Abstract
A series of ethylene copolymers with long-chain α-olefins [LCAOs, 1-dodecene (DD), 1-tetradecene (TD), 1-hexadecene (HD)] and various LCAO contents were prepared, and their thermal properties, including effects of LCAO content and side chain length, were explored. The Cp*TiCl2(O-2,6-iPr2-4-SiEt3-C6H2)–MAO catalyst system afforded rather high-molecular-weight copolymers with unimodal molecular weight distributions and uniform compositions (confirmed by DSC thermograms). In addition to the melting temperatures (Tm values) corresponding to the so-called main chain crystallization (samples with low LCAO contents, the Tm value decreased upon increasing the LCAO content) and the side chain crystallization [polymer samples with high LCAO contents, by intermolecular interaction of side chains as observed in poly(DD), poly(TD), and poly(HD)], the other Tm value was observed, especially in poly(ethylene-co-HD)s (assumed to be due to co-crystallization of the branch and the main chain through an interaction of the main chain and the long side chains). The presence of another crystalline phase in poly(ethylene-co-HD)s was also suggested by a wide-angle X-ray diffraction (WAXD) analysis. These Tm values in poly(ethylene-co-TD)s and poly(ethylene-co-DD)s with rather high TD or DD contents were affected by the heating conditions in the measurement of DSC thermograms (5 or 10 °C/min), suggesting that the driving force for formation of the crystal packing (observed as Tm) is weak and affected by the alkyl side chain lengths.
Introduction
Ethylene/α-olefin copolymers (linear low-density polyethylene, LLDPE) have been widely used as commodity plastics in our daily life, and it is well known that the material property is affected by the chain length of the incorporated α-olefin, the α-olefin (comonomer) contents, and the composition (and molecular weight and the distribution, monomer sequence distribution, etc.). It is also known that α-olefin incorporation affects the crystallinity of polyethylene lamellae formed during the crystallization process (main chain crystallization, Figure 1a), and the lamellae thickness, percentage of semicrystalline lamellae, and tie molecules play an important role in the material property. Many studies have thus been conducted for the analysis of ethylene/α-olefin copolymers1−12 prepared by conventional catalysts including metallocene catalysts5−10 or the model system.11,12 Reports with the designed copolymers by the placement of certain alkyl side chains in a controlled manner (the same methylene spacing unit), which were prepared by adopting acyclic diene metathesis polymerization and subsequent hydrogenation, are known.13−22 Most of these studies were analyses of semicrystalline copolymers with rather low α-olefin contents, and the copolymers (with 1-butene, 1-hexene, 1-octene, etc.) became amorphous by loss of crystallinity with incorporation of the α-olefin in the main chain. No additional melting temperatures, as described in this text, were observed in these copolymers.
Figure 1.
Schematic models for (a) main chain crystallization (observed in semicrystalline polyethylene and ethylene/α-olefin copolymers with low α-olefin content), (b) side chain crystallization [observed in semicrystalline homopolymers of long-chain α-olefins (LCAOs, 1-dodecene, 1-tetradecene, 1-hexadecene, 1-octadecene, etc.), end-to-end model (major), and interdigitating model],26,27 and (c, d) assumed chain packing models22 in the model ethylene copolymers containing C21 alkyl chain in every 15th or 19th methylene unit. The dashed-line range depicts the lamellar thickness/layers in each model.
Amorphous poly(α-olefin)s are used in hot melt applications due to their inherent stickiness and softness (high melt-flow rate with low density). Ultrahigh-molecular-weight polymers, possessing highly entangled bottlebrush architectures, are used as drag reducing agents for improved transport (piping capacity) of crude oil and petroleum products in pipeline methods.23,24 In contrast, poly(α-olefin)s, especially those consisting of long-chain α-olefins [LCAOs, 1-dodecene (DD), 1-tetradecene (TD), 1-hexadecene (HD), 1-octadecene, etc.],25−29 are semicrystalline branched (highly entangled) macromolecules with a high graft density, and the polymers are thus recognized as the simplest bottlebrush polymers. The melting temperatures in poly(LCAO)s are affected by the branch length and the stereoregularity (Table 1).25−28 Recent reports revealed their melt structure, linear rheology, and interchain friction mechanism;26,27 the polymers crystallize by fully extending their coiled side chains with an arrangement of side chains from adjacent bottlebrush chains (side chain crystallization, Figure 1b).26 Although the Tm values in the ethylene copolymers also decreased upon increasing the LCAO incorporation,10 thermal properties of copolymers with high LCAO contents, transition between the main chain crystallization (the ethylene copolymer with low LCAO contents) and the side crystallization observed in poly(LCAO)s, have not yet been studied. One of the probable reasons for this is that homopolymerization of LCAO by the ordinary metallocene catalysts affords oligomers,25,28 and the Mn values in the resultant ethylene copolymers with LCAO thus generally decreased upon increasing the LCAO content.5−9
Table 1. Summary of the Reported Melting Temperatures (Tm Values) in Poly(α-olefin)s25−28.
| α-olefin | regularity | Tm/°C | references |
|---|---|---|---|
| 1-dodecene | atactic | –24 | (25, 28) |
| 1-dodecene | atactic | –25.2 | this paper |
| 1-dodecene | isotactic | 26 | (27) |
| 1-tetradecene | atactic | 7.5 | this paper |
| 1-tetradecene | isotactic | 43 | (27) |
| 1-hexadecene | atactic | 26 | (25, 28) |
| 1-hexadecene | atactic | 25.6 | this paper |
| 1-octadenene | atactic | 42 | (25, 28) |
| 1-octadecene | atactic | 42 | (26) |
| 1-octadecene | isotactic | 54 | (27) |
One report shows that the model ethylene copolymers placing C21 alkyl branch on every 15th or 19th methylene units (corresponding to incorporation of linear C22H44 with 14.3 or 11.1 mol %, respectively) showed two melting temperatures (ca. 26 and 37 °C), and the ratio was affected by the number of the ethylene spacer.22 The results were explained using an assumption of formation of side chain crystallization and co-crystallization of the branch and the main chain; two kinds of chain packing models, the end-to-end (interdigitating) model (Figure 1c) and the inclusion model (Figure 1d), were assumed for the explanation.22
As described in this article, we observed two different melting temperatures in poly(ethylene-co-HD)s that are different from those observed in poly(HD), and we also obtained the polymer samples possessing three melting temperatures (including the one corresponding to the main chain crystallization). Therefore, we prepared a series of ethylene copolymers with LCAOs with different chain lengths (DD, TD, and HD, Chart 1) and studied their thermal properties including wide-angle X-ray diffraction (WAXD) analysis of poly(ethylene-co-HD)s films with different HD contents. Through this study, we wish to show the existence of transition between the main chain crystallization and the side chain crystallization, presumable co-crystallization behaviors.
Chart 1. Ethylene Copolymers with 1-Dodecene [Poly(E-co-DD)s], 1-Tetradecene [Poly(E-co-TD)s], and 1-Hexadecene [Poly(E-co-HD)s].
