Significance
The zinc metalloprotease ZMPSTE24 removes the last 15 amino acids of prelamin A, including a farnesylated cysteine, to produce mature lamin A. The premature aging disorder Hutchinson–Gilford progeria syndrome is caused by a permanently farnesylated prelamin A variant lacking the ZMPSTE24 cleavage site. ZMPSTE24 loss of function leads to the accumulation of farnesylated prelamin A and causes progeroid disorders. Some studies have implicated prelamin A in physiological aging. We describe mice with an amino acid substitution in prelamin A that blocks the ZMPSTE24-catalyzed cleavage. These mice develop progeroid phenotypes but, in contrast to those modeling Hutchinson–Gilford progeria syndrome or ZMPSTE24 deficiency, have near-normal lifespans, thus providing a model to study the effects of farnesylated prelamin A during aging.
Keywords: aging, bone, lamin, nuclear envelope, progeria
Abstract
Prelamin A is a farnesylated precursor of lamin A, a nuclear lamina protein. Accumulation of the farnesylated prelamin A variant progerin, with an internal deletion including its processing site, causes Hutchinson–Gilford progeria syndrome. Loss-of-function mutations in ZMPSTE24, which encodes the prelamin A processing enzyme, lead to accumulation of full-length farnesylated prelamin A and cause related progeroid disorders. Some data suggest that prelamin A also accumulates with physiological aging. Zmpste24−/− mice die young, at ∼20 wk. Because ZMPSTE24 has functions in addition to prelamin A processing, we generated a mouse model to examine effects solely due to the presence of permanently farnesylated prelamin A. These mice have an L648R amino acid substitution in prelamin A that blocks ZMPSTE24-catalyzed processing to lamin A. The LmnaL648R/L648R mice express only prelamin and no mature protein. Notably, nearly all survive to 65 to 70 wk, with ∼40% of male and 75% of female LmnaL648R/L648R mice having near-normal lifespans of 90 wk (almost 2 y). Starting at ∼10 wk of age, LmnaL648R/L648R mice of both sexes have lower body masses than controls. By ∼20 to 30 wk of age, they exhibit detectable cranial, mandibular, and dental defects similar to those observed in Zmpste24−/− mice and have decreased vertebral bone density compared to age- and sex-matched controls. Cultured embryonic fibroblasts from LmnaL648R/L648R mice have aberrant nuclear morphology that is reversible by treatment with a protein farnesyltransferase inhibitor. These novel mice provide a model to study the effects of farnesylated prelamin A during physiological aging.
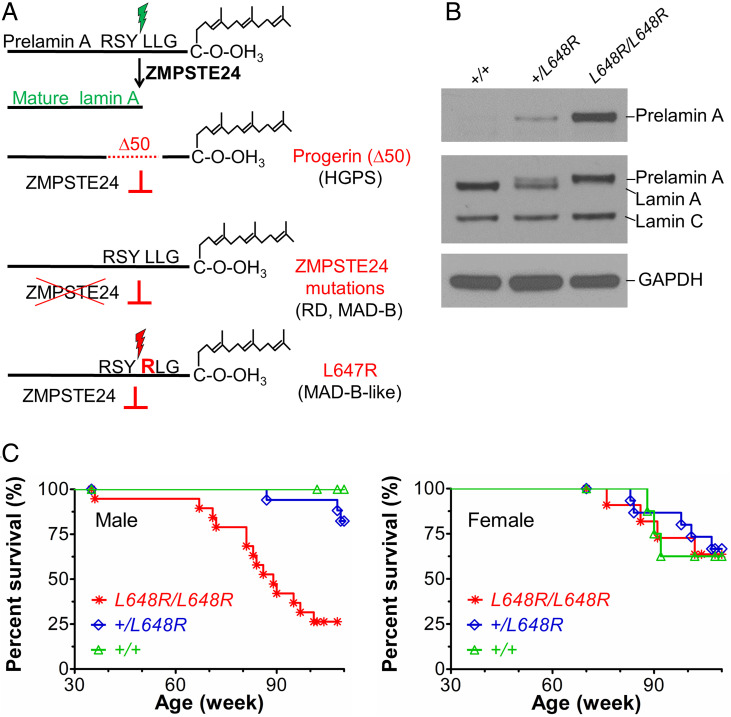
The lamin A/C gene (LMNA) encodes the splice variants lamin A and lamin C, which are intermediate filament building blocks of the nuclear lamina that differ in their carboxyl-terminal domains. Prelamin A, but not lamin C, has a carboxyl-terminal cysteine-aliphatic-aliphatic-any amino acid (CAAX) motif that initiates a series of posttranslational processing reactions to generate mature lamin A. In addition to CAAX processing (farnesylation, cleavage of -AAX, and carboxymethylation), prelamin A undergoes a final cleavage reaction, uniquely catalyzed by the zinc metalloprotease ZMPSTE24. This removes the last 15 amino acids of prelamin A, including its farnesylated cysteine, resulting in the production of mature, unfarnesylated lamin A. Defects in the posttranslational processing of prelamin to lamin A cause progeroid disorders (Fig. 1A) (1–3).
Fig. 1.
Survival of mice with a Lmna L648R mutation corresponding to the human mutation LMNA L647R that encodes an uncleavable variant of prelamin A. (A) Prelamin A is normally processed to lamin A after proteolytic cleavage catalyzed by the zinc metalloprotease ZMPSTE24 between tyrosine (Y) and leucine (L) 647 (648 in mouse), removing a peptide containing the carboxyl-terminal farnesylated cysteine (C). HGPS-causing LMNA mutations generate a prelamin A variant with an internal deletion of 50 amino acids (Δ50) called progerin, which lacks the ZMPSTE24 cleavage site and retains a farnesylated carboxyl-terminal cysteine. ZMPSTE24 loss-of-function mutations cause RD or MAD-B, in which unprocessed farnesylated prelamin A accumulates. LMNA mutation causing a MAD-B-like disorder generates a leucine (L) to arginine (R) substitution at residue 647 of prelamin A blocks ZMPSTE24 processing, leading to expression of a farnesylated variant with only a single amino acid difference. (B) Immunoblots of protein extracts from livers of Lmna+/+ (+/+), Lmna+/L648R (+/L648R), and LmnaL648R/L648R (L648R/L648R) mice. Blots were probed with an antibody specific for prelamin A (Top), an anti-lamin A/C antibody that recognized prelamin A, lamin A, and lamin C (Middle), or anti-GAPDH antibody as loading control (Bottom). (C) Survival curves for male L648R/L648R (n = 19), +/L648R (n = 17), and +/+ (n = 10) mice and female L648R/L648R (n = 11), +/L648R (n = 15), and +/+ (n = 8) mice.
Hutchinson–Gilford progeria syndrome (HGPS) results from a splicing mutation in LMNA that generates a variant with a 50-amino acid internal deletion (Δ50) called progerin, which retains its CAAX motif but lacks the ZMPSTE24 cleavage site, and thus progerin’s carboxyl-terminal cysteine remains permanently farnesylated (4, 5). Children with HGPS manifest numerous premature aging symptoms, including failure to thrive, bone loss, and early-onset severe atherosclerosis leading to death typically in the second decade. Progeroid disorders also result from mutations in ZMPSTE24 that lead to accumulation of full-length farnesylated prelamin A. Mandibuloacral dysplasia type B (MAD-B) is generally less severe than HGPS and caused by partial loss-of-function mutations in ZMPSTE24, with disease severity correlating with residual proteolytic activity (6, 7). Restrictive dermopathy (RD), caused by complete loss-of-function mutations in ZMPSTE24, is neonatal lethal (8).
