Abstract
Q fever is a zoonosis with a worldwide distribution with the exception of New Zealand. The disease is caused by Coxiella burnetii, a strictly intracellular, gram-negative bacterium. Many species of mammals, birds, and ticks are reservoirs of C. burnetii in nature. C. burnetii infection is most often latent in animals, with persistent shedding of bacteria into the environment. However, in females intermittent high-level shedding occurs at the time of parturition, with millions of bacteria being released per gram of placenta. Humans are usually infected by contaminated aerosols from domestic animals, particularly after contact with parturient females and their birth products. Although often asymptomatic, Q fever may manifest in humans as an acute disease (mainly as a self-limited febrile illness, pneumonia, or hepatitis) or as a chronic disease (mainly endocarditis), especially in patients with previous valvulopathy and to a lesser extent in immunocompromised hosts and in pregnant women. Specific diagnosis of Q fever remains based upon serology. Immunoglobulin M (IgM) and IgG antiphase II antibodies are detected 2 to 3 weeks after infection with C. burnetii, whereas the presence of IgG antiphase I C. burnetii antibodies at titers of ≥1:800 by microimmunofluorescence is indicative of chronic Q fever. The tetracyclines are still considered the mainstay of antibiotic therapy of acute Q fever, whereas antibiotic combinations administered over prolonged periods are necessary to prevent relapses in Q fever endocarditis patients. Although the protective role of Q fever vaccination with whole-cell extracts has been established, the population which should be primarily vaccinated remains to be clearly identified. Vaccination should probably be considered in the population at high risk for Q fever endocarditis.
Because Q fever is rarely a notifiable disease, the incidence of human Q fever cannot be assessed in most countries. Current epidemiological studies indicate, however, that Q fever should be considered a public health problem in many countries, including France, the United Kingdom, Italy, Spain, Germany, Israel, Greece, and Canada (Nova Scotia), as well as in many countries where Q fever is prevalent but unrecognized because of poor surveillance of the disease. Q fever remains primarily an occupational hazard in persons in contact with domestic animals such as cattle, sheep and, less frequently, goats. Persons at risk from Q fever include farmers, veterinarians, abattoir workers, those in contact with dairy products, and laboratory personnel performing Coxiella burnetii culture and more importantly working with C. burnetii-infected animals. However, there has been an increase in reports of sporadic cases in people living in urban areas after occasional contact with farm animals or after contact with infected pets such as dogs and cats.
C. burnetii infection in humans usually is asymptomatic or manifests as a mild disease with spontaneous recovery. However, Q fever may lead to serious complications and even death in patients with acute disease, especially those with meningoencephalitis or myocarditis, and more frequently in chronically infected patients with endocarditis. Patients at risk from chronic Q fever include persons with previous cardiac valve defects and to a lesser extent immunocompromised hosts and pregnant women. Q fever during pregnancy has been associated with abortion, premature birth, and low weight in newborn babies.
The clinical manifestations of Q fever may be so variable that the disease is often diagnosed only if it has been systematically considered. However, when evoked, a definite diagnosis of the disease is easy and remains based upon serology, with phase I and phase II antibodies distinguishing acute from chronic disease. However, cell culture systems (especially the shell vial method) have led to the more frequent isolation of C. burnetii from human sources.
The possibility of studying larger series of clinical C. burnetii strains by molecular biological techniques has improved genetic and antigenic characterization of the bacterium and helped to develop a better understanding of the pathophysiology of Q fever. In particular, recent experimental data indicate that host factors rather than specific genetic bacterial determinants are the main factors influencing the clinical course of C. burnetii infection.
Tetracyclines are still the best for treating acute Q fever. Although the prognosis of Q fever endocarditis has recently been improved by the use of the combination of doxycycline with chloroquine, a definite antibiotic regimen has still to be established for treating Q fever endocarditis. Therefore, prevention of chronic Q fever in the “at-risk” population has to be considered. Effective vaccines exist for humans but are currently not available in most countries. We have recently reviewed diagnostic techniques for Q fever (106). The present review deals with recent advances in the microbiological, clinical, epidemiological, diagnostic, and therapeutic aspects of Q fever.
HISTORICAL BACKGROUND
The term “Q fever” (for query fever) was proposed in 1937 by Edward Holbrook Derrick to describe febrile illnesses in abattoir workers in Brisbane, Queensland, Australia (75). In 1935, as the Director of the Laboratory of Microbiology and Pathology of the Queensland Health Department at Brisbane, he was invited to investigate an outbreak of undiagnosed febrile illness among abattoir workers in Brisbane. Since sporadic cases of the illness continued to occur regularly, he first carefully described the disease. He then attempted to isolate the etiological agent of the disease by inducing a febrile illness in guinea pigs. However, he did not succeed in isolating or even visualizing the etiological agent and speculated that the Q fever agent was a virus. The probable rickettsial origin of the disease was hypothesized by Macfarlane Burnet and his associate Mavis Freeman, to which Derrick had sent some infectious material. They reproduced the disease in guinea pigs and also in other animals including mice and monkeys. Examining hematoxylin-and-eosin-stained spleen sections from infected mice, Burnet and Freeman observed intracellular vacuoles filled with granular material, whereas staining by Castaneda’s method or Giemsa allowed visualization of numerous small rods which appeared rickettsial in nature (45). With these results, Derrick and his collaborators investigated the epidemiology of the disease, especially the potential role of an arthropod vector. They concluded that wild animals were the natural reservoir of Q fever, with domestic animals being a secondary reservoir, and that the disease may be transmitted by ticks or other arthropods.
In 1935, and independently of Derrick’s work, Gordon Davis, at the Rocky Mountain Laboratory in Hamilton, Mont., was investigating the ecology of Rocky Mountain spotted fever. Ticks collected in Nine Mile, Mont., were allowed to feed on guinea pigs, and a febrile illness was established in some animals (70). However, the observed symptoms in these animals, including a lack of marked testicular swelling, were not suggestive of Rocky Mountain spotted fever. In addition, the disease could be transmitted to uninfected guinea pigs by intraperitoneal inoculation of blood collected from infected animals, and the etiological agent could not be grown in axenic media. In 1936, Herald Rea Cox joined Davis at the Rocky Mountain Laboratory to further characterize the “Nine Mile agent.” Burnet and Freeman, as well as Davis and Cox, demonstrated that the etiological agent was filterable and displayed properties of both viruses and rickettsiae (63, 70). A major advance was obtained in 1938, when Cox succeeded in propagating the infectious agent in embryonated eggs (64).
The connection between the groups in Montana and Brisbane arose when a laboratory-acquired Q fever infection occurred in the Rocky Mountain Laboratory in 1938. Rolla Eugene Dyer, Director of the National Institutes of Health, went to Hamilton to verify the possibility of growing the Nine Mile agent in eggs. He then became infected with the organism that the laboratory was working with. A febrile illness was reproduced in guinea pigs inoculated with Dyer’s blood, and rickettsiae were identified in spleen samples from the infected animals. Also, cross-immunity was demonstrated between microorganisms isolated from Dyer’s blood and the Nine Mile agent. Dyer then established a definitive link between the Nine Mile agent and the Australian Q fever agent. Burnet sent him some spleen samples which had been removed from mice infected with the Q fever agent. After inoculation of the Q fever agent into guinea pigs, Dyer demonstrated that such animals were protected from a new challenge with the strain isolated from his blood. Such cross-immunity was highly indicative that the Q fever agent, Dyer’s blood isolate, and the Nine Mile agent were in fact isolates of a single microorganism. The etiological agent of Q fever was first named Rickettsia burnetii. However, in 1938, Cornelius B. Philip proposed the creation of a new genus called Coxiella and the renaming of the etiological agent as C. burnetii, a name which honours both Cox and Burnet, who had identified the Q fever agent as a new rickettsial species.
BACTERIOLOGY
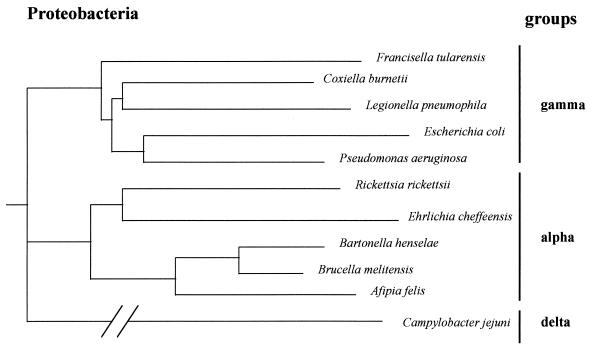
C. burnetii is an obligate intracellular, small gram-negative bacterium (0.2 to 0.4 μm wide, 0.4 to 1 μm long). Although possessing a membrane similar to that of a gram-negative bacterium, it is usually not stainable by the Gram technique. The Gimenez method (120) is usually used to stain C. burnetii in clinical specimens or laboratory cultures. Since C. burnetii cannot be grown in axenic medium and has long been recovered from ticks, it has been classified in the Rickettsiales order, the Rickettsiaceae family, and the Rickettsiae tribe together with the genera Rickettsia and Rochalimaea (396). However, recent phylogenetic investigations, based mainly on 16S rRNA sequence analysis, have shown that the Coxiella genus belongs to the gamma subdivision of Proteobacteria (347, 393, 394, 410), with the genera Legionella, Francisella, and Rickettsiella as its closest relatives (Fig. 1). Bacteria of the Rickettsia genus belong to the alpha-1 subgroup of Proteobacteria, whereas species of the genus Rochalimaea have recently been reclassified within the genus Bartonella and the family Bartonellaceae and belong to the alpha-2 subgroup of Proteobacteria.
FIG. 1.
Phylogenetic tree showing the relationships of C. burnetii to other species belonging to the Proteobacteria. The tree was constructed by the neighbor-joining method with 16S rRNA gene sequences.
C. burnetii expresses a low degree of genetic heterogeneity among strains by DNA-DNA hybridization (384). However, when DNA from 38 C. burnetii isolates was examined by restriction fragment length polymorphism (RFLP) analysis, six genomic groups (I to VI) were described (146). Later, analysis of NotI and SfiI C. burnetii DNA restriction fragments by pulsed-field gel electrophoresis (PFGE) resulted in the characterization of four different DNA fragment patterns representing isolates from genomic groups I, IV, V, and VI (140). Genomic variation in C. burnetii has been more recently further characterized (359, 402). By using PFGE and NotI, 16 additional restriction groups were identified among 80 C. burnetii isolates collected worldwide. By using different banding patterns and the unweighted pair group method with arithmetic mean (UPGMA), a phylogenetic tree, involving strains from various sources including goats, sheep, and humans with acute or chronic Q fever, was constructed. The dendrogram showed that most French isolates (groups 12 to 16) formed a separate cluster from the Russian isolates (groups 10 and 11). The other isolates were located in one main cluster, with large genetic distances, ranging from 7 to 58%, between the different groups. Thus, considerable heterogeneity was observed among C. burnetii total genomic RFLP patterns, an unexpected result. Restriction site redistribution may correspond to significant chromosomal rearrangements such as translocations, inversions, insertions, or deletions.
The genome size of C. burnetii Nine Mile strain is 2.1 Mb (403); the genome size is highly variable among different C. burnetii strains, ranging from 1.5 to 2.4 Mb (403). A locus structurally and functionally suggestive of the origin of replication (oriC) of the C. burnetii genome has been isolated (52). However, it is considered only putative at this time because, although functionally expressed in plasmids in an Escherichia coli host, it did not initiate DNA synthesis in the C. burnetii chromosome (354). The inability to localize origin function by standard methods could well be related to the fact that C. burnetii probably has a linear rather than a circular chromosome and thus may not have conventional bidirectional replication (403). A physical macrorestriction map of C. burnetii Nine Mile phase I was constructed by Willems et al. (402, 403). Twenty-five DNA fragments were distinguished by PFGE after restriction of total DNA with NotI. Such a physical map may serve as a basis for constructing a genetic map and comparing gene loci and genetic organization among different C. burnetii isolates (402, 403). The inability to clone the ends of the 240- and 7.3-kb fragments from C. burnetii Nine Mile phase I strain into a compatible vector, after excision of these fragments from a PFGE gel and digestion with a second enzyme (402, 403), favored the hypothesis that the chromosome of this organism may be linear.
Eleven chromosomal genes have been cloned and expressed in E. coli: gltA, the citrate synthase gene (139); sodB, the superoxide dismutase gene (141); htpA, the 14-kDa heat shock protein gene (385); htpB, the 62-kDa heat shock protein gene (385); omp, a 27-kDa surface antigen gene (145); pyrB, the aspartate carbamoyl transferase gene (151); qrsA, a sensor protein gene (237); dnaJ, a heat shock protein gene (424); mucZ, the capsule induction protein gene (425); serS, the seryl-tRNA synthase gene (402); and algC, the phosphomannomutase gene (402). C. burnetii gene sequences partially or completely available in the GenBank or EMBL databases include 23 chromosomal sequences and 17 plasmid sequences. Genes organized into operons have been described previously (151, 385). Genetic transformation has been achieved in C. burnetii (355). Plasmid pSKO(+)1000, containing a previously characterized C. burnetii autonomous replication sequence (354) cloned into a ColE1-type replicon encoding β-lactamase, was introduced into C. burnetii by electroporation. Transformants stably maintained the pSKO(+)1000 bla DNA sequence in the chromosome, and the bla gene was expressed in C. burnetii during acid activation (355).
The C. burnetii genome comprises facultatively a 36- to 42-kb plasmid, whose function remains undetermined. The first to be described was the QpH1 plasmid (36 kb, one to three copies per cell) (313). The entire nucleotide sequence of this plasmid has been determined (361). Such a plasmid was found in genomic groups I, II, and III. A 39-kb plasmid was then characterized in genomic group IV (197). This plasmid, designated QpRS, was found in C. burnetii Priscilla, obtained from an aborted goat fetus. The QpRS plasmid was also found in human Q fever endocarditis isolates, all belonging to the genomic group IV. A third plasmid, of 42 kb, designated QpDG, was found in genomic group VI isolates, obtained from feral rodents (197). Finally, plasmidless C. burnetii isolates from Q fever endocarditis patients were found to contain DNA sequences with homology to the QpRS plasmid (316). This chromosome-integrated plasmid DNA fragment has recently been cloned and sequenced (402). Such strains corresponded to genomic group V. More recently, a new 33-kb plasmid has been found in a French strain isolated from an endocarditis patient and designated QpDV by Valkova and Kazar (380). These plasmids all share a 30-kb region (196), whereas plasmid type-specific regions have been characterized (235, 236).
C. burnetii displays antigenic variations similar to the smooth-rough variation in the family Enterobacteriaceae. Phase variation is related mainly to mutational variation in the lipopolysaccharide (LPS) (129–131). Phase I is the natural phase found in infected animals, arthropods, or humans. It is highly infectious and corresponds to smooth LPS. In contrast, phase II is not very infectious and is obtained only in laboratories after serial passages in cell cultures or embryonated egg cultures. It corresponds to rough LPS. Compared to phase I, phase II displays a truncated LPS and lacks some protein cell surface determinants (8). The sugar composition of LPS is also different in the two phases. LPS in phase I contains sugars such as l-virenose, dihydrohydroxystreptose, and galactosamine uronyl-α-(1,6)-glucosamine, which are lacking in phase II LPS (10, 132, 321, 322). Although the genetic evidence supporting phase variation in C. burnetii remains unsettled, large chromosomal deletions were demonstrated in the attenuated phase II C. burnetii Nine Mile strain (383). Recently, a NotI C. burnetii DNA restriction fragment common to all C. burnetii isolates has been cloned and sequenced (402). One open reading frame displayed significant homology to the algC gene encoding phosphomannomutase (PMM) in Pseudomonas aeruginosa. In the later species, PMM is involved in the synthesis of LPS and the surface polysaccharide alginate (423). P. aeruginosa with PMM activity corresponds to the smooth phenotype, whereas P. aeruginosa with no PMM due to mutations in algC corresponds to the rough phenotype. However, more experimental data are needed before making a connection between the P. aeruginosa alginate pathway and the C. burnetii LPS.
Genetic variability among different C. burnetii strains, as demonstrated by different RFLP-based genomic groups (146), specific plasmid regions (314), and LPS variations (130), were tentatively related to virulence. Genomic groups I, II, and III were associated with animal, tick, or acute Q fever human isolates, referred to as acute strains, whereas groups IV and V were associated with human Q fever endocarditis isolates, referred to as chronic strains. Group VI isolates, obtained from feral rodents in Dugway (Utah), were of unknown pathogenicity. QpH1 plasmid was found in genomic groups I, II, and III and thus was associated with acute C. burnetii strains, whereas QpRS plasmid was found in genomic group IV and was associated with chronic strains (197, 313, 314). Comparison of the various isolates for LPS variations, using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting, resulted in isolates being placed into groups similar to the genomic groups (130). These findings, however, were not confirmed in larger series of C. burnetii strains by using genomic RFLP analysis (360), plasmid typing with specific primers (348), or LPS analysis with specific monoclonal antibodies (421). Recent investigations suggest that predisposing host factors are more important than genomic strain variation in the explanation of the occurrence of acute or chronic Q fever diseases in humans (181, 351, 421). Moreover, recent data shows that genetic variation has an apparently closer connection with the geographical source of the isolate than with the clinical presentation.
LIFE IN THE HOST CELL
Susceptible Cells
C. burnetii can be grown in vitro in a number of cell types, including mouse macrophage-like cells (including the P388D1 and J774 cell lines), fibroblast cells (including L929 cell line), and Vero cells (5, 21, 23, 127, 221, 287, 306). Embryonated eggs and laboratory animals such as mice and guinea pigs have been used extensively for in vivo propagation of C. burnetii. In humans (41, 124, 217, 281, 343) and animals (183, 219), however, monocytes-macrophages are the only known target cells. When infection occurs via the respiratory route, alveolar macrophages in the lungs are supposedly the primary cells to be infected during acute Q fever. Kupffer cells in the liver are also susceptible and may be infected via the bloodstream or from the digestive route; only a few patients are supposedly infected via the digestive route.
Entry into Eukaryotic Cells
Microorganisms, including intracellular microorganisms, use specific eukaryotic receptors such as integrins to invade host cells. Many of these agents, including Legionella pneumophila and Mycobacterium tuberculosis, use CR3 as a receptor for entry into phagocytes. C. burnetii phase II enters human monocyte-derived macrophages by engaging the CR3 receptor (233). In contrast, the infectious phase I C. burnetii blocks entry via the CR3 receptor and binds to human monocytes via the complex of LRI (leukocyte response integrin, αvβ3) and IAP (integrin-associated protein) (233). The natural phase I C. burnetii is only poorly internalized by monocytes and macrophages but can survive within these cells. In contrast, phase II C. burnetii is readily internalized by monocytes and macrophages (233) but is rapidly killed via the phagolysosomal pathway. The receptor used by each C. burnetii phase for entry into monocytes and macrophages is probably critical for its survival within these phagocytic cells.
Intracellular Location and Multiplication
After passive entry into the host cell, C. burnetii is internalized within eukaryotic cells in phagosomes, which fuse rapidly with lysosomes to form phagolysosomes. The early phagolysosomes fuse progressively to form a large unique vacuole (127). The lysosomal origin of C. burnetii-containing vacuoles is evidenced by the presence within this compartment of lysosomal markers such as proton-ATPase, acid phosphatase, cathepsin D, and the lysosomal glycoproteins LAMP-1 and LAMP-2 (143). C. burnetii has adapted to the phagolysosomes of eukaryotic cells (127), multiplying in acidic vacuoles (pH 4.7 to 5.2) (5, 221). Such acidity was stable in L929 fibroblast cells persistently infected with C. burnetii Nine Mile over a period of 6 months (221). C. burnetii is an acidophilic bacterium whose metabolism is enhanced at acidic pH. Its multiplication can be stopped by raising the phagolysosomal pH using lysosomotropic agents such as chloroquine (5, 289). The only microorganism known to have similarly adapted to such an acidic compartment of monocytes-macrophages is the amastigote form of Leishmania species (14). Acidity is needed for C. burnetii to assimilate nutrients necessary for its metabolism, including synthesis of nucleic acids (53) and amino acids (128, 144, 426). For example, the penetration of glutamate (128) and proline (144) into C. burnetii was shown to be pH dependent. It has been hypothesized that the protein encoded by the qrsA gene, classified as a sensor protein, may be involved in the adaptation mechanism of C. burnetii to the acidic milieu of the phagolysosomes (402).
Intracellular Cycle and Sporulation-Like Process
C. burnetii displays a complex intracellular cycle, leading to the formation of spore-like forms (227). McCaul and Williams (228) have proposed the terms “small-cell variant” (SCV) and “large-cell variant” (LCV) to differentiate the two C. burnetii cell forms observed in persistently infected cells (48, 251, 388, 398). SCVs and LCVs correspond to different intracellular development stages of C. burnetii (227). They can be differentiated by their morphology, size, peptidoglycan content, and resistance to osmotic pressure (9, 228, 229). SCVs are 204 by 450 nm in size and rod shaped, with densely stained walls and electron-dense nucleoids. LCVs are up to 2 μm long, more pleomorphic, rounded, granular, sometimes with fibrillar cytoplasm and with dispersed nucleoid filaments. Heinzen and Hackstadt (142) characterized in SCV but not in LCV a 20-kDa DNA-binding protein (termed Hq1) with primary amino acid sequence similarities to the eukaryotic histone H1. Hq1 is supposed to control nucleoid structure in the different C. burnetii cell variants. Both LCVs and SCVs have a typical eubacterial gram-negative cell wall with two layers separated by the periplasmic space. However, a dense material fills the periplasmic space in SCVs. This material corresponds to proteins and peptidoglycan and may explain the increased resistance of SCVs to environmental conditions.
SCVs are metabolically inactive and resistant to osmotic pressure and correspond to the extracellular form of the bacterium. SCVs attach to the eukaryotic cell membrane to enter phagocytic cells. After phagolysosomal fusion, acid activation of the metabolism of SCVs may lead to the formation of LCVs. Thus, LCVs correspond to the metabolically active intracellular form of C. burnetii. A sporogenic differentiation has been characterized in LCVs, leading to the formation of resistant, spore-like forms of bacteria (227, 228). Both activated SCVs and LCVs divide by binary fission. This sporulation-like process in LCVs may be distinguished morphologically from the cell division process by the asymmetrical position of the septum, close to one of the LCV pole in the former process (227, 230). McCaul showed that DNA was present in both the spore and the mother cells, suggesting the presence of newly replicated chromosome on each side of the septum (227). The endogenous spore-like forms undergo further development to the metabolically inactive SCVs, which are then released from the infected host cell either by cell lysis or possibly by exocytosis. Physical or biochemical factors which may induce the sporulation-like process in C. burnetii are unknown. However, nutrient deprivation is commonly the signal for the initiation of sporulation in sporulated gram-positive bacteria such as Bacillus or Clostridium species. The extracellular forms of C. burnetii resist environmental conditions such as desiccation and low or high pH and chemical products such as ammonium chloride, disinfectants such as 0.5% sodium hypochlorite, and UV radiation (20, 324). Only exposure to high concentrations of formalin (i.e., ≥5%) for a prolonged time (at least 24 to 48 h) may allow killing of C. burnetii (324). We have tested the resistance of C. burnetii to formalin in our laboratory (184). A C. burnetii inoculum of 108 IU/ml was exposed overnight to different concentrations of formalin and then subcultured in uninfected HEL cells grown in shell vials to determine the residual viable bacterial inoculum. Undetectable viability in shell vial cultures (i.e., less than 1 microorganism per ml) was obtained only after overnight incubation in 10% formalin.
Persistent Cell Infection
C. burnetii has the ability to induce persistent infections both in humans and animals (22). Chronically infected animals shed bacteria in feces and urine. Such persistent infections are mostly asymptomatic but may occur in pregnant females in the form of massive contamination of the placenta with C. burnetii, leading to abortion or low fetal birth weight. C. burnetii also induces chronic infections in humans, especially in immunocompromised patients or during pregnancy (350). In vitro, C. burnetii-infected eukaryotic cells may be maintained in persistent cultures for several months or even years, provided that the cell incubation medium is changed regularly (5, 21, 23, 46, 306). The slow intracellular multiplication of C. burnetii, with a doubling time of ∼20 h, which is similar to that of eukaryotic cells, may partly explain why the bacterium does not damage infected cells despite prolonged infection. Roman et al. (306) have proposed a model for persistent cell infection in which when an infected cell divides, one daughter cell receives the unique C. burnetii vacuole whereas the other remains uninfected. The finding that uninfected cells are still present in infected cell cultures after several months of infection is compatible with such model.
EPIDEMIOLOGY
Mode of Transmission
The aerosol route (inhalation of infected fomites) is the primary mode of human contamination with C. burnetii (212). Contamination by C. burnetii aerosols may occur directly from parturient fluids of infected animals, which may contaminate newborn animals, placenta, or wool (365). C. burnetii is very resistant to killing in nature and may survive for several weeks in areas where animals have been present; the organism may also be spread by the wind (220, 367). Thus, Q fever may occur in patients without any evident contact with animals. We have recently infected amoebae with C. burnetii Nine Mile and demonstrated that C. burnetii remained viable in amoebae for 6 weeks, as demonstrated by the ability to subculture bacteria in HEL cells (184). Thus, amoebae may serve as a reservoir for C. burnetii in nature, as has been demonstrated for Legionella species (308), and could represent a protected niche in the wild.
Ingestion (mainly drinking raw milk) is probably a minor factor in the transmission of C. burnetii (30, 102, 177) and is now even a point of controversy. Person-to-person transmission is probably extremely rare. Although infrequent, sporadic human Q fever cases have occurred following contact with an infected parturient woman (in an obstetrician who performed an abortion on the pregnant woman) (297), via transplacental transmission resulting in congenital infections (350), during autopsies (135, 200), via intradermal inoculation (13a), or via blood transfusion (13b). Although C. burnetii has been isolated from arthropods, mainly ticks, arthropod-borne transmission of Q fever in humans is unlikely to be significant (25, 92). However, we have reported two cases of coinfection with Rickettsia conorii and C. burnetii diagnosed in patients living near Montpellier and suspect that they were caused by a tick bite (160). Sexual transmission of C. burnetii was demonstrated experimentally in infected mice (377); however, this mode of transmission remains to be established in humans and wild animals.
Reservoirs
Q fever is a zoonosis with a worldwide distribution. The reservoir is large and includes many wild and domestic mammals, birds, and arthropods such as ticks (20). Babudieri, in a large review, reported that C. burnetii was detected in virtually all the animal kingdoms (20). However, domestic ruminants represent the most frequent source of human C. burnetii infection (212). Animals are often chronically infected but do not experience symptoms of C. burnetii infection. The uterus and mammary glands of females are sites of chronic C. burnetii infection (20). Shedding of C. burnetii into the environment occurs mainly during parturition; over 109 bacteria per g of placenta are released at the time of delivery (20). Milk may also contain large amounts of C. burnetii, although this is probably a minor route of Q fever acquisition.
Livestock.
Early studies have shown that coxiellosis in livestock is widespread. However, most seroepidemiological studies in livestock were performed in the 1960s, and in most areas the real prevalence of C. burnetii infection in such animals is currently unknown. Recent seroepidemiological studies available for cattle have indicated that C. burnetii antibody seroprevalence in these animals is higher nowadays than 20 or 30 years ago (175, 179). Cattle, goats, and sheep are considered the primary reservoirs from which human contamination occurs. Infected mammals shed C. burnetii in their urine, feces, milk, and birth products, from which humans may be contaminated. Although C. burnetii infection is usually not harmful in infected animals, abortions in sheep and goats (261, 390) and lower birth weight and infertility in cattle, have been associated with chronic C. burnetii infection (149, 319). Epidemiological data indicate that dairy cows are more frequently chronically infected than sheep and thus may represent the most important source of human infection. Studies performed in California in 1951 showed that when imported into an area of endemic infection, 40% of uninfected cows became C. burnetii infected within 6 months, as evidenced by seroconversion to C. burnetii antigens (156). Prolonged detection of specific antibodies in the sera of infected dairy cows and the longer shedding of C. burnetii in the milk of these animals compared to sheep have been reported (20, 38, 95, 179). Goats share a predisposition with dairy cows to remain chronically infected (179). Transmission of C. burnetii to humans from infected goats may be significant in areas where they replace cows as a source of milk.
Pets.
Cats and dogs may represent reservoirs of C. burnetii. Dogs may be infected by tick bite (198), by consumption of placentas or milk from infected ruminants, and by the aerosol route. C. burnetii infection in parturient dogs may lead to the early death of pups (44). The possibility of human Q fever acquired from infected dogs has been reported (44, 185, 205, 302), and we have isolated two strains from the uterus of Canadian dogs (282). Human Q fever cases were described in Nova Scotia after contact with parturient cats (171, 180, 205, 210); these included 12 patients who developed a febrile illness 2 weeks after playing poker in a room where a cat had given birth to kittens (171). All the infected persons had handled the cat or its litter, and specific antibodies were demonstrated in the cat serum.
Ticks and other arthropods.
In many animals, a transient bacteremia with C. burnetii occurs early after infection. Thus, ticks have the opportunity to become infected with C. burnetii during feeding. Over 40 tick species are naturally infected with C. burnetii, including Rhipicephalus sanguineus found in dogs (198), Haemaphysalis humerosa found in the marsupial bandicoot (334), Amblyomma triguttatum found in kangaroos (278), and several ticks collected in different parts of the United States. These included Dermacentor occidentalis, Amblyomma americanum, Haemaphysalis leporis-palustris, Ixodes dentatus, and Otobius magnini (63, 65, 71). Experimental transmission of C. burnetii from infected to uninfected guinea pigs via tick bite has been performed with Ixodes holocyclus, Haemaphysalis bispinosa, and Rhipicephalus sanguineus (271, 335, 336). Experimental infection with C. burnetii has also been performed in Dermacentor andersoni (262). C. burnetii multiplies in the cells of the middle gut or stomach of infected ticks. These arthropods expel heavy loads of C. burnetii with their feces onto the skin of the animal host at the time of feeding. Infection of tick ovaries has been demonstrated and may lead to germinative infection of the offspring, allowing C. burnetii infection to persist in the tick population (20). C. burnetii organisms in ticks, as in mammals, are in phase I and thus are highly infectious. However, ticks are not considered essential in the natural cycle of C. burnetii infection in livestock (20), and animals which live in close contact have many other opportunities to become infected with C. burnetii. In contrast, ticks may play a significant role in the transmission of coxiellosis among the wild vertebrates, especially in rodents, lagomorphs, and wild birds (20, 179, 207). Anecdotal reports indicate that C. burnetii may be isolated from other arthropods including chiggers (20), lice (121), and flies (274). However, other extensive investigations of lice, fleas, mites, flies, mosquitoes, and other arthropods collected from cows, sheep, and rodents have not resulted in isolation of C. burnetii from these arthropods. The role of these arthropods in the natural cycle of C. burnetii remains unknown. The possibility of C. burnetii being transmitted to humans via a tick bite has seldom been reported (92), and human Q fever as opposed to other rickettsial diseases is rarely if ever an arthropod-borne disease.
Others.
C. burnetii infection has been reported less frequently in a number of other domestic or wild mammals, including horses, rabbits, swine, camels, water buffalo, rats, and mice (20). A recent seroepidemiological study of rats in the United Kingdom has shown anti-phase II antibody seroprevalences ranging from 7 to 53% among wild brown rat populations (391). The authors hypothesized that wild rats may represent a major reservoir of C. burnetii from which domestic animals, especially cats, which are natural predators of these animals, may become contaminated. Birds may also be infected, and C. burnetii was isolated from pigeons, chickens, ducks, geese, and turkeys (20). Humans may acquire Q fever from infected domestic poultry by consumption of raw eggs or inhalation of infected fomites. Anti-C. burnetii antibodies have been found in snakes and tortoises in India, but C. burnetii has not been isolated from these animals (20).
Geographic Distribution
Q fever has been described in almost every country (20), with New Zealand remaining an exception (148). In most countries, Q fever is not included in the list of nationally notifiable diseases. Thus, its epidemiology may only be extrapolated from investigations of defined outbreaks, from serosurveys conducted in humans or in animals in some areas, or from data obtained from public health laboratories or reference laboratories for rickettsial diseases. In this review, only recently reported epidemiological situations are addressed.
France.
Although Q fever cases are reported to occur sporadically in various parts of France (365), the disease is diagnosed predominantly in the south, near Marseille. However, this probably reflects the influence of the presence of the National Reference Center (NRC) for rickettsial diseases in this area rather than a higher prevalence of the disease. In a serosurvey reported by Tissot Dupont et al. in 1992 (365) with 942 serum samples collected from blood donors in Marseille, specific anti-C. burnetii antibodies were detected in 38 samples, corresponding to a seroprevalence of 4.03 per 100 inhabitants. The highest prevalence reported in France was in a rural population in the Alps, with specific antibodies detected in 30% of the village population (43). Of the 22,496 serum samples tested at the NRC from January 1982 to December 1990, anti-C. burnetii immunoglobulin G (IgG) phase II was detected in 5,166 (23%) at titers of ≥1:25 and in 1,754 (7.8%) at titers of >1:200 (365). During the same period, 323 acute Q fever cases were diagnosed at the NRC. Among the 149 acutely infected Q fever patients whose occupation was known, only 9.4% were farmers and 29.8% lived in a rural area; contact with farm animals and ingestion of raw milk or unpasteurized cheese were rarely reported. However, among reported risk factors, contact with sheep or placenta from ewes or goats was predominant. Thus, Q fever is common in France. Since the rural population has decreased extensively over the last few decades, the disease is now often found in the urban population, often after occasional exposure to infected animals or potentially after ingestion of contaminated raw milk (365). Most cases are diagnosed in spring or early summer.
