Abstract
Background
Intra-abdominal hypertension (IAH) in acute pancreatitis (AP) is associated with deterioration in organ function. This trial aimed to assess the efficacy of neostigmine for IAH in patients with AP.
Methods
In this single-center, randomized trial, consenting patients with IAH within 2 weeks of AP onset received conventional treatment for 24 h. Patients with sustained intra-abdominal pressure (IAP) ≥ 12 mmHg were randomized to receive intramuscular neostigmine (1 mg every 12 h increased to every 8 h or every 6 h, depending on response) or continue conventional treatment for 7 days. The primary outcome was the percent change of IAP at 24 h after randomization.
Results
A total of 80 patients were recruited to neostigmine (n = 40) or conventional treatment (n = 40). There was no significant difference in baseline parameters. The rate of decrease in IAP was significantly faster in the neostigmine group compared to the conventional group by 24 h (median with 25th–75th percentile: −18.7% [− 28.4 to − 4.7%] vs. − 5.4% [− 18.0% to 0], P = 0.017). This effect was more pronounced in patients with baseline IAP ≥ 15 mmHg (P = 0.018). Per-protocol analysis confirmed these results (P = 0.03). Stool volume was consistently higher in the neostigmine group during the 7-day observational period (all P < 0.05). Other secondary outcomes were not significantly different between neostigmine and conventional treatment groups.
Conclusion
Neostigmine reduced IAP and promoted defecation in patients with AP and IAH. These results warrant a larger, placebo-controlled, double-blind phase III trial.
Trial registration Clinical Trial No: NCT02543658 (registered August /27, 2015).
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-022-03922-4.
Keywords: Neostigmine, Acute pancreatitis, Intra-abdominal hypertension, Acute compartment syndrome
Introduction
Acute pancreatitis (AP) is a common disease of the digestive system [1]. Severe acute pancreatitis (SAP) with persistent organ failure is associated with an increased risk of death [2–5]. Intra-abdominal hypertension (IAH) is defined as a persistent increase in intra-abdominal pressure (IAP) ≥ 12 mmHg, according to the World Society for Abdominal Compartment Syndrome (WSACS) [6]. IAH is considered to be an early risk factor in the development of SAP [7]. In a prospective study, IAH was diagnosed in 17% of patients with AP, resulting in a mortality rate of 37% [8].
The inflammatory state of AP sparks a cascade of pancreatic and visceral edema, acute peripancreatic fluid collections, ascites, gut injury with paralytic ileus and gastric dilatation, leading to elevated IAP [9]. When IAP rises above 20 mmHg, abdominal compartment syndrome (ACS) and organ failure ensue [6]. AP complicated by ACS is associated with a mortality rate of 49% [9]. Although surgical decompression can promptly improve ACS, it causes substantial morbidity [9]. Thus, non-operative strategies for reducing IAH in AP patients are preferred, including nasogastric decompression, promotility agents and percutaneous catheter drainage (PCD), etc. [6, 7]. Neostigmine is an anti-cholinesterase drug that can enhance intestinal peristalsis, promoting the passage of flatus and defecation. Treatment with neostigmine effectively induces colonic decompression among those patients with colonic pseudo-obstruction [10–14]. The WSACS has suggested neostigmine be used for the treatment of established colonic ileus associated with IAH that does not respond to other simple measures [6]. No robust evidence exists, however, on the effects of pharmacological promotility therapy for IAP or outcomes among those with IAH/ACS [6]. Whether neostigmine can effectively reduce IAP and is beneficial in AP is unclear. In addition, confirmation of colonic ileus requires an abdominal X-ray typically in a standing position or computed tomography (CT) of the abdomen, likely to be unsuitable for patients with organ failure or hemodynamical instability during the early phase of AP. Paralytic ileus is a common risk factor for IAH in patients with AP [7]. If conventional treatment fails to correct IAH, there may be persistent paralytic ileus contributing to IAH, in which case neostigmine treatment may be beneficial.
This trial aimed to evaluate the efficacy of neostigmine in reducing IAP in patients with AP and persistent IAH following 24 h of conventional treatment, whether or not colonic pseudo-obstruction was established by X-ray or computerized tomography.
Methods
Study design and participants
This single-center, two-armed, parallel-group, superiority, randomized controlled phase II clinical trial was conducted between September 2015 and August 2017 in the Pancreatic Intensive Care Unit (ICU) of the Department of Gastroenterology at the First Affiliated Hospital of Nanchang University. This trial was registered (ClinicalTrials.gov, No. NCT02543658) and conducted adhering to a protocol that was approved by the Medical Ethics Research Committee of our hospital (No. 2015-011).
Patients aged between 18 and 70 years old who were within two weeks of AP onset and diagnosed with IAH during their Pancreatic ICU stay were assessed for eligibility. IAP was measured indirectly, using intravesicular pressure measured through a bladder catheter [6]. Briefly, the patient was in the supine position, 25 ml sterile normal saline was injected through the bladder before measurement, and with the transducer zeroed at the level where the midaxillary line crosses the iliac crest. For mechanically ventilated patients, IAP was measured under sedation. For patients with spontaneous breathing, IAP was measured at end expiration and ensuring that abdominal muscle contractions are absent.
When IAP remained ≥ 12 mmHg after conventional treatment (including sedation and analgesia, nasogastric decompression, glycerin enema for defecation, negative fluid balance and PCD for ascites) for 24 h, participants were considered to be enrolled in the trial when they met no exclusion criteria. Exclusion criteria included: (1) history of laparotomy; (2) intra-abdominal bleeding; (3) contraindications to neostigmine: angina pectoris, myocardial infarction, ventricular tachycardia, bradycardia, acute circulatory failure, epilepsy, bronchial asthma, mechanical intestinal obstruction, hyperthyroidism, serious arrhythmia, intestinal fistula or allergy to neostigmine; (4) urinary tract infection, or previous bladder surgery; (5) pregnancy or lactation. All patients or their legal representatives provided written informed consent before randomization.
Randomization and concealment
Patients were enrolled in this trial by gastroenterologists who evaluated the study participants in the Pancreatic ICU. A statistician generated a randomization list with a computer program for use in sealed, opaque envelopes. The allocation sequence was concealed from the researchers. Once the patient was included in the study, the sealed envelope was opened by one of the study investigators to determine the treatment allocation. Participants were allocated to the neostigmine group or conventional group in a 1:1 ratio. As the dosing schedule of neostigmine depended on the response in an unpredictable manner, the clinicians, outcome assessors, and patients were not blinded from assignment to intervention.
Intervention and follow-up
In the neostigmine group, patients received an initial dose of neostigmine (given intramuscularly within minutes of randomization) of 1 mg every 12 h. If there was no defecation after 12 h, the dose was increased to 1 mg every 8 h; if there was no defecation after 24 h, the dose was increased to 1 mg every 6 h. Neostigmine was stopped if the IAP dropped below 12 mmHg; otherwise, it was administered continuously for 7 days. Both the neostigmine and conventional groups received concomitant treatments as follows: (1) gastrointestinal decompression with a nasogastric and/or rectal tube; (2) paraffin oil and liquid soaked preparation of rheum officinale (rhubarb) and glauber salt by a nasogastric or nasojejunal tube. These traditional Chinese medicine components are widely used in China to alleviate gut dysmotility and have been shown to mitigate the severity of AP in patients [15, 16]; (3) glycerin enema to promote defecation; (4) PCD for ascites; (5) intravenous albumin, diuretics and when indicated renal replacement therapy for fluid overload; (6) sedation and analgesia to avoid agitation and patient-ventilator asynchrony.
