Abstract
Acute lung injury is one of major complications associated with sepsis, responsible for morbidity and mortality. Patients who suffer from acute lung injury often require respiratory support under sedations, and it would be important to know the role of sedatives in lung injury. We examined volatile anesthetic isoflurane, which is commonly used in surgical setting, but also used as an alternative sedative in intensive care settings in European countries and Canada. We found that isoflurane exposure attenuated neutrophil recruitment to the lungs in mice suffering from experimental polymicrobial abdominal sepsis. We found that isoflurane attenuated one of major neutrophil chemoattractants LTB4 mediated response via its receptor BLT1 in neutrophils. Furthermore, we have shown that isoflurane directly bound to BLT1 by a competition assay using newly developed labeled BLT1 antagonist, suggesting that isoflurane would be a BLT1 antagonist.
Keywords: Lung injury, volatile anesthetic, BLT1
Introduction
Sepsis, defined as a life-threatening organ dysfunction caused by an abnormal host response to infection, is a significant health care burden with high morbidities and mortalities. Despite continuous medical advances such as the development of new antibiotics and vaccines and intensive care management, sepsis remains the leading cause of death in the non-cardiac intensive care units[1,2]. It costs a staggering $20.3 billion per year, occupying 40% of intensive care expenditures and no specific therapy is yet available[1]. Respiratory dysfunction is a hallmark of sepsis and acute lung injury (ALI) is among the leading causes of morbidities and mortalities in sepsis [3–6]. Although sepsis-induced ALI can be caused by primary respiratory tract infection, sepsis originating from extra-pulmonary infection is associated with the highest risk of progression to ALI [4]. Abdominal sepsis is the most common presentation after respiratory tract infection [7,8] and is associated with extremely high morbidities and mortalities [9,10]. Treatment of ALI remains unsolved problems of intensive care medicine.
Endothelial and epithelial injury followed by an increased permeability of the alveolar-capillary barrier is responsible for the impairment of arterial oxygenation in ALI. Neutrophils are the first-line defense cells with potent antimicrobial tools such as oxidants and proteinases and are recruited to the site of infection and inflammation. However, they can provide unregulated release of these compounds into host tissues, leading into endothelial and epithelial injury.
Because of no established target therapy, ALI management remains conservative. Often these patients receive sedatives during their care. We and others have previous reported that volatile anesthetics (VAs), often used for surgical anesthesia, possess potent immunomodulatory properties. VAs have been used as alternative ICU sedatives in Canada and Europe. The beneficial property of VAs on lung injury has been reported in direct lipopolysaccharide (LPS) lung injury model [11–16], and recently we showed the protective effect of VAs on lung injury model induced by flagellin tracheal instillation [17]. However, the effect of VAs on lung injury in extrapulmonary sepsis has been studied to a limited extent. In this study we aim to investigate the effect of volatile anesthetic isoflurane on extrapulmonary sepsis-induced lung injury and the mechanism through which this drug mediates the effect. Specifically, we investigated the effect of isoflurane on sepsis-induced lung injury and on lipid mediators using a murine model of polymicrobial abdominal sepsis induced by cecal ligation and puncture (CLP) surgery.
Materials and Methods
CLP model and animal care use
Male mice on the C57BL/6J background aged 8–10 week were purchased from Jackson Laboratory (Bar Harbor, Maine, USA) and inbred in our animal facility. They were housed under 12-hour day and night cycles and specific pathogen free conditions. All the experimental protocols complied with institutional and Animal Research Reporting of In Vivo Experiment guidelines regarding the use of animals in research [18] and were approved by Boston Children’s Hospital Institutional Animal Care and Use Committee. Polymicrobial abdominal sepsis was induced by CLP surgery as we previously performed [14]. Briefly, mice were anesthetized with intraperitoneal injection of ketamine (60 mg/kg) and xylazine (5 mg/kg). Following exteriorization, the cecum was ligated at 1.0 cm from its tip and subjected to a single, through-and-through puncture using an 18-gauge needle. A small amount of fecal material was expelled with gentle pressure to maintain the patency of puncture sites. The cecum was inserted into the abdominal cavity. Warmed saline (0.1 ml/gm) was administered subcutaneously to alleviate postoperative surgical pain. Some groups of mice were placed on the nose cone and continuously exposed to 1% isoflurane using vaporizer (VetQuip, Eastern Creek, NSW, Australia) for 6 hours. Mice were euthanized at indicated time points in the Result section and subjected to analysis. Bronchoalveolar lavage [19] was performed by inserting 20G angiocatheter via trachea and flushing with 1 mL of PBS 2 times.
