Abstract
Molecular dynamics (MD) simulations of biological membranes have achieved such level of sophistication that are commonly used to predict unresolved structures and various properties of lipids, and to substantiate experimental data. While achieving sufficient sampling of lipid dynamics remains a major challenge, a commonly used method to improve lipid sampling, e.g., in terms of specific interactions with membrane-associated proteins, is to randomize the initial arrangement of lipid constituents in multiple replicas of simulations, without changing the overall lipid composition of the membrane of interest. Here, we introduce a method that can rapidly generate multiple replicas of lipid bilayers with different spatial and conformational configurations for any given lipid composition. The underlying algorithm, which allows one to shuffle lipids at any desired level, relies on the application of an external potential, here referred to as the “carving potential”, that removes clashes/entanglements before lipid positions are exchanged (shuffled), thereby minimizing the energy penalty due to abrupt lipid repositioning. The method is implemented as “Membrane Mixer Plugin (MMP) 1.0” in VMD, with a convenient graphical user interface that guides the user in setting various options and parameters. The plugin is fully automated and generates new membrane replicas more rapidly and conveniently than other analogous tools. The plugin and its capabilities introduced here can be extended to include additional features in future versions.
Graphical Abstract

Rapid lipid shuffling with Membrane Mixer Plugin (MMP). This VMD plugin uses external potentials (mesh) to catalyze clash-free exchange of lipids in a membrane of any composition, thereby generating randomly configured membrane systems for multi-replica MD simulations.
Introduction
Biological membranes are cellular components that primarily separate the inner of a living cell from its surroundings.1 At the same time they provide a major platform for some of the most fundamental processes in a cell.2,3 For example, and despite being only a few nanometers thick, they provide an effective permeation barrier against water soluble material, thereby channeling controlled transport of material to specialized membrane channels and transporters in the membrane.4,5
Biological membranes are heterogeneous and composed of diverse lipid types, lending them complexity in both physical and chemical characteristics and in biological behavior.6 Lipids have evolved to include different shapes, sizes, and charges, in order to optimize properties of the membranes for specific functions and processes, most prominently in connection to thermodynamic and kinetic properties of membrane proteins.7,8 Cellular membranes vary in different cells, and even in various cellular compartments/organelles,9 or during different phases of the life of a cell, again, pointing at their effect on the function.10
Experimental techniques such as mass spectrometry and optical spectroscopy have been used to determine the overall lipid compositions (molar ratios) of different biological membranes,9,11,12 though the precise spatial distribution of lipids within a membrane and their change during different biological activities of the cell are much more challenging to obtain experimentally.9,13 Although advances in experimental techniques have provided invaluable information about structure, dynamics, and roles of membranes, atomic-level details and how they achieve particular properties remain unresolved, as they require techniques that offer higher spatial and temporal resolutions at the same time.14,15 Examples of such properties include how asymmetric transmembrane distribution of anionic phospholipids or cholesterol might affect protein dynamics and function,16 the details governing formation of nanodomains, 17 or spatial distribution of different lipid types around membrane proteins.18
Molecular dynamics (MD) simulation has been among the most effective biophysical tools to extract high-resolution information on lipids in membranes, to interpret experimental observations, and to characterize novel phenomena in these complex molecular systems.14,15,19,20 The level of details provided by MD outperforms experimental technique.14 Substantial improvements in force fields and the simulation methodologies have allowed for most accurate descriptions of complex membranes in the last decade.19,21-23 The slow dynamics of lipids on MD timescales, especially when simulated at an atomic level, however remain a challenge, preventing one from reaching fully equilibrium states on affordable timescales. In order to alleviate this problem to some degrees, one can randomize the initial arrangement of lipids in multiple replicas of a simulation, thereby, sampling more of the configuration space, a protocol that has become common among researchers, especially motivated by availability of computational resources that promote multi-replica simulations.
In modeling biological membranes by MD, several steps are taken before the simulations.24 The first is to construct an initial configuration for the constituent lipids, taking into account the target molar ratios of lipids and their positions.14,21 A number of existing tools can assist the user to generate such initial configurations for complex membranes with various shapes and at different resolutions (e.g., all-atom or coarse-grained).25,26 These tools rely on a variety of techniques for initial placement of lipids within the membrane.26
A widely used web-based platform, CHARMM-GUI, for example, interactively guides the user in building the system and conveniently prepares simulation input files for a variety of MD engines.22,27 PACKMOL-Memgen28 is another tool that can generate complex membrane systems using Packmol29 as the packing engine. MemBuilder is yet another web-based tool that generates lipid bilayers, micelles, and liposomes, though only for a limited selection of lipids and force fields.30 VMD,31 a popular molecular visualization and analysis environment, can be employed through its Membrane Builder plugin to generate single-lipid bilayers as well. Coarse-grained membrane systems can be constructed with INSANE19 or through web-based utilities such as MERMAID.32 Furthermore, there are online databases that store ready-to-use and even pre-equilibrated lipid bilayers.33,34 Such plethora of tools have been helpful in generating the initial configurations of a membrane for MD simulations. However, most of these require long preparation procedures and often many “mouse clicks” through the graphical user interfaces (GUI), especially when the user attempts to include proteins in the bilayer. The difficulty becomes more evident for web-based resources when a high level of optimization for the final membrane is requested.
As stated above, modern MD studies have adopted an approach in which lipid sampling can be improved by replicating the simulation system using membranes with different initial lipid placements (same lipid composition but different initial lipid configurations).15,35,36 Such a practice reduces bias due to the initial lipid configuration and conformation, e.g., in cases where one aims at probing the interaction between a membrane-embedded protein and specific signaling lipids, and to characterize structural and functional implications associated with the phenomenon.37 However, generating a large number of initial lipid configurations using currently available tools can be time consuming.
