Abstract
Spaghetti meat (SM), woody breast (WB), and white striping (WS) are myopathies affecting breast muscle of broiler chickens, and are characterized by a loss of myofibers and an increase in fibrous tissue. The conditions develop in intensive broiler chicken production systems, and cause poor meat process-ability and negative customer perception leading to monetary losses. The objectives of the present study were to describe the physical and histological characteristics of breast myopathies from commercial broiler chicken flocks in Ontario, Canada, and to assess the associations between the severity of myopathies with the physical and histological characteristics of the affected breast muscle fillets. Chicken breast fillets (n = 179) were collected over 3 visits from a processing plant and scored macroscopically to assess the severity of myopathies, following an established scoring scheme. For each fillet, the surface area, length, width, thickness, weight, and hardness (compression force) were measured. A subset of 60 fillets was evaluated microscopically. Multinomial logistic regression models were built to evaluate associations between physical parameters and macroscopic scores. The odds of SM co-occurring with severe WB (SM1WB2) were significantly associated with increased fillet thickness (OR = 1.59, 95% CI 1.31–1.94) and weight (OR = 1.06, 95% CI 1.03–1.09). Histologically, myopathies had overlapping lesions consisting of polyphasic myodegeneration, perivascular inflammatory cuffing and accumulation of fibrous tissue and fat. The pairwise correlation between macroscopic and microscopic scores was moderate (rho 0.45, P < 0.001). This is the first study to characterize breast myopathies in Canadian broiler flocks. Results show that the morphologic and microscopic changes of fillets from this cohort are similar to data from other countries, and provide database to benchmark these parameters in future studies. Our standardized categorization can be applied to broiler breast fillets in other regions of the world.
Key words: broiler chicken, breast muscle, emerging myopathy, fibrosis, woody breast
INTRODUCTION
Over the past decades chicken meat consumption has significantly increased worldwide due to its relatively low cost, ease of meal preparation, nutritional and dietary perception, as well as wide cultural acceptance (Petracci and Cavani, 2012). To meet this growing demand, broiler chickens are selectively bred for fast growth and high yield. Compared to 50 yr ago, broiler chickens today are marketed in about half the time will have consumed approximately 50% less feed, and gained twice as much bodyweight (Zuidhof et al., 2014). Since the early 2010s, selection for fast growth has been associated with the emergence of myopathies in broiler chickens that mainly affect the breast muscle fillets (Kuttappan et al., 2012a). These conditions are referred to as spaghetti meat (SM), woody breast (WB), and white striping (WS), and are respectively characterized by the loss and unraveling of myofibers, increased toughness of the meat, and development of white striations parallel to the orientation of the myofibers (Kuttappan et al., 2017; Sihvo et al., 2017; Baldi et al., 2019).
The underlying histological changes of these conditions are nonspecific and often overlapping. They include multifocal polyphasic myodegeneration and necrosis, myofiber regeneration, interstitial fibrosis with the accumulation of fat, and perivascular cuffing with mononuclear cells and heterophils. (Kuttappan et al., 2013b; Papah et al., 2017; Sihvo et al., 2017; Baldi et al., 2018; Chen et al., 2019). Multiple studies have shown that breast fillets are often affected by more than one myopathy, with WS often co-occurring with SM and WB (Radaelli et al., 2017; Sihvo et al., 2017; Baldi et al., 2018).
The affected fillets may be removed from the food chain due to rejection by consumers because of negative visual appearance and undesirable sensory properties of the meat (Kuttappan et al., 2012b; Petracci et al., 2019; de Carvalho et al., 2020). The resulting economic loss can be substantial, and conservative estimates place it at $1 billion annual loss in North America alone (Barbut, 2020).
Although automated systems based on spectrometry have started to appear to selectively remove affected fillets (Wold et al., 2017, 2019; Wold and Løvland, 2020) at the processing plant, breast myopathies are still most commonly identified by visual and tactile inspection by trained personnel. For research purposes, published macroscopic scoring systems divide SM, WB, and WS into 3 and 4 tiers of severity (Kuttappan et al., 2013b; Sihvo et al., 2017; Baldi et al., 2018; Malila et al., 2018). Since these scoring systems suffer from subjective bias, efforts have been made to correlate physical parameters with the presence and severity of breast myopathies. For instance, studies in the United States indicated that the relative length, width, and thickness of affected fillets were good indicators of predicting WB and WS (Griffin et al., 2018). Ideally, such information can be used to train automated image analysis algorithms that may be applied in-line at processing plants.
Although the presence of WB and WS have been reported in some slow and fast growing Canadian broilers (Santos et al., 2021), no systematic studies have addressed the morphological characteristics and severity of breast myopathies (SM, WB, and WS) in commercial Canadian broilers. Therefore, the objectives of the study were to describe the physical and histological characteristics of SM, WB, and WS from commercial broiler chicken flocks in Ontario, Canada, and to assess associations between the severity of myopathies and physical and histological characteristics of the affected breast muscle fillets.
MATERIALS AND METHODS
Sample Collection and Macroscopic Scoring
A total of 179 skinless breast fillets from 3 broiler chicken flocks (60 fillets × 3 flocks, 1 fillet missing) were collected at a large Ontario processing plant during 3 visits (April to May 2019). They were male Ross708 (37 d; average weight at slaughter, 2.2 kg); mixed-sex Cobb500 (36 d, 2.4 kg); and mixed-sex Ross708 (40 d, 2.1 kg). Exsanguination to deboning time was 3 h. After deboning, representative samples for each myopathy were collected at the plant. Fillets were placed in plastic bags and, within 5 h of collection (total 10–12 h from exsanguination), transported on ice to the Ontario Veterinary College to score myopathies and measure physical parameters.
Myopathies were scored by visual examination and palpation of fillets by 2 trained members of the investigative team to minimize scoring variations. In the rare instances when fillets were scored differently, the 2 scorers re-evaluated the fillets together to reach an agreement. SM was rated as absent (SM0) or present (SM1) based on presence of myofiber separation. WB and WS were scored using previously established criteria, with modifications to better capture the range of lesion severity (Kuttappan et al., 2016; Sihvo et al., 2017): WB was scored as absent (WB0), moderate (WB1) or severe (WB2) based on tissue hardness upon palpation; and WS was scored as absent (WS0), mild (WS1), moderate (WS2), or severe (WS3) based on the frequency and thickness of white lines (Supplementary Table 1A). Each fillet received a compound score indicative of each myopathy (e.g., SM1WB1WS1), which stratified the data into multiple categories (referred to as myopathy score). However, since WS0, fillets without distinct white lines, was present only in a small number of fillets (n = 10), and WS1, in a large majority (n = 130), the WS category was not included in the statistical analysis. Thus, only WB and SM scores were used (e.g., SM0WB1) regardless of WS scores.
