Abstract
Background
Lack of robust data on economic burden due to enteric fever in India has made decision making on typhoid vaccination a challenge. Surveillance for Enteric Fever network was established to address gaps in typhoid disease and economic burden.
Methods
Patients hospitalized with blood culture-confirmed enteric fever and nontraumatic ileal perforation were identified at 14 hospitals. These sites represent urban referral hospitals (tier 3) and smaller hospitals in urban slums, remote rural, and tribal settings (tier 2). Cost of illness and productivity loss data from onset to 28 days after discharge from hospital were collected using a structured questionnaire. The direct and indirect costs of an illness episode were analyzed by type of setting.
Results
In total, 274 patients from tier 2 surveillance, 891 patients from tier 3 surveillance, and 110 ileal perforation patients provided the cost of illness data. The mean direct cost of severe enteric fever was US$119.1 (95% confidence interval [CI], US$85.8–152.4) in tier 2 and US$405.7 (95% CI, 366.9–444.4) in tier 3; 16.9% of patients in tier 3 experienced catastrophic expenditure.
Conclusions
The cost of treating enteric fever is considerable and likely to increase with emerging antimicrobial resistance. Equitable preventive strategies are urgently needed.
Keywords: cost of illness, economic burden, enteric fever, health expenditure, ileal perforation, India, out of pocket expenditure, typhoid
Enteric fever is a public health concern in many low and middle-income countries (LMICs). In 2017, 14.3 million enteric fever episodes resulted in an estimated 178 000 deaths globally, with 70% in South Asia alone. Enteric fever was also the cause of 6 737 500 years of life lost in South Asia [1]. With the emergence of antimicrobial resistance and slow progress on water and sanitation interventions, the burden of enteric fever may increase [2, 3].
Typhoid vaccination could provide control of the disease until water, sanitation, and hygiene interventions reap dividends [4]. The World Health Organization (WHO) recommended introducing typhoid conjugate vaccine (TCV) in LMIC countries [5]. However, this requires substantial investment for these economies. With only 1.15% of gross domestic product public spending towards health care, India’s vaccination program has been slow to introduce new vaccines and relies heavily on out-of-pocket payments for treatment [6]. As India transitions out of Gavi Alliance support, a policy decision on introducing a new vaccine is urgent and necessitates substantial epidemiological and economic evidence of enteric fever burden [7–9].
Disease-specific spending in India is not well documented. There are very few data sources of individual health care expenditure, with only 3 studies reporting typhoid fever costs [10]. The Diseases of the Most Impoverished (DOMI) program reported that the total cost incurred by a hospitalized case to be US$129 [11]. In Kolkata, the average treatment cost of a hospitalized patient was US$99.36 in 2 hospitals [12]. In an urban slum in New Delhi, the mean total cost (patient and provider) of typhoid fever was US$126, with a hospitalized case costing much higher, US$636 [13]. Pooling these data has been challenging due to the different settings, health care facilities, and methodologies. Hence there was a need to assess the out-of-pocket burden of patients suffering from enteric fever in rural and urban areas.
The Surveillance for Enteric Fever in India (SEFI) network was established to obtain reliable contemporaneous epidemiological data on enteric fever [14–16]. This article presents the out-of-pocket costs incurred by patients hospitalized with severe enteric fever and nontraumatic ileal perforation, a common complication of enteric fever [17], from the SEFI surveillance.
METHODS
Study Design and Setting
The SEFI network conducted surveillance at 18 sites across India for 2 years between November 2017 and March 2020. In tier 1 surveillance (4 sites), a large cohort of children younger than 15 years were followed for 24 months to measure the community incidence of typhoid fever. However, because the study supported the diagnostics and management of illness, tier 1 was not included in the cost estimation. The tier 2 surveillance was done in smaller hospitals in 5 rural sites and 1 urban site, combining facility-based surveillance with a health care utilization survey to estimate the incidence of severe enteric fever. The tier 3 surveillance was done in 8 key tertiary care hospitals in India. All blood culture-confirmed enteric fever patients in these hospitals were enrolled to assess patterns of enteric fever caused by Salmonella Typhi and Salmonella Paratyphi and estimate their antimicrobial resistance [14]. The 14 hospitals in tiers 2 and 3 captured cost of illness data for this study.
Data Collection
Severe enteric fever was defined as a hospitalized case with blood culture-confirmation for enteric fever. Nontraumatic ileal perforation was defined as a case diagnosed with ileal perforation by the operating surgeon.
