Abstract
The Rift Valley fever virus (RVFV) is an emerging high-priority pathogen endemic in Africa with pandemic potential. There is no specific treatment or approved antiviral drugs for the RVFV. We previously developed a cell-based high-throughput assay to screen small molecules targeting the RVFV and identified a potential effective antiviral compound (1-N-(2-(biphenyl-4-yloxy)ethyl)propane-1,3-diamine) as a lead compound. Here, we investigated how structural modifications of the lead compound affected the biological properties and the antiviral effect against the RVFV. We found that the length of the 2-(3-aminopropylamino)ethyl chain of the compound was important for the compound to retain its antiviral activity. The antiviral activity was similar when the 2-(3-aminopropylamino)ethyl chain was replaced with a butyl piperazine chain. However, we could improve the cytotoxicity profile of the lead compound by changing the phenyl piperazine linker from the para-position (compound 9a) to the meta-position (compound 13a). Results from time-of-addition studies suggested that compound 13a might be active during virus post-entry and/or the replication phase of the virus life cycle and seemed to affect the K+ channel. The modifications improved the properties of our lead compound, and our data suggest that 13a is a promising candidate to evaluate further as a therapeutic agent for RVFV infection.
Introduction
Rift Valley fever (RVF) is an acute viral infection caused by the emerging mosquito-borne Rift Valley fever virus (RVFV) (genus Phlebovirus, family Phenuiviridae), which infects domestic animals and humans. The RVFV causes deadly infection among ruminants with high fever, hepatitis, acute deaths of newborns, and abortions in pregnant animals. Abortion storms are considered as a hallmark of RVFV outbreaks.1−3 Humans are infected by mosquito bites as well as handling contaminated animal tissues and fluids while working with slaughter or taking care of infected animals in herds. In humans, RVFV infection ranges from a mild illness associated with fever and liver abnormalities to much more severe symptoms such as retinitis, encephalitis, and hemorrhagic fever.4,5 The association of RVFV infection with miscarriage in humans has also been reported.6,7
The RVFV causes recurrent outbreaks throughout the African countries.8 The RVF epidemic in Yemen and Saudi Arabia in 2000 was the first of this kind outside Africa. This epidemic affected both livestock and humans, and approximately 200 humans died.9,10 The spread of the RVFV outside Africa was mainly due to import of infected animals from epidemic countries. Notably, more than 30 different mosquito species have been identified to carry the RVFV, of which several are competent vectors and are distributed globally.11 The RVFV has been endemic in the African subcontinent for decades, but outbreaks in Saudi Arabia, Yemen, Madagascar, and the Comoros Archipelago suggest that the geographical distribution of the RVFV is changing.12−14 Recent studies have reported the presence of RVFV-seropositive animals in Iran and Turkey,15,16 and other Asian countries are also at risk. Due to the presence of competent mosquito vectors in Europe, studies have emphasized that the RVFV can pose a major threat there.17−19
Currently, there are no safe and effective treatments to prevent or cure RVFV-infected humans or livestock. So far, several natural products and synthetic chemical compounds have been reported as potent RVFV inhibitors in vitro, but none of them progressed further to become an RVFV-specific drug candidate.20−26 Favipiravir is not RVFV-specific but has broad-spectrum activity against a number of RNA viruses, including the RVFV, and has gone through human clinical trials.27−29 However, there is a need to develop potent efficacious antiviral compounds against RVFV infection.
Previously, we have developed a high-throughput screening method for identifying potent inhibitors of RVFV infection in vitro and identified several compounds.30 The parent compound (N1-(2-(biphenyl-4-yloxy)ethyl)propane-1,3-diamine) was identified as a promising hit for further evaluation (Figure 1). The structure of the parent compound (designated as compound 1) consists of a biphenyl group that is connected, through an ether bond, to a 2-(3-aminopropylamino)ethyl chain. To investigate the important structural factors of parent compound 1, we aimed to synthesize a set of first-generation compounds with modification of the 2-(3-aminopropylamino)ethyl chain and keep the biphenyloxy part conserved. Depending on the result after the first investigation, the intention was to investigate the biphenyl part by synthesizing a set of second-generation compounds with modifications of the biphenyl part and keep the modified alkyl amine chain of interest conserved.
Figure 1.
Structure of parent compound 1, identified from high-throughput screening of RVFV infection in vitro.
Results and Discussion
Chemistry: First-Generation Compounds
To determine the importance of the 2-(3-aminopropylamino)ethyl chain for the parent compound 1, a set of first-generation compounds were synthesized. The approach for synthesizing the compounds 4d–e, 5a–c, and 5f–g is described in Scheme 1. Ether bond formation between alcohols 3a–g and 4-biphenol 2 was performed under standard Mitsunobu conditions using di-isopropyl azodicarboxylate (DIAD) and triphenylphosphine (PPh3) in tetrahydrofuran (THF), which gave compounds 4a–g.31 N-Boc removal of N-Boc-protected compounds 4a–c and 4f–g was performed under acidic conditions with either trifluoroacetic acid (TFA) or HCl, which gave the corresponding deprotected compounds 5a–c and 5f–g as free amines or HCl salts.
Scheme 1. Synthesis of Compounds 4d–e, 5a–c, and 5f–g: (a) PPh3, DIAD, THF, RT; (b) 4a–c, TFA, DCM at RT; 4f–g, 4 M HCl (dioxane), DCM, RT.
The synthetic approach for alkyl-piperazine analogs 8c and 9a–b is described in Scheme 2. For synthesis of compounds 8a–c, N-Boc piperazine 6a or N-methyl piperazine 6b was treated with an equivalent amount of 1,4-dibromobutane or 1,5-dibromopentane in acetonitrile and excess cesium carbonate as a base. Increasing the temperature to 115 °C for 20 min using microwave heating allowed the spiro salt intermediate 7a–7c to form in situ. Addition of 4-biphenol 2 in small excess and increased microwave heating to 170 °C resulted in nucleophilic ring opening of the spiro salt intermediate, which gave the alkyl piperazine analogs 8a–c in a one-pot fashion in <57% yield. The initial attempt was performed using potassium carbonate as a base instead of cesium carbonate, and the reaction worked but a higher temperature was necessary for completion. In addition, an attempt of changing the solvent to dimethylformamide (DMF) was also performed but without any success. Finally, acidic N-Boc removal of compounds 8a–b with HCl gave 9a–b as di-HCl salts.
Scheme 2. One-Pot Synthesis of Compounds 8a–c and Deprotection of the Boc Group: (a) Cs2CO3, 1,4-Dibromobutane, MeCN, Microwave Heating (115 °C) Followed by Addition of 4-Biphenol 2 and Microwave Heating (170 °C); (b) 4 M HCl (dioxane), DCM.

For synthesis of the N-phenyl-substituted butyl piperazine 11, the N-phenyl piperazine spiro salt 7c was isolated and reacted as a substrate with 4-biphenol. The synthetic approach for compound 11 is described in Scheme 3. The N-phenyl piperazine spiro salt 7c was synthesized from N-phenyl piperazine 6c, which was reacted with an equivalent amount of 1,4-dibromobutane in acetonitrile and potassium carbonate as a base. The reaction was performed at 115 °C using microwave heating for 15 min, which gave 7c in 79% yield after isolation. Nucleophilic ring opening of the spiro salt 7c was performed using acetonitrile as a solvent in excess of cesium carbonate as a base at 170 °C accomplished using microwave heating. After 25 min, the reaction was completed, and compound 11 was isolated in 89% yield.
Scheme 3. Synthesis of the Phenyl-Substituted Piperazine 11 by Nucleophilic Ring Opening of the Spiro Salt 6c: (a) K2CO3, 1,4-Dibromobutane, MeCN, Microwave Heating (115 °C); (b) Cs2CO3, MeCN, Microwave Heating (170 °C).
Biology: First-Generation Compounds
To evaluate the first-generation compounds, their antiviral activities were analyzed using the rRVFVΔNSs::Katushka virus assay essentially as described previously.30 Briefly, A549 cells were infected with rRVFVΔNSs::Katushka (multiplicity of infection (MOI) = 0.1) together with serial dilutions of test compounds and incubated for 16 h. Then, the number of virus-infected cells was quantified by monitoring the expression of Katushka fluorescent proteins in a Trophos plate runner HD (Trophos, Roche Group). To determine the cytotoxic concentration (CC)50 values, A549 cells were treated with three-fold serially diluted compounds for 24 h, and the cytotoxicity was measured using a resazurin cell viability assay (Sigma-Aldrich). The results from the antiviral and cytotoxicity assays are summarized in Table 1. Changing the 2-(3-aminopropylamino) ethyl chain in compound 1 to a shorter ethyl (compound 5b) or propyl amine (compound 5c) chain resulted in reduction of antiviral activity but improved the cytotoxicity profile. On the other hand, replacing the 2-(3-aminopropylamino) ethyl chain with a hexyl amine chain (compound 5a) showed a similar antiviral effect to compound 1 but increased the cytotoxicity. Introducing a heterocycle instead of the amino functionality for the ethylamine-substituted compound 5b to an N-piperidine (compound 4d) or N-morpholine (compound 4e) decreased the antiviral potency but showed an improved cytotoxicity profile. However, the N-piperazine-substituted compound 5f showed similar antiviral potency to the ethylamine-substituted compound 5b. Increasing the length of the alkyl piperazine chain to butyl (compound 9a) and pentyl (compound 9b) showed increased antiviral activity with an increased length of the alkyl ligand, but the propyl (compound 5g) showed reduced antiviral activity. Butyl and pentyl piperazine compounds 9a and 9b showed similar potency with EC50 = 12.8 ± 0.2 μM and EC50 = 11.7 ± 1.8 μM to the parent compound 1 (12 μM), and butyl piperazine compound 9a showed a similar cytotoxicity profile (CC50 = 74.8 μM ± 1.2) to compound 1 (CC50 = 86 μM ± 9); meanwhile, pentyl piperazine compound 9b showed increased cytotoxicity. Introducing an N-methyl in the piperazine moiety (8c) decreased the antiviral potency, and for the N-phenyl (11), the antiviral activity was completely eliminated.
Table 1. The Antiviral Activity, Cytotoxicity, and Selectivity Index of First-Generation Compoundsa.
EC50, half-maximum effective concentration; CC50, cytotoxicity concentration at 50%. Both values are representatives of two independent experiments with two replicates each time. ND, not detectable.
