Significance
To date, no cure or preventative treatment for Parkinson’s disease (PD) has yet been developed. Here, we show that kurarinone, a natural flavonoid, alleviated parkinsonism-like symptoms induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in mice. Using a proteomics approach, we identified the soluble epoxide hydrolase (sEH) as a possible target of kurarinone’s reduction of neuroinflammation. This was supported using complementary biochemical approaches, which demonstrated that kurarinone is a high nanomolar uncompetitive inhibitor of sEH. Our findings suggest that natural products could attenuate the development of PD through inhibition of sEH.
Keywords: soluble epoxide hydrolase, kurarinone, Sophora flavescens, Parkinson’s disease
Abstract
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders and is characterized by loss of dopaminergic neurons in the substantia nigra (SN), causing bradykinesia and rest tremors. Although the molecular mechanism of PD is still not fully understood, neuroinflammation has a key role in the damage of dopaminergic neurons. Herein, we found that kurarinone, a unique natural product from Sophora flavescens, alleviated the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)–induced behavioral deficits and dopaminergic neurotoxicity, including the losses of neurotransmitters and tyrosine hydroxylase (TH)–positive cells (SN and striatum [STR]). Furthermore, kurarinone attenuated the MPTP-mediated neuroinflammation via suppressing the activation of microglia involved in the nuclear factor kappa B signaling pathway. The proteomics result of the solvent-induced protein precipitation and thermal proteome profiling suggest that the soluble epoxide hydrolase (sEH) enzyme, which is associated with the neuroinflammation of PD, is a promising target of kurarinone. This is supported by the increase of plasma epoxyeicosatrienoic acids (sEH substrates) and the decrease of dihydroxyeicosatrienoic acids (sEH products), and the results of in vitro inhibition kinetics, surface plasmon resonance, and cocrystallization of kurarinone with sEH revealed that this natural compound is an uncompetitive inhibitor. In addition, sEH knockout (KO) attenuated the progression of PD, and sEH KO plus kurarinone did not further reduce the protection of PD in MPTP-induced PD mice. These findings suggest that kurarinone could be a potential natural candidate for the treatment of PD, possibly through sEH inhibition.
Parkinson’s disease (PD) is the second-most common neurodegenerative disorder after Alzheimer’s disease (AD) and affects 1.7% of the population over 65 y old, especially people over 80 y old (1, 2). PD is caused by the loss of dopaminergic neurons in the substantia nigra (SN), and it is associated with accumulation of Lewy bodies (LBs) in neuronal somata and Lewy neurites in neuronal processes with fibrillar α-synuclein (3). PD is characterized by the classical motor features of parkinsonism, including bradykinesia, rest tremor, and rigidity as well as postural instability (4, 5). Advances have been made in understanding PD neurodegenerative pathophysiology (4–6). However, translation into patients’ care is still lagging well behind. So far, the treatment of PD in recent trials still depends on strategies for neuroprotection, motor symptoms, and nonmotor symptoms (6). However, PD symptoms have proven elusive to slow down or reverse through the aforementioned interventions (6); therefore, a cure for PD (no symptoms, no side effects, to borrow a phrase from the epilepsy field) has not yet been achieved.
Although the molecular mechanism resulting in the neuronal degeneration of PD is not fully understood, some factors are associated with the damage of dopaminergic neurons in PD, including mitochondrial dysfunction, oxidative stress, endoplasmic reticulum stress, and especially neuroinflammation (7, 8). Extensive recent evidence revealed that the release of α-synuclein from dopaminergic neurons activated microglia cells to cause the neuroinflammation, allowing the increase of inflammatory cytokines interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) (9, 10). These changes have been found in postmortem brain tissue from PD patients (11, 12). Therefore, neuroinflammation plays a central role in the development of PD and is the target of some recent investigations for treating PD (13–16).
Natural products are an important resource of innovational drugs since they possess complex and changeable structures and remarkable biological effects. A great body of evidence has indicated the effect of natural products from traditional Chinese medicines in the neuroinflammation of PD (17–19), such as genistein, resveratrol, and alaternin. Kurarinone is one of the major constituents of the traditional Chinese medicine Sophorae Flavescentis Radix, or Kushen in Chinese (the root of Sophora flavescens), which is often used to treat diarrhea, bacterial and fungal infections, eczema, and inflammation-related diseases (20). Kurarinone shares a flavanone core with a characteristic lavandulyl moiety at C-8 (21, 22) and possesses several pharmacological effects, such as anti-inflammatory and antioxidative activities (23, 24), as well as activation on the large-conductance Ca2+-activated K+ channel (25, 26).
Therefore, in this study, we first tested the ability of kurarinone to reduce neuroinflammation and improve behavioral deficits in a PD mice model induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). To understand how kurarinone decreases inflammation and because its treatment resulted in elevated levels of epoxyeicosatrienoic acids (EETs), endogenous signaling molecules that control inflammation (27), we used several biochemical methods to determine the molecular target of kurarinone. The interactions between kurarinone and soluble epoxide hydrolase, the main enzyme metabolizing EETs (28), were confirmed using enzyme kinetics and cocrystallization. Our findings suggest that kurarinone could be a potential natural candidate for the treatment of PD through sEH inhibition and other mechanisms, as well as being a lead to develop a new family of sEH inhibitors.
Results
Kurarinone from S. flavescens Alleviated MPTP-Induced Gait Disorders in Mice.
The extract of S. flavescens (SFE) displayed significant anti-PD effect in MPTP-induced PD in mice (SI Appendix, Figs. S1–S4). In order to discover the active constituents of S. flavescens, the brain and plasma of the mice were collected 20 min after administration of SFE and analyzed by liquid chromatography–tandem mass spectrometry (LC-MS/MS). As shown in SI Appendix, Fig. S5, six and four peaks were identified in the LC-MS/MS plot of plasma and brain, namely kushenols F, N, and S, flemiphilippinin D, sophoraflavanone G, and kurarinone, respectively.