Myeloperoxidase activity measurement
Myeloperoxidase (MPO) activity was measured as we previously performed [14,20]. Briefly, mice were euthanized 12 hours after CLP procedure and the body was flushed with phosphate-buffered saline (PBS) through the pulmonary artery. Lung was removed and immediately snap-frozen and stored at −80°C until assay. Frozen lung was thawed, homogenized and resuspended in 50 mM KPO4 buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide and were incubated at 60°C for 2 hours. Following three freeze-thaw cycles, samples were centrifuged and supernatant was subject to analysis with the addition of o-dianisodine and H2O2. Absorbance was measured at 450 nm.
RNA purification and qPCR
Following the harvest, tissues were snap frozen and stored at −80°C for a short period. Total RNA was extracted using TRIzol reagent (Life Technologies, Carlsbad, CA, USA) and subjected to DNase digestion. 1 μg of RNA was subjected to reverse transcription using Superscript III RNase reverse transcriptase (Life Technologies). Real time quantitative polymerase chain reaction (RT-qPCR) was performed using SYBR green master mix (Applied Biosystems, South Francisco, CA, USA). We quantitated mRNA level of tumor necrosis factor-α (TNF-α), interleukin-10 (IL-10), interferon-γ (IFN-γ) and glyceraldehyde-3-phsphate dehydrogenase (GAPDH). The sequences of primers used for this assay were listed in Supplemental Table 1.
Measurement of eicosanoids
Reverse-phase mass spectrometry (MS)-based quantitation technique for eicosanoids was previously described [21]. The lipids were extracted with methanol and diluted with water containing 0.1% formic acid to yield a final methanol concentration of 20%. After addition of deuterium-labeled internal standards, the samples were loaded on Oasis HLB cartridge (Waters, Milford, MA, USA). The column was washed with 1 ml of water, 1 ml of 15% methanol and 1 ml of petroleum ether and then eluted with 0.2 ml of methanol containing 0.1% formic acid. Eicosanoids were quantified by reverse-phase HPLC-electrospray ionization-tandem MS method.
Neutrophil chemotaxis
Mouse bone marrow neutrophils were purified by a three-layer Percoll gradient of 78%, 69% and 52% with the purity of > 90% as previously described [22]. Neutrophil chemotaxis was examined using μ chemotaxis slide [23]. Neutrophils suspended in the modified Hanks’ buffer containing 0.1% BSA were applied into the slide channel of μ-Slide chemotaxis chamber (Ibidi, Fitchburg, Wisconsin, USA) and incubated for 10 min at room temperature. Following cell attachment, chamber reservoirs were filled with the modified Hanks’ buffer containing 0.1% BSA, and leukotriene B4 (LTB4) (100 μg/mL), or C5a (1 μg/mL) was added in the upper reservoir according to the manufacturer’s instruction. After 30 min, cell movement was recorded at 20s intervals for 20 min using EVOS cell imaging microscope (Thermofischer, Waltham, MA, USA). Tracks from each cell were determined with Manual Tracking plug-in of ImageJ software. The mean velocity (motility) was calculated.
LTB4 mediated calcium mobilization using fluorescence microscopy and FlexStation
CHO-K1 cells stably transfected with human BLT1 (BLT1-CHO cells) were previously described [24]. CHO-K1 cells transfected with mock vector and BLT1-CHO cells were maintained in Ham F12, 10% FBS, P/S and G418. Calcium mobilization experiment using BLT1-CHO cells were done on fluorescence microscopy or FlexStation as previously performed [25]. Once cell supernatant was aspirated, cells were suspended in PBS containing Fluo-3 (4 μM) and probenecid (2.5 mM) for 30 min. Then, cells were washed and replenished with PBS with probenecid (2.5 mM). Cells were incubated with or without isoflurane for 15 min. Then, calcium mobilization was tested in the BLT1 CHO cells in the presence of 100 nM LTB4. For fluorescence microscopy, cell imaging was obtained every 10 sec following the addition of 100 nM LTB4. For FlexStation analysis, 4 × 104 cells stably expressing BLT1 were seeded onto 96-well plates. After 24 h, cells were loaded with 5 μM Fluo-8 AM (ABD Bioquest) in 100 μl of HBSS buffer (HBSS containing 2.5 mM probenecid and 20 mM HEPES, pH 7.4) supplemented with 0.04% Pluronic F-127 and 1% FCS at 37°C for 30 min, followed by a further incubation at room temperature for 1 hr. The cells were incubated with isoflurane in HBSS buffer for 30 min or BIIL260 compounds in HBSS buffer with 0.5% BSA for 1.5 hr, and LTB4-induced intracellular calcium mobilization was determined by monitoring the fluorescence intensity (excitation at 490 nm, emission at 525 nm) using a FlexStation 3 plate reader (Molecular Devices, Sunnyvale, CA).