The economic data was obtained from the hybrid and laboratory surveillance using an incidence-based approach with an additional recall component for collecting pre- and posthospitalization costs using a structured questionnaire [18]. It included sociodemographic characteristics, income-expenditure details, illness-specific expenses, productive time lost, alternatives used for productivity, and related costs. Patients were followed up until 28 days to understand the costs incurred for the entire episode of illness. All collected information was entered electronically into a cloud-based server via a secure data management system. Data validation was done at the point of entry with inbuilt range and internal consistency checks. Simultaneously, the data management team performed standard logic checks and resolved issues using a closed query redressal system.
Data Analysis
Data analysis was done with Stata 15.1 software package. Incomplete data were excluded from the final analysis. All cost-related information was reported in Indian Rupees (Rs) and US dollars, wherein 2 January 2019 (mid-year of surveillance) currency conversion rate (1 US$ = Rs 69.6089) was applied [19].
The direct cost of 1 episode of severe enteric fever included hospitalization charges and outpatient charges pre- and posthospitalization. It comprises medical costs, including bed/consultation, diagnostic tests, procedure costs, prescribed medicines, and nonmedical costs such as food, transport, lodging, other expenses, and productive time lost by patients and caregivers. Indirect costs were computed from the patient and caregiver’s income loss and costs related to alternative productivity arrangements. The effective income loss was computed, assuming the patient or caregiver would work an 8-hour shift for 26 days a month. The total indirect cost was the sum of income lost and the payments related to alternate productivity used. Income lost was equal to the total time lost in professional work (hours) multiplied by the gross hourly income.
All costs related to enteric fever were stratified by setting (tier 2/tier 3). However, nontraumatic ileal perforation costs were presented together, assuming costs would be similar across settings as it is often identified at surgery. Moreover, these ileal perforations were classified by 2 investigators (J. J. and S. K.) independently based on causality using available clinical data, laboratory evidence, and surgical or histopathological evidence. The cost for confirmed enteric ileal perforations were also presented [20]. Financial impact indicators such as distress financing and catastrophic health expenditure were computed. A household was said to be “distress financing” when they sourced their finances by borrowing (with or without interest) and selling assets [21, 22]. A household experienced catastrophic health expenditure if the household spent beyond 40% of their annual nonsubsistence expenditure on health care [23, 24]. Univariate analysis was done to explore the determinants of catastrophic health expenditure, while multivariate analysis was done to adjust for confounding.
Ethical Considerations
The institutional review boards of the Christian Medical College, Vellore, as the coordinating institution, and all participating institutions approved the study. All patient details were collected after obtaining written informed consent from the patient/caregiver.
RESULTS
Overall, 1275 patients provided costing information with 274 enteric fever patients from tier 2 sites, 891 from tier 3 sites, and 110 ileal perforation patients (n = 18 confirmed enteric fever perforations) from both tiers (Table 1). Of tier 2 and tier 3 patients, 31.1% and 62.9%, respectively, were younger than 15 years. More than 80% of the enteric fevers were due to S. Typhi in both settings. The mean duration of hospitalization was 5.6 days in tier 2, 7.95 days in tier 3, and 19.06 days among all-cause ileal perforation patients (Table 1).
Table 1.
Demographic Characteristics of Blood Culture-Confirmed Enteric Fever Patients Included in the Study (n = 1275)
| Characteristics | Tier 2 | Tier 3 | Ileal Perforation, All-Cause |
|---|---|---|---|
| Total | 274 | 891 | 110 |
| Organism | |||
| Typhoid | 221 (80.7) | 782 (87.8) | … |
| Paratyphoid | 53 (19.3) | 109 (12.2) | … |
| Age, y | |||
| 0–4 | 28 (10.2) | 228 (25.6) | 22 (20) |
| 5–14 | 60 (21.9) | 332 (37.3) | 8 (7.3) |
| 15–29 | 148 (54) | 243 (27.3) | 34 (30.9) |
| 30–44 | 33 (12) | 61 (6.8) | 20 (18.2) |
| 45–59 | … | … | … |
| ≥60 | 5 (1.8) | 27 (3) | 26 (23.6) |
| Sex | |||
| Male | 176 (64.2) | 545 (61.2) | 70 (63.6) |
| Female | 98 (35.8) | 346 (38.8) | 40 (36.4) |
| Duration of hospitalization, d, mean (95% CI) | 5.6 (5.2–5.9) | 7.95 (7.63–8.27) | 19.06 (16.56–21.57) |
| Delay in hospitalization, d, mean (95% CI) | 7.2 (6.1–8.3) | 9.5 (8.4–10.6) | 11.3 (7.4–15.1) |
| Annual income, Rs, mean (95% CI) | 2 52 924.5 (2 22 006.5–2 83 842.4) |
4 80 806.1 (4 35 837.6–5 25 774.5) |
4 62 392.7 (3 49 387.7–5 75 397.8) |
| Annual expenditure, Rs, mean (95% CI) | 1 36 775.5 (1 27 120.4–1 46 430.7) |
2 43 832.6 (2 29 837.8–2 57 827.4) |
187 374.8 (1 60 526.6–2 14 222.9) |
| Capacity to pay, Rs, mean (95% CI) | 99 712.96 (90 804.2–10 8621.7) | 1 88 603.3 | 1 35 896.3 |
| (1 75 087.3–2 02 119.4) | (1 10 816.8–1 60 975.8) | ||
| Health insurance, yes | 43 (15.7) | 202 (22.7) | 2 (1.8) |
Data are No. (%) except where indicated.
