Significance
Maternal obesity is a growing public health concern and is linked to an increased risk of neurodevelopmental and psychiatric disorders in humans. Despite accumulating evidence for the important role maternal microbes play during gestation and nursing, the longitudinal effect of maternal nutrition on offspring metabolism across the gut–brain axis at different ages remains unexplored. We provide evidence for the protective efficacy of perinatal probiotic exposure against increased anxiety-like behaviors induced by maternal obesity. Protection was maintained into adulthood, which may be mediated by the enhancement of brain energy metabolites and an increase in gut butyrate. These findings reveal the importance and long-lasting role of maternally derived microbiota and metabolites in increasing resilience to mood disorders in the offspring.
Keywords: metabolomics, anxiety, lactate, SCFA, maternal obesity
Abstract
Maternal obesity disturbs brain–gut–microbiota interactions and induces negative affect in the offspring, but its impact on gut and brain metabolism in the offspring (F1) are unknown. Here, we tested whether perinatal intake of a multispecies probiotic could mitigate the abnormal emotional behavior in the juvenile and adult offspring of obese dams. Untargeted NMR-based metabolomic profiling and gene-expression analysis throughout the gut–brain axis were then used to investigate the biology underpinning behavioral changes in the dams and their offspring. Prolonged high-fat diet feeding reduced maternal gut short-chain fatty acid abundance, increased markers of peripheral inflammation, and decreased the abundance of neuroactive metabolites in maternal milk during nursing. Both juvenile (postnatal day [PND] 21) and adult (PND112) offspring of obese dams exhibited increased anxiety-like behavior, which were prevented by perinatal probiotic exposure. Maternal probiotic treatment increased gut butyrate and brain lactate in the juvenile and adult offspring and increased the expression of prefrontal cortex PFKFB3, a marker of glycolytic metabolism in astrocytes. PFKFB3 expression correlated with the increase in gut butyrate in the juvenile and adult offspring. Maternal obesity reduced synaptophysin expression in the adult offspring, while perinatal probiotic exposure increased expression of brain-derived neurotrophic factor. Finally, we showed that the resilience of juvenile and adult offspring to anxiety-like behavior was most prominently associated with increased brain lactate abundance, independent of maternal group. Taken together, we show that maternal probiotic supplementation exerts a long-lasting effect on offspring neuroplasticity and the offspring gut–liver–brain metabolome, increasing resilience to emotional dysfunction induced by maternal obesity.
Depression is a common and debilitating disorder affecting all ages and demographics in a manner that is largely independent of economic factors (1, 2). Similarly, maternal obesity is a growing public health concern in Europe (3) and in the United States (4), and, in low- and middle-income countries, its prevalence is rapidly increasing (5–7). Though accumulating evidence points to a biological linkage between obesity and depression (8), few thorough investigations have been undertaken into how maternal obesity affects offspring behavior (9).
In humans, affective behavioral problems in childhood, such as depression and anxiety, are more likely to occur after gestational and early life exposure to maternal obesity (10, 11). To better understand the relationship between maternal diet-induced obesity and the behavior of offspring, animal models are used to allow greater control over nutrition and eliminate confounding genetic, environmental, and socioeconomic factors. Such preclinical studies have progressed our understanding of the underlying mechanisms linking maternal high-fat diet (HFD) intake and offspring neurodevelopment (12). For example, the important research of Sullivan et al. (13) has linked changes in maternal HFD consumption during gestation with decreased cerebrospinal fluid levels of 5-hydroxytryptamine and increased anxiety-like behavior in juvenile, female nonhuman primate (NHP) offspring, but abnormalities were not assessed in adulthood. Neuroplastic changes may be linked to early exposure to hepatic inflammation as a consequence of maternal HFD-induced obesity (14).
The last decade has seen the microbiota–gut–brain axis emerge as a pivotal regulator of neurodevelopment (15–17) and obesity (18). Maternal diet and obesity affect the composition of the maternal and neonatal gut microbiome in humans (19–21) and rodents (22). Moreover, maternal gut dysbiosis induced by diet (23) or antimicrobial treatment (24) has been shown to affect the offspring microbiome, as well as the brain and behavior of the offspring. Despite these discoveries, no studies have investigated whether maternal probiotic intake may counter the adverse effects of maternal obesity via changes to the microbiota–gut–brain axis. Lipid-lowering drugs remain strongly contraindicated during pregnancy (25), whereas probiotics are safe to administer (26).
In unchallenged, wild-type mice, maternal perinatal prebiotic intake reduces offspring anxiety-like behaviors, alters brain N-methyl-d-aspartate receptor (NMDAR) gene expression, and increases fecal short-chain fatty acid (SCFA) levels (27). In the offspring of obese dams, microbial reconstitution with Lactobacillus reuteri can normalize brain dopaminergic activity and social behavior (23), although no insight into downstream changes in microbial, or brain, metabolism was presented. Subsequent rodent (24) and human (28) studies have identified neuromodulatory roles for specific microbial metabolites, as opposed to microbial species, whose metabolic potentials converge considerably (29). Furthermore, preliminary research also indicates a role for early life gut microbiota in the regulation of central astrocyte–neuron energy metabolism (30). Lactate, in particular, which is produced in the brain by astrocytes, makes an important contribution to neuronal energy homeostasis (31–33) and brain signaling (33, 34). Astrocyte dysfunction is a feature of clinical depression and generates depressive-like phenotypes in rodents (35), although it remains untested whether interventions targeting the early life gut–brain axis can restore or enhance brain energy metabolism, alter markers of neuronal plasticity, or increase emotional resilience.
Here, we confirm that maternal obesity induces negative affect in their offspring and show that these effects are long-lasting. Markers of immune activation were quantified in the liver of dams and offspring, and the relative expression of inflammatory and plasticity-related genes were evaluated in the prefrontal cortex (PFC). The protracted structural and functional plasticity of the PFC throughout life (36) renders it a prime target to investigate the adverse consequences of maternal obesity on offspring brain and behavior at juvenile and adult ages. Using untargeted NMR-based metabolomic profiling, we also sought to discover how maternal obesity disturbs brain–gut metabolic interactions in male and female offspring during development and maturity and whether these effects could be mitigated by perinatal exposure to a multispecies probiotic. Our findings reveal a long-lasting protective effect of maternal probiotic intake on offspring anxiety-like behavior, which we show exhibits a persistent association with offspring brain lactate levels.
Results
Chronic HFD Exposure Induces Maternal Obesity and Perturbs the Microbiota–Gut–Brain-Axis Metabolome.
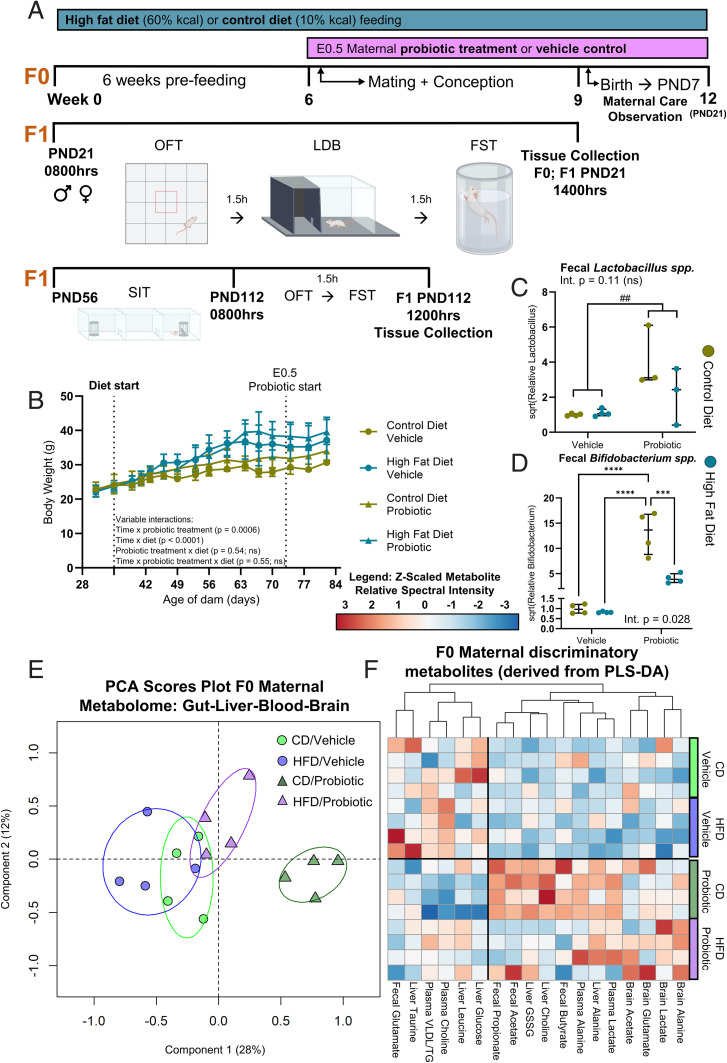
Mixed-effects modeling of time, HFD, and probiotic treatment grouping between 4 and 12 wk of age showed that pregravid mice fed an HFD gained significantly more weight over time than mice fed a control diet (CD) (Fig. 1), independent of probiotic treatment, which led to an obese phenotype (>20% heavier than CD) at the time of mating (SI Appendix, Fig. S1). Litter size was reduced in obese dams, relative to lean dams (SI Appendix, Fig. S2). Two-way ANOVA revealed a main effect of probiotic treatment, which increased fecal Lactobacillus spp. levels in probiotic-treated dams at the time of pup weaning relative to vehicle (control) dams, independent of maternal diet (Fig. 1C). For Lactobacillus spp. levels, there was no main effect of diet (high fat or control) and no diet × treatment (probiotic or vehicle) interaction; however, a significant diet × probiotic treatment interaction was observed for fecal Bifidobacterium spp. levels, and Tukey post hoc tests revealed that CD/probiotic dams had significantly increased levels relative to all other groups (Fig. 1D). Unsupervised principal component analysis (PCA) of the gut, liver, brain, and blood metabolome revealed distinct metabolic profiles in dams with and without probiotic treatment, particularly in those that were not fed the HFD (Fig. 1E and PCA loadings plot, SI Appendix, Fig. S3). Supervised partial least-squares discriminant analysis (PLS-DA) confirmed these group differences and identified additional, tissue-specific variation in the metabolome between obese and lean dams with and without probiotic treatment (SI Appendix, Figs. S4–S8 and Table S1). The top (ranked variable importance in projection [VIP]) discriminatory metabolites identified by PLS-DA (SI Appendix, Tables S2–S5) for each tissue are summarized in Fig. 1F. Gut acetate and butyrate and liver choline levels were increased in CD/probiotic dams relative to all other groups (two-way ANOVA applying the Benjamini and Hochberg false discovery rate [FDR] correction; SI Appendix, Table S1). Plasma lactate levels were increased in probiotic-treated dams relative to vehicle controls (SI Appendix, Fig. S9A), independent of diet.
