Highlights
-
•
Prostate SBRT are treated using MR-guided adaptive IMRT (A-IMRT) and VMAT based on translation correction (3DOF-VMAT) at our institution.
-
•
Comparison of reference and delivered dose between adaptive-IMRT and 3DOF-VMAT to assess the effect of interfractional motion.
-
•
Despite large interfractional changes, prostate received clinically acceptable dose with a margin of 5 mm through either A-IMRT or 3DOF-VMAT.
-
•
A-IMRT was more superior than 3DOF-VMAT in sparing the rectum in the high dose region; no difference between the two systems was observed for bladder.
Keywords: MR-guided adaptive radiotherapy, IMRT, VMAT, Prostate SBRT
Abstract
Introduction
To compare the dosimetry of prostate stereotactic radiotherapy (SBRT) delivered by adaptive intensity modulated radiotherapy (A-IMRT) and 3 degree of freedom volumetric modulated arc therapy (3DOF-VMAT).
Methods & Materials
Twenty-five prostate patients treated with High Dose Rate (HDR) brachytherapy followed by SBRT were included (fifteen with hydrogel spacer in place for treatment). Interfraction changes in the volume of prostate, rectum and bladder were measured. Fractional dose to these structures was estimated for A-IMRT and 3DOF-VMAT for comparison against the corresponding reference dose and between each other.
Results
Clinically acceptable dose was delivered to prostate in all 125 fractions through A-IMRT and 3DOF-VMAT. A-IMRT was better than 3DOF-VMAT in reducing dose to 1 cm3 of rectum. Conversely, 3DOF-VMAT was superior in sparing 50% and 20% of rectum. When comparing the reference and delivered dose, there was no significant difference for Bladder D5cm3 for either technique. However, rectum in the high dose region benefited more from A-IMRT by being irradiated to a lower than reference dose in more fractions than 3DOF-VMAT. Hydrogel spacer reduced the rectal dose and was associated with a smaller deviation from reference dose for rectum D50% for A-IMRT.
Conclusions
Despite the presence of large interfraction organ volumes changes, clinically acceptable dose was delivered to the prostate by both systems. A-IMRT facilitated a greater rectal sparing from the high dose region than 3DOF-VMAT. Further reduction in rectal dose could be achieved by hydrogel spacer to displace the rectum, or by adaptation delivered by VMAT.
Introduction
There is increasing evidence to support the adoption of ultrahypofractionated schedules (≥4Gy/fraction) delivered with Stereotactic Body Radiation Therapy (SBRT) for the treatment of low/intermediate risk prostate cancer [1]. Geometric precision is paramount when a small margin is used to minimize irradiation of the surrounding organs at risk (OARs). The integration of Conebeam-CT on a linear accelerator for image guidance to correct for translational displacement has demonstrated efficacy in margin reduction without compromising tumor control probability [2]. To further improve precision, adapting the radiotherapy distribution based on information derived from images acquired during the course of radiotherapy was proposed [3]. In a study comparing the efficacy between translational correction and adaptation for prostate, the latter was shown to achieve a better therapeutic ratio [4].
The superior soft tissue contrast of Magnetic Resonance Imaging (MRI) makes visualization and delineation of the prostate easier and more consistent among different observers when compared to CT [5], [6]. In addition to reducing delineation uncertainty in the planning process, MR-integrated linear accelerator (MRL) is clinically available to deliver real-time adaptive treatment using step and shoot Intensity Modulated Radiation Therapy (IMRT) [7], [8].
Several studies have reported feasibility and early clinical outcome of delivering adaptive radiotherapy for prostate cancer on the MRL [9], [10], [11]. However, there are two major factors in the existing configuration of the MRL that could limit the potential of MR-guided adaptive radiotherapy: 1) Longer treatment session due to the need for recontouring, re-optimization and delivery using IMRT [12], [13], [14], [15] and 2) Inferior plan quality by IMRT when compared to Volumetric Modulated Arc Therapy (VMAT) [14], [16]. Although comparison of Adaptive-IMRT (A-IMRT) on the MRL and 3-degree-of-freedom image-guided VMAT (3DOF-VMAT) on a standard linear accelerator have been conducted for conventional or hypofractionated schedules [10], [15], [17], [18], it is uncertain if the finding could be generalized for ultrahypofractionation, when interfraction motion could have a greater effect on dose delivered over less fractions. The purpose of this study was to quantify changes in the target volumes and OARs between different sessions, and to assess their effect on dose delivered through A-IMRT and 3DOF-VMAT.
Materials and Methods
Twenty-five prostate patients recruited to a prospective trial evaluating the efficacy of High Dose Rate (HDR) brachytherapy to partial prostate and MR-guided stereotactic body radiotherapy (SBRT) treated between September 2019 and June 2021 were included. At the discretion of the treating physician, patients with intraprostatic lesion in proximity of the anterior rectal wall had Hydrogel Spacer (SpaceOAR system; Boston Scientific, Marlborough, MA, USA) inserted to increase rectal sparing. Approval has been granted by the institutional research ethics board.
