Abstract
The toxicity of single pesticides is likely underestimated when considering complex pesticide mixtures found in agricultural runoff and this is especially true for newer pesticides with little toxicity data on non-target species. The goal of our study was to compare the toxicity of two newer pesticides, imidacloprid (IMI) and chlorantraniliprole (CHL), when an invertebrate and fish were exposed to single compounds, binary mixtures or surface water collected near agricultural fields. A secondary goal was to determine whether changes in select subcellular molecular pathways correspond to the insecticides’ mechanisms of activity in aquatic organisms. We conducted acute (96 h) exposures using a dilution series of field water and environmentally relevant concentrations of single and binary mixtures of IMI and CHL. We then evaluated survival, gene expression and the activity of IMI toward the n-acetylcholine receptor (nAChR) and CHL activity toward the ryanodine receptor (RyR). Both IMI and CHL were detected at all sampling locations for May 2019 and September 2019 sampling dates and exposure to field water led to high invertebrate but not fish mortality. Fish exposed to field collected water had significant changes in the relative expression of genes involved with detoxification and neuromuscular function. Exposure of fish to single compounds or binary mixtures of IMI and CHL led to increased relative gene expression of RyR in fish. Furthermore, we found that IMI targets the nAChR in aquatic invertebrates and that CHL can cause overactivation of the RyR in invertebrates and fish. Overall, our finding suggests that IMI and CHL may impact neuromuscular health in fish. Expanding monitoring efforts to include sublethal and molecular assays would allow the detection subcellular level effects due to complex mixtures present in surface water near agricultural areas.
Keywords: Neonicotinoid, Anthranilic diamide, N-acetylcholine receptor, Ryanodine receptor, Mixture toxicity
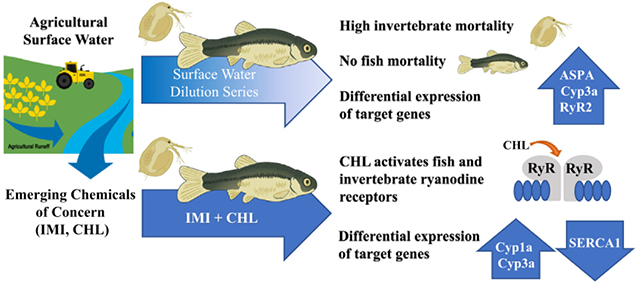
GRAPHICAL ABSTRACT
1. Introduction
The diversity and quantity of pesticides being applied globally are increasing at a rapid pace (Bernhardt et al., 2017; Pisa et al., 2021; Stehle and Schulz, 2015). As the variety of pesticides being applied increases, so does the complexity of the resulting mixtures. Runoff enters waterways from agricultural and urban areas, resulting in complex chemical mixtures that have the potential to cause rapid changes in water quality. These dynamic mixtures often include chemicals of concern that are known to have adverse biological effects in single chemical laboratory exposures. In fact, a recent meta-analysis reported that pesticides exceeded aquatic life benchmarks in 63.5% of agricultural stream sites surveyed across the U.S. (Wolfram et al., 2018). Evaluating the survival of sensitive model species after an acute exposure is a common benchmark for assessing toxicity of surface waters (Goh et al., 2019); however, this may not represent ecologically relevant impacts seen in runoff-impacted waterways (Connon et al., 2019; Spurgeon et al., 2010). Studies of multiple stressors demonstrate complex, nonlinear and often synergistic effects (Spurgeon et al., 2010; Todgham and Stillman, 2013), suggesting that the effects of multiple stressors are often worse than that predicted from results obtained from single stressor studies (Crain et al., 2008; Yang et al., 2007). Therefore, using single stressor data to infer physiological effects occurring in the natural environment may underestimate toxicity.
Pesticide resistance will continue to drive the discovery of new insecticides, and concurrently drive the environmental impacts of overuse (Bass et al., 2015; Weston et al., 2013; Wolfram et al., 2018; Zhang, 2018). Recent shifts in the use trends for various classes of insecticides include phasing out first-generation insecticides in favor of new cost-efficient, effective chemicals. There is a clear global trend showing an increase in the use of neonicotinoids, as well as chemicals with novel mechanisms of action like anthranilic diamides (Bentley et al., 2010; Wolfram et al., 2018). Two such chemicals of emerging concern are imidacloprid (IMI) and chlorantraniliprole (CHL). Imidacloprid is a neonicotinoid pesticide with a mechanism of action on postsynaptic nicotinic acetylcholine receptors (nAChR), impacting the nervous system (Duke et al., 1993). Neonicotinoids display lower nAChR activity in vertebrates as compared to invertebrates (Liu and Casida, 1993) but have been suggested to cause neurotoxicity in zebrafish as evidenced by changes in expression of the key neurotoxic genes c-fos and Brain Derived Neurotrophic Factor (BDNF; Özdemir et al., 2018). Additionally, neonicotinoids have been suggested to cause neurotoxicity in mammals, which may be due to neonicotinoid metabolites (see review by Zhao et al., 2020). Chlorantraniliprole is an anthranilic diamide insecticide that increases the activity of the ryanodine receptor (RyR) impacting muscle contraction (Bentley et al., 2010; Cordova et al., 2007). Diamide insecticides were developed to display high affinity for invertebrate species with significantly reduced affinity in vertebrates species (Cordova et al., 2007; Lahm et al., 2007; Qi and Casida, 2013). However, more recent research suggests that CHL may also target the RyR in mammals (Truong and Pessah, 2019) supporting potential impacts in vertebrates. Taken together the mechanisms of action of IMI and CHL suggest that they would exert toxicity on sensitive aquatic invertebrate and potentially on vertebrate species.
Both IMI and CHL are now being utilized across the globe (Teixeira and Andaloro, 2013; Bakker et al., 2020). One such example is the Central Coast region of California, which contains some of the most intensively farmed agricultural land in the United States (Hunt et al., 2003). Recent data from the CA Department of Pesticide (CDPR) Pesticide Use Report (PUR) database (https://calpip.cdpr.ca.gov/main.cfm) show that approximately 97,026 and 20,620 pounds of IMI and CHL were applied in the Central Coast region (Monterey and San Luis Obispo counties) between 2017 and 2019, respectively. This has led to increased detections of these pesticides in waterways that surround agricultural areas in the Central Coast, namely, the Salinas River, its tributaries, and other associated waterbodies. These waterways transect the Central Coast receiving runoff from nearby agricultural fields and urbanized areas. The detection of pesticides in these waterways leads to potentially harmful impacts on water quality where chemicals of concern, including IMI and CHL (Table S1), are frequently detected in the region at levels that may be toxic to sensitive organisms (Anderson et al., 2003; Goh et al., 2019; Hunt et al., 2003; Kuivila et al., 2012). As a result, the Salinas River was placed on the U.S.A. Federal Clean Water Act 303(d) list of impaired water bodies (Hunt et al., 2003).
Poor water quality threatens the vast number of species present in the Salinas region, including many species of economic and conservation concern. For example, the river and its tributaries have been designated by the National Marine Fisheries Service as critical habitat for southern steelhead trout (Oncorhynchus mykiss) serving as a migration corridor and spawning habitat (Anderson et al., 2003). Additionally, pink salmon (O. gorbuscha), a commercially harvested and abundant species in the North Pacific are considered imperiled in California and spawn in the Salinas River (Skiles et al., 2013). Toxicity studies have also shown that water collected in the Salinas River and its tributaries causes high rates of mortality in sensitive invertebrate species (Anderson et al., 2006,2003; Hunt et al., 2003). Invertebrate community structure was also highly impacted downstream of monitoring sites that receive runoff from nearby agricultural fields (Anderson et al., 2006). Together these studies support the impact of agricultural runoff on nearby receiving water. While survival of model invertebrate or fish species is an established endpoint for ecotoxicology assessments, sublethal endpoints are more sensitive and have greater ecological relevance, revealing a more complete picture of site toxicity (Beggel et al., 2011; Hasenbein et al., 2019).
The goal of our study was to compare the toxicity of IMI and CHL single and binary exposures to that elicited by agricultural surface water collected from the Salinas, CA area in both a sensitive invertebrate and a model fish species. A secondary goal was to determine whether changes in select subcellular molecular pathways correspond to the insecticides’ mechanisms of activity in aquatic organisms. We collected water samples at monitoring stations near agricultural fields in Salinas waterways and tributaries, then screened them for pesticides and used standard toxicity assays to evaluate effects in Daphnia magna and the fathead minnow (Pimephales promelas). We confirmed the insecticides’ mechanism of action using radioligand binding in the fathead minnow and three invertebrates (Daphnia magna, Chironomus dilutus, Hyalella azteca). We then evaluated differential gene responses for specific pathways in fish to determine if organisms exposed to agricultural water display similar signs of disruption as those exposed to single or binary mixtures of pure IMI and CHL. This work addresses IMI and CHL toxicity in ecologically relevant aquatic organisms, helping to determine the impacts of their use near important waterways.
2. Materials and methods
2.1. Field water sampling
2.1.1. Study sites
Chemical monitoring sites have been established throughout the Salinas River, nearby tributaries and other waterbodies as previously described (Deng et al., 2019; Goh et al., 2019). Chemical detection data have been collected from these sites for over a decade (Deng et al., 2019; Goh et al., 2019). Long-term monitoring sites near Salinas, CA were initially chosen based on reported nearby pesticide use, detections from previous monitoring (often determined to be out-of-compliance with water quality levels), and proximity to ecologically sensitive areas (Luo et al., 2018). We sampled water from select, existing long-term monitoring sites (Table S2). The sampling sites for this study included six sites in or around Salinas, CA that included four sites that directly receive surface water runoff from adjacent agricultural fields: Quail Creek (Sal_Quail), Chualar Creek (Sal_Chualar), Alisal Creek (Sal_Hartnell), and a reclamation ditch (Sal_SanJon); the main channel of Tembladero Slough (Sal_Haro) and the Salinas River (Sal_Davis). These sites are located immediately downstream of high use agricultural areas, where there is an increased risk of contamination from agricultural runoff.