Patients with IAP < 15 mmHg received enteral nutrition (EN) through a nasojejunal tube. The rate was initiated at 20 ml/h and increased gradually by 15 ml every 8 h to the goal rate (25–35 kcal/kg/d), depending on patient tolerance [17, 18]. EN was stopped temporarily when the IAP ≥ 15 mmHg and parenteral nutrition was initiated. When any patient’s IAP rose above 25 mmHg, or there were progressive organ dysfunction and fulminant ACS [19], a multidisciplinary seminar was held, including gastroenterologists, surgeons, interventional physicians and intensive care physicians, to decide whether to perform a surgical decompression. All patients were followed-up at 1, 3 and 6 months after discharge through the outpatient interview or telephone connection. Patient demographics, hospitalization and follow-up data were recorded on standardized case record forms by an investigator or coordinator who was unaware of study-group assignments.
Outcomes
Definitions of the primary and secondary endpoints are displayed in Table 1. During the internal review process, we found neostigmine has the most significant effect in reducing IAP within 24 h. Therefore, instead of the “percent change of IAP from randomization to 7 days” that registered in ClinicalTrials.gov., we modified the primary endpoint as “percent change of IAP at 24 h after randomization”. We measured IAP at 3 h after randomization, then every 6 h for the following 3 days (72 h). After this period, IAP was measured as clinically indicated; patients who remained in Pancreatic ICU with IAP ≥ 12 mmHg had IAP measured every 6 h, while those transferred to general wards with normal IAP had IAP measured once every other day until 7 days after randomization. The following secondary endpoints were analyzed: (1) stool volumes at 24 h and 7 days after randomization; (2) timing of the start of EN; (3) deterioration of IAH; (4) new-onset ACS; (5) new-onset organ failure; (6) mortality for index hospital stay and within 6 months of follow-up; (7) other complications, adverse events and costs. Known local and systemic complications of AP, including new-onset organ failure occurring after neostigmine treatment or in the absence of neostigmine treatment, were not recorded as adverse events.
Table 1.
Definition of the primary and secondary endpoints
| Endpoint | Definition |
|---|---|
| IAH | A sustained or repeated pathological elevation in IAP ≥ 12 mmHg |
| IAH grade | Grade I, IAP 12–15 mmHg |
| Grade II, IAP 16–20 mmHg | |
| Grade III, IAP 21–25 mmHg | |
| Grade IV, IAP > 25 mmHg | |
| ACS | A sustained IAP > 20 mmHg (with or without an APP < 60 mmHg) that is associated with new organ dysfunction/failure |
| Increase in stool volume | Increase in 24 h stool volume on a designated day (day 1, day 2, day 3, day 5, and day 7) after randomization above the baseline 24 h stool volume before randomization |
| New-onset ACS | ACS occurring after randomization (not present at any time before it), assessed for up to 4 weeks |
| Deterioration of IAH | IAP that rebounds ≥ 5 mmHg or increases to ≥ 20 mmHg within 7 days after randomization |
| New-onset organ failure | Organ failure occurring after randomization (not present at any time before randomization) |
| Multiple-organ failure | Failure of two or more organs |
| Respiratory failure | PaO2/FiO2 ≤ 300, or requirement for mechanical ventilation |
| Circulatory failure | Circulatory systolic blood pressure < 90 mmHg, despite adequate fluid resuscitation, or requirement for inotropic catecholamine support |
| Renal failure | Creatinine level > 177 μmol/L after rehydration or new need for haemofiltration or hemodialysis |
| Timing of EN | Time from randomization to the initiation of tolerated EN |
| Intra-abdominal bleeding | Intra-abdominal bleeding that requires surgical, radiologic, or endoscopic intervention |
| Enterocutaneous or enteric fistula | Secretion of fecal material from a percutaneous drain or inflow into a necrotic cavity, either from small or large bowel, confirmed by endoscopy, imaging, or during surgery |
| Adverse event | The following events occurred during the use of neostigmine: drug eruption, ataxia, convulsions, coma, slurred speech, anxiety, fear, cardiac arrest, or other untoward events not characteristic of or expected from AP; diarrhea was excluded as this was part of the therapeutic effect to reduce IAP |
ACS abdominal compartment syndrome, APP intraperitoneal perfusion pressure, EN enteral nutrition, IAH intra-abdominal hypertension, IAP intra-abdominal pressure
Statistical analysis
The sample size was calculated from our observational data in which IAP decreased by 30% after treatment with neostigmine for 24 h (10 patients), compared to 5% after conventional treatment for 24 h (10 patients) (unpublished data), predicting an absolute reduction in IAP of 25%. Allowing for the possibility that neostigmine treatment might be better than conventional treatment and 10% loss to follow-up, we set the power at 80% and alpha at 5%, requiring a sample size of 40 patients in each of the two groups, i.e., a total of 80 cases. Primary and secondary endpoints were compared between treatment groups, and both intention-to-treat and per-protocol analyses were performed. Furthermore, patients with baseline IAP above 15 mmHg were selected for intention-to-treat subgroup analysis. Student’s t-test was performed for continuous variables with normal distribution, and the Kruskal–Wallis H test in the absence of normal distribution. IAP at each time point was analyzed as post values in the intervention group vs post values in the control group by ANCOVA. The X2 test or Fisher exact test was performed for categorical variables, and relative risk (RR) was calculated for dichotomous variables. Two-tailed P < 0.05 was considered statistically significant. The analyses were performed using the SPSS25.0 statistical software (IBM Corp, Armonk, NY).