Genetically modified mouse models provide valuable tools to study these human progeroid disorders and may have the potential to illuminate the role of permanently farnesylated prelamin A in physiologic aging. Zmpste24−/− mice accumulate farnesylated prelamin A and develop progeroid phenotypes, including severe growth retardation, craniofacial abnormalities, spontaneous bone fractures, and have a median survival of only ∼20 wk (9, 10). In Zmpste24−/− mice, disease severity is ameliorated by genetic reduction of prelamin A or pharmacological treatment to block protein farnesylation (11–13). These findings, along with those showing a correlation between disease severity and residual enzyme activity in humans with ZMPSTE24 mutations, suggest a “dose–response” between the amount of farnesylated prelamin A and the degree of pathology. However, ZMPSTE24 has at least one other function besides prelamin A processing, namely clearing clogged translocons (14, 15). Hence, some of the organismal pathology caused by ZMPSTE24 deficiency may result from defects in protein translocation into the endoplasmic reticulum (ER). ZMPSTE24 may also have additional functions, inferred from its contribution to viral defense in mice (16, 17) and from genetic studies in yeast that have implicated it in ER protein quality control, membrane stress, and establishing membrane protein topology (18–22).
Substitution of a hydrophobic residue with an arginine at the ZMPSTE24 cleavage site in prelamin A (V637R in chickens and L647R in humans) blocks its proteolysis (23, 24), resulting in the accumulation of permanently farnesylated prelamin A. We have previously reported a patient with a heterozygous LMNA mutation generating the L647R amino acid substitution in prelamin A. This patient had a relatively mild progeroid disorder with clinical features similar to those of MAD-B (25). We therefore generated and characterized mice with the corresponding L648R amino acid substitution in prelamin A to determine if LmnaL648R/L648R mice have the same or different phenotype and disease severity as Zmpste24−/− mice.
Results
Generation and Survival of Lmna L648R Mice.
Normally, farnesylated prelamin A is a transient species, essentially undetectable in cells because of its efficient conversion to mature lamin A. However, LMNA mutations causing HGPS, ZMPSTE24 mutations causing RD and MAD-B, and the LMNA L647R mutation causing a MAD-B–like disorder, lead to accumulation of farnesylated prelamin A or variants (Fig. 1A). Whereas several mouse lines with HGPS-causing and Zmpste24 loss-of-function mutations have been generated and characterized (9, 10, 26–31), a mouse with a single amino acid substitution in prelamin A that causes a MAD-B–like disease in humans does not exist. Such a mouse could provide valuable information on the impact of prelamin A on promoting aging phenotypes, absent the complications in Zmpste24−/− mice, where altered processing of substrates in addition to prelamin A or other disrupted pathways may contribute to the phenotypes.
We therefore used a recombination-mediated genetic engineering strategy to generate mice carrying a LmnaL648R allele (SI Appendix, Fig. S1A). It should be noted that L648R in mice is equivalent to L647R in humans. Founder mice carried one mutant and one wild-type allele (SI Appendix, Fig. S1B). We crossed founder male mice to female Lmna+/+ C57BL/6J mice and intercrossed the heterozygous male and female offspring to generate heterozygous Lmna+/L648R and homozygous LmnaL648R/L648R mice. Male and female LmnaL648R/L648R mice were able to breed and female mice were able to become pregnant up to ∼24 wk of age.
As expected, Lmna+/+ mice expressed mature lamin A and lamin C, Lmna+/L648R mice expressed prelamin A, lamin A, and lamin C, and LmnaL648R/L648R mice expressed prelamin A and lamin C, at roughly the same levels, but no mature lamin A (Fig. 1B and SI Appendix, Fig. S1C). For analysis of survival and growth, as well as all subsequent experiments, we used mice that were crossed several generations onto a >90% pure C57BL/6J background. The homozygous LmnaL648R/L648R mice had unexpectedly long lifespans, with nearly all living 65 to 70 wk and ∼40% of male and ∼75% of female LmnaL648R/L648R alive at 90 wk (almost 2 y) of age (Fig. 1C).
Growth and Metabolic Features of Lmna L648R Mice.
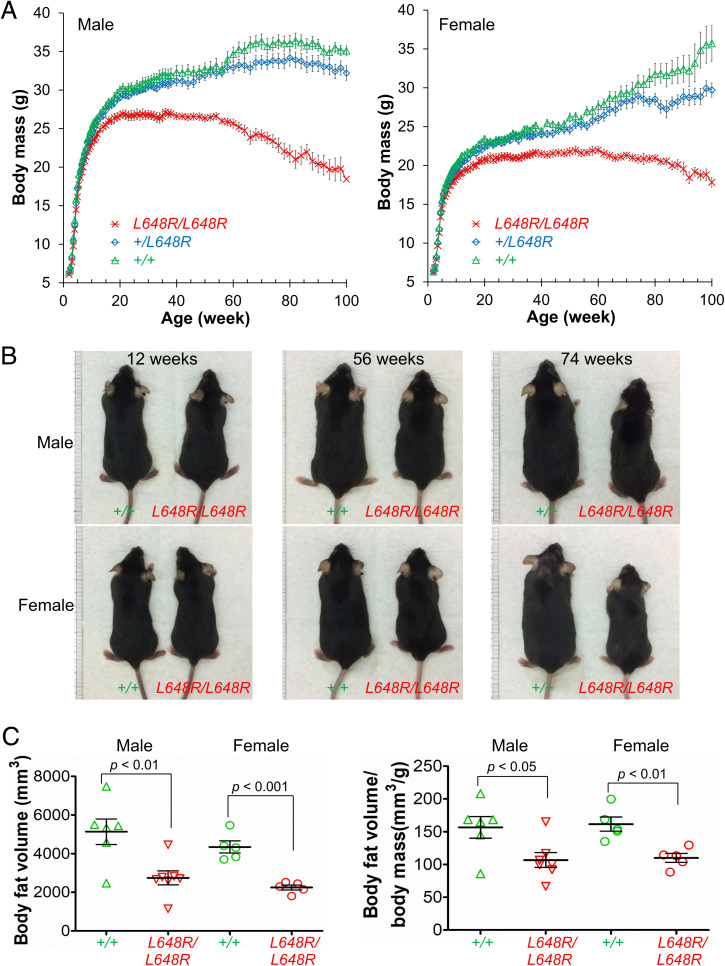
Heterozygous and homozygous Lmna L648R mice were grossly indistinguishable from their wild-type littermates early in life. Starting at ∼10 wk of age, however, LmnaL648R/L648R mice had decreased body masses and this difference became accentuated over time (Fig. 2A). Given the normal growth and survival of heterozygous Lmna+/L648R mice, we performed more detailed phenotypic analyses only on the homozygous LmnaL648R/L648R mice compared to Lmna+/+ wild-type mice. Similarly, many other heterozygous Lmna mutant mice, including some heterozygous for the HGPS mutation, have modest, minimal, or in many cases no abnormal phenotypes (32, 33). Consistent with their decreased body mass compared to wild-type mice with aging, male and female LmnaL648R/L648R mice were visibly smaller than Lmna+/+ animals of the same age at 56 and 74 wk (Fig. 2B).
Fig. 2.
Growth of LmnaL648R/L648R mice. (A) Body mass versus age of male LmnaL648R/L648R (L648R/L648R) (n = 20), Lmna+/L648R (+/L648R) (n = 17), and Lmna+/+ (+/+) (n = 13) mice and female L648R/L648R (n = 13), +/L648R (n = 15), and +/+ (n = 11) mice. Values are means and error bars indicate SEM. (B) Photographs comparing the sizes of male and female +/+ and L648R/L648R mice at the indicated ages. (C) Body fat volume (Left) and body fat volume normalized to body mass (Right) of 52-wk-old male and female +/+ and L648R/L648R mice. Each triangle or circle represents value for an individual animal; long horizontal bars represent mean and errors bars indicate SEM.