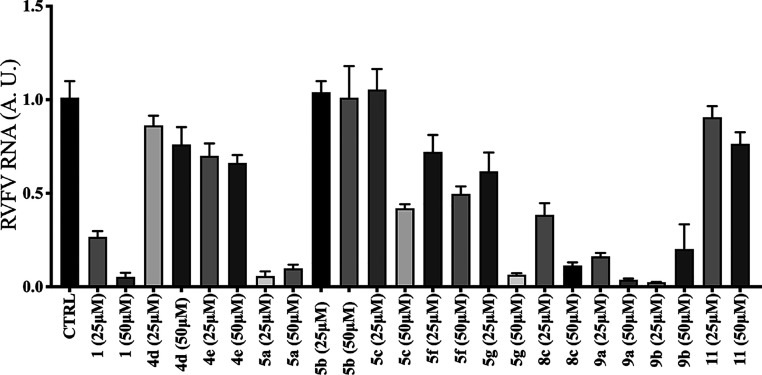
The antiviral activity identified from the fluorescence assay was further confirmed with an orthogonal qRT-PCR assay. A549 cells were infected with rRVFVΔNSs::Katushka (MOI = 1) and treated and incubated with the compound for 16 h. Thereafter, viral RNA was purified, and the amount of synthesized viral RNA was quantified by qRT-PCR (Figure 2). As shown in Figure 2, the qRT-PCR results resembled the results obtained from the fluorescence assay. Analogues (4d–e) were unable to inhibit the RNA expression at the concentrations used in the qRT-PCR assay. Also, the analogues 5b–c, 5f–g, and 8c were also unable to inhibit the viral RNA expressions. In the fluorescence assay, we observed that analogues 5a and 9a–b had similar EC50 values to compound 1. These three analogues also had similar patterns to compound 1 regarding inhibition of viral RNA expression. When taking into account the CC50 values, butyl piperazine compound 9a was considered the best among the first-generation analogues with a similar cytotoxicity profile to compound 1. The N-phenyl piperazine analogue 11 did not show any inhibition during the fluorescence assay, and a similar phenomenon was also observed by the qRT-PCR assay.
Figure 2.
Effect of first-generation compounds on RVFV RNA expression. A549 cells were infected with rRVFVΔNSs::Katushka (MOI = 1) and treated with compounds 1, 4d–e, 5a–c, 5f–g, 8c, 9a–b, and 11 at 25 and 50 μM concentrations. The viral load was measured at 16 h post infection using qRT-PCR targeted against the RVFV L segment, which encodes the RVFV RNA-dependent RNA polymerase, and these values were normalized to β-actin mRNA (A.U. = arbitrary units). Each bar represents the mean ± SEM and is representative of two independent experiments with two replicates each time.
Chemistry: Second-Generation Compounds
Based on the first-generation compounds, we concluded that the butyl piperazine ligand in compound 9a was the best choice for further modification of the biphenyl part. Compound 9a with a butyl piperazine ligand showed an equal antiviral activity and toxicity profile to the parent compound 1 with a 2-(3-aminopropylamino)ethyl ligand. For synthesis of the butyl piperazine analogs, the spiro salt 7a was synthesized and isolated and used as a substrate for further synthesis of the butyl piperazine analogs 12a–m by nucleophilic ring opening of the spiro salt. Acidic removal of the Boc group using HCl gave 13a–m as di-HCl salts in 25–72% yields calculated over two steps. The synthetic approach for compounds 13a–m is described in Scheme 4. For synthesis of compound 16, the above approach was not successful. Instead, compound 16 was synthesized in two steps described in Scheme 5. First, diphenylmethanol 14 was O-alkylated with 1,4-dibromobutane and sodium hydride as a base to give compound 15 in 22% yield. Second, N-alkylation of piperazine with 1 equiv of compound 15 and excess of cesium carbonate gave compound 16 in 57% yield.
Scheme 4. Synthesis of Compounds 13a–m by Nucleophilic Ring Opening of the Spiro Salt 6a: (a) K2CO3, 1,4-Dibromobutane, MeCN, Microwave Heating (115 °C); (b) Cs2CO3, MeCN, Microwave Heating (170 °C).

Scheme 5. (a) NaH, THF, RT; (b) Cs2CO3, MeCN, Microwave Heating (115 °C).
Biology: Second-Generation Compounds
The butyl piperazine analogues with modification of the biphenyl part showed a wide range of antiviral and cytotoxic activities (Table 2). Changing the biphenyl part to a smaller phenyl group (13f) completely removed the antiviral activity. In addition, introducing a more bulky group such as diphenyl methyl (16) instead of the biphenyl reduced the antiviral activity significantly. For the investigation of the structural isomers of the biphenyl part, the antiviral activity was similar for both the para-position of 9a (EC50 = 12.8 μM ± 0.2) and the meta-position of 13a (EC50 = 13.8 ± 5.3 μM) but reduced when the phenyl was located in the ortho-position of 13b (EC50 = 63.5 μM ± 11.1). On the contrary, an improved cytotoxicity profile was observed when the phenyl was located in the meta-position (CC50 = 144.8 ± 5.5 μM). Then, the phenyl was changed to benzyl (13c–e), and among them, the highest antiviral activity was observed for the benzyl located in the para-position of 13c (EC50 = 15.4 ± 4.7 μM) compared to the ortho-position of 13d (EC50 = 22.9 ± 5.2) and the meta-position of 13e (EC50 = 31.2 ± 15.8). Benzyl-substituted compounds (13c–e) showed a similar cytotoxicity profile to the meta-biphenyl analog 13a. Introducing a more bulky substituent such as cyclohexyl (13l) instead of phenyl (9a) in the para-position increased the antiviral activity (EC50 = 8.8 ± 0.5 μM) but also increased the cytotoxicity. Extreme toxicity was observed when the outer phenyl ring was replaced with adamantine (13m). To investigate if addition of an electron-donating, electron-withdrawing, or steric group could affect the antiviral activity, an acetyl (13g), dimethylamine (13h), or isopropyl (13j) substituent was introduced in the meta-position of the outer ring of the biphenyl part. For the meta-acetyl-substituted compound 13g, the antiviral activity decreased (EC50 = 41.9 ± 27.3 μM), while the meta-N,N-dimethylamine-substituted compound 13h (EC50 = 15.5 ± 0.7 μM) displayed improved antiviral activity but showed high toxicity. Good antiviral activity was detected when the isopropyl substituent was introduced to either the para- (13i) or ortho-position (13k). In contrast, addition of an isopropyl group to the biphenyl ring may have enhanced the antiviral activity for 13i–k but diminished the cytotoxicity profile. One interesting observation was that analogues with low EC50 values (13i–m) exhibited high toxicity. This suggested that the observed antiviral activities of these compounds were actually cytotoxic effects.
Table 2. The Antiviral Activity, Cytotoxicity, and Selectivity Index of Second-Generation Compoundsa.
EC50, half-maximum effective concentration; CC50, cytotoxicity concentration at 50%. Both values are representatives of two independent experiments with two replicates each time. ND, not detectable.
Similar to the first-generation compounds, the antiviral activity of the second-generation compounds identified from the fluorescence assay was further confirmed by qRT-PCR. Compared to 13a, compounds (13b–e and 13 g–h) showed low or no inhibitory effect on RVFV RNA expression. We had similar observation for compounds 13b–e and 13 g–h when we detected the EC50 values by the fluorescence assay. In contrast, compounds (13i–m) having lower EC50 values than 13a in the fluorescence assay also efficiently inhibited the RVFV RNA expression in qRT-PCR. However, as mentioned earlier, these compounds were highly cytotoxic; therefore, the observed antiviral potency was most probably due to their toxic effect on the cells and not because they inhibited the virus itself (Figure 3). Taking into account both antiviral activity and cytotoxicity of all the compounds, 13a was considered the best to be further explored in a mode-of-action study, although 13a has similar antiviral activity to compound 1. However, 13a had a better cytotoxicity profile than compound 1.
Figure 3.
Effects of second-generation compounds on RVFV RNA expression. A549 cells were infected with rRVFVΔNSs::Katushka (MOI = 1) and treated with compounds 13a–m and 16 at 25 and 50 μM concentrations. The viral load was measured at 16 h post infection using qRT-PCR targeted against the RVFV L segment, which encodes the RVFV RNA-dependent RNA polymerase, and these values were normalized to β-actin mRNA (A.U. = arbitrary units). Each bar represents the mean ± SEM and is representative of two independent experiments with two replicates each time.
Mode-of-Action Studies
The time-of-addition assay was performed to determine at which stage in the RVFV infection cycle compound 13a had an effect. Compound 13a (50 μM) was added at different time points of virus (rRVFVΔNSs::Katushka) infection: preinfection (−1 h before infection), during virus addition (0 h), at early post-entry (2 h post infection (hpi) and 4 hpi), and at late stages of virus infection (6 and 8 hpi). The compound was also added to the cells 1 h before infection, incubated for 1 h, and then removed just before virus addition (−1 to 0 h) as indicated in Figure 4. The experiment was terminated at 13 hpi, and infectivity was assessed by the fluorescence assay, as described previously. As demonstrated in Figure 4, data suggested that the highest antiviral potency was observed when compound 13a was added in the beginning of virus infection. These results suggested that compound 13a was active at early stages of the viral replication cycle, most likely during or just after virus entry. The compound 13a progressively lost its potency when added at late stages of the infection cycle.
Figure 4.

Time-of-addition study. Pre- and post treatment of rRVFVΔNSs::Katushka-infected cells with compound 13a (50 μM). A549 cells were inoculated with rRVFVΔNSs::Katushka at MOI = 0.1 (time point 0 h). Compound 13a was added 1 h before infection and then removed at the time of infection (time point −1 to 0 h); the compound was added 1 h prior to infection (time point −1 h), at the same time as infection (time point 0 h), or at the indicated time points post infection and incubated for 13 h, and the percentage of viral infection was determined by a fluorescent cell focus assay. (A) Cells infected with the virus (red) and stained with DAPI (blue). (B) Quantification of the time-of-addition assay in (A). The percentage of infection shown here is relative to the infected control where no antiviral compound was added (CTRL). Each bar represents the mean ± SD and is representative of two independent experiments with at least two replicates each time.
It is now well-evident that virion fusion and entry to the host cells are largely regulated by ion channels. Studies have shown that the functionality of ion channels plays a crucial role during entry or post-entry stages of several viruses.34 For example, the hepatitis C virus (HCV) requires ion channels for its successful infection cycle.35,36 Therefore, ion channels could be new targets to counteract virus infections, with a potentially broad-spectrum antiviral activity useful for future pandemics. Previous antiviral screens of chemical compounds identified several clinically approved ion channel inhibitors as membrane fusion blockers of the HCV.37−39 Many of them were closely related to our compound 13a. Recently, it has been reported that the K+ channel regulates the post-entry stages of the Bunyamwera virus (BUNV), a closely related virus to the RVFV, and the authors showed that blocking the K+ channel with chemical compounds inhibits BUNV infection.40,41 Based on data from our time-of-addition experiments and the above-mentioned facts, we hypothesized that compound 13a might influence ion channels. Therefore, we performed resting membrane potential experiments to evaluate if compound 13a has effects on the ion channel (i.e., the K+ channel).
