Abstract
Background
The World Health Organization declared the outbreak of the novel coronavirus (COVID-19) as a global health emergency on January 30, 2020, and as a pandemic disease on March 11, 2020. This review highlights the international situation, risk factors, and related protections to be taken as prerequisite measures and probable treatment options for the COVID-19-infected population in the current scenario.
Main text
The SARS-CoV-2 viruses and their variants caused mild-to-severe respiratory tract infection and used airborne pathways as a way of contagion. Human-to-human transmission led to an exponential growth in the rise in the number of cases making it a real burden to immobilize the rapid spread of the virus while asymptomatic patients created ambiguity for confirmation in the community. It was clear from the case studies of patients that most of them were asymptomatic but still vulnerable to the people around, and hence, in a flash, many countries around the globe went into a complete lockdown, influencing the economy and thrashing industrial outputs. On the other hand, numerous researches were made to counteract the spread through studies in antiviral therapy, immune-based therapy, vaccination development, and natural remedies.
Conclusion
Although exploration for a specific drug required for the COVID-19 treatment is under extensive research worldwide and some of them are in clinical trial now. Virtual drug library screening is one of the current techniques for repurposing accessible compounds. This review could provide beneficial information about the potential current and future treatment strategies to treat the pandemic COVID-19 infection.
Keywords: COVID-19, SARS-CoV-2, Coronavirus, Pandemic, Respiratory, Airborne, Treatment of COVID-19
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the coronavirus strain responsible for the novel coronavirus 2019 (COVID-19) infection. COVID-19 emerged in the Wuhan city of China in late 2019 and swiftly occupied many of the individuals across the city with symptoms of atypical pneumonia, resulting in an outbreak of epidemic phase (Zhu et al. 2020a, b). Later, World Health Organization (WHO) declared the outbreak as a pandemic after assessing the situation around the world on March 11, 2020 (Cucinotta and Vanelli 2020). By the end of July 2021, more than 192 million cases had been recorded worldwide, resulting in the deaths of more than 4.1 million individuals worldwide from the beginning of the pandemic (WHO 2022a). Evidence till now supports the statement that COVID-19 has a zoonotic source as a constructed virus would have shown genomic sequences of known elements. The other forms of coronavirus include severe acute respiratory syndrome coronavirus (SARS-CoV) and middle East respiratory syndrome coronavirus (MERS-CoV), which has severe effects on the respiratory health of humans, while human coronavirus stains such as (HCoV) HKU1, NL63, OC43, and 229E show mild symptoms (Chilamakuri and Agarwal 2021; Corman et al. 2018). Throughout the COVID-19 pandemic, SARS-CoV-2 genetic variants have emerged and spread across the world. Airborne transmission is a major concern of SARS-CoV-2 as the expiratory activities (i.e., coughing, sneezing) of an infected person can generate respiratory droplets and infect individuals within a radius of 6ft (Ghinai et al. 2020). The front portion of the mouth is where atomization of droplets occurs; thus, covering the mouth by the use of a surgical facemask is essential (Johnson et al. 2011).
The incubation period of a virus is the period between the exposure and the potential earliest date of symptoms, and current research showed that the incubation period for COVID-19 ranges from 2 to 7 days while the median estimate is 4 days (Guan et al. 2020). Fever, dry cough, dyspnea, myalgia, tiredness, regular or reduced leukocyte counts, and radiographic indications of pneumonia are all common signs of COVID-19 infection. Symptoms of lung abnormalities, lymphopenia, and thrombocytopenia have also been observed in certain COVID-19 individuals. The pathophysiological feature of COVID-19 is governed by proliferative and exudative stages of alveolar damage, necrosis of pneumocytes, inflammatory infiltrates, and microvascular damage (Carsana et al. 2020). To successfully combat current and possible future pandemics, detailed investigations of this new coronavirus, its mode of infection, and replication are required. This review focuses on the global situation, clinical manifestation, precautions to be taken as a precautionary measure, and various treatment approaches for COVID-19.
Main text
History and classification
Coronaviruses are a family of hundreds of viruses, and it was seen that the majority of these viruses showed their harmful effect on different animals like bats, chickens, camels, and cats. In the 1960s, human coronaviruses 229E and OC43 were first discovered that were able to infect humans (Andersen et al. 2020). Among human coronaviruses, four are endemic (229E, OC43, NL63, and HKU1) and are well known for causing mild diseases (Kahn and McIntosh 2005; Saxena et al. 2020).
In November 2002, the first SARS-CoV virus was identified, resulting in severe acute respiratory syndrome (SARS) (Lau et al. 2020). In 2003, the members of Canada's National Microbiology Laboratory identified this virus's genome and confirmed the reason for this outbreak (Pal et al. 2020). Since 2005, several novel coronaviruses have been recognized from bats, and the evidence showed that human respiratory coronaviruses, SARS coronavirus, and MERS coronavirus were initially derived from bat viruses ancestral (Paden et al. 2018; Burrell et al. 2017).
Another deadlier coronavirus MERS-CoV (Middle East respiratory syndrome) was discovered in 2012. In MERS, the first case was from Saudi Arabia. Later another two MERS outbreak was identified in 2015 and 2018 in South Korea and Saudi Arabia, respectively. Then the first SARS-CoV-2 or COVID-19 infection was reported in December 2019 in Wuhan city of China. The virus was first discovered in bats and then pangolins (Panyod et al. 2020; Zhang et al. 2020). The genomic structure of virus SARS-CoV and SARS-CoV-2 bears many common characteristics and shows almost similar symptoms. This disease turns life-threatening if people are suffering from SARS. A total of 774 people was died from 2002 to 2014, according to the last reported case (Abdul-Fattah et al. 2021).
On account of their genus, there are four main subgroups of coronaviruses, known as alpha (α), beta (β), gamma (γ), and delta (δ) coronaviruses. Among them α and β coronaviruses are known to infect mammals, and other two γ and δ coronaviruses are known to create infection on birds (Wertheim et al. 2013; Guo et al. 2020).
Structure
Coronaviruses are large, roughly spherical, and consisting of particles with bulbous surface projections (Goldsmith et al. 2004). It is a single-stranded RNA-enveloped virus with the largest genomes (26.4–31.7 kb) among all known RNA viruses belonging to the Coronaviridae family. The virus envelope appears to be two separate electron-dense shells in electron micrographs (Huang et al. 2020; Mousavizadeh and Ghasemi 2020; Fehr and Perlman 2015).
