Abstract
Opsins, the protein moieties of animal visual photo-pigments, have emerged as moonlighting proteins with diverse, light-dependent and -independent physiological functions. This raises the need to revise some basic assumptions concerning opsin expression, structure, classification, and evolution.
Keywords: sensory transduction, photosensitivity, vision, moonlighting proteins, G protein-coupled receptors, sensory cell evolution
Introduction: Expanded Functions of Opsins
Opsins are G protein-coupled receptors (GPCRs) that mediate animal vision (Terakita 2005). Their light-sensitivity relies on a covalently bound visual chromophore—usually 11-cis-retinal—that isomerizes upon photon absorption. Besides initiating visual phototransduction, opsins serve nonvisual light-dependent roles in various tissues including the skin and the brain, contributing to processes such as pigmentation changes, avoidance behaviors, hair growth, gonad maturation, and the entrainment of circadian rhythms (Leung and Montell 2017).
Over the past decade, various additional functions have been ascribed to opsins that are light-independent. For example, opsins are expressed in vertebrate taste buds (Sukumaran et al. 2017) and mechanosensory neuromasts of the lateral line (Backfisch et al. 2013; Baker et al. 2015), and they contribute to sperm thermotaxis (Pérez-Cerezales et al. 2015). In the marine bristleworm Platyneries, opsins are expressed in cells with both photo- and mechanosensory signatures (Revilla-I-Domingo et al. 2021), whereas in Drosophila, opsins are involved in thermal behaviors (Shen et al. 2011), hearing (Senthilan et al. 2012), proprioception (Zanini et al. 2018), and taste sensation (Leung et al. 2020). In Drosophila thermoreceptor cells, opsins, though thermostable, might act as thermo-activated receptor proteins (Shen et al. 2011; Leung and Montell 2017), and, in taste transduction, opsins seem to function as chemically activated gustatory receptor proteins (Leung et al. 2020). The latter chemosensory function is independent of chromophore-binding. Instead, bitter compounds can activate opsins, indicating that besides photosensitive chromophores, opsins can have other ligands.
In addition to contributing to sensory stimulus transduction, opsins also serve housekeeping functions within cells. For example, unconjugated opsins, without a chromophore, contribute to the maintenance of mechanosensory cilia in Drosophila mechanoreceptors; within these cells, opsins localize to the base of the cilia, and, without opsins, these cilia degenerate (Zanini et al. 2018). This opsin-dependence of mechanosensory cilia is remarkable given that it involves nonciliary, rhabdomeric opsins. Opsins can be subdivided into main subfamilies (Terakita 2005; Feuda et al. 2012; Ramirez et al. 2016), that, besides the placozoan-specific placopsins (Feuda et al. 2012), traditionally include the rhabdomeric (R) opsins, the ciliary (C) opsins, and the Go-coupled plus retinochrome, retinal G protein-coupled receptor (Go/RGR) opsins (fig. 1) (for additional discussion, see Ramirez et al. [2016]). Ciliary photoreceptors typically use C opsins, and rhabdomeric photoreceptors R opsins, so the R opsin-dependence of mechanosensory cilia seems unconventional in more than one sense. Opsins can also scramble phospholipids, that is, they act as lipid transport proteins to facilitate rapid, bidirectional movement of lipids from one side of the membrane to the opposing side (Menon et al. 2011), presumably contributing to homeostasis of the disc membranes of mammalian rod photoreceptor cells (Ernst and Menon 2015). The scramblase activity, which opsins share with other rhodopsin-class GPCRs such as the β1- and β2-adrenergic receptors, is particular in that it is ATP-independent and requires neither light nor chromophore-binding (Goren et al. 2014). Finally, opsins regulate melanin levels in skin melanocytes in a light-independent manner (Ozdeslik et al. 2019) and they also serve as trafficking guides for other proteins, physically binding—and acting as a vehicle for—guanylate cyclase 1, the second messenger-generating enzyme of the vertebrate phototransduction cascade (Pearring et al. 2015).