Abbreviation: CI, confidence interval; Rs, Indian Rupee.
The mean direct cost of enteric fever was Rs8292.3 (US$119.1) in tier 2 settings, while the same in tier 3 was Rs28 237.7 (US$405.7) (Table 2). All-cause nontraumatic ileal perforation cost was Rs84 227.5 (US$1210) while those perforations confirmed to be enteric cause (n = 18) cost Rs90 869.2 (US$1305.4). Approximately three-quarters of these costs were during the hospital stay in all patients.
Table 2.
Distribution of Total Costs of Severe Enteric Fever
| Costs | Tier 2, Rs (US$) (n = 274) | Tier 3, Rs (US$) (n = 891) | ||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| Direct medical costs | ||||
| User charges | 1626.6 (23.4) | 306.9 (4.4) | 9995.5 (143.6) | 463.0 (6.7) |
| Diagnostic charges | 1402.2 (20.1) | 114.4 (1.6) | 5999.5 (86.2) | 270.3 (3.9) |
| Drugs and consumables | 3168.3 (45.5) | 218.5 (3.1) | 6242.0 (89.7) | 496.8 (7.1) |
| Procedure/surgery | 819.7 (11.8) | 663.9 (9.5) | 1279.3 (18.4) | 193.5 (2.8) |
| Total direct medical costs | 7016.7 (100.8) | 1169.7 (16.8) | 23 516.2 (337.8) | 1087.9 (15.6) |
| Direct nonmedical costs | ||||
| Travel cost | 336.4 (4.8) | 39 (0.6) | 1233.6 (17.7) | 70.9 (1) |
| Meal cost | 473.4 (6.8) | 34.5 (0.5) | 1347.6 (19.4) | 55.4 (0.8) |
| Lodging charges | 6.2 (0.1) | 4.7 (0.1) | 117.0 (1.7) | 33.7 (0.5) |
| Informal costs | 31 (0.4) | 11.1 (0.2) | 19.9 (0.3) | 6.4 (0.1) |
| Other costs | 428.5 (6.2) | 40.1 (0.6) | 685.9 (9.9) | 34.1 (0.5) |
| Total direct nonmedical costs | 1275.6 (18.3) | 84.9 (1.2) | 3404.1 (48.9) | 128.0 (1.8) |
| Uncategorized othersa | … | … | 1317.4 (18.9) | 687.9 (9.9) |
| Total direct cost | 8292.3 (119.1) | 1178 (16.9) | 28 237.7 (405.7) | 1373.4 (19.7) |
| Indirect cost | ||||
| Income lost by patient | 1653.2 (23.8) | 243 (3.5) | 3415.2 (49.1) | 510 (7.3) |
| Income lost by caretakers | 2935.6 (42.2) | 234.3 (3.4) | 6972.7 (100.2) | 565.7 (8.1) |
| Payment to substitute | 116.9 (1.7) | 58.6 (0.8) | 822.9 (11.8) | 321.4 (4.6) |
| Total indirect cost | 4705.8 (67.6) | 318.8 (4.6) | 11 210.8 (161.1) | 982.1 (14.1) |
Abbreviation: Rs, Indian Rupee; SE, standard error.
aUncategorized costs are costs wherein the patient/ bill carried only the total direct costs and therefore were left uncategorized.
Among the determinants of expenditure, drugs and consumables were the highest in tier 2 (38.2%), while hospital charges like consultation, bed, and administrative charges formed the highest (35.4%) in tier 3 (Table 2). Children (<15 years) with enteric fever had lower expenses than adults in both settings (Table 3). Enteric fever patients with a private hospitalization spent at least 3 times as much as patients hospitalized in a public hospital in both settings. Patients who needed intensive care (Rs54 592.7/US$784.3) spent 8 times more than regular hospitalization in tier 2 settings (Table 3).
Table 3.