A-IMRT clinical workflow
CT and 3.0 T MR images were acquired for both HDR brachytherapy and SBRT reference planning at the simulation session. Patients were instructed to have an empty rectum and a full bladder. One week post simulation, patients received a single 15 Gy fraction to the intraprostatic lesion by HDR brachytherapy. Details of the HDR brachytherapy has previously been reported [19].
MR-guided SBRT was commenced within 8 days after HDR brachytherapy as per the clinical study protocol and delivered on every other day.
For MR-guided SBRT planning, CT was registered to the 3D T2 weighted MR acquired on the Unity MR-Linac (Elekta Unity, Stockholm, Sweden). The CT image provides patient specific average densities for use in dose calculation. The following Volumes of Interest (VOIs) were delineated on the reference MRI: Clinical Target Volume (CTV) (defined as prostate +/- seminal vesicles), rectum (from the anal verge to the rectosigmoid junction), bladder, urethra, penile bulb, and femurs. A Planning Target Volume (PTV) was generated by applying a 5 mm uniform expansion to the CTV. The average electron density of the PTV, femurs and the entire image set was derived from the CT and assigned onto the corresponding volumes on the MR. A reference plan was generated in the Monaco treatment planning system (v5.4, Elekta AB, Stockholm, Sweden) using 9 fields (Gantry angles: 215, 265, 295, 325, 0, 35, 65, 95 and 145), with a maximum of 70 segments and a minimum segment size of 4 cm2, to deliver 30 Gy in 5 fractions to the dose specification point. The Monaco Monte Carlo dose computation engine was used with dose grid size of 0.3 cm3. Planning goals per fraction are as follow: CTV D95% ≥ 660 cGy; PTV D95% ≥ 600 cGy; rectum D50% ≤ 200 cGy, D20% ≤ 400 cGy, D1cm3 ≤ 600 cGy; bladder D5cm3 ≤ 600 cGy
At each treatment session, the beam configuration and the optimization objectives used to generate the reference plan was used as a starting point to re-optimize an adapted plan based on the VOIs redefined on the 2-minutes T2-weighted localization MR (MRLoc). Quality controls check on the adapted plan, along with the acquisition of a second MR to verify the position of the prostate were completed prior to beam delivery. An additional T2-weighted MR was collected during beam delivery (MRBeamON) to assess any motion or anatomical changes exhibited during the session.
Estimation of delivered dose through A-IMRT
The MRLoc, MRBeamON and the dose distributions of the A-IMRT adapted plans of each treatment fraction were exported from Monaco into RayStation (v8, RaySearch, Stockholm, Sweden) for analysis. The CTV, rectum and bladder were contoured on all MRBeamON to provide geometric information of these volumes at the time of beam delivery. Dose cubes of the A-IMRT adapted plans of all treated fractions were then transferred onto MRBeamON for estimation of delivered dose to these VOIs.
3DOF-VMAT plan generation and delivered dose estimation
For comparison with the A-IMRT approach, a single arc VMAT distribution using a collimator angle of 15 degrees and 180 segments was generated in RayStation using the TrueBeam 6MV flattening-filter-free beam model (Varian Medical Systems, Palo Alto, CA). Dose was calculated using collapsed cone convolution superposition algorithm, with a calculation grid size of 0.25 cm3. The same planning goals were used for optimization and evaluation.
Delivered dose through 3DOF-VMAT was estimated by first registering the five MRBeamON of each patient to the corresponding reference image using the CTV to simulate the clinical workflow for 3DOF correction. Afterwards, the same procedure of transferring dose cubes of the 3DOF-VMAT reference plan onto the MRBeamON was repeated to retrieve the delivered dose.
Data collection & statistical analysis
Changes in the volume of the prostate, rectum and bladder in between and during the treatment sessions was measured. Reference plan dose and delivered dose to 95% of CTV, 50%, 20% and 1 cm3 of rectum, and 5 cm3 of bladder were retrieved from the A-IMRT and 3DOF-VMAT dose on the MRBeamON. Delivered dose to the OARs was considered clinically acceptable when it met the corresponding clinical goal. For the CTV, delivered dose was checked against the PTV goal for acceptability. Major violation was defined as a > 10% deviation from clinical goals. Due to the presence of hydrogel spacer in selective patients, dose to rectum was analyzed and compared between the two groups. Differences between dose parameters of the A-IMRT and the 3DOF-VMAT were assessed using paired two tail t-test (p < 0.05).