The K+ channel plays a vital role to maintain the charge difference across the cell membrane (the resting membrane potential). Due to the changes of the ion channel’s status (either open or closed), the membrane potential can either become more positive (depolarization) or more negative (hyperpolarization). This scenario can be monitored using a membrane potential-sensitive dye, bis(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC4(3)).40,42 An increased DiBAC4(3) fluorescence intensity indicates cell depolarization, while a decreased fluorescence intensity means cellular hyperpolarization. We measured the membrane potential of A549 cells when treated with compound 13a or quinidine as a positive control to validate the assay. Quinidine is a known K+ channel blocker that leads to depolarization. Before performing the membrane potential experiment, we examined the antiviral activity of quinidine and confirmed that it inhibits RVFV infection (EC50 value = 146 μM) (Figure 5a). We then performed the membrane potential assay, and A549 cells treated with compound 13a (25 and 50 μM) exhibited an increased DiBAC4(3) fluorescence intensity (depolarization) similar to quinidine (200 μM), compared to cells that remained untreated, which showed a very low or decreased fluorescence intensity (hyperpolarization) (Figure 5b). This indicated that compound 13a either blocked directly or had an indirect effect on the K+ channel.
Figure 5.
Blocking of the K+ channel inhibited RVFV infection. (A) Dose–response curves of inhibition of RVFV infection by quinidine and compound 13a. A549 cells were infected with rRVFVΔNSs::Katushka (MOI 0.1) in the presence of the compounds. Katushka expression, as a measure of infectivity, was determined 16 h after infection. (B) A549 cells were treated with quinidine (200 μM) or compound 13a (25 and 50 μM) for 16 h. Cells were fixed and stained with DiBAC4(3) (green) and DAPI (blue) and imaged on an Olympus CKX53 fluorescence microscope.
Conclusions
The highly pathogenic RVFV has great health and socioeconomic impacts on endemic countries and could spread to new regions, with potentially devastating consequences. It is crucial to develop better therapeutics to prevent public and animal health threats. In this study, we investigated the SAR of the novel antiviral compound 1, previously identified from chemical library screening specifically for the RVFV.30 In the first-generation compounds, we examined the importance of the (3-aminopropylamino)ethyl chain of compound 1. Here, we showed that the length of the alkyl amine chain seemed to be important to retain the antiviral activity, but it was also affecting the cytotoxic profile. A general observation was that for compounds with shorter alkyl amine chains (5b–c and 5f–g), the antiviral efficacy and toxicity against A549 cells decreased, and for compounds with longer alkyl amine chains (5a and 9a–b), the antiviral efficacy and toxicity increased. In addition, the presence of a hydrogen bond-donating amine functionality seemed to be necessary for the antiviral activity. When N–H for the ethyl piperazine analog 5f was replaced with −CH2 (4d) or oxygen (4e), the antiviral activity was dramatically reduced. Similarly, when the hydrogen bond-donating amine functionality of the butyl piperazine analog 9a was substituted with N-methyl (8c) or N-phenyl (11), the antiviral activity was reduced or completely abolished. The butyl piperazine compound 9a and the parent compound 1 showed similar antiviral activity and toxicity to A549 cells, but taking into account the CC50 values, butyl piperazine compound 9a was considered the best candidate for further modifications.
In the second-generation compounds, we examined the importance of the biphenyl part of the butyl piperazine compound 9a. An investigation of the structural isomers by exchanging the para-biphenyl (9a) with ortho-biphenyl (13a) or meta-biphenyl (13b) or substitution of phenyl to benzyl (13c–e) resulted in no improvement in the antiviral activity. However, when the biphenyl was located in the ortho-position (13a), the CC50 value was improved to almost double compared to the biphenyl in the para-position (9a). When the biphenyl group was replaced with a phenyl (13f) or 1,2-diphenyl methyl (16), the antiviral activity was completely removed or significantly reduced. Addition of an isopropyl group to the biphenyl ring induced antiviral activity but showed more toxicity (13i–k). Therefore, considering both the antiviral activity and the cytotoxicity profile, compound 13a was the best compound with a selectivity index (SI) of 10. In addition, mode-of-action studies suggested that 13a could inhibit the post-entry or the early replication phase of the RVFV life cycle, and it affected the K+ channel. In the future, it would be interesting to investigate the pharmacokinetics and antiviral efficiency of compound 13a in an animal model. To conclude, our studies have identified a novel compound with the potential to be further developed as an antiviral drug against the emerging and potentially deadly RVFV infection for which there are no available therapeutics.
Experimental Section
Chemistry: General Experimental Procedures
H1 and C13 NMR spectra were recorded on a Bruker DRX-400 spectrometer (Bruker, Billerica, MA, USA) at 298 K. 1H and 13C chemical shifts are reported relative to CHCl3 (δH 7.26 ppm) or CDCl3 (δC 77.16 ppm), DMSO-d6 (δH 2.50 ppm or δC 39.52 ppm), and MEOH (δH 3.33 ppm) or MeOD (δC 49.0 ppm) as an internal reference. High-resolution mass spectral (HRMS) data were recorded with an Agilent 1290 binary LC system connected to an Agilent 6230 Accurate-Mass TOF LC/MS (ESI+), calibrated with an Agilent G1969-85001 ES-TOF reference mix containing ammonium trifluoroacetate, purine, and hexakis(1H,1H,3H-tetrafluoropropoxy)phosphazine in 90:10 acetonitrile:water. TLC was performed on silica gel 60 F254 (Merck Millipore) with detection of UV light. Flash column chromatography [the eluent for flash chromatography is given between brackets in the Experimental Section] was carried out on silica gel (particle size, 60 Å; 230–400 mesh; Sigma-Aldrich). Preparative HPLC was performed using a VP 250/21 Nucleodur C-18, HTEC, 5 μm column (Macherey-Nagel) on a Gilson 333/334 Prep-Scale system with a flow rate of 210 mL/min, detection at 210 nm (Gilson 151), and a CH3CN (0.005% HCO2H)/H2O (0.005% HCO2H) eluent system. Compounds 2, 10c, 10e, 10f, 10l, and 10b were purchased from Sigma-Aldrich. Compounds 10d(32) and 10m(31) were synthesized according to previously described literature procedures.
General Synthetic Procedure
Procedure A: Synthesis of Compounds 4a–g (R2 = Boc) Using the Mitsunobu Reaction, Exemplified for Compound 4a
Biphenyl-4-ol (86.2 mg, 0.506 mmol), tert-butyl 6-hydroxyhexylcarbamate 3a (100 mg, 0.46 mmol), and PPh3 (145 mg, 0.55mmol) were dissolved in 1 mL of THF. To the solution, DIAD (0.11 mL, 0.55 mmol) was added, and the reaction was stirred at rt for 9 days. The resulting mixture was diluted with EtOAc and washed two times with brine. The organic phase was dried with Na2SO4, filtrated, and concentrated. Purification with flash chromatography [P:E 6:1] over silica gave 172 mg of the Boc-protected compound 4a, which was taken directly to the next reaction.
Procedure B: Synthesis of Compounds 5a–c and Deprotection of the Boc Group Using TFA, Exemplified for Compound 5a
Boc-protected compound 4a dissolved in 1.5 mL of DCM and 1.5 mL of TFA was added. After ca. 2.5 h of reaction, it was diluted with water and DCM. NaOH (aq) (2 M) was added until basic pH. The aqueous phase was extracted three times with DCM. Organic phases were combined and washed with brine, dried with anhydrous Na2SO4 (s), filtrated, and concentrated. Free amine was redissolved in Et2O, and 4 M HCl (dioxane) was added until acidic pH. The resulting mixture was concentrated and triturated with Et2O three times, which gave 5a as a HCl salt (52 mg, 0.17 mmol) in 37% yield.
Procedure C: Deprotection of the Boc Group Using HCl, Exemplified for Compound 5g
Boc-protected 4g (160 mg, 0.404 mmol) was dissolved in DCM (1 mL). HCl (4 M) in dioxane (1.6 mL) was added, and the reaction was stirred at rt for 3 h. The resulting mixture was concentrated and triturated three times with Et2O, which gave 5g (116 mg, 0.315 mmol) as a solid in 60% yield calculated over two steps.
Procedure D: One-Pot Synthesis of Compounds 8a–c, Exemplified for Compound 8a
Boc-piperazine 6a (100 mg, 0.54 mmol) was dissolved in MeCN (3 mL) in a microwave vessel, and Na2CO3 (525 mg, 1.61 mmol) was added. To the mixture, 1,4-dibromobutane (67.3 μL, 0.564 mmol) was added, and the reaction was heated in a microwave at 115 °C. After 20 min, 4-biphenol 2 (100 mg, 0.59 mmol) was added, and the reaction was further heated at 170 °C. The resulting mixture was diluted with EtOAc and washed two times with brine. The organic phase was dried with anhydrous Na2SO4 (s), filtrated, and concentrated. Flash chromatography over silica [2:1 P:E] gave compound 8a (125 mg, 0.30 mmol) in 56% yield.
Procedure E: Spiro Salt Formation, Exemplified for Compound 7a
Boc-protected piperazine 6a (500 mg, 2.685 mmol) was dissolved in MeCN (3 mL), and K2CO3 (1.113 g, 8.054 mmol) and 1,4-dibromobutane (337 μL, 2.819 mmol) were added. The reaction was capped (microwave vessel) and heated using a sand batch at 120 °C for 40 min. The resulting mixture was diluted with CHCl3 (product soluble in CHCl3) and filtrated to remove K2CO3 solids. The organic phase was extracted with Milli-Q water twice. The combined water phases were co-concentrated using absolute EtOH. The resulting colorless precipitate was dissolved in CHCl3 and dried with anhydrous Na2SO4 (s), filtrated, and evaporated. After drying under vacuum, it gave 7a (757 mg, 2.356 mmol) as a colorless solid in 87% yield.
Procedure F: Synthesis of Substituted Biphenols Using the Suzuki Coupling Reaction, Exemplified for Compound 10a
3-Bromophenol (1.26 g, 7.29 mmol), benzeneboronic acid (1,78 g, 14.57 mmol), K2CO3 (2.52 g, 18.22 mmol), and Pd(PPh3)2Cl2 (26 mg, 0.037 mmol) were mixed with a solution of 20 mL of dioxane and 5 mL of water followed by reflux overnight. The resulting black mixture was diluted with Et2O and washed with H2O. The water phase was extracted with additional Et2O. The combined organic phases were dried with Na2SO4, filtrated, and concentrated. Flash chromatography over silica [8.5:1 H:E] gave 10a (0.91 g, 5.35 mmol) as a colorless solid in 73% yield.