2.1.2. Water sampling
We collected water samples from six sites (listed above) on May 14th 2019 and from a subset of those sites (Quail Creek, Alisal Creek and the Salinas River) on September 17th 2019, following standard sampling protocols (Jones, 1999). In brief, we collected samples from well-mixed, wadable waters using 1-liter amber glass bottles certified to meet current US EPA guidelines then sealed with Teflon-lined lids. Immediately after collection, we placed samples in coolers on wet ice for transportation, then refrigerated them at 4 °C upon arrival in the lab. We measured water quality parameters in situ using a YSI EXO1 multi-parameter water quality Sonde (Doo and He, 2008), where parameters recorded including ambient water pH, specific conductance, dissolved oxygen, temperature, total dissolved solids, salinity, and total suspended solids. Results of water quality parameters are shown in Table S3.
2.1.3. Geometric dilution series for field water treatments
Based on high invertebrate mortality from several previous, preliminary exposure studies from the same field sites (CDPR Technical Report Hasenbein et al., 2018, Grant # 16-C0084), we created a geometric dilution series to better capture sublethal effects. We mixed field water with standard US EPA control water for each test species (see Methods Section 2.4.1) to create the dilution series. For our exposures conducted in May, we included 100%, 60% and 35% field water, where dilutions were conducted using control water for a given species (see Methods Section 2.2). Based on the high levels of invertebrate mortality observed in our first exposure event, we added additional lower concentrations (20%, 12%) to the subsequent sampling event in September. Immediately before initiating the test, we thoroughly mixed each sample by agitation to homogenize and distribute any remaining sediment particles, then diluted it into control water to obtain the desired concentrations. Once aliquoted into beakers, we allowed the dilutions to reach the desired test temperature for each organism prior to loading organisms into beakers. We repeated this procedure on day 2 of the test to prepare each treatment for the 80% water change. Acute exposure test conditions were identical for both single/binary and field exposures (see Methods Section 2.4.2).
2.2. Single/binary chemical treatments
We purchased chemicals (IMI and CHL; >97.5% purity) from AccuStandard (New Haven, CT, USA) and dissolved them in deionized water (IMI) or acetone (CHL). Pesticide-grade acetone (Fisher Chemical, USA) was used as a solvent carrier for the CHL treatments, and in solvent controls, to a final concentration of 0.01% in exposure water. Our stock solutions were then spiked into control water according to target concentrations, keeping acetone at 0.01%, and mixed thoroughly. Our exposure concentrations matched range-finding experiments and environmentally relevant concentrations (Table S1). In total, D. magna were exposed to six single concentrations (25, 50, 100, 500, 1000, 10,000 ng/L) of each pesticide and three mixture concentrations (25 × 25 ng/L, 500 × 500 ng/L, 10,000 × 10,000 ng/L), a solvent control (for CHL exposures only), and a negative control (control water only). P. promelas were exposed to three single concentrations (25, 500, 10,000 ng/L) of each pesticide and three mixture concentrations (25 × 25 ng/L, 500 × 500 ng/L, 10,000 × 10,000 ng/L), a solvent control, and a negative control. Acute exposure test conditions were identical for both single/binary and field exposures (see Methods Section 2.4.2).
2.3. Chemical analyses
Chemical analysis was completed at the Center for Analytical Chemistry, California Department of Food and Agriculture (Sacramento, CA) using multi-residue liquid chromatography tandem mass spectrometry (LC-MS/MS) and gas chromatography–mass spectrometry (GC–MS/MS) methods. For field water, 47 pesticides were included for screening based on the procedures described in the Monitoring Prioritization Model (Luo et al., 2018). For single and binary chemical treatments, IMI and CHL concentrations were measured to confirm target exposure concentrations. Laboratory QA/QC followed CDPR guidelines provided in the Standard Operating Procedure CDPR SOP QAQC012.00 (Teerlink and DaSilva, 2017). Extractions included laboratory blanks and matrix spikes (method detection limit and reporting limit for each analyte available upon request).
2.4. Toxicity testing
2.4.1. Test organisms
We obtained D. magna from Aquatic Research Organisms Inc. (Hampton, NH, USA), and cultured them in our laboratory at the University of California, Davis (USA). Groups of 20 individuals were maintained at 20 ± 2 °C and a 16-h light: 8-h dark photoperiod in 2L beakers of reconstituted control water (USEPA, 2002), which was prepared by dissolving 23.04 g NaHCO3, 14.40 g CaSO·42H2O, 14.40 g MgSO4, and 0.96 g KCl in 120 L of deionized water to achieve a hardness of 160–180 mg/L CaCO3 and alkalinity of 110–120 mg/L CaCO3. We obtained P. promelas larvae from Aquatic Biosystems, Inc. (Ft. Collins, Colorado, USA) at 7 days post-hatch on the day of arrival. We habituated the fish to control water at a temperature of 25 °C over a period of 8 h. Control water consisted of deionized water, modified with salts to meet USEPA specifications (specific conductivity (EC): 265–293 μS/cm; hardness: 80–100 mg/L CaCO3; alkalinity: 57–64 mg/L CaCO3 (USEPA, 2002). During the habituation period <1% mortality was observed, and the fish fed and swam normally. We conducted all studies in accordance with national and institutional guidelines for animal welfare and are described under the University of California Davis, Institutional Animal Care and Use Committee protocol #19690.
2.4.2. Acute exposure conditions
Organismal exposures followed acute toxicity procedures outlined by the US Environmental Protection Agency (USEPA, 2002). For 96 h acute exposures we used third brood D. magna neonates (< 24 h-old) and P. promelas larvae (7 days post hatch; dph). Test exposure temperatures were maintained in separate environmental chambers under fluorescent light with a 16-h light: 8-h dark photoperiod, at 20 °C and 25 °C for D. magna neonates and P. promelas larvae, respectively. For D. magna, we placed twenty individuals into each of the 250-mL replicate beakers containing 200 mL of treatment water, with four replicates per treatment. For P. promelas-, each treatment consisted of four replicate 600 mL beakers containing 500 mL test solution and 10 fish larvae. At test initiation, we gently added organisms to each replicate beaker and treatment in a random order. Beaker locations were then randomized within the environmental chamber. We fed D. magna at test initiation and at water renewal, using a suspension of concentrated (i.e., 3 × 107 cells/mL) Raphidocelis subcapitata (obtained from Aquatic Research Organism Inc), and YCT (yeast, cerophyl, trout chow mixture, total solids > 1.9 g solids/L of final YCT mixture) (USEPA, 2002). We fed fish larvae ad libitum with newly hatched Artemia franciscana, twice daily.
We recorded mortality daily for all test species, and immediately removed any dead organisms from the test vessels. After 48 h, new treatment waters were prepared, and an 80% water change was performed. At the time of water renewal, we measured water quality parameters using a YSI EXO1 multi-parameter water quality Sonde (Doo and He, 2008), where parameters recorded including pH, specific conductance, dissolved oxygen, temperature. Test vessels were randomly distributed after each water renewal. At test termination we euthanized surviving fish from each replicate beaker in an overdose of tricaine methanesulfonate (500 mg/L MS-222, buffered with 500 mg/L sodium bicarbonate). We then pooled remaining fish within each replicate beaker into 1.5 mL microcentrifuge tubes, and immediately froze them in liquid nitrogen for subsequent gene expression analysis (see Methods Section 2.6).
2.5. Confirmation of IMI and CHL mechanism of action in aquatic model species
2.5.1. Protein preparations
We obtained non-exposed invertebrates and larval fish (7-14 dph) used in in vitro assays from Aquatic Research Organisms Inc. (Hampton, NH, USA) and cultured or habituated as described previously. For each species separately, we pooled whole individuals (n > 50) into 15-mL conical tubes and immediately flash frozen in liquid nitrogen until use in molecular analyses. The pooled tissue was then used to create crude microsomal protein homogenates enriched in RyR or nAChR following previously published methods (Bass et al., 2011; Fritsch and Pessah, 2013; Qi and Casida, 2013; Wiesner and Kayser, 2000). Briefly, we placed tissue into a homogenization buffer consisting of 300 mM Sucrose, 20 mM Hepes, leupeptin (2 μg/mL), phenylmethanesulfonyl fluoride (PMSF,1 mM), sodium orthovanadate (0.5 mM) NaF (10 mM), β-glycerol (2 mM) and NaP2O7 (5 mM) adjusted to a pH of 7.2. Tissue was then homogenized, on ice, utilizing a Polytron 1200 E (Kinematica, Bohemia, NY) for 2 bursts of 20 s with 2 min on ice between bursts. The homogenate underwent centrifugation at 8000 rpm for 10 min at 4 °C and we collected supernatant into an ultracentrifugation tube. We resuspended the pellet in 5 mL of homogenization buffer and repeated the homogenization and centrifugation steps. Supernatants were combined and underwent ultracentrifugation at 100,000 g for 1 h at 4 °C. The microsomal pellet was then suspended in a 300 mM Sucrose 20 mM Hepes buffer (pH = 7.2) and we it placed into 100 μL aliquots to avoid multiple freeze thaw cycles after storage at −80 °C. We determined protein concentrations in triplicate using a BCA assay (Pierce, Rockford, IL).