Zmpste24−/− mice have severe lipodystrophy along with growth retardation (9, 10). We therefore examined body fat composition and various metabolic parameters in LmnaL648R/L648R mice. Male and female LmnaL648R/L648R mice both had significantly reduced body fat at 52 wk of age, compared to wild-type mice (Fig. 2C). However, fasting blood glucose concentration at 30 wk was not significantly different between LmnaL648R/L648R and wild-type mice (SI Appendix, Fig. S2A). Likewise, glucose tolerance testing showed no difference between male LmnaL648R/L648R and Lmna+/+ mice, but the female mutant mice had decreased glucose tolerance compared to wild-type controls (SI Appendix, Fig. S2B). Female LmnaL648R/L648R also had lower plasma insulin concentrations compared to sex-matched Lmna+/+ mice (SI Appendix, Fig. S2C). There were no significant differences in other routine blood biochemical parameters between LmnaL648R/L648R and Lmna+/+ mice (SI Appendix, Table S1). Thus, while LmnaL648R/L648R mice clearly have low body mass and body fat, these phenotypes are not associated with any major prominent serum metabolic abnormality, except possible mild insulin resistance in female mice.
Cranial and Mandibular Defects in LmnaL648R/L648R Mice.
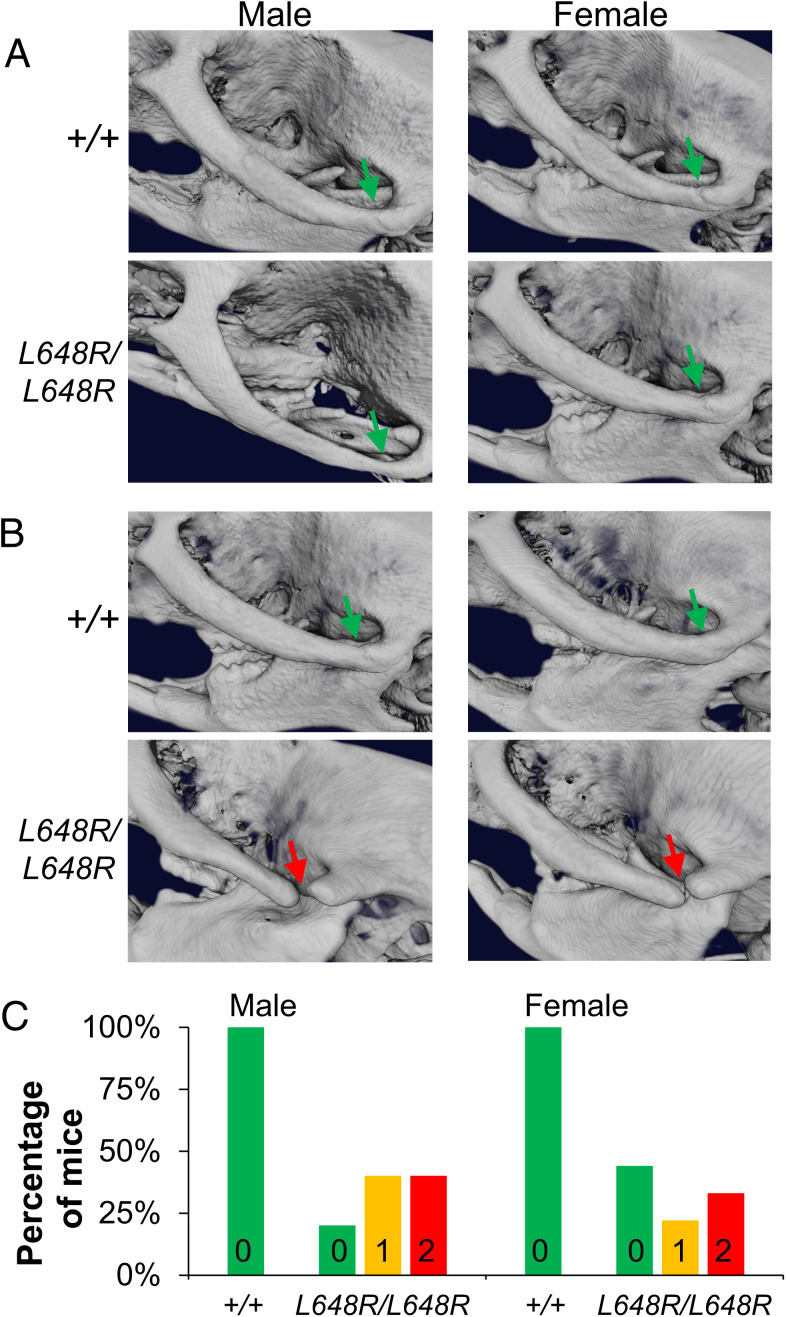
Zmpste24−/− mice have profound cranial and mandibular abnormalities of the zygomatic arch, mandible, and dentition (9, 10). The human patient with the LMNA L647R mutation has microcephaly, micrognathia, and dental crowding (25). We therefore examined the skulls of LmnaL648R/L648R mice by microcomputed tomography (micro-CT). At 4 wk of age, skulls of male and female LmnaL648R/L648R mice were essentially indistinguishable from Lmna+/+ mice. The zygomatic arches developed normally with the proper formation of both zygomaticotemporal and zygomaticomaxillary sutures in LmnaL648R/L648R mice (Fig. 3A). When rescanned at 30 wk of age, however, male and female LmnaL648R/L648R mice exhibited wide-open zygomaticotemporal sutures in one or both zygomatic arches (Fig. 3B). These defects were present in one or both zygomatic arches in the majority of male and female LmnaL648R/L648R mice but not in any Lmna+/+ mice at 30 wk of age (Fig. 3C). The normal zygomatic arches 4 wk after birth and defects at older ages implies that these are degenerative but not developmental deformities.
Fig. 3.
Degenerative deformities in zygomatic arches of LmnaL648R/L648R mice. (A) Representative 3D renderings of the micro-CT images of male and female Lmna+/+ (+/+) and LmnaL648R/L648R (L648R/L648R) mice showing the formation of the zygomatic arches (green arrows) at 4 wk. (B) Degenerative deformity (red arrows) in the zygomaticotemporal suture of male and female L648R/L648R mice compared to the proper maintenance (green arrows) in the +/+ mice at 30 wk. (C) Percentages of male +/+ (n = 7), female +/+ (n = 7), male L648R/L648R (n = 10), and female L648R/L648R (n = 9) mice with degenerative deformity in 0, 1, or 2 zygomatic arches at 30 wk of age.
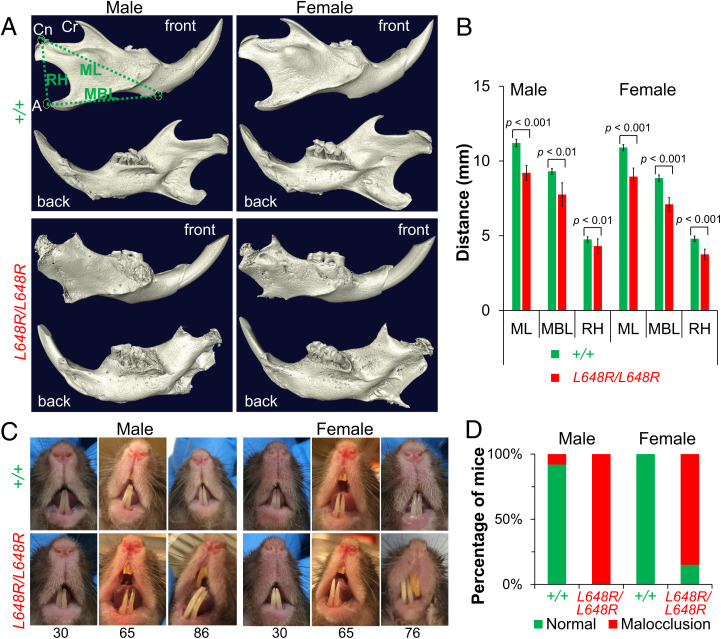
By 20 wk of age, and more prominently at 30 wk of age, micro-CT imaging and three-dimensional (3D)-reconstruction analyses revealed profound mandibular degeneration in male and female LmnaL648R/L648R mice (SI Appendix, Fig. S3). The 3D rendering of micro-CT–scanned skull images after segmentation of mandible further examined the degenerative defects. The condylar, coronoid, and angular processes were severely deformed, resulting in decreased mandibular length, mandibular body length, and ramus height. (Fig. 4A). Mandible dimensions were significantly decreased in both male and female LmnaL648R/L648R mice compared to age- and sex-matched Lmna+/+ controls (Fig. 4B). Dental malocclusion occurs in mice when the incisors overgrow because the mandibular and maxillary teeth are not normally aligned. Although oral examination showed the male and female LmnaL648R/L648R had minimal to mild dental malocclusion at 30 wk of age, this condition was quite evident at ages older than 70 wk (Fig. 4C). This was confirmed by micro-CT scanning (SI Appendix, Fig. S4). By 65 wk of age, virtually all male and 85% of female LmnaL648R/L648R mice had dental malocclusion (Fig. 4D). This was likely attributed to the mandibular degeneration, leading to the improper alignment of the upper and lower jaws. LmnaL648R/L648R mice clearly have mandibular and dental defects, which could compromise dietary intake and may contribute to decreased body mass observed with advancing age. The mandibular and dental abnormalities in LmnaL648R/L648R mice resemble those of Zmpste24−/− mice and those with HGPS mutations but appear less severe and are not prominent until later in life.
