Abstract
Among all possible systemic sclerosis internal organ complications, kidney involvement is frequently neglected or underestimated, except for the life-threatening scleroderma renal crisis. Fortunately, this severe clinical presentation is nowadays better controlled with available treatments, in particular angiotensin-converting enzyme inhibitors, and this has led to a reduction in its short- and longer-term mortality. Pathogenetic determinants are not well understood and many different other kidney involvements are possible in systemic sclerosis, including proteinuria, albuminuria, reduction of renal filtration, autoantibodies-related glomerulonephritis, and drug-related side effects. Different serological and radiological methods of evaluations are nowadays available, some representing promising diagnostic tool and prognostic outcome measure. Except for angiotensin-converting enzyme inhibitors in scleroderma renal crisis, no other treatment is currently recommended for treatment of kidney involvement in systemic sclerosis. For this reason, further studies are necessary to investigate its prognostic impact, in particular in combination with other systemic sclerosis–related internal organ manifestations. This review summarizes current available literature on kidney involvement in systemic sclerosis.
Keywords: Systemic sclerosis, Kidney, Scleroderma renal crisis, Renal function
Introduction
Among kidney involvement in systemic sclerosis (SSc), scleroderma renal crisis (SRC) represents the principal manifestation of kidney damage (1). Reflecting the vasculopathy that characterizes SSc, even in the absence of a renal crisis, chronic kidney diseases (CKDs) other than renal crisis may occur (2); indeed, hypertension, abnormal kidney function, or proteinuria are found in up to 60% of patients, but the majority of this prevalence may reflect other non-SSc determinants, such as comorbidities and aging. In addition, other renal manifestations such as abnormal glomerular filtration and decreased blood flow can be detected using sensitive measures of renal function or Doppler studies (3). This review summarizes current knowledge on pathogenesis, clinical manifestation, and assessment and treatment of kidney involvement in SSc: we performed a literature search in MEDLINE/PubMed and Thomson Reuter’s Web of Science for articles published from inception to June 2017.
Pathogenesis and risk factors
The multifactorial pathogenesis of kidney involvement in SSc, in particular of SRC, is not completely understood (summarized in Fig. 1). The primary event of kidney damage is an injury to the endothelial cells, causing intimal thickening and proliferation of intralobular and arcuate arteries. Histopathologic studies and renal plasma flow measurements suggest that the vascular intima proliferation leads to the typical vascular “onion bulb-like” lesion, characterized by vessel lumen narrowing and resting renal blood flow. In this context, thickened abnormal vessel wall facilitates platelet aggregation, adhesion, and platelet factor release; these participate to the increased vascular permeability and to the production and deposition of extracellular matrix (ECM—i.e. collagen and fibrin) that contribute to the luminal narrowing (4). Therefore, these modifications reduce renal perfusion, in particular cortical blood flow, with possible subsequent renal ischemia (4).
Fig. 1.
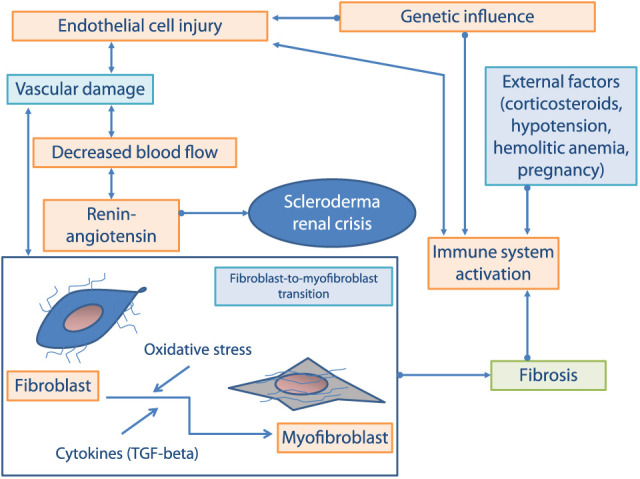
Mediators and processes involved in scleroderma renal crisis pathogenesis.
An episodic vasospasm of unclear significance, also called “renal Raynaud’s phenomenon,” has been demonstrated in early SSc patients (5). However, though vascular involvement has a major effect on the development of SRC, several authors believe that something more than just a decreased blood flow triggers the acute episode of SRC.
The renin–angiotensin system (RAS) is composed of several components and membrane receptors; some of them have been characterized as responsible for the vascular remodeling process occurring after vascular endothelial injury. This system mainly modulates blood pressure (BP) and maintains hydro-mineral homeostasis (6). Several authors suggested that continually increased local RAS production contributes to vascular remodeling, and it has been well documented that increased levels of angiotensin II elicit several dangerous effects on vessel in the context of atheromatous diseases (7). A marked increase in peripheral levels of renin, at the time of SRC, leading to the onset of malignant hypertension and rapidly progressive renal failure has also been demonstrated (8); however, what triggers the acute onset of hyperreninemia is not known.
Recent evidences demonstrated that different ECM-derived fragments play a role in the vascular remodeling process which is strictly related to endothelial cell (EC) apoptosis and ECM degradation. The fragment LG3 of perlecan derives from the degradation of a proteoglycan embedded within the vascular basement membrane and is released upon others from metalloproteinase degradation and interacts with α2β1 integrins on mesenchymal stem cells (MSC) and fibroblasts, resulting in a state of resistance to apoptosis. Binding of LG3 to α2β1 integrin on EC is able to inhibit angiogenesis. Therefore, it is conceivable that LG3 may contribute to vascular remodeling in conditions associated with EC apoptosis (9).
Although hormonal factors within the kidney, such as catecholamines, kinins, and prostaglandins, were suspected to be involved in the pathogenesis of SSc, only few data sustain this hypothesis, suggesting that the pathophysiology of SSc renal crisis is mostly due to the dysfunction of factors controlling renal circulation (10). Endothelin-1 (ET-1), the most potent endogenous vasoconstrictor produced within the vasculature (11), is also responsible for proliferation, inflammation, fibrosis, as well as for the development and progression of CKD (12). ET-1 is produced within the kidney and its action is mediated through endothelin-A (ETA) and -B (ETB) receptors. In 1991, the evidence provided by Kahaleh (13) suggested increased circulating levels of ET-1 in SSc patients and in particular in those with severe complications such as pulmonary arterial hypertension (PAH) (14). Moreover in 1999, Kobayashi et al. demonstrated the expression of ET-1 and ETB in kidney biopsies obtained from two patients died of SRC (15). More recently, in order to investigate the role of ET-1 in the pathogenesis of SRC, Mouthon et al. (13) compared the distribution of ET-1 in patients affected by SSc with SRC with that of other vascular diseases involving the kidney, using immunohistochemistry on renal biopsies. The data revealed ET-1 overexpression in glomeruli, arterioles, and interlobular arteries in kidney biopsies from SRC patients (16). In 2013, a total of 27 renal biopsy samples from SRC were analyzed to investigate the expression of the two high-affinity receptors ETA and ETB: the results were in agreement with previous findings by Kobayashi et al. (17) supporting the role of the endothelin axis into SRC pathogenic mechanisms.
Genetic studies provided evidences for a deregulation of the immune system as a contributing factor in the SSc development. In particular, circulating functional autoantibodies targeting vascular receptors and potentially responsible of the development of vascular complications have been found in SSc patients (18): autoantibodies against ACE2 and ETA receptor, in fact, may play a role in different aspects of SSc, including SRC (19). An unfavorable prognosis has been observed when anti-RNA polymerase III antibodies (anti-RNAP-III) coexist with SRC (20). A retrospective analysis from nine Italian centers including patients with SRC and contemporary available serum sample identified ANA positivity in 44 patients (96%), anti-topo I antibodies in 30 (65%), and anti-RNAP I–III in 7 (15%). The anti-topoisomerase I reactivity was more frequent than anti-RNAP I–III in this study and was associated with delayed onset and high mortality rates (21).