The incidence of acute Q fever is estimated at 50 per 100,000 inhabitants per year, and that of Q fever endocarditis is estimated at 1 per 106 inhabitants per year (365). Q fever represents 5% of cases of nationally diagnosed endocarditis (104). We have been involved in the investigation of three outbreaks over the last few years. An outbreak of Q fever was reported in the Alps after exposure to goats or consumption of milk from these animals (102). In 1996, an outbreak of 204 cases was diagnosed in Briançon, a town with 1,500 inhabitants. Investigations revealed an open abattoir in the center of the town to be the cause. Aerosols were apparently generated by helicopters landing close to the abattoir (367). More recently, a family outbreak was diagnosed and linked to pigeons (282).
United Kingdom.
From 1975 to 1995, 67 to 169 Q fever cases were reported annually to the Communicable Disease Surveillance Center by laboratories in England and Wales (364). This represents a stable incidence ranging from 0.15 to 0.35 case per 100,000 population per year. Among 641 Q fever cases reported between 1991 and 1995 in England, Wales, Northern Ireland, and the Channel Islands, Q fever most frequently involved adult men (485 cases versus 151 in women; sex ratio, 4:1) with a mean age of 46.2 years and occurred most frequently in May (364). Most cases were reported in Northern Ireland and southwestern England. In a series reporting 90 cases, endocarditis represented 11% of Q fever cases diagnosed (261). Q fever accounts for about 3% of all endocarditis cases in England and Wales (261).
Between 1980 and 1996, eight outbreaks in the United Kingdom were reported in the literature: 29 cases in Wales in 1982 in the community (312); 14 cases in southwestern England in the same year in laboratory staff after exposure to experimentally infected sheep (134); 25 cases in postal workers in Oxford in 1983 (411); 2 laboratory-acquired cases in Northern Ireland in 1986 (162); 5 cases in school students, presumably infected from school animals including poultry and goats, in southwestern England in 1987 (337); 147 cases in the Midlands and 47 cases in Northern Ireland in 1989 in the community (58, 162); and, more recently, 4 cases in 1992 on the Isle of Wight in waste disposal workers (12).
To determine the occupational risk of Q fever in farm workers, the prevalence and incidence of IgG anti-phase II C. burnetii were determined in a representative cohort of 404 farm workers and compared to the prevalence and incidence in a cohort of 395 police and emergency service personnel (362, 363). The prevalence was three times higher in the farm worker cohort than in the nonfarming populations (105 of 385 [27.3%] versus 43 of 395 [10.9%]). No seroconversion was observed among farm workers during the first year of the survey period, whereas two seroconversions occurred during the second year, representing an incidence of 813 per 100,000 per year. The presence of anti-C. burnetii IgG antibodies in patient sera was significantly associated with exposure to cattle and especially to pregnant animals or their birth products. However, the risk of acquiring Q fever was always dependent on total farm animal contact, suggesting a wide dissemination of C. burnetii in the farm environment. More recently, the prevalence of Q fever was established in a rural population in the west of Wales (69). Among 265 patients randomly selected from the “working population,” an overall low prevalence of 7.9% was found (IgG anti-phase II C. burnetii, >1:32 by indirect immunofluorescence assay [IFA]). However, antibody prevalence was 15.1% in farmers and 4.2% in persons working in sectors unrelated to farming. Consumption of unpasteurized milk and contact with mechanized milking were also risk factors for the presence of anti-C. burnetii antibodies, although these risk factors were usually associated with farming.
Spain.
From 1981 to 1985, 249 Q fever cases from different Spanish regions were serologically diagnosed at the Centro Nacional de Microbiologia, Virologia e Immunologia Sanitarias (357). Most cases were sporadic, although cases from two outbreaks (51 cases) were included in the study. These included 234 and 15 acute and chronic Q fever cases, respectively (6%), including 14 cases diagnosed as endocarditis. Most acute cases occurred in hospitalized patients with atypical pneumonia (75%) or a febrile illness (18%). Hepatic involvement was documented in 7.4 and 19% of patients with pneumonia or febrile illness, respectively. The majority of cases were in men (77.1%) between 15 and 44 years old, which supposedly reflected occupational risk.
The geographical distribution of cases showed that Q fever was diagnosed predominantly in Northern Spain, especially in the Basque and Navarra provinces, supposedly because of the greater cattle-raising activities in these areas (357). A large number of cases was also diagnosed in the Madrid area, although this may only reflect proximity of the reference center. The disease seems less prevalent in the central and southern regions of the country (357). Interestingly, the major clinical manifestation of acute Q fever seems to vary in different regions of Spain. Q fever most often presents as a pneumonia in the Basque region in northern Spain (238, 357), whereas hepatitis is predominant in Andalusia in southern Spain (299).
Q fever is strongly endemic in the Basque Country (238, 315). Many Q fever outbreaks have been recorded in the last 20 years in this region, representing over 300 cases (315). Montejo Baranda et al. (238) reported 130 Q fever pneumonia cases serologically diagnosed between June 1981 and June 1984. This is the largest series of Q fever pneumonia reported anywhere in the world. Of these cases, 76 were sporadic whereas 54 occurred in three epidemic outbreaks. Most cases were in men (94 cases; male/female ratio, 3:1). The majority of infected men and women (86.9%) were between 11 and 40 years old. Most cases were diagnosed during three different Q fever outbreaks, whereas 52 cases were sporadic and occurred in patients who had regular or occasional contact with cattle, sheep, or goats or who had ingested unpasteurized milk. A high seasonal variation in incidence was noted, with the majority of cases occurring between March and July, corresponding to the peak in the lambing season. Most patients presented with fever and headaches, often accompanied by myalgia, whereas respiratory symptoms and lung consolidation on chest X rays were recorded in 85 (65.4%) and 98 (75.4%) of patients, respectively. Elevated hepatic transaminase levels were recorded in 80 patients (61.5%). Evolution to chronic Q fever was not detected in any patients. Q fever is considered to be the second most common cause of community-acquired pneumonia in the Basque region, with Streptococcus pneumoniae being the first (238, 338). In a series of 164 cases, Sobradillo et al. (338) showed that Q fever was responsible for 18.8% of the cases of community-acquired pneumonia in the Basque region, with most cases occurring between January and June.
Switzerland.
Only 30 to 90 Q fever cases are reported annually to the Federal Office of Public Health in Switzerland. A large outbreak of Q fever occurred in the Val de Bagnes (Valais, Switzerland) in the autumn of 1983 (86). This outbreak was investigated after eight Q fever cases were diagnosed concomitantly at the Marigny hospital in patients who lived in the valley. Epidemiological investigation revealed that the outbreak had started 3 weeks after about 850 to 900 sheep descended from the alpine pasture and crossed several villages of the Val de Bagnes. Between October and December 1983, Q fever occurred in 21.1% of the more exposed population residing in villages situated in the lower part of the valley but in only 2.9% of the population of the higher villages away from the road crossed by the sheep. Altogether, Q fever was diagnosed serologically in 415 of the 3,036 inhabitants examined, including 240 men and 175 women. Most cases (224 of 415 [54%]) were asymptomatic, whereas more than 75% of the 191 patients with acute Q fever examined by physicians presented with prolonged fever, shivering, and headaches (84, 86). However, only 8 patients (4%) required hospitalization, and no Q fever endocarditis cases had been diagnosed in 1987 at the time of the outbreak. A high prevalence of antibodies to C. burnetii antigens was found (38%) in sera from 448 sheep examined.
Israel.
Q fever is endemic in Israel. Between 1981 and 1990, 758 Q fever cases were reported to the Ministry of Health (415). A series of 34 patients with Q fever endocarditis was reported more recently (332). Patients were from 25 to 74 years old, and most were males (23 of 34 [68%]). The national incidence of Q fever endocarditis in Israel was estimated to be 3.5 cases per year or approximately 0.75 cases per 1 million population per year. Possible exposure to cattle or sheep was recorded in 10 patients (29%), 8 of whom lived in rural areas.
Greece.
A study performed in 1990 by Alexiou-Daniil (7) in northern Greece demonstrated that 4.7% of 3686 patients with “atypical pneumonia” had antibodies against C. burnetii antigens. The high seroprevalences of anti-C. burnetii antibodies found in two villages in Crete (38.1% in 1987) encouraged physicians from the island to send sera to the National Reference Center of Parasitology, Zoonoses and Geographical Medicine in Heraklion (Crete) (372). From 1989 to 1993, 98 Q fever cases were diagnosed serologically by IFA. Most patients were men (72 of 98 [73.5%]). Patients aged 20 to 39 years and 80 to 89 years had a higher risk of acquiring Q fever. A seasonal occurrence was noted, with the majority of cases being diagnosed between January and June. Contact with animals or ingestion of unpasteurized milk or fresh cheese was recorded for 35.4% of the patients. Most patients presented with fever (91.7%) and respiratory symptoms (88.5%), whereas hepatitis was present in 52%. Interestingly, 11 patients (11.5%) presented with neurological symptoms and 2 (2.1%) had cutaneous rash.
Italy.
A large outbreak of Q fever occurred in the summer and autumn of 1993 near Vicenza in northeastern Italy (326). The outbreak followed the crossing of populated areas near Vicenza by several flocks of sheep on their way to higher prealpine pastures. Whereas only 3 Q fever cases were officially reported in the province of Vicenza between 1983 and 1992, 58 cases were diagnosed serologically by the complement fixation test during the 5-month study period. Most cases were men (sex ratio, 2.8:1). The majority of patients presented with fever (100%), weakness (81%), headaches (76%), and chills (72%). Cough was recorded in 47% of patients, whereas abnormalities were present in 39 (81%) of the 48 chest X-rays performed. Hospitalization was necessary in 48% of patients. The only risk factor for acquisition of Q fever in this population was exposure to the migrating flocks of sheep. Of the 100 flocks investigated, 30 were found to be infected with C. burnetii, with seroprevalences ranging from 12 to 55% in a given flock.
Germany.
Q fever is a notifiable disease in Germany, and 27 to 100 cases are reported annually (13). In May 1996, a Q fever outbreak occurred in Rollshausen and five surrounding towns in the district of Lohra (13, 194). In this rural area, two flocks of sheep (1,000 to 2,000 and 20 animals, respectively) had been kept near Rollshausen before the Q fever outbreak. Lambing occurred in December 1995 and January 1996. The Robert Koch Institute was invited to investigate the outbreak. A retrospective cohort study was conducted in Rollshausen residents who were older than 15 years of age. Sera from 200 inhabitants were tested by enzyme-linked immunosorbent assay for the presence of anti-C. burnetii antibodies. Of the 200 residents, 45 (23%) were considered to be infected with C. burnetii on the basis of clinical and/or serological investigation. The attack rates were similar in men and women and in the different age populations. Most patients suffered from fatigue (80%), fever (78%), malaise (76%), and chills (71%). All 35 symptomatic patients had pneumonia confirmed by chest X rays, and 4 (11%) were admitted to hospital. Living near the flocks of sheep was significantly associated with an increased risk of acquiring Q fever, and the predominant mode of contamination was considered to be by air. Anti-C. burnetii antibodies were found in the sera of 15 of 20 sheep from the largest flock investigated.
Russia.
Official Russian statistics indicate that between 1957 and 1995, 11,058 Q fever cases were reported in 37 administrative territories, including 39% in Povolzhje, 31% in West Siberia, and 14% in central Chernozemje, mostly in the regions of Astrakhan, Novosibirsk, and Voronezh (309). However, Q fever is underreported in Russia because of diagnostic difficulties and insufficient laboratory equipment. As in other countries, cattle, sheep and goats are the main reservoirs from which humans become contaminated, especially at the time of parturition. Q fever cases acquired from goat and sheep reservoirs have been reported over recent years mainly in the European and Asiatic parts of Russia, including outbreaks in the regions of Novosibirsk, Voronezh, and Altai. Reports of goats as sources of Q fever in Russia have increased over recent years, mainly due to their increased number.
United States.
The prevalence of C. burnetii infection in humans and animals is poorly defined in the United States. The first major Q fever outbreaks were reported in 1946 in packing houses in Amarillo, Tex. (371), and in Chicago, Ill. (328). Studies performed between 1947 and 1950 (27, 56, 371) showed that California was an area of endemic infection for Q fever. Between 1948 and 1977, a total of 1,169 human Q fever cases were reported to the Centers for Disease Control (67), including 785 (67%) from California. Fewer than 30 cases were reported annually between 1978 and 1986 (318).
A cat-associated Q fever outbreak occurred in 1989 in Goldsboro, Maine (276). Fifteen members of one family developed acute Q fever after exposure to a parturient cat that delivered in the family home about 2 weeks before the first Q fever case was diagnosed. Of the 15 family members, 11 presented clinical manifestations compatible with acute Q fever, including fever, headache, and myalgia in all cases. All members reported contact with the parturient cat, whereas none reported recent contact with cattle, sheep, goats, or rabbits. Interestingly, this outbreak occurred in Maine, a state near maritime Canada, where the majority of cat-related Q fever outbreaks have been described (171, 180, 210).
Nova Scotia.
Q fever was first reported in Nova Scotia in 1981 (204). The disease was unexpectedly diagnosed while investigating the usual causes of atypical pneumonia in this province of Canada (204). Marrie recorded 174 Q fever cases between 1980 and 1987 (208). The mean age of the 174 patients was 40.1 years (212), although most cases occurred in patients aged from 20 to 49 years. The sex ratio (men/women) was 2:1. Most Q fever cases recently reported were acquired from exposure to parturient cats (171, 180, 210). Exposure to infected dogs, wild hares, and deer has also been reported as a risk factor (44, 185, 207). Q fever cases are not seasonal in Nova Scotia (220). Eleven Q fever endocarditis cases were diagnosed between 1979 and 1993 (220) in Nova Scotia, which represents an incidence of 0.73 per million inhabitants per year. C. burnetii is the etiological agent of approximately 3% of all endocarditis cases in this province of Canada (213).
Active surveillance of the role of Q fever as an etiological agent of community-acquired pneumonia exists in Nova Scotia (204, 218), a situation which is extremely rare worldwide. The most recent investigation (218) was performed in 149 non-human immunodeficiency virus (HIV)-infected patients presenting with atypical pneumonia. An etiological diagnosis was established in 49.7% of the patients. The most frequent etiological agents were Mycoplasma pneumoniae (22.8% of cases), Chlamydia pneumoniae (10.7%), both (3.4%), C. burnetii (2.7%), Chlamydia psittaci (1.3%), Legionella spp. (0.7%), and influenza A virus (2.7%). Thus, C. burnetii was the third most frequently recognized bacterial etiological agent of “atypical pneumonia” in Nova Scotia and was more frequently diagnosed than psittacosis and legionellosis.
Japan.
Serosurveys have shown that Q fever is endemic in animals in Japan (154, 241, 414, 420). C. burnetii was first isolated from a patient’s blood in 1989 (253). In 1996, To et al. (368) retrospectively investigated the role of C. burnetii in children with “atypical pneumonia”. Acute-phase sera collected between 1982 and 1983 in Gifu Prefecture from 58 children (from 2 to 10 years old) were tested for the presence of phase II C. burnetii antibodies and for the presence of C. burnetii DNA by a PCR-based assay. Specific IgM antibodies were found in 20 of 58 patients (34.5%) who were diagnosed with Q fever pneumonia, whereas IgG antibodies were found in 7 of 58 (12%). The PCR-based assay was positive in 23 of 58 patients (39.6%). In all patients, C. burnetii infection was confirmed by infection of mice with the patient serum samples. Since convalescent-phase sera were not available, diagnosis was essentially based upon PCR. In our experience, PCR-based methods to diagnose Q fever are not adequate for blood samples, and results obtained with such a technique should be interpreted with caution (282). In the same year, Nagaoka et al. (250) reported the isolation of C. burnetii in sera from children with influenza-like symptoms. Paired sera (acute phase and convalescent phase) collected from 1992 to 1993 in 55 schoolchildren (7 to 11 years old) in Shizuoka Prefecture during an influenza epidemic were examined for the presence of phase II C. burnetii antibodies by IFA. Of the 55 convalescent-phase sera, 18 (32.7%) were positive. C. burnetii was isolated from 13 children by inoculation of acute-phase serum samples into mice. Thus, both studies indicate that Q fever may be widespread and present at a high incidence in Japan. The prevalence of Q fever among children with “atypical pneumonia” (i.e., 39.6%) (368) and those with influenza symptoms (i.e., 32.7%) (250) seems high and should be confirmed by further studies.
Yuasa et al. (422) have recently reported the first cases of chronic Q fever in Japan. Q fever endocarditis was retrospectively diagnosed in patients with culture-negative endocarditis by using PCR to detect C. burnetii in paraffin-embedded endocardial and liver tissue removed from these patients. The high prevalence of C. burnetii infection in animals in Japan suggests that the prevalence of human Q fever will probably increase in this country. In 1993, Htwe et al. (153) studied 626 human serum samples collected from 1978 to 1991 for the presence of phase II C. burnetii antibodies by IFA. The overall seroprevalence was 16.5%, but the value was 22.5% in 275 veterinarians, 11.2% in 107 meat-processing workers, 15.2% in 184 respiratory disorder patients, and only 1.6% in 60 healthy controls. Thus, Q fever is probably common in Japan, as in most other countries, in individuals in contact with animals and animal products.
Australia.
Since the description of Q fever by Derrick in 1937 in abattoir workers in Brisbane (Queensland), the disease has continued to be prevalent in Australia. Q fever has been a notifiable disease in Australia since 1977. In 1982, Spelman (341) reported 111 consecutive Q fever cases occurring between June 1962 and June 1981. All but one were in males. The only woman in the series may have been infected outside Australia, since she presented symptoms 6 days after returning from a 3-month holiday in Greece. Most of the infected men (102 of 110) had recently worked in abattoirs. Most of the infected patients presented with an acute febrile illness (81 of 111 [72.9%]), but prolonged fever was recorded in 18 (16.2%), “atypical pneumonia” was found in 8 (7.2%), and acute hepatitis was found in only 3 (2.7%). Only one patient (0.9%) was diagnosed with Q fever endocarditis.
Between 1977 and 1994, 202 to 860 cases were reported annually despite the development of an effective vaccine, which had been available since 1989 (114). Garner et al. (114) have reviewed national notifications of Q fever which were made between 1991 and 1994 in Australia; this is the most recent evaluation of Q fever epidemiology in Australia. Data was collected by the National Notifiable Diseases Surveillance System (2,635 records) and the Laboratory Virus and Serology Reporting Scheme (1,407 records). Most cases were recorded from Queensland and New South Wales, and no cases were recorded from northern Tasmania. The national notification rates ranged from 3.11 to 4.99 per 100,000 population in the study period. Notification was 2.4 times greater in Queensland than in New South Wales and 8 times greater than in southern Australia.
This data shows that Q fever usually affects adult males (sex ratio, 5:1) aged from 20 to 50 years, has no apparent seasonal incidence, and is found predominantly in eastern Australia, especially in southern Queensland and northern New South Wales. Q fever activity is still significantly associated with livestock and the meat industry. The incidence of chronic Q fever is unknown in Australia, and only five deaths attributed to Q fever were reported between 1982 and 1994. As in many other countries, Q fever is probably underreported in Australia because diagnosis of the disease often remains based upon the complement fixation test, which has poor sensitivity (270).
New Zealand.
A seroepidemiological study was conducted in 2,181 cattle and 12,556 sheepdogs in New Zealand in 1993 (148). Sera collected from these animals were examined for the presence of anti-C. burnetii antibodies. Since cattle and sheep are the major ruminant population of New Zealand, the study was considered to be reliable indicator of the presence of C. burnetii in this country. All sera tested were negative, and the authors argued that New Zealand may be considered to be free from coxiellosis and thus from human Q fever.
Incidence and Seasonal Variation
Although the number of Q fever cases reported in humans may vary from one area to another, this may not accurately reflect the true incidence of the disease, especially since the clinical manifestations of the disease are often nonspecific or even absent. Of note, the incidence of Q fever is often very high in areas where rickettsiologists are working (75). In Europe, acute Q fever cases are more frequently reported in spring and early summer (365). The maximum incidence of Q fever during spring is supposedly related to the “outside” lambing season, which corresponds to heavy contamination of the environment with C. burnetii. The “major” lambing season in October is not related to a higher incidence.
Sex and Age of Host
Q fever is usually an occupational hazard. Persons at greatest risk are those in contact with farm animals and include farmers, abattoir workers, and veterinarians. Laboratory personnel are also at risk for Q fever infection, especially when manipulating animal or human products containing phase I C. burnetii. However, persons in contact with pets (e.g., cats and dogs), especially when they give birth, are also at risk. The seroprevalence of Q fever was found to be three times higher in HIV-positive patients than in blood donors (295), suggesting higher susceptibility or higher exposure of this population to C. burnetii infection. The fact that the population investigated consisted mainly of drug addicts also suggests the possibility of blood transmission. Patients at risk for chronic Q fever include those with previous valvulopathy (75, 286, 299, 300), immunocompromised patients (294, 295), and pregnant women (297).
The sex ratio and age of infection with C. burnetii may vary from one area to another according to the most predominant animal reservoir from which patients become contaminated and the opportunity for exposure to these infected animals. In areas where exposure to infected cattle is the predominant risk factor for Q fever, including most European countries, California, and Australia (69, 114, 238, 315, 341, 357, 362, 363), the disease most often supervenes in the active population of from 30 to 60 years old and more frequently in men. In contrast, exposure to parturient cats is the primary mode of C. burnetii contamination in Nova Scotia, and a sex ratio of 1:1 was reported in this province of Canada (216). However, the sex ratio of patients with Q fever illness may not accurately reflect the risk of C. burnetii contamination. Of the 323 hospitalized patients with acute Q fever diagnosed in France from 1982 to 1990, infection was more frequently reported in men than in women (sex ratio, 2.5:1) (365). However, among 942 sera collected from blood donors in the same country (365), anti-C. burnetii antibodies were detected in 38, with a sex ratio of 1:1. Thus, although men and women may have been at equal risk from C. burnetii contamination, Q fever was more severe in men than in women.
PATHOGENESIS AND PATHOLOGY
Humans
The aerosol route is the principal mode of acquisition of C. burnetii infection in humans (20). Ingestion of high doses of C. burnetii via the digestive route (especially by consumption of contaminated dairy products) is considered a rare alternative for acquiring infection (102). The incubation period of acute Q fever may range from 1 to 3 weeks, depending on the C. burnetii-inoculating dose (211). In the majority of cases, C. burnetii infection remains asymptomatic or presents as a nonspecific flu-like illness; thus, the disease remains undiagnosed (86). Acute Q fever has two primary clinical presentations: atypical pneumonia and hepatitis. It has been hypothesized that the route of acquisition C. burnetii infection may influence the clinical presentation of the disease in a particular area. Pneumonia is the primary clinical manifestation of acute Q fever in Nova Scotia, where C. burnetii infection is supposed to occur predominantly via contaminated aerosols from infected cats (210). In contrast, ingestion of raw milk supposedly plays a more significant role in many parts of Europe, where acute Q fever manifests mostly as a granulomatous hepatitis (102). Most infected patients, however, will experience a transient bacteremia with C. burnetii, usually late in the incubation period. Thus, whatever the mode of acquisition of C. burnetii, hematogenous spread of the pathogen may lead to involvement of other organs including the liver, spleen, lungs, bone marrow, and female genital tract. Life-threatening complications may occur, including meningoencephalitis, myocarditis, or pericarditis. C. burnetii infection is usually controlled by the T-cell immune response. However, it is likely that cell-mediated immunity usually does not lead to eradication of C. burnetii from the infected host. The possibility of Q fever resurgence has been documented in patients with acquired immunosuppression, including those with cancers, lymphomas, or HIV infection, and in pregnant women (286, 294, 295, 297, 350). In patients with previous valvulopathy (290) and, to a lesser extent, in pregnant women (350) and immunocompromised patients (138, 286, 294, 295), Q fever may become a chronic and often spontaneously fatal disease. Chronic Q fever may be defined by a clinical evolution lasting longer than 6 months, and is biologically characterized by the presence of IgG and IgA to phase I C. burnetii antigens (263). Q fever endocarditis is the most frequent clinical manifestation of chronic Q fever. Typically a disseminated disease, it is often associated with multiorgan involvement including chronic hepatitis. Less frequently, chronic infections of vascular aneurysms or prosthesis (40, 93, 97, 105, 299), chronic osteomyelitis (61, 93, 277) and osteoarthritis (267, 285), lung tumors (161, 191), pneumonic fibrosis (3), and chronic hepatitis without endocarditis (397, 419) have been described.
Pathological lesions of Q fever in humans have been previously described in organ biopsy specimens or in autopsy specimens performed for diagnostic purposes (214). Liver biopsy specimens were frequently obtained in patients with hepatitis. In contrast, because Q fever pneumonia is rarely fatal, pathological descriptions of the disease in humans are scarce. Organ biopsies are no longer performed for Q fever diagnosis which may be established accurately by serological methods. Typical pulmonary histopathological lesions in patients with Q fever pneumonia correspond to a gross consolidation, microscopic interstitial pneumonia, and alveolar exudates (189, 268, 412). Interstitial infiltrates are composed of mostly macrophages and lymphocytes and to a lesser extent polymorphonuclear leukocytes (161, 268). Fibrin and erythrocytes, together with mononuclear cells, are found in alveolar exudates. Such pathological findings are not specific to Q fever pneumonia and may be encountered with other etiological agents of atypical pneumonia, including Legionella pneumophila, Chlamydia pneumoniae, and Chlamydia psittaci. By using specific antibodies, C. burnetii may be revealed intracellularly within alveolar macrophages.
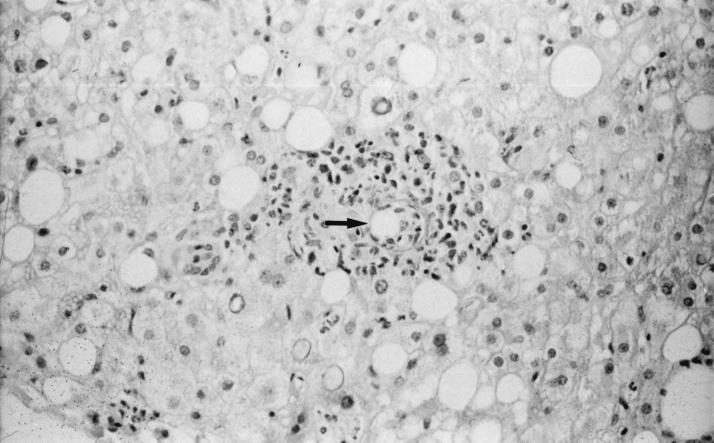
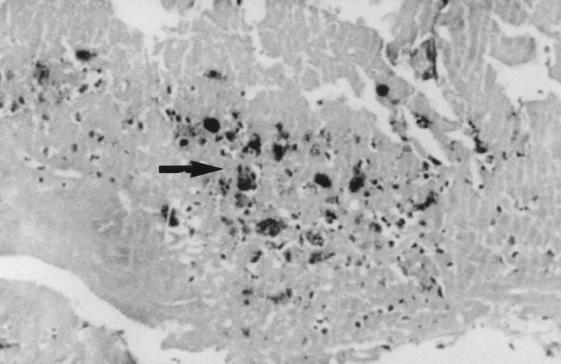
Because a granulomatous hepatitis is the most frequent indication of the presence of C. burnetii in the liver (33, 72, 83, 117, 150, 265, 275, 281, 333, 374, 386, 392), Q fever hepatitis was frequently confused with tuberculosis. Other less specific hepatic lesions, including portal triaditis, Kupffer cell hyperplasia, and moderate fatty change, have been described. Kupffer cells are considered to be the target cells for C. burnetii infection in liver tissue. This may initiate local inflammation and the formation of granulomas. Histological examination of liver tissue sections reveal the presence of focal hepatocellular necrosis and cell infiltrates composed of macrophages, lymphocytes and polymorphonuclear leukocytes. Macrophages with epithelioid morphology, multinucleated giant cells, and fibrin may also be present. A characteristic feature is noted when a central clear space and a fibrin ring are observed within the granuloma or at its periphery, which is referred to as a doughnut granuloma (83, 124, 265, 281, 343) (Fig. 2). Although doughnut granulomas have occasionally been found in patients with Hodgkin’s disease, typhoid fever, cytomegalovirus infection, infectious mononucleosis, or allopurinol hypersensitivity, it is considered to be characteristic of Q fever. C. burnetii is not generally detected in the liver, although isolation or visualization by direct immunofluorescence of bacteria has occasionally been successful. Although liver involvement is often associated with Q fever endocarditis, granulomas have rarely been reported in chronically infected patients and the typical doughnut granulomas have never been reported (290, 397). The predominant histological pattern of the liver in chronic Q fever patients corresponds to a nonspecific reactive hepatitis with lymphocytic infiltration with foci of spotty necrosis (161). The lack of T-cell immune response in chronically infected patients may explain the absence of typical granuloma.
FIG. 2.
Doughnut granuloma (arrow) in a liver section from a patient with acute Q fever hepatitis. Hematoxylin-phloxin-saffron stain. Magnification, ×250.
Bone marrow lesions usually correspond to granulomas similar to those found in the liver (59, 74, 150, 254, 333, 343, 386). Doughnut granulomas with macrophages, lymphocytes, polymorphonuclear leukocytes, and multinucleated giant cells surrounding a central clear space and a fibrinoid ring may also be found in bone marrow. Indeed, liver and bone marrow granulomas are concurrently present in many Q fever patients (150, 333, 386).
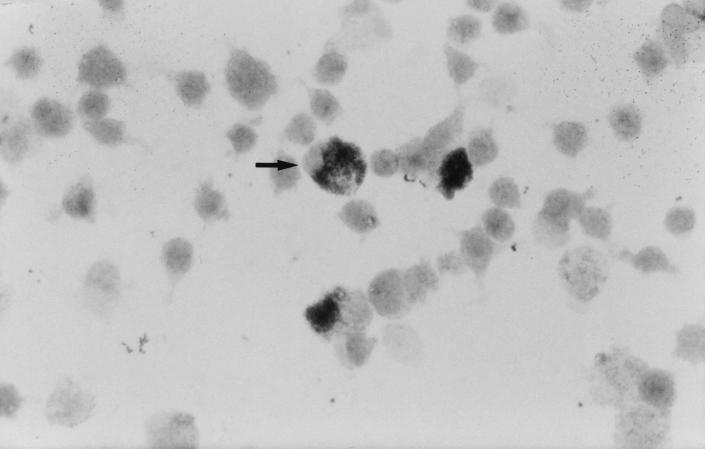
Q fever endocarditis usually involves the aortic and mitral valves, although prosthetic valve endocarditis is increasingly reported (98, 240, 307, 332). The typical vegetation is small and often difficult to visualize by transthoracic echocardiography. Gross examination of cardiac valves may reveal the infectious vegetation with variable destruction of the relevant cardiac valve. Perforation of valvular cusps and the Valsalva sinus and the formation of aneurysms at the site of the valvular annulus, the membranous portion of the interventricular septum, or the subvalvular myocardium have occasionally been described. Histological findings in the infected cardiac valve are nonspecific. They include the presence of thrombi composed of fibrin and platelets, necrosis and necrotic cellular debris, foci of calcification or ossification, and fibroblasts laying down collagen (11, 40, 41, 60, 96, 369, 374, 409). Inflammatory-cell infiltrates are mainly composed of lymphocytes, histiocytes, and occasionally plasma cells. Foamy macrophages are frequently observed. Focal microabscesses may also be found. C. burnetii may be visualized in the infected valve by immunohistochemistry (41). C. burnetii is found intracellularly as coccoid organisms, clustered as a single intracytoplasmic mass, almost exclusively in foamy macrophages and in histiocytes (41). Arterial emboli from the vegetation may cause infarcts, especially in the spleen, kidneys, and brain. Q fever endocarditis may also induce circulating immune complex-related complications, including immune complex glomerulonephritis.
Naturally Infected Animals
C. burnetii-infected animals do not usually experience symptomatic C. burnetii infections. In the acute phase, the presence of C. burnetii may be demonstrated in the blood, lungs, spleen, and liver. Most animals remain totally asymptomatic, including a lack of fever. C. burnetii infection often becomes chronic, with persistent shedding of C. burnetii in feces and urine. However, animals do not develop chronic endocarditis as is observed in humans. The female uterus and mammary glands are primary sites of chronic C. burnetii infection (20). Thus, the shedding of C. burnetii into the environment occurs mainly during parturition. Birth products, mainly the placenta, are heavily contaminated with C. burnetii. C. burnetii can also be recovered from milk. The only pathological manifestations that have been associated with chronic C. burnetii infection in animals are abortion, mainly in sheep and goats (260, 390), and lower birth weight and infertility in cattle (149, 319).
Laboratory Animals
Animal models of acute Q fever.
C. burnetii infection may be induced in a number of laboratory animals, including mice, rats, rabbits, guinea pigs and monkeys. C. burnetii infection in laboratory animals may be totally asymptomatic, induce fever and granuloma formation, or be fatal, especially when heavy C. burnetii inocula are used.
(i) Mice.
Mice injected intranasally or intraperitoneally with C. burnetii usually remain asymptomatic, including a lack of fever (179). However, these animals develop granulomatous lesions with mononuclear cells in the spleen, liver, kidneys, and adrenals (45, 269). Numerous bacteria are seen in the liver and spleen. Granulomatous lesions persist for several weeks or months. Mice remain chronically infected, most often with persistent shedding of C. burnetii in feces and urine. The possibility of resurgence of latent infection was demonstrated in white mice receiving steroids 3 months after C. burnetii infection (331). The same effect was obtained after whole-body irradiation of previously infected white mice (330). In contrast, reactivation of C. burnetii infection did not occur in previously infected deer mice, suggesting natural resistance of this species to chronic C. burnetii infection (330, 331). The role of immunity in the evolution of Q fever has also been assessed in mice. Euthymic mice resist phase I C. burnetii infection, whereas athymic mice become chronically infected (170). The route of C. burnetii inoculation in mice may determine various pathological manifestations. In a study reported by Marrie et al. (219), BALB/c mice were injected intraperitoneally or intranasally with four C. burnetii strains: MPZ, obtained from a human placenta; NSCI, obtained from a cat uterus; Q229, obtained from a human endocarditis case; and Nine Mile phase I and II. Liver and spleen enlargement were visible only in intraperitoneally injected mice. Hepatic granulomas were predominant in intraperitoneally injected animals, although they were also visible in those infected intranasally. Likewise, interstitial alveolitis was predominant in intranasally infected mice.