In silico docking simulation
Potential isoflurane binding site on BLT1 was predicted in silico. First BIIL260 was removed from the protein data bank (PDB) 5X33 [26] to create apo-BLT1 structure. An estimated binding site was determined using SwissDock software and embedded into the grid with the size of 20 × 20 × 20 cubic angstroms for docking with isoflurane using Autodock Vina (The Scripps Research Institute, La Jolla, CA, USA).
Competition assay using biotinylated BIIL260 compound
BIIL260 biotinylated compounds (will be published elsewhere) and streptavidin-allophycocyanin (APC) were co-incubated for multimer formation. The multimer was co-incubated with either CHO-K1 cells or BLT1-CHO cells for 30 min at 37°C. Some cells were co-incubated with the multimer under isoflurane for 30 min using an air-tight chamber as previously described [27]. After washing, cells were subjected to flow cytometry analysis using Accuri 6 (BD Biosciences, Franklin Lakes, NJ, USA). Binding percentage of BIIL260 under isoflurane was calculated as [mean fluorescence intensity (MFI) of BLT1-CHO cells – MFI of CHO-K1 cells]/ [average MFI of BLT1-CHO cells without isoflurane – average MFI of CHO-K1 cells without isoflurane] × 100 (%).
Statistical analysis
Data were analyzed as indicated in the corresponding figure legends. Statistical significance was defined as P<0.05. All the statistical calculations were performed using PRISM5 software (GraphPad Software, La Jolla, CA, USA).
Results
Isoflurane attenuated neutrophil recruitment to the lungs and subsequently lung injury during sepsis
We used a polymicrobial abdominal sepsis model induced by CLP surgery. First, we validated the development of lung injury in this model. By 12 hours, the infiltration of leukocytes along with hemorrhage in the alveolar space was observed, consistent with lung injury (Fig. 1a). This was further worsened by 24 hours after CLP surgery (Fig. 1a). Total protein and IgM levels in BAL fluid also supported that isoflurane attenuated lung injury induced by CLP (Fig. 1b). Isoflurane exposure attenuated the degree of accumulated leukocytes as well as hemorrhage in the alveolar space (Fig.1a). Isoflurane exposure was associated with a reduction in total protein and IgN levels in BAL fluid compared to no exposure (Fig. 1b). Isoflurane exposure was associated with less proinflammatory cytokines TNF-α and IFN-γ levels in the lungs, along with higher level of anti-inflammatory IL-10 (Fig.1c). Because TNF-α and IFN-γ are associated with lung injury [28,29], and IL-10 is associated with the protection of lung injury, these results are in line with the histology and BAL results. The MPO activity was used as a surrogate of neutrophil numbers. MPO levels were lower in the isoflurane treated group at 6 and 12 hours after CLP surgery (Fig. 1d). No difference in MPO levels were observed at 24 hours between the groups. MPO also exists in macrophages, though much less than in neutrophils. Given that macrophages become dominant responders at the later time point, this could likely represent macrophages. The presence of neutrophils was also examined using flow cytometry. A significantly lower number of neutrophils was observed after isoflurane exposure at 12h post-CLP compared to WT mice in BAL fluid (Fig. 1e).
Figure 1. Isoflurane attenuated neutrophil recruitment and lung injury in CLP sepsis.

After mice were subjected to CLP surgery, a group of mice were further exposed to 1% isoflurane for 6 hours. Mice were euthanized at 12 and 24 hours after CLP. a. H&E analysis of the lungs. Scale bar is 100 μm. b. Total protein and IgM levels in BAL fluid. Data are shown as mean +/− S.D. of 4 samples. c. TNF-α, IFN-γ and IL-10 qPCR of lung samples. Data are shown as mean +/− S.D. of 4 samples. d. MPO analysis. Data are shown as mean +/− S.D. of 4 mice per group. e. Neutrophil counts in BAL fluid. Data are shown as mean +/− S.D. of 4 samples. (b-e) Statistical analysis was performed using one-way ANOVA with Bonferroni post hoc analysis. *, ** and *** p< 0.05, < 0.01, and < 0.001, respectively.