Results and Discussion
Synthesis of Ethylene Copolymers with 1-Dodecene (DD), 1-Tetradecene (TD), and 1-Hexadecene (HD) and Microstructural Analysis Using 13C NMR Spectra
Ethylene (E) copolymers with 1-dodecene (DD), 1-tetradecene (TD), and 1-hexadecene (HD) were prepared by copolymerization in toluene using Cp*TiCl2(O-2,6-iPr2-4-SiEt3C6H2) (Cp* = C5Me5) catalyst in the presence of methylaluminoxane (MAO) cocatalyst. The titanium catalyst has been chosen in this study because the catalyst exhibited notable catalytic activities for E/DD copolymerization with efficient DD incorporation to afford the copolymers.30,31 The catalysts of this type are known to be effective for the synthesis of new ethylene copolymers32−34 with sterically encumbered olefins (disubstituted olefins, branched α-olefins), cyclic olefins, and aromatic vinyl monomers, as well as the synthesis of poly(LCAO)s.25,28,29 These copolymerizations were conducted with the termination of low comonomer conversions (less than 10%, short period) to obtain the copolymer with uniform compositions (to avoid changes in the LCAO concentrations during the polymerization runs). The results are summarized in Table 2.
Table 2. Ethylene Copolymerization with 1-Dodecene (DD), 1-Tetradecene (TD), and 1-Hexadecene (HD) by the Cp*TiCl2(O-2,6-iPr2-4-SiEt3C6H2)–MAO Catalyst Systema.
| run | cat./μmol | α-olefin | conc.b/M | E/atm | yield/mg | activityc | Mnd × 10–4 | Mw/Mnd | cont.e/mol % |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.001 | 6 | 18.1 | 181 000 | insoluble | ||||
| 2 | 0.01 | DD | 0.75 | 2 | 329 | 329 000 | 8.9 | 1.86 | 18.2 |
| 3 | 0.0025 | DD | 0.75 | 4 | 123 | 492 000 | 11.4 | 1.81 | 15.6 |
| 4 | 0.001 | DD | 0.30 | 6 | 83.5 | 835 000 | 25.0 | 2.05 | 5.1 |
| 5 | 0.001 | DD | 0.45 | 6 | 88.9 | 889 000 | 18.5 | 1.97 | 6.6 |
| 6f | 0.001 | DD | 0.75 | 6 | 104 | 1 040 000 | 16.7 | 1.62 | 15.0 (15.5)g |
| 7 | 0.0025 | DD | 1.13 | 6 | 309 | 1 240 000 | 11.4 | 1.74 | 16.0 |
| 8 | 0.0025 | DD | 1.50 | 6 | 363 | 1 450 000 | 11.6 | 1.76 | 17.6 |
| 9 | 0.001 | DD | 0.30 | 8 | 163 | 1 630 000 | 30.1 | 2.09 | 4.0 |
| 10 | 0.001 | DD | 0.45 | 8 | 177 | 1 770 000 | 23.6 | 1.95 | 5.3 |
| 11 | 0.001 | DD | 0.60 | 8 | 177 | 1 770 000 | 20.6 | 1.96 | 6.1 |
| 12 | 0.001 | DD | 0.75 | 8 | 235 | 2 350 000 | 16.9 | 1.89 | 7.2 (8.0)g |
| 13 | 0.001 | DD | 0.90 | 8 | 283 | 2 830 000 | 14.7 | 1.98 | 8.5 |
| 14 | 1.0 | DD | 0.75 | 477 | 954 | 12.7 | 1.94 | 100 | |
| 15 | 0.01 | TD | 0.67 | 2 | 130 | 130 000 | 12.2 | 1.74 | 15.4 |
| 16 | 0.0025 | TD | 0.67 | 4 | 58.3 | 233 000 | 13.1 | 1.98 | 12.0 |
| 17 | 0.001 | TD | 0.27 | 8 | 120 | 1 200 000 | 43.5 | 2.95 | 3.0 |
| 18 | 0.001 | TD | 0.40 | 8 | 299 | 2 990 000 | 35.5 | 2.49 | 4.5 |
| 19 | 0.001 | TD | 0.27 | 6 | 387 | 3 870 000 | 29.4 | 2.31 | 4.8 |
| 20 | 0.001 | TD | 0.40 | 5 | 169 | 1 690 000 | 17.6 | 2.05 | 8.8 |
| 21 | 0.001 | TD | 0.40 | 6 | 222 | 2 220 000 | 23.4 | 2.25 | 6.3 |
| 22 | 0.001 | TD | 0.40 | 7 | 234 | 2 340 000 | 29.1 | 2.10 | 5.2 |
| 23 | 0.001 | TD | 0.54 | 6 | 207 | 2 070 000 | 18.1 | 1.95 | 8.1 |
| 24 | 0.001 | TD | 0.67 | 6 | 122 | 1 220 000 | 14.0 | 1.87 | 11.4 (12.0)g |
| 25h | 0.001 | TD | 0.67 | 6 | 136 | 1 360 000 | 16.6 | 1.96 | 10.0 |
| 26 | 0.001 | TD | 0.67 | 8 | 442 | 4 420 000 | 15.2 | 2.00 | 6.6 |
| 27 | 0.0025 | TD | 1.01 | 6 | 250 | 1 000 000 | 11.2 | 1.78 | 13.5 |
| 28 | 0.005 | TD | 1.34 | 6 | 162 | 323 000 | 12.1 | 1.97 | 15.2 |
| 29 | 1.0 | TD | 0.67 | 329 | 658 | 17.6 | 1.98 | ||
| 30 | 0.0005 | HD | 0.58 | 2 | 70.8 | 142 000 | 11.0 | 1.66 | 15.0 |
| 31 | 0.001 | HD | 0.58 | 4 | 176 | 1 760 000 | 13.8 | 1.86 | 11.4 |
| 32 | 0.0005 | HD | 0.35 | 6 | 97.1 | 1 942 000 | 25.9 | 1.98 | 6.1 |
| 33 | 0.0005 | HD | 0.58 | 6 | 95.2 | 1 900 000 | 18.7 | 1.96 | 9.7 |
| 34h | 0.0005 | HD | 0.58 | 6 | 156 | 3 120 000 | 15.2 | 1.96 | 9.3 |
| 35 | 0.001 | HD | 0.87 | 6 | 190 | 1 900 000 | 14.2 | 1.90 | 12.5 |
| 36 | 0.001 | HD | 1.16 | 6 | 257 | 2 570 000 | 14.1 | 1.83 | 14.0 |
| 37 | 0.001 | HD | 0.23 | 8 | 180 | 1 800 000 | 43.3 | 2.57 | 2.7 |
| 38 | 0.001 | HD | 0.35 | 8 | 244 | 2 440 000 | 26.9 | 2.57 | 4.4 |
| 39 | 0.0005 | HD | 0.58 | 8 | 180 | 3 600 000 | 21.4 | 1.96 | 6.4 (7.1)g |
| 40 | 1.0 | HD | 0.58 | 134 | 268 | 7.8 | 1.51 |
Conditions: total volume, 30 mL; MAO, 2.0 mmol; 6 min (runs 14, 29, 40: total 6.0 mL, 30 min); and 25 °C.
