Abstract
Introduction:
Pericardial effusion is a common manifestation of systemic sclerosis, but its pathogenesis has been poorly investigated. Adipokines and interleukins may play a role in the pathophysiology of pericardial effusion. This study aimed at evaluating serum levels of adipokines and interleukins in systemic sclerosis patients with and without pericardial effusion.
Methods:
A total of 87 systemic sclerosis patients (age 52.6 ± 14 years; disease duration 8.2 ± 6.7 years) were recruited in this study. Demographics, body mass index, and clinical characteristics were recorded in each patient. Pericardial effusion was considered pathologic when ≥50 mL was detected by echocardiography. Serum levels of adiponectin, leptin, resistin, visfatin, tumor necrosis factor-α, interferon-γ, interlueukin-2, interlueukin-10, and interlueukin-17 were measured using Multiplex Immunoassay (Bioplex 200 System).
Results:
In all, 11 (13%) systemic sclerosis patients had pericardial effusion. Systemic sclerosis patients with and without pericardial effusion did not differ in age, sex, and body mass index. Systemic sclerosis patients with pericardial effusion had significantly higher levels of visfatin (median/interquartile range: 1546 pg/mL (interquartile range: 8590) vs 388 pg/mL (interquartile range: 103), p = 0.03) and interlueukin-17 (1.33 pg/mL (interquartile range: 3.5) vs 0.05 pg/mL (interquartile range: 0.56), p = 0.04), but lower levels of adiponectin (2,845,000 pg/mL (interquartile range: 4,132,900) vs 5,272,100 pg/mL (interquartile range 8,243,600), p = 0.02) than patients without pericardial effusion. Interstitial lung disease, pulmonary arterial hypertension, and “limited” or “diffuse” cutaneous subset did not correlate to adipokines or interleukin levels.
Conclusion:
Visfatin and adiponectin may play an important role in the pathogenesis of systemic sclerosis–related pericardial effusion. Further longitudinal studies are needed to unravel a possible role of these molecules as biomarkers of pericardial effusion in systemic sclerosis patients.
Keywords: Visfatin, leptin, adiponectin, interlueukin-17
Introduction
There is emerging evidence that adipokines, such as adiponectin, leptin, resistin, and visfatin, first believed to be peculiar in the fat environment, may play a role in inflammatory autoimmune diseases, including systemic sclerosis (SSc). 1 Adiponectin has been reported to be inversely correlated to skin involvement in SSc patients. 2 A protective role of adiponectin in skin fibrosis has been hypothesized and it has been proposed as a biomarker of disease activity. 3 The literature on the expression of leptin in SSc is controversial as either a decrease 4 or an increase in leptin serum levels 1 has been reported.
Visfatin is produced by white adipose tissue, but also by lymphocytes and macrophages, and is regulated by several factors, including glucocorticoids, tumor necrosis factor (TNF), interleukin (IL)-6, and growth hormone. 5 Conflicting data are published on visfatin expression in SSc. The visfatin serum levels seemed to be not different between healthy controls and SSc patients. 6 Instead, it was observed that a higher level of visfatin in SSc patients correlated with longer disease duration, and in vitro, visfatin reversed the profibrotic phenotype of SSc dermal fibroblasts. 7 Interestingly, visfatin has been detected to be significantly higher in patients with idiopathic recurrent acute pericarditis while flares than in those during symptom-free intervals. 8 The pericardial involvement in SSc is described between 33% and 72% in necroscopic studies. 9 Although an inflammatory hypothesis is supposed in SSc-associated pericarditis pathogenesis, 10 studies have never been focused on it. This study was carried out to evaluate the levels of adipokines (visfatin, leptin, adiponectin, and resistin) and ILs (TNF-α, IFN-γ, IL-2, IL-10, and IL-17) in SSc-related pericardial effusion (PE).
Patients and methods
Patients
A total of 87 outpatients (84 female, mean age of 52.6 ± 14.2 years), who fulfilled the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) 2013 SSc classification criteria, 11 were consecutively recruited in this study. The disease duration was computed from the onset of the first clinical manifestation of SSc other than Raynaud’s phenomenon. In each patient was assessed a comprehensive evaluation that included the following: history and physical examination, rheumatologic serologic testing, pulmonary function testing, thoracic, high-resolution computed tomography (HRCT) imaging, and the anthropometric measures, such as body mass index (BMI); demographic, clinical, and laboratory characteristics; SSc-related manifestations; and comorbidity (diabetes, systemic arterial hypertension, and dyslipidemia). The diagnosis of interstitial lung disease (ILD) was based on the presence of respiratory symptoms, abnormal pulmonary function tests, and diffuse parenchymal lung disease on thoracic HRCT. Pulmonary arterial hypertension (PAH) was diagnosed by right heart catheterization. The severity of skin involvement by the modified Rodnan skin score and global disease activity were measured in each patient. 12 The study obtained the approval of the local ethics committees (Ethics Review Board of Policlinico of Bari, comitatoetico@policlinico.ba.it , protocol number 588/CE), and all patients were informed about the nature and aim of the study and gave and signed their consent to participate in this study.
