes for drug-controlled release. Nanoscale Res. Lett. 2017;12:355. doi: 10.1186/s11671-017-2119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ibrahim S., Tagami T., Kishi T., Ozeki T. Curcumin marinosomes as promising nano-drug delivery system for lung cancer. Int. J. Pharm. 2018;540:40–49. doi: 10.1016/j.ijpharm.2018.01.051. [DOI] [PubMed] [Google Scholar]
- 100.Habib L., Jraij A., Khreich N., Fessi H., Charcosset C., Greige-Gerges H. Morphological and physicochemical characterization of liposomes loading cucurbitacin E, an anti-proliferative natural tetracyclic triterpene. Chem. Phys. Lipids. 2014;177:64–70. doi: 10.1016/j.chemphyslip.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 101.Wang H., Liu M., Du S. Optimization of madecassoside liposomes using response surface methodology and evaluation of its stability. Int. J. Pharm. 2014;473:280–285. doi: 10.1016/j.ijpharm.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 102.Zhang L., Sheng D., Wang D., Yao Y., Yang K., Wang Z., Deng L., Chen Y. Bioinspired multifunctional melanin-based nanoliposome for photoacoustic/magnetic resonance imaging-guided efficient photothermal ablation of cancer. Theranostics. 2018;8:1591–1606. doi: 10.7150/thno.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ren J., Fang Z., Jiang L., Du Q. Quercetin-containing self-assemble proliposome preparation and evaluation. J. Liposome Res. 2017;27:335–342. doi: 10.1080/08982104.2016.1239635. [DOI] [PubMed] [Google Scholar]
- 104.Shao P., Wang P., Niu B., Kang J. Environmental stress stability of pectin-stabilized resveratrol liposomes with different degree of esterification. Int. J. Biol. Macromol. 2018;119:53–59. doi: 10.1016/j.ijbiomac.2018.07.139. [DOI] [PubMed] [Google Scholar]
- 105.Liu Y., Sun C., Li W., Adu-Frimpong M., Wang Q., Yu J., Xu X. Preparation and characterization of syringic acid–loaded TPGS liposome with enhanced oral bioavailability and in vivo antioxidant efficiency. AAPS PharmSciTech. 2019;20 doi: 10.1208/s12249-019-1290-6. [DOI] [PubMed] [Google Scholar]
- 106.Xia H., Cheng Z., Cheng Y., Xu Y. Investigating the passage of tetramethylpyrazine-loaded liposomes across blood-brain barrier models in vitro and ex vivo. Mater. Sci. Eng. C. 2016;69:1010–1017. doi: 10.1016/j.msec.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 107.Zhou W., Liu W., Zou L., Liu W., Liu C., Liang R., Chen J. Storage stability and skin permeation of vitamin C liposomes improved by pectin coating. Colloids Surf. B Biointerfaces. 2014;117:330–337. doi: 10.1016/j.colsurfb.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 108.Sugasini D., Lokesh B.R. Curcumin and linseed oil co-delivered in phospholipid nanoemulsions enhances the levels of docosahexaenoic acid in serum and tissue lipids of rats. Prostaglandins Leukot. Essent. Fatty Acids. 2017;119:45–52. doi: 10.1016/j.plefa.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 109.Liang X., Chen X., Zhao G., Tang T., Dong W., Wang C., Zhang J., Liao Z. Preparation, characterization, and pharmacokinetic evaluation of imperatorin lipid microspheres and their effect on the proliferation of MDA-MB-231 cells. Pharmaceutics. 2018;10:236. doi: 10.3390/pharmaceutics10040236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ha T.V.A., Kim S., Choi Y., Kwak H.-S., Lee S.J., Wen J., Oey I., Ko S. Antioxidant activity and bioaccessibility of size-different nanoemulsions for lycopene-enriched tomato extract. Food Chem. 2015;178:115–121. doi: 10.1016/j.foodchem.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 111.Khorsandi K., Hosseinzadeh R., Fateh M. Curcumin intercalated layered double hydroxide nanohybrid as a potential drug delivery system for effective photodynamic therapy in human breast cancer cells. RSC Adv. 2015;5:93987–93994. [Google Scholar]
- 112.Khorsandi K., Hosseinzadeh R., Shahidi F.K. Photodynamic treatment with anionic nanoclays containing curcumin on human triple-negative breast cancer cells: cellular and biochemical studies. J. Cell. Biochem. 2019;120:4998–5009. doi: 10.1002/jcb.27775. [DOI] [PubMed] [Google Scholar]
- 113.Hosseinzadeh R., Khorsandi K. Methylene blue, curcumin and ion pairing nanoparticles effects on photodynamic therapy of MDA-MB-231 breast cancer cell. Photodiagnosis Photodyn. Ther. 2017;18:284–294. doi: 10.1016/j.pdpdt.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 114.Chopra D., Ray L., Dwivedi A., Tiwari S.K., Singh J., Singh K.P., Kushwaha H.N., Jahan S., Pandey A., Gupta S.K., Chaturvedi R.K., Pant A.B., Ray R.S., Gupta K.C. Photoprotective efficiency of PLGA-curcumin nanoparticles versus curcumin through the involvement of ERK/AKT pathway under ambient UV-R exposure in HaCaT cell line. Biomaterials. 2016;84:25–41. doi: 10.1016/j.biomaterials.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 115.Zeng K., Xu Q., Ouyang J., Han Y., Sheng J., Wen M., Chen W., Liu Y.-N. Coordination nanosheets of phthalocyanine as multifunctional platform for imaging-guided synergistic therapy of cancer. ACS Appl. Mater. Interfaces. 2019;11:6840–6849. doi: 10.1021/acsami.8b22008. [DOI] [PubMed] [Google Scholar]
- 116.Wang Y., Wu Y., Li K., Shen S., Liu Z., Wu D. Ultralong circulating lollipop-like nanoparticles assembled with gossypol, doxorubicin, and polydopamine via π–π stacking for synergistic tumor therapy. Adv. Funct. Mater. 2019;29:1805582. [Google Scholar]
- 117.Fang J., Zhang S., Xue X., Zhu X., Song S., Wang B., Jiang L., Qin M., Liang H., Gao L. Quercetin and doxorubicin co-delivery using mesoporous silica nanoparticles enhance the efficacy of gastric carcinoma chemotherapy. Int. J. Nanomed. 2018;13:5113–5126. doi: 10.2147/IJN.S170862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rao M.P., Manjunath K., Bhagawati S.T., Thippeswamy B.S. Bixin loaded solid lipid nanoparticles for enhanced hepatoprotection – preparation, characterisation and in vivo evaluation. Int. J. Pharm. 2014;473:485–492. doi: 10.1016/j.ijpharm.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 119.Rahman H., Rasedee A., How C.W., Abdul A.B., Allaudin Z.N., Othman H.H., Saeed M., Yeap S.K. Zerumbone-loaded nanostructured lipid carriers: preparation, characterization, and antileukemic effect. Int. J. Nanomed. 2013;8:2769–2781. doi: 10.2147/IJN.S45313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mehta M., Dureja H., Garg M. Development and optimization of boswellic acid-loaded proniosomal gel. Drug Deliv. 2016;23:3072–3081. doi: 10.3109/10717544.2016.1149744. [DOI] [PubMed] [Google Scholar]