Total Direct Cost of Severe Enteric Fever by the Causative Organism, Age, Sex, and Sector
| Characteristic | Tier 2 | Tier 3 | ||
|---|---|---|---|---|
| n | Mean, Rs (US$) | n | Mean, Rs (US$) | |
| Enteric Fever | 274 | 8292.3 (119.1) | 891 | 28 237.7 (405.7) |
| Organism | ||||
| Salmonella Typhi | 221 | 9061.9 (130.2) | 782 | 28 337.3 (407.1) |
| Salmonella Paratyphi | 53 | 5083.7 (73) | 109 | 27 523.3 (395.4) |
| Age, y | ||||
| <15 | 88 | 6975.7 (100.2) | 560 | 18 396.2 (264.3) |
| ≥ 15 | 186 | 8915.3(128.1) | 331 | 44 888.1 (644.9) |
| Sector | ||||
| Public sector | 133 | 4308.8 (61.9) | 331 | 9205.9 (132.3) |
| Private/charity | 141 | 12 049.8 (173.1) | 560 | 39 486.9 (567.3) |
| Sex | ||||
| Male | 176 | 7069.5 (101.6) | 545 | 28 335.4 (407.1) |
| Female | 98 | 10 488.5 (150.7) | 346 | 28 083.8 (403.5) |
| ICU admission | ||||
| No | 264 | 6538.5 (93.9) | 823 | 25 169.9 (361.6) |
| Yes | 10 | 54592.7 (784.3) | 68 | 65 367.2 (939.1) |
| Outcome | ||||
| Recovered without complications | 247 | 7008 (100.7) | 857 | 28 043.9 (402.9) |
| Sequelae/death/referred | 23 | 21 722.9 (312.1) | 12 | 68 443.3 (983.3) |
| Duration of stay, d | ||||
| ≤3 | 56 | 4582.8 (65.8) | 48 | 15 170.2 (217.9) |
| 3–7 | 168 | 7086.1 (101.8) | 492 | 24 996.6 (359.1) |
| >7 | 50 | 16 500 (237) | 351 | 34 567.8 (496.6) |
| Insurance | ||||
| No | 231 | 8270.4 (118.8) | 689 | 27 132.4 (389.8) |
| Yes | 43 | 8410.1 (120.8) | 202 | 32 007.7 (459.8) |
Abbreviation: ICU, intensive care unit; Rs, Indian Rupee.
The average productive time lost by the patient and caregivers together in different activities was 207 hours in tier 2, 263.5 hours in tier 3, and 535 hours for ileal perforations confirmed to be due to enteric fever. The indirect cost of enteric fever was Rs4705.8 (US$67.6) among patients in tier 2, while it was Rs11 210.8 (US$161.1) in tier 3 settings (Table 2). The indirect cost of ileal perforation of any cause was Rs26 488.2 (US$380.5) while the same among ileal perforations confirmed to be due to enteric fever was Rs46 770 (US$671.9).
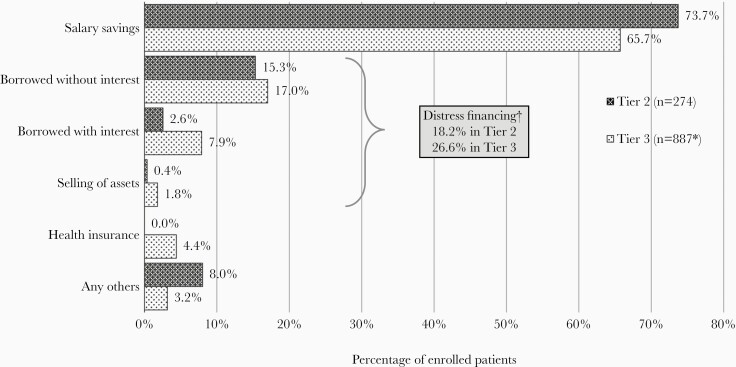
Patients with enteric fever predominantly used their salary or savings (73.7% and 65.7% in tier 2 and tier 3, respectively) to finance their treatment costs (Figure 1). No patients in tier 2 settings utilized health insurance, although 15.7% (n = 43) reported having some form of health insurance. In tier 3, 4.4% availed health insurance, although 22.7% (n = 202) reported having health insurance coverage. In tier 2 and tier 3 settings, 18.2% (n = 50) and 26.6% (n = 237), respectively, of the enteric fever patients were forced to distress finance their expenses (Figure 1). The costs for all-cause nontraumatic ileal perforation forced 39% of the households into distress financing their expenses while the costs due to confirmed enteric perforations forced 66.7% of the households into distress financing.
Figure 1.
Source of financing for severe enteric fever. Tier 2; surveillance in smaller hospitals in 5 rural sites and 1 urban site; tier 3, surveillance in 8 key tertiary care hospitals. *Four patients in tier 3 did not report their source of finance. †A person is said to be under distress financing when they spend by borrowing or selling of assets.
