Significance
The Hippo pathway plays critical roles in controlling cell proliferation, and its dysregulation is widely implicated in numerous human cancers. YAP, a Hippo signaling effector, often acts as a nexus and integrator for multiple prominent signaling networks. In this study, we discover NF-κB cross talk with the Hippo pathway and identify p65 as a critical regulator for YAP nuclear retention and transcriptional activity. Furthermore, we find that p65-induced YAP activation is essential for maintaining the proliferation of ATL cells in vitro and in vivo. Our findings unravel the functional interplay between NF-κB and YAP signaling and provide mechanistic insights into the YAP-dependent growth control pathway and tumorigenesis.
Keywords: HTLV-1, ATL, Tax, YAP, p65
Abstract
Adult T-cell leukemia/lymphoma (ATL) is an aggressive malignancy caused by human T-cell leukemia virus type 1 (HTLV-1) infection. HTLV-1 exerts its oncogenic functions by interacting with signaling pathways involved in cell proliferation and transformation. Dysregulation of the Hippo/YAP pathway is associated with multiple cancers, including virus-induced malignancies. In the present study, we observe that expression of YAP, which is the key effector of Hippo signaling, is elevated in ATL cells by the action of the HTLV-1 Tax protein. YAP transcriptional activity is remarkably enhanced in HTLV-1–infected cells and ATL patients. In addition, Tax activates the YAP protein via a mechanism involving the NF-κB/p65 pathway. As a mechanism for this cross talk between the Hippo and NF-κB pathways, we found that p65 abrogates the interaction between YAP and LATS1, leading to suppression of YAP phosphorylation, inhibition of ubiquitination-dependent degradation of YAP, and YAP nuclear accumulation. Finally, knockdown of YAP suppresses the proliferation of ATL cells in vitro and tumor formation in ATL-engrafted mice. Taken together, our results suggest that p65-induced YAP activation is essential for ATL pathogenesis and implicate YAP as a potential therapeutic target for ATL treatment.
Adult T-cell leukemia/lymphoma (ATL) is an aggressive T-cell malignancy caused by human T-cell leukemia virus type 1 (HTLV-1) (1, 2). HTLV-1 infection is associated with the clonal proliferation and transformation of T lymphocytes (1, 3). The oncogenic potential of HTLV-1 is attributed to the ability of viral genes to regulate cellular transcription factors and signaling pathways that are involved in cell growth, cell cycle progression, and apoptosis (4). The HTLV-1 genome encodes several structural and enzymatic proteins (gag, pol, and env) and regulatory and accessory proteins (Tax, Rex, HBZ, p12, p13, and p30) (5, 6). Among these, Tax was thought to play a pivotal role in the development of ATL because of its pleiotropic effects (7, 8). Indeed, Tax not only activates viral transcription but also deregulates a wide range of cell signaling pathways, leading to immortalization of T lymphocytes and tumor formation in vitro and in vivo (1, 7, 8).
Tax-mediated nuclear factor-κB (NF-κB) activation plays a central role in the initiation and maintenance of the malignant phenotype of ATL (9, 10). Tax was reported to interact with IKKγ and induce the phosphorylation of IKKα and IKKβ. Phosphorylated IKKα and IKKβ then trigger the proteasome-mediated degradation of IκB, leading to the release of p50/p65 from IκB and nuclear translocation of the activated p65 (10). In ATL, the activation of the NF-κB pathway stimulates the expression of cellular genes which are essential for cell proliferation (11). Evidence suggests that NF-κB signaling does not exist in isolation, and it closely coordinates with other signaling pathways to support the development of cancer (12). Further studies are ongoing to understand the complex interaction between NF-κB and other parallel signaling networks.
The Hippo tumor suppressor pathway, originally discovered in Drosophila, is an evolutionarily conserved pathway that regulates organ size, cell proliferation, and tumorigenesis (13, 14). In mammals, the Hippo pathway can be initiated by phosphorylation and activation of the MST1/2 kinases (15). Through forming a complex with SAV1, activated MST1/2 phosphorylates and activates the LATS1/2 kinases (16). Activated LATS1/2, in turn, phosphorylates the key factors of Hippo pathway, yes-associated protein (YAP), and transcriptional coactivator with PDZ binding (TAZ) (17). Further study indicates that LATS1/2 phosphorylates YAP at five sites (Ser61, Ser109, Ser127, Ser164, and Ser381) (18). The two residues in YAP most relevant to its function are Ser127 and Ser381 (17, 18). Phosphorylation of Ser127 in YAP by LATS1/2 induces its interaction with 14-3-3 proteins, resulting in cytoplasmic retention of YAP (17). Phosphorylation of YAP on Ser381 triggers its ubiquitination and subsequent proteasome-dependent degradation (18). Thus, activation of the Hippo pathway and phosphorylation of YAP prevent its activity in the nucleus. On the other hand, when the upstream kinases are repressed, hypophosphorylated YAP is localized to the nucleus where it acts as a transcriptional coactivator, interacting with the TEADs (TEAD1–4 transcription factors) and stimulating the expression of target genes associated with cell proliferation and resistance to apoptosis (19). Therefore, activation of the Hippo pathway suppresses cell proliferation. Unsurprisingly, dysregulation of the Hippo pathway and overexpression of YAP have been found to contribute to the development of many malignancies (13, 20).
Accumulating evidence shows that a variety of human viruses exert their carcinogenic function via the Hippo pathway (21). Known mechanisms of Hippo dysregulation in virus-infected cells include 1) up-regulation of YAP expression through activating its promoter (22–24), 2) inhibition of proteasome-dependent degradation of YAP protein (25, 26), 3) inhibition of YAP phosphorylation to increase nuclear localization (26, 27), and 4) suppression of the Hippo pathway through regulating the kinase activity of MST1/2 or LATS1/2 (28).
To date, whether the Hippo pathway plays a role in the genesis of HTLV-1–induced ATL has been unknown. In this study, we report that the Hippo tumor suppressor pathway is inhibited in ATL cells. HTLV-1 infection stimulates YAP activity in two ways: Tax increases the transcription of the YAP gene, and Tax activates NF-κB, which in turn activates the YAP protein. YAP activation supports the proliferation of ATL cells in vitro and in vivo. Thus, our study indicates that p65-mediated YAP activation is associated with the leukemogenesis of ATL.
Results
YAP Is Up-Regulated and Activated in ATL.