Procedure G: Nucleophilic Ring Opening of the Spiro Salt, Exemplified for Compound 12a
The spiro salt 7a (50 mg, 0.156 mmol) and 3-biphenol 10a (29.13 mg, 0.171 mmol) were mixed in 0.8 mL of MeCN. Cs2CO3 (152 mg, 0.467 mmol) was added, and the reaction was microwave heated at 170 °C for 25 min. The resulting mixture was diluted with EtOAc and washed two times with brine. The organic phase was dried with anhydrous Na2SO4, filtrated, and concentrated. Flash chromatography over silica [1:1 P:E] gave compound 12a (46 mg, 0.112 mmol) as a sticky oil in 72% yield.
N1-(2-(Biphenyl-4-yloxy)ethyl)propane-1,3-diamine·2HCl (1)
Biphenyl-4-ol (178 mg, 1.05 mmol), N,N-di-Boc-2-(3-aminopropylamino)ethanol (500 mg, 1.57 mmol), and PPh3 (412 mg, 1.57 mmol) were dissolved in 0.4 mL of THF and 0.1 mL of DMF. An increased temperature was needed for complete solvation. To the solution, DIAD (0.3 mL, 1.57 mmol) was added, and the reaction was stirred at 55 °C. After 16 h, additional PPh3 (137 mg, mmol) and DIAD (0.1 mL, mmol) were added, and the reaction was further stirred at 70 °C for 24 h. The resulting mixture was diluted with EtOAc and washed two times with brine. The organic phase was dried with Na2SO4, filtrated, and concentrated. Purification using flash chromatography [E:P 1:7] gave 222 mg of the Boc-protected compound 1 in 45% yield. For removal of the Boc groups, 33 mg of the product was dissolved in 0.4 mL of DCM and 0.5 mL of 4 M HCl (dioxane was added). After 8 h, the resulting mixture was concentrated, and the retained solid was triturated three times with Et2O, which gave compound 1 (mg, mmol) as a di-HCl salt in 75% yield. 1H NMR (400 MHz, DMSO-d6): δ 9.34 (broad s, 2H), 8.10 (broad s, 3H) 7.67–7.59 (m, 4H), 7.47–7.41 (m, 2H), 7.35–7.29 (m, 1H), 7.13–7.07 (m, 2H), 4.35 (t, J = 5.2 Hz, 2H), 3.39–3.31 (apparent broad s, 2H), 3.14–3.06 (apparent broad s, 2H), 2.97–2.88 (apparent broad s, 2H); 13C NMR (100 MHz, DMSO-d6): δ 157.4, 139.7, 133.3, 128.9, 127.8, 126.9, 126.2, 115.2, 63.4, 46.0, 44.2, 36.1, 23.6; HRMS [M + H]+ calculated for C17H23N2O: 271.1810; found, 271.1807.
1-(2-(Biphenyl-4-yloxy)ethyl)piperidine (4d)
4d was synthesized by procedure A in 37% yield. 1H NMR (400 MHz, CDCl3): δ 7.57–7.49 (m, 4H), 7.41 (t, J = 7.8 Hz, 2H), 7.32–7.27 (m, 1H), 6.98 (d, J = 8.7 Hz, 2H), 4.15 (t, J = 6.1 Hz, 2H), 2.80 (t, J = 6.1 Hz, 2H), 2.57–2.49 (m, 4H), 1.16 (p, J = 5.5 Hz, 4H), 1.50–1.41 (m, 2H); 13C NMR (100 Hz, CDCl3): δ 158.6, 141.1, 134.0, 129.0, 128.4, 127.0, 126.9, 115.1, 66.3, 58.2, 55.3, 26.2, 24.5; HRMS [M + H]+ calculated for C19H24NO: 282.1858; found, 282.1853.
4-(2-(Biphenyl-4-yloxy)ethyl)morpholine (4e)
4e was synthesized by procedure A in 44% yield. 1H NMR (400 MHz, CDCl3): δ 7.57–7.50 (m, 4H), 7.42 (t, J = 7.8 Hz, 2H), 7.33–7.27 (m, 1H), 7.01–6.96 (m, 2H), 4.16 (t, J = 5.75 Hz, 2H), 3.78–3.72 (m, 4H), 2.83 (t, 5.75 Hz, 2H), 2.62–2.58 (m, 4H); 13C NMR (100 Hz, CDCl3): δ 158.4, 140.9, 134.2, 128.9, 128.3, 126.9, 115.1, 67.1, 66.1, 57.8, 54.3; HRMS [M + H]+ calculated for C18H22NO2: 284.1651; found, 284.1648.
6-(Biphenyl-4-yloxy)hexan-1-amine·HCl (5a)
5a was synthesized by procedures A and B in 37% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.89 (broad s, 2H), 7.63–7.56 (m, 4H), 7.43 (t, J = 7.59 Hz, 2H), 7.33–7.27 (m, 1H), 7.04–6.99 (m, 2H), 4.01 (t, J = 6.5 Hz, 2H), 2.83–2.72 (m, 2H), 1.78–1.68 (m, 2H), 1.63–1.53 (m, 2H), 1.49–1.34 (m, 2H); 13C NMR (100 MHz, DMSO-d6): δ 158.4, 139.9, 132.5, 128.9, 127.8, 126.7, 126.2, 114.9, 67.3, 38.7, 28.5, 26.9, 25.6, 25.1; HRMS [M + H]+ calculated for C18H24NO: 270.1858; found, 270.1856.
2-(Biphenyl-4-yloxy)ethanamine·HCl (5b)
5b was synthesized by procedures A and B in 50% yield.
1H NMR (400 MHz, CD3OD): δ 7.62–7.54 (m, 4H), 7.44–7.37 (m, 2H), 7.32–7.26 (m, 1H), 7.12–7.07 (m, 2H), 4.27 (t, J = 5.18 Hz, 2H), 3.39 (t, J = 5.2 Hz, 2H); 13C NMR (100 MHz, CD3OD): δ 159.0, 141.8, 136.1, 129.9, 129.2, 127.9, 127.6, 116.0, 65.4, 40.4; HRMS [M + H]+ calculated for C14H16NO: 214.1232; found, 214.1211.
3-(Biphenyl-4-yloxy)propan-1-amine (5c)
5c was synthesized by procedures A and B in 42% yield as a free amine. 1H NMR (400 MHz, MeOD): δ 7.57–7.50 (m, 4H), 7.41–7.36 (m, 2H), 7.29–7.24 (m, 1H), 7.01–6.96 (m, 2H), 4.09 (t, J = 6.12 Hz, 2H), 2.85 (t, J = 7.2 Hz, 2H), 1.96 (p, J = 6.6 Hz, 2H); 13C NMR (100 MHz, MeOD): δ 160.0, 142.1, 135.0, 129.8, 129.0, 127.6, 127.5, 115.9, 67.1, 39.8, 33.3; HRMS [M + H]+ calculated for C15H18NO: 228.1388; found, 228.1381.
1-(2-(Biphenyl-4-yloxy)ethyl)piperazine (5f)
5f was synthesized by procedures A and B in 41% yield as a free amine. 1H NMR (400 MHz, CDCl3): δ 7.58–7.49 (m, 4H), 7.45–7.37 (m, 2H), 7.33–7.27 (m, 1H), 7.01 (m, 2H), 4.16 (t, J = 5.8 Hz, 2H), 2.93 (t, J = 5.0 Hz, 2H), 2.82 (t, J = 5.9 Hz, 2H), 2.57 (apparent broad s, 4H), 1.57 (apparent broad s, 1H). 13C NMR (100 Hz, CDCl3): δ 158.5, 140.9, 134.0, 128.9, 128.3, 126.9, 126.8, 115.0, 66.0, 56.0, 55.2, 46.3; HRMS [M + H]+ calculated for C18H23N2O: 283.1810; found, 283.1819.
1-(3-(Biphenyl-4-yloxy)propyl)piperazine (5g)
5g was synthesized by procedures A and C in 60% yield. 1H NMR (400 MHz, DMSO-d6): δ 12.08 (broad s, 1H), 9.92 (broad s, 2H), 7.65–7.56 (m, 4H), 7.43 (t, J = 7.7 Hz, 2H), 7.34–7.27 (m, 1H), 7.07–7.01 (m, 2H), 4.12 (t, J = 6.15 Hz, 2H), 3.82–3.64 (m, 2H), 3.56–3.22 (m, 8H) 2.28–2.17 (m, 2H); 13C NMR (100 MHz, DMSO-d6): δ 157.9, 139.8, 132.8, 128.9, 127.8, 126.8, 126.2, 115.0, 65.0, 53.1, 47.8, 39.5, 23.2: HRMS [M + H]+ calculated for C19H25N2O: 297.1967; found, 297.1967.
8-(tert-Butoxycarbonyl)-8-aza-5-azoniaspiro[4.5]decane Bromide (7a)
7a was synthesized by procedure E in 87% yield. 1H NMR (600 MHz, CDCl3): δ 4.09–3.93 (m, 4H), 3.81–3.77 (m, 4H), 3.77–3.65 (m, 4H), 2.39–3.30 (m, 4H), 1.45 (s, 9H); 13C NMR (150 MHz, CDCl3): δ 153.7, 81.9, 62.7, 59.3, 59.3, 28.4, 21.7; HRMS [M]+ calculated for C13H25N2O2: 241.1916; found, 241.1915.
8-Phenyl-8-aza-5-azoniaspiro[4.5]decane Bromide (7d)
7d was synthesized by procedure E in 89% yield. 1H NMR (600 MHz, CDCl3): δ 7.31–7.25 (m, 2H), 6.98–6.91 (m, 3H), 4.01–3.95 (m, 4H), 3.93–3.3.87 (m, 4H), 3.53–3.46 (m, 4H), 2.35–3.28 (m, 4H), 1.45 (s, 9H); 13C NMR (150 MHz, CDCl3): δ 149.1, 129.8, 122.1, 117.0, 62.8, 59.6, 45.6, 21.8; HRMS [M]+ calculated for C14H21N2: 217.1705; found, 217.1720.
1-(4-(Biphenyl-4-yloxy)butyl)-4-methylpiperazine (8c)
8c was synthesized by procedure D in 55% yield and converted to a HCl salt. 1H NMR (400 MHz, DMSO-d6): δ 11.85 (broad s, 2H), 7.65–7.57 (m, 4H), 7.47–7.4 (m, 2H) 7.34–7.28 (m, 1H), 7.06–7.01 (m, 2H), 4.05 (t, J = 6.0 Hz, 2H), 3.84–3.3 (m, 8H), 3.28–3.10 (m, 2H), 2.85 (broad s, 3H), 1.95–1.85 (m, 2H); 13C NMR (100 MHz, DMSO-d6): δ 158.1, 139.8, 132.6, 128.9, 127.7, 126.8, 126.2, 114.9, 66.9, 55.4, 49.6, 48.1, 42.1, 25.8, 20.2; HRMS [M + H]+ calculated for C21H29N2O: 325.2280; found, 325.2273.