In an international observational cohort SRC survey, anti-RNAP-III positivity was detected in about 20% of patients (22), and this increased risk association was also confirmed by Terras et al. (23). In the Australian Scleroderma cohort study, the presence of anti-RNAP-III was associated with 25% risk of SRC development and was detectable in 59% of SRC patients (24). By contrast, different studies concluded that anti-RNAP-III antibodies are independently associated with SRC development. A systematic literature review and meta-analysis to enable comparison with a French cohort highlighted the wide variability of anti-RNAP-III positivity among patients with SRC, suggesting how patient’s geographical origin and environmental factors modulate anti-RNAP-III positivity prevalence (25). Finally, it was also reported that anti-RNAP-III-positive SRC patients are more likely to carry genetic polymorphisms of the gene encoding ETA, thus suggesting that the ETA-anti-RNAP-III axis could contribute to SRC susceptibility (26).
Although these autoantibodies have been recognized as a risk factor for the development of renal damage, there is currently no evidence for their specific role in the pathogenesis of SRC; a potential pathogenic role of these autoantibodies targeting vascular receptors in SRC is currently under evaluation, and the results applied to PAH could be used as a model for SRC (19).
A strong association with specific MHC class I has been recently demonstrated, in particular with HLA-DRB1*0407 and *1304 haplotypes. Gene screening studies showed an association of SRC with genes in the complement region. Finally, polymorphism in the endothelin ligand receptor axis but not in the ACE axis has been associated with increased risk of SRC (27).
Interleukin (IL)-9 is a pleiotropic cytokine produced mainly by Th9 cells, which is involved in the pathogenesis of different autoimmune disorders (28). IL-4, transforming growth factor-beta, and the epithelial cytokine thymic stromal lymphopoietin (TSLP) are cytokines involved in the Th9 differentiation process. Accordingly, it has been demonstrated that SSc patients have an increased expression of IL-25 and of Th9 polarizing cytokines. An overexpression of IL-9 was also found in renal biopsy of SRC patients, supporting the hypothesis that IL-9 and Th9 cells represent a player in its pathogenesis (28).
In SSc, it has been observed that an inappropriate fibroblast activation, accumulation of myofibroblasts in affected tissues, and the persistence of their functions are critical determinants of the extent and rate of fibrosis, able to significantly influence also patients’ prognosis and the overall mortality. Recently, the fibroblast-to-myofibroblast transition has emerged as another possible cause of fibrosis and deeply involved in the pathogenesis of fibrotic diseases and therefore of SRC (29). Indeed, fibroblasts are the principal effector cells responsible for ECM production and deposition, leading to the progressive loss of kidney function through renal parenchyma disruption (30). Recently, adiponectin, a multifunctional cytokine that modulates inflammation and metabolism, and lipocalin-2, a 25-kDa secretory glycoprotein, have been demonstrated to be related to angiogenesis, fibrosis, kidney injury, and immunity, playing a potential role in the pathogenesis of SRC (31, 32).
Among other possible risk factors, different conditions have been analyzed in which renal blood flow is further compromised, such as cardiac dysfunction (i.e. congestive heart failure arrhythmias, pericardial effusions), anemia, sepsis, dehydration, and drug abuse (4, 16, 18, 19); although it is not established if these may represent early consequences or real risk factors for SRC, they represent useful alerts. It is known that chronic use of calcineurin inhibitors is marred by several side effects among vascular toxicity. Among them, cyclosporine and tacrolimus, which cause vasospasm, have been associated with SRC (33) and increase the production of proinflammatory cytokines and endothelial activation markers both in vivo and ex vivo (34).
Corticosteroids (CS) are capable of inhibiting prostacyclin production and increasing ACE activity, and this could contribute to the pathogenesis of SRC. As of today, in vitro studies that evaluate the mechanism underlying the effects of the use of CS on the effector cells involved in the vascular damage observed in SRC are still lacking; whereas the use of CS as a potent risk factor in the development of SRC has been evaluated by Steen and Medsger (35) in their retrospective case-control study and in several case reports and a systematic review (33, 36-38).
Among other SSc-related clinical variables, major risk factors for the development of SRC include rapidly progressive skin disease (39), tendon friction rubs (40), and large joint contractures (41).
Clinical presentations
Together with SRC, kidney manifestations of SSc include normotensive SRC, isolated reduced glomerular filtration rate (GFR), reduced renal functional reserve (RFR), proteinuria, microalbuminuria, abnormal renal vascular resistance indices (Tab. I), and a clinical picture due to coexistent of myeloperoxidase antineutrophil cytoplasmic antibody (MPO-ANCA)-associated glomerulonephritis (GN) and vasculitis and SSc. Renal involvement may present with different patterns (2), and whether some patients might develop is still controversial (3). Most patients with SSc and renal involvement have a rapidly progressive or acute onset of renal failure when related to SRC. Less frequently, renal impairment is secondary to extrarenal visceral dysfunction such as SSc heart failure or to side effects of medications.
Table I.
Reported renal manifestations of scleroderma
| Scleroderma renal crisis (5, 17, 42-48) |
| Normotensive scleroderma renal crisis (49-53) |
| Myeloperoxidase-antineutrophil cytoplasmic antibody (MPO-ANCA)-associated glomerulonephritis and vasculitis (54-56) |
| Isolated reduced glomerular filtration rate (57) |
| Reduced renal functional reserve (58-60) |
| Microalbuminuria and proteinuria (61-63) |
| Scleroderma-associated vasculopathy manifested by abnormal renal vascular resistance indices and endothelial markers (64-72) |
SRC is characterized by the acute onset of severe arterial hypertension (often described as accelerated or malignant) together with acute kidney injury (5). Penn et al. (17) estimated the frequency of SRC as in 12% of diffuse cutaneous systemic sclerosis (dcSSc) patients and 2% of limited cutaneous systemic sclerosis (lcSSc) patients, with an odds ratio (OR) above 7 for dcSSc, reproducing data from an earlier studies (42-44). The reported median duration of SSc at the time of SRC was 7.5 months (0–200); 66% within 1 year of SSc diagnosis, rising to 86% at 4 years (29, 36). Conversely, lcSSc patients typically develop SRC later in the disease course (44). Avouac et al. (45) reported the characteristics of SSc patients presenting who were included in the EUSTAR database up to April 2008, and the prevalence of SRC was in 2%, clustered in 4.2% of dcSSc and 1.2% of lcSSc.
If a patient with SSc has an elevated BP of >150/85 mmHg or an increase of ≥20 mmHg from their usual systolic BP on two occasions in 24 h, they should be assessed urgently with blood tests and urinalysis in suspicion of SRC. If there is a significant increase in serum creatinine (either an absolute increase of 26.5 µmol/L or an increase of 50% from the baseline value) or urine dipstick shows proteinuria (> 2+) and/or hematuria 1+, they should be started on an angiotensin-converting enzyme inhibitors (ACEi) immediately and admitted to hospital for further assessment (46).
To aid early identification of the occurrence of SRC in high-risk patients, home BP monitoring was recommended twice weekly for all patients with dcSSc who are within 4 years of diagnosis (47) and targets should be individualized according to the patients’ own normal values.
Most patients with SRC complain of non-specific symptoms including fatigue and dyspnea. Other typical clinical features are those seen in accelerated hypertension of any cause: headache, hypertensive retinopathy with blurred vision, or other encephalopathic symptoms, including seizures (48). In addition, there may be evidence of microangiopathic hemolytic anemia (MAHA), oliguria, cardiac failure, and tachy-arrhythmias. MAHA or intravascular hemolysis is present in approximately 50% of cases and is evidenced by reduced platelet counts, reduced serum haptoglobin levels, red cell fragments, and schistocytes on blood film, together with elevated lactate dehydrogenase levels. Pulmonary edema is common and reflects the large afterload and oliguria, inducing salt and water retention. Pericarditis, myocarditis, and arrhythmias may supervene and may be associated with a poorer prognosis (48). However, these findings typically result from dramatically increased peripheral resistance and effective outflow tract obstruction rather than primary myocardial dysfunction. Tachycardia and tachyarrhythmias are also seen in this group, which has a high prevalence of concomitant myocardial fibrosis. Not all these symptoms needed to be present to diagnose SRC, and it can even occur in normotensive patients (44).
