Abstract
Background:
Light has powerful effects on mood, sleep, and the circadian system. Humans evolved in an environment with a clear distinction between day and night, but our modern environments have blurred this distinction. Negative effects of light exposure at night have been well characterized. The importance of daytime light exposure has been less well characterized. Here we examine the cross-sectional and longitudinal associations of time spent in daytime outdoor light with mood, sleep, and circadian-related outcomes.
Methods:
Participants were drawn from the UK Biobank cohort, a large study of UK adults (n = 502,000; 37–73 years old; 54% women).
Results:
UK Biobank participants reported spending a median of 2.5 daylight hours (IQR = 1.5–3.5 h) outdoors per day. Each additional hour spent outdoors during the day was associated with lower odds of lifetime major depressive disorder (95% CI OR:0.92–0.98), antidepressant usage (OR:0.92–0.98), less frequent anhedonia (OR:0.93–0.96) and low mood (OR:0.87–0.90), greater happiness (OR:1.41–1.48) and lower neuroticism (incident rate ratio, IRR:0.95–0.96), independent of demographic, lifestyle, and employment covariates. In addition, each hour of daytime light was associated with greater ease of getting up (OR:1.46–1.49), less frequent tiredness (OR:0.80–0.82), fewer insomnia symptoms (OR:0.94–0.97), and earlier chronotype (adjusted odds ratio; OR:0.75–0.77). Auto-Regressive Cross-Lagged (ARCL) models were used to examine the longitudinal association of time spent in outdoor light at baseline with later mood-, sleep- and circadian-related outcomes reported at time point 2. Overall, longitudinal associations support cross-sectional findings, though generally with smaller effect sizes.
Limitations:
Future studies that examine the intensity of daytime light exposure at the ocular level are needed.
Conclusions:
Our findings suggest that low daytime light exposure is an important environmental risk factor for mood, sleep, and circadian-related outcomes.
Keywords: Daylight, Circadian rhythms, Mood, Depression, Sleep, Natural light
1. Introduction
Circadian rhythms are fundamental to general health, including mood and sleep (Allada and Bass, 2021). A distinct light-dark rhythm, with dim light at night and bright light during the day is essential for both the robust amplitude of circadian rhythms and its appropriate alignment with the waking day (Roenneberg et al., 2010). Humans evolved under such conditions (Stothard et al., 2017; Wright et al., 2013). Modern human light exposure has dramatically altered this pattern. Ubiquitous electric lighting in homes and workplaces and the use of light-emitting electronic devices at night has blurred the distinction between day and night (Cain et al., 2020). People now spend most waking hours in intermediate, artificial lighting conditions, due to reduced sunlight exposure and relatively bright nighttime light exposure (Goulet et al., 2007; Martinez-Nicolas et al., 2019).
A blunted circadian rhythm amplitude has been consistently reported in depressive disorders, which are characterized by symptoms including low mood, fatigue, and disturbances in sleep quality (Germain and Kupfer, 2008; Souêtre et al., 1989; Wirz-Justice, 2006). Postmortem brain tissue of patients with Major Depressive Disorder has been shown to have profoundly disturbed circadian rhythms throughout the brain consistent with a low amplitude clock (Li et al., 2013). Modern lighting likely contributes to disturbed amplitude. Light exposure during the night can suppress circadian amplitude (Jewett et al., 1994), while bright light exposure during the daytime enhances circadian amplitude (Park and Tokura, 1999; Winfree, 1980). Thus, our modern exposure to more light at night and less light in the day will tend to reduce amplitude, which may be of particular concern in depression.
Insufficient exposure to daytime light could be a key factor contributing to poor mood and sleep outcomes in depressive disorders. This hypothesis is supported by the recent findings that individuals with depression are less sensitive to the effects of light on the circadian system (McGlashan et al., 2019), that bright light therapy is efficacious in treating mood disorders (Geoffroy et al., 2019; Golden et al., 2005), and that selective serotonin reuptake inhibitors (SSRIs) enhance light sensitivity (McGlashan et al., 2018). Furthermore, targeted light exposure, in combination with an SSRI, has been shown to increase SSRI efficacy in the treatment of major depressive disorder (Lam et al., 2016) and cross-sectional studies report that workers exposed to natural sunlight report fewer insomnia symptoms, better sleep quality and fewer depressive symptoms (Harb et al., 2015; Leger et al., 2011).
Despite the well-established role of light in the regulation of circadian rhythms, sleep, and mood, there is little epidemiological data relating these outcomes to free-living daytime light exposure in the general adult population. Here, we report the first large-scale cohort study of cross-sectional and longitudinal associations of daytime light exposure with mood-, sleep-and circadian-related outcomes.
2. Methods
2.1. Participants
The UK Biobank prospective general population cohort contains more than 502,000 UK residents (aged 37–73 years; ~54% women) recruited via National Health Service (NHS) patient registers from 2006 to 2010, with the study population described in detail elsewhere (Collins, 2012; Sudlow et al., 2015). Participants provided detailed demographic, lifestyle, health, mood and physical information via assessment and touch-screen questionnaire. For longitudinal analyses, individuals who participated in both wave 0 (T0) and wave 1 (T1) of the UK Biobank study were included (ntotal = 20,336). Additionally, from 2016 to 2017 approximately 160,000 participants completed an extended online mental health questionnaire (MHQ). Participants who accepted the invitation to join the UK Biobank cohort provided written, informed consent and the UK Biobank has generic ethical approval from the North West Multi-Center Research Ethics Committee (Eysenck and Eysenck, 1984/NW/03820). The current analyses were done under UK Biobank application number 6818 (Martin Rutter).
