Abstract
Acute and chronic hepatitis B virus (HBV) infection is a leading cause of liver disease worldwide. It is estimated that approximately 350 million people worldwide have chronic HBV infection and that 1 million persons die each year from HBV-related chronic liver disease. In the past decade, significant progress in the understanding of the molecular virology and pathogenesis of HBV infection has been made. In addition, effective treatment modalities have been developed for persons with chronic infection. Worldwide, prevention of HBV transmission has become a high priority. In 1992, the Global Advisory Group to the World Health Organization recommended that all countries integrate hepatitis B vaccine into national immunization programs by 1997. Currently, 80 countries have done so and several others are planning to. Many countries have reported dramatic reductions in the prevalence of chronic HBV infection among children born since the hepatitis B vaccine was introduced into infant immunization schedules. Recent reports from Taiwan indicate a reduction in the incidence of liver cancer among children as a result of widespread hepatitis B vaccination programs.
Viral hepatitis is a disease with multiple causes that was first described in the fifth century BC. When Hippocrates described epidemic jaundice, he was undoubtedly referring to persons infected with acute hepatitis B virus (HBV) as well as other agents capable of infecting the liver. Epidemics of jaundice have been described throughout history and were particularly common during various wars in the 19th and 20th centuries. While many of these outbreaks were due to hepatitis A, it is likely that epidemic transmission of hepatitis B also occurred in settings where the use of blood-containing products was common.
The recognition of a form of hepatitis that was transmitted by direct inoculation of blood or blood products was first documented by Lurman in Bremen, Germany, in 1883, during a smallpox immunization campaign (99). Thousands of persons received vaccine that had been prepared from human lymph. Of 1,289 shipyard workers who received this vaccine, 191 (15%) developed jaundice several weeks to 8 months later; jaundice did not occur among unvaccinated workers. In the first part of the 20th century, outbreaks of “long-incubation” hepatitis were described in a variety of risk groups including persons who attended clinics for venereal diseases, diabetes, and tuberculosis; patients who received blood transfusions; persons inoculated with mumps or measles convalescent-phase serum; and military personnel who received yellow fever vaccine during World War II (37, 123). The outbreak in yellow fever vaccine recipients was linked to a specific lot of vaccine that contained human serum (139). A follow-up study in the 1980s demonstrated that 97% of recipients of the serum-containing vaccine had serologic evidence of HBV infection compared to 13% of persons who received yellow fever vaccine that did not contain human serum, confirming that HBV was the cause of this outbreak.
The discovery of the etiologic agent of hepatitis B and the development of safe and effective vaccines constitute one of the remarkable scientific achievements of the 20th century. In the 19th century, Virchow had proposed that the pathogenesis of acute hepatitis was due to a plug of mucus in the ampulla of Vater (152). This hypothesis was disputed when pathologic studies revealed diffuse hepatic inflammation in persons with acute jaundice, suggesting an infectious cause (36, 136). Studies of human volunteers in the 1930s and 1940s provided convincing evidence of a viral cause with at least two etiologic agents (60, 100, 101). In 1947, MacCallum and Bauer (100) proposed the current nomenclature of hepatitis A for infectious hepatitis and hepatitis B for “homologous serum” hepatitis. At that time, it was known that the epidemiology of the two diseases differed. Hepatitis A was transmitted by the fecal-oral route, had an incubation period of 2 to 6 weeks, and was primarily a disease of younger children. In contrast, hepatitis B was transmitted by percutaneous exposure to blood products, had a longer incubation period (ranging from 2 to 6 months), and occurred more often in adults.
These observations were confirmed in a series of studies by Krugman and collaborators at the Willowbrook Institute in the 1960s and 1970s (47, 80, 81). Krugman described two types of viral hepatitis, which were referred to as MS-1 and MS-2. MS-1 resembled what MacCallum had classified as hepatitis A. Studies of human volunteers confirmed that this agent was transmitted by the fecal-oral route and had an incubation period of 30 to 38 days. MS-2 resembled hepatitis B in that it had a longer incubation period (41 to 108 days) and was transmitted by percutaneous exposure. These studies also confirmed the existence of homologous immunity after hepatitis A or hepatitis B infection.
Around the same time of the Willowbrook studies, Blumberg and Alter described an immunoprecipitin that was present in the serum of a leukemic patient and was detected in a gel diffusion experiment (13). The patient was an Australian aborigine, and the antigen was named the Australian antigen. Subsequent studies revealed that the antigen had a variable distribution in different populations and occurred more commonly among patients who received multiple transfusions and blood products (14, 15, 132). Extensive studies described the distribution of Australia antigen in various population groups and in patients whose diseases did not appear to be related to hepatitis. The association of the Australian antigen with acute hepatitis B was subsequently demonstrated and led to the development of specific tests for the identification of HBV infection (12, 133).
The viral etiology of hepatitis B was firmly established by electron microscopy and the detection of several viral particles (referred to as Dane particles) that reacted with antisera to Australia antigen (29). It was demonstrated that the Dane particle was HBV, and its surface component was designated hepatitis B surface antigen (HBsAg). The core component contained endogenous DNA and hepatitis core antigen (HBcAg) (hepatitis B nomenclature is given in Table 1). The differential presence of HBsAg, antibodies to HBsAg (anti-HBs), and antibodies to HBcAg (anti-HBc) were used to classify patients as having acute or chronic infections. A third antigen related to infectivity, hepatitis e antigen (HBeAg), was first described in 1972 by Magnius and Espmark (102).
TABLE 1.
Hepatitis nomenclature
| Abbreviation | Term | Definition and comments |
|---|---|---|
| Hepatitis B | ||
| HBV | Hepatitis B virus | Etiologic agent of “serum” hepatitis; also known as Dane particle |
| HBsAg | Hepatitis B surface antigen | Surface antigen(s) of HBV detectable in large quantity in serum; several subtypes identified |
| HBeAg | Hepatitis B e antigen | Soluble antigen; correlates with HBV replication, high-titer HBV in serum, and infectivity of serum |
| HBcAg | Hepatitis B core antigen | Core antigen of HBV; no commercial test available |
| Anti-HBs | Antibody to HBsAg | Indicates past infection with and immunity to HBV, passive antibody from HBIG, or immune response to hepatitis B vaccine |
| Anti-HBe | Antibody to HBeAg | Presence in serum of persons with chronic HBV infection indicates low titer of HBV |
| Anti-HBc | Antibody to HBcAg | Indicates prior infection at some undefined time in past |
| Pre-S1, pre-S2 | Envelope protein epitopes | Envelope proteins containing pre-SHBs epitopes |
| SHBs | Major surface antigen protein | The major protein that forms HBsAg particle is the smallest gene product (SHBs) |
| MHBs | Middle HBs protein | The middle HBsAg protein, containing pre-S2 gene product |
| LHBs | Large HBs protein | The large HBsAg protein, containing pre-S1 and pre-S2 gene products |
| Immune globulin | ||
| HBIG | Hepatitis B immune globulin | Contains high titers of antibody to HBsAg |
The development of sensitive and specific tests to detect HBV infection allowed investigators to define the natural history of HBV infection and develop strategies to prevent transmission. The development of assays to screen blood for HBsAg led to procedures to prevent transfusion-associated hepatitis B. In addition, units of blood that tested positive for anti-HBs were used for the preparation of hepatitis B immune globulin (HBIG). Early studies indicated that HBIG was effective in preventing or modifying the course of HBV infection (83).
In 1970, in the course of studies on the natural history of HBV infection at Willowbrook, Krugman et al. boiled a preparation of MS-2 serum for 1 min to determine the effect of heat on the infectivity of the virus (82). The 1-min boiling step destroyed the infectivity of the preparation, but the heat-inactivated material proved to be antigenic. It was subsequently shown that the preparation was immunogenic and partially protective when volunteers were challenged with MS-2 after receiving the boiled preparation (84). The description of this “inactivated” vaccine soon led to the development of plasma-derived subunit vaccines.
The development of diagnostic assays to distinguish hepatitis A and hepatitis B soon led observers to recognize the existence of other agents that were the etiologic agents of non-A, non-B hepatitis. In 1977, Rizetto and colleagues detected a new antigen in serum samples of patients with severe chronic liver disease (136a). The delta antigen was subsequently shown to be a core protein of a defective virus, which has been classified as hepatitis D virus (HDV). HDV is defective in that it requires HBV to replicate. Thus, prevention of HBV infection prevents HDV transmission and the associated morbidity and mortality. In the late 1980s, studies of patients with non-A, non-B hepatitis led to the discovery and development of serologic assays for HCV and HEV (24, 135).
VIROLOGY
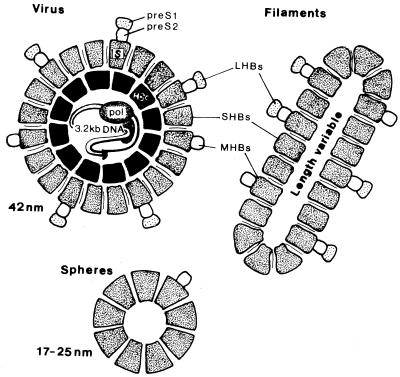
HBV is a double-stranded, enveloped DNA virus of the Hepadnaviridae family, which replicates in the liver and causes hepatic dysfunction. HBsAg is found on the surface of the virus and is also produced in excess amounts, circulating in the blood as 22-nm spherical and tubular particles (Fig. 1). The inner core of the virus contains HBcAg, HBeAg, a single molecule of partially double-stranded DNA, and a DNA-dependent DNA polymerase. HBsAg can be identified in serum 30 to 60 days after exposure to HBV and persists for variable periods.
FIG. 1.
Schematic diagram of hepadnavirus particles. Individual subunits containing SHBs protein only, HBs protein plus pre-S2 (MHBs), and HBs protein plus pre-S1 and pre-S2 (LHBs) are shown in intact virus, among filamensts and spheres. The virus particles contain an internal nucleocapsid (HBc) and viral genome. pol, polymerase. Reprinted from reference 77 with permission of the publisher.
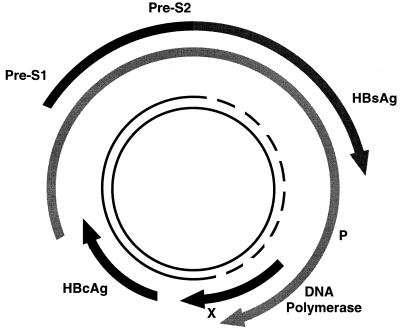
HBV is the smallest DNA virus known: it has only 3,200 bp in its genome, which is uniquely organized in a partly double-stranded, circular pattern (Fig. 2). The minus strand of the DNA is almost a complete circle and contains overlapping genes that encode both structural proteins (pre-S, surface, and core) and replicative proteins (polymerase and X protein). The plus strand of the DNA is shorter and variable in length.
FIG. 2.
Structure and organization of the HBV genome.
Four mRNA transcripts of known function have been identified as being involved in HBV transcription and translation (Fig. 3) (85). The longest (3.5 kb) is the template for genome replication and the expression of precore/core and polymerase proteins. A 2.4-kb transcript encodes pre-S1, pre-S2, and HBsAg, while a 2.1-kb transcript encodes only pre-S2 and HBsAg. The smallest transcript (0.7 kb) encodes the X protein.
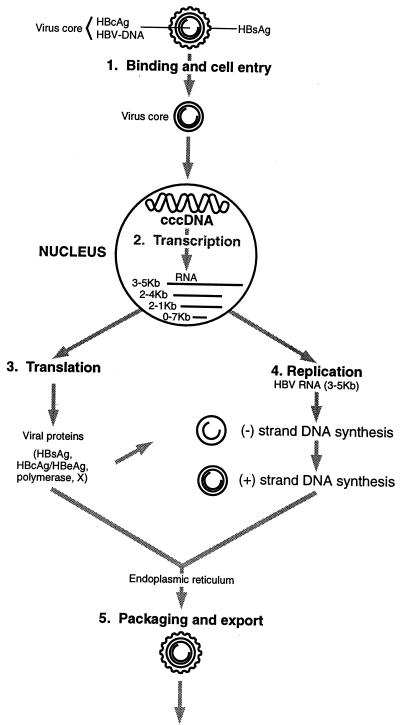
FIG. 3.
Binding and replication of HBV. Reprinted from reference 85 with permission of the publisher.
HBcAg and HBeAg are translated from a common gene. When transcribed, HBcAg is targeted to the endoplasmic reticulum; here it is cleaved, and HBeAg (the precore fragment) is secreted. HBcAg is essential for viral packaging and is an integral part of the nucleocapsid. It is not detectable in serum by conventional techniques; however, it can be detected in liver tissue in patients with acute or chronic HBV infection. HBeAg is a soluble protein that can be detected in the serum of patients with high virus titers; it is not essential for viral replication.
The surface/pre-S gene encodes for the virus envelope. The major protein that forms the HBsAg particles is the smallest gene product (SHBs). The middle protein (MHBs), which contains the pre-S2 component, and the large surface protein (LHBs), which contains pre-S1, are also incorporated into HBsAg particles but are found in larger proportions in the intact virus particles. The specialized functions of these proteins have been the subject of intense study.
It is suggested that the pre-S proteins play an important role in the attachment of HBV to hepatocytes (44). Liver-specific attachment sites have been identified in vitro for pre-S1 and pre-S2 (45, 124, 129). In addition, pre-S2 attaches to artificially polymerized human serum albumin (129). Since binding of this albumin has also been observed on hepatocytes, it has been hypothesized that binding of HBV to its host cell might be mediated by a bridge of modified albumin.
