Abstract
Background
Undernutrition during critical periods of neurodevelopment can hinder the developing brain with lasting negative consequences for brain size, structure and function. In this study, we describe self-perceived cognitive problems of men and women who were born around the time of the Dutch famine of 1944–45.
Methods
We compared self-perceived cognitive problems between men and women who had been exposed to the 1944–45 Dutch famine in late, mid or early gestation and those who were born before or conceived after the famine (and had thus not been exposed prenatally). We included 595 participants aged 71–74 years.
Results
Women who had been exposed to famine in late gestation more often reported cognitive problems compared to those who had not been exposed (OR 2.2 [95% CI 1.1–4.4]), whereas for men, this was the case for those exposed in early gestation (OR 2.3 [0.9–5.5]). Furthermore, men and women exposed in early gestation more often reported consulting a healthcare practitioner for cognitive problems in the past 12 months (OR 3.2 [1.3–8.1]). Especially men exposed in early gestation reported having consulted a healthcare practitioner more often than unexposed men (OR 4.4 [1.2–16.0]).
Conclusions
These findings suggest that prenatal undernutrition does not only have lasting effects on brain size, but also on its function, with more self-perceived cognitive problems at older age, which also require more medical attention. Also, the effects of undernutrition depend on sex and its timing during gestation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-022-02820-2.
Keywords: Prenatal undernutrition, Cognitive aging, Self-reported
Background
The significance of early-life factors in age-related cognitive decline and risk for dementia is becoming increasingly clear from several studies in humans and animals [1–4]. Adverse events during the prenatal and early postnatal period may directly affect late-life cognitive function and dementia risk by lowering brain and cognitive reserve capacity or by changing the rate at which dementia-related pathologies can accumulate (for instance by limiting the brains’ ability to clear these pathologies) [1, 2]. Indirectly, adverse early-life factors may increase the risk for mediating adverse health outcomes, which in turn have been associated with cognitive decline (e.g. cardiovascular disease) [4–6]. Additionally, adverse early-life factors may accelerate the general aging process and thereby accelerate brain aging [2].
Studies of men and women born around the time of the 1944–45 Dutch famine have provided direct evidence in humans that undernutrition during critical periods of prenatal development increases the risk of age-related diseases such as type 2 diabetes and coronary heart disease, as well as overall mortality [5]. Furthermore, men and women exposed to famine in early gestation performed worse on a selective attention task in their late fifties, which may be an early manifestation of accelerated cognitive aging [6]. Because self-reported cognitive problems may be an indicator of cognitive aging and can be a predictor for future dementia [7], we here describe findings of self-reported cognitive problems at a mean age of 72 years as a further investigation of the effects of prenatal undernutrition on future cognitive decline.
Methods
Participants
The Dutch famine birth cohort (DFBC) consists of individuals born alive as term singleton babies in the Wilhelmina Gasthuis (a teaching hospital in Amsterdam, the Netherlands) between November 1, 1943 and February 28, 1947. In 1994, 2155 (89.3%) individuals could be included in the DFBC (Fig. 1). In the current study, 1207 individuals were eligible and invited to participate, of which 595 (49.3%) gave written informed consent and completed the questionnaire. The Medical Ethics Review Committee of the Academic Medical Center concluded that a full review and official approval of this study was not required according to Dutch law.
Fig. 1.