Previously we have shown that isoflurane directly interacted with and inhibited CD11a and CD11b, major adhesion molecules involved in neutrophil recruitment to various organs [13,15,16]. However, we also showed that CD11a and CD11b were not involved in neutrophil recruitment to the lungs [14], suggesting that the reduction in neutrophil recruitment to the lungs by isoflurane would be due to other factors. LTB4 is a major neutrophil chemoattractant and a derivative of arachidonic acids produced by 5-lipoxygenase. Its importance in neutrophil recruitment and lung injury is well illustrated by the report that the deficiency of 5-lipoxygenase led to the protection from the lung injury in the CLP model [30]. Because isoflurane significantly attenuated neutrophil recruitment to the lungs, we hypothesized that isoflurane exposure was associated with less LTB4 level in the lungs between isoflurane exposure and non-exposure groups. Contrary to our hypothesis, lung LTB4 levels were not different at 6 and 12 hours between the groups (Fig. 2). At 24 hours, LTB4 level was rather higher in the isoflurane exposed arm, though not statistically significant (Fig. 2). LTC4 and LTE4 levels in the lungs did not differ throughout. Of note, 15-hydroxyeicosatetraenoic acid (HETE) facilitates the production of resolvins [31], a lipid mediator that facilitates the active resolution of inflammation. At both 12 and 24 hours, 15-HETE levels were higher in isoflurane arm (Fig. 2), indicating that isoflurane exposure enhanced the resolution of inflammation, compatible with the previous report [32]. This is in line with higher IL-10 mRNA level in the lungs of isoflurane-exposed mice.
Figure 2. Lipidomics of lung samples.

Eicosanoid levels in the lungs from mice with or without isoflurane exposure at different time points after CLP sepsis. Data are shown as mean +/− S.D. of 6 samples. Two-way ANOVA with Bonferroni post hoc analysis was performed. * P< 0.05. In the figures, mock, no isoflurane exposure; ISO, isoflurane exposure; AA, arachidonic acid.
Isoflurane attenuated LTB4 mediated neutrophil chemotaxis
Major neutrophil chemoattractants include LTB4 and C5a. Here we examined to see if isoflurane attenuated LTB4, C5a, -mediated neutrophil chemotaxis using bone marrow neutrophils. Isoflurane exposure attenuated neutrophil chemotaxis by LTB4, but not by C5a (Fig. 3a). LTB4 stimulation induces calcium mobilization. Using BLT1-CHO cells, we examined the effect of isoflurane on calcium mobilization upon LTB4 stimulation using both FlexStation and fluorescence microscopy. Isoflurane exposure even at 0.3 and 0.5% attenuated calcium mobilization in FlexStation (Fig. 3b), consistent with the chemotaxis data. On fluorescence microscopy, LTB4 stimulation immediately induced calcium mobilization denoted by green fluorescence, but isoflurane (1%) attenuated (Fig. 3c). Thus, our data showed that isoflurane directly attenuated LTB4-mediated neutrophil recruitment, not LTB4 production.