(ii) Guinea pigs.
Guinea pigs were used extensively for C. burnetii isolation before cell cultures were available (179). They were preferred for the isolation of C. burnetii from infected samples because C. burnetii infection in mice is often asymptomatic. Mature animals (weighing 550 to 650 g) were used because they have a more uniform response to C. burnetii infection. Guinea pigs may be infected intranasally or intraperitoneally. C. burnetii infection is indicated in these animals by hyperthermy (≥40°C) 1 to 2 weeks following inoculation. Unless the initial C. burnetii inoculum is high (>106 infecting units), all animals recover from C. burnetii infection within 2 to 3 weeks without sequelae. Animals are bacteremic for 5 to 7 days following C. burnetii challenge; the bacteremia is then cleared as specific antibodies are detected. Phase II antibodies are detected 15 days postinfection or later, and both phase I and phase II antibodies are found within the second month of infection. Pathological changes in different organs are found a few days after C. burnetii challenge and persist for 2 to 3 weeks (190). The spleen and mesenteric lymph nodes may be enlarged. Histological examination of lung tissue specimens shows the presence of mononuclear cell infiltrate, whereas granulomas are found in liver, spleen and bone marrow specimens. Such pathological changes regress during convalescence, without sequelae. Infected guinea pigs often remain latently infected, as evidenced by the resurgence of the disease on induction of immunosuppression with cyclophosphamide, whole-body irradiation, or steroids (17, 330, 331). Thus, the natural course of C. burnetii infection in guinea pigs corresponds to an acute illness with formation of granulomas in the liver, spleen, bone marrow, and other organs and with rapid regression of clinical signs and clearance of granuloma. This type of evolution closely mimics acute Q fever disease in humans, and experimental models with guinea pigs may therefore be more relevant than mouse models.
In a recent experiment, the influence of the route of C. burnetii administration on pathological lesions was assessed (183). Cell infiltrates in the lungs were predominant when the intranasal route was used as compared to the intraperitoneal route. Granulomas were more frequently observed in liver sections when the intraperitoneal route was used. On the other hand, only animals challenged with high doses of C. burnetii (105 IU) displayed the histological lesions of myocarditis. These results suggest that both the route of C. burnetii inoculation and the dose of the C. burnetii inoculum may have influenced the pathological lesions in infected guinea pigs.
(iii) Other animals.
C. burnetii infection in hamsters, rabbits, and monkeys has been less frequently studied. Hamsters are considered to be easy targets for infection, with high yields of bacteria in their spleens (352). Rabbits have seldom been used for C. burnetii isolation. Pregnant females infected with C. burnetii often deliver dead fetuses. The pathogenesis of C. burnetii infection was studied in cynomolgus monkeys (122). Animals infected by the aerosol route developed pneumonia with fever, cough, and dyspnea 4 to 7 days postinfection. Bacteremia was demonstrated between days 7 and 13 following C. burnetii challenge, and pathological examination of lung and liver tissue revealed the presence of interstitial pneumonia and subacute hepatitis. The presence of C. burnetii was demonstrated in lung, spleen, liver, kidney, heart, and testis sections. Phase II and phase I anti-C. burnetii antibodies were detected by IFA on days 7 and 14 postinoculation, respectively.
(iv) Embryonated eggs.
Since Cox et al. (64) demonstrated that C. burnetii could be grown in the yolk sac of chicken embryos, this culture system has been extensively used for C. burnetii isolation and propagation (256, 395). C. burnetii infection leads to death of the embryo within 14 days. However, although phase I C. burnetii organisms are recovered from infected animals, bacteria collected after several passages in embryonated eggs are in phase II.
Animal models of chronic Q fever.
Animals infected with C. burnetii never develop endocarditis spontaneously, whatever the strain used. The role of immunity in the prevention of the evolution of Q fever into a chronic disease has been demonstrated in mice and guinea pigs. Athymic mice systematically develop a chronic disease (170). Chronic infection can also be established in guinea pigs receiving steroids (331) or after whole-body irradiation (330). Four animal models of Q fever endocarditis have recently been developed. The disease was established in immunocompromised mice (17), pregnant mice (351), rabbits with intracardiac catheters (239), and guinea pigs with cardiac valves previously damaged by electrocoagulation (181).
The first model was developed in mice immunocompromised with cyclophosphamide (17). Mice were infected intraperitoneally with high doses of C. burnetii, and their hearts were removed within the following 15 days, at the time of spontaneous death or after sacrifice. Histopathological examination of the removed organs revealed disseminated C. burnetii infection, including involvement of heart valves. Pathological manifestations, including cardiac valve involvement, resolved in surviving animals after several weeks, when immune system suppression due to cyclophosphamide had regressed.
C. burnetii endocarditis was established by using the rabbit experimental model of Garrison and Freeman (115). Briefly, the introduction of a catheter into the left ventricle induced aortic valve lesions and the formation of thrombotic vegetation. The rabbits were then injected intraperitoneally with a C. burnetii inoculum, which led to colonization of the thrombotic vegetation with bacteria and establishment of Q fever endocarditis.
Catheter-induced endocarditis does not, however, reproduce the pathophysiology of Q fever endocarditis in humans, which most often occurs in damaged native valves. More recently, a native-valve endocarditis model was developed in guinea pigs, in which aortic valve lesions were induced by electrocoagulation before C. burnetii challenge (181). This model did not therefore use an intracardiac catheter for induction of the formation of thrombotic vegetation. Of 20 guinea pigs, with damaged aortic valves, 10 (50%) developed endocarditis 7 to 10 days following C. burnetii challenge. C. burnetii endocarditis was documented by the presence of positive blood cultures for C. burnetii, characteristic histological lesions in heart valves, and culture of C. burnetii from valve tissue. The most important limitation of the cyclophosphamide-treated mouse and guinea pig models was that most infected animals died soon after C. burnetii challenge. These models must be improved in order to better reproduce the natural long-lasting disease observed in chronically infected humans. In particular, anti-phase I C. burnetii antibodies which are characteristic of chronic disease in humans, were not detected in animals with Q fever endocarditis.
A fourth model was recently developed in pregnant mice (351). The mice were inoculated intraperitoneally with C. burnetii Nine Mile. Although infected mice remained well and continued to feed normally, chronic C. burnetii infection was revealed by the birth of underweight offspring. Surprisingly, examination of valves of sacrificed mice showed endocarditis lesions.
Immune System Modulation
T-cell immunity is effective in the control of Q fever, although in most cases, it does not completely eradicate bacteria; some patients therefore experience a chronic infection. The intracellular survival of C. burnetii and establishment of persistent infection probably correspond to subversion of microbicidal functions of macrophages, as well as impairment of the T-cell immune response (233). In vitro experiments have shown that the natural phase I C. burnetii resists the killing action of monocytes-macrophages (233). First, it may resist the microbicidal effects of reactive oxygen intermediates through the action of oxygen scavengers such as superoxide dismutase and catalase (6). However, C. burnetii does not induce the release of reactive oxygen intermediates by monocytes and macrophages in vitro (233). Surprisingly, virulent phase I C. burnetii strongly stimulates the synthesis of tumor necrosis factor (TNF) by human monocytes (50). This usually increases the microbicidal potential of infected monocytes and thus may restrict the intracellular growth of C. burnetii. However, TNF-α may also enhance ingestion of the poorly internalized phase I C. burnetii by monocytes-macrophages by upregulating adhesion receptors on these cells (233).
The down modulation of macrophage responses to lymphokines may also allow C. burnetii to survive within these cells (233). However, the priming of microbicidal functions of mouse fibroblasts by gamma interferon (IFN-γ) in vitro limits the intracellular multiplication of C. burnetii (376). C. burnetii-infected monocytes cannot be primed by IFN-γ for oxidative metabolism (233). Recent experimentations have shown that IFN-γ promotes the killing of C. burnetii in THP-1 monocytes through an apoptotic mechanism mediated in part by TNF (73). A/J mice, which are defective in IFN-γ-mediated priming for cytotoxicity, are highly susceptible to C. burnetii infection (323). Impairment of protective T-cell responses by C. burnetii may correspond to a decrease in specific T lymphocytes, down-modulation of Th1 lymphokines (e.g. interleukin-2 [IL-2] and IFN-γ), and induction of suppressor factors (233). Q fever endocarditis patients exhibit CD4+-T-cell lymphopenia which preferentially affect unprimed lymphocytes (310). Peripheral blood lymphocytes (PBL) from patients convalescing from or with active acute Q fever disease manifest a marked proliferative response when cultured in vitro with C. burnetii antigens (173). A lymphoproliferative response is also noted in persons early after vaccination with formalin-inactivated C. burnetii (16, 157). In contrast, PBL from Q fever endocarditis patients do not proliferate in the presence of C. burnetii antigen, whereas they still proliferate in the presence of Candida albicans antigens or mitogens (172). Prostaglandins may suppress T-cell-mediated immunity against C. burnetii. Large amounts of prostaglandin E2 are produced in response to C. burnetii by peripheral blood mononuclear cells (PBMC) from patients with endocarditis. Cytokines such as transforming growth factor β and IL-10 are produced in excess by PBMC and monocytes from patients with Q fever endocarditis. Moreover, the increase in IL-10 production is associated with the Q fever relapses (49).
Induction of suppressive mediators by monocytes may result from the action of specific bacterial determinants. Monocytes and T cells from vaccinated individuals with phase I C. burnetii corresponded to only residual production of IFN-γ, whereas phase II C. burnetii and pretreatment of phase I LPS with periodate (leading to artificial phase II LPS) strongly stimulated IFN-γ production (158). It was hypothesized that LPS probably promotes the suppression of IFN-γ production by PBL from patients with Q fever endocarditis by masking bacterial determinants critical for lymphokine production (233).
In conclusion, the data currently available for humans and animal models leads to several hypotheses regarding the pathogenesis of Q fever (Table 1). Acute Q fever in humans may range from asymptomatic infection to fatal disease; symptomatic patients may present with various clinical manifestations such as pneumonia, hepatitis, encephalitis, or myocarditis. Four contributing factors may explain such variation in clinical presentation of Q fever: (i) the route of C. burnetii infection, including the aerosol or digestive routes; (ii) the C. burnetii inoculating dose; (iii) the infecting C. burnetii strain, which may present various virulence potentials; and (iv) host factors, including the immune status of the infected patient. In both the mouse and guinea pig models, the route of infection determines the predominant clinical and pathological manifestations of Q fever (183, 219). The route of C. burnetii inoculation in humans may also determine in part the predominant clinical manifestation, with pneumonia being the most frequent clinical manifestation when C. burnetii infection occurs predominantly via contaminated aerosols (210) and granulomatous hepatitis being predominant when transmission occurs via ingestion of raw milk (102). In the guinea pig model, only the animals given a high C. burnetii inoculum developed myocarditis (183); and in humans, the severity of acute Q fever has also been linked experimentally to the dose of the infecting C. burnetii inoculum (183). We may also hypothesize that geographical variations in the clinical expression of acute Q fever may be related to genetic C. burnetii strain variations, but this has not yet been established. Finally, host factors, especially the immune status of infected patients, may influence the course of C. burnetii infection, including evolution to chronic disease (286).
TABLE 1.
Current hypothesis on pathogenesis of Q fever from clinical and experimental data
| Pathogenesis factor | Data in humans and experimental animals | Reference(s) |
|---|---|---|
| Acute Q fever (factors influencing clinical manifestations) | ||
| Host factors | Unknown | |
| C. burnetii strain | Unknown | |
| Route of infection | Aerosol versus intraperitoneal inoculation in BALB/c mouse and guinea pig models | 183, 219 |
| Inoculum dose | Myocarditis in guinea pigs | 183 |
| Chronic Q fever (factors influencing evolution to chronic Q fever) | ||
| Host factors | ||
| Immunosuppression | Patients with acquired immunosuppression (cancers, lymphomas, or HIV infection) | 138, 286, 294, 295 |
| Persistent infection in athymic mice | 170 | |
| Reactivation of infection with steroids or whole-body irradiation in mice and guinea pigs | 330, 331 | |
| Endocarditis in mice receiving cyclophosphamide | 17 | |
| Valvulopathy | Human endocarditis and previous valvulopathy | 286, 290 |
| Endocarditis in guinea pigs with damaged cardiac valves | 181 | |
| Pregnancy | Endocarditis in pregnant mice | 351 |
| Chronic Q fever in pregnant mammals and women | 20, 350 | |
| C. burnetii strain | Genetic heterogeneity among “acute” and “chronic” strains but lack of pathotype-specific gene in human strains | 314, 348 |
| Experimental endocarditis with Nine Mile “acute” strain | 17, 181, 239, 351 |
The pathogenesis of chronic Q fever has also begun to be clarified recently, although a number of questions remain unanswered. In animals (17, 170, 181, 351), as in humans (75, 286, 294, 295, 297, 299, 300), conditions such as cardiac valve defect, immunosuppression, or pregnancy may encourage the development of Q fever endocarditis. The role of the immune status in the course of Q fever is also demonstrated by the ability to reactivate latent C. burnetii infection in guinea pigs and white mice following administration of steroids or after whole-body irradiation 3 months following C. burnetii infection (330, 331). Likewise, exacerbation of Q fever has been described in humans with Q fever endocarditis following administration of steroids (186). Genetic factors may also influence the evolution of C. burnetii infection to chronicity, as evidenced by the greater resistance to chronic infection of deer mice than of white mice (330, 331). Interestingly, in all animal endocarditis models (17, 115, 181, 351), infection of cardiac valves was established with C. burnetii Nine Mile, which is considered by some authors to be a reference strain for acute Q fever (314). These findings contradict the hypothesis that specific C. burnetii strains induce either acute disease or chronic endocarditis (314). In fact, this postulate was based upon genetic analysis of only a few C. burnetii strains, including strains of nonhuman origin (314), and was not confirmed when a larger series of human C. burnetii strains was studied (348, 360). In addition, we have diagnosed Q fever endocarditis in patients with predisposing factors such as valvulopathy or immunodeficiency several months or years after they had experienced acute Q fever (234), without evidence of new exposure to C. burnetii infection, which suggests that the same C. burnetii strain may induce both acute and chronic Q fever in the same patient.
Immunocompetent patients with acute Q fever develop a transient bacteremia, with C. burnetii being found intracellularly in circulating monocytes and not free in the plasma. It can be hypothesized that in patients with a cardiac valve defect and/or persistent bacteremia due to immunodeficiency, C. burnetii-infected monocytes in blood may attach to the damaged endothelial surface of valvular tissue, leading to colonization of cardiac valves and endocarditis (233). Recent pathological findings in Q fever endocarditis patients are compatible with such a hypothesis (41). Since few circulating C. burnetii-infected monocytes are detected during Q fever endocarditis, however, additional factors are likely to be involved in the pathogenesis of Q fever endocarditis, including immune complexes which are constant during chronic Q fever (233).
CLINICAL FINDINGS
C. burnetii infection may present with acute or chronic clinical manifestations. However, almost 60% of Q fever cases are asymptomatic. Among the 40% of patients who are symptomatic, the majority (38% of the 40%) will experience a mild disease without the need for hospitalization. Hospitalized patients represent only 2% of infected individuals, whereas 1/10 of them (0.2%) suffer from chronic Q fever. These proportions correspond to the deduced data from Southern France, where the incidence of Q fever is reported to be 50 of 100,000 inhabitants and that of Q fever endocarditis to be 1 of 1,000,000 (282). In a series from Switzerland (86), 224 of the 415 serologically diagnosed patients (54%) were asymptomatic and only 8 (4%) required hospitalization. After 10 years of follow-up, no chronic Q fever cases have been diagnosed. Symptomatic acute Q fever manifests primarily as a self-limited febrile illness, atypical pneumonia, or a granulomatous hepatitis, whereas endocarditis is the more common presentation of chronic Q fever. However, because the clinical manifestations of Q fever are often unspecific, the disease should be systematically considered in febrile patients with recent contact with parturient animals.
Acute Q Fever
Common clinical presentation.
The incubation period of Q fever may last from 2 to 3 weeks, depending on the C. burnetii inoculum. In symptomatic patients, the onset is usually abrupt, with severe fever, fatigue, chills, and headaches (Table 2). The most frequent clinical manifestation of acute Q fever is probably a self-limited febrile illness associated with severe headaches. Atypical pneumonia is also a major clinical presentation. Clinical symptoms may range from clinically asymptomatic pneumonia diagnosed on the chest X ray (Fig. 3) to acute respiratory distress, although the latter presentation remains extremely rare. Clinical presentation and radiographic findings (multiple rounded opacities of both lungs) mostly suggest the diagnosis of atypical pneumonia due to virus, Mycoplasma pneumoniae, or Chlamydia pneumoniae. Hepatitis is another frequent presentation of acute Q fever and is usually revealed on laboratory investigation showing enhanced hepatic enzyme levels (aspartate aminotransferase [AST], alanine aminotransferase [ALT], and alkaline phosphatase). Hepatomegaly may be clinically detectable, but jaundice is rare.
TABLE 2.
Clinical symptoms and signs, and acute complications in symptomatic acute Q fever patientsa and biological findings in patients with acute Q fever whether symptomatic or notb
| Clinical findings | % of patients with indicated findings | Biological findings | % of patients with indicated findings |
|---|---|---|---|
| Fever | 88–100 | Normal leukocyte count | 90 |
| Fatigue | 97–100 | Thrombocytopenia | 25 |
| Chills | 68–88 | Increased transaminase levels | 45–85 |
| Headache | 68–98 | Increased bilirubin levels | 9–14.3 |
| Myalgia | 47–69 | Increased alkaline phosphatase levels | 27.7–57 |
| Sweats | 31–98 | Increased γ-glutamyl transferase levels | 25–75 |
| Cough | 24–90 | Increased creatine phosphokinase levels | 29 |
| Nausea | 22–49 | Increased lactate-dehydrogenase levels | 33.3–40 |
| Vomiting | 13–42 | Increased creatinine levels | 29–40 |
| Chest pain | 10–45 | Elevated erythrocyte sedimentation rate | 43–87.5 |
| Diarrhea | 5–22 | Smooth muscle antibodies | 65 |
| Skin rash | 5–21 | Antiphospholipase antibodies | 50 |
| Myocarditis | 0.5–1 | ||
| Pericarditis | 1 | ||
| Meningoencephalitis | 1 | ||
| Death | 1–2 |
FIG. 3.
Chest X ray of a patient with Q fever pneumonia.
The most frequent clinical presentation of acute Q fever may vary from one area to another. Pneumonia is the major clinical presentation in Nova Scotia (208) and in Switzerland (84). In contrast, hepatitis is observed more frequently than pneumonia in France (365), Ontario (382), and California (55). A febrile illness was the most frequent manifestation of acute Q fever in a series of 111 consecutive patients in Australia (341). The major clinical presentation of acute Q fever may also vary between different areas in the same country: pneumonia is more frequently observed in the Basque region in Spain (2), whereas hepatitis is predominant in Andalusia in the same country (299, 339).
The three main symptoms of fever, pulmonary signs, and elevated liver enzyme levels can coexist. Of 323 hospitalized patients with acute Q fever in France, 25% presented with the three symptoms, 40% presented with fever and elevated liver enzyme levels, 17% presented with fever and pulmonary signs, and 8, 6, and 4%, respectively, presented with only fever, pulmonary signs, or elevated liver enzyme levels (365). The disease is usually mild and resolves spontaneously within 2 to 3 weeks. Only a few patients (about 5% of symptomatic patients) experience complications leading to hospitalization, and fatal diseases are extremely rare.
Prolonged fever.
In acute Q fever patients, the fever may reach from 39 to 40°C, usually remaining elevated all day. Fever typically increases to a plateau within 2 to 4 days, and then after 5 to 14 days the temperature returns rapidly to normal. The fever is usually accompanied by severe headaches. However, in untreated patients, fever may last from 5 to 57 days (76). Thus, acute Q fever is a cause of prolonged fever of unknown etiology. One-quarter of acute Q fever patients experience a biphasic fever. The first phase is as described above. The second phase corresponds to the reappearance of fever, usually to lower levels and intermittently, and lasts from 1 to 19 days (76). Fever has been reported to be of longer duration in older patients (55, 76), lasting for over 14 days in 60% of patients older than 40 years and in only 29% of patients younger than 40 years (55).
Pneumonia.
Almost all patients suffering from acute Q fever pneumonia present with a fever, usually associated with fatigue, chills, headaches, myalgia, and sweats (217). Headaches are often severe and retroorbital. Cough was recorded in 24 to 90% of infected patients in different series of Q fever patients (55, 340). Nausea, vomiting, chest pain, diarrhea, sore throat, and rash have been less frequently reported. The disease is usually mild, as is typical for viral atypical pneumonia. Clinical signs are often lacking on physical examination. The most frequent finding is inspiratory crackles. About 5% of acute Q fever pneumonia patients have splenomegaly. Radiographic findings may include single or multiple opacities of rounded configuration, increased reticular markings, atelectasis, and pleural effusion (123, 217). Complications associated with acute Q fever pneumonia are rare and may include encephalitis, renal failure, congestive heart failure, respiratory failure, and myocarditis. The fatality rate is lower than 3%, and death often occurs in patients with previous pulmonary or cardiac defects.
Hepatitis.
Q fever hepatitis is usually only revealed by an increase in hepatic enzyme levels (209). Alkaline phosphatase, AST, and ALT levels are usually mildly elevated to two to three times the normal level (209). Q fever hepatitis is usually accompanied clinically by fever and less frequently by abdominal pain (especially in the right hypochondrium), anorexia, nausea, vomiting, and diarrhea. Progressive jaundice and palpation of a mass in the right hypochondrium have also been reported. Extensive destruction of liver tissue leading to hepatic coma and death have occasionally been reported (32, 76). If liver biopsy is performed, histology typically reveals a granulomatous hepatitis, even in asymptomatic patients (Fig. 2). Frequently, patients with hepatitis exhibit autoantibodies, including antibodies directed to smooth muscle, anticardiolipin antibodies, antiphospholipid antibodies, circulating anticoagulant, and antinuclear antibodies (187, 293). The presence of circulating antibodies should be checked before a hepatic biopsy is performed, because of the risk of hemorrhage.
Myocarditis.
Myocarditis is a rare but life-threatening clinical manifestation of acute Q fever (54, 279, 329, 350). It represents 0.5 to 1% of Q fever cases diagnosed in our laboratory. In most patients, myocarditis is revealed only by abnormalities on the electrocardiogram (279). Thus, it is probable that myocarditis is an underestimated clinical manifestation of Q fever disease. The most frequent electrocardiogram abnormality is T-wave change. Myocarditis may also be revealed clinically (350) through tachycardia, hypoxemia requiring ventilatory support, and cardiac failure, which may lead to death.
A fulminant case of Q fever myocarditis was described by Chevalier et al. (54). A 15-year-old child presented with acute myocardial dysfunction with fever. Despite supportive care, intractable heart failure occurred and a cardiac transplantation was performed. The patient died of multiple-organ failure 2 months after admission, despite antibiotic therapy with doxycycline combined with pefloxacin. Q fever myocarditis was diagnosed by serology showing the presence of anti-phase II antibodies (IgG titer, 1:400; IgM titer, 1:50), by culture of C. burnetii from a myocardial biopsy specimen, and by demonstration of the presence of C. burnetii within myocardial biopsy specimens by immunohistology. The only risk factor recognized was exposure to a parturient cat 4 months prior to hospitalization. Myocarditis may be associated with pericarditis, and a pericardial effusion may be observed on chest radiographs.
Pericarditis.
Pericarditis has been infrequently reported in Q fever patients (25, 51, 77, 89, 118, 199, 242, 243, 244, 304, 379), and most cases have been recorded in Spain. Pericarditis represents approximately 1% of Q fever cases diagnosed in our laboratory. Since chest pain is often noted in patients suffering from Q fever, it is possible that Q fever pericarditis is underdiagnosed. Pericarditis usually occurs as a clinical manifestation of acute Q fever and may be associated with concomitant myocarditis or pleuritis. Clinical manifestations of Q fever pericarditis are not specific and most often correspond to a fever with thoracic pain. An electrocardiogram may reveal abnormalities, especially on T wave, whereas an echocardiogram may show the presence of pericardial effusion. Although the disease usually resolves spontaneously, recurrent forms have been described (89, 304). Pericarditis may also be associated with Q fever endocarditis in chronically infected patients.
Skin rash.
Although C. burnetii was first considered to be a rickettsial pathogen, Q fever was differentiated clinically from spotted-fever-group rickettsiosis (including Rocky Mountain spotted fever) or typhus group rickettsiosis because of the lack of cutaneous eruption. More recent clinical descriptions have emphasized the possibility of skin rash in Q fever disease, whereas spotted-fever-group rickettsiosis occasionally presents as spotless fever (39, 42). Skin lesions have been found in 5 to 21% of Q fever patients in different series (84, 341, 365). The Q fever rash is nonspecific and may correspond to pink macular lesions or purpuric red papules of the trunk.
Meningoencephalitis.
A few cases of encephalitis, meningoencephalitis, and encephalomyelitis have been reported to occur late in the course of acute Q fever (76, 206, 279, 340, 375). Meningoencephalitis is present in approximately 1% of Q fever patients diagnosed in our laboratory (Fig. 4). In the series reported by Derrick (76), 1 (0.3%) of 273 patients examined had encephalomyelitis. Cerebrospinal fluid examination may reveal the presence of leukocytes, composed mainly of mononuclear cells (206), increased protein concentration, and usually normal glucose concentration. Such clinical manifestations, however, should be differentiated from embolic manifestations in Q fever endocarditis patients. The most common residual disorder of Q fever meningitis is disturbance of vision (99).
FIG. 4.
Magnetic resonance image of Q fever meningoencephalitis.
Others.
Less common manifestations of acute Q fever include hemolytic anemia, mediastinal lymphadenopathy, erythema nodosum, thyroiditis, pancreatitis, mesenteric paniculitis, epididymitis, orchitis, priapism, inappropriate secretion of antidiuretic hormone, optic neuritis, Guillain-Barré syndrome (in our experience, 1 in 1500 cases diagnosed), and extrapyramidal neurological disease (211).
Biological findings.
The biological findings are listed in Table 2. Acute manifestations of Q fever are associated with a normal leukocyte count in over 90% of infected patients (217, 220, 280, 293, 338, 341). However, both leukocytosis and leukopenia have been described (217, 341). Leukocytosis was reported in 2.7 to 29% of acute Q fever patients (217, 341). Thrombocytopenia is the most frequent abnormality found on the hemogram and may occur in up to 25% of patients at initial presentation (293). Thrombocytopenia may also occur during the course of hospitalization (211, 217). The platelet count is usually between 100 × 109 and 150 × 109/liter. Abnormal liver function tests are the primary manifestation of Q fever hepatitis but are also common in patients presenting with Q fever pneumonia. Altered liver function tests were recorded in 85% of 103 abattoir workers in Australia, most of them presenting with an acute febrile illness (341), and in 45% of 164 patients with Q fever pneumonia in Spain (338). Elevated transaminase levels have been reported respectively in 38.3 to 60% of patients for AST and 33.3 to 80% for ALT (81, 211, 293). However, transaminase levels are usually only moderately elevated, ranging from 2 to 10 times the normal level. Bilirubin levels were elevated in 9 to 14.3% of patients (217, 341), alkaline phosphatase levels were elevated in 27.7 to 57% (81, 217, 341), γ-glutamyl-transferase levels were elevated in 72 to 75% (293, 341), creatine phosphokinase levels were elevated in 29% (217), and lactate dehydrogenase levels were elevated in 33.3 to 40% (81, 293). Elevated creatinine levels were recorded in 29 to 40% of patients with acute Q fever (217, 293). Haematuria and proteinuria have often been reported (211, 279, 341). An erythrocyte sedimentation rate over 50 mm/h was reported in 43 to 87.5% of patients (217, 338). Autoantibodies, including smooth muscle antibodies and less frequently cold agglutinins, anti-prothrombinase, anti-hemophilic B antibodies, and a positive Coombs test, are also frequently found in acutely infected patients (187, 293). Acute hemolytic anemia, with or without cold agglutinins or a positive Coombs test, has been reported (187). The possibility of blood coagulation abnormalities should prompt appropriate biological investigations before performing a liver biopsy or surgery in Q fever patients. Hypoxemia was recorded in up to 26.6% of patients with Q fever pneumonia (217).
Chronic Q Fever
Endocarditis.
Endocarditis is the major clinical presentation of chronic Q fever (85, 93, 98, 125, 133, 283, 307, 311, 317, 349, 369, 374, 381, 409). It accounts for 60 to 70% of all chronic Q fever cases. Q fever endocarditis represents 3% of all cases of endocarditis diagnosed in England and Wales (260) and at least 5% in France (104). Although it can be spontaneously fatal when untreated, mortality from Q fever endocarditis is less than 10% when appropriate antibiotic therapy is administered. However, most clinical studies have shown relapse rates of over 50% after antibiotic therapy is withdrawn. Q fever endocarditis supervenes almost exclusively in patients with previous cardiac valve defects (over 90% of Q fever endocarditis patients). The underlying heart disease may be congenital, rheumatic, degenerative, or syphilitic. The aortic and mitral valves are mostly involved. Q fever prosthetic valve endocarditis has been increasingly reported over recent years (98, 240, 307, 332). The male/female ratio is 75%, and most patients are older than 40 years. Immunosuppression such as is observed in organ transplant recipients, patients with cancer, lymphoma, or chronic renal insufficiency, and AIDS patients is also associated, although less frequently, with the evolution of Q fever to chronicity (286, 294, 295). This may be related, in part, to valvular damages due to chronic inflammation and cardiotoxic drug administration in this population.
Q fever endocarditis patients usually present with symptoms suggestive of cardiac involvement including heart failure or cardiac valve dysfunction. They may also present with a less specific general disease characterized by a low-grade fever (present at the beginning of the disease in 68% of patients), often remittent and well tolerated, which may be associated with malaise, weakness, fatigue, weight loss, chills, anorexia, and night sweats (Table 3). Cardiac symptoms are related to heart failure in 67% of infected patients with previous valvulopathy, dyspnea, acute pulmonary edema, angina, and palpitations. A heart valve murmur may be present but often corresponds to a previously diagnosed valvulopathy. A chest X ray may show cardiomegaly. Electrocardiography may reveal arrhythmia and ventricular hypertrophy. Cardiac vegetation is visible on the echocardiogram in only 12% of patients and is often small. Transesophageal echography is superior to transthoracic echography. Worsening valvular dysfunction is the most frequently observed abnormality.
TABLE 3.
Clinical symptoms and signs and biological findings in Q fever endocarditis patientsa
| Clinical findings | % of patients with indicated findings | Biological findings | % of patients with indicated findings |
|---|---|---|---|
| Male sex | 76 | Leukocytosis | 25 |
| Valve involved: | Leukopenia | 15 | |
| Aortic | 33 | Increased transaminase levels | 40–83 |
| Mitral | 50 | Thrombocytopenia | 26–56 |
| Both | 17 | Anemia | 40–55 |
| Fever | 68 | Increased creatinine level | 65–73 |
| Cardiac failure | 67 | Elevated sedimentation rate | 88 |
| Hepatomegaly | 56 | Increased gamma globulin concentration | 94 |
| Splenomegaly | 55 | Circulating immune complexes | 90 |
| Clubbing of digits | 37 | Antinuclear antibodies | 35 |
| Purpuric rash | 19 | Rheumatoid factor | 60 |
| Arterial embolism | 21 | Smooth muscle antibodies | 40 |
| Death | 37 |
Peripheral manifestations of endocarditis are common. These include digital clubbing (in 37% of patients) and a purpuric rash (in 19% of patients), usually on the extremities and mucosa, which corresponds to immune complex vasculitis (290). Splenomegaly and hepatomegaly are frequent, especially in patients with long-term evolution of the disease (283). Renal involvement with microscopic haematuria is frequent and corresponds to immune complex glomerulonephritis, which may evolve to renal insufficiency. Embolic manifestations are observed in about 20% of patients and may involve the brain (with stroke) and arm or leg vessels. Whereas diagnosis of Q fever endocarditis may be missed in the early stage of the disease, symptoms progressively complement one another after several months and evolve into the more evident clinical presentation including considerable hepatomegaly, renal insufficiency, purpuric rash, and embolic manifestations, which may result in death.
