Abstract
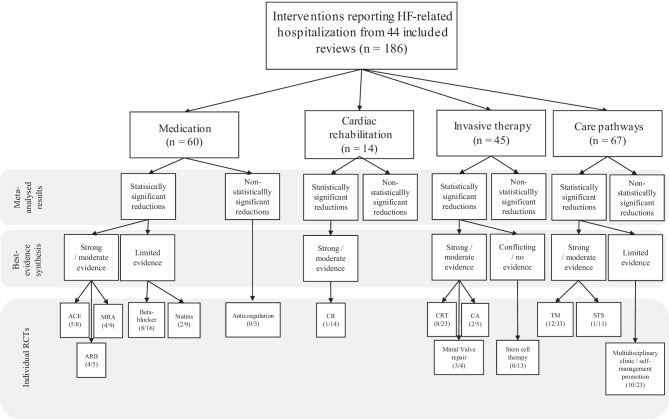
Heart failure (HF) is a major health concern, which accounts for 1–2% of all hospital admissions. Nevertheless, there remains a knowledge gap concerning which interventions contribute to effective prevention of HF (re)hospitalization. Therefore, this umbrella review aims to systematically review meta-analyses that examined the effectiveness of interventions in reducing HF-related (re)hospitalization in HFrEF patients. An electronic literature search was performed in PubMed, Web of Science, PsycInfo, Cochrane Reviews, CINAHL, and Medline to identify eligible studies published in the English language in the past 10 years. Primarily, to synthesize the meta-analyzed data, a best-evidence synthesis was used in which meta-analyses were classified based on level of validity. Secondarily, all unique RCTS were extracted from the meta-analyses and examined. A total of 44 meta-analyses were included which encompassed 186 unique RCTs. Strong or moderate evidence suggested that catheter ablation, cardiac resynchronization therapy, cardiac rehabilitation, telemonitoring, and RAAS inhibitors could reduce (re)hospitalization. Additionally, limited evidence suggested that multidisciplinary clinic or self-management promotion programs, beta-blockers, statins, and mitral valve therapy could reduce HF hospitalization. No, or conflicting evidence was found for the effects of cell therapy or anticoagulation. This umbrella review highlights different levels of evidence regarding the effectiveness of several interventions in reducing HF-related (re)hospitalization in HFrEF patients. It could guide future guideline development in optimizing care pathways for heart failure patients.
Keywords: Heart failure related hospitalizations, Interventions, Medication, Invasive therapy, Rehabilitations, Care pathways
Introduction
Heart failure (HF) is a major health concern, with mortality ranging from 5 to 40% [1], corresponding with a fivefold increased risk of death, compared to the general population [2]. It is even estimated that HF patients have a worse life expectancy than the majority of cancer patients, with a median survival of approximately 2 to 3 years [3, 4]. More than 400,000 patients in the USA are being diagnosed with HF, annually [5]. Moreover, prevalence rates are progressively rising and are expected to increase with 46% from 2012 to 2030 [6, 7].
In addition, heart failure is the diagnosis with the highest readmission rates among all diseases [8–11], as it accounts for 1 to 2% of all hospital admissions [12, 13]. In elderly people, it is the major cause of hospitalization [8]. Most patients are hospitalized at least once a year after diagnosis (i.e., 68 to 78% of patients) [8, 14, 15], and more than one-fourth is at risk of being readmitted within 30 days after initial diagnosis [8, 15–18]. Comparatively to prevalence rates, the total number of hospitalizations is also expected to rise, by 50% in the near future [19, 20].
Hospitalization places a great burden on patients [21]. Patients may experience various limitations in their activities of daily living [22–24], which highly impact their quality of life and level of satisfaction [21, 25]. Moreover, aside from a reduced quality of life, patients who are hospitalized have a significantly higher risk of death than non-hospitalized patients [26, 27]. Additionally, hospitalization due to HF places a great burden on the healthcare system, as it accounts for more than half of total healthcare costs [28, 29] corresponding with more than > 15 billion dollars a year for the American healthcare system [24, 30, 31]. HF is the most costly condition in western countries and since long time hospitalization for HF even exceeds the hospitalization costs for both cancer and myocardial infarction combined [32, 33]. Accordingly, hospitalization is judged as a highly important outcome measure in (inter)national literature and registries [34, 35].
Nevertheless, despite the rising prevalence rates, it seems that up to 40% of hospitalizations could be classified as preventable [36–40]. Therefore, the reduction of hospitalizations is the most promising factor as target to improve patients’ reported experiences or outcomes and to reduce the costs of HF management [25, 41, 42]. The combined measure of patient outcomes and costs are the main goal in value based healthcare, a well-known and promising strategy in healthcare in order to improve patient value [43–45].
Multiple previous studies examined the effect of various interventions to reduce (re)hospitalization in HF, mostly in patients with an left ventricular ejection fraction (LVEF) < 40% (i.e., patients with HFrEF) [46], but contrasting findings are found within the literature regarding the effectiveness of these interventions in reducing hospital admissions [47, 48]. Moreover, there is some considerable heterogeneity in strategies and methods used in previous studies [49]. Some studies, for example, focused on remote monitoring to prevent readmissions, while others examined quality improvement of interventions or transitional care systems [36, 37, 50–52]. Therefore, there remains a gap in information concerning which interventions could effectively contribute to effective prevention of HF hospitalization or readmission [47, 48, 53, 54].
Hence, even though multiple interventions have been included in the guidelines for treatment of HF [46, 55], there is a compelling need of a comprehensive overview of which types of interventions prove effective specifically in reducing HF hospitalizations, especially in HFrEF patients. This umbrella review therefore aims to systematically review all published meta-analyses conducted in the past 10 years that examined the incremental effect of different interventions in addition to standard care, to reduce (re)hospitalization in HFrEF patients, in order to highlight different levels of evidence regarding their effectiveness.
Methods
The systematic review protocol of this review was registered, in accordance with the PRISMA guidelines, at the International Prospective Register of Systematic Reviews (PROSPERO) on July 6, 2020 (registration number: 247872).
Search strategy
An electronic literature search was performed in PubMed, Web of Science, PsycInfo, Cochrane Reviews, CINAHL, and Medline to identify eligible studies published in the English language from January 2010 up to the end of June 2020. Search terms were developed using MeSH terms. Key words were related to (1) interventions, (2) heart failure, (3) hospitalization, and (4) meta-analysis (Table 1).
Table 1.
Search strategy for each database
| Database | Search terms |
|---|---|
| PubMed | (((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((telemedicine[Title/Abstract]) OR (telecare[Title/Abstract])) OR (teleconsultation[Title/Abstract])) OR (telecommunication[Title/Abstract])) OR (home monitoring[Title/Abstract])) OR (monitoring[Title/Abstract])) OR (tele*[Title/Abstract])) OR (tele med*[Title/Abstract])) OR (tele-med*[Title/Abstract])) OR (telehealth[Title/Abstract])) OR (tele-health[Title/Abstract])) OR (remote consult*[Title/Abstract])) OR (remote monitoring[Title/Abstract])) OR (remote patient monitoring[Title/Abstract])) OR (structured telephone support[Title/Abstract])) OR (structured scheduled telephone support[Title/Abstract])) OR (telephone support[Title/Abstract])) OR (telecardiol*[Title/Abstract])) OR (home care services[Title/Abstract])) OR (disease management[Title/Abstract])) OR (patient care team[Title/Abstract])) OR (patient discharge[Title/Abstract])) OR (patient education[Title/Abstract])) OR (patient aftercare[Title/Abstract])) OR (patient care planning[Title/Abstract])) OR (home care services[Title/Abstract])) OR (manage*[Title/Abstract])) OR (comprehensive discharge planning[Title/Abstract])) OR (discharge planning[Title/Abstract])) OR (hospital discharge[Title/Abstract])) OR (patient care planning[Title/Abstract])) OR (multidisciplinary care[Title/Abstract])) OR (care management[Title/Abstract])) OR (transition*[Title/Abstract])) OR (comprehensive health care[Title/Abstract])) OR (process of care[Title/Abstract])) OR (comprehensive care[Title/Abstract])) OR (multidisciplinary care[Title/Abstract])) OR (improve*[Title/Abstract])) OR (promot*[Title/Abstract])) OR (enhanc*[Title/Abstract])) OR (optimi*[Title/Abstract])) OR (quality of health care[Title/Abstract])) OR (improvement initiative[Title/Abstract])) OR (process* improvement[Title/Abstract])) OR (management quality circles[Title/Abstract])) OR (total quality management[Title/Abstract])) OR (guideline adherence[Title/Abstract])) OR (clinical competence[Title/Abstract])) OR (rehabilitation centers[Title/Abstract])) OR (exercise therapy[Title/Abstract])) OR (rehabilitation[Title/Abstract])) OR (sports[Title/Abstract])) OR (physicial exertion[Title/Abstract])) OR (exertion[Title/Abstract])) OR (exercise[Title/Abstract])) OR (rehabilit*[Title/Abstract])) OR (lifestyle intervent*[Title/Abstract])) OR (life-style intervent*[Title/Abstract])) OR (psychotherapy[Title/Abstract])) OR (psychotherap*[Title/Abstract])) OR (psycholog*[Title/Abstract])) OR (psycholog* intervent*[Title/Abstract])) OR (self-care[Title/Abstract])) OR (relaxation therapy[Title/Abstract])) OR (counseling[Title/Abstract])) OR (cognitive therapy[Title/Abstract])) OR (behaviour therapy[Title/Abstract])) OR (behavior therapy[Title/Abstract])) OR (meditation[Title/Abstract])) OR (hypnotherap*[Title/Abstract])) OR (psycho-educat*[Title/Abstract])) OR (psychoeducat*[Title/Abstract])) OR (motiv* intervent*[Title/Abstract])) OR (health education[Title/Abstract])) OR (self-management[Title/Abstract])) OR (action plan*[Title/Abstract])) OR (medication[Title/Abstract])) OR (medication* treatment[Title/Abstract])) OR (pharmacotherapy[Title/Abstract])) OR (device* implantation[Title/Abstract])) OR (medication adherence[Title/Abstract])) OR (patient compliance[Title/Abstract])) OR (adherent[Title/Abstract])) OR (non-compliant[Title/Abstract])) OR (noncompliance[Title/Abstract])) OR (nonadherent[Title/Abstract])) OR (nonadherence[Title/Abstract])) OR (prescription drug[Title/Abstract])) OR (dosage forms[Title/Abstract])) OR (prescribed[Title/Abstract])) OR (pill*[Title/Abstract])) OR (invasisve HF monitoring[Title/Abstract])) OR (implanted monitoring devices[Title/Abstract])) OR (CRT[Title/Abstract])) OR (biventricular pacing[Title/Abstract])) OR (drug therapy[Title/Abstract])) OR (intervention[Title/Abstract])) OR (interven*[Title/Abstract]))OR (immunization[Title/Abstract]))) OR (e-health[Title/Abstract])) OR (program[Title/Abstract])) OR (mobile health[Title/Abstract])) OR (mhealth[Title/Abstract])) OR (after-hours care[Title/Abstract])) OR (integrated delivery of health care[Title/Abstract])) OR (managed care programs[Title/Abstract])) OR (technological interventions[Title/Abstract])) OR (inventions[Title/Abstract])) OR (automation[Title/Abstract])) OR (program evaluation[Title/Abstract])) OR (standard of care[Title/Abstract])) AND (((((heart failure[Title/Abstract]) OR (cardiac failure[Title/Abstract])) OR (congestive*[Title/Abstract])) OR (left ventricular dysfunction[Title/Abstract])) OR (CHF[Title/Abstract]))) AND ((((((readmission*hospitalization*[Title/Abstract]) OR (rehospitalization*[Title/Abstract])) OR (admission*[Title/Abstract])) OR (re-admission*[Title/Abstract])) OR (readmission*[Title/Abstract])) OR (length of stay[Title/Abstract]))) AND (((((meta analysis[Title/Abstract]) OR (meta-analysis[Title/Abstract])) OR (meta analy*[Title/Abstract])) OR (metaanaly*[Title/Abstract])) OR (meta-analy*[Title/Abstract])) |
| Cochrane library |
#1(Telemedicine):ti,ab,kw OR (telecare):ti,ab,kw OR (teleconsultation):ti,ab,kw OR (telecommunication):ti,ab,kw OR (home monitoring):ti,ab,kw OR (monitoring):ti,ab,kw OR (tele*):ti,ab,kw OR (tele med):ti,ab,kw OR (tele-med*):ti,ab,kw OR (telehealth*):ti,ab,kw OR (tele-health*):ti,ab,kw OR (remote consult*):ti,ab,kw OR (remote monitoring):ti,ab,kw OR (remote patient monitoring):ti,ab,kw OR (structured telephone support):ti,ab,kw OR (structured scheduled telephone support):ti,ab,kw OR (telephone support):ti,ab,kw OR (telecardiol*):ti,ab,kw OR (home care services):ti,ab,kw OR (disease management):ti,ab,kw OR (patient care team):ti,ab,kw OR (patient discharge):ti,ab,kw OR (patient education):ti,ab,kw OR (patient aftercare):ti,ab,kw OR (patient care planning):ti,ab,kw OR (home care services):ti,ab,kw OR (manage*):ti,ab,kw OR (comprehensive discharge planning):ti,ab,kw OR (discharge planning):ti,ab,kw OR (hospital discharge):ti,ab,kw OR (patient care planning):ti,ab,kw OR (multidisciplinary care):ti,ab,kw OR (care management):ti,ab,kw OR (transition*):ti,ab,kw OR (comprehensive health care):ti,ab,kw OR (process of care):ti,ab,kw OR (comprehensive care):ti,ab,kw OR (multidisciplinary care):ti,ab,kw OR (improve*):ti,ab,kw OR (promot*):ti,ab,kw OR (enhanc*):ti,ab,kw OR (optimi*):ti,ab,kw OR (quality of health care):ti,ab,kw OR (improvement initiative):ti,ab,kw OR (process* improvement):ti,ab,kw OR (management quality circles):ti,ab,kw OR (total quality management):ti,ab,kw OR (guideline adherence):ti,ab,kw OR (clinical competence):ti,ab,kw OR (*rehabilitation centers):ti,ab,kw OR (exercise therapy):ti,ab,kw OR (*rehabilitation):ti,ab,kw OR (sports):ti,ab,kw OR (physical exertion):ti,ab,kw OR (exertion):ti,ab,kw OR (exercise):ti,ab,kw OR (rehabilit*):ti,ab,kw OR (lifestyle intervent*):ti,ab,kw OR (life-style intervent*):ti,ab,kw OR (psychotherapy):ti,ab,kw OR (psychotherap*):ti,ab,kw OR (psycholog*):ti,ab,kw OR (psycholog* intervent*):ti,ab,kw OR (self-care):ti,ab,kw OR (relaxation therapy):ti,ab,kw OR (counseling):ti,ab,kw OR (cognitive therapy):ti,ab,kw OR (behaviour therapy):ti,ab,kw OR (behavior therapy):ti,ab,kw OR (meditation):ti,ab,kw OR (hypnotherap*):ti,ab,kw OR (psycho-educat*):ti,ab,kw OR (psychoeducat*):ti,ab,kw OR (motiv* intervent*):ti,ab,kw OR (health education):ti,ab,kw OR ( self-management):ti,ab,kw OR (action plan*):ti,ab,kw OR (Medication):ti,ab,kw OR (medication* treatment):ti,ab,kw OR (pharmacotherapy):ti,ab,kw OR (device* implantation):ti,ab,kw OR (medication adherence):ti,ab,kw OR (patient compliance):ti,ab,kw OR (adherent):ti,ab,kw OR (non-compliant):ti,ab,kw OR (noncompliance):ti,ab,kw OR (nonadherent):ti,ab,kw OR (nonadherence):ti,ab,kw OR (prescription drugs):ti,ab,kw OR (dosage forms):ti,ab,kw OR (prescribed):ti,ab,kw OR (pill* ORinvasive HF monitoring):ti,ab,kw OR (implanted monitoring devices):ti,ab,kw OR (CRT):ti,ab,kw OR (biventricular pacing):ti,ab,kw OR (drug therapy):ti,ab,kw OR (intervention):ti,ab,kw OR (interven*):ti,ab,kw OR (e-health):ti,ab,kw OR (program):ti,ab,kw OR (mobile health):ti,ab,kw OR (mhealth):ti,ab,kw OR (after-hours care):ti,ab,kw OR (integrated delivery of health care):ti,ab,kw OR (managed care programs):ti,ab,kw OR (technological interventions):ti,ab,kw OR (inventions):ti,ab,kw OR (automation):ti,ab,kw OR (program evaluation):ti,ab,kw OR (standard of care):ti,ab,kw OR (OR influenza):ti,ab,kw #2(meta analysis):ti,ab,kw OR (meta-analysis):ti,ab,kw OR (meta analy*):ti,ab,kw OR (metaanaly*):ti,ab,kw OR (meta-analy*):ti,ab,kw #3(hospitalization*):ti,ab,kw OR (rehospitalization*):ti,ab,kw OR (admission*):ti,ab,kw OR (re-admission*):ti,ab,kw OR (readmission*):ti,ab,kw OR (length of stay):ti,ab,kw #4(*Heart failure):ti,ab,kw OR (cardiac failure):ti,ab,kw OR (congestive*):ti,ab,kw OR (left ventricular dysfunction):ti,ab,kw OR (CHF):ti,ab,kw #5#1 AND #2 AND #3 AND #4 |
| Web of Science |
#1: TS = (Telemedicine OR telecare OR teleconsultation OR telecommunication OR home monitoring OR monitoring OR tele* OR tele med OR telemed* OR telehealth* OR telehealth* OR remote consult* OR remote monitoring OR remote patient monitoring OR structured telephone support OR structured scheduled telephone support OR telephone support OR telecardiol* OR home care services OR disease management OR patient care team OR patient discharge OR patient education OR patient aftercare OR patient care planning OR home care services OR manage* OR comprehensive discharge planning OR discharge planning OR hospital discharge OR patient care planning OR multidisciplinary care OR care management OR transition* OR comprehensive health care OR process of care OR comprehensive care OR multidisciplinary care OR improve* OR promot* OR enhanc* OR optimi* OR quality of health care OR improvement initiative OR process* improvement OR management quality circles OR total quality management OR guideline adherence OR clinical competence OR *rehabilitation centers OR exercise therapy OR *rehabilitation OR sports OR physical exertion OR exertion OR exercise OR rehabilit* OR lifestyle intervent* OR life-style intervent* OR psychotherapy OR psychotherap* OR psycholog* OR psycholog* intervent* OR self-care OR relaxation therapy OR counseling OR cognitive therapy OR behaviour therapy OR behavior therapy OR meditation OR hypnotherap* OR psycho-educat* OR psychoeducat* OR motiv* intervent* OR health education OR self-management OR action plan* OR Medication OR medication* treatment OR pharmacotherapy OR device* implantation OR medication adherence OR patient compliance OR adherent OR non-compliant OR noncompliance OR nonadherent OR nonadherence OR prescription drugs OR dosage forms OR prescribed OR pill* ORinvasive HF monitoring OR implanted monitoring devices OR CRT OR biventricular pacing OR drug therapy OR intervention OR interven* OR e-health OR program OR mobile health OR mhealth OR after-hours care OR integrated delivery of health care OR managed care programs OR technological interventions OR inventions OR automation OR program evaluation OR standard of care) #2: TS = (meta analysis OR meta-analysis OR meta analy* OR metaanaly* OR meta-analy*) #3: TS = (hospitalization* OR rehospitalization* OR admission* OR re-admission* OR readmission* OR length of stay) #4: TS = (*Heart failure OR cardiac failure OR congestive* OR left ventricular dysfunction OR CHF) #5: #4 AND #3 AND #2 AND #1 |
| Psycinfo | TX ( Telemedicine OR telecare OR teleconsultation OR telecommunication OR home monitoring OR monitoring OR tele* OR tele med OR tele-med* OR telehealth* OR tele-health* OR remote consult* OR remote monitoring OR remote patient monitoring OR structured telephone support OR structured scheduled telephone support OR telephone support OR telecardiol* OR home care services OR disease management OR patient care team OR patient discharge OR patient education OR patient aftercare OR patient care planning OR home care services OR manage* OR comprehensive discharge planning OR discharge planning OR hospital discharge OR patient care planning OR multidisciplinary care OR care management OR transition* OR comprehensive health care OR process of care OR comprehensive care OR multidisciplinary care OR improve* OR promot* OR enhanc* OR optimi* OR quality of health care OR improvement initiative OR process* improvement OR management quality circles OR total quality management OR guideline adherence OR clinical competence OR *rehabilitation centers OR exercise therapy OR *rehabilitation OR sports OR physical exertion OR exertion OR exercise OR rehabilit* OR lifestyle intervent* OR life-style intervent* OR psychotherapy OR psychotherap* OR psycholog* OR psycholog* intervent* OR self-care OR relaxation therapy OR counseling OR cognitive therapy OR behaviour therapy OR behavior therapy OR meditation OR hypnotherap* OR psycho-educat* OR psychoeducat* OR motiv* intervent* OR health education OR self-management OR action plan* OR Medication OR medication* treatment OR pharmacotherapy OR device* implantation OR medication adherence OR patient compliance OR adherent OR non-compliant OR noncompliance OR nonadherent OR nonadherence OR prescription drugs OR dosage forms OR prescribed OR pill* ORinvasive HF monitoring OR implanted monitoring devices OR CRT OR biventricular pacing OR drug therapy OR intervention OR interven* OR e-health OR program OR mobile health OR mhealth OR after-hours care OR integrated delivery of health care OR managed care programs OR technological interventions OR inventions OR automation OR program evaluation OR standard of care) AND TX ( meta analysis OR meta-analysis OR meta analy* OR metaanaly* OR meta-analy*) AND TX ( hospitalization* OR rehospitalization* OR admission* OR re-admission* OR readmission* OR length of stay) AND TX ( *Heart failure OR cardiac failure |
| Medline | AB ( Telemedicine OR telecare OR teleconsultation OR telecommunication OR home monitoring OR monitoring OR tele* OR tele med OR tele-med* OR telehealth* OR tele-health* OR remote consult* OR remote monitoring OR remote patient monitoring OR structured telephone support OR structured scheduled telephone support OR telephone support OR telecardiol* OR home care services OR disease management OR patient care team OR patient discharge OR patient education OR patient aftercare OR patient care planning OR home care services OR manage* OR comprehensive discharge planning OR discharge planning OR hospital discharge OR patient care planning OR multidisciplinary care OR care management OR transition* OR comprehensive health care OR process of care OR comprehensive care OR multidisciplinary care OR improve* OR promot* OR enhanc* OR optimi* OR quality of health care OR improvement initiative OR process* improvement OR management quality circles OR total quality management OR guideline adherence OR clinical competence OR *rehabilitation centers OR exercise therapy OR *rehabilitation OR sports OR physical exertion OR exertion OR exercise OR rehabilit* OR lifestyle intervent* OR life-style intervent* OR psychotherapy OR psychotherap* OR psycholog* OR psycholog* intervent* OR self-care OR relaxation therapy OR counseling OR cognitive therapy OR behaviour therapy OR behavior therapy OR meditation OR hypnotherap* OR psycho-educat* OR psychoeducat* OR motiv* intervent* OR health education OR self-management OR action plan* OR Medication OR medication* treatment OR pharmacotherapy OR device* implantation OR medication adherence OR patient compliance OR adherent OR non-compliant OR noncompliance OR nonadherent OR nonadherence OR prescription drugs OR dosage forms OR prescribed OR pill* ORinvasive HF monitoring OR implanted monitoring devices OR CRT OR biventricular pacing OR drug therapy OR intervention OR interven* OR e-health OR program OR mobile health OR mhealth OR after-hours care OR integrated delivery of health care OR managed care programs OR technological interventions OR inventions OR automation OR program evaluation OR standard of care OR) AND AB ( meta analysis OR meta-analysis OR meta analy* OR metaanaly* OR meta-analy) AND AB ( hospitalization* OR rehospitalization* OR admission* OR re-admission* OR readmission* OR length of stay) AND AB ( Heart failure OR cardiac failure OR congestive* OR left ventricular dysfunction OR CHF) |
| CINAHL | AB ( Telemedicine OR telecare OR teleconsultation OR telecommunication OR home monitoring OR monitoring OR tele* OR tele med OR tele-med* OR telehealth* OR tele-health* OR remote consult* OR remote monitoring OR remote patient monitoring OR structured telephone support OR structured scheduled telephone support OR telephone support OR telecardiol* OR home care services OR disease management OR patient care team OR patient discharge OR patient education OR patient aftercare OR patient care planning OR home care services OR manage* OR comprehensive discharge planning OR discharge planning OR hospital discharge OR patient care planning OR multidisciplinary care OR care management OR transition* OR comprehensive health care OR process of care OR comprehensive care OR multidisciplinary care OR improve* OR promot* OR enhanc* OR optimi* OR quality of health care OR improvement initiative OR process* improvement OR management quality circles OR total quality management OR guideline adherence OR clinical competence OR *rehabilitation centers OR exercise therapy OR *rehabilitation OR sports OR physical exertion OR exertion OR exercise OR rehabilit* OR lifestyle intervent* OR life-style intervent* OR psychotherapy OR psychotherap* OR psycholog* OR psycholog* intervent* OR self-care OR relaxation therapy OR counseling OR cognitive therapy OR behaviour therapy OR behavior therapy OR meditation OR hypnotherap* OR psycho-educat* OR psychoeducat* OR motiv* intervent* OR health education OR self-management OR action plan* OR Medication OR medication* treatment OR pharmacotherapy OR device* implantation OR medication adherence OR patient compliance OR adherent OR non-compliant OR noncompliance OR nonadherent OR nonadherence OR prescription drugs OR dosage forms OR prescribed OR pill* ORinvasive HF monitoring OR implanted monitoring devices OR CRT OR biventricular pacing OR drug therapy OR intervention OR interven* OR e-health OR program OR mobile health OR mhealth OR after-hours care OR integrated delivery of health care OR managed care programs OR technological interventions OR inventions OR automation OR program evaluation OR standard of care) AND AB ( meta analysis OR meta-analysis OR meta analy* OR metaanaly* OR meta-analy*) AND AB ( hospitalization* OR rehospitalization* OR admission* OR re-admission* OR readmission* OR length of stay) AND AB ( Heart failure OR cardiac failure OR congestive* OR left ventricular dysfunction OR CHF) |
Ample differences existed in the classification of categories of interventions depicted in the existing literature. For example, previous reviews classified interventions in either educational interventions, pharmacological interventions, telemonitoring (TM), structured telephone support (STS), nurse home visits, nurse care management, and disease management clinics [41]; or discharge planning protocols, comprehensive geriatric assessments, discharge support arrangements, and educational interventions [56]; or case management interventions, clinical interventions, and multidisciplinary interventions [53]; or predischarge interventions, postdischarge interventions, and interventions bridging the transition [57]. A list of 4 categories of interventions was derived following a scoping review that combine the most common interventions aimed at reducing hospital (re)admissions, cardiac rehabilitation, care pathways, medication, and invasive treatment. Both general terms linked to the concept of interventions (e.g., programs, inventions, therapy) and terms for specific examples of (categories of) interventions were included in the search strategy.
Eligibility criteria
Search results of all databases were combined, and duplicates were removed. Titles and abstracts were screened against the following inclusion criteria: (1) a meta-analysis was conducted, on (2) randomized controlled trials (RCTs), (3) that examined the effectiveness of (3.a) cardiac rehabilitation, or (3.b) care pathways, or (3.c) medication, or (3.d) invasive therapy, (4) in patients with an established diagnosis of chronic heart failure, (5) with an LVEF < 40, (6) with a primary or secondary objective to evaluate the effect on reduction of (7) HF-related hospitalization or readmissions, (8) as compared to usual care, (9) conducted in the past 10 years, (10) followed patients for at least three months, and (11) were reported in English. Meta-analyses that included both RCTs and observational or cohort studies were not excluded. Yet only the included RCTs (and corresponding meta-analyzed effect sized) were extracted and used for our analyses. Only meta-analyses that reported at least one meta-analyzed effect estimate for HF-related admissions were included. In order to assure objective assessment, the title and abstract screening were independently conducted by two researchers (FH, TG). In case of disagreement between reviewers, points of disagreement were discussed in order to reach consensus. For full-text screening, inter-rate reliability was calculated using Cohen’s kappa.
Studies were excluded when the patient population was not primarily diagnosed with heart failure (e.g., patients with diabetes and comorbid heart failure). Additionally, if studies examined HF patients in combination with other patient groups yet did not report data on the individual patient groups, the study was excluded, as we would otherwise be unable to make a distinction between the differences in patient groups. Furthermore, studies that only reported data on a combined endpoint (e.g., mortality in conjunction with HF-hospitalization) and meta-analyses that examined risk stratification, prognostic factors, or lifestyle advice in patients were excluded. Moreover, meta-analyses were also excluded when examining a specific subgroup of HF patients (e.g., patients with and LVAD) or when examining a broader category of patients that could possibly include HF patients (e.g., “older patients” in general).
Quality assessment
The “A MeaSurement Tool to Assess systematic Reviews 2” (AMSTAR 2) was used to assess the methodological quality of included meta-analyses [58]. AMSTAR 2 consists of 16 items, of which 10 items were retained from the original AMSTAR tool. Response options for the items were “yes,” “partial yes,” and “no,” with “yes” responses denoting a positive result. The overall score of this tool was converted to high quality, moderate quality, low quality, and critically low quality. High quality was achieved when studies possessed no or one non-critical weakness; moderate quality was achieved when studies had more than one non-critical weakness; low quality was achieved when studies had one critical flaw, with or without a non-critical weakness; and critically low quality was achieved when studies exhibited more than one critical flaw with or without non-critical weaknesses. Critical domains are depicted in Table 2 [58]. In order to assure objective assessment, the quality assessment was independently conducted by two researchers (GS, TG). In case of disagreement between reviewers, points of disagreement were discussed in order to reach consensus (RT).
Table 2.
