Abstract
Objective:
To compare dementia incidence and prevalence after age 50 by HIV status
Design:
Observational cohort, 2000 to 2016
Methods:
People with HIV (PWH) on antiretroviral therapy (ART) and demographically-similar people without HIV (PWoH), all aged 50 years and older, were identified from Kaiser Permanente healthcare systems in Northern California, Southern California, and Mid-Atlantic States (Maryland, Virginia, Washington D.C.). Dementia diagnoses were obtained from electronic health records. Incidence and prevalence of dementia, overall and by time period (i.e., 2000–2002, 2003–2004, …, 2015–2016), were calculated using Poisson regression. Trends were examined using Joinpoint regression. Rate ratios were used to compare dementia by HIV status with adjustment for sociodemographics, substance use, and clinical factors.
Results:
The study included 13,296 PWH and 155,354 PWoH (at baseline: for both, mean age=54 years, 89% male; for PWH, 80% with HIV RNA <200 copies/ml). From 2000 to 2016, overall incidence of dementia was higher among PWH (adjusted incidence rate ratio [aIRR]=1.80, 95% CI=1.60–2.04). Dementia incidence decreased among both PWH and PWoH (−8.0% and −3.1% per period, respectively), but remained higher among PWH in the most recent time period, 2015–2016 (aIRR=1.58, 95% CI=1.18–2.12). The overall prevalence of dementia from 2000–2016 was higher among PWH (adjusted prevalence ratio [aPR]=1.86, 95% CI=1.70–2.04) and was also higher among PWH in 2015–2016 (aPR=1.75, 95% CI=1.56–1.97).
Conclusion:
Reductions in dementia incidence are encouraging and may reflect ART improvement, but PWH are still more likely to have dementia than PWoH. Monitoring the burden of dementia among PWH is important as this population ages.
Keywords: Dementia, neurocognitive disorder, antiretroviral therapy, cognitive impairment, comorbidity, substance use, aging
Introduction
With advances in HIV treatment, the characteristics of neurocognitive disorders among people with HIV (PWH) have changed [1–3]. Prior to the availability of antiretroviral therapy (ART), HIV-associated dementia was one of the most frequent diagnoses among people with uncontrolled HIV infection and was often associated with late-stage HIV disease, neurologic opportunistic infection, and poor prognosis [2, 4, 5]. The neurological benefits of ART are clear, as HIV-associated dementia rarely develops among those with well-controlled HIV [2]. However, cognitive impairments continue to affect 30–50% of PWH, indicating that ART use may not completely prevent or resolve neurologic complications associated with HIV infection [1, 6–10]. Most research to-date has focused on a spectrum of cognitive impairments among people with HIV, collectively called HIV-associated neurocognitive disorders (HAND) and ranging from asymptomatic impairment detectable only upon neuropsychological testing to severe impairment interfering with everyday activities [11]. Fewer studies have evaluated age-associated neurodegenerative disease including dementias such as Alzheimer’s disease and vascular dementia among PWH, but this is an important emerging concern given that most PWH in the United States (U.S.) are now greater than 50 years old [2, 12, 13].
While several studies report that the incidence and prevalence of dementia among older adults in the general U.S. population have decreased in recent years, possibly due to improvements in dementia-related risk factors such as cardiovascular disease [14, 15], these trends may not be reflected in the HIV population. In fact, given that PWH have a higher prevalence of dementia risk factors such as smoking, unhealthy alcohol use, and cardiovascular disease, the burden of neurocognitive impairment in this population is anticipated to increase as a greater proportion of PWH reach older ages [16, 17]. However, few studies have directly compared the epidemiology of dementia among PWH and age-matched uninfected individuals from the general population.
Improved clarity on the temporal trends and current burden of dementia among PWH will be vital to meet the cognitive care needs of an aging HIV population. ART-treated PWH now have life expectancies nearing those of people without HIV (PWoH) [18], but approaches to dementia prevention for the general population may not apply to cognitive impairments in the HIV context. Observational studies of HIV cohorts report that HAND is common despite ART use, but its characteristics and predictors have changed since the pre-ART era [1, 3, 7, 9, 19, 20]. Also, the clinical relevance of HAND has been debated as it encompasses a broad spectrum of cognitive impairments and may have less clinical importance than symptomatic dementia reported or detected in primary care [11, 21–23]. Further, studies on temporal changes in the epidemiology of cognitive impairments among PWH were conducted over 10 years ago, prior to recent ART advances, and were not powered nor designed to evaluate age-associated dementias given the young median age of the study populations and focus on the heterogeneous outcome of HAND [1, 24, 25].
In this study, we use data from three large healthcare delivery systems to describe the incidence and prevalence of clinically-apparent dementia diagnosed in routine primary care among ART-treated PWH aged 50 years and older.
Methods
Study design, setting, and participants.
This observational cohort study of PWH and PWoH included members of Kaiser Permanente (KP) health plans in Northern California, Southern California, and Mid-Atlantic States (Maryland, Virginia, Washington D.C.) between 2000 and 2016. These integrated healthcare systems provide comprehensive medical services to approximately 9.5 million members who are demographically similar to the insured adult population in the underlying catchment areas [26]. PWH were identified using regional KP HIV registries which capture all known cases of HIV/AIDS among KP members by monitoring laboratory databases, pharmacy records, and International Classification of Diseases (ICD)-coded diagnoses for indicators of HIV infection.
Study participants were selected from an established cohort of PWH and PWoH who were frequency-matched 1:10 by age, sex, race/ethnicity, medical facility, and year at baseline (i.e. start of follow-up) [18]. Follow-up began on the earliest date on or after January 1, 2000 that all study eligibility criteria were met. People were eligible for inclusion if they were ≥50 years old and had ≥1 year of continuous KP membership in the year before baseline. PWH were required to be on ART, defined as having ≥1 prescription fill for ART from a KP pharmacy in the year before baseline.
