Abstract
Zeolite-based molecular sieves are applied in industrial dehydration units for their high water uptake capacities and extremely low equilibrium pressure of water vapor. During their operational life, they tend to lose their water vapor adsorption capacity slowly. To optimize the usage of molecular sieves in dryer units, it is vital to understand the mechanism(s) leading to deactivation. In this work, the capacity loss was studied by exposing LTA- and FAU-type zeolites to methanol and heptane vapors under relatively harsh conditions using repetitive adsorption/regeneration cycles. A simple microflow unit was designed and used for the deactivation experiments. The water vapor adsorption capacity of the resulting samples was measured using a gravimetric analyzer. In addition, they were characterized by classic XRD, 13C NMR, and TGA techniques. The crystallinity of fresh and spent zeolite XRD patterns was not drastically affected even after exposure to the contaminants. It was found that methanol easily gave rise to a severe loss of water vapor adsorption capacity, much more so than heptane. Water vapor uptake in the methanol exposed samples is ∼50% lower than that for the fresh zeolites. This is attributed to nonvolatile, residual hydrocarbons.
1. Introduction
Dehydration is a process widely applied in the natural gas processing industry. Tight control over the water content of the product streams, either gaseous or liquid, is essential to prevent operational problems in the downstream sections.1,2 In combination with CO2, it leads to corrosion issues, and under high-pressure/low-temperature conditions, it can form hydrates with methane, leading to blockages. Also in the production of biofuels, often derived from materials rich in oxygen,3,4 water is an undesirable byproduct that needs to be removed.
Absorbents or adsorbents suitable for dehydration are either hygroscopic liquids (like glycerol) or porous solids.5 Well-known and widely applied solid adsorbents include silica and alumina pellets.6,7 In addition, zeolites, especially from the LTA and FAU families, are chosen when to meet more stringent dehydration requirements. Water contents less than 0.1 ppmv (dew points in the range of −80 to −100 °C)8,9 are reached in standard operation. For natural gas liquids, these conditions are slightly relaxed. However, zeolites are still used there too.10 High affinity for water has a reverse side though. Previously adsorbed water will desorb only completely in the range of 275–325 °C.11 The zeolite frameworks withstand these high-temperature process steps quite well though. In practice, this implies cyclic operation: the reactors swing from the adsorption stage to the regeneration stage and back.
At the end of an adsorption cycle, the pellets are saturated with water. Other components that were present in the feed may be adsorbed as well. This depends on the feed composition and purity in terms of the presence and nature of contaminants. Another condition to meet is regeneration without the formation of liquid water in parts of the packed bed. The presence of liquid water can happen if the design heating protocol could not properly be followed.3
Practice teaches that “molsieves” do lose their adsorption capacity over time. In this context, the term “molsieve” refers to pellets (beads or extrudates) composed of a zeolite (LTA or FAU) and a binder, usually a clay.12−14 Two main classes of performance degradation are commonly referred to as caking and coking. Caking relates to the loss of mechanical integrity of the pellets, while coking refers to the buildup of carbonaceous residue inside the pellets.15,16
On an industrial scale, dehydration of gas or natural gas liquids (NGL) is done using multiple (2–4) parallel vessels loaded with molsieves. The feed gas enters the vessels from the top. For a “four-vessel” dryer train, one vessel will always be in the regeneration mode. Part of the dried gas from the other three is heated to the regeneration temperature. This hot gas (275–325 °C) is fed to the bottom section of the fourth vessel being regenerated. Once all adsorbed components are desorbed, the vessel is cooled and lined up for dehydration duty again. Then, the next vessel is put up for regeneration and so on. Regeneration is repeated every 24–36 h. Over the total operational life of the molsieves, usually 3–5 years, this implies hundreds of heating/cooling cycles. Deactivation leads to ever shorter runs. When the run time before breakthrough becomes shorter than the time needed for regeneration, the plant is due for a molsieve change-out.17−20
This study focuses on the effect of contaminants on the water adsorption capacity of molsieves. Practical molsieve pellets for water removal are based on 4A and 5A zeolites. Large pore zeolites like 13X are often used in combination with A zeolites for the removal of larger-size contaminants such as mercaptans. However, 13X also has a significant capacity for water adsorption.
We report on the impact of methanol and heptane injected into a stream of inert gas, in this case, nitrogen. In industrial gas dehydration operation, methanol is occasionally injected. This prevents the formation of hydrates that can form with methane at high pressures and low temperatures during start-up or process upsets.21,22 Solid hydrates inevitably lead to operational problems resulting from blockages.
While deactivation by coke deposition during the conversion of methanol to hydrocarbons over commercial acid zeolite catalysts, such as HZSM-523−25 and SAPO-34,26,27 has been widely studied, a few studies are reported in the literature on the deactivation by coke deposition on industrial zeolite adsorbents. Coke formation due to propene exposure on a pure 5A zeolite and an industrial adsorbent (80 wt % 5A zeolite and 20 wt % kaolinite as a binder) was investigated by Misk et al. in a flow reactor.28 They found that coking at 623 K on the material with binder was more pronounced than on the 5A powder. Coke was present as heavy polyaromatics. Sun et al.29 investigated the deactivation of 5A zeolite in a fixed-bed adsorber during 1-hexene adsorption and studied the effects of binder and operating conditions on coke formation. Again, it was found that the binder accelerates coke formation. Uguina et al.30 studied deactivation by coke formation on 5A molecular sieves by carrying out 10 adsorption–desorption cycles of a mixture of n-decane and iso-octane in a fixed-bed apparatus. The previously published literature on the adsorption of different hydrocarbons on 5A molecular sieves has shown that the loss of catalyst adsorption capacity is a result of coke formation in the zeolite leading to site coverage and pore blockages. In natural gas liquids, hydrocarbons this heavy do not occur.
To the best of our knowledge, the deactivation of industrial molecular sieves (4A, 5A, and 13X zeolites) due to coke formation because of the presence of methanol during dehydration has not been explored yet.