Figure 3. The effect of isoflurane on neutrophil chemotaxis and calcium mobilization.
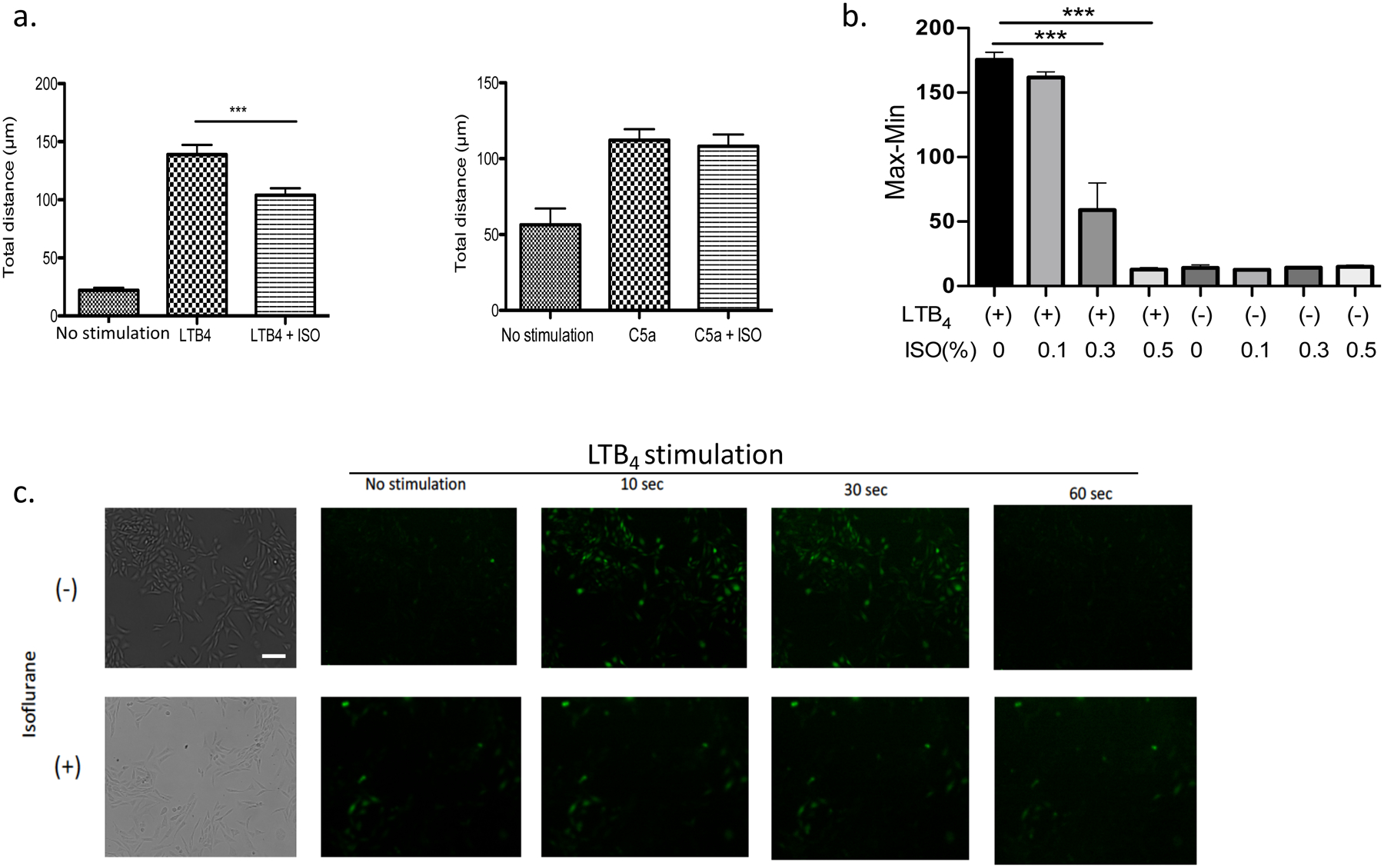
a. The chemotaxis of bone marrow neutrophils toward LTB4 was examined with or without isoflurane exposure. Data are shown as mean +/− S.D. of 100 neutrophils. b. The effect of isoflurane (0.1–0.5%) on calcium mobilization in CHO BLT1 cells under LTB4 (100 nM) stimulation using FLEXStation. Data are shown as mean +/− S.D. of 6 samples. c. The effect of isoflurane (1%) on calcium mobilization in CHO BLT1 cells under LTB4 (100 nM) stimulation using fluorescence microscope. Scale bar is 50 μm. (a, b) Statistical analysis was performed using one-way ANOVA with Bonferroni post hoc analysis. *** P< 0.001.
Docking simulation suggested that isoflurane binds to BLT1
BLT1 is a primary receptor for LTB4. LTB4-BLT1 binding activates phosphoinositide 3-kinase (PI3K), then phospholipase Cγ (PLCγ), followed by Ca2+ activation [33,34]. Similarly, C5a uses PI3K pathway as in BLT1 [35]. Because only LTB4-BLT1 axis was affected by isoflurane, we hypothesized that isoflurane would directly bind to BLT1. Using the reported X-ray crystallographic structure of BLT1 (PDB: 5X33), we performed docking simulation to determine the interaction between isoflurane and BLT1. Isoflurane bound to the same pocket that the BLT1 inhibitor BIIL260 bound to, indicating that isoflurane might serve as BLT1 inhibitor (Fig. 4a).
Figure 4. In silico docking of isoflurane on BLT1 receptor and displacement assay.

a. In silico docking of isoflurane onto BLT1 protein structure (PDB. 5X33). b. The structure of BLT1 antagonist BIIL260 and its modification to acquire biotin tag. c. The effect of BIIL260 biotinylated compounds on calcium mobilization using FlexStation. d. Displacement assay of BIIL260 T3 compounds by isoflurane. Statistical analysis was performed using Student t test. *** p< 0.001.
Isoflurane directly bound to BLT1
BIIL260 compound was modified to contain biotin (T1–T5) (Fig. 4b). The effectiveness of biotin-labeled BIIL260 compounds on calcium mobilization was examined using FLEX station. At high concentrations, T3, T4 and T5 were able to inhibit calcium mobilization induced by LTB4 (Fig. 4c). Next, using APC-streptavidin multimer, we performed FACS-based BIIL260 binding assay to WT CHO-K1 cells and BLT1-CHO cells using T3–5. Among them, T3 was suitable for subsequent experiment. Isoflurane exposure attenuated the binding of T3, suggesting that isoflurane directly bound to BLT1 at the BIIL260 binding pocket (Fig. 4d) as predicted in silico.
Discussion
In this report, we reported that isoflurane directly interacted with BLT1, attenuating neutrophil recruitment and acute lung injury indirectly induced by the experimental polymicrobial abdominal sepsis. This result is consistent with our previous data of the favorable role of isoflurane in attenuating direct lung injury [17].