Individuals with pre-existing dementia at baseline were excluded from analyses of dementia incidence but included in analyses of dementia prevalence. For analyses of dementia incidence, follow-up ended on the earliest of incident dementia, death, health plan disenrollment, or end of the study (December 31, 2016). For analyses of dementia prevalence, follow-up ended on the earliest of death, health plan disenrollment, or end of the study. People who developed incident dementia during the study remained prevalent cases until the end of follow-up. A flow chart of PWH included in the study is shown in Supplemental Figure 1. The study protocol was approved by the Institutional Review Board at KP Northern California.
Dementia ascertainment.
Dementia diagnoses were identified from the electronic health record (EHR) using ICD codes, which included diagnoses of Alzheimer’s disease, vascular dementia, Parkinson’s dementia, dementia with Lewy bodies, frontotemporal dementia, and other/unspecified dementias (Supplemental Table 1). In a prior KP Northern California study, these ICD codes were confirmed via chart review to have comparable positive predictive value (PPV) for dementia in PWH (PPV=93%; 64/69) and PWoH (PPV=97%; 114/117; p=0.21), regardless of the diagnosing provider [27]. Incident dementia was defined as the first dementia diagnosis of any type occurring after ≥1 year of continuous KP membership during which time the patient received no dementia diagnosis.
Covariates.
Data were gathered from the EHR on: 1) sociodemographic factors, including age, sex, race/ethnicity, and Census-based neighborhood-level education, 2) substance use, including ever smoking, alcohol use disorder, and other substance use disorder, 3) cardiovascular disease, including cerebrovascular disease, peripheral vascular disease, heart failure, and coronary heart disease, and 4) other clinical factors, including hypertension, dyslipidemia, diabetes mellitus, obesity (body mass index ≥30 kg/m2), depression, and healthcare utilization (number of outpatient visits in the past year). For PWH, data were also gathered on CD4 and HIV RNA levels. Covariates are described in more detail in Supplemental Table 2.
Statistical analyses.
Dementia incidence and prevalence were evaluated by HIV status for the entire study period (i.e., 2000–2016) and by time period (i.e. 2000–2002, 2003–2004, 2005–2006, 2007–2008, 2009–2010, 2011–2012, 2013–2014, 2015–2016).
First, HIV-specific dementia incidence and prevalence were calculated in each time period using Poisson regression. To control for changing demographics over time, incidence and prevalence in each time period were standardized to the age and sex distribution of the overall study population (i.e., PWH and PWoH combined) in the first time period, 2000–2002. Temporal trends in standardized incidence rate (sIR) were evaluated using the Joinpoint Regression Program, version 4.8.0.1[28]. This method uses least squares regression to fit line segments to the natural log of the sIR, joined at discrete points identified by the software to represent statistically significant changes in direction of trend [29]. The best-fitting and simplest model was selected using a sequence of permutation tests with Monte Carlo sampling and Bonferroni correction for multiple testing. The average percentage change in sIR per period was calculated and pairwise comparability tests were performed to determine whether trends in dementia incidence and prevalence differed by HIV status [30].
Then, dementia incidence and prevalence were compared by HIV status overall and within individual time periods using rate ratios from unadjusted and covariate-adjusted Poisson regression models. To assess differences in the association of HIV with dementia incidence and prevalence by demographic subgroup, we examined covariate-adjusted models with terms for HIV*sex and HIV*race/ethnicity.
In sensitivity analyses, rate ratios were recalculated: 1) excluding PWH with detectable HIV RNA (>200 copies/ml) at baseline, as these individuals may have had suboptimal adherence to ART; and 2) excluding PWH with prior advanced immunodeficiency (CD4 cell count <200 cells/μl), which has been associated with long-term neurologic effects [12] and may be a marker for historical risk factors, such as prior untreated HIV infection, not captured by our adjustment for baseline clinical covariates. Analyses were conducted using Stata 17 (College Station, Texas, USA).
Results
The study included 13,296 PWH and 155,354 PWoH (Table 1). Participants were similar on the matching factors of age, sex, and race/ethnicity. The average age at baseline was 54 years (standard deviation [SD]=6 years), 89% of participants were male, and 53% of PWH and 51% of PWoH were non-Hispanic White. PWH were more likely than PWoH to have a history of substance use, cardiovascular disease, dyslipidemia, or depression, and were less likely to have hypertension, diabetes or to be obese. All PWH were on ART prior to baseline. At baseline, 80% of PWH were virally suppressed, 51% had a CD4 cell count of ≥500 cells/μl, and 36% had prior advanced immunodeficiency (CD4 cell count <200 cells/μl). PWH in the study had been living with HIV for an average of 9 years (SD=8 years).
Table 1.
Baseline characteristics of study population
| Characteristic | With HIV N=13,296 n (%) | Without HIV N=155,354 n (%) |
|---|---|---|
| Age, mean years (SD) | 53.9 (5.5) | 53.5 (5.5) |
|
| ||
| Male | 11,854 (89.2) | 137,990 (88.8) |
|
| ||
| Race/ethnicity | ||
|
| ||
| White, non-Hispanic | 7,073 (53.2) | 79,095 (50.9) |
| Black, non-Hispanic | 2,660 (20.0) | 32,237 (20.8) |
| Hispanic | 2,241 (16.9) | 25,835 (16.6) |
| Asian, non-Hispanic/Other | 1,322 (9.9) | 18,187 (11.7) |
|
| ||
| Lower neighborhood-level educationa | 2,109 (20.8) | 25,748 (20.3) |
|
| ||
| Ever smoking | 6,895 (51.9) | 64,917 (41.8) |
|
| ||
| Alcohol use disorder | 1,116 (8.4) | 9,473 (6.1) |
|
| ||
| Other substance use disorder | 1,430 (10.8) | 5,642 (3.6) |
|
| ||
| Cardiovascular diseaseb | 1,275 (9.6) | 12,865 (8.3) |
|
| ||
| Hypertension | 3,903 (29.4) | 47,955 (30.9) |
|
| ||
| Dyslipidemia | 6,219 (46.8) | 62,144 (40.0) |
|
| ||
| Diabetes | 1,444 (10.9) | 18,949 (12.2) |
|
| ||
| Obesityc | 1,704 (21.4) | 32,492 (41.4) |
|
| ||
| Depression | 3,944 (29.7) | 19,733 (12.7) |
|
| ||
| Number of outpatient visits in prior year, mean (SD) | 13.6 (16.6) | 5.7 (10.5) |
|
| ||
| Pre-existing dementia diagnosis | 249 (1.9) | 734 (0.5) |
|
| ||
| CD4 count (cells/μl)d | - | |
| ≥500 | 6,280 (50.9) | |
| 200–499 | 4,763 (38.6) | |
| <200 | 1,305 (10.6) | |
|
| ||
| Prior advanced immunodeficiencye | 4,821 (36.3) | - |
|
| ||
| HIV suppressionf | 9,977 (80.1) | - |
|
| ||
| Duration of HIV infection, mean years (SD) | 9.1 (7.7) | - |
Abbreviation: SD=standard deviation
Lower neighborhood-level education defined as ≥25% of the population in the patient’s Census block group having no high school diploma; % of known; 23.8% of people with HIV and 18.5% of people without HIV had unknown education.