Echocardiography
The presence of PE was evaluated by means of two-dimensional (2D; M-mode, Doppler, and color flow Doppler) transthoracic echocardiography (TTE) equipped with a 1–2 MHz transducer. The presence of effusion in pericardial sac was evaluated by parasternal long-axis view and M-mode measurement of the size of anterior and posterior telediastolic echo-free spaces. Simple semiquantitative assessment of a nonlocalized effusion as an echo-free space (anterior plus posterior) <10 mm during diastole was defined as a small PE.13,14 The presence of heart kinetic abnormalities, the sonographic characteristic of pericardial layers, and the estimation of pressure in pulmonary artery were simultaneously assessed by an expert TTE cardiologist as per the 2015 European Society of Cardiology/European Association for Cardio-Thoracic Surgery (ESC/EACTS) guidelines. 13
Methods
Patients’ fasting blood samples, taken into plain tubes, were centrifuged at room temperature at 1000g for 15 min and then aliquoted and stored at −30°C until use. Serum levels of adiponectin, leptin, resistin, visfatin, TNF-α, interferon γ (IFN-γ), IL-2, IL-10, and IL-17A were measured using Multiplex Immunoassay (Bioplex 200 System; Bio-Rad Laboratories, Hercules, CA, USA) by means of two kits (Bioplex ProTM Cytokine/Chemokine and Growth Factor Assay and Bioplex Pro Diabetes Assay). The analysis was set according to the manufacturer’s protocol. The serum levels of adipokines and ILs were expressed as pictogram per milliliter.
Statistical analysis
Normal distribution of data was assessed using Kolmogorov–Smirnov Test. The comparisons between SSc patient groups were evaluated by Mann–Whitney U test and Student’s t-test, where appropriate. The relationship between the adipokines and IL serum levels and other variables was analyzed by linear regression. The predictors of PE were evaluated by binary logistic regression. Statistical significance was set at p ≤ 0.05. Data are expressed as median and interquartile range (IQR) or means ± 1 standard deviation (SD), where appropriate. The data analysis was assessed using IBM SPSS statistic 23.
Results
A total of 87 (84 females) outpatients were recruited in this study. The mean age of patients was 52.6 ± 14.2 years, and the disease duration was 8.2 ± 6.7 years. The mean modified Rodnan skin scoring was 2.8 ± 1.3, with 72 patients classified as having “limited” cutaneous subset. According to the disease activity score, 35 (40%) patients had high activity SSc. Further demographic characteristics are shown in Table 1.
Table 1.
Demographic characteristics of 87 SSc patients.
| Age (years), mean ± SD | 52.6 ± 14.2 |
| Female, n (%) | 84 (97) |
| Smokers, n (%) | 4 (5) |
| BMI (kg/m2), mean ± SD | 23.7 ± 4.3 |
| BMI subgroups | |
| <18.5 | 8 (10) |
| ≥18.5 and <25 | 40 (52) |
| ≥25 and <30 | 24 (31) |
| ≥30 | 5 (7) |
| Disease duration (years), mean ± SD | 8.2 ± 6.7 |
| Disease activity score, mean ± SD | 2.8 ± 1.3 |
| Disease activity | |
| Remission (disease activity score <3) | 52 (60) |
| High activity (disease activity score ≥3) | 35 (40) |
| Modified Rodnan skin score, mean ± SD | 4.6 ± 4.9 |
| Skin subset | |
| Limited, n (%) | 72 (82) |
| Diffuse, n (%) | 15 (18) |
| Nailfold video capillaroscopy pattern | |
| Early, n (%) | 4 (5) |
| Active, n (%) | 46 (53) |
| Late, n (%) | 33 (42) |
| Interstitial lung disease, n (%) | 51 (59) |
| Pulmonary arterial hypertension, n (%) | 7 (8) |
| Systemic arterial hypertension, n (%) | 8 (9) |
| Diabetes mellitus, n (%) | 4 (5) |
| Hypercholesterolemia, n (%) | 7 (8) |
| Erythrocyte sedimentation rate (mm/h), mean ± SD | 19.25 ± 14.8 |
| C-reactive protein (mg/L), mean ± SD | 4.4 ± 5.1 |
SSc: systemic sclerosis; BMI: body mass index; SD: standard deviation.