- 121.Obeid M.A., Khadra I., Albaloushi A., Mullin M., Alyamani H., Ferro V.A. Microfluidic manufacturing of different niosomes nanoparticles for curcumin encapsulation: physical characteristics, encapsulation efficacy, and drug release. Beilstein J. Nanotechnol. 2019;10:1826–1832. doi: 10.3762/bjnano.10.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chin G.S., Todo H., Kadhum W.R., Hamid M.A., Sugibayashi K. In vitro permeation and skin retention of α-mangostin proniosome. C Chem. Pharm. Bull. 2016;64:1666–1673. doi: 10.1248/cpb.c16-00425. [DOI] [PubMed] [Google Scholar]
- 123.Artiga-Artigas M., Lanjari-Pérez Y., Martín-Belloso O. Curcumin-loaded nanoemulsions stability as affected by the nature and concentration of surfactant. Food Chem. 2018;266:466–474. doi: 10.1016/j.foodchem.2018.06.043. [DOI] [PubMed] [Google Scholar]
- 124.Saralkar P., Dash A.K. Alginate nanoparticles containing curcumin and resveratrol: preparation, characterization, and in vitro evaluation against DU145 prostate cancer cell line. AAPS PharmSciTech. 2017;18:2814–2823. doi: 10.1208/s12249-017-0772-7. [DOI] [PubMed] [Google Scholar]
- 125.Cragg G.M., Pezzuto J.M. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med. Princ. Pract. 2016;25(suppl 2):41–59. doi: 10.1159/000443404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cragg G.M., Grothaus P.G., Newman D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009;109:3012–3043. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]
- 127.Demain A.L., Vaishnav P. Natural products for cancer chemotherapy. Biotechnol. 2011;4:687–699. doi: 10.1111/j.1751-7915.2010.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tachibana H., Koga K., Fujimura Y., Yamada K. A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 2004;11:380–381. doi: 10.1038/nsmb743. [DOI] [PubMed] [Google Scholar]
- 129.Garbisa S., Biggin S., Cavallarin N., Sartor L., Benelli R., Albini A. Tumor invasion: molecular shears blunted by green tea. Nat. Med. 1999;5 doi: 10.1038/15145. 1216-1216. [DOI] [PubMed] [Google Scholar]
- 130.Chung J.E., Tan S., Gao S.J., Yongvongsoontorn N., Kim S.H., Lee J.H., Choi H.S., Yano H., Zhuo L., Kurisawa M., Ying J.Y. Self-assembled micellar nanocomplexes comprising green tea catechin derivatives and protein drugs for cancer therapy. Nat. Nanotechnol. 2014;9:907–912. doi: 10.1038/nnano.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hale J.J., Mills S.G., MacCoss M., Dorn C.P., Finke P.E., Budhu R.J., Reamer R.A., Huskey S.-E.W., Luffer-Atlas D., Dean B.J., McGowan E.M., Feeney W.P., Chiu S.-H.L., Cascieri M.A., Chicchi G.G., Kurtz M.M., Sadowski S., Ber E., Tattersall F.D., Rupniak N.M.J., Williams A.R., Rycroft W., Hargreaves R., Metzger J.M., MacIntyre D.E. Phosphorylated morpholine acetal human neurokinin-1 receptor antagonists as water-soluble prodrugs. J. Med. Chem. 2000;43:1234–1241. doi: 10.1021/jm990617v. [DOI] [PubMed] [Google Scholar]
- 132.Senter P.D., Schreiber G.J., Hirschberg D.L., Ashe S.A., Hellström K.E., Hellström I. Enhancement of the in vitro and in vivo antitumor activities of phosphorylated mitomycin C and etoposide derivatives by monoclonal antibody-alkaline phosphatase conjugates. Cancer Res. 1989;49:5789. [PubMed] [Google Scholar]
- 133.Hu K., Miao L., Goodwin T.J., Li J., Liu Q., Huang L. Quercetin remodels the tumor microenvironment to improve the permeation, retention, and antitumor effects of nanoparticles. ACS Nano. 2017;11:4916–4925. doi: 10.1021/acsnano.7b01522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fahlman B.M., Krol E.S. UVA and UVB radiation-induced oxidation products of quercetin. J. Photochem. Photobiol. B Biol. 2009;97:123–131. doi: 10.1016/j.jphotobiol.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 135.Lin C., Yu Y., Zhao H.-G., Yang A., Yan H., Cui Y. Combination of quercetin with radiotherapy enhances tumor radiosensitivity in vitro and in vivo. Radiother. Oncol. 2012;104:395–400. doi: 10.1016/j.radonc.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 136.Ma T., Liu Y., Wu Q., Luo L., Cui Y., Wang X., Chen X., Tan L., Meng X. Correction to quercetin-modified metal–organic frameworks for dual sensitization of radiotherapy in tumor tissues by inhibiting the carbonic anhydrase IX. ACS Nano. 2020;14 doi: 10.1021/acsnano.9b09760. 2553-2553. [DOI] [PubMed] [Google Scholar]
- 137.Vane J., Botting R. Inflammation and the mechanism of action of anti-inflammatory drugs. Faseb. J. 1987;1:89–96. [PubMed] [Google Scholar]
- 138.Azab A., Nassar A., Azab A.N. Anti-inflammatory activity of natural products. Molecules. 2016;21:1321. doi: 10.3390/molecules21101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Du J., Zhang Y.S., Hobson D., Hydbring P. Nanoparticles for immune system targeting, Drug Discov. Today Off. 2017;22:1295–1301. doi: 10.1016/j.drudis.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 140.Brusini R., Varna M., Couvreur P. Advanced nanomedicines for the treatment of inflammatory diseases. Adv. Drug Deliv. Rev. 2020;157:161–178. doi: 10.1016/j.addr.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dacoba T.G., Olivera A., Torres D., Crecente-Campo J., Alonso M.J. Modulating the immune system through nanotechnology. Semin. Immunol. 2017;34:78–102. doi: 10.1016/j.smim.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kannaiyan R., Shanmugam M.K., Sethi G. Molecular targets of celastrol derived from Thunder of God Vine: potential role in the treatment of inflammatory disorders and cancer. Cancer Lett. 2011;303:9–20. doi: 10.1016/j.canlet.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 143.Guo L., Luo S., Du Z., Zhou M., Li P., Fu Y., Sun X., Huang Y., Zhang Z. Targeted delivery of celastrol to mesangial cells is effective against mesangioproliferative glomerulonephritis. Nat. Commun. 2017;8:878. doi: 10.1038/s41467-017-00834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li Z., Geng Y.-N., Jiang J.-D., Kong W.-J. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid. Based Complement Alternat. Med. 2014 doi: 10.1155/2014/289264. (2014) 289264-289264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Allijn I.E., Czarny B.M.S., Wang X., Chong S.Y., Weiler M., Da Silva A.E., Metselaar J.M., Lam C.S.P., Pastorin G., De Kleijn D.P.V., Storm G., Wang J.-W., Schiffelers R.M. Liposome encapsulated berberine treatment attenuates cardiac dysfunction after myocardial infarction. J. Contr. Release. 2017;247:127–133. doi: 10.1016/j.jconrel.2016.12.042. [DOI] [PubMed] [Google Scholar]