In this work, we introduce a novel protocol to rapidly generate membrane replicas with the same ratio but shuffled positions of lipids, allowing one to obtain many initial configurations within minutes. Once an initial membrane with the desired lipid composition is generated (e.g., by one of the tools described above), our algorithm generates as many replicates as desired, each with a different initial lipid positioning while maintaining the composition. This is achieved by exchanging the positions of lipid pairs at a level specified by the user, which maintains the lipid ratio of the membrane. Our fully-automated tool generates and uses a specifically designed external potential (termed here the “carving potential”) to remove steric clashes before exchanging the position of the lipids. Given its robust design, the tool allows for exchange of any lipid types in the membrane, irrespective of their shapes, molecular weights, conformations, and charge states. The employed carving potential is key to this process, as it generates the space required for a successful exchange without encountering high energy penalties. It is noteworthy that the protocol described here is only intended to provide different initial membrane configurations for MD simulations. Further production simulations of the generated systems will be needed to represent thermodynamical properties of the membrane.
We report several example applications of the technique including commonly used MD systems, namely, a protein-embedded membrane, a Nanodisc, and a ternary lipid bilayer with nanodomains. Analyses performed on the shuffled membranes show that the method can reliably generate lipid bilayers with virtually indistinguishable physicochemical properties from the initial membranes. The method has been implemented as a plugin in the latest version of VMD: “Membrane Mixer Plugin” (MMP).
Methods
The underlying protocol
MMP is designed to generate multiple initial configurations (replicas) of a membrane, with any lipid composition, for all-atom MD simulations, rapidly and conveniently. Besides lipids, the simulation system may contain other species such as water, ions, ligands, and even proteins. The protocol randomly shuffles lipids, and even lipid-like molecules when specified, in the membrane to generate new arrangements while preserving the lipid composition. Currently, the MMP plugin is only compatible with flat bilayers, as during the protocol the membrane normal is aligned with the z axis of the simulation cell, a common setting in MD simulations of membranes and membrane proteins.
The MMP lipid shuffling protocol consists of several steps, each involving geometrical and/or configurational modifications of lipids assisted by MD simulations (Figure 1):
Figure 1:
Different steps of the MMP shuffling protocol. (1) An initial lipid bilayer is generated outside MMP with a desired lipid composition. (2) Lipid pairs for exchange are selected based on a degree of shuffling and lipid selection specified by the user. (3) MMP generates a carving potential based on the shape of the incoming lipid. (4) Subsequently, minimization and MD simulation with the carving potentials remove potential clashes and entanglements before the incoming lipid is moved to its new location. (5) The selected lipid pair is shuffled. (6) The external carving potentials are removed gradually in a post-shuffling MD simulation to produce a relaxed, clash-free bilayer. Molecular images exemplify a lipid bilayer composed of two lipid types, A (orange) and B (cyan). Shuffling is illustrated for a single lipid pair (red). The carving potential is shown in yellow.
(1) The protocol starts with a series of sanity checks, ensuring all the input files and parameters provided by the user are in the correct format and range. Furthermore, at this step the initial membrane is centered (midplane set to z = 0), in order to facilitate the detection of the two leaflets. This treatment is needed as the lipid shuffling events are intentionally restricted to only lipids within the same leaflet, preserving the lipid composition of each leaflet, and therefore any desired asymmetry.
(2) Next, a number of lipid pairs (specified by the user) are randomly selected and tagged for shuffling. Any two lipids (of any type) within the same leaflet can be selected for exchange; the lipids in an exchange pair can have different types (e.g., cholesterol/phospholipid) or similar types (e.g., two phospholipids with different conformations).
(3) Simple swapping of the lipid pairs would almost always result in significant steric clashes, and even entanglements or ring piercing, which can be impossible to resolve by brute force MD. In order to avoid such issues, each lipid shuffling is preceded by a “carving” step involving minimization and a short (a few picoseconds) MD simulation, during which a repulsive potential (corresponding to the shape/conformation of the incoming lipid) is placed on the target position of the incoming lipid, thereby promoting overlapping atoms from the surrounding molecules to leave the space. The interpolation38 between these repulsive potentials provides a volumetric potential representing the shape of incoming lipid. The carving potentials is generated in VMD by employing the Molecular Dynamics Flexible Fitting (MDFF) plugin.39 The repulsive forces produced by the carving potential during minimization and the short MD simulations do not act on the shuffled lipids, rather on the surrounding molecules, and are tuned by a scaling factor, which is gradually reduced to zero during subsequent steps of the protocol. This key step ensures that the shuffling is successful even for lipids of very different sizes/shapes.
(4) The carving step is then followed by the shuffling step in which positions of lipid pairs are switched. During the shuffling step and for each lipid pair, MMP measures the center of mass (COM) of both molecules in the last configuration of the MD simulation at Step (3) and shuffles the two lipids by swapping the xy components of their COM. By default, the COM z coordinate is kept unchanged to avoid forcing different lipid types to unnatural insertion depths in the membrane (e.g., when exchanging a cholesterol with a phospholipid). If desired, the user can override this and exchange all components of the COM at this step.
(5) The shuffling step is followed by a short MD simulation (on the order of picoseconds) during which the carving potential is gradually removed. This step partially relaxes the membranes and fills out the potential gaps introduced during the shuffling step.
(6) As the last step, MMP examines the system for remaining ring-piercing issues by checking for unusually long bonds (longer than 2.5 Å), a common symptom of ring piercing. If found, MMP attempts fixing the issue by moving the two atoms of the piercing bond by 3 Å in a random direction, so they move out of the pierced ring, and then minimizing the whole system. The procedure is repeated until all piercing issues are removed. Furthermore, at this step the plugin uses tools available in VMD to check for other potential problems, such as inversion of chiral centers or cis peptide bonds in proteins, and reports them in the log file.
All the steps are fully automated, and an extensive progress is logged in a text file for the user.