2.5.2. Radioligand binding assays
To measure the activity of CHL at the RyR, we incubated microsomal preparations in the presence of varying concentrations of CHL together with tritiated ryanodine ([3H]Ry; Bass et al., 2011; Fritsch and Pessah, 2013; Qi and Casida, 2013). Here, 100 μg/mL microsomal preparation from a given species was incubated in a binding buffer consisting of 140 mM KCL, 20 mM Hepes, and 15 mM NaCl (pH = 7.1) with 10 nM [3H]Ry and 0.5% DMSO or 0.01-100 μM CHL in 0.5% DMSO. Non-specific binding was run under the same assay conditions but also included 10 μM unlabeled ryanodine and 200 μM EGTA. We ran each treatment in 300 μL of buffer, in triplicate, and incubated assays in a shaking water bath held at 25 °C for 16 h. After incubation, we filtered samples using Whatman GF/B filters and washed three times with 5 mL ice cold buffer containing 140 mM KCl, 10 mM Hepes and 0.1 mM CaCl2 adjusted to pH = 7.3. The filters were exposed to 5 mL of a scintillation cocktail, stored overnight and radioactivity measured in a liquid scintillation counter. We tested assays for CHL RyR activity at least twice and ran them on two separate protein homogenates.
For the activity of IMI at the nAChR, we assessed the pesticide’s ability to displace tritiated IMI ([3H]IMI) in competitive binding assays following methods of Wiesner and Kayser (2000). Here, we incubated 100 μg/mL microsomal preparation from a given species in a binding buffer consisting of 20 mM Na2HPO4, 150 mM NaCl, 1 mM EDTA, 0.1 mM PMSF and 2 μg/mL (pH = 7.0) that contained 1 nM [3H]IMI and 0.5% DMSO or 0.01-100 μM IMI in 0.5% DMSO. Non-specific binding was run under the same assay conditions but also included 10 μM unlabeled IMI. We ran assays in a total of 300 μL, in triplicate, and incubated them in a shaking water bath at 20 °C for 3 h. After incubation, we filtered samples using Whatman GF/B filters and washed them three times with 5 mL ice cold buffer containing 20 mM Na2HPO4, 150 mM NaCl, 1 mM EDTA adjusted to pH = 7.0. The filters were exposed to 5 mL of a scintillation cocktail, stored overnight and radioactivity measured in a liquid scintillation counter. We conducted assays for IMI at least twice on two separate protein homogenates. Due to our findings in D. magna and P. promelas (see Results Section 3.4), we conducted additional studies to investigate the mechanism of action of CHL and IMI in other important aquatic model species H. azteca and C. dilutus. We conducted protein preparations, binding conditions and analysis as described for D. magna and P. promelas.
2.6. Evaluation of relative gene expression
We extracted total RNA from ten pooled fish larvae per replicate (n = 4) using a QIAcube system (Qiagen, Hilden, Germany) and QIAGEN RNeasy Plus Mini Kits according to manufacturer’s instructions. We confirmed RNA concentrations using a Qubit 4 fluorimeter/broad range RNA assay kit (Invitrogen, Carlsbad, CA), then verified total RNA quality and integrity through nanodrop (Invitrogen, Carlsbad, CA) and electrophoresis on a 1% (wt/vol) agarose gel, respectively. We synthesized complementary DNA (cDNA) from 1 μg of total RNA using Superscript III Reverse Transcriptase, a 100 mM dNTP set, and random primers (Invitrogen, Carlsbad, CA) following manufacturer’s instructions. Next, we carried out a 1:16 dilution with nuclease free water to generate sufficient template for qPCR analysis, following dilution series analysis during primer validation. We used primer pairs designed for a suite of target genes of interest and three reference genes (Table 1). This suite of target genes were selected because they are involved in detoxification, neurological function, or are related to the presumed mechanisms of action for IMI or CHL (Soderlund, 2012; Zanger and Schwab, 2013).
Table 1.
Genes of interest and reference genes for qPCR analyses.
| Reference genes | Abbrev. | Forward | Reverse | Primer efficiency % |
|---|---|---|---|---|
|
| ||||
| Elongation Factor 1-alpha | EF1a | CTCTTTCTGTTACCTGGCAAAGG | TCCCATGATTGATTAGTTTCAGGAT | 97 |
| L8 ribosomal protein | L8 | GGCTAAGGTGGTTTTCCGTGA | CTTCAGCTGCAATGAACAGCTC | 99 |
| beta-actin | B-ACTIN | CAACACCGTGCTGTCTGGAG | TCTTTCTGCATACGGTCAGCAA | 93 |
| Gene of interest | Abbrev. | Forward | Reverse | Primer efficiency % |
|
| ||||
| Acetylcholinesterase | AChE | ATGACCAATAGGCCAAAGCATT | ACGGAAAATTCCATCGATCTCA | 101 |
| Aspartoacylase | ASPA | TCTGGTAATGGATGTCCCGATT | GACCTCTATGGAAAAGCCATGC | 100 |
| Cytochrome P4501A | CYP1a | GCTTCTCGAGGCCTTTATCC | ACAGTGAGGGATGGTGAACG | 99 |
| CYP3A126 | CYP3a | CAACCCAGAGGCCATGAAGA | GGGCCTTATTTGGGAAGGTCT | 92 |
| Ryanodine Receptor, 1 | RyR1 | AAGATGACGATGAAGGGTTTGTC | CATGGCAGGTTCCATATATCCAG | 99 |
| Ryanodine Receptor, 2 | RyR2 | CCACCTTCTCGAGGTCAGGTT | CCGCCTCAGTGACGGATAATAA | 99 |
| Sarco/Endoplasmic Reticulum ATPase | SERCA1 | CAACATTGGCCACTTCAACG | GAGCCACAGCGATCTTFAAGT | 98 |
Primers for qPCR analyses for target genes of interest designed using Roche Universal Library (UPL) Assay Design Center.
We obtained lyophilized primers from IDT (Integrated DNA Technologies, Inc., Germany) and rehydrated them to 100 μmol with RNase-free water. We performed all PCR reactions using QuantiTect SYBR® Green PCR Kit 2× concentration, (Bio-Rad, California, USA) per the manufacturer’s protocol, using 5 μL of cDNA in a final reaction volume of 12 μL. Fluorescence was detected (ABI PRISM 7900 Sequence Detection System, Applied Biosystems, Carlsbad, CA,) over 40 cycles, with cycling conditions of 15 min initial heat inactivation at 95 °C, 15 s denaturation at 94°C, 30 s at an annealing temperature of 55 °C, and extension at 72 °C. Fluorescence of samples was measured every 7 s and signals were considered positive if fluorescence intensity exceeded 10 times the standard deviation of the baseline fluorescence (threshold cycle, CT). SDS 2.2.1 software (Applied Biosystems, Carlsbad, CA, USA) was used to quantify transcription. Using the computational algorithm geNorm (Vandesompele et al., 2002), we assessed the expression stability of each gene. Based on the standard curves, all primer pair efficiencies were within acceptable range, from 92% (Cyp3a) to 101% (AChE). We examined melt curves for each sample to verify single product amplification and consistency among samples.
2.7. Statistical analysis
2.7.1. Mortality
For the dilution series of field water and IMI/CHL single/binary treatments, acute toxicity was defined as a statistically significant difference (P < 0.05) in mortality compared to the laboratory control water within 96 h of test initiation. We determined significance by Analysis of Variance (ANOVA) followed by Dunnett’s test for multiple comparisons using GraphPad Prism software (version 8.0). For single/binary exposures, we calculated the median lethal concentration (LC) toxicity thresholds (96 h LC50 values) with 95% confidence intervals (CI) for single/binary exposures using Probit Analysis in the ‘ecotox’ package of RStudio statistical software (version 1.3.1073, R Core Team, 2020). We also generated dose-response plots to display treatment effects using RStudio (R Core Team, 2020).
2.7.2. Radioligand binding
We calculated specific binding by subtracting the non-specific binding from the total observed binding in a given assay. Specific binding due to chemical concentration, in disintegrations per minute (DPM), was then represented as percent binding relative to control binding. We then determined direct impacts of IMI or CHL on the RyR and nAChR using sigmoidal-dose response curves or a one-way ANOVA if necessary (GraphPad Prism version 8.0). For activity of CHL at the RyR, we calculated an effective concentration that would cause 50% of the maximum response (EC50; relative EC50 where maximum effects are not scaled to 100%). Due to the nature of the RyR binding assay (see Results and Discussion), we also calculated the CHL concentration needed to cause a 200% (2-fold change, EC2X; an absolute value) over activation at the RyR of a given species. For IMI activity at the nAChR we calculated an inhibition concentration to 50% of control binding (IC50).
2.7.3. Relative gene expression
For gene expression analyses, we used the mean cycle threshold (Ct) of triplicate technical replicates to calculate relative quantification using the 2−ΔΔCt method (Livak and Schmittgen, 2001) relative to three reference genes and control samples for each treatment. For single and binary mixture treatments requiring acetone solvent controls, we calculated the mean ΔCt using the solvent treatment control group. We analyzed differential expression using one-way ANOVA followed by Dunnett’s multiple comparisons test. To test homogeneity of variances and normality, we used Levene’s test and the Shapiro-Wilk test, respectively. When data were not normally distributed, we applied a In-transformation to achieve normality. When a significant interaction was detected, we used one-way ANOVA followed by Dunnett’s multiple comparisons test to determine significant differences between treatments and controls. All analyses were performed using the statistical software GraphPad Prism (version 8.0) with a significance level at α = 0.05.