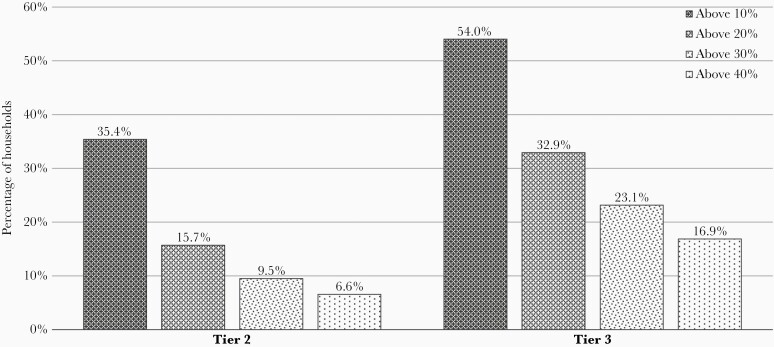
In tier 2, 35.4% (n = 97) of households spent over one-tenth of their nonsubsistence expenses on enteric fever treatment, while it was 54% (n = 481) of the households in tier 3 for the same (Figure 2). In tier 2 and tier 3 settings, 6.6% (n = 18) and 16.9% (n = 150), respectively, experienced catastrophic health expenditure (Figure 2). With regard to ileal perforations, 49.1% of the households with all-cause perforations and 61.1% of households with confirmed enteric fever as the cause experienced catastrophic health expenditure.
Figure 2.
Percentage of households spending above the cutoff on health care due to enteric fever. Distribution of direct medical expenses for enteric fever as a proportion of capacity to pay.. Catastrophic health expenditure was not computed for 1 subject with no expenses.
Multivariate analysis showed that, in both settings, hospitalization in the private sector compared to the public sector (tier 2, adjusted odds ratio [aOR], 12; 95% confidence interval [CI], 1.5–95.2: tier 3, aOR, 2.7; 95% CI, 1.7–4.3) and lowest income quintile compared to higher-income quintiles (tier 2, aOR, 7.0; 95% CI, 2.3–21.5: tier 3, aOR, 5.3; 95% CI, 3.5–8.2) had higher odds of experiencing financial catastrophe. Furthermore, families with lower than primary education (aOR, 7.6; 95% CI, 2.1–27.6) in tier 2 and patients older than 15 years (aOR, 2.9; 95% CI, 1.9–4.3) in tier 3 had a higher risk of catastrophic health expenditure compared to their counterparts (Table 4).
Table 4.
Determinants of Catastrophic Health Expenditure
| Determinant | Tier 2 (n = 274) | Tier 3 (n = 890a) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | % | Adjusted Odds Ratio | P Value | n | % | Adjusted Odds Ratio | P Value | |
| Total | 18 | 6.6 | 150 | 16.8 | ||||
| Highest education of the household | ||||||||
| Less than Primary | 6 | 28.6 | 7.56 (95% CI 2.07–27.57) | .002 | Education details not collected in this surveillance | |||
| More than Primary | 12 | 4.7 | Ref | |||||
| Household size | ||||||||
| Up to 3 members | 2 | 3.8 | 40 | 22.1 | 1.33 (95% CI 0.86–2.06) | .203 | ||
| More than 3 members | 16 | 7.2 | 110 | 15.5 | Ref | |||
| Sector | ||||||||
| Public | 1 | 0.8 | Ref | .019 | 30 | 9.1 | Ref | <.0001 |
| Private/Charity | 17 | 12.1 | 12.02 (95% C 1.52–95.19) | 120 | 21.5 | 2.69 (95% CI 1.69–4.28) | ||
| Age, y | ||||||||
| <15 | 7 | 8.0 | 65 | 11.6 | Ref | <.0001 | ||
| ≥15 | 11 | 5.9 | 85 | 25.8 | 2.87 (95% CI 1.91–4.3) | |||
| Sex | ||||||||
| Male | 10 | 5.7 | 97 | 17.8 | ||||
| Female | 8 | 8.2 | 53 | 15.3 | ||||
| Insurance | ||||||||
| No | 14 | 6.1 | 118 | 17.2 | ||||
| Yes | 4 | 9.3 | 32 | 15.8 | ||||
| Income quintile | ||||||||
| Q1, poorest | 12 | 18.5 | 6.95 (95% CI 2.25–21.52) | .001 | 60 | 33.3 | 5.31 (95% CI 3.45–8.17) | <.0001 |
| Q2 and above | 6 | 2.87 | Ref | 90 | 12.7 | Ref |
Univariate logistic regression was used to find early predictors and only those found to have an association was built into the regression model to identify predictors.
aCatastrophic health expenditure could not be computed for 1 patient in tier 3 as he/she did not report any expenses.
