Introduction
Scientists have explored gene drive technologies with the aim of controlling vector-borne diseases, including malaria, which killed an estimated 386,000 people in 2019.1 Gene drives are genetic modifications that can be used to create populations where a particular gene is passed from a parent organism to its offspring at a higher rate than would be expected under natural inheritance conditions.2 For example, mosquitoes that transmit malaria may be modified with a gene drive so that surviving offspring inherit and spread a gene that inhibits or alters the mosquitoes' infection potential. The drive would spread over subsequent generations, resulting in limited malaria transmission. Other gene drive strategies are designed to dramatically reduce the population of the carrying organism, such as a mosquito. Due to the broad-acting and potentially irreversible nature of these tools, current regulations may not be sufficient to mitigate the unique risks posed by gene drive technologies.3
A great deal of attention has been focused on the potential risks of gene drives, the kinds of biosafety protections they may require, and how they may be reversed; however, less attention has been paid to the systems that would be useful to have in place in the future, when multiple gene drives may be fielded in multiple species, environments, and countries.4-7 The need for coordinated governance of these technologies will become more pressing as gene drive technologies advance and more drives are created to address other vector-borne diseases like the West Nile virus, agricultural pest management, or invasive species. Gene drives carry different inherent risks compared with other genetically modified organisms (GMOs). Existing governance mechanisms for traditional GMOs are insufficient for oversight of gene drives, which require different systems to assess their usefulness and safety. To address the needs for enhanced oversight, we propose a tiered registry system, similar to the clinical trials databases, which can provide government officials, researchers, biotechnology companies, and the public with useful information about ongoing gene drive research or previously released gene drives. Such a resource would enable scientists to confirm that new gene drives would not interfere with existing drives, provide the public with the information needed to make informed decisions concerning consent for release of gene drives, provide researchers with technical information needed to prevent collisions of independent projects modifying the same organism, and provide regulators with information critical for effective oversight. We propose that the US government should implement such a registry for gene drives in the United States, which does not have a robust gene drive regulatory system in place and is not party to the international treaty most relevant for international gene drive regulation, the Convention on Biological Diversity.8 In this commentary, we describe current efforts to safely regulate gene drive and similar genetic technologies worldwide and how the United States could build a tiered registry database that is specifically designed to regulate such technologies throughout a drive's life cycle.
The Growing Need for Gene Drive Governance
In May 2021, Oxitec released genetically modified Aedes Aegypti mosquitoes into the wild in the United States, despite objections from some local community members.9 These mosquitoes, while not containing a gene drive, represent a GMO forerunner to gene drives that may be released in the future and serve as an important lesson in the consequences of a lack of regulation and oversight. The mosquitoes released by Oxitec are known as OX513A. They are male A Aegypti that have been genetically modified so all female progeny die, thus creating a severe imbalance between sexes in the local community that can lead to population decline.10 These GMO mosquitoes must be continually released to maintain population suppression, whereas gene drive organisms are self-propagating. Although the Oxitec GMO mosquitoes were ultimately approved by the US Environmental Protection Agency for a field trial release with an experimental use permit following an environmental impact assessment,11 the controversy surrounding this event underscores the need for a specific regulatory category for gene drives in the United States.
The United States regulatory environment is such that several federal agencies could potentially have regulatory authority over a gene drive depending on its application. US jurisdictional regulation is product-based; the regulatory process is more influenced by the population the product is meant to be used in or the desired outcome of the product, rather than the use of a gene drive or similar technological approach. The US Food and Drug Administration, US Department of Agriculture, and US Environmental Protection Agency all have regulations on GMOs, and based on those regulations, are currently the most relevant agencies to regulate gene drives, although other agencies may become relevant as biotechnology's role expands with time. The exact regulations that dictate jurisdiction are extensive and complex, but none explicitly define or mention gene drive technologies. Because gene drives are distinct from GMOs, a clear legal definition is needed, which other regulatory bodies worldwide have recognized.
Although gene drives are technically GMOs, their potential for self-spread and persistence in the environment and their ability to alter wild species sets them apart from other GMOs. Despite the unique issues gene drives present compared with other GMOs, gene drives do not have a clear definition or delineation from other GMOs in current US regulatory language.12 Gene drives can be designed for use in both cultivated and wild organisms, but their primary comparative advantage is for use in wild species where there is limited or no human capability to spread the desired gene throughout an ecosystem via other means, as in the malaria use case. Conversely, GMO species are typically designed to live in a cultivated setting where they will not spread through, nor dominate the genetics of, a closely related wild species. The United States has extensive regulatory oversight of GMOs, but clear and defined regulations are also needed for gene drives and other GMOs that are designed to spread through wild populations, to ensure environmental protection and safety.