Physical Measurements
On arrival at the laboratory, samples were photographed and weighed using a digital scale (PM600, Mettler Toledo, Columbus, OH). Pictures were used to measure cranial surface area (2-dimensional), length, and width, using an image processing software (ImageJ, National Institutes of Health, Bethesda, MD). Length and width were measured at the longest and widest axis, respectively, of the surface of each fillet. Thickness was measured at the thickest point of the cranial part of the fillet, using a digital caliper (58-6800-4, Mastercraft, Brantford, ON, CA). Compression force was determined at the cranial region of raw fillets, using a texture analyzer (TA-XT2, Texture Technologies Corp., Hamilton, MA) set to compress by applying a force perpendicular to the fillets using a 10-mm round probe attached to a 30-kg load cell, set at a trigger force of 1 Newton and speed of 5 mm/s for both pre- and post-test. Each fillet was compressed to 20% of its height at 3 compression points at the cranial region approximately 10-h postmortem.
Histological Evaluation
Microscopic lesions were assessed on a subset of 60 fillets collected on a single day of sampling. The 60 samples were chosen according to the macroscopic presence of myopathy. Tissue samples were collected by cutting one core (1 × 1 × 1 cm) from the cranial, medial, and caudal third portion of each fillet (Supplementary Figure 1A); to account for previous reports indicating that the cranial area is most severely affected (Mudalal et al., 2015; Baldi et al., 2018). Tissues were fixed in 10% formalin for 24 to 48 h, embedded in paraffin (3 cores from one fillet per cassette), and processed for hematoxylin and eosin (H&E) and Masson's trichrome staining. The histological scoring was conducted with a light microscope (BX45, Olympus Canada, Richmond Hill, ON, CA), and facilitated using disposable microscope slide-grids of 20 mm × 20 mm with 1 mm spacing (Z688533, Sigma-Aldrich, St. Louis, MO) that were placed over the sections during observation (Supplementary Figure 1B). Considering that the tissue sections on the slide were roughly rectangular, scoring was carried out in six, 4-mm2 windows (each encompassing four, 1 mm2 squares) corresponding to the upper left, upper right, middle right, middle left, lower left, and lower right areas of the tissue, as shown in Supplementary Figure 1C. For each of the 24 squares of the grid, the microscopic scores considered myodegeneration (defined as loss of striation, fragmentation of myofibers, and accumulation of inflammatory cells around myofibers), perivascular infiltrates with inflammatory cells (PVI) and endomysial accumulation of fat and fibrous tissue (lipidosis/fibrosis, LF) on a scale of 0 to 3, which is a slight modification from a previous report (Sihvo et al., 2017; Supplementary Table 1B). For each of the 3 areas (i.e., cranial, medial, and caudal), a final histological score was calculated by adding the individual scores for each of the 24 squares. The score could range from 0 to 216 (24 areas × 3 [maximal score] × 3 criteria). All histological assessments were performed by a veterinarian (S.C.) in a blind manner, implemented by disguising slide identification and randomizing slide order. To test interobserver agreement, a random subset of slides (33.3%, n = 20) was independently scored by a pathologist who also participated in the study (M.I.) (Pavlides et al., 2017).
Statistical Analysis
Descriptive Statistics of Variables
All physical measurements (area, hardness, length, thickness, weight, and width) and the histological score of the fillets were tested for normal distribution using the Shapiro-Wilk normality test. Fillets with scores of 0 for SM and WB were designated as the normal controls (SM0WB0). Since data were normally distributed, an ANOVA with parametric post-hoc Dunnett's test was used to compare the area, length, thickness, weight, and width of fillets from each myopathy score to the normal controls. The Kruskal-Wallis test and Dunn's multiple range test were used for post-hoc multiple comparisons to assess the mean differences of hardness between fillets from each myopathy score with the normal controls. Also, the Kruskal-Wallis test and Dunn's multiple range test were used for post-hoc multiple comparisons to assess the mean differences of histological scores among cranial, medial, and caudal areas.
Exploratory Statistics
Regression analyses using a multinomial logistic regression model were employed to identify associations between the occurrence of breast myopathies (as defined by myopathy scores, outcome variable) and physical parameters (independent predictor variables).
To select variables to include in the final model, univariable analyses using a relaxed P-value (P < 0.20) were built to screen the effect of each independent variable on the dependent variable using the SM0WB0 group as the referent category. Pair-wise correlation coefficients, using the Spearman's rank test, were examined among all significant independent variables to prevent the inclusion of co-linear variables. When 2 variables were highly correlated (rho ≥ 0.70; P < 0.05), the one with the smallest P-value was considered for inclusion in the multivariable model.
All unconditionally significant variables identified on univariable screening were offered to a multivariable model, and a manual backward elimination process was performed. Variables with P > 0.05, as calculated using the likelihood ratio test, were removed for a better fit of a model. For a suspected confounder, when the removal of a variable changed the coefficients of the remaining variables from the final model by more than 20%, the variable was kept in the final model (Dohoo et al., 2014). The final multivariable model included all significant independent variables that affected the occurrence of specific myopathy categories, using the normal controls (SM0WB0) as a the referent category.
Also, a univariable multinomial logistic regression model was employed to identify associations between the occurrence of breast myopathies (myopathy scores as outcome variables) and histological scores (independent predictor variables). Histological scores from the cranial area only were selected as a predictor variable, since the medial area had aponeurosis, an internal sheet of connective tissue, resulting in artifactually increased fibrous tissue, and the histological scores from the caudal area were not significantly different among myopathy groups.
The results from the logistic regression analysis were reported as odds ratios including 95% CI and the respective P-values. An odds ratio >1 indicates an increased odds of an outcome (e.g., SM0WB2) compared to the normal fillet (e.g., SM0WB0) as a result of an increase in the continuous predictor variable by one unit, whereas the odds ratio <1 denotes a decreased odd.
