Abstract
Primary bone cancers (PBC) belong to the family of mesenchymal tumors classified based on their cellular origin, extracellular matrix, genetic regulation, and epigenetic modification. The three major PBC types, Ewing sarcoma, osteosarcoma, and chondrosarcoma, are frequently aggressive tumors, highly metastatic, and typically occur in children and young adults. Despite their distinct origins and pathogenesis, these sarcoma subtypes rely upon common signaling pathways to promote tumor progression, metastasis, and survival. The IGF/PI3K/mTOR and AXL/YAP/TAZ pathways, in particular, have gained significant attention recently given their ties to oncogenesis, cell fate and differentiation, metastasis, and drug resistance. Naturally, these pathways – and their protein constituents – have caught the eye of the pharmaceutical industry, and a wide array of small molecule inhibitors and antibody drug-conjugates have emerged. Here, we review how the IGF/PI3K/mTOR and AXL/YAP/TAZ pathways promote PBC and highlight the drug candidates under clinical trial investigation.
Keywords: AXL, Bone cancer, Ewing sarcoma, Osteosarcoma, Chondrosarcoma, IGF-1R, PI3K, mTOR
1. Introduction
Osteosarcoma (OS) and Ewing sarcoma (ES) are the most frequently occurring malignant primary bone cancers (PBC) of childhood and adolescence[1]. Chondrosarcoma (CS) tends to occur later in the 4th-7th decades. Standard treatment with chemotherapy, radiation, and surgery has variable success across these tumor types, but the cure rate for those with metastatic or relapsed disease has changed little over the last five decades[2].
Fortunately, molecular characterization of these tumors has revealed potential pathways for drug intervention. Though IGF-1 regulates osteogenesis and bone homeostasis in normal bones [3], [4], it also instigates an aberrant IGF/PI3K/mTOR pathway signaling cascade in PBC[5]. Downstream of IGF-1R, PI3K activation has been shown in several solid tumors to affect cell metabolism, cell survival, proliferation, and protein synthesis [6], [7]. Due to the frequent activation of the IGF/PI3K/mTOR pathway in PBC and other solid tumors, which intersects a range of mechanical and chemical signaling mechanisms in cancer, this pathway has been identified as an attractive target in OS, ES, and CS[8], [9], [10]. Though IGF-1R-targeted therapies have been the subject of many clinical trials for sarcoma, with or without mTOR inhibitors [11], [12], their impact on cell differentiation, plasticity, and drug resistance mechanisms remain largely unexplored. The high inter-patient heterogeneity among PBC patients also suggests the need for response biomarkers to select and steer patients toward the agents most likely to provide clinical benefit [13], [14].
The dysregulated AXL-ABL2-YAP/TAZ feedback loop – comprised of Yes-associated protein 1 (YAP), transcriptional coactivator with PDZ-binding motif (TAZ), and the oncogenic receptor tyrosine kinase (RTK) AXL – have been observed in ES and OS. Activation of YAP, TAZ, and AXL can induce cell proliferation, resistance to biologically targeted therapy, and metastasis of bone sarcoma [7], [15], [16], [17], [18], [19]. The importance of this pathway in tumorigenesis has naturally led scientists to investigate whether its protein constituents are druggable targets in bone sarcomas[19]. Interestingly, Dupont et al. showed that YAP and TAZ also regulate biomechanical signals independent of the Hippo pathway, which in turn have striking effects on cell fate determination, stem cell properties, and proliferation rates. [20] Colocalization of nuclear YAP and TAZ in mesenchymal stem cells (MSCs) correlates with the environment’s tensegrity. An increased propensity toward osteogenic lineage commitment is induced through high tensegrity. In contrast, less stiff environments favor an adipogenic phenotype and cytosolic YAP/TAZ expression[21]. In human OS cell lines, knockdown of AXL leads to decreased proliferation and increased apoptosis[22]. In addition, the clinical activity of cabozantinib, an inhibitor of AXL and other kinases, has been recently reported in phase 2 clinical trials for patients with OS and ES[23].
Though other pathways almost certainly contribute to bone sarcomagenesis, this review focuses exclusively on two important ones, IGF/PI3K/mTOR and AXL/YAP/TAZ, that have immediate clinical relevance given the exponential rise of new investigational agents that have reached the clinic.
2. Clinical description
PBCs are included in the broader category of sarcomas and correspond to 0.2% of diagnosed cancers, which are primarily composed of osteosarcoma (OS), Ewing sarcoma (ES), and chondrosarcoma (CS) [24]. These malignant bone tumors are clinically aggressive and often need extensive multi-modality treatment. Despite their low incidence, PBCs are associated with excessive morbidity and mortality, greatly affecting the children and young adult population[25]. The lack of specific symptoms at disease onset can delay the diagnosis and allow time for local tumor invasion and distant metastasis to bone and lung. Though the 5-year survival rate for those presenting with localized OS and ES reaches 50–70%, only 20–30% of those presenting with lung or bone metastases at diagnosis survive[26]. While slower growing, CS is notoriously resistant to chemotherapy and often recurs locally[27].
2.1. Osteosarcoma
OS is the most common PBC that usually affects children and adolescents[25]. The etiology of OS is complex and may occur due to widespread chromosomal errors or specific gene mutation in p53, RB1, or the RECQL4 genes that, respectively, cause Li-Fraumeni syndrome, hereditary retinoblastoma, and Rothmund-Thomas syndrome[28]. Interestingly, whole-genome sequencing data suggests that one-third of primary OSs – compared with just 2%–3% of other cancer types – have high mutation burdens thought to result from chromothripsis[29]. The phenomenon of chromothripsis (i.e., shattered DNA) occurs when one or more chromosomes are broken into many pieces and then chaotically rejoined [29], [30]. Since the resulting DNA damage is unpredictable, one frequently observes marked inter-patient genetic and phenotypic heterogeneity [29], [30].
From a clinical perspective, the World health organization classifies OS subtypes by their location relative to the bone cortex and by their grade (high, intermediate, or low). High-grade OS can occur in any bone, but usually it originates near the distal femur (43%) or proximal tibia (23%). These bones experience rapid cell division during the adolescent growth spurt, though it remains to be determined if the stimulatory signals during puberty contribute to tumorigenesis[31]. The main prognostic factor in OS is the presence or absence of metastatic disease. Though beyond the scope of this review, treatment often relies upon chemotherapy (mainly cisplatin, doxorubicin, methotrexate, or ifosfamide) and surgery[28]. Complementing traditional chemotherapy, several molecularly and pathway-targeted therapies have been developed, including immune modulators [32], [33], bone signaling regulators, receptor activator of nuclear factor k-B ligand blockade [34], [35], and receptor or non-receptor tyrosine kinases inhibitors [14], [31], [36], [37].
2.2. Ewing sarcoma
ES can occur in the bone (mainly in the pelvis, femur, tibia, and ribs) or soft tissue sites (e.g., thoracic wall, gluteal muscle, cervical muscles, and pleura cavities). The cell of origin remains unknown, but many suspect this sarcoma subtype emerges from neural crest cells or mesoderm-derived mesenchymal stem cells[38], [39], [40]. Phenotypically, ES is characterized by a small round cell appearance, positive expression of the surface marker CD99[41], and chromosomal translocation of the EWSR1 gene to ETS family genes[42]. This fusion protein acts as an oncogenic transcription factor that controls ES progression. 70–80% of patients with localized ES, and ∼30% for those with metastatic disease, survive. All patients receive chemotherapy, which typically consists of vincristine, doxorubicin, cyclophosphamide alternated with ifosfamide and etoposide [38], [43]. When practical, surgery is preferred, though unresectable tumors in the spine or pelvis can successfully be treated with radiation.
Several next-generation EWSR1-FLI1 targeted therapeutics are in preclinical development, with at least one compound (TK-216) that has entered phase clinical testing in patients (NCT02657005) [44]. In addition, several other genes were also reported to promote the recurrence and progression of ES, such as VEGF, IGF-1, CAV1, GLI1, RB, and p53, which might be used as therapeutic targets for ES. Several proteins appear to enable resistance to IGF-1R/mTOR-directed treatment, including IRS1, PI3K, STAT3, YAP-1, and TAZ[7].
2.3. Chondrosarcoma
CS is a malignant tumor of bone characterized by cartilage matrix production and a diverse histopathological and clinical behavior[45]. The exact etiology of CS is not known. There may be a genetic or chromosomal component that predisposes certain individuals to this type of PBC. However, the somatic mutations of isocitrate dehydrogenase (IDH) genes that encode for proteins catalyzing the oxidative decarboxylation of isocitrate, producing αKG and CO2 in the Krebs cycle [46], [47], are present in 50% of primary conventional CS [48], [49]. The CS is subclassified in primary central, secondary peripheral, and periosteal (aka juxtacortical) suptypes [14], [50]. The most common primary location is the pelvis, followed by the femur, humerus, and ribs[51]. Conventional CS is a low or intermediate grade (90%), characterized by a slow clinical course and low metastatic potential.
In contrast, the high-grade CS (10%) is associated with high metastatic potential and poor prognosis [48], [52]. Among patients with primary CS of bone and metastasis at presentation, low tumor grade, surgical treatment, tumor size <10 cm, and first primary tumor predict prolonged survival[53]. CS is characterized by a resistance to chemo- and radiotherapies mainly due to a high ECM deposition and low neovascularization, which blocks drug diffusion and activity[54]. The IDH1/2 mutations in CS make the development of IDH targeted therapy a promising treatment option, and there are several ongoing clinical trials in Phase I/II assessing the clinical activity of IDH blockades (NCT02273739, NCT02481154/NCT02073994, and NCT02496741).
3. IGF1/PI3K/mTOR pathway
Insulin-like growth factor 1 (IGF-1) is important for several different growth and differentiation processes of normal bone physiology through endocrine mechanisms[55]. IGF-1 receptor (IGF-1R) blockade in chondrocytes, osteoblasts, and osteocytes has shown that IGF-1 signaling is required for controlling cell proliferation and differentiation.