Results
Participant characteristics
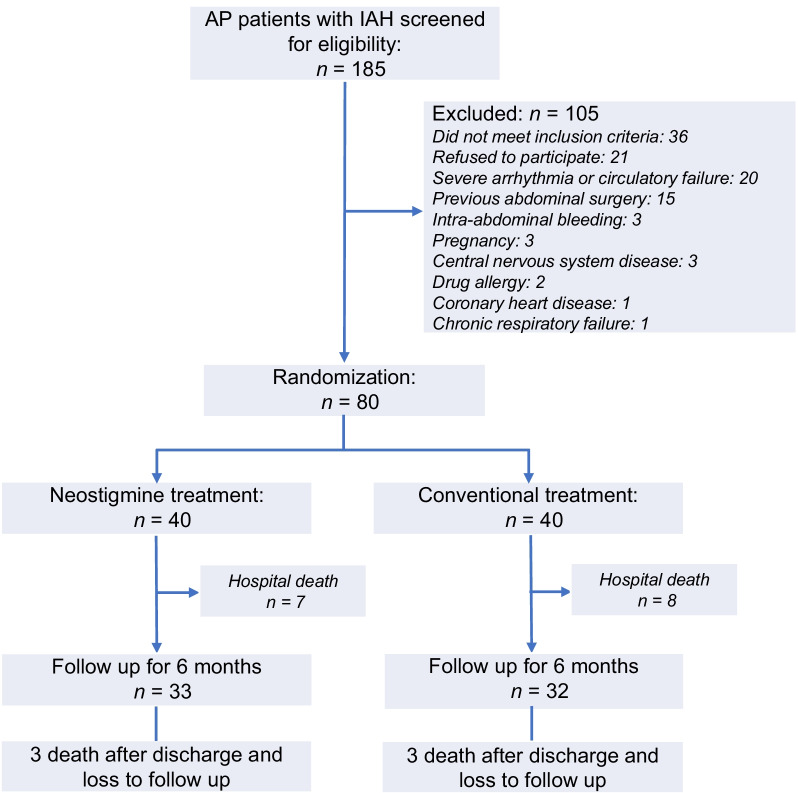
From 1 September 2015 to 15 August 2017, 552 AP patients admitted to Pancreatic ICU in our hospital were screened, of whom 185 patients with IAH were assessed for eligibility and 80 patients were included and randomized to neostigmine (n = 40) or conventional (n = 40) treatment (Fig. 1). The etiology of AP in 41 (51%) patients was hypertriglyceridemia, defined as admission serum triglyceride level > 1000 mg/dL (11.3 mmol/L) and/or lipemic serum excluding other causes [20, 21], followed by 26 (32.5%) biliary and 8 (10%) alcohol excess. Before randomization, 38 (47.5%) patients had respiratory failure and 48 (60%) had IAP > 15 mmHg (15 (18.8%) with ACS). Four patients in the neostigmine group and 1 patient in the conventional group used opioids. In the neostigmine group, 3 patients had colonic ileus, 1 of whom used opioids.
Fig. 1.
Study flowchart. AP, acute pancreatitis; IAH, intra-abdominal hypertension
Baseline characteristics were equally distributed between the two treatment groups (Table 2). There was no significant difference in baseline IAP between neostigmine and conventional groups (16.3 ± 2.7 vs. 15.9 ± 2.4, P = 0.63). In the neostigmine group, there were 33 (82.5%), 9 (22.5%) and 23 (57.5%) patients who received neostigmine every 12 h, for 3 days or for 7 days, respectively (Additional file 1: Table S1). In the conventional group, 4 patients were eventually given neostigmine because of a continuous increase in IAP. Therefore, separate per-protocol analyses were also conducted after excluding the aforementioned 4 patients. The baseline parameters remained comparable between the two groups in the per-protocol analysis (Additional file 1: Table S2).
Table 2.
Intention-to-treat analysis of baseline characteristics
| Characteristic | Neostigmine (n = 40) | Conventional (n = 40) | P value |
|---|---|---|---|
| Age (year) | 46 ± 13 | 49 ± 14 | 0.85 |
| Sex (m/f) | 27/13 | 34/6 | 0.11 |
| Etiology | |||
| Biliary | 12 (30.0%) | 14 (35.0%) | 0.95 |
| Hypertriglyceridemiaa | 21 (52.5%) | 20 (50.0%) | |
| Alcohol excess | 4 (10.0%) | 4 (10.0%) | |
| Idiopathic | 3 (7.5%) | 2 (5.0%) | |
| AP onset to hospital admission (d) | 3 (1–4) | 2 (1–3) | 0.06 |
| AP onset to randomization (d) | 5 (3–7) | 5 (4–6) | 0.55 |
| Comorbidity | |||
| Diabetes mellitus | 3 (7.5%) | 6 (15.0%) | 0.48 |
| Hypertension | 2 (5.0%) | 7 (17.5%) | 0.15 |
| Coronary heart disease | 1 (2.5%) | 0 | 1.00 |
| Chronic renal insufficiency | 0 | 1 (2.5%) | 1.00 |
| Admission clinical severity | |||
| SIRS | 2 (2–3) | 2 (2–3) | 0.70 |
| APACHE II | 9 (7–9) | 9 (7–12) | 0.79 |
| C-reactive protein (mg/L) | 228.6 ± 144.1 | 295.8 ± 125.8 | 0.70 |
| White cell count (× 109/L) | 14.7 ± 5.9 | 14.2 ± 5.6 | 0.45 |
| Procalcitonin (ng/mL) | 1.7 (0.6–13.7) | 2.8 (1.3–6.7) | 0.40 |
| Serum lactate | 2.0 ± 1.3 | 1.7 ± 0.9 | 0.16 |
| Organ failureb | 32 (80.0%) | 27 (67.5%) | 0.31 |
| Single organ failure | |||
| Respiratory | 21 (52.5%) | 17 (42.5%) | 0.50 |
| Renal | 3 (7.5%) | 1 (2.5%) | 0.61 |
| Multiple organ failure | 8 (20.9%) | 9 (22.5%) | 1.00 |
| CTSI within 1 week of AP onsetc | 5 (3–7) | 5 (3–7) | 0.99 |
| ANC | 28 (73.7%) | 26 (76.4%) | 0.63 |
| APFC | 10 (26.3%) | 8 (23.5%) | 0.59 |
| IAH level before randomization, mmHg | 16.3 ± 2.7 | 15.9 ± 2.4 | 0.63 |
| Grade I | 15 (37.5%) | 17 (42.5%) | |
| Grade II | 22 (55.0%) | 21 (52.5%) | |
| Grade III | 3 (7.5%) | 2 (5.0%) | |
| Grade IV | 0 | 0 | |
| ACS | 9 (22.5%) | 6 (15.0%) | 0.56 |
| Use of opioids | 4 (10.0%) | 1 (2.5%) | 0.36 |
| Colonic ileusc,d | 3 (7.9%) | 0 | 0.11 |
| 24 h of defecation (mL) | 450 (10–1050) | 800 (520–990) | 0.14 |
| PCD of ascites | 10 (25.0%) | 6 (15.0%) | 0.40 |
| Admitted to the ICU at randomization | 40 (100%) | 40 (100%) | 1.00 |
ACS abdominal compartment syndrome, AP acute pancreatitis, APACHE II acute physiology and chronic health evaluation II, APFC acute peripancreatic fluid collection, ANC acute necrotic collection, CTSI computed tomography severity index, IAH intra-abdominal hypertension, ICU Intensive Care Unit, PCD percutaneous catheter drainage, RAC Revised Atlanta Classification, SAP severe acute pancreatitis, SIRS systemic inflammatory response syndrome
aDefined as admission serum triglyceride level > 1000 mg/dL and/or lipemic serum after ruling out biliary and alcohol excess etiologies
bPatients with circulatory failure were excluded because neostigmine may affect the circulation
cThere were 38 and 34 cases in the neostigmine group and conventional group, respectively, underwent CT within the first week after AP onset
dOpioids were used in 2 of the 3 patients with colonic ileus
Intra-abdominal pressure after randomization
The IAP decreased after randomization in both groups, but dropped significantly faster as assessed at multiple time points in the neostigmine group than the conventional group (Table 3). IAP decreased from 16.3 ± 2.7 to 13.8 ± 3.5 mmHg after 9 h of neostigmine administration. IAP levels were significantly lower in neostigmine group than conventional treatment group at 9 h (13.8 ± 3.5 vs. 15.0 ± 3.1, P = 0.038), 15 h (13.3 ± 3.4 vs. 14.7 ± 3.1, P = 0.015), and 168 h (12.2 ± 2.7 vs. 13.6 ± 3.5, P = 0.045) after randomization.