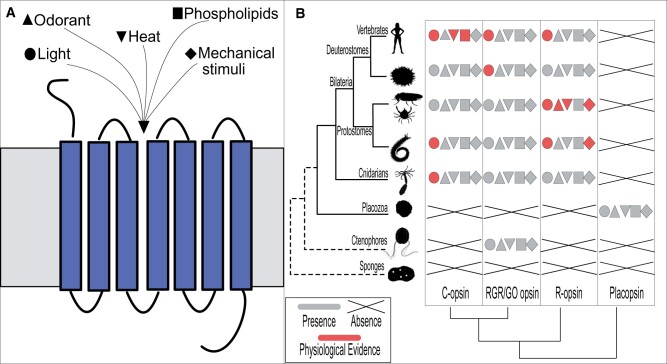
Fig. 1.
Synopsis of the functional diversity of opsins. (A) Schematic representation of functional diversity of opsins. Opsin is depicted generically as a polytopic membrane protein with seven transmembrane spans, in the context of a membrane bilayer. (B) Phylogenetic distribution of main metazoan opsin paralogs (according to Feuda et al. [2012]) and their respective functions. The species tree reflects that reported by Feuda et al. (2017), and the dashed lines indicate the uncertainty in the phylogenetic position of ctenophores (Feuda et al. 2017, Li et al. 2021). Symbols as in panel (A). Echinoderm R-opsins are expressed in photoreceptors (Ullrich-Lüter et al. 2011), yet physiological evidence implicating them in light detection has not been reported, so the respective symbol is left gray.
Given these diverse functions, opsins can no longer be considered exclusively as molecular light sensors—they are moonlighting proteins (Jeffery 2014, 2018) that, analogous to moonlighting people, have several, apparently unrelated jobs. Here we argue that this expanded functional diversity challenges some basic assumptions concerning opsins, five of which are spotlighted here.
Opsin Presence Does Not Necessarily Imply Photosensitivity
Opsin expression alone does not suffice to delineate photosensitive cells. Especially when opsins occur in cells outside eyes, establishing photosensitivity requires physiological studies demonstrating cellular light-responses electrophysiologically or by functional imaging, whereas gene expression and cell morphology can only provide hints. A striking example of this is the expression of opsin genes in sound receptors of the antennal hearing organ of Drosophila. Initially, it seemed reasonable to assume that these sound receptors use opsins to detect light—a notion that seems supported by the presence of additional components of the phototransduction machinery in these cells (Senthilan et al. 2012), along with visual cycle proteins (Katana et al. 2019). Multimodal cells that respond to both mechanical stimuli and light occur in Drosophila (Xiang et al. 2010), yet the fly’s sound-receptors turned out to be light-unresponsive, requiring opsins to transduce sound stimuli (Senthilan et al. 2012). Genetic manipulations showed that mechanosensory opsin function does not require chromophore-binding (Katana et al. 2019) and that opsins, rather that functioning directly in mechanosensory stimulus transduction, have structural roles, contributing to mechanoreceptor cell integrity (Zanini et al. 2018). Clearly, many cells use opsins for photon-detection, yet there are exceptions that must be kept in mind when inferring cellular functions from the presence of opsins.
There May Be No Visual and Nonvisual Opsins
Opsins are commonly categorized as visual and nonvisual, depending on whether they contribute to retinal image formation. Functional data now suggest that this dichotomy is far from accurate. In Drosophila, opsins that mediate vision also have thermo-, mechano-, and chemosensory roles, documenting nonvisual functions for “visual opsins” (Leung and Montell 2017). Drosophila Rh1 for example, the major visual opsin of the fly’s compound eye, contributes to the proprioceptive control of locomotion (Zanini et al. 2018) and the sensation of temperature (Shen et al. 2011) and taste (Leung et al. 2020). This convergence of visual and nonvisual functions on single opsins seems not to be insect-specific. One of the three opsins contributing to human color vision, the short-wavelength-sensitive cone opsin OPN1-SW, occurs in epidermal cells of the skin where it presumably serves nonvisual functions (Haltaufderhyde et al. 2015). The same applies to the human opsin OPN2 that, besides mediating dim-light photoreception in retinal rods, is expressed in the skin, in melano- and keratinocytes (Haltaufderhyde et al. 2015). Even the photoreceptors in the mammalian retina not only serve retinal image formation. In addition, rods and cones contribute to the pupillary reflex and circadian light entrainment, together with retinal ganglion cells that are intrinsically photosensitive, expressing the R opsin melanopsin (OPN4) (Hattar et al. 2002, 2003). Vertebrate melanopsins seems to serve nonvisual functions only, whereas vision in invertebrates such as insects and cephalopods relies on melanopsin-related R opsins (Koyanagi and Terakita 2008). Obviously, the strict dichtonomy between visual and nonvisual opsins does not reflect the complex functions of opsins and their cooption by different types of cells in different organisms. In praxis, opsins serving vision may be referred to as “visual opsins,” yet when doing so one should not forget that additional functions might exist that are equally important for the organisms.