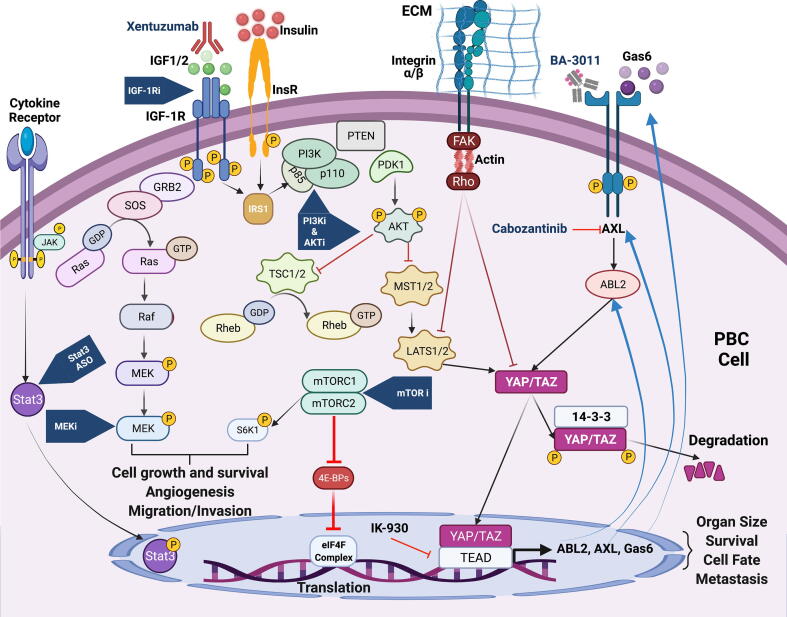
IGF1/PI3K/mTOR pathway activation begins when IGF-1 binds to IGF1R, prompting phosphorylation at several tyrosine residues in the kinase domain (e.g., 1131, 1135, 1136) or membrane domain (e.g., tyrosine 950). IGF-1R phosphorylation, in turn, activates downstream substrates, such as insulin receptor substrate (IRS) and Shc[56]. IRS1 activates phosphatidylinositol 3 kinase (PI3K) [57] and the extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) network by binding to Shc and Grb2 (Fig. 1) [14], [58], [59]. PI3K phosphorylates phosphatidylinositol 4,5 bisphosphate, which leads to phosphatidylinositol 3,4,5, trisphosphate. Ultimately, serine/threonine kinase (PDK1) is recruited and AKT is partially activated at threonine-308 (Fig. 1). Full AKT activation is accomplished by Ser-473 phosphorylation by mTORC [28], [60]. At the terminal end of this cascade, the mammalian target of rapamycin (mTOR) regulates several processes critical for cell proliferation and protein synthesis.
Fig. 1.
Activation of the IGF/PI3K/mTOR and AXL/YAP/TAZ pathways in PBC.
mTOR subsists in two distinct entities, mTORC1 and mTORC2, a serine/threonine tyrosine kinase that regulates normal cell growth, development, metabolism, and angiogenesis. In response to nutrients and growth factor receptor signals, the downstream effectors (p70S6K and 4E-BP1) profoundly affect cellular growth, proliferation, and protein synthesis (translation) [8]. mTORC1 is sensitive to rapamycin and other so-called rapalogs (e.g., temsirolimus, everolimus, ridaforolimus, or rapamycin) [8]. mTORC2, which is not sensitive to rapamycin, can enhance AKT activity [61], [62].
The IGF/PI3K/mTOR pathway is linked to the pathogenesis and progression of PBC [63], [64]. Comparing PBCs with normal bone cells at the gene expression levels validated a loss of all the intracellular IGF inhibitors, IGFBPs [7], [14], [65]. This pathway actively modulates cell migration, cell cycle progression, EMT, and tumor growth in preclinical models of PBCs [7], [14], [66]. Increased IGF1/IGF1R expression has been demonstrated in OS, ES, and CS patients’ tumors, and it was associated with a poor prognosis [14], [67], [68]. Despite this evidence, IGF-1R blockade in PBC cell lines with mAb against IGF/IGF1R was rarely successful when used as monotherapy [7], [69]. Activation of the downstream PI3K/Akt pathway has also been demonstrated in OS and CS cells, though again, single-agent targeting was generally unsuccessful [10], [70]. More encouraging results were achieved in combination studies, and partial responses were shown in PBC cases treated with dual IGF1R/mTOR blockade [12], [14].
4. Therapeutic opportunities targeting the IGF/PI3K/mTOR pathway in PBC
The IGF/PI3K/mTOR pathway can be therapeutically targeted conceptually at three different levels: 1) blocking its proximal components (either IGF ligands or IGF-1R using antagonistic humanized monoclonal antibodies), 2) impeding its mid constituents at either PI3K or AKT using small molecules tyrosine kinase inhibitors, and 3) blocking its distal mTOR components using rapamycin and its analogs. Given the prominent role this pathway has on vital cellular processes, feedback loops abound and quickly counter the therapeutic effect of most drugs. For that reason, it is increasingly common to see combination strategies employed in the preclinical and clinical setting to block the IGF/PI3K/mTOR pathway at two or more levels in an attempt to mitigate acquired drug resistance [7], [14].
4.1. IGF ligand inhibitors
IGF ligand-blockade demonstrated a complementary therapeutic approach that overcame the upregulation of insulin receptors as a mechanism of resistance to IGF-1R neutralization. Humanized monoclonal antibodies against IGF-1 and IGF-2 have been developed by Medimmune (MEDI-573, dusigitumab) and Boehringer Ingelheim (BI836845, xentuzumab). Both antibodies bind and neutralize IGF-1 and IGF-2, thereby preventing activation of IGF-1R and IR-α while avoiding unwanted effects upon insulin[71]. These ligand-directed antibodies were generally well tolerated within phase 1 trials in patients with solid tumors. To date, the sole clinical experience treating PBC patients with ligand-targeted drugs includes a ES patient who received a subtherapeutic dose of MEDI-573 while enrolled in an early dose cohort[72]. Xentuzumab, which also binds and neutralizes murine IGFs, has shown strong preclinical activity in ES. Despite this, both IGF ligand inhibitors (dusigitumab and xentuzumab) have been discontinued due to poor activity in common cancer types.
4.2. IGF-1R blockade
The IGF-1R pathway has been studied extensively in PBCs [7], [65], [66]. IGF-1R antagonists have generally consisted of antibodies or small molecule TKIs, as summarized in Table 1 [73], [74], [75]. To date, six mAbs had been investigated in clinical trials: R1507 (robatumumab, Roche), cixutumumab (IMC-A12, ImClone), SCH-717454 (19D12, Schering-Plough), MK-0646 (dalotuzumab, Merck), AMG479 (Ganitumab, Amgen), figitumumab (CP-751-871, Pfizer). Single-agent activity ranged between 9 and 14%, and most responses lasted <2 months [73], [75], [76]. Small molecule IGF-1R antagonists, including NVP-AEW541, BMS-536924, GSK-183870A, and OSI-906 showed promising preclinical activity. However, cross-reactivity to the insulin receptor led to unacceptable toxicity[77], [78], [79], [80], and these compounds were subsequently abandoned.Table 2.
Table 1.
IGF-1R blockade strategies in ES and OS.
| IGF-1R Blockade | Phase | Company | Clinical activities (ORR & SD) | |
|---|---|---|---|---|
| Monoclonal Antibodies | SCH-717454 | II | Schering-Plough | PRs seen in ES patients, Phase II in relapsed OS & ES[81] |
| Figitumumab (CP-751871) | I-II | Pfizer | ES (14% CR + PR & 24% SD) [82] | |
| Cixutumumab (IMC-A12) | I-II | ImClone | Phase II with ES (PD: 66.7%) [83] | |
| Dalotuzumab (MK-0646) | II | Merck | Phase I- dose escalations in 1 ES that had a mixed response[84] | |
| Teprotumumab (R1507) | II | Roche | Several PRs in ES (11, CR/PR) [75] & OS (PR: 2, SD: 10, PD: 26) [85] patients | |
| Ganitumab (AMG-479) | II | Amgen | ES (6% CR + PR & 49% SD) [86] | |
| TKIs | Linsitinib (OSI-906) | II | Astellas Pharma | ES (16 patients; no CR/PR) (NCT02546544) |
| BSM-754807 | Preclinical | BMS | No Clinical testing | |
| BMS-554417 | Preclinical | BMS | No Clinical testing | |
| NVP-AEW541 | Preclinical | Novartis | No Clinical testing | |
| GSK1904529A | Preclinical | GSK | No Clinical testing |
ORR: overall response rate; SD: stable disease; CR: complete response; PR: partial response; PD: progressive disease; ES: Ewing sarcoma; OS: osteosarcoma.
Table 2.
PI3K blockade strategies in PBCs.
| PI3K Blockade | Name | Phase | Company | MOA on PBC |
|---|---|---|---|---|
| PI3Kα | Alpelisib (BYL719) | Preclinical | Novartis | Cell migration inhibition Tumor progression inhibition[94] |
| Pan-PI3K | BKM120 Buparlisib | Preclinical | Novartis | Inhibition of Cell proliferation inhibits activation of Akt[96] |
| LY294002 | Preclinical | Eli Lilly | Inhibition of Cell proliferation[97] | |
| Copanlisib (BAY80-6946) | I/II | Bayer | Inhibition of cell survival[98]. Ongoing trial to investigate the safety and feasibility in pediatric patients with recurrent or refractory OS, ES (NCT03458728) |
4.3. IRS-1 inhibitors
The IGF-1R/PI3K/mTOR pathway has been the target of preclinical studies for OS PBC[87] and has a vital role in various mitogenic and antiapoptotic signaling through other components like insulin receptor substrates 1 and 2 (IRS1/2), which play central roles in cancer cell proliferation, resistance to anticancer drugs, and tumor metastasis[88], [89], [90]. A selective inhibitor of IRS1/2, NT157 was evaluated as a dose-dependent inhibitor of growth in several OS cell lines by downregulating the expression of IRS1/2 and principal downstream mediators of the IGF pathway. NT157 affected the OS cell migratory ability. In addition, the same IRS1/2 inhibitor was combined with mTOR and PI3K/mTOR inhibitors, and significant synergistic effects were obtained[87]. This IRS1/2 inhibitor is not yet been tested clinically.
4.4. PI3Ks inhibitors
These targets are downstream of the IGF-1R/IR but also of other RTKs. Their effective targeting can eliminate the activation of downstream signaling, reduce cell proliferation and survival that are inducted by other RTKs, and downgrade cell activation caused by PI3K mutations. Impaired PI3K signaling can trigger compensatory alterations at the cellular and whole-body level, the latter occurring via drug-induced hyperglycemia and hyperinsulinemia that activates IR-A[91]
There are two broad types of PI3K inhibitors, the pan-PI3K and the isoform-specific inhibitors. Pan-PI3K inhibitor has been reported to inhibit autophagy and enhance OS cell apoptosis [92], [93]. Despite the effective inhibition of the PI3K pathway and PBC tumor growth in PBC preclinical studies, these pan-inhibitors may never be fully developed as clinical therapeutic drugs because of their poor pharmacokinetic parameters, including low solubility, instability, and excessive toxicity. However, the development of isoform-specific inhibitors of PI3K p110 α,β,δ or γcatalytic subunits have emerged and might offer the opportunity to lower these side effects, thus granting their tolerability. For example, alpelisib, a specific PI3Kα inhibitor that blocks the ATP site, is proposed to reduce cell proliferation and block tumor bone formation in murine preclinical models of OS[94]. Another PI3Kα inhibitor, BKM120, can decrease OS cell invasion and survival[95].
4.5. mTOR inhibitors
As a central hub of cell biology, mTOR profoundly affects whole-organism carbohydrate physiology. mTORC1 and mTORC2 contribute to tumorigenesis differently [99], [100] and elicit unique behaviors when targeted. The rapamycin analogs ridaforolimus, everolimus, and temsirolimus, which inhibit mTORC1, were first approved to treat renal malignancies[101].