Fig. 4.
Mandibular defects in LmnaL648R/L648R mice. (A) Representative 3D renderings of the segmented micro-CT–scanned images showing mandibles of male and female Lmna+/+ (+/+) and LmnaL648R/L648R (L648R/L648R) mice. A: angular process; Cn: condylar process; Cr: coronoid process; MBL: mandibular body length; ML: mandibular length; RH: ramus height. (B) Comparisons of ML, MBL, and RH between male +/+ (n = 4) and L648R/L648R (n = 4) mice and between female +/+ (n = 4) and L648R/L648R (n = 4) mice. Values are means and error bars indicate SEM. (C) Representative photographs of teeth of male and female +/+ and L648R/L648R mice at the ages in weeks indicated. Dental malocclusion is minimal to mild at 30 wk of age and more severe at older ages. (D) Percentages of male +/+ (n = 13), female +/+ (n = 10), male L648R/L648R (n = 18) and female L648R/L648R (n = 13) mice with malocclusion at ≥65 wk of age.
Decreased Vertebral Bone Density and Long Bone Defects in LmnaL648R/L648R Mice.
Zmpste24−/− mice suffer from osteoporosis and bone fractures (9, 10, 12, 34). We therefore used micro-CT–scanned images to analyze vertebral bone density in Lmna+/+ and LmnaL648R/L648R mice. From the two-dimensional (2D) images, we first segmented the vertebra L5 by selecting the trabecular bone area. Next, we generated the 3D-rendered image and computed statistical information of the 3D model to obtain bone and nonbone volumes (Fig. 5A). At 30 wk of age, both male and female LmnaL648R/L648R mice had significantly reduced vertebral bone density compared to sex-matched Lmna+/+ controls (Fig. 5B). Tibias of both male and female LmnaL648R/L648R mice were thinner and had a more irregular surface than those of Lmna+/+ mice (Fig. 5C). These bone density abnormalities are similar to, but apparently less severe and of later onset, than those reported in Zmpste24−/− mice.
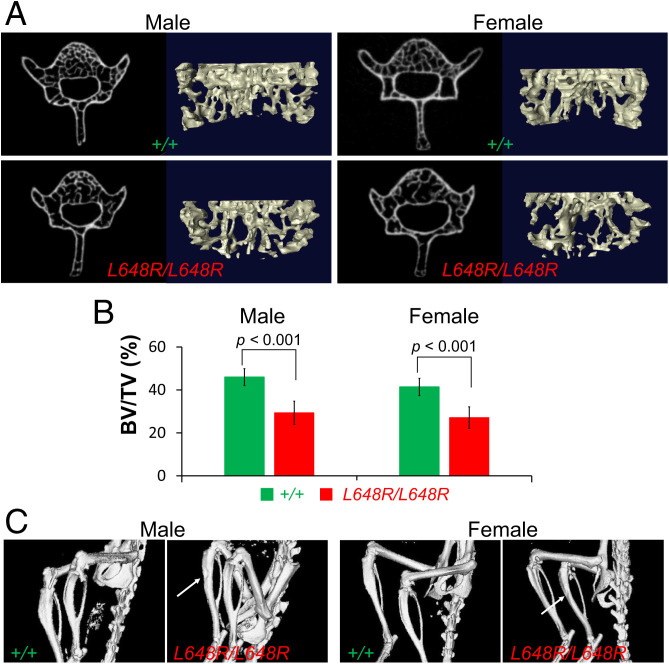
Fig. 5.
Decreased vertebral bone density and tibial defects in LmnaL648R/L648R mice. (A) Representative micro-CT–scanned transverse sections and 3D reconstructions of the L5 vertebrae from male and female Lmna+/+ (+/+) and LmnaL648R/L648R (L648R/L648R) mice. (B) Comparison of vertebra L5 bone density (bone volume/total volume; BV/TV %) between male +/+ (n = 5) and L648R/L648R (n = 5) mice and between female +/+ (n = 7) and L648R/L648R (n = 6) mice. Values are means and error bars indicate SEM. (C) Micro-CT–generated representative images of hind legs of living male and female +/+ and L648R/L648R mice. Arrows indicate thinner and more irregular surfaces of tibias of L648R/L648R mice.
We also examined grip strength and ribs of LmnaL648R/L648R mice. At 35 wk of age, there was no difference in grip strength between LmnaL648R/L648R and Lmna+/+ mice of both male and female sex (SI Appendix, Fig. S5A). Even in three female LmnaL648R/L648R mice that survived to 104 to 120 wk of age, grip strength was no different from age-matched controls (SI Appendix, Fig. S5B). We used micro-CT to examine the middle thoracic spines and ribs of dissected and fixed skeletons from five male and six female LmnaL648R/L648R mice killed at 30 wk of age and did not observe any rib fractures (SI Appendix, Fig. S5C). Micro-CT of seven male and five female live LmnaL648R/L648R mice at 52 wk of age also did not detect rib factures. Micro-CT scans of dissected and fixed skeletons from three male and one female LmnaL648R/L648R mouse that died or had to be killed at 76 to 84 wk of age identified two broken ribs in the vicinity of the costovertebral junction with hypertrophic calluses at the fracture sites in one mouse (SI Appendix, Fig. S5D). In contrast to Zmpste24−/− mice, which have grip abnormalies and nearly every rib broken by 24 to 30 wk of age (9), LmnaL648R/L648R mice have preserved grip strength throughout their lifetimes and only rare rib fractures at old, preterminal ages.
Farnesylation-Dependent Abnormal Nuclear Morphology in LmnaL648R/L648R Mouse Embryonic Fibroblasts.
We isolated fibroblasts from LmnaL648R/L648R and Lmna+/+ mouse embryos. Immunoblotting confirmed that LmnaL648R/L648R MEFs produce only prelamin A and no mature lamin A, whereas the reverse is the case for Lmna+/+ mouse embryonic fibroblasts (MEFs). (Fig. 6A). Immunostaining similarly reveals that prelamin A accumulates in LmnaL648R/L648R MEFs but not in Lmna+/+ MEFs (Fig. 6B). A high percentage of misshapen nuclei are apparent in mutant cells and this trend is amplified with passage number (Fig. 6C). Treatment of LmnaL648R/L648R MEFs with the protein farnesyltransferase inhibitor (FTI) lonafarnib corrected the abnormal nuclear morphology (Fig. 6D). The percentage of mutant MEFs with abnormal nuclear morphology was significantly less in cultures treated with FTI compared to vehicle only (Fig. 6E). These results strongly suggest that accumulation of the farnesylated form of prelamin A is responsible for abnormal nuclear morphology. Given the correlations between FTI-induced reversal of abnormal nuclear morphology in MEFs from Zmpste24−/− and HGPS mutant mice and the beneficial effects of these drugs on the corresponding whole animals (11, 31, 35–37), the farnesylated form of prelamin A is also likely responsible for the abnormal phenotypes in LmnaL648R/L648R mice.
