Abstract
Systemic sclerosis is an autoimmune connective tissue disease characterized by early and persistent microvascular impairment which leads to functional and organic manifestations, with progressive fibrosis of the skin and internal organs. Morphological and functional assessment of the peripheral microvasculature is a must, not only for diagnosis but also for the prognosis and therapeutical follow-up of systemic sclerosis patients, as reported in recent studies. Nailfold videocapillaroscopy is the validated technique for the study of scleroderma microangiopathy as it is able to detect peripheral microvascular morphology and both classify and score the capillary abnormalities into different microangiopathy patterns (‘Early’, ‘Active’ and ‘Late’). Indeed, the possibility to early diagnose and follow the microvascular changes and the safety of the technique have made nailfold videocapillaroscopy a mandatory tool for patient evaluation and included its assessment in the new systemic sclerosis classification criteria. Important links between nailfold videocapillaroscopy patterns and systemic sclerosis clinical manifestations have been described.
Keywords: Systemic sclerosis, microangiopathy, nailfold videocapillaroscopy, scleroderma patterns, diagnostic tools, connective tissue diseases
Introduction
Systemic sclerosis (SSc) is a complex disease characterized by early microvascular abnormalities, immune dysregulation and chronic inflammation, with subsequent progressive fibrosis of the skin and internal organs. 1 Scleroderma microangiopathy is characterized by structural and functional capillary alterations, including dilated and giant capillaries, microhaemorrhages, a progressive reduction in the number of capillaries, branched capillaries (expression of angiogenesis), resulting in microvascular architecture alteration.2–4
The history of the capillaroscopic analysis in SSc dates back to more or less 40 years ago when Maricq et al. made the first interpretation of these microvascular alterations using a wide-field microscopy technique. They described these microvascular alterations as having a ‘scleroderma-type’ capillaroscopic pattern.5,6
These ‘scleroderma-type’ abnormalities make it possible to distinguish the wide spectrum of scleroderma diseases (e.g. SSc, mixed connective tissue disease, dermatopolymyositis and, at times, specific overlapping subsets of systemic lupus erythematosus) from healthy controls or other diseases.2,4,7–9 At first, the capillaroscopic aspects of the vascular damage observed in SSc were placed into one of two major microangiopathy patterns, named ‘Active’ and ‘Slow’.6,10
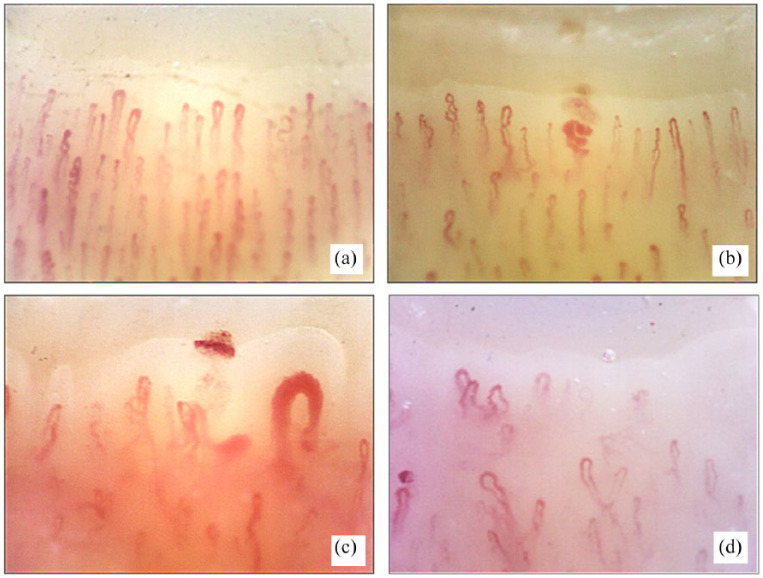
This classification was revised in 2000, when Cutolo et al. introduced a new concept, based on selected morphological characterization of the microangiopathy progression. The microvascular lesions detected by nailfold videocapillaroscopy (NVC) in SSc patients have been classified into three distinct patterns. These patterns are clearly distinguishable from a normal pattern and reflect various capillary alteration phases, showing an evolutive trend from the ‘Early’ stage pattern, to the ‘Active’ one, up to the ‘Late’ pattern3,4,11,12 (Figure 1). Each microangiopathy pattern is characterized by specific capillaroscopic abnormalities and correlates with disease duration, serum autoantibody profile and several clinical aspects of the disease.3,4,13 A very recent survey assessed the reproducibility of these patterns. 12
Figure 1.
Nailfold videocapillaroscopy images (×200) in (a) a healthy subject, (b) ‘Early’, (c) ‘Active’ and (d) ‘Late’ patterns of scleroderma microangiopathy.
This report reviews the studies that have investigated the progressive improvement in capillaroscopic analysis and the correlations between scleroderma NVC patterns and several SSc clinical aspects.
Normal and scleroderma nailfold capillary patters
The detection of the early microvascular damage seems to be the best approach to evidence the early development of SSc and is usually evident many years before other signs and symptoms. The alterations in the microvessel structure are easily detected by nailfold capillaroscopy, particularly in their early stages. This has high differential diagnostic value, even in the presence of isolated Raynaud’s phenomenon (RP).2,14,15
More than 90% of SSc patients have the characteristic capillaroscopic abnormalities of scleroderma microangiopathy and although nailfold capillary morphology has a wide inter- and intra-individual variability, the scleroderma pattern is almost easily distinguishable from that of normal subjects.16,17
The most important characteristics of a normal nailfold capillaroscopy are as follows: the ‘U’ or ‘hairpin’ shape capillary morphology; the morphological homogeneity of capillary diameters (the diameters of capillary branches are <20 μm, and the afferent/efferent limb ratio is 2:1); the homogeneous capillary distribution with at least 9 capillaries/mm counted at the distal capillary row; one capillary inside each dermal papilla; the main capillary axis perpendicular to the distal row; and the high level of skin transparency with good capillary visibility (Figure 1(a)).17–19
Really, ‘isolated’ either variations or abnormalities in morphology, distribution and/or orientation of the capillaries (due to individual variability, manicures, onychophagia, localized traumas and contact with chemical substances) may be observed in healthy subjects.17,18 The most frequently described variations in the normal framework in healthy subjects are tortuosity and capillary crossing. 20 Moreover, non-specific abnormalities like homogeneous enlarged loops, capillary neoformation or micro-bleeding have a low prevalence in only a few fingers in disease-free subjects.16,17 Conversely, non-homogeneous enlarged loops may be observed in connective tissue diseases, even in a pre-clinic stage. 21 A normal capillary pattern is characterized by the absence of either capillary loss or giant capillaries.17,18,21–23
The NVC pattern in primary RP is one of regular capillary loops along the nailfold bed without abnormal enlargements or capillary loss. A clear understanding of the range of variability of ‘normal’ nailfold capillary patterns is a must if a normal pattern is to be distinguished from a scleroderma pattern.17,20 Of note, enlarged loops may be observed in SSc patients also in a pre-clinical stage, while the progression to secondary RP is unlikely in subjects affected by isolated RP in the presence of a mean capillary diameter <30 μm. 21
On the contrary, the observation of giant capillaries (diameter > 50 μm) and microhaemorrhages at NVC suffices to identify the ‘Early’ scleroderma pattern of microangiopathy. With the progression of the microvascular damage, the more advanced ‘Active’ pattern is characterized by an increase in giant capillaries and microhaemorrhages and furthers capillary loss. Finally, typical features of the ‘Late’ pattern of microangiopathy are the absence of giant capillaries and the presence of abnormal-shaped and dilated capillaries, neoangiogenesis and capillary ‘desertification’2–4,24–28 (Figure 1).