The viral envelope of coronaviruses consists of three main structural proteins. These are the very large glycoprotein S (for spike), glycoprotein M (membrane protein), and the internal phosphorylated nucleocapsid protein (N), and there is another minor transmembrane protein E also present (Fig. 1) (Burrell et al. 2017; Huang et al. 2020). Each type of protein in the virions of coronaviruses shows different characteristics (Table 1). Moreover, some coronaviruses further contain a shorter spikelike surface protein termed hemagglutinin esterase (HE) (Haque et al. 2020). The surface spikes of coronavirus are homotrimers of the protein S. It comprises two subunits S1 and S2, which are associated with cell recognition and the combination of viral and cellular membranes, respectively (Coutard et al. 2020). The S1 subunit takes place at the head of the spike, and there is a receptor-binding domain (RBD). The S2 subunit creates a stem that attaches to the viral envelope's spike (Huang et al. 2020). The nucleocapsid (N) protein is linked to positive-sense single-stranded RNA in a continuous beads-on-a-string type fabric. The M proteins and nucleocapsid protect the virus skeleton when placed outside the host cell, which is responsible for the shape of the virion (Mousavizadeh and Ghasemi 2020; Agrahari et al. 2021).
Fig. 1.
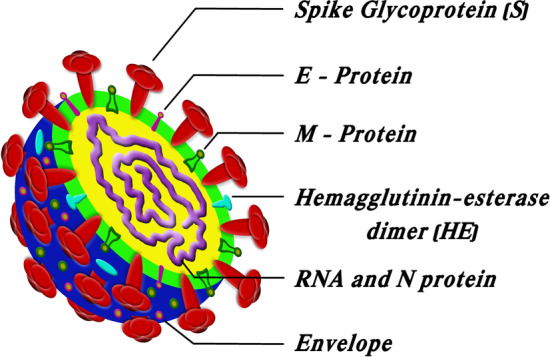
Structure of coronavirus
Table 1.
Characteristics of different types of structural protein (Haque et al. 2020; Huang et al. 2020; Boopathi et al. 2021; Mousavizadeh and Ghasemi 2020)
| Type of structural protein | Characteristics |
|---|---|
| Nucleocapsid protein (N) | N-protein coats the viral RNA genome which plays a vital role in its replication and transcription. It is responsible for encapsulating and protecting (+)-RNA, which contains the virus genome |
| Spike protein (S) |
Type I membrane glycoprotein is made of 1160–1400 amino acids S-protein facilitates viral entrance into the host cell by mediating receptor-binding and membrane fusion between the virus and the host cell |
| Envelope protein (E) | E-protein is a tiny membrane protein with 76–109 amino acids that is a minor component of the viral particle. It is involved in virus assembly, host cell membrane permeability, and virus–host cell contact |
| Membrane protein (M) |
The M-protein is most prevalent on the viral surface and is thought to be the coronavirus's major organizer. It neutralizes the virus-specific antibodies that have formed inside the host cell Determines the shape of the viral envelope |
| HE protein | The HE protein may have a role in viral entrance; it is not necessary for virus replication, but it appears to be important for natural host cell infection |
SARS-CoV-2 variants
SARS-CoV-2 is susceptible to genetic evolution, causing several variants with different features than their ancestral strains. Several variations of SARS-CoV-2 have been reported throughout this pandemic, of which only a handful are considered variants of concern (VOCs) by the WHO, considering their propensity to produce greater transmissibility or virulence, reduction in neutralization by antibodies obtained via natural infection or vaccination, the capacity to avoid detection, or a decline in treatments or vaccine efficacy (Cascella et al. 2022; WHO 2022b). Variants having particular genetic markers that have been linked to alterations are known as variants of interest (VOIs). The Centers for Disease Control and Prevention (CDC) and WHO have developed a categorization method for separating SARS-CoV-2 variations into VOCs and VOIs. An updated list (Table 2) of VOCs and VOIs has been prepared by WHO to assist in establishing monitoring and research priorities (WHO 2022b).
Table 2.
List of SARS-CoV-2 variants of concern (VOCs) and variants of interest (VOIs) (Cascella et al. 2022; WHO 2022b)
| Name of the variant | Lineage | First reported | Date of the first report |
|---|---|---|---|
| Variants of concern (VOCs) | |||
| Alpha | B.1.1.7 | UK, September 2020 | December 18, 2020 |
| Beta | B.1.351 | South Africa, May 2020 | December 18, 2020 |
| Gamma | P.1 | Brazil, November 2020 | January 11, 2021 |
| Delta | B.1.617.2 | India, October 2020 | VOI: April 4, 2021 VOC: May 11, 2021 |
| Omicron | B.1.1.529 | South Africa, November 2021 | November 26, 2021 |
| Variants of interest (VOIs) | |||
| Epsilon | B.1.427/B.1.429 | USA, March 2020 | March 5, 2021 |
| Zeta | P.2 | Brazil, April 2020 | March 17, 2021 |
| Eta | B.1.525 | Multiple countries, December 2020 | March 17, 2021 |
| Theta | P.3 | Philippines, January 2021 | March 24, 2021 |
| Iota | B.1.526 | USA, November 2020 | March 24, 2021 |
| Kappa | B.1.617.1 | India, October 2020 | April 4, 2021 |
| Lambda | C.37 | Peru, August 2020 | June 14, 2021 |
| Mu | B.1.621 | Colombia, January 2021 | August 30, 2021 |
Global situation
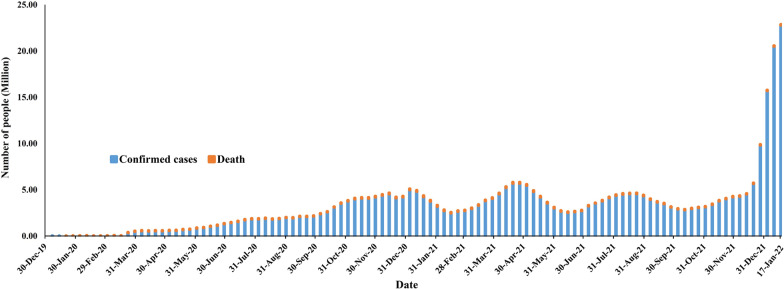
COVID-19 confirmed cases were increased within a few months from its emergence, then by the mid of February 2021, confirmed cases were reduced to some extent, and in April and August 2021, confirmed cases have increased. In January 2022, confirmed cases increased enormously. As per the WHO report, by January 25, 2022, there have been 364,191,494 confirmed cases of COVID-19, including 5,631,457 deaths. According to the WHO report, the world's confirmed cases and mortality with time during this pandemic has been explained with a graphical presentation in Fig. 2 (WHO 2022a).
Fig. 2.