Critical domains of the AMSTAR 2
| Registered protocol before commencement of the review |
| Risk of bias from individual studies being included in the review |
| Appropriateness of meta-analytical methods |
| Consideration of risk of bias when interpreting the results of the review |
| Assessment of presence and likely impact of publication bias |
Data extraction
A standardized extraction form was used to extract data from the included studies. Sociodemographic data (e.g., age, sex), number of participants, left ventricular ejection fraction, type of intervention and control, follow-up period, effect size, and conclusion were extracted from either the individual RCT or the meta-analysis in which the RCT was included. Only the most recent meta-analysis was included when multiple articles were written by the same authors on the same dataset. Comparisons were made between the different categories of interventions in terms of effectiveness in reducing HF-related (re)hospitalization. Interventions were classified as having a significant effect on HF-related (re)hospitalization (as compared to usual care) based on their own reported RR statistics, findings, and conclusions.
Data synthesis
Interventions were first classified into the four predefined categories (i.e., cardiac rehabilitation, care pathways, medication, and invasive therapy) and subsequently divided into more detailed classes of interventions (e.g., TM and STS) to examine the exact effect of all unique interventions.
Primary analysis: meta-analyses
To synthesize the data, a best-evidence synthesis was used as primary analysis, in which meta-analyses were classified based on level of internal and external validity [59]. The levels of evidence regarding the significance or non-significance of a relationship between the intervention and HF-related hospitalization among studies were ranked according to the following statements: (1) strong evidence: consistent findings (> 75% of the studies reported consistent findings) in multiple high quality studies; (2) moderate evidence: consistent findings (> 75% of the studies reported consistent findings) in one high-quality study and two or more moderate quality studies or in three or more weak quality studies; (3) limited evidence: generally consistent findings (> 75% of the studies reported consistent findings) in a high quality study or in two or fewer moderate quality studies; (4) no evidence: no studies could be found; (5) conflicting evidence: conflicting findings.
Secondary analysis: extracted RCTs
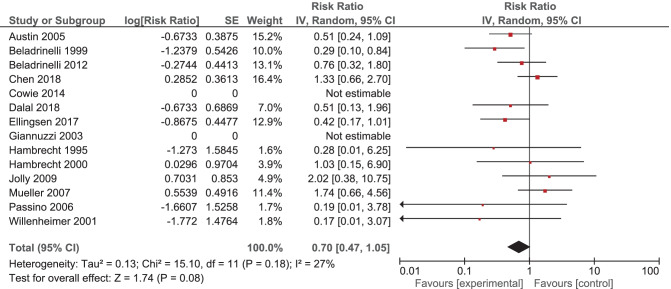
It was expected that multiple meta-analyses would report identical RCTs, as it was previously found that the amount of redundancy and duplication among reviews is substantial [60, 61]. Therefore, the corrected covered area (CCA) was calculated, which is a measure of duplicates in meta-analyses divided by the frequency of duplicates, reduced by the number of original publications [62]. A CCA of 0–5% is considered as slight overlap, while 6–10%, 11–15%, > 15% are respectively regarded as moderate, high, and very high overlap. In order to prevent bias as a result of duplicated data, a secondary analysis was conducted to control for the effects of overlap. All unique RCTs were extracted from the meta-analyses. Individual risk ratios (RRs) and 95% CIs for each intervention were calculated using Review Manager V.5.4. or extracted from the meta-analyses. The I2-statistic was used to present the heterogeneity of intervention effect. When the I2-statistic was statistically significant, a random-effects model was used in analyses. The RR-statistics found in our own meta-analyses were compared to the reported effects in the published meta-analyses.
Results
Search results
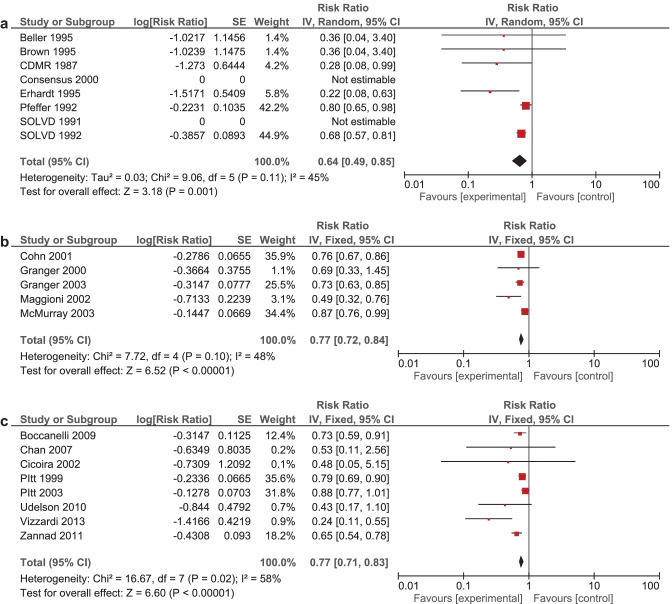
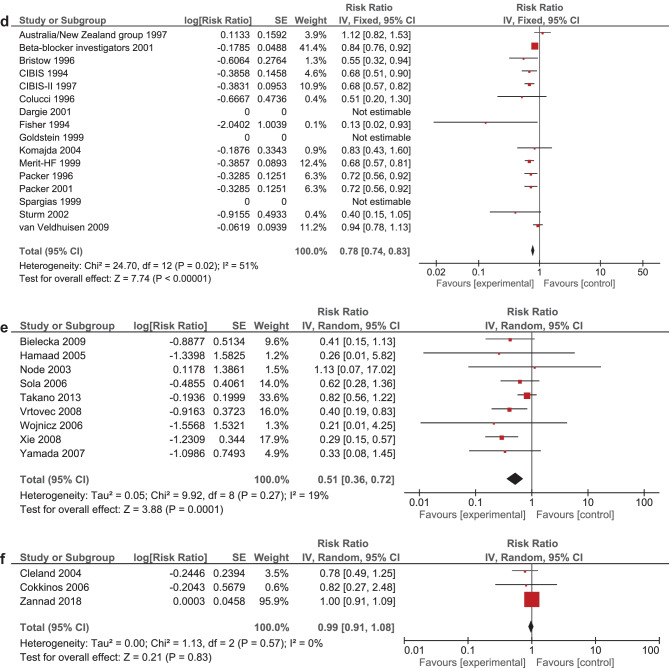
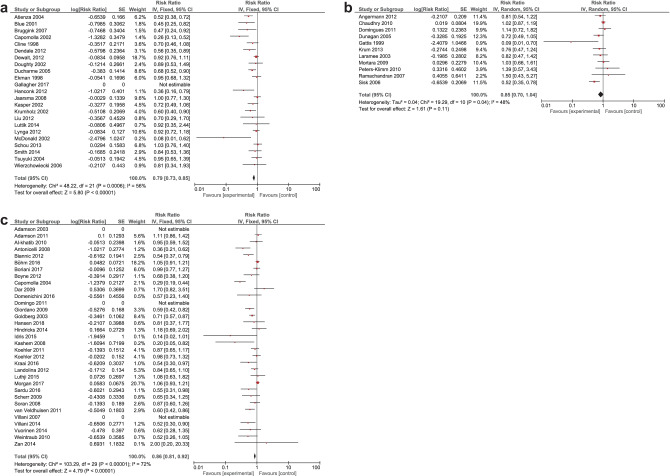
After removal of duplicate meta-analyses, 639 titles and abstracts were screened (see Fig. 1). A total of 202 full-text articles were assessed for eligibility, of which 44 were included in our analyses. Cohen’s kappa for full-text screening was 0.76, indicating substantial agreement [63]. Median year of publication of all included meta-analyses was 2018. The 44 included meta-analyses encompassed 348 RCTs of which 186 were unique RCTs regarding interventions to prevent HF hospitalization (Table 3). Of these 186 unique RCTs, 44 were classified as invasive therapy, 14 as cardiac rehabilitation, 60 as medication, and 67 as care pathways (Table 4). The CCA for cardiac revalidation was , the CCA for invasive therapy was , the CCA for medication was , and the CCA for care pathways was . This indicates a moderate to very high overlap in included RCTs [62].
Fig. 1.
Flow diagram of study inclusion. RCT: randomized controlled trial
Table 3.
Overlap between different meta-analyses in included RCTs
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abraham et al. [87] | 2002 | x | |||||||||||||||||||||
| Abraham et al. [88] | 2004 | ||||||||||||||||||||||
| Adamson et al. [89] | 2003 | x | |||||||||||||||||||||
| Adamson et al. [90] | 2011 | x | x | x | |||||||||||||||||||
| Al-khatib et al. [91] | 2010 | x | |||||||||||||||||||||
| Angermann et al. [92] | 2012 | x | |||||||||||||||||||||
| Antonicelli et al. [93] | 2008 | ||||||||||||||||||||||
| Asgar et al. [94] | 2017 | x | |||||||||||||||||||||
| Assmus et al. [95] | 2006 | x | |||||||||||||||||||||
| Assmus et al. [96] | 2013 | x | x | ||||||||||||||||||||
| Atienza et al. [97] | 2004 | ||||||||||||||||||||||
| Austin et al. [98] | 2005 | x | |||||||||||||||||||||
| Australia/New Zealand Heart Failure Group [99] | 1997 | ||||||||||||||||||||||
| Bartunek et al. [100] | 2013 | x | |||||||||||||||||||||
| Belardinelli et al. [101] | 1999 | x | |||||||||||||||||||||
| Belardinelli et al. [102] | 2012 | x | |||||||||||||||||||||
| Beller et al. [103] | 1995 | ||||||||||||||||||||||
| Bentkover et al. [104] | 2007 | ||||||||||||||||||||||
| Beta-Blocker evaluation of survival trial [105] | 2001 | ||||||||||||||||||||||
| Biannic et al. [106] | 2012 | x | |||||||||||||||||||||
| Bielecka-Dabrowa et al. [107] | 2009 | x | |||||||||||||||||||||
| Blue et al. [108] | 2001 | ||||||||||||||||||||||
| Boccanelli et al. [109] | 2009 | x | x | ||||||||||||||||||||
| Böhm et al. [110] | 2016 | x | |||||||||||||||||||||
| Bolli et al. [111] | 2011 | x | |||||||||||||||||||||
| Boriani et al. [112] | 2017 | x | x | ||||||||||||||||||||
| Boyne et al. [113] | 2012 | ||||||||||||||||||||||
| Bristow et al. [114] | 1996 | ||||||||||||||||||||||
| Brown et al. [115] | 1995 | ||||||||||||||||||||||
| Lok et al. [116] | 2007 | x | |||||||||||||||||||||
| Capomolla et al. [117] | 2002 | x | |||||||||||||||||||||
| Cazeau et al. [118] | 2001 | x | |||||||||||||||||||||
| CDMR [119] | 1988 | ||||||||||||||||||||||
| Chan et al. [120] | 2007 | x | x | ||||||||||||||||||||
| Chaudhry et al. [69] | 2010 | x | |||||||||||||||||||||
| Chen et al. [121] | 2018 | ||||||||||||||||||||||
| Chung [122] | 2021 | ||||||||||||||||||||||
| CIBIS [123] | 1994 | ||||||||||||||||||||||
| CIBIS-II [124] | 1999 | ||||||||||||||||||||||
| Cicoira et al. [125] | 2002 | x | |||||||||||||||||||||
| Cleland et al. [126] | 2004 | ||||||||||||||||||||||
| Cline et al. [127] | 1998 | x | |||||||||||||||||||||
| Cohn and Tognoni [128] | 2001 | x | |||||||||||||||||||||
| Cokkinos et al. [129] | 2006 | ||||||||||||||||||||||
| Colucci et al. [130] | 1996 | ||||||||||||||||||||||
| Consensus et al. [241] | 2000 | ||||||||||||||||||||||
| Cowie et al. [131] | 2014 | ||||||||||||||||||||||
| Dalal et al. [132] | 2019 | x | |||||||||||||||||||||
| Dar et al. [133] | 2009 | ||||||||||||||||||||||
| Dargie [134] | 2001 | ||||||||||||||||||||||
| Daubert et al. [135] | 2009 | ||||||||||||||||||||||
| Dendale et al. [136] | 2012 | x | |||||||||||||||||||||
| Dewalt et al. [137] | 2012 | ||||||||||||||||||||||
| Di Biase et al. [138] | 2016 | x | x | ||||||||||||||||||||
| DIG [139] | 1997 | ||||||||||||||||||||||
| Domenichini et al. [140] | 2016 | x | x | ||||||||||||||||||||
| Domingo et al. [141] | 2011 | ||||||||||||||||||||||
| Domingues et al. [142] | 2011 | ||||||||||||||||||||||
| Doughty et al. [143] | 2002 | x | |||||||||||||||||||||
| Ducharme et al. [144] | 2005 | x | |||||||||||||||||||||
| Dunagan et al. [145] | 2005 | ||||||||||||||||||||||
| Ekman et al. [146] | 1998 | x | |||||||||||||||||||||
| Ellingsen et al. [147] | 2017 | x | |||||||||||||||||||||
| Erhardt et al. [148] | 1995 | ||||||||||||||||||||||
| Fisher et al. [149] | 1994 | ||||||||||||||||||||||
| Fox et al. [150] | 2008 | x | |||||||||||||||||||||
| Fragasso et al. [151] | 2006 | ||||||||||||||||||||||
| Gallagher et al. [152] | 2017 | ||||||||||||||||||||||
| Gasparini et al. [153] | 2006 | x | |||||||||||||||||||||
| Gattis et al. [154] | 1999 | x | x | ||||||||||||||||||||
| Giannini et al. [155] | 2016 | x | |||||||||||||||||||||
| Giannuzzi et al. [156] | 2003 | x | |||||||||||||||||||||
| Giordano et al. [157] | 2009 | x | x | ||||||||||||||||||||
| Goldberg et al. [158] | 2003 | ||||||||||||||||||||||
| Goldstein et al. [159] | 1999 | ||||||||||||||||||||||
| Granger et al. [160] | 2000 | ||||||||||||||||||||||
| Granger et al. [161] | 2003 | x | |||||||||||||||||||||
| Hamaad et al. [162] | 2005 | x | |||||||||||||||||||||
| Hambrecht et al. [163] | 1995 | ||||||||||||||||||||||
| Hambrecht et al. [164] | 2000 | x | |||||||||||||||||||||
| Hamshere et al. [165] | 2015 | x | |||||||||||||||||||||
| Hanconk et al. [166] | 2012 | x | |||||||||||||||||||||
| Hansen et al. [167] | 2018 | x | |||||||||||||||||||||
| Heldman et al. [168] | 2014 | x | x | ||||||||||||||||||||
| Heldman et al. [168] | 2014 | x | |||||||||||||||||||||
| Higgins et al. [169] | 2003 | x | |||||||||||||||||||||
| Hindricks et al. [170] | 2014 | x | x | ||||||||||||||||||||
| Idris et al. [171] | 2015 | ||||||||||||||||||||||
| Jaarsma et al. [47] | 2008 | x | |||||||||||||||||||||
| Jolly et al. [172] | 2009 | x | |||||||||||||||||||||
| Jones and Wong [173] | 2013 | x | |||||||||||||||||||||
| Kashem et al. [174] | 2008 | ||||||||||||||||||||||
| Kasper et al. [175] | 2002 | x | |||||||||||||||||||||
| Koehler et al. [176] | 2011 | x | x | ||||||||||||||||||||
| Komajda [177] | 2004 | ||||||||||||||||||||||
| Kraai et al. [178] | 2016 | ||||||||||||||||||||||
| Krum et al. [179] | 2013 | x | |||||||||||||||||||||
| Krumholz et al. [180] | 2002 | ||||||||||||||||||||||
| Landolina et al. [181] | 2012 | x | x | ||||||||||||||||||||
| Laramee et al. [182] | 2003 | x | x | ||||||||||||||||||||
| Linde et al. [183] | 2002 | x | |||||||||||||||||||||
| Leclercq et al. [184] | 2007 | x | |||||||||||||||||||||
| Linde et al. [185] | 2008 | x | |||||||||||||||||||||
| Liu et al. [186] | 2012 | x | |||||||||||||||||||||
| Lüthje et al. [187] | 2015 | x | x | ||||||||||||||||||||
| Luttik et al. [188] | 2014 | x | |||||||||||||||||||||
| Lyngå et al. [68] | 2012 | ||||||||||||||||||||||
| MacDonald et al. [189] | 2011 | x | x | ||||||||||||||||||||
| Maggioni et al. [190] | 2002 | ||||||||||||||||||||||
| Margulies et al. [191] | 2016 | ||||||||||||||||||||||
| Marrouche et al. [192] | 2018 | x | x | ||||||||||||||||||||
| Martinelli et al. [193] | 2010 | x | |||||||||||||||||||||
| Mathiasen et al. [194] | 2015 | x | |||||||||||||||||||||
| Menasché [195] | 2008 | x | |||||||||||||||||||||
| Mcdonald et al. [196] | 2002 | ||||||||||||||||||||||
| McMurray et al. [197] | 2003 | x | |||||||||||||||||||||
| MERIT-HF [198] | 1999 | ||||||||||||||||||||||
| Morgan et al. [199] | 2017 | x | |||||||||||||||||||||
| Mortara et al. [200] | 2009 | x | x | ||||||||||||||||||||
| Moss et al. [201] | 2002 | ||||||||||||||||||||||
| Moss et al. [202] | 2009 | x | |||||||||||||||||||||
| Mozid et al. [203] | 2014 | x | |||||||||||||||||||||
| Mueller et al. [204] | 2007 | x | |||||||||||||||||||||
| Node et al. [205] | 2003 | x | |||||||||||||||||||||
| Obadia et al. [206] | 2018 | x | x | ||||||||||||||||||||
| Packer et al. [207] | 1993 | ||||||||||||||||||||||
| Packer et al. [208] | 1996 | ||||||||||||||||||||||
| Packer et al. [209] | 1996 | ||||||||||||||||||||||
| Packer et al. [210] | 2001 | ||||||||||||||||||||||
| Passino et al. [211] | 2006 | ||||||||||||||||||||||
| Patel et al. [212] | 2015 | x | |||||||||||||||||||||
| Pätilä et al. [213] | 2014 | x | x | ||||||||||||||||||||
| Perin et al. [214] | 2012 | x | |||||||||||||||||||||
| Peters-klimm et al. [215] | 2010 | ||||||||||||||||||||||
| Pfeffer et al. [216] | 1992 | ||||||||||||||||||||||
| Piepoli et al. [217] | 2008 | x | |||||||||||||||||||||
| Pinter et al. [218] | 2009 | ||||||||||||||||||||||
| Pitt et al. [219] | 1999 | x | |||||||||||||||||||||
| Pitt et al. [220] | 2003 | x | x | ||||||||||||||||||||
| Pokushalov et al. [221] | 2010 | ||||||||||||||||||||||
| Pokushalov et al. [222] | 2011 | ||||||||||||||||||||||
| Prabhu et al. [223] | 2017 | x | |||||||||||||||||||||
| Ramachandran et al. [224] | 2007 | x | x | ||||||||||||||||||||
| Rosano et al. [225] | 2003 | ||||||||||||||||||||||
| Ruschitzka et al. [226] | 2013 | ||||||||||||||||||||||
| Sardu et al. [227] | 2016 | ||||||||||||||||||||||
| Scherr et al. [228] | 2009 | ||||||||||||||||||||||
| Schou et al. [229] | 2013 | x | |||||||||||||||||||||
| Sisk et al. [230] | 2006 | x | x | x | |||||||||||||||||||
| Smith et al. [231] | 2014 | x | |||||||||||||||||||||
| Sola et al. [232] | 2006 | x | |||||||||||||||||||||
| Yusuf et al. [233] | 1991 | x | |||||||||||||||||||||
| Yusuf et al. [234] | 1992 | x | |||||||||||||||||||||
| Spargias et al. [235] | 1999 | x | |||||||||||||||||||||
| Stone et al. [236] | 2018 | x | x | ||||||||||||||||||||
| Sturm et al. [237] | 2000 | ||||||||||||||||||||||
| Swedberg et al. [238] | 2010 | x | |||||||||||||||||||||
| Takano et al. [239] | 2013 | x | |||||||||||||||||||||
| Tang et al. [240] | 2010 | x | |||||||||||||||||||||
| Consensus et al. [241] | 2000 | ||||||||||||||||||||||
| Thibault et al. [242] | 2011 | x | |||||||||||||||||||||
| Thibault et al. [243] | 2013 | ||||||||||||||||||||||
| Tsuyuki et al. [244] | 2004 | x | x | ||||||||||||||||||||
| Tuunanen et al. [245] | 2008 | ||||||||||||||||||||||
| Udelson et al. [246] | 2010 | x | |||||||||||||||||||||
| Uretsky et al. [247] | 1993 | ||||||||||||||||||||||
| van Veldhuisen et al. [248] | 2009 | ||||||||||||||||||||||
| van Veldhuisen et al. [249] | 2011 | x | x | ||||||||||||||||||||
| Villani et al. [250] | 2007 | ||||||||||||||||||||||
| Villani et al. [251] | 2014 | x | |||||||||||||||||||||
| Vitale et al. [252] | 2004 | ||||||||||||||||||||||
| Vizzardi et al. [253] | 2010 | x | |||||||||||||||||||||
| Vrtovec et al. [254] | 2008 | x | |||||||||||||||||||||
| Vuorinen et al. [255] | 2014 | x | |||||||||||||||||||||
| Weintraub et al. [256] | 2010 | ||||||||||||||||||||||
| Wierzchowiecki et al. [257] | 2006 | ||||||||||||||||||||||
| Willenheimer et al. [258] | 2001 | ||||||||||||||||||||||
| Wojnicz et al. [259] | 2006 | x | |||||||||||||||||||||
| Xie et al. [260] | 2010 | x | |||||||||||||||||||||
| Yamada et al. [261] | 2007 | x | |||||||||||||||||||||
| Young et al. [262] | 2003 | x | |||||||||||||||||||||
| Zan [263] | 2020 | x | |||||||||||||||||||||
| Zannad et al. [264] | 2011 | x | x | x | x | ||||||||||||||||||
| Zannad et al. [265] | 2018 |
| 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abraham et al. [87] | 2002 | x | x | ||||||||||||||||||||
| Abraham et al. [88] | 2004 | x | |||||||||||||||||||||
| Adamson et al. [89] | 2003 | ||||||||||||||||||||||
| Adamson et al. [90] | 2011 | ||||||||||||||||||||||
| Al-khatib et al. [91] | 2010 | ||||||||||||||||||||||
| Angermann et al. [92] | 2012 | x | x | ||||||||||||||||||||
| Antonicelli et al. [93] | 2008 | x | x | ||||||||||||||||||||
| Asgar et al. [94] | 2017 | ||||||||||||||||||||||
| Assmus et al. [95] | 2006 | ||||||||||||||||||||||
| Assmus et al. [96] | 2013 | ||||||||||||||||||||||
| Atienza et al. [97] | 2004 | x | x | ||||||||||||||||||||
| Austin et al. [98] | 2005 | ||||||||||||||||||||||
| Australia/New Zealand Heart Failure Group [99] | 1997 | x | |||||||||||||||||||||
| Bartunek et al. [100] | 2013 | ||||||||||||||||||||||
| Belardinelli et al. [101] | 1999 | x | |||||||||||||||||||||
| Belardinelli et al. [102] | 2012 | ||||||||||||||||||||||
| Beller et al. [103] | 1995 | x | |||||||||||||||||||||
| Bentkover et al. [104] | 2007 | x | |||||||||||||||||||||
| Beta-Blocker evaluation of survival trial [105] | 2001 | x | |||||||||||||||||||||
| Biannic et al. [106] | 2012 | ||||||||||||||||||||||
| Bielecka-Dabrowa et al. [107] | 2009 | ||||||||||||||||||||||
| Blue et al. [108] | 2001 | x | x | x | x | x | |||||||||||||||||
| Boccanelli et al. [109] | 2009 | x | x | ||||||||||||||||||||
| Böhm et al. [110] | 2016 | ||||||||||||||||||||||
| Bolli et al. [111] | 2011 | ||||||||||||||||||||||
| Boriani et al. [112] | 2017 | ||||||||||||||||||||||
| Boyne et al. [113] | 2012 | x | |||||||||||||||||||||
| Bristow et al. [114] | 1996 | x | |||||||||||||||||||||
| Brown et al. [115] | 1995 | x | |||||||||||||||||||||
| Lok et al. [116] | 2007 | x | x | ||||||||||||||||||||
| Capomolla et al. [117] | 2002 | x | x | x | x | ||||||||||||||||||
| Cazeau et al. [118] | 2001 | x | |||||||||||||||||||||
| CDMR [119] | 1988 | x | |||||||||||||||||||||
| Chan et al. [120] | 2007 | x | |||||||||||||||||||||
| Chaudhry et al. [69] | 2010 | x | x | ||||||||||||||||||||
| Chen et al. [121] | 2018 | x | |||||||||||||||||||||
| Chung [122] | 2021 | x | |||||||||||||||||||||
| CIBIS [123] | 1994 | x | x | ||||||||||||||||||||
| CIBIS-II [124] | 1999 | x | |||||||||||||||||||||
| Cicoira et al. [125] | 2002 | x | |||||||||||||||||||||
| Cleland et al. [126] | 2004 | x | |||||||||||||||||||||
| Cline et al. [127] | 1998 | ||||||||||||||||||||||
| Cohn and Tognoni [128] | 2001 | x | |||||||||||||||||||||
| Cokkinos et al. [129] | 2006 | x | |||||||||||||||||||||
| Colucci et al. [130] | 1996 | x | |||||||||||||||||||||
| Consensus et al. [241] | 2000 | x | |||||||||||||||||||||
| Cowie et al. [131] | 2014 | x | |||||||||||||||||||||
| Dalal et al. [132] | 2019 | x | |||||||||||||||||||||
| Dar et al. [133] | 2009 | x | x | x | x | ||||||||||||||||||
| Dargie [134] | 2001 | x | |||||||||||||||||||||
| Daubert et al. [135] | 2009 | x | |||||||||||||||||||||
| Dendale et al. [136] | 2012 | x | x | x | |||||||||||||||||||
| Dewalt et al. [137] | 2012 | x | |||||||||||||||||||||
| Di Biase et al. [138] | 2016 | x | x | ||||||||||||||||||||
| DIG [139] | 1997 | x | |||||||||||||||||||||
| Domenichini et al. [140] | 2016 | ||||||||||||||||||||||
| Domingo et al. [141] | 2011 | x | |||||||||||||||||||||
| Domingues et al. [142] | 2011 | x | |||||||||||||||||||||
| Doughty et al. [143] | 2002 | x | |||||||||||||||||||||
| Ducharme et al. [144] | 2005 | ||||||||||||||||||||||
| Dunagan et al. [145] | 2005 | x | x | x | |||||||||||||||||||
| Ekman et al. [146] | 1998 | x | |||||||||||||||||||||
| Ellingsen et al. [147] | 2017 | ||||||||||||||||||||||
| Erhardt et al. [148] | 1995 | x | |||||||||||||||||||||
| Fisher et al. [149] | 1994 | x | |||||||||||||||||||||
| Fox et al. [150] | 2008 | x | |||||||||||||||||||||
| Fragasso et al. [151] | 2006 | x | x | ||||||||||||||||||||
| Gallagher et al. [152] | 2017 | x | |||||||||||||||||||||
| Gasparini et al. [153] | 2006 | ||||||||||||||||||||||
| Gattis et al. [154] | 1999 | ||||||||||||||||||||||
| Giannini et al. [155] | 2016 | ||||||||||||||||||||||
| Giannuzzi et al. [156] | 2003 | x | |||||||||||||||||||||
| Giordano et al. [157] | 2009 | x | x | ||||||||||||||||||||
| Goldberg et al. [158] | 2003 | x | x | ||||||||||||||||||||
| Goldstein et al. [159] | 1999 | x | |||||||||||||||||||||
| Granger et al. [160] | 2000 | x | |||||||||||||||||||||
| Granger et al. [161] | 2003 | x | |||||||||||||||||||||
| Hamaad et al. [162] | 2005 | ||||||||||||||||||||||
| Hambrecht et al. [163] | 1995 | x | |||||||||||||||||||||
| Hambrecht et al. [164] | 2000 | ||||||||||||||||||||||
| Hamshere et al. [165] | 2015 | ||||||||||||||||||||||
| Hanconk et al. [166] | 2012 | ||||||||||||||||||||||
| Hansen et al. [167] | 2018 | ||||||||||||||||||||||
| Heldman et al. [168] | 2014 | ||||||||||||||||||||||
| Heldman et al. [168] | 2014 | ||||||||||||||||||||||
| Higgins et al. [169] | 2003 | x | x | ||||||||||||||||||||
| Hindricks et al. [170] | 2014 | ||||||||||||||||||||||
| Idris et al. [171] | 2015 | x | |||||||||||||||||||||
| Jaarsma et al. [47] | 2008 | x | |||||||||||||||||||||
| Jolly et al. [172] | 2009 | x | x | ||||||||||||||||||||
| Jones and Wong [173] | 2013 | x | x | x | x | ||||||||||||||||||
| Kashem et al. [174] | 2008 | x | x | x | |||||||||||||||||||
| Kasper et al. [175] | 2002 | x | x | x | x | x | |||||||||||||||||
| Koehler et al. [176] | 2011 | x | x | ||||||||||||||||||||
| Komajda et al. [177] | 2004 | x | |||||||||||||||||||||
| Kraai et al. [178] | 2016 | x | |||||||||||||||||||||
| Krum et al. [179] | 2013 | x | |||||||||||||||||||||
| Krumholz et al. [180] | 2002 | x | x | x | |||||||||||||||||||
| Landolina et al. [181] | 2012 | ||||||||||||||||||||||
| Laramee et al. [182] | 2003 | x | x | x | x | ||||||||||||||||||
| Linde et al. [183] | 2002 | ||||||||||||||||||||||
| Leclercq et al. [184] | 2007 | ||||||||||||||||||||||
| Linde et al. [185] | 2008 | x | x | ||||||||||||||||||||
| Liu et al. [186] | 2012 | ||||||||||||||||||||||
| Lüthje et al. [187] | 2015 | ||||||||||||||||||||||
| Luttik et al. [188] | 2014 | ||||||||||||||||||||||
| Lyngå et al. [68] | 2012 | x | x | ||||||||||||||||||||
| MacDonald et al. [189] | 2011 | x | x | x | x | ||||||||||||||||||
| Maggioni et al. [190] | 2002 | x | |||||||||||||||||||||
| Margulies et al. [191] | 2016 | x | |||||||||||||||||||||
| Marrouche et al. [192] | 2018 | x | x | x | x | ||||||||||||||||||
| Martinelli et al. [193] | 2010 | ||||||||||||||||||||||
| Mathiasen et al. [194] | 2015 | ||||||||||||||||||||||
| Menasché [195] | 2008 | ||||||||||||||||||||||
| Mcdonald et al. [196] | 2002 | x | |||||||||||||||||||||
| McMurray et al. [197] | 2003 | x | |||||||||||||||||||||
| MERIT-HF [198] | 1999 | x | x | ||||||||||||||||||||
| Morgan et al. [199] | 2017 | ||||||||||||||||||||||
| Mortara et al. [200] | 2009 | x | x | x | |||||||||||||||||||
| Moss et al. [201] | 2002 | x | |||||||||||||||||||||
| Moss et al. [202] | 2009 | x | x | x | |||||||||||||||||||
| Mozid et al. [203] | 2014 | ||||||||||||||||||||||
| Mueller et al. [204] | 2007 | x | |||||||||||||||||||||
| Node et al. [205] | 2003 | ||||||||||||||||||||||
| Obadia et al. [206] | 2018 | ||||||||||||||||||||||
| Packer et al. [207] | 1993 | x | |||||||||||||||||||||
| Packer et al. [208] | 1996 | x | |||||||||||||||||||||
| Packer et al. [209] | 1996 | x | |||||||||||||||||||||
| Packer et al. [210] | 2001 | x | |||||||||||||||||||||
| Passino et al. [211] | 2006 | x | |||||||||||||||||||||
| Patel et al. [212] | 2015 | ||||||||||||||||||||||
| Pätilä et al. [213] | 2014 | ||||||||||||||||||||||
| Perin et al. [214] | 2012 | ||||||||||||||||||||||
| Peters-klimm et al. [215] | 2010 | x | |||||||||||||||||||||
| Pfeffer et al. [216] | 1992 | x | |||||||||||||||||||||
| Piepoli et al. [217] | 2008 | x | |||||||||||||||||||||
| Pinter et al. [218] | 2009 | x | |||||||||||||||||||||
| Pitt et al. [219] | 1999 | x | x | ||||||||||||||||||||
| Pitt et al. [220] | 2003 | x | |||||||||||||||||||||
| Pokushalov et al. [221] | 2010 | x | |||||||||||||||||||||
| Pokushalov et al. [222] | 2011 | x | |||||||||||||||||||||
| Prabhu et al. [223] | 2017 | x | x | x | x | ||||||||||||||||||
| Ramachandran et al. [224] | 2007 | x | |||||||||||||||||||||
| Rosano et al. [225] | 2003 | x | x | ||||||||||||||||||||
| Ruschitzka et al. [226] | 2013 | x | |||||||||||||||||||||
| Sardu et al. [227] | 2016 | x | |||||||||||||||||||||
| Scherr et al. [228] | 2009 | x | x | ||||||||||||||||||||
| Schou et al. [229] | 2013 | ||||||||||||||||||||||
| Sisk et al. [230] | 2006 | x | |||||||||||||||||||||
| Smith et al. [231] | 2014 | ||||||||||||||||||||||
| Sola et al. [232] | 2006 | ||||||||||||||||||||||
| Yusuf et al. [233] | 1991 | x | x | ||||||||||||||||||||
| Yusuf et al. [234] | 1992 | x | x | ||||||||||||||||||||
| Spargias et al. [235] | 1999 | ||||||||||||||||||||||
| Stone et al. [236] | 2018 | ||||||||||||||||||||||
| Sturm et al. [237] | 2000 | x | |||||||||||||||||||||
| Swedberg et al. [238] | 2010 | x | x | ||||||||||||||||||||
| Takano et al. [239] | 2013 | ||||||||||||||||||||||
| Tang et al. [240] | 2010 | x | x | x | |||||||||||||||||||
| Consensus et al. [241] | 2000 | x | |||||||||||||||||||||
| Thibault et al. [242] | 2011 | x | |||||||||||||||||||||
| Thibault et al. [243] | 2013 | x | |||||||||||||||||||||
| Tsuyuki et al. [244] | 2004 | x | x | ||||||||||||||||||||
| Tuunanen et al. [245] | 2008 | x | x | ||||||||||||||||||||
| Udelson et al. [246] | 2010 | ||||||||||||||||||||||
| Uretsky et al. [247] | 1993 | x | |||||||||||||||||||||
| van Veldhuisen et al. [248] | 2009 | x | |||||||||||||||||||||
| van Veldhuisen et al. [249] | 2011 | ||||||||||||||||||||||
| Villani et al. [250] | 2007 | x | |||||||||||||||||||||
| Villani et al. [251] | 2014 | ||||||||||||||||||||||
| Vitale et al. [252] | 2004 | x | x | ||||||||||||||||||||
| Vizzardi et al. [253] | 2010 | x | |||||||||||||||||||||
| Vrtovec et al. [254] | 2008 | ||||||||||||||||||||||
| Vuorinen et al. [255] | 2014 | x | |||||||||||||||||||||
| Weintraub et al. [256] | 2010 | x | |||||||||||||||||||||
| Wierzchowiecki et al. [257] | 2006 | x | |||||||||||||||||||||
| Willenheimer et al. [258] | 2001 | x | |||||||||||||||||||||
| Wojnicz et al. [259] | 2006 | ||||||||||||||||||||||
| Xie et al. [260] | 2010 | ||||||||||||||||||||||
| Yamada et al. [261] | 2007 | ||||||||||||||||||||||
| Young et al. [262] | 2003 | ||||||||||||||||||||||
| Zan [263] | 2020 | ||||||||||||||||||||||
| Zannad et al. [264] | 2011 | x | x | x | |||||||||||||||||||
| Zannad et al. [265] | 2018 | x |
1, Adamson et al. [266]; 2, Agasthi et al. [267]; 3, Al-Majed et al. [268]; 4, Alotaibi et al. [269]; 5, AlTurki et al. [270]; 6, Benito-González et al. [271]; 7, Bertaina et al. [272]; 8, Bjarnason-Wehrens et al. [273]; 9, Bonsu et al. [274]; 10, Carbo et al. [275]; 11, de Vecchis et al. [276]; 12, Driscoll et al. [277]; 13, Emdin et al. [278]; 14, Fisher et al. [279]; 15, Fisher et al. [280]; 16, Gandhi et al. [281]; 17, Halawa et al. [282]; 18, Hartmann et al. [283]; 19, Hu et al. [284]; 20, Inglis et al. [285]; 21, Inglis et al. [286]; 22, Japp et al. [287]; 23, Jonkman et al. [288]; 24, Kang et al. [289]; 25, Klersy et al. [290]; 26, Komajda et al. [291]; 27, Le et al. [292]; 28, Ma et al. [293]; 29, Malik et al. [294]; 30, Moschonas et al. [295]; 31, Pandor et al. [296]; 32, Shah et al. [297]; 33, Sulaica et al. [298]; 34, Taylor et al. [299]; 35, Thomas et al. [300]; 36, Thomsen et al. [301]; 37, Tse et al. [302]; 38, Tu et al. [303]; 39, Turagam et al. [304]; 40, Uminski et al. [305]; 41, Xiang et al. [306]; 42, Zhang et al. [307]; 43, Zhang et al. [308]; 44, Zhou and Chen [309]
Table 4.
