Significance
Parkinson’s disease (PD) is a progressive disorder of the central nervous system that affects motor control. While subcutaneous injection of apomorphine (Apokyn) is clinically used to alleviate intermittent episodes of dyskinesia, the treatment requires multiple injections of the drug per day, significantly deterring patient compliance. We introduce a deep eutectic-based ternary solvent system that self-emulsifies in situ following subcutaneous injection and entraps apomorphine, allowing a 48-h duration of detectable drug concentration in the plasma of pigs, which is a remarkable improvement over the clinical comparator. The results from the animal studies support the self-emulsifying system as a potent, long-lasting therapeutic for PD patients and potentially for other therapeutics that have a similar delivery challenge.
Keywords: Parkinson’s, depot, apomorphine, deep eutectic, ionic liquid
Abstract
Apomorphine, a dopamine agonist, is a highly effective therapeutic to prevent intermittent off episodes in advanced Parkinson’s disease. However, its short systemic half-life necessitates three injections per day. Such a frequent dosing regimen imposes a significant compliance challenge, especially given the nature of the disease. Here, we report a deep eutectic-based formulation that slows the release of apomorphine after subcutaneous injection and extends its pharmacokinetics to convert the current three-injections-a-day therapy into an every-other-day therapy. The formulation comprises a homogeneous mixture of a deep eutectic solvent choline-geranate, a cosolvent n-methyl-pyrrolidone, a stabilizer polyethylene glycol, and water, which spontaneously emulsifies into a microemulsion upon injection in the subcutaneous space, thereby entrapping apomorphine and significantly slowing its release. Ex vivo studies with gels and rat skin demonstrate this self-emulsification process as the mechanism of action for sustained release. In vivo pharmacokinetics studies in rats and pigs further confirmed the extended release and improvement over the clinical comparator Apokyn. In vivo pharmacokinetics, supported by a pharmacokinetic simulation, demonstrate that the deep eutectic formulation reported here allows the maintenance of the therapeutic drug concentration in plasma in humans with a dosing regimen of approximately three injections per week compared to the current clinical practice of three injections per day.
Parkinson’s disease (PD), the second most common neurodegenerative disease, is characterized by dopamine deficiency arising from the progressive loss of dopaminergic neurons in the pars compacta of the substantia nigra. Multiple motor as well as nonmotor symptoms, such as rigidity, tremor, bradykinesia, and cognitive dysfunction, are associated with PD (1). While a number of disease-modifying therapies to treat PD are currently in clinical trials (2, 3), the approved therapies comprise only those that treat the symptoms. Among them, apomorphine (APO) is a leading drug given to patients to alleviate short intermittent periods of motor complications like dyskinesia, which often develop in advanced PD after long-term prior treatment with oral levodopa (4). APO has poor oral bioavailability and high first pass metabolism, thus leaving subcutaneous injections as the only viable administration mode (Apokyn). However, the short half-life (69.7 ± 25.8 min) of APO in the systemic circulation necessitates frequent injections of Apokyn, namely, three times a day, at the onset of individual off episodes (5). This poses a significant challenge with patient compliance in terms of pain, infection, emetic side effects, inaccurate dosing, lack of manual dexterity, or even inability to self-inject (6–9).
Two notable strategies have been evaluated in clinical trials to mitigate the shortcomings of frequent subcutaneous APO injections. Kynmobi, a Food and Drug Administration (FDA)-approved sublingual film containing APO, allows rapid absorption of the drug via buccal administration (10, 11). It consists of two layers, as follows: one labeled as a buffer layer that neutralizes acid generation following drug absorption and another as an active layer that contains APO to allow rapid drug diffusion and absorption. However, it lacks a mechanism for sustained release and thus still requires repeated on-demand administration and induces fluctuations in blood concentrations. The continuous subcutaneous infusion of APO has also been explored as an alternative. The subcutaneous pump continuously injects Apokyn from a prefilled syringe and aims to maintain therapeutic concentrations of APO in systemic circulation and has shown a shortened duration of total daily off episodes in clinical trials. However, this approach is hindered by the complexity of use and local site reactions (12–14). Furthermore, this product recently received Refusal to File from the FDA. Many other preclinical and clinical formulations of APO have attempted to achieve noninvasive administration and prolonged pharmacokinetics (15, 16), for example, prodrug modification for oral delivery (17) or encapsulation in microemulsion for transdermal delivery (18); however, the utility of these approaches is limited by low bioavailability compared to subcutaneous injections. Thus, the development of a safe and simple sustained release formulation of APO remains an unmet clinical need.
From a scientific perspective, APO represents one of the most challenging drugs to formulate; it has limited water solubility, is highly susceptible to oxidation, exhibits short plasma half-life (5, 19), and has a tight therapeutic window with a minimum effective concentration (MEC) of 4 ng/mL and a maximum tolerated concentration (MTC) of 10 ng/mL in humans. The use of a large number of sustained release technologies, including microspheres (20, 21), depots (11), liposomes (22), and polymeric as well as solid lipid nanoparticles (23, 24) among others, has been attempted with APO. However, the multiple physicochemical, biological, and clinical constraints have posed a hurdle in delivering APO in a safe and sufficiently sustained manner. Consequently, no long-acting formulation of APO is currently available.
Here, we report a strategy for achieving an extended release of APO based simultaneously on the differential miscibility of a deep eutectic solvent, namely, choline and geranic acid (CAGE1:2) in two solvents, water and n-methyl pyrrolidone (NMP), as well as the differential solubility of APO in each of these three solvents. We designed the formulation to be a homogenous, stable solution of APO in a three-component system (CAGE1:2/NMP/water). However, upon subcutaneous injection, NMP rapidly diffuses away, and the formulation self-emulsifies into a dispersion of CAGE1:2 in water while trapping APO in it, thereby achieving sustained release (Fig. 1). This design increased the timescale of APO pharmacokinetics, converting the current clinical standard of three-times-a-day formulation into an every-other-day formulation. While achieving this goal, we satisfied three essential constraints including the following: increased solubility of APO from 10 mg/mL in Apokyn to 30 mg/mL to support long-lasting delivery from a single dose; stability of APO against oxidation; and the use of all components, other than CAGE1:2, at concentrations already listed in other FDA-approved subcutaneous products, thus facilitating the potential for translation of this strategy. We refer to this formulation as self-emulsifying, APO-releasing therapeutic (SEAPORT). We report the design strategy, ex vivo assessment, in vivo pharmacokinetics in rats, safety in rats, and in vivo pharmacokinetics in pigs.