Graphical user interface
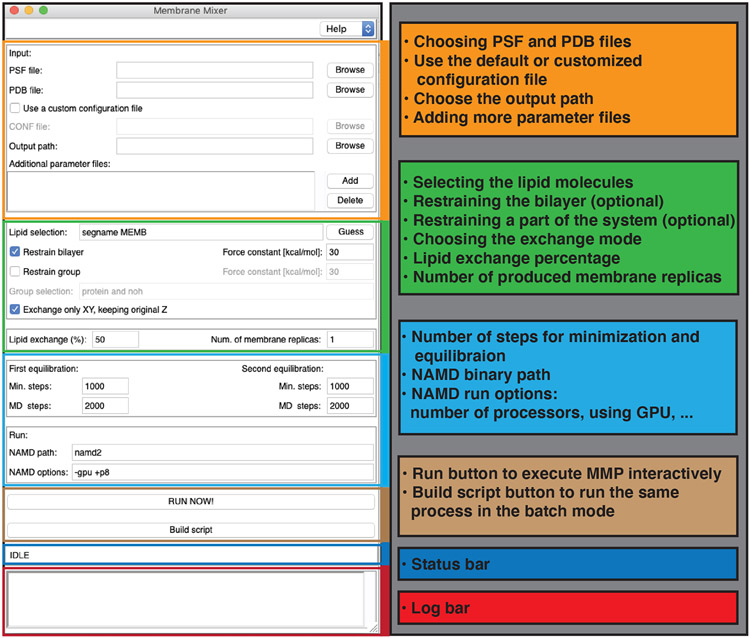
The GUI environment of MMP can be accessed from the Extensions → Modeling menu in VMD (Figure 2). The GUI is designed and built around a series of TCL routines. It provides a comprehensive interface, e.g., for modifying default options and settings and specifying the required files, via common software interaction paradigms using buttons, menus, and file dialogs. Tasks are grouped in different sections within MMP (Figure 2).
Figure 2:
The MMP GUI in VMD. The first section (orange) sets the basic files (PSF and PDB files, any additional parameter files, and NAMD configuration file for MD simulations) and chooses an output path. The second section (green) is used to select the pool of lipids considered for shuffling, to restrain the bilayer and/or additional groups (e.g., protein), and selects the exchange mode (full COM or COMxy-only exchange). The lipid exchange percentage and number of membrane replicas can also be specified here. MD simulation parameters and NAMD runtime options are set in the next section (light blue). In the next section (brown) the user specifies the interactive or batch mode of lipid shuffling. The last two sections are used for the status bar (dark blue) and log reports (red), e.g., warnings and errors.
MD simulations are performed using NAMD,40,41 which needs to be available in the user’s computer environment, but is automatically started in the background. Generally, only PSF (topology) and PDB (coordinates) files are required for MMP, as other files (e.g., NAMD configuration file and simulation parameter files) are generated automatically within the plugin. However, the I/O section of the GUI (Figure 2, orange box) allows the user to specify the location of the files to be used in the MD steps, e.g., additional CHARMM force field parameters. Customized files are especially beneficial for systems with non-standard residues, or for applying special restraints and biases. The user can also provide a path where all the generated files will be stored.
The second section (Figure 2, green box) is used to specify the lipid pool for shuffling. To make the lipid pool known to MMP, users can either specify the lipids through specific selections in their simulated system, or use the “Guess” button (Figure 2, the green section) to automatically select lipids according to their conventional names (CHARMM parlance). The z positions of lipid headgroups are restrained by default throughout the protocol, in order to prevent potential artifacts by the carving potential, e.g., separation of the two bilayer leaflets. In addition, other groups of atoms can be tagged for restrains, an option highly desirable, e.g., for restraining membrane proteins or preventing their large conformational change during membrane embedding. Alternatively, the user can provide a NAMD configuration file with the restraining potentials. The shuffling protocol can be performed either by exchanging only the xy components of the COM (recommended setting) or by exchanging the full COM of the lipids. Furthermore, in this section, the user is asked to specify the exchange percentage, which determines the number of lipids from the “Lipid Pool” to be shuffled. For instance, if the lipid pool includes a total of 100 lipids, a “Lipid Exchange” of 80% will shuffle 80 lipid pairs (40 pairs per leaflet). Here, the total number of membrane replicas to be generated is also specified.
A section of the GUI is dedicated to setting up the minimization and MD steps used during the protocol (Figure 2, cyan box). The settings may be tuned according to the size of the system and available compute resources. While longer relaxation simulations are generally preferred and can generate better membranes, one should limit the simulations performed under carving potentials, which can cause artifacts when run for too long. Other simulation details, the path to the NAMD binary, and additional command-line options are specified in this section.
The algorithm can be invoked interactively in a live VMD session, or alternatively, the user can store the details in a full batch script and run it on other platforms, e.g., on a cluster (Figure 2, brown box), to gain additional performance. The last section of the GUI provides status and log windows, which communicate on the fly information on the execution progresses and any errors or warnings (Figure 2, blue and red boxes).
MMP runs until the desired number of membrane replicas are generated. It skips replicas encountering problems during the process (e.g., if NAMD crashes in an early stage). Any skipped replica or detected issues are reported in the log file. New membrane configurations and associated files are organized into separate folders created automatically.
The interactivity of the VMD environment is not affected by the MMP execution, allowing the user to continue manipulating the molecular system. In addition, the algorithm will display the lipid selection for exchanges, the grid potentials used for the carving step, and ring piercing issues. Furthermore, it updates the geometry of the system with the final coordinates once the the MD steps are completed.
It is advised that the final models be inspected for complicated geometrical conflicts that cannot be automatically detected and resolved, for example, when lipid tails cross over protein loops. In the case of these rare events, it might be easier to simply discard the problematic membrane replica and generate a new one by additional MMP iterations.