RESULTS
Physical Characteristics
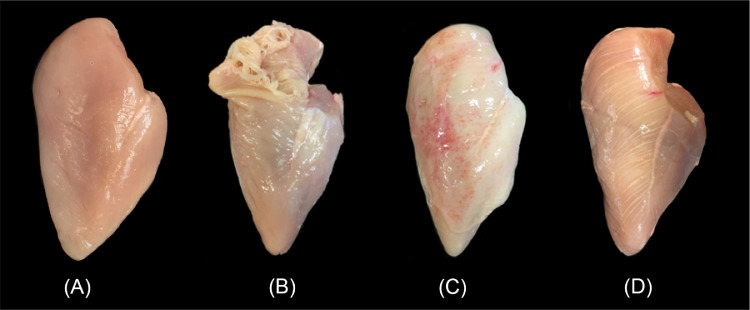
The macroscopic appearance of representative cases of SM, WB, and WS is shown in Figure 1. Fillets with SM were soft and friable when palpated and showed separation of myofiber bundles affecting primarily the cranial area of the breast muscle, starting from the superficial layer. Fillets with WB had a tougher texture, were often pale, and sometimes showed petechial hemorrhage and edema on the surface. Areas of tougher texture could be localized and/or diffuse. Occasionally, fillets with severe WB were presented with multifocal areas of increased thickness (bulging) on the cranial or caudal area. Breasts affected by WS exhibited white lines parallel to the myofibers, most frequently affecting the cranial area of the fillet and rarely extending to the caudal area. Overall, changes associated with each myopathy appeared to be more severe in the cranial area compared with the caudal portion of the fillets. No other changes, such as discoloration or necrosis, were recorded in the samples.
Figure 1.
Representative macroscopic appearance of myopathies in breast fillets. (A) Normal breast fillet, represents SM0WB0WS0; (B) spaghetti meat with separation of myofibers in the cranial area, represents SM1WB0WS1; (C) woody breast with gelatinous exudate and petechial hemorrhage, represents SM0WB2WS1; (D) white striping consisting of white lines parallel to myofibers, represents SM0WB1WS2. Abbreviations: SM, spaghetti meat; SM0, SM absent; SM1, SM present; WB, woody breast; WB0, WB absent; WB1, WB moderate; WB2, WB severe.
The co-occurrence of SM, WB, and WS is summarized in Table 1. Of the 179 fillets, 18.4% (n = 33) did not reveal a macroscopic myopathy (i.e., SM0WB0WS0). The remaining 146 were assigned into 15 different categories, according to the myopathy score. Only 13.4% (24) samples had a single myopathy, whereas 68.2% (n = 122) were affected by more than one. A score of WS3 was not identified among the samples.
Table 1.
Distribution of myopathy scores, as assessed by macroscopic inspection, in 179 fillets from a processing plant in Ontario, Canada. Spaghetti meat (SM), woody breast (WB), white striping (WS) were evaluated.
| Myopathy scores | WS0 |
WS1 |
WS2 |
WS3 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WB0 | WB1 | WB2 | WB0 | WB1 | WB2 | WB0 | WB1 | WB2 | WB0 | WB1 | WB2 | |
| SM0 | 33 | 5 | 3 | 14 | 28 | 37 | 1 | 1 | 2 | 0 | 0 | 0 |
| SM1 | 1 | 1 | 0 | 12 | 19 | 20 | 0 | 1 | 1 | 0 | 0 | 0 |
SM0, SM absent, SM1, SM present.
WB0, WB absent, WB1, WB moderate, WB2, WB severe.
WS0, WS absent, WS1, WS mild, WS2, WS moderate, WS3, WS severe.
Comparisons of Physical Parameters Among Myopathy Groups
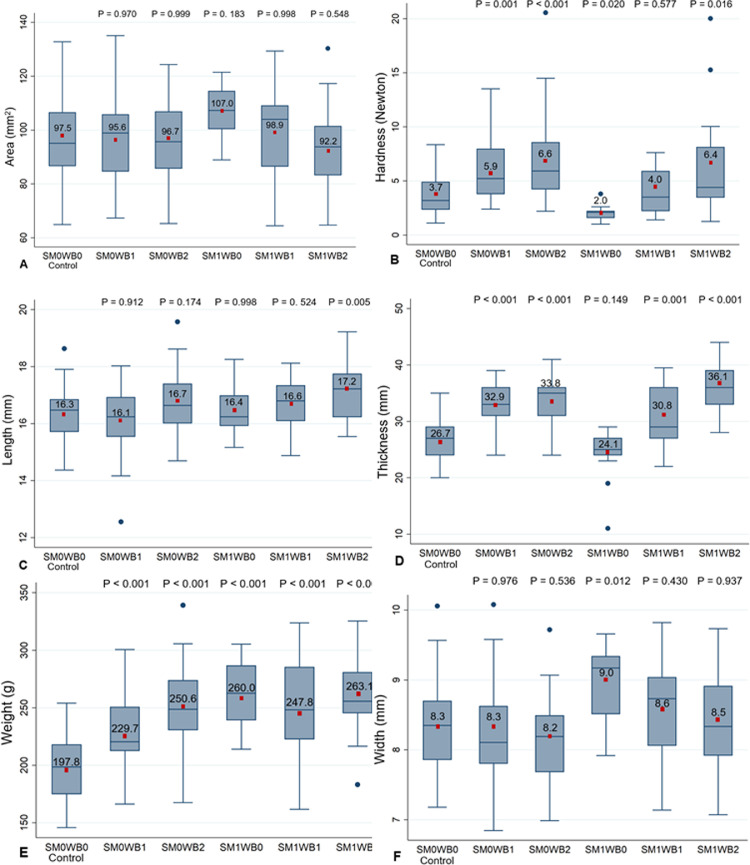
No statistically significant differences between the myopathy groups and the normal controls were observed in the surface area of fillets (Figure 2A). There were significant differences in hardness between the fillets with myopathy and normal controls (Figure 2B). Specifically, when the severity of WB increased, fillets affected by WB required higher compression force: SM0WB1 (P = 0.001), SM0WB2 (P < 0.001), and SM1WB2 (P = 0.016). On the other hand, fillets with only SM (SM1WB0) required significantly (P = 0.020) less compression force compared to the normal controls. Only fillets affected by both SM and severe WB (SM1WB2) were significantly longer than the normal controls (P = 0.005, Figure 2C). Fillets affected by WB of any severity, or in any combination, were significantly thicker than the normal controls (P ≤ 0.001), while SM1WB0 fillets were not (P = 0.149, Figure 2D). Fillets with myopathies were significantly heavier than fillets without myopathies, regardless of the severity of score (P < 0.001, Figure 2E). Only SM1WB0 fillets were significantly wider than fillets without myopathies (P = 0.012, Figure 2F).