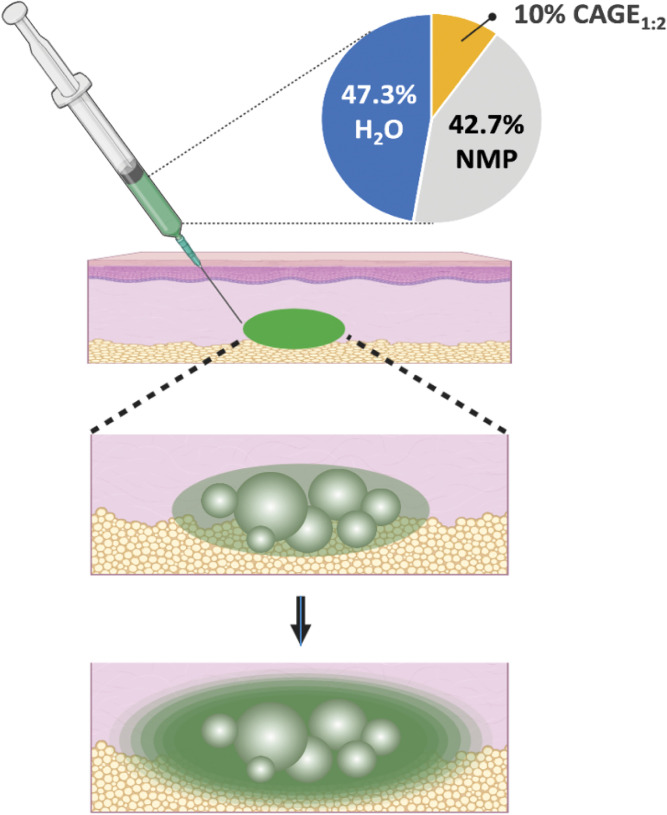
Fig. 1.
Schematic diagram of SEAPORT principle. APO, PEG3350, and SMB are solubilized in a CAGE1:2/NMP/water (10:42.7:47.3% vol/vol) mixture in SEAPORT, and then upon subcutaneous injection, water-miscible NMP diffuses away quickly and the remaining CAGE1:2 emulsifies with APO to form a depot, allowing the sustained release of the drug.
Results
High Solubility and Stability of APO in SEAPORT.
A ternary phase diagram shows that CAGE1:2 exhibits concentration-dependent miscibility with water (SI Appendix, Fig. S1A). Specifically, a CAGE1:2/water mixture forms an emulsion at a concentration of 10% vol/vol (inset images), which was selected as the concentration of choice. The phase diagram also shows that an addition of greater than 35% vol/vol NMP, an organic solvent in the FDA list of inactive ingredients in approved subcutaneous products, completely solubilizes 10% vol/vol CAGE1:2-in-water emulsion to form a homogenous solution. An NMP concentration of 42.7% vol/vol was selected based on its concentration used in an FDA-approved product (ELIGARD). This ternary system was used as the base of SEAPORT. APO was solubilized at a concentration of 30 mg/mL in a mixture of 10% vol/vol CAGE1:2, 42.7% vol/vol NMP, and 47.3% vol/vol water (SEAPORT). While 5% vol/vol CAGE1:2-in-water also forms an emulsion, the final formulation consisting of 5% vol/vol CAGE1:2 and 42.7% vol/vol NMP with 30 mg/mL APO was opaque and unstable (SI Appendix, Fig. S1B), thus supporting 10% vol/vol as the optimal CAGE1:2 concentration in SEAPORT. A total of 3 mg/mL sodium metabisulfite (SMB) and 28.8 mg/mL polyethylene glycol molecular weight 3350 (PEG 3350) were added as an antioxidant and a stabilizer to SEAPORT, respectively (Fig. 2A).
Fig. 2.
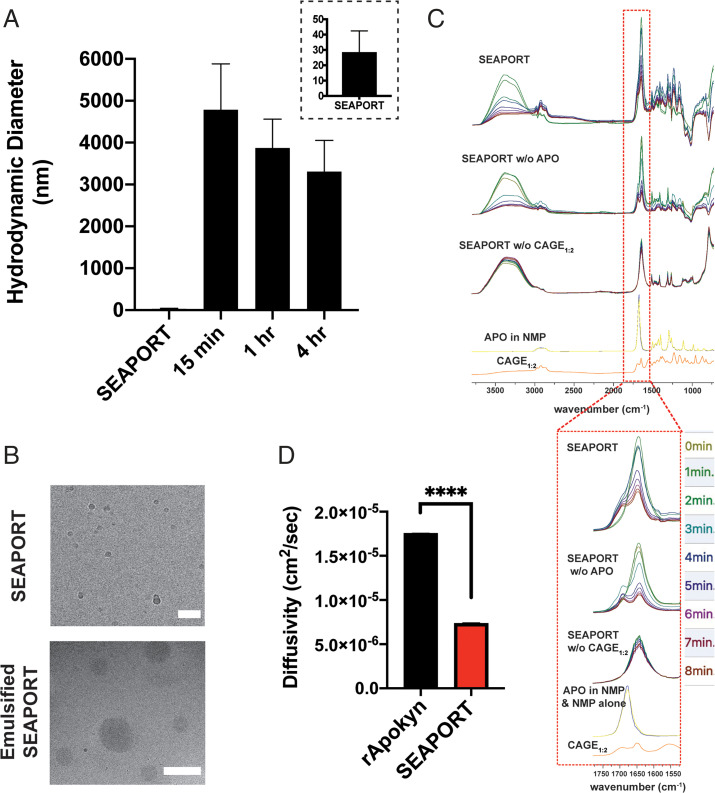
Solubility and stability of APO in SEAPORT. (A) Photograph image of SEAPORT formulation. (B) APO concentration in SEAPORT immediately following formulation detected by LC-MS (n = 3, mean ± SD). (C) Long-term stability determined by APO concentration using LC-MS on days 1, 2, 4, and 7 after incubation at 4 °C (n = 3, mean ± SD).
The concentration of APO in SEAPORT was confirmed using liquid chromatography-mass spectrometry (LC-MS), which remained consistent at over 90% of the initial concentration over 7 d of incubation at 4 °C, 25 °C, and 37 °C (Fig. 2 B and C) with one exception of 37 °C on day 2 (SI Appendix, Fig. S1C). SEAPORT remained stable and homogenous with no apparent phase separation over 7 d of incubation at 4 °C, 25 °C, and 37 °C (SI Appendix, Fig. S1D). A stress-aging condition at 37 °C darkened the color of SEAPORT, but a similar color change was observed in SEAPORT without (w/o) APO, thus suggesting that the color was due to the inactive ingredients in the formulation. The absence of antioxidant SMB resulted in oxidation of APO and a color change to dark blue/green, which was indirectly measured via a colorimetric absorbance assay (SI Appendix, Fig. S1 D and E). SEAPORT containing 3 mg/mL SMB was sufficient to prevent oxidation of APO for at least 7 d even under the stress-aging 37 °C condition (SI Appendix, Fig. S1F).
Sustained Release of APO from SEAPORT Ex Vivo.
