Highlights
-
•
Ultrasound increased the secondary structure stability of SPI-HF.
-
•
Ultrasound decreased the zeta potential, particle size, and turbidity of complexes.
-
•
Ultrasound treatment enhanced the emulsifying properties of SPI-HF.
Keywords: Ultrasound, Soy protein isolate, Hawthorn flavonoids, Physicochemical property, Emulsifying property, Molecular docking
Abstract
In recent years, more and more attention had been paid to the combination of proteins and flavonoids, and several flavonoids had been reported to improve the physicochemical and emulsifying properties of proteins. This study investigated the effects of ultrasonic treatment (450 W for 10 min, 20 min, and 30 min) on the physicochemical properties, antioxidant activity, and emulsifying properties of soy protein isolate (SPI) -hawthorn flavonoids (HF) non-covalent complexes. The results showed that the addition of HF to SPI and 20 min of ultrasound could reduce α-helix and random coil, increase β-sheet and β-turn, and enhance fluorescence quenching. In addition, it decreased the particle size, zeta potential, surface hydrophobicity, and turbidity to 88.43 or 95.27 nm, −28.80 mV, 1250.42, and 0.23, respectively. The protein solubility, free sulfhydryl group, antioxidant activity, emulsifying activity index, and emulsifying stability index all increased to 73.93%, 15.07 μmol/g, 71.00 or 41.91%, 9.81 m2/g, and 67.71%, respectively. Moreover, high-density small and low-flocculation droplets were formed. Therefore, the combined ultrasound treatment and addition of HF to SPI is a more effective method for protein modification compared to ultrasound treatment alone. It provides a theoretical basis for protein processing and application in the future.
1. Introduction
Soy protein isolate (SPI) is a complete protein with a protein content of over 90%, and it is obtained from defatted soybean meal by removing non-protein components. SPI is widely used as a food additive in food processing because of its functional characteristics, high nutritional value, and low cost [1], [2]. However, SPI can be partially denatured during the extraction process, reducing its physicochemical properties and emulsifying properties. Therefore, it is necessary to develop modification processes that avoid denaturing SPI [3]. Non-covalent interaction primarily occurs at the hydrogen acceptor on proteins and the phenolic hydroxyl group on polyphenols, forming hydrogen bonds. Some amino acid groups or residues on protein can also be combined with phenolic hydroxyl groups or benzene rings to produce ionic bonds, hydrophobicity, van der Waals force, etc. [4]. Recent studies have investigated the complexation of proteins with flavonoids in order to change the protein’s functional properties. Chen et al. [5] found that tea polyphenols and SPI could improve the solubility of protein through non-covalent interaction. Jiang et al. [6] found that when whey protein and chlorogenic acid formed a complex, the solubility and foaming performance of whey protein were significantly improved. In addition, the complex could synergistically scavenge free radicals and improve oxidation stability.
Hawthorn flavonoids (HF) are functional components extracted from hawthorn leaves that promote digestion, reduce blood stasis, regulate blood lipids, and resist cancer [7], [8]. They primarily contain vitexin-2-O-rhamnoside, glucosylvitexin, apigenin8-C-glucoside, and hyperoside [9]. A study by Perez et al. [10] found that hawthorn could improve the physicochemical, sensory, and rheological properties of yogurt. Niu et al. [11] showed that hawthorn and wolfberry compounds had antibacterial effects on Staphylococcus aureus and Klebsiella pneumoniae. Although hawthorn leaves are often discarded as waste because they are inedible, they contain a high quantity of beneficial flavonoids. HF rarely binds to proteins, so the combination of HF with proteins is a promising area for future research.
Ultrasound is a very effective method for modifying the functional characteristics of proteins [12]. Its techniques can destroy the intramolecular protein bonds and change the molecular structure of foods through cavitation, mechanical, and thermal effects [13]. In a study by Qin et al. [14], an SPI-wheat gluten complex was treated by ultrasound, and the molecular particle size was reduced. Ma et al. [15] studied between SPI and citrus pectin after ultrasonic treatment and found that the emulsion droplets were smaller and more uniform, thereby improving its emulsifying properties. Tong et al. [2] showed that the structure and antioxidant activity of the soybean protein improved after ultrasound. Ma et al. [16] observed that under ultrasonic conditions of 270 W, 360 W, 450 W, 540 W, and 630 W, the grafting degree of SPI to apple pectin was the highest at 450 W. The solubility and antioxidant capacity of SPI hydrolysate could be effectively improved after 450 W ultrasonic treatment [17]. Sui et al. also reported that under the condition of 450 W ultrasound, SPI and lecithin composite emulsion were more stable after ultrasound [18]. The above research showed that the structure and function of SPI have been improved well under the condition of ultrasonic treatment with 450 W, so this experiment chose SPI-HF non-covalent complexes to experiment under the condition of 450 W.
Based on this evidence, the current study investigated the effects of various durations of ultrasonic treatment on the physicochemical properties, antioxidant activity, and emulsifying properties of SPI-HF non-covalent complexes. The objective of the experiment was to understand the interaction between the protein and HF under an ultrasonic field and provide data for the application of HF in the food and pharmaceutical industries.
2. Materials and methods
2.1. Materials
Defatted soybean flakes were provided by Qinhuangdao Yihai Kerry Cereals, Oils and Foods Co., Ltd. (Qinhuangdao, China). HFs were provided by Linyi Aikang Pharmaceutical Co., Ltd. (Linyi, China). Corn oil (Xiwang, Binzhou, China) was obtained from Wal-Mart (Hangzhou, China). A bicinchoninic acid (BCA) protein concentration determination kit was purchased from Shanghai Beyotime Biotechnology Co., Ltd. (Shanghai, China). Phosphate buffered saline (PBS), 8-anilino-1-naphthalenesulfonic acid (ANS), Tris, glycine, ethylene diamine tetraacetic acid (EDTA), 5,5′-dithiobis-2-nitrobenzoic acid (DTNB), 1,1-diphenyl-2-picrylhydrazyl (DPPH), potassium persulfate, 2, 2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic (ABTS), sodium dodecyl sulfate (SDS), and Nile blue were purchased from Sigma-Aldrich Chemical Co. (MO, USA). All other chemicals and solvents were analytical-grade.
2.2. Preparation of SPI
The SPI was performed based on the slightly adjusted procedure of Tong et al. [2]. The defatted soybean flakes were ground into a powder and then dispersed in deionized water at a ratio of 1:10 (w/v). The solution was adjusted to a pH of 8.0 with 2 M NaOH, stirred at room temperature for 1 h, centrifuged at 4 ℃ (10,000 g, 30 min), and the supernatant was collected. Next, the pH of supernatant was adjusted to 4.5 with 2 M HCl, and it was kept overnight at 4 ℃. It was then centrifuged at 4 ℃ (6,000 g, 30 min) to obtain the precipitate. After washing the precipitate with water, it was dispersed in deionized water and the pH was adjusted to 7.5. The suspension was placed in a dryer, then freeze-dried, ground, and stored. Based on the Kjeldahl method, the protein content was 93.12 ± 1.01 %.