Normotensive SRC was defined by a 50% increase in serum creatinine over baseline or serum creatinine >20% of upper limit of normal and one of the following five features: proteinuria >2+ by dipstick, thrombocytopenia <100,000 mm−3, hemolysis (defined as anemia not due to other causes), and either schistocytes or other red blood cell fragments seen on blood smear or increased reticulocyte count or renal biopsy findings consistent with SRC (49). Normotensive SRC represent 10% of SRC and are often associated with CS exposure, thrombotic microangiopathy, and poor prognosis (50). Penn and colleagues (44, 51) suggested that this presentation might be more common in patients receiving ACEi, although the mechanism by which this occurs is not well understood; moreover, SRC can also occur in patients without skin involvement (52, 53).
Coexistence of MPO-ANCA-associated GN and SSc has been reported by several authors, resulting in renal failure (54, 55). In contrast to classical SRC, these patients tend to have established lcSSc, and the process has a subacute presentation with normal or mild elevated BP, worsening renal failure, active urinary sediment with casts, and proteinuria (sometimes in nephrotic range). Disease duration at the onset of renal failure is usually much longer in ANCA-related GN than in typical SRC (54, 56).
Isolated reduced GFR can be present in SSc despite normal serum creatinine (57). The principal determinants of creatinine are muscle mass and GFR, and it is well recognized that serum creatinine may not be elevated until the GFR is less than 50% of normal.
Proteinuria and albuminuria are useful markers of vasculopathy and are known to be independent predictors of cardiovascular morbidity and mortality in patients with and without other vasculopathic diseases, such as diabetes and hypertension (61, 62). Seiberlich et al. analyzed urine albumin, urine total protein, and urine electrophoresis to assess protein excretion in 80 SSc patients and 18 healthy age- and gender-matched controls, all with normal GFR. Increased total protein excretion was seen in 17.5% of SSc patients, and albuminuria was identified in 25% (22.5% microalbuminuria and 2.5% macroalbuminuria). Albuminuria correlated with disease duration >4 years and elevation of systolic BP, suggesting it may be reflective of chronic vascular injury (63).
Assessment
Various different methodologies have been described in assessing kidney involvement in SSc (Tab. II), mostly as research tools with a non-specific clinical significance, thus no specific recommendation is given regarding routine patient assessment. Highly invasive methods, such as angiography (73, 74) and scintigraphy, are been replaced by ultrasound and biomarkers, for which future research agenda is still open and encouraging.
Table II.
Methods of investigation and assessment of kidney involvement in SSc
Scintigraphy and RFR
The 99 m-technetium-diethylentriaminepentaacetic acid (99 m-Tc-DTPA) renal scintigraphy was first studied in five normotensive SSc patients showing that cold challenge was able to induce renal vasospasm, identified by a significant reduction in 99 m-Tc-DTPA clearance, thus leading to the hypothesis of a renal vessel Raynaud’s phenomenon (75). Similarly, poor blood perfusion and reduced-to-absent DTPA parenchymal uptake were seen in hypertensive SSc patients presenting SRC, suggesting a significant loss of renal functionality during this life-threatening complication (76).
The 99 m-Tc-DTPA scintigraphy also showed 80% abnormality of RFR in 30 SSc patients without renal involvement and regardless creatinine clearance levels, thus leading to the possibility of diagnosing preclinical renal involvement also in case of normal serum creatinine (58). This is in line with previous results from Livi et al. (59) who showed that RFR was significantly reduced in SSc patients, in particular if they already presented arterial hypertension and reduced basal GFR. Moreover, a defective RFR was also significantly predictive for further GFR reduction, development of microalbuminuria, and arterial hypertension (60).
Renal function and physiology
Given the high prevalence of preclinical renal involvement in SSc, testing for renal functionality should be performed as a routine assessment, although this might not represent current practice. Back in late 1950s, Urai et al. (77) showed that more than 80% of SSc patients had reduced renal plasma flow by para-aminohippurate clearance (despite a normal creatinine clearance). Plasma renin activity was also found increased in SSc patient as an indicator of chronic renal dysfunction, although not paralleled by significant proteinuria or decreased 24 h creatinine clearance (78). These findings support the vascular nature of renal involvement, mainly located at the pre-glomerular arteries; this is further indicated by the recent demonstration of decreased values of creatinine clearance having a strong negative correlation with the increasing severity of nailfold videocapillaroscopy (NVC) microangiopathy and being associated with history of digital ulcers (79).
Although considered a gold standard to determine mean GFR, renal scintigraphy is time-consuming and not commonly available, thus silver standards such as formulae to calculate GFR were created. In SSc patient, while Gigante et al. showed significant correlation between scintigraphy-measured GFR and CKD epidemiology collaboration equation (CKD-EPI) (80), Suebmee et al. confirmed statistically significant correlation with CKD-EPI, but also with Cockroft–Gault formula and Modification of Diet in Renal Disease (MDRD) study equation (81), The differences among these results could be possibly related to the different population size (41 vs 76 patients), female gender prevalence (90.2% vs 65.8%), and age distribution (median 46 vs 55).
Other studies have confirmed the high prevalence of subclinical renal impairment in SSc (57) and demonstrated a usually good prognostic impact on patient survival: in fact, Caron et al. showed that abnormal and normal baseline creatinine clearance presented a similar annual decline to the general population (82). Moreover, Scheja et al. demonstrated that this long-term decline was paralleled by new diagnosis of arterial hypertension or SSc-related heart involvement or non-SRC nephropathies (83).
Ultrasound and Doppler ultrasound
Ultrasound is a non-invasive gold standard for assessing kidney and the urinary tract, with the Doppler technique allowing the evaluation of renal arteries and parenchymal vascularization up to the smaller interlobar and arcuate arteries (64). Kidney arteries Doppler ultrasound allows the measurement of the renal resistive index (RRI), a semi-quantitative index measuring the distal resistance encountered by the blood flow (65). Even in the general population, age, renal vascular resistances and compliance, parenchymal fibrosis, arterial hypertension, arteriosclerosis, diabetes mellitus, hyperuricemia, and low-grade inflammation are possible determinants of renal vasculopathy or tubule-interstitial nephropathy, thus influencing RRI value (66, 67). Regarding SSc patients, Rivolta et al. first showed increased RRI values in 25 SSc patients compared to healthy controls, being correlated with disease duration. More recently, other studies showed that RRI has a positive significant correlation with both measured and estimated GFRs (69). Moreover, the progression of capillary damage on NVC and history of digital ulcers were associated with higher RRI values (69), also predictive for the development of a new digital ulcer (70). Finally, RRI changes in time were also presented as a reflection of the patient clinical status: this was presented in a case report of SRC treated with ACEi, in which RRI gradually decreased (71), and also in a case series of eight patients treated with repeated iloprost infusions for 6 months, showing a significant reduction of RRI compared to baseline and also compared to a chronic nifedipine treatment group (72). Despite these preliminary reports, the usefulness of RRI measurement in SSc patients still needs validation.