Figure 2.
Box plots of the mean values of fillet physical parameters (area, hardness, length, thickness, weight, and width) divided by the myopathy scores. An ANOVA with parametric post-hoc Dunnett's test was used to compare the area, length, thickness, weight, and width of fillets from each myopathy score to the normal controls (SM0WB0). The Kruskall-Wallis test with Dunn's post-hoc method for multiple comparisons was used for hardness (n = 179; SM0WB0 = 48, SM0B1 = 34, SM0WB2 = 42, SM1WB0 = 13, SM1WB1 = 21, SM1WB2 = 21). Dots indicate outlying values which are outside 1.5 times the interquartile range above the upper quartile and below the lower quartile. Red squares inside the box plots indicate the mean values of area, hardness, length, thickness, weight, and width of the different groups. Abbreviations: SM, spaghetti meat; SM0, SM absent; SM1, SM present; WB, woody breast; WB0, WB absent; WB1, WB moderate; WB2, WB severe.
Associations Between Myopathies and Physical Parameters
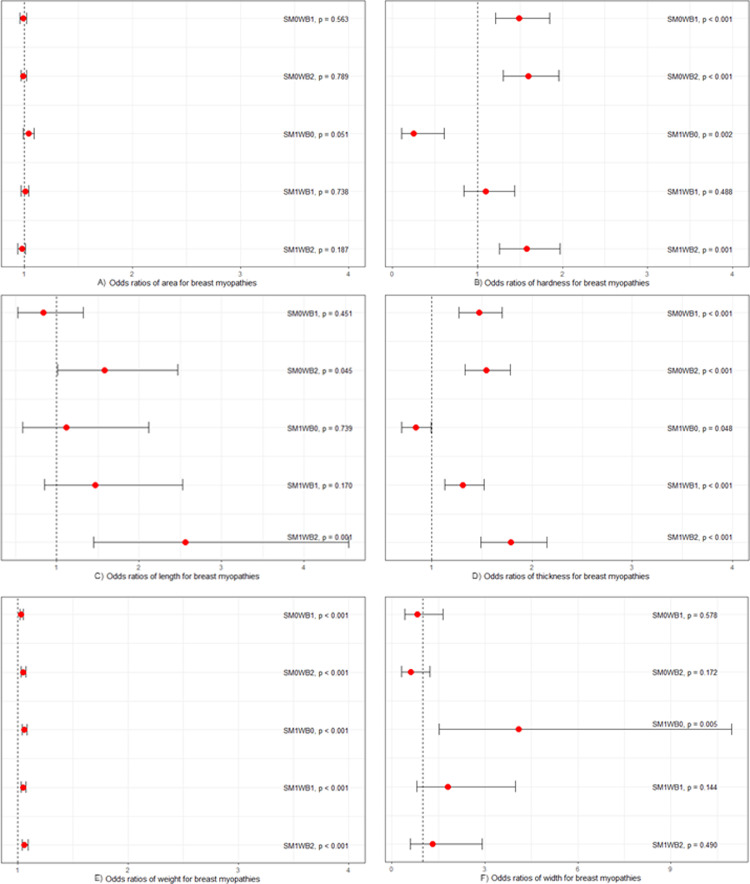
A multinomial regression model was employed to test unconditional associations between physical parameters and the myopathy score (Figure 3), using the normal controls (SM0WB0) as the referent category. There were no significant associations between surface area and myopathy categories (Figure 3A). The hardness (Figure 3B) was positively associated with SM0WB1, SM0WB2, and SM1WB2 fillets (P < 0.001), and negatively associated with SM1WB0 (P = 0.002) fillets. The length (Figure 3C) was positively associated with SM0WB2 (P = 0.045) and SM1WB2 (P = 0.001) fillets. The thickness (Figure 3D) of fillets was positively associated with the SM0WB1, SM0WB2, SM1WB1, and the SM1WB2 categories (P < 0.001) and negatively associated with the SM1WB0 category (P = 0.048). The weight (Figure 3E) of fillets was positively associated with all categories of breast myopathies (P < 0.001) and the width (Figure 3F) was positively associated with the SM1WB0 category (P = 0.005).
Figure 3.
Unconditional association between physical parameters and myopathy scores. For each model, the myopathy score was the outcome variable, and the normal controls (SM0WB0) were set as the referent category (dotted line). The total number of fillets: n = 179. (A) The area was not significantly associated with breast myopathies. (B) Hardness was positively associated with SM0WB1, SM0WB2, and SM1WB2. (C) The length was positively associated with SM1WB2. (D) Thickness was positively associated with SM0WB1, SM0WB2, SM1WB1, SM1WB2, whereas negatively associated with SM1WB0. (E) Weight was positively associated with SM0WB1, SM0WB2, SM1WB0, SM1WB1, SM1WB2. (F) The width was positively associated with SM1WB0. Abbreviations: SM, spaghetti meat; SM0, SM absent; SM1, SM present; WB, woody breast; WB0, WB absent; WB1, WB moderate; WB2, WB severe.
The area variable was excluded during the unconditional regression analysis. Correlations among unconditionally significant variables were further evaluated before the inclusion of variables in the final model. Hardness and thickness were strongly correlated (rho 0.71, P < 0.001), and other variables (length, weight, and width) were weak or moderately correlated (rho < 0.45, Table 2). Therefore, the hardness variable was excluded from the final model to prevent multicollinearity. Later, the length variable was excluded during the likelihood ratio test.
Table 2.
Spearman's rank correlation coefficients to test multicollinearity among predictor variables that were significantly correlated by univariable regression analyses. There was a strong correlation between hardness and thickness (n = 179)
| Parameters | Hardness | Length | Thickness | Weight | Width |
|---|---|---|---|---|---|
| Hardness | 1 | ||||
| Length | 0.21* | 1 | |||
| Thickness | 0.71* | 0.22* | 1 | ||
| Weight | 0.12 | 0.42* | 0.37* | 1 | |
| Width | −0.29* | 0.37* | −0.19* | 0.45* | 1 |
Statistically significant (P < 0.05) Spearman's rank correlation coefficients.