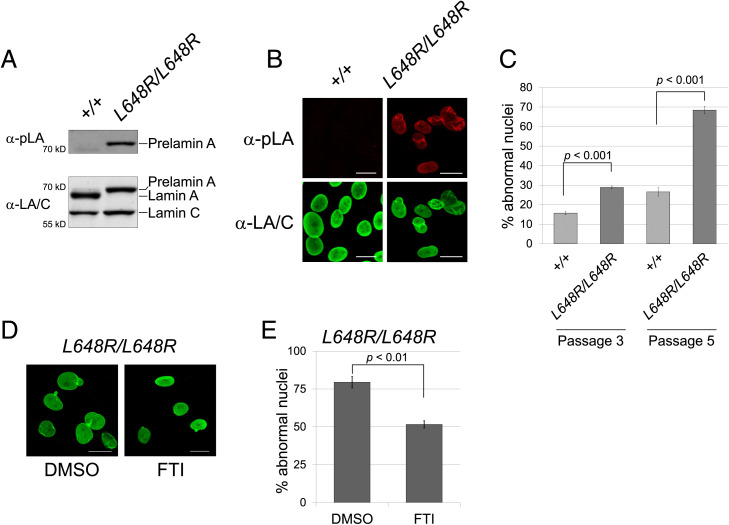
Fig. 6.
Farnesylation-dependent abnormal nuclear morphology in LmnaL648R/L648R MEFs. (A) Immunoblots of protein extracts from MEFs generated from Lmna+/+ (+/+) and LmnaL648R/L648R (L648R/L648R) mouse embryos. Blots were probed with an antibody specific for prelamin A (α-pLA, Upper) and anti-lamin A/C antibody that recognizes prelamin A, lamin A, and lamin C (α-LA/C, Lower). (B) Immunofluorescence photomicrographs showing the indicated MEFs at passage 5 stained with antibodies that specifically recognize prelamin A (Upper) or prelamin A, lamin A, and lamin C (Lower). (Scale bar, 20 µm.) (C) Quantification of aberrant nuclear morphology in MEFs at passage 3 and passage 5. Three independently grown MEF cultures were fixed, stained, and ∼100 nuclei counted from each. Values are means (n = 3) and error bars indicate SEM. Representative images of nuclei that are counted as abnormal—including those that are blebbed, misshapen, or highly crenylated—are shown in SI Appendix, Fig. S6. (D) Immunofluorescence photomicrographs showing LmnaL648R/L648R MEFs at passage 6 treated with DMSO vehicle or FTI as described in Materials and Methods. (Scale Bar, 20 µm.) (E) Percentages of nuclei with aberrant nuclear morphology in LmnaL648R/L648R MEFs treated with DMSO or FTI. For quantification, three independently grown MEF cultures were fixed, stained, and ∼100 nuclei counted from each. Values are means (n = 3) and error bars indicate SEM.
Discussion
HGPS, MAD-B, and other progeroid disorders arise from genetic mutations that lead to the accumulation of permanently farnesylated prelamin A or variant proteins (1–3). We have generated LmnaL648R/L648R mice that accumulate a farnesylated prelamin A variant (and no mature lamin A) with a single amino acid substitution at the ZMPSTE24 cleavage site that blocks processing. Like mice with Lmna mutations that lead to production of progerin and Zmpste24−/− mice that accumulate prelamin A, the LmnaL648R/L648R mice develop progeroid phenotypes. Fibroblasts from these mice also have abnormal nuclear morphology that is reversible by pharmacological blockage of protein farnesylation, which is also the case for human and mouse cells expressing progerin or deficient in ZMPSTE24 (38). Notably, however, LmnaL648R/L648R mice have dramatically greater longevity (∼1.5 to > 2 y) as compared to Zmpste24−/− mice (4 to 7 mo) (9, 10) and Lmna mouse models of HGPS that have two mutant alleles encoding progerin (4 to 10 mo) (26, 27, 29, 39). Because LmnaL648R/L648R mice do not die prematurely, and can live more than 2 y, they are an ideal model for studying the effects of permanently farnesylated prelamin A in the context of physiologic aging.
The phenotype of LmnaL648R/L648R mice in some ways resembles that of the one human patient described to date with the corresponding, albeit heterozygous, LMNA L647R mutation (25). The patient was small for chronological age, microcephalic, and had a “MAD-B–like” condition with micrognathia, dental crowding, decreased subcutaneous fat and bone defects, including acro-osteolysis and other radiological findings suggestive of abnormal bone resorption or deposition. Fibroblasts from this patient expressed prelamin A to lamin A at a ratio of ∼1:1; however, this individual was not nearly as severely affected as children with HGPS that are heterozygous for the LMNA G608G allele and whose fibroblasts generally express progerin to lamin A at a <1:1 ratio. Although the human data are from only one individual, they suggest that full-length prelamin A may be less “toxic” to cells than progerin, which contains a 50-amino acid deletion.
To a large extent, LmnaL648R/L648R mice phenocopy what has been reported for Zmpste24−/− mice in terms of growth, skull, and other bone defects (9, 10, 12, 34). However, their phenotype is far less severe than that of Zmpste24−/− mice and they do not exhibit premature lethality. Both mice have severe mandibular and dental abnormalities that could potentially lead to malnourishment, causing the observed failure to thrive. However, LmnaL648R/L648R mice have decreased body mass compared to wild-type mice at ages before dental malocclusion becomes prominent, suggesting that factors other than dental abnormalities resulting in decreased caloric intake may contribute to their failure to thrive. LmnaL648R/L648R mice also have decreased whole body fat content, as do Zmpste24−/− mice and humans with MAD-B (7, 9, 10, 40, 41). Decreased body fat content of LmnaL648R/L648R mice was not associated with insulin resistance. Female LmnaL648R/L648R mice had modest hyperglycemia after glucose administration, but a lower-than-normal plasma insulin concentration. Zmpste24−/− mice also have plasma insulin concentrations lower than wild-type mice (42). Conditional deletion of Zmpste24 from adipocytes leads to only modest body fat loss only in male mice (43). These findings suggest that decreased body fat in LmnaL648R/L648R mice is not caused by a direct effect of prelamin A on adipocytes.
Why are LmnaL648R/L648R mice similar to, but less severely affected than Zmpste24−/− mice, given that both express solely farnesylated prelamin A and no mature lamin A? One possibility is that they are exposed to a lower “dose” of prelamin A. Zmpste24−/− mice heterozygous for an Lmna knockout allele, which express half the amount of prelamin A, have significantly less severe phenotypes than Zmpste24−/− mice with two wild-type Lmna alleles (12, 13). However, LmnaL648R/L648R mice like Zmpste24−/− mice with two wild-type Lmna alleles express roughly equal amounts of prelamin A relative to lamin C, so this explanation seems unlikely (12, 13). Another possibility is that the single amino acid substitution renders the L648R variant less “toxic” than native prelamin A, and determining whether binding of this variant to the known prelamin A interactors, such as AKT and SUN1 (44–46), is diminished would be of interest to test in the future. However, this explanation is difficult to reconcile with the wealth of data suggesting that it is the farnesyl and carboxylmethyl moieties of prelamin A that are primarily responsible for its adverse effects in cultured cells and mice (45–47). Furthermore, mice engineered to express only a nonfarnesylated prelamin A, without any lamin C, develop cardiomyopathy but do not have progeroid phenotypes and live much longer and have less severe growth retardation than Zmpste24−/− mice (38).
We favor the hypothesis that ZMPSTE24’s other crucial cellular functions, in addition to prelamin A cleavage, contribute to the severity of phenotypes and limited lifespan of the Zmpste24−/− mice. ZMPSTE24 has a key role in clearing clogged translocons, as well as other less mechanistically well-understood roles in viral defense, protein secretion, ER protein quality control, membrane stress, and establishing membrane protein topology (14–22). While genetic reduction of prelamin A clearly improves the abnormal phenotypes of Zmpste24−/− mice (12, 48), loss of one or several of these other functions may make them more susceptible to a “toxic dose” of prelamin A or create additional pathologies that exacerbate those resulting from this dose. LmnaL648R/L648R mice also live significantly longer and have milder phenotypes than reported for various mouse models expressing progerin, the prelamin A variant in HGPS (26–29, 31, 37). Hence, the loss of 50 amino acids in progerin may make it more toxic than full-length prelamin A. In any case, much research has focused on Zmpste24−/− and HGPS mouse models to understand the role of prelamin A in aging; the significant longevity of LmnaL648R/L648R mice makes them an alternative, and potentially an ideal one to study how prelamin A impacts several aging tissues. It may also be informative to study Zmpste24−/− mice heterozygous for a knockout Lmna allele as they age beyond 28 to 30 wk, the oldest age to which have been studied in previous reports (12, 48).