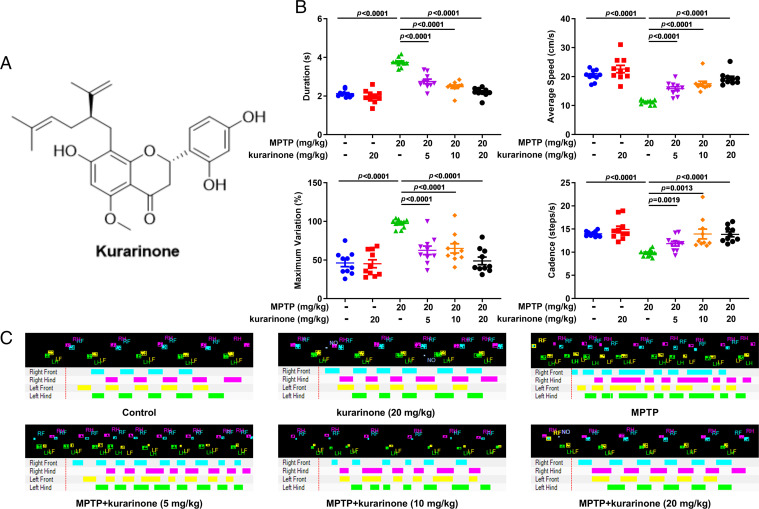
Kurarinone (Fig. 1A), the major constituent of SFE (22), was used to assay for its anti-PD effect in MPTP-treated mice. To assess the effect of kurarinone on MPTP-induced gait disorders, some behavioral parameters were also measured on the CatWalk Automated Gait Analysis System, including duration, maximum variation, average speed, and cadence. Administration of kurarinone (5, 10, and 20 mg/kg) dose-dependently decreased the duration and maximum variation and enhanced the average speed and cadence of mice compared with the MPTP-only group (Fig. 1 B and C).
Fig. 1.
Kurarinone from S. flavescens alleviated the behavior of MPTP-induced PD mice. (A) The structure of kurarinone. (B) The behavioristic parameters. Data represent mean ± SEM, n = 10. The significance was determined by one-way ANOVA followed by Tukey’s test. (C) The representative footprints. LF, left front; LH, left hind; RF, right front; RH, right hind; NO, noise.
Kurarinone Alleviated MPTP-Induced Neurotoxicity.
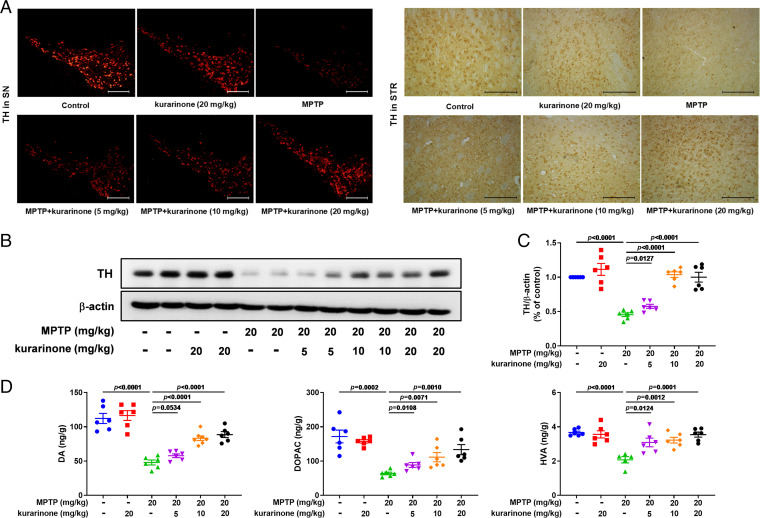
Tyrosine hydroxylase (TH) is a critical marker of dopaminergic neurons; therefore, cells (SN and STR) were immunostained to quantify the neuroprotective effect of kurarinone in MPTP-induced mice. Compared with the control group, MPTP treatment led to the decrease of TH-positive cells (SN and STR; Fig. 2A) and dopamine transporter (DAT, STR; SI Appendix, Fig. S7), respectively. In addition, the TH expression level in the SN was also decreased by MPTP treatment (Fig. 2B and SI Appendix, Fig. S8 A and B). Administration of kurarinone (5, 10, and 20 mg/kg) dose-dependently reversed these changes in MPTP-induced PD mice (Fig. 2 A and B and SI Appendix, Figs. S7 and S8 A and B). Finally, levels of dopamine (DA) and its metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) were detected to investigate the neuroprotective effect of kurarinone in MPTP-induced mice. The levels of DA, DOPAC, and HVA were decreased in MPTP-induced mice, whereas kurarinone (5, 10, and 20 mg/kg) treatment significantly attenuated the reduction of DOPAC and HVA in the STR. The DA level was also increased after administration of kurarinone (10 and 20 mg/kg), except for 5 mg/kg of kurarinone treatment. This result suggests that kurarinone significantly reduced MPTP-induced neurotoxicity.
Fig. 2.
Kurarinone enhanced TH expression and neurotransmitter levels in MPTP-induced PD mice. (A) The representative staining of TH in the SN (scale bar: 300 μm) and STR (scale bar: 100 μm). (B) Effect of kurarinone on the TH expression level in the SN. (C) Quantitative data of TH. Data represent mean ± SEM, n = 6. The significance of difference was determined by one-way ANOVA followed by Tukey’s test. (D) Effects of kurarinone on neurotransmitters DA, DOPAC, and HVA. Data represent mean ± SEM, n = 6. The significance of difference was determined by one-way ANOVA followed by Tukey’s test.
Kurarinone Reversed the Activation of MPTP-Induced Microglia Cells and MPTP-Induced Neuroinflammation.