Fig. 1.
Effect of chronic HFD and perinatal probiotic treatment on F0 maternal Bifidobacterium spp., Lactobacillus spp., and gut, liver, plasma and brain metabolites. (A) Study design schematic. F0, maternal generation; F1, offspring generation. (B) Mixed-effects modeling of time, HFD, and probiotic treatment grouping on maternal body weight. Significant main effects of time [F(2.1,25.6) = 82.98, P < 0.0001] and diet [F(1,12) = 9.62, P = 0.0092] with significant time × probiotic [F(15,180) = 2.82] and time × diet [F(15,180) = 11.09] interactions. No main effect of probiotic treatment [F(1,12) = 1.24, P = 0.29]. (C) Maternal fecal Lactobacillus spp. levels. Probiotic treatment increased Lactobacillus spp. levels relative to vehicle controls [two-way ANOVA, main effect, F(1,10) = 12.51] independent of diet and without interaction [F(1,10) = 3.01]. (D) Maternal fecal Bifidobacterium spp. levels. Significant diet × probiotic interaction [Q(1,12) = 9.58]. Post hoc Tukey tests revealed that levels of Bifidobacterium spp. were highest in the CD/probiotic dams compared to all other groups. (E) PCA scores plot of all maternal gut, liver, plasma, and brain metabolites by 1H NMR spectroscopy. R 2X = 0.52 with three principal components. (F) Heatmap of key discriminatory maternal metabolites determined by PLS-DA. Data are presented as median ± interquartile range. ##P < 0.01 (indicating main effect of maternal probiotic treatment); ***P < 0.001; ****P < 0.0001 (indicating significant post hoc difference, computed only in the case of a significant diet × probiotic treatment interaction). Int., interaction; ns, not significant. n = 4 per group. Metabolomic descriptive statistics are reported in SI Appendix, Figs. S4–S7 and Tables S1–S5.
Maternal Obesity Alters Maternal Behaviors and Markers of Innate Immunity Postpartum.
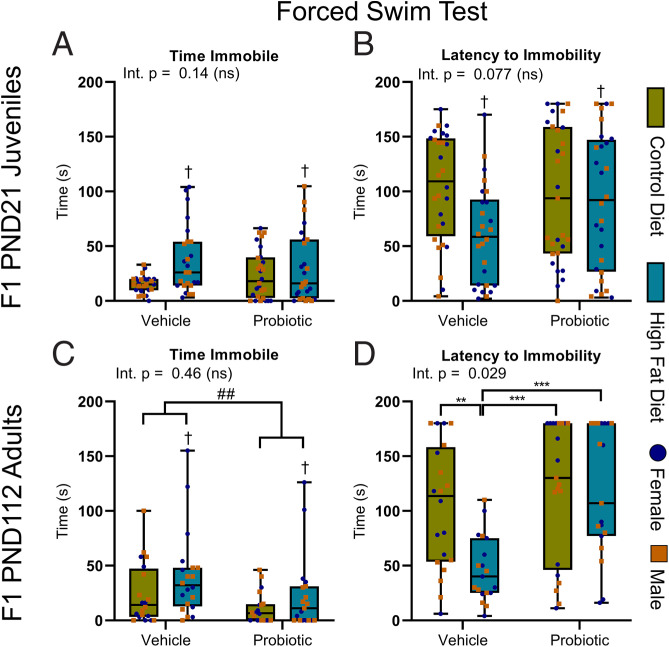
During the first week of nursing, maternal HFD-induced obesity led to a significant increase in time spent away from the nest and reduced the frequency of nest building (SI Appendix, Fig. S10) relative to dams fed the CD, independent of probiotic treatment and with no interaction. In the forced swim test (FST), a significant diet × probiotic treatment interaction was identified, whereby HFD/vehicle dams exhibited a significantly reduced latency to immobility compared to CD/vehicle and CD/probiotic dams, whereas HFD/probiotic dams displayed a reduced latency to immobility only compared to CD/vehicle dams (SI Appendix, Fig. S11). No behavioral changes were seen in the light–dark box (LDB) or open field test (OFT) (SI Appendix, Fig. S11).
Two-way ANOVA revealed that brain TLR4 (SI Appendix, Table S6) and liver TLR4 and IL-1B (SI Appendix, Table S7) were elevated in obese dams relative to lean dams in a manner that was independent of probiotic treatment and with no significant interaction. There was a significant interaction for liver SAA-2 expression, which exhibited elevated expression in HFD/vehicle offspring moreso than HFD/probiotic offspring (SI Appendix, Table S7). TNF and IL-6 cytokines were reduced in the liver and increased in the brain in probiotic-treated dams relative to vehicle dams, independent of diet and with no interaction.
Perinatal Probiotic Intake Mitigated Anxiety-Like Behaviors in the Juvenile and Adult Offspring of Obese Dams.
OFT.
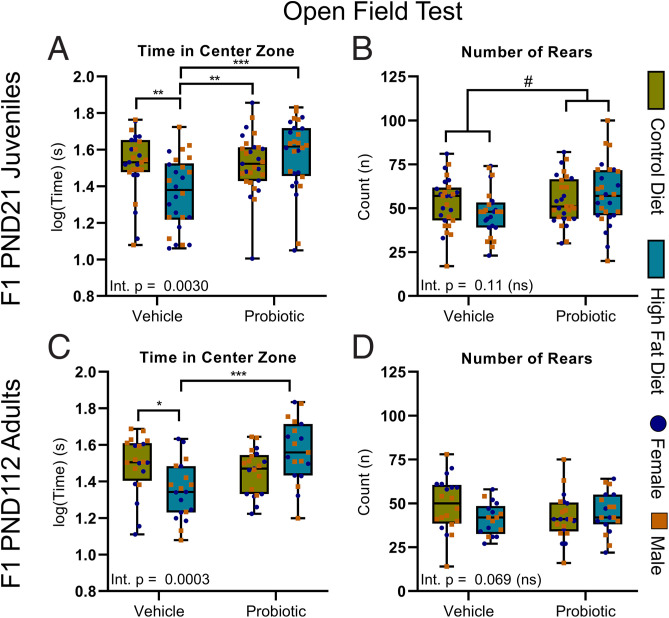
Throughout our studies, where three-way ANOVA revealed that the outcomes were independent of sex, we only report the results of the subsequent consolidated two-way ANOVA. However, for completeness, the figures show how the offspring (F1) females and males are distributed. In the OFT, two-way ANOVA revealed a significant maternal diet/probiotic treatment interaction. Tukey post hoc testing revealed that the HFD/vehicle offspring spent less time in the center of the open field than any other group (Fig. 2A). No interaction was observed for offspring rearing events, but there was a main effect of maternal probiotic treatment, whereby perinatal probiotic exposure increased rearing events (Fig. 2B) independent of maternal diet with no interaction.
Fig. 2.
Maternal probiotic treatment reverses anxiety-like behavior in the young and adult offspring in the OFT. (A) There was a significant interaction for time in the center zone in juvenile offspring [F(1,106) = 9.23]. In post hoc Tukey tests, HFD/vehicle offspring spent less time in center than the juvenile offspring of all other groups. (B) Maternal probiotic treatment increased juvenile offspring rearing behavior [main effect, F(1,107) = 4.14] independent of maternal diet with no interaction. (C) In the adult offspring, there was a significant interaction for time in the center zone [F(1,75) = 14.20]. Post hoc Tukey tests revealed that HFD/vehicle offspring spent less time in center than CD/vehicle and HFD/probiotic adult offspring. (D) Rearing behavior in adult offspring was unchanged. Two-way ANOVA; data are presented as boxplots showing median, interquartile range, and min/max points. #P < 0.05 (indicating main effect of maternal probiotic treatment); *P < 0.05; **P < 0.01; ***P < 0.001 (indicating significant Tukey post hoc comparisons, computed only in the case of a significant diet × probiotic treatment interaction. Int., interaction; ns, not significant. n = 26 to 29 for juvenile offspring (13 to 15 male, 13 or 14 female) and 20 or 21 for adult offspring (10 or 11 male, 9 or 10 female) per group.
In the adult offspring, there was also a significant maternal diet × probiotic interaction for time in the center zone of the OFT. Post hoc testing showed that the reduced time in center zone persisted in HFD/vehicle offspring relative to CD/vehicle and HFD/probiotic offspring (Fig. 2C), but there were no enduring effects on rearing behavior (Fig. 2D). Two-way ANOVA confirmed that, in both juvenile (SI Appendix, Fig. S12) and adult (SI Appendix, Fig. S13) offspring, overall locomotor activity was unaltered by maternal diet or probiotic treatment.
LDB.
There was no interaction between maternal diet and probiotic treatment on anxiety-like behavior in the LDB in the juvenile offspring (SI Appendix, Fig. S12D–F). Perinatal probiotic exposure reduced latency to enter the light compartment (SI Appendix, Fig. S12D) and increased the number of light–dark crossing (SI Appendix, Fig. S12F) in the juvenile offspring relative to vehicle offspring, independent of maternal obesity.
FST.