- 146.Jung E., Noh J., Kang C., Yoo D., Song C., Lee D. Ultrasound imaging and on-demand therapy of peripheral arterial diseases using H2O2-Activated bubble generating anti-inflammatory polymer particles. Biomaterials. 2018;179:175–185. doi: 10.1016/j.biomaterials.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 147.Chung C.H., Jung W., Keum H., Kim T.W., Jon S. Nanoparticles derived from the natural antioxidant rosmarinic acid ameliorate acute inflammatory bowel disease. ACS Nano. 2020;14:6887–6896. doi: 10.1021/acsnano.0c01018. [DOI] [PubMed] [Google Scholar]
- 148.Lee N.-Y., Ko W.-C., Hsueh P.-R. Nanoparticles in the treatment of infections caused by multidrug-resistant organisms. Front. Pharmacol. 2019;10 doi: 10.3389/fphar.2019.01153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Baptista P.V., McCusker M.P., Carvalho A., Ferreira D.A., Mohan N.M., Martins M., Fernandes A.R. Nano-strategies to fight multidrug resistant bacteria—“A battle of the titans”. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pelgrift R.Y., Friedman A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013;65:1803–1815. doi: 10.1016/j.addr.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 151.Sun D., Zhang W., Mou Z., Chen Y., Guo F., Yang E., Wang W. Transcriptome analysis reveals silver nanoparticle-decorated quercetin antibacterial molecular mechanism. ACS Appl. Mater. Interfaces. 2017;9:10047–10060. doi: 10.1021/acsami.7b02380. [DOI] [PubMed] [Google Scholar]
- 152.Ji H., Dong K., Yan Z., Ding C., Chen Z., Ren J., Qu X. Bacterial hyaluronidase self-triggered prodrug release for chemo-photothermal synergistic treatment of bacterial infection. Small. 2016;12:6200–6206. doi: 10.1002/smll.201601729. [DOI] [PubMed] [Google Scholar]
- 153.Montanari J., Maidana C., Esteva M.I., Salomon C., Morilla M.J., Romero E.L. Sunlight triggered photodynamic ultradeformable liposomes against Leishmania braziliensis are also leishmanicidal in the dark. J. Contr. Release. 2010;147:368–376. doi: 10.1016/j.jconrel.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 154.Finkel T., Holbrook N.J. Oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 155.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 156.Li Y.J., Luo L.J., Harroun S.G., Wei S.C., Unnikrishnan B., Chang H.T., Huang Y.F., Lai J.Y., Huang C.C. Synergistically dual-functional nano eye-drops for simultaneous anti-inflammatory and anti-oxidative treatment of dry eye disease. Nanoscale. 2019;11:5580–5594. doi: 10.1039/c9nr00376b. [DOI] [PubMed] [Google Scholar]
- 157.Loguercio C., Federico A. Oxidative stress in viral and alcoholic hepatitis. Free Radic. Biol. Med. 2003;34:1–10. doi: 10.1016/s0891-5849(02)01167-x. [DOI] [PubMed] [Google Scholar]
- 158.Okuda M., Li K., Beard M.R., Showalter L.A., Scholle F., Lemon S.M., Weinman S.A. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 159.Davis-Searles P.R., Nakanishi Y., Kim N.C., Graf T.N., Oberlies N.H., Wani M.C., Wall M.E., Agarwal R., Kroll D.J. Milk thistle and prostate cancer: differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res. 2005;65:4448–4457. doi: 10.1158/0008-5472.CAN-04-4662. [DOI] [PubMed] [Google Scholar]
- 160.Gazak R., Walterova D., Kren V. Silybin and silymarin--new and emerging applications in medicine. Curr. Med. Chem. 2007;14:315–338. doi: 10.2174/092986707779941159. [DOI] [PubMed] [Google Scholar]
- 161.Liu C.-H., Lin C.-C., Hsu W.-C., Chung C.-Y., Lin C.-C., Jassey A., Chang S.-P., Tai C.-J., Tai C.-J., Shields J., Richardson C.D., Yen M.-H., Tyrrell D.L.J., Lin L.-T. Highly bioavailable silibinin nanoparticles inhibit HCV infection. Gut. 2017;66:1853. doi: 10.1136/gutjnl-2016-312019. [DOI] [PubMed] [Google Scholar]
- 162.Chen H., Yoshioka H., Kim G.S., Jung J.E., Okami N., Sakata H., Maier C.M., Narasimhan P., Goeders C.E., Chan P.H. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxidants Redox Signal. 2011;14:1505–1517. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chan P.H. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cerebr. Blood Flow Metabol. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 164.Cho S., Park W., Kim D.H. Silica-coated metal chelating-melanin nanoparticles as a dual-modal contrast enhancement imaging and therapeutic agent. ACS Appl. Mater. Interfaces. 2017;9:101–111. doi: 10.1021/acsami.6b11304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lin J., Wang M., Hu H., Yang X., Wen B., Wang Z., Jacobson O., Song J., Zhang G., Niu G., Huang P., Chen X. Multimodal-imaging-guided cancer phototherapy by versatile biomimetic theranostics with UV and γ-irradiation protection. Adv. Mater. 2016;28:3273–3279. doi: 10.1002/adma.201505700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jathoul A.P., Laufer J., Ogunlade O., Treeby B., Cox B., Zhang E., Johnson P., Pizzey A.R., Philip B., Marafioti T., Lythgoe M.F., Pedley R.B., Pule M.A., Beard P. Deep in vivo photoacoustic imaging of mammalian tissues using a tyrosinase-based genetic reporter. Nat. Photonics. 2015;9:239–246. [Google Scholar]
- 167.Liu Y., Ai K., Liu J., Deng M., He Y., Lu L. Dopamine-melanin colloidal nanospheres: an efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013;25:1353–1359. doi: 10.1002/adma.201204683. [DOI] [PubMed] [Google Scholar]
- 168.Brenner M., Hearing V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008;84:539–549. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Panzella L., Gentile G., D'Errico G., DellaVecchia N.F., Errico M.E., Napolitano A., Carfagna C., d 'Ischia M. Atypical structural and π -ElectronFeatures of a melanin polymer that lead to superior free -Radical-Scavenging Properties. Angew. Chem. Int. Ed. 2013;52:12684–12687. doi: 10.1002/anie.201305747. [DOI] [PubMed] [Google Scholar]
- 170.Ju K.-Y., Lee Y., Lee S., Park S.B., Lee J.-K. Bioinspired polymerization of dopamine to generate melanin-like nanoparticles having an excellent free-radical-scavenging property. Biomacromolecules. 2011;12:625–632. doi: 10.1021/bm101281b. [DOI] [PubMed] [Google Scholar]