Baseline characteristics of RCTs
| Included RCTs | N (intervention) | N (control) | Total | Mean age | % Male | Mean LVEF | Intervention | Control | Follow-up period |
|---|---|---|---|---|---|---|---|---|---|
| Beller et al. [103] | 130 | 63 | 193 | 61 | 76 | 28 | Initial oral dose of 5 mg of lisinopril. The dose of diuretic therapy was adjusted based on the clinical condition of the patient, particularly to control edema | Matching placebo | 3 months |
| Brown et al. [115] | 116 | 125 | 241 | 62 | 82 | 25 | The 24-week double-blind treatment period beginning with 10 mg of fosinopril. In the ensuing 3 weeks, patients were titrated to 20 mg of study medication (level TI), as tolerated | Matching placebo | N/R |
| CDMR [119] | 200 | 100 | 300 | 57 | 83 | 25 | Captopril (25 to 50 mg, three times a day) | Placebo | 6 months |
| Consensus et al. [241] | 127 | 126 | 263 | 71 | 56 | < 40 | Enalapril (2.5 to 40 mg/day) | Placebo | 12 months |
| Erhardt et al. [148] | 155 | 153 | 308 | 64 | 76 | 27 | Fosinopril 10 mg | Matching placebo | 12 weeks |
| Pfeffer et al. [216] | 1115 | 1116 | 2231 | 60 | 83 | 31 | Captopril | Placebo | 36 months |
| Yusuf et al. [233] | 1285 | 1284 | 2569 | 61 | 81 | 25 | Enalapril | Placebo | 41 months |
| Yusuf et al. [234] | 2111 | 2117 | 4228 | 59 | 89 | 28 | Enalapril | Placebo | 42 months |
| Cleland et al. [126] | 89 | 190 | 279 | 63 | 74 | < 35 | Warfarin with INR of 2.5 | Aspirin or no antithrombotic therapy | 27 months |
| Cokkinos et al. [129] | 92 | 105 | 197 | 59 | 85 | 28 | Warfarin was supplied as 5-mg tablets. The daily dose was 2.5–10 mg, with a target INR of 2–3 | Placebo | 19.5 months |
| Zannad et al. [265] | 2507 | 2515 | 5022 | 66 | 77 | 34 | Rivaroxaban 2.5 mg twice daily | Placebo | 21 months |
| Cohn and Toghoni [128] | 2511 | 2499 | 5010 | 63 | 80 | 27 | Valsartan was initiated at a dose of 40 mg twice daily, and the dose was doubled every 2 weeks until a target dose of 160 mg twice daily was reached | Placebo | 23 months |
| Granger et al. [160] | 179 | 91 | 270 | 66 | 25 | 26 | Candesartan, 4 mg, 8 mg and 16 mg | Matching placebo | 12 months |
| Granger et al. [161] | 1013 | 1015 | 2028 | 66 | 68 | 30 | Candesartan, 4 mg, 8 mg, 16 mg, 32 mg | Matching placebo | 34 months |
| Maggioni et al. [190] | 185 | 181 | 67 | 63 | 71 | 28 | Valsartan | Placebo | 12 months |
| McMurray et al. [197] | N/R | N/R | 7599 | 67 | 40 | 54 | Candesartan | Matching placebo | N/R |
| Spargias et al. [235] | 1734 | 243 | 1977 | 67 | 74 | 40 | Ramipril | Placebo | N/R |
| Sturm [237] | 51 | 49 | 100 | 52 | 90 | 17 | Atenolol | Placebo | 24 months |
| Australia/New Zealand Heart Failure Research Collaborative Group [99] | 208 | 207 | 415 | 67 | 80 | 29 | Carvedilol | Matching placebo | 19 months |
| Beta-Blocker evaluation of survival trial [105] | 1354 | 1354 | 2708 | 60 | 79 | 23 | Initial oral dose of 3 mg of bucindolol, which was repeated twice daily for 1 week | Placebo | 24 months |
| Bristow et al. [114] | 261 | 84 | 345 | 60 | 78 | 23 | Low-dose Carvedilol (6.25 mg BID), medium-dose Carvedilol (12.5 mg BID), and high-dose Carvedilol (25 mg BID) | Placebo | 6 months |
| CIBIS [123] | 320 | 321 | 641 | N/R | N/R | 25 | 2.5 mg Bisoprolol | 2,5 mg placebo | 1.9 years |
| CIBIS-II [124] | N/R | N/R | N/R | N/R | N/R | 28 | Bisoprolol 1.25 mg | Placebo | 1.3 years |
| Colucci et al. [130] | 232 | 134 | 366 | 55 | 86 | 23 | Carvedilol | Placebo | 213 days |
| Dargie [134] | 975 | 984 | 1959 | 63 | 74 | 33 | Carvedilol | Identical looking placebo | 1.3 years |
| Fisher et al. [149] | 25 | 25 | 50 | 63 | 100 | 22 | Metoprolol, from 6.25 to 12.5 mg twice a day to 12.5 mg three times a day to 25 mg twice a day | Placebo | 6 months |
| Goldstein et al. [159] | 40 | 20 | 60 | N/R | N/R | 27 | The initial dose of approximately 12.5 mg Metoprolol (one half of a 25 mg tablet) was administered once daily. The dose of metoprolol was increased to 25 mg and subsequently increased in steps of 50 mg to 100 mg and finally to 150 mg once daily | Matching placebo | 26 weeks |
| Komajda [177] | N/R | N/R | 572 | N/R | N/R | < 40 | Enalapril | Matching placebo | N/R |
| Merit-HF [198] | 1990 | 2001 | 3991 | 64 | 78 | 28 | Metoprolol | Placebo | 1 year |
| Packer et al. [208] | 133 | 145 | 278 | 61 | 73 | 22 | Carvedilol, 25–50 mg BID | Placebo | 6 months |
| Packer et al. [209] | 696 | 398 | 1094 | 58 | 77 | 23 | Carvedilol | Placebo | 6.5 months |
| Packer et al. [210] | 1156 | 1133 | 2289 | 63 | 80 | 20 | Carvedilol | Placebo | 10.4 months |
| van Veldhuisen et al. [248] | 678 | 681 | 1359 | 76 | 70 | 29 | Nebivolol | Placebo | 21 months |
| Di Biase [138] | 102 | 101 | 203 | 74 | 60 | 29 | PVI + LAPWI + SVCI + CFAE | AMIO therapy | 24 months |
| Jones and Wong [173] | 26 | 26 | 52 | 63 | 87 | 22 | PVI + linear then CFAEs | Rate control | 12 months |
| MacDonald et al. [189] | 22 | 19 | 41 | 63 | 78 | 20 | PVI ± linear lesions + CFAEs | Rate control | 6 months |
| Marrouche et al. [192] | 179 | 184 | 363 | 85 | 61 | 32 | PVI + / − additional lesions at discretion of operator | Rate and/or rhythm control | 38 months |
| Prabhu et al. [223] | 33 | 33 | 66 | 91 | 61 | 35 | PVI + LAPWI | Rate control | 6 months |
| DIG [139] | 3397 | 3403 | 6800 | 64 | 78 | 29 | Digoxin | Placebo | 37 months |
| Packer et al. [207] | 85 | 93 | 178 | 61 | 76 | 28 | Digoxin | Placebo | 3 months |
| Uretsky et al. [247] | 42 | 46 | 88 | 64 | 90 | 29 | Digoxin | Withdrawal of digoxin | 3 months |
| Assmus et al. [95] | 24 | 23 | 47 | 61 | 100 | 39–41 | Intracoronary infusion of BMC or CPC | No cell infusion | 3 months |
| Assmus et al. [96] | 64 | 39 | 103 | 65 | 90 | 32–37 | Intracoronary infusion of BMCs | Cell-free medium (placebo) | 45.7 months |
| Bartunek et al. [100] | 32 | 15 | 47 | 59 | 91 | 28 | Patients in the cell therapy arm received bone marrow–derived cardiopoietic stem cells meeting quality release criteria | Standard of care comprising a beta-blocker, an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and a diuretic with dosing and schedule tailored for maximal benefit and tolerability in accordance with practice guidelines for heart failure management | 2 years |
| Bolli et al. [111] | 16 | 7 | 23 | 57 | 100 | 30 | Autologous CSCs were isolated from the right atrial appendage and re-infused intracoronarily 4 ± 1 months after surgery; | No treatment | 12 months |
| Hamshere et al. [165] | 15 | 15 | 30 | 56 | 86 | 42 | G-CSF + BMSC | Peripheral placebo (saline) | 12 months |
| Heldman et al. [168] | 22 | 11 | 33 | 60 | 95 | 38–40 | Mesenchymal stem cell group or bone marrow mononuclear cell group | Placebo | 12 months |
| Heldman et al. [168] | 38 | 21 | 59 | 61 | 100 | 36 | Mesenchymal stem cell group or bone marrow mononuclear cell group | Placebo | 12 months |
| Mathiasen et al. [194] | 40 | 20 | 60 | 66 | 90 | 28 | BMSC | Placebo | 6 months |
| Menasché [195] | 63 | 34 | 97 | 61 | 100 | 29 | Cell suspension | Placebo solution consisting of the suspension medium without skeletal myoblasts | 72 months |
| Mozid et al. [203] | 14 | 2 | 16 | 70 | 94 | 31 | G-CSF + BMSC | Placebo | 6 months |
| Patel et al. [212] | 24 | 6 | 30 | 59 | 100 | 26 | BMAC infusion | Standard heart failure care | 12 months |
| Pätilä et al. [213] | 20 | 19 | 39 | 65 | 95 | 37 | Injections of BMMC or vehicle intra-operatively into the myocardial infarction border area | Controls received only vehicle medium by syringes | 12 months |
| Perin et al. [214] | 20 | 10 | 30 | 61 | 80 | 39 | Transendocardial delivery of ABMMNCs | Placebo | 6 months |
| Austin et al. [98] | 100 | 100 | 200 | 60 | 66 | 85% < 35 | An 8-week cardiac rehabilitation program that was coordinated by the clinical nurse specialist. Patients attended classes twice weekly for a period of 2.5 h | Eight weekly monitoring of clinical status (functional performance, fluid status, cardiac rhythm, laboratory assessment) in the cardiology outpatients by the clinical nurse specialist | 8 weeks |
| Belardinelli et al. [101] | 50 | 49 | 99 | 59 | 88 | 28 | The exercise group underwent exercise training for 14 months | The control group did not exercise | 14 months |
| Belardinelli et al. [102] | 63 | 60 | 123 | 59 | 78 | 37 | The trained group underwent an ET program for 10 years. The training program consisted of 3 sessions per week at the hospital for 2 months, then 2 supervised sessions the rest of the year. Every 6 months, patients exercised at the hospital, and then they returned to a coronary club, where they exercised the rest of the year | The nontrained group was not provided with a formal ET program | 120 months |
| Chen et al. [121] | 19 | 18 | 27 | 61 | 36 | 36 | Outpatient cardiac rehabilitation for 1 week, before starting home-based cardiac rehabilitation. Home-based cardiac rehabilitation was conducted by requesting the interventional group to carry out aerobic exercise at least 3 times per week, for a duration of at least 30 min each time | Instructed to maintain both their standard medical care and previous activity levels | 3 months |
| Cowie et al. [131] | 30 | 16 | 46 | 64 | 91 | The hospital group attended a physiotherapist-led class | A DVD and booklet (replicating the class) was created for home use. Controls followed their usual HFNS care | 5 years | |
| Dalal et al. [132] | 107 | 109 | 216 | 70 | 78 | 35 | REACH-HF manual for patients with a choice of two structured exercise programs | No cardiac rehabilitation approach that included medical management according to national and local guidelines, including specialist heart failure nurse care | 12 weeks |
| Ellingsen et al. [147] | 78 | 81 | 261 | 60 | 81 | 29 | HIIT and MCT had 3 supervised sessions per week on a treadmill or bicycle. HIIT included four 4-min intervals aiming at 90 to 95% of maximal heart rate separated by 3-min active recovery periods of moderate intensity. MCT sessions aimed at 60 to 70% of maximal heart rate | Patients were advised to exercise at home according to current recommendations and attended a session of moderate-intensity training at 50 to 70% of maximal heart rate every 3 weeks | 3 months |
| Giannuzzi et al. [156] | 45 | 45 | 90 | 60 | N/R | 25 | The exercise protocol consisted of supervised continuous sessions of 30-min bicycle ergometry > 3 times a week (3 to 5 times) at 60% of the peak V˙ O2 achieved at the initial symptom-limited exercise testing. In addition to supervised sessions, patients were asked to take a brisk daily walk for > 30 min and intermittent unsupervised sessions of calisthenics (30 min) as part of the home-based exercise program | Educational support, but no formal exercise protocol | 6 months |
| Hambrecht et al. [163] | 12 | 10 | 22 | 52 | 27 | 26 | Patients assigned to the training program remained in an intermediate care ward for the initial 3 weeks. Training sessions were conducted individually under strict supervision for the first 3 weeks. Patients exercised six times daily for 10 min on a bicycle ergometer | Patients assigned to the control group spent 3 days in an intermediate care ward for baseline evaluation. After discharge, medical therapy was continued, and patients were supervised by their private physicians | 6 months |
| Hambrecht et al. [164] | 36 | 37 | 73 | 54 | 100 | 27 | 2 weeks of in-hospital ergometer exercise for 10 min 4 to 6 times per day, followed by 6 months of home-based ergometer exercise training for 20 min per day at 70% of peak oxygen uptake | No intervention | 6 months |
| Jolly et al. [172] | 84 | 85 | 169 | 66 | 75 | < 40 | Three supervised exercise sessions to plan an individualized exercise program. These were followed by a home-based program, with home visits at 4, 10, and 20 weeks, telephone support at 6, 15, and 24 weeks, and a manual with details about safe progressive exercise and self-monitoring of frequency, duration, and intensity | Specialist heart failure nurse input in primary and secondary care through clinic and home visits that included the provision of information about heart failure, advice about self-management and monitoring of their condition, and titration of beta-blocker therapy | 3 months |
| Mueller et al. [204] | 25 | 25 | 50 | 55 | 100 | < 40 | Five indoor cycling sessions were performed weekly for a duration of 30 min, and all subjects walked outdoors for 45 min twice daily. Training duration was one month | Control subjects received usual clinical care, including verbal encouragement to remain physically active | 1 month |
| Passino et al. [211] | 44 | 41 | 85 | N/R | N/R | 35 | The training group underwent a nine-month training program. The training program consisted of cycling on a bike for a minimum of 3 days per week, 30 min per day | Control patients continued their usual lifestyle | 9 months |
| Willenheimer et al. [258] | 27 | 27 | 54 | N/R | N/R | 35 | Patients carried out cycle ergometer interval training at a heart rate corresponding to 80% of peak-VO2 ± 5 beats/min, for as long as possible during each interval | Control patients were asked not to change their degree of physical activity during the active study period | 6 months |
| Abraham et al. [87] | 228 | 225 | 453 | 64 | 68 | 22 | Atrial-synchronized biventricular pacing | No pacing for six months, during which time medications for heart failure were to be kept constant | 6 months |
| Abraham et al. [88] | 101 | 85 | 186 | 64 | 89 | 25 | Optimal medical treatment with active CRT and active ICD therapy | Optimal medical treatment and active ICD therapy | 6 months |
| Bentkover et al. [104] | 36 | 36 | 72 | 79 | 79 | < 35 | Biventricular pacing and ICD | ICD alone | 6 months |
| Cazeau et al. [118] | 29 | 29 | 58 | 63 | 75 | 23 | Atriobiventricular (active) pacing | Ventricular inhibited (inactive) pacing | 3 months |
| Chung [122] | 9 | 9 | 18 | 76 | 76 | 30 | A CRT–defibrillator device with LV coronary venous lead system | A dual-chamber ICD | 12 months |
| Daubert et al. [135] | 82 | 180 | 262 | 81 | 81 | 28 | Patients who had undergone successful implantation were randomly assigned in a 2-to-1 scheme to a CRT ON group for 24 months | CRT OFF | 24 months |
| Gasparini et al. [153] | 33 | 36 | 69 | 67 | 94 | 26 | BiV CRT | LV | 12 months |
| Higgins et al. [169] | 245 | 245 | 490 | 66 | 84 | 22 | CRT-D | ICD | 6 months |
| Linde et al. [183] | 25 | 18 | 43 | 66 | 84 | 30 | Biventricular VVIR pacing during two 3-month periods | Right-univentricular VVIR pacing during two 3-month periods | 3 months |
| Leclercq et al. [184] | 25 | 19 | 44 | 74 | 100 | 27 | Biventricular VVIR pacing during two 3-month periods | Right-univentricular VVIR pacing during two 3-month periods | 3 months |
| Linde et al. [185] | 419 | 191 | 610 | 79 | 79 | 27 | Active CRT | Control | 12 months |
| Martinelli et al. [193] | 27 | 27 | 54 | 59 | 68 | 30 | Device was initially programmed to BiVP, crossed to RVP and crossed back to BiVP | Device was initially programmed to RVP, crossed to BiVP and crossed back to RVP | 18 months |
| Moss et al. [201] | 742 | 490 | 1232 | 65 | 85 | 23 | ICD | Conventional medical therapy | 20 months |
| Moss et al. [202] | 1089 | 731 | 1820 | 75 | 75 | 24 | Cardiac-resynchronization therapy with biventricular pacing | ICD alone | 2.4 years |
| Piepoli et al. [217] | 44 | 45 | 89 | 72 | 72 | 24 | CRT-P/CRT-D | Medical | 12 months |
| Pinter et al. [218] | 36 | 36 | 72 | 79 | 79 | 23 | CRT-D | ICD | 6 months |
| Pokushalov et al. [221] | 91 | 87 | 178 | 90 | 90 | 29 | CRT-P + CABG | CABG | 18 months |
| Pokushalov et al. [222] | 13 | 13 | 26 | 96 | 96 | 27 | BMMC + active CRT | BMMC + inactive CRT | 6 months |
| Ruschitzka et al. [226] | 404 | 405 | 809 | 72 | 72 | 27 | CRT capability turned on | CRT capability turned off | 19.4 months |
| Tang et al. [240] | 894 | 904 | 1798 | 83 | 83 | 23 | ICD + CRT | ICD alone | 40 months |
| Thibault et al. [242] | 60 | 61 | 121 | 75 | 75 | 24 | biventricular CRT | LV CRT | 6 months |
| Thibault et al. [243] | 44 | 41 | 85 | 71 | 71 | 25 | CRT-D | ICD | 12 months |
| Young et al. [262] | 182 | 187 | 369 | 68 | 78 | 24 | Combined CRT and ICD capabilities | ICD activated, CRT off | 6 months |
| Fragasso et al. [151] | 34 | 31 | 65 | 65 | 96 | 35 | Trimetazidine, 20 mg three times daily | Placebo | 13 months |
| Rosano et al. [225] | 16 | 16 | 32 | 66 | 75 | 33 | 20 mg t.d.s. trimetazidine | Placebo t.d.s | 6 months |
| Tuunanen et al. [245] | 12 | 7 | 19 | 58 | 79 | 34 | Trimetazidine | Placebo | 3 months |
| Vitale et al. [252] | 23 | 24 | 47 | 78 | 85 | 29 | Trimetazidine | Placebo | 6 months |
| Margulies et al. [191] | 154 | 146 | 300 | 62 | 69 | 25 | Liraglutide | Placebo | 6 months |
| Fox et al. [150] | 5479 | 5438 | 10,917 | 60 | 83 | 32 | Ivabradine 7.5 MG BID | Placebo | 19 months |
| Swedberg et al. [238] | 3241 | 3264 | 6505 | 65 | 76 | 29 | Ivabradine 7.5 MG BID | Placebo | 22.9 months |
| Asgar et al. [94] | 50 | 42 | 92 | 75 | 77 | 38 | Treated with the MitraClip | This retrospective comparator group consisted of medically managed patients | 22–33 months |
| Giannini et al. [155] | 60 | 60 | 120 | 76 | 70 | 34 | MitraClip | Optimal medical therapy | 17 months |
| Obadia et al. [206] | 152 | 152 | 304 | 71 | 79 | 33 | Percutaneous mitral-valve repair | medical therapy alone | 12 months |
| Stone et al. [236] | 302 | 312 | 614 | 73 | 67 | 31 | Transcatheter mitral-valve repair plus medical therapy | Medical therapy alone | 16.5 months |
| Boccanelli et al. [109] | 188 | 193 | 381 | 63 | 84 | 40 | Canrenone | Placebo | 12 months |
| Chan et al. [120] | 23 | 25 | 48 | 63 | 83 | 27 | Candesartan 8 mg and spironolactone 25 mg once daily | Candesartan 8 mg and a matching identical placebo once daily | 12 months |
| Cicoira et al. [125] | 54 | 52 | 106 | 67 | 86 | 33 | Spironolactone treatment, at an initial dose of 25 mg once daily | Placebo | 12 months |
| Pitt et al. [219] | 822 | 841 | 1663 | 65 | 73 | 25 | Spironolactone, 25 mg | Matching placebo | 24 months |
| Pitt et al. [220] | 3319 | 3313 | 6632 | 64 | 71 | 33 | Eplerenone | Placebo | 16 months |
| Udelson et al. [246] | 116 | 109 | 225 | 63 | 84 | 27 | Eplerenone, 50 mg/d | Placebo | 9 months |
| Vizzardi et al. [253] | 65 | 65 | 130 | 65 | N/R | 36 | 25 mg of spironolactone once daily | Matching placebo | 44 months |
| Zannad et al. [264] | 1364 | 1373 | 2737 | 69 | 78 | 26 | Eplerenone 50 mg/d | Placebo | 21 months |
| Atienza et al. [97] | 164 | 174 | 338 | 68 | 60 | 36 | 1 individual session prior to discharge by nurse, 1 visit to physician, 3-monthly follow-up visits and tele-monitoring | Usual care (discharge planning according to protocol) | 509 days |
| Blue et al. [108] | 84 | 81 | 165 | 75 | 48 | Severe 40% | Planned home visits of decreasing frequency, supplemented by telephone contact as needed. The aim was to educate the patient about heart failure and its treatment, optimize treatment (drugs, diet, exercise), monitor electrolyte concentrations, teach self-monitoring and management, liaise with other health care and social workers as required, and provide psychological support | Patients in the usual care group were managed as usual by the admitting physician and, subsequently, general practitioner. They were not seen by the specialist nurses after hospital discharge | 12 months |
| Lok et al. [116] | 118 | 122 | 240 | 71 | 79 | 31 | An intensive follow-up of the patients during 1 year at a HF outpatient clinic led by a HF physician and a cardiovascular nurse. Verbal and written comprehensive education was imparted about the disease and the aetiology, medication, compliance and possible adverse events. Patients were advised about individualized diet with salt and fluid restriction, weight control, early recognition of worsening HF, when to call a healthcare provider, and about physical exercise and rest. An appointment with a dietician was made. The nurse asked the patient about his or her social and medical circumstances and performed a short physical examination. The physician assessed the clinical condition of the patient, the laboratory results and ECG, performed a physical examination, and, together with the nurse, proposed a treatment regimen | Their routine care was no doubt largely according to the guideline of the European Society of Cardiology prevailing at that time (version 2001), with optimal application of medical therapy including the target dose or high dose of HF medication | 12 months |
| Capomolla et al. [117] | 112 | 122 | 234 | 56 | 84 | 31 | The objectives of the multidisciplinary staff are prevention and functional recovery of consequences of acute hemodynamic instabilization | Patients were referred to their primary care physician and cardiologist. During follow-up the process of care was driven by the patient’s needs into a heterogeneous range of emergency room management, hospital admission, and outpatient access | 12 months |
| Cline et al. [127] | 80 | 110 | 190 | 76 | 55 | 36 | The education program consisted of two 30-min information visits by a nurse during primary hospitalization and a 1-h information visit for patients and family 2 weeks after discharge | Routine clinical practice | 1 year |
| Dendale et al. [136] | 80 | 80 | 160 | 76 | 65 | 33 | Patients were seen in the outpatient heart failure clinic with additional planned visits at 3 and 6 months. Daily patient telemonitoring was conducted with specified alert limits set for each patient. Alterations in patient status were forwarded to the general practitioner and heart failure clinic for subsequent patient follow-up and management | Usual care | 6 months |
| Dewalt et al. [137] | 303 | 302 | 605 | 61 | 52 | < 40 | The intervention began with a 1-h educational session with a clinical pharmacist or health educator during a regular clinic visit. Patients were given an educational booklet designed for low literacy patients and a digital scale. As part of the educational session, patients were taught to identify signs of heart failure exacerbation, perform daily weight assessment, and adjust their diuretic dose. The program coordinator then made scheduled follow-up phone calls and monthly during months | Patients enrolled in the control group received a general heart failure education pamphlet written at approximately the 7th grade level and continued with usual care from their primary physician | 12 months |
| Doughty et al. [143] | 100 | 97 | 197 | 74 | 56 | 34 | One-on-one education with the study nurse was initiated at the first clinic visit. A patient diary, for daily weights, medication record, clinical notes and appointments, and education booklet were provided. Group education sessions (each lasting 1.5–2 h) were offered, two within 6 weeks of hospital discharge and a further after 6 months | Continued under the care of their GP with additional follow-up measures as usually recommended by the medical team responsible for their in-patient care | 12 months |
| Ducharme et al. [144] | 115 | 115 | 230 | 70 | 73 | 35 | Patients in the intervention group were referred to a multidisciplinary specialized heart failure outpatient clinic where they were evaluated by the study team within 2 weeks of hospital discharge | Received treatment and appropriate follow-up according to the standards of the attending physicians but without further direct contact with the research team or the planned intervention | 6 months |
| Ekman et al. [146] | 79 | 79 | 158 | N/R | N/R | 43 | The structured-care program was based on a nurse monitored, outpatient clinic, run in cooperation with the study doctors, who were responsible for optimal pharmacological treatment | Usual care | 5 months |
| Gallagher et al. [152] | 20 | 20 | 40 | 64 | 75 | 25 | A licensed clinical social worker reviewed adherence data daily during the first 7 days after discharge and weekly thereafter and contacted participants who were nonadherent for two or more days per week. During these phone calls, the social worker inquired about consequences of nonadherence, and assessed and responded to reasons for missed doses | For participants assigned to passive monitoring, adherence data were recorded but not monitored by the study team | 1 months |
| Hancock et al. [166] | 16 | 12 | 28 | 85 | 44 | 43 | An assessment visit by a consultant cardiologist who initiated a plan of treatment, followed by visits at one to two weekly intervals within the home by heart failure specialist nurses. The HFSNs enacted the plan, including blood tests, assessment of symptoms and signs, educational advice, and medication titration | Routine care | 6 months |
| Jaarsma et al. [47] | 340 | 339 | 679 | 72 | 66 | 34 | (A) 2 individual session by cardiologist, 9 visits to nurse, possibility to contact nurse (B) 2 individual sessions by cardiologist, 18 visits to nurse, 2 home visits, 2 multidisciplinary sessions, follow-up telephone contact by nurse | Usual care (standard management by cardiologist) | 18 months |
| Kasper et al. [175] | 102 | 98 | 200 | 62 | 61 | 27 | Patients received nurse-led care coordination linked to a multidisciplinary team composed of a heart failure nurse, cardiologist, and patient’s primary care physician. Patients were contacted via telephone at preplanned intervals after discharge, in addition to scheduled visits within the community | Patients received unrestricted follow-up care from their primary physicians, who received a baseline heart failure management plan, as documented in the patient's chart | 6 months |