In the juvenile offspring, maternal obesity increased passive stress coping independent of maternal probiotic treatment, with no interaction (Fig. 3 A and B). Exposure to both perinatal HFD and probiotic increased offspring body weights in early life (SI Appendix, Fig. S14A), including at weaning age (SI Appendix, Fig. S14B), and, thus, there was no relationship between juvenile body weight and immobility in the FST, nor between OFT activity and the FST.
Fig. 3.
Persistent effects of maternal obesity on depressive-like behavior in young and adult offspring. In the juvenile offspring, maternal obesity increased immobility time [main effect, F(1,102) = 5.75] (A) and reduced latency to immobility [main effect, Q(1,102) = 4.54] (B) in the FST relative to the offspring of lean dams. Note there were no significant interactions. (C) In the adult offspring, the time spent immobile was reduced by maternal probiotic treatment [main effect, Q(1,75) = 7.42] and was increased by maternal HFD-induced obesity [main effect, Q(1,75) = 4.12]. (D) However, in the adults, a significant interaction [F(1,75) = 5.06] for latency to immobility was observed, and post hoc tests revealed that the latency in HFD/vehicle was significantly shorter than for all other groups. Two-way ANOVA; data are presented as boxplots showing median, interquartile range, and min/max points. †P < 0.05 (indicating main effect of maternal diet); ##P < 0.01 (indicating main effect of maternal probiotic treatment); **P < 0.01; ***P < 0.001 (indicating significant post hoc comparison, computed only in the case of a significant diet × probiotic treatment interaction). Int., interaction; ns, not significant. n = 26 to 29 for juvenile offspring (13 to 15 male, 13 or 14 female) and 20 or 21 for adult offspring (10 or 11 male, 9 or 10 female) per group.
In the adult offspring, two-way ANOVA did not reveal an interaction for total time immobile (Fig. 3C), but there were independent main effects for both maternal diet and probiotic treatment, whereby maternal HFD increased time immobile in adult offspring relative to CD adult offspring independent of probiotic, and maternal probiotic treatment reduced time immobile relative to offspring exposed to vehicle, independent of maternal diet. Regarding latency to immobility, a significant interaction did occur. Tukey post hoc tests indicated that HFD/vehicle offspring exhibited a significantly reduced latency to immobility compared to adult offspring from each other group (Fig. 3D). No weight differences persisted in adult male or female offspring (SI Appendix, Fig. S14C–E), and no relationship was identified between FST immobility and body weight.
Social interaction test.
Behavior in the social interaction test (SIT) was assessed, in place of the LDB, in the adult offspring. Two-way ANOVA revealed that maternal obesity reduced social interaction at 8 wk of age, independent of maternal probiotic treatment and with no significant interaction between maternal diet and probiotic treatment (SI Appendix, Fig. S15).
Maternal Perinatal Probiotic Treatment Increases the Delivery of Lactate and SCFAs to Offspring during Nursing.
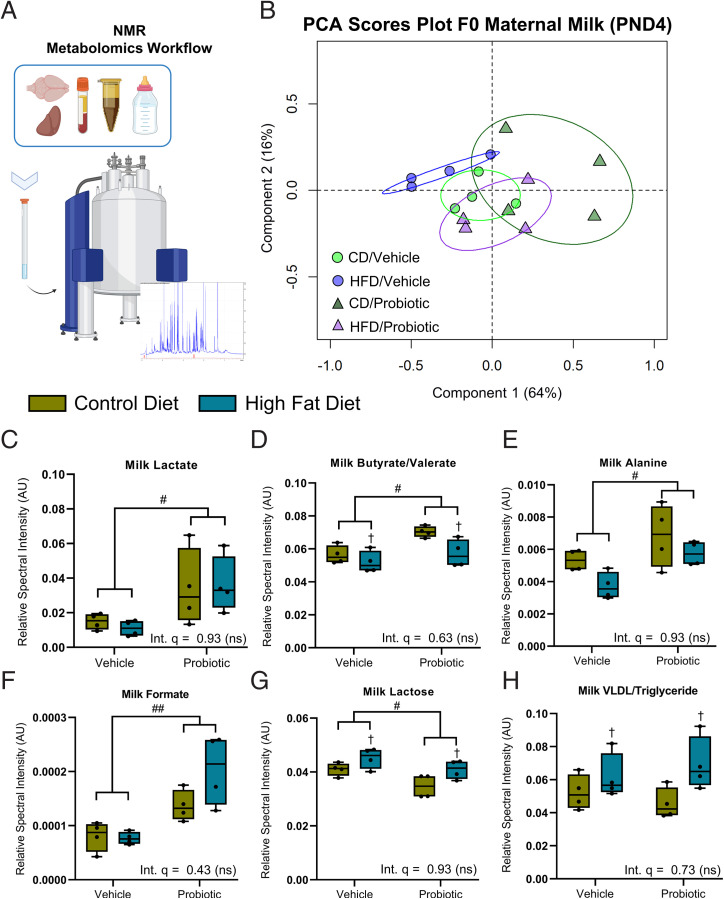
The composition of maternal milk during nursing was found to reflect the varying abundance of plasma and gut (microbial) metabolites of dams (Figs. 1F and 4 A and B and SI Appendix, Figs. S16 and S17 and Tables S8 and S9). While two-way ANOVA of the key milk metabolites did not identify any significant interactions between diet and probiotic treatment (SI Appendix, Table S9), main effects of both diet and probiotic were identified. Specifically, we observed that milk from probiotic-fed dams contained a significantly elevated relative abundance of lactate, alanine, and SCFAs compared to milk from dams fed the vehicle control, independent of maternal diet (Fig. 4C–F). Lactose levels were reduced in the milk of probiotic-fed dams compared to the milk of vehicle-fed dams that was also independent of maternal diet (Fig. 4G). Reciprocally, maternal obesity significantly reduced the milk content of butyrate/valerate and increased lactose and lipid content (Fig. 4H) relative to dams fed a CD, independent of maternal probiotic treatment.
Fig. 4.
Maternal perinatal probiotic treatment increases the delivery of lactate and SCFAs to offspring during nursing. (A) Metabolomics workflow schematic. (B) PCA scores plot of all maternal milk metabolites, collected on PND4. R2X = 0.80 with two principal components. (C) Milk lactate levels were significantly increased by probiotic treatment relative to vehicle [F(1,12) = 9.70]. (D) Milk butyrate/valerate were increased by probiotic treatment relative to vehicle [F(1,12) = 9.65] and decreased by HFD relative to CD [F(1,12) = 8.07]. (E and F) Milk alanine [F(1,12) = 9.22] (E) and formate [F(1,12) = 22.78] (F) were increased by probiotic treatment relative to vehicle. (G) Milk lactose was reduced by probiotic treatment relative to vehicle [F(1,12) = 8.84] and increased by HFD relative to CD [F(1,12) = 8.51]. (H) Milk very-low-density lipoprotein and triglyceride were increased by HFD relative to CD [F(1,12) = 6.77]. (C–H) Significance by main effect only with no interaction. Data are presented as boxplots showing median, interquartile range, and min/max points. Two-way ANOVA with Benjamini and Hochberg FDR correction. †q < 0.05 (indicating main effect of maternal diet); #q < 0.05; ##q < 0.01 (indicating main effect of maternal probiotic treatment). AU, arbitrary units; Int., interaction; ns, not significant. n = 4 per group. Metabolomic descriptive statistics are reported in SI Appendix, Fig. S17 and Tables S8 and S9.
Perinatal Probiotic Exposure Increases Brain Lactate and Gut Butyrate in the Juvenile and Adult Offspring.
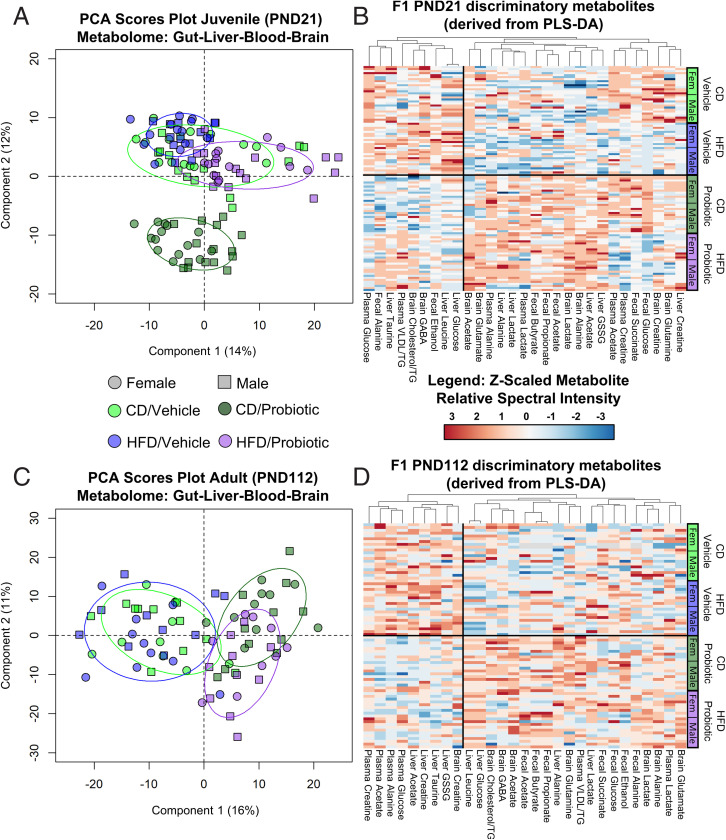
Juvenile and adult offspring brain, liver, plasma, and fecal samples were interrogated for metabolite composition by using 1H NMR. No sex differences were observed in the F1 juvenile offspring (SI Appendix, Fig. S18 A and B), and group differences in metabolites were unrelated to sex differences in the F1 adults (SI Appendix, Fig. S18C and D). PCA of all metabolites (relative abundance, z-scaled by sex) identified unsupervised group differences, in particular, as a result of maternal probiotic intake, in juvenile (Fig. 5A and PCA loadings plot, SI Appendix, Fig. S19) and adult (Fig. 5C and PCA loadings plot, SI Appendix, Fig. S20) offspring. PLS-DA was employed to identify the most important discriminatory metabolites. These are depicted in the PLS-DA loading plots (SI Appendix, Figs. S21–S28), complementary VIP scores in SI Appendix, Tables S10–S17, and summary atlases (SI Appendix, Fig. S16). From this analysis, metabolite heatmaps were generated for the juvenile (Fig. 5B) and adult (Fig. 5D) offspring, with corresponding analysis by two-way ANOVA in SI Appendix, Tables S18–S25.
