Abstract
Background
Arthroscopic knee surgery remains a common treatment for symptomatic knee osteoarthritis, including for degenerative meniscal tears, despite guidelines strongly recommending against its use. This Cochrane Review is an update of a non‐Cochrane systematic review published in 2017.
Objectives
To assess the benefits and harms of arthroscopic surgery, including debridement, partial menisectomy or both, compared with placebo surgery or non‐surgical treatment in people with degenerative knee disease (osteoarthritis, degenerative meniscal tears, or both).
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and two trials registers up to 16 April 2021, unrestricted by language.
Selection criteria
We included randomised controlled trials (RCTs), or trials using quasi‐randomised methods of participant allocation, comparing arthroscopic surgery with placebo surgery or non‐surgical interventions (e.g. exercise, injections, non‐arthroscopic lavage/irrigation, drug therapy, and supplements and complementary therapies) in people with symptomatic degenerative knee disease (osteoarthritis or degenerative meniscal tears or both). Major outcomes were pain, function, participant‐reported treatment success, knee‐specific quality of life, serious adverse events, total adverse events and knee surgery (replacement or osteotomy).
Data collection and analysis
Two review authors independently selected studies for inclusion, extracted data, and assessed risk of bias and the certainty of evidence using GRADE. The primary comparison was arthroscopic surgery compared to placebo surgery for outcomes that measured benefits of surgery, but we combined data from all control groups to assess harms and knee surgery (replacement or osteotomy).
Main results
Sixteen trials (2105 participants) met our inclusion criteria. The average age of participants ranged from 46 to 65 years, and 56% of participants were women. Four trials (380 participants) compared arthroscopic surgery to placebo surgery. For the remaining trials, arthroscopic surgery was compared to exercise (eight trials, 1371 participants), a single intra‐articular glucocorticoid injection (one trial, 120 participants), non‐arthroscopic lavage (one trial, 34 participants), non‐steroidal anti‐inflammatory drugs (one trial, 80 participants) and weekly hyaluronic acid injections for five weeks (one trial, 120 participants). The majority of trials without a placebo control were susceptible to bias: in particular, selection (56%), performance (75%), detection (75%), attrition (44%) and selective reporting (75%) biases. The placebo‐controlled trials were less susceptible to bias and none were at risk of performance or detection bias. Here we limit reporting to the main comparison, arthroscopic surgery versus placebo surgery.
High‐certainty evidence indicates arthroscopic surgery leads to little or no difference in pain or function at three months after surgery, moderate‐certainty evidence indicates there is probably little or no improvement in knee‐specific quality of life three months after surgery, and low‐certainty evidence indicates arthroscopic surgery may lead to little or no difference in participant‐reported success at up to five years, compared with placebo surgery.
Mean post‐operative pain in the placebo group was 40.1 points on a 0 to 100 scale (where lower score indicates less pain) compared to 35.5 points in the arthroscopic surgery group, a difference of 4.6 points better (95% confidence interval (CI) 0.02 better to 9 better; I2 = 0%; 4 trials, 309 participants). Mean post‐operative function in the placebo group was 75.9 points on a 0 to 100 rating scale (where higher score indicates better function) compared to 76 points in the arthroscopic surgery group, a difference of 0.1 points better (95% CI 3.2 worse to 3.4 better; I2 = 0%; 3 trials, 302 participants).
Mean post‐operative knee‐specific health‐related quality of life in the placebo group was 69.7 points on a 0 to 100 rating scale (where higher score indicates better quality of life) compared with 75.3 points in the arthroscopic surgery group, a difference of 5.6 points better (95% CI 0.36 better to 10.68 better; I2 = 0%; 2 trials, 188 participants). We downgraded this evidence to moderate certainty as the 95% confidence interval does not rule in or rule out a clinically important change.
After surgery, 74 out of 100 people reported treatment success with placebo and 82 out of 100 people reported treatment success with arthroscopic surgery at up to five years (risk ratio (RR) 1.11, 95% CI 0.66 to 1.86; I2 = 53%; 3 trials, 189 participants). We downgraded this evidence to low certainty due to serious indirectness (diversity in definition and timing of outcome measurement) and serious imprecision (small number of events).
We are less certain if the risk of serious or total adverse events increased with arthroscopic surgery compared to placebo or non‐surgical interventions. Serious adverse events were reported in 6 out of 100 people in the control groups and 8 out of 100 people in the arthroscopy groups from eight trials (RR 1.35, 95% CI 0.64 to 2.83; I2 = 47%; 8 trials, 1206 participants). Fifteen out of 100 people reported adverse events with control interventions, and 17 out of 100 people with surgery at up to five years (RR 1.15, 95% CI 0.78 to 1.70; I2 = 48%; 9 trials, 1326 participants). The certainty of the evidence was low, downgraded twice due to serious imprecision (small number of events) and possible reporting bias (incomplete reporting of outcome across studies). Serious adverse events included death, pulmonary embolism, acute myocardial infarction, deep vein thrombosis and deep infection.
Subsequent knee surgery (replacement or high tibial osteotomy) was reported in 2 out of 100 people in the control groups and 4 out of 100 people in the arthroscopy surgery groups at up to five years in four trials (RR 2.63, 95% CI 0.94 to 7.34; I2 = 11%; 4 trials, 864 participants). The certainty of the evidence was low, downgraded twice due to the small number of events.
Authors' conclusions
Arthroscopic surgery provides little or no clinically important benefit in pain or function, probably does not provide clinically important benefits in knee‐specific quality of life, and may not improve treatment success compared with a placebo procedure. It may lead to little or no difference, or a slight increase, in serious and total adverse events compared to control, but the evidence is of low certainty. Whether or not arthroscopic surgery results in slightly more subsequent knee surgery (replacement or osteotomy) compared to control remains unresolved.
Keywords: Aged; Female; Humans; Middle Aged; Arthroscopy; Arthroscopy/adverse effects; Osteoarthritis, Knee; Osteoarthritis, Knee/surgery; Pain Measurement; Pain, Postoperative; Quality of Life
Plain language summary
Arthroscopic surgery for degenerative knee disease
Background
Degenerative knee disease (osteoarthritis in the knee which affects the joint lining and menisci) is the most common cause of knee pain, swelling and stiffness in the knee joint which leads to difficulty in walking. The cartilage in the knee joint is damaged, resulting in friction in the joint surfaces and formation of new bone in severe cases. Arthroscopic knee surgery removes damaged cartilage and loose tissue and smooths the knee joint surfaces.
Study characteristics
We included 16 randomised trials (2105 participants) published up to 16 April 2021. Trials were conducted in Canada, Denmark, Finland, Italy, Norway, Pakistan, South Korea, Spain, Sweden, Netherlands and USA.
Overall, 56% of participants were women. The average age of participants ranged from 46 to 65 years and the average duration of symptoms ranged from 1.6 months to 4.4 years. Of the nine trials reporting their funding source, none received funding from industry. The other seven trials did not report any funding source.
We limit reporting to the main comparison, arthroscopic surgery versus placebo (dummy or sham) surgery.
Key results
Compared with placebo surgery, arthroscopic surgery had little benefit:
Pain (lower scores mean less pain)
Improvement in pain was 4.6 points better (0.02 better to 9 better) on a 0 to 100 point scale with arthroscopic surgery than with placebo, 3 months after surgery.
• People who had arthroscopic surgery rated their post‐operative pain as 35.5 points.
• People who had placebo surgery rated their post‐operative pain as 40.1 points.
Knee function (higher scores mean better function)
Improvement in knee function was 0.1 points better (3.2 worse to 3.4 better) on a 0 to 100 point scale with arthroscopic surgery than with placebo, 3 months after surgery.
• People who had arthroscopic surgery rated their post‐operative knee function as 76.0 points.
• People who had placebo surgery rated their post‐operative knee function as 75.9 points.
Knee‐specific quality of life (higher scores mean better quality of life)
Improvement in knee‐specific quality of life was 5.6 points better (0.4 better to 10.7 better) on a 0 to 100 point scale with arthroscopic surgery than with placebo, 3 months after surgery.
• People who had arthroscopic surgery rated their post‐operative quality of life as 75.3 points.
• People who had placebo surgery rated their post‐operative quality of life as 69.7 points.
Treatment success (rated by participants)
8% more people rated their treatment a success (25% fewer to 63% more), or 8 more people out of 100, at up to 5 years after surgery.
• 82 out of 100 people reported treatment success with arthroscopic surgery.
• 74 out of 100 people reported treatment success with placebo surgery.
Serious adverse events
2% more people (2% fewer to 10% more) had serious adverse events, or 2 more people out of 100, at up to 5 years after surgery.
• 8 out of 100 people reported serious adverse events with arthroscopic surgery.
• 6 out of 100 people reported serious adverse events with placebo surgery.
Total adverse events
2% more people (3% fewer to 11% more), had adverse events, or 2 more people out of 100, at up to 5 years after surgery.
• 17 out of 100 people reported adverse events with arthroscopic surgery.
• 15 out of 100 people reported adverse events with placebo surgery.
Subsequent knee surgery
2% more people (0.1% fewer to 9% more), had subsequent knee surgery, or 2 more people out of 100, at up to 5 years.
• 4 out of 100 people had knee replacement or osteotomy (knee surgery that reshapes bone) with arthroscopic surgery.
• 2 out of 100 people had knee replacement or osteotomy with placebo surgery.
Certainty of the evidence
We are confident that knee arthroscopy does not provide any clinically important benefits in terms of pain and function. We are moderately confident that knee arthroscopy probably does not provide any clinically important benefits in knee‐specific quality of life over a placebo procedure. Knee arthroscopy may not increase participant‐reported success compared with placebo. We have little confidence in the evidence because of differences across trials in reporting success and the small number of events. We are less certain of the risk of serious and total adverse events in arthroscopy versus placebo surgery: the evidence was uncertain because of the small number of events and incomplete reporting of study information.
Adverse events associated with surgery include total knee replacement, osteotomy, repeat arthroscopy, arthroscopy in opposite knee, cutaneous nerve lesion (damage to nerves in the skin), deep or superficial infection, general knee pain, swelling, instability, stiffness or decreased range of motion in the affected or opposite knee, haemarthrosis (bleeding into the knee joint), death, acute myocardial infarction (heart attack), hypoxaemia (decreased oxygen in the blood), deep vein thrombosis (blood clot in the deep veins), tendonitis (inflammation of tendons), pain from fall or other trauma, rupture of a Baker's cyst (a fluid‐filled sac behind the knee), and back or hip or foot pain.
Arthroscopic surgery may or may not lead to slightly more subsequent knee surgery (replacement or osteotomy) than the placebo procedure.
Summary of findings
Summary of findings 1. Summary of findings.
| Arthroscopic surgery compared to placebo for degenerative knee disease | |||||||
| Patient or population: people with degenerative knee disease (osteoarthritis including degenerative meniscal tears) Setting: surgical Intervention: arthroscopic surgery Comparison: placebo for benefits and all control groups for adverse events including knee replacement | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Placebo | Arthroscopic surgery | Difference | |||||
|
Paina Scale: 0 to 100, 0 is no pain Follow‐up: 3 months |
The mean pain in the placebo group was 40.1 pointsb | The mean pain in the arthroscopic surgery group was 35.5 points | 4.6 points better (0.02 better to 9 better)c | 309 (4 studies) | ⊕⊕⊕⊕ Highd | SMD ‐0.23 (‐0.45 to ‐0.001). Knee arthroscopic surgery results in little or no clinically important improvement in pain. Absolute change 5% better (0.02% better to 9% better) Relative change 8% better (0.03% better to 15% better)e |
|
|
Knee functiona Scale: 0 to 100, 100 is best function Follow‐up: 3 months |
The mean knee function in the placebo group was 75.9 pointsb | The mean knee function in the arthroscopic surgery group was 76 points | 0.1 points better (3.2 worse to 3.4 better) | 302 (3 studies) | ⊕⊕⊕⊕ Highd | SMD 0.01 (‐0.22 to 0.23). Knee arthroscopic surgery results in little or no improvement in function. Absolute change 0.1% better (3% worse to 3% better) Relative change 0.2% better (5% worse to 6% better)e |
|
|
Knee‐specific health‐related quality of lifea Scale: 0 to 100, 100 is best quality of life Follow‐up: 3 months |
The mean quality of life in the placebo group was 69.7 pointsb | The mean quality of life in the arthroscopic surgery group was 75.3 points | 5.6 points better (0.4 better to 10.7 better) | 188 (2 studies) | ⊕⊕⊕⊝ Moderatef | SMD 0.31 (0.02 to 0.59). Knee arthroscopic surgery probably provides little or no clinically important improvement in knee‐specific quality of life. Absolute change 6% better (0.4% to 11% better). Relative change 11% better (0.8% better to 20% better) |
|
|
Participant‐reported success Last follow‐up |
74% | 82% (49% to 100%) |
8% more (25% fewer to 63% more) | RR 1.11 (0.66 to 1.86) | 189 (3 studies) | ⊕⊕⊝⊝ Lowf,g | Knee arthroscopic surgery may result in little or no improvement in the number of people reporting success. Relative change 11% more reported success (34% fewer to 86% more) |
|
Serious adverse events
Last follow‐upi Events include repeat arthroscopy, pulmonary embolism, deep vein thrombosis, heart attack, death, knee surgery, post‐operative knee infection, anterior cruciate ligament reconstruction |
5.6% | 7.6% (3.6% to 15.8%) |
2% more (2% fewer to 10% more) | RR 1.35 (0.64 to 2.83) | 1206 (8 studies)j |
⊕⊕⊝⊝ Lowf,h | Knee arthroscopy may or may not lead to more serious adverse events. Relative change 35% more (36% fewer to 183% more) |
|
Total adverse events Last follow‐upi Events include serious events and less serious transient pain in the back, hip, foot, tendonitis, syncope, rupture of Baker's cyst, pain and swelling in index knee after surgery, superficial infection, haemarthrosis, cutaneous nerve lesion, nausea, dizziness |
15.0% | 17.2% (11.7% to 25.5%) |
2% more (3% fewer to 11% more) | RR 1.15 (0.78 to 1.70) | 1326 (9 studies)j |
⊕⊕⊝⊝ Lowf,h | Knee arthroscopy may or may not slightly increase total adverse events. Relative change 15% more (22% fewer to 70% more) |
| Knee surgery (replacement or osteotomy) Last follow‐upi | 1.5% | 5% (1.4% to 10.8%) |
2% more (0.1% fewer to 9% more) | RR 2.63 (0.94 to 7.34) | 864 (4 studies)j |
⊕⊕⊕⊝ Lowk | Knee arthroscopy may or may not lead to slightly more knee surgery. Relative change 163% more (6% fewer to 634% more) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
aPain measured onnumerical rating scale (Sihvonen 2013), Knee‐Specific Pain Scale (KSPS) (Moseley 2002), questionnaire designed specifically for the trial (Moseley 1996); Knee Injury and Osteoarthritis Outcome Score (KOOS) pain subscale (Roos 2018). Knee function measured on Knee Injury and Osteoarthritis Outcome Score (KOOS) (Roos 2018), Lysholm knee score (Sihvonen 2013), Short Form 36‐item questionnaire (SF‐36) bodily pain (Moseley 2002). Knee‐related quality of life (QoL) measured on the KOOS Knee‐related QoL subscale (Roos 2018), and the Western Ontario Meniscal Evaluation tool (WOMET) (Sihvonen 2013) bControl group risk was estimated from the placebo value at follow‐up for pain, knee function and knee‐related quality of life in Sihvonen 2013 cStandardised mean difference (SMD) back‐translated to typical scales by multiplying the SMD by the standard deviation (SD) at baseline in the placebo group as reported in Sihvonen 2013: mean (SD) for knee pain (0 to 100 scale): 60.1 (20.0); mean (SD) knee function (0 to 100 scale): 60.1 (14.6); mean (SD) generic quality of life (15D): 0.90 (0.06); mean (SD) knee‐specific quality of life (WOMET 0 to 100 scale): 52.8 (18.1). dOverall, the certainty of evidence was high at 3‐month follow‐up for pain and function. One trial measuring pain was at potential risk of selection bias, but this probably did not change our confidence in the effect estimates. The 95% confidence intervals exclude a clinically important change (defined as 12 points (minimum, maximum: 2, 30) on a 0 to 100 point pain scale; and 13 (3, 34) on a 0 to 100 point WOMAC function scale). Further research is likely to strengthen the conclusion that there was no important differences in pain and function between groups, rather than change the conclusion eRelative change: absolute change (mean difference) divided by mean at baseline in the placebo group (values were: 60.1 points on 0 to 100 point pain scale; 60.1 points on 0 to 100 knee function scale; and 52.8 points on 0 to 100 quality of life scale; from Sihvonen 2013). fDowngraded due to imprecision: the 95% confidence intervals do not rule in or rule out a clinically important change (defined as 10 points on the 0 to 100 point quality of life scale); or for dichotomous outcomes the total number of participants was small, or number of events was small (< 200); or data were from a single trial only. gDowngraded due to indirectness for participant‐reported success as there was diversity in definition and timing of measurement: reported at 6 months, 24 months and 5 years across trials. hDowngraded due to possible reporting bias: incomplete reporting of outcome across studies. iTotal and serious adverse events were reported at 24 months (Roos 2018; Van de Graaf 2018); 25 months (Merchan 1993); and 5 years (Gauffin 2014; Herrlin 2007; Katz 2013; Kise 2016; Sihvonen 2013). Total adverse events only were reported at 6 months in one study (Saeed 2015). jFor serious adverse events, adverse events and subsequent knee surgery (replacement or osteotomy), we included trials that compared arthroscopy to placebo or to non‐surgical interventions. For serious adverse events, comparison groups were placebo in 2 trials, exercise in 5 trials and oral non‐steroidal anti‐inflammatory drugs (NSAIDs) in 1 trial. For adverse events, the comparison groups included placebo in 2 trials, exercise in 5 trials, and oral NSAIDs and hyaluronic acid injections in single trials. For knee surgery, the comparison groups included placebo in 1 trial and exercise in 3 trials. kDowngraded twice due to imprecision: the 95% confidence intervals do not rule in or rule out a clinically important change as the total number of events was small (< 200).
Background
Description of the condition
Degenerative knee disease (osteoarthritis of the knee, which can include the joint lining, menisci, or both) is a prevalent musculoskeletal condition and a major contributor to disability globally. Its prevalence continues to increase with population growth, ageing and rising global obesity. It affects one or more compartments of the knee joint and its periarticular structures, including the articular cartilage, menisci, underlying bone and synovial lining. In the USA, it is estimated that about 25% of people aged 45 years or older have symptoms of degenerative knee disease that impact on their quality of life (Alkan 2014; Mahir 2016), while about 10% of the world's population aged 60 years or older have symptomatic osteoarthritis (Zhang 2010).
The major symptoms of knee osteoarthritis are pain, stiffness and swelling, which in turn can lead to impaired mobility and physical function. Symptoms commonly fluctuate, and may or may not progressively worsen. A symptomatic meniscal tear may be suggested by new onset of knee pain accompanied by mechanical symptoms, such as locking or catching, with medial or lateral joint line pain. The presence of a collection of symptoms, including localised pain, clicking, catching and giving way or buckling, has been found to more likely indicate the presence of a symptomatic meniscal tear on the basis of orthopaedic surgeon and magnetic resonance imaging (MRI) assessment, compared to the absence of all of these symptoms (Niu 2011). However, these symptoms are non‐specific, and there is no current consensus on what defines a symptomatic meniscal tear (Buchbinder 2015). Similarly, signs such as medial or lateral joint line tenderness and loss of full extension, and named physical tests such as the McMurray test, purported to be useful in making a diagnosis of a symptomatic meniscal tear, have been found to have limited diagnostic accuracy (Hegedus 2007).
International guidelines and clinical care standards recommend that the diagnosis of degenerative knee disease be made on the basis of clinical features alone, unless an alternative diagnosis is suspected (ACQSHC 2017; NICE 2014; RACGP 2018; Zhang 2009). For example, the UK's National Institute for Health and Care Excellence (NICE) 2014 clinical guideline recommends that osteoarthritis be diagnosed clinically without investigations if a person is over 45 years and has activity‐related joint pain and either has no morning joint‐related stiffness or morning stiffness that lasts no longer than 30 minutes (NICE 2014).
When there is a suspicion of an alternate diagnosis (e.g. malignancy, insufficiency fracture), plain radiographs are the first line investigation in routine care (ACQSHC 2017), followed by MRI if there is continued suspicion of serious pathology not detected by X‐ray. Weight‐bearing X‐rays are also indicated for severe symptoms that have not responded to non‐operative treatment when contemplating joint replacement.
The Kellgren and Lawrence classification is widely used to grade the severity of osteoarthritis (Kellgren 2000). It includes five grades, ranging from 0 to 4, based on the increasing severity of osteoarthritis, where grade 0 indicates no osteoarthritis and grade 4 indicates severe osteoarthritis (Kellgren 2000).
MRI findings of degenerative knee disease are common in asymptomatic people and may be present even when plain radiographs are normal (Englund 2008). For example, one population‐based study that included 710 people under 50 years old who had no radiographic evidence of knee osteoarthritis found the presence of at least one type of abnormality in 90% to 97% of those with symptoms and 86% to 88% of those with painless knees (Guermazi 2012). In a related study, one or more meniscal tears was present in 23% of those without symptoms, 32% in people with symptoms and 24% of those with no or equivocal radiographic evidence of osteoarthritis (Englund 2008). Most often it is the medial meniscus that is torn, and multiple tears are present in more than a third of patients (Bhattacharyya 2003; Englund 2008).
Description of the intervention
Currently, treatment of degenerative knee disease can be grouped broadly into two types: non‐operative and operative management. Non‐operative non‐pharmacologic treatment includes achieving and maintaining a normal weight (Christensen 2007), and supervised land‐based or aquatic exercise (Bartel 2016; Fransen 2015). Simple analgesia provides very minimal benefit (Leopoldino 2019), while non‐steroidal anti‐inflammatory drugs provide limited benefits (Puljak 2017), and may only be suitable for a subset of people without comorbidities that preclude their use. Intra‐articular glucocorticoid injections may provide short‐term pain relief (Jüni 2015). Joint replacement surgery, which is the only definitive treatment, is reserved for people with severe disease who have failed non‐operative management (Brignardello‐Petersen 2017).
Arthroscopic surgery is the most widely performed surgical procedure for degenerative knee disease. It involves the insertion of an arthroscope into the knee joint under either local or general anaesthesia through two portals on the front of the knee joint (Kise 2016). The two most frequently used arthroscopic procedures for managing degenerative knee disease are debridement and partial meniscectomy (Katz 2013). Arthroscopic debridement involves the removal of damaged cartilage, irrigation of the knee joint to wash out all debris, including cartilage fragments and loose bodies, and smoothing of the joint surfaces (Kirkley 2008). Arthroscopic partial meniscectomy involves the removal of torn meniscal fragments and trimming the meniscus back to a stable rim (Sihvonen 2013).
How the intervention might work
The overall mechanism of action of arthroscopic surgery is hypothesised to be via identification and removal of the mechanical components that contribute to the symptoms of osteoarthritis, such as damaged cartilage and loose bodies, while preserving the knee joint (Howell 2014; Kirkley 2008; Shin 2012). Theoretically, this process would reduce inflammation of the joint lining and improve joint motion, resulting in decreased pain and improved knee function (Mounsey 2009). Partial debridement of the torn meniscus is also hypothesised to lead to improvements in pain and mechanical symptoms (Howell 2014; Steadman 2007).
Why it is important to do this review
Evidence from randomised controlled trials that have included a placebo or exercise control, accumulating over two decades, indicates that arthroscopic surgery may provide limited benefit for people with degenerative knee disease, irrespective of osteoarthritis grade or the presence or absence of meniscal tears (Moseley 2002; Herrlin 2007; Kirkley 2008; Katz 2013; Sihvonen 2013; Kise 2016).
Since 2013, evidence‐based guidelines have consistently recommended against the use of arthroscopic debridement and lavage for symptomatic osteoarthritis of the knee, but have been inconsistent in their recommendations regarding treatment of degenerative meniscal tears (Australian Knee Society 2016; Brown 2013; McAlindon 2014; NICE 2014). Both the 2013 and updated 2015 second edition of the American Academy of Orthopaedic Surgeons (AAOS) guidelines for the treatment of knee osteoarthritis made a strong recommendation against performing arthroscopy with lavage, debridement, or both, in people with a primary diagnosis of symptomatic osteoarthritis of the knee (Brown 2013). However, the 2015 guidelines made an inconclusive recommendation regarding arthroscopic partial meniscectomy, stating that the lack of compelling evidence has resulted in an unclear balance between benefits and potential harm. The 2014 NICE clinical guideline for the care and management of osteoarthritis recommends against referral for arthroscopic lavage and debridement as part of treatment for osteoarthritis, "unless the person has knee osteoarthritis with a clear history of mechanical locking (as opposed to morning joint stiffness), 'giving way' or X‐ray evidence of loose bodies"; meniscal tears are not specifically mentioned (NICE 2014). The 2014 Osteoarthritis Research Society International (OARSI) guideline does not make any recommendation regarding arthroscopy, citing the consistent evidence of ineffectiveness (McAlindon 2014). The 2016 Australian Knee Society Position Statement also indicates that arthroscopic debridement or lavage, or both, are not indicated as a primary treatment in the management of knee osteoarthritis, but indicates arthroscopy may be appropriate for symptomatic meniscal tears that have failed an appropriate trial of a structured rehabilitation program (Australian Knee Society 2016).
In contrast to previous guidelines, the updated 2018 Royal Australian College of General Practitioners (RACGP) guideline for the management of knee and hip osteoarthritis recommends against offering meniscectomy and cartilage repair for people with knee osteoarthritis unless the person also has mechanical symptoms of a clinically locked knee (RACGP 2018). This is also consistent with the 2017 Australian Osteoarthritis of the Knee Clinical Care Standard, which indicates that arthroscopic procedures should only be offered if the individual has true mechanical locking or another appropriate indication for these procedures (e.g. septic arthritis or as an investigation when MRI is not possible), on the basis that these treatments are ineffective (ACQSHC 2017).
In 2017, we published a Clinical Practice Guideline as part of the BMJ Rapid Recommendation series, in which we made a strong recommendation against the use of arthroscopy in nearly all people with degenerative knee disease, including those with or without imaging evidence of osteoarthritis, mechanical symptoms, or sudden symptom onset (Siemieniuk 2017). Triggered by a 2016 randomised controlled trial that found that knee arthroscopy was no better than exercise therapy for treating people with a degenerative medial meniscus tear (Kise 2016), the recommendation was based upon a systematic review that included 13 randomised controlled trials (1688 participants) with placebo and non‐operative care controls that assessed benefits and harms, and 12 observational studies (> 1.8 million participants) that also contributed to assessment of potential harms (Brignardello‐Petersen 2017). The review identified:
high‐certainty evidence that knee arthroscopy provides a very small reduction in pain up to three months (mean difference (MD) = 5.4 on a 100‐point scale, 95% CI 2.0 to 8.8), and very small or no pain reduction up to two years (MD = 3.1, 95% CI ‐0.2 to 6.4); and
moderate‐certainty evidence that knee arthroscopy results in a very small improvement in function in the short term (MD = 4.9 on a 100‐point scale, 95% CI 1.5 to 8.4) and very small or no improvement in function up to two years (MD = 3.2, 95% CI ‐0.5 to 6.8)
when compared to conservative management (which included various controls, including placebo surgery). There was low‐certainty evidence of a very low probability of serious complications after knee arthroscopy.
Despite the availability of evidence of a lack of clinically relevant benefit from arthroscopic partial meniscectomy over placebo surgery or a structured exercise program, there continues to be a lack of consensus among orthopaedic surgeons regarding the current place of this procedure in the routine management of people with knee symptoms putatively attributed to a degenerative meniscal tear (Lohmander 2019). Recently, the British Association for Surgery of the Knee (BASK) Meniscal Consensus Project published a guideline specifically focused on meniscal tears (Abram 2019a). Informed by evidence and based upon a consensus approach to management of common clinical presentations, it recommended against arthroscopic meniscal surgery in people with advanced osteoarthritis, except in rare special cases, but recommended offering it to people with 'meniscal' or 'possible meniscal' symptoms and signs and a 'meniscal target' who fail to respond to a period of non‐operative treatment. Earlier surgery could also be considered if deemed appropriate by an experienced colleague acting as a second opinion. However, a recent post hoc analysis of a randomised controlled trial that found no benefit of partial meniscectomy over placebo failed to identify a subgroup who might benefit (Sihvonen 2018), while others have failed to identify specific patient characteristics that might predict a more favourable outcome following meniscal surgery (Pihl 2020).
While our review only considered potential harms of arthroscopy up to three months post surgery, accumulating evidence from both observational studies and randomised controlled trials suggest that arthroscopic partial meniscectomy may be associated with worsening of the underlying osteoarthritis, accompanied by an increased risk of joint replacement surgery, particularly in older people (Abram 2019b; Dearing 2010; Harris 2013; Hawker 2008; Katz 2013; Roemer 2017; Wai 2002).
Synthesis of all the available evidence is therefore warranted to determine the balance of benefits to harms of arthroscopic surgery for degenerative knee disease. This Cochrane Review is an update of an earlier systematic review (Brignardello‐Petersen 2017). The updated review has been conducted according to the guidelines recommended by the Cochrane Musculoskeletal Editorial Board (Ghogomu 2014). The Cochrane format emphasises assessment of placebo‐controlled trials separately from other controls, to enable more discriminating estimates of benefits of arthroscopic surgery per se, from any differences compared to other treatments, and to determine if there are any differences in outcomes between those with meniscal tear and those without. Other updates to the methods are described where relevant.
Objectives
To assess the benefits and harms of arthroscopic surgery, including debridement, partial menisectomy or both, compared with placebo surgery or non‐surgical treatment in people with degenerative knee disease (osteoarthritis, degenerative meniscal tears, or both).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), or trials using quasi‐randomised methods of participant allocation. Studies reported as full‐text, those published as abstract only, and unpublished data were all eligible for inclusion. There were no language or date restrictions. In contrast to our original review (Brignardello‐Petersen 2017), which excluded trials with fewer than 10 participants, we did not exclude trials based upon their size. We also limited our review of harms to trial data.
Types of participants
We included people with symptomatic (defined as persistent knee pain that affects quality of life) degenerative knee disease (osteoarthritis, degenerative meniscal tears, or both). There was no age limit.
We excluded participants with acute traumatic knee pain.
Types of interventions
We included trials comparing arthroscopic surgery that included debridement or partial meniscectomy, or both, with:
placebo surgery (primary comparison as it is least prone to bias); and
non‐surgical interventions, including: exercise and other physical therapy interventions; injections (including glucocorticoid injection, platelet‐rich plasma or cell‐based therapies such as stem cell therapy); non‐arthroscopic lavage/irrigation, drug therapy (including simple analgesia and non‐steroidal anti‐inflammatory drugs); and supplements and complementary therapies.
We excluded arthroscopic joint lavage alone as this intervention is covered by a separate Cochrane Review (Reichenbach 2010), as is osteotomy (Brouwer 2014). We also excluded studies that compared one type of arthroscopic procedure to another type, or to another type of surgery.
Co‐interventions were allowed, provided they were applied equally in all treatment groups.
Types of outcome measures
Major outcomes
Overall pain measured on a visual analogue scale (VAS), numerical rating scale or other scales, or pain subscales of composite scales if separate pain scales were not reported.
Function measured on a visual analogue scale (VAS), numerical rating scale or other scales, or function or activities of daily living scales (e.g. Lysholm Knee Scoring Scale) or subscales of composite scales (e.g. Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) physical function scale, or Knee Injury and Osteoarthritis Outcome Score (KOOS) Activities of Daily Living (ADL) subscale), if separate function scales were not reported.
Knee‐specific health‐related quality of life using scales such as the Western Ontario Meniscal Evaluation Tool (WOMET) if available.
Participant‐reported treatment success as defined by the trialists.
Serious adverse events: proportion in each group with serious adverse events, defined as an event that leads to hospitalisation, disability or death (such as deep vein thrombosis, cardiovascular or pulmonary events and including knee surgery).
Total adverse events: proportion in each group experiencing any adverse event, mild or serious in nature, including deep vein thrombosis, infections, cardiovascular events and pulmonary embolism.
Knee surgery (replacement or osteotomy): proportion in each group who subsequently had a knee replacement or osteotomy.
Minor outcomes
Generic health‐related quality of life (HRQoL) measured on a generic scale (e.g. SF‐36 (36‐item Short Form Health Survey); EQ‐5D (EuroQoL 5‐dimension instruments); 15D (a 15‐dimensional, self‐administered HRQoL instrument).
Progression of knee osteoarthritis as defined by the trialists (e.g. Kellgren‐Lawrence classification (Kellgren 1957), or Ahlback classification (Ahlback 1968)).
Time points
We stratified the analysis for pain, function and health‐related quality of life by these follow‐up time frames:
up to three months;
between three and six months;
between six months and two years;
between two and five years;
between five and 10 years.
For participant‐reported treatment success, serious adverse events, total adverse events, progression of knee osteoarthritis, and subsequent knee surgery (replacement or osteotomy), we extracted and reported events at the final follow‐up.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases on 16 April 2021.
The Cochrane Central Register of Controlled Trials (CENTRAL) (via Ovid EBM Reviews, 16 April 2021).
MEDLINE (via Ovid from 1946 to 16 April 2021).
Embase (via Ovid from 1947 to 16 April 2021).
The search strategies are shown in Appendix 1, Appendix 2 and Appendix 3.
We also searched ClinicalTrials.gov (www.clinicaltrials.gov/) and the World Health Organization (WHO) trials portal (www.who.int/clinical-trials-registry-platform) for ongoing studies, using the terms 'arthroscopic' or 'arthroscopy' or 'debridement' and 'knee osteoarthritis' or 'meniscal degeneration' on 16 April 2021 (see Appendix 4).
No language restrictions were applied.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We also searched Scopus (www.elsevier.com/solutions/scopus) for subsequent publications relating to the included trials on 16 April 2021.
Data collection and analysis
Selection of studies
Two review authors (RJ, SC) independently screened the titles and abstracts of the studies identified from the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. Two review authors (RJ, SC) independently screened the full‐text versions of potentially eligible records and identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreement through discussion or consulted a third author (DOC). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We completed a PRISMA flow diagram and 'Characteristics of excluded studies' table.
Data extraction and management
Two review authors (DOC, SC) extracted study characteristics from included studies. We extracted the following study characteristics.
Methods: study design, setting, total duration of study, details of any 'run in' period, number of study centres and location, withdrawals and date of study.
Participants: N, mean (SD) age, age range, sex, disease duration, severity of condition, diagnostic criteria, important condition‐specific baseline data, inclusion criteria and exclusion criteria.
Interventions: intervention, comparison, concomitant medications and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Characteristics of the design of the trial, as outlined below in the Assessment of risk of bias in included studies section.
Notes: trial registration, funding for trial, and notable declarations of interest of trial authors.
Two review authors (DOC, SC) extracted outcome data from included studies, including the number of events and number of participants per treatment group for dichotomous outcomes, and means and standard deviations and number of participants per treatment group for continuous outcomes. We noted in the 'Characteristics of included studies' tables if outcome data were not reported in a usable way and when data were transformed or estimated from a graph. We resolved disagreements by consensus or by involving a third author (RB). One review author (DOC) transferred data into Review Manager (Review Manager 2020). A second author (RJ) double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports.
In keeping with our previous review (Brignardello‐Petersen 2017), where multiple measures of pain were reported, we extracted the measure highest on the following hierarchy recommended by Juhl 2012: (1) Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale; (2) pain during activity (visual analogue scale (VAS)); (3) pain during walking (VAS); (4) general knee pain (VAS); (5) pain at rest (VAS); (6) other composite pain scales (e.g. SF‐36 bodily pain subscale, Arthritis Impact Measurement Scale (AIMS) pain subscale); and (7) other single item measures. The Knee Injury and Osteoarthritis Outcome Score (KOOS) pain subscale is considered equivalent to the WOMAC pain subscale. Where pain subscales of composite scales were not presented, we did not use total scores.
Similarly, where multiple measures of overall function were reported, we extracted the measure highest on the hierarchy recommended by Juhl 2012: (1) WOMAC function subscale; (2) SF‐36 physical function subscale; (3) SF‐36 (physical composite score); and (4) other composite disability scores. The KOOS ADL subscale is considered equivalent to the WOMAC function subscale. Where function subscales of composite scales were not presented, we used the total scores for function.
To prevent selective inclusion of data based on the results, we used the following a priori defined decision rules to select data from trials.
Where trials reported outcomes at multiple time points, we extracted data from the latest time point within the period of time we were interested in.
Where trialists reported both final values and change from baseline values for the same outcome, we extracted final values.
Where trialists reported both unadjusted and adjusted values for the same outcome, we extracted unadjusted values.
We extracted intention‐to‐treat (ITT)‐analysed data for outcomes assessing benefits (pain, function, knee‐specific and generic quality of life and participant‐reported treatment success) if reported, or extracted the number of participants analysed at that time point, if data were not available for missing participants. For outcomes assessing harms (serious adverse events, total adverse events, progression of knee osteoarthritis, knee surgery (replacement or osteotomy)), we extracted data for those randomised to, and receiving, allocated treatment.
The primary comparison was arthroscopic surgery versus placebo surgery for outcomes that measured benefits of surgery (pain, function and health‐related quality of life at three months, and treatment success at last follow‐up), but we combined data from all control groups to assess harms (serious adverse events, total adverse events, progression of knee osteoarthritis, knee surgery (replacement or osteotomy)) at last follow‐up.
Assessment of risk of bias in included studies
Two review authors (DOC, SC) independently assessed risk of bias for each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). Any disagreements were resolved through discussion or by involving another review author (RJ or RB). We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias: unexplained baseline imbalance (i.e. not explained by suboptimal randomisation), unit of analysis issues, inappropriate or unequal application of co‐interventions across treatment groups, whether the number of cross‐overs from the control group to arthroscopic surgery group biased the analysis.
We graded each potential source of bias as high, low or unclear risk, and gave a justification for our judgment, in the risk of bias tables. We summarised the risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes (e.g. self‐reported outcomes such as pain, function, participant‐reported treatment success, health‐related quality of life; and objective outcomes such as adverse events and knee surgery (replacement or osteotomy)). We considered the impact of missing data by key outcomes, where possible.
Where information on risk of bias relates to unpublished data or correspondence with a trialist, we noted this in the risk of bias table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
We present the figures generated by the risk of bias tool to provide summary assessments of the risk of bias.
Assessment of bias in conducting the systematic review
We conducted the review according to our prior published review (Brignardello‐Petersen 2017), reporting any deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We used the Cochrane Collaboration statistical software, Review Manager 5.4 (Review Manager 2020), to perform data analysis. We analysed dichotomous data as risk ratios (RR), with 95% confidence intervals (CI). Continuous data were analysed as mean difference (MD) or standardised mean difference (SMD), depending on whether the same scale was used to measure an outcome, with 95% CI. We entered data presented as a scale with a consistent direction of effect across studies.
When different scales were used to measure the same conceptual outcome (e.g. function), we calculated SMD, with corresponding 95% CIs. SMDs were back‐translated to a typical scale by multiplying the SMD by a typical among‐person standard deviation (SD) (e.g. the standard deviation of the control group at baseline from the most representative trial (Higgins 2021)). This contrasts with our original review which converted pain and function to 0 to 100 scales before meta‐analysis, then calculated MD. For pain, we converted pooled SMD results to a 0 to 100 scale using the SD of 20 from Sihvonen 2013. For function, we converted SMD results to the KOOS 0 to 100 scale using the SD of 14.6 from Sihvonen 2013. For knee‐specific health‐related quality of life, we converted SMD results to the WOMET 0 to 100 scale using the SD of 18.1 from Sihvonen 2013. For generic health‐related quality of life, we converted SMD results to the 15D 0 to 1 scale using the SD of 0.06 from Sihvonen 2013 (and also the SF‐36 Mental Component Summary (MCS) score 0 to 100 scale using SD of 10 from Roos 2018).
In the 'Comments' column of the summary of findings table, we reported the absolute percent difference and the relative percent change from baseline. The number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) will only be reported when the outcome shows a clinically important difference between treatment groups.
For dichotomous outcomes (treatment success, adverse events, progression of knee osteoarthritis, knee replacement or osteotomy), we calculated NNTB or NNTH from the control group event rate and the relative risk using the Visual Rx NNT calculator (Cates 2008). We calculated the NNTB for continuous measures (pain, function, health‐related quality of life) using the Wells calculator (available at the Cochrane Musculoskeletal Group (CMSG) Editorial office, musculoskeletal.cochrane.org/).
In keeping with our previous review (Brignardello‐Petersen 2017), we used the minimal important differences (MIDs) for pain, function and health‐related quality of life from a linked systematic review performed to establish the most credible MIDs for each of the instruments used to measure these outcomes (Devji 2017). The most credible MID was the median of all the credible MIDs. For pain, we used the MID for WOMAC pain which was found to be 12 points (minimum, maximum: 2, 30) on a 0 to 100 point scale (noting that the MID for KOOS pain was also 12 (4, 20) also on a 0 to 100 point scale). For function, we used WOMAC function MID of 13 (3, 34) on a 0 to 100 point scale (noting that the MID for KOOS ADL was 8 (3, 9) also on a 0 to 100 point scale). For health‐related quality of life, we used the MID for the EQ‐5D which was 0.15 (minimum and maximum were not reported) on a ‐0.59 to 1 scale.
For dichotomous outcomes, we calculated the absolute percent change from the difference in the risks between the intervention and control group using GRADEpro (GRADEPro GDT) and expressed this as a percentage. The relative percent change was calculated as the risk ratio − 1 and expressed as a percentage. For continuous outcomes, we calculated the absolute benefit as the improvement in the intervention group minus the improvement in the control group (mean difference), in the original units, and expressed this as a percentage.
The relative percent change for dichotomous data was calculated as the Risk Ratio ‐ 1 and expressed as a percentage. For continuous outcomes, the relative difference in the change from baseline was calculated as the absolute benefit divided by the baseline mean of the control group, expressed as a percentage.
Unit of analysis issues
The participant was the unit of analysis wherever possible. If a trial randomised participants to different treatment groups, but treated two knees in a single participant without adjusting for the lack of independence in the analysis, we reported this as a potential source of other bias. We assessed the impact of including these trials in a sensitivity analysis. When the data for these studies were extracted, the number of knees was taken as the population for the study.
Dealing with missing data
When required, we contacted trial authors to obtain data missing from the trial reports. For outcomes assessing benefit (pain, function, knee‐specific and generic health‐related quality of life and participant‐reported treatment success), we used the number of participants per group analysed at that time point. If the number of participants per group analysed was not presented for each time point, the number of randomised participants in each group at baseline was used. For outcomes assessing harms (severe adverse events, total adverse events, progression of knee osteoarthritis, knee surgery (replacement or osteotomy)), we used the number of participants receiving the allocated intervention as the denominator.
Where possible, we calculated missing standard deviations from other statistics, such as standard errors, confidence intervals or P values, according to the methods recommended in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). If we could not calculate standard deviations, we imputed them from other studies in the meta‐analysis, as per Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021). Where data were calculated or imputed, we reported this in the notes section of the Characteristics of included studies.
Assessment of heterogeneity
We assessed the clinical and methodological diversity of the included studies, in terms of participants, interventions, outcomes and study characteristics, to determine whether a meta‐analysis was appropriate. We assessed statistical heterogeneity by visually inspecting forest plots to check for obvious differences in results between the studies, and by using the I² and Chi² tests.
As recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021), we interpreted an I² statistic for heterogeneity of 0% to 40% as 'might not be important'; 30% to 60% may represent 'moderate' heterogeneity; 50% to 90% may represent 'substantial' heterogeneity; and 75% to 100% represents 'considerable' heterogeneity. As noted in the Cochrane Handbook for Systematic Reviews of Interventions, the importance of I2 depends on (i) the magnitude and direction of effects, and (ii) the strength of evidence for heterogeneity.
We interpreted the Chi² test so that P ≤ 0.10 indicates evidence of statistical heterogeneity. Where we identified substantial heterogeneity, we reported it and investigated possible causes by following the recommendations in section 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021).