Total number of confirmed cases and deaths globally at the end of every week from its emergence
Clinical manifestations
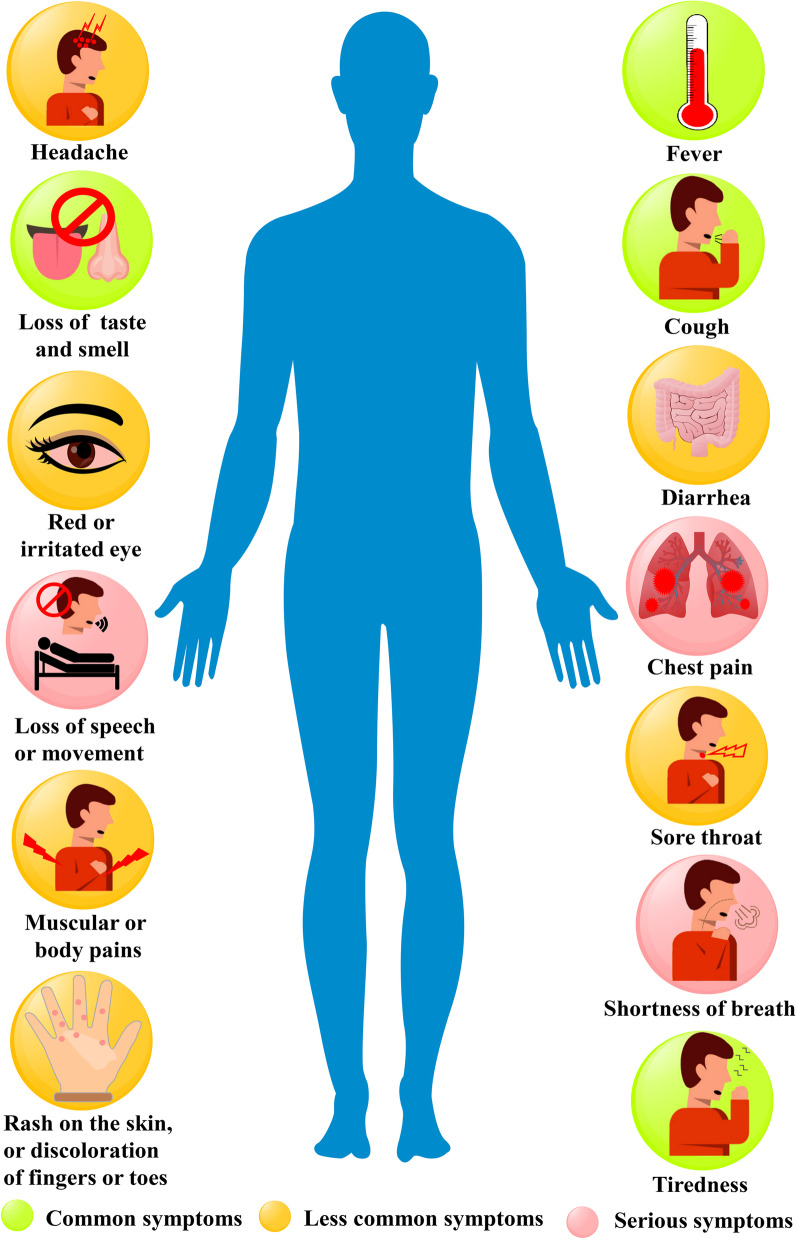
COVID-19 is a contiguous disease and is transmittable from an infected person to healthy individuals via air droplets, and the virus can affect different people in peculiar ways. Mild-to-moderate illness occurs in the infected people, while some recover without prior hospitalization. The most prevalent symptoms of COVID-19 patients are fever, muscular or body pains, dry cough, loss of taste or smell, myalgia or tiredness, pneumonia, and complex dyspnea, and less common symptoms are designated by aches and pains, sore throat, diarrhea, conjunctivitis, headache, skin rash, discolouration of fingers or toes, nausea, and vomiting. Breathing difficulties, shortness of breath, chest discomfort or pressure, and loss of speech or movement are all severe symptoms. Different symptoms of COVID-19 patients have been graphically represented in Fig. 3. Most symptoms appear 2–14 days after virus exposure in most symptomatic patients (Huang et al. 2020). For patients with health comorbidities, such as cardiovascular illnesses, renal impairment, liver dysfunction, diabetes, Parkinson's disease, and cancer, both young and old individuals' health might get complicated. Most people with mild-to-moderate illness may recover without requiring special treatment. Within 2–4 weeks of therapy, healthy people may recover from the viral illness (Harcourt et al. 2020).
Fig. 3.
Different symptoms of COVID-19 patients
Preventions
Prevention is better than cure; hence, the spread of this disease can be controlled by paying constant attention to some basic preventative measures (Fig. 4) given below.
Get vaccine on time, and follow the local vaccination guideline.
Maintain social distancing of at least 1-m space between yourself and others to reduce your threat of infection when others cough, sneeze, or talk (Huang et al. 2021).
Use a face mask when being around other people (Clapham and Cook 2021).
Frequently, in a proper manner, clean and rub your hands (at least 20 s.) with an alcohol-based hand wash or usage soap, followed by rinsing with water. If hand wash or soap is not available, use alcohol-based hand sanitizer (minimum 60% alcohol).
Avoid going to crowded, congested, and/or involving close touch areas.
Surfaces that are often handled, such as doorknobs, faucets, and phone displays, should be cleaned and disinfected regularly.
Cover the nose and the mouth with the bent elbow or tissue during coughing or sneezing. After that, throw away the used tissue in a closed container and wash hands to maintain good respiratory hygiene.
If anyone is feeling unwell with some COVID-19 mild symptoms, they should stay home and self-isolate until they recover. If the person develops fever, cough, or difficulty breathing, get medical treatment, call the doctor ahead of time if possible, and follow your local health authority's instructions (WHO 2022c).
Fig.4.
Preventive measures to control COVID-19 infection
Present treatments of COVID-19
Since the beginning of the COVID-19 pandemic, different measures have been taken to treat COVID-19 infection. Still, there is no clinically validated and specific antiviral medication available to treat COVID-19 infection at this time. Patients are usually provided medical treatment or supportive therapy, such as oxygen supplementation and mechanical ventilation, to alleviate symptoms (Cucinotta and Vanelli 2020). For the treatment of COVID-19 infection, many strategies have been explored, and repurposing drugs is one of them. Some of the antivirals that have been repurposed include remdisivir, lopivir, lopinavir–ritonavir, ribavirin, baloxavirmarboxil, favipiravir, and arbidol/umifenovir. Other drugs that show potential action against COVID-19 but are not antivirals include chloroquine, hydroxychloroquine, corticosteroids, losartan, statins, interferons, nitric oxide, and epoprostenol, which are instances for the repurposing strategy. Some medications have been suggested for treatment in critically sick patients. Tocilizumab, siltuximab, sarilumab, anakinra, and ruxolitinib were used to treat COVID-19 individuals who developed cytokine release syndrome (CRS). Antibiotics like azithromycin are frequently used to treat secondary infections (Ginsburg and Klugman 2020). The vaccine is another better approach for the prevention of COVID-19 infection. Though various vaccines against the SARS-CoV-2 virus have recently been approved, availability remains a major barrier, and public acceptance has become a contentious issue. But day by day, SARS-CoV-2 virus becomes more contagious and harmful due to their new mutants called variants. Recently the omicron variant's capacity to evade vaccine-elicited immunity is a major concern. So, there is a requirement for potential therapeutic molecules to treat the infection. Several antiviral drugs might be potentially repurposed or developed into viable treatments for this novel coronavirus. However, several clinical trials exploring possible therapies are now underway.