In the final multivariable model, a total of 3 variables (thickness, weight, width) were included, and associations between these variables and myopathy scores were tested using the normal fillet (SM0WB0) as the referent category (Table 3). The odds ratios obtained from the analysis using weight as an independent variable were relatively small (1.03–1.07, P < 0.007), whereas the odds ratios obtained from the analysis using thickness as an independent variable were relatively large (1.22–1.59, P < 0.015) except for SM1WB0 category.
Table 3.
Multivariable multinomial logistic regression models showing associations between physical parameters and myopathy scores, as assessed in a cohort of 179 breast fillets collected at a processing plant in Ontario, Canada.
| Outcomea | Exposure | Odds ratio (95% CI) | P value |
|---|---|---|---|
| SM0WB1 | Thickness | 1.41 (1.20–1.65) | <0.001 |
| Weight | 1.03 (1.01–1.05) | 0.007 | |
| Width | 0.37 (0.12–1.15) | 0.085 | |
| SM0WB2 | Thickness | 1.35 (0.15–1.59) | <0.001 |
| Weight | 1.06 (1.04–1.09) | <0.001 | |
| Width | 0.13 (0.04–0.43) | 0.001 | |
| SM1WB0 | Thickness | 0.82 (0.67–1.00) | 0.054 |
| Weight | 1.07 (1.03–1.10) | <0.001 | |
| Width | 0.29 (0.05–1.54) | 0.145 | |
| SM1WB1 | Thickness | 1.22 (1.04–1.44) | 0.015 |
| Weight | 1.05 (1.02–1.08) | <0.001 | |
| Width | 0.44 (0.13–1.53) | 0.195 | |
| SM1WB2 | Thickness | 1.59 (1.31–1.94) | <0.001 |
| Weight | 1.06 (1.03–1.09) | <0.001 | |
| Width | 0.31 (0.08–1.20) | 0.090 |
For each model, SM0WB0 was the referent group.
Abbreviations: SM, spaghetti meat; SM0, SM absent; SM1, SM present; WB, woody breast; WB0, WB absent; WB1, WB moderate; WB2, WB severe.
Histological Characteristics
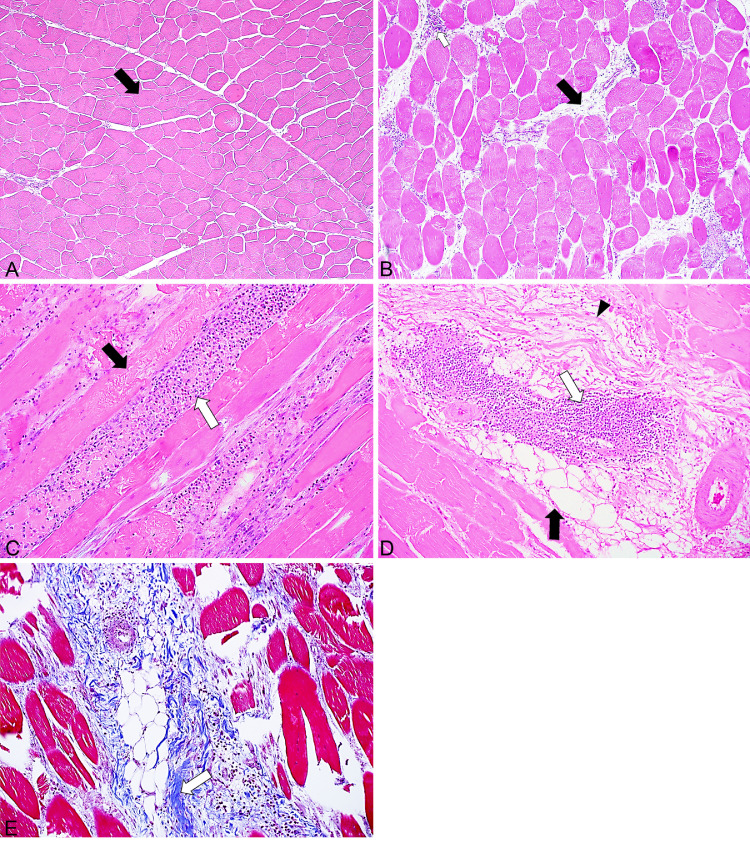
A total of 60 fillets were evaluated histologically. Samples macroscopically classified without myopathy (SM0WB0, n = 17) showed a typical polygonal shape of myofibers on cross-section, with peripherally located nuclei in many areas (Figure 4A). However, myofibers in some areas were surrounded by scant amounts of connective tissue (endomysium) with few, scattered inflammatory cells and rare areas of fat accumulation (Figure 4B). Samples that were macroscopically classified as SM0WB1 (n = 8), SM0WB2 (n = 11), SM1WB0 (n =13), SM1WB1 (n = 10), and SM1WB2 (n = 1), regardless of the type of combined score, revealed similar and overlapping histological lesions, without distinctive features of one particular myopathy. Fillets exhibited changes that directly affected the myofibers (myodegeneration and necrosis), or the interstitium (inflammation, lipid infiltration, fibrosis) or both (Figure 4C). The degeneration and necrosis of myofibers was characterized by hypereosinophilia, loss of cross striation, fragmentation of myofibers, and sarcoplasmic accumulation of macrophages and fewer heterophils (Figure 4D). Rare regenerating fibers showed nuclear rowing in hyperchromatic myotubules. Inflammation was characterized by the interstitial accumulation of lymphocytes, macrophages and scattered heterophils and perivenular accumulation of mononuclear leukocytes. Interstitial lesions were characterized by the accumulation of loose connective tissue, in some areas associated with adipocytes, that expanded the endomysium and occasionally replaced myofibers (Figure 4E). Presence of collagen from interstitial fibrosis was confirmed with Masson's trichrome stain (Figure 4F). Bacterial infection was not observed in any of the examined fillets.
Figure 4.
Pectoralis major tissue: (A) polygonal shape (black arrow) of myofibers from non-affected area, score SM0WB0; (B) scattered inflammatory cells (white arrow) and accumulation of fibro-fatty tissue in non-affected area (black arrow), score SM0WB0; (C) degenerating and necrotic myofiber (white arrow) and fragmentation of myofiber (black arrow), score SM1WB1; (D) changes in interstitium with inflammatory cells (white arrow), lipid infiltration (black arrow), and fibrosis (black arrowhead), score SM1WB2; (E) interstitial fibrosis (white arrow), score SM1WB2; (A–D) H&E stain, (E) Masson's trichrome stain. Abbreviations: SM, spaghetti meat; SM0, SM absent; SM1, SM present; WB, woody breast; WB0, WB absent; WB1, WB moderate; WB2, WB severe.