The ability of SEAPORT to provide the sustained release of APO was tested ex vivo using rat skin as a model. The release was compared against an Apokyn-mimicking formulation, that is, 10 mg/mL APO in water with antioxidants (referred to as rApokyn) as the reference formulation. A variant of SEAPORT without CAGE1:2 (SEAPORT w/o CAGE1:2) was also used as a control to assess whether CAGE1:2 played a role in achieving the sustained release from SEAPORT. The apparatus was designed to detect APO diffusing out of the formulation, through the surrounding tissue, and into the saline medium (Fig. 3A). rApokyn exhibited a burst release with nearly 50% of the injected dose released in the first 6 h (Fig. 3B and SI Appendix, Fig. S2A). In contrast, SEAPORT suppressed the early burst release and allowed nearly zero order release kinetics, leading up to 40% of the injected dose released after 24 h. SEAPORT w/o CAGE1:2 demonstrated a release profile comparable to rApokyn, suggesting that CAGE1:2 plays a critical role in the observed controlled release of APO from SEAPORT. These quantitative measurements of release were also consistent with the visual observations in the rat skin injected with the formulations, where an accumulation of oxidized APO in the controls was clearly observed at the boundary of the skin by 48 h, while only minimal color was observed in the SEAPORT group (SI Appendix, Fig. S2B).
Fig. 3.
Ex vivo diffusion of APO. (A) Schematic illustration of ex vivo apparatus for APO release study from harvested rat skin. (B) Percent release of APO into saline from harvested rat skin subcutaneously injected with 50 μL rApokyn, SEAPORT, and SEAPORT w/o CAGE1:2, as determined by LC-MS (n = 5, mean ± SD). (C) Photograph images of 36% wt/vol agarose gel prepared in 20-mL glass scintillation vial (white arrows, hollow channel at the center of agarose gel). (D) Photograph image of opaque, emulsified SEAPORT after incubation in the agarose gel. (E) Percent of APO remaining at the center of agarose gel after incubation at 37 °C for specified timepoint, determined by LC-MS (n = 3, mean ± SD).
To gain further insights into the behavior of APO in the subcutaneous tissue, SEAPORT was also studied in an agarose gel with high water content to mimic the subcutaneous space. A hollow channel was created at the center of the agarose gel and filled with the formulations to allow spontaneous mixing with water from the agarose gel (Fig. 3C). Delivery of SEAPORT in the gel led to the formation of an emulsified depot that was visible by the transition of the clear formulation to an opaque mixture (Fig. 3D). The clearance of APO from the center channel of the agarose gel was significantly slower for SEAPORT than that for SEAPORT w/o CAGE1:2 (Fig. 3E). Release from rat skin as well as gel confirmed that SEAPORT slows the release of APO from the injected site.
Emulsification of SEAPORT Slows the Diffusion of APO.
SEAPORT incubated in the agarose gel model was used to assess the behavior of the subcutaneously injected formulation. Dynamic light scattering (DLS) and cryogenic transmission electron microscopy (cryo-TEM) were used to characterize the formulation. Prior to injection, SEAPORT exhibited 10- to 30-nm nanoclusters, possibly from the association between APO and CAGE1:2 (Fig. 4 A and B). Upon incubation in the agarose gel, the formulation generated large, micrometer-sized emulsions (Fig. 4 A and B and Movie S1). The transition of aggregates from 10- to 30-nm sized to micrometer-sized clusters upon injection into the gel was consistent with the visual observation that the formulation transitioned from transparent to milky (Fig. 3D). Consistent with the ex vivo release, there was no apparent generation of emulsions from SEAPORT w/o CAGE1:2 (Movie S2). Furthermore, when CAGE1:2 concentration in the formulation was increased to either 20% or 30% vol/vol, the resulting product failed to emulsify upon incubation in the agarose gel (SI Appendix, Fig. S3A), which is in agreement with the ternary phase diagram (SI Appendix, Fig. S1A).
Fig. 4.
Self-emulsification of SEAPORT. (A) Hydrodynamic diameter of the SEAPORT formulation pre- and postincubation in 36% wt/vol agarose gel in water determined by DLS (n = 3, formulation replicates, mean ± SD, inset: zoomed in on SEAPORT). (B) Cryo-TEM image of SEAPORT formulation before and following 20-min incubation in agarose gel (scale bar: Top = 50 nm, Bottom = 1 µm). (C) ATR-FTIR spectra of SEAPORT, SEAPORT w/o APO, SEAPORT w/o CAGE1:2, APO in NMP, NMP alone, and CAGE1:2 (each colored FTIR trace depicts a different timepoint). (D) Diffusion coefficients of APO in rApokyn and SEAPORT at 20 min postincubation in 36% wt/vol agarose gel, as determined by DOSY NMR (n = 3, mean ± SD, ****P < 0.0001).
The emulsification process of SEAPORT was further characterized using Fourier transform infrared spectroscopy-attenuated total reflectance (ATR-FTIR) spectra based on the characteristic peaks of NMP and CAGE1:2. NMP exhibited a ν(C = O) peak at 1,679 cm−1 in neat NMP and at 1,645 cm−1 in SEAPORT. CAGE1:2 showed a characteristic v(C = C) peak at 1,650 cm−1 that overlapped with the NMP peak and an additional distinct peak at 1,675 cm−1 (Fig. 4C). The intensities of peaks at 1,645 cm−1 (NMP and CAGE1:2) and 1,675 cm−1 (CAGE1:2) can thus be used to assess the evolution of the formulation. In both SEAPORT and SEAPORT w/o APO, the intensity of the peak at 1,645 cm−1 decreased over the course of 8 min of incubation in an agarose gel. A much smaller decrease in the peak intensity at 1,675 cm−1 was observed, resulting in a comparable ratio of peak heights at 1,645 cm−1 and 1,675 cm−1 between that of SEAPORT and neat CAGE1:2 within 8 min of incubation (SI Appendix, Fig. S3B). Meanwhile, SEAPORT w/o CAGE1:2 showed no decrease in the 1,645-cm−1 peak, which is indicative of no change in the formulation composition. This suggests that upon injection into the gel, SEAPORT undergoes displacement of NMP with a high amount of CAGE1:2 potentially due to phase separation of CAGE1:2 from SEAPORT into emulsions.
Diffusion-ordered spectroscopy (DOSY) further showed a significant decrease in the diffusivity of APO from emulsified SEAPORT in the agarose gel compared to that from rApokyn in the agarose gel (Fig. 4D and SI Appendix, Fig. S3 C and D). Interestingly, the SEAPORT formulation itself also decreased the diffusivity of APO without emulsification in comparison to rApokyn, potentially due to nanoclustering of APO with CAGE1:2 (SI Appendix, Fig. S3 E–G). Collectively, these data confirm a slower diffusion of APO from emulsified SEAPORT and provide a potential mechanism of its sustained release.
Extended Pharmacokinetics of APO from SEAPORT in Rats.