At the molecular level, data supporting SHBs as the binding site for HBV to hepatocytes have been the subject of intense study and debate. Studies have demonstrated binding of SHBs to Vero cells and recombinant SHBs to hepatocytes (78, 90). However, a direct interaction of natural SHBs with hepatocytes or a role of SHBs in the penetration of hepatocytes has not been convincingly demonstrated. Thus, the remarkable success of hepatitis B vaccines that do not contain pre-S components has been something of an enigma.
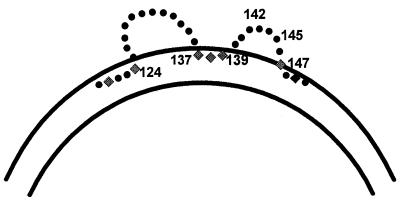
One of the key reasons for the success of currently available vaccines is the existence of a neutralizing epitope on the SHBs protein. This epitope is dependent on the conformation of SHBs proteins determined by cross-linked disulfide bonds. Although the precise disulfide linkages between the cysteine residues of the envelope proteins remain undefined, the protein conformation endowed by the linkages determines the neutralizing epitopes. Antibodies to the common “a” determinant(s) are found in the sera of immunized persons who remain protected against different subtypes of HBV, whereas antibodies to the subtype determinants do not confer protection (133). The “a” determinant(s) is located within the domains bordered by amino acids 120 and 147, which contain a predicted double-loop structure projecting from the surface of the virus (Fig. 4). The major determinant for neutralizing antibodies is located predominantly on the second of these two loops, between amino acids 139 and 147 (11).
FIG. 4.
Schematic diagram of two loops of the “a” determinant of HBs protein. The major epitope is located in the second loop between amino acids 139 and 147.
Two other determinants of HBs have been described. One determinant has either a d or y specificity, and the other has w or r. All combinations of these determinants have been found, resulting in nine subtypes: ayw1, ayw2, ayw3, ayw4, ayr, adw2, adw4, adrq+, adrq−. The most common subtypes among persons with HBV infection in the United States are those with adw specificity (34). HBV has also been classified into six genotypes, A to F.
HBV replication begins with binding of the virus to the cell surface and penetration (45, 77, 85) (Fig. 3). The virus is transported without processing to the nucleus, where the relaxed circular DNA is converted to a covalently closed circular DNA (cccDNA), which in turn acts as the template for viral RNA synthesis. HBV DNA does not integrate into the host genome during the normal course of replication. Transcription results in RNA of various sizes. The 3.5-kb genomic RNA serves as a template for reverse transcription and DNA synthesis, which produces an open circular DNA molecule. The mature core particles are packed into HBsAg and pre-S proteins in the endoplasmic reticulum and exported from the cell. A pool of cccDNA is maintained in the nuclei by the transport of newly synthesized HBV DNA back to the nucleus. Because HBsAg can inhibit the formation of cccDNA, this may represent negative feedback to HBV replication (144).
CLINICAL DISEASE
Acute Infection
The consequences of acute HBV infection are highly variable. The incubation period ranges from 6 weeks to 6 months, and development of clinical manifestations is highly age dependent. Newborns generally do not develop any clinical signs or symptoms, and infection produces typical illness in only 5 to 15% of children 1 to 5 years of age (115). Older children and adults are symptomatic in 33 to 50% of infections. Symptomatic infections vary in severity from mild to fulminant forms. Clinical signs and symptoms of acute HBV infection include fever, anorexia, nausea, malaise, vomiting, jaundice, dark urine, clay-colored or pale stools, and abdominal pain. Occasionally, extrahepatic manifestations occur and include skin rashes, arthralgias, and arthritis. Fulminant hepatitis occurs in about 1 to 2% of persons with reported acute disease and has a case-fatality ratio of 63 to 93%.
Chronic Infection
Chronic HBV infection is defined as the presence of HBsAg in serum for at least 6 months or the presence of HBsAg and the absence of anti-HBc immunoglobulin M (IgM). The risk of developing chronic infection varies inversely with age and is highest (up to 90%) for infants infected in the perinatal period (71, 115). Between 25 and 50% of children infected between 1 and 5 years of age develop chronic infection, compared to 6 to 10% of acutely infected older children and adults.
Persons with chronic HBV infection are at substantially increased risk of developing chronic liver diseases, including cirrhosis of the liver and primary hepatocellular carcinoma (5, 91, 98, 114). The age at which chronic infection occurs may alter the risk of developing the disease. Prospective studies indicate that up to 25% of persons who acquire HBV infection as infants and young children develop either hepatocellular carcinoma or cirrhosis compared to 15% of adolescents and young adults who acquire chronic HBV infection (6, 67). The risk of hepatocellular carcinoma diminishes with resolution of chronic HBV infection (96). Several host-related factors are associated with increased risk of developing chronic infection, including the presence of chronic diseases such as renal failure, human immunodeficiency virus infection, and diabetes (71, 127).
Persons with chronic HBV infection are generally classified as having one of three histologic patterns on liver biopsy: chronic persistent hepatitis, chronic active hepatitis, and cirrhosis. The degree of histologic injury is often not reflected by the symptoms, and persons with severe chronic liver disease are often asymptomatic until late in the course of their illness (64). Persons with chronic active hepatitis develop fibrosis of the liver and are predisposed to the development of cirrhosis. Cirrhosis is an irreversible form of liver injury that may lead to the development of hepatocellular carcinoma through the promotional effect of hepatocyte regeneration (32). No specific HBV oncogene sequence has been identified for the development of hepatocellular carcinoma, which typically appears 25 to 30 years after the onset of infection.
PATHOGENESIS
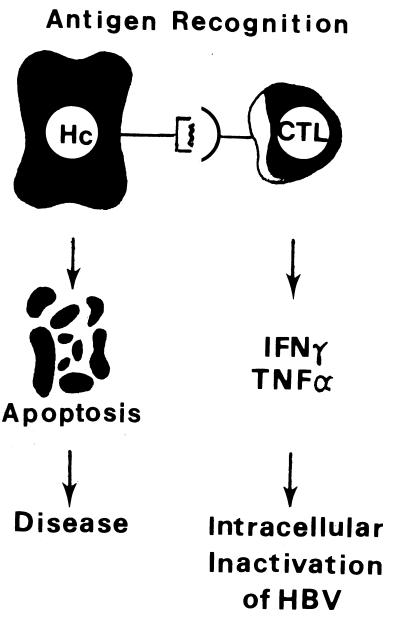
The cellular and humoral immune responses to HBV infection are complex. Most studies suggest that HBV is not directly cytopathic to infected hepatocytes and that the cellular response to several viral proteins correlates with the severity of clinical disease and viral clearance (22). It is believed that the antibody response to viral envelope antigens contributes to clearance of the virus and that cytotoxic T cells mediate viral clearance by killing infected cells. In addition, it has been shown that cytotoxic T lymphocytes inhibit HBV gene expression through the secretion of antiviral cytokines and that the expression of these cytokines may be the principal mechanism of viral clearance during HBV infection (23) (Fig. 5). It is hypothesized that chronic infection is related to a weak T-cell response to viral antigens. While neonatal immune tolerance to viral antigens appears to play an important role in viral persistence among persons infected at birth, the basis of a poor T-cell response in adults is not well understood.
FIG. 5.
T-cell response to HBV-infected hepatocytes. Inhibition of viral replication through the release of cytokines gamma interferon (IFNγ) and tumor necrosis factor alpha (TNFα) may be a key mechanism of viral clearance during acute HBV infection. CTL, cytotoxic T lymphocyte. Reprinted from reference 23 with permission of the publisher.
DIAGNOSIS
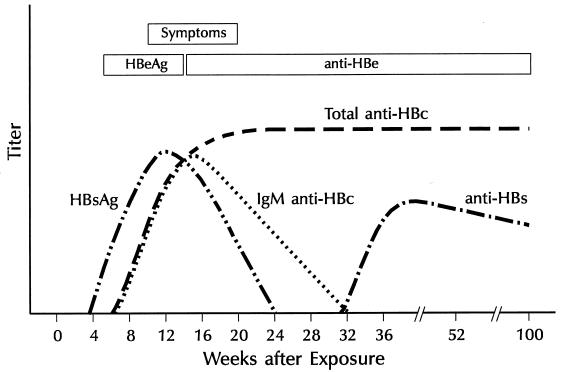
Because the clinical symptoms of HBV infection are indistinguishable from other forms of viral hepatitis, definitive diagnosis is dependent on serologic testing for HBV infection. A variety of tests are available to make the diagnosis of HBV infection (61). Acute HBV infection is characterized by the presence of HBsAg in serum and the development of IgM class antibody (IgM anti-HBc) (Table 2) (20). Detection of HBsAg has evolved from immunodiffusion methods to reversed passive hemagglutination assays and to the more sensitive enzyme immunoassays and radioimmunoassays, which can detect HBsAg at concentrations of ≥0.1 ng/ml. HBeAg is also detectable during acute infection. During convalescence, HBsAg and HBeAg are cleared, and anti-HBs, anti-HBc, and anti-HBe develop (Fig. 6). Anti-HBs is a protective antibody that neutralizes the virus. The presence of anti-HBs following acute infection indicates recovery and immunity from reinfection. Anti-HBs is also detected among persons who have received hepatitis B vaccine. Immunoassays for the detection of total anti-HBc involve both IgM and IgG class antibody to the core protein and indicate current or past exposure to virus and viral replication. IgG anti-HBc appears shortly after HBsAg among persons with acute disease and generally persists for life; therefore, total anti-HBc is not a good marker for persons with acute disease. The detection of IgM anti-HBc is diagnostic of acute HBV infection.
TABLE 2.
Interpretations of available serologic test results for HBV
| HBsAg | IgM anti-HBc | Total anti-HBc | Anti-HBs | Interpretation |
|---|---|---|---|---|
| + | − | − | − | Early HBV infection before anti-HBc response. |
| + | + | + | − | Early HBV infection. Because IgM anti-HBc is positive, the onset is within 6 months. IgG antibody usually appears shortly after IgM; therefore, both are usually posi-tive when IgM is positive. |
| − | + | + | − or + | Recent acute HBV infection (within 4–6 months) with resolution; i.e., HBsAg has already disappeared. Anti-HBs usually appears within a few weeks or months of HBsAg disappearance. |
| + | − | + | − | HBV infection, onset at least 6 months earlier because IgM anti-HBc has disappeared. Prob-able chronic HBV infection. |
| − | − | − | + | Response to hepatitis B vaccine. No evidence of infection. |
| − | − | + | + | Past HBV infection, recovered. |
FIG. 6.
Characteristics of acute hepatitis B with recovery.
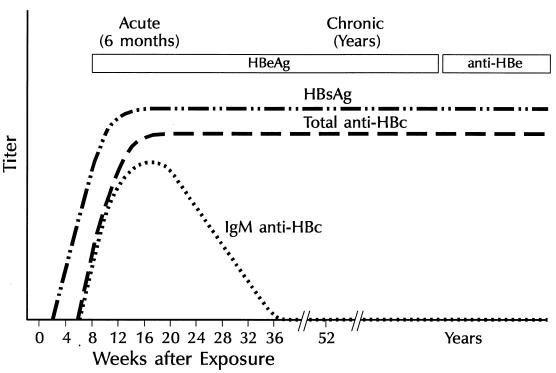
In persons with chronic HBV infection, HBsAg remains persistently detectable, generally for life (Fig. 7). HBeAg is variably present, and IgM anti-HBc generally becomes undetectable 6 months after acute infection.
FIG. 7.
Characteristics of progression to chronic HBV infection.
Detection of HBV DNA has limited usefulness for diagnostic purposes. HBV DNA is detectable in the serum of persons with acute and chronic HBV infection (56, 63, 76, 151, 153). Most slot or dot blot hybridization assays can detect HBV DNA levels as low as 5 pg/ml, which corresponds to 1.5 × 106 genomes per ml. A commercial liquid hybridization assay (Abbott) detects 1.5 pg of HBV DNA per ml (4.0 × 105 genomes per ml), and the branched-DNA hybridization assay detects 2.5 pg of HBV DNA per ml. PCR is much more sensitive than direct hybridization and detects HBV DNA levels of 10−3 pg/ml (approximately 100 to 1,000 genomes); however, PCR assays are prone to false-positive results.
The clinical significance of detecting HBV DNA by hybridization and by PCR is quite different. Generally, detection by PCR has the same significance as detection of HBsAg and indicates current HBV infection. In contrast, detection by hybridization indicates significant viral replication and a high probability of active liver disease (similar to HBeAg). Monitoring HBV DNA levels is useful in determining the response of chronic HBV infection to treatment (see below). Nucleic acid sequence analysis has been used to identify genetic variants of the virus and to investigate common-source outbreaks of HBV infection (17, 58).
CLINICAL MANAGEMENT
No specific treatment is available for persons with acute HBV infection; supportive and symptomatic care is the mainstay of therapy. In the past decade, numerous antiviral agents have been investigated as candidates for the treatment of chronic HBV infection. In 1976, two studies, one with leukocyte interferon and one with beta interferon, suggested that interferon can affect the serologic profile of persons with chronic HBV infection (31, 51). Follow-up studies revealed the most promising agent to be alpha-2b interferon, which has been licensed for this purpose by the Food and Drug Administration. The goals of interferon treatment for patients with chronic HBV infection are to clear serologic markers of HBV replication and to improve the liver disease (by normalization of alanine aminotransferase levels and liver histologic test results). In a meta-analysis of 15 clinical trials, the overall response rate (as measured by clearance of HBeAg from serum) was 33% among patients treated with interferon compared with 12% among untreated controls (159). Long-term follow-up of treated patients suggests that remission of chronic hepatitis induced by alpha interferon is of long duration (79). The use of a brief course of corticosteroids with rapid tapering before interferon therapy has had conflicting results (62).