2.2. Measurement of time spent in outdoor light
Participants self-reported how many hours they spent outdoors during the day on a typical day in both summer and winter (UK Biobank data-fields 1050 and 1060, respectively). Participants reported an integer using a touch-screen number pad or selected among alternate options including “Less than an hour a day”, “Do not know”, or “Prefer not to answer”. Initial cleaning of the data involved excluding participants that rated “Prefer not to answer” or “Do not know”, re-coding “Less than an hour a day” as zero (n = 98,431), and excluding values larger than the typical day length in the UK during summer (16 h; n = 253) and winter (8 h; n = 5,474). Summer and winter reports were strongly correlated (Pearson’s r = .65), indicating people who spent more time in outdoor light in winter also tended to do so in summer. Furthermore, a subset of approximately 20,000 participants completed a repeat assessment of this question from 2012–2013. Both summer and winter reports were strongly correlated (both r > .6) across repeat assessments, indicating good reliability of the measure. To yield a single measure of time spent in outdoor light, winter and summer reports were averaged within-participants. For analysis, extreme bins (9 12 h spent outdoors) with low density (total n = 1,016 (0.2%) were collapsed into a single bin. Finally, due to the right-ward skew of the time spent in outdoor light distribution a log transformation was applied before being entered into regression analyses.
2.3. Measurement of mood, sleep, and circadian-related outcomes
We extracted self-reported circadian-related outcomes from the UK Biobank dataset. Chronotype (“Morningness-Eveningness”; data-field 1180) was measured with the question “Do your consider yourself to be?” with six options: “Definitely a ‘morning’ person”, “More a ‘morning’ than ‘evening’ person”, “More an ‘evening’ than a ‘morning’ person”, “Definitely an ‘evening’ person”, “Do not know” or “Prefer not to answer”. The latter two categories were coded as missing and the remaining four categories were treated as an ordinal variable. Difficulty getting up in the morning (data-field 1170) was extracted as a second circadian-related metric. The use of this metric was informed by both theory and empirical evidence for its putative circadian underpinnings. Delayed circadian phase and difficulty getting up in the morning are canonical symptoms of delayed sleep-wake phase disorder (DSWPD) (American Academy of Sleep Medicine, 2005). Delayed phase results in individuals awakening “early” relative to their internal rhythm and closer to the nadir in the circadian arousal rhythm, leading to feelings of grogginess upon awakening. Further, in the present data set this phenotype correlates with chronotype strongly at both the phenotypic (r = .47) and genetic level (rg = .79).
Sleep-related metrics were self-reported insomnia and tiredness. Insomnia symptoms (data-field 1200) were measured with the question “Do you have trouble falling asleep at night or do you wake up in the middle of the night?" with available responses “never/rarely”, “sometimes”, “usually”, “prefer not to answer”. The latter category was coded as missing. Tiredness (data-field 2080) was assessed with the question "Over the past two weeks, how often have you felt tired or had little energy?" with available responses “not at all”, “several days”, “more than half the days”, “nearly every day”. Participants were also able to select “do not know” and “prefer not to answer, these categories were treated as missing. Sleep duration (data-field 1160) was also extracted as a key variable, however, given the potential confounding role of this variable on time spent in outdoor light (as it directly impacts available time to spend outdoors) this variable was used as a covariate for adjustment instead (see below for details).
Mood-related outcomes extracted were subjective happiness, frequency of recent low mood, frequency of recent anhedonia, neuroticism, use of common SSRI medication and lifetime incidence of recurrent major depressive disorder. Frequency of recent low mood (data-field 2050) and anhedonia (2060) were measured with the questions "Over the past two weeks, how often have you felt down, depressed or hopeless?" and "Over the past two weeks, how often have you had little interest or pleasure in doing things?" with available responses “not at all”, “several days”, “more than half the days”, “nearly every day”. For the above questions, participants were able to select “do not know” and “prefer not to answer, these categories were treated as missing. An aggregate neuroticism score was calculated for participants who completed all 12 questions from the Eysenck Personality Questionnaire Revised (Short Form; EPQ-R-S) Neuroticism Scale at baseline (Eysenck and Eysenck, 1984). The score represents the number of questions answered in the affirmative (range 0–12). Participants self-reported medications (data-field 20003) was used to identify the use of common anti-depressant medications from three broad drug classes that are used to treat the symptoms of depression: SSRIs, MAOIs and TCAs (see Supplement eTable 1 for drug names and UK Biobank codes). Medications in these classes were drawn from a published, derived list (Howard et al., 2018). A single, binary variable was derived with “cases” reporting the use of at least one anti-depressant medication and “controls” who did not report the use of any anti-depressant medications. A subset of UK Biobank participants completed the additional online Mental Health Questionnaire (MHQ; n = 161,467) that measured the incidence of symptoms of mental disorders across the lifetime. Subjective happiness and variables necessary to determine major depressive disorder status were extracted for this subset of participant who completed the MHQ. Consistent with Smith et al. (2013), participants met criteria for lifetime recurrent major depressive disorder if they reported having two or more periods of low mood or anhedonia (lasting at least two weeks) as well as having visited either a doctor or psychiatrist for “nerves, anxiety, tension or depression”. This resulted in a binary variable consisting of “control” participants who did not meet the above criteria (with participants reporting a single episode of depression or missing information on criteria excluded) and “recurrent major depressive disorder” participants. Subjective happiness (data-field 4526) were measured with the question “In general how happy are you?” and available responses ranged from 1 “extremely happy” to 6 “extremely unhappy”. For ease of interpretation, happiness scores were reversed so that higher scores reflected greater happiness.