Remdesivir
Remdesivir is a broad-spectrum antiviral drug with antiviral efficacy in vitro against SARS-CoV-2 by inhibiting viral replication (Wang et al. 2020). According to Holshue et al. (2020), the first instance in the USA (reported in Washington State) was treated with an intravenous injection of remdesivir, which alleviated symptoms and had no adverse effects. In randomized controlled clinical studies, remdesivir was shown to be superior to placebo in decreasing the time to recovery in patients hospitalized with mild-to-severe COVID-19 infection (Beigel et al. 2020; Goldman et al. 2020). Remdesivir was approved by US Food and Drug Administration on October 22, 2020, for the treatment of COVID-19 necessitating hospitalization in adult and children patients 12 years old and weighing a minimum of 40 kg (US FDA 2021).
Favipiravir
Favipiravir is a broad-spectrum antiviral and anti-influenza drug that restricts viral RNA replication by inhibition of RNA polymerase (Fang et al. 2020). Several studies have revealed that favipiravir can effectively treat COVID-19, particularly in patients with mild-to-moderate disease. Favipiravir has been demonstrated in certain investigations to lower viral load in the upper respiratory tract and the lungs (Shirali and Daikoku 2020). Additional well-designed trials, including therapy dosage and duration investigations, are required before conclusive findings can be reached (Manabe et al. 2021).
Lopinavir–Ritonavir
It is an FDA-approved antiretroviral medication for HIV disease that was recommended as an antiviral treatment for COVID-19 during the early stages of the pandemic. A randomized control study in individuals hospitalized with severe COVID-19 found no benefit from lopinavir–ritonavir therapy compared to the standard of care (Cao et al. 2020).
Arbidol
Arbidol is also known as umifenovir, a Russian antiviral drug that seems to be effective against many viruses, including influenza, respiratory syncytial virus (RSV), poliovirus, rhinovirus, Zika virus, hepatitis and SARS-CoV, and MERS-CoV coronaviruses (Gao et al. 2020). The trimerization of SARS-CoV-2 spike glycoprotein, which is important for cell adhesion and penetration, may be effectively blocked or hampered by arbidol. When the trimerization of the SARS-CoV-2 spike glycoprotein is blocked, a bare or immature virus is formed, which is less infectious (Vankadari 2020). As a host-targeting drug, Arbidol also disrupts many steps of viral cycle replication, including entrance, attachment, internalization, and membrane fusion. Arbidol substantially improved the clinical state of hospitalized COVID-19 patients, including peripheral oxygen saturation, the need for ICU admissions, the length of hospitalization, chest computed tomography involvements, white blood cells, and erythrocyte sedimentation rate, according to a randomized controlled trial (Nojomi et al. 2020).
Ivermectin
Ivermectin is a broad-spectrum antiparasitic drug that the FDA has authorized. Based on an in vitro research that indicated the reduction of SARS-CoV-2 replication, it was utilized globally to treat COVID-19. Later on, a randomized control study including 476 adult patients with moderate COVID-19 disease could not produce substantial improvement or remission of symptoms after being randomized to take ivermectin for 5 days or a placebo (Caly et al. 2020).
Chloroquine and hydroxychloroquine
Chloroquine and hydroxychloroquine have a long history of use in the inhibition and treatment of malaria and the treatment of chronic inflammatory disorders such as rheumatoid arthritis. Hydroxychloroquine is derived from chloroquine, and initially, they have been proposed as an antiviral treatment for COVID-19 (Gasmi et al. 2021; Horby et al. 2020). Later on, a randomized controlled trial reported that hydroxychloroquine was administered as a postexposure prophylactic within 4 days of a high-risk or moderate-risk COVID-19 exposure, and hydroxychloroquine did not protect against COVID-19-related illness or infection (Boulware et al. 2020; Mitjà et al. 2021). Another open-label randomized controlled trial reported that chloroquine/hydroxychloroquine treatment in patients brought to the hospital with severe COVID-19 resulted in clinical deterioration and increased rates of invasive mechanical ventilation and renal failure (Réa-Neto et al. 2021). The Food and Drug Administration (FDA) granted emergency use authorization (EUA) to chloroquine and hydroxychloroquine in May 2020 to treat severe cases of COVID-19 in hospital settings. The FDA, however, removed the emergency use authorization for chloroquine and hydroxychloroquine on June 15, 2020, in situations where clinical studies were lacking (Omokhua-Uyi and Staden 2021).
Anakinra
Anakinra is a 17-kD recombinant human IL-1 receptor antagonist (blocking both IL-1α and IL-1β), with a short half-life of around 3–4 h and a favorable safety profile, authorized for the treatment of rheumatoid arthritis, gouty arthritis, and other uncommon auto-inflammatory disorders. Anakinra is a safe and effective treatment strategy for delaying mechanical ventilation, reducing the need for supplemental oxygen, and regulating SARS-CoV-2-triggered inflammation in patients with severe COVID-19 pneumonia and a high oxygen need (Balkhair et al. 2021). Later on, Tharaux et al. reported that the patients with COVID-19 and mild-to-moderate pneumonia, a randomized clinical study found that anakinra was ineffective in lowering the requirement for noninvasive or mechanical ventilation or mortality. More research is needed to determine the effectiveness of anakinra in additional categories of individuals with more severe COVID-19 and in different settings (Tharaux et al. 2021).
Tocilizumab
Tocilizumab, a monoclonal anti-interleukin-6 (IL-6) antibody, has been identified as a possible therapeutic option for COVID-19 patients at risk of cytokine storms. IL‐6 is an essential cytokine in inflammatory reaction and immune response and is one of the most significant cytokines involved in COVID‐19-induced cytokine storms (Luo et al. 2020). However, a different study report showed that it is an effective treatment preference for critically ill COVID-19 patients, as it substantially reduces their oxygen requirements and their ICU stay, median hospital stay, and death. COVID-19-induced cytokine storms are effectively treated with this drug by decreasing the level of IL-6 (Chachar et al. 2021; Luo et al. 2020).
Sotrovimab
Sotrovimab, a monoclonal antibody, has been developed to treat various types of coronaviruses, including COVID-19. It is primarily used to treat mild and moderate COVID-19 infection and prevent the progression of the disease condition from critical to severe. A retrospective study reported that the use of sotrovimab significantly improved symptom resolution, outcome, laboratory marker, and decreased hospitalization rate in individuals with mild and moderate COVID-19. This study suggests the use of sotrovimab in the early stages of COVID-19 treatment (Elesdoudy 2021). Later on, Guta and his group reported that the sotrovimab lowered the probability of disease progression in high-risk patients with mild-to-moderate COVID-19. And there were no threatening signs found during the study (Gupta et al. 2021). The use of sotrovimab for treating mild or moderate COVID-19 in patients at high risk of hospitalization has also been conditionally recommended by the WHO (Kmietowicz 2022).