A downstream target of the IGF/PI3K/mTOR pathway, activated S6 kinase is readily detectable in PBC specimens. In a subgroup analysis, ridaforolimus led to a non-statistically significant improvement PFS for bone tumors (HR 0.70, 95% CI upper limit >1); this study was not adequately powered for subgroup analyses[102]. Everolimus has demonstrated some activity in osteosarcoma; within a pediatric phase 1 study, one of two enrolled osteosarcoma patients showed prolonged stable disease for several courses[103]. Several drug combinations, rapamycin with cyclophosphamide and temsirolimus with cixutumumab, failed to demonstrate significant activity in OS [12], [104]. Ten CS patients were treated with a sirolimus/cyclophosphamide combination, which was well tolerated and had modest clinical activity[105]. Temsirolimus may also potentiate the cytotoxicity of liposomal doxorubicin[106]. Last, neoadjuvant everolimus has been tried in CS (NCT02008019) was suspended due to limited activity.
4.6. Cotargeting IGF/PI3K/mTOR pathway
Given the limited or short-lived antineoplastic response observed when individual proteins are targeted, many have hypothesized that effective blockade of the IGF/PI3K/mTOR will require co-targeting two or more proteins concurrently to avoid counter-productive feedback loop activation. Preclinical data indicate that the combination of mTOR with IGF-1R blockade results in greater Akt downregulation and enhanced antiproliferative effects[7]. This therapeutic approach guided the design of numerous early phase clinical trials assessing mTORC1 and IGF-1R blockade in sarcoma patients [14], [107], [108], [109]. Dual IGF-1R/mTOR therapies were generally more successful than monotherapy and were reasonably well tolerated compared to traditional cytotoxic chemotherapy [11], [14].
While single-agent IGF-1R targeted monoclonal antibodies delivered a clear activity signal, neither the response rate (10–14%) nor response duration (2 months) offers enough clinical benefit to expand into the next clinical trial[75]. During the last decade, our research team has demonstrated that combined IGF-1R/mTOR-targeted therapy was synergistic, with a 29% response rate and, significantly, with a standard response duration enduring more than one year[11]. Later, other trials developed by Schwartz et al. and COG (Children’s Oncology Group) were unsuccessful when trying to replicate these promising results, principally because the mTOR dose was decreased in a sizable fraction for patients due to mucositis or hepatotoxicity [104], [110]. Since those studies were conducted, investigators have become adept at palliating mucositis and other mTOR-associated symptoms and thereby have extended the safety profile of mTOR combinations [111], [112].
Harmonizing these clinical experiences, our lab and others throughout the country have made considerable advances shaping how to efficiently cotarget the IGF-1/PI3K/mTOR pathway to significantly succeed in managing PBC clinically. Despite exhibiting little single-agent activity, our results suggest mTOR suppression is central to meaningful clinical action. Of critical importance, because mTOR inhibition quickly upregulates IRS-1 and PI3K (due to derepression of the p70S6K → IRS-1 feedback loop) [113], [114], [115], [116], it is imperative to simultaneously block the proximal portion of the IGF-1/PI3K/mTOR pathway (Fig. 1).
In our experience, this can be achieved by co-targeting mTOR together with the IGF-1/IGF-2 ligands (plus the hybrid IR-α/IGF-1R), IGF-1R, or PI3K. As shown in our preclinical studies, IGF-1R targeting in animal models leads to compensatory changes in Smad3, STAT3, and other proteins that can be explored for additional synergy[7]. In the clinic, data from one ES patient who had responded to therapy and then progressed 14 months later on IGF-1R/mTOR-based therapy revealed similar compensatory changes[7]. Preliminary data using alpelisib (a p110α-selective PI3K inhibitor) in ES patient-derived tumor explants (PDX) models suggest a xentuzumab/alpelisib two-drug combination might also be effective. They could serve to expand future clinical trial options if neutralizing antibody programs are revived.
Though antagonists of IGF, IGF-1R, IRS-1, and mTOR have largely been abandoned as a single-agent treatment for PBC, PI3K inhibitors remain under clinical investigation. Alpelisib has been FDA-approved for specific PI3K-mutant breast cancer subtypes. Pan-PI3K inhibitors, such as copanlisib with a broader affinity for the p110-α p110-δ catalytic PI3K subunit, have received approval for lymphoma subtypes. To date, PI3K inhibitors have not been studied in PBC. If and when they are, Hopkins et al. have suggested in other cancers that their antineoplastic effect can be magnified by reducing hyperinsulinemia via adherence to a ketogenic diet[117]. Whether that strategy proves beneficial in PBC remains to be determined.
5. AXL-YAP/TAZ positive feedback loop
5.1. The Hippo and YAP/TAZ pathway is conserved across species.
YAP (encoded by Yes-associated protein 1) [118], [119] and TAZ (encoded by WWTR1) [118], [119], [120] are transcriptional activators that regulate cell proliferation, survival, and differentiation [121], [122]. The biological response is directly tied to the location of YAP/TAZ in the cell. For instance, activation leads to the shuttling of YAP/TAZ into the nucleus, and suppression of YAP/TAZ maintains its presence in the cytoplasm. When YAP and TAZ were first discovered, the biological function was unclear. Later, key insights regarding YAP and TAZ function were inferred from the effects of Yorkie, a homolog within the Drosophila Hippo signaling pathway[123].
The Hippo signaling pathway is a conserved cascade regulating the growth and size of tissues, involving the translocation of Yorkie, the YAP/TAZ homolog, between the cytoplasm and nucleus. When Hippo signaling is inactivated in Drosophila, it causes enlargement of larval tissue and tumor initiation, indicating the Hippo signaling pathway is a key tumor suppressor[124]. Similar findings emerged in mammalian cells, where YAP/TAZ was shown to regulate organ size[125] and tumor progression. The Hippo signaling pathway is mediated through the kinases MST1/2 and LATS1/2 in mammals. When activated, MST1/2 kinases form a complex with SAV1 to phosphorylate and activate LATS1/2. Subsequently, LATS1/2 phosphorylates YAP and TAZ, marking them for ubiquitination and subsequent degradation[124]. Since the nuclear-localized YAP/TAZ paralogs act as transcription factors, upstream Hippo signaling can inhibit cell proliferation.
Conversely, Hippo inactivation promotes YAP/TAZ nuclear shuttling. Notably, because YAP/TAZ lack DNA-binding domains themselves, they must first complex with one of several TEA-binding domains (TEAD) to exert their diverse epigenetic effects. As discussed shortly, obligate binding to TEAD1 or other TEAD proteins has created a therapeutic opportunity to target TEAD as a potential antineoplastic agent.
5.2. Diverse regulation of YAP/TAZ via mechanotransduction
Though canonical YAP/TAZ activation was first linked to Hippo mutations, Hippo downregulation can have similar effects. Notably, key findings from the regenerative medicine field revealed that YAP/TAZ activity is also critically dependent upon biomechanical cues imparted on cells by their surroundings. In vivo, this mostly occurs through cell-cell adhesion, mediated at the subcellular level by adherens junctions (AJ), and cell-ECM associated focal adhesions (FA) that rely upon integrin/ECM pairings[126]. Besides their role as structural anchors that preserve tissue shape and polarity, AJ and FA serve as critical macro-molecular hubs that regulate cytoskeletal architecture and recruit signaling proteins. Through both direct and indirect interactions with the Hippo pathway, cytoskeleton, FAK, and cadherin-catenin complexes, YAP/TAZs acts as a cell’s molecular ‘rheostat’ of mechanotransduction.
Cadherin-catenin complexes, FAK-Src kinases, and cytoskeletal tension influence Hippo signaling [127], [128]. In normal cells and tissues, cell-cell contact inhibition gradually reduces the rate of proliferation[129]. Cadherins, such as E-cadherin, participate in homophilic binding that initiates a cascade through β-catenin and α-catenin, leading to the interaction with the Merlin and Kibra complex. This complex activates MST1/2 initiating the canonical Hippo signaling. Apical and basolateral cell polarity can regulate the Hippo signaling pathway via AJs, tight junctions (TJ), and gap junctions (GJ) [130]. When correctly engaged, AJs and TJs bind and retain YAP and TAZ at the cell membrane, thereby preventing their activity.
Cell shape and cytoskeletal dynamics also regulate YAP/TAZ[131]. Mechanical cues and activation of RhoA promote increased F-actin polymerization, which inhibits LATS1/2 and activates YAP/TAZ. For example, when cells are placed on larger two-dimensional surfaces or micropatterned culture substrates that promote increased cytoskeletal tension, YAP/TAZ becomes activated. Conversely, rounded cells with low cytoskeletal tension maintain YAP/TAZ within the cytoplasm in an inactive state. This mechanism is influenced by the adhesive area, substrate stiffness, cell density, and shear stress. Taken together, by modulating YAP/TAZ activity, physical cues strongly influence cell proliferation, differentiation, and survival.
5.3. The critical role of YAP/TAZ in connective tissues and PBC
Given the impact of YAP/TAZ and TEAD[118], [119], [120] upon stemness[132], cell migration[133], organ size[125], and epithelial-to-mesenchymal transition (EMT) [119], it is not surprising that YAP/TAZ plays a vital role in osteogenesis [134], [135] and chondrogenesis[136], [137], [138]. Human mesenchymal stem cells (hMSCs), when grown on substrates of varying stiffnesses, adopted distinct cell fates dictated by the specific stiffness. For instance, hMSCs on rigid substrate differentiated toward an osteogenic cell fate while hMSCs on soft substrate differentiated toward an adipocytic cell fate[21].
YAP and TAZ are overexpressed and activated in several cancers and are linked to sustained proliferation, cell survival, EMT[119], and tumor progression. In OS, a recent study showed that 75% of patient histology samples (n = 175) had high YAP expression, with 46% of patients demonstrating YAP nuclear localization[139]. YAP nuclear localization and B1-integrin expression have been linked to adverse metastatic events and worse prognosis [140], [141]. Verteporfin, a small molecule TEAD inhibitor, impaired the growth and migration of OS cell lines. ROCK2 silencing in the preclinical setting had a similar effect[139]. While knockdown of YAP in OS cell lines suppressed in vitro tumor cell proliferation, invasion, and tumor formation in mice [142], [143], the role of TAZ in these PBCs requires further investigation. To that end, recent work showed that U2OS and HOS human OS cell lines cultured under a migratory ability had increased expression in TAZ and EMT-TFs, including N-cadherin, vimentin, and SNAIL[144]. This EMT-TF phenotypic induction was reversed through inhibition of TAZ[144]. Thus, YAP and TAZ might play divergent roles in OS pathogenesis and progression.
The precise role of YAP and TAZ in ES is less well understood. A recent study comparing YAP expression between ES and normal tissue demonstrated only a moderate increase in ES[145]. Another study found an association of YAP/TAZ expression with disease progression. Knockdown of YAP led to decreased cell proliferation in ES cell lines and decreased tumor growth in an ES xenograft[146]. Further, in ES cell lines, YAP and TAZ regulated the expression of secreted ECM proteins proteoglycan four and tenascin C downstream of CDC42 signaling[147]. Counterintuitively though, in OS, tenascin C complexed to α9β1 integrin to foster metastasis by blocking YAP nuclear translocation and target gene activation[148]. Tenascin C also complexed to α5β1 integrin in ES promoted metastasis by triggering YAP[149] and tyrosine phosphorylation through SRC kinase[150].