Results
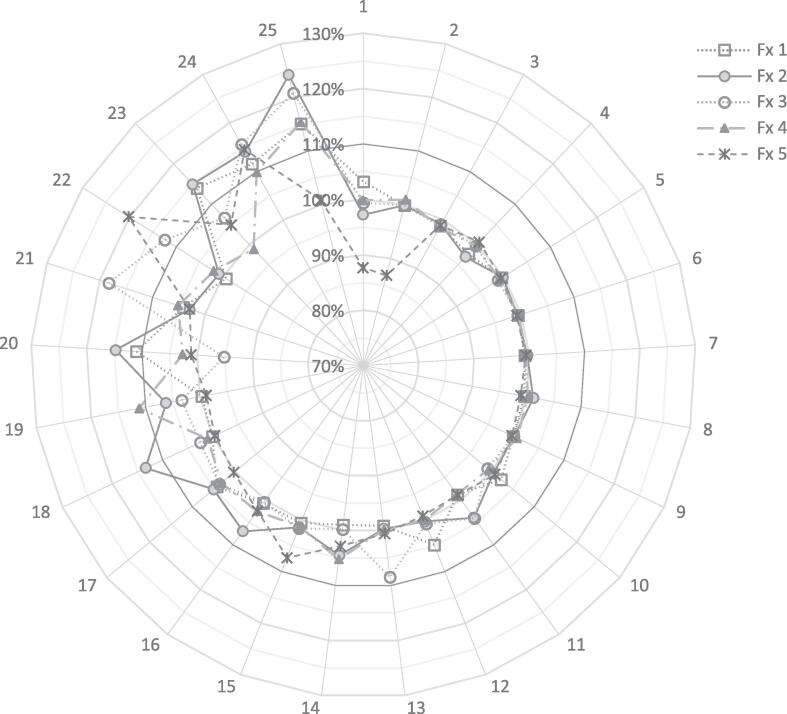
A total of 125 fractions from 25 patients was available for analysis. Fig. 1 displays the prostate treatment volume relative to planning for each fraction. Twenty-three patients received SBRT with a median of 4 days after HDR brachytherapy. Patients 13 and 22 received SBRT first, followed by HDR within 5 days. Overall, there was a mean increase of 4% (SD: 5%) in prostate volume during treatment when compared to planning. There were 17 fractions (13.6%) in which a > 10% increase in prostate volume from planning was observed (Fig. 1). Two patients (Patient 24 and 25) had an increase of > 10% for 4 or more treatment fractions.
Fig. 1.
Relative prostate volume of each fraction compared to the prostate at the time of planning. Prostate change was < 10% for patient 1–17 for all 5 fractions.
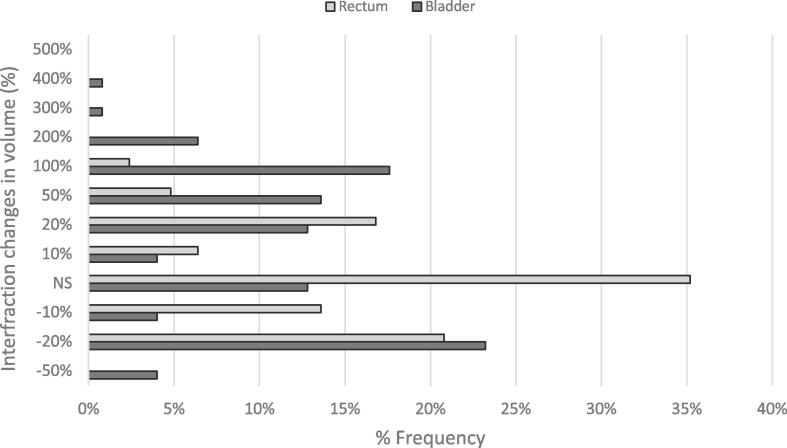
The volumes of OARs exhibited significant variation from fraction to fraction, with 89% and 63% of fractions exhibiting a ± 10% difference from the reference volumes for bladder and rectum, respectively (Fig. 2).
Fig. 2.
Frequency and magnitude of changes of bladder and rectum volume relative to planning. NS: Non-Significant (≤±10%).
Comparison of reference planned dose between A-IMRT and 3DOF-VMAT
Among the 25 patients, 15 patients were treated with the hydrogel spacer in place. The rectal dose to 20% and 1 cm3 was significantly lower than those who did not have one (p ≤ 0.02). Mean dose was reduced from 346 cGy to 218 cGy to 20% and from 603 cGy to 455 cGy to 1 cm3 of rectum through A-IMRT, and from 327 cGy to 173 cGy and 598 cGy to 418 cGy through 3DOF-VMAT (Table 1).
Table 1.
Mean and range of reference and delivered dose (cGy) per fraction to VOIs by A-IMRT and 3DOF-VMAT. *denotes significance difference.