- 171.Liu Y., Ai K., Ji X., Askhatova D., Du R., Lu L., Shi J. Comprehensive insights into the multi-antioxidative mechanisms of melanin nanoparticles and their application to protect brain from injury in ischemic stroke. J. Am. Chem. Soc. 2017;139:856–862. doi: 10.1021/jacs.6b11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mandel S., Youdim M.B.H. Catechin polyphenols: neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic. Biol. Med. 2004;37:304–317. doi: 10.1016/j.freeradbiomed.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 173.Singh B.N., Shankar S., Srivastava R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011;82:1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li Y.-J., Luo L.-J., Harroun S.G., Wei S.-C., Unnikrishnan B., Chang H.-T., Huang Y.-F., Lai J.-Y., Huang C.-C. Synergistically dual-functional nano eye-drops for simultaneous anti-inflammatory and anti-oxidative treatment of dry eye disease. Nanoscale. 2019;11:5580–5594. doi: 10.1039/c9nr00376b. [DOI] [PubMed] [Google Scholar]
- 175.Gitler A.D., Dhillon P., Shorter J. Neurodegenerative disease: models, mechanisms, and a new hope. Dis. Model Mech. 2017;10:499–502. doi: 10.1242/dmm.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hussain R., Zubair H., Pursell S., Shahab M. Neurodegenerative diseases: regenerative mechanisms and novel therapeutic approaches. Brain Sci. 2018;8:177. doi: 10.3390/brainsci8090177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jin K., Peel A.L., Mao X.O., Xie L., Cottrell B.A., Henshall D.C., Greenberg D.A. Increased hippocampal neurogenesis in Alzheimer's disease. Proc. Natl. Acad. Sci. U.S.A. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yang C., Zhang X., Fan H., Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009;1282:133–141. doi: 10.1016/j.brainres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 179.Kim S.J., Son T.G., Park H.R., Park M., Kim M.-S., Kim H.S., Chung H.Y., Mattson M.P., Lee J. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult Hippocampus. J. Biol. Chem. 2008;283:14497–14505. doi: 10.1074/jbc.M708373200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liao K.-K., Wu M.-J., Chen P.-Y., Huang S.-W., Chiu S.-J., Ho C.-T., Yen J.-H. Curcuminoids promote neurite outgrowth in PC12 cells through MAPK/ERK- and PKC-dependent pathways. J. Agric. Food Chem. 2012;60:433–443. doi: 10.1021/jf203290r. [DOI] [PubMed] [Google Scholar]
- 181.Tiwari S.K., Agarwal S., Seth B., Yadav A., Nair S., Bhatnagar P., Karmakar M., Kumari M., Chauhan L.K., Patel D.K., Srivastava V., Singh D., Gupta S.K., Tripathi A., Chaturvedi R.K., Gupta K.C. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer's disease model via canonical Wnt/beta-catenin pathway. ACS Nano. 2014;8:76–103. doi: 10.1021/nn405077y. [DOI] [PubMed] [Google Scholar]
- 182.Ittner L.M., Gotz J. Amyloid-beta and tau--a toxic pas de deux in Alzheimer's disease. Nat. Rev. Neurosci. 2011;12:65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- 183.Yao L., Gu X., Song Q., Wang X., Huang M., Hu M., Hou L., Kang T., Chen J., Chen H., Gao X. Nanoformulated alpha-mangostin ameliorates Alzheimer's disease neuropathology by elevating LDLR expression and accelerating amyloid-beta clearance. J. Contr. Release. 2016;226:1–14. doi: 10.1016/j.jconrel.2016.01.055. [DOI] [PubMed] [Google Scholar]
- 184.Dauer W., Przedborski S. Parkinson's Disease. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 185.Pei J., Lv Q., Han J., Li X., Jin S., Huang Y., Jin S., Yuan H. Schisandra lignans-loaded enteric nanoparticles: preparation, characterization, and in vitro-in vivo evaluation. J. Drug Target. 2013;21:180–187. doi: 10.3109/1061186X.2012.737000. [DOI] [PubMed] [Google Scholar]
- 186.Chen T., Li C., Li Y., Yi X., Wang R., Lee S.M.-Y., Zheng Y. Small-sized mPEG–PLGA nanoparticles of schisantherin A with sustained release for enhanced brain uptake and anti-parkinsonian activity. ACS Appl. Mater. Interfaces. 2017;9:9516–9527. doi: 10.1021/acsami.7b01171. [DOI] [PubMed] [Google Scholar]
- 187.Wolfram J., Suri K., Huang Y., Molinaro R., Borsoi C., Scott B., Boom K., Paolino D., Fresta M., Wang J., Ferrari M., Celia C., Shen H. Evaluation of anticancer activity of celastrol liposomes in prostate cancer cells. J. Microencapsul. 2014;31:501–507. doi: 10.3109/02652048.2013.879932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gomes E.R., Novais M.V.M., Silva I.T., Barros A.L.B., Leite E.A., Munkert J., Frade A.C.M., Cassali G.D., Braga F.C., Pádua R.M., Oliveira M.C. Long-circulating and fusogenic liposomes loaded with a glucoevatromonoside derivative induce potent antitumor response. Biomed. Pharmacother. 2018;108:1152–1161. doi: 10.1016/j.biopha.2018.09.109. [DOI] [PubMed] [Google Scholar]
- 189.Yang L., Xin J., Zhang Z., Yan H., Wang J., Sun E., Hou J., Jia X., Lv H. TPGS-modified liposomes for the delivery of ginsenoside compound K against non-small cell lung cancer: formulation design and its evaluation in vitro and in vivo. J. Pharm. Pharmacol. 2016;68:1109–1118. doi: 10.1111/jphp.12590. [DOI] [PubMed] [Google Scholar]
- 190.Rezaei-Sadabady R., Eidi A., Zarghami N., Barzegar A. Intracellular ROS protection efficiency and free radical-scavenging activity of quercetin and quercetin-encapsulated liposomes, Artificial Cells, Nanomed. Biotechnol. 2016;44:128–134. doi: 10.3109/21691401.2014.926456. [DOI] [PubMed] [Google Scholar]
- 191.Mielczarek L., Krug P., Mazur M., Milczarek M., Chilmonczyk Z., Wiktorska K. In the triple-negative breast cancer MDA-MB-231 cell line, sulforaphane enhances the intracellular accumulation and anticancer action of doxorubicin encapsulated in liposomes. Int. J. Pharm. 2019;558:311–318. doi: 10.1016/j.ijpharm.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 192.Tian J., Wang L., Wang L., Ke X. A wogonin-loaded glycyrrhetinic acid-modified liposome for hepatic targeting with anti-tumor effects. Drug Deliv. 2014;21:553–559. doi: 10.3109/10717544.2013.853850. [DOI] [PubMed] [Google Scholar]
- 193.Tsai Y.-J., Chen B.-H. Preparation of catechin extracts and nanoemulsions from green tea leaf waste and their inhibition effect on prostate cancer cell PC-3. Int. J. Nanomed. 2016;11:1907–1926. doi: 10.2147/IJN.S103759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Najlah M., Kadam A., Wan K.-W., Ahmed W., Taylor K.M.G., Elhissi A.M.A. Novel paclitaxel formulations solubilized by parenteral nutrition nanoemulsions for application against glioma cell lines. Int. J. Pharm. 2016;506:102–109. doi: 10.1016/j.ijpharm.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 195.Pham J., Nayel A., Hoang C., Elbayoumi T. Enhanced effectiveness of tocotrienol-based nano-emulsified system for topical delivery against skin carcinomas. Drug Deliv. 2016;23:1514–1524. doi: 10.3109/10717544.2014.966925. [DOI] [PubMed] [Google Scholar]