Dexamethasone
Dexamethasone is a glucocorticoid drug used to treat COVID-19 infection. In hospitalized patients with COVID-19 and respiratory failure who needed supplementary oxygen or mechanical ventilation, a low dose of dexamethasone (6 mg daily for 10 days) reduced death. Data also suggest that mortality may be higher in hospitalized patients who do not receive oxygen (Matthay and Thompson 2020).
Ruxolitinib
Ruxolitinib is an inhibitor of JAK 1 and 2 used to treat myelofibrosis (Wang et al. 2020). JAK inhibitors reduced the need for invasive mechanical ventilation and improved survival in people with COVID-19, most significantly baricitinib (Chen et al. 2021). Ruxolitinib, a JAK inhibitor, is under phase III clinical trial of patients with COVID-19 associated with cytokine storm and acute respiratory disorder syndrome (Valenzuela-Almada et al. 2021).
Baricitinib
Baricitinib is a Janus kinase (JAK) 1 and 2 inhibitor used to treat rheumatoid arthritis (Cantini et al. 2020). One of the most usually prescribed medicines for COVID-19-related pneumonia is baricitinib. WHO also recommends it for the treatment of COVID-19 (Kmietowicz 2022). It is a well-known medication with a strong affinity for infected cells (Richardson et al. 2020). According to statistics, creatine kinase in patients who took bacitracin surpassed the allowed threshold. Alternatively, high creatine kinase levels might make it difficult to start baricitinib therapy (Praveen et al. 2020). A randomized, double-blind, placebo-controlled study found that combining the baricitinib with remdesivir to treat hospitalized patients with COVID-19 pneumonia was safe and preferable to remdesivir alone. The confluence was associated with a lower risk of severe side effects (Kalil et al. 2021).
Convalescent plasma therapy
Convalescent plasma treatment is a type of adoptive immunotherapy that can treat a wide range of illnesses. Passive immunity can be created by using antiviral antibodies from recovered individuals to treat additional patients with a specific infectious illness. Other respiratory viral diseases, including SARS-CoV-1, H1N1 influenza, MERS-CoV, West Nile virus, and Ebola virus, have recently been treated with this technique (Marano et al. 2016). Hyperimmune immunoglobulin showed a statistically significant reduction in the risk of death among those treated with convalescent plasma or serum in all of the investigations (Al-Tawfiq and Arabi 2020). This treatment has played an essential role in treating COVID-19 patients when no effective antiviral drugs are available. In an initial uncontrolled case series, five critically sick patients with COVID-19 and acute respiratory distress syndrome underwent convalescent plasma treatment, and the result showed improvement in their clinical status (Shen et al. 2020). Later on, Duan et al. (2020) reported that a single dosage of CP (200 mL) was well tolerated and could considerably raise or sustain neutralizing antibodies at a high level, resulting in viremia disappearing in 7 days. However, clinical symptoms improved quickly over 3 days. This suggests that CP might be a viable rescue strategy for severe COVID-19 and that a randomized study is necessary. Due to sample and experimental design constraints, a definitive conclusion on the potential efficacy of this form of treatment cannot be made, and further clinical observations will be required.
Azithromycin
Antibiotic azithromycin belongs to the macrolide family used to treat a wide range of Gram-positive bacterial infections. By interfering with protein synthesis and mRNA translation, it inhibits bacterial growth. This antibiotic is useful in the treatment of bacterial pneumonia. In addition to its antibacterial properties, it possesses immunomodulatory and anti-inflammatory properties, which may help to reduce the difficulties caused by respiratory viral infections such as COVID-19 (Pani et al. 2020; Echeverría-Esnal et al. 2021; Oldenburg and Doan 2020). However, according to the meta-analysis study by Mangkuliguna et al. (2021), in COVID-19 patients, azithromycin did not provide significant clinical improvement, although it was well tolerated and safe to use. Because of the absence of therapeutic advantages, azithromycin should be avoided to use routinely unless bacterial pneumonia is present. So, further clinical studies are required to elucidate the role of azithromycin in COVID-19 treatment.
Vaccines
The world has taken different significant actions to control the COVID-19 pandemic from its beginning. However, the disease spreads unabated, wreaking havoc on people's health, social lives, and economies. Therefore, the prevention and control of the COVID-19 pandemic were immediately needed. Several vaccines have been studied, produced, tested, and assessed at a breakneck speed, and in 2021 many vaccines have been approved. According to the WHO, more than 9.8 billion vaccination doses had been delivered as of January 27, 2022 (WHO 2022a). The immune system is triggered by vaccination, resulting in the generation of neutralizing antibodies against SARS-CoV-2. But the variants of the virus are always a major cause of concern. Vaccines have long been known to lose their potency over time. As a result, various countries have authorized the administration of an additional dose of vaccine (known as a booster) to people 3–5 months after their vaccination cycle is completed. This method appears to be efficient in preserving SARS-CoV-2 immunity. Some important vaccines of the world are listed in Table 3.
Table 3.
Some approved COVID-19 vaccines with their developer, origin, type, dosage and storage conditions
| Name of the vaccine | Developer | Developer country | Vaccine type | No. of doses | Efficacy (%) | Storage (°C) | References |
|---|---|---|---|---|---|---|---|
| BNT162b2 (Comirnaty) | Pfizer-BioNtech | USA and Germany | mRNA | IM (2) | 95 | − 80 to – 60 | Polack et al. (2020) |
| mRNA-1273 (Moderna COVID-19 Vaccine) | Moderna | USA | mRNA | IM (2) | 94.0 | − 25 to − 15 | Baden et al. (2021) |
| Ad26.COV2.S (Janssen COVID-19 vaccine) | Janssen Pharmaceuticals | USA and Germany | Viral vector | IM 1 | 81.7 | 2–8 | Sadoff et al. (2021) |
| ChAdOx1 nCoV-19 (AZD-1222) (Covishield) | Oxford-AstraZeneca | UK, Sweden and India | Viral vector | IM (2) | 81 | 2–8 | Voysey et al. (2021) |
| Sputnik V | Gamaleya Research Institute | Russia | Viral vector | IM (2) | 91.6 | 2–8 | Logunov et al. (2021) |
| BBV152 (Covaxin) | Bharat Biotech | India | Inactivated | IM (2) | 78 | 2–8 | Ella et al. (2021) |
| CronaVac | Sinovac | China | Inactivated | IM (2) | 50.4 | 2–8 | Zhang et al. (2021) |
| BBIBP-CorV | Sinopharm | China | Inactivated | IM (2) | 79.3 | 2–8 | Xia et al. (2021) |
| NVX-CoV2373 | Novavax | USA | Protein subunit | IM (2) | 96 | 2–8 | Shinde et al. (2021) |
| CVnCoV | CureVac | Germany | mRNA | IM (2) | 48.2 | 2–8 | Hadj Hassine (2021) |
| AD5-nCOV (Convidecia) | CanSino Biologics | China | Viral vector | IM, IN (1) | 63 | 2–8 | Wu et al. (2021) |
| SCB-2019 | Clover Biopharmaceu ticals | China | Protein subunit | IM | 67 | 2–8 | Bravo et al. (2022) |
| ZyCoV-D | Cadila Healthcare | India | DNA plasmid | ID (3) | 66.6 | 2–8 | Chavda et al. (2021) |
| ZF2001 (Zifivax) | Anhui Zhifei Longcom | China | Protein subunit | IM (3) | 82 | 2–8 | Yang et al. (2021) |
IM intramuscular, IN intranasal, ID intradermal
Conclusions
Extensive research has been being carried out on SARS-CoV- 2 and its different variants to combat them with the new treatment strategies. With the continued enormously hard efforts to prevent the spread of SARS-CoV- 2 globally, we hope that this pandemic disease will subside in the coming couple of months like SARS and MERS. Some strategies like vaccination, social distancing, self-quarantine, stay home, stay safe, night curfew, partial or complete lockdown, maintaining hygiene, wearing masks, and using hand sanitizer frequently have been imposed to control the transmission of COVID-19. Moreover, parallel clinical trials have been conducted on the proposed therapeutic options, including vaccines and potent drugs repurposed for COVID. The global availability of COVID-19 vaccines has been compensated for and overcome by international collaborations and competitions among pharmaceutical industries and researchers in scientific institutions, starting with the historically fast clinical trials. To produce safe and effective vaccines, various technologies have been used. Presently, multiple vaccines have been approved, which are significantly efficacious toward prevention of COVID-19. Some of them are also showing promising results against newer strains. This review would help the readers to understand the current scenario of the COVID-19 pandemic.