Molecular dynamics protocol
MD simulations described in Results and Discussion were performed using NAMD2.40,41 The fully atomistic CHARMM36 force field42,43 was used in all simulations. TIP3 44 was employed for water molecules. A 12-Å cutoff was used for short-range nonbonded interactions, with a switching function starting at 10 Å. Long-range electrostatic interactions were calculated using the particle mesh Ewald (PME) method45 with a grid density of 1 Å−3 and a PME interpolation order of 6. All bonds involving hydrogen atoms were kept rigid with the SHAKE algorithm.46 Temperature was set to 310K by using Langevin thermostat with a damping coefficient of 1.0 ps−1. Pressure was maintained at 1 atm by Nosé-Hoover Langevin piston barostat (period: 50 fs, decay: 25 fs).47,48 Nanodisc simulations were performed in a fixed box while all other simulations were run in a flexible cell, allowing the dimensions of the periodic cell to change independently while keeping the cell aspect ratio in the xy plane fixed. The simulation timestep was set to 2 fs. Lennard-Jones and PME forces were updated every timestep. Carving potentials were generated using the MDFF plugin of VMD with resolution and spacing of 6 and 0.8 Å, respectively.39 The carving potential is applied only to tagged atoms that have a force multiplier of 1 (occupancy column) and a charge of −1 (repulsion from the carving potential, β column) specified in an ad hoc PDB file. Biased simulations with carving potentials were performed using the grid-steered MD module38 in NAMD.40,41 During the first MD relaxation of the MMP protocol, a scaling factor of 10 was applied to the grid-potential values. The force scaling was lowered to 4 at the beginning of the MD simulation in the second step, and then gradually reduced to zero in the following MD steps (Figure 2, cyan box). MD trajectories were visualized with VMD.31
Simulation system preparation
A total of four systems were prepared for test purposes: i) an N-palmitoyl-D-erythro-sphingosylphosphorylcholine/cholesterol (SM/CL) membrane (7:3 molar ratio, 23K atoms); ii) a P-glycoprotein (Pgp) embedded in a 1-palmitoyl-2-oleoylphosphatidylcholine (POPC)/CL membrane (1:1 molar ratio, 289K atoms); iii) a nanodisc composed of POPC/1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE) binary mixture (1:1 molar ratio, 286K atoms); and, iv) a phase-separated ternary lipid bilayer containing 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), SM and CL lipids (50:39:11 molar ratio, 186K atoms).
The initial membrane configurations for Systems i-iii, including water and 150 mM salt, were generated using CHARMM-GUI,24,27,49 whereas iv was obtained from a previous work.50 Before executing MMP, Systems i-iii were simulated using the default CHARMM-GUI protocol, as follows. membranes were first minimized for 10,000 steps and pre-equilibrated for 2.5 ns with restraints on protein backbones and lipid headgroups, in order to relax lipid tails and protein side chains. This step was followed by a 1-ns unrestrained simulation. The resulting configurations were then used as starting snapshots for MMP.
The exchange protocol was applied three times to each of Systems ii and iii with a lipid exchange percentage of 50%. System iv was subjected to various exchange percentages (0, 20, 40, 60, and 80%), with applying the shuffling protocol three times for each specific exchange percentage, generating a total of 15 independent systems with different lipid configurations. Default MMP settings were used for all membrane generations: 1,000 steps of minimization before each MD simulation, followed by 2,000 and 12,000 MD steps in the first and second MMP relaxation steps, respectively, along with positional restraints on lipid headgroups and protein heavy atoms (k = 30 kcal/mol/Å2). In order to better examine the configurations generated by MMP, control simulations were performed in which the original systems were simulated without lipid shuffling. All MMP-generated replicas and control systems were simulated for 20 ns without any restraints, and the last 10 ns of each simulation was used for analysis, except from the mass distributions which were calculated using the entire trajectory. We intentionally avoided long simulations here, since at long timescales differences between the initial membrane models might be completely obscured. The employed 20-ns simulations should provide sufficient sampling to allow meaningful comparison of the original and MMP-generated membranes.
Analysis
Various membrane properties including area per lipid, interdigitation, thickness, mass density, and radius of gyration (rg, only for the Nanodisc system) were extracted using MEM-BPLUGIN51 in VMD or customized scripts. Analysis of membranes was performed on trajectories after centering the membrane along the z axis. Mass interdigitation reported here is a correlation-based fraction that accounts for mass overlap between the two leaflets:
with ρi(z) representing the mass density of each leaflet (a or b) along z.
A single Gaussian was fit to the distribution of each property, both for MMP-generated and control systems. Three-fold replicates of each system were merged together into one histogram before performing the fit. In order to compare the cases more readily, membrane properties are reported relative to control membranes (no shuffling) by subtracting control means from histograms. Therefore, the generated replicas should produce distributions with (or close to) zero means if they are indistinguishable from control membranes.
To further illustrate the shuffling efficiency of MMP, the entropy of mixing Smix was calculated for MMP-generated membranes starting from a phase-separated membrane at different exchange percentages. For this purpose, the xy plane of the simulation box was divided into 17 × 17 = 289 cells (17 cells per axis). Lipid molecules in each cell were counted and Smix was calculated as:
where Ncell is the number of cells and pi is the probability of finding a lipid type in cell i.52
Results and Discussion
Effective lipid shuffling empowered by a carving potential
To gauge the effectiveness of the MMP protocol in generating healthy membranes suitable for MD simulations, we start with one lipid exchange per leaflet in an SM/CL binary membrane. MMP was performed on all three lipid-pair combinations, i.e, SM/SM, CL/CL, and SM/CL exchanges, in separate runs. A key methodological step implemented in MMP is the use of an external (carving) potential to substantially reduce clashes/entanglements between molecules during the lipid shuffling. To test the effect of this step, in a separate set of systems (control), we deactivated the carving potential (turning off the external potential) during the lipid shuffling process. Thus, a total of six membranes were generated to compare the membrane behavior after lipid shuffling.
The total energy of the system (Figure 3A), which may be viewed as a metric of steric clashes, can substantially increase (e.g., by ~1,000 kcal/mol for the SM/SM and SM/CL exchanges in our example), if the carving potential is not used during lipid shuffling (Figure 3A). This considerable increase is caused by multiple clashes that appear near the shuffled lipids (Figure 3B). By repelling the surrounding lipids, the external carving potential prepares the space for the incoming lipid, thus reducing substantially the total energy of the system (Figure 3C). In the example described here, the exchange of one CL with an SM lipid also resulted in a ring piercing, which further increases the energy, and is often impossible to remove by simple MD (Figure 3B).
Figure 3:
Single lipid exchange (per leaflet) in an SM/CL membrane (molar ratio 7:3) using MMP. A) Total energy of the system during MD simulation after lipid shuffling using the MMP protocol (1,000 minimization and 12 ps MD relaxation) with (green) or without (orange) applying the carving potential, for SM/SM, CL/CL, and SM/CL exchanges. An offset of 70,000 kcal/mol was used to ensure all energy values are positive. The yellow and purple backgrounds correspond to the minimization and MD steps, respectively. An enlarged view of the last 2 ps is shown on the right. Application of the carving potential generates a more clash-free membranes as indicated by ~1,000 kcal/mol lower total energies in SM/SM and SM/CL systems. Molecular views of a exchanged SM (yellow) and CL (blue) lipids without (B) and with (C) the carving potential (red mesh), along with other lipids within 3 Å.