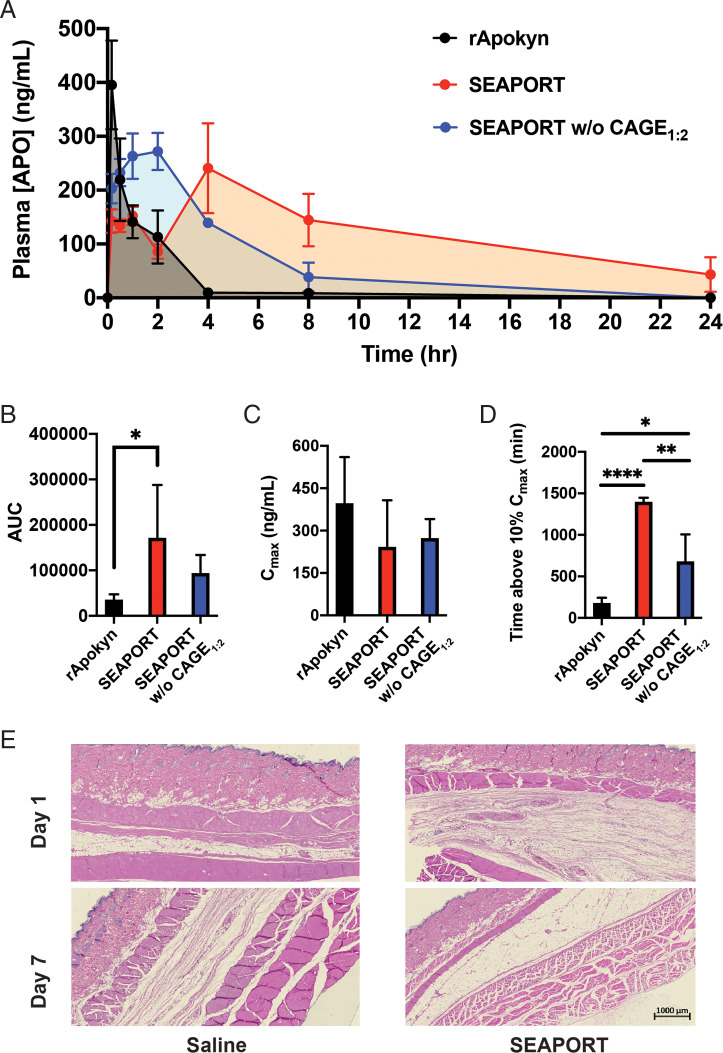
The in vivo pharmacokinetics of APO from subcutaneously injected SEAPORT was measured in rats with rApokyn and SEAPORT w/o CAGE1:2 as controls. rApokyn exhibited a burst release with a time to maximum plasma drug concentration (tmax) of 10 min, and a nearly complete elimination by 4 h postadministration (Fig. 5A). In contrast, SEAPORT exhibited two peaks of plasma APO concentration. The time of the first peak corresponded closely to the tmax of rApokyn, but the second peak appeared at 4 h postinjection and represented the maximum concentration. In addition, the second peak led to a sustained delivery of APO with plasma concentrations detectable even at 24 h. The existence of two peaks is likely attributed to the presence of two forms of APO in the subcutaneous SEAPORT depot, namely, the smaller burst of free APO present outside the emulsions, followed by a slower release of APO trapped inside the emulsions. This is further supported by the absence of a second peak in the SEAPORT w/o CAGE1:2 group. The maximum plasma drug concentration (Cmax) was comparable between all three groups, but the area under the curve (AUC) of SEAPORT was significantly greater than that of rApokyn (Fig. 5 B and C). Moreover, the time at which the APO concentration dropped to 10% of Cmax was significantly greater in SEAPORT than that in either rApokyn or SEAPORT w/o CAGE1:2, indicating that SEAPORT exhibits a sustained release in vivo via CAGE1:2-mediated extended pharmacokinetics and can potentially maintain effective APO concentrations in plasma longer than Apokyn (Fig. 5D).
Fig. 5.
Pharmacokinetics and toxicity in rats. (A) Pharmacokinetics of APO in male Wistar rats subcutaneously injected with rApokyn (2.5 mg APO/kg), SEAPORT (7.5 mg APO/kg), and SEAPORT w/o CAGE1:2 (7.5 mg APO/kg) (n = 4/group, mean ± SEM), detected with triple-quadrupole LC-MS/MS. AUC (B), Cmax (C), duration of plasma [APO] above 10% Cmax (n = 4/group, mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001) (D), and representative images of hematoxylin and eosin-stained skin sections at the site of injection, collected on day 1 and 7 postinjection (scale bar = 1 mm) (E).
The safety of SEAPORT was assessed by blinded histological evaluation of subcutaneous tissues on days 1 and 7 postadministration at a dose of 7.5 mg APO/kg (corresponding to 0.25 mL SEAPORT/kg). This dose is substantially higher than the expected dose in humans (0.01 mL/kg or 1 mL per 70-kg subject), thus providing a large margin in safety assessment. No toxicity from SEAPORT was noted in the tissue based on several criteria, including inflammation, fibrosis, ulceration, necrosis, edema, and hyperkeratosis (Fig. 5E).
Prolonged Duration of APO above MEC with SEAPORT in Pigs.
The SEAPORT formulation was scaled to 5 mL to facilitate an evaluation in pigs. Pigs were used as a preferred large animal since they have been previously used in a preclinical study of subcutaneous infusion of APO leading to phase 1 human clinical studies (25).
SEAPORT was used as the test formulation with rApokyn (APO at 10 mg/mL) as the reference formulation. Both formulations were injected at a dose of 1 mL per animal. No injection site reactions, clinical signs, or adverse reactions were observed in the study. Both the reference formulation and SEAPORT exhibited a similar Cmax of ∼20 ng/mL at tmax of 30 min (Fig. 6 A and B and SI Appendix, Table S1). However, when the pharmacokinetic data are normalized to a dose of 1 mg, SEAPORT was able to blunt the dose-normalized Cmax by 4.9-fold compared to the reference formulation. Moreover, SEAPORT exhibited a second phase of release starting at around 2 h postadministration, followed by a slower decay with APO concentration above the MEC of 4 ng/mL at 5 h, and with detectable APO concentration extending up to 48 h. As a result, SEAPORT showed a significant increase over the reference formulation in both indices, namely, AUC and time spent above the MEC (Fig. 6 C and D). Plasma APO concentration in the SEAPORT group dropped below the MEC at 5 h in contrast to 2 h in the reference formulation group. The prolonged duration of APO in circulation illustrates the potential of SEAPORT to reduce the frequency of injection necessary to prevent off episodes in PD.
Fig. 6.
Pharmacokinetics of APO in pigs. (A) Pharmacokinetics of APO from pigs subcutaneously injected with rApokyn (injected dose, 6 mg APO) and SEAPORT (injected dose, 30 mg APO) (n = 3/group, mean ± SEM, inset: zoomed in on first 6 h), detected by triple-quadrupole LC-MS/MS. Cmax (B), AUC (C) and duration of plasma [APO] above the MEC (4 ng/mL) (D) (n = 3/group, mean ± SD, **P < 0.01).
Compartmental Modeling and Simulation of Multiple SEAPORT Doses in Humans.