The ‘Early’ NVC scleroderma pattern is fundamental for an early SSc diagnosis and that of other diseases of the scleroderma spectrum, and it has been defined as having a combination of a few enlarged/giant capillaries, a few capillary microhaemorrhages, a relatively well-preserved capillary distribution and no evident capillary loss3,4 (Figure 1(b)). Noteworthy is the fact that a recent report has evidenced that the ‘Early’ scleroderma NVC pattern is also the predominant pattern in VEDOSS (very early diagnosis of systemic sclerosis) patients. 15
As mentioned above, the ‘Active’ NVC scleroderma pattern indicates disease progression, and it is characterized by frequent giant capillaries, frequent capillary microhaemorrhages, a moderate capillary loss, mild disorganization of the capillary architecture and absent or mild ramified capillaries3,4,27 (Figure 1(c)). Finally, the ‘Late’ scleroderma NVC pattern includes all the aspects of a severe capillary loss, tissue fibrosis and neoangiogenesis3,4,27 (Figure 1(d)).
The ‘scleroderma-like’ pattern has been defined as a capillary pattern that contains a mixture of the microvascular markers for the various scleroderma patterns that do not fully fit into any single definition as such, for example, ‘Early’, ‘Active’ or ‘Late’ scleroderma patterns.2–4,11,22 This ‘mixture’ of microvascular changes may be observed, for example, either in patients with dermatomyositis and mixed connective tissue disease or in SSc patients under treatment.29,30
Practically, the scleroderma-spectrum disease category is defined as having an ‘Early’, ‘Active’ or ‘Late’ scleroderma pattern or a ‘scleroderma-like’ pattern.2–4,11
In 2008, the Genova School proposed that the NVC parameters belonging to the scleroderma pattern (enlarged capillaries, giant capillaries, microhaemorrhages, capillary loss, disorganization of the microvascular array and capillary ramifications) should be assessed in a quantitative manner. 31 Therefore, a semiquantitative rating scale was adopted to score these microvascular parameters, which was validated and published by a two-center study.16,31
Currently, the same group headed by Cutolo et al. has set up and presented an automatic system able to count the nailfold capillaries by analysing the capillaroscopic images. 32 This is an important practical achievement, as it has been established that the most indicative parameter for the evaluation of progressive microvascular damage in scleroderma is the simple capillaries count.32,33
Sulli et al. proposed the evaluation of the vascular damage be based on an ‘microangiopathy evolution score’ (MES). This score is a sum of the score given to the three parameters, that is, capillary loss, disorganization of the microvascular array and capillary ramifications. 31 Indeed, the proposed MES showed statistically significant changes throughout the follow-up period, confirming its efficacy in quantifying and monitoring SSc microvascular damage over time 31 (Table 1).
Table 1.
Important milestones in the study of microcirculatory damage by nailfold videocapillaroscopy in systemic sclerosis patients and healthy subjects.
| Study | Milestones in the study of microvascular damage analysed by nailfold capillaroscopy |
|---|---|
| Maricq and LeRoy
5
Maricq 6 |
Description of a ‘scleroderma-type’ capillaroscopic
pattern Two major patterns of microangiopathy: ‘Active’ and ‘Slow’ |
| Cutolo et al. 4 | Definition of ‘scleroderma capillaroscopic pattern’ Three patterns of microangiopathy: ‘Early’, ‘Active’ and ‘Late’ |
| Sulli et al. 31 | Definition of semiquantitative rating scale to score altered microvascular parameters and ‘microangiopathy evolution score’ (MES) |
| Smith et al. 16 | Reliability of the qualitative and semiquantitative nailfold videocapillaroscopy assessment |
| Ingegnoli et al. 17 | Nailfold capillaroscpic patterns in healthy subjects: a real issue in capillaroscopy |
| Smith et al. 20 | EULAR Study Group definition on reliability of simple capillaroscopic definitions to describe capillary morphology |
EULAR: European League Against Rheumatism.
Correlations between scleroderma patterns and internal organ involvement
Recently, several studies have demonstrated that SSc microangiopathy correlates with disease subsets and their severity. Furthermore, some authors have shown cross-sectional and predictive associations between progressive capillaroscopic changes and the impairment of internal organ function, that is, lung disease, skin fibrosis and digital ulcers.33–44 It has now become clear that SSc patients with a ‘Late’ NVC pattern have an increased risk of a complex disease and moderate to severe skin or visceral involvement, compared to patients with an ‘Early’ and ‘Active’ NVC pattern33,37–41 (Tables 1 and 2).
Table 2.