Table 3.
Intra-abdominal pressure from randomization to 7 days
| Time (h) | Intention-to-treat analysis | Subgroup analysis (IAP > 15 mmHg at baseline) | Per-protocol analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Neostigmine (n = 40) | Conventional (n = 40) | P value† | Neostigmine (n = 25) | Conventional (n = 23) | P value† | Neostigmine (n = 40) | Conventional (n = 36) | P value† | |
| IAP | IAP | IAP | IAP | IAP | IAP | ||||
| 0 | 16.3 ± 2.7 | 15.9 ± 2.4 | 17.9 ± 2.0 | 17.6 ± 1.7 | 16.3 ± 2.7 | 15.9 ± 2.5 | |||
| 3 | 14.6 ± 3.0 | 15.0 ± 3.1 | 0.205 | 15.0 ± 3.0 | 16.7 ± 2.6 | 0.010 | 14.6 ± 3.0 | 14.9 ± 2.9 | 0.322 |
| 9 | 13.8 ± 3.5 | 15.0 ± 3.1 | 0.038 | 14.2 ± 3.3 | 16.0 ± 3.4 | 0.018 | 13.8 ± 3.5 | 14.7 ± 2.8 | 0.079 |
| 15 | 13.3 ± 3.4 | 14.7 ± 3.1 | 0.015 | 13.2 ± 3.7 | 15.8 ± 3.0 | 0.001 | 13.3 ± 3.4 | 14.7 ± 3.1 | 0.015 |
| 24 | 13.7 ± 3.6 | 14.7 ± 3.2 | 0.083 | 13.7 ± 3.6 | 15.2 ± 3.2 | 0.020 | 13.7 ± 3.6 | 14.5 ± 3.1 | 0.152 |
| 30 | 13.7 ± 3.5 | 14.3 ± 3.1 | 0.323 | 13.4 ± 3.2 | 15.0 ± 2.9 | 0.038 | 13.7 ± 3.5 | 14.0 ± 3.1 | 0.533 |
| 36 | 14.3 ± 3.6 | 13.8 ± 3.0 | 0.619 | 14.2 ± 3.5 | 14.1 ± 3.1 | 0.849 | 14.3 ± 3.6 | 13.7 ± 3.0 | 0.521 |
| 42 | 13.8 ± 3.1 | 14.2 ± 2.6 | 0.454 | 13.7 ± 3.4 | 14.5 ± 2.4 | 0.183 | 13.8 ± 3.1 | 14.2 ± 2.5 | 0.465 |
| 48 | 14.1 ± 3.2 | 13.7 ± 2.9 | 0.767 | 14.8 ± 3.0 | 14.4 ± 2.2 | 0.895 | 14.1 ± 3.2 | 13.4 ± 2.9 | 0.489 |
| 54 | 13.6 ± 3.4 | 13.4 ± 2.8 | 0.961 | 14.1 ± 3.4 | 14.0 ± 2.1 | 0.845 | 13.6 ± 3.4 | 13.2 ± 2.6 | 0.790 |
| 60 | 13.0 ± 3.1 | 13.6 ± 2.6 | 0.280 | 13.0 ± 3.2 | 14.1 ± 2.1 | 0.097 | 13.0 ± 3.1 | 13.3 ± 2.4 | 0.522 |
| 66 | 13.6 ± 3.1 | 13.3 ± 3.9 | 0.853 | 14.0 ± 2.8 | 13.7 ± 3.4 | 0.904 | 13.6 ± 3.1 | 12.9 ± 3.7 | 0.468 |
| 72 | 13.2 ± 2.9 | 13.8 ± 2.9 | 0.237 | 13.8 ± 3.1 | 14.2 ± 2.4 | 0.490 | 13.2 ± 2.9 | 13.4 ± 2.6 | 0.589 |
| 120 | 13.2 ± 3.2 | 13.8 ± 2.9 | 0.213 | 13.6 ± 3.2 | 14.4 ± 2.8 | 0.261 | 13.2 ± 3.2 | 13.2 ± 2.2 | 0.731 |
| 168 | 12.2 ± 2.7 | 13.6 ± 3.5 | 0.045 | 11.9 ± 2.8 | 14.2 ± 3.6 | 0.013 | 12.2 ± 2.7 | 13.0 ± 2.7 | 0.199 |
IAP, intra-abdominal pressure
†IAP at each time point were analyzed as post values in the intervention group versus post values in the control group by ANCOVA
Subgroup analysis restricting patient inclusion to baseline IAP > 15 mmHg showed that the mean IAP levels in the neostigmine group were significantly lower than conventional group at 3 h, 9 h, 24 h, 30 h, and 168 h after randomization (all P ≤ 0.038) (Table 3). In per-protocol analysis, IAP at 15 h remained markedly lower in neostigmine group (P = 0.015) (Table 3).
Primary outcome
Intension-to-treat analysis (Table 4) showed that the rate of decrease in IAP was significantly faster in the neostigmine group compared to the conventional group by 24 h (median with 25th–75th percentile: − 18.7% [− 28.4 to − 4.7%) vs − 5.4% [− 18.0% to 0], P = 0.017). Intension-to-treat subgroup analysis of AP patients with baseline IAP > 15 mmHg and per-protocol analysis also showed that IAP decreased significantly faster in the neostigmine group than in the conventional group (Additional file 1: Tables S3 and S4).
Table 4.