Alpha interferon treatment of patients with chronic hepatitis B is recommended for patients with persistent elevations in aminotransferase concentrations in serum; detectable levels of HBsAg, HBeAg, and HBV DNA in serum; chronic hepatitis on liver biopsy; and compensated liver disease (62). Patients with normal aminotransferase levels should not be treated. Biopsy of the liver should be performed before therapy to assess the degree of fibrosis present. The recommended regimen is either 5 million units daily or 10 million units three times a week, given subcutaneously for 4 months. Patient characteristics associated with a favorable response to therapy include low pretherapy HBV DNA levels (<200 pg/ml), high pretherapy alanine aminotransferase levels (>100 IU/ml), short duration of infection, acquisition of disease in adulthood, active histologic profile, and absence of complicating diseases such as renal failure or human immunodeficiency virus infection.
Because relatively few patients respond to interferon therapy, considerable research has been conducted on other antiviral and immune system-modulating agents. Nucleoside analogues such as famciclovir and lamivudine have been extensively evaluated and shown to be well tolerated after oral administration (16, 33, 35). Both drugs lead to rapid decreases in HBV DNA levels; clearance of HBeAg and decreases in serum aminotransferase levels have been observed in some patients. However, short courses of therapy have been followed by a rapid return of viral DNA to pretreatment levels and no sustained improvement in chronic liver disease. Clinical trials of long-term therapy are under way.
EPIDEMIOLOGY
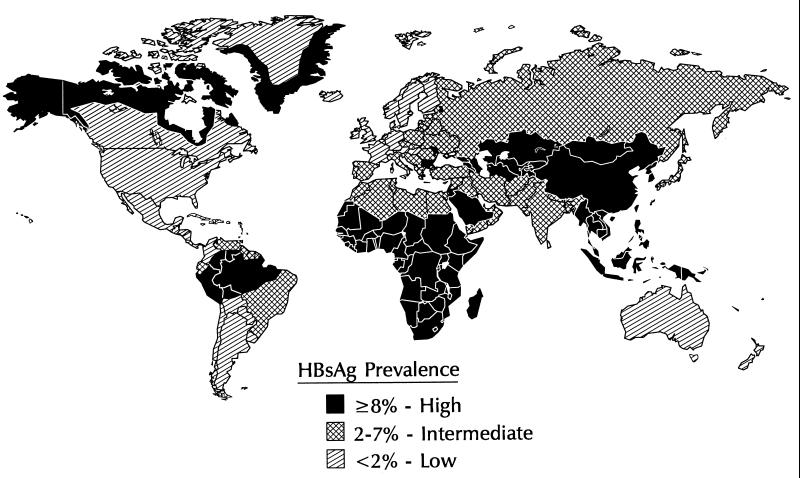
HBV is transmitted by percutaneous or permucosal exposure to infectious body fluids, by sexual contact with an infected person, and perinatally from an infected mother to her infant. The frequency of HBV infection and patterns of HBV transmission vary markedly in different parts of the world. Approximately 45% of the world’s population live in areas where the prevalence of chronic HBV infection is high (≥8% of the population is HBsAg positive), 43% live in areas where the prevalence is moderate (2 to 7% of the population is HBsAg positive), and 12% live in areas of low endemicity (<2% of the population is HBsAg positive (Fig. 8).
FIG. 8.
Geographic distribution of chronic HBV infection.
In areas of high endemicity, the lifetime risk of HBV infection is >60% and most infections occur at birth or during early childhood, when the risk of chronic infection is greatest. Because most early childhood HBV infections are asymptomatic, there is little recognition of acute disease, but rates of chronic liver disease and liver cancer are high. Areas of high endemicity include most of Asia (except Japan and India), most of the Middle East, the Amazon Basin of South America, most Pacific Island Groups, Africa, and other special populations such as Native Alaskans, Australian Aborigines, and Maoris in New Zealand (4, 11, 54, 68, 72, 104, 115, 118, 145, 149, 150, 158).
The mechanisms of early childhood transmission in areas of high endemicity are variable. Generally, infections cluster in households of persons with chronic infection (97). The major determinants of infection include exposure to a HBsAg-positive mother or sibling. The contribution of perinatal transmission to the overall burden of disease is related to the prevalence of HBeAg among pregnant women. If a mother is HBsAg positive and HBeAg positive, 70 to 90% of her infants will become infected if not given immunoprophylaxis (140, 163). Of infants born to HBsAg-positive mothers who are HBeAg negative, approximately 5 to 20% are infected at birth. Infants of HBsAg-positive women who are not infected at birth are at increased risk of HBV infection during early childhood because of household contact with infected persons (7).
In East and Southeast Asian countries, as well as the Pacific, 35 to 50% of HBsAg-positive women are HBeAg positive (7, 68, 89, 93, 11, 140). It is estimated that 3 to 5% of all infants in these countries may develop chronic HBV infection at birth and that up to 30 to 50% of all chronic infections among children may result from perinatal transmission. In areas of high endemicity where the prevalence of HBeAg among pregnant women is low (Africa, South America, and the Middle East), perinatal HBV transmission contributes less to the pool of children with chronic infection than does postnatal early childhood transmission (11, 73, 110, 149). Generally, in these areas, 1 to 2% of infants develop chronic infection and 10 to 20% of all chronic infections among children result from perinatal exposures.
In areas of moderate endemicity, the lifetime risk of HBV infection is 20 to 60% and infections occur in all age groups. Recognition of acute disease is common because many infections occur in adolescents and young adults. In addition, high rates of HBV-related chronic liver disease occur due to the high prevalence of chronic HBV infection. Generally, in areas of moderate endemicity, 2 to 7% of pregnant women are HBsAg positive and <20% of HBsAg-positive women are HBeAg positive; thus, perinatal transmission accounts for a small proportion (10 to 20%) of the persons with chronic infection. In these areas, early childhood HBV transmission may be quite variable in different regions or among different ethnic groups within a country. Acute disease among adults tends to occur in the same risk groups as in developed countries.
In areas of low endemicity, the lifetime risk of infection is <20% and most infections occur among adults in well-defined risk groups. In the United States, the prevalence of chronic HBV infection is 0.35% and 5% of the general population has evidence of prior HBV infection (108, 117). High-risk groups for HBV infection include intravenous-drug users, homosexual men, persons who have heterosexual contact with multiple partners, household contacts of persons with chronic HBV infection, hemophiliacs, hemodialysis patients and staff, inmates of long-term correctional facilities, persons with occupational exposure to blood and infectious body fluids, and institutionalized persons with developmental disabilities.
While most acute HBV infections in the United States occur among young adults, about one-third of the chronic infections are acquired through perinatal and early childhood exposures (108). It is estimated that 20,000 HBsAg-positive women give birth each year in the United States and that 9,500 infants would become infected if prophylaxis were not provided. In addition, a number of well-defined populations with high rates of early childhood HBV transmission reside in the United States, including Alaskan Natives, children of Pacific Island communities, and children of first-generation immigrants from countries where HBV is of high or intermediate endemicity. Among U.S.-born children of first-generation immigrants during the first decade of life, infection rates average 1 to 2% per year and the prevalence of chronic HBV infection ranges from 1 to 7% (40, 69, 105).
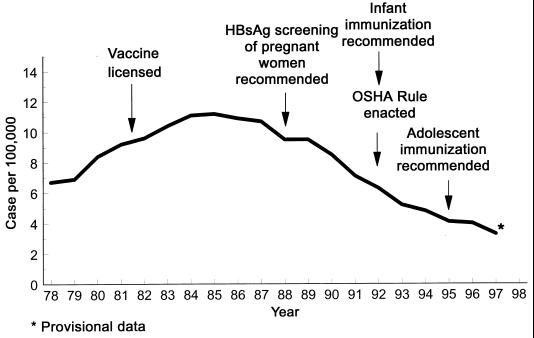
In the United States, there was a 1.7-fold increase in the incidence of reported cases from 1979 to 1985, but there has been a 3-fold decrease since 1986 (Fig. 9). It is estimated that approximately 100,000 to 130,000 persons are infected each year and that 5,000 persons die each year of HBV-related liver disease, including 150 to 200 deaths from fulminant hepatitis, 3,000 to 4,000 from cirrhosis, and 600 to 1,000 from primary hepatocellular carcinoma (17a, 108).
FIG. 9.
Reported cases of hepatitis B in the United States from 1975 to 1997.
Worldwide, the consequences of acute and chronic HBV infection are major public health problems. Approximately 5% of the world’s population (300 million persons) have chronic HBV infection, which is the leading cause of chronic hepatitis, cirrhosis, and hepatocellular carcinoma worldwide (111). It is estimated that 500,000 to 1,000,000 persons die annually of HBV-related liver disease.
PREVENTION
Passive Immunization
The discovery that passively acquired anti-HBs could protect individuals from acute clinical hepatitis B and chronic HBV infection if given soon after exposure led to the development of a specific Ig containing high titer of anti-HBs (HBIG). HBIG is prepared by the Cohn Oncly fractionation procedure from serum containing high titers of anti-HBs and is standardized to 100,000 IU of anti-HBs/ml. HBIG is effective, often in combination with hepatitis B vaccine, as postexposure prophylaxis following (i) perinatal exposure for an infant born to an HBsAg-positive mother, (ii) percutaneous or mucous membrane exposure to HBsAg-positive blood, or (iii) sexual exposure to an HBsAg-positive person (9, 49, 134). HBIG is also used to protect patients from severe recurrent HBV infection following liver transplantation.
Active Immunization
Safe, immunogenic, and effective hepatitis B vaccines have been commercially available in the United States since 1981. Hepatitis B vaccines are composed of highly purified preparations of HBsAg. The vaccines are prepared either by harvesting HBsAg from the plasma of persons with chronic infection (plasma-derived vaccine) or by inserting plasmids containing the HBs gene, and in some cases the pre-S1 and/or pre-S2 gene, into yeast or mammalian cells. The vaccines undergo various inactivation steps, are highly purified, and then are adjuvanted with aluminum phosphate or aluminum hydroxide and preserved with thimerosal.
In the United States, vaccines are licensed for all age groups as a three-dose series consisting of two priming doses given 1 month apart and a third dose given 5 months after the second dose. The recommended dose varies with the product, the recipient’s age, and, for infants, the mother’s HBsAg serologic status. In general, the vaccine dose for infants and adolescents is 50 to 75% lower than that for adults.
Immunogenicity and Efficacy of Vaccines
The protective efficacy of hepatitis B vaccination is directly related to the development of anti-HBs (28, 39, 52, 146). Persons who develop anti-HBs titers of >10 mIU/ml after a primary vaccination series are virtually 100% protected against clinical illness and chronic infection. The vaccines produced by each manufacturer have been evaluated in clinical trials to determine the age-specific dose that achieves the maximum seroprotection rate. The priming doses induce detectable levels of antibody in 70 to 90% of healthy infants, adolescents, and adults. The final dose induces protective levels of anti-HBs in >95% of infants of HBsAg-negative women and in adolescents (1, 50, 55, 112, 155, 157).
The recommended series of three intramuscular doses of hepatitis B vaccine induces a protective antibody response in >90% of healthy adults younger than 40 years. After age 40, the cumulative age-specific decline in immunogenicity drops below 90%, and by age 60 only 75% of vaccinees develop protective levels of anti-HBs (137, 161). Although other host factors such as smoking, obesity, human immunodeficiency virus infection, and the presence of a chronic disease contribute to decreased immunogenicity of the primary vaccination series, age is the major determinant of poor vaccine response (3).
The efficacy of hepatitis B vaccines has been demonstrated in clinical trials involving several high-risk groups including homosexual men, health care workers and hemodialysis staff, children living in areas of high endemicity, and infants of HBeAg-positive (highly infectious) mothers (8, 28, 39, 146, 163). These studies demonstrated virtually complete protection in persons who developed anti-HBs titers of >10 mIU following vaccination. Cases of clinical hepatitis B and, rarely, of chronic HBV infection were observed in persons who developed anti-HBs responses <10 mIU. Studies among children living in areas of high endemicity have also demonstrated excellent vaccine efficacy in persons who develop anti-HBs titers of >10 mIU/ml. For these reasons, an anti-HBs response of >10 mIU by commercial radioimmunoassay or enzyme immunoassay is considered to be the lower limit of adequate response to vaccine.
In infants born to HBeAg positive mothers, combined treatment with either plasma or recombinant vaccines and HBIG is 79 to 98% efficacious in preventing chronic HBV infection (Table 3) (2, 9, 74, 87, 95, 128, 131, 141, 142, 148, 160). One longitudinal study found significantly lower rates of chronic infection in infants treated with recombinant vaccines and HBIG (3.8%) than in infants treated with plasma-derived vaccine and HBIG (10.5 to 13.9%) (143). Several studies have demonstrated a high efficacy of vaccine alone in preventing perinatal HBV transmission, including reports where the administration of HBIG provided no additional protection (131). However, lower doses of vaccine alone appear to be less efficacious in preventing perinatal HBV transmission (2).
TABLE 3.