Fig. 5.
Offspring gut–brain metabolome reflects maternal metabotype influenced by diet and perinatal probiotic supplementation. (A) PCA scores plot of all juvenile offspring gut, liver, plasma, and brain metabolites at PND21. R2X = 0.51 with six principal components. (B) Heatmap of discriminatory metabolites contributing to the F1 juvenile (PND21) tissue-specific PLS-DA models. (C) PCA scores plot of all adult offspring gut, liver, plasma, and brain metabolites at PND112. R2X = 0.55 with six principal components. (D) Heatmap of metabolites in the F1 adult (PND112) offspring, based on those metabolites altered in juvenile offspring. Top VIP metabolites that were significantly altered in at least one tissue were investigated in all tissues. n = 26 to 29 for juvenile offspring (13 to 15 male, 13 or 14 female) and 20 or 21 for adult offspring (10 or 11 male, 9 or 10 female) per group. Descriptive statistics are reported in SI Appendix, Figs. S19–S28 and Tables S10–S25.
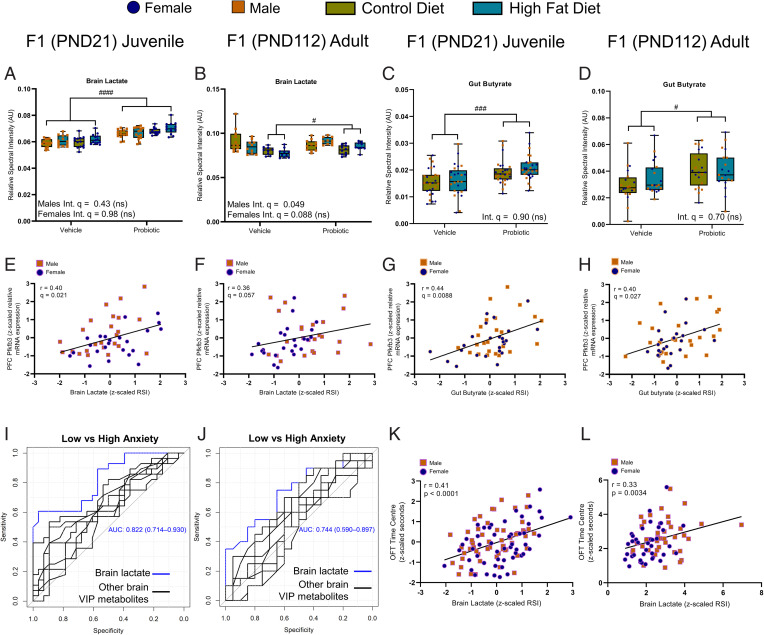
Lactate levels, which were increased in the milk of probiotic-fed dams at postnatal day (PND) 4 (Fig. 4C), were also significantly elevated in the brain (see Fig. 8A), liver (SI Appendix, Table S19), and plasma (SI Appendix, Table S9B) of the juvenile (PND21) offspring relative to the offspring of vehicle-fed dams. This was independent of maternal diet and without a significant interaction. In the plasma, the increased lactate levels were within range of those previously reported during high-intensity treadmill exercise in mice (37–39). As in their mothers, the juvenile offspring exposed to the probiotic also exhibited elevated propionate and butyrate in the gut relative to vehicle offspring (see main effect, Fig. 8C and SI Appendix, Table S21 and Fig. S24). Other key brain metabolites altered by maternal diet and/or probiotic treatment are summarized in SI Appendix, Fig. S16 and Table S18. Briefly, there were significant interactions for brain creatine and brain acetate levels, where both HFD/vehicle and HFD/probiotic offspring had reduced brain creatine compared to CD/vehicle offspring, while only HFD/vehicle offspring had reduced levels relative to CD/probiotic offspring. Acetate levels were increased in the brain of HFD/probiotic offspring relative to all other groups, while both the HFD/vehicle and CD/probiotic offspring had reduced levels compared to the other groups.
Fig. 8.
Key gut and brain metabolites altered by maternal probiotic treatment show correlations with a genetic marker of astrocytic metabolism and anxiety behavior. (A) In the juvenile offspring, two-way ANOVA revealed that maternal probiotic treatment increased brain lactate levels in both male [main effect, F(1,53) = 44.57] and female [main effect, F(1,48) = 60.07] relative to vehicle. (B) In the adult male offspring, there was a significant maternal diet × probiotic treatment interaction [F(1,38) = 5.32] for brain lactate. Note that post hoc Tukey analysis did not identify significant group differences (P > 0.05). In the adult female offspring, maternal probiotic intake increased brain lactate independent of diet [F(1,34) = 6.97]. Maternal probiotic treatment increased gut butyrate levels in both the juvenile offspring [main effect, F(1,101) = 13.83] (C) and the adult offspring [F(1,72) = 8.71] (D) independent of maternal diet and sex. A significant correlation (Spearman’s rank correlation coefficient) between brain lactate levels and PFC PFKFB3 expression was found for juvenile (r = 0.40, n = 48 [24F, 24M]) (E), but not for adult (r = 0.36, n = 48 [24F, 24M]) (F), offspring. Correlations for gut butyrate and PFC PFKFB3 expression were found for both juvenile (r = 0.44, n = 48 [24M, 24F]) (G) and adult (r = 0.40, n = 48 [24M, 24F]) (H) offspring. Receiver operating characteristic curves (I, juvenile; J, adult) for brain lactate concentration compared to other discriminatory brain metabolites as classifiers for high and low anxiety-like behavior in the OFT in juvenile (n = 28 low anxiety [10M, 18F], 28 high anxiety [15M, 13F]) and adult (n = 20 low anxiety [12M, 8F], high anxiety [5M, 15F]) offspring, respectively. Significant correlations between brain lactate levels and OFT time in center were found for juvenile (r = 0.41, n = 111 [57M, 54F]) (K) and adult (r = 0.33, n = 80 [42M, 38F]) (L) offspring. (A–D) n = 13 to 15 juvenile offspring, n = 9 to 11 adult offspring per sex per group. Data are presented as boxplots showing median, interquartile range, and min/max points. AU, arbitrary units; Int., interaction; ns, not significant. #P < 0.05; ###P < 0.001; ####P < 0.0001 (indicating main effect of maternal probiotic treatment). Where sex differences occurred, separate two-way ANOVAs were performed.
In the adult female offspring (PND112), two-way ANOVA revealed that in the absence of a significant interaction, brain lactate remained elevated in the offspring exposed to maternal probiotic intake relative to vehicle controls. In the male adult offspring, there was a significant maternal diet × probiotic interaction, although no post hoc differences reached significance (see Fig. 8B). In the plasma (SI Appendix, Fig. S9C), lactate levels were not altered by maternal diet or probiotic treatment. There was, however, a main effect of maternal probiotic intake on gut butyrate (see Fig. 8D) and propionate levels, which remained increased in the adult offspring of probiotic-treated dams relative to vehicle offspring (Fig. 5D and SI Appendix, Table S25). Interestingly, tissue-specific PLS-DA suggested that maternal probiotic intake exerted the most lasting influence on the adult brain metabolome (SI Appendix, Fig. S25), as opposed to the liver, gut, or plasma metabolites (SI Appendix, Figs. S26–S28). Two-way ANOVA confirmed that brain alanine, acetate, and glutamine were increased in the adult offspring of probiotic dams relative to vehicle controls, independent of maternal diet and sex with no interaction (main effect, SI Appendix, Table S22). Lastly, there was a significant maternal diet × probiotic treatment interaction affecting brain GABA levels in the adult offspring, whereby abundance was increased in CD/probiotic offspring relative to all other groups (SI Appendix, Table S22).
Perinatal Probiotic Intake Has Long-Lasting Effects on Neuroplasticity Gene Expression in the Offspring PFC.
Expression of NMDAR subunits.
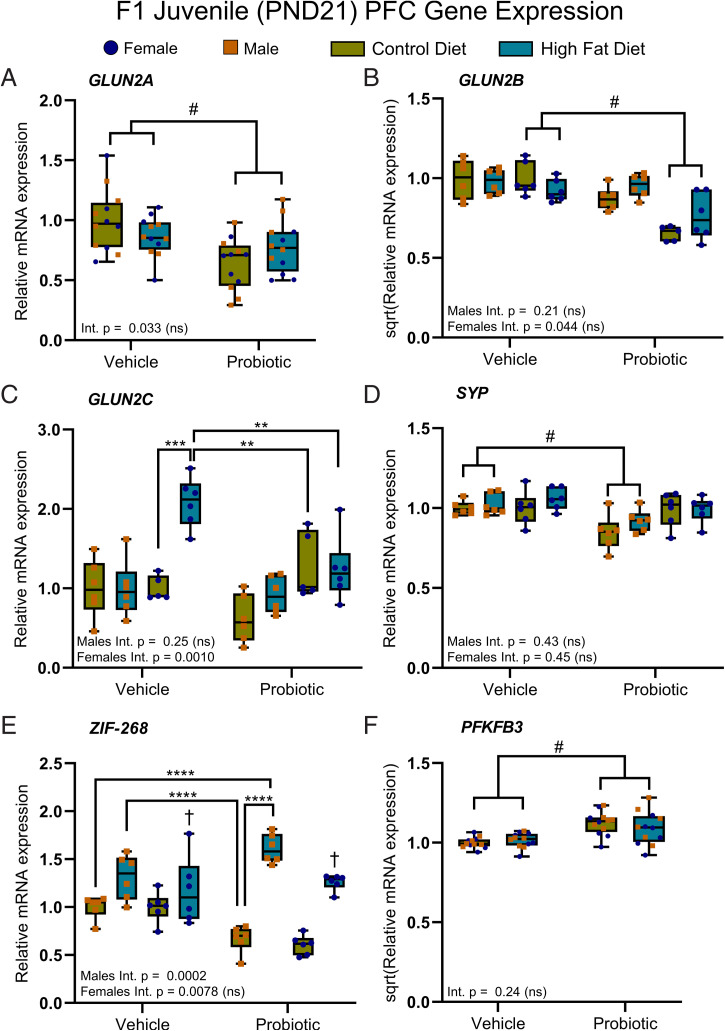
In the juvenile offspring, two-way ANOVA (with the Bonferroni correction) revealed a main effect of maternal probiotic intake on GLUN2A expression, where expression was reduced in the offspring of probiotic-fed dams relative to the offspring of vehicle controls, independent of maternal diet with no interaction (Fig. 6A). In the same way, GLUN2B expression was also reduced, but only in the female offspring (Fig. 6B). A significant maternal diet × probiotic interaction occurred for GLUN2C expression in the female juvenile offspring, whereby expression was increased only in HFD/vehicle female offspring compared to the juvenile female offspring from all other groups (Fig. 6C). GLUN1 and PSD-95, constituent postsynaptic NMDAR components, were unaltered in the juvenile offspring (SI Appendix, Table S26), and there were no significant effects or interactions between maternal diet and probiotic treatment on GLUN1/2A/2B/2C or PSD-95 gene expression in the F1 adults (SI Appendix, Table S27).