- 196.Yadav R., Kumar D., Kumari A., Yadav S.K. PLA nanovectors with encapsulated betulin: plant leaf extract-synthesized nanovectors are more efficacious than PVA-synthesized nanovectors. Biotechnol. Lett. 2016;38:259–269. doi: 10.1007/s10529-015-1981-3. [DOI] [PubMed] [Google Scholar]
- 197.Gulcur E., Thaqi M., Khaja F., Kuzmis A., Onyuksel H. Curcumin in VIP-targeted sterically stabilized phospholipid nanomicelles: a novel therapeutic approach for breast cancer and breast cancer stem cells. Drug Deliv. Transl. Res. 2013;3 doi: 10.1007/s13346-013-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yang F., Xiao W., Ma X., Huang R., Yu R., Li G., Huang X., Chen C., Ding P. Optimization of a novel chelerythrine-loaded magnetic Fe3O4/chitosan alpha-ketoglutaric acid system and evaluation of its anti-tumour activities. J. Pharm. Pharmacol. 2016;68:1030–1040. doi: 10.1111/jphp.12564. [DOI] [PubMed] [Google Scholar]
- 199.Luo J., Meng X., Su J., Ma H., Wang W., Fang L., Zheng H., Qin Y., Chen T. Biotin-modified polylactic-co-glycolic acid nanoparticles with improved antiproliferative activity of 15,16-dihydrotanshinone I in human cervical cancer cells. J. Agric. Food Chem. 2018;66:9219–9230. doi: 10.1021/acs.jafc.8b02698. [DOI] [PubMed] [Google Scholar]
- 200.Mary Lazer L., Sadhasivam B., Palaniyandi K., Muthuswamy T., Ramachandran I., Balakrishnan A., Pathak S., Narayan S., Ramalingam S. Chitosan-based nano-formulation enhances the anticancer efficacy of hesperetin. Int. J. Biol. Macromol. 2018;107:1988–1998. doi: 10.1016/j.ijbiomac.2017.10.064. [DOI] [PubMed] [Google Scholar]
- 201.Baksi R., Singh D.P., Borse S.P., Rana R., Sharma V., Nivsarkar M. In vitro and in vivo anticancer efficacy potential of Quercetin loaded polymeric nanoparticles. Biomed. Pharmacother. 2018;106:1513–1526. doi: 10.1016/j.biopha.2018.07.106. [DOI] [PubMed] [Google Scholar]
- 202.Matthaiou E.-I., Barar J., Sandaltzopoulos R., Li C., Coukos G., Omidi Y. Shikonin-loaded antibody-armed nanoparticles for targeted therapy of ovarian cancer. Int. J. Nanomed. 2014;9:1855–1870. doi: 10.2147/IJN.S51880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lee J.E., Kim M.G., Jang Y.L., Lee M.S., Kim N.W., Yin Y., Lee J.H., Lim S.Y., Park J.W., Kim J., Lee D.S., Kim S.H., Jeong J.H. Self-assembled PEGylated albumin nanoparticles (SPAN) as a platform for cancer chemotherapy and imaging. Drug Deliv. 2018;25:1570–1578. doi: 10.1080/10717544.2018.1489430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lu J., Huang Y., Zhao W., Marquez R.T., Meng X., Li J., Gao X., Venkataramanan R., Wang Z., Li S. PEG-derivatized embelin as a nanomicellar carrier for delivery of paclitaxel to breast and prostate cancers. Biomaterials. 2013;34:1591–1600. doi: 10.1016/j.biomaterials.2012.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Haron A.S., Syed Alwi S.S., Saiful Yazan L., Abd Razak R., Ong Y.S., Zakarial Ansar F.H., Roshini Alexander H. Cytotoxic effect of thymoquinone-loaded nanostructured lipid carrier (TQ-NLC) on liver cancer cell integrated with hepatitis B genome, Hep3B, evid. Based Compl. Alternat. Med. 2018:1549805. doi: 10.1155/2018/1549805. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ong Y.S., Saiful Yazan L., Ng W.K., Abdullah R., Mustapha N.M., Sapuan S., Foo J.B., Tor Y.S., How C.W., Abd Rahman N., Zakarial Ansar F.H. Thymoquinone loaded in nanostructured lipid carrier showed enhanced anticancer activity in 4T1 tumor-bearing mice. Nanomedicine. 2018;13:1567–1582. doi: 10.2217/nnm-2017-0322. [DOI] [PubMed] [Google Scholar]
- 207.Ong Y.S., Saiful Yazan L., Ng W.K., Noordin M.M., Sapuan S., Foo J.B., Tor Y.S. Acute and subacute toxicity profiles of thymoquinone-loaded nanostructured lipid carrier in BALB/c mice. Int. J. Nanomed. 2016;11:5905–5915. doi: 10.2147/IJN.S114205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kapoor R., Singh S., Tripathi M., Bhatnagar P., Kakkar P., Gupta K.C. O-Hexadecyl-Dextran entrapped berberine nanoparticles abrogate high glucose stress induced apoptosis in primary rat hepatocytes. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Badamaranahalli S.S., Kopparam M., Bhagawati S.T., Durg S. Embelin lipid nanospheres for enhanced treatment of ulcerative colitis – preparation, characterization and in vivo evaluation. Eur. J. Pharmaceut. Sci. 2015;76:73–82. doi: 10.1016/j.ejps.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 210.Chamcheu J.C., Siddiqui I.A., Adhami V.M., Esnault S., Bharali D.J., Babatunde A.S., Adame S., Massey R.J., Wood G.S., Longley B.J., Mousa S.A., Mukhtar H. Chitosan-based nanoformulated (-)-epigallocatechin-3-gallate (EGCG) modulates human keratinocyte-induced responses and alleviates imiquimod-induced murine psoriasiform dermatitis. Int. J. Nanomed. 2018;13:4189–4206. doi: 10.2147/IJN.S165966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Peng L.-H., Xu S.-Y., Shan Y.-H., Wei W., Liu S., Zhang C.-Z., Wu J.-H., Liang W.-Q., Gao J.-Q. Sequential release of salidroside and paeonol from a nanosphere-hydrogel system inhibits ultraviolet B-induced melanogenesis in Guinea pig skin. Int. J. Nanomed. 2014:1897–1908. doi: 10.2147/IJN.S59290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wang J., Tan J., Luo J., Huang P., Zhou W., Chen L., Long L., Zhang L.-m., Zhu B., Yang L., Deng D.Y. Enhancement of scutellarin oral delivery efficacy by vitamin B12-modified amphiphilic chitosan derivatives to treat type II diabetes induced-retinopathy. J. Nanobiotechnol. 2017;15:18. doi: 10.1186/s12951-017-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Abdelwahab S.I., Sheikh B.Y., Taha M.M., How C.W., Abdullah R., Yagoub U., El-Sunousi R., Eid E.E. Thymoquinone-loaded nanostructured lipid carriers: preparation, gastroprotection, in vitro toxicity, and pharmacokinetic properties after extravascular administration. Int. J. Nanomed. 2013;8:2163–2172. doi: 10.2147/IJN.S44108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mazumder A., Dwivedi A., du Preez J.L., du Plessis J. In vitro wound healing and cytotoxic effects of sinigrin-phytosome complex. Int. J. Pharm. 2016;498:283–293. doi: 10.1016/j.ijpharm.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 215.Abd El-Fattah A.I., Fathy M.M., Ali Z.Y., El-Garawany A.E.A., Mohamed E.K. Enhanced therapeutic benefit of quercetin-loaded phytosome nanoparticles in ovariectomized rats. Chem. Biol. Interact. 2017;271:30–38. doi: 10.1016/j.cbi.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 216.Sun Q., Li W., Li H., Wang X., Wang Y., Niu X. Preparation, characterization and anti-ulcer efficacy of sanguinarine loaded solid lipid nanoparticles. Pharmacology. 2017;100:14–24. doi: 10.1159/000454882. [DOI] [PubMed] [Google Scholar]