2.3. Preparation of SPI-HF solutions
The SPI-HF solutions and ultrasonic power were determined according to Ma et al., Tian et al., and Ma et al., [16], [17], [18] with a slight modification. SPI (1:25, w/v) was dispersed in PBS (5 mg/mL, pH 8.0). HF (SPI: HF = 1:1, w/w) was then added to the SPI dispersion and stirred for 2 h. Next, the pH of the SPI-HF solution was adjusted to 7.0 with 2 M NaOH. The resulting SPI and SPI-HF solutions were ultrasonically treated at 20 kHz and 450 W for 10 min, 20 min, and 30 min. The ultrasonic samples were then freeze-dried and analyzed. The SPI solutions that underwent ultrasonic treatment for 0 min, 10 min, 20 min, and 30 min were labeled SA-0, SA-10, SA-20, and SA-30, respectively. The SPI-HF solutions that underwent ultrasonic treatment for 0 min, 10 min, 20 min, and 30 min were labeled SHA-0, SHA-10, SHA-20, and SHA-30, respectively.
2.4. Characterization of SPI-HF solutions
2.4.1. Circular dichroism (CD)
A slightly modified procedure of Chen et al. [5] was used for the CD. The freeze-dried samples were diluted to a concentration of 0.1 mg/mL with PBS. Deionized water was used as a blank. Chirascan V100 (Applied Photophysics, England) was used to detect a wavelength range of 190–260 nm. The secondary structure contents of the detected samples were obtained using CDNN software analysis.
2.4.2. Fluorescence spectrum
The fluorescence spectrum was performed based on the slightly adjusted procedure of Tong et al. [2]. The freeze-dried samples were diluted with PBS to a concentration of 0.2 mg/mL. The fluorescence spectrum was recorded using an RF-6000 (Shimadzu, Japan) with an excitation wavelength of 280 nm and an emission wavelength of 300–400 nm (slit width = 5.0 nm).
2.4.3. Particle size
The Particle size was carried out according to the work of Ma et al. [15]. The freeze-dried samples were dissolved in PBS, and each group was diluted properly before measurement. The particle sizes were measured using a Zetasizer Nano ZS90 (Malvern, England).
2.4.4. Zeta potential
The zeta potential was evaluated according to Tong et al. [2]. The freeze-dried samples were dissolved in PBS and diluted properly. Then, the zeta potential was measured using a Zetasizer Nano ZS90 (Malvern, England) at 25 ℃. The sample loading amount, refractive index, and temperature equilibrium time was 1 mL, 1.450, and 2 min, respectively.
2.4.5. Protein solubility
The protein solubility was determined based on the method described by Jiang et al. [6]. The freeze-dried samples were diluted to 1 mg/mL with PBS and centrifuged (12,000 g, 15 min). Then, the protein content of the supernatant was measured using a UV-2600 (Shimadzu, Japan) at an absorbance wavelength of 562 nm. The protein content was calculated according to the standard curve (y = 0.6965x + 0.013, R2 = 0.9979). Protein solubility was calculated based on the following formula:
| (1) |
2.4.6. Surface hydrophobicity (H0)
The H0 was performed based on the slightly adjusted procedure of Dong et al. [12]. The freeze-dried samples were prepared into solutions of different concentrations (0.02, 0.04, 0.06, 0.08, 0.1 mg/mL) using PBS. Next, 20 μL ANS solution (8.0 mmol/L) was added to 4 mL protein solution and reacted in the dark for 15 min. The fluorescence intensity of the mixed solutions was measured with an RF-6000 (Shimadzu, Japan). The excitation wavelength, emission wavelength, and excitation and emission slit width were 390 nm, 470 nm, and 5 nm, respectively. The fluorescence intensity was then plotted against the sample concentration, and the initial slope of the curve is H0 of the protein.
2.4.7. Free sulfhydryl group (SH)
Qin et al. [14] procedure with a minor modification was applied for SH. The freeze-dried samples were dissolved in Tris-glycine buffer (0.086 mol/L Tris, 0.09 mol/L glycine, 4 mmol/L EDTA, pH 8.0), diluted to 2 mg/mL, and centrifuged (10,000 g, 15 min). Then, 0.05 mL Ellman reagent (4 mg/mL, DTNB dissolved in Tris-glycine buffer) was added to the 5 mL of supernatant, incubated at room temperature for 15 min, and absorbance was measured at 412 nm by a UV-2600 (Shimadzu, Japan). Lastly, the SH was calculated according to the following formula:
| (2) |
where A412 is the absorbance of the samples, C is the SPI concentration (1 mg/mL), and D is the dilution factor (1). The factor 73.53 comes from 106/(1.36 × 104), with 1.36 × 104 being the molar extinction coefficient.
2.4.8. Turbidity
The turbidity was determined by modification of the methods described by Martini et al. [19]. The freeze-dried samples were dissolved in PBS and diluted to 5 mg/mL. The absorbance was then measured at 600 nm with a UV-2600 (Shimadzu, Japan).
2.5. Antioxidant activity
2.5.1. DPPH radical scavenging activity
A slightly modified procedure of Zhang et al. [3] was used to determine the antioxidant activity. The freeze-dried samples were dissolved in PBS to obtain a concentration of 2 mg/mL. Secondly, DPPH was dissolved in 95% ethanol to obtain a 0.1 mmol/L solution. Next, 2 mL DPPH solution was added to the 2-mL sample solutions and reacted in the dark for 30 min. The absorbance was measured by a UV-2600 (Shimadzu, Japan) at 517 nm. The DPPH was calculated according to the following formula:
| (3) |
where Ai is the absorbance of the sample in DPPH, Aj is the absorbance of the sample in 95% ethanol, and A0 is the absorbance of DPPH in 95% ethanol.
2.5.2. ABTS radical scavenging activity
Potassium persulfate (2.6 mmol/L) and ABTS (7.4 mmol/L) were mixed at a ratio of 1:1 (v/v) and stored in the dark for 16 h to obtain ABTS free radical cations. The freeze-dried samples were dissolved in PBS to obtain a concentration of 2 mg/mL. Then, 60 µL sample solution was added to 4 mL ABTS solution and reacted in the dark for 6 min. The absorbance was measured by a UV-2600 (Shimadzu, Japan) at 734 nm. The ABTS was calculated according to the following formula:
| (4) |
where Ai is the absorbance of the sample in ABTS, Aj is the absorbance of the sample in PBS, and A0 is the absorbance of ABTS in PBS.