Elevated YAP/TAZ expression has been reported in some CS. A recent study showed that less than half of CS specimens harbored activated YAP/TAZ[151]. While little is known of the role of YAP/TAZ in CS, recent studies of chondrogenesis showed that TAZ-deficient mice have impaired chondrogenic differentiation and development[152]. In that regard, the dysregulation of YAP/TAZ may contribute to pathogenesis and aggressiveness in CS[153]. In addition, blockade of the YAP/TAZ-activating kinases SRC and RAC was described to impede chondrosarcoma cells migration[154], and nuclear accumulation of YAP as a consequence of LATS1 inactivation by protein arginine methyltransferase one was shown as the worst prognostic factor in chondrosarcoma[155]. Finally, downregulation of YAP/TAZ and LATS1 in chondrosarcoma cells treated with BRD4 inhibitor, JQ1, led to cell cycle arrest, senescence, and apoptosis[156]. It is likely that the function of YAP/TAZ in the migration or metastasis of bone cancers at least partially recapitulates their role during normal development of the respective tissues of the origin.
5.4. AXL and its impact upon YAP/TAZ
In addition to the YAP/TAZ transcriptional activators, the AXL protein can profoundly affect cell viability, dedifferentiation, cell fate, and metastasis in human bone sarcoma [19], [157]. As a TAM (Tyro3, AXL, and MER) RTK family member, AXL also regulates cell survival, proliferation, migration, invasion, and angiogenesis[158], [159], [160], [161], [162], [163], [164], [165], [166]. In carcinomas, including hepatocellular carcinoma[167] and lung adenocarcinoma brain metastasis[168], AXL promotes tumorigenesis. In addition to activating YAP/TAZ via ABL2 in some cancers, AXL – along with CTGF, CYR61, and MYC1 – is also a downstream YAP/TAZ gene target [169], [170]. In lung adenocarcinomas, for instance, YAP was overexpressed and positively correlated with AXL expression[171]. In vitro YAP knockdown significantly reduced AXL expression[171].
6. Therapeutic opportunities targeting AXL, YAP, or TAZ in PBC
Though IGF, PI3K, and mTOR inhibitors have been extensively tested as anticancer agents, drug candidates targeting the AXL/YAP/TAZ pathway remain in their infancy; see clinical trial data summarized in Table 3. In the preclinical setting, Fleuren et al. showed for the first time that AXL is a potential novel and druggable therapeutic target in ES[157]. They demonstrated that AXL and Gas6 are abundantly expressed in ES tumors and that high AXL protein expression is an independent prognostic marker of poor overall survival[157]. They also blocked AXL function using BGB324, which reduced ES cell viability and migration in all cell lines in vitro[157]; however, there have not been any animal preclinical efficacy studies of the AXL inhibitor, BGB324. Preclinical acellularized lung (ACL) models have been used to investigate how these proteins affect OS metastasis[19]. In that system, AXL inhibition attenuated proliferation, migration, and metastatic potential in vitro and in vivo.
Table 3.
AXL blockade strategies in PBCs.
| AXL Blockade | Name | Company | Sarcoma type | Phase | Clinical Activities |
|---|---|---|---|---|---|
| Antibody-drug Conjugate | BA3011 | BioAtla, Inc. | Osteosarcoma Ewing Sarcoma |
I-II | No results. (NCT03425279) |
| TKIs | Cabozantinib | Exelixis | Osteosarcoma Ewing Sarcoma |
II | ES (26% PR + 49% SD) OS (12% PR + 33% SD) (NCT02243605)[174] |
| Osteosarcoma | II | No results. (NCT05019703) | |||
| Osteosarcoma Ewing Sarcoma |
I | No results. (NCT04661852) | |||
| Osteosarcoma | II | No results. (NCT02867592) |
SD: stable disease; PR: partial response; ES: Ewing sarcoma; OS: osteosarcoma.
In the clinic, the recently opened sarcoma-specific phase 1/2 trial testing BioAtla’s AXL-targeted therapy (NCT03425279) is currently enrolling patients with ES, OS, CS, and several other sarcoma subtypes. Small molecule oral TEAD inhibitors such as IK-930 are completing IND-enabling studies and are expected to reach the clinic in early 2022. Several multi-targeted FDA-approved TKIs known to antagonize AXL, such as cabozantinib, have shown promising activity in ES and OS (NCT02867592) [23], [172], [173].
7. Crosstalk between IGF-1R/PI3K/mTOR pathway and AXL-YAP/TAZ positive feedback loop
Our group has previously shown that mechanical stress and culture architecture can affect PBC drug sensitivity in vitro to chemotherapy and IGF-1R/mTOR targeted therapy[175], [176], [177]. Increased mechanical stimulation through focal adhesions and actin stress fibers interface with the IGF/PI3K/mTOR pathway and AXL/YAP/TAZ feedback loop in at least two ways. First, FAK activates Akt through the canonical IGF/PI3K/mTOR pathway by stimulating the activity of PI3K [178], [179] (Fig. 1). Downstream, Akt then negatively regulates MST1/2, leading to a downregulation of the cytosolic YAP/TAZ and afterward boosted nuclear YAP[180]. Second, a substrate stiffness signal cascade is initiated by Rho GTPases through its interaction with the distal AXL-YAP/TAZ feedback loop by suppressing LATS1/2 and directly inhibiting the phosphorylation and subsequent degradation of YAP and TAZ [20], [181]. Nuclear YAP/TAZ then potentiate the IGF/PI3K/mTOR pathway through a PTEN inhibition caused by miRNA-29[182]. Thus, the mechanical cues can potentiate the IGF-1R/mTOR cascade either by promoting the activity of PI3K through FAK-mediated signaling or by inhibiting PTEN through nuclear YAP induction [20], [178], [179], [180], [181], [182]. Additionally, recent gain-of-function screens identified connections between AXL-YAP/TAZ and the MAPK and PI3K signaling pathways[183]. Further research is needed to determine whether cotargeting the MAPK or PI3K pathways and AXL/YAP/TAZ will prove synergistic as an anticancer strategy for treating patients with high-risk PBCs.
8. Conclusion and future perspectives
Recent research investigating the molecular drivers of PBCs has suggested that the IGF/PI3K/mTOR and AXL/YAP/TAZ pathways are critically important. Nevertheless, despite strong antitumor activity in the preclinical setting, anticancer agents directed against IGF-1R or mTOR have had limited clinical utility when used individually. Optimal studies co-targeting the IGF/PI3K/mTOR pathway at two or more levels within the pathway are still being explored to prevent the activation of feedback loops that promote acquired tumor drug resistance. Multi-targeted TKIs with partial AXL activity, like cabozantinib, have demonstrated early success in PBCs, and confirmatory studies are ongoing. However, newer selective inhibitors of AXL, YAP, or TAZ have just reached the clinic, and their effect on PBCs remains to be seen.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Wan-Ibrahim W.I., Singh V.A., Hashim O.H., Abdul-Rahman P.S. Biomarkers for Bone Tumors: Discovery from Genomics and Proteomics Studies and Their Challenges. Mol Med. 2016;21(1):861–872. doi: 10.2119/molmed.2015.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skubitz K.M., D'Adamo D.R. Sarcoma. Mayo Clin Proc. 2007;82(11):1409–1432. doi: 10.4065/82.11.1409. [DOI] [PubMed] [Google Scholar]
- 3.Kawai M., Rosen C.J. The insulin-like growth factor system in bone: basic and clinical implications. Endocrinol Metab Clin North Am. 2012;41(2):323–333. doi: 10.1016/j.ecl.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolb E.A., Gorlick R. Development of IGF-IR Inhibitors in Pediatric Sarcomas. Current oncology reports. 2009;11(4):307–313. doi: 10.1007/s11912-009-0043-1. [DOI] [PubMed] [Google Scholar]
- 5.Helman L.J., Meltzer P. Mechanisms of sarcoma development. Nat Rev Cancer. 2003;3(9):685–694. doi: 10.1038/nrc1168. [DOI] [PubMed] [Google Scholar]
- 6.Wan X., Helman L.J. The biology behind mTOR inhibition in sarcoma. Oncologist. 2007;12(8):1007–1018. doi: 10.1634/theoncologist.12-8-1007. [DOI] [PubMed] [Google Scholar]
- 7.Lamhamedi-Cherradi SE, Menegaz BA, Ramamoorthy V, et al. IGF-1R and mTOR Blockade: Novel Resistance Mechanisms and Synergistic Drug Combinations for Ewing Sarcoma. Journal of the National Cancer Institute 2016;108(12). 10.1093/jnci/djw182. [DOI] [PMC free article] [PubMed]
- 8.Ludwig J.A., Lamhamedi-Cherradi S.E., Lee H.Y., Naing A., Benjamin R. Dual targeting of the insulin-like growth factor and collateral pathways in cancer: combating drug resistance. Cancers (Basel) 2011;3(3):3029–3054. doi: 10.3390/cancers3033029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurmasheva R.T., Dudkin L., Billups C., Debelenko L.V., Morton C.L., Houghton P.J. The insulin-like growth factor-1 receptor-targeting antibody, CP-751,871, suppresses tumor-derived VEGF and synergizes with rapamycin in models of childhood sarcoma. Cancer Res. 2009;69(19):7662–7671. doi: 10.1158/0008-5472.CAN-09-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y.-X., van Oosterwijk J.G., Sicinska E., Moss S., Remillard S.P., van Wezel T., Bühnemann C., Hassan A.B., Demetri G.D., Bovée J.V.M.G., Wagner A.J. Functional profiling of receptor tyrosine kinases and downstream signaling in human chondrosarcomas identifies pathways for rational targeted therapy. Clin Cancer Res. 2013;19(14):3796–3807. doi: 10.1158/1078-0432.CCR-12-3647. [DOI] [PubMed] [Google Scholar]
- 11.Naing A., LoRusso P., Fu S., et al. Insulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing's sarcoma family tumors. Clin Cancer Res. 2012;18(9):2625–2631. doi: 10.1158/1078-0432.CCR-12-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz G.K., Tap W.D., Qin L.X., et al. Cixutumumab and temsirolimus for patients with bone and soft-tissue sarcoma: a multicentre, open-label, phase 2 trial. The lancet oncology. 2013;14(4):371–382. doi: 10.1016/S1470-2045(13)70049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleuren E.D., Versleijen-Jonkers Y.M., van de Luijtgaarden A.C., et al. Predicting IGF-1R therapy response in bone sarcomas: immuno-SPECT imaging with radiolabeled R1507. Clin Cancer Res. 2011;17(24):7693–7703. doi: 10.1158/1078-0432.CCR-11-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amin H.M., Morani A.C., Daw N.C., et al. IGF-1R/mTOR Targeted Therapy for Ewing Sarcoma: A Meta-Analysis of Five IGF-1R-Related Trials Matched to Proteomic and Radiologic Predictive Biomarkers. Cancers (Basel) 2020;12(7) doi: 10.3390/cancers12071768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katschnig A.M., Kauer M.O., Schwentner R., et al. EWS-FLI1 perturbs MRTFB/YAP-1/TEAD target gene regulation inhibiting cytoskeletal autoregulatory feedback in Ewing sarcoma. Oncogene. 2017;36(43):5995–6005. doi: 10.1038/onc.2017.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed AA, Abedalthagafi M, Anwar AE, Bui MM. Akt and Hippo Pathways in Ewing's Sarcoma Tumors and Their Prognostic Significance. Journal of Cancer 2015;6(10):1005-1010. (In English). 10.7150/jca.12703. [DOI] [PMC free article] [PubMed]