Renal biopsy
Histopathology studies have been performed in SSc patients with and without renal involvement, mostly directed at further understanding the pathogenetic mechanisms of disease-related renal impairment. Back in 1978, Lapenas et al. (84) compared renal biopsy specimens of SSc versus severe arterial hypertension patients: he found that SSc patients with recent onset of arterial hypertension had intimal thickening of arcuate and interlobular arteries determining lumen narrowing, associated with initial interstitial and adventitial fibrosis, which were strong prominent features in longer disease course. Moreover, deposits of immunoglobulins and complement fractions bound to the vascular walls were typical features of SSc patients, representing an ongoing immune process. Subsequently, Trostle et al. compared renal specimens of SSc patients with age- and gender-matched controls, identifying different features between CREST syndrome and diffuse cutaneous SSc with or without SRC. They showed that intimal thickening and consequent luminal occlusion were significant only in small arteries of CREST patients, while significant in small, medium, and large vessels of diffuse cutaneous SSc patients. This was particularly evident for diffuse SSc patients with history of SRC, in which intimal thickening was mucoid and edematous, compared to fibroelastic and sclerotic in the CREST and non-SRC diffuse patients, supporting the importance of classifying patients (85). Going into details, thrombotic microangiopathy affecting mainly interlobular arteries with fibrinoid necrosis of the vessel wall, mucoid and edematous swelling of the intima, and the subsequent “onion skin” intimal proliferation are the hallmark of SRC histology. Chronic changes also include glomerular involvement, tubular atrophy, and interstitial fibrosis (86). From a prognostic perspective, few studies have investigated the predictive role of histopathological changes: Penn et al. (44, 87) first showed that myxoid intimal thickening and thrombosis, as acute vascular changes, were associated with worse prognosis, while a better outcome was noticed when chronic changes were present, although conceptually controversial. First, data regarding acute changes were also strengthened by Batal et al. (88) showing that the presence/extent of vascular thrombosis, fibrinoid changes, and also severe glomerular ischemic collapse and acute tubular necrosis (as secondary modifications to the acute phase) were predictors of negative renal outcomes (such as non-recovering renal failure, necessity for dialysis, or renal failure–related death).
Biomarkers
Different circulating biomarkers have been studied in the recent years, although their usefulness has been only partially proven and validated. For example, a cutoff of 50 AU/mL was proposed for anti-Annexin V (expressed on various cell types, inhibiting prothrombin activity), with higher values being significantly correlated with increased RRI (89). Similarly, a 4.7 mg/dL cutoff for serum uric acid was tested as a good predictor for increased RRI (90). Soluble T-cell immunoglobulin and mucin domain 3 (sTIM3) was found to be more frequently elevated in patients with history of SRC (91); this complication was extensively studied by Chighizola et al. (92) identifying N-terminal (NT)-pro hormone BNP (NT-pro-BNP) cutoff level of 360 pmol/L as a strong independent predictor for necessity for renal supplementation or dialysis (OR = 7.6).
Therapy and prognosis
Recent studies suggest a strong association between renal involvement and outcomes in SSc; therefore, it becomes mandatory to pay attention to all the markers of renal vasculopathy, from a mild renal insufficiency to the most compromised forms, for the increased risk of mortality which can result in the presence of kidney damage (2). SRC has a severe morbidity and high mortality, although the advent of ACEi has dramatically changed the survival, turning lung disease into the main cause of death in SSc patients: it still remains a frequent cause of SSc-related death mostly in dcSSc patients, and at least half of SRC patients require dialysis, either temporary or long term (44). No randomized controlled trial has proven the efficacy of ACEi, but they represent the first choice of therapy in the management of a hypertensive state associated with raised amounts of renin (see Tab. III for summary of current evidences) (93).
Table III.
Recommendations from current evidences on the treatment of scleroderma renal crisis
| Frequent blood pressure monitoring with target therapy to maintain blood pressure to <130/90 mmHg (avoid β-blockers not to worsen peripheral vasculopathy) |
| Early treatment with angiotensin-converting enzyme inhibitors (ACEi) with increasing doses up to blood pressure target |
| Add other anti-hypertensive (e.g. calcium channel blockers) as needed in refractory patients or consider association with Bosentan (pay attention to possible drug-related fluid retention, optimizable by drug dosing and adding diuretics) in selected patients |
| In case of severe renal failure and/or end-stage renal disease (ESRD), consider dialysis as required (temporary or permanent but without interrupting the administration of ACEi) |
| Consider renal transplantation in dialysis-dependent eligible patients (usually within 2 years) |
| Corticosteroids and/or nephrotoxic drugs should be avoided in patients with diffuse scleroderma; use the lowest dose of corticosteroids and for the minimum duration if necessary |
In patients with established SRC, it is mandatory to control blood pressure early with increasing doses of ACEi in association with other anti-hypertensive drugs, if necessary. In these refractory patients and in case of severe renal failure, dialysis should be considered, and if the condition does not reverse despite aggressive treatment, eligible patients should be considered for renal transplantation (usually up to 2 years as suggested by evidence and experts, as recovery of intrinsic renal function after SRC may occur during a period of 1–3 years) (44, 94). The prevention of SRC lacks consensus, but CSs and/or nephrotoxic drugs should be avoided in patients with dcSSc (95).
Prior to the late 1970s, only <10% of SRC patients survived more than 3 months and the therapeutic approach was essentially arterial hypertension control (96), peritoneal, or hemodialysis plus bilateral nephrectomy or renal transplantation, as suggested by Leroy et al. (97) to increase SRC patients’ survival rate. The ‘70s could be named the “captopril era”’, as first reports demonstrated the beneficial effect of ACEi in SRC in terms of prevention of the renal deterioration, control of BP, and survival prolongation (98, 99). This was prospectively confirmed in larger population of SRC patients followed between 1972 and 1987, showing a fall in mortality rate 76% to less than 15% at 1 year and from 66% to 10% at 5 years. Successful discontinuation of dialysis was possible in 55% of ACEi-treated patients, with older age and congestive heart failure being significantly associated with poor outcome (42). Within a large dcSSc population including 145 SRC, Steen and Medsger demonstrated that 61% of SRC patients with renal crisis had good outcomes at 5 and 10 years, with few patients receiving temporary dialysis and more than half discontinuing it in 3–18 months; these data led to a conclusive message encouraging the prosecution of taking ACEi even after beginning dialysis, aiming at its discontinuation (48).
Early SRC diagnosis and aggressive ACEi use were demonstrated to prevent or even reverse renal failure, reducing SRC deaths from 42% to 6% of SSc-related deaths and making 5-year cumulative survival equivalent to that of patients with dcSSc without SRC in case of good SRC outcome (100).
Results in favor of immediate ACEi use also come from the studies of Loïc Guillevin et al. (38) though confirming elevated early mortality rate. Their results hypothesized that ACEi prescription for patients with early dcSSc might contribute to containing SRC occurrence, confirming the pivotal importance of prompt anti-hypertensive treatment to achieve BP reduction of 10–20 mmHg systolic pressure per 24 h and stressing the need for establishing new therapeutic agents supporting ACEi in steroid-treated patients (43). Regarding the prophylactic ACEi use, they showed no beneficial effect against the development of SRC (43). The International Scleroderma Renal Crisis Study Investigators (ISRCS) group investigated on the prophylactic use of ACEi: 21% of 75 SRC cases were exposed to low dose with ACEi prior the onset of SRC regardless the indication (Raynaud’s phenomenon, systemic hypertension, chronic renal insufficiency, and prophylaxis because of concurrent CS use, but none of the subjects were on ACEi as proper prophylaxis for SRC) (22). The authors concluded that exposure to ACEi prior to the onset of SRC was associated with worse outcomes, in agreement with retrospective data from previous studies (43, 44), and that it was associated with less than two-fold increase in death at 1 year after the SRC onset, especially in patients taking prednisone (for every milligram of daily prednisone, the risk of death increased by 4%). Moreover, the rates of mortality and dialysis were similar in subjects exposed and not exposed to ACE inhibitors prior to the onset of SRC, as previously shown (38).
In light of all these data, European League Against Rheumatism (EULAR) has been able to draw up evidence-based C-level recommendation on the use of ACEi, suggesting immediate initiation of high doses to improve SRC outcome and their continuation as long as there is chance for renal functional recovery (101). The experts also highlighted that published evidence does not support the preventive use of ACEi to decrease risk of development or to improve outcome of SRC (101).