Studies of the efficacy of hepatitis B vaccines in neonates born to HBeAg-positive mothers
| Study | Vaccinea | Dose (μg) | Schedule (mo) | HBIG at birth | No. studied | % HBsAg positive | % Efficacy |
|---|---|---|---|---|---|---|---|
| Beasley et al. (8) | MSD-P | 20 | 0, 1, 6 | Yes | 159 | 2.0–8.6 | 91.4–98.0 |
| Wong et al. (160) | DRC-P | 3 | 0, 1, 2, 6 | Yes | 124 | 9.2–14.4 | 79.3–86.8 |
| Lo et al. (95) | Pasteur | 5 | 0, 1, 2, 12 | Yes | 72 | 8.1–11.4 | 85.5–89.7 |
| Stevens et al. (143) | MSD-P | 20 | 0, 1, 6 | Yes | 158 | 13.9 | |
| MSD-P | 10 | 0, 1, 6 | Yes | 152 | 10.9 | ||
| MSD-R | 5 | 0, 1, 6 | Yes | 351 | 5.4 | ||
| Pongpiat et al. (128) | MSD-R | 5 | 0, 1, 6 | Yes | 20 | 10.0 | 89.2 |
| Lee et al. (87) | SKB | 20 | 0, 1, 2, 12 | Yes | 54 | 7.4 | 91.6 |
| SKB | 10 | 0, 1, 2, 12 | Yes | 56 | 1.8 | 98.0 | |
| SKB | 20 | 0, 1, 6 | Yes | 60 | 3.3 | 96.3 | |
| Poovorawan et al. (131) | SKB | 10 | 0, 1, 2, 12 | Yes | 65 | 1.5 | 97.6 |
| SKB | 10 | 0, 1, 6 | Yes | 60 | 0 | >97 | |
| Beasley et al. (8) | MSD-P | 20 | 0, 1, 6 | No | 40 | 22.5 | 75.0 |
| Wong et al. (160) | DRC-P | 3 | 0, 1, 2, 6 | No | 64 | 24.3 | 65.1 |
| Lo et al. (95) | Pasteur | 5 | 0, 1, 2, 12 | No | 36 | 19.4 | 75.3 |
| Poovorawan et al. (131) | SKB | 10 | 0, 1, 2, 12 | No | 59 | 3.4 | 94.8 |
| SKB | 10 | 0, 1, 6 | No | 59 | 3.4 | 94.8 | |
| Tin (148) | MSD-P | 20 | 0, 1, 2, 6 | No | 113 | 17.7 | 70.4 |
| MSD-P | 10 | 0, 1, 6 | No | 58 | 12.1 | 79.8 | |
| MSD-R | 5 | 0, 1, 6 | No | 60 | 5 | 91.6 | |
| Assateerawatt et al. (2) | MSD-R | 2.5 | 0, 1, 2, 6 | No | 24 | 29 | 66.0 |
| Moulia-Pelat et al. (121) | Pasteur-R | 20 | Mixed | No | 16 | 6.2 | 93 |
P, plasma-derived vaccine; R, recombinant vaccine; MSD, Merck vaccine; DRC, Dutch Red Cross; SKB, SmithKline Beecham.
Long-Term Protection
The duration of immunity after hepatitis B vaccination has been the subject of considerable study in the 15 years since vaccine licensure. Decline in anti-HBs titers after vaccination has been well quantitated in several studies (25, 30, 46, 48, 53, 75, 147, 154). Generally, there is a rapid decline in protective antibody in the first 12 months after the third dose and a more gradual decline over time. Among adult vaccinees, anti-HBs levels decline to <10 mIU/ml in 7 to 50% of vaccinees 5 years after vaccination and in 30 to 60% of vaccinees by 9 to 11 years. No studies have reported acute cases of hepatitis B among vaccine responders; however, a few studies have detected asymptomatic infections by serologic testing. Of 1,786 persons monitored for 5 to 11 years, 63 (3.5%) became anti-HBc positive; none of these persons had evidence of chronic HBV infection. It is thought that these mild, clinically inapparent infections will not produce sequelae associated with chronic HBV infections. These studies indicate that protection against serious HBV infection persists for at least 11 years despite the decline in antibody titer (Table 4).
TABLE 4.
Long-term protection among adults who respondeda to a primary hepatitis B vaccination series
| Group (reference) | No. of patients | Length of follow-up (yr) | % of patients showing anti- HBs loss (%) | No. of late infections
|
|
|---|---|---|---|---|---|
| Allb | Chronic | ||||
| Health care workers (106) | 144 | 11 | 31 | 0 | 0 |
| Homosexual men (143) | 127 | 11 | 61 | 0 | 0 |
| Eskimos (154)c | 1,194 | 9–10 | 24 | 13 | 0 |
| Homosexual men (53)d | 634 | 9 | 54 | 48 | 0 |
| Military (48) | 190 | 6 | 45 | 4 | 0 |
| Medical students (48) | 100 | 5 | 19 | 1 | 0 |
| Health care workers (147) | 41 | 5 | 32 | 0 | 0 |
| Health care workers (25) | 143 | 5 | 7 | 4 | 0 |
| Health care workers (46) | 32 | 5 | 24 | 0 | 0 |
| Health care workers (30) | 31 | 5–7 | 52 | 0 | 0 |
| Health care workers (75) | 72 | 5 | 30 | 0 | 0 |
Peak anti-HBs titer, ≥10mIU/ml.
Persons who have antibody to HBcAg.
Includes children.
Includes follow-up unpublished data.
Studies of infants and young children have also shown excellent long-term protection in those who initially responded to hepatitis B vaccination; however, breakthrough infections with viremia have been observed in several studies of vaccinated children born to HBsAg-positive women (Table 5) (26, 27, 55, 70, 88, 92, 94, 130, 162, 165). Pooling data from follow-up studies of 1,993 infants born to chronically infected women and monitored for 5 to 11 years show that 20 (1.0%) became HBsAg positive more than 1 year after vaccination.
TABLE 5.
Long-term protection from HBV infection after hepatitis B vaccination in persons who responded to a primary vaccination series
| Group and location (reference) | No. of patients | Length of follow-up (yr) | % of patients showing anti-HBs loss | No. of carriers | HBsAg | anti-HBc |
|---|---|---|---|---|---|---|
| Infants of HBeAg-positive mothers | ||||||
| U.S.A. (143)a | 315 | 4–11 | 12 | 0 | 0 | 30 |
| China (92) | 74 | 9 | 49 | 0 | 0 | 7 |
| China (162) | 536 | 9 | 44 | 14 | 14 | NA |
| China (165) | 50 | 5 | 17 | 1 | 3 | |
| Taiwan (94) | 199 | 6 | 3 | 0 | 0 | 0 |
| Taiwan (70) | 654 | 5 | 9 | 3 | 3 | 46 |
| Taiwan (65) | 165 | 5 | 17 | 0 | 0 | 11 |
| Routine infant or early childhood vaccine | ||||||
| Senegal (26) | 327 | 2–12 | NA | 9 | 7 | |
| China (162) | 536 | 8 | 39 | 3 | 3 | NA |
| Venezuela (55)b | 280 | 6 | 29 | 0 | 0 | 6 |
Contains unpublished follow-up data.
NA, data not available.
The proposed mechanism for continued protection against clinically significant HBV infection, despite declining antibody titers, is an anamnestic immune response after HBV exposure (156). The phenomenon of immunologic memory has been demonstrated by the presence of a rapid increase in anti-HBs titers following a booster dose of vaccine in persons given a primary vaccination series several years earlier (18, 30, 65, 119, 120). Booster-dose studies of adults have demonstrated that >90% of vaccinees have such immune memory when challenged with hepatitis B vaccine; they also suggest that the immune system would be able to respond rapidly to HBV exposure. The long incubation period of HBV infection (60 to 120 days), coupled with the excellent anamnestic antibody response to low levels of HBsAg in previously immunized persons, appears to limit breakthrough infections to those who do not develop detectable viremia, symptomatic disease, or chronic infection.
The need for booster doses of hepatitis B vaccine after a primary vaccine series has been the subject of considerable debate. For children living in areas of high endemicity, there is no difference in the prevalence of HBV infection at 12 years of age in children who receive a booster dose of vaccine at school entry and in children who do not (26, 131). Currently, vaccine advisory groups in the United States do not recommend routine booster doses of hepatitis B vaccine in persons who have responded to vaccination. Ongoing studies should provide information on the need for booster doses during the second decade after vaccination.
Recommendations for Vaccination
The epidemiology of HBV infection indicates that multiple age groups must be targeted to provide widespread immunity and effectively prevent HBV transmission and HBV-related chronic liver disease. Since the licensure of hepatitis B vaccine, the Advisory Committee on Immunization Practices (ACIP) of the U.S. Public Health Service has developed a comprehensive strategy to eliminate HBV transmission in the United States (Table 6).
TABLE 6.
Evolving strategy of the ACIP to eliminate HBV transmission in the United States
| Strategy | Yr first rec-ommended |
|---|---|
| Preexposure prophylaxis | |
| Vaccination of high-risk groups | 1982 |
| Routine vaccination of high-risk infants and childrena | 1986 |
| Routine vaccination of all infants | 1992 |
| Routine vaccination of adolescents at 11–12 yr of age | 1995 |
| Vaccination of high-risk children born after 1983a | 1995 |
| Catch-up vaccination of all children and adolescents | 1997 |
| Postexposure prophylaxis | |
| Accidental or percutaneous exposure to blood | 1982 |
| Sexual partners of persons with acute infection | 1982 |
| Infants born to HBsAg-positive women | 1982 |
| Household contacts of persons with chronic HBV infection | 1982 |
| Universal HBsAg screening of all pregnant women | 1988 |
Children born to first-generation immigrants from countries where HBV infection is of high or intermediate endemicity.
The initially recommended strategy in the United States was selective vaccination of individuals with identified risk factors. However, programs to vaccinate high-risk persons were not developed, most persons with identified risk factors were difficult to target for vaccination, and many infected persons had no identifiable risk factors. Thus, vaccinating persons prior to exposure was generally not feasible, and the incidence of acute hepatitis B remained high during the first decade after vaccine licensure.
Recommendations to vaccinate all newborns were the subject of considerable debate among primary-care providers in the United States, many of whom considered patients in their practice to be at low risk for acquiring HBV infection (41, 103). In addition, providers expressed concerns about the cost-effectiveness of infant vaccination and long-term protection after hepatitis B vaccination of infants (43). Some providers believed that routine vaccination of adolescents would be a more effective primary immunization strategy. However, cost-effectiveness analysis demonstrated that routine immunization of infants was the most effective strategy to prevent HBV transmission in the United States because of the additional benefit in preventing early childhood transmission (109). Despite the initial concerns of providers, hepatitis B vaccination coverage of infants increased rapidly and reached levels as high as that observed for Haemophilus influenzae type b vaccine within 3 years after its introduction (42). In 1997, >80% of 2- to 3-year-old children had received three doses of hepatitis B vaccine.
The implementation of routine infant immunization will eventually produce broad population-based immunity to HBV infection and prevent HBV transmission among all age groups. However, since most HBV infections in the United States occur among young adults, it will take 2 to 3 decades before infant immunization affects the incidence of hepatitis B in the United States. To hasten the development of population-based immunity and to decrease more rapidly the incidence of hepatitis B, catch-up vaccination of 11- to 12-year-old adolescents was recommended by the ACIP in 1995. This strategy was expanded to include all adolescents in 1997. Vaccination of high-risk adults remains a high priority, and efforts to vaccinate health care workers have resulted in a dramatic decline in the number of cases of acute disease (106). Enhanced efforts are being made to vaccinate other high-risk adult populations.
Disease Control
Unlike other vaccine-preventable diseases, the efficacy of hepatitis B prevention programs is not based solely on surveillance of acute disease. Because most HBV infections in children younger than 10 years are asymptomatic, evaluations based on surveillance data would not reliably measure the effectiveness of hepatitis B vaccination programs, especially those directed at infants. Because most of the HBV-associated morbidity and mortality is related to chronic infection, population-based surveys demonstrating a reduction in the prevalence of chronic infection form a key indicator of vaccination program success and disease reduction. Trends in disease incidence are useful to evaluate the effectiveness of programs directed at adolescents and high-risk adults who are more likely to have symptomatic infections after HBV exposure (106).
Numerous studies have demonstrated the effectiveness of routine infant immunization. Population-based surveys among Alaskan Native and U.S.-affiliated Pacific Island communities have demonstrated a remarkable reduction in the prevalence of chronic HBV infection in children born after implementation of routine infant immunization (Table 7) (57, 107, 154). In areas where vaccination coverage was high, the prevalence of chronic HBV infection declined to <1% and residual transmission occurred among children who did not start the vaccination series at birth. Similar studies in Taiwan, Indonesia, and The Gambia have also demonstrated similar reductions in the prevalence of chronic HBV infection (21, 38, 138).
TABLE 7.
Population-based studies comparing the prevalence of chronic HBV infection in children born before and after the introduction of hepatitis B vaccine into infant immunization schedules
| Location (reference) | No. testeda | Age (yr) at testing | Vaccine coverage (%) | % with chronic HBV infection
|
|
|---|---|---|---|---|---|
| Before program | After program | ||||
| Alaska (57) | 268 | 1–10 | 96 | 16 | 0 |
| Taiwan (21) | 424 | 7–10 | 73 | 10 | 1.1 |
| Samoa | 435 | 7–8 | 87 | 7 | 0.5 |
| Indonesia (138) | 2519 | 4 | >90 | 6.2 | 1.9 |
| Pacific (107) | 200 | 3–4 | 94 | 9 | 0.5 |
| Micronesia (107) | 364 | 3–4 | 82 | NAb | 1.1 |
| Micronesia (107) | 544 | 2 | 37 | 12 | 3 |
| Polynesia (121) | 582 | 1–2 | 66 | 6.5 | 0.7 |
Number of children tested in follow-up seroprevalence surveys at the age indicated.
NA, data not available.
The ultimate goal of hepatitis B vaccination programs is to decrease the incidence of HBV-related chronic liver disease and hepatocellular carcinoma. Recent studies in Taiwan have demonstrated a reduction in the incidence of primary liver cancer in children born after the implementation of routine infant hepatitis B vaccination programs (Fig. 10) (19, 86).