Opsin Structures Might Reflect Nonlight-Sensing Demands
Like other GPCRs, opsins are heptahelical proteins. Their seventh transmembrane domain (TM7) usually contains a conserved lysine-residue that binds the retinal chromophore via a Schiff-base linkage (Terakita 2005). The structural basis of opsin photo-activation has been studied extensively, with high-resolution structures and biophysical analyses providing detailed insights into light-induced conformational changes (Menon et al. 2001). As opsin function is not restricted to photon-detection, it seems reasonable to assume that additional selective pressures have left their marks on opsin structures. Phospholipid scrambling by bovine opsin, for example, involves conformational changes to TM5-7, which together form a hydrophilic cavity, or groove (Morra et al. 2018; Khelashvili and Menon 2022). With this polar groove, the protein acts like a credit card reader, allowing phospholipids to swipe their way between membrane leaflets with the headgroup moving through the groove, dragging the hydrophobic tails through the membrane core (Pomorski and Menon 2006). Deformations of the membrane that are associated with groove dilation bring together the two bilayer leaflets, additionally facilitating phospholipid flip-flop outside the groove, at the protein-membrane interface (Malvezzi et al. 2018; Khelashvili and Menon 2022). The conformational changes necessary for scrambling are unrelated to those that accompany the photoisomerization of retinal during light transduction (Morra et al. 2018; Khelashvili and Menon 2022). The chemoactivation of opsins represents another pathway for structure evolution unrelated to light-sensing. Certain tastants seem to fit into the chromophore binding pocket of opsins (Leung et al. 2020), suggesting that alternative activation mechanisms and functions must be taken into account when assessing opsin structure-function relations.
Ancestral Opsin Function Might Not Have Been the Detection of Light
Opsins evolved through duplication in stem eumetazoans, giving rise to melatonin receptors and proto-opsin genes (Feuda et al. 2012).The ancestral function of opsins is often thought to have been the detection of light (light first), even though ancestral opsins such as the placopsins lack the key lysine necessary for chromophore-binding (Feuda et al. 2012). Considering the multiple roles of opsins, it seems possible that light-independent opsin functions evolutionarily preceded light-dependent ones, (nonlight first), or that the first opsins served both light-dependent- and -independent roles (light and nonlight first). Support for a “light first” scenario comes from the extensive association between opsin genes and visual systems, with almost all metazoans using opsins for detecting light. Furthermore, light-independent functions of opsin such as those described in Drosophila rely on opsins uniquely present in Brachyceran flies, suggesting that their light-independent sensory functions are derived (Pisani et al. 2020). Residues required for phospholipid scrambling appear to be conserved across GPCRs (Morra et al. 2018; Khelashvili and Menon 2022). Accordingly, opsins presumably inherited their phospholipid scrambling activity from their GPCR ancestor, implying that the first opsins scrambled phospholipids, without (“nonlight first” scenario) or in addition to detecting light (“light and nonlight first” scenario). Even though Drosophila opsins are evolutionarily derived, it also seems possible that their light-independent sensory functions have deep evolutionary roots given the presence of opsins in vertebrate taste buds (Haltaufderhyde et al. 2015) and mechanosensory neuromasts (Backfisch et al. 2013; Baker et al. 2015). To discriminate between these three scenarios, more information will be needed about the molecular and physiological functions of opsins outside visual systems, in particular in nonbilaterian metazoans (fig. 1).