Fig. 6.
Maternal probiotic treatment alters synaptic plasticity in juvenile offspring. (A) Maternal probiotic treatment reduced PFC expression of GLUN2A in the juvenile offspring [main effect, F(1,44) = 13.16] independent of maternal diet with no interaction. (B) GLUN2B expression was reduced in the females [main effect, F(1,19) = 35.26] of probiotic dams, but not males [main effect, F(1,20) = 4.22, P = 0.053], relative to the juvenile offspring of vehicle-fed dams. (C) For PFC GLUN2C expression, a significant maternal diet × probiotic treatment interaction was noted for females [F(1,18) = 15.28]. Post hoc tests revealed that GLUN2C expression was increased in the female offspring of HFD/vehicle dams relative to female offspring in the other groups. (D) SYP expression was reduced in the male probiotic offspring [main effect, F(1,20) = 16.77], but not females [main effect, F(1,20) = 1.00, P = 0.33], relative to vehicle offspring. (E) Expression of PFC ZIF-268 was increased in the female offspring of obese dams relative to female offspring of lean dams, independent of maternal probiotic [main effect, F(1,20) = 24.99]. In the juvenile male offspring, a significant interaction was identified [F(1,20) = 21.41]. Tukey post hoc tests revealed that male CD/probiotic offspring had lower expression than HFD/vehicle and HFD/probiotic offspring, while male CD/vehicle offspring had lower expression relative to HFD/vehicle offspring only. (F) Lastly, maternal probiotic treatment increased juvenile PFC PFKFB3 expression relative to vehicle offspring [main effect, F(1,44) = 23.96]. Two-way ANOVA; data are presented as boxplots showing median, interquartile range, and min/max points. Int., interaction. †P < 0.001 (indicating main effect of maternal diet); #P < 0.001 (indicating main effect of maternal probiotic treatment); **P < 0.01; ***P < 0.001; ****P < 0.0001 (indicating a significant post hoc comparison, performed only if a significant interaction was identified). Ns, not significant. For all comparisons note: Bonferroni corrected significance level (α) = 0.001. n = 6 per sex per group. Where sex differences occurred, separate two-way ANOVAs were performed. Descriptive statistics are reported in SI Appendix, Tables S26 and S28.
Expression of serotonin receptor subunits.
There were no interactions or main effects of maternal obesity and probiotic on young or adult offspring serotonergic receptor expression (SI Appendix, Tables S26 and S27).
Plasticity gene expression.
Two-way ANOVA revealed a main effect of maternal probiotic intake on expression of the presynaptic density marker SYP, which was reduced in the male juvenile offspring of probiotic-fed dams relative to vehicle offspring, independent of maternal diet with no interaction (Fig. 6D). In the same way, CREB1 expression was increased in the male offspring (SI Appendix, Table S26). A significant interaction occurred for ZIF-268 expression in the male juvenile offspring. Post hoc Tukey tests showed that HFD/probiotic offspring had increased expression relative to both CD/probiotic and CD/vehicle offspring, while HFD/vehicle offspring had increased expression only relative to CD/probiotic offspring (Fig. 6E). With the absence of an interaction in the female offspring, there was a main effect of maternal diet, whereby expression was increased in the offspring of HFD-fed dams relative to CD (Fig. 6E). Other plasticity-related genes were unchanged at juvenile age (SI Appendix, Table S26).
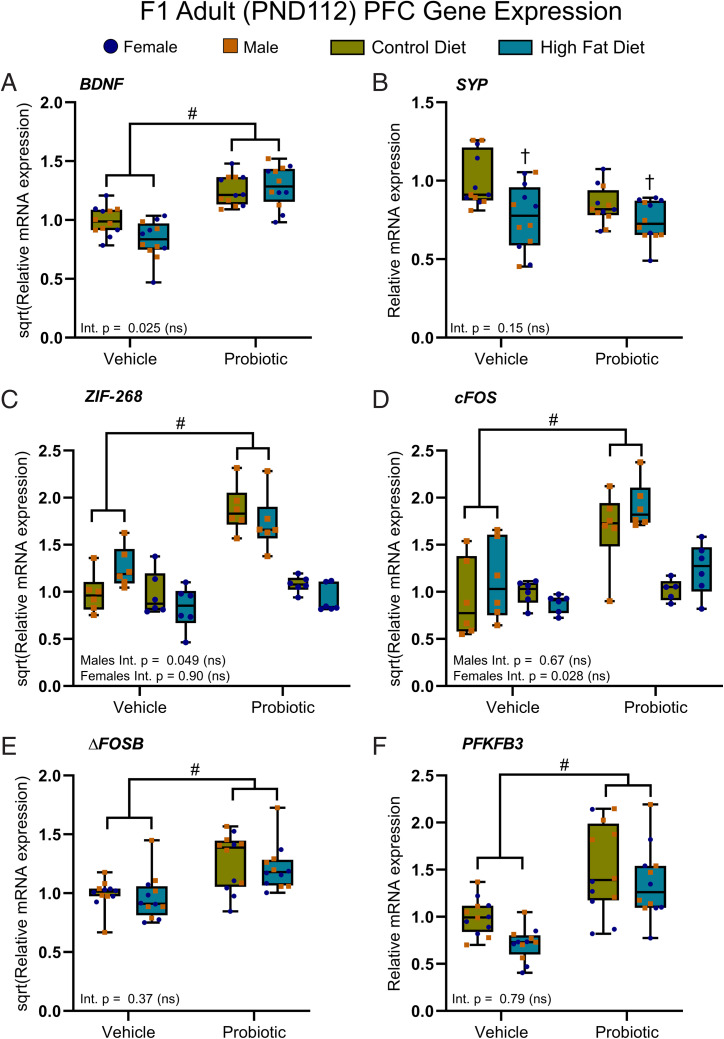
In the adult offspring, there was a main effect of maternal probiotic intake on BDNF (Fig. 7A) and FOSB (Fig. 7E) expression, where expression was increased in the offspring of probiotic-fed dams relative to vehicle, independent of maternal diet with no significant interaction or sex differences. SYP was decreased in the adult offspring of obese dams relative to lean dams as a main effect, independent of maternal probiotic treatment with no sex differences or interaction (Fig. 7B). Both ZIF-268 (Fig. 7C) and cFOS (Fig. 7D) expression were increased in the adult male offspring of probiotic-fed dams relative to the adult male offspring of vehicle-fed dams as a main effect without interaction. GSK3B and CREB1 expression were unaltered in the adult offspring (SI Appendix, Table S27).
Fig. 7.
Maternal probiotic treatment has lasting effects on astrocytic metabolism and neuronal plasticity. (A) Maternal probiotic treatment increased BDNF expression in the adult offspring of probiotic-fed dams relative to the adult offspring of dams given the vehicle [main effect, F(1,44) = 70.53], independent of maternal diet. (B) Maternal HFD reduced SYP expression relative to maternal CD [main effect, F(1,44) = 12.86]. The expression of ZIF-268 [F(1,20) = 34.99] (C) and cFos (F(1,20) = 25.17) (D) were significantly elevated in the male adult offspring of probiotic-treated dams, relative to vehicle offspring via a main effect of maternal probiotic treatment. FosB (E) and PFKFB3 (F) expression were both increased by maternal probiotic treatment, relative to the adult offspring of dams given the vehicle [main effects, FosB F(1,42) = 33.60; PFKFB3 Q(1,42) = 29.96]. (A–F) No significant maternal diet × probiotic treatment interactions reached significance. Data are presented as boxplots showing median, interquartile range, and min/max points. Int., interaction; ns, not significant. †P < 0.001 (indicating main effect of maternal diet); #P < 0.001 (indicating main effect of maternal probiotic treatment). For all comparisons, note: Bonferroni-corrected significance level (α) = 0.001. n = 6 per sex per group. Where sex differences occurred, separate two-way ANOVAs were performed. Descriptive statistics are reported in SI Appendix, Tables S27 and S29.
Astrocytic metabolic activity.
Two-way ANOVA revealed a main effect of maternal probiotic intake on PFC PFKFB3 expression. PFKFB3 was increased in both the juvenile (Fig. 6F) and adult (Fig. 7F) offspring of dams treated with the probiotic compared to the offspring of vehicle controls, independent of maternal obesity and with no interaction or sex differences. ATP1A2 expression was not altered in the juvenile or adult offspring (SI Appendix, Tables S26 and S27).
Proinflammatory cytokine expression.