In fact, the clinical manifestations leading to diagnosis of Q fever endocarditis are protean and not specific, explaining why in many cases the diagnosis is established only after several months or even years of evolution (283). Thus, chronic Q fever has been diagnosed in the following clinical settings: chronic renal insufficiency, stroke, chronic hepatomegaly or splenomegaly, chronic eruption, chronic elevation of liver enzyme levels (ALT and AST), necessity for early valve replacement after cardiac valve surgery, postoperative fever, and unexplained chronic inflammatory syndrome (290). It is now clear that a delay in diagnosing Q fever endocarditis has a significant effect on the prognosis. For the first 15 patients diagnosed by the authors (in 1987) (283), the mean time taken to diagnose Q fever endocarditis was 18 months; six patients (40%) died. However, for the most recent 10 cases, diagnosed between 1996 and 1998, the mean time taken for diagnosis was only 5 months and all remain alive. Furthermore, we have noted that a delay in diagnosis may lead to complications. Seven of the 15 patients discussed above presented with hepatosplenomegaly, whereas none of the 10 most recent patients, for whom the diagnosis was made more rapidly, had any signs of liver or spleen enlargement. These observations emphasize the serious complications that can arise from diagnostic delays. Also, since the clinical symptoms of Q fever endocarditis progressively complement one another during evolution of the disease, the time at which the diagnosis is established may influence the type and frequency of observed clinical signs and symptoms and thus may explain how series could be discrepant in reporting the usual clinical presentation of Q fever endocarditis. It also emphasizes the importance of recognizing Q fever disease as soon as possible (300). Duke’s criteria are used worldwide for calculation of a diagnostic score for infective endocarditis (87). Such criteria are helpful when a definite diagnosis for infective endocarditis (i.e., histological and microbiological demonstration of infection in removed cardiac valve tissue) is lacking. These criteria include major criteria such as isolation of a typical microorganism for infective endocarditis from two separate blood cultures or cardiac vegetation on echocardiogram (87); minor criteria include fever, previous valvulopathy, etc. (87). The combination of two major criteria or one major and three minor criteria is considered diagnostic for infective endocarditis (87). We have recently reported that Duke’s criteria may also be helpful in the diagnosis of Q fever endocarditis when the isolation of C. burnetii from blood and/or the presence of an anti-phase I IgG titer of ≥800 is considered a major criterion (104).
Biological findings in Q fever endocarditis patients (Table 3) have been recently summarized (290) from data reported in the literature (85, 93, 98, 125, 133, 283, 307, 311, 317, 369, 381, 409). A severe inflammatory syndrome is associated in almost every patient, including an increased erythrocyte sedimentation rate in 88% of patients and increased gamma globulin concentrations in 94% (up to 60 to 70 g/liter), which correspond to a polyclonal increase in IgG, IgM, and IgA levels. Anemia and thrombocytopenia are present in 55 and 56% of patients, respectively. Anemia due to autoimmune mechanisms has been reported (283, 374). Most patients present with altered hepatic function tests, including elevated hepatic transaminase levels (AST in 83% of patients and ALT in 37%), and elevated alkaline phosphatase levels (in 74% of patients). Lactate dehydrogenase and creatine phosphokinase levels are also frequently elevated. Rheumatoid factor, circulating immune complexes, and cryoglobulin are frequently present (133, 283). A microscopic hematuria is found in one-third of patients. Anti-smooth muscle antibodies, circulating anticoagulant antibodies, antimitochondrial antibodies, low titers of antinuclear antibodies, and a positive Coombs’ test have also been reported (187).
Vascular infections.
C. burnetii vascular infection is a rare but life-threatening condition. Infections of aneurysms and vascular grafts have been reported (40, 93, 97, 105, 299). Diagnosis is frequently established by serology, which corresponds to an antibody profile of chronic Q fever with the presence of anti-phase I C. burnetii antibodies. Diagnosis may also be established by culture of C. burnetii from blood and vascular tissue specimens. Most cases have been reported over recent years because of improved knowledge and recognition of the disease by clinicians. In our laboratory, however, many cases were diagnosed because removed aortic aneurysm specimens were systematically examined for the presence of C. burnetii by culture and PCR. Thus, among the 13 C. burnetii vascular infection cases reported in the literature, 10 have been diagnosed in our laboratory and 7 have been diagnosed in the past 7 years. The patients were usually men aged of 66.5 ± 11.5 years (mean ± standard deviation) with a previous aortic abnormality (mainly infrarenal aneurysm or vascular graft), and a history of environmental exposure (105). Almost all patients presented with a severe inflammatory syndrome, including a highly elevated erythrocyte sedimentation rate and high levels of C-reactive protein and fibrinogen in serum. A total of 70% were febrile at presentation. Weight loss and abdominal pain were frequently reported. In contrast, hyperleukocytosis, thrombocytopenia, and elevated hepatic enzyme levels were less frequently observed than in Q fever endocarditis patients. No specific symptoms of vascular involvement were found. Since the clinical manifestations of C. burnetii vascular infection are nonspecific, the disease may be recognized by physicians only if C. burnetii serology is performed systematically, especially in patients with aneurysm or vascular graft with unexplained fever, abdominal pain, or weight loss. In our experience, 7 (3%) of 200 cases of chronic Q fever diagnosed in our laboratory were vascular infections; however, the disease is probably underreported.
Osteoarticular infections.
Three types of C. burnetii osteoarticular infections, including osteomyelitis (61, 93), osteoarthritis (267, 285), and aortic graft infection with contiguous spinal osteomyelitis (93, 277), have been reported. C. burnetii infection of bones has been found more frequently in children suffering from coxitis or spondylodiskitis and is not associated with specific host factors in this population. Bone infections have also been reported in adults who are immunocompromised or have a joint prosthesis. Cottalorda et al. (61) described C. burnetii osteoarticular infection in three children: L3 spondylitis and coxitis of the right hip joint in a 7-year-old boy, osteomyelitis of the talus in a 9-year-old boy, and osteomyelitis of the fibula with an accompanying skin abscess in a 2-year-old girl. Diagnosis was established by serology, showing the presence of high levels of anti-phase I C. burnetii antibodies in all cases, and isolation of C. burnetii from tissue specimens in the first and third patients. Exposure factors for C. burnetii infection were contact with goats in the first and third children and owning a cat in the second.
C. burnetii osteoarticular infection is probably an underreported disease. Five (4%) of the 200 chronic Q fever cases diagnosed in our laboratory were osteomyelitis (282). It should be suspected specifically when pathological examination of tissue biopsy specimens suggests tuberculoid bone lesions and no mycobacterial pathogens are found.
Chronic hepatitis.
Although chronic Q fever involvement of the liver is frequently associated with endocarditis (397), a few cases of chronic hepatitis without Q fever endocarditis have been described. Yebra et al. (419) described an acute case of hepatitis in a 31-year-old man that became chronic with persistence of pathological liver abnormalities 2 years after the initial diagnosis. Phase I antibody titers by IFA were 1:2,048. The fact that diagnosis of Q fever endocarditis based on echocardiographic examination of cardiac valves is often difficult to establish means that diagnosis of chronic hepatitis without endocarditis should always be regarded with caution. Only one case of chronic hepatitis without endocarditis was diagnosed in our laboratory among 200 chronic Q fever cases (282).
Chronic pulmonary infections.
Chronic lung involvement is rare and may correspond to pulmonary fibrosis or pseudotumors. Pneumonic fibrosis has been reported as a complication of chronic Q fever in the former USSR (3). Inflammatory pseudotumor of the lung was first described by Janigan and Marrie (161). The disease may radiologically mimic lung neoplasm and may lead to lung tissue resection. In the case described by Janigan and Marrie (161), histological examination of resected lung specimens revealed the presence of mononuclear cells obstructing the bronchioles and infiltrating the alveoli and septa. Lipton et al. (191) reported another case in a 36-year-old patient in Canada. The patient was admitted to hospital after a 4-week evolution of fever, weight loss, and anorexia, with elevated alkaline phosphatase and AST levels despite penicillin therapy. On admission, the physical examination was unremarkable, although a chest X ray revealed a mass lesion in the right lower lobe which suggested malignancy. Bronchoscopy with cytologic examination was normal. Q fever serology was performed and revealed a high anti-phase II C. burnetii complement fixation titer (1:1,024). Tetracycline therapy was initiated, and complete resolution of the lung mass was found on a repeat chest X ray after 1 month of therapy. The patient had no apparent risk factor for Q fever, including lack of contact with animals in the last 6 months. In our series of 200 chronic Q fever patients, only 2 (1%) presented with pseudotumor of the lungs and none presented with lung fibrosis (282).
Chronic fatigue syndrome.
Chronic fatigue syndrome has been reported infrequently as a possible clinical manifestation following acute Q fever (18, 19, 137, 203). The disease was first documented in Australia in abattoir workers who had probable recurrent contact with C. burnetii-infected animals (203). Following acute Q fever disease, patients presented with prolonged fatigue, arthralgia, myalgia, muscle fasciculation, blurred vision, sweats and enlarged painful lymph nodes. A case-control study was conducted more recently in the United Kingdom with 102 patients from a 1989 Q fever outbreak and pneumonia controls (19). Significantly, chronic fatigue syndrome was observed more frequently in patients convalescing from acute Q fever disease. Characteristically, such patients complained of fatigue, sweating, breathlessness on exertion, and blurring of vision; in some patients, these symptoms persisted for many years. Harvey-Sutton (137) also reported the possibility of bradycardia. Thus, human C. burnetii infection may induce a persistent debilitating syndrome in convalescing patients, as is occasionally observed in patients with chronic typhoid fever or chronic brucellosis. The post-Q fever chronic fatigue syndrome should, however, be distinguished from chronic Q fever disease (especially Q fever endocarditis), including the demonstration of the absence or low level of anti-phase I antibodies in the former patients.
Q Fever during Pregnancy
Twenty-seven Q fever cases during pregnancy have been reported so far in France, the United Kingdom, the United States, Italy, Czechoslovakia, Israel, and Canada (20, 31, 78, 93, 110, 113, 192, 215, 232, 297, 303, 350, 356, 389). The cases were in females aged between 18 and 39 years. Premature birth was reported in eight cases (i.e., 25.9%) (31, 192, 215, 303, 350, 356), and one healthy premature infant (1.88 kg) was born after cesarean delivery because of maternal illness (192). Chronic Q fever in a woman was responsible for recurrent cases of premature birth (350). Spontaneous abortion was reported in six cases (i.e., 22.2%) (78, 297, 350). Death in utero was reported in two cases (110, 232), including in one patient with concomitant Chlamydia psittaci infection (232). Pregnancy was terminated in one case because of concomitant rubella (356). Finally, only five healthy infants were born at term (20, 215, 350, 356).
Q fever during pregnancy corresponds mainly to placentitis. C. burnetii was recovered by culture from 12 of 19 placentas examined (20, 31, 215, 297, 303, 350, 356, 389). The pathogenesis of fetal disease remains unexplained. Immune complexes, which are frequent during Q fever disease, may lead to vasculitis or vascular thrombosis and placental insufficiency. However, C. burnetii may cause direct fetal injury. C. burnetii infection of the fetus has been demonstrated by detecting C. burnetii in the tissue from aborted fetuses by culture, immunohistology, or PCR-based techniques (350, 356).
Most infected pregnant women present with fever. Flu-like illness (192), severe thrombocytopenia (303, 350), and atypical pneumonia (350, 356) have also been reported. However, Q fever in pregnant women may also be asymptomatic (215). Serological profiles at the time of diagnosis were suggestive of acute Q fever in 14 (58.3%) of 24 pregnant women for whom serology was performed and of chronic Q fever in 10 (41.7%). Thus, pregnancy should be considered a favoring condition leading to chronic Q fever. Contact with cattle, goats, sheep, cats, and dogs were the main risk factors for C. burnetii infection in pregnant women diagnosed with Q fever. Interestingly, a cat belonging to one patient (350) gave birth 2 months before the patient aborted. Contact with infected animals at the time of delivery is a major risk factor for C. burnetii infection (180, 210, 276). Thus, pregnant women should avoid contact with domestic animals or pets, especially cats, to prevent both toxoplasmosis and Q fever. Medical staff in contact with C. burnetii-infected pregnant women are also at risk for acquiring Q fever. In the series previously mentioned (350), the obstetrician who extracted the fetus from one patient experienced acute Q fever pneumonia 7 days later.
Q fever should be added to the etiological agents of intrauterine infections associated with morbidity and mortality during pregnancy, which are grouped under the term TORCH for Toxoplasma, “others” (including Listeria, hepatitis B, and HIV), rubella, cytomegalovirus, and herpes. Q fever serology should be performed in pregnant women suffering from fever associated with pneumonia, hepatitis, and severe thrombocytopenia and in those undergoing abortion or premature delivery. Q fever serology should be monitored in pregnant women with acute Q fever because of the possibility of developing chronic Q fever (350). Q fever serology should also be performed in previously infected women who become pregnant because of the risk or resurgence of the disease and fetal contamination (350).
LABORATORY DIAGNOSIS
Collection and Handling of Clinical Specimens
C. burnetii is a highly infectious agent, and many laboratory-acquired cases of Q fever have been described. Thus, clinical materials from patients supposedly infected with C. burnetii should be handled with care by experienced personnel wearing gloves and masks and only in biosafety level 3 laboratories. The same measures should be applied when manipulating C. burnetii-infected cell cultures or C. burnetii-infected animals. In laboratory personnel working with C. burnetii-infected animals, vaccination might prove valuable.
Pathological Findings and Immunohistology
Typical histological lesions of acute and chronic Q fever are described above (see “Pathogenesis and Pathology”). C. burnetii in involved organs may be detected by immunodetection techniques. In our laboratory, these techniques are currently used in combination with C. burnetii culture only for the confirmation of Q fever endocarditis when the cardiac valve has been removed. Tissue biopsy specimens are tested either fresh or following formalin fixation and paraffin embedding. Immunodetection may be performed by the immunoperoxidase technique (41), capture ELISA/ELIFA systems (358), or immunofluorescence with polyclonal or monoclonal antibodies (226, 245, 358). Only the last technique may be used with paraffin-embedded tissues (298). Brouqui et al. (41) used an immunohistochemical technique to study valve specimens from 17 Q fever endocarditis patients (Fig. 5). They found that in infected cardiac valves, C. burnetii was visible as a voluminous intracytoplasmic mass within infected mononuclear cells and not extracellularly as for most other etiological agents of infective endocarditis. Intracellular multiplication of C. burnetii may explain why cardiac valve vegetations are small or nonexistent in Q fever endocarditis patients and thus are most often not visualized by echocardiography.
FIG. 5.
Q fever endocarditis. Immunohistochemistry on cardiac valve, using immunoperoxidase staining, reveals the presence of C. burnetii (arrow). Magnification, ×100.
C. burnetii Culture
Isolation of C. burnetii by culture is done in very few laboratories because of the risk of transmission to laboratory workers and the lack of sensitivity of the technique. Since C. burnetii is a strict intracellular bacterium, culture cannot be obtained in axenic medium. Although C. burnetii has been successfully isolated in guinea pigs, mice, and embryonated eggs (256, 269, 405), such techniques have been abandoned because they are more hazardous than in vitro cell cultures. There is also a high risk of cross-contamination between infected and uninfected animals. However, the guinea pig model remains useful when attempting to isolate C. burnetii from specimens contaminated with other bacteria. Clinical specimens are injected intraperitoneally into guinea pigs, which become febrile and are sacrificed after 5 to 8 days. Spleen extracts from infected animals are the most valuable specimens for recovery of C. burnetii. Interestingly, Legionnaires’ disease was first discovered by animal inoculation while looking for Q fever (231).
A number of cell lines can be used for in vitro cultures (Fig. 6). Human embryonic lung fibroblasts (HEL cells) grown in shell vials are used routinely in our laboratory because of their high susceptibility to C. burnetii infection and easy maintenance (287). Several human specimens, including blood, cerebrospinal fluid, bone marrow, cardiac valve, vascular aneurysm or graft, bone biopsy, liver biopsy, milk, placenta, and fetal specimens after abortion, are suitable for C. burnetii culture (119, 247, 287). Cell monolayers in shell vials are inoculated with 1 ml of clinical specimen and centrifuged (700 × g at 20°C) for 1 h to enhance attachment and penetration of C. burnetii into cells. Inoculated monolayers are incubated at 37°C in 5% CO2 for 5 to 7 days. C. burnetii is usually observed by microscopic examination of cell monolayers after Gimenez or immunofluorescence staining.
FIG. 6.
P388D1 macrophage-like cells chronically infected with C. burnetii Nine Mile strain. Cells containing large vacuoles full of bacteria are visible on the Gimenez stain (arrow). Magnfication, ×400.
DNA Probes and DNA Amplification
Radiolabeled DNA probes were first used for detection and identification of C. burnetii strains (107, 108, 195, 400) from clinical samples or cultures. More recently, C. burnetii DNA amplification from clinical specimens by PCR has been used successfully (345). By using this method, C. burnetii DNA can be detected retrospectively in frozen samples and even in paraffin-embedded tissues (345, 346). The technique may allow the quantification of C. burnetii in tissue samples (112). C. burnetii genes such as 16S rDNA, sodB, and gltA may be amplified. More recently, an open reading frame encoding a polypeptide of 367 amino acids was found downstream of the heat shock proteins genes (htpAB genes) (152). Primers derived from htpAB-associated sequence were employed in a PCR assay for detection of C. burnetii in cow’s milk (401). About 19 copies of this sequence were found in the genome of the C. burnetii Nine Mile phase I strain (401). The presence of multiple copies of the target sequence in C. burnetii genome may increase the sensitivity of this PCR assay. We currently use primers derived from the htpAB-associated repetitive element for routine diagnosis of C. burnetii infection in humans. However, in our experience, PCR-based methods are not adequate for blood samples and are prone to false-positive results. Because of these limitations, we are very skeptical about the report by To et al. (368) in Japan showing the presence of C. burnetii DNA in sera from 23 of 58 (39.6%) children with atypical pneumonia.
PCR-based techniques are also used to detect C. burnetii DNA within infected cultures in shell vial supernatants (247). By using this technique, positive blood cultures were detected in 11 of 66 Q fever pneumonia patients (17%) and 9 of 17 Q fever endocarditis patients (53%) when blood specimens were collected before initiation of the antibiotic therapy (247). In contrast, blood cultures remained negative in 160 acute Q fever patients and in 145 patients with endocarditis in whom antibiotic therapy had been administered before blood collection (247).
Serology
Q fever diagnosis remains based upon serological methods because culture and molecular biology techniques have low sensitivity and are available only in reference laboratories. Serological diagnosis is easy to establish, although antibodies are mostly detected only after 2 to 3 weeks from the onset of the disease. Thus, serological tests should be performed on both acute- and convalescent-phase sera. Moreover, serology allows the differentiation of acute and chronic Q fever infections. Methods which have been used include microagglutination (103, 163, 252), complement fixation (147, 246, 270), radioimmunoassay (80), IFA (100, 270), indirect hemolysis test (370), ELISA (174, 272, 378, 387, 406), enzyme-linked immunosorbent fluorescence assay (ELIFA) (320), dot immunoblotting, and Western blotting (35, 399). The techniques most commonly used include complement fixation, IFA, ELISA, and microagglutination. Only the first two methods are commercially available.
In the past, the complement fixation test was extensively used. Heat-inactivated sera were tested against either phase I or phase II C. burnetii antigens (147). A complement fixation anti-phase II antibody titer of ≥40 indicated a diagnosis of acute Q fever (126), whereas an anti-phase I antibody titer of >200 indicated a diagnosis of chronic Q fever (273). The complement fixation test is specific but has a lower rate of sensitivity and is more time-consuming than IFA or ELISA (270). Moreover, seroconversion is detected by complement fixation at a later date: 2 to 3 weeks for the complement fixation test, compared to 10 to 15 days for IFA and ELISA (126, 270). In addition, false-negative results have been described with the complement fixation test in chronically infected patients with high antibody titers due to a prozone phenomenon, as well as false-positive results due to cross-reactions with hen egg antigens.
The IFA remains the reference technique for Q fever diagnosis (263, 366). The microimmunofluorescence test has the advantage of requiring only small amounts of antigen. Both phase I and phase II C. burnetii Nine Mile strain are used as antigens. Phase II antigen is obtained by growing C. burnetii in cell culture, while phase I antigen is obtained from spleens of infected mice (366). Antibodies of the IgG, IgM, and IgA subclasses can be determined. IFA techniques may be improved by using an absorbent rheumatoid factor to remove IgG before determination of IgM or IgA titers (366). In our laboratory, sera are screened by microimmunofluorescence at a 1:50 dilution with phase II antigens. Positive sera found on screening are serially diluted and then tested on both phase II and phase I antigens for the presence of IgG, IgM, and IgA. During acute Q fever, seroconversion is usually detected from 7 to 15 days after the onset of clinical symptoms and antibodies are detected by the third week in about 90% of cases. An IgG anti-phase II antibody titer of ≥200 and an IgM anti-phase II antibody titer of ≥50 are considered significant (366), but the choice of negative cutoff titers depends on the amount of background antigen stimulation in the population studied and may vary from one area to another (216). Chronic Q fever is characterized by the presence of anti-phase I antibodies, and an IgG anti-phase I antibody titer of ≥800 is considered to be highly predictive of Q fever endocarditis (366). The Duke criteria for diagnosis of infective endocarditis have recently been modified to include either the isolation of C. burnetii from blood or the presence of an anti-phase I IgG titer of ≥800 as new major criteria (104).
ELISA has been described as being more sensitive than the complement fixation test and IFA (62, 100, 272). Both anti-phase I and anti-phase II antibodies can be detected. Waag et al. (387) proposed cutoff values of ≥1,024 for anti-phase II IgG and ≥512 for anti-phase II IgM for acute Q fever and ≥128 for anti-phase I IgG and IgM antibodies for chronic Q fever. Since the results obtained with ELISA are more difficult to interpret by inexperienced persons than those obtained by IFA, ELISA is not widely used for Q fever diagnosis. It has been proposed as a useful tool for seroepidemiological purposes (271).
Microagglutination is a simple and sensitive test that may detect antibodies early after the onset of clinical symptoms (163). However, the main limitation of the technique is that much larger amounts of antigen are required than for IFA or ELISA. More recently, the high-density particle agglutination has proven to be more highly specific and much more sensitive than microagglutination (252).
Other techniques proposed for Q fever diagnosis include dot immunoblotting, Western blotting, indirect hemolysis test, and radioimmunoassay. Dot immunoblotting was reported to be sensitive and specific and may be useful as a screening test (62). Western blotting has also been reported to be sensitive and specific (35). The molecular masses of the different antigens detected vary from 10 to 100 kDa. Antibodies reacting with 50-, 80-, and 160-kDa antigens are considered indicative of chronic Q fever (35). However, in our experience, Western blotting is a time-consuming technique and the results obtained by this technique are not reproducible. Tokarevich et al. (370) have proposed an indirect hemolysis test which was highly sensitive and specific. Radioimmunoassay has been proposed by Doller et al. (80) to be a sensitive and specific technique. However, it can be performed only in radioactivity-equipped laboratories.
Cross-reactions are the biggest source of confusion when interpreting serological results, and these vary according to the serological technique. Cross-reactions have been described between C. burnetii and either Legionella pneumophila (88, 101), Legionella micdadei (79, 249), and Bartonella quintana or Bartonella henselae (182). Such cross-reactivity should be considered in the etiological diagnosis of atypical pneumonia due to C. burnetii and Legionella species or culture-negative endocarditis due to C. burnetii and Bartonella species. A differential diagnosis is easily established when quantitative antibody titers against both anti-phase I and anti-phase II C. burnetii antigens are determined.
In conclusion, diagnosis of Q fever continues to be based upon serology. In our experience, the microimmunofluorescence test is the most useful technique, allowing determination of both phase I and phase II antibodies. Because cross-reactions are not significant when quantitative serology is performed, seroconversion or a fourfold rise in antibody titers can be considered diagnostic of Q fever. However, the disease may often be readily diagnosed with a single serum sample; the currently proposed cutoff values are summarized in Table 4 (366). In acutely infected patients, the most limiting factor is late seroconversion. If phase II IgG antibody titers are ≤1:100 in a single serum sample, active Q fever is highly improbable (366). The diagnosis of Q fever can definitely be ruled out if the serum sample was collected 1.5 months after the onset of symptoms or later. The presence of both an anti-phase II IgG titer of ≥1:200 and IgM titer of ≥1:50 is 100% predictive of acute Q fever (366). However, such results are observed only in 10% of patients during the second week following the onset of symptoms, 50% during the third week, and 70% during the fourth week. When intermediate titers are detected, serology cannot be interpreted accurately unless a second serum sample is collected after an interval of at least 2 weeks. Phase I IgG titers of ≥1:800 are highly indicative of chronic Q fever, with a 98% positive predictive value and 100% sensitivity, whereas titers of ≥1:1,600 are 100% predictive for chronic Q fever. Phase I IgA, which were first considered useful for the diagnosis of chronic Q fever, is now used only for serological follow-up. C. burnetii culture from blood samples or tissue biopsy specimens, although relatively insensitive, remains of interest because larger series of C. burnetii strains can be obtained and antibiotic susceptibilities of an individual strain in chronically infected patients can be tested. PCR-based methods are currently applicable only to tissue samples, especially cardiac valve specimens. These techniques occasionally confirm a diagnosis of Q fever disease when cultures are negative, but they are not usually necessary for routine diagnosis. Results obtained by PCR-based techniques should also be considered with caution because of the risk of false-positive results.
TABLE 4.
Cutoff proposal for Q fever diagnosis by using microimmunofluorescence and interpretation of serological results obtained with a single serum sample
| Phase II antibody titer
|
Phase I antibody titer (IgG) | Interpretation | |
|---|---|---|---|
| IgG | IgM | ||
| ≤100 | Active Q fever improbable | ||
| ≥200 | ≥50 | Acute Q fever (100% predictive) | |
| ≥1:800 | Chronic Q fever (98% predictive) | ||
| ≥1:1,600 | Chronic Q fever (100% predictive) | ||
TREATMENT
C. burnetii In Vitro Antibiotic Susceptibility
Since C. burnetii does not multiply in axenic medium, conventional assays for antibiotic susceptibility testing are not suitable. Three different experimental systems have been developed to evaluate the antibiotic susceptibility of this bacterium, including the embryonated-egg model, the animal model, and the cell culture model, which is now the most commonly used.
Embryonated-egg model.
C. burnetii can infect embryonated eggs and multiply within the yolk sac. This multiplication leads to death of the embryo, usually within 14 days postinoculation. The activity of antibiotics against C. burnetii can be deduced from their ability to prolong the median survival time (MST) of infected eggs when administered into the yolk sac, compared to untreated controls. The ability of the antibiotics to cure infected eggs has also been evaluated by subculturing the contents of the yolk sacs of surviving eggs to uninfected ones. Huebner et al. (155) reported that high doses of streptomycin (10 mg/egg) prolonged the MST of eggs infected with C. burnetii Nine Mile strain by 6 days. However, bacteria were not eradicated by this compound, since treated yolk sacs yielded a heavy growth of bacteria on subculture. Jackson (159) reported the efficacy of oxytetracycline (Terramycin), aureomycin, and chloramphenicol (administered individually). Ormsbee and Pickens (255) proposed another model in which the number of C. burnetii within the yolk sacs was evaluated by means of the complement fixation test. Antibiotic activity was determined by a delay in the development of positive complement fixation values and by complement fixation titers obtained with treated eggs compared to untreated controls. In this model, penicillin G (6 mg/egg) had no inhibitory effect whereas chloramphenicol (1 mg/egg) and streptomycin (5 mg/egg) were slightly effective. Oxytetracycline and to a lesser extent aureomycin were the most effective compounds tested. Similar results were obtained with the California, Adohr, and Idaho strains (257). Oxytetracycline and aureomycin were effective, whereas chloramphenicol, erythromycin, and thiomycetin were not. Spicer et al. reported that the Nine Mile, Ohio, Cyprus, and Scottish isolates were resistant to erythromycin, clindamycin, viomycin, cycloserine, and cephalothin but susceptible to rifampin, trimethoprim, doxycycline, and oxytetracycline (342). However, none of these drugs was bactericidal, as determined by subculture. Using the Cyprus isolate, the authors also reported the first natural resistance to tetracyclines (342). Thus, the embryonated-egg model allowed early description of the susceptibility of intracellular C. burnetii to antibiotics. In this model, β-lactam compounds, chloramphenicol, and erythromycin were not effective whereas tetracyclines and rifampin were bacteriostatic.
More recent investigations with the embryonated-egg model have shown that the new fluoroquinolone compounds, pefloxacin and ofloxacin, are effective against the C. burnetii Nine Mile isolate (284). Ofloxacin prolonged the MST to 1.7 days at 0.025 mg/egg and 3.1 days at 0.050 mg/egg. Pefloxacin was ineffective at 0.025 mg/egg but prolonged the MST to 2.4 days at 0.050 mg/egg and to 3.5 days at 0.1 mg/egg. Thus, both quinolones were considered to be effective, with ofloxacin being the more effective.
Animal models.
Antibiotic activity against C. burnetii was evaluated in guinea pigs, which may be considered a suitable model for acute Q fever since infected animals develop fever and granulomatous hepatitis as in humans. In early experiments, Huebner et al. (155) evaluated the activity of streptomycin in guinea pigs inoculated with C. burnetii Henzerling or Dyer. The efficacy of the antibiotic therapy was evaluated by determination of the morbidity and mortality rates of animals receiving antibiotics for 16 days compared with untreated controls. Of the 24 inoculated animals, 19 survived in the streptomycin-treated group, whereas of the 28 untreated animals, only 1 survived in the control group. However, streptomycin was effective only when used at a high dosage (40 to 50 mg/kg), which is well above the toxic range for humans.
Several other animal species, including mice, rabbits and monkeys, can be infected with C. burnetii. Two experimental models have recently been described for Q fever endocarditis. In a mouse model, animals that were immunocompromised by cyclophosphamide before C. burnetii infection developed C. burnetii-disseminated infection and cardiac valve lesions. More recently, La Scola et al. (181) proposed an experimental model for C. burnetii endocarditis in immunocompetent guinea pigs. Q fever endocarditis was established by producing cardiac valve lesions by electrocoagulation before C. burnetii infection. Although these models are promising, experimental data on antibiotic activity in these animals is not yet available.
Cell culture models.
A number of cell lines have been used to test antibiotic activity against intracellular C. burnetii, including murine macrophage-like cell lines (P388D1 and J774) and a murine fibroblast cell line (L929) (5, 21, 23, 46, 306). Similar experimental procedures are used with these cell models. Cell monolayers grown in flasks or shell vials are infected with a predetermined C. burnetii inoculum and incubated for various times at 37°C to allow penetration of bacteria into cells. Antibiotics are then added to the culture media, whereas antibiotic-free cultures serve as growth controls. During antibiotic challenges, infected cells are revealed by immunofluorescence with anti-C. burnetii antibodies. In most models (289, 291, 416, 417), antibiotic activity is evaluated by determination of the percentage of infected cells in antibiotic-containing cultures compared to that in drug-free controls. More recently, a quantitative model allowing the determination of the C. burnetii inoculum dose in cultures before and after antibiotic exposure has been developed (222).
(i) Acute infections.
Yeaman et al. (417) described an acute C. burnetii infection model in acutely infected L929 cells. In this model, the percentage of infected cells was determined during antibiotic challenge by microscopic examination of cell smears stained by the Gimenez technique. Antibiotic efficacy was determined by daily determination of the percentage of infected cells in cultures receiving antibiotics compared to drug-free controls. Bacteriostatic activity was demonstrated against C. burnetii Nine Mile and Priscilla isolates with doxycycline (10 μg/ml), rifampin (1 μg/ml), and ofloxacin (5 μg/ml). The last two compounds were more effective than doxycycline.
Raoult et al. (291) proposed an acute model allowing more rapid determination of the bacteriostatic activity of antibiotics against C. burnetii. HEL cells were grown in shell vials at 37°C in a 5% carbon dioxide atmosphere. Cell monolayers were infected with a C. burnetii inoculum that would infect 30 to 50% of the cells after a 6-day incubation of cultures in the absence of antibiotics. The percentage of infected cells in cultures was determined by counting fluorescent cells after staining monolayers by IFA (using anti-C. burnetii rabbit serum and a goat anti-rabbit globulin coupled to fluorescein). Bacteriostatic activity was deduced from the reduction of the percentage of infected cells in cultures receiving antibiotics as compared with drug-free controls. The C. burnetii isolate was considered to be resistant to the antibiotic tested when infection in treated cultures was comparable to that in drug-free controls, of intermediate susceptibility when fewer than 10% of cells were infected in antibiotic-containing cultures, and susceptible when no infected cells or few isolated bacteria were seen after a 6-day antibiotic treatment. The authors evaluated the antibiotic activity on 12 C. burnetii isolates including the Nine Mile isolate (the reference strain for acute Q fever), the Priscilla and Q212 isolates (reference strains for chronic infection), and 10 human isolates from patients suffering from chronic Q fever. Amoxicillin at 4 μg/ml and amikacin at 8 μg/ml were ineffective. Chloramphenicol at 8 μg/ml was bacteriostatic against most C. burnetii strains. Erythromycin at 1 μg/ml was not very effective. Ofloxacin, pefloxacin, and ciprofloxacin at 1 μg/ml were effective to various degrees, with ofloxacin being the most effective compound. All isolates were susceptible to co-trimoxazole (2 μg/ml), rifampin (4 μg/ml), tetracycline (4 μg/ml), doxycycline (4 μg/ml), and minocycline (4 μg/ml).
By using the same method, ceftriaxone (4 μg/ml) displayed a bacteriostatic activity against 6 of the 13 strains tested, suggesting that certain β-lactam compounds may have some activity against intracellular C. burnetii (291). More recent investigations have shown that clarithromycin, a new macrolide compound, is more effective than erythromycin (223). Among the newly available fluoroquinolones, levofloxacin was superior to the racemic mixture of ofloxacin (225). Sparfloxacin (292) was the most effective compound of this class tested. The shell vial model is a convenient technique which allows rapid determination of the antibiotic activity of several antibiotics against C. burnetii. This technique would potentially be helpful to determine acquired antibiotic resistance in a particular C. burnetii strain. This might prove most useful for Q fever endocarditis patients, allowing more accurate determination of the optimum antibiotic treatment to be administered.
(ii) Chronic infections.