The most important relationship between nailfold capillaroscopy and organ involvements in systemic sclerosis (SSc) patients.
| Study | The relationship between nailfold capillaroscopy and organ involvement in SSc | |
|---|---|---|
| NVC links with global SSc impairment | Caramaschi et al. 34 | Nailfold videocapillaroscopic patterns are associated with disease subset and severity |
| Smith et al.38,39 | Nailfold capillaroscopy for the prediction of novel future severe organ involvement in systemic sclerosis | |
| Ingegnoli et al. 40 | Higher number of SSc clinical manifestations in patients with progressively worsening of microangiopathy pattern | |
| Avouac et al. 36 | Sequential nailfold videocapillaroscopy examinations detect organ progression in systemic sclerosis | |
| NVC links with PAH | Ong et al. 43 | A reduction in nailfold capillary density (‘Late’ SSc pattern) is correlated with the PAH severity |
| Hofstee et al. 45 | Nailfold capillary density is associated with the presence and severity of PAH | |
| Riccieri et al. 41 | Microangiopathy severity is correlated with PAH | |
| Corrado et al. 42 | Patients with IPAH have significantly lower capillary density compared to healthy subjects | |
| NVC links with DUs | Sebastiani et al.50,51 | Capillaroscopic DUs risk index |
| Smith et al. 49 | NVC may provide a clinical prognostic index for DUs | |
| Silva et al. 46 | NVC patterns as predictors of DUs | |
| Cutolo et al. 33 | NVC feature risk factors for DUs | |
| NVC links with DT | Sulli et al. 55 | NVC correlates with DT evaluated by mRSS and US at the level of the periungual region |
| Sulli et al. 57 | NVC correlates with total DT (by mRSS and US) | |
| Ruaro et al. 53 | NVC correlates with DT (by mRSS and US) at the level of the fingers | |
| NVC links with PBF | Murray et al. 59 | NVC, LDI and thermal imaging independently provide good
discrimination between SSc, primary RP and healthy
controls NVC being the most suitable technique to classify patients |
| Cutolo et al.47,81 | Peripheral blood perfusion, evaluated by LDF, correlates with microvascular abnormalities studied by NVC | |
| Rosato et al. 62 | LDPI and PPG can provide useful information to distinguish patients with PRP from those with SSc but NVC is the best method to analyse microvascular damage | |
| Ruaro et al.63,61 | NVC patterns of microangiopathy and MES is negatively correlated with skin blood perfusion, as evaluated by LASCA | |
| Della Rossa et al. 64 | PORH peak flow, assessed by LASCA, decreases on the basis of the NVC pattern | |
| Ingegnoli et al. 93 | NVC is the best method to analyse microvascular damage in rheumatic diseases |
NVC: nailfold videocapillaroscopy; PAH: pulmonary arterial hypertension; IPAH: idiopathic pulmonary arterial hypertension; DUs: digital ulcers; DT: dermal thickness; PBF: peripheral blood flow; LDF: laser Doppler flowmetry; LDI: laser Doppler imaging; RP: Raynaud’s phenomenon; LDPI: laser Doppler perfusion imaging; PPG: photoplethysmography; PRP: primary Raynaud’s phenomenon; mRSS: modified Rodnan skin score; US: ultrasound; MES: microangiopathy evolution score; LASCA: laser speckle contrast analysis; PORH: post-occlusive reactive hyperaemia.
Correlations between scleroderma patterns and pulmonary arterial hypertension
Interestingly, cross-sectional and predictive associations between progressive capillaroscopic changes (detecting microvascular damage) and pulmonary arterial hypertension (PAH) in SSc have been described in the last few years and confirmed very recently. 42 One early study reported that reduced nailfold capillary density in SSc patients with established PAH might have had a pathogenic significance and could allow for the early identification of a subset of patients with this severe disease. 43
Another study demonstrated that healthy subjects have a statistically higher number of capillaries than SSc patients without PAH, SSc patients with PAH and patients with idiopathic PAH. 45 Interestingly, capillary density was negatively and significantly correlated with the average pulmonary arterial pressure at rest in patients with SSc and PAH and in patients with idiopathic PAH. 45
In summary, a reduction in nailfold capillary density (‘Late’ SSc pattern) appears to be correlated with the severity of PAH in both SSc and idiopathic PAH. 45 Recently, these aforementioned results have been supported by studies from two different groups. Smith et al. described the role NVC plays in the prediction of organ involvement, in particular PAH, as well as Ricceri et al. who showed that NVC damage is correlated with the grade of PAH39,41 (Table 2).
Correlations between scleroderma patterns and digital ulcers
Skin ulcers are a common vascular complication of progressive scleroderma, can occur in association with all the NVC scleroderma patterns and are characterized by avascular areas, indicative of, or consistent to, tissue necrosis (Figure 1(d)).46–48 An association between trophic lesions of the skin and capillary loss, as assessed by semiquantitative scoring, has also been reported in a wide study. 33 Indeed, capillary loss may be important in tissue hypoxia, and the observation of rapidly progressive capillary loss at capillaroscopy may represent the first signs of severe SSc complication, that is, digital ulcers.23,33
As mentioned above, a recent international multicenter study (the CAP Study), that linked the NVC parameters to the progression of digital ulcers in SSc (700 patients, 14 countries, 59 centres), has confirmed the value of NVC analysis as a predictive tool. 33 Again the number of capillaries was the most statistically significant datum or even the only parameter associated with the risk of new digital ulcers in established SSc patients. Other studies also attest the predictive role of capillaroscopy for digital trophic lesions in SSc.39,49 Several indices have been proposed to evaluate the predictive role of NVC in the development of skin ulcers in SSc patients. One such study proposed a risk index that might be able to predict new digital ulcers through NVC analysis in SSc patients. 50 Although this index has been recently validated, it seems to be quite complex for routine use, as it is not able to asses SSc patients with the ‘Late’ microangiopathy.50,51 Another study reported that capillary dimensions and capillary loss are strongly associated with digital ulceration. 52
Noteworthy is the fact that a routine prognostic index, based exclusively on the capillary count, was used in SSc day hospital care in consecutive patients for the prediction of digital trophic lesions. Based on receiver operating characteristic curve (ROC) analysis, a cut-off value of 1.67 was found for the prognostic index (mean score of capillary loss as calculated over eight fingers), with a sensitivity of 72.22/70.00, specificity of 70.59/69.77, positive likelihood ratio of 2.46/2.32 and a negative likelihood ratio of 0.39/0.43, respectively, for present/future digital trophic lesions 49 (Table 2).