Initial concentration (mmol/mL).
Activity = kg-polymer/mol-Ti·h.
GPC data in o-dichlorobenzene vs polystyrene standards.
α-Olefin in copolymer (mol %) estimated using 1H NMR spectra (selected data are shown in SI).
Polymerization data cited from ref (31).
α-Olefin in copolymer (mol %) estimated using 13C NMR spectra (selected data are shown in SI).
Polymerization at 50 °C.
As reported recently,30,31 the ethylene/DD copolymerizations afforded poly(ethylene-co-DD)s possessing rather high molecular weights with unimodal molecular weight distributions (Mn = 8.9–30.1 × 104, Mw/Mn = 1.62–2.09) and uniform compositions (confirmed by DSC thermograms shown below). As also reported previously in ethylene/1-hexene copolymerization,35 the activity (on the basis of polymer yield) was affected by the ethylene pressure and DD concentration charge: the activity increased upon increasing the ethylene pressure. Moreover, the catalytic activity increased upon increasing the DD concentration charge, whereas a decrease in the activity was observed in copolymerization by a conventional catalyst such as linked half-titanocene catalyst [Me2Si(C5Me4)(NtBu)]TiCl2 (called constrained geometry catalyst, CGC) and ordinary metallocene catalysts.36 As reported recently,30,31 the activities by Cp*TiCl2(O-2,6-iPr2–4-SiEt3-C6H2) were higher than those by Cp*TiCl2(O-2,6-iPr2C6H3).36 The DD content in the copolymer increased upon increasing the DD concentration charge, and the Mn value decreased with an increase in the DD content. The polymerization of DD resulted in poly(DD) with a rather high molecular weight (run 14, Mn = 1.27 × 105).
Similar trends were observed in ethylene copolymerizations with TD and HD, and copolymerization afforded poly(ethylene-co-TD)s (Mn = 1.12–4.35 × 105, Mw/Mn = 1.74–2.95) and poly(ethylene-co-HD)s (Mn = 1.10–4.33 × 105, Mw/Mn = 1.66–2.57) possessing uniform compositions (confirmed by DSC thermograms shown below). As observed in the ethylene copolymerization with DD, the activity increased upon increasing the TD or HD concentration charge along with increasing the TD or HD content in the copolymers. The activities conducted at 50 °C were higher than those conducted at 25 °C, as observed in copolymerization with DD and 9-decen-1-ol.30 The ethylene copolymers (with DD, TD, and HD) with various contents were thus prepared by adopting this catalyst system. Homopolymerizations with TD and HD also afforded poly(TD) and poly(HD) (runs 29 and 40, respectively).
Figure 2 shows selected 13C NMR spectra (1,1,2,2-tetrachloroethane-d2 at 110 °C) for poly(E-co-DD) (run 6, DD 15.5 mol %), poly(E-co-TD) (run 24, TD 12.0 mol %), and poly(E-co-HD) (run 39, HD 7.1 mol %), and their triad sequence distributions, the dyads, the (relative monomer) reactivity ratios [rE, rC values (C = comonomer, DD, TD, HD)], and rE·rC values on the basis of microstructure analysis estimated by the NMR spectra are summarized in Table 3.
Figure 2.
13C NMR spectra (in 1,1,2,2-tetrachloroethane-d2 at 110 °C) for (a) poly(E-co-DD) (run 6, DD 15.5 mol %), (b) poly(E-co-TD) (run 24, TD 12.0 mol %), and (c) poly(E-co-HD) (run 39, HD 7.1 mol %). The polymerization data are shown in Table 2, and selected NMR spectra of the ethylene copolymers (for estimations of the comonomer contents) are shown in the SI.
Table 3. Monomer Sequence Distributions of Poly(ethylene-co-LCAO)s [LCAO = 1-Dodecene (DD), 1-Tetradecene (TD), 1-Hexadecene (HD)] Prepared by the Cp*TiCl2(O-2,6-iPr2-4-SiEt3-C6H2)–MAO Catalyst Systemad.
| triad
sequence distributionc (%) |
dyadsd (%) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| run | α-olefin | contentb/mol % | EEE | EEC+CEE | CEC | ECE | CCE+ECC | CCC | EE | EC+CE | CC | rEe | rCe | rE·rCf |
| 6 | DD | 15.3 | 52.4 | 29.0 | 3.04 | 13.2 | 2.29 | 66.9 | 31.9 | 1.14 | 4.32 | 0.07 | 0.30 | |
| 24 | TD | 12.0 | 64.0 | 23.6 | 0.4 | 11.7 | 0.3 | trace | 75.8 | 24.0 | 0.2 | 5.81 | 0.02 | 0.11 |
| 37 | HD | 7.1 | 74.1 | 18.8 | 0.06 | 7.0 | 0.08 | trace | 83.5 | 16.5 | 0.04 | 4.56 | 0.01 | 0.05 |
For detailed polymerization conditions, see Table 2; C = comonomer [1-dodecene (DD), 1-tetradecene (TD), 1-hexadecene (HD)].
Comonomer contents in copolymer estimated using 13C NMR spectra.
Calculated using 13C NMR spectra; E = ethylene and C = comonomer (DD, TD, HD).
[EE] = [EEE] + 1/2[EEC+CEE], [EC] = [CEC] + [ECE] + 1/2{[EEC+CEE] + [CCE+ECC]}, [CC] = [CCC] + 1/2[CCE+ECC].
rE = [C]0/[E]0 × 2[EE]/[EC+CE], rC = [E]0/[C]0 × 2[CC]/[EC+CE].
rE·rC = 4[EE][CC]/[EC+CE]2.
The rE and the rC values can be used to evaluate efficiency in the comonomer incorporation, especially in ethylene/α-olefin copolymerization; the large rE value (also defined as kEE/kEC, Table 3) expresses less comonomer incorporation. The rE values are 4.32 (run 6, DD), 5.81 (run 24, TD), and 4.56 (run 39, HD), and the values are close to those of Cp*TiCl2(O-2,6-iPr2C6H3) and [Me2Si(C5Me4)(NtBu)]TiCl2 (linked half-titanocene, constrained geometry type);36 the values are much smaller than those by Cp2ZrCl2.36 It was revealed that the rE·rC values, especially in poly(E-co-TD) and poly(E-co-HD), are small (rE·rC = 0.11, 0.05, respectively), suggesting that the percentage of repeated TD or HD insertion was very low. This means that copolymerization proceeds with favored isolated and/or alternating TD and HD insertions. This reflects the low CC values (in dyad level) even with a rather high TD content (CC 0.2%, run 24, 12.0 mol %). This also reflects the observed low catalytic activities in TD or HD polymerizations, as described above (runs 29, 40).
Analysis of Thermal Properties by DSC Thermograms: Effect of Long Side Chains toward Thermal Behaviors
Figure 3 shows DSC thermograms (second heating, 10 °C/min) for poly(E-co-HD)s with various HD contents (2.7–15.0 mol %), and the thermograms for polyethylene (run 1) and poly(HD) (run 40) were also placed. The corresponding plots of the melting temperatures (Tm values) vs the HD content are shown in Figure 4, and the Tm values in the copolymers are summarized in Table 4.