Discussion
Overall, the direct cost of enteric fever requiring hospitalization was estimated to be US$119.1 in tier 2 surveillance and US$405.7 in tier 3 surveillance, which was higher than the DOMI study (2003) estimates from Kolkata, India (US$29) [11]. Similarly, the direct costs in tier 3 settings were higher than the total cost of typhoid fever requiring in-patient care from the Delhi study (1995–1996) done in urban slums. It is noted here that the total costs in the Delhi study included the patient costs and the cost borne by the health system [13]. Both these previous studies were >2 decades old, and health care costs are likely to have changed over this time.
Studies from the Surveillance for Enteric Fever in Asia Project (SEAP 2016–2018) from Pakistan, Nepal, and Bangladesh (Ranged between US$ 168.72 - 316.94) done in teaching hospitals, reported slightly lower directs costs among inpatients and indirect costs comparable to our estimates from similar tier 3 surveillance (direct cost US$405.7; indirect cost US$161.1) [25–27]. These studies excluded medical costs that were not related to enteric fever. In our study all costs related to the Acute Febrile Illness were captured. Also, the use of telephonic data collection could run the risk of underestimating the costs in these studies.
The cost of nontraumatic ileal perforation due to enteric fever has not been previously studied in India. Unsurprisingly this complication of enteric fever led to approximately 3 times the costs of a noncomplicated enteric fever in our study. This shows the direct costs for a hospitalized case of enteric fever could range from US$119.1 to US$1305.4, depending on the setting and severity.
None of the tier 2 and only 4.4% (n = 39) of tier 3 patients could access health insurance for their treatment costs. The Rashtriya Swasthya Bima Yojana (RSBY) and Ayushman Bharath (AB-PMJAY-2018) are national schemes aimed to enable the “poorest 40 percent of the population to meet the expenses for quality secondary and tertiary care. Thereby it was aimed to reduce catastrophic expenditure and remove the financial risk arising out of such episodes” [28–31]. However, the study participants could not access these schemes and had to pay for care largely out of pocket. Consequently, 45% (n = 287) of enteric fever households resorted to distress financing. The lowest income quintile group in both the tiers (18.5% and 33.3%) had the highest proportion of catastrophic spending (Table 4). Therefore, national health insurance schemes need to expand coverage and enroll a wider network of health care providers to protect the poor. Universal health insurance schemes and universal basic income approaches could pave the way for equitable access to health care and also reduce impoverishment in the country.
Patients who sought care in the private sector had 3 times greater expenditure compared to the public sector and therefore had higher odds (aOR, 12.0 and 2.7 for tier 2 and tier 3, respectively) of experiencing catastrophic health expenditure. The cost of treatment for enteric fever requiring hospitalization in tier 3 (US$405.7) was higher than the cost of dengue hospitalization (US$197.03) in teaching hospitals in India [32]. This shows that enteric fever treatment is expensive and results in high out-of-pocket payments, similar to ailments due to other infectious causes.
The data collected through the SEFI study shows that out-of-pocket patient expenditure for the treatment of enteric fever in India remains high in both tiers. The disease continues to disproportionately coerce households into financial catastrophe. To prevent this, control measures against the disease, such as the typhoid conjugate vaccines, must be deployed after appropriate cost-effectiveness studies.
Our study is the first large study to report patient-level costs of enteric fever requiring hospitalization in India, studying costs from 1275 patients from 14 sites. Previous studies in India were from 1 or 2 sites, and the costs were derived from a significantly lower study sample [11, 13].
There were limitations to the study. Firstly, while we attempted to represent the costs in different risk settings, we recognize the limitation in being truly representative of the country. This study does not provide adequate information on the costs related to enteric fever in very poor urban slums where most patients tend to get treated in informal care as outpatients with prophylactic antibiotics. Secondly, we recognize that a variable proportion of health care costs are absorbed by the health system in both the government and private charitable facilities, and a comprehensive economic burden requires estimation of the health system costs. However, collecting costs from health care providers require multiple levels of approval, particularly in the government sector. Therefore, these costs were not computed and our focus remained predominantly on out-of-pocket expenditure. Further, consumption expenditure details obtained in our study might be affected by recall bias as participants were required to provide their last month’s complete expense details. Also, we assumed an 8-hour schedule of work per day for computation of indirect costs and did not collect actual work timings. We did not monetize wages for children, unemployed, and homemakers, which could underestimate the indirect cost of the disease.
