Abstract
Purpose:
To assess global, zonal, and local correlations between vessel density changes measured by optical coherence tomography angiography (OCTA) and retinal sensitivity measured by microperimetry across diabetic retinopathy severity.
Methods:
Diabetic patients with and without retinopathy, and age matched non-diabetic controls, underwent OCTA imaging and microperimetry testing. Skeletonized vessel density (SVD) was computed from 3 mm x 3 mm OCTA images of the superficial capillary plexus, deep capillary plexus, and full retinal layer, excluding larger retinal vessels and the foveal avascular zone (FAZ). Pearson’s correlation was used to assess associations between average sensitivity and vessel density globally and in four parafoveal zones; centrally, average sensitivity was correlated with the FAZ area. Linear mixed effects modeling was used to assess relationships between local SVD measurements and their spatially corresponding retinal sensitivity measurements.
Results:
Thirty-nine eyes from 39 participants were imaged. In all slabs, there was a statistically significant positive correlation between retinal sensitivities and SVDs on both global and zonal scales. No statistically significant correlation was found between central retinal sensitivities and the FAZ areas. Assessment of 1,136 spatially paired retinal sensitivity and SVD measurements revealed a statistically significant local relationship; this appeared to be driven by eyes with proliferative diabetic retinopathy that had reduced retinal sensitivities.
Conclusions:
Data from this study supports positive correlations between SVD and retinal sensitivity at global and zonal spatial scales in the parafovea of diabetic eyes. However, our analysis did not find evidence of statistically significant correlations between retinal sensitivity and SVD on a local scale until advanced diabetic retinopathy.
Keywords: Diabetic retinopathy, microperimetry, optical coherence tomography angiography, OCTA, retina
Summary statement:
Vessel density changes measured by optical coherence tomography angiography and microperimetry-derived retinal sensitivity from patients with diabetic retinopathy correlated globally and zonally, as well as locally at reduced sensitivities in late disease.
Diabetic retinopathy (DR) is a common and potentially blinding microvascular complication of diabetes mellitus (DM).1 Optical coherence tomography angiography (OCTA) is a non-invasive imaging modality that has been utilized to evaluate macular capillary impairment in DR. Compared to the standard assessment of retinal vasculature with two-dimensional fluorescein angiography (FA), OCTA allows for distinct visualization of the different retinal capillary plexuses. Studies using OCTA have yielded insights into the microvascular changes secondary to DR, including enlargement of the foveal avascular zone (FAZ), increased areas of capillary non-perfusion, and reduced vessel density compared to non-diabetic subjects.2-6 Additionally, it has been demonstrated that the severity of DR correlates with changes in these OCTA metrics.2,7-12 Microvascular abnormalities have even been identified in diabetic patients prior to clinical visualization of signs of retinopathy.13,14
Although many OCTA metrics have been correlated with DR severity, it remains unclear to what extent OCTA-measured blood flow changes correlate with functional parameters. Studies have shown correlation of enlarging FAZ on OCTA with worsening of visual acuity in diabetic patients.15-19 However, FAZ size has been found to be highly variable on OCTA and to enlarge with age in healthy eyes without affecting visual acuity.20-24 There are also mixed findings regarding correlations between retinal vessel density metrics and visual acuity in DR. Vessel density in the deep capillary plexus (DCP) and the superficial capillary plexus (SCP) appear to correlate with visual acuity in DR,16,17,19 although visual acuity only represents impairment of foveal visual function, and is not necessarily a good proxy of global visual function.18
Microperimetry (MP) is a visual function test that generates a topographic retinal sensitivity map of the level of light measured in decibels (dB) actively detected across the retina by the participant. The fundus-registration of MP enables correlations of morphologic and functional changes that are not possible with other functional assessment techniques, such as electroretinogram and perimetry.25 Using microperimetry and OCT and/or FA, the reduction of retinal sensitivity with increasing DR severity has been well documented in conjunction with various morphologic features of DR including diabetic macular edema, reduced ganglion cell complex thickness, hard exudates, and capillary nonperfusion.26-37
There are several recent studies comparing OCTA metrics to retinal sensitivity measured by microperimetry in diabetic retinopathy.38-41 While these studies report a positive association between OCTA parameters related to retinal ischemia (such as reduced vessel density and increased areas of capillary nonperfusion) and reduced MP sensitivity in DR, these comparisons have been predominantly qualitative.38,40 If correlation was performed, it was done so only globally41 or without comparing across DR severity.39 The aim of this study is to quantitatively assess the relationships between vessel density and retinal sensitivity at global, zonal, and local spatial scales across DR severity.
Methods
Subjects
This study received institutional review board approval from Tufts Medical Center and was performed in accordance with the Declaration of Helsinki and the Health Insurance Portability and Accountability Act.
Diabetic individuals without diabetic retinopathy (DM no DR), with mild, moderate, or severe nonproliferative diabetic retinopathy (NPDR), and with proliferative diabetic retinopathy (PDR) were prospectively recruited from and imaged at the New England Eye Center, Boston, MA between October 2019 and April 2021. A group of age matched control subjects without diabetes and lacking retinal disease was also recruited and imaged with the same protocols. Informed consent was obtained from the subjects after explanation of the nature and possible consequences of the study. Diabetic patients underwent clinical assessment by a retinal specialist (CB, AW, NK, JSD) to determine the severity of retinopathy, if any. Exclusion criteria included any concomitant posterior segment pathology such as age-related macular degeneration, retinal detachment, vitreomacular traction, vascular occlusions, and myopic refractive error ≥ 6 diopters, as well as any pathology hindering a clear view to the retina including clinically significant cataracts. Diabetic macular edema was not an exclusion criterion. Participants were excluded from the final analysis if OCTA images had significant image artifact or color fundus photographs on the microperimetry exam were of low quality.
Microperimetry acquisition and sensitivity calculations
Microperimetry was performed using the MP-3 microperimeter (Nidek, Gamagori, Japan). Only one eye was selected for study inclusion per participant. If OCTA imaging was acquired first that day for routine clinical use prior to the microperimetry assessment, the eye with a better subjective image (i.e. less artifact) was selected. Patients were also imaged prior to treatment, if any. Otherwise, the eye was selected at random. Microperimetry assessments were conducted in a dark room under pharmacologic pupil dilation while the contralateral non-study eye was patched. The MP-3 microperimeter utilizes an automated eye tracking system that corrects for eye movement to ensure the projected stimuli are properly aligned to the fundus image. Prior to MP for study use, suprathreshold pretest training was performed using a sparse grid of 9 stimulus points centered at the fovea. The goal of this practice exam was to normalize against any learning curve that might reduce the participant’s ability to accurately respond to stimuli during the study MP examination. A shortened practice test of 9 points, rather than the the 37 points used in the study examination, was used with the aim of reducing the participants’ time burden and minimizing fatigue.