Data are presented as number (n) and percentage (%) or mean ± SD.
A PE was found in 11 patients (13%), of whom 3 patients also had PAH. The SSc patient groups with and without PE did not differ in terms of age (46.5 ± 13.8 and 53.5 ± 14.2 years, respectively), sex, and BMI (24.4 ± 5.5 vs 23.62 ± 4.1, respectively); 2 out of 11 obese patients had PE. Furthermore, no differences were observed in the frequency of PAH and ILD in patients with and without PE, and no association was observed between PE and disease duration or disease (early, active, or late) stage (data not shown). No demographic characteristics correlated with PE, neither did disease duration. Of the 11 patients with PE, 8 were on active disease (score ≥3), while 3 patients were not (p = 0.02). In SSc-PE patients, we found a slight but not significant increase in erythrocyte sedimentation rate (ESR; 24 mm/h ± 20 vs 19.3 mm/h ± 14; p = 0.36) and C-reactive protein (CRP) serum levels (5.7 mg/L ± 4 vs 4.2 mg/L ± 5, p = 0.16) compared to SSc patients without PE.
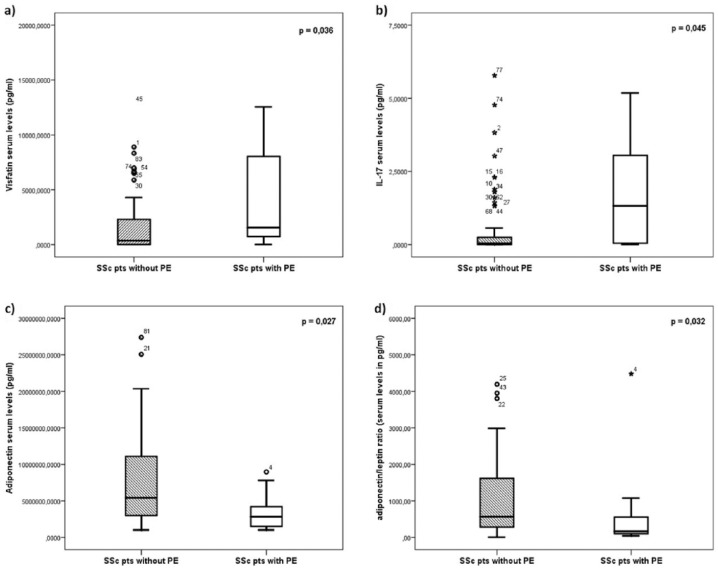
As shown in Table 2, serum levels of TNF-α, IFN-γ, IL-2, IL-10, leptin, and resistin were not significantly different between patients with and without PE. However, compared to SSc patients without PE, those with PE had significantly higher serum levels of visfatin (p = 0.03; Figure 1(a)) and IL-17 (p = 0.04; Figure 1(b)). However, patients with PE showed significantly lower levels of adiponectin (p = 0.02; Figure 1(c)) and adiponectin/leptin ratio (p = 0.03; Figure 1(d)) than those without PE (raw data are also shown in Table 2).
Table 2.
Comparison of adipokine and interleukin serum levels between the SSc patient subgroups with and without PE.
| SSc without PE | SSc with PE | p value | |
|---|---|---|---|
| TNF-α (pg/mL) | 0.14 (10.23) | 1.7 (9.18) | 0.880 |
| IFN-γ (pg/mL) | 14.11 (48.6) | 15.37 (66.82) | 0.851 |
| IL-2 (pg/mL) | 0.05 (4.76) | 0.05 (8.32) | 0.878 |
| IL-10 (pg/mL) | 1.45 (7.2) | 2.01 (12.6) | 0.944 |
| Leptin (pg/mL) | 7514 (15,363) | 16,831 (23,229) | 0.574 |
| IL-17 (pg/mL) | 0.05 (0.39) | 1.33 (3.5) | 0.045 |
| Resistin (pg/mL) | 7275 (2894) | 7473 (4049) | 0.939 |
| Visfatin (pg/mL) | 352 (2296) | 1546 (8590) | 0.036 |
| Adiponectin (pg/mL) | 5,566,300 (7,976,400) | 2,845,000 (4,132,900) | 0.027 |
| Adiponectin/leptin | 569 (1415) | 166 (504) | 0.032 |
PE: pericardial effusion; SSc: systemic sclerosis; TNF: tumor necrosis factor; IFN: interferon; IL: interleukin.