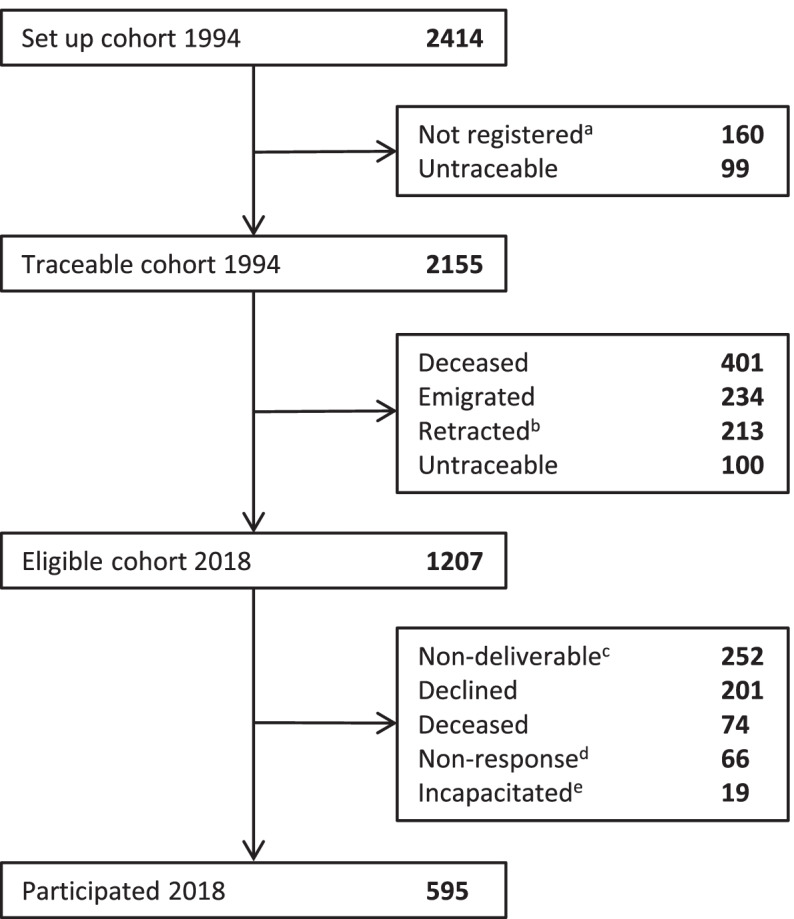
Derivation of study participants. The questionnaire was sent to all 1207 eligible participants. aThese babies had not been registered in Amsterdam at birth. bThese individuals refused permission to record their address or retracted their permission later. cInvalid home address and could not be reached by phone or no correct phone number and validity of home address unknown. dNo telephone contact established (5 attempts) or contact established but no subsequent reaction. eParticipants were physically or mentally ill, or cognitively impaired and therefore declined participation
Exposure
The Dutch famine was a consequence of events occurring at the end of World War II, this cascade of events has been described in detail elsewhere [5, 6]. Daily food rations dropped steeply, reaching a level below 1000 kcal per person on November 26, 1944 and improved relatively quickly after the liberation of the Netherlands in May 1945. There was a more or less proportionate drop of protein, carbohydrate and fat. In correspondence with previous publications on the DFBC, we considered three different 16-week exposure periods: individuals mainly exposed in late gestation (born between January 7 and April 28, 1945), mid-gestation (born between April 29 and August 18, 1945) or early gestation (born between August 19 and December 8, 1945) [6]. Individuals born in these periods were prenatally exposed to at least 13 weeks of famine during which their mother’s daily food-ration contained on average fewer than 1000 kcal. As children younger than 1 year of age were relatively protected against the famine, both individuals born before (born before January 7, 1945) and conceived after the famine (born after December 8, 1945) were considered unexposed and acted as a control group.
Self-perceived cognitive problems
Self-perceived cognitive problems were assessed with two questions as part of a larger questionnaire (including mental and physical health, daily life functioning, quality of life and stressful life events). One question was selected from The Older Persons and Informal Caregivers Survey – Minimum DataSet (TOPICS-MDS) validated questionnaire (version before 2017) [8]. Participants had to select the answer that best described their health at that time: “1) I have no problems with my memory, attention and thinking; 2) I have some problems with my memory, attention and thinking; 3) I have severe problems with my memory, attention and thinking.” The second question was adapted from the TOPICS-MDS (version 2017): “Did you consult a doctor or other healthcare practitioner for problems with your memory, attention and thinking in the past 12 months?” [8].
Study parameters
Maternal, pregnancy and birth characteristics were collected from participants’ medical birth records. Hospital anxiety and depression scale (HADS) scores [9], highest achieved education and living situation were derived from the current questionnaire. Socioeconomic status (SES) was derived from individuals’ four-digit current postal code as reported in the questionnaire by matching with Statistics Netherlands (CBS) data. For individuals who had participated in the DFBC studies in 2002 or 2008, we retrieved HADS scores and information about diabetes, stroke and cardiovascular health status at a mean age of 58 and/or 63 years.
Statistical analyses
We performed logistic regression analyses to investigate associations between famine exposure during late, mid or early gestation and self-perceived cognitive problems, compared to the control groups (Table 2). For each analysis, we ran a crude model and a model adjusted for covariates (sex, birth weight, education and SES). As we have repeatedly observed sex-specific results in previous DFBC-studies, we subsequently performed the analyses separately for men and women. To evaluate potential participation bias we explored differences in birth weight among those not eligible, those eligible participating and those eligible not participating in the current study.
Table 2.