Two-way ANOVA revealed that maternal probiotic supplementation, as a main effect, increased juvenile IL-6 in the PFC relative to the juvenile offspring of vehicle controls, independent of maternal obesity with no interaction or sex differences (SI Appendix, Table S28). Expression of TNF, TLR4, and IL-1B expression was unchanged, and there were no persistent changes in PFC cytokine expression in the adult offspring (SI Appendix, Table S29).
In contrast to PFC expression, a significant maternal diet × probiotic interaction was identified for liver IL-6 in the female juvenile offspring, with no significant interaction or main effects in male offspring. Post hoc tests revealed that expression was elevated in the offspring of HFD/vehicle dams relative to the offspring of all other conditions (SI Appendix, Table S28). There was also a main effect of maternal probiotic supplementation on reducing TLR4 expression in the liver of the juvenile offspring, relative to juvenile offspring from dams fed the vehicle, independent of maternal diet and with no sex differences and no significant maternal diet × probiotic interaction. In the adult male offspring, maternal obesity increased liver IL-1B and IL-6 expression relative to the adult male offspring of lean dams (main effect, SI Appendix, Table S29). Conversely, in the female adult offspring, maternal diet had no effect on proinflammatory gene expression, whereas maternal probiotic intake increased expression of IL-6 relative to vehicle controls, with no significant interaction (main effect, SI Appendix, Table S29).
Brain Lactate and Gut SCFAs Correlate with PFC Gene Expression.
We further investigated how brain (SI Appendix, Table S30) and gut (SI Appendix, Table S31) metabolite levels (top three or four ranked by VIP score from the corresponding PLS-DA) correlated with altered PFC gene expression. In the juvenile offspring, there was a significant negative correlation between brain lactate levels and expression of GLUN2A (r = –0.47, q = 0.0080), GLUN2B (r = –0.50, q = 0.0080), and SYP (r = –0.40, q = 0.021). A positive correlation existed between lactate and PFKFB3 (r = 0.40, q = 0.021; Fig. 8E). Increased alanine was associated with increased expression of IL-6 in the PFC (r = 0.44, q = 0.013). Creatine, the top brain metabolite reduced in the juvenile offspring of obese dams, was not significantly correlated with gene expression.
An increase in fecal SCFAs acetate (r = 0.38, q = 0.025), propionate (r = 0.53, q = 0.0013), and butyrate (r = 0.44, q = 0.0088; Fig. 8G) all significantly correlated with increased expression of PFKFB3 (SI Appendix, Table S31). Propionate negatively correlated with GLUN2A (r = –0.35, q = 0.029), GLUN2B (r = –0.43, q = 0.0088), and SYP (r = –0.31, q = 0.047) expression, while butyrate correlated negatively with GLUN2C (r = –0.34, q = 0.038) and SYP (r = –0.37, q = 0.025) expression.
For the same metabolite correlation analyses where PFC gene expression was also altered between groups in adult offspring, only fecal butyrate remained significantly correlated with PFC PFKFB3 (r = 0.40, q = 0.027; Fig. 8H and SI Appendix, Table S32). Lactate was not significant after correcting for the FDR (r = 0.36, q = 0.057; Fig. 8F and SI Appendix, Table S33).
Elevated Brain Lactate Accompanies the Therapeutic Effect of Early Life Probiotic Exposure on Anxiety-Like Behavior.
Finally, we investigated how brain and gut metabolites—in particular, those that were discriminatory between offspring of lean, obese, and probiotic-supplemented dams—correlated with patterns of anxiety-like behavior. In both juvenile (area under the curve [AUC] 0.82) and adult (AUC 0.74) offspring, lactate was the most discriminatory brain metabolite between high (first quartile) and low (fourth quartile) anxiety-like behavior, independent of experimental groupings (Fig. 8 I and J, respectively). Brain lactate levels correlated with reduced anxiety-like behavior in the open field across all juvenile (r = 0.41, P < 0.0001) and adult (r = 0.33, P = 0.0034) offspring (Fig. 8 K and L, respectively).
Discussion
The current study demonstrates that maternal obesity alters brain metabolism in the juvenile and adult offspring, with long-term effects on behavior and PFC plasticity gene expression. Maternal, perinatal multispecies probiotic supplementation ameliorated offspring anxiety-like behavior associated with maternal obesity in both the young (PND21) and adult (PND112) offspring. Additionally, early life probiotic exposure increased gut butyrate and brain lactate, reduced GLUN2A/2B messenger RNA (mRNA), and elevated PFKFB3 expression [a marker of increased astrocytic metabolism through glycolysis (33)] in the PFC of young offspring relative to vehicle controls. PFKFB3 was also elevated in adult offspring exposed to perinatal probiotic supplementation relative to vehicle controls and remained correlated with gut butyrate levels. BDNF and other plasticity-related genes were increased in adulthood after early life probiotic exposure, whereas SYP levels were reduced in the adult offspring of obese dams relative to the offspring of lean dams. Together, these data show that the intake of a multispecies probiotic supplement during gestation and nursing has a lasting influence on behavior, neuroplasticity, and neurometabolism and can mitigate some of the behavioral symptoms of maternal obesity in the offspring.
The anxiolytic and antidepressant effects of perinatal probiotic exposure in this study are concordant with reports describing similar behavioral responses in rodent offspring: Lactobacillus helveticus NS8 intake in normal-weight dams from embryonic day (E) 13 to E22 during gestation has been shown to reduce offspring anxiety (40), and maternal perinatal prebiotic intake (which encourages the growth of endogenous Bifidobacterium spp.) reduced passive stress coping in the FST in adulthood (27). Reduced social behavior observed in the adult offspring of obese dams is associated with reduced Bifidobacterium spp. and Lactobacillus spp. (23), but reconstitution with microbiota of control offspring through coprophagia reversed these deficits. These findings, in combination with our results, emphasize the important role of maternal microbiota and metabolites on early life neurodevelopment and behavior (24).
In this study, expression of the GLUN2A and GLUN2B NMDAR subunits was reduced in the PFC of juvenile probiotic offspring. We previously found that maternal prebiotic supplementation reduced these subunits in the juvenile offspring hippocampus (27), and others have shown that prebiotic supplementation reduced GLUN2B protein in the frontal cortex (41) of adult mice. Consistent with our present data in juvenile mice, these reported changes in gene expression were associated with reduced anxiety-like (41) behavior. Thus, despite prebiotics and probiotics having distinct actions on the gut flora, the central mechanisms underlying their psychotropic effect may be similar. NMDAR antagonists, including those selective to the GLUN2B subunit, have antidepressant activity in mice (42) and humans (43, 44). While alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor (AMPAR) expression was not assessed in this study, a decrease in NMDAR expression may increase AMPAR (non-NMDA glutamatergic) throughput relative to NMDAR throughput in the PFC, which is one of the mechanisms thought to underly the persistent antidepressant effect of NMDAR antagonism (42). This is supported by the idea that antidepressant NMDAR antagonists affect presynaptic, rather than postsynaptic, circuits (45, 46), with antagonism ultimately enhancing PFC synaptic efficiency and glutamate release (46). We show that expression of postsynaptic density marker PSD-95 was stable, while SYP expression, a presynaptic density marker, decreased in the male juvenile offspring of probiotic-fed dams alongside reductions in GLUN2A and GLUN2B expression relative to vehicle controls. These observations, together with the overall increase in cortical glutamate shown here in probiotic offspring, are largely consistent with the downstream effects of NMDAR antagonism in vivo (47).
Besides the increase in cortical glutamate, NMR-based metabolomic profiling revealed a substantial increase in l-lactate in the brain, liver, and plasma of juvenile offspring after maternal probiotic intake compared to vehicle controls, with no overt sex differences. Analysis of maternal milk at PND4 indicated increased lactate ingestion in probiotic compared to vehicle offspring during the first week of life, which may stem from increased levels of Lactobacillus spp. and Bifidobacterium spp. in the colon of dams receiving the probiotic (48). While a direct link between maternal microbiota and increased brain lactate has not been reported, the importance of maternal microbiota and microbial metabolites in neurodevelopment during the perinatal period is recognized (24). l-lactate has antidepressant-like properties when administered to adult mice (49, 50), and the selective serotonin reuptake inhibitor fluoxetine has been shown to increase lactate release from astrocytes in vitro (51). Functioning independently as both a signaling molecule (33) and neuronal energy source (52, 53), the pleiotropic properties of lactate may have contributed to the early life changes in NMDAR gene expression (54) and behavior (49, 50). Here, the abundance of brain lactate, but not other brain metabolites, correlated highly with GLUN2A/2B and PFKFB3 expression changes across all young male and female offspring and correlated with reduced anxiety-like behavior in both young and adult offspring. Lactate has been shown to directly modulate histone deacetylase (HDAC) activity in the mouse hippocampus, which was associated with reduced GLUN2A mRNA expression and resilience to chronic social defeat stress (49). PFKFB3 is a master regulator of lactate production through the glycolytic pathway in astrocytes (55) and may be regulated in part by the microbiome (30). Here, we provide evidence supporting the role of microbes and microbial metabolites, in particular, brain lactate and gut butyrate, in regulating mood behavior and the expression of plasticity-related genes.
In juveniles, maternal obesity reduced brain and liver creatine levels, which were normalized after perinatal probiotic intake in the liver, but not the brain. A systematic review (56) collated data from studies employing in vivo brain proton magnetic resonance spectroscopy in major depressive disorder (MDD) patients and found that creatine and glutamate were significantly down-regulated in MDD. This is consistent with the brain metabolite changes we found in the juvenile offspring of obese dams. Low dietary creatine intake is a clinical risk factor for depression (57), and creatine supplementation has shown promise as an adjunct supplement for MDD (58). A promising theory for the pathophysiology of synaptic dysfunction in psychiatric disorders is that plastic changes are constrained by brain energy deficits (59). This has been supported by preclinical (60) and clinical evidence (61) implicating reduced creatine in depression and reduced creatine kinase flux in bipolar disorder, respectively. The results from our metabolomic study support accumulating evidence connecting maternal obesity during pregnancy to long-term changes in central metabolism and behavior (12). We suggest that the increase in cortical lactate, the source of which may be astrocytic (via increased PFC PFKFB3 expression), microbial (via increased milk lactate and Lactobacillus spp. in probiotic dams), or both, in the juvenile probiotic offspring may have compensated for the creatine energy deficit imposed by maternal obesity (62).