Assessment of reporting biases
To assess small study effects, we planned to generate funnel plots for meta‐analyses including at least 10 trials of varying size. If we detected asymmetry in the funnel plot, we planned to review the characteristics of the trials to assess whether the asymmetry was likely due to publication bias or other factors, such as the methodological or clinical diversity of the trials. Where we were able to pool more than 10 trials, we conducted formal statistical tests to investigate funnel plot asymmetry, and followed the recommendations in section 13 of the Cochrane Handbook for Systematic Reviews of Interventions (Page 2021).
To assess outcome reporting bias, we checked trial protocols against published reports. For studies published after 1 July 2005, we searched the Clinical Trial Register at the International Clinical Trials Registry Platform of the World Health Organization (www.who.int/clinical-trials-registry-platform) for the a priori trial protocol. If trial protocols were unavailable, we compared the outcomes reported in the methods and results sections of the trial reports.
Data synthesis
We pooled outcomes that assessed the benefits of treatment across studies with a similar comparator and stratified the primary analysis by follow‐up time, as follows:
arthroscopic surgery versus placebo surgery;
arthroscopic surgery versus exercise;
arthroscopic surgery versus glucocorticoid injections;
arthroscopic surgery versus non‐arthroscopic lavage;
arthroscopic surgery versus anti‐inflammatory drugs; and
arthroscopic surgery versus hyaluronic acid injections.
We pooled outcomes that assessed the harms of treatment (serious and total adverse events, progression of knee osteoarthritis, knee surgery (replacement or osteotomy)) across all studies (arthroscopic surgery versus any control).
Expecting some differences in the effect of the intervention across studies, we used a random‐effects model as the default.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses to assess if there were differences in pain and function for the primary comparison of arthroscopic surgery versus placebo:
between participants with and without meniscal tear;
between studies that describe arthroscopy with supervised exercise compared to studies that do not (or are unsupervised).
We performed subgroup analyses only for studies of arthroscopic surgery compared with placebo. We used the formal test for subgroup interactions in Review Manager (Review Manager 2020). We also compared the magnitude of the effects between the subgroups by means of assessing the overlap of the confidence intervals of the summary estimates. Non‐overlap of the confidence intervals indicates statistical significance, and we used the formal test for differences in subgroups in Review Manager.
Sensitivity analysis
We performed sensitivity analyses to investigate the robustness of the effect on pain and function to potential selection and detection biases for the primary comparison of arthroscopic surgery versus placebo, at the primary time point (three months).
We performed a sensitivity analysis with fixed‐effect rather than random‐effects model for pain and function for the primary comparison of arthroscopic surgery versus placebo, at the primary time point (three months).
We also performed a sensitivity analysis by pooling outcomes that assessed benefit across all studies (arthroscopic surgery versus any control) as per our original review (Brignardello‐Petersen 2017), for all time points.
We planned to investigate the robustness of the effect on pain and function to unit of analysis errors for the primary comparison of arthroscopic surgery versus placebo at the primary time point (three months), but no unit of analysis errors were identified.
Interpreting results and reaching conclusions
We followed guidance in Chapter 15 of the Cochrane Handbookfor Systematic Reviews of Interventions for interpreting results (Schünemann 2021a), and were aware of distinguishing a lack of evidence of effect from a lack of effect. We based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this review. We avoided making recommendations for practice and our implications for research recommend priorities for future research and outline the remaining uncertainties in this area.
Summary of findings and assessment of the certainty of the evidence
We created a summary of findings table for arthroscopic surgery compared to placebo surgery using the following outcomes: pain, function, knee‐specific health‐related quality of life, treatment success, proportion experiencing serious and total adverse events, and proportion undergoing subsequent knee surgery (replacement or osteotomy). For benefits of surgery, we included pain, function and knee‐specific quality of life measured at three months, and treatment success at last follow‐up. For serious adverse events, total adverse events and knee replacements or osteotomies, we included combined data from all control groups at last follow‐up.
Two review authors (DOC, RJ) independently assessed the certainty of the evidence across all studies contributing to the meta‐analysis for each outcome, using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias), as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2021a; Schünemann 2021b). We developed the summary of findings table using GRADEpro software (GRADEPro GDT). We justified decisions to downgrade the quality of studies in the footnotes of the table.
We reported the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH), absolute and relative percent change in the 'Comments' column of the summary of findings table, as described in the Measures of treatment effect section above.
Results
Description of studies
Results of the search
Overall, there are 16 included studies, 15 excluded studies, two studies awaiting classification and four ongoing studies.
The results of the search are shown in Figure 1. The search identified 3404 records (3044 from electronic databases, 359 from trial registries, 1 from reference checking). After removal of duplicates, we screened 2262 records. We retrieved 37 studies for full‐text screening.
1.
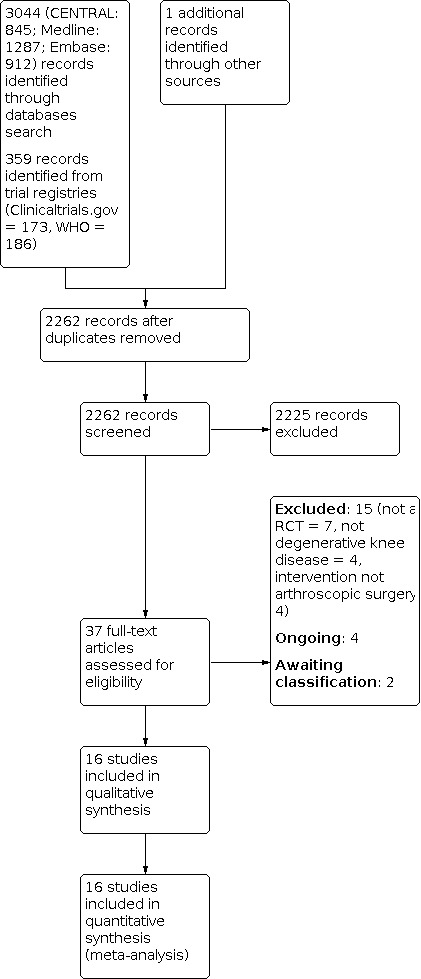
Study flow diagram
We excluded 15 studies (seven were not RCTs, four examined interventions other than arthroscopic surgery and four did not include participants with degenerative knee disease).
Sixteen trials met our criteria for inclusion (Chang 1993; Gauffin 2014; Herrlin 2007; Katz 2013; Kirkley 2008; Kise 2016; Merchan 1993; Moseley 1996; Moseley 2002; Osteras 2012; Roos 2018; Saeed 2015; Sihvonen 2013; Van de Graaf 2018; Vermesan 2013; Yim 2013).
Our previous review included 12 of these trials (i.e. Brignardello‐Petersen 2017 included: Chang 1993; Gauffin 2014; Herrlin 2007; Katz 2013; Kirkley 2008; Kise 2016; Moseley 2002; Osteras 2012; Saeed 2015; Sihvonen 2013; Vermesan 2013; Yim 2013). Two other trials were published after our previous search cut‐off date (Roos 2018; Van de Graaf 2018). In this update, we included two trials that were excluded from our previous review: Moseley 1996 was previously excluded as it included fewer than ten participants, while Merchan 1993 was previously erroneously excluded. Stensrud 2015 was included in our original review but was an interim preliminary report of a subset of 82 out of 140 participants from Kise 2016 (same clinical trial registry number NCT01002794).
We identified four ongoing trials in clinical trials registries (NCT02113280; NCT02995551; NCT04313569; NCT04837456).
We also note that an included study, Sihvonen 2013, has a separate trial registration for an ongoing 10 year follow‐up (NCT01052233), but includes the same participants enrolled earlier. Thus, we have not counted this as a separate ongoing study, but grouped it as a secondary report of Sihvonen 2013.
Included studies
We provide a full description of the 16 included trials in the Characteristics of included studies tables. We contacted the authors of eight trials to retrieve (1) information about study design, participants, interventions and outcomes of the trial, (2) information required to complete the risk of bias assessments or (3) missing data for unreported or partially reported outcomes (Chang 1993; Herrlin 2007; Kise 2016; Moseley 1996; Roos 2018; Saeed 2015; Sihvonen 2013; Yim 2013). We received replies from the authors of four trials (Herrlin 2007; Kise 2016; Roos 2018; Sihvonen 2013).
Studies awaiting classification
Two studies are awaiting classification (Kang 2005; NCT00562822).
Ongoing studies
We identified four ongoing studies that did not have study results available at the time of submission of this review (NCT02113280; NCT02995551; NCT04313569; NCT04837456). One study compares arthroscopic meniscectomy to conservative treatment in people with degenerative meniscal tears (NCT04313569). The other trials are comparing knee arthroscopy to exercise (NCT02113280; NCT02995551; NCT04837456). We provide a description of these trials in the Characteristics of ongoing studies table.
Study design and setting
All 16 included trials were parallel‐group RCTs. Fourteen trials included two intervention arms and two trials included three intervention arms (Moseley 1996; Moseley 2002).
The trials were conducted in Canada (Kirkley 2008), the USA (Chang 1993; Katz 2013; Moseley 1996; Moseley 2002), Denmark (Roos 2018), Finland (Sihvonen 2013), Sweden (Gauffin 2014; Herrlin 2007), Norway (Kise 2016; Osteras 2012), the Netherlands (Van de Graaf 2018), Spain (Merchan 1993), Italy (Vermesan 2013), Pakistan (Saeed 2015), and South Korea (Yim 2013).
Participant and intervention characteristics
Participant characteristics are detailed in the Characteristics of included studies tables, and age, osteoarthritis and meniscal tear criteria are shown in Table 2. A total of 2105 participants were included in the 16 trials. The number of participants per trial ranged from 10 in Moseley 1996 to 351 in Katz 2013. The minimum age requirements were 35 years in Roos 2018 to under 70 years in the Moseley 1996 and Moseley 2002 studies. The mean age of participants ranged from 46.4 years in Roos 2018 to 65 years in Chang 1993 (age reported for all trials except Moseley 1996 and Saeed 2015). Fifty‐six percent of participants were female (gender reported for all trials except Moseley 1996). The mean duration of symptoms, reported in nine trials, ranged from 1.6 months in Osteras 2012 to 53 months in Chang 1993.
1. Characteristics of participants.
| Study ID | Age range (years) | Osteoarthritis and criteria | Meniscal tear and criteria |
| Trials with a placebo control | |||
| Moseley 1996 | < 70 | ACR‐defined or clinically diagnosed | No criteria re presence/absence specified |
| Moseley 2002 | < 70 | ACR‐defined or clinically diagnosed | No criteria re presence/absence specified |
| Sihvonen 2013 | 35 to 65 | KL grade 0 to 1 | Medial meniscal tear on MRI |
| Roos 2018 | 35 to 55 | KL grade 0 to 2 | Medial meniscal tear on MRI |
| Trials with an exercise control | |||
| Gauffin 2014 | 45 to 64 | < 50% joint narrowing | Clinically suspected meniscal injury (66/75 randomised to surgery received surgery but only 56 had partial meniscectomies (1 loose bodies removed, 1 synovectomy, 1 partial resection ACL remnant, 8 deemed surgery unnecessary; of the 16/75 who crossed over to surgery, 11 had 11 partial meniscectomies (1 loose bodies removed, 1 microfracture, 1 partial resection ACL remnant, 1 deemed surgery unnecessary and 2 unknown). 3 ACL total ruptures were found (2 surgical group, 1 in non‐surgery group who crossed over toe surgery) |
| Herrlin 2007 | 45 to 65 | Grade 0 to 1 Ahlbacks classification | Medial meniscal tear |
| Katz 2013 | 45 or older meniscal tear, mild to mod OA, KL grade 0‐3 | KL grade 0 to 3 | Meniscal tear |
| Kirkley 2008 | 18 or older | KL grade 2 to 4 except grade 4 if involves both compartments | Exclude large meniscal tear (bucket handle tear) mainly clinical, few had MRIs |
| Kise 2016 | 35 to 60 | KL grade 0 to 2 | Medial meniscal tear |
| Osteras 2012 | 35 to 60 | KL grade 0 to 2 | Meniscal tear |
| Van de Graaf 2018 | 45 to 70 | KL grade 0 to 3 | Meniscal tear |
| Yim 2013 | no age restriction specified | KL grade 0 to 1 | Medial posterior horn horizontal meniscal tear on MRI |
| Trials with other controls | |||
| Saeed 2015 | >40 | KL grade 2 and 3 | No criteria re presence/absence specified |
| Vermesan 2013 | Not specified | medial compartment cartilage and meniscus lesions on MRI | Medial compartment cartilage and meniscus lesions on MRI |
| Chang 1993 | >20 | KL grades 1 to 3 | No criteria re presence/absence specified |
| Merchan 1993 | Not specified | minimal joint space narrowing and formation of small osteophytes | No criteria re presence/absence specified |
ACR: American College of Rheumatology; ACL: anterior cruciate ligament; KL grade: Kellgren‐Lawrence classification grade; MRI: magnetic resonance imaging
The required duration of knee pain varied between trials. Two trials specified persistent knee pain for more than two months (Kise 2016; Roos 2018); seven trials included participants with pain for more than three months (Chang 1993; Gauffin 2014; Herrlin 2007; Moseley 1996; Moseley 2002; Osteras 2012; Sihvonen 2013); and one trial specified symptoms of torn menisci for at least four weeks (Katz 2013).
The inclusion criteria regarding the presence/absence of osteoarthritis and degenerative meniscal tears varied across trials (Table 2). We describe these below, grouped according to the comparator to arthroscopic surgery.
Detailed descriptions of the interventions delivered in each trial are summarised in the Characteristics of included studies tables. We present a summary of the arthroscopic technique and comparison in each trial in Table 3. Arthroscopic procedures varied from debridement of torn menisci to surgical resection of proliferative synovium and excision of loose cartilage fragments. We describe the interventions below, grouped according to the comparator to arthroscopic surgery.
2. Characteristics of interventions used in included trials.
| Study ID | Description of arthroscopic surgery | Description of post‐surgical exercise (in arthroscopic surgery arm) | Description of control | Co‐interventions |
| Trials with a placebo control | ||||
| Moseley 1996 | Arthroscopic debridement: diagnostic arthroscopy, joint lavage, shaving of rough articular cartilage, removal of loose debris, trimming of torn/degenerated menisci | Participants were instructed to resume walking and other activities of daily living as soon as their symptoms would allow. No other exercises were given. | Skin incisions without insertion of arthroscope, knee manipulation, saline splashing over the joint. Surgeon asked for all instruments. Simulation of standard arthroscopic debridement as close as possible. Time spent in the operation theatre: 1 hour | Oral analgesia (acetaminophen with codeine), crutches until able to walk comfortably without a limp. NSAIDs taken pre‐operatively could be resumed after the first follow‐up at 10 days. Applied equally in all treatment groups: yes |
| Moseley 2002 | Arthroscopic debridement: diagnostic arthroscopy, joint lavage, shaving of rough articular cartilage, removal of loose debris, trimming of torn or degenerated meniscal fragments, and smoothening of the remaining meniscus to a firm and stable rim. Shaving of spurs from the tibial spine area that blocked full extension. | Participants were given a graduated exercise program after surgery; details of the program were not reported. | Simulated debridement with three 1 cm skin incisions but without insertion of the arthroscope. Knee manipulation, surgeon asked for all instruments, saline splashing. Time spent in the operation theatre: same as debridement group. | Walking aids, graduated exercise program and analgesics. Applied equally in all treatment groups: yes |
| Roos 2018 | Partial meniscectomy with preservation of as much meniscus as possible. Documentation of findings in cartilage, ligaments, synovium and the medial and lateral menisci. Registration of the type and extent of meniscus lesion and ICRS classification of articular cartilage changes. | Post‐operative home‐based exercise program. At 1 week, biking, swimming and fast walking, and at 2 to 3 weeks, more intense biking and jogging were recommended. For the first post‐operative week, 7 different non‐weight‐bearing exercises to improve lower extremity function and knee range of motion were suggested, and an additional 3 weight‐bearing thereafter. All exercises were recommended to be performed 10 to 15 times three times daily. | Skin incisions in same location as in arthroscopic surgery without insertion of arthroscope, knee manipulation, spillage of water, use of all equipment needed for arthroscopic surgery. Surgeon asked for all instruments. Simulation of arthroscopic surgery as close as possible. | Weight‐bearing and non‐weight‐bearing exercises. Applied equally in all treatment groups: yes |
| Sihvonen 2013 | Arthroscopic partial meniscectomy ‐ removal of damaged menisci with arthroscopic instruments (mechanised shaver and meniscal punches) until solid meniscus tissue was reached. Resection of loose, unstable meniscal fragments while preserving as much of the meniscus tissue as possible. | Post‐operative graduated home exercise program for both legs for 10 to 15 minutes at a time, 5 days a week. | The surgeon asked for all instruments, knee manipulation, simulation of a standard arthroscopic partial meniscectomy procedure by using a mechanised shaver (without the blade) outside the knee, suction was also used to drain the joint and saline was splashed. Time spent in the operation theatre: same as the surgery group. | Walking aids, graduated home exercise program, over‐the‐counter analgesics. Applied equally in all treatment groups: yes |
| Trials with an exercise control | ||||
| Gauffin 2014 | Arthroscopic surgery: inspection of joint, meniscal resection performed if needed (but not performed if not needed) | Post‐operatively all participants were allowed immediate, full weight‐bearing activity. They were advised to resume the exercise programme according to phase 1 for 1 week, and then switch to phase 2. | Unsupervised exercise program lasted 3 months, performed twice a week and comprised two phases. Phase 1 was performed for the first 3 weeks and included 20 to 30 min brisk walk, 10 x 2 sets of the following: squats, pelvic lifts, pelvic lifts with ball between knees, heel raise, wall squats and standing on a pillow on one leg; Phase 2: 20 to 30 min brisk walk, 10 x 3 sets of all exercises done in phase 1. Frequency, intensity and duration: phase 1 ‐ daily, 2 sets; phase 2 ‐ twice per week 3 sets each for 3 months. Supervised: no Setting: home |
None specified |
| Herrlin 2007 | Arthroscopic partial meniscectomy: arthroscopic joint inspection, registration of meniscal lesions and Outerbridge classification of changes in the articular cartilage. | Twice a week during a period of 8 weeks each participant followed a standardised exercise program similar to the exercise group. This was followed by a written unsupervised home program twice a week. |
A. Supervised exercise.Description: all exercises for 3 x 10 sets. 0 to 8 weeks: stationary bicycling 7 to 15 min, knee extensions concentrically with two legs and eccentrically with one leg, stair walking and balance on wobble boards (3 min), jogging, jumps, landing on a rebounder (5 min), stretching of knee extensors and flexors (1 min/muscle group). 0 to 4 weeks: calf raise on leg press, knee flexions concentrically with two legs and eccentrically with one leg. 1 to 4 weeks: leg press. 5 to 8 weeks: calf raises standing on one leg, lunges with < 80 of knee flexion with or without weight in the hands, knee flexions with one leg, knee extensions with one leg. Frequency, intensity and duration: twice a week for 8 weeks Supervised: yes. Setting: research centre. B. Unsupervised exercise.Description: 3 x 10 sets of one‐leg standing during 1 min and a step down exercise. Frequency, intensity and duration: twice a week for 8 weeks. Supervised: no. Setting: home |
None specified |
| Katz 2013 | Arthroscopic partial meniscectomy: trimming of damaged meniscus to a stable rim, removal of loose fragments of cartilage and bone without any penetration of the subchondral bone | Post‐operative standardised physical therapy program, as described in the exercise group. | Supervised exercise.Description:phase I: acute phase (1 to 10 days post‐op) Retrograde Massage, Cryotherapy E‐Stim: NMES or IFC, Joint Mobilisation Soft Tissue Mobilisation Stretching LE Muscles, Quad Sets SAQ/LAQ/HS Curls Hip‐4 way, Bicycle, Elliptical, Treadmill, Leg Press, Balance/Proprioception. Phase II: Subacute Phase (10 days to 4 weeks post‐op) Retrograde Massage Cryotherapy E‐Stim: NMES or IFC, Joint Mobilisation Soft Tissue Mobilisation Stretching LE Muscles, Concentric/Eccentric Hip/Knee progressive resistive exercises, ROM, Resisted terminal knee extension, modified mini squats, step up/down progressions, toe raises, functional and agility training. Phase III: Advanced Activity Phase (4 to 7 weeks post‐op) ‐ continued stretching program, continued PRE therapeutic exercises program, closed chain program with progression to dynamic single leg stance, plyometrics, running, and sport specificity training. Frequency, intensity and duration: 8 exercises, 12 to 15 repetitions, 1 to 2 sets. Supervised: yes for once or twice weekly in the initial sessions in each phase, after which exercises were done at home Setting: clinic for 1/2 sessions then home for the rest of the phase. | Acetaminophen, non‐steroidal anti‐inflammatory agents and intra‐articular injections of glucocorticoids as required. Applied equally in all treatment groups: yes |
| Kirkley 2008 | Arthroscopic surgery: saline irrigation of medial, lateral, and patellofemoral joint compartments, based on joint findings one of the following was done ‐ synovectomy; debridement; or excision of degenerative tears of the menisci, fragments of articular cartilage, or chondral flaps and osteophytes that prevented full extension. | Optimized physical and medical therapy for 12 weeks followed by home exercises and arthritis education similar to the exercise group were given post‐operatively. |
A. Physical therapy.Description: not provided. Frequency intensity and duration: 1 hour once a week for 12 consecutive weeks. Supervised: yes Setting: clinic. B. Home exercise along with physical therapy.Description: range‐of‐motion and strengthening exercises. Frequency intensity and duration: twice daily and once on the day of a scheduled physical‐therapy session for 12 weeks along with the physical therapy. Supervised: no Setting: home. C. Unsupervised home exercise.Description: not provided. Frequency intensity and duration: duration of the study. Supervised: no. Setting: home |
Step‐wise use of acetaminophen and non‐steroidal anti‐inflammatory drugs, intra‐articular injection of hyaluronic acid and oral glucosamine. Arthritis education ‐ attendance at local Arthritis Society workshops, The Arthritis Helpbook and an educational videotape. Applied equally in all treatment groups: yes |
| Kise 2016 | Arthroscopic partial meniscectomy: joint inspection and lavage, probing of menisci and resection of unstable meniscal tissue | Participants were advised to use crutches until normal weight‐bearing, and were given written and oral instructions for simple home exercises to be performed two to four times daily. | Supervised exercise.Description: stationary cycle (20 min) 3 x 10 sets of the following: squat, single‐leg squat, step‐up, knee stability in pull loop and skating; hamstring on fitball (3 x 8); 2‐4 x 15‐6 sets of: single‐leg leg press, single‐leg knee extension, single‐leg leg curl; limping cross (3 x 3 rounds). Frequency intensity and duration: minimum of two and a maximum of three sessions each week (24 to 36 sessions). Each session lasted approximately 60 to 80 minutes for a total of 12 weeks. Supervised: yes Setting: clinic | None specified |
| Osteras 2012 | Arthroscopic partial meniscectomy | None specified | Supervised exercise.Description: 15 to 20 min of aerobic work on a stationary ergometer cycle. After 4 exercises each of 3 sets of 30 repetitions halfway through the exercise program, the participants cycled for 10 min and again after the last 4 exercises, the participants did another 10 min on a stationary ergometer cycle. Frequency intensity and duration: 3 times per week for 3 months. Supervised: yes. Setting: clinic | None specified |
| Van de Graaf 2018 | Arthroscopic partial meniscectomy: standard anteromedial and anterolateral portals were introduced for inspection of the knee joint. The affected meniscus was partially removed until a stable and solid meniscus remained. | Post‐operatively, participants received instructions for a home exercise program which consisted of one leg standing for 60 seconds and a step‐down exercise comprising 3, 9, 10 repetitions, twice a week. | Physical therapy (PT). Participants were referred to PT clinics which were instructed about the exercise protocol by a knee‐specialised physical therapist or the primary investigator, prior to the first participant’s referral. The PT exercise protocol developed by a knee‐specialised physical therapist consisted of 16 sessions of 30 minutes each conducted over 8 weeks. The PT protocol comprised cardiovascular, coordination/balance, and closed kinetic chain strength exercises (in which the distal part of the extremity is fixed to an object that is stationary). If PT failed, the participant was allowed to attend additional PT sessions or have APM, depending on their preference | Home exercise program. Applied equally in all treatment groups: yes |
| Yim 2013 | Arthroscopic meniscectomy: meniscal resection with limited debridement of the articular surface lesion. | Post‐operatively, all participants were provided with a home exercise program, which was conducted unsupervised, using the same protocol as the non‐operative group for 8 weeks. |
A. Supervised exercise.Description: scheduled physical exercise to improve muscle strength, endurance, and flexibility. Frequency intensity and duration: 60 minutes per session, 3 times weekly, for 3 weeks. Supervised: yes Setting: clinic B. Unsupervised exercise.Description: 3 x 10 sets of the following: half squats with < 45 degrees of flexion with weights, squats with full flexion with weights, knee extension in sitting position, knee flexion in sitting position; stretching of knee extensors and flexors 1 min/muscle group, stationary bicycling (gradual increase every 15 min). Frequency intensity and duration: daily for 8 weeks Supervised: no Setting: home |
Analgesics, NSAIDs or muscle relaxants for the first 2 weeks. Applied equally in all treatment groups: yes |
| Other trials | ||||
| Chang 1993 | Joint inspection followed by either debridement of torn meniscus and removal of meniscal and cruciate ligament fragments, removal of proliferative synovium, excision of loose articular cartilage fragments, based on the joint findings | Participants were routinely instructed in partial weight‐bearing precautions for 10 days post‐operatively, followed by physical therapy, consisting of strengthening and flexibility exercises and gait training. | Non‐arthroscopic (closed‐needle joint) lavage: tidal knee lavage was done under local anaesthesia. A total of 1 litre of saline was injected into and aspirated from the knee in aliquots of 40‐120 cc, depending on the size of the knee capsule. | Non‐narcotic analgesia and physical therapy, consisting of strengthening and flexibility exercises and gait training. Applied equally in all treatment groups: yes |
| Saeed 2015 | Arthroscopic debridement performed using two portals in all cases and under spinal anaesthesia | None reported | Intra‐articular hyaluronic acid injections under intradermal anaesthesia given weekly for 5 weeks with a 24‐gauge needle under strict aseptic conditions in the operation theatre. In case of joint effusion, aspiration was done before the injection to prevent dilution of the injection. | None specified |
| Vermesan 2013 | Arthroscopic debridement | None reported | A single intra‐articular glucocorticoid injection using 1 mL of betamethasone in 4 mL of 1% lidocaine was administered. | None specified |
| Merchan 1993 | Arthroscopic surgery: debridement of synovial tissue; removal of degenerative menisci, osteophytes, and loose bodies; limited debridement of cartilage defects | Post‐operatively, a compression bandage was used with early exercises, motion, and weight‐bearing as tolerated. Physiotherapy consisting of quadriceps exercises and knee flexion exercises was practiced for 4 weeks after surgery. | The non‐operative treatment consisted of non‐steroidal anti‐inflammatory drugs and a decrease in the intensity of the activities of daily living for a pain‐free knee. Physiotherapy was practiced as in the operative group (i.e. quadriceps and knee flexion exercises for 4 weeks). | None specified |
APM: arthroscopic partial meniscectomy; ICRS: International Cartilage Repair Society; NSAIDS: non‐steroidal anti‐inflammatory drugs
Arthroscopic surgery versus placebo surgery
Four trials compared arthroscopic surgery to placebo (Moseley 1996; Moseley 2002; Roos 2018; Sihvonen 2013). Two trials specifically included people with knee pain and a medial meniscal tear on MRI with minimal radiographic evidence of osteoarthritis (either Kellgren‐Lawrence (KL) classification grade 0 to 1 (Sihvonen 2013), or KL grade 0 to 2 (Roos 2018)). The other two trials included people with American College of Rheumatology (ACR)‐defined or clinically diagnosed knee osteoarthritis and did not make any distinction based upon the presence/absence of degenerate meniscal tears (Moseley 1996; Moseley 2002).
All four placebo‐controlled trials debrided degenerative or torn menisci if present. Two performed partial medial meniscectomy alone (Roos 2018; Sihvonen 2013), while the other two trials debrided degenerate articular cartilage as well as any torn or degenerate meniscal fragments (Moseley 1996; Moseley 2002). The placebo surgery control was similar across all four trials (see Table 3). Two trials included an additional arthroscopic lavage control arm (Moseley 1996; Moseley 2002), but these data were not extracted for this review.
Arthroscopic surgery versus exercise
Five trials compared arthroscopic surgery and exercise to exercise alone (Gauffin 2014; Herrlin 2007; Katz 2013; Kirkley 2008; Yim 2013), while three trials compared arthroscopic surgery to exercise therapy (Kise 2016; Osteras 2012; Van de Graaf 2018). We have considered these eight trials together.
One trial was performed primarily to investigate the value of arthroscopic treatment versus exercise for osteoarthritis (KL grades 2 to 4 excluding grade 4 if involving both compartments) (Kirkley 2008). This trial excluded people with large meniscal tears (bucket handle tears) detected by clinical examination or MRI in a minority of cases.
The remaining seven trials included participants with meniscal tears and excluded people with severe osteoarthritis (KL grade 4 (Katz 2013; Van de Graaf 2018), KL grade 3 or more (Kise 2016; Osteras 2012), KL grade 2 or more (Yim 2013), Ahlbacks classification grade 2 or more (Herrlin 2007), and 50% or more joint space narrowing (Gauffin 2014). Three trials specified a medial meniscal tear (Herrlin 2007; Kise 2016; Yim 2013), while the other four trials did not specify the site of the meniscal tear (Gauffin 2014; Katz 2013; Osteras 2012; Van de Graaf 2018). Gauffin 2014 included people with suspected meniscal injury but not all participants were found to have meniscal tears (see Table 2).
One trial performed a meniscal resection only if participants were found to have a meniscal tear at arthroscopy (Gauffin 2014). All other trials performed a partial meniscectomy (Herrlin 2007; Katz 2013, Kirkley 2008; Kise 2016; Osteras 2012; Van de Graaf 2018; Yim 2013). Three trials reported that they also performed limited debridement of articular cartilage if needed (Katz 2013; Kirkley 2008; Yim 2013).
Exercises varied across the trials, in both the control arm and in the post‐operative exercise prescribed in the arthroscopy groups (see Table 3). Co‐interventions were applied equally across both treatment groups.
Arthroscopic surgery versus other interventions
Vermesan 2013 included participants with medial compartment cartilage and meniscus lesions in MRI in a trial that compared arthroscopic surgery to a single intra‐articular glucocorticoid injection.
Chang 1993 included participants with osteoarthritis KL grades 1 to 3 and did not specify criteria regarding the menisci in a trial that compared arthroscopic surgery to non‐arthroscopic lavage.
Merchan 1993 included participants with mild osteoarthritis only (minimal joint space narrowing and formation of small osteophytes and did not specify criteria regarding the menisci) in a trial that compared arthroscopic surgery to non‐steroidal anti‐inflammatory drugs (NSAIDs).
Saeed 2015 included participants with osteoarthritis KL grade 2 and 3 only and did not specify criteria regarding presence/absence of meniscal tears in a trial that compared arthroscopic surgery to five intra‐articular hyaluronic acid injections given at weekly intervals.
No trials compared arthroscopic surgery to supplements or complementary therapies, or both.
Outcomes
The pain, function, health‐related quality of life and participant‐reported success outcomes that were extracted for the purpose of analyses are summarised in Table 4.
3. Outcomes included in analyses.
| Study ID | Pain | Function | Knee‐specific and generic health‐related quality of life | Participant‐reported treatment success | Knee surgery (replacement or osteotomy) | Serious and total adverse events | Progression of knee OA |
| Chang 1993 | AIMS Pain | AIMS Physical Function | ‐ | Patient global assessment measured on VAS | ‐ | ‐ | ‐ |
| Gauffin 2014 | KOOS Pain | KOOS ADL | KOOS QoL (knee‐specific), EQ‐5D (generic) |
Improvement in the KOOS‐Pain score of > 10 points from baseline | ‐ | Yes | Yes |
| Herrlin 2007 | KOOS Pain | KOOS ADL | KOOS QoL (knee‐specific) | ‐ | ‐ | ‐ | Yes |
| Katz 2013 | KOOS Pain | WOMAC Physical Function | ‐ | Improvement in the WOMAC‐Physical Function score of at least 8 points | Yes | Yes | ‐ |
| Kirkley 2008 | WOMAC Pain | WOMAC Physical Function | Standard‐gamble utility score (generic) | ‐ | ‐ | ‐ | ‐ |
| Kise 2016 | KOOS Pain | KOOS ADL | KOOS QoL (knee‐specific), SF‐36 MCS (generic) |
‐ | Yes | Yes | Yes |
| Merchan 1993 | ‐ | ‐ | ‐ | Increase in post‐treatment modified Hospital for Special Surgery Knee Rating Score of at least 10 points | ‐ | Yes | ‐ |
| Moseley 1996 | Average intensity of knee pain (NRS) | ‐ | ‐ | Satisfaction with surgery measured as number of participants reporting 'strongly agree' or 'slightly agree' for item 'do you feel the operation was worthwhile?' | ‐ | ‐ | ‐ |
| Moseley 2002 | SF‐36 Pain | SF‐36 Physical Function | ‐ | ‐ | ‐ | ‐a | ‐ |
| Osteras 2012 | VAS Pain at rest | KOOS | ‐ | ‐ | ‐ | ‐ | ‐ |
| Roos 2018 | KOOS Pain | KOOS ADL | KOOS QoL (knee‐specific), SF‐36 MCS (generic) |
Global perceived effect (rating of 'better' or 'much better') | ‐ | Total adverse events onlya | ‐ |
| Saeed 2015 | Pain on Knee Society Score System (KSSS) | ‐ | ‐ | ‐ | ‐ | Total adverse events only | ‐ |
| Sihvonen 2013 | Knee pain after exercise (NRS) | Lysholm Knee Score | WOMET score (knee‐specific), 15D (generic) | Number of participants reporting 'much better' or 'better' for item 'Is your knee better than before the intervention? | Yes | Yes | Yes |
| Van de Graaf 2018 | VAS Pain on weight‐bearing | International Knee Documentation Committee (IKDC) Subjective Knee Form | ‐ | ‐ | Yes | Yes | Yes |
| Vermesan 2013 | ‐ | Oxford Knee Score | ‐ | ‐ | ‐ | ‐ | ‐ |
| Yim 2013 | VAS Pain during activity | Lysholm Knee Score | ‐ | Satisfaction with management, measured as number of participants reporting 'very satisfied' or 'satisfied' | ‐ | ‐ | Yes |
aUnclear whether serious adverse events occurred in both treatment groups or only in the arthroscopy group
ADL: activities of daily living; AIMS: Arthritis Impact Measurement Scale; NRS: numerical rating scale; EQ‐5D 3L: EuroQoL 5‐dimension 3‐level quality of life questionnaire; OA: osteoarthritis; KOOS: Knee injury and Osteoarthritis Outcome Score; QoL/QOL: quality of life; SF‐36: 36‐item Short Form Health Survey; VAS: visual analogue scale; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index
All but one trial measured pain (Merchan 1993), although a second trial measured pain as part of the Oxford Knee Score but did not report the pain subscale result separately (Vermesan 2013). One trial used the WOMAC pain subscale to measure pain (Kirkley 2008), while five trials used the KOOS pain subscale (KOOS‐P) (Gauffin 2014; Herrlin 2007; Katz 2013; Kise 2016; Roos 2018). Osteras 2012 measured pain using KOOS but did not report the pain subscale result separately.
Four trials measured pain using a Visual Analogue Scale (VAS) (Herrlin 2007; Osteras 2012; Van de Graaf 2018; Yim 2013), and two used a numerical rating scale (Moseley 1996; Sihvonen 2013). Two trials used the pain subscale of the Arthritis Impact Measurement Scale (AIMS2‐P) (Chang 1993; Moseley 2002), one used the Knee Society Score System (KSSS) (Saeed 2015), and one used the Knee‐Specific Pain Scale (KSPS) and the pain subscale of the SF‐36 (Moseley 2002).
All but one trial measured function (Saeed 2015). Three trials used the composite KOOS score (Kise 2016; Osteras 2012; Roos 2018), and four used the KOOS ADL subscale (Gauffin 2014; Herrlin 2007; Kise 2016; Roos 2018) (to note: Osteras 2012 did not report the KOOS ADL subscale result separately). Three trials used the Lysholm Knee Score (Herrlin 2007; Sihvonen 2013; Yim 2013), and two used the WOMAC physical function subscale (Katz 2013; Kirkley 2008). Other measures of function included the WOMAC total score (Kirkley 2008), the Physical Functioning Scale (Moseley 2002), the Oxford Knee Score (Vermesan 2013), the Subjective Knee Form of the International Knee Documentation Committee (IKDC) (Van de Graaf 2018), the modified Hospital for Special Surgery Knee Rating Scale (mHSSKRS) (Merchan 1993), the McMaster‐Toronto Arthritis Patient Preference Disability Questionnaire (MACTAR) (Kirkley 2008), the Arthritis Self‐Efficacy Scale (ASES) (Kirkley 2008), AIMS physical function subscale (Chang 1993), the AIMS2 walking‐bending subscale (Moseley 2002), the Tegner Activity Scale (Herrlin 2007; Yim 2013; Van de Graaf 2018), SF‐36 physical function subscale (Moseley 2002; Katz 2013), SF‐36 Physical Component Summary (Kirkley 2008; Kise 2016; Roos 2018; Van de Graaf 2018), a mobility (activity) scale developed for the trial (Moseley 1996), and other physical performance tests (e.g. hop test, bend test) (Kise 2016; Roos 2018; Van de Graaf 2018).
Five trials measured knee‐specific health‐related quality of life (Gauffin 2014; Herrlin 2007; Kise 2016; Roos 2018; Sihvonen 2013). Four used the KOOS quality of life (QoL) subscale (Gauffin 2014; Herrlin 2007; Kise 2016; Roos 2018), and one used the Western Ontario Meniscal Evaluation Tool (WOMET) QoL score (Sihvonen 2013).
Seven trials measured generic health‐related quality of life (Gauffin 2014; Katz 2013; Kirkley 2008; Kise 2016; Moseley 1996; Roos 2018; Sihvonen 2013). Two used the SF‐36 Mental Component Summary (Kise 2016; Roos 2018), and four used the EQ‐5D (Gauffin 2014; Katz 2013; Roos 2018; Van de Graaf 2018). Van de Graaf 2018 reported in their protocol that they measured the SF‐36 but did not report the Mental Component Summary results. Other measures included the EuroQol Visual Analogue Scale (EQ VAS) (Gauffin 2014), the 15D (Sihvonen 2013), the standard‐gamble utility technique (Kirkley 2008), and a general well‐being scale developed for the trial (details of measurement scale not reported) (Moseley 1996).
Eight trials included participant‐reported treatment success (Chang 1993; Gauffin 2014; Katz 2013; Merchan 1993; Moseley 1996; Roos 2018; Sihvonen 2013; Yim 2013). Various ways of measuring treatment success were used, including: improvement in the KOOS pain score of more than 10 points (Gauffin 2014); improvement in the WOMAC physical function score of at least 8 points (Katz 2013); improvement in the modified Hospital for Special Surgery Knee Rating Score of at least 10 points (Merchan 1993); improvement of at least 2 points on a 7‐point Global Perceived Effect scale (Roos 2018); reduction of 1 cm or more in baseline overall well‐being rated on a 10 cm visual analogue scale (0 = best to 10 = worst) (Chang 1993); participants reporting being 'much better' or 'better' on 5‐point Likert scale in response to the question 'Is your knee better than before the intervention?' (Sihvonen 2013); participants reporting being 'very satisfied' or 'satisfied' with treatment (Yim 2013); participants reporting 'strongly agree' or 'slightly agree' to the question 'Do you feel the operation was worthwhile?' (Moseley 1996); and participant rating on a 6‐point Likert scale ranging from 'delighted' to 'terrible' (Gauffin 2014).
Nine trials reported adverse events (Gauffin 2014; Katz 2013; Kise 2016; Merchan 1993; Moseley 2002; Roos 2018; Saeed 2015; Sihvonen 2013; Van de Graaf 2018). Of these, eight studies reported non‐serious (other) adverse events (Gauffin 2014; Katz 2013; Kise 2016; Moseley 2002; Saeed 2015; Merchan 1993; Roos 2018; Van de Graaf 2018). Five of the nine studies reported serious adverse events, defined as an event that leads to hospitalisation, disability or death (Katz 2013; Merchan 1993; Roos 2018; Sihvonen 2013; Van de Graaf 2018). Seven studies did not report adverse events (Chang 1993; Herrlin 2007; Kirkley 2008; Moseley 1996; Osteras 2012; Vermesan 2013; Yim 2013). Moseley 2002 did not report adverse events details per group.
Six trials reported progression of knee osteoarthritis (Gauffin 2014; Herrlin 2007; Kise 2016; Sihvonen 2013; Van de Graaf 2018; Yim 2013), reporting it as a dichotomous outcome in all studies except Van de Graaf 2018. In three trials (Gauffin 2014; Kise 2016; Yim 2013), radiographic osteoarthritis was defined as equal to or higher than Kellgren‐Lawrence (KL) grade 2 (definite osteophytes and possible joint space narrowing) (Kellgren 1957). Herrlin 2007 defined progression of osteoarthritis post hoc as at least one grade worse on the Ahlback classification (Ahlback 1968). Sihvonen 2013 defined progression as at least one grade progression in radiographic tibiofemoral knee osteoarthritis using the Kellgren‐Lawrence classification. Van de Graaf 2018 reported mean Kellgren‐Lawrence classification scores and not number of participants with osteoarthritis progression.
Four trials reported the proportion of participants undergoing subsequent knee surgery (replacement or osteotomy) (Katz 2013; Kise 2016; Sihvonen 2013; Van de Graaf 2018).
We contacted the authors of trials to retrieve missing data where outcome data were not fully reported. We received missing outcome data from the authors of four trials (Herrlin 2007; Kise 2016; Roos 2018; Sihvonen 2013).
Trial funding
Of the nine trials reporting their funding source (Chang 1993; Katz 2013; Kirkley 2008; Kise 2016; Merchan 1993; Moseley 2002; Roos 2018; Sihvonen 2013; Van de Graaf 2018), none received funding from industry. The other seven trials did not report any funding source (Gauffin 2014; Herrlin 2007; Moseley 1996; Osteras 2012; Saeed 2015; Vermesan 2013; Yim 2013).
Excluded studies
A full description of all excluded trials is provided in the Characteristics of excluded studies table. As noted above, we excluded 15 studies (seven were not RCTs, four examined interventions other than arthroscopic surgery and four did not include participants with degenerative knee disease).
Risk of bias in included studies
All trials were susceptible to bias. Overall, 9 out of 16 trials (56%) were at risk of selection bias, 12 of 16 (75%) were susceptible to performance bias, 12 (75%) were at risk of detection bias; 7 (44%) were at risk of attrition bias, and 12 (75%) at risk of selective reporting bias (see Figure 2).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Seven out of 16 trials (44%) reported using adequate methods of randomisation and allocation concealment and were judged to be at low risk of selection bias (Katz 2013; Kirkley 2008; Kise 2016; Moseley 2002; Roos 2018; Sihvonen 2013; Van de Graaf 2018). Eight trials did not report the method of sequence generation (Gauffin 2014; Herrlin 2007; Merchan 1993; Moseley 1996; Osteras 2012; Saeed 2015; Vermesan 2013; Yim 2013), and seven trials did not clearly describe methods for concealing the allocation sequence (Herrlin 2007; Merchan 1993; Moseley 1996; Osteras 2012; Saeed 2015; Vermesan 2013; Yim 2013). Therefore, we judged the risk of selection bias as unclear in these trials. Additionally, baseline differences in outcome measures between the treatment groups were found in one trial (Merchan 1993).
Chang 1993 redesigned their study to a 'pre‐randomised' design due to poor recruitment. In this method, also called the Zelen method of randomisation, participants were asked if they would be willing to undergo arthroscopic surgery, and those who indicated they would be, were allocated to either arthroscopic surgery or conservative treatment, then asked to consent to their allocated treatment. The authors did not report if the randomisation sequence was concealed.
Blinding
Only 4 of 16 (25%) trials were judged at low risk of performance bias, where both participants and study personnel were blinded to group assignment (Moseley 1996; Moseley 2002; Roos 2018; Sihvonen 2013). We judged the remaining 12 trials (75%) to be at high risk of performance bias as participants and study personnel were not blinded to group assignment (Chang 1993; Gauffin 2014; Herrlin 2007; Katz 2013; Kirkley 2008; Kise 2016; Osteras 2012; Van de Graaf 2018; Yim 2013), or blinding was not reported and probably was not done (Merchan 1993; Saeed 2015; Vermesan 2013).
All 16 trials reported using one or more self‐reported outcomes. Four trials (25%) were at low risk of detection bias because participants were blinded to group allocation (Moseley 1996; Moseley 2002; Roos 2018; Sihvonen 2013). We judged the remaining 12 trials (75%) to be at high risk of detection bias, either because the studies reported that participants were not blinded to group allocation or blinding was not reported and probably was not done (Chang 1993; Gauffin 2014; Herrlin 2007; Katz 2013; Kirkley 2008; Kise 2016; Merchan 1993; Osteras 2012; Saeed 2015; Van de Graaf 2018; Vermesan 2013; Yim 2013).
Assessor‐rated outcomes of interest (e.g. knee replacement, progression of knee osteoarthritis, adverse events) were measured in 10 of 16 (63%) trials (Gauffin 2014; Herrlin 2007; Katz 2013; Kise 2016; Merchan 1993; Roos 2018; Saeed 2015; Sihvonen 2013; Van de Graaf 2018; Yim 2013). There was a low risk of detection bias for assessor‐rated outcomes in 5 of these 10 trials (50%), as assessors were effectively blinded to the treatment assignment (Kise 2016; Sihvonen 2013; Roos 2018; Van de Graaf 2018; Yim 2013). We judged a further three trials (30%) to be at low risk of detection bias despite no or unclear blinding of outcome assessment, as the outcomes were unlikely to be influenced by a lack of blinding (Gauffin 2014; Herrlin 2007; Saeed 2015). We assessed two trials (20%) as having a high risk of detection bias as outcome assessors were not adequately blinded (Katz 2013; Merchan 1993).
Incomplete outcome data
Nine out of 16 trials (56%) were judged at low risk of attrition bias because they had no withdrawals or losses to follow‐up or the attrition was so small it was unlikely to have biased the results (Chang 1993; Herrlin 2007; Katz 2013; Kise 2016; Roos 2018; Saeed 2015; Sihvonen 2013; Van de Graaf 2018; Yim 2013). In three trials (19%), there was differential dropout across groups, we judged these studies to be at high risk of attrition bias (Gauffin 2014; Kirkley 2008; Moseley 1996). In the remaining four trials (25%), the reasons for incomplete outcome data were not reported, and we judged the risk of attrition bias as unclear (Merchan 1993; Moseley 2002; Osteras 2012; Vermesan 2013).
Selective reporting
We assessed 4 out of 16 trials (25%) to be at low risk of reporting bias (Kirkley 2008; Moseley 2002; Roos 2018; Van de Graaf 2018). Seven trials (44%) were judged at high risk of reporting bias (Gauffin 2014; Herrlin 2007; Katz 2013; Moseley 1996; Saeed 2015; Vermesan 2013; Yim 2013), while the remaining five trials (31%) were judged as having an unclear risk (Chang 1993; Kise 2016; Merchan 1993; Osteras 2012; Sihvonen 2013). Nine trials (56%) were not prospectively registered (Chang 1993; Herrlin 2007; Merchan 1993; Moseley 1996; Moseley 2002; Osteras 2012; Saeed 2015; Vermesan 2013; Yim 2013), and seven trials (44%) did not report one or more pre‐specified outcomes or did not report outcomes as described in methods (Gauffin 2014; Herrlin 2007; Katz 2013; Kise 2016; Moseley 1996; Saeed 2015; Vermesan 2013). Two trials (13%) had incomplete reporting of outcome data (i.e. no standard deviations or confidence intervals) (Moseley 1996; Yim 2013).
Other potential sources of bias
Thirteen trials (81%) had no apparent other sources of bias (Chang 1993; Gauffin 2014; Herrlin 2007; Kirkley 2008; Kise 2016; Merchan 1993; Moseley 1996; Moseley 2002; Roos 2018; Saeed 2015; Sihvonen 2013; Van de Graaf 2018; Vermesan 2013). We judged the remaining three trials as having an unclear risk of other bias. In Katz 2013, 30.2% of participants assigned to physical therapy crossed over to arthroscopy within six months of randomisation, potentially underestimating any effect of surgery. It was unclear whether or not Osteras 2012 performed an unplanned interim analysis. In Yim 2013, an unspecified number of participants in the arthroscopic surgery group were not prescribed exercise. We did not identify any unit of analysis errors.
Effects of interventions
See: Table 1
See Table 1 for the main comparison.
Benefits
Arthroscopic surgery versus placebo surgery
Four trials compared arthroscopic surgery to placebo (Moseley 1996; Moseley 2002; Roos 2018; Sihvonen 2013).
Pain
All four trials reported pain at up to three months. Three trials reported pain between three and six months (Moseley 1996; Moseley 2002; Sihvonen 2013), and three trials reported pain between six months and two years) (Moseley 2002; Roos 2018; Sihvonen 2013). Sihvonen 2013 reported pain at five years. No trials reported pain between 5 and 10 years. Moseley 1996 used a 10‐point numerical rating scale (lower score = less pain) to measure pain. Moseley 2002 used three pain scales: the Knee‐Specific Pain Scale (KSPS) (0 to 100 scale, lower score = less pain; included in this review), the pain subscale of the Arthritis Impact Measurement Scales (AIMS2‐P) (0 to 100 scale, lower score = less pain) and a 2‐item pain subscale of the SF‐36 (SF‐36‐P) (0 to 100 scale, higher score = less pain). Roos 2018 used the pain subscale of the Knee Injury and Osteoarthritis Outcome Score (KOOS‐P) (0 to 100 scale, higher score = less pain). Sihvonen 2013 used an 11‐point numerical rating scale (lower score = less pain).
High‐certainty evidence indicates arthroscopic surgery leads to little or no difference in pain at up to three months compared with placebo surgery (standardised mean difference (SMD) ‐0.23, 95% confidence interval (CI) ‐0.45 to ‐0.001; I2 = 0%; 4 trials, 309 participants; high‐certainty evidence; Analysis 1.1; Figure 3). Mean post‐operative pain in the placebo group at up to three months was 40.1 points on a 0 to 100 scale (where lower score indicates less pain) compared to 35.5 points in the arthroscopic surgery group, a difference of 4.6 points (95% CI 0.02 better to 9 better). This is an absolute improvement of 5% (95% CI 0.02% better to 9% better) and relative improvement of 8% (95% CI 0.03% better to 15% better). As the 95% confidence intervals do not include any appreciable benefit, we did not downgrade for imprecision.
1.1. Analysis.