Association of prenatal exposure to famine with self-reported cognitive problems compared to individuals born before or conceived after the famine
| Exposure to famine | |||||
|---|---|---|---|---|---|
| Born Before | In late gestation | In mid gestation | In early gestation | Conceived after | |
| Question 1: I have some/severe problems with my memory, attention and thinking | |||||
| Full Cohort: N (%) | 41 (22.5) | 21 (21.4) | 22 (28.6) | 15 (30.0) | 37 (20.1) |
| OR (95% CI) Model 1a | Reference | 1.0 (0.6–1.7) | 1.5 (0.8–2.6) | 1.6 (0.8–3.1) | Reference |
| OR (95% CI) Model 2b | Reference | 1.1 (0.6–1.9) | 1.7 (0.9–2.9) | 1.7 (0.9–3.3) | Reference |
| Women: N (%) | 18 (18.8) | 16 (33.3) | 12 (24.0) | 5 (19.2) | 16 (18.6) |
| OR (95% CI) Model 3c | Reference | 2.2 (1.1–4.4) | 1.4 (0.7–2.9) | 1.0 (0.4–2.9) | Reference |
| OR (95% CI) Model 4d | Reference | 2.5 (1.2–5.4) | 1.6 (0.7–3.4) | 1.1 (0.4–3.4) | Reference |
| Men: N(%) | 23 (26.7) | 5 (10.0) | 10 (37.0) | 10 (41.7) | 21 (21.4) |
| OR (95% CI) Model 3c | Reference | 0.4 (0.1–0.9) | 1.9 (0.8–4.4) | 2.3 (0.9–5.5) | Reference |
| OR (95% CI) Model 4d | Reference | 0.4 (0.1–1.0) | 2.0 (0.8–4.8) | 2.2 (0.9–5.7) | Reference |
| Question 2: I consulted a doctor or other healthcare practitioner for problems with your memory, attention and thinking in the past 12 months | |||||
| Full Cohort: N (%) | 9 (4.9) | 8 (8.2) | 5 (6.6) | 7 (14.0) | 9 (4.8) |
| OR (95% CI) Model 1a | Reference | 1.7 (0.7–4.1) | 1.4 (0.5–3.8) | 3.2 (1.3–8.1) | Reference |
| OR (95% CI) Model 2b | Reference | 1.9 (0.8–4.6) | 1.3 (0.5–3.8) | 2.9 (1.1–7.5) | Reference |
| Women: N (%) | 5 (5.2) | 4 (8.3) | 4 (8.0) | 3 (11.5) | 21 (6.8) |
| OR (95% CI) Model 3c | Reference | 1.6 (0.5–5.3) | 1.5 (0.5–5.1) | 2.3 (0.6–8.9) | Reference |
| OR (95% CI) Model 4d | Reference | 1.8 (0.5–6.2) | 1.4 (0.4–4.7) | 2.4 (0.6–9.7) | Reference |
| Men: N (%) | 4 (4.7) | 4 (8.0) | 1 (3.8) | 4 (16.7) | 4 (4.1) |
| OR (95% CI) Model 3c | Reference | 1.9 (0.6–6.7) | 0.9 (0.1–7.4) | 4.4 (1.2–16.0) | Reference |
| OR (95% CI) Model 4d | Reference | 2.6 (0.7–9.5) | 1.0 (0.1–8.4) | 4.0 (1.0–15.5) | Reference |
Significant (p < 0.05) findings are depicted in bold
SES socioeconomic status
aModel 1: Logistic regression model, unadjusted
bModel 2: Logistic regression model, adjusted for sex, birth weight, education and SES
cModel 3: Sex-specific logistic regression model, unadjusted
Results
In total, 134 (22.7%; excluding 4 missing) participants reported to have some problems with their cognition. Only two (0.3%) participants reported severe problems, therefore, we merged some/severe problems in further analyses. Thirty-eight individuals (6.4%; excluding 1 missing) reported to have consulted a healthcare practitioner for cognitive problems in the past 12 months (12 individuals did not report these problems in the first question). Age, sex, birth characteristics, education and SES were not associated with reporting cognitive problems or consulting a healthcare practitioner for these problems (Additional file 1). Individuals who were living alone or scored more than 7 points on either the anxiety or depression scale (either at a mean age of 58, 63 or 72) reported significantly more cognitive problems than individuals who were not living alone or scored 7 or less points on the anxiety or depression scale. The same applied to men and women with hypertension, hypercholesterolemia, vascular problems or diabetes at a mean age of 58 or 63, however, only some of these associations were statistically significant (Additional file 1). Most baseline characteristics were similar, however, some pregnancy, birth and adult characteristics differed among the exposure and control groups (Table 1).
Table 1.