Several countries have acknowledged and addressed the need to specifically regulate gene drives and similar technologies to minimize potential harms, caused directly or indirectly, to local ecology and public health. Despite gaps in regulatory structures, there have been extensive nonregulatory efforts to mitigate potential risks associated with gene drives, including stringent biosafety rules for creating and testing potential drives, broad multidisciplinary teams to provide oversight to gene drive projects, comprehensive risk assessment guidelines, and calls for creating reversal drives in tandem with the gene drive of interest.5,13,14 The Cartagena Protocol on Biosafety to the Convention on Biological Diversity covers living modified organisms (LMOs) within its regulatory framework, and the organisms carrying gene drives are LMOs.15 The Cartagena Protocol established the Biosafety Clearing-House to “facilitate the exchange of information on LMOs and assist the Parties to better comply with their obligations under the Protocol.”16 While information about gene drives may be reported to this clearinghouse as part of an LMO, there is currently no requirement to demonstrate stakeholder engagement. The European Union similarly hosts a GMO registry that would include gene drives, but it does not include provisions needed to account for the unique ecological risk gene drives pose compared with other GMOs.17 Neither of these international registries adequately address the need for coordination and detailed information sharing between scientists, regulators, and the public for gene drives. Additionally, the United States is not a party to the Convention on Biological Diversity nor a member of the European Union, leaving the United States without any registry for gene drive technologies.
Why a Registry and Why Tiered?
Effective governance for gene drives, and similar genetic technologies with the intention of widespread population modification, will rely on decision makers having access to critical information to inform risk assessments and assess liability. We, among others, propose a registry as an ideal mechanism for governance of these technologies.18,19 Registries have been successful in providing critical information for both regulators and the public in the past, such as with the comprehensive ClinicalTrials.gov website. Registries, when implemented well, are not just a tool for oversight and regulation, but also a resource that improves transparency, empowers the public, and enables communication between technologists.
Risk Assessments
In the United States, regulators conduct risk assessments when reviewing a product for approval or use to determine the risks the technology may pose to the environment, health, and public safety. Risk assessments, while never perfect, are critical exercises that inform decision makers by providing information about the potential impacts of a technology. This information is critical when weighing the benefits and risks of approving a technology.20 A registry would supply much of the input data for a risk assessment and make it available to regulators and the general public to conduct their own risk assessments. A registry could also act as a resource to investigators as a starting point should there be ecological or public health issues suspected to be caused by a gene drive.
Communication Between Technologists
Policymakers and decision makers are not the only stakeholders that could benefit from a gene drive registry. It would also provide technology developers with a forum where they could communicate. If gene drives become a common biotechnological intervention, it could be possible for different constructs in the same species to coexist or interact with each other. Developing a mandatory registry would enable technologists to check if their proposed drive systems could potentially interact with (or be redundant to) a previously released drive in the same target population, or a drive that might plausibly interbreed with their target population, either through genetic or ecological mechanisms. A major risk of gene drives is the potential unknown consequences of unpredicted spread or interactions; avoiding interactions between different gene drive organisms should be a paramount priority as potential interactions expand the risk of unknown consequences.
Empowering the Public
Many of the current gene drive projects discuss the importance of stakeholder engagement, public approval, and transparency. Going forward, all gene drive and gene drive-adjacent technologists should do the same. A registry enables such transparency by making the data available to the public. Having the data publicly available, however, is only the first step in educating public stakeholders on the benefits and risks of gene drives. The public must be empowered to make informed decisions concerning the testing and release of such technologies within their communities. A successfully implemented and advertised registry is the first step for true stakeholder engagement and public oversight. If more for-profit companies enter the gene drive field, it would be helpful to have a mandate for this information to be available to the public, and in a standardized format, as financial pressures could otherwise encourage companies to keep technical and release details proprietary.