Comparison 1: Arthroscopic surgery versus placebo surgery, Outcome 1: Pain (lower score=less pain)
3.

Forest plot of comparison 1: arthroscopic surgery versus placebo surgery, outcome: 1.1 Pain (lower score = less pain)
There was little to no difference in pain with arthroscopic surgery compared with placebo: between three and six months (SMD ‐0.12, 95% CI ‐0.37 to 0.12; or 2.4 points better (7.4 points better to 2.4 points worse) on a 0 to 100 point scale; I2 = 0%; 3 trials, 265 participants); between six months and two years (SMD ‐0.20, 95% CI ‐0.48 to 0.09; or equivalent to 4 points better (9.6 points better to 1.8 points worse) on a 100‐point scale; I2 = 30%; 3 trials, 295 participants); or between two and five years (last follow‐up) (SMD ‐0.08, 95% CI ‐0.41 to 0.24; or 1.6 points better (8.2 points better to 4.8 points worse) on a 0 to 100 points scale; 1 study, 142 participants).
Subgroup analyses based on the presence or absence of meniscal tear and use or not of supervised exercise did not alter the estimates of treatment effect to a clinically important level (Analysis 8.1; Analysis 8.2; Analysis 8.3; Analysis 8.4; Analysis 9.1; Analysis 9.2; Analysis 9.3; Analysis 9.4).
8.1. Analysis.

Comparison 8: Subgroup analysis: presence of meniscal tear, Outcome 1: Pain up to 3 months (lower score=less pain)
8.2. Analysis.

Comparison 8: Subgroup analysis: presence of meniscal tear, Outcome 2: Pain at >3 months up to 6 months (lower score=less pain)
8.3. Analysis.

Comparison 8: Subgroup analysis: presence of meniscal tear, Outcome 3: Pain at >6 months up to 2 years (lower score=less pain)
8.4. Analysis.

Comparison 8: Subgroup analysis: presence of meniscal tear, Outcome 4: Pain at >2 years up to 5 years (lower score=less pain)
9.1. Analysis.

Comparison 9: Subgroup analysis: arthroscopy with supervised exercise, Outcome 1: Pain up to 3 months (lower score=less pain)
9.2. Analysis.

Comparison 9: Subgroup analysis: arthroscopy with supervised exercise, Outcome 2: Pain at >3 months up to 6 months (lower score=less pain)
9.3. Analysis.

Comparison 9: Subgroup analysis: arthroscopy with supervised exercise, Outcome 3: Pain at >6 months up to 2 years (lower score=less pain)
9.4. Analysis.

Comparison 9: Subgroup analysis: arthroscopy with supervised exercise, Outcome 4: Pain at >2 years up to 5 years (lower score=less pain)
Sensitivity analyses indicated there was little change in effect estimates when restricting studies to those at low risk of selection bias. As no studies were at risk of detection biases, no sensitivity analysis for this bias could be performed. Use of a fixed‐effect model also did not alter the results.
Sensitivity analysis comparing arthroscopic surgery to any control indicates arthroscopic surgery leads to little reduction in pain at up at three months (SMD ‐0.21, 95% CI ‐0.32 to ‐0.10; equivalent to 4.2 points better (95% CI 2 to 6.4 points better) on a 0 to 100 scale; I2 = 0%; 12 trials, 1283 participants; high‐certainty evidence), between three and six months (SMD ‐0.19, 95% CI ‐0.30 to ‐0.07; or 3.8 points better (1.4 to 6 points better) on a 0 to 100 scale); I2 = 0%; 8 trials, 1252 participants; high‐certainty evidence), between six months and two years (SMD ‐0.11, 95% CI ‐0.22 to ‐0.01; or 2.2 points better (0.2 to 4.4 points better) on a 100 point scale; I2 = 0%; 11 trials, 1505 participants; high‐certainty evidence), and leads to no difference in pain between two and five years (SMD ‐0.01, 95% CI ‐0.30 to 0.29; I2 = 49%; 3 trials, 361 participants), compared with any control (Analysis 13.1).
13.1. Analysis.

Comparison 13: Sensitivity analysis: arthroscopic surgery versus any control, Outcome 1: Pain (lower score=less pain)
Function
Three trials reported function at up to three months (Moseley 2002; Roos 2018; Sihvonen 2013). These same three trials reported function between six months and two years, using a variety of scales: the SF‐36 Physical Function subscale and the Arthritis Impact Measurement Scales walking‐bending subscale (AIMS2‐WB) (Moseley 2002); the composite Knee Injury and Osteoarthritis Outcome Score (KOOS5), the KOOS ADL subscale and the SF‐36 Physical Component Summary (Roos 2018); and Lysholm Knee Score (Sihvonen 2013). Two trials reported function between three and six months (Moseley 2002; Sihvonen 2013). Sihvonen 2013 reported function at five years. No trials reported function between 5 and 10 years.
High‐certainty evidence indicates arthroscopic surgery leads to little or no difference in knee function at up to three months compared with placebo (SMD 0.01, 95% CI ‐0.22 to 0.23; I2 = 0%; 3 trials, 302 participants; high‐certainty evidence; Analysis 1.2; Figure 4). Mean post‐operative function in the placebo group at up to three months was 75.9 points on a 0 to 100 rating scale (where higher score indicates better function) compared to 76 points in the arthroscopic surgery group, a difference of 0.1 points better (95% CI 3.2 worse to 3.4 better). This is an absolute improvement of 0.1% (95% CI 3% worse to 3% better) and relative improvement of 0.2% (95% CI 5% worse to 6% better). As the 95% confidence intervals do not include any appreciable benefit, we did not downgrade for imprecision. There was little or no difference in knee function with arthroscopic surgery compared with placebo: between three and six months (SMD 0.05, 95% CI ‐0.20 to 0.29; equivalent to 0.7 points better (2.9 worse to 4.2 better) on a 0 to 100 point scale; I2 = 0%; 2 trials, 257 participants); between six months and two years (SMD 0.10, 95% CI ‐0.27 to 0.47; or 1.5 points better (3.9 points worse to 6.9 points better) on a 0 to 100 point scale; I2 = 56%; 3 trials, 293 participants); or between two and five years (last follow‐up) (SMD ‐0.15, 95% CI ‐0.48 to 0.18; equivalent to 2.2 points worse (7 points worse to 2.6 points better) on a 0 to 100 point scale; 1 trial, 142 participants).
1.2. Analysis.

Comparison 1: Arthroscopic surgery versus placebo surgery, Outcome 2: Function (higher score=better function)
4.

Forest plot of comparison 1: arthroscopic surgery versus placebo surgery, outcome: 1.2 Function (higher score = better function)
Subgroup analyses based on the presence or absence of meniscal tear and use or no use of supervised exercise did not alter the estimates of treatment effect to a clinically important level (Analysis 8.5; Analysis 8.6; Analysis 8.7; Analysis 8.8; Analysis 9.5; Analysis 9.6; Analysis 9.7; Analysis 9.8).
8.5. Analysis.

Comparison 8: Subgroup analysis: presence of meniscal tear, Outcome 5: Function up to 3 months (higher score=better function)
8.6. Analysis.

Comparison 8: Subgroup analysis: presence of meniscal tear, Outcome 6: Function at >3 months up to 6 months (higher score=better function)
8.7. Analysis.

Comparison 8: Subgroup analysis: presence of meniscal tear, Outcome 7: Function at >6 months up to 2 years (higher score=better function)
8.8. Analysis.

Comparison 8: Subgroup analysis: presence of meniscal tear, Outcome 8: Function at >2 years up to 5 years (higher score=better function)
9.5. Analysis.

Comparison 9: Subgroup analysis: arthroscopy with supervised exercise, Outcome 5: Function up to 3 months (higher score=better function)
9.6. Analysis.

Comparison 9: Subgroup analysis: arthroscopy with supervised exercise, Outcome 6: Function at >3 months up to 6 months (higher score=better function)
9.7. Analysis.

Comparison 9: Subgroup analysis: arthroscopy with supervised exercise, Outcome 7: Function at >6 months up to 2 years (higher score=better function)
9.8. Analysis.

Comparison 9: Subgroup analysis: arthroscopy with supervised exercise, Outcome 8: Function at >2 years up to 5 years (higher score=better function)
Sensitivity analyses to investigate the robustness of the effects to potential selection and detection biases or use of a fixed‐effect model did not alter the results. Sensitivity analysis comparing arthroscopic surgery to any control indicates arthroscopic surgery probably leads to a very little improvement in function at up to three months (SMD 0.19, 95% CI 0.04 to 0.34; 2.8 points better (0.6 to 5.0 better) on a 0 to 100 point scale; I2 = 45%; 12 trials, 1403 participants; moderate‐certainty evidence), and little or no difference between three and six months (SMD 0.10, 95% CI ‐0.01 to 0.21; or 1.5 points better (0.1 points worse to 3.1 better) on a 0 to 100 point scale; I2 = 0%; 7 trials, 1245 participants; high‐certainty evidence), between six months and two years (SMD 0.11, 95% CI 0.01 to 0.20; or 1.6 points better (0.1 to 2.9 points better) on a 0 to 100 point scale; I2 = 0%; 12 trials, 1651 participants; high‐certainty evidence), and between six months and two years (SMD ‐0.09, 95% CI ‐0.30 to 0.12; I2 = 0%; 3 trials, 361 participants) compared with any control (Analysis 13.2).
13.2. Analysis.

Comparison 13: Sensitivity analysis: arthroscopic surgery versus any control, Outcome 2: Function (higher score=better function)
Knee‐specific health‐related quality of life
Knee‐specific health‐related quality of life was reported in two trials at up to three months (Roos 2018; Sihvonen 2013), and between six months and two years, using the KOOS quality of life subscale and the WOMET score, respectively. The Sihvonen 2013 study also reported knee‐related quality of life between three and six months, and between two and five years. Neither trial reported knee‐related quality of life between 5 and 10 years.
Moderate‐certainty evidence indicates arthroscopic surgery probably leads to little or no difference, or a very small improvement, in knee‐specific quality of life at up to three months compared with placebo (SMD 0.31, 95% CI 0.02 to 0.59; translates to 5.6 points better (0.36 better to 10.7 points better) on a 0 to 100 point scale; I2 = 0%; 2 trials, 188 participants; moderate‐certainty evidence; Analysis 1.3). Mean post‐operative knee‐specific quality of life in the placebo group at up to three months was 69.7 points on a 0 to 100 rating scale (where higher score indicates better quality of life) compared with 75.3 points in the arthroscopic surgery group, a difference of 5.6 points better (95% CI 0.4 better to 10.7 better), an absolute improvement of 6% (0.4% better to 11% better) and a relative improvement of 11% (95% CI 0.8% better to 20% better). We downgraded the certainty of the evidence due to imprecision (the 95% confidence intervals include both an unimportant improvement and the clinically important improvement threshold of 10%). Moderate‐certainty evidence also indicates little or no difference at other time frames: between three and six months (MD 2.60, 95% CI ‐4.27 to 9.47; absolute improvement 3%, 95% CI 4% worse to 9% better; I2 = 0%; 1 trial, 146 participants); between six months and two years (SMD 0.23, 95% CI ‐0.24 to 0.70; translates to 4.2 points better (4.3 points worse to 12.7 points better) on a 0 to 100 point scale; absolute improvement 6% (6% worse to 18% better); I2 = 50%; 2 trials, 188 participants); or between two and five years (last follow‐up) (SMD ‐0.02, 95% CI ‐0.35 to 0.31; equivalent to 0.4 points worse (6 points worse to 5.6 points better); 1 trial, 142 participants).
1.3. Analysis.

Comparison 1: Arthroscopic surgery versus placebo surgery, Outcome 3: Knee‐specific quality of life (higher score=better)
Sensitivity analysis comparing arthroscopic surgery to any control did not alter the estimates of treatment effect to a clinically important level (Analysis 13.3).
13.3. Analysis.

Comparison 13: Sensitivity analysis: arthroscopic surgery versus any control, Outcome 3: Knee‐specific quality of life (higher score=better)
Generic health‐related quality of life
Generic health‐related quality of life was reported in one trial at 3 and 24 months (Roos 2018), using the SF‐36 Mental Component Summary (MCS) and the EQ‐5D. One additional trial reported generic quality of life using the 15D at 12 months of follow‐up (Sihvonen 2013). No trials reported generic health‐related quality of life between three and six months, or between two and 10 years.
Based on Roos 2018, arthroscopic surgery probably results in little or no difference in generic quality of life at up to three months compared with placebo (MD ‐3.50, 95% CI ‐7.20 to 0.20; 1 trial, 42 participants; moderate‐certainty evidence; Analysis 1.4). The certainty of the evidence was downgraded due to serious imprecision (data from a single trial).
1.4. Analysis.

Comparison 1: Arthroscopic surgery versus placebo surgery, Outcome 4: Generic quality of life (higher score=better)
Moderate‐certainty evidence from two trials ‐ Roos 2018 and Sihvonen 2013 ‐ indicates arthroscopic surgery probably results in little or no difference in generic quality of life between six months and two years compared with placebo (SMD 0.15, 95% CI ‐0.28 to 0.58; I2 = 42%; 2 trials, 188 participants; moderate‐certainty evidence). This translates to a mean difference in generic quality of life of 1.5 points (95% CI ‐2.8 to 5.8 points) on a 0 to 100 SF‐36 scale (higher score = better quality of life) or 0.01 points (95% CI ‐0.2 to 0.03) on the 15D generic quality of life scale from 0 to 1 (higher score = better quality of life). We downgraded the certainty of the evidence due to serious indirectness (outcome dissimilarity).
Sensitivity analysis comparing arthroscopic surgery to any control did not alter the estimates of treatment effect to a clinically important level (Analysis 13.4).
13.4. Analysis.

Comparison 13: Sensitivity analysis: arthroscopic surgery versus any control, Outcome 4: Generic quality of life (higher score=better)
Participant‐reported treatment success
Three trials reported participant‐reported treatment success at up to five years (Moseley 1996; Roos 2018; Sihvonen 2013). Moseley 1996 used the number of participants reporting they 'strongly agree' or 'slightly agree' for the question 'Do you feel the operation was worthwhile?' at three and six months. Roos 2018 used the number of participants rating their overall improvement in knee symptoms after the operation as 'better' or 'much better' at 3 and 24 months. Sihvonen 2013 used the number of participants reporting being 'much better' or 'better' for the question 'Is your knee better than before the intervention?' at 12, 24 and 60 months. No trials reported participant‐reported treatment success between 5 and 10 years.
Low‐certainty evidence indicates arthroscopic surgery may lead to little or no difference in participant‐reported treatment success compared with placebo at up to five years (68 out of 91 participants in the arthroscopic surgery group (75%) versus 72 out of 98 participants in the placebo group (74%), RR 1.11, 95% CI 0.66 to 1.86; I2 = 53%; 3 trials, 189 participants; low‐certainty evidence; Analysis 1.5). We downgraded the certainty of the evidence due to serious indirectness (some diversity in definition and timing of outcome measurement: reported at 6 months, 24 months and 5 years across trials) and serious imprecision (small number of events).
1.5. Analysis.

Comparison 1: Arthroscopic surgery versus placebo surgery, Outcome 5: Participant‐reported success
Sensitivity analysis comparing arthroscopic surgery to any control did not alter the estimate of treatment effect to a clinically important level (RR 1.24, 95% CI 0.96 to 1.60; I2 = 83%; 8 trials, 851 participants; Analysis 13.5).
13.5. Analysis.

Comparison 13: Sensitivity analysis: arthroscopic surgery versus any control, Outcome 5: Participant‐reported success
Arthroscopic surgery versus exercise
Five trials compared arthroscopic surgery plus exercise therapy to exercise therapy alone (Gauffin 2014; Herrlin 2007; Katz 2013; Kirkley 2008; Yim 2013), and three trials compared arthroscopic surgery to exercise therapy (Kise 2016; Osteras 2012; Van de Graaf 2018). We have considered these eight trials together.
Pain
Seven trials reported pain at up to three months (Gauffin 2014; Herrlin 2007; Kirkley 2008; Kise 2016; Osteras 2012; Van de Graaf 2018; Yim 2013), five trials reported pain between three and six months (Herrlin 2007; Katz 2013; Kirkley 2008; Van de Graaf 2018; Yim 2013), seven trials between six months and two years (Gauffin 2014; Herrlin 2007; Katz 2013; Kirkley 2008; Kise 2016; Van de Graaf 2018; Yim 2013), and three trials between two and five years (Gauffin 2014; Katz 2013; Kise 2016). No trials reported pain between 5 and 10 years.
Moderate‐certainty evidence indicates arthroscopic surgery leads to little or no benefit at any time point compared with exercise. At up to three months, the SMD was ‐0.21 (95% CI ‐0.33 to ‐0.08; I2 = 0%; 7 trials, 942 participants). This translates to a small, clinically unimportant mean difference in pain of ‐4.2 points (‐6.6 points to ‐1.6 points) on a 0 to 100 point pain scale, below the clinically important threshold of 15 points on 0 to 100 scale (or 15% absolute change). Between three and six months, the SMD was ‐0.20 (95% CI ‐0.33 to ‐0.08; I2 = 0%; 5 trials, 987 participants; back‐translated MD ‐4.0 points (‐6.6 to ‐1.6), on a 0 to 100 scale). Between six months and two years, the SMD was ‐0.11 (95% CI ‐0.22 to 0.01; I2 = 0%; 7 trials, 1178 participants; which translates to a mean difference of ‐2.2 points (‐4.4 to 0.2 points) on a 0 to 100 scale). In the longer term (i.e. between two and five years), arthroscopic surgery may lead to little or no difference in pain compared with exercise (MD 1.27 points, 95% CI ‐8.50 to 11.03; I2 = 75%; 2 trials, 219 participants; Analysis 2.1). We downgraded the certainty of the evidence by one grade due to serious risk of bias (largely detection bias). Other biases probably did not result in an overestimate of effect. We were unable to include data on pain at five years from Katz 2013 as it was not reported in a useable way.
2.1. Analysis.

Comparison 2: Arthroscopic surgery versus exercise, Outcome 1: Pain (lower score=less pain)
Function
Seven trials reported function at up to three months (Gauffin 2014; Herrlin 2007; Kirkley 2008; Kise 2016; Osteras 2012; Van de Graaf 2018; Yim 2013), five trials reported function between three and six months (Herrlin 2007; Katz 2013; Kirkley 2008; Van de Graaf 2018; Yim 2013), seven trials between six months and two years (Gauffin 2014; Herrlin 2007; Katz 2013; Kirkley 2008; Kise 2016; Van de Graaf 2018; Yim 2013), and three trials between two and five years (Gauffin 2014; Katz 2013; Kise 2016). There was no important heterogeneity at any time point. No trials reported function between 5 and 10 years.
Moderate‐certainty evidence indicates arthroscopic surgery may lead to little or no difference in function compared with exercise: at up to three months (SMD 0.13, 95% CI 0.00 to 0.26; I2 = 0%; 7 trials, 949 participants; translates to 1.9 points on a 0 to 100 point scale (0.01 to 3.8 points)); between three and six months (SMD 0.12, 95% CI ‐0.01 to 0.24; I2 = 0%; 5 trials, 988 participants; translates to 1.8 (‐0.1 to 3.5) points on 0 to 100 point scale); between six months and two years (SMD 0.10, 95% CI ‐0.01 to 0.21; I2 = 0%; 7 trials, 1227 participants; 1.5 (‐0.1 to 3.1 points) on 0 to 100 scale); and between two and five years (MD ‐0.79 points, 95% CI ‐5.50 to 3.91; I2 = 20%; 2 trials, 219 participants; Analysis 2.2). We downgraded the certainty of the evidence by one grade due to serious risk of bias (largely detection bias). Other biases probably did not result in an overestimate of effect. We were unable to include data on function at five years from Katz 2013 as it was not reported in a useable way.
2.2. Analysis.

Comparison 2: Arthroscopic surgery versus exercise, Outcome 2: Function (higher score=better function)
Knee‐specific health‐related quality of life
Three trials reported knee‐specific health‐related quality of life on the KOOS quality of life (QoL) subscale at up to three months (Gauffin 2014; Herrlin 2007; Kise 2016), one trial reported this outcome between three and six months (Herrlin 2007), three trials between six months and two years (Gauffin 2014; Herrlin 2007; Kise 2016), and two trials between two and five years (Gauffin 2014; Kise 2016). There was no important heterogeneity at any time point. No trials reported knee‐specific health‐related quality of life between 5 and 10 years.
Low‐certainty evidence indicates arthroscopic surgery may lead to little or no difference, or a very small improvement, in knee‐specific quality of life compared with exercise at up to three months (MD 6.87, 95% CI 2.55 to 11.19; I2 = 0%; 3 trials, 347 participants). We downgraded the certainty of the evidence due to serious risk of bias (largely detection bias) and serious imprecision (the 95% confidence intervals include both an unimportant improvement and the clinically important improvement threshold of 10%). There was little or no difference in knee‐specific quality of life with arthroscopic surgery compared with exercise between three and six months (MD 0.49, 95% CI ‐8.28 to 9.26; 1 trial, 96 participants), between six months and two years (MD 4.47, 95% CI ‐1.33 to 10.28; I2 = 32%; 3 trials, 348 participants), and between two and five years (MD 2.13, 95% CI ‐5.74 to 10.00; I2 = 35%; 2 trials, 220 participants; Analysis 2.3).
2.3. Analysis.

Comparison 2: Arthroscopic surgery versus exercise, Outcome 3: Knee‐specific quality of life (higher score=better)
Generic health‐related quality of life
Two trials reported generic health‐related quality of life at up to three months (Gauffin 2014; Kirkley 2008), one trial between three and six months (Kirkley 2008), three trials between six months and two years (Gauffin 2014; Kirkley 2008; Kise 2016), and one trial between two and five years (Gauffin 2014). These trials used different measurement scales: EQ‐5D (Gauffin 2014), standard‐gamble utility score (Kirkley 2008), and SF‐36 MCS (Kise 2016). There was no important heterogeneity. None of the trials reported generic health‐related quality of life between 5 and 10 years.
We downgraded the evidence to low certainty at each time point due to the potential for biased and imprecise estimates. Low‐certainty evidence (downgraded due to serious risk of bias and imprecision) indicates arthroscopic surgery may lead to little or no improvement in generic quality of life compared with exercise: at up to three months (SMD 0.09, 95% CI ‐0.14 to 0.32; I2 = 0%; 2 trials, 290 participants), translates to 0.01 points (‐0.01 to 0.02) on the 15D 0 to 1 scale, and 0.9 points (‐1.4 to 3.2) points on the 0 to 100 SF‐36 scale); between three and six months (MD 0.03, 95% CI ‐0.04 to 0.10, 15D 0 to 1 scale; I2 = 0%; 1 trial, 163 participants); between six months and two years (SMD 0.03, 95% CI ‐0.16 to 0.22; I2 = 0%; 3 trials, 425 participants; translates to 0.001 points (‐0.01 to 0.01) on 0 to 1 scale and 0.3 points (‐1.6 to 2.2) points on 0 to 100 scale); and between two and five years (MD ‐0.05, 95% CI ‐0.12 to 0.02, EQ‐5D 0 to 1 scale; 1 trial, 101 participants; Analysis 2.4).
2.4. Analysis.

Comparison 2: Arthroscopic surgery versus exercise, Outcome 4: Generic quality of life (higher score=better)
Participant‐reported treatment success
Three trials reported participant‐reported treatment success at up to five years (Gauffin 2014; Katz 2013; Yim 2013). No trials reported participant‐reported treatment success between 5 and 10 years.
There was considerable heterogeneity in results (I2 = 88%; Chi2 = 16.04; P < 0.001), due to a larger treatment effect in one study (Katz 2013), but the studies are consistent in the effect direction. It is uncertain whether arthroscopic surgery leads to participant‐reported treatment success at up to five years compared with exercise because the certainty of the evidence is low (RR 1.17, 95% CI 0.86 to 1.59; Analysis 2.5). The certainty was downgraded due to serious risk of bias (all trials had high risk of performance and detection bias, and some concerns with selection bias). There was also serious statistical inconsistency (I2 = 88%; Chi2 = 16.04; P < 0.001), which could be explained by small and large treatment effects across only three studies, and possible indirectness (diversity in outcome measurement across trials).
2.5. Analysis.

Comparison 2: Arthroscopic surgery versus exercise, Outcome 5: Participant‐reported success
Arthroscopic surgery versus glucocorticoid injection
One trial compared arthroscopic surgery to a single intra‐articular glucocorticoid injection (Vermesan 2013).
Function
Vermesan 2013 reported function at one month and one year using the Oxford Knee Score. Mean function at one month in the glucocorticoid injection group was 39.9 (0 to 48 scale, higher is better) compared with 42.8 in the arthroscopic surgery group, a difference of 2.9 points (95% CI 1.64 to 4.16; 1 trial, 120 participants; low‐certainty evidence; Analysis 3.1). There was no difference in function with arthroscopic surgery compared with a single intra‐articular glucocorticoid injection at one year (MD 1.40, 95% CI ‐0.07 to 2.87; 1 trial, 98 participants; low‐certainty evidence). Based on this study, arthroscopic surgery may slightly improve function compared with a single intra‐articular glucocorticoid injection at one month but leads to little or no difference in function at one year. We downgraded the evidence due to serious risk of bias (some concerns with selection, performance and detection bias, and high risk of reporting bias) and serious imprecision (only one study).
3.1. Analysis.