General, maternal, pregnancy, birth and adult characteristics by timing of prenatal exposure to the Dutch famine
| Exposure to famine | |||||||
|---|---|---|---|---|---|---|---|
| N | Born before | In late gestation | In mid gestation | In early gestation | Conceived after | Total | |
| General characteristics | |||||||
| N | 183 | 98 | 77 | 50 | 187 | 595 | |
| N Women (%) | 595 | 96 (52.5) | 48 (49.0) | 50 (64.9)* | 26 (52.0) | 89 (47.6) | 309 (51.9) |
| Age (years) | 595 | 73.8 (0.4)*** | 73.1 (0.1)** | 72.8 (0.1) | 72.5 (0.1)* | 71.9 (0.4) | 72.8 (0.8) |
| Maternal and pregnancy characteristics | |||||||
| Pregnancy duration (days) | 520 | 284 (11) | 282 (10) | 287 (13) | 285 (9.6) | 286 (12) | 285 (11) |
| Maternal age at birth (years) | 595 | 28.8 (6.3) | 31.1 (6.1)*** | 29.6 (6.3) | 27.0 (5.9) | 28.4 (6.2) | 29.0 (6.3) |
| N Primiparous (%) | 595 | 70 (38.3) | 19 (19.4)*** | 23 (29.9) | 19 (38.0) | 77 (41.2) | 208 (35.0) |
| N Manual occupation head of family (%) | 475 | 108 (79.4)* | 52 (59.8)* | 49 (74.2) | 27 (64.3) | 97 (67.4) | 333 (70.1) |
| Birth characteristics | |||||||
| Birth weight (g) | 595 | 3373 (458) | 3222 (498)*** | 3204 (470)*** | 3503 (377) | 3446 (478) | 3360 (476) |
| Head circumference (cm) | 588 | 32.8 (1.4) | 32.5 (1.7)** | 32.2 (1.4)*** | 32.9 (1.4) | 33.1 (1.6) | 32.8 (1.5) |
| Adult characteristics | |||||||
| 2002a | |||||||
| N Hypercholesterolemia (%) | 483 | 46 (30.1) | 21 (25.3) | 24 (32.9) | 11 (29.7) | 37 (27.0) | 139 (28.8) |
| N Hypertension (%) | 483 | 49 (32.0) | 24 (28.9) | 30 (41.1) | 10 (27.0) | 51 (37.2) | 164 (34.0) |
| N Stroke or TIA (%) | 483 | 0 | 0 | 3 (4.1) | 0 | 0 | 3 (0.6) |
| N Diabetes type 2 (%) | 483 | 26 (17.0) | 12 (14.5) | 9 (12.3) | 5 (13.5) | 14 (10.2) | 66 (13.7) |
| N Vascular problemsc (heart infarction or angina pectoris) (%) | 483 | 7 (4.6) | 5 (6.0) | 4 (5.5) | 1 (2.7) | 4 (2.9) | 21 (4.3) |
| N HADS anxiety >7b (%) | 463 | 20 (13.4) | 10 (13.2) | 14 (19.7) | 10 (29.4) | 29 (21.8) | 83 (17.9) |
| N HADS depression >7b (%) | 461 | 7 (4.7) | 5 (6.5) | 4 (5.6) | 5 (15.2) | 12 (9.2) | 33 (7.2) |
| 2008a | |||||||
| N Hypercholesterolemia (%) | 387 | 57 (47.1) | 16 (24.2)** | 27 (47.4) | 15 (50.0) | 43 (38.1) | 158 (40.8) |
| N Hypertension (%) | 388 | 43 (35.5) | 23 (34.3) | 27 (47.4) | 14 (46.7) | 51 (45.1) | 158 (40.7) |
| N Stroke or TIA (%) | 388 | 3 (2.5) | 3 (4.5) | 3 (5.3) | 2 (6.7) | 2 (1.8) | 13 (3.4) |
| N Diabetes type 2 (%) | 388 | 16 (13.2) | 11 (16.4) | 4 (7.0) | 6 (20.0) | 13 (11.5) | 50 (12.9) |
| N Vascular problemsc (heart infarction or angina pectoris) (%) | 387 | 14 (11.6) | 5 (7.6) | 4 (7.0) | 4 (13.3) | 11 (9.7) | 38 (9.8) |
| N HADS anxiety >7b (%) | 369 | 17 (15.2) | 7 (11.1) | 7 (12.3) | 8 (30.8) | 18 (16.2) | 57 (15.4) |
| N HADS depression >7b (%) | 372 | 7 (6.2) | 6 (9.2) | 5 (8.9) | 7 (25.0)** | 9 (8.2) | 34 (9.1) |
| 2018 | |||||||
| Level of educationc | 589 | 4.8 (1.4) | 4.9 (1.5) | 4.8 (1.3) | 4.5 (1.2) | 4.9 (1.4) | 4.8 (1.4) |
| Socioeconomic statusd | 590 | 0.08 (1.02) | 0.16 (1.07) | 0.13 (0.98) | 0.14 (1.05) | 0.08 (1.14) | 0.10 (1.06) |
| N Living alone (%) | 591 | 65 (35.5)* | 24 (24.7) | 23 (29.9) | 17 (34.0) | 43 (23.4) | 172 (29.1) |
| N HADS anxiety >7b (%) | 584 | 21 (11.8) | 11 (11.7) | 9 (12.0) | 8 (16.0) | 32 (17.1) | 81 (13.9) |
| N HADS depression >7b (%) | 586 | 14 (7.8) | 6 (6.3) | 10 (13.2) | 8 (16.0) | 17 (9.1) | 55 (9.4) |
Numbers represent frequencies(%) or means (SD). Asterisks represent p-values of linear and logistic regression analyses compared with participants unexposed to famine in gestation, or, for individuals born before the famine, compared to individuals conceived after the famine
SES socioeconomic status, TIA Transient Ischemic Attack, HADS hospital anxiety and depression scale