CONCLUSION
This study shows that out-of-pocket expenses for enteric fever are high in urban areas and for hospitalizations. Patients and their households bear high indirect costs, particularly detrimental for those from lower strata who access public services intended to provide free health care. The study shows that enteric fever continues to push families into impoverishment and that the government health insurance schemes for the poor have not proven very effective in averting their financial risk. It is hoped that these results will help build a case for developing and implementing effective control measures to minimize the effects it bears on enteric fever patients due to out-of-pocket expenditures.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We acknowledge with gratitude the participants and their families for their partnership over the 2 years of surveillance. We acknowledge Miriam Thankam George, Vijay Anand Ismavel, Anna P. Alexander, Dennis Martin, Pradeep Zacharia, Balaji Veeraraghavan, Anuradha De, Sachee Agrawal, and Deepak S. Singh for their support at various stages of the surveillance. In addition to members of the Surveillance for Enteric Fever consortium, we acknowledge Jason Andrews, Nathan Lo, Gordon Dougan, Robert Breiman, Megan Carey, and Thomas Cherian for their contribution to the design and analytic plans. Supriya Kumar, Duncan Steele, and Anita Zaidi of the Bill and Melinda Gates Foundation have been steadfast in their support of the study with constructive comments and encouragement. Our gratitude is also due to Soumya Swaminathan, former Secretary, Department of Health Research, Government of India, for providing leadership and participation from the Government in the design and conduct of the surveillance.
Author contributions. J. J., G. K., and V. R. conceived and designed the study. J. J., G. K., D. K., S. P., A. S. C., A. S. K., K. R., P. S. P., and V. R. developed the study protocol and analysis plan. J. J. and G. K. coordinated the project. R. R., S. K., D. K., K. R., A. S. K., S. E. E., M. S. E., M. G., A. S. H., D. R. J., S. T., R. M. K., C. D. S., A. K., J. S., K. S., S. P. P., S. N., S. A., M. T., and P. B. coordinated data collection at the sites. D. K., P. S. P., A. S., S. K. R., and S. P. analyzed the data. D. K., A. S., and S. K. R. drafted the manuscript. All authors have reviewed the manuscript and approved it. J. J. and G. K. had complete access to data and guaranteed the manuscript.
Disclaimer . The funder had no role in the design analysis or interpretation of the findings.
Financial support. This work was supported by Bill and Melinda Gates Foundation (grant number INV-009497-OPP1159351) as part of the National Surveillance System for Enteric Fever in India.
Supplement sponsorship. This supplement is sponsored by the Christian Medical College Vellore Association.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Stanaway JD, Reiner RC, Blacker BF, et al. The global burden of typhoid and paratyphoid fevers: a systematic analysis for the global burden of disease study 2017. Lancet Infect Dis 2019; 19:369–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Browne AJ, Kashef Hamadani BH, Kumaran EAP, et al. Drug-resistant enteric fever worldwide, 1990 to 2018: a systematic review and meta-analysis. BMC Med 2020; 18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luthra K, Watts E, Debellut F, Pecenka C, Bar-Zeev N, Constenla D. A review of the economic evidence of typhoid fever and typhoid vaccines. Clin Infect Dis 2019; 68:S83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Institute of Medicine (US) Roundtable on Environmental Health Sciences, Research, and Medicine. Global environmental health: research gaps and barriers for providing sustainable water, sanitation, and hygiene services: workshop summary. Washington, DC: National Academies Press, 2009. [PubMed] [Google Scholar]
- 5. World Health Organization. Typhoid vaccines: WHO position paper, March 2018—recommendations. Vaccine 2019; 37:214–6. [DOI] [PubMed] [Google Scholar]
- 6. Government of India, Ministry of Finance. Economic survey 2019–20: volume 2. New Delhi, India: Department of Economic Affairs, 2020. [Google Scholar]
- 7. Balaji V, Kapil A, Shastri J, et al. Longitudinal typhoid fever trends in India from 2000 to 2015. Am J Trop Med Hyg 2018; 99:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mogasale V, Ramani E, Park IY, Lee JS. A forecast of typhoid conjugate vaccine introduction and demand in typhoid endemic low- and middle-income countries to support vaccine introduction policy and decisions. Hum Vaccines Immunother 2017; 13:2017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gavi. Historic partnership between Gavi and India to save millions of lives.https://www.gavi.org/news/media-room/historic-partnership-between-gavi-and-india-save-millions-lives. Accessed 9 June 2020.