Brayson et al. (49) reported that transgenic cardiomyocyte-specific expression of human prelamin A with the L647R amino acid substitution causes cardiomyopathy in mice, with abnormal heart function detected as early as 4 wk of age. In contrast, our mice expressing the mouse prelamin A L648R variant from the endogenous Lmna alleles lived almost 2 y without evidence of cardiomyopathy. Although only a few MAD-B patients have been thoroughly described in the literature (7), early-onset primary cardiomyopathy has not been described as a feature of these individuals. One patient with MAD-B and a compound heterozygous ZMPSTE24 mutation reportedly has a greater carotid intima media thickness relative to chronological age, and a raised proteomic classifier for the diagnosis of coronary artery disease (50). Cardiac complications of cardiovascular disease, rather than primary cardiomyopathy, lead to early death in HGPS (51). Arteries from patients with HGPS show loss of medial smooth-muscle cells, adventitial thickening, and other pathological alterations that lead to sclerotic plaques in coronary arteries (52–55). This vascular pathology is the cause of myocardial infarctions and ischemic heart disease invariably present in individuals with HGPS.
Several mouse models of HGPS develop vascular alterations that mimics this human pathology (26–30, 56). Zmpste24−/− mice may die at too young an age to determine if prelamin A accumulation causes vascular pathology. LmnaL648R/L648R mice therefore provide a model to determine the effects of prelamin A in the development of cardiovascular disease. We did not observe vascular pathology when we examined these mice at 7 mo of age; however, we will continue to monitor them to see if it develops with advancing age or in combination with genetic susceptibility to atherosclerosis, as has been done with HGPS model mice (56). Indeed, Ragnauth et al. (57) have reported that prelamin A accumulation is prevalent in the medial vascular smooth muscle cells of human arteries from old individuals, but not young ones, and in atherosclerotic lesions. This prelamin A accumulation is associated with reduced ZMPSTE24, suggesting that loss of its activity may change with aging. This same group also reported that prelamin A was present in vascular smooth muscle cells in calcified arteries of subjects receiving renal dialysis, a model for vascular aging (58). This raises the possibility that prelamin A accumulation contributes to pathology associated with physiological aging. Because of the near-normal lifespan of the LmnaL648R/L648R mice, they will provide a model to determine the impact of prelamin A on the vasculature during aging.
Materials and Methods
Mice.
The Institutional Animal Care and Use committee at Columbia University Irving Medical Center approved all protocols. We used recombination-mediated genetic engineering or recombineering to construct vectors for manipulation of the mouse genome (59). Bacterial artificial chromosome clone RP23-281P8 carrying Lmna was purchased from BACPAC Resource. We engineered the T > G transversion at Lmna c.2176 in exon 11, which changed codon 648 CTC encoding Leu to CGC encoding Arg. We then inserted a Frt-Neo-Frt (FNF) cassette into intron 11 (SI Appendix, Fig. S1A). A gene-targeting vector was constructed by retrieving the 1.6-kb left homology arm (5′ to L648R mutation), the FNF cassette, and the 1.45-kb right homology arm (end of FNF cassette to 3′) into pMCS-DTA vector carrying the Diphtheria toxin α-chain (DTA)-negative selection marker. The FNF cassette conferred G418 resistance during gene targeting in KV1 (129B6 hybrid) embryonic stem cells and the DTA cassette provided an autonomous negative selection to reduce random integration events during gene targeting. Several targeted embryonic stem cell clones were identified and injected into C57BL/6J blastocysts to generate chimeric mice. Male chimeras were bred to C57BL/6J female mice to transmit the Lmna-L648R-FNF allele. The FNF cassette was then removed by crossing mice with the Lmna-L648R-FNF allele to B6.Cg-Tg(ACTFLPe)9205Dym/J mice (The Jackson Laboratory; stock no: 005703). The offspring were crossed to wild-type C57BL/6J mice and heterozygous offspring subsequently crossed to wild type C57BL/6J mice for another several genenrations before intercrossing male and female heterozygous mice to generate LmnaL648R/L648R, Lmna+/L648R, and Lmna+/+ mice used in the experiments on a >90% pure C57BL/6J background. Mice were housed in a barrier facility with 12/12-h light/dark cycles, fed a chow diet, and genotyped by PCR using genomic DNA isolated from tail clippings.
Immunoblotting of Proteins Isolated from Mouse Tissues.
Proteins were extracted from mouse tissues, separated by electrophoresis in SDS-polyacrylamide slab gels, transferred to nitrocellulose membranes, and analyzed by immunoblotting using methods described previously (60). Primary antibodies for immunoblotting were rabbit anti-lamin A/C (Santa Cruz) at 1:5,000 dilution, rat monoclonal anti-prelamin A 3C8 (61) (kindly provided by Loren Fong and Stephen G. Young, University of California, Los Angeles) at 1:3,000 dilution, and mouse anti-GAPDH (Ambion) at 1:3,000 dilution. Secondary antibodies were ECL-horseradish peroxidase-conjugated anti-rabbit, anti-rat, and anti-mouse antibodies (GE Healthcare) used at a dilution of 1:5,000. Signals were detected using SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Fisher Scientific) and Autoradiography film (LabScientific). For quantification, films were scanned with a digital scanner and the band signal intensities were quantified using Fiji ImageJ (https://imagej.net/software/fiji/) and analyzed using Excel (Microsoft).
Growth and Survival Analyses.
Mice were weighed twice per week in the first 12 wk, once per week up to 40 wk, and once every 2 wk for the remainder of their lifespans. For survival analyses, a combined endpoint of death or distress severe enough that a staff veterinarian blinded to genotype determined that killing was necessary. Killing was performed according to the approved protocol of the Institute of Comparative Medicine at Columbia University Irving Medical Center.
Blood Biochemical Parameters and Glucose Tolerance Tests.
Mice were fasted for 5 h, and blood was collected and centrifuged at 3,000 × g at 4 °C for 10 min. Plasma was collected and stored at −80 °C until analysis. Blood biochemistry analysis was performed on a Heska Element DC Chemistry Analyzer at the Institute of Comparative Medicine, Columbia University Irving Medical Center. Plasma insulin was measured using Ultra-Sensitive Mouse Insulin ELISA Kit (Crystal Chem). For glucose tolerance tests, mice were fasted in clean cages with no food and ad libitum drinking water for 15 h; then blood glucose was measured using One Touch Ultra (LifeScan) at time 0, and then 15, 30, 60, 90, and 120 min after intraperitoneal injection of a 2-g/kg bolus of sterile glucose solution.
Micro-CT and Image Analysis.
Mice and dissected bones were scanned using the Perkin-Elmer Quantum FX micro-CT imaging system (PerkinElmer) at the Oncology Precision Therapeutics and Imaging Core at Columbia University Irving Medical Center. For imaging mice, the animal was anesthetized with 2 to 3% isoflurane. The skeletal structure of the mouse was scanned in the axial plane placed in a cylindrical sample holder at the field of view of 20 to 60 mm for lower-high resolution. A single scan of 4 min was taken, and the animal then was removed from the micro-CT machine, returned to its cage, and kept warm for homoeothermic stability until consciousness returned. Dissected bone was placed on the sample holder and scanned at the same settings as for mice. Micro-CT VOX files were analyzed using Analyze 14.0 (AnalyzeDirect) and images were created using a surface rendering technique. The VOX files were also transferred to DICOM format and analyzed using AMIRA software. To obtain high-resolution images (6.6 μm), the dissected heads were scanned by SkyScan 1272 (Bruker BioSpin). For mandible size analysis, the scanned 2D images were segmented to select the mandibular area, followed by rendering of a 3D model from the segmented images, and computed statistical information of the 3D model analyzed to obtain the structure measurements using AMIRA software (Thermo Fisher Scientific). For bone density analysis, the top 60 images of vertebra L5 were segmented by selecting the trabecular bone area in all 60 2D images, and then the 60 segmented images were used to construct a 3D surface model. The computed statistical information of the 3D model was used to obtain bone volume and nonbone volume, followed by statistical analysis.