2.4. Statistical analyses
The association between time spent in outdoor light during the day and mood, sleep and circadian-related outcome measures was examined via a series of regression models. We report three models for each outcome: first, an unadjusted model; second, adjusted for age (continuous), sex (male/female), employment status (employed/unemployed) and season of assessment (winter/spring/summer/autumn); third, additional adjustment for, physical activity (MET-minutes/week; continuous), frequency of socialization (continuous), and sleep duration (continuous). Frequency of socialization was measured with the question “how often do you visit friends or family or have them visit you”, per week, with six levels ranging from “never or almost never” to “almost daily”. Physical activity was measured using the short International Physical Activity Questionnaire (IPAQ) and metabolic equivalent (MET) minutes of physical activity per week were calculated according to published guidelines (Craig et al., 2003). Ordinal logistic regression was used for all outcomes excepting neuroticism for which negative binomial regression was used; and lifetime major depressive disorder status and anti-depressant medication use for which binary logistic regression were used. Odds ratios (OR; ordinal and binary logistic regression) and incidence rate ratios (IRRs; negative binomial regression) are reported with 95% confidence intervals. To obtain the genetic correlation between chronotype and difficulty getting up, representing the degree of overlap in their genetic architecture, Linkage Disequilibrium Score Regression (LDSC) was used (Bulik-Sullivan et al., 2015).
The longitudinal association between time spent in outdoor light and mood and sleep outcomes was examined using auto-regressive, cross-lagged (ARCL) models (Kenny and Harackiewicz, 1979). This method permits the use of repeated measures data to simultaneously examine: the within-person stability of predictor and outcome variables over time (termed auto-regressive effects), the cross-sectional association between predictors and outcomes variables within a time point (termed synchronous effects) and, importantly, the longitudinal association between predictors at earlier time points and outcomes at later time points as a measure of temporal precedence (termed cross-lagged effects). Our main hypothesis focused on the longitudinal, cross-lagged effect of earlier time spent in outdoor light on later mood and sleep outcomes. The R package lavaan (Rosseel, 2012) was used to build the models and data from the subset of individuals who participated in both wave 0 (T0) and wave 1 (T1) of the UK Biobank study were included (ntotal = 20,336). The distance between two time points was normally distributed with a mean of 4.3 years and an SD of 0.93 years. Strong consistency (large and positive auto-regressive effects) of all measures between waves was observed as a pre-requisite for the ARCL models. For each outcome, the ARCL model simultaneously tested six paths: two autoregressive (e.g. Depressed Mood T0 -> Depressed Mood T1), two synchronous (Time Spent in Outdoor Light T0 -> Depressed Mood T0) and two cross-lagged (e.g. Time Spent in Outdoor Light T0 -> Depressed Mood T1). As such, the ARCL model allows the assessment of the influence of time spent in outdoor light on mood and sleep outcomes across time while controlling for their within-person stability. Standardized betas and their associated p values are reported for all models.
3. Results
UK Biobank participants reported spending a median of 2.5 h (IQR = 1.5–3.5 h) in outdoor light during the daytime, per day. Cross-sectional associations between time spent in outdoor light and mood, sleep, and circadian-related outcomes for three models of increasing multivariable adjustment are presented in Table 1. Analysis of circadian-related variables in the fully-adjusted regression models (model 3) found greater time spent in outdoor light during the day was associated with both earlier chronotype (OR 0.76, 95% CI 0.75–0.77) and greater ease of getting up (OR 1.47, 95% CI 1.46–1.49), after adjusting for age, sex, season of assessment, employment status, exercise, socialization and sleep duration. Greater time spent in outdoor light during the day was also associated with less insomnia symptoms (OR 0.96, 95% CI 0.94–0.97) and less tiredness (OR 0.81, 95% CI 0.80–0.82) in the fully adjusted model.
Table 1.