Intention-to-treat analysis of primary endpoint and secondary endpoints
| Endpoint | Neostigmine (n = 40) | Conventional (n = 40) | RR (95% CI) | P value |
|---|---|---|---|---|
| Primary endpoint | ||||
| Percent change of IAP at 24 h, % | − 18.7 ([− 28.4]-[− 4.7]) | − 5.4 ([− 18.0]− 0) | 0.017 | |
| Secondary endpoint | ||||
| Increase in stool volume at 24 h after randomization (mL) | 870 (250–2070) | 60 ([− 30]− 770) | 0.00 | |
| Increase in stool volume at 7 d after randomization (mL) | 1025 (450–1520) | 370 (150–1200) | 0.02 | |
| Serum lactate | 1.7 ± 0.8 | 1.6 ± 0.7 | 0.97 | |
| Timing of ENa | 2 (0–3) | 2 (0–3) | 1.00 | |
| Deterioration of IAHb | 4 (10.0%) | 8 (20.0%) | 0.50 (0.16–1.53) | 0.35 |
| New-onset ACS | 2 (5.0%) | 4 (10.0%) | 0.50 (0.10–2.58) | 0.68 |
| New-onset organ failure | 12 (30.0%) | 16 (40.0%) | 0.75 (0.41–1.38) | 0.48 |
| Single organ failure | ||||
| Respiratory | 2 (5.0%) | 6 (15.0%) | 0.33 (0.07–1.55) | 0.26 |
| Circulatory | 3 (7.5%) | 3 (7.5%) | 1.00 (0.21–4.66) | 1.00 |
| Renal | 0 | 3 (7.5%) | – | 0.24 |
| Multiple organ failure | 7 (15.0%) | 4 (10.0%) | 1.75 (0.56–5.52) | 0.52 |
| Invasive interventionsc | ||||
| Percutaneous catheter drainage | 8 (20.0%) | 5 (12.5%) | 1.60 (0.58–4.48) | 0.55 |
| Endoscopic transmural drainage | 3 (7.5%) | 4 (10.0%) | 0.75 (0.18–3.04) | 1.00 |
| Endoscopic necrosectomyd | 1 (2.5%) | 2 (5.0%) | 0.50 (0.05–5.30) | 1.00 |
| Surgical laparotomy | 3 (7.5%) | 4 (10.0%) | 0.75 (0.18–3.14) | 1.00 |
| Intra-abdominal bleeding (requiring intervention) | 2 (5.0%) | 4 (10.0%) | 0.50 (0.10–2.58) | 0.68 |
| Enterocutaneous fistula (requiring intervention) | 2 (5.0%) | 0 | – | 0.49 |
| Septicemia | 11 (27.5%) | 11 (27.5%) | 1.00 (0.49–2.04) | 1.00 |
| Vascular complicationse | 4 (11.8%) | 5 (13.5%) | 0.87 (0.26–2.98) | 0.56 |
| Portal vein thrombosis | 0 | 3 (7.5%) | 0.24 | |
| Splenic vein thrombosis / splenic infarction | 3 (7.5%) | 1 (2.5%) | 0.53 | |
| Portal vein and splenic vein thrombosis | 0 | 1 (2.5%) | 0.49 | |
| Superior mesenteric vein and splenic vein thrombosis | 1 (2.5%) | 0 | 0.49 | |
| RAC disease severity | ||||
| MSAP | 3 (7.5%) | 5 (12.5%) | 0.60 (0.15–2.34) | 0.71 |
| SAP | 37 (92.5%) | 35 (87.5%) | 1.05 (0.91–1.22) | 0.71 |
| Death in index hospital stay | 7 (17.5%) | 8 (20%) | 0.88 (0.35–2.18) | 0.77 |
| Length of ICU stay (d) | 14 ± 9 | 15 ± 14 | 0.94 | |
| Length of hospital stay (d) | 23 ± 13 | 22 ± 16 | 0.48 | |
| Medical expenses (1000 RMB) | 125.9 ± 83.6 | 134.3 ± 128.8 | 0.80 | |
| Follow-up (6 M) | N = 33 | N = 32 | ||
| Pancreatic pseudocyst | 2 (6.1%) | 1 (3.1%) | 1.94 (0.18–20.35) | 1.00 |
| Needing elective intervention | 0 | 1 (3.1%) | – | 0.49 |
| Walled-off necrosis | 14 (42.4%) | 11 (34.4%) | 1.23 (0.66–2.30) | 0.61 |
| Needing elective intervention | 3 (9.1%) | 1 (3.1%) | 2.91 (0.32–26.52) | 0.61 |
| Portal thrombosis | 1 (3.1%) | 1 (3.1%) | 0.97 (0.06–14.85) | 1.00 |
| Pancreatogenic portal hypertension | 1 (3.1%) | 2 (6.1%) | 0.48 (0.04–5.62) | 1.00 |
| New onset diabetes | 9 (27.3%) | 5 (15.6%) | 2.03 (0.60–6.88) | 0.37 |
| Impaired glucose tolerance | 3 (9.1%) | 2 (6.3%) | 1.55 (0.23–19.63) | 1.00 |
| External secretion dysfunction | 7 (24.1%) | 4 (13. 3%) | 2.06 (0.53–8.00) | 0.33 |
| Recurrent AP | 4 (12.2%) | 1 (3.1%) | 4.28 (0.45–40.53) | 0.36 |
| Death after discharge | 3 (9.1%) | 3 (9.4%) | 0.97 (0.18–5.19) | 1.00 |
ACS abdominal compartment syndrome, AP acute pancreatitis, CI confidence interval, EN enteral nutrition, IAH intra-abdominal hypertension, ICU Intensive Care Unit, MSAP moderately severe acute pancreatitis, RR relative risk, SAP severe acute pancreatitis
aTime from randomization to initiation of EN
bIAP that rebounded ≥ 5 mmHg or increased ≥ 20 mmHg in 1–7 days after randomization
cAll interventions after randomization were counted
dIn the neostigmine group, 1 case underwent endoscopic debridement; in the conventional group, 1 case underwent percutaneous retroperitoneal endoscopic debridement and 1 case underwent endoscopic debridement
e30 cases in the neostigmine group, and 32 cases in conventional group received CT enhancement and vascular imaging
Secondary outcomes
Stool volumes (above baseline) were consistently higher in the neostigmine group compared to the conventional group from day 1 (median with 25th–75th percentile: 870 ml [250–2070] vs. 60 ml [30–770], P = 0.00) to day 7 [1025 ml [450–1520] vs. 370 ml [150–1200], P = 0.02; Fig. 2 and Table 4). The timing of EN (2 [0–3] vs. 2 [0–3] days), deterioration of IAH (4/40 vs. 8/40), new-onset ACS (2/40 vs 4/40), new-onset organ failure (12/40 vs. 16/40), mortality (7/40 vs. 8/40) and other outcome measures including follow-up for 6 months were not significantly different. These results remained unchanged by per-protocol analysis (Additional file 1: Table S4). In the subgroup analysis for baseline IAP > 15 mmHg, only stool volumes (above baseline) at day 1 were higher in the neostigmine group compared to the conventional group; all the remaining results were not statistically different between the two groups (Additional file 1: Table S3).
Fig. 2.
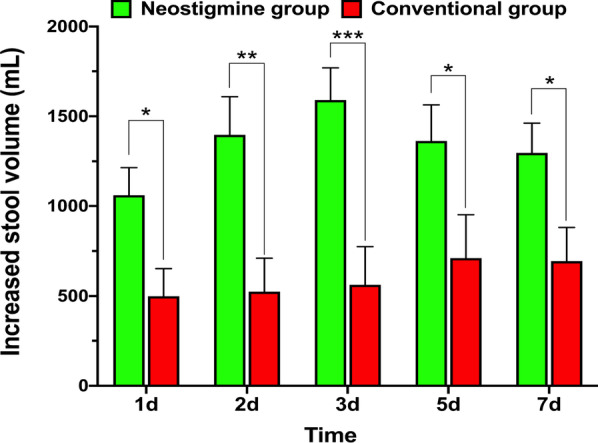
Intention-to-treat plots of increased stool volumes (mL/24 h minus baseline 24 h stool volume before randomization). *P < 0.05, **P < 0.01, ***P < 0.001)
Among 12 patients with worsening IAH, 3 patients had intra-abdominal bleeding, 2 of whom underwent successful hemostasis and IAH subsequently decreased. Hemostasis could not be achieved in 1 patient who eventually died from uncontrollable progressive ACS. Five of the 12 patients with worsening IAH were fluid overloaded, so diuretics, albumin and/or renal replacement therapy were instituted: IAP reduced in 1, who survived; 2 developed ACS and multiple organ failure and died, while 2 had persistent respiratory failure, sepsis and died. The remaining 4 patients with worsening IAH had > 50% pancreatic necrosis and fluid accumulation in the retroperitoneum: 1 did not develop ACS or infection and survived, while 3 developed ACS, infected pancreatic necrosis, multiple organ failure and died.