Heptane is a natural component of NGL’s and many gas streams, be it in (very) low concentrations. It was selected as it is the smallest linear alkane that cracks relatively easily compared to hexane and pentane. Cracking reactions imply the generation of olefins, which in turn can lead to the formation of carbonaceous residues. This is particularly the case in the absence of hydrogen and a hydrogenation function. The effect of other contaminants such as amines or corrosion inhibitors will be reported separately. The deactivation of acidic catalysts as a result of cracking reactions is an extensively researched area. The main criterion to prevent deactivation is the presence of high-pressure hydrogen (∼30 bar) and a hydrogenating function like Pt. Heptane cracking without hydrogen leads to the formation of olefinic intermediates, which quickly give rise to coke. For adsorbents, which are nonacidic, this is less well described. A recent publication addresses this issue though.31 The authors studied in cycled operation the water adsorption capacity loss of zeolite 13X in the presence of water, CO2, and methane. It was observed that both in the presence and absence of heptane, capacity was lost but to a comparable degree. This is attributed to a change in the textural properties of the material. Yet in the presence of heptane at the end of the 10-cycle experiment, more than 2% w carbon was deposited, but that did not have a significant effect on the adsorption capacity under these conditions. In our studies, different zeolites were tested with pure components rather than mixtures.
The final purpose is to come to a ranking of contaminants in terms of their impact on the adsorption capacity over time. In addition, this work provides information on the resilience of various materials to the deactivation protocol (short duration and high intensity) applied. The samples were analyzed by a variety of methods as described in the following sections, both before and after the deactivation experiments.
2. Results and Discussion
For desiccants in operation, the water adsorption capacity is the key property. Consequently, it is the first property determined after the termination of aging runs.
2.1. Water Vapor Adsorption Capacity
The water vapor adsorption capacity is expressed as milligrams (mg) of adsorbed water per gram dry desiccant. In the case of various zeolites type A, this leads to small differences in equilibrium values. If the zeolite framework is considered as a large anion, the corresponding cations can vary. For 3A, 4A, and 5A, this is potassium, sodium, and calcium, respectively. This implies that a gram of 3A contains less molsieve anion than, for example, a gram of 4A. This will lead to an apparent difference in adsorption capacity on a mass basis. When comparing 4A and 5A zeolites, this is not much of an issue. Although calcium has a higher atomic weight than sodium, only half the amount is needed as it has double the valency. Apart from this, the adsorption capacities can still be different due to, for example, the available space and affinity of water molecules for the different cations.
The isotherms in Figures 1–4 demonstrate that even at the lowest pressure achievable in our equipment (1 mbar), the materials have an adsorption capacity close to the maximum. In materials with a binder, there is also pore volume outside the zeolite crystals, but still inside the pellets. These will only fill if conditions for capillary condensation are met or if liquid water is present. These conditions cannot be exploited in the microbalances for the risk of condensation in an uncontrolled way.
Figure 1.

Water vapor adsorption isotherms at 30 °C on 4A powder, fresh and exposed to MeOH for one and three cycles.
Figure 4.

Water vapor adsorption isotherms at 30 °C on crushed 13X pellets, fresh and exposed to MeOH for one and three cycles.
In practice, for gas dehydration operation, the feed is usually saturated with water vapor pressure depending on the pretreatment facilities upstream of the dryers. While passing through the adsorber bed, the water vapor partial pressure drops in the mass transfer zone to almost zero. Upon usage of the bed, the total adsorption capacity will slowly decrease over time. The equilibrium pressure over a fresh or spent molsieve for that matter, in the initial stages of adsorption, is extremely low (μbar range) and beyond our experimental abilities. For the final part, that is, to attain at 0.1 ppmv water, from 10 ppmv (which would not be acceptable in the product stream), only a very small amount of molsieve is needed. Therefore, the effect of capacity loss is not seen as an increase of the humidity level in the product stream but rather as a reduced cycle time.
Figure 1 shows the effect of methanol treatment on the water adsorption capacity of 4A powder. Even at the highest pressure (29 mbar; ∼70% of the dewpoint pressure) at 30 °C, the zeolite does not saturate. For the base 4A powder, a water uptake of 278–282 mg/g was observed. For mutual comparison, it is often sufficient to select just one standard condition: 30 °C, ≈29 mbar in our case.
The water vapor adsorption capacity for fresh 4A pellets at 28 mbar is 208 mg/g, shown in Figure 2. The difference with the powder results mainly from the weight of the binder material. The total pore volume of these pellets was found to be 0.371 mL/g, which means that there is an interstitial pore volume present corresponding to 0.163 mL/g.
Figure 2.

Water vapor adsorption isotherms at 30 °C on 4A crushed pellets, fresh and exposed to MeOH and heptane for three cycles.
In Figures 1 and 2, the impact of exposure to methanol vapor on the adsorption capacity of 4A powder and pellets is demonstrated. Figure 2 also shows results for exposure to heptane for 4A pellets. The impact of methanol under these specific sets of conditions is significant. For the powder sample, a reduction of 11% capacity (from 282 to 251 mg/g at 29 mbar) is seen after a three-cycle treatment with methanol. For the pelletized material, the effect is significantly more pronounced. A capacity loss of 70% (from 208 to 64 mg/g at 29 mbar) was found after three-cycle treatment with methanol. This is in contrast to the exposure to heptane for which hardly any performance loss was noted even after the three-cycle treatment.
The same experiments were done using 5A-based molsieve pellets. The isotherms are presented in Figure 3. Again, the impact of methanol exposure is larger than that of heptane. After three cycles of heptane treatment, a reduction of only 8% resulted (from 210 to 194 mg/g at 29 mbar), while for methanol treatment, the loss in water adsorption capacity was 62% (from 210 to 80 mg/g at 29 mbar).
Figure 3.

Water vapor adsorption isotherms at 30 °C on crushed 5A pellets, fresh and exposed to MeOH or heptane for one and three cycles.