Comparison 3: Arthroscopic surgery versus glucocorticoid injection, Outcome 1: Function (OKS, 0‐48, higher score=better function)
Outcomes not measured
Pain, knee‐specific and generic health‐related quality of life, treatment success, serious and total adverse events and progression of knee osteoarthritis were not measured.
Arthroscopic surgery versus non‐arthroscopic lavage
One trial compared arthroscopic surgery plus physical therapy and analgesia to non‐arthroscopic joint lavage plus physical therapy and analgesia (Chang 1993).
Pain
Chang 1993 reported pain at 3 and 12 months using the Arthritis Impact Measurement Scales pain subscale (AIMS‐P; 0 to 10 scale; lower score indicates less pain). Based on this study, arthroscopic surgery may lead to little or no difference in pain compared with non‐arthroscopic lavage at 3 months (MD ‐0.40, 95% CI ‐1.66 to 0.86; 1 trial, 32 participants; low‐certainty evidence) and 12 months (MD 0.30, 95% CI ‐1.15 to 1.75; 1 trial, 32 participants; low‐certainty evidence; Analysis 4.1). We downgraded the evidence due to serious risk of bias (some concerns with selection and reporting bias, and high risk of performance and detection bias) and serious imprecision (only one study).
4.1. Analysis.

Comparison 4: Arthroscopic surgery versus non‐arthroscopic lavage, Outcome 1: Pain (AIMS‐P subscale, 0‐10, lower score=less pain)
Function
Chang 1993 reported function at 3 and 12 months using the Arthritis Impact Measurement Scales physical function subscale (AIMS‐PF; 0 to 10 scale; lower score is better). Based on this study, arthroscopic surgery may lead to little or no difference in function compared with non‐arthroscopic lavage at 3 months (MD 0.50, 95% CI ‐0.25 to 1.25; 1 trial, 32 participants; low‐certainty evidence) and 12 months (MD 0.30, 95% CI ‐0.50 to 1.10; 1 trial, 32 participants; low‐certainty evidence; Analysis 4.2). We downgraded the evidence due to serious risk of bias (some concerns with selection and reporting bias, and high risk of performance and detection bias) and serious imprecision (only one study).
4.2. Analysis.

Comparison 4: Arthroscopic surgery versus non‐arthroscopic lavage, Outcome 2: Function (AIMS‐PF subscale, 0‐10, higher score=better function)
Participant‐reported treatment success
Chang 1993 reported participant‐reported treatment success, defined as 1 cm improvement or greater from baseline global assessment of overall well‐being (measured on a 0 to 10 visual analogue scale; lower is better). Seven of 16 participants in the arthroscopic surgery group and seven of 12 participants in the non‐arthroscopic lavage group reported treatment success according to this definition. Based on this study, arthroscopic surgery may result in little or no difference in participant‐reported treatment success compared with non‐arthroscopic lavage at 12 months (last follow‐up) (RR 0.75, 95% CI 0.36 to 1.56; 1 trial, 28 participants; low‐certainty evidence; Analysis 4.3). We downgraded the evidence due to serious risk of bias (some concerns with selection and reporting bias, and high risk of performance and detection bias) and serious imprecision (only one study).
4.3. Analysis.

Comparison 4: Arthroscopic surgery versus non‐arthroscopic lavage, Outcome 3: Participant‐reported success (≥1cm improvement in VAS)
Outcomes not measured
Knee‐specific and generic health‐related quality of life, progression of knee osteoarthritis and knee surgery (replacement or osteotomy) were not measured. It was unclear if serious and total adverse events were measured as they were not reported.
Arthroscopic surgery versus non‐steroidal anti‐inflammatory drugs
One trial compared arthroscopic surgery and physiotherapy to non‐steroidal anti‐inflammatory drugs (NSAIDs) and physiotherapy (Merchan 1993).
Function
Merchan 1993 measured function at an average of 25 months follow‐up (range 12 to 36 months) using the modified Hospital for Special Surgery Knee Rating Score (0 to 100; higher score indicates better function). However, only group means were reported and our attempts to obtain missing data from authors were unsuccessful.
Participant‐reported treatment success
Merchan 1993 defined treatment success as a 10‐point or greater increase in the post‐treatment modified Hospital for Special Surgery Knee Rating Score (0 to 100; higher is better; contains a subjective and objective subscale). Twenty‐six of 35 participants in the arthroscopic surgery plus physiotherapy group and six of 38 participants in the NSAIDs plus physiotherapy group were improved at last follow‐up according to this definition. Based on this study, arthroscopic surgery plus physiotherapy may improve participant‐reported treatment success compared with anti‐inflammatory drugs plus physiotherapy at last follow‐up (RR 4.70, 95% CI 2.20 to 10.06; 1 trial, 73 participants; low‐certainty evidence; Analysis 5.1). We downgraded the evidence due to serious risk of bias (some concerns with selection and reporting bias, and high risk of performance and detection bias) and serious imprecision (only one study, wide confidence intervals).
5.1. Analysis.

Comparison 5: Arthroscopic surgery versus NSAIDs, Outcome 1: Participant‐reported success
Outcomes not measured
Pain, knee‐specific and generic health‐related quality of life, progression of knee osteoarthritis and knee surgery (replacement or osteotomy) were not measured.
Arthroscopic surgery versus hyaluronic acid injection
One trial compared arthroscopic surgery to receipt of five hyaluronic acid injections given at weekly intervals (Saeed 2015).
Pain
Saeed 2015 reported pain at one, three and six months using the Knee Society Scoring System (KSSS pain score of 30 or higher; higher score = less pain). Based on this study, it is uncertain whether arthroscopic surgery reduces pain at three and six months compared to hyaluronic acid injections because the certainty of the evidence is very low. We downgraded the evidence due to serious risk of bias (some concerns with selection, performance and detection bias, and high risk of reporting bias), serious imprecision (wide confidence intervals) and serious indirectness (outcome dissimilarity).
Outcomes not measured
Function, knee‐specific and generic quality of life, treatment success, progression of knee osteoarthritis and knee surgery (replacement or osteotomy) were not measured.
Harms
Knee arthroscopy versus all control groups
Serious adverse events
Serious adverse events, defined as those necessitating hospitalisation (including subsequent knee surgery), prolonged inpatient hospital care, or those that are life threatening or result in death or disability, were reported in nine trials. This includes two placebo‐controlled trials (Roos 2018; Sihvonen 2013), five trials with exercise control (Gauffin 2014; Herrlin 2007; Katz 2013; Kise 2016; Van de Graaf 2018), one trial with NSAIDs control (Merchan 1993), and one trial with a single intra‐articular glucocorticoid injection control (Vermesan 2013).
In total, serious adverse events were reported in 32 of 574 participants (5.6%) in the control groups and in 41 of 632 participants (6.5%) in the arthroscopy groups from eight trials. Events included repeat arthroscopy, pulmonary embolism, deep vein thrombosis, heart attack, death, knee surgery, post‐operative knee infection and anterior cruciate ligament reconstruction. Based on these studies, the risk of serious adverse events may increase with arthroscopic surgery at up to five years compared with control (RR 1.35, 95% CI 0.64 to 2.83; I2 = 47%; 8 trials, 1206 participants; low‐certainty evidence; Analysis 7.1; Table 1). We downgraded the certainty of the evidence due to serious imprecision (small number of events) and possible reporting bias (incomplete reporting of outcomes across studies).
7.1. Analysis.

Comparison 7: Harms: arthroscopic surgery versus control, Outcome 1: Serious adverse events
Roos 2018 reported that two of 22 (9%) participants from the arthroscopic surgery group had serious knee‐related adverse events (one partial meniscectomy, one anterior cruciate ligament reconstruction). Two other serious adverse events were reported (abdominal surgery, malignant melanoma) but as these two events were likely unrelated to the intervention, we excluded the events from Analysis 7.1.
Sihvonen 2013 reported that eight of 76 (10.5%) participants from the placebo group had serious knee‐related adverse events (one proximal tibial osteotomy, seven arthroscopic partial meniscectomies) and no other serious adverse events. Sihvonen 2013 reported that seven of 70 (10%) participants from the arthroscopic surgery group had a serious knee‐related adverse event (three knee replacements, four arthroscopies) and that one (1.4%) participant from this group had a serious adverse events (one deep infection at four months), giving a total of eight of 70 (11.4%) participants with serious adverse events in this group.
Gauffin 2014 reported no serious adverse events in the exercise group, and that three of 66 (4.5%) participants in the arthroscopic surgery group had serious adverse events (two repeat arthroscopies, one death after three years).
Herrlin 2007 reported no serious adverse events with exercise. However, 13 of 49 (26.5%) participants crossed over from the exercise group and had an arthroscopic procedure on average 6.5 months following treatment, and three of 47 (6.4%) participants from the arthroscopy group underwent an additional arthroscopic procedure at between 13 and 40 months following their original surgery. No other serious adverse events were reported.
Katz 2013 reported that, at 12 months, three of 109 (2.8%) participants from the physical therapy group had a serious knee‐related adverse event (subsequent knee replacement) and two of 109 (1.8%) participants from this group had other serious adverse events (one stroke; one sudden death), giving a total of five of 109 (4.6%) participants with serious adverse events. In the arthroscopic surgery group, five of 164 (3%) participants had a serious knee‐related adverse event (subsequent knee replacement) and three of 164 (1.8%) participants from this group had other serious adverse events (one acute myocardial infarction; one pulmonary embolism resulting in death; one vascular disorder), giving a total of eight of 164 (4.9%) participants with serious adverse events. At five years' follow‐up, Katz 2013 reported that two of 109 (1.8%) participants randomised to and receiving physical therapy had a serious knee‐related adverse event (subsequent knee replacement), and 16 of 164 participants randomised to and receiving arthroscopic surgery plus seven of 68 cross‐over participants (for a total of 23 of 232 (10%) participants) had a serious knee‐related adverse event (subsequent knee replacement). Other serious adverse events were not reported at five years so only the 12‐month follow‐up data could be included in Analysis 7.1.
Kise 2016 reported no serious knee‐related or other serious adverse events in the exercise group. Kise 2016 reported that five of 64 (7.8%) participants randomised to and receiving arthroscopic surgery and two of 14 cross‐over participants (for a total of seven of 78 (9%) participants) had serious knee‐related adverse events (one knee replacement at 34 months; one osteotomy at four months; three partial meniscectomies of those randomised to arthroscopic surgery; one osteotomy at nine months; one partial meniscectomy). No other serious adverse events were reported with arthroscopic surgery.
Van de Graaf 2018 reported that eight of 162 (4.9%) participants had serious adverse events with physical therapy (one acute myocardial infarction; one sudden death; one neurological event; one alcoholic pancreatitis; three knee replacements; one arthroscopy). Van de Graaf 2018 reported that nine of 159 (5.7%) participants had serious adverse events with arthroscopic surgery (one intracranial malignancy; one lymph node malignancy; one rectal polyp; two knee replacements; two subsequent arthroscopies in affected knee; one arthroscopy in opposite knee; one other knee surgery). We included the apparent intervention‐related adverse events in Analysis 7.1 (4/162 (2.5%) with physical therapy; 5/159 (3.1%) with arthroscopic surgery).
Merchan 1993 reported that two of 40 (5%) participants in the NSAIDs plus physiotherapy group had died after randomisation and that there were no other serious adverse events in this group. Merchan 1993 reported that seven of 40 (17.5%) participants in the arthroscopic surgery plus physiotherapy group had serious adverse events (five died after randomisation; two had deep vein thrombosis).
Vermesan 2013 reported that five study participants (4.2%) had serious knee‐related adverse events (subsequent knee replacement) but the authors did not specify to which treatment group the participants belonged, so these data were not included in Analysis 7.1.
Total adverse events
Eleven trials reported total adverse events, defined as any adverse event, mild or serious in nature. This includes three placebo‐controlled trials (Moseley 2002; Roos 2018; Sihvonen 2013), five trials with exercise control (Gauffin 2014; Herrlin 2007; Katz 2013; Kise 2016; Van de Graaf 2018), one trial with NSAIDs control (Merchan 1993), one trial with hyaluronic acid injection control (Saeed 2015), and one trial with a single intra‐articular glucocorticoid injection control (Vermesan 2013).
Overall, total adverse events were reported in 95 of 634 (15%) participants in the control groups and in 114 of 692 (16.5%) participants in the arthroscopy groups from nine trials with usable data. Based on these trials, arthroscopic surgery may, or may not, slightly increase the risk of experiencing total adverse events at up to five years compared with control (RR 1.15, 95% CI 0.78 to 1.70; I2 = 48%; 9 trials, 1326 participants; low‐certainty evidence; Analysis 7.2; Table 1). We downgraded the certainty of the evidence due to serious imprecision (small number of events) and possible reporting bias (incomplete reporting of outcomes across studies).
7.2. Analysis.

Comparison 7: Harms: arthroscopic surgery versus control, Outcome 2: Total adverse events
Roos 2018 reported that three of 22 (13.6%) participants in the placebo group and six of 22 (27.3%) in the arthroscopic surgery group had adverse events (including four knee‐related and two other adverse events).
Sihvonen 2013 reported that eight of 76 (10.5%) participants from the placebo group had serious knee‐related adverse events (one proximal tibial osteotomy; seven arthroscopic partial meniscectomies) and no other serious adverse events in this group. Sihvonen 2013 reported that seven of 70 (10%) participants from the arthroscopic surgery group had a serious knee‐related adverse event (three knee replacements; 4 arthroscopies) and that one (1.4%) participant from this group had a serious adverse events (one deep infection at four months) (for a total of 8/70 (11.4%) participants).
Moseley 2002 reported that two participants had minor adverse events (incisional erythema treated with antibiotics; calf swelling but no thrombolysis on venography), but did not specify to which treatment group the participants belonged, so the data could not be included in Analysis 7.2.
Gauffin 2014 reported no adverse events in the exercise group, and that three of 66 (4.5%) participants in the arthroscopic surgery group had adverse events (two repeat arthroscopies; one death after three years).
Herrlin 2007 reported no serious adverse events with exercise. However, 13 of 49 (26.5%) participants crossed over from the exercise group and had an arthroscopic procedure on average 6.5 months following treatment, and three of 47 (6.4%) participants from the arthroscopy group underwent an additional arthroscopic procedure at between 13 and 40 months following their original surgery. No other adverse events were reported.
Katz 2013 reported that three of 109 (2.8%) participants from the physical therapy group had a knee‐related adverse event (subsequent knee replacement) and that 15 of 109 (13.8%) participants from this group had other adverse events at 12 months (one stroke; one sudden death; one atrial fibrillation; one skin problem; four pain from fall or other trauma; one knee bursitis; one knee pain; four back/hip/foot pain; one other) (for a total of 18/109 (16.5%) participants). Katz 2013 reported that five of 164 (3%) participants from the arthroscopic surgery group had a knee‐related adverse event (subsequent knee replacement) and that 18 of 164 (11%) participants from this group had other adverse events at 12 months (one acute myocardial infarction; one pulmonary embolism resulting in death; one hypoxaemia; two deep vein thrombosis; one syncope; two skin problems; two pain from fall or other trauma; three tendonitis; one rupture of Baker's cyst; one knee pain; two back/hip/foot pain; one other) (for a total of 23/164 (14%) participants). At five years' follow‐up, Katz 2013 reported that two of 109 (1.8%) participants randomised to and receiving physical therapy had a knee‐related adverse event (subsequent knee replacement) and that 16 of 164 participants randomised to and receiving arthroscopic surgery plus seven of 68 cross‐over participants (for a total of 23/232 (9.9%) participants) had a knee‐related adverse event (subsequent knee replacement). Other adverse events were not reported at five years so only the 12‐month follow‐up data could be included in Analysis 7.2.
Kise 2016 reported that 31 of 60 (51.7%) participants had adverse events with exercise (16 with pain, swelling, instability, stiffness or decreased range of motion in index knee and 15 with similar symptoms in the contralateral knee), and that 31 of 64 (48.4%) participants randomised to and receiving arthroscopic surgery and two of 14 cross‐over participants (for a total of 33/78 (42.3%) participants) had adverse events (one knee replacement; one osteotomy; three partial meniscectomies; 16 knee pain, swelling, instability, stiffness or decreased range of motion; 10 with similar symptoms in the contralateral knee in those randomised to arthroscopic surgery; one osteotomy; one partial meniscectomy).
Van de Graaf 2018 reported that 12 of 162 (7.4%) participants had adverse events with physical therapy (one acute myocardial infarction, one sudden death, one neurological event; one alcoholic pancreatitis; three knee replacements; one arthroscopy; two knee pain resulting in extra consultations; two other musculoskeletal events). Van de Graaf 2018 reported that 18 of 159 (11.3%) participants had adverse events with arthroscopic surgery (one intracranial malignancy; one lymph node malignancy; one rectal polyp; two total knee replacements; two subsequent arthroscopies in affected knee; one arthroscopy in opposite knee; one other knee surgery; one reactive arthritis; six knee pain resulting in extra consultations; two back/hip/foot pain).
Merchan 1993 reported that two of 40 (5%) participants in the NSAIDs plus physiotherapy group had died after randomisation and no other adverse events. Merchan 1993 reported that nine of 40 (22.5%) participants in the arthroscopic surgery plus physiotherapy group had adverse events (five died after randomisation, two deep vein thrombosis, one superficial infection, one haemarthrosis).
Saeed 2015 reported that eight of 60 (13.3%) participants in the hyaluronic acid injection group had pain at the injection site, and that 13 of 60 (21.7%) participants in the arthroscopic surgery group experienced adverse events (pain and mild effusion) at six months' follow‐up.
Vermesan 2013 reported that five study participants (4.2%) had a subsequent knee replacement but the authors did not specify to which treatment group the participants belonged, so the data could not be included in Analysis 7.2.
Adverse events in the surgery groups included seven deaths; one acute myocardial infarction; one hypoxaemia; four deep vein thrombosis; one intracranial malignancy; one lymph node malignancy; one syncope; one rectal polyp; one reactive arthritis; two skin problems; three tendonitis; two pain from fall or other trauma; one rupture of Baker's cyst; four back/hip/foot pain; and three other unspecified adverse events. Other knee‐related adverse events included nine total knee replacements; two osteotomies; 13 repeat arthroscopies; one other knee surgery; one arthroscopy in opposite knee; one cutaneous nerve lesion; one deep infection; one superficial infection; 37 with knee pain, swelling, instability, stiffness or decreased range of motion; 10 with pain, swelling, instability, stiffness or decreased range of motion in the contralateral knee; and one haemarthrosis.
Adverse events in the control groups included four deaths; one acute myocardial infarction; one stroke; one neurological event (unspecified); one atrial fibrillation; one alcoholic pancreatitis; one skin problem; four pain from fall or other trauma; four back/hip/foot pain; and four other unspecified adverse events. Other knee‐related adverse events included six knee replacements; one high tibial osteotomy; 14 arthroscopies; one knee bursitis; 27 with knee pain, swelling, instability, stiffness or decreased range of motion in index knee; 15 with pain, swelling, instability, stiffness or decreased range of motion in the contralateral knee; and two other unspecified musculoskeletal events.
Progression of knee osteoarthritis
Progression of knee osteoarthritis was reported in one placebo‐controlled trial (Sihvonen 2013), and five exercise‐controlled trials (Gauffin 2014; Herrlin 2007; Kise 2016; Van de Graaf 2018; Yim 2013).
In total, progression of knee osteoarthritis was reported in 69 of 256 (27.0%) participants in the control groups, and in 98 of 277 (35.4%) participants in the arthroscopic surgery groups from five trials where progression of knee osteoarthritis was reported as a dichotomous outcome and the data could be combined. Based on these studies, arthroscopic surgery may lead to greater progression of knee osteoarthritis at up to five years compared with control (RR 1.25, 95% CI 1.01 to 1.54; I2 = 0%; 5 trials, 533 participants; low‐certainty evidence; Analysis 7.3). We downgraded the certainty of the evidence two levels due to serious imprecision (small number of events).
7.3. Analysis.

Comparison 7: Harms: arthroscopic surgery versus control, Outcome 3: Progression of knee osteoarthritis
Gauffin 2014 reported that 10 of 27 (37%) participants from the exercise group and 33 of 55 (60%) participants from the arthroscopy group had radiographic deterioration from baseline to the 5‐year follow‐up according to the Kellgren‐Lawrence classification.
Herrlin 2007 reported that two of 45 (4.4%) participants from the exercise group and two of 43 (4.7%) participants from the arthroscopy group who underwent radiographic examination at the 5‐year follow‐up had evidence of osteoarthritis progression in the medial compartment from baseline, according to the Ahlback classification. In three cases, progression was from grade 1 to grade 2, and in one case from grade 1 to grade 3 (although the authors did not specify to which treatment group the participant with osteoarthritis progression from grade 1 to grade 3 belonged).
Kise 2016 reported that 10 of 58 (17.2%) participants from the exercise group and 13 of 62 (21.0%) participants from the arthroscopy group had radiographic knee osteoarthritis consistent with grade 2 or more on the Kellgren‐Lawrence classification at five years.
Sihvonen 2013 reported that 44 of 74 (59.5%) participants from the placebo group and 48 of 67 (71.6%) participants from the arthroscopy group had at least one grade progression in radiographic tibiofemoral knee osteoarthritis on the Kellgren‐Lawrence classification at five years.
Van de Graaf 2018 reported knee osteoarthritis severity progressed from 1.3 points at baseline to 1.5 points at 24 months on the Kellgren‐Lawrence classification in the physical therapy group (MD 0.18 points, 95% CI 0.04 to 0.31), and from 1.3 points at baseline to 1.6 points at 24 months in the arthroscopy group (MD 0.37 points, 95% CI 0.25 to 0.49). The authors reported no significant between‐group difference (0.10 points more progression in the arthroscopy group, 95% CI ‐0.05 to 0.26, P = 0.18).
Yim 2013 reported that three of 52 (5.8%) participants from the exercise group and two of 50 (4%) participants from the arthroscopy group had radiographic deterioration of grade 2 or more on the Kellgren‐Lawrence classification at two years.
Subsequent knee surgery (replacement or high tibial osteotomy)
The need for subsequent knee surgery was reported in five trials: one placebo‐controlled trial (Sihvonen 2013); three exercise‐controlled trials (Katz 2013; Kise 2016; Van de Graaf 2018); and one glucocorticoid injection‐controlled trial (Vermesan 2013).
In total, subsequent knee surgery (replacement or high tibial osteotomy) was reported in six of 407 (1.5%) participants in the control groups and in 23 of 457 (5%) participants in the arthroscopy groups from four trials with usable data. There is some imprecision due to the small event rate and the 95% confidence interval including both no difference between groups and a large increase in risk (RR 2.63, 95% CI 0.94 to 7.34; I2 = 11%; 4 studies, 864 participants; low‐certainty evidence; Analysis 7.4; Table 1). The certainty of the evidence was low, downgraded twice due to serious imprecision (small event rate).
7.4. Analysis.

Comparison 7: Harms: arthroscopic surgery versus control, Outcome 4: Subsequent knee surgery (replacement or high tibial osteotomy)
Sihvonen 2013 reported that one of 76 (1%) participants from the placebo surgery group had a subsequent high tibial osteotomy and that three of 70 (4%) participants from the arthroscopic surgery group had a subsequent knee replacement at five years.
Katz 2013 reported that two of 109 (1.8%) randomised to and receiving physical therapy, and 16 of 164 (9.8%) randomised to and receiving arthroscopic surgery group plus seven of 68 (10.3%) cross‐over participants from physical therapy to arthroscopy (for a total of 23/232 (10%) participants) had a subsequent total knee replacement.
Kise 2016 reported that zero of 60 participants randomised to and receiving exercise and two of 64 (3%) participants randomised to and receiving arthroscopic surgery plus one of 14 cross‐over participants (for a total of 3 of 78 (4%) participants) had subsequent knee surgery (one total knee replacement at 34 months and one osteotomy at four months of those randomised to arthroscopic surgery, plus one osteotomy at nine months).
Van de Graaf 2018 reported that three of 162 (2%) participants from the exercise group and two of 159 (1%) participants from the arthroscopic surgery group had a subsequent knee replacement.
Vermesan 2013 reported that five study participants (4.2%) had a subsequent knee replacement, but the authors did not specify to which treatment group the participants belonged, so the data could not be included in Analysis 7.4.
Discussion
Summary of main results
We found 16 randomised trials, including 2105 participants, that compared arthroscopic surgery with placebo surgery (4 trials, 380 participants) or non‐surgical treatment (12 trials, 1725 participants) in people with degenerative knee disease, with or without degenerative meniscal tears. Our findings demonstrate that arthroscopic knee surgery provides little or no clinically important benefit in pain or function in the short or longer term, and probably provides no clinically important benefit in knee‐specific or generic quality of life, and may not improve treatment success, in the short or longer term compared with a placebo procedure (Table 1). Arthroscopic surgery may lead to little or no difference in, or slightly more, serious adverse events and total adverse events compared to placebo or non‐surgical interventions. Few events may occur in this population even without surgery; yet most observed serious adverse events were likely attributable to the index procedure.
Arthroscopic surgery may lead to greater progression of knee osteoarthritis and it may or may not lead to a slight increase in subsequent knee surgery (replacement or osteotomy), although the 95% confidence intervals for subsequent knee surgery includes no difference between groups and a large increase in risk. We concluded that arthroscopy may increase the risk of osteoarthritis progression on the basis that the relative risk estimate indicated that there could be a 25% increased risk, although the 95% confidence interval included almost no increased risk to a greater than 50% increase in risk, and this result was consistent across studies (no statistical heterogeneity). For subsequent knee surgery, we concluded that there may or may not be an increased risk: while the relative risk estimate indicated that the increased risk could be 2.5 times greater and the upper bound of the 95% confidence interval indicated that there could be a large increase in risk, the lower bound of the 95% confidence interval also included no difference between groups (and there was likely no important heterogeneity across studies). However, the observed overall risk of progression of knee osteoarthritis and risk of subsequent knee surgery both increased by about 2% following arthroscopic surgery compared with control, estimates that are consistent with those reported in larger population‐based studies (Winter 2017).
Compared to exercise, arthroscopic knee surgery probably provides little or no clinically important benefit in pain or function, and may provide little or no improvements in knee‐specific and generic quality of life in the short or longer term. We are uncertain whether arthroscopic surgery leads to a difference in treatment success and progression of knee osteoarthritis because the certainty of the evidence is very low.
Compared to a single intra‐articular glucocorticoid injection, arthroscopic knee surgery may slightly improve function in the short term but leads to little or no difference in the longer term. Pain, knee‐specific and generic quality of life, treatment success and progression of knee osteoarthritis were not assessed in the one trial comparing these treatments.
Compared to non‐arthroscopic lavage, arthroscopic knee surgery may lead to little or no difference in pain or function in the short or longer term, or treatment success. Knee‐specific and generic quality of life, adverse events, progression of knee osteoarthritis and knee surgery (replacement or osteotomy) were not reported in the trial evaluating these treatments.
Compared to non‐steroidal anti‐inflammatory drugs (NSAIDs), arthroscopic knee surgery may improve participant‐reported treatment success but its effect on function is uncertain because the certainty of the evidence is very low. Pain, knee‐specific and generic quality of life, progression of knee osteoarthritis and knee surgery (replacement or osteotomy) were not assessed in the trial comparing these treatments.
It is uncertain whether arthroscopic knee surgery reduces pain in the short term compared to five hyaluronic acid injections given at weekly intervals because the certainty of the evidence is very low. Function, knee‐specific quality of life, treatment success and progression of knee osteoarthritis were not assessed in the single trial comparing these treatments.
No trials compared arthroscopic surgery to complementary therapies.
Overall completeness and applicability of evidence
Overall, the trials included participants and interventions that are largely reflective of clinical practice, indicating that the results of the review can be broadly applied to practice. A quarter of studies were placebo‐controlled trials with minimal biases, while half were open‐label trials comparing arthroscopic surgery to common conservative care in the form of exercise or rehabilitation programmes. Two further trials compared surgery to other common conservative care modalities: intra‐articular glucocorticoid injection and non‐steroidal anti‐inflammatory medication. Two trials used less common comparators: non‐arthroscopic lavage and hyaluronic acid injections.
Trials were conducted across several countries, the majority in Europe, followed by North America, with single trials conducted in Pakistan and South Korea. The trials varied in their eligibility criteria regarding the presence of osteoarthritis and degenerative meniscal tears. For our primary comparison of arthroscopic surgery versus placebo surgery, half of the trials included participants with meniscal tears (and it was unclear if participants in the remaining trials had meniscal tears). Overall, trials included participants with symptoms of knee pain or torn menisci in the preceding few months. Some trials required evidence of degeneration or tears of the meniscus or cartilage on imaging or arthroscopy, and some trials included only participants with radiographic evidence of osteoarthritis, ranging from mild to severe disease. It is likely this variation reflects conditions seen in clinical practice.
Arthroscopic procedures varied from debridement of rough cartilage and trimming of torn/degenerated menisci to surgical resection of damaged menisci and excision of loose fragments of cartilage and bone. These procedures are reflective of those used in clinical practice.
The majority of trials were designed to assess the benefits of knee arthroscopy in terms of important patient‐relevant outcomes: pain and knee function. Fewer trials (50%) measured treatment success and only five (31%) trials reported knee‐specific quality of life, so we are less certain of the applicability of results for these outcomes.
We were unable to reliably estimate the harms associated with arthroscopic surgery from the included trials, as event rates were low, and trials were likely not large enough to detect important differences between groups. Our risk estimates were further hampered by the failure of nearly half the trials to report adverse events. Serious surgical‐related adverse events may increase by an absolute risk of 2% with arthroscopic surgery, but due to the low number of events, there is some uncertainty around the estimate ‐ the 95% confidence intervals include both an increase and small decrease in risk.
Longer‐term outcomes, including progression of knee osteoarthritis and subsequent knee replacement, are also important. Six trials reported progression of knee osteoarthritis and five measured knee surgery (replacement or osteotomy) as an outcome. These trials were not large or long enough to reliably assess these outcomes, but the absolute risk of subsequent knee replacement or knee surgery was about 2% greater following arthroscopic surgery than with control, which is consistent with the incidence of knee replacement after arthroscopic surgery reported in observational studies. A systematic review of 20 observational cohort and cross‐sectional studies indicates the yearly incidence of knee replacement after arthroscopic surgery for osteoarthritis is 2.62% (95% CI 1.26% to 3.46%) and the median interval between arthroscopy and knee replacement is 2.0 years (Winter 2017). A long‐term trial, an extension of Sihvonen 2013, is underway, with plans for a 10‐year follow‐up to assess the incidence of radiographically‐confirmed osteoarthritis following knee arthroscopy, with results expected in 2024 (NCT01052233).
Quality of the evidence
Arthroscopic surgery versus placebo surgery
We did not downgrade the evidence for pain or function. We downgraded the evidence for knee‐specific quality of life to moderate certainty due to serious imprecision, as the 95% confidence intervals did not rule in or rule out a clinically important change. We downgraded the evidence for participant‐reported treatment success to low certainty due to serious indirectness ‐ treatment success was defined variably across trials and measured at different time points ‐ and serious imprecision, as there were small numbers reported.
We downgraded the evidence for serious adverse events and total adverse effects to low certainty due to serious imprecision (small number of events) and likely reporting bias (incomplete reporting of outcome across studies). Few events may occur in this population even without surgery; most observed serious adverse events were likely attributable to the index procedure.
We downgraded the evidence for both progression of knee osteoarthritis and subsequent knee surgery by two levels ‐ to low certainty ‐ due to the small number of events. The trials were not large or long enough to detect many events (98 with knee arthroscopy and 69 with control for progression of knee osteoarthritis; 23 with knee arthroscopy and 6 with control for subsequent knee surgery). Although only six and five studies reported progression of knee osteoarthritis and subsequent knee replacement, respectively, we did not downgrade for reporting bias as it appears the remaining studies did not intend to measure these outcomes.
Arthroscopic surgery versus exercise
We downgraded the evidence for pain and function to moderate certainty due to serious risk of bias (largely detection bias). We downgraded the evidence for knee‐specific and generic quality of life to low certainty due to serious risk of bias and serious imprecision. We downgraded the evidence for treatment success to very low certainty due to serious risk of bias (all trials had high risk of performance and detection bias, and some concerns with selection bias), serious inconsistency and serious indirectness (diversity in outcome measurement across trials). We downgraded the evidence for progression of knee osteoarthritis to very low certainty due to very serious imprecision (very few events reported) and likely reporting bias.
Arthroscopic surgery versus glucocorticoid injection
We downgraded the evidence for function to low certainty due to serious risk of bias (some concerns with selection, performance and detection bias, high risk of reporting bias) and serious imprecision (only one study).
Arthroscopic surgery versus non‐arthroscopic lavage
We downgraded the evidence for pain, function and treatment success to low certainty due to serious risk of bias (some concerns with selection and reporting bias, high risk of performance and detection bias) and serious imprecision (only one study).
Arthroscopic surgery versus anti‐inflammatory drugs
We downgraded the evidence for treatment success to low certainty due to serious risk of bias (some concerns with selection and reporting bias, high risk of performance and detection bias) and serious imprecision (only one study with wide confidence intervals). We downgraded the evidence for function to very low certainty because of serious risk of bias, serious imprecision and reporting bias.
Arthroscopic surgery versus hyaluronic acid injections
We downgraded the evidence for pain to very low certainty due to serious risk of bias (some concerns with selection, performance and detection bias, high risk of reporting bias), serious imprecision (wide confidence intervals and only one study) and serious indirectness (outcome dissimilarity).
Potential biases in the review process
To the best of our knowledge, we identified all relevant trials meeting the review's eligibility criteria through a comprehensive search of major electronic databases and trial registries without language restrictions. We used up to three independent assessors to screen and select studies and perform risk of bias judgements. None of the review authors have been involved in the conduct of the included trials.
There were too few studies to formally assess the presence of publication bias. We identified four ongoing trials comparing arthroscopic surgery to exercise (NCT02113280; NCT02995551; NCT04837456; NCT04313569). As these trials are unblinded and thus subject to detection biases, it is unlikely the results, when available, will change the conclusions of this review for the comparison of arthroscopic surgery versus exercise.
Agreements and disagreements with other studies or reviews
In addition to our earlier systematic review (Brignardello‐Petersen 2017), we identified 11 other systematic reviews (Abram 2020; Barlow 2015; Health Quality Ontario 2014; Hohmann 2018; Khan 2014; Lamplot 2016; Lee 2018; Li 2020; Monk 2017; Thorlund 2015; Van de Graaf 2018), and one narrative review (Ha 2016), assessing the effects of arthroscopic surgery compared to non‐surgical interventions. Two focused exclusively on arthroscopic surgery versus exercise (Hohmann 2018; Li 2020), and eight included only studies with participants with meniscal tears (Abram 2020; Ha 2016; Hohmann 2018; Khan 2014; Lee 2018; Li 2020; Monk 2017; Van de Graaf 2016). Three reviews included studies other than randomised trials (Abram 2020; Lamplot 2016; Monk 2017); one excluded trials because of no or insufficient data on pain or functional outcomes (Thorlund 2015); and eight reviews failed to identify one or more eligible trials that are included in this review update (Barlow 2015; Ha 2016; Health Quality Ontario 2014; Lamplot 2016; Lee 2018; Li 2020; Monk 2017; Van de Graaf 2016).
Abram 2020 included 10 trials that appear in this review update (Gauffin 2014; Herrlin 2007; Katz 2013; Kise 2016; Osteras 2012; Roos 2018; Sihvonen 2013; Van de Graaf 2018; Vermesan 2013; Yim 2013), and their conclusions are largely in agreement with this review update. They reported little or no difference in pain, function, knee‐specific quality of life and generic quality of life with arthroscopic surgery compared to placebo in the short or longer term and no difference in presence of mechanical knee symptoms between groups. They reported low event rates for subsequent knee surgery (2.9% with arthroscopic surgery, 6.6% with placebo) and adverse events.
Lee 2018 included eight trials that appear in this review update (Chang 1993; Gauffin 2014; Herrlin 2007; Katz 2013; Kirkley 2008; Moseley 2002; Vermesan 2013; Yim 2013), but failed to identify three eligible trials at the time of their search (Kise 2016; Osteras 2012; Sihvonen 2013). They reported no significant differences between arthroscopic surgery and conservative management (including placebo surgery).
Monk 2017 included six trials that are included in this review update (Herrlin 2007; Katz 2013; Moseley 2002; Sihvonen 2013; Vermesan 2013; Yim 2013), but failed to identify Gauffin 2014 and Osteras 2012. They reported no clear benefit of arthroscopic surgery over non‐operative treatment for participants with degenerative tears in the presence or absence of osteoarthritic changes.
Van de Graaf 2016 included five trials that are included in this review update (Herrlin 2007; Katz 2013; Osteras 2012; Sihvonen 2013; Yim 2013), but failed to identify Gauffin 2014 and Vermesan 2013. They reported a small statistically significant but clinically unimportant benefit in pain (MD 0.56, 95% CI 0.28 to 0.83 on VAS) and function (MD 3.56, 95% CI 0.24 to 6.88 on KOOS) with arthroscopic surgery compared with conservative treatment at up to six months, and no difference between groups at longer follow‐up.
Lamplot 2016 included five trials included in this review update (Gauffin 2014; Katz 2013; Kirkley 2008; Merchan 1993; Sihvonen 2013), but failed to identify seven eligible trials at the time of their search that are included in this review update (Chang 1993; Herrlin 2007; Moseley 1996; Osteras 2012; Saeed 2015; Vermesan 2013; Yim 2013. The review authors vote counted based on statistical significance and reported two trials showed benefit with arthroscopic surgery compared to conservative treatment and three found no difference.
Ha 2016 included five trials that are included in this review update (Gauffin 2014; Herrlin 2007; Katz 2013; Sihvonen 2013; Yim 2013), but failed to identify Osteras 2012 and Vermesan 2013. The authors provided a narrative summary of individual trial findings (no synthesis) and said they could not draw any conclusions on the optimal treatment for meniscal tears.
Thorlund 2015 included eight trials that are included in this review update (Chang 1993; Herrlin 2007; Katz 2013; Kirkley 2008; Moseley 2002; Osteras 2012; Sihvonen 2013; Yim 2013). The authors reported a small clinically unimportant benefit in knee pain in the short term with arthroscopic surgery compared to control but no difference in the longer term or in function between groups. Harms reported included symptomatic deep venous thrombosis, pulmonary embolism, infection and death.
Barlow 2015 included three trials included in this review update (Chang 1993; Kirkley 2008; Moseley 2002), but failed to identify Merchan 1993 and Moseley 1996. The authors provided a narrative summary of individual trial findings and concluded that none of the trials support use of arthroscopy in people with osteoarthritis.
Khan 2014 included six trials included in this review update (Herrlin 2007; Katz 2013; Osteras 2012; Sihvonen 2013; Vermesan 2013; Yim 2013), and reported no clinically important difference in pain and function between arthroscopic surgery and non‐operative or sham treatments in the short and longer term.
Health Quality Ontario 2014 included seven trials included in this review update (Herrlin 2007; Katz 2013; Kirkley 2008; Moseley 2002; Osteras 2012; Sihvonen 2013; Yim 2013), but failed to identify four eligible trials at the time of their search (Chang 1993; Merchan 1993; Moseley 1996; Vermesan 2013). They reported no difference in pain or function between arthroscopic surgery and placebo, and between arthroscopic surgery and usual care (e.g. physical therapy).
Li 2020 included six trials included in this review update (Herrlin 2007; Katz 2013; Kise 2016; Osteras 2012; Van de Graaf 2018; Yim 2013), but failed to identify Gauffin 2014. They reported a small benefit in pain and function with arthroscopic surgery compared with physical exercise in the short term but no difference between groups in the longer term. Li 2020 reported no difference in osteoarthritis progression between arthroscopic surgery and exercise based on two trials (Herrlin 2007; Van de Graaf 2018).
Hohmann 2018 included six trials included in this review update (Gauffin 2014; Herrlin 2007; Katz 2013; Kise 2016; Osteras 2012; Yim 2013), and concluded that there is no compelling evidence to support arthroscopic surgery compared to physical therapy.
Only Li 2020 assessed the effect of arthroscopic surgery on the progression of knee osteoarthritis compared with exercise, but failed to report on this outcome in the results of the review.
Authors' conclusions
Implications for practice.
The findings of this review demonstrate that arthroscopic surgery for people with symptomatic degenerative knee disease (average age ranging from 46 to 65 years; 56% women), provides little or no clinically important benefit in pain or function, probably does not provide clinically important benefits in knee‐specific quality of life, and may not improve treatment success compared with placebo surgery. These results apply to people with knee osteoarthritis with or without meniscal tears, as well as people with meniscal tears alone. Arthroscopic surgery may or may not increase serious and total adverse events compared to control. Serious adverse events include deep venous thrombosis, myocardial infarction, pulmonary embolism, infection and death, and are likely mostly attributable to the arthroscopic surgery. Arthroscopic surgery may lead to greater progression of knee osteoarthritis and may or may not slightly increase subsequent knee surgery (replacement or osteotomy).
Participants in the included trials experienced improvement in pain and function over time whether or not they received surgery, placebo surgery or other control treatment. People contemplating arthroscopic surgery should be informed about the findings of this evidence synthesis to help them make an evidence‐informed decision. They should also be informed that their symptoms are likely to improve slowly over time irrespective of treatment and that surgery has the potential for short‐term harms related to the surgery and long‐term harms of greater progression of knee osteoarthritis and the need for further knee surgery.
Implications for research.
Given there are no benefits in pain and function, probably no benefit in quality of life, and maybe no difference in treatment success with arthroscopic surgery compared to placebo surgery, more randomised placebo‐controlled trials assessing benefits of knee arthroscopic surgery are likely unnecessary. If proponents of the procedure consider there may still be one or more subgroups who may benefit from arthroscopic surgery, then the onus is on them to provide this evidence. However, to date, several studies have failed to find evidence of these subgroups (Sihvonen 2018; Pihl 2020).
We are less certain if arthroscopic surgery leads to more serious and total adverse events, earlier progression of knee osteoarthritis and more knee surgery (replacement or osteotomy) compared with placebo. Longer‐term follow‐up of participants in the included placebo‐controlled trials or data from prospectively designed registries would provide more precise estimates of the risk of adverse events, progression of knee osteoarthritis and subsequent knee replacement with arthroscopic surgery. However, given there is high‐certainty evidence of no benefit, the value of assessing if there is more harm may be limited.
Future updates of this review may be considered if further placebo‐controlled trials are published that are likely to increase the certainty of effect estimates of harms.
Further trials comparing arthroscopic surgery with non‐surgical interventions are likely to be of limited value, and we are unlikely to include such studies in future updates.
Acknowledgements
We thank the authors of the following articles, who supplied data missing from their published articles, upon request: Herrlin 2007; Kise 2016; Roos 2018; Sihvonen 2013.
This is an update of a previous review (Brignardello‐Petersen 2017), and we acknowledge the authors of that review: Romina Brignardello‐Petersen, Gordon Guyatt, Rachelle Buchbinder, Rudolf Poolman, Stefan Schandelmaier, Yaping Chang, Behnam Sadeghirad, Nathan Evaniew and Per Vandvik.
We acknowledge peer reviewers Dr Aleksi Reito, Department of Musculoskeletal Diseases, Tampere University Hospital, School of Medicine and Health Technology, Tampere University, Finland; Professor of Orthopaedic Surgery Ian Harris, University of New South Wales, Sydney; consumer reviewer Ms Catherine Hofstetter, and copy editor Faith Armitage.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
Database: EBM Reviews ‐ Cochrane Central Register of Controlled Trials <April 2021>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp osteoarthritis/
2 osteoarthr$.tw.
3 (degenerative adj2 arthritis).tw.
4 arthrosis.tw.
5 Menisci, Tibial/
6 menisc$.tw.
7 Arthroscopy/
8 Debridement/
9 (arthroscop$ adj5 debridement).tw.
10 (arthroscop$ adj5 meniscectomy).tw.
11 (arthroscop$ and knee$).tw.
12 1 or 2 or 3 or 4 or 5 or 6
13 7 or 8 or 9 or 10 or 11
14 12 and 13
Appendix 2. MEDLINE search strategy
Database: Ovid MEDLINE(R) <1946 to Present with Daily Update>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp osteoarthritis/
2 osteoarthr$.tw.
3 (degenerative adj2 arthritis).tw.
4 arthrosis.tw.
5 Menisci, Tibial/
6 menisc$.tw.
7 Arthroscopy/
8 Debridement/
9 (arthroscop$ adj5 debridement).tw.
10 (arthroscop$ adj5 meniscectomy).tw.
11 (arthroscop$ and knee$).tw.
12 1 or 2 or 3 or 4 or 5 or 6
13 7 or 8 or 9 or 10 or 11
14 12 and 13
15 randomized controlled trial.pt.
16 controlled clinical trial.pt.
17 randomized.ab.
18 placebo.ab.
19 drug therapy.fs.
20 randomly.ab.
21 trial.ab.
22 groups.ab.
23 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22
24 exp animals/ not humans.sh.
25 23 not 24
26 14 and 25
Appendix 3. EMBASE search strategy
Database: Embase Classic+Embase <1947 to Present>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 osteoarthritis/
2 osteoarthr$.tw.
3 (degenerative adj2 arthritis).tw.
4 arthrosis.tw.
5 exp knee meniscus/
6 menisc$.tw.
7 meniscal repair/
8 exp knee arthroscopy/
9 arthroscopic debridement/
10 (arthroscop$ adj5 debridement).tw.
11 (arthroscop$ adj5 meniscectomy).tw.
12 (arthroscop$ and knee$).tw.
13 or/1‐6
14 or/7‐12
15 13 and 14
16 random$.tw.
17 factorial$.tw.
18 crossover$.tw.
19 cross over.tw.
20 cross‐over.tw.
21 placebo$.tw.
22 (doubl$ adj blind$).tw.
23 (singl$ adj blind$).tw.
24 assign$.tw.
25 allocat$.tw.
26 volunteer$.tw.
27 crossover procedure/
28 double blind procedure/
29 randomized controlled trial/
30 single blind procedure/
31 or/16‐30
32 15 and 31
33 limit 32 to exclude medline journals
Appendix 4. Trial registries
ClinicalTrials.Gov
('arthroscopic' or 'arthroscopy' or 'debridement') and ('knee osteoarthritis' or 'meniscal degeneration')
World Health Organization: International Clinical Trials Registry Platform Search Portal
('arthroscopic' or 'arthroscopy' or 'debridement') and ('knee osteoarthritis' or 'meniscal degeneration')
Data and analyses
Comparison 1. Arthroscopic surgery versus placebo surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pain (lower score=less pain) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 Up to 3 months | 4 | 309 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.45, ‐0.00] |
| 1.1.2 >3 months up to 6 months | 3 | 265 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.37, 0.12] |
| 1.1.3 >6 months up to 2 years | 3 | 295 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.48, 0.09] |
| 1.1.4 >2 years up to 5 years | 1 | 142 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.41, 0.24] |
| 1.2 Function (higher score=better function) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 Up to 3 months | 3 | 302 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.22, 0.23] |
| 1.2.2 >3 months up to 6 months | 2 | 257 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.20, 0.29] |
| 1.2.3 >6 months up to 2 years | 3 | 293 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.27, 0.47] |
| 1.2.4 > 2 years up to 5 years | 1 | 142 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.48, 0.18] |
| 1.3 Knee‐specific quality of life (higher score=better) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 Up to 3 months | 2 | 188 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [0.02, 0.59] |
| 1.3.2 >3 months up to 6 months | 1 | 146 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.20, 0.45] |
| 1.3.3 >6 months up to 2 years | 2 | 188 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.24, 0.70] |
| 1.3.4 >2 years up to 5 years | 1 | 142 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.35, 0.31] |
| 1.4 Generic quality of life (higher score=better) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.4.1 Up to 3 months | 1 | 42 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.18, 0.06] |
| 1.4.2 >6 months up to 2 years | 2 | 188 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.28, 0.58] |
| 1.5 Participant‐reported success | 3 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.66, 1.86] |
Comparison 2. Arthroscopic surgery versus exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Pain (lower score=less pain) | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1.1 Up to 3 months | 7 | 942 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.33, ‐0.08] |
| 2.1.2 >3 months up to 6 months | 5 | 987 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.33, ‐0.08] |
| 2.1.3 >6 months up to 2 years | 7 | 1178 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.22, 0.01] |
| 2.1.4 >2 years up to 5 years | 2 | 219 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.48, 0.58] |
| 2.2 Function (higher score=better function) | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.2.1 Up to 3 months | 7 | 949 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [0.00, 0.26] |
| 2.2.2 >3 months up to 6 months | 5 | 988 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.01, 0.24] |
| 2.2.3 >6 months up to 2 years | 7 | 1228 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.01, 0.21] |
| 2.2.4 >2 years up to 5 years | 2 | 219 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.34, 0.23] |
| 2.3 Knee‐specific quality of life (higher score=better) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.3.1 Up to 3 months | 3 | 347 | Mean Difference (IV, Random, 95% CI) | 6.87 [2.55, 11.19] |
| 2.3.2 >3 months up to 6 months | 1 | 96 | Mean Difference (IV, Random, 95% CI) | 0.49 [‐8.28, 9.26] |
| 2.3.3 >6 months up to 2 years | 3 | 348 | Mean Difference (IV, Random, 95% CI) | 4.47 [‐1.33, 10.28] |
| 2.3.4 >2 years up to 5 years | 2 | 220 | Mean Difference (IV, Random, 95% CI) | 2.13 [‐5.74, 10.00] |
| 2.4 Generic quality of life (higher score=better) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.4.1 Up to 3 months | 2 | 290 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.14, 0.32] |
| 2.4.2 >3 months up to 6 months | 1 | 163 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.17, 0.45] |
| 2.4.3 >6 months up to 2 years | 3 | 425 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.16, 0.22] |
| 2.4.4 >2 years up to 5 years | 1 | 101 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.67, 0.15] |
| 2.5 Participant‐reported success | 3 | 532 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.86, 1.59] |
Comparison 3. Arthroscopic surgery versus glucocorticoid injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Function (OKS, 0‐48, higher score=better function) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1.1 Up to 3 months | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 2.90 [1.64, 4.16] |
| 3.1.2 >6 months up to 2 years | 1 | 98 | Mean Difference (IV, Random, 95% CI) | 1.40 [‐0.07, 2.87] |
Comparison 4. Arthroscopic surgery versus non‐arthroscopic lavage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Pain (AIMS‐P subscale, 0‐10, lower score=less pain) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1.1 Up to 3 months | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.66, 0.86] |
| 4.1.2 >6 months up to 2 years | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐1.15, 1.75] |
| 4.2 Function (AIMS‐PF subscale, 0‐10, higher score=better function) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.2.1 Up to 3 months | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.25, 1.25] |
| 4.2.2 >6 months up to 2 years | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.50, 1.10] |
| 4.3 Participant‐reported success (≥1cm improvement in VAS) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 5. Arthroscopic surgery versus NSAIDs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Participant‐reported success | 1 | 73 | Risk Ratio (M‐H, Random, 95% CI) | 4.70 [2.20, 10.06] |
Comparison 6. Arthroscopic surgery versus hyaluronic acid injections.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Pain (KSSS pain score of 30 or higher; higher=less pain) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1.1 Up to 3 months | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.67, 0.98] |
| 6.1.2 >3 months up to 6 months | 1 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.65, 0.92] |
| 6.1.3 >6 months up to 2 years | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.63, 0.86] |
6.1. Analysis.