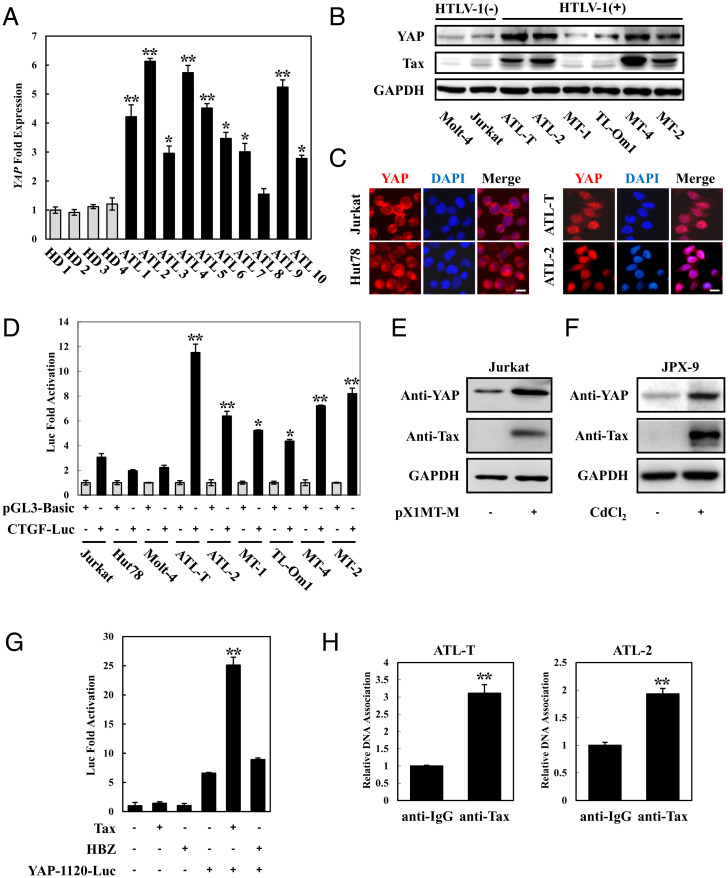
To determine the status of Hippo signaling in ATL, we first examined the expression of YAP, which is the core effector of the Hippo pathway, in primary T cells isolated from ATL patients. Compared with T cells from healthy subjects, primary ATL cells expressed high levels of YAP (Fig. 1A). Next, we examined the expression of YAP in HTLV-1–infected cell lines. Quantitative real-time PCR results demonstrated that YAP expression was up-regulated in many ATL cell lines (SI Appendix, Fig. S1A). Consistent with these results, we found that the protein levels of YAP were significantly elevated in HTLV-1–infected cell lines, relative to noninfected ones (Fig. 1B).
Fig. 1.
YAP is up-regulated and activated in ATL. (A) High expression of YAP in ATL patients. CD4-positive cells were isolated from PBMCs of healthy donors and ATL patients. qPCR was performed to analyze the expression of YAP. HD 1 to 4 indicates healthy donors, and ATL 1 to 10 indicates ATL patients. (B) YAP protein was up-regulated in ATL cell lines. Total protein was extracted from HTLV-1–negative and HTLV-1–positive cell lines and subjected to Western blot using YAP and Tax antibodies. (C) YAP localized in the nucleus of ATL cell lines. YAP subcellular localization was determined by immunofluorescence staining for YAP (red) and 4',6-diamidino-2-phenylindole (DAPI) for DNA (blue). (Scale bar, 10 μm.) (D) The Hippo pathway was suppressed in ATL cells. Cells were cotransfected with pGL3-Basic or CTGF-Luc together with phRL-TK. Forty-eight hours after transfection, cells were harvested and analyzed for luciferase activity. (E) HTLV-1 induced YAP expression. Jurkat cells were transfected with an infectious molecular clone of HTLV-1 (pX1MT-M). Forty-eight hours posttransfection, the expression of YAP was analyzed by immunoblotting. (F) YAP protein was up-regulated in Tax-expressing JPX-9 cells. JPX-9 cells were treated with 30 μmol/L of CdCl2 for 18 h. After incubation, the expression of Tax and YAP protein was examined by immunoblot analysis. (G) Tax enhanced YAP promoter activity. Jurkat cells were transfected with YAP reporter vector (YAP-1120-Luc) and Tax or HBZ expression plasmid. Luciferase activity was measured 48 h after transfection. (H) ChIP analysis of the association of endogenous Tax and YAP promoters in ATL cells (ATL-T and ATL-2). The statistical analyses were performed by unpaired two-tailed Student's t test. *P < 0.05, **P < 0.01.
Nuclear translocation is required for YAP to regulate its target gene expression (29). We next examined the subcellular localization of YAP protein in ATL cell lines. An immunofluorescence assay indicated that YAP was enriched in the nucleus of the HTLV-1–infected cell lines, ATL-T and ATL-2, while it was distributed in the cytoplasm in the control Jurkat and Hut78 cells (Fig. 1C). To further investigate the activation of YAP in ATL, we transfected the Hippo pathway reporter construct CTGF–Luc into HTLV-1 noninfected and infected cell lines. As shown in Fig. 1D, CTGF reporter activity was higher in all tested HTLV-1–infected cell lines compared to the HTLV-1–negative T cells. Analysis of the well-characterized YAP target genes’ expression revealed that the transcription of the CYR61 and CTGF genes was enhanced, and the expression of the RASSF4 and TIMP1 genes was suppressed in HTLV-1–positive cell lines, as would be consistent with the activation of YAP (SI Appendix, Fig. S1B).
Taken together, these results indicate that YAP was elevated and activated in ATL and in HTLV-1–infected cell lines.
HTLV-1 Tax Up-Regulates YAP Expression.
To investigate the mechanism of YAP up-regulation in ATL cells, we transfected an infectious molecular clone of HTLV-1 (pX1MT-M) into Jurkat cells. The result showed that overexpression of HTLV-1 up-regulated the level of YAP protein (Fig. 1E). It is well established that Tax and HBZ are the two viral genes that play a central role in the pathogenesis of HTLV-1. Thus, we next analyzed whether Tax or HBZ could control YAP expression. As shown in Fig. 1F and SI Appendix, Fig. S1C, the protein level of YAP was elevated in CdCl2-treated JPX-9 cells by enforced expression of Tax rather than CdCl2 treatment itself. In addition, we found that HBZ had no significant effect on YAP level in Jurkat cells (SI Appendix, Fig. S1D). Indeed, the levels of YAP correlated with the Tax expression in ATL cells (Fig. 1B).
To clarify the mechanism by which Tax enhanced the expression of YAP in ATL cells, we constructed a luciferase reporter that contains the putative promoter region of YAP (encompassing the −1003 to +117 region) and performed a luciferase assay. As shown in Fig. 1G, overexpression of Tax led to an increase of YAP-1120-luciferase expression in Jurkat cells, whereas HBZ had no such effect. In addition, we verified the binding of Tax to the YAP promoter using chromatin immunoprecipitation (ChIP) assays in ATL cell lines (Fig. 1H).
These results demonstrate that the enhanced expression of YAP in ATL can be attributed to the association of Tax with the YAP promoter.