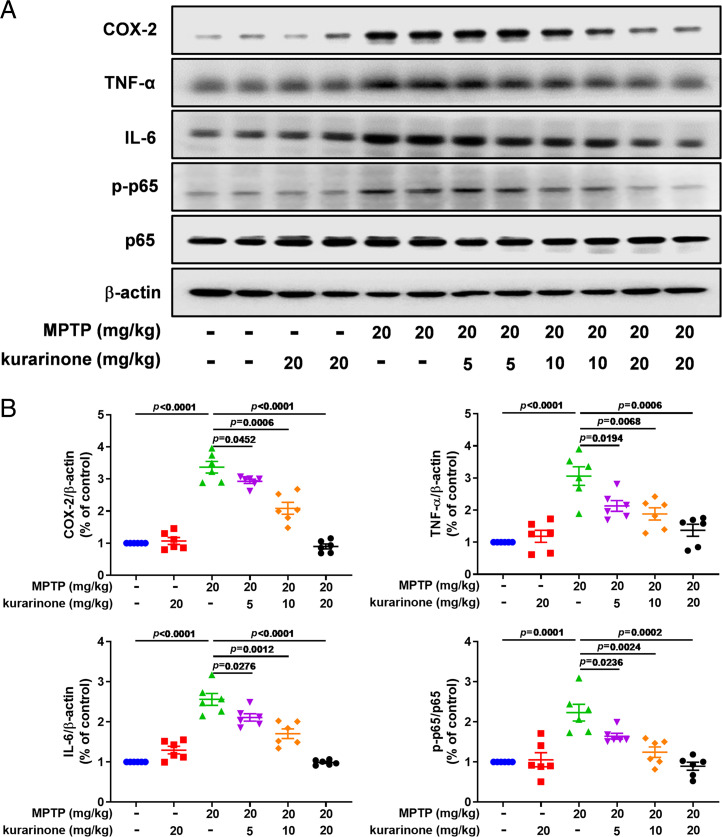
Neuroinflammation usually leads to the production of cytotoxic substances, further aggravating PD development; therefore, we investigated the effect of kurarinone on microglia and the inflammatory nuclear factor kappa B (NF-κB) signaling pathway. Firstly, ionized calcium-binding adaptor molecule-1 (Iba-1), a marker of microglia, was used to inspect the effect of kurarinone on its activation (Fig. 3). As shown in SI Appendix, Fig. S9, MPTP treatment promoted the activation of microglia stained by Iba-1 in the SN compared with the control group, whereas administration of kurarinone (5, 10, and 20 mg/kg) significantly inhibited its activation (SI Appendix, Fig. S8C). Furthermore, MPTP treatment up-regulated expression levels of NF-κB–related proteins in the SN, including COX-2, TNF-α, IL-6, and p-p65 (Fig. 3 A and B), resulting in the activation of the NF-κB signaling pathway. However, administration of kurarinone (5, 10, and 20 mg/kg) down-regulated these protein expression levels in a dose-dependent manner, which demonstrated that kurarinone effectively alleviates the neuroinflammation in MPTP-induced PD mice.
Fig. 3.
Kurarinone form S. flavescens inhibited neuroinflammation in MPTP-induced PD mice. (A) Effects of kurarinone on expression levels of COX-2, TNF-α, IL-6, p-p65, and p65. (B) Quantitative data on COX-2, TNF-α, IL-6, and p-p65/p65 levels. Data represent mean ± SEM, n = 6. The significance of difference was determined by one-way ANOVA followed by Tukey’s test.
Target Profiling of Kurarinone by the TPP and SIP Methods.
The thermal proteome profiling (TPP) and solvent-induced protein precipitation (SIP) methods depend on the principle that the stability of the target proteins bound with a ligand will change. These ligand-induced stability shifts have led to possible target proteins having different resistance to denaturization and thereby differential precipitation when the bound and free target proteins are subjected to heat or solvent treatment (29–31). Recently, TPP and SIP methods have been widely used to map the target proteins of different ligands such as drugs, nucleotides, and metabolites (31–34). Therefore, to ensure reliability in identification of the protein targets of kurarinone, the TPP and SIP methods were employed to reveal potential targets of kurarinone.
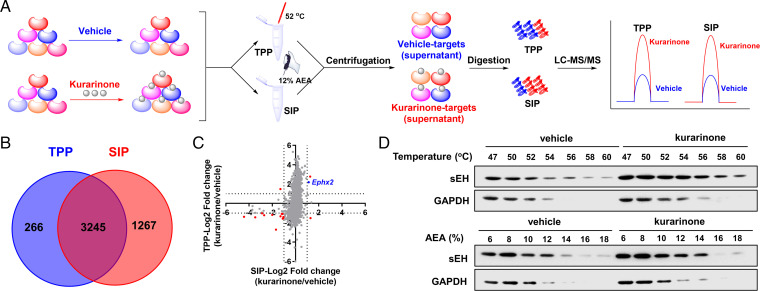
The workflows of the TPP and SIP methods used for screening protein targets of kurarinone are pictured in Fig. 4A. As described in Fig. 4B, 3,245 proteins were quantified in both the TPP and SIP methods. The fold change of each protein between vehicle and kurarinone-treated groups was calculated. The fold change of overlapping proteins identified by the TPP and SIP methods was compared. The fold change for each protein reflected the extent of stability shifts of a protein bound with or without kurarinone, with a large −log2 (fold change) corresponding to greater stability shift. After applying a cutoff of ±1 for −log2 (fold change) both in the TPP and SIP methods, 14 proteins were identified as candidate targets of kurarinone (Fig. 4C), such as sEH (gene name, Ephx2), keratin 17, desmoplakin, adgrb1, slc18a2, and adhesion G protein–coupled receptor B1 (SI Appendix, Table S1). The gene ontology analysis suggests that these proteins are involved in lipid metabolic process, inflammatory response, metabolic process, cell surface receptor signaling pathway, G protein–coupled receptor signaling pathway, negative regulation of angiogenesis, and so on (SI Appendix, Table S1). The above in vivo results showed kurarinone could alleviate MPTP-induced neuroinflammation, and 14 differential proteins, including sEH, were identified by the TPP and SIP assays. According to a previous investigation on PD, sEH plays a role in neuroinflammation (28), which suggests that sEH is a possible target of kurarinone.