In search for additional improvement in kidney function, therapeutic advances for other SSc vascular complications have been translated to SRC treatment; the rational use of ERA (Endothelin-1 receptor antagonists) in SRC comes from the experimental data on the endothelin system in renal physiology (102): endogenous renal ET modulates and regulates renal sodium and water excretion, blood flow, mesangial contraction, podocyte function, and acid/base handling, all relevant in CKD (103, 104). Different physiological and pathological factors can increase renal ET-1 production, thus promoting renal injury, inflammation, and fibrosis (105). Preliminary data from a pilot, open-label safety study by Penn et al. (17) measuring ET-1 serum levels in 20 healthy controls and 80 SSc with or without SRC and performing renal biopsies in the 27 SRC patients, showed ET-1 and both ETA and ETB receptors expression in glomeruli, interstitium, and hallmark vascular lesions of SRC. The overexpression of ET-1 in podocytes and arterioles, in addition to other known pathological findings of SRC, was also reported by Izzedine et al. in a short case report, stressing that ET-1 may be considered a therapeutic target of this severe vascular injury, as it is implicated in the development of vascular smooth muscle cells in patients with SRC (116). Despite the encouraging preclinical studies (106-109) and the initial enthusiasm supporting ERA treatment in those cases with renal disease progression under hypertensive as well as normotensive conditions, some trials were discontinued and few clinical studies had relatively inconclusive or unavailable results, thus requiring more data (including access to unpublished), more translational research, as well as outcome studies with approachable and defined clinical endpoints (109). Data from the interventional open-label ScS-REINBO trial (NCT01241383), exploring safety and efficacy of Bosentan on renal function in patients with SRC at 6 and 12 months, have been presented during the 2017 Meeting of the American College of Rheumatology. Despite the expectations, it was not possible to draw conclusions regarding kidney and patients’ survival. Bosentan was well tolerated, and no serious adverse effects requiring drug withdrawal was observed (105). A well-designed study named ZEBRA (phase II, randomized and placebo-controlled; NCT02047708) is ongoing to assess safety, tolerability, and efficacy of adding Zibotentan (a selective ETA antagonist) to conventional treatments in adult patients with renal disease secondary to SSc. This three-part study involves patients with mild or moderate kidney disease (ZEBRA-1), patients who experienced SRC not requiring dialysis (ZEBRA-2A), and patients who had SRC and are on dialysis (ZEBRA-3). Soluble vascular cell adhesion molecule-1 (sVCAM1) is also tested as biomarker of renal involvement in scleroderma in the three patient cohorts (110).
SRC is often treatment controlled, although up to 50% of patients get to end-stage renal disease (ESRD) and may still require long-term peritoneal dialysis, hemodialysis, and/or transplantation. Cozzi et al. reported that 55% of SRC patients developed ESRD after 1 year on ACEi. The survival rate was 70% and 50% at 1 and 5 years, respectively, increasing to 90% at 1 year and 70% at 5 years in a subgroup of patients treated with plasma exchange in addition to ACEi because of concomitant MAHA or intolerance to high doses of ACEi (111). Chronic dialysis condition engraves on mortality rate in SRC (48), in particular for longer disease duration and older ages (22), with higher impact than kidney transplantation (112). Cumulating hemodialysis and transplantation, however, patients’ survival was shown to be worse than overall dialysis and transplanted non-SSc population, with 20% 5 years of survival in Europe and 33% 3 years of survival in the United States (113, 114). Conversely, considering only kidney transplantation in a French multicenter study, patient survival was 100%, 90.3%, and 82.5% in 1, 3, and 5 years, respectively, while death-censored graft survival was 97.2% after 1 and 3 years and 92.8% after 5 years. Interestingly, cardiac and gastrointestinal involvements worsened after kidney transplantation in 45% and 26% of cases, while pulmonary involvement was an independent risk factor for post-transplantation death (115).
Conclusion
Although there are different possible SSc-related kidney complications, the majority of pathogenetic and treatment-oriented studies focus on SRC, obviously given its huge impact on patient morbidity and mortality. Once the treatment of this threatening condition will be managed comprehensively and optimally, treatment of chronic renal function impairment and the added value of kidney Doppler ultrasound should be the main research area on this topic, as having high impact on the SSc population and promising role as outcome measure.
Footnotes
Disclosures: Financial support: No grants or funding have been received for this study.
Conflict of interest: None of the authors has financial interest related to this study to disclose.
References
- 1. Muangchan C, Canadian Scleroderma Research G, Baron M, et al. The 15% rule in scleroderma: the frequency of severe organ complications in systemic sclerosis: a systematic review. J Rheumatol. 2013;40(9):1545-1556. [DOI] [PubMed] [Google Scholar]
- 2. Shanmugam VK, Steen VD. Renal manifestations in scleroderma: evidence for subclinical renal disease as a marker of vasculopathy. Int J Rheumatol. Epub ahead of print 14 May 2010. DOI: 10.1155/2010/538589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Steen VD, Syzd A, Johnson JP, et al. Kidney disease other than renal crisis in patients with diffuse scleroderma. J Rheumatol. 2005;32(4):649-655. [PubMed] [Google Scholar]
- 4. Steen VD. Kidney involvement in systemic sclerosis. Presse Med. 2014;43(10 Pt. 2):e305-114. [DOI] [PubMed] [Google Scholar]
- 5. Cannon PJ, Hassar M, Case DB, et al. The relationship of hypertension and renal failure in scleroderma (progressive systemic sclerosis) to structural and functional abnormalities of the renal cortical circulation. Med (Baltimore). 1974;53(1):1-46. [DOI] [PubMed] [Google Scholar]
- 6. Wu SJ, Soulez M, Yang YH, et al. Local augmented angiotensinogen secreted from apoptotic vascular endothelial cells is a vital mediator of vascular remodelling. PLoS ONE. 2015;10(7):e0132583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kranzhofer R, Schmidt J, Pfeiffer CA, et al. Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arterioscler Thromb Vas Biol. 1999;19(7):1623-1629. [DOI] [PubMed] [Google Scholar]
- 8. Soulez M, Pilon EA, Dieude M, et al. The perlecan fragment LG3 is a novel regulator of obliterative remodeling associated with allograft vascular rejection. Circ Res. 2012;110(1):94-104. [DOI] [PubMed] [Google Scholar]
- 9. Turk M, Pope JE. The frequency of scleroderma renal crisis over time: a meta analysis. J Rheumatol. 2016;43(7):1350-1355. [DOI] [PubMed] [Google Scholar]
- 10. Guillevin L, Mouthon L. Scleroderma renal crisis. Rheum Dis Clin N Am. 2015;41(3):475-488. [DOI] [PubMed] [Google Scholar]
- 11. Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988;332(6163):411-415. [DOI] [PubMed] [Google Scholar]