The results for the same experiment performed on 13X pellets are presented in Figure 4. Again, methanol exposure leads to momentous capacity losses. Even after one treatment cycle, capacity tumbles from 263 to 126 mg/g at 29 mbar, and after three treatment cycles, it drops to 64 mg/g (a loss of 76% capacity).
The question of whether this capacity loss is permanent or temporary was addressed for a sample of 5A pellets. Results are shown in Figure 5. The water vapor adsorption isotherms were determined at 30 °C for fresh material and the same after exposure to three heating/cooling cycles in methanol. Again, a significant loss in adsorption capacity was observed. As described in Section 4, prior to the start of the adsorption run, the samples are heated to 350 °C in vacuum for 6 h. Any volatile materials residing inside the structure are expected to have desorbed under these conditions. Still, the uptake capacity suffered a loss. Calcination in the air at 450 °C for 3 h was shown to restore the adsorption capacity almost to the full. Clearly, the material deposited inside the pores is combustible and could be removed by a fairly straightforward treatment. In the laboratory, this is no issue, but in a full-sized gas dryer, this will not be a feasible operation.
Figure 5.

Water vapor adsorption isotherms at 30 °C on crushed 5A pellets, fresh and exposed to MeOH for three cycles and the same calcined at 450 °C in the air for 3 h.
2.2. XRD
Figures 6 and 7 show the [200] peaks of the 4A and 5A pelletized molsieves, both fresh and after exposure to methanol or heptane. For 4A and 5A, the powders are also shown. The position of the [200] peak for the 4A powder after calcination in the air for 6 h at 350 °C (to remove any residuals from the synthesis) was 2θ = 7.19°. For the calcined 5A powder, the peak was found at 2θ = 7.16°. Under the conditions of the measurements, the samples can be considered as hydrated. The IZA database33 gives the [200] peak maximum at 2θ = 7.18° for hydrated 4A. The [200] peak maxima for the fresh and spent pellets were all found at the expected position (2θ ≈ 7.2°) for the 4A and 5A samples. The full-range (2θ 5–45°) XRD patterns are provided in the Supporting Information (Figures S1–S3). The deactivation experiments do not result in significant changes in the XRD patterns of pelletized 4A, except for some variation in intensity.
Figure 6.
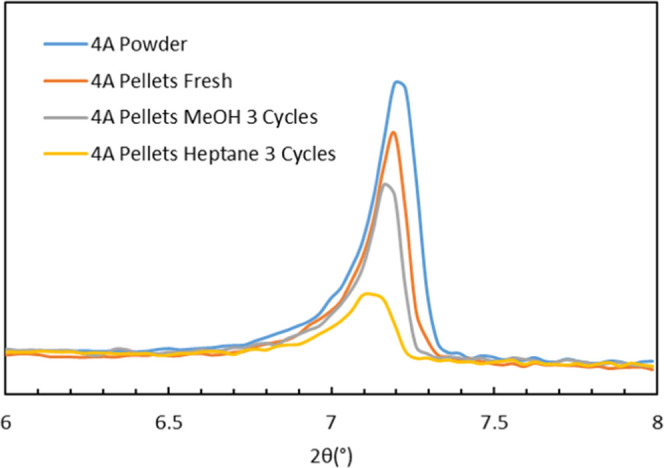
XRD [200] reflections of 4A powder and pellets: as such (fresh), after exposure to three cycles of methanol (MeOH), and after exposure to three cycles of heptane.
Figure 7.

XRD [200] reflections of 5A powder and pellets: as such (fresh), after exposure to three cycles of methanol (MeOH), and after exposure to three cycles of heptane.
Figure 8 shows that the [111] reflection for the fresh 13X pellets (in the hydrated state, the theoretical maximum is at 2θ = 6.16°) was found at 2θ = 6.07°. After exposure to three methanol deactivation cycles, the peak shifted slightly to 2θ = 6.11°. The intensities vary, but no conclusions are drawn from that. These results show that the spent samples did not change their crystalline phase significantly but possibly to a small extent.
Figure 8.
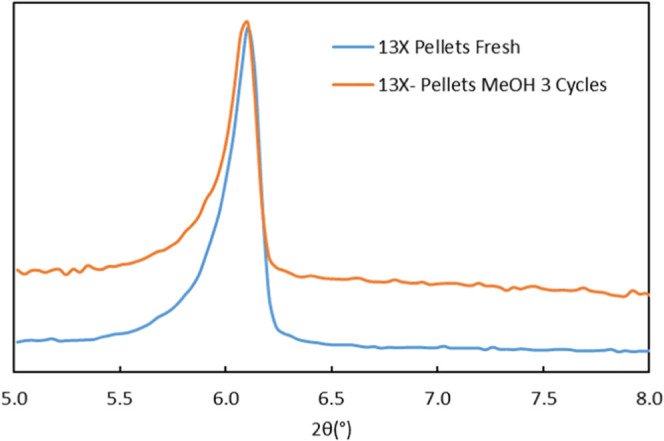
XRD [111] reflections of 13X pellets: as such (fresh) and after exposure to three cycles of methanol (MeOH).
2.3. 13C CP MAS NMR
The nature of carbon-containing residue formed on the 4A and 5A samples after methanol and heptane treatment was analyzed by 13C CP MAS NMR experiments. Figure 9 shows the spectra of both the three-cycle methanol and the three-cycle heptane runs. In all spectra, carbon-related features were observed. For the 13X sample, only the methanol test was analyzed.
Figure 9.