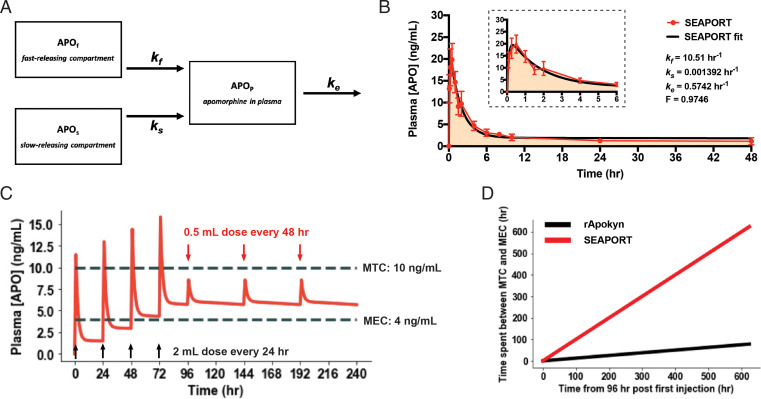
Pharmacokinetic modeling of SEAPORT was performed to assess how the observed pharmacokinetics can be used to design a long-lasting dosing regimen of APO in humans with the goal of minimizing the number of injections and time spent outside the therapeutic window. We utilized compartmental modeling consisting of fast- and slow-acting compartments of SEAPORT in the subcutaneous space evidenced by pharmacokinetics in both rats and pigs and the vasculature compartment (Fig. 7A). The parameters of the model were the fraction of APO in the slow-releasing compartment (F), two distinct rates of APO release from the two depot compartments (kf and ks), and the rate of APO elimination from the bloodstream (ke). First-order kinetics were assumed for all compartments. We then employed the least-squares fitting of the model’s analytical solution to SEAPORT’s pharmacokinetics data in pigs to determine the rate constants (Fig. 7B). We also fitted the model to the pharmacokinetics data for the reference group to determine its absorption rate constant as a comparison (SI Appendix, Fig. S4A).
Fig. 7.
Modeling and simulation of pharmacokinetics from repeated dosing of SEAPORT. (A) Compartmental model diagram used to generate pharmacokinetics model. (B) Least-squares fitting of pharmacokinetic data for SEAPORT in pigs modeled with the analytical solution from the model using GraphPad Prism9 (inset, zoomed in on first 6 h). (C) Simulation of SEAPORT pharmacokinetics in human over a 10-d period from four initial injections of 60 mg APO 24 h apart (2 mL SEAPORT), followed by injections of 15 mg APO (0.5 mL SEAPORT) every 48 h thereafter. (D) Time spent between MTC and MEC during the time window between 96 and 720 h (day 4 and 30) after initial injection (SEAPORT, 15 mg APO dose every 48 h; rApokyn, 10 mg APO dose every 8 h).
The model indicated that the release from SEAPORT is characterized by two time constants, as follows: a fast-release rate (kf) of 10.51 h−1 and a slow-release rate of 0.001392 h−1. The kf of 10.51 h−1 is in good agreement with the reported tmax of 10 min. The ke was 0.5742 h−1, which also closely matched the reported half-life (69.7 ± 25.8 min) of APO. The model suggests that >95% of the delivered APO is in the slow-release phase, thus demonstrating the benefit of CAGE1:2 in SEAPORT. The residual plot showed the presence of randomly distributed residuals without a clear pattern, confirming a good fit of the model to the data without over-fitting (SI Appendix, Fig. S4B).
Using the rate constants, a dosing regimen was designed to achieve a satisfactory pharmacokinetic profile of APO in humans with SEAPORT compared to rApokyn (Fig. 7C and SI Appendix, Fig. S4C). According to the simulation, a regimen based on four priming injections of a 60-mg dose (2 mL SEAPORT) every 24 h and then subsequent doses of 15 mg (0.5 mL SEAPORT) every 48 h thereafter is sufficient to maintain long-term APO concentration in the blood above the MEC. A 30-d simulation showed injections of the reference formulation (aqueous APO at 10 mg/mL) every 8 h is able to maintain APO concentration between the MEC and MTC only for a total of less than 100 h over 26 d starting at day 4 postinjection (Fig. 7D and SI Appendix, Fig. S4 D and E). Meanwhile, the APO concentration from SEAPORT never fell below the MEC or rose above the MTC during the same time window. This represents a significant advance in the dosing frequency to achieve patient compliance compared to Apokyn that requires multiple injections per day.
Discussion
APO exhibits rapid drug absorption from the subcutaneous tissue, which has driven subcutaneous injection as the major administration route for the drug (5). However, there are still critical hurdles to the subcutaneous administration of APO. APO exhibits rapid metabolism and clearance following its Cmax at 10 min with a half-life of around 33 min and duration of clinical response of around 45 to 60 min (5, 26, 27). This requires intermittent, frequent injections and leads to patient noncompliance, which calls for a formulation of APO allowing a sustained release and thus less frequent injection to subcutaneous tissue.
Self-emulsifying drug delivery systems (SEDDS) are homogeneous mixtures of oils, surfactants, and cosolvents that emulsify upon aqueous dilution to improve lipophilic drug solubility, absorption, and controlled release (28, 29). The majority of SEDDS have been developed to increase oral bioavailability, including FDA-approved Sandimmune Neoral. Here, we report SEDDS using a deep eutectic solvent as the emulsifier for sustained release in subcutaneous tissue.
SEAPORT exploits differential miscibility of three solvents, namely, CAGE1:2, NMP, and water, and ,simultaneously, differential solubility of APO in these solvents. At the concentrations used in SEAPORT, these solvents form a homogenous clear solution, although nanoclusters of APO and CAGE1:2 were observed in DLS. Upon injection in the subcutaneous space, the deep eutectic solvent in SEAPORT self-emulsifies in situ due to the quick diffusion of cosolvent NMP and leads to the formation of a depot comprised largely of CAGE1:2 that traps APO for sustained release.
NMP has been used in previous literature as a water-miscible cosolvent for a similar purpose, such as in situ-forming implants of the poly(lactic-coglycolic acid) polymer (30). The safety of NMP as an injectable solvent has previously been reported (31), and the concentration of NMP in SEAPORT falls within the specified concentration in the FDA’s database for inactive ingredients in approved drug products. It is also important to note that all excipients with the exception of CAGE1:2 are also included in the FDA-approved inactive ingredient list and are used below the specified concentrations. CAGE1:2 itself has been tested for safety in topical applications and has been tested in the clinic (32). This facilitates the clinical translation of SEAPORT formulation.
The pharmacokinetics of APO in SEAPORT from both rats and pigs showed the presence of two distinct rates of release. The second release is characterized by a slower decay in the plasma concentration of APO, confirming the slow-releasing component. This sustained release in pigs with a 5-h duration of plasma concentration above MEC is superior to other APO formulations for sustained release, such as the hydrogel-based buccal prolonged delivery device that showed approximately a 2.5-h duration (33). The first release closely overlapped with the initial burst from rApokyn, indicating that SEAPORT may also have the fast-releasing component responsible for partial burst release. While SEAPORT was developed to provide the sustained release of APO, the early absorption of APO outside emulsion also offers an important clinical benefit. Specifically, the fast onset in addition to extended pharmacokinetics would provide patients with a therapeutic outcome over a wider time window that includes the time period immediately after injection.