Cross-sectional associations of time spent in outdoor light during the day with mood, sleep, and circadian-related outcomes in the UK Biobank. Odds (OR) and Incidence Rate Ratios (IRR) are presented with 95% CIs across three models of increasing covariate adjustment. McFadden’s pseudo-R2 is reported for all models.
| Model 1 (Unadjusted) |
Model 2 (+ age, sex, season, employment) |
Model 3 (+ exercise, socialization, sleep duration) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | OR/IRR (95% CI) | P value | Model Pseudo R2 | N | OR/IRR (95% CI) | P value | Model Pseudo R2 | N | OR/IRR (95% CI) | P value | Model Pseudo R2 | |
|
| ||||||||||||
| Chronotype (Eveningness) | 411,875 | 0.71 (0.70–0.72) | < 5 × 10−200 | 0.076 | 411,875 | 0.72 (0.71–0.73) | < 5 × 10−200 | 0.08 | 381,396 | 0.76 (0.75–0.77) | < 5 × 10−200 | 0.15 |
| Ease of Getting Up | 456,674 | 1.94 (1.92–1.97) | < 5 × 10−200 | 0.096 | 456,674 | 1.65 (1.63–1.67) | < 5 × 10−200 | 0.12 | 421,154 | 1.47 (1.46–1.49) | < 5 × 10−200 | 0.20 |
| Insomnia Symptoms | 456,843 | 0.94 (0.93–0.95) | 3 × 10−31 | 0.088 | 456,843 | 0.90 (0.89–0.91) | 3 × 10−78 | 0.10 | 421,216 | 0.96 (0.94–0.97) | 3 × 10−11 | 0.20 |
| Tiredness | 445,181 | 0.66 (0.65–0.67) | < 5 × 10−200 | 0.096 | 445,181 | 0.74 (0.73–0.75) | < 5 × 10−200 | 0.11 | 411,510 | 0.81 (0.80–0.82) | 8 × 10−197 | 0.19 |
| Happiness | 158,133 | 1.64 (1.61–1.67) | < 5 × 10−200 | 0.085 | 158,133 | 1.61 (1.59–1.64) | < 5 × 10−200 | 0.09 | 146,540 | 1.45 (1.41–1.48) | < 5 × 10−200 | 0.17 |
| Neuroticism | 374,277 | 0.90 (0.89–0.90) | < 5 × 10−200 | 0.071 | 374,277 | 0.93 (0.93–0.94) | 7 × 10−122 | 0.08 | 348,877 | 0.96 (0.95–0.96) | 2 × 10−41 | 8 × 10−197 |
| Low Mood | 439,228 | 0.74 (0.73–0.75) | < 5 × 10−200 | 0.096 | 439,228 | 0.81 (0.80–0.82) | 6 × 10−166 | 0.11 | 406,347 | 0.88 (0.87–0.90) | 2 × 10−49 | 0.21 |
| Anhedonia | 443,306 | 0.81 (0.80–0.83) | 2 × 10−167 | 0.097 | 443,306 | 0.85 (0.83–0.86) | 4 × 10−101 | 0.11 | 410,144 | 0.95 (0.93–0.96) | 6 × 10−10 | 0.21 |
| Lifetime Recurrent Major Depressive Disorder | Cases: 23,515 Control:118,927 |
0.86 (0.84–0.89) | 3 × 10−24 | 0.054 | Cases: 23,515 Control: 118,927 |
0.95 (0.92–0.98) | 9 × 10−4 | 0.07 | Cases: 21,743 Control: 110,650 |
0.96 (0.92–0.98) | 0.002 | 0.14 |
| Antidepressant Usage | Cases: 29,858 Control:427,277 |
0.88 (0.86–0.90) | 3 × 10−25 | 0.10 | Cases: 29,858 Control: 427,277 |
0.85 (0.83–0.88) | 7 × 10−34 | 0.13 | Cases: 25,016 Control:396,417 |
0.95 (0.92–0.98) | 3 × 10−4 | 0.25 |
Finally, analysis of mood-related variables revealed that greater time spent in outdoor light during the day was associated with lower odds of lifetime recurrent major depressive disorder (OR 0.96, CI 0.92–0.98), lower odds of using antidepressant medication (OR 0.95, 95% CI 0.92–0.98), greater self-reported happiness (OR 1.45, 95% CI 1.41–1.48), lower neuroticism (IRR 0.96, 95% CI 0.95–0.96), less frequent low mood (OR 0.88, 95% CI 0.87–0.89) and anhedonia (OR 0.95, 95% CI 0.93–0.96), after adjusting for age, sex, season of assessment, employment status, exercise, socialization and sleep duration in the fully adjusted model.
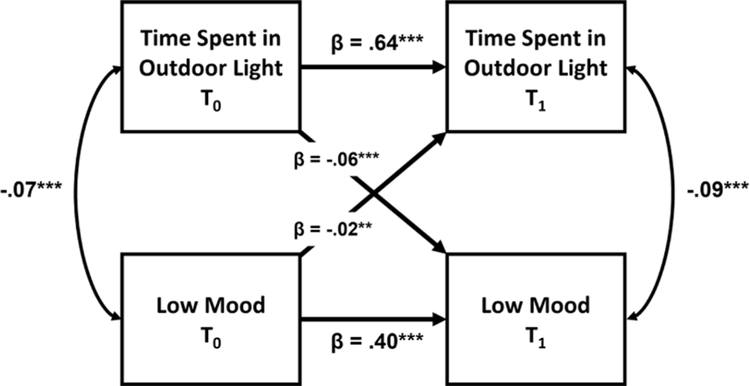
Auto-regressive, cross-lagged (ARCL) models were used to assess the main hypothesis of a longitudinal, cross-lagged association between time spent in outdoor light on mood, sleep, and circadian rhythm-related outcomes across wave 0 (T0) and wave 1 (T1) of the UK Biobank study (ns between 13,565–18,011) as a measure of temporal precedence. Fig. 1 displays the ARCL model for the frequency of low mood outcome (see Fig. S1 for ARCL models of other outcomes). For all models, the autoregressive path measuring the consistency of the timespent in outdoor light variable between T0 and T1, a pre-requisite of the model, was positive and large (β = .63–.66, p < 1 × 10−4), indicating strong within-person consistency of this measure between waves. The autoregressive paths for mood-related variables of frequency of low mood (β = .40, p < 1 × 10−4), frequency of anhedonia (β = .37, p < 1 × 10−4), neuroticism (β = .82, p < 1 × 10−4) and the use of anti-depressant medications (β = .65, p < 1 × 10−4) also showed good consistency between waves. In a test of our main hypothesis, the cross-lagged paths showed that greater time spent in outdoor light at T0 predicted less frequent low mood (β = −.06, p < 1 × 10−4), less frequent anhedonia (β = −.04, p < 1 × 10−4), lower neuroticism (β = −.01, p = .01) and lower odds of taking anti-depressant medications (β = −.02, p = .03) at T1. The alternate cross-lagged paths indicated that more frequent low mood (β = −.02, p = .001), more frequent anhedonia (β = −.01, p = .03), greater neuroticism (β = −.03, p < 1 × 10−4) and using anti-depressant medications (β = −.04, p = .001) at T0 predicted lower time spent in outdoor light at T1.