| Volumes of Interest | Metrics | Mean (Range) Dose/Fraction (cGy) |
|||||
|---|---|---|---|---|---|---|---|
| Reference |
Delivered |
||||||
| A-IMRT | 3DOF-VMAT | p | A-IMRT | 3DOF-VMAT | p | ||
| CTV | D95% | 661 (658 – 674) | 661 (660 – 665) | 0.173 | 657 (603 – 672) | 656 (621 – 666) | 0.250 |
| Rectum | D50% | 143 (85 – 215) | 68 (28 – 152) | 0.003* | 147 (77 – 263) | 77 (28 – 142) | <0.001* |
| Rectum (with SpaceOAR) | 120 (66 – 233) | 62 (32 – 137) | <0.001* | 125 (43 – 310) | 56 (32 – 186) | <0.001* | |
| Rectum | D20% | 346 (238 – 426) | 327 (70 – 431) | 0.112 | 306 (173 – 510) | 295 (58 – 424) | 0.053 |
| Rectum (with SpaceOAR) | 218 (159 – 426) | 173 (53 – 340) | 0.133 | 194 (120 – 406) | 159 (40 – 421) | <0.001* | |
| Rectum | D1cm3 | 603 (543 – 625) | 598 (590 – 616) | 0.896 | 573 (372 – 678) | 593 (59 – 661) | 0.062 |
| Rectum (with SpaceOAR) | 455 (259 – 594) | 418 (136 – 593) | 0.508 | 345 (206 – 360) | 443 (103 – 611) | <0.001* | |
| Bladder | D5cm3 | 601 (524 – 661) | 590 (469 – 621) | <0.001* | 600 (322 – 673) | 574 (276 – 641) | <0.001* |
The reference dose to the evaluated metrics were comparable between A-IMRT and 3DOF-VMAT, except for rectum D50% (p < 0.003) and bladder D5cm3 (p = 0.0003). For plan acceptability, reference 3DOF-VMAT achieved all planning goals for all patients, and a single major violation in one patient by A-IMRT (Rectum D50% = 233 cGy)
Comparison of delivered dose between A-IMRT and 3DOF-VMAT
Clinically acceptable dose (D95% ≥ 600 cGy) was delivered to CTV in all fractions through A-IMRT and 3DOF-VMAT (Table 1). Delivered A-IMRT did not meet at least one or more rectal dose constraints in 10 fractions and bladder D5cm3 in 6 fractions. Delivered 3DOF-VMAT met all dose constraints except for one fraction (Rectum D1cm3 = 661 cGy). Major violations in rectal D20% and D1cm3 were only observed in patients without the hydrogel spacer.
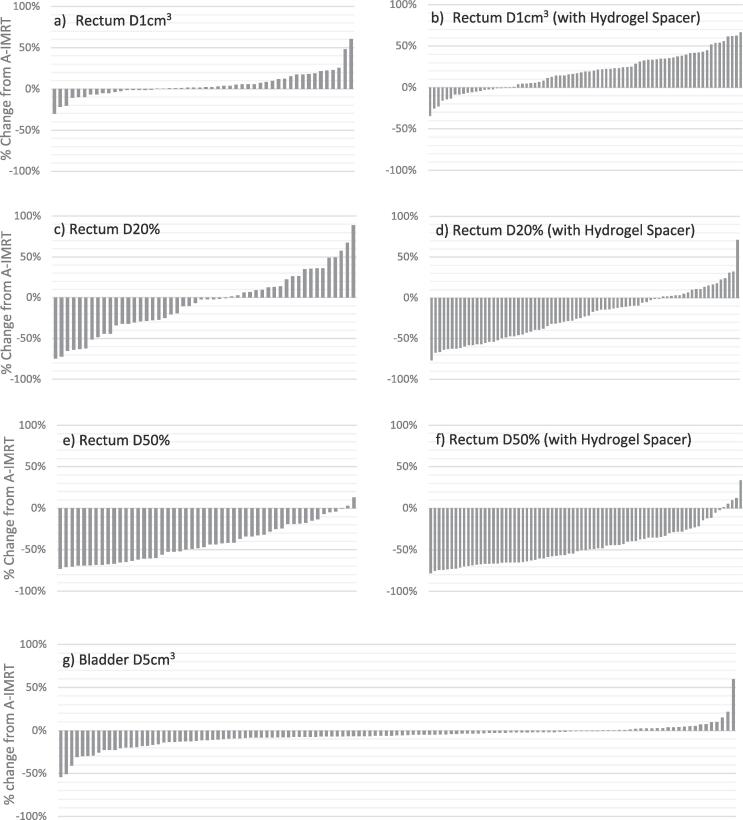
Fig. 3 shows the percent difference between doses delivered through 3DOF-VMAT relative to A-IMRT over 125 fractions for individual OARs dose constraints. 3DOF-VMAT delivered a higher dose to 1 cm3 of rectum than A-IMRT in 66% of fractions, and the increase occurred more frequently among those with hydrogel spacer (Fig. 3a & b). Therefore, the difference in the overall mean rectum D1cm3 only reaches significance in the hydrogel spacer group (3DOF-VMAT vs IMRT: 483 cGy vs 345 cGy, p < 0.05) but not in the other group (593 cGy vs. 554 cGy, p = 0.06). Conversely, 3DOF-VMAT was superior to A-IMRT in sparing 20% and 50% of rectum and 5 cm3 of bladder, and the differences reached statistical significance except for rectum D20% in the absence of hydrogel spacer (p = 0.053) (Table 1 & Fig. 3c-g).
Fig. 3.
Waterfall plot displaying the % change in delivered dose by 3DOF-VMAT from A-IMRT of individual fractions for Rectum D1cm3, D20% and D50% without hydrogel spacer (a, c, e) and with hydrogel spacer (b, d, and f) and Bladder D5cm3 (g).