- 17.Luu AK, Viloria-Petit AM. Targeting Mechanotransduction in Osteosarcoma: A Comparative Oncology Perspective. International Journal of Molecular Sciences 2020;21(20) (In English). ARTN 7595. 10.3390/ijms21207595. [DOI] [PMC free article] [PubMed]
- 18.Zhang HT, Gui T, Sang Y, et al. The BET Bromodomain Inhibitor JQ1 Suppresses Chondrosarcoma Cell Growth via Regulation of YAP/p21/c-Myc Signaling. Journal of Cellular Biochemistry 2017;118(8):2182-2192. (In English). 10.1002/jcb.25863. [DOI] [PubMed]
- 19.Lamhamedi-Cherradi SE, Mohiuddin S, Mishra DK, et al. Transcriptional activators YAP/TAZ and AXL orchestrate dedifferentiation, cell fate, and metastasis in human osteosarcoma. Cancer Gene Therapy 2021 (In English). 10.1038/s41417-020-00281-6. [DOI] [PMC free article] [PubMed]
- 20.Dupont S., Morsut L., Aragona M., et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 21.Engler A.J., Sen S., Sweeney H.L., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y., Tang Y.J., Man Y., Pan F., Li Z.H., Jia L.S. Knockdown of AXL receptor tyrosine kinase in osteosarcoma cells leads to decreased proliferation and increased apoptosis. Int J Immunopathol Pharmacol. 2013;26(1):179–188. doi: 10.1177/039463201302600117. [DOI] [PubMed] [Google Scholar]
- 23.Schoffski P., Blay J.Y., Ray-Coquard I. Cabozantinib as an emerging treatment for sarcoma. Curr Opin Oncol. 2020;32(4):321–331. doi: 10.1097/CCO.0000000000000644. [DOI] [PubMed] [Google Scholar]
- 24.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 25.Miller K.D., Fidler-Benaoudia M., Keegan T.H., Hipp H.S., Jemal A., Siegel R.L. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin. 2020;70(6):443–459. doi: 10.3322/caac.21637. [DOI] [PubMed] [Google Scholar]
- 26.Ferguson JL, Turner SP. Bone Cancer: Diagnosis and Treatment Principles. Am Fam Physician 2018;98(4):205-213. (https://www.ncbi.nlm.nih.gov/pubmed/30215968). [PubMed]
- 27.Brown H.K., Schiavone K., Gouin F., Heymann M.F., Heymann D. Biology of Bone Sarcomas and New Therapeutic Developments. Calcif Tissue Int. 2018;102(2):174–195. doi: 10.1007/s00223-017-0372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rainusso N., Wang L.L., Yustein J.T. The adolescent and young adult with cancer: state of the art – bone tumors. Curr Oncol Rep. 2013;15(4):296–307. doi: 10.1007/s11912-013-0321-9. [DOI] [PubMed] [Google Scholar]
- 29.Stephens P.J., Greenman C.D., Fu B., et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144(1):27–40. doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyerson M., Pellman D. Cancer genomes evolve by pulverizing single chromosomes. Cell. 2011;144(1):9–10. doi: 10.1016/j.cell.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 31.Gill J., Ahluwalia M.K., Geller D., Gorlick R. New targets and approaches in osteosarcoma. Pharmacol Ther. 2013;137(1):89–99. doi: 10.1016/j.pharmthera.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Chou A.J., Kleinerman E.S., Krailo M.D., et al. Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma: a report from the Children's Oncology Group. Cancer. 2009;115(22):5339–5348. doi: 10.1002/cncr.24566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arndt C.A., Koshkina N.V., Inwards C.Y., et al. Inhaled granulocyte-macrophage colony stimulating factor for first pulmonary recurrence of osteosarcoma: effects on disease-free survival and immunomodulation. a report from the Children's Oncology Group. Clin Cancer Res. 2010;16(15):4024–4030. doi: 10.1158/1078-0432.CCR-10-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyers P.A., Healey J.H., Chou A.J., et al. Addition of pamidronate to chemotherapy for the treatment of osteosarcoma. Cancer. 2011;117(8):1736–1744. doi: 10.1002/cncr.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jia Q., Ivanov I., Zlatev Z.Z., et al. Dietary fish oil and curcumin combine to modulate colonic cytokinetics and gene expression in dextran sodium sulphate-treated mice. Br J Nutr. 2011;106(4):519–529. doi: 10.1017/S0007114511000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manetti F., Santucci A., Locatelli G.A., et al. Identification of a novel pyrazolo[3,4-d]pyrimidine able to inhibit cell proliferation of a human osteogenic sarcoma in vitro and in a xenograft model in mice. J Med Chem. 2007;50(23):5579–5588. doi: 10.1021/jm061449r. [DOI] [PubMed] [Google Scholar]
- 37.Spreafico A., Schenone S., Serchi T., et al. Antiproliferative and proapoptotic activities of new pyrazolo[3,4-d]pyrimidine derivative Src kinase inhibitors in human osteosarcoma cells. FASEB J. 2008;22(5):1560–1571. doi: 10.1096/fj.07-9873com. [DOI] [PubMed] [Google Scholar]
- 38.Balamuth N.J., Womer R.B. Ewing's sarcoma. The lancet oncology. 2010;11(2):184–192. doi: 10.1016/S1470-2045(09)70286-4. [DOI] [PubMed] [Google Scholar]
- 39.von Levetzow C., Jiang X., Gwye Y., et al. Modeling initiation of Ewing sarcoma in human neural crest cells. PLoS One. 2011;6(4) doi: 10.1371/journal.pone.0019305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tirode F., Laud-Duval K., Prieur A., Delorme B., Charbord P., Delattre O. Mesenchymal stem cell features of Ewing tumors. Cancer Cell. 2007;11(5):421–429. doi: 10.1016/j.ccr.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 41.Riggi N., Stamenkovic I. The Biology of Ewing sarcoma. Cancer letters. 2007;254(1):1–10. doi: 10.1016/j.canlet.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 42.Ludwig J.A. Ewing sarcoma: historical perspectives, current state-of-the-art, and opportunities for targeted therapy in the future. Curr Opin Oncol. 2008;20(4):412–418. doi: 10.1097/CCO.0b013e328303ba1d. [DOI] [PubMed] [Google Scholar]
- 43.Gaspar N., Hawkins D.S., Dirksen U., et al. Ewing Sarcoma: Current Management and Future Approaches Through Collaboration. J Clin Oncol. 2015;33(27):3036–3046. doi: 10.1200/JCO.2014.59.5256. [DOI] [PubMed] [Google Scholar]
- 44.Zollner S.K., Amatruda J.F., Bauer S., et al. Ewing Sarcoma-Diagnosis, Treatment, Clinical Challenges and Future Perspectives. J Clin Med. 2021;10(8) doi: 10.3390/jcm10081685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bovee J.V., Cleton-Jansen A.M., Taminiau A.H., Hogendoorn P.C. Emerging pathways in the development of chondrosarcoma of bone and implications for targeted treatment. The lancet oncology. 2005;6(8):599–607. doi: 10.1016/S1470-2045(05)70282-5. [DOI] [PubMed] [Google Scholar]
- 46.Liu L., Ulbrich J., Muller J., et al. Deregulated MYC expression induces dependence upon AMPK-related kinase 5. Nature. 2012;483(7391):608–612. doi: 10.1038/nature10927. [DOI] [PubMed] [Google Scholar]
- 47.Wang P.Y., Ma W., Park J.Y., et al. Increased oxidative metabolism in the Li-Fraumeni syndrome. N Engl J Med. 2013;368(11):1027–1032. doi: 10.1056/NEJMoa1214091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meijer D., de Jong D., Pansuriya T.C., et al. Genetic characterization of mesenchymal, clear cell, and dedifferentiated chondrosarcoma. Genes Chromosomes Cancer. 2012;51(10):899–909. doi: 10.1002/gcc.21974. [DOI] [PubMed] [Google Scholar]
- 49.Schaap F.G., French P.J., Bovee J.V. Mutations in the isocitrate dehydrogenase genes IDH1 and IDH2 in tumors. Adv Anat Pathol. 2013;20(1):32–38. doi: 10.1097/PAP.0b013e31827b654d. [DOI] [PubMed] [Google Scholar]
- 50.Schajowicz F. Juxtacortical chondrosarcoma. J Bone Joint Surg Br. 1977;59-B(4):473–80. doi: 10.1302/0301-620X.59B4.270475. [DOI] [PubMed] [Google Scholar]
- 51.Mavrogenis A.F., Angelini A., Drago G., Merlino B., Ruggieri P. Survival analysis of patients with chondrosarcomas of the pelvis. J Surg Oncol. 2013;108(1):19–27. doi: 10.1002/jso.23351. [DOI] [PubMed] [Google Scholar]
- 52.Chow WA. Chondrosarcoma: biology, genetics, and epigenetics. F1000Res 2018;7. 10.12688/f1000research.15953.1. [DOI] [PMC free article] [PubMed]
- 53.Wang Z., Chen G., Chen X., et al. Predictors of the survival of patients with chondrosarcoma of bone and metastatic disease at diagnosis. J Cancer. 2019;10(11):2457–2463. doi: 10.7150/jca.30388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mery B., Espenel S., Guy J.B., et al. Biological aspects of chondrosarcoma: Leaps and hurdles. Critical reviews in oncology/hematology. 2018;126:32–36. doi: 10.1016/j.critrevonc.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Efstratiadis A. Genetics of mouse growth. Int J Dev Biol 1998;42(7):955-76. (https://www.ncbi.nlm.nih.gov/pubmed/9853827). [PubMed]
- 56.Hernandez-Sanchez C., Blakesley V., Kalebic T., Helman L., LeRoith D. The role of the tyrosine kinase domain of the insulin-like growth factor-I receptor in intracellular signaling, cellular proliferation, and tumorigenesis. J Biol Chem. 1995;270(49):29176–29181. doi: 10.1074/jbc.270.49.29176. [DOI] [PubMed] [Google Scholar]
- 57.Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 1993;75(1):73-82. (https://www.ncbi.nlm.nih.gov/pubmed/8402902). [PubMed]
- 58.Ling Y., Maile L.A., Lieskovska J., Badley-Clarke J., Clemmons D.R. Role of SHPS-1 in the regulation of insulin-like growth factor I-stimulated Shc and mitogen-activated protein kinase activation in vascular smooth muscle cells. Mol Biol Cell. 2005;16(7):3353–3364. doi: 10.1091/mbc.e04-10-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ornitz D.M., Marie P.J. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 2002;16(12):1446–1465. doi: 10.1101/gad.990702. [DOI] [PubMed] [Google Scholar]
- 60.Hresko R.C., Mueckler M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005;280(49):40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- 61.Laplante M., Sabatini D.M. mTOR signaling at a glance. J Cell Sci. 2009;122(Pt 20):3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laplante M., Sabatini D.M. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci. 2013;126(Pt 8):1713–1719. doi: 10.1242/jcs.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anderson W.J., Doyle L.A. Updates from the 2020 World Health Organization Classification of Soft Tissue and Bone Tumours. Histopathology. 2021;78(5):644–657. doi: 10.1111/his.14265. [DOI] [PubMed] [Google Scholar]