Future prospective
The pandemic situation by SARS-CoV-2 is still wreaking havoc all over the world due to the rapid spreading ability of the virus, the lack of risk assessment, and the inclination to precipitate severe illness in co-morbid conditions. The associated enhancement in the mortality rate is genuinely an alarming public health issue. Disease control has become more complex with the increasing number of COVID-19 cases per day, particularly because no effective drugs against COVID-19 are being identified. In the current review, we presented a brief overview of SARS-CoV-2 infection ongoing treatment options based on the significant recent findings on some therapeutic strategies that could be used for future treatment of SARS-CoV-2 infection. Nonetheless, additional advanced research in the area and clinical studies would reveal the significance of the findings of the current review.
Acknowledgements
The authors sincerely acknowledge the University of North Bengal, India for providing support and facilities, and e-resources to conduct this work.
Abbreviations
- WHO
World Health Organization
- COVID-19
Novel coronavirus
- US FDA
US Food and Drug Administration
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SARS-CoV
Severe acute respiratory syndrome coronavirus
- SARS
Severe acute respiratory syndrome
- MERS
Middle East respiratory syndrome
- HE
Hemagglutinin esterase
- RBD
Receptor-binding domain
- VOCs
Variants of concern
- VOIs
Variants of interest
- CDC
Centers for Disease Control and Prevention
- CRS
Cytokine release syndrome
Authors' contributions
S.G. conducted literature review, extracted relevant information, prepared the tables, and reviewed and revised the manuscript; S.S. conducted literature review, extracted relevant information, drafted a part of the manuscript, and reviewed; R.S. conducted literature review, extracted relevant information, and drafted a part of the manuscript; P.P. compiled the data and critically reviewed the manuscript, prepared figures, and revised the manuscript; G.N. and R.S. critically reviewed the manuscript for important intellectual content; T.K.D. conceived and designed the study, critically reviewed the manuscript for important intellectual content, and revised the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdul-Fattah S, Pal A, Kaka N, Kakodkar P. History and recent advances in coronavirus discovery. In: Roy K, editor. In silico modeling of drugs against coronaviruses. Methods in pharmacology and toxicology. New York: Humana; 2021. [Google Scholar]
- Agrahari R, Mohanty S, Vishwakarma K, Nayak SK, Samantaray D, Mohapatra S. Update vision on COVID-19: structure, immune pathogenesis, treatment and safety assessment. Sens Int. 2021;2:100073. doi: 10.1016/j.sintl.2020.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tawfiq JA, Arabi Y. Convalescent plasma therapy for coronavirus infection: experience from MERS and application in COVID-19. Hum Vaccines Immunother. 2020;16(12):2973–2979. doi: 10.1080/21645515.2020.1793712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/nejmoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkhair A, Al-Zakwani I, Al Busaidi M, Al-Khirbash A, Al Mubaihsi S, BaTaher H, Al Aghbari J, Al Busaidi I, Al Kindi M, Baawain S, Al Alawi A. Anakinra in hospitalized patients with severe COVID-19 pneumonia requiring oxygen therapy: results of a prospective, open-label, interventional study. Int J Infect Dis. 2021;103:288–296. doi: 10.1016/j.ijid.2020.11.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/nejmoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boopathi S, Poma AB, Kolandaivel P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J Biomol Struct Dyn. 2021;39(9):3409–3418. doi: 10.1080/07391102.2020.1758788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, Skipper CP, Nascene AA, Nicol MR, Abassi M, Engen NW. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020;383(6):517–525. doi: 10.1056/nejmoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo L, Smolenov I, Han HH, Li P, Hosain R, Rockhold F, Clemens SA, Roa C, Jr, Borja-Tabora C, Quinsaat A, Lopez P. Efficacy of the adjuvanted subunit protein COVID-19 vaccine, SCB-2019: a phase 2 and 3 multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2022;399(10323):461–472. doi: 10.1016/s0140-6736(22)00055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell CJ, Howard CR, Murphy FA. Fenner and White's medical virology. 5. London: Academic Press; 2017. pp. 437–446. [Google Scholar]
- Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantini F, Blandizzi C, Niccoli L, Petrone L, Goletti D. Systematic review on tuberculosis risk in patients with rheumatoid arthritis receiving inhibitors of Janus Kinases. Expert Opin Drug Saf. 2020;19(7):861–872. doi: 10.1080/14740338.2020.1774550. [DOI] [PubMed] [Google Scholar]
- Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X. A trial of Lopinavir–Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/nejmoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, Rech R, Colombo R, Antinori S, Corbellino M, Galli M. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20(10):1135–1140. doi: 10.1016/s1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella M, Rajnik M, Aleem A, Dulebohn S, Di Napoli R (2022) Features, evaluation, and treatment of coronavirus (COVID-19). In: Stat Pearls. Stat Pearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK554776. Accessed 29 Jan 2022 [PubMed]
- Chachar AZ, Khan KA, Iqbal J, Shahid AH, Asif M, Fatima SA, Khan AA, Younis BB. "Tocilizumab-an option for patients with COVID-19 associated cytokine release syndrome: a single center experience", a retrospective study-original article. Ann Med Surg. 2021;63:102165. doi: 10.1016/j.amsu.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavda VP, Hossain MK, Beladiya J, Apostolopoulos V. Nucleic acid vaccines for COVID-19: a paradigm shift in the vaccine development arena. Biologics. 2021;1(3):337–356. doi: 10.3390/biologics1030020. [DOI] [Google Scholar]
- Chen CX, Wang JJ, Li H, Yuan LT, Gale RP, Liang Y. JAK-inhibitors for coronavirus disease-2019 (COVID-19): a meta-analysis. Leukemia. 2021 doi: 10.1038/s41375-021-01266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilamakuri R, Agarwal S. COVID-19: characteristics and therapeutics. Cells. 2021;10(2):206. doi: 10.3390/cells10020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham HE, Cook AR. Face masks help control transmission of COVID-19. Lancet Digit Health. 2021;3(3):e136–137. doi: 10.1016/s2589-7500(21)00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman VM, Muth D, Niemeyer D, Drosten C. Hosts and sources of endemic human coronaviruses. Adv Virus Res. 2018;100:163–188. doi: 10.1016/bs.aivir.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutard B, Valle C, de Lamballerie X, Canard B, Seidah N, Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176:104742. doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, Zhou M, Chen L, Meng S, Hu Y, Peng C. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. PNAS. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverría-Esnal D, Martin-Ontiyuelo C, Navarrete-Rouco ME, De-Antonio Cuscó M, Ferrández O, Horcajada JP, Grau S. Azithromycin in the treatment of COVID-19: a review. Expert Rev Anti Infect Ther. 2021;19(2):147–163. doi: 10.1080/14787210.2020.1813024. [DOI] [PubMed] [Google Scholar]