| Krumholz et al. [180] | 44 | 44 | 88 | 74 | 57 | 38 | The study intervention was based on five sequential care domains for chronic illness, including patient knowledge of the illness, the relation between medications and illness, the relation between health behaviors and illness, knowledge of early signs and symptoms of decompensation and where and when to obtain assistance | Patients assigned to the control group received all usual care treatments and services ordered by their physicians | 12 months |
| Liu et al. [186] | 53 | 53 | 200 | 63 | 66 | 29 | The patient was cared for by an HF team consisting of 3 cardiologists specialized in HF care, one psychologist, one dietary assistant, and two case managers | The primary care physician was responsible for patient evaluation, treatment and clinic visits. Neither scheduled follow-up nor specialized HF nurses were available | 6 months |
| Luttik et al. [188] | 92 | 97 | 200 | 73 | 63 | 32 | Follow-up by the HF clinic | Follow-up by their GP | 12 months |
| Lyngå et al. [68] | 166 | 153 | 344 | 73 | 75 | 57% < 30 | Patients randomized to the IG were given an electronic scale to install in their homes | The patients in the CG were informed to contact the HF clinic on a special telephone in the case of a weight gain of .2 kg in 3 days | 12 months |
| Mcdonald et al. [196] | 51 | 47 | 98 | 71 | 66 | 37 | Patients systematically received specialist nurse-led education and specialist dietitian consults on three or more occasions during the index admission. The education program focused on daily weight monitoring, disease and medication understanding, and salt restriction | Patients underwent investigations for HF, including echocardiography and right and left heart catheterization where indicated. Optimal medical therapy was administered | 3 months |
| Schou et al. [229] | 460 | 460 | 200 | 69 | 63 | 32 | Patients allocated to an extended follow-up completed the following program: visits at 1–3-month intervals at the discretion of the investigators | Usual care by a GP | 9 months |
| Smith et al. [231] | 92 | 106 | 198 | 63 | 66 | 30 | The intervention began with four weekly group visit appointments followed by a 5th “booster” appointment held 6 months after randomization | HF care from their existing treatment team both during and after hospitalization | 12 months |
| Tsuyuki et al. [244] | 140 | 136 | 276 | 74 | 55 | 31 | The essential components of the patient support program were simplified into 5 basic areas: salt and fluid restriction, daily weighing, exercise alternating with rest periods, proper medication use, and knowing when to call their physician (early recognition of worsening symptoms) | Usual care | 6 months |
| Wierzchowiecki et al. [257] | 64 | 65 | 129 | 81 | N/R | 36 | Multidisciplinary care | Routine care | 6 months |
| Bielecka-Dabrowa et al. [107] | 41 | 27 | 68 | 57 | 85 | 29 | Atorvastatin 40 mg daily for 2 months (8 weeks) and next 10 mg for 4 months | DCM was treated according to present standards without statin therapy | 6 months |
| Hamaad et al. [162] | 12 | 9 | 23 | 67 | 86 | 32 | Atorvastatin, 40 mg once daily | Placebo | 32.8 months |
| Node et al. [205] | 23 | 25 | 48 | 48 | 69 | 34 | Simvastatin | Placebo | 3.5 months |
| Sola et al. [232] | 54 | 54 | 108 | 33 | 63 | 33 | Atorvastatin | No statin treatment | 12 months |
| Takano et al. [239] | 288 | 286 | 577 | 63 | N/R | 34 | Pitavastatin | Control | 35.5 months |
| Vrtovec et al. [254] | 55 | 55 | 110 | 62 | 61 | 25 | Atorvastatin (10 mg/day) | No statins | 12 months |
| Wojnicz et al. [259] | 36 | 38 | 74 | 38 | 81 | 28 | Atorvastatin | Placebo | 6 months |
| Xie et al. [260] | N/R | N/R | 81 | N/R | N/R | 38 | Atorvastatin (10–20 mg/day) | Routine treatment | 12 months |
| Yamada et al. [261] | 19 | 19 | 38 | 64 | 79 | 34 | Atorvastatin 10 mg/day | Conventional treatment | 31 months |
| Angermann et al. [92] | 352 | 363 | 715 | 69 | 71 | 30 | Included the following elements: (1) in-hospital face-to-face contact between specialist nurse, patient, and relatives to explain the intervention, practice supervision of blood pressure, heart rate and symptoms; (2) telephone-based structured monitoring; (3) up titration of heart failure medication; (4) needs-adjusted specialist care, which nurses coordinated with patients’ physician(s); (5) measures for appropriate education and supervision of interveners to ensure high intervention quality | Standard postdischarge planning, which typically included treatment plans, comprehensive discharge letters, and fixed appointments with GPs or cardiologists within 7–14 days | 6 months |
| Chaudhry et al. [69] | 826 | 827 | 1653 | 61 | 52 | 71% < 40 | Structured (daily) telephone-based monitoring (of symptoms and weight) via an interactive voice response system | Standard optimal care. Followed by local physician. Guideline based therapy | 6 months |
| Domingues et al. [142] | 48 | 63 | 111 | 63 | 68 | 29 | Structured (weekly for 1st month, every 15 days for following 2 months) telephone-based education and monitoring signs and symptoms of decompensation | Usual care that consisted of the follow-up of the patient at the return appointment at the outpatient clinic without any telephone contact | 3 months |
| Dunagan et al. [145] | 76 | 75 | 151 | 70 | 44 | 75% < 40 | The intervention group received additional education from study nurses during scheduled telephone contact | Educational packet describing the causes of HF, the basic principles of treatment, their role in routine care and monitoring of their condition, and appropriate strategies for managing a HF exacerbation | 12 months |
| Gattis et al. [154] | 90 | 91 | 181 | 67 | 68 | 30 | Clinical pharmacist-led medication review and patient education. Regularly scheduled telephone contact (at 2, 12 and 24 weeks) to detect clinical deterioration early | Usual care | 6 months |
| Krum et al. [179] | 188 | 217 | 405 | 73 | 61 | 36 | Nurse-led telephone monitoring. Participant responded to computer-generated CHF self-monitoring questions by pressing the numbers on the touch-phone keypad. Nurse survey incoming calls daily and responded to preset variations to participant's parameters | Usual care involved standard general practice management of heart failure | 12 months |
| Laramee et al. [182] | 141 | 146 | 287 | 71 | 54 | < 40 | Four major components were (1) early discharge planning and coordination of care, (2) individualized and comprehensive patient and family education, (3) 12 weeks of enhanced telephone follow-up and surveillance, and (4) promotion of optimal CHF medications and medication doses (ACEIs or ARBs and BBs) | Standard care, typical of a tertiary care hospital, and all conventional treatments requested by the attending physician | 3 months |
| Mortara et al. [200] | 301 | 160 | 461 | 60 | 85 | 29 | The patients enrolled in HT strategies 2 and 3 transmitted weekly records of the following data to the coordinating center via an automated interactive voice response system: weight; heart rate; systolic arterial pressure; dyspnea score; asthenia score; oedema score; changes in therapy; and blood results | Patients allocated to the control arm were discharged as normal from the hospital | 12 months |
| Peters-klimm et al. [215] | 97 | 100 | 197 | 70 | 72 | 38 | The design of the intervention addressed 4 elements: delivery system design, self-management support, decision support, clinical information systems | No case management was applied | 12 months |
| Ramachandran et al. [224] | 25 | 25 | 50 | 45 | 78 | 21 | Intervention group participants were managed in the heart failure clinic and received disease, medication and self-management education and telephonic disease management which consisted of reinforcement of information and drug dose modification | Usual care in the heart failure clinic | 6 months |
| Sisk et al. [230] | 203 | 203 | 406 | N/R | N/R | < 40 | An in-person appointment was arranged for each intervention participant, which included symptom and disease education and referral to additional patient services (if required). Follow-up telephone calls consisted of participant assessment, recording of admission information reinforcement of self-monitoring and administration of a food-frequency questionnaire | Usual care patients received federal consumer guidelines for managing systolic dysfunction but no other intervention | 12 months |
| Adamson et al. [89] | N/R | N/R | 32 | 59 | 38 | 29 | Permanent right-ventricular implantable hemodynamic monitor system similar to a single-lead pacemaker | Historical controls | 17 months |
| Adamson et al. [90] | 198 | 202 | 400 | 55 | 69 | 23 | Expert disease management conforming to consensus recommendations coupled with hemodynamic information from the IHM | The control group received expert disease management with frequent and random nursing calls | 12 months |
| Al-khatib et al. [91] | 76 | 75 | 151 | 63 | 62 | 25 | Remote monitoring of ICDs using the Medtronic CareLink transmission monitor | Quarterly ICD interrogations in clinic classified as standard of care | 12 months |
| Antonicelli et al. [93] | 28 | 29 | 57 | 78 | 61 | 35 | Patients were contacted by telephone at least once a week by the team to obtain information on symptoms and adherence to prescribed treatment, as well as blood pressure, heart rate, bodyweight and 24-h urine output data for the previous day. A weekly ECG transmission was also required. Evaluation of these parameters was followed by reassessment of the therapeutic regimen and modification whenever needed | Standard care based on routinely scheduled clinic visits from a team specialized in CHF patient management | 12 months |
| Biannic et al. [106] | 35 | 38 | 73 | 78 | 79 | 32 | TM during 3 months, after which participants all received usual care up until 1 year | Usual care | 3 months |
| Böhm et al. [110] | 497 | 505 | 1002 | 66 | 80 | 27 | Telemedicine alerts enabled, triggered by intrathoracic fluid index threshold crossing, which was programmed at the investigator’s discretion. The fluid status monitoring algorithm detects changes in thoracic impedance resulting from accumulation of intrathoracic fluid as an early sign of developing cardiac decompensation | To not transmit alerts | 23 months |
| Boriani et al. [112] | 428 | 437 | 865 | 66 | 76 | 27 | Received a monitor for scheduled remote device checks, and automatic alerts for lung fluid accumulation atrial tachyarrhythmia, and system integrity were enabled. In-office device checks were requested to re-arm alerts which had been temporarily inactivated due to previous transmissions | In-office follow-ups alone | 24 months |
| Boyne et al. [113] | 185 | 197 | 382 | 71 | 59 | 36 | The patients in the intervention arm received a device, with a liquid crystal display and four keys, connected to a landline phone. Daily pre-set dialogues were communicated about symptoms, knowledge, and behaviour, being answered by touching one of the keys and sent to a server and to the nurses’ desktop | Nurse-led usual care was given according to the latest European Society of Cardiology guidelines, including oral and written educational information, and psychosocial support as needed | 12 months |
| Capomolla et al. [310] | 67 | 66 | 133 | 57 | 88 | 29 | The objectives of the multidisciplinary staff are prevention and functional recovery of consequences of acute hemodynamic instabilization. The team members also have the task of creating, analyzing, and correcting the organization that supports the process of treatment identified in an individual care plan | Patients were referred to their primary care physician and cardiologist. During follow-up the process of care was driven by the patient’s needs into a heterogeneous range of emergency room management, hospital admission, and outpatient access | 11 months |
| Dar et al. [133] | 91 | 91 | 182 | 72 | 66 | 61% < 40 | Home telemonitoring. Daily measurement, manual transmission of weight, blood pressure, heart rate, oxygen saturation and symptoms | Standard care | 6 months |
| Domenichini et al. [140] | 39 | 41 | 80 | 68 | 94 | 29 | OptiVolw or CorVueTM functions and alarms activated | The OptiVolw or CorVueTM functions switched on, as Group 1, whereas the alarms were not activated | 12 months |
| Domingo et al. [141] | 44 | 48 | 92 | 66 | 71 | 36 | Motiva System with educational videos, motivational messages | Patients were instructed to record their weight, blood pressure, and heart rate each morning before breakfast | 12 months |
| Giordano et al. [157] | 230 | 230 | 460 | 57 | 85 | 28 | Patient telemonitoring involving medical and nursing professionals. Daily transmission of cardiac parameters was monitored by a cardiologist, general practitioner and nurse, who assessed the patient's clinical status, providing consultation or triage. Nurse-driven telephone contacts to assess patient status and treatment regimen adherence were conducted weekly, or biweekly, dependent on patient status | Referred to their primary care physician. A structured follow-up with the cardiologist at 12 months in the hospital outpatient department and the appointment with the primary care physician within 2 weeks from the discharge were planned | 12 months |
| Goldberg et al. [158] | 138 | 142 | 280 | N/R | N/R | 22 | The system includes an electronic scale placed in patients’ homes. Patients were instructed to weigh themselves and respond to yes/no questions about heart failure related symptoms twice daily. The attending physician individualized the symptom questions and weight goals for each patient at the time of enrollment | Patients were instructed to contact their physician for weight increases of more than a prespecified amount or if their symptoms of heart failure worsened. These patients were asked to bring a copy of their home weight log to study visits | 6 months |
| Hansen et al. [167] | 102 | 108 | 210 | 63 | 83 | 28 | Receive quarterly automated follow-up via telemetry | Receive quarterly personal contact with a physician | 13 months |
| Hindricks et al. [170] | 331 | 333 | 664 | 66 | 81 | 26 | In the telemonitoring group, transmitted data were reviewed by study investigators according to their clinical routine. In parallel, transmitted data were reviewed by a central monitoring unit composed of trained study nurses and supporting physicians | In the control group, no study participant had access to telemonitoring data until study completion. All patients were treated according to European guidelines | 12 months |
| Idris et al. [171] | 14 | 14 | 28 | 63 | 39 | 23 | Daily remote monitoring of blood pressure, heart rate, oxygen saturation, and weight via the telemonitoring system for 3 months | Standard care | 3.6 months |
| Kashem et al. [174] | 24 | 24 | 48 | 54 | 74 | 25 | Blood pressure, pulse, steps/day, and weight together with symptoms were entered. The most recent laboratory data and medication were entered by the practice staff, and the patient was instructed to review medications and laboratory values and transmit any questions to the practice | Usual care | 12 months |
| Koehler et al. [176] | 354 | 356 | 710 | 67 | 82 | 27 | The system is based on a wireless Bluetooth device, together with a personal digital assistant, as the central structural element. Data transfer was performed with the use of cell phone technologies. The patient performed a daily self-assessment and the data were transferred to the responsible telemedical center | Usual care | 26 months |
| Koehler et al. [311] | N/R | N/R | 710 | 67 | 81 | < 30 | The system is based on a wireless Bluetooth device together with a personal digital assistant as the central structural element. The patient performed a daily self-assessment and the data was transferred to the telemedical center which provided physician-led medical support 24 h a day, 7 days a week for the entire study period | Usual care | 24 months |
| Kraai et al. [178] | 94 | 83 | 177 | 69 | 37 | 27 | Patients in the telemonitoring group received telemonitoring devices at home consisting of a weighing scale, blood pressure equipment, an ECG-device and a health-monitor. The instruction was to record weight and blood pressure once a day and an ECG in case of starting or up-titration of Beta-blockers. After receiving the data from the above-mentioned devices, the health-monitor generated standard health-related questions regarding the patients’ health status | The ICT-guided-DSM group followed the normal HF-routine of the individual hospitals, like any other HF-patient, without limitations to the visits | 9 months |
| Landolina et al. [181] | 101 | 99 | 200 | 68 | 79 | 31 | ICD-OptiVol | Remote transmission off | 16 months |
| Lüthje et al. [187] | 89 | 87 | 176 | 66 | 77 | 32 | The device determines a representative impedance daily and compares this with a roving reference value. Whenever daily impedance drops below the reference, a cumulative, absolute difference is calculated, and called fluid index | Standard in-office visits were performed every 3 months | 15 months |
| Morgan et al. [199] | 824 | 826 | 1650 | 70 | 86 | 30 | Remote monitoring via an electronic care record form management system | Usual care | 34 months |
| Sardu et al. [227] | 89 | 94 | 183 | 72 | 76 | < 35 | CRT-D with TM | CRT-D with traditional ambulatory monitoring | 12 months |
| Scherr et al. [228] | 54 | 54 | 108 | 66 | 79 | 25 | Pharmacological treatment with telemedical surveillance for 6 months | Pharmacological treatment | 6 months |
| Soran et al. [312] | 160 | 155 | 315 | 76 | 31 | 24 | Home-based disease management program to monitor and to detect early signs and symptoms of heart failure using telecommunication equipment | Patient 1-on-1 education, an effort to use evidenced-based optimal medical treatment, and a commercially available digital home scale with management by primary physician | 6 months |
| van Veldhuisen [249] | 167 | 168 | 335 | 86 | 86 | 25 | Information available to physicians and patients as an audible alert in case of preset threshold crossings | Information and an alert were not available | 15 months |
| Villani et al. [250] | 30 | 30 | 60 | 69 | 75 | 31 | N/A | N/A | 12 months |
| Villani et al. [251] | 40 | 40 | 80 | 72 | 74 | 32 | The patient front-end operated through a personal digital assistant given to each patient leaving hospital. The cardiologist decided what variables should be followed up (e.g., heart rate, body weight, blood pressure, ECG) and the frequency of monitoring (e.g., daily for blood pressure and body weight, weekly for the ECG) according to the patient’s clinical characteristics | Usual care | 1 year |
| Vuorinen et al. [255] | 47 | 47 | 94 | 58 | 83 | 27 | A patient regularly reported their most important health parameters to the nurse using a mobile phone app. At the beginning of the study, the patients were given a home-care package including a weight scale, a blood pressure meter, a mobile phone, and self-care instructions. The patients were advised to carry out and report the measurements together with the assessment of symptoms once a week | A multidisciplinary care approach including patient guidance and support for self-care has been adopted at the clinic | 6 months |
| Weintraub et al. [256] | 95 | 93 | 188 | 69 | 66 | 32 | Specialized primary and networked care in heart failure disease management program | Disease management program in conjunction with the AHM system | 3 months |
| Zan [263] | N/R | N/R | 40 | 53 | N/R | 32 | Intervention for heart failure self-management over a 90-day study period. Patients were instructed to take their weight, blood pressure, and heart rate measurements each morning | Matched controls | 3 months |
ABMMNC autologous bone marrow mononuclear cell, ACE angiotensin-converting enzyme, AMIO amiodarone, ARB angiotensin II receptor blockers, BB beta-blocker, BID twice a day, BiV biventricular, BMAC bone marrow aspirate stem cell concentrate, BMC bone marrow cells, BMMC bone marrow–derived mast cell, BMSC bone marrow stromal cells, CA catheter ablation, CABG coronary artery bypass graft, CFAE complex fractionated atrial electrogram, CPC circulating blood, CPET cardiopulmonary exercise test, CR cardiac rehabilitation, CRT cardiac resynchronization therapy, CRT cardiac resynchronization therapy, CRT-D cardiac resynchronization therapy defibrillator, CSC cardiac stem cells, ET exercise training, G-CSF granulocyte-colony stimulating factor, HIIT high-intensity interval training, ICD implantable cardioverter defibrillator, INR international normalized ratio, LAPWI left atrial posterior wall isolation, LVEF left ventricular ejection fraction, MCT moderate-intensity continuous training, MDC multidisciplinary clinics, MRA mineralocorticoid receptor antagonists, PVI pulmonary vein isolation, RVP right ventricular pacing, STS structured telephone support, SVCI systemic vascular conductance index, TM telemonitoring, VVIR ventricular rate modulated pacing
Quality assessment
Overall, risk of bias was classified as relatively low (Table 5). Of the 44 meta-analyses, 11 scored critically low, 15 low, 1 moderate, and 17 high. Almost all meta-analyses registered their protocol before commencement of the review (item 2) and used appropriate meta-analytical methods (item 11). Reviews were mostly downgraded based on the lack of an adequate investigation of publication bias (item 15).
Table 5.
AMSTAR 2 scores
| Critical domains | Non-critical domains | Judgment | |||||
|---|---|---|---|---|---|---|---|
| Item 2a | Item 9b | Item 11c | Item 13d | Item 14e | |||
| Adamson et al. [266] | ●●● | ● | ●●● | ● | ●●● | ●● | Critically low |
| Agasthi et al. [267] | ●●● | ●●● | ●●● | ●●● | ●●● | ●● | High |
| Al-Majed et al. [268] | ●●● | ●●● | ●●● | ● | ●●● | ●● | Low |
| Alotaibi et al. [269] | ●●● | ●●● | ●●● | ●●● | ●●● | ●● | High |
| AlTurki et al. [270] | ●●● | ●●● | ●●● | ●●● | ●●● | ●● | High |
| Benito-González et al. [271] | ●●● | ●●● | ●●● | ●●● | ●●● | ●● | High |
| Bertaina et al. [272] | ●●● | ●●● | ●●● | ●●● | ● | ●● | Low |
| Bjarnason-Wehrens et al. [273] | ●●● | ●●● | ●●● | ●●● | ●●● | ●● | High |
| Bonsu et al. [274] | ●●● | ●●● | ●●● | ●●● | ●●● | ●● | High |
| Carbo et al. [275] | ●●● | ●●● | ●●● | ●●● | ●●● | ●● | High |
| De Vecchis et al. [276] | ●●● | ●●● | ●●● | ● | ●●● | ●● | Low |
| Driscoll et al. [277] | ●●● | ●●● | ●●● | ●●● | ●●● | ●● | High |
| Emdin et al. [278] | ●●● | ●●● | ●●● | ●●● | ● | ●● | Low |
| Fisher et al. [279] | ●●● | ●●● | ●●● | ●●● | ●●● | ●● | High |
| Fisher et al. [280] | ●●● | ●●● | ●●● | ●●● | ●●● | ●● | High |
| Gandhi et al. [281] | ●●● | ●●● | ●●● | ●●● | ●●● | ●● | High |
| Halawa et al. [282] | ●●● | ●●● | ●●● | ●●● | ●●● | ●● | High |
| Hartmann et al. [283] | ●●● | ●●● | ● | ● | ●●● | ●● | Critically low |
| Hu et al. [284] | ●●● | ●●● | ●●● | ●●● | ● | ●● | Low |
| Inglis et al. [285] | ●●● | ●●● | ●●● | ● | ● | ●● | Critically low |
| Inglis et al. [286] | ●●● | ●●● | ●●● | ●●● | ●●● | ●● | High |
| Japp et al. [287] | ●● | ● | ●●● | ● | ● | ●● | Critically low |
| Jonkman et al. [288] | ●●● | ●●● | ●●● | ● | ● | ●● | Critically low |
| Kang et al. [289] | ● | ● | ●●● | ● | ● | ●● | Critically low |
| Klersy et al. [290] | ● | ●●● | ●●● | ● | ●●● | ●● | Critically low |
| Komajda et al. [291] | ●●● | ●●● | ●●● | ●●● | ● | ●● | Low |
| Le et al. [292] | ●●● | ● | ●●● | ● | ● | ●● | Critically low |
| Ma et al. [293] | ●●● | ●●● | ●●● | ● | ●●● | ●● | Low |
| Malik et al. [294] | ●●● | ●●● | ●●● | ●●● | ●●● | ●● | High |
| Moshonas et al. [295] | ●●● | ●●● | ●●● | ●●● | ●●● | ●● | High |
| Pandor et al. [296] | ●●● | ●●● | ●●● | ●●● | ● | ●● | Low |
| Shah et al. [297] | ●●● | ● | ●●● | ● | ● | ●● | Critically low |
| Sulaica et al. [298] | ●● | ●●● | ●●● | ●●● | ●●● | ●● | Moderate |
| Taylor et al. [299] | ●●● | ●●● | ●●● | ●●● | ●●● | ●● | High |
| Thomas et al. [300] | ●●● | ●●● | ●●● | ●●● | ● | ●● | Low |
| Thomsen et al. [301] | ●●● | ● | ●●● | ● | ●●● | ●● | Critically low |
| Tse et al. [302] | ●●● | ●●● | ●●● | ●●● | ●●● | ●● | High |
| Tu et al. [303] | ●●● | ●●● | ●●● | ●●● | ● | ●● | Low |
| Turagam et al. [304] | ●●● | ●●● | ●●● | ●●● | ● | ●● | Low |
| Uminski et al. [305] | ●●● | ●●● | ●●● | ●●● | ● | ●● | Low |
| Xiang et al. [306] | ●●● | ● | ●●● | ● | ●●● | ●● | Critically low |
| Zhang et al. [307] | ●●● | ●●● | ●●● | ● | ●●● | ●● | Low |
| Zhang et al. [308] | ●●● | ●●● | ●●● | ●●● | ● | ●● | Low |
| Zhou and Chen [309] | ●●● | ●●● | ●●● | ●●● | ● | ●● | Low |
aRegistered protocol before commencement of the review
bRisk of bias from individual studies being included in the review
cAppropriateness of meta-analytical method
dConsideration of risk of bias when interpreting the results of the review
eAssessment of presence and likely impact of publication bias
Study characteristics
A total of 425,220 patients were included in the 44 meta-analyses and 186 RCTs (Table 4). RCTs included between 16 and 10,917 patients. The mean age of patients ranged from 33 to 96 years. Mean LVEF varied between 17 and 40%. Percentage of male patients ranged from 25 to 100%. Follow-up period ranged widely from 30 days to 10 years. Studies that tried to prevent hospital admissions with cardiac rehabilitation focused on either exercise only or multicomponent cardiac rehabilitation. Care pathways could be divided into either TM, STS, and self-management promotion programs or multidisciplinary clinics. Invasive therapy encompassed catheter ablation (CA), cardiac resynchronization therapy (CRT), mitral valve repair, or stem cell therapy. Medication subtypes were angiotensin-converting enzyme inhibitors (ACE), angiotensin II receptor blockers (ARBs), mineralocorticoid receptor antagonists (MRAs), beta-blockers, statins, anticoagulation, and a miscellaneous subcategory.