Comparison 6: Arthroscopic surgery versus hyaluronic acid injections, Outcome 1: Pain (KSSS pain score of 30 or higher; higher=less pain)
Comparison 7. Harms: arthroscopic surgery versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Serious adverse events | 8 | 1206 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.64, 2.83] |
| 7.2 Total adverse events | 9 | 1326 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.78, 1.70] |
| 7.3 Progression of knee osteoarthritis | 5 | 533 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.01, 1.54] |
| 7.4 Subsequent knee surgery (replacement or high tibial osteotomy) | 4 | 864 | Risk Ratio (M‐H, Random, 95% CI) | 2.63 [0.94, 7.34] |
Comparison 8. Subgroup analysis: presence of meniscal tear.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Pain up to 3 months (lower score=less pain) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1.1 Participants with meniscal tear | 2 | 188 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.66, ‐0.08] |
| 8.1.2 Unclear if participants had meniscal tear | 2 | 121 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.36, 0.36] |
| 8.2 Pain at >3 months up to 6 months (lower score=less pain) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.2.1 Participants with meniscal tear | 1 | 146 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.58, 0.08] |
| 8.2.2 Unclear if participants had meniscal tear | 2 | 119 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.33, 0.39] |
| 8.3 Pain at >6 months up to 2 years (lower score=less pain) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.3.1 Participants with meniscal tear | 2 | 188 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.88, 0.25] |
| 8.3.2 Unclear if participants had meniscal tear | 1 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.49, 0.27] |
| 8.4 Pain at >2 years up to 5 years (lower score=less pain) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.4.1 Participants with meniscal tear | 1 | 142 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.41, 0.24] |
| 8.4.2 Unclear if participants had meniscal tear | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
| 8.5 Function up to 3 months (higher score=better function) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.5.1 Participants with meniscal tear | 2 | 188 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.20, 0.37] |
| 8.5.2 Unclear if participants had meniscal tear | 1 | 114 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.48, 0.25] |
| 8.6 Function at >3 months up to 6 months (higher score=better function) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.6.1 Participants with meniscal tear | 1 | 145 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.32, 0.33] |
| 8.6.2 Unclear if participants had meniscal tear | 1 | 112 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.27, 0.47] |
| 8.7 Function at >6 months up to 2 years (higher score=better function) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.7.1 Participants with meniscal tear | 2 | 187 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.48, 0.96] |
| 8.7.2 Unclear if participants had meniscal tear | 1 | 106 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.42, 0.34] |
| 8.8 Function at >2 years up to 5 years (higher score=better function) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.8.1 Participants with meniscal tear | 1 | 142 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.48, 0.18] |
| 8.8.2 Unclear if participants had meniscal tear | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
Comparison 9. Subgroup analysis: arthroscopy with supervised exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Pain up to 3 months (lower score=less pain) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1.1 Unsupervised/ home exercises | 4 | 309 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.45, ‐0.00] |
| 9.1.2 Supervised exercise | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
| 9.2 Pain at >3 months up to 6 months (lower score=less pain) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.2.1 Unsupervised/ home exercises | 3 | 265 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.37, 0.12] |
| 9.2.2 Supervised exercise | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
| 9.3 Pain at >6 months up to 2 years (lower score=less pain) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.3.1 Unsupervised/ home exercises | 3 | 295 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.48, 0.09] |
| 9.3.2 Supervised exercise | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
| 9.4 Pain at >2 years up to 5 years (lower score=less pain) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.4.1 Unsupervised/ home exercises | 1 | 142 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.41, 0.24] |
| 9.4.2 Supervised exercise | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
| 9.5 Function up to 3 months (higher score=better function) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.5.1 Unsupervised/ home exercises | 3 | 302 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.22, 0.23] |
| 9.5.2 Supervised exercise | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
| 9.6 Function at >3 months up to 6 months (higher score=better function) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.6.1 Unsupervised/ home exercises | 2 | 257 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.20, 0.29] |
| 9.6.2 Supervised exercise | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
| 9.7 Function at >6 months up to 2 years (higher score=better function) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.7.1 Unsupervised/ home exercises | 3 | 293 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.27, 0.47] |
| 9.7.2 Supervised exercise | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
| 9.8 Function at >2 years up to 5 years (higher score=better function) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.8.1 Unsupervised/ home exercises | 1 | 142 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.48, 0.18] |
| 9.8.2 Supervised exercise | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
Comparison 10. Sensitivity analysis: low risk of selection bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Pain up to 3 months (lower score=less pain) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1.1 Low risk of bias | 3 | 302 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.50, 0.07] |
| 10.1.2 Risk of bias | 1 | 7 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.74, 1.54] |
| 10.2 Function up to 3 months (higher score=better function) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.2.1 Low risk of bias | 3 | 302 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.24, 0.46] |
10.1. Analysis.

Comparison 10: Sensitivity analysis: low risk of selection bias, Outcome 1: Pain up to 3 months (lower score=less pain)
10.2. Analysis.

Comparison 10: Sensitivity analysis: low risk of selection bias, Outcome 2: Function up to 3 months (higher score=better function)
Comparison 11. Sensitivity analysis: low risk of detection bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Pain up to 3 months (lower score=less pain) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1.1 Low risk of bias | 4 | 309 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.44, 0.01] |
| 11.1.2 Risk of bias | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
| 11.2 Function up to 3 months (higher score=better function) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.2.1 Low risk | 3 | 302 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.24, 0.46] |
| 11.2.2 Risk of bias | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
11.1. Analysis.

Comparison 11: Sensitivity analysis: low risk of detection bias, Outcome 1: Pain up to 3 months (lower score=less pain)
11.2. Analysis.

Comparison 11: Sensitivity analysis: low risk of detection bias, Outcome 2: Function up to 3 months (higher score=better function)
Comparison 12. Sensitivity analysis: fixed‐effect model.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Pain (lower score=less pain) | 4 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1.1 Up to 3 months | 4 | 309 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.45, ‐0.00] |
| 12.2 Function (higher score=better function) | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.2.1 Up to 3 months | 3 | 302 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.22, 0.23] |
12.1. Analysis.

Comparison 12: Sensitivity analysis: fixed‐effect model, Outcome 1: Pain (lower score=less pain)
12.2. Analysis.