Correlations between scleroderma patterns and dermal thikness
A very recent study has demonstrated a correlation between the morphological and functional aspect of peripheral microangiopathy and skin involvement, another important and typical aspect of SSc 53 (Table 2). The modified Rodnan skin score (mRSS) is the validated method used to assess the severity of the skin damage in SSc. 54 However, recently, several studies have reported that skin high frequency ultrasound (HF-US) is able to detect earlier skin impairment.55–57
In one study carried out with the use of both HF-US and the mRSS, the authors reported that SSc patients with a ‘Late’ NVC pattern of microangiopathy and/or a high MES score had a propensity for a higher dermal thickness (DT) than patients with ‘Active’ or ‘Early’ SSc patterns.53,55 Moreover, a statistically significant positive correlation between MES and DT was demonstrated, as was the fact that patients with limited cutaneous systemic sclerosis (lcSSc) had lower DT and MES than those with diffuse cutaneous SSc.55,56
Some skin areas that were deemed normal on the basis of a negative mRSS were shown to be already involved by an early stage fibrotic process at HF-US analysis. 57 This finding has important implications, as it could be also a sign of disease progression in patients with an ‘Early’ NVC pattern of microangiopathy.56,57 The study also demonstrated that the sum of the DTs measured by HF-US in the 17 areas usually evaluated by mRSS was significantly higher in the lcSSc patients who had an NVC ‘Late’ microangiopathy pattern and an elevated MES score. 57 These results confirm the importance of evaluating microvascular damage, as it mirrors numerous clinical aspects of SSc, like skin involvement, both at disease onset and during follow-up.55–57
Correlations between scleroderma patterns and skin blood perfusion
Although NVC is not able to measure blood perfusion under standard conditions, in some cases it may show the blood flow intensity 58 (Table 2). However, there are various techniques capable of making a direct/indirect assessment of blood flow/perfusion in SSc, including laser techniques, thermography or photoplethysmography.59–68 Yet, another breakthrough in the field of microvascular dynamics in SSc has been demonstrated in several studies, that is, the microvascular involvement (assessed either qualitatively by NVC patterns of microangiopathy or quantitatively by NVC scoring) is negatively correlated with skin blood perfusion.47,63,66 Indeed, studies that evaluated skin blood perfusion in a single point (laser Doppler flowmetry) or over a large area (laser Doppler imaging or laser speckle contrast analysis (LASCA)) have demonstrated that SSc patients with a ‘Late’ NVC pattern had a lower blood flow than those with an ‘Active’ or ‘Early’ NVC pattern.47,63
Recent studies reported that when blood perfusion was assessed by the LASCA technique, it was significantly lower in SSc patients than in healthy subjects at the fingertip level, in the periungual areas and the palm of the hands.53,63,68 Moreover, there was a statistically significant negative correlation between the extent of the nailfold microangiopathy and blood perfusion values at the level of the same skin areas.53,63,68
Other studies observed that the combination of NVC, with other techniques that provide dynamic information on microvascular involvement, for example, Laser Doppler imaging, thermal imaging or photoplethysmography, improve SSc patient classification and provide useful information to distinguish patients with primary RP from SSc patients. 59 A very recent survey, together with previous studies, confirmed that NVC is used by at least 72% of capillaroscopy users, and that NVC is the best method to investigate and classify an SSc patient.59,62
Another technique to evaluate functional vascular damage in SSc patients is power Doppler ultrasonography (PDUS).69,70 Recently, some authors reported that macrovascular features such as ulnar artery occlusion, assessed by PDUS, and microvascular damage, evaluated by nailfold capillaroscopy, are associated with the most common digital manifestations of SSc, such as digital ulcers.69,70
Microangiopathy follow-up during SSc treatment
Although various treatment options are available for the management of RP, these approaches at most are able to reduce the severity of the symptoms but do not resolve the clinical situation.71–74 Microangiopathy evaluated by NVC (alone or with functional techniques) has also been used to evaluate the response to specific therapies in SSc patients.75–84 Interestingly, some studies used NVC to detect the microvascular changes in terms of likely response markers to immunosuppressive/anti-fibrosing treatment and most of these studies considered endotelin-1 receptor antagonists effective therapeutical agents.75–83
Early studies on the effect of cyclosporin have shown a moderate improvement in clinical symptoms and SSc nailfold microangiopathy, after a 12-month treatment cycle.76,77 Similarly, cyclophosphamide was reported to have a positive significant association with an improvement in microvascular damage and regression of the capillaroscopic pattern severity. 78
Recently, a study reported no progression of the microvascular damage (i.e. no further capillary loss), during the 12-month follow-up in patients on Rituximab with early SSc and diffuse skin involvement. 79
Other studies reported morphologic modifications in the scleroderma microangiopathy following vasoactive therapy. The development of nailfold microvascularization, characterized by an increase in the loop number and a reduction in avascular areas, was described in four patients with SSc after being on iloprost for 3 years. 85 Various studies used NVC to assess the results in SSc patients treated with a combination of intravenous prostanoids and endothelin-1 receptor blockers and reported a significant reduction in capillary loss.80–83 Interestingly, autologus haemopoietic stem cell transplants in patients with severe diffuse SSc has recently been reported and were demonstrated to have improved microangiopathy to the point where the NVC pattern changed from ‘Late’ to ‘Active’. 84
In conclusion, the possibility of using NVC to make a detailed analysis of the microvascular damage may well represent a promising, safe and inexpensive tool for the early monitoring of the efficacy of a therapeutic intervention in SSc and its clinical complications.
Microangiopathy as a criteria for SSc classification
In 2001, nailfold microangiopathy was included as a parameter in the LeRoy criteria for the classification of early SSc. 86 Furthermore, another study reported that the 1980 American College of Rheumatology (ACR) criteria for the identification of patients with a limited SSc had a statistically higher sensitivity, that is, from 34% to 89%, when nailfold capillary abnormalities and evaluation of the presence of visible telangiectasia were also taken into consideration.86,87
In 2007, the NVC evaluation was proposed by Cutolo and Matucci-Cerinic to be introduced in the classification criteria of SSc and more recently in the VEDOSS criteria for very early SSc diagnosis and later on included after a Delphi consensus in 2011.88,89 Finally, in 2013, the evaluation of nailfold microangiopathy was included in the new European League Against Rheumatism (EULAR) and ACR criteria for SSc. 90 Furthermore, the use of capillaroscopy by physicians in their routine clinical practice was encouraged by the same authors. 90
In addition, in 2014, the NVC evaluation was included in the diagnostic criteria for primary RP 91 (Table 3).
Table 3.
Use of capillaroscopy in various criteria for systemic sclerosis (SSc) and primary Raynaud’s phenomenon (RP) classification.
| Study | Nailfold capillaroscopy/microangiopathy in the criteria for SSc and RP |
|---|---|
| LeRoy et al. 86 | NVC included as a parameter in the criteria for the classification of early systemic sclerosis |
| Lonzetti et al. 87 Cutolo and Matucci Cerinic 88 | NVC increases the sensitivity of American College of Rheumatology classification criteria for limited scleroderma |
| Avouac et al. 89 | NVC included in the VEDOSS criteria for very early SSc diagnosis |
| Van den Hoogen et al. 90 | NVC patterns included in the new European League Against Rheumatism and American College of Rheumatology criteria for SSc |
| Maverakis et al. 91 | NVC included in the diagnostic criteria for primary RP |
| Cutolo et al. 74 | The presence of SSc-specific autoantibodies and/or abnormal nailfold capillaroscopy (LeRoy and Medsger Criteria) may be used to select patients for clinical trials |
NVC: nailfold videocapillaroscopy; VEDOSS: very early diagnosis of systemic sclerosis.