FIG. 10.
Liver cancer death rates in children from birth to 9 years old in Taiwan. Programs to prevent perinatal HBV transmission were initiated in 1984, and routine infant vaccination was begun in 1986. Mass campaigns to vaccinate older children and adults were conducted from 1987 to 1990. Adapted from data in reference 86.
EMERGING ISSUES
Mutant Viruses
HBV infection with molecular variants of the virus has been found in vaccinated persons in Italy, Singapore, The Gambia, and the United States and in liver transplant recipients who received HBIG for prophylaxis of relapse of HBV infection (17, 59, 66, 116, 122, 164, 166). It has been proposed that these variants contain HBsAg that is not recognized by vaccine-induced antibodies and that acute HBV infection occurs in the presence of protective levels of anti-HBs. Several investigators have reported a mutation in the genome that causes a change in one amino acid in the “a” determinant of the HBs protein, which is the proposed conformational epitope essential for recognition and neutralization by anti-HBs antibodies. The most common alteration described is a replacement of glycine by arginine at amino acid 145, but other mutations such as replacement of aspartic acid by alanine at amino acid 144 have also been described. Similar variants have also been found in unvaccinated persons with chronic HBV infection, suggesting that they occur naturally. The incidence of infection with variant strains of HBV among vaccinated infants has been reported in a population-based study of 1,092 infants born to HBeAg-positive pregnant women between 1981 and 1993 (122). Overall, 94 (8.6%) of the infants became infected and HBV variant strains were isolated from 22 of the infected children. As found in other studies, the most common amino acid changes occurred at positions 143 to 145; however, mutations were also found across most of the region. In addition, mixed infections involving wild-type and mutant viruses were often detected in both mothers and infants, suggesting that infants acquire their mutant strain from the mother.
At present, the public health importance of the HBV molecular variants is debatable. Studies of vaccinated household members living with persons chronically infected with variants have not demonstrated intrahousehold transmission of the variant (126). In addition, preexposure vaccination of chimpanzees with currently licensed vaccines (not containing pre-S epitopes) conferred protection following intravenous challenge with the 145-HBV mutant (125). Further studies and enhanced surveillance to detect the emergence of these variants remain high priorities in evaluating the effectiveness of current immunization strategies.
Public Health Considerations
Worldwide, prevention of chronic HBV infection has become a high priority. In 1992, the Global Advisory Group to the World Health Organization recommended that all countries integrate hepatitis B vaccine into national immunization programs by 1997. For countries where the prevalence of chronic HBV infection is >2%, the Global Advisory Group recommended incorporating hepatitis B vaccine into infant immunization schedules. The strategy for low-endemicity countries could include routine immunization of adolescents in addition to or instead of immunization of infants.
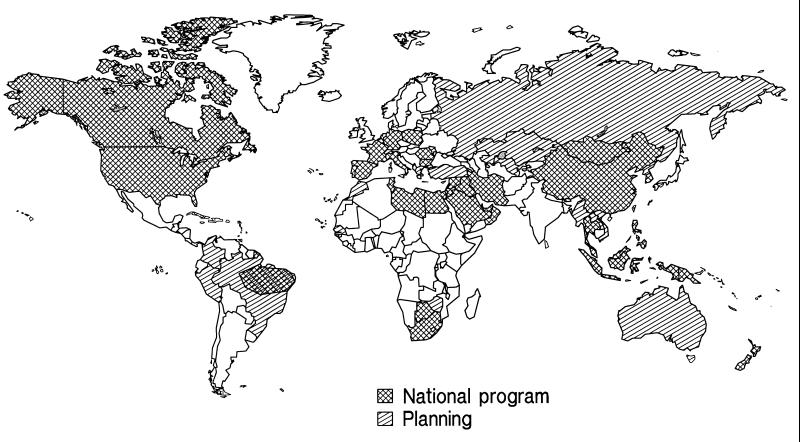
Currently, 80 countries have integrated hepatitis B vaccine into national immunization programs, and several others are planning to do so (Fig. 11). Infant vaccination programs have been implemented in regions of Southeast Asia, China, the Pacific, and the Middle East with high endemicity, and there are plans to implement programs in several other regions of high endemicity, including India. Most of the countries of high endemicity in sub-Saharan Africa have not made plans to incorporate hepatitis B vaccine into Expanded Program on Immunization schedules. The primary obstacle to implementing programs in these countries has been the high cost of vaccine compared to other Expanded Program on Immunization antigens. Increased global production of vaccine has decreased the cost of vaccine in the past decade. It is anticipated that increased production of vaccine and the development of combination vaccines containing hepatitis B vaccine will facilitate more widespread use of the vaccine in economically disadvantaged countries.
FIG. 11.
Countries that have integrated hepatitis B vaccine into national immunization programs, 1996. Source, Mark Kane, Global Program on Immunization, World Health Organization.
Vaccine advisory groups in the United States have recommended a combination of routine infant immunization and catch-up vaccination of all older children as the primary strategy to eliminate HBV transmission in the United States. Numerous studies have shown that this combination can virtually eliminate HBV transmission in a community (19, 21, 107, 113). In fact, many long-term programs have demonstrated a greater than expected benefit in terms of disease reduction compared to three-dose vaccination coverage. It is likely that this effect is related to a number of factors. Widespread use of vaccine creates immunized cohorts of children who no longer serve as reservoirs of virus. Thus, successive cohorts of children are less likely to be exposed to the HBV in early childhood, when the risk of chronic infection is greatest. Other prevention efforts, such as safe injection practices, screening of blood products, and the use of Universal Precautions for infectious body fluid exposures, have also been implemented. Finally, in settings where vaccination coverage is low, it appears that vaccination of children with one or two doses of vaccine may provide some degree of protection in preventing HBV infection and chronic infection.
The serious adverse outcomes related to acquisition of chronic HBV infection occur several decades after exposure; therefore, the benefit of infant immunization will not be realized for several decades. Commitment of public health resources to eliminate HBV transmission requires recognition of the importance of this disease, persistent efforts to ensure that populations are protected, and patience to realize the goals of disease reduction. The recent studies in Taiwan demonstrating a reduction of liver cancer deaths in children provide assurance that the strategy of routine infant immunization is a well-conceived public health practice that will benefit generations to come.
ACKNOWLEDGMENTS
I thank S. Tewfik and L. Peruski at NAMRU-3 for assistance with figures. In addition, Gwen Lewis provided valuable editorial comments. Thanks are due to Mark Kane, Expanded Program on Immunization of the World Health Organization, who provided Fig. 11.
REFERENCES
- 1.Andre F E. Summary of safety and efficacy data on yeast-derived hepatitis B vaccine. Am J Med. 1989;87(Suppl.):39–45. doi: 10.1016/0002-9343(89)90525-1. [DOI] [PubMed] [Google Scholar]
- 2.Assateerawatt A, Tanphaichitr V S, Suvatte V, Ingarm L. Immunogenicity and protective efficacy of low dose recombinant DNA hepatitis B vaccine in normal and high-risk neonates. Asian Pac J Allergy Immunol. 1991;9:89–93. [PubMed] [Google Scholar]
- 3.Averhoff F, Mahoney F J, Coleman P J, Schatz G, Hurwitz E, Margolis H S. Risk factors for lack of response to hepatitis B vaccine: a randomized trial comparing the immunogenicity of recombinant hepatitis B vaccines in an adult population. Am J Prev Med. 1998;1:73–77. doi: 10.1016/s0749-3797(98)00003-8. [DOI] [PubMed] [Google Scholar]
- 4.Barin F, Perrin J, Chotard J, Denis F, N’Doye R, Diop Mar I, Chiron J P, Coursaget P. Cross sectional and longtitudinal epidemiology of hepatitis B in Senegal. Prog Med Virol. 1981;27:148–162. [PubMed] [Google Scholar]
- 5.Beasley R P. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942–1956. doi: 10.1002/1097-0142(19880515)61:10<1942::aid-cncr2820611003>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 6.Beasley R P, Hwang L-Y. Overview of the epidemiology of hepatocellular carcinoma. Baltimore, Md: The Williams & Wilkins Co.; 1991. [Google Scholar]
- 7.Beasley R P, Hwang L Y. Postnatal infectivity of hepatitis B surface antigen-carrier mothers. J Infect Dis. 1983;147:185–190. doi: 10.1093/infdis/147.2.185. [DOI] [PubMed] [Google Scholar]
- 8.Beasley R P, Hwang L Y, Lee G C, Lan C C, Roan C H, Huang F Y, Chen C L. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet. 1983;ii:1099–1102. doi: 10.1016/s0140-6736(83)90624-4. [DOI] [PubMed] [Google Scholar]
- 9.Beasley R P, Hwang L Y, Stevens C E, Lin C C, Hsieh F J, Wang K Y, Sun T S, Szmuness W. Efficacy of hepatitis B immune globulin for prevention of perinatal transmission of the hepatitis B virus carrier state: final report of a randomized double-blind, placebo-controlled trial. Hepatology. 1983;3:135–141. doi: 10.1002/hep.1840030201. [DOI] [PubMed] [Google Scholar]
- 10.Bensabeth G, Hadler S C, Soares M C, Fields H, Maynard J E. Epidemiologic and serologic studies of acute viral hepatitis in Brazil’s Amazon Basin. Bull Pan Am Health Org. 1987;21:16–27. [PubMed] [Google Scholar]
- 11.Bhatnagar P K, Papas E, Blum H E, Milich D R, Nitecki D, Karels M J, Vyas G N. Immune response to synthetic peptide analogues of hepatitis B surface antigen specific for the a determinant. Proc Natl Acad Sci USA. 1982;79:4400–4404. doi: 10.1073/pnas.79.14.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blumberg B S. Australia antigen and the biology of hepatitis B. Science. 1977;197:17–25. doi: 10.1126/science.325649. [DOI] [PubMed] [Google Scholar]
- 13.Blumberg B S, Alter H J. A “new” antigen in leukemic serum. JAMA. 1967;191:541–546. doi: 10.1001/jama.1965.03080070025007. [DOI] [PubMed] [Google Scholar]
- 14.Blumberg B S, Gerstley B J, Hungerford D A, London W T, Sutnick A I. A serum antigen (Australia antigen) in Down’s syndrome, leukemia, and hepatitis. Ann Intern Med. 1967;66:924–931. doi: 10.7326/0003-4819-66-5-924. [DOI] [PubMed] [Google Scholar]
- 15.Blumberg B S, Mazzur S, Hertzog K, Millman I, Bloom J, Damon A. Australia antigen in the Solomon Islands. Hum Biol. 1974;46:239–262. [PubMed] [Google Scholar]
- 16.Boker K H, Ringe B, Kruger M P, Manns M P. Prostaglandin E plus famciclovir—a new concept for the treatment of severe hepatitis B after liver transplantation. Transplantation. 1995;57:1706–1708. [PubMed] [Google Scholar]
- 17.Carman W F, Zanetti A R, Karayiannis P, Waters J, Manzillo G, Tanzi E, Zuckerman A J, Thomas H C. Vaccine-induced escape mutant of hepatitis B virus. Lancet. 1990;336:325–329. doi: 10.1016/0140-6736(90)91874-a. [DOI] [PubMed] [Google Scholar]
- 17a.Centers for Disease Control and Prevention. Unpublished data.