In the adult offspring, gene-expression analysis revealed a long-term effect of maternal probiotic administration on synaptic plasticity. Increased BDNF and the immediate early genes ZIF-268 and cFOS indicate an increase in the maintenance of BDNF-mediated synaptic plasticity in adult probiotic offspring (63). Because this mechanism is held to underlie response to traditional antidepressant activity and mood resilience in adults (63, 64), it may account for the long-term reduction in depressive-like behavior in these offspring. The elevation of FOSB in the adult offspring of probiotic dams may also contribute to the antidepressant-like behavior, as induced overexpression of FOSB has been shown to counteract the behavioral effects of chronic social defeat stress in mice (65).
To further investigate the etiology of the enduring behavioral and molecular changes in offspring, we explored how maternal obesity and probiotic supplementation affected maternal care behavior (MCB), immunology, and metabolism during nursing. Obesity increased IL-1B expression in dams, whereas probiotic treatment reduced IL-6. In humans, increased maternal IL-6 during pregnancy is predictive of reduced brain connectivity and working memory in 2-y-old offspring (66). Maternal obesity has been directly linked to placental inflammation in NHPs (67), and it is conceivable that the divergent maternal proinflammatory activity in obese versus probiotic-supplemented obese and lean dams may have contributed to different neurodevelopment trajectories in the offspring (68).
Maternal probiotic intake increased the abundance of SCFAs in the milk and gut of probiotic-fed dams during nursing, as well as in the gut of their juvenile and adult offspring relative to the vehicle controls in each age group. This may have contributed to the long-term changes in gene expression and behavior exhibited by these offspring. While not detected in brain tissue using NMR, the SCFAs butyrate and propionate are brain-permeable and, like lactate, capable of modulating gene transcription through HDAC modification in vivo (49, 69). We found that increased gut propionate and butyrate coincided with increased anxiety-like behavior in adult offspring. Moreover, these metabolites correlated highly with reduced PFC SYP expression in juvenile offspring, while propionate also correlated with reduced PFC GLUN2A/B. Given this observation, it would be interesting to determine the direct contribution of these metabolites—in parallel to lactate—to long-term transcriptome and mood changes.
In this study, we used a probiotic containing Bifidobacterium spp. and Lactobacillus spp. (SI Appendix, Table S34), both of which produce lactate as well as the SCFAs propionate, butyrate, and acetate (70). We determined the sum therapeutic effect of these maternally ingested strains on the offspring metabolome, with the aim of identifying beneficial metabolites involved in resilience to anxiety-like behavior induced by maternal obesity. While the rationale of employing any one strain as a potential antidepressant is limited, we did not resolve the precise source of the microbially derived metabolites or the contribution of individual strains and species to ameliorating the adverse effects of maternal obesity in the offspring. Similarly, we did not determine whether metabolite changes in the brain differed between isolated brain regions. Future studies will investigate whether individual strains or metabolites are sufficient to reproduce the long-lasting effects on behavior and plasticity gene expression in the offspring and will resolve the metabolic changes of individual brain regions.
In summary, maternal obesity increased anxiety- and depressive-like behavior in young and adult offspring. Anxiety-like behavior was prevented in the juvenile offspring of obese dams by maternal perinatal probiotic treatment, and both anxiety- and depressive-like behavior were prevented in the adult offspring of obese dams that were exposed to perinatal probiotic treatment. We identified reduced brain energy metabolites—creatine and glutamate—as being associated with behavioral dysfunction in the juvenile offspring of obese dams and increased cortical lactate as a potential source for the remedial effects of early life probiotic exposure. As pharmacotherapy, including weight-loss therapy, during pregnancy raises safety concerns, the use of probiotics represents a safe alternative that appears to have real promise for mitigating the effects of maternal obesity on offspring neurodevelopment and behavior. We have also demonstrated, independent of maternal obesity, the long-term effects of maternal probiotic treatment on promoting plasticity gene expression and reducing anxiety- and depressive-like behaviors in adult offspring, which may be mediated by the early life increases in neuroactive lactate and SCFA metabolites.
Materials and Methods
Detailed methods may be found in SI Appendix.
Animals.
CD-1 IGS mice (Charles River) were housed in a pathogen-free facility under standard conditions. Food and water, including specific dietary administrations, were provided ad libitum. All licensed procedures were performed with UK Home Office approval, under license P996B4A4E.
The study design is summarized in Fig. 1A. Dams were a fed an HFD or CD for 12 wk, from 6 wk prior to the start of pregnancy (E0.5) until offspring were of weaning age (PND21). Mice were checked at 0800 each day for the presence of a vaginal plug. The date of a vaginal plug first appearing was designated E0.5. Males were removed from the cage the following day. Parent (F0) mice were weighed twice a week prior to the start of gestation (Fig. 1B). Offspring (F1) pups were weighed every third day until weaning (SI Appendix, Fig. S14A and B), and for the adult cohort, weekly thereafter (SI Appendix, Fig. S14C–E). Probiotic supplementation began at E0.5 and continued until PND21. At this point, dams and half the juvenile offspring were subject to the OFT, LDB, and FST, and tissue was immediately harvested for qPCR (left brain PFC, liver, and fecal) and metabolomics (right brain cerebrum, liver, plasma, and fecal). The remaining offspring were weaned onto regular chow and run through the SIT at 8 wk of age. At PND112, adult offspring were run through the OFT and FST, and tissue was harvested for qPCR and metabolomics.
Treatments.
Probiotic administration.
Bio-Kult Advanced (ADM Protexin) is a multistrain probiotic consisting of 14 live strains of bacteria (SI Appendix, Table S34). At E0.5, female F0 mice were randomly assigned to receive either 4 × 107 colony-forming units per milliliter or vehicle only (Fig. 1A). This corresponded to intake of ∼6.4 million live bacteria per gram of body weight per day, based on the average daily water consumption of an adult mouse (0.12 mL/g per mouse per day).
Diets.
Female F0 mice were randomly assigned to be maintained on either an HFD consisting of 60% kilocalories from fat or a carbohydrate-matched CD with 10% kilocalories from fat (diets from Research Diets Inc.). This resulted in four dams per group (CD/vehicle, HFD/vehicle, CD/probiotic, and HFD/probiotic).
Behavior.
F0 dams and F1 juvenile mice were subject to an OFT (50 × 50 × 50 cm3, length × width × height [lxwxh]), LDB (black compartment, 21 × 16 × 16 cm3, lxwxh; white compartment, 46.5 × 21 × 21 cm3, lxwxh), and FST (32 × 17 × 12 cm3, lxwxh) as described (27). F1 adult mice were tested for social behavior in the SIT (65 × 30 × 20 cm3, lxwxh) at 8 wk of age and the OFT and FST at 16 wk. All behavior was scored while blinded to the identity of treatment groups. Behavioral testing was completed with minimal noise disruption under controlled lighting conditions. Apparatuses were cleaned with 70% ethanol between animals. More detail, including the evaluation of MCB, can be found in SI Appendix.
Tissue Collection.
Following behavioral testing, animals were terminally anesthetized under isoflurane gas at the same time each day, to minimize potential circadian variation in metabolic profiles. To collect plasma, blood was collected into lithium heparin-coated tubes via cardiac puncture with a heparinized 23G needle. Blood was left to stand for 30 min at room temperature, then centrifuged at 1,300 × g for 10 min at 4 ∘C. Plasma lactate was determined with a lactate monitor (EKF Diagnostics). Plasma was stored at –80 ∘C. Animals were transcardially perfused with cold saline (0.9% m/v) containing heparin (5,000 USP/L) until the liver was clear of blood. Whole brain and liver portions were immediately dissected and snap frozen in isopentane. Fecal matter from the distal colon was collected, and all samples were stored at –80 ∘C until further use.
RNA Extraction, Complementary DNA Conversion, qPCR, and Metabolomic Profiling.
Refer to SI Appendix, Methods. List of primers for qPCR can be found in SI Appendix, Table S35.
Statistical Analysis.
Statistical tests were performed in Prism 8 (GraphPad) and R (version 3.3.1). Initially, three-way ANOVAs were performed to identify whether sex interacted with maternal diet and/or maternal perinatal probiotic intake, affecting the dependent (outcome) variable. If a statistically significant interaction with sex occurred, males and females were analyzed separately with two two-way ANOVAs. Where no main effects of, or interactions with, sex occurred, male and female data were consolidated into a single two-way ANOVA. These processes served to simplify the interpretation of the main effects and their interaction. Prior to three-way or two-way ANOVA, Grubbs’ test for outliers was performed on each group, and statistically significant outliers (P < 0.05) were removed from downstream analyses. Normality of residuals was tested with the Shapiro–Wilk test (P < 0.01). Alternative tests were used for nonparametric data (SI Appendix, Methods). All univariate data were visualized as boxplots showing all data points, where the upper and lower bounds of the box indicate the interquartile range, the line within the box as the median, and the error bars denoting the full range of values. The significance threshold was set at P < 0.05 for behavior and q < 0.05 for univariate metabolomic and Spearman rank correlation analyses adjusted for multiple testing using the FDR method (Benjamini Hochberg). For offspring gene-expression data, significance was set at P < 0.001 using the Bonferroni method based on 50 independent tests.
For information on the multivariate analysis performed for the metabolomics data, refer to SI Appendix, Methods.
Supplementary Material
Acknowledgments
D.E.R.-S. is supported by the Newton Abraham Studentship (Oxford) and the Clarendon Fund in association with the Lincoln College Kingsgate award (Oxford).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. J.C. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2108581119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix. The raw metabolomics spreadsheet data have been deposited in the Oxford Research Archive (https://doi.org/10.5287/bodleian:ZOb4VbEqn).