Comparison 12: Sensitivity analysis: fixed‐effect model, Outcome 2: Function (higher score=better function)
Comparison 13. Sensitivity analysis: arthroscopic surgery versus any control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Pain (lower score=less pain) | 13 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1.1 Up to 3 months | 12 | 1283 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.32, ‐0.10] |
| 13.1.2 >3 months up to 6 months | 8 | 1252 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.30, ‐0.07] |
| 13.1.3 >6 months up to 2 years | 11 | 1505 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.22, ‐0.01] |
| 13.1.4 >2 years up to 5 years | 3 | 361 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.30, 0.29] |
| 13.2 Function (higher score=better function) | 13 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.2.1 Up to 3 months | 12 | 1403 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [0.04, 0.34] |
| 13.2.2 >3 months up to 6 months | 7 | 1245 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.01, 0.21] |
| 13.2.3 >6 months up to 2 years | 12 | 1651 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [0.01, 0.20] |
| 13.2.4 >2 years up to 5 years | 3 | 361 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.30, 0.12] |
| 13.3 Knee‐specific quality of life (higher score=better) | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.3.1 Up to 3 months | 5 | 535 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [0.16, 0.50] |
| 13.3.2 >3 months up to 6 months | 2 | 242 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.17, 0.33] |
| 13.3.3 >6 months up to 2 years | 5 | 536 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.00, 0.38] |
| 13.3.4 >2 years up to 5 years | 3 | 362 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.16, 0.26] |
| 13.3.5 >5 years up to 10 years | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
| 13.4 Generic quality of life (higher score=better) | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.4.1 Up to 3 months | 3 | 332 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.36, 0.30] |
| 13.4.2 >3 months up to 6 months | 1 | 163 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.17, 0.45] |
| 13.4.3 >6 months up to 2 years | 5 | 613 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.08, 0.24] |
| 13.4.4 >2 years up to 5 years | 1 | 101 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.67, 0.15] |
| 13.5 Participant‐reported success | 8 | 851 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.96, 1.60] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chang 1993.
| Study characteristics | ||
| Methods |
Study design: multicentre, parallel‐group, two‐arm randomised controlled trial Setting: the Rheumatology‐Orthopedic Knee Clinic of the Northwestern Medical Faculty Foundation and the Division of Rheumatology of the Lutheran General Medical Group, Illinois, USA Trial time period: not reported Interventions: arthroscopic surgery versus non‐arthroscopic joint lavage Sample size calculations: authors did not describe how the sample size was estimated Analysis: intention‐to‐treat analysis |
|
| Participants |
Number of participants
Inclusion criteria
In participants with bilateral disease, the more symptomatic knee was designated the study knee. Exclusion criteria
Baseline characteristics Arthroscopic surgery group (N = 18 included in analyses)
Joint lavage group (N = 14 included in analyses)
Pre‐treatment group differences: the baseline demographic, clinical and functional characteristics were similar between the two groups except for the initial AIMS Physical Activity Score (a statistically significant difference between groups (P < 0.05) was identified). |
|
| Interventions |
Arthroscopic surgery group Arthroscopic surgery plus physical therapy and analgesia. Arthroscopy was done under general anaesthesia. A diagnostic evaluation was performed, and the anatomic findings were recorded on a standardised form. Following this evaluation, any of the following interventions were performed under arthroscopic guidance: (1) debridement of torn meniscus and removal of meniscal and cruciate ligament fragments; (2) removal of proliferative synovium; and (3) excision of loose articular cartilage fragments. Osteochondral lesions were not drilled. All participants received continuous saline lavage during the procedure and were routinely instructed in partial weightbearing precautions to continue for 10 days following the procedure. If an osteochondral lesion was detected in a weight‐bearing area, this period of protection was increased to 3 weeks. Prior to and following surgery, participants assigned to this group received only non‐narcotic analgesia and physical therapy, consisting of strengthening and flexibility exercises and gait training. Non‐arthroscopic (closed‐needle joint) lavage group Closed‐needle joint lavage plus physical therapy and analgesia. Participants assigned to this group received non‐narcotic analgesia and physical therapy identical to the arthroscopy group. In addition, participants received a tidal knee lavage procedure which was chosen to offset the potentially strong placebo effect of a surgical procedure and to control for the effects on pain and disability of the lavage procedure that occur during the arthroscopic procedure. Tidal knee lavage was performed as described by Ike and colleagues (Ike 1992) under local anaesthesia. A total of 1 litre of saline was injected into and aspirated from the knee in aliquots of 40 cc to 120 cc, depending on the size of the knee capsule. Post‐intervention Participants in both groups received non‐narcotic analgesia and physical therapy. |
|
| Outcomes | Outcomes were measured at baseline and at 3 and 12 months of follow‐up Clinical parameters
Pain and functional status measures
Global measures
Economic measures
Outcomes included in this review at 3 and 12 months
|
|
| Notes |
Funding: supported by grant 9040 from the Robert Wood Johnson Foundation, by MAC grant AR‐30692 from the NIH (NIAMS), and by the Percy Surgical Research Trust of Lutheran General Hospital Trial registration: not reported Adverse events: unclear if measured; not reported Knee surgery (replacement or osteotomy): not reported Progression of knee OA: not reported Withdrawals: 2/34 (6%), 1 (5%) from arthroscopy group and 1 (7%) from lavage group, dropped out after randomisation but before treatment commenced and were excluded from analysis at 3 and 12 months. Missing data at 12 months imputed by trialists using 3‐month outcomes : 7/32 (22%), 5 (28%) from arthroscopy group, 2 (14%) from lavage group.
Treatment non‐adherence: 3/18 participants from arthroscopy group withdrew before surgery; one improved between enrolment and planned surgery so cancelled surgery, and two developed other medical illnesses that precluded surgery. Data analysis: missing standard deviations for outcomes used in this review: contacted authors but no response received. We imputed SDs. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Used a modification of the pre‐randomisation design described by Zelen (Zelen 1981). Eligible participants were asked if they would accept an arthroscopic procedure if it was offered. Subjects who answered 'yes' were randomly assigned to arthroscopy or lavage and then asked to accept the assigned therapy. The randomisation plan was stratified by study site. No description of sequence generation process provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient description of the method of concealment. Use of assignment 'envelopes' described but unclear if safeguards used (e.g. sealed, sequentially numbered, opaque) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and investigators was not done |
| Blinding of outcome assessor Self‐reported outcomes | High risk | Blinding of participants was not done; hence, there was a risk of bias in the measurement of subjective outcomes of pain, physical function, physical activity, social activity, depression, and anxiety (AIMS subscales) and participants' global assessment |
| Blinding of outcome assessor Assessor‐reported outcome (knee replacement) | Low risk | Blinding of outcome assessors was done. Participants were asked not to disclose their treatment assignment to assessors and to cover actual, or potential, arthroscopy scars |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals (n = 2) were balanced in number across groups and for similar reasons. Missing data at 12 months were imputed using appropriate methods |
| Selective reporting (reporting bias) | Unclear risk | Trial registration not done and study protocol not available. Insufficient information to judge high or low risk |
| Other bias | Low risk | No other biases apparent |
Gauffin 2014.
| Study characteristics | ||
| Methods |
Study design: single centre, parallel‐group, two‐arm, randomised, controlled trial Setting: orthopaedic department at the Linkoping University hospital, Sweden Trial time period: participants were enrolled between 4 March 2010 and 5 April 2012 Interventions: arthroscopic surgery plus unsupervised exercise versus unsupervised exercise Sample size calculations: the article did not describe how the sample size was estimated in this study. Analysis: intention‐to‐treat analysis was used; for those crossing over to the other group, an 'as‐treated' analysis was performed. |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Knee arthroscopy (N = 75)
Exercise therapy (N=75)
Pre‐treatment group differences: there were no differences in the baseline characteristics between the two groups |
|
| Interventions |
Arthroscopic surgery Arthroscopic surgery plus unsupervised exercise program. All operations were performed with full or local anaesthetics by an experienced arthroscopist at an independent daycare clinic. After the arthroscope was inserted in the joint and the joint was visually inspected, the surgeon judged, according to their experience, whether a meniscal resection or any other surgical treatment was indicated. After surgery, all participants were allowed immediate, full weight‐bearing activity. The participants were advised to resume the exercise programme according to phase 1 for 1 week, and then switch to phase 2. Phase 1 program was for 1 week and consisted of a brisk walk 20‐30 min, 10 x 2 sets of squats, pelvic lifts, pelvic lifts with ball between knees and extension of one knee, heel raise, wall squat, and standing on a pillow on one leg (30 sec x 2). Phase 2 was done twice a week for 3 months and consisted of 3 sets of all of the above‐mentioned exercises. Exercise Unsupervised exercise program. At an independent clinic, five physiotherapists experienced in knee rehabilitation gave individual instructions for the exercise programme. The exercise programme aimed to increase muscle function and postural control and was done twice a week for 3 months, unsupervised. Participants could exercise either at the gym or at home. Compliance was monitored with self‐reported exercise diaries. |
|
| Outcomes | Outcomes were measured at baseline, at 3 and 12 months, 3 years and 5 years of follow‐up. Primary outcome KOOS (Knee injury and Osteoarthritis Outcome Score) Pain subscale at 12 months, where scores range from 0 to 100 (100 indicates good knee function and less pain) Secondary outcomes
Outcomes included in this review at 3 and 12 months and at 5 years:
|
|
| Notes |
Funding: no funding source reported Trial registration: NCT01288768 Adverse events: Arthroscopic surgery Serious adverse events: No.(%): 3/66 (4.5%) Nature of event: two repeat arthroscopies in the surgery group ‐ one at 10 months and the other at 21 months after intervention. One participant died three years after surgery; it was not reported whether it was related to the intervention. Other adverse events: none reported Total adverse events: No.(%): 3/66 (4.5%) Exercise therapy Serious adverse events: none reported Other adverse events: none reported Total adverse events: none reported Knee surgery (replacement or osteotomy): not reported Progression of knee OA: radiographic deterioration from baseline to the 5‐year follow‐up, assessed according to the Kellgren‐Lawrence grade, occurred in 33/55 (60%) of the arthroscopy group and 10/27 (37%) of the exercise group. Withdrawals: no withdrawals were reported in this study; however, 9/75 in the arthroscopy group and 18/75 in the exercise group did not complete the 3‐month analysis; 5/75 in the arthroscopy group and 15/75 in the exercise group did not complete the 12‐month analysis; 9/75 in the arthroscopy group and 39/75 in the exercise group did not complete the 5‐year questionnaire and 20/75 in the arthroscopy group and 48/75 in the exercise group did not complete the 5‐year weight‐bearing radiographs. Reasons for lack of outcome data were not reported by group. Data analysis: ITT data were used for 3‐ and 12‐month follow‐up but only as‐treated data were reported at 5‐year follow‐up for outcomes: pain (Analysis 2.1), function (Analysis 2.2) and knee‐specific and generic quality of life (Analysis 2.3; Analysis 2.4) and participant reported success (Analysis 2.5). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation process provided |
| Allocation concealment (selection bias) | Low risk | "The orthopaedic surgeon who enrolled and assessed participants was blinded to the allocation sequence. The allocations were placed in sequentially numbered, opaque, sealed envelopes in 15 blocks, block size 10" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded to group assignments. Blinding of physiotherapists was attempted but some participants revealed their group. Surgeons were not blinded |
| Blinding of outcome assessor Self‐reported outcomes | High risk | Participants were not blinded to group assignments; thus, there was a risk of bias in the measurement of pain, function, knee‐specific quality of life, generic quality of life |
| Blinding of outcome assessor Assessor‐reported outcome (knee replacement) | Low risk | It is unclear whether outcome assessors checking electronic medical files for adverse events at 12 months were blind to group allocation, but we judged outcome measurement unlikely to be influenced by lack of blinding. Blinding of radiologist assessing X‐rays not reported. Blinding of surgeon assessing X‐rays not done. Blinding of physiotherapists assessing functional tests (not used in our analysis) attempted but broken |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/75 in the arthroscopy group and 18/75 in the exercise group did not complete the 3‐month analysis; 5/75 in the arthroscopy group and 15/75 in the exercise group did not complete the 12‐month analysis; 8/74 in the arthroscopy group and 36/72 in the exercise group did not complete the 5‐year analysis (4 participants underwent surgery in the affected knee and were excluded from the study after 1 year). In the 3‐month assessment, 14 participants (per group information was not given) completed the questionnaire at 5 months and these data were excluded |
| Selective reporting (reporting bias) | High risk | Authors have not collected or reported Tegner activity scale and have collected but not reported KOOS total score (secondary outcomes in the trial registration form) |
| Other bias | Low risk | No other bias apparent |
Herrlin 2007.
| Study characteristics | ||
| Methods |
Study design: single centre, parallel‐group, two‐arm, randomised, controlled trial Setting: Capio Artro Clinic, Stockholm Sports Trauma Research Center, Sweden Trial time period: participants were enrolled between June 2003 and April 2005 Interventions: arthroscopic surgery plus supervised exercise versus supervised exercise Sample size calculations: sample size calculations reported that 40 participants per group were needed to detect an average difference of 10 points in the KOOS with 80% power. P = 0.05 was considered to be statistically significant. Analysis: intention‐to‐treat |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Arthroscopy (N = 47)
Exercise (N = 49)
Pre‐treatment group differences: there were no differences in baseline characteristics between the two groups |
|
| Interventions |
Arthroscopic surgery
Arthroscopic partial meniscectomy plus supervised exercise. Arthroscopy was performed on an outpatient basis by two experienced surgeons, majority under local anaesthesia. A 5.5 mm, 30, arthroscope was used with a pressure‐controlled irrigation system. A standard operation protocol was used to document possible findings in cartilage, ligaments, synovium and the medial and lateral meniscus. Meniscal lesions were registered and changes in the articular cartilage were classified according to the Outerbridge classification: grade 0 = intact articular surfaces, grade I = softening of the surfaces, grade II = partial‐thickness defects less than 1.5 cm, grade III = partial‐thickness tears greater than 1.5 cm/fragmentation, grade IV = exposed bone. Twice a week during a period of 8 weeks, each participant followed a standardised exercise programme which consisted of exercises for improving muscle strength and endurance, muscle flexibility, balance and proprioception. The goal of the exercise programme was to reduce pain, restore full ROM and improve knee function. The participants were informed to exert the exercises with some strain but perform them almost pain‐free and without having any negative influence in the affected knee at the following day. If the participant could tolerate the exercises without any problems, he/she performed the exercises with increasing weights and higher resistance. Exercise Supervised exercise programme. Each participant followed a standardised exercise programme with the possibility for individual adaptation twice a week for a period of 8 weeks, as described above. |
|
| Outcomes | Outcomes were measured at baseline and at 2, 6, 12, 24 and 60 months' follow‐up (the published papers reported at 2, 6, 24 and 60 months; author supplied 12‐month data upon request). Outcomes
Outcomes used in this review at 2 and 6 months, 2 and 5 years
|
|
| Notes |
Funding: not reported Trial registration: not done Adverse events: Arthroscopic surgery Serious adverse events: No. (%): 3/47 (6.4%) Three participants had an additional arthroscopy between 13 and 40 months after the initial operation. Re‐arthroscopy showed degenerative articular changes. Other adverse events: 0/47 (0%) Total adverse events: 3/47 (6.4%) 3 participants from the arthroscopy group had an additional arthroscopic procedure between 13 and 40 months following the original surgery. Exercise Serious adverse events: 13/49 (26.5%) 13 participants presented with medial meniscal tears on arthroscopic examination and were treated with partial meniscectomy. Other adverse events: 0/49 (0%) Total adverse events: 13/49 (26.5%) Cross‐overs: 13 participants from the exercise group had an arthroscopy at an average of 6.5 months after the intervention. Knee surgery (replacement or osteotomy): not reported Progression of knee OA: 2/43 participants from the arthroscopy group and 2/45 participants from the exercise group who had radiographic examination at 60 months after the intervention had progression of knee osteoarthritis in the medial compartment. In three cases, progression was from grade 1 to grade 2 and in one case from grade 1 to grade 3 (the authors did not specify which treatment group the latter participant was from) Withdrawals: none Data analysis: KOOS Pain, KOOS ADL and KOOS QoL were extracted at 2, 6, 12 and 24 months based on data supplied by the author upon request; progression of knee osteoarthritis was extracted from published paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation process provided |
| Allocation concealment (selection bias) | Unclear risk | There was no reporting of allocation concealment in this study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and study personnel was not done |
| Blinding of outcome assessor Self‐reported outcomes | High risk | As blinding of participants was not done, there is a risk of bias in the measurement of knee pain and function |
| Blinding of outcome assessor Assessor‐reported outcome (knee replacement) | Low risk | No blinding of study personnel but the assessor‐reported outcomes, adverse events and progression of knee OA based on radiographic examination, are unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who completed baseline measurements completed follow‐up assessments at 8 weeks and 6 months. 4 participants were lost to follow‐up at 24 months (1 from arthroscopy group and 3 from exercise group) and 4 participants were lost to follow‐up at 60 months (2 from arthroscopy group and 2 from exercise group) |
| Selective reporting (reporting bias) | High risk | Trial registration not done. 12‐month outcomes were not reported in the trial publications |
| Other bias | Low risk | No other bias apparent |
Katz 2013.
| Study characteristics | ||
| Methods |
Study design: multicentre, parallel‐group, two‐arm, randomised, controlled trial Setting: seven US tertiary referral centres ‐ Brigham and Women’s Hospital, Boston; Hospital for Special Surgery, New York; Cleveland Clinic, Cleveland; Vanderbilt University, Nashville; Mayo Clinic, Rochester; Rush University Medical Center, Chicago; Washington University, St. Louis, USA Trial time period: participants were enrolled between June 2008 and August 2011 Interventions: arthroscopic surgery plus supervised physical therapy versus supervised physical therapy Sample size calculations: the study was powered to detect a 10‐point difference on the WOMAC functional scale between the operative and non‐operative arms, The study team adopted a Type I error rate of 5% and power of 80% and the target sample size was set at 340 participants. The sample size calculation took into account two sources of sample degradation: losses to follow‐up and cross‐over from the assigned arm to the other arm prior to the primary outcome assessment at six months. The study was also powered for one pre‐planned subgroup analysis in which participants with Kellgren‐Lawrence (KL) Grade 3 (joint space narrowing) would be analysed in one subgroup and those with KL Grades 0 to 2 in the other. Analysis: outcome analysis only included participants who did not withdraw from the study. |
|
| Participants |
Number of participants
Criteria for defining knee osteoarthritis with meniscal tear: symptomatic participants 45 years of age or older with a meniscal tear and evidence of mild‐to‐moderate osteoarthritis on imaging Inclusion criteria.
Exclusion criteria
Baseline characteristics Arthroscopic Partial Meniscectomy (N = 161)
Physical Therapy (N = 169)
Pre‐treatment group differences: no differences were reported between the two groups. |
|
| Interventions |
Arthroscopic surgery Arthroscopic partial meniscectomy plus physical therapy. The damaged meniscus was trimmed back to a stable rim. Loose fragments of cartilage and bone were removed without any penetration of the subchondral bone. Preoperative antibiotics were used routinely. Postoperatively, participants were allowed to bear weight as they were able. Bracing was not used. Participants were referred to a physical therapist for a postoperative standardized physical therapy program, as described below. Exercise: Supervised physical therapy provided to both groups. Phase I‐Acute Phase (1‐10 days post‐op) 8 exercises, 12‐15 repetitions, 1‐2 sets of the following types of exercises:
Phase II‐Subacute Phase (10 days ‐ 4 weeks post‐op) Participant must meet 3 of the 4 criteria: Knee A/PROM 0>=115 degrees, moderate to minimal effusion, knee Pain = 3/5) 8 exercises, 12‐15 repetitions, 1‐2 sets of the following types of exercises:
Phase III‐Advanced Activity Phase (4‐7 weeks post‐op) 8 exercises, 12‐15 repetitions, 1‐2 sets of the following types of exercises:
In both the arthroscopic partial meniscectomy plus physical therapy and physical therapy alone groups, participants were permitted to receive acetaminophen and non‐steroidal anti‐inflammatory agents as needed, and intra‐articular injections of glucocorticoids were permitted over the course of the trial. |
|
| Outcomes | Outcomes were measured at baseline and at 3 and 6 months and every 6 months thereafter up to 5 years follow‐up (protocol states outcomes measured at baseline, 3, 6, 12, 18 and 24 months, but data in published papers report outcomes at baseline, 6 and 12 months and up to 5 years. Authors did not respond to requests for data). Primary outcome Physical function on the physical‐function scale of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) assessed at 6 months. Scores range from 0 to 100, with higher scores indicating worse physical function Secondary outcomes
Outcomes included in this review at 3, 6 and 24 months and 5 years
|
|
| Notes |
Funding: this study was funded by grants (R01AR055557, K24AR057827, and P60AR047782) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health. Trial registration: ClinicalTrials.gov number, NCT00597012 (METEOR study) Adverse Events Arthroscopic partial meniscectomy (APM): Serious adverse events ‐ no. (%)
Total serious adverse events ‐ 8/164 (4.9%) Other adverse events ‐ no. (%)
Total other adverse events ‐ 15 (8.62%) Total ALL adverse events ‐ 23/164 (14.0%) Physical therapy (PT): Serious adverse events ‐ no. (%)
Total serious adverse events ‐ 5/109 (4.59%) Other adverse events ‐ no.(%)
Total other adverse events ‐ 13 (7.34%) Total ALL adverse events ‐ 18/109 (16.5%) There was no group differences in the frequency of adverse events. Knee surgery (replacement or osteotomy) (12 months' follow‐up): Arthroscopic Partial Meniscectomy: No. of participants = 5 (2.8%) Physical Therapy: No. of participants = 3 (1.7%) Knee surgery (replacement or osteotomy) (5 years' follow‐up): Randomised to and receiving Arthroscopic Partial Meniscectomy: No. of participants = 16/164 (9.8%) Cross‐overs from Physical Therapy to Arthroscopic Partial Meniscectomy: No. of participants = 7/68 (10.3%) Randomised to and receiving Physical Therapy: No. of participants = 2/109 (1.8%) Progression of knee OA: not reported Withdrawals: 7/174 from APM group and 4/177 from physical therapy group at 6 months and 9/174 from the APM group and 7/177 from physical therapy group at 12 months Report of study results: Attempts to obtain KOOS Pain and WOMAC PF 5‐year follow‐up data from authors were unsuccessful. For total knee replacement, adverse events and serious adverse events, we reported 109 in the PT group (i.e. those who did not cross over to APM). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was done using a secure program on the trial website" in blocks of varying size within each site, stratified by the extent of osteoarthritis at baseline (Kellgren‐Lawrence grade 0–2 (no joint space narrowing) versus Kellgren‐Lawrence grade 3 (< 50% joint space narrowing). Probably low risk |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by a research coordinator in real time using a secure website, thus ensuring concealment until the time of allocation to treatment group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Our study was not blinded, since our investigative group did not consider a sham comparison group feasible" |
| Blinding of outcome assessor Self‐reported outcomes | High risk | As participants were aware of their treatment, assessment of self‐reported outcomes including WOMAC and KOOS were at risk of detection bias |
| Blinding of outcome assessor Assessor‐reported outcome (knee replacement) | High risk | Radiographs of the knee were assessed by surgeons (who were aware of the treatment assignment) as well as musculoskeletal radiologists, whose knowledge of treatment assignment is not reported. KL grading and assessment of radiographs are subject to bias as blinding of outcome assessors was not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The proportion of loss to follow‐up and reasons for incomplete outcome data are similar between groups |
| Selective reporting (reporting bias) | High risk | SF‐36 5‐item mental health index and EQ‐5D were measured, according to the protocol paper, but were not listed as outcomes in the trial registration form. No results data for these outcomes are available |
| Other bias | Unclear risk | 33.8% (60/177) of participants assigned to physical therapy crossed over to arthroscopy within 6 months of randomisation, but their outcome data for pain, function and treatment success was included in the analysis for the physical therapy group potentially underestimating any effect of surgery |
Kirkley 2008.
| Study characteristics | ||
| Methods |
Study design: single centre, parallel‐group, two‐arm, randomised, controlled trial Setting: Fowler Kennedy Sport Medicine Clinic, University of Western Ontario, London, Ontario, Canada Trial time period: participants were enrolled between January 1999 and August 2007 Interventions: arthroscopic surgery plus optimised physical and medical therapy, home exercises and arthritis education versus optimised physical and medical therapy, home exercises and arthritis education Sample size calculations: assignment of 186 participants to treatment would provide 80% statistical power to detect a 200‐point difference in the WOMAC score between the two treatment groups with allowance of 15% of participants whose data cannot be evaluated Analysis: intention‐to‐treat analysis was conducted |
|
| Participants |
Number of participants
Criteria for defining knee osteoarthritis: participants with idiopathic or secondary osteoarthritis of the knee with grade 2, 3, or 4 radiographic severity, as defined by the modified Kellgren–Lawrence classification Inclusion criteria
Exclusion criteria
Baseline characteristics Arthroscopic Surgery (N = 92)
Physical and Medical Therapy (N = 86)
Pre‐treatment group differences: participants in the surgery group had slightly higher total WOMAC scores compared to those in the physical and medical therapy group. Other baseline characteristics were similar across the two groups. |
|
| Interventions |
Arthroscopic surgery: Arthroscopic surgery plus optimised physical and medical therapy, home exercises and arthritis education. Arthroscopic debridement was performed under general anaesthesia. Irrigation of the medial, lateral, and patellofemoral joint compartments was done using 1 litre of saline. One or more of the following procedures was done ‐ synovectomy; debridement; or excision of degenerative tears of the menisci, fragments of articular cartilage, or chondral flaps and osteophytes. Optimised physical and medical therapy was initiated within 7 days after surgery and followed an identical program in both groups. Exercise: Physical and medical therapy, home exercises and arthritis education provided to both groups. Physical therapy was provided for 1 hour once a week for 12 consecutive weeks. Information regarding a home exercise program that emphasised range‐of‐motion and strengthening exercises was provided to all participants. These exercises were done twice daily and once on the day of a scheduled physical‐therapy session. After 12 weeks of supervised activity, participants continued an unsupervised exercise program at home for the duration of the study. The participants received additional education from attendance at local Arthritis Society workshops, from a copy of The Arthritis Helpbook that was provided to them, and from an educational videotape. The medical therapy for both groups involved the administration of intra‐articular injection of hyaluronic acid, oral glucosamine and step‐wise use of acetaminophen and non‐steroidal anti‐inflammatory drugs. |
|
| Outcomes | Outcomes were measured at baseline and at 3, 6, 12, 18 and 24 months of follow‐up Primary outcome The total Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score (range, 0 to 2400; higher scores indicate more severe symptoms and poorer physical function) at 2 years of follow‐up. Secondary outcomes
Outcomes used in this review at 3, 6 and 24 months
|
|
| Notes |
Funding: this study was sponsored by the Fowler Kennedy Sport Medicine Clinic and supported by the Canadian Institutes of Health Research. Trial registration: Clinical trials number NCT00158431 Adverse events: unclear if measured; not reported Knee surgery (replacement or osteotomy): not reported Progression of knee OA: not reported Withdrawals: 6/94 in the arthroscopy group and 14/94 in the physical and medical therapy group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned using a computer‐generated schedule. |
| Allocation concealment (selection bias) | Low risk | To minimise the risk of predicting the treatment assignment of the next eligible participant, randomisation was performed in permuted blocks of two or four with random variation of the blocking number. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study staff was done ‐ "To preserve blinding, each patient wore a neoprene sleeve over the knee so that the study nurse could not identify a surgical scar". Blinding of participants was not done. |
| Blinding of outcome assessor Self‐reported outcomes | High risk | As blinding of participants was not done, there could be bias in the assessment of pain, stiffness, disability, quality of life and functional status. |
| Blinding of outcome assessor Assessor‐reported outcome (knee replacement) | Low risk | "At each visit, the patients were evaluated by a nurse who was unaware of the treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6/94 (6%) (withdrawal of consent ‐ 2; withdrawal from study ‐ 1; death ‐ 1; loss to follow‐up ‐ 2) in the arthroscopy group and 14/94 (15%) (withdrawal of consent ‐ 8; loss to follow‐up ‐ 6) in the physical and medical therapy group did not complete the study |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the trial registration and protocol have data reported in the results publication. |
| Other bias | Low risk | No other bias apparent |
Kise 2016.
| Study characteristics | ||
| Methods |
Study design: multicenter, parallel‐group, two‐arm, randomised controlled trial Setting: orthopaedic departments at two public hospitals and two physiotherapy clinics in Norway Trial time period: participants were enrolled between October 2009 and September 2012 Interventions: arthroscopic surgery versus supervised exercise Sample size calculations: the sample size calculation was based on the change in KOOS4 (defined as the average score for 4/5 KOOS subscale scores) from baseline to 2‐year follow‐up. To detect a 10‐point difference with a standard deviation of 15, with a level of power of 90%, level of significance of 0.05, and an estimated 15% dropout rate at two years, 56 participants were required in each group. To factor in a 20% cross‐over rate, 140 participants were recruited. Analysis: intention‐to‐treat |
|
| Participants |
Number of participants
Definition of degenerative meniscal tear: degenerative meniscal tear was defined as an intrameniscal linear magnetic resonance imaging signal penetrating one or both surfaces of the meniscus. Inclusion criteria
Exclusion criteria
Baseline characteristics Arthroscopic partial meniscectomy (N = 70)
Supervised exercise therapy (N = 70)
Pre‐treatment group differences: there were no obvious differences in baseline characteristics between the groups. |
|
| Interventions |
Arthroscopic surgery Arthroscopic partial meniscectomy. Six orthopaedic surgeons with at least 10 years of clinical experience performed the operations. Surgery was performed with the participant under general anaesthesia, with or without thigh tourniquet, antibiotic prophylaxis, or antithrombotic prophylaxis. Arthroscopes with 30 degree optics and standard arthroscopic instruments were used. Ringer acetate was used for lavage. Normal procedure involved two portals: anteromedial and anterolateral. Additional injuries (ligaments, cartilage) preceded systematic probing of both menisci, and finally, all unstable meniscal tissue was resected. The participants were discharged from hospital on the day of surgery and were advised to use two crutches post‐operatively until gait normalised and no swelling or discomfort occurred during weight bearing. Before hospital discharge, the participants were given written and oral instructions for simple home exercises, aimed at regaining knee range of motion and reducing swelling. They were encouraged to perform the exercises two to four times daily. Exercise The supervised exercise therapy programme consisted of progressive neuromuscular and strength exercises over 12 weeks, performed during a minimum of two and a maximum of three sessions each week (24 to 36 sessions). Each session lasted approximately 60 to 80 minutes. About 20 minutes was spent warming up and cooling down on a stationary cycle, 20 to 30 minutes was spent on neuromuscular exercise, and 20 to 30 minutes was spent on strength training. |
|
| Outcomes | Outcomes were measured at baseline and at 3, 12 and 24 months and 5 years of follow‐up. Primary outcomes
Secondary outcomes
Outcomes included in this review at 3 and 24 months and 5 years
|
|
| Notes |
Funding: this study was funded by Sophies Minde Ortopedi AS, Swedish Rheumatism Association, Swedish Scientific Council, Region of Southern Denmark, Danish Rheumatism Association, and the Health Region of South‐East Norway. Trial Registration: www.clinicaltrials.gov (NCT01002794) Adverse events: Arthroscopic partial meniscectomy Serious adverse events
Other adverse events
Total ALL adverse events: 31/64 (48.4%) Supervised exercise therapy Serious adverse events
Other adverse events
Total ALL adverse events: 31/60 (51.7%) Knee surgery (replacement or osteotomy) (5 year follow‐up): 1 participant in the surgery group received a total knee arthroplasty 34 months after the index surgery. 2 participants (one allocated to surgery and one cross‐over) received an osteotomy 4 to 6 months after the index procedure Progression of knee OA: 10/58 (17.2%) participants from the exercise group and 13/62 (21.0%) participants from the arthroscopy group had radiographic knee OA consistent with grade 2 or more on the Kellgren‐Lawrence classification at five years. Withdrawals: 6/70 in the arthroscopic partial meniscectomy group and 8/70 in the supervised exercise therapy group Cross‐overs: in the final report (Berg 2020, secondary publication of Kise 2016), 14/70 crossed over from exercise to arthroscopy group in contrast to earlier report (Kise 2016) which reported 13/70 cross‐overs. Report of study results: 3‐month study findings reported in Stensrud 2015 (secondary publication of Kise 2016). Data analysis: the authors supplied outcome data for knee‐related quality of life measured on KOOS QoL subscale (0 to 100, higher score = better) upon request |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence was used. |
| Allocation concealment (selection bias) | Low risk | The allocations were kept in sequentially‐numbered, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded to the group assignments; however, strength and function test assessors were blinded. |
| Blinding of outcome assessor Self‐reported outcomes | High risk | Participants were not blinded to group assignments, thus, there was a risk of bias in the measurement of pain, function and knee‐related quality of life. |
| Blinding of outcome assessor Assessor‐reported outcome (knee replacement) | Low risk | The outcome assessors were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8/70 in the exercise group and 6/70 in the arthroscopic group did not have outcome data (questionnaires not returned). |
| Selective reporting (reporting bias) | Unclear risk | One outcome (incident and enlarging marginal tibiofemoral osteophytes at 5 years) was pre‐specified as a co‐primary outcome but moved to secondary outcome and redefined as progression of both osteophytes and tibiofemoral joint space narrowing separately at 5 years. The rationale for the change was provided (to describe radiographic changes in knee osteoarthritis development) and the change was made before 5‐year data collection and analysis. Additional secondary outcomes that were not pre‐specified were reported (e.g. total radiographic progression score, severity of knee OA using modified Kellgren‐Lawrence classification, adverse events, knee surgery). Although the paper did not publish the KOOS summary data for each treatment group, the authors provided them upon request and they were used in the analysis. |
| Other bias | Low risk | No other bias apparent |
Merchan 1993.
| Study characteristics | ||
| Methods |
Study design: single centre, parallel‐group, two‐arm, open‐label, randomised controlled trial Setting: participants recruited from outpatient clinic of an orthopaedic hospital in Spain Trial time period: January 1988 to December 1990 Interventions: arthroscopic surgery plus physiotherapy versus non‐steroidal anti‐inflammatory drugs (NSAIDs) plus physiotherapy Sample size calculations: a priori sample size calculation not described Analysis: intention‐to‐treat |
|
| Participants |
Number of participants Number of participants screened: not reported Number of participants at enrolment: not reported Number randomised: 80: 40 to arthroscopic surgery and physiotherapy group and 40 to non‐steroidal anti‐inflammatory drugs and physiotherapy group Number included in the analysis: data for 35/40 (87.5%) for the arthroscopic surgery and physiotherapy group and 38/40 (95%) for the non‐steroidal antiinflammatory drugs and physiotherapy group were available at the 1‐, 2‐ and 3‐year follow‐ups Inclusion criteria
Exclusion criteria
Baseline characteristics Arthroscopic surgery and physiotherapy group (N = 35) Mean (range) age: 57.1 (50 to 63) years No. of men/women: 7/28 Mean Inital Knee Score (IKS) calculated using the modified Hospital for Special Surgery Knee Rating Score: 26.85 Mean Final Knee Score (FKS) calculated using the modified Hospital for Special Surgery Knee Rating Score: 37.00 Mean Knee Score Difference (KSD = FKS‐IKS): 10.14 Non‐steroidal anti‐inflammatory drugs and physiotherapy group (N = 38) Mean (range) age: 56.9 (50 to 65) years No. of men/women: 13/25 Mean IKS: 29.86 Mean FKS: 32.76 Mean KSD: 2.89 Pre‐treatment group differences: the initial knee score (IKS), age and sex did not show statistical differences between the two groups. |
|
| Interventions |
Arthroscopic surgery Arthroscopic surgery and physiotherapy. The surgical technique included debridement of synovial tissue; removal of degenerative menisci, osteophytes, and loose bodies; and limited debridement of cartilage defects. Osseous debridement to bleeding bone (abrasion arthroplasty) was not performed. The mean operative time was 50 minutes (range 35 to 70). Post‐operatively, a compression bandage was used with early exercises, motion, and weight bearing as tolerated. The operative findings of articular cartilage changes were graded according to Outerbridge (Outerbridge 1961). Superficial fibrillation was present in 8 participants, fragmentation of < 1.3cm2 in 16, fragmentation of > 1.3cm2 in 7, and eburnation to subchondral bone in 4. There were meniscal tears in 31 of the 35 knees with a ratio of medial to lateral of 4 to 1. The most common tear was a flap of the posterior horn of the medial meniscus. Osteophytes were excised from the intercondylar notch in 6 knees, loose bodies were removed in 7, and 4 were noted to have chondrocalcinosis. Physiotherapy was practiced for 4 weeks after surgery. The physiotherapy regimen included quadriceps exercises and knee flexion exercises immediately postoperatively. Non‐steroidal anti‐inflammatory drugs Non‐steroidal anti‐inflammatory drugs and physiotherapy. The non‐operative treatment consisted of non‐steroidal anti‐inflammatory drugs and a decrease in the intensity of the activities of daily living for a pain‐free knee. Physiotherapy was practiced as in the operative group (i.e. quadriceps and knee flexion exercises for 4 weeks). This non‐operative group had no further courses of treatment during the follow‐up period. |
|
| Outcomes | The mean follow‐up time was 25 months (range 12 to 36) in the arthroscopic surgery and physiotherapy group and 23 months (range 12 to 36) in the non‐steroidal anti‐inflammatory drugs and physiotherapy group. Outcomes
Outcomes used in this review at mean of 23 to 25 months
Measures of variability for function outcomes using mHSSKRS were not reported so could not be used in this review. |
|
| Notes |
Funding: Fundacion Caja de Madrid Trial registration: not done Adverse events: Arthroscopic surgery and physiotherapy group Serious adverse events: Death: 5/40 (12.5%) participants died after randomisation and were excluded from follow‐up. No further information provided. Deep vein thrombosis: 2/40 (5%) Other adverse events: Superficial infection: 1/40 (2.5%) Haemarthrosis: 1/40 (2.5%) Total ALL adverse events: 9/40 (22.5%) Non‐steroidal anti‐inflammatory drugs and physiotherapy group Serious adverse events: Death: 2/40 (5%) participants died after randomisation and were excluded from follow‐up. No further information provided. No other serious adverse events reported Other adverse events: none reported Total ALL adverse events: 2/40 (5%) Knee surgery (replacement or osteotomy): not reported Progression of knee OA: not reported Withdrawals: 5/40 in the arthroscopic surgery and physiotherapy group and 2/40 in the non‐steroidal anti‐inflammatory drugs and physiotherapy group due to death |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation process provided. There were some baseline differences in outcome measures between the treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | Allocation performed by "pulling consecutively numbered envelopes that had previously been randomly placed on a bulletin board". Unclear if sealed or opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided. Probably not done |
| Blinding of outcome assessor Self‐reported outcomes | High risk | No information provided. Probably not done. Measurement of mHSSKRS subjective score likely to be influenced by lack of blinding |
| Blinding of outcome assessor Assessor‐reported outcome (knee replacement) | High risk | No information provided. Probably not done. Measurement of mHSSKRS objective score and treatment success likely to be influenced by lack of blinding. Adverse events unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imbalance in withdrawals across groups: 12.5% (5/40) due to death in the arthroscopic surgery plus physiotherapy group vs. 5% (2/40) due to death in the NSAIDs plus physiotherapy group. The cause of deaths in both groups were not reported. |
| Selective reporting (reporting bias) | Unclear risk | Trial registration not done and protocol not available. Insufficient information to judge high or low risk |
| Other bias | Low risk | No other bias apparent |
Moseley 1996.
| Study characteristics | ||
| Methods |
Study design: single centre, three‐arm, double‐blind, randomised, placebo‐controlled trial Setting: Houston Veterans Administration (VA) Medical Center, Texas, USA Trial time period: June 1992 Interventions: arthroscopic debridement versus arthroscopic lavage versus placebo surgery Sample size calculations: a priori sample size calculation was not done Analysis: statistical analysis not done |
|
| Participants |
Number of participants Number of participants screened for eligibility: not reported Number of participants enrolled: 10 Number randomised: 10 participants: 5 participants were randomised to the placebo group, 3 were randomised to the arthroscopic lavage group and 2 to the arthroscopic debridement group Number included in analysis: a statistical analysis was not performed because of the small number of subjects and responses. However, data were available from all 10 participants at 2 and 6 weeks and from 9 participants at 3 and 6 months and reported as means. Inclusion criteria
Exclusion criteria Not reported Baseline characteristics Arthroscopic debridement (N = 2) Mean intensity of worst knee pain on a scale where 1 = no pain, 10 = severe pain: 9.0 Mean average intensity of knee pain on a scale where 1 = no pain, 10 = severe pain: 7.0 Mean intensity of today's pain on a scale where 1 = no pain, 10 = severe pain: 6.0 Number of days this week with knee pain: 6 Average knee extension (negative values refer to flexion contracture): ‐12.0 Average knee flexion: 111 Average knee crepitus on a scale: 0 = none, 3 = severe: 1.5 Average knee effusion on a scale: 0 = none, 3 = severe: 0 Average global knee tenderness on a scale: 0 = none, 9 = severe: 5.0 50‐foot walk in seconds: 12.0 Arthroscopic Lavage (N = 3) Mean intensity of worst knee pain on a scale where 1 = no pain, 10 = severe pain: 8.5 Mean average intensity of knee pain on a scale where 1 = no pain, 10 = severe pain: 7.5 Mean intensity of today's pain on a scale where 1 = no pain, 10 = severe pain: 5.5 Number of days this week with knee pain: 6 Average knee extension (negative values refer to flexion contracture): ‐5.0 Average knee flexion: 105 Average knee crepitus on a scale: 0 = none, 3 = severe: 1.5 Average knee effusion on a scale: 0 = none, 3 = severe: 1.0 Average global knee tenderness on a scale: 0 = none, 9 = severe: 4.0 50‐foot walk in seconds: 11.0 Placebo surgery (N = 5) Mean intensity of worst knee pain on a scale where 1 = no pain, 10 = severe pain: 8.4 Mean average intensity of knee pain on a scale where 1 = no pain, 10 = severe pain: 6.8 Mean intensity of today's pain on a scale where 1 = no pain, 10 = severe pain: 6.8 Number of days this week with knee pain: 5.6 Average knee extension (negative values refer to flexion contracture): ‐5.2 Average knee flexion: 125 Average knee crepitus on a scale: 0 = none, 3 = severe: 1.0 Average knee effusion on a scale: 0 = none, 3 = severe: 0 Average global knee tenderness on a scale: 0 = none, 9 = severe: 2.8 50‐foot walk in seconds: 11.5 Pre‐treatment group differences: there were no obvious differences in baseline characteristics between the three groups except for greater flexion contracture in the debridement group compared to the other two groups. |
|
| Interventions | The arthroscopic debridement and arthroscopic lavage groups both received a standard general endotracheal anaesthetic that was routinely used at the medical centre. The placebo arthroscopy group received a lesser anaesthetic, an intravenous tranquilliser (benzodiazepine, droperidol, or both) in conjunction with an opioid (fentanyl or sufentanil), without having placement of an endotracheal tube. This combination of drugs, along with the local anaesthetic injected at the stab wound sites, sedated the participant and rendered them insensitive to pain. The placebo participants breathed spontaneously throughout the procedure, and they inhaled oxygen supplemented by a nasal cannula. End‐tidal carbon dioxide was continuously monitored throughout the procedure. Using this type of anaesthesia for the placebo group minimised the potential complications from induction of general anaesthesia and placement of an endotracheal tube. Once anaesthetised, all participants had their knees examined and then prepared and draped in the usual manner. All participants had a gram of cephalosporin antibiotic administered intravenously as prophylaxis against infection. Bupivacaine (0.25%) with epinephrine was then injected into the skin where the arthroscopy portals were to be made. Arthroscopic debridement The arthroscopic debridement participants had three stab wounds made and an arthroscope inserted in the inferolateral portal; an inflow cannula was inserted in the superomedial portal, and the various operating instruments were inserted from the inferomedial portal. The knee was distended with sterile saline from the inflow cannula, and a constant flow of fluid was lavaged through the knee as is typical for arthroscopic surgery. A minimum of 10 litres of fluid was lavaged through the knee. After a diagnostic arthroscopic examination, the arthroscopic instruments were used to shave the rough articular cartilage (chondroplasty), remove loose debris, trim torn or degenerated meniscal fragments, and correct any other soft tissue abnormalities that could interfere with the mechanical function of the knee. However, no abrasion arthroplasty or removal of bone spurs was performed. At the end of the procedure, the instruments were removed, the portals were closed with absorbable suture, and a sterile compression dressing was applied. The average time for the surgery was 45 minutes. Arthroscopic lavage The arthroscopic lavage participants had a procedure identical to that of the debridement participants, except that no operating instruments were used to remove or trim the various parts of the knee. A diagnostic arthroscopic examination was performed, and a minimum of 10 litres of fluid was lavaged through the knee. A minimum of 30 minutes was spent performing the lavage procedure, and the total time spent in the operating room was approximately 1 hour. Placebo surgery Participants undergoing placebo arthroscopy were prepared, draped, examined and injected with local anaesthetic in the same manner as the other two groups. Three stab wounds were made in the skin with a scalpel, but no instruments of any kind were placed into the knee. The knee was manipulated, instruments were requested and passed, saline was splashed, and a standard arthroscopic debridement was simulated as closely as possible in the event the participant was not totally unaware during the event. A minimum of 30 minutes was spent performing the placebo surgery, and the typical time spent in the operating room was 1 hour. Participants in all three groups spent approximately an hour in the operating theatre. All participants were taken to the recovery room and treated the same as any participant having any arthroscopic procedure. Once the participant returned to the orthopaedic ward from the recovery room, he/she was observed until stable enough to be sent home. All participants were discharged from the hospital the afternoon of surgery or the next morning with an oral narcotic analgesic (typically acetaminophen with codeine) for use as required. Before discharge, participants were fitted with crutches and instructed to discontinue using them as soon as they could walk comfortably without a limp. At the first post‐operative visit, participants were instructed to resume their pre‐operative anti‐inflammatory medications and to resume walking and other activities of daily living as soon as their symptoms would allow. |