2.6. Preparation of SPI-HF emulsions
The SPI-HF emulsions were determined according to Dong et al. [12]. The freeze-dried samples were dissolved in PBS and corn oil (oil/water ratio = 1:9). These solutions were mixed and homogenized at 10,000 r/min for 2 min using a homogenizer (T18DS25, IKA, Germany) to form the emulsions. The SPI emulsions that underwent ultrasonic treatment for 0 min, 10 min, 20 min, and 30 min were labeled SE-0, SE-10, SE-20, and SE-30, respectively. The SPI-HF emulsions that received ultrasonic treatment for 0 min, 10 min, 20 min, and 30 min were labeled SHE-0, SHE-10, SHE-20, and SHE-30, respectively.
2.7. Characterization of SPI-HF emulsions
2.7.1. Emulsifying properties
The emulsifying activity index (EAI) and emulsifying stability index (ESI) were according to Zhang et al. [3] with some modifications. The fresh emulsion (50 µL) was dispersed in 0.1% SDS solution at a ratio of 1:100 and shaken in a vortex mixer for 30 s. The absorbance was then measured at 500 nm with a spectrophotometer (UV-2600, Shimadzu, Japan) and recorded as A0. The emulsion was left to stand for 10 min, and the previous steps were repeated. This absorbance was recorded as A10. The EAI and ESI were calculated using the following formulas:
| (5) |
| (6) |
where, A0 is the absorbance of the emulsion measured at 0 min, A10 is the absorbance of the emulsion measured at 10 min, DF is the dilution multiple (1 0 0), θ is the volume fraction of oil in the emulsion (10%), L is the thickness of the cuvette (1 cm), and C is the protein concentration in the solution used to prepare the emulsion (0.005 g/mL).
2.7.2. Particle size
Each group was diluted properly before measurement. The particle size was measured using a Zetasizer Nano ZS90 (Malvern, England).
2.7.3. Optical microscope
To obtain optical microscope images, 20 μL of emulsion was placed on a glass slide and covered with a cover glass. The images were obtained using a DM IL LED microscope (Leica, Germany).
2.7.4. Confocal laser scanning microscope (CLSM)
The CLSM was observed according to the method of Sui et al. [18] with slight modifications. For the CLSM imaging, Nile blue (0.1%, w/v) was dissolved in isopropanol as a dyeing solution. Next, 40 μL dyeing solution and 1 mL emulsion were mixed and shaken. Then 20 μL dyed emulsion was placed on the glass slide and covered by a glass slide. The excitation wavelength was 633 nm, and the CLSM images were obtained using a TCS SP8 (Leica, Germany).
2.8. Molecular docking
SPI is primarily composed of 7S and 11S, which account for 80–90% of the total protein. The crystal structures of 7S protein and 11S protein were obtained from the Protein Data Bank (7S: 3AUP, 11S: 2D5F), and molecular docking was conducted using AutoDock Tools-1.5.6 software.
2.9. Statistical analysis
All the data points in the experiment were acquired in triplicate, and the results were expressed as the mean ± standard deviation (SD). SPSS version 22 (IBM, USA) and Origin 2019 (OriginLab, USA) were used to analyze the data. Then the statistical significance (P < 0.05) was determined using a one-way ANOVA with Duncan’s test.
3. Results and discussion
3.1. Circular dichroism
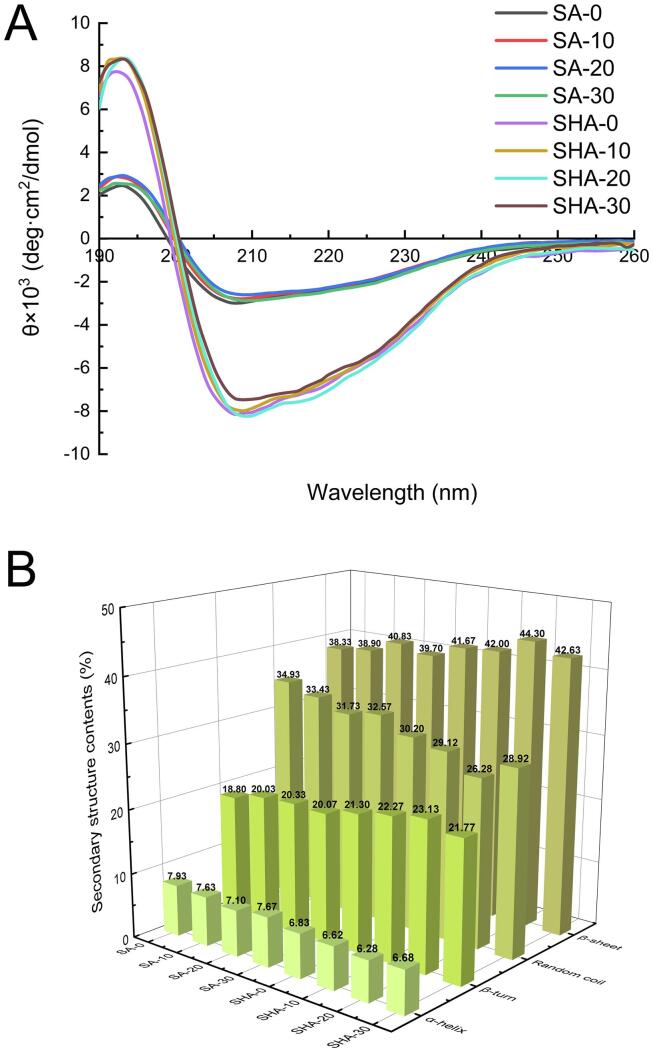
CD can characterize the protein’s ligand-induced conformational changes. As shown in Fig. 1A, all of the samples had a positive peak at approximately 193 nm, a zero crossing at around 199 nm, and a broad negative peak at approximately 207 nm.
Fig. 1.
Effect of HF and ultrasound duration on the (A) circular dichroic graph and (B) secondary structures of SA and SHA aqueous solutions.
As shown in Fig. 1B, the α-helix and random coil of SA-0 were significantly lower compared to SHA-0, and the β-sheet and β-turn were significantly increased (P < 0.05). This result indicates that the addition of HF changed the secondary structure of SPI. Previous studies have shown that decreasing the α-helix of protein and increasing the β-turn improves the stability of protein [20]. A study by Zou et al. [21] found that ultrasound could reduce the α-helix and random coil of protein and increase the β-sheet and β-turn. In the current study, the α-helix and random coil of SA-10, SA-20, SA-30, SHA-10, SHA-20, and SHA-30 decreased after ultrasonic treatment, while the β-sheet and β-turn increased significantly (P < 0.05). The α-helix and random coil of SA and SHA first decreased under ultrasonic treatment for 10–20 min and then increased from 20 to 30 min, but the β-sheet and β-turn presented an opposite trend. The SHA-20 samples had the lowest α-helix and random coil and the highest β-sheet and β-turn compared to the other groups (P < 0.05). This may be due to the cavitation effect induced by the ultrasound accelerated mechanical movement and collision of SPI molecules, which led to the denaturation of the protein from a stereoscopic structure to an amorphous structure, and change of conformation leads to the rearrangement of the protein polypeptide chains [22]. In a study by Tang et al. [23], Moringa oleifera seed protein showed similar results with increased duration of ultrasonic treatment.