aThese data were only available for participants participating at a mean age of 58 (2002) or 63 (2008) in the Dutch Famine Birth Cohort. Hypercholesterolemia, Hypertension and stroke or TIA were derived from questions asking if participants had ever had these health conditions. Diabetes was also based on the questionnaire (2002 and 2008) or having a fasting plasma glucose of ≥7 mmol/l or a 2-h plasma glucose of ≥11.1 mmol/l following a 75 g oral glucose load (2002). Vascular problems were defined as either having a history of heart infarction or probable angina pectoris (based on multiple questions related to chest pain)
bAnxiety and depression score > 7 on the hospital anxiety and depression scale (HADS) [9]
cLevel of education as the highest level of finished schooling measured on a 7-point scale; 1) Less than 6 years of primary school, 2) 6 years of primary school, 3) more than primary school, without an additional diploma, 4) craft school, 5) (pre-)secondary vocational education, 6) pre-university education, 7) higher professional education/university
dBased on zip code and data from Statistics Netherlands from 2017 (CBS) (Range: −4.76-2.78, mean: 0.10 in our dataset)
* < 0.05, ** < 0.01, *** < 0.001
Overall, the prevalence of reporting cognitive problems did not differ between those unexposed and those prenatally exposed to famine (Table 2). However, those exposed in early gestation more often reported having consulted a healthcare practitioner for cognitive problems in the past 12 months than controls (OR: 3.2 [95% CI 1.3–8.1]). Looking at men and women separately showed that women exposed in late gestation more often reported cognitive problems (OR 2.2 [1.1–4.4]), whereas men exposed in early gestation more often reported cognitive problems (OR 2.3 [0.9–5.5]) than those who had not been exposed to famine prenatally. Men exposed in early gestation four times more often reported having consulted a healthcare practitioner for cognitive problems than men who had not been exposed prenatally (OR 4.4 [1.2–16.0]). Adjusting for covariates did not attenuate the estimates.
Discussion
Our findings suggest that prenatal undernutrition increases the risk of cognitive problems in later life, which also leads to more often consulting a healthcare practitioner for these problems. This increased risk appears to depend on sex and timing during gestation. Particularly men who had been exposed to famine in early gestation more often reported cognitive problems and consulting a healthcare practitioner for these problems. This is in line with the poorer cognitive function in their late fifties [5]. Interestingly, women exposed to famine in late gestation more often reported cognitive problems.
Agreement with other studies
To our knowledge, no other studies have investigated associations between prenatal undernutrition and self-perceived cognitive problems later in life. Studies regarding the Chinese Great Leap Forward famine of 1959–61 did show that men and women prenatally exposed to undernutrition had more cognitive impairments (measured with cognitive tests) and dementia than controls [10, 11]. However, most Chinese famine studies are hampered by only selecting controls born after the famine, thereby not accounting for age differences (a major risk factor) and potentially overestimating their results. In the DFBC, we account for this bias by also including controls born before the famine. Furthermore, studies have linked other adverse prenatal and early-life circumstances to late-life cognitive problems and dementia [1–4, 6]. Good economic conditions at the time of birth have for instance been associated with a higher chance of good cognitive functioning at age 60 and older, while a recession was associated with lower cognitive functioning at those ages [12]. Only one study investigated, and found, an association between a prenatal exposure (maternal hypertensive disorder) and subjective cognitive complaints [13].