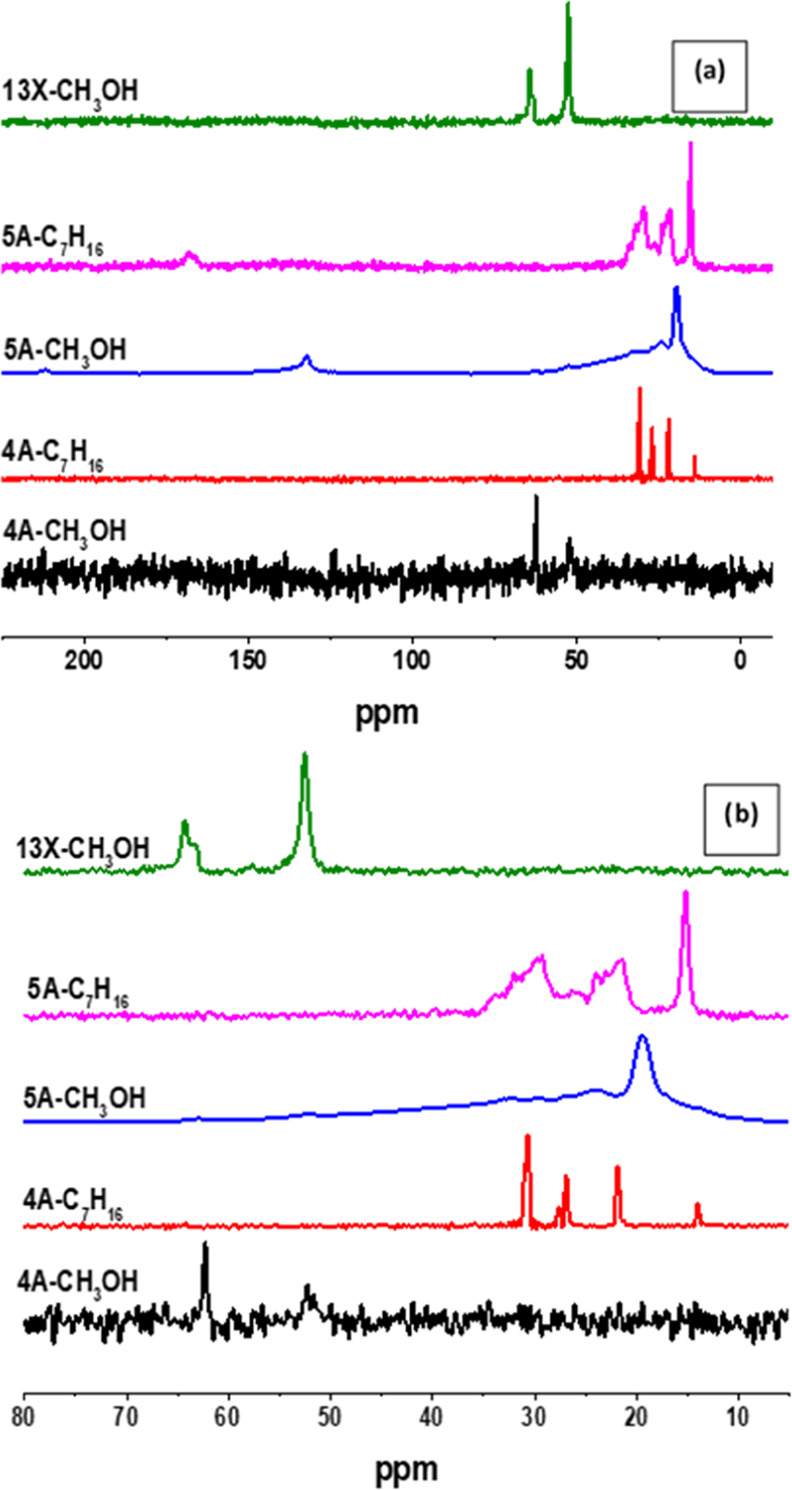
13C CP MAS NMR spectra of 4A, 5A, and 13X beads: (a) full spectra and (b) enlarged spectra.
The spectrum of the heptane-treated 5A sample shows chemical shifts corresponding to −CH3 groups at 15 ppm. The two broad peaks with multiplets centered at 21 and 29 ppm are attributed to −CH2 and −CH groups of aliphatic carbons, respectively. The peak with a chemical at 165 ppm indicates carbon bound to a heteroatom.34,35 The same was seen in the FTIR spectra. This remains unexplained.
The chemical shifts that appear for the heptane-treated 4A sample were completely different from the 5A sample. Four sharp peaks appeared at 14, 22, 27, and 31 ppm. These are attributed to the CH3, CH2 (2,6), CH2 (4), and CH2 (3,5) groups of the heptane molecule. This indicates that heptane got trapped inside the 4A zeolite pores but it did not suffer any chemical changes visible by NMR during the three-cycle test. Heptane molecules that moved into the 5A zeolite pores underwent chemical conversion steps generating other compounds than heptane. Heptane that passed through the 4A zeolite pores remained intact but got trapped inside the zeolite pores. This suggests that “coke” formation could occur in the absence of steric hindrance. Because of the larger pores of 5A samples, the steric hindrance is less significant. Hence, a higher rate of “coke” formation is observed in 5A zeolite in comparison to that in the smaller pore 4A zeolite. Another explanation could be that 5A is slightly more acidic than 4A, although LTA zeolites are nonacidic in nature.
All methanol-treated samples showed two separate well-distinguished peaks at 52 and 64 ppm. The former one is attributed to unreacted methanol, and the latter one is contributed from dimethyl ether (DME) groups.36 It is reported that the resonance at 64 ppm can also be contributed to from the CH3O– groups attached to the surface produced by acidic sites with methanol.34 Unreacted methanol peak at 52 ppm is more prominent for 13X sample, and its contribution on the 4A sample was very low and on the 5A sample, it is almost negligible. The chemical shift at 64 ppm is apparent for the 13X and 4A samples, and it appeared as a small hump in the 5A sample. The methanol-treated 4A and 13X samples showed a different behavior compared to the methanol-treated 5A sample. Chemical shifts of both aliphatic and aromatic carbons were absent for 4A and 13X samples.
The sharp peak at 19 ppm for the methanol-treated 5A sample was attributed to −CH2 groups, and the shoulder peaks between 10–15 and 20–40 ppm were attributed to −CH3 and −CH groups of aliphatic molecules, respectively.34,35 Aromatic or olefinic carbon was observed as a small peak at a chemical shift of 130 ppm.