- 12. Dhaun N, MacIntyre IM, Bellamy CO, et al. Endothelin receptor antagonism and renin inhibition as treatment options for scleroderma kidney. Am J Kidney Dis. 2009;54(4):726-731. [DOI] [PubMed] [Google Scholar]
- 13. Kahaleh MB. Endothelin, an endothelial-dependent vasoconstrictor in scleroderma: enhanced production and profibrotic action. Arthritis Rheum. 1991;34(8):978-983. [DOI] [PubMed] [Google Scholar]
- 14. Vancheeswaran R, Magoulas T, Efrat G, et al. Circulating endothelin-1 levels in systemic sclerosis subsets–a marker of fibrosis or vascular dysfunction? J Rheumatol. 1994;21(10):1838-1844. [PubMed] [Google Scholar]
- 15. Kobayashi H, Nishimaki T, Kaise S, et al. Immunohistological study endothelin-1 and endothelin-A and B receptors in two patients with scleroderma renal crisis. Clin Rheumatol. 1999;18(5):425-427. [DOI] [PubMed] [Google Scholar]
- 16. Mouthon L, Bussone G, Berezne A, et al. Scleroderma renal crisis. J Rheumatol. 2014;41(6):1040-1048. [DOI] [PubMed] [Google Scholar]
- 17. Penn H, Quillinan N, Khan K, et al. Targeting the endothelin axis in scleroderma renal crisis: rationale and feasibility. QJM. 2013;106(9):839-848. [DOI] [PubMed] [Google Scholar]
- 18. Ghossein C, Varga J, Fenves AZ. Recent developments in the classification, evaluation, pathophysiology, and management of scleroderma renal crisis. Curr Rheumatol Rep. 2016;18(1):5. [DOI] [PubMed] [Google Scholar]
- 19. Becker MO, Kill A, Kutsche M, et al. Vascular receptor autoantibodies in pulmonary arterial hypertension associated with systemic sclerosis. Am J Resp Crit Care Med. 2014;190(7):808-817. [DOI] [PubMed] [Google Scholar]
- 20. Meyer O, De Chaisemartin L, Nicaise-Roland P, et al. Anti-RNA polymerase III antibody prevalence and associated clinical manifestations in a large series of French patients with systemic sclerosis: a cross-sectional study. J Rheumatol. 2010;37(1):125-130. [DOI] [PubMed] [Google Scholar]
- 21. Codullo V, Cavazzana I, Bonino C, et al. Serologic profile and mortality rates of scleroderma renal crisis in Italy. J Rheumatol. 2009;36(7):1464-1469. [DOI] [PubMed] [Google Scholar]
- 22. Hudson M, Baron M, Tatibouet S, et al. Exposure to ACE inhibitors prior to the onset of scleroderma renal crisis-results from the international scleroderma renal crisis survey. Semin Arthritis Rheu. 2014;43(5):666-672. [DOI] [PubMed] [Google Scholar]
- 23. Terras S, Hartenstein H, Hoxtermann S, et al. RNA polymerase III autoantibodies may indicate renal and more severe skin involvement in systemic sclerosis. Int J Dermatol. 2016;55(8):882-885. [DOI] [PubMed] [Google Scholar]
- 24. Nikpour M, Hissaria P, Byron J, et al. Prevalence, correlates and clinical usefulness of antibodies to RNA polymerase III in systemic sclerosis: a cross-sectional analysis of data from an Australian cohort. Arthritis Res Ther. 2011;13(6):R211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sobanski V, Dauchet L, Lefevre G, et al. Prevalence of anti-RNA polymerase III antibodies in systemic sclerosis: new data from a French cohort and a systematic review and meta-analysis. Arthritis Rheumatol. 2014;66(2):407-417. [DOI] [PubMed] [Google Scholar]
- 26. Woodworth TG, Suliman YA, Furst DE, et al. Scleroderma renal crisis and renal involvement in systemic sclerosis. Nat Rev Nephrol. 2016;12(11):678-691. [DOI] [PubMed] [Google Scholar]
- 27. Agarwal SK. The genetics of systemic sclerosis. Discov Med. 2010;10(51):134-143. [PMC free article] [PubMed] [Google Scholar]
- 28. Guggino G, Lo Pizzo M, Di Liberto D, et al. Interleukin-9 over-expression and T helper 9 polarization in systemic sclerosis patients. Clin Exp Immunol. 2017;190(2):208-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rossi FW, Napolitano F, Pesapane A, et al. Upregulation of the N-formyl Peptide receptors in scleroderma fibroblasts fosters the switch to myofibroblasts. J Immunol. 2015;194(11):5161-5173. [DOI] [PubMed] [Google Scholar]
- 30. Grande MT, Sanchez-Laorden B, Lopez-Blau C, et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med. 2015;21(9):989-997. [DOI] [PubMed] [Google Scholar]
- 31. Takahashi T, Asano Y, Noda S, et al. A possible contribution of lipocalin-2 to the development of dermal fibrosis, pulmonary vascular involvement and renal dysfunction in systemic sclerosis. Brit J Dermatol. 2015;173(3):681-689. [DOI] [PubMed] [Google Scholar]
- 32. Yang J, Lin SC, Chen G, et al. Adiponectin promotes monocyte-to-fibroblast transition in renal fibrosis. J Am Soc Nephrol 2013;24(10):1644-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nunokawa T, Akazawa M, Yokogawa N, et al. Late-onset scleroderma renal crisis induced by tacrolimus and prednisolone: a case report. Am J Ther. 2014;21(5):e130-113. [DOI] [PubMed] [Google Scholar]
- 34. Rodrigues-Diez R, Gonzalez-Guerrero C, Ocana-Salceda C, et al. Calcineurin inhibitors cyclosporine A and tacrolimus induce vascular inflammation and endothelial activation through TLR4 signaling. Sci Rep. Epub ahead of print 13 June 2016. DOI: 10.1038/srep27915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Steen VD, Medsger TA., Jr. Case-control study of corticosteroids and other drugs that either precipitate or protect from the development of scleroderma renal crisis. Arthritis Rheum. 1998;41(9):1613-1619. [DOI] [PubMed] [Google Scholar]
- 36. Trang G, Steele R, Baron M, et al. Corticosteroids and the risk of scleroderma renal crisis: a systematic review. Rheumatol Int. 2012;32(3):645-653. [DOI] [PubMed] [Google Scholar]
- 37. Toescu SM, Mansell A, Dinneen E, et al. Steroid-induced scleroderma renal crisis in an at-risk patient. BMJ Case Rep. Epub ahead of print 13 November 2014. DOI: 10.1136/bcr-2014-206675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guillevin L, Berezne A, Seror R, et al. Scleroderma renal crisis: a retrospective multicentre study on 91 patients and 427 controls. Rheumatology (Oxford). 2012;51(3):460-467. [DOI] [PubMed] [Google Scholar]
- 39. Bryan C, Howard Y, Brennan P, et al. Survival following the onset of scleroderma: results from a retrospective inception cohort study of the UK patient population. Brit J Rheumatol. 1996;35(11):1122-1126. [DOI] [PubMed] [Google Scholar]
- 40. Bunn CC, Denton CP, Shi-Wen X, et al. Anti-RNA polymerases and other autoantibody specificities in systemic sclerosis. Brit J Rheumatol. 1998;37(1):15-20. [DOI] [PubMed] [Google Scholar]
- 41. DeMarco PJ, Weisman MH, Seibold JR, et al. Predictors and outcomes of scleroderma renal crisis: the high-dose versus low-dose D-penicillamine in early diffuse systemic sclerosis trial. Arthritis Rheum. 2002;46(11):2983-2989. [DOI] [PubMed] [Google Scholar]
- 42. Steen VD, Costantino JP, Shapiro AP, et al. Outcome of renal crisis in systemic sclerosis: relation to availability of angiotensin converting enzyme (ACE) inhibitors. Ann Intern Med. 1990;113(5):352-357. [DOI] [PubMed] [Google Scholar]
- 43. Teixeira L, Mouthon L, Mahr A, et al. Mortality and risk factors of scleroderma renal crisis: a French retrospective study of 50 patients. Ann Rheum Dis. 2008;67(1):110-116. [DOI] [PubMed] [Google Scholar]
- 44. Penn H, Howie AJ, Kingdon EJ, et al. Scleroderma renal crisis: patient characteristics and long-term outcomes. QJM. 2007;100(8):485-494. [DOI] [PubMed] [Google Scholar]