References
- 1.Malhi G. S., Mann J. J., Depression . Lancet 392, 2299–2312 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro W. S., et al., Income inequality and mental illness-related morbidity and resilience: A systematic review and meta-analysis. Lancet Psychiatry 4, 554–562 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Devlieger R., et al., Maternal obesity in Europe: Where do we stand and how to move forward? A scientific paper commissioned by the European Board and College of Obstetrics and Gynaecology (EBCOG). Eur. J. Obstet. Gynecol. Reprod. Biol. 201, 203–208 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Yogev Y., Catalano P. M., Pregnancy and obesity. Obstet. Gynecol. Clin. North Am. 36, 285–300 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Jaacks L. M., Slining M. M., Popkin B. M., Recent underweight and overweight trends by rural-urban residence among women in low- and middle-income countries. J. Nutr. 145, 352–357 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Arana S., Avendano M., van Lenthe F. J., Burdorf A., Trends in overweight among women differ by occupational class: Results from 33 low- and middle-income countries in the period 1992–2009. Int. J. Obes. 38, 97–105 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poston L., et al., Preconceptional and maternal obesity: Epidemiology and health consequences. Lancet Diabetes Endocrinol. 4, 1025–1036 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Milaneschi Y., Simmons W. K., van Rossum E. F. C., Penninx B. W., Depression and obesity: Evidence of shared biological mechanisms. Mol. Psychiatry 24, 18–33 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Rivera H. M., Christiansen K. J., Sullivan E. L., The role of maternal obesity in the risk of neuropsychiatric disorders. Front. Neurosci. 9, 194 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez A., Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. J. Child Psychol. Psychiatry 51, 134–143 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Robinson M., et al., Pre-pregnancy maternal overweight and obesity increase the risk for affective disorders in offspring. J. Dev. Orig. Health Dis. 4, 42–48 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Sullivan E. L., Nousen E. K., Chamlou K. A., Maternal high fat diet consumption during the perinatal period programs offspring behavior. Physiol. Behav. 123, 236–242 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan E. L., et al., Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J. Neurosci. 30, 3826–3830 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCurdy C. E., et al., Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J. Clin. Invest. 119, 323–335 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinan T. G., Cryan J. F., Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 595, 489–503 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson A. L., et al., Infant gut microbiome associated with cognitive development. Biol. Psychiatry 83, 148–159 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratsika A., Codagnone M. C., O’Mahony S., Stanton C., Cryan J. F., Priming for life: Early life nutrition and the microbiota-gut-brain axis. Nutrients 13, 423 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres-Fuentes C., Schellekens H., Dinan T. G., Cryan J. F., The microbiota-gut-brain axis in obesity. Lancet Gastroenterol. Hepatol. 2, 747–756 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Chu D. M., et al., The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 8, 77 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collado M. C., Isolauri E., Laitinen K., Salminen S., Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 88, 894–899 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Santacruz A., et al., Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 104, 83–92 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Paul H. A., Bomhof M. R., Vogel H. J., Reimer R. A., Diet-induced changes in maternal gut microbiota and metabolomic profiles influence programming of offspring obesity risk in rats. Sci. Rep. 6, 20683 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buffington S. A., et al., Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 165, 1762–1775 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vuong H. E., et al., The maternal microbiome modulates fetal neurodevelopment in mice. Nature 586, 281–286 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haramburu F., Daveluy A., Miremont-Salamé G., Statins in pregnancy: New safety data are reassuring, but suspension of treatment is still advisable. BMJ 350, h1484 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Allen S. J., et al., Dietary supplementation with lactobacilli and bifidobacteria is well tolerated and not associated with adverse events during late pregnancy and early infancy. J. Nutr. 140, 483–488 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Hebert J. C., et al., Mom’s diet matters: Maternal prebiotic intake in mice reduces anxiety and alters brain gene expression and the fecal microbiome in offspring. Brain Behav. Immun. 91, 230–244 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Valles-Colomer M., et al., The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4, 623–632 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Visconti A., et al., Interplay between the human gut microbiome and host metabolism. Nat. Commun. 10, 4505 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Margineanu M. B., et al., Gut microbiota modulates expression of genes involved in the astrocyte-neuron lactate shuttle in the hippocampus. Eur. Neuropsychopharmacol. 41, 152–159 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Rabinowitz J. D., Enerbäck S., Lactate: The ugly duckling of energy metabolism. Nat. Metab. 2, 566–571 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barros L. F., Metabolic signaling by lactate in the brain. Trends Neurosci. 36, 396–404 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Magistretti P. J., Allaman I., Lactate in the brain: From metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 19, 235–249 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Mosienko V., Teschemacher A. G., Kasparov S., Is L-lactate a novel signaling molecule in the brain? J. Cereb. Blood Flow Metab. 35, 1069–1075 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q., Jie W., Liu J. H., Yang J. M., Gao T. M., An astroglial basis of major depressive disorder? An overview. Glia 65, 1227–1250 (2017). [DOI] [PubMed] [Google Scholar]
- 36.McEwen B. S., Morrison J. H., The brain on stress: Vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 79, 16–29 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pederson B. A., et al., Exercise capacity of mice genetically lacking muscle glycogen synthase: In mice, muscle glycogen is not essential for exercise. J. Biol. Chem. 280, 17260–17265 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Oh S. L., et al., Effect of HX108-CS supplementation on exercise capacity and lactate accumulation after high-intensity exercise. J. Int. Soc. Sports Nutr. 10, 21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panajatovic M. V., et al., PGC-1α plays a pivotal role in simvastatin-induced exercise impairment in mice. Acta Physiol. (Oxf.) 228, e13402 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niu Y., et al., Pre-gestational intake of Lactobacillus helveticus NS8 has anxiolytic effects in adolescent Sprague Dawley offspring. Brain Behav. 10, e01714 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savignac H. M., et al., Prebiotic administration normalizes lipopolysaccharide (LPS)-induced anxiety and cortical 5-HT2A receptor and IL1- β levels in male mice. Brain Behav. Immun. 52, 120–131 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maeng S., et al., Cellular mechanisms underlying the antidepressant effects of ketamine: Role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol. Psychiatry 63, 349–352 (2008). [DOI] [PubMed] [Google Scholar]
- 43.C. A. Zarate, Jr, et al., A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 63, 856–864 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Berman R. M., et al., Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 47, 351–354 (2000). [DOI] [PubMed] [Google Scholar]
- 45.Moghaddam B., Adams B., Verma A., Daly D., Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 17, 2921–2927 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Razoux F., Garcia R., Léna I., Ketamine, at a dose that disrupts motor behavior and latent inhibition, enhances prefrontal cortex synaptic efficacy and glutamate release in the nucleus accumbens. Neuropsychopharmacology 32, 719–727 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Duman R. S., Aghajanian G. K., Sanacora G., Krystal J. H., Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med. 22, 238–249 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X., et al., The composition and concordance of lactobacillus populations of infant gut and the corresponding breast-milk and maternal gut. Front. Microbiol. 11, 597911 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karnib N., et al., Lactate is an antidepressant that mediates resilience to stress by modulating the hippocampal levels and activity of histone deacetylases. Neuropsychopharmacology 44, 1152–1162 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carrard A., et al., Peripheral administration of lactate produces antidepressant-like effects. Mol. Psychiatry 23, 488 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allaman I., Fiumelli H., Magistretti P. J., Martin J. L., Fluoxetine regulates the expression of neurotrophic/growth factors and glucose metabolism in astrocytes. Psychopharmacology (Berl.) 216, 75–84 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Pellerin L., Magistretti P. J., Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. U.S.A. 91, 10625–10629 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bélanger M., Allaman I., Magistretti P. J., Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metab. 14, 724–738 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Jourdain P., et al., Dual action of L-lactate on the activity of NR2B-containing NMDA receptors: From potentiation to neuroprotection. Sci. Rep. 8, 13472 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bolaños J. P., Bioenergetics and redox adaptations of astrocytes to neuronal activity. J. Neurochem. 139, 115–125 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacDonald K., et al., Biomarkers for major depressive and bipolar disorders using metabolomics: A systematic review. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 180, 122–137 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Bakian A. V., Huber R. S., Scholl L., Renshaw P. F., Kondo D., Dietary creatine intake and depression risk among U.S. adults. Transl. Psychiatry 10, 52 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kondo D. G., et al., Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: A 31-phosphorus magnetic resonance spectroscopy study. J. Affect. Disord. 135, 354–361 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harper D. G., et al., Tissue type-specific bioenergetic abnormalities in adults with major depression. Neuropsychopharmacology 42, 876–885 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Della F. P., et al., Tianeptine treatment induces antidepressive-like effects and alters BDNF and energy metabolism in the brain of rats. Behav. Brain Res. 233, 526–535 (2012). [DOI] [PubMed] [Google Scholar]
- 61.Song X., et al., Bioenergetics and abnormal functional connectivity in psychotic disorders. Mol. Psychiatry 26, 2483–2492 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stork C., Renshaw P. F., Mitochondrial dysfunction in bipolar disorder: Evidence from magnetic resonance spectroscopy research. Mol. Psychiatry 10, 900–919 (2005). [DOI] [PubMed] [Google Scholar]
- 63.Bramham C. R., Messaoudi E., BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 76, 99–125 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Castrén E., Rantamäki T., The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev. Neurobiol. 70, 289–297 (2010). [DOI] [PubMed] [Google Scholar]
- 65.Donahue R. J., Muschamp J. W., Russo S. J., Nestler E. J., Carlezon W. A., Jr, Effects of striatal ΔFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol. Psychiatry 76, 550–558 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rudolph M. D., et al., Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat. Neurosci. 21, 765–772 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frias A. E., et al., Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology 152, 2456–2464 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van der Burg J. W., et al., The role of systemic inflammation linking maternal BMI to neurodevelopment in children. Pediatr. Res. 79, 3–12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arpaia N., et al., Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ríos-Covián D., et al., Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 7, 185 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix. The raw metabolomics spreadsheet data have been deposited in the Oxford Research Archive (https://doi.org/10.5287/bodleian:ZOb4VbEqn).