Balancing Intellectual Property Protections with Transparency
There may be concerns from researchers that their technologies could be used without authorization if details are made publicly available too early in the research and development process. On the other hand, the public has a right to information about ongoing research and development of technologies that have the potential to cause harm to public health and the surrounding environment. The tension between intellectual property protection and public engagement can be mitigated through careful design of mandated information at different tiered levels within the registry.
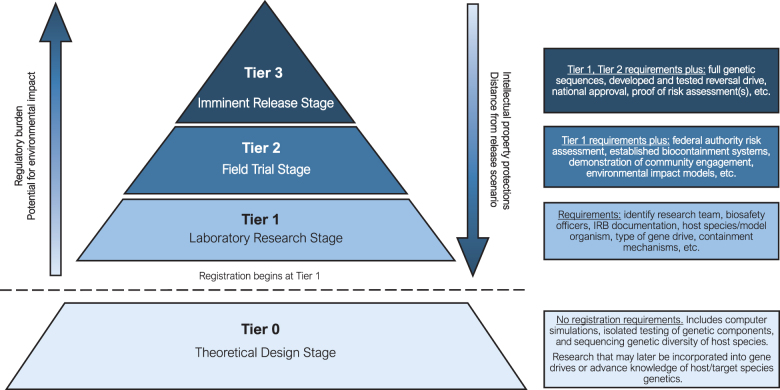
The tiering scheme we propose is designed to balance transparency with intellectual property protection by increasing the level of detail provided by the registry as a project advances toward application. Researchers and technologists would be able to keep their work private and protected while in early phases, but would be mandated to provide more information as a gene drive or similar product moves closer to a field release stage (Figure 1). As the possibility of an authorized release of a technology becomes more imminent, more information on the specifics of the product must be made available to inform independent risk assessments. The proposed tiered system also distinguishes at which level a gene drive research project should even be included within the registry at all. In the early stages, when investigators are only beginning to conceptualize the details of a technology or model a system, no information would need to be shared as the potential risk of release is negligible. As the project matures, researchers must share certain technical details that will enable them to avoid collisions of each other's efforts, such as interactions between different gene drives in the same host. Such collisions are not low-probability events given the amount of interest in using mosquitoes as host organisms. Once a gene drive cassette is inserted into a living organism, the basic information about the cassette, host species, and research lead would be shared. As the development proceeds to field trials and release, more detailed technical information, risk assessments conducted by the investigators and any external parties, and evidence of stakeholder engagement and approval would be made publicly available through the registry.
Figure 1.
Proposed tiered registry for gene drive governance, with descriptions of the requirements associated with each tier. Abbreviation: IRB, institutional review board. Color images are available online.
Historical Example: ClinicalTrials.gov
The ClinicalTrials.gov registry for clinical trials has been successful in enabling better governance, coordination, and transparency for clinical trials. In 1997, the US Congress passed the Food and Drug Administration Modernization Act of 1997, which required the US National Institutes of Health to create a publicly available resource to track clinical trials regulated by the US Food and Drug Administration to provide trial participants and their family members, the medical community, researchers, and the public with relevant information about ongoing and previous clinical trials.21 The need for such information was raised to the national stage by gay rights advocates. Widespread prejudice and miscommunication concerning HIV drug trials contributed to many gay men not being able to access information about such trials, which resulted in the development of the AIDS Clinical Trials Information Service database. The AIDS Clinical Trials Information Service was used as a model to create the subsequent database for tracking most clinical trials in the United States, ClinicalTrials.gov. Since ClinicalTrials.gov was launched in 2000, the information provided on the site and types of trials required to be listed has expanded. Today, ClinicalTrials.gov enables the public, medical professionals, researchers, and regulators to easily find information about any clinical trial conducted or supported by several federal agencies. It has been invaluable for standardizing the type of information reported, reducing redundancy, empowering the public to find useful information, and guiding the development for more transparent norms and governance of clinical trials in the country. ClinicalTrials.gov has contributed to developing international norms for clinical trials governance and reporting. A similar database for gene drives and related technologies could also contribute to international efforts toward creating norms for governance of these technologies.