Neutrophil recruitment is controlled by chemoattractants such as chemokines and lipid mediators. A growing literature suggests that lipid mediators orchestrate neutrophil recruitment with these chemokines. LTB4 is a major neutrophil chemoattractant and binds to its G protein-coupled receptors (GPCRs) on neutrophil cell surface to transmit signals. LTB4 receptor, BLT1 is one of GPCRs. We have shown that isoflurane attenuated BLT1 function, and directly bound to BLT1, supporting the idea that isoflurane is a BLT1 antagonist. Previously VA was shown to interact with rhodopsin, another GPCR [36] and BLT1 is the second GPCR shown to interact with VA.
In this study, we primarily focused on the degree of neutrophil recruitment under isoflurane. 15-HETE level was significantly increased in isoflurane-treated mice compared to WT. 15-HETE produces specialized pre-resolving mediators (SPMs). Resolution of inflammation is a dynamic process that is coordinated by biochemical and cellular mechanisms. Lipoxins and MaR1 are one of potent in vitro and in vivo SPMs. SPMs orchestrate anti-inflammatory and proresolving actions that are cell type specific. Chiang et al. previously demonstrated isoflurane facilitated the resolution of inflammation in zymosan injection model [32]. Our data also supported that isoflurane could facilitate the resolution of inflammation. Crosstalk between 15-HETE and LTB4 has been reported. For example, Ternowiz et al. reported that 15-HETE inhibits LTB4-induced skin response [37]. Whether or not isoflurane directly affects SPM production or affect some interaction between LTB4 and 15-HETE needs further investigation. Although we did not see a statistical significance in some of eicosanoid mediators, isoflurane treated samples showed a trend to show higher levels overall. Phospholipase A2 (PLA2) is an enzyme that releases arachidonic acid from cell membrane [38]. Because arachidonic acid also showed a trend to be higher in isoflurane arm, isoflurane may affect PLA2 function. This should be studied in the future.
In conclusion, we demonstrated that isoflurane attenuated lung injury in extrapulmonary sepsis model by a novel target BLT1.
Supplementary Material
Supplemental Table 1. Primer sequences used for RT-qPCR
Highlights.
Volatile anesthetic isoflurane exposure attenuated experimental sepsis mediated lung injury.
Isoflurane attenuated leukotriene B4 mediated neutrophil chemotaxis.
Isoflurane directly targeted BLT1 and compete its antagonist.
Financial support:
This study was in part supported by NIH R01 GM118277 (K.Y.) and MEXT/JSPS KAKENHI Grant Number 19K07357 (T.O.).
List of abbreviation:
- ALI
Acute lung injury
- VA
Volatile anesthetic
- LPS
Lipopolysaccharide
- CLP
Cecal ligation and puncture
- MPO
Myeloperoxidase
- TNF
Tumor necrosis factor
- IL
Interleukin
- IFN
Interferon
- MS
Mass spectrometry
- LT
Leukotriene
- APC
Allophycocyanin
- HETE
Hydroxyeicosatertraenoic acid
- PI3K
Phosphoinositide 3-kinase
- PL
Phospholipase
- GPCR
G protein-coupled receptor
- SPM
Specialized pre-resolving mediator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R, S. Surviving Sepsis Campaign Guidelines Committee including The Pediatric, Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012, Intensive Care Med 39 (2013) 165–228. 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yuki K, Murakami N, Sepsis pathophysiology and anesthetic consideration, Cardiovasc Hematol Disord Drug Targets 15 (2015) 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Phua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, Singh JM, Scales DC, Stather DR, Li A, Jones A, Gattas DJ, Hallett D, Tomlinson G, Stewart TE, Ferguson ND, Has mortality from acute respiratory distress syndrome decreased over time?: A systematic review, Am J Respir Crit Care Med 179 (2009) 220–227. 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- [4].Erickson SE, Martin GS, Davis JL, Matthay MA, Eisner MD, N.N.A. Network, Recent trends in acute lung injury mortality: 1996–2005, Crit Care Med 37 (2009) 1574–1579. 10.1097/CCM.0b013e31819fefdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Randolph AG, Management of acute lung injury and acute respiratory distress syndrome in children, Crit Care Med 37 (2009) 2448–2454. 10.1097/CCM.0b013e3181aee5dd. [DOI] [PubMed] [Google Scholar]