Cerebrovascular disease, peripheral vascular disease, heart failure, and coronary heart disease.
Body mass index ≥30 kg/m2; % of known; 40.1% of people with HIV and 49.4% of people without HIV had unknown body mass index.
% of known; 7.1% of people with HIV had unknown CD4 count.
Ever CD4 count <200 cells/μl
HIV RNA <200 copies/ml; % of known; 7.3% of people with HIV had unknown HIV RNA levels.
Dementia incidence
At baseline, 249 (1.9%) PWH and 734 (0.5%) PWoH had pre-existing dementia and were therefore excluded from analyses of dementia incidence, resulting in a total of 13,047 PWH and 154,620 PWoH. During follow-up, 326 (2.5%) PWH and 2,006 (1.3%) PWoH were diagnosed with dementia, 3,896 (29.9%) PWH and 48,084 (31.1%) PWoH ended their KP membership, and 1,135 (8.7%) PWH and 6,332 (4.1%) PWoH died. At the end of follow-up, 7,690 (58.9%) PWH and 98,198 (63.5%) PWoH were still alive and without a diagnosis of dementia. PWH were followed for an average of 5.4 years (SD=4.6 years), and PWoH were followed for an average of 6.0 years (SD=4.8 years).
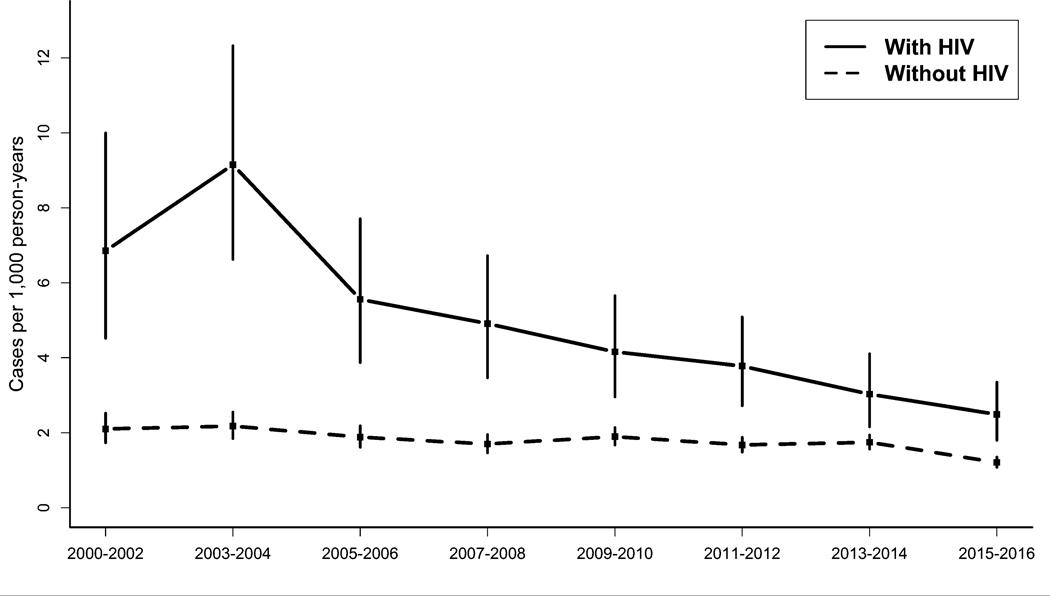
From 2000 to 2016, dementia incidence was higher among PWH than PWoH in all time periods but declined for both groups over time (Figure 1). Among PWH, dementia incidence decreased from an age- and sex-standardized IR (sIR) of 6.9 cases per 1,000 person-years [95% confidence interval=4.5 to 10.0] in 2000–2002 to 2.5 cases [1.8 to 3.4] in 2015–2016. Among PWoH, dementia incidence decreased from a sIR of 2.1 [1.7 to 2.5] in 2000–2002 to 1.2 [1.1 to 1.4] in 2015–2016. The average decrease in dementia incidence per period was significantly greater among PWH (−8.0% [−10.6% to −5.3%]) than PWoH (−3.1% [−5.4% to −0.8%], p-interaction <0.001).
Figure 1. Incidence of dementia by HIV status – Kaiser Permanente, 2000–2016.
Incidence estimates were standardized to the overall age and sex distribution in the time period 2000–2002. Vertical bars depict 95% confidence intervals.
The overall incidence of dementia (i.e. all time periods combined from 2000–2016) was higher among PWH after adjustment for sociodemographics, substance use, cardiovascular disease, and other clinical factors (adjusted incidence rate ratio [aIRR]= 1.8 [1.6 to 2.0]; Table 2). In comparisons of dementia incidence by HIV status within individual time periods, dementia incidence was 1.2 to 3.7 times higher among PWH in each period, and despite decreases in dementia incidence over time, remained higher among PWH in the most recent period, 2015–2016 (aIRR=1.6 [1.2 to 2.1]). The higher dementia incidence among PWH was similar by sex (p-interaction=0.84) and by race/ethnicity (p-interaction=0.36).