3.2. Fluorescence spectrum
Intrinsic fluorescence is often used to detect the spatial structure changes of hydrophobic amino acids (such as tryptophan). As shown in Fig. 2, the fluorescence intensity of SHA-0 is lower than that of SA-0. It is possible that the addition of HF causes an increase in hydrogen bonds and fluorescence quenching [5]. After ultrasonic treatment, the fluorescence intensity of the SA group and SHA group both decreased and redshifted. This may be due to the ultrasound treatment promoting the non-covalent interaction between SPI and HF molecules, increasing the number of tryptophan bound to HF in SPI, thus reducing the fluorescence intensity [24]. Moreover, the λmax of the SA group increased from 318 nm (SA-0) to 319 nm (SA-20) and then decreased to 318 nm (SA-30), while the λmax of the SHA group increased from 319 nm (SHA-0) to 321 nm (SHA-20) and then decreased to 320 nm (SHA-30). These results indicate that the polar microenvironment of tryptophan changed, and amino acids tended to move to the hydrophilic environment. This may have been induced by the stretched and loosened protein molecules [15], [25].
Fig. 2.
Effect of HF and ultrasound duration on the fluorescence spectrum of SA and SHA aqueous solutions.
3.3. Particle size and zeta potential
Particle size is one of the main factors affecting the functional characteristics of a protein. As shown in Fig. 3A, the average particle size in the SHA group is significantly lower than that of the SA group (P < 0.05), indicating that the addition of HF reduced the particle size of SPI in aqueous solutions regardless of ultrasonic treatment. Meanwhile, the average particle size in the SA group and SHA group decreased after ultrasonic treatment, showing a two-way adjustment effect of first decreasing (0–20 min) and then increasing (20–30 min). The SHA-20 group reached the lowest particle size compared to the other groups (88.43 nm) (P < 0.05). These results may be attributed to ultrasonic cavitation, which then induced the collapse and dissociation of large aggregated proteins [23]. Consistent with our results, previous studies have demonstrated that longer durations of ultrasonic treatment would lead to aggregation of protein molecules in an aqueous solution, resulting in poor solution stability and promoting the transformation of small molecule aggregates to medium-sized aggregates [26]. As shown in Fig. 3B, the particle size of the SA and SHA group showed a unimodal distribution. However, compared to the SA group, the peak of the SHA group was farther to the left, and the ultrasonic treatment exacerbated this phenomenon. Based on these data, the addition of HF and ultrasonic treatment may have synergically expanded the protein structure, weakened the interaction between protein molecules, and dissociated subunits, thereby reducing the particle size [27].
Fig. 3.
Effect of HF and ultrasound duration on the (A) particle size and zeta potential and (B) particle distribution of SA and SHA aqueous solutions.
Zeta potential characterizes the mutual attraction between particles. As shown in Fig. 3A, the negative charge of SHA-0 was significantly lower than that of SA-0 in the absence of ultrasonic treatment (P < 0.05). SPI had a negative charge because its isoelectric point was lower than the pH of the solution, and the addition of HF increased the density of negative charge, making the zeta potential lower [15]. Under the condition of ultrasonic treatment for 20 min, the negative charge of SHA-20 reached its lowest point (-28.80 mV) (P < 0.05). It may be that ultrasound promoted the interaction between SPI and HF, the ability of the protein to bind flavonoids is enhanced, and HF itself has a negative charge, so the complexes reached the lowest value after 20 min of ultrasound [28]. When the duration of ultrasonic treatment was too long, it caused the re-aggregation of protein and an increase in zeta potential. In a study that was consistent with our results, Blayo et al. [29] called this phenomenon the “over-processing” effect. The current study demonstrated that the addition of HF and ultrasonic treatment can reduce the zeta potential of SPI-HF non-covalent complexes. Since the zeta potential affects the solubility and H0 of protein, we then analyzed these properties.
3.4. Protein solubility
The solubility of protein is an important functional characteristic of protein in food. As shown in Fig. 4A, the solubility of SHA-0 was significantly higher than that of SA-0 (P < 0.05). The addition of HF may have increased hydrogen bonds, leading to increased protein solubility [27]. After ultrasonic treatment, the solubility of SHA-20 reached the highest value compared to other groups (73.93%) (P < 0.05). This may have been caused by ultrasonic cracking of the aggregates, leading to the formation of smaller particles. The SPI molecule was partially unfolded during ultrasonication, becoming more soluble and more prone to interacting with HF [30], [31]. Cao et al. [26] found that the stability and solubility of the solution decreased significantly under excessive ultrasonic treatment, which is similar to the results in our research.
Fig. 4.
Effect of HF and ultrasound duration on the (A) protein solubility, (B) H0, and (C) SH of SA and SHA aqueous solutions.
3.5. Surface hydrophobicity
H0 is an index that characterizes the intermolecular interactions of the protein. As shown in Fig. 4B, the H0 of SHA-0 was significantly lower than that of SA-0 (P < 0.05), and the addition of HF reduced H0 by forming a large number of hydrophilic hydroxyl groups [27]. After ultrasonic treatment, the H0 of the SA group and SHA group decreased overall. Both groups exhibited a two-way adjustment effect of first decreasing (10–20 min) and then increasing (20–30 min), in which the H0 of SHA-20 reached the lowest value compared to the other groups (1250.42) (P < 0.05). The ultrasound treatment may have led to an increase in hydrophilic amino acids, thus burying more of the hydrophobic parts. Zhou et al. [32] found that under excessive ultrasound durations, the molecular structure of protein rearranged and the H0 increased.
3.6. Free sulfhydryl group (SH)
SH can reflect the degree of denaturation of a protein. As shown in Fig. 4C, the number of SH in the SHA-0 group was significantly higher compared to that of SA-0 (P < 0.05). SPI combined with HF may have had a hydrophobic effect, which destroyed intermolecular disulfide bonds and formed SH [27]. After ultrasonic treatment, the number of SH in the SA group and SHA group both increased, exhibiting a two-way regulating effect of first increasing (10–20 min) and then decreasing (20–30 min). The number of SH in the SHA-20 group reached the highest value after ultrasonic treatment for 20 min (15.07 μmol/g). The buried SH residues of the protein may become exposed during the period when disulfide bonds were broken because of the high pressure, shear force, and turbulent flow of the cavitation phenomenon [33], [34]. Consistent with our results, Zhang et al. [35] found that the high frequency of ultrasound promoted protein aggregation and decreased the SH content.