- [6].Dahlem P, van Aalderen WM, Bos AP, Pediatric acute lung injury, Paediatr Respir Rev 8 (2007) 348–362. 10.1016/j.prrv.2007.03.001. [DOI] [PubMed] [Google Scholar]
- [7].Finfer S, Bellomo R, Lipman J, French C, Dobb G, Myburgh J, Adult-population incidence of severe sepsis in Australian and New Zealand intensive care units, Intensive Care Med 30 (2004) 589–596. 10.1007/s00134-004-2157-0. [DOI] [PubMed] [Google Scholar]
- [8].Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K, E.I.G.o. Investigators, International study of the prevalence and outcomes of infection in intensive care units, JAMA 302 (2009) 2323–2329. 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- [9].Herzog T, Chromik AM, Uhl W, Treatment of complicated intra-abdominal infections in the era of multi-drug resistant bacteria, Eur J Med Res 15 (2010) 525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pearse RM, Moreno RP, Bauer P, Pelosi P, Metnitz P, Spies C, Vallet B, Vincent JL, Hoeft A, Rhodes A, European M Surgical Outcomes Study group for the Trials groups of the European Society of Intensive Care, A. the European Society of, Mortality after surgery in Europe: a 7 day cohort study, Lancet 380 (2012) 1059–1065. 10.1016/S0140-6736(12)61148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bedirli N, Demirtas CY, Akkaya T, Salman B, Alper M, Bedirli A, Pasaoglu H, Volatile anesthetic preconditioning attenuated sepsis induced lung inflammation, The Journal of surgical research 178 (2012) e17–23. 10.1016/j.jss.2011.12.037. [DOI] [PubMed] [Google Scholar]
- [12].Xiong XQ, Lin LN, Wang LR, Jin LD, Sevoflurane attenuates pulmonary inflammation and ventilator-induced lung injury by upregulation of HO-1 mRNA expression in mice, International journal of nanomedicine 6 (2013) 1075–1081. 10.2147/IJN.S41625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jung S, Yuki K, Differential effects of volatile anesthetics on leukocyte integrin macrophage-1 antigen, J Immunotoxicol 13 (2016) 148–156. 10.3109/1547691X.2015.1019596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Koutsogiannaki S, Schaefers MM, Okuno T, Ohba M, Yokomizo T, Priebe GP, DiNardo JA, Sulpicio SG, Yuki K, From the Cover: Prolonged Exposure to Volatile Anesthetic Isoflurane Worsens the Outcome of Polymicrobial Abdominal Sepsis, Toxicol Sci 156 (2017) 402–411. 10.1093/toxsci/kfw261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yuki K, Astrof NS, Bracken C, Yoo R, Silkworth W, Soriano SG, Shimaoka M, The volatile anesthetic isoflurane perturbs conformational activation of integrin LFA-1 by binding to the allosteric regulatory cavity, FASEB J 22 (2008) 4109–4116. 10.1096/fj.08-113324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yuki K, Bu W, Xi J, Sen M, Shimaoka M, Eckenhoff RG, Isoflurane binds and stabilizes a closed conformation of the leukocyte function-associated antigen-1, FASEB J 26 (2012) 4408–4417. 10.1096/fj.12-212746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yuki K, Mitsui Y, Shibamura-Fujiogi M, Hou L, Odegard KC, Soriano SG, Priebe GP, Koutsogiannaki S, Anesthetics isoflurane and sevoflurane attenuate flagellin-mediated inflammation in the lung, Biochem Biophys Res Commun 557 (2021) 254–260. 10.1016/j.bbrc.2021.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, N.C.R.R.G.W. Group, Animal research: reporting in vivo experiments: the ARRIVE guidelines, Br J Pharmacol 160 (2010) 1577–1579. 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dunne JL, Ballantyne CM, Beaudet AL, Ley K, Control of leukocyte rolling velocity in TNF-alpha-induced inflammation by LFA-1 and Mac-1, Blood 99 (2002) 336–341. 10.1182/blood.v99.1.336. [DOI] [PubMed] [Google Scholar]
- [20].Liu JR, Han X, Soriano SG, Yuki K, The role of macrophage 1 antigen in polymicrobial sepsis, Shock 42 (2014) 532–539. 10.1097/SHK.0000000000000250. [DOI] [PubMed] [Google Scholar]
- [21].Okuno T, Koutsogiannaki S, Hou L, Bu W, Ohto U, Eckenhoff RG, Yokomizo T, Yuki K, Volatile anesthetics isoflurane and sevoflurane directly target and attenuate Toll-like receptor 4 system, FASEB J 33 (2019) 14528–14541. 10.1096/fj.201901570R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Boxio R, Bossenmeyer-Pourie C, Steinckwich N, Dournon C, Nusse O, Mouse bone marrow contains large numbers of functionally competent neutrophils, J Leukoc Biol 75 (2004) 604–611. 10.1189/jlb.0703340. [DOI] [PubMed] [Google Scholar]