Figure 3.
(a) DSC thermograms of poly(E-co-HD)s with different HD contents (second heating at 10 °C/min). (b) Expanded thermograms from −20 to 70 °C. Polymerization data are shown in Table 2, and the additional thermograms are shown in the SI.
Figure 4.
Plots of melting temperatures (Tm values) vs HD contents in poly(E-co-HD)s. ● shows plots corresponding to the main chain crystallization. ○ and ⧫ show plots due to other crystallizations (side chain and co-crystallization of side chain and main chain, respectively).
Table 4. Summary of DSC Data (Melting Temperatures, Tm Values) in Poly(E-co-LCAO)s [LCAO = 1-Dodecene (DD), 1-Tetradecene (TD), 1-Hexadecene (HD)]a.
| run | α-olefin | contentb/mol % | Tmc/°C | Mnd × 10–4 | Mw/Mnd |
|---|---|---|---|---|---|
| 1 | none | 0 | 134 | ||
| 9 | DD | 4.0 | 81.6 | 30.1 | 2.09 |
| 4 | DD | 5.1 | 63.3 | 25.0 | 2.05 |
| 10 | DD | 5.3 | 59.4 | 23.6 | 1.95 |
| 11 | DD | 6.1 | 53.2 | 20.6 | 1.96 |
| 5 | DD | 6.6 | 44.1 | 18.5 | 1.97 |
| 12 | DD | 7.2 | 28.6 | 16.9 | 1.89 |
| 13 | DD | 8.5 | 24.1 | 14.7 | 1.98 |
| 6 | DD | 15.0 | –21.4 | 16.7 | 1.62 |
| 3 | DD | 15.6 | –38.8 (−39.9)e | 11.4 | 1.81 |
| 7 | DD | 16.0 | –41.3, −50.1 (−42.2, −52.0)e | 11.4 | 1.74 |
| 8 | DD | 17.6 | –43.1, −50.8 (−43.4, −52.1)e | 11.6 | 1.76 |
| 2 | DD | 18.2 | –43.0, −53.2 (−43.0, −54.1)e | 8.9 | 1.86 |
| 14 | DD | 100 | –25.2 | 12.7 | 1.94 |
| 17 | TD | 3.0 | 81.2 | 43.5 | 2.95 |
| 18 | TD | 4.5 | 67.9 | 35.5 | 2.49 |
| 19 | TD | 4.8 | 68.0, −19.7 | 29.4 | 2.31 |
| 22 | TD | 5.2 | 58.5, −13.2 (59.1, −16.6)e | 29.1 | 2.1 |
| 21 | TD | 6.3 | (52.7, −21.6) | 23.4 | 2.25 |
| 26 | TD | 6.6 | 40.4, 6.0, −10.7 (40.8, 6.9)e | 15.2 | 2 |
| 23 | TD | 8.1 | 36.9, −11.5 (38.7, −11.4, −14.7)e | 18.1 | 1.95 |
| 20 | TD | 8.8 | 36.2, −9.1 | 17.6 | 2.05 |
| 24 | TD | 11.4 | 23.5, −10.0 (26.2, −8.2)e | 14 | 1.87 |
| 16 | TD | 12.0 | –14.6, −10.7 (−18.1, −11.6)e | 13.1 | 1.98 |
| 27 | TD | 13.5 | –11.3 (−11.2)e | 11.2 | 1.78 |
| 28 | TD | 15.2 | –11.3 | 12.1 | 1.97 |
| 15 | TD | 15.4 | –10.4 | 12.2 | 1.74 |
| 29 | TD | 100 | 7.5 | 17.6 | 1.98 |
| 37 | HD | 2.7 | 81.8, 1.1 | 43.3 | 2.57 |
| 38 | HD | 4.4 | 67.6, 1.4 | 26.9 | 2.57 |
| 32 | HD | 6.1 | 56.9, 24.9, 2.0 | 25.9 | 1.98 |
| 39 | HD | 6.4 | 42.8, 3.6 | 21.4 | 1.96 |
| 33 | HD | 9.7 | 28.1, 5.1 | 18.7 | 1.96 |
| 31 | HD | 11.4 | 10.9, 3.5 | 13.8 | 1.86 |
| 35 | HD | 12.5 | 22.9, 10.4, 5.6 | 14.2 | 1.90 |
| 36 | HD | 14.0 | 6.0 | 14.1 | 1.83 |
| 30 | HD | 15.0 | 9.8 | 11.0 | 1.66 |
| 40 | HD | 100 | 25.6 | 7.8 | 1.51 |
As observed in ordinary ethylene copolymers with α-olefin (such as 1-hexene and 1-octene), the Tm values in poly(E-co-HD)s initially decreased upon increasing the HD content [Figure 3a, plotted as ● in Figure 4, Tm = 134 °C (PE, run 1), 81.8 °C (run 37, HD 2.7 mol %), 67.6 °C (run 38, HD 4.4 mol %), 56.9 °C (run 32, HD 6.1 mol %), 42.8 °C (run 39, HD 6.4 mol %), and 28.1 °C (run 33, HD 9.7 mol %)] with broadening due to loss of crystallinity in the main chain packing.
It was revealed that the copolymers containing 6.1 mol % (and 12.5 mol %) HD possessed three Tm values, although one of these (due to the main chain crystallization) was slightly broad. Importantly, the Tm value observed to be the lowest seems to increase slightly upon increasing the HD content [Figure 3b, plotted as ○ in Figure 4, e.g., Tm = 1.1 °C (run 37, HD 2.7 mol %), 1.4 °C (run 38, HD 4.4 mol %), 2.0 °C (run 32, HD 6.1 mol %), 3.6 °C (run 39, HD 6.4 mol %), 5.1 °C (run 33, HD 9.7 mol %), 5.6 °C (run 35, HD 12.5 mol %), 6.0 °C (run 36, HD 14.0 mol %), and 9.8 °C (run 30, HD 15.0 mol %)]. Since it seems likely that the Tm value probably reaches that of poly(HD) (25.6 °C, run 40) eventually on increasing the HD content, it is thus assumed that the observed Tm values could be probably due to the so-called side chain crystallization (intermolecular interaction of side chain in the copolymer, as depicted in Figure 1b).25−28 On the basis of the microstructure analysis (described above), the resultant poly(E-co-HD)s possessed isolated and/or an alternating HD inserted unit in the main chain and the percentage of the HD repeated insertion was very low (Table 3), and the distance between the HD side chain (n-C14H25) is thus great compared to poly(HD). This suggests that the considered side chain crystallization through the intermolecular interaction could be rather weak (observed as lower Tm values depending upon the HD content). Furthermore, it seems likely that the thermograms with HD contents of 11.4 mol % (run 31) and 12.5 mol % (run 35) were bimodal and became unimodal upon increasing the HD content (Figure 3b, HD 14.0 and 15.0 mol %); a probable linear correlation was also observed (plotted as ⧫ in Figure 4). As described in the introduction, two possibilities could probably be considered, the interdigitating model and the inclusion model, as the co-crystallization of the main chain and side chain (in the assumed explanation for the observed Tm value, as also suggested by the WAXD analysis, shown in Figure 9), the latter model may be a probable possibility with speculation of the HD content (such as 11.4–12.5 mol %).