At the minimization stage, the energies seem to converge, regardless of the use of the external potential (Figure 3A left panels). Note, however, that the example exchange here is only applied to one lipid pair per leaflet, while a typical MMP application may shuffle tens of lipids. Without the application of the carving potential, such exchange percentages will result in substantially higher-energy systems.
Conventional protein-membrane system
Key processes in a cell, e.g., selective transport of material, are mediated by membrane proteins. The structure and function of these proteins are particularly sensitive to and affected by the lipids in a bilayer, either directly or indirectly.15 Pgp is a membrane protein from the ATP-binding cassette (ABC) transporters superfamily which is involved in active export of molecules from the cell and a key component of resistance to chemotherapeutic agents.53-55 Here, MMP was used to replicate a Pgp-embedded POPC/CL membrane with an exchange rate of 50% (Figure 4A, B). Membrane thickness, interdigitation, total area of the simulation box in xy plane, and normalized mass density were calculated in order to examine the effectiveness of the MMP lipid shuffling protocol in producing healthy membranes (Figure 4C-F and S1A-C).
Figure 4:
Lipid shuffling in a protein-embedded membrane. (A) Top and (B) bottom views of the Pgp-embedded binary membranes composed of POPC (green) and CL (blue) in the original (blue box) and in three MMP-generated configurations (orange box). The two halves of Pgp are shown in red and yellow. (C) membrane thickness, (D) interdigitation, and (E) Total area, fitted to Gaussians, are shown for the control system (dashed lines) and for the MMP-generated membranes (solid lines). Histograms for the distribution of these properties are also shown for the MMP systems. (F) Normalized mass densities for the phosphorus atoms of phospholipids, oxygen atoms of cholesterols, and water for the control (dashed lines) and MMP generated systems averaged over all three replicas (solid lines).
Average (μ) membrane thickness of the MMP replicas deviates from the control system by less than 0.16 Å (0.35%). Similarly, interdigitation and total area show negligible differences with respect to the control (μ = −0.012 (5.7%) and −0.106 Å2 (0.21%), respectively). In all cases, average distributions of the control membrane fall within one standard deviation (σ) of the distribution of the replicas generated by MMP (Table S1). This indicates that MMP preserves the basic physico-chemical properties of the original membrane. The small deviations in thickness, interdigitation, and total area are likely caused by the carving potential during the MMP protocol. Although a restraint on the z position of the lipid headgroups was imposed, a repulsive grid potential can cause a slight separation of the two leaflets, which will however be readily relaxed with a short MD simulation. To avoid such a case, a higher force constant for headgroup restraints can be opted.
Finally, the distributions for phosphorus atoms of phospholipids and oxygen atoms of cholesterols match almost perfectly between the original and the MMP membranes (Figure 4F), with typical peaks and troughs highlighting the lipid boundaries (Figure 4F). Water density profiles are also similar for the systems and go to zero at the center of the membrane, corroborating the absence of water penetration into the membrane during the MMP shuffling protocol (Figure 4F cyan lines). In general, membrane properties of MMP membranes largely coincide with and are indistinguishable from those of the original membrane.
The original Pgp system was built in CHARMM-GUI (all steps, including generation of NAMD input files) in about 1 hour and 40 minutes, whereas each MMP replica here was generated in ~17 minutes (on a TESLA K80 GPU with 16 Intel Xeon Processor E5 v3 CPUs).
Lipid shuffling in Nanodiscs
Nanodiscs offer a convenient platform to study membrane-associated phenomena and proteins in solution, due to their bilayer-like structure.56,57 Nanodiscs are perhaps best described as lipids forming a bilayer patch whose hydrophobic edges are covered by a belt provided by a scaffold protein. They can be composed of a wide range of lipid types and ratios.56,57 Given their importance, MMP was designed to also work with Nanodiscs. Here, we use MMP to mix the lipids in a pre-constructed Nanodisc and replicate its POPC/POPE binary mixture in three additional Nanodiscs with the same lipid composition (Figure 5A, B).
Figure 5:
MMP replication of Nanodiscs. (A) Top and (B) bottom views of a Nanodisc composed of POPC (green) and POPE (red) in the original (blue box) and MMP-generated configurations (orange box, three replicas). The two Nanodisc scaffold proteins are shown in orange and blue. (C) Membrane thickness, (D) interdigitation, (E) radius of gyration, and (F) normalized mass density are shown as Gaussian fits for the control (dashed lines) and MMP-generated replicas (solid lines). Histograms of data from all three MMP replicas (collectively) are also shown. (F) Normalized mass densities of the phosphorus atoms of phospholipids for the control (dashed lines) and MMP generated systems averaged over all three replicas (solid lines).
MMP effectiveness and accuracy in generating simulation-ready Nanodisc replicates were tested by analyzing thickness, interdigitation, radius of gyration (rg) and normalized mass density in the generated Nanodiscs with 50% shuffling rate (Figure 5C-F and S2A-C). Average differences (with respect to control) for thickness and interdigitation are only −0.178 Å (0.46%) and 0.028 (7.8%), respectively, indicating negligible differences between the MMP replicas and the original Nanodisc. rg, used as an additional geometric parameter for the Nanodiscs, shows a variation of only 0.180 Å (0.36%), which is within one standard deviation of the Gaussian fit (Table S2). These small deviations from the original system will be readily and rapidly eliminated by short MD simulations (Figure S2). We note that the pseudo-bimodal distribution observed for rg (Figure 5E) is due to the low number of replicas in the sample (3 MMP replicas). The distribution would converge to a single-peaked one after adding more replicas. Important is that all rg values are within one standard deviating from the control system, indicating close similarity of MMP and control membranes.
Normalized mass densities of phosphorus atoms were extracted from the Nanodisc simulations (Figure 5F). The density is symmetric with respect to z due to the symmetry of the Nanodisc. The densities peak at −20 Å and 20 Å for both control and MMP replicas. Each Nanodisc structure was generated by MMP in only ~9 minutes (compared to ~29 minutes with CHARMM-GUI).