Grip Strength Measurements.
Forelimb grip strength was measured using a previously published protocol (62). Each mouse was placed in front of a Chatillon digital force gauge pull meter (Columbus Instruments) with angled mesh assembly and allowed to grasp the pull bar with forelimbs and then pulled backward by the tail until the grip was broken. Force applied to the bar the moment the grasp was released was recorded. Each mouse pulled the grid three times in a row and then returned in the cage for a resting period of at least 1 min. This was repeated five times for a total of 15 pulls. Maximum grip strength was determined by averaging the three highest pull measurements of the 15 values, and normalized to body mass.
MEF Isolation, Culture, Immunoblotting, Immunofluorescence Microscopy, and FTI Treatment.
Fibroblasts were isolated from 13.5-d embryos of female Lmna+/L648R mice that were crossed with male Lmna+/L648R mice using previously described methods (63). Primary MEFs were cultured in Dulbecco’s modified Eagle medium (Invitrogen or Thermo Fisher Scientific) containing 10% fetal bovine serum (FBS; Gemini) with 5% CO2 at 37 °C and propagated in the same media containing penicillin-streptomycin-glutamine (1:100 dilution of Gibco Penicillin-Streptomycin-Glutamine 100X; Thermo Fisher Scientific).
For immunoblotting, SDS sample buffer was added to MEFs and the lysates sonicated and then heated for 10 min at 65 °C. Proteins in lysates were separated by electrophoresis in 10% SDS-polyacrylamide slab gels and transferred to nitrocellulose using methods described previously (64), except that blocking was done with phosphate-buffered saline plus 0.1% Triton X-100 (PBS-T) containing 5% nonfat dry milk (Bio-Rad Blotting-grade Blocker) and 10 mM NaN3. Primary antibodies were mouse monoclonal anti-lamin A/C (Santa Cruz) at 1:1,000 dilution and rat monoclonal anti-prelamin A clone 3C8) at 1:2,000 dilution. Secondary antibodies were goat anti-rat IRDye 680RD and goat anti-mouse IRDye 800C (LI-COR), and imaging was performed using a LI-COR Odyssey Imaging system.
For immunofluorescence microscopy, MEFs at the indicated passages were fixed in 4% freshly prepared formaldehyde for 20 min, permeabilized for 10 min in PBS-T, blocked for 1 h in PBS-T containing 2% bovine serum albumin, and colabeled with the mouse monoclonal anti-lamin A/C antibodies at 1:500 dilution, and the rat monoclonal anti-prelamin A antibody 3C8 at 1:300 dilution for 1 h. Cells were then incubated for 1 h with IRDye680RD goat anti-mouse (LI-COR) and Alexa Fluor 647 donkey anti-rat (Abcam) secondary antibodies. Images were captured on a ZEISS LSM700 system with ZEISS Plan-NEOFLUAR 40×/1.3 oil submersion objective and analyzed by ZEN (black edition) software. Florescence intensities were quantified using Fiji ImageJ. To test the effects of a FTI, MEFs were treated for 48 h with 2 µg/mL lonafarnib (The Progeria Research Foundation) dissolved in dimethyl sulfoxidie (DMSO), or DMSO-only as the vehicle control.
Statistics.
Student’s t tests of comparisons between two means were performed using Excel (Microsoft); bar graphs and body mass growth curves were generated using the same software. Statistical significance was set at P < 0.05. The Kaplan–Meier estimator in statistical software GraphPad Prism 5 (Prism Software) was used to generate the survival curves.
Supplementary Material
Acknowledgments
We thank Drs. Loren Fong and Stephen G. Young (University of California, Los Angeles) for antibodies against prelamin A, and the Genetically Modified Mouse Model Shared Resource of the Herbert Irving Comprehensive Cancer Center at Columbia University for assistance in generating mice. Research reported in this publication was supported by the National Institute of Aging, National Institue of General Medical Sciences, and National Institute of Dental and Craniofacial Research of the NIH under Awards R21AG058032 (to S.M. and H.J.W.), R35GM127073 (to S.M.), and R01DE015654 and R01DE026936 (to W.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Competing interest statement: H.J.W. has received consulting income from Eiger BioPharmaceuticals.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2118695119/-/DCSupplemental.
Data Availability
All study data are included in the main text and SI Appendix.
References
- 1.Davies B. S., Fong L. G., Yang S. H., Coffinier C., Young S. G., The posttranslational processing of prelamin A and disease. Annu. Rev. Genomics Hum. Genet. 10, 153–174 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Worman H. J., Michaelis S., Permanently farnesylated prelamin A, progeria, and atherosclerosis. Circulation 138, 283–286 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young S. G., Meta M., Yang S. H., Fong L. G., Prelamin A farnesylation and progeroid syndromes. J. Biol. Chem. 281, 39741–39745 (2006). [DOI] [PubMed] [Google Scholar]
- 4.De Sandre-Giovannoli A., et al. , Lamin a truncation in Hutchinson-Gilford progeria. Science 300, 2055 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Eriksson M., et al. , Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423, 293–298 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrowman J., Wiley P. A., Hudon-Miller S. E., Hrycyna C. A., Michaelis S., Human ZMPSTE24 disease mutations: Residual proteolytic activity correlates with disease severity. Hum. Mol. Genet. 21, 4084–4093 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navarro C. L., et al. , New ZMPSTE24 (FACE1) mutations in patients affected with restrictive dermopathy or related progeroid syndromes and mutation update. Eur. J. Hum. Genet. 22, 1002–1011 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moulson C. L., et al. , Homozygous and compound heterozygous mutations in ZMPSTE24 cause the laminopathy restrictive dermopathy. J. Invest. Dermatol. 125, 913–919 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergo M. O., et al. , Zmpste24 deficiency in mice causes spontaneous bone fractures, muscle weakness, and a prelamin A processing defect. Proc. Natl. Acad. Sci. U.S.A. 99, 13049–13054 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pendás A. M., et al. , Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat. Genet. 31, 94–99 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Fong L. G., et al. , A protein farnesyltransferase inhibitor ameliorates disease in a mouse model of progeria. Science 311, 1621–1623 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Fong L. G., et al. , Heterozygosity for Lmna deficiency eliminates the progeria-like phenotypes in Zmpste24-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 101, 18111–18116 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varela I., et al. , Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature 437, 564–568 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Ast T., Michaelis S., Schuldiner M., The protease Ste24 clears clogged translocons. Cell 164, 103–114 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kayatekin C., et al. ; AMP T2D-GENES Consortium, Translocon declogger Ste24 protects against IAPP oligomer-induced proteotoxicity. Cell 173, 62–73 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu B., Wang L., Li S., Dorf M. E., ZMPSTE24 defends against influenza and other pathogenic viruses. J. Exp. Med. 214, 919–929 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S., Fu B., Wang L., Dorf M. E., ZMPSTE24 is downstream effector of interferon-induced transmembrane antiviral activity. DNA Cell Biol. 36, 513–517 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costanzo M., et al. , The genetic landscape of a cell. Science 327, 425–431 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosomi A., et al. , The ER-associated protease Ste24 prevents N-terminal signal peptide-independent translocation into the endoplasmic reticulum in Saccharomyces cerevisiae. J. Biol. Chem. 295, 10406–10419 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonikas M. C., et al. , Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science 323, 1693–1697 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Runnebohm A. M., et al. , Overlapping function of Hrd1 and Ste24 in translocon quality control provides robust channel surveillance. J. Biol. Chem. 295, 16113–16120 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tipper D. J., Harley C. A., Yeast genes controlling responses to topogenic signals in a model transmembrane protein. Mol. Biol. Cell 13, 1158–1174 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hennekes H., Nigg E. A., The role of isoprenylation in membrane attachment of nuclear lamins. A single point mutation prevents proteolytic cleavage of the lamin A precursor and confers membrane binding properties. J. Cell Sci. 107, 1019–1029 (1994). [DOI] [PubMed] [Google Scholar]