Figure 9.

Wide-angle X-ray diffraction (WAXD) spectra of poly(ethylene-co-HD)s [HD content 2.7 mol % (run 37), 6.1 mol % (run 32), 12.5 mol % (run 35)] and poly(HD) (run 40) films measured at 263 K. Detailed polymerization data are summarized in Table 2, and the corresponding DSC thermograms are shown in Figure 3. The DSC data are summarized in Table 4.
Figure 5 shows DSC thermograms for poly(E-co-TD)s with various TD contents (3.0–15.4 mol %) and the thermogram for poly(TD) (run 29). Corresponding plots of the Tm values vs the TD content are shown in Figure 6, and the Tm values are also summarized in Table 4.
Figure 5.
(a) DSC thermograms of poly(E-co-TD)s with different TD contents (second heating at 10 °C/min). (b) Expanded thermograms from −40 to 80 °C. Polymerization data are shown in Table 2, and additional thermograms are shown in the SI.
Figure 6.
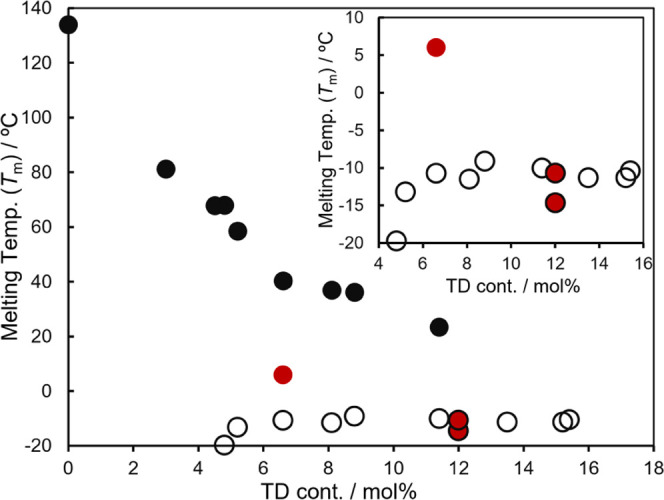
Plots of melting temperatures (Tm values) vs TD contents in poly(E-co-TD)s. ● shows plots corresponding to the main chain crystallization. ○ (partly filled in red) shows plots due to the other crystallization.
As observed in poly(E-co-HD)s, Tm values in poly(E-co-TD)s decreased upon increasing the TD content [Figure 5a, plotted as ● in Figure 6, e.g., Tm = 81.2 °C (run 17, TD 3.0 mol %), 67.9 °C (run 18, TD 4.5 mol %), 58.5 °C (run 22, TD 5.2 mol %), 40.4 °C (run 26, TD 6.6 mol %), 23.5 °C (run 24, TD 11.4 mol %)] with broadening, especially the TD contents higher than 5.2 mol %. It was revealed that the Tm values were not observed even in a slight trace in the copolymer with 6.3 mol % (run 21) when the thermograms were measured under conventional conditions (10 °C/min, second heating); the value was, however, observed under different conditions (Figure 5b). The values were observed with samples possessing higher TD contents (up to 11.4 mol %) as slight traces (Figure 5a), probably due to weakened crystal packing (main chain). This assumption may also be suggested from the fact that the observed Tm values were affected slightly by the conditions (5, 10 °C/min, Table 4, the thermograms are shown in the SI).
As also observed in poly(E-co-HD)s, three Tm values were observed in the copolymer with TD 6.6 mol % (run 26), and the lowest Tm value (−10.7 °C) was affected by the TD content. The Tm value was initially low (−16.6, −21.6 °C, runs 21, 22) but seemed to reach a constant value (or slightly increasing) even upon increasing the TD content (plotted as ○ in Figure 6) within these TD content regions. Moreover, the thermogram of the sample with TD 12.0 mol % was bimodal and became unimodal with an increase of TD content (13.5, 15.4 mol %, runs 27, 15, respectively, Figure 5a). Since there is a similar observation in poly(E-co-HD)s (Figures 3 and 4), these Tm values could also be assumed to be due to a different packing model (from the main chain and side chain, plotted as red circles in Figure 6) although the interaction seems to be weak compared to HD due to a rather short side chain branching.
Figure 7 shows DSC thermograms (second heating, 10 °C/min) for poly(E-co-DD)s with various DD contents (4.0–18.2 mol %) and the thermogram for poly(DD) (run 14). Corresponding plots of the Tm values vs the DD content are shown in Figure 8, and the values are also summarized in Table 4. As observed in poly(E-co-HD)s and poly(E-co-TD)s, the Tm values initially decreased upon increasing the DD content [Figure 7a, plotted as ● in Figure 8, e.g., Tm = 81.6 °C (run 9, DD 4.0 mol %), 63.3 °C (run 4, DD 5.1 mol %), 59.4 °C (run 10, DD 5.3 mol %), 44.1 °C (run 5, DD 6.6 mol %), 24.1 °C (run 13, DD 8.5 mol %)] with broadening, especially DD contents higher than 6.1 mol %.
Figure 7.

DSC thermograms (between −70 and 80 °C) of poly(E-co-DD)s with different DD contents (second heating at 10 °C/min). Polymerization data are shown in Table 2, and the additional thermograms are shown in the SI.
Figure 8.

Plots of melting temperatures (Tm values) vs DD contents in poly(E-co-DD)s. ● shows plots corresponding to the main chain crystallization. ○ (partly filled in red) shows plots that are probably due to the other crystallization (or side chain crystallization in the sample with DD content of 15.6 mol %).
In contrast to the thermograms of the copolymers with HD, TD, the sample with DD 15.0 mol % (and DD 15.6 mol %) showed one broad Tm value (plotted as red circles, Figure 8), and the thermogram became bimodal on increasing the DD content; the values were also affected by the heating conditions (5, 10 °C/min, Table 4, the thermograms are shown in the SI). The thermogram of the Tm value at −43 °C seemed to become sharp upon increasing the DD content (runs 2, 8, Figure 7). The observed difference could probably be explained due to the rather short alkyl branching in DD compared to those in HD and TD, which showed an apparent low Tm value with poly(DD) (−25.2 °C) compared to poly(TD) (7.5 °C) and poly(HD) (25.6 °C); the observed fact seems to be explained as an effect of the side chain length in the crystallization in poly(LCAO)s.26,27 However, we only have a simple assumption (probably due to side chain and co-crystallization of the main chain and side chain) as the explanation for the observed bimodal thermograms.
WAXD Analysis of Poly(E-co-HD) Films
Wide-angle X-ray diffraction measurements were performed at BL-6A in the Photon Factory of High Energy Accelerator Research Organization (Tsukuba, Japan), and the diffraction angle was calibrated with a diffraction pattern of silver behenate. Samples were sandwiched between thin polyimide films with a 1 mm aluminum spacer and placed on a temperature-controlled hot stage. After being heated at 120 °C, the samples were cooled to −20 °C at a rate of 10 °C/min. The spectra measured at 263 K (−10 °C) are shown in Figure 9.