3.7. Turbidity
Turbidity analysis can directly detect the interaction between proteins and flavonoids, which correlates to the color of the solution and the number of aggregate complex polymers. As shown in Fig. 5, the turbidity of SHA-0 was significantly higher than that of SA-0 (P < 0.05), which indicates that flavonoids easily formed brown quinones with the protein under neutral conditions [36]. After ultrasonic treatment, the turbidity of the SA and SHA group decreased, showing a two-way adjustment effect of first increasing (10–20 min) and then decreasing (20–30 min). Under the condition of 20-min ultrasonic treatment, the turbidity of SHA-20 reached the lowest value compared to the other SHA groups (P < 0.05). This showed that ultrasonic treatment can enhance the dispersion of non-covalent complexes to a certain extent and make SPI-HF non-covalent complexes have good solubility in the water system to prevent the formation of large aggregates [15]. Previous studies have also shown that the conformation of proteins changes with increasing duration of ultrasound, which increases the turbidity of the protein solution [26].
Fig. 5.
Effect of HF and ultrasound duration on the (A) turbidity and (B) visual observations of SA and SHA aqueous solutions.
3.8. Antioxidant activity
The scavenging ability of DPPH and ABTS has been widely used to detect the scavenging effect of various antioxidants on free radicals. As shown in Fig. 6, the DPPH and ABTS values of SHA-0 were significantly higher than that of SA-0 (P < 0.05). This result indicates that the SPI-HF complex inhibited free radicals and improved the antioxidant capacity of SPI [37]. After ultrasonic treatment, the free radical scavenging rates of DPPH and ABTS in the SA group and SHA group both increased, showing a two-way adjustment effect of first increasing (10–20 min) and then decreasing (20–30 min). These results indicate that the addition of HF and ultrasonic treatment may destroy macromolecules and release small molecules with high antioxidant capacity, thus improving the free radical scavenging ability [17]. Moreover, the SHA-20 group reached the highest DPPH and ABTS scavenging rates compared to the other groups (71.00%, 41.91%) (P < 0.05). This may be because ultrasonic treatment promoted the combination of SPI and HF to provide more hydrogen donors, which can react with DPPH and ABTS free radicals to transform them into more stable products [38]. Consistent with the results of this study, Ruan et al. [39] also found that “excessive treatment”, induced by long durations of ultrasound, denatured the protein, which did not improve antioxidant activity.
Fig. 6.
Effect of HF and ultrasound times on the (A) DPPH and (B) ABTS of SA and SHA aqueous solutions.
3.9. Characterization of SPI-HF emulsions
3.9.1. Emulsifying properties
EAI and ESI are important indices for characterizing the emulsifying ability of protein and the ability to stabilize emulsions. As shown in Fig. 7, the EAI and ESI of the SHE-0 group were significantly higher than that of SE-0 (P < 0.05). This may be because HF enlarged the polypeptide chain structure of the protein, thereby increasing the EAI and ESI [40]. After 10–20 min of ultrasound, EAI and ESI increased over time, showing a strong time-effect relationship. When the ultrasound duration increased to 30 min, EAI and ESI then decreased. This showed that ultrasound reduced de-folded structure, which promoted the adsorption of protein at the water–oil interface and more stable structure, resulting in increased EAI and ESI [41]. However, excessive ultrasound may destroy the spherical structure of SPI, and the hydrophobic groups would be aggregated together [15].
Fig. 7.
Effect of HF and ultrasound duration on the (A) EAI and (B) ESI of the SE and SHE emulsions.
3.9.2. Particle size
As shown in Fig. 8A, the average particle size in the SHE-0 group was significantly lower than that of SE-0 (P < 0.05). The addition of HF may have enhanced the interaction between oil and water, reduced the particle size of the emulsion, and produced more uniform emulsion droplets [39]. After ultrasonic treatment, the average particle size of the SE and SHE group decreased, showing a two-way regulation effect of first decreasing (10–20 min) and then increasing (20–30 min). The average particle size of the SHE-20 group had the lowest value compared to other groups (95.27 nm) (P < 0.05). As shown in Fig. 8B, the particle sizes of the SE and SHE group exhibited a unimodal distribution. However, compared to the SE group, the peak of the SHE group was farther to the left, and after ultrasonic treatment, the peak of the two groups were narrower.
Fig. 8.
Effect of HF and ultrasound duration on the (A) particle size and (B) particle distribution of the SE and SHE emulsions.
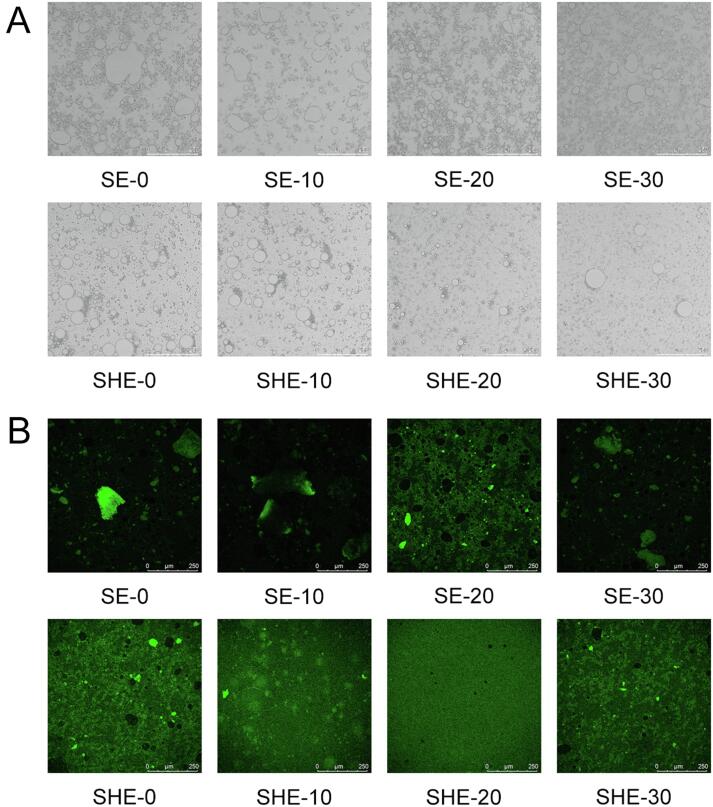
3.9.3. Microscopic observation
Optical microscopes can be used to observe the microstructure of each emulsion under different treatment conditions. As shown in Fig. 9A, SE-0 showed irregular aggregation, while SHE-0 did not. The addition of HF may have reduced the particle size of the emulsion and distributed the protein evenly [42]. After ultrasonic treatment, small and low-flocculating emulsion droplets with high density formed, especially in the SHE-20 group. This result was consistent with the emulsifying activity, emulsifying stability, and average particle size results (3.9.1, 3.9.2). The results indicated that the ultrasonic treatment may change the rigid structure of the protein to a more flexible structure, preventing the flocculation and aggregation of emulsions [43]. Moreover, the ultrasonic treatment complexes produced more uniform emulsion, the droplet size was significantly reduced and the distribution was more uniform. Cao et al. [25] found that with increased time under ultrasound, protein molecules interact with each other and form aggregates again.
Fig. 9.