Effect of interventions
Primary analysis: meta-analyses
Meta-analytic results of the 44 included meta-analyses are demonstrated in Table 6 and Fig. 2. According to our best-evidence synthesis, strong evidence suggests that CA, CR, and TM could prevent heart failure hospitalization. Furthermore, moderate evidence was found for the effectiveness of RAAS inhibitors, and CRT in reducing HF-related hospitalizations, while only limited evidence suggests the beneficial effects of beta-blockers, statins, mitral valve therapy, and multidisciplinary clinics or self-management promotion programs. There is conflicting evidence regarding the effect of cell therapy on HF hospitalization, and no evidence was found that anticoagulation should reduce HF-related hospitalizations.
Table 6.
Effectiveness of interventions
| Author, year | Category | Sig | Conclusion | Statistics |
|---|---|---|---|---|
| Adamson et al. [266] | Care pathways | √ | Haemodynamic-guided HF management is superior in reducing long-term HF-hospitalization risk | HR: 0.63 (0.54–0.73) |
| Alotaibi et al. [269] | Care pathways | √ | A significant reduction in HF-hospitalizations in patients undergoing catheter ablation | RR: 0.56 (0.44–0.71) |
| Carbo et al. [275] | Care pathways | √ | We found reduction trends in HF-related admissions due to m-Health | SMD: − 0.43 (–0.83|–0.02) |
| Driscoll et al. [277] | Care pathways | √ | Nuse-led titration may result in a significant reduction in hospital admissions | RR: 0.51 (0.36–0.72) |
| Gandhi et al. [281] | Care pathways | × | Multidisciplinary heart failure clinics failed to show a reduction in HF hospitalization | OR: 0.68 |
| Halawa et al. [282] | Care pathways | × | Usage of intra-cardiac devices is not linked to improving rates of HF admission | OR: 1.25 (0.92–1.69) |
| Inglis et al. [285] | Care pathways | √ | Both STS and TM reduced HF-related hospitalizations | RR: 0.77 (0.68–0.87)c |
| RR: 0.79 (0.67–0.94)d | ||||
| Inglis et al. [286] | Care pathways | √ | STS and TM improve outcomes for patients with CHF | RR: 0.77 (0.68–0.87)c |
| RR: 0.79 (0.67– 0.94)d | ||||
| Jonkman et al. [288] | Care pathways | × | No specific program characteristics were consistently associated with better effects of self-management interventions | RR: 0.96 (0.92–0.995) |
| Klersy et al. [290] | Care pathways | √ | TM was associated with a significantly lower number of hospitalizations for HF | IRR: 0.77 (0.65–0.91) |
| Pandor et al. [296] | Care pathways | × | There were no major effects on HF-related hospitalization for STS HM (HR: 1.03, 95% CrI: 0.66, 1.54) or TM with medical support during office hours | HR: 1.03, (0.66, 1.54)c |
| HR: 0.95, (0.70, 1.34)d | ||||
| Thomas et al. [300] | Care pathways | √ | Specialist clinics for patients with HF can reduce the risk of unplanned admissions | RR: 0.51 (0.41–0.63) |
| Tse et al. [302] | Care pathways | √ | Hospitalization rates can be reduced by remote patient monitoring using either TM or hemodynamic monitoring | HR: 0.73 (0.65–0.83)d |
| HR: 0.60 (0.53–0.69)m | ||||
| Uminski et al. [305] | Care pathways | √ | A post-discharge virtual ward can provide added benefits to usual care to reduce HF-related hospital admissions | RR: 0.61 (0.49–0.76) |
| Xiang et al. [306] | Care pathways | √ | Telehealth had a significant overall effect on CHF hospitalization | RR: 0.72 (0.61–0.85) |
| Bjarnason-Weherens et al. [273] | CR | √ | Exercise-based intervention reduces the level of hospitalizations due to HF | RR: 0.59 (0.12–2.91) |
| Taylor et al. [299] | CR | √ | ExCR did reduce HF-specific hospitalization | RR: 0.59 (0.42–0.84) |
| Agasthi et al. [267] | Invasive therapy | √ | CA was associated with significantly lower rate of HF-readmission | RR: 0.58 (0.46–0.81) |
| Al-Majed et al. [268] | Invasive therapy | √ | CRT reduces HF-hospitalization in patients | RR: 0.69 (0.58–0.82) |
| AlTurki et al. [270] | Invasive therapy | √ | RM showed benefit in reducing HF-related hospitalization when compared to standard of care | RR: 0.95 (0.78–1.16) |
| Benito-González et al. [271] | Invasive therapy | √ | TMVR with MitraClip® system was related to a significant reduction in hospitalizations for HF | HR: 0.65 (0.46–0.92) |
| Bertaina et al. [272] | Invasive therapy | √ | MitraClip for FMR in patients with LV dysfunction is associated with a considerable reduction of HF-hospitalization | OR: 0.49 (0.24–1.00) |
| Fisher et al. [279] | Invasive therapy | √ | Cell treatment is associated with a significant reduction of rehospitalization caused by worsening HF | RR: 0.39 (0.22–0.70) |
| Fisher et al. [280] | Invasive therapy | × | Cell therapy does not appear to reduce the risk of rehospitalization for HF | RR: 0.62 (0.36–1.04) |
| Ma et al. [293] | Invasive therapy | √ | CA reduced risks of HF readmission | RR: 0.58 (0.46–0.66) |
| Malik and Aronow [294] | Invasive therapy | √ | CA was effective in reducing hospitalization for HF | OR: 0.41 (0.28–0.59) |
| Moschonas et al. [295] | Invasive therapy | √ | In patients randomized to AFA, there were significant improvements in unplanned hospitalization rates | RR: 0.58 (0.46–0.73) |
| Tu et al. [303] | Invasive therapy | √ | CRT had a marked effect in reducing new hospitalizations for worsening HF | RR: 0.69 (0.60–0.79) |
| Turagam et al. [304] | Invasive therapy | √ | CA was associated with reductions in HF hospitalizations | RR: 0.60 (0.39–0.93) |
| Bonsu et al. [274] | Medication | √ | Superiority of lipophilic statin treatment in decreasing hospitalization for worsening HF | OR:0. 49 (0.36–0.67)a |
| OR: 0.94 (0. 86–1.03)b | ||||
| De Vecchis and Ariano [276] | Medication | √ | ARA use in patients with heart failure was associated with a significant reduction in hospitalization | OR: 0.73 (0.61–0.89) |
| Emdin et al. [278] | Medication | √ | RAAS inhibition overall reduces the risk for hospitalization for HF | RR: 0.80, (0.77–0.83) |
| Gandhi et al. [313] | Medication | √ | In patients with acute advanced CHF concomitant hypertonic saline administration decreased HF-rehospitalization | RR: 0.50 (0.33–0.76) |
| Hartmann et al. [283] | Medication | × | Ivabradine showed no significant effect for hospitalization due to HF | RR: 0.87 (0.68–1.12) |
| lTurki al. [284] | Medication | √ | The use of AldoAs may exert beneficial effects in reducing re-hospitalization for cardiac causes | RR: 0.62 (0.52–0.74) |
| Japp et al. [287] | Medication | √ | MRAs did improve hospitalizations | HR: 0.62 (0.47–0.82) |
| Kang et al. [289] | Medication | √ | There was a trend towards reduced HF hospitalization risk with RAS inhibitors | RR: 0.91 (0.83–1.01) |
| Komajda et al. [291] | Medication | √ | Disease-modifying medications resulted in the progressive improvement in hospitalization outcomes | HR: 0.25 (0.07–0.99) |
| Le et al. [292] | Medication | √ | Significant relative risk reduction of CV hospitalization was observed in those assigned to AAs | RR: 0.79 (0.68–0.91) |
| Shah et al. [297] | Medication | × | Pooled analysis of these trials suggests no consistent benefit of RAS inhibition with regard to HF hospitalization | OR: 0.90 (0.80–1.02) |
| Sulaica et al. [298] | Medication | × | No difference was noted between the anticoagulation and placebo group in regard to hospitalization for HF | OR: 0.97 (0.80–1.18) |
| Thomsen et al. [301] | Medication | √ | Drugs targeting the renin–angiotensin–aldosterone system, beta-blockers, digoxin, and CRT significantly reduced the risk of HF hospitalization | RR: 0.71 (0.65–0.78)e |
| RR: 0.63 (0.44–0.91)f | ||||
| RR: 0.76 (0.64–0.90)g | ||||
| RR: 0.78 (0.73–0.82)h | ||||
| RR: 0.40 (0.20–0.78)i | ||||
| RR: 0.87 (0.68–1.11)j | ||||
| RR: 0.64 (0.57–0.71)k | ||||
| RR 1.34 (1.04–1.73)l | ||||
| Zhang et al. [307] | Medication | √ | The beneficial effects of TMZ have been demonstrated by the decrease of hospitalization | RR: 0.43 (0.21–0.91) |
| Zhang et al. [308] | Medication | × | Our meta-analysis suggests that liraglutide treatment has no important influence on hospitalization for HF | RR: 1.18 (0.88–1.58) |
| Zhou and Chen [309] | Medication | √ | TMZ treatment in CHF patients may reduce hospitalization for cardiac causes | RR: 0.43 (0.21–0.91) |
HF heart failure, CA catheter ablation, CR cardiac rehabilitation, CRT cardiac resynchronization therapy, STS structured telephone support, UF ultrafiltration, TMZ Trimetazidine, TM telemonitoring
a Lipostatin
b Rosuvastatin
c Structured telephone support
d Telemonitoring
e ACE
f ARB
g ARA
h Beta-blocker
i Digoxin
j Ivabradine
k CRT
l ICD
m Hemodynamic monitoring
Fig. 2.
Effects of different interventions on HF-related hospitalization in meta-analyzed and single-study results. ACE, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; MRA, mineralocorticoid receptor antagonists; CR, cardiac rehabilitation; CRT, cardiac resynchronization therapy; CA, catheter ablation; TM, telemonitoring; STS, structured telephone support
Secondary analysis: extracted RCTs
In order to prevent bias as a result of duplicated data, all unique RCTs (N = 186) were extracted in a secondary analysis from the meta-analyses and compared to the results from our primary analysis.
Cardiac rehabilitation
A total of 14 studies examined the effects of cardiac rehabilitation. Of these individual studies, 1 reported a significant effect. When examined in a meta-analysis, a significant positive effect of cardiac rehabilitation was found (RR: 0.66, 95% CI: 0.44 | 0.97) (Fig. 3). This is in accordance with the general findings reported by the studied meta-analyses. Upon visual inspection, the funnel plots suggest no publication bias (Fig. 7).
Fig. 3.
Forest plot of RR for HF-related hospitalization between cardiac rehabilitation and control. Random effects model
Fig. 7.
(A–D) Funnel plots of the effects of (A) cardiac rehabilitation, (B) telemonitoring, (C) medication, and (D) invasive therapy
Invasive therapy
There were 5 studies examining the effect of CA. Of these studies, 2 studies reported a significant effect. A positive effect of CA on HF-related hospitalization was found in our meta-analyses (RR: 0.57, 95% CI: 0.46 | 0.72) (Fig. 4). This is consistent with the general findings reported by the studied meta-analyses.
Fig. 4.
(A–D) Forest plots of RR for HF-related hospitalization between (A) catheter ablation, (B) cardiac resynchronization therapy, (C) mitral valve therapy, and (D) stem cell therapy, and control. Fixed effects model
A total of 23 studies examined CRT to prevent HF-related hospitalization. Of these, 8 studies found a positive effect. Our meta-analysis suggested a positive effect of CRT (RR: 0.85, 95% CI: 0.78 | 0.92). This is in line with the general findings reported by the studied meta-analyses.
Of the 4 studies that examined mitral valve repair, 3 reported an effective reduction in HF-related hospitalization. Our meta-analyses suggested a positive effect (RR: 0.74, 95% CI: 0.64 | 0.86), which is in agreement with the general findings reported by the studied meta-analyses.
Stem cell therapy was in 0 of the 13 studies related to reduced HF-related hospitalization, which is in line with our meta-analyzed result (RR: 0.71, 95% CI: 0.45 | 1.14) and the conflicting evidence suggested by the studied meta-analyses.
The funnel plots indicate no, or only minimal publication bias (Fig. 7).
Medication
ACE inhibitors (5/18 studies; RR: 0.64, 95% CI: 0.49 | 0.85), MRAs (4/9 studies; RR: 0.77, 95% CI: 0.71 | 0.83), ARBs (4/5 studies; RR: 0.77, 95% CI: 0.72 | 0.84), beta-blockers (8/16 studies; RR: 0.78, 95% CI: 0.74 | 0.83), and statins (2/9 studies; RR: 0.51, 95% CI: 0.36 | 0.72) showed a significant effect of reduced hospitalizations in our meta-analyses (Fig. 5). This is in line with the general findings reported by the studied meta-analyses.
Fig. 5.
(A–F) Forest plots of RR for HF-related hospitalization between (A) angiotensin-converting enzyme inhibitors, (B) angiotensin II receptor blockers, (C) mineralocorticoid receptor antagonists, (D) beta-blockers, (E) statins, and (F) anticoagulation, and control. Fixed effects model
Anticoagulation (RR: 0.99, 95% CI: 0.91 | 1.08) was in none of the studies (0/3) able to reduce HF-related hospitalizations. This absence of an effect was also reported by the studied meta-analyses.
The asymmetry in the medication funnel plots suggests some publication bias towards significant effectiveness of medication in reducing HF-related hospitalizations (Fig. 7).
Care pathways
Multidisciplinary clinics or self-management promotion programs (10/23 studies; RR: 0.79, 95% CI: 0.73 | 0.85) and TM (12/33 studies; RR: 0.86, 95% CI: 0.81 | 0.92) were related to less HF-related hospitalizations (Fig. 6). This is in agreement with findings reported by the studied meta-analyses. STS (1/11 studies; RR: 0.85, 95% CI: 0.85 | 1.04) was not related to reductions in HF-related hospitalizations. This is in contrast to findings from the meta-analyses. Visual inspection of the funnel plots did not suggest publication bias (Fig. 7).
Fig. 6.
(A–C) Forest plot of RR for HF-related hospitalization between (A) multidisciplinary clinics or self-management promotion programs, (B) structured telephone support, and (C) telemonitoring, and control. Fixed effects model
Discussion
Heart failure is a major health concern, with the highest readmission rates among all diseases [8–11]. Yet, up to 40% of hospitalizations could be classified as preventable [36–40]. This umbrella review therefore aimed to systematically review all published meta-analyses conducted in the past 10 years that examined the incremental benefit of interventions in addition to standard care, in reducing HF-related (re)hospitalization, in order to provide a comprehensive overview of different levels of evidence with regard to the different interventions that aim to reduce HF-related (re)hospitalization.
Even though previous studies did examine the effectiveness of interventions in treatment for heart failure in general, this umbrella review highlights different levels of evidence regarding the effectiveness of several interventions in reducing HF-related hospitalization. All different categories of interventions (i.e., cardiac rehabilitation, invasive treatment, medication, and care pathways) entail interventions that prove able to statistically significantly reduce HF-related hospitalizations. Strong or at least moderate evidence was found for the beneficial effects of CA, CRT, ACE inhibitors, MRAs, ARBs, CR, TM, and STS. Limited evidence was found for the ability of beta-blockers,, statins, mitral valve repair, and multidisciplinary clinics or self-management promotion programs to reduce hospitalization rates. Conflicting or no evidence was found for the effects of anticoagulation and stem cell therapy.
The findings of this umbrella review were generally supported by the American Heart Association and European Society of Cardiology heart failure guidelines [46, 64]. Yet, evidence for effectiveness was still lacking for several interventions in these guidelines. A couple of interventions proposed in the guidelines had low levels of evidence, as they were only supported by a single randomized clinical trial. Although these guidelines do not solely focus on the prevention of (re)hospitalization, this umbrella review now provides additional evidence for the effectiveness of ARBs (e.g., Valsartan) and telemonitoring as effective in the prevention of (re)hospitalization in heart failure. Therefore, the results of this review may be used in addition in clinical practice, as well as by policymakers, as a guideline in deciding what treatment option might help prevent hospitalization in at risk heart failure patients.
Effectiveness of reported interventions was measured in terms of a reduced risk for heart failure related hospitalizations. However, it would be naïve to suggest that this equals the clinical, genuine effect of treatment. Non-effectiveness of treatment could also be related to non-adherence or non-acceptance of the intervention by the patient, since it is estimated that non-adherence ranges between 30 and 50% in patients with chronic illnesses [65]. And non-adherence not only holds for medication, yet also for cardiac rehabilitation [66, 67] and telemonitoring [68, 69]. It has been shown that worsening of HF is often related to non-adherence of patients [70] and is in fact associated with 10% of hospitalizations [65, 71] and a 10% increased risk of readmission [72]. The other way around, reductions in non-adherence are found to result in less hospital admissions [73].
Differences in non-adherence to different forms of interventions were also found. For example, patients are found to be more adherent to ACE-inhibitors (77.8%) as compared to beta-blockers (69.8%) [74]. These differences could be explained by cognitions of patients regarding the efficacy of the intervention and the usability of the intervention [75]. Moreover, low health literacy or simply a lack of knowledge about the syndrome could also contribute to non-adherence [76–78]. In clinical practice, one should therefore educate patients about the importance of disease management with medication, invasive therapy, cardiac rehabilitation, and care pathways [65, 79].
Moreover, when implementing interventions in practice, one should not only focus on effectiveness, yet also incorporate, for example, the costs of the intervention. Especially, since HF is the most costly condition in western countries, with at least twice the costs of the estimated consumption of healthcare in the general population in a year [32, 33, 80], mainly due to HF-related hospitalization [28, 29]. Research has shown that, in terms of cost-effectiveness, medication treatment with beta-blockers, ARBs, or ACE inhibitors could be preferred over more cost expensive therapies as device therapy with CRT [81, 82]. More specifically, with regard to specific forms of medication, ivabradine seems a cost-effective treatment option, while this does not hold for valsartan [82]. In addition, general HF treatment combined with telemonitoring has been found to be between 27 and 52% more cost-efficient than usual care alone [83, 84]. Furthermore, telemonitoring seems not only cost-efficient; but nowadays, with the pandemic consequences of COVID-19 it seems more desired than ever [85]. The pandemic served as a catalyst, as both healthcare professionals as patients wanted optimal care in a time of reduced ambulatory outpatient clinics, with being compliant to social distancing [84]. Our review shows, in addition, that, even though the terms are interchangeably used to both describe some form of “remote care,” telemonitoring and structured telephone support have different levels of effectiveness with regard to prevention of heart failure related (re)hospitalizations, which should be accounted for in clinical practice.
In this umbrella review, we only aimed to provide an overview of effective treatment options for prevention of heart failure (re)hospitalization. Consequently, no conclusions could be drawn regarding the hierarchy of effectiveness based upon this review. In future research, it should be examined what factors contribute to effectiveness of interventions, as our study only showed that particular interventions could reduce heart failure hospitalizations, but not why per se. Research should focus on the effective mechanisms of care pathway programs, for example, or on determinants of successful implementations of interventions for heart failure.
The aim of our review was to assess interventions which are currently used in clinical practice and examined in large populations. Our results are based upon meta-analyses performed within the past 10 years. Yet, most recent innovative treatment options are probably underrepresented. For example, no study examined the effects of SGLT-II inhibitors, while the European Society of Cardiology stated that SGLT-II inhibitors could be preferred in heart failure patients [86]. Future studies should examine whether the use of SGLT-II inhibitors could show effective in reducing hospitalization. Moreover, as the aim of our review was to assess interventions which are currently used in clinical practice and examined in large populations, we expected to find multiple meta-analyses examining the same interventions. A large amount of overlap in RCTs in included meta-analyses was found. This stresses the importance of registration of protocols and knowing whether the intended research subject has a significantly different research objective than existing, or outdated reviews [62].
To conclude, this umbrella review highlights different levels of evidence regarding the effectiveness of several interventions in reducing HF-related hospitalization in HFrEF patients. It provides an overview of all, known, meta-analyses conducted in the past 10 years that examined interventions to prevent heart failure related hospitalizations. All different categories of interventions entail interventions that prove able to statistically significantly reduce HF-related hospitalizations. Most evidence was found for the beneficial effects of angiotensin-converting enzyme inhibitors (ACE), mineralocorticoid receptor antagonists (MRAs), angiotensin II receptor blockers (ARBs), cardiac rehabilitation, and telemonitoring. The results of this review may be used in clinical practice, as well as by policymakers, to guide treatment for heart failure patients at risk of hospitalization.
Acknowledgements
The authors thank the nurses and nurse practitioners of the cardiology departments of the Catharina Hospital, Màxima Medisch Centrum, Anna Hospital, and Elkerliek Hospital for their valuable assistance in, and discussion, of this research.
Author contribution
All authors made substantial contributions to conception and design, and/or acquisition of data, and/or analysis and interpretation of data. All authors participated in drafting the article or revising it critically for important intellectual content gave final approval of the version to be submitted and any revised version.
Declarations
Ethical approval
The study has been performed in accordance with the ethical standards in the 1964 Declaration of Helsinki and with relevant regulations of the US Health Insurance Portability and Accountability Act (HIPAA).
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Frederique J. Hafkamp, Email: Frederique.hafkamp@nederlandshartnetwerk.nl
Rene A. Tio, Email: rene.tio@catharinaziekenhuis.nl
Luuk C. Otterspoor, Email: luuk.otterspoor@catharinaziekenhuis.nl
Tineke de Greef, Email: tineke.d.greef@catharinaziekenhuis.nl.
Gijs J. van Steenbergen, Email: gijs.v.steenbergen@catharinaziekenhuis.nl
Arjen R. T. van de Ven, Email: a.vande.ven@st-anna.nl
Geert Smits, Email: g.smits@pozob.nl.
Hans Post, Email: hans.post@catharinaziekenhuis.nl.
Dennis van Veghel, Email: dennis.v.veghel@catharinaziekenhuis.nl.