Conclusion
The correlations between microvascular damage as detectable by different non-invasive methods (in primis NVC) and clinical progression of SSc have currently been well established. The information provided by the morphological and functional analysis of the microvascular bed are not only able to provide valuable information for the assessment of early SSc diagnosis and prognosis but may also reflect the efficacy of therapy in SSc patients.
One of the aims set by the EULAR study group on microcirculation in Rheumatic Diseases is of further disseminating and standardizing the use of capillaroscopy and of investigating new aspects of its putative roles in SSc and other rheumatic diseases.92,93
Acknowledgments
The authors thank Barbara Wade, contract Professor at the University of Torino, for her linguistic advice. The authors also thank Dr Sara De Gregorio from the Division of Rheumatology, University of Genova, for her support in the graphic reproduction. Barbara Ruaro is supported by a grant from the Italian Society of Rheumatology. Vanessa Smith is a Senior Clinical Investigator of the Research Foundation – Flanders (Belgium) (FWO) (1802915N). All authors are members of the EULAR Study Group on Microcirculation in Rheumatic Diseases.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was supported by funding from the Research Laboratory and Academic Division of Rheumatology of the University of Genova, Italy.
References
- 1. Varga J, Trojanowska M, Kuwana M. Pathogenesis of systemic sclerosis: recent insights of molecular and cellular mechanisms and therapeutic opportunities. J Scleroderma Relat Disord 2017; 2: 135–234, e7–e11. [Google Scholar]
- 2. Cutolo M, Smith V. State of the art on nailfold capillaroscopy: a reliable diagnostic tool and putative biomarker in rheumatology? Rheumatology 2013; 52(11): 1933–1940. [DOI] [PubMed] [Google Scholar]
- 3. Cutolo M, Pizzorni C, Tuccio M, et al. Nailfold videocapillaroscopic patterns and serum autoantibodies in systemic sclerosis. Rheumatology 2004; 43(6): 719–726. [DOI] [PubMed] [Google Scholar]
- 4. Cutolo M, Sulli A, Pizzorni C, et al. Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. J Rheumatol 2000; 27(1): 155–160. [PubMed] [Google Scholar]
- 5. Maricq HR, LeRoy EC. Patterns of finger capillary abnormalities in connective tissue disease by ‘wide-field’ microscopy. Arthritis Rheum 1973; 16(5): 619–628. [DOI] [PubMed] [Google Scholar]
- 6. Maricq HR. Wide-field capillary microscopy. Arthritis Rheum 1981; 24(9): 1159–1165. [DOI] [PubMed] [Google Scholar]
- 7. Ingegnoli F. Capillaroscopy abnormalities in relation to disease activity in juvenile systemic lupus erythematosus. Microvasc Res 2013; 87: 92–94. [DOI] [PubMed] [Google Scholar]
- 8. Ruaro B, Sulli A, Alessandri E, et al. Kikuchi-Fujimoto’s disease associated with systemic lupus erythematosus: difficult case report and literature review. Lupus 2014; 23(9): 939–944. [DOI] [PubMed] [Google Scholar]
- 9. Ruaro B, Sulli A, Alessandri E, et al. Coexistence of osteopoikilosis with seronegative spondyloarthritis and Raynaud’s phenomenon: first case report with evaluation of the nailfold capillary bed and review of the literature. Reumatismo 2012; 64(5): 335–339. [DOI] [PubMed] [Google Scholar]
- 10. Carpentier PH, Maricq HR. Microvasculature in systemic sclerosis. Rheum Dis Clin North Am 1990; 16(1): 75–91. [PubMed] [Google Scholar]
- 11. Sulli A, Pizzorni C, Smith V, et al. Timing of transition between capillaroscopic patterns in systemic sclerosis. Arthritis Rheum 2012; 64(3): 821–825. [DOI] [PubMed] [Google Scholar]
- 12. Boulon C, Devos S, Mangin M, et al. Reproducibility of capillaroscopic classifications of systemic sclerosis: results from the SCLEROCAP study. Rheumatology 2017; 56(10): 1713–1720. [DOI] [PubMed] [Google Scholar]
- 13. Sulli A, Ruaro B, Smith V, et al. Progression of nailfold microvascular damage and antinuclear antibody pattern in systemic sclerosis. J Rheumatol 2013; 40(5): 634–639. [DOI] [PubMed] [Google Scholar]
- 14. Ingegnoli F, Boracchi P, Gualtierotti R, et al. Improving outcome prediction of systemic sclerosis from isolated Raynaud’s phenomenon: role of autoantibodies and nail-fold capillaroscopy. Rheumatology 2010; 49(4): 797–805. [DOI] [PubMed] [Google Scholar]
- 15. Cutolo M, Smith V, Distler O, et al. Preliminary analysis of nailfold capillaroscopy in the very early diagnosis of systemic sclerosis (VEDOSS): the capi-vedoss experience. Ann Rheum Dis 2017; 76(Suppl. 2): 65.26905864 [Google Scholar]
- 16. Smith V, Pizzorni C, De Keyser F, et al. Reliability of the qualitative and semiquantitative nailfold videocapillaroscopy assessment in a systemic sclerosis cohort: a two-centre study. Ann Rheum Dis 2010; 69(6): 1092–1096. [DOI] [PubMed] [Google Scholar]
- 17. Ingegnoli F, Gualtierotti R, Lubatti C, et al. Nailfold capillary patterns in healthy subjects: a real issue in capillaroscopy. Microvasc Res 2013; 90: 90–95. [DOI] [PubMed] [Google Scholar]
- 18. Grassi W, De Angelis R. Capillaroscopy: questions and answers. Clin Rheumatol 2007; 26(12): 2009–2016. [DOI] [PubMed] [Google Scholar]
- 19. Andrade LE, Gabriel Júnior A, Assad RL, et al. Panoramic nailfold capillaroscopy: a new reading method and normal range. Semin Arthritis Rheum 1990; 20(1): 21–31. [DOI] [PubMed] [Google Scholar]
- 20. Smith V, Beeckman S, Herrick AL, et al. An EULAR study group pilot study on reliability of simple capillaroscopic definitions to describe capillary morphology in rheumatic diseases. Rheumatology 2016; 55(5): 883–890. [DOI] [PubMed] [Google Scholar]