|
| Outcomes | Outcomes were measured at 2 and 6 weeks, and 3 and 6 months. Study outcomes
The subjective data (pain, mobility, general well‐being, satisfaction with surgery) were collected from a questionnaire designed specifically for this study (not included in trial report). The questionnaire was reported to be based on the AIMS‐2, the SF‐36, the Wisconsin Brief Pain Questionnaire and the Knee Society's Knee Rating Scale. Outcomes used in this review at 3 and 6 months
|
|
| Notes |
Funding: not reported Trial registration: not reported Adverse events: unclear if measured; not reported Knee surgery (replacement or osteotomy): not reported Progression of knee OA: not reported Withdrawals: 1/10 in total. 1/2 (50%) from the arthroscopic debridement group Analysis: we included arthroscopic debridement versus placebo and we excluded the lavage treatment arm from this review. Data imputations: SDs for pain were not reported at 3 and 6 months (and no baseline values were reported). We used SD values from Sihvonen 2013 for pain at 3 and 6 months for this study in Analysis 2.1 as Sihvonen and colleagues also used a pain numerical rating scale. For satisfaction with surgery, we assumed dropouts (one in arthroscopic debridement group) were unsatisfied and used the number randomised as the denominator. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation process given, other than stating it was randomised. "An unbalanced randomization scheme was used to determine if physician and patient blinding could truly be maintained in the placebo surgery group." |
| Allocation concealment (selection bias) | Unclear risk | Sealed 'randomisation envelopes' were used, which were opened in the operating theatre, to reveal which procedure the participant was to receive. Insufficient information provided about whether appropriate safeguards were used (e.g. opaque, sequentially‐numbered envelopes) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and study personnel (other than the operating surgeon) were blinded to the treatment assignment group. Identical preparation, stab wounds and post‐operative care were performed in all groups so it is unlikely that participants could guess their group assignment. "All postoperative care was performed by orthopaedic residents, nurses and other personnel who were blinded to the type of treatment that the patient received." |
| Blinding of outcome assessor Self‐reported outcomes | Low risk | Blinding of participants was done; low risk of bias in the measurement of pain and satisfaction with surgery |
| Blinding of outcome assessor Assessor‐reported outcome (knee replacement) | Low risk | Knee replacement not measured |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1/2 (50%) participants withdrew from the arthroscopic debridement group and 0/5 withdrew from the placebo surgery group. The reason for withdrawal was not reported. |
| Selective reporting (reporting bias) | High risk | Trial registration not done and protocol not available. Data on mobility and general well‐being were reported as being collected in the methods but results not reported. Pain measured but outcome data not adequately reported (no measure of variance) |
| Other bias | Low risk | No other bias apparent |
Moseley 2002.
| Study characteristics | ||
| Methods |
Study design: single centre, three‐arm, randomised, placebo‐controlled trial Setting: Houston Veterans Affairs Medical Center, Houston, Texas, USA Trial time period: participants were enrolled from October 1995 through September 1998 Interventions: arthroscopic debridement versus arthroscopic lavage versus placebo surgery Sample size calculations: a total sample of 180 participants (60 participants in each arm) would provide 90% statistical power and a two‐sided type I error of 0.04 to detect a moderate effect size (0.55) between the placebo group and the combined arthroscopic‐treatment groups Analysis: no information on intention‐to‐treat analysis or analysis on data from those who did not complete the 24‐month follow‐up |
|
| Participants |
Number of participants
Criteria for defining knee osteoarthritis: the definition was according to the American College of Rheumatology. The severity of osteoarthritis in the study knee (that with the greatest pain‐induced limitation of function) was assessed radiographically and graded on a scale of zero to four. The scores for the three compartments were added together to generate a severity grade of 0 to 12. Inclusion criteria
Exclusion criteria
Baseline characteristics Arthroscopic debridement
Arthroscopic Lavage
Placebo Surgery
Pre‐treatment group differences: although baseline characteristics appeared to be similar across all three groups, the lavage group showed higher scores for depression compared to the other two groups and the use of prescription analgesics was lower in the debridement group. |
|
| Interventions | One orthopedic surgeon (board‐certified, fellowship‐trained in arthroscopy and sports medicine, in practice for 10 years in academic medical centre) performed all the operations. Post‐operative care was delivered according to a protocol specifying that all participants should receive the same walking aids, graduated exercise program and analgesics. The protocol was not provided in the publication or supplementary appendix. Arthroscopic debridement After diagnostic arthroscopy, the joint was lavaged with at least 10 litres of fluid, rough articular cartilage was shaved (chrondroplasty was performed), loose debris was removed, all torn or degenerated meniscal fragments were trimmed, and the remaining meniscus was smoothed to a firm and stable rim. No abrasion arthroplasty or microfracture was performed. Typically, bone spurs were not removed, but any spurs from the tibial spine area that blocked full extension were shaved smooth. Participants received standard general anaesthesia with endotracheal intubation. Arthroscopic lavage After diagnostic arthroscopy, the joint was lavaged with at least 10 litres of fluid. Anything that could be flushed out through arthroscopic cannulas was removed. Normally, no instruments were used to mechanically debride or remove tissue. However, if a mechanically important, unstable tear in the meniscus (e.g. a displaced “bucket‐handle” tear) was encountered, the torn portion was removed and the remaining meniscus was smoothed to a firm, stable rim. No other debridement was performed. Participants received standard general anaesthesia with endotracheal intubation. Placebo surgery Simulated debridement with three 1‐cm skin incisions but without insertion of the arthroscope. The surgeon asked for all instruments and manipulated the knee as if arthroscopy were being performed. Saline was splashed to simulate the sounds of lavage. No instrument entered the portals for arthroscopy. The participant was kept in the operating room for the amount of time required for a debridement. Participants received a short‐acting intravenous tranquilliser and an opioid and spontaneously breathed oxygen‐enriched air. Participants spent the night after the procedure in the hospital and were cared for by nurses who were unaware of the treatment‐group assignment. |
|
| Outcomes | Outcomes were measured at baseline and at 2 and 6 weeks, and at 3, 6, 12, 18 and 24 months after the procedure. Primary outcome Pain in the study knee assessed by a 12‐item self‐reported Knee‐Specific Pain Scale (KSPS) at 24 months' follow‐up. Scores range from 0 to 100 with higher scores indicating more severe pain. Secondary outcomes (measured at all time points)
Outcomes included in this review at 3 and 6 months and 2 years
NB. Adverse events could not be included as the details of the group to which the adverse events belonged were not reported. Quality of life outcome data could not be included as SF‐36 Mental Component Summary (MCS) scores were not reported. |
|
| Notes |
Funding: supported by a grant from the Department of Veterans Affairs Trial registration: not reported Serious adverse events: none reported Other adverse events
Details of the group to which these participants belonged were not reported. Knee surgery (replacement or osteotomy): not reported Progression of knee OA: not reported Withdrawals: 6/59 in the arthroscopic debridement group, 5/61 in the lavage group and 5/60 in the placebo group Data analysis: we included arthroscopic debridement versus placebo in Analysis 1.1 and Analysis 1.2 and we excluded the lavage treatment arm from this review. We included the SF‐36‐P and SF‐36‐PF scores for pain and function, respectively, but other measures for these outcome domains were available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned using a stratified randomisation process in blocks of six based on the severity of osteoarthritis (grade 1, 2 or 3; grade 4, 5 or 6; and grade 7 or 8) using sealed, sequentially‐numbered, stratum‐specific envelopes. Probably low risk |
| Allocation concealment (selection bias) | Low risk | Sealed, sequentially‐numbered, stratum‐specific envelopes containing treatment assignments were prepared by research staff and handed to the surgeon in the operating suite. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants was done ("the treatment assignment was not revealed to the patient"). Study personnel who were unaware of the treatment‐group assignments performed all post‐operative outcome assessments; the operating surgeon did not participate in outcome assessment. To assess whether participants remained unaware of their treatment‐group assignment, they were asked at each follow‐up visit to guess which procedure they had undergone. Participants in the placebo group were no more likely than participants in the other two groups to guess that they had undergone a placebo procedure. For example, at 2 weeks, 13.8% of participants in the placebo group guessed that they had undergone a placebo procedure, and 13.2% of participants in the lavage and debridement groups guessed that they had undergone a placebo procedure. |
| Blinding of outcome assessor Self‐reported outcomes | Low risk | Blinding of participants was done and there is low risk of bias in the measurement of pain and function. |
| Blinding of outcome assessor Assessor‐reported outcome (knee replacement) | Low risk | Knee replacement not measured. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6/59 (10%) in the debridement group, 5/61 (8%) in the lavage group and 5/60 (8%) in the placebo group did not complete the study. No data were given on the reasons for loss to follow‐up or withdrawals. |
| Selective reporting (reporting bias) | Low risk | Trial registration not done and protocol not available. The published article included results on all study outcomes as described in methods |
| Other bias | Low risk | No other bias apparent |
Osteras 2012.
| Study characteristics | ||
| Methods |
Study design: multicentre, two‐arm, randomised controlled trial Setting: two orthopedic clinics in two hospitals in Norway Trial time period: participants were recruited over a period of one year; however, the exact time frame was not mentioned. Interventions: arthroscopic surgery versus exercise Sample size calculations: based on a pre‐determined difference between treatment groups of 20% change in pain on a 10‐cm visual analogue scale and a standard deviation of 1.5 cm, 10 participants were required in each group to have 80% power to detect the 20% difference as statistically significant at the level of P < 0.05. Analysis: intention‐to‐treat |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Arthroscopic partial meniscectomy
Medical exercise therapy
Pre‐treatment group differences: there were no obvious differences in baseline characteristics between the two groups. |
|
| Interventions |
Arthroscopic surgery Arthroscopic partial meniscectomy. A standard arthroscopic partial meniscectomy was applied as a surgical intervention, which was carried out at two hospitals in Trondheim, Norway, and performed on participants who fulfilled inclusion criteria and were randomised to surgical treatment. Normal procedures for this surgery at the respective hospitals were followed, the protocols did not differ between the hospitals, and there were two surgeons involved. Exercise Supervised (medical) exercise therapy. The exercise program was developed for this particular study, with a focus on co‐ordination and muscle function training, along with pain modification exercise therapy. The program was for 3 months, and participants exercised 3 times per week. Each treatment in the exercise group started with 15 to 20 minutes of aerobic work on a stationary ergometer cycle. After 4 exercises each of 3 sets of 30 repetitions halfway through the exercise program, the subjects cycled for 10 minutes and again after the last 4 exercises, the participants did another 10 minutes on a stationary ergometer cycle. |
|
| Outcomes | Outcomes were measured at baseline and at 3 months of follow‐up. Primary outcome Pain in the last week measured with a visual analogue scale (VAS) at rest and recorded on a 0 to 10 cm line (0 = no pain and 10 = maximal pain) Secondary outcomes
Outcomes used in this review at 3 months
|
|
| Notes |
Funding: no information on funding was provided in this article Trial registration: not reported Adverse events: unclear if measured; not reported Knee surgery (replacement or osteotomy): not reported Progression of knee OA: not reported Withdrawals: unclear; pilot study results published in conference abstract (Osteras 2011, secondary publication of Osteras 2012) includes more participants Conference abstract (n = 22 participants); not reported if same participants as in Osteras 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on sequence generation process provided |
| Allocation concealment (selection bias) | Unclear risk | "The randomisation procedure was concealed from the experimenters and treating physiotherapist". There was no information on how the allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The interventionists were blinded to the group assignment; however, participants were unable to be blinded due to the nature of the intervention (surgery versus physiotherapy). |
| Blinding of outcome assessor Self‐reported outcomes | High risk | Participants were not blinded and there is risk of bias in the measurement of pain, other symptoms, activities of daily living, functioning in sport and recreation, knee‐related quality of life, depression and anxiety. |
| Blinding of outcome assessor Assessor‐reported outcome (knee replacement) | High risk | As the outcome assessor was not blinded to the intervention group assignment, there is risk of bias in the measurement of the quadriceps muscle strength tests. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Different numbers of participants were included in two reports (n = 17 versus n = 22). These are likely to be from a single study (trial registration not reported). |
| Selective reporting (reporting bias) | Unclear risk | Trial registration not done. While outcomes described in methods were reported in the results of the main publication, there is a discrepancy in participant numbers across two reports so the risk of bias is unclear. |
| Other bias | Unclear risk | Unclear if there was an unplanned interim analysis performed |
Roos 2018.
| Study characteristics | ||
| Methods |
Study design: multicentre, prospective, double‐blind, randomised, placebo‐controlled trial Setting: outpatient departments of the orthopaedic clinics in Region Zealand; Slagelse Hospital; Næstved Hospital, Denmark Trial time period: 21 February 2011 to March 2015 Interventions: arthroscopic surgery versus placebo surgery Sample size calculations: 36 individuals per group would be required to obtain a power of at least 80% to detect a minimal important change (MIC) of 10 KOOS 5 score units, assuming a common standard deviation of 15. The study team decided to include 80 individuals in total (40 participants in each group), allowing for a 10% dropout rate. Analysis: intention‐to‐treat analysis was planned and both a per‐protocol and ITT analysis (using best observation carried forward for missing data) were executed. |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Arthroscopic partial meniscectomy (n = 22) Mean (SD) age: 47.2 (5.9) No. (%) male/female: 13/9 (59/41) Mean (SD) BMI: 27.6(3.6) No. (%) Kellgren‐Lawrence grading: 0 ‐ 9 (41), 1 ‐ 9 (41), 2 ‐ 4 (18), 3 ‐ 0, 4 ‐ 0 No. (%) joint line tenderness: 21 (100) No. (%) positive McMurray test: 17 (81) No. (%) swelling present: 11 (52) No. (%) full knee extension: 18 (86) No. (%) small extension deficit < 10o: 3 (14) No. (%) full knee flexion: 17 (81) No. (%) small flexion deficit < 10o: 3 (14) No. (%) large flexion deficit: 1 (5) Median (IQR) duration of pain (months): 5 (2‐6) Mean (SD) KOOS5 (0–100 worst to best): 51.2 (15.6) Mean (SD) KOOS Pain (0–100): 55.1 (15.4) Mean (SD) KOOS Symptoms (0–100): 62.8 (17.7) Mean (SD) KOOS Function in daily living (0–100): 64.9 (19.9) Mean (SD) KOOS Function in sport and recreation (0–100): 35.0 (23.0) Mean (SD) KOOS Knee‐related quality of life (0–100): 38.7 (15.4) Mean (SD) EQ‐5D VAS score: 69 (14) Mean (SD) EQ‐5D 3L index value (0–1): 0.749 (0.108) Mean (SD) SF‐36 Physical Component Summary (0–100): 38 (10) Mean (SD) SF‐36 Mental Component Summary (0–100): 59 (7) Placebo surgery (n = 22) Mean (SD) age: 46.4 (5.5) No. (%) male/female: 10/12 (45/55) Mean (SD) BMI: 26 (3.9) No. (%) Kellgren‐Lawrence grading: 0 ‐ 10 (45), 1 ‐ 8 (36), 2 ‐ 4 (18), 3 ‐ 0, 4 ‐ 0 No. (%) joint line tenderness: 20 (91) No. (%) positive McMurray test: 17 (77) No. (%) swelling present: 8 (36) No. (%) full knee extension: 21 (95) No. (%) small extension deficit < 10o: 1 (5) No. (%) full knee flexion: 14 (64) No. (%) small flexion deficit < 10o: 6(27) No. (%) large flexion deficit: 2 (9) Median (IQR) duration of pain (months): 3.5 (2.0 – 6.0) Mean (SD) KOOS5 (0–100): 44.8 (19.9) Mean (SD) KOOS Pain (0–100): 45.9 (22.0) Mean (SD) KOOS Symptoms (0–100): 59.9 (20.6) Mean (SD) KOOS Function in daily living (0–100): 56.5 (22.3) Mean (SD) KOOS Function in sport and recreation (0–100): 25.2 (26.3) Mean (SD) KOOS Knee‐related quality of life (0–100): 36.6 (20.2) Mean (SD) EQ‐5D VAS score: 63 (SD not reported) Mean (SD) EQ‐5D 3L index value (0–1): 0.642 (SD not reported) Mean (SD) SF‐36 Physical component summary (0–100): 35 (SD not reported) Mean (SD) SF‐36 Mental component summary (0–100): 57 (SD not reported) Pre‐treatment group differences: the KOOS scores (KOOS5 and subscales) were higher in the arthroscopic group compared to the placebo surgery group. |
|
| Interventions |
Arthroscopic surgery The arthroscopic partial meniscectomy was performed on an outpatient basis under general anaesthesia combined with local anaesthesia. Arthroscopic surgery was performed by experienced surgeons in their final year of residency or attending orthopaedic surgeons. Two standard portals on the lateral and medial sides of the ligamentum patella were created but no outflow cannula inserted. An arthroscope was used with a pressure‐controlled irrigation system. Tourniquet use was at the discretion of the surgeon. The strategy for the meniscectomy was to preserve as much tissue as possible. A standard operation protocol was used to document possible findings in cartilage, ligaments, synovium and the medial and lateral menisci. The type, and extent of meniscus lesion was registered and changes in the articular cartilage was classified according to the ICRS (International Cartilage Repair Society) classification. Placebo surgery The placebo procedure (skin incision only) was performed under the same conditions as the arthroscopic surgery. Participants were fully sedated with general anaesthesia. Local anaesthetic was applied and two skin incisions made at the same locations and of the same size as in the arthroscopic surgery group. Then the knee was manipulated as if a real arthroscopy was performed, the spillage of water and all other equipment needed for an arthroscopy was used. No instruments entered the arthroscopy portals to avoid the possibility of deep infection, osteochondral lesions or unwanted interventions by the surgeon. A pre‐recorded video of a standard arthroscopic partial meniscectomy was planned to be played during the placebo procedure (but was not done). All participants in both intervention groups were given a folder including an exercise program for post‐operative participants after knee arthroscopy or placebo procedure. The exercise program included 7 different non‐weight‐bearing exercises to improve lower extremity function and knee range of motion (for the first week after surgery) and a further 3 weight‐bearing exercises thereafter. The exercises were for the participants to carry out at home and were recommended to be performed 10 to 15 times three times daily. The participants were also advised to start unloaded cycling, swimming or walking after 1 week, and jogging or loaded cycling after 2 to 3 weeks. |
|
| Outcomes | Outcomes were measured at baseline, 3 and 24 months. Primary outcome
Secondary outcomes
Outcomes used in this review at 3 and 24 months
Serious adverse events could not be included as the details of the group to which some serious adverse events belonged were not reported. |
|
| Notes |
Funding: University of Southern Denmark, Odense; the Orthopedic Departments of Slagelse and Næstved Hospital; the Research Unit of Hospital South, Region Zealand; Edith and Henrik Henriksens Memorial Fund; Region Zealand Health Scientific Research Fund; Research Fund of Hospital South Clinical trial registration: NCT01264991 Adverse events: 4/22 knee‐related adverse events in the surgery group, of which two were serious (two re‐arthroscopies comprising one partial meniscectomy and one anterior cruciate ligament reconstruction), two were not serious (one cutaneous nerve lesion and one mild knee swelling). No reported knee‐related adverse events reported in the placebo group. Total other adverse events ‐ 7 adverse events in 5 participants (2 participants in the surgery group and 3 participants from placebo group). The nature of events included: chest pain, finger injury, nausea, dizziness and kidney stone, and included two regarded as serious (abdominal surgery and malignant melanoma). Details of the group to which some serious adverse events belonged were not reported. Knee surgery (replacement or osteotomy): none Progression of knee OA: not reported Withdrawals: 1/22 in the arthroscopy group and 1/22 in the placebo group at 3 months, and 2/22 participants in the placebo group only at 24 months Post‐protocol changes: a pre‐recorded video of a standard arthroscopic partial meniscectomy was planned to be played during the procedure for both the surgery and placebo group, but was not performed in the placebo group. Statistical power: trial was underpowered due to inability to recruit 40 participants per group as planned Data analysis: SMD for generic quality of life at > 6 months up to 2 years (Analysis 1.4) back‐translated to SF‐36 PCS by multiplying the SMD by the standard deviation at baseline in the surgery group (as standard deviation in the placebo group was not reported) (SD = 10) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a computer‐generated table of random numbers, prepared by an external co‐investigator |
| Allocation concealment (selection bias) | Low risk | Used consecutively‐numbered, sealed envelopes stored in a briefcase outside the operating theatre |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded to group allocation at short‐term follow‐up, there was low risk of bias at primary time point. Blinding broken for 16 participants (36%; 6/22 from arthroscopy group and 10/22 from placebo group) prior to 2‐year follow‐up. Prior to 3 months, two additional participants from the arthroscopy group (due to adverse events) and one from the placebo group (persisting pain) were unblinded by the treating surgeon. Between 3 and 24 months, two from the arthroscopy group and eight from the placebo group were unblinded by the treating surgeon because of persisting pain. In total, 10/22 in the arthroscopy group and 19/22 in the placebo group were unblinded. Operating surgeons and theatre staff were not blinded. |
| Blinding of outcome assessor Self‐reported outcomes | Low risk | Low risk at short‐term follow‐up, unclear if knowledge of treatment in 6/22 and 10/22 influenced assessment of pain and function at 2 years |
| Blinding of outcome assessor Assessor‐reported outcome (knee replacement) | Low risk | Outcome assessors were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Self‐reported outcome data were missing for 2/22 participants in the placebo group at baseline. Data missing for 1/22 in the arthroscopy group and 1/22 in the placebo group at 3 months. 2/22 participants in the placebo group were lost to follow‐up at 2 years (crossed over to arthroscopic surgery between 3 and 24 months) |
| Selective reporting (reporting bias) | Low risk | 3‐month follow‐up data were provided upon request from the authors |
| Other bias | Low risk | No other bias apparent |
Saeed 2015.
| Study characteristics | ||
| Methods |
Study design: single centre, two‐arm, randomised controlled trial Setting: Department of Orthopedics, Ch. Rehmat Ali Memorial Trust Hospital attached to Continental Medical College Lahore, Pakistan Trial time period: January 2012 to December 2014 Interventions: arthroscopic surgery versus five intra‐articular hyaluronic acid injections given at weekly intervals Sample size calculations: not reported Analysis: intention‐to‐treat |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Arthroscopic surgery
Intra‐articular hyaluronic acid injections
Pre‐treatment group differences: there were some differences between groups in pain at baseline: in the intra‐articular hyaluronic acid injection group, 13% of participants had pain score 10, 43% had 20 and 43% had 30, while in the arthroscopic group, 7% of participants had pain score 10, 70% had 20 and 23% had 30. |
|
| Interventions |
Arthroscopic surgery Arthroscopic debridement. Participants were admitted to the hospital, arthroscopic debridement was performed in the operation theatre by using two portals in all cases under spinal anaesthesia. Participants were discharged on the next day. Monitoring of electrocardiogram (ECG) and blood pressure was standard in all cases during the entire duration of the procedure. All the debridements were done by a single surgeon to minimise the bias for the study. Intra‐articular hyaluronic acid injections Participants were injected with intra‐articular hyaluronic acid after being given intradermal anaesthesia. The injections were given weekly for five weeks with a 24‐gauge needle under strict aseptic conditions in the operation theatre as an outpatient. In case of joint effusion, aspiration was done before the injection to prevent dilution of the injection. |
|
| Outcomes | Outcome were assessed at baseline and at 1, 3 and 6 months' follow‐up Outcomes
Outcomes used in this review at 3 and 6 months
|
|
| Notes |
Funding: not reported Trial registration: not reported Adverse events: Arthroscopic debridement group Serious adverse events: none reported Other adverse events: No.(%): 13 (26) Nature of event: pain and mild effusion Total adverse events: No.(%): 13 (26) Intra‐articular hyaluronic acid injection group Serious adverse events: none reported Other adverse events: No.(%): 8 (13.4) Nature of event: pain at injection site Total adverse events: No.(%): 8 (13.4) The frequency of adverse events in the arthroscopy group was higher than the injection group Knee surgery (replacement or osteotomy): not reported Progression of knee OA: not reported Withdrawals: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as 'randomized experimental study' but no information about sequence generation process provided |
| Allocation concealment (selection bias) | Unclear risk | No information on whether allocation concealment was done or not |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and study personnel was not reported. Probably not done due to the nature of the intervention |
| Blinding of outcome assessor Self‐reported outcomes | High risk | Risk of detection bias in assessment of participant‐reported knee pain and adverse events as blinding of participants was not reported but probably not done |
| Blinding of outcome assessor Assessor‐reported outcome (knee replacement) | Low risk | Blinding of study personnel not reported but adverse effects unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Trial registration not done and protocol not available. The authors measured overall knee function (using the Knee Society Score System), but it is unclear if they reported overall scores or a pain subscore. |
| Other bias | Low risk | No other bias apparent |
Sihvonen 2013.
| Study characteristics | ||
| Methods |
Study design: multicentre, randomised, parallel‐arm, double‐blind, placebo‐controlled trial Setting: five orthopedic clinics in Finland Trial time period: participants were enrolled between December 2007 and January 2013 Interventions: arthroscopic partial meniscectomy (APM) plus home exercises versus placebo surgery plus home exercises Sample size calculations: a total sample of 134 participants with 40, 54 and 40 participants per group for the Lysholm score, WOMET score and pain assessment, respectively, with 80% power to show a clinically meaningful advantage of APM over placebo, based on a two‐sided type 1 error rate of 5%. Anticipating a loss to follow‐up of at least 20%, the study planned to recruit 70 participants per group. Analysis: intention‐to‐treat |
|
| Participants |
Number of participants
Criteria for defining study participants: those who have knee symptoms consistent with a degenerative medial meniscus tear and no knee osteoarthritis Inclusion criteria
Exclusion criteria
Baseline characteristics Arthroscopic partial meniscectomy
Placebo surgery
Pre‐treatment group differences: there were no differences in the baseline characteristics between the two groups. |
|
| Interventions | Arthroscopic examination of the knee was first performed in all participants with the use of standard anterolateral and anteromedial portals and a 4‐mm arthroscope. The orthopedic surgeon evaluated the medial, lateral and patellofemoral joint compartments and graded the intra‐articular pathologic changes. Arthroscopic surgery Arthroscopic partial meniscectomy plus home exercises. The damaged and loose part of the meniscus tissue was removed with arthroscopic instruments (mechanised shaver and meniscal punches) until solid meniscus tissue was reached. The meniscus was then probed to ensure that all loose and weak fragments and unstable meniscus tissue had been successfully resected, preserving as much of the meniscus tissue as possible. Placebo surgery Placebo surgery plus home exercises. A standard arthroscopic partial meniscectomy procedure was simulated. The surgeon asked for all instruments and manipulated the knee as if an arthroscopic partial meniscectomy was being performed. The mechanised shaver (without the blade) was pushed firmly against the patella, outside of the knee, to mimic as closely as possible the feelings and sounds of the normal use of the arthroscopic shaver. Further, to simulate the sounds of normal arthroscopic partial meniscectomy, suction was also used to drain the joint and saline was splashed. The participant was kept in the operating room for the amount of time required to perform an actual arthroscopic partial meniscectomy. All procedures were standardised and recorded on video. In both arthroscopic partial meniscectomy and placebo surgery groups, the post‐operative care was delivered according to a standard protocol specifying that all participants received the same walking aids and graduated home exercise programme. Participants were instructed to take over‐the‐counter analgesic agents as required. |
|
| Outcomes | Outcomes were assessed at baseline and 2, 6, 12, 24, 36, 48 and 60 months' follow‐up. Primary outcomes
Secondary outcomes
Outcomes used in this review at 2, 6 and 12 months, and 2 and 5 years
|
|
| Notes |
Funding: funded by the Sigrid Juselius Foundation, the Competitive Research Fund of Pirkanmaa Hospital District, and the Academy of Finland. Trial registration: ClinicalTrials.gov number NCT00549172; and NCT01052233 trial number for 10‐year follow‐up which is ongoing Adverse events Arthroscopic partial meniscectomy: 7/70 (10%) participants from the arthroscopic surgery group had a serious knee‐related adverse event (3 knee replacement, 4 arthroscopies) and 1/70 (1%) participants from this group had other serious adverse events (1 deep infection of the index knee at 4 months) (8/70 (11.43%) participants) Placebo surgery: 8/76 (10.52%) participants from the placebo group had serious knee‐related adverse events (1 proximal tibial osteotomy, 7 arthroscopic partial meniscectomies) and no other serious adverse events in this group Knee surgery (replacement or osteotomy) (5‐year follow‐up) Arthroscopic partial meniscectomy: No. (%) of participants = 3/70 (4%) participants from the arthroscopic surgery group had a subsequent knee replacement Placebo surgery: 1/76 (1%) participants from the placebo surgery group had a subsequent high tibial osteotomy [trial authors reported 3/68 (4%) in the arthroscopy group and 1/74 (1%) in the placebo group at 5 years. Number receiving allocated intervention was used as denominator in meta‐analysis] Progression of knee OA At 5 years, 44/74 (59.5%) participants from the placebo group and 48/67 (71.6%) participants from the arthroscopy group had at least one grade progression in radiographic tibiofemoral knee OA on the Kellgren‐Lawrence classification. Withdrawals At 12 months, there was no loss to follow‐up and all randomised participants completed the study. Lysholm Knee Score data for one participant in the placebo surgery group were missing at 6 months' follow‐up (no reason given) and data were not imputed. At 24 months, 2 participants from the placebo group were lost to follow‐up (one not responding to contact attempts and one deceased). At 60 months, 4 participants (2 placebo and 2 arthroscopic surgery) were lost to follow‐up (two not responding and 2 deceased ‐ reasons not given per group) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation was performed by a statistician using a computer‐generated schedule |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, opaque, sealed envelopes were prepared by a statistician with no involvement in the clinical care of participants in the trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The operating surgeon and other staff in the operating room were aware of group assignment before the procedure but did not participate in further treatment or follow‐up of participants. Participants were blinded. At 12 months, 2/70 (3%) participants in the arthroscopic surgery group and 5/76 (7%) participants in the placebo surgery group reported persistent symptoms after surgery that were sufficiently severe to lead to revealing of the study‐group assignment at an average of 8 months after surgery. At 24 months, 5/70 (7%) in the arthroscopic surgery group and 7/74 (9%) participants in the placebo group reported symptoms so severe to lead to unblinding. At 60 months, 8/68 (12%) in the arthroscopic surgery group and 8/74 (11%) participants in the placebo group reported symptoms so severe to lead to unblinding |
| Blinding of outcome assessor Self‐reported outcomes | Low risk | Participants were blinded to the group assignment. Participants in the placebo surgery group were not significantly more likely than participants in arthroscopic surgery group to guess that they had undergone a placebo procedure (47% and 38% respectively, P = 0.39) |
| Blinding of outcome assessor Assessor‐reported outcome (knee replacement) | Low risk | Outcome assessors were blinded; low risk of bias for assessment of knee replacement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 12 months, there was no loss to follow‐up and all randomised participants completed the study. Lysholm Knee Score data for one participant in the placebo surgery group were missing at 6 months' follow‐up (no reason given) and data were not imputed. However, it is unlikely that this would have had a clinically important impact on the observed effect size. At 24 months, 2 participants from the placebo group were lost to follow‐up (one not responding to contact attempts and one deceased). At 60 months, 4 participants (2 placebo and 2 arthroscopic surgery) were lost to follow‐up (two not responding and 2 deceased ‐ reasons not given per group) |
| Selective reporting (reporting bias) | Unclear risk | One primary outcome (WOMET at 12 months) was not pre‐specified and was added after data collection but before data analysis (trial registration was amended and revised protocol published). The rationale for adding this primary outcome was provided (the score was validated in the participant population). Two other pre‐specified outcomes were changed: (1) pain at rest at 12 months was pre‐specified as a primary outcome but moved to a secondary outcome after data collection but before data analysis. The rationale was that reporting both pain at rest and after exercise was 'somewhat ambiguous' and that pain after exercise was the more important of the two; (2) secondary cost‐utility analysis based on 15D score and healthcare resource utilisation at 12 months was removed before data analysis (no reason given). The 2‐year follow‐up paper (Sihvonen 2018) reported the primary time point as 24 months (but protocol states primary time point as 12 months). A new statistical analysis plan for 5‐ and 10‐year follow‐up was published in 2020. Results on revised primary and secondary outcomes were reported |
| Other bias | Low risk | No other bias apparent |
Van de Graaf 2018.
| Study characteristics | ||
| Methods |
Study design: non‐inferiority, parallel‐arm, multicentre, randomised controlled trial Setting: nine hospitals in the Netherlands Trial time period: July 2013 to November 2015 Interventions: arthroscopic partial meniscectomy (APM) versus physical therapy (PT) Sample size calculations: initially a sample size of 402 participants was estimated to have power of 90%, an α of 0.05 and SD of 20 points; a clinically relevant difference of 8.8 points on the International Knee Documentation Committee (IKDC) ‘Subjective Knee Form’ was rounded down to a non‐inferiority threshold of 8 to increase the power. However, an interim analysis led to recalculation of SD to 18 points resulting in a sample size requirement of 320 participants (120 per group). Analysis: intention‐to‐treat. As‐treated analysis was also conducted and results reported in 3 groups ‐ APM group, PT group and delayed APM group i.e. those who were randomised to the PT group but received APM during follow‐up. |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Arthroscopic partial meniscectomy group Mean (SD) age: 57.6 (6.5) No. of male/female: 78/80 Mean (SD) BMI: 26.7 (3.8) No. (%) mechanical complaints: 56 (35.4) No. (%) medial meniscal involvement: 126 (79.7) No. (%) osteoarthritis score (KL classification): 0 = 18 (12.0); 1 = 81 (54.0); 2 = 45 (30.0); 3 = 6 (4.0) Mean (SD) Knee function International Knee Documentation Committee (IKDC) score (0 = most limitations to 100 = no limitations): 44.8 (16.6) Median (IQR) Knee pain on VAS (0 = no pain to 100 = worst pain): 61.1 (44.9‐83.4) Physical therapy group Mean (SD) age: 57.3 (6.8) No. of male/female: 79/81 Mean (SD) BMI: 27.2 (4.0) No. (%) mechanical complaints: 67 (41.6) No. (%) medial meniscal involvement: 136 (84.5) No. (%) osteoarthritis score (KL classification): 0 = 15 (10.1); 1 = 74 (49.7); 2 = 55 (36.9); 3 = 5 (3.3) Mean (SD) Knee function IKDC score (0 = most limitations to 100 = no limitations): 46.5 (14.6) Median (IQR) Knee pain on VAS (0 = no pain to 100 = worst pain): 59.3 (44.9‐77.4) Pre‐treatment group differences: there are no pre‐treatment group differences between the groups. |
|
| Interventions |
Arthroscopic partial meniscectomy (APM) Arthroscopic partial meniscectomy was performed within 4 weeks of randomisation in an outpatient clinic under general or spinal anaesthesia by orthopaedic surgeons experienced in arthroscopic surgery, or orthopaedic residents skilled in arthroscopic surgery under supervision of an orthopaedic surgeon. Standard anteromedial and anterolateral portals were introduced for inspection of the knee joint. The affected meniscus was partially removed until a stable and solid meniscus remained. All participants received perioperative instructions and a home exercise program. Participants were only referred to PT after APM if they did not recover as anticipated as defined by the Dutch Orthopedic Association guidelines. Physical Therapy (PT) Participants were referred to PT clinics and their initial PT session was scheduled within 2 weeks after randomisation. Participating PT clinics were instructed about the exercise protocol by a knee‐specialised physical therapist or the primary investigator, prior to the first participant’s referral. The PT exercise protocol was developed by a knee‐specialised physical therapist and consisted of 16 sessions of 30 minutes each conducted over 8 weeks. The PT protocol comprised cardiovascular, coordination/balance, and closed kinetic chain strength exercises (in which the distal part of the extremity is fixed to an object that is stationary). If PT failed, the participant was allowed to attend additional PT sessions or have APM, depending on their preference. Post‐intervention: both groups received the same home exercise instructions. The home exercise program consisted of one leg standing during 60 seconds and a step‐down exercise comprising 3, 9, 10 repetitions, twice a week. |
|
| Outcomes | Outcomes were measured at 3, 6, 12 and 24 months Primary outcome
Secondary outcomes
Outcomes used in this review at 3, 6 and 24 months
|
|
| Notes |
Funding: this study was funded by the Netherlands Organization for Health Research and Development (in Dutch: ZonMw; grant 837002009), Zilverenkruis Health Insurance (grant Z436), and the Foundation of Medical Research of the OLVG, Amsterdam (grant 15u.025). Trial registration: ClinicalTrials.gov number NCT01850719 Adverse events Arthroscopic partial meniscectomy Serious adverse events: No.of events = 9 No. (%) of participants affected = 9/159 (5.7) Nature of event: neurological events including intracranial malignancy (1), lymph node malignancy (1), rectal polyp (1), knee replacement (2), arthroscopy in affected knee (2), arthroscopy in opposite knee (1), other knee surgery (1) Other adverse events: No.of events = 9 No. (%) of participants affected = 9 (5.6) Nature of event: reactive arthritis (1), knee pain resulting in extra consultation (6), Pain in back, hip or foot (2) Total adverse events: No. (%): 18/159 (11.32) Physical therapy Serious adverse events: No.of events = 8 No. (%) of participants affected = 8/162 (4.94) Nature of event: acute myocardial infarction (1), sudden death (1), neurological event (1), alcoholic pancreatitis (1), arthroscopy (1), knee replacement (3) Other adverse events: No.of events = 4 No. (%) of participants affected = 4 (2.4) Nature of event: Knee pain resulting in extra consultation (2) other musculoskeletal (2) Total adverse events: No. (%): 12/162 (7.41) Knee surgery (replacement or osteotomy) Arthroscopic partial meniscectomy: No. (%) of participants = 2/159 (1.2) Other surgery: 3/159 had a re‐arthroscopy, 1/159 'other' surgery Physical therapy: No. (%) of participants = 3/162 (1.8) Other surgery: 1/162 arthroscopy Progression of knee OA: OA severity in the arthroscopy group progressed from 1.3 points at baseline to 1.6 points at 24 months (MD 0.37 points, 95% CI 0.25 to 0.49) and in the physical therapy group from 1.3 points at baseline to 1.5 points at 24 months (MD 0.18 points, 95% CI 0.04 to 0.31). Mixed‐model analysis found no significant between‐group difference (0.10 points more progression in the arthroscopy group, 95% CI ‐0.05 to 0.26, P = 0.18) Withdrawals: two participants (1 from each group) withdrew immediately after randomisation; 18/158 in the APM group and 14/161 in the PT group due to loss to follow‐up. Cross‐overs: 47/161 (29%) participants assigned to the exercise group had arthroscopic surgery within two years' follow‐up, and 8/159 (5%) participants assigned to the arthroscopy group did not have the procedure |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a computerised software program (TENALEA Clinical Trial Data Management system) in a 1:1 ratio using random blocks with a maximum block size of 6. |
| Allocation concealment (selection bias) | Low risk | Randomisation to groups was concealed as it was through an online program. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, physicians, and physical therapists were not blinded. |
| Blinding of outcome assessor Self‐reported outcomes | High risk | As participants were unable to be blinded due to the nature of the intervention, there could be a risk of bias in the reporting of pain and function. |
| Blinding of outcome assessor Assessor‐reported outcome (knee replacement) | Low risk | The radiologists assessing X‐rays were blinded to treatment allocation so there is low risk of bias in the assessment of osteoarthritis. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants (1 from each group) withdrew immediately after randomisation without providing a reason. 18/158 (11%) in the APM group and 14/161 (8.6%) in the PT group were lost to follow‐up at 24 months and excluded from the final analysis. Missing outcome data reasonably balanced across groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Trial registered and protocol published. Four deviations from the protocol were clearly outlined in the publication of results ‐ recalculation of SD, inclusion of all time points in the measurement of primary outcome, change to mixed‐model analysis from longitudinal analyses and correction of protocol error of 10% loss to follow‐up to 20%. Some secondary outcomes listed in the protocol such as resource utilisation, health‐related quality of life, patient‐specific complaints, participant expectations, and participant satisfaction were not analysed and authors reported they will be analysed and reported separately. The authors reported median and IQR data; however, upon request, they provided mean and SD data which has been used in the analysis |
| Other bias | Low risk | No other bias apparent |
Vermesan 2013.
| Study characteristics | ||
| Methods |
Study design: single centre, parallel‐group, two‐arm, randomised controlled trial Setting: hospital in Italy Trial time period: not reported Interventions: arthroscopic surgery versus a single intra‐articular glucocorticoid injection Sample size calculations: not reported Analysis: not reported |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria: not reported Baseline characteristics Arthroscopic surgery
Glucocorticoid injection
Pre‐treatment group differences: there were no differences in the baseline characteristics between the two groups. |
|
| Interventions |
Arthroscopic surgery Arthroscopic debridement. No description of this procedure was provided. Intra‐articular glucocorticoid injection A single intra‐articular glucocorticoid injection (1 mL of betamethasone in 4 mL of 1% lidocaine) was administered. |
|
| Outcomes | Outcome was measured at 1 month and 1 year of follow‐up. Oxford Knee Scores which range from: 0 to 19 = severe knee arthritis; 20 to 29 = moderate to severe arthritis; 30 to 39 = mild to moderate arthritis; 40 to 48 = satisfactory joint function (Dawson 1998) Outcomes used in this review at 1 month and 1 year
|
|
| Notes |
Funding: no information on the funding source was provided Trial registration: not done Adverse events: only knee‐related adverse events reports (total of 5 participants had knee replacement); unclear if trial measured other adverse events Knee surgery (replacement or osteotomy): a total of 5 participants (4.2%) in both groups had a knee replacement. Per‐group data not given. Progression of knee OA: not reported Withdrawals: 12/60 in the glucocorticoid injection group and 10/60 in the arthroscopic group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation process provided |
| Allocation concealment (selection bias) | Unclear risk | There was no information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and study personnel was not reported. Probably not done |
| Blinding of outcome assessor Self‐reported outcomes | High risk | As blinding of participants was not reported and probably was not done, there is likely to be a risk of bias in the measurement of knee pain and function using the Oxford Knee Score. |
| Blinding of outcome assessor Assessor‐reported outcome (knee replacement) | Low risk | No assessor‐reported outcomes were measured in this trial. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was no loss to follow‐up for the 1 month follow‐up. 12/60 (20%) participants in the glucocorticoid injection group and 10/60 (16%) participants in the arthroscopic group did not have outcome data at the 12 month follow‐up. The reasons for loss to follow‐up were not reported. |
| Selective reporting (reporting bias) | High risk | Trial registration not done and protocol not available. The published article had results for one study outcome ‐ the Oxford Knee Scores. Correlation analysis was reported in the methods but this was not reported in the results |
| Other bias | Low risk | No other bias apparent |
Yim 2013.
| Study characteristics | ||
| Methods |
Study design: single centre, parallel‐group, two‐arm, single‐blinded, randomised controlled trial Setting: Center for Joint Disease, Chonnam National University Hwasun Hospital, Jeonnam, South Korea Trial time period: participants were enrolled between January 2007 and July 2009 Interventions: arthroscopic surgery plus home exercise versus non‐operative care with physical therapy plus home exercise Sample size calculations: the sample size was calculated based on Lysholm Knee Score data obtained from 30 prior cases, where the standard deviation was approximately 18. To test the difference in the minimal clinical relevance of 10 between the 2 groups with 80% power and a significance level of P < 0.05, these values were estimated with 54 participants in each group Analysis: intention‐to‐treat |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Arthroscopic meniscectomy
Non‐operative group
Pre‐treatment group differences: there were no differences in baseline characteristics between the two groups. |
|
| Interventions |
Arthroscopic surgery Arthroscopic meniscectomy plus home exercises. Arthroscopic meniscectomy was carried out by a single experienced orthopaedic surgeon, using a 5.5‐mm, 30 degree arthroscope and a pressure‐controlled irrigation system. The procedure in each case was limited to resection with limited debridement of the articular surface lesion. Participants who underwent additional procedures, such as curettage, abrasion arthroplasty, or subchondral drilling for any articular lesions, were excluded from this study (n = 3). No participant underwent total meniscectomy or peripheral meniscal repair. All participants were discharged on the day after surgery. Subsequently, participants were permitted to use co‐interventions, such as analgesics or NSAIDs, within 2 weeks. All participants were then provided with a home exercise program, which was conducted unsupervised, using the same protocol as the non‐operative group, for 8 weeks. Exercise Non‐operative care with physical therapy plus home exercises. All participants in the non‐operative group were prescribed drugs, such as analgesics, non‐steroidal anti‐inflammatory drugs (NSAIDs), or muscle relaxants, depending on clinical symptoms for the first 2 weeks. In addition, they underwent scheduled physical exercise to improve muscle strength, endurance, and flexibility for 60 minutes per session, 3 times weekly, for 3 weeks under the supervision of a physical therapist. After an early, intensive, supervised rehabilitation program to strengthen muscles during the first 3 weeks, all participants were provided with a home exercise program, which they conducted unsupervised for 8 weeks. The home exercise program consisted of daily isometric and isotonic muscle exercises. This included: 1. Stretching of knee extensors and flexors (0‐8 times per week; 3 times per day; 1min/muscle group); 2. Knee extension in sitting position (0‐8 times per week; 3 times per day; 3 x 10 repetitions); 3. Knee flexion in sitting position (0‐8 times per week; 3 times per day; 3 x 10 repetitions); 4. Stationary bicycling (0‐8 times per week; 3 times per day; gradual increase every 15 minutes); 5. Half squats with < 45 degrees of flexion with weights (5‐8 times per week; 3 times per day; 3 x 10 repetitions); 6. Squats with full flexion with weights (5‐8 times per week; 3 times per day; 3 x 10 repetitions). Participants were instructed to perform the exercises with some strain but almost pain‐free and not adversely influencing the affected knee. Post‐intervention Participants in both groups were permitted to use co‐interventions, such as analgesics, muscle relaxants or NSAIDs, within 2 weeks. |