References
- 1.Massie BM, Shah NB. Evolving trends in the epidemiologic factors of heart failure: rationale for preventive strategies and comprehensive disease management. Am Heart J Mosby. 1997;133:703–712. doi: 10.1016/S0002-8703(97)70173-X. [DOI] [PubMed] [Google Scholar]
- 2.Magnussen C, Niiranen TJ, Ojeda FM, Gianfagna F, Blankenberg S, Vartiainen E, Sans S, Pasterkamp G, Hughes M, Costanzo S, Donati MB, Jousilahti P, Linneberg A, Palosaari T, de Gaetano G, Bobak M, den Ruijter HM, Jørgensen T, Söderberg S, Kuulasmaa K, Zeller T, Iacoviello L, Salomaa V, Schnabel RB. Sex-specific epidemiology of heart failure risk and mortality in Europe: results from the BiomarCaRE consortium. JACC Hear Fail. 2019;7:204–213. doi: 10.1016/j.jchf.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Mamas MA, Sperrin M, Watson MC, Coutts A, Wilde K, Burton C, Kadam UT, Kwok CS, Clark AB, Murchie P. Do patients have worse outcomes in heart failure than in cancer? a primary care-based cohort study with 10-year follow-up in Scotland. Eur J Heart Fail Wiley Online Library. 2017;19:1095–1104. doi: 10.1002/ejhf.822. [DOI] [PubMed] [Google Scholar]
- 4.Ho KKL, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham study. J Am Coll Cardiol. 1993;22:A6–A13. doi: 10.1016/0735-1097(93)90455-A. [DOI] [PubMed] [Google Scholar]
- 5.Data from the American Heart Association (2000) Heart and stroke statistical update. Am Hear Assoc Dallas. TX
- 6.Mosterd A, Hoes AW (2007) Clinical epidemiology of heart failure. Heart 93:1137 LP – 1146 [DOI] [PMC free article] [PubMed]
- 7.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM. Forecasting the impact of heart failure in the United States: a policy statement from the american heart association. Circ Hear Fail Am Heart Assoc. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the medicare fee-for-service program. N Engl J Med Mass Medical Soc. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 9.Cheng RK, Cox M, Neely ML, Heidenreich PA, Bhatt DL, Eapen ZJ, Hernandez AF, Butler J, Yancy CW, Fonarow GC. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the medicare population. Am Heart J Elsevier. 2014;168:721–730. doi: 10.1016/j.ahj.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto-Filho JA, Kim N, Bernheim SM, Suter LG. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. Jama American Medical Association. 2013;309:355–363. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patil S, Shah M, Patel B, Agarwal M, Ram P, Alla VM (2019) Readmissions among patients admitted with acute decompensated heart failure based on income quartiles. In: Mayo clinic proceedings. Elsevier, p 1939–1950 [DOI] [PubMed]
- 12.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R. American heart association council on epidemiology and prevention statistics committee and stroke statistics subcommittee. Hear Dis stroke Stat Updat a Rep from Am Hear Assoc Circ. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 13.Braunwald E. The war against heart failure: the Lancet lecture. Lancet Elsevier. 2015;385:812–824. doi: 10.1016/S0140-6736(14)61889-4. [DOI] [PubMed] [Google Scholar]
- 14.Dunlay SM, Redfield MM, Weston SA, Therneau TM, Long KH, Shah ND, Roger VL. Hospitalizations after heart failure diagnosis: a community perspective. J Am Coll Cardiol. 2009;54:1695–1702. doi: 10.1016/j.jacc.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stauffer BD, Fullerton C, Fleming N, Ogola G, Herrin J, Stafford PM, Ballard DJ. Effectiveness and cost of a transitional care program for heart failure: a prospective study with concurrent controls. Arch Intern Med. 2011;171:1238–1243. doi: 10.1001/archinternmed.2011.274. [DOI] [PubMed] [Google Scholar]
- 16.Bernheim SM, Grady JN, Lin Z, Wang Y, Wang Y, Savage SV, Bhat KR, Ross JS, Desai MM, Merrill AR. National patterns of risk-standardized mortality and readmission for acute myocardial infarction and heart failure: update on publicly reported outcomes measures based on the 2010 release. Circ Cardiovasc Qual Outcomes Am Heart Assoc. 2010;3:459–467. doi: 10.1161/CIRCOUTCOMES.110.957613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krumholz HM, Merrill AR, Schone EM, Schreiner GC, Chen J, Bradley EH, Wang Y, Wang Y, Lin Z, Straube BM. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes Am Heart Assoc. 2009;2:407–413. doi: 10.1161/CIRCOUTCOMES.109.883256. [DOI] [PubMed] [Google Scholar]
- 18.Ross JS, Chen J, Lin Z, Bueno H, Curtis JP, Keenan PS, Normand S-LT, Schreiner G, Spertus JA, Vidán MT (2010) Recent national trends in readmission rates after heart failure hospitalization. Circ Hear Fail Am Heart Assoc 3:97–103 [DOI] [PMC free article] [PubMed]
- 19.Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC (2012) Get with the guidelines scientific advisory committee and investigators. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 126:65–75 [DOI] [PubMed]
- 20.Guideline N (2018) Chronic heart failure in adults: diagnosis and management. Natl Inst Heal Care Excell [PubMed]
- 21.Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Young JB, Michelson EL. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation Citeseer. 2007;116:1482–1487. doi: 10.1161/CIRCULATIONAHA.107.696906. [DOI] [PubMed] [Google Scholar]
- 22.Mahoney JE, Eisner J, Havighurst T, Gray S, Palta M. Problems of older adults living alone after hospitalization. J Gen Intern Med Wiley Online Library. 2000;15:611–619. doi: 10.1046/j.1525-1497.2000.06139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waters KR. Outcomes of discharge from hospital for elderly people. J Adv Nurs Wiley Online Library. 1987;12:347–355. doi: 10.1111/j.1365-2648.1987.tb01341.x. [DOI] [PubMed] [Google Scholar]
- 24.Michels N. The transition from hospital to home: an exploratory study. Home Health Care Serv Q Taylor & Francis. 1988;9:29–44. doi: 10.1300/J027v09n01_03. [DOI] [PubMed] [Google Scholar]
- 25.Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17:41. [PubMed] [Google Scholar]
- 26.Dharmarajan K, Hsieh AF, Kulkarni VT, Lin Z, Ross JS, Horwitz LI, Kim N, Suter LG, Lin H, Normand S-LT (2015) Trajectories of risk after hospitalization for heart failure, acute myocardial infarction, or pneumonia: retrospective cohort study. bmj British Medical Journal Publishing Group 350 [DOI] [PMC free article] [PubMed]
- 27.Koudstaal S, Pujades-Rodriguez M, Denaxas S, Gho JMIH, Shah AD, Yu N, Patel RS, Gale CP, Hoes AW, Cleland JG (2017) Prognostic burden of heart failure recorded in primary care, acute hospital admissions, or both: a population-based linked electronic health record cohort study in 2.1 million people. Eur J Heart Fail Wiley Online Library 19:1119–1127 [DOI] [PMC free article] [PubMed]
- 28.Smith P, McKeon A, Blunt I, Edwards N (2014) NHS hospitals under pressure: trends in acute activity up to 2022. London Nuff Trust
- 29.Stewart S, Jenkins A, Buchan S, McGuire A, Capewell S, McMurray JJJV. The current cost of heart failure to the national health service in the UK. Eur J Heart Fail Wiley Online Library. 2002;4:361–371. doi: 10.1016/S1388-9842(01)00198-2. [DOI] [PubMed] [Google Scholar]
- 30.Commission MPA (2012) Report to the congress: creating greater efficiency in medicare
- 31.Fingar K, Washington R (2016) Trends in hospital readmissions for four high-volume conditions. 2009–2013:55–79 [PubMed]
- 32.O’CONNELL JB, Bristow MR (1994) Economic impact of heart failure in the United States: time for a different approach. J Hear lung Transplant 13:S107–S112 [PubMed]
- 33.Lee WC, Chavez YE, Baker T, Luce BR. Economic burden of heart failure: a summary of recent literature. Hear lung Elsevier. 2004;33:362–371. doi: 10.1016/j.hrtlng.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Burns DJP, Arora J, Okunade O, Beltrame JF, Bernardez-Pereira S, Crespo-Leiro MG, Filippatos GS, Hardman S, Hoes AW, Hutchison S. International consortium for health outcomes measurement (ICHOM): standardized patient-centered outcomes measurement set for heart failure patients. JACC Hear Fail JACC: Heart Failure. 2020;8:212–222. doi: 10.1016/j.jchf.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 36.Phelan D, Smyth L, Ryder M, Murphy N, O’Loughlin C, Conlon C, Ledwidge M, McDonald K. Can we reduce preventable heart failure readmissions in patients enrolled in a disease management programme? Ir J Med Sci Springer. 2009;178:167–171. doi: 10.1007/s11845-009-0332-6. [DOI] [PubMed] [Google Scholar]
- 37.Hernandez AF, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW, Peterson ED, Curtis LH. Relationship between early physician follow-up and 30-day readmission among medicare beneficiaries hospitalized for heart failure. Jama American Medical Association. 2010;303:1716–1722. doi: 10.1001/jama.2010.533. [DOI] [PubMed] [Google Scholar]
- 38.Van WC, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. Cmaj Can Med Assoc. 2011;183:E391–E402. doi: 10.1503/cmaj.101860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jessup M, Brozena S. Heart failure. N Engl J Med Massachusetts Medical Society. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 40.Horowitz JD. Home-based intervention: the next step in treatment of chronic heart failure? Eur Heart J. 2000;21:1807–1809. doi: 10.1053/euhj.2000.2112. [DOI] [PubMed] [Google Scholar]
- 41.Van SHGC, Rahman T, Mytton O, Ramasundarahettige C, Ibrahim Q, Kabali C, Coppens M, Brian Haynes R, Connolly S. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta-analysis. Eur J Heart Fail John Wiley & Sons, Ltd. 2017;19:1427–1443. doi: 10.1002/ejhf.765. [DOI] [PubMed] [Google Scholar]
- 42.Seto E (2008) Cost comparison between telemonitoring and usual care of heart failure: a systematic review. Telemed e-Health Mary Ann Liebert, Inc. 140 Huguenot Street 3rd Floor New Rochelle, NY 10801 USA 14:679–686 [DOI] [PubMed]
- 43.Porter ME. What is value in health care. N Engl J Med. 2010;363:2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 44.Porter ME, Teisberg EO (2006) Redefining health care: creating value-based competition on results. Harvard business press
- 45.Porter ME, Lee TH. The strategy that will fix health care. Harv Bus Rev. 2013;91:24. [Google Scholar]
- 46.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the european society of cardiology (ESC) developed with the special contribution of. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 47.Jaarsma T, van der Wal MHL, Lesman-Leegte I, Luttik M-L, Hogenhuis J, Veeger NJ, Sanderman R, Hoes AW, van Gilst WH, Lok DJA. Effect of moderate or intensive disease management program on outcome in patients with heart failure: coordinating study evaluating outcomes of advising and counseling in heart failure (COACH) Arch Intern Med American Medical Association. 2008;168:316–324. doi: 10.1001/archinternmed.2007.83. [DOI] [PubMed] [Google Scholar]
- 48.Stewart S, Carrington MJ, Marwick TH, Davidson PM, Macdonald P, Horowitz JD, Krum H, Newton PJ, Reid C, Chan YK. Impact of home versus clinic-based management of chronic heart failure: the WHICH? (which heart failure intervention is most cost-effective & consumer friendly in reducing hospital care) multicenter, randomized trial. J Am Coll Cardiol. 2012;60:1239–1248. doi: 10.1016/j.jacc.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 49.Bradley EH, Curry L, Horwitz LI, Sipsma H, Thompson JW, Elma M, Walsh MN, Krumholz HM. Contemporary evidence about hospital strategies for reducing 30-day readmissions: a national study. J Am Coll Cardiol. 2012;60:607–614. doi: 10.1016/j.jacc.2012.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Danz MS, Rubenstein LV, Hempel S, Foy R, Suttorp M, Farmer MM, Shekelle PG. Identifying quality improvement intervention evaluations: is consensus achievable? BMJ Qual Saf BMJ Publishing Group Ltd. 2010;19:279–283. doi: 10.1136/qshc.2009.036475. [DOI] [PubMed] [Google Scholar]
- 51.Kitsiou S, Paré G, Jaana M (2015) Effects of home telemonitoring interventions on patients with chronic heart failure: an overview of systematic reviews. J Med Internet Res JMIR Publications Inc., Toronto, Canada 17:e63 [DOI] [PMC free article] [PubMed]
- 52.Naylor MD, Aiken LH, Kurtzman ET, Olds DM, Hirschman KB. The importance of transitional care in achieving health reform. Health Aff Health Affairs. 2011;30:746–754. doi: 10.1377/hlthaff.2011.0041. [DOI] [PubMed] [Google Scholar]
- 53.Takeda A, Taylor SJC, Taylor RS, Khan F, Krum H, Underwood M (2012) Clinical service organisation for heart failure. Cochrane database Syst Rev John Wiley & Sons, Ltd [DOI] [PubMed]
- 54.Stewart S, Carrington M, Marwick T, Davidson P, Macdonald P, Horowitz J, Krum H, Newton P, Reid C, Chan Y, Scuffham P. Which Heart failure Intervention is most Cost-effective and consumer friendly in reducing Hospital care (WHICH?): a multicentre randomised controlled trial. Hear Lung Circ Elsevier. 2012;21:S98–S99. doi: 10.1016/j.hlc.2012.05.249. [DOI] [PubMed] [Google Scholar]
- 55.van der Meer P, Gaggin HK, Dec GW. ACC/AHA versus ESC guidelines on heart failure: JACC guideline comparison. J Am Coll Cardiol American College of Cardiology Foundation Washington, DC. 2019;73:2756–2768. doi: 10.1016/j.jacc.2019.03.478. [DOI] [PubMed] [Google Scholar]
- 56.Parker SG, Peet SM, McPherson A, Cannaby AM, Abrams K, Baker R, Wilson A, Lindesay J, Parker G, Jones DR (2002) A systematic review of discharge arrangements for older people. Database of abstracts of reviews of effects (DARE): Quality-assessed reviews [Internet] Centre for reviews and dissemination (UK) [DOI] [PubMed]
- 57.Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med American College of Physicians. 2011;155:520–528. doi: 10.7326/0003-4819-155-8-201110180-00008. [DOI] [PubMed] [Google Scholar]
- 58.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E (2017) AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. bmj British Medical Journal Publishing Group 358:j4008 [DOI] [PMC free article] [PubMed]
- 59.Slavin RE. Best evidence synthesis: an intelligent alternative to meta-analysis. J Clin Epidemiol Elsevier. 1995;48:9–18. doi: 10.1016/0895-4356(94)00097-A. [DOI] [PubMed] [Google Scholar]
- 60.Doundoulakis I, Antza C, Apostolidou-Kiouti F, Akrivos E, Karvounis H, Kotsis V, Haidich A-B, Giannakoulas G (2018) Overview of systematic reviews of non-vitamin K oral anticoagulants in atrial fibrillation: evidence of publication overlap. Circ Cardiovasc Qual Outcomes Am Heart Assoc 11:e004769 [DOI] [PubMed]
- 61.Siontis KC, Ioannidis JPA (2018) Replication, duplication, and waste in a quarter million systematic reviews and meta-analyses. Am Heart Assoc [DOI] [PubMed]
- 62.Pieper D, Antoine S-L, Mathes T, Neugebauer EAM, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol Elsevier. 2014;67:368–375. doi: 10.1016/j.jclinepi.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 63.McHugh ML. Interrater reliability: the kappa statistic. Biochem medica Croatian Society of Medical Biochemistry and Laboratory Medicine. 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 64.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the american college of cardiology foundation/american heart association task force on practice guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 65.Kini V, Ho PM. Interventions to improve medication adherence: a review. Jama American Medical Association. 2018;320:2461–2473. doi: 10.1001/jama.2018.19271. [DOI] [PubMed] [Google Scholar]
- 66.van Engen-Verheul M, de Vries H, Kemps H, Kraaijenhagen R, de Keizer N, Peek N. Cardiac rehabilitation uptake and its determinants in the Netherlands. Eur J Prev Cardiol Oxford University Press. 2013;20:349–356. doi: 10.1177/2047487312439497. [DOI] [PubMed] [Google Scholar]
- 67.Turk-Adawi KI, Oldridge NB, Tarima SS, Stason WB, Shepard DS. Cardiac rehabilitation enrollment among referred patients: patient and organizational factors. J Cardiopulm Rehabil Prev LWW. 2014;34:114–122. doi: 10.1097/HCR.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 68.Lyngå P, Persson H, Hägg-Martinell A, Hägglund E, Hagerman I, Langius-Eklöf A, Rosenqvist M (2012) Weight monitoring in patients with severe heart failure (WISH). A randomized controlled trial. Eur J Heart Fail England 14:438–444 [DOI] [PubMed]
- 69.Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, Phillips CO, Hodshon BV, Cooper LS, Krumholz HM. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363:2301–2309. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Der WMHL, Jaarsma T, Moser DK, Veeger NJGM, van Gilst WH, van Veldhuisen DJ. Compliance in heart failure patients: the importance of knowledge and beliefs. Eur Heart J Oxford University Press. 2006;27:434–440. doi: 10.1093/eurheartj/ehi603. [DOI] [PubMed] [Google Scholar]
- 71.Komajda M, Cowie MR, Tavazzi L, Ponikowski P, Anker SD, Filippatos GS, Investigators Q. Physicians’ guideline adherence is associated with better prognosis in outpatients with heart failure with reduced ejection fraction: the QUALIFY international registry. Eur J Heart Fail Wiley Online Library. 2017;19:1414–1423. doi: 10.1002/ejhf.887. [DOI] [PubMed] [Google Scholar]
- 72.Rosen OZ, Fridman R, Rosen BT, Shane R, Pevnick JM (2017) Medication adherence as a predictor of 30-day hospital readmissions. Patient Prefer Adherence Dove Press 11:801 [DOI] [PMC free article] [PubMed]
- 73.Hood SR, Giazzon AJ, Seamon G, Lane KA, Wang J, Eckert GJ, Tu W, Murray MD. Association between medication adherence and the outcomes of heart failure. Pharmacother J Hum Pharmacol Drug Ther Wiley Online Library. 2018;38:539–545. doi: 10.1002/phar.2107. [DOI] [PubMed] [Google Scholar]
- 74.Viana M, Laszczynska O, Mendes S, Friões F, Lourenço P, Bettencourt P, Lunet N, Azevedo A. Medication adherence to specific drug classes in chronic heart failure. J Manag Care Pharm Academy of Managed Care Pharmacy. 2014;20:1018–1026. doi: 10.18553/jmcp.2014.20.10.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ware P, Dorai M, Ross HJ, Cafazzo JA, Laporte A, Boodoo C, Seto E (2019) Patient adherence to a mobile phone–based heart failure telemonitoring program: a longitudinal mixed-methods study. JMIR mHealth uHealth JMIR Publications Inc., Toronto, Canada 7:e13259 [DOI] [PMC free article] [PubMed]
- 76.Horowitz CR, Rein SB, Leventhal H. A story of maladies, misconceptions and mishaps: effective management of heart failure. Soc Sci Med Elsevier. 2004;58:631–643. doi: 10.1016/S0277-9536(03)00232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haynes SC, Pallin R, Tong K, Henderson S, Romano PS. Understanding adherence to the CardioMEMS pulmonary artery pressure monitoring system for heart failure: a qualitative study. Hear Lung Elsevier. 2020;49:251–258. doi: 10.1016/j.hrtlng.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 78.Piotrowicz E, Baranowski R, Bilinska M, Stepnowska M, Piotrowska M, Wójcik A, Korewicki J, Chojnowska L, Malek LA, Klopotowski M. A new model of home-based telemonitored cardiac rehabilitation in patients with heart failure: effectiveness, quality of life, and adherence. Eur J Heart Fail Wiley Online Library. 2010;12:164–171. doi: 10.1093/eurjhf/hfp181. [DOI] [PubMed] [Google Scholar]
- 79.Ruppar TM, Cooper PS, Mehr DR, Delgado JM, Dunbar - Jacob JM (2016) Medication adherence interventions improve heart failure mortality and readmission rates: systematic review and meta-analysis of controlled trials. J Am Heart Assoc Malden, Massachusetts: Wiley-Blackwell 5:1-N.PAG [DOI] [PMC free article] [PubMed]
- 80.Mejhert M, Lindgren P, Schill O, Edner M, Persson H, Kahan T. Long term health care consumption and cost expenditure in systolic heart failure. Eur J Intern Med Elsevier. 2013;24:260–265. doi: 10.1016/j.ejim.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 81.Rohde LE, Bertoldi EG, Goldraich L, Polanczyk CA (2013) Cost-effectiveness of heart failure therapies. Nat Rev Cardiol Nature Publishing Group 10:338 [DOI] [PubMed]
- 82.Pina IL, Desai NR, Allen LA, Heidenreich P. Managing the economic challenges in the treatment of heart failure. Prog Cardiovasc Dis Elsevier. 2018;61:476–483. doi: 10.1016/j.pcad.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 83.Hameed AS, Modre-Osprian R, Schreier G (2017) Identification of cost indicators with significant economic impact on the total treatment costs of chronic heart vailure patients-a meta-analysis. eHealth 161–168 [PubMed]
- 84.Cleland JGF, Clark RA, Pellicori P, Inglis SC (2020) Caring for people with heart failure and many other medical problems through and beyond the COVID-19 pandemic: the advantages of universal access to home telemonitoring. Eur J Heart Fail Wiley-Blackwell [DOI] [PMC free article] [PubMed]
- 85.Gorodeski EZ, Goyal P, Cox ZL, Thibodeau JT, Reay RE, Rasmusson K, Rogers JG, Starling RC. Virtual visits for care of patients with heart failure in the era of COVID-19: a statement from the heart failure society of America. J Card Fail Elsevier. 2020;26:448–456. doi: 10.1016/j.cardfail.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seferović PM, Fragasso G, Petrie M, Mullens W, Ferrari R, Thum T, Bauersachs J, Anker SD, Ray R, Çavuşoğlu Y. Heart failure association of the european society of cardiology update on sodium–glucose co-transporter 2 inhibitors in heart failure. Eur J Heart Fail Wiley Online Library. 2020;22:1984–1986. doi: 10.1002/ejhf.2026. [DOI] [PubMed] [Google Scholar]
- 87.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL. Cardiac resynchronization in chronic heart failure. N Engl J Med Mass Medical Soc. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 88.Abraham WT, Young JB, León AR, Adler S, Bank AJ, Hall SA, Lieberman R, Liem LB, O’Connell JB, Schroeder JS, Wheelan KR. Effects of cardiac resynchronization on disease progression in patients with left ventricular systolic dysfunction, an indication for an implantable cardioverter-defibrillator, and mildly symptomatic chronic heart failure. Circulation. 2004;110:2864–2868. doi: 10.1161/01.CIR.0000146336.92331.D1. [DOI] [PubMed] [Google Scholar]
- 89.Adamson PB, Kleckner KJ, VanHout WL, Srinivasan S, Abraham WT. Cardiac resynchronization therapy improves heart rate variability in patients with symptomatic heart failure. Circulation. 2003;108:266–269. doi: 10.1161/01.CIR.0000083368.75831.7A. [DOI] [PubMed] [Google Scholar]
- 90.Adamson PB, Gold MR, Bennett T, Bourge RC, Stevenson LW, Trupp R, Stromberg K, Wilkoff BL, Costanzo MR, Luby A, Aranda JM, Heywood JT, Baldwin HA, Aaron M, Smith A, Zile M. Continuous hemodynamic monitoring in patients with mild to moderate heart failure: results of the reducing decompensation events utilizing intracardiac pressures in patients With chronic heart failure (REDUCEhf) trial. Congest Heart Fail United States. 2011;17:248–254. doi: 10.1111/j.1751-7133.2011.00247.x. [DOI] [PubMed] [Google Scholar]
- 91.Al-Khatib SM, Piccini JP, Knight D, Stewart M, Clapp-Channing N, Sanders GD. Remote monitoring of implantable cardioverter defibrillators versus quarterly device interrogations in clinic: results from a randomized pilot clinical trial. J Cardiovasc Electrophysiol United States. 2010;21:545–550. doi: 10.1111/j.1540-8167.2009.01659.x. [DOI] [PubMed] [Google Scholar]
- 92.Angermann CE, Störk S, Gelbrich G, Faller H, Jahns R, Frantz S, Loeffler M, Ertl G. Mode of action and effects of standardized collaborative disease management on mortality and morbidity in patients with systolic heart failure: the interdisciplinary network for heart failure (INH) study. Circ Heart Fail United States. 2012;5:25–35. doi: 10.1161/CIRCHEARTFAILURE.111.962969. [DOI] [PubMed] [Google Scholar]
- 93.Antonicelli R, Testarmata P, Spazzafumo L, Gagliardi C, Bilo G, Valentini M, Olivieri F, Parati G. Impact of telemonitoring at home on the management of elderly patients with congestive heart failure. J Telemed Telecare England. 2008;14:300–305. doi: 10.1258/jtt.2008.071213. [DOI] [PubMed] [Google Scholar]
- 94.Asgar AW, Khairy P, Guertin M-C, Cournoyer D, Ducharme A, Bonan R, Basmadjian A, Tardif J-C, Cohen DJ. Clinical outcomes and economic impact of transcatheter mitral leaflet repair in heart failure patients. J Med Econ England. 2017;20:82–90. doi: 10.1080/13696998.2016.1227828. [DOI] [PubMed] [Google Scholar]
- 95.Assmus B, Schächinger V, Zeiher AM (2006) Regenerative therapy in cardiology: how distant is it from reality?. Internist (Berl) Germany 47:1177–1182 [DOI] [PubMed]
- 96.Assmus B, Walter DH, Seeger FH, Leistner DM, Steiner J, Ziegler I, Lutz A, Khaled W, Klotsche J, Tonn T, Dimmeler S, Zeiher AM. Effect of shock wave-facilitated intracoronary cell therapy on LVEF in patients with chronic heart failure: the CELLWAVE randomized clinical trial. JAMA United States. 2013;309:1622–1631. doi: 10.1001/jama.2013.3527. [DOI] [PubMed] [Google Scholar]
- 97.Atienza F, Anguita M, Martinez-Alzamora N, Osca J, Ojeda S, Almenar L, Ridocci F, Vallés F, de Velasco JA. Multicenter randomized trial of a comprehensive hospital discharge and outpatient heart failure management program. Eur J Heart Fail England. 2004;6:643–652. doi: 10.1016/j.ejheart.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 98.Austin J, Williams R, Ross L, Moseley L, Hutchison S. Randomised controlled trial of cardiac rehabilitation in elderly patients with heart failure. Eur J Heart Fail England. 2005;7:411–417. doi: 10.1016/j.ejheart.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 99.Australia/New Zealand Heart Failure Research Collaborative Group (1997) Randomised, placebo-controlled trial of carvedilol in patients with congestive heart failure due to ischaemic heart disease. Lancet 349(9049):375–380. PMID: 9033462 [PubMed]
- 100.Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, El NB, Banovic M, Beleslin B, Vrolix M, Legrand V, Vrints C, Vanoverschelde JL, Crespo-Diaz R, Homsy C, Tendera M, Waldman S, Wijns W, Terzic A. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol United States. 2013;61:2329–2338. doi: 10.1016/j.jacc.2013.02.071. [DOI] [PubMed] [Google Scholar]
- 101.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999;99(9):1173–1182. doi: 10.1161/01.CIR.99.9.1173. [DOI] [PubMed] [Google Scholar]
- 102.Belardinelli R, Georgiou D, Cianci G, Purcaro A. 10-year exercise training in chronic heart failure: a randomized controlled trial. J Am Coll Cardiol. 2012;60(16):1521–1528. doi: 10.1016/j.jacc.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 103.Beller B, Bulle T, Bourge RC, Colfer H, Fowles RE, Giles TD, Grover J, Whipple JP, Fisher MB, Jessup M. Lisinopril versus placebo in the treatment of heart failure: the Lisinopril Heart Failure Study Group. J Clin Pharmacol England. 1995;35:673–680. doi: 10.1002/j.1552-4604.1995.tb04107.x. [DOI] [PubMed] [Google Scholar]
- 104.Bentkover JD, Dorian P, Thibault B, Gardner M. Economic analysis of a randomized trial of biventricular pacing in Canada. Pacing Clin Electrophysiol United States. 2007;30:38–43. doi: 10.1111/j.1540-8159.2007.00577.x. [DOI] [PubMed] [Google Scholar]
- 105.Eichhorn EJ, Domanski MJ, Krause-Steinrauf H, Bristow MR, Lavori PW. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med United States. 2001;344:1659–1667. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 106.Biannic C, Coutance G, Calus J, Belin A, Loiselet P, Michel L (2012) Educational home follow-up by telemedicine in cases of cardiac insufficiency. Randomised, multicentric study from the Basse-Normandie region. Preliminary results. Eur Res Telemed 1:40–48
- 107.Bielecka-Dabrowa A, Goch JH, Mikhailidis DP, Rysz J, Maciejewski M, Banach M (2009) The influence of atorvastatin on parameters of inflammation and function of the left ventricle in patients with dilated cardiomyopathy. Med Sci Monit Int Med J Exp Clin Res United States 15:MS12–23 [PubMed]
- 108.Blue L, Lang E, McMurray JJ, Davie AP, McDonagh TA, Murdoch DR, Petrie MC, Connolly E, Norrie J, Round CE, Ford I, Morrison CE. Randomised controlled trial of specialist nurse intervention in heart failure. BMJ. 2001;323:715–718. doi: 10.1136/bmj.323.7315.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boccanelli A, Mureddu GF, Cacciatore G, Clemenza F, Lenarda A Di, Gavazzi A, Porcu M, Latini R, Lucci D, Maggioni A Pietro, Masson S, Vanasia M, de Simone G (2009) Anti-remodelling effect of canrenone in patients with mild chronic heart failure (AREA IN-CHF study): final results. Eur J Heart Fail England 11:68–76 [DOI] [PubMed]
- 110.Böhm M, Drexler H, Oswald H, Rybak K, Bosch R, Butter C, Klein G, Gerritse B, Monteiro J, Israel C, Bimmel D, Käab S, Huegl B, Brachmann J. Fluid status telemedicine alerts for heart failure: a randomized controlled trial. Eur Heart J England. 2016;37:3154–3163. doi: 10.1093/eurheartj/ehw099. [DOI] [PubMed] [Google Scholar]
- 111.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet (London, England) 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 112.Boriani G, Costa A Da, Quesada A, Ricci R Pietro, Favale S, Boscolo G, Clementy N, Amori V, Mangoni di S Stefano L, Burri H (2017) Effects of remote monitoring on clinical outcomes and use of healthcare resources in heart failure patients with biventricular defibrillators: results of the MORE-CARE multicentre randomized controlled trial. Eur J Heart Fail England 19:416–425 [DOI] [PubMed]
- 113.Boyne JJJ, Vrijhoef HJM, Crijns HJGM, De WG, Kragten J, Gorgels APM. Tailored telemonitoring in patients with heart failure: results of a multicentre randomized controlled trial. Eur J Heart Fail England. 2012;14:791–801. doi: 10.1093/eurjhf/hfs058. [DOI] [PubMed] [Google Scholar]
- 114.Bristow MR, Gilbert EM, Abraham WT, Adams KF, Fowler MB, Hershberger RE, Kubo SH, Narahara KA, Ingersoll H, Krueger S, Young S, Shusterman N. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators Circulation United States. 1996;94:2807–2816. doi: 10.1161/01.cir.94.11.2807. [DOI] [PubMed] [Google Scholar]
- 115.Brown EJJ, Chew PH, MacLean A, Gelperin K, Ilgenfritz JP, Blumenthal M (1995) Effects of fosinopril on exercise tolerance and clinical deterioration in patients with chronic congestive heart failure not taking digitalis. Fosinopril Heart Failure Study Group. Am J Cardiol United States 75:596–600 [DOI] [PubMed]
- 116.Lok DJA, Veldhuisen DJ van, Wijngaarden J van, Cornel JH, Zuithoff NPA, Badings E, Hoes AW (2007) Added value of a physician-and-nurse-directed heart failure clinic: results from the Deventer-Alkmaar heart failure study. Heart 93:819–825 [DOI] [PMC free article] [PubMed]