Table 2.
Incidence rate ratios of dementia in people with and without HIV, overall and by time period – Kaiser Permanente, 2000–2016
| With HIV | Without HIV | Incidence rate ratio (95% CI) | ||||
|---|---|---|---|---|---|---|
| Time period | Cases of dementia | Person-years of follow-up | Cases of dementia | Person-years of follow-up | Unadjusted | Adjusteda |
| 2000–2002 | 27 | 3,856 | 113 | 53,811 | 3.33 (2.19–5.08) | 2.54 (1.60–4.05) |
| 2003–2004 | 43 | 4,672 | 153 | 67,184 | 4.04 (2.88–5.67) | 3.66 (2.58–5.19) |
| 2005–2006 | 35 | 6,208 | 173 | 84,055 | 2.74 (1.90–3.94) | 2.37 (1.63–3.45) |
| 2007–2008 | 38 | 7,611 | 198 | 101,770 | 2.57 (1.81–3.63) | 2.04 (1.43–2.91) |
| 2009–2010 | 41 | 9,001 | 275 | 119,727 | 1.98 (1.43–2.75) | 1.64 (1.16–2.31) |
| 2011–2012 | 45 | 10,806 | 313 | 143,169 | 1.90 (1.39–2.60) | 1.69 (1.23–2.32) |
| 2013–2014 | 45 | 12,947 | 393 | 168,466 | 1.49 (1.09–2.03) | 1.24 (0.90–1.70) |
| 2015–2016 | 52 | 15,236 | 388 | 196,300 | 1.73 (1.29–2.31) | 1.58 (1.18–2.12) |
| Overall | 326 | 70,337 | 2,006 | 934,482 | 2.16 (1.92–2.43) | 1.80 (1.60–2.04) |
Adjusted for current age, sex, race/ethnicity, neighborhood-level education, ever smoking, alcohol use disorder, other substance use disorder, cerebrovascular disease, peripheral vascular disease, coronary heart disease, heart failure, hypertension, dyslipidemia, diabetes mellitus, obesity, depression, and number of outpatient visits in the year before baseline.
In sensitivity analyses excluding PWH with detectable HIV RNA at baseline or prior advanced immunodeficiency, overall dementia incidence was similarly elevated among PWH (aIRR=1.6 [1.4 to 1.9] and aIRR=1.7 [1.5 to 2.0], respectively; Supplemental Table 3).
Dementia prevalence
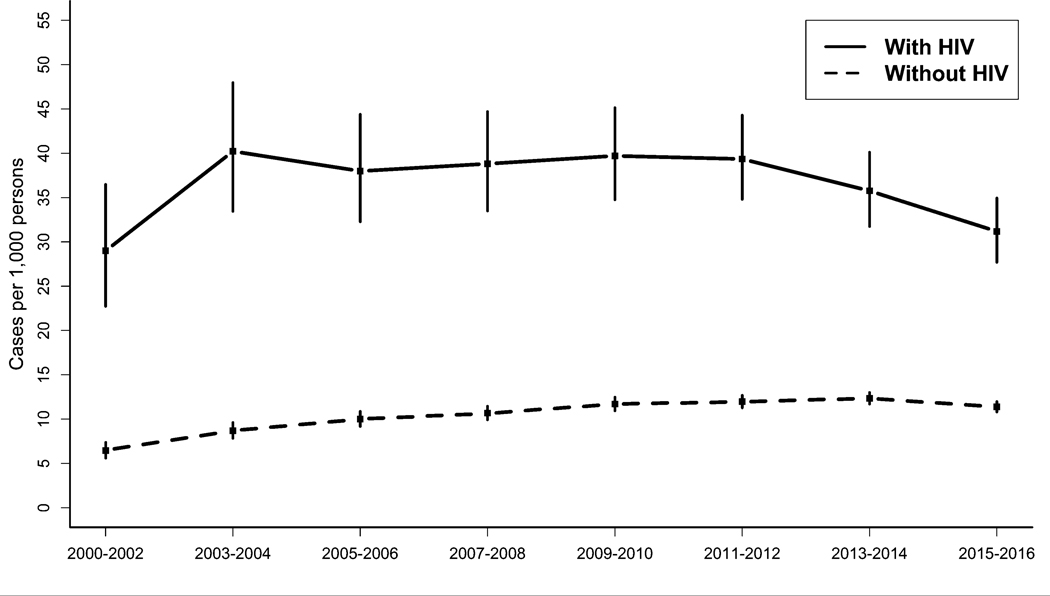
From 2000 to 2016, dementia prevalence increased from 29.0 [22.7 to 36.5] to 31.2 [27.7 to 35.0] cases per 1,000 persons among PWH and from 6.5 [5.6 to 7.4] to 11.4 [10.8 to 12.0] cases per 1,000 persons among PWoH (Figure 2). Although both PWH and PWoH experienced overall increases in dementia prevalence, temporal trends differed by HIV status (p-interaction=0.02). Among PWH, dementia prevalence increased on average 1.5% [−2.8 to 6.0] per period from 2000 to 2012 and decreased on average 6.4% [−18.7 to 7.9] per period from 2013 to 2016, although neither of these trends were significant (p=0.4 and p=0.2, respectively). Among PWoH, dementia prevalence increased on average 6.2% [1.6 to 11.0, p<0.01] per period from 2000 to 2010 and was fairly stable from 2011 to 2016 (−0.6% [−4.7 to 3.7], p=0.7).
Figure 2. Prevalence of dementia by HIV status – Kaiser Permanente, 2000–2016.
Prevalence estimates were standardized to the overall age and sex distribution in the time period 2000–2002. Vertical bars depict 95% confidence intervals.