These results indicate that the 5A sample is involved in the conversion of methanol into aliphatic and aromatic components. This process was not observed in 4A and 13X samples. For the latter two samples, methanol is seen to form methoxy species, probably with surface hydroxyl groups. Prior to the NMR recording, the samples are exposed to water vapor. This may induce hydrolysis, and free methanol is formed again. For both zeolites, no indications were found for the formation of aliphatic or aromatic compounds.
Similarly, heptane 5A is active for the conversion of the alkane into coke-like molecules. But nothing happened to the heptane molecules that were trapped inside the 4A zeolites. Based on these observations, it appears that 5A is more reactive to both methanol and heptane conversion than the 4A and 13X counterparts.
2.4. TGA
Figures 10–12 show the TGA thermographs of fresh and spent (“coked”) zeolites. The overall weight loss of all samples ranged from 12% wt to 22% wt. Initial loss occurs at a temperature below 200 °C. This is due to the water desorption from the zeolite pores.37 These numbers vary as the degree of hydration was not the same for the samples. The second weight loss occurs between 200 and 450 °C.
Figure 10.
TGA profiles of fresh and spent (MeOH and heptane) 4A pellets.
Figure 12.
TGA profiles of fresh and spent (MeOH) 13X pellets.
Figure 11.
TGA profiles of fresh and spent (MeOH and heptane) 5A pellets.
The fresh zeolite 4A sample shows an initial weight loss of 8% wt, which is related to water desorption. The second weight loss, only 3% wt, is probably due to water formed by dihydroxylation and water loss from the interparticle voids.38 The spent 4A zeolites from methanol and heptane deactivation cycles show almost 15% wt initial weight loss for both samples corresponding to the desorption of water. A second weight loss of 4% wt around 350 °C occurs for the heptane-treated samples. This is due to trapped heptane combusting. For the methanol-treated sample, no such weight loss was seen.
The 5A sample showed different behavior. There is no trapped heptane, and both the fresh and spent samples show the same weight loss of 19% wt. At about 300 °C, a very small difference is seen between the fresh and heptane-treated samples. The 5A methanol-treated sample shows a 6% wt initial weight loss of water desorbing and a second weight loss of 14% wt at about 400 °C. This is thought to be the result of residing carbon materials combusting. This is not seen for 4A and is consistent with the NMR results.
The 13X fresh sample shows a 15% wt loss due to the desorption of water. The initial gain of weight is due to the adsorption of residual water vapor in the TGA voids. The 13X methanol sample shows an initial loss of 17% wt due to water desorption, but no other features other than a very small shoulder at about 350–400 °C. This may be due to a small amount of methoxy groups residing in the structure. This is comparable to the 5A findings.
2.5. Methanol Adsorption
Examples of adsorption isotherms for methanol39,40 are shown in Figure S4. For comparison, adsorption on 3A zeolite is also shown. As the pore diameter of the latter (3 Å) is smaller than the kinetic diameter of methanol (3.6 Å), the adsorption capacity is low. Zeolites 4A and 5A adsorb roughly the same amount at 247 mbar; 157 vs 167 mg/g, respectively. The shape of the isotherms is classic Langmuir-like, type I.
In Figures S5 and S6, methanol and water adsorption isotherms are compared. The uptake capacity is expressed as mL/g rather than mg/g. For the conversion from mg to mL, simply the liquid densities were used at the given temperature. At the nanoscale of the pores sizes, this is presumably a too simplistic approach. Yet the results are remarkable. For both the 4A and 5A zeolites, the volume of the adsorbed material at saturation is almost the same for methanol and water. The entire pore structure is filled with the adsorbent.
3. Conclusions
At this point, it is obvious that the impact of methanol bigger than that of heptane. It may be clear that in an industrial plant, these particular conditions do not occur. Still, the message is clear. Exposure to methanol is to be avoided if possible. But methanol exposure is incidental, while heptane may be of continuous presence leading to tiny amounts of coke building up over time.
This implies that if methanol is present in the feed, it may cause problems. Alternatively, methanol can be selected as a probe molecule to test for differences between various (commercial) materials. Conversion reactions of methanol over zeolites were studied exhaustively. This led to commercial processes like MTG and MTO. Catalysts for these reactions are based on ZSM-5, an acidic low-alumina material. This is quite unlike molsieves from the LTA family.
The impact of two contaminants (methanol and heptane) on the water adsorption capacity of zeolites 4A, 5A, and 13X was assessed. For all combinations, a reduction of water adsorption capacity was observed. Under the given conditions, the effect of methanol was more significant than that of heptane. In some experiments, about half of the adsorption capacity was lost.
The XRD patterns clearly show that fresh, methanol-, and heptane-exposed zeolite patterns were not changed drastically. The TGA profile shows the gradual decreases in mass due to the burning out of carbonaceous materials at high temperatures.
13C CP MAS-NMR spectra showed the presence of different carbonaceous materials in the spent samples. On 5A, both heptane and methanol underwent conversion reactions. On 4A, heptane got trapped inside the structure, but no evidence was found for any reaction. Both 4A and 13X methanol led to the formation of methoxy groups and after hydrolysis seemed to produce methanol. This leads to a very significant loss of adsorption capacity, but likely not a permanent one. For 5A, this is different though.
4. Experimental Section
4.1. Materials
Zeolites (3A, 4A, 5A, and 13X), both powders and pellets, were obtained from commercial vendors such as Sigma-Aldrich, Alfa Aesar, and Chemiewerk Bad, Germany. The pellets (beads or extrudates) contain about 80% w zeolite and 20% w binder material. The nature of the binder material is not specified. Methanol (>99.9%) and heptane (>99%) were obtained from Sigma-Aldrich. Nitrogen gas (99.999% purity) was provided by Air Products.
4.2. Methods
4.2.1. Deactivation Cycles
It may be clear from the introduction that mirroring the dehydration operation in the field to the laboratory is not possible. Experiments cannot last for years. The approach chosen was to expose the desiccants to a series of high-temperature cycles in the presence of a volatile contaminant. The effect on water adsorption capacity on these samples could be mapped to desiccants samples obtained from the plants after unloading.