Tax Stimulates YAP Activity via p65.
We next investigated whether the HTLV-1 encoded viral proteins Tax and HBZ can modulate YAP activity. As shown in Fig. 2A, Tax stimulated YAP-induced transcriptional activation in a dose-dependent manner. In contrast, HBZ did not have any positive or negative impact on YAP-induced luciferase expression (Fig. 2B and SI Appendix, Fig. S1E).
Fig. 2.
Tax activates YAP via p65. (A) Tax stimulated YAP transcriptional activation. Jurkat cells were cotransfected with 8×GTIIC–Luc (0.5 μg), phRL-TK (20 ng), and pCG-Tax (1 or 3 μg) with or without pCMV-FLAG-YAP (0.1 μg). Forty-eight hours after transfection, the cells were harvested and analyzed for luciferase activity. (B) HBZ had no effect on YAP activity. Jurkat cells were cotransfected with 8×GTIIC–Luc, phRL-TK, pCMV-FLAG-YAP, and pME18Sneo-HBZ. After 48 h, a dual-luciferase reporter assay was performed. (C) Analysis of Tax mutants for their effect on YAP-induced transcriptional activation. Jurkat cells were cotransfected with 8×GTIIC–Luc, phRL-TK, pCMV-FLAG-YAP, and pCG-Tax or its mutants. Luciferase activity was measured 48 h after transfection. (D) Tax activated YAP transcription depending on NF-κB. Jurkat cells were cotransfected with 8×GTIIC–Luc, phRL-TK, pCMV-FLAG-YAP, pCG-Tax, and expression plasmid for IκB (S32A/S36A) or ACREB. At 48 h after transfection, cell lysates were subjected to luciferase assay. (E) p65 enhanced YAP activity. Jurkat cells were cotransfected with 8×GTIIC–Luc, phRL-TK, pCMV-FLAG-YAP, and pSG-p65. At 48 h after transfection, the cells were harvested, and luciferase activity was measured. The statistical analyses were performed by unpaired two-tailed Student's t test. *P < 0.05, **P < 0.01.
To further elucidate the mechanism by which Tax enhances the YAP transcriptional response, we employed two Tax mutants: Tax M47, which can activate NF-κB but not the CREB/ATF pathway, and Tax M22, which is capable of activating CREB/ATF but not NF-κB signaling (30). Luciferase assays demonstrated that Tax M47 significantly enhanced YAP-mediated transcriptional activation, whereas Tax M22 was ineffective at doing so (Fig. 2C and SI Appendix, Fig. S1E). In addition, we found that the activation effect of Tax on YAP was suppressed by a cotransfected dominant negative mutant of IκB (S32A/S36A), whereas ACREB, which is a dominant negative inhibitor of CREB, had no effect on these responses (Fig. 2D). These observations indicate that Tax stimulates YAP activity via the NF-κB pathway. Next, we cotransfected the expression vectors of YAP and p65 into Jurkat cells. Fig. 2E demonstrated that the expression of p65 can enhance YAP-dependent transcription.
Overall, these results indicate that Tax-mediated activation of NF-κB pathway is responsible for the YAP activation in ATL.
p65 Interacts with YAP and Enhances Its Activity.
By what mechanism does p65 activate YAP? Accumulating evidence shows that the interaction between cellular factors and the main components of the Hippo pathway may affect their subcellular localization and modulate transcription activation (14). Therefore, we next explored whether p65 can physically interact with Hippo-associated proteins. YAP, LATS1, MST1, 14-3-3, and p65 expression plasmids were cotransfected into 293T cells. As shown in Fig. 3A and SI Appendix, Fig. S2A , p65 interacted with YAP, and to a lesser extent with MST1 and 14-3-3, but could not bind to LATS1. Moreover, this YAP–p65 interaction was also confirmed to occur endogenously in the ATL cell lines, ATL-T and ATL-2 (Fig. 3B and SI Appendix, Fig. S2B). The association between YAP and p65 was further analyzed by confocal microscopy. As shown in Fig. 3C, the YAP and p65 protein colocalized well in the nucleus.
Fig. 3.
p65 interacts with YAP to enhance YAP activity. (A) p65 interacted with YAP but not LAST1. The 293T cells were transfected with the indicated expression vectors. Cell lysates were subjected to immunoprecipitation (IP) using anti-FLAG followed by immunoblotting (IB) using anti-HA. The expression levels of YAP, p65, and LATS1 were detected. (B) YAP interacted with p65 endogenously. Whole-cell lysate of ATL-T cells was subjected to immunoprecipitation with anti-YAP or control IgG, and immunoprecipitates were probed with anti-p65 antibody. The band of p65 which coimmunoprecipitated with YAP is indicated with an asterisk. (C) YAP colocalizated with p65 in the cell nucleus. The 293T cells were transfected with pCMV-FLAG-YAP together with (v–vii) or without (i and ii) pcDNA-MycHis-p65. p65 was detected using anti-MYC Cy3 antibody (iii and vi). YAP was detected using anti–FLAG-biotin and secondary Streptavidin-Alexa 488 antibody (i and v). The overlay of YAP and p65 is shown (vii). DAPI (4,6 diamidino-2-phenylindole) was used to counterstain the nucleus (ii and iv). (Scale bar, 10 μm.) (D) Rel domain of p65 was responsible for interacting with YAP. (Top) Schema of p65 deletion mutants. NLS, nuclear localization signal. (Bottom) The 293T cells were transfected with HA-YAP and full-length or mutant FLAG-p65. Forty-eight hours after transfection, total cell lysates were subjected to immunoprecipitation using anti-FLAG followed by IB using anti-HA. (E) Analysis of p65 deletion mutants for their effect on YAP-mediated signaling. Jurkat cells were cotransfected with 8×GTIIC–Luc, phRL-TK, pCMV-FLAG-YAP, and pCMV-FLAG-p65 mutants. Luciferase activity was measured 48 h after transfection. (F) Analysis of p65 mutants for their effect on NF-κB activation. Jurkat cells were cotransfected with κB-Luc, phRL-TK, and full-length or FLAG-p65 mutants. Luciferase activity was measured 48 h after transfection. The statistical analyses were performed by unpaired two-tailed Student's t test. *P < 0.05, **P < 0.01.
We next evaluated the p65 deletion mutants shown in Fig. 3D to determine which region of p65 is responsible for interacting with YAP. Immunoprecipitation assays indicated that wild-type p65 and mutant p65 (1–320) interacted with YAP, while the p65 (313–551) mutant that lacks the Rel homology domain could not bind to YAP (Fig. 3D). Consistent with this observation, a luciferase assay demonstrated that p65 (1–320) could enhance YAP-induced reporter activity about as well as wild-type p65 could, whereas p65 (313–551) lost the transcriptional activation function (Fig. 3E). Neither of the p65 mutants could activate the NF-κB pathway (Fig. 3F). This observation suggests that the Rel homology domain of p65 is essential for activating YAP.