The stimulation pattern for study-eye examination consisted of 37 total points covering the central 10-degree field, with 12 stimulus points in rings of 1-degree, 3-degree, and 5-degree radii, and one stimulus point at the central fovea (Figure 1A). The stimulation pattern was always mapped to the physiologic foveal center at 0 degrees, not to the patients’ fixation point (Figure 1A). This custom grid thus aligned with the central and inner sectors of the standard ETDRS grid for correlative zonal analysis.32,38,42,43 The stimulus size was Goldman III with a duration of 200 milliseconds (ms) and the dynamic range of the stimulus intensity was 0-34 dB. Examinations were performed with a background luminosity of 4 apostilbs. The standard 1-degree red cross was used as the fixation target and the stimulation algorithm followed the 4-2 threshold strategy to evaluate sensitivity. Participants were instructed to look at the fixation target even when they saw a flash of light and were encouraged throughout the exam to maintain fixation of the target.
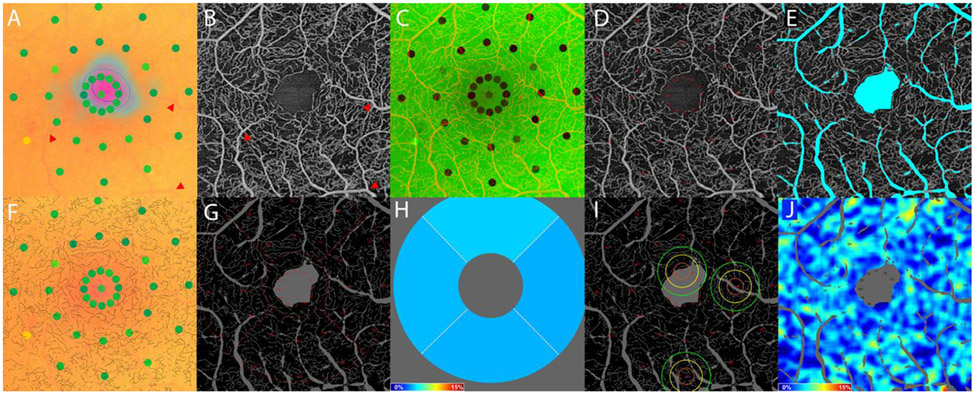
Figure 1.
Image analysis methodology using images from the right eye of a patient with severe non-proliferative diabetic retinopathy. The microperimetry exam output was transformed so as to be spatially aligned with its corresponding 3 mm x 3 mm OCTA image. (A) The microperimetry exam does not map to the center of the fixation cloud. (A-B) Registration was performed using large vessel bifurcations (e.g., those pointed to with red arrows). (C) Microperimetry exam and OCTA image registration overlay. (D) Microperimetry sensitivity positions were transformed from the microperimetry coordinate frame into the 3x3mm OCTA coordinate frame. (E) Large vessels and the FAZ were masked prior to OCTA image processing to exclude from analysis. (F) The 3 mm x 3 mm OCTA images were binarized using Otsu’s method, then skeletonized, and overlaid onto the registered microperimetry image to confirm registration accuracy. (G) Global and zonal skeletonized vessel density were computed from the skeletonized vessel image, excluding the large vessels and FAZ. (H) The zonal skeletonized vessel density was computed within the superior, inferior, nasal, and temporal inner subfields of the ETDRS grid centered on the FAZ. (I) Three examples of local skeletonized vessel density calculations centered at microperimetry points #1, #16, and #31 with kernel isocontours at 1σ (orange), 2σ (yellow), and 3σ (green). (J) Skeletonized vessel densities were extracted from each position of sensitivity measurement.
OCTA image acquisition
Study eyes were imaged on the swept-source OCTA PLEX Elite 9000 (Carl Zeiss Meditec, Dublin, CA). The PLEX Elite 9000 operates at 100,000 A-scans per second with a central wavelength of 1,060 nm, an axial resolution of approximately 6 μm, and a depth of 3 mm. The macula-centered 3 mm x 3 mm en face images comprise 300 A-scans per B-scan and 300 B-scans per volume, while the 6 mm x 6 mm images comprise 500 A-scans per B-scan and 500 B-scans per volume. Automated segmentation of the superficial capillary plexus (SCP), deep capillary plexus (DCP), and full retinal layer (FRL) slab was performed using the device’s proprietary segmentation scheme. Images requiring manual segmentation (i.e. secondary to DME aberrations) were excluded from the study. Images were resized to a standard pixel resolution upon export based on proprietary device-manufacturer settings.
Registration of microperimetry and OCTA images
All image registration and analyses were performed in MATLAB 2019b (Mathworks, Inc., Natick, MA). Microperimetry images (sensitivity maps superimposed on their respective color fundus photographs) were registered with 3 mm x 3 mm en face FRL OCTA images using fiducial markers manually positioned at corresponding bifurcations of the retinal vasculature (Figure 1A-D). In cases in which a 6 mm x 6 mm OCTA image was available, the microperimetry image was first registered to the 6 mm x 6 mm image and then to the 3 mm x 3 mm image. The rationale for this two-step approach is that the 6 mm x 6 mm OCTA images contains larger vessel bifurcations that can more easily be visualized in the color fundus image of the MP acquisition. There were only 5 cases in which an adequate 6 mm x 6 mm OCTA image was not available for this two-step registration approach, in which case, the 3 mm x 3 mm image was utilized for registration.
OCTA image processing and analysis
Global, zonal, and local measurements (defined below) were performed on en face 3 mm x 3 mm OCTA images from the SCP, DCP, and FRL slabs. For all analyses, the FAZ was excluded, as were regions corresponding to larger retinal vasculature. The FAZ area was manually traced using the FRL slab, which was selected for tracing because the FAZ structure extends through the entire foveal thickness.44 Larger retinal vasculature was identified using Frangi vesselness filtering of the SCP images (Figure 1E; see Appendix I for details). An adaptive, global binarization level was automatically selected using Otsu’s method, which was then applied to binarize the 3 mm x 3 mm retinal OCTA projections.45-46 Binarized images were then skeletonized, thereby reducing all vessel widths to 1 pixel. Vessel skeletonization has the advantage of mitigating the effects of optical aberrations. Skeletonized vessel density (SVD) measurements were computed from the skeletonized vessel images as the ratio of vascular length to the field-of view area. In particular, global SVDs were computed over the entire (non-excluded) field-of-view, while the zonal SVDs were was computed within the (non-excluded) superior, inferior, nasal, and temporal inner subfields of the ETDRS grid (Figure 1G-H). Local SVDs were computed using a Nadaraya-Watson estimator on the skeletonized vessel image with the FAZ and large retinal vessels treated as missing data (Figure 1I; see Appendix I for details). For the local analysis, local SVDs were extracted at each sensitivity measurement, yielding a set of spatially paired sensitivity and SVD measurements (Figure 1J). Sensitivity measurements positioned within the FAZ were excluded from the local analysis.
Statistical analysis
One-way ANOVA with Tukey’s test for post-hoc analysis was utilized to compare average retinal sensitivity, SVD, and FAZ area measurements across disease severity. Pearson’s correlation was computed between SVD and retinal sensitivity measurements for the global and zonal analyses. Since the central sensitivity points primarily encompass the FAZ, in which vessel density is not meaningful, Pearson’s correlation was also computed between the FAZ area and the average sensitivity points from the 1-degree ring and the central sensitivity point.