The deactivation of molecular sieves using methanol and heptane was carried out in a home-built microflow fixed-bed reactor operating at atmospheric pressure as shown in Figure 13. The setup consists of two sections: a feed section and a reaction section. In the feed section, nitrogen at a constant flow rate carries the chosen hydrocarbon into the reaction section. The flow rate of nitrogen is controlled using a mass flow controller (Cole-Parmer: EW-32907-63), and the hydrocarbon is introduced into the nitrogen stream using a syringe pump (KD Scientific-Legato 210 Dual syringe) operating at a constant infusion rate. The pump holds two parallel 50 mL syringes that infuse at the same rate simultaneously. The line that introduces the hydrocarbon into the nitrogen stream and the line that delivers the feed mixture into the reaction zone are both heated to a temperature of 20 °C above the boiling point of the hydrocarbon to evaporate the liquid and avoid condensation in the lines. In the reaction section, the mixture is fed to a reactor, a horizontal stainless steel pipe (1/4 in.) that contains a molecular sieve bed. The molecular sieve bed is preceded by 10 cm of small stainless steel rings to bring the feed to the reaction temperature. The reactor is fixed inside a tubular furnace that incorporates a temperature controller. The exhaust stream from the reactor is vented.
Figure 13.
Simplified flow diagram of the microflow unit used for deactivation cycles.
The fresh molecular sieves, about 5 g, were loaded as a packed bed in the middle of the stainless steel reactor tube. The ratio of the length of the bed to its diameter was set at 10:1. Therefore, the lengths of molecular sieve beds in all experiments were fixed at 5 cm. Occasionally, powders were tested. These were spatulated in small ceramic “boats” in a thin layer. Prior to each experiment, the lines were heated at 120 °C and flushed with nitrogen bypassing the reactor. Subsequently, the molecular sieves were pretreated for more than 3 h by heating the bed at 350 °C inside the tubular furnace while flushing it with a nitrogen stream preheated at 120 °C. The bed temperature was then reduced to 40 °C, and the temperature of the lines was fixed at 20 °C above the hydrocarbon boiling point to start the molecular sieve deactivation experiment. During the experiment, the nitrogen flow rate was fixed at 50 mL/min and the syringe pump dosage rate was fixed at 5 mL/h, which corresponds to a total of 10 mL/h hydrocarbon infusion rate for 10 h. As the hydrocarbon dosing was initiated, the molecular sieve bed was heated from 40 to 325 °C at a rate of 5 °C/min for around 1 h and then maintained at 325 °C for 9 h. After 10 h of exposure, the hydrocarbon stream was terminated and the bed was allowed to cool down to room temperature. Depending on the experiment, this step was repeated several times. The deactivated molecular sieves were then characterized to study their physicochemical properties.
4.2.2. Water Adsorption
The water vapor adsorption capacities of the samples were measured as a function of temperature and pressure using a gravimetric sorption analyzer (STATIC) from Rubotherm, Germany. The latter is a so-called magnetic suspension balance (MSB), type Isosorp.32 The equipment can be operated in a temperature range of 20–150 °C using a liquid circulator or 50–350 °C using an electrical heater at pressures up to 50 bars. To prevent any risk of unwanted condensation, maximum partial vapor pressures were limited to 70% of the dewpoint pressure. Prior to each measurement, the samples were pretreated under vacuum at 350 °C for 6 h. After pretreatment, the samples were cooled to, usually 30 °C, to measure the adsorption isotherm. The three positions of MSB allow us to measure the zero point (position 1), the sample weight plus the sample holder (position 2), and in position 3, a titanium sinker, with a known volume, is added to the weight. By means of the weight change of the sinker, the density of the gas can be measured in situ. With this density, and the known volume of the sample, a buoyancy correction was applied to the sample weight.
4.2.3. Characterization Methods
Wide-angle X-ray diffraction (WAXRD) analysis of the fresh and spent zeolites was performed on an analytical powder diffractometer (Panalytical X’Pert PRO) using Cu Kα radiation (λ = 1.5406 Å) in reflection mode. The fresh and spent zeolites were subjected to thermogravimetric analyses (TGA) using a Waters-SDT Q600 (TA Instruments). The temperature was increased from room temperature to 900 °C with a ramping rate of 10 °C/min under dry airflow (20 mL/min).
Solid-state 13C NMR was carried out in a Bruker 400 AVANCE III spectrometer (9.40 T) under magic angle spinning (MAS). This technique provides details about the chemical nature of coke formed and the state of the inorganic matrix of the samples. Coke formed on zeolites was analyzed by 13C CP MAS measurements at a resonance frequency of 100.628 MHz with a spinning rate of 8 kHz. Cross-polarization measurements on 13C were conducted using a 90° pulse for protons of 2.30 μs, 2.0 ms of contact time, and 5 s of recycling delay. Before the analysis, zeolite samples were powdered and kept in a humidity chamber for over 10 h at 25 °C and 90% humidity for water saturation. Samples of approximately 100 mg were packed into a 4 mm diameter cylindrical zirconia rotor for the NMR analysis.
Acknowledgments
The authors thank the Gas Processing and Materials Science Research Center (GRC) of the Petroleum Institute of Khalifa University for financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c03370.