3. Results
3.1. Chemical analysis of field collected water samples
Out of the 47 pesticides that were screened for, 17 were detected in the surface waters sampled in May 2019, with a minimum of 11 pesticides detected at each site (Table S4). IMI and CHL were detected at all six sites (Table 2). IMI ranged in concentration from 0.019 μg/L to 1.19 μg/L. Concentrations of IMI exceeded the EPA benchmarks for acute invertebrate (0.385 μg/L) and/or chronic (0.01 μg/L) exposure in all six sites, with higher concentrations detected at Sal_Hartnell (1.01 μg/L), Sal_Chualar (1.19 μg/L) and Sal_Quail (0.759 μg/L). Additionally, several pyrethroids were detected in the May 2019 samples and were often found at levels at, or above, EPA benchmarks. This included permethrin, lambda cyhalothrin and bifenthrin, analytes of particular concern (Table S4). CHL ranged in concentration from trace detection to a max of 10.2 μg/L. The concentration of CHL detected at Sal_Hartnell (10.2 μg/L) exceeded both the LC50 for a sensitive invertebrate species, D. magna (7.1 μg/L), and the EPA benchmark for aquatic life (USEPA, 2020) for acute invertebrate exposure (5.8 μg/L) (Table S1).
Table 2.
Chemical Analysis of agricultural surface water samples collected 5/14/2019 and 9/17/2019 from CDPR long-term monitoring sites in Salinas, CA. liquid chromatograph multi-analyte and pyrethroid screen were performed at the Center for Analytical Chemistry, California Department of Food and Agriculture, Sacramento, CA.
| Sal_Quail | Sal_Hartnell | Sal_Davis | Sal_SanJon | Sal_Chualar | Sal_Haro | ||
|---|---|---|---|---|---|---|---|
| 05/14/2019 | Chlorantraniliprole (μg/L) | 0.466 | 10.200 | Trace | 0.634 | 0.236 | 0.258 |
| Imidacloprid (μg/L) | 0.759 | 1.010 | 0.019 | 0.495 | 1.190 | 0.292 | |
| 09/17/2019 | Chlorantraniliprole (μg/L) | 0.350 | 0.504 | 0.021 | 0.368 | 0.159 | 0.156 |
| Imidacloprid (μg/L) | 0.293 | 0.513 | 0.014 | 2.100 | 4.050 | 0.697 |
Laboratory QA/QC followed CDPR guidelines, and Laboratory blanks and matrix spikes were included in each extraction set. Samples from 9/17/2019 for Sal_SanJon, Sal_Chualar and Sal_Haro (shown in gray) were screened for pesticides as part of CDPR’s routine monitoring but, these sites were not included in the biological assessments for the September exposures.
Overall, 18 of the 47 analyzed pesticides were detected in the surface water samples collected in September 2019, 7 of which were detected at each sampling site (Table S5). IMI and CHL were detected at all sites (Table 2). IMI concentrations were above the EPA benchmark for chronic invertebrate exposure (0.01 μg/L), and above the acute invertebrate level (0.385 μg/L) at Sal_Hartnell (0.513 μg/L). CHL concentrations were below the acute lethality benchmarks for invertebrate species exposure (LC50 = 7.1 μg/L; EPA benchmark for acute, 5.8 μg/L, and chronic, 4.47 μg/L). Several other chemical detections exceeded threshold values. Notably, methomyl was detected at Sal_Quail (29.9 μg/L) at nearly three times the limit for chronic fish exposure (12 μg/L), and above the EPA benchmark for chronic invertebrate exposure (0.7 μg/L) at all sites. Thiamethoxam (neonicotinoid) was present in Sal_Quail (3.99 μg/L) and Sal_Hartnell (0.827 μg/L) at levels exceeding the EPA benchmark for chronic invertebrate exposure (0.74 μg/L) and was detected below EPA thresholds at Sal_Davis (0.064 μg/L). Additionally, several pyrethroids were detected in the September 2019 samples and were often found at levels at or above EPA benchmarks. This included permethrin, lambda cyhalothrin and bifenthrin, which are analytes of particular concern (Table S5).
3.2. Mortality of fish and invertebrates exposed to dilutions of field collected water samples
No significant mortality occurred for P. promelas for any samples. For D. magna May 2019 exposures, significant mortality (p < 0.001) occurred for the [100] and [60] exposure dilutions. For D. magna no significant mortality occurred in any dilution for sites Sal_Haro, Sal_Chualar or Sal_Davis. For Sal_SanJon [100], 100% mortality of D. magna occurred at 96 h. For Sal_SanJon [60], 25% mortality occurred. For Sal_Quail [100], 97.5% mortality occurred, and 100% mortality occurred in [60] and 60% in [35]. For Sal_Hartnell, 100% mortality occurred at all dilutions of field water (Table S6).
Due to the high mortality of D. magna exposed to water samples collected at Salinas monitoring stations in May 2019, two additional dilutions ([20], [12]) were added to the September 2019 exposure study. Exposures targeted two previously toxic sites (Sal_Hartnell and Sal_Quail) and one non-toxic site located downstream in the main Salinas River (Sal_Davis) (Table S7). For Sal_Quail, all dilutions had significant (p < 0.001) levels of mortality: 100% was observed at all dilutions of field water except [12], which had 87.5% mortality. For Sal_Hartnell, all dilutions had significant (p < 0.001) levels of mortality, except the lowest dilution [12], with; 100% mortality was observed at [100] and [60], 62.5% mortality at [35], 28% at [20]. For Sal_Davis, no significant mortality was observed at any dilution (Table S7). All water quality parameters (pH, specific conductance (SC), dissolved oxygen (DO), temperature (T)) of renewal and wastewater for May and September acute exposures fell within acceptable ranges (USEPA, 2002).
3.3. Mortality of fish and invertebrates exposed to IMI and CHL
Single and binary exposures to IMI and CHL did not cause mortality of P. promelas for any treatment (Table S8). For D. magna, the highest treatment concentrations of CHL (10,000 ng/L) resulted in significant mortality (p < 0.0001), with 100% mortality (Fig. S1, Table S9). No significant D. magna mortality was observed for IMI for any concentration tested. Mortality for the two highest binary mixture concentrations (500 ng/L and 10,000 ng/L IMI/CHL) was also significant (p = 0.0001, p < 0.0001, respectively). Analytical chemistry data of nominal test concentrations for IMI/CHL exposures are shown in Table S10.
3.4. IMI and CHL receptor binding in model aquatic species
The plant alkaloid ryanodine, for which the RyR is named, binds preferentially to the open state of the RyR (Meissner, 1986). Therefore, increased [3H]Ry binding in the presence of CHL would signify increased activity due to chemical perturbation. Here, we found that CHL activated the RyR present in the invertebrate model D. magna and the fish model P. promelas (Fig. 1) causing an approximate 500% maximal response in both species. The RyR in D. magna displayed a higher sensitivity to CHL experiencing a 200% overactivation (EC2X) at 0.48 μM compared to the EC2X seen in fish at 3.61 μM. We also saw that CHL activates the RyR in the important ecotoxicology species H. azteca and C. dilutus (Fig. S2). We observed insignificant binding of [3H]IMI at the nAChR in D. magna and P. promelas where total binding was equal to radioligand binding under non-specific binding conditions (data not shown). This was observed under a wide array of assay conditions including those experiments run with protein preparations created under different homogenization techniques and with varying binding assay conditions including altered buffers, temperature, and incubation periods. Interestingly, despite the lack of binding in D. magna and P. promelas we did find that [3H]IMI displays a high affinity for the nicotinic receptor found in H. azteca and C. dilutus (Fig. 2), with IC50 values of 8.86 nM and 8.04 nM, respectively.
Fig. 1.

Binding of [3H]Ry to D. magna and P. promelas ryanodine receptors in the presence of chlorantraniliprole. A) Binding curves with specific binding relative to DMSO control (100%); mean ± SEM, n = 3-6. B) Potency and efficacy of chlorantraniliprole observed by species. Abbreviations; EC50, Effect Concentration to 50% maximal; EC2X, concentration needed to cause 200% overactivation.
Fig. 2.

[3H]IMI binding in H. azteca and C. dilutus protein preparations in the presence of competitive concentrations of non-labeled imidacloprid. A) Binding curves with specific binding relative to DMSO control (100%); mean ± SEM, n = 6-9. B) Inhibitory concentrations to 50% of maximal inhibition (IC50) observed by species.
3.5. Relative gene expression of fish exposed to IMI and CHL
Differential expression of target genes involved in detoxification response and neuromuscular signaling pathways, comparing treated to non-treated control fish after 96 h exposures to single and binary mixtures of IMI and CHL is shown in Fig. 3. Gene expression was determined after 96 h exposure to low (25 ng/L), medium (500 ng/L), and high (10,000 ng/L) concentrations of IMI and CHL individually and as binary mixtures. Acetylcholinesterase (AChE) was upregulated in fish exposed to IMI, CHL, and binary mixtures at the lowest concentration, although changes did were not significantly from controls. Aspartoacylase (ASPA) was significantly upregulated in CHL exposed fish for all concentrations, and for the highest concentration of the binary mixture. Cytochrome P4501A (Cyp1a) and Cytochrome P4503A126 (Cyp3a) displayed a non-monotonic change in expression in fish exposed to CHL and the binary CHL/IMI mixtures and a log-linear dose response in IMI exposed fish. Ryanodine receptor 1 (RyR1) and Ryanodine receptor 2 (RyR2) were upregulated at the low and mid concentrations for both CHL and binary mixtures, although this was only significant for RyR2 at the CHL medium concentration (500 ng/L) and at lowest mixture concentration (25 ng/L). Sarco(endo)plasmic reticulum 1 (SERCA1) showed minor changes in expression in CHL, IMI and CHL/IMI exposed fish but these changes were not significantly different from the controls.
Fig. 3.

Log2 Fold-change of gene expression in P. promelas after exposure to chlorantraniliprole (2A), imidacloprid (2B), and binary mixtures (2C) for genes of interest: Acetylcholinesterase (AChE), Aspartoacylase (ASPA), Cytochrome P4501A (Cyp1a), Cyp3A126 (Cyp3a), Ryanodine receptor 1 (RyR1), Ryanodine receptor 2 (RyR2) and Sarco/Endoplasmic Reticulum ATPase (SERCA1). P-values are reported as * = P ≤ 0.05, ** = P ≤ 0.01, *** = P ≤ 0.001.