Interpretation and potential mechanisms
In previous DFBC-studies, men exposed in early gestation were shown to have smaller total brain volume and increased BrainAGE at age 68 [14, 15]. These findings may point at a compromised brain reserve, which may result in an earlier crossing of the threshold below which normal cognitive functioning cannot be sustained [4]. Furthermore, animal studies have indicated that early-life circumstances can modify the rate of accumulation of dementia-related pathologies [2]. Additionally, as hypothesized previously [6], our current observations could be due to vascular effects. Prior DFBC-studies showed increased risks for cardiovascular and metabolic diseases in exposed individuals, as well as overall worse brain perfusion [5, 16]. Building on this, the association we observed is likely not a direct effect alone. We lacked statistical power to formally explore mediators, however, we observed more reporting of cognitive problems in individuals with hypercholesterolemia or hypertension around age 58 and 63 in our cohort. Furthermore, high anxiety and depression scores measured at a mean age of 58, 63 and 72 were strongly associated with self-perceived cognitive problems. Anxiety and depression symptoms seem more prevalent among men and women who had been exposed in early gestation compared to controls, similar to what we have observed before in men exposed in early gestation [17]. Associations with anxiety and depression symptoms reported 10 years earlier would suggest a possible role for these symptoms as mediating factors. Some studies have indicated that earlier depressive episodes may confer increased risk for later pathologic cognitive decline [18]. However, the relationship between depression and anxiety - especially in older adults - and self-perceived cognitive problems is complex [7]. Anxiety and depression may influence the awareness and occurrence of cognitive problems, yet, anxiety and depression in older adults have been associated with objective measures of brain aging and increased risk for dementia [7].
There are differences in the prenatal development of men and women, men are, for instance, more vulnerable to certain adverse prenatal circumstances [19]. In earlier studies in the same cohort, we have observed sex-specific effects similar to the ones described in the current study, the effects of exposure to famine in early gestation were more pronounced in men than in women. These results could be due to the increased vulnerability of men due to their early life growth strategy [20]). Alternatively, excess mortality in women exposed early in gestation, may have led to an underestimation of the effect in women as relatively healthier women remain [21].
Strengths and limitations
Strengths of the current study include that the results are in line with previous findings and seem relatively robust. We made use of a unique cohort with a quasi-experimental set up, which limits the risks of bias and confounding [5, 6].
This study has limitations. The number of participants was small, thus we had limited power in our analyses, especially regarding consulting a healthcare practitioner for cognitive problems and the sex-stratified analyses. A limitation of the current study is the lack of objective information about cognitive status and/or dementia diagnosis in the cohort. Regardless, we do observe significant associations. Furthermore, only 50.0% of the initial cohort was eligible to participate in 2018, and of this group, 49.3% participated in the current study. Birth weights were similar among these groups, however, adult characteristics may differ and selective participation may have caused bias. Individuals with (early stage) dementia may, for instance, be less likely to participate in questionnaires, and when they do, may fail to recognize their cognitive problems [7]. However, as our study sample is relatively healthy and relatively young we expect to mainly see early and less severe symptoms of cognitive decline. As prenatal famine exposure has previously been associated with poorer health outcomes, we expect a relatively greater loss of participants with poorer health in the exposed groups [22]. Both participation bias and possible misrepresentation of dementia diagnoses would most likely lead to an underestimation of the associations, this may especially be true for people who had been exposed in early gestation who seemed most affected by brain aging and health problems [5, 6, 14–16]. Furthermore, adding covariates to the models did not substantially attenuate our estimates, which adds to the robustness of our findings, however, the possibility of residual confounding remains.
Regarding the exposure, we cannot be absolutely sure that our associations are solely due to prenatal undernutrition. Maternal stress due to the food scarcity and war conditions may have also played a role [23]. The stress experienced by pregnant women during the famine was likely more extreme than the stress experienced by women before or after the famine [23]. Regarding the outcome, the current study focused on a subjective measure of cognitive aging. Although objective measures of cognitive and brain aging remain the gold standard, self-perceived cognitive problems are of importance. Self-perceived cognitive problems usually occur before any other objective measures of cognitive problems and can be predictive for future objective cognitive decline and the development of mild cognitive impairment (MCI) and dementia [7, 24]. Studies have suggested that self-perceived cognitive problems represent one of the earliest symptoms of Alzheimer’s disease [7, 24]. Furthermore, subjective cognitive problems have been associated with measures of brain aging, like hippocampal atrophy and greater white matter hyperintensity volume [25]. Self-perceived cognitive problems, in our sample, show - to be expected - associations with measures of depression and anxiety, strengthening our confidence in their validity [7]. As described earlier the relationship between self-perceived cognitive problems and anxiety and depression is complex. One study showed associations between self-perceived cognitive problems and more objective cognitive decline and higher risk of MCI and dementia at the end of follow-up, while excluding participants with clinical depression [24]. These findings would indicate that the predictive value of self-perceived cognitive problems is not solely dependent on the presence of depressive symptoms.