Adverse events
Neostigmine treatment was considered unlikely to be related to all 6 adverse events (Additional file 1: Tables S5 and S6). The new-onset circulatory failure occurred in 3 patients in the neostigmine group and 1 patient in the conventional group given neostigmine because of continuous increase in IAP. All 4 had respiratory and/or renal failure prior to randomization, and circulatory failure was attributed to progression of AP. One patient in the neostigmine group developed new-onset respiratory failure after rebound elevation in IAP leading to ACS. Overall, 12 patients in the neostigmine group compared to 16 patients in the conventional group developed new-onset organ failure after randomization (new-onset organ failure after cessation or in the absence of neostigmine administration was not recorded as an adverse event). One patient in the neostigmine group developed bradycardia while also receiving esmolol, a cardio-selective beta1 receptor blocker with rapid onset and short duration of action; the bradycardia ceased after withdrawal of esmolol during continuation of neostigmine.
Discussion
This first randomized controlled trial of neostigmine as a promotility agent for AP patients with IAH/ACS showed neostigmine treatment was significantly more effective than conventional treatment in reducing IAP in AP patients with persistent IAH after 24 h of conventional treatment (including gastrointestinal decompression, glycerin enema, negative fluid balance, and ascites drainage). The decrease in IAP occurred within 3 h of neostigmine administration and was most pronounced within the first 15 h and more evident in patients with severe IAP (> 15 mmHg) at baseline. The reduction in IAP appears relatively modest in neostigmine group; the first reason is that the patients included in this study were difficult to reduce the IAP to normal after 24 h of other simple measures; secondly, for ethical reasons, patients in the conventional group also used Chinese traditional medicine—rheum officinale and glauber salt to promote gastrointestinal peristalsis after randomization. A small pilot study on SAP patients found that dachengqi decoction with rheum officinale and glauber salt as the main components can significantly reduce IAP on days 4–8 after admission [11]. We believe that the use of neostigmine in patients with IAH above grade II (> 15 mmHg) is more clinically significant, since the effect of neostigmine on reducing IAP is more profound in patients with IAP greater than 15 mmHg. Subclinical organ injury develops at levels of IAP previously deemed to be safe (IAP between 12 and 15 mmHg), but as IAP increases, organ dysfunction will become more pronounced, and there is a dose-dependent relationship between IAP and organ dysfunction [22].
As IAH/ACS is associated with a higher incidence of organ dysfunction [23], higher mortality and longer hospitalization in patients with SAP [24], reducing IAP as promptly as possible may improve outcomes in AP. We found no statistically significant difference, however, in the incidence of new organ failure or other complications between neostigmine and conventional treatment, although patients receiving neostigmine showed a trend toward less new-onset respiratory (2 of 40 vs. 6 of 40), renal (0 vs. 3) and overall organ (12 vs. 16) failure.
Neostigmine treatment resulted in significantly increased stool volumes compared to conventional treatment, confirming enhanced intestinal peristalsis, although this did not significantly hasten the initiation of EN. Early EN has been shown to enhance recovery in AP, likely by protecting the gut mucosal barrier and reducing bacterial translocation, infected pancreatic necrosis and other severe outcomes [25]. The optimal time to initiate EN in patients with AP is considered to be within 24–72 h of admission, allowing for early intolerance to oral feeding [26]. IAH/ACS impedes early EN and is commonly associated with food intolerance. A prospective pilot study found early EN to hinder the development of IAH and reduce the severity of SAP compared with delayed EN [17]. In our study, neostigmine treatment promoted gut motility and thus reduced IAP, which may increase tolerance to EN. The median time to initiation of EN in the neostigmine and conventional groups was, however, comparable at the second day after randomization (third day after admission to Pancreatic ICU), within the period recommended in guidelines [27]. European Society of Intensive Care Medicine (ESICM) clinical practice guidelines recommend EN should be administered with caution when IAP reaches ≥ 15 mmHg [27]. The high proportion (48, i.e., 60%) of our patients who had IAP ≥ 15 mmHg at entry into a trial may partially explain the lack of any significant effect of neostigmine on the timing of EN initiation.
Neostigmine blocks the active site of anticholinesterase, increasing the availability of acetylcholine to ligate nicotinic ion channel receptors and muscarinic G-protein receptors that elicit second messenger cascades [28]. Muscarinic receptors predominate in the enteric nervous system, which response to vagal parasympathetic preganglionic activation, as well as operating independently to drive motility, secretion, the cholinergic anti-inflammatory pathway, epithelial proliferation and repair [28, 29]. Vagal nerve stimulation has shown consistent anti-inflammatory effects in colitis models, whereas vagotomy increases inflammatory markers in these models [28] and greater severity of pancreatic injury in experimental AP [30]. The evidence from our trial indicates a direct beneficial effect of neostigmine on the gut function that may include reduction in gut injury, which might contribute to improved outcomes from AP. Despite the predominance of hypertriglyceridemia etiology in our trial (41 of 80 patients), which has a high incidence in China [31–34], and induces more SAP [20, 34, 35], the mechanisms of action of neostigmine are likely to apply equally to other etiologies of human AP, although this remains to be tested further.
Patients in both the neostigmine and conventional groups were given rheum officinale (rhubarb) and glauber salt to promote defecation. Rhubarb and glauber salt are principal constituents of dachengqi decoction and its derivatives, commonly used in China to treat AP [15, 16], but they are rarely used in other countries in the world. Modified dachengqi decoction significantly decreased IAP 4–8 days after admission and improved clinical outcomes in a randomized trial conducted in predicted SAP patients [36]. A recent meta-analysis [37] of 11 randomized trials has shown that the combination of rhubarb and early EN compared to early EN alone improves gut motility, enhances the efficacy of EN and reduces AP severity in predicted SAP patients. Despite the use of rhubarb and glauber salt in both our treatment groups; however, neostigmine had significant beneficial effects on IAP and gut function, indicative of an independent action that may be widely applicable to patients with AP and IAH ± ACS.
Neostigmine was not effective in reducing IAH in all AP patients. In the neostigmine group, IAP rebounded by ≥ 5 mmHg or rose to > 20 mmHg in 4 patients, 2 of whom developed new ACS. In addition to ileus, distention, inflammation and gut wall edema, extra-luminal factors may contribute to the development of IAH in AP. These factors include third space fluid losses, acute fluid collections, fluid overload, pancreatic/peri-pancreatic necrosis and/or intra-abdominal hemorrhage, each of which may contribute to the failure of treatment for IAH, notwithstanding additional water losses in stool from neostigmine treatment. A previous randomized controlled trial found controlled fluid resuscitation reduced the incidence of ACS in patients with SAP [38], although optimal protocols are yet to be worked out.