Observing of the effect of HF and ultrasound duration on the SE and SHE emulsions via (A) optical microscope and (B) CLSM images. Original magnification: ×100.
The microstructure of the emulsions was observed via CLSM. As shown in Fig. 9B, obvious aggregation occurred in the SE-0 group compared to SHE-0, potentially because the addition of HF helped more protein particles adsorb at the water–oil interface and stabilize more and smaller oil droplets [44]. After ultrasonic treatment, the aggregation phenomenon reduced, and the SHE-20 group had the best effect. These results indicate that ultrasonic cavitation can effectively decompose protein aggregates, improve the dispersion of SPI molecules and change the aggregation state of protein [45], [46]. Moreover, when ultrasonic durations continued to increase, soluble protein aggregates reaggregated into insoluble aggregates through covalent bond and non-covalent bond combination, which reduced the emulsion stability [47].
3.10. Molecular docking
Molecular docking is an effective method for analyzing molecular interaction. The interactions between SPI and HF were investigated by docking the 7S and 11S proteins with the simulated molecules of vitexin-2-O-rhamnoside and glucosylvitexin, which are the main components of hawthorn flavonoids.
As shown in Fig. 10A, vitexin-2-O-rhamnoside interacted with 15 amino acids (Ser 267, Met 263, Asn 43, Met 97, Gln104, Ser 98, His 46, Ala 164, Trp 48, Asn 45, Gly 44, Val 158, Gly 228, Gln 227, and Leu 226) in the 7S protein via weak van der Waals forces. Vitexin-2-O-rhamnoside also interacted with 13 amino acids (Trp 384, Val 458, Ala 461, Ser 453, Gly 321, Asp457, Pro 86, Val 322, Leu 386, Ser 452, Val 451, Glu 324, and Glu 323) in the 11S protein through hydrogen bonds, electrostatic forces, hydrophobic interactions, and van der Waals forces. Hydrogen bonds were observed between vitexin-2-O-rhamnoside and Ser 453, Ser 452, Glu 324, and Glu 323, and a Pi-Cation electrostatic interaction was observed between vitexin-2-O-rhamnoside and Gly 321. Two hydrophobic effects were also observed, including Pi-Sigma (between vitexin-2-O-rhamnoside and Val 322) and Pi-Alkyl (between vitexin-2-O-rhamnoside and Pro 86, Leu 386, and Val 322). As shown in Fig. 10B, glucosylvitexin interacted with 10 amino acids (Leu 226, Asp 41, Pro 101, Ser 267, Gln 227, Glu 229, Arg 356, Met 97, His 46, and Gln 104) in the 7S protein. These interactions included hydrogen bonds, electrostatic forces, carbon-hydrogen bonds, and hydrophobic interactions. Five hydrogen bonds were observed between glucosylvitexin and Leu 22, Asp 4, Gln 227, Ser 267, and Gln 104. Two electrostatic forces were found, which were Pi-Cation (between glucosylvitexin and Arg 356) and Pi-Anion (between glucosylvitexin and Glu 229). Some weak carbon-hydrogen bonds were observed, such as Met 97 and His 46. A hydrophobic effect from Pi-Alkyl interaction between glucosylvitexin and Pro 101 was also observed. Glucosylvitexin interacted with 11 different amino acids in the 11S protein: Gln 155, Asn 154, Asn 153, Pro 64, Phe163, Pro 127, Tyr 62, Pro 83, Val 129, Gly 128, and Pro 130. Four hydrogen bonds formed with Asn 153, Pro 127, Gly 128, and Tyr 62. The hydrophobic interactions included Pi-Pi T-shaped (between glucosylvitexin and Phe 163 or Tyr 62) and Pi-alkyl (between glucosylvitexin and Pro 127). The remaining interactions were van der Waals forces.
Fig. 10.
Schematic diagram of the molecular docking of (A) vitexin-2-O-rhamnoside and (B) glucosylvitexin with 7S and 11S protein molecules.
Overall, the non-covalent complex interactions between SPI and HF were mainly hydrogen bonds and hydrophobic interactions. The binding behavior of HF occurred in the hydrophobic pocket of the protein, and hydrophobic amino acid residues provided important hydrophobic interactions. This played a key role in the formation of the non-covalent protein–flavonoid complex [48]. The formation of hydrogen bonds and hydrophobic interactions promoted the hydrophilic ability in the non-covalent composite system and enhanced other physicochemical properties by improving the protein solubility [49]. The molecular docking results verified that the main forces in the non-covalent composite are hydrogen bonds and hydrophobic interactions.
4. Conclusion
This experiment investigated the effects of different durations of ultrasound on the physicochemical properties, antioxidant activity, and emulsifying properties of SPI-HF non-covalent complexes. When ultrasound was conducted at 450 W for 20 min, the addition of HF decreased the zeta potential, H0, and turbidity and improved the protein solubility, SH, secondary structure stability, and antioxidant activity. In addition, this experimental group exhibited better EAI, ESI, and smaller particle sizes. The emulsion, observed by optical microscope and CLSM, was uniform and stable. The main forces in the non-covalent complexes were hydrogen bonding and hydrophobic interaction. This study can provide useful data for the application of non-covalent complexes of protein and flavonoids in the field of functional food ingredients and ultrasonic protein modification.
CRediT authorship contribution statement
Yi-Lun Wang: Methodology, Writing – original draft, Writing – review & editing. Jin-Jie Yang: Data curation, Writing – original draft. Shi-Cheng Dai: Formal analysis, Writing – review & editing. Xiao-Hong Tong: Conceptualization. Tian Tian: Data curation. Chu-Cheng Liang: Investigation. Liang Li: Formal analysis. Huan Wang: Project administration, Supervision, Writing – original draft, Writing – review & editing. Lian-Zhou Jiang: Resources, Conceptualization, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by Heilongjiang Province Postdoctoral Special Funding Project (LBH-TZ2117) and the Opening Project of Key Laboratory of Soybean Biology of Chinese Education Ministry (SBKF13).
Contributor Information
Huan Wang, Email: whname@neau.edu.cn.
Lian-Zhou Jiang, Email: jlzname@neau.edu.cn.