- [23].Kamakura S, Nomura M, Hayase J, Iwakiri Y, Nishikimi A, Takayanagi R, Fukui Y, Sumimoto H, The cell polarity protein mInsc regulates neutrophil chemotaxis via a noncanonical G protein signaling pathway, Dev Cell 26 (2013) 292–302. 10.1016/j.devcel.2013.06.008. [DOI] [PubMed] [Google Scholar]
- [24].Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T, A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis, Nature 387 (1997) 620–624. 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- [25].Okuno T, Ishitani T, Yokomizo T, Biochemical characterization of three BLT receptors in zebrafish, PLoS One 10 (2015) e0117888. 10.1371/journal.pone.0117888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hori T, Okuno T, Hirata K, Yamashita K, Kawano Y, Yamamoto M, Hato M, Nakamura M, Shimizu T, Yokomizo T, Miyano M, Yokoyama S, Na(+)-mimicking ligands stabilize the inactive state of leukotriene B4 receptor BLT1, Nat Chem Biol 14 (2018) 262–269. 10.1038/nchembio.2547. [DOI] [PubMed] [Google Scholar]
- [27].Zha H, Matsunami E, Blazon-Brown N, Koutsogiannaki S, Hou L, Bu W, Babazada H, Odegard KC, Liu R, Eckenhoff RG, Yuki K, Volatile anesthetics affect macrophage phagocytosis, PLoS One 14 (2019) e0216163. 10.1371/journal.pone.0216163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mukhopadhyay S, Hoidal JR, Mukherjee TK, Role of TNFalpha in pulmonary pathophysiology, Respir Res 7 (2006) 125. 10.1186/1465-9921-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Liu B, Bao L, Wang L, Li F, Wen M, Li H, Deng W, Zhang X, Cao B, Anti-IFN-gamma therapy alleviates acute lung injury induced by severe influenza A (H1N1) pdm09 infection in mice, J Microbiol Immunol Infect 54 (2021) 396–403. 10.1016/j.jmii.2019.07.009. [DOI] [PubMed] [Google Scholar]
- [30].Monteiro AP, Soledade E, Pinheiro CS, Dellatorre-Teixeira L, Oliveira GP, Oliveira MG, Peters-Golden M, Rocco PR, Benjamim CF, Canetti C, Pivotal role of the 5-lipoxygenase pathway in lung injury after experimental sepsis, Am J Respir Cell Mol Biol 50 (2014) 87–95. 10.1165/rcmb.2012-0525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Serhan CN, On the relationship between leukotriene and lipoxin production by human neutrophils: evidence for differential metabolism of 15-HETE and 5-HETE, Biochim Biophys Acta 1004 (1989) 158–168. 10.1016/0005-2760(89)90264-6. [DOI] [PubMed] [Google Scholar]
- [32].Chiang N, Schwab JM, Fredman G, Kasuga K, Gelman S, Serhan CN, Anesthetics impact the resolution of inflammation, PLoS One 3 (2008) e1879. 10.1371/journal.pone.0001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dixit N, Wu DJ, Belgacem YH, Borodinsky LN, Gershwin ME, Adamopoulos IE, Leukotriene B4 activates intracellular calcium and augments human osteoclastogenesis, Arthritis Res Ther 16 (2014) 496. 10.1186/s13075-014-0496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ichiki T, Koga T, Okuno T, Saeki K, Yamamoto Y, Yamamoto H, Sakaguchi M, Yokomizo T, Modulation of leukotriene B4 receptor 1 signaling by receptor for advanced glycation end products (RAGE), FASEB J 30 (2016) 1811–1822. 10.1096/fj.201500117. [DOI] [PubMed] [Google Scholar]
- [35].Newton K, Dixit VM, Signaling in innate immunity and inflammation, Cold Spring Harb Perspect Biol 4 (2012). 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ishizawa Y, Pidikiti R, Liebman PA, Eckenhoff RG, G protein-coupled receptors as direct targets of inhaled anesthetics, Mol Pharmacol 61 (2002) 945–952. 10.1124/mol.61.5.945. [DOI] [PubMed] [Google Scholar]
- [37].Ternowitz T, Andersen PH, Bjerring P, Fogh K, Schroder JM, Kragballe K, 15-hydroxyeicosatetraenoic acid (15-HETE) specifically inhibits the LTB4-induced skin response, Arch Dermatol Res 281 (1989) 401–405. 10.1007/BF00455325. [DOI] [PubMed] [Google Scholar]
- [38].Dennis EA, Norris PC, Eicosanoid storm in infection and inflammation, Nat Rev Immunol 15 (2015) 511–523. 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Primer sequences used for RT-qPCR