Selection Acting on Light-Independent Functions Might Have Contributed to Opsin Diversification
Since their first appearance, opsins have witnessed an almost bewildering evolutionary diversification, turning into the largest class of GPCRs with more than 1,000 members (Terakita 2005) and with several lineage-specific expansions in some taxa (Davies et al. 2015; Futahashi et al. 2015; Beaudry et al. 2017; Musilova et al. 2019). The main driving force for this diversification is thought to be the demands of light-sensing, in particular color vision (Futahashi et al. 2015; Melin et al. 2017; Musilova et al. 2019; Hauser et al. 2021; van der Kooi et al. 2021); opsins are rendered light-sensitive by chromophore-binding, yet spectral tuning is determined by the opsins themselves (Terakita 2005). Most animals use only two to four opsins for color vision, and a few classes of photoreceptor cells expressing different opsins are sufficient for encoding light in the visible spectrum (Barlow 1982; Cronin et al. 2014). This number contrasts with the large number of opsins that are found in some species (Davies et al. 2015; Futahashi et al. 2015; Beaudry et al. 2017), with the water flea Daphnia pulex holding the record of no less than 46 opsins encoded in the genome (Colbourne et al. 2011). The disparity between the numbers of opsins encoded by some genomes and those required for vision indicates that additional factors have contributed to opsin diversification. Clearly, more work is needed to decipher the range of selective pressures contributing to this evolutionary drive and its physiological consequences.
Acknowledgments
This work was supported by a Royal Society University Research Fellowship and Royal Society Grants (UF160226 and RGF\R1\181012) to R.F.; a National Institutes of Health Grant (R01 EY027969) to A.K.M.; the German Science Foundation (DFG, SFB 889 “Cellular Mechanisms of Sensory Processing,” A1, to M.C.G.); and the Multiscale Bioimaging Cluster of Excellence (MBExC), University of Göttingen (M.C.G.).
References
- Backfisch B, Veedin Rajan VB, Fischer RM, Lohs C, Arboleda E, Tessmar-Raible K, Raible F.. 2013. Stable transgenesis in the marine annelid Platynereis dumerilii sheds new light on photoreceptor evolution. Proc Natl Acad Sci U S A. 110(1):193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker GE, de Grip WJ, Turton M, Wagner HJ, Foster RG, Douglas RH.. 2015. Light sensitivity in a vertebrate mechanoreceptor? J Exp Biol. 218:2826–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB. 1982. What causes trichromacy? A theoretical analysis using comb-filtered spectra. Vision Res. 22(6):635–643. [DOI] [PubMed] [Google Scholar]
- Beaudry FEG, Iwanicki TW, Mariluz BRZ, Darnet S, Brinkmann H, Schneider P, Taylor JS.. 2017. The non-visual opsins: eighteen in the ancestor of vertebrates, astonishing increase in ray-finned fish, and loss in amniotes. J Exp Zool B Mol Dev Evol. 328(7):685–696. [DOI] [PubMed] [Google Scholar]
- Colbourne JK, Pfrender ME, Gilbert D, Thomas WK, Tucker A, Oakley TH, Tokishita S, Aerts A, Arnold GJ, Basu MK, et al. 2011. The ecoresponsive genome of Daphnia pulex. Science 331(6017):555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin TW, Johnsen S, Marshall NJ, Warrant EJ.. 2014. Visual ecology. Princeton (NJ): Princeton University Press. [Google Scholar]
- Davies WI, Tamai TK, Zheng L, Fu JK, Rihel J, Foster RG, Whitmore D, Hankins MW.. 2015. An extended family of novel vertebrate photopigments is widely expressed and displays a diversity of function. Genome Res. 25(11):1666–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst OP, Menon AK.. 2015. Phospholipid scrambling by rhodopsin. Photochem Photobiol Sci. 14(11):1922–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuda R, Dohrmann M, Pett W, Philippe H, Rota-Stabelli O, Lartillot N, Wörheide G, Pisani D.. 2017. Improved modeling of compositional heterogeneity supports sponges as sister to all other animals. Curr Biol. 27(24):3864–3870. [DOI] [PubMed] [Google Scholar]