- 18.Chan C Y, Lee S D, Tsai Y T, Lo K J. Booster response to recombinant yeast-derived hepatitis B vaccine in vaccinees whose anti-HBs responses were elicited by a plasma-derived vaccine. Vaccine. 1991;9(Suppl. A):765–767. doi: 10.1016/0264-410x(91)90294-g. [DOI] [PubMed] [Google Scholar]
- 19.Chang M H, Chen C J, Lai M S, Hsu H M, Wu T C, Kong M S, Liang D C, Shau W Y, Chen D S. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855–1859. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 20.Chau K, Hargie M P, Decker R H, Mushahwar I K, Overby L R. Serodiagnosis of recent hepatitis B virus infection by IgM class anti-HBc. Hepatology. 1983;3:142–149. doi: 10.1002/hep.1840030202. [DOI] [PubMed] [Google Scholar]
- 21.Chen H L, Chang M H, Ni Y, Hsu H Y, Lee P I, Lee C Y, Chen D S. Seroepidemiology of hepatitis B virus infection in children: ten years of mass vaccination in Taiwan. JAMA. 1996;276:906–908. [PubMed] [Google Scholar]
- 22.Chisari F, Ferrari C. Hepatitis B virus immunapathogenesis. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 23.Chisari F V. Cytotoxic T cells and viral hepatitis. J Clin Investig. 1997;99:1472–1477. doi: 10.1172/JCI119308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choo Q L, Kuo G, Weiner A J, Overby L R, Bradley D W, Houghton M. Isolation of cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 25.Courouce A M, Loplanche A, Benhamou E, Jungers P. Long term efficacy of hepatitis B vaccination in healthy adults. New York, N.Y: Alan R. Liss; 1988. [Google Scholar]
- 26.Coursaget P, Leboulleux D, Soumare M, le Cann P, Yvonnet B, Chiron J P, Coll-Seck A M, Diop-Mar I. Twelve-year follow-up study of hepatitis B immunization of Senegalese infants. J Hepatol. 1994;21:250–254. doi: 10.1016/s0168-8278(05)80404-0. [DOI] [PubMed] [Google Scholar]
- 27.Coursaget P, Yvonnet B, Chotard J, Sarr M, Vincelot P, N’doye R, Diop-Mar I, Chiron J P. Seven-year study of hepatitis B vaccine efficacy in infants from an endemic area (Senegal) Lancet. 1986;ii:1143–1145. doi: 10.1016/s0140-6736(86)90543-x. [DOI] [PubMed] [Google Scholar]
- 28.Crosnier J, Jungers P, Courouce A M, Laplanche A, Benhamou E, Degos F, Lacour B, Prunet P, Cerisier Y, Guesry P. Randomised placebo-controlled trial of hepatitis B surface antigen vaccine in French haemodialysis units. I. Medical staff. Lancet. 1981;i:455–459. doi: 10.1016/s0140-6736(81)91847-x. [DOI] [PubMed] [Google Scholar]
- 29.Dane D S, Cameron C H, Briggs M. Virus-like particles in serum of patients with Australia-antigen-associated hepatitis. Lancet. 1970;i:695–698. doi: 10.1016/s0140-6736(70)90926-8. [DOI] [PubMed] [Google Scholar]
- 30.Davidson M, Krugman S. Recombinant yeast hepatitis B vaccine compared with plasma-derived vaccine: immunogenicity and effect of a booster dose. J Infect. 1986;13(Suppl. A):31–38. doi: 10.1016/s0163-4453(86)92653-8. [DOI] [PubMed] [Google Scholar]
- 31.Desmyter J, De Groote J, Desmet V J, Billiau A, Ray M B, Bradburne A F, Edy V G, De Somer P. Administration of human fibroblast interferon in chronic hepatitis B infection. Lancet. 1976;ii:645–647. doi: 10.1016/s0140-6736(76)92460-0. [DOI] [PubMed] [Google Scholar]
- 32.Di Bisceglie A M. Hepatocellular carcinoma. Ann Intern Med. 1988;108:390–401. doi: 10.7326/0003-4819-108-3-390. [DOI] [PubMed] [Google Scholar]
- 33.Dientstag J L, Perillo R P, Schiff E R, Barthomomew M, Vicary C, Rubin M. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med. 1995;333:1657–1661. doi: 10.1056/NEJM199512213332501. [DOI] [PubMed] [Google Scholar]
- 34.Dodd R Y, Holland P V, Ni L Y, Smith H M, Greenwalt T J. Hepatitis B antigen: regional variation in incidence and subtype ratio in the American Red Cross donor population. Am J Epidemiol. 1973;97:111–115. doi: 10.1093/oxfordjournals.aje.a121487. [DOI] [PubMed] [Google Scholar]
- 35.Doong S-L, Tsai C-H, Schinazi E R, Liotta D C, Cheng Y-C. Inhibition of the replication of hepatitis B virus in vitro by 2′,3′-dideoxy-3′-thiacytidine and related analogues. Proc Natl Acad Sci USA. 1991;88:8495–8499. doi: 10.1073/pnas.88.19.8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eppinger H. Die pathogenesis des icterus. Verh Dtsch Ges Inn Med. 1922;34:15. [Google Scholar]
- 37.Flaum A, Malmros H, Persson E. Eine nosocomiale icterus-epidemic. Acta Med Scand. 1926;16:544. [Google Scholar]
- 38.Fortuin M, Chotard J, Jack A D, Maine N P, Mendy M, Hall A J, Inskip H M, George M O, Whittle H C. Efficacy of hepatitis B vaccine in the Gambian expanded programme on immunisation. Lancet. 1993;341:1129–1131. doi: 10.1016/0140-6736(93)93137-p. [DOI] [PubMed] [Google Scholar]
- 39.Francis D P, Halder S C, Thompson S E, Maynard J E, Ostrow D G, Altman N, Braff E H, O’Malley P, Hawkins D, Judson F N, Penley K, Nylund T, Christie G, Meyers F, Moore J N, Gardner A, Doto I L, Miller J H, Reynolds G H, Murphy B L, Schable C A, Clark B T, Curran J W, Redeker A G. Prevention of hepatitis B with vaccine. Report from the Centers for Disease Control multi-center trial among homosexual men. Ann Intern Med. 1982;97:362–366. doi: 10.7326/0003-4819-97-3-362. [DOI] [PubMed] [Google Scholar]
- 40.Franks A L, Berg C J, Kane M A, Browne B B, Sikes R K, Elsea W R, Burton A H. Hepatitis B infection among children born in the U.S. to southeast Asian refugees. N Engl J Med. 1989;321:1301–1305. doi: 10.1056/NEJM198911093211905. [DOI] [PubMed] [Google Scholar]
- 41.Freed G L, Bordley W C, Clark S J, Konrad T R. Reactions of pediatricians to a new CDC recommendation for universal immunization of infants with hepatitis B vaccine. Pediatrics. 1993;91:699–702. [PubMed] [Google Scholar]
- 42.Freed G L, Konrad T R, Lohr J A. Adoption of new haemophilus influenza type b vaccine recommendations. Am J Dis Child. 1993;147:124–128. doi: 10.1001/archpedi.1993.02160260014004. [DOI] [PubMed] [Google Scholar]
- 43.Ganiats T R. Are we jumping on the bandwagon too early. J Fam Pract. 1993;36:147–149. [PubMed] [Google Scholar]
- 44.Gerlich W H, Bruss V. Functions of hepatitis B virus proteins and molecular targets for protective immunity. New York, N.Y: Marcel Dekker, Inc.; 1993. [Google Scholar]
- 45.Gerlich W H, Lu X, Heerman K H. Studies on the attachment and penetration of hepatitis B virus. J Hepatol. 1993;17:S10–S14. doi: 10.1016/s0168-8278(05)80417-9. [DOI] [PubMed] [Google Scholar]
- 46.Gibas A, Watkins E, Hinkle C, Dienstag J L. Long term persistence of protective antibody after hepatitis B vaccination of healthy adults. New York, N.Y: Alan R. Liss; 1988. [Google Scholar]
- 47.Giles J P. Viral hepatitis: relationship of Australia-SH antigen to the Willowbrook MS-2 strain. N Engl J Med. 1969;281:119–122. doi: 10.1056/NEJM196907172810302. [DOI] [PubMed] [Google Scholar]
- 48.Goh K T, Oon C J, Heng B H, Lim B K. Long term immunogenicity and efficacy of a reduced dose of plasma-derived hepatitis B vaccine in young adults. Bull W H O. 1995;73:523–527. [PMC free article] [PubMed] [Google Scholar]
- 49.Grady G F, Lee V A, Prince A M, Gitnick G L, Fawaz K A, Vyas G N, Levitt M D, Senior J R, Galambos J T, Bynum T E, Singleton J W, Clowdus B F, Akdamar K, Aach R D, Winkelman E I, Schiff G M, Hersh T. Hepatitis B immune globulin for accidental exposures among medical personnel: final report of a multicenter controlled trial. J Infect Dis. 1978;138:625–638. doi: 10.1093/infdis/138.5.625. [DOI] [PubMed] [Google Scholar]
- 50.Greenberg D P, Vadheim C M, Wong V K, Marcy S M, Partridge S, Greene T, Chiu C Y, Margolis H S, Ward J I. Comparative safety and immunogenicity of two recombinant hepatitis B vaccines given to infants at two, four and six months of age. Pediatr Infect Dis J. 1996;15:590–596. doi: 10.1097/00006454-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Greenberg H B, Pollard R B, Lutwick L I, Gregory P B, Robinson W S, Merigan T S. Effect of human leukocyte interferon on hepatitis B virus infection in patients with chronic active hepatitis. N Engl J Med. 1976;295:517–522. doi: 10.1056/NEJM197609022951001. [DOI] [PubMed] [Google Scholar]
- 52.Hadler S C, Francis D P, Maynard J E, Thompson S E, Judson F N, Echenberg D F, Ostrow D G, O’Malley P M, Penley K A, Altman N L. Long-term immunogenicity and efficacy of hepatitis B vaccine in homosexual men. N Engl J Med. 1986;315:209–214. doi: 10.1056/NEJM198607243150401. [DOI] [PubMed] [Google Scholar]
- 53.Hadler S C, Judson F N, O’Malley P M. Studies of hepatitis B vaccine in homosexual men. London, United Kingdom: John Libby, Eurotext; 1990. [Google Scholar]
- 54.Hadler S C, Margolis H S. Epidemiology of hepatitis B virus infection. New York, N.Y: Marcel Dekker, Inc.; 1993. [Google Scholar]
- 55.Hadler S C, Margolis H S. Hepatitis B immunization: vaccine types, efficacy, and indications for immunization. Vol. 12. Boston, Mass: Blackwell Scientific Publications; 1992. [PubMed] [Google Scholar]
- 56.Hadziyannis S J, Lieberman H M, Karvountzis G G, Shafritz D A. Analysis of liver disease, nuclear HBcAg, viral replication, and hepatitis B virus DNA in liver and serum of HBeAg vs anti-HBe positive carriers of the virus. Hepatology. 1983;3:656–662. doi: 10.1002/hep.1840030505. [DOI] [PubMed] [Google Scholar]
- 57.Harpaz, R. Personal communication.
- 58.Harpaz R, Von Seidlein L, Averhoff F M, Tormey M P, Sinha S D, Kotsopoulou K, Lambert S B, Robertson B H, Cherry J D, Shapiro C N. Transmission of hepatitis B virus to multiple patients from a surgeon without evidence of inadequate infection control. N Engl J Med. 1996;334:549–554. doi: 10.1056/NEJM199602293340901. [DOI] [PubMed] [Google Scholar]
- 59.Harrison T J, Hopes E A, Oon C J, Zanetti A R, Zuckerman A J. Independent emergence of a vaccine-induced escape mutant of hepatitis B virus. J Hepatol. 1991;13(Suppl.):S105–S107. doi: 10.1016/0168-8278(91)90037-c. [DOI] [PubMed] [Google Scholar]
- 60.Havens W. Period of infectivity of patients with experimentally induced infectious hepatitis. J Exp Med. 1946;83:251–258. [PubMed] [Google Scholar]
- 61.Hoofnagle J H, Di Bisceglie A M. Serologic diagnosis of acute and chronic viral hepatitis. Semin Liver Dis. 1991;11:73–83. doi: 10.1055/s-2008-1040426. [DOI] [PubMed] [Google Scholar]
- 62.Hoofnagle J H, Di Bisceglie A M. The treatment of chronic viral hepatitis. N Engl J Med. 1997;336:347–356. doi: 10.1056/NEJM199701303360507. [DOI] [PubMed] [Google Scholar]
- 63.Hoofnagle J H, Di Biscelgie A M. Serologic diagnosis of acute and chronic viral hepatitis. Semin Liver Dis. 1991;11:73–83. doi: 10.1055/s-2008-1040426. [DOI] [PubMed] [Google Scholar]
- 64.Hoofnagle J H, Seef L B. Natural history of chronic type B hepatitis. Prog Liver Dis. 1982;7:469–479. [PubMed] [Google Scholar]
- 65.Horowitz M M, Ershler W B, McKinney W P, Battiola R J. Duration of immunity after hepatitis B vaccination: efficacy of low-dose booster vaccine. Ann Intern Med. 1988;108:185–189. doi: 10.7326/0003-4819-108-2-185. [DOI] [PubMed] [Google Scholar]
- 66.Howard C R. The structure of hepatitis B envelope and molecular variants of hepatitis B virus. J Viral Hepatitis. 1995;2:165–170. doi: 10.1111/j.1365-2893.1995.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 67.Hsieh C C, Tzonou A, Zavitsanos X, Kalamani E, Lan S J, Trichopoulos D. Age at first establishment of chronic hepatitis B virus infection and hepatocellular carcinoma risk: a birth order study. Am J Epidemiol. 1992;136:1115–1121. doi: 10.1093/oxfordjournals.aje.a116577. [DOI] [PubMed] [Google Scholar]
- 68.Hu M D, Schenzle D, Dienhart F, Scheid R. Epidemiology of hepatitis A and B in Shanghai area: prevalence of serum markers. Am J Epidemiol. 1984;120:404–413. doi: 10.1093/oxfordjournals.aje.a113905. [DOI] [PubMed] [Google Scholar]
- 69.Hurie M B, Mast E E, Davis J P. Horizontal transmission of hepatitis B virus infection to U.S. born children of Hmong refugees. Pediatrics. 1992;89:269–273. [PubMed] [Google Scholar]