- 217.Pinilla C.M.B., Noreña C.P.Z., Brandelli A. Development and characterization of phosphatidylcholine nanovesicles, containing garlic extract, with antilisterial activity in milk. Food Chem. 2017;220:470–476. doi: 10.1016/j.foodchem.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 218.Singh R.P., Gangadharappa H.V., Mruthunjaya K. Phytosome complexed with chitosan for gingerol delivery in the treatment of respiratory infection: in vitro and in vivo evaluation. Eur. J. Pharmaceut. Sci. 2018;122:214–229. doi: 10.1016/j.ejps.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 219.Ahmad N., Ahmad R., Alam M.A., Ahmad F.J. Quantification and brain targeting of eugenol-loaded surface modified nanoparticles through intranasal route in the treatment of cerebral ischemia. Drug Res. 2018;68:584–595. doi: 10.1055/a-0596-7288. [DOI] [PubMed] [Google Scholar]
- 220.Li L., Wang Y., Guo R., Li S., Ni J., Gao S., Gao X., Mao J., Zhu Y., Wu P., Wang H., Kong D., Zhang H., Zhu M., Fan G. Ginsenoside Rg3-loaded, reactive oxygen species-responsive polymeric nanoparticles for alleviating myocardial ischemia-reperfusion injury. J. Contr. Release. 2020;317:259–272. doi: 10.1016/j.jconrel.2019.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ahmad N., Ahmad R., Naqvi A.A., Alam M.A., Ashafaq M., Samim M., Iqbal Z., Ahmad F.J. Rutin-encapsulated chitosan nanoparticles targeted to the brain in the treatment of Cerebral Ischemia. Int. J. Biol. Macromol. 2016;91:640–655. doi: 10.1016/j.ijbiomac.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 222.Ishak R.A.H., Mostafa N.M., Kamel A.O. Stealth lipid polymer hybrid nanoparticles loaded with rutin for effective brain delivery – comparative study with the gold standard (Tween 80): optimization, characterization and biodistribution. Drug Deliv. 2017;24:1874–1890. doi: 10.1080/10717544.2017.1410263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ackroyd R., Kelty C., Brown N., Reed M. The history of photodetection and photodynamic therapy. Photochem. Photobiol. 2007;74:656–669. doi: 10.1562/0031-8655(2001)074<0656:thopap>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 224.Han H.S., Choi K.Y., Lee H., Lee M., An J.Y., Shin S., Kwon S., Lee D.S., Park J.H. Gold-nanoclustered hyaluronan nano-assemblies for photothermally maneuvered photodynamic tumor ablation. ACS Nano. 2016;10:10858–10868. doi: 10.1021/acsnano.6b05113. [DOI] [PubMed] [Google Scholar]
- 225.N V.R., Han H.S., Lee H., Nguyen V.Q., Jeon S., Jung D.-W., Lee J., Yi G.-R., Park J.H. ROS-responsive mesoporous silica nanoparticles for MR imaging-guided photodynamically maneuvered chemotherapy. Nanoscale. 2018;10:9616–9627. doi: 10.1039/c8nr00888d. [DOI] [PubMed] [Google Scholar]
- 226.Liu C., Shin J., Son S., Choe Y., Farokhzad N., Tang Z., Xiao Y., Kong N., Xie T., Kim J.S., Tao W. Pnictogens in medicinal chemistry: evolution from erstwhile drugs to emerging layered photonic nanomedicine. Chem. Soc. Rev. 2021;50:2260–2279. doi: 10.1039/d0cs01175d. [DOI] [PubMed] [Google Scholar]
- 227.Muniyandi K., George B., Parimelazhagan T., Abrahamse H. Role of photoactive phytocompounds in photodynamic therapy of cancer. Molecules. 2020;25 doi: 10.3390/molecules25184102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ren G., Miao Z., Liu H., Jiang L., Limpa-Amara N., Mahmood A., Gambhir S.S., Cheng Z. Melanin-targeted preclinical PET imaging of melanoma metastasis. J. Nucl. Med. 2009;50:1692–1699. doi: 10.2967/jnumed.109.066175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhang R., Fan Q., Yang M., Cheng K., Lu X., Zhang L., Huang W., Cheng Z. Engineering melanin nanoparticles as an efficient drug–delivery system for imaging-guided chemotherapy. Adv. Mater. 2015;27:5063–5069. doi: 10.1002/adma.201502201. [DOI] [PubMed] [Google Scholar]
- 230.Chu M., Hai W., Zhang Z., Wo F., Wu Q., Zhang Z., Shao Y., Zhang D., Jin L., Shi D. Melanin nanoparticles derived from a homology of medicine and food for sentinel lymph node mapping and photothermal in vivo cancer therapy. Biomaterials. 2016;91:182–199. doi: 10.1016/j.biomaterials.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 231.Jeong S., Lee J., Im B.N., Park H., Na K. Combined photodynamic and antibiotic therapy for skin disorder via lipase-sensitive liposomes with enhanced antimicrobial performance. Biomaterials. 2017;141:243–250. doi: 10.1016/j.biomaterials.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 232.Oh I.-h., Min H.S., Li L., Tran T.H., Lee Y.-k., Kwon I.C., Choi K., Kim K., Huh K.M. Cancer cell-specific photoactivity of pheophorbide a–glycol chitosan nanoparticles for photodynamic therapy in tumor-bearing mice. Biomaterials. 2013;34:6454–6463. doi: 10.1016/j.biomaterials.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 233.Lee M.M.S., Zheng L., Yu B., Xu W., Kwok R.T.K., Lam J.W.Y., Xu F., Wang D., Tang B.Z. A highly efficient and AIE-active theranostic agent from natural herbs. Mater. Chem. Front. 2019;3:1454–1461. [Google Scholar]
- 234.Barras A., Boussekey L., Courtade E., Boukherroub R. Hypericin-loaded lipid nanocapsules for photodynamic cancer therapy in vitro. Nanoscale. 2013;5:10562. doi: 10.1039/c3nr02724d. [DOI] [PubMed] [Google Scholar]
- 235.Lima A.M., Pizzol C.D., Monteiro F.B.F., Creczynski-Pasa T.B., Andrade G.P., Ribeiro A.O., Perussi J.R. Hypericin encapsulated in solid lipid nanoparticles: phototoxicity and photodynamic efficiency. J. Photochem. Photobiol. B Biol. 2013;125:146–154. doi: 10.1016/j.jphotobiol.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 236.Ebermann R., Alth G., Kreitner M., Kubin A. Natural products derived from plants as potential drugs for the photodynamic destruction of tumor cells. J. Photochem. Photobiol. B Biol. 1996;36:95–97. doi: 10.1016/s1011-1344(96)07353-8. [DOI] [PubMed] [Google Scholar]
- 237.Dwivedi V.P., Bhattacharya D., Singh M., Bhaskar A., Kumar S., Fatima S., Sobia P., Kaer L.V., Das G. Allicin enhances antimicrobial activity of macrophages during Mycobacterium tuberculosis infection. J. Ethnopharmacol. 2019;243:111634. doi: 10.1016/j.jep.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 238.Madunić J., Madunić I.V., Gajski G., Popić J., Garaj-Vrhovac V. Apigenin: a dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018;413:11–22. doi: 10.1016/j.canlet.2017.10.041. [DOI] [PubMed] [Google Scholar]