The overall prevalence of dementia from 2000 to 2016 was higher among PWH (adjusted prevalence ratio [aPR]=1.9 [1.7 to 2.0]). Within individual time periods, covariate-adjusted dementia prevalence was 1.8 to 3.5 times higher among PWH compared with PWoH (Table 3) and remained higher among PWH in the most recent period, 2015–2016 (aPR=1.8 [1.6 to 2.0]). The higher dementia prevalence among PWH was similar by sex (p-interaction=0.39) and by race/ethnicity (p-interaction=0.31).
Table 3.
Prevalence ratios of dementia in people with and without HIV, overall and by time period – Kaiser Permanente, 2000–2016
| With HIV | Without HIV | Prevalence ratio (95% CI) | ||||
|---|---|---|---|---|---|---|
| Time period | Cases of dementia | Number of persons | Cases of dementia | Number of persons | Unadjusted | Adjusteda |
| 2000–2002 | 73 | 2,482 | 212 | 32,706 | 4.54 (3.49–5.90) | 3.13 (2.33–4.20) |
| 2003–2004 | 124 | 3,041 | 370 | 41,152 | 4.54 (3.71–5.54) | 3.49 (2.82–4.32) |
| 2005–2006 | 157 | 4,058 | 545 | 50,553 | 3.59 (3.01–4.27) | 2.79 (2.32–3.35) |
| 2007–2008 | 193 | 4,851 | 724 | 60,391 | 3.32 (2.84–3.88) | 2.44 (2.06–2.88) |
| 2009–2010 | 240 | 5,723 | 982 | 71,143 | 3.04 (2.65–3.49) | 2.25 (1.94–2.61) |
| 2011–2012 | 286 | 6,860 | 1,259 | 84,626 | 2.80 (2.47–3.18) | 2.14 (1.88–2.43) |
| 2013–2014 | 316 | 8,053 | 1,583 | 98,494 | 2.44 (2.17–2.75) | 1.87 (1.65–2.11) |
| 2015–2016 | 340 | 9,352 | 1,834 | 113,179 | 2.24 (2.00–2.51) | 1.75 (1.56–1.97) |
| Overall | 574 | 13,296 | 2,732 | 155,354 | 2.45 (2.25–2.68) | 1.86 (1.70–2.04) |
Adjusted for current age, sex, race/ethnicity, neighborhood-level education, ever smoking, alcohol use disorder, other substance use disorder, cerebrovascular disease, peripheral vascular disease, coronary heart disease, heart failure, hypertension, dyslipidemia, diabetes mellitus, obesity, depression, and number of outpatient visits in the year before baseline.
In sensitivity analyses excluding PWH with detectable HIV RNA at baseline or prior advanced immunodeficiency, overall dementia prevalence was similarly elevated among PWH (aPR=1.8 [1.6 to 2.0] and aPR=1.6 [1.4 to 1.8], respectively; Supplemental Table 4).
Discussion
In this study of ART-treated PWH and demographically-similar PWoH, the incidence of dementia decreased from 2000 to 2016 for both PWH and PWoH. Reductions in incidence were greater among PWH, but dementia incidence remained 58% higher among PWH in the most recent period, 2015–2016. The overall prevalence of dementia increased from 2000 to 2016 for both PWH and PWoH. In recent years, trends in dementia prevalence are suggestive of a decrease among PWH and stabilization among PWoH. In the 2015–2016 period, dementia prevalence was 75% higher among PWH compared with PWoH. In all time periods, dementia incidence and prevalence were higher among PWH even after accounting for sociodemographics, substance use, comorbidities, and frequency of healthcare utilization. Higher incidence and prevalence of dementia among PWH were not significantly different by sex or racial/ethnic groups.
Reductions in dementia incidence over time may be due to a combination of factors. In the general U.S. population, decreasing dementia incidence has been hypothesized to be due in part to improved prevention and management of cardiovascular risk factors for dementia as well as population-level improvements in educational attainment [14, 15, 31]. Our observation that reductions in incident dementia were more pronounced among PWH may indicate better chronic disease management and attention to modifiable risk factors such as smoking cessation and hypertension control in this population [32, 33], which typically has more frequent contact with the healthcare system than the average adult. It could also reflect improvements in ARTs as well as increased options for treatment simplification [34], all of which may enhance adherence to newer regimens and lead to more effective HIV suppression over time. Moreover, broader ART options are now available for PWH with specific comorbidities such as cardiovascular, liver, or renal disease, and in general, virologic failure has become increasingly rare with newer, more potent ART regimens [34]. Another possibility is that observed trends reflect a survivor effect whereby PWH who live longer and stay in HIV care may be generally healthier and at lower risk for developing dementia.
For the most part, increasing trends in dementia prevalence among PWH mirror trends among PWoH. In more recent years, the data are suggestive of diverging trends by HIV status, with decreasing prevalence among PWH and more stable prevalence among PWoH. It is unclear what may have been driving differential trends by HIV status towards the end of the study. Temporal analyses controlled for changes in the age and sex distribution of the study population, excluding this as a likely explanation. Also, the average duration of HIV infection increased incrementally with each subsequent time period, making differential inclusion or attrition of PWH by duration of HIV infection another unlikely reason. More follow-up time will be needed to confirm this initial finding and shed light on potential explanations – for example, whether divergent trends may be due to differential survival by HIV status following dementia diagnosis, changing epidemiology of dementia-related comorbidities such as smoking [32], or perhaps attrition of PWH with greater lifetime exposure to HIV viremia due to less effective ART in earlier years.