- 64.Lilienthal I., Herold N. Targeting Molecular Mechanisms Underlying Treatment Efficacy and Resistance in Osteosarcoma: A Review of Current and Future Strategies. Int J Mol Sci. 2020;21(18) doi: 10.3390/ijms21186885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang R., Piperdi S., Zhang Y., et al. Transcriptional Profiling Identifies the Signaling Axes of IGF and Transforming Growth Factor-b as Involved in the Pathogenesis of Osteosarcoma. Clin Orthop Relat Res. 2016;474(1):178–189. doi: 10.1007/s11999-015-4578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao D, Lei Y, Ye Z, et al. Blockade of IGF/IGF-1R signaling axis with soluble IGF-1R mutants suppresses the cell proliferation and tumor growth of human osteosarcoma. Am J Cancer Res 2020;10(10):3248-3266. (https://www.ncbi.nlm.nih.gov/pubmed/33163268). [PMC free article] [PubMed]
- 67.Jentzsch T, Robl B, Husmann M, Bode-Lesniewska B, Fuchs B. Worse prognosis of osteosarcoma patients expressing IGF-1 on a tissue microarray. Anticancer Res 2014;34(8):3881-9. (https://www.ncbi.nlm.nih.gov/pubmed/25075009). [PubMed]
- 68.Wang Y.H., Han X.D., Qiu Y., et al. Increased expression of insulin-like growth factor-1 receptor is correlated with tumor metastasis and prognosis in patients with osteosarcoma. J Surg Oncol. 2012;105(3):235–243. doi: 10.1002/jso.22077. [DOI] [PubMed] [Google Scholar]
- 69.Benini S., Baldini N., Manara M.C., et al. Redundancy of autocrine loops in human osteosarcoma cells. Int J Cancer. 1999;80(4):581–588. doi: 10.1002/(sici)1097-0215(19990209)80:4<581::aid-ijc16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 70.Zvi Y., Ugur E., Batko B., et al. Prognostic and Therapeutic Utility of Variably Expressed Cell Surface Receptors in Osteosarcoma. Sarcoma. 2021;2021:8324348. doi: 10.1155/2021/8324348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao J., Chesebrough J.W., Cartlidge S.A., et al. Dual IGF-I/II-neutralizing antibody MEDI-573 potently inhibits IGF signaling and tumor growth. Cancer Res. 2011;71(3):1029–1040. doi: 10.1158/0008-5472.CAN-10-2274. [DOI] [PubMed] [Google Scholar]
- 72.Haluska P., Menefee M., Plimack E.R., et al. Phase I dose-escalation study of MEDI-573, a bispecific, antiligand monoclonal antibody against IGFI and IGFII, in patients with advanced solid tumors. Clin Cancer Res. 2014;20(18):4747–4757. doi: 10.1158/1078-0432.CCR-14-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olmos D., Postel-Vinay S., Molife L.R., et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing's sarcoma: a phase 1 expansion cohort study. The lancet oncology. 2010;11(2):129–135. doi: 10.1016/S1470-2045(09)70354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.von Mehren M., Britten C.D., Pieslor P., et al. A phase 1, open-label, dose-escalation study of BIIB022 (anti-IGF-1R monoclonal antibody) in subjects with relapsed or refractory solid tumors. Invest New Drugs. 2014;32(3):518–525. doi: 10.1007/s10637-014-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pappo A.S., Patel S.R., Crowley J., et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for Research through Collaboration study. J Clin Oncol. 2011;29(34):4541–4547. doi: 10.1200/JCO.2010.34.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tolcher A.W., Sarantopoulos J., Patnaik A., et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. 2009;27(34):5800–5807. doi: 10.1200/JCO.2009.23.6745. [DOI] [PubMed] [Google Scholar]
- 77.Scotlandi K., Manara M.C., Nicoletti G., et al. Antitumor activity of the insulin-like growth factor-I receptor kinase inhibitor NVP-AEW541 in musculoskeletal tumors. Cancer Res. 2005;65(9):3868–3876. doi: 10.1158/0008-5472.CAN-04-3192. [DOI] [PubMed] [Google Scholar]
- 78.Manara M.C., Landuzzi L., Nanni P., et al. Preclinical in vivo study of new insulin-like growth factor-I receptor–specific inhibitor in Ewing's sarcoma. Clin Cancer Res. 2007;13(4):1322–1330. doi: 10.1158/1078-0432.CCR-06-1518. [DOI] [PubMed] [Google Scholar]
- 79.Sabbatini P., Rowand J.L., Groy A., et al. Antitumor activity of GSK1904529A, a small-molecule inhibitor of the insulin-like growth factor-I receptor tyrosine kinase. Clin Cancer Res. 2009;15(9):3058–3067. doi: 10.1158/1078-0432.CCR-08-2530. [DOI] [PubMed] [Google Scholar]
- 80.Duan Z., Choy E., Harmon D., et al. Insulin-like growth factor-I receptor tyrosine kinase inhibitor cyclolignan picropodophyllin inhibits proliferation and induces apoptosis in multidrug resistant osteosarcoma cell lines. Mol Cancer Ther. 2009;8(8):2122–2130. doi: 10.1158/1535-7163.MCT-09-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anderson P.M., Bielack S.S., Gorlick R.G., et al. A phase II study of clinical activity of SCH 717454 (robatumumab) in patients with relapsed osteosarcoma and Ewing sarcoma. Pediatr Blood Cancer. 2016;63(10):1761–1770. doi: 10.1002/pbc.26087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Juergens H., Daw N.C., Geoerger B., et al. Preliminary efficacy of the anti-insulin-like growth factor type 1 receptor antibody figitumumab in patients with refractory Ewing sarcoma. J Clin Oncol. 2011;29(34):4534–4540. doi: 10.1200/JCO.2010.33.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schoffski P., Adkins D., Blay J.Y., et al. An open-label, phase 2 study evaluating the efficacy and safety of the anti-IGF-1R antibody cixutumumab in patients with previously treated advanced or metastatic soft-tissue sarcoma or Ewing family of tumours. Eur J Cancer. 2013;49(15):3219–3228. doi: 10.1016/j.ejca.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 84.Atzori F., Tabernero J., Cervantes A., et al. A phase I pharmacokinetic and pharmacodynamic study of dalotuzumab (MK-0646), an anti-insulin-like growth factor-1 receptor monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res. 2011;17(19):6304–6312. doi: 10.1158/1078-0432.CCR-10-3336. [DOI] [PubMed] [Google Scholar]
- 85.Pappo A.S., Vassal G., Crowley J.J., et al. A phase 2 trial of R1507, a monoclonal antibody to the insulin-like growth factor-1 receptor (IGF-1R), in patients with recurrent or refractory rhabdomyosarcoma, osteosarcoma, synovial sarcoma, and other soft tissue sarcomas: results of a Sarcoma Alliance for Research Through Collaboration study. Cancer. 2014;120(16):2448–2456. doi: 10.1002/cncr.28728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tap W.D., Demetri G., Barnette P., et al. Phase II study of ganitumab, a fully human anti-type-1 insulin-like growth factor receptor antibody, in patients with metastatic Ewing family tumors or desmoplastic small round cell tumors. J Clin Oncol. 2012;30(15):1849–1856. doi: 10.1200/JCO.2011.37.2359. [DOI] [PubMed] [Google Scholar]
- 87.Garofalo C., Capristo M., Mancarella C., Reunevi H., Picci P., Scotlandi K. Preclinical Effectiveness of Selective Inhibitor of IRS-1/2 NT157 in Osteosarcoma Cell Lines. Front Endocrinol (Lausanne) 2015;6:74. doi: 10.3389/fendo.2015.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dalmizrak O., Wu A., Chen J., et al. Insulin receptor substrate-1 regulates the transformed phenotype of BT-20 human mammary cancer cells. Cancer Res. 2007;67(5):2124–2130. doi: 10.1158/0008-5472.CAN-06-3954. [DOI] [PubMed] [Google Scholar]
- 89.Baserga R. The insulin receptor substrate-1: a biomarker for cancer? Exp Cell Res. 2009;315(5):727–732. doi: 10.1016/j.yexcr.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 90.Hoang C.D., Zhang X., Scott P.D., et al. Selective activation of insulin receptor substrate-1 and -2 in pleural mesothelioma cells: association with distinct malignant phenotypes. Cancer Res. 2004;64(20):7479–7485. doi: 10.1158/0008-5472.CAN-04-1898. [DOI] [PubMed] [Google Scholar]
- 91.Chandarlapaty S., Sawai A., Scaltriti M., et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19(1):58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee J.W., Kim K.S., An H.K., Kim C.H., Moon H.I., Lee Y.C. Dendropanoxide induces autophagy through ERK1/2 activation in MG-63 human osteosarcoma cells and autophagy inhibition enhances dendropanoxide-induced apoptosis. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0083611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu B., Wu Y., Peng D. Astrocyte elevated gene-1 regulates osteosarcoma cell invasion and chemoresistance via endothelin-1/endothelin A receptor signaling. Oncol Lett. 2013;5(2):505–510. doi: 10.3892/ol.2012.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gobin B., Huin M.B., Lamoureux F., et al. BYL719, a new alpha-specific PI3K inhibitor: single administration and in combination with conventional chemotherapy for the treatment of osteosarcoma. Int J Cancer. 2015;136(4):784–796. doi: 10.1002/ijc.29040. [DOI] [PubMed] [Google Scholar]
- 95.Liu B., Wu Y., Zhou Y., Peng D. Endothelin A receptor antagonism enhances inhibitory effects of anti-ganglioside GD2 monoclonal antibody on invasiveness and viability of human osteosarcoma cells. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0093576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Anderson J.L., Park A., Akiyama R., Tap W.D., Denny C.T., Federman N. Evaluation of In Vitro Activity of the Class I PI3K Inhibitor Buparlisib (BKM120) in Pediatric Bone and Soft Tissue Sarcomas. PloS one. 2015;10(9) doi: 10.1371/journal.pone.0133610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Long X.H., Zhong Z.H., Peng A.F., et al. LY294002 suppresses the malignant phenotype and sensitizes osteosarcoma cells to pirarubicin chemotherapy. Mol Med Rep. 2014;10(6):2967–2972. doi: 10.3892/mmr.2014.2617. [DOI] [PubMed] [Google Scholar]
- 98.Jiang N., Dai Q., Su X., Fu J., Feng X., Peng J. Role of PI3K/AKT pathway in cancer: the framework of malignant behavior. Mol Biol Rep. 2020;47(6):4587–4629. doi: 10.1007/s11033-020-05435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Conciatori F., Ciuffreda L., Bazzichetto C., et al. mTOR Cross-Talk in Cancer and Potential for Combination Therapy. Cancers (Basel) 2018;10(1) doi: 10.3390/cancers10010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rad E., Murray J.T., Tee A.R. Oncogenic Signalling through Mechanistic Target of Rapamycin (mTOR): A Driver of Metabolic Transformation and Cancer Progression. Cancers (Basel) 2018;10(1) doi: 10.3390/cancers10010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abdel-Rahman O., Fouad M. Risk of oral and gastrointestinal mucosal injury in patients with solid tumors treated with everolimus, temsirolimus or ridaforolimus: a comparative systematic review and meta-analysis. Expert Rev Anticancer Ther. 2015;15(7):847–858. doi: 10.1586/14737140.2015.1047350. [DOI] [PubMed] [Google Scholar]