Fig. 4.
sEH was identified as the potential target of kurarinone by TPP and SIP assays. (A) Schematic representation of the TPP and SIP methods for screening of target proteins of kurarinone. (B) The Venn diagram of potential target proteins identified by the TPP and SIP methods. (C) Fourteen candidate targets were screened by filtering the log2 (fold change) of each protein identified in the TPP and SIP assays. Red and blue dots are differential proteins affected by kurarinone in the TPP and SIP assays, respectively. (D) Western blotting demonstrated that sEH was stabilized after the incubation with kurarinone by using the TPP and SIP methods. AEA, an organic solvent mixture of acetone: ethanol: acetic acid; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
To verify the stabilization of sEH by kurarinone as detected by mass spectrometry, we used different temperatures and percentages of solvent mixtures to denature proteins in the vehicle and kurarinone-treated groups and measured the supernatants by immunoblotting. The Western blotting result in Fig. 4D revealed that sEH in the kurarinone-treated group was gradually decreased with the elevation of temperature and percentages of solvent mixture, whereas that in the vehicle group rapidly declined. Comparison of these two groups demonstrated that sEH was stabilized by kurarinone and agreed with the result, supporting our hypothesis that sEH is a potential target protein of kurarinone.
Kurarinone Suppressed sEH to Stabilize the Level of EETs in MPTP-Induced PD Mice, Resulting in the Inhibition of GSK3β.
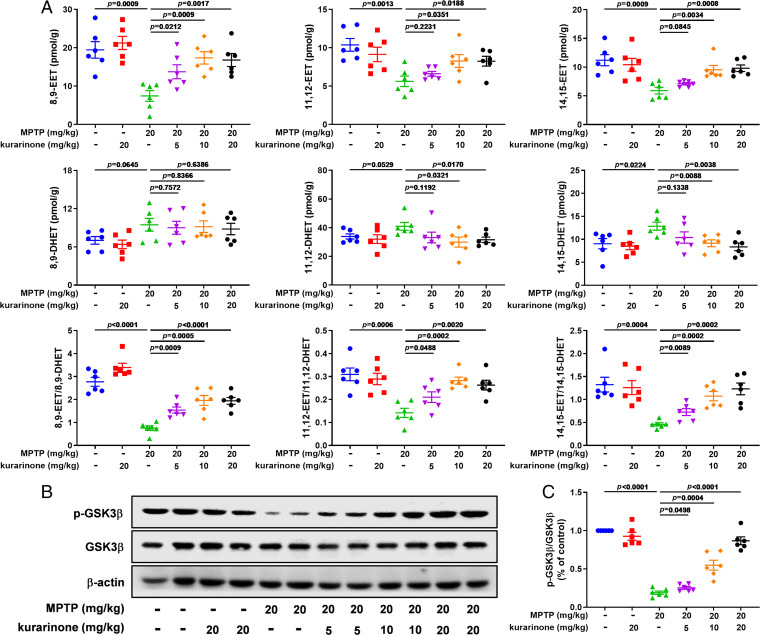
The result of TPP and SIP methods indicated that sEH is a promising target of kurarinone, which encouraged us to investigate the effect of kurarinone toward sEH activity and its related metabolites. sEH is an α/β hydrolase fold protein and hydrolyzes EETs to produce dihydroxyeicosatrienoic acids (DHETs) (35). EETs and their corresponding diols (DHETs) are important indexes to reflect sEH activity; therefore, we used LC-MS/MS to study the content of EETs and DHETs in STR of PD mice. As shown in Fig. 5A, except for 8,9-EET, 5 mg/kg of kurarinone treatment did not show significant influence toward levels of 11,12-EET, 14,15-EET, 8,9-DHET, 11,12-DHET, and 14,15-DHET. It is worth noting that administration of kurarinone (10 and 20 mg/kg) attenuated the reduction of MPTP-mediated endogenous 8,9-EET, 11,12-EET, and 14,15-EET and reserved the increase of their corresponding diols 11,12-DHET and 14,15-DHET, except for 8,9-DHET (Fig. 5A). The ratio of EETs and DHETs suggests that kurarinone inhibited endogenous sEH activity (Fig. 5A).
Fig. 5.
Kurarinone alleviated the increase of sEH activity and suppressed the GSK3β signaling pathway via stabilizing the level of EETs in MPTP-induced PD mice. (A) Effect of kurarinone on 8,9-EET; 11,12-EET; 14,15-EET; 8,9-DHET; 11,12-DHET; 14,15-DHET; 8,9-EET/8,9-DHET; 11,12-EET/11,12-DHET; and 14,15-EET/14,15-DHET in MPTP-induced PD mice. Data represent mean ± SEM, n = 6. The significance of difference was determined by one-way ANOVA followed by Tukey’s test. (B) Effects of kurarinone on p-GSK3β and GSK3β expression levels in MPTP-induced PD mice. (C) Quantitative data of phosphorylated GSK3β (p-GSK3β)/GSK3β. Data represent mean ± SEM, n = 6. The significance of difference was determined by one-way ANOVA followed by Tukey’s test.
Glycogen synthase kinase 3 beta (GSK3β) is an important serine/threonine kinase involved in neuroinflammation; meanwhile, EETs are able to regulate its activity (36, 37). Therefore, the effect of the inhibition of sEH by kurarinone toward GSK3β was also determined. As shown in Fig. 5 B and C, the phosphorylated level of GSK3β was significantly decreased in MPTP-induced PD mice, while administration of kurarinone (5, 10, and 20 mg/kg) significantly improved its phosphorylated level. These results suggest that sEH activity was inhibited to enhance the endogenous level of EETs, permitting the stabilization of GSK3β phosphorylation.
In order to increase the clinical relevance of the efficacy for kurarinone in PD, MPTP and rotenone (ROT)-induced PD mice were administered kurarinone (20 mg/kg) after the beginning of MPTP and ROT treatment. As described above, kurarinone also alleviated PD after MPTP and ROT administration (SI Appendix, Figs. S10 and S11).
The Interaction of Kurarinone with sEH.