- 21. Trombetta AC, Smith V, Pizzorni C, et al. Quantitative alterations of capillary diameter have a predictive value for development of the capillaroscopic systemic sclerosis pattern. J Rheumatol 2016; 43(3): 599–606. [DOI] [PubMed] [Google Scholar]
- 22. Cutolo M, Pizzorni C, Sulli A. Identification of transition from primary Raynaud’s phenomenon to secondary Raynaud’s phenomenon by nailfold videocapillaroscopy: comment on the article by Hirschl et al. Arthritis Rheum 2007; 56(6): 2102–2103. [DOI] [PubMed] [Google Scholar]
- 23. Bernero E, Sulli A, Ferrari G, et al. Prospective capillaroscopy-based study on transition from primary to secondary Raynaud’s phenomenon: preliminary results. Reumatismo 2013; 65(4): 186–191. [DOI] [PubMed] [Google Scholar]
- 24. Smith V, Cutolo M. When and how to perform the capillaroscopy. In: Cutolo M. (ed.) Atlas of capillaroscopy in rheumatic diseases. Philadelphia, PA: Elsevier, 2010, pp. 33–42. [Google Scholar]
- 25. Ingegnoli F, Smith V, Sulli A, et al. Capillaroscopy in routine diagnostics: potentials and limitations. Curr Rheumatol Rev. Epub ahead of print 14 June 2017. DOI: 10.2174/1573397113666170615084229. [DOI] [PubMed] [Google Scholar]
- 26. Cutolo M, Sulli A, Smith V. Assessing microvascular changes in systemic sclerosis diagnosis and management. Nat Rev Rheumatol 2010; 6(10): 578–587. [DOI] [PubMed] [Google Scholar]
- 27. Cutolo M, Sulli A, Smith V. How to perform and interpret capillaroscopy. Best Pract Res Clin Rheumatol 2013; 27(2): 237–248. [DOI] [PubMed] [Google Scholar]
- 28. Herrick AL, Cutolo M. Clinical implications from capillaroscopic analysis in patients with Raynaud’s phenomenon and systemic sclerosis. Arthritis Rheum 2010; 62(9): 2595–2604. [DOI] [PubMed] [Google Scholar]
- 29. Bertolazzi C, Cutolo M, Smith V, et al. State of the art on nailfold capillaroscopy in dermatomyositis and polymyositis. Semin Arthritis Rheum 2017; 47: 432–444. [DOI] [PubMed] [Google Scholar]
- 30. De Angelis R, Cutolo M, Gutierrez M, et al. Different microvascular involvement in dermatomyositis and systemic sclerosis. A preliminary study by a tight videocapillaroscopic assessment. Clin Exp Rheumatol 2012; 30(2 Suppl. 71): S67–S70. [PubMed] [Google Scholar]
- 31. Sulli A, Secchi ME, Pizzorni C, et al. Scoring the nailfold microvascular changes during the capillaroscopic analysis in systemic sclerosis patients. Ann Rheum Dis 2008; 67(6): 885–887. [DOI] [PubMed] [Google Scholar]
- 32. Cutolo M, Melsens K, Trombetta AC, et al. Reliability of a new automated system for absolute capillary number counting (autocapi) on systemic sclerosis nailfold video-capillaroscopic images. Ann Rheum Dis 2017; 76(Suppl. 2): 914.27965260 [Google Scholar]
- 33. Cutolo M, Herrick AL, Distler O, et al. Nailfold videocapillaroscopic features and other clinical risk factors for digital ulcers in systemic sclerosis: a multicenter, prospective cohort study. Arthritis Rheum 2016; 68(10): 2527–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Caramaschi P, Canestrini S, Martinelli N, et al. Scleroderma patients nailfold videocapillaroscopic patterns are associated with disease subset and disease severity. Rheumatology 2007; 46(10): 1566–1569. [DOI] [PubMed] [Google Scholar]
- 35. Arana-Ruiz JC, Silveira LH, Castillo-Martínez D, et al. Assessment of nailfold capillaries with a handheld dermatoscope may discriminate the extent of organ involvement in patients with systemic sclerosis. Clin Rheumatol 2016; 35(2): 479–482. [DOI] [PubMed] [Google Scholar]
- 36. Avouac J, Lepri G, Smith V, et al. Sequential nailfold videocapillaroscopy examinations have responsiveness to detect organ progression in systemic sclerosis. Semin Arthritis Rheum 2017; 47(1): 86–94. [DOI] [PubMed] [Google Scholar]
- 37. Pizzorni C, Sulli A, Paolino S, et al. Progression of organ involvement in systemic sclerosis patients with persistent ‘late’ nailfold capillaroscopic pattern of microangiopathy: a prospective study. J Rheumatol 2017; 44(12): 1941–1942. [DOI] [PubMed] [Google Scholar]
- 38. Smith V, Decuman S, Sulli A, et al. Do worsening scleroderma capillaroscopic patterns predict future severe organ involvement? A pilot study. Ann Rheum Dis 2012; 71(10): 1636–1639. [DOI] [PubMed] [Google Scholar]
- 39. Smith V, Riccieri V, Pizzorni C, et al. Nailfold capillaroscopy for prediction of novel future severe organ involvement in systemic sclerosis. J Rheumatol 2013; 40(12): 2023–2028. [DOI] [PubMed] [Google Scholar]
- 40. Ingegnoli F, Ardoino I, Boracchi P, et al. Nailfold capillaroscopy in systemic sclerosis: data from the EULAR scleroderma trials and research (EUSTAR) database. Microvasc Res 2013; 89: 122–128. [DOI] [PubMed] [Google Scholar]
- 41. Riccieri V, Vasile M, Iannace N, et al. Systemic sclerosis patients with and without pulmonary arterial hypertension: a nailfold capillaroscopy study. Rheumatology 2013; 52(8): 1525–1528. [DOI] [PubMed] [Google Scholar]
- 42. Corrado A, Correale M, Mansueto N, et al. Nailfold capillaroscopic changes in patients with idiopathic pulmonary arterial hypertension and systemic sclerosis-related pulmonary arterial hypertension. Microvasc Res 2017; 114: 46–51. [DOI] [PubMed] [Google Scholar]
- 43. Ong YY, Nikoloutsopoulos T, Bond CP, et al. Decreased nailfold capillary density in limited scleroderma with pulmonary hypertension. Asian Pac J Allergy Immunol 1998; 16(2–3): 81–86. [PubMed] [Google Scholar]
- 44. Bredemeier M, Xavier RM, Capobianco KG, et al. Nailfold capillary microscopy can suggest pulmonary disease activity in systemic sclerosis. J Rheumatol 2004; 31(2): 286–294. [PubMed] [Google Scholar]
- 45. Hofstee HM, Noordegraaf AV, Voskuyl AE, et al. Nailfold capillary density is associated with the presence and severity of pulmonary arterial hypertension in systemic sclerosis. Ann Rheum Dis 2009; 68(2): 191–195. [DOI] [PubMed] [Google Scholar]