Local relationships between sensitivity and SVD were evaluated using linear mixed effects models (LMM). A random intercept was added at the subject level to account for the dependence among multiple measures performed for each subject, and a random intercept for the sensitivity rings was nested under the subject level to account for dependence among measures with respect to distance from the foveal center. Disease severity and SVD were included as fixed effects. ANOVA was used to test the fixed effects, and the Kenward-Rogers approximation was used to evaluate significance.47 A p-value of < 0.05 was considered significant. All statistical analyses were performed using RStudio version 1.1.463 with the lme4 library for mixed effects modeling, the lmerTest library for the Kenward-Rogers approximation, and the ggeffects and ggplot2 libraries for graphing.48-52
Results
Fifty-four participants were recruited for this study but only 39 participants met study inclusion criteria with same day PLEX Elite 9000 OCTA imaging and MP-3 microperimeter testing of adequate quality.
Twenty-two participants were male and 17 were female; 24 right eyes and 15 left eyes were included. The mean age of participants was 58 years old (range: 32-87 years). Table 1 shows the demographics of study participants stratified by DR severity.
Table 1.
Participant demographics and global measurements by diabetic retinopathy severity. DCP = deep capillary plexus, DM = diabetes mellitus, DR = diabetic retinopathy, FRL = full retinal layer, IVI = intravitreal injection, LP = laser photocoagulation, NPDR = non-proliferative diabetic retinopathy, PDR = proliferative diabetic retinopathy, SCP = superficial capillary plexus.
| Normal | DM no DR | Mild NPDR | Moderate NPDR |
Severe NPDR |
PDR | ||
|---|---|---|---|---|---|---|---|
| n | 7 | 8 | 6 | 4 | 6 | 8 | |
| Average age (range) | 55 (42-78) | 59 (49-79) | 66 (51-87) | 64 (53-72) | 56 (51-74) | 52 (32-70) | |
| Male/Female | 3/4 | 4/4 | 2/4 | 2/2 | 5/1 | 6/2 | |
| OD/OS | 4/3 | 5/3 | 4/2 | 2/2 | 4/2 | 5/3 | |
| Mean visual acuity, Snellen | 20/21 | 20/33 | 20/34 | 20/40 | 20/33 | 20/40 | |
| Pseudophakic | 1/7 | 0/7 | 2/6 | 3/4 | 2/6 | 2/8 | |
| DME | - | - | 1/6 | 4/4 | 2/6 | 2/8 | |
| Prior treatment | - | - | IVI x 1 IVI x 5 |
IVI x 45 IVI x 1 IVI x 2, LP x 2 |
LP x 1 IVI x 7, LP x 1 |
IVI x 3 IVI x 2 LP x 3 LP x 1 IVI x 2, LP x 3 IVI x 6, LP x 2 IVI x 9, LP x 1 |
Consistent with previous reports, in this cohort retinal sensitivity measurements generally decreased as DR severity increased. Post hoc analysis of one-way ANOVA revealed a statistically significant difference in retinal sensitivity between normal versus PDR eyes globally (p = 0.017), zonally (superior: p = 0.018; nasal: p = 0.018; inferior: p = 0.015; and temporal: p = 0.05), and within the central 1-degree ring (p < 0.001), as well as between PDR versus DM no DR (p = 0.001), mild NPDR (p = 0.008) and severe NPDR (p = 0.008) again within the central 1-degree ring (Figure 2). Additionally, in general, SVD decreased as disease severity increased, with the exception of the SVD measurements from the FRL, as well as SVD measurements from the DCP globally and in the superior and nasal sectors. Post hoc analysis of one-way ANOVA revealed statistically significant differences between normal and PDR eyes in the inferior sector of the DCP slab; between normal and mild NPDR eyes, normal and moderate NPDR eyes, and normal and PDR eyes in the nasal sector of the FRL slab; and between DM no DR and mild NPDR eyes as well as DM no DR and moderate NPDR eyes in the nasal sector of the FRL slab. (Figure 3). There was no statistically significant difference in FAZ area across disease severity in this cohort (p = 0.982).
Figure 2.
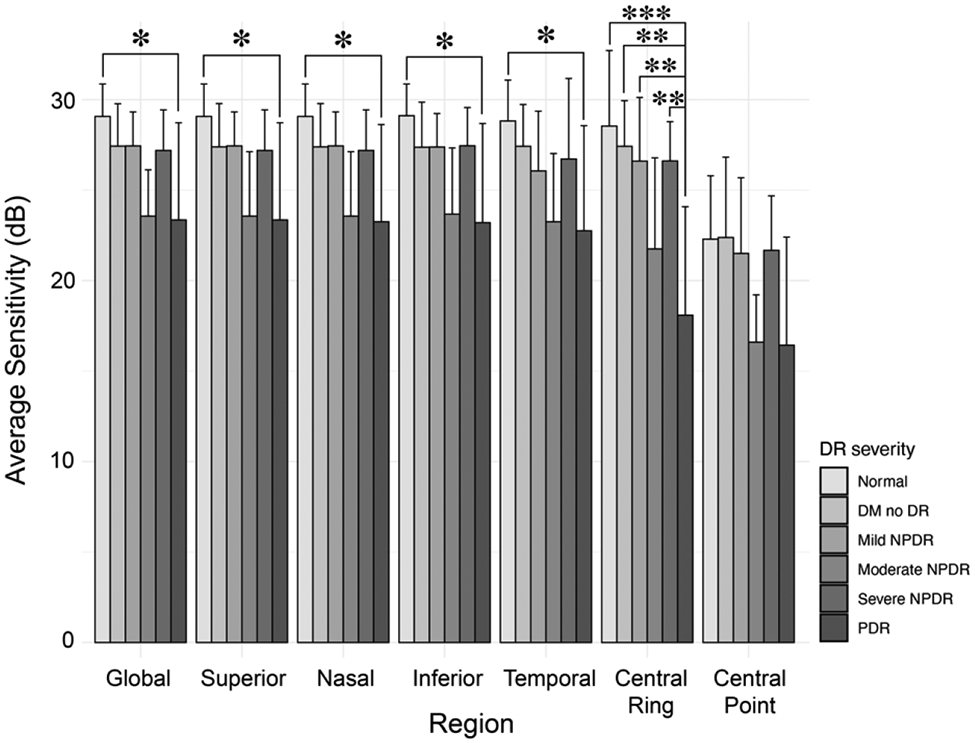
Average retinal sensitivity by region per diabetic retinopathy severity group. *p ≤ 0.05, **p ≤ 0.01, *** p ≤ 0.001. DM = diabetes mellitus, DR = diabetic retinopathy, NPDR = non-proliferative diabetic retinopathy, PDR = proliferative diabetic retinopathy
Figure 3.