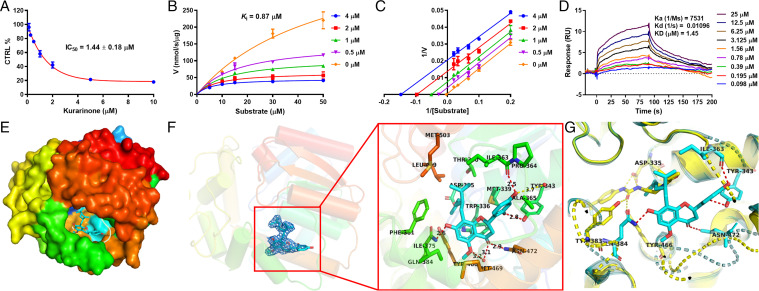
To understand how kurarinone affects sEH, human and mouse recombinant sEH and PHOME were used to assay the inhibitory activity and inhibition kinetics in vitro. Kurarinone displayed dose-dependent inhibitory effects against human sEH with an inhibition constant (Ki) value of 0.87 μM (Fig. 6 A–C). The kinetic analysis suggests that kurarinone is an uncompetitive inhibitor of sEH. The half-maximal inhibitory concentration (IC50) value of kurarinone was 0.42 μM toward the mouse sEH activity (SI Appendix, Table S2). To ensure that the inhibitory effect was not an artifact, a radioactive substrate (t-DPPO) and another fluorescent substrate (MNPC) were also used to test the inhibitory effect of kurarinone against sEH activity (SI Appendix, Tables S3 and S4). The results support sEH inhibition by kurarinone. In order to reveal the interaction kinetics of kurarinone with sEH in vitro, a surface plasmon resonance (SPR) technique was utilized. Kurarinone was able to increase its binding to sEH in a dose-dependent manner, as described in Fig. 6D. The association constant (Ka) and dissociation constant (Kd) for kurarinone and sEH binding were 7531 M−1 s−1 and 0.01096 s−1, respectively, and its equilibrium dissociation constant (KD) was 1.45 μM, which is close to the Ki obtained.
Fig. 6.
Kurarinone inhibited the sEH activity in vitro and interacted with sEH. (A) IC50 plot of kurarinone against sEH. (B) Michaelis-Menten plot of kurarinone against sEH. (C) Lineweaver-Burk plot of kurarinone against sEH. (D) SPR plot of kurarinone with sEH. (E) Kurarinone bound to the cavity of sEH (PDB code 7EBA). (F) Electronic cloud plot of kurarinone and the interaction of kurarinone with sEH. (G) Interaction differences of kurarinone (cyan) and TPPU (yellow, PDB code 4OCZ) with sEH. CTRL, control.
In order to investigate the binding of kurarinone with sEH, the natural compound was added to a solution of human recombinant sEH and manipulated to obtain a cocrystal. The resolution of this cocrystal was 2.10 Å with rmsd of 0.0152 Å (SI Appendix, Table S5). This cocrystal indicated that kurarinone bound in the catalytic tunnel of sEH but not at the catalytic site through van der Waals’ interactions, water hydrogen bond, conventional hydrogen bond, carbon hydrogen bond, π-δ, π-π T-shaped, alkyl, and π-alkyl interactions (SI Appendix, Fig. S12 and Fig. 6E). Compared with the competitive sEH inhibitor TPPU (Fig. 6G), kurarinone displayed a different binding pattern with sEH. As shown in Fig. 6F, kurarinone formed four conventional hydrogen bond interactions with amino acid residues Tyr343, Ile363, Gln384, and Asn472 and OH-7, OH-2′, OH-4′, and the carbonyl group at C-4, respectively.
The Anti-PD Effect of Kurarinone Was Abolished by Ephx2 Genetic Deletion.
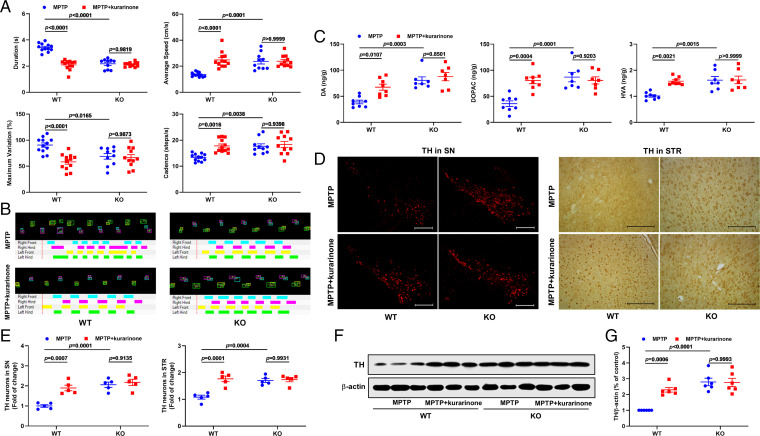
To test the role of sEH in the treatment of PD with kurarinone, wild-type (WT) and Ephx2 KO mice were administered MPTP and kurarinone. The CatWalk Automated Gait Analysis System was used to assay the behavior of treated mice (Fig. 7 A and B). Compared with the MPTP-treated WT group, Ephx2 genetic deletion significantly attenuated the MPTP-induced gait disorders (Fig. 7 A and B). After administration of kurarinone (20 mg/kg), its therapeutic effect was not observed in the MPTP-treated KO mice (Fig. 7 A and B). The LC-MS/MS results of DA and its metabolites DOPAC and HVA demonstrated that sEH KO attenuated their reduction induced by MPTP, and the effect of kurarinone (20 mg/kg) was abolished by Ephx2 genetic deletion (Fig. 7C). Furthermore, we also investigated TH-positive cells in the SN and STR and TH expression level in the SN. In the SN, Ephx2 genetic abolishment up-regulated the TH expression level and increased TH-positive cells compared with the MPTP-treated WT group. It is worth noting that administration of kurarinone (20 mg/kg) did not further improve the decrease of TH-positive cells and TH expression level induced by MPTP in KO mice (Fig. 7 D–G). A similar result was also observed in STR. These findings suggest that Ephx2 genetic deletion abolished the anti-PD effect of kurarinone, which further supports our hypothesis that sEH is a key target of kurarinone in the treatment of PD.
Fig. 7.