- 117.Capomolla S, Febo O, Ceresa M, Caporotondi A, Guazzotti G, La RM, Ferrari M, Lenta F, Baldin S, Vaccarini C, Gnemmi M, Pinna G, Maestri R, Abelli P, Verdirosi S, Cobelli F. Cost/utility ratio in chronic heart failure: comparison between heart failure management program delivered by day-hospital and usual care. J Am Coll Cardiol United States. 2002;40:1259–1266. doi: 10.1016/S0735-1097(02)02140-X. [DOI] [PubMed] [Google Scholar]
- 118.Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, Garrigue S, Kappenberger L, Haywood GA, Santini M, Bailleul C, Daubert JC. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med United States. 2001;344:873–880. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 119.Captopril-Digoxin Multicenter Research Group (1988) Comparative effects of therapy with captopril and digoxin in patients with mild to moderate heart failure. JAMA 259(4):539–544. PMID: 2447297 [PubMed]
- 120.Chan AKY, Sanderson JE, Wang T, Lam W, Yip G, Wang M, Lam Y-Y, Zhang Y, Yeung L, Wu EB, Chan WWM, Wong JTH, So N, Yu C-M. Aldosterone receptor antagonism induces reverse remodeling when added to angiotensin receptor blockade in chronic heart failure. J Am Coll Cardiol United States. 2007;50:591–596. doi: 10.1016/j.jacc.2007.03.062. [DOI] [PubMed] [Google Scholar]
- 121.Chen Y-W, Wang C-Y, Lai Y-H, Liao Y-C, Wen Y-K, Chang S-T, Huang J-L, Wu T-J (2018) Home-based cardiac rehabilitation improves quality of life, aerobic capacity, and readmission rates in patients with chronic heart failure. Medicine (Baltimore) 97:e9629 [DOI] [PMC free article] [PubMed]
- 122.Chung ES, Menon SG, Weiss R, Schloss EJ, Chow T, Kereiakes DJ, Pastore JM. Feasibility of biventricular pacing in patients with recent myocardial infarction: impact on ventricular remodeling. CHF. 2007;13(1):9–15. doi: 10.1111/j.1527-5299.2007.05868.x. [DOI] [PubMed] [Google Scholar]
- 123.CIBIS Investigators and Committees (1994) A randomized trial of beta-blockade in heart failure. The Cardiac Insufficiency Bisoprolol Study (CIBIS). Circulation 90(4):1765–1773. 10.1161/01.cir.90.4.1765. PMID: 7923660 [DOI] [PubMed]
- 124.(1999) The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 353(9146):9–13. PMID: 10023943 [PubMed]
- 125.Cicoira M, Zanolla L, Rossi A, Golia G, Franceschini L, Brighetti G, Marino P, Zardini P. Long-term, dose-dependent effects of spironolactone on left ventricular function and exercise tolerance in patients with chronic heart failure. J Am Coll Cardiol United States. 2002;40:304–310. doi: 10.1016/S0735-1097(02)01965-4. [DOI] [PubMed] [Google Scholar]
- 126.Cleland JGF, Findlay I, Jafri S, Sutton G, Falk R, Bulpitt C, Prentice C, Ford I, Trainer A, Poole-Wilson PA. The Warfarin/Aspirin Study in Heart failure (WASH): a randomized trial comparing antithrombotic strategies for patients with heart failure. Am Heart J United States. 2004;148:157–164. doi: 10.1016/j.ahj.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 127.Cline CM, Israelsson BY, Willenheimer RB, Broms K, Erhardt LR. Cost effective management programme for heart failure reduces hospitalisation. Heart. 1998;80:442–446. doi: 10.1136/hrt.80.5.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med United States. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 129.Cokkinos DV, Haralabopoulos GC, Kostis JB, Toutouzas PK. Efficacy of antithrombotic therapy in chronic heart failure: the HELAS study. Eur J Heart Fail England. 2006;8:428–432. doi: 10.1016/j.ejheart.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 130.Colucci WS, Packer M, Bristow MR, Gilbert EM, Cohn JN, Fowler MB, Krueger SK, Hershberger R, Uretsky BF, Bowers JA, Sackner-Bernstein JD, Young ST, Holcslaw TL, Lukas MA (1996) Carvedilol inhibits clinical progression in patients with mild symptoms of heart failure. US Carvedilol Heart Failure Study Group. Circulation United States 94:2800–2806 [DOI] [PubMed]
- 131.Cowie MR, Anker SD, Cleland JGF, Felker GM, Filippatos G, Jaarsma T, Jourdain P, Knight E, Massie B, Ponikowski P, López-Sendón J. Improving care for patients with acute heart failure: before, during and after hospitalization. ESC Hear Fail England. 2014;1:110–145. doi: 10.1002/ehf2.12021. [DOI] [PubMed] [Google Scholar]
- 132.Dalal HM, Taylor RS, Jolly K, Davis RC, Doherty P, Miles J, van Lingen R, Warren FC, Green C, Wingham J, Greaves C, Sadler S, Hillsdon M, Abraham C, Britten N, Frost J, Singh S, Hayward C, Eyre V, Paul K, Lang CC, Smith K. The effects and costs of home-based rehabilitation for heart failure with reduced ejection fraction: the REACH-HF multicentre randomized controlled trial. Eur J Prev Cardiol. 2019;26:262–272. doi: 10.1177/2047487318806358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dar O, Riley J, Chapman C, Dubrey SW, Morris S, Rosen SD, Roughton M, Cowie MR. A randomized trial of home telemonitoring in a typical elderly heart failure population in North West London: results of the Home-HF study. Eur J Heart Fail. 2009;11:319–325. doi: 10.1093/eurjhf/hfn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dargie HJ (2001) Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet (London, England) England 357:1385–1390 [DOI] [PubMed]
- 135.Daubert C, Gold MR, Abraham WT, Ghio S, Hassager C, Goode G, Szili-Török T, Linde C. Prevention of disease progression by cardiac resynchronization therapy in patients with asymptomatic or mildly symptomatic left ventricular dysfunction: insights from the European cohort of the REVERSE (Resynchronization Reverses Remodeling in Systolic L. J Am Coll Cardiol United States. 2009;54:1837–1846. doi: 10.1016/j.jacc.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 136.Dendale P, De KG, Troisfontaines P, Weytjens C, Mullens W, Elegeert I, Ector B, Houbrechts M, Willekens K, Hansen D. Effect of a telemonitoring-facilitated collaboration between general practitioner and heart failure clinic on mortality and rehospitalization rates in severe heart failure: the TEMA-HF 1 (TElemonitoring in the MAnagement of Heart Failure) study. Eur J Heart Fail England. 2012;14:333–340. doi: 10.1093/eurjhf/hfr144. [DOI] [PubMed] [Google Scholar]
- 137.DeWalt DA, Schillinger D, Ruo B, Bibbins-Domingo K, Baker DW, Holmes GM, Weinberger M, Macabasco-O’Connell A, Broucksou K, Hawk V, Grady KL, Erman B, Sueta CA, Chang PP, Cene CW, Wu J-R, Jones CD, Pignone M (2012) Multisite randomized trial of a single-session versus multisession literacy-sensitive self-care intervention for patients with heart failure. Circulation 125:2854–2862 [DOI] [PMC free article] [PubMed]
- 138.Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, Natale A. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637–1644. doi: 10.1161/CIRCULATIONAHA.115.019406. [DOI] [PubMed] [Google Scholar]
- 139.Digitalis Investigation Group The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336(8):525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 140.Domenichini G, Rahneva T, Diab IG, Dhillon OS, Campbell NG, Finlay MC, Baker V, Hunter RJ, Earley MJ, Schilling RJ (2016) The lung impedance monitoring in treatment of chronic heart failure (the LIMIT-CHF study). Europace 18(3):428–435. 10.1093/europace/euv293. Epub 2015 Dec 18. PMID: 26683599 [DOI] [PubMed]
- 141.Domingo M, Lupón J, González B, Crespo E, López R, Ramos A, Urrutia A, Pera G, Verdú JM, Bayes-Genis A (2011) Noninvasive remote telemonitoring for ambulatory patients with heart failure: effect on number of hospitalizations, days in hospital, and quality of life. CARME (CAtalan Remote Management Evaluation) study. Rev Esp Cardiol Spain 64:277–285 [DOI] [PubMed]
- 142.Domingues FB, Clausell N, Aliti GB, Dominguez DR, Rabelo ER. Education and telephone monitoring by nurses of patients with heart failure: randomized clinical trial. Arq Bras Cardiol Brazil. 2011;96:233–239. doi: 10.1590/S0066-782X2011005000014. [DOI] [PubMed] [Google Scholar]
- 143.Doughty RN, Wright SP, Pearl A, Walsh HJ, Muncaster S, Whalley GA, Gamble G, Sharpe N. Randomized, controlled trial of integrated heart failure management: the Auckland Heart Failure Management Study. Eur Heart J England. 2002;23:139–146. doi: 10.1053/euhj.2001.2712. [DOI] [PubMed] [Google Scholar]
- 144.Ducharme A, Doyon O, White M, Rouleau JL, Brophy JM (2005) Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial. C Can Med Assoc J = J l’Association medicale Can 173:40–45 [DOI] [PMC free article] [PubMed]
- 145.Dunagan WC, Littenberg B, Ewald GA, Jones CA, Emery VB, Waterman BM, Silverman DC, Rogers JG. Randomized trial of a nurse-administered, telephone-based disease management program for patients with heart failure. J Card Fail United States. 2005;11:358–365. doi: 10.1016/j.cardfail.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 146.Ekman I, Andersson B, Ehnfors M, Matejka G, Persson B, Fagerberg B. Feasibility of a nurse-monitored, outpatient-care programme for elderly patients with moderate-to-severe, chronic heart failure. Eur Heart J England. 1998;19:1254–1260. doi: 10.1053/euhj.1998.1095. [DOI] [PubMed] [Google Scholar]
- 147.Ellingsen Ø, Halle M, Conraads V, Støylen A, Dalen H, Delagardelle C, Larsen A-I, Hole T, Mezzani A, Van CEM, Videm V, Beckers P, Christle JW, Winzer E, Mangner N, Woitek F, Höllriegel R, Pressler A, Monk-Hansen T, Snoer M, Feiereisen P, Valborgland T, Kjekshus J, Hambrecht R, Gielen S, Karlsen T, Prescott E, Linke A. High-intensity interval training in patients with heart failure with reduced ejection fraction. Circulation. 2017;135:839–849. doi: 10.1161/CIRCULATIONAHA.116.022924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Erhardt L, MacLean A, Ilgenfritz J, Gelperin K, Blumenthal M (1995) Fosinopril attenuates clinical deterioration and improves exercise tolerance in patients with heart failure. Fosinopril Efficacy/Safety Trial (FEST) Study Group. Eur Heart J England 16:1892–1899 [DOI] [PubMed]
- 149.Fisher ML, Gottlieb SS, Plotnick GD, Greenberg NL, Patten RD, Bennett SK, Hamilton BP. Beneficial effects of metoprolol in heart failure associated with coronary artery disease: a randomized trial. J Am Coll Cardiol. 1994;23(4):943–950. doi: 10.1016/0735-1097(94)90641-6. [DOI] [PubMed] [Google Scholar]
- 150.Fox K, Ford I, Steg PG, Tendera M, Ferrari R (2008) Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet (London, England) England 372:807–816 [DOI] [PubMed]
- 151.Fragasso G, Palloshi A, Puccetti P, Silipigni C, Rossodivita A, Pala M, Calori G, Alfieri O, Margonato A. A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. J Am Coll Cardiol United States. 2006;48:992–998. doi: 10.1016/j.jacc.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 152.Gallagher J, James S, Keane C, Fitzgerald A, Travers B, Quigley E, Hecht C, Zhou S, Watson C, Ledwidge M, McDonald K. Heart failure virtual consultation: bridging the gap of heart failure care in the community - a mixed-methods evaluation. ESC Hear Fail. 2017;4:252–258. doi: 10.1002/ehf2.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gasparini M, Bocchiardo M, Lunati M, Ravazzi PA, Santini M, Zardini M, Signorelli S, Passardi M, Klersy C (2006) Comparison of 1-year effects of left ventricular and biventricular pacing in patients with heart failure who have ventricular arrhythmias and left bundle-branch block: the bi vs left ventricular pacing: an international pilot evaluation on heart failure. Am Heart J United States 152:155.e1–7 [DOI] [PubMed]
- 154.Gattis WA, Hasselblad V, Whellan DJ, O’Connor CM. Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team: results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) Study. Arch Intern Med United States. 1999;159:1939–1945. doi: 10.1001/archinte.159.16.1939. [DOI] [PubMed] [Google Scholar]
- 155.Giannini C, Fiorelli F, De CM, Guarracino F, Faggioni M, Giordano P, Spontoni P, Pieroni A, Petronio AS. Comparison of percutaneous mitral valve repair versus conservative treatment in severe functional mitral regurgitation. Am J Cardiol United States. 2016;117:271–277. doi: 10.1016/j.amjcard.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 156.Giannuzzi P, Saner H, Björnstad H, Fioretti P, Mendes M, Cohen-Solal A, Dugmore L, Hambrecht R, Hellemans I, McGee H, Perk J, Vanhees L, Veress G. Secondary prevention through cardiac rehabilitation: position paper of the Working Group on Cardiac Rehabilitation and Exercise Physiology of the European Society of Cardiology. Eur Heart J England. 2003;24:1273–1278. doi: 10.1016/S0195-668X(03)00198-2. [DOI] [PubMed] [Google Scholar]
- 157.Giordano A, Scalvini S, Zanelli E, Corrà U, Longobardi GL, Ricci VA, Baiardi P, Glisenti F. Multicenter randomised trial on home-based telemanagement to prevent hospital readmission of patients with chronic heart failure. Int J Cardiol Netherlands. 2009;131:192–199. doi: 10.1016/j.ijcard.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 158.Goldberg LR, Piette JD, Walsh MN, Frank TA, Jaski BE, Smith AL, Rodriguez R, Mancini DM, Hopton LA, Orav EJ, Loh E. Randomized trial of a daily electronic home monitoring system in patients with advanced heart failure: the Weight Monitoring in Heart Failure (WHARF) trial. Am Heart J United States. 2003;146:705–712. doi: 10.1016/S0002-8703(03)00393-4. [DOI] [PubMed] [Google Scholar]
- 159.Goldstein S, Kennedy HL, Hall C, Anderson JL, Gheorghiade M, Gottlieb S, Jessup M, Karlsberg RP, Friday G, Haskell L. Metoprolol CR/XL in patients with heart failure: a pilot study examining the tolerability, safety, and effect on left ventricular ejection fraction. Am Heart J United States. 1999;138:1158–1165. doi: 10.1016/S0002-8703(99)70083-9. [DOI] [PubMed] [Google Scholar]
- 160.Granger CB, Ertl G, Kuch J, Maggioni AP, McMurray J, Rouleau JL, Stevenson LW, Swedberg K, Young J, Yusuf S, Califf RM, Bart BA, Held P, Michelson EL, Sellers MA, Ohlin G, Sparapani R, Pfeffer MA. Randomized trial of candesartan cilexetil in the treatment of patients with congestive heart failure and a history of intolerance to angiotensin-converting enzyme inhibitors. Am Heart J United States. 2000;139:609–617. doi: 10.1016/S0002-8703(00)90037-1. [DOI] [PubMed] [Google Scholar]
- 161.Granger CB, McMurray JJ V, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K (2003) Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet (London, England) England 362:772–776 [DOI] [PubMed]
- 162.Hamaad A, Sosin M, Lip GYH, MacFadyen RJ. Short-term adjuvant atorvastatin improves frequency domain indices of heart rate variability in stable systolic heart failure. Cardiovasc drugs Ther United States. 2005;19:183–187. doi: 10.1007/s10557-005-2219-8. [DOI] [PubMed] [Google Scholar]
- 163.Hambrecht R, Niebauer J, Fiehn E, Kälberer B, Offner B, Hauer K, Riede U, Schlierf G, Kübler W, Schuler G. Physical training in patients with stable chronic heart failure: effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J Am Coll Cardiol United States. 1995;25:1239–1249. doi: 10.1016/0735-1097(94)00568-B. [DOI] [PubMed] [Google Scholar]
- 164.Hambrecht R, Gielen S, Linke A, Fiehn E, Yu J, Walther C, Schoene N, Schuler G. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. JAMA United States. 2000;283:3095–3101. doi: 10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- 165.Hamshere S, Arnous S, Choudhury T, Choudry F, Mozid A, Yeo C, Barrett C, Saunders N, Gulati A, Knight C, Locca D, Davies C, Cowie MR, Prasad S, Parmar M, Agrawal S, Jones D, Martin J, McKenna W, Mathur A. Randomized trial of combination cytokine and adult autologous bone marrow progenitor cell administration in patients with non-ischaemic dilated cardiomyopathy: the REGENERATE-DCM clinical trial. Eur Heart J. 2015;36:3061–3069. doi: 10.1093/eurheartj/ehv390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hancock HC, Close H, Mason JM, Murphy JJ, Fuat A, de Belder M, Hunt T, Baker A, Wilson D, Hungin APS. Feasibility of evidence-based diagnosis and management of heart failure in older people in care: a pilot randomised controlled trial. BMC Geriatr. 2012;12:70. doi: 10.1186/1471-2318-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hansen C, Loges C, Seidl K, Eberhardt F, Tröster H, Petrov K, Grönefeld G, Bramlage P, Birkenhauer F, Weiss C. INvestigation on Routine Follow-up in CONgestive HearT FAilure Patients with Remotely Monitored Implanted Cardioverter Defibrillators SysTems (InContact) BMC Cardiovasc Disord. 2018;18:131. doi: 10.1186/s12872-018-0864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Higgins SL, Hummel JD, Niazi IK, Giudici MC, Worley SJ, Saxon LA, Boehmer JP, Higginbotham MB, De MT, Foster E, Yong PG. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol United States. 2003;42:1454–1459. doi: 10.1016/S0735-1097(03)01042-8. [DOI] [PubMed] [Google Scholar]
- 170.Hindricks G, Taborsky M, Glikson M, Heinrich U, Schumacher B, Katz A, Brachmann J, Lewalter T, Goette A, Block M, Kautzner J, Sack S, Husser D, Piorkowski C, Søgaard P (2014) Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet (London, England) England 384:583–590 [DOI] [PubMed]
- 171.Idris S, Degheim G, Ghalayini W, Larsen TR, Nejad D, David S. Home telemedicine in heart failure: a pilot study of integrated telemonitoring and virtual provider appointments. Rev Cardiovasc Med United States. 2015;16:156–162. doi: 10.3909/ricm0760. [DOI] [PubMed] [Google Scholar]
- 172.Jolly K, Taylor RS, Lip GYH, Davies M, Davis R, Mant J, Singh S, Greenfield S, Ingram J, Stubley J, Bryan S, Stevens A. A randomized trial of the addition of home-based exercise to specialist heart failure nurse care: the Birmingham Rehabilitation Uptake Maximisation study for patients with Congestive Heart Failure (BRUM-CHF) study. Eur J Heart Fail. 2009;11:205–213. doi: 10.1093/eurjhf/hfn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jones DG, Wong T (2013) Catheter ablation versus rate control for atrial fibrillation: what have we learnt from the ARC-HF trial? Future Cardiol England 599–602 [DOI] [PubMed]
- 174.Kashem A, Droogan MT, Santamore WP, Wald JW, Bove AA. Managing heart failure care using an Internet-based telemedicine system. J Card Fail United States. 2008;14:121–126. doi: 10.1016/j.cardfail.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 175.Kasper EK, Gerstenblith G, Hefter G, Van AE, Brinker JA, Thiemann DR, Terrin M, Forman S, Gottlieb SH. A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission. J Am Coll Cardiol United States. 2002;39:471–480. doi: 10.1016/S0735-1097(01)01761-2. [DOI] [PubMed] [Google Scholar]
- 176.Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Böhm M, Boll H, Baumann G, Honold M, Koehler K, Gelbrich G, Kirwan B-A, Anker SD. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation United States. 2011;123:1873–1880. doi: 10.1161/CIRCULATIONAHA.111.018473. [DOI] [PubMed] [Google Scholar]
- 177.Komajda M. Prevalence of anemia in patients with chronic heart failure and their clinical characteristics. J Card Fail United States. 2004;10:S1–4. doi: 10.1016/j.cardfail.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 178.Kraai I, de Vries A, Vermeulen K, van Deursen V, van der Wal M, de Jong R, van Dijk R, Jaarsma T, Hillege H, Lesman I. The value of telemonitoring and ICT-guided disease management in heart failure: Results from the IN TOUCH study. Int J Med Inform Ireland. 2016;85:53–60. doi: 10.1016/j.ijmedinf.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 179.Krum H, Forbes A, Yallop J, Driscoll A, Croucher J, Chan B, Clark R, Davidson P, Huynh L, Kasper EK, Hunt D, Egan H, Stewart S, Piterman L, Tonkin A. Telephone support to rural and remote patients with heart failure: the Chronic Heart Failure Assessment by Telephone (CHAT) study. Cardiovasc Ther England. 2013;31:230–237. doi: 10.1111/1755-5922.12009. [DOI] [PubMed] [Google Scholar]
- 180.Krumholz HM, Amatruda J, Smith GL, Mattera JA, Roumanis SA, Radford MJ, Crombie P, Vaccarino V. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. J Am Coll Cardiol United States. 2002;39:83–89. doi: 10.1016/S0735-1097(01)01699-0. [DOI] [PubMed] [Google Scholar]
- 181.Landolina M, Perego GB, Lunati M, Curnis A, Guenzati G, Vicentini A, Parati G, Borghi G, Zanaboni P, Valsecchi S, Marzegalli M. Remote monitoring reduces healthcare use and improves quality of care in heart failure patients with implantable defibrillators: the evolution of management strategies of heart failure patients with implantable defibrillators (EVOLVO) study. Circulation United States. 2012;125:2985–2992. doi: 10.1161/CIRCULATIONAHA.111.088971. [DOI] [PubMed] [Google Scholar]
- 182.Laramee AS, Levinsky SK, Sargent J, Ross R, Callas P. Case management in a heterogeneous congestive heart failure population: a randomized controlled trial. Arch Intern Med United States. 2003;163:809–817. doi: 10.1001/archinte.163.7.809. [DOI] [PubMed] [Google Scholar]
- 183.Linde C, Leclercq C, Rex S, Garrigue S, Lavergne T, Cazeau S, McKenna W, Fitzgerald M, Deharo J-C, Alonso C, Walker S, Braunschweig F, Bailleul C, Daubert J-C. Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol United States. 2002;40:111–118. doi: 10.1016/S0735-1097(02)01932-0. [DOI] [PubMed] [Google Scholar]
- 184.Leclercq C, Cazeau S, Lellouche D, Fossati F, Anselme F, Davy J-M, Sadoul N, Klug D, Mollo L, Daubert J-C (2007) Upgrading from single chamber right ventricular to biventricular pacing in permanently paced patients with worsening heart failure: the RD-CHF Study. Pacing Clin Electrophysiol United States 30(Suppl 1):S23–30 [DOI] [PubMed]
- 185.Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, Daubert C (2008) Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol United States 52:1834–1843 [DOI] [PubMed]
- 186.Liu M-H, Wang C-H, Huang Y-Y, Tung T-H, Lee C-M, Yang N-I, Wang J-S, Kuo L-T, Cherng W-J. Edema index-guided disease management improves 6-month outcomes of patients with acute heart failure. Int Heart J Japan. 2012;53:11–17. doi: 10.1536/ihj.53.11. [DOI] [PubMed] [Google Scholar]
- 187.Lüthje L, Vollmann D, Seegers J, Sohns C, Hasenfuß G, Zabel M. A randomized study of remote monitoring and fluid monitoring for the management of patients with implanted cardiac arrhythmia devices. Eur Eur pacing, arrhythmias, Card Electrophysiol J Work groups Card pacing, arrhythmias, Card Cell Electrophysiol Eur Soc Cardiol England. 2015;17:1276–1281. doi: 10.1093/europace/euv039. [DOI] [PubMed] [Google Scholar]
- 188.Luttik MLA, Jaarsma T, van Geel PP, Brons M, Hillege HL, Hoes AW, Jong R de, Linssen G, Lok DJA, Berge M, van Veldhuisen DJ (2014) Long-term follow-up in optimally treated and stable heart failure patients: primary care vs. heart failure clinic. Results of the COACH-2 study. Eur J Heart Fail England 16:1241–1248 [DOI] [PubMed]
- 189.MacDonald MR, Connelly DT, Hawkins NM, Steedman T, Payne J, Shaw M, Denvir M, Bhagra S, Small S, Martin W, McMurray JJV, Petrie MC. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart England. 2011;97:740–747. doi: 10.1136/hrt.2010.207340. [DOI] [PubMed] [Google Scholar]
- 190.Maggioni AP, Anand I, Gottlieb SO, Latini R, Tognoni G, Cohn JN. Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin-converting enzyme inhibitors. J Am Coll Cardiol United States. 2002;40:1414–1421. doi: 10.1016/S0735-1097(02)02304-5. [DOI] [PubMed] [Google Scholar]
- 191.Margulies KB, Hernandez AF, Redfield MM, Givertz MM, Oliveira GH, Cole R, Mann DL, Whellan DJ, Kiernan MS, Felker GM, McNulty SE, Anstrom KJ, Shah MR, Braunwald E, Cappola TP. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2016;316:500–508. doi: 10.1001/jama.2016.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, Schunkert H, Christ H, Vogt J, Bänsch D. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med United States. 2018;378:417–427. doi: 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]
- 193.Martinelli Filho M, De Siqueira SF, Costa R, Greco OT, Moreira LF, D'avila A, Heist EK. Conventional versus biventricular pacing in heart failure and bradyarrhythmia: the COMBAT study. J Card Fail. 2010;16(4):293–300. doi: 10.1016/j.cardfail.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 194.Mathiasen AB, Qayyum AA, Jørgensen E, Helqvist S, Fischer-Nielsen A, Kofoed KF, Haack-Sørensen M, Ekblond A, Kastrup J. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial) Eur Heart J England. 2015;36:1744–1753. doi: 10.1093/eurheartj/ehv136. [DOI] [PubMed] [Google Scholar]
- 195.Menasché P. Cardiac cell therapy trials: chronic myocardial infarction and congestive heart failure. J Cardiovasc Transl Res United States. 2008;1:201–206. doi: 10.1007/s12265-008-9017-1. [DOI] [PubMed] [Google Scholar]
- 196.McDonald K, Ledwidge M, Cahill J, Quigley P, Maurer B, Travers B, Ryder M, Kieran E, Timmons L, Ryan E. Heart failure management: multidisciplinary care has intrinsic benefit above the optimization of medical care. J Card Fail United States. 2002;8:142–148. doi: 10.1054/jcaf.2002.124340. [DOI] [PubMed] [Google Scholar]
- 197.McMurray JJ V, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, Olofsson B, Yusuf S, Pfeffer MA (2003) Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet (London, England) England 362:767–771 [DOI] [PubMed]
- 198.(1999) Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 353(9169):2001–2007. PMID: 10376614 [PubMed]
- 199.Morgan JM, Kitt S, Gill J, McComb JM, Ng GA, Raftery J, Roderick P, Seed A, Williams SG, Witte KK, Wright DJ, Harris S, Cowie MR. Remote management of heart failure using implantable electronic devices. Eur Heart J. 2017;38:2352–2360. doi: 10.1093/eurheartj/ehx227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mortara A, Pinna GD, Johnson P, Maestri R, Capomolla S, La RMT, Ponikowski P, Tavazzi L, Sleight P. Home telemonitoring in heart failure patients: the HHH study (Home or Hospital in Heart Failure) Eur J Heart Fail. 2009;11:312–318. doi: 10.1093/eurjhf/hfp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Moss AJ, Daubert J, Zareba W. MADIT-II: clinical implications. Card Electrophysiol Rev United States. 2002;6:463–465. doi: 10.1023/A:1021104929368. [DOI] [PubMed] [Google Scholar]
- 202.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NAM, 3rd, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med United States. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 203.Mozid A, Yeo C, Arnous S, Ako E, Saunders N, Locca D, Brookman P, Archbold RA, Rothman M, Mills P, Agrawal S, Martin J, Mathur A. Safety and feasibility of intramyocardial versus intracoronary delivery of autologous cell therapy in advanced heart failure: the REGENERATE-IHD pilot study. Regen Med England. 2014;9:269–278. doi: 10.2217/rme.14.3. [DOI] [PubMed] [Google Scholar]
- 204.Mueller L, Myers J, Kottman W, Oswald U, Boesch C, Arbrol N, Dubach P. Exercise capacity, physical activity patterns and outcomes six years after cardiac rehabilitation in patients with heart failure. Clin Rehabil England. 2007;21:923–931. doi: 10.1177/0269215507079097. [DOI] [PubMed] [Google Scholar]
- 205.Node K, Fujita M, Kitakaze M, Hori M, Liao JK. Short-term statin therapy improves cardiac function and symptoms in patients with idiopathic dilated cardiomyopathy. Circulation. 2003;108:839–843. doi: 10.1161/01.CIR.0000084539.58092.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Obadia J-F, Messika-Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, Lefèvre T, Piot C, Rouleau F, Carrié D, Nejjari M, Ohlmann P, Leclercq F, Etienne C Saint, Teiger E, Leroux L, Karam N, Michel N, Gilard M, Donal E, Trochu J-N, Cormier B, Armoiry X, Boutitie F, Maucort-Boulch D, Barnel C, Samson G, Guerin P, Vahanian A, Mewton N (2018) Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med United States 379:2297–2306
- 207.Packer M, Gheorghiade M, Young JB, Costantini PJ, Adams KF, Cody RJ, Smith LK, Van Voorhees L, Gourley LA, Jolly MK (1993) Withdrawal of digoxin from patients with chronic heart failure treated with angiotensin-converting-enzyme inhibitors. RADIANCE Study. N Engl J Med United States 329:1–7 [DOI] [PubMed]
- 208.Packer M, Colucci WS, Sackner-Bernstein JD, Liang CS, Goldscher DA, Freeman I, Kukin ML, Kinhal V, Udelson JE, Klapholz M, Gottlieb SS, Pearle D, Cody RJ, Gregory JJ, Kantrowitz NE, LeJemtel TH, Young ST, Lukas MA, Shusterman NH (1996) Double-blind, placebo-controlled study of the effects of carvedilol in patients with moderate to severe heart failure. The PRECISE Trial. Prospective Randomized Evaluation of Carvedilol on Symptoms and Exercise. Circulation United States 94:2793–2799 [DOI] [PubMed]
- 209.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH (1996) The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med United States 334:1349–1355 [DOI] [PubMed]
- 210.Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, DeMets DL. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344(22):1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 211.Passino C, Severino S, Poletti R, Piepoli MF, Mammini C, Clerico A, Gabutti A, Nassi G, Emdin M. Aerobic training decreases B-type natriuretic peptide expression and adrenergic activation in patients with heart failure. J Am Coll Cardiol United States. 2006;47:1835–1839. doi: 10.1016/j.jacc.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 212.Patel AN, Silva F, Winters AA. Stem cell therapy for heart failure. Heart Fail Clin United States. 2015;11:275–286. doi: 10.1016/j.hfc.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 213.Pätilä T, Lehtinen M, Vento A, Schildt J, Sinisalo J, Laine M, Hämmäinen P, Nihtinen A, Alitalo R, Nikkinen P, Ahonen A, Holmström M, Lauerma K, Pöyhiä R, Kupari M, Kankuri E, Harjula A. Autologous bone marrow mononuclear cell transplantation in ischemic heart failure: a prospective, controlled, randomized, double-blind study of cell transplantation combined with coronary bypass. J Hear lung Transplant Off Publ Int Soc Hear Transplant United States. 2014;33:567–574. doi: 10.1016/j.healun.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 214.Perin EC, Silva G V, Zheng Y, Gahremanpour A, Canales J, Patel D, Fernandes MR, Keller LH, Quan X, Coulter SA, Moore WH, Herlihy JP, Willerson JT (2012) Randomized, double-blind pilot study of transendocardial injection of autologous aldehyde dehydrogenase-bright stem cells in patients with ischemic heart failure. Am Heart J United States 163:415–421, 421.e1 [DOI] [PubMed]