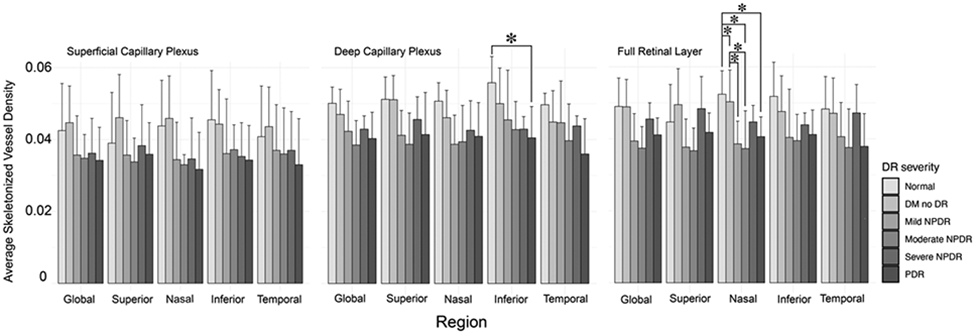
Average skeletonized vessel density by region per diabetic retinopathy severity group. *p ≤ 0.05. DM = diabetes mellitus, DR = diabetic retinopathy, NPDR = non-proliferative diabetic retinopathy, PDR = proliferative diabetic retinopathy
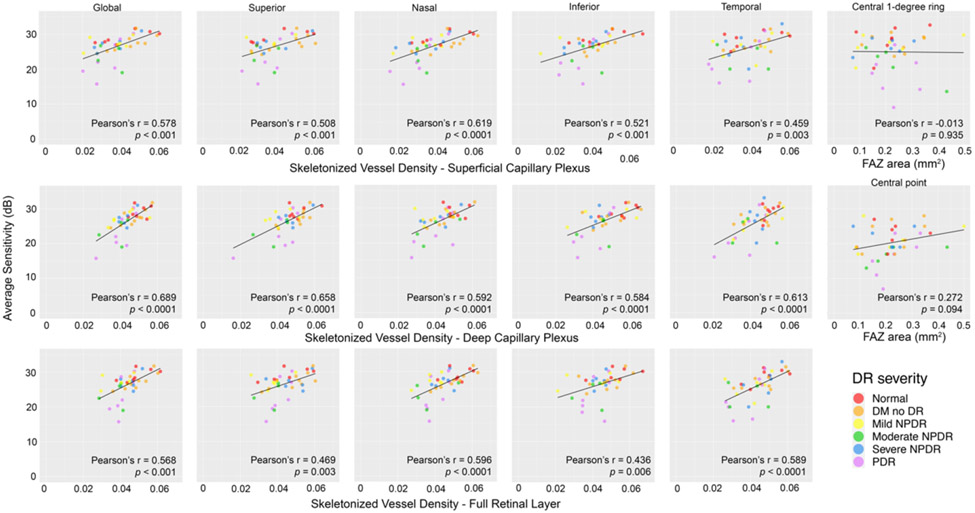
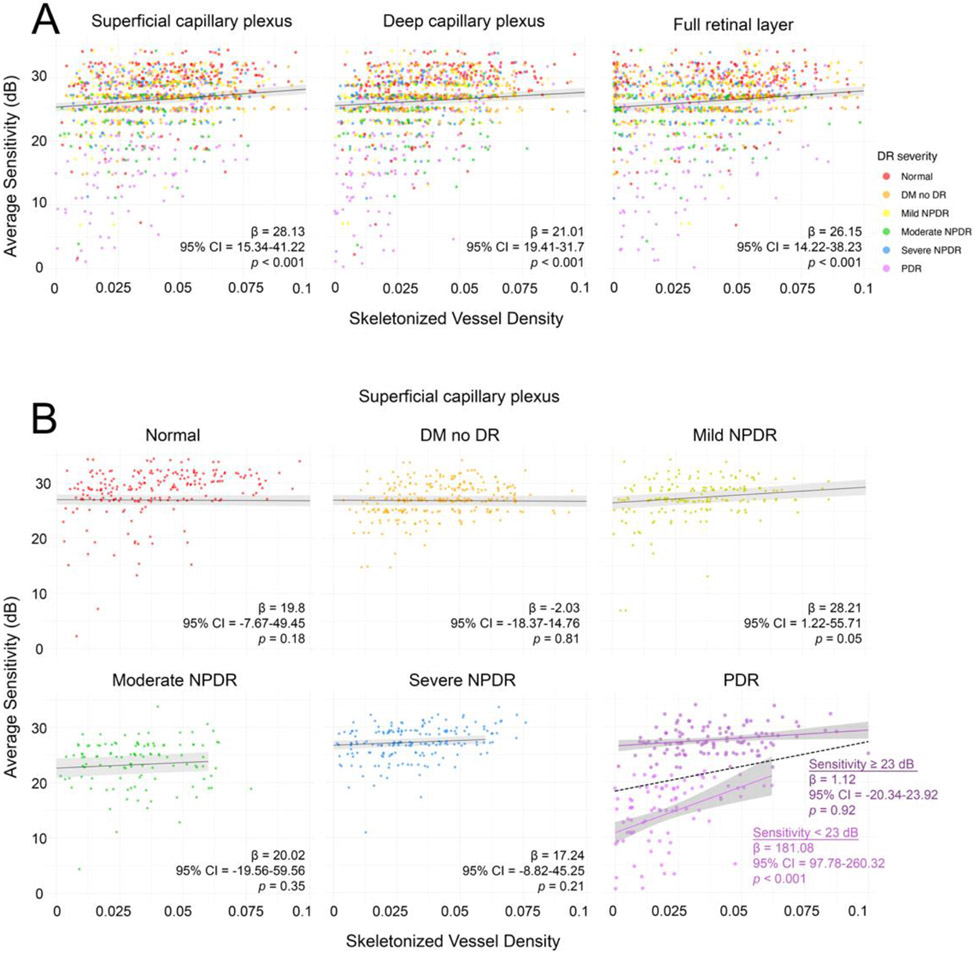
There was a statistically significant positive correlation between retinal sensitivity and SVD measurements in all slabs globally and in the parafoveal regions, but not between retinal sensitivity of the 1-degree ring and central point with FAZ area (Figure 4). Paired retinal sensitivity and SVD measurements were analyzed at 1,136-point coordinates per slab. There was a statistically significant positive linear relationship found between local retinal sensitivity and SVD with the 100 μm kernel in all slabs when all disease severities were pooled (Figure 5A). There was also a statistically significant negative linear relationship between point sensitivity and disease severity by linear mixed effects modeling in the SCP (β = −1.07, 95% CI = −1.73 to −0.42, p = 0.003), DCP (β = −1.1, 95% CI = −1.77 to −0.42, p = 0.003), and FRL (β = −1.09, 95% CI = −1.76 to −0.42, p = 0.003). When each severity category was evaluated individually, statistical significance between sensitivity and SVD was only maintained among PDR eyes (Figure 5B). The PDR datapoints (n = 252) were then bisected into two groups at the average sensitivity of the PDR group (23 dB), with 120-point coordinates containing a sensitivity below this average and 132-point coordinates containing a sensitivity equal to or above this average. In this bisected analysis, a statistically significant positive linear relationship between sensitivity and SVD was only maintained in the group with reduced sensitivity (Figure 5B). The average SVD was statistically significantly lower in the reduced sensitivity group compared to the average or higher sensitivity group (SCP = 0.02 versus 0.039, DCP = 0.027 versus 0.039, and FRL = 0.03 versus 0.041, respectively; all p < 0.001).
Figure 4.
Global and zonal scale assessment of Pearson’s correlation between average retinal sensitivity and skeletonized vessel density (SVD) in the superficial capillary plexus, deep capillary plexus, and full retinal layer across diabetic retinopathy severity globally and in the superior, nasal, inferior, and temporal perifoveal regions (1st through 5th columns). Pearson’s correlation of average retinal sensitivity in the central 1-degree ring and central point with foveal avascular zone area across diabetic retinopathy severity (right-most column). DR = diabetic retinopathy, DM = diabetes mellitus, FAZ = foveal avascular zone, NPDR = non-proliferative diabetic retinopathy, PDR = proliferative diabetic retinopathy.