Comparison of differences between delivered vs reference dose for A-IMRT and 3DOF-VMAT
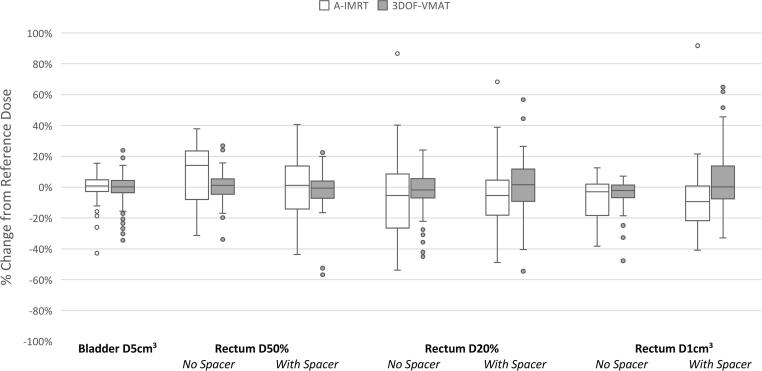
Fig. 4 provides box plots of the % change from reference dose for the rectum and bladder dose constraints. Despite a large range of bladder variation between planning and treatment (Fig. 2), dose to 5 cm3 of bladder for both A-IMRT or 3DOF-VMAT did not differ significantly from the reference dose, with a median deviation of 1% (95% CI: −1%, 2%) and 0% (95% CI: −2%, 2%), respectively.
Fig. 4.
Boxplot comparing the median and the range of change from reference dose of individual OAR dose constraints for A-IMRT and 3DOFVMAT.
A-IMRT facilitated a reduction in dose delivered to 20% and 1 cm3 of rectum compared to the reference plan. The reduction was greater in the presence of hydrogel spacer for D1cm3. The median difference from reference dose with and without hydrogel spacer were: −5% (95% CI: −10%, −1%) and −5% (95% CI: −12%, 1%) for D20%; −9% (95% CI: −13%, −6%) and −3 (95% CI: −7%, 1%) for D1cm3. Meanwhile rectal D50% was increased from reference by a median of 14% (95% CI: 11%, 17%) for patients without a hydrogel spacer, but was significantly reduced to 1% (95% CI: −2%, 4%) for patients with one (p = 0.009).
Compared to A-IMRT, 3DOF-VMAT was less effective in delivering a lower than reference dose to the rectum in the high dose region. Rectum D20% and 1 cm3 received a lower than reference dose in 50% and 46% of fractions through 3DOF-VMAT, compared to 59% and 66% through A-IMRT. The variability of changes from reference dose was noted to be larger among patients with hydrogel spacer than those without. The interquartile range of the difference from reference dose with and without hydrogel spacer were 21% vs. 12% for D20%, and 20% vs. 8% for D1cm3, respectively.
Discussion
Using data from 25 prostate patients treated with MR-guided SBRT after HDR brachytherapy to the intraprostatic lesion, this study measured the volumes changes of prostate, bladder and rectum between treatment fractions, and compared its effect on dosimetry between A-IMRT and 3DOF-VMAT.
Two of 25 patients had a > 10% increase in prostate volume from the time of planning for 4 or more treatment fractions (Fig. 1). This could be attributed to the changes induced by edema after HDR brachytherapy [20] and persistent swelling from the external beam radiotherapy. A transient increase of > 10% in prostate volume during a course of ultrahypofractionated radiotherapy has been reported and suggests the need to account this in the PTV margin [21]. In this cohort, despite a maximum 24% prostate enlargement, the prostate received a clinically acceptable dose delivered through 3DOF-VMAT with a 5 mm PTV margin. Further margin reduction could affect the clinically acceptable delivered 3DOF-VMAT dose and volume changes would have to be monitored closely during treatment. In contrast, A-IMRT adapted plans accommodate both prostate volume and interfraction position changes, and as such, would facilitate margin reduction.
3DOF-VMAT achieved a significantly lower rectum D50% than A-IMRT, which agrees with findings from two other studies that compared 3DOF-VMAT and A-IMRT (7–9 beams) [10], [18]. VMAT has the potential to generate plans with increased conformality compared with IMRT due to the efficient delivery of dose with variable dose rate from full range of gantry angles [13], [14], [16]. In a comparison study using IMRT only, differences between 3DOF and adaptation in the rectum being spared from lower dose was minimal [4], suggesting a more dominant role of VMAT over adaptation in OAR sparing in the current work. IMRT plans with 17 beams have been shown to produce a significant reduction in rectal volume receiving roughly 65% of the prescription as compared to dual-arc VMAT [15]. While increasing number of beams to resemble a VMAT distribution can be a contributing factor, the superiority of IMRT over VMAT in that study could also be attributed to the difference in MLC travelling direction and narrower leaf width (4 mm vs 7 mm for this study) [8], [23]. The latter has been shown to have a significant effect in the low dose region and on dose homogeneity [24]. This hypothesis could be tested by a comparison on plan quality between the different MRL system configurations.