XRD pattern from fresh and spent 4A-based materials (Figure S1); XRD pattern from fresh and spent 5A-based materials (Figure S2); XRD pattern from fresh and spent 13X-based materials (Figure S3); methanol vapor adsorption isotherms at 33 °C on crushed 3A, 4A, and 5A pellets (Figure S4); comparison between MeOH and H2O vapor adsorption on crushed 4A pellets at 33 °C and the adsorption capacity is expressed as mL/g (Figure S5); and comparison between MeOH and H2O vapor adsorption on crushed 5A pellets at 33 °C and the adsorption capacity is expressed as mL/g (Figure S6) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Fulcheri L.; Schwob Y. From methane to hydrogen, carbon black and water. Int. J. Hydrogen Energy 1995, 20, 197–202. 10.1016/0360-3199(94)E0022-Q. [DOI] [Google Scholar]
- Gudmundsson J. S.; Parlaktuna M.; Khokhar A. Storage of natural gas as frozen hydrate. SPE Prod. Facil. 1994, 9, 69–73. 10.2118/24924-PA. [DOI] [Google Scholar]
- Sie S.; Senden M.; Van Wechem H. Conversion of natural gas to transportation fuels via the shell middle distillate synthesis process (SMDS). Catal. Today 1991, 8, 371–394. 10.1016/0920-5861(91)80058-H. [DOI] [Google Scholar]
- Abhari R.; Havlik P.. Process for producing bio-derived fuel with alkyl ester and iso-paraffin components. Google Patents: 2008.
- Nunley M. A.; Bhasin M. M.; Etzkorn W. G.; Keller I. G. E.; Wadia P. H.. Methods for integrated natural gas purification and products produced therefrom. Google Patents: 2017.
- Goldsworthy M. Measurements of water vapour sorption isotherms for RD silica gel, AQSOA-Z01, AQSOA-Z02, AQSOA-Z05 and CECA zeolite 3A. Microporous Mesoporous Mater. 2014, 196, 59–67. 10.1016/j.micromeso.2014.04.046. [DOI] [Google Scholar]
- Xiao Y.; He G.; Yuan M. Adsorption equilibrium and kinetics of methanol vapor on zeolites NaX, KA, and CaA and activated alumina. Ind. Eng. Chem. Res. 2018, 57, 14254–14260. 10.1021/acs.iecr.8b04076. [DOI] [Google Scholar]
- Kim K.-M.; Oh H.-T.; Lim S.-J.; Ho K.; Park Y.; Lee C.-H. Adsorption equilibria of water vapor on zeolite 3A, zeolite 13X, and dealuminated Y zeolite. J. Chem. Eng. Data 2016, 61, 1547–1554. 10.1021/acs.jced.5b00927. [DOI] [Google Scholar]
- Kim J.-H.; Lee C.-H.; Kim W.-S.; Lee J.-S.; Kim J.-T.; Suh J.-K.; Lee J.-M. Adsorption equilibria of water vapor on alumina, zeolite 13X, and a zeolite X/activated carbon composite. J. Chem. Eng. Data 2003, 48, 137–141. 10.1021/je0201267. [DOI] [Google Scholar]
- Lercher J. A.; Seshan K. Sorption and activation of hydrocarbons by molecular sieves. Curr. Opin. Solid State Mater. Sci. 1997, 2, 57–62. 10.1016/S1359-0286(97)80106-4. [DOI] [Google Scholar]
- Wang L.; Tao L.; Xie M.; Xu G.; Huang J.; Xu Y. Dehydrogenation and aromatization of methane under non-oxidizing conditions. Catal. Lett. 1993, 21, 35–41. 10.1007/BF00767368. [DOI] [Google Scholar]
- Kłosek-Wawrzyn E.; Małolepszy J.; Murzyn P. Sintering behavior of kaolin with calcite. Procedia Eng. 2013, 57, 572–582. 10.1016/j.proeng.2013.04.073. [DOI] [Google Scholar]
- Sulaymon A.; Mahdi A. Spherical zeolite-binder agglomerates. Chem. Eng. Res. Des. 1999, 77, 342–350. 10.1205/026387699526179. [DOI] [Google Scholar]
- Jänchen J.; Schumann K.; Thrun E.; Brandt A.; Unger B.; Hellwig U. Preparation, hydrothermal stability and thermal adsorption storage properties of binderless zeolite beads. Int. J. Low Carbon Technol. 2012, 7, 275–279. 10.1093/ijlct/cts037. [DOI] [Google Scholar]
- Liu B.; Slocombe D.; Wang J.; Aldawsari A.; Gonzalez-Cortes S.; Arden J.; Kuznetsov V.; AlMegren H.; AlKinany M.; Xiao T.; Edwards P. P. Microwaves effectively examine the extent and type of coking over acid zeolite catalysts. Nat. Commun. 2017, 8, 514 10.1038/s41467-017-00602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz H. “Coking” of zeolites during methanol conversion: Basic reactions of the MTO-, MTP-and MTG processes. Catal. Today 2010, 154, 183–194. 10.1016/j.cattod.2010.05.012. [DOI] [Google Scholar]
- Bandiera J.; Taarit Y. B. Catalytic investigation of the dehydrogenation properties of pentasil type zeolites as compared with their cracking properties. Appl. Catal. 1990, 62, 309–316. 10.1016/S0166-9834(00)82254-X. [DOI] [Google Scholar]
- Choudhary V.; Devadas P.; Kinage A.; Sivadinarayana C.; Guisnet M. Acidity, catalytic activity, and deactivation of H-gallosilicate (MFI) in propane aromatization: Influence of hydrothermal pretreatments. J. Catal. 1996, 158, 537–550. 10.1006/jcat.1996.0052. [DOI] [Google Scholar]
- Herold R.; Mokhatab S.. Optimal Design and Operation of Molecular Sieve Gas Dehydration Units—Part 1. Gas Processing & LNG. August 2017. www.gasprocessingnews.com/features/201708/optimaldesign-and-operation-of-molecular-sieve-gas-dehydrationunits%E2.