Ephx2 genetic deletion abolished the an-PD effect of kurarinone. (A) Ephx2 genetic deletion abolished the effect of kurarinone on gait disorders. Data represent mean ± SEM, n = 10 to 12. (B) The representative footprints. (C) Ephx2 genetic deletion abolished the effect of kurarinone on neurotransmitters DA, DOPAC, and HVA. Data represent mean ± SEM, n = 7 or 8. (D) Ephx2 genetic deletion abolished the effect of kurarinone on TH-positive neurons in the SN (scale bar: 300 μm) and STR (scale bar: 100 μm). The representative staining of TH in the SN and STR. (E) The data of TH-positive neurons in the SN and STR. Data represent mean ± SEM, n = 5. The significance of difference was determined by two-way ANOVA followed by Tukey’s test. (F) Ephx2 genetic deletion abolished the effect of kurarinone on the TH expression level in the SN. (G) Quantitative data of TH. Data represent mean ± SEM, n = 6. The significant difference was determined by two-way ANOVA followed by Tukey’s test. CTRL, control.
Discussion
This study demonstrated that kurarinone from S. flavescens alleviated MPTP-induced gait disorders and dopaminergic neurotoxicity by increasing the neurotransmitters and expression of TH-positive cells (SN and STR). Furthermore, it also attenuated the MPTP-mediated neuroinflammation via suppressing the activation of microglia involved in the NF-κB signaling pathway. It is worth noting that administration of kurarinone increased levels of the 8,9-EET; 11,12-EET; and 14,15-EET substrates of sEH and decreased levels of 11,12-DHET and 14,15-DHET products in MPTP-induced mice, and its KD value with sEH was 1.45 μM, which suggests that kurarinone could significantly inhibit sEH activity in vivo to attenuate MPTP-induced PD in conjunction with the proteomics results of the SIP and TPP assays. Cocrystallization of kurarinone with sEH (Protein Data Bank [PDB] code 7EBA) revealed that amino acid residues Tyr343, Ile363, Gln384, and Asn472 played a key role in its inhibition on sEH. In addition, Ephx2 genetic deletion attenuated the progression of PD, and sEH KO plus kurarinone did not further reduce the protection of PD in MPTP-induced PD mice. These findings suggest that kurarinone suppressed sEH activity via interacting with Tyr343, Ile363, Gln384, and Asn472 to enhance levels of endogenous EETs, evoking its anti-PD effect through the regulation of neuroinflammation, which demonstrates that kurarinone and similar structures could be developed as sEH inhibitors in the treatment of PD.
PD is a progressive neurodegenerative disorder characterized by the loss of dopaminergic neurons in the SN (3), further causing the parkinsonism-like behaviors (4, 5). A great body of studies indicate that the damage of dopaminergic neurons leads to the release of α-synuclein and activates microglia to produce proinflammatory cytokines IL-6 and TNF-α and up-regulate iNOS and COX-2 expression levels, allowing the development of neuroinflammation in PD (10, 38). Studies by Imamura et al. (12, 38) revealed that the major histocompatibility complex class II–positive microglia significantly increased in the SN of PD patients, and the number of positive microglia in the SN of PD level III patients was 10-fold more than that of the normal controls. Consistent with previous studies, the activation of microglia was also detected in our study, while kurarinone dose-dependently inhibited its activation (Fig. 4A). The inflammatory mediators IL-6, TNF-α, and ICAM-1 cause neuroinflammation and dopaminergic neuronal loss in PD, and are all regulated by a “master switch” for inflammatory gene expression—NF-κB (39). In the in vitro and in vivo PD models, expression levels of IL-6, TNF-α, p-p65, and MCP-1 were increased (40, 41), changes that are found in PD brains as well (42–45). Previous studies showed that kurarinone, a lavandulyl flavanone from S. flavescens (21), inhibits iNOS-dependent nitric oxide release, reactive oxygen species generation, and expression of inflammatory cytokines (e.g., TNF-α, IL-1β, and iNOS) in lipopolysaccharide-induced RAW264.7 macrophages (24), which reveals that kurarinone might possess anti-inflammatory potential in PD. In this study, we demonstrated that kurarinone could attenuate behavioral deficits of MPTP-induced PD mice via alleviating the damage of dopaminergic neurons, suppressing the activation of microglia and the NF-κB signaling pathway (Figs. 1–3), possibly by multiple mechanisms.
sEH is an α/β hydrolase fold protein containing two 62.5-kDa monomers and possessing two catalytic functions in mammals comprising a C-terminal hydrolase and N-terminal phosphatase (35). EETs and other epoxy–fatty acids (EpFAs) are formed from unsaturated fatty acids by cytochrome P450 (CYP) oxidases (e.g., CYP2J2 and CYP2C8). The sEH C-terminal activity can hydrolyze EpFAs to produce diols (46), resulting in the elimination of the beneficial effects of EETs and other EpFAs, such as vasodilation and resolution of inflammation (47–49). However, inhibition of sEH stabilizes the level of EETs, which activates peroxisome proliferator-activated receptor gamma (PPAR-γ) and inhibits NF-κB and GSK3β, resulting in increased anti-inflammatory effects. Therefore, sEH plays a key role in inflammation-related neurodegenerative diseases (50), especially PD and AD. The expression level of sEH was significantly increased in MPTP-induced PD mice (8), and its overexpression was also detected in STR of dementia patients with LBs (8). Therefore, chemical inhibition of sEH or sEH deficiency could attenuate dopamine neuron loss and neurotoxicity (8, 51, 52), and thus, sEH is a promising target for the treatment of PD. In this study, we found an increase of sEH and a decrease of its inflammation-resolving substrates in MPTP-induced PD mice as previously reported (Fig. 5 A–C). In addition, the proteomics result of the SIP and TPP indicated that sEH was a specific target of kurarinone in the treatment of PD, which was supported by the increase of 8,9-EET; 11,12-EET; and 14,15-EET and the decrease of 11,12-DHET and 14,15-DHET (Fig. 5C). Moreover, sEH deficiency attenuated the progression of PD, and the anti-PD effect of kurarinone was not observed in MPTP-induced sEH KO mice.