The pharmacokinetic assessment of SEAPORT using the model provides important insights about the therapeutic. We employed a simple, classical compartmental model to characterize pharmacokinetics data from pigs following subcutaneous administration of SEAPORT. In general, the fast-release component of SEAPORT behaves very much like rApokyn in terms of absorption and tmax. While the fitted model showed a mere 2.5% of APO in the fast-releasing compartment in SEAPORT, it demonstrated a similar pharmacokinetic profile compared to rApokyn in the early timepoints, possibly due to the presence of NMP that facilitates diffusion and absorption. This is also evidenced by greater kf in SEAPORT than in rApokyn. The slow-release phase in SEAPORT, on the other hand, is significantly slower than rApokyn and provides a long-lasting release.
The studies reported here demonstrate that a deep eutectic-based formulation offers a potential solution to the long-lasting challenge of APO delivery for treating PD. SEAPORT is a homogenous, clear solution that can be prepared in a single-step process at a large scale. Pharmacokinetic studies in rats and pigs both showed prolonged duration of higher APO concentration in plasma in comparison to the clinical comparator. Simulations based on modeling with pharmacokinetics data from pigs showed that an alternate-day dosing schedule following an everyday-priming schedule for the first 4 d can maintain therapeutically effective concentrations of APO in pigs over at least 30 d, which is in stark contrast to multiple injections required per day to achieve a similar effect with Apokyn. Safety studies reported here demonstrate that a single administration of SEAPORT administered at a large excess was well tolerated in rats. The principle of SEAPORT can be potentially extended to other drugs, thus enabling the sustained release of many additional drugs. Upon further studies focused on safety and dosing evaluation, SEAPORT offers a strategy for the treatment of PD.
Materials and Methods
Materials.
Choline bicarbonate, geranic acid, SMB, poly(ethylene glycol) Mn 3350, boldine, N-methyl-pyrrolidinone, methanol (high-performance liquid chromatography [HPLC] grade, 99.8% purity), and acetonitrile (HPLC grade, 99.8% purity) were obtained from Millipore Sigma. D2O and N-methyl-2-pyrrolidinone-d9 were obtained from Cambridge Isotope Laboratories. Pierce formic acid, agarose (Thermo Fisher Scientific), and benzyl alcohol (Alfa Aesar) were used as received. APO hydrochloride was provided by Dr. Reddy’s Laboratories, Ltd.
Deep Eutectic Solvent Synthesis.
Choline bicarbonate and geranic acid were reacted via salt metathesis reaction in 1:2 molar ratio at 40 °C for 12 h. Water produced from the reaction was removed using rotary evaporator at 20 mbar at 60 °C for 4 h and additional drying in a vacuum oven at 60 °C for 48 h. The resulting product was stored at room temperature until used. 1H NMR showed a spectrum consistent with previous literature (34).
Reference Apokyn Formulation (rApokyn).
Apokyn was reproduced using the composition listed in Apokyn’s FDA label and used as the clinical comparator (reference formulation). Briefly, 10 mg of APO hydrochloride, 1 mg SMB, and 5 mg benzyl alcohol were dissolved in 1 mL of water. The pH of the final formulation was adjusted to pH 4.0 using hydrochloric acid.
SEAPORT Formulation.
SEAPORT formulation was prepared by dissolving 30 mg of APO hydrochloride, 3 mg SMB, and 28.8 mg poly(ethylene glycol) Mn 3350 in 42.7% N-methyl-2-pyrrolidinone, 10% CAGE1:2, and 47.3% H2O by volume. The mixture was vigorously vortexed for 1 min to yield a homogeneous preparation. SEAPORT w/o CAGE1:2 control formulation was prepared by replacing 10% vol/vol CAGE1:2 with H2O.
SEAPORT Solubility and Stability.
The amount of APO solubilized in the formulation as well as the remaining soluble APO following incubation at 4 °C, 25 °C, and 37 °C were detected via Agilent 1290 μltra high-performance liquid chromatography equipped with Agilent G6135B electrospray ionization–mass spectrometry detector (UPLC-MS). Agilent SB-Phenyl reversed-phase column (4.6 × 150 mm, 5 µm) protected by a guard column (ZORBAX, 4.6 × 12.5 mm, 5 μm) was maintained at 30 °C. Samples were prepared in 50:50 vol/vol 0.9% NaCl in water:100 ng/mL boldine (internal standard) in methanol. APO and boldine were eluted using 0.1% vol/vol formic acid in water as mobile phase A and 0.1% vol/vol formic acid in acetonitrile as mobile phase B in isocratic mode at 0.5 mL/min with 65% mobile phase A. Positive selected ion monitoring (SIM) mode was used to detect APO and boldine at a mass-to-charge ratio (m/z) of 268.3 and 327.4, respectively.
To determine the level of APO oxidation over time, the changes in color of formulations incubated at 4 °C, 25 °C, and 37 °C were observed on day 1, 3, 5, and 7. Absorbance due to dark-blue color from oxidized APO was detected at 600 nm using a plate reader (Biotek Neo2).
Ex Vivo APO Release.
Skin (5 cm × 10 cm) harvested from the dorsal side of euthanized Wistar rats was used to study APO release from SEAPORT ex vivo (SI Appendix, Fig. S2C). A total of 50 µL of specified formulations was injected using an insulin syringe in the fat, subcutaneous layer by inserting the needle (28G) from the stratum corneum until the tip of the needle was visible through the translucent layer of fat tissue (Fig. 3A and Movie S3). Injection resulted in immediate formation of a small bleb (SI Appendix, Fig. S2D). Following injection, the skin was cut around the injection site with an ∼3- to 5-mm margin in all directions, placed in each well of a 12-well plate containing 1 mL of 0.9% wt/vol NaCl and 0.01% wt/vol gentamicin in water, and incubated at 37 °C with a parafilm cover. The entire 1 mL was collected and replaced with a fresh 1 mL of 0.9% wt/vol NaCl and 0.01% gentamicin in water at predetermined timepoints. The amount of APO in the release medium was quantified via UPLC-MS with the same method used in SEAPORT characterization.
SEAPORT Emulsion Characterization.
An agarose gel was used to mimic self-emulsification of SEAPORT in subcutaneous tissue. A total of 3 mL of a 36% wt/vol agarose gel was prepared by heating 1,080 mg of agarose with 3 mL H2O absorbed in a 20-mL glass scintillation vial for 35 s and placing it in a 65 °C oven overnight. N-methyl-2-pyrollidinone-d9 and D2O were used in the preparation of both SEAPORT and agarose gel for NMR. A 100-µL, hollow compartment at the center of the agarose channel was created by punching the gel removed from the oven with the back end of a glass Pasteur pipette. A similar method was followed to create the agarose gel with a hollow center channel in a 24-well plate to capture a video of the emulsification following the injection of SEAPORT via inverted microscope with a charge-coupled device camera (Olympus CKX53/HCImage software).