With 63% of rectum exhibiting > 10% change in volume from the time of planning, the use of adaptation is better than 3DOF in delivering a lower than reference dose to 20% and 1 cm3 of rectum. Despite the use of image guidance, large variation in delivered dose to rectum due to interfractional changes have been reported [25], [26]. Plan adaptation based on re-contoured rectum and other VOIs facilitates maximization of the therapeutic ratio for each fraction [27]. This is particularly effective when the anatomy at the time of planning is unfavorable (e.g., close proximity of OARs to CTV), which could result in generating a reference plan that fail to meet all clinical goals. Dunlop et al. reported that online adaptation was able to deliver a higher dose to the CTV when compared to 3DOF due to the displacement of the bowel further away from the CTV at time of treatment [10].
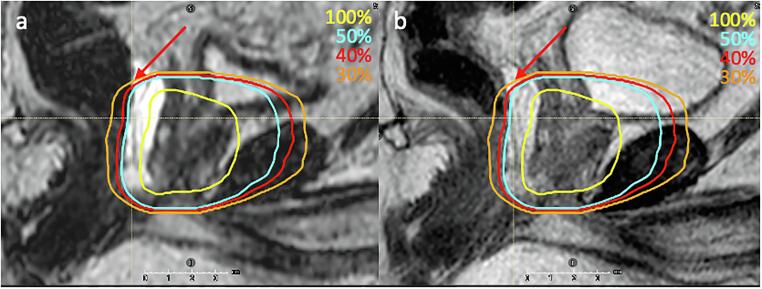
While sparing of rectum in the high dose region was improved by adaptation, upon further investigation, the reduction in some fractions was also found to be caused by intrafraction rectal motion exhibited during the online adaptive sessions (on average 25–30 min long between acquisition of the MRLoc and MRBeamON. Fig. 5 shows one of the fractions in which the rectum at the time of beam delivery was displaced posteriorly from the time of adaptive planning, moving towards the dose fall-off direction and hence received a lower delivered dose. Displacement in the opposite direction was also observed, resulting in major violations in a few fractions. Reducing the session time through contour autosegmentation, using a faster delivery system such as VMAT, or better motion management and monitoring strategy could potentially improve alignment between planned and delivered dose [1], [28], [29].
Fig. 5.
Sagittal view showing a) MRLoc and b) MRBeamON with 100%, 50%, 40% and 30% isodose lines. Rectum was displaced posteriorly towards the lower isodose region at the time of beam delivery, most significant in the superior aspect where the red arrow is pointing. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In addition to reducing rectal dose [30], [31], the presence of a hydrogel spacer minimized the variance between reference and delivered dose to 50% of rectum for A-IMRT plans. While inter- and intrafraction changes in rectum location and shape may play a role, the magnitude of these change was found not to be significantly different between the presence or absence of hydrogel spacer in this and other studies [30], [32]. Instead, the presence of the hydrogel spacer allows the rectum to be located farther from the high dose and dose-gradient region, making rectal metrics less sensitive to rectal volume and position changes. The competing bladder constraint could also cause the optimizer to distribute dose differently from the reference plan during online adaptation, affecting the rectal dose more among those without a hydrogel spacer.
Limiting the volume of bladder receiving high dose is of interest due to data showing its relationship with acute and late urinary toxicity [33]. As absolute volume parameters has been shown to be representative for the actual treatment [34], bladder D5cm3 was selected for reporting and comparison in this study. Unlike rectum, difference between reference and delivered to the high dose region of the bladder was minimal despite a large variation in bladder volume and the use of adaptation. This implies that the bladder trigone is relatively stable and that performing 3DOF correction is adequate in delivering the intended planned dose near the prostate. Further dose reduction to potentially decrease urinary toxicity could be facilitated by margin reduction and the use of full bladder to spare more normal bladder tissue from irradiation [35].
We acknowledge that the calculated delivered dose by 3DOF-VMAT may have been under/overestimated by registering the MRBeamON and the reference image, with the assumption of zero motion between image acquisition and beam delivery. Previous work has reported this period to be around 5 min in a real clinical workflow [36] and intrafraction of the prostate could be up to 2 mm at this timepoint, and increases further as the period lengthens [29].