References
- 1.Tong X., Lian Z., Miao L., Qi B., Jiang L. An innovative two-step enzyme-assisted aqueous extraction for the production of reduced bitterness soybean protein hydrolysates with high nutritional value. LWT- Food Science and Technology. 2020;134 [Google Scholar]
- 2.Tong X., Cao J., Tian T., Lyu B., Miao L., Lian Z., Cui W., Liu S., Wang H., Jiang L. Changes in structure, rheological property and antioxidant activity of soy protein isolate fibrils by ultrasound pretreatment and EGCG. Food Hydrocolloids. 2022;122 [Google Scholar]
- 3.Zhang W., Huang L., Chen W., Wang J., Wang S. Influence of ultrasound-assisted ionic liquid pretreatments on the functional properties of soy protein hydrolysates. Ultrason. Sonochem. 2021;73 doi: 10.1016/j.ultsonch.2021.105546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H., Sun Y., Li Y., Tong X., Regenstein J.M., Huang Y., Ma W., Sami R., Qi B., Jiang L. Effect of the condition of spray-drying on the properties of the polypeptide-rich powders from enzyme-assisted aqueous extraction processing. Drying Technol. 2019;37(16):2105–2115. [Google Scholar]
- 5.Chen G., Wang S., Feng B., Jiang B., Miao M. Interaction between soybean protein and tea polyphenols under high pressure. Food Chem. 2019;277:632–638. doi: 10.1016/j.foodchem.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 6.Jiang J., Zhang Z., Zhao J., Liu Y. The effect of non-covalent interaction of chlorogenic acid with whey protein and casein on physicochemical and radical-scavenging activity of in vitro protein digests. Food Chem. 2018;268:334–341. doi: 10.1016/j.foodchem.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Wu J., Peng W., Qin R., Zhou H. Crataegus pinnatifida: chemical constituents, pharmacology, and potential applications. Molecules (Basel, Switzerland) 2014;19(2):1685–1712. doi: 10.3390/molecules19021685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li T., Zhu J., Guo L., Shi X., Liu Y., Yang X. Differential effects of polyphenols-enriched extracts from hawthorn fruit peels and fleshes on cell cycle and apoptosis in human MCF-7 breast carcinoma cells. Food Chem. 2013;141(2):1008–1018. doi: 10.1016/j.foodchem.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 9.Mraihi F., Hidalgo M., de Pascual-Teresa S., Trabelsi-Ayadi M., Chérif J.-K. Wild grown red and yellow hawthorn fruits from Tunisia as source of antioxidants. Arabian J. Chem. 2015;8(4):570–578. [Google Scholar]
- 10.Pérez J., Arteaga M., Andrade R., Durango A., Salcedo J. Effect of yam (Dioscorea spp.) starch on the physicochemical, rheological, and sensory properties of yogurt. Heliyon. 2021;7(1):e05987. doi: 10.1016/j.heliyon.2021.e05987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niu Y., Nan Y., Yuan L., Wang R. Study on antibacterial effect of medlar and hawthorn compound extract in vitro. Afr. J. Tradit. Complement. Altern. Med. 2013;10:567–573. doi: 10.4314/ajtcam.v10i3.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong Z.Y., Li M.Y., Tian G., Zhang T.H., Ren H., Quek S.Y. Effects of ultrasonic pretreatment on the structure and functionality of chicken bone protein prepared by enzymatic method. Food Chem. 2019;299:125103. doi: 10.1016/j.foodchem.2019.125103. [DOI] [PubMed] [Google Scholar]
- 13.Kentish S., Feng H. Applications of power ultrasound in food processing. Annual review of food science and technology. 2014;5(1):263–284. doi: 10.1146/annurev-food-030212-182537. [DOI] [PubMed] [Google Scholar]
- 14.Qin X.-S., Luo S.-Z., Cai J., Zhong X.-Y., Jiang S.-T., Zhao Y.-Y., Zheng Z. Transglutaminase-induced gelation properties of soy protein isolate and wheat gluten mixtures with high intensity ultrasonic pretreatment. Ultrason. Sonochem. 2016;31:590–597. doi: 10.1016/j.ultsonch.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Ma X., Yan T., Hou F., Chen W., Miao S., Liu D. Formation of soy protein isolate (SPI)-citrus pectin (CP) electrostatic complexes under a high-intensity ultrasonic field: Linking the enhanced emulsifying properties to physicochemical and structural properties. Ultrason. Sonochem. 2019;59:104748. doi: 10.1016/j.ultsonch.2019.104748. [DOI] [PubMed] [Google Scholar]
- 16.Ma X., Hou F., Zhao H., Wang D., Chen W., Miao S., Liu D. Conjugation of soy protein isolate (SPI) with pectin by ultrasound treatment. Food Hydrocolloids. 2020;108 [Google Scholar]
- 17.Tian R., Zhu G., Feng J., Tian B., Sui X. Ultrasound driven conformational and physicochemical changes of soy protein hydrolysates. Ultrason. Sonochem. 2020;68 doi: 10.1016/j.ultsonch.2020.105202. [DOI] [PubMed] [Google Scholar]
- 18.Sui X., Bi S., Qi B., Wang Z., Zhang M., Li Y., Jiang L. Impact of ultrasonic treatment on an emulsion system stabilized with soybean protein isolate and lecithin: Its emulsifying property and emulsion stability. Food Hydrocolloids. 2017;63:727–734. [Google Scholar]
- 19.Martini S., Potter R., Walsh M. Optimizing the use of power ultrasound to decrease turbidity in whey protein suspensions. Food Res. Int. 2010;43:2444–2451. [Google Scholar]
- 20.Tian Z., Tian L., Shi M., Zhao S., Guo S., Luo W., Wang C., Tian Z. Investigation of the interaction of a polyamine-modified flavonoid with bovine serum albumin (BSA) by spectroscopic methods and molecular simulation. J. Photochem. Photobiol., B. 2020;209 doi: 10.1016/j.jphotobiol.2020.111917. [DOI] [PubMed] [Google Scholar]
- 21.Zou Y., Xu P., Wu H., Zhang M., Sun Z., Sun C., Wang D., Cao J., Xu W. Effects of different ultrasound power on physicochemical property and functional performance of chicken actomyosin. Int. J. Biol. Macromol. 2018;113:640–647. doi: 10.1016/j.ijbiomac.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 22.Hu H., Wu J., Li-Chan E.C., Zhu L., Zhang F., Xu X., Fan G., Wang L., Huang X., Pan S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocolloids. 2013;30:647–655. [Google Scholar]
- 23.Tang S.Q., Du Q.H., Fu Z. Ultrasonic treatment on physicochemical properties of water-soluble protein from Moringa oleifera seed. Ultrason Sonochem. 2021;71 doi: 10.1016/j.ultsonch.2020.105357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang F., Liu X., Huang Y., Huang C., Zhang K. Swirling cavitation improves the emulsifying properties of commercial soy protein isolate. Ultrason. Sonochem. 2018;42:471–481. doi: 10.1016/j.ultsonch.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Z., Zhu W., Yi J., Liu N., Cao Y., Lu J., Decker E.A., McClements D.J. Effects of sonication on the physicochemical and functional properties of walnut protein isolate. Food Res. Int. 2018;106:853–861. doi: 10.1016/j.foodres.2018.01.060. [DOI] [PubMed] [Google Scholar]