A total of 100 µL of specified formulations was placed in the hollow compartment, incubated for predetermined timepoints, and removed for further characterization. The physical structure of the formulation and emulsion was measured using DLS (Malvern ZetaSizer Nano ZS) and visualized with cryo-TEM (FEI Tecnai Arctica operated at 200 kV) without any dilution of samples. DOSY NMR (Bruker AVANCE NEO 400B, 16 scans, 3-s delay) was used to measure the diffusion coefficient of APO in rApokyn and SEAPORT immediately after their formulation as well as 20 min postincubation in the agarose gel. A smaller and thinner 36% wt/vol agarose gel was prepared to obtain FTIR spectra of the formulation and emulsion in real-time. The agarose gel was mounted on Bruker ALPHA ATR-FTIR, and the formulation was placed in the hollow compartment for time-course detection (64 scans at each timepoint). To determine the peak heights at 1,645 cm−1 and 1,675 cm−1, the IR spectrum was deconvoluted using the FITYK 1.3.1 software with Voigt type function for mathematical processing.
Pharmacokinetics and Toxicity in Rats.
All experiments were performed according to the approved protocols by the Institutional Animal Care and Use Committee of the Faculty of Arts and Sciences, Harvard University. Male Wistar rats weighing between 300 and 350 g were subcutaneously injected with rApokyn (2.5 mg APO/kg), SEAPORT (7.5 mg APO/kg), or SEAPORT w/o CAGE1:2 (7.5 mg APO/kg) in the dorsal flank, and blood was collected at predetermined timepoints. A total of 95 µL of separated plasma was mixed with 5 µL of 15% wt/vol SMB in 0.2N HCl, and APO was extracted using 1:3 vol/vol plasma:50 ng/mL boldine in methanol. The separation of APO with boldine as an internal standard was performed using an Agilent 1290 HPLC system with the same column as that used in the SEAPORT characterization. Mobile phase A was water with 0.1% (vol/vol) formic acid, and mobile phase B was acetonitrile with 0.1% (vol/vol) formic acid. Online mass spectrometry detection was performed using an Agilent 6460 triple quadrupole mass spectrometer in positive electrospray ionization mode. Quantification was accomplished employing multiple reaction monitoring by monitoring the transitions for APO 268.1/191.1 and boldine 328.2/265.1. The amount of APO in the samples was quantified using corresponding external calibration standard curves generated with pure standards. Detailed LC conditions are provided in supplemental information. In addition, tissue around the injection site was harvested from the stratum corneum to muscle layer on day 1 and 7, fixed with paraformaldehyde, sectioned, and stained with hematoxylin and eosin to observe toxicity from SEAPORT and SEAPORT w/o CAGE1:2 formulations.
Pharmacokinetics in Pigs.
This pharmacokinetic study was performed as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals and institutional animal ethics committee of the respective animal facility. The study data are from two independent studies. Both formulations were administered at a dose volume of 1 mL/dose via subcutaneous route. Pigs were physically restrained, and the dose was administered by inserting a 21-gauge needle at the inguinal region (loose skin behind the ear). Blood samples were collected from each animal at different timepoints up to 72 h postinjection of SEAPORT (APO 30 mg/mL) and up to 24 h postinjection of the reference formulation (10 mg/mL APO). Collected blood was immediately transferred to prelabeled 0.5-mL amber-colored tubes containing 20 μL of 1,000 IU/mL heparin sodium as an anticoagulant and centrifuged at 4,000 rpm for 10 min at 4 °C within 45 min of collection. Plasma was separated and transferred into two sets of prelabeled, amber-colored tubes containing 10 μL of 15% wt/vol of SMB with or without 0.1% wt/vol ascorbic acid in 0.2 N HCl as a stabilizer. Approximately 200 μL of the plasma sample was added to all tubes in each set and stored at –70 ± 10 °C until bioanalysis. Plasma samples were analyzed using LC-tandem MS with a linearity range of 0.2 to 200 ng/mL using API 4000, and pharmacokinetic parameters were estimated by noncompartmental analysis using Phoenix WinNonlin software. Quantifiable APO concentration of 2.85 ± 0.37 ng/mL (mean ± SD) was observed at predose in the SEAPORT-treated group and was considered as baseline and subtracted from all datapoints for pharmacokinetic analysis.
Pharmacokinetics Modeling and Simulation.
A compartmental model was used in the analysis of pharmacokinetics. A total of three compartments were included (Fig. 7A) with the following system of ordinary differential equations (ODE):
Initial conditions:
where APOf and APOs are the mass of APO in the fast- and slow-releasing compartments of injected depot, respectively; kf and ks are the release rate constants from the fast- and slow-releasing compartments; APOp is the mass of APO in the plasma compartment; ke is the elimination rate constant from plasma; APOInj is the mass of APO injected; and F is the fraction of APOinj in APOs. ODEs were solved to generate an analytical solution for the concentration of APOp using 2,000 mL as the volume of blood in a pig. The solution was fitted against SEAPORT pharmacokinetics in pig by least-squares fitting using GraphPad Prism9. Python was used to create a simulation of multiple dosing using the rate constants determined by fitting and the volume of blood in an average human (5,000 mL). Python code is provided in SI Appendix.
Statistical Analysis.
All results are expressed as mean ± SD unless specified otherwise. All statistical analyses were performed in GraphPad Prism9. The two-tailed Student’s t test was used for comparisons between two groups, while one-way ANOVA with post hoc testing (Dunnett’s multiple comparison) was performed to either compare between multiple groups or to compare each group to a control group. Statistical significance is defined as follows: *P < 0.05, **P < 0.01, and ***P < 0.001.
Supplementary Material
Acknowledgments
The work was financially supported by Promius Pharma (a subsidiary of Dr. Reddy’s Laboratories) and School of Engineering and Applied Sciences at Harvard University. The authors acknowledge the Harvard Center for Mass Spectrometry; the Harvard Center for Biological Imaging; the Allston-Science and Engineering Complex’s Molecular and Cellular Biology Core; and Harvard University Center for Nanoscale Systems (CNS), a member of the National Nanotechnology Coordinated Infrastructure Network (NNCI) supported by National Science Foundation ECCS-2025158, for infrastructure and support. We also thank the Dana-Farber/Harvard Cancer Center in Boston, MA, for the use of the Rodent Histopathology Core and its histological section preparation service. Dana-Farber/Harvard Cancer Center is supported in part by the National Cancer Institute Cancer Center Support Grant National Institutes of Health 5P30CA06516.
Footnotes
Competing interest statement: J.K. and S.M. are inventors on patent applications that cover formulations discussed in this manuscript. S.M. is a shareholder/board member/consultant of Liquideon LLC, Cage Bio and i2O Therapeutics. K.I. is an employee of i2O Therapeutics. P.K.S., R.J., and S.A. are employees of Dr. Reddy’s Laboratories.