Implementation of the Tiered Registry
There are many options for what kind of organization could administer and fund a tiered registry for gene drives and similar technologies in the United States. In this section, we explore the advantages, disadvantages, and tradeoffs of such options. Broadly, a registry could be administered through one of the following entities:
An industry-led body – such as the International Gene Synthesis Consortium in the synthetic biology space
A nongovernment organization – such as the Foundation for the National Institutes of Health, which has relevant expertise in the gene drive space
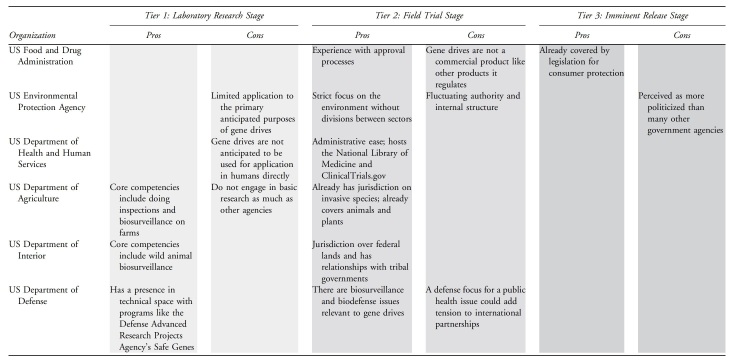
One or more government agencies – see Table 1 for a partial list of likely candidates
Table 1.
Pros and Cons of Different Agencies to Manage a Tiered Registry for Gene Drives
|
In considering the advantages and disadvantages of various administrative entities for a tiered gene drive registry, it is important to remember that the multiple tiers of the registry do not correspond only to differing degrees of technological disclosure, but they also accomplish various underlying goals of the registry itself (Figure 1). Tier 1 is the laboratory research stage, with the primary purpose of preventing technical collisions and ensuring technical best practices. Tier 2, the field trial stage, enables regulatory oversight and, while still technical in scope, would focus more on ecological modeling and risk assessment. Tier 3, the imminent release stage, emphasizes public engagement and transparency—this should be sought throughout all tiers, but in anticipation of near-term release it would become the overriding priority as technical and safety questions begin to be more firmly answered.
An industry-led consortium could have all of the diverse and specific scientific and technical expertise needed to test and evaluate individual gene drives. Although this technical competency would make them well suited to administer Tier 1, and to some degree Tier 2, the companies' inherent focus on also making a profit could cause the public to assume that the consortium has research bias or a conflict of interest, thus sabotaging the purpose of Tier 3. Even if the companies and/or their researchers do not consider the impacts of their risk assessments on their profits, it will be hard for the public to accept them as fair or impartial stewards of the registry.
A nongovernmental organization (NGO) might not have the automatic assumption of conflict of interest as an industry consortium might, which may encourage more open sharing of information from development teams. NGOs fall into many classifications, however, including those with openly political advocacy agendas, so their perceived neutrality is not assured.22 Still, an initiative led by an NGO could recruit the relevant technical expertise and have markedly more flexibility to adapt to changing needs of the database than a government entity might.
The government has access to many specialists who can be loaned across agencies, or hired as contractors from outside, to provide domain-specific expertise as needed. However, individual government agencies are restricted to operate within predefined jurisdictions, both geographic and by application or subject matter. This makes the specific agencies participating in the administration of a gene drive agency potentially limiting in its roles by tier or its applicability to specific gene drive projects. Regardless of the primary administrative organization of a tiered gene drive registry, it would benefit from broad input from organizations and entities outside its own confines. If the registry will be mandatory, then a federal agency will likely be the most appropriate choice for host.
Funding for a tiered registry could come from several sources such as the US federal government or through philanthropic societies interested in governance of genetic technologies. The appropriate funder will likely depend on the host of the registry. The amount of funding needing to maintain the registry will also depend on the host and how the registry is implemented. If the registry is mandatory, and developers have a responsibility to conduct the risk assessments and ensure appropriate stakeholder engagement, then the host of the registry does not necessarily have to employ or contract experts to complete these steps. Alternatively, if the registry is voluntary with support from technologists, funders, the public, and government officials, then there may be political will to provide funding for the host of the registry to also conduct the risk assessments and help direct community outreach.
Conclusion
A tiered registry, as proposed in this commentary, would improve transparency for gene drive and similar genetic technologies research and development. Building trust with communities that would host gene drive organisms will require honesty and communication to the public and among technologists. It is an ethical imperative that the research and development of these technologies be transparent. A registry would serve this purpose while enabling such work to advance.