Figure 5.
Local relationships between retinal sensitivities and SVD. (A) Local relationships for pooled disease severities across the superficial capillary plexus, deep capillary plexus, and full retinal layer. (B) Local relationships for separated disease severities across the superficial capillary plexus. The data in the PDR plot shows additional analysis with data bisected at the average PDR sensitivity (23 dB), revealing a significant linear relationship between retinal sensitivity and SVD in only the reduced sensitivities group. Dashed line reflects the trendline prior to data bisection. DR = diabetic retinopathy, NPDR = non-proliferative diabetic retinopathy, PDR = proliferative diabetic retinopathy.
Discussion
This study investigated relationships between retinal sensitivities measured by MP and skeletonized vessel density measured with OCTA in eyes stratified by DR severity. Our results corroborate findings of prior studies demonstrating that vessel density correlates with retinal sensitivity measured by MP on a global scale and in perifoveal regions.38-41 This finding was true for all three retinal vasculature slabs tested. We believe, however, that this is the first paper to demonstrate a quantitative, point-wise structure-function relationship between retinal sensitivity and vessel density in DR. Prior studies have qualitatively observed that points of reduced retinal sensitivity spatially align with local areas of vessel loss in DR. Scarinci et al. noted a correspondence between areas of capillary non-perfusion with areas of reduced retinal sensitivity in patients with severe NPDR and PDR, although these results were not quantified.40 Pereira et al., too, suggested areas of lower retinal sensitivity subjectively aligned with areas of capillary dropout on OCTA in a case series of patients with diabetic macular ischemia.38 Similar findings have also been reported using FA instead of OCTA.27,53
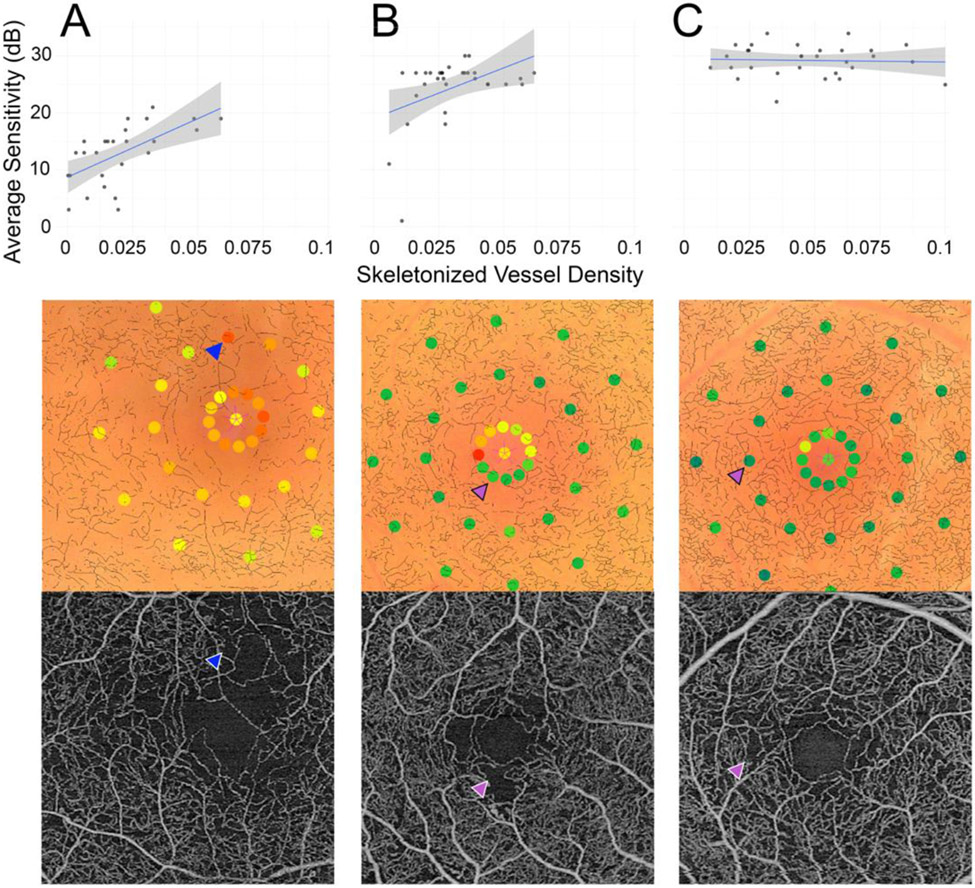
An interesting finding from our study is that the local relationship between retinal sensitivity and SVD appears to be driven by the points of reduced sensitivity in PDR eyes. In other words, a region of retinal flow impairment most often accompanied a region of functional loss but not vice versa, and this was only evident in late-stage disease. Figure 6 depicts three individual PDR cases further illustrating this relationship. Figure 6A is a case of PDR wherein reduced sensitivity points correspond to areas of reduced vessel density, while Figure 6B-C show cases where not all areas of reduced sensitivity were associated with points of reduced sensitivity (purple arrows).
Figure 6.
Three representative PDR cases illustrated by local structure-function graphs, the microperimetry exam with registered skeletonized vessel overlay, and 3 mm x 3 mm OCTA image (all data from the superficial capillary plexus). (A) The right eye of a 59-year-old woman. Blue arrows delineate an example of reduced sensitivity accompanied by a flow deficit. (B) The right eye of a 62-year-old man. Purple arrows show a high sensitivity point that corresponded to a flow deficit area. (C) The right eye of a 32-year-old woman. Purple arrows show a high sensitivity point that corresponded to a flow deficit area.