1-(4-(Biphenyl-4-yloxy)butyl)piperazine (9a)
9a was synthesized by procedures D and C in 51% yield as a di-HCl salt. 1H NMR (400 MHz, DMSO-d6): δ 11.72 (broad s, 1H), 9.62 (broad s, 2H), 7.64–7.56 (m, 4H), 7.43 (t, J = 7.5 Hz, 2H), 7.34–7.28 (m, 1H), 7.06–7.01 (m, 2H), 4.05 (t, J = 5.9 Hz, 2H), 3.77–3.60 (m, 2H), 3.58–3.36 (m, 4H), 3.33–3.13 (m, 4H), 1.95–1.83 (m, 2H), 1.83–1.74 (m, 2H); 13C NMR (100 MHz, DMSO-d6): δ 158.1, 139.8, 132.6, 128.9, 127.8, 126.7, 126.2, 114.9, 66.9, 55.3, 47.7, 39.7, 25.8, 20.1; HRMS [M + H]+ calculated for C20H27N2O: 311.2123; found, 311.2119.
1-(5-(Biphenyl-4-yloxy)pentyl)piperazine (9b)
9b was synthesized by procedures D and C in 5% yield after purification with HPLC as a free amine. 1H NMR (400 MHz, DMSO-d6): δ 7.63–7.55 (m, 4H), 7.46–7.39 (m, 2H), 7.33–7.27 (m, 1H), 7.03–6.98 (m, 2H), 4.00 (t, J = 6.41 Hz, 2H), 2.97–2.91 (m, 4H), 2.49–2.44 (m, 4H), 2.35–2.29 (m, 2H), 1.53–1.37 (m, 4H); 13C NMR (100 MHz, DMSO-d6): δ 158.3, 139.9, 132.4, 128.9, 127.7, 126.7, 126.1, 114.9, 67.5, 57.7, 50.7, 43.7, 28.6, 25.7, 23.4; HRMS [M + H]+ calculated for C21H28N2O: 325.2274 ; found 325.2280.
Biphenyl-3-ol (10a)
10a was synthesized by procedure F in 73% yield. 1H NMR (400 MHz, CDCl3): δ 7.61–7.55 (m, 2H), 7.47–7.41 (m, 2H), 7.40–7.29 (m, 2H), 7.21–7.17 (m, 1H), 7.09–7.07 (m, 1H), 6.84 (ddd, J1 = 8.0 Hz, J2 = 2.5 Hz, J3 = 0.85 Hz, 1H), 5.14 (s, 1H); 13C NMR (100 Hz, CDCl3): δ 155.9, 143.1, 140.9, 130.1, 128.9, 127.6, 127.3, 119.9, 114.3, 114.2; HRMS [M–H]− calculated for C12H10O: 169.0653; found, 169.0679.
1-(4′-Hydroxybiphenyl-3-yl)ethanone (10g)
10 g was synthesized by procedure F in 71% yield. 1H NMR (400 MHz, CDCl3): δ 8.14 (apparent t, J = 1.8 Hz, 1H), 7.91–7.87 (m, 1H), 7.76–7.73 (m, 1H), 7.54–7.47 (m, 3H), 6.95 (apparent d, J = 8.7 Hz, 2H), 5.43 (s, 1H), 2.67 (s, 3H); 13C NMR (100 Hz, CDCl3): δ 198.9, 155.9, 141.5, 137.7, 132.9, 131.6, 129.2, 128.7, 126.9, 126.6, 116.0, 27.0; HRMS [M–H]− calculated for C14H12O2: 211.0759; found, 211.0788.
4′-Isopropylbiphenyl-4-ol (10i)
10i was synthesized by procedure F in 70% yield. 1H NMR (400 MHz, CDCl3): δ 7.54–7.47 (m, 4H), 7.32 (apparent d, J = 8.3 Hz, 2H), 6.92 (apparent d, J = 8.7 Hz, 2H), 5.16 (broad s, 1H), 3.04–2.92 (m, 1H), 1.33 (d, J = 6.94 Hz, 6H); 13C NMR (100 Hz, CDCl3): δ 154.9, 147.6, 138.4, 134.2, 128.4, 126.9, 126.8, 115.8, 33.9, 24.2; HRMS [M–H]− calculated for C15H16O: 211.1128; found, 211.1127.
3′-Isopropylbiphenyl-4-ol (10j)
10j was synthesized by procedure F in 82% yield. 1H NMR (400 MHz, CDCl3): δ 7.49 (apparent d, J = 8.6 Hz, 2H), 7.42–7.32 (m, 3H), 7.22–7.16 (m, 1H), 6.91 (apparent d, J = 8.6 Hz, 2H), 4.84 (s, 1H), 3.04–2.91 (m, 1H), 1.31 (d, J = 6.9 Hz, 6H); 13C NMR (100 Hz, CDCl3): δ 155.1, 149.5, 140.9, 134.5, 128.8, 128.6, 125.2, 125.0, 124.4, 115.7, 34.4, 24.2; HRMS [M–H]− calculated for C15H16O: 211.1128; found, 211.1128.
2′-Isopropylbiphenyl-4-ol (10k)
10k was synthesized by procedure F in 68% yield. 1H NMR (400 MHz, CDCl3): δ 7.44–7.32 (m, 2H), 7.25–7.16 (m, 4H), 6.90 (apparent d, J = 8.62 Hz), 5.12 (broad s, 1H), 3.17–3.04 (m, 1H), 1.19 (d, J = 6.9 Hz, 6H); 13C NMR (100 Hz, CDCl3): δ 154.5, 146.7, 140.7, 134.8, 130.7, 130.3, 127.6, 125.7, 125.4, 115.0, 29.4, 24.4; HRMS [M–H]− calculated for C15H16O: 211.1128; found, 211.1127.
3′-(Dimethylamino)biphenyl-4-ol (10h)
10 h was synthesized by procedure F in 29% yield. 1H NMR (400 MHz, CDCl3): δ 7.48 (apparent d, 8.8 Hz, 2H), 7.3 (t, J = 7.7 Hz, 1H), 6.95–6.85 (m, 4H), 6.77–6.72 (m, 1H), 5.34–4-70 (broad s, 1H), 3.01 (s, 6H); 13C NMR (100 Hz, CDCl3): δ 155.2, 151.1, 142.1, 135.1, 129.6, 128.7, 115.9, 115.6, 111.60, 111.57, 41.0.
1-(4-(Biphenyl-4-yloxy)butyl)-4-phenylpiperazine (11)
11 was synthesized by procedure G in 89% yield: 1H NMR (400 MHz, DMSO-d6): δ 11.0 (broad s, 1H), 7.64–7.57 (m, 4H), 7.46–7.39 (m, 2H), 7.34–7.23 (m, 3H), 7.06–6.98 (m, 4H), 6.89–6.83 (m, 1H), 4.06 (t, J = 6.12 Hz, 2H), 3.83–3.77 (m, 2H), 3.59–3.53 (m, 2H), 3.24–3.06 (m, 6H), 1.99–1.87 (m, 2H), 1.85–1.75 (2H); 13C NMR (100 Hz, CDCl3): δ 158.1, 149.6, 139.8, 132.6, 129.2, 128.9, 127.8, 126.8, 126.2, 120.0, 116.0, 115.0, 66.9, 55.1, 50.6, 45.4, 26.0, 20.1; HRMS [M + H]+ calculated for C26H30N2O: 387.2436; found, 387.2436.
1-(4-(Biphenyl-3-yloxy)butyl)piperazine·2HCl (13a)
13a was synthesized by procedures G and C as a di-HCl salt in 67% yield. 1H NMR (400 MHz, DMSO-d6): δ 11.82 (broad s, 1H), 9.77 (broad s, 2H), 7.72–7.63 (m, 2H), 7.50–7.44 (m, 2H), 7.41–7.35 (m, 2H), 7.25–7.21 (m, 2H), 7.21–7.17 (m, 1H), 6.95 (dd, J = 8.2, 2.2 Hz, 1H), 4.09 (d, J = 6.0 Hz, 1H), 3.82–3.61 (m, 2H), 3.60–3.336 (m, 4H), 3.36–3.11 (m, 4H), 1.99–1.86 (m, 2H), 1.86–1.75 (m, 2H); 13C NMR (100 MHz, DMSO-d6): δ 159.0, 141.7, 140.1, 130.0, 128.9, 127.6, 126.8, 119.1, 113.7, 112.8, 66.9, 55.3, 47.7, 39.6, 25.9, 20.1; HRMS [M + H]+ calculated for C20H27N2O: 311.2123; found, 311.2130.
1-(4-(Biphenyl-2-yloxy)butyl)piperazine·2HCl (13b)
13b was synthesized by procedures G and C as a di-HCl salt in 50% yield. 1H NMR (400 MHz, DMSO-d6): δ 11.8 (broad s, 1H), 9.73 (apparent broad s, 2H), 7.55–7.42 (m, 4H), 7.37–7.27 (m, 2H), 7.11 (d, J = 7.9 Hz. 1H), 7.03 (dt, J = 7.5, 0.7 Hz, 1H), 4.00 (t, J = 6 Hz, 2H), 3.79–3.33 (m, 6H), 3.28–3.14 (m, 2H), 3.14–3.03 (m, 2H), 1.85–1.75 (m, 2H), 1.75–1.66 (m, 2H); 13C NMR (100 MHz, DMSO-d6): δ 155.3, 138.2, 130.4, 130.0, 129.3, 128.8, 128.1, 126.8, 120.9, 112.7, 67.0, 55.1, 47.5, 39.5, 25.8, 19.9; HRMS [M + H]+ calculated for C20H27N2O: 311.2123; found, 311.2122.
1-(4-(4-Benzylphenoxy)butyl)piperazine·2HCl (13c)
13c was synthesized by procedures G and C as a di-HCl salt in 65% yield. 1H NMR (400 MHz, DMSO-d6): δ 11.62 (broad s, 1H), 9.61 (broad s, 2H), 7.30–7.24 (m, 2H), 7.22–7.09 (m, 5H), 6.88 (m, 2H), 3.95 (t, J = 6.0 Hz, 2H), 3.86 (s, 2H), 3.75–3.59 (m, 2H), 3.52–3.36 (m, 4H), 3.28–3.09 (m 4H), 1.89–1.79 (m, 2H), 1.78–1.69 (m, 2H); 13C NMR (100 MHz, DMSO-d6): δ 156.8, 141.8, 133.3, 129.7, 128.5, 128.4, 125.8, 114.4, 66.7, 55.3, 47.7, 40.0, 39.6, 25.8, 20.1; HRMS [M + H]+ calculated for C21H29N2O: 325,2280; found, 325.2283.