Fig. 1.
Autoregressive, cross-lagged (ARCL) model diagram for the longitudinal effect of time spent in outdoor light during the day on the frequency of low mood symptoms between the first (T0) and second (T1) waves of the UK Biobank study. Auto-regressive paths occur between waves of the same measure (e. g. Depressed Mood T0 -> Depressed Mood T1) as a measure of consistency, synchronous paths occur between measures within a wave (e.g. Time Spent in Outdoor Light T0 -> Depressed Mood T0) as a measure of cross-sectional association, and cross-lagged paths occur between measures across waves (e.g. Time Spent in Outdoor Light T0 -> Depressed Mood T1) as a measure of longitudinal association and temporal precedence.
Sleep-related outcomes of insomnia symptom frequency (β = .64, p < 1 × 10−4) and frequency of tiredness (β = .51, p < 1 × 10−4) showed strong consistency between waves. Cross-lagged paths indicated that greater time spent in outdoor light at T0 predicts lower frequency of insomnia symptoms (β = −.02, p = .002) and less frequent tiredness (β = −.04, p p < 1 × 10−4) at T1. Further, greater insomnia symptoms and tiredness at T0 predicted less time spent in outdoor light at T1 (β = −.02, p = .007; β = −.04, p < 1 × 10−4, respectively).
Both circadian-related metrics of chronotype (β = .82, p < 1 × 10−4) and ease of getting up (β = .68, p < 1 × 10−4) also showed strong consistency between waves. The cross-lagged paths for these outcomes indicated that greater time spent in outdoor light during the day at T0 predicted greater ease of getting up (β = −.05, p < 1 × 10−4) at T1 and a non-significant trend for earlier chronotype at T1 (β = −.01, p = .09). Finally, later chronotype and lower ease of getting up at T0 predicted lower time spent in outdoor light at T1 (β = −.03, p < 1 × 10 −4; β = −.05, p < 1 × 10−4, respectively).
4. Discussion
We examined the associations between self-reported time spent in outdoor light during the day and mood, sleep, and circadian-related outcomes in the UK Biobank cohort. After adjusting for demographic, employment, and lifestyle covariates we observed that greater time spent in outdoor light during the day was associated with fewer depressive symptoms, lower odds of using antidepressant medication and lower odds of lifetime recurrent major depressive disorder, greater ease of getting up in the morning, less tiredness, better sleep, and earlier chronotype. These relationships held longitudinally, such that greater time spent in outdoor light during the day in the first time point predicted less frequent depressive symptoms, lower odds of using antidepressant medications, greater ease of getting up in the morning, less tiredness, and better sleep at the second time point. These results may be explained by the impacts of light on the circadian system as well as non-circadian, direct effects of light on mood centers in the brain.
We observed that greater time spent in outdoor light was associated with better mood outcomes. These associations held longitudinally, such that daytime light exposure at the first time point predicted better mood outcomes at the second time point, though with a smaller effect size compared to the cross-sectional results. This is consistent with an expected decay in the effect of earlier light on later mood with a long lagtime between the waves. There are potential circadian mechanisms which could account for these effects. First, daytime light exposure may improve mood by correcting abnormal circadian timing relative to sleep/wake behavior. Delayed circadian timing is associated with poorer mood and correcting this has been associated with recovery (Emens et al., 2009). Thus, daytime light, by helping to correct delayed timing, could be beneficial for mood. Second, daytime light exposure may have euthymic effects by strengthening the signal (amplitude) from the circadian clock. Blunted circadian rhythms are a key feature of depressive disorders. Daytime light therapy, which can boost circadian amplitude, is an effective treatment of depressive disorders (Geoffroy et al., 2019; Golden et al., 2005; Park and Tokura, 1999). Beyond effects on the clock, circadian photoreception is now appreciated to influence areas of the brain involved in emotional regulation. Intrinsically photosensitive retinal ganglion cells containing the photopigment melanopsin are the primary light input to the suprachiasmatic nucleus, but also directly project to the amygdala and habenula (Fernandez et al., 2018; Rupp et al., 2019), brain areas associated with negative emotionality and implicated in depression (Korgaonkar et al., 2019). Daytime light likely has positive effects on mood through circadian photoreception, both by effects on the clock and by direct effects on emotional processing.