One plausible explanation for this observation is that local ischemia is not the primary local causative driver of retinal dysfunction in DR. This explanation would be consistent with the neurodegenerative theory of DR, which posits that retinal neurons are chiefly affected in DR and it is their reaction to microenvironment stressors that then subsequently causes vascular complications.52-56 The neurodegenerative theory of DR contrasts with the classic hypothesis of DR as primarily a retinal vascular disorder with a pathogenesis rooted in glycosylation-induced microvascular damage, leading to ischemic neuronal injury—a theory that appears more consistent with a pattern of functional loss accompanying flow deficits. There exists a large body of work suggesting both neurodegeneration and glial dysfunction precede microvascular changes in DR.56, 57, 59 Studies assessing neuroglial dysfunction using multifocal ERG have shown that increased implicit times can predict the development of retinal vascular dysfunction in specific retinal locations.60-62 However, a recent study did not find a correlation with OCTA vascular metrics and mfERG in DM.63 Importantly, our study was not designed to investigate the causal relationship between vascular and neuroretinal damage in DR. In fact, an alternative hypothesis to that discussed above would be that local effects are visible only when there is very profound ischemia, which would support the hypothesis that DR is primarily a retinal vascular disease with functional deficits becoming apparent later in disease course. This seems less plausible, however, given that we do see broad functional loss early in disease. We nevertheless hope that some of the methods demonstrated in this study may find usage in future studies examining these causal relationships.64
Montesano et al. recently published a paper showing a significant relationship between both microperimetry and frequency doubling technology (FDT) perimetry with ganglion cell count in diabetic patients without DR and healthy controls.65 They argue that by excluding patients with DR, their results support early neuronal loss ahead of clinically evident vascular alterations – the neurodegenerative theory – in diabetes.65 However, it is known that patients without clinically evident DR still have apparent microvascular derangements as evidenced by OCTA studies, and therefore further work is required to integrate OCTA image analysis into a similar investigation to fully support this claim.13,14 Still, they interestingly did not find a significant difference in sensitivity between healthy and diabetic patients using microperimetry but did with FDT perimetry. They subsequently outline an important mathematical model that suggests modifying the microperimetric test to use a smaller stimulus size (Goldmann I) could improve detection of neuronal damage, which may be important to consider in future studies. Their work might help to explain why this study did not find a consistent pattern in retinal sensitivity reduction as DR severity progressed, as perhaps the larger Goldmann III stimulus size utilized in this study only produced partial summation conditions, which would reduce the sensitivity in picking up differences across the spectrum of disease severity.65
We used FAZ area as a proxy for vessel loss in our comparison to central sensitivity points encompassing the FAZ. While FAZ area has been shown to increase with DR severity, this trend was not evident in our cohort.9,10 Subsequently, there was no apparent trend in central foveal sensitivity with FAZ area. Although FAZ area has also been reported to be highly variable, the lack of a correlation to DR severity in this study underscores the small sample size, which is a primary limitation to this study.22-24 Previously, Tsai et al. did find a correlation between retinal sensitivity and increasing FAZ area measured from the superficial capillary plexus.41
Our study has several limitations that are important to consider when interpreting our findings. One notable limitation to this study is that our choice of Gaussian kernel size (100 μm standard deviation) imposes a definition of what spatial relationships are—and are not—considered “local” by our analysis. In particular, choices of larger kernel sizes would capture spatial relationships occurring at larger spatial scales, and vice versa. Because there lacks data supporting the usage of a particular spatial scale, the kernel size of our study was chosen so as to balance the need for: (1) a plausible physiologic scale of local interaction; (2) a sufficiently large scale so as not to be affected by small errors in data registration; and (3) a sufficiently small scale so as to avoid substantial overlap between measurements at neighboring positions, which would reduce their independence. Recognizing the constraints imposed by the selection of a single scale, we repeated the local analysis using Gaussian kernels having standard deviations of 50, 150, 200, 250 and 300 μm. Applying the same LMM structure as in the primary analysis, a significant relationship between vessel density and retinal sensitivity point coordinates from severe NPDR eyes began to appear with a kernel size of 200 μm and was maintained in PDR eyes at a kernel size of 150 and onward (data not shown). However, it is difficult to compare findings from different kernel sizes because they have differing degrees of sample overlap and therefore violate, to differing degrees, the assumption of independent samples, as well as the assumptions of grouping accounted for by the model. We believe that further exploration is warranted to investigate the spatial level at which vessel density and retinal sensitivity first begin to correlate.
Other limitations to this study include utilizing a small cohort of eyes, which undermines the generalizability of these results, and the potential for variability across microperimetry measurements. We attempted to reduce the latter by employing a training exam before the study test. Prior studies have generally shown low variability in global measurements among macular diseases but greater variability in pointwise sensitivity, as was used in the local analysis.66-68 However, the local analysis was performed on a large sample of point coordinates, which may help reduce the effects of noise within the sample set. Given the small sample of total eyes, a global and zonal analysis per disease severity category could not be performed, limiting our conclusions. Finally, we recognize that our chosen metric of vascular change – SVD – does not fully capture the ischemic process or other vascular alterations that might be relevant to assess alongside retinal function.
In conclusion, in our cohort of DR eyes, OCTA-measured SVD correlated with retinal sensitivity at global and zonal spatial scales in the parafovea; statistically significant associations at local spatial scales were only observed at measurement points of reduced sensitivities in late disease. The methods used for assessing local structure-function relationships may find use in future studies of DR and other retinal pathologies.
Financial Support:
This work was funded in part by the Massachusetts Lions Clubs; a Research to Prevent Blindness Challenge Grant; the Champalimaud Vision Award; the Beckman-Argyros Award in Vision Research; NIH R01EY015130, R01EY017011, EY014800, and R01EY011289 grants; Retina Research Foundation Awards; and the Yale School of Medicine Student Research Fellowship.
Appendix I: Image Processing
Exclusion of Larger Retinal Vasculature via Frangi Vesselness Filtering
Larger retinal vessels were identified using Frangi vessel filtering of the SCP OCTA images.69 Referring to equation (15) of Frangi et al.,69 vesselness scores were computed using parameters β = 0.5, c = 12, where the former was set as in their paper, and the latter was chosen by subjective inspection of the filtered images. The minimum and maximum filter scales were set at smin = 75 μm and smax = 500 μm, respectively, and were chosen to match the larger retinal vasculature present in the 3 mm x 3 mm SCP image. Computations were performed at filter scales spaced at 12 μm increments between smin and smax. Image pixels having vesselness scores ≥ 0.7, a threshold chosen subjectively, were considered to belong to larger retinal vasculature, and were removed from the analysis.
Computation of Local Skeletonized Vessel Density
The local skeletonized vessel density (SVD) at a pixel position x was computed as:
where T is the digital filter template (900 μm × 900 μm), Kσ is a Gaussian kernel with standard deviation σ = 100 μm, and Is is the skeletonized en face OCTA image.
Footnotes
Meeting Presentation: Preliminary results from this study were presented at the Association for Research in Vision and Ophthalmology Annual Meeting, 2020 (virtual).
Disclosures: EMM: Gyroscope (Consulting); VISTA (Patents). CRB: Carl Zeiss Meditec, Genentech, Novartis (speaker fees). AJW: Allergan (consulting); Genentech, Novartis (Investigator). JSD: Carl Zeiss Meditec, Optovue (funding), Aura Biosciences (consulting); Sesen Bio (Director), Eye-Point Pharma (Employee). JGF: Topcon (grants); Optovue, Carl Zeiss Meditech (patents); Optovue (consultant, personal financial interest). NKW: Apellis, Boehringer Ingelheim, Nidek (Consulting); Carl Zeiss Meditec, Heidelberg, Nidek, Optovue, Topcon, Regeneron (Research Support); Gyroscope (Officer of entity); Ocudyne (Shareholder). ESL, ECG, YZ, VP, IG, AYA, and AJW: no disclosures.