To identify the region of YAP responsible for the binding with p65, we tested the ability of a series of FLAG-tagged truncated YAP proteins to interact with p65 (SI Appendix, Fig. S3A). As shown in SI Appendix, Fig. S3B, YAP mutants without the TAD and WW domains could not bind to p65, indicating that the interaction with p65 is mediated by the TAD segment of YAP.
p65 Inhibits Ubiquitination-Dependent Degradation of YAP and Induces YAP Nuclear Translocation.
In the Hippo signaling pathway, the LATS1 tumor suppressor binds to and further phosphorylates YAP, resulting in the inactivation of YAP (31). The observations shown in Fig. 3 prompted us to ask whether p65 influenced YAP/LATS1 complex formation because p65 interacted with YAP. The 293T cells were transfected with the expression vector of YAP and LATS1 with or without p65. As shown in Fig. 4A, p65 protein inhibited the interaction between YAP and LATS1. As phosphorylation at the serine 127 and 381 sites of YAP by LATS are key events for YAP inhibition, we next performed experiments to determine the effect of p65 on YAP phosphorylation. The results showed that enforced expression of p65 suppressed the phosphorylation of YAP protein at its Ser127 and Ser381 sites, and this inhibition was dose-dependent (Fig. 4B). In addition, the phosphorylation of YAP in ATL-T cells was up-regulated by treatment with the p65 inhibitor SN50 (Fig. 4C).
Fig. 4.
p65 inhibits YAP degradation and induces YAP nuclear localization. (A) p65 suppressed the interaction between YAP and LATS1. The 293T cells were cotransfected with FLAG-YAP, HA-LATS1, and HA-p65. Cell lysates were subjected to immunoprecipitation (IP) using anti-FLAG followed by immunoblotting (IB) with anti-HA. (B) p65 inhibited YAP phosphorylation. The 293T cells were transfected with FLAG-YAP and increasing amounts of HA-p65. After 48 h, the cell lysates were subjected to immunoblotting using YAP phosphorylation antibodies. (C) NF-κB contributed to the activation of YAP in ATL. After the treatment of ATL-T cells with SN50, cell lysates were subjected to Western blot using the indicated antibodies. (D) p65 inhibited the YAP/14-3-3 interaction. The 293T cells were cotransfected with FLAG-YAP, HA-p65, and Myc-14-3-3. Cell lysates were subjected to immunoprecipitation using anti-FLAG followed by immunoblotting with anti-Myc. (E) p65 induced YAP nuclear translocation. The 293T cells were transfected with pCMV-FLAG-YAP together with or without pcDNA-MycHis-p65. YAP was detected using anti-FLAG-biotin and secondary Streptavidin-Alexa 488 antibody. (Scale bar, 10 μm.) (F) SN50 treatment inhibited nuclear localization of YAP protein. After treating the ATL-T cells with SN50, cells were fixed and subjected to immunofluorescence analysis to determine the localization of YAP protein. (Scale bar, 10 μm.) (G) p65 could not inhibit the YAP-S127D/14-3-3 interaction. The 293T cells were cotransfected with FLAG-YAP-S127D, HA-p65, and Myc-14-3-3. Cell lysates were subjected to immunoprecipitation using anti-FLAG followed by immunoblotting with anti-Myc. (H) p65 inhibited YAP degradation. The 293T cells were transfected with FLAG-YAP and HA-p65 and then subjected to determination of YAP protein degradation in the presence of CHX (50 μg/mL) by Western blot. (I) p65 suppressed polyubiquitination of YAP. The 293T cells were transfected with FLAG-YAP, FLAG-YAP-S381D, and HA-ubiquitin, together with or without pSG-p65. After 24 h, cells were treated with MG132 for 12 h. Cell lysates were subjected to IP using anti-FLAG followed by IB using anti-HA.
It has been reported that phosphorylation of YAP at Ser127 induces its binding with 14-3-3 leading to its cytoplasmic sequestration, while phosphorylation on YAP Ser381 triggers its ubiquitination and degradation (17, 18). We therefore investigated whether the p65-induced down-regulation of YAP phosphorylation at Ser127 could influence the interaction between YAP and 14-3-3. As shown in Fig. 4D, overexpression of p65 suppressed YAP/14-3-3 complex formation. An immunofluorescence assay indicated that YAP was largely localized in the cytoplasm of control 293T cells. When p65 protein was coexpressed with YAP, YAP was enriched in the nucleus, indicating that p65 caused the localization of YAP to shift from the cytoplasm to the nucleus (Fig. 4E). Treatment of ATL-T cells with SN50, an inhibitor of p65, significantly changed the location of YAP protein from the nucleus to the cytoplasm (Fig. 4F). However, p65 could not inhibit the interaction between 14-3-3 and the YAP-S127D mutant, which is a phosphorylation mimic form of YAP (Fig. 4G).
To examine the effect of p65 on YAP degradation, we tested YAP protein stability by CHX chase experiment. As shown in Fig. 4H, FLAG-YAP protein expressed in 293T cells was degraded quickly. Enforced expression of p65 dramatically stabilized YAP protein (Fig. 4H). Protein ubiquitination is a crucial modification that induces degradation of YAP proteins in the cytoplasm. We therefore studied whether p65 could inhibit the ubiquitination of YAP. The result shows that YAP immunoprecipitated from MG132-treated HA-Ub–expressing 293T cells exhibited incorporation of ubiquitin. When coexpressed with p65, the polyubiquitination of YAP was abrogated (Fig. 4I). This p65-induced suppression of K48-linked ubiquitination of YAP was abrogated by replenishment with Ub-K48R (SI Appendix, Fig. S4). p65 could not inhibit ubiquitination of the YAP-S381D mutant (the YAP phosphorylation mimic) (Fig. 4I). Collectively, these results demonstrate that p65 stimulates YAP activity by inhibiting its degradation and by localizing it to the nucleus.
Having shown that Tax activates YAP via a mechanism involving p65 and that p65 activates YAP by binding to it, we next investigated whether Tax affected the binding of p65 to YAP. SI Appendix, Fig. S5 A and B demonstrated that Tax binds to YAP and enhances the interaction between YAP and p65. Moreover, Tax inhibits the phosphorylation of YAP protein through the NF-κB/p65 pathway (SI Appendix, Fig. S5C). These observations suggest that the Tax/p65/YAP axis might be essential in the dysregulation of the Hippo pathway in ATL.
YAP Promotes Proliferation of ATL Cells In Vitro and In Vivo.