C. burnetii was reported to be more resistant to antibiotics, especially to doxycycline, when chronically infected cells (i.e., infected for more than 400 days) were used in antibiotic challenges, compared to acutely infected cells (i.e., infected for less than 30 days) (417). Thus, persistently infected cell cultures have been used in antibiotic challenges as models for chronic Q fever infection. Results obtained with such models are considered to be more predictive of the antibiotic activity in chronically infected patients. However, since the goal of the antibiotic treatment of patients with Q fever endocarditis is to eradicate C. burnetii from the infected valve, these models were designed to test the bactericidal activity of antibiotics against C. burnetii.
Yeaman et al. (416) used chronically infected L929 cells to test the bactericidal activity of antibiotics against C. burnetii. Antibiotic activity was determined by the reduction of the percentage of infected cells in antibiotic-containing cultures compared to 100%-infected untreated controls over a period of 10 days. The percentage of infected cells was determined by direct microscopic examination of cells after staining by the Gimenez technique. In this model, the percentage of infected cells was not reduced by tetracycline, erythromycin, or sulfamethoxazole at concentrations up to 10 μg/ml and was only slightly reduced by chloramphenicol, doxycycline, and trimethoprim, suggesting that these drugs were not bactericidal. In contrast, the quinolone compounds difloxacin (10 μg/ml), ciprofloxacin (10 μg/ml), oxolinic acid (10 μg/ml), and rifampin (1 μg/ml) reduced the percentage of infected cells from 100% to 2, 2, 7, and 4%, respectively, after 10 days of continuous culture treatment. Such results were interpreted as demonstrating that these drugs were bactericidal.
However, clinical data did not support the in vitro rickettsiacidal activity of fluoroquinolones. Relapses after discontinuation of antibiotic therapy remained frequent in chronic Q fever patients treated with the combination of a fluoroquinolone plus doxycycline or rifampin (188). Raoult et al. (289) proposed a new cell model of P388D1 macrophage-like cells or L929 cells persistently infected with C. burnetii Nine Mile isolate, in which cell division was blocked with cycloheximide during antibiotic challenges. In this model, doxycycline (4 μg/ml), pefloxacin (1 μg/ml), and rifampin (4 μg/ml) prevented cell death due to the inhibition of C. burnetii multiplication, whereas in cultures receiving antibiotics without a bacteriostatic effect (such as erythromycin), C. burnetii infection resulted in detachment of cell monolayers and cell lysis. However, none of the antibiotics tested reduced the percentage of infected cells, indicating a lack of bactericidal activity. In contrast, combinations of doxycycline with a lysosomotropic alkalinizing agent (chloroquine or amantadine) reduced the percentage of infected cells to 0% on day 9 of experiments (289).
A new chronically infected cell model allowing quantitative analysis of antibiotic bactericidal activity against C. burnetii without the use of cycloheximide has been recently proposed (222). In this model, persistently infected P388D1 cells were used. Antibiotic activity was evaluated quantitatively by enumeration of C. burnetii inoculum before and after antibiotic exposure, and bactericidal activity corresponded to a significant reduction in bacterial titers in antibiotic-containing cultures compared to the initial intracellular C. burnetii inoculum. After a 24-h antibiotic exposure of C. burnetii-infected P388D1 cells, cell monolayers were harvested and lysed by thermal shock. Various dilutions of each C. burnetii suspension were inoculated into uninfected HEL cells and incubated at 37°C in 5% CO2 atmosphere for 6 days. HEL cells were then stained in a direct fluorescence assay. The number of infected cells (containing a fluorescent vacuole) at the appropriate dilution was recorded, and bacterial titers were determined as infecting units per milliliter of medium. In this model, we confirmed the lack of bactericidal activity of doxycycline (4 μg/ml), rifampin (4 μg/ml), and pefloxacin (1 μg/ml). The synergistic activity of doxycycline and the lysosomotropic agent chloroquine was also confirmed. Similar activity was obtained with other lysosomotropic agents such as amantadine and ammonium chloride. The pH of the C. burnetii-containing phagolysosomes and its variations when using the lysosomotropic agents were determined by a spectrofluorometric method (325). We demonstrated that increased antibiotic activity correlated well with increased phagolysosomal pH, suggesting that lysosomotropic agents act by producing an alkaline environment within cells.
Acquired resistance in C. burnetii.
Differences in susceptibility to erythromycin among different C. burnetii strains have been described (291). Differences in susceptibility to doxycycline, ciprofloxacin, and rifampin among various C. burnetii “chronic” isolates were also reported (418). In vitro selection of C. burnetii strains resistant to tetracyclines (37) has been performed. However, the mechanisms of antibiotic resistance in C. burnetii and the genetic determinants for such resistance remain poorly defined. Porins have been demonstrated in C. burnetii cells (24), but their potential role in antibiotic resistance associated with impermeability remain undefined. High-level resistance to fluoroquinolones by nucleotide mutation leading to an amino acid substitution of Gly in place of Glu at position 87 of the GyrA amino acid sequence has recently been reported (248). The heterogeneity in antibiotic susceptibilities among different C. burnetii strains, which cannot be assessed routinely, probably explains in part the variations in efficacy of the antibiotic therapy in patients with Q fever endocarditis. Improved definition of the mechanisms of antibiotic resistance in C. burnetii, and thereby improved detection of such mechanisms, remains essential to improve the prognosis in chronically infected patients.
Clinical Data
Acute Q fever.
Acute Q fever is most often a mild disease that resolves spontaneously within 2 weeks. Additionally, confirmation of the diagnosis by serology is usually available only for convalescent patients, after 2 to 3 weeks of the disease. Thus, clinical evaluation of the efficacy of antibiotic therapy is difficult and comparative studies are scarce. Most clinical data were obtained from patients with Q fever pneumonia. A randomized study was carried out with tetracycline alone compared to placebo (280). Although tetracycline administered at 500 mg four times a day (q.i.d.) reduced the duration of fever by 50%, antibiotic treatment had to be started during the first 3 days of the illness to be effective. Thus, empirical therapy is recommended in severely ill patients because of the possibility of a delay in diagnosis. In a nonrandomized comparison of acute Q fever treatments, the mean duration of fever was 3.3 days in untreated patients, 2 days in patients treated with tetracycline at 500 mg q.i.d. and 1.7 days in patients receiving doxycycline at 100 mg twice a day (b.i.d.) (341). Doxycycline therapy is now recommended instead of tetracycline due to its improved pharmacokinetic properties and less frequent gastric intolerance.
Although doxycycline remains the preferred antibiotic in the treatment of acute Q fever, its administration should be limited in patients with gastric intolerance and is contraindicated in children of less than 8 years old and in pregnant women. Thus, clinical trials have been performed with newly available antimicrobials. Fluoroquinolones such as ofloxacin (200 mg three times a day [t.i.d.]) and pefloxacin (400 mg b.i.d.) have been used successfully to treat acutely infected patients (34). However, these antibiotics had to be administered for 14 to 21 days to be effective. Fluoroquinolones are also contraindicated in children and pregnant women. Macrolides may represent a potential alternative in this population. Erythromycin (500 mg q.i.d.) has been used successfully to treat Q fever pneumonia cases (68, 266). Patients made a rapid clinical improvement and were apyretic by day 4 of antibiotic treatment. However, Marrie et al. (214) reported that erythromycin was ineffective in the treatment of severe cases of Q fever pneumonia, even with daily intravenous dosage of 4 g. Although erythromycin is currently recommended for the antibiotic treatment of atypical pneumonia, it is still unclear if such a regimen is adequate for Q fever pneumonia. Erythromycin susceptibility varies among different C. burnetii strains (291) in vitro, and such antibiotic susceptibility may correlate with discrepancies in the clinical efficiency of this drug to treat acute Q fever cases. In most patients, lack of isolation of the infecting C. burnetii strain precludes the assessment of its susceptibility to erythromycin. Thus, erythromycin should not be considered a reliable alternative for Q fever treatment. In vitro experiments have shown that clarithromycin and roxithromycin are more effective than erythromycin (223), but clinical trials with these newer macrolides are lacking.
Anecdotal reports indicate that other antibiotics, including lincomycin (374), co-trimoxazole (133, 325) and chloramphenicol (57), may be effective in the treatment of Q fever pneumonia. Since Q fever pneumonia is usually a self-limited febrile illness, it is difficult to ascertain the clinical effectiveness of such antibiotic regimens. In vitro experiments have shown that co-trimoxazole may represent a potential alternative in patients for whom both tetracyclines and fluoroquinolones are contraindicated, but more clinical data is needed. Co-trimoxazole was administered for 3 weeks in a 26-year old pregnant women who presented with acute Q fever pneumonia at 8 weeks of gestation. The antibiotic regimen failed to cure the C. burnetii infection, resulting in abortion due to infection of the placenta and fetus (297, 350).
The slow regression of symptoms in patients with Q fever hepatitis has led to anecdotal reports mentioning the clinical benefit of the combination of prednisone with an antibiotic therapy (187). In our experience, these patients typically are men about 50 years old, with persistent fever despite appropriate antibiotic therapy, a sedimentation rate of ≥100/h, and a high level autoantibodies. The body temperature usually decreases to about 38°C on administration of doxycycline and returns rapidly to its normal level when corticoid therapy is added. Prednisone should be administered, at a dose of 40 mg for 48 h, then 20 mg for 48 h, and then 10 mg for an additional 48 h, in such patients when apyrexia is not obtained after 3 days of antibiotic therapy.
In conclusion, doxycycline at 200 mg daily for 14 days is the current recommended regimen for acute Q fever. Fluoroquinolones are considered to be a reliable alternative and have been advocated for patients with Q fever meningoencephalitis, because they penetrate the cerebrospinal fluid (82). Although a macrolide compound or co-trimoxazole may be potential effective alternatives, no reliable antibiotic regimen can currently be recommended for children and pregnant women.
Chronic Q fever.
Q fever endocarditis is the most frequent clinical presentation of chronic Q fever and also the most severe, with a spontaneous death rate which may exceed 65% (40, 93, 133, 136, 169, 260, 280, 283, 307, 311, 341, 353, 369, 381, 409). In fact, most patients with Q fever endocarditis will die of the disease without antibiotic therapy, although its evolution is slow and may last for years. C. burnetii is resistant in vitro to both β-lactam compounds and aminoglycosides (291, 417), and these antibiotics, alone or in combination, are not effective in the treatment of Q fever endocarditis. Tetracyclines used alone are effective (93, 374, 409), but most patients relapse when antibiotic therapy is stopped and death remains frequent. C. burnetii was recovered from cardiac valve tissue removed from a patient with Q fever endocarditis despite 4 years of antibiotic therapy with tetracycline (374). Monotherapy with alternative drugs, including co-trimoxazole, rifampin, and fluoroquinolones, has been administered to Q fever endocarditis patients, especially because of gastric intolerance to tetracyclines (47, 109, 353, 369). Although an initial clinical response was noted, most patients relapsed on antibiotic withdrawal, and these drugs are not considered to be safe antibiotic regimens for the treatment of chronic Q fever. Thus, combination antibiotic therapies have been proposed.
The first antibiotic combinations used were tetracycline with either lincomycin (369, 373, 374) or co-trimoxazole (109, 133, 369, 381), but no significant clinical improvement over tetracycline alone was noted. In one patient, a phase I C. burnetii antibody titer of 1:1,280 persisted and C. burnetii was detected in the removed cardiac valve tissue despite 24 months of administration of tetracycline and co-trimoxazole (381). Also, in patients requiring valve replacement, C. burnetii was detected in the removed valve despite prolonged administration of various antibiotic regimens, all including co-trimoxazole (98, 133, 353, 381). Antibiotic therapy failure and death occurred in a 38 year-old man who received 11 months of therapy with co-trimoxazole followed by 5 months of therapy with co-trimoxazole and rifampin (353). Rifampin was also combined with doxycycline or pefloxacin (47, 188). Although the combination of rifampin with a fluoroquinolone was shown to be effective in vitro (417), its use in vivo is problematic because of its interaction with anticoagulants, which are usually prescribed to patients with cardiac valve defects or with prosthetic valves. More recently, the high in vitro activity of fluoroquinolones against C. burnetii prompted Raoult and coworkers to investigate the combination of doxycycline with either pefloxacin or ofloxacin in 16 patients with Q fever endocarditis (188). The doxycycline-fluoroquinolone combination was significantly superior to doxycycline alone. Q fever endocarditis was initially controlled in all patients, and the global mortality rate was reduced to about 6%. However, relapse rates of over 50% were still observed on antibiotic withdrawal, and a minimum of 3 years of antibiotic therapy was recommended (224, 296). Of these 16 patients, 11 underwent valve replacement because of hemodynamic failure a few weeks to 4 years following diagnosis of Q fever endocarditis. In two cases, viable C. burnetii was recovered from removed cardiac valve tissue despite 9 and 12 months of continuous therapy with doxycycline and a fluoroquinolone, respectively (188). These results show that antibiotics, even when used in combination and for prolonged periods, do not cure most chronically infected hosts. They also confirm the recent demonstration of the lack of antibiotic bactericidal activity against intracellular C. burnetii (222). In vitro experiments have shown that the acidity within the phagolysosomal compartment may partially explain the lack of antibiotic bactericidal activity against intracellular C. burnetii (222). These experiments demonstrated that the combination of doxycycline and an alkalinizing agent of phagolysosomes such as chloroquine displays in vitro bactericidal activity against C. burnetii (222). This combination was recently compared to doxycycline-ofloxacin in 35 patients with Q fever endocarditis (301). In this limited series, the death rate was less than 5% with both regimens. However, relapse occurred in 64.3% of patients receiving doxycycline-ofloxacin but significantly less frequently (14.3%) in those treated with doxycycline-chloroquine. Patients improved more rapidly when given the latter regimen. Treatment duration of 18 months with doxycycline-chloroquine was sufficient to prevent most relapses.
The optimum duration of the antibiotic therapy cannot be accurately determined because no definite criteria for C. burnetii cure are currently available. Suggestions have ranged from 1 year of antibiotic therapy (409) to indefinite administration of antibiotics (264). Since C. burnetii culture from blood samples from patients receiving appropriate antibiotic therapy remains difficult (247), blood cultures cannot be used to predict clinical efficacy of therapy. In contrast, phase I IgG and IgA antibody titers can be easily monitored. Raoult and coworkers have adopted a decrease of phase I IgG and IgA antibody titers to 1:200 or less as the main predictive criterion of clinical cure (288, 296). In our experience, this represents the only currently available objective criterion for the surveillance of chronically infected patients and it is well correlated with the risk of relapse when antibiotic therapy is stopped. When doxycycline-ofloxacin was used, phase I antibody titers of ≤1:200 were not obtained until after at least three years of antibiotic therapy, whereas they were obtained after 18 months of therapy with doxycycline-chloroquine. Thus, our current recommendations for the antibiotic treatment of Q fever endocarditis are at least 18 months of therapy with doxycycline (100 mg b.i.d.) and chloroquine (200 mg t.i.d.) or at least 3 years of therapy doxycycline (100 mg b.i.d.) and ofloxacin (200 mg t.i.d.) in patients to whom the first antibiotic regimen cannot be administered.
Clinical and biological evaluation should be performed on a monthly basis during antibiotic therapy, including blood cell numeration, measurement of hepatic enzyme levels in serum and creatinine levels in serum, and Q fever serology. In our laboratory, lymphocyte typing is also performed because, in our experience, inversion of the T4/T8 lymphocytic ratio indicates relapse in 50% of cases (282). More recently, upregulation of IL-10 gene transcription by PBMC from Q fever endocarditis patients has been demonstrated, and the presence of IL-10 in PBMC supernatants has been proposed as a marker of disease relapse (49). An echocardiogram should be performed every 3 months. Chloroquine used at therapeutic dosages may have some deleterious effects, including the risk of retinopathy, necessitating a regular ophthalmologic examination. Chloroquine levels in serum should be monitored to ensure that they are maintained at of 1 ± 0.2 mg/liter. However, in our experience, the chloroquine dose often has to be adjusted because of intolerance. In most patients, chloroquine was administered at 200 mg three times a day for the first 3 to 6 months and then decreased to 200 mg twice a day or even once a day.
A clinical and biological evaluation should also be performed after antibiotic treatment has been stopped. We perform such an evaluation (including blood cell enumeration, hepatic enzyme level measurement, lymphocytic typing and Q fever serology) each month for the first 6 months following antibiotic withdrawal, then every 6 months for 2 years, and once a year thereafter. An echocardiogram is also performed every 6 months for the first 2 years in patients without symptoms.
Valve replacement has been proposed in addition to antibiotic treatment in Q fever endocarditis (98, 176). Cardiac surgery was proposed mainly as a result of hemodynamic failure. In such cases, the presence of C. burnetii in removed cardiac tissue should be systematically assessed by both culture and PCR. Interestingly, C. burnetii has been recovered from macroscopically unremarkable cardiac valve tissue (133). In a previous study (188), valve replacement was performed in 19 patients with hemodynamic failure and C. burnetii was grown from the cardiac valve tissue removed from 9 patients. In one patient who underwent two valve replacements, C. burnetii was cultured from the removed cardiac valve tissue on both occasions, demonstrating that surgery itself does not eradicate Q fever endocarditis. Since Q fever endocarditis is a disseminated disease, surgery should be reserved for patients with hemodynamic complications and should be combined with an antibiotic therapy to prevent relapse. In patients undergoing valve replacement, antibiotics are also needed, because of the risk of infection of the prosthetic valve from a dormant site, including from the other cardiac valves (264). The increasing use of cardiac surgery for valve replacement over recent years has led to an increase in the number of prosthetic valve endocarditis cases reported worldwide, especially endocarditis due to Staphylococcus species. Q fever prosthetic valve endocarditis has also been increasingly reported (98, 240, 307, 332, 344).
In conclusion, experimental models have led to the determination of the intracellular susceptibility of C. burnetii to antibiotics. Only tetracyclines, rifampin, co-trimoxazole, fluoroquinolones, and the newer macrolides (especially clarithromycin) have displayed significant in vitro activity. The in vitro activity of tetracyclines, fluoroquinolones, and co-trimoxazole correlates well with clinical data. However, it should be noted that few C. burnetii isolates have been used in these antibiotic experiments and that heterogeneity in antibiotic susceptibility has been reported (342, 416, 417, 418). Difficulty in isolating C. burnetii means that the antibiotic susceptibility of each clinical isolate is not usually determined. Thus, results obtained in in vitro models with reference strains may not accurately reflect the clinical situation. Relapses or failures despite appropriate antibiotic therapy may reflect resistance in individual C. burnetii strains rather than poor intracellular antibiotic activity, at least in some patients. Additionally, contradictory results have been obtained when investigating the bactericidal activity of antibiotics against C. burnetii, and this has to be considered when treating chronic Q fever. The lack of bactericidal activity of most antibiotics against intracellular C. burnetii is indicative of the difficulty in eradicating C. burnetii in chronically infected patients.
VACCINE PROPHYLAXIS
Since Q fever is enzootic among wild and domestic animals, controlling C. burnetii infection in susceptible animals is difficult. Q fever vaccines were first tested in animals for this purpose (4, 408). Since C. burnetii infection in animals bred for consumption has been associated with abortions (sheep and goats) and infertility (cattle) (149, 261, 319, 390), prevention of Q fever infection may have an economic impact. The first vaccines available were composed of inactivated whole C. burnetii cells, whereas a chloroform-methanol residue of C. burnetii cells have been proposed more recently (38, 319, 404). Chloroform-methanol residue vaccines were shown to be better tolerated in animals than C. burnetii whole-cell vaccines (94, 408). Q fever vaccines varied in their composition, including the strain and phase of C. burnetii used. Vaccines prepared from phase I C. burnetii organisms were more protective than those prepared from phase II bacteria, whereas cross-protection among various C. burnetii strains was found in vaccinated guinea pigs (258). When tested on cattle and sheep, these vaccines showed different protective effects against experimental and natural C. burnetii infection in seronegative animals (4, 26, 38, 319, 408). Q fever vaccination was also shown to protect cattle against abortion (26), low fetal weight (38), and chronic infertility (319). In contrast, vaccination did not eradicate C. burnetii in animals naturally infected prior to vaccination and C. burnetii shedding persisted unchanged (319). In Europe, a vaccine containing both phase II C. burnetii and Chlamydia psittaci has been marketed to protect cattle and goats against fertility problems caused by these two agents (175). However, a Q fever outbreak was reported in France in persons in contact with vaccinated goats (102). Moreover, this phase II-containing vaccine was suspected of increasing the shedding of C. burnetii in milk for several months when administered to previously infected animals (319). Q fever vaccination of domestic animals (mainly cattle, sheep, and goats) is currently not widely used because it is protective and safe only in animals that are uninfected at the time of vaccination. Extensive evaluation of the protective effect of such a vaccination on animal and human health compared to the cost of such prophylactic measures is lacking.
Q fever vaccines used in humans consist of killed, purified phase I C. burnetii whole cells, which contain LPS-protein complex antigens (168, 408). Available vaccines are prepared with a single strain and are therefore monovalent. Because the genetic and antigenic heterogeneity among C. burnetii strains has been demonstrated (130, 140, 146, 359, 402), the protective potency of such vaccines in various geographic areas remains to be established. The use of pooled phase I corpuscular or soluble Q fever vaccine prepared from representative strains of different antigenic groups may represent an alternative if polyvalent vaccines are needed.
An attenuated M-44 C. burnetii strain was proposed for prophylaxis of Q fever in the USSR as early as 1965 (116). However, this vaccine has been abandoned because of concern about its toxicity and the possibility of reactivation. A corpuscular vaccine that consisted of formalin-killed phase I C. burnetii cells grown in chicken embryo yolk sac was proposed (168). A formalin-inactivated Q fever vaccine (Q-Vax; Commonwealth Serum Laboratories), prepared from phase I C. burnetii Henzerling strain, was approved for the general market in Australia in March 1989. Although highly immunogenic, this type of vaccine may induce adverse effects, especially when administered in previously infected populations (28). In addition, decreased lymphocyte responsiveness to mitogens (66), immunopathological changes including hepatosplenomegaly, and death have been reported in mice receiving this vaccine (66, 165, 404). Such adverse effects could be abolished by pretreatment of phase I C. burnetii cells with chloroform-methanol while maintaining the immunogenicity of the vaccine (404, 407). Such a chloroform-methanol residue-based vaccine has recently been proposed for vaccination in humans (111). A soluble vaccine containing trichloroacetic acid-extracted antigen from phase I C. burnetii Nine Mile strain was also proposed (36) and successfully used in people whose work was likely to lead to their exposure to C. burnetii in Czechoslovakia (164). Trichloroacetic acid-extract antigen treated with chloroform-methanol was also tested and displayed fewer side effects but less immunogenicity than the same antigen without chloroform-methanol treatment (167).
The postvaccination immune response can be assessed both by evaluation of the antibody response and cell-mediated immunity against C. burnetii antigens (15, 28, 29, 413). Since cell-mediated immunity plays a predominant role in resistance to C. burnetii infection, tests exploring such immune system mechanisms are considered to be more predictive of protection against Q fever. The skin test antigen was found to be a highly effective indicator of the postvaccination immune response (15, 166, 193). Other tests, i.e., lymphocyte blast transformation, migration of PBL, formation of IFN-γ by PBL after stimulation by C. burnetii antigens and phagocytosis of phase I C. burnetii by PBL, have also been described (168).
The efficacy of Q fever vaccines in humans has been demonstrated primarily in vaccinated laboratory personnel or abattoir workers (1, 36, 164, 201, 202, 327). Among 924 nonimmune abattoir workers in Australia who volunteered for a formalin-inactivated Q fever vaccine (phase I C. burnetii Henzerling strain, now referred to as the Q-Vax vaccine), no Q fever case was diagnosed within 18 months of vaccination whereas 34 cases were recorded among 1,349 unvaccinated controls (201). Protection occurred despite detected seroconversion in only 64.3% of 409 vaccinated patients for whom serological evaluation was performed. Erythema or tenderness at the site of vaccine inoculation was the most common adverse effect, whereas transient headache, shivering, or flu-like symptoms were observed in 10 to 18% of vaccinated subjects. Recent investigations of abattoir workers in the same country (1, 202), using the Q-Vax vaccine, have shown 100% protection for at least 5 years, although natural Q fever infection supervened in 2 of 2,553 vaccinated subjects in the latter investigation (1) a few days following vaccination, before specific immunity had developed. A Q fever chemovaccine (trichloroacetic acid-extracted antigen from phase I C. burnetii Nine Mile strain) was evaluated in agricultural workers in Central Slovakia between 1977 and 1978 and in laboratory personal (State Veterinary Institute) in East Slovakia and workers of a cotton-processing plant in Moravia in 1980 (164). Of the 1,256 volunteers tested, 202 (16.1%) had positive skin tests prior to vaccination, indicating previous Q fever infection. The immunogenicity of the vaccine was shown by seroconversion 5 weeks after vaccination in 393 of 714 patients (55%) receiving a single dose of vaccine and even more frequently (74.4%) in those receiving two doses. Local reactions of minor severity and systemic reactions (flu-like symptoms) occurred in 39.6 and 4.3% of vaccinated persons, respectively, although reactogenicity was more frequent in previously infected individuals. The protective effect of the vaccine was not, however, evaluated.
Since Q fever in humans is often an occupational hazard, vaccination should be considered primarily in exposed populations (259). Thus, Q fever vaccine should be recommended for livestock handlers, processors of animal products (including abattoir workers), persons in contact with dairy products, veterinarians, and laboratory personnel working with C. burnetii-infected animals, especially pregnant sheep. However, recent epidemiological investigations have shown that Q fever cases are increasingly reported in urban areas, especially in persons in contact with pets during parturition. Thus, vaccination should probably be considered in persons not professionally exposed but at risk for chronic Q fever, including patients with cardiac valve defects, vascular aneurysms, or prostheses and immunocompromised patients. However, investigations into the prophylactic effectiveness and safety of a Q fever vaccine in such populations are lacking. A single-dose vaccine is currently recommended because more frequent local and systemic reactions are likely when repeat injections are performed (259). To prevent severe postvaccination reactions (including local erythema, induration, granulomas, sterile abscesses, and systemic reactions), vaccination should not be performed in patients previously sensitized by natural Q fever infection (28, 178). Ideally, both humoral and cellular immune responses to C. burnetii should be assessed prior to vaccination. Skin and lymphocyte proliferation tests have been reported to be more predictive of postvaccination adverse effects than is serology (15, 164).
ACKNOWLEDGMENTS
We thank Richard Birtles and Emma Birtles for help in the preparation of the manuscript.