Our finding that ART-treated PWH had higher incidence and prevalence of dementia than PWoH across all time periods is consistent with growing evidence that cognitive aging may be premature and/or accelerated among PWH [35–42]. These results also align with a recent study within an independent KP Northern Californian cohort from 2013 to 2019 which found elevated dementia risk among ART-treated PWH compared with demographically-matched uninfected PWoH, even after controlling for traditional dementia risk factors [27]. Persistently elevated dementia risk among ART-treated PWH suggests that the current strategy of primarily encouraging ART initiation and adherence may be insufficient to fully protect the cognitive function of older PWH, particularly in the context of higher baseline dementia risk from the contributing effects of HIV disease on neurocognition. Indeed, unlike most HIV/AIDS-related conditions which have decreased with ART use, cognitive impairment appears less impacted [33, 38, 43, 44]. Notably, in this study, dementia incidence and prevalence remained elevated among PWH even in sensitivity analyses which restricted PWH to those with HIV RNA <200 copies/ml (a proxy for optimal ART adherence) and no prior advanced immunodeficiency (a proxy for timely ART initiation and consistent usage).
Overall, our findings underline the importance of identifying effective strategies to prevent or delay the onset of dementia in an aging HIV population, especially since cognitive impairments among PWH could have adverse impacts on ART adherence and HIV outcomes [45]. It remains unclear whether PWH may require dementia prevention measures distinct from those recommended for the general population [46]. The American Academy of Neurology currently recommends annual cognitive screening of patients aged 65 years and older[47], but some evidence indicate that PWH may develop dementia at earlier ages [27, 42]. There are currently limited data on age-associated dementias in PWH, and consensus cognitive screening guidelines for older PWH in routine clinical care have not been developed. The validation of cognitive screening tools for PWH also remains a developing area of research [2, 45, 48–50]. As the population with HIV continues to experience improved survival on suppressive ART[18], expanding our understanding of dementia risk among PWH may help identify subgroups that could benefit from enhanced cognitive surveillance or early intervention.
This study had some limitations. First, dementia diagnoses based on clinical workup are subject to variation across providers. Providers may be less likely to overlook cognitive complaints in PWH or more likely to refer them for follow-up evaluation, resulting in higher diagnosis rates in this group. However, chart review confirmed comparable PPV of our ICD-based definition of dementia in PWH and PWoH and similar ICD coding patterns by HIV status [27]. Second, provider patterns in dementia diagnosis may have changed over time resulting in over- or under-estimation of dementia in some time periods. However, the ICD codes used to identify dementia were confirmed by review of charts sampled across the entire study period. These changes are therefore unlikely to have substantially affected comparisons by HIV status since we restricted the definition of dementia to ICD codes with the highest PPV among both PWH and PWoH. Third, there were insufficient numbers of cases to conduct analyses by dementia subtype, and without biomarker data (e.g. neuroimaging, cerebrospinal fluid [CSF]), we could not investigate dementia etiology and whether some cases of dementia identified among PWH may have been due to CSF viral escape [51–53]. However, this phenomenon is uncommon and would likely have accounted for few, if any, of the dementia diagnoses among PWH in this study, all of whom were ART-treated. Fourth, the generalizability of this study may be limited since it was conducted in an insured U.S. population that was primarily male. However, our HIV population was representative of the HIV population in the service areas for each of the KP health systems and that would most likely be seen in clinical practice [54–58]. Over 90% of the U.S. population is currently insured[59] and this percentage may be even higher among PWH who qualify for publicly-funded insurance which is accepted by KP health systems. Therefore, our results may generalize to other insured PWH in the U.S. and in other settings with similar access to care. Lastly, some cases of HAND may have been misclassified as dementia given variable clinical presentation of cognitive impairments in PWH[3, 12, 19] and lack of widely-recognized HIV-specific diagnostic guidelines for dementia in primary care settings [60]. However, the detection of HAND in primary care is less sensitive than the more extensive neuropsychological assessments conducted in research settings which are more likely to detect mild or moderate cases. Therefore, our estimates of dementia are likely to be conservative and reflective of clinically-apparent dementia, which is what this study intended to capture.
A major strength of our study was the large cohort of people with well-controlled HIV and demographically-similar comparator population of PWoH from the same setting. Also, few studies have been powered to examine dementia among PWH older than 50 years, with individual-level adjustment for dementia-related comorbidities and risk factors. In this study, pooling EHR data across multiple healthcare systems capitalized on the availability of harmonized clinical covariates and the existence of long-standing HIV registries. Further, the “closed” nature and integrated delivery design of the health systems allowed for comprehensive capture of ART use among PWH.
Conclusions
Despite effective ART use and declining dementia incidence among PWH, dementia incidence and prevalence remain higher among PWH compared with PWoH. Dementia can cause significant disability and dependence, diminishing the benefits of achieving near-normal life expectancy with HIV treatment. To maintain the health and quality of life of older PWH, further research is needed to determine factors contributing to persistently elevated dementia risk among ART-treated PWH.
Supplementary Material
The figure shows people with HIV (PWH) who were included in the study. PWH were from Kaiser Permanente (KP) health systems in Northern California, Southern California, and Mid-Atlantic States (Maryland, Virginia, and Washington, D.C.).
ACKNOWLEDGEMENTS
ROLE OF EACH AUTHOR
JOL conceptualized the study, conducted the data analysis, drafted the manuscript, and provided funding support. CL provided biostatistical support and critical input on the manuscript. PG, CEH, DDS, JAF, WJT, and MAH provided critical input on the manuscript. WAL collected data and provided critical input on the manuscript. MJS provided supervision and critical input on the manuscript.
Footnotes
Conflicts of Interest and Source of Funding: JOL, WJT, MAH and MJS report a previous grant from Gilead Sciences, Inc., outside the submitted work. No conflicts of interest were declared for the remaining authors. This work was supported by grants from the Kaiser Permanente Northern California Community Benefit Grants Program (PI: Jennifer Lam) and the National Institute of Allergy and Infectious Diseases (K01AI157849, PI: Jennifer Lam).