- 102.Demetri G.D., Chawla S.P., Ray-Coquard I., et al. Results of an international randomized phase III trial of the mammalian target of rapamycin inhibitor ridaforolimus versus placebo to control metastatic sarcomas in patients after benefit from prior chemotherapy. J Clin Oncol. 2013;31(19):2485–2492. doi: 10.1200/JCO.2012.45.5766. [DOI] [PubMed] [Google Scholar]
- 103.Fouladi M., Laningham F., Wu J., et al. Phase I study of everolimus in pediatric patients with refractory solid tumors. J Clin Oncol. 2007;25(30):4806–4812. doi: 10.1200/JCO.2007.11.4017. [DOI] [PubMed] [Google Scholar]
- 104.Wagner L.M., Fouladi M., Ahmed A., et al. Phase II study of cixutumumab in combination with temsirolimus in pediatric patients and young adults with recurrent or refractory sarcoma: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2015;62(3):440–444. doi: 10.1002/pbc.25334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bernstein-Molho R., Kollender Y., Issakov J., et al. Clinical activity of mTOR inhibition in combination with cyclophosphamide in the treatment of recurrent unresectable chondrosarcomas. Cancer Chemother Pharmacol. 2012;70(6):855–860. doi: 10.1007/s00280-012-1968-x. [DOI] [PubMed] [Google Scholar]
- 106.Thornton K.A., Chen A.R., Trucco M.M., et al. A dose-finding study of temsirolimus and liposomal doxorubicin for patients with recurrent and refractory bone and soft tissue sarcoma. Int J Cancer. 2013;133(4):997–1005. doi: 10.1002/ijc.28083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Naing A., Kurzrock R., Burger A., et al. Phase I trial of cixutumumab combined with temsirolimus in patients with advanced cancer. Clin Cancer Res. 2011;17(18):6052–6060. doi: 10.1158/1078-0432.CCR-10-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Subbiah V., Naing A., Brown R.E., et al. Targeted morphoproteomic profiling of Ewing's sarcoma treated with insulin-like growth factor 1 receptor (IGF1R) inhibitors: response/resistance signatures. PloS one. 2011;6(4) doi: 10.1371/journal.pone.0018424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Quek R., Wang Q., Morgan J.A., et al. Combination mTOR and IGF-1R inhibition: phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumors. Clin Cancer Res. 2011;17(4):871–879. doi: 10.1158/1078-0432.CCR-10-2621. [DOI] [PubMed] [Google Scholar]
- 110.Gary K. Schwartz WDT, Li-Xuan Qin, Michael B. Livingston, Samir D. Undevia, Bartosz Chmielowski, Mark Agulnik, Scott Schuetze, Damon R. Reed, Scott H. Okuno, Joseph A. Ludwig, Kenneth R. Hande, Petra Rietschel, Andrew S. Kraft, Douglas Adkins, Bruce Brockstein, Vincent Yim, Christiana Bitas, Cristina Antonescu, Robert G. Maki; Memorial Sloan-Kettering Cancer Center, New York, NY; Carolinas Hematology-Oncology Associates, Charlotte, NC; University of Chicago Medical Center, Chicago, IL; University of California, Los Angeles Translational Oncology Research Laboratory, Los Angeles, CA; Northwestern University, Feinberg School of Medicine, Chicago, IL; University of Michigan, Ann Arbor, MI; H. Lee Moffitt Cancer Canter & Research Institute, Tampa, FL; Mayo Clinic, Rochester, MN; University of Texas M. D. Anderson Cancer Center, Houston, TX; Vanderbilt University, Nashville, TN; Albert Einstein Cancer Center, Bronx, NY; Medical University of South Carolina Hollings Cancer Center, Charleston, SC; Washington University, St. Louis, MO; NorthShore University HealthSystem, Chicago, IL; Mount Sinai School of Medicine, New York, NY. A phase II multicenter study of the IGF-1 receptor antibody cixutumumab (A12) and the mTOR inhibitor temsirolimus (TEM) in patients (pts) with refractory IGF-1R positive (+) and negative (-) bone and soft tissue sarcomas (STS). American Society of Clinical Conference Annual Meeting. Chicago: ASCO; 2012.
- 111.de Oliveira M.A., Martins E.M.F., Wang Q., et al. Clinical presentation and management of mTOR inhibitor-associated stomatitis. Oral Oncol. 2011;47(10):998–1003. doi: 10.1016/j.oraloncology.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 112.Vadhan-Raj S., Trent J., Patel S., et al. Single-dose palifermin prevents severe oral mucositis during multicycle chemotherapy in patients with cancer: a randomized trial. Annals of internal medicine. 2010;153(6):358–367. doi: 10.7326/0003-4819-153-6-201009210-00003. [DOI] [PubMed] [Google Scholar]
- 113.Yoneyama Y., Inamitsu T., Chida K., et al. Serine Phosphorylation by mTORC1 Promotes IRS-1 Degradation through SCFbeta-TRCP E3 Ubiquitin Ligase. iScience. 2018;5:1–18. doi: 10.1016/j.isci.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wan X., Harkavy B., Shen N., Grohar P., Helman L.J. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26(13):1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 115.Hartley D, Cooper GM. Role of mTOR in the degradation of IRS-1: regulation of PP2A activity. J Cell Biochem 2002;85(2):304-14. (https://www.ncbi.nlm.nih.gov/pubmed/11948686). [DOI] [PubMed]
- 116.Haruta T., Uno T., Kawahara J., et al. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol Endocrinol. 2000;14(6):783–794. doi: 10.1210/mend.14.6.0446. [DOI] [PubMed] [Google Scholar]
- 117.Hopkins B.D., Pauli C., Du X., et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature. 2018;560(7719):499–503. doi: 10.1038/s41586-018-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Harvey K.F., Zhang X., Thomas D.M. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13(4):246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 119.Moroishi T., Hansen C.G., Guan K.L. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15(2):73–79. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moya I.M., Halder G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat Rev Mol Cell Biol. 2019;20(4):211–226. doi: 10.1038/s41580-018-0086-y. [DOI] [PubMed] [Google Scholar]
- 121.Zanconato F., Cordenonsi M., Piccolo S. YAP and TAZ: a signalling hub of the tumour microenvironment. Nat Rev Cancer. 2019;19(8):454–464. doi: 10.1038/s41568-019-0168-y. [DOI] [PubMed] [Google Scholar]
- 122.Low B.C., Pan C.Q., Shivashankar G.V., Bershadsky A., Sudol M., Sheetz M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014;588(16):2663–2670. doi: 10.1016/j.febslet.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 123.Huang J., Wu S., Barrera J., Matthews K., Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122(3):421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 124.Piccolo S., Dupont S., Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94(4):1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 125.Yu F.X., Zhao B., Guan K.L. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell. 2015;163(4):811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Harris T.J., Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11(7):502–514. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- 127.Ma H., Wang J., Zhao X., et al. Periostin Promotes Colorectal Tumorigenesis through Integrin-FAK-Src Pathway-Mediated YAP/TAZ Activation. Cell Rep. 2020;30(3):793–806 e6. doi: 10.1016/j.celrep.2019.12.075. [DOI] [PubMed] [Google Scholar]
- 128.Lamar J.M., Xiao Y., Norton E., et al. SRC tyrosine kinase activates the YAP/TAZ axis and thereby drives tumor growth and metastasis. J Biol Chem. 2019;294(7):2302–2317. doi: 10.1074/jbc.RA118.004364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141(8):1614–1626. doi: 10.1242/dev.102376. [DOI] [PubMed] [Google Scholar]
- 130.Elbediwy A., Vincent-Mistiaen Z.I., Thompson B.J. YAP and TAZ in epithelial stem cells: A sensor for cell polarity, mechanical forces and tissue damage. Bioessays. 2016;38(7):644–653. doi: 10.1002/bies.201600037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 2004;6(4):483-95. (https://www.ncbi.nlm.nih.gov/pubmed/15068789). [DOI] [PubMed]
- 132.Kim T., Yang S.J., Hwang D., et al. A basal-like breast cancer-specific role for SRF-IL6 in YAP-induced cancer stemness. Nat Commun. 2015;6:10186. doi: 10.1038/ncomms10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Feng X., Liu P., Zhou X., et al. Thromboxane A2 Activates YAP/TAZ Protein to Induce Vascular Smooth Muscle Cell Proliferation and Migration. J Biol Chem. 2016;291(36):18947–18958. doi: 10.1074/jbc.M116.739722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hong J.H., Hwang E.S., McManus M.T., et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309(5737):1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- 135.Yang J.Y., Cho S.W., An J.H., et al. Osteoblast-targeted overexpression of TAZ increases bone mass in vivo. PloS one. 2013;8(2) doi: 10.1371/journal.pone.0056585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Karystinou A., Roelofs A.J., Neve A., Cantatore F.P., Wackerhage H., De Bari C. Yes-associated protein (YAP) is a negative regulator of chondrogenesis in mesenchymal stem cells. Arthritis Res Ther. 2015;17:147. doi: 10.1186/s13075-015-0639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Goto H., Nishio M., To Y., et al. Loss of Mob1a/b in mice results in chondrodysplasia due to YAP1/TAZ-TEAD-dependent repression of SOX9. Development. 2018;145(6) doi: 10.1242/dev.159244. [DOI] [PubMed] [Google Scholar]
- 138.Deng Y., Wu A., Li P., et al. Yap1 Regulates Multiple Steps of Chondrocyte Differentiation during Skeletal Development and Bone Repair. Cell Rep. 2016;14(9):2224–2237. doi: 10.1016/j.celrep.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 139.Zucchini C., Manara M.C., Cristalli C., et al. ROCK2 deprivation leads to the inhibition of tumor growth and metastatic potential in osteosarcoma cells through the modulation of YAP activity. J Exp Clin Cancer Res. 2019;38(1):503. doi: 10.1186/s13046-019-1506-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bouvier C., Macagno N., Nguyen Q., et al. Prognostic value of the Hippo pathway transcriptional coactivators YAP/TAZ and beta1-integrin in conventional osteosarcoma. Oncotarget. 2016;7(40):64702–64710. doi: 10.18632/oncotarget.11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Morice S., Mullard M., Brion R., et al. The YAP/TEAD Axis as a New Therapeutic Target in Osteosarcoma: Effect of Verteporfin and CA3 on Primary Tumor Growth. Cancers (Basel) 2020;12(12) doi: 10.3390/cancers12123847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang Y.H., Li B., Shen L., Shen Y., Chen X.D. The role and clinical significance of YES-associated protein 1 in human osteosarcoma. Int J Immunopathol Pharmacol. 2013;26(1):157–167. doi: 10.1177/039463201302600115. [DOI] [PubMed] [Google Scholar]