- 46. Silva I, Teixeira A, Oliveira J, et al. Endothelial dysfunction and nailfold videocapillaroscopy pattern as predictors of digital ulcers in systemic sclerosis: a Cohort Study and review of the literature. Clin Rev Allergy Immunol 2015; 49(2): 240–252. [DOI] [PubMed] [Google Scholar]
- 47. Cutolo M, Ferrone C, Pizzorni C, et al. Peripheral blood perfusion correlates with microvascular abnormalities in systemic sclerosis: a laser-Doppler and nailfold videocapillaroscopy study. J Rheumatol 2010; 37(6): 1174–1180. [DOI] [PubMed] [Google Scholar]
- 48. Suliman YA, Bruni C, Johnson SR, et al. Defining skin ulcers in systemic sclerosis: systematic literature review and proposed World Scleroderma Foundation (WSF) definition. J Scleroderma Relat Disord 2017; 2(2): 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smith V, De Keyser F, Pizzorni C, et al. Nailfold capillaroscopy for day-to-day clinical use: construction of a simple scoring modality as a clinical prognostic index for digital trophic lesions. Ann Rheum Dis 2011; 70(1): 180–183. [DOI] [PubMed] [Google Scholar]
- 50. Sebastiani M, Manfredi A, Colaci M, et al. Capillaroscopic skin ulcer risk index: a new prognostic tool for digital skin ulcer development in systemic sclerosis patients. Arthritis Rheum 2009; 61(5): 688–694. [DOI] [PubMed] [Google Scholar]
- 51. Sebastiani M, Manfredi A, Cestelli V, et al. Validation study of predictive value of capillaroscopic skin ulcer risk index (CSURI) in scleroderma patients treated with bosentan. Clin Exp Rheumatol 2015; 33(4 Suppl. 91): S196. [PubMed] [Google Scholar]
- 52. Kim HS, Park MK, Kim HY, et al. Capillary dimension measured by computer-based digitalized image correlated with plasma endothelin-1 levels in patients with systemic sclerosis. Clin Rheumatol 2010; 29(3): 247–254. [DOI] [PubMed] [Google Scholar]
- 53. Ruaro B, Sulli A, Pizzorni C, et al. Correlation between blood perfusion values and dermal thickness in different skin areas of systemic sclerosis patients. Microvasc Res 2017; 115: 28–33. [DOI] [PubMed] [Google Scholar]
- 54. Khanna D, Furst DE, Clements J, et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J Scleroderma Relat Disord 2017; 2(1): 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sulli A, Ruaro B, Alessandri E, et al. Correlations between nailfold microangiopathy severity, finger dermal thickness and fingertip blood perfusion in systemic sclerosis patients. Ann Rheum Dis 2014; 73(1): 247–251. [DOI] [PubMed] [Google Scholar]
- 56. Ruaro B, Sulli A, Alessandri E, et al. Correlation between three different methods to assess dermal thickness in systemic sclerosis patients with different patterns of nailfold microangiopathy. Ann Rheum Dis 2017; 76(Suppl. 2): 915. [Google Scholar]
- 57. Sulli A, Ruaro B, Smith V, et al. Subclinical dermal involvement is detectable by high frequency ultrasound even in patients with limited cutaneous systemic sclerosis. Arthritis Res Ther 2017; 19(1): 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mugii N, Hasegawa M, Hamaguchi Y, et al. Reduced red blood cell velocity in nailfold capillaries as a sensitive and specific indicator of microcirculation injury in systemic sclerosis. Rheumatology 2009; 48(6): 696–703. [DOI] [PubMed] [Google Scholar]
- 59. Murray AK, Moore TL, Manning JB, et al. Non-invasive imaging techniques in the assessment of scleroderma spectrum disorders. Arthritis Rheum 2009; 61(8): 1103–1111. [DOI] [PubMed] [Google Scholar]
- 60. Roustit M, Cracowski JL. Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharmacol Sci 2013; 34(7): 373–384. [DOI] [PubMed] [Google Scholar]
- 61. Ruaro B, Paolino S, Pizzorni C, et al. Assessment of treatment effects on digital ulcer and blood perfusion by laser speckle contrast analysis in a patient affected by systemic sclerosis. Reumatismo 2017; 69(3): 134–136. [DOI] [PubMed] [Google Scholar]
- 62. Rosato E, Molinaro I, Rossi C, et al. The combination of laser Doppler perfusion imaging and photoplethysmography is useful in the characterization of scleroderma and primary Raynaud’s phenomenon. Scand J Rheumatol 2011; 40(4): 292–298. [DOI] [PubMed] [Google Scholar]
- 63. Ruaro B, Sulli A, Smith V, et al. Laser speckle contrast analysis: a new method to evaluate peripheral blood perfusion in systemic sclerosis patients. Ann Rheum Dis 2014; 73(6): 1181–1185. [DOI] [PubMed] [Google Scholar]
- 64. Della Rossa A, D’Ascanio A, Barsotti S, et al. Post-occlusive reactive hyperaemia (POHR) in systemic sclerosis: very early disease (VEDOSS) represents a separate entity compared to established disease. Scand J Rheumatol 2016; 45(5): 408–411. [DOI] [PubMed] [Google Scholar]
- 65. Lambrecht V, Cutolo M, De Keyser F, et al. Reliability of the quantitative assessment of peripheral blood perfusion by laser speckle contrast analysis in a systemic sclerosis cohort. Ann Rheum Dis 2016; 75(6): 1263–1264. [DOI] [PubMed] [Google Scholar]
- 66. Ruaro B, Sulli A, Pizzorni C, et al. Correlations between skin blood perfusion values and nailfold capillaroscopy scores in systemic sclerosis patients. Microvasc Res 2016; 105: 119–124. [DOI] [PubMed] [Google Scholar]
- 67. Ruaro B, Sulli A, Smith V, et al. Short-term follow-up of digital ulcers by laser speckle contrast analysis in systemic sclerosis patients. Microvasc Res 2015; 101: 82–85. [DOI] [PubMed] [Google Scholar]
- 68. Sulli A, Ruaro B, Cutolo M. Evaluation of blood perfusion by laser speckle contrast analysis in different areas of hands and face in patients with systemic sclerosis. Ann Rheum Dis 2014; 73(11): 2059–2061. [DOI] [PubMed] [Google Scholar]