References
- 1.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of neurovirology 2011; 17(1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clifford DB, Ances BM. HIV-associated neurocognitive disorder. The Lancet Infectious diseases 2013; 13(11):976–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sacktor N. Changing clinical phenotypes of HIV-associated neurocognitive disorders. Journal of neurovirology 2018; 24(2):141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, Becker JT, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology 1993; 43(11):2245–2252. [DOI] [PubMed] [Google Scholar]
- 5.Mateen FJ, Shinohara RT, Carone M, Miller EN, McArthur JC, Jacobson LP, et al. Neurologic disorders incidence in HIV+ vs HIV- men: Multicenter AIDS Cohort Study, 1996–2011. Neurology 2012; 79(18):1873–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. Aids 2007; 21(14):1915–1921. [DOI] [PubMed] [Google Scholar]
- 7.Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010; 75(23):2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cysique LA, Heaton RK, Kamminga J, Lane T, Gates TM, Moore DM, et al. HIV-associated neurocognitive disorder in Australia: a case of a high-functioning and optimally treated cohort and implications for international neuroHIV research. Journal of neurovirology 2014; 20(3):258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sacktor N, Skolasky RL, Seaberg E, Munro C, Becker JT, Martin E, et al. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology 2016; 86(4):334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makinson A, Dubois J, Eymard-Duvernay S, Leclercq P, Zaegel-Faucher O, Bernard L, et al. Increased Prevalence of Neurocognitive Impairment in Aging People Living With Human Immunodeficiency Virus: The ANRS EP58 HAND 55–70 Study. Clin Infect Dis 2020; 70(12):2641–2648. [DOI] [PubMed] [Google Scholar]
- 11.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69(18):1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valcour V, Paul R. HIV infection and dementia in older adults. Clin Infect Dis 2006; 42(10):1449–1454. [DOI] [PubMed] [Google Scholar]
- 13.Wing EJ. HIV and aging. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases 2016; 53:61–68. [DOI] [PubMed] [Google Scholar]
- 14.Langa KM. Is the risk of Alzheimer’s disease and dementia declining? Alzheimer’s research & therapy 2015; 7(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C, Seshadri S. Incidence of Dementia over Three Decades in the Framingham Heart Study. N Engl J Med 2016; 374(6):523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guaraldi G, Palella FJ Jr., Clinical implications of aging with HIV infection: perspectives and the future medical care agenda. Aids 2017; 31 Suppl 2:S129–s135. [DOI] [PubMed] [Google Scholar]
- 17.Valcour VG. HIV, aging, and cognition: emerging issues. Top Antivir Med 2013; 21(3):119–123. [PMC free article] [PubMed] [Google Scholar]
- 18.Marcus JL, Leyden WA, Alexeeff SE, Anderson AN, Hechter RC, Hu H, et al. Comparison of Overall and Comorbidity-Free Life Expectancy Between Insured Adults With and Without HIV Infection, 2000–2016. JAMA network open 2020; 3(6):e207954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sacktor N, Robertson K. Evolving clinical phenotypes in HIV-associated neurocognitive disorders. Curr Opin HIV AIDS 2014; 9(6):517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cole MA, Margolick JB, Cox C, Li X, Selnes OA, Martin EM, et al. Longitudinally preserved psychomotor performance in long-term asymptomatic HIV-infected individuals. Neurology 2007; 69(24):2213–2220. [DOI] [PubMed] [Google Scholar]
- 21.Saloner R, Cysique LA. HIV-Associated Neurocognitive Disorders: A Global Perspective. Journal of the International Neuropsychological Society : JINS 2017; 23(9–10):860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gisslen M, Price RW, Nilsson S. The definition of HIV-associated neurocognitive disorders: are we overestimating the real prevalence? BMC Infect Dis 2011; 11:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Underwood J, De Francesco D, Leech R, Sabin CA, Winston A. Medicalising normality? Using a simulated dataset to assess the performance of different diagnostic criteria of HIV-associated cognitive impairment. PLoS One 2018; 13(4):e0194760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sacktor N, Lyles RH, Skolasky R, Kleeberger C, Selnes OA, Miller EN, et al. HIV-associated neurologic disease incidence changes: Multicenter AIDS Cohort Study, 1990–1998. Neurology 2001; 56(2):257–260. [DOI] [PubMed] [Google Scholar]
- 25.Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. Journal of neurovirology 2004; 10(6):350–357. [DOI] [PubMed] [Google Scholar]
- 26.Gordon N. Similarity of adult Kaiser Permanente Members to the adult population in Kaiser Permanente’s Northern California service area: Comparisons based on the 2017/2018 cycle of the California Health Interview Survey. Oakland, CA: Kaiser Permanente Division of Research; 2020. [Google Scholar]
- 27.Lam JO, Hou CE, Hojilla JC, Anderson AN, Gilsanz P, Alexeeff SE, et al. Comparison of dementia risk after age 50 between individuals with and without HIV infection. Aids 2021; 35(5):821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Statistical Methodology and Applications Branch - Surveillance Research Program. Joinpoint Regression Program, Version 4.8.0.1 - April 2020. National Cancer Institute. [Google Scholar]
- 29.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Statistics in medicine 2000; 19(3):335–351. [DOI] [PubMed] [Google Scholar]
- 30.Kim HJ, Fay MP, Yu B, Barrett MJ, Feuer EJ. Comparability of segmented line regression models. Biometrics 2004; 60(4):1005–1014. [DOI] [PubMed] [Google Scholar]
- 31.Bancks M, Alonso A, Allen N, Yaffe K, Carnethon M. Temporal trends in cognitive function of older US adults associated with population changes in demographic and cardiovascular profiles. J Epidemiol Community Health 2019; 73(7):612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lam JO, Levine-Hall T, Hood N, Alexeeff SE, Horberg MA, Young-Wolff KC, et al. Smoking and cessation treatment among persons with and without HIV in a U.S. integrated health system. Drug Alcohol Depend 2020; 213:108128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein DB, Leyden WA, Xu L, Chao CR, Horberg MA, Towner WJ, et al. Declining relative risk for myocardial infarction among HIV-positive compared with HIV-negative individuals with access to care. Clin Infect Dis 2015; 60(8):1278–1280. [DOI] [PubMed] [Google Scholar]