This article is a PNAS Direct Submission. A.A. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2110450119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information. The Python code used in pharmacokinetics simulation is available at GitHub (https://github.com/mitragotrilab/PNAS_2021).
References
- 1.Jankovic J., Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 79, 368–376 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Dawson V. L., Dawson T. M., Promising disease-modifying therapies for Parkinson’s disease. Sci. Transl. Med. 11, eaba1659 (2019). [DOI] [PubMed] [Google Scholar]
- 3.McFarthing K., et al. , Parkinson’s disease drug therapies in the clinical trial pipeline: 2020. J. Parkinsons Dis. 10, 757–774 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jenner P., Katzenschlager R., Apomorphine—Pharmacological properties and clinical trials in Parkinson’s disease. Parkinsonism Relat. Disord. 33 (suppl. 1), S13–S21 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Nicolle E., et al. , Pharmacokinetics of apomorphine in parkinsonian patients. Fundam. Clin. Pharmacol. 7, 245–252 (1993). [DOI] [PubMed] [Google Scholar]
- 6.Deffond D., Durif F., Tournilhac M., Apomorphine in treatment of Parkinson’s disease: Comparison between subcutaneous and sublingual routes. J. Neurol. Neurosurg. Psychiatry 56, 101–103 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lees A. J., The on-off phenomenon. J. Neurol. Neurosurg. Psychiatry 52, 29–37 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pietz K., Hagell P., Odin P., Subcutaneous apomorphine in late stage Parkinson’s disease: A long term follow up. J. Neurol. Neurosurg. Psychiatry 65, 709–716 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi P., Colosimo C., Moro E., Tonali P., Albanese A., Acute challenge with apomorphine and levodopa in Parkinsonism. Eur. Neurol. 43, 95–101 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Bilbault T., et al. , Buccal mucosal irritation studies of sublingual apomorphine film (APL-130277) in Syrian golden hamsters. Ther. Deliv. 7, 611–618 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Olanow C. W., et al. , CTH-300 Study investigators, Apomorphine sublingual film for off episodes in Parkinson’s disease: A randomised, double-blind, placebo-controlled phase 3 study. Lancet Neurol. 19, 135–144 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Katzenschlager R., et al. , Apomorphine subcutaneous infusion in patients with Parkinson’s disease with persistent motor fluctuations (TOLEDO): A multicentre, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 17, 749–759 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Bhidayasiri R., Garcia Ruiz P. J., Henriksen T., Practical management of adverse events related to apomorphine therapy. Parkinsonism Relat. Disord. 33 (suppl. 1), S42–S48 (2016). [DOI] [PubMed] [Google Scholar]
- 14.García Ruiz P. J., et al. , Efficacy of long-term continuous subcutaneous apomorphine infusion in advanced Parkinson’s disease with motor fluctuations: A multicenter study. Mov. Disord. 23, 1130–1136 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Borkar N., Mu H., Holm R., Challenges and trends in apomorphine drug delivery systems for the treatment of Parkinson’s disease. Asian J Pharm Sci 13, 507–517 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta H. V., Lyons K. E., Pahwa R., Old drugs, new delivery systems in Parkinson’s disease. Drugs Aging 36, 807–821 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Borkar N., et al. , Lipophilic prodrugs of apomorphine I: Preparation, characterisation, and in vitro enzymatic hydrolysis in biorelevant media. Eur. J. Pharm. Biopharm. 89, 216–223 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Priano L., et al. , Controlled-release transdermal apomorphine treatment for motor fluctuations in Parkinson’s disease. Neurol. Sci. 23 (suppl. 2), S99–S100 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Gancher S. T., Nutt J. G., Woodward W. R., Absorption of apomorphine by various routes in parkinsonism. Mov. Disord. 6, 212–216 (1991). [DOI] [PubMed] [Google Scholar]
- 20.Regnier-Delplace C., et al. , PLGA microparticles with zero-order release of the labile anti-Parkinson drug apomorphine. Int. J. Pharm. 443, 68–79 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Ikechukwu Ugwoke M., Kaufmann G., Verbeke N., Kinget R., Intranasal bioavailability of apomorphine from carboxymethylcellulose-based drug delivery systems. Int. J. Pharm. 202, 125–131 (2000). [DOI] [PubMed] [Google Scholar]
- 22.Wen C. J., Zhang L. W., Al-Suwayeh S. A., Yen T. C., Fang J. Y., Theranostic liposomes loaded with quantum dots and apomorphine for brain targeting and bioimaging. Int. J. Nanomedicine 7, 1599–1611 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan J. P. K., et al. , Effective encapsulation of apomorphine into biodegradable polymeric nanoparticles through a reversible chemical bond for delivery across the blood-brain barrier. Nanomedicine (Lond.) 17, 236–245 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Tsai M. J., et al. , Oral apomorphine delivery from solid lipid nanoparticles with different monostearate emulsifiers: Pharmacokinetic and behavioral evaluations. J. Pharm. Sci. 100, 547–557 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Ramot Y., et al. , ND0701, a novel formulation of apomorphine for subcutaneous infusion, in comparison to a commercial apomorphine formulation: 28-day pharmacokinetic study in minipigs and a phase I study in healthy volunteers to assess the safety, tolerability, pharmacokinetics and relative bioavailability. CNS Drugs 32, 443–454 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Gancher S. T., Woodward W. R., Boucher B., Nutt J. G., Peripheral pharmacokinetics of apomorphine in humans. Ann. Neurol. 26, 232–238 (1989). [DOI] [PubMed] [Google Scholar]
- 27.LeWitt P. A., Subcutaneously administered apomorphine: Pharmacokinetics and metabolism. Neurology 62(6, suppl. 4)S8–S11 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Gursoy R. N., Benita S., Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed. Pharmacother. 58, 173–182 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Malkawi A., et al. , Self-emulsifying drug delivery systems: Hydrophobic drug polymer complexes provide a sustained release in vitro. Mol. Pharm. 17, 3709–3719 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benhabbour S. R., et al. , Ultra-long-acting tunable biodegradable and removable controlled release implants for drug delivery. Nat. Commun. 10, 4324 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poet T. S., et al. , Quantitative risk analysis for N-methyl pyrrolidone using physiologically based pharmacokinetic and benchmark dose modeling. Toxicol. Sci. 113, 468–482 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Ko J., et al. , Clinical translation of choline and geranic acid deep eutectic solvent. Bioeng. Transl. Med. 6, e10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Itin C., Komargodski R., Barasch D., Domb A. J., Hoffman A., Prolonged delivery of apomorphine through the buccal mucosa, towards a noninvasive sustained administration method in Parkinson’s disease: In vivo investigations in pigs. J. Pharm. Sci. 110, 1824–1833 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Shi Y., et al. , Oral delivery of sorafenib through spontaneous formation of ionic liquid nanocomplexes. J. Control. Release 322, 602–609 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information. The Python code used in pharmacokinetics simulation is available at GitHub (https://github.com/mitragotrilab/PNAS_2021).