Finally, to determine the physiological significance of the up-regulated YAP expression and activity in ATL cells, we investigated whether YAP plays a role in ATL cell growth or tumorigenesis. As shown in Fig. 5A, knockdown of YAP by short hairpin RNA (shRNA) in ATL-T and ATL-2 cells led to a 60% reduction of the expression levels of YAP protein. MTT (3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide) assays indicated that suppression of YAP significantly inhibited proliferation of HTLV-1–infected cell lines (ATL-T and ATL-2) but not an HTLV-1–noninfected cell line (Jurkat) (Fig. 5B and SI Appendix, Fig. S6A). In addition, the colony-forming ability of ATL-T and ATL-2 cells was strongly suppressed when YAP was depleted (Fig. 5C and SI Appendix, Fig. S6B). To further investigate the effect of YAP, we analyzed the expression of Hippo target genes in YAP knockdown cells. As shown in SI Appendix, Fig. S6C, YAP silencing resulted in a down-regulation of CYR61, CTGF, and NRP1 genes and up-regulation of RASSF4 and TIMP1 genes in ATL cells, indicating that YAP might support the proliferation of ATL cells through modulating the expression of its target genes involved in proliferation and apoptosis.
Fig. 5.
YAP supports proliferation of ATL cells in vitro and in vivo. (A) Lentivirus-based shRNA system was used to stably knockdown YAP in ATL-T and ATL-2 cells. The knockdown efficiency was confirmed by Western blot. (B) Knockdown of YAP suppressed ATL cell proliferation. ATL-T and ATL-2 cells were transfected with a recombinant lentivirus expressing the pLKO-shYAP. After puromycin selection, cell proliferation was detected by MTT assay. (C) Effect of YAP shRNAs on proliferation of ATL-T cells was determined by colony-formation assay. (D) YAP was important for tumorigenesis of ATL cells. YAP knockdown ATL-T cells or negative cells were injected into the NCG mice (n = 8). Three weeks after injection, all mice were killed. Photographs of dissected tumors from NCG mice. (E) Diagram of average volumes and weight of tumors from ATL-T-shYAP injected NCG mice. (F) Overexpression of p65 rescued cell proliferation inhibition by YAP silencing. ATL-T cells were transfected with a recombinant lentivirus expressing the pLKO-shYAP plasmid together with pCSII-CMV-p65 or pCSII-CMV-p65 (1-320). After puromycin selection, cell proliferation was determined by MTT assay. (G) Schematic model that illustrates Tax/p65/YAP signaling axis in ATL. In normal T cells, phosphorylation of YAP by MST-LATS cascade induces cytoplasmic retention of YAP and triggers ubiquitination-dependent degradation of YAP. In ATL, Tax-induced p65 activation abrogated YAP-LATS1 interaction, leading to the suppression of YAP phosphorylation. It results in the inhibition of YAP degradation and induction of YAP nuclear translocalization. Hyperactivated YAP supports the proliferation of ATL cells. The statistical analyses were performed by unpaired two-tailed Student's t test. *P < 0.05, **P < 0.01.
We then investigated if the activation of YAP contributed to the tumorigenesis of ATL in vivo. An equivalent number of control and YAP knockdown ATL-T or ATL-2 cells were injected into NCG mice, and tumor growth was analyzed. Consistent with the in vitro results, silencing YAP expression attenuated the expansion of ATL cells in this xenograft mouse model, as shown by both tumor size and weight (Fig. 5 D and E and SI Appendix, Fig. S6 D and E). Moreover, the expression of YAP and its target genes CYR61 and NRP1 was down-regulated in the tumor nodules originating from YAP knockdown cells compared to nodules originating from negative control cells (SI Appendix, Fig. S6F).
Furthermore, we tested whether p65 contributes to the oncogenic functions of YAP. As shown in Fig. 5F and SI Appendix, Fig. S7, decreased cell proliferation ability induced by knockdown of YAP was partially rescued by wild-type p65 or the p65 (1-320) mutant which can interact with YAP.
Overall, we conclude that YAP enhances the proliferation of ATL cells in vitro and in vivo. Tax activates the transcription of YAP, and p65 activates the YAP protein by binding to YAP and inhibiting its interaction with LATS1. Thus, p65 suppresses YAP phosphorylation, resulting in the inhibition of ubiquitination-dependent degradation of YAP and permitting YAP nuclear translocation (Fig. 5G).
Discussion
Accumulating evidence shows that the Hippo/YAP signaling pathway plays critical roles in a wide range of biological processes, including cell proliferation, apoptosis, and tumorigenesis (13, 14, 20). Dysregulation of YAP protein, the key factor in the Hippo pathway, has been implicated in viral transformation (21). Examples include hepatitis B virus (HBV), hepatitis C virus (HCV), human papillomavirus (HPV), Epstein–Barr virus, and Kaposi sarcoma-associated herpesvirus (21). The expression level of YAP is remarkably elevated in HBV-infected hepatocellular carcinoma (HCC) cells (22). Knockdown of YAP/TAZ abolishes the proliferation of HCC cells induced by viral HBx, HBXIP, and preS2 proteins, indicating that HBV contributes to the growth of hepatoma cells through dysregulating the Hippo pathway (22, 23, 32). In the case of HCV infection, HCV NS4B protein suppresses the Hippo pathway, resulting in epithelial–mesenchymal transition (EMT) and HCC development through activation of the PI3K/AKT pathway (33). After HPV infection, the E6 viral protein prevents the proteasome-dependent degradation of YAP and induces YAP nuclear localization. Activation of YAP maintains the proliferation of HPV-infected cervical cancer cells and facilitates HPV infection (25, 34). These findings indicate that modulation of the Hippo pathway is common among different viruses, suggesting that these activities are important for viral oncogenesis. This study addresses the regulatory role of the Hippo pathway in ATL. Moreover, all reported instances of virus-induced dysregulation of Hippo signaling so far have been due to direct regulation by viral proteins (21). In this study, we demonstrate that HTLV-1 employs a unique mechanism to enhance YAP-mediated transcriptional activation through the NF-κB pathway.
Extensive research on signal transduction reveals that a single pathway cannot operate independently and that pathways are small parts of integrated networks within the cell. Several studies have demonstrated that the Hippo pathway activity is dysregulated through cross talk with multiple cancer-associated signaling pathways, such as the MAPK, Wnt, TGFβ/BMP, GPCR, PI3K-mTOR, Notch, Hedgehog, Mevalonate, Androgen Receptor (AR), and Hypoxia pathways (13, 35–38). Moreover, YAP/TAZ often acts as a nexus and integrator for multiple prominent signaling networks (38). Recently, several studies demonstrated that the Hippo/YAP pathway can activate or suppress the effects of the NF-κB pathway on gene transcription. Gao et al. reported that TNFα or macrophage conditional medium (CM) treatment increases the interaction between p65 and YAP. YAP/TEAD and p65 proteins then synergistically regulate the transcription of hexokinase 2 (HK2) (39). Another study showed that YAP is necessary to attenuate osteoarthritis progression by inhibiting inflammatory responses triggered by the NF-κB pathway (40). In soft tissue sarcomas, YAP was found to suppress the expression of ubiquitin-specific peptidase 31 (USP31), a negative regulator of NF-κB signaling, leading to the promotion of sarcomagenesis (41). However, to date there has been no evidence to show that the NF-κB pathway could regulate Hippo signaling directly. Our study reports experimental evidence of such a direct mechanism of cross talk between these two pathways.