REFERENCES
- 1.Ackland J R, Worswick D A, Marmion B P. Vaccine prophylaxis of Q fever. A follow-up study of the efficacy of Q-Vax (CSL) 1985–1990. Med J Aust. 1994;160:704–708. [PubMed] [Google Scholar]
- 2.Aguirre Errasti C, Montejo Baranda M, Hernandez Almaraz J L, de la Hoz Torres C, Martinez Gutierrez E, Villate Navarro J C, Sobradillo Pena V. An outbreak of Q fever in the Basque country. Can Med Assoc J. 1984;131:48–49. [PMC free article] [PubMed] [Google Scholar]
- 3.Aitken I D, Bögel K, Cracea E, Edlinger E, Houwers D, Krauss H, Rady M, Rehacek J, Schiefer H G, Schmeer N, Tarasevich I V, Tringali G. Q fever in Europe: current aspects of aetiology, epidemiology, human infection, diagnosis and therapy. Infection. 1987;15:323–327. doi: 10.1007/BF01647731. [DOI] [PubMed] [Google Scholar]
- 4.Aitken I D. Clinical aspects and prevention of Q fever in animals. Eur J Epidemiol. 1989;5:420–424. doi: 10.1007/BF00140132. [DOI] [PubMed] [Google Scholar]
- 5.Akporiaye E T, Rowatt J D, Aragon A A, Baca O G. Lysosomal response of a murine macrophage-like cell line persistently infected with Coxiella burnetii. Infect Immun. 1983;40:1155–1162. doi: 10.1128/iai.40.3.1155-1162.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akporiaye E T, Baca O G. Superoxide production and superoxide dismutase and catalase activities in Coxiella burnetii. J Bacteriol. 1983;154:520–523. doi: 10.1128/jb.154.1.520-523.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexiou-Daniil S, Antiniadis A, Pappas K, Doutsos J, Malisiovas N, Papapanagiotou I. Incidence of Coxiella burnetii infections in Greece. Hell Iatriki. 1990;56:251–255. [Google Scholar]
- 8.Amano K-I, Williams J C. Chemical and immunological characterization of lipopolysaccharides from phase I and phase II Coxiella burnetii. J Bacteriol. 1984;160:994–1002. doi: 10.1128/jb.160.3.994-1002.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amano K I, Williams J C, McCaul T F, Peacock M G. Biochemical and immunological properties of Coxiella burnetii cell wall and peptidoglycan-protein complex fractions. J Bacteriol. 1984;160:982–988. doi: 10.1128/jb.160.3.982-988.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amano K I, Williams J C, Missler S R, Reinhold V N. Structure and biological relationships of Coxiella burnetii lipopolysaccharides. J Biol Chem. 1987;262:4740–4747. [PubMed] [Google Scholar]
- 11.Andrews P S, Marmion B P. Chronic Q fever. Br Med J. 1959;2:983–985. doi: 10.1136/bmj.2.5158.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anonymous. Q fever in the Isle of Wight. Commun Dis Rep. 1992;2:65. [PubMed] [Google Scholar]
- 13.Anonymous. Q fever outbreak—Germany, 1996. Morbid Mortal Weekly Rep. 1997;46:29–32. [PubMed] [Google Scholar]
- 13a.Anonymous. Experimental Q fever in man. Br Med J. 1950;1:1000. . (Editorial.) [Google Scholar]
- 13b.Anonymous. Comment on Q fever transmitted by blood transfusion—United States. Can Dis Weekly Rep. 1977;3:210. . (Editorial.) [Google Scholar]
- 14.Antoine J C, Prina E, Jouanne C, Bongrand P. Parasitophorous vacuoles of Leishmania amazonensis infected macrophages maintain an acidic pH. Infect Immun. 1990;58:779–787. doi: 10.1128/iai.58.3.779-787.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ascher M S, Berman M A, Ruppanner R. Initial clinical and immunologic evaluation of a new phase I Q fever vaccine and skin test in humans. J Infect Dis. 1983;148:214–222. doi: 10.1093/infdis/148.2.214. [DOI] [PubMed] [Google Scholar]
- 16.Ascher M S, Williams J C, Berman M A. Dermal granulomatous hypersensitivity in Q fever: comparative studies of the granulomatous potential of whole cell of Coxiella burnetii phase I and subfractions. Infect Immun. 1983;42:887–889. doi: 10.1128/iai.42.3.887-889.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atzopien E, Baumgärtner W, Artelt A, Thiele D. Valvular endocarditis occurs as a part of a disseminated Coxiella burnetii infection in immunocompromised BALB/cJ (H-2d) mice infected with the Nine Mile isolate of C. burnetii. J Infect Dis. 1994;170:223–226. doi: 10.1093/infdis/170.1.223. [DOI] [PubMed] [Google Scholar]
- 18.Ayres J G, Smith E G, Flint N. Protracted fatigue and debility after acute Q fever. Lancet. 1996;347:978–979. doi: 10.1016/s0140-6736(96)91470-1. [DOI] [PubMed] [Google Scholar]
- 19.Ayres J G, Flint N, Smith E G, Tunnicliffe W S, Fletcher T J, Hammond K, Ward D, Marmion B P. Post-infection fatigue syndrome following Q fever. Q J Med. 1998;91:105–123. doi: 10.1093/qjmed/91.2.105. [DOI] [PubMed] [Google Scholar]
- 20.Babudieri B. Q fever: a zoonosis. Adv Vet Sci. 1959;5:81. [Google Scholar]
- 21.Baca O G, Akporiaye E T, Aragon A S, Martinez I L, Robles M V, Warner N L. Fate of phase I and phase II Coxiella burnetii in several macrophage-like tumor cell lines. Infect Immun. 1981;33:258–266. doi: 10.1128/iai.33.1.258-266.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baca O G, Paretsky D. Q fever and Coxiella burnetii: a model for host-parasite interactions. Microbiol Rev. 1983;47:127–149. doi: 10.1128/mr.47.2.127-149.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baca O G, Scott T O, Akporiaye E T, DeBlassie R, Crissman H A. Cell cycle distribution patterns and generation times of L929 fibroblast cells persistently infected with Coxiella burnetii. Infect Immun. 1985;47:366–369. doi: 10.1128/iai.47.2.366-369.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banerjee-Bhatnagar N, Bolt C R, Williams J C. Pore-forming activity of Coxiella burnetii outer membrane protein oligomer comprised of 29.5- and 31-Kda polypeptides. Inhibition of porin activity by monoclonal antibodies 4E8 and 4D6. Ann N Y Acad Sci. 1996;791:378–401. doi: 10.1111/j.1749-6632.1996.tb53545.x. [DOI] [PubMed] [Google Scholar]
- 25.Beaman M H, Hung J. Pericarditis associated with tick-borne Q fever. Aust N Z J Med. 1989;19:254–256. doi: 10.1111/j.1445-5994.1989.tb00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behymer D E, Biberstein E L, Riemann H P, Franti C E, Sawyer M, Ruppanner R, Crenshaw G L. Q fever (Coxiella burnetii) investigations in dairy cattle: challenge of immunity after vaccination. Am J Vet Res. 1976;37:631–634. [PubMed] [Google Scholar]
- 27.Bell J A, Beck M D, Huebner R J. Epidemiologic studies of Q fever in southern California. JAMA. 1950;142:868–872. doi: 10.1001/jama.1950.02910300006002. [DOI] [PubMed] [Google Scholar]
- 28.Bell J F, Lackman D B, Meis A, Hadlow W J. Recurrent reaction at site of Q fever vaccination in a sensitized person. Mil Med. 1964;7:591–595. [PubMed] [Google Scholar]
- 29.Bell J F, Luoto L, Casey M, Lackman D B. Serologic and skin test response after Q fever vaccination by the intracutaneous route. J Immunol. 1964;93:403–408. [PubMed] [Google Scholar]
- 30.Benson W W, Brock D W, Mather J. Serologic analysis of a penitentiary group using raw milk from a Q fever infected herd. Public Health Rep. 1963;78:707–710. [PMC free article] [PubMed] [Google Scholar]
- 31.Bental T, Fejgin M, Keysary A, Rzotkiewicz S, Oron C, Nachum R, Beyth Y, Lang R. Chronic Q fever of pregnancy presenting as Coxiella burnetii placentitis: successful outcome following therapy with erythromycin and rifampin. Clin Infect Dis. 1995;21:1318–1321. doi: 10.1093/clinids/21.5.1318. [DOI] [PubMed] [Google Scholar]
- 32.Berkovitch M, Aladjem M, Beer S, Cohar K. A fatal case of Q fever hepatitis in a child. Helv Paediatr Acta. 1985;40:87–91. [PubMed] [Google Scholar]
- 33.Berstein M, Edmondson H A, Barbour B H. The liver lesion in Q fever. Arch Intern Med. 1965;116:491–494. [PubMed] [Google Scholar]
- 34.Bertrand A, Janbon F, Jonquet O, Reynes J. Infections par les rickettsiales et fluoroquinolones. Pathol Biol (Paris) 1988;36:493–495. [PubMed] [Google Scholar]
- 35.Blondeau J M, Williams J C, Marrie T J. The immune response to phase I and II Coxiella burnetii antigens as measured by western immunoblotting. Ann N Y Acad Sci. 1990;590:187–202. doi: 10.1111/j.1749-6632.1990.tb42220.x. [DOI] [PubMed] [Google Scholar]
- 36.Brezina R, Schramek S, Kazar J, Urvolgyi J. Q fever chemovaccine for human use. Acta Virol. 1974;18:269. [Google Scholar]
- 37.Brezina R, Schramek S, Kazar J. Selection of chlortetracycline-resistant strain of Coxiella burnetii. Acta Virol. 1975;19:496. [PubMed] [Google Scholar]
- 38.Brooks D L, Ermel R W, Franti C E, Ruppanner R, Behymer D E, Williams J C, Stephenson J C. Q fever vaccination of sheep: challenge of immunity in ewes. Am J Vet Res. 1986;47:1235–1238. [PubMed] [Google Scholar]
- 39.Brouqui P, Dupont H T, Drancourt M, Bourgeade A, Raoult D. Spotless boutonneuse fever. Clin Infect Dis. 1992;14:114–116. doi: 10.1093/clinids/14.1.114. [DOI] [PubMed] [Google Scholar]
- 40.Brouqui P, Tissot Dupont H, Drancourt M, Berlan Y, Etienne J, Leport C, Goldstein F, Massip P, Micoud M, Bertrand A, Raoult D. Epidemiologic and clinical features of chronic Q fever: 92 cases from France (1982–1990) Arch Intern Med. 1993;153:642–648. doi: 10.1001/archinte.153.5.642. [DOI] [PubMed] [Google Scholar]
- 41.Brouqui P, Dumler J S, Raoult D. Immunohistologic demonstration of Coxiella burnetii in the valves of patients with Q fever endocarditis. Am J Med. 1994;97:451–458. doi: 10.1016/0002-9343(94)90325-5. [DOI] [PubMed] [Google Scholar]
- 42.Brouqui, P., J. R. Harle, J. Delmont, C. Frances, P. J. Weller, and D. Raoult. 1997. African tick-bite fever. An imported spotless rickettsiosis. 157:119–124. [PubMed]
- 43.Bru J P, Stahl J P, Gaillat J, Favier A, Micoud M. Enquete épidémiologique de la fièvre Q dans une commune rurale. Lyon Med. 1983;249:459–461. [Google Scholar]
- 44.Buhariwalla F, Cann B, Marrie T J. A dog related outbreak of Q fever. Clin Infect Dis. 1996;23:753–755. doi: 10.1093/clinids/23.4.753. [DOI] [PubMed] [Google Scholar]
- 45.Burnet F M, Freeman M. Experimental studies on the virus of Q fever. Med J Aust. 1937;2:299–302. [Google Scholar]
- 46.Burton P R, Stueckemann J, Welsh R M, Paretsky D. Some ultrastructural effects of persistent infections by the rickettsia Coxiella burnetii in mouse L cells and green monkey kidney (Vero) cells. Infect Immun. 1978;21:556–566. doi: 10.1128/iai.21.2.556-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cacoub P, Wechsler B, Chapelon C, Thibon M, Godeau P. Q-fever endocarditis and treatment with the fluoroquinolones. Arch Intern Med. 1991;151:816–818. doi: 10.1001/archinte.151.4.816a. [DOI] [PubMed] [Google Scholar]
- 48.Canonico P G, van Zwieten M J, Christmas W A. Purification of large quantities of Coxiella burnetii by density gradient centrifugation. Appl Microbiol. 1972;23:1015–1022. doi: 10.1128/am.23.5.1015-1022.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Capo C, Zaffran Y, Zugun F, Houpikian P, Raoult D, Mege J L. Production of interleukin-10 and transforming growth factor β by peripheral blood mononuclear cells in Q fever endocarditis. Infect Immun. 1996;64:4143–4147. doi: 10.1128/iai.64.10.4143-4147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Capo C, Zugun F, Stein A, Tardei G, Lepidi H, Raoult D, Mege J L. Upregulation of tumor necrosis factor alpha and interleukin-1β in Q fever endocarditis. Infect Immun. 1996;64:1638–1642. doi: 10.1128/iai.64.5.1638-1642.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caughey J E. Pleuropericardial lesion in Q fever. Br Med J. 1977;1:1447. doi: 10.1136/bmj.1.6074.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen S-Y, Hoover T A, Thompson H A, Williams J C. Characterization of the origin of DNA replication of the Coxiella burnetii chromosome. Ann N Y Acad Sci. 1990;590:491–503. doi: 10.1111/j.1749-6632.1990.tb42259.x. [DOI] [PubMed] [Google Scholar]
- 53.Chen S-Y, Vodkin M, Thompson H A, Williams J C. Isolated Coxiella burnetii synthesizes DNA during acid activation in the absence of host cells. J Gen Microbiol. 1990;136:89–96. doi: 10.1099/00221287-136-1-89. [DOI] [PubMed] [Google Scholar]
- 54.Chevalier P, Vandenesch F, Brouqui P, Kirkorian G, Tabib A, Etienne J, Raoult D, Loire R, Touboul P. Fulminant myocardial failure in a previously healthy young man. Circulation. 1997;95:1654–1657. doi: 10.1161/01.cir.95.6.1654. [DOI] [PubMed] [Google Scholar]
- 55.Clark W H, Lennette E H, Railsback O C, Romer M S. Q fever studies in California. VII. Clinical features in one hundred and eighty cases. Arch Intern Med. 1951;88:155–167. doi: 10.1001/archinte.1951.03810080023003. [DOI] [PubMed] [Google Scholar]
- 56.Clark W H, Lennette E H, Romer M S. Q fever studies in California. XI. An epidemiologic summary of 350 cases occurring in northern California in 1948–1949. Am J Hyg. 1951;54:319–330. [PubMed] [Google Scholar]
- 57.Clark W H, Lennette E H. Treatment of Q fever with antibiotics. Ann N Y Acad Sci. 1952;55:1004–1006. doi: 10.1111/j.1749-6632.1952.tb22660.x. [DOI] [PubMed] [Google Scholar]
- 58.Connolly J H, Coyle P V, Adgey A A, O’Neill H J, Simpson D M. Clinical Q fever in Northern Ireland. Ulster Med J. 1990;59:137–44. [PMC free article] [PubMed] [Google Scholar]
- 59.Constans J, Mathieu F, Bergeaud S, Ducloux G, Fleury B, De Mascarel A, Conri C. Q fever: diagnosis contribution of bone marrow biopsy. A propos of a case. Rev Med Interne. 1987;8:511–512. doi: 10.1016/s0248-8663(87)80202-3. [DOI] [PubMed] [Google Scholar]
- 60.Constantinidis K, Jenkins J P R. Chronic Q fever endocarditis. Practitioner. 1979;222:533–536. [PubMed] [Google Scholar]
- 61.Cottalorda J, Jouve J L, Bollini G, Touzet P, Poujol A, Kelberine F, Raoult D. Osteoarticular infection due to Coxiella burnetii in children. J Pediatr Orthop. 1995;4:219–221. doi: 10.1097/01202412-199504020-00018. [DOI] [PubMed] [Google Scholar]
- 62.Cowley R, Fernandez F, Freemantle W, Rutter D. Enzyme immunoassay for Q fever: comparison with complement fixation and immunofluorescence tests and dot immunoblotting. J Clin Microbiol. 1992;30:2451–2455. doi: 10.1128/jcm.30.9.2451-2455.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cox H R. A filter-passing infectious agent isolated from ticks. III. Description of organism and cultivation experiments. Public Health Rep. 1938;53:2270–2276. [Google Scholar]
- 64.Cox H R, Bell E J. The cultivation of Rickettsia diaporica in tissue culture and in the tissues of developing chicken embryos. Public Health Rep. 1939;54:2171–2175. [Google Scholar]
- 65.Cox H R. Rickettsia diaporica and American Q fever. Am J Trop Med Hyg. 1940;20:463–465. [Google Scholar]
- 66.Damrow T A, Williams J C, Waag D M. Suppression of in vitro lymphocyte proliferation in C57BL/10ScN mice vaccinated with phase I Coxiella burnetii. Infect Immun. 1985;47:149–156. doi: 10.1128/iai.47.1.149-156.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.D’Angelo J L, Baker E F, Schlosser W. Q fever in the United States, 1948–1977. J Infect Dis. 1979;139:613–615. doi: 10.1093/infdis/139.5.613. [DOI] [PubMed] [Google Scholar]
- 68.D’Angelo J J, Hetherington R. Q fever treated with erythromycin. Br Med J. 1979;2:305–306. doi: 10.1136/bmj.2.6185.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davies T R, Edwards Y, Morgan A, Caul E O. Prevalence of Q fever in a rural practice. J Public Health Med. 1997;19:324–327. doi: 10.1093/oxfordjournals.pubmed.a024638. [DOI] [PubMed] [Google Scholar]
- 70.Davis G E, Cox H R. A filter-passing infectious agent isolated from ticks. I. Isolation from Dermacentor andersoni, reactions in animals, and filtration experiments. Public Health Rep. 1938;53:2259–2261. [Google Scholar]
- 71.Davis G E. Rickettsia diaporica: recovery of three strains from Dermacentor andersoni collected in southeastern Wyoming: their identity with Montana strain 1. Public Health Rep. 1939;54:2219–2225. [Google Scholar]
- 72.Delaney J C, Roberts H L. Q fever endocarditis and chronic liver involvement. Practitioner. 1975;214:243–245. [PubMed] [Google Scholar]
- 73.Dellacasagrande J, Capo C, Raoult D, Mege J L. IFN-γ-mediated control of Coxiella burnetii survival in monocytes: the role of cell apoptosis and TNF1. J Immunol. 1999;162:2259–2265. [PubMed] [Google Scholar]
- 74.Delsol G, Pellegrin M, Familiades J, Auvergnat J C. Bone marrow lesions in Q fever. Blood. 1978;52:637–638. [PubMed] [Google Scholar]
- 75.Derrick E H. “Q” fever, new fever entity: clinical features, diagnosis and laboratory investigation. Med J Aust. 1937;2:281–299. doi: 10.1093/clinids/5.4.790. [DOI] [PubMed] [Google Scholar]
- 76.Derrick E H. The course of infection with Coxiella burnetii. Med J Aust. 1973;1:1051–1057. [PubMed] [Google Scholar]
- 77.Diaz Morant V, Mateo Sanchez J I, Lara Fernandez A, Cabello Rueda F. Pleuropericarditis caused by Q fever. An Med Interna. 1995;12:568–569. [PubMed] [Google Scholar]
- 78.Dindinaud G, Aigius G, Burucoa C, Senet J M, desahyes M, Magnin G. Q fever and fetal death in utero. Two cases. J Gynecol Obstet Biol Reprod. 1991;20:969–972. [PubMed] [Google Scholar]
- 79.Dobija-Domaradzki M, Hausser J L, Gosselin F. Coexistence of Legionnaire’s disease and Q fever in a single patient. Can Med Assoc J. 1984;130:1022–1023. [PMC free article] [PubMed] [Google Scholar]
- 80.Doller G, Doller P C, Gerth H J. Early diagnosis of Q fever: detection of immunoglobulin M by radioimmunoassay and enzyme immunoassay. Eur J Clin Microbiol Infect Dis. 1984;3:550–553. doi: 10.1007/BF02013617. [DOI] [PubMed] [Google Scholar]
- 81.Domingo P, Orobitg J, Colomina J, Alvarez E, Cadafalch J. Liver involvement in acute Q fever. Chest. 1988;94:895–896. doi: 10.1378/chest.94.4.895. [DOI] [PubMed] [Google Scholar]
- 82.Drancourt M, Raoult D, Xeridat B, Milandre L, Nesri M, Dano P. Q fever meningoencephalitis in five patients. Eur J Epidemiol. 1991;7:134–138. doi: 10.1007/BF00237356. [DOI] [PubMed] [Google Scholar]
- 83.DuPont H L, Hornick R B, Levin H S, Rapoport M I, Woodward T E. Q fever hepatitis. Ann Intern Med. 1971;74:198–206. doi: 10.7326/0003-4819-74-2-198. [DOI] [PubMed] [Google Scholar]
- 84.Dupuis G, Peter O, Pedroni D, Petite J. Clinical aspects observed during an epidemic of 415 cases of Q fever. Schweiz Med Wochenschr. 1985;115:814–818. [PubMed] [Google Scholar]
- 85.Dupuis G, Peter O, Lüthy R, Nicolet J, Peacock M, Burgdorfer W. Serological diagnosis of Q fever endocarditis. Eur Heart J. 1986;7:1062–1066. doi: 10.1093/oxfordjournals.eurheartj.a062016. [DOI] [PubMed] [Google Scholar]
- 86.Dupuis G, Petite J, Peter O, Vouilloz M. An important outbreak of human Q fever in a Swiss alpine valley. Int J Epidemiol. 1987;16:282–287. doi: 10.1093/ije/16.2.282. [DOI] [PubMed] [Google Scholar]
- 87.Durack D T, Lukes A S, Bright D K the Duke Endocarditis Service. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med. 1994;96:200–209. doi: 10.1016/0002-9343(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 88.Dyer D E, Gibbons V L, Brady L M, Cunningham A L. Serological reaction to Legionella pneumophila group 4 in a patient with Q fever. J Infect Dis. 1988;158:499–500. . (Letter.) [PubMed] [Google Scholar]
- 89.Echaniz A, Miguez E, Llinarez P, Diz-Lois F. Recurrent pericarditis caused by Q fever. Rev Clin Esp. 1992;191:170–171. [PubMed] [Google Scholar]
- 90.Reference deleted.
- 91.Reference deleted.
- 92.Eklund C M, Parker R R, Lackman D B. A case of Q fever probably contracted by exposure to ticks in nature. Public Health Rep. 1947;62:1413–1416. [PubMed] [Google Scholar]
- 93.Ellis M E, Smith C C, Moffat M A. Chronic and fatal Q-fever infection: a review of 16 patients seen in North-East Scotland (1967–1980) Q J Med. 1983;52:54–66. [PubMed] [Google Scholar]
- 94.Elliott J J, Ruble D L, Zaucha G M, Jaax G P, Waag D M. Comparison of Q fever cellular and chloroform-methanol residue vaccines as skin test antigens in the sensitized guinea pig. Acta Virol. 1998;42:147–155. [PubMed] [Google Scholar]
- 95.Enright J B, Franti C R, Longhurst W M, Behymer D E, Wright M E, Dutson V J. Coxiella burnetii in a wildlife-livestock environment: antibody response of ewes and lambs in an endemic Q fever area. Am J Epidemiol. 1971;94:62–71. doi: 10.1093/oxfordjournals.aje.a121296. [DOI] [PubMed] [Google Scholar]
- 96.Ferguson I C, Craik J E, Grist N R. Clinical, virological, and pathological findings in a fatal case of Q fever endocarditis. J Clin Pathol. 1962;15:235–237. doi: 10.1136/jcp.15.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fergusson R J, Shaw T R D, Kitchin A H, Matthews M B, Inglis J M, Peutherer J F. Subclinical chronic Q fever. Q J Med. 1985;57:669–676. [PubMed] [Google Scholar]
- 98.Fernandez Guerrero M L, Muelas Aguado J M, Renedo G, Fraile J, Soriano F, De Villalobos E. Q fever endocarditis on porcine bioprosthetic valves. Clinicopathologic features and microbiologic findings in three patients treated with doxycycline, cotrimoxazole, and valve replacement. Ann Intern Med. 1988;108:209–213. doi: 10.7326/0003-4819-108-2-209. [DOI] [PubMed] [Google Scholar]
- 99.Ferrante M A, Dolan M J. Q fever meningoencephalitis in a soldier returning from the Persian Gulf War. Clin Infect Dis. 1993;16:489–496. doi: 10.1093/clind/16.4.489. [DOI] [PubMed] [Google Scholar]
- 100.Field P R, Hunt J G, Murphy A M. Detection and persistence of specific IgM antibody to Coxiella burnetii by enzyme-linked immunosorbent assay: a comparison with immunofluorescence and complement fixation tests. J Infect Dis. 1983;148:477–487. doi: 10.1093/infdis/148.3.477. [DOI] [PubMed] [Google Scholar]
- 101.Finidori J P, Raoult D, Bornstein N, Fleurette J. Study of cross reactions between Coxiella burnetii and Legionella pneumophila using indirect immunofluorescence assay and immunoblotting. Acta Virol. 1992;36:459–465. [PubMed] [Google Scholar]
- 102.Fishbein D B, Raoult D. A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products. Am J Trop Med Hyg. 1992;47:35–40. doi: 10.4269/ajtmh.1992.47.35. [DOI] [PubMed] [Google Scholar]
- 103.Fiset P, Ormsbee R A, Silberman R, Peacock M, Spielman S H. A microagglutination technique for detection and measurement of rickettsial antibodies. Acta Virol. 1969;13:60–66. [PubMed] [Google Scholar]
- 104.Fournier P E, Casalta J P, Habib G, Messana T, Raoult D. Modification of the diagnostic criteria proposed by the Duke endocarditis service to permit improved diagnosis of Q fever endocarditis. Am J Med. 1996;100:629–633. doi: 10.1016/s0002-9343(96)00040-x. [DOI] [PubMed] [Google Scholar]
- 105.Fournier P E, Casalta J P, Piquet P, Tournigand P, Branchereau A, Raoult D. Coxiella burnetii infection of aneurysms or vascular grafts: report of seven cases and review. Clin Infect Dis. 1998;26:116–121. doi: 10.1086/516255. [DOI] [PubMed] [Google Scholar]
- 106.Fournier P E, Marrie T J, Raoult D. Diagnosis of Q fever. J Clin Microbiol. 1998;36:1823–1834. doi: 10.1128/jcm.36.7.1823-1834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Frazier M E, Mallavia L P, Samuel J E, Baca O G. DNA probes for the identification of Coxiella burnetii strains. Ann N Y Acad Sci. 1990;590:445–458. doi: 10.1111/j.1749-6632.1990.tb42253.x. [DOI] [PubMed] [Google Scholar]
- 108.Frazier M E, Heinzen R A, Mallavia L P, Baca O G. DNA probes detecting Coxiella burnetii strains. Acta Virol. 1992;36:83–89. [PubMed] [Google Scholar]
- 109.Freeman R, Hodson M E. Q fever endocarditis treated with trimethoprim and sulphamethoxazole. Br Med J. 1972;1:419–420. doi: 10.1136/bmj.1.5797.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Friedland J S, Jeffrey I, Griffin G E, Booker M, Courtenay-Evans R. Q fever and intrauterine death. Lancet. 1994;343:288. doi: 10.1016/s0140-6736(94)91131-2. [DOI] [PubMed] [Google Scholar]
- 111.Fries L F, Waag D M, Williams J C. Safety and immunogenicity of a chloroform-methanol residue vaccine for Q fever. Infect Immun. 1993;61:1251–1258. doi: 10.1128/iai.61.4.1251-1258.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fritz E, Thiele D, Willems H, Wittenbrink M M. Quantification of Coxiella burnetii by polymerase chain reaction (PCR) and a colorimetric microtiter plate hybridization assay (CMHA) Eur J Epidemiol. 1995;11:549–557. doi: 10.1007/BF01719307. [DOI] [PubMed] [Google Scholar]
- 113.Gaburro D, del Campo A. Considerazioni epidemiologiche e cliniche su un’infezione da Coxiella burnetii in tre gemelle immature. Mal Infect Parsit. 1956;8:384. [Google Scholar]
- 114.Garner M G, Longbottom H M, Cannon R M, Plant A J. A review of Q fever in Australia 1991–1994. Aust N Z Public Health. 1997;21:722–730. doi: 10.1111/j.1467-842x.1997.tb01787.x. [DOI] [PubMed] [Google Scholar]
- 115.Garrison P K, Freeman L R. Experimental endocarditis. I. Staphylococcal endocarditis in rabbits resulting from placement of a polyethylene catheter in the right side of the heart. Yale J Biol Med. 1970;42:394–410. [PMC free article] [PubMed] [Google Scholar]
- 116.Genig V A, Knyazeva E N, Chelnikov P S, Miroshnichenko M M. Experience on the mass immunization of humans with the M-44 vaccine against Q fever. 1. Subcutaneous method of immunization. Vopr Virusol. 1965;10:319–323. [PubMed] [Google Scholar]
- 117.Gerstl B, Movitt E R, Shaken J R. Liver function and morphology in “Q” fever. Gastroenterology. 1956;30:813–816. [PubMed] [Google Scholar]
- 118.Gikas A M, Froudarakis, Nicolaidis G, Tselentis Y. Myopericarditis complicating Q fever. Ann Med Intern. 1994;145:149–151. [PubMed] [Google Scholar]
- 119.Gil-Grande R, Aguado J M, Pastor C, Garcia-Bravo M, Gomez-Pellico C, Soriano F, Noriega A. Conventional viral cultures and shell vial assay for diagnosis of apparently culture-negative Coxiella burnetii endocarditis. Eur J Clin Microbiol Infect Dis. 1995;14:64–67. doi: 10.1007/BF02112624. [DOI] [PubMed] [Google Scholar]
- 120.Gimenez D F. Staining rickettsiae in yolk sac cultures. Stain Technol. 1964;30:135–137. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- 121.Giroud P, Jardin J. Infection latente et conservation de Rickettsia burnetii chez l’homme: le rôle du pou. Bull Soc Pathol Exot. 1954;45:764–765. [PubMed] [Google Scholar]
- 122.Gonder J C, Kishimoto R A, Kastello M D, Pedersen C E, Larson E W. Cynomolgus monkey model for experimental Q fever infection. J Infect Dis. 1979;139:191–196. doi: 10.1093/infdis/139.2.191. [DOI] [PubMed] [Google Scholar]
- 123.Gordon J D, MacKeen A D, Marrie T J, Fraser D B. The radiographic features of epidemic and sporadic Q fever pneumonia. J Can Assoc Radiol. 1984;35:293–296. [PubMed] [Google Scholar]
- 124.Greiner T C, Mitros F A, Sapleton J, van Rybroek J. Fine-needle aspiration findings of the liver in a case of Q fever. Diagn Cytopathol. 1992;8:181–184. doi: 10.1002/dc.2840080218. [DOI] [PubMed] [Google Scholar]
- 125.Grist N R, Ross C A. Q fever. Br Med J. 1968;2:119–120. doi: 10.1136/bmj.2.5597.119-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guigno D, Coupland B, Spith E G, Farrell I D, Desselberger U, Caul E O. Primary humoral antibody response to Coxiella burnetii, the causative agent of Q fever. J Clin Microbiol. 1992;30:1958–1967. doi: 10.1128/jcm.30.8.1958-1967.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hackstadt T, Williams J C. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc Natl Acad Sci USA. 1981;78:3240–3244. doi: 10.1073/pnas.78.5.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hackstadt T, Williams J C. pH dependence of the Coxiella burnetii glutamate transport system. J Bacteriol. 1983;154:598–603. doi: 10.1128/jb.154.2.598-603.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hackstadt T, Peacock M G, Hitchcock P J, Cole R L. Lipopolysaccharide variation in Coxiella burnetii: intrastrain heterogeneity in structure and antigenicity. Infect Immun. 1985;48:359–365. doi: 10.1128/iai.48.2.359-365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hackstadt T. Antigenic variation in the phase I lipopolysaccharide of Coxiella burnetii isolates. Infect Immun. 1986;52:337–340. doi: 10.1128/iai.52.1.337-340.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hackstadt T. Steric hindrance of antibody binding to surface proteins of Coxiella burnetii by phase I lipopolysaccharide. Infect Immun. 1988;56:802–807. doi: 10.1128/iai.56.4.802-807.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hackstadt T. The role of lipopolysaccharides in the virulence of Coxiella burnetii. Ann N Y Acad Sci. 1990;590:27–32. doi: 10.1111/j.1749-6632.1990.tb42203.x. [DOI] [PubMed] [Google Scholar]
- 133.Haldane E V, Marrie T J, Faulkner R S, Lee S H, Cooper J H, MacPherson D D, Montague T J. Endocarditis due to Q fever in Nova Scotia: experience with five patients in 1981–1982. J Infect Dis. 1983;148:978–985. doi: 10.1093/infdis/148.6.978. [DOI] [PubMed] [Google Scholar]
- 134.Hall C J, Richmond S J, Caul E O, Pearce N H, Silver I A. Laboratory outbreak of Q fever acquired from sheep. Lancet. 1982;i:1004–1006. doi: 10.1016/s0140-6736(82)92001-3. [DOI] [PubMed] [Google Scholar]
- 135.Harman J B. Q fever in Great Britain; clinical account of eight cases. Lancet. 1949;ii:1028–1030. doi: 10.1016/s0140-6736(49)91600-1. [DOI] [PubMed] [Google Scholar]
- 136.Hart R J, Shaw D B. Q-fever endocarditis. Br Med J. 1973;3:233. doi: 10.1136/bmj.3.5873.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Harvey-Sutton P L. Post-Q fever syndrome. Med J Aust. 1995;162:168. doi: 10.5694/j.1326-5377.1995.tb138500.x. [DOI] [PubMed] [Google Scholar]
- 138.Heard S R, Ronalds C J, Hearth R B. Coxiella burnetii infection in immunocompromised hosts. J Infect Dis. 1985;11:15–18. doi: 10.1016/s0163-4453(85)90870-9. [DOI] [PubMed] [Google Scholar]
- 139.Heinzen R A, Mallavia L P. Cloning and functional expression of the Coxiella burnetii citrate synthase gene in Escherichia coli. Infect Immun. 1987;55:848–855. doi: 10.1128/iai.55.4.848-855.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Heinzen R A, Stiegler G L, Whiting L L, Schmitt S A, Mallavia L P, Frazier M E. Use of pulsed field gel electrophoresis to differentiate Coxiella burnetii strains. Ann N Y Acad Sci. 1990;590:504–513. doi: 10.1111/j.1749-6632.1990.tb42260.x. [DOI] [PubMed] [Google Scholar]
- 141.Heinzen R A, Frazier M E, Mallavia L P. Coxiella burnetii superoxide dismutase gene: cloning, sequencing, and expression in Escherichia coli. Infect Immun. 1992;60:3814–3823. doi: 10.1128/iai.60.9.3814-3823.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Heinzen R A, Hackstadt T. A developmental stage-specific histone H1 homolog of Coxiella burnetii. J Bacteriol. 1996;178:5049–5052. doi: 10.1128/jb.178.16.5049-5052.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Heinzen R A, Scidmore M A, Rockey D D, Hackstadt T. Differential interaction with endocytic and exocytic pathways distinguish parasitphorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect Immun. 1996;64:796–809. doi: 10.1128/iai.64.3.796-809.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hendrix L, Mallavia L P. Active transport of proline by Coxiella burnetii. J Gen Microbiol. 1984;130:2857–2863. doi: 10.1099/00221287-130-11-2857. [DOI] [PubMed] [Google Scholar]
- 145.Hendrix L R, Samuel J E, Mallavia L P. Identification and cloning of a 27-kDa Coxiella burnetii immunoreactive protein. Ann N Y Acad Sci. 1990;590:534–540. doi: 10.1111/j.1749-6632.1990.tb42263.x. [DOI] [PubMed] [Google Scholar]
- 146.Hendrix L R, Samuel J E, Mallavia L P. Differentiation of Coxiella burnetii isolates by analysis of restriction-endonuclease-digested DNA separated by SDS-PAGE. J Gen Microbiol. 1991;137:269–276. doi: 10.1099/00221287-137-2-269. [DOI] [PubMed] [Google Scholar]
- 147.Herr S, Huchzermeyer H F, Te Brugge L A, Williamson C C, Roos J A, Schiele G J. The use of a single complement fixation test technique in bovine brucellosis, Johne’s disease, dourine, equine piroplasmosis and Q fever serology. Oderstepoort J Vet Res. 1985;52:279–282. [PubMed] [Google Scholar]
- 148.Hilbink F, Penrose M, Kovacova E, Kazar J. Q fever is absent from New Zealand. Int J Epidemiol. 1993;22:945–949. doi: 10.1093/ije/22.5.945. [DOI] [PubMed] [Google Scholar]
- 149.Ho T, Htwe K K, Yamasaki N, Zhang G Q, Ogawa M, Yamagushi T, Fukushi H, Hirai K. Isolation of Coxiella burnetii from dairy cattle and ticks, and some characteristics of the isolates in Japan. Microbiol Immunol. 1995;39:663–671. doi: 10.1111/j.1348-0421.1995.tb03254.x. [DOI] [PubMed] [Google Scholar]
- 150.Hofmann C E, Heaton J W. Q fever hepatitis: clinical manifestations and pathological findings. Gastroenterology. 1982;83:474–479. [PubMed] [Google Scholar]
- 151.Hoover T A, Williams J C. Characterization of Coxiella burnetii pyrB. Ann N Y Acad Sci. 1990;590:485–490. doi: 10.1111/j.1749-6632.1990.tb42258.x. [DOI] [PubMed] [Google Scholar]
- 152.Hoover T A, Vodkin M H, Williams J. A Coxiella burnetii repeated DNA element resembling a bacterial insertion sequence. J Bacteriol. 1992;174:5540–5548. doi: 10.1128/jb.174.17.5540-5548.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Htwe K K, Yoshida T, Hayashi S, Miyake T, Amano K-I, Morita C, Yamagushi T, Fukushi H, Hirai K. Prevalence of antibodies to Coxiella burnetii in Japan. J Clin Microbiol. 1993;31:722–723. doi: 10.1128/jcm.31.3.722-723.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Htwe K K, Amano K, Sugiyama Y, Yagami K, Minamoto N, Hashimoto A, Yamagushi T, Fukushi H, Hirai K. Seroepidemiology of Coxiella burnetii in domestic and companion animals in Japan. Vet Rec. 1992;131:490. doi: 10.1136/vr.131.21.490. [DOI] [PubMed] [Google Scholar]
- 155.Huebner R J, Hottle G A, Robinson E B. Action of streptomycin in experimental infection with Q fever. Public Health Rep. 1948;63:357–362. [PMC free article] [PubMed] [Google Scholar]
- 156.Huebner R J, Bell J A. Q fever studies in southern California: summary of current results and a discussion of possible control measures. JAMA. 1951;145:301–305. doi: 10.1001/jama.1951.02920230025005. [DOI] [PubMed] [Google Scholar]
- 157.Izzo A A, Marmion B P, Worswick D A. Markers of cell-mediated immunity after vaccination with an inactivated, whole-cell Q fever vaccine. J Infect Dis. 1988;157:781–789. doi: 10.1093/infdis/157.4.781. [DOI] [PubMed] [Google Scholar]
- 158.Izzo A A, Marmion B P. Variations in interferon-gamma responses to Coxiella burnetii antigens with lymphocytes from vaccinated or naturally infected subjects. Clin Exp Immunol. 1993;94:507–515. doi: 10.1111/j.1365-2249.1993.tb08226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jackson E R. Comparative efficacy of several antibiotics on experimental rickettsial infections in embryonnated eggs. Antibiot Chemother. 1951;1:231–235. [PubMed] [Google Scholar]
- 160.Janbon F, Raoult D, Reynes J, Bertrand A. Concomitant human infection due to Rickettsia conorii and Coxiella burnetii. J Infect Dis. 1989;160:354–355. doi: 10.1093/infdis/160.2.354. [DOI] [PubMed] [Google Scholar]
- 161.Janigan D T, Marrie T J. An inflammatory pseudotumor of the lung in Q fever pneumonia. N Engl J Med. 1983;308:86–87. doi: 10.1056/NEJM198301133080207. [DOI] [PubMed] [Google Scholar]
- 162.Jorm L R, Lightfood N F, Morgan K L. An epidemiological study of an outbreak of Q fever in a secondary school. Epidemiol Infect. 1990;104:467–477. doi: 10.1017/s0950268800047476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kazar J, Brezina R, Schramek S, Palanova A, Tvrda B. Suitability of the microagglutination test for detection of post-infection and post-vaccination Q fever antibodies in human sera. Acta Virol. 1981;25:235–240. [PubMed] [Google Scholar]
- 164.Kazar J, Brezina R, Palanova A, Turda B, Schramek S. Immunogenicity and reactogenicity of a Q fever chemovaccine in persons professionally exposed to Q fever in Czechoslovakia. Bull W H O. 1982;60:389–394. [PMC free article] [PubMed] [Google Scholar]
- 165.Kazar J, Schramek S, Zajacova S. Induction of splenomegaly in mice by killed Coxiella burnetii cells. Acta Virol. 1983;27:65–70. [PubMed] [Google Scholar]
- 166.Kazar J, Schramek S, Brezina R. The value of skin test in Q fever convalescents and vaccinees as indicator of antigen exposure and inducer of antibody recall. Acta Virol. 1984;28:134–140. [PubMed] [Google Scholar]
- 167.Kazar J, Schramek S, Lisak V, Brezina R. Antigenicity of chloroform-methanol-treated Coxiella burnetii preparations. Acta Virol. 1987;31:158–167. [PubMed] [Google Scholar]
- 168.Kazar J, Rehacek J. Q fever vaccines: present status and application in man. Zentbl Bakteriol Mikrobiol Hyg. 1987;267:74–78. doi: 10.1016/s0176-6724(87)80190-6. [DOI] [PubMed] [Google Scholar]
- 169.Kimbrough R C, Ormsbee R A, Peacock M G, Rogers W R, Bennetts R W, Raaf J, Krause A, Gardner C. Q fever endocarditis in the United States. Ann Intern Med. 1979;91:400–402. doi: 10.7326/0003-4819-91-3-400. [DOI] [PubMed] [Google Scholar]
- 170.Kishimoto R A, Rozmiarek H, Larson E W. Experimental Q fever infection in congenitally athymic nude mice. Infect Immun. 1978;22:69–71. doi: 10.1128/iai.22.1.69-71.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kosatsky T. Household outbreak of Q-fever pneumonia related to a parturient cat. Lancet. 1984;ii:1447–1449. doi: 10.1016/s0140-6736(84)91633-7. [DOI] [PubMed] [Google Scholar]
- 172.Koster F T, Williams J C, Goodwin J S. Cellular immunity in Q fever: specific lymphocyte unresponsiveness in Q fever endocarditis. J Infect Dis. 1985;152:1283–1289. doi: 10.1093/infdis/152.6.1283. [DOI] [PubMed] [Google Scholar]
- 173.Koster F T, Williams J C, Goodwin J S. Cellular immunity in Q fever: modulation of responsiveness by a suppressor T cell-monocyte circuit. J Immunol. 1985;135:1067–1072. [PubMed] [Google Scholar]
- 174.Kovacova E, Gallo J, Schramek S, Kazar J, Brezina R. Coxiella burnetii antigens for detection of Q fever antibodies by ELISA in human sera. Acta Virol. 1987;31:254–259. [PubMed] [Google Scholar]
- 175.Krauss H. Clinical aspects and prevention of Q fever in animals. Eur J Epidemiol. 1989;5:454–455. doi: 10.1007/BF00140140. [DOI] [PubMed] [Google Scholar]
- 176.Kristinsson A, Bentall H H. Medical and surgical treatment of Q fever endocarditis. Lancet. 1967;ii:693–695. doi: 10.1016/s0140-6736(67)90975-0. [DOI] [PubMed] [Google Scholar]
- 177.Krumbiegel E R, Wisniewski H J. Q fever in Milwaukee. II. Consumption of infected raw milk by human volunteers. Arch Environ Health. 1970;21:63–65. doi: 10.1080/00039896.1970.10667193. [DOI] [PubMed] [Google Scholar]
- 178.Lackman D B, Bell E J, Bell J F, Pickens E G. Intradermal sensibility testing in man with a purified vaccine for Q fever. Am J Public Health. 1962;52:87–91. doi: 10.2105/ajph.52.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lang G H. Coxiellosis (Q fever) in animals. In: Marrie T J, editor. Q fever. I. The disease. Boca Raton, Fla: CRC Press, Inc.; 1990. [Google Scholar]
- 180.Langley J M, Marrie T J, Covert A, Waag D M, Williams J C. Poker players’ pneumonia: an urban outbreak of Q fever following exposure to a parturient cat. N Engl J Med. 1988;319:354–356. doi: 10.1056/NEJM198808113190607. [DOI] [PubMed] [Google Scholar]
- 181.La Scola B, Lepidi H, Maurin M, Raoult D. A guinea pig model for Q fever endocarditis. J Infect Dis. 1998;178:278–281. doi: 10.1086/517453. [DOI] [PubMed] [Google Scholar]
- 182.La Scola B, Raoult D. Serological cross reactions between Bartonella quintana, Bartonella henselae, and Coxiella burnetii. J Clin Microbiol. 1996;34:2270–2274. doi: 10.1128/jcm.34.9.2270-2274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.La Scola B, Lepidi H, Raoult D. Pathologic changes during acute Q fever: influence of the route of infection and inoculum size in infected guinea pigs. Infect Immun. 1997;65:2443–2447. doi: 10.1128/iai.65.6.2443-2447.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.La Scola, B., and D. Raoult. 1998. Unpublished data.