Histological changes were evaluated with a scoring system that took into account myodegeneration, inflammation, and interstitial changes (fibrosis and/or accumulation of fat tissue) in 3 different areas of the fillet (cranial, medial, and caudal). When applying the histological score, there was a high level of interobserver agreement (= 0.75) between 2 independent observers (S.C. and M.I.). Table 4 summarizes the histological scores stratified by the grossly identified myopathies. The histological scores were not normally distributed (Shapiro-Wilk normality test, P < 0.006) and the ranges of histological scores of each area varied between 0 and 79. The histological scores of each area never reached the maximum value (216) because there were lesions without pathological changes. The histological scores were higher in samples from the cranial and medial areas than in those from the caudal area (P < 0.001), whereas there were no statistically significant differences in scores of the cranial and medial area (P = 0.069).
Table 4.
Histological scores of the cranial, medial, and caudal area, segregated by myopathy score, from 60 breast fillets sampled at a broiler processing plant in Ontario, Canada.
| Area | N | Min | Mean | Median | Max | SD |
|---|---|---|---|---|---|---|
| Cranial | 60 | 0 | 25.3a | 20 | 76 | 17.9 |
| Medial | 60 | 2 | 30.4a | 29 | 79 | 17.4 |
| Caudal | 60 | 0 | 8.2b | 4 | 50 | 9.8 |
Mean values followed by different superscript letters indicate significant differences (P < 0.001, Kruskal Wallis test with Dunn's multiple comparisons).
Pairwise correlation analysis of the myopathy scores and histological scores using Spearman's rho correlation test showed a moderate correlation (rho 0.45; P < 0.001). Correlation between myodegeneration vs. lipidosis and fibrosis was strong (rho 0.79; P < 0.001), whereas correlation between myodegeneration vs. perivascular infiltration was moderate (rho 0.56; P < 0.001). Likewise, correlation between perivascular infiltration vs. lipidosis and fibrosis was moderate (rho 0.53; P < 0.001).
Table 5 shows the results of the univariable multinomial logistic regression model between histological scores from the cranial area and myopathy scores. The odds ratios obtained from the analyses using histological scores from the cranial area as an independent variable were the same for the outcome variables of SM0WB1, SM0WB2, an SM1WB1 (OR = 1.11, P < 0.006), whereas the odds ratio obtained from the analysis using the outcome variable of SM1WB0 was 1.08 (P = 0.024).
Table 5.
A univariable multinomial logistic regression model showing associations between histological scores from the cranial area and myopathy scores, as assessed in a cohort of 60 breast fillets collected at a processing plant in Ontario, Canada.
| Outcomea | Exposure | Odds ratio (95% CI) | P value |
|---|---|---|---|
| SM0WB1 | Histological scores from the cranial area | 1.11 (1.03–1.19) | 0.006 |
| SM0WB2 | // | 1.11 (1.04 –1.19) | 0.002 |
| SM1WB0 | // | 1.08 (1.01–1.15) | 0.024 |
| SM1WB1 | // | 1.11 (1.04–1.19) | 0.003 |
SM0WB0 was the referent category.
Abbreviations: SM, spaghetti meat; SM0, SM absent; SM1, SM present; WB, woody breast; WB0, WB absent; WB1, WB moderate; WB2, WB severe.
DISCUSSION
The present study offers a standardized categorization of physical and histologic features that are associated with breast myopathies (SM, WB, WS) encountered in a cohort of commercial broiler chickens from Ontario, Canada.
The broiler breeds in our study were Ross and Cobb which represent 2 breeds used around the world. Therefore, our standardized categorization should apply in other regions in the world. In the study, we characterized the myopathies through a macroscopic scoring scheme to classify the fillets into a myopathy score, which was used to further correlate to physical parameters (area, hardness, length, thickness, weight, and width) and severity of histological changes. Quantitiative relationships between the occurrence of macroscopic categories and the values of physical parameters and histological scores were further evaluated by regression and pairwise correlation analyses.
In agreement with literature from different countries, the type and severity of myopathies had an effect on the physical aspects of the affected fillets in our cohort, although some results differed from those in previous reports. In our study, breast fillets with WB were overall harder (required higher compression force) than unaffected fillets, most probably as a results of fibrosis causing the accumulation of cross-linked collagen fibrils in the tissue (Velleman et al., 2017). Conversely, SM-affected fillets required much less force to compress, and therefore, are more comparable to unaffected fillets. This is consistent with evidence showing that the typical appearance of SM-affected fillets (i.e., an unraveling of myofibers) may be caused by a reduction in the integrity of the connective tissue (Petracci et al., 2019). Although WB-affected fillets were thicker than unaffected fillets, this difference was not observed between SM-affected and unaffected fillets. This is in disagreement with an Italian study reporting that SM fillets were thicker than the normal controls (Baldi et al., 2018). This difference is likely the result of variations in age, sex, strain, and body weight of the broilers in the 2 experimental cohorts. In our study, breast fillets became heavier with increasing severity of either SM or WB, a finding that is supported by numerous previous reports (Kuttappan et al., 2013a; Tijare et al., 2016; Papah et al., 2017; Sihvo et al., 2017). Increased weight of breast fillets could be the consequence of the selecting high breast-yield broiler chickens (Petracci et al., 2015).
In agreement with Mudalal et al. (2015), the length of WB-affected fillets did not differ from the normal controls; however, in our study fillets affected by both SM and severe WB (SM1WB2) were longer compared to the normal controls. These findings are different from Baldi et al. (2018), where no difference between normal and SM fillet were found, and could result from differences in demographics and husbandry practices between the two populations under study. In agreement with other studies (Mudalal et al., 2015; Baldi et al., 2018), no differences were seen between the width of affected and normal fillets in our cohort.
Given the fact that most fillets in our cohort were affected by WS1, a morphological characterization for this phenotype alone could not be clearly assessed. While WS1 could have been divided into additional categories, this was not considered relevant to the situation in the field, where mild WS in many production systems is considered to be a new normal, without the need to further differentiate between degrees of mild striation. Our WS classification was adopted from a previous study (Malila et al., 2018). While this is an objective and accepted method of WS classification in research, it should be noted that the need to carefully count and measure striations makes it unsuitable for in-line diagnosis at the processing plant, due to the high line speed (15,000/h).