NF-κB is a major regulator of cell proliferation and is involved in tumorigenesis. It is possible that the NF-κB pathway exerts some of its prosurvival effects in HTLV-1–infected cells through cross talk with Hippo signaling. Characterization of the mechanism(s) of cross talk between these two signaling pathways can add new possibilities for the development of anti-ATL therapies. Through the elucidation of the crystal structures of the p65/YAP protein complexes, we can design novel small molecular inhibitors to block the interaction between YAP and p65 and to interfere with the resulting signal transduction cascades.
The NF-κB signaling pathway is persistently activated in HTLV-1–infected T cells and freshly isolated ATL cells (11). Constitutively activated NF-κB contributes to the abnormal proliferation and genetic changes of ATL cells and thus drives the progression of ATL (9). It is well established that Tax acts as an oncogenic mediator of HTLV-1 and induces cell immortalization and transformation mainly through activating the NF-κB pathway (10). However, in 60% of ATL cases, ATL cells have lost Tax expression because of genetic and epigenetic changes in the HTLV-1 provirus and subsequent selection by immune pressure. In such ATL cells lacking functional Tax, other viral and/or cellular factors may also be essential for the maintenance and/or progression of ATL (1). NF-κB is constitutively activated even in ATL cells without Tax expression by several mechanisms (42, 43). In this study, we demonstrate that p65-mediated NF-κB signaling dysregulates the Hippo pathway and activates YAP activity, resulting in the maintenance of ATL cells’ proliferation. Thus, we have identified abnormally regulated Hippo/YAP signaling as a mechanism for initiating and/or maintaining ATL regardless of Tax.
Accumulating evidence shows that HBZ counteracts Tax in many signaling pathways, such as NF-κB, AP-1, TGF-β, and Wnt (6). However, we found in this study that while Tax is an activator of YAP via p65, HBZ has no opposite effect on the Hippo pathway. In addition, HBZ selectively inhibits cell senescence induced by a hyperactivated NF-κB pathway, while HBZ does not influence NF-κB triggered cell proliferation (44). Thus, the YAP protein is constitutively activated in ATL cells and supports their proliferation.
The Hippo pathway exerts a significant impact on tumorigenesis due to its regulation not only of cell proliferation but also of DNA damage-induced apoptosis, EMT, and both innate and adaptive immunity (13, 20, 45). It was reported that YAP could enhance the expression of Bcl-2 family members in cancer cell lines, thus suppressing mitochondrial induced apoptosis (46). Overexpression of YAP induces resistance to the apoptosis cascade induced by tumor necrosis factor alpha (TNF-α), FAS, and chemotherapeutic agents (47). Additionally, YAP can trigger the transition of normal epithelial cells into metastatic cells via EMT, which is a key step for tumor metastasis (48). In HTLV-1–infected cells, Tax engages two signaling pathways, AKT and NF-κB, to inhibit apoptosis (1). However, as mentioned above, in >60% of ATL patients the leukemic cells contain mutations that silence the Tax gene. The absence of Tax in these cells implicates other cellular factors or signaling pathways in the suppression of apoptosis in ATL. We present evidence that YAP is overexpressed and hyperactivated after HTLV-1 infection. Moreover, Hippo pathway target genes associated with cellular apoptosis, such as RASSF4 and BCL2, are abnormally expressed in ATL cells. As mentioned above, YAP may remain activated in ATL cells even in the absence of Tax. Thus, the constitutive activation of YAP may explain how apoptosis is quelled in the absence of Tax. Recently, emerging evidence has shown that the Hippo pathway also participates in the immune response, acting as a negative regulator of innate immunity against human viruses (21, 45). As shown in SI Appendix, Fig. S8, YAP protein indeed represses IFN-β innate immune signaling in ATL cells. We suggest that HTLV-1 might facilitate its escape from the host immune system and maintain cell survival via Hippo/YAP signaling. Further research is needed to fully elucidate the cellular functions and mechanisms of the Hippo pathway in HTLV-1 infection and ATL development.
In conclusion, we have shown that HTLV-1 infection dysregulates the Hippo signaling pathway and induces the activation of YAP through two mechanisms: Tax-mediated stimulation of YAP gene transcription and Tax-mediated NF-κB activation of the YAP protein. The activated YAP supports the proliferation of ATL cells in vitro and in vivo. HTLV-1 may take advantage of these mechanisms to allow infected cells to proliferate.
Materials and Methods
Cell Culture.
HTLV-1 transformed human T-cell lines (MT-1, MT-2, MT-4, ATL-2, ATL-T, and TL-Om1), HTLV-1–negative human T-cell lines (Jurkat, Hut78, and Molt-4), and JPX-9 (a modified Jurkat line that expresses Tax under a metallothionein promoter) were grown in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and antibiotics. Human embryonic kidney cell line 293T was cultured in Dulbecco’s Modified Eagle medium supplemented with 10% FBS and antibiotics.
Clinical Samples.
Peripheral blood mononuclear cells (PBMCs) were isolated from ATL patients (n = 10) and healthy volunteers (n = 4). CD4+ T cells were enriched by human naive CD4 T-cell enrichment set (BD Biosciences). Clinical samples were obtained and used according to the principles expressed in the Declaration of Helsinki, and the experiments were approved by the Ethics Committee of Zhejiang Normal University and Kyoto University (approval no. RT2019001). All ATL patients and healthy individuals provided written informed consent for the collection of samples and subsequent analysis.
Plasmids and Reagents.
Expression vectors for YAP, LATS1, 14-3-3, MST1, and CTGF luciferase reporter construct (CTGF-Luc) were kindly given by Bin Zhao of Zhejiang University, Hangzhou, China. Expression plasmids for Tax, Tax deletion mutants, HBZ, p65, p65 deletion mutants, pX1MT-M, IκB (S32A/S36A), ACREB, and phRL-TK were prepared as previously described (49). The Ubiquitin and its mutant plasmids (HA-Ub, HA-Ub-K48, and HA-Ub-K48R) were a gift from Hong Zhu of Zhejiang University, Hangzhou, China. Expression vectors for YAP deletion and point mutants were generated by PCR. The YAP promoter was cloned into the pGL4.1 basic vector. The 8×GTIIC luciferase reporter was purchased from Addgene (no. 34615). SN50, CdCl2, MG132, puromycin, and chlorhexidine (CHX) were purchased from Millipore.