References
- 1.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376: 124–136. [DOI] [PubMed] [Google Scholar]
- 2.Agemy SA, Scripsema NK, Shah CM, et al. RETINAL VASCULAR PERFUSION DENSITY MAPPING USING OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY IN NORMALS AND DIABETIC RETINOPATHY PATIENTS. Retina. 2015. pp. 2353–2363. doi: 10.1097/iae.0000000000000862 [DOI] [PubMed] [Google Scholar]
- 3.Hwang TS, Jia Y, Gao SS, et al. OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY FEATURES OF DIABETIC RETINOPATHY. Retina. 2015;35: 2371–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hwang TS, Gao SS, Liu L, et al. Automated Quantification of Capillary Nonperfusion Using Optical Coherence Tomography Angiography in Diabetic Retinopathy. JAMA Ophthalmol. 2016;134: 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freiberg FJ, Pfau M, Wons J, et al. Optical coherence tomography angiography of the foveal avascular zone in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254: 1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen S, Moult EM, Zangwill LM, et al. Geometric Perfusion Deficits: A Novel OCT Angiography Biomarker for Diabetic Retinopathy Based on Oxygen Diffusion. Am J Ophthalmol. 2021;222:256–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sim DA, Keane PA, Fung S, et al. Quantitative Analysis of Diabetic Macular Ischemia Using Optical Coherence Tomography. Investigative Opthalmology & Visual Science. 2014. p. 417. doi: 10.1167/iovs.13-12677 [DOI] [PubMed] [Google Scholar]
- 8.Al-Sheikh M, Akil H, Pfau M, Sadda SR. Swept-Source OCT Angiography Imaging of the Foveal Avascular Zone and Macular Capillary Network Density in Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2016;57: 3907–3913. [DOI] [PubMed] [Google Scholar]
- 9.Mastropasqua R, Toto L, Mastropasqua A, et al. Foveal avascular zone area and parafoveal vessel density measurements in different stages of diabetic retinopathy by optical coherence tomography angiography. Int J Ophthalmol. 2017;10: 1545–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nesper PL, Roberts PK, Onishi AC, et al. Quantifying Microvascular Abnormalities With Increasing Severity of Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci. 2017;58: BIO307–BIO315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durbin MK, An L, Shemonski ND, et al. Quantification of Retinal Microvascular Density in Optical Coherence Tomographic Angiography Images in Diabetic Retinopathy. JAMA Ophthalmology. 2017. p. 370. doi: 10.1001/jamaophthalmol.2017.0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pramil V, Levine ES, Waheed NK. Macular Vessel Density in Diabetic Retinopathy Patients: How Can We Accurately Measure and What Can It Tell Us? Clin Ophthalmol. 2021;15:1517–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Carlo TE, Chin AT, Bonini Filho MA, et al. DETECTION OF MICROVASCULAR CHANGES IN EYES OF PATIENTS WITH DIABETES BUT NOT CLINICAL DIABETIC RETINOPATHY USING OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY. Retina. 2015;35: 2364–2370. [DOI] [PubMed] [Google Scholar]
- 14.Alibhai AY, Moult EM, Shahzad R, et al. Quantifying Microvascular Changes Using OCT Angiography in Diabetic Eyes without Clinical Evidence of Retinopathy. Ophthalmol Retina. 2018;2: 418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balaratnasingam C, Inoue M, Ahn S, et al. Visual Acuity Is Correlated with the Area of the Foveal Avascular Zone in Diabetic Retinopathy and Retinal Vein Occlusion. Ophthalmology. 2016;123: 2352–2367. [DOI] [PubMed] [Google Scholar]
- 16.Samara WA, Shahlaee A, Adam MK, et al. Quantification of Diabetic Macular Ischemia Using Optical Coherence Tomography Angiography and Its Relationship with Visual Acuity. Ophthalmology. 2017;124: 235–244. [DOI] [PubMed] [Google Scholar]
- 17.Dupas B, Minvielle W, Bonnin S, et al. Association Between Vessel Density and Visual Acuity in Patients With Diabetic Retinopathy and Poorly Controlled Type 1 Diabetes. JAMA Ophthalmol. 2018;136: 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DaCosta J, Bhatia D, Talks J. The use of optical coherence tomography angiography and optical coherence tomography to predict visual acuity in diabetic retinopathy. Eye . 2019. doi: 10.1038/s41433-019-0606-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AttaAllah HR, Mohamed AAM, Ali MA. Macular vessels density in diabetic retinopathy: quantitative assessment using optical coherence tomography angiography. Int Ophthalmol. 2019;39: 1845–1859. [DOI] [PubMed] [Google Scholar]
- 20.Laatikainen L, Larinkari J. Capillary-free area of the fovea with advancing age. Invest Ophthalmol Vis Sci. 1977;16: 1154–1157. [PubMed] [Google Scholar]
- 21.Tan CS, Lim LW, Chow VS, et al. Optical Coherence Tomography Angiography Evaluation of the Parafoveal Vasculature and Its Relationship With Ocular Factors. Invest Ophthalmol Vis Sci. 2016;57: OCT224–34. [DOI] [PubMed] [Google Scholar]
- 22.Shahlaee A, Pefkianaki M, Hsu J, Ho AC. Measurement of Foveal Avascular Zone Dimensions and its Reliability in Healthy Eyes Using Optical Coherence Tomography Angiography. Am J Ophthalmol. 2016;161: 50–5.e1. [DOI] [PubMed] [Google Scholar]
- 23.Magrath GN, Say EAT, Sioufi K, et al. VARIABILITY IN FOVEAL AVASCULAR ZONE AND CAPILLARY DENSITY USING OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY MACHINES IN HEALTHY EYES. Retina. 2017;37: 2102–2111. [DOI] [PubMed] [Google Scholar]
- 24.Linderman RE, Muthiah MN, Omoba SB, et al. Variability of Foveal Avascular Zone Metrics Derived From Optical Coherence Tomography Angiography Images. Transl Vis Sci Technol. 2018;7: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molina-Martín A, Pérez-Cambrodí RJ, Piñero DP. Current Clinical Application of Microperimetry: A Review. Semin Ophthalmol. 2018;33: 620–628. [DOI] [PubMed] [Google Scholar]
- 26.Vujosevic S, Midena E, Pilotto E, et al. Diabetic macular edema: correlation between microperimetry and optical coherence tomography findings. Invest Ophthalmol Vis Sci. 2006;47: 3044–3051. [DOI] [PubMed] [Google Scholar]
- 27.Unoki N, Nishijima K, Sakamoto A, et al. Retinal Sensitivity Loss and Structural Disturbance in Areas of Capillary Nonperfusion of Eyes with Diabetic Retinopathy. American Journal of Ophthalmology. 2007. pp. 755–760.e1. doi: 10.1016/j.ajo.2007.07.011 [DOI] [PubMed] [Google Scholar]
- 28.Deák GG, Bolz M, Ritter M, et al. A systematic correlation between morphology and functional alterations in diabetic macular edema. Invest Ophthalmol Vis Sci. 2010;51: 6710–6714. [DOI] [PubMed] [Google Scholar]
- 29.Midena E, Vujosevic S. Microperimetry in diabetic retinopathy. Saudi J Ophthalmol. 2011;25: 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatef E, Colantuoni E, Wang J, et al. The relationship between macular sensitivity and retinal thickness in eyes with diabetic macular edema. Am J Ophthalmol. 2011;152: 400–405.e2. [DOI] [PubMed] [Google Scholar]
- 31.Nittala MG, Gella L, Raman R, Sharma T. Measuring retinal sensitivity with the microperimeter in patients with diabetes. Retina. 2012;32: 1302–1309. [DOI] [PubMed] [Google Scholar]
- 32.Soliman W, Hasler P, Sander B, Larsen M. Local retinal sensitivity in relation to specific retinopathy lesions in diabetic macular oedema. Acta Ophthalmol. 2012;90: 248–253. [DOI] [PubMed] [Google Scholar]