- 10. Kastor A, Mohanty SK. Disease-specific out-of-pocket and catastrophic health expenditure on hospitalization in India: do Indian households face distress health financing? PLoS One 2018; 13:e0196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Poulos C, Riewpaiboon A, Stewart JF, et al. Cost of illness due to typhoid fever in five Asian countries. Trop Med Int Health 2011; 16:314–23. [DOI] [PubMed] [Google Scholar]
- 12. Sur D, Chatterjee S, Riewpaiboon A, Manna B, Kanungo S, Bhattacharya SK. Treatment cost for typhoid fever at two hospitals in Kolkata, India. J Health Popul Nutr 2009; 27:725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bahl R, Sinha A, Poulos C, et al. Costs of illness due to typhoid fever in an Indian urban slum community: implications for vaccination policy. J Health Popul Nutr 2004; 22:304–10. [PubMed] [Google Scholar]
- 14. Carey ME, MacWright WR, Im J, et al. The surveillance for enteric fever in Asia project (SEAP), severe typhoid fever surveillance in Africa (SETA), surveillance of enteric fever in India (SEFI), and strategic typhoid alliance across Africa and Asia (STRATAA) population-based enteric fever studies: a review of methodological similarities and differences. Clin Infect Dis 2020; 71:S102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Srinivasan M, Sindhu KN, John J, Kang G. Opportunities for typhoid vaccination in India. Indian Pediatr 2019; 56:453–8. [PubMed] [Google Scholar]
- 16. John J, Bavdekar A, Rongsen-Chandola T, Dutta S, Kang G. Estimating the incidence of enteric fever in children in India: a multi-site, active fever surveillance of pediatric cohorts. BMC Public Health 2018; 18:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wani RA, Parray FQ, Bhat NA, Wani MA, Bhat TH, Farzana F. Nontraumatic terminal ileal perforation. World J Emerg Surg 2006; 1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prinja S, Jagnoor J, Sharma D, et al. Out-of-pocket expenditure and catastrophic health expenditure for hospitalization due to injuries in public sector hospitals in North India. PLoS One 2019; 14:e0224721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. International Monetary Fund. Representative exchange rates for selected currencies for January 2019. https://www.imf.org/external/np/fin/data/rms_mth.aspx?SelectDate=2019-01-31&reportType=REP. Accessed 9 June 2020.
- 20. Njarekkattuvalappil SK, Thomas M, Kapil A, et al. Ileal perforation and enteric fever: implications for burden of disease estimation. J Infect Dis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Joe W. Distressed financing of household out-of-pocket health care payments in India: incidence and correlates. Health Policy Plan 2015; 30:728–41. [DOI] [PubMed] [Google Scholar]
- 22. Kruk ME, Goldmann E, Galea S. Borrowing and selling to pay for health care in low- and middle-income countries. Health Aff 2017. [DOI] [PubMed] [Google Scholar]
- 23. Xu K, Evans DB, Kawabata K, Zeramdini R, Klavus J, Murray CJL. Household catastrophic health expenditure: a multicountry analysis. Lancet 2003; 362:111–7. [DOI] [PubMed] [Google Scholar]
- 24. Xu K, Evans DB, Carrin G, Aguilar-Rivera AM, Musgrove P, Evans T. Protecting households from catastrophic health spending. Health Aff 2007; 26:972–83. [DOI] [PubMed] [Google Scholar]
- 25. Mejia N, Qamar F, Yousafzai MT, et al. Typhoid and paratyphoid cost of illness in Pakistan: patient and health facility costs from the surveillance for enteric fever in Asia project II. Clin Infect Dis 2020; 71:S319–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mejia N, Abimbola T, Andrews JR, et al. Typhoid and paratyphoid cost of illness in Nepal: patient and health facility costs from the surveillance for enteric fever in Asia project II. Clin Infect Dis 2020; 71:S306–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mejia N, Pallas SW, Saha S, et al. Typhoid and paratyphoid cost of illness in Bangladesh: patient and health facility costs from the surveillance for enteric fever in Asia project II. Clin Infect Dis 2020; 71:S293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Government of India. National Portal of India. Ayushman Bharat National Health Protection Mission. https://www.india.gov.in/spotlight/ayushman-bharat-national-health-protection-mission. Accessed 23 February 2021.
- 29. Karan A, Yip W, Mahal A. Extending health insurance to the poor in India: an impact evaluation of Rashtriya Swasthya Bima Yojana on out of pocket spending for healthcare. Soc Sci Med 2017; 181:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prinja S, Bahuguna P, Gupta I, Chowdhury S, Trivedi M. Role of insurance in determining utilization of healthcare and financial risk protection in India. PLoS One 2019; 14:e0211793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ved RR, Gupta G, Singh S. India’s health and wellness centres: realizing universal health coverage through comprehensive primary health care. WHO South-East Asia J Public Health 2019; 8:18–20. [DOI] [PubMed] [Google Scholar]
- 32. Shepard DS, Halasa YA, Tyagi BK, et al. Economic and disease burden of dengue illness in India. Am J Trop Med Hyg 2014; 91:1235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]