In addition to the diffractions at 20.79 and 22.97° [corresponding to (110) and (200) diffractions observed in polyethylene, main chain crystallization] in the poly(ethylene-co-HD) sample with low HD content (HD 2.7 mol %, run 37) and similar diffraction at 20.85° [corresponding to (100) in poly(HD), side chain crystallization], another diffraction (shown by the red arrow in Figure 9) was observed at 19.16° in poly(ethylene-co-HD) samples (HD 2.7–12.5 mol %), especially in the sample with HD 6.1 mol %, which was observed to have three melting temperatures in the DSC thermograms. The results thus clearly suggest the presence of another crystalline phase in these copolymer samples.
Concluding Remarks
A series of ethylene (E) copolymers with α-olefin containing rather long side chains [LCAO, 1-dodecene (DD), 1-tetradecene (TD), 1-hexadecene (HD)] with various comonomer contents were prepared, and their thermal properties, including the effects of LCAO contents and side chain length, were explored. The Cp*TiCl2(O-2,6-iPr2-4-SiEt3-C6H2)–MAO catalyst system enabled the synthesis of the copolymers with unimodal molecular weight distributions (Table 2) and with uniform compositions (confirmed by DSC thermograms). In addition to the melting temperatures (Tm values) corresponding to the so-called main chain crystallization (samples with low LCAO contents) and side chain crystallization [as observed in poly(DD), poly(TD), and poly(HD), polymer samples with high LCAO contents],25−29 the other Tm values were observed, especially in poly(E-co-HD)s; the wide-angle X-ray diffraction (WAXD) data clearly suggest the presence of another crystalline phase. These Tm values in poly(E-co-TD)s and poly(E-co-DD)s with rather high TD or DD contents were affected by the heating conditions in the measurement of DSC thermograms (5, 10 °C/min, Table 4, the additional thermograms are shown in the SI), suggesting that the driving force for generating crystal packing (observed Tm) should be weak and affected by the length of the alkyl side chains. Presently, as described in the text, we speculate using the inclusion model (Figure 1d) rather than the interdigitating model (Figure 1c), proposed as a model polyethylene containing C21H43 alkyl branching placed at every 15th or 19th carbon,22 for the observed additional Tm values on the basis of rather high HD (TD) contents; the presence, observed as a different diffraction peak, could be strongly suggested by the WAXD analysis. As far as we know, the study on thermal properties in a series of poly(E-co-LCAO)s with various LCAO contents has never been reported, and the results should thus be helpful for a better understanding, further study of details (by a physical approach), and the design of new polymers by the copolymerization approach.
Experimental Section
General Procedure
All experiments were conducted under the atmospheric pressure of nitrogen in a dry box (Vacuum Atmospheres Co.). All chemicals used in these experiments were of reagent grade and were purified using standard purification procedures. 1-Dodecene (DD, Tokyo Chemical Industry Co., Ltd.), 1-tetradecene (TD, Aldrich Chemical Co.), and 1-hexadecene (HD, Tokyo Chemical Industry Co., Ltd.) were stored in bottles in a dry box upon addition of molecular sieves and were passed through an alumina short column prior to use. Ethylene (purity >99.9%; Sumitomo Seika Co., Ltd.) of polymerization grade was used as received. Toluene (anhydrous grade, Kanto Kagaku Co., Ltd.) was transferred into a bottle containing molecular sieves (mixture of 3A and 4A 1/16 and 13X) in a dry box and was used without further purification. AlMe3-Free MAO (d-MAO) white solids employed as a cocatalyst were prepared by removing toluene and AlMe3 from commercially available methylaluminoxane (MAO) [TMAO-S, 9.5 wt % (Al) toluene solution, Tosoh Finechem Co.] in vacuo in a dry box (at ca. 50 °C for removing toluene and AlMe3 and then heated at >100 °C for 1 h for completion).25,30,31,35,36 Cp*TiCl2(O-2,6-iPr2-4-SiEt3-C6H2) was prepared according to a previous report.30
All 1H and 13C NMR spectra for analysis of the resultant copolymers were recorded on a Bruker AV500 spectrometer (500.13 MHz, 1H; 125.77 MHz, 13C), and all chemical shifts are given in ppm and are in reference to SiMe4. 13C NMR spectra for the resultant copolymers were recorded with proton decoupling (the pulse interval was 5.2 s, the acquisition time was 0.8 s, the pulse angle was 90°) by ca. 6000 accumulation of transients. The polymer samples were dissolved in 1,1,2,2-tetrachloroethane-d2 at 110 °C. Molecular weights (Mn) and molecular weight distributions (Mw/Mn) of the resultant copolymers were measured by gel permeation chromatography (GPC, Tosoh HLC-8121GPC/HT) in o-dichlorobenzene (containing 0.05 wt/v % 2,6-di-tert-butyl-p-cresol) at 140 °C (column TSK gel GMHHR-H HT × 2, 30 cm × 7.8 mm i.d., molecular weight ranging from <102 to <2.8 × 108; RI-8022 detector). The molecular weights were calculated using a standard procedure based on the calibration with standard polystyrene samples. Thermal properties of the resultant copolymer were evaluated using a differential scanning calorimeter (DSC, Hitachi DSC-7020) under a nitrogen atmosphere. First, the polymer samples were preheated from 30 to 250 °C with an increasing rate at 20 °C/min and then cooled to −100 °C with a rate of 10 °C/min. Then, the samples were second-heated from −100 to 250 °C at a rate of 5 or 10 °C/min and then cooled to 30 °C at a rate of 20 °C/min. The Tm values were determined from the middle of the phase transition of the second heating scan. Wide-angle X-ray diffraction measurements were performed at BL-6A in the Photon Factory of High Energy Accelerator Research Organization. The diffraction angle was calibrated with a diffraction pattern of silver behenate. Diffraction data were collected using a PILATUS 100 K (DECTRIS Ltd., Switzerland). Samples were sandwiched between thin polyimide films with a 1 mm aluminum spacer and placed on a temperature-controlled hot stage (Linkam Scientific Instruments Ltd., THMS-600). After being heated at 120 °C, the samples were cooled to −20 °C at a rate of 10 °C/min, and the spectra were measured at 263 K.
Ethylene Copolymerization with Long-Chain α-Olefins [LCAOs, 1-Dodecene (DD), 1-Tetradecene (TD), 1-Hexadecene (HD)]
Copolymerization of ethylene with long-chain α-olefins (LCAOs) was conducted in toluene. A typical procedure is as follows. Into a 100 mL scale stainless steel autoclave placed in a dry box, d-MAO (116 mg, 2.0 mmol), LCAO (prescribed amount), and toluene (total 29.0 mL) were added. The reaction apparatus was taken out and the reactor was then filled with ethylene (1 atm). A toluene solution (1.0 mL) containing Cp*TiCl2(O-2,6-iPr2-4-SiEt3-C6H2) (prescribed amount) was then added into the reactor at a prescribed temperature. Ethylene was immediately pressurized (prescribed amount) by closing the valve, and the reaction mixture was magnetically stirred for 6 min. After the reaction, the reactor was immediately cooled in an ice bath and the remaining ethylene was purged. The reaction mixture was then poured into a mixed HCl (10 mL) and isopropanol (iPrOH) (100 mL) solution and stirred at room temperature for 15 min. The obtained polymer was washed with hot iPrOH and stirred for 30 min to remove the remaining LCAO monomers. The precipitates in the mixed solution were then collected through suction filtration and washed with iPrOH. The resultant copolymer was then dried in vacuo at room temperature for 6 h.