Regression models were built to better understand the associations between physical parameters and the occurrence of myopathies. WS was not included as a dependent variable because only 2.4% of fillets showed WS2 and it resulted in substantial standard errors and P-values. Our multinomial logistic regression model showed that an increase in the weight of breast fillets increases the odds that these may be affected by either SM or WB or both. Even though the odds ratios obtained from the analyses which used the weight as an independent variable were relatively small, they still show the positive association with dependent variables of different groups of myopathies: for example, for a 1-unit increase in weight (g), we expect a 1.06 increase in the odds of SM1WB2. This suggests that myopathies also come with increased weight of affected fillets. This finding is similar to those of previous studies (Alnahhas et al., 2016; Aguirre et al., 2020) which reported the association between WS and weight. The odds ratios obtained from the analyses which used the thickness as an independent variable was relatively larger than those from weight except for the SM1WB0 group. Thickness and SM0WB1, SM0WB2, SM1WB1, and SM1WB2 were positively associated. This finding indicates that thicker fillets increased the odds of WB or SM or both, which is similar to a US study reporting an association between WB and the thickness of affected fillets (Griffin et al., 2018).
In addition to the physical parameters, we assessed the microscopic features of fillets affected by breast myopathies. In our cohort, SM, WB, and WS showed similar microscopic features (myodegeneration, inflammation, interstitial accumulation of fat, and fibrous tissue) and overlapping severity. Our model shows that the odds of having breast myopathies increased when the histological scores increased. While the severity of microscopic changes was found to increase in fillets macroscopically affected by myopathies, the histological score did not segregate myopathies according to the macroscopic features. This could indicate a common pathogenesis, or it could be a reflection of the fact that most myopathies occur together, yielding a mixed phenotype. Additionally, the histological scores and myopathy scores were found to be moderately correlated, despite this finding being highly statistically significant (P < 0.001). This indicates that microscopic changes may precede the development of a macroscopic phenotype. Overall, our results are in agreement with previous studies (Radaelli et al., 2017; Sihvo et al., 2017), which indicate a lack of distinctive microscopic features between myopathies that are macroscopically well defined. Lastly, it should be noted that mild microscopic lesions were also observed in macroscopically normal muscle, a finding consistent with previous observations (Papah et al., 2017; Sihvo et al., 2017).
The potential limitation of our work is that we employed targeted sampling to find fillets with representative features of breast myopathies. It might be somewhat difficult to generalize our findings to some local flocks. In any case, we collected samples from a large Ontario processing plant, and the samples can be used to identify the characteristics of fillets with breast myopathies.
CONCLUSIONS
This is the first systematic study to characterize breast myopathies in Canadian broiler chickens. Our results show that fillets in this cohort are affected by myopathies similar to those described in other countries with intensive poultry production, and that the myopathies affect both the physical (macroscopic) and microscopic features of fillets. The strongest associations were observed between myopathies and heavier and thicker breast fillets, however, microscopic lesions were similar among all affected fillets in the cohort and could not be used to discriminate between the types of myopathy. Some of the most severe muscle changes described in this study is likely to result in negative visual and sensorial appeal for consumers, and additional research is needed to improve in-line detection of such myopathies.
ACKNOWLEDGMENTS
The authors are grateful to the Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA) for providing financial support (Grant No: UofG2017-2922).
DISCLOSURES
The authors have no conflicts if interest to report.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2022.101747.
Appendix. Supplementary materials
REFERENCES
- Aguirre M.E., Leyva-Jimenez H., Travis R., Lee J.T., Athrey G., Alvarado C.Z. Evaluation of growth production factors as predictors of the incidence and severity of white striping and woody breast in broiler chickens. Poult. Sci. 2020;99:3723–3732. doi: 10.1016/j.psj.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnahhas N., Berri C., Chabault M., Chartrin P., Boulay M., Bourin M.C., Le Bihan-Duval E. Genetic parameters of white striping in relation to body weight, carcass composition, and meat quality traits in two broiler lines divergently selected for the ultimate pH of the pectoralis major muscle. BMC Genet. 2016;17:1–9. doi: 10.1186/s12863-016-0369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi G., Soglia F., Laghi L., Tappi S., Rocculi P., Tavaniello S., Prioriello D., Mucci R., Maiorano G., Petracci M. Comparison of quality traits among breast meat affected by current muscle abnormalities. Food Res. Int. 2019;115:369–376. doi: 10.1016/j.foodres.2018.11.020. [DOI] [PubMed] [Google Scholar]
- Baldi G., Soglia F., Mazzoni M., Sirri F., Canonico L., Babini E., Laghi L., Cavani C., Petracci M. Implications of white striping and spaghetti meat abnormalities on meat quality and histological features in broilers. Animal. 2018;12:164–173. doi: 10.1017/S1751731117001069. [DOI] [PubMed] [Google Scholar]
- Barbut S. Understanding the woody breast syndrome and other myopathies in modern broiler chickens. 31st Annual Australian Poultry Science Symposium; Sydney; 2020. [Google Scholar]
- Chen L.R., Suyemoto M.M., Sarsour A.H., Cordova H.A., Oviedo-Rondón E.O., Wineland M., Barnes H.J., Borst L.B. Temporal characterization of wooden breast myopathy (“woody breast”) severity and correlation with growth rate and lymphocytic phlebitis in three commercial broiler strains and a random-bred broiler strain. Avian Pathol. 2019;48:319–328. doi: 10.1080/03079457.2019.1598541. [DOI] [PubMed] [Google Scholar]