|
| Outcomes | Outcomes were assessed at baseline and at 3, 6, 12 and 24 months follow‐up. Study outcomes
Outcomes used in this review at 3, 6 and 24 months
|
|
| Notes |
Funding: not reported Trial registration: not registered Adverse events: unclear if measured; not reported Knee surgery (replacement or osteotomy): not reported Progression of knee OA: 2/50 (4.0%) participants in the meniscectomy group and 3/52 (5.8%) in the non‐operative group at 2‐year follow‐up Withdrawals: 4/54 (7.4%) in the meniscectomy group and 2/54 (3.7%) in the non‐operative group Data imputations: SDs for VAS pain and Lysholm Knee Function Score not reported at follow‐up and not provided by authors on request. We used Yim 2013 baseline SDs in analyses Analysis 2.1; Analysis 2.2; Analysis 13.1; and Analysis 13.2. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation process provided |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment unclear. "Closed‐envelope technique" reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and study personnel not done |
| Blinding of outcome assessor Self‐reported outcomes | High risk | Participants were aware of treatment allocation; thus, there is risk of bias in measurements of pain, knee function and activity and participant satisfaction with treatment |
| Blinding of outcome assessor Assessor‐reported outcome (knee replacement) | Low risk | Outcome assessors for progression of knee OA probably blinded: "Clinical outcome measures and physical examinations were conducted by independent authors not involved in the treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/54 (7.4%) (not meeting inclusion criteria = 3, loss to follow‐up = 1) in the arthroscopic meniscectomy group and 2/54 (3.7%) (cross‐over to the other group = 1, loss to follow‐up = 1) in the non‐operative exercise group were excluded from the analysis at 24 months |
| Selective reporting (reporting bias) | High risk | Trial registration not done and protocol not available. The authors reported mean and range for study outcomes but standard deviations and confidence intervals were not reported. Adverse events were not reported |
| Other bias | Unclear risk | An unspecified number of participants in the arthroscopic surgery group were not prescribed exercise |
ACR: American College of Rheumatology; ADL: activities of daily living; AIMS: Arthritis Impact Measurement Scale; AIMS2‐P: pain subscale of the Arthritis Impact Measurement Scale; BMI: body mass index; cc: cubic centimetres; DVT: deep vein (venous) thrombosis; EQ‐5D 3L: EuroQoL 5‐dimension 3‐level quality of life questionnaire; IQR: interquartile range; ITT: intention‐to‐treat; KL grade: Kellgren‐Lawrence classification grade; KOOS: Knee injury and Osteoarthritis Outcome Score; KOOS 4/5: derived from 4 or 5 KOOS subscale scores; MI: myocardial infarction; MRI: magnetic resonance imaging; NSAIDS: non‐steroidal anti‐inflammatory drugs; OA: osteoarthritis; OARSI: Osteoarthritis Research Society International; PT: physical therapy; QoL/QOL: quality of life; ROM: range of motion; SD: standard deviation; SMD: standardised mean difference; VAS: visual analogue scale; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index; WOMET: Western Ontario Meniscal Evaluation Tool
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahn 2015 | Study population did not have degenerative knee disease |
| Biedert 2000 | Study population did not have degenerative knee disease |
| Bisson 2015 | Study population did not have degenerative knee disease |
| Bradley 2002 | Intervention was not arthroscopic surgery |
| Hubbard 1996 | Study population did not have degenerative knee disease or osteoarthritis |
| Kalunian 2000 | Intervention was not arthroscopic surgery |
| Lee 2020 | Not an RCT |
| Lu 2018 | Not an RCT |
| Ma 2020 | Not an RCT |
| Marsh 2016 | Study examined cost‐effectiveness of arthroscopic surgery |
| Pan 2020 | Not an RCT |
| Rimington 2009 | Not an RCT |
| Wijn 2020 | Not an RCT |
| Zhang 2018 | Intervention was arthroscopic joint lavage |
| Zhao 2018 | Not an RCT |
Characteristics of studies awaiting classification [ordered by study ID]
Kang 2005.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Article is in Chinese awaiting translation into English |
NCT00562822.
| Methods |
Study design: prospective single‐blind, single‐centre, parallel, two‐arm randomised controlled trial Setting: London Health Sciences Centre ‐ University Hospital, London, Ontario, Canada Trial time period: January 1999 to August 2007 Interventions: arthroscopic surgery plus optimised physical and medical therapy versus optimised physical and medical therapy Sample size calculations: estimated enrolment of 188 participants, no sample size calculations reported Analysis: not reported |
| Participants |
Inclusion Criteria Idiopathic or secondary osteoarthritis of the knee with Grade 2 to 4 radiographic severity as defined by the modified Kellgren‐Lawrence classification Exclusion Criteria
|
| Interventions |
Arthroscopic surgery plus optimised physical and medical therapy: arthroscopic surgery to treat unresolved symptoms of osteoarthritis of the knee Optimised physical and medical therapy: treatment with physical and medical therapy alone |
| Outcomes |
Primary outcome measures: Function, pain and quality of life based on the WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) scores (time frame: 2 years) Secondary outcome measures: Health utility using the standard‐gamble technique (time frame: 24 months) |
| Notes |
Clinical trial registration:NCT00562822 Trial status: recruitment completed Estimated study completion date: recruitment completed in August 2007; however, results not available yet |
Characteristics of ongoing studies [ordered by study ID]
NCT02113280.
| Study name | DEMAND ‐ DEgenerative Meniscal Tears ‐ Arthroscopy vs. Dedicated Exercise Official title: Randomised Controlled Trial Comparing Arthroscopy With Physiotherapy for Degenerative Meniscal Tears |
| Methods |
Study design: prospective parallel‐arm randomised controlled trial Setting: North Tyneside General Hospital, UK Trial time period: December 2015 to December 2018 Interventions: arthroscopic surgery versus physiotherapy Sample size calculations: not reported Analysis: not reported |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Arthroscopy: participants to receive knee arthroscopy and meniscal debridement Physiotherapy: outpatient standardised physiotherapy regime with focus on exercise therapy |
| Outcomes | Outcomes will be measured at baseline, 6 weeks and 6, 12 and 24 months. Primary outcome
Secondary outcomes
|
| Starting date | December 2015 |
| Contact information | Sarah Johnson‐Lynn, MBBS Specialty Registrar, Northumbria Healthcare NHS Foundation Trust 08448118111 ext 2508 selynn@doctors.org.uk Derek Kramer, MBBS 08448118111 ext 2508 derek.kramer@northumbria‐healthcare.nhs.uk |
| Notes |
Clinical trial registration:NCT02113280 Trial status: withdrawn (failed funding application) Expected completion date: December 2018 |
NCT02995551.
| Study name | Danish RCT on Exercise versus Arthroscopic Meniscal Surgery for Young Adults (DREAM) Official title: Danish Rct on Exercise versus Arthroscopic Meniscal Surgery for Young Adults (DREAM) ‐ A Randomized Controlled Trial of Meniscal Tear Treatment in Young Adults |
| Methods |
Study design: prospective, parallel‐arm, multicentre, randomised controlled trial Setting: Denmark Trial time period: January 2017 to June 2018 Interventions: arthroscopic meniscal surgery versus individualised supervised exercise therapy and education Sample size calculations: 59 participants in each of the intervention groups is needed (assuming a common standard deviation (SD) of 16.5, power = 90%, alpha level = 0.05) to detect a clinically relevant difference of 10 points in the primary outcome (KOOS4) from baseline to 12 months' follow‐up. A total of 140 participants will be recruited to account for loss to follow‐up (19%). Analysis: intention‐to‐treat |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Arthroscopic meniscal surgery Arthroscopic meniscal repair or resection will be conducted at the discretion of the operating surgeon at one of the six hospitals. The specific surgical procedure (i.e. repair or resection) cannot be determined before the surgeon has visual confirmation about the exact knee pathology and extent of the meniscal tear at arthroscopy. Exercise therapy and patient education Participants allocated to exercise therapy and participant education will twice weekly participate in a 12‐week individualised, supervised exercise programme (approximately 60 to 90 minutes/session) tailored to 18 to 40 years old participants with meniscal tear |
| Outcomes | Outcomes will be measured at 3, 6 and 12 months' follow‐up Primary outcome Change in Knee injury and Osteoarthritis Outcome Score (KOOS) scores from baseline to follow‐up. KOOS4 is the mean score for the KOOS subscales pain, symptoms, function in sports and recreational activities (Sport/Rec) and quality of life (QOL) Secondary outcomes:
|
| Starting date | January 2017 |
| Contact information | Principal Investigator: Søren Thorgaard Skou, PT, PhD, Assistant Professor, University of Southern Denmark Tel: +4523708640; Email: stskou@health.sdu.dk |
| Notes |
Clinical trial registration:NCT02995551 Trial status: recruiting participants Expected completion date: December 2020 |
NCT04313569.
| Study name | Arthroscopic Versus Conservative Treatment of Degenerative Meniscal Tear in Middle Aged Patients in Regard to Pain & Knee Function Official title: Arthroscopic Versus Conservative Treatment of Degenerative Meniscal Tear in Middle Aged Patients in Regard to Pain & Knee Function: Comparative Study |
| Methods |
Study design: single‐centre, parallel‐group, two‐arm, randomised controlled trial Setting: Erbil teaching hospital, Iran Trial time period: August 2017 to September 2019 Interventions: arthroscopic meniscectomy versus conservative treatment Sample size calculations: 60 participants, 30 in each group Analysis: not reported |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention ‐ arthroscopic meniscectomy Control ‐ conservative treatment Use of medications, like analgesics, muscle relaxants, non‐steroidal anti‐inflammatory drugs (NSAID) and local painkillers, depending on participants' condition, and physiotherapy, lifestyle and daily activity modification, participant education about positioning of the knee. |
| Outcomes |
Primary outcomes: Lysholm Knee Scoring Scale at 1 year: Lysholm Knee score scale has 8 parts (swelling, pain, squatting, support, stair climbing, limping, locking and instability). Score of 100 means no problems in knee. Visual analogue score at 1 year: 10‐point Visual Analogue Score scale used in order to measure the severity of knee pain during the study. 0 = no pain; 10 = most severe pain Secondary outcomes: None specified |
| Starting date | 1 August 2017 |
| Contact information | Sherwan Ahmed Ali Hamawandi, Assistant Professor of Orthopedic Surgery, Hawler Medical University |
| Notes |
Clinical trial registration:NCT04313569 Trial status: recruitment completed Expected completion date: 30 September 2019. No results available. |
NCT04837456.
| Study name | Metabolic Syndrome and Degenerate Meniscus Tears |
| Methods |
Study design: single centre, double‐blind, parallel‐group, four‐arm randomised controlled trial Setting: First Affiliated Hospital of Jinzhou Medical University, China Trial time period: June 2017 to March 2020 Interventions: calorie‐restricted diet and exercise intervention group; libitum diet and waiting list control group; early arthroscopic partial meniscectomy (APM) (syndrome within 3 to 6 months) group or a delayed APM (syndrome more than 6 months) group Sample size calculations: 180 participants Analysis: not reported |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Calorie restricted diet and exercise intervention: A balanced diet that provided an energy deficit of 800 kcal/day from their daily energy requirement. Macronutrient content of low caloric diet, expressed as percentage of ingested energy with carbohydrates 45‐65%; fat 20‐35%; and protein 10‐35%. Each session was approximately 150 minutes one week for six months and consisted of aerobic exercises, resistance training, and exercises to improve flexibility and balance. Libitum diet and waiting list control group: participants then underwent a calorie of 2000 calorie above based on libitum free diets recommended to adults and normal physical activity without exercise during the program. Early APM group: Early APM group participants received APM with symptoms within 3 to 6 months. Delayed APM group: Delayed APM group recruit participants with symptoms lasting for more than 6 months. |
| Outcomes |
Primary outcomes Knee KOOS 4 WOMAC International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form The WOMET score Kellgren‐Lawrence grade BMI Lysholm knee score Blood tests |
| Starting date | 1 June 2017 |
| Contact information | Hongyu Wang, chief resident, The First People's Hospital of Jingzhou |
| Notes |
Clinical trial registration:NCT04837456 Trial status: recruitment completed Expected completion date: 1 March 2021 |
Differences between protocol and review
We extracted end‐of‐treatment mean (SD) for pain, function, quality of life (QoL), used SMD as scales differed, then back‐translated using SD from control at baseline (in comparison, the earlier review converted all to change‐from‐baseline using a common scale, then calculated difference in change scores between groups, and used MD).
Our planned outcome was subsequent knee replacement but we changed it to subsequent knee surgery (including knee replacement and any other surgery to treat severe knee osteoarthritis; e.g. high tibial osteotomy).
Contributions of authors
RB conceived the review. DOC, RJ and RB drafted the protocol. RJ and SC screened titles and abstracts. RJ, SC and DOC made decisions about inclusion of trials. DOC and SC extracted data. DOC, SC and RJ assessed risk of bias of included trials. DOC and RJ applied GRADE to draw conclusions about the certainty of the body of evidence. SC sought missing data from trial authors and calculated/imputed data if required. DOC analysed data and drafted the review. RB and RJ contributed to writing the review. All authors reviewed and approved the final version.
Sources of support
Internal sources
-
Monash Department of Clinical Epidemiology, Cabrini Institute, Australia
In kind support
-
Department of Epidemiology and Preventive Medicine, School of Public Health and Preventive Medicine, Monash University, Australia
In kind
External sources
-
National Health and Medical Research Council (NHMRC), Australia
Denise O'Connor and Rachelle Buchbinder are supported by NHMRC fellowships
Declarations of interest
Denise O'Connor is an Editor, Renea Johnston a Managing Editor, Sheila Cyril an Assistant Managing Editor and Rachelle Buchbinder the Coordinating Editor with Cochrane Musculoskeletal but they were not involved in editorial decisions regarding this review. Denise O'Connor is also Editor with Cochrane Effective Practice and Organisation of Care (EPOC). They are recipients of an Australian National Health and Medical Research Council (NHMRC) Cochrane Collaboration Round 7 Funding Program Grant, which supports the activities of Cochrane Musculoskeletal ‐ Australia and Cochrane Australia, but the funders do not participate in the conduct of this review. Denise O'Connor is supported by an Australian NHMRC Translating Research into Practice (TRIP) Fellowship (APP1168749). Rachelle Buchbinder is supported by an Australian NHMRC Investigator Grant (APP1194483).
RBP: none known
RWP: none known
POV: none known
New
References
References to studies included in this review
Chang 1993 {published and unpublished data}
- Chang RW, Facloner J, Stulberg SD, Arnold WJ, Dyer AR.Prerandomization: an alternative to classic randomization. Journal of Bone and Joint Surgery 1990;72-A:1451-5. [PubMed] [Google Scholar]
- Chang RW, Falconer J, Stulberg SD, Arnold WJ, Manheim LM, Dyer AR.A randomized, controlled trial of arthroscopic surgery versus closed-needle joint lavage for patients with osteoarthritis of the knee. Arthritis and Rheumatism 1993;36(3):289–96. [DOI] [PubMed] [Google Scholar]
Gauffin 2014 {published and unpublished data}
- Gauffin H, Kvist J, Hedevik H, Sonesson S.Knee arthroscopic surgery in middle-aged patients with meniscal symptoms: a 5-year follow-up of a prospective, randomized study. Osteoarthritis and Cartilage 2019;27:S-231. [Google Scholar]
- Gauffin H, Sonesson S, Meunier A, Magnusson H, Kvist J.Knee arthroscopic surgery in middle-aged patients with meniscal symptoms: a 3-year follow-up of a prospective, randomized study. American Journal of Sports Medicine 2017;45(9):2077-84. [DOI] [PubMed] [Google Scholar]
- Gauffin H, Tagesson S, Meunier A, Magnusson H, Kvist J.Knee arthroscopic surgery is beneficial to middle-aged patients with meniscal symptoms: a prospective, randomised, single-blinded study. Osteoarthritis and Cartilage 2014;22:1808–16. [DOI] [PubMed] [Google Scholar]
- Sonesson S, Kvist J, Yacob J, Hedevik H, Gauffin H.Knee arthroscopic surgery in middle-aged patients with meniscal symptoms: a 5-year follow-up of a prospective, randomized study. Orthopaedic Journal of Sports Medicine 2020;8(1):[12 p.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herrlin 2007 {published and unpublished data}
- Herrlin S, Hallander M, Wange P, Weidenhielm L, Werner S.Arthroscopic or conservative treatment of degenerative medial meniscal tears: a prospective randomised trial. Knee Surgery, Sports Traumatology, Arthroscopy 2007;15:393–401. [DOI] [PubMed] [Google Scholar]
- Herrlin SV, Wange PO, Lapidus G, Hallander M, Werner S, Weidenhielm L.Is arthroscopic surgery beneficial in treating non-traumatic, degenerative medial meniscal tears? A five year follow-up. Knee Surgery, Sports Traumatology, Arthroscopy 2013;21(2):358-364. [DOI] [PubMed] [Google Scholar]
Katz 2013 {published and unpublished data}
- Collins JE, Losine E, Marx RG, Guermazi A, Jarraya M, Jones MH, et al, MeTeOR Investigator Group.Early magnetic resonance imaging-based changes in patients with meniscal tear and osteoarthritis: eighteen-month data from a randomized controlled trial of arthroscopic partial meniscectomy versus physical therapy. Arthritis Care & Research 2020;72:630-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JN, Brophy RH, Chaisson CE, Chaves L, Cole BJ, Dahm DL, et al.Surgery versus physical therapy for a meniscal tear and osteoarthritis. New England Journal of Medicine 2013;368(18):1675–84. [NCT00597012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JN, Chaisson CE, Cole B, Guermazi A, Hunter DJ, Jones M, et al.The MeTeOR trial (Meniscal Tear in Osteoarthritis Research): rationale and design features. Contemporary Clinical Trials 2012;33(6):1189-96. [NCT00597012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JN, Shrestha S, Losina E, Jones MH, Marx RG, Mandl LA, et al, METEOR Investigators.Five-year outcome of operative and nonoperative management of meniscal tear in persons older than forty-five years. Arthritis and Rheumatology 2020;72(2):273-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JN, Shrestha S, Losina E, Jones MH, Marx RG, Mandl LA, METEOR Investigators.Five-year outcome of operative and non-operative management of meniscal tear in persons greater than 45 years old. Arthritis and Rheumatology 2020;72(2):273-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JN, Shrestha S, Losina E, Mandl LA, Levy B, Spindler K, et al.Five-year outcome of operative and nonoperative management of meniscal tear in the presence of osteoarthritic changes [abstract]. Arthritis and Rheumatology 2018;70(Suppl 10):Abstract no. 1816. [Google Scholar]
- Katz JN, Spindler K, Safran-Norton C, Martin S, Mandl L, Jones M, et al.Predictors and outcomes of cross-over to surgery in a randomized trial of surgery vs. physical therapy for meniscal tear and osteoarthritis. Osteoarthritis and Cartilage 2015;23(Suppl 2):A36. [Google Scholar]
- Shrestha S, Katz J, Losina E, Collins J.Five year structural changes in patients with meniscal tear and osteoarthritis from an RCT of arthroscopic partial meniscectomy vs. physical therapy. Arthritis and Rheumatology 2019;71(Suppl 10):4975-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkley 2008 {published and unpublished data}
- Kirkley A, Birmingham TB, Litchfield RB, Giffin JR, Willits KR, Wong CJ, et al.A randomized trial of arthroscopic surgery for osteoarthritis of the knee. New England Journal of Medicine 2008;359(11):1097–107. Erratum in: New England Journal of Medicine 2009; 361(20):2004. [DOI] [PubMed] [Google Scholar]
- Marsh J, Birmingham TB, Giffin JR, Isaranuwatchai W, Hoch JS, Litchfield R, et al.Cost-effectiveness analysis of arthroscopic surgery compared to non-operative management for osteoarthritis of the knee. Osteoarthritis and Cartilage 2015;23(Suppl 2):A31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh JD, Birmingham TB, Giffin JR, Isaranuwatchai W, Hoch JS, Feagan BG, et al.Cost-effectiveness analysis of arthroscopic surgery compared with non-operative management for osteoarthritis of the knee. BMJ Open 2016;6(1):e009949. [NCT00158431] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kise 2016 {published and unpublished data}
- Berg B, Roos EM, Englund M, Kise NJ, Tiulpin A, Saarakkala S, et al.Development of osteoarthritis in patients with degenerative meniscal tears treated with exercise therapy or surgery: a randomized controlled trial. Osteoarthritis and Cartilage 2020;28(7):897-906. [DOI] [PubMed] [Google Scholar]
- Berg B, Roos EM, Englund M, Kise NJ, Tiulpin A, Saarakkala S, et al.Knee osteoarthritis development five years following arthroscopic partial meniscectomy or exercise therapy for degenerative meniscal tears: the Odense-Oslo meniscectomy versus exercise trial. Osteoarthritis and Cartilage 2020;28(Suppl):S28. [DOI: 10.1016/j.joca.2020.02.045] [DOI] [Google Scholar]
- Kise NJ, Risberg MA, Stensrud S, Ranstam J, Engebretsen L, Roos EM.Exercise therapy versus arthroscopic partial meniscectomy for degenerative meniscal tear in middle aged patients: randomised controlled trial with two year follow-up. BMJ 2016;354:i3740. [NCT01002794] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensrud S, Risberg MA, Roos EM.Effect of exercise therapy compared with arthroscopic surgery on knee muscle strength and functional performance in middle-aged patients with degenerative meniscus tears: a 3-mo follow-up of a randomized controlled trial. American Journal of Physical Medicine and Rehabilitation 2015;94(6):460–73. [NCT01002794] [DOI] [PubMed] [Google Scholar]
Merchan 1993 {published and unpublished data}
- Merchan EC and Galindo E.Arthroscope-guided surgery versus nonoperative treatment for limited degenerative osteoarthritis of the femorotibial joint in patients over 50 years of age: a prospective comparative study. Arthroscopy 1993;9(6):663-7. [DOI] [PubMed] [Google Scholar]
Moseley 1996 {published data only}
- Moseley JB Jr, Wray NP, Kuykendall D, Willis K, Landon G.Arthroscopic treatment of osteoarthritis of the knee: a prospective, randomized, placebo-controlled trial. Results of a pilot study. American Journal of Sports Medicine 1996;24(1):28-34. [DOI] [PubMed] [Google Scholar]
Moseley 2002 {published data only}
- Moseley JB, O'Malley K, Petersen NJ, Menke TJ, Brody BA, Kuykendall DH, et al.A controlled trial of arthroscopic surgery for osteoarthritis of the knee. New England Journal of Medicine 2002;347(2):81-8. [DOI] [PubMed] [Google Scholar]
Osteras 2012 {published and unpublished data}
- Osteras H, Osteras B, Torstensen TA.Medical exercise therapy, and not arthroscopic surgery, resulted in decreased depression and anxiety in patients with degenerative meniscus injury. Journal of Bodywork and Movement Therapies 2012;16(4):456–63. [DOI] [PubMed] [Google Scholar]
- Osteras H, Torstensen TA, Selven E, Haugerud L.High dosage medical exercise therapy or arthroscopic treatment for patients with degenerative meniscus injury: a pilot study. Physiotherapy 2011;97(Suppl 1):eS946-7. [Google Scholar]
Roos 2018 {published data only}
- Hare KB, Lohmander LS, Christensen R, Roos EM.Arthroscopic partial meniscectomy in middle-aged patients with mild or no knee osteoarthritis: a protocol for a double-blind, randomized sham-controlled multi-centre trial. BMC Musculoskeletal Disorders 2013;14:71. [NCT01264991] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare KB, Lohmander LS, Roos EM.The challenge of recruiting patients into a placebo-controlled surgical trial. Trials 2014;15:167. [NCT01264991] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare KB, Lohmander S, Roos EM.The challenges of recruiting patients into a sham surgery trial. Osteoarthritis and Cartilage 2012;20:S173-4. [NCT01264991] [Google Scholar]
- Roos EM, Hare KB, Nielsen SB, Christensen R, Lohmander LS.Better outcome from arthroscopic partial meniscectomy than skin incisions only? A sham-controlled randomised trial in patients aged 35–55 years with knee pain and an MRI-verified meniscal tear. BMJ Open 2018;8(2):[9 p.]. [DOI: ] [NCT01264991] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saeed 2015 {published data only}
- Saeed K, Khan SA, Ahmed I.Efficacy of intra articular hyaluronic acid versus arthroscopic debridement in terms of improvement in pain score in Kellgran-Lawrence Grading II & III osteoarthritis of knee joint. Pakistan Journal of Medical and Health Sciences 2015;9(3):1011–5. [Google Scholar]
Sihvonen 2013 {published and unpublished data}
- Jarvinen T, Sihvonen R, Paavola M, Malmivaara A, Itala A, Joukainen A, et al.Arthroscopic partial meniscectomy vs sham surgery for degenerative meniscus tear. Arthroscopy 2014;30(6 Supp 1):e38. [Google Scholar]
- Sihvonen R, Englund M, Turkiewicz A, Järvinen TL, Finnish Degenerative Meniscal Lesion Study Group.Mechanical symptoms and arthroscopic partial meniscectomy in patients with degenerative meniscus tear: a secondary analysis of a randomized trial. Annals of Internal Medicine 2016;164(7):449-55. [DOI: 10.7326/M15-0899] [NCT00549172] [DOI] [PubMed] [Google Scholar]
- Sihvonen R, Kalske R, Englund M, Turkiewics A, Toivonen P, Taimela S, Finnish Degenerative Meniscal Lesion Study (FIDELITY) Investigators.Statistical analysis plan for the 5-year and 10-year follow-up assessments of the FIDELITY trial: study protocol. Trials 2020;21:76. [NCT01052233] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihvonen R, Paavola M, Malmivaara A, Itälä A, Joukainen A, Kalske J, FIDELITY (Finnish Degenerative Meniscus Lesion Study) Investigators.Arthroscopic partial meniscectomy for a degenerative meniscus tear: a 5 year follow-up of the placebo-surgery controlled FIDELITY (Finnish Degenerative Meniscus Lesion Study) trial. British Journal of Sports Medicine 2020;54(22):1332-9. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihvonen R, Paavola M, Malmivaara A, Itala A, Joukainen A, Nurmi H, et al.Arthroscopic partial meniscectomy versus placebo surgery for a degenerative meniscus tear: a 2-year follow-up of the randomised controlled trial. Annals of the Rheumatic Diseases 2018;77:188-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihvonen R, Paavola M, Malmivaara A, Itala A, Joukainen A, Nurmi H, et al.Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. New England Journal of Medicine 2013;369(26):2515–24. [NCT00549172] [DOI] [PubMed] [Google Scholar]
- Sihvonen R, Paavola M, Malmivaara A, Järvinen TL.Finnish Degenerative Meniscal Lesion Study (FIDELITY): a protocol for a randomised, placebo surgery controlled trial on the efficacy of arthroscopic partial meniscectomy for patients with degenerative meniscus injury with a novel 'RCT within-a-cohort' study design. BMJ Open 2013;3(3):e002510. [DOI: 10.1136/bmjopen-2012-002510] [NCT00549172] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van de Graaf 2018 {published data only}
- Noorduyn JC, Glastra van Loon T, Van de Graaf VA, Willigenburg NW, Butter IK, Scholten-Peeters GG, et al.Functional outcomes of arthroscopic partial meniscectomy versus physical therapy for degenerative meniscal tears using a patient-specific score: a randomized controlled trial. Orthopaedic Journal of Sports Medicine 2020;8(10):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Graaf VA, Noorduyn JC, Willigenburg NW, et al.Effect of early surgery vs physical therapy on knee function among patients with nonobstructive meniscal tears: the ESCAPE randomized clinical trial. JAMA 2018;320(13):1328–37. [DOI: 10.1001/jama.2018.13308] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Graaf VA, Scholtes VA, Wolterbeek N, Noorduyn JC, Neeter C, Van Tulder MW, et al, Escape Research Group.Cost-effectiveness of early surgery versus conservative treatment with optional delayed meniscectomy for patients over 45 years with non-obstructive meniscal tears (ESCAPE study): protocol of a randomised controlled trial. BMJ Open 2016;6:e014381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Graaf VA, Van Dongen JM, Willigenburg NW, Noorduyn JC, Butter IK, De Gast A, et al, ESCAPE Research Group.How do the costs of physical therapy and arthroscopic partial meniscectomy compare? A trial-based economic evaluation of two treatments in patients with meniscal tears alongside the ESCAPE study. British Journal of Sports Medicine 2020;54:538-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vermesan 2013 {published and unpublished data}
- Vermesan D, Prejbeanu R, Laitin S, Damian G, Deleanu B, Abbinante A, et al.Arthroscopic debridement compared to intra-articular steroids in treating degenerative medial meniscal tears. European Review for Medical and Pharmacological Sciences 2013;17:3192–6. [PubMed] [Google Scholar]
Yim 2013 {published and unpublished data}
- Yim JH, Seon JK, Song EK, Choi JL, Kim MC, Lee KB, et al.A comparative study of meniscectomy and nonoperative treatment for degenerative horizontal tears of the medial meniscus. American Journal of Sports Medicine 2013;41(7):1565–70. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahn 2015 {published data only}
- Ahn JH, Jeong HJ, Lee YS, Park JH, Lee JW, Park J-H, et al.Comparison between conservative treatment and arthroscopic pull-out repair of the medial meniscus root tear and analysis of prognostic factors for the determination of repair indication. Archives of Orthopaedic and Trauma Surgery 2015;135:1265–76. [DOI] [PubMed] [Google Scholar]
Biedert 2000 {published data only}
- Biedert RM.Treatment of intrasubstance meniscal lesions: a randomized prospective study of four different methods. Knee Surgery, Sports Traumatology, Arthroscopy 2000;8(2):104-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bisson 2015 {published data only}
- Bisson LJ, Kluczynski MA, Wind WM, Fineberg MS, Bernas GA, Rauh MA, et al.Design of a randomized controlled trial to compare debridement to observation of chondral lesions encountered during partial meniscectomy: the ChAMP (Chondral Lesions And Meniscus Procedures) trial. Contemporary Clinical Trials 2015;45(Pt B):281-6. [DOI] [PubMed] [Google Scholar]
- Bisson LJ, Kluczynski MA, Wind WM, Fineberg MS, Bernas GA, Rauh MA, et al.Patient outcomes after observation versus debridement of unstable chondral lesions during partial meniscectomy. Journal of Bone and Joint Surgery 2017;99(13):1078-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bradley 2002 {published data only}
- Bradley JD, Heilman DK, Katz BP, Gsell P, Wallick JE, Brandt KD.Tidal irrigation as treatment for knee osteoarthritis: a sham-controlled, randomized, double-blinded evaluation. Arthritis and Rheumatology 2002;46(1):100-8. [DOI] [PubMed] [Google Scholar]
Hubbard 1996 {published data only}
- Hubbard MJ.Articular debridement versus washout for degeneration of the medial femoral condyle. Journal of Bone and Joint Surgery 1996;78:B:217-9. [PubMed] [Google Scholar]
Kalunian 2000 {published data only}
- Kalunian KC, Moreland LW, Klashman DJ, Brion PH, Concoff AL, Myers S, et al.Visually-guided irrigation in patients with early knee osteoarthritis: a multicenter randomized, controlled trial. Osteoarthritis and Cartilage 2000;8:412–8. [DOI] [PubMed] [Google Scholar]
Lee 2020 {published data only}
- Lee SH, Lee OS, Kim ST, Lee YS.Revisiting arthroscopic partial meniscectomy for degenerative tears in knees with mild or no osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Clinical Journal of Sports Medicine 2020;30(3):195-202. [DOI: 10.1097/JSM.0000000000000585] [DOI] [PubMed] [Google Scholar]
Lu 2018 {published data only}
- Lu Y-C, Bi B, Xiang Y-S, Du X-Y.Effectiveness and safety of arthroscopic debridement for treatment of degenerative knee osteoarthritis in elderly patients: study protocol for a non-randomized controlled trial. Clinical Trials in Degenerative Diseases 2018;3(1):15-21. [Google Scholar]
Ma 2020 {published data only}
- Ma J, Chen H, Liu A, Cui Y, Ma X.Medical exercise therapy alone versus arthroscopic partial meniscectomy followed by medical exercise therapy for degenerative meniscal tear: a systematic review and meta-analysis of randomized controlled trials. Journal of Orthopaedic Surgery and Research 2020;15(1):219. [DOI: 10.1186/s13018-020-01741-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marsh 2016 {published data only}
- Marsh JD, Birmingham TB, Giffin JR, Isaranuwatchai W, Hoch JS, Feagan BG, et al.Cost-effectiveness analysis of arthroscopic surgery compared with non-operative management for osteoarthritis of the knee. BMJ Open 2016;6(5):e009949. [DOI: 10.1136/bmjopen-2015-009949] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pan 2020 {published data only}
- Pan H, Zhang P, Zhang Z, Yang Q.Arthroscopic partial meniscectomy combined with medical exercise therapy versus isolated medical exercise therapy for degenerative meniscal tear: a meta-analysis of randomized controlled trials. International Journal of Surgery 2020;79:222-32. [DOI: 10.1016/j.ijsu.2020.05.035] [DOI] [PubMed] [Google Scholar]
Rimington 2009 {published data only}
- Rimington T, Mallik K, Evans D, Mroczek K, Reider B.A prospective study of the non-operative treatment of degenerative meniscus tears. Orthopedics 2009;32(8):[no pagination]. [DOI: 10.3928/01477447-20090624-06] [DOI] [PubMed] [Google Scholar]
Wijn 2020 {published data only}
- Wijn SR, Rovers MM, Rongen JJ, Østerås H, Risberg MA, Roos EM, et al.Arthroscopic meniscectomy versus non-surgical or sham treatment in patients with MRI confirmed degenerative meniscus lesions: a protocol for an individual participant data meta-analysis. BMJ Open 2020;10:e031864. [DOI: 10.1136/bmjopen-2019-031864] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2018 {published data only}
- Zhang Y-F, Liu H.Clinical efficacy of knee arthroscopy in the treatment of degenerative knee osteoarthritis. Biomedical Research 2018;29(5):958-61. [DOI: 10.4066/biomedicalresearch.29-17-3351] [DOI] [Google Scholar]
Zhao 2018 {published data only}
- Zhao B, Yu Y, Liu W, Du J.Efficacy of arthroscopic loose body removal for knee osteoarthritis. Experimental and Therapeutic Medicine 2018;15(2):1666-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Kang 2005 {published data only}
- Kang JG.Treatment of knee osteoarthritis with arthroscopic debridement and intra-articular sodium hyaluronate injection. Journal of Jilin University Medicine Edition 2005;31(5):802-5. [Google Scholar]
NCT00562822 {published data only}
- NCT00562822.Surgery versus no surgery for osteoarthritis (OA) of the knee (MRC Knee). clinicaltrials.gov/ct2/show/NCT00562822 (first received 27 November 2007). [NCT00562822]
References to ongoing studies
NCT02113280 {published data only}
- NCT02113280.DEMAND - DEgenerative Meniscal Tears - Arthroscopy vs. Dedicated Exercise (DEMAND). clinicaltrials.gov/ct2/show/NCT02113280 (first received 14 April 2014). [NCT02113280]
NCT02995551 {published data only}
- NCT02995551.Danish RCT on Exercise versus Arthroscopic Meniscal surgery for young adults (DREAM). clinicaltrials.gov/ct2/show/NCT02995551 (first received 16 December 2016). [NCT02995551]
- Skou ST, Lind M, Hölmich P, Jensen HP, Jensen C, Afzal M, et al.Study protocol for a randomised controlled trial of meniscal surgery compared with exercise and patient education for treatment of meniscal tears in young adults. BMJ Open 2017;7(8):e017436. [DOI: 10.1136/bmjopen-2017-017436] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT04313569 {published data only}
- NCT04313569.Arthroscopic versus conservative treatment of degenerative meniscal tear in middle aged patients in regard to pain and knee function. clinicaltrials.gov/ct2/show/NCT04313569 (first received 18 March 2020).
NCT04837456 {published data only}
- NCT04837456.Metabolic syndrome and degenerate meniscus tears. clinicaltrials.gov/ct2/show/NCT04837456 (first received 8 April 2021).
Additional references
Abram 2019a
- Abram SG, Beard DJ, Price AJ, on behalf of the BASK Meniscal Working Group.Arthroscopic meniscal surgery: a national society treatment guideline and consensus statement. Bone and Joint Journal 2019;101-B:652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Abram 2019b
- Abram SG, Judge A, Beard D, Price A.Rates of knee arthroplasty within one-year of undergoing arthroscopic partial meniscectomy in England: temporal trends, regional and age-group variation in conversion rate. Osteoarthritis and Cartilage 2019;27(10):1420-9. [DOI] [PubMed] [Google Scholar]
Abram 2020
- Abram SG, Hopewell S, Monk AP, Bayliss LE, Beard DJ, Price AJ.Arthroscopic partial meniscectomy for meniscal tears of the knee: a systematic review and meta-analysis. British Journal of Sports Medicine 2020;54(11):652-63. [DOI] [PubMed] [Google Scholar]
ACQSHC 2017
- Australian Commission on Safety and Quality in Health Care.Osteoarthritis of the Knee Clinical Care Standard. Sydney, Australia: ACSQHC 2017.
Ahlback 1968
- Ahlback S.Osteoarthritis of the knee. A radiographic investigation. Acta Radiologica 1968;277:7–72. [PubMed] [Google Scholar]
Alkan 2014
- Alkan BM, Fidan F, Tosun A, Ardıçoğlu O.Quality of life and self-reported disability in patients with knee osteoarthritis. Modern Rheumatology 2014;24:166–71. [DOI] [PubMed] [Google Scholar]
Australian Knee Society 2016
- Australian Knee Society.Position Statement from the Australian Knee Society on Arthroscopic Surgery of the Knee, including reference to the presence of Osteoarthritis or Degenerative Joint Disease. Australian Orthopaedic Association October 2016. [DOI] [PMC free article] [PubMed]
Barlow 2015
- Barlow T, Downham C, Griffin D.Arthroscopy in knee osteoarthritis: a systematic review of the literature. Acta Orthopaedica Belgica 2015;81(1):1-8. [PubMed] [Google Scholar]
Bartel 2016
- Bartels EM, Juhl CB, Christensen R, Hagen KB, Danneskiold‐Samsøe B, Dagfinrud H, et al.Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database of Systematic Reviews 2016, Issue 3. Art. No: CD005523. [DOI: 10.1002/14651858.CD005523.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhattacharyya 2003
- Bhattacharyya T, Gale D, Dewire P, Totterman S, Gale ME, McLaughlin S, et al.The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. Journal of Bone and Joint Surgery. American Volume 2003;85:4-9. [DOI] [PubMed] [Google Scholar]
Brignardello‐Petersen 2017
- Brignardello-Petersen R, Guyatt GH, Buchbinder R, Poolman RW, Schandelmaier S, Chang Y, et al.Knee arthroscopy versus conservative management in patients with degenerative knee disease: a systematic review. BMJ Open 2017;7:e016114. [DOI: 10.1136/bmjopen-2017-016114] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brouwer 2014
- Brouwer RW, Huizinga MR, Duivenvoorden T, Van Raaij TM, Verhagen AP, Bierma-Zeinstra SM, et al.Osteotomy for treating knee osteoarthritis.. Cochrane Database of Systematic Reviews 2014, Issue 12. Art. No: CD004019. [DOI: 10.1002/14651858.CD004019.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2013
- Brown GA.AAOS clinical practice guideline: treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. Journal of the American Academy of Orthopedic Surgeons 2013;21:577–9. [DOI] [PubMed] [Google Scholar]
Buchbinder 2015
- Buchbinder R, Harris IA, Sprowson A.Management of degenerative meniscal tears and the role of surgery. BMJ 2015;350:h2212. [DOI: 10.1136/bmj.h2212] [DOI] [PubMed] [Google Scholar]
Cates 2008 [Computer program]
- Visual Rx.Cates C, Version 3. Dr. Christopher Cates EBM website, 2008. Available at www.nntonline.net.
Christensen 2007
- Christensen R, Bartels EM, Astrup A, Bliddal H.Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta‐analysis. Annals of the Rheumatic Diseases 2007;66(4):433-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dawson 1998
- Dawson J, Fitzpatrick R, Murray D, Carr A.Questionnaire on the perceptions of patients about total knee replacement. Journal of Bone and Joint Surgery. British Volume 1998;80(1):63-9. [DOI] [PubMed] [Google Scholar]
Dearing 2010
- Dearing J, Brenkel I.Incidence of knee arthroscopy in patients over 60 years of age in Scotland. Surgeon 2010;8:144-50. [DOI] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG (editors).Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021), Cochrane, 2021. Available from www.training.cochrane.org/handbook/archive/v6.2/chapter-10.
Devji 2017
- Devji T, Guyatt GH, Lytvyn L, Brignardello-Petersen R, Foroutan F, Sadeghirad B, et al.Application of minimal important differences in degenerative knee disease outcomes: a systematic review and case study to inform BMJ Rapid Recommendations. BMJ Open 2017;7:e015587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Englund 2008
- Englund M, Guermazi A, Gale D, Hunter DJ, Aliabadi P, Clancy M, et al.Incidental meniscal findings on knee MRI in middle-aged and elderly persons. New England Journal of Medicine 2008;359:1108-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fransen 2015
- Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL.Exercise for osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2015, Issue 1. Art. No: CD004376. [DOI: 10.1002/14651858.CD004376.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghogomu 2014
- Ghogomu EA, Maxwell LJ, Buchbinder R, Rader T, Pardo Pardo J, Johnston RV, et al.Updated method guidelines for Cochrane musculoskeletal group systematic reviews and meta-analyses. Journal of Rheumatology 2014;41(2):194-205. [DOI] [PubMed] [Google Scholar]
GRADEPro GDT [Computer program]
- GRADEpro GDT.Version accessed prior to 14 February 2022. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available from www.gradepro.org.
Guermazi 2012
- Guermazi A, Niu J, Hayashi D, Roemer FW, Englund M, Neogi T, et al.Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study). BMJ 2012;345:e5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ha 2016
- Ha AY, Shalvoy RM, Voisinet A, Racine J, Aaron RK.Controversial role of arthroscopic meniscectomy of the knee: a review. World Journal of Orthopedics 2016;7(5):287-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2013
- Harris IA, Madan NS, Naylor JM, Chong S, Mittal R, Jalaludin BB.Trends in knee arthroscopy and subsequent arthroplasty in an Australian population: a retrospective cohort study. BMC Musculoskeletal Disorders 2013;14:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawker 2008
- Hawker G, Guan J, Judge A, Dieppe P.Knee arthroscopy in England and Ontario: patterns of use, changes over time, and relationship to total knee replacement. Journal of Bone and Joint Surgery 2008;90:2337-45. [DOI] [PubMed] [Google Scholar]
Health Quality Ontario 2014
- Evidence Development and Standards Branch, Health Quality Ontario.Arthroscopic debridement of the knee: an evidence update. Ontario Health Technology Assessment Series 2014;14(13):1-43. [PMC free article] [PubMed]
Hegedus 2007
- Hegedus EJ, Cook C, Hasselblad V, Goode A, McCrory DC.Physical examination tests for assessing a torn meniscus in the knee: a systematic review with meta-analysis. Journal of Orthopaedic and Sports Physical Therapy 2007;37:541-50. [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s).Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2021
- Higgins JP, Li T, Deeks JJ, editor(s).Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Hohmann 2018
- Hohmann E, Glatt V, Tetsworth K, Cote M.Arthroscopic partial meniscectomy versus physical therapy for degenerative meniscus lesions: how robust is the current evidence? A critical systematic review and qualitative synthesis. Journal of Arthroscopic and Related Surgery 2018;34(9):2699-708. [DOI] [PubMed] [Google Scholar]
Howell 2014
- Howell R, Kumar NS, Patel N, Tom J.Degenerative meniscus: pathogenesis, diagnosis, and treatment options. World Journal of Orthopedics 2014;5(5):597-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ike 1992
- Ike RW, Arnold WJ, Rothschild E, Shaw HL, the Tidal Irrigation Cooperating Group.Tidal irrigation versus conservative medical management in patients with osteoarthritis of the knee: a prospective, randomized study. Journal of Rheumatology 1992;19:772-9. [PubMed] [Google Scholar]
Juhl 2012
- Juhl C, Lund H, Roos EM, Zhang W, Christensen R.A hierarchy of patient-reported outcomes for meta-analysis of knee osteoarthritis trials: empirical evidence from a survey of high impact journals. Arthritis 2012;2012:136245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jüni 2015
- Jüni P, Hari R, Rutjes AW, Fischer R, Silletta MG, Reichenbach S, et al.Intra‐articular corticosteroid for knee osteoarthritis. Cochrane Database of Systematic Reviews 2015, Issue 10. Art. No: CD005328. [DOI: 10.1002/14651858.CD005328.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kellgren 1957
- Kellgren JH, Lawrence JS.Radiological assessment of osteo-arthrosis. Annals of Rheumatic Disease 1957;16:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kellgren 2000
- Kellgren JH, Lawrence JS.Radiological assessment of osteo-arthrosis. Annals of Rheumatic Disease 2000;16(4):494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2014
- Khan M, Evaniew N, Bedi A, Ayeni O, Bhandari M.Arthroscopic surgery for degenerative tears of the meniscus: a systematic review and meta-analysis. CMAJ : Canadian Medical Association Journal 2014;186:1057–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamplot 2016
- Lamplot JD, Brophy RH.The role for arthroscopic partial meniscectomy in knees with degenerative changes: a systematic review. Bone and Joint Journal 2016;98-B:934-8. [DOI] [PubMed] [Google Scholar]
Lee 2018
- Lee DY, Park YJ, Kim HJ, Nam DC, Park JS, Song SY, et al.Arthroscopic meniscal surgery versus conservative management in patients aged 40 years and older: a meta-analysis. Archives of Orthopaedic and Trauma Surgery 2018;138(12):1731-9. [DOI] [PubMed] [Google Scholar]
Leopoldino 2019
- Leopoldino AO, Machado GC, Ferreira PH, Pinheiro MB, Day R, McLachlan AJ, et al.Paracetamol versus placebo for knee and hip osteoarthritis. Cochrane Database of Systematic Reviews 2019, Issue 2. Art. No: CD013273. [DOI: 10.1002/14651858.CD013273] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2020
- Li J, Zhu W, Gao X, Li X.Comparison of arthroscopic partial meniscectomy to physical therapy following degenerative meniscus tears: a systematic review and meta-analysis. BioMed Research International 2020;2020:1709415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lohmander 2019
- Lohmander LS, Jarvinen TL.The importance of getting it right the first time. Osteoarthritis and Cartilage 2019;27(10):1405-7. [DOI] [PubMed] [Google Scholar]
Mahir 2016
- Mahir L, Belhaj K, Zahi S, Azanmasso H, Lmidmani F, El Fatimi A.Impact of knee osteoarthritis on the quality of life. Annals of Physical and Rehabilitation Medicine 2016;59s:e159. [Google Scholar]
McAlindon 2014
- McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al.OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis and Cartilage 2014;22:363-88. [DOI] [PubMed] [Google Scholar]
Monk 2017
- Monk AP, Garfjeld Roberts P, Palmer AJ, Bayliss LE, Beard DJ, Hopewell S, et al.The urgent need for evidence in arthroscopic meniscal surgery. American Journal of Sports Medicine 2017;45(4):965-73. [DOI] [PubMed] [Google Scholar]
Mounsey 2009
- Mounsey A, Ewigman B.Arthroscopic surgery for knee osteoarthritis? Just say no. Journal of Family Practice 2009;58(3):143-5. [PMC free article] [PubMed] [Google Scholar]
NICE 2014
- National Institute for Health and Care Excellence.Osteoarthritis: Care and management. London, UK: NICE 2014.
Niu 2011
- Niu NN, Losina E, Martin SD, Wright J, Solomon DH, Katz JN.Development and preliminary validation of a meniscal symptom index. Arthritis Care and Research 2011;63(2):208-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Outerbridge 1961
- Outerbridge RE.The etiology of chondromalacia patellae. Journal of Bone and Joint Surgery. British Volume 1961;43:752-7. [DOI] [PubMed] [Google Scholar]
Page 2021
- Page MJ, Higgins JP, Sterne JA.Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Pihl 2020
- Pihl K, Ensor J, Peat G, Englund M, Lohmander S, Jørgensen U, et al.Wild-goose chase - no predictable patient subgroups who benefit from meniscal surgery: patient-reported outcomes of 641 patients 1 year after surgery. British Journal of Sports Medicine 2020;54(1):13-22. [DOI] [PubMed] [Google Scholar]
Puljak 2017
- Puljak L, Marin A, Vrdoljak D, Markotic F, Utrobicic A, Tugwell P.Celecoxib for osteoarthritis. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD009865. [DOI: 10.1002/14651858.CD009865.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
RACGP 2018
- Royal Australian College of General Practitioners.Guideline for the management of knee and hip osteoarthritis. 2nd edition. East Melbourne, Australia: RACGP 2018.
Reichenbach 2010
- Reichenbach S, Rutjes AW, Nüesch E, Trelle S, Jüni P.Joint lavage for osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2010, Issue 5. Art. No: CD007320. [DOI: 10.1002/14651858.CD007320.pub2] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5.4 (RevMan 5).Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Roemer 2017
- Roemer FW, Kwoh CK, Hannon MJ, Hunter DJ, Eckstein F, Grago J, et al.Partial meniscectomy is associated with increased risk of incident radiographic osteoarthritis and worsening cartilage damage in the following year. European Radiology 2017;27:404-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2021a
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al.Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Schünemann 2021b
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al.Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Shin 2012
- Shin CS, Lee JH.Arthroscopic treatment for osteoarthritic knee. Knee Surgery & Related Research 2012;24(4):187-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siemieniuk 2017
- Siemieniuk RA, Harris IA, Agoritsas T, Poolman RW, Brignardello-Petersen R, Velde SV, et al.Arthroscopic surgery for degenerative knee arthritis and meniscal tears: a clinical practice guideline. BMJ 2017;257:j1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sihvonen 2018
- Sihvonen R, Paavola M, Malmivaara A, Itala A, Joukainen A, Nurmi H, et al.Arthroscopic partial meniscectomy versus placebo surgery for a degenerative meniscus tear: a 2-year follow-up of the randomised controlled trial. Annals of the Rheumatic Diseases 2018;77:188-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steadman 2007
- Steadman JR, Ramappa AJ, Maxwell RB, Briggs KK.An arthroscopic treatment regimen for osteoarthritis of the knee. Arthroscopy 2007;23(9):948-55. [DOI] [PubMed] [Google Scholar]
Stensrud 2015
- Stensrud S, Risberg MA, Roos EM.Effect of exercise therapy compared with arthroscopic surgery on knee muscle strength and functional performance in middle-aged patients with degenerative meniscus tears: a 3-mo follow-up of a randomized controlled trial. American Journal of Physical Medicine and Rehabilitation 2015;94(6):460–73. [NCT01002794] [DOI] [PubMed] [Google Scholar]
Thorlund 2015
- Thorlund JB, Juhl CB, Roos EM, Lohmander LS.Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. British Journal of Sports Medicine 2015;49:1229-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van de Graaf 2016
- Van de Graaf VA, Wolterbeek N, Mutsaerts EL, Scholtes VA, Saris DB, De Gast A, et al.Arthroscopic partial meniscectomy or conservative treatment for nonobstructive meniscal tears: a systematic review and meta-analysis of randomized controlled trials. Arthroscopy 2016;32:1855–65. [DOI] [PubMed] [Google Scholar]
Wai 2002
- Wai E, Kreder H, Williams J.Arthroscopic debridement of the knee for osteoarthritis in patients fifty years of age or older: utilization and outcomes in the Province of Ontario. Journal of Bone and Joint Surgery. American Volume 2002;84:17-22. [DOI] [PubMed] [Google Scholar]
Winter 2017
- Winter AR, Collins JE, Katz JN.The likelihood of total knee arthroplasty following arthroscopic surgery for osteoarthritis: a systematic review. BMC Musculoskeletal Disorders 2017;18:408. [DOI: 10.1186/s12891-017-1765-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zelen 1981
- Zelen M.Alternatives to classic randomized trials. Surgical Clinics of North America 1981;61:1425-32. [DOI] [PubMed] [Google Scholar]
Zhang 2009
- Zhang W, Doherty M, Peat G, Bierma-Zeinstra MA, Arden NK, Bresnihan B, et al.EULAR evidence based recommendations for the diagnosis of knee osteoarthritis. Annals of the Rheumatic Diseases 2009;69(3):483-9. [DOI] [PubMed] [Google Scholar]
Zhang 2010
- Zhang Y, Jordan JM.Epidemiology of osteoarthritis. Clinics in Geriatric Medicine 2010;26(3):355-69. [DOI] [PMC free article] [PubMed] [Google Scholar]