- 70.Hwang L Y, Lee C Y, Beasley R P. Five year follow-up of HBV vaccination with plasma-derived vaccine in neonates: evaluation of immunogenicity and efficacy against perinatal transmission. In: Hollinger F, Lemon S, Margolis H, editors. Viral hepatitis and liver disease. Baltimore, Md: The Williams & Wilkins Co.; 1991. [Google Scholar]
- 71.Hyams K C. Risk of chronicity following acute hepatitis B virus infection. Clin Infect Dis. 1995;20:992–1000. doi: 10.1093/clinids/20.4.992. [DOI] [PubMed] [Google Scholar]
- 72.Hyams K C, al-Arabi M A, al-Tagani A A, Messiter J F, al-Guali A A, George J F. Epidemiology of hepatitis B in the Gezira region of Sudan. Am J Trop Med Hyg. 1989;40:200–206. doi: 10.4269/ajtmh.1989.40.200. [DOI] [PubMed] [Google Scholar]
- 73.Hyams K C, Osman N M, Khaled E M, Koraa A A, Imam I Z, el Ghorab N M, Dunn M A, Woody J N. Maternal infant transmission of hepatitis B in Egypt. J Med Virol. 1988;24:191–197. doi: 10.1002/jmv.1890240208. [DOI] [PubMed] [Google Scholar]
- 74.Ip N M H, Lelie P N, Wong V C W, Kuhns M C, Reesink H W. Prevention of hepatitis B virus carrier state in infants according to maternal serum levels of HBV DNA. Lancet. 1989;152:817–822. doi: 10.1016/s0140-6736(89)90003-2. [DOI] [PubMed] [Google Scholar]
- 75.Jilg W, Schmidt M, Deinhardt F. Persistence of specific antibodies after hepatitis B vaccination. J Hepatol. 1988;6:201–207. doi: 10.1016/s0168-8278(88)80032-1. [DOI] [PubMed] [Google Scholar]
- 76.Kaneko S, Miller R H, Di Bisceglie A M. Detection of hepatitis B virus DNA in serum by polymerase chain reaction. Gastroenterology. 1990;99:799–804. doi: 10.1016/0016-5085(90)90971-3. [DOI] [PubMed] [Google Scholar]
- 77.Kann M, Gerlich W H. Replication of hepatitis B virus. In: Harrison T J, Zuckerman A J, editors. The molecular medicine of viral hepatitis. New York, N.Y: John Wiley & Sons, Inc.; 1997. pp. 63–116. [Google Scholar]
- 78.Komani K, Peeples M E. Physiology and function of the Vero cell receptor for the hepatitis B small S protein. Virology. 1990;177:332–338. doi: 10.1016/0042-6822(90)90488-d. [DOI] [PubMed] [Google Scholar]
- 79.Korenman J, Baker B, Wagonner J, Everhart J E, Di Bisceglie A M, Hoofnagle J H. Long term remission of chronic hepatitis B after alpha-interferon therapy. Ann Intern Med. 1991;114:629–634. doi: 10.7326/0003-4819-114-8-629. [DOI] [PubMed] [Google Scholar]
- 80.Krugman S, Giles J P. Viral hepatitis, type B (MS-2 strain): further observations on natural history and prevention. N Engl J Med. 1973;288:755–760. doi: 10.1056/NEJM197304122881503. [DOI] [PubMed] [Google Scholar]
- 81.Krugman S, Giles J P, Hammond J. Infectious hepatitis. Evidence for two distinctive clinical, epidemiological, and immunological types of infection. JAMA. 1967;200:365–373. doi: 10.1001/jama.200.5.365. [DOI] [PubMed] [Google Scholar]
- 82.Krugman S, Giles J P, Hammond J. Viral hepatitis type B: heat on the infectivity and antigenicity of the MS-2 strains. J Infect Dis. 1970;122:432–436. doi: 10.1093/infdis/122.5.432. [DOI] [PubMed] [Google Scholar]
- 83.Krugman S, Giles J P, Hammond J. Viral hepatitis type B: prevention with specific hepatitis B immune serum globulin. JAMA. 1971;218:1665–1670. [PubMed] [Google Scholar]
- 84.Krugman S, Giles J P, Hammond J. Viral hepatits type B MS-2 strain): studies on active immunization. JAMA. 1971;217:41–45. [PubMed] [Google Scholar]
- 85.Lau J Y N, Wright T L. Molecular virology and pathogenesis of hepatitis B. Lancet. 1993;342:1335–1340. doi: 10.1016/0140-6736(93)92249-s. [DOI] [PubMed] [Google Scholar]
- 86.Lee C-L, Ko Y-C. Hepatitis B vaccination and hepatocellular carcinoma in Taiwan. Pediatrics. 1997;99:351–353. doi: 10.1542/peds.99.3.351. [DOI] [PubMed] [Google Scholar]
- 87.Lee C Y, Huang L M, Chang M H, Hsu C Y, Wu S J, Sung J L, Safary A. The protective efficacy of recombinant hepatitis B vaccine in newborn infants of hepatitis B e antigen-positive-hepatitis B surface antigen carrier mothers. Pediatr Infect Dis J. 1991;10:299–303. doi: 10.1097/00006454-199104000-00007. [DOI] [PubMed] [Google Scholar]
- 88.Lee P I, Lee C Y, Huang L M, Chang M H. Long term efficacy of recombinant hepatitis B vaccine and risk of natural infection in infants born to mothers with hepatitis B e antigen. J Pediatr. 1995;126:716–721. doi: 10.1016/s0022-3476(95)70398-5. [DOI] [PubMed] [Google Scholar]
- 89.Lee S D, Lo K J, Wu J C, Tsai Y T, Wang J Y, Ting L P, Tong M J. Prevention of maternal-infant hepatitis B transmission by immunization: the role of serum hepatitis B virus DNA. Hepatology. 1986;6:369–373. doi: 10.1002/hep.1840060306. [DOI] [PubMed] [Google Scholar]
- 90.Leenders W H, Glansbeek H L, Bruin W C, Yap S H. Binding of the major and large HBsAg to human hepatocytes and liver plasma membranes: putative external and internal receptors for infection and secretion of hepatitis B virus. Hepatology. 1990;12:141–147. doi: 10.1002/hep.1840120122. [DOI] [PubMed] [Google Scholar]
- 91.Liaw Y-F, Tai D-I, Chu C-M, Chen T-J. The development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Hepatology. 1988;8:493–496. doi: 10.1002/hep.1840080310. [DOI] [PubMed] [Google Scholar]
- 92.Lieming D, Mintai Z, Yinfu W, Shaochin Z, Weiqin K, Smego A. A 9-year follow-up study of the immunogenicity and long-term efficacy of plasma-derived hepatitis B vaccine in high-risk Chinese neonates. Clin Infect Dis. 1993;17:475–479. [PubMed] [Google Scholar]
- 93.Lingao A L, Domingo E O, West S, Reyes C M, Gasmen S, Viterbo G, Tiu E, Langsang M A. Seroepidemiology of hepatitis B virus in the Phillipines. Am J Epidemiol. 1986;123:473–480. doi: 10.1093/oxfordjournals.aje.a114262. [DOI] [PubMed] [Google Scholar]
- 94.Lo K J, Lee S D, Tsai Y T, Wu T C, Chan C Y, Chen G H, Yeh C L. Long-term immunogenicity and efficacy of hepatitis B vaccine in infants born to HBeAg positive HBsAg-carrier mothers. Hepatology. 1988;8:1647–1650. doi: 10.1002/hep.1840080629. [DOI] [PubMed] [Google Scholar]
- 95.Lo K J, Tsai Y T, Lee S D, Wu T C, Wang J Y, Chen G H, Yeh C L, Chiang B N, Yeh S H, Goudeau A. Immunoprophylaxis of infection with hepatitis B virus in infants born to hepatitis B surface antigen-positive carrier mothers. J Infect Dis. 1985;152:817–822. doi: 10.1093/infdis/152.4.817. [DOI] [PubMed] [Google Scholar]
- 96.Lok A S F, Chung H-T, Liu V W S, Ma O C K. Long-term follow-up of chronic hepatitis B patients treated with interferon alpha. Gastroenterology. 1993;105:1833–1838. doi: 10.1016/0016-5085(93)91082-s. [DOI] [PubMed] [Google Scholar]
- 97.Lok A S F, Lai C, Wu P C, Wong V C, Yeoh E K, Lin H J. Hepatitis B virus infection in Chinese families in Hong Kong. Am J Epidemiol. 1987;126:191–197. doi: 10.1093/oxfordjournals.aje.a114681. [DOI] [PubMed] [Google Scholar]
- 98.Lok A S F, Lai C-L. A longitudinal follow-up of asymptomatic hepatitis B surface antigen-positive children. Hepatology. 1988;8:1130–1133. doi: 10.1002/hep.1840080527. [DOI] [PubMed] [Google Scholar]
- 99.Lurman A. Eine icterus Epidemic. Berlin Klin Wochenschr. 1855;22:20–23. [Google Scholar]
- 100.MacCallum F O, Bauer D J. Homologous serum hepatitis. Lancet. 1947;ii:691–692. [Google Scholar]
- 101.MacCallum F O, Bauer D J. Homologous serum jaundice: transmission experiments with human volunteers. Lancet. 1944;i:622–627. [Google Scholar]
- 102.Magnius L O, Espmark J A. New specificities in Australia antigen-positive sera distinct from the Le Bouvier determinants. J Immunol. 1972;109:1017–1021. [PubMed] [Google Scholar]
- 103.Mahoney F, Smith N, Allter M J, Margolis H. Progress towards the elimination of hepatitis B virus transmission in the United States. Viral Hepatitis Rev. 1997;3:105–119. [Google Scholar]
- 104.Mahoney F, Woodruff B, Erben J, Coleman P, Reid E, Schatz G, Kane M. Evaluation of a hepatitis B immunization program on the prevalence of hepatitis B in virus infection. J Infect Dis. 1993;167:203–207. doi: 10.1093/infdis/167.1.203. [DOI] [PubMed] [Google Scholar]
- 105.Mahoney F J, Lawrence M, McFarland L, Scott C, Le Q, Farley T. Continuing transmission of hepatitis B virus infection among U.S.-born Southeast Asian children. Pediatrics. 1995;96:1113–1116. [PubMed] [Google Scholar]
- 106.Mahoney F J, Stewart K, Hu H, Coleman P, Alter M J. Progress toward the elimination of hepatitis B virus transmission among health care workers in the United States. Arch Intern Med. 1997;157:2601–2605. [PubMed] [Google Scholar]
- 107.Mahoney F J, Woodruff B, Auerbach A, Polloi A, McCready J, Durand M, Aniol K, Reed E, Williams I, Pretrick E. Progress on the elimination of hepatitis B virus transmission in Micronesia and American Samoa. Pac Health Dialog. 1996;1996:140–46. [Google Scholar]
- 108.Margolis H S, Alter M J, Hadler S C. Hepatitis B: evolving epidemiology and implications for control. Semin Liver Dis. 1991;11:84–92. doi: 10.1055/s-2008-1040427. [DOI] [PubMed] [Google Scholar]
- 109.Margolis H S, Coleman P J, Brown R E, Mast E E, Sheingold S H, Arevalo J A. Prevention of hepatitis B virus transmission by immunization. An economic analysis of current recommendations. JAMA. 1995;274:1201–1208. [PubMed] [Google Scholar]
- 110.Marinier E, Barrois V, Larouze B, London W T, Cofer A, Diakhate L, Blumberg B S. Lack of perinatal transmission of hepatitis B virus infection in Senegal, West Africa. J Pediatr. 1985;106:843–849. doi: 10.1016/s0022-3476(85)80371-1. [DOI] [PubMed] [Google Scholar]
- 111.Maynard J E, Kane M A, Alter M J, Hadler S C. Control of hepatitis B by immunization: global perspectives. New York, N.Y: Grune and Stratton Inc.; 1988. [Google Scholar]
- 112.McLean A A, Hilleman M R, McAleer W J, Buynak E B. Summary of worldwide clinical experience with H-B-Vax (B, MSD) J Infect. 1983;1(Suppl.):95–104. doi: 10.1016/s0163-4453(83)96879-2. [DOI] [PubMed] [Google Scholar]
- 113.McMahon B, Rhoades E R, Heyward W L, Tower E, Ritter D, Lanier A P, Wainwright R B, Helminiak C. A comprehensive programme to reduce the incidence of hepatitis B virus infection and its sequelae in Alaskan natives. Lancet. 1987;ii:1134–1136. doi: 10.1016/s0140-6736(87)91557-1. [DOI] [PubMed] [Google Scholar]
- 114.McMahon B J, Alberts S R, Wainwright R B, Bulkow L, Lanier A P. Hepatitis B-related sequelae: prospective study in 1400 hepatitis B surface antigen-positive Alaska native carriers. Arch Intern Med. 1990;150:1051–1054. doi: 10.1001/archinte.150.5.1051. [DOI] [PubMed] [Google Scholar]
- 115.McMahon B J, Alward W L M, Hall D B, Heyward W L, Bender T R, Francis D P, Maynard J E. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis. 1985;151:599–603. doi: 10.1093/infdis/151.4.599. [DOI] [PubMed] [Google Scholar]
- 116.McMahon G, Ehrlich P H, Moustafa Z A, McCarthy L A, Dottavio D, Tolpin M D, Nadler P I, Ostberg L. Genetic alterations in the gene encoding the major HBsAg: DNA and immunological analysis of recurrent HBsAg derived from monoclonal antibody-treated liver transplant patients. Hepatology. 1992;15:757–766. doi: 10.1002/hep.1840150503. [DOI] [PubMed] [Google Scholar]
- 117.McQuillan G M, Townsend T R, Fields H A, Carrol M, Leahy M, Polk B F. Seroepidemiology of hepatitis B virus in the United States, 1976 to 1980. Am J Med. 1989;87(Suppl.):5–10. doi: 10.1016/0002-9343(89)90523-8. [DOI] [PubMed] [Google Scholar]
- 118.Milne A, Allwood G K, Moyes C D, Pearce N E, Lucas C R. Prevalence of hepatitis B infections in a multiracial New Zealand community. N Z Med J. 1985;10:529–532. [PubMed] [Google Scholar]
- 119.Milne A, Krugman S, Waldon J A, Hadler S C, Lucas C R, Moyes C D, Pearce N E. Hepatitis B vaccination in children: five year booster study. N Z Med J. 1992;105:336–338. [PubMed] [Google Scholar]
- 120.Milne A, Waldon J. Recombinant DNA hepatitis B vaccination in teenagers: effect of a booster does at 5 and a half years. J Infect Dis. 1992;166:942. doi: 10.1093/infdis/166.4.942. [DOI] [PubMed] [Google Scholar]