Quantitative and Semiquantitative PCR.
Total RNA was extracted using TRIzol Reagent (Thermo Fisher Scientific) as previously described (42). Reverse transcription was performed using the SuperScript III first-strand synthesis system (Life Technologies). Real-time qPCR was carried out using Power SYBR Green PCR Master Mix and StepOnePlus Real-Time PCR System (Thermo Fisher Scientific). Semiquantitative PCR was performed as previously described (42). Primers used in this study are shown in SI Appendix, Table 1.
Luciferase Assay.
Jurkat T cells were seeded on 12-well plates at 1.0 × 105 cells per well. After 24 h, cells were transfected with luciferase reporter plasmid together with the indicated expression plasmids using Lipofectamine LTX with Plus Reagent (Thermo Fisher Scientific). Forty-eight hours later, the luciferase assay was performed using the Dual-Luciferase Reporter Assay System (Promega). Luciferase values were normalized to Renilla activity, and the data represent the mean and SD of a triplicate set of experiments.
Immunoprecipitation and Immunoblotting.
The 293T cells were transfected with the indicated plasmids using Lipofectamine 2000 Reagent (Thermo Fisher Scientific). After 48 h, cells were collected, and immunoprecipitation and Western blot assay were performed as previously described (50). For the endogenous interaction between YAP and p65, ATL cell lysate was incubated with anti-YAP antibody (Cell Signaling Technology) or anti-mouse immunoglobulin G (IgG) (Santa Cruz Biotechnology), and the following procedures were the same as described above in 293T cells. Other antibodies used were as follows: anti-FLAG M2, anti-HA, anti–c-Myc (Sigma-Aldrich), anti-p65, anti–phospho-YAP (Ser127), anti–phospho-YAP (Ser381), anti-GAPDH (Cell Signaling Technology), and anti-Tax was used as previously described (51).
Knockdown.
Knockdown of YAP in HTLV-1–infected cells lines was performed with a lentiviral vector pLKO.1-based shRNA system (Open Biosystems) as described previously (42). The shRNA sequence specifically targeting YAP was shYAP#1, 5′-CTGGTCAGAGATACTTCTTAA-3′; and shYAP#2, 5′-AAGCTTTGAGTTCTGACATCC-3′.
Cell Proliferation Assay.
Cells were seeded in 96-well plates, and at indicated time points, 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to access the proliferation of cells as described previously (52). For colony formation assay, ATL cells were placed into each well of six-well plates and kept in complete medium for 2 wk. Colonies were fixed with methanol and stained with methylene blue.
Immunofluorescence Analysis.
The 293T cells were transfected with expression vectors using Lipofectamine 2000 (Thermo Fisher Scientific). Forty-eight hours after transfection, cells were fixed with 4% paraformaldehyde and then permeabilized with 0.2% Triton X-100. After blocking by 5% BSA, p65 protein was detected using anti–c-MYC Cy3 antibody (Sigma-Aldrich). YAP was detected using anti–FLAG-biotin (Sigma-Aldrich) and secondary Streptavidin-Alexa 488 antibody (Thermo Fisher Scientific). Fluorescence was observed under a fluorescence confocal microscopy (Leica TCS SP5 AOBS). For the detection of YAP subcellular localization in ATL cells, YAP protein was stained using anti-YAP and secondary Cy3-conjugated Goat anti-mouse IgG antibody (Sigma-Aldrich).
ChIP Assay.
The 293T cells were transfected with the indicated expression vectors together with reporter plasmid. Forty-eight hours after transfection, a ChIP assay was done according to the manufacturer's protocol (Upstate Biotechnology) as reported previously (42). Precipitated DNA was purified and amplified by real-time PCR using primers specific for the YAP promoter. All primers are listed in SI Appendix, Table 1.
In Vivo Tumorigenicity Assay.
ATL cells were harvested and resuspended with sterile phosphate-buffered saline; 4 × 106 cells were injected into the either side of the flank area of female NCG mice (NOD-Prkdcem26Cd52Il2rgem26Cd22/Nju, NOD/ShiLtJNju based immune-deficient mice) (Model Animal Research Center of Nanjing University). Three weeks after injection, all mice were killed. Tumor weights and volumes were determined in mice from the shYAP and control groups as described (42). The expression of YAP and its target genes were determined by real-time PCR. All animals used in this study were maintained and handled according to protocols approved by Zhejiang Normal University (approval no. DW2019001).
Statistical Analyses.
Each experiment was repeated at least three times. The statistical significance of differences between different groups was determined using the unpaired two-tailed Student’s t test. Significance was assumed for *P < 0.05 and **P < 0.01.