1-(4-(3-Benzylphenoxy)butyl)piperazine·2HCl (13d)
13d was synthesized by procedures G and C as a di-HCl salt in 53% yield. 1H NMR (400 MHz, DMSO-d6): δ 11.68 (broad s, 1H), 9.67 (broad s, 2H), 7.35–7.13 (m, 6H), 6.82–6.72 (m, 3H), 3.95 (t, J = 5.9 Hz, 2H), 3.76–3.58 (m, 2H), 3.55–3.34 (m, 4H), 3.30–3.06 (m, 4H), 1.91–1.78 (m, 2H), 1.79–1.68 (m, 2H); 13C NMR (100 MHz, DMSO-d6): δ 158.6, 142.9, 141.2, 129.5, 128.7, 128.4, 126.0, 121.1, 115.1, 111.8, 66.6, 55.3, 47.7, 41.1, 39.5, 25.8, 20.1; HRMS [M + H]+ calculated for C21H29N2O: 325,2280; found, 325.2272.
1-(4-(2-Benzylphenoxy)butyl)piperazine·2HCl (13e)
13e was synthesized by procedures G and C as a di-HCl salt in 70% yield. 1H NMR (400 MHz, DMSO-d6): δ 11.81 (broad s, 1H), 9.70 (apparent broad s, 2H), 7.31–7.10 (m, 7H), 6.95 (d, J = 8.0 Hz, 1H), 6.86 (t, J = 7.4 Hz, 1H), 3.97 (t, J = 5.9 Hz, 2H), 3.90 (s, 2H), 3.70–3.58 (m, 2H), 3.54–3.36 (m, 4H), 2.29–3.09 (m, 4H), 1.90–1.80 (m, 2H), 1.80–1.71 (m, 2H); 13C NMR (100 MHz, DMSO-d6): δ 156.1, 140.9, 130.2, 129.2, 128.7, 128.2, 127.6, 125.7, 120.3, 11.6, 66.7, 55.3, 47.6, 39.5, 35.5, 26.0, 20.0; HRMS [M + H]+ calculated for C21H29N2O: 325,2280; found, 325.2291.
1-(4-Phenoxybutyl)piperazine·2HCl (13f)
13f was synthesized by procedures G and C as a di-HCl salt in 63% yield. 1H NMR (400 MHz, DMSO-d6): δ 11.85 (broad s, 1H), 9.83 (broad s, 2H), 7.35–7.22 (m, 2H), 7.00–6.86 (m, 3H), 3.99 (t, J = 6 Hz, 2H), 3.75–3.61 (m, 2H), 3.56–3.36 (m, 4H), 3.36–3.15 (m, 4H), 1.93–1.82 (m, 2H), 1.82–1.71 (m, 2H); 13C NMR (100 MHz, DMSO-d6): δ 158.5, 129.5, 120.6, 114.5, 66.6, 55.2, 47.6, 39.5, 25.9, 20.0; HRMS [M + H]+ calculated for C14H23N2O: 235.1810; found, 235.1809.
1-(4′-(4-(Piperazin-1-yl)butoxy)biphenyl-3-yl)ethanone·2HCl (13g)
13g was synthesized by procedures G and C as a di-HCl salt in 67% yield. 1H NMR (400 MHz, DMSO-d6): δ 11.67 (broad s, 1H), 9.59 (broad s, 2H), 8.13 (t, J = 1.71 Hz, 1H), 7.91–7.86 (m, 2H), 7.68 (apparent d, J = 8.8 Hz, 2H), 7.59 (t, 7.8 Hz, 1H), 7.06 (apparent d, J = 8.8 Hz, 2H), 4.06 (t, J = 8 Hz, 2H), 3.70–3.60 (m, 2H), 3.55–3.40 (m, 4H), 3.29–3.13 (m, 4H), 2.65 (s, 3H), 1.94–1.75 (m, 4H); 13C NMR (100 MHz, DMSO-d6): δ 198.1, 158.5, 140.3, 137.5, 131.7, 130.8, 129.3, 128.0, 126.5, 125.7, 115.1, 66.9, 55.3, 47.7, 40.2, 26.9, 25.9, 20.1; HRMS [M + H]+ calculated for C22H28N2O2: 353.2229; found, 353.2228.
N,N-Dimethyl-4′-(4-(piperazin-1-yl)butoxy)biphenyl-3-amine·3HCl (13h)
13h was synthesized by procedures G and C as a tri-HCl salt in 53% yield. 1H NMR (400 MHz, DMSO-d6): δ 11.82 (broad s, 1H), 9.83 (broad s, 1H), 9.71 (broad s, 1H), 7.86–7.06 (broad m, 4H), 7.63 (d, J = 8.63 Hz, 2H), 7.05 (d, J = 8.63 Hz, 2H), 4.05 (t, J = 6.19 Hz, 2H), 3.72–3.67 (m, 2H), 3.55–3.42 (m, 4H), 3.31–3.26 (m, 2H), 3.25–3.20 (m, 2H), 1.93–185 (m, 2H), 1.83–1.77 (m, 2H); 13C NMR (100 MHz, DMSO-d6): δ 158.5, 130.0, 128.0, 115.0, 66.9, 55.2, 47.7, 40.1, 25.8, 20.0; HRMS [M + H]+ calculated for C22H31N3O: 354.2545; found, 354.2550.
1-(4-(4′-Isopropylbiphenyl-4-yloxy)butyl)piperazine·2HCl (13i)
13i was synthesized by procedures G and C as a di-HCl salt in 72% yield. 1H NMR (400 MHz, DMSO-d6): δ 11.88 (broad s, 1H), 9.84 (broad s, 2H), 7.60–7.49 (m, 4H), 7.30 (d, J = 8.6 Hz, 2H), 7.02 (d, J = 8.6 Hz, 2H), 4.04 (t, J = 6 Hz, 2H), 3.74–3.25 (m, 8H), 3.25–3.16 (m, 2H), 2.91 (m, 1H), 1.96–1.84 (m, 2H), 1.84–1.75 (m, 2H); 13C NMR (100 MHz, DMSO-d6): δ 157.5, 145.9, 140.3, 133.8, 129.8, 127.5, 125.5, 125.4, 114.1, 66.8, 55.2, 47.7, 40.1, 28.9, 25.9, 24.1, 20.1; HRMS [M + H]+ calculated for C23H32N2O: 353.2593; found, 353.2595.
1-(4-(3′-Isopropylbiphenyl-4-yloxy)butyl)piperazine·2HCl (13j)
13j was synthesized by procedures G and C as a di-HCl salt in 45% yield. 1H NMR (400 MHz, DMSO-d6): δ 11.82 (broad s, 1H), 9.78 (broad s, 2H), 7.59 (apparent d, J = 8.68 Hz, 2H), 7.47–7.43 (m, 1H), 7.42–7.33 (m, 1H), 7.34 (t, J = 7.58 Hz, 1H), 7.20–7.16 (m, 1H), 7.02 (apparent d, J = 8.68 Hz, 2H), 4.04 (t, J = 6.13 Hz, 2H), 3.75–3.63 (m, 2H), 3.56–3.40 (m, 4H), 3.33–3.09 (m, 4H), 2.94 (m, 1H), 1.94–1.81 (m, 2H), 1.84–1.74 (m, 2H), 1.24 (d, J = 6.92 Hz, 6H); 13C NMR (100 MHz, DMSO-d6): δ 158.1, 149.1, 139.9, 133.0, 128.9, 127.9, 124.7, 124.3, 123.8, 114.9, 66.9, 55.3, 47.7, 39.8, 33.6, 25.9, 24.0, 20.1; HRMS [M + H]+ calculated for C23H32N2O: 353.2593; found, 353.2595.
1-(4-(2′-Isopropylbiphenyl-4-yloxy)butyl)piperazine·2HCl (13k)
13k was synthesized by procedures G and C as a di-HCl salt in 50% yield. 1H NMR (400 MHz, DMSO-d6): δ 11.77 (broad s, 1H), 9.74 (broad s, 2H), 7.42–7.37 (m, 1H), 7.35–7.29 (m, 1H), 7.22–7.15 (m, 1H), 7.22–7.15 (m, 3H), 7.11–7.06 (m, 1H), 7.02–76.96 (m, 2H), 4.04 (t, J = 5.8 Hz, 2H), 3.74–3.65 (m, 2H), 3.33–3.16 (m, 4H), 2.99 (m, J = 7 Hz, 1H), 1.94–1.85 (m, 2H), 1.85–1.76 (m, 2H), 1.11 (d, J = 7 Hz, 6H); 13C NMR (100 MHz, DMSO-d6): δ 157.9, 146.9, 137.4, 132.6, 127.5, 126.8, 126.1, 114.9, 66.8, 55.2, 47.6, 40.2, 33.0, 25.8, 23.9, 20.0; HRMS [M + H]+ calculated for C23H32N2O: 353.2593; found, 353.2592.
1-(4-(4-Cyclohexylphenoxy)butyl)piperazine·2HCl (13l)
13l was synthesized by procedures G and C as a di-HCl salt in 25% yield. 1H NMR (400 MHz, DMSO-d6): δ 11.64 (broad s, 1H), 9.64 (broad s, 1H), 7.11 (d, J = 8.64 Hz, 2H), 6.83 (d, J = 8.64 Hz, 2H), 3.95 (t, J = 6.03 Hz, 2H), 3.73–3.64 (m, 2H), 3.54–3.38 (m, 4H), 3.30–3.11 (m, 4H), 2.46–2.38 (m, 1H), 1.91–1.64 (m, 8H), 1.41–1.14 (m, 4H); 13C NMR (100 MHz, DMSO-d6): δ 156.7, 139.8, 127.5, 114.3, 66.7, 55.3, 47.7, 42.9, 40.1, 34.3, 26.4, 25.9, 25.6, 20.1; HRMS [M + H]+ calculated for C20H32N2O: 317.2593; found, 317.2599.
1-(4-(4-Adamantylphenoxy)butyl)piperazine·2HCl (13m)
13m was synthesized by procedures G and C as a di-HCl salt in 59% yield. 1H NMR (400 MHz, DMSO-d6): δ 11.83 (s, 1H), 9.85 (m, 2H), 7.24 (d, J = 8.88 Hz, 2H), 6.86 (d, J = 8.88 Hz, 2H), 3.95 (t, J = 6.15 Hz, 2H), 3.72–3.62 (m, 2H) 3.54–3.39 (m, 4H), 3.335–3.12 (m, 4H), 2.03 (broad s, 3H), 1.92–1.64 (m, 14H); 13C NMR (100 MHz, DMSO-d6): δ 156.9, 154.9, 143.8, 125.9, 114.0, 79.7, 67.7, 58.4, 53.1, 43.5, 37.0, 35.7, 29.1, 28.6, 27.5, 23.5; HRMS [M + H]+ calculated for C24H36N2O: 369.2906; found, 369.2903.