References
- 1. World Health Organization (WHO). World Malaria Report 2020. Geneva: WHO; 2020. Accessed July 27, 2021. https://www.who.int/publications-detail-redirect/9789240015791
- 2. Wedell N, Price TAR, Lindholm AK. Gene drive: progress and prospects. Proc Biol Sci. 2019;286(1917):20192709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frieß JL, von Gleich A, Giese B. Gene drives as a new quality in GMO releases—a comparative technology characterization. PeerJ. 2019;7:e6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Delborne J, Kuzma J, Gould F, Frow E, Leitschuh C, Sudweeks J. Mapping research and governance needs for gene drives. J Responsible Innov. 2018;5(suppl 1):S4-S12. [Google Scholar]
- 5. Oye KA, Esvelt K, Appleton E, et al. Regulating gene drives. Science. 2014;345(6197):626-628. [DOI] [PubMed] [Google Scholar]
- 6. Akbari OS, Bellen HJ, Bier E, et al. Safeguarding gene drive experiments in the laboratory. Science. 2015;349(6251):927-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Champer J, Chung J, Lee YL, et al. Molecular safeguarding of CRISPR gene drive experiments. eLife. 2019;8:e41439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thizy D, Coche I, de Vries J. Providing a policy framework for responsible gene drive research: an analysis of the existing governance landscape and priority areas for further research. Wellcome Open Res. 2020;5:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Filosa G. In Florida Keys, a controversial project releases genetically modified mosquitoes. Miami Herald. April 27, 2021. Accessed July 27, 2021. https://www.miamiherald.com/news/local/community/florida-keys/article250977309.html
- 10. Phuc HK, Andreasen MH, Burton RS, et al. Late-acting dominant lethal genetic systems and mosquito control. BMC Biol. 2007;5(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kofler N, Kuzma J. Before genetically modified mosquitoes are released, we need a better EPA. Boston Globe. June 22, 2020. Accessed October 27, 2021. https://www.bostonglobe.com/2020/06/22/opinion/before-genetically-modified-mosquitoes-are-released-we-need-better-epa/
- 12. Alphey LS, Crisanti A, Randazzo F, Akbari OS. Opinion: standardizing the definition of gene drive. Proc Natl Acad Sci U S A. 2020;117(49):30864-30867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Target Malaria. Our approach. Accessed July 27, 2021. https://targetmalaria.org/what-we-do/our-approach/
- 14. Devos Y, Bonsall MB, Firbank LG, Mumford J, Nogué F, Wimmer EA. Gene drive-modified organisms: developing practical risk assessment guidance. Trends Biotechnol. 2021;39(9):853-856. [DOI] [PubMed] [Google Scholar]
- 15. Keiper F, Atanassova A. Regulation of synthetic biology: developments under the Convention on Biological Diversity and its protocols. Front Bioeng Biotechnol. 2020;8:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Convention on Biological Diversity. Information sharing. Accessed December 3, 2021. https://bch.cbd.int/protocol/cpb_art20.shtml
- 17. Bruetschy C. The EU regulatory framework on genetically modified organisms (GMOs). Transgenic Res. 2019;28(suppl 2):169-174. [DOI] [PubMed] [Google Scholar]
- 18. African Union Development Agency, Foundation for the National Institutes of Health. 4th meeting of the Gene Drive Research Forum. Accessed November 30, 2021. https://fnih.org/sites/default/files/pdf/SUMMARY%20-%204th%20Gene%20Drive%20Research%20Forum%20FINAL.pdf
- 19. Warmbrod KL, Kobokovich A, West R, Ray G, Trotochaud M, Montague M. Gene Drives: Pursuing Opportunities, Minimizing Risk. Baltimore, MD: Johns Hopkins Center for Health Security; 2020. Accessed December 10, 2021. https://www.centerforhealthsecurity.org/our-work/pubs_archive/pubs-pdfs/2020/200518-Gene-Drives-Report.pdf
- 20. Golnar AJ, Ruell E, Lloyd AL, Pepin KM. Embracing dynamic models for gene drive management. Trends Biotechnol. 2021;39(3):211-214. [DOI] [PubMed] [Google Scholar]
- 21. Food and Drug Administration Modernization Act of 1997, Pub L No. 105-115 (1997). Accessed December 10, 2021. https://www.congress.gov/105/plaws/publ115/PLAW-105publ115.pdf
- 22. Piotrowicz M, Cianciara D. The role of non-governmental organizations in the social and the health system. Przegl Epidemiol. 2013;67(1):69-74,151-155. [PubMed] [Google Scholar]