- 69. Lescoat A, Coiffier G, de Carlan M, et al. Combination of capillaroscopic and ultrasonographic evaluations in systemic sclerosis: results of a cross-sectional study. Arthritis Care Res. Epub ahead of print 12 September 2017. DOI: 10.1002/acr.23413. [DOI] [PubMed] [Google Scholar]
- 70. Cutolo M, Ruaro B, Smith V. Macrocirculation versus microcirculation and digital ulcers in systemic sclerosis patients: macro-microcirculation and scleroderma. Rheumatology 2017; 56(11): 1834–1836. [DOI] [PubMed] [Google Scholar]
- 71. Khanna D, Distler JHW, Sandner P, et al. Emerging strategies for treatment of systemic sclerosis. J Scleroderma Relat Disord 2016; 1(2): 186–193. [Google Scholar]
- 72. Gladue H, Berrocal V, Harris R, et al. A randomized controlled trial of acupressure for the treatment of Raynaud’s phenomenon: the difficulty of conducting a trial in Raynaud’s phenomenon. J Scleroderma Relat Disord 2016; 1(2): 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cutolo M, Sulli A. Therapy: optimized treatment algorithms for digital vasculopathy in systemic sclerosis. Nat Rev Rheumatol 2015; 11(10): 569–571. [DOI] [PubMed] [Google Scholar]
- 74. Cutolo M, Smith V, Furst DE, et al. Points to consider-Raynaud’s phenomenon in systemic sclerosis. Rheumatology 2017; 56(Suppl. 5): v45–v48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Aschwanden M, Daikeler T, Jaeger KA, et al. Rapid improvement of nailfold capillaroscopy after intense immunosuppression for systemic sclerosis and mixed connective tissue disease. Ann Rheum Dis 2008; 67(7): 1057–1059. [DOI] [PubMed] [Google Scholar]
- 76. Filaci G, Cutolo M, Scudeletti M, et al. Cyclosporin A and iloprost treatment of systemic sclerosis: clinical results and interleukin-6 serum changes after 12 months of therapy. Rheumatology 1999; 38(10): 992–996. [DOI] [PubMed] [Google Scholar]
- 77. Filaci G, Cutolo M, Basso M, et al. Long-term treatment of patients affected by systemic sclerosis with cyclosporin A. Rheumatology 2001; 40(12): 1431–1434. [DOI] [PubMed] [Google Scholar]
- 78. Caramaschi P, Volpe A, Pieropan S, et al. Cyclophosphamide treatment improves microvessel damage in systemic sclerosis. Clin Rheumatol 2009; 28(4): 391–395. [DOI] [PubMed] [Google Scholar]
- 79. Smith V, Pizzorni C, Riccieri V, et al. Stabilization of microcirculation in patients with early systemic sclerosis with diffuse skin involvement following rituximab treatment: an Open-label Study. J Rheumatol 2016; 43(5): 995–996. [DOI] [PubMed] [Google Scholar]
- 80. Cutolo M, Zampogna G, Vremis L, et al. Longterm effects of endothelin receptor antagonism on microvascular damage evaluated by nailfold capillaroscopic analysis in systemic sclerosis. J Rheumatol 2013; 40(1): 40–45. [DOI] [PubMed] [Google Scholar]
- 81. Cutolo M, Ruaro B, Pizzorni C, et al. Longterm treatment with endothelin receptor antagonist bosentan and iloprost improves fingertip blood perfusion in systemic sclerosis. J Rheumatol 2014; 41(5): 881–886. [DOI] [PubMed] [Google Scholar]
- 82. Trombetta AC, Pizzorni C, Ruaro B, et al. Effects of longterm treatment with bosentan and iloprost on nailfold absolute capillary number, fingertip blood perfusion, and clinical status in systemic sclerosis. J Rheumatol 2016; 43(11): 2033–2041. [DOI] [PubMed] [Google Scholar]
- 83. Guiducci S, Bellando Randone S, Bruni C, et al. Bosentan fosters microvascular de-remodelling in systemic sclerosis. Clin Rheumatol 2012; 31(12): 1723–1725. [DOI] [PubMed] [Google Scholar]
- 84. Miniati I, Guiducci S, Conforti ML, et al. Autologous stem cell transplantation improves microcirculation in systemic sclerosis. Ann Rheum Dis 2009; 68(1): 94–98. [DOI] [PubMed] [Google Scholar]
- 85. Faggioli P, Giani L, Mazzone A. Possible role of iloprost (stable analogue of PGI2) in promoting neoangiogenesis in systemic sclerosis. Clin Exp Rheumatol 2006; 24(2): 220–221. [PubMed] [Google Scholar]
- 86. LeRoy EC, Medsger TA., Jr. Criteria for the classification of early systemic sclerosis. J Rheumatol 2001; 28(7): 1573–1576. [PubMed] [Google Scholar]
- 87. Lonzetti LS, Joyal F, Raynauld JP, et al. Updating the American College of Rheumatology preliminary classification criteria for systemic sclerosis: addition of severe nailfold capillaroscopy abnormalities markedly increases the sensitivity for limited scleroderma. Arthritis Rheum 2001; 44(3): 735–736. [DOI] [PubMed] [Google Scholar]
- 88. Cutolo M, Matucci Cerinic M. Nailfold capillaroscopy and classification criteria for systemic sclerosis. Clin Exp Rheumatol 2007; 25(5): 663–665. [PubMed] [Google Scholar]
- 89. Avouac J, Fransen J, Walker UA, et al. Preliminary criteria for the very early diagnosis of systemic sclerosis: results of a Delphi Consensus Study from EULAR Scleroderma Trials and Research Group. Ann Rheum Dis 2011; 70(3): 476–481. [DOI] [PubMed] [Google Scholar]
- 90. Van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheum 2013; 65(11): 2737–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Maverakis E, Patel F, Kronenberg DG, et al. International consensus criteria for the diagnosis of Raynaud’s phenomenon. J Autoimmun 2014; 48: 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Burmester GR, Bijlsma JWJ, Cutolo M, et al. Managing rheumatic and musculoskeletal diseases – past, present and future. Nat Rev Rheumatol 2017; 13(7): 443–448. [DOI] [PubMed] [Google Scholar]
- 93. Ingegnoli F, Ughi N, Dinsdale G, et al. An international SUrvey on non-iNvaSive tecHniques to assess the mIcrocirculation in patients with RayNaud’s phEnomenon (SUNSHINE survey). Rheumatol Int 2017; 37: 1879–1890. [DOI] [PubMed] [Google Scholar]