- 239.Piboonprai K., Khumkhrong P., Khongkow M., Yata T., Ruangrungsi N., Chansriniyom C., Iempridee T. Anticancer activity of arborinine from Glycosmis parva leaf extract in human cervical cancer cells. Biochem. Biophys. Res. Commun. 2018;500:866–872. doi: 10.1016/j.bbrc.2018.04.175. [DOI] [PubMed] [Google Scholar]
- 240.Liu H., Dong Y., Gao Y., Du Z., Wang Y., Cheng P., Chen A., Huang H. The fascinating effects of baicalein on cancer: a review. Int. J. Mol. Sci. 2016;17:1681. doi: 10.3390/ijms17101681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Luo J., Kong J.-L., Dong B.-Y., Huang H., Wang K., Wu L.-H., Hou C.-C., Liang Y., Li B., Chen Y.-Q. Baicalein attenuates the quorum sensing-controlled virulence factors of Pseudomonas aeruginosa and relieves the inflammatory response in P. aeruginosa-infected macrophages by downregulating the MAPK and NFκB signal-transduction pathways. Drug Des. Dev. Ther. 2016;10:183–203. doi: 10.2147/DDDT.S97221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.de Oliveira Júnior R.G., Bonnet A., Braconnier E., Groult H., Prunier G., Beaugeard L., Grougnet R., da Silva Almeida J.R.G., Ferraz C.A.A., Picot L. Bixin, an apocarotenoid isolated from Bixa orellana L., sensitizes human melanoma cells to dacarbazine-induced apoptosis through ROS-mediated cytotoxicity. Food Chem. Toxicol. 2019;125:549–561. doi: 10.1016/j.fct.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 243.Kumar Y., Phaniendra A., Periyasamy L. Bixin triggers apoptosis of human Hep3B hepatocellular carcinoma cells: an insight to molecular and in silico approach. Nutr. Cancer. 2018;70:971–983. doi: 10.1080/01635581.2018.1490445. [DOI] [PubMed] [Google Scholar]
- 244.Venkatesha S.H., Dudics S., Astry B., Moudgil K.D. Control of autoimmune inflammation by celastrol, a natural triterpenoid. Pathog. Dis. 2016;74:ftw059. doi: 10.1093/femspd/ftw059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.He N., Wang P., Wang P., Ma C., Kang W. Antibacterial mechanism of chelerythrine isolated from root of Toddalia asiatica (Linn) Lam. BMC Complement. Altern. Med. 2018;18 doi: 10.1186/s12906-018-2317-3. 261-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhou F., Ji B., Zhang H., Jiang H., Yang Z., Li J., Li J., Yan W. The antibacterial effect of cinnamaldehyde, thymol, carvacrol and their combinations against the foodborne pathogen Salmonella typhimurium. J. Food Saf. 2007;27:124–133. [Google Scholar]
- 247.Sun J., Blaskovich M.A., Jove R., Livingston S.K., Coppola D., Sebti S.M., Cucurbitacin Q. A selective STAT3 activation inhibitor with potent antitumor activity. Oncogene. 2005;24:3236–3245. doi: 10.1038/sj.onc.1208470. [DOI] [PubMed] [Google Scholar]
- 248.Tsai S.-L., Suk F.-M., Wang C.-I., Liu D.-Z., Hou W.-C., Lin P.-J., Hung L.-F., Liang Y.-C. Anti-tumor potential of 15,16-dihydrotanshinone I against breast adenocarcinoma through inducing G1 arrest and apoptosis. Biochem. Pharmacol. 2007;74:1575–1586. doi: 10.1016/j.bcp.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 249.Ke Z., Yang L., Wu H., Li Z., Jia X., Zhang Z. Evaluation of in vitro and in vivo antitumor effects of gambogic acid-loaded layer-by-layer self-assembled micelles. Int. J. Pharm. 2018;545:306–317. doi: 10.1016/j.ijpharm.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 250.Seeram N.P., Adams L.S., Henning S.M., Niu Y., Zhang Y., Nair M.G., Heber D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005;16:360–367. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 251.Jeong C.-H., Bode A.M., Pugliese A., Cho Y.-Y., Kim H.-G., Shim J.-H., Jeon Y.-J., Li H., Jiang H., Dong Z. [6]-Gingerol suppresses colon cancer growth by targeting leukotriene A4 hydrolase. Cancer Res. 2009;69:5584–5591. doi: 10.1158/0008-5472.CAN-09-0491. [DOI] [PubMed] [Google Scholar]
- 252.Xiao J., Chen D., Lin X.-X., Peng S.-F., Xiao M.-F., Huang W.-H., Wang Y.-C., Peng J.-B., Zhang W., Ouyang D.-S., Chen Y. Screening of drug metabolizing enzymes for the ginsenoside compound K in vitro: an efficient anti-cancer substance originating from panax ginseng. PLoS One. 2016;11 doi: 10.1371/journal.pone.0147183. e0147183-e0147183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Parhiz H., Roohbakhsh A., Soltani F., Rezaee R., Iranshahi M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother Res. 2015;29:323–331. doi: 10.1002/ptr.5256. [DOI] [PubMed] [Google Scholar]
- 254.Plastina P., Benincasa C., Perri E., Fazio A., Augimeri G., Poland M., Witkamp R., Meijerink J. Identification of hydroxytyrosyl oleate, a derivative of hydroxytyrosol with anti-inflammatory properties, in olive oil by-products. Food Chem. 2019;279:105–113. doi: 10.1016/j.foodchem.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 255.Fuccelli R., Fabiani R., Rosignoli P. Hydroxytyrosol exerts anti-inflammatory and anti-oxidant activities in a mouse model of systemic inflammation. Molecules. 2018;23:3212. doi: 10.3390/molecules23123212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lin C.-L., Hsiao G., Wang C.-C., Lee Y.-L. Imperatorin exerts antiallergic effects in Th2-mediated allergic asthma via induction of IL-10-producing regulatory T cells by modulating the function of dendritic cells. Pharmacol. Res. 2016;110:111–121. doi: 10.1016/j.phrs.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 257.Nasser M.I., Zhu S., Hu H., Huang H., Guo M., Zhu P. Effects of imperatorin in the cardiovascular system and cancer. Biomed. Pharmacother. 2019;120:109401. doi: 10.1016/j.biopha.2019.109401. [DOI] [PubMed] [Google Scholar]