Shuffling lipids in a phase-separated ternary bilayer
Sufficient mixing of lipids in phase-separated membranes can be a challenging task due to atomistic MD simulations and the slow diffusion of lipids.52,58 By providing a tool to rapidly shuffle lipids and generate simulation ready membranes, MMP can be used to alleviate the problem, for example, bringing a phase-separated membrane to a more mixed configuration. Highly dynamic nanodomains, composed of sterols and sphingolipids, have been shown to behave differently from their surrounding lipids and can affect cellular processes.17,59
We started from an initial lipid arrangement with a clear nanodomain representing a phase-separated system (Figure 6A). This test bilayer included a disordered DOPC phase along with an ordered SM/CL nanodomain.50 We used MMP to replicate the system at different exchange percentages (Figure 6A-E and S3A-E, exchange percentages: 0%, 20%, 40%, 60%, and 80%). The mean entropy (Smix), obtained from Gaussian fits to histograms, increases from 0.16 to 0.22 for MMP exchange percentages of 0% to 80%, respectively (Figure 6F and Table S3). The increase reflects more homogeneous and entropically-favorable distribution of lipids in the ternary bilayer after the MMP mixing, which results in removal of the nanodomain (e.g., in Figure 6E). Since MMP exchange protocol selects randomly the lipids to shuffle, the change in Smix with respect to the change in exchange percentage is not linear (Figure 6F). However, MMP is effective in disrupting clusters of lipids at high lipid exchange percentages and in generating homogeneously-distributed lipid bilayers, and therefore, it can be used in general to quickly scramble lipids in unmixed membranes.
Figure 6:
Lipid mixing in a phase-separated ternary mixture. Lipid mixing entropy (Smix) and top views of snapshots for phase-separated mixtures obtained applying MMP with different exchange percentages: A) 0%, B) 20%, C) 40%, D) 60%, and E) 80% (DOPC: gray, SM: yellow, CL: blue). Each distribution was obtained by merging the data from three independent replicas. F) The mean value obtained from the Gaussian fit to Smix distributions (solid lines in histograms) for each exchange percentage, with error bars representing the standard deviation.
Concluding Remarks
Multi-replicate simulations have become a common practice in the MD simulation community. They can improve the sampling of the configuration space and reduce the bias introduced by the initial modeling. Of particular importance to this work, membrane systems can significantly benefit from this practice, given the relatively slow diffusion of lipids, which often prevents one from reaching convergence in describing their positions and mixing during atomistic simulations. The slow diffusion of lipids in a single simulation can, for example, result conclusions about interaction of specific lipid types with certain regions of the protein that are biased by the initial placement of lipids. We have developed a new strategy to generate multiple replicas for any membrane, conveniently and rapidly, based on the concept of randomly shuffling positions of existing lipids, thereby preserving the lipid composition of the system. In order to minimize steric clashes, entanglements, and ring piercing, which might arise during shuffling of large, complex, lipid molecules, we devise an external carving potential that prepares the lipid sites for clash-free exchange before the lipid positions are switched.
The method is implemented as a plugin in VMD under the name “Membrane Mixer 1.0”, with a convenient GUI to set options and parameters, while taking advantage of the interactive, comprehensive molecular environment of VMD. The plugin is fully automated and generates any desired number of membranes at a faster rate than analogous tools. The functionality and effectiveness of the method is demonstrated in a few test cases, including a binary lipid bilayer, a protein-embedded system, a Nanodisc, and a phase-separated membrane. The properties of the MMP-generated membranes are virtually identical to their reference membranes, as shown by the analysis of common membrane properties. Efforts are ongoing to extend the plugin to support other simulation systems, such as monolayers, vesicles, and curved membranes.
Supplementary Material
Acknowledgement
This research is supported by National Institutes of Health grants R01-GM123455 and P41-GM104601 (to E.T.). Simulations in this study have been performed using allocations at National Science Foundation Supercomputing Centers (XSEDE grant number MCA06N060), and the Blue Waters Petascale Computing Facility of National Center for Supercomputing Applications at University of Illinois at Urbana-Champaign, which is supported by the National Science Foundation (awards OCI-0725070 and ACI-1238993) and the state of Illinois.
Footnotes
Data and Software Availability
MMP and its source code are available through VMD (version 1.9.4). The simulation data and analysis codes reported in this study are openly available at https://doi.org/10.5281/zenodo.5703715.