Data are expressed as median and interquartile range.
Figure 1.
Comparison between SSc patients with and without pericardial effusion (PE) of serum levels of (a) visfatin, (b) IL-17, (c) adiponectin, and (d) adiponectin/leptin ratio. Boxplots show median and the lower and upper quartiles (defined as the 25th and 75th percentiles).
Within the SSc patients with PE, no difference in adipocytokines/ILs serum levels according to the menopausal status was found. Serum levels of adipocytokines and ILs did not change in the subgroups of SSc patients with or without ILD or PAH, different video capillaroscopy patterns, limited or diffuse skin subset, and different autoantibodies profile (data not shown).
Discussion
Our study has been focused on SSc-related PE and aimed at searching for a possible correlation of PE with peculiar clinical patterns and adipokines/IL expression. A pericardial involvement is detected in 33%–72% of SSc patients at autopsy. 9 In a case series study, it was excluded that the SSc-related PE was secondary to renal failure, heart failure, or infection. 15 SSc-related pericardial abnormalities more commonly described are PE, thickness of the anterior pericardial recess, and pericardial thickening. Pericardial involvement is more frequently detected in patients with SSc-ILD, and its presence seems to be strongly associated with PAH. 16 The pathogenesis of small PE and its association with other SSc-related clinical manifestations are unknown. In our study, we detect a low frequency of PE, and the rate of patients with PAH, ILD, or severe skin involvement was not significantly higher in SSc patients with PE. However, in Fischer et al.’s study, 16 the association of PE with PAH was shown in 41 selected SSc patients with definite ILD, whereas we studied unselected patients consecutively attending our scleroderma clinic. Although we did not perform specific examination of the pericardium, we can exclude the presence of significant heart fibrosis in our patients on the basis of the absence of heart kinetic abnormalities at echocardiography and on the chest findings.
Recently, adipokines are emerging as possible players in the complex scenario of SSc pathogenesis. Of note, an increase in visfatin levels has been found in patients with idiopathic recurrent pericarditis, but no data are available on the role of adipokines in SSc-related PE. 8 In our study, we observed increased serum levels of visfatin in patients with PE in comparison with patients without PE, but no correlation was found with other clinical manifestations, including skin involvement and disease duration. Indeed, a relationship with the extension of skin fibrosis had been previously reported as elevated visfatin levels were detected in SSc patients with “diffuse” cutaneous subset and longer disease duration. 7 Furthermore, visfatin reversed the profibrotic phenotype of SSc dermal fibroblasts and induced the expression of IL-12p70 in THP-1 cells treated with IFN-γ plus lipopolysaccharide in vitro. 7 Findings on leptin in the literature were not univocal1,4 and also our study did not the resolve the question as serum leptin tended to be higher in SSc patients with PE, but without reaching the significance level. As adiponectin and leptin have counteracting effects, the adiponectin/leptin ratio should be taken into consideration as an index of the balance between anti-inflammatory and proinflammatory response.17,18 We found low adiponectin serum levels and lower adiponectin/leptin ratio in patients with PE than in patients without, suggesting an active inflammatory process in SSc-related PE.
IL-17 is secreted by CD4 + T cells and by innate immune cells, and it is demonstrated to prompt the activation of different cell subset as stroma-type cells, fibroblasts, and endothelial cells to secrete proinflammatory cytokines in the early stage of SSc. 19 However, lower serum levels of IL-17 in SSc patients than in healthy controls have been also reported. 20 Our study demonstrated increased IL-17 serum levels in patients with PE, without further correlation with other clinical manifestations.
Some limitations of this study need to be taken into account, such as the low frequency of PE findings and the lack of pericardial fluid examination. Furthermore, TTE was performed by only one cardiologist without confirmation by a different investigator, and we cannot exclude that some patients had missed TTE on their own decision. Nevertheless, visceral fat biopsy could be informative about whether the source of vistatin increase is the adipose tissue and if fat proinflammatory activation is a pathogenic key in SSc or just an epiphenomenon of a systemic inflammation state. Further studies are necessary to unravel the role of visfatin and adiponectin as biomarkers of SSc and the role of the adipose tissue and the immune system in PE related to SSc pathology.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Pehlivan Y, Onat AM, Ceylan N, et al. Serum leptin, resistin and TNF-α levels in patients with systemic sclerosis: the role of adipokines in scleroderma. Int J Rheum Dis 2012; 15(4): 374–379. [DOI] [PubMed] [Google Scholar]
- 2. Masui Y, Asano Y, Shibata S, et al. Serum adiponectin levels inversely correlate with the activity of progressive skin sclerosis in patients with diffuse cutaneous systemic sclerosis. J Eur Acad Dermatol Venereol 2012; 26(3): 354–360. [DOI] [PubMed] [Google Scholar]
- 3. Tomčík M, Arima K, Hulejová H, et al. Adiponectin relation to skin changes and dyslipidemia in systemic sclerosis. Cytokine 2012; 58(2): 165–168. [DOI] [PubMed] [Google Scholar]
- 4. Kotulska A, Kucharz EJ, Brzezińska-Wcisło L, et al. A decreased serum leptin level in patients with systemic sclerosis. Clin Rheumatol 2001; 20(4): 300–302. [DOI] [PubMed] [Google Scholar]
- 5. Tilg H, Moschen AR. Role of adiponectin and PBEF/visfatin as regulators of inflammation: involvement in obesity-associated diseases. Clin Sci 2008; 114(4): 275–288. [DOI] [PubMed] [Google Scholar]
- 6. Ozgen M, Koca SS, Aksoy K, et al. Visfatin levels and intima-media thicknesses in rheumatic diseases. Clin Rheumatol 2011; 30(6): 757–763. [DOI] [PubMed] [Google Scholar]
- 7. Masui Y, Asano Y, Shibata S, et al. A possible contribution of visfatin to the resolution of skin sclerosis in patients with diffuse cutaneous systemic sclerosis via a direct anti-fibrotic effect on dermal fibroblasts and Th1 polarization of the immune response. Rheumatology 2013; 52(7): 1239–1244. [DOI] [PubMed] [Google Scholar]
- 8. Cantarini L, Brucato A, Simonini G, et al. Leptin, adiponectin, resistin, visfatin serum levels and idiopathic recurrent pericarditis: biomarkers of disease activity? A preliminary report. Clin Exp Rheumatol 2013; 31(2): 207–212. [PubMed] [Google Scholar]
- 9. Byers RJ, Marshall DA, Freemont AJ. Pericardial involvement in systemic sclerosis. Ann Rheum Dis 1997; 56(6): 393–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Champion HC. The heart in scleroderma. Rheum Dis Clin North Am 2008; 34(1): 181–190; viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013; 65(11): 2737–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Valentini G, Bencivelli W, Bombardieri S, et al. European Scleroderma Study Group to define disease activity criteria for systemic sclerosis. III. Assessment of the construct validity of the preliminary activity criteria. Ann Rheum Dis 2003; 62(9): 901–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adler Y, Charron P, Imazio M, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC)endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015; 36(42): 2921–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Imazio M, Adler Y. Management of pericardial effusion. Eur Heart J 2013; 34(16): 1186–1197. [DOI] [PubMed] [Google Scholar]
- 15. Uhl GS, Koppes GM. Pericardial tamponade in systemic sclerosis (scleroderma). Br Heart J 1979; 42(3): 345–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fischer A, Misumi S, Curran-Everett D, et al. Pericardial abnormalities predict the presence of echocardiographically defined pulmonary arterial hypertension in systemic sclerosis-related interstitial lung disease. Chest 2007; 131(4): 988–992. [DOI] [PubMed] [Google Scholar]
- 17. Frühbeck G, Catalán V, Rodríguez A, et al. Involvement of the leptin-adiponectin axis in inflammation and oxidative stress in the metabolic syndrome. Sci Rep 2017; 7(1): 6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gandhi R, Takahashi M, Smith H, et al. The synovial fluid adiponectin-leptin ratio predicts pain with knee osteoarthritis. Clin Rheumatol 2010; 29(11): 1223–1228. [DOI] [PubMed] [Google Scholar]
- 19. Kurasawa K, Hirose K, Sano H, et al. Increased interleukin-17 production in patients with systemic sclerosis. Arthritis Rheum 2000; 43(11): 2455–2463. [DOI] [PubMed] [Google Scholar]
- 20. Gourh P, Arnett FC, Assassi S, et al. Plasma cytokine profiles in systemic sclerosis: associations with autoantibody subsets and clinical manifestations. Arthritis Res Ther 2009; 11(5): R147. [DOI] [PMC free article] [PubMed] [Google Scholar]