Interestingly, 12 individuals reported to have consulted a healthcare practitioner for cognitive problems while they did not report having cognitive problems. Possibly, this discrepancy is due to others worrying about the participants’ cognitive health. Excluding these individuals from the analyses regarding consulting a healthcare practitioner led to slightly higher effect estimates regarding the association between prenatal undernutrition and self-perceived cognitive problems, suggesting we might underestimate our findings by including these individuals.
Conclusion
Our results suggest that prenatal undernutrition increases the risk of self-perceived cognitive problems in men and women, and that this effect depends on sex and the timing during gestation. Possibly, these self-perceived cognitive problems are predictive of further cognitive decline and dementia [7]. Future studies will investigate dementia prevalence in these men and women as they age.
Supplementary Information
Additional file 1. Association of covariates with self-perceived cognitive problems. Description: We explored associations between covariates and self-perceived cognitive problems with logistic regression analyses.
Acknowledgments
We thank the participants of the Dutch famine birth cohort for their willing cooperation in this study.
Abbreviations
- DFBC
Dutch Famine Birth Cohort
- CI
Confidence Interval
- HADS
Hospital Anxiety and Depression Scale
- OR
Odds Ratio
- SES
Socioeconomic Status
- TIA
Transient Ischemic Attack
- TOPICS-MDS
The Older Persons and Informal Caregivers Survey – Minimum DataSet
Authors’ contributions
Study concept and design (TR, SR), acquisition of data (SR), analysis (AMW) and interpretation of data (AMW, AB, TR, SR). AMW drafted the manuscript and all authors critically reviewed and revised the manuscript. The author(s) read and approved the final manuscript.
Funding
This work was supported by the European Commission Horizon 2020, project DynaHealth (633595) and project LongITools (874739); and a Dutch Research Council (NWO) Aspasia grant (015014039) awarded to S.R. de Rooij. The funding organizations had no role in any part of this study.
Availability of data and materials
The datasets supporting the conclusion of this article are not available in a public repository.
Data can be made available by the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The Medical Ethics Review Committee of the Academic Medical Center concluded that a full review and official approval of this study was not required according to Dutch law. All participants provided written informed consent.
All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Seifan A, Schelke M, Obeng-Aduasare Y, Isaacson R. Early life epidemiology of Alzheimer’s disease--a critical review. Neuroepidemiology. 2015;45:237–254. doi: 10.1159/000439568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lesuis SL, Hoeijmakers L, Korosi A, de Rooij SR, Swaab DF, Kessels HW, et al. Vulnerability and resilience to Alzheimer’s disease: early life conditions modulate neuropathology and determine cognitive reserve. Alzheimers Res Ther. 2018;10:95. doi: 10.1186/s13195-018-0422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raikkonen K, Kajantie E, Pesonen AK, Heinonen K, Alastalo H, Leskinen JT, et al. Early life origins cognitive decline: findings in elderly men in the Helsinki birth cohort study. PLoS One. 2013;8:e54707. doi: 10.1371/journal.pone.0054707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borenstein AR, Copenhaver CI, Mortimer JA. Early-life risk factors for Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:63–72. doi: 10.1097/01.wad.0000201854.62116.d7. [DOI] [PubMed] [Google Scholar]
- 5.Roseboom TJ, Painter RC, van Abeelen AFM, Veenendaal MVE, de Rooij SR. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas. 2011;70:141–145. doi: 10.1016/j.maturitas.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 6.de Rooij SR, Wouters H, Yonker JE, Painter RC, Roseboom TJ. Prenatal undernutrition and cognitive function in late adulthood. Proc Natl Acad Sci. 2010;107:16881–16886. doi: 10.1073/pnas.1009459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabin LA, Smart CM, Amariglio RE. Subjective cognitive decline in preclinical Alzheimer’s disease. Annu Rev Clin Psychol. 2017;13:369–396. doi: 10.1146/annurev-clinpsy-032816-045136. [DOI] [PubMed] [Google Scholar]
- 8.TOPICS-MDS. https://topics-mds.eu/survey-instrument/. Accessed 8 Sep 2020.