Acknowledgments
This project was partly supported by Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS, Grant Nos. 18H01982 and 21H01942). The WAXD experiments at Photon Factory were performed under the approval of the Photon Factory Program Advisory Committee (Proposal No. 2021G570). The authors express their heartfelt thanks to Tosoh Finechem Co. for donating MAO, and S.K. expresses her thanks to the Tokyo Metropolitan Government (Tokyo Human Resources Fund for City Diplomacy) for the predoctoral fellowship. K.N. expresses his heartfelt thanks to Professor K. Kikuchi (Tokyo Metropolitan University), Dr. H. Hirano (Osaka Research Institute of Industrial Science and Technology, ORIST), Dr. Kazuo Takaoki and Dr. Y. Nozue (Sumitomo Chemical Co., Ltd.), Professor M. Yamaguchi (Japan Advanced Institute of Science and Technology, JAIST), and Japan Polychem Corporation (especially Dr. T. Sakuragi) for helpful discussions concerning the thermal analysis of ethylene copolymers.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c06560.
Experimental procedure for the synthesis of ethylene copolymers with 1-dodecene, 1-tetradecene, and 1-hexedecene including general procedures; selected NMR spectra of the copolymers; and additional DSC thermograms of the copolymers (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- For selected examples, refs (1−4):; Alamo R. G.; Mandelkern L. Thermodynamic and structural properties of ethylene copolymers. Macromolecules 1989, 22, 1273–1277. 10.1021/ma00193a045. [DOI] [Google Scholar]
- Hosoda S.; Nomura H.; Gotoh Y.; Kihara H. Degree of branch inclusion into the lamellar crystal for various ethylene/α-olefin copolymers. Polymer 1990, 31, 1999–2005. 10.1016/0032-3861(90)90030-3. [DOI] [Google Scholar]
- Müller A. J.; Arnal M. L.; Spinelli A. L.; Cañizales E.; Puig C. C.; Wang H.; Han C. C. Morphology and crystallization kinetics of melt miscible polyolefin blends. Macromol. Chem. Phys. 2003, 204, 1497–1513. 10.1002/macp.200350011. [DOI] [Google Scholar]
- Stadler J. F.; Takahashi T.; Yonetake K. Lattice sizes, crystallinities, and spacing between amorphous chains - characterization of ethene-/ α -olefin copolymers with various comonomers and comonomer contents measured by wide angle X-ray scattering. e-Polymers 2009, 9, 040 10.1515/epoly.2009.9.1.479. [DOI] [Google Scholar]
- Selected reports by the metallocene (and linked half-titanocene) catalysts, refs (5−10):Walter P.; Trinkle S.; Suhm J.; Mäder D.; Friedrich C.; Mülhaupt R. Short and long chain branching of polyethene prepared by means of ethene copolymerization with 1-eicosene using MAO activated Me2Si(Me4Cp)(NtBu)TiCl2. Macromol. Chem. Phys. 2000, 201, 604–612. . [DOI] [Google Scholar]
- Pollard M.; Klimke K.; Graf R.; Spiess H. W.; Wilhelm M.; Sperber O.; Piel C.; Kaminsky W. Observation of chain branching in polyethylene in the solid state and melt via 13C NMR spectroscopy and melt NMR relaxation time measurements. Macromolecules 2004, 37, 813–825. 10.1021/ma0349130. [DOI] [Google Scholar]
- Kaminsky W.; Piel C.; Scharlach K. Polymerization of ethene and longer chained olefins by metallocene catalysis. Macromol. Symp. 2005, 226, 25–34. 10.1002/masy.200550803. [DOI] [Google Scholar]
- Piel C.; Starck P.; Seppälä J. V.; Kaminsky W. Thermal and mechanical analysis of metallocene-catalyzed ethene−α-olefin copolymers: The influence of the length and number of the crystallizing side chains. J. Polym. Sci., Part A: Polym. Chem. 2006, 44, 1600–1612. 10.1002/pola.21265. [DOI] [Google Scholar]
- Stadler F. J.; Piel C.; Klimke K.; Kaschta J.; Parkinson M.; Wilhelm M.; Kaminsky W.; Münstedt H. Influence of type and content of various comonomers on long-chain branching of ethene/α-olefin copolymers. Macromolecules 2006, 39, 1474–1482. 10.1021/ma0514018. [DOI] [Google Scholar]
- Janicek M.; Cermak R.; Obadal M.; Piel C.; Ponizil P. Ethylene copolymers with crystallizable side chains. Macromolecules 2011, 44, 6759–6766. 10.1021/ma201017m. [DOI] [Google Scholar]
- Lohse D. J.; Milner S. T.; Fetters L. J.; Xenidou M.; Hadjichristidis N.; Mendelson R. A.; García-Franco C. A.; Lyon M. K. Well-Defined, Model Long Chain Branched Polyethylene. 2. Melt Rheological Behavior. Macromolecules 2002, 35, 3066–3075. 10.1021/ma0117559. [DOI] [Google Scholar]
- Hadjichristidis N.; Xenidou M.; Iatrou H.; Pitsikalis M.; Poulos Y.; Avgeropoulos A.; Sioula S.; Paraskeva S.; Velis G.; Lohse D. J.; Schulz D. N.; Fetters L. J.; Wright P. J.; Mendelson R. A.; García-Franco C. A.; Sun T.; Ruff C. J. Well-defined, model long chain branched polyethylene. 1. Synthesis and characterization. Macromolecules 2000, 33, 2424–2436. 10.1021/ma991670w. [DOI] [Google Scholar]
- Selected reports by the acyclic diene metathesis (ADMET) polymerization, refs (13−22):; Pribyl J.; Wagener K. B.; Rojas G. ADMET polymers: synthesis, structure elucidation, and function. Mater. Chem. Front. 2021, 5, 14–43. 10.1039/D0QM00273A. [DOI] [Google Scholar]
- Wei Y.; Graf R.; Sworen J. C.; Cheng C.-Y.; Bowers C. R.; Wagener K. B.; Spiess H. W. Local and collective motions in precise polyolefins with alkyl branches: A combination of 2H and 13C solid-state NMR spectroscopy. Angew. Chem., Int. Ed. 2009, 48, 4617–4620. 10.1002/anie.200900377. [DOI] [PubMed] [Google Scholar]
- Rojas G.; Inci B.; Wei Y.; Wagener K. Precision polyethylene: Changes in morphology as a function of alkyl branch size. J. Am. Chem. Soc. 2009, 131, 17376–17386. 10.1021/ja907521p. [DOI] [PubMed] [Google Scholar]
- Inci B.; Wagener K. B. Decreasing the alkyl branch frequency in precision polyethylene: Pushing the limits toward longer run lengths. J. Am. Chem. Soc. 2011, 133, 11872–11875. 10.1021/ja2040046. [DOI] [PubMed] [Google Scholar]