In this study, we did not observe any adverse event likely to be related to neostigmine treatment, but a potential safety signal remains, since neostigmine may increase cardiac output by lowering IAP and thus systemic vascular resistance, yet result in an increased cholinergic drive that may slow heart rate [13]. Symptomatic bradycardia has been observed in patients treated with neostigmine for acute colonic pseudo-obstruction, but the bradycardia that developed in our patient resolved on cessation of the β-adrenergic antagonist esmolol. Patients with underlying brady-arrhythmias or those receiving β-adrenergic antagonists are likely to be more susceptible to bradycardia during the treatment of neostigmine [10]. In addition to bradycardia, neostigmine may also cause bronchoconstriction and increase airway resistance [13], although this has not been observed in previous studies [10–13] and was not in our study. A further caution would be the late use of neostigmine 4–6 weeks after the onset of AP, because of a risk of acute or sub-acute intestinal obstruction in the presence of adhesions caused by fibrosis of necrotic tissue.
Limitations
This trial was not blinded, allowing for flexibility to alter the frequency of neostigmine administration depending on changes in IAP, but introducing potential bias, e.g., timing the start of EN. As neostigmine treatment every 12 h was sufficient for the vast majority of (35 of 40) patients, this frequency or specific rules could be adopted in any future double-blind trial. Our trial had a relatively small sample size, designed as a phase II trial to test the efficacy of neostigmine on the reduction in IAP, but not on the complications of AP.
Conclusion
This preliminary study found neostigmine to reduce IAP effectively in patients with AP and IAH who were not responding to conventional treatment, by enhancing intestinal peristalsis and promoting defecation, especially in patients with baseline IAP > 15 mmHg. These results warrant a larger, multi-center, phase III trial designed to assess the impact of neostigmine on the complications of AP from multiple etiologies.
Supplementary Information
Additional file 1: Table S1. Schedule of neostigmine administration in the neostigmine group: (a) frequency and (b) duration, Table S2. Per-protocol analysis of baseline characteristics, Table S3. Per-protocol analysis of secondary endpoints, Table S4. Subgroup analysis of secondary endpoints (IAP ≥ 15 mmHg at randomization), Table S5. Characteristics of patients who developed adverse events, Table S6. Adverse events, causes and outcomes.
Acknowledgements
We would like to thank all of the doctors, nurses, technicians and patients involved at the participating centers for their dedication to the study.
Abbreviations
- ACS
Abdominal compartment syndrome
- AP
Acute pancreatitis
- CT
Computed tomography
- EN
Enteral nutrition
- IAH
Intra-abdominal hypertension
- IAP
Intra-abdominal pressure
- PCD
Percutaneous catheter drainage
- SAP
Severe acute pancreatitis
Authors' contributions
WHE, YZ and NL designed the research. LX, YZ, PL, HZ, YW, HK and XH performed the research. PC, YL, and YZ collected data. WHE, XS, and WC analyzed the data. WHE, PC, YL and WC drafted of the manuscript. WHU, RS, YZ, and NL reviewed and revised the manuscript. All authors read and approved the final manuscript.
Funding
National Key Clinical Specialist Project [No. (2011)872]; National Natural Science Foundation of China (No. 81660114, No. 81860122); and Jiangxi Province Outstanding Youth Talent Funding Program (No. 20192BCBL23021); and Science and Technology Research Project of Jiangxi Provincial Department of Education (No. 60125). RS is supported by an NIHR Senior Investigator award. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This trial was registered (ClinicalTrials.gov, No. NCT02543658) and conducted adhering to a protocol that was approved by the Medical Ethics Research Committee of our hospital (2015, No. 011). Written informed consent was obtained from a relative prior to inclusion.
Consent for publication
Not applicable.
Competing interests
Robert Sutton has received research support and/or funding from Calcimedica, Cypralis, GlaxoSmithKline, MSD/Merck, and Novartis, has been a consultant for AbbVie, Calcimedica, Cypralis, Eagle Pharmaceuticals, Novartis, and Takeda (all funds to the University of Liverpool), and is collaborating in the IMI2 TransBioLine project with multiple public and private institutions including Janssen, Lilly, MSD/Merck, Novartis, Pfizer, Roche, and Sanofi-Aventis. The other authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenhua He, Peng Chen, and Yupeng Lei contributed equally to this work.
Contributor Information
Yin Zhu, Email: zhuyin27@sina.com.
Nonghua Lu, Email: lunonghua@ncu.edu.cn.
References
- 1.Mederos MA, Reber HA, Girgis MD. Acute pancreatitis: a review. JAMA. 2021;325:382–390. doi: 10.1001/jama.2020.20317. [DOI] [PubMed] [Google Scholar]
- 2.Guo Q, Li A, Xia Q, et al. The role of organ failure and infection in necrotizing pancreatitis: a prospective study. Ann Surg. 2014;259:1201–1207. doi: 10.1097/SLA.0000000000000264. [DOI] [PubMed] [Google Scholar]
- 3.Schepers NJ, Bakker OJ, Besselink MG, et al. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut. 2019;68:1044–1051. doi: 10.1136/gutjnl-2017-314657. [DOI] [PubMed] [Google Scholar]
- 4.Garg PK, Singh VP. Organ failure due to systemic injury in acute pancreatitis. Gastroenterology. 2019;156:2008–2023. doi: 10.1053/j.gastro.2018.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi N, Liu T, de la Iglesia-Garcia D, et al. Duration of organ failure impacts mortality in acute pancreatitis. Gut. 2020;69:604–605. doi: 10.1136/gutjnl-2019-318241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkpatrick AW, Roberts DJ, De Waele J, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190–1206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trikudanathan G, Vege SS. Current concepts of the role of abdominal compartment syndrome in acute pancreatitis: an opportunity or merely an epiphenomenon. Pancreatology. 2014;14:238–243. doi: 10.1016/j.pan.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Aitken EL, Gough V, Jones A, et al. Observational study of intra-abdominal pressure monitoring in acute pancreatitis. Surgery. 2014;155:910–918. doi: 10.1016/j.surg.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 9.van Brunschot S, Schut AJ, Bouwense SA, et al. Abdominal compartment syndrome in acute pancreatitis: a systematic review. Pancreas. 2014;43:665–674. doi: 10.1097/MPA.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 10.Ponec RJ, Saunders MD, Kimmey MB. Neostigmine for the treatment of acute colonic pseudo-obstruction. N Engl J Med. 1999;341:137–141. doi: 10.1056/NEJM199907153410301. [DOI] [PubMed] [Google Scholar]
- 11.Amaro R, Rogers AI. Neostigmine infusion: new standard of care for acute colonic pseudo-obstruction? Am J Gastroenterol. 2000;95:304–305. doi: 10.1111/j.1572-0241.2000.01737.x. [DOI] [PubMed] [Google Scholar]