Greater time spent in outdoor light during the day was associated with earlier chronotype and greater ease of awakening. This relationship is expected based on the known effects of light on the human circadian system. Light exposure has a different impact on the timing of circadian rhythms, such that exposure to morning light shifts rhythms earlier, whereas exposure to evening and early night light shifts rhythms later (Khalsa et al., 2003). Sunlight tends to shift the clock earlier, compensating for the fact that the average intrinsic period of the human circadian clock is > 24 h (speeds up a slightly slow clock). Greater daytime light exposure would thus be expected to contribute to an earlier chronotype, as is predicted by models of the human circadian system (Skeldon et al., 2017). The greater ease of awakening observed with more daytime light exposure is consistent with daytime light advancing the circadian clock. The core body temperature trough (when there is the strongest signal for sleep from the circadian clock) tends to occur in the late night, but can overlap with wake time in those who are delayed (Duffy et al., 1999). If more outdoor light tends to advance timing, there would be less overlap of the circadian signal for sleep and wake time. Our results are consistent with other cross-sectional studies of adults in smaller cohorts, such that greater time in outdoor light is associated with earlier mid-sleep and chronotype (Martin et al., 2012; Roenneberg et al., 2003). In addition to this cross-sectional association, we describe here a predicted longitudinal association, such that greater light at earlier time points predicted earlier chronotype and greater ease of awakening, though the chronotype cross-lagged path was a non-significant, trend level association. These results provide evidence for daytime light exposure having an active effect on the circadian system.
Our analysis of sleep outcomes is consistent with the expected downstream effects of daytime light on the circadian system. Greater daytime outdoor light exposure was associated with fewer symptoms of insomnia and less frequent tiredness, even when controlling for individual sleep duration and other relevant covariates. These findings concur with smaller cross-sectional studies that reported exposure to natural daylight (Leger et al., 2011) and, in general, greater daytime light exposure predicts better sleep quality and fewer insomnia symptoms (Wams et al., 2017). There are plausible circadian mechanisms that could explain this pattern of results, likely in combination. Firstly, advancing phase of the circadian rhythm may lead to improved sleep via improving the alignment between sleep-onset and the circadian signal for sleep (Dijk and Czeisler, 1994). Secondly, outdoor light exposure may improve sleep outcomes via the enhancement of the amplitude of the circadian rhythm in arousal, whereby a larger rhythm would result in both a stronger signal for alertness during the day and a stronger signal for sleep at night. Such an explanation would be consistent with previous work in both humans and animal models showing daytime light bolsters circadian rhythms and concomitantly improves the consolidation of sleep in such a manner (Ancoli-Israel et al., 2003; González and Aston-Jones, 2006).
5. Limitations
Despite the proposed evidence for a circadian-related mechanism for the sleep and mood associations with time spent outdoors during the day, it should be noted that there are limitations of both the sample and the subjective nature of predictors and outcome measures used. Firstly, the UK Biobank sample consists of largely healthy, middle-older aged adults. Given the changes in circadian rhythms, sleep, and mood across the lifespan, the associations we found would conceivably be for younger ages. Secondly, our light exposure measure is a subjective report of the length of time spent outdoors in the day. While this could measure the time spent in outdoor light, it fails to capture objective intensity and duration at the ocular level of light exposure. An advantage of this measure, however, over other studies that utilize objective satellite measures of light exposure is that we have obtained individual-level light exposure measures for the use of both cross-sectional and longitudinal analyses (Paksarian et al., 2020). Finally, we relied on subjective reports for our circadian-related outcome measures. Future studies may seek to address the above issues by sampling across the lifespan and by utilizing objective measures of both light exposure and circadian phase, with a view to enabling the parsing of circadian-mediated and direct effects of light on mood and sleep.
6. Conclusions
Here we report the cross-sectional and longitudinal association between daytime light exposure and mood, sleep, and circadian-related outcomes in the UK Biobank. Together, these results are consistent with the impact of daytime light exposure on the circadian system, via advancing circadian phase, enhancing amplitude or a combination of these factors, as well as expected downstream effects of these circadian impacts: improved mood and sleep. These findings, while observational in nature, point toward future research that may improve our understanding of the role of light in the pathophysiology of mood and sleep disorders by directly measuring circadian physiology and daytime light exposure in a well-controlled longitudinal design.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Dr. Joshua Wiley for his advice on longitudinal structural equation models and Irma Vlasac for her early guidance. This work utilized the UK Biobank resource (application 26209; Martin Rutter).
Funding
AC Burns was supported by Research Training Program (RTP) scholarships from the Australian Government.
Declaration of Competing Interest
AC Burns, R Saxena & JM Lane declare no relevant conflict. C Vetter is part of the Circadian Light Therapy (Inc.), and Chronsulting Scientific Advisory Boards. C Vetter has also acted as a consultant to NIOSH, and the US DoE, and has received funding for unrelated projects by the NIH, CU Boulder, and the Colorado Clinical and Translational Sciences Institute. SW Cain and AJK Phillips are both investigators on projects funded by the Alertness Safety and Productivity CRC, have received research funds from Versalux and Delos, and consulted for Beacon Lighting. SWC has consulted for Versalux and Dyson.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jad.2021.08.056.