- 34.Saag MS, Gandhi RT, Hoy JF, Landovitz RJ, Thompson MA, Sax PE, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2020 Recommendations of the International Antiviral Society-USA Panel. Jama 2020; 324(16):1651–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aung HL, Aghvinian M, Gouse H, Robbins RN, Brew BJ, Mao L, et al. Is There Any Evidence of Premature, Accentuated and Accelerated Aging Effects on Neurocognition in People Living with HIV? A Systematic Review. AIDS Behav 2021; 25(3):917–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aung HL, Bloch M, Vincent T, Quan D, Jayewardene A, Liu Z, et al. Cognitive ageing is premature among a community sample of optimally treated people living with HIV. HIV Med 2021; 22(3):151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen RA, Seider TR, Navia B. HIV effects on age-associated neurocognitive dysfunction: premature cognitive aging or neurodegenerative disease? Alzheimer’s research & therapy 2015; 7(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nookala AR, Mitra J, Chaudhari NS, Hegde ML, Kumar A. An Overview of Human Immunodeficiency Virus Type 1-Associated Common Neurological Complications: Does Aging Pose a Challenge? Journal of Alzheimer’s disease : JAD 2017; 60(s1):S169–s193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alford K, Vera JH. Cognitive Impairment in people living with HIV in the ART era: A Review. British medical bulletin 2018; 127(1):55–68. [DOI] [PubMed] [Google Scholar]
- 40.Pfefferbaum A, Rogosa DA, Rosenbloom MJ, Chu W, Sassoon SA, Kemper CA, et al. Accelerated aging of selective brain structures in human immunodeficiency virus infection: a controlled, longitudinal magnetic resonance imaging study. Neurobiology of aging 2014; 35(7):1755–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brew BJ, Cysique L. Does HIV prematurely age the brain? The lancet HIV 2017; 4(9):e380–e381. [DOI] [PubMed] [Google Scholar]
- 42.Goodkin K, Miller EN, Cox C, Reynolds S, Becker JT, Martin E, et al. Effect of ageing on neurocognitive function by stage of HIV infection: evidence from the Multicenter AIDS Cohort Study. The lancet HIV 2017; 4(9):e411–e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eggers C, Arendt G, Hahn K, Husstedt IW, Maschke M, Neuen-Jacob E, et al. HIV-1-associated neurocognitive disorder: epidemiology, pathogenesis, diagnosis, and treatment. Journal of neurology 2017; 264(8):1715–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lerner AM, Eisinger RW, Fauci AS. Comorbidities in Persons With HIV: The Lingering Challenge. Jama 2019. [DOI] [PubMed] [Google Scholar]
- 45.Valcour V, Paul R, Chiao S, Wendelken LA, Miller B. Screening for cognitive impairment in human immunodeficiency virus. Clin Infect Dis 2011; 53(8):836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anstey KJ, Peters R, Clare L, Lautenschlager NT, Dodge HH, Barnes DE, et al. Joining forces to prevent dementia: The International Research Network On Dementia Prevention (IRNDP). International psychogeriatrics 2017; 29(11):1757–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foster NL, Bondi MW, Das R, Foss M, Hershey LA, Koh S, et al. Quality improvement in neurology: Mild cognitive impairment quality measurement set. Neurology 2019; 93(16):705–713. [DOI] [PubMed] [Google Scholar]
- 48.Carroll A, Brew B. HIV-associated neurocognitive disorders: recent advances in pathogenesis, biomarkers, and treatment. F1000Research 2017; 6:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Underwood J, Winston A. Guidelines for Evaluation and Management of Cognitive Disorders in HIV-Positive Individuals. Curr HIV/AIDS Rep 2016; 13(5):235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brew BJ, Chan P. Update on HIV dementia and HIV-associated neurocognitive disorders. Current neurology and neuroscience reports 2014; 14(8):468. [DOI] [PubMed] [Google Scholar]
- 51.Ferretti F, Gisslen M, Cinque P, Price RW. Cerebrospinal Fluid HIV Escape from Antiretroviral Therapy. Curr HIV/AIDS Rep 2015; 12(2):280–288. [DOI] [PubMed] [Google Scholar]
- 52.Mastrangelo A, Turrini F, de Zan V, Caccia R, Gerevini S, Cinque P. Symptomatic cerebrospinal fluid escape. Aids 2019; 33 Suppl 2:S159–s169. [DOI] [PubMed] [Google Scholar]
- 53.Pérez-Valero I, Ellis R, Heaton R, Deutsch R, Franklin D, Clifford DB, et al. Cerebrospinal fluid viral escape in aviremic HIV-infected patients receiving antiretroviral therapy: prevalence, risk factors and neurocognitive effects. Aids 2019; 33(3):475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.California Department of Health - Office of AIDS. Epidemiology of HIV in California. 2017. [Google Scholar]
- 55.Maryland Department of Health - Center for HIV Surveillance Epidemiology and Evaluation. Gender and HIV in Maryland, 2019. [Google Scholar]
- 56.Maryland Department of Health - Center for HIV Surveillance Epidemiology and Evaluation. HIV by Race/Ethnicity in Maryland, 2019. [Google Scholar]
- 57.Virginia Department of Health. Virginia HIV Surveillance Annual Report, 2017. [Google Scholar]
- 58.District of Columbia Department of Health - HIV/AIDS Hepatitis STD and TB Administration (HAHSTA). Annual Epidemiology & Surveillance Report - Surveillance Data Through December 2015. [Google Scholar]
- 59.Keisler-Starkey K, Bunch LN. Health insurance coverage in the United States: 2020. Current Population Reports. Washington, D.C.: United States Census Bureau; 2021. [Google Scholar]
- 60.Nightingale S, Winston A, Letendre S, Michael BD, McArthur JC, Khoo S, et al. Controversies in HIV-associated neurocognitive disorders. The Lancet Neurology 2014; 13(11):1139–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The figure shows people with HIV (PWH) who were included in the study. PWH were from Kaiser Permanente (KP) health systems in Northern California, Southern California, and Mid-Atlantic States (Maryland, Virginia, and Washington, D.C.).