1-(4-(Benzhydryloxy)butyl)-4-phenylpiperazine (16)
Diphenyl methanol (500 mg, 2.71 mmol) was dissolved in 15 mL of dry THF, and NaH (119.4 mg, 2.98 mmol) was added under stirring at rt. After 15 min, 1,4-dibromobutane (1.347 mg, 6.24 mmol) was added, and the reaction was stirred over the weekend at a temperature of 65 °C. The resulting mixture was diluted with EtOAc and washed two times with brine. The organic phase was dried with anhydrous Na2SO4, filtrated, and concentrated. Purification with flash chromatography [1:40, H:E] gave 190 mg of ((4-bromobutoxy)methylene)dibenzene 15 in 22% yield. Compound 15 (50 mg, 0.156 mmol), piperazine (134.9 mg, 1.566 mmol), and Cs2CO3 (325.8 mg, 0.469 mmol) were mixed in acetonitrile (0.4 mL). The reaction was done using microwave heating at 115 °C for 20 min. The resulting mixture was diluted with EtOAc and washed two times with brine. The organic phase was dried with anhydrous Na2SO4, filtrated, and concentrated. Purification by flash chromatography gave compound 16 (29 mg, 0.089 mmol) in 57% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.37–7.27 (m, 8H), 7.25–7.19 (m, 2H), 5.32 (s, 1H), 3.50–3.42 (m, 3H), 2.93 (apparent t, J = 4.8 Hz, 4H), 2.50–2.38 (m, 4H), 2.36–2.30 (m, 2H), 1.70–1.55 (m, 4H); 13C NMR (100 MHz, DMSO-d6): δ 142.7, 128.5, 127.4, 127.0, 83.7, 69.0, 59.0, 53.9, 45.8, 28.0, 23.6; HRMS [M + H]+ calculated for C21H28N2O: 325.2280; found, 325.2282.
Cells and the Virus
Human lung adenocarcinoma basal epithelial cells, A549, were cultured in a cell culture medium (Dulbecco’s modified Eagle’s medium [DMEM], Sigma-Aldrich, St. Louis, MO) containing 0.75 g NaHCO3/L, 20 mM HEPES (4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid) (EuroClone, Milan, Italy), penicillin G (100 IU/mL) and streptomycin sulfate (100 μg/mL) combined (1× PEST, Gibco, Carlsbad, CA), and 5% fetal bovine serum (FBS, Gibco) at 37 °C. For virus infection, a cell maintenance medium was used containing the same components, except at a lower FBS concentration (2%). The replication-competent recombinant RVFV expressing the far-red fluorescent protein Katushka instead of the NSs protein (rRVFVΔNSs::Katushka) was used for the whole study.30
Effective Concentration 50 (EC50) Assay
To determine the EC50 value of the compounds, the fluorescence intensity of individual infectious cell foci was quantified in a dose-dependent manner for all compounds, described previously.30 Briefly, approximately 10,000 A549 cells/well were seeded in 96-well black-wall plates with a transparent bottom on the day before infection. Just before infection, compounds were serially diluted in three-fold steps from 100 to 0.045 μM and mixed with 1000 plaque forming units of the rRVFVΔNSs::Katushka virus in a total volume of 100 μL of DMEM containing 2% FBS, with multiplicity of infection (MOI) = 0.1. The growth medium was removed, 100 μL of the virus and the compound mixture was added to the cells, and the plate was incubated for 16 h at 37 °C in 5% CO2. Later, the medium was removed, and cells were fixed for 1 h with 3% paraformaldehyde (PFA); then, the cellular nuclei were stained with 0.1% DAPI for 15 min. The wells were washed with PBS, and the number of infected cells/well was counted by a Trophos plate runner HD (Trophos, Roche Group) following the expression of the Katushka protein by the virus. Simultaneously, the total number of cells/well was also counted following the DAPI staining. GraphPad Prism software version 9.2.0 (GraphPad Software, La Jolla, CA, USA) was used to calculate the EC50 value with nonlinear regression analysis with a variable slope. All laboratory work with the rRVFVΔNSs::Katushka virus was performed under biosafety level 2 conditions as approved by the Swedish Work Environment Authority.
Cellular Toxicity Assay
The resazurin cell viability assay (Sigma-Aldrich) was used to analyze the cellular toxicity of synthesized analogues, described previously. This assay measures the metabolic activity of living cells and is based on the oxidoreduction of the nontoxic indicator blue dye resazurin. Viable cells with active metabolism can reduce resazurin into resorufin, which is pink and fluorescent. Briefly, A549 cells (approximately 10,000/well) were seeded in a black-wall transparent-bottom 96-well plate and incubated at 37 °C in 5% CO2 overnight. Cells were then treated with compound concentrations starting from 300 μM with 2-fold serial dilutions down to 2.34 μM and incubated at 37 °C in 5% CO2 for 24 h. To analyze the cell survival/toxicity, 10 μL (40 μM final concentration) of resazurin was added per well and incubated for 3–4 h at 37 °C in a 5% CO2 incubator, and the resorufin fluorescence intensity was measured by a Trophos plate runner HD (Trophos, Roche Group). The CC50 value was then calculated with GraphPad Prism software version 9.2.0 (GraphPad Software, La Jolla, CA, USA) following the nonlinear regression analysis with a variable slope.
Viral RNA Extraction and qRT-PCR
Cell seeding, virus infection with rRVFVΔNSs::Katushka (MOI = 1.0), and compound addition were carried out in the same way as previously described.33 Briefly, A549 cells (approximately 10,000/well) were seeded in transparent 24-well plates and incubated at 37 °C in 5% CO2 overnight. Cells were then infected with rRVFVΔNSs::Katushka (MOI = 1.0) together with the compound (50 and 25 μM) and incubated at 37 °C in 5% CO2 for 16 h. Then, the virus inoculum was discarded, cells were washed with PBS and lysed with proteinase K, and the total cellular RNA was extracted. Extraction of viral RNA and quantification of the viral load were performed as previously described.33
Time-of-Addition Assay
The fluorescent cell focus assay was used for the time-of-addition assay, as previously described.33 In short, A549 cells were infected at an MOI of 0.1, and 50 μM compound 13a was added prior to infection (−1 h), at the time of infection (0 h), and at 2, 4, 6, and 8 h after infection. An additional experiment was to treat cells with 50 μM of the compound 1 h before infection (−1 h) and then remove the cell medium, containing the compound, at the time of infection (−1 to 0 h). The infection was assayed by the fluorescent cell focus assay at 13 h post infection. The cellular nucleoli were stained with 0.1% DAPI and counted as described in the previous section.
Resting Membrane Potential Assay
Before performing the resting membrane potential assay, we performed the dose–response activity of quinidine and compound 13a, similar to the effective concentration assay. The only exception was that both compounds were serially diluted in two-fold steps, from 400 to 3.12 μM for quinidine and from 100 to 1.56 μM for compound 13a. The resting membrane potential assay was performed as previously described.40 Briefly, A549 cells were treated with 20 μM DiBAC4(3) (Sigma) for 20 min at 37 °C in the dark. After labeling, cells were washed and treated with either quinidine (200 μm) as a positive control or compound 13a (25 and 50 μM) for 16 h. Labeled cells were also left untreated as the DiBAC4(3) control. Wide-field images were taken with an Olympus CKX53 fluorescence microscope.
Acknowledgments
The authors thank Göran Bucht and Jonas Näslund at the Swedish Defence Research Agency, Umeå, Sweden for assistance. The study was supported by grants from the Swedish Research Council grant 2016-06251, the Erling-Persson Foundation, and the Medical Faculty, Umeå University and through a regional agreement between the Umeå University and the Västerbotten County Council (ALF).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c06513.
Nuclear magnetic resonance (NMR) spectra and high-performance liquid chromatography (HPLC) analysis of the compounds; purity of the compounds used in the experiments analyzed by NMR and HPLC (PDF)
Author Contributions
# K.I. and M.C. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Gerdes G. H. Rift Valley fever. Rev Sci Tech 2004, 23, 613–623. 10.20506/rst.23.2.1500. [DOI] [PubMed] [Google Scholar]
- Smithburn K. C. Rift Valley fever; the neurotropic adaptation of the virus and the experimental use of this modified virus as a vaccine. Br J Exp Pathol 1949, 30, 1–16. [PMC free article] [PubMed] [Google Scholar]
- Easterday B. C. Rift valley fever. Adv Vet Sci 1965, 10, 65–127. [PubMed] [Google Scholar]
- Madani T. A.; Al-Mazrou Y. Y.; Al-Jeffri M. H.; Mishkhas A. A.; Al-Rabeah A. M.; Turkistani A. M.; Al-Sayed M. O.; Abodahish A. A.; Khan A. S.; Ksiazek T. G.; Shobokshi O. Rift Valley fever epidemic in Saudi Arabia: epidemiological, clinical, and laboratory characteristics. Clin Infect Dis 2003, 37, 1084–1092. 10.1086/378747. [DOI] [PubMed] [Google Scholar]
- Nanyingi M. O.; Munyua P.; Kiama S. G.; Muchemi G. M.; Thumbi S. M.; Bitek A. O.; Bett B.; Muriithi R. M.; Njenga M. K. A systematic review of Rift Valley Fever epidemiology 1931-2014. Infect Ecol Epidemiol 2015, 5, 28024. 10.3402/iee.v5.28024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudin M.; Jumaa A. M.; Jomma H. J.; Karsany M. S.; Bucht G.; Naslund J.; Ahlm C.; Evander M.; Mohamed N. Association of Rift Valley fever virus infection with miscarriage in Sudanese women: a cross-sectional study. Lancet Glob Health 2016, 4, e864–e871. 10.1016/S2214-109X(16)30176-0. [DOI] [PubMed] [Google Scholar]
- Abdel-Aziz A. A.; Meegan J. M.; Laughlin L. W. Rift Valley fever as a possible cause of human abortions. Trans R Soc Trop Med Hyg 1980, 74, 685–686. 10.1016/0035-9203(80)90167-4. [DOI] [PubMed] [Google Scholar]
- WHO fact sheet on Rift Valley fever: Emergencies preparedness, response- Disease outbreak news, Rift Valley fever. http://www.who.int/csr/don/archive/disease/rift_valley_fever/en/.