References
- Allada R, Bass J, 2021. Circadian mechanisms in medicine. New Eng. J. Med. 384, 550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine, 2005. International classification of sleep disorders. diagnostic and coding manual, 51–55. [Google Scholar]
- Ancoli-Israel S, Gehrman P, Martin JL, Shochat T, Marler M, Corey-Bloom J, Levi L, 2003. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe alzheimer’s disease patients. Behav. Sleep Med. 1, 22–36. [DOI] [PubMed] [Google Scholar]
- Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh PR, Duncan L, Perry JR, Patterson N, Robinson EB, 2015. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain SW, McGlashan EM, Vidafar P, Mustafovska J, Curran SP, Wang X, Mohamed A, Kalavally V, Phillips AJ, 2020. Evening home lighting adversely impacts the circadian system and sleep. Sci. Rep. 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins R, 2012. What makes UK biobank special? Lancet 9822, 1173–1174. [DOI] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, 2003. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 35, 1381–1395. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Czeisler CA, 1994. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci. Lett. 166, 63–68. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ, Hall EF, Czeisler CA, 1999. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J. Investig. Med. 47, 141–150. [PMC free article] [PubMed] [Google Scholar]
- Emens J, Lewy AJ, Mark J, Arntz D, Rough J, 2009. Circadian misalignment in major depressive disorder. Psychiatry Res. 168, 259–261. [DOI] [PubMed] [Google Scholar]
- Eysenck H, Eysenck S, 1984. Eysenck personality questionnaire-revised. [Google Scholar]
- Fernandez DC, Fogerson PM, Lazzerini Ospri L, Thomsen MB, Layne RM, Severin D, Zhan J, Singer JH, Kirkwood A, Zhao H, Berson DM, Hattar S, 2018. Light affects mood and learning through distinct retina-brain pathways. Cell 175, 71–84.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy PA, Schroder CM, Reynaud E, Bourgin P, 2019. Efficacy of light therapy versus antidepressant drugs, and of the combination versus monotherapy, in major depressive episodes: a systematic review and meta-analysis. Sleep Med. Rev. 48, 101213. [DOI] [PubMed] [Google Scholar]
- Germain A, Kupfer DJ, 2008. Circadian rhythm disturbances in depression. Hum. Psychopharmacol. Clin. Exp. 23, 571–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden RN, Gaynes BN, Ekstrom RD, Hamer RM, Jacobsen FM, Suppes T, Wisner KL, Nemeroff CB, 2005. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am. J. Psychiatry 162, 656–662. [DOI] [PubMed] [Google Scholar]
- González MMC, Aston-Jones G, 2006. Circadian regulation of arousal: role of the noradrenergic locus coeruleus system and light exposure. Sleep 29, 1327–1336. [DOI] [PubMed] [Google Scholar]
- Goulet G, Mongrain V, Desrosiers C, Paquet J, Dumont M, 2007. Daily light exposure in morning-type and evening-type individuals. J. Biol. Rhythms 22, 151–158. [DOI] [PubMed] [Google Scholar]
- Harb F, Hidalgo MP, Martau B, 2015. Lack of exposure to natural light in the workspace is associated with physiological, sleep and depressive symptoms. Chronobiol. Int. 32, 368–375. [DOI] [PubMed] [Google Scholar]
- Howard DM, Adams MJ, Shirali M, Clarke T-K, Marioni RE, Davies G, Coleman JRI, Alloza C, Shen X, Barbu MC, Wigmore EM, Gibson J, Agee M, Alipanahi B, Auton A, Bell RK, Bryc K, Elson SL, Fontanillas P, Furlotte NA, Hinds DA, Huber KE, Kleinman A, Litterman NK, McCreight JC, McIntyre MH, Mountain JL, Noblin ES, Northover CAM, Pitts SJ, Sathirapongsasuti JF, Sazonova OV, Shelton JF, Shringarpure S, Tian C, Tung JY, Vacic V, Wilson CH, Hagenaars SP, Lewis CM, Ward J, Smith DJ, Sullivan PF, Haley CS, Breen G, Deary IJ, McIntosh AM, Me Research T, 2018. Genome-wide association study of depression phenotypes in UK biobank identifies variants in excitatory synaptic pathways. Nat. Commun. 9, 1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett ME, Kronauer RE, Czeisler CA, 1994. Phase-Amplitude resetting of the human circadian pacemaker via bright light: a further analysis. J. Biol. Rhythms 9, 295–314. [DOI] [PubMed] [Google Scholar]
- Kenny DA, Harackiewicz JM, 1979. Cross-lagged panel correlation: practice and promise. J. Appl. Psychol. 64, 372. [Google Scholar]
- Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA, 2003. A phase response curve to single bright light pulses in human subjects. J. Physiol. 549, 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar MS, Erlinger M, Breukelaar IA, Boyce P, Hazell P, Antees C, Foster S, Grieve SM, Gomes L, Williams LM, Harris AWF, Malhi GS, 2019. Amygdala activation and connectivity to emotional processing distinguishes asymptomatic patients with bipolar disorders and unipolar depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4, 361–370. [DOI] [PubMed] [Google Scholar]