- Hosoda S.; Nozue Y.; Kawashima Y.; Suita K.; Seno S.; Nagamatsu T.; Wagener K. B.; Inci B.; Zuluaga F.; Rojas G.; Leonard J. K. Effect of the sequence length distribution on the lamellar crystal thickness and thickness distribution of polyethylene: Perfectly equisequential ADMET polyethylene vs ethylene/α-olefin copolymer. Macromolecules 2011, 44, 313–319. 10.1021/ma102072p. [DOI] [Google Scholar]
- Nozue Y.; Kawashima Y.; Seno S.; Nagamatsu T.; Hosoda S.; Berda E. B.; Rojas G.; Baughman T. W.; Wagener K. B. Unusual crystallization behavior of polyethylene having precisely spaced branches. Macromolecules 2011, 44, 4030–4034. 10.1021/ma200145k. [DOI] [Google Scholar]
- Inci B.; Lieberwirth I.; Steffen W.; Mezger M.; Graf R.; Landfester K.; Wagener K. Decreasing the alkyl branch frequency in precision polyethylene: Effect of alkyl branch size on nanoscale morphology. Macromolecules 2012, 45, 3367–3376. 10.1021/ma3002577. [DOI] [Google Scholar]
- Matsui K.; Seno S.; Nozue Y.; Shinohara Y.; Amemiya Y.; Berda E. B.; Rojas G.; Wagener K. B. Influence of branch incorporation into the lamella crystal on the crystallization behavior of polyethylene with precisely spaced branches. Macromolecules 2013, 46, 4438–4446. 10.1021/ma400608q. [DOI] [Google Scholar]
- Li H.; Rojas G.; Wagener K. B. Precision long-chain branched polyethylene via acyclic diene metathesis polymerization. ACS Macro Lett. 2015, 4, 1225–1228. 10.1021/acsmacrolett.5b00641. [DOI] [PubMed] [Google Scholar]
- Matsui K.; Li H.; Nozue Y.; Rojas G.; Bell M.; Shinohara Y.; Amemiya Y.; Wagener K. B. A Study of ADMET polyethylene with 21-carbon branches on Every 15th compared to every 19th Carbon: What a difference four extra backbone methylenes make. J. Polym. Sci., Part A: Polym. Chem. 2017, 55, 3090–3096. 10.1002/pola.28649. [DOI] [Google Scholar]
- Ivchenko P. V.; Nifant’ev I. E.; Tavtorkin A. V. Polyolefin drag reducing agents. Pet. Chem. 2016, 56, 775–787. 10.1134/S096554411609005X. [DOI] [Google Scholar]
- Gómez Cuenca F.; Marín M. G.; Folgueras Díaz M. B. Energy-savings modeling of oil pipelines that use drag-reducing additives. Energy Fuels 2008, 22, 3293–3298. 10.1021/ef800364a. [DOI] [Google Scholar]
- Nomura K.; Pengoubol S.; Apisuk W. Synthesis of ultrahigh molecular weight polymers by homopolymerisation of higher α-olefins catalysed by aryloxo-modified half-titanocenes. RSC Adv. 2016, 6, 16203–16207. 10.1039/C5RA27797C. [DOI] [Google Scholar]
- López-Barrón C. R.; Tsou A. H.; Younker J. M.; Norman A. I.; Schaefer J. J.; Hagadorn J. R.; Throckmorton J. A. Microstructure of crystallizable α-olefin molecular bottlebrushes: Isotactic and atactic poly(1-octadecene). Macromolecules 2018, 51, 872–883. 10.1021/acs.macromol.7b02524. [DOI] [Google Scholar]
- López-Barrón C. R.; Tsou A. H.; Hagadorn J. R.; Throckmorton J. A. Highly entangled α-olefin molecular bottlebrushes: Melt structure, linear rheology, and interchain friction mechanism. Macromolecules 2018, 51, 6958–6966. 10.1021/acs.macromol.8b01431. [DOI] [Google Scholar]
- Nomura K.; Pengoubol S.; Apisuk W. Synthesis of ultrahigh molecular weight polymers with low PDIs by polymerizations of 1-decene, 1-dodecene, and 1-tetradecene by Cp*TiMe2(O-2,6-iPr2C6H3)–borate catalyst. Molecules 2019, 24, 1634 10.3390/molecules24081634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura K.; Pengoubol S.; Apisuk W. Synthesis of ultrahigh molecular weight polymers containing reactive functionality with low PDIs by polymerizations of long-chain α-olefins in the presence of their nonconjugated dienes by Cp*TiMe2(O-2,6-iPr2C6H3)–borate catalyst. Polymers 2020, 12, 3 10.3390/polym12010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitphaitun S.; Yan Q.; Nomura K. Effect of SiMe3, SiEt3para-substituents for exhibiting high activity, introduction of hydroxy group in ethylene copolymerization catalyzed by phenoxide-modified half-titanocenes. Angew. Chem., Int. Ed. 2020, 59, 23072–23076. 10.1002/anie.202010559. [DOI] [PubMed] [Google Scholar]
- Kitphaitun S.; Yan Q.; Nomura K. Effect of para-substituents in ethylene copolymerizations with 1-decene, 1-dodecene, and with 2-methyl-1-pentene using phenoxide modified half-titanocenes–MAO catalyst systems. ChemistryOpen 2021, 10, 867–876. 10.1002/open.202100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura K.; Liu J.; Sudhakar P.; Kitiyanan B. Nonbridged half-metallocenes containing anionic ancillary donor ligands: New promising candidates as catalysts for precise olefin polymerization. J. Mol. Catal. A: Chem. 2007, 267, 1–29. 10.1016/j.molcata.2006.11.006. [DOI] [Google Scholar]
- Nomura K. Half-titanocenes containing anionic ancillary donor ligands as promising new catalysts for precise olefin polymerization. Dalton Trans. 2009, 34, 8811–8823. 10.1039/B910407K. [DOI] [PubMed] [Google Scholar]
- Nomura K.; Liu J. Half-titanocenes for precise olefin polymerisation: Effects of ligand substituents and some mechanistic aspects. Dalton Trans. 2011, 40, 7666–7682. 10.1039/C1DT10086F. [DOI] [PubMed] [Google Scholar]
- Nomura K.; Oya K.; Imanishi Y. Ethylene/α-olefin copolymerization by various nonbridged (cyclopentadienyl)(aryloxy) titanium(IV) complexes – MAO catalyst system. J. Mol. Catal. A: Chem. 2001, 174, 127–140. 10.1016/S1381-1169(01)00196-0. [DOI] [Google Scholar]
- Kakinuki K.; Fujiki M.; Nomura K. Copolymerization of ethylene with α-olefins containing various substituents catalyzed by half-titanocenes: Factors affecting the monomer reactivities. Macromolecules 2009, 42, 4585–4595. 10.1021/ma900576v. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.