3.6. Relative gene expression of fish exposed to field collected water samples
Differential expression for target genes (detoxification and neuromuscular pathways) in fish after 96 h exposures to a geometric dilution of field water collected in May 2019 are shown in Fig. 4. Relative to controls, expression of Cyp1a was upregulated for Sal_Quail, Sal_Hartnell and Sal_Davis in a log-linear dose-response, increasing with increasing concentration of field water. Cyp1a was significantly upregulated for all sites at [100], Sal_Quail and Sal_Hartnell at [60], and for Sal_Quail at [35]. Expression of Cyp3a also followed a log-linear dose-response curve, increasing with increasing concentration of field water for each site. Cyp3a was significantly upregulated for Sal_Quail and Sal_Hartnell at all concentrations but was not significant for Sal_Davis at any concentration. Interestingly, in fish exposed to water collected in the field, Cyp1a and Cyp3a were upregulated in the two field sites that demonstrated high invertebrate mortality. We also observed significant upregulation of Cyp1a in the highest concentration of water from Sal_Davis in May 2019, which is considered a non-toxic site based on repeated assessments (CDPR Technical Report Hasenbein et al., 2018, Grant #16-C0084) and the mortality data from this study.
Fig. 4.

Log2 Fold-change of gene expression in P. promelas after acute exposure to a geometric dilution series of agricultural surface water ([100], [60] and [35]) collected in May 2019. Target genes of interest are: Acetylcholinesterase (AChE), Aspartoacylase (ASPA), Cytochrome P4501A (Cyp1a), Cyp3A126 (Cyp3a), Ryanodine receptor 1 (RyR1), Ryanodine receptor 2 (RyR2) and Sarco/Endoplasmic Reticulum ATPase (SERCA 1). Field sites shown: Sal_Quail (2A), Sal_Hartnell (2B) and Sal_Davis (2C). P-values are reported as * = P ≤ 0.05, ** = P ≤ 0.01, *** = P ≤ 0.001.
Differential expression for target genes (detoxification and neuromuscular pathways) of fish after 96 h exposures to a geometric dilution of field water collected in September 2019 are shown in Fig. 5. Cyp1a was upregulated at all sites but differential expression was not statistically significant relative to the control. Cyp3a was significantly upregulated for Sal_Quail at [100]. SERCA1 was strongly downregulated for Sal_Hartnell and Sal_Davis at all concentrations and downregulated for Sal_Quail at [60]. Fish exposed to water collected in Sept. 2019 displayed a significantl downregulation of SERCA1 relative gene expression, especially at the Sal_Hartnell and Sal_Davis locations.
Fig. 5.

Log2 Fold change of gene expression in P. promelas after acute exposure to a geometric dilution series of agricultural surface water ([100], [60], [35] and [20]) collected in Sept.2019. Target genes of interest are: Acetylcholinesterase (AChE), Aspartoacylase (ASPA), Cytochrome P4501A (Cyp1a), Cyp3A126 (Cyp3a), Ryanodine receptor 1 (RyR1), Ryanodine receptor 2 (RyR2) and Sarco/Endoplasmic Reticulum ATPase (SERCA1). Field sites shown: Sal_Quail (3A), Sal_Hartnell (3B) and Sal_Davis (3C). P-values are reported as * = P ≤ 0.05, ** = P ≤ 0.01, *** = P ≤ 0.001.
4. Discussion
We compared the effects of the insecticides IMI and CHL in single and binary mixtures and as components in field water exposures, on D. magna and P. promelas, two commonly used aquatic toxicology model species. Exposure to surface water collected near high use agricultural areas resulted in high invertebrate mortality even at the most diluted field waters. We did not observe any changes to survival of P. promelas exposed to surface water and single or binary mixtures containing the insecticides IMI and/or CHL, which suggests low acute toxicity to the model fish. However, exposed fish had significant changes in the relative expression of genes involved in detoxification and neuromuscular function, showing potential sublethal impacts. We also investigated the activity of IMI and CHL at the nAChR and RyR, respectively. Taken together, the survival, gene expression and binding activity data suggest that CHL and mixtures containing CHL have biologically important effects in both invertebrates and fish.
Chemical analyses of field water samples show repeated detections of IMI and CHL. Imidacloprid had the highest detection frequency among all the pesticides monitored between 2007 and 2016, making it a main pesticide of concern in Salinas and throughout California (Deng et al., 2019). Imidacloprid along with other neonicotinoids are used ubiquitously in over 120 countries worldwide and have been detected in the environment since their introduction (Jeschke et al., 2011). Chlorantraniliprole is an emerging chemical of concern that has proven to be extremely effective against many insect pests, and has subsequently experienced a rapid increase in use around the world (Teixeira and Andaloro, 2013). In many of the evaluated samples, both pesticides were present at concentrations that would be expected to affect sensitive species, where September 2019 chemistry data had lower concentrations of IMI and CHL than those seen in May 2019. Additionally, several other pesticides of concern exceeded benchmark levels and/or LC50s for sensitive species likely contributing to invertebrate mortality and sublethal effects in fish. Notably, methomyl a carbamate pesticide, was detected at concentrations many times the level expected to impact fish and is likely contributing to the toxicity for these samples (Van Scoy et al., 2013).
There was considerable overlap in the pesticides detected during both sampling periods, with few exceptions. The neonicotinoid thiamethoxam, which has been shown to interactively increase toxicity of CHL and esfenvalerate (Jones et al., 2012), was only detected in the September sampling event. Previous studies on thiamethoxam have shown that acute exposure can alter locomotor activity in zebrafish larvae (Liu et al., 2018) and cause neurotoxicity in catfish (Baldissera et al., 2018), albeit at concentrations above those detected in our sampled sites. In September, there were also several pyrethroids present at higher levels, compared to that detected in May 2019, including lambda cyhalothrin, permethrin and malathion. This finding is consistent with a recent study examining the lag time between pesticide application during the growing season and subsequent detections in California surface water due to the pattern of dry summers followed by winter rain events typical of this region (DeMars et al., 2021). In addition to the 47 pesticides included in our analysis, it is possible that other, untargeted pesticides could be contributing to the observed toxicity. Pesticide use patterns in the area surrounding Salinas waterways and tributaries have been shifting away from organophosphate pesticides toward pyrethroid and neonicotinoid pesticides (Anderson et al., 2003), emphasizing the importance of monitoring a wide variety of pesticides at regular intervals. Previous toxicity studies using field water from multiple sites in the Salinas waterways and tributaries have shown high rates of mortality in sensitive invertebrate species including D. magna (unpublished data; Anderson et al., 2006). In these studies, macroinvertebrate community structure was also highly impacted downstream of the sampling sites, suggesting that multitrophic assessments are crucial to understanding the ecological impacts of contaminants on a larger geographical scale (Anderson et al., 2006).
The current study is the first to address CHL activity at the RyR in model organisms commonly used in aquatic ecotoxicology. We show that CHL activates the RyR in the crustaceans H. azteca, and D. magna, insect C. dilutus and the vertebrate fish model P. promelas. The high CHL affinity for H. azteca and D. magna RyR was not observed in the other crustacean (Maine lobster; Homarus americanus) tested to date (Qi and Casida, 2013), suggesting differences in sensitivity in diverse crustacean species. Notably, we also observed significant activation of RyR found in the vertebrate fish model P. promelas suggesting CHL may impact neuromuscular health in fish. This is in line with more recent data regarding the impact of CHL, and related pesticides, on mice. Specifically, CHL caused a 200% over activation of RyR in P. promelas at 3.61 μM (current study) and was found to cause a ~ 200% over activation of RyR in wildtype mice at 1 μM (Truong and Pessah, 2019) showing similar levels of vertebrate sensitivity. It should be noted, however, that the fish binding assays completed in the current study were run in crude microsomal preparations compared to the junctional sarcoplasmic reticulum preparations run in mice (Truong and Pessah, 2019). We also observed high IMI affinity toward the nAChR in the aquatic toxicology model species H. azteca and C. dilutus at 8.86 nM and 8.04 nM, respectively, which is similar to that seen in other invertebrates such as the house fly (Musca domestica; 1.2 nM; Liu and Casida, 1993). The lack of binding in D. magna was surprising, which may have been due to the binding conditions utilized in the current study. However, there are conflicting results of IMI affinity across closely related invertebrate species, where there are still many questions regarding the interaction of IMI and related compounds with the nAChR (Crosswaithe et al. 2017). For example, insects, mainly hemipteran species that are particularly sensitive to neonicotinoids, display numerous IMI binding sites on the nAChR including a very high affinity site sensitive to sub-nM concentrations of IMI. Other insect species may lack the very high affinity site, possibly explaining lower neonicotinoid whole organism toxicity (Crosswaithe et al. 2017). The lack of IMI binding toward the nAChR in P. promelas is consistent with the lack of binding seen in other vertebrate species including the electric eel electric organ and numerous mammalian species (Liu and Casida, 1993; Tomizawa et al. 2000). The current work is one of the few studies looking at the direct interaction of IMI with the nAChR in a fish species and future research with varying assay conditions and the inclusion of IMI metabolites may better explain neurotoxic effects in neonicotinoid exposed fish.