- 185.Laughlin T, Wang D, Williams J, Marrie T J. Q fever: from deer to dog to man. Lancet. 1991;337:676–677. doi: 10.1016/0140-6736(91)92494-m. [DOI] [PubMed] [Google Scholar]
- 186.Lev B I, Shachar A, Segev S, Weiss P, Rubinstein E. Quiescent Q fever endocarditis exacerbated by cardiac surgery and corticosteroid therapy. Arch Intern Med. 1988;148:1531–1532. [PubMed] [Google Scholar]
- 187.Levy P, Raoult D, Razongles J J. Q fever and autoimmunity. Eur J Epidemiol. 1989;5:447–453. doi: 10.1007/BF00140139. [DOI] [PubMed] [Google Scholar]
- 188.Levy P Y, Drancourt M, Etienne J, Auvergnat J C, Beytout J, Sainty J M, Goldstein F, Raoult D. Comparison of different antibiotic regimens for therapy of 32 cases of Q fever endocarditis. Antimicrob Agents Chemother. 1991;35:533–537. doi: 10.1128/aac.35.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lillie R D, Perrin T L. Histopathology of pneumonitis due to “Q” fever virus. Public Health Rep. 1941;56:149–152. [Google Scholar]
- 190.Lillie R D. Pathologic histology in guinea pigs following intraperitoneal inoculation with the virus of “Q” fever. Public Health Rep. 1942;57:296–304. [Google Scholar]
- 191.Lipton J H, Fong T C, Gill M J, Burgess K, Elliott P D. Q fever inflammatory pseudotumor of the lung. Chest. 1987;92:756–757. doi: 10.1378/chest.92.4.756. [DOI] [PubMed] [Google Scholar]
- 192.Ludlam H, Wreghitt T G, Thornton S, Thomson B J, Bishop N J, Coomber S, Cunniffe J. Q fever in pregnancy. J Infect. 1997;34:75–78. doi: 10.1016/s0163-4453(97)80014-x. [DOI] [PubMed] [Google Scholar]
- 193.Luoto L, Bell F J, Casey M, Lackman D B. Q fever vaccination of human volunteers. 1. Serologic and skin test response following subcutaneous injection. Am J Hyg. 1963;78:1–15. [PubMed] [Google Scholar]
- 194.Lyytikainen O, Ziese T, Schwartlander B, Matzdorff P, Kuhnhen C, Jager C, Petersen L. An outbreak of sheep-associated Q fever in a rural community in Germany. Eur J Epidemiol. 1998;14:193–199. doi: 10.1023/a:1007452503863. [DOI] [PubMed] [Google Scholar]
- 195.Mallavia L P, Whiting L L, Minnick M F, Heinzen R A, Reschke D, Foreman M, Baca O G, Frazier M E. Strategy for detection and differentiation of Coxiella burnetii strains using the polymerase chain reaction. Ann N Y Acad Sci. 1990;590:572–581. doi: 10.1111/j.1749-6632.1990.tb42268.x. [DOI] [PubMed] [Google Scholar]
- 196.Mallavia L P, Samuel J E, Frazier M E. The genetics of Coxiella burnetii: etiologic agent of Q fever and chronic endocarditis. In: Williams J C, Thompson H A, editors. Q fever: the biology of Coxiella burnetii. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 259–284. [Google Scholar]
- 197.Mallavia L P. Genetic of rickettsiae. Eur J Epidemiol. 1991;7:213–221. doi: 10.1007/BF00145669. [DOI] [PubMed] [Google Scholar]
- 198.Mantovani A, Benazzi P. The isolation of Coxiella burnetii from Rhipicephalus sanguineus on naturally infected dogs J. Am Vet Med Assoc. 1953;122:117–120. [PubMed] [Google Scholar]
- 199.Manuel Cruz J, Martinez R, Varela A, Hernandez R. Pericarditis and Q fever. An Med Intern. 1994;11:515. [PubMed] [Google Scholar]
- 200.Marmion B P, Stoker M G P. Q fever in Great Britain: epidemiology of an outbreak. Lancet. 1950;ii:611–616. doi: 10.1016/s0140-6736(50)91582-0. [DOI] [PubMed] [Google Scholar]
- 201.Marmion B P, Ormsbee R A, Kyrkou M, Wright J, Worswick D, Cameron S, Esterman A, Feery B, Collins W. Vaccine prophylaxis of abattoir-associated Q fever. Lancet. 1984;ii:1411–1414. doi: 10.1016/s0140-6736(84)91617-9. [DOI] [PubMed] [Google Scholar]
- 202.Marmion B P, Ormsbee R A, Kyrkou M, Wright J, Worswick D, Izzo A, Esterman A, Feery B, Shapiro R A. Vaccine prophylaxis of abattoir-associated Q fever: eight years’ experience in Australian abattoirs. Epidemiol Infect. 1990;104:275–287. doi: 10.1017/s0950268800059458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Marmion B P, Shannon M, Maddocks I, Storm P, Pentilla I. Protracted fatigue and debility after acute Q fever. Lancet. 1996;347:978–979. doi: 10.1016/s0140-6736(96)91469-5. [DOI] [PubMed] [Google Scholar]
- 204.Marrie T J, Haldane E V, Faulkner R S, Martin R S, Lee S H S. Causes of atypical pneumonia. Results of a 1-year prospective study. Can Med Assoc J. 1981;125:1118–1123. [PMC free article] [PubMed] [Google Scholar]
- 205.Marrie T J, Van Buren J, Fraser J, Haldane E V, Faulkner R S, Williams J C, Kwan C. Seroepidemiology of Q fever among domestic animals in Nova Scotia. Am J Public Health. 1985;75:763–766. doi: 10.2105/ajph.75.7.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Marrie T J. Pneumonia and meningoencephalitis due to Coxiella burnetii. J Infect. 1985;11:59–61. doi: 10.1016/s0163-4453(85)91066-7. [DOI] [PubMed] [Google Scholar]
- 207.Marrie T J, Schlech W F, Williams J C, Yates L. Q fever pneumonia associated with exposure to wild rabbits. Lancet. 1986;i:427–429. doi: 10.1016/s0140-6736(86)92380-9. [DOI] [PubMed] [Google Scholar]
- 208.Marrie T J. Q fever, 1979–1987—Nova Scotia. Can Dis Weekly Rep. 1988;14:69–70. [PubMed] [Google Scholar]
- 209.Marrie T J. Liver involvement in acute Q fever. Chest. 1988;94:896–898. doi: 10.1378/chest.94.4.895. [DOI] [PubMed] [Google Scholar]
- 210.Marrie T J, Durant H, Williams J C, Mintz E, Waag D M. Exposure to parturient cats is a risk factor for acquisition of Q fever in Maritime Canada. J Infect Dis. 1988;158:101–108. doi: 10.1093/infdis/158.1.101. [DOI] [PubMed] [Google Scholar]
- 211.Marrie T J. Acute Q fever. In: Marrie T J, editor. Q fever. 1. The disease. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 125–160. [Google Scholar]
- 212.Marrie T J. Epidemiology of Q fever. In: Marrie T J, editor. Q fever. 1. The disease. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 49–70. [Google Scholar]
- 213.Marrie T J. A comparison of Q fever endocarditis with native valve endocarditis. Ann N Y Acad Sci. 1990;590:61–67. doi: 10.1111/j.1749-6632.1990.tb42207.x. [DOI] [PubMed] [Google Scholar]
- 214.Marrie T J. Coxiella burnetii (Q fever) In: Mandell G L, Bennett J E, editors. Principles and practice of infectious diseases. 3rd ed. New York, N.Y: Churchill Livingstone, Inc.; 1990. pp. 1727–1734. [Google Scholar]
- 215.Marrie T J. Q fever in pregnancy: report of two cases. Infect Dis Clin Pract. 1993;2:207–209. [Google Scholar]
- 216.Marrie T J, Pollak P T. Seroepidemiology of Q fever in Nova Scotia: evidence for age dependent cohorts and geographical distribution. Eur J Epidemiol. 1995;11:47–54. doi: 10.1007/BF01719945. [DOI] [PubMed] [Google Scholar]
- 217.Marrie T J. Coxiella burnetii (Q fever) pneumonia. Clin Infect Dis. 1995;21(Suppl. 3):5253–5264. doi: 10.1093/clind/21.supplement_3.s253. [DOI] [PubMed] [Google Scholar]
- 218.Marrie T J, Peeling R W, Fine M J, Singer D E, Coley C M, Kapoor W N. Ambulatory patients with community-acquired pneumonia: the frequency of atypical agents and clinical course. Am J Med. 1996;101:508–515. doi: 10.1016/s0002-9343(96)00255-0. [DOI] [PubMed] [Google Scholar]
- 219.Marrie T J, Stein A, Janigan D, Raoult D. Route of infection determines the clinical manifestations of acute Q fever. J Infect Dis. 1996;173:484–487. doi: 10.1093/infdis/173.2.484. [DOI] [PubMed] [Google Scholar]
- 220.Marrie T J, Raoult D. Q fever—a review and issues for the next century. Int J Antimicrob Agents. 1997;8:145–161. doi: 10.1016/s0924-8579(96)00369-x. [DOI] [PubMed] [Google Scholar]
- 221.Maurin M, Benoliel A M, Bongrand P, Raoult D. Phagolysosomes of Coxiella burnetii-infected cells maintain an acidic pH during persistent infection. Infect Immun. 1992;60:5013–5016. doi: 10.1128/iai.60.12.5013-5016.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Maurin M, Benoliel A M, Bongrand P, Raoult D. Phagolysosomal alkalinization and the bactericidal effect of antibiotics: the Coxiella burnetii paradigm. J Infect Dis. 1992;166:1097–1102. doi: 10.1093/infdis/166.5.1097. [DOI] [PubMed] [Google Scholar]
- 223.Maurin M, Raoult D. In vitro susceptibility of spotted fever group Rickettsiae and Coxiella burnetii to clarithromycin. Antimicrob Agents Chemother. 1993;37:2633–2637. doi: 10.1128/aac.37.12.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Maurin M, Raoult D. Current concepts and perspectives in Q fever treatment. In: Raoult D, editor. Antimicrobial agents and intracellular pathogens. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 153–179. [Google Scholar]
- 225.Maurin M, Raoult D. Bacteriostatic and bactericidal activity of levofloxacin against Rickettsia rickettsii, Rickettsia conorii, “Israeli spotted fever group rickettsia” and Coxiella burnetii. J Antimicrob Chemother. 1997;39:725–730. doi: 10.1093/jac/39.6.725. [DOI] [PubMed] [Google Scholar]
- 226.McCaul T F, Williams J C. Localization of DNA in Coxiella burnetii by post-embedding immunoelectron microscopy. Ann N Y Acad Sci. 1990;590:136–147. doi: 10.1111/j.1749-6632.1990.tb42216.x. [DOI] [PubMed] [Google Scholar]
- 227.McCaul T F. The development cycle of Coxiella burnetii. In: Williams J C, Thompson H A, editors. Q fever: the biology of Coxiella burnetii. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 223–258. [Google Scholar]
- 228.McCaul T F, Williams J C. Developmental cycle of Coxiella burnetii: structure and morphogenesis of vegetative and sporogenic differentiations. J Bacteriol. 1981;147:1063–1076. doi: 10.1128/jb.147.3.1063-1076.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.McCaul T F, Hackstadt T, Williams J C. Ultrastructural and biological aspects of Coxiella burnetii under physical disruptions. In: Burgdorfer W, Anacker R L, editors. Rickettsiae and rickettsial diseases. New York, N.Y: Academic Press, Inc.; 1981. pp. 267–280. [Google Scholar]
- 230.McCaul T F, Williams J C, Thompson H A. Electron microscopy of Coxiella burnetii in tissue culture. Induction of cell types as products of developmental cycle. Acta Virol. 1991;35:545–556. [PubMed] [Google Scholar]
- 231.McDade J E, Brenner D J, Bozeman F M. Legionnaire’s disease bacterium isolated in 1947. Ann Intern Med. 1979;90:659–661. doi: 10.7326/0003-4819-90-4-659. [DOI] [PubMed] [Google Scholar]
- 232.McGivern D, White R, Paul I D, Caul E O, Roome A P, Westmoreland D. Concomitant zoonotic infections with ovine Chlamydia and Q fever in pregnancy: clinical features, diagnosis, management and public health implications. Case report. Br J Obstet Gynaecol. 1988;95:294–298. doi: 10.1111/j.1471-0528.1988.tb06872.x. [DOI] [PubMed] [Google Scholar]
- 233.Mege J L, Maurin M, Capo C, Raoult D. Coxiella burnetii: the ‘query’ fever bacterium. A model of immune subversion by a strictly intracellular microorganism. FEMS Microbiol Rev. 1997;19:209–217. doi: 10.1111/j.1574-6976.1997.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 234.Micoud M, Brion J P, Boulard J C, Magne J L, Gratacap B, Stahl J P, Farah I, Raoult D. Infection of aortic aneurysm with Coxiella burnetii. Lancet. 1991;338:584. doi: 10.1016/0140-6736(91)91161-m. [DOI] [PubMed] [Google Scholar]
- 235.Minnick M F, Heinzen R A, Douthart R, Mallavia L P, Frazier M E. Analysis of QpRS-specific sequences from Coxiella burnetii. Ann N Y Acad Sci. 1990;590:514–522. doi: 10.1111/j.1749-6632.1990.tb42261.x. [DOI] [PubMed] [Google Scholar]
- 236.Minnick M E, Heinzen R A, Frazier M E, Mallavia L P. Characterization and expression of the cbbE′ gene of Coxiella burnetii. J Gen Microbiol. 1990;136:1099–1107. doi: 10.1099/00221287-136-6-1099. [DOI] [PubMed] [Google Scholar]
- 237.Mo Y Y, Mallavia L P. A Coxiella burnetii gene encodes a sensor-like protein. Gene. 1994;15:185–190. doi: 10.1016/0378-1119(94)90654-8. [DOI] [PubMed] [Google Scholar]
- 238.Montejo Baranda M, Corral Carranceja J, Aguirre Errasti C. Q fever in the Basque country: 1981–1984. Rev Infect Dis. 1985;7:700–701. doi: 10.1093/clinids/7.5.700. [DOI] [PubMed] [Google Scholar]
- 239.Moos A, Vishwanath S, Hackstadt T. Abstracts of the Seventh National Meeting of the American Society for Rickettsiology and Rickettsial Diseases 1988. Santa Fe, N.M: American Society for Rickettsiology and Rickettsial Diseases; 1988. Experimental Q fever endocarditis in rabbits. [Google Scholar]
- 240.Morgans C M, Cartwright R Y. Case of Q fever endocarditis at site of aortic valve prosthesis. Br Heart J. 1969;31:520–522. doi: 10.1136/hrt.31.4.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Morita C, Katsuyama J, Yanase T, Ueno H, Muramatsu Y, Hohdatsu T, Koyama H. Seroepidemiological survey of Coxiella burnetii in domestic cats in Japan. Microbiol Immunol. 1994;38:1001–1003. doi: 10.1111/j.1348-0421.1994.tb02159.x. [DOI] [PubMed] [Google Scholar]
- 242.Mosquera Lozano J D, Brea Hernando A J, Lopez Bonilla A, Marquez de Prado Urquia M. Pleuropericarditis caused by Q fever. Ann Med Interna. 1994;11:366–367. [PubMed] [Google Scholar]
- 243.Moughabghab A V, Fenides A, Woelffle D, Socolovsky C. Coxiella burnetii pleuropericarditis. Rev Med Interne. 1994;15:838–840. doi: 10.1016/s0248-8663(05)82842-5. [DOI] [PubMed] [Google Scholar]
- 244.Moustaghfir A, Deharo J C, Dupont H T, Macaluso G, Raoult D, Djiane P. Acute pericarditis in Coxiella burnetii infection. A propos of a case. Arch Mal Cœur Vaiss. 1995;88:1657–1659. [PubMed] [Google Scholar]
- 245.Muhlemann K, Matter L, Meyer B, Schoper K. Isolation of Coxiella burnetii from heart valves of patients treated for Q fever endocarditis. J Clin Microbiol. 1995;33:428–431. doi: 10.1128/jcm.33.2.428-431.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Murphy A M, Field P R. The persistence of complement-fixing antibodies to Q fever (Coxiella burnetii) after infection. Med J Aust. 1970;1:1148–1150. doi: 10.5694/j.1326-5377.1970.tb84481.x. [DOI] [PubMed] [Google Scholar]
- 247.Musso D, Raoult D. Coxiella burnetii blood cultures from acute and chronic Q fever patients. J Clin Microbiol. 1995;33:3129–3132. doi: 10.1128/jcm.33.12.3129-3132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Musso D, Drancourt M, Osscini S, Raoult D. Sequence of quinolone resistance-determining region of gyrA gene for clinical isolates and for an in vitro-selected quinolone-resistant strain of Coxiella burnetii. Antimicrob Agents Chemother. 1996;40:870–873. doi: 10.1128/aac.40.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Musso D, Raoult D. Serological cross-reactions between Coxiella burnetii and Legionella micdadei. Clin Diagn Lab Immunol. 1997;4:208–212. doi: 10.1128/cdli.4.2.208-212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Nagaoka H, Akiyama M, Sugieda M, Nishio T, Akahane S, Hattori H, Ho T, Fukushi H, Hirai K. Isolation of Coxiella burnetii from children with influenza-like symptoms in Japan. Microbiol Immunol. 1996;40:147–151. doi: 10.1111/j.1348-0421.1996.tb03330.x. [DOI] [PubMed] [Google Scholar]
- 251.Nermut M V, Schramek S, Brezina R. Electron microscopy of Coxiella burnetii phase I and II. Acta Virol. 1968;12:446–452. [PubMed] [Google Scholar]
- 252.Nguyen S V, Otsuka H, Zhang G Q, To H, Yamaguchi T, Fukushi H, Noma A, Hirai K. Rapid method for detection of Coxiella burnetii antibodies using high-density particle agglutination. J Clin Microbiol. 1996;34:2947–2951. doi: 10.1128/jcm.34.12.2947-2951.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Oda H, Yoshiie K. Isolation of a Coxiella burnetii strain that has low virulence for mice from a patient with acute Q fever. Microbiol Immunol. 1989;33:969–973. doi: 10.1111/j.1348-0421.1989.tb00984.x. [DOI] [PubMed] [Google Scholar]
- 254.Okun D B, Sun N C, Tanaka K R. Bone marrow granulomas in Q fever. Am J Clin Pathol. 1979;71:117–121. doi: 10.1093/ajcp/71.1.117. [DOI] [PubMed] [Google Scholar]
- 255.Ormsbee R A, Pickens E G. A comparison by means of the complement-fixation test of the relative potencies of chloramphenicol, aureomycin, and terramycin in experimental Q fever infections in embryonated eggs. J Immunol. 1951;67:437–448. [PubMed] [Google Scholar]
- 256.Ormsbee R A. The growth of Coxiella burnetii in embryonnated eggs. J Bacteriol. 1952;63:73. doi: 10.1128/jb.63.1.73-86.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ormsbee R A, Parker H, Pickens E G. The comparative effectiveness of aureomycin, terramycin, chloramphenicol, erythromycin, and thiomycetin in suppressing experimental rickettsial infections in chick embryos. J Infect Dis. 1955;96:162–167. doi: 10.1093/infdis/96.2.162. [DOI] [PubMed] [Google Scholar]
- 258.Ormsbee R A, Bell E J, Lackman D B, Tallent G. The influence of phase on the protective potency of Q fever vaccine. J Immunol. 1964;92:404–412. [PubMed] [Google Scholar]
- 259.Ormsbee R A, Marmion B P. Prevention of Coxiella burnetii infection: vaccines and guidelines for those at risk. In: Marrie T J, editor. Q fever. 1. The disease. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 225–248. [Google Scholar]
- 260.Palmer N C, Kierstaed M, Key D W. Placentitis and abortion in goats and sheep in Ontario caused by Coxiella burnetii. Can Vet J. 1983;24:60–63. [PMC free article] [PubMed] [Google Scholar]
- 261.Palmer S R, Young S E J. Q fever endocarditis in England and Wales, 1975–81. Lancet. 1982;ii:1148–1149. doi: 10.1016/s0140-6736(82)91341-1. [DOI] [PubMed] [Google Scholar]
- 262.Parker R R, Davis G E. A filter-passing infectious agent isolated from ticks. II. Transmission by Dermacentor andersoni. Public Health Rep. 1938;53:2267–2269. [Google Scholar]
- 263.Peacock M G, Philip R N, Williams J C, Faulkner R S. Serological evaluation of Q fever in humans: enhanced phase I titers of immunoglobulins G and A are diagnostic for Q fever endocarditis. Infect Immun. 1983;41:1089–1098. doi: 10.1128/iai.41.3.1089-1098.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Pedoe H D. Apparent recurrence of Q fever endocarditis following homograft replacement of aortic valve. Br Heart J. 1970;32:568–570. doi: 10.1136/hrt.32.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Pellegrin M, Delsol G, Auvergnat J C, Familiades J, Faure H, Guiu M, Voigt J. Granulomatous hepatitis in Q fever. Hum Pathol. 1980;11:51–57. doi: 10.1016/s0046-8177(80)80105-5. [DOI] [PubMed] [Google Scholar]
- 266.Perez-del-Molino A, Aguado J M, Riancho J A, Sampredo I, Matorras P, Gonzales-Macias J. Erythromycin and the treatment of Coxiella burnetii pneumonia. J Antimicrob Chemother. 1991;28:455–459. doi: 10.1093/jac/28.3.455. [DOI] [PubMed] [Google Scholar]
- 267.Perez Ortola R, Fernandez Penalba G, Cia Ruiz J, Merino A. Osteoarthritis por Coxiella burnetii. Rev Clin Esp. 1992;191:25–26. [PubMed] [Google Scholar]
- 268.Perin T L. Histopathologic observations in a fatal case of Q fever. Arch Pathol. 1949;47:361–365. [PubMed] [Google Scholar]
- 269.Perrin T K, Bengtson I A. The histopathology of experimental Q fever in mice. Public Health Rep. 1942;57:790–794. [Google Scholar]
- 270.Peter O, Dupuis G, Burgdorfer W, Peacock M. Evaluation of the complement fixation and indirect immunofluorescence tests in the early diagnosis of primary Q fever. Eur J Clin Microbiol Infect Dis. 1985;4:394–396. doi: 10.1007/BF02148690. [DOI] [PubMed] [Google Scholar]
- 271.Peter O, Dupuis G, Peacock M G, Burgdorfer W. Comparison of enzyme-linked immunosorbent assay and complement fixation and indirect fluorescent-antibody tests for detection of Coxiella burnetii antibody. J Clin Microbiol. 1987;25:1063–1067. doi: 10.1128/jcm.25.6.1063-1067.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Peter O, Dupuis G, Bee D, Luthy R, Nicolet J, Burgdorfer W. Enzyme-linked immunosorbent assay for diagnosis of chronic Q fever. J Clin Microbiol. 1988;26:1978–1982. doi: 10.1128/jcm.26.10.1978-1982.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Peter O, Flepp M, Bestetti G, Nicolet J, Luthy R, Dupuis G. Q fever endocarditis: diagnostic approaches and monitoring of therapeutic effects. Clin Investig. 1992;70:932–937. doi: 10.1007/BF00180442. [DOI] [PubMed] [Google Scholar]
- 274.Philip C B. Observations on experimental Q fever. J Parasitol. 1948;34:457–464. [PubMed] [Google Scholar]
- 275.Picchi J, Nelson A R, Waller E E, Razavi M, Clizer E E. Q fever associated with granulomatous hepatitis. Ann Intern Med. 1960;53:1965–1967. doi: 10.7326/0003-4819-53-5-1065. [DOI] [PubMed] [Google Scholar]
- 276.Pinsky R L, Fishbein D B, Greene C R, Gensheimer K F. An outbreak of cat-associated Q fever in the United States. J Infect Dis. 1991;164:202–204. doi: 10.1093/infdis/164.1.202. [DOI] [PubMed] [Google Scholar]
- 277.Piquet P, Raoult D, Tranier P, Mercier C I. Coxiella burnetii infection of pseudoaneurysm of an aortic bypass graft with contiguous vertebral osteomyelitis. J Vasc Surg. 1994;19:165–168. doi: 10.1016/s0741-5214(94)70131-8. [DOI] [PubMed] [Google Scholar]
- 278.Pope J H, Scott W, Dweyer R. Coxiella burnetii in kangoroos and kangorro-ticks in Western Queenland. Aust J Exp Biol. 1960;38:17–19. doi: 10.1038/icb.1960.3. [DOI] [PubMed] [Google Scholar]
- 279.Powell O. “Q” fever: clinical features in 72 cases. Aust Ann Med. 1960;9:214–216. doi: 10.1111/imj.1960.9.3.214. [DOI] [PubMed] [Google Scholar]
- 280.Powell O W, Kennedy K P, McIver M, Silverstone H. Tetracycline in the treatment of Q fever. Aust Ann Med. 1962;11:184–188. doi: 10.1111/imj.1962.11.3.184. [DOI] [PubMed] [Google Scholar]
- 281.Qizilbash A H. The pathology of Q fever as seen on liver biopsy. Arch Pathol Lab Med. 1983;107:364–367. [PubMed] [Google Scholar]
- 282.Raoult, D. Unpublished data.
- 283.Raoult D, Etienne J, Massip P, Iaocono S, Prince M A, Beaurain P, Benichou S, Auvergnat J C, Mathieu P, Bachet P. Q fever endocarditis in the south of France. J Infect Dis. 1987;155:570–573. doi: 10.1093/infdis/155.3.570. [DOI] [PubMed] [Google Scholar]
- 284.Raoult D, Yeaman M R, Baca O G. Susceptibility of Coxiella burnetii to pefloxacin and ofloxacin in ovo and in persistently infected L929 cells. Antimicrob Agents Chemother. 1989;33:621–623. doi: 10.1128/aac.33.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Raoult D, Bollini G, Gallais H. Osteoarticular infection due to Coxiella burnetii. J Infect Dis. 1989;159:1159–1160. doi: 10.1093/infdis/159.6.1159. [DOI] [PubMed] [Google Scholar]
- 286.Raoult D. Host factors in the severity of Q fever. Ann N Y Acad Sci. 1990;590:33–38. doi: 10.1111/j.1749-6632.1990.tb42204.x. [DOI] [PubMed] [Google Scholar]
- 287.Raoult D, Vestris G, Enea M. Isolation of 16 strains of Coxiella burnetii from patients by using a sensitive centrifugation cell culture system and establishment of strains in HEL cells. J Clin Microbiol. 1990;28:2482–2484. doi: 10.1128/jcm.28.11.2482-2484.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Raoult D, Levy P Y, Harle J R, Etienne J, Massip P, Goldstein F, Micoud M, Beytout J, Gallais H, Remy G, Capron J P. Chronic Q fever: Diagnosis and follow up. Ann NY Acad Sci. 1990;590:51–60. doi: 10.1111/j.1749-6632.1990.tb42206.x. [DOI] [PubMed] [Google Scholar]
- 289.Raoult D, Drancourt M, Vestris G. Bactericidal effect of doxycycline associated with lysosomotropic agents on Coxiella burnetii in P388D1 cells. Antimicrob Agents Chemother. 1990;34:1512–1514. doi: 10.1128/aac.34.8.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Raoult D, Raza A, Marrie T J. Q fever endocarditis and other forms of chronic Q fever. In: Marrie T J, editor. Q fever. 1. The disease. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 179–199. [Google Scholar]
- 291.Raoult D, Torres H, Drancourt M. Shell-vial assay: Evaluation of a new technique for determining antibiotic susceptibility, tested in 13 isolates of Coxiella burnetii. Antimicrob Agents Chemother. 1991;35:2070–2077. doi: 10.1128/aac.35.10.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Raoult D, Bres P, Drancourt M, Vestris G. In vitro susceptibilities of Coxiella burnetii, Rickettsia rickettsii, and Rickettsia conorii to the fluoroquinolone sparfloxacin. Antimicrob Agents Chemother. 1991;35:88–91. doi: 10.1128/aac.35.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Raoult D. Diagnostic biologique de la fièvre Q. Rev Fr Lab. 1991;227:68–70. [Google Scholar]
- 294.Raoult D, Brouqui P, Marchon B, Gastaut J A. Acute and chronic Q fever in patients with cancer. Clin Infect Dis. 1992;14:127–130. doi: 10.1093/clinids/14.1.127. [DOI] [PubMed] [Google Scholar]
- 295.Raoult D, Levy P Y, Tissot Dupont H, Chicheportiche C, Tamalet C, Gastaut J A, Salducci J. Q fever and HIV infection. AIDS. 1993;7:81–86. doi: 10.1097/00002030-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 296.Raoult D. Treatment of Q fever. Antimicrob Agents Chemother. 1993;37:1733–1736. doi: 10.1128/aac.37.9.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Raoult D, Stein A. Q fever during pregnancy, a risk for women, foetuses and obstetricians. N Engl J Med. 1994;330:371. doi: 10.1056/nejm199402033300518. [DOI] [PubMed] [Google Scholar]
- 298.Raoult D, Laurent J C, Multillod M. Monoclonal antibodies to Coxiella burnetii for antigenic detection in cell cultures and in paraffin embedded tissues. Am J Clin Pathol. 1994;101:318–320. doi: 10.1093/ajcp/101.3.318. [DOI] [PubMed] [Google Scholar]
- 299.Raoult D, Marrie T. Q fever. Clin Infect Dis. 1995;20:489–496. doi: 10.1093/clinids/20.3.489. [DOI] [PubMed] [Google Scholar]
- 300.Raoult D. Q fever: still a query after all these years. J Med Microbiol. 1996;44:77–78. doi: 10.1099/00222615-44-2-77. [DOI] [PubMed] [Google Scholar]
- 301.Raoult D, Houpikian P, Tissot Dupont H, Riss J M, Arditi-Djiane J, Brouqui P. Comparison of 2 regimens containing doxycycline and ofloxacin or hydroxychloroquine. Arch Intern Med. 1999;159:167–173. doi: 10.1001/archinte.159.2.167. [DOI] [PubMed] [Google Scholar]
- 302.Rauch A M, Tanner M, Pacer R E, Barrett M J, Brokopp C D, Schonberger L B. Sheep-associated outbreak of Q fever—Idaho. Arch Intern Med. 1987;147:341–344. [PubMed] [Google Scholar]
- 303.Riechman N, Raz R, Keysary A, Goldwasser R, Flatan E. Chronic Q fever and severe thrombocytopenia in a pregnant women. Am J Med. 1988;85:253–254. doi: 10.1016/s0002-9343(88)80355-3. [DOI] [PubMed] [Google Scholar]
- 304.Ripoll M M, Gomez-Mendieta M A, Gomez-Cerezo J, Belichon J C, Molina F. Pericarditis recidicante por fiebre Q. Enferm Infecc Microbiol Clin. 1997;15:168. [PubMed] [Google Scholar]
- 305.Reference deleted.