- Feuda R, Hamilton SC, McInerney JO, Pisani D.. 2012. Metazoan opsin evolution reveals a simple route to animal vision. Proc Natl Acad Sci U S A. 109(46):18868–18872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futahashi R, Kawahara-Miki R, Kinoshita M, Yoshitake K, Yajima S, Arikawa K, Fukatsu T.. 2015. Extraordinary diversity of visual opsin genes in dragonflies. Proc Natl Acad Sci U S A. 112(11):E1247–E1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren MA, Morizumi T, Menon I, Joseph JS, Dittman JS, Cherezov V, Stevens RC, Ernst OP, Menon AK.. 2014. Constitutive phospholipid scramblase activity of a G protein-coupled receptor. Nat Commun. 5:5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltaufderhyde K, Ozdeslik RN, Wicks NL, Najera JA, Oancea E.. 2015. Opsin expression in human epidermal skin. Photochem Photobiol. 91(1):117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW.. 2002. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295(5557):1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, et al. 2003. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature 424(6944):76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser FE, Ilves KL, Schott RK, Alvi E, López-Fernández H, Chang BSW.. 2021. Evolution, inactivation and loss of short wavelength-sensitive opsin genes during the diversification of Neotropical cichlids. Mol Ecol. 30(7):1688–1703. [DOI] [PubMed] [Google Scholar]
- Jeffery CJ. 2014. An introduction to protein moonlighting. Biochem Soc Trans. 42(6):1679–1683. [DOI] [PubMed] [Google Scholar]
- Jeffery CJ. 2018. Protein moonlighting: what is it, and why is it important? Phil Trans R Soc B. 373(1738):20160523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katana R, Guan C, Zanini D, Larsen ME, Giraldo D, Geurten BRH, Schmidt CF, Britt SG, Göpfert MC.. 2019. Chromophore-independent roles of opsin apoproteins in Drosophila mechanoreceptors. Curr Biol. 29(17):2961–2969. [DOI] [PubMed] [Google Scholar]
- Khelashvili H, Menon AK.. 2022. Phospholipid scrambling by G protein-coupled receptors. Annu Rev Biophys. 51:39–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M, Terakita A.. 2008. Gq-coupled rhodopsin subfamily composed of invertebrate visual pigment and melanopsin. Photochem Photobiol. 84(4):1024–1030. [DOI] [PubMed] [Google Scholar]
- Leung NY, Montell C.. 2017. Unconventional roles of opsins. Annu Rev Cell Dev Biol. 33:241–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung NY, Thakur DP, Gurav AS, Kim SH, Di Pizio A, Niv MY, Montell C.. 2020. Functions of opsins in Drosophila taste. Curr Biol. 30(8):1367–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Shen XX, Evans B, Dunn CW, Rokas A.. 2021. Rooting the animal tree of life. Mol Biol Evol. 38(10):4322–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvezzi M, Andra KK, Pandey K, Lee BC, Falzone ME, Brown A, Iqbal R, Menon AK, Accardi A.. 2018. Out-of-the-groove transport of lipids by TMEM16 and GPCR scramblases. Proc Natl Acad Sci U S A. 115(30):E7033–E7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melin AD, Chiou KL, Walco ER, Bergstrom ML, Kawamura S, Fedigan LM.. 2017. Trichromacy increases fruit intake rates of wild capuchins (Cebus capucinus imitator). Proc Natl Acad Sci U S A. 114(39):10402–10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon I, Huber T, Sanyal S, Banerjee S, Barré P, Canis S, Warren JD, Hwa J, Sakmar TP, Menon AK.. 2011. Opsin is a phospholipid flippase. Curr Biol. 21(2):149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon ST, Han M, Sakmar TP.. 2001. Rhodopsin: structural basis of molecular physiology. Physiol Rev. 81(4):1659–1688. [DOI] [PubMed] [Google Scholar]