We also observed changes in the expression of genes involved in target pathways after acute exposure to agricultural surface water and environmentally relevant chemical mixtures of IMI and CHL. In both field and single/binary exposures, genes in the Cytochrome P450 (Cyp450) family were differentially expressed, including Cyp1a and Cyp3a, which are involved in the metabolism of diverse chemicals as a first line of detoxification (De Montellano, 2005; Stegeman, 1994; Zanger and Schwab, 2013). In the single/binary exposures, Cyp1a and Cyp3a expression was consistent with responses of Cyp450 family proteins in other studies (Vandenberg et al., 2012). We did not observe changes in Cyp450 genes in IMI exposed fish. IMI displays low acute toxicity to fish, although it has been shown to cause immune system suppression and neurobehavioral impairment in larval zebrafish exposed to mg/L concentrations (Crosby et al., 2015). Our exposure concentrations did not approach the mg/L scale and could have been too low in single compound exposures to observe differential expression for Cyp450 markers. Upregulation of Cyp family genes is well-documented after exposure to several pesticides present in our field samples. The Cyp450 family proteins can be induced by a wide variety of xenobiotics making them particularly useful indicators for mixtures containing multiple classes of pesticides (Crain et al., 2008).
A gene involved in neurologic function was differentially expressed in both field and single/binary exposures. ASPA specifically maintains myelin sheet integrity in nerve cells (Baslow, 2002). Differential expression of ASPA has been measured in delta smelt (Hypomesus transpacificus) and P. promelas after sub-lethal exposure to insecticides, and may be implicated in impairing neurological function (Beggel et al., 2011; Connon et al., 2009). Physiological changes to the myelin-like structure (medullary sheath) of target pest invertebrates after exposure to CHL have also been observed (Ma et al., 2017). To our knowledge, no literature exists on the mechanism by which CHL may affect expression of ASPA.
Genes related to cellular Ca2+ homeostasis and signaling were altered in P. promelas exposed to water collected in the field and single/binary IMI and CHL exposures. Specifically, we investigated changes in relative gene expression in RyR1, RyR2 and SERCA1, an ATPase that pumps Ca2+ into the sarco(endo)plasmic reticulum (SR/ER) to restore SR/ER Ca2+ stores needed for muscle contraction and neuronal signaling. We saw changes in RyR2 gene expression when fish were exposed to CHL alone as would be suggested by CHL’s mechanism of action. We also found increased RyR2 in the IMI and CHL binary mixtures. IMI and its metabolites affect intracellular Ca2+ concentrations through their action at voltage-gated Ca2+ channels (VGCCs) (Jepson et al., 2006; Simon-Delso et al., 2015), which are well-known signaling partners of RyR. The combination of CHL with IMI may have led to altered Ca2+ homeostasis contributing to changes in RyR2 expression. Interestingly, we saw a large decrease in SERCA1 gene expression in fish exposed to field waters from Sal_Hartnell and Sal_Davis collected in September 2019. Pyrethroids have been documented to change Ca2+ homeostasis via interactions with VGCCs and a high affinity to the SERCA pump (Cao et al., 2011; Dusza et al., 2018). Pesticides that cause SERCA pump inhibition can further enhance the effect of compounds that cause an opening of the RyR by decreasing SR/ER Ca2+ stores (Dusza et al., 2018; Yao et al., 2011). CHL is more toxic when used in combination with some pyrethroids (Jones et al., 2012), and could have an increased contribution to site toxicity when present in combination with pyrethroids. Together, these findings support the conclusion that the observed mixture toxicity exceeded predictions based on single chemical assessments, and that altered gene expression could potentially impact fish.
Acute single chemical exposure assessments have been an integral part of the regulatory framework but cannot predict organismal responses to environmentally relevant mixtures. Synergistic effects of complex chemical mixtures are well documented in previous studies (Crain et al., 2008; Todgham and Stillman, 2013). Furthermore, the interaction of contaminants in combination with other environmental stressors can result in synergistic, additive and/or antagonistic effects. This illustrates the limitations of extrapolating toxicity from single stressor studies for comparison to environmentally relevant mixtures. As the complexity of mixtures increases, non-targeted, effect-based evaluations become necessary for determining biological outcomes. This is especially relevant for mixtures that include new and emerging contaminants of concern, where data on their biological effects may be limited to acute exposures on target and model organisms. The development of gene expression assays for use as monitoring and diagnostic tools depend on a clear understanding of the mechanisms underlying a molecular response, and more research is needed particularly for chemicals of emerging concern and their specific mechanisms of activity.
Climate change is expected to influence pest dynamics and pesticide applications globally (Wolfram et al., 2018). There is a pressing need to expand monitoring efforts to include effects-based assays to determine the biological effects of complex mixtures (e.g., binding assays, gene expression, Connon et al., 2019, 2012; Mehinto et al., 2021; Schuijt et al., 2021). Such efforts would allow the detection of subcellular level effects before they are apparent at higher levels of biological organization, particularly at low but environmentally relevant insecticide concentrations. Furthermore, additional endpoints such as development and behavior would provide for greater understanding of the consequences of pesticide exposure on invertebrate and fish populations (Ford et al., 2021; von Hellfeld et al., 2020; Wlodkowic and Campana, 2021).
5. Conclusions
In this study we targeted subcellular molecular pathways known to coincide with insecticides’ mechanisms of activity in aquatic organisms, then compared the relative degree of subcellular stress induced by IMI and CHL with responses to environmental mixtures. This combined approach helped evaluate species-specific responses and tolerance thresholds to IMI and CHL exposure. We demonstrated that CHL activates RyR in fathead minnow and in several model invertebrates commonly used in aquatic ecotoxicology. This finding is important for understanding how CHL may impact neuromuscular health in fish. Exposure to agricultural surface waters resulted in invertebrate toxicity that exceeded predictions based on single chemical assessments, and elicited detoxification responses and impacted neuromuscular function pathways in fish. In the absence of sublethal endpoints, our findings would have excluded important effects on fish. By conducting geometric dilution series and examining differential gene expression, we obtained a more comprehensive understanding of the sublethal effects of agricultural surface water on aquatic life. Pesticide contamination is a serious issue in agricultural and urban areas worldwide, and particularly in the central coast region of California. The implications of the current study may serve to inform management efforts and highlight the importance of continued research on chemicals of emerging concern.
Supplementary Material
HIGHLIGHTS.
Novel pesticides in agricultural water have unknown effects on aquatic species.
We exposed D. magna and P. promelas to imidacloprid, chlorantraniliprole and agricultural water.
We assessed survival, gene expression and ryanodine receptor binding activity.
Fish exposed to CHL or CHL/IMI mixture had altered Cyp3a and RyR2 gene expression.
Chlorantraniliprole altered ryanodine receptor activity in fish and invertebrates.
Imidacloprid activated the n-acetylcholine receptor in invertebrates.
Acknowledgements
We thank members of the Connon laboratory at UC Davis: Hadeel Bader, Trinity Burnham-Pohlmann, Nicole Egan, Nathaniel Gonzalez, Claudia Mortyn, Ignacio Ognian, Paola Perez, Anna Remstedt, and Celeste Valdiva; CDPR staff Anson Main and Mason Zoerner for their assistance conducting these studies. We thank the Center for Analytical Chemistry, at the California Department of Food and Agriculture (Sacramento, CA) for chemical analyses.
Funding
This work was supported by the California Department of Pesticide Regulation grant numbers 16-C0084 to EBH, SH, REC, and #18-C0042 to SAS, SPL and REC, and National Institute of General Medical Sciences of the NIH under Award numbers; 8UL1GM1189798TL4GM118980-02, 8RL5GM118978-02 CSULB Research Stimulation Grant to EBH.
Footnotes
CRediT authorship contribution statement
Sarah A. Stinson: Funding acquisition, Investigation, Project administration, Resources, Data curation, Formal analysis, Writing – original draft, Visualization. Simone Hasenbein: Funding acquisition, Investigation, Resources, Writing – review & editing. Richard E. Connon: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. Xin Deng: Resources, Writing – review & editing. Jordan S. Alejo: Investigation. Sharon P. Lawler: Funding acquisition, Writing – review & editing. Erika B. Holland: Conceptualization, Funding acquisition, Investigation, Formal analysis, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.150920.