- de Carvalho L.M., Ventanas S., Olegario L.S., Madruga M.S., Estévez M. Consumers awareness of white-striping as a chicken breast myopathy affects their purchasing decision and emotional responses. LWT. 2020;131 [Google Scholar]
- Dohoo I.R., Martin W., Stryhn H. AVC Incorporated; Charlottetown, Canada: 2014. Veterinary Epidemiologic Research. [Google Scholar]
- Griffin J.R., Moraes L., Wick M., Lilburn M.S. Onset of white striping and progression into wooden breast as defined by myopathic changes underlying Pectoralis major growth. Estimation of growth parameters as predictors for stage of myopathy progression. Avian Pathol. 2018;47:2–13. doi: 10.1080/03079457.2017.1356908. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Brewer V.B., Apple J.K., Waldroup P.W., Owens C.M. Influence of growth rate on the occurrence of white striping in broiler breast fillets. Poult. Sci. 2012;91:2677–2685. doi: 10.3382/ps.2012-02259. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Brewer V.B., Mauromoustakos A., McKee S.R., Emmert J.L., Meullenet J.F., Owens C.M. Estimation of factors associated with the occurrence of white striping in broiler breast fillets. Poult. Sci. 2013;92:811–819. doi: 10.3382/ps.2012-02506. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Hargis B.M., Owens C.M. White striping and woody breast myopathies in the modern poultry industry: a review. Poult. Sci. 2016;95:2724–2733. doi: 10.3382/ps/pew216. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Lee Y.S., Erf G.F., Meullenet J.F.C., Mckee S.R., Owens C.M. Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping. Poult. Sci. 2012;91:1240–1247. doi: 10.3382/ps.2011-01947. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Owens C.M., Coon C., Hargis B.M., Vazquez-A Non M. Research note incidence of broiler breast myopathies at 2 different ages and its impact on selected raw meat quality parameters. Poult. Sci. 2017;96:3005–3009. doi: 10.3382/ps/pex072. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Shivaprasad H.l., Shaw D.P., Valentine B.A., Hargis B.M., Clark F.D., McKee S.R., Owens C.M. Pathological changes associated with white striping in broiler breast muscles. Poult. Sci. 2013;92:331–338. doi: 10.3382/ps.2012-02646. [DOI] [PubMed] [Google Scholar]
- Malila Y., U-Chupaj J., Srimarut Y., Chaiwiwattrakul P., Uengwetwanit T., Arayamethakorn S., Punyapornwithaya V., Sansamur C., Kirschke C.P., Huang L., Tepaamorndech S., Petracci M., Rungrassamee W., Visessanguan W. Monitoring of white striping and wooden breast cases and impacts on quality of breast meat collected from commercial broilers (Gallus gallus) Asian-Australas. J. Anim. Sci. 2018;31:1807–1817. doi: 10.5713/ajas.18.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudalal S., Lorenzi M., Soglia F., Cavani C., Petracci M. Implications of white striping and wooden breast abnormalities on quality traits of raw and marinated chicken meat. Animal. 2015;9:728–734. doi: 10.1017/S175173111400295X. [DOI] [PubMed] [Google Scholar]
- Papah M.B., Brannick E.M., Schmidt C.J., Abasht B. Evidence and role of phlebitis and lipid infiltration in the onset and pathogenesis of wooden breast disease in modern broiler chickens. Avian Pathol. 2017;46:623–643. doi: 10.1080/03079457.2017.1339346. [DOI] [PubMed] [Google Scholar]
- Pavlides M., Birks J., Fryer E., Delaney D., Sarania N., Banerjee R., Neubauer S., Barnes E., Fleming K.A., Wang L.M. Interobserver variability in histologic evaluation of liver fibrosis using categorical and quantitative scores. Am. J. Clin. Pathol. 2017;147:364–369. doi: 10.1093/ajcp/aqx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracci M., Cavani C. Muscle growth and poultry meat quality issues. Nutrients. 2012;4:1–12. doi: 10.3390/nu4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracci M., Mudalal S., Soglia F., Cavani C. Meat quality in fast-growing broiler chickens. Worlds Poult. Sci. J. 2015;71:363–374. [Google Scholar]
- Petracci M., Soglia F., Madruga M., Carvalho L., Ida E., Estévez M. Wooden-breast, white striping, and spaghetti meat: causes, consequences and consumer perception of emerging broiler meat abnormalities. Compr. Rev. Food Sci. Food Saf. 2019;18:565–583. doi: 10.1111/1541-4337.12431. [DOI] [PubMed] [Google Scholar]
- Radaelli G., Piccirillo A., Birolo M., Bertotto D., Gratta F., Ballarin C., Vascellari M., Xiccato G., Trocino A. Effect of age on the occurrence of muscle fiber degeneration associated with myopathies in broiler chickens submitted to feed restriction. Poult. Sci. 2017;96:309–319. doi: 10.3382/ps/pew270. [DOI] [PubMed] [Google Scholar]
- Santos M.N., Rothschild D., Widowski T.M., Barbut S., Kiarie E.G., Mandell I., Guerin M.T., Edwards A.M., Torrey S. In pursuit of a better broiler: carcass traits and muscle myopathies in conventional and slower-growing strains of broiler chickens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihvo H.K., Lindén J., Airas N., Immonen K., Valaja J., Puolanne E. Wooden breast myodegeneration of Pectoralis major muscle over the growth period in broilers. Vet. Pathol. 2017;54:119–128. doi: 10.1177/0300985816658099. [DOI] [PubMed] [Google Scholar]
- Tijare V.V., Yang F.L., Kuttappan V.A., Alvarado C.Z., Coon C.N., Owens C.M. Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies. Poult. Sci. 2016;95:2167–2173. doi: 10.3382/ps/pew129. [DOI] [PubMed] [Google Scholar]
- Velleman S.G., Clark D.L., Tonniges J.R. Fibrillar collagen organization associated with broiler wooden breast fibrotic myopathy. Avian Dis. 2017;61:481–490. doi: 10.1637/11738-080217-Reg.1. [DOI] [PubMed] [Google Scholar]
- Wold J.P., Løvland A. NIR spectroscopic techniques for quality and process control in the meat industry. Meat Muscle Biol. 2020;4:1–8. [Google Scholar]
- Wold J.P., Mage I., Løvland A., Sanden K.W., Ofstad R. Near-infrared spectroscopy detects woody breast syndrome in chicken fillets by the markers protein content and degree of water binding. Poult. Sci. 2019;98:480–490. doi: 10.3382/ps/pey351. [DOI] [PubMed] [Google Scholar]
- Wold J.P., Veiseth-Kent E., Høst V., Løvland A. Rapid on-line detection and grading of wooden breast myopathy in chicken fillets by near-infrared spectroscopy. PLoS One. 2017;12:1–16. doi: 10.1371/journal.pone.0173384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuidhof M.J., Schneider B.L., Carney V.L., Korver D.R., Robinson F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 20051. Poult. Sci. 2014;93:2970–2982. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.