- Lam RW, Levitt AJ, Levitan RD, Michalak EE, Cheung AH, Morehouse R, Ramasubbu R, Yatham LN, Tam EM, 2016. Efficacy of bright light treatment, fluoxetine, and the combination in patients with nonseasonal major depressive disorder: a randomized clinical trial. JAMA Psychiatry 73, 56–63. [DOI] [PubMed] [Google Scholar]
- Leger D, Bayon V, Elbaz M, Philip P, Choudat D, 2011. Underexposure to light at work and its association to insomnia and sleepiness: a cross-sectional study of 13 296 workers of one transportation company. J. Psychosom. Res. 70, 29–36. [DOI] [PubMed] [Google Scholar]
- Li SX, Liu LJ, Xu LZ, Gao L, Wang XF, Zhang JT, Lu L, 2013. Diurnal alterations in circadian genes and peptides in major depressive disorder before and after escitalopram treatment. Psychoneuroendocrinology 38, 2789–2799. [DOI] [PubMed] [Google Scholar]
- Martin JS, Hébert M, Ledoux É,Gaudreault M, Laberge L, 2012. Relationship of chronotype to sleep, light exposure, and work-related fatigue in student workers. Chronobiol. Int. 29, 295–304. [DOI] [PubMed] [Google Scholar]
- Martinez-Nicolas A, Martinez-Madrid MJ, Almaida-Pagan PF, Bonmati-Carrion MA, Madrid JA, Rol MA, 2019. Assessing chronotypes by ambulatory circadian monitoring. Front Physiol. 10, 1396–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan EM, Coleman MY, Vidafar P, Phillips AJK, Cain SW, 2019. Decreased sensitivity of the circadian system to light in current, but not remitted depression. J. Affect. Disord. 256, 386–392. [DOI] [PubMed] [Google Scholar]
- McGlashan EM, Nandam LS, Vidafar P, Mansfield DR, Rajaratnam SMW, Cain SW, 2018. The SSRI citalopram increases the sensitivity of the human circadian system to light in an acute dose. Psychopharmacology 235, 3201–3209. [DOI] [PubMed] [Google Scholar]
- Paksarian D, Rudolph KE, Stapp EK, Dunster GP, He J, Mennitt D, Hattar S, Casey JA, James P, Merikangas KR, 2020. Association of outdoor artificial light at night with mental disorders and sleep patterns among US adolescents. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Tokura H, 1999. Bright light exposure during the daytime affects circadian rhythms of urinary melatonin and salivary immunoglobulin A. Chronobiol. Int. 16, 359–371. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Hut R, Daan S, Merrow M, 2010. Entrainment concepts revisited. J. Biol. Rhythms 25, 329–339. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Wirz-Justice A, Merrow M, 2003. Life between clocks: daily temporal patterns of human chronotypes. J. Biol. Rhythms 18, 80–90. [DOI] [PubMed] [Google Scholar]
- Rosseel Y, 2012. Lavaan: an R package for structural equation modeling and more. version 0.5–12 (BETA). J. Statist. Softw. 48, 1–36. [Google Scholar]
- Rupp AC, Ren M, Altimus CM, Fernandez DC, Richardson M, Turek F, Hattar S, Schmidt TM, 2019. Distinct ipRGC subpopulations mediate light’s acute and circadian effects on body temperature and sleep. eLife 8, e44358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeldon AC, Phillips AJ, Dijk DJ, 2017. The effects of self-selected light-dark cycles and social constraints on human sleep and circadian timing: a modeling approach. Sci. Rep. 7, 45158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DJ, Nicholl BI, Cullen B, Martin D, Ul-Haq Z, Evans J, Gill JM, Roberts B, Gallacher J, Mackay D, 2013. Prevalence and characteristics of probable major depression and bipolar disorder within UK biobank: cross-sectional study of 172,751 participants. PloS One 8, e75362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souêtre E, Salvati E, Belugou JL, Pringuey D, Candito M, Krebs B, Ardisson JL, Darcourt G, 1989. Circadian rhythms in depression and recovery: evidence for blunted amplitude as the main chronobiological abnormality. Psychiatry Res. 28, 263–278. [DOI] [PubMed] [Google Scholar]
- Stothard ER, McHill AW, Depner CM, Birks BR, Moehlman TM, Ritchie HK, Guzzetti JR, Chinoy ED, LeBourgeois MK, Axelsson J, Wright KP, 2017. Circadian entrainment to the natural light-dark cycle across seasons and the weekend. Curr. Biol. 27, 508–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, 2015. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLOS Med. 12, e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wams EJ, Woelders T, Marring I, van Rosmalen L, Beersma DGM, Gordijn MCM, Hut RA, 2017. Linking light exposure and subsequent sleep: a field polysomnography study in humans. Sleep 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfree AT, 1980. The Geometry of Biological Time. Springer, New York. [Google Scholar]
- Wirz-Justice A, 2006. Biological rhythm disturbances in mood disorders. Int. Clin. Psychopharmacol. 21, S11–S15. [DOI] [PubMed] [Google Scholar]
- Wright KP, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED, 2013. Entrainment of the human circadian clock to the natural light-dark cycle. Curr. Biol. 23, 1554–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.