- Balkhy H. H.; Memish Z. A. Rift Valley fever: an uninvited zoonosis in the Arabian peninsula. Int. J. Antimicrob. Agents 2003, 21, 153–157. 10.1016/S0924-8579(02)00295-9. [DOI] [PubMed] [Google Scholar]
- Shoemaker T.; Boulianne C.; Vincent M. J.; Pezzanite L.; Al-Qahtani M. M.; Al-Mazrou Y.; Khan A. S.; Rollin P. E.; Swanepoel R.; Ksiazek T. G.; Nichol S. T. Genetic analysis of viruses associated with emergence of Rift Valley fever in Saudi Arabia and Yemen, 2000-01. Emerg Infect Dis 2002, 8, 1415–1420. 10.3201/eid0812.020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepin M.; Bouloy M.; Bird B. H.; Kemp A.; Paweska J. Rift Valley fever virus(Bunyaviridae: Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet Res 2010, 41, 61. 10.1051/vetres/2010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancelot R.; Beral M.; Rakotoharinome V. M.; Andriamandimby S. F.; Heraud J. M.; Coste C.; Apolloni A.; Squarzoni-Diaw C.; de La Rocque S.; Formenty P. B.; Bouyer J.; Wint G. R.; Cardinale E. Drivers of Rift Valley fever epidemics in Madagascar. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 938–943. 10.1073/pnas.1607948114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellagi K.; Salez N.; Maquart M.; Larrieu S.; Yssouf A.; Silai R.; Leparc-Goffart I.; Tortosa P.; de Lamballerie X. Serological Evidence of Contrasted Exposure to Arboviral Infections between Islands of the Union of Comoros (Indian Ocean). PLoS neglected tropical diseases 2016, 10, e0004840 10.1371/journal.pntd.0004840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger M.; Beral M.; Licciardi S.; Soule M.; Faharoudine A.; Foray C.; Olive M. M.; Maquart M.; Soulaimane A.; Madi Kassim A.; Cetre-Sossah C.; Cardinale E. Evidence for circulation of the rift valley fever virus among livestock in the union of Comoros. PLoS neglected tropical diseases 2014, 8, e3045 10.1371/journal.pntd.0003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gür S.; Kale M.; Erol N.; Yapici O.; Mamak N.; Yavru S. The first serological evidence for Rift Valley fever infection in the camel, goitered gazelle and Anatolian water buffaloes in Turkey. Trop Anim Health Prod 2017, 49, 1531–1535. 10.1007/s11250-017-1359-8. [DOI] [PubMed] [Google Scholar]
- Fakour S.; Naserabadi S.; Ahmadi E. The first positive serological study on rift valley fever in ruminants of Iran. J Vector Borne Dis 2017, 54, 348–352. 10.4103/0972-9062.225840. [DOI] [PubMed] [Google Scholar]
- Chevalier V.; Pepin M.; Plee L.; Lancelot R. Rift Valley fever--a threat for Europe?. Eurosurveillance 2010, 15, 19506. [PubMed] [Google Scholar]
- Mansfield K. L.; Banyard A. C.; McElhinney L.; Johnson N.; Horton D. L.; Hernandez-Triana L. M.; Fooks A. R. Rift Valley fever virus: A review of diagnosis and vaccination, and implications for emergence in Europe. Vaccine 2015, 33, 5520–5531. 10.1016/j.vaccine.2015.08.020. [DOI] [PubMed] [Google Scholar]
- Tran A.; Ippoliti C.; Balenghien T.; Conte A.; Gely M.; Calistri P.; Goffredo M.; Baldet T.; Chevalier V. A geographical information system-based multicriteria evaluation to map areas at risk for Rift Valley fever vector-borne transmission in Italy. Transbound Emerg Dis 2013, 60, 14–23. 10.1111/tbed.12156. [DOI] [PubMed] [Google Scholar]
- Broce S.; Hensley L.; Sato T.; Lehrer-Graiwer J.; Essrich C.; Edwards K. J.; Pajda J.; Davis C. J.; Bhadresh R.; Hurt C. R.; Freeman B.; Lingappa V. R.; Kelleher C. A.; Karpuj M. V. Biochemical and biophysical characterization of cell-free synthesized Rift Valley fever virus nucleoprotein capsids enables in vitro screening to identify novel antivirals. Biol Direct 2016, 11, 25. 10.1186/s13062-016-0126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbecker M.; Lanchy J. M.; Lodmell J. S. Inhibition of Rift Valley fever virus replication and perturbation of nucleocapsid-RNA interactions by suramin. Antimicrob. Agents Chemother. 2014, 58, 7405–7415. 10.1128/AAC.03595-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler J. W.; Smith J. M.; Ripoll D. R.; Spik K. W.; Taylor S. L.; Badger C. V.; Grant R. J.; Ogg M. M.; Wallqvist A.; Guttieri M. C.; Garry R. F.; Schmaljohn C. S. A fusion-inhibiting peptide against Rift Valley fever virus inhibits multiple, diverse viruses. PLoS neglected tropical diseases 2013, 7, e2430 10.1371/journal.pntd.0002430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudhasani R.; Kota K. P.; Retterer; Tran; Whitehouse; Bavari S. High content image-based screening of a protease inhibitor library reveals compounds broadly active against Rift Valley fever virus and other highly pathogenic RNA viruses. PLoS neglected tropical diseases 2014, 8, e3095 10.1371/journal.pntd.0003095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan A.; Kehn-Hall K.; Senina S.; Lundberg L.; Van Duyne R.; Guendel I.; Das R.; Baer A.; Bethel L.; Turell M.; Hartman A. L.; Das B.; Bailey C.; Kashanchi F. Curcumin inhibits Rift Valley fever virus replication in human cells. The Journal of biological chemistry 2012, 287, 33198–33214. 10.1074/jbc.M112.356535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S. A.; Patil V.; Patil R.; Beaman K.; Patil S. A. Identification of novel 5,6-dimethoxyindan-1-one derivatives as antiviral agents. Med. Chem. 2017, 787. 10.2174/1573406413666170330094822. [DOI] [PubMed] [Google Scholar]
- Westover J. B.; Mathis A.; Taylor R.; Wandersee L.; Bailey K. W.; Sefing E. J.; Hickerson B. T.; Jung K. H.; Sheridan W. P.; Gowen B. B. Galidesivir limits Rift Valley fever virus infection and disease in Syrian golden hamsters. Antiviral Res. 2018, 156, 38–45. 10.1016/j.antiviral.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroline A. L.; Powell D. S.; Bethel L. M.; Oury T. D.; Reed D. S.; Hartman A. L. Broad spectrum antiviral activity of favipiravir (T-705): protection from highly lethal inhalational Rift Valley Fever. PLoS neglected tropical diseases 2014, 8, e2790 10.1371/journal.pntd.0002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y.; Gowen B. B.; Takahashi K.; Shiraki K.; Smee D. F.; Barnard D. L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013, 100, 446–454. 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharton D.; Bailey K. W.; Vest Z.; Westover J. B.; Kumaki Y.; van Wettere A.; Furuta Y.; Gowen B. B. Favipiravir (T-705) protects against peracute Rift Valley fever virus infection and reduces delayed-onset neurologic disease observed with ribavirin treatment. Antiviral Res. 2014, 104, 84–92. 10.1016/j.antiviral.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. K.; Baudin M.; Eriksson J.; Oberg C.; Habjan M.; Weber F.; Overby A. K.; Ahlm C.; Evander M. High-Throughput Screening Using a Whole-Cell Virus Replication Reporter Gene Assay to Identify Inhibitory Compounds against Rift Valley Fever Virus Infection. Journal of biomolecular screening 2016, 21, 354–362. 10.1177/1087057115625184. [DOI] [PubMed] [Google Scholar]
- Hardy A. D.; MacNicol D. D.; Wilson D. R. A new approach for the design of inclusion compounds. J. Chem. Soc., Perkin Trans. 2 1979, 7, 1011–1019. [Google Scholar]
- Tucker T. J.; Saggar S.; Sisko J. T.; Tynebor R. M.; Williams T. M.; Felock P. J.; Flynn J. A.; Lai M. T.; Liang Y.; McGaughey G.; Liu M.; Miller M.; Moyer G.; Munshi V.; Perlow-Poehnelt R.; Prasad S.; Sanchez R.; Torrent M.; Vacca J. P.; Wan B. L.; Yan Y. The design and synthesis of diaryl ether second generation HIV-1 non-nucleoside reverse transcriptase inhibitors (NNRTIs) with enhanced potency versus key clinical mutations. Bioorg. Med. Chem. Lett. 2008, 18, 2959–2966. 10.1016/j.bmcl.2008.03.064. [DOI] [PubMed] [Google Scholar]
- Islam M. K.; Strand M.; Saleeb M.; Svensson R.; Baranczewski P.; Artursson P.; Wadell G.; Ahlm C.; Elofsson M.; Evander M. Anti-Rift Valley fever virus activity in vitro, pre-clinical pharmacokinetics and oral bioavailability of benzavir-2, a broad-acting antiviral compound. Sci. Rep. 2018, 8, 1925. 10.1038/s41598-018-20362-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hover S.; Foster B.; Barr J. N.; Mankouri J. Viral dependence on cellular ion channels – an emerging anti-viral target?. J Gen Virol 2017 Mar, 98, 345–351. 10.1099/jgv.0.000712. [DOI] [PubMed] [Google Scholar]
- Feske S.; Skolnik E. Y.; Prakriya M. Ion channels and transporters in lymphocyte function and immunity. Nat. Rev. Immunol. 2012, 12, 532–547. 10.1038/nri3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igloi Z.; Mohl B. P.; Lippiat J. D.; Harris M.; Mankouri J. Requirement for chloride channel function during the hepatitis C virus life cycle. J. Virol. 2015, 89, 4023–4029. 10.1128/JVI.02946-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chockalingam K.; Simeon R. L.; Rice C. M.; Chen Z. A cell protection screen reveals potent inhibitors of multiple stages of the hepatitis C virus life cycle. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 3764–3769. 10.1073/pnas.0915117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin P. M.; Hadi S.; Brown R. J. P.; Doerrbecker J.; Schulze K.; Zeilinger C.; Schaewen M. V.; Heller B.; Vercauteren K.; Luxenburger E.; Baktash Y. M.; Vondran F. W. R.; Speerstra S.; Awadh A.; Mukhtarov F.; Schang L. M.; Kirschning A.; Müller R.; Guzman C. A.; Kaderali L.; Randall G.; Meuleman P.; Ploss A.; Pietschmann T. Flunarizine prevents hepatitis C virus membrane fusion in a genotype-dependent manner by targeting the potential fusion peptide within E1. Hepatology. 2016, 63, 49–62. 10.1002/hep.28111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietschmann T. Inhibitors Close Gates for Hepatitis C Virus and Open Doors for Drug Repurposing in Infectious Viral Diseases. J. Virol. 2017, 91, 01914–01916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hover S.; King B.; Hall B.; Loundras E.-A.; Taqi H.; Daly J.; Dallas M.; Peers C.; Schnettler E.; McKimmie C.; Kohl A.; Barr J. N.; Mankouri J. Modulation of Potassium Channels Inhibits Bunyavirus Infection. J Biol Chem. 2016, 291, 3411–3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hover S.; Foster B.; Fontana J.; Kohl A.; Goldstein S. A. N.; Barr J. N.; Mankouri J. Bunyavirus requirement for endosomal K+ reveals new roles of cellular ion channels during infection. PLoS Pathog. 2018, 14, e1006845 10.1371/journal.ppat.1006845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräuner T.; Hülser D. F.; Strasser R. J. Comparative measurements of membrane potentials with microelectrodes and voltage-sensitive dyes. Biochim. Biophys. Acta 1984, 771, 208–216. 10.1016/0005-2736(84)90535-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.