- 12.Loftus CG, Harewood GC, Baron TH. Assessment of predictors of response to neostigmine for acute colonic pseudo-obstruction. Am J Gastroenterol. 2002;97:3118–3122. doi: 10.1111/j.1572-0241.2002.07108.x. [DOI] [PubMed] [Google Scholar]
- 13.Korsten MA, Rosman AS, Ng A, et al. Infusion of neostigmine-glycopyrrolate for bowel evacuation in persons with spinal cord injury. Am J Gastroenterol. 2005;100:1560–1565. doi: 10.1111/j.1572-0241.2005.41587.x. [DOI] [PubMed] [Google Scholar]
- 14.Mehta R, John A, Nair P, et al. Factors predicting successful outcome following neostigmine therapy in acute colonic pseudo-obstruction: a prospective study. J Gastroenterol Hepatol. 2006;21:459–461. doi: 10.1111/j.1440-1746.2005.03994.x. [DOI] [PubMed] [Google Scholar]
- 15.Lu X, Xiao W, Kang X, et al. The effect of Chinese herbal medicine on non-biliogenic severe acute pancreatitis: a systematic review and meta-analysis. J Ethnopharmacol. 2014;155:21–29. doi: 10.1016/j.jep.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 16.Qiong W, Yiping W, Jinlin Y, et al. Chinese medicinal herbs for acute pancreatitis. Cochrane Database Syst Rev. 2005;2005(1):CD003631. doi: 10.1002/14651858.CD003631.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun JK, Li WQ, Ke L, et al. Early enteral nutrition prevents intra-abdominal hypertension and reduces the severity of severe acute pancreatitis compared with delayed enteral nutrition: a prospective pilot study. World J Surg. 2013;37:2053–2060. doi: 10.1007/s00268-013-2087-5. [DOI] [PubMed] [Google Scholar]
- 18.Mirtallo JM, Forbes A, McClave SA, et al. International consensus guidelines for nutrition therapy in pancreatitis. JPEN J Parenter Enteral Nutr. 2012;36:284–291. doi: 10.1177/0148607112440823. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen SB, Langsted A, Nordestgaard BG. Nonfasting mild-to-moderate hypertriglyceridemia and risk of acute pancreatitis. JAMA Intern Med. 2016;176:1834–1842. doi: 10.1001/jamainternmed.2016.6875. [DOI] [PubMed] [Google Scholar]
- 20.Nawaz H, Koutroumpakis E, Easler J, et al. Elevated serum triglycerides are independently associated with persistent organ failure in acute pancreatitis. Am J Gastroenterol. 2015;110:1497–1503. doi: 10.1038/ajg.2015.261. [DOI] [PubMed] [Google Scholar]
- 21.Adiamah A, Psaltis E, Crook M, et al. A systematic review of the epidemiology, pathophysiology and current management of hyperlipidaemic pancreatitis. Clin Nutr. 2018;37:1810–1822. doi: 10.1016/j.clnu.2017.09.028. [DOI] [PubMed] [Google Scholar]
- 22.De Laet IE, Malbrain M, De Waele JJ. A clinician's guide to management of intra-abdominal hypertension and abdominal compartment syndrome in critically Ill patients. Crit Care. 2020;24(1):97. doi: 10.1186/s13054-020-2782-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Bahrani AZ, Abid GH, Holt A, et al. Clinical relevance of intra-abdominal hypertension in patients with severe acute pancreatitis. Pancreas. 2008;36:39–43. doi: 10.1097/mpa.0b013e318149f5bf. [DOI] [PubMed] [Google Scholar]
- 24.Bhandari V, Jaipuria J, Singh M, et al. Intra-abdominal pressure in the early phase of severe acute pancreatitis: canary in a coal mine? Results from a rigorous validation protocol. Gut Liver. 2013;7:731–738. doi: 10.5009/gnl.2013.7.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crockett SD, Wani S, Gardner TB, et al. American Gastroenterological Association Institute guideline on initial management of acute pancreatitis. Gastroenterology. 2018;154:1096–1110. doi: 10.1053/j.gastro.2018.01.032. [DOI] [PubMed] [Google Scholar]
- 26.Arvanitakis M, Ockenga J, Bezmarevic M, et al. ESPEN guideline on clinical nutrition in acute and chronic pancreatitis. Clin Nutr. 2020;39:612–631. doi: 10.1016/j.clnu.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Reintam Blaser A, Starkopf J, Alhazzani W, et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017;43:380–398. doi: 10.1007/s00134-016-4665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaknine S, Soreq H. Central and peripheral anti-inflammatory effects of acetylcholinesterase inhibitors. Neuropharmacology. 2020;168:108020. doi: 10.1016/j.neuropharm.2020.108020. [DOI] [PubMed] [Google Scholar]
- 29.Brinkman DJ, Ten Hove AS, Vervoordeldonk MJ, et al. Neuroimmune interactions in the gut and their significance for intestinal immunity. Cells. 2019;8(7):670. doi: 10.3390/cells8070670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Westerloo DJ, Giebelen IA, Florquin S, et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–1830. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 31.Zhang R, Deng L, Jin T, et al. Hypertriglyceridaemia-associated acute pancreatitis: diagnosis and impact on severity. HPB (Oxford) 2019;21:1240–1249. doi: 10.1016/j.hpb.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Ding Y, Zhang M, Wang L, et al. Association of the hypertriglyceridemic waist phenotype and severity of acute pancreatitis. Lipids Health Dis. 2019;18:93. doi: 10.1186/s12944-019-1019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng X, Li L, Zhu Y, et al. Superoxide dismutase predicts persistent circulation failure and mortality in the early stage of acute pancreatitis. Dig Dis Sci. 2020;65:3551–3557. doi: 10.1007/s10620-020-06069-w. [DOI] [PubMed] [Google Scholar]
- 34.Wan J, He W, Zhu Y, et al. Stratified analysis and clinical significance of elevated serum triglyceride levels in early acute pancreatitis: a retrospective study. Lipids Health Dis. 2017;16:124. doi: 10.1186/s12944-017-0517-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pascual I, Sanahuja A, Garcia N, et al. Association of elevated serum triglyceride levels with a more severe course of acute pancreatitis: cohort analysis of 1457 patients. Pancreatology. 2019;19:623–629. doi: 10.1016/j.pan.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Wan MH, Li J, Huang W, et al. Modified Da-Cheng-Qi decoction reduces intra-abdominal hypertension in severe acute pancreatitis: a pilot study. Chin Med J (Engl) 2012;125:1941–1944. [PubMed] [Google Scholar]
- 37.Chen X, Yang K, Jing G, et al. Meta-analysis of efficacy of rhubarb combined with early enteral nutrition for the treatment of severe acute pancreatitis. JPEN J Parenter Enteral Nutr. 2020;44:1066–1078. doi: 10.1002/jpen.1789. [DOI] [PubMed] [Google Scholar]
- 38.Mao EQ, Tang YQ, Fei J, et al. Fluid therapy for severe acute pancreatitis in acute response stage. Chin Med J (Engl) 2009;122:169–173. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Schedule of neostigmine administration in the neostigmine group: (a) frequency and (b) duration, Table S2. Per-protocol analysis of baseline characteristics, Table S3. Per-protocol analysis of secondary endpoints, Table S4. Subgroup analysis of secondary endpoints (IAP ≥ 15 mmHg at randomization), Table S5. Characteristics of patients who developed adverse events, Table S6. Adverse events, causes and outcomes.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.