- 258.Imran M., Salehi B., Sharifi-Rad J., Aslam Gondal T., Saeed F., Imran A., Shahbaz M., Tsouh Fokou P.V., Umair Arshad M., Khan H., Guerreiro S.G., Martins N., Estevinho L.M. Kaempferol: a key emphasis to its anticancer potential. Molecules. 2019;24:2277. doi: 10.3390/molecules24122277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Aziz N., Kim M.-Y., Cho J.Y. Anti-inflammatory effects of luteolin: a review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018;225:342–358. doi: 10.1016/j.jep.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 260.Jung E., Lee J.-A., Shin S., Roh K.-B., Kim J.-H., Park D. Madecassoside inhibits melanin synthesis by blocking ultraviolet-induced inflammation. Molecules. 2013;18:15724–15736. doi: 10.3390/molecules181215724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Gutierrez-Orozco F., Chitchumroonchokchai C., Lesinski G.B., Suksamrarn S., Failla M.L. α-Mangostin: anti-inflammatory activity and metabolism by human cells. J. Agric. Food Chem. 2013;61:3891–3900. doi: 10.1021/jf4004434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Alberdi E., Sánchez-Gómez M.V., Ruiz A., Cavaliere F., Ortiz-Sanz C., Quintela-López T., Capetillo-Zarate E., Solé-Domènech S., Matute C. Mangiferin and morin attenuate oxidative stress, mitochondrial dysfunction, and neurocytotoxicity, induced by amyloid beta oligomers. Oxid. Med. Cell Longev. 2018;2018 doi: 10.1155/2018/2856063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Shetty P.K., Venuvanka V., Jagani H.V., Chethan G.H., Ligade V.S., Musmade P.B., Nayak U.Y., Reddy M.S., Kalthur G., Udupa N., Rao C.M., Mutalik S. Development and evaluation of sunscreen creams containing morin-encapsulated nanoparticles for enhanced UV radiation protection and antioxidant activity. Int. J. Nanomed. 2015;10:6477–6491. doi: 10.2147/IJN.S90964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Salehi B., Fokou P.V.T., Sharifi-Rad M., Zucca P., Pezzani R., Martins N., Sharifi-Rad J. The therapeutic potential of naringenin: a review of clinical trials. Pharmaceuticals. 2019;12:11. doi: 10.3390/ph12010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Omar S.H. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 2010;78:133–154. doi: 10.3797/scipharm.0912-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Lorenz P., Roychowdhury S., Engelmann M., Wolf G., Horn T.F.W. Oxyresveratrol and resveratrol are potent antioxidants and free radical scavengers: effect on nitrosative and oxidative stress derived from microglial cells. Nitric Oxide. 2003;9:64–76. doi: 10.1016/j.niox.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 267.Zhang L., Li D.-c., Liu L.-f. Paeonol: pharmacological effects and mechanisms of action. Int. Immunopharm. 2019;72:413–421. doi: 10.1016/j.intimp.2019.04.033. [DOI] [PubMed] [Google Scholar]
- 268.Bui-Xuan N.-H., Tang P.M.-K., Wong C.-K., Chan J.Y.-W., Cheung K.K.Y., Jiang J.L., Fung K.-P. Pheophorbide a: a photosensitizer with immunostimulating activities on mouse macrophage RAW 264.7 cells in the absence of irradiation. Cell. Immunol. 2011;269:60–67. doi: 10.1016/j.cellimm.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 269.Qu Z.-q., Zhou Y., Zeng Y.-s., Lin Y.-k., Li Y., Zhong Z.-q., Chan W.Y. Protective effects of a Rhodiola crenulata extract and salidroside on hippocampal neurogenesis against streptozotocin-induced neural injury in the rat. PLoS One. 2012;7 doi: 10.1371/journal.pone.0029641. e29641-e29641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Croaker A., King G.J., Pyne J.H., Anoopkumar-Dukie S., Simanek V., Liu L. Carcinogenic potential of sanguinarine, a phytochemical used in ‘therapeutic’ black salve and mouthwash. Mutat. Res. Rev. Mutat. Res. 2017;774:46–56. doi: 10.1016/j.mrrev.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 271.Yang X.-J., Miao F., Yao Y., Cao F.-J., Yang R., Ma Y.-N., Qin B.-F., Zhou L. In vitro antifungal activity of sanguinarine and chelerythrine derivatives against phytopathogenic fungi. Molecules. 2012;17:13026–13035. doi: 10.3390/molecules171113026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Sa F., Zhang L.Q., Chong C.M., Guo B.J., Li S., Zhang Z.J., Zheng Y., Hoi P.M., Lee S.M.Y. Discovery of novel anti-parkinsonian effect of schisantherin A in in vitro and in vivo. Neurosci. Lett. 2015;593:7–12. doi: 10.1016/j.neulet.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 273.Yang B., Zhao Y.-L., Yang X., Liao X.-L., Yang J., Zhang J.-H., Gao C.-Z. Scutellarin-cyclodextrin conjugates: synthesis, characterization and anticancer activity. Carbohydr. Polym. 2013;92:1308–1314. doi: 10.1016/j.carbpol.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 274.Guo C., He J., Song X., Tan L., Wang M., Jiang P., Li Y., Cao Z., Peng C. Pharmacological properties and derivatives of shikonin—a review in recent years. Pharmacol. Res. 2019;149:104463. doi: 10.1016/j.phrs.2019.104463. [DOI] [PubMed] [Google Scholar]
- 275.Jie M., Cheung W.M., Yu V., Zhou Y., Tong P.H., Ho J.W.S. Anti-proliferative activities of sinigrin on carcinogen-induced hepatotoxicity in rats. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110145. e110145-e110145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Mazumder A., Dwivedi A. J. du Plessis, Sinigrin and Its Therapeutic Benefits, Molecules (Basel, Switzerland) 2016;21 doi: 10.3390/molecules21040416. 416-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Yuan C., Zhang X., Long X., Jin J., Jin R. Effect of β-sitosterol self-microemulsion and β-sitosterol ester with linoleic acid on lipid-lowering in hyperlipidemic mice. Lipids Health Dis. 2019;18 doi: 10.1186/s12944-019-1096-2. 157-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Gargouri O.D., Gargouri B., Trabelsi S.K., Bouaziz M., Abdelhédi R. Synthesis of 3-O-methylgallic acid a powerful antioxidant by electrochemical conversion of syringic acid. Biochim. Biophys. Acta Gen. Subj. 2013;1830:3643–3649. doi: 10.1016/j.bbagen.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 279.Sunil C., Xu B. An insight into the health-promoting effects of taxifolin (dihydroquercetin) Phytochemistry. 2019;166:112066. doi: 10.1016/j.phytochem.2019.112066. [DOI] [PubMed] [Google Scholar]
- 280.Li X.-Y., He J.-L., Liu H.-T., Li W.-M., Yu C. Tetramethylpyrazine suppresses interleukin-8 expression in LPS-stimulated human umbilical vein endothelial cell by blocking ERK, p38 and nuclear factor-κB signaling pathways. J. Ethnopharmacol. 2009;125:83–89. doi: 10.1016/j.jep.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 281.Bhagya N., Chandrashekar K.R. Tetrandrine – a molecule of wide bioactivity. Phytochemistry. 2016;125:5–13. doi: 10.1016/j.phytochem.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 282.Kim M., Miyamoto S., Yasui Y., Oyama T., Murakami A., Tanaka T. Zerumbone, a tropical ginger sesquiterpene, inhibits colon and lung carcinogenesis in mice. Int. J. Cancer. 2009;124:264–271. doi: 10.1002/ijc.23923. [DOI] [PubMed] [Google Scholar]
- 283.Pushpakom S., Iorio F., Eyers P.A., Escott K.J., Hopper S., Wells A., Doig A., Guilliams T., Latimer J., McNamee C., Norris A., Sanseau P., Cavalla D., Pirmohamed M. Drug repurposing: progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]