- 306.Roman M J, Coriz P D, Baca O G. A proposed model to explain persistent infection of host cells with Coxiella burnetii. J Gen Microbiol. 1986;132:1415–1422. doi: 10.1099/00221287-132-5-1415. [DOI] [PubMed] [Google Scholar]
- 307.Ross P J, Jacobson J, Muir J R. Q fever endocarditis of porcine xenograft valves. Am Heart J. 1983;105:151–153. doi: 10.1016/0002-8703(83)90293-4. [DOI] [PubMed] [Google Scholar]
- 308.Rowbotham T J. Preliminary report on the pathogenicity of Legionella pneumophila for fresh-water and soil amoebae. J Clin Pathol. 1980;33:1179–1183. doi: 10.1136/jcp.33.12.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Rudakov N V. Ecologico-epidemiological observations of Q fever in Russia. In: Kazar J, Toman R, editors. Rickettsiae and rickettsial diseases. Bratislava, Slovakia: Slovak Academy of Sciences; 1996. pp. 524–527. [Google Scholar]
- 310.Sabatier F, Dignat-George F, Mege J L, Brunet C, Raoult D, Sampol J. CD4+ T cell lymphopenia in Q fever endocarditis. Clin Diagn Lab Immunol. 1997;4:89–92. doi: 10.1128/cdli.4.1.89-92.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Saginur R, Silver S S, Bonin R, Carlier M, Orizaga M. Q-fever endocarditis. Can Med Assoc J. 1985;133:1228–1230. [PMC free article] [PubMed] [Google Scholar]
- 312.Salmon M M, Howells B, Glencross E J G, Evans A D, Palmer S R. Q fever in an urban area. Lancet. 1982;i:1002–1004. doi: 10.1016/s0140-6736(82)92000-1. [DOI] [PubMed] [Google Scholar]
- 313.Samuel J E, Frazier M E, Kahn M LE, Thomashow L S, Mallavia L P. Isolation and characterization of a plasmid from phase I Coxiella burnetii. Infect Immun. 1983;41:448–493. doi: 10.1128/iai.41.2.488-493.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Samuel J E, Frazier M E, Mallavia L P. Correlation of plasmid type and disease caused by Coxiella burnetii. Infect Immun. 1985;49:775–779. doi: 10.1128/iai.49.3.775-779.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Sanzo J M, Garcia-Calabuig M A, Audicana A, Dehesa V. Q fever: prevalence of antibodies to Coxiella burnetii in the Basque Country. Int J Epidemiol. 1993;22:1183–1187. doi: 10.1093/ije/22.6.1183. [DOI] [PubMed] [Google Scholar]
- 316.Savinelli E A, Mallavia L P. Comparison of Coxiella burnetii plasmids to homologous chromosomal sequences present in a plasmidless endocarditis-causing isolate. Ann N Y Acad Sci. 1990;590:523–533. doi: 10.1111/j.1749-6632.1990.tb42262.x. [DOI] [PubMed] [Google Scholar]
- 317.Sawyer L A, Fishbein D B, McDade J E. Q fever: current concepts. Rev Infect Dis. 1987;9:935–946. doi: 10.1093/clinids/9.5.935. [DOI] [PubMed] [Google Scholar]
- 318.Sawyer L A, Fishbein D B, MacDade J E. Q fever in patients with hepatitis and pneumonia: results of laboratory-based surveillance in the United States. J Infect Dis. 1988;158:497–498. doi: 10.1093/infdis/158.2.497. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 319.Schmeer N, Muller P, Langel J, Krauss H, Frost J W, Wieda J. Q fever vaccines for animals. Zentbl Bakteriol Microbiol Hyg Ser A. 1987;267:79–88. doi: 10.1016/s0176-6724(87)80191-8. [DOI] [PubMed] [Google Scholar]
- 320.Schmeer N, Muller H P, Baumgartner W, Wieda J, Krauss H. Enzyme-linked immunosorbent fluorescence assay and high-pressure liquid chromatography for analysis of humoral immune responses to Coxiella burnetii proteins. J Clin Microbiol. 1988;26:2520–2525. doi: 10.1128/jcm.26.12.2520-2525.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Schramek S, Mayer H. Different sugar compositions of lipopolysaccharides isolated from phase I and pure phase II cells of Coxiella burnetii. Infect Immun. 1982;38:53–57. doi: 10.1128/iai.38.1.53-57.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Schramek S, Radziejewski-Lebrecht J, Mayer H. 3-C-branched aldoses in lipopolysaccharide of phase I Coxiella burnetii and their role as immunodominant factors. Eur J Biochem. 1985;148:455–461. doi: 10.1111/j.1432-1033.1985.tb08861.x. [DOI] [PubMed] [Google Scholar]
- 323.Scott G H, Williams J C, Stephenson E H. Animal models in Q fever: pathological response of inbred mice to phase I Coxiella burnetii. J Gen Microbiol. 1987;133:691–700. doi: 10.1099/00221287-133-3-691. [DOI] [PubMed] [Google Scholar]
- 324.Scott G H, Williams J C. Susceptibility of Coxiella burnetii to chemical disinfectants. Ann N Y Acad Sci. 1990;590:291–296. doi: 10.1111/j.1749-6632.1990.tb42235.x. [DOI] [PubMed] [Google Scholar]
- 325.Seggev J S, Levin S, Schey G. Unusual radiological manifestations of Q fever. Eur J Respir Dis. 1986;69:120–122. [PubMed] [Google Scholar]
- 326.Selvaggi T M, Rezza G, Scagnelli M, Rigoli R, Massu M, De Lalla F, Pellizzer G P, Tramarin A, Bettini C, Zampieri L, Belloni M, Dalla Pozza E, Marangon S, Marchioretto N, Togni G, Giacobbo M, Todescato A, Binkin N. Investigation of a Q-fever outbreak in Northern Italy. Eur J Epidemiol. 1996;12:403–408. doi: 10.1007/BF00145305. [DOI] [PubMed] [Google Scholar]
- 327.Shapiro R A, Siskind V, Schofield F D, Stallman N, Worswick D A, Marmion B P. A randomized, controlled, double-blind, cross-over, clinical trial of Q fever vaccine in selected Queensland abattoirs. Epidemiol Infect. 1990;104:267–273. doi: 10.1017/s0950268800059446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Shepard C C. An outbreak of Q fever in Chicago packing house. Am J Hyg. 1947;46:185–192. doi: 10.1093/oxfordjournals.aje.a119162. [DOI] [PubMed] [Google Scholar]
- 329.Sheridan P, MacCaig J N, Hart R J C. Myocarditis complicating Q fever. Br Med J. 1974;2:155–156. doi: 10.1136/bmj.2.5911.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Sidwell R W, Thorpe B D, Gebhardt L P. Studies of latent Q fever infections. I. Effects of whole body X irradiation upon latently infected guinea pigs, white mice, and deer mice. Am J Hyg. 1964;79:113–124. [PubMed] [Google Scholar]
- 331.Sidwell R W, Thorpe B D, Gebhardt L P. Studies of latent Q fever infections. II. Effects of multiple cortisone injections. Am J Hyg. 1964;79:320–327. doi: 10.1093/oxfordjournals.aje.a120386. [DOI] [PubMed] [Google Scholar]
- 332.Siegman-Igra Y, Kaufman O, Keysary A, Rzotkiewicz S, Shalit I. Q fever endocarditis in Israel and a worldwide review. Scand J Infect Dis. 1997;29:41–49. doi: 10.3109/00365549709008663. [DOI] [PubMed] [Google Scholar]
- 333.Silver S S, McLeish W A. “Doughnut” granulomas in Q fever. Can Med Assoc J. 1984;130:102–104. [PMC free article] [PubMed] [Google Scholar]
- 334.Smith D J W, Derrick E H. Studies on the epidemiology of Q fever. I. The isolation of six strains of Rickettsia burneti from the tick Haemaphysalis humerosa. Aust J Exp Biol Med Sci. 1940;18:1–5. [Google Scholar]
- 335.Smith D J W. Studies on the epidemiology of Q fever. III. The transmission of Q fever by the tick Haemaphysalis humerosa. Aust J Exp Biol Med Sci. 1940;18:103–106. [Google Scholar]
- 336.Smith D J W. Studies on the epidemiology of Q fever. VIII. The transmission of Q fever by the tick Rhipicephalus sanguineus. Aust J Exp Biol Med Sci. 1941;19:119–122. [Google Scholar]
- 337.Smith D L, Ayres J G, Blair I, Burge P S, Carpenter M J, Caul E O, Coupland B, Desselberger U, Evans M, Farrell I D. A large Q fever outbreak in the West Midlands: clinical aspects. Respir Med. 1993;87:509–516. doi: 10.1016/0954-6111(93)90006-l. [DOI] [PubMed] [Google Scholar]
- 338.Sobradillo V, Ansola P, Baranda F, Corral C. Q fever pneumonia: a review of 164 community-acquired cases in the Basque country. Eur Respir J. 1989;2:263–266. [PubMed] [Google Scholar]
- 339.Sobradillo V, Zalacain R, Capelastegui A, Uresandi F, Corral J. Antibiotic treatment in pneumonia due to Q fever. Thorax. 1992;47:276–278. doi: 10.1136/thx.47.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Somma-Moreira R E, Caffarena R M, Somma S, Pérez G, Monteiro M. Analysis of Q fever in Uruguay. Rev Infect Dis. 1987;9:386–387. doi: 10.1093/clinids/9.2.386. [DOI] [PubMed] [Google Scholar]
- 341.Spelman D W. Q fever: a study of 111 consecutive cases. Med J Aust. 1982;1:547–553. doi: 10.5694/j.1326-5377.1982.tb124169.x. [DOI] [PubMed] [Google Scholar]
- 342.Spicer A J, Peacock M G, Williams J C. Effectiveness of several antibiotics in suppressing chick embryo lethality during experimental infections by Coxiella burnetii, Rickettsia typhi, and R. rickettsii. In: Burgdorfer W, Anacker R L, editors. Rickettsiae and rickettsial diseases. New York, N.Y: Academic Press, Inc.; 1981. pp. 375–383. [Google Scholar]
- 343.Srigley J R, Vellend H, Palmer N, Phillips M J, Geddie W R, van Nostrand A W, Edwards V D. Q-fever. The liver and bone marrow pathology. Am J Surg Pathol. 1985;9:752–758. [PubMed] [Google Scholar]
- 344.Stchepinsky O, Papo T, Amoyal P, Huisman J P, Théodose Y, Gaultier Y, Alexandre L, Piette J C. Endocardite àCoxiella burnetii sur prothèse valvulaire mécanique. A propos de deux cas. Arch Mal Cœur. 1995;88:511–515. [PubMed] [Google Scholar]
- 345.Stein A, Raoult D. Detection of Coxiella burnetii by DNA amplification using polymerase chain reaction. J Clin Microbiol. 1992;30:2462–2466. doi: 10.1128/jcm.30.9.2462-2466.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Stein A, Raoult D. A simple method for amplification of DNA from paraffin-embedded tissues. Nucleic Acids Res. 1992;20:5237–5238. doi: 10.1093/nar/20.19.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Stein A, Saunders N A, Taylor A G, Raoult D. Phylogenic homogeneity of Coxiella burnetii strains as determined by 16S ribosomal RNA sequencing. FEMS Microbiol Lett. 1993;113:339–344. doi: 10.1111/j.1574-6968.1993.tb06537.x. [DOI] [PubMed] [Google Scholar]
- 348.Stein A, Raoult D. Lack of pathotype specific gene in human Coxiella burnetii isolates. Microb Pathog. 1993;15:177–185. doi: 10.1006/mpat.1993.1068. [DOI] [PubMed] [Google Scholar]
- 349.Stein A, Raoult D. Q fever endocarditis. Eur Heart J. 1995;16(Suppl. B):19–23. doi: 10.1093/eurheartj/16.suppl_b.19. [DOI] [PubMed] [Google Scholar]
- 350.Stein A, Raoult D. Q fever during pregnancy: a public health problem in Southern France. Clin Infect Dis. 1998;27:592–596. doi: 10.1086/514698. [DOI] [PubMed] [Google Scholar]
- 351.Stein, A., and D. Raoult. Submitted for publication.
- 352.Stoenner H G, Lackman D B. The biologic properties of Coxiella burnetii isolated from rodents collected in Utah. Am J Hyg. 1960;71:45–48. doi: 10.1093/oxfordjournals.aje.a120088. [DOI] [PubMed] [Google Scholar]
- 353.Subramanya N I, Wright J S, Khan M A. Failure of rifampicin and co-trimoxazole in Q fever endocarditis. Br Med J. 1982;285:343–344. doi: 10.1136/bmj.285.6338.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Suhan M, Chen S-Y, Thompson H A, Hoover T A, Hill A, Williams J C. Cloning and characterization of an autonomous replication sequence from Coxiella burnetii. J Bacteriol. 1996;176:5233–5243. doi: 10.1128/jb.176.17.5233-5243.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Suhan M L, Chen S-Y, Thompson H A. Transformation of Coxiella burnetii to ampicillin resistance. J Bacteriol. 1996;178:2701–2708. doi: 10.1128/jb.178.9.2701-2708.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Syrucek L, Sobeslavsky O, Gutvirth I. Isolation of Coxiella burnetii from human placentas. J Hyg Epidemiol Microbiol Immunol. 1958;2:29–35. [PubMed] [Google Scholar]
- 357.Tellez A, Sainz C, Echevarria C, De Carlos S, Fernandez M V, Leon P, Brezina R. Q fever in Spain: acute and chronic cases 1981–1985. Rev Infect Dis. 1988;10:198–202. doi: 10.1093/clinids/10.1.198. [DOI] [PubMed] [Google Scholar]
- 358.Thiele D, Karo M, Krauss H. Monoclonal antibody based capture ELISA/ELIFA for detection of Coxiella burnetii in clinical specimens. Eur J Epidemiol. 1992;8:568–574. doi: 10.1007/BF00146378. [DOI] [PubMed] [Google Scholar]
- 359.Thiele D, Willems H, Köpf G, Krauss H. Polymorphism in DNA restriction patterns of Coxiella burnetii isolates investigated by pulsed field gel electrophoresis and image analysis. Eur J Epidemiol. 1993;9:419–425. doi: 10.1007/BF00157400. [DOI] [PubMed] [Google Scholar]
- 360.Thiele D, Willems H. Is plasmid based differentiation of Coxiella burnetii in “acute” and “chronic” isolates still valid? Eur J Epidemiol. 1994;10:427–434. doi: 10.1007/BF01719667. [DOI] [PubMed] [Google Scholar]
- 361.Thiele D, Willems H, Haas M, Krauss H. Analysis of the entire nucleotide sequence of the cryptic plasmid QpH1 from Coxiella burnetii. Eur J Epidemiol. 1994;10:413–420. doi: 10.1007/BF01719665. [DOI] [PubMed] [Google Scholar]
- 362.Thomas D R, Salmon R L, Kench S M, Meadows D, Coleman T J, Morgan-Capner P, Morgan K L. Zoonotic illness: determining risks and measuring effects of the association between current animal exposure and a history of illness in a well characterized rural population. J Epidemiol Community Health. 1994;48:151–155. doi: 10.1136/jech.48.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Thomas D R, Treweek L, Salmon R L, Kench S M, Coleman T J, Meadows D, Morgan-Capner P, Caul E O. The risk of acquiring Q fever on farms: a seroepidemiological study. Occup Environ Med. 1995;52:644–647. doi: 10.1136/oem.52.10.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Thomas D R, Salmon R L, Smith R M M, Caul E O, Treweek L, Kench S M, Coleman T J, Meadows D, Morgan-Capner P, Sillis M. Epidemiology of Q fever in the UK. In: Kazar J, Toman R, editors. Rickettsiae and rickettsial diseases. Bratislava, Slovakia: Slovak Academy of Sciences; 1996. pp. 512–517. [Google Scholar]
- 365.Tissot Dupont H, Raoult D, Brouqui P, Janbon F, Peyramond D, Weiller P J, Chicheportiche C, Nezri M, Poirier R. Epidemiologic features and clinical presentation of acute Q fever in hospitalized patients—323 French cases. Am J Med. 1992;93:427–434. doi: 10.1016/0002-9343(92)90173-9. [DOI] [PubMed] [Google Scholar]
- 366.Tissot Dupont H, Thirion X, Raoult D. Q fever serology: cutoff determination for microimmunofluorescence. Clin Diagn Lab Immunol. 1994;1:189–196. doi: 10.1128/cdli.1.2.189-196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Tissot Dupont H, Torres S, Nezri M, Raoult D. A hyperendemic focus of Q fever related to sheep and wind. Amer J Epidemiol. 1999;150:67–74. doi: 10.1093/oxfordjournals.aje.a009920. [DOI] [PubMed] [Google Scholar]
- 368.To H, Kako N, Zhang G Q, Otsuka H, Ogawa M, Ochiai O, Nguyen SA V, Yamaguchi T, Fukushi H, Nagaoka N, Akiyama M, Amano K, Hirai K. Q fever pneumonia in children in Japan. J Clin Microbiol. 1996;34:647–651. doi: 10.1128/jcm.34.3.647-651.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Tobin M J, Cahill N, Gearty G, Maurer B, Blake S, Daly K, Hone R. Q fever endocarditis. Am J Med. 1982;72:396–400. doi: 10.1016/0002-9343(82)90495-8. [DOI] [PubMed] [Google Scholar]
- 370.Tokarevich N K, Schramek S, Daiter A B. Indirect haemolysis test in Q fever. Acta Virol. 1990;34:358–360. [PubMed] [Google Scholar]
- 371.Topping N H, Shepard C C, Irons J V. Q fever in the United States. I. Epidemiologic studies of an outbreak among stock handlers and slaughter house workers. JAMA. 1947;133:813–821. doi: 10.1001/jama.1947.02880120001001. [DOI] [PubMed] [Google Scholar]
- 372.Tselentis Y, Gikas A, Kofteridis D, Kyriakakis E, Lydatakis N, Bouros D, Tsaparas N. Q fever in the Greek island of Crete: epidemiologic, clinical, and therapeutic data from 98 cases. Clin Infect Dis. 1995;20:1311–1316. doi: 10.1093/clinids/20.5.1311. [DOI] [PubMed] [Google Scholar]
- 373.Turck W P, Matthews M B. Rickettsial endocarditis. Br Med J. 1969;1:185–186. doi: 10.1136/bmj.1.5637.185-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Turck W P G, Howitt G, Turnberg L A, Fox H, Longson M, Matthews M B, Das Gupta R. Chronic Q fever. Q J Med. 1976;45:193–217. [PubMed] [Google Scholar]
- 375.Turck W P G. Q fever. In: Braude A I, Davis C E, Fierer J, editors. medical microbiology and infectious diseases. Toronto, Canada: The W. B. Saunders Co.; 1981. pp. 932–938. [Google Scholar]
- 376.Turco J, Thompson H A, Winkler H H. Gamma interferon inhibits growth of Coxiella burnetii in mouse fibroblasts. Infect Immun. 1984;45:781–783. doi: 10.1128/iai.45.3.781-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Tylewska-Wierbanowska S K, Kruszewka D. Q-fever sexually transmitted infection? J Infect Dis. 1990;161:368–369. doi: 10.1093/infdis/161.2.368. [DOI] [PubMed] [Google Scholar]
- 378.Uhaa I J, Fishbein D B, Olson J G, Rives C C, Waag D M, Williams J C. Evaluation of specificity of indirect enzyme-linked immunosorbent assay for diagnosis of human Q fever. J Clin Microbiol. 1994;32:1560–1565. doi: 10.1128/jcm.32.6.1560-1565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Urrutia A, et al. Acute myopericarditis as a manifestation of Q fever. Med Clin. 1991;96:319. [PubMed] [Google Scholar]
- 380.Valkova D, Kazar J. A new plasmid (QpDV) common to Coxiella burnetii isolates associated with acute and chropnic Q fever. FEMS Microbiol Lett. 1995;125:275–280. doi: 10.1111/j.1574-6968.1995.tb07368.x. [DOI] [PubMed] [Google Scholar]
- 381.Varma M P, Adgey A A, Connolly J H. Chronic Q fever endocarditis. Br Heart J. 1980;43:695–699. doi: 10.1136/hrt.43.6.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Vellend H, Salit I E, Spence L, McLaughlin B, Carlson J, Palmer N, Van Dreumel A A, Hodgkinson J R. Q fever—Ontario. Can Dis Weekly Rep. 1982;8:171–173. [Google Scholar]
- 383.Vodkin M H, Williams J C. Overlapping deletion in two spontaneous phase variants of Coxiella burnetii. J Gen Microbiol. 1986;132:2587–2594. doi: 10.1099/00221287-132-9-2587. [DOI] [PubMed] [Google Scholar]
- 384.Vodkin M H, Williams J C, Stephenson E H. Genetic heterogeneity among isolates of Coxiella burnetii. J Gen Microbiol. 1986;132:455. doi: 10.1099/00221287-132-2-455. [DOI] [PubMed] [Google Scholar]
- 385.Vodkin M H, Williams J C. A heat shock operon in Coxiella burnetii produces a major antigen homologous to a protein to both mycobacteria and Escherichia coli. J Bacteriol. 1988;170:1227–1234. doi: 10.1128/jb.170.3.1227-1234.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Voigt J J, Delsol G, Fabre J. Liver and bone marrow granulomas in Q fever. Gastroenterology. 1983;84:887–888. [PubMed] [Google Scholar]
- 387.Waag D, Chulay J, Marrie T, England M, Williams J C. Validation of an enzyme immunoassay for serodiagnosis of acute Q fever. Eur J Clin Microbiol Infect Dis. 1995;14:421–427. doi: 10.1007/BF02114898. [DOI] [PubMed] [Google Scholar]
- 388.Wachter R F, Briggs G P, Gangemi J D, Pedersen C E. Changes in buoyant density relationships of 2 cell types of Coxiella burnetii phase I. Infect Immun. 1975;12:433–436. doi: 10.1128/iai.12.2.433-436.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Wagstaff D J, Janney J H, Crawford K J, Dimijian G G, Joseph J M. Q fever studies in Maryland. Public Health Rep. 1965;80:1095–1099. [PMC free article] [PubMed] [Google Scholar]
- 390.Waldhalm D G, Stoenner H G, Simmons R E, Thomas L A. Abortion associated with Coxiella burnetii infection in dairy goats. J Am Vet Med Assoc. 1978;137:1580–1581. [PubMed] [Google Scholar]
- 391.Webster J P, Lloyd G, Macdonald D W. Q fever (Coxiella burnetii) reservoir in wild brown rat (Rattus norvegicus) populations in the UK. Parasitology. 1995;110:31–35. doi: 10.1017/s0031182000081014. [DOI] [PubMed] [Google Scholar]
- 392.Weir W R C, Bannister B, Chambers S, DeCock K, Mistry H. Chronic Q fever associated with granulomatous hepatitis. J Infect. 1984;8:56–60. doi: 10.1016/s0163-4453(84)93354-1. [DOI] [PubMed] [Google Scholar]
- 393.Weisburg W G, Woese C R, Dobson M E, Weiss E. A common origin of rickettsiae and certain plant pathogens. Science. 1985;230:556–558. doi: 10.1126/science.3931222. [DOI] [PubMed] [Google Scholar]
- 394.Weisburg W G, Dobson M E, Samuel J E, Dasch G A, Mallavia L P, Baca O, Mandelco L, Sechrest J E, Weiss E, Woese C R. Phylogenetic diversity of the Rickettsiae. J Bacteriol. 1989;171:4202–4206. doi: 10.1128/jb.171.8.4202-4206.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Weiss E, Pietryk H. Growth of Coxiella burnetii in monolayer cultures of chicken embryo entodermal cells. J Bacteriol. 1957;72:235–238. doi: 10.1128/jb.72.2.235-241.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Weiss E, Moulder J W. Genus III. Coxiella. In: Krieg N R, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 1. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 701–704. [Google Scholar]
- 397.Westlake P, Price L M, Russell M, Kelly J K. The pathology of Q fever hepatitis: a case diagnosed by liver biopsy. J Clin Gastroenterol. 1987;9:357–363. doi: 10.1097/00004836-198706000-00024. [DOI] [PubMed] [Google Scholar]
- 398.Wiebe M E, Burton P R, Shankel D M. Isolation and characterization of two cell types of Coxiella burnetii phase I. J Bacteriol. 1972;110:368–377. doi: 10.1128/jb.110.1.368-377.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Willems H, Thiele D, Glas-Adollah-Baik Kashi M, Krauss H. Immunoblot technique for Q fever. Eur J Epidemiol. 1992;8:103–107. doi: 10.1007/BF03334980. [DOI] [PubMed] [Google Scholar]
- 400.Willems H, Thiele D, Krauss H. Plasmid based differentiation and detection of Coxiella burnetii in clinical samples. Eur J Epidemiol. 1993;9:411–417. doi: 10.1007/BF00157399. [DOI] [PubMed] [Google Scholar]
- 401.Willems H, Thiele D, Frolich-Ritter R, Krauss H. Detection of Coxiella burnetii in cow’s milk using the polymerase chain reaction. J Vet Med Ser B. 1994;41:580–587. doi: 10.1111/j.1439-0450.1994.tb00267.x. [DOI] [PubMed] [Google Scholar]
- 402.Willems H, Thiele D, Burger C, Ritter M, Oswald W, Krauss H. Molecular biology of Coxiella burnetii. In: Kazar J, Toman R, editors. Rickettsiae and rickettsial diseases. Bratislava, Slovakia: Slovak Academy of Sciences; 1996. pp. 363–378. [Google Scholar]
- 403.Willems H, Jäger C, Baljer G. Physical and genetic map of the obligate intracellular bacterium Coxiella burnetii. J Bacteriol. 1998;180:3816–3822. doi: 10.1128/jb.180.15.3816-3822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Williams J C, Cantrell J L. Biological and immunological properties of Coxiella burnetii vaccines in C57BL/10ScN endotoxin-non responder mice. Infect Immun. 1982;35:1091–1102. doi: 10.1128/iai.35.3.1091-1102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Williams J C, Thomas L A, Peacock M G. Humoral immune response to Q fever: enzyme-linked immunosorbent assay antibody response to Coxiella burnetii in experimentally infected guinea pigs. J Clin Microbiol. 1986;24:935–939. doi: 10.1128/jcm.24.6.935-939.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Williams J C, Thomas L A, Peacock M G. Identification of phase-specific antigenic fractions of Coxiella burnetii by enzyme-linked immunosorbent assay. J Clin Microbiol. 1986;24:929–934. doi: 10.1128/jcm.24.6.929-934.1986. . (Erratum, 25:588, 1987.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Williams J C, Damrow T A, Waag D M, Amano K I. Characterization of a phase I Coxiella burnetii chloroform-methanol residue vaccine that induces active immunity against Q fever in C57BL/10ScN mice. Infect Immun. 1986;51:851–858. doi: 10.1128/iai.51.3.851-858.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Williams J C, Peacock M G, Waag D M, Kent G, England M J, Nelson G, Stephenson E H. Vaccines against coxiellosis and Q fever. Development of a chloroform-methanol residue subunit of phase I Coxiella burnetii for the immunization of animals. Ann N Y Acad Sci. 1993;653:88–111. doi: 10.1111/j.1749-6632.1992.tb19633.x. [DOI] [PubMed] [Google Scholar]
- 409.Wilson H G, Neilson G H, Galea E G, Stafford G, O’Brien M F. Q fever endocarditis in Queensland. Circulation. 1976;53:680–684. doi: 10.1161/01.cir.53.4.680. [DOI] [PubMed] [Google Scholar]
- 410.Wilson K H, Blitchington R, Shah P, McDonald G, Gilmore R D, Mallavia L P. Probe directed at a segment of Rickettsia rickettsii rRNA amplified with polymerase chain reaction. J Clin Microbiol. 1989;27:2692–2696. doi: 10.1128/jcm.27.12.2692-2696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Winner S J, Eglin R P, Moore V I, Mayon-White R T. An outbreak of Q fever affecting postal workers in Oxfordshire. J Infect. 1987;14:255–261. doi: 10.1016/s0163-4453(87)93560-2. [DOI] [PubMed] [Google Scholar]
- 412.Whittick J W. Necropsy findings in a case of Q fever in Britain. Br Med J. 1950;1:979–982. doi: 10.1136/bmj.1.4660.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Worswick D, Marmion B P. Antibody response in acute and chronic Q fever and in subjects vaccinated against Q fever. J Med Microbiol. 1985;19:281–296. doi: 10.1099/00222615-19-3-281. [DOI] [PubMed] [Google Scholar]
- 414.Yanase T, Muramatsu Y, Ueno H, Morita C. Seasonal variations in the presence of antibodies against Coxiella burnetii in dairy cattle in Hokkaido, Japan. Microbiol Immunol. 1997;41:73–75. doi: 10.1111/j.1348-0421.1997.tb01184.x. [DOI] [PubMed] [Google Scholar]
- 415.Yarrow A, Slater P E, Costin C. Q fever in Israel. Public Health Rev. 1990;18:129–137. [PubMed] [Google Scholar]
- 416.Yeaman M R, Mitscher L A, Baca O G. In vitro susceptibility of Coxiella burnetii to antibiotics, including several quinolones. Antimicrob Agents Chemother. 1987;31:1079–1084. doi: 10.1128/aac.31.7.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Yeaman M R, Roman M J, Baca O G. Antibiotic susceptibilities of two Coxiella burnetii isolates implicated in distinct clinical syndromes. Antimicrob Agents Chemother. 1989;33:1052–1057. doi: 10.1128/aac.33.7.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Yeaman M R, Baca O G. Unexpected antibiotic susceptibility of a chronic isolate of Coxiella burnetii. Ann N Y Acad Sci. 1990;590:297–305. doi: 10.1111/j.1749-6632.1990.tb42236.x. [DOI] [PubMed] [Google Scholar]
- 419.Yebra M, Marazuela M, Albarran F, Moreno A. Chronic Q fever hepatitis. Rev Infect Dis. 1988;10:1229–1230. doi: 10.1093/clinids/10.6.1229-b. [DOI] [PubMed] [Google Scholar]
- 420.Yoshiie K, Oda H, Nagano N, Matayoshi S. Serological evidence that the Q fever agent (Coxiella burnetii) has spread widely among dairy cattle of Japan. Microbiol Immunol. 1991;35:577–581. doi: 10.1111/j.1348-0421.1991.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 421.Yu X, Raoult D. Serotyping Coxiella burnetii isolates from acute and chronic Q fever patients by using monoclonal antibodies. FEMS Microbiol Lett. 1994;117:15–19. doi: 10.1111/j.1574-6968.1994.tb06735.x. [DOI] [PubMed] [Google Scholar]
- 422.Yuasa Y, Yoshiie K, Takasaki T, Yoshida H, Oda H. Retrospective survey of chronic Q fever in Japan by using PCR to detect Coxiella burnetii DNA in paraffin-embedded clinical samples. J Clin Microbiol. 1996;34:824–827. doi: 10.1128/jcm.34.4.824-827.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Zielinski N A, Chakrabarty A M, Berry A. Characterization and regulation of the Pseudomonas aeruginosa algC gene encoding phosphomannomutase. J Biol Chem. 1991;266:9754–9763. [PubMed] [Google Scholar]
- 424.Zuber M, Hoover T A, Court D L. Cloning, sequencing and expression of the dnaJ gene of Coxiella burnetii. Gene. 1995;152:99–102. doi: 10.1016/0378-1119(94)00687-n. [DOI] [PubMed] [Google Scholar]
- 425.Zuber M, Hoover T A, Court D L. Analysis of a Coxiella burnetii gene product that activates capsule synthesis in Escherichia coli: requirement for the heat shock chaperone DnaK and the two-component regulator RcsC. J Bacteriol. 1995;177:4238–4244. doi: 10.1128/jb.177.15.4238-4244.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Zuerner R L, Thompson H A. Protein synthesis by intact Coxiella burnetii cells. J Bacteriol. 1983;156:186–191. doi: 10.1128/jb.156.1.186-191.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]