- 26.Cao H., Sun R., Shi J., Li M., Guan X., Liu J., Huang K., Zhang Y. Effect of ultrasonic on the structure and quality characteristics of quinoa protein oxidation aggregates. Ultrason Sonochem. 2021;77 doi: 10.1016/j.ultsonch.2021.105685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xue F., Li C., Adhikari B. Physicochemical properties of soy protein isolates-cyanidin-3-galactoside conjugates produced using free radicals induced by ultrasound. Ultrason Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2020.104990. [DOI] [PubMed] [Google Scholar]
- 28.Avramenko N.A., Low N.H., Nickerson M.T. The effects of limited enzymatic hydrolysis on the physicochemical and emulsifying properties of a lentil protein isolate. Food Res. Int. 2013;51:162–169. [Google Scholar]
- 29.Blayo C., Vidcoq O., Lazennec F., Dumay E. Effects of high pressure processing (hydrostatic high pressure and ultra-high pressure homogenisation) on whey protein native state and susceptibility to tryptic hydrolysis at atmospheric pressure. Food Res. Int. 2016;79:40–53. [Google Scholar]
- 30.Caa C., Kma D., Pza B., Aa A., Oepa D., Amrpa D. Comparative study of high intensity ultrasound effects on food proteins functionality - ScienceDirect. J. Food Eng. 2012;108:463–472. [Google Scholar]
- 31.Malik M.A., Sharma H.K., Saini C.S. High intensity ultrasound treatment of protein isolate extracted from dephenolized sunflower meal: Effect on physicochemical and functional properties. Ultrason. Sonochem. 2017;39:511–519. doi: 10.1016/j.ultsonch.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 32.Zhou X., Wang C., Sun X., Zhao Z., Guo M. Effects of High Intensity Ultrasound on Physiochemical and Structural Properties of Goat Milk β-Lactoglobulin. Molecules (Basel, Switzerland) 2020;25:3637. doi: 10.3390/molecules25163637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao H., Li-Chan E., Li W., Ming T., Pan S. The effect of high intensity ultrasonic pre-treatment on the properties of soybean protein isolate gel induced by calcium sulfate. Food Hydrocolloids. 2013;32:303–311. [Google Scholar]
- 34.Zhang P., Hu T., Feng S., Xu Q., Zheng T., Zhou M., Chu X., Huang X., Lu X., Pan S. Effect of high intensity ultrasound on transglutaminase-catalyzed soy protein isolate cold set gel. Ultrason. Sonochem. 2016;29:380. doi: 10.1016/j.ultsonch.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z., Wang Y., Jiang H., Dai C., Xing Z., Kumah Mintah B., Dabbour M., He R., Ma H. Effect of dual-frequency ultrasound on the formation of lysinoalanine and structural characterization of rice dreg protein isolates. Ultrason Sonochem. 2020;67 doi: 10.1016/j.ultsonch.2020.105124. [DOI] [PubMed] [Google Scholar]
- 36.Pan X., Fang Y., Wang L., Shi Y., Pei F., Li P., Xia J., Xiong W., Shen X., Hu Q. Covalent Interaction between Rice Protein Hydrolysates and Chlorogenic Acid: Improving the Stability of Oil-in-Water Emulsions. J. Agric. Food. Chem. 2019 doi: 10.1021/acs.jafc.8b06898. [DOI] [PubMed] [Google Scholar]
- 37.J. Li, B. Wang, Y. He, L. Wen, H. Nan, F. Zheng, H. Liu, S. Lu, M. Wu, H. Zhang, A review of the interaction between anthocyanins and proteins, Food science and technology international = Ciencia y tecnologia de los alimentos internacional, 27 (2021) 470-482. [DOI] [PubMed]
- 38.Amadou G.W., Le Y.H., Shi S. Jin, Reducing, Radical Scavenging, and Chelation Properties of Fermented Soy Protein Meal Hydrolysate by Lactobacillus plantarum LP6. Int. J. Food Prop. 2011;14:654–665. [Google Scholar]
- 39.Ruan S., Li Y., Wang Y., Huang S., Ma H. Analysis in protein profile, antioxidant activity and structure-activity relationship based on ultrasound-assisted liquid-state fermentation of soybean meal with Bacillus subtilis. Ultrason. Sonochem. 2019;64 doi: 10.1016/j.ultsonch.2019.104846. [DOI] [PubMed] [Google Scholar]
- 40.Sui X., Sun H., Qi B., Zhang M., Li Y., Jiang L. Functional and conformational changes to soy proteins accompanying anthocyanins: Focus on covalent and non-covalent interactions. Food Chem. 2017;245:871–878. doi: 10.1016/j.foodchem.2017.11.090. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J.B., Wu N.N., Yang X.Q., He X.T., Wang L.J. Improvement of emulsifying properties of Maillard reaction products from β-conglycinin and dextran using controlled enzymatic hydrolysis. Food Hydrocolloids. 2012;28:301–312. [Google Scholar]
- 42.Liu F., Tang C.H. Emulsifying properties of soy protein nanoparticles: influence of the protein concentration and/or emulsification process. J. Agric. Food. Chem. 2014;62:2644. doi: 10.1021/jf405348k. [DOI] [PubMed] [Google Scholar]
- 43.Shanmugam A., Ashokkumar M. Ultrasonic preparation of stable flax seed oil emulsions in dairy systems – Physicochemical characterization. Food Hydrocolloids. 2014;39:151–162. [Google Scholar]
- 44.Chen X., Julian M., Zhu Y., Yan C., Zou L., Wei L., Cheng C., Fu D., Liu C. Enhancement of the solubility, stability and bioaccessibility of quercetin using protein-based excipient emulsions. Food Res. Int. 2018;114:30–37. doi: 10.1016/j.foodres.2018.07.062. [DOI] [PubMed] [Google Scholar]
- 45.Liu F., Ma C., Mcclements D.J., Gao Y. Development of polyphenol-protein-polysaccharide ternary complexes as emulsifiers for nutraceutical emulsions: Impact on formation, stability, and bioaccessibility of β-carotene emulsions. Food Hydrocolloids. 2016:578–588. [Google Scholar]
- 46.Stepišnik Perdih T., Zupanc M., Dular M. Revision of the mechanisms behind oil-water (O/W) emulsion preparation by ultrasound and cavitation. Ultrason Sonochem. 2019;51:298–304. doi: 10.1016/j.ultsonch.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Li Z., Zheng Y., Sun Q., Wang J., Zheng B., Guo Z. Structural characteristics and emulsifying properties of myofibrillar protein-dextran conjugates induced by ultrasound Maillard reaction. Ultrason Sonochem. 2021;72 doi: 10.1016/j.ultsonch.2020.105458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma C.-M., Zhao X.-H. Depicting the non-covalent interaction of whey proteins with galangin or genistein using the multi-spectroscopic techniques and molecular docking. Foods. 2019;8:360. doi: 10.3390/foods8090360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y., Wang Q., Lei L., Li F., Zhao J., Zhang Y., Li L., Wang Q., Ming J. Molecular interaction of soybean glycinin and β-conglycinin with (−)-epigallocatechin gallate induced by pH changes. Food Hydrocolloids. 2020;108 [Google Scholar]