References
- (1).Wilkins M; Blaurock A; Engelman D Bilayer structure in membranes. Nature New Biology 1971, 230, 72–76. [DOI] [PubMed] [Google Scholar]
- (2).Casares D; Escribá PV; Rosselló CA Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. International Journal of Molecular Sciences 2019, 20, 2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Jacobson K; Liu P; Lagerholm BC The Lateral Organization and Mobility of Plasma Membrane Components. Cell 2019, 177, 806–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Anderson AG; Goddard III WA; Schröder P Quantum Monte Carlo on graphical processing units. Computational Physics Communications 2007, 177, 298–306. [Google Scholar]
- (5).Dehghani-Ghahnaviyeh S; Kapoor K; Tajkhorshid E Conformational changes in the nucleotide-binding domains of P-glycoprotein induced by ATP hydrolysis. FEBS Letters 2021, 595, 735–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).van Meer G; Voelker DR; Feigenson GW Membrane lipids: where they are and how they behave. Nature Reviews Molecular Cell Biology 2008, 9, 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Booth PJ; Riley ML; Flitsch SL; Templer RH; Farooq A; Curran AR; Chadborn N; Wright P Evidence that bilayer bending rigidity affects membrane protein folding. Biochemistry 1997, 36, 197–203. [DOI] [PubMed] [Google Scholar]
- (8).Kleinschmidt JH Folding kinetics of the outer membrane proteins OmpA and FomA into phospholipid bilayers. Chemistry and Physics of Lipids 2006, 141, 30–47. [DOI] [PubMed] [Google Scholar]
- (9).Brügger B Lipidomics: analysis of the lipid composition of cells and subcellular organelles by electrospray ionization mass spectrometry. Annual Review of Biochemistry 2014, 83, 79–98. [DOI] [PubMed] [Google Scholar]
- (10).Harayama T; Riezman H Understanding the diversity of membrane lipid composition. Nature Reviews Molecular Cell Biology 2018, 19, 281–296. [DOI] [PubMed] [Google Scholar]
- (11).Hidaka H; Hanyu N; Sugano M; Kawasaki K; Yamauchi K; Katsuyama T Analysis of human serum lipoprotein lipid composition using MALDI-TOF mass spectrometry. Annals of Clinical & Laboratory Science 2007, 37, 213–221. [PubMed] [Google Scholar]
- (12).Gidden J; Denson J; Liyanage R; Ivey DM; Lay JO Jr Lipid compositions in Escherichia coli and Bacillus subtilis during growth as determined by MALDI-TOF and TOF/TOF mass spectrometry. International journal of mass spectrometry 2009, 283, 178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Spector AA; Yorek MA Membrane lipid composition and cellular function. Journal of Lipid Research 1985, 26, 1015–1035. [PubMed] [Google Scholar]
- (14).Egberts E; Marrink S-J; Berendsen HJ Molecular dynamics simulation of a phospholipid membrane. European Biophysics Journal 1994, 22, 423–436. [DOI] [PubMed] [Google Scholar]
- (15).Muller MP; Jiang T; Sun C; Lihan M; Pant S; Mahinthichaichan P; Trifan A; Tajkhorshid E Characterization of Lipid–Protein Interactions and Lipid-Mediated Modulation of Membrane Protein Function Through Molecular Simulation. Chemical Reviews 2019, 119, 6086–6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Kobayashi T; Menon AK Transbilayer Lipid Asymmetry. Current Biology 2018, 28, R386–R391. [DOI] [PubMed] [Google Scholar]
- (17).Lingwood D; Simons K Lipid Rafts as a Membrane-Organizing Principle. Science 2010, 327, 46–50. [DOI] [PubMed] [Google Scholar]
- (18).Ge J; Elferich J; Dehghani-Ghahnaviyeh S; Zhao Z; Meadows M; von Gersdorff H; Tajkhorshid E; Gouaux E Molecular mechanism of prestin electromotive signal amplification. Cell 2021, 184, 4669–4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Wassenaar TA; Ingólfsson HI; Böckmann RA; Tieleman DP; Marrink SJ Computational Lipidomics with Insane : A Versatile Tool for Generating Custom Membranes for Molecular Simulations. Journal of Chemical Theory and Computation 2015, 11, 2144–2155. [DOI] [PubMed] [Google Scholar]
- (20).Velasco CA; Ghahnaviyeh SD; Pishkenari HN; Auth T; Gompper G Complex self-propelled rings: a minimal model for cell motility. Soft Matter 2017, 13, 5865–5876. [DOI] [PubMed] [Google Scholar]
- (21).Van der Ploeg P; Berendsen H Molecular dynamics simulation of a bilayer membrane. Journal of Chemical Physics 1982, 76, 3271–3276. [Google Scholar]
- (22).Wu E; Cheng X; Jo S; Rui H; Song K; Dávila-Contreras E; Qi Y; Lee J; Monje-Galvan V; Venable R; Klauda J; Im W CHARMM-GUI Membrane Builder Toward Realistic Biological Membrane Simulations. Journal of Computational Chemistry 2014, 35, 1997–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Souza PC; Alessandri R; Barnoud J; Thallmair S; Faustino I; Grünewald F; Patmanidis I; Abdizadeh H; Bruininks BM; Wassenaar TA, et al. Martini 3: a general purpose force field for coarse-grained molecular dynamics. Nature Methods 2021, 18, 382–388. [DOI] [PubMed] [Google Scholar]
- (24).Lee J et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. Journal of Chemical Theory and Computation 2016, 12, 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Javanainen M; Martinez-Seara H Efficient Preparation and Analysis of Membrane and Membrane Protein Systems. Biochimica et Biophysica Acta – Biomembranes 2016, 1858, 2468–2482. [DOI] [PubMed] [Google Scholar]
- (26).Sommer B Membrane Packing Problems: a Short Review on Computational Membrane Modeling Methods and Tools. Computational and Structural Biotechnology Journal 2013, 5, e201302014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Jo S; Kim T; Iyer VG; Im W CHARMM-GUI: a Web-based Graphical User Interface for CHARMM. Journal of Computational Chemistry 2008, 29, 1859–1865. [DOI] [PubMed] [Google Scholar]
- (28).Schott-Verdugo S; Gohlke H PACKMOL-Memgen: a Simple-To-Use, Generalized Workflow for Membrane-Protein–Lipid-Bilayer System Building. Journal of Chemical Information and Modeling 2019, 59, 2522–2528. [DOI] [PubMed] [Google Scholar]
- (29).Martínez L; Andrade R; Birgin EG; Martínez JM PACKMOL: a Package for Building Initial Configurations for Molecular Dynamics Simulations. Journal of Computational Chemistry 2009, 30, 2157–2164. [DOI] [PubMed] [Google Scholar]
- (30).Ghahremanpour MM; Arab SS; Aghazadeh SB; Zhang J; van der Spoel D MemBuilder: a Web-based Graphical Interface to Build Heterogeneously Mixed Membrane Bilayers for the GROMACS Biomolecular Simulation Program. Bioinformatics 2013, 30, 439–441. [DOI] [PubMed] [Google Scholar]