- 45. Avouac J, Walker U, Tyndall A, et al. Characteristics of joint involvement and relationships with systemic inflammation in systemic sclerosis: results from the EULAR scleroderma trial and research group (EUSTAR) database. J Rheumatol. 2010;37(7):1488-1501. [DOI] [PubMed] [Google Scholar]
- 46. Lynch BM, Stern EP, Ong V, et al. UK scleroderma study group (UKSSG) guidelines on the diagnosis and management of scleroderma renal crisis. Clin Exp Rheumatol. 2016;34(Suppl. 100(5)):106-109. [PubMed] [Google Scholar]
- 47. Traub YM, Shapiro AP, Rodnan GP, et al. Hypertension and renal failure (scleroderma renal crisis) in progressive systemic sclerosis: review of a 25-year experience with 68 cases. Med (Baltimore). 1983;62(6):335-352. [DOI] [PubMed] [Google Scholar]
- 48. Steen VD, Medsger TA, Jr. Long-term outcomes of scleroderma renal crisis. Ann Intern Med. 2000;133(8):600-603. [DOI] [PubMed] [Google Scholar]
- 49. Steen VD, Mayes MD, Merkel PA. Assessment of kidney involvement. Clin Exp Rheumatol. 2003;21(3 Suppl. 29): S29-31. [PubMed] [Google Scholar]
- 50. Helfrich DJ, Banner B, Steen VD, et al. Normotensive renal failure in systemic sclerosis. Arthritis Rheum. 1989;32(9):1128-1134. [DOI] [PubMed] [Google Scholar]
- 51. Penn H, Denton CP. Diagnosis, management and prevention of scleroderma renal disease. Curr Opin Rheumatol. 2008;20(6):692-696. [DOI] [PubMed] [Google Scholar]
- 52. Molina JF, Anaya JM, Cabrera GE, et al. Systemic sclerosis sine scleroderma: an unusual presentation in scleroderma renal crisis. J Rheumatol. 1995;22(3):557-560. [PubMed] [Google Scholar]
- 53. Gonzalez EA, Schmulbach E, Bastani B. Scleroderma renal crisis with minimal skin involvement and no serologic evidence of systemic sclerosis. Am J Kidney Dis. 1994;23(2):317-319. [DOI] [PubMed] [Google Scholar]
- 54. Mimura I, Hori Y, Matsukawa T, et al. Noncrescentic ANCA-associated renal crisis in systemic sclerosis. Clin Nephrol. 2008;70(2):183-185. [DOI] [PubMed] [Google Scholar]
- 55. Endo H, Hosono T, Kondo H. Antineutrophil cytoplasmic autoantibodies in 6 patients with renal failure and systemic sclerosis. J Rheumatol. 1994;21(5):864-870. [PubMed] [Google Scholar]
- 56. Wutzl AL, Foley RN, O’Driscoll BR, et al. Microscopic polyangiitis presenting as pulmonary-renal syndrome in a patient with long-standing diffuse cutaneous systemic sclerosis and antibodies to myeloperoxidase. Arthritis Rheum. 2001;45(6):533-536. [DOI] [PubMed] [Google Scholar]
- 57. Kingdon EJ, Knight CJ, Dustan K, et al. Calculated glomerular filtration rate is a useful screening tool to identify scleroderma patients with renal impairment. Rheumatology (Oxford). 2003;42(1):26-33. [DOI] [PubMed] [Google Scholar]
- 58. Amin A, El-Sayed S, Taher N, et al. Tc-99m diethylenetriamine pentaacetic acid (DTPA) renal function reserve estimation: is it a reliable predictive tool for assessment of preclinical renal involvement in scleroderma patients? Clin Rheumatol. 2012;31(6):961-966. [DOI] [PubMed] [Google Scholar]
- 59. Livi R, Teghini L, Pignone A, et al. Renal functional reserve is impaired in patients with systemic sclerosis without clinical signs of kidney involvement. Ann Rheum Dis. 2002;61(8):682-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Livi R, Guiducci S, Perfetto F, et al. Lack of activation of renal functional reserve predicts the risk of significant renal involvement in systemic sclerosis. Ann Rheum Dis. 2011;70(11):1963-1967. [DOI] [PubMed] [Google Scholar]
- 61. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296-1305. [DOI] [PubMed] [Google Scholar]
- 62. Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110(1):32-35. [DOI] [PubMed] [Google Scholar]
- 63. Seiberlich B, Hunzelmann N, Krieg T, et al. Intermediate molecular weight proteinuria and albuminuria identify scleroderma patients with increased morbidity. Clin Nephrol. 2008;70(2):110-117. [DOI] [PubMed] [Google Scholar]
- 64. Veglio F, Frascisco M, Melchio R, et al. Assessment of renal resistance index after captopril test by Doppler in essential and renovascular hypertension. Kidney Int. 1995;48(5):1611-1616. [DOI] [PubMed] [Google Scholar]
- 65. Knapp R, Plotzeneder A, Frauscher F, et al. Variability of Doppler parameters in the healthy kidney: an anatomic-physiologic correlation. J Ultras Med. 1995;14(6):427-429. [DOI] [PubMed] [Google Scholar]
- 66. Boddi M, Sacchi S, Lammel RM, et al. Age-related and vasomotor stimuli-induced changes in renal vascular resistance detected by Doppler ultrasound. Am J Hypertens. 1996;9(5):461-466. [DOI] [PubMed] [Google Scholar]
- 67. Boddi M, Natucci F, Ciani E. The internist and the renal resistive index: truths and doubts. Intern Emerg Med. 2015;10(8):893-905. [DOI] [PubMed] [Google Scholar]
- 68. Rivolta R, Mascagni B, Berruti V, et al. Renal vascular damage in systemic sclerosis patients without clinical evidence of nephropathy. Arthritis Rheum. 1996;39(6):1030-1034. [DOI] [PubMed] [Google Scholar]
- 69. Rosato E, Gigante A, Barbano B, et al. Intrarenal hemodynamic parameters correlate with glomerular filtration rate and digital microvascular damage in patients with systemic sclerosis. Semin Arthritis Rheum. 2012;41(6):815-821. [DOI] [PubMed] [Google Scholar]
- 70. Rosato E, Barbano B, Gigante A, et al. Increased intrarenal arterial stiffness may predict the occurrence of new digital ulcers in systemic sclerosis. Arthrit Care Res (Hoboken). 2014;66(9):1380-1385. [DOI] [PubMed] [Google Scholar]
- 71. Rosato E, Gigante A, Barbano B, et al. Doppler indices of intrarenal arterial stiffness are useful in monitoring scleroderma renal crisis. Scand J Rheumatol. 2013;42(1):80-81. [DOI] [PubMed] [Google Scholar]
- 72. Scorza R, Rivolta R, Mascagni B, et al. Effect of iloprost infusion on the resistance index of renal vessels of patients with systemic sclerosis. J Rheumatol. 1997;24(10):1944-1948. [PubMed] [Google Scholar]
- 73. Lester PD, Koehler PR. The renal angiographic changes in scleroderma. Radiology. 1971;99(3):517-521. [DOI] [PubMed] [Google Scholar]
- 74. Winograd J, Schimmel DH, Palubinskas AJ. The spotted nephrogram of renal scleroderma. AJR Am J Roentgenol. 1976;126(4):734-738. [DOI] [PubMed] [Google Scholar]
- 75. Yamauchi K, Arimori S. Alterations of glomerular filtration rate during cold exposure in progressive systemic sclerosis: measurement with technetium-99m DTPA. Jpn J Med. 1990;29(2):208-211. [DOI] [PubMed] [Google Scholar]
- 76. Woolfson RG, Cairns HS, Williams DJ, et al. Renal scintigraphy in acute scleroderma: report of three cases. J Nucl Med. 1993;34(7):1163-1165. [PubMed] [Google Scholar]
- 77. Urai L, Nagy Z, Szinay G, et al. Renal function in scleroderma. Brit Med J. 1958;2(5107):1264-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Clements PJ, Lachenbruch PA, Furst DE, et al. Abnormalities of renal physiology in systemic sclerosis: a prospective study with 10-year followup. Arthritis Rheum. 1994;37(1):67-74. [DOI] [PubMed] [Google Scholar]
- 79. Gigante A, Barbano B, Granata G, et al. Evaluation of estimated glomerular filtration rate and clinical variables in systemic sclerosis patients. Clin Nephrol. 2016;85(6):326-331. [DOI] [PubMed] [Google Scholar]