- 143.Yang Z., Zhang M., Xu K., et al. Knockdown of YAP1 inhibits the proliferation of osteosarcoma cells in vitro and in vivo. Oncol Rep. 2014;32(3):1265–1272. doi: 10.3892/or.2014.3305. [DOI] [PubMed] [Google Scholar]
- 144.Shen S., Huang K., Wu Y., et al. A miR-135b-TAZ positive feedback loop promotes epithelial-mesenchymal transition (EMT) and tumorigenesis in osteosarcoma. Cancer letters. 2017;407:32–44. doi: 10.1016/j.canlet.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 145.Bierbaumer L., Katschnig A.M., Radic-Sarikas B., et al. YAP/TAZ inhibition reduces metastatic potential of Ewing sarcoma cells. Oncogenesis. 2021;10(1):2. doi: 10.1038/s41389-020-00294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rodriguez-Nunez P., Romero-Perez L., Amaral A.T., et al. Hippo pathway effectors YAP1/TAZ induce an EWS-FLI1-opposing gene signature and associate with disease progression in Ewing sarcoma. J Pathol. 2020;250(4):374–386. doi: 10.1002/path.5379. [DOI] [PubMed] [Google Scholar]
- 147.Delve E, Co V, Regmi SC, Parreno J, Schmidt TA, Kandel RA. YAP/TAZ regulates the expression of proteoglycan 4 and tenascin C in superficial-zone chondrocytes. Eur Cell Mater 2020;39:48-64. 10.22203/eCM.v039a03. [DOI] [PubMed]
- 148.Sun Z., Schwenzer A., Rupp T., et al. Tenascin-C Promotes Tumor Cell Migration and Metastasis through Integrin alpha9beta1-Mediated YAP Inhibition. Cancer Res. 2018;78(4):950–961. doi: 10.1158/0008-5472.CAN-17-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.He S., Huang Q., Hu J., et al. EWS-FLI1-mediated tenascin-C expression promotes tumour progression by targeting MALAT1 through integrin alpha5beta1-mediated YAP activation in Ewing sarcoma. Br J Cancer. 2019;121(11):922–933. doi: 10.1038/s41416-019-0608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hawkins A.G., Julian C.M., Konzen S., Treichel S., Lawlor E.R., Bailey K.M. Microenvironmental Factors Drive Tenascin C and Src Cooperation to Promote Invadopodia Formation in Ewing Sarcoma. Neoplasia. 2019;21(10):1063–1072. doi: 10.1016/j.neo.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fullenkamp C.A., Hall S.L., Jaber O.I., et al. TAZ and YAP are frequently activated oncoproteins in sarcomas. Oncotarget. 2016;7(21):30094–30108. doi: 10.18632/oncotarget.8979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li Y., Yang S., Qin L., Yang S. TAZ is required for chondrogenesis and skeletal development. Cell Discov. 2021;7(1):26. doi: 10.1038/s41421-021-00254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tan M.L., Choong P.F., Dass C.R. Anti-chondrosarcoma effects of PEDF mediated via molecules important to apoptosis, cell cycling, adhesion and invasion. Biochemical and biophysical research communications. 2010;398(4):613–618. doi: 10.1016/j.bbrc.2010.05.098. [DOI] [PubMed] [Google Scholar]
- 154.Xu W., Wan Q., Na S., Yokota H., Yan J.L., Hamamura K. Suppressed invasive and migratory behaviors of SW1353 chondrosarcoma cells through the regulation of Src, Rac1 GTPase, and MMP13. Cell Signal. 2015;27(12):2332–2342. doi: 10.1016/j.cellsig.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 155.Chen J.C., Yang S.T., Lin C.Y., et al. BMP-7 enhances cell migration and alphavbeta3 integrin expression via a c-Src-dependent pathway in human chondrosarcoma cells. PloS one. 2014;9(11) doi: 10.1371/journal.pone.0112636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang H.T., Gui T., Sang Y., et al. The BET Bromodomain Inhibitor JQ1 Suppresses Chondrosarcoma Cell Growth via Regulation of YAP/p21/c-Myc Signaling. J Cell Biochem. 2017;118(8):2182–2192. doi: 10.1002/jcb.25863. [DOI] [PubMed] [Google Scholar]
- 157.Fleuren E.D., Hillebrandt-Roeffen M.H., Flucke U.E., et al. The role of AXL and the in vitro activity of the receptor tyrosine kinase inhibitor BGB324 in Ewing sarcoma. Oncotarget. 2014;5(24):12753–12768. doi: 10.18632/oncotarget.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lemmon M.A., Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gay C.M., Balaji K., Byers L.A. Giving AXL the axe: targeting AXL in human malignancy. Br J Cancer. 2017;116(4):415–423. doi: 10.1038/bjc.2016.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhu C., Wei Y., Wei X. AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications. Mol Cancer. 2019;18(1):153. doi: 10.1186/s12943-019-1090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Scaltriti M., Elkabets M., Baselga J. Molecular Pathways: AXL, a Membrane Receptor Mediator of Resistance to Therapy. Clin Cancer Res. 2016;22(6):1313–1317. doi: 10.1158/1078-0432.CCR-15-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Antony J., Huang R.Y. AXL-Driven EMT State as a Targetable Conduit in Cancer. Cancer Res. 2017;77(14):3725–3732. doi: 10.1158/0008-5472.CAN-17-0392. [DOI] [PubMed] [Google Scholar]
- 163.Pinato D.J., Chowdhury S., Stebbing J. TAMing resistance to multi-targeted kinase inhibitors through Axl and Met inhibition. Oncogene. 2016;35(21):2684–2686. doi: 10.1038/onc.2015.374. [DOI] [PubMed] [Google Scholar]
- 164.Schoumacher M., Burbridge M. Key Roles of AXL and MER Receptor Tyrosine Kinases in Resistance to Multiple Anticancer Therapies. Current oncology reports. 2017;19(3):19. doi: 10.1007/s11912-017-0579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Miller M.A., Sullivan R.J., Lauffenburger D.A. Molecular Pathways: Receptor Ectodomain Shedding in Treatment, Resistance, and Monitoring of Cancer. Clin Cancer Res. 2017;23(3):623–629. doi: 10.1158/1078-0432.CCR-16-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Akalu Y.T., Rothlin C.V., Ghosh S. TAM receptor tyrosine kinases as emerging targets of innate immune checkpoint blockade for cancer therapy. Immunol Rev. 2017;276(1):165–177. doi: 10.1111/imr.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xu M.Z., Chan S.W., Liu A.M., et al. AXL receptor kinase is a mediator of YAP-dependent oncogenic functions in hepatocellular carcinoma. Oncogene. 2011;30(10):1229–1240. doi: 10.1038/onc.2010.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hoj J.P., Mayro B., Pendergast A.M. A TAZ-AXL-ABL2 Feed-Forward Signaling Axis Promotes Lung Adenocarcinoma Brain Metastasis. Cell Rep. 2019;29(11):3421–3434 e8. doi: 10.1016/j.celrep.2019.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Azzolin L., Panciera T., Soligo S., et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158(1):157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 170.Zanconato F., Forcato M., Battilana G., et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol. 2015;17(9):1218–1227. doi: 10.1038/ncb3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zanconato F., Cordenonsi M., Piccolo S. YAP/TAZ at the Roots of Cancer. Cancer Cell. 2016;29(6):783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schallier D., Lesfevre P., Everaert H. Antitumor Activity of Cabozantinib in Metastatic Adult Ewing Sarcoma: A Case Report. Anticancer Res. 2020;40(4):2265–2269. doi: 10.21873/anticanres.14190. [DOI] [PubMed] [Google Scholar]
- 173.Akshintala S, Widemann BC, Barkauskas DA, et al. Phase 2 trial of cabozantinib in children and young adults with refractory sarcomas, Wilms tumor, and rare tumors: Children's Oncology Group Study (ADVL1622). Journal of Clinical Oncology 2021;39(15_suppl):10010-10010. 10.1200/JCO.2021.39.15_suppl.10010.
- 174.Italiano A., Mir O., Mathoulin-Pelissier S., et al. Cabozantinib in patients with advanced Ewing sarcoma or osteosarcoma (CABONE): a multicentre, single-arm, phase 2 trial. The lancet oncology. 2020;21(3):446–455. doi: 10.1016/S1470-2045(19)30825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Santoro M., Lamhamedi-Cherradi S.E., Menegaz B.A., Ludwig J.A., Mikos A.G. Flow perfusion effects on three-dimensional culture and drug sensitivity of Ewing sarcoma. Proc Natl Acad Sci U S A. 2015;112(33):10304–10309. doi: 10.1073/pnas.1506684112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fong E.L., Lamhamedi-Cherradi S.E., Burdett E., et al. Modeling Ewing sarcoma tumors in vitro with 3D scaffolds. Proc Natl Acad Sci U S A. 2013;110(16):6500–6505. doi: 10.1073/pnas.1221403110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Molina E.R., Chim L.K., Salazar M.C., et al. Mechanically tunable coaxial electrospun models of YAP/TAZ mechanoresponse and IGF-1R activation in osteosarcoma. Acta biomaterialia. 2019;100:38–51. doi: 10.1016/j.actbio.2019.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kim N.G., Gumbiner B.M. Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway. The Journal of cell biology. 2015;210(3):503–515. doi: 10.1083/jcb.201501025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jang S.W., Yang S.J., Srinivasan S., Ye K. Akt phosphorylates MstI and prevents its proteolytic activation, blocking FOXO3 phosphorylation and nuclear translocation. J Biol Chem. 2007;282(42):30836–30844. doi: 10.1074/jbc.M704542200. [DOI] [PubMed] [Google Scholar]
- 180.Chan E.H., Nousiainen M., Chalamalasetty R.B., Schafer A., Nigg E.A., Sillje H.H. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24(12):2076–2086. doi: 10.1038/sj.onc.1208445. [DOI] [PubMed] [Google Scholar]
- 181.Zhao B., Li L., Wang L., Wang C.Y., Yu J., Guan K.L. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26(1):54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tumaneng K., Schlegelmilch K., Russell R.C., et al. YAP mediates crosstalk between the Hippo and PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nat Cell Biol. 2012;14(12):1322–1329. doi: 10.1038/ncb2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Azad T., Nouri K., Janse van Rensburg H.J., et al. A gain-of-functional screen identifies the Hippo pathway as a central mediator of receptor tyrosine kinases during tumorigenesis. Oncogene. 2020;39(2):334–355. doi: 10.1038/s41388-019-0988-y. [DOI] [PubMed] [Google Scholar]