Conclusions
Despite the presence of notable interfraction motion, A-IMRT and 3DOF-VMAT could deliver clinically acceptable dose to CTV. While there was no difference in the delivered dose to the bladder neck between online adaptation and 3DOF correction, the former facilitated a greater rectal sparing from the high dose region. Further reduction in rectal dose could be achieved by hydrogel spacer to displace the rectum away from the prostate, or by adaptation delivered by VMAT to create a steeper dose gradient. The clinical efficacy of MR-guided adaptation needs to be further evaluated by comparing patients’ outcome and toxicity against the current standard of practice.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Wolf F., Sedlmayer F., Aebersold D., Albrecht C., Böhmer D., Flentje M., et al. Ultrahypofractionation of localized prostate cancer. Strahlenther Onkol. 2021;197(2):89–96. doi: 10.1007/s00066-020-01723-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maund I.F., et al. Image-guided radiotherapy of the prostate using daily CBCT: the feasibility and likely benefit of implementing a margin reduction. The British Journal of Radiology. 2014;87(1044):20140459. doi: 10.1259/bjr.20140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sonke J.-J., Aznar M., Rasch C. Adaptive Radiotherapy for Anatomical Changes. Seminars in Radiation Oncology. 2019;29(3):245–257. doi: 10.1016/j.semradonc.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Qin A.n., Sun Y., Liang J., Yan D.i. Evaluation of Online/Offline Image Guidance/Adaptation Approaches for Prostate Cancer Radiation Therapy. International Journal of Radiation Oncology*Biology*Physics. 2015;91(5):1026–1033. doi: 10.1016/j.ijrobp.2014.12.043. [DOI] [PubMed] [Google Scholar]
- 5.Villeirs G.M., Van Vaerenbergh K., Vakaet L., Bral S., Claus F., De Neve W.J., et al. Interobserver delineation variation using CT versus combined CT+ MRI in intensity–modulated radiotherapy for prostate cancerQuantifizierung der Interobserver–Variation im CT im Vergleich zur Kombination CT und MRT bei intensitätsmodulierter Strahlentherapie. Strahlenther Onkol. 2005;181(7):424–430. doi: 10.1007/s00066-005-1383-x. [DOI] [PubMed] [Google Scholar]
- 6.Murray J., Tree A.C. Prostate cancer – Advantages and disadvantages of MR-guided RT. Clinical and Translational Radiation Oncology. 2019;18:68–73. doi: 10.1016/j.ctro.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winkel D., Bol G.H., Kroon P.S., van Asselen B., Hackett S.S., Werensteijn-Honingh A.M., et al. Adaptive radiotherapy: The Elekta Unity MR-linac concept. Clinical and Translational Radiation Oncology. 2019;18:54–59. doi: 10.1016/j.ctro.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klüter S. Technical design and concept of a 0.35 T MR-Linac. Clinical and Translational. Radiation Oncology. 2019;18:98–101. doi: 10.1016/j.ctro.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuccia F., Corradini S., Mazzola R., Spiazzi L., Rigo M., Bonù M.L., et al. MR-Guided Hypofractionated Radiotherapy: Current Emerging Data and Promising Perspectives for Localized Prostate Cancer. Cancers. 2021;13(8):1791. doi: 10.3390/cancers13081791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunlop A., Mitchell A., Tree A., Barnes H., Bower L., Chick J., et al. Daily adaptive radiotherapy for patients with prostate cancer using a high field MR-linac: Initial clinical experiences and assessment of delivered doses compared to a C-arm linac. Clinical and translational radiation oncology. 2020;23:35–42. doi: 10.1016/j.ctro.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J., Vedam S., Lee B., Castillo P., Sobremonte A., Hughes N., et al. Online adaptive planning for prostate stereotactic body radiotherapy using a 1.5 Tesla magnetic resonance imaging-guided linear accelerator. Physics and Imaging. Radiation Oncology. 2021;17:20–24. doi: 10.1016/j.phro.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corradini S., Alongi F., Andratschke N., Azria D., Bohoudi O., Boldrini L., et al. ESTRO-ACROP recommendations on the clinical implementation of hybrid MR-linac systems in radiation oncology. Radiother Oncol. 2021;159:146–154. doi: 10.1016/j.radonc.2021.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008;35(1):310–317. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 14.Quan E.M., Li X., Li Y., Wang X., Kudchadker R.J., Johnson J.L., et al. A Comprehensive Comparison of IMRT and VMAT Plan Quality for Prostate Cancer Treatment. International Journal of Radiation Oncology*Biology*Physics. 2012;83(4):1169–1178. doi: 10.1016/j.ijrobp.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendes V.D.S., et al. Dosimetric comparison of MR-linac-based IMRT and conventional VMAT treatment plans for prostate cancer. Radiation Oncology. 2021;16(1):1–12. doi: 10.1186/s13014-021-01858-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palma D., Vollans E., James K., Nakano S., Moiseenko V., Shaffer R., et al. Volumetric Modulated Arc Therapy for Delivery of Prostate Radiotherapy: Comparison With Intensity-Modulated Radiotherapy and Three-Dimensional Conformal Radiotherapy. International Journal of Radiation Oncology*Biology*Physics. 2008;72(4):996–1001. doi: 10.1016/j.ijrobp.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 17.Christiansen R.L., Hansen C.R., Dahlrot R.H., Bertelsen A.S., Hansen O., Brink C., et al. Plan quality for high-risk prostate cancer treated with high field magnetic resonance imaging guided radiotherapy. Physics and Imaging in Radiation Oncology. 2018;7:1–8. doi: 10.1016/j.phro.