- 9.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 10.Kang Y, Zhang Y, Feng Z, Liu M, Li Y, Yang H, et al. Nutritional deficiency in early life facilitates aging-associated cognitive decline. Curr Alzheimer Res. 2017;14:841–849. doi: 10.2174/1567205014666170425112331. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, An Y, Yu H, Feng L, Liu Q, Lu Y, et al. Association between exposure to the Chinese famine in different stages of early life and decline in cognitive functioning in adulthood. Front Behav Neurosci. 2016;10:146. doi: 10.3389/fnbeh.2016.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doblhammer G, van den Berg GJ, Fritze T. Economic conditions at the time of birth and cognitive abilities late in life: evidence from ten European countries. PLoS One. 2013;8:e74915. doi: 10.1371/journal.pone.0074915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuovinen S, Eriksson JG, Kajantie E, Lahti J, Pesonen AK, Heinonen K, et al. Maternal hypertensive disorders in pregnancy and self-reported cognitive impairment of the offspring 70 years later: the Helsinki Birth Cohort Study. Am J Obstet Gynecol. 2013;208:200.e1–200.e9. doi: 10.1016/j.ajog.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 14.De Rooij SR, Caan MWA, Swaab DF, Nederveen AJ, Majoie CB, Schwab M, et al. Prenatal famine exposure has sex-specific effects on brain size. Brain. 2016;139:2136–2142. doi: 10.1093/brain/aww132. [DOI] [PubMed] [Google Scholar]
- 15.Franke K, Gaser C, Roseboom TJ, Schwab M, de Rooij SR. Premature brain aging in humans exposed to maternal nutrient restriction during early gestation. Neuroimage. 2018;173:460–471. doi: 10.1016/j.neuroimage.2017.10.047. [DOI] [PubMed] [Google Scholar]
- 16.de Rooij SR, Mutsaerts HJMM, Petr J, Asllani I, Caan MWA, Groot P, et al. Late-life brain perfusion after prenatal famine exposure. Neurobiol Aging. 2019;82:1–9. doi: 10.1016/j.neurobiolaging.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 17.De Rooij SR, Painter RC, Phillips DI, Rikknen K, Schene AH, Roseboom TJ. Self-reported depression and anxiety after prenatal famine exposure: mediation by cardio-metabolic pathology? J Dev Orig Health Dis. 2011;2:136–143. doi: 10.1017/S2040174411000055. [DOI] [PubMed] [Google Scholar]
- 18.Butters MA, Young JB, Lopez O, Aizenstein HJ, Mulsant BH, Reynolds CF, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10:345. [DOI] [PMC free article] [PubMed]
- 19.Aiken CE, Ozanne SE. Sex differences in developmental programming models. Reproduction. 2013;145:R1–13. doi: 10.1530/REP-11-0489. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJP. Boys live dangerously in the womb. Am J Hum Biol. 2010;22:330–335. doi: 10.1002/ajhb.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Abeelen AFM, Veenendaal MVE, Painter RC, De Rooij SR, Dijkgraaf MGW, Bossuyt PMM, et al. Survival effects of prenatal famine exposure. Am J Clin Nutr. 2012;95:179–183. doi: 10.3945/ajcn.111.022038. [DOI] [PubMed] [Google Scholar]
- 22.Bleker LS, De Rooij SR, Painter RC, Ravelli ACJ, Roseboom TJ. Cohort profile: the Dutch famine birth cohort (DFBC) -a prospective birth cohort study in the Netherlands. BMJ Open. 2021;11:e042078. doi: 10.1136/bmjopen-2020-042078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Rooij SR, Bleker LS, Painter RC, Ravelli AC, Roseboom TJ. Lessons learned from 25 years of research into long term consequences of prenatal exposure to the Dutch famine 1944–45: the Dutch famine birth cohort. Int J Environ Health Res. 2021;1-15. [DOI] [PubMed]
- 24.Kielb S, Rogalski E, Weintraub S, Rademaker A. Objective features of subjective cognitive decline in a United States national database. Alzheimers Dement. 2017;13:1337–1344. doi: 10.1016/j.jalz.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Rooden S, Van Den Berg-Huysmans AA, Croll PH, Labadie G, Hayes JM, Viviano R, et al. Subjective cognitive decline is associated with greater white matter Hyperintensity volume. J Alzheimers Dis. 2018;66:1283-94. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Association of covariates with self-perceived cognitive problems. Description: We explored associations between covariates and self-perceived cognitive problems with logistic regression analyses.
Data Availability Statement
The datasets supporting the conclusion of this article are not available in a public repository.
Data can be made available by the corresponding author upon reasonable request.