Supplementary Material
Acknowledgments
We thank Dr. Kun-Liang Guan (University of California San Diego) and Dr. Guangyong Ma (China Pharmaceutical University) for providing plasmids and for helpful discussion. We also thank Dr. Linda Kingsbury for proofreading. This work was supported by a grant from the National Natural Science Foundation of China to T.Z. (Grant 31970173) and a grant from the Natural Science Foundation of Zhejiang Province to T.Z. (Grant LY21C010001). This study was also supported by the Project for Cancer Research and Therapeutic Evolution (P-CREATE) to M.M. (Grant 20cm0106306h0005), the Research Program on Emerging and Re-emerging Infectious Diseases to M.M. (Grant 20fk0108088h0002) from the Japan Agency for Medical Research and Development (AMED), and Japan Society for the Promotion of Science (JSPS) KAKENHI to M.M. (Grant 19H03689).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2115316119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Matsuoka M., Jeang K. T., Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat. Rev. Cancer 7, 270–280 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Watanabe T., Adult T-cell leukemia: Molecular basis for clonal expansion and transformation of HTLV-1-infected T cells. Blood 129, 1071–1081 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangham C. R. M., Matsuoka M., Human T-cell leukaemia virus type 1: Parasitism and pathogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372, 20160272 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall W. W., Fujii M., Deregulation of cell-signaling pathways in HTLV-1 infection. Oncogene 24, 5965–5975 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Nicot C., Harrod R. L., Ciminale V., Franchini G., Human T-cell leukemia/lymphoma virus type 1 nonstructural genes and their functions. Oncogene 24, 6026–6034 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Giam C. Z., Semmes O. J., HTLV-1 infection and adult T-cell leukemia/lymphoma—A tale of two proteins: Tax and HBZ. Viruses 8, E161 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boxus M., et al. , The HTLV-1 Tax interactome. Retrovirology 5, 76 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohanty S., Harhaj E. W., Mechanisms of oncogenesis by HTLV-1 Tax. Pathogens 9, E543 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harhaj E. W., Giam C. Z., NF-κB signaling mechanisms in HTLV-1-induced adult T-cell leukemia/lymphoma. FEBS J. 285, 3324–3336 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kfoury Y., et al. , The multifaceted oncoprotein Tax: Subcellular localization, posttranslational modifications, and NF-κB activation. Adv. Cancer Res. 113, 85–120 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Qu Z., Xiao G., Human T-cell lymphotropic virus: A model of NF-κB-associated tumorigenesis. Viruses 3, 714–749 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oeckinghaus A., Hayden M. S., Ghosh S., Crosstalk in NF-κB signaling pathways. Nat. Immunol. 12, 695–708 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Harvey K. F., Zhang X., Thomas D. M., The Hippo pathway and human cancer. Nat. Rev. Cancer 13, 246–257 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Yu F. X., Zhao B., Guan K. L., Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 163, 811–828 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng Z., Moroishi T., Guan K. L., Mechanisms of Hippo pathway regulation. Genes Dev. 30, 1–17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan E. H., et al. , The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene 24, 2076–2086 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Zhao B., et al. , Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao B., Li L., Tumaneng K., Wang C. Y., Guan K. L., A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 24, 72–85 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein C., et al. , YAP1 exerts its transcriptional control via TEAD-mediated activation of enhancers. PLoS Genet. 11, e1005465 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panciera T., Azzolin L., Cordenonsi M., Piccolo S., Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 18, 758–770 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z., et al. , The Hippo pathway and viral infections. Front. Microbiol. 10, 3033 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang T., et al. , Hepatitis B virus X protein modulates oncogene Yes-associated protein by CREB to promote growth of hepatoma cells. Hepatology 56, 2051–2059 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Wang Y., et al. , The oncoprotein HBXIP up-regulates YAP through activation of transcription factor c-Myb to promote growth of liver cancer. Cancer Lett. 385, 234–242 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Seo H. M., et al. , Expression of YAP and TAZ in molluscum contagiosum virus infected skin. Br. J. Dermatol. 179, 188–189 (2018). [DOI] [PubMed] [Google Scholar]
- 25.He C., et al. , The Hippo/YAP pathway interacts with EGFR signaling and HPV oncoproteins to regulate cervical cancer progression. EMBO Mol. Med. 7, 1426–1449 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang J. H., et al. , Polyomavirus small T antigen interacts with yes-associated protein to regulate cell survival and differentiation. J. Virol. 88, 12055–12064 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu G., et al. , Kaposi sarcoma-associated herpesvirus promotes tumorigenesis by modulating the Hippo pathway. Oncogene 34, 3536–3546 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He J., et al. , Positive regulation of TAZ expression by EBV-LMP1 contributes to cell proliferation and epithelial-mesenchymal transition in nasopharyngeal carcinoma. Oncotarget 8, 52333–52344 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Totaro A., Panciera T., Piccolo S., YAP/TAZ upstream signals and downstream responses. Nat. Cell Biol. 20, 888–899 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith M. R., Greene W. C., Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 4, 1875–1885 (1990). [DOI] [PubMed] [Google Scholar]
- 31.Zhang J., Smolen G. A., Haber D. A., Negative regulation of YAP by LATS1 underscores evolutionary conservation of the Drosophila Hippo pathway. Cancer Res. 68, 2789–2794 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Liu P., et al. , HBV preS2 promotes the expression of TAZ via miRNA-338-3p to enhance the tumorigenesis of hepatocellular carcinoma. Oncotarget 6, 29048–29059 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu B., et al. , Hepatitis C virus NS4B protein induces epithelial-mesenchymal transition by upregulation of Snail. Virol. J. 14, 83 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webb Strickland S., Brimer N., Lyons C., Vande Pol S. B., Human Papillomavirus E6 interaction with cellular PDZ domain proteins modulates YAP nuclear localization. Virology 516, 127–138 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quinn H. M., et al. , YAP and β-catenin cooperate to drive oncogenesis in basal breast cancer. Cancer Res. 81, 2116–2127 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Gan W., et al. , LATS suppresses mTORC1 activity to directly coordinate Hippo and mTORC1 pathways in growth control. Nat. Cell Biol. 22, 246–256 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cotton J. L., et al. , YAP/TAZ and hedgehog coordinate growth and patterning in gastrointestinal mesenchyme. Dev. Cell 43, 35–47.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang K., et al. , YAP and TAZ take center stage in cancer. Biochemistry 54, 6555–6566 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Gao Y., et al. , TNFα-YAP/p65-HK2 axis mediates breast cancer cell migration. Oncogenesis 6, e383 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deng Y., et al. , Reciprocal inhibition of YAP/TAZ and NF-κB regulates osteoarthritic cartilage degradation. Nat. Commun. 9, 4564 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye S., et al. , YAP1-mediated suppression of USP31 enhances NFκB activity to promote sarcomagenesis. Cancer Res. 78, 2705–2720 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song Z., et al. , Long noncoding RNA ANRIL supports proliferation of adult T-cell leukemia cells through cooperation with EZH2. J. Virol. 92, e00909-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saitoh Y., et al. , Overexpressed NF-kappaB-inducing kinase contributes to the tumorigenesis of adult T-cell leukemia and Hodgkin Reed-Sternberg cells. Blood 111, 5118–5129 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Zhi H., et al. , NF-κB hyper-activation by HTLV-1 tax induces cellular senescence, but can be alleviated by the viral anti-sense protein HBZ. PLoS Pathog. 7, e1002025 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White S. M., Murakami S., Yi C., The complex entanglement of Hippo-Yap/Taz signaling in tumor immunity. Oncogene 38, 2899–2909 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenbluh J., et al. , β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell 151, 1457–1473 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong J., et al. , Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120–1133 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Overholtzer M., et al. , Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc. Natl. Acad. Sci. U.S.A. 103, 12405–12410 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao T., et al. , Human T-cell leukemia virus type 1 bZIP factor selectively suppresses the classical pathway of NF-kappaB. Blood 113, 2755–2764 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Zhao T., et al. , HTLV-1 bZIP factor supports proliferation of adult T cell leukemia cells through suppression of C/EBPα signaling. Retrovirology 10, 159 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma G., Yasunaga J. I., Ohshima K., Matsumoto T., Matsuoka M., Pentosan polysulfate demonstrates anti-human T-cell leukemia virus type 1 activities in vitro and in vivo. J. Virol. 93, e00413-19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu L., et al. , Hypericin-photodynamic therapy inhibits the growth of adult T-cell leukemia cells through induction of apoptosis and suppression of viral transcription. Retrovirology 16, 5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.