- Elesdoudy A. Sotrovimab: is it effective in early treatment of mild and moderate COVID-19 infections? A retrospective study. Egypt J Bronchol. 2021;15:56. doi: 10.1186/s43168-021-00104-8. [DOI] [Google Scholar]
- Ella R, Reddy S, Jogdand H, Sarangi V, Ganneru B, Prasad S, Das D, Raju D, Praturi U, Sapkal G, Yadav P. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021;21(7):950–961. doi: 10.1016/s1473-3099(21)00070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang QQ, Huang WJ, Li XY, Cheng YH, Tan MJ, Liu J, Wei HJ, Meng Y, Wang DY. Effectiveness of favipiravir (T-705) against wild-type and oseltamivir-resistant influenza B virus in mice. Virology. 2020;545:1–9. doi: 10.1016/j.virol.2020.02.005. [DOI] [PubMed] [Google Scholar]
- Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Chen S, Wang K, Chen R, Guo Q, Lu J, Wu X, He Y, Yan Q, Wang S, Wang F. Clinical features and efficacy of antiviral drug, Arbidol in 220 nonemergency COVID-19 patients from East-West-Lake Shelter Hospital in Wuhan: a retrospective case series. Virol J. 2020;17(1):162. doi: 10.1186/s12985-020-01428-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasmi A, Peana M, Noor S, Lysiuk R, Menzel A, Benahmed AG, Bjørklund G. Chloroquine and hydroxychloroquine in the treatment of COVID-19: the never-ending story. Appl Microbiol Biotechnol. 2021;105(4):1333–1343. doi: 10.1007/s00253-021-11094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghinai I, McPherson TD, Hunter JC, Kirking HL, Christiansen D, Joshi K, Rubin R, Morales-Estrada S, Black SR, Pacilli M, Fricchione MJ. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. The Lancet. 2020;395(10230):1137–1144. doi: 10.1016/s0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg AS, Klugman KP. COVID-19 pneumonia and the appropriate use of antibiotics. Lancet Glob Health. 2020;8(12):e1453–e1454. doi: 10.1016/s2214-109x(20)30444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, Spinner CD, Galli M, Ahn MY, Nahass RG, Chen YS. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020;383(19):1827–1837. doi: 10.1056/nejmoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith CS, Tatti KM, Ksiazek TG, Rollin PE, Comer JA, Lee WW, Rota PA, Bankamp B, Bellini WJ, Zaki SR. Ultrastructural characterization of SARS coronavirus. Emerg Infect Dis. 2004;10(2):320–326. doi: 10.3201/eid1002.030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DS, Du B. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y-R, Cao Q-D, Hong Z-S, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Mil Med Res. 2020;7(1):1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Gonzalez-Rojas Y, Juarez E, Crespo Casal M, Moya J, Falci DR, Sarkis E, Solis J, Zheng H, Scott N, Cathcart AL. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385(21):1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- Hadj Hassine I. Covid-19 vaccines and variants of concern: a review. Rev Med Virol. 2021 doi: 10.1002/rmv.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque SM, Ashwaq O, Sarief A, Azad John Mohamed AK. A comprehensive review about SARS-CoV-2. Future Virol. 2020;15(9):625–648. doi: 10.2217/fvl-2020-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt J, Tamin A, Lu X, Kamili S, Sakthivel SK, Murray J, Queen K, Tao Y, Paden CR, Zhang J, Li Y. Severe acute respiratory syndrome coronavirus 2 from patient with coronavirus disease, United States. Emerg Infect Dis. 2020;26(6):1266. doi: 10.3201/eid2606.200516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/nejmoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P, Mafham M, Linsell L, et al. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383(21):2030–2040. doi: 10.1056/nejmoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Yang C, Xu X-f, Xu W, Liu S-w. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41(9):1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Wang J, Cai J, Yao S, Chan PK, Tam TH, Hong YY, Ruktanonchai CW, Carioli A, Floyd JR, Ruktanonchai NW. Integrated vaccination and physical distancing interventions to prevent future COVID-19 waves in Chinese cities. Nat Hum Behav. 2021;5(6):695–705. doi: 10.1038/s41562-021-01063-2. [DOI] [PubMed] [Google Scholar]
- Johnson G, Morawska L, Ristovski Z, Hargreaves M, Mengersen K, Chao CY, Wan MP, Li Y, Xie X, Katoshevski D, Corbett S. Modality of human expired aerosol size distributions. J Aerosol Sci. 2011;42(12):839–851. doi: 10.1016/j.jaerosci.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn JS, McIntosh K. History and recent advances in coronavirus discovery. Pediatr Infect Dis J. 2005;24(11):S223–S227. doi: 10.1097/01.inf.0000188166.17324.60. [DOI] [PubMed] [Google Scholar]
- Kalil AC, Patterson TF, Mehta AK, Omashek KM, Wolfe CR, Ghazaryan V, Marconi VC, Ruiz-Palacios GM, Hsieh L, Kline S, Tapson V. Baricitinib plus Remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384(9):795–807. doi: 10.1056/nejmoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmietowicz Z. Covid-19: WHO recommends baricitinib and sotrovimab to treat patients. BMJ. 2022;376:o97. doi: 10.1136/bmj.o97. [DOI] [PubMed] [Google Scholar]
- Lau SKP, Luk HKH, Wong ACP, Li KS, Zhu L, He Z, Fung J, Chan TT, Fung KS, Woo PC. Possible bat origin of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7):1542–1547. doi: 10.3201/eid2607.200092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, Kovyrshina AV, Lubenets NL, Grousova DM, Erokhova AS, Botikov AG. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/s0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe T, Kambayashi D, Akatsu H, Kudo K. Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2021;21(1):489. doi: 10.1186/s12879-021-06164-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangkuliguna G, Glenardi SN, Pramono LA. Efficacy and safety of Azithromycin for the treatment of COVID-19: a systematic review and meta-analysis. Tuberc Respir Dis (Seoul) 2021 doi: 10.4046/trd.2021.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marano G, Vaglio S, Pupella S, Facco G, Catalano L, Liumbruno GM, Grazzini G. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016;14(2):152–157. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay MA, Thompson BT. Dexamethasone in hospitalised patients with COVID-19: addressing uncertainties. Lancet Respir Med. 2020;8(12):1170–1172. doi: 10.1016/s2213-2600(20)30503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitjà O, Corbacho-Monné M, Ubals M, Alemany A, Suñer Navarro C, Tebé C, Tobias A, Peñafiel J, Ballana E, Pérez CA, Admella P. A cluster-randomized trial of hydroxychloroquine for prevention of Covid-19. N Engl J Med. 2021;384(5):417–427. doi: 10.1056/nejmoa2021801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavizadeh L, Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis. J Microbiol Immunol Infect. 2020;54(2):159–163. doi: 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojomi M, Yassin Z, Keyvani H, Makiani MJ, Roham M, Laali A, Dehghan N, Navaei M, Ranjbar M. Effect of Arbidol (Umifenovir) on COVID-19: a randomized controlled trial. BMC Infect Dis. 2020;20(1):954. doi: 10.1186/s12879-020-05698-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg CE, Doan T. Azithromycin for severe COVID-19. Lancet. 2020;396(10256):936–937. doi: 10.1016/S0140-6736(20)31863-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omokhua-Uyi AG, Van Staden J. Natural product remedies for COVID-19: a focus on safety. S Afr J Bot. 2021;139:386–398. doi: 10.1016/j.sajb.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paden CR, Yusof M, Al Hammadi ZM, Ueen K, Tao Y, Eltahir YM, Elsayed EA, Marzoug BA, Bensalah OK, Khalafalla AI, Al Mulla M. Zoonotic origin and transmission of Middle East respiratory syndrome coronavirus in the UAE. Zoonoses Public Health. 2018;65(3):322–333. doi: 10.1111/zph.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal M, Berhanu G, Desalegn C, Kandi V. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): an update. Cureus. 2020;12(3):e7423. doi: 10.7759/cureus.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani A, Lauriola M, Romandini A, Scaglione F. Macrolides and viral infections: focus on azithromycin in COVID-19 pathology. Int J Antimicrob Agents. 2020;56(2):106053. doi: 10.1016/j.ijantimicag.2020.106053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyod S, Ho CT, Sheen LY. Dietary therapy and herbal medicine for COVID-19 prevention: a review and perspective. J Tradit Complement Med. 2020;10(4):420–427. doi: 10.1016/j.jtcme.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Marc GP, Moreira ED, Zerbini C, Bailey R. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/nejmoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praveen D, Puvvada RC, Vijey Aanandhi M. Janus kinase inhibitor baricitinib is not an ideal option for management of COVID-19. Int J Antimicrob Agents. 2020;55(5):105967. doi: 10.1016/j.ijantimicag.2020.105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réa-Neto Á, Bernardelli RS, Câmara BMD, Reese FB, Queiroga MVO, Oliveira MC. An open-label randomized controlled trial evaluating the efficacy of chloroquine/hydroxychloroquine in severe COVID-19 patients. Sci Rep. 2021;11(1):9023. doi: 10.1038/s41598-021-88509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, Rawling M, Savory E, Stebbing J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30–e31. doi: 10.1016/s0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B, Offergeld K. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena SK, Kumar S, Maurya VK, Sharma R, Dandu HR, Bhatt MLB. Current insight into the novel coronavirus disease 2019 (COVID-19) In: Saxena SK, editor. Coronavirus disease 2019 (COVID-19) Singapore: Springer; 2020. pp. 1–8. [Google Scholar]
- Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F, Li D, Yang M, Xing L, Wei J. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde V, Bhikha S, Hoosain Z, Archary M, Bhorat Q, Fairlie L, Lalloo U, Masilela MS, Moodley D, Hanley S, Fouche L. Efficacy of NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384(20):1899–1909. doi: 10.1056/nejmoa2103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraki K, Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Ther. 2020;209:107512. doi: 10.1016/j.pharmthera.2020.107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharaux P-L, Pialoux G, Pavot A, Mariette X, Hermine O, Resche-Rigon M, Porcher R, Ravaud P, Bureau S, Dougados M, Tibi A. Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet Respir Med. 2021;9(3):295–304. doi: 10.1016/s2213-2600(20)30556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US FDA (2021) Coronavirus (COVID-19) drugs. https://www.fda.gov/drugs/emergency-preparedness-drugs/coronavirus-covid-19-drugs. Accessed 18 July 2021
- Valenzuela-Almada MO, Putman MS, Duarte-García A. The protective effect of rheumatic disease agents in COVID-19. Best Pract Res Clin Rheumatol. 2021;35(1):101659. doi: 10.1016/j.berh.2021.101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankadari N. Arbidol: A potential antiviral drug for the treatment of SARS-CoV-2 by blocking trimerization of the spike glycoprotein. Int J Antimicrob Agents. 2020;56(2):105998. doi: 10.1016/j.ijantimicag.2020.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/s0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheim JO, Chu DK, Peiris JS, Kosakovsky Pond SL, Poon LL. A case for the ancient origin of coronaviruses. J Virol. 2013;87(12):7039–7045. doi: 10.1128/jvi.03273-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2022a) Coronavirus (COVID-19) Dashboard. https://covid19.who.int/. Accessed 28 Jan 2022a
- WHO (2022b) Tracking SARS-CoV-2 variants. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants. Accessed 28 Jan 2022b
- WHO (2022c) Coronavirus disease (COVID-19) advice for the public. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed Jan 29 2022c
- Wu S, Huang J, Zhang Z, Wu J, Zhang J, Hu H, Zhu T, Zhang J, Luo L, Fan P, Wang B. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect Dis. 2021;21(12):1654–1664. doi: 10.1016/s1473-3099(21)00396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, Tan W, Wu G, Xu M, Lou Z, Huang W. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39–51. doi: 10.1016/s1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Li Y, Dai L, Wang J, He P, Li C, Fang X, Wang C, Zhao X, Huang E, Wu C. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021;21(8):1107–1119. doi: 10.1016/s1473-3099(21)00127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Wu Q, Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr Biol. 2020;30(7):1346–1351.e1342. doi: 10.1016/j.cub.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W, Chen X. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi: 10.1016/s1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu FC, Li YH, Guan XH, Hou LH, Wang WJ, Li JX, Wu SP, Wang BS, Wang Z, Wang L, Jia SY. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395(10240):1845–1854. doi: 10.1016/s0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Wei L, Niu P. The novel coronavirus outbreak in Wuhan, China. Glob Health Res Policy. 2020;5:6. doi: 10.1186/s41256-020-00135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.