References
- Anderson BS, Hunt JW, Phillips BM, Nicely PA, de Vlaming V, Connor V, Richard N, Tjeerdema RS, 2003. Integrated assessment of the impacts of agricultural drainwater in the Salinas River (California, USA). Environ. Pollut 124, 523–532. 10.1016/S0269-7491(03)00012-5. [DOI] [PubMed] [Google Scholar]
- Anderson BS, Phillips BM, Hunt JW, Connor V, Richard N, Tjeerdema RS, 2006. Identifying primary stressors impacting macroinvertebrates in the Salinas River (California, USA): relative effects of pesticides and suspended particles. Environ. Pollut 141, 402–408. 10.1016/j.envpol.2005.08.056. [DOI] [PubMed] [Google Scholar]
- Bakker L, van der Werf W, Tittonell P, Wyckhuys KAG, Bianchi FJJA, 2020. Neonicotinoids in global agriculture: evidence for a new pesticide treadmill? Ecol. Soc 25, art26. 10.5751/ES-11814-250326. [DOI] [Google Scholar]
- Baldissera MD, Souza CF, Golombieski JI, Seben D, Sippert LR, Salbego J, Marchesan E, Zanella R, Baldisserotto B, 2018. Purinergic signaling as potential target of thiamethoxam-induced neurotoxicity using silver catfish (Rhamdia quelen) as experimental model. Mol. Cell. Biochem 449, 39–45. 10.1007/s11010-018-3340-x. [DOI] [PubMed] [Google Scholar]
- Baslow MH, 2002. Functions of N-acetyl-l-aspartate and N-acetyl-l-aspartylglutamate in the vertebrate brain: role in glial cell-specific signaling. J. Neurochem 75, 453–459. 10.1046/j.1471-4159.2000.0750453.x. [DOI] [PubMed] [Google Scholar]
- Bass C, Puinean AM, Andrews M, Cutler P, Daniels M, Elias J, Paul VL, Crossthwaite AJ, Denholm I, Field LM, Foster SP, Lind R, Williamson MS, Slater R, 2011. Mutation of a nicotinic acetylcholine receptor β subunit is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. BMC Neurosci. 12, 51. 10.1186/1471-2202-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass C, Denholm I, Williamson MS, Nauen R, 2015. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol 121, 78–87. 10.1016/j.pestbp.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Beggel S, Connon R, Werner I, Geist J, 2011. Changes in gene transcription and whole organism responses in larval fathead minnow (Pimephales promelas) following short-term exposure to the synthetic pyrethroid bifenthrin. Aquat. Toxicol 105, 180–188. 10.1016/j.aquatox.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Bentley KS, Fletcher JL, Woodward MD, 2010. Chlorantraniliprole. Hayes’ Handbook of Pesticide Toxicology. Elsevier, pp. 2231–2242 10.1016/B978-0-12-374367-1.00102-6. [DOI] [Google Scholar]
- Bernhardt ES, Rosi EJ, Gessner MO, 2017. Synthetic chemicals as agents of global change. Front. Ecol. Environ 15, 84–90. 10.1002/fee.1450. [DOI] [Google Scholar]
- Cao Z, Shafer TJ, Murray TF, 2011. Mechanisms of pyrethroid insecticide-induced stimulation of calcium influx in neocortical neurons. J. Pharmacol. Exp. Ther 336, 197–205. 10.1124/jpet.110.171850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connon RE, Geist J, Pfeiff J, Loguinov AV, D’Abronzo LS, Wintz H, Vulpe CD, Werner I, 2009. Linking mechanistic and behavioral responses to sublethal esfenvalerate exposure in the endangered delta smelt; Hypomesus transpacificus (Fam. Osmeridae). BMC Genomics 10, 608. 10.1186/1471-2164-10-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connon RE, Geist J, Werner I, 2012. Effect-based tools for monitoring and predicting the ecotoxicological effects of Chemicals in the Aquatic Environment. Sensors 12, 12741–12771. 10.3390/s120912741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connon R, Hasenbein S, Brander S, Poynton H, Holland E, Schlenk D, Orlando J, Hladik M, Collier T, Scholz N, Incardona J, Denslow N, Hamdoun A, Nicklisch S, Garcia-Reyero N, Perkins E, Gallagher E, Deng X, Wang D, Fong S, Breuer R, Hajibabei M, Brown J, Colbourne J, Young T, Cherr G, Whitehead A, Todgham A, 2019. Review of and recommendations for monitoring contaminants and their effects in the San Francisco Bay–Delta San Franc. 17, 2. 10.15447/sfews.2019v17iss4art2. [DOI] [Google Scholar]
- Cordova D, Benner EA, Sacher MD, Rauh JJ, Sopa JS, Lahm GP, Selby TP, 2007. Elucidation of the Mode of Action of Rynaxypyr®, a Selective Ryanodine Receptor Activator. 10.1002/9783527611249.ch12. [DOI] [Google Scholar]
- Crain CM, Kroeker K, Halpern BS, 2008. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett 11, 1304–1315. 10.111l/j.1461-0248.2008.01253.x. [DOI] [PubMed] [Google Scholar]
- Crosby EB, Bailey JM, Oliveri AN, Levin ED, 2015. Neurobehavioral impairments caused by developmental imidacloprid exposure in zebrafish. Neurotoxicol. Teratol 49, 81–90. 10.1016/j.ntt.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Montellano PRO (Ed.), 2005. Cytochrome P450: Structure, Mechanism, and Biochemistry. Kluwer Academic/Plenum Publishers, New York: . https://link.springer.com/book/10.1007%2Fb139087a. [Google Scholar]
- DeMars C, Wang R, Grieneisen ML, Steggall J, Zhang M, 2021. Assessment of pyrethroid contamination and potential mitigation strategies in California central coast surface waters. J. Environ. Manag 278, 111507. 10.1016/j.jenvman.2020.111507. [DOI] [PubMed] [Google Scholar]
- Deng X, Wagner S, Wang D, Luo Y, Goh KS, 2019. Pesticide detections, benchmark exceedances, and temporal trends in surface water of California’s Imperial, Salinas, and Santa Maria Valleys. In: Goh KS, Gan J, Young DF, Luo Y (Eds.), ACS Symposium Series. American Chemical Society, Washington, DC, pp. 119–142 10.1021/bk-2019-1308.ch008. [DOI] [Google Scholar]
- Doo S, He L-M, 2008. Calibration, Field Measurement, Cleaning, and Storage of the YSI 6920 V2-2 Multiparameter Sonde. (No. SOP EQWA010.00). California Department of Pesticide Regulation, http://www.cdpr.ca.gov/docs/emon/pubs/sopequip.htm. [Google Scholar]
- Imidacloprid: a new nitroguanidine insecticide. In: Duke SO, Menn JJ, Plimmer JR (Eds.), Pest Control with Enhanced Environmental Safety, ACS Symposium Series. American Chemical Society, Washington, DC 10.1021/bk-1993-0524. [DOI] [Google Scholar]
- Dusza HM, Cenijn PH, Kamstra JH, Westerink RHS, Leonards PEG, Hamers T, 2018. Effects of environmental pollutants on calcium release and uptake by rat cortical microsomes. NeuroToxicology 69, 266–277. 10.1016/j.neuro.2018.07.015. [DOI] [PubMed] [Google Scholar]
- Ford AT, Ågerstrand M, Brooks BW, Allen J, Bertram MG, Brodin T, Dang Z, Duquesne S, Sahm R, Hoffmann F, Hollert H, Jacob S, Klüver N, Lazorchak JM, Ledesma M, Melvin SD, Mohr S, Padilla S, Pyle GG, Scholz S, Saaristo M, Smit E, Steevens JA, van den Berg S, Kloas W, Wong BBM, Ziegler M, Maack G, 2021. The role of behavioral ecotoxicology in environmental protection. Environ. Sri. Technol 55, 5620–5628. 10.1021/acs.est.0c06493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch EB, Pessah IN, 2013. Structure-activity relationship of non-coplanar polychlorinated biphenyls toward skeletal muscle ryanodine receptors in rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol 140–141, 204–212. 10.1016/j.aquatox.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh KS, Luo Y, Singhasemanon N, 2019. Surface water protection program for pesticide use in California. In: Goh KS, Gan J, Young DF, Luo Y (Eds.), ACS Symposium Series. American Chemical Society, Washington, DC, pp. 1–10 10.1021/bk-2019-1308.ch001. [DOI] [Google Scholar]
- Hasenbein S, Holland E, Stinson SA, Connon RE, 2018. Developing Molecular Biomarkers to Assess Chlorantraniliprole and Imidacloprid Impacts in Aquatic Species (No. 16-C0084). CA Department of Pesticide Regulation, https://www.cdpr.ca.gov/docs/emon/surfwtr/contracts/final_report_16-c0084.pdf. [Google Scholar]
- Hasenbein S, Holland EB, Connon RE, 2019. Eyes to the future: approaches to assess pesticide impact on surface waters in a changing climate. In: Goh KS, Gan J, Young DF, Luo Y (Eds.), ACS Symposium Series. American Chemical Society, Washington, DC: pp. 189–214 10.1021/bk-2019-1308.ch010. [DOI] [Google Scholar]
- von Hellfeld R, Brotzmann K, Baumann L, Strecker R, Braunbeck T, 2020. Adverse effects in the fish embryo acute toxicity (FET) test: a catalogue of unspecific morphological changes versus more specific effects in zebrafish (Danio rerio) embryos. Environ. Sri. Eur 32, 122. 10.1186/sl2302-020-00398-3. [DOI] [Google Scholar]
- Hunt JW, Anderson BS, Phillips BM, Nicely PN, Tjeerdema RS, Puckett HM, Stephenson M, Worcester K, de Vlaming V, 2003. Ambient toxicity due to chlorpyrifos and diazinon in a Central California coastal watershed. Environ. Monit. Assess 82, 83–112. 10.1023/A1021677914391. [DOI] [PubMed] [Google Scholar]
- Jepson JEC, Brown LA, Sattelle David B., 2006. The actions of the neonicotinoid imidacloprid on cholinergic neurons of Drosophila melanogaster. Invert. Neurosci 6, 33–40. 10.1007/s10158-005-0013-8. [DOI] [PubMed] [Google Scholar]
- Jeschke P, Nauen R, Schindler M, Elbert A, 2011. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem 59, 2897–2908. 10.1021/jf101303g. [DOI] [PubMed] [Google Scholar]
- Jones, 1999. Standard operating procedure QAQC004. 01-Transporting, Packaging and Shipping Samples From the Field to the Warehouse or Laboratory. https://www.cdpr.ca.gov/docs/emon/pubs/sop_qaqc.htm.