- Forzatti P.; Lietti L. Catalyst deactivation. Catal. Today 1999, 52, 165–181. 10.1016/S0920-5861(99)00074-7. [DOI] [Google Scholar]
- Nasir Q.; Suleman H.; Elsheikh Y. A. A review on the role and impact of various additives as promoters/inhibitors for gas hydrate formation. J. Nat. Gas Sci. Eng. 2020, 76, 103211 10.1016/j.jngse.2020.103211. [DOI] [Google Scholar]
- Teixeira A. M.; de Oliveira Arinelli L.; de Medeiros J. L.; Ofélia de Queiroz F. A. Recovery of thermodynamic hydrate inhibitors methanol, ethanol and MEG with supersonic separators in offshore natural gas processing. J. Nat. Gas Sci. Eng. 2018, 52, 166–186. 10.1016/j.jngse.2018.01.038. [DOI] [Google Scholar]
- Zhang J.; Zhang H.; Yang X.; Huang Z.; Cao W. Study on the deactivation and regeneration of the ZSM-5 catalyst used in methanol to olefins. J. Nat. Gas Chem. 2011, 20, 266–270. 10.1016/S1003-9953(10)60183-1. [DOI] [Google Scholar]
- Aguayo A. T.; Castaño P.; Mier D.; Gayubo A. G.; Olazar M.; Bilbao J. Effect of cofeeding butane with methanol on the deactivation by coke of a HZSM-5 zeolite catalyst. Ind. Eng. Chem. Res. 2011, 50, 9980–9988. 10.1021/ie200946n. [DOI] [Google Scholar]
- Lee K.-Y.; Kang M.-Y.; Ihm S.-K. Deactivation by coke deposition on the HZSM-5 catalysts in the methanol-to-hydrocarbon conversion. J. Phys. Chem. Solids 2012, 73, 1542–1545. 10.1016/j.jpcs.2012.09.005. [DOI] [Google Scholar]
- Castellanos-Beltran I. J.; Assima G. P.; Lavoie J.-M. Effect of temperature in the conversion of methanol to olefins (MTO) using an extruded SAPO-34 catalyst. Front. Chem. Sci. Eng. 2018, 12, 226–238. 10.1007/s11705-018-1709-8. [DOI] [Google Scholar]
- Gao S.; Xu S.; Wei Y.; Qiao Q.; Xu Z.; Wu X.; Zhang M.; He Y.; Xu S.; Liu Z. Insight into the deactivation mode of methanol-to-olefins conversion over SAPO-34: Coke, diffusion, and acidic site accessibility. J. Catal. 2018, 367, 306–314. 10.1016/j.jcat.2018.09.010. [DOI] [Google Scholar]
- Misk M.; Joly G.; Magnoux P.; Guisnet M.; Jullian S. Formation of coke from propene over 5A adsorbents–influence of the binder on the coke composition, location and removal. Microporous Mesoporous Mater. 2000, 40, 197–204. 10.1016/S1387-1811(00)00260-2. [DOI] [Google Scholar]
- Sun H.; Shen B. Experimental study on coking, deactivation, and regeneration of binderless 5A zeolite during 1-hexene adsorption. Adsorption 2013, 19, 111–120. 10.1007/s10450-012-9426-y. [DOI] [Google Scholar]
- Uguina M.; Sotelo J.; Calleja G.; Díaz J.; Castillo E. Effects of the operating conditions on coke deactivation of 5A molecular sieve in n-decane adsorption/desorption. Adsorption 2002, 8, 133–140. 10.1023/A:1020430417911. [DOI] [Google Scholar]
- Gomes Santiago R.; Ferreira dos Santos B.; Gomes Lima I.; Oliveira Moura K.; Carrijo Melo D.; Mantovani Grava W.; Bastos-Neto M.; Pereira de Lucena S. M.; Cristina Silva de Azevedo D. Investigation of premature aging of zeolites used in the drying of gas streams. Chem. Eng. Commun. 2019, 206, 1367–1374. 10.1080/00986445.2018.1533468. [DOI] [Google Scholar]
- Opatokun S. A.; Prabhu A.; Al Shoaibi A.; Srinivasakannan C.; Strezov V. Food wastes derived adsorbents for carbon dioxide and benzene gas sorption. Chemosphere 2017, 168, 326–332. 10.1016/j.chemosphere.2016.10.083. [DOI] [PubMed] [Google Scholar]
- Treacy M. M.; Higgins J. B.. Collection of Simulated XRD Powder Patterns for Zeolites Fifth (5th) Revised Edition; Elsevier, 2007. [Google Scholar]
- Derouane E. G.; Gilson J.-P.; Nagy J. B. In situ characterization of carbonaceous residues from zeolite-catalysed reactions using high resolution solid state 13C-nmr spectroscopy. Zeolites 1982, 2, 42–46. 10.1016/S0144-2449(82)80039-0. [DOI] [Google Scholar]
- Bonardet J.; Barrage M.; Fraissard J. Use of NMR techniques for studying deactivation of zeolites by coking. J. Mol. Catal. A: Chem. 1995, 96, 123–143. 10.1016/1381-1169(94)00030-1. [DOI] [Google Scholar]
- Zhou L.; Li S.; Qi G.; Su Y.; Li J.; Zheng A.; Yi X.; Wang Q.; Deng F. Methanol carbonylation over copper-modified mordenite zeolite: A solid-state NMR study. Solid State Nucl. Magn. Reson. 2016, 80, 1–6. 10.1016/j.ssnmr.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Petkowicz D. I.; Rigo R. T.; Radtke C.; Pergher S. B.; dos Santos J. H. Zeolite NaA from Brazilian chrysotile and rice husk. Microporous Mesoporous Mater. 2008, 116, 548–554. 10.1016/j.micromeso.2008.05.014. [DOI] [Google Scholar]
- Akçay K.; Sirkecioğlu A.; Tatlıer M.; Savaşçı Ö. T.; Erdem-Şenatalar A. Wet ball milling of zeolite HY. Powder Technol. 2004, 142, 121–128. 10.1016/j.powtec.2004.03.012. [DOI] [Google Scholar]
- Aittomäki A.; Härkönen M. Zeolite heat pump-adsorption of methanol in synthetic zeolites 13X, 4A and 5A. Int. J. Refrig. 1986, 9, 240–244. 10.1016/0140-7007(86)90097-6. [DOI] [Google Scholar]
- Yarulina I.; Chowdhury A. D.; Meirer F.; Weckhuysen B. M.; Gascon J. Recent trends and fundamental insights in the methanol-to-hydrocarbons process. Nat. Catal. 2018, 1, 398–411. 10.1038/s41929-018-0078-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