- 24.Mallampalli M. P., Huyer G., Bendale P., Gelb M. H., Michaelis S., Inhibiting farnesylation reverses the nuclear morphology defect in a HeLa cell model for Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. U.S.A. 102, 14416–14421 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y., et al. , A mutation abolishing the ZMPSTE24 cleavage site in prelamin A causes a progeroid disorder. J. Cell Sci. 129, 1975–1980 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cabral W. A., et al. , Genetic reduction of mTOR extends lifespan in a mouse model of Hutchinson-Gilford progeria syndrome. Aging Cell 20, e13457 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim P. H., et al. , Disrupting the LINC complex in smooth muscle cells reduces aortic disease in a mouse model of Hutchinson-Gilford progeria syndrome. Sci. Transl. Med. 10, eaat7163 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J. M., et al. , Modulation of LMNA splicing as a strategy to treat prelamin A diseases. J. Clin. Invest. 126, 1592–1602 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osorio F. G., et al. , Splicing-directed therapy in a new mouse model of human accelerated aging. Sci. Transl. Med. 3, 106ra107 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Varga R., et al. , Progressive vascular smooth muscle cell defects in a mouse model of Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. U.S.A. 103, 3250–3255 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang S. H., et al. , A farnesyltransferase inhibitor improves disease phenotypes in mice with a Hutchinson-Gilford progeria syndrome mutation. J. Clin. Invest. 116, 2115–2121 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart C. L., Kozlov S., Fong L. G., Young S. G., Mouse models of the laminopathies. Exp. Cell Res. 313, 2144–2156 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H., Kieckhaefer J. E., Cao K., Mouse models of laminopathies. Aging Cell 12, 2–10 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Rivas D., Li W., Akter R., Henderson J. E., Duque G., Accelerated features of age-related bone loss in zmpste24 metalloproteinase-deficient mice. J. Gerontol. A Biol. Sci. Med. Sci. 64, 1015–1024 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Capell B. C., et al. , A farnesyltransferase inhibitor prevents both the onset and late progression of cardiovascular disease in a progeria mouse model. Proc. Natl. Acad. Sci. U.S.A. 105, 15902–15907 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toth J. I., et al. , Blocking protein farnesyltransferase improves nuclear shape in fibroblasts from humans with progeroid syndromes. Proc. Natl. Acad. Sci. U.S.A. 102, 12873–12878 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang S. H., et al. , Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson-Gilford progeria syndrome mutation. Proc. Natl. Acad. Sci. U.S.A. 102, 10291–10296 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davies B. S., et al. , An accumulation of non-farnesylated prelamin A causes cardiomyopathy but not progeria. Hum. Mol. Genet. 19, 2682–2694 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cubria M. B., et al. , Evaluation of musculoskeletal phenotype of the G608G progeria mouse model with lonafarnib, pravastatin, and zoledronic acid as treatment groups. Proc. Natl. Acad. Sci. U.S.A. 117, 12029–12040 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agarwal A. K., Fryns J. P., Auchus R. J., Garg A., Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum. Mol. Genet. 12, 1995–2001 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Miyoshi Y., et al. , Severe mandibuloacral dysplasia caused by novel compound heterozygous ZMPSTE24 mutations in two Japanese siblings. Clin. Genet. 73, 535–544 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mariño G., et al. , Premature aging in mice activates a systemic metabolic response involving autophagy induction. Hum. Mol. Genet. 17, 2196–2211 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Heizer P. J., et al. , Deficiency in ZMPSTE24 and resulting farnesyl-prelamin A accumulation only modestly affect mouse adipose tissue stores. J. Lipid Res. 61, 413–421 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Z. J., et al. , Dysregulated interactions between lamin A and SUN1 induce abnormalities in the nuclear envelope and endoplasmic reticulum in progeric laminopathies. J. Cell Sci. 127, 1792–1804 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Ibrahim M. X., et al. , Targeting isoprenylcysteine methylation ameliorates disease in a mouse model of progeria. Science 340, 1330–1333 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao H., et al. , Targeting RAS-converting enzyme 1 overcomes senescence and improves progeria-like phenotypes of ZMPSTE24 deficiency. Aging Cell 19, e13200 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meta M., Yang S. H., Bergo M. O., Fong L. G., Young S. G., Protein farnesyltransferase inhibitors and progeria. Trends Mol. Med. 12, 480–487 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Varela I., et al. , Combined treatment with statins and aminobisphosphonates extends longevity in a mouse model of human premature aging. Nat. Med. 14, 767–772 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Brayson D., et al. , Prelamin A mediates myocardial inflammation in dilated and HIV-associated cardiomyopathies. JCI Insight 4, e126315 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lucas-Herald A. K., et al. , Proteomic evidence of biological aging in a child with a compound heterozygous ZMPSTE24 mutation. Proteomics Clin. Appl. 13, e1800135 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Merideth M. A., et al. , Phenotype and course of Hutchinson-Gilford progeria syndrome. N. Engl. J. Med. 358, 592–604 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamczyk M. R., del Campo L., Andrés V., Aging in the cardiovascular system: Lessons from Hutchinson-Gilford progeria syndrome. Annu. Rev. Physiol. 80, 27–48 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Olive M., et al. , Cardiovascular pathology in Hutchinson-Gilford progeria: Correlation with the vascular pathology of aging. Arterioscler. Thromb. Vasc. Biol. 30, 2301–2309 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stehbens W. E., Delahunt B., Shozawa T., Gilbert-Barness E., Smooth muscle cell depletion and collagen types in progeric arteries. Cardiovasc. Pathol. 10, 133–136 (2001). [DOI] [PubMed] [Google Scholar]
- 55.Stehbens W. E., Wakefield S. J., Gilbert-Barness E., Olson R. E., Ackerman J., Histological and ultrastructural features of atherosclerosis in progeria. Cardiovasc. Pathol. 8, 29–39 (1999). [DOI] [PubMed] [Google Scholar]
- 56.Hamczyk M. R., et al. , Vascular smooth muscle-specific progerin expression accelerates atherosclerosis and death in a mouse model of Hutchinson-Gilford progeria syndrome. Circulation 138, 266–282 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ragnauth C. D., et al. , Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation 121, 2200–2210 (2010). [DOI] [PubMed] [Google Scholar]
- 58.Liu Y., Drozdov I., Shroff R., Beltran L. E., Shanahan C. M., Prelamin A accelerates vascular calcification via activation of the DNA damage response and senescence-associated secretory phenotype in vascular smooth muscle cells. Circ. Res. 112, e99–e109 (2013). [DOI] [PubMed] [Google Scholar]
- 59.Warming S., Costantino N., Court D. L., Jenkins N. A., Copeland N. G., Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 33, e36 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y., et al. , Postnatal development of mice with combined genetic depletions of lamin A/C, emerin and lamina-associated polypeptide 1. Hum. Mol. Genet. 28, 2486–2500 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tu Y., Sánchez-Iglesias S., Araújo-Vilar D., Fong L. G., Young S. G., LMNA missense mutations causing familial partial lipodystrophy do not lead to an accumulation of prelamin A. Nucleus 7, 512–521 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aartsma-Rus A., van Putten M., Assessing functional performance in the mdx mouse model. J. Vis. Exp. 85, 51303 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Durkin M. E., Qian X., Popescu N. C., Lowy D. R., Isolation of mouse embryo fibroblasts. Bio Protoc. 3, e908 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Babatz T. D., et al. , Site specificity determinants for prelamin A cleavage by the zinc metalloprotease ZMPSTE24. J. Biol. Chem. 296, 100165 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the main text and SI Appendix.