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Schoot A.J.A.J., van den Wollenberg W., Carbaat C., de Ruiter P., Nowee M.E., Pos F., et al. Evaluation of plan quality in radiotherapy planning with an MR-linac. Physics and Imaging in Radiation Oncology. 2019;10:19–24. doi: 10.1016/j.phro.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murgic J., Chung P., Berlin A., Bayley A., Warde P., Catton C., et al. Lessons learned using an MRI-only workflow during high-dose-rate brachytherapy for prostate cancer. Brachytherapy. 2016;15(2):147–155. doi: 10.1016/j.brachy.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Rink A., Borg J., Simeonov A., O'Leary G., Helou J., Ménard C., et al. Dosimetric impact of intrafraction changes in MR-guided high-dose-rate (HDR) brachytherapy for prostate cancer. Brachytherapy. 2018;17(1):59–67. doi: 10.1016/j.brachy.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Gunnlaugsson A., Kjellén E., Hagberg O., Thellenberg-Karlsson C., Widmark A., Nilsson P. Change in prostate volume during extreme hypo-fractionation analysed with MRI. Radiation Oncology. 2014;9(1) doi: 10.1186/1748-717X-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raaymakers B.W., Jürgenliemk-Schulz I.M., Bol G.H., Glitzner M., Kotte A.N.T.J., van Asselen B., et al. First patients treated with a 1.5 T MRI-Linac: clinical proof of concept of a high-precision, high-field MRI guided radiotherapy treatment. Phys Med Biol. 2017;62(23):L41–L50. doi: 10.1088/1361-6560/aa9517. [DOI] [PubMed] [Google Scholar]
- 24.Wang L., Movsas B., Jacob R., Fourkal E., Chen L., Price R., et al. Stereotactic IMRT for prostate cancer: Dosimetric impact of multileaf collimator leaf width in the treatment of prostate cancer with IMRT. Journal of Applied Clinical Medical Physics. 2004;5(2):29–41. doi: 10.1120/jacmp.v5i2.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wahl M., Descovich M., Shugard E., Pinnaduwage D., Sudhyadhom A., Chang A., et al. Interfraction anatomical variability can lead to significantly increased rectal dose for patients undergoing stereotactic body radiotherapy for prostate cancer. Technol Cancer Res Treat. 2017;16(2):178–187. doi: 10.1177/1533034616649495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devlin L., et al. Dosimetric impact of organ at risk daily variation during prostate stereotactic ablative radiotherapy. The British journal of radiology. 2020;93(1108):20190789. doi: 10.1259/bjr.20190789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Timmeren J.E., Chamberlain M., Krayenbuehl J., Wilke L., Ehrbar S., Bogowicz M., et al. Treatment plan quality during online adaptive re-planning. Radiation Oncology. 2020;15(1) doi: 10.1186/s13014-020-01641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pathmanathan A.U., et al. Magnetic resonance imaging-guided adaptive radiation therapy: a “game changer” for prostate treatment? International Journal of Radiation Oncology* Biology*. Physics. 2018;100(2):361–373. doi: 10.1016/j.ijrobp.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 29.Shelton J., Rossi P.J., Chen H., Liu Y., Master V.A., Jani A.B. Observations on prostate intrafraction motion and the effect of reduced treatment time using volumetric modulated arc therapy. Practical radiation oncology. 2011;1(4):243–250. doi: 10.1016/j.prro.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Farjam R., et al. Quantifying the impact of SpaceOAR hydrogel on inter-fractional rectal and bladder dose during 0.35 T MR-guided prostate adaptive radiotherapy. Journal of applied clinical medical physics. 2021;22(9):49–58. doi: 10.1002/acm2.13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alongi F., et al. Rectal spacer hydrogel in 1.5 T MR-guided and daily adapted SBRT for prostate cancer: dosimetric analysis and preliminary patient-reported outcomes. The British Journal of Radiology. 2021;94(1117):20200848. doi: 10.1259/bjr.20200848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juneja P., Kneebone A., Booth J.T., Thwaites D.I., Kaur R., Colvill E., et al. Prostate motion during radiotherapy of prostate cancer patients with and without application of a hydrogel spacer: a comparative study. Radiation Oncology. 2015;10(1) doi: 10.1186/s13014-015-0526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rancati T., Palorini F., Cozzarini C., Fiorino C., Valdagni R. Understanding Urinary Toxicity after Radiotherapy for Prostate Cancer: First Steps Forward. Tumori Journal. 2017;103(5):395–404. doi: 10.5301/tj.5000681. [DOI] [PubMed] [Google Scholar]
- 34.Hoogeman M.S., Peeters S.T.H., Bois J.d., Lebesque J.V. Absolute and relative dose–surface and dose–volume histograms of the bladder: which one is the most representative for the actual treatment? Phys Med Biol. 2005;50(15):3589–3597. doi: 10.1088/0031-9155/50/15/007. [DOI] [PubMed] [Google Scholar]
- 35.Grün A., Kawgan-Kagan M., Kaul D., Badakhshi H., Stromberger C., Budach V., et al. Impact of bladder volume on acute genitourinary toxicity in intensity modulated radiotherapy for localized and locally advanced prostate cancerEinfluss des Harnblasenvolumens auf uro-genitale akut-Nebenwirkungen unter hochdosierter intensitätsmodulierter Strahlentherapie (IMRT) bei lokal begrenztem und fortgeschrittenem Prostatakarzinom. Strahlenther Onkol. 2019;195(6):517–525. doi: 10.1007/s00066-018-1398-8. [DOI] [PubMed] [Google Scholar]
- 36.Li W., Jaffray D.A., Wilson G., Moseley D. How long does it take? An analysis of volumetric image assessment time. Radiother Oncol. 2016;119(1):150–153. doi: 10.1016/j.radonc.2016.01.015. [DOI] [PubMed] [Google Scholar]