- 121.Moulia-Pelat J P, Spiegel A, Martin P M, Cardines R, Boutin J P, Roux J F, Excler J L, Saliou P. A 5-year immunization field trial against hepatitis B using a Chinese hamster ovary cell recombinant vaccine in French Polynesian newborns: results at 3 years. Vaccine. 1994;12:499–502. doi: 10.1016/0264-410x(94)90306-9. [DOI] [PubMed] [Google Scholar]
- 122.Nainan O V, Stevens C E, Margolis H S. Ninth Triennial International Symposium on Viral Hepatitis and Liver Disease. 1996. Hepatitis B virus (HBV) antibody resistant mutants: frequency and significance; p. 29. [Google Scholar]
- 123.Neefe J R, Gellis S S, Stokes J. Homologous serum hepatitis and infectious (epidemic) hepatitis: studies in volunteers bearing on immunological and other characteristics of etiologic agents. Am J Med. 1946;1:3–22. doi: 10.1016/0002-9343(46)90017-4. [DOI] [PubMed] [Google Scholar]
- 124.Neurath A R, Kent S B, Strick N, Parker K. Identification and chemical synthesis of a host cell receptor binding cite on hepatitis B virus. Cell. 1986;46:429–436. doi: 10.1016/0092-8674(86)90663-x. [DOI] [PubMed] [Google Scholar]
- 125.Ogata N, Miller R H, Ishak K G, Zanetti A R, Purcell R H. Genetic and biologic characterization of two hepatitis B virus variants: a precore mutant implicated in fulminant hepatitis and a surface mutant resistant to immunoprophylaxis. Tokyo, Japan: Springer Verlag; 1993. [Google Scholar]
- 126.Oon C J. Ninth Triennial International Symposium on Viral Hepatitis and Liver Disease. 1996. Studies on the transmissability of HBV vaccine escape 145 Gly to Arg mutants in immune and hepatitis B carriers; p. 265. [Google Scholar]
- 127.Polish L B, Shapiro C N, Bauer F, Klotz P, Ginier P, Roberto R R, Margolis H S, Alter M J. Nosocomial transmission of hepatitis B virus associated with the use of a spring-loaded finger-stick device. N Engl J Med. 1992;326:721–725. doi: 10.1056/NEJM199203123261101. [DOI] [PubMed] [Google Scholar]
- 128.Pongpipat D, Suvatte V, Assateerawats A. Hepatitis B immunization in high risk neonates born from HBsAg positive mothers: comparison between plasma derived and recombinant DNA vaccine. Asian Pac J Allergy Immunol. 1989;7:37–40. [PubMed] [Google Scholar]
- 129.Pontisso P, Petit M A, Bankowski M J, Peeples K. Human liver plasma membranes contain receptors for the hepatitis B virus pre-s1 region and, via polymerized human serum albumin, for the pre-s2 region. J Virol. 1989;63:1981–1988. doi: 10.1128/jvi.63.5.1981-1988.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Poovorawan Y, Sanpavat S, Chumdermpadetsuk S, Safary A. Long-term hepatitis B vaccine in infants born to hepatitis B e antigen positive mothers. Arch Dis Child Fetal Neonatal Ed. 1997;77:F47–F51. doi: 10.1136/fn.77.1.f47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Poovorawan Y, Sanpavat S, Pongpunglert W, Chumdermpadetsuk S, Sentrakul P, Vandepapeliere P, Safary A. Long term efficacy of hepatitis B vaccine in infants born to hepatitis B e antigen-positive mothers. Pediatr Infect Dis J. 1992;11:816–821. doi: 10.1097/00006454-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 132.Prince A M. An antigen detected in the blood of patients during the incubation of serum hepatitis. Proc Natl Acad Sci USA. 1968;60:814–821. doi: 10.1073/pnas.60.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Prince A M, Ikram H, Hopp T P. Hepatitis B virus vaccine: identification of HBsAg/a and HBsAg/d but not HBsAg/y subtype antigenic determinants on a synthetic immunogenic peptide. Proc Natl Acad Sci USA. 1982;79:579–582. doi: 10.1073/pnas.79.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Redeker A G, Mosley J W, Gocke D J, McKee A P, Pollack W. Hepatitis B immune globulin as a prophylactic measure for spouses exposed to acute type B hepatitis. N Engl J Med. 1975;293:1055–1059. doi: 10.1056/NEJM197511202932101. [DOI] [PubMed] [Google Scholar]
- 135.Reyes G R, Purdy M A, Kim J P, Luk K C, Young L M, Fry K E, Bradley D W. Isolation of a cDNA clone from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science. 1990;248:1335–1339. doi: 10.1126/science.2107574. [DOI] [PubMed] [Google Scholar]
- 136.Rich A R. The pathogenesis of the forms of jaundice. Bull Johns Hopkins Hosp. 1930;47:338–377. [Google Scholar]
- 136a.Rizzetto M, Canese M G, Arico J, Crivelli O, Bonino F, Trepo C G, Verme G. Immunoflourescent detection of a new antigen-antibody system (delta-antidelta) associated to the hepatitis B virus in the liver and serum of HBsAg carriers. Gut. 1977;18:997–1003. doi: 10.1136/gut.18.12.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roome A J, Walsh S J, Cartter M L, Hadler J L. Hepatitis B vaccine responsiveness in Connecticut public safety personnel. JAMA. 1993;270:2931–2934. [PubMed] [Google Scholar]
- 138.Ruff T A, Gertig D M, Otto B F, Gust I D, Sutanto A, Soewarso T I, Kandun N, Marschner I C, Maynard J E. Lombok Hepatitis B Model Immunization Project: toward universal infant hepatitis B immunization in Indonesia. J Infect Dis. 1995;171:290–296. doi: 10.1093/infdis/171.2.290. [DOI] [PubMed] [Google Scholar]
- 139.Seeff L B, Beebe G W, Hoofnagle J H, Norman J E, Buskell-Bales Z, Waggoner J G, Kaplowitz N, Koff R S, Petrini J L, Jr, Schiff E R, et al. A serologic follow-up of the 1942 epidemic of post-vaccination hepatitis in the United States Army. N Engl J Med. 1987;316:965–970. doi: 10.1056/NEJM198704163161601. [DOI] [PubMed] [Google Scholar]
- 140.Stevens C E, Neurath R A, Beasley R P, Szmuness W. HBeAg and anti-HBe detection with radioimmunoassay: correlation with vertical transmission of hepatitis B virus in Taiwan. J Med Virol. 1979;3:237–241. doi: 10.1002/jmv.1890030310. [DOI] [PubMed] [Google Scholar]
- 141.Stevens C E, Taylor P E, Tong M J. Viral hepatitis and liver disease. New York, N.Y: Alan R. Riss; 1988. [Google Scholar]
- 142.Stevens C E, Taylor P E, Tong M J, Toy P T, Vyas G N, Nair P V, Weissman J Y, Krugman S. Yeast-recombinant hepatitis B vaccine. Efficacy with hepatitis B immune globulin in prevention of perinatal hepatitis B virus transmission. JAMA. 1987;257:2612–2616. doi: 10.1001/jama.257.19.2612. [DOI] [PubMed] [Google Scholar]
- 143.Stevens C E, Toy P T, Taylor P E, Lee T, Yip H Y. Prospects for control of hepatitis B virus infection: implications of childhood vaccination and long term protection. Pediatrics. 1992;90(Suppl.):170–173. [PubMed] [Google Scholar]
- 144.Summers J, Smith P M, Horwich A L. Hepadnavirus envelope proteins regulate covalently closed circular DNA amplification. J Virol. 1990;64:2819–2824. doi: 10.1128/jvi.64.6.2819-2824.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sung J L. Hepatitis B eradication strategy for Asia. Vaccine. 1990;8(Suppl.):96–99. doi: 10.1016/0264-410x(90)90227-d. [DOI] [PubMed] [Google Scholar]
- 146.Szmuness W, Stevens C E, Harley E J, Zang E A, Olesko W R, Williams D C, Sadovsky R, Morrison J M, Kellner A. Hepatitis B vaccine: demonstration of efficacy in a controlled trial in a high risk population in the U.S. N Engl J Med. 1980;303:833–841. doi: 10.1056/NEJM198010093031501. [DOI] [PubMed] [Google Scholar]
- 147.Taylor P E, Stevens C E. Persistence of antibody to hepatitis B surface antigen after vaccination in healthy adults. New York, N.Y: Alan R. Liss; 1988. [Google Scholar]
- 148.Tin K. Symposium on Control of Hepatitis B in Infants and Children in High Risk Areas of the World, Whakatane, New Zealand. 1987. [Google Scholar]
- 149.Toukan A U, Sharaiha Z K, Abu-el-Rub O A, Hmoud M K, Dahbour S S, Abu-Hassan H, Yacoub S M, Hadler S C, Margolis H S, Coleman P J, Maynard J E. The epidemiology of hepatitis B virus among family members in the Middle East. Am J Epidemiol. 1990;132:220–232. doi: 10.1093/oxfordjournals.aje.a115651. [DOI] [PubMed] [Google Scholar]
- 150.Tsega E, Mengesha B, Hansson B-G, Lindberg J, Nordenfelt E. Hepatitis A, B, and delta infection in Ethiopia: a serologic survey with demographic data. Am J Epidemiol. 1986;123:344–351. doi: 10.1093/oxfordjournals.aje.a114243. [DOI] [PubMed] [Google Scholar]
- 151.Ulrich P P, Bhat R A, Seto B, Mack D, Sninsky J, Vyas G N. Ezymatic amplification of hepatitis B virus DNA in serum compared with infectivity testing in chimpanzees. J Infect Dis. 1989;160:37–43. doi: 10.1093/infdis/160.1.37. [DOI] [PubMed] [Google Scholar]
- 152.Virchow R. Uber das Vorkommen und den Nachweiss des hepatogenen, insebesondere des Katarrhalischen Icterus. Virchows Ser A. 1865;32:117–125. [Google Scholar]
- 153.Vyas G N, Ulrich P O. Molecular characterization of genetic variants of hepatitis B virus. Baltimore, Md: The Williams & Wilkins Co.; 1991. [Google Scholar]
- 154.Wainwright R, Buklow L R, Parkinson A J, Zanis C, McMahon B J. Protection provided by hepatitis B vaccine in a Yupik Eskimo population—results of a 10-year study. J Infect Dis. 1997;175:674–677. doi: 10.1093/infdis/175.3.674. [DOI] [PubMed] [Google Scholar]
- 155.West D J. Clinical experience with hepatitis B vaccines. Am J Infect Control. 1989;17:172–180. doi: 10.1016/0196-6553(89)90213-7. [DOI] [PubMed] [Google Scholar]
- 156.West D J, Calandra G B. Vaccine induced memory for hepatitis B surface antigen: implications for policy on booster caccination. Vaccine. 1996;14:1019–1027. doi: 10.1016/0264-410x(96)00062-x. [DOI] [PubMed] [Google Scholar]
- 157.West D J, Calandra G B, Hesley T M, Idoli V, Miller W J. Control of hepatitis B through routine immunization of infants: the need for flexible schedules, and new combination vaccine formulations. Vaccine. 1993;11:S21–S27. doi: 10.1016/0264-410x(93)90154-p. [DOI] [PubMed] [Google Scholar]
- 158.Wong D C, Purcell R H, Rosen L. Prevalence of antibody to hepatitis A and B in selected Pacific populations of the South Pacific. Am J Epidemiol. 1979;110:227–236. doi: 10.1093/oxfordjournals.aje.a112807. [DOI] [PubMed] [Google Scholar]
- 159.Wong D K H, Cheung A M, O’Rourke K, Naylor C D, Dersky A S, Heathcote J. Effect of alpha-interferon treatment in patients with B e antigen-positive chronic hepatitis B: a metanalysis. Ann Intern Med. 1993;119:312–323. doi: 10.7326/0003-4819-119-4-199308150-00011. [DOI] [PubMed] [Google Scholar]
- 160.Wong V C, Ip H M, Reesink H W, Lelie P N, Reerink-Brongers E E, Yeung C Y, Ma H K. Prevention of the HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and HBeAg by administration of hepatitis-B vaccine and hepatitis-B immunoglobulin. Double-blind randomised placebo-controlled study. Lancet. 1984;i:921–926. doi: 10.1016/s0140-6736(84)92388-2. [DOI] [PubMed] [Google Scholar]
- 161.Wood R C, MacDonald K L, White K E, Hedberg C W, Hanson M, Osterholm M T. Risk factors for lack of detectable antibody following hepatitis B vaccination of Minnesota health care workers. JAMA. 1993;270:2935–2972. [PubMed] [Google Scholar]
- 162.Xia G, Liu C, Yan T, et al. Prevalence of hepatitis B virus markers in children vaccinated by hepatitis b vaccine in five hepatitis B vaccine experimental areas of China. Chin J Exp Clin Virol. 1995;9:17–23. [Google Scholar]
- 163.Xu Z-Y, Liu C-B, Francis D P, Purcell R H, Gun Z L, Duan S C, Chen R J, Margolis H S, Huang C H, Maynard J E. Prevention of perinatal acquisition of hepatitis B virus carriage using vaccine: preliminary report of a randomized double-blind placebo-controlled and comparative trial. Pediatrics. 1985;76:713–718. [PubMed] [Google Scholar]
- 164.Zanetti A R, Tanzi E, Manzillo G, Maio G, Sbreglia C, Caporaso N, Thomas H, Zuckerman A J. Hepatitis B variant in Europe. Lancet. 1988;ii:1132–1133. doi: 10.1016/s0140-6736(88)90541-7. [DOI] [PubMed] [Google Scholar]
- 165.Zhi-yi X U, Duan S-D, Margolis H. Long term efficacy of postexposure immunization of infants for prevention of hepatitis B virus infection. J Infect Dis. 1995;171:54–60. doi: 10.1093/infdis/171.1.54. [DOI] [PubMed] [Google Scholar]
- 166.Zuckerman A J, Harrison T J, Oon C J. Mutation in S region of hepatitis B virus. Lancet. 1994;343:737–738. doi: 10.1016/s0140-6736(94)91622-5. [DOI] [PubMed] [Google Scholar]