- (31).Humphrey W; Dalke A; Schulten K VMD: Visual Molecular Dynamics. Journal of Molecular Graphics 1996, 14, 33–38. [DOI] [PubMed] [Google Scholar]
- (32).Damre M; Marchetto A; Giorgetti A MERMAID: Dedicated Web Server to Prepare and Run Coarse-Grained Membrane Protein Dynamics. Nucleic Acids Research 2019, 47, W456–W461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Ermilova I; Lyubartsev AP Extension of the Slipids Force Field to Polyunsaturated Lipids. Journal of Physical Chemistry B 2016, 120, 12826–12842. [DOI] [PubMed] [Google Scholar]
- (34).Jämbeck JPM; Lyubartsev AP Another Piece of the Membrane Puzzle: Extending Slipids Further. Journal of Chemical Theory and Computation 2013, 9, 774–784. [DOI] [PubMed] [Google Scholar]
- (35).Chavent M; Duncan AL; Sansom MS Molecular Dynamics Simulations of Membrane Proteins and Their Interactions: from Nanoscale to Mesoscale. Current Opinion in Structural Biology 2016, 40, 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Hedger G; Sansom MS Lipid Interaction Sites on Channels, Transporters and Receptors: Recent Insights from Molecular Dynamics Simulations. Biochimica et Biophysica Acta 2016, 1858, 2390–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Wu EL; Fleming PJ; Yeom MS; Widmalm G; Klauda JB; Fleming KG; Im WE coli Outer Membrane and Interactions with OmpLA. Biophysical Journal 2014, 106, 2493–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Wells DB; Abramkina V; Aksimentiev A Exploring Transmembrane Transport Through α-Hemolysin with Grid-Steered Molecular Dynamics. Journal of Chemical Physics 2007, 127, 125101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Trabuco LG; Villa E; Schreiner E; Harrison CB; Schulten K Molecular Dynamics Flexible Fitting: a Practical Guide to Combine Cryo-Electron Microscopy and X-ray Crystallography. Methods 2009, 49, 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Phillips JC; Braun R; Wang W; Gumbart J; Tajkhorshid E; Villa E; Chipot C; Skeel RD; Kale L; Schulten K Scalable molecular dynamics with NAMD. Journal of Computational Chemistry 2005, 26, 1781–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Phillips JC et al. Scalable molecular dynamics on CPU and GPU architectures with NAMD. Journal of Chemical Physics 2020, 153, 044130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Hart K; Foloppe N; Baker CM; Denning EJ; Nilsson L; Mackerell AD Jr. Optimization of the CHARMM Additive Force Field for DNA: Improved Treatment of the BI/BII Conformational Equilibrium. Journal of Chemical Theory and Computation 2012, 8, 348–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Klauda JB; Venable RM; Freites JA; O’Connor JW; Tobias DJ; Mondragon-Ramirez C; Vorobyov I; MacKerell AD Jr.; Pastor RW Update of the CHARMM all-atom additive force field for lipids: Validation on six lipid types. Journal of Physical Chemistry B 2010, 114, 7830–7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Jorgensen W; Chandrasekhar J; Maudura JD; Impey RW; Klein ML Comparison of Simple Potential Functions for Simulating Liquid Water. Journal of Chemical Physics 1983, 79, 926–935. [Google Scholar]
- (45).Darden T; York D; Pedersen L Particle mesh Ewald: an N·log(N) method for Ewald sums in large systems. Journal of Chemical Physics 1993, 98, 10089–10092. [Google Scholar]
- (46).Ryckaert J-P; Ciccotti G; Berendsen HJC Numerical Integration of the Cartesian Equations of Motion of a System With Constraints: Molecular Dynamics of n-Alkanes. Journal of Computational Physics 1977, 23, 327–341. [Google Scholar]
- (47).Martyna GJ; Tobias DJ; Klein ML Constant pressure molecular dynamics algorithms. Journal of Chemical Physics 1994, 101, 4177–4189. [Google Scholar]
- (48).Feller SE; Zhang Y; Pastor RW Constant pressure molecular dynamics simulation: the Langevin piston method. Journal of Chemical Physics 1995, 103, 4613–4621. [Google Scholar]
- (49).Qi Y; Lee J; Klauda JB; Im W CHARMM-GUI Nanodisc Builder for Modeling and Simulation of Various Nanodisc Systems. Journal of Computational Chemistry 2019, 40, 893–899. [DOI] [PubMed] [Google Scholar]
- (50).Licari G; Strakova K; Matile S; Tajkhorshid E Twisting and Tilting of a Mechanosensitive Molecular Probe Detects Order in Membranes. Chemical Science 2020, 11, 5637–5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Guixa-Gonzalez R; Rodriguez-Espigares I; Ramirez-Anguita JM; Carrio-Gaspar P; Martinez-Seara H; Giorgino T; Selent J MEMBPLUGIN: Studying Membrane Complexity in VMD. Bioinformatics 2014, 30, 1478–80. [DOI] [PubMed] [Google Scholar]
- (52).Fathizadeh A; Elber R A Mixed Alchemical and Equilibrium Dynamics to Simulate Heterogeneous Dense Fluids: Illustrations for Lennard-Jones Mixtures and Phospholipid Membranes. Journal of Chemical Physics 2018, 149, 072325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Kim Y; Chen J Molecular Structure of Human P-glycoprotein in the ATP-Bound, Outward-Facing Conformation. Science 2018, 359, 915–919. [DOI] [PubMed] [Google Scholar]
- (54).Thangapandian S; Kapoor K; Tajkhorshid E Probing Cholesterol Binding and Translocation in P-glycoprotein. Biochimica et Biophysica Acta 2020, 1862, 183090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Dehghani-Ghahnaviyeh S; Kapoor K; Tajkhorshid E Conformational Changes in the Nucleotide-Binding Domains of P-glycoprotein Induced by ATP Hydrolysis. FEBS Letters 2020, [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Nath A; Atkins WM; Sligar SG Applications of Phospholipid Bilayer Nanodiscs in the Study of Membrane and Membrane Proteins. Biochemistry 2007, 46, 2059–2069. [DOI] [PubMed] [Google Scholar]
- (57).Denisov IG; Sligar SG Nanodiscs in Membrane Biochemistry and Biophysics. Chemical Reviews 2017, 117, 4669–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Chiu S-W; Jakobsson E; Subramaniam S; Scott HL Combined Monte Carlo and Molecular Dynamics Simulation of Fully Hydrated Dioleyl and Palmitoyl-oleyl Phosphatidylcholine Lipid Bilayers. Biophysical Journal 1999, 77, 2462–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).M’Baye G; Mély Y; Duportail G; Klymchenko AS Liquid Ordered and Gel Phases of Lipid Bilayers: Fluorescent Probes Reveal Close Fluidity but Different Hydration. Biophysical Journal 2008, 95, 1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.