- 215.Peters-Klimm F, Campbell S, Hermann K, Kunz CU, Müller-Tasch T, Szecsenyi J. Case management for patients with chronic systolic heart failure in primary care: the HICMan exploratory randomised controlled trial. Trials. 2010;11:56. doi: 10.1186/1745-6215-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJJ, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC (1992) Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl JMed United States 327:669–677 [DOI] [PubMed]
- 217.Piepoli MF, Villani GQ, Corrà U, Aschieri D, Rusticali G. Time course of effects of cardiac resynchronization therapy in chronic heart failure: benefits in patients with preserved exercise capacity. Pacing Clin Electrophysiol United States. 2008;31:701–708. doi: 10.1111/j.1540-8159.2008.01073.x. [DOI] [PubMed] [Google Scholar]
- 218.Pinter A, Mangat I, Korley V, Connolly S, Connors S, Gardner M, Philippon F, Sterns L, Thibault B, Dorian P. Assessment of resynchronization therapy on functional status and quality of life in patients requiring an implantable defibrillator. Pacing Clin Electrophysiol United States. 2009;32:1509–1519. doi: 10.1111/j.1540-8159.2009.02543.x. [DOI] [PubMed] [Google Scholar]
- 219.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J (1999) The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med United States 341:709–717 [DOI] [PubMed]
- 220.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med United States. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 221.Pokushalov E, Romanov A, Prohorova D, Cherniavsky A, Karaskov A, Gersak B. Coronary artery bypass grafting with and without concomitant epicardial cardiac resynchronization therapy in patients with ischemic cardiomyopathy: a randomized study. Heart Surg Forum United States. 2010;13:E177–E184. doi: 10.1532/HSF98.20091149. [DOI] [PubMed] [Google Scholar]
- 222.Pokushalov E, Romanov A, Corbucci G, Prohorova D, Chernyavsky A, Larionov P, Terekhov I, Artyomenko S, Kliver E, Shirokova N, Karaskov A, Dib N. Cardiac resynchronization therapy and bone marrow cell transplantation in patients with ischemic heart failure and electromechanical dyssynchrony: a randomized pilot study. J Cardiovasc Transl Res United States. 2011;4:767–778. doi: 10.1007/s12265-011-9283-1. [DOI] [PubMed] [Google Scholar]
- 223.Prabhu S, Taylor AJ, Costello BT, Kaye DM, McLellan AJA, Voskoboinik A, Sugumar H, Lockwood SM, Stokes MB, Pathik B, Nalliah CJ, Wong GR, Azzopardi SM, Gutman SJ, Lee G, Layland J, Mariani JA, Ling L-H, Kalman JM, Kistler PM. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA-MRI Study. J Am Coll Cardiol United States. 2017;70:1949–1961. doi: 10.1016/j.jacc.2017.08.041. [DOI] [PubMed] [Google Scholar]
- 224.Ramachandran K, Husain N, Maikhuri R, Seth S, Vij A, Kumar M, Srivastava N, Prabhakaran D, Airan B, Reddy KS. Impact of a comprehensive telephone-based disease management programme on quality-of-life in patients with heart failure. Natl Med J India India. 2007;20:67–73. [PubMed] [Google Scholar]
- 225.Rosano GMC, Vitale C, Sposato B, Mercuro G, Fini M. Trimetazidine improves left ventricular function in diabetic patients with coronary artery disease: a double-blind placebo-controlled study. Cardiovasc Diabetol. 2003;2:16. doi: 10.1186/1475-2840-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, Brugada J, Dickstein K, Ford I, Gorcsan J, 3rd, Gras D, Krum H, Sogaard P, Holzmeister J. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med United States. 2013;369:1395–1405. doi: 10.1056/NEJMoa1306687. [DOI] [PubMed] [Google Scholar]
- 227.Sardu C, Santamaria M, Rizzo MR, Barbieri M, di Marino M, Paolisso G, Santulli G, Marfella R. Telemonitoring in heart failure patients treated by cardiac resynchronisation therapy with defibrillator (CRT-D): the TELECART Study. Int J Clin Pract. 2016;70:569–576. doi: 10.1111/ijcp.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Scherr D, Kastner P, Kollmann A, Hallas A, Auer J, Krappinger H, Schuchlenz H, Stark G, Grander W, Jakl G, Schreier G, Fruhwald FM (2009) Effect of home-based telemonitoring using mobile phone technology on the outcome of heart failure patients after an episode of acute decompensation: randomized controlled trial. J Med Internet Res 11:e34 [DOI] [PMC free article] [PubMed]
- 229.Schou M, Gustafsson F, Videbaek L, Tuxen C, Keller N, Handberg J, Sejr Knudsen A, Espersen G, Markenvard J, Egstrup K, Ulriksen H, Hildebrandt PR. Extended heart failure clinic follow-up in low-risk patients: a randomized clinical trial (NorthStar) Eur Heart J England. 2013;34:432–442. doi: 10.1093/eurheartj/ehs235. [DOI] [PubMed] [Google Scholar]
- 230.Sisk JE, Hebert PL, Horowitz CR, McLaughlin MA, Wang JJ, Chassin MR. Effects of nurse management on the quality of heart failure care in minority communities: a randomized trial. Ann Intern Med. 2006;145:273–283. doi: 10.7326/0003-4819-145-4-200608150-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Smith CE, Piamjariyakul U, Wick JA, Spertus JA, Russell C, Dalton KM, Elyachar A, Vacek JL, Reeder KM, Nazir N, Ellerbeck EF. Multidisciplinary group clinic appointments: the Self-Management and Care of Heart Failure (SMAC-HF) trial. Circ Heart Fail. 2014;7:888–894. doi: 10.1161/CIRCHEARTFAILURE.113.001246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sola S, Mir MQS, Lerakis S, Tandon N, Khan BV. Atorvastatin improves left ventricular systolic function and serum markers of inflammation in nonischemic heart failure. J Am Coll Cardiol United States. 2006;47:332–337. doi: 10.1016/j.jacc.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 233.Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med United States. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 234.Yusuf S, Pitt B, Davis CE, Hood WBJ, Cohn JN. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med United States. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 235.Spargias KS, Hall AS, Greenwood DC, Ball SG. beta blocker treatment and other prognostic variables in patients with clinical evidence of heart failure after acute myocardial infarction: evidence from the AIRE study. Heart. 1999;81:25–32. doi: 10.1136/hrt.81.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med United States. 2018;379:2307–2318. doi: 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]
- 237.Sturm B, Pacher R, Strametz-Juranek J, Berger R, Frey B, Stanek B. Effect of β1 blockade with atenolol on progression of heart failure in patients pretreated with high-dose enalapril. Eur J Heart Fail John Wiley & Sons, Ltd. 2000;2:407–412. doi: 10.1016/S1388-9842(00)00120-3. [DOI] [PubMed] [Google Scholar]
- 238.Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L (2010) Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet (London, England) England 376:875–885 [DOI] [PubMed]
- 239.Takano H, Mizuma H, Kuwabara Y, Sato Y, Shindo S, Kotooka N, Fujimatsu D, Kobayashi Y, Inoue T, Node K, Komuro I. Effects of pitavastatin in Japanese patients with chronic heart failure: the Pitavastatin Heart Failure Study (PEARL Study) Circ J Japan. 2013;77:917–925. doi: 10.1253/circj.CJ-12-1062. [DOI] [PubMed] [Google Scholar]
- 240.Tang ASL, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, Hohnloser SH, Nichol G, Birnie DH, Sapp JL, Yee R, Healey JS, Rouleau JL. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med United States. 2010;363:2385–2395. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 241.CONSENSUS Trial Study Group. Swedberg K, Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study CONSENSUS) Am J Cardiol. 1988;62(2):60A–66A. doi: 10.1016/S0002-9149(88)80087-0. [DOI] [PubMed] [Google Scholar]
- 242.Thibault B, Harel F, Ducharme A, White M, Frasure-Smith N, Roy D, Philippon F, Dorian P, Talajic M, Dubuc M, Gagné P, Guerra PG, Macle L, Rivard L, Khairy P. Evaluation of resynchronization therapy for heart failure in patients with a QRS duration greater than 120 ms (GREATER-EARTH) trial: rationale, design, and baseline characteristics. Can J Cardiol England. 2011;27:779–786. doi: 10.1016/j.cjca.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 243.Thibault B, Harel F, Ducharme A, White M, Ellenbogen KA, Frasure-Smith N, Roy D, Philippon F, Dorian P, Talajic M, Dubuc M, Guerra PG, Macle L, Rivard L, Andrade J, Khairy P. Cardiac resynchronization therapy in patients with heart failure and a QRS complex <120 milliseconds: the Evaluation of Resynchronization Therapy for Heart Failure (LESSER-EARTH) trial. Circulation United States. 2013;127:873–881. doi: 10.1161/CIRCULATIONAHA.112.001239. [DOI] [PubMed] [Google Scholar]
- 244.Tsuyuki RT, Fradette M, Johnson JA, Bungard TJ, Eurich DT, Ashton T, Gordon W, Ikuta R, Kornder J, Mackay E, Manyari D, O’Reilly K, Semchuk W. A multicenter disease management program for hospitalized patients with heart failure. J Card Fail United States. 2004;10:473–480. doi: 10.1016/j.cardfail.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 245.Tuunanen H, Engblom E, Naum A, Någren K, Scheinin M, Hesse B, Juhani Airaksinen KE, Nuutila P, Iozzo P, Ukkonen H, Opie LH, Knuuti J. Trimetazidine, a metabolic modulator, has cardiac and extracardiac benefits in idiopathic dilated cardiomyopathy. Circulation United States. 2008;118:1250–1258. doi: 10.1161/CIRCULATIONAHA.108.778019. [DOI] [PubMed] [Google Scholar]
- 246.Udelson JE, Feldman AM, Greenberg B, Pitt B, Mukherjee R, Solomon HA, Konstam MA. Randomized, double-blind, multicenter, placebo-controlled study evaluating the effect of aldosterone antagonism with eplerenone on ventricular remodeling in patients with mild-to-moderate heart failure and left ventricular systolic dysfunction. Circ Heart Fail United States. 2010;3:347–353. doi: 10.1161/CIRCHEARTFAILURE.109.906909. [DOI] [PubMed] [Google Scholar]
- 247.Uretsky BF, Young JB, Shahidi FE, Yellen LG, Harrison MC, Jolly MK (1993) Randomized study assessing the effect of digoxin withdrawal in patients with mild to moderate chronic congestive heart failure: results of the PROVED trial. PROVED Investigative Group. J Am Coll Cardiol United States 22:955–962 [DOI] [PubMed]
- 248.van Veldhuisen DJ, Cohen-Solal A, Böhm M, Anker SD, Babalis D, Roughton M, Coats AJS, Poole-Wilson PA, Flather MD. Beta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: Data From SENIORS (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure) J Am Coll Cardiol United States. 2009;53:2150–2158. doi: 10.1016/j.jacc.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 249.van Veldhuisen DJ, Braunschweig F, Conraads V, Ford I, Cowie MR, Jondeau G, Kautzner J, Aguilera RM, Lunati M, Yu CM, Gerritse B, Borggrefe M. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation United States. 2011;124:1719–1726. doi: 10.1161/CIRCULATIONAHA.111.043042. [DOI] [PubMed] [Google Scholar]
- 250.Villani A, Malfatto G, Rosa F Della, Branzi G, Boarin S, Borghi C, Cosentino E, Gualerzi M, Coruzzi P, Molinari E, Compare A, Cassi M, Collatina S, Parati G (2007) Disease management for heart failure patients: role of wireless technologies for telemedicine. The ICAROS project. G Ital Cardiol (Rome) Italy 8:107–114 [PubMed]
- 251.Villani A, Malfatto G, Compare A, Rosa F Della, Bellardita L, Branzi G, Molinari E, Parati G (2014) Clinical and psychological telemonitoring and telecare of high risk heart failure patients. J Telemed Telecare England 20:468–475 [DOI] [PubMed]
- 252.Vitale C, Wajngaten M, Sposato B, Gebara O, Rossini P, Fini M, Volterrani M, Rosano GMC. Trimetazidine improves left ventricular function and quality of life in elderly patients with coronary artery disease. Eur Heart J England. 2004;25:1814–1821. doi: 10.1016/j.ehj.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 253.Vizzardi E, D’Aloia A, Giubbini R, Bordonali T, Bugatti S, Pezzali N, Romeo A, Dei Cas A, Metra M, Dei CL. Effect of spironolactone on left ventricular ejection fraction and volumes in patients with class I or II heart failure. Am J Cardiol United States. 2010;106:1292–1296. doi: 10.1016/j.amjcard.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 254.Vrtovec B, Okrajsek R, Golicnik A, Ferjan M, Starc V, Schlegel TT, Radovancevic B. Atorvastatin therapy may reduce the incidence of sudden cardiac death in patients with advanced chronic heart failure. J Card Fail United States. 2008;14:140–144. doi: 10.1016/j.cardfail.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 255.Vuorinen A-L, Leppänen J, Kaijanranta H, Kulju M, Heliö T, Gils M van, Lähteenmäki J (2014) Use of home telemonitoring to support multidisciplinary care of heart failure patients in Finland: randomized controlled trial. J Med Internet Res 16:e282 [DOI] [PMC free article] [PubMed]
- 256.Weintraub A, Gregory D, Patel AR, Levine D, Venesy D, Perry K, Delano C, Konstam MA. A multicenter randomized controlled evaluation of automated home monitoring and telephonic disease management in patients recently hospitalized for congestive heart failure: the SPAN-CHF II trial. J Card Fail United States. 2010;16:285–292. doi: 10.1016/j.cardfail.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 257.Wierzchowiecki M, Poprawski K, Nowicka A, Kandziora M, Piatkowska A, Jankowiak M, Michałowicz B, Stawski W, Dziamska M, Kaszuba D, Szymanowska K, Michalski M. A new programme of multidisciplinary care for patients with heart failure in Poznań: one-year follow-up. Kardiol Pol Poland. 2006;64:1062–1063. [PubMed] [Google Scholar]
- 258.Willenheimer R, Rydberg E, Cline C, Broms K, Hillberger B, Oberg L, Erhardt L. Effects on quality of life, symptoms and daily activity 6 months after termination of an exercise training programme in heart failure patients. Int J Cardiol Netherlands. 2001;77:25–31. doi: 10.1016/S0167-5273(00)00383-1. [DOI] [PubMed] [Google Scholar]
- 259.Wojnicz R, Wilczek K, Nowalany-Kozielska E, Szyguła-Jurkiewicz B, Nowak J, Poloński L, Dyrbuś K, Badziński A, Mercik G, Zembala M, Wodniecki J, Rozek MM. Usefulness of atorvastatin in patients with heart failure due to inflammatory dilated cardiomyopathy and elevated cholesterol levels. Am J Cardiol United States. 2006;97:899–904. doi: 10.1016/j.amjcard.2005.09.142. [DOI] [PubMed] [Google Scholar]
- 260.Xie R, Cui W, Liu F, Yang C, Pei W, Lu J (2010) Statin therapy shortens QTc, QTcd, and improves cardiac function in patients with chronic heart failure. Int J Cardiol Netherlands 255–257 [DOI] [PubMed]
- 261.Yamada T, Azuma A, Sasaki S, Sawada T, Matsubara H. Randomized evaluation of atorvastatin in patients with coronary heart disease: a serial intravascular ultrasound study. Circ J Japan. 2007;71:1845–1850. doi: 10.1253/circj.71.1845. [DOI] [PubMed] [Google Scholar]
- 262.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, Canby RC, Schroeder JS, Liem LB, Hall S, Wheelan K. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA United States. 2003;289:2685–2694. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 263.Zan S, Agboola S, Moore SA, Parks KA, Kvedar JC, Jethwani K (2015) Patient engagement with a mobile web-based self-management: a pilot study. JMIR mHealth uHealth 3(2):e3789 [DOI] [PMC free article] [PubMed]
- 264.Zannad F, McMurray JJV, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med United States. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [Google Scholar]
- 265.Zannad F, Anker SD, Byra WM, Cleland JGF, Fu M, Gheorghiade M, Lam CSP, Mehra MR, Neaton JD, Nessel CC, Spiro TE, van Veldhuisen DJ, Greenberg B. Rivaroxaban in patients with heart failure, sinus rhythm, and coronary disease. N Engl J Med United States. 2018;379:1332–1342. doi: 10.1056/NEJMoa1808848. [DOI] [PubMed] [Google Scholar]
- 266.Adamson PB, Ginn G, Anker SD, Bourge RC, Abraham WT (2017) Remote haemodynamic-guided care for patients with chronic heart failure: a meta-analysis of completed trials. Eur J Heart Fail Hoboken, New Jersey: John Wiley & Sons, Inc. 19:426–433 [DOI] [PubMed]
- 267.Agasthi P, Lee JZ, Amin M, Al-Saffar F, Goel V, Tseng A, Almader-Douglas D, Killu AM, Deshmukh AJ, Del-Carpio Munoz F, Mulpuru SK. Catheter ablation for treatment of atrial fibrillation in patients with heart failure with reduced ejection fraction: a systematic review and meta-analysis. J Arrhythm. 2019;35:171–181. doi: 10.1002/joa3.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Al-Majed NS, McAlister FA, Bakal JA, Ezekowitz JA (2011) Meta-analysis: cardiac resynchronization therapy for patients with less symptomatic heart failure. Ann Intern Med 154:401-+ [DOI] [PubMed]
- 269.Alotaibi S, Hernandez-Montfort J, Ali OE, El-Chilali K, Perez BA. Remote monitoring of implantable cardiac devices in heart failure patients: a systematic review and meta-analysis of randomized controlled trials. Heart Fail Rev. 2020;25(3):469–479. doi: 10.1007/s10741-020-09923-1. [DOI] [PubMed] [Google Scholar]
- 270.AlTurki A, Proietti R, Dawas A, Alturki H, Huynh T, Essebag V. Catheter ablation for atrial fibrillation in heart failure with reduced ejection fraction: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2019;19(1):1–13. doi: 10.1186/s12872-019-0998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Benito-González T, Estévez-Loureiro R, Villablanca PA, Armeni P, Iglesias-Gárriz I, Minguito C, Garrote C, Prado AP de, Tundidor-Sanz E, Gualis J, Fernández-Vázquez F (2020) Percutaneous Mitral Valve Repair Vs. Stand-alone medical therapy in patients with functional mitral regurgitation and heart failure. Cardiovasc Revasc Med United States: Elsevier 21:52–60 [DOI] [PubMed]
- 272.Bertaina M, Galluzzo A, D’Ascenzo F, Conrotto F, Grosso Marra W, Frea S, Alunni G, Crimi G, Moretti C, Montefusco A, D’Amico M, Perl L, Rinaldi M, Giustetto C, De FGM. Prognostic impact of MitraClip in patients with left ventricular dysfunction and functional mitral valve regurgitation: a comprehensive meta-analysis of RCTs and adjusted observational studies. Int J Cardiol Netherlands: Elsevier. 2019;290:70–76. doi: 10.1016/j.ijcard.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 273.Bjarnason-Wehrens B, Nebel R, Jensen K, Hackbusch M, Grilli M, Gielen S, Schwaab B, Rauch B, German Soc Of C (2020) Exercise-based cardiac rehabilitation in patients with reduced left ventricular ejection fraction: the Cardiac Rehabilitation Outcome Study in Heart Failure (CROS-HF): a systematic review and meta-analysis. Eur J Prev Cardiol 27:929–952 [DOI] [PMC free article] [PubMed]
- 274.Bonsu KO, Reidpath DD, Kadirvelu A. Lipophilic statin versus rosuvastatin (hydrophilic) treatment for heart failure: a meta-analysis and adjusted indirect comparison of randomised trials. Cardiovasc Drugs Ther. 2016;30:177–188. doi: 10.1007/s10557-015-6636-z. [DOI] [PubMed] [Google Scholar]
- 275.Carbo A, Gupta M, Tamariz L, Palacio A, Levis S, Nemeth Z, Dang S. Mobile technologies for managing heart failure: a systematic review and meta-analysis. Telemed J E Health United States: Mary Ann Liebert, Inc; 2018. [DOI] [PubMed] [Google Scholar]
- 276.De Vecchis R, Ariano C (2017) Differential efficacy profile of aldosterone receptor antagonists, depending on the type of chronic heart failure, whether with reduced or preserved left ventricular ejection fraction-results of a meta-analysis of randomized controlled trials. Cardiovasc Diagn Ther China (Republic : 1949- ): AME Publishing Company 7:272–287 [DOI] [PMC free article] [PubMed] [Retracted]
- 277.Driscoll A, Currey J, Tonkin A, Krum H (2015) Nurse-led titration of angiotensin converting enzyme inhibitors, beta-adrenergic blocking agents, and angiotensin receptor blockers for people with heart failure with reduced ejection fraction. Cochrane Database Syst Rev John Wiley & Sons, Ltd [DOI] [PMC free article] [PubMed]
- 278.Emdin CA, Callender T, Cao J, McMurray JJ V, Rahimi K (2015) Meta-analysis of large-scale randomized trials to determine the effectiveness of inhibition of the renin-angiotensin aldosterone system in heart failure. Am J Cardiol Philadelphia, Pennsylvania: Elsevier B.V 116:155–161 [DOI] [PubMed]
- 279.Fisher SA, Doree C, Mathur A, Martin-Rendon E. Meta-analysis of cell therapy trials for patients with heart failure. Circ Res. 2015;116:1361–1377. doi: 10.1161/CIRCRESAHA.116.304386. [DOI] [PubMed] [Google Scholar]
- 280.Fisher SA, Doree C, Mathur A, Taggart DP, Martin-Rendon E (2016) Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst Rev John Wiley & Sons, Ltd [DOI] [PMC free article] [PubMed]
- 281.Gandhi S, Mosleh W, Sharma UC, Demers C, Farkouh ME, Schwalm JD. Multidisciplinary heart failure clinics are associated with lower heart failure hospitalization and mortality: systematic review and meta-analysis. Can J Cardiol. 2017;33(10):1237–1244. doi: 10.1016/j.cjca.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 282.Halawa A, Enezate T, Flaker G. Device monitoring in heart failure management: outcomes based on a systematic review and meta-analysis. Cardiovasc Diagn Ther. 2019;9(4):386. doi: 10.21037/cdt.2019.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Hartmann C, Bosch NL, Miguita LD, Tierie E, Zytinski L, Baena CP. The effect of ivabradine therapy on heart failure patients with reduced ejection fraction: a systematic review and meta-analysis. Int J Clin Pharm. 2018;40:1443–1453. doi: 10.1007/s11096-018-0715-8. [DOI] [PubMed] [Google Scholar]
- 284.Hu LJ, Chen YQ, Deng SB, Du JL, She Q. Additional use of an aldosterone antagonist in patients with mild to moderate chronic heart failure: a systematic review and meta-analysis. Br J Clin Pharmacol. 2013;75(5):1202–1212. doi: 10.1111/bcp.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Inglis SC, Clark RA, McAlister FA, Stewart S, Cleland JG. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: Abridged Coc. Eur J Hear Fail. 2011;13:1028–1040. doi: 10.1093/eurjhf/hfr039. [DOI] [PubMed] [Google Scholar]
- 286.Inglis SC, Clark RA, Dierckx R, Prieto-Merino D, Cleland JGF (2015) Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev [DOI] [PMC free article] [PubMed]
- 287.Japp D, Shah A, Fisken S, Denvir M, Shenkin S, Japp A. Mineralocorticoid receptor antagonists in elderly patients with heart failure: a systematic review and meta-analysis. Age Ageing. 2017;46:18–25. doi: 10.1093/ageing/afw138. [DOI] [PubMed] [Google Scholar]
- 288.Jonkman NH, Westland H, Groenwold RHH, Ågren S, Anguita M, Blue L, Bruggink-André de la Porte PWF, DeWalt DA, Hebert PL, Heisler M, Jaarsma T, Kempen GIJM, Leventhal ME, Lok DJA, Mårtensson J, Muñiz J, Otsu H, Peters-Klimm F, Rich MW, Riegel B (2016) What are effective program characteristics of self-management interventions in patients with heart failure? An individual patient data meta-analysis. J Card Fail Philadelphia, Pennsylvania: W B Saunders 22:861–871 [DOI] [PMC free article] [PubMed]
- 289.Kang S-H, Oh I-Y, Kang D-Y, Cha M-J, Cho Y, Choi E-K, Hahn S, Oh S (2015) Cardiac resynchronization therapy and QRS duration: systematic review, meta-analysis, and meta-regression. J Korean Med Sci Korea (South): Korean Academy of Medical Science 30:24–33 [DOI] [PMC free article] [PubMed]
- 290.Klersy C, De SA, Gabutti G, Raisaro A, Curti M, Regoli F, Auricchio A. Economic impact of remote patient monitoring: an integrated economic model derived from a meta-analysis of randomized controlled trials in heart failure. Eur J Hear Fail. 2011;13:450–459. doi: 10.1093/eurjhf/hfq232. [DOI] [PubMed] [Google Scholar]
- 291.Komajda M, Böhm M, Borer JS, Ford I, Tavazzi L, Pannaux M, Swedberg K (2018) Incremental benefit of drug therapies for chronic heart failure with reduced ejection fraction: a network meta-analysis. Eur J Heart Fail Hoboken, New Jersey: John Wiley & Sons, Inc 20:1315–1322 [DOI] [PubMed]
- 292.Le H-H, El-Khatib C, Mombled M, Guitarian F, Al-Gobari M, Fall M, Janiaud P, Marchant I, Cucherat M, Bejan-Angoulvant T, Gueyffier F (2016) Impact of aldosterone antagonists on sudden cardiac death prevention in heart failure and post-myocardial infarction patients: a systematic review and meta-analysis of randomized controlled trials. PLoS One United States: Public Library of Science 11:e0145958 [DOI] [PMC free article] [PubMed]
- 293.Ma Y, Bai F, Qin F, Li Y, Tu T, Sun C, Zhou S, Liu Q. Catheter ablation for treatment of patients with atrial fibrillation and heart failure: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2018;18:165. doi: 10.1186/s12872-018-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Malik AH, Aronow WS. Comparative therapeutic assessment of atrial fibrillation in heart failure with reduced ejection fraction-a network meta-analysis. Am J Ther United States: Lippincott Williams & Wilkins. 2020;27:e286–e296. doi: 10.1097/MJT.0000000000000892. [DOI] [PubMed] [Google Scholar]
- 295.Moschonas K, Nabeebaccus A, Okonko DO, McDonagh TA, Murgatroyd FD, Dhillon P, Scott PA. The impact of catheter ablation for atrial fibrillation in heart failure. J Arrhythmia. 2019;35:33–42. doi: 10.1002/joa3.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Pandor A, Gomersall T, Stevens JW, Wang J, Al-Mohammad A, Bakhai A, Cleland JG, Cowie MR, Wong R. Remote monitoring after recent hospital discharge in patients with heart failure: a systematic review and network meta-analysis. Heart. 2013;99:1717–1726. doi: 10.1136/heartjnl-2013-303811. [DOI] [PubMed] [Google Scholar]
- 297.Shah RV, Desai AS, Givertz MM. The effect of renin-angiotensin system inhibitors on mortality and heart failure hospitalization in patients with heart failure and preserved ejection fraction: a systematic review and meta-analysis. J Card Fail. 2010;16:260–267. doi: 10.1016/j.cardfail.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 298.Sulaica EM, Macaulay TE, Helbing RR, Abo-Aly M, Abdel-Latif A, Wanat MA. A comparison of anticoagulation, antiplatelet, and placebo treatment for patients with heart failure reduced ejection fraction in sinus rhythm: a systematic review and meta-analysis. Heart Fail Rev. 2020;25:207–216. doi: 10.1007/s10741-019-09818-w. [DOI] [PubMed] [Google Scholar]
- 299.Taylor R, Long LD, Mordi IR, Madsen MT, Davies EJ, Dalai H, Rees K, Singh SJ, Gluud C, Zwisler AD. Exercise-based rehabilitation for heart failure cochrane systematic review, meta-analysis, and trial sequential analysis. Jacc-Heart Fail. 2019;7:691–705. doi: 10.1016/j.jchf.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 300.Thomas R, Huntley A, Mann M, Huws D, Paranjothy S, Elwyn G, Purdy S, Thomas R, Huntley A, Mann M, Huws D, Paranjothy S, Elwyn G, Purdy S. Specialist clinics for reducing emergency admissions in patients with heart failure: a systematic review and meta-analysis of randomised controlled trials. Heart BMJ Publishing Group. 2013;99:233–239. doi: 10.1136/heartjnl-2012-302313. [DOI] [PubMed] [Google Scholar]
- 301.Thomsen MM, Lewinter C, Køber L. Varying effects of recommended treatments for heart failure with reduced ejection fraction: meta-analysis of randomized controlled trials in the ESC and ACCF/AHA guidelines. Esc Hear Fail England: John Wiley & Sons Ltd on behalf of the European Society of Cardiology. 2016;3:235–244. doi: 10.1002/ehf2.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Tse G, Chan C, Gong MQ, Meng L, Zhang J, Su XL, Ali-Hasan-Al-Saegh S, Sawant AC, Bazoukis G, Xia YL, Zhao JC, Lee APW, Roever L, Wong MCS, Baranchuk A, Liu T, Int Hlth Informatics Study I (2018) Telemonitoring and hemodynamic monitoring to reduce hospitalization rates in heart failure: a systematic review and meta-analysis of randomized controlled trials and real-world studies. J Geriatr Cardiol 15:298–309 [DOI] [PMC free article] [PubMed]
- 303.Tu RH, Zhong GQ, Zeng ZY, Wu WF, Wu H, Cao XL, Aung LHH. Cardiac resynchronization therapy in patients with mild heart failure: a systematic review and meta-analysis of randomized controlled trials. Cardiovasc Drugs Ther. 2011;25:331–340. doi: 10.1007/s10557-011-6313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Turagam MK, Garg J, Whang W, Sartori S, Koruth JS, Miller MA, Langan N, Sofi A, Gomes A, Choudry S, Dukkipati SR, Reddy VY (2019) Catheter ablation of atrial fibrillation in patients with heart failure a meta-analysis of randomized controlled trials. Ann Intern Med 170:41-+ [DOI] [PubMed]
- 305.Uminski K, Komenda P, Whitlock R, Ferguson T, Nadurak S, Hochheim L, Tangri N, Rigatto C (2018) Effect of post-discharge virtual wards on improving outcomes in heart failure and non-heart failure populations: a systematic review and meta-analysis. PLoS One Public Library of Science San Francisco, CA USA 13:e0196114 [DOI] [PMC free article] [PubMed]
- 306.Xiang R, Li L, Liu SX (2013) Meta-analysis and meta-regression of telehealth programmes for patients with chronic heart failure. J Telemed Telecare Thousand Oaks, California: Sage Publications Inc 19:249–259 [DOI] [PubMed]
- 307.Zhang L, Lu Y, Jiang H, Zhang L, Sun A, Zou Y, Ge J. Additional use of trimetazidine in patients with chronic heart failure: a meta-analysis. J Am Coll Cardiol United States: Elsevier Biomedical. 2012;59:913–922. doi: 10.1016/j.jacc.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 308.Zhang Y, Chen Q, Huang G, Wang L. The influence of liraglutide for heart failure: a meta-analysis of randomized controlled trials. Heart Surg Forum United States: Forum Multimedia Pub. 2019;22:E438–E444. doi: 10.1532/hsf.2513. [DOI] [PubMed] [Google Scholar]
- 309.Zhou X, Chen J (2014) Is treatment with trimetazidine beneficial in patients with chronic heart failure? PLoS One United States: Public Library of Science 9:e94660 [DOI] [PMC free article] [PubMed]
- 310.Capomolla S, Pinna G, La Rovere MT, Maestri R, Ceresa M, Ferrari M, ... Cobelli F (2004) Heart failure case disease management program: a pilot study of home telemonitoring versus usual care. Eur Heart J Suppl 6(suppl_F):F91–F98
- 311.Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Böhm M, Anker SD. Telemedicine in heart failure: pre-specified and exploratory subgroup analyses from the TIM-HF trial. Int J Cardiol. 2012;161(3):143–150. doi: 10.1016/j.ijcard.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 312.Soran OZ, Piña IL, Lamas GA, Kelsey SF, Selzer F, Pilotte J, Feldman AM. A randomized clinical trial of the clinical effects of enhanced heart failure monitoring using a computer-based telephonic monitoring system in older minorities and women. J Card Fail. 2008;14(9):711–717. doi: 10.1016/j.cardfail.2008.06.448. [DOI] [PubMed] [Google Scholar]
- 313.Gandhi S, Mosleh W, Myers RB. Hypertonic saline with furosemide for the treatment of acute congestive heart failure: a systematic review and meta-analysis. Int J Cardiol. 2014;173(2):139–145. doi: 10.1016/j.ijcard.2014.03.020. [DOI] [PubMed] [Google Scholar]