- Morra G, Razavi AM, Pandey K, Weinstein H, Menon AK, Khelashvili G.. 2018. Mechanisms of lipid scrambling by the G protein-coupled receptor opsin. Structure 26(2):356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musilova Z, Cortesi F, Matschiner M, Davies WIL, Patel JS, Stieb SM, de Busserolles F, Malmstrøm M, Tørresen OK, Brown CJ, et al. 2019. Vision using multiple distinct rod opsins in deep-sea fishes. Science 364(6440):588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdeslik RN, Olinski LE, Trieu MM, Oprian DD, Oancea E.. 2019. Human nonvisual opsin 3 regulates pigmentation of epidermal melanocytes through functional interaction with melanocortin 1 receptor. Proc Natl Acad Sci U S A. 116(23):11508–11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearring JN, Spencer WJ, Lieu EC, Arshavsky VY.. 2015. Guanylate cyclase 1 relies on rhodopsin for intracellular stability and ciliary trafficking. Elife 4:e12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Cerezales S, Boryshpolets S, Afanzar O, Brandis A, Nevo R, Kiss V, Eisenbach M.. 2015. Involvement of opsins in mammalian sperm thermotaxis. Sci Rep. 5(1):16146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani D, Rota-Stabelli O, Feuda R.. 2020. Sensory neuroscience: a taste for light and the origin of animal vision. Curr Biol. 30(13):R773–R775. [DOI] [PubMed] [Google Scholar]
- Pomorski T, Menon AK.. 2006. Lipid flippases and their biological functions. Cell Mol Life Sci. 63(24):2908–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez MD, Pairett AN, Pankey MS, Serb JM, Speiser DI, Swafford AJ, Oakley TH.. 2016. The last common ancestor of most bilaterian animals possessed at least nine opsins. Genome Biol Evol. 8(12):3640–36522016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revilla-I-Domingo R, Rajan VBV, Waldherr M, Prohaczka G, Musset H, Orel L, Gerrard E, Smolka M, Stockinger A, Farlik M, et al. 2021. Characterization of cephalic and non-cephalic sensory cell types provides insight into joint photo- and mechanoreceptor evolution. Elife 10:e66144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthilan PR, Piepenbrock D, Ovezmyradov G, Nadrowski B, Bechstedt S, Pauls S, Winkler M, Möbius W, Howard J, Göpfert MC.. 2012. Drosophila auditory organ genes and genetic hearing defects. Cell 150(5):1042–1054. [DOI] [PubMed] [Google Scholar]
- Shen WL, Kwon Y, Adegbola AA, Luo J, Chess A, Montell C.. 2011. Function of rhodopsin in temperature discrimination in Drosophila. Science 331(6022):1333–1336. [DOI] [PubMed] [Google Scholar]
- Sukumaran SK, Lewandowski BC, Qin Y, Kotha R, Bachmanov AA, Margolskee RF.. 2017. Whole transcriptome profiling of taste bud cells. Sci Rep. 7(1):7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terakita A. 2005. The opsins. Genome Biol. 6(3):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich-Lüter EM, Dupont S, Arboleda E, Hausen H, Arnone MI.. 2011. Unique system of photoreceptors in sea urchin tube feet. Proc Natl Acad Sci U S A. 108(20):8367–8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooi CJ, Stavenga DG, Arikawa K, Belušič G, Kelber A.. 2021. Evolution of insect color vision: from spectral sensitivity to visual ecology. Annu Rev Entomol. 66:435–461. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, Jan YN.. 2010. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature 468(7326):921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanini D, Giraldo D, Warren B, Katana R, Andrés M, Reddy S, Pauls S, Schwedhelm-Domeyer N, Geurten BRH, Göpfert MC.. 2018. Proprioceptive opsin functions in Drosophila larval locomotion. Neuron 98(1):67–74. [DOI] [PubMed] [Google Scholar]