- 80. Gigante A, Rosato E, Massa R, et al. Evaluation of chronic kidney disease epidemiology collaboration equation to estimate glomerular filtration rate in scleroderma patients. Rheumatology (Oxford). 2012;51(8):1426-1431. [DOI] [PubMed] [Google Scholar]
- 81. Suebmee P, Foocharoen C, Mahakkanukrauh A, et al. Correlation of glomerular filtration rate between renal scan and estimation equation for patients with scleroderma. Am J Med Sci. 2016;352(2):166-171. [DOI] [PubMed] [Google Scholar]
- 82. Caron M, Hudson M, Baron M, et al. Longitudinal study of renal function in systemic sclerosis. J Rheumatol. 2012;39(9):1829-1834. [DOI] [PubMed] [Google Scholar]
- 83. Scheja A, Bartosik I, Wuttge DM, et al. Renal function is mostly preserved in patients with systemic sclerosis. Scand J Rheumatol. 2009;38(4):295-298. [DOI] [PubMed] [Google Scholar]
- 84. Lapenas D, Rodnan GP, Cavallo T. Immunopathology of the renal vascular lesion of progressive systemic sclerosis (scleroderma). Am J Pathol. 1978;91(2):243-258. [PMC free article] [PubMed] [Google Scholar]
- 85. Trostle DC, Bedetti CD, Steen VD, et al. Renal vascular histology and morphometry in systemic sclerosis: a case-control autopsy study. Arthritis Rheum. 1988;31(3):393-400. [DOI] [PubMed] [Google Scholar]
- 86. Lusco MA, Najafian B, Alpers CE, et al. AJKD atlas of renal pathology: systemic sclerosis. Am J Kidney Dis. 2016;67(4): e19-20. [DOI] [PubMed] [Google Scholar]
- 87. Penn H, Howie AJ, Stratton RJ, et al. The prognostic value of renal biopsy in scleroderma renal crisis. 2007, Available at: https://acr.confex.com/acr/2007/webprogram/Paper7446.html
- 88. Batal I, Domsic RT, Shafer A, et al. Renal biopsy findings predicting outcome in scleroderma renal crisis. Hum Pathol. 2009;40(3):332-340. [DOI] [PubMed] [Google Scholar]
- 89. Habeeb RA, Mansour HE, Abdeldayem AM, et al. Anti-Annexin v antibodies: association with vascular involvement and disease outcome in patients with systemic sclerosis. Clin Med Insights Arthritis Musculoskelet Disord. 2010;3:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gigante A, Barbano B, Barilaro G, et al. Serum uric acid as a marker of microvascular damage in systemic sclerosis patients. Microvasc Res. 2016;106:39-43. [DOI] [PubMed] [Google Scholar]
- 91. Chiba M, Yanaba K, Hayashi M, et al. Clinical significance of serum soluble T-cell immunoglobulin and mucin domain 3 levels in systemic sclerosis: association with disease severity. J Dermatol. 2017;44(2):194-197. [DOI] [PubMed] [Google Scholar]
- 92. Chighizola CB, Pregnolato F, Meroni PL, et al. N-terminal pro brain natriuretic peptide as predictor of outcome in scleroderma renal crisis. Clin Exp Rheumatol. 2016;34(Suppl. 100(5)):122-128. [PubMed] [Google Scholar]
- 93. Charles C, Clements P, Furst DE. Systemic sclerosis: hypothesis-driven treatment strategies. Lancet. 2006;367(9523):1683-1691. [DOI] [PubMed] [Google Scholar]
- 94. Denton CP, Lapadula G, Mouthon L, et al. Renal complications and scleroderma renal crisis. Rheumatology (Oxford). 2009;48(Suppl. 3): iii32-35. [DOI] [PubMed] [Google Scholar]
- 95. Bussone G, Berezne A, Pestre V, et al. The scleroderma kidney: progress in risk factors, therapy, and prevention. Curr Rheumatol Rep. 2011;13(1):37-43. [DOI] [PubMed] [Google Scholar]
- 96. Mitnick PD, Feig PU. Control of hypertension and reversal of renal failure in scleroderma. N Engl J Med. 1978;299(16):871-872. [DOI] [PubMed] [Google Scholar]
- 97. LeRoy EC, Fleischmann RM. The management of renal scleroderma: experience with dialysis, nephrectomy and transplantation. Am J Med. 1978;64(6):974-978. [DOI] [PubMed] [Google Scholar]
- 98. Zawada ET, Jr., Clements PJ, Furst DA, et al. Clinical course of patients with scleroderma renal crisis treated with captopril. Nephron. 1981;27(2):74-78. [DOI] [PubMed] [Google Scholar]
- 99. Whitman HH, 3rd, Case DB, Laragh JH, et al. Variable response to oral angiotensin-converting-enzyme blockade in hypertensive scleroderma patients. Arthritis Rheum. 1982;25(3):241-248. [DOI] [PubMed] [Google Scholar]
- 100. Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis. 2007;66(7):940-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kowal-Bielecka O, Fransen J, Avouac J, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis. 2017;76(8):1327-1339. [DOI] [PubMed] [Google Scholar]
- 102. Kohan DE, Barton M. Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int. 2014;86(5):896-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kohan DE, Rossi NF, Inscho EW, et al. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev. 2011;91(1):1-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Stuart D, Chapman M, Rees S, et al. Myocardial, smooth muscle, nephron, and collecting duct gene targeting reveals the organ sites of endothelin A receptor antagonist fluid retention. J Pharmacol Exp Ther. 2013;346(2):182-189. [DOI] [PubMed] [Google Scholar]
- 105. Bérezné AAH, Karras A, Marie I, et al. Bosentan in scleroderma renal crisis: a national open label prospective study [abstract]. San Diego, CA: Annual Meeting of the American College of Rheumatology; 2007. [Google Scholar]
- 106. Benigni A, Zola C, Corna D, et al. Blocking both type A and B endothelin receptors in the kidney attenuates renal injury and prolongs survival in rats with remnant kidney. Am J Kidney Dis. 1996;27(3):416-423. [DOI] [PubMed] [Google Scholar]
- 107. Benigni A, Perico N, Remuzzi G. The potential of endothelin antagonism as a therapeutic approach. Expert Opin Inv Drug. 2004;13(11):1419-1435. [DOI] [PubMed] [Google Scholar]
- 108. Nakamura T, Ebihara I, Fukui M, et al. Effect of a specific endothelin receptor A antagonist on mRNA levels for extracellular matrix components and growth factors in diabetic glomeruli. Diabetes. 1995;44(8):895-899. [DOI] [PubMed] [Google Scholar]
- 109. Kelland NF, Webb DJ. Clinical trials of endothelin antagonists in heart failure: publication is good for the public health. Heart. 2007;93(1):2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. https://clinicaltrials.gov/ct2/show/NCT02047708
- 111. Cozzi F, Marson P, Cardarelli S, et al. Prognosis of scleroderma renal crisis: a long-term observational study. Nephrol Dial Transpl. 2012;27(12):4398-4403. [DOI] [PubMed] [Google Scholar]
- 112. Gibney EM, Parikh CR, Jani A, et al. Kidney transplantation for systemic sclerosis improves survival and may modulate disease activity. Am J Transpl. 2004;4(12):2027-2031. [DOI] [PubMed] [Google Scholar]
- 113. Hesselstrand R, Scheja A, Wuttge DM. Scleroderma renal crisis in a Swedish systemic sclerosis cohort: survival, renal outcome, and RNA polymerase III antibodies as a risk factor. Scand J Rheumatol. 2012;41(1):39-43. [DOI] [PubMed] [Google Scholar]
- 114. Batal I, Domsic RT, Medsger TA, et al. Scleroderma renal crisis: a pathology perspective. Int J Rheumatol. Epub ahead of print 28 July 2010. DOI: 10.1155/2010/543704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bertrand D, Dehay J, Ott J, et al. Kidney transplantation in patients with systemic sclerosis: a nationwide multicentre study. Transpl Int. 2017;30(3):256-265. [DOI] [PubMed] [Google Scholar]
- 116. Izzedine H, Rouvier P, Deray G. Endothelin receptor antagonism-based treatment for scleroderma renal crisis. Am J Kidney Dis. 2013;62(2):394-395. [DOI] [PubMed] [Google Scholar]