Some sEH inhibitors, such as 12-(3-adamantan-1-yl-ureido)-dodecanoic acid (AUDA) and 1-(1-propanoylpiperidin-4-yl)-3-[4-(trifluoromethoxy)phenyl]urea (TPPU), are commercially available and are used to investigate the effect of sEH in biological studies. Moreover, sEH inhibitors AR9281 and GSK2256294 have finished phase 1 or phase 2 clinical trials (50, 53), and a TPPU analog finished a human phase 1a trial recently with no adverse effects. All of the synthetic sEH inhibitors belong to the family of ureas or amides (50) and interact with amino acid residues Asp333 of the catalytic triad Asp333–Asp495–His523, responsible for opening the epoxide ring, and two tyrosines, Tyr381 and Tyr465, in charge of the fixation of the oxygen atom of the epoxide; therefore, they are defined as competitive inhibitors (50). In this study, kurarinone was found to be an uncompetitive inhibitor (Fig. 6C), which was supported by its cocrystallization with sEH showing the interactions with Tyr343, Ile363, Gln384, and Asn472 (Fig. 6F). This finding indicates the binding of kurarinone toward sEH.
In summary, kurarinone was found to significantly attenuate MPTP-induced dopaminergic neurotoxicity, such as the loss of DA and its metabolites and the decrease in TH level, resulting in the alleviation of PD-like behaviors in MPTP-induced mice. Furthermore, kurarinone ameliorated neuroinflammation via selective suppression of sEH activity to enhance levels of EETs. In addition, Ephx2 genetic deletion alleviated the development of PD, and sEH KO plus kurarinone did not further reduce the protection of PD. These findings demonstrated that sEH plays an important role in the development of PD and that kurarinone could be a potential natural candidate for the treatment of PD through sEH inhibition, as well as being a lead to develop a new family of sEH inhibitors.
Materials and Methods
The protocol using animals was approved by the Institutional Animal Care and Use Committee of Dalian Medical University. Details of the experimental protocols, including materials, animals, MPTP-induced model, high-performance liquid chromatography analysis, immunohistochemistry, Western blot, LC-MS/MS analysis for EETs and DHETs, and statistical analysis, are given in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
This work is supported by the Distinguished Professor of Liaoning Province ( Grant XLYC2002008), Natural Science Foundation of Liaoning Province (Grant 2020-MS-256), Dalian Science and Technology Leading Talents Project (Grant 2019RD15), Revolutionizing Innovative, Visionary Environmental Health Research Program of the National Institute of Environmental Health Sciences (Grant R35 ES030443), Superfund Basic Research Program of the National Institutes of Environmental Health Sciences (Grant P42 ES04699), and Dalian Young Star of Science and Technology (Grant 2019RQ123). We are thankful for the help of Prof. Ming-Liang Ye (Dalian Institute of Chemical Physics, Chinese Academy of Sciences) and his postdoctorate Xiao-Lei Zhang (Dalian Institute of Chemical Physics, Chinese Academy of Sciences) in protein identification.
Footnotes
Reviewers: J.R.C., Purdue University; and R.D., Emory University School of Medicine
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2118818119/-/DCSupplemental.
Data Availability
Cocrystallization data are available in the PDB (7EBA). All study data are included in the article and/or SI Appendix.
References
- 1.Dickson D. W., Parkinson’s disease and parkinsonism: Neuropathology. Cold Spring Harb. Perspect. Med. 2, a009258 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selikhova M., et al. , A clinico-pathological study of subtypes in Parkinson’s disease. Brain 132, 2947–2957 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Dickson D. W., et al. , Neuropathological assessment of Parkinson’s disease: Refining the diagnostic criteria. Lancet Neurol. 8, 1150–1157 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Grayson M., Parkinson’s disease. Nature 538, S1 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Stoker T. B., Barker R. A., Recent developments in the treatment of Parkinson’s disease. F1000 Res. 9, 862 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulisevsky J., Oliveira L., Fox S. H., Update in therapeutic strategies for Parkinson’s disease. Curr. Opin. Neurol. 31, 439–447 (2018). [DOI] [PubMed] [Google Scholar]
- 7.De Virgilio A., et al. , Parkinson’s disease: Autoimmunity and neuroinflammation. Autoimmun. Rev. 15, 1005–1011 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Ren Q., et al. , Soluble epoxide hydrolase plays a key role in the pathogenesis of Parkinson’s disease. Proc. Natl. Acad. Sci. U.S.A. 115, E5815–E5823 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunot S., et al. , Nitric oxide synthase and neuronal vulnerability in Parkinson’s disease. Neuroscience 72, 355–363 (1996). [DOI] [PubMed] [Google Scholar]
- 10.Rocha N. P., de Miranda A. S., Teixeira A. L., Insights into neuroinflammation in Parkinson’s disease: From biomarkers to anti-inflammatory based therapies. BioMed Res. Int. 2015, 628192 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zemtsova I., et al. , Microglia activation in hepatic encephalopathy in rats and humans. Hepatology 54, 204–215 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Imamura K., et al. , Cytokine production of activated microglia and decrease in neurotrophic factors of neurons in the hippocampus of Lewy body disease brains. Acta Neuropathol. 109, 141–150 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Mani S., Sevanan M., Krishnamoorthy A., Sekar S., A systematic review of molecular approaches that link mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurol. Sci. 42, 4459–4469 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Hirsch E. C., Standaert D. G., Ten unsolved questions about neuroinflammation in Parkinson’s disease. Mov. Disord. 36, 16–24 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Kwon H. S., Koh S. H., Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 9, 42 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasheed M., Liang J., Wang C., Deng Y., Chen Z., Epigenetic regulation of neuroinflammation in Parkinson’s disease. Int. J. Mol. Sci. 22, 4956 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X. Z., Zhang S. N., Liu S. M., Lu F., Recent advances in herbal medicines treating Parkinson’s disease. Fitoterapia 84, 273–285 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Feng S. T., et al. , Mangiferin: A multipotent natural product preventing neurodegeneration in Alzheimer’s and Parkinson’s disease models. Pharmacol. Res. 146, 104336 (2019). [DOI] [PubMed] [Google Scholar]
- 19.González-Burgos E., Fernandez-Moriano C., Gómez-Serranillos M. P., Potential neuroprotective activity of Ginseng in Parkinson’s disease: A review. J. Neuroimmune Pharmacol. 10, 14–29 (2015). [DOI] [PubMed] [Google Scholar]