- Jones MM, Robertson Jacqueline L., Weinzierl Richard L., 2012. Toxicity of thiamethoxam and mixtures of chlorantraniliprole plus acetamiprid, esfenvalerate, or thiamethoxam to neonates of oriental fruit moth (Lepidoptera: Tortricidae). J. Econ. Entomol 105, 1426–1431. 10.1603/EC11349. [DOI] [PubMed] [Google Scholar]
- Kuivila KM, Hladik ML, Ingersoll CG, Kemble NE, Moran PW, Calhoun DL, Nowell LH, Gilliom RJ, 2012. Occurrence and potential sources of pyrethroid insecticides in stream sediments from seven U.S Metropolitan Areas. 46, 4297–4303. 10.1021/es2044882. [DOI] [PubMed] [Google Scholar]
- Lahm GP, Stevenson TM, Selby TP, Freudenberger JH, Cordova D, Flexner L, Bellin CA, Dubas CM, Smith BK, Hughes KA, Hollingshaus JG, Clark CE, Benner EA, 2007. RynaxypyrTM: a new insecticidal anthranilic diamide that acts as a potent and selective ryanodine receptor activator. Bioorg. Med. Chem. Lett 17, 6274–6279. 10.1016/j.bmcl.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Liu MY, Casida JE, 1993. High affinity binding of [3H]Imidacloprid in the insect acetylcholine receptor. Pestic. Biochem. Physiol 46 (1), 40–46. 10.1006/pest.1993.1034. [DOI] [Google Scholar]
- Liu X, Zhang Q, Li S, Mi P, Chen D, Zhao X, Feng X, 2018. Developmental toxicity and neurotoxicity of synthetic organic insecticides in zebrafish (Danio rerio): a comparative study of deltamethrin, acephate, and thiamethoxam. Chemosphere 199, 16–25. 10.1016/j.chemosphere.2018.01.176. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luo Y, Deng X, Ensminger M, Budd R, 2018. Methodology for prioritizing pesticides for surface water monitoring in agricultural and urban areas of California. In: Zhang M, Jackson S, Robertson MA, Zeiss MR (Eds.), ACS Symposium Series. American Chemical Society, Washington, DC, pp. 307–322 10.1021/bk-2018-1283.ch014. [DOI] [Google Scholar]
- Ma S, Liu L, Ma Z, Zhang X, 2017. Microstructural and ultrastructural changes in the muscle cells of the oriental armyworm mythimna separata Walker (Lepidoptera: Noctuidae) on treatment with wilforine. Pestic. Biochem. Physiol 139, 60–67. 10.1016/j.pestbp.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Mehinto AC, Schoenfuss HL, Wenger E, Diehl D, Bay SM, 2021. Application of an effects-based monitoring strategy to assess the impact of contaminants on fish health in an urbanized watershed. Environ. Toxicol. Chem 40, 402–412. 10.1002/etc.4921. [DOI] [PubMed] [Google Scholar]
- Meissner G, 1986. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J. Biol. Chem 261, 6300–6306. 10.1016/S0021-9258(19)84563-5. [DOI] [PubMed] [Google Scholar]
- Özdemir Selçuk, Altun Serdar, Özkaraca Mustafa, Ghosi Atena, Toraman Emine, Arslan Harun, 2018. Cypermethrin, chlorpyrifos, deltamethrin, and imidacloprid exposure up-regulates the mRNA and protein levels of bdnf and c-fos in the brain of adult zebrafish (Danio rerio). Chemosphere 203, 318–326. 10.1016/j.chemosphere.2018.03.190. [DOI] [PubMed] [Google Scholar]
- Pisa L, Goulson D, Yang E-C, Gibbons D, Sánchez-Bayo F, Mitchell E, Aebi A, van der Sluijs J, MacQuarrie CJK, Giorio C, Long EY, McField M, Bijleveld van Lexmond M, Bonmatin J-M, 2021. An update of the worldwide integrated assessment (WIA) on systemic insecticides, part 2: impacts on organisms and ecosystems. Environ. Sci. Pollut Res 28, 11749–11797. 10.1007/s11356-017-0341-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi S, Casida JE, 2013. Species differences in chlorantraniliprole and flubendiamide insecticide binding sites in the ryanodine receptor. Pestic. Biochem. Physiol 107, 321–326. 10.1016/j.pestbp.2013.09.004. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2020. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. http://r.meteo.uni.wroc.pl/web/packages/dplR/vignettes/intro-dplR.pdf. [Google Scholar]
- Schuijt LM, Peng F-J, van den Berg SJP, Dingemans MML, Van den Brink PJ, 2021. (Eco)toxicological tests for assessing impacts of chemical stress to aquatic ecosystems: facts, challenges, and future. Sci. Total Environ 795, 148776. 10.1016/j.scitotenv.2021.148776. [DOI] [PubMed] [Google Scholar]
- Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C, Furlan L, Gibbons DW, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke CH, Liess M, Long E, McField M, Mineau P, Mitchell EAD, Morrissey CA, Noome DA, Pisa L, Settele J, Stark JD, Tapparo A, Van Dyck H, Van Praagh J, Van der Sluijs JP, Whitehorn PR, Wiemers M, 2015. Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 22, 5–34. 10.1007/s11356-014-3470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiles TD, Yoshiyama RM, P.B., 2013. Pink salmon (Oncorhynchus gorbuscha) in the Salinas River, California: new record and historical perspectives. Calif. Fish Game 99, 55–59. https://nrm.dfg.ca.gov/FileHandler.ashx?DocumentID=69448. [Google Scholar]
- Soderlund DM, 2012. Molecular mechanisms of pyrethroid insecticide neurotoxicity: recent advances. Arch. Toxicol 86, 165–181. 10.1007/s00204-011-0726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurgeon DJ, Jones OAH, Dorne J-LCM, Svendsen C, Swain S, Stürzenbaum SR, 2010. Systems toxicology approaches for understanding the joint effects of environmental chemical mixtures. Sci. Total Environ 408, 3725–3734. 10.1016/j.scitotenv.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Stegeman JJ, 1994. Biochemistry and molecular biology of monooxygenases: current perspectives on forms, functions, and regulation of cytochrome P450 in aquatic species. Aquat. Toxicol. Mol. Biochem. Cell. Perspect, 87–206. 10.1201/9781351069878/aquatic-toxicology-donald-malins. [DOI] [Google Scholar]
- Stehle S, Schulz R, 2015. Agricultural insecticides threaten surface waters at the global scale. Proc. Natl. Acad. Sci 112, 5750–5755. 10.1073/pnas.1500232112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teerlink J, DaSilva A, 2017. Standard Operating Procedure QAQC012.00 - Guide for Analytical Method Development for Surface Water and Sediment Samples, https://www.cdpr.ca.gov/docs/emon/pubs/sop_qaqc.htm.
- Teixeira LA., Andaloro JT, 2013. Diamide insecticides: global efforts to address insect resistance stewardship challenges. Pestic. Biochem. Physiol 106, 76–78. 10.1016/j.pestbp.2013.01.010. [DOI] [Google Scholar]
- Todgham AE, Stillman JH, 2013. Physiological responses to shifts in multiple environmental stressors: relevance in a changing world. Integr. Comp. Biol 53, 539–544. 10.1093/icb/ict086. [DOI] [PubMed] [Google Scholar]
- Truong KM, Pessah IN, 2019. Comparison of chlorantraniliprole and flubendiamide activity toward wild-type and malignant hyperthermia-susceptible ryanodine receptors and heat stress intolerance. Toxicol. Sci 167, 509–523. 10.1093/toxsci/kfy256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA, 2002. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms, https://www.epa.gov/sites/default/files/2015-08/documents/acute-freshwater-and-marine-wet-manual_2002.pdf.
- USEPA, 2020. Aquatic Life Benchmarks and Ecological Risk Assessments for Registered Pesticides.
- Van Scoy AR, Yue M, Deng X, Tjeerdema RS, 2013. Environmental Fate and Toxicology of Methomyl. In: Whitacre DM (Ed.), Reviews of Environmental Contamination and Toxicology, Reviews of Environmental Contamination and Toxicology. Springer New York, New York, NY, pp. 93–109 10.1007/978-1-4614-4717-7_3. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee D-H, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP, 2012. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev 33, 378–455. 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F, 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3 (research0034), 1. 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston DP, Poynton HC, Wellborn GA, Lydy MJ, Blalock BJ, Sepulveda MS, Colbourne JK, 2013. Multiple origins of pyrethroid insecticide resistance across the species complex of a nontarget aquatic crustacean, Hyalella azteca. Proc Natl. Acad. Sci 110, 16532–16537. 10.1073/pnas.1302023110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner P, Kayser H, 2000. Characterization of nicotinic acetylcholine receptors from the insects Aphis craccivora, Myzus persicae, and locusta migratoria by radioligand binding assays: relation to thiamethoxam action. J. Biochem. Mol. Toxicol 14, 221–230. . [DOI] [PubMed] [Google Scholar]
- Wlodkowic D, Campana O, 2021. Toward high-throughput fish embryo toxicity tests in aquatic toxicology. Environ. Sci. Technol 55, 3505–3513. 10.1021/acs.est.0c07688. [DOI] [PubMed] [Google Scholar]
- Wolfram J, Stehle S, Bub S, Petschick LL, Schulz R, 2018. Meta-analysis of insecticides in United States surface waters: status and future implications. Environ. Sci. Technol 52, 14452–14460. 10.1021/acs.est.8b04651. [DOI] [PubMed] [Google Scholar]
- Yang L, Kemadjou JR, Zinsmeister C, Bauer M, Legradi J, Müller F, Pankratz M, Jäkel J, Strähle U, 2007. Transcriptional profiling reveals barcode-like toxicogenomic responses in the zebrafish embryo. Genome Biol. 8, R227. 10.1186/gb-2007-8-10-r227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Gallenkamp D, Wölfel K, Lüke B, Schindler M, Scherkenbeck J, 2011. Synthesis and SERCA activities of structurally simplified cyclopiazonic acid analogues. Bioorg. Med. Chem 19, 4669–4678. 10.1016/j.bmc.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Zanger UM, Schwab M, 2013. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther 138, 103–141. 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Zhang W, 2018. Global pesticide use: Profile, trend, cost/benefit and more. Proc. Int. Acad. Ecol. Environ. Sci 8. http://www.iaees.org/publications/journals/piaees/articles/2018-8(1)/global-pesticide-use-Profile-trend-cost-benefit.pdf. [Google Scholar]
- Zhao G, Yang F, Li J, Xing H, Ren F, Pang G, Li Y, 2020. Toxicities of neonicotinoid-containing pesticide mixtures on nontarget organisms. Environ. Toxicol. Chem 39, 1884–1893. 10.1002/etc.4842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