- 33.Cennamo G, Vecchio EC, Finelli M, et al. Evaluation of ischemic diabetic maculopathy with Fourier-domain optical coherence tomography and microperimetry. Can J Ophthalmol. 2015;50: 44–48. [DOI] [PubMed] [Google Scholar]
- 34.Raman R, Nittala MG, Gella L, et al. Retinal Sensitivity over Hard Exudates in Diabetic Retinopathy. J Ophthalmic Vis Res. 2015;10: 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montesano G, Gervasoni A, Ferri P, et al. Structure–function relationship in early diabetic retinopathy: a spatial correlation analysis with OCT and microperimetry. Eye . 2017;31: 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugiura Y, Dolz-Marco R, Fernández-Avellaneda P, et al. Clinical utility of morphofunctional correlation of microperimetry and optical coherence tomography. Retina. 2020. doi: 10.1097/IAE.0000000000003009 [DOI] [PubMed] [Google Scholar]
- 37.Orunda-Hospital E, Otero-Rodríguez J, Perdices L, et al. Microperimetry and Optical Coherence Tomography Changes in Type-1 Diabetes Mellitus without Retinopathy. Diagnostics, 2021:11,136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pereira F, Godoy BR, Maia M, Regatieri CV. Microperimetry and OCT angiography evaluation of patients with ischemic diabetic macular edema treated with monthly intravitreal bevacizumab: a pilot study. Int J Retina Vitreous. 2019;5: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alonso-Plasencia M, Abreu-González R, Gómez-Culebras MA. Structure-Function Correlation Using OCT Angiography And Microperimetry In Diabetic Retinopathy. Clin Ophthalmol. 2019;13: 2181–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scarinci F, Varano M, Parravano M. Retinal Sensitivity Loss Correlates with Deep Capillary Plexus Impairment in Diabetic Macular Ischemia. J Ophthalmol. 2019;2019: 7589841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai ASH, Gan ATL, Ting DSW, et al. DIABETIC MACULAR ISCHEMIA: Correlation of Retinal Vasculature Changes by Optical Coherence Tomography Angiography and Functional Deficit. Retina. 2019. doi: 10.1097/IAE.0000000000002721 [DOI] [PubMed] [Google Scholar]
- 42.Roh M, Laíns I, Shin HJ, et al. Microperimetry in age-related macular degeneration: association with macular morphology assessed by optical coherence tomography. Br J Ophthalmol. 2019;103: 1769–1776. [DOI] [PubMed] [Google Scholar]
- 43.Ro-Mase T, Ishiko S, Omae T, et al. Association Between Alterations of the Choriocapillaris Microcirculation and Visual Function and Cone Photoreceptors in Patients With Diabetes. Invest Ophthalmol Vis Sci. 2020;61: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borrelli E, Sadda SR, Uji A, Querques G. Pearls and Pitfalls of Optical Coherence Tomography Angiography Imaging: A Review. Ophthalmol Ther. 2019;8: 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nobuyuki O. A threshold selection method from gray-level histograms. IEEE Trans Sys Man Cybern. 1979;9(1):62–66. [Google Scholar]
- 46.Mehta N, Braun PX, Gendelman I, et al. Repeatability of binarization thresholding methods for optical coherence tomography angiography image quantification. Sci Rep, 2020:10(15368). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luke SG. Evaluating significance in linear mixed-effects models in R. Behav Res Methods. 2017;49: 1494–1502. [DOI] [PubMed] [Google Scholar]
- 48.R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2013. http://www.R-project.org/. [Google Scholar]
- 49.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015;67(1):1–48. [Google Scholar]
- 50.Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest Package: Tests in Linear Mixed Effects Models. Journal of Statistical Software. 2017;82(13):1–26. [Google Scholar]
- 51.Lüdecke D. ggeffects: Tidy Data Frames of Marginal Effects from Regression Models. Journal of Open Source Software. 2018;3(26):772. [Google Scholar]
- 52.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York, 2016. [Google Scholar]
- 53.Hammouda LMM, Eid AM, AttaAllah HR, Ali ES. Macular sensitivity in areas of capillary nonperfusion in nonproliferative diabetic retinopathy. Journal of the Egyptian Ophthalmological Society. 2016;109: 26. [Google Scholar]
- 54.Antonetti DA, Barber AJ, Bronson SK, et al. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55: 2401–2411. [DOI] [PubMed] [Google Scholar]
- 55.Hernández C, Simó-Servat O, Simó R. Somatostatin and diabetic retinopathy: current concepts and new therapeutic perspectives. Endocrine. 2014;46: 209–214. [DOI] [PubMed] [Google Scholar]
- 56.Sohn EH, van Dijk HW, Jiao C, et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci U S A. 2016;113: E2655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lynch SK, Abràmoff MD. Diabetic retinopathy is a neurodegenerative disorder. Vision Res. 2017;139: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rossino MG, Dal Monte M, Casini G. Relationships Between Neurodegeneration and Vascular Damage in Diabetic Retinopathy. Front Neurosci. 2019;13: 1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abcouwer SF, Gardner TW. Diabetic retinopathy: loss of neuroretinal adaptation to the diabetic metabolic environment. Ann N Y Acad Sci. 2014;1311: 174–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han Y, Bearse MA Jr, Schneck ME, et al. Multifocal electroretinogram delays predict sites of subsequent diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004;45: 948–954. [DOI] [PubMed] [Google Scholar]
- 61.Ng JS, Bearse MA Jr, Schneck ME, et al. Local diabetic retinopathy prediction by multifocal ERG delays over 3 years. Invest Ophthalmol Vis Sci. 2008;49: 1622–1628. [DOI] [PubMed] [Google Scholar]
- 62.Harrison WW, Bearse MA Jr, Ng JS, et al. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Invest Ophthalmol Vis Sci. 2011;52: 772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frizziero L, Midena G, Longhin E, et al. Early Retinal Changes by OCT Angiography and Multifocal Electroretinography in Diabetes. J Clin Med. 2020;9:3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pfau M, Kaur Jolly J, Wu Z, et al. Fundus-controlled perimetry (microperimetry): Application as outcome measure in clinical trials. Progress in Retinal and Eye Research, 2020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 65.Montesano G, Ometto G, Higgins BE, et al. Evidence for Structural and Functional Damage of the Inner Retina in Diabetes with No Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2021;62(3):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen FK, Patel PJ, Xing W, et al. Test–Retest Variability of Microperimetry Using the Nidek MP1 in Patients with Macular Disease. Invest Ophthalmol Vis Sci. 2009;50: 3464–3472. [DOI] [PubMed] [Google Scholar]
- 67.Palkovits S, Hirnschall N, Georgiev S, et al. Test–Retest Reproducibility of the Microperimeter MP3 With Fundus Image Tracking in Healthy Subjects and Patients With Macular Disease. Transl Vis Sci Technol. 2018;7: 17–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alibhai AY, Mehta N, Hickson-Curran S, et al. Test–retest variability of microperimetry in geographic atrophy. International Journal of Retina and Vitreous. 2020. doi: 10.1186/s40942-020-00217-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frangi AF, Niessen WJ, Vincken KL, Viergever MA. Multiscale vessel enhancement filtering In International Conference on Medical Image Computing and Computer-Assisted Intervention. MICCAI. 1998. p. 130. [Google Scholar]