- 20.He X., Fang J., Huang L., Wang J., Huang X., Sophora flavescens Ait.: Traditional usage, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 172, 10–29 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Ryu S. Y., Lee H. S., Kim Y. K., Kim S. H., Determination of isoprenyl and lavandulyl positions of flavonoids from Sophora flavescens by NMR experiment. Arch. Pharm. Res. 20, 491–495 (1997). [DOI] [PubMed] [Google Scholar]
- 22.Ma X. C., et al. , Simultaneous determination of nine major flavonoids in Sophora flavescens by RP-LC. Chromatographia 68, 471–474 (2008). [Google Scholar]
- 23.Nishikawa S., et al. , Anti-inflammatory activity of kurarinone involves induction of HO-1 via the KEAP1/Nrf2 pathway. Antioxidants 9, E842 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han J. M., et al. , Lavandulyl flavonoids from Sophora flavescens suppress lipopolysaccharide-induced activation of nuclear factor-kappaB and mitogen-activated protein kinases in RAW264.7 cells. Biol. Pharm. Bull. 33, 1019–1023 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Lee S., et al. , Urinary bladder-relaxant effect of kurarinone depending on potentiation of large-conductance Ca2+-activated K+ channels. Mol. Pharmacol. 90, 140–150 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Lee S., Choi J. S., Park C. S., Direct activation of the large-conductance calcium-activated potassium channel by flavonoids isolated from Sophora flavescens. Biol. Pharm. Bull. 41, 1295–1298 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Ni K. D., Liu J. Y., The functions of cytochrome P450 ω-hydroxylases and the associated eicosanoids in inflammation-related diseases. Front. Pharmacol. 12, 716801 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kodani S. D., Morisseau C., Role of epoxy-fatty acids and epoxide hydrolases in the pathology of neuro-inflammation. Biochimie 159, 59–65 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savitski M. M., et al. , Tracking cancer drugs in living cells by thermal profiling of the proteome. Science 346, 1255784 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Franken H., et al. , Thermal proteome profiling for unbiased identification of direct and indirect drug targets using multiplexed quantitative mass spectrometry. Nat. Protoc. 10, 1567–1593 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Zhang X., et al. , Solvent-induced protein precipitation for drug target discovery on the proteomic scale. Anal. Chem. 92, 1363–1371 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Becher I., et al. , Thermal profiling reveals phenylalanine hydroxylase as an off-target of panobinostat. Nat. Chem. Biol. 12, 908–910 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Sridharan S., et al. , Proteome-wide solubility and thermal stability profiling reveals distinct regulatory roles for ATP. Nat. Commun. 10, 1155 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huber K. V. M., et al. , Proteome-wide drug and metabolite interaction mapping by thermal-stability profiling. Nat. Methods 12, 1055–1057 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newman J. W., Morisseau C., Hammock B. D., Epoxide hydrolases: Their roles and interactions with lipid metabolism. Prog. Lipid Res. 44, 1–51 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Zhao W. Y., et al. , Inula japonica ameliorated bleomycin-induced pulmonary fibrosis via inhibiting soluble epoxide hydrolase. Bioorg. Chem. 102, 104065 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Deng B. Q., et al. , Epoxide metabolites of arachidonate and docosahexaenoate function conversely in acute kidney injury involved in GSK3β signaling. Proc. Natl. Acad. Sci. U.S.A. 114, 12608–12613 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imamura K., et al. , Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 106, 518–526 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Tsoulfas G., Geller D. A., NF-kappaB in transplantation: Friend or foe? Transpl. Infect. Dis. 3, 212–219 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Campolo M., et al. , TLR4 absence reduces neuroinflammation and inflammasome activation in Parkinson’s diseases in vivo model. Brain Behav. Immun. 76, 236–247 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Yan J., et al. , Nur77 attenuates inflammatory responses and oxidative stress by inhibiting phosphorylated IκB-α in Parkinson’s disease cell model. Aging (Albany NY) 12, 8107–8119 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mogi M., et al. , Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci. Lett. 180, 147–150 (1994). [DOI] [PubMed] [Google Scholar]
- 43.Mogi M., et al. , Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci. Lett. 165, 208–210 (1994). [DOI] [PubMed] [Google Scholar]
- 44.Soós J., Engelhardt J. I., Siklós L., Havas L., Majtényi K., The expression of PARP, NF-κB and parvalbumin is increased in Parkinson disease. Neuroreport 15, 1715–1718 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Teismann P., et al. , Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc. Natl. Acad. Sci. U.S.A. 100, 5473–5478 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morisseau C., Hammock B. D., Epoxide hydrolases: Mechanisms, inhibitor designs, and biological roles. Annu. Rev. Pharmacol. Toxicol. 45, 311–333 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Falck J. R., et al. , Comparison of vasodilatory properties of 14,15-EET analogs: Structural requirements for dilation. Am. J. Physiol. Heart Circ. Physiol. 284, H337–H349 (2003). [DOI] [PubMed] [Google Scholar]
- 48.Imig J. D., Navar L. G., Roman R. J., Reddy K. K., Falck J. R., Actions of epoxygenase metabolites on the preglomerular vasculature. J. Am. Soc. Nephrol. 7, 2364–2370 (1996). [DOI] [PubMed] [Google Scholar]
- 49.Roman R. J., P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol. Rev. 82, 131–185 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Sun C. P., et al. , Discovery of soluble epoxide hydrolase inhibitors from chemical synthesis and natural products. J. Med. Chem. 64, 184–215 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qin X., et al. , Soluble epoxide hydrolase deficiency or inhibition attenuates MPTP-induced parkinsonism. Mol. Neurobiol. 52, 187–195 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Huang H. J., Wang Y. T., Lin H. C., Lee Y. H., Lin A. M., Soluble epoxide hydrolase inhibition attenuates MPTP-induced neurotoxicity in the nigrostriatal dopaminergic system: Involvement of α-synuclein aggregation and ER stress. Mol. Neurobiol. 55, 138–144 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Lazaar A. L., et al. , Pharmacokinetics, pharmacodynamics and adverse event profile of GSK2256294, a novel soluble epoxide hydrolase inhibitor. Br. J. Clin. Pharmacol. 81, 971–979 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Cocrystallization data are available in the PDB (7EBA). All study data are included in the article and/or SI Appendix.