Abstract
The role of pericytes in systemic sclerosis (SSc) is unclear because of the difficulty in phenotyping them. They are mainly distributed in the pre-capillary, capillary and post-capillary abluminal side of non-muscular micro-vessels, express platelet-derived growth factor receptors (PDGFRs), and preside over vascular integrity and regeneration. By establishing close contact with many endothelial cells, a single pericyte can regulate ion influx, mechanical stress, leukocyte diapedesis, and platelet activation. Moreover, under pathological conditions such as SSc, pericytes may acquire a contractile phenotype and respond to various stimuli, including endothelin, angiotensin II and reactive oxygen species. The pericytes of SSc patients share some molecular patterns with myofibroblasts or fibroblasts, including A disintegrin and metalloproteinase domain 12 (ADAM-12), α-smooth muscle actin (α-SMA), the extra domain A (ED-A) variant of fibronectin, and Thy-1. Following stimulation with PDGF-β or transforming growth factor-β (TGF-β), pericytes may acquire a myofibroblast phenotype, and produce extracellular matrix or indirectly promote fibroblast activation. They may also contribute to fibrosis by means of epigenetic regulation. The pericyte plasmalemma is particularly rich in caveolae containing caveolin-1, a deficit of which has been associated with defective vessel tone control and lung fibrosis in mice. Consequently, dysfunctional pericytes may underlie the microangiopathy and fibrosis observed in SSc patients. However, given its variability in biological behaviour and the lack of a pan-pericyte marker, the exact role of these cells in SSc warrants further investigation.
Keywords: Micro-vessels, Pathogenesis, Pericytes, Systemic sclerosis
Introduction
Systemic sclerosis (SSc) is a connective tissue disease characterized by micro-vessel dysfunction, immune activation, and fibrosis of the skin and visceral organs. Unlike other autoimmune diseases, SSc seems to be mainly driven by vascular insult, and the immune system seems to play a less important role in its pathogenesis (1). Raynaud’s phenomenon, digital ulcers, pulmonary artery hypertension (PAH) and telangiectasias, which are some of the particular manifestations of SSc, are due to profound changes in micro-vessel architecture (including tight junction disruption, cytosol vacuolisation, and the apoptosis of endothelial cells). Skin and visceral fibrosis are due to the abnormal deposition of extracellular matrix, and connective tissue remodelling by myofibroblasts and fibroblasts. Pericytes establish close connections with vascular or stromal cells, and may acquire various distinct phenotypes as they are capable of transforming themselves into myofibroblasts (2). Furthermore, although there is little concrete evidence, the activation of pericytes may largely contribute to the pathogenesis of SSc by favouring micro-vessel dysfunction and fibrosis. The aim of this review is to summarize the biological properties of pericytes and their hypothetical role in the development of SSc.
The pericyte: a multifaceted cell
Pericytes are a pool of vascular cells that mainly populate the pre-capillary, capillary and post-capillary abluminal side of non-muscular micro-vessels, although they have also been detected in human large vessels, vasa vasorum, and lymphatic vessels. The efficiency of the vascular barrier depends on the number of pericytes surrounding each vessel, which varies depending on the anatomical district of the vessels themselves: many in the central nervous system (CNS) and lungs, and much fewer in skeletal muscles (3).
Pericytes are intimately associated with endothelial and vascular smooth muscle cells, with which they share some phenotypical characteristics. Pericytes and pericyte-like cells such as myofibroblasts and fibroblasts belong to a heterogeneous population derived from mesenchymal tissue. During embryogenesis, pericytes originate from stem cells mainly as a result of transforming growth factor-beta (TGF-β) stimulation (4). It has been shown that the stimulation of different cytokines (e.g., TGF-β or platelet-derived growth factor-beta, PDGF-β) may lead to the divergent differentiation of stem cells into pericytes or endothelial cells. Furthermore, stimulation with TGF-β and PDGF-β may promote the initiation of neoangiogenesis at a different rate and the transition of pericytes into fibroblasts with different biological properties (5). Both TGF-β and PDGF-β are abundantly expressed in SSc, and may contribute to protecting vessel stability or encouraging extracellular matrix deposition (6, 7). It has also been shown that there is a high degree of transition from other mesenchymal cells into pericytes and vice versa during adult angiogenesis, and pericytes may also derive from circulating progenitor cells, fibroblasts or myofibroblasts. Under certain conditions, neural crests may be a further local source of pericyte progenitors in the CNS (8).
After maturation, pericytes express PDGF-β receptors (PDGFβR). The pericytes derived from tissue stem cells also express alpha-smooth muscle actin (α-SMA), whereas those derived from blood pluripotent stem cells express cluster of differentiation (CD)45 and CD11b (3).
Given their heterogeneity and high degree of phenotypical transition, as well as the fact that no specific pericyte marker is available, identifying pericytes in tissue sections is quite difficult because they may express different proteins depending on the species, vessels, state of activation, pathological condition and culture medium. Alpha-SMA, desmin and tropomyosin characterise a contractile phenotype, whereas regulator of G protein signalling 5 (RGS-5), nestin, high-molecular weight melanoma-associated antigen (HMWMAA) are mainly expressed during angiogenesis (9). Similarly, the phenotype of pericytes (including their size, form, distribution, extracellular processes) varies according to their location.
Various markers have been used to detect pericytes in histological studies that have particularly focused on perivascular cells in the blood-brain and blood-retina barriers, including PDGFβR, nerve/glial antigen-2 (NG2), CD13, desmin, vimentin, Kir 6.1 (10). Renal pericytes express α-SMA and NG2 in new-borns and after a kidney injury, whereas adult kidney pericytes become negative for α-SMA and positive for Col1a1-GFP, PDGFRβ and CD73 (11). In renal fibrosis, pericytes have been investigated as the source of the myofibroblasts responsible for the deposition of extracellular matrix in the interstitium and glomeruli; however, the results are heterogeneous as pericytes have been variously identified as CD73-, PDGFR-β-, NG2- and/or α-SMA-positive stromal cells, and different transgenic mice models have been used (12).
The expression of some of these markers may therefore vary in vitro and in vivo, and depending on their anatomical site. The expression of α-SMA has been detected in pre- and post-capillary pericytes populating the microvascular beds of rat mesentery and bovine retina, but not in capillary pericytes in vivo, whereas large amounts have been reported in in vitro pericyte experiments involving large vessels, thus underlining the differences in biological functions depending on localisation (13).
Biological properties of pericytes
A single pericyte, generally located at the gap between two consecutive endothelial cells and far from the gas-exchange site, can interact with multiple endothelial cells, with the pericyte/endothelial cell ratio depending on activation, the angiogenic process, the type of vessel, and its location. Adherent gap junctions between pericytes and endothelial cells allow a dense exchange of molecular and ionic signals, and redistribute mechanical stresses. An abnormal interaction between pericytes and endothelial cells has been observed in diabetic microangiopathy, leading to vessel dilatation, rigidity and the formation of micro-aneurysms (14), and similar findings are usually observed in the terminal nailfold circulation of SSc patients during a nailfold capillaroscopy examination (15).
Pericytes are fundamental for the control of vessel tone, integrity, reparation or regeneration. They may also help the chemotaxis and diapedesis of peripheral blood leukocytes by expressing adhesive molecules, such as intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1) and E-selectin, and seem to be involved to some extent in immune defence and coagulation processes (3). As they share the characteristics of contractile and phagocytic cells, pericytes not only respond to endothelin, norepinephrine, angiotensin II (ATII), nitric oxide (NO), adenosine and reactive oxygen species (ROS), but also to complement residuals, cytokines and antigen ligands. Pericytes show active phagocytosis and pinocytosis, and may recognise antigens as a result of class I and II major histocompatibility complex (MHC) activating the pool of naïve lymphocytes. Furthermore, pericytes may synthesise tissue factor, and participate in extrinsic coagulation by recruiting and activating platelets, which in turn may induce pericyte activation and fibrosis by synthesising PDGF-β and TGF-β (16). In a murine model of kidney tubule interstitial fibrosis, it has been demonstrated that the over-expression of TGF-β precedes and amplifies the infiltration of PDGFβR−positive perivascular cells, including myofibroblasts and pericytes (17). Moreover, treatment with PDGF-β antagonists prevents fibrosis in mouse models (18), thus underlining the concept that blocking pericyte functions may help to counteract fibroblast activation and extracellular matrix deposition.
Pericytes contribute to vascular stability, and any reduction in their number or activity may compromise the regenerative properties of small vessels. It has been shown that pre-treatment with an antagonist of TGF-β, one of the strongest pericyte stimulators, can impair neo-angiogenesis in experimental models of kidney fibrosis (17). During the first phases of angiogenesis, pericytes lose contact with endothelial cells, grow and proliferate, and increase their metabolic activity. They also acquire an amoeboid-fibroblastic phenotype that enables them to guide and scaffold the advance of the future endothelial wall. Angiogenesis is governed by a specific interplay of molecular signals between endothelial cells and pericytes that is ordered in a time-frame that proceeds from pericyte recruitment and vessel coating to pericyte detachment. The expression of TGF-β seems to be crucial in the first phase of vessel sprouting, whereas PDGF-β and vascular endothelial growth factor (VEGF) are involved later. Following a complex cascade that is partly mediated by the Ang protein family, VEGF seems to antagonise TGF-β signalling, prevent pericyte covering, and favour vessel destabilisation (7, 19). Animal experiments have shown that pericytes are actively involved in cutaneous angiogenesis following third-degree skin burns, during which they may progressively transit from a pro-angiogenetic to a contractile and pro-fibrotic phenotype as they are also capable of becoming part of the new vessel muscle tunic and producing collagen IV (20). At the same time, it has been demonstrated that pericytes may also participate in vessel involution. In the early phase of diabetic retinopathy, pericytes and endothelial cells may disappear from retinal capillaries, giving rise to the formation of acellular tubes, occlusion, micro-aneurysms and haemorrhages that contribute to visual loss. The mechanism underlying the disappearance of pericytes has been explained on the basis of direct hyperglycaemic toxicity, although other causes such as oxidative and mechanical stress, the participation of the renin-angiotensin system, pericyte migration into perivascular space, and the presence of autoantibodies against pericyte markers have also been suggested (21). Pericyte degeneration in other districts such as the brain has also been investigated. Various neurodegenerative disorders (including dementia, Alzheimer’s disease, amyotrophic lateral sclerosis and ischemic stroke) are characterised by a loss of the pericyte coating of brain vessels, and one experiment involving pericyte-deficient mice showed reduced cerebral blood flow in response to neuronal stimuli, thus favouring metabolic stress and alterations in neuronal excitability over time (22).
Pericytes also seem to participate actively in fibrotic processes. In an experiment using mice models, Birbrair et al found two distinct pericyte subsets in the pulmonary, renal, cardiac and brain vascular tree: type-1 (Nestin-GFP-/NG2-DsRed+) and type-2 (Nestin-GFP+/NG2-DsRed+). These responded differently to tissue injuries insofar as type-1 pericytes were involved in collagen deposition in the lungs and brain but not in other districts (23). Other authors have demonstrated that murine lungs are populated by a pool of pericyte progenitors expressing the transcriptional factor forkhead transcription factor-d1 (Foxd1), PDGFβR and NG2, which may proliferate after exposure to bleomycin and favour the deposition of collagen (24).
In brief, pericytes seem to play a key role in vasculogenesis, fibrosis and scar tissue repair (25), and show considerable plasticity when trans-differentiating from one mesenchymal phenotype to another. Pericyte dysfunction could therefore underlie many pathological conditions and the clinical manifestations of SSc.
Pericytes in systemic sclerosis
Despite all the evidence suggesting that pericytes play a central role in angiogenesis and tissue repair, their exact contribution to the pathogenesis of SSc is still a matter of debate. Given their particular characteristics of presiding over micro-vessel integrity and controlling vessel tone, participating in fibrosis, and being involved in immune cell recruitment and tissue repair, there is a clear rationale underlying the hypothesis that they participate in each step of the pathogenesis of the disease. There are some certainties concerning the origin of the cells that give rise to skin and visceral fibrosis in SSc: pericytes share a common derivation from mesenchymal cells with endothelial cells, myofibroblasts and fibroblasts, are highly plastic, and lack a single distinctive marker. The bone marrow mesenchymal stem cells (BM-MSCs) of SSc patients can be induced to differentiate and behave as pericytes after stimulation with TGF-β and PDGF-BB and, depending on the micro-environment, can be reprogrammed from a more pro-fibrotic phenotype to a more vasculogenic phenotype (26). Some authors have hypothesized that the fibroblasts and myofibroblasts actively involved in the pathogenesis SSc may derive from a common pericyte progenitor (27). Upon stimulation with TGF-β, pericytes from skin and bone marrow samples of SSc patients express A disintegrin and metalloproteinase domain 12 (ADAM-12), which is a marker of a pro-fibrotic phenotype. The activated pericyte phenotype in SSc is characterised by the expression of PDGF-β receptors, which are not seen in normal skin. The activation of cutaneous pericytes in affected and unaffected skin is characterised by an increased expression of PDGF-AB/BB, and PDGFβRs represent a distinctive trait of early SSc that cannot be seen in skin samples of subjects affected by primary Raynaud’s phenomenon (28). Moreover, the blockade of PDGF receptors by means of crenolanib, an inhibitor of PDGF receptor signalling, attenuates collagen deposition and fibroblast proliferation in cultured fibroblasts and murine models of angiotensin II-induced skin and heart fibrosis (29). It has also been demonstrated that myofibroblasts and pericytes in skin sections taken from SSc patients share the same pattern of molecular markers (α−SMA, the extra-domain A [ED-A] splice variant of fibronectin, and Thy-1), which is not observed in pericytes obtained from normal skin samples (30). The expression of the ED-A variant of fibronectin and Thy-1 in SSc pericytes (two markers of myofibroblast activation) seems to strengthen the hypothesis of the common origin of pericytes and myofibroblasts in SSc patients.
Telocytes, a newly described population of stromal cells involved in tissue repair, immunomodulation and angiogenesis, may establish close interactions with pericytes, and activate them by means of a paracrine or a cell-cell contact pathway (31). Although telocytes are characterised by the expression of other molecular markers than those of pericytes (essentially CD34) (32), it has been demonstrated that they may lose the expression of CD34 and acquire α−SMA during some pathological conditions such as tumours (30). Telocyte levels have recently been found to be considerably reduced in SSc patients depending on the severity of fibrosis in the skin and visceral organs (33, 34).
The origin of myofibroblasts from epithelial and endothelial cells has also been recently described. During endothelial to mesenchymal transition (EndoMT), endothelial cells modify their phenotype, acquiring the expression α-SMA, vimentin, and type I collagen, and losing that of CD31/PECAM-1, von Willebrand Factor (vWF), and VE-cadherin (35). This cell population has been associated with endothelial dysfunction, pulmonary artery hypertension, and lung fibrosis in SSc patients (36-38) but, like telocytes, EndoMT cells seem to be distinct from pericytes and may synergistically contribute to vasculopathy and fibrosis.
Pericytes may control angiogenesis by favouring or blocking new vessel sprouting by means of a C-X-C motif chemokine receptor 3 (CXCR3) pathway (39). Pericytes may express CXCR3 ligands IP-9 and IP-10, which limit endothelial cell migration and promote vessel involution. Under normal conditions, mature and pericyte-coated vessels do not express CXCR3, but it is highly expressed by endothelial cells following vascular injury. The final vascular tree architecture could therefore be guided by the expression of CXCR3 and its ligands by endothelial cells and pericytes. A number of studies have underlined the association between the IP-10/CXCR3 pathway and fibrotic involvement in SSc (40, 41), and the delayed healing of digital ulcers observed in SSc patients may be associated with impaired pericyte functioning in terms of a disturbed chemokine pathway (42). However, the IP-10/CXCR3 pathway is not unique to SSc, as it is also associated with other pathological conditions such as malignancies or infections characterized by tissue injury and attempted repair (43).
A recent study by Toyama et al has revealed a deficit in Friend leukaemia virus integration 1 (Fli-1) in SSc patients, which has been related to defective angiogenesis, fibrosis and immune system abnormalities, and prevents the migration of endothelial cells or fibroblasts in response to VEGF-A and PDGF-BB stimulation (44). More specifically, the reduced expression of Fli-1 has been associated with a reduced pericyte coating of micro-vessels, which reflects an acceleration of the first four steps of angiogenesis (during which vessels lose pericyte cover) and a defect in the last two steps, when mature vessels re-acquire pericyte cover. It has been demonstrated in in vitro human and animal models that pericytes selectively express miRNA-145, which targets and regulates the expression of Fli-1 (45). However, an epigenetic study of SSc fibroblasts and skin tissues has found a reduced concentration of miRNA-145 in comparison with normal fibroblasts (46). It is unclear whether the expression of miRNA-145 is concomitantly altered in pericytes and fibroblasts taken from SSc subjects, but it can be hypothesized that an epigenetic imbalance in Fli-1 expression in the pericytes of SSc subjects would favour the development of a pro-fibrotic phenotype, thus reducing the contribution of these cells to angiogenesis.
One of the distinctive characteristics of pericytes is the presence of plasmalemma caveolae, rich in caveolin-1. It has been extensively pointed out in several studies of SSc that a possibly genetically caused deficit in caveolin-1 may be a triggering factor of the disease. According to these data, the caveolin-1 rs959173 C minor allele seems to confer protection against the limited form of SSc in Caucasian populations (47). Caveolin-1, which is located in specialised microdomains of the plasma membrane known as lipid rafts, promotes endocytosis, lipid transport and signal transduction by means of plasmalemma invaginations (48). Mice lacking the expression of caveolin-1 gene experience alterations in vessel tone control in the cardiovascular system and progressive lung fibrosis, thus mimicking the pathogenetic pathway of SSc (49). As studies of mice and humans have shown a ubiquitous distribution of caveolin-1 with a similar level of expression on epithelial and vascular cells (50, 51), a genetic deficit in its synthesis may account for the combination of micro-vessel dysfunction and fibrosis. Although no clear evidence is yet available, pericytes lacking caveolin-1 may have impaired interactions with other vascular cells, including endothelial cells. Experiments involving murine models have shown a reduced expression of caveolin-1 in lung fibroblasts undergoing proliferation when exposed to hyperoxic conditions (52). Other authors have demonstrated that a deficit in caveolin-1 may induce mitochondrial dysfunction and accelerate the senescence of fibroblasts, thus leading in the generation of ROS and endothelial damage (53). On the other hand, oxidative stress may induce epithelial cell and fibroblast senescence by up-regulating the expression of caveolin-1, which arrests the cell cycle and inhibits mitosis (54, 55). It can be hypothesized that a genetically determined deficit in caveolin-1 in SSc subjects promotes the proliferation of fibroblasts following an oxidative insult. Some experiments involving humans and animal models have found that the administration of caveolin scaffolding domain peptide can prevent monocyte migration via the chemokine receptor type 4-positive (CXCR4+)/stromal cell-derived factor 1 (CXCL12) pathway and fibroblast accumulation in a bleomycin model of lung fibrosis (56). A recent study by Cipriani et al showed that bone marrow mesenchymal stem cells (SSc-MSCs) of SSc patients (used as surrogates for pericytes) had lower levels of caveolin-1 that were significantly associated with a reduced degradation of the VEGF/VEGFR2 complex, and led to their more pronounced transition towards a pro-fibrotic phenotype (57).
Pericytes are abundantly expressed in the vasculature of the CNS, where they contribute to reducing vascular permeability. However, the presence of neurological vascular sufferance in SSc is still uncertain, although many studies have demonstrated the involvement of both the CNS and the peripheral nervous system during the course of the disease, and white matter lesions have often been documented in asymptomatic SSc patients (58). Several studies have more recently found a close interaction between the peripheral terminations of nerve fibres and the microvasculature. It has been shown that the down-regulation of neuropilin-1 (NRP-1),which was initially identified as an axonal growth factor (59), may reduce angiogenesis in endothelial cells by complexing with the VEGFR-2 co-receptor (60). Following stimulation with basic fibroblast growth factor (b-FGF), human vascular smooth cells may become a source of NRP-1 (61), and topically applied recombinant b-FGF has been successful in healing SSc ulcers (62). There is some evidence that, after stimulation with NRP-1 and PDGF-BB, pericytes may develop from mesenchymal cells in tumorigenesis models (63), and that their correct interaction with endothelial cells depends on co-stimulation with NRP-1 and VEGFR-2 (64). Moreover, stimulation with PDGF-D (mainly produced by arterial endothelial cells) but not PDGF-B may favour the formation of a receptor complex involving NRP-1 and PDGFRβ co-expressed in trans on pericytes and endothelial cells, thus giving rise to the more efficient recruitment of pericytes and the formation of pericyte-coated new vessels (65).
Finally, given their ability to differentiate and adapt their phenotype to the requirements of their microenvironment, pericytes may be key cells in the regenerative medicine that is currently being applied to the therapeutic algorithm of SSc. The autologous transplantation of adipose tissue injected into the subcutaneous layer of the fingers has led to promising outcomes in the case of digital ulcers (66). Adipose tissue contains a large number of perivascular cells, including pericytes, and it has been shown that pericytes isolated from adipose tissue retain stem cell regenerative properties insofar as they are capable of de-differentiating into bone and cartilage under specific conditions (67, 68). However, the final phenotype of the transplanted mesenchymal cells and their local biological properties still need further elucidation.
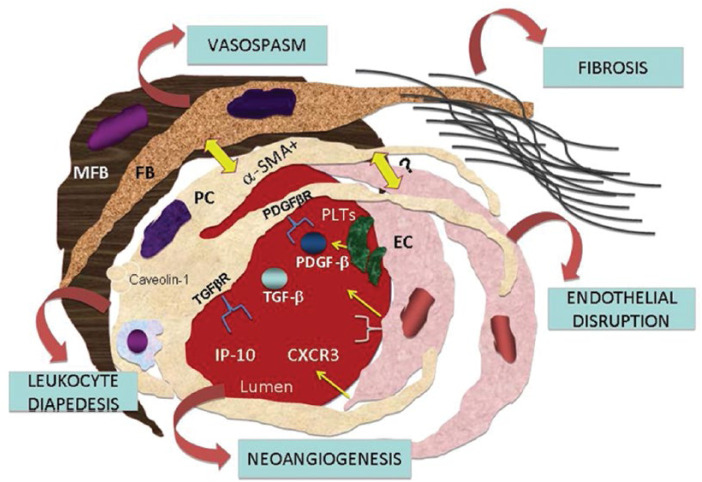
Figure 1 shows the network of pericytes, endothelial cells, myofibroblasts, fibroblasts and blood cells in a hypothetical micro-vessel.
Fig. 1.
The interplay between pericytes (PC) and other neighbouring mesenchymal cells in a micro-vessel. A single pericyte embraces several endothelial cells (EC), establishes contacts with myofibroblasts (MFB) and fibroblasts (FB), and may be involved in controlling endothelial integrity, vessel tone and fibrosis. Under particular conditions, pericytes may transit to an endothelial or myofibroblast/fibroblast phenotype and vice versa (yellow arrows). Pericytes express receptors for transforming-growth factor-beta (TGFβR) and platelet-derived growth factor-beta (PDGFβR), which may be released by activated platelets (PLTs). Like contractile cells, pericytes also express cytosolic α-smooth actin (α-SMA) and caveolin-1 on the plasmalemma. The interaction of pericytes binding IP-10 to the chemokine receptor CXCR3 on endothelial cells may control vessel regeneration. Pericytes may help white blood cells to transmigrate to extra-vascular sites, where they contribute to inflammation. On the basis of this scenario, pericytes seem to be crucially involved in the microvascular damage and fibrotic changes observed in patients with systemic sclerosis.
Conclusions
The biological and anatomical properties of pericytes make them candidates for being active participants in the onset and progression of SSc, although it is still not clear to what extent they contribute to the pathogenesis of the disease. Many of the unanswered questions concerning their exact role in SSc arise from the difficulty of identifying and isolating them, which indicates that their phenotypes and behaviours may vary in different biological and pathological situations. Further studies electively focusing on pericytes from different anatomical sites and in different stages or forms of the disease are now required.
Footnotes
Disclosures: Financial support: No grants or funding have been received for this study.
Conflict of interest: None of the authors has financial interest related to this study to disclose.
References
- 1. Matucci-Cerinic M, Kahaleh B, Wigley FM. Review: evidence that systemic sclerosis is a vascular disease. Arthritis Rheum. 2013;65(8):1953-1962. [DOI] [PubMed] [Google Scholar]
- 2. Herrick AL. Vascular function in systemic sclerosis. Curr Opin Rheumatol. 2000;12(6):527-533. [DOI] [PubMed] [Google Scholar]
- 3. Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193-215. [DOI] [PubMed] [Google Scholar]
- 4. Yamazaki T, Nalbandian A, Uchida Y, et al. Tissue myeloid progenitors differentiate into pericytes through TGF-β signaling in developing skin vasculature. Cell Reports. 2017;18(12):2991-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodriguez A, Friman T, Kowanetz M, van Wieringen T, Gustafsson R, Sundberg C. Phenotypical differences in connective tissue cells emerging from microvascular pericytes in response to overexpression of PDGF-B and TGF-β1 in normal skin in vivo. Am J Pathol. 2013;182(6):2132-2146. [DOI] [PubMed] [Google Scholar]
- 6. Cipriani P, Di Benedetto P, Ruscitti P, et al. Impaired endothelium-mesenchymal stem cells cross-talk in systemic sclerosis: a link between vascular and fibrotic features. Arthritis Res Ther. 2014;16(5):442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312(5):623-629. [DOI] [PubMed] [Google Scholar]
- 8. Trost A, Schroedl F, Lange S, et al. Neural crest origin of retinal and choroidal pericytes. Invest Ophthalmol Vis Sci. 2013;54(13):7910-7921. [DOI] [PubMed] [Google Scholar]
- 9. Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-oncol. 2005;7(4):452-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trost A, Lange S, Schroedl F, et al. Brain and retinal pericytes: origin, function and role. Front Cell Neurosci. 2016;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pan SY, Chang YT, Lin SL. Microvascular pericytes in healthy and diseased kidneys. Int J Nephrol Renovasc Dis. 2014;7:39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun YB, Qu X, Caruana G, Li J. The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation. 2016;92(3):102-107. [DOI] [PubMed] [Google Scholar]
- 13. Nehls V, Drenckhahn D. Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol. 1991;113(1):147-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lorenzi M, Cagliero E. Pathobiology of endothelial and other vascular cells in diabetes mellitus. Call for data. Diabetes. 1991;40(6):653-659. [DOI] [PubMed] [Google Scholar]
- 15. Rossi D, Russo A, Manna E, et al. The role of nail-videocapillaroscopy in early diagnosis of scleroderma. Autoimmun Rev. 2013;12(8):821-825. [DOI] [PubMed] [Google Scholar]
- 16. Pattanaik D, Brown M, Postlethwaite BC, Postlethwaite AE. Pathogenesis of Systemic Sclerosis. Front Immunol. 2015;6:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pozdzik AA, Giordano L, Li G, et al. Blocking TGF-β signaling pathway preserves mitochondrial proteostasis and reduces early activation of PDGFRβ+ Pericytes in Aristolochic Acid Induced Acute Kidney Injury in Wistar Male Rats. PLoS ONE. 2016;11(7):e0157288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kavian N, Batteux F. Macro- and microvascular disease in systemic sclerosis. Vascul Pharmacol. 2015;71:16-23. [DOI] [PubMed] [Google Scholar]
- 19. Ribatti D, Nico B, Crivellato E. The role of pericytes in angiogenesis. Int J Dev Biol. 2011;55(3):261-268. [DOI] [PubMed] [Google Scholar]
- 20. Busuioc CJ, Mogoşanu GD, Popescu FC, Lascăr I, Pârvănescu H, Mogoantă L. Phases of the cutaneous angiogenesis process in experimental third-degree skin burns: histological and immunohistochemical study. Rom J Morphol Embryol. 2013;54(1):163-171. [PubMed] [Google Scholar]
- 21. Beltramo E, Porta M. Pericyte loss in diabetic retinopathy: mechanisms and consequences. Curr Med Chem. 2013;20(26):3218-3225. [DOI] [PubMed] [Google Scholar]
- 22. Kisler K, Nelson AR, Rege SV, et al. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat Neurosci. 2017;20(3):406-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Birbrair A, Zhang T, Files DC, et al. Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem Cell Res Ther. 2014;5(6):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hung C, Linn G, Chow YH, et al. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med. 2013;188(7):820-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U. Skin wound healing: an update on the current knowledge and concepts. Eur Surg Res. 2017;58(1-2):81-94. [DOI] [PubMed] [Google Scholar]
- 26. Cipriani P, Marrelli A, Benedetto PD, et al. Scleroderma mesenchymal stem cells display a different phenotype from healthy controls; implications for regenerative medicine. Angiogenesis. 2013;16(3):595-607. [DOI] [PubMed] [Google Scholar]
- 27. Cipriani P, Di Benedetto P, Ruscitti P, et al. Perivascular cells in diffuse cutaneous systemic sclerosis overexpress activated ADAM12 and are involved in myofibroblast transdifferentiation and development of fibrosis. J Rheumatol. 2016;43(7):1340-1349. [DOI] [PubMed] [Google Scholar]
- 28. Rajkumar VS, Sundberg C, Abraham DJ, Rubin K, Black CM. Activation of microvascular pericytes in autoimmune Raynauds phenomenon and systemic sclerosis. Arthritis Rheum. 1999;42(5):930-941. [DOI] [PubMed] [Google Scholar]
- 29. Makino K, Makino T, Stawski L, et al. Blockade of PDGF receptors by crenolanib has therapeutic effect in patient fibroblasts and in preclinical models of systemic sclerosis. J Invest Dermatol. 2017;137(8):1671-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rajkumar VS, Howell K, Csiszar K, Denton CP, Black CM, Abraham DJ. Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis. Arthritis Res Ther. 2005;7(5):R1113-R1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Díaz-Flores L, Gutiérrez R, Díaz-Flores LJr, Goméz MG, Sáez FJ, Madrid JF. Behaviour of telocytes during physiopathological activation. Semin Cell Dev Biol. 2016;55:50-61. [DOI] [PubMed] [Google Scholar]
- 32. Bei Y, Zhou Q, Fu S, et al. Cardiac telocytes and fibroblasts in primary culture: different morphologies and immunophenotypes. PLoS ONE. 2015;10(2):e0115991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manetti M, Guiducci S, Ruffo M, et al. Evidence for progressive reduction and loss of telocytes in the dermal cellular network of systemic sclerosis. J Cell Mol Med. 2013;17(4):482-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Manetti M, Rosa I, Messerini L, Guiducci S, Matucci-Cerinic M, Ibba-Manneschi L. A loss of telocytes accompanies fibrosis of multiple organs in systemic sclerosis. J Cell Mol Med. 2014;18(2):253-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Piera-Velazquez S, Mendoza FA, Jimenez SA. Endothelial to mesenchymal transition (EndoMT) in the pathogenesis of human fibrotic diseases. J Clin Med. 2016;5(4)pii:E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jimenez SA. Role of endothelial to mesenchymal transition in the pathogenesis of the vascular alterations in systemic sclerosis. ISRN Rheumatol. 2013;2013:835948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Good RB, Gilbane AJ, Trinder SL, et al. Endothelial to mesenchymal transition contributes to endothelial dysfunction in pulmonary arterial hypertension. Am J Pathol. 2015;185(7):1850-1858. [DOI] [PubMed] [Google Scholar]
- 38. Mendoza FA, Piera-Velazquez S, Farber JL, Feghali-Bostwick C, Jiménez SA. Endothelial cells expressing endothelial and mesenchymal cell gene products in lung tissue from patients with systemic sclerosis-associated interstitial lung disease. Arthritis Rheum (Munch). 2016;68(1):210-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bodnar RJ, Satish L, Yates CC, Wells A. Pericytes: a newly recognized player in woundn healing. Wound Repair Regen. 2016;24(2):204-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Corrado A. The Th1 chemokine IP-10 in Systemic sclerosis. Clin Ter. 2014;165(6):e436-e441. [DOI] [PubMed] [Google Scholar]
- 41. Rabquer BJ, Tsou PS, Hou Y, et al. Dysregulated expression of MIG/CXCL9, IP-10/CXCL10 and CXCL16 and their receptors in systemic sclerosis. Arthritis Res Ther. 2011;13(1):R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yates CC, Whaley D, Wells A. Transplanted fibroblasts prevents dysfunctional repair in a murine CXCR3-deficient scarring model. Cell Transplant. 2012;21(5):919-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lazzeri E, Romagnani P. CXCR3-binding chemokines: novel multifunctional therapeutic targets. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5(1):109-118. [DOI] [PubMed] [Google Scholar]
- 44. Toyama T, Asano Y, Miyagawa T, et al. The impact of transcription factor Fli1 deficiency on the regulation of angiogenesis. Exp Dermatol. 2017. [DOI] [PubMed] [Google Scholar]
- 45. Larsson E, Fredlund Fuchs P, Heldin J, et al. Discovery of microvascular miRNAs using public gene expression data: miR-145 is expressed in pericytes and is a regulator of Fli1. Genome Med. 2009;1(11):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhou B, Zuo XX, Li YS, et al. Integration of microRNA and mRNA expression profiles in the skin of systemic sclerosis patients. Sci Rep. 2017;7:42899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Manetti M, Allanore Y, Saad M, et al. Evidence for caveolin-1 as a new susceptibility gene regulating tissue fibrosis in systemic sclerosis. Ann Rheum Dis. 2012;71(6):1034-1041. [DOI] [PubMed] [Google Scholar]
- 48. Fujimoto T. Cell biology of caveolae and its implication for clinical medicine. Nagoya J Med Sci. 2000;63(1-2):9-18. [PubMed] [Google Scholar]
- 49. Drab M, Verkade P, Elger M, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293(5539):2449-2452. [DOI] [PubMed] [Google Scholar]
- 50. Williams TM, Lisanti MP. The Caveolin genes: from cell biology to medicine. Ann Med. 2004;36(8):584-595. [DOI] [PubMed] [Google Scholar]
- 51. Kogo H, Fujimoto T. Caveolin-1 isoforms are encoded by distinct mRNAs. Identification Of mouse caveolin-1 mRNA variants caused by alternative transcription initiation and splicing. FEBS Lett. 2000;465(2-3):119-123. [DOI] [PubMed] [Google Scholar]
- 52. Wang X, Fu JH, Xue XD. Expression dynamics of caveolin-1 in fibroblasts of newborn rats with chronic lung disease and its impact on lung fibroblast proliferation. Acta Cir Bras. 2017;32(5):359-368. [DOI] [PubMed] [Google Scholar]
- 53. Yu DM, Jung SH, An HT, et al. Caveolin-1 deficiency induces premature senescence with mitochondrial dysfunction. Aging Cell. 2017;16(4):773-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dasari A, Bartholomew JN, Volonte D, Galbiati F. Oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements. Cancer Res. 2006;66(22):10805-10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Volonte D, Zhang K, Lisanti MP, Galbiati F. Expression of caveolin-1 induces premature cellular senescence in primary cultures of murine fibroblasts. Mol Biol Cell. 2002;13(7):2502-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tourkina E, Bonner M, Oates J, et al. Altered monocyte and fibrocyte phenotype and function in scleroderma interstitial lung disease: reversal by caveolin-1 scaffolding domain peptide. Fibrogenesis Tissue Repair. 2011;4(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cipriani P, Di Benedetto P, Capece D, et al. Impaired Cav-1 expression in SSc mesenchymal cells upregulates VEGF signaling: a link between vascular involvement and fibrosis. Fibrogenesis Tissue Repair. 2014;7(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Amaral TN, Peres FA, Lapa AT, Marques-Neto JF, Appenzeller S. Neurologic involvement in scleroderma: a systematic review. Semin Arthritis Rheum. 2013;43(3):335-347. [DOI] [PubMed] [Google Scholar]
- 59. Kawakami A, Kitsukawa T, Takagi S, Fujisawa H. Developmentally regulated expression of a cell surface protein, neuropilin, in the mouse nervous system. J Neurobiol. 1996;29(1):1-17. [DOI] [PubMed] [Google Scholar]
- 60. Kahaleh B, Guiducci S, Kuwana M. Recent updates in experimental protocols for endothelial cells. J Scleroderma Relat Disord. 2016;1(3):257-265. [Google Scholar]
- 61. Liu W, Parikh AA, Stoeltzing O, et al. Upregulation of neuropilin-1 by basic fibroblast growth factor enhances vascular smooth muscle cell migration in response to VEGF. Cytokine. 2005;32(5):206-212. [DOI] [PubMed] [Google Scholar]
- 62. Yamanaka K, Inaba T, Nomura E, et al. Basic fibroblast growth factor treatment for skin ulcerations in scleroderma. Cutis. 2005;76(6):373-376. [PubMed] [Google Scholar]
- 63. Dhar K, Dhar G, Majumder M, et al. Tumor cell-derived PDGF-B potentiates mouse mesenchymal stem cells-pericytes transition and recruitment through an interaction with NRP-1. Mol Cancer. 2010;9(1):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kawamura H, Li X, Goishi K, et al. Neuropilin-1 in regulation of VEGF-induced activation of p38MAPK and endothelial cell organization. Blood. 2008;112(9):3638-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Muhl L, Folestad EB, Gladh H, et al. Neuropilin 1 binds PDGF-D and is a co-receptor in PDGF-D-PDGFRβ signaling. J Cell Sci. 2017;130(8):1365-1378. [DOI] [PubMed] [Google Scholar]
- 66. Del Papa N, Di Luca G, Sambataro D, et al. Regional implantation of autologous adipose tissue-derived cells induces a prompt healing of long-lasting indolent digital ulcers in patients with systemic sclerosis. Cell Transplant. 2015;24(11):2297-2305. [DOI] [PubMed] [Google Scholar]
- 67. Zhang JX, Du CY, Guo WM, et al. Adipose tissue-derived pericytes for cartilage tissue engineering. Curr Stem Cell Res Ther. 2017;12(999):1. [DOI] [PubMed] [Google Scholar]
- 68. James AW, Zara JN, Zhang X, et al. Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Transl Med. 2012;1(6):510-519. [